Abstract
Background
Tumor angiogenesis is essential for solid tumor progression, invasion and metastasis. The aim of this study was to identify potential signaling pathways involved in tumor angiogenesis.
Methods
Genetically engineered mouse models were used to investigate the effects of endothelial ARL13B(ADP-ribosylation factor-like GTPase 13B) over-expression and deficiency on retinal and cerebral vasculature. An intracranially transplanted glioma model and a subcutaneously implanted melanoma model were employed to examine the effects of ARL13B on tumor growth and angiogenesis. Immunohistochemistry was used to measure ARL13B in glioma tissues, and scRNA-seq was used to analyze glioma and endothelial ARL13B expression. GST-fusion protein-protein interaction and co-immunoprecipitation assays were used to determine the ARL13B-VEGFR2 interaction. Immunobloting, qPCR, dual-luciferase reporter assay and functional experiments were performed to evaluate the effects of ARL13B on VEGFR2 activation.
Results
Endothelial ARL13B regulated vascular development of both the retina and brain in mice. Also, ARL13B in endothelial cells regulated the growth of intracranially transplanted glioma cells and subcutaneously implanted melanoma cells by controlling tumor angiogenesis. Interestingly, this effect was attributed to ARL13B interaction with VEGFR2, through which ARL13B regulated the membrane and ciliary localization of VEGFR2 and consequently activated its downstream signaling in endothelial cells. Consistent with its oncogenic role, ARL13B was highly expressed in human gliomas, which was well correlated with the poor prognosis of glioma patients. Remarkably, ARL13B, transcriptionally regulated by ZEB1, enhanced the expression of VEGFA by activating Hedgehog signaling in glioma cells.
Conclusions
ARL13B promotes angiogenesis and tumor growth by activating VEGFA-VEGFR2 signaling. Thus, targeting ARL13B might serve as a potential approach for developing an anti-glioma or anti-melanoma therapy.
Keywords: ARL13B, intercellular communication, tumor angiogenesis, VEGFR2, vascular development
Graphical Abstract
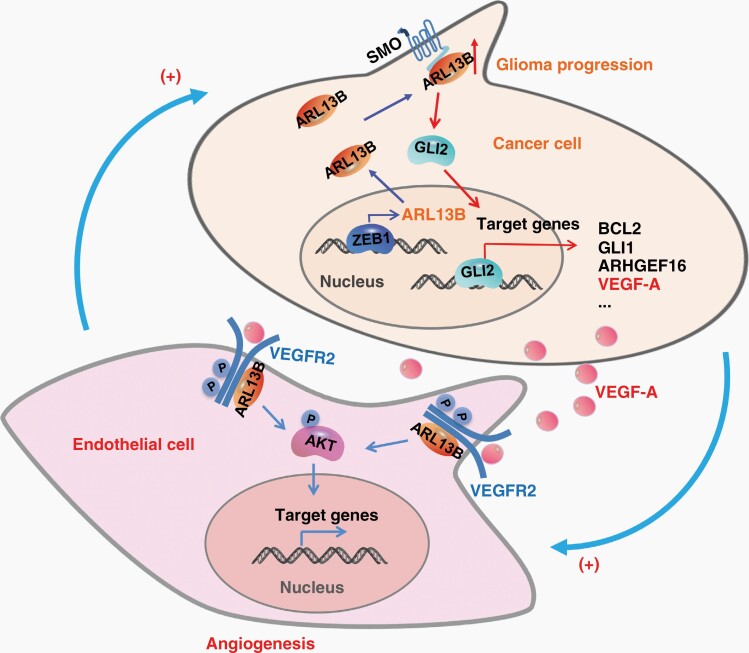
Graphical Abstract.
Key Points.
ARL13B promotes vascular development and tumor angiogenesis.
ARL13B activates VEGFR2 signaling in endothelial cells.
ARL13B in glioma cells promotes tumor angiogenesis by triggering VEGFR2 signaling and intercellular communication.
Importance of the Study.
Angiogenesis is often hijacked by solid tumors for their own growth benefit. Therefore, a better understanding of the molecular mechanisms underlying the regulation of tumor angiogenesis would offer crucial and useful information for the development of anticancer therapies. In our attempt to do so, we found that ARL13B interacts with VEGFR2 and mediates VEGF signaling in endothelial cells and cancer cells. Also, ARL13B augmented angiogenesis in both normal tissues and cancer tissues, such as glioma and melanoma tissues, by activating VEGFR2 signaling. Consistently, ARL13B, highly expressed in human gliomas, was well correlated with the poor prognosis of glioma patients. Interestingly, ARL13B,transcriptionally activated by the oncogenic transcription factor ZEB1, enhanced VEGFA expression by activating Hedgehog signaling in glioma cells. Together, our findings unveil a novel role for ARL13B in angiogenesis via activation of the VEGFA-VEGFR2 signaling and suggest ARL13B as a potential target for the development of an anti-tumor angiogenesis therapy.
Angiogenesis, the formation of new capillary blood vessels essential for normal human development and reproduction, is often hijacked by solid tumors for their own growth benefit.1 Eradicating tumor angiogenesis has become an attractive strategy for developing anticancer therapies. Therefore, a better understanding of the molecular mechanisms underlying the regulation of tumor angiogenesis would provide useful information for the development of such a strategy.
Vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs) play pivotal roles in tumor angiogenesis.2 VEGFR2, activated by VEGFA, is primarily expressed in endothelial cells as a key receptor that regulates vascular permeability and leads to angiogenesis, including sprouting and vessel elongation, and mediates VEGF-stimulated cell proliferation, migration and survival.2 The VEGF signaling is highly regulated at the levels of gene expression, endocytosis, trafficking, and proteolytic degradation.3 Targeting the VEGF/VEGFR2 signaling has been explored as a therapeutic approach to disrupt tumor vessel formation and to inhibit tumor growth.4 The anti-VEGF antibody bevacizumab has been effectively used for several cancers.4 However, its clinical efficacy for glioma is limited, as glioma possesses prominent vascular abnormalities.5 Although several small-molecule inhibitors of VEGFR2, including sunitinib, sorafenib, and vandetanib, have been approved for cancer therapy,4 adverse effects have been observed in their clinical application. Therefore, identifying new molecular targets for the development of improved anti-angiogenic therapies is necessary.
ARL13B, a member of the Arf-like Ras superfamily of small GTPases, is highly enriched within cilium and serves as a signaling hub for the extracellular environment.6 It’s required for ciliogenesis.7 Genetic mutation of ARL13B is highly associated with Joubert syndrome,8 and null mutation of Arl13b results in alteration of the ciliary axoneme structure and neural tubes defects.9 Recently, ARL13B was implicated in cancer progression.10–12 Our group found that ARL13B promotes tumorigenesis in gastric cancer by regulating Smoothened trafficking and activating Hedgehog(Hh) signaling.10 In medulloblastoma, deletion of Arl13b in ptch1-deleted mice inhibited tumor formation by suppressing Hh signaling overactivation.11 Knockdown of ARL13B sensitized glioblastoma cells to chemotherapy.12 It’s worth noting that Arl13b/Arl13b+ cilia are expressed on endothelial cells lining the vasculature and act as sensors of blood flow,13 and have recently implicated in vascular stability in the zebrafish brain.14 However, role of ARL13B in tumor angiogenesis is unclear. Our identification of ARL13B in VEGA-VEGFR2 signaling pathway suggested that it might play a role in angiogenesis in both normal and tumor tissues.
