Abstract
The runner’s high is an ephemeral feeling some humans experience during and after endurance exercise. Recent evidence in mice suggests that a runner’s high depends on the release of endocannabinoids (eCBs) during exercise. However, little is known under what circumstances eCBs are released during exercise in humans. This systematic review sampled all data from clinical trials in humans on eCB levels following exercise from the discovery of eCBs until April 20, 2021. PubMed/NCBI, Ovid MEDLINE, and Cochrane library were searched systematically and reviewed following the PRISMA guidelines. From 278 records, 21 met the inclusion criteria. After acute exercise, 14 of 17 studies detected an increase in eCBs. In contrast, after a period of long-term endurance exercise, four articles described a decrease in eCBs. Even though several studies demonstrated an association between eCB levels and features of the runner’s high, reliable proof of the involvement of eCBs in the runner’s high in humans has not yet been achieved due to methodological hurdles. In this review, we suggest how to advance the study of the influence of eCBs on the beneficial effects of exercise and provide recommendations on how endocannabinoid release is most likely to occur under laboratory conditions.
Keywords: endocannabinoid, runner’s high, exercise, euphoria, endurance, running
Introduction
The search for the neurobiological causes of the runner’s high has fascinated scientists and laymen for the past decades. A runner’s high is defined as an emotional state during or after endurance training characterized by reduced pain sensitivity, sedation, euphoria, and reduced anxiety. Some have also emphasized a lost sense of time and feelings of effortlessness (Dietrich and Audiffren 2011).
Historically, studies of the 1980s (Appenzeller 1981; Carr and others 1981) claimed that the release of endorphins, a hydrophilic molecule binding to opioid receptors, is responsible for the runner’s high. The endorphin hypothesis was poorly supported by evidence, although it was widely perpetrated by the media (Dietrich and McDaniel 2004). Several findings speak against this hypothesis: First, peripheral endorphins do not have a major effect on the brain, as they cannot cross the blood-brain barrier due to their hydrophilic structure (Dietrich and McDaniel 2004). In line, a connection between peripheral endorphin levels during endurance exercise and elevated mood could not be found (Kraemer and others 1989). Second, blockage of the opioid system did not affect the subjective experience during endurance exercise (Farrell and others 1986; Markoff and others 1982).
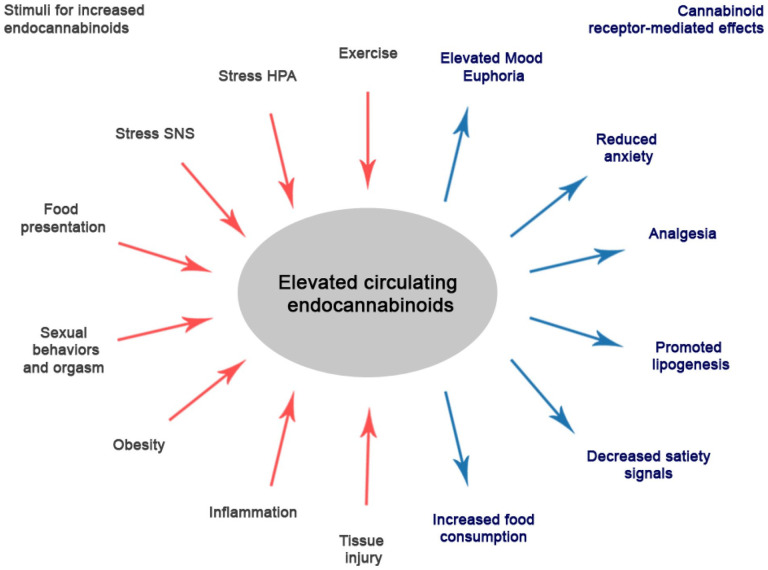
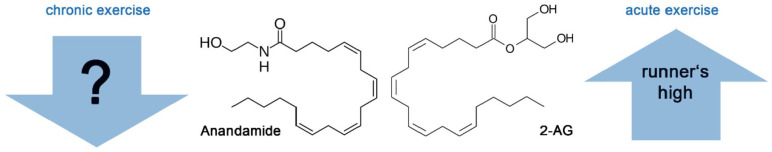
In the 1990s, two main endogenous endocannabinoids (eCBs) were discovered: arachidonoyl ethanolamide, which was termed “anandamide” (AEA) in 1992 (Devane and others 1992), and 2-arachidonoyl glycerol (2-AG) in 1995 (Mechoulam and others 1995). Their discovery led to the so-called endocannabinoid hypothesis of the runner’s high. In comparison to endorphins, eCBs are lipophilic molecules and can penetrate the blood-brain barrier easily, making them better candidates to explain the runner’s high (Dietrich and McDaniel 2004; Fuss and others 2015; Siebers and others 2021; Watkins 2018). The eCB system is a potent endogenous system involved in various physiological functions in the nervous system. Some involved physiological processes are synaptic transmission, mood, reward, anxiety, appetite, memory processing, neuroprotection, and neuroinflammation (Hillard 2018; Watkins 2018; Fig. 1). Furthermore, they also play important roles during neural development, for example, neuronal proliferation, neuronal migration, and axonal growth (Hillard 2015). The two main eCB compounds, AEA and 2-AG, bind to G-protein-coupled cannabinoid receptors CB1 and CB2. In addition, various related biogenic lipids are often described and sampled in addition to the eCBs since they involve the same precursors, such as N-acylated ethanolamine phospholipids (N-oleoylethanolamine [OEA], N-palmitoylethanolamine [PEA], N-stearoylethanolamine [SEA]) and diacylglycerol (2-oleoylglycerol [2-OG]).
Figure 1.
The release of endocannabinoids is triggered by various stimuli (red arrows). Higher levels of endocannabinoids in turn were associated with a plethora of (neuro)biological consequences (blue arrows). The figure is adapted and updated from Hillard (2018).
Interestingly, it seems that not every human can experience a runner’s high. For example, studies with endurance runners reported that only 69% to 77% of the participants experienced a runner’s high at least once in the past (Hinton and Taylor 1986; Siebers and others 2021). This finding may however also stem from a poor conceptualization of the runner’s high as participants are usually only asked whether they ever experienced this phenomenon without further description. This complicates research into the neurobiology of a runner’s high.
Despite the inconsistent occurrence in humans, the neurobiological basis of the endophenotypes of the runner’s high can be studied in animal models. Various studies indicated that eCB signaling is essential for voluntary wheel running in mice and rats (Dubreucq and others 2010; Dubreucq and others 2013; Fuss and others 2015; Galdino and others 2014). Studies also showed that two criteria of the runner’s high, hypoalgesia and anxiolysis, were indeed related to an increase in eCBs postexercise in animal models (Fuss and others 2015). However, another aspect of the runner’s high, euphoria, is yet not possible to study in animal models. Therefore, in recent years, there have been increasing efforts to investigate the relationship between characteristics of the runner’s high and eCBs in humans. This systematic review aims to provide a contemporary overview of the relation between endurance exercise and eCBs, and also addresses the four main features of the runner’s high: reduced pain sensitivity, sedation, euphoria, and reduced anxiety.
Method
A systematic search for trials concerning endurance exercise and the eCB system was conducted in accordance with the PRISMA guidelines (Shamseer and others 2015).
Inclusion Criteria
All trials that met the following criteria were included: (1) published in English in a peer-reviewed journal; (2) original article; (3) experimental trials with human participants; (4) containing aerobic exercise, namely, cycling, running, or hiking; (5) with a duration of at least 20 minutes; (6) low- to high-intensity levels; (7) measurement of eCB and eCB-like lipid levels in the blood before and after exercise or comparing eCBs in an exercise and control group.
Procedure
To provide an overview of studies regarding eCBs and the runner’s high, a keyword list was defined (endocannabinoid AND (running OR cycling OR hiking OR exercise OR physical activity)). First, PubMed/NCBI and Ovid MEDLINE, and Cochrane library were checked systematically until April 20, 2021. The Mesh Terms “Exercise” and “Endocannabinoids” were used if possible. For example, the following search was used in PubMed: (“Exercise”[Mesh]) AND (“Endocannabinoids”[Mesh]).
