Abstract
Introduction
A variety of industry composite indices are employed within health research in risk-adjusted outcome measures and to assess health-related social needs. During the COVID-19 pandemic, the relationships among risk adjustment, clinical outcomes, and composite indices of social risk have become relevant topics for research and healthcare operations. Despite the widespread use of these indices, composite indices are often comprised of correlated variables and therefore may be affected by information duplicity of their underlying risk factors.
Methods
A novel approach is proposed to assign outcome- and disease group−driven weights to social risk variables to form disease and outcome−specific social risk indices and apply the approach to the county-level Centers for Disease Control and Prevention social vulnerability factors for demonstration. The method uses a subset of principal components reweighed through Poisson rate regressions while controlling for county-level patient mix. The analyses use 6,135,302 unique patient encounters from 2021 across 7 disease strata.
Results
The reweighed index shows reduced root mean squared error in explaining county-level mortality in 5 of the 7 disease strata and equivalent performance in the remaining strata compared with the reduced root mean squared error using the current Centers for Disease Control and Prevention Social Vulnerability Index as a benchmark.
Conclusions
A robust method is provided, designed to overcome challenges with current social risk indices, by accounting for redundancy and assigning more meaningful disease and outcome−specific variable weights.
INTRODUCTION
There is increasing demand in the healthcare industry to understand and adjust for social factors that may be associated with disparities in health outcomes.1, 2, 3, 4, 5 A more informed understanding of the relationship between social determinants and clinical outcomes may improve fairness in the risk adjustment of metrics within regulatory programs that impact Inpatient Prospective Payment System payment.5, 6, 7, 8, 9 Other programs, although not tied to hospital payment, publish grades or hospital rankings that can affect an organization's industry reputation.10, 11, 12, 13, 14
Outcome measures included in these programs are risk-adjusted using patient comorbidities and other patient characteristics; however, none adjust for social factors known to be associated with disparities in health outcomes. Hospitals serving historically marginalized communities may be unfairly penalized in such programs.15, 16, 17 Such redistribution of resources brings to light policy issues related to social determinants of equity, which Camara Jones aptly defines as, “interventions on the structures, policies, practices, norms, and values, that differently distribute resources and risks…”.15 , 16 , 18
A wide range of social risk indices has been put forth in the literature, such as the Centers for Disease Control and Prevention Agency for Toxic Substances and Disease Registry Social Vulnerability Index (SVI), Minority Health Social Vulnerability Index, Social Deprivation Index, and Area Deprivation Index. Such indices are comprised of social risk variables across various socioeconomic and sociodemographic domains.19, 20, 21, 22, 23, 24 Although absent in hospital ranking programs, the utility of such indices in risk adjustment to help explain aspects of disparity as they relate to health outcomes has also been shown.22 , 25, 26, 27, 28, 29
Index development continues to mature, overcoming various methodologic concerns along the way. A primary concern is the potential to over- or under-represent the influence of underlying aspects of disparity owing to the aggregation of variables with similar information content into a singular index. As Krieger et al. state in reference to the use of overall indices, “One concern is that combining measures of income and education into one index… can conflate pathways and obscure each component's distinct-and conceivably different-contribution to specified health outcomes.”30 More robust methods have been shown in recent research, such as the use of principal component analysis (PCA) to identify latent aspects of social risk spanning the range of variables used within the composite. Singh et al., for example, developed an index on the basis of the first principal component derived from a curated set of SES variables.25
The second concern relates to methods used to weigh variables or principal components in the case of PCA within an overall composite. The National Quality Foundation (NQF) has put forth 10 recommendations relating to the use of social risk factors within clinical measurement.5, 31 Among these recommendations, the NQF suggests that social risk factors should demonstrate (1) “clinical/conceptual relationship with the outcome of interest”; (2) “empirical association with the outcome of interest,” and (3) “contribution of unique variation in the outcome (i.e., not redundant).”31 Braveman et al. corroborate this position by advocating for the use of outcome- and social group−specific measures of SES.32 Kolak et al. employed a PCA index method but recommends the use of multiple components (i.e., principal components) to capture the unique association between the latent aspects of social risk and the outcome being measured rather than a singular index.26
It has long been established that disease states can be sensitive to social risk factors. For instance, although housing air quality and other social factors may correlate with asthma hazards,33 , 34 the models may fail without a disease-specific approach.35 By identifying the social risk factors most relevant to each health outcome, policies can be designed to overcome the disparities in those health outcomes by factor. Thus, such outcome-specific identification can help to allocate resources efficiently where disparities due to such factors may be most influential to enhance health outcomes. Although it is true that those factors may relate to health outcomes in intricate, complex forms, it is essential to identify root causes to address these disparities.