In our investigation to address this possibility, as detailed below, we found that ARL13B indeed interacted with VEGFR2 and mediated VEGF signaling in endothelial and cancer cells. Additionally, by employing several genetically engineered mouse model systems, we demonstrated that ARL13B augmented angiogenesis in both normal and cancer tissues, such as glioma and melanoma tissues, by promoting cell-to-cell communication and activating VEGFR2 signaling. Further analyses showed that ARL13B is highly expressed in human gliomas, which is highly associated with the poor prognosis of glioma patients. In glioma cells, ARL13B was transcriptionally regulated by transcription factor Zinc finger E-box binding homeobox 1(ZEB1) to enhance VEGFA expression by activating Hh signaling. Together, our results unveil a novel role for ARL13B in angiogenesis via activating VEGFA-VEGFR2 signaling and suggest ARL13B as a potential target for the development of an anti-tumor angiogenesis therapy.
Methods
For detailed Material and Methods, see Supplementary Information.
Ethics Statements
All mouse experiments and human glioma specimens collection were approved by Institutional Review Board of The First Affiliated Hospital of Nanchang University [(2020)Medical Research Ethical Review No. 12-134].
Mice
Arl13b conditional knockout mice B6-Arl13bEm1(flox)NCU(Arl13bfl/+) and inducible forced expression mice B6-Gt(ROSA)26SorEm1(CAG-LSL-Arl13b-IRES-eGFP)NCU(Rosa26LSL-Arl13b/+) were generated using CRISPR/Cas9 technology.
Immunofluorescence Staining of Retinas
The delivery day was defined as postnatal day 0 (P0). The retinas were dissected and fixed. After blocking and permeabilization, anti-Arl13b antibody and Isolectin B4, and followed secondary antibodies were incubated.
Enrichment of Brain Endothelial Cells
Brain tissues were digested and the single-cell suspensions were enriched with biotin-conjugated anti-CD31 antibody.
Immunofluorescence Staining of Vasculature
Brain or tumor tissues were dissected, embedded and cut into 20-μm sections. Tissue sections were fixed and incubated with indicated primary antibodies and followed secondary antibodies.
Intracranial Transplanted Glioma Model
GL261-Red-Fluc cells (2 × 105 cells for Arl13b-iΔEC and control mice, 1 × 105 cells for Arl13b-iEC and control mice) were harvested, resuspended and slowly injected into the right hemispheres of mice.
Assessment of Tumor Vascular Permeability
Tumor Matrigel plug implant in mice was performed and vascular leakage was determined with Evans blue.
Subcutaneous Implanted Melanoma Model
B16F10 cells (1 × 105 cells for Arl13b-ΔEC and control mice, 0.6 × 105 cells for Arl13b-EC and control mice) were resuspended and injected subcutaneously into the right flank of mice.
Immunohistochemistry (IHC)
IHC staining was performed as described previously.10
Single-Cell RNA-seq Data
The single-cell RNA sequencing data was downloaded from GEO database(GSE84465) and analyzed with t-distributed stochastic neighbor embedding(t-SNE) projection.
Yeast Two-Hybrid Screening
Yeast two-hybrid screening was conducted as manufacturer’s instructions. The full-length human ARL13B cDNA(NM_182896) was used as bait.
Protein–Protein Interaction and Immunoblotting Assays
ARL13B-VEGFR2 interaction was determined using GST-fusion protein-protein interaction and co-immunoprecipitation (co-IP) assays, as described previously.10
Cell Surface Biotinylation Assay
The cell surface protein labeled with sulfo-NHS-SS-Biotin were immunoprecipitated and analyzed by immunoblotting.
SMA560 Tumor Model
SMA560 cells (1.5 × 106 cells for Lv-Arl13b and Lv-Con group) were injected subcutaneously into the two flanks of BALB/c-nu mice.
Results
Endothelial ARL13B is Involved in Vascular Development in the Postnatal Mouse Retina
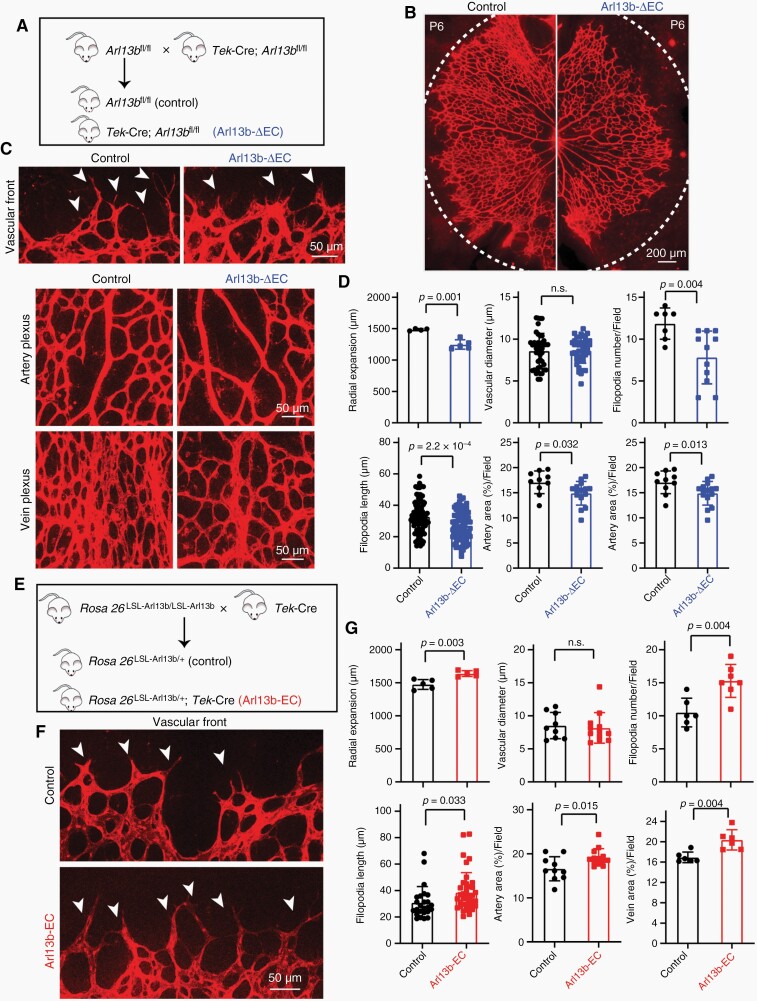
To investigate whether Arl13b is involved in vascular development in vivo, we generated conditional Arl13b-knockout mice (Arl13bfl/+, cKO) by CRISPR/Cas9 technology (Supplementary Figure S1A–C). The positive F0-generation cKO mice were mated with wild-type mice to obtain F1-generation cKO mice, and genomic screening of F1-generation cKO mice was carried out by PCR-sequencing and Southern-blot assays, which indicated integration of the transgene (Supplementary Figure S1B). We then interbred the Arl13bfl/fl mice with Tek-Cre+; Arl13bfl/fl mice to generate endotheial cell-Arl13b-deficient mutant mice, called Arl13b-ΔEC (Tek-Cre+; Arl13bfl/fl) mice, and corresponding control mice (Figure 1A). The endothelial Arl13b deficiency of retinas from Arl13b-ΔEC mice was confirmed (Supplementary Figure S1D). Arl13b deletion in endothelial cells did not result in embryonic lethality, and the mice were viable. Compared with control littermates, retinas from Arl13b-ΔEC mice exhibited wider vascular mesh with impaired vascular radial expansion, reduced number and length of filopodia, and area of artery and vein, but no difference in the vascular diameter at P6 was observed (Figure 1B–D). The results suggested that ARL13B in endothelial cells plays roles in vascular development.
Figure 1.
Endothelial ARL13B promoted retinal vascular angiogenesis in mice. (A) Breeding scheme for the production of endothelium-specific Arl13b-knockout mice(Arl13b-ΔEC). (B–D) Retinal vascular angiogenesis was examined in Arl13b-ΔEC mice at P6. Representative images of isolectin B4(IB4)-stained retinal vessels, amplifying vascular fronts (arrows indicate filopodia) and vascular plexuses of arteries and veins are showed (C). Retinal vascular radial expansion, vessel diameter, filopodia number, filopodia length, and vascular area of arteries and veins were quantified (D). (E) Breeding scheme for the production of endothelium-specific Arl13b overexpressing mice (Arl13b-EC). (F, G) Alterations in retinal vascular angiogenesis in Arl13b-EC mice at P6 were examined. Representative images of IB4-stained retinal vascular fronts (arrows indicate filopodia) are shown. Quantification of the radial expansion, vessel diameter, filopodia number, filopodia length, and vascular area of artery and vein were shown (G). (D, G) Data are mean ± SD. P values were determined by independent-samples t-test.