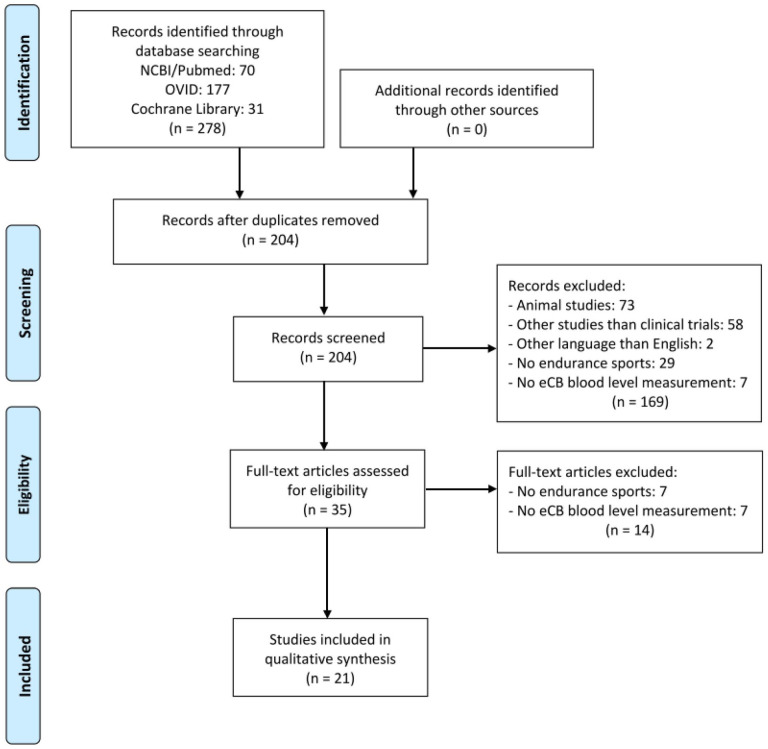
The list was complemented by studies from past reviews and personal libraries. First, by reading the titles and, if necessary, by reading the abstracts, all studies were ordered into two groups: animal studies and human studies by two independent reviewers (Fig. 2). Next, every unique article was examined to determine whether the studies follow the inclusion criteria by reading the abstracts. Especially, attention was paid to the criteria of time series eCB and eCB-like lipids comparison in blood and endurance exercise. After the articles’ discard, 35 studies met inclusion criteria and were further analyzed. In the end, 21 articles met the inclusion criteria with 31 samples (26 on acute exercise; 5 on long-term exercise). The studies were ordered alphabetically in two tables regarding acute exercise (Table 1) or long-term exercise training (Table 2) including 571 participants (243 women, 306 men, 22 not reported). In total, this systematic research included 378 participants in the study of acute exercise and 193 individuals who were studied regarding long-term exercise training. Nineteen of the 378 and 46 of the 193 participants did not perform exercise as they were part of a control group.
Figure 2.
Flow chart of literature search including all steps performed according to the PRISMA statement (Moher and others 2009).
Table 1.
Studies Regarding Acute exercise.
| Investigators | Participants (n) | Average Age (in Years) | Primary Exercise Stimulus | Exercise Intensity | Additional Warm-Up and Cool-Down | Control Condition | Blood Sampling | Further Detecting Methods | Time Main Effect of Postexercise Blood Levels | Further Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AEA | 2-AG | Other eCB | ||||||||||
| Antunes and others (2016) | n = 18 physically active men (aerobic exercise 5×/week) divided into a group with symptoms of sports addiction (SA, n = 8) and CON (n = 10) | SA: 32.9 ± 4.7 CON: 30.2 ± 3.1 |
60 minutes of running on a treadmill after 2 weeks of exercise deprivation period | Ventilatory threshold | NA | NA | At baseline, after 7 days of exercise deprivation, after 14 days of exercise deprivation, and postexercise | Q: POMS (version Brunel) at the same time-points as blood
sampling. Negative Addiction Scale and Exercise Dependence Scale were questioned at the first visit |
↑* only in CON | NA | NA | AEA was decreased significantly at all time points in SA compared to CON and
did not increase after the exercise. Depressive mood symptoms, fatigue, confusion, anger, and vigor loss increased in SA during withdrawal. There was a mood improvement after training in SA. No significant mood changes were detected in CON at all time points. |
| Marin Bosch and others (2020) | n = 15 healthy and recreational fit male participants (VO2max above 40 mL/kg/min and below 65 mL/kg/min) | 23.7 ± 4 | 45 minutes cycling on an ergometer (30 minutes at a moderate intensity and 15 minutes at a high intensity) | Moderate intensity: 70% AAMHR corresponding to 60%
VO2max High intensity: 80% AAMHR corresponding to 70% VO2max |
3 minutes warm-up; 3 minutes cool-down | 30 minutes resting | Before and after exercise/resting | Q: STAI, questionnaires for depression, and circadian typology M: A serial reaction time task before and after the condition, and an fMRI analysis 45 minutes after the condition |
↑# | NA | NA | AEA increased significantly after moderate and high intensity in comparison
with resting. High-intensity exercise increased the motor sequence memory significantly and a trend was visible for moderate-intensity exercise. The increase in motor sequence memory correlated with AEA expansion and coincided with local expansions in caudate nucleus and hippocampus activity. |
| Brellenthin and others (2017) | n = 36 healthy adults (18 women and 18 men) divided into three groups after their weekly physical activity (low: n = 11, moderate: n = 12, and high: n = 13) | 21.1 ± 3.8 | 45 minutes running on a treadmill | 70% to 75% VO2max | 10 minutes warm-up | 45 minutes running on a treadmill at preferred intensity and time | Before and after exercise | Q: PAR at the beginning POMS, STAI, CES before and after running |
↑* | ↑# | OEA: ↑* PEA: ↑# |
AEA and OEA increases were higher in prescribed running (P
< 0.05). There were significant decreases in tension (P < 0.05), depression (P < 0.01), anger (P < 0.05), TMD (P < 0.05), and increases in vigor (P < 0.01) after both exercise conditions. The decrease in state anxiety, TMD, and confusion was greater in the preferred condition (P < 0.05). |
| Brellenthin and others (2019) | n = 21 participants (12 men; 9 women) with substance use disorder divided into two groups: intensive outpatient treatment (IOP; n = 10) or IOP plus aerobic exercise treatment (IOP-EX; n = 11) | IOP: 35.0 ± 7.1 IOP-EX: 35.1 ± 0.2 |
6 weeks IOP plus exercise (30 minutes incline walking on a treadmill 3 times a week) | Rising intensities from the first 3 weeks from 65% to 70% till the last 3 weeks at an intensity of 75% of AAMHR | 5 minutes warm-up | IOP with 30 minutes sitting on a chair three times a week | Once a week before and after exercise or sitting | Q: POMS, Craving Questionnaires–Short Forms before and after treatment once a
week after exercise or sitting PHQ-9, GAD-7, SCQ, and PSS asked at baseline and after 6 weeks of treatment |
↑# only in IOP-EX (NR long-term effects of exercise) | ↔ (NR long-term effects of exercise) | NA | Both treatments reduced perceived stress (P < 0.01),
craving, tension, depression, anger, confusion, and TMD (all Ps
< 0.05). The exercise group experienced acute increases in vigor. |
| Cedernaes and others (2016) | n = 16 healthy men with a regular sleep rhythm of 7–9 hours | 22.9 ± 0.7 | 30 minutes ergometer cycling after three nights of sleep restriction (4.25-hour sleep opportunity) | Cycling: 75% of the VO2 reserve capacity | 5 minutes warm-up | 30 minutes of ergometer cycling after three nights of normal sleep (8.5-hour sleep opportunity) | At five time points: day 3 at 7:30 p.m.; day 4 at 8:30 a.m., ∼10 a.m. (pretreatment), ∼15 minutes postexercise, and ∼4 hours postexercise | NA | ↔ | ↑* only after 8.5 hours sleep and only after 15 minutes postexercise | OEA: ↑# (only after 4 hours postexercise) PEA: ↔ |
NA |
| Crombie and others (2018) | n = 24 participants (6 men, 18 women) divided into PTSD (n = 12; 3 men, 9 women) and CON (n = 12; 3 men, 9 women) | 25.0 ± 5.6 | 30 minutes running or walking on a treadmill | 70% to 75% AAMHR | 10 minutes warm-up | NA | Before and after exercise | Q: POMS, STAI, PANAS, MPQ-SF before and after running | ↑# | ↑* | OEA: ↑* PEA: ↔ 2-OG: ↔ |
Pain decreased after running (P = 0.001). State anxiety, negative affect, tension, fatigue, confusion, and TMD were reduced (all Ps = 0.001 to 0.050). Higher reductions in negative mood states occurred after exercise in the PTSD group than in the control group. |
| Crombie and others (2019) | n = 20 participants (5 men, 15 women) divided into PTSD (n = 10) and CON (n = 10) | 23.7 ± 7.2 | 30 minutes running or walking on a treadmill | 70% to 75% AAMHR | 5 minutes warm-up 5 minutes cool-down |
The Trier Social Stress Test (TSST) | Before and after exercise/TSST | Q: Before and after running/TSS, POMS, STAI, PANAS | ↑# | ↑* only in CON | OEA: ↑# PEA: ↔ |
Vigor (P = 0.023) and positive affect (P =
0.038) were increased after running. There were significant group × time interactions for tension (P = 0.025), depression (P = 0.040), fatigue (P = 0.005), confusion (P = 0.023), TMD (P = 0.05), state anxiety (P = 0.05), and negative affect (P = 0.05) with general reduction in the PTSD group after running. AEA increased after the TSST. |