Robust social indices should employ methods to ensure that unique aspects are appropriately captured and meaningfully weighed in the context of health outcomes and disease states, in line with how clinical patient characteristics are weighed differently when constructing outcome and disease−specific risk indicators, such as validated clinical determinants of cardiovascular disease events.36 , 37 With these best practices in mind, a methodologic approach to index formulation is proposed that (1) identifies unique latent aspects of social risk and (2) appropriately weighs aspects of social risk on the basis of their unique association to an outcome within a specific disease group. Although the methodologic approach in this study was applied to variables used within the SVI, the approach is generalizable and can be applied to other sets of social factors, such as those included within other composite indices (Area Deprivation Index, Minority Health Social Vulnerability Index, Social Deprivation Index, and others).
METHODS
Study Sample
Acute inpatient hospitalizations from the year 2021 were extracted from the Premier Healthcare Database (PHD), a private all-payer administrative database.38 All patient data are deidentified, so this study is exempt from IRB approval. To show the proposed approach, clinical cohorts frequently used within hospital regulatory and private hospital ratings programs were evaluated, including acute myocardial infarction (AMI), heart failure (HF), perinatal and related conditions (PR), pneumonia (PN), stroke (STK), and total hip and knee arthroplasty (THA/TKA).7 , 10 , 12 , 13 These cohorts were identified by the ICD-10 principal diagnosis associated with the hospitalization. A coronavirus disease (COVID-19) cohort was also included, given its relevance to current research. A principal or secondary diagnosis of COVID-19 was used to identify COVID-19 hospitalizations. The number of hospitalizations varied by cohort (Table 1) .
Table 1.
County and Patient Hospitalization Counts by Disease Group
| Disease group | PHD counties (n) | PHD percentage of all U.S. counties | PHD hospitalizations (n) |
|---|---|---|---|
| AMI | 2,843 | 90% | 364,094 |
| COVID-19 | 3,023 | 96% | 1,398,265 |
| HF | 2,852 | 91% | 774,251 |
| PN | 2,872 | 91% | 738,269 |
| PR | 2,896 | 92% | 2,207,649 |
| STK | 2,829 | 90% | 429,232 |
| THA/TKA | 2,629 | 84% | 223,542 |
AMI, acute myocardial infarction; HF, heart failure; PN, pneumonia; PR, perinatal and related conditions; STK, stroke; THA/TKA, total hip and knee arthroplasty.
The extracted data elements included patient age, sex, Federal Information Processing Standards (FIPS) county code of the primary patient residence, Clinical Classification Software Refined (CCSR)39 grouping of the principal ICD-10 code associated with the hospitalization, and an indicator of mortality during hospitalization.
County factors describing social risk across 3,142 counties were extracted from the 2018 Centers for Disease Control and Prevention vulnerability data set.23 These 15 variables represent aspects of social risk across 4 data domains (Table 2 ).23 The overall SVI index, an equally weighted aggregation of these social factors, was also extracted.23, 40
Table 2.
CDC SVI Variable Descriptions by Domain
| Variable name | Variable description |
|---|---|
| SES | |
| ep_pov | Percentage of persons below poverty |
| ep_unemp | Percentage of civilians (aged 16+ years) unemployed |
| ep_pci_ra | Per capita income |
| ep_nohsdp | Percentage of persons with no high-school diploma (aged 25+ years) |
| Household composition/disability | |
| ep_age_65 | Percentage of persons aged ≥65 years |
| ep_age_17 | Percentage of persons aged ≤17 years |
| ep_disabl | Percentage of civilian non-institutionalized population with a disability |
| ep_sngpnt | Percentage of single-parent households with children aged <18 years |
| ep_minrty | Percentage of minority population (all persons except White, non-Hispanic) |
| Minority status and language | |
| ep_limeng | Percentage of persons (aged 5+ years) who speak English less than well |
| ep_munit | Percentage housing in structures with 10 or more units |
| Housing type and transportation | |
| ep_mobile | Percentage of mobile homes |
| ep_crowd | Percentage of households with more people than rooms |
| ep_noveh | Percentage of households with no vehicle available |
| ep_groupq | Percentage of persons in institutionalized group quarters |
Source: CDC SVI Documentation 2018 | Place and Health | Agency for Toxic Substances and Disease Registry. Accessed January 21, 2022.
