Abstract
Objective
Tirzeptide is a novel glucagon-like peptide-1 receptor (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) drug, which shows good efficiency for weight loss. Therefore, we aim to investigate the efficacy and safety of tirzepatide for weight loss in type 2 diabetes mellitus (T2DM) and obesity patients in this meta-analysis study.
Methods
Cochrane Library, PubMed, Embase, Clinical Trials, and Web of Science were searched from inception to October 5, 2022. All randomized controlled trials (RCTs) were included. The odds ratio (OR) was calculated using fixed-effects or random-effects models by Review Manager 5.3 software.
Results
In total, ten studies (12 reports) involving 9,873 patients were identified. A significant loss body weight in the tirzepatide group versus the placebo by -9.81 kg (95% CI (-12.09, -7.52), GLP-1 RAs by -1.05 kg (95% CI (-1.48, -0.63), and insulin by -1.93 kg (95% CI (-2.81, -1.05), respectively. In sub-analysis, the body weight of patients was significantly reduced in three tirzepatide doses (5 mg, 10 mg, and 15 mg) when compared with those of the placebo/GLP-1 RA/insulin. In terms of safety, the incidence of any adverse events and adverse events leading to study drug discontinuation was higher in the tirzepatide group, but the incidence of serious adverse events and hypoglycaemia was lower. Additionally, the gastrointestinal adverse events (including diarrhea, nausea, vomiting and decreased appetite) of tirzepatide were higher than those of placebo/basal insulin, but similar to GLP-1 RAs.
Conclusion
In conclusion, tirzeptide can significantly reduce the weight of T2DM and patient with obesity, and it is a potential therapeutic regimen for weight-loss, but we need to be vigilant about its gastrointestinal reaction.
Introduction
Obesity is a metabolic disease, which is related to a variety of chronic diseases in addition to affecting the quality of life [1]. Recent statistics indicate that overweight/obesity and its relentless global rise, with the number of people with excess body weight reaching > 2 billion, approximately 30% of the world population [2]. Some researchers reckon that overweight and obesity are major risk factors for cardiovascular disease [3]. Thus, weight loss can reduce the incidence of cardiovascular events and all-cause mortality in cardiovascular patients [3, 4], and lessen the incidence of diabetes [5, 6]. Currently, a growing number of drugs are used for weight loss, such as glucagon-like peptide-1 receptor agonists (GLP-1 RAs). Liraglutide was the first GLP-1RAs to be approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of obesity [7]. Additionally, more evidence supports the use of the GLP-1RAs semaglutide in people with obesity without type 2 diabetes mellitus (T2DM) [8].
As time goes on, an increasing number of drugs have been developed for the treatment of T2DM or obesity. In recent years, Glucagon-like peptide-1 receptor (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are known as incretins among the many hormones in the body that has attracted the attention of researchers, which can promote insulin release after meals, lowering blood sugar and making the body more sensitive to insulin [9, 10]. Moreover, it also contributed to weight loss by slowing gastric emptying. GLP-1RAs are now considered the choice of injectable therapy for many people with T2DM and obesity, with several members of the class having weight loss efficacy [11–13]. Building on that concept, the combined GIP and GLP-1 RAs have been proposed as a novel therapeutic option for T2DM and obesity.
Tirzepatide (LY3298176, Mounjaro) is the first dual GIP and GLP-1 RAs for the treatment of T2DM, obesity, and nonalcoholic steatohepatitis [14]. It is a first-in-class GLP-1/GIP receptor agonists that FDA approved on May 13, 2022, to improve blood sugar control in adults with T2DM as an adjunct to diet and exercise [15]. Tirzepatide can lower the hemoglobin A1C level more than other medications to which it was compared [16, 17]. At the same time, there is growing evidence that tirzepatide plays a role in the weight loss of T2DM patients. Furthermore, another study showed that tirzepatide did not increase the risk of major cardiovascular events in participants with T2DM versus controls [18]. Tirzepatide also supported substantial weight loss in a recent clinical trial, potentially supporting its use as an obesity treatment [19].
In this paper, we performed a comprehensive systematic review and meta-analysis of all currently available randomized controlled trials (RCTs) of tirzepatide in individuals with T2DM and obesity to evaluate weight loss and adverse events when they were treated with tirzepatide.