| Crombie and others (2020) | n = 40 trauma-free women (n = 12), trauma-exposed women without PTSD (n = 14), and trauma-exposed women with PTSD (n = 14) | 24.1 ± 6.0 | 30 minutes running or walking on a treadmill | 70% to 75% AAMHR | 5 minutes warm-up 5 minutes cool-down |
40 minutes sitting | Before and after exercise/resting | Q: Before and after running/resting POMS, STAI, PANAS; RPE every 5 min during
exercise; after Neutral-Predictable-Unpredictable threat task (NPU)
AFRQ M: after exercise/resting NPU |
↑# | ↑# | OEA: ↑# PEA: ↑# |
Anxiety and fear ratings to NPU were significantly lower following exercise
compared to resting (P < 0.05). Participants with higher increases in peripheral sampled eCBs had higher reductions in anxiety and fear ratings to NPU following exercise. There were significant reductions in fatigue, confusion, TMD, and increases in positive affect after exercise in all groups (Ps < 0.05). A significant postexercise increase in all eCBs (Ps < 0.05) was measured in comparison to sitting. |
| Feuerecker and others (2012) | n = 12 young healthy men | 27.6; range: 24–38 | Protocol A (PA): physical exercise at a lower altitude Protocol B (PB): physical exercise by an active ascent to high altitude |
NR | NA | Protocol C (PC): passive ascent by a helicopter | Before the start, at the summit cottage 60 to 90 minutes upon arrival, in the morning after the overnight stay, and 60 to 90 minutes after returning to base camp the next day | NA | ↑* only in PA and PB | ↔ | NA | A more pronounced increase was found under high-altitude conditions in
PB. PC demonstrated no significant eCB level changes. |
| Heyman and others (2012) | n = 11 young well-trained male cyclists | 23.3 ± 5.1 | 60 minutes pedaling on an ergometer followed by a 30-minute intense endurance performance test (TT) | 55% of Wmax, and TT with a known endpoint equal to 30 minutes at 75% of Wmax | NA | NA | Before exercise, after 15 minutes sitting, after 60 minutes constant pedaling, after the end of the exercise, and 15 minutes after recovery | NA | ↑* only after TT | ↔ | OEA: ↑# after 60 minutes ↑# after TT PEA: ↑* after 60 minutes ↑# after TT |
AEA did not increase significantly after 60 minutes pedaling at an intensity
of 55% Wmax. Cortisol increased at TT and recovery in comparison to
baseline (Ps < 0.001). AEA correlated positively with cortisol. AEA, OEA and PEA were significantly higher after 15 minutes of recovery compared to the end of the exercise. |
| Meyer and others (2019) | n = 17 women with mayor depressive disorder | 40.8 ± 14.8 | 20 minutes prescribed ergometer cycling (PRI) | Intensity of 13 rated on the RPE | 5 minutes warm-up 5 minutes cool-down |
20 minutes of ergometer cycling with preferred intensity (PRE) | Serum before and within 10 minutes postexercise | Q: POMS, STAI before, 10 and 30 minutes postexercise; RPE every 5 minutes during exercise | ↑* only in PRI | ↔ | OEA:↑* only
in PRI PEA: ↔ |
All subscales of the POMS (Ps < 0.05) and TMD
(Ps < 0.01) were improved after exercise. STAI was just increased after PRI (P < 0.001). Significant negative correlations (Ps < 0.05) were observed 10 minutes after the PRE between AEA and depression, confusion, fatigue, TMD, and STAI. 30 minutes postexercise negative correlation between AEA and confusion, TMD, and state anxiety were detected. There were no significant associations between PRE and mood changes. |
| Raichlen and others (2012) | n = 10 recreationally fit humans, 8 mixed-breed dogs, and 8 ferrets | NR | 30 minutes running on a treadmill | 70% to 80% of AAMHR | NA | 30 minutes of walking calculated by Froude number | Before and after treatment | Q: PANAS before and after treatment | ↑* | ↔ | NA | Pre- and postpositive affect of the PANAS was positively correlated to the increase in AEA in humans. |
| Raichlen and others (2013) | n = 10 healthy regular runners (6 men and 4 women) | 31.9 ± 12.1 | 30 minutes running on a treadmill on four different days | Four different intensities: (I) HR < 50% AAMHR (II) HR ~ 70% AAMHR (III) HR ~ 80% AAMHR (IV) HR ~ 90% AAMHR |
NA | Walking on a treadmill (I) | Before and after treatment | NA | ↑* only in II and III | ↔ | NA | NA |
| Siebers and others (2021) | n = 63 recreationally active participants (32 female; 31 male) divided into a placebo (n = 31) and a naltrexone group (50 mg; n = 32) | Placebo group: 26.5 ± 1.0 Naltrexone group: 28.1 ± 1.1 |
45 minutes running on a treadmill | Running at a moderate-intensity of 70% to 85% AAMHR | 5 minutes warm-up | 45 minutes of walking on a treadmill with an intensity less than 50% of AAMHR | Before and after treatment | Q: STAI, VAS, IPAQ, AQ, Anxiety VAS after Human Elevated plus-maze M: Human Elevated plus-maze |
↑# | ↑# | PEA: ↑# Arachidonic acid: ↑# |
Endorphin blockade by naltrexone did not inhibit eCB release, anxiolytic effects, or euphoria after running. Euphoria was nearly twofold higher after running but remained roughly unchanged after walking. Anxiolytic effects were observed after the run condition (P = 0.024). |
| Sparling and others (2003) | n = 24 male athletes divided into a group of running (R; n = 8), a group of cycling (C; n = 8), and CON (n = 8) | 23.7 ± 9.4 | 45 minutes running or cycling | 70% to 80% AAMHR | 5 minutes warm-up | 50 minutes of resting seated | Before and after treatment | N/A | ↑# after R ↑* after C |
↔ | NA | Plasma levels of 2-AG showed a similar trend during sports, but not in sedentary controls. |
| Stensson and Grimby-Ekman (2019) | n = 32 participants (22 women, 10 men) divided into a group with chronic neck pain (CNP; n = 21) and CON (n = 11) | CNP: 50.8 ± 12.9 CON: 37.7 ± 15.9 |
30 minutes dynamic arm cycling | Increasing load and a steady pace of 25 laps/min | NA | NA | Before and 60 minutes after arm cycling | M: PPT using a handheld electronic pressure algometer and rated on a numeric rating scale before, immediately after, and 60 minutes after arm cycling | ↓* only in
CON ↔ only in CNP |
↔ | OEA: ↔ PEA: ↔ |
Pain intensity demonstrated no statistically significant change during the whole study. |
| Stone and others (2018) | n = 9 women (mean 61 years) recruited from a local choir | 61; range 55–67 | 30 minutes cycling (n = 8) performed in a group activity | NR | NA | 30 minutes of dancing, reading, singing performed in a group activity on various dates | Before and after activity | Q: PANAS before and after activity | ↔ | NA | OEA:↑* PEA: ↔ |
AEA, OEA (Ps < 0.05), and PEA (P <
0.01) increased after singing significantly. There was a significant improvement
in positive mood and emotions after singing (Ps < 0.01). All
activities included, OEA was correlated with positive mood effects
(P = 0.0025). Cycling had no impact on mood rating. |
CON = control group; NA = not applicable; NR = not reported; Q = questionnaires; M = further measurements; AEA = anandamide; 2-AG = 2-arachidonoyl glycerol; OEA = oleoylethanolamine; PEA = palmitoylethanolamine; 2-OG = 2-oleoylglycerol; SEA = stearoylethanolamide; POMS = Profile of Mood States; AQ = Acrophobia Questionnaire; IPAQ = International Physical Activity Questionnaire; TMD = Total Mood Disturbance; PANAS = Positive and Negative Affect Schedule; STAI = State-Trait Anxiety Inventory; AFRQ = Anxiety and Fear Rating Questionnaire; PAR = Physical Activity Recall; CES = Commitment to Exercise Scale; GAD-7 = Generalized Anxiety Disorder Scale-7; MPQ-SF = Short-form McGill Pain Questionnaire; PPT = pressure-pain threshold; VAS = visual analog scale; AAMHR = age-adjusted maximum heart rate; PTSD = posttraumatic stress disorder; RPE = rate of perceived exertion.
P < 0.05, #P < 0.01.
Table 2.