Log transformed and reversed.
CDC, Centers for Disease Control and Prevention; SVI, Social Vulnerability Index.
The per capita income variable (ep_pci), exhibiting a strong rightward skew, was the only variable used in the SVI that was not reported as a percentage and was expected to be inversely correlated with social vulnerability. The ep_pci variable was therefore log transformed and reversed by subtracting each value from the maximum log value in the data set, with the resulting variable renamed as epi_pci_r. The values for ep_pov, ep_unemp, ep_pci, and rpl_themes were missing for Rio Arriba County, NM (FIPS County Code=35,039), and it was the only county excluded from the analyses owing to missing data. The resulting SVI county dataset is comprised of 3,141 counties.
Patient CCSR groupings, included as control variables, were highly imbalanced for many cohorts, with most of the patient volume represented by a small subset of CCSR categories. To reduce dimensionality, CCSR groupings with an occurrence frequency <0.05% within each disease stratum were set to an Other CCSR category. The percentages of data grouped to the Other CCSR category were 0.43% (AMI), 15.4% (COVID-19), 1.16% (HF), 6.72% (PN), 17.6% (PR), 11.5% (STK), and 5.36% (THA/TKA) for each of the 7 cohorts evaluated in this study.
Statistical Analysis
A disease and outcome−specific SVI (DOS-SVI) was produced on the basis of an outcome-driven reweighing of the 15 individual SVI factors while controlling for patient mix within each county. A risk-adjustment model developed at the patient hospitalization level was first developed to assess patient-level risk, which was subsequently aggregated at the county level. The second county-level model was designed to measure the association between the 15 social risk factors and county-level mortality while controlling for expected mortality extracted from the previous hospitalization-level model.
Patient-level risk was estimated through generalized additive models, stratified by clinical cohort. For each generalized additive model, the binary occurrence of mortality was regressed on patient age, sex, and CCSR grouping. Without loss of generality, other patient-level characteristics can be added to the model, if available. Age was modeled as a thin plate regression spline owing to its potential nonlinear relationship with the probability of mortality for some disease strata. Observed mortality outcomes and fitted mortality probabilities were then summed by county, using the FIPS county code, which served as inputs to the subsequent county-level model. County-added observed outcomes represent actual county mortality counts by stratum, whereas county-added probabilities represent the corresponding expected mortality counts.
Total observed and expected cases by county were linked to the SVI variables, such that each observation represents a unique FIPS county code. Although the SVI data include county-level information for 3,141 distinct counties, patient data were only available for a subset of the total U.S. counties by cohort within the PHD (Table 1).
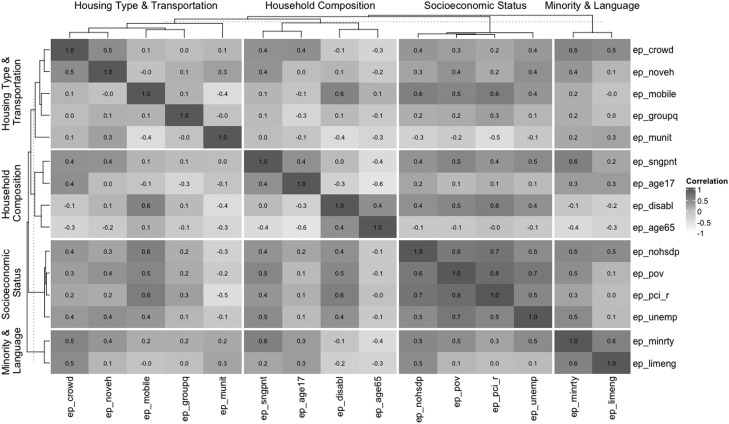
As shown in Figure 1 , the SVI variables exhibit varying degrees of multicollinearity. To reduce information redundancy, social vulnerability principal components were extracted and used as inputs in the analysis. To extract additional sources of variability of mortality counts at the county level, Poisson rate regression models were fit for each disease stratum, regressing total observed county-level cases of mortality on 9 principal components, with expected cases as the offset variable. The choice of 9 principal components was made through an arbitrary inclusion threshold of 90% of the cumulative SVI variance, with 9 principal components explaining 91.46% of the variation of the SVI variables.