Methods
Study search and selection
To conduct our study, we systematically searched PubMed, EMBASE, Cochrane library, Web of Science, and Clinical Trails databases from their inception to October 5, 2022, in the English language. "Tirzepatide" [MeSH] OR "LY3298176" OR "Mounjaro" were among the search phrases used. According to the inclusion and exclusion criteria, two researchers independently read the title and abstract of the literature for preliminary screening and also read the full text of literature that potentially met the inclusion criteria. Any disagreement was discussed and decided by the third researcher.
Studies were included for this meta-analysis if they met the following criteria: only RCT; adults of obesity patients with or without T2DM; tirzepatide is the intervention drug; comparison is placebo or antidiabetic; and outcome of efficacy and safety. Authorship; year of publication; randomization; intervention; and patient number; study design; study duration; study site; study population; therapy duration; body weight; and risk of AEs were extracted from all included studies.
Outcome indicators and the risk of bias assessment
The primary outcome indicators included body weight, glycosylated hemoglobin, type A1C (HbA1c) and the incidence of any AEs. The secondary outcome indicators included the incidence of SAEs, AE leading to study drug discontinuation, hypoglycemia, and other AEs. The Cochrane Collaboration bias assessment tool was used to evaluate the risk bias of the included studies by two researchers independently [20]. According to the tool the risk was categorized as “high risk”, “low risk”, or “unclear”. Review Manager 5.3 was used to carry out quality assessment and an investigation of publication bias.
Statistical analysis
Review Manager 5.3 was utilized to perform statistical analysis. The mean difference (MD) was used as the effect analysis statistic for continuous measurement data; Oddi ratio (OR) was used as the effect analysis statistic for dichotomous variables, and 95%CI was considered for each effect. Statistical heterogeneity between the results was analyzed by Chi-square (χ2) test, and the heterogeneity was quantitatively judged by I2. When I2 ≤ 50% and P > 0.1, the fixed effect model was applied, and when I2 > 50% and P < 0.1, the random effect model was applied. Additionally, we also investigated the source of heterogeneity with a sensitivity analysis when I2 was higher than 50%. The meta-analysis level was set as 0.05.
Results
Searching results and study characteristics
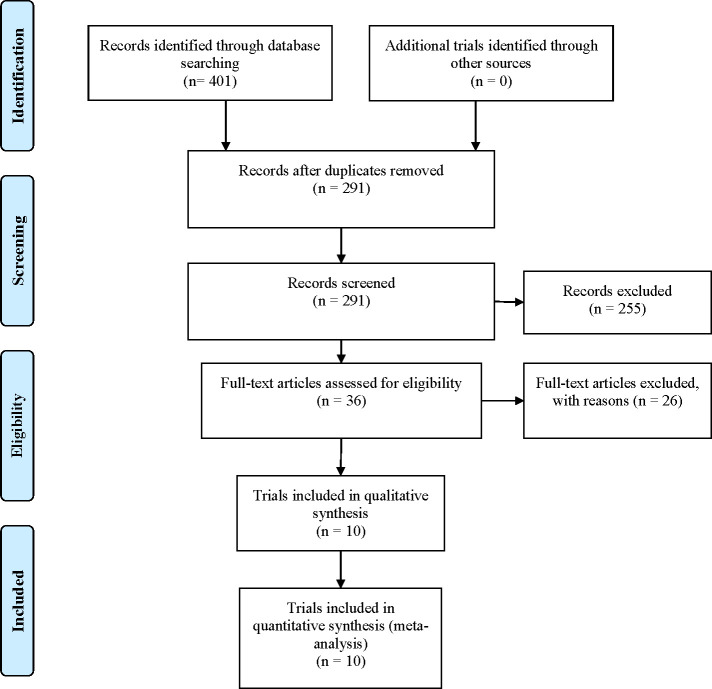
The initial 401 articles were searched, including Cochrane library (n = 38), PubMed (n = 74), Embase (n = 156), Clinical Trails (n = 25) and Web of Science (n = 108). The duplicate literature (n = 140) was first removed with EndNote X8 software, then the rest literature was further read for screening, and finally, the 10 studies that conformed to the inclusion criteria were included. A total of 9873 T2DM patients were involved. All studies were published in English. In this study, three