Studies Regarding the Long-Term Effects of Exercise Training.
| Investigators | Participants (n) | Age (in Years) | Primary Exercise Stimulus | Exercise Intensity | Additional Warm-Up and Cool-Down | Control Condition | Blood Sampling | Other Detecting Methods | Time Main Effect of Postexercise Blood Levels | Further Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AEA | 2-AG | ||||||||||
| Di Marzo and others (2009) | n = 49 viscerally obese men | Average age 49 years | One-year intervention of lifestyle modification program including nutrition changes and physical activity | NR | NA | NA | Before and after the 1-year lifestyle program | M: BMI and waist circumference; visceral adipose tissue (VAT) by computer tomography, and detection of triacylglycerol levels HDL3-cholesterol in blood before and after the program. | ↓# | ↓# | Levels of 2-AG correlated with decreases in VAT and triacylglycerol levels and
the increase in HDL3-cholesterol levels. BMI and waist circumference decreased significantly (Ps < 0.0001). |
| Oliveira and others (2019a) | n = 34 participants (18 women, 12 men, 4 NR) divided into exercise group (n = 17) and CON (n = 17) | 38 ± 11.5 | 12-week program of 30 minutes running or walking on a treadmill three times a week | VT | 5-minute warm-up; 5-minute cool-down | Routine sports activity defined as ≤1 day/week | Before and after 12 weeks of treatment/control after Q | Q: POMS before and after 12 weeks of treatment/control | ↓* only in the exercise group | NA | Anxiety, anger, TMD, and body weight decreased significantly in the exercise
group (Ps < 0.05). AEA decrease was associated with weight loss. |
| Oliveira and others (2019b) | n = 58 participants (41 women, 9 men, 8 NR) with or without migraine divided into migraine waitlist group (n = 15), migraine exercise group (M-EX; n = 15), control exercise group (CON-EX; n = 14), and control waitlist group (n = 14) | 36.2 ± 10.9 | A 12-week program of 30 minutes running on a treadmill three times a week | VT | 5-minute warm-up; 5-minute cool-down | Waiting list continuing routine activity | Before and after 12 weeks of treatment/control condition after Q | Q: POMS before and after 12 weeks of treatment/control condition, neurological
examination and checking of headache diaries every 4 weeks M: Cardiorespiratory fitness test at baseline and end 1 week after Q and blood sampling |
↓# only in CON-EX ↓* only in M-EX |
NA | The number of days with migraine (P < 0.01), migraine
attacks (P < 0.05), and abortive medication used
(P < 0.05) were decreased in the
M-EX. Cardiorespiratory fitness increased in M-EX and CON-EX (Ps < 0.05). Anxiety, depression, anger, and fatigue scores decreased in the M-EX (Ps < 0.05). There was a correlation between the reduction of abortive medication used and cardiorespiratory fitness (P < 0.001) as well as reduced AEA (P < 0.05). |
| Koay and others (2020) | n = 52 newly enlisted male soldiers | 22 ± 4 | 80-day exercise intervention as part of the Army Recruit Course | NR | NA | NA | Before and after the 80-day exercise intervention early in the morning | M: BMI, body fat, blood pressure, estimated VO2max | NA | ↓* | BMI (P = 0.02), body fat (P < 0.001),
blood pressure (P < 0.0001) decreased. The estimated VO2 max was increased (P < 0.0001) post-training intervention. |
NA = not applicable; NR = not reported; CON = control group; Q = questionnaires; M = further measurements; AEA = anandamide; 2-AG = 2-arachidonoyl glycerol; POMS = Profile of Mood States; TMD = Total Mood Disturbance; VT = ventilatory threshold; BMI = body mass index.
P < 0.05, #P < 0.01.
Results
Blood Sampling Methods and Exercise Time
Most of the articles focused on eCB blood levels before and after acute exercise (81%; Table 1). More than half of the studies observed eCB levels after exercise on a treadmill (57%) followed by cycling (29%). Only one study examined eCB levels during hiking in nature (Feuerecker and others 2012). In general, participants were performing exercise from 20 to 60 minutes (warm-up and cool-down excluded; see Tables 1 and 2) with an average time of 37 minutes. Mostly, blood was sampled before and immediately after acute exercise. One study measured eCBs in blood serum (Meyer and others 2019), whereas all other studies measured eCBs in blood plasma. Two studies sampled only blood after a break of more than 10 minutes after exercise (Cedernaes and others 2016; Stensson and Grimby-Ekman 2019). Articles that studied chronic exercise sampled blood before and after the long-term exercise program. Nearly all included articles reported on AEA levels (95%), 76% reported on 2-AG, 43% on OEA, 48% on PEA, and one study reported on AA (arachidonic acid) or 2-OG levels in blood (5%).
Acute Exercise and AEA Levels
The rise of AEA was a robust finding after a bout of exercise across studies. Fourteen of 17 articles (82% of the articles) detected an increase in AEA after acute exercise. Four samples did not find any difference (15%), and one sample observed a decrease in AEA (4%). Participants performed moderate-intensity exercise described as 70% to 85% of the age-adjusted maximum heart rate (AAMHR) in seven of the 17 studies and were controlled by a less intense condition (e.g., walking on a treadmill <50% AAMHR, or remaining seated for the same time). All studies with a control group described an increase in AEA compared to the control condition.
Acute Exercise and 2-AG Levels
The impact of acute exercise on 2-AG levels was less consistent. Five of 14 articles (12 of 23 samples; 52%) found an increase of 2-AG after acute exercise, and eight of 14 articles demonstrated no changes (11 of 23 samples; 48%). In six of the 14 studies, participants performed moderate-intensity exercise and were controlled by a less intense condition. Only two studies described an increase in 2-AG compared to the control condition.
Acute Exercise and Other eCB-Like Molecules
Seven of 10 studies (13 of 17 samples; 76%) found an increase in OEA after exercise, but only four of 10 studies (9 of 19 samples; 47%) found an increase in PEA. Furthermore, one study detected no change of 2-OG after exercise, and one study observed an increase in AA after exercise. Just two studies contained a moderate intensity and were controlled by a less intense condition. Both studies described an increase in OEA, PEA, or AA in comparison to the control condition.
Long-Term Endurance Exercise and eCBs
All four studies regarding exercise programs of at least 12 weeks detected a decrease in the eCBs studied, namely, AEA (3 studies; 100%) or 2-AG (2 studies; 100%). There were no measurements of other eCB-like molecules.
Anxiolytic Effects after Sport
Eight of 14 acute exercise studies and two of four long-term exercise studies observed changes in anxiety using either a questionnaire (Profile of Mood States [POMS] = 9, 90%; State-Trait Anxiety Inventory [STAI] = 5, 50%), Neutral-Predictable-Unpredictable threat task (NPU; n = 1), or a human elevated plus-maze (n = 1). The results were inconsistent. Eight studies (80%) and 12 samples (63%) described a decrease in anxiety after acute and long-term physical activity, whereas seven samples (37%) could not find any changes. Two studies described contradicting results. Brellenthin and others (2017) described a decrease in anxiety during preferred in contrary to prescribed exercise, in contrast to Meyer and others (2019), who reported that only prescribed exercise decreased anxiety.
Positive Mood Effects after Exercise
Eleven studies assessed mood via questionnaires. Eight studies used the POMS, four the positive affect subscale of the PANAS questionnaire and one study used a visual analog scale (VAS). Twelve samples found a decrease in Total Mood Disturbance (TMD) in the POMS, three found increased positive mood in the PANAS, and one study described euphoria after exercise on a VAS. In total, nine of 11 studies (82%) and 17 of 20 samples (85%) reported a positive effect of acute exercise on mood.
Hypoalgesia after Exercise
Only two studies focused on hypoalgesia effects after exercise. The results were inconsistent. One study described less pain after exercise (Crombie and others 2018), and one study described no pain changes in a pressure-pain threshold (PPT) task after exercise (Stensson and Grimby-Ekman 2019).
Sedation after Exercise
No study researched postexercise sedation effects.
Description of Selected Studies
Acute Endurance Exercise and Endocannabinoids
The first to demonstrate that physical exercise activates the eCB system were Sparling and colleagues in 2003 (Sparling and others 2003). They studied 24 male volunteers that regularly performed endurance exercise. Those that indicated to run or cycle were also assigned to their preferred exercise regime (i.e., running or cycling, respectively) or to a control condition. Participants performed exercise in a range of 70% to 80% of maximum heart rate (140–160 bpm) for 45 minutes, whereas control subjects remained seated for 50 minutes. Sparling and others (2003) found that AEA levels significantly increased compared to controls in both exercise groups. Data for 2-AG levels were not reported in the article, but the authors stressed that 2-AG levels showed a similar trend but did not reach statistical significance.
Nine years later, Heyman and colleagues (2012) investigated 11 young, well-trained male cyclists. They used a more standardized protocol than Sparling and others (2003), allowing no exercise, alcohol, or coffee 24 hours before testing and provided a standardized breakfast. The exercise started 150 minutes after breakfast with 60 minutes pedaling at 55% of their maximal power output (Wmax), immediately followed by a 30-minute endurance performance task at an intensity equal to 75% Wmax. They found that AEA levels increased after intense exercise and continued to rise after recovery, while 2-AG levels remained stable throughout the experiment. Moreover, the eCB-like lipids PEA and OEA gradually increased after moderate exercise, intense exercise, and recovery. However, 60 minutes of cycling at 55% of Wmax did not significantly increase AEA levels.