Figure 1.
Correlation matrix of social risk factors.
ep_pov denotes the percentage of persons below poverty, ep_unemp denotes the percentage of civilians (aged 16+ years) unemployed, ep_pci_r denotes per capita income, ep_nohsdp denotes the percentage of persons with no high school diploma (aged 25+ years), ep_age_65 denotes the percentage of persons aged ≥65 years, ep_age_17 denotes the percentage of persons aged ≤17 years, ep_disabl denotes the percentage of civilian non-institutionalized population with a disability, ep_sngpnt denotes the percentage of single-parent households with children aged <18 years, ep_minrty denotes the percentage of minority population (all persons except White, non- Hispanic), ep_limeng denotes the percentage of persons (aged 5+ years) who speak English less than well, ep_munit denotes the percentage housing in structures with 10 or more units, ep_mobile denotes the percentage of mobile homes, ep_crowd denotes the percentage of households with more people than rooms, ep_noveh denotes the percentage of households with no vehicle available, and ep_groupq denotes the percentage of persons in institutionalized group quarters.
The coefficients for each of the principal components were normalized, multiplied by their respective principal components, and summed, resulting in the reweighed overall composites. Analyses were conducted using R statistical software, Version 3.6.2 (R Foundation for Statistical Computing).41
To measure the benefit of the DOS-SVI, 2 benchmarks were calculated. The first benchmark, designed to measure the impact of excluding social risk factors altogether, was calculated as a model-free estimate through the root mean squared error (RMSE) between the county-level expected and observed cases—referred to as the null model in this paper. The second benchmark was based on disease-specific Poisson rate regression models, using the SVI as the single explanatory variable while adjusting for expected county-level mortality. The resulting RMSE was designed to measure the fit of the current SVI compared with the proposed alternative within a common Poisson model.
RESULTS
Table 3 lists the RMSEs for the proposed model and the 2 benchmark values across strata. Among the 3 models, the principal component model reduces RMSE across all the 7 cohorts compared with the null model. In comparison with the benchmark model, the principal component model reduces RMSE for 5 of the 7 cohorts, with equivalent performance in the remaining 2 cohorts. The most salient shifts from the null model can be seen in the COVID-19 and PN cohorts, with RMSE reduced from 48.85 to 40.94 and 17.61 to 13.85, respectively.
Table 3.
RMSE by Model Type and Disease Group
| Disease group | Null model RMSE | SVI RMSE | Principal component RMSE | RMSE percent reductiona |
|---|---|---|---|---|
| AMI | 4.33 | 4.23 | 3.93 | 7.09% |
| COVID-19 | 48.85 | 50.00 | 40.94 | 18.1% |
| HF | 6.68 | 6.64 | 6.00 | 9.64% |
| PN | 17.61 | 17.90 | 13.85 | 22.6% |
| PR | 0.28 | 0.27 | 0.27 | 0.0% |
| STK | 7.87 | 7.96 | 7.09 | 10.9% |
| THA/TKA | 0.24 | 0.23 | 0.23 | 0.0% |
RMSE reduction column corresponds to the change from the SVI to principal component model.
AMI, acute myocardial infarction; HF, heart failure; PN, pneumonia; PR, perinatal and related condition; RMSE, root mean squared error; STK, stroke; SVI, Social Vulnerability Index; THA/TKA, total hip and knee arthroplasty.
The DOS-SVI deviated from the SVI at varying degrees depending on the disease stratum being evaluated. The SVI is a percentile rank of the domain aggregates, and therefore to compare fairly with the DOS-SVI, the difference in the percentile rank of the DOS-SVI and SVI is evaluated in Appendix Figure 1 (available online). This comparison shows that the AMI, COVID-19, PN, PR, and STK cohorts aligned most closely with the SVI, with residual standard deviations of 0.19, 0.23, 0.26, 0.28, and 0.28, respectively. The HF and THA/TKA cohorts varied to a greater degree with SDs of 0.34 and 0.36, respectively.