We further generated conditional Arl13b-knock-in mice(Rosa26LSL-Arl13b/+, cKI) by inserting the coding sequence of Arl13b into the ROSA26 locus via CRISPR/Cas9 technology (Supplementary Figure S2A–C), PCR-sequencing and Southern-blot assays were used to identify and verify the transgene (Supplementary Figure S2B). We then interbred the Rosa26LSL-Arl13b/LSL-Arl13b mice with Tek-Cre mice to generate endothelial cell Arl13b-knock-in mutant mice, called Arl13b-EC (Tek-Cre+; Rosa26LSL-Arl13b/+) mice, and control littermates (Figure 1E). GFP expression was confirmed in retinas vessel from Arl13b-EC mice, indicating that ARL13B was expressed in the vascular front and plexus (Supplementary Figure S2D). Constitutive expression of endothelial Arl13b did not result in embryonic lethality, and the mice were viable. We further examined the effects of endothelial ARL13B over-expression on the postnatal maturation of retinal vasculature. Retinas from Arl13b-EC mice at P6 didn’t show significantly different in vessel diameter but exhibited increased vascular radial expansion and overgrowth of the vascular network (Figure 1F, G; Supplementary Figure S2E). The vascular front of Arl13b-EC mice displayed increased filopodia number (Figure 1F, G). The vascular plexus of Arl13b-EC showed increased vascular areas of artery and vein (Figure 1G; Supplementary Figure S2E).
Next, we assessed the retinal vascular chronological development using P3, P9, and P15 mice and also examined the deeper vascular layer of retina using P15 mice. The decreased radial expansion of retinal vascular network at P3 and P6 was restored in retinas from Arl13b-ΔEC mice at P9 and P15 (Supplementary Figure S1E). The deeper vascular layer density of retina at P15 displayed no significant difference between control and Arl13b-ΔEC mice (Supplementary Figure S1F). Also, the increased radial expansion of retinal vascular network was restored in Arl13b-EC mice at P9 and P15, and the vascular density at the secondary plexus was no different between Arl13b-EC mice and control littermates (Supplementary Figure S2F–H). These results suggest that the phenotype of retinal vasculature in endothelial Arl13b-overexpression and -deficiency mice was transient.
Endothelial ARL13B Regulates Angiogenesis in Brain
To determine whether Arl13b affects cerebrovascular development in adult mice, we analyzed the effects of endothelial ARL13B upregulation on vascular angiogenesis in brain tissues. Arl13b-iEC (Tek-CreERT2+; Rosa26LSL-Arl13b/+) mice with inducible specific Arl13b-knock-in in endothelial cells and control littermates were generated and treated with tamoxifen (Supplementary Figure S3A). Vascular endothelial Arl13b over-expression was detected in enriched brain endothelial cells after tamoxifen administration (Supplementary Figure S3B). The vascular morphology of brain tissues in Arl13b-iEC mice was similar to that in control littermates (Supplementary Figure S3C). However, Arl13b-iEC brain showed increased vessel number, area and branches (Supplementary Figure S3C, D). Vessel sprouts were increased in Arl13b-iEC mice(Supplementary Figure S3E). Junctional VE-cadherin, a major determinant of endothelial cell contact integrity, was decreased in the vessels of Arl13b-iEC mouse brain tissue (Supplementary Figure S3F). However, no significant difference in Col4-positive basement membrane or Pdgfrβ-positive pericytes between Arl13b-iEC and control group was observed (Supplementary Figure S3F).
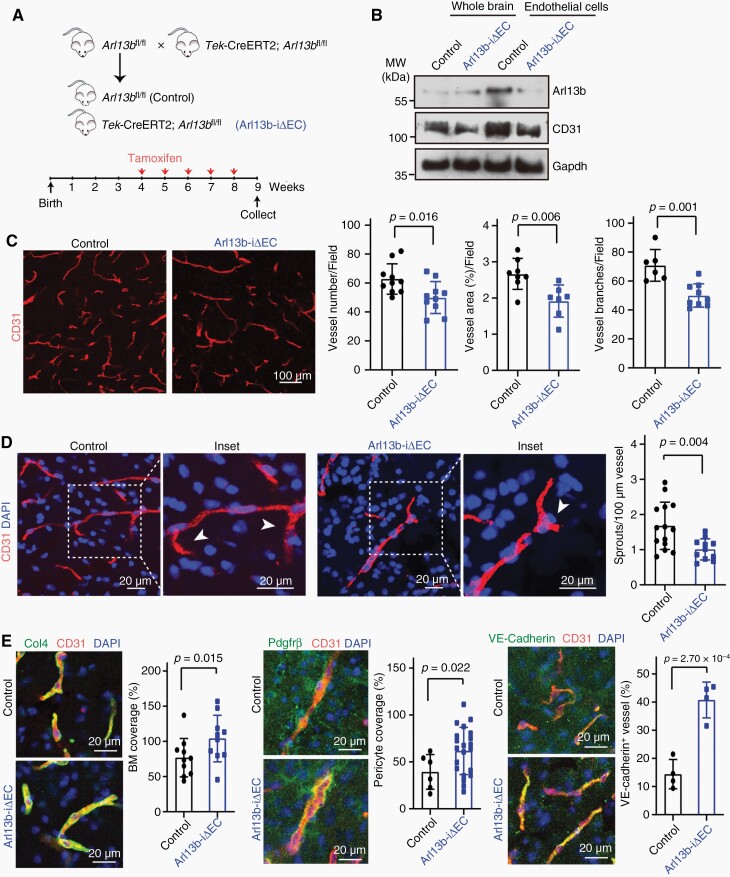
Additionally, inducible deletion of endothelial cell Arl13b alleles of mutant mice, called Arl13b-iΔEC (Tek-CreERT2+; Arl13bfl/fl), and control littermates were generated (Figure 2A). Inducible depletion of Arl13b after tamoxifen administration was determined (Figure 2B). Compared with control littermates, Arl13b-iΔEC mice showed decreased vessel counts, areas and branches (Figure 2C). The sprouts of vessels were also reduced in Arl13b-iΔEC mice (Figure 2D). Interestingly, the basement membrane, pericytes, and junctional VE-cadherin levels were increased in the vessels of brain tissue from Arl13b-iΔEC mice (Figure 2E). These results demonstrate that ARL13B plays a role in regulating vascular morphology and function in brain.
Figure 2.
Brain vessels were regressed in endothelial Arl13b-deficient mice. (A) Breeding scheme for the production of inducible endothelial Arl13b-deficient mice(Arl13b-iΔEC) and the time course for tamoxifen injection. (B) Arl13b expression was inhibited in brain endothelial cells from Arl13b-iΔEC mice. (C) Vessel count, area, and branches were decreased in brain tissues from Arl13b-iΔEC. (D) Vascular sprouting(arrows) was decreased in brain tissues from Arl13b-iΔEC mice. Insets show magnified areas. (E) Col4-positive basement membrane (BM), Pdgfrβ-positive pericytes and VE-cadherin-positive vessels were examined and quantified. (C–E) Data are mean ± SD. P values were determined by independent-samples t-test.