Another interesting study was performed by Raichlen and others (2012), who hypothesized that cursorial mammals and humans are equipped with a neurobiological make-up that facilitates moderate-intensity endurance exercise. They studied the eCB system’s response to exercise in a cursorial mammal species (dogs), a non-cursorial species (ferrets), and humans during 30 minutes of treadmill walking or running. They found that only dogs and humans showed an increase in AEA in response to exercise, while this was absent in non-cursorial ferrets. 2-AG showed no reaction to exercise. Interestingly, changes in AEA significantly correlated with changes in the positive affect subscale of the PANAS questionnaire. Moreover, under control conditions, both eCBs remained stable. Thus, the authors concluded that humans and dogs achieve physiological and psychological improvements through exercise-induced AEA release and hypothesized that this biological mechanism is more broadly evolved in some mammalian species to provide them with the ability to run long distances.
Raichlen and others (Raichlen and others 2013) further investigated in human runners how exercise intensity affects eCB levels in a follow-up study. The 10 participants were analyzed on four different days simultaneously on a treadmill, walking or running at four different intensities for 30 minutes in random order. Interestingly, Raichlen and colleagues found that only endurance exercise at ~70% and ~80% of AAMHR significantly affected AEA levels. This suggests that neither walking-speed training nor high-intensity running affects AEA. Following their arguments, 70% to 85% of AAMHR is the perfect range to achieve a runner’s high.
One study (Brellenthin and others 2017) examined the impact of mood response on prescribed or preferred exercise among individuals with various physical activity levels per week. They invited 36 participants and categorized them into low, moderate, and high physical activity groups based on their weekly physical activity. Participants performed prescribed running for 45 minutes (70% to 75% VO2max) and, on a second day, preferred running with participants’ choice of intensity. Running on a treadmill in prescribed and preferred conditions increased AEA, 2-AG, OEA, and PEA significantly in all groups. Intensity of weekly physical activity did not influence mood or eCB release. However, OEA and AEA were higher in the prescribed condition. There were significant effects on tension, depression, anger, and increases in vigor after both exercise conditions in questionnaires. In comparison to the prescribed condition, state anxiety, TMD, and confusion were higher in the preferred condition.
The specific influence of the surroundings on the runner’s high is not fully understood. In a less standardized paradigm, just one study (Feuerecker and others 2012) investigated in nature how hiking in the alps under hypoxic conditions affects the eCB system. Healthy young male volunteers (n = 12) performed hiking for several hours at two different altitudes. They found that AEA increases after exercise, and they discovered a more pronounced increase under high altitude conditions. However, the high altitude conditions alone did not affect AEA levels under control conditions (no-exercise) when participants were transported with a helicopter to the same altitude. Moreover, 2-AG levels were not affected by hiking.
Including a functional magnetic resonance task, the effects of exercise on motor sequence memory in brain and its correlation to AEA were studied (Marin Bosch and others 2020). In a crossover design, on three different time points, 15 participants cycled for 30 minutes at moderate intensity (70% VO2max), for 15 minutes at a high intensity (80% VO2max), or rested for 30 minutes. Before and after the condition, a serial reaction time task took place where participants needed to execute a sequence of keypresses with four fingers during an fMRI analysis to investigate memory for motor sequences. As a result, again, AEA increased significantly after high and moderate intensity. High-intensity cycling enhanced the motor sequence memory significantly and a trend was observed for moderate-intensity exercise. This improvement correlated with AEA increase and coincided with local expansions in caudate nucleus and hippocampus activity. For the first time, this article implies that eCBs interact with brain signaling after exercise in humans involving hippocampus-related functions.
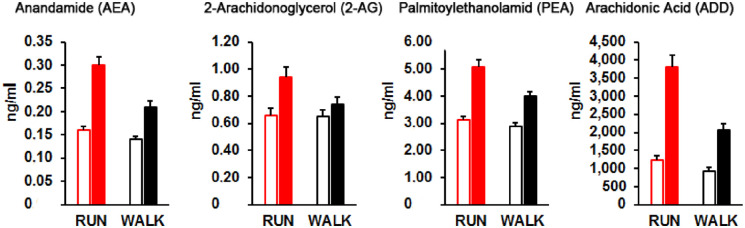
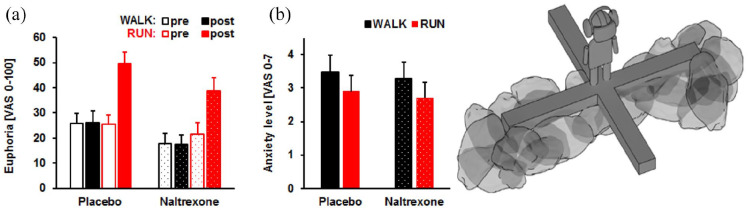
In a double-blind, randomized within-subject study, 63 recreationally active participants received 50 mg of the opioid antagonist naltrexone or an identical-looking placebo to further clarify whether endorphins or eCBs are essential for the runner’s high (Siebers and others 2021). Next, on two different dates, they ran 45 minutes at an intensity of 70% to 85% of AAMHR or walked 45 minutes (<50% AAMHR) on a treadmill. Blood samples and a VAS about the participant’s emotional state were acquired directly before and after each condition. Anxiety was assessed in a human elevated plus-maze using virtual reality. All eCBs increased significantly after both conditions but were twofold higher after running (Fig. 3). Euphoria was also nearly twofold higher after running but remained roughly unchanged after walking. Moreover, anxiolytic effects were observed after running (Fig. 4). Opioid blockade did not inhibit anxiolytic effects or euphoria after running. Also, the release of eCBs was not prevented by opioid blockage after running and walking. In conclusion, this study suggests that the runner’s high does not depend on endorphins (Siebers and others 2021).
Figure 3.
Moderate-intensity running (RUN) stimulates endocannabinoid-release significantly more pronounced than low-intensity walking (WALK). Left open columns represent Meanpre + SEM and solid (red and black) columns Meanpost + SEM. Adapted from Siebers and others (2021).
Figure 4.
(A) Euphoria levels increase in the running (RUN) compared to walking (WALK) condition in the placebo (n = 31) and naltrexone-treated (n = 32) groups. (B) Anxiety levels on the human elevated plus-maze are lower after RUN compared to WALK in both groups (NAL vs. PLA). Columns represent Means + SEM. The left panels are reprinted from Siebers and others (2021), with permission from Elsevier. The right panel shows a schematic depiction of the human EPM adapted from Biedermann and others (2016).
It is important to emphasize that some studies did not detect an increase in AEA after exercise. Cedernaes and others (2016) were studying 16 young and healthy male volunteers who performed 30 minutes of exercise on an ergometer at 75% of VO2 reserve capacity. The participants exercised after three nights with 8 hours sleep opportunity and, on another period, with three nights of 4.25 hours sleep opportunity. As a result, no increase in AEA, but 2-AG was found in the study. Furthermore, OEA increased significantly 4 hours postexercise with and without sleep restriction. Of note, between the termination of exercise and blood sampling was a 15-minute time gap, which may have affected the results. In contrast, after three nights of sleep restriction, the group observed no significant 2-AG release.
Moreover, in a study where 21 participants with chronic pain were compared with 11 healthy controls in 30 minutes of arm cycling with an increasing workload, no increase in eCBs was found (Stensson and Grimby-Ekman 2019). PPT was tested before, immediately, and 60 minutes after the physical activity. Pain intensity demonstrated no significant changes during all time points within both groups. Conversely, AEA was significantly decreased 60 minutes after the exercise in healthy controls. No other time-condition changes were detected in the eCB system. Importantly, the study’s intensity was not focused on individual aspects, and the blood was drawn 60 minutes after the bout of exercise. Thus, the study might have analyzed the homeostatic downregulation of the eCB system.
In line, Stone and others (2018) did not detect significant AEA changes after 30 minutes of cycling in a spin class. They recruited nine women from a choir and observed AEA, OEA, and PEA in four different conditions of group activity: 30 minutes of dancing, reading, singing, or spinning. Interestingly, OEA was the only eCB-like lipid, which increased after 30 minutes of spin class. Notably, the heart frequency measured immediately after the bout of exercise was less than the 70% AAMHR suggested by Raichlen and others (2013). One further explanation for the absence of an eCB increase might be that the eight participating women from the choir were not used to spinning class or dancing class. In contrast, the participants showed a significant increase in all measured eCBs and eCB-like substances during a regular singing class in the choir. Also, mood and positive emotions increased significantly after singing but not after cycling. Thus, this study suggests that the experience of positive emotions may somehow be necessary to release eCBs. Furthermore, the study indicates that other activities than endurance sports can activate the eCB system.