Owing to the orthogonal nature of the PCA, results in each component capture different latent aspects of social risk. The scree plot in Appendix Figure 2 (available online) shows the cumulative variability of the underlying data explained by the principal components derived from the SVI variables, with the first 2 principal components explaining more than 53% of the variance of the 15 variables. Appendix Figure 3 (available online) shows the correlation between each of the derived principal components and the raw SVI variables.
The correlation between the derived DOS-SVIs and the raw social risk variables (Appendix Figures 4–10, available online) can help with the interpretability of the results. The AMI, COVID-19, and PR cohorts have a clear set of social risk factors associated with mortality risk. The AMI DOS-SVI is correlated with the percentage of households with more people than rooms (r=0.754, p<0.001) and unemployment (r=0.732, p<0.001). The COVID DOS-SVI is correlated with income (r=0.667, p<0.001) and the percentage of households with more people than rooms (r=0.678, p<0.001). The PR DOS-SVI is associated with income (r=0.724, p<0.001), percentage of mobile homes (r=0.724, p<0.001), disability (r=0.743, p<0.001), unemployment (r=0.703, p<0.001), poverty (r=0.757, p<0.001), and completion of high school (r=0.618, p<0.001). The PN DOS-SVI is correlated with the percentage of households with more people than rooms (r=0.816, p<0.001). The HF, STK, and THA/TKA DOS-SVIs are not largely correlated with raw risk factors, with an arbitrary correlation threshold of 0.6.
The beta coefficients for each principal component can give additional insight into the differing disease-specific association between the unique latent aspects of social risk and inpatient mortality (Appendix Table 1, available online). In addition to the residual index difference shown in Appendix Figure 1 (available online), a geographic representation of the county-level indices is provided in Appendix Figure 11 (available online), using the state of North Carolina as an example.
In-sample counties (i.e., counties with patient volume in the PHD) comprised a large proportion of the national total (Table 1). While out-of-sample counties had a relatively lower total population (Appendix Figure 12, available online). The DOS-SVI distributions for in- and out-of-sample counties are evenly distributed across disease strata (Appendix Figure 13, available online).
Significance of the disease-specific coefficients and the correlation between social risk factors and their respective indices emphasize the utility of disease-specific variable weights because an index comprising equally weighted variables would suffer from the impacts of multicollinearity when capturing the unique disease-specific association between mortality and aspects of social risk.
County population size is a factor in the alignment between the SVI and DOS-SVI. Appendix Table 1 (available online) shows the RMSE between the SVI and DOS-SVI across counties grouped by population quartiles. Apart from the HF disease group, the first and fourth quartiles have the lowest RMSE relative to the second and third quartiles, indicating stronger alignment at the population extremes. In the case of HF, the alignment between the 2 indices generally increases as county population size increases. The full set of DOS-SVI and their respective percentiles by cohort type are included in Appendix Tables 3−9 (available online).
DISCUSSION
Enhanced approaches for weighing the underlying aspects of social risk factors can help explain variation in mortality outcomes upon controlling for patient, age, sex, and disease group. In this application of disease-specific indices, the associations between social risk factors and mortality within disease strata are consistent with previous research, corroborating the importance of considering social risk in the context of disease strata.27 , 42, 43, 44, 45, 46 Although it is common in the literature to consider disease-specific clinical risk factors, it is still less common to consider social vulnerability factors or indices that are also disease specific. The proposed method addresses this gap in the literature. The approach put forth in this study is a computationally tractable model that is generalizable to other disease groups and outcomes. In addition, the approach can be implemented at different levels, such as the census block or patient level, providing data availability. This approach can also serve to reveal and/or empirically demonstrate the heterogeneity of mechanisms linking social determinants of health with specific diseases or health outcomes, especially because such associations are not expected to be homogeneous across diseases or health outcomes. This aligns with the NQF call for disease-specific metrics.5 When appropriate, the index formulation method shown in this study can be used to adjust for social factors within risk-adjusted outcomes and as a distribution to stratify populations.
Accounting for social factors in risk adjustment is necessary from a benchmarking perspective to ensure that hospitals and physicians are not unfairly penalized for serving historically disadvantaged communities.15, 16, 17 This is especially important in pay for performance and other publicly reported programs.10, 11, 12, 13, 14
Limitations
Despite these advantages, there are limitations to the proposed approach. Counties can be heterogeneous, and therefore county-level SVI variables can mask considerable disparities within counties.47 In these cases, the resulting principal component weights will be diluted through the county-level aggregation. To mitigate this limitation, future studies may need to include census tract−level variables.