Endothelial ARL13B Enhances Glioma Growth by Promoting Tumor Angiogenesis
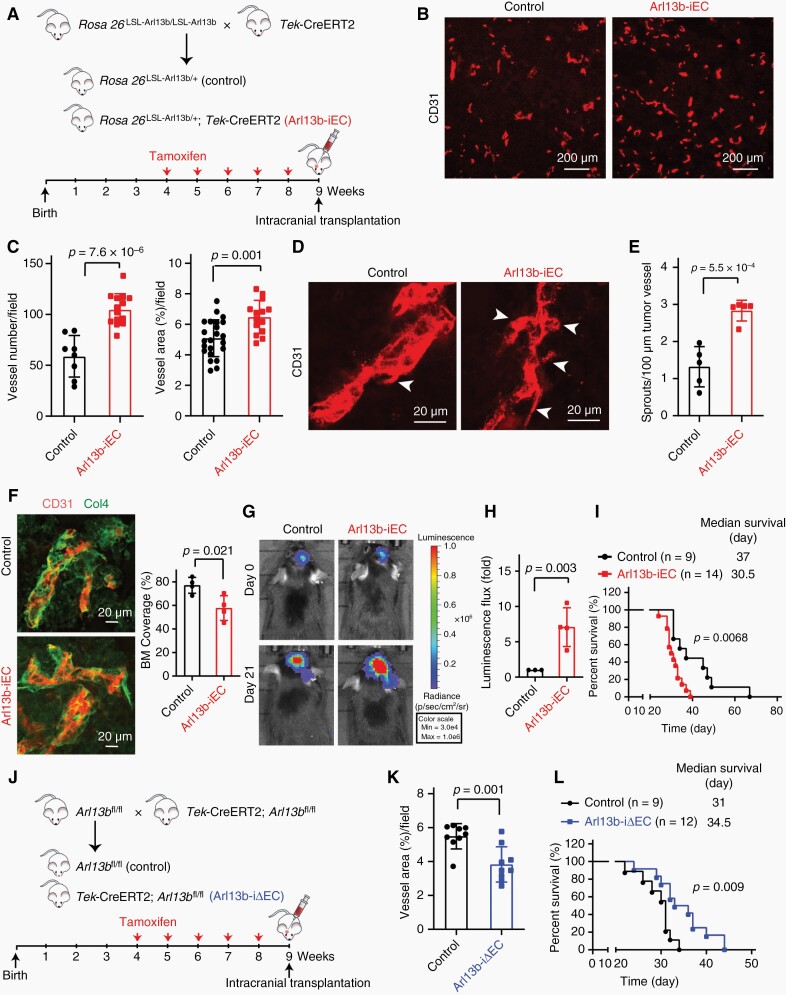
Glioma tissues are abnormally rich with blood vessels.15 Our studies above suggested that ARL13B might promote tumor angiogenesis and consequently glioma growth. To test this idea, we explored the role of endothelial Arl13b in glioma progression by employing Arl13b-iEC mice bearing orthotopic glioma derived from GL261-RFP-Rluc cells (Figure 3A). Glioma vessels in Arl13b-iEC mice were increased in number and area (Figure 3B, C). Tumor vessels in Arl13b-iEC mice displayed more abundant sprouts and a marked decrease in Col4-positive basement membranes (Figure 3D–F). Consistently, Arl13b-iEC mice also exhibited increased tumor growth and shorter lifetimes than control mice (Figure 3G–I). Next, we tested whether endothelial Arl13b deficiency affects glioma growth by employing Arl13b-iΔEC mice bearing orthotopic gliomas derived from GL261-RFP-Rluc cells (Figure 3J). Glioma vessels in Arl13b-iΔEC mice were reduced in number and area (Figure 3K; Supplementary Figure S4A, B). Arl13b-iΔEC mice tumor vessels exhibited a marked increase in Col4-positive basement membranes (Supplementary Figure S4C, D). Remarkably, Arl13b-iΔEC mice showed extended life-span (Figure 3L). Furthermore, we employed Arl13b-ΔEC mice bearing subcutaneous GL261-RFP-Rluc cells to determine the tumor vascular permeability, and found that endothelial Arl13b deficiency leads to reduced leakage of Evans blue (Supplementary Figure S4E, F). So, these results demonstrate that endothelial ARL13B could augment glioma growth by promoting angiogenesis in tumor tissues.
Figure 3.
ARL13B regulates glioma growth by controlling tumor angiogenesis. (A) Breeding scheme for production of inducible endothelium-specific Arl13b-overexpressing mice(Arl13b-iEC) and the time course for tamoxifen injection and intracranial transplantation of GL261-RFP-Rluc cells. (B–E) Representative images of CD31-stained tumor vessels (B) and sprouts (D) of glioma tissues from Arl13b-iEC and control mice. Bar graphs show quantification of the tumor vessel count, area (C), and sprouts (E). Arrows indicate the tumor sprouts. (F) Col4-positive BM was determined and quantified. (G, H) Glioma cells grew faster in Arl13b-iEC than control mice. Luminescence flux was detected (G) and quantified (H). (I) Survival curves for the GL261-RFP-Rluc bearing mice. (J) Breeding scheme for the production of inducible endothelium-specific Arl13b-deficient mice (Arl13b-iΔEC) and the time course for tamoxifen injection and intracranial transplantation. (K) Tumor vessel areas of Arl13b-iΔEC and control mice were quantified. (L) Survival curves for the GL261-RFP-Rluc-bearing mice. (C, E, F, H, K) Data are mean ± SD. P values were calculated by independent-samples t-test. (I, L) P values were determined by the log-rank (Mantel–Cox) test.
Endothelial ARL13B Depletion Inhibits Melanoma Growth by Impairing Tumor Angiogenesis
To confirm the aforementioned effect of ARL13B on tumor angiogenesis, we subcutaneously transplanting B16F10 mouse melanoma cells into Arl13b-ΔEC mice to study the effects of ARL13B on melanoma growth and tumor angiogenesis. As shown in Supplementary Figure S5A–C, Arl13b-ΔEC mice displayed decrease of tumor size and weight 13 days after transplantation. Compared with melanoma vessels in control mice, melanoma vessels in Arl13b-ΔEC mice exhibited marked decrease in vascular count and area (Supplementary Figure S5C, D). The basement membrane, pericytes and junctional VE-cadherin were increased in melanoma vessels from Arl13b-ΔEC mice (Supplementary Figure S5E, F). Whereas the tumor size and vascular count and area were increased in Arl13b-EC mice (Supplementary Figure S6A–E), the basement membrane, pericytes and junctional VE-cadherin were reduced in melanoma vessels of Arl13b-EC mice (Supplementary Figure S6F, G). These results demonstrate that ARL13B also plays crucial roles in vessel formation in melanoma consequently promoting tumor growth.
Increased ARL13B Levels are Associated with a Poorer Prognosis in Glioma
To determine the clinical relevance of the above results, we analyzed CCLE and GEO dataset consisting of data from preclinical human cancer cell models and found that ARL13B is excessively upregulated in glioma cell lines and this upregulation is highly correlated with glioma grade (Supplementary Figure S7A, B). To validate this result, we examined ARL13B expression in 128 glioma patients by IHC. As shown in Supplementary Figure S7C, D, ARL13B expression was higher in high-grade glioma than in lower-grade glioma, which was confirmed in 9 randomly selected glioma tissues by immunoblotting (Supplementary Figure S7E, F). Univariate analysis of disease-free survival and overall survival using the Kaplan–Meier method showed that glioma (including lower- and high-grade) patients with high levels of ARL13B had a poor prognosis (Supplementary Figure S8A, B; Table S1). Also, we found that high level of ARL13B is well correlated with poor progression both in the lower (<45 years) and higher (≥45 years) age groups (Supplementary Figure S8C–F). The level of ARL13B in human glioma specimens was positively correlated with the degree of tumor malignancy (Supplementary Table S2). Furthermore, to analyze scRNA-seq data, we characterized the distribution of cells from peripheral normal brain tissues and glioma tissues. And, the transcriptional accumulation of ARL13B across fourteen unsupervised cell cluster were generated in separate plots. Our results revealed that ARL13B is highly expressed in glioma cells but not significantly increased in endothelial cells from glioma tissues (Supplementary Figure S8G–I). Collectively, the high level of ARL13B is closely associated with the poor prognosis of glioma patients, suggesting that high level of ARL13B in glioma cells may promote glioma progression by promoting tumor angiogenesis.