Long-Term Consequences of Exercise on the Endocannabinoid System
Less is known about the effects of long-term training on the eCB system. Koay and others (2020) studied how an 80-day exercise intervention as part of the Army Recruit Course affects various metabolic substrates. In this study, 52 young, male, and lately enlisted soldiers were included. Laboratory parameters (e.g., 2-AG), body mass index (BMI), body fat, blood pressure, and estimated VO2max were measured before and after an 80-day exercise program containing moderate-intensity aerobic and strength exercise. Among various changes after the exercise program, 2-AG was 1.11-fold decreased. Furthermore, BMI, body fat, and blood pressure were reduced, and VO2max was increased after the Army Recruit Course.
Compared with a control group, one study (Antunes and others 2016) found decreased AEA levels in a group of exercise addiction at all time points during a 2-week exercise deprivation period. For this trial, they invited 18 participants who regularly performed five times per week exercise and divided participants into a control group (n = 10) and an exercise-addicted group (n = 8). Next, an exercise withdrawal for 2 weeks took place, followed by running on a treadmill for 60 minutes at ventilatory threshold intensity. Blood was sampled at baseline, after 7 days, after 14 days, and post running. Strikingly, lower AEA levels at all time points in the exercise-addicted group compared with the control group were found. Running did elevate AEA levels in the control group, only. Within the exercise-addicted group, withdrawal of exercise increased depressive mood symptoms, fatigue, confusion, anger, and a loss of vigor. Because of these results, Antunes and colleagues hypothesized that individuals with exercise addiction might have a dysfunctional eCB system.
Meanwhile, in a study of 49 obese men, which took part in a 1-year lifestyle modification program, including nutrition changes and physical activity, various anthropometric and metabolic risk factors, as well as the eCBs AEA and 2-AG before and after the 1-year lifestyle change, were measured. Unfortunately, the intensity and modality of the physical activity were not described further. However, AEA and 2-AG decreased significantly after a 1-year lifestyle change, while 2-AG correlated with decreased visceral adipose tissue and triacylglycerol levels (Di Marzo and others 2009).
Moreover, in a secondary analysis changes in mood after a 12 weeks program (anxiety, anger, TMD) as well as body weight were at least partly attributed to a significant decrease in AEA, which was measured at the beginning and end of the study (Oliveira and others 2019a). For this study, 30 healthy inactive men were divided into two groups. One group performed a 12-week sports program running three times a week for 40 minutes at the ventilatory threshold. The control group remained in their physically inactive lifestyle, defined as ≤1 day/week of leisure-time physical activity.
Discussion
This systematic review demonstrates that most studies published up to date reported a significant increase in eCBs after acute exercise. Those studies that did not find a significant increase in AEA used a low exercise intensity (Raichlen and others 2013) or a high latency until blood was sampled after the exercise task (Stensson and Grimby-Ekman 2019). Furthermore, habituation to exercise might play a role (Stone and others 2018). Meanwhile, an increase in 2-AG was only found in six out of 14 studies. One possible explanation could be the fact that several studies had small sample sizes, ranging from 8 to 21 exercising participants, suggesting they may have been insufficiently powered to detect changes in 2-AG. While acute exercise increases eCBs and eCB-like lipids, the contrary was found for long-term endurance exercise programs, which consistently found a decrease in eCB levels. However, the finding that long-term exercise programs decrease eCBs must be interpreted with caution. Up to date, only four studies were published on that topic and several possible biases must be considered. Apart from a possible reporting bias, changes in BMI and fat tissue following long-term exercise may have an impact on the eCB system.
One aim of this review was to summarize the current evidence regarding an association between the runner’s high and the eCB system. From the core features of a runner’s high (euphoria, anxiolysis, hypoalgesia, and sedation), sedation was the only one not assessed in human studies.
Exercise consistently had a positive effect on mood. For example, euphoria and feelings of happiness were reported after acute exercise (Siebers and others 2021). Another study found a significant association between positive affect and AEA levels after acute exercise, even though no significant increase in AEA through the exercise intervention was detected (Raichlen and others 2012). While the self-reported positive effects of endurance exercise are a robust finding across studies (Berger and Motl 2000; Reed and Ones 2006; Yeung 1996), the possible role of eCBs, and particularly AEA, has come into focus only recently.
The anxiolytic effect of exercise is well documented (Ensari and others 2015; Petruzzello and others 1991). In this review, 80% of the articles found an anxiolytic effect after a bout of exercise (see Table 1). Studies that investigated endurance exercise in vulnerable groups also detected significant effects on anxiety, for example, for people with major depression (Meyer and others 2019), migraine (Oliveira and others 2019b), substance use disorder (Brellenthin and others 2019), and posttraumatic stress disorder (Crombie and others 2018; Crombie and others 2019; Crombie and others 2020). The anxiolytic effects of a bout of exercise were also detected in an anxiety-provoking virtual reality paradigm (Siebers and others 2021). Moreover, associations with the eCB system were found in several studies. In exercising women suffering from major depression, a negative correlation between AEA levels and state anxiety (STAI) after exercise was found. In a study where participants received predictable and unpredictable electric shocks in an NPU task a higher increase in eCBs was associated with a higher decrease in anxiety and fear ratings (Crombie and others 2020).
Whether or not or under which circumstances exercise leads to hypoalgesia is still a matter of debate. From the studies reviewed here, one could find a hypoalgesic effect through pain measurement after 30 minutes of running (Crombie and others 2018), while in a group of patients with fibromyalgia and a control group no such effect was detected (Stensson and Grimby-Ekman 2019). These inhomogeneous findings are also reflected in two reviews on this topic (Dannecker and Koltyn 2014; Koltyn 2000). Various variables seem to impact the detection of hypoalgesia after endurance exercise, namely, the method of pain testing, time-points of detection, instruments used for assessing pain (questionnaire, VAS), participants’ health condition, exercise form, exercise intensity, and exercise duration.
A milestone in describing exercise-induced hypoalgesia in relation to the eCB system was the study of Koltyn and others (2014). This study was not included in this review because they used short-duration isometric exercise to study eCBs. They detected less pain after 3 minutes of submaximal isometric handgrip exercise. Furthermore, opioid blockade by naloxone did not demonstrate changes in pain perception. AEA, 2-AG, OEA, PEA, 2-OG, and N-docsahexaenoylethanolamine increased significantly postexercise. The latter showed a significant association with exercise-induced hypoalgesia (Koltyn and others 2014).
Even though a sedative effect of running is often assumed, none of the 21 studies reviewed here reported an effect of exercise on alertness. Thus, there is still no evidence that the fourth criterion of a runner’s high, namely, sedation, is indeed associated with the eCB system. Meanwhile, a study in mice suggests that sedation is an unspecific consequence of exercise that does not require eCB signaling (Fuss and others 2015).
Provoking a runner’s high might be challenged by several factors. Lactate thresholds might differ between individuals, and it is not possible to determine the lactate threshold for all persons at 85% of AAMHR or 75% VO2max (Meyer and others 1999). Moreover, a rise in lactate levels in the blood can affect metabolization in the brain which might influence eCB signaling (Basso and Suzuki 2017). Thus, different exercise intensities and individual cardiorespiratory fitness levels might impact responses of the endocannabinoid system. Future research into endocannabinoid-mediated mechanisms might address these variations by measuring individual lactate changes or cardiorespiratory fitness.
These factors, as well as heterogeneities in how blood sampling and processing were performed, could explain the negative findings in some studies that investigated eCB after acute exercise.
The Hemostasis Theory
Several studies described a decrease in eCBs as a long-term consequence of an exercise program (Di Marzo and others 2009; Koay and others 2020; Oliveira and others 2019a; Oliveira and others 2019b) and 60 minutes postexercise (Stensson and Grimby-Ekman 2019). This was found for 2-AG after the 80-day exercise intervention (Koay and others 2020), as well as for AEA after a 12-week exercise program (Oliveira and others 2019a; Oliveira and others 2019b). The decrease in eCBs as a long-term consequence was also detected in a study in mice where the level of AEA even correlated negatively with the daily running distance after long-term wheel running (Biedermann and others 2016).
One possible mechanism is an increase in FAAH (fatty acid amide hydrolase) activity in lymphocytes, which was found in physically active men compared to sedentary controls (Gasperi and others 2014). In an in vitro experiment, IL-6 led to activation of the FAAH promoter in human lymphocytes. Thus, FAAH activity is enhanced and might modulate eCB levels in the plasma of physically active people. The authors hypothesized that the interaction between IL-6 (interleukin-6) and FAAH might be an adaptation process to cope with increased eCB levels in individuals performing endurance sports regularly (Gasperi and others 2014). This interesting mechanism should be further investigated in humans performing endurance exercise.