Although the principal component model overcomes challenges with correlated variables, there is greater opacity in interpreting the principal components themselves. Conversely, the extracted PCA weights are disease-specific, aligning with NQF's recommendation, and further combine highly correlated variables, making the resulting composite a mixture of latent components with disease-agnostic undefined weightings by uncorrelated latent factor.
Finally, although the clinical mechanisms in which risk factors affect specific diseases are not explored, the proposed data-driven approach offers a unique opportunity to build social vulnerability indices that are disease appropriate and avoid the dilution implicit in one-size-fits-all approaches. The proposed approach can in fact be used as a stepping stone to explore such mechanisms upon demonstrating the relevant risk factors and associated principal components.
CONCLUSIONS
The proposed index formulation method has been designed to account for areas of increased disease-specific mortality risk associated with social risk factors. Regressing observed county-level mortality frequencies as a broader set of social risk factors while controlling for patient clinical and demographic characteristics reduces errors in risk-adjusted mortality estimates more than the county-level SVI. Social risk indices such as DOS-SVI and SVI may be associated with areas of risk surrounding a hospital or within a population associated with a health system. Applying the DOS-SVI index formulation method may enable further study of specific regions or populations adversely affected by social risk factors or potentially neglected by health structures. In turn, the DOS-SVI approach may enhance control for social factors in risk-adjustment modeling for population health or reveal specific gaps in healthcare operations.
Acknowledgments
ACKNOWLEDGMENTS
The Premier Healthcare Database is considered exempt from IRB oversight as dictated by Title 45 Code of Federal Regulations, Part 46 of the U.S., specifically 45 CFR 46.101(b)(4). In accordance with the Health Insurance Portability and Accountability Act Privacy Rule, disclosed data from the Premier Healthcare Database are considered deidentified per 45 CFR 164.506(d)(2)(ii)(B) through the Expert Determination method.
The authors received no specific funding for this work.
JM and MK are employed by and have stock in Premier, Inc. No other financial disclosures were reported.
CRediT AUTHOR STATEMENT
Michael Korvink: Conceptualization, Methodology, Formal analysis, Data curation, Writing–original draft, Writing–review & editing. Laura H. Gunn: Conceptualization, Methodology, Writing–review & editing, Supervision. German Molina: Conceptualization, Methodology, Writing–review & editing, Supervision. Dani Hackner: Writing–review & editing, Supervision. John Martin: Conceptualization, Methodology, Writing–original draft, Writing–review & editing, Supervision.
Footnotes
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2023.05.002.
Appendix. SUPPLEMENTAL MATERIAL
REFERENCES
- 1.CMS proposes policies to advance health equity and maternal health, Support Hospitals. https://www.cms.gov/newsroom/press-releases/cms-proposes-policies-advance-health-equity-and-maternal-health-support-hospitals. Accessed 31 October 2022.
- 2.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Care Services, Board on Population Health and Public Health Practice . In: Committee on accounting for socioeconomic status in medicare payment programs. Steinwachs DM, Stratton K, Kwan LY, editors. National Academies Press; U.S.: 2017. http://www.ncbi.nlm.nih.gov/books/NBK436064/ Accounting for Social Risk Factors in Medicare Payment. [PubMed] [Google Scholar]
- 3.Meddings J, Reichert H, Smith SN, et al. The impact of disability and social determinants of health on condition-specific readmissions beyond medicare risk adjustments: A cohort study. J Gen Intern Med. 2017;32(1):71–80. doi: 10.1007/s11606-016-3869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight–reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874–882. doi: 10.1056/NEJMms2004740. [DOI] [PubMed] [Google Scholar]
- 5.NQF. Risk adjustment for socioeconomic status or other sociodemographic factors–description. https://www.qualityforum.org/ProjectDescription.aspx?projectID=73517. Accessed 26 September 2022.
- 6.Senathirajah M, Dankwa-Mullan I, Pickens G, Benevent R, Spurlock B. A hospital social needs index would help hospitals collaborate to address social needs and health equity. Health Aff Forefront. 10.1377/forefront.20220729.222359. Accessed 2 August 2022. [DOI]
- 7.Hospital value-based purchasing program. CMS. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Hospital-Value-Based-Purchasing-. Accessed 31 January 2022.