ARL13B Interacts with VEGFR2
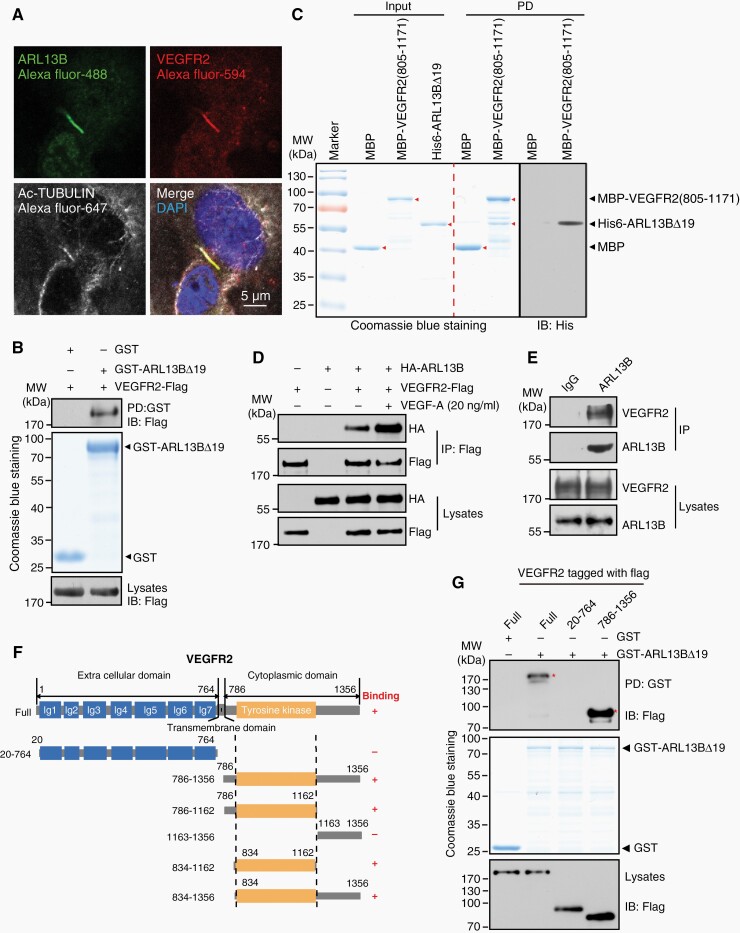
To elucidate the mechanism underlying the regulatory role of ARL13B in angiogenesis, we performed yeast two-hybrid screen using full-length ARL13B as a bait to identify novel ARL13B-interacting partners. Through this screening, a positive clone encoding an intracellular fragment of VEGFR2 was found, suggesting that VEGFR2 is a potential ARL13B-binding protein. Since ARL13B acts as a marker of primary cilium, we tested whether it resides with VEGFR2 within cilium. Indeed, ARL13B and VEGFR2 were co-localized within and outside the primary cilium of endothelial cell ECV304 (Figure 4A; Supplementary Figure S9A). ARL13B downregulation resulted in deconstruction of primary cilium with a shorter length and decrease in the ciliary localization of VEGFR2 (Supplementary Figure S9B, C).
Figure 4.
ARL13B interacts with VEGFR2. (A) The colocalization of ARL13B and VEGFR2 in primary cilium. (B) GST-fusion protein-protein interaction assays revealed the interaction between ARL13B and VEGFR2. (C) ARL13B binds VEGFR2. Arrowheads indicate target proteins. (D) Co-IP-IB assays with indicated antibodies to detect the ARL13B-VEGFR2 interaction in mammalian cells. (E) The endogenous ARL13B-VEGFR2 interaction in endothelial cells was determined by co-IP-IB assay. (F) Diagrammatic representation of VEGFR2 and various truncated mutants used for mapping the ARL13B-binding domain. (G) GST-fusion protein-protein interaction assays were conducted to map the ARL13B-binding domain of VEGFR2. Asterisks indicate domains that interact with ARL13B.
To confirm the interaction between ARL13B and VEGFR2, we conducted in vitro GST-fusion protein-protein assays. As shown in Figure 4B, GST-ARL13BΔ19, a truncated form of recombinant GST-ARL13B lacking the N-terminal hydrophobic region to facilitate protein purification from bacteria, interacted with VEGFR2. Their interaction was also validated by using purified His-ARL13BΔ19 and MBP-VEGFR2(805-1171) (Figure 4C) as well as co-IP experiments with both exogenous and endogenous proteins in endothelial cells (Figure 4D, E). Interestingly, their interaction was enhanced in response to VEGFA ligand (Figure 4D). Taken together, these results demonstrate that Arl13b interacts with VEGFR2 in and outside of the primary cilia of endothelial cells.
To map their binding domains, we generated truncated mutants of VEGFR2 (Figure 4F) and performed GST-fusion protein-protein interaction assays. As shown in Figure 4G, ARL13B interacted with the intracellular region of VEGFR2, specifically with the tyrosine kinase domain within this region (Supplementary Figure S9D). These results suggest that ARL13B may be required for the activation of VEGFA-VEGFR2 signaling.
ARL13B Activates VEGFR2 Signaling in Endothelial Cells
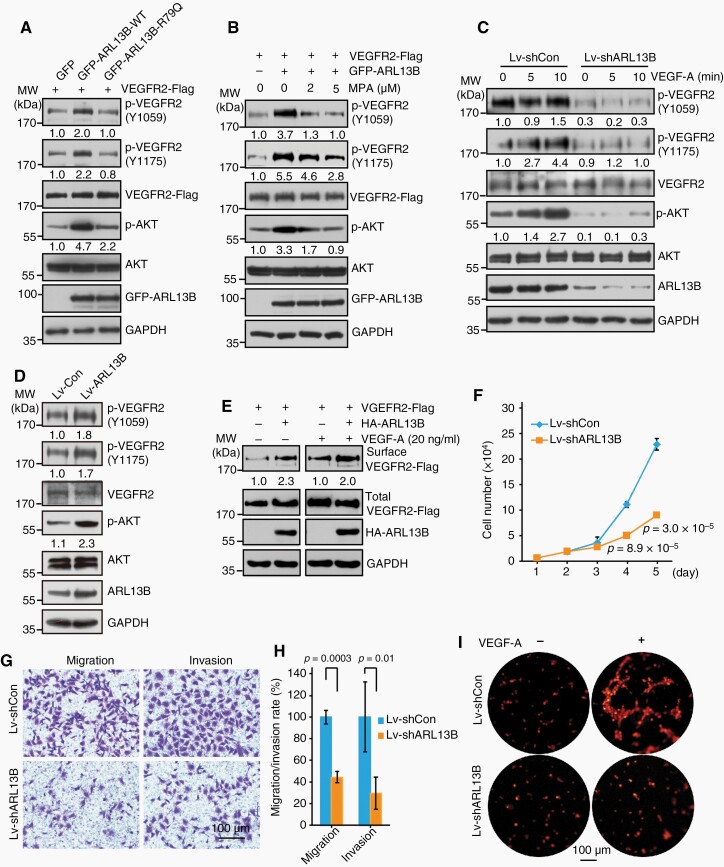
Since ARL13B interact with the VEGFR2 tyrosine kinase domain (Figure 4G; Supplementary Figure S9D), we next determined whether ARL13B regulates VEGFR2 signaling. As shown in Figure 5A, ARL13B increased the phosphorylation of VEGFR2 and AKT. Consistently, the inactive R79Q-mutant of ARL13B had a reduced effect on VEGFR2 signaling activation (Figure 5A). Furthermore, an inhibitor of inosine monophosphate dehydrogenase (IMPDH) mycophenolic acid (MPA)16 was used to reduce intracellular GTP levels, which resulted in a marked decrease in VEGFR2 and AKT phosphorylation (Figure 5B). ARL13B knockdown markedly reduced autophosphorylation of VEGFR2 and downstream phosphorylation of AKT in human endothelial cells ECV304 and suppressed VEGFA (20-ng/ml) responsive activation of this signaling (Figure 5C). In contrast, ARL13B over-expression drastically enhanced the VEGFR2 signaling pathway as demonstrated by increased phosphorylation levels of VEGFR2 and AKT (Figure 5D). It has been shown that in response to VEGFA, phosphorylated VEGFR2 can be internalized.3 Using cell surface biotinylation assays, we revealed that ARL13B overexpression leads to the accumulation of VEGFR2 on cell membrane (Figure 5E), suggesting that ARL13B can activate the VEGFA-VEGFR2 signaling.