ECBs and the Influence on Stress
In the past, various studies demonstrated an association between the hypothalamic-pituitary-adrenal axis and eCBs. For example, a stress task can increase AEA levels (Crombie and others 2019). Furthermore, an increase in cortisol after exercise correlated with AEA levels (Heyman and others 2012). A further striking approach was made by Strewe and colleagues analyzing eCB levels in cosmonauts during spaceflight (Strewe and others 2012). ECBs were significantly increased during a parabolic flight and life onboard the International Space Station (ISS) in cosmonauts without motion sickness. These cosmonauts were in low-stress conditions. Conversely, in cosmonauts with motion sickness as well as higher-rated stress levels, an eCB increase was absent, and a massive rise in cortisol was detected.
Following the concept of allostasis helps us interpret the results. McEwen and Gianaros (2010) described that the body processes a stressor by physiological alterations of the HPA axis, hormones, autonomic nervous system, or cytokines to realize short-term adaptation, that is, allostasis. These mechanisms can lead to long-term dysregulation, contributing to chronic maladaptation, so-called allostatic load. Endurance sports, especially in clinical trials, might be a stressor. Thus, the increase in cortisol in the study of Heyman and others (2012) and Crombie and others (2019) is not surprising. Interestingly, there was a correlation between eCBs and cortisol, as well as increased eCB levels leading to the hypothesis that eCBs modulate stress responses (Hillard 2018). The release of eCBs following exercise may thus be an important factor in shaping how the stress inflicted by exercise is perceived, which may be crucial in the long-term motivation to perform endurance exercise.
Limitations
Three studies did not report any intensity measures during endurance sports (Di Marzo and others 2009; Feuerecker and others 2012; Koay and others 2020). However, the description of the endurance exercise regime (1-year lifestyle program, 80-day Army Recruit Course, and hiking in the Alps) indicated that the intensity was rather high and should thus meet our endurance exercise inclusion criteria.
Future Research
An ongoing and open question is where and how exercise-induced eCBs may affect the brain. Using an fMRI task, increased activity in the caudate nucleus and hippocampus was found in a previous study also investigating eCBs (Marin Bosch and others 2020). However, no correlation between AEA release and brain activity was detected. Furthermore, all studies included in this work focus on eCB ligands in blood. However, there might be significant changes in the eCB receptor expression and activity in the brain as were shown in animal studies (de Chiara and others 2010; Di Marzo and others 2009; Gomes da Silva and others 2010; Zhou and Shearman 2004). A study protocol with a positron emission tomography and an eCB-ligand would be an important step toward the understanding of the brain structures involved with the positive effects of exercise-induced eCBs in humans (Boecker and others 2008).
We proposed that FAAH activity in lymphocytes may be responsible for the downregulation of the eCB system after long-term exercise, for example, to protect the body from high eCB levels (Gasperi and others 2014). This mechanism needs to be further studied.
Furthermore, there is still a lack of research regarding exercise-induced hypoalgesia during acute exercise. While some evidence suggests that exercise-induced hypoalgesia may be explained through endocannabinoid release during short-duration isometric exercise (Koltyn and others 2014), studies included in this review that investigated acute endurance exercise were inconsistent (Crombie and others 2018; Stensson and Grimby-Ekman 2019). Future research should address this topic.
Even though some people perform endurance exercise over several hours, up until today, no study evaluated eCB levels after 60 minutes at 70% to 85% of the AAMHR. It might be that the eCB system is also affected when energy metabolization processes of the body change during longer exercise regimes. A trial with sampling blood during a marathon would be an elegant path to evaluate the eCB system over time.
Next, just one study in this review was performed outside the laboratory (Feuerecker and others 2012). Surroundings during exercise might impact the eCB system and might help produce a runner’s high. Future research should focus on such contextual factors.
Conclusion and a Recipe to Stimulate Endocannabinoid Release under Laboratory Conditions
Acute aerobic exercise was found to activate the eCB system. There were significant increases in AEA and less frequently in 2-AG after both preferred and prescribed exercise. Exercise-induced increases in eCBs seem to be associated with features of a runner’s high, namely, decreased levels of anxiety and increased euphoria. There is some evidence that eCBs are associated with decreased perception of pain after exercise. Meanwhile, evidence for associations between eCBs and sedation postexercise are scarce, yet. Chronic aerobic exercise, on the other hand, is associated with decreased levels of eCBs and the neurobiological consequences of the supposed downregulation of the eCB system are not clear yet (Fig. 5).
Figure 5.
The endocannabinoids anandamide and 2-AG are increased after acute exercise and the increase is associated with features of the runner’s high. In contrast, chronic exercise leads to a downregulation of the endocannabinoids and the neurobiological consequences of this downregulation are not yet clear.
After reviewing the existing literature, we suggest the following recommendations on how to stimulate endocannabinoid release under laboratory conditions and thus produce a runner’s high:
Running seems to be the best way to increase eCB levels in the blood, followed by cycling (Sparling and others 2003).
Intensities of 70% to 85% of AAMHR imply to be the best range to achieve an increase in AEA and less frequently in 2-AG (Heyman and others 2012; Marin Bosch and others 2020; Raichlen and others 2013; Siebers and others 2021).
Duration should be at least 20 minutes to achieve anxiolytic (Petruzzello and others 1991), analgesic (Rice and others 2019), and positive mood effects (Berger and Motl 2000). The highest positive mood effects can be expected after 30 to 35 minutes (Reed and Ones 2006).
Surroundings, like exercising in nature, might play a significant role (Feuerecker and others 2012).
Prior experience in the chosen exercise performance might play an essential role (Stone and others 2018).
The highest eCB levels can be sampled immediately after exercise. An eCB increase can be detected up to 15 minutes postexercise (Cedernaes and others 2016; Heyman and others 2012).
Positive affect can be detected at least 30 minutes postexercise (Reed and Ones 2006).
Adequate questionnaires to analyze a runner’s high are for euphoria a VAS, PANAS, or POMS, for anxiety the STAI, and for pain a standard numerical scale (Koltyn and others 2014).
Methods for measuring individual fitness are the IPAQ (Siebers and others 2021) or, more invasive, lactate threshold measurement as well as VO2max measurement.
Footnotes
Author Contributions: MS and JF performed the searches and the screening of manuscripts. MS performed the data analysis. All authors assisted with data interpretation and drafting of the manuscript, as well as reading and approving the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Johannes Fuss  https://orcid.org/0000-0003-0445-5021
https://orcid.org/0000-0003-0445-5021
References
- Antunes HKM, Leite GSF, Lee KS, Barreto AT, Santos RVTD, Souza HdS, and others. 2016. Exercise deprivation increases negative mood in exercise-addicted subjects and modifies their biochemical markers. Physiol Behav 156:182–90. [DOI] [PubMed] [Google Scholar]
- Appenzeller O. 1981. What makes us run? N Engl J Med 305(10):578–80. [DOI] [PubMed] [Google Scholar]
- Basso JC, Suzuki WA. 2017. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: a review. Brain Plast 2(2):127–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger BG, Motl RW. 2000. Exercise and mood: a selective review and synthesis of research employing the profile of mood states. J Appl Sport Psychol 12(1):69–92. [Google Scholar]
- Biedermann SV, Auer MK, Bindila L, Ende G, Lutz B, Weber-Fahr W, and others. 2016. Restricted vs. unrestricted wheel running in mice: effects on brain, behavior and endocannabinoids. Horm Behav 86:45–54. [DOI] [PubMed] [Google Scholar]
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, and others. 2008. The runner’s high: opioidergic mechanisms in the human brain. Cereb Cortex 18(11):2523–31. [DOI] [PubMed] [Google Scholar]
- Brellenthin AG, Crombie KM, Hillard CJ, Brown RT, Koltyn KF. 2019. Psychological and endocannabinoid responses to aerobic exercise in substance use disorder patients. Subst Abus 42(3):272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brellenthin AG, Crombie KM, Hillard CJ, Koltyn KF. 2017. Endocannabinoid and mood responses to exercise in adults with varying activity levels. Med Sci Sports Exerc 49(8):1688–96. [DOI] [PubMed] [Google Scholar]
- Carr DB, Bullen BA, Skrinar GS, Arnold MA, Rosenblatt M, Beitins IZ, and others. 1981. Physical conditioning facilitates the exercise-induced secretion of beta-endorphin and beta-lipotropin in women. N Engl J Med 305(10):560–3. [DOI] [PubMed] [Google Scholar]