- 8.Hospital-acquired condition reduction program. CMS. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/HAC-Reduction-Program. Accessed 31 January 2022.
- 9.Hospital Readmissions Reduction Program (HRRP), CMS. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program. Accessed 30 September 2022.
- 10.Overall hospital quality star ratings, CMS. https://qualitynet.cms.gov/inpatient/public-reporting/overall-ratings. Accessed 30 September 2022.
- 11.FAQ. How and why we rank and rate hospitals. U.S. News & World Report. https://health.usnews.com/health-care/best-hospitals/articles/faq-how-and-why-we-rank-and-rate-hospitals. Accessed 21 January 2022.
- 12.100 top hospitals. PINC ai. https://www.pinc-ai.com/100-top-hospitals. Accessed 9 March 2023.
- 13.Methodology for the fortune/Merative 100 top hospitals. Fortune. 2022 https://fortune.com/franchise-list-page/methodology-merative-100-top-hospitals-2022/ [Google Scholar]
- 14.Austin JM, D'Andrea G, Birkmeyer JD, et al. Safety in numbers: the development of leapfrog's composite patient safety score for U.S. hospitals. J Patient Saf. 2014;10(1):64–71. doi: 10.1097/PTS.0b013e3182952644. [DOI] [PubMed] [Google Scholar]
- 15.Ryan AM. Will value-based purchasing increase disparities in care? N Engl J Med. 2013;369(26):2472–2474. doi: 10.1056/NEJMp1312654. [DOI] [PubMed] [Google Scholar]
- 16.Chien AT, Wroblewski K, Damberg C, et al. Do physician organizations located in lower socioeconomic status areas score lower on pay-for-performance measures? J Gen Intern Med. 2012;27(5):548–554. doi: 10.1007/s11606-011-1946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffery JB, Safran DG. Addressing social risk factors in value-based payment: adjusting payment not performance to optimize outcomes and fairness. Health Aff Forefront. https://www.healthaffairs.org/do/10.1377/forefront.20210414.379479/. Accessed 19 May 2023.
- 18.Jones CP, Jones CY, Perry GS, Barclay G, Jones CA. Addressing the social determinants of children's health: a cliff analogy. J Health Care Poor Underserved. 2009;20(4)(suppl):1–12. doi: 10.1353/hpu.0.0228. [DOI] [PubMed] [Google Scholar]
- 19.Tipirneni R, Karmakar M, O'Malley M, Prescott HC, Chopra V. Contribution of individual- and neighborhood-level social, demographic, and health factors to COVID-19 hospitalization outcomes. Ann Intern Med. 2022;175(4):505–512. doi: 10.7326/M21-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Kind AJH, Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual. 2018;33(5):493–501. doi: 10.1177/1062860617753063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible–the neighborhood atlas. N Engl J Med. 2018;378(26):2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. 2013;48(2 Pt 1):539–559. doi: 10.1111/j.1475-6773.2012.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC. SVI documentation . 2018. Place and Health | ATSDR.https://www.atsdr.cdc.gov/placeandhealth/svi/documentation/SVI_documentation_2018.html Published January 21, 2022. [Google Scholar]
- 24.Maizlish N, Delaney T, Dowling H, et al. California healthy places index: frames matter. Public Health Rep. 2019;134(4):354–362. doi: 10.1177/0033354919849882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh GK, Siahpush M. Increasing inequalities in all-cause and cardiovascular mortality among US adults aged 25–64 years by area socioeconomic status, 1969–1998. Int J Epidemiol. 2002;31(3):600–613. doi: 10.1093/ije/31.3.600. [DOI] [PubMed] [Google Scholar]
- 26.Kolak M, Bhatt J, Park YH, Padrón NA, Molefe A. Quantification of neighborhood-level social determinants of health in the continental United States. JAMA Netw Open. 2020;3(1) doi: 10.1001/jamanetworkopen.2019.19928. e1919928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freese KE, Vega A, Lawrence JJ, Documet PI. Social vulnerability is associated with risk of COVID-19 related mortality in U.S. Counties with confirmed cases. J Health Care Poor Underserved. 2021;32(1):245–257. doi: 10.1353/hpu.2021.0022. [DOI] [PubMed] [Google Scholar]
- 28.Hyer JM, Tsilimigras DI, Diaz A, et al. High social vulnerability and “textbook outcomes” after cancer operation. J Am Coll Surg. 2021;232(4):351–359. doi: 10.1016/j.jamcollsurg.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 29.An J, Hoover S, Konda S, Kim SJ. Effectiveness of the COVID-19 Community Vulnerability Index in explaining COVID-19 deaths. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.953198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 31.NQF: Social Risk Trial Final Report. https://www.qualityforum.org/Publications/2017/07/Social_Risk_Trial_Final_Report.aspx. Accessed 30 September 2022.