Figure 5.
ARL13B promotes VEGFR2 signaling and endothelial cell malignancy. (A) The GTPase activity of ARL13B is indispensable to its activating effect on VEGFR2 signaling. (B) VEGFR2 signaling activity was controlled by cellular GTP levels. (C) Downregulation of ARL13B markedly inhibited the activation of VEGFR2 signaling in endothelial cells. (D) Upregulation of ARL13B promoted VEGFR2 signaling activation in endothelial cells. (E) ARL13B regulates membrane localization of VEGFR2. (F) Downregulation of ARL13B inhibited endothelial cells proliferation. (G, H) Downregulation of ARL13B inhibited endothelial cells migration and invasion. (I) Downregulation of ARL13B inhibited the tube formation capacity of endothelial cells. (F, H) Data are mean ± SD. P values were calculated by independent-samples t-test.
Activation of VEGFR2 signaling is crucial for the proliferation, migration and tube formation capacity of endothelial cells.17 Then, we tested whether ARL13B affects these cellular processes. As shown in Figure 5F–H, in endothelial cell medium containing serum, ARL13B depletion inhibited the proliferation of endothelial cells (Figure 5F). ARL13B knockdown drastically inhibited cell migration and invasion (Figure 5G, H). Consistently, ARL13B downregulation markedly reduced the ability of endothelial cells to form VEGFA-induced cellular tubes (Figure 5I). Taken together, these results demonstrated that ARL13B promotes the proliferation, migration and tube formation of endothelial cells, supporting its roles in tumor angiogenesis, by activating the VEGFA-VEGFR2 signaling.
ARL13B Accelerates Glioma Growth and Angiogenesis by Activating Hh-VEGFA Signaling
To investigate the effects of high ARL13B expression in glioma cells, we assessed the ARL13B expression in various human and mouse glioma cell lines (Supplementary Figure S10A–C). U87-MG, H4, U251-MG and SMA560 cells were infected with lentivirus over-expressing ARL13B. ARL13B upregulation led to the increase expression of an Hh target gene BCL2 (Supplementary Figure S10D–L). Also, glioma cells with ARL13B over-expression contained higher levels of VEGFA (Supplementary Figure S10E–L). To determine whether GLI2, a transcription factor of Hh signaling, transcriptionally regulates VEGFA expression, we stably expressed the activated form of GLI2(GLI2A) in glioma cells as previously reported,18 and found that GLI2A upregulated VEGFA expression (Supplementary Figure S11A). The VEGFA level was higher in the medium of GLI2A over-expressing cells than control cells (Supplementary Figure S11B). Also, GLI2A increased the expression of VEGFA in glioma cells (Supplementary Figure S11C). By constrast, the Hh signaling inhibitor GANT61 reduced the expression of VEGFA (Supplementary Figure S11D–F). We also identified potential Gli2 binding sites (GBS) within −3000-bp to 500-bp of transcriptional start site (TSS) of the VEGFA gene via Matlnspector software (Genomatix) and selected four putative GBSs with matrix similarity greater than 0.92 (Supplementary Figure S11G, H). Four fragments with potential GBS were amplified for constructing luciferase reporter plasmids. The luciferase assays showed that Gli2 transcriptionally regulated VEGFA expression by binding to GBS3 element upstream of the TSS (Supplementary Figure S11I). Also, we found that the conditional medium of GLI2A over-expressing glioma cells enhances endothelial cells growth (Supplementary Figure S11J). These results indicate that ARL13B promotes the expression of VEGFA by activating Hh signaling and secreted VEGFA from glioma cells to promote endothelial cells growth.
Then, we subcutaneously transplanted SMA560 cells with Arl13b-overexpression and control cells into the flank of BALB/c-nu mice, and found that Arl13b upregulation accelerates tumor growth, promotes the expression of Bcl2 and Vegfa, and increased the vascular count and area in tumor tissue (Supplementary Figure S12). Taken together, these results demonstrate that high level of ARL13B in glioma cells can accelerate glioma growth by activating Hh-VEGFA signaling and consequently promoting tumor angiogenesis (Supplementary Figure S15).
ZEB1 Activates ARL13B Gene Transcription
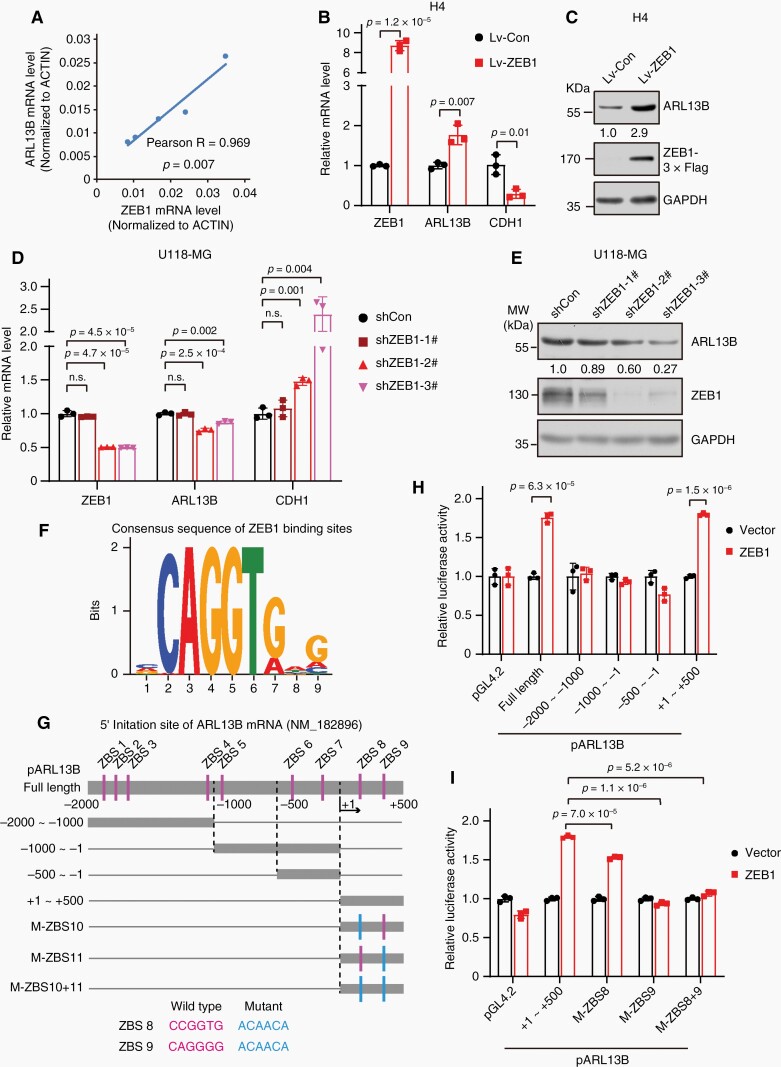
Finally, we attempted to dissect how ARL13B expression is upregulated in glioma cells. First, we analyzed the correlation between ZEB1 and Arl13b in GEPIA2 database and detected ARL13B and ZEB1 mRNA level in glioma cells. As results, we found that the transcription factor ZEB1 is highly co-expressed with ARL13B (Figure 6A; Supplementary Figure S13A–C). Upregulation of ZEB1 promoted ARL13B expression (Figure 6B, C; Supplementary Figure S13D–G), whereas knockdown of ZEB1 inhibited ARL13B expression (Figure 6D, E; Supplementary Figure S13H–K). The region −2000-bp to +500-bp of TSS of ARL13B gene harbors potential binding sites for ZEB1 (ZBS) (Figure 6F, G). Our luciferase reporter assays showed that ZEB1 binds to the fragment +1 to +500-bp to mediate ARL13B transcription (Figure 6H). Also, we mutated ZBS8 and 9 within fragment +1 to +500-bp. By comparing these mutated promoters-driven luciferase activity with the wild-type promoter, we found that ZEB1 mainly bind to ZBS9 to regulate the transcription of ARL13B (Figure 6G and I). These data demonstrate that ARL13B expression is transcriptionally activated by ZEB1.