- Cedernaes J, Fanelli F, Fazzini A, Pagotto U, Broman JE, Vogel H, and others. 2016. Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology 74:258–68. [DOI] [PubMed] [Google Scholar]
- Crombie KM, Brellenthin AG, Hillard CJ, Koltyn KF. 2018. Psychobiological responses to aerobic exercise in individuals with posttraumatic stress disorder. J Trauma Stress 31(1):134–45. [DOI] [PubMed] [Google Scholar]
- Crombie KM, Cisler JM, Hillard CJ, Koltyn KF. 2020. Aerobic exercise reduces anxiety and fear ratings to threat and increases circulating endocannabinoids in women with and without PTSD. Mental Health Phys Act 20(12):100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie KM, Leitzelar BN, Brellenthin AG, Hillard CJ, Koltyn KF. 2019. Loss of exercise- and stress-induced increases in circulating 2-arachidonoylglycerol concentrations in adults with chronic PTSD. Biol Psychol 145:1–7. [DOI] [PubMed] [Google Scholar]
- Dannecker EA, Koltyn KF. 2014. Pain during and within hours after exercise in healthy adults. Sports Med 44(7):921–42. [DOI] [PubMed] [Google Scholar]
- de Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, and others. 2010. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology 35(2):374–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, and others. 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258(5090):1946–9. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Côté M, Matias I, Lemieux I, Arsenault BJ, Cartier A, and others. 2009. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia 52(2):213–7. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Audiffren M. 2011. The reticular-activating hypofrontality (RAH) model of acute exercise. Neurosci Biobehav Rev 35(6):1305–25. [DOI] [PubMed] [Google Scholar]
- Dietrich A, McDaniel WF. 2004. Endocannabinoids and exercise. Br J Sports Med 38(5):536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq S, Durand A, Matias I, Bénard G, Richard E, Soria-Gomez E, and others. 2013. Ventral tegmental area cannabinoid type-1 receptors control voluntary exercise performance. Biol Psychiatry 73(9):895–903. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. 2010. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol 224(1):106–13. [DOI] [PubMed] [Google Scholar]
- Ensari I, Greenlee TA, Motl RW, Petruzzello SJ. 2015. Meta-analysis of acute exercise effects on state anxiety: an update of randomized controlled trials over the past 25 years. Depress Anxiety 32(8):624–34. [DOI] [PubMed] [Google Scholar]
- Farrell PA, Gustafson AB, Garthwaite TL, Kalkhoff RK, Cowley AW, Morgan WP. 1986. Influence of endogenous opioids on the response of selected hormones to exercise in humans. J Appl Physiol 61(3):1051–7. [DOI] [PubMed] [Google Scholar]
- Feuerecker M, Hauer D, Toth R, Demetz F, Hölzl J, Thiel M, and others. 2012. Effects of exercise stress on the endocannabinoid system in humans under field conditions. Eur J Appl Physiol 112(7):2777–81. [DOI] [PubMed] [Google Scholar]
- Fuss J, Steinle J, Bindila L, Auer MK, Kirchherr H, Lutz B, and others. 2015. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci U S A 112(42):13105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdino G, Romero TRL, Silva JFP, Aguiar DC, de Paula AM, Cruz JS, and others. 2014. The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology 77:313–24. [DOI] [PubMed] [Google Scholar]
- Gasperi V, Ceci R, Tantimonaco M, Talamonti E, Battista N, Parisi A, and others. 2014. The fatty acid amide hydrolase in lymphocytes from sedentary and active subjects. Med Sci Sports Exerc 46(1):24–32. [DOI] [PubMed] [Google Scholar]
- Gomes da Silva S, Araujo BHS, Cossa AC, Scorza FA, Cavalheiro EA, Naffah-Mazzacoratti MdG, and others. 2010. Physical exercise in adolescence changes CB1 cannabinoid receptor expression in the rat brain. Neurochem Int 57(5):492–6. [DOI] [PubMed] [Google Scholar]
- Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, and others. 2012. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—possible implications for reward and depression. Psychoneuroendocrinology 37(6):844–51. [DOI] [PubMed] [Google Scholar]
- Hillard CJ. 2015. The endocannabinoid signaling system in the CNS: a primer. Int Rev Neurobiol 125:1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ. 2018. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 43(1):155–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton ER, Taylor S. 1986. Does placebo response mediate runner’s high? Percept Mot Skills 62(3):789–90. [DOI] [PubMed] [Google Scholar]
- Koay YC, Stanton K, Kienzle V, Li M, Yang J, Celermajer DS, and others. 2020. Effect of chronic exercise in healthy young male adults: a metabolomic analysis. Cardiovasc Res 117(2):613–22. [DOI] [PubMed] [Google Scholar]
- Koltyn KF. 2000. Analgesia following exercise: a review. Sports Med 29(2):85–98. [DOI] [PubMed] [Google Scholar]
- Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, Hillard C. 2014. Mechanisms of exercise-induced hypoalgesia. J Pain 15(12):1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer RR, Blair S, Kraemer GR, Castracane VD. 1989. Effects of treadmill running on plasma beta-endorphin, corticotropin, and cortisol levels in male and female 10K runners. Eur J Appl Physiol Occup Physiol 58(8):845–51. [DOI] [PubMed] [Google Scholar]
- Marin Bosch B, Bringard A, Logrieco MG, Lauer E, Imobersteg N, Thomas A, and others. 2020. Effect of acute physical exercise on motor sequence memory. Sci Rep 10(1):15322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoff RA, Ryan P, Young T. 1982. Endorphins and mood changes in long-distance running. Med Sci Sports Exerc 14(1):11–5. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. 2010. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 1186:190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, and others. 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50(1):83–90. [DOI] [PubMed] [Google Scholar]
- Meyer JD, Crombie KM, Cook DB, Hillard CJ, Koltyn KF. 2019. Serum endocannabinoid and mood changes after exercise in major depressive disorder. Med Sci Sports Exerc 51(9):1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Gabriel HH, Kindermann W. 1999. Is determination of exercise intensities as percentages of VO2max or HRmax adequate? Med Sci Sports Exerc 31(9):1342–5. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira AB, de Mello MT, Tufik S, Peres MFP. 2019. Weight loss and improved mood after aerobic exercise training are linked to lower plasma anandamide in healthy people. Physiol Behav 201:191–7. [DOI] [PubMed] [Google Scholar]
- de Oliveira AB, Ribeiro RT, Mello MT, Tufik S, Peres MFP. 2019. Anandamide is related to clinical and cardiorespiratory benefits of aerobic exercise training in migraine patients: a randomized controlled clinical trial. Cannabis Cannabinoid Res 4(4):275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzello SJ, Landers DM, Hatfield BD, Kubitz KA, Salazar W. 1991. A meta-analysis on the anxiety-reducing effects of acute and chronic exercise. Outcomes and mechanisms. Sports Med 11(3):143–82. [DOI] [PubMed] [Google Scholar]
- Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A. 2012. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high”. J Exp Biol 215(Pt 8):1331–6. [DOI] [PubMed] [Google Scholar]
- Raichlen DA, Foster AD, Seillier A, Giuffrida A, Gerdeman GL. 2013. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol 113(4):869–75. [DOI] [PubMed] [Google Scholar]
- Reed J, Ones DS. 2006. The effect of acute aerobic exercise on positive activated affect: a meta-analysis. Psychol Sport Exer 7(5):477–514. [Google Scholar]
- Rice D, Nijs J, Kosek E, Wideman T, Hasenbring MI, Koltyn K, and others. 2019. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J Pain 20(11):1249–66. [DOI] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, and others. 2015. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647. [DOI] [PubMed] [Google Scholar]
- Siebers M, Biedermann SV, Bindila L, Lutz B, Fuss J. 2021. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology 126:105173. [DOI] [PubMed] [Google Scholar]
- Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. 2003. Exercise activates the endocannabinoid system. Neuroreport 14(17):2209–11. [DOI] [PubMed] [Google Scholar]
- Stensson N, Grimby-Ekman A. 2019. Altered relationship between anandamide and glutamate in circulation after 30 min of arm cycling: a comparison of chronic pain subject with healthy controls. Mol Pain 15:1744806919898360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone NL, Millar SA, Herrod PJJ, Barrett DA, Ortori CA, Mellon VA, and others. 2018. An analysis of endocannabinoid concentrations and mood following singing and exercise in healthy volunteers. Front Behav Neurosci 12:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strewe C, Feuerecker M, Nichiporuk I, Kaufmann I, Hauer D, Morukov B, and others. 2012. Effects of parabolic flight and spaceflight on the endocannabinoid system in humans. Rev Neurosci 23(5–6):673–80. [DOI] [PubMed] [Google Scholar]
- Watkins BA. 2018. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Aspects Med 64:68–78. [DOI] [PubMed] [Google Scholar]
- Yeung RR. 1996. The acute effects of exercise on mood state. Journal of Psychosomatic Research. 40(2):123–41. [DOI] [PubMed] [Google Scholar]
- Zhou D, Shearman LP. 2004. Voluntary exercise augments acute effects of CB1-receptor inverse agonist on body weight loss in obese and lean mice. Pharmacol Biochem Behav 77(1):117–25. [DOI] [PubMed] [Google Scholar]