- 32.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in Health Research: one size does not fit all. JAMA. 2005;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 33.Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC. Indoor air pollution and asthma in children. Proc Am Thorac Soc. Proc Ann Am Thorac Soc. 2010;7(2):102–106. doi: 10.1513/pats.200908-083RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nayak SS, Borkar R, Ghozy S, et al. Social vulnerability, medical care access and asthma related emergency department visits and hospitalization: an observational study. Heart Lung. 2022;55:140–145. doi: 10.1016/j.hrtlng.2022.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Kolling J, Strosnider H, Wilt GE, Berens A, Devine O. In: American Public Health Association; 2017. Social and environmental risk factors to county-level asthma emergency department visits.https://apha.confex.com/apha/2017/meetingapp.cgi/Paper/377408 [Google Scholar]
- 36.McKay AJ, Gunn LH, Ference BA, et al. Is the SMART risk prediction model ready for real-world implementation? A validation study in a routine care setting of approximately 380 000 individuals. Eur J Prev Cardiol. 2022;29(4):654–663. doi: 10.1093/eurjpc/zwab093. [DOI] [PubMed] [Google Scholar]
- 37.Hageman SHJ, McKay AJ, Ueda P, et al. Estimation of recurrent atherosclerotic cardiovascular event risk in patients with established cardiovascular disease: the updated SMART2 algorithm. Eur Heart J. 2022;43(18):1715–1727. doi: 10.1093/eurheartj/ehac056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Premier healthcare database white paper: data that informs and performs. https://learn.premierinc.com/white-papers/premier-healthcare-database-whitepaper. Accessed 22 November 2022.
- 39.Clinical Classifications Software Refined (CCSR). https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp. Accessed 21 September 2021.
- 40.Flanagan BE, Gregory EW, Hallisey EJ, Heitgerd JL, Lewis B. A social vulnerability index for disaster management. J Homel Secur Emerg Manag. 2011;8(1) doi: 10.2202/1547-7355.1792. [DOI] [Google Scholar]
- 41.R Development Core Team; 2020. R: A Language and Environment for Statistical Computing.http://www.r-project.org/ [Google Scholar]
- 42.Daley S, Kajendrakumar B, Nandhakumar S, et al. County-level socioeconomic status adjustment of acute myocardial infarction mortality hospital performance measure in the U.S. Healthcare (Basel) 2021;9(11):1424. doi: 10.3390/healthcare9111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckman M, Grant A, Henson S, et al. A review of socioeconomic factors associated with acute myocardial infarction-related mortality and hospital readmissions. Hosp Pract (1995) 2022;50(1):1–8. doi: 10.1080/21548331.2021.2022357. [DOI] [PubMed] [Google Scholar]
- 44.Khan SU, Hagan KK, Javed Z. Disproportionate impact of COVID-19 among socially vulnerable patients. Circ Cardiovasc Qual Outcomes. 2022;15(8) doi: 10.1161/CIRCOUTCOMES.122.009294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basile Ibrahim B, Interrante JD, Fritz AH, Tuttle MS, Kozhimannil KB. Inequities in availability of evidence-based birth supports to improve perinatal health for socially vulnerable rural residents. Children (Basel) 2022;9(7):1077. doi: 10.3390/children9071077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amjad S, MacDonald I, Chambers T, et al. Social determinants of health and adverse maternal and birth outcomes in adolescent pregnancies: A systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2019;33(1):88–99. doi: 10.1111/ppe.12529. [DOI] [PubMed] [Google Scholar]
- 47.Cottrell EK, Hendricks M, Dambrun K, et al. Comparison of community-level and patient-level social risk data in a network of community health centers. JAMA Netw Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.