Figure 6.
ZEB1 transcriptionally activates ARL13B expression. (A) The correlation between ARL13B and ZEB1 mRNA level in various glioma cells (A172, H4, U87-MG, U118-MG, and U251-MG) was analyzed by qPCR. (B, C) ARL13B levels were increased in ZEB1 over-expression cells. (D, E) ARL13B levels are reduced in ZEB1-depleted cells. (F) The consensus sequence of the ZEB1 binding sites (ZBS). (G) The distribution of putative ZBS candidates within −2000-bp to +500-bp of TSS of ARL13B. Luciferase reporter constructs are shown in schematic illustration. (H) ZEB1 enhanced ARL13B promoter activity. (I) ZEB1 regulates ARL13B transcription primarily through ZBS9 element. (B, D, H, I) Data are mean ± SD. P values were calculated by independent-samples t-test.
Discussion
As a process required for invasion and metastasis, tumor angiogenesis plays critical roles in cancer progression.15 Thus, completely understanding the mechanisms underlying tumor angiogenesis is essential for developing an effective approach to intervent cancer progression. In our attempt to understand this phenomenon, we identified ARL13B as a new player in regulating tumor angiogenesis. As discussed below, our further analyses demonstrate that ARL13B can interact with VEGFR2, consequently triggering VEGFA-VEGFR2 signaling and promoting tumor angiogenesis and growth.
ARL13B Activates VEGFR2 Signaling in Endothelial Cells
VEGFR2 is a key cytoplasmic membrane receptor for VEGFA in endothelial cells. Its endocytosis and trafficking are crucial for executing this signaling.3 A review present that VEGFR2 was proposed to be localized within cilium.19 Here, we found that ARL13B plays roles in the regulation of VEGFA-VEGFR2 signaling. Previously ARL13B was shown to be critical for membrane trafficking, as it is highly enriched on the ciliary membrane, and is present on the cell surface.20 Interestingly, ARL13B interacted with the intracellular domain of VEGFR2 (Figure 4). Recent studies reported that ARL13B functions outside of primary cilium to regulate Hh signaling,21,22 our results showed that ARL13B colocalized with VEGFR2 on the membrane of endothelial cells (Supplementary Figure S9A). Endogenous ARL13B interacted with the intracellular domain of VEGFR2, triggering its membrane localization and downstream signaling (Figure 5). Thus, our studies identify ARL13B as a novel player in VEGFR2 signaling.
ARL13B Promotes Vascular Development and Tumor Angiogenesis
Endothelial cilia play crucial roles in the regulation of vascular barriers23 that line blood vessels and function as a mechanosensor of blood flow.24 Primary cilia endow endothelial cells with sensitivity to BMP9 and prevent excessive vascular regression.25 Here, we observed primary cilium in several glioma cells, and the short cilia in vessels from Arl13b-ΔEC mice (Supplementary Figure S14). We also found that endothelial ARL13B plays a role in vascular development. High level of ARL13B in endothelial cells promoted overgrowth of both retina and brain vessels, whereas endothelial ARL13B deficiency impaired vascular network (Figures 1 and 2; Supplementary Figures S1–S3). These results are consistent with the report showing that ARL13B is critical for early postnatal proliferation of retinal progenitors, development of photoceptor cilia, and morphogenesis of photoreceptor outer segment discs in retinal development.26 Further supporting the role of ARL13B in retinal angiogenesis is the fact that ARL13B is mutated in Joubert syndrome, which is characterized by severe defects in retinogenesis.8 We showed that the effect of endothelial Arl13b knocking-out or overexpressing on postnatal retinal vessel was transient (Figures 1 and 2; Supplementary Figures S1–S3). This transient phenotype of Arl13b-deficiency mice is consistent with mutant mice deficient in IFT88 (an essential component of cilia), which might due to the dynamical formation and disappearance of cilia during vascular development.25 However, whether is there a compensatory mechanism could be further uncovered.
In addition to its role in normal vascular development, ARL13B plays roles in tumor angiogenesis, as revealed by our studies with two different tumor model systems (Figure 3; Supplementary Figures S4–S6). Glioma and melanoma are highly lethal malignant tumors characterized by a marked increase in blood vessel formation.15,27 Because of this feature, conventional therapies are ineffective, and the prognosis is quite poor.28 Thus, it is critical to identify the crucial player in pathological vascular formation. Our identification of ARL13B as a novel player in angiogenesis in these tumors could offer a new target for further development of therapies for these malignancies. We found that intracranially transplanted gliomas in Arl13b-iEC mice grow faster and display more tumor vessels, while in Arl13b-iΔEC mice show smaller intracranial gilomas and less angiogenesis (Figure 3). Additionally, the subcutaneous transplantation of melanoma cells in Arl13b-ΔEC led to the inhibition of melanoma cell growth by reducing angiogenesis (Supplementary Figure S5). These results demonstrate that ARL13B or ARL13B-VEGFR2 signaling plays curcial roles in angiogenesis and consequent growth of glioma and melanoma.
ARL13B Regulates Secretion of VEGFA from Tumor Cells to Promote Tumor Angiogenesis
Tumor vessels develop by sprouting or intussusception from the host vascular network, mainly driven by the VEGF-VEGFR2 signaling.29 VEGFs and their primary receptor VEGFR2 are primarily drivers of the angiogenesis process that transduce signals to direct endothelial cell proliferation, migration, tube formation and sprouting.30 Endothelial tip cells harbor abundant and dynamic filopodia localized at the growing end of a nascent vascular sprout. High expression of VEGFR2 allows endothelial tip cells to extend filopodia and detect VEGFA to promote migration along the VEGFA gradient.31 A previous study showed that VEGFA was downregulated by an inhibitor of Hh signaling in hepatocellular carcinoma.32 The Hh signaling pathway promotes tumor angiogenesis by inducing VEGFA in stromal perivascular cells.33 We found that GLI2 transcriptionally regulates the expression and secretion of VEGFA in glioma cells and ARL13B activates Hh signaling pathway (Supplementary Figures S10 and S11). Our study demonstrated that ARL13B can augment tumor angiogenesis by inducing the secretion of VEGFA in tumor cells.
ZEB1 Transcriptionally Activates ARL13B Expression
ARL13B is involved in ciliogenesis, protein and membrane trafficking, and signal transduction, which play critical roles under normal physiological conditions and pathological conditions including kidney cysts, Joubert syndrome and cancer.8,10,11 Studies reported that palmitoylation of ARL13B regulates its stability, and the degradation of ARL13B is dependent on the proteasome.34 ARL13B is also epigenetically regulated by EZH2.12 However, transcriptional regulation of ARL13B has not yet been reported. Here, we report that ZEB1 transcriptionally activates ARL13B expression, and ZEB1 level is closely related to ARL13B level in glioma cells (Figure 6; Supplementary Figure S13). This is in line with previous observations that ZEB1+ cells in patient GBM are also ciliated.35 Our study reveals ZEB1 as a direct transcriptional activator of ARL13B in glioma cells.
Our studies demonstrated that ARL13B can promote angiogenesis and tumor growth by facilitating cell-to-cell communication and activating VEGFA-VEGFR2 signaling (Supplementary Figure S15). These findings not only reveal a novel function of this protein, but also suggest that ARL13B and ARL13B-VEGFR2 signaling pathway could serve as potential targets for the future development of therapies against malignant solid tumors.
Supplementary material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Acknowledgments
We thank Dr. Jianghong Man at National Center of Biomedical Aanlysis for providing the valuable reagents for this work; Drs. Minzhang Cheng and Hailong Wang for insightful discussions and suggestions regarding this work; Ms. Gelian Li, Dandan Zhang, and Jiankun Guo for technical assistance.
Contributor Information
Limin Chen, Center for Experimental Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, China.
Xinsheng Xie, Center for Experimental Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, China.
Tiantian Wang, Center for Experimental Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, China.
Linlin Xu, Center for Experimental Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, China.
Zhenyu Zhai, Center for Experimental Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, China.
Haibin Wu, Center for Experimental Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, China.
Libin Deng, Department of Epidemiology and Biostatistics, School of Public Health, Nanchang University, Nanchang, China.
Quqin Lu, Department of Epidemiology and Biostatistics, School of Public Health, Nanchang University, Nanchang, China.
Zhengjun Chen, Center for Excellence in Molecular Cell Science, Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China.
Xiao Yang, Genetic Laboratory of Development and Disease, Institute of Lifeomics, National Center for Protein Sciences, Beijing, China.
Hua Lu, Department of Biochemistry and Molecular Biology, Tulane University School of Medicine, New Orleans, USA.
Ye-Guang Chen, School of Life Sciences, Tsinghua University, Beijing, China.
Shiwen Luo, Center for Experimental Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, China.
Funding
This work was supported by grants from National Natural Science Foundation of China (32070783 to S.L., 81660461 to L.C.), National Key R&D Program of China (2022YFA1105200 to S.L.) and Natural Science Foundation of Jiangxi Province (20202BBG72003 to S.L., 20202ACBL206032 to L.C.). H.L. was supported by the Reynolds and Ryan Families Chair Fund of Transitional Cancer.
Conflict of Interest Statement
The authors declare no potential conflicts of interest.
Authorship Statement
L.C., X.X., and T.W. performed experiments and analyzed data. L.X. performed IHC assay. L.D. conducted scRNA-seq analysis. Z.Z. supervised yeast two-hybrid screening. Q.L. supervised statistical analysis. H.W., Z.C. and X.Y. contributed materials and reviewed the manuscript. H.L advised some experiments. H.L. and Y.C. edited the manuscript. S.L. planned and supervised the study. L.C. and S.L. drafted the manuscript.
References
- 1. De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–474. [DOI] [PubMed] [Google Scholar]
- 2. Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. [DOI] [PubMed] [Google Scholar]
- 3. Simons M. An inside view: VEGF receptor trafficking and signaling. Physiology. 2012;27(4):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17(7):347–362. [DOI] [PubMed] [Google Scholar]
- 5. Wick W, Osswald M, Wick A, Winkler F. Treatment of glioblastoma in adults. Ther Adv Neurol Disord. 2018;11:1756286418790452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cevik S, Hori Y, Kaplan OI, et al. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J Cell Biol. 2010;188(6):953–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol. 2010;189(6):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cantagrel V, Silhavy JL, Bielas SL, et al. ; International Joubert Syndrome Related Disorders Study Group. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83(2):170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horner VL, Caspary T. Disrupted dorsal neural tube BMP signaling in the cilia mutant Arl13b hnn stems from abnormal Shh signaling. Dev Biol. 2011;355(1):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shao J, Xu L, Chen L, et al. Arl13b promotes gastric tumorigenesis by regulating Smo trafficking and activation of the Hedgehog signaling pathway. Cancer Res. 2017;77(15):4000–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bay SN, Long AB, Caspary T. Disruption of the ciliary GTPase Arl13b suppresses Sonic hedgehog overactivation and inhibits medulloblastoma formation. Proc Natl Acad Sci USA. 2018;115(7):1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shireman JM, Atashi F, Lee G, et al. De novo purine biosynthesis is a major driver of chemoresistance in glioblastoma. Brain. 2021;144(4):1230–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dinsmore C, Reiter JF. Endothelial primary cilia inhibit atherosclerosis. EMBO Rep. 2016;17(2):156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thirugnanam K, Prabhudesai S, Van Why E, et al. Ciliogenesis mechanisms mediated by PAK2-ARL13B signaling in brain endothelial cells is responsible for vascular stability. Biochem Pharmacol. 2022;202:115143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Das S, Marsden PA. Angiogenesis in glioblastoma. New Engl J Med. 2013;369(16):1561–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lowe JK, Brox LW, Henderson JF. Consequences of inhibition of guanine nucleotide synthesis by mycophenolic acid and virazole. Cancer Res. 1977;37(3):736–743. [PubMed] [Google Scholar]
- 17. Shibuya M. VEGFR and type-V RTK activation and signaling. CSH Perspect Biol. 2013;5(10):a009092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang D, Wang Y, Xu L, et al. GLI2 promotes cell proliferation and migration through transcriptional activation of ARHGEF16 in human glioma cells. J Exp Clin Cancer Res. 2018;37(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shivanna M, Anand M, Chakrabarti S, Khanna H. Ocular ciliopathies: genetic and mechanistic insights into developing therapies. Curr Med Chem. 2019;26(17):3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7(5):347–358. [DOI] [PubMed] [Google Scholar]
- 21. Ferent J, Constable S, Gigante ED, et al. The ciliary protein Arl13b functions outside of the primary cilium in Shh-mediated axon guidance. Cell Rep. 2019;29(11):3356–3366.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gigante ED, Taylor MR, Ivanova AA, Kahn RA, Caspary T. ARL13B regulates Sonic hedgehog signaling from outside primary cilia. eLife. 2020;9:e50434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma N, Zhou J. Functions of endothelial cilia in the regulation of vascular barriers. Front Cell Dev Biol. 2020;8:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luu VZ, Chowdhury B, Al-Omran M, Hess DA, Verma S. Role of endothelial primary cilia as fluid mechanosensors on vascular health. Atherosclerosis. 2018;275:196–204. [DOI] [PubMed] [Google Scholar]
- 25. Vion AC, Alt S, Klaus-Bergmann A, et al. Primary cilia sensitize endothelial cells to BMP and prevent excessive vascular regression. J Cell Biol. 2018;217(5):1651–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dilan TL, Moye AR, Salido EM, et al. ARL13B, a Joubert syndrome-associated protein, is critical for retinogenesis and elaboration of mouse photoreceptor outer segments. J Neurosci. 2019;39(8):1347–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–446. [DOI] [PubMed] [Google Scholar]
- 28. Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. [DOI] [PubMed] [Google Scholar]
- 29. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. [DOI] [PubMed] [Google Scholar]
- 30. Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–275. [DOI] [PubMed] [Google Scholar]
- 31. Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pinter M, Sieghart W, Schmid M, et al. Hedgehog inhibition reduces angiogenesis by downregulation of tumoral VEGF-A expression in hepatocellular carcinoma. United Eur Gastroenterol. 2013;1(4):265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen W, Tang T, Eastham-Anderson J, et al. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc Natl Acad Sci USA. 2011;108(23):9589–9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roy K, Jerman S, Jozsef L, et al. Palmitoylation of the ciliary GTPase ARL13b is necessary for its stability and its role in cilia formation. J Biol Chem. 2017;292(43):17703–17717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarkisian MR, Siebzehnrubl D, Hoang-Minh L, et al. Detection of primary cilia in human glioblastoma. J Neurooncol. 2014;117(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.