tirzepatide doses has been giving (5 mg, 10 mg and 15 mg, subcutaneous injection, once a week), and a comparator, including placebo (two study by Frías [21, 22], SURPASS-1 [23], SURPASS-5 [24], SURMOUNT-1 [19], study by Heise [25], basal insulin [10 U/day insulin degludec (SURPASS–3) [26], and 10 U/day insulin glargine (SURPASS-4) [27]], GLP-1 RAs [1mg semaglutide (SURPASS-2) [28], and 1.5mg dulaglutide [21], 0.75 mg dulaglutide (SURPASS J–mono) [29]]. The study by Frias [21] with five groups, including tirzepatide (5 mg, 10 mg, 15 mg), placebo, and dulaglutide (1.5 mg) groups. Another one study by Frías [22] had four groups, we just including tirzepatide (15−2 mg) and placebo. The 15−2 mg tirzepatide dose-escalation regimens were 2.5 mg weeks 0–3; 7.5 mg weeks 4–7; and 15 mg weeks 8–11. Meanwhile, the duration of intervention in 4 studies was 40 weeks, 4 studies was 52 weeks, one study was 26 weeks, and another duration was 12 weeks. Ten studies were published from 2018 to 2022. The literature screening process and results are shown in Fig 1. Table 1 shows the baseline characteristics of the selected studies.
Fig 1. Flow diagram of studies searched in this meta-analysis.
Table 1. General baseline of included studies.
| Study, year published | Intervention | Patient number | Study duration | Therapy duration | Study population | Study design | Study site | Male (%) | Mean ±S.D. age |
|---|---|---|---|---|---|---|---|---|---|
| Frías JP, et al. 2018 | 5mg | 55 | Between May, 2017 and March, 2018 | 26–week | 18–75 years with 2 type diabetes for at least 6 months, HbA1c (7.0–10.5), BMI 23–50 kg/m². | phase 2b | 47 sites in 4 countries | 34 (62) | 57.9 ± 8.2 |
| 10mg | 51 | 30 (59) | 56.5 ± 9.9 | ||||||
| 15mg | 53 | 22 (42) | 56.0 ± 7.6 | ||||||
| 1.5mg dulaglutide | 54 | 24 (44) | 58.7 ± 7.8 | ||||||
| placebo | 51 | 29 (57) | 56.6 ± 8.9 | ||||||
| Frías JP, et al. 2020 | 15mg | 28 | Between November, 2017 and April, 2018 | 12–week | Type 2 diabetes for at least 6 months HbA1c 7.0–10.5, BMI 23–45 kg/m2. | phase 2 | 13 sites in United States | 23 (82.1) | 56.6 ± 9.21 |
| placebo | 26 | 12 (46.2) | 56.0 ± 10.13 | ||||||
| Rosenstock J, et al. 2021 (SURPASS–1) | 5mg | 121 | Between June, 2019 and Oct, 2020 | 40–week | ≥18 years with type 2 diabetes. HbA1c 7.0–9.5, BMI≥23 kg/m², and stable weight (±5) during the previous 3 months | phase 3 | 52 sites in 4 countries | 56 (46) | 54.1 ± 11.9 |
| 10mg | 121 | 72 (60) | 55.8 ± 10.4 | ||||||
| 15mg | 121 | 63 (52) | 52.9 ± 12.3 | ||||||
| placebo | 115 | 56 (49) | 53.6 ± 12.8 | ||||||
| Frías JP, et al. 2021 (SURPASS–2) | 5mg | 470 | Between July, 2019 and February, 2021 | 40–week | ≥18 years with type 2 diabetes, metformin≥ 1500 mg/d. HbA1c 7.0–10.5, BMI ≥25 kg/m², stable weight (±5) during the previous 3 months. | open–label, phase 3 | 128 sites in 8 countries | 205 (43.6) | 56.3 ±10.0 |
| 10mg | 469 | 238(50.7) | 57.2 ±10.5 | ||||||
| 15mg | 470 | 214(45.5) | 55.9 ±10.4 | ||||||
| 1 mg semaglutide | 469 | 225(48.0) | 56.9 ±10.8 | ||||||
| Ludvik B, et al. 2021 (SURPASS–3) | 5mg | 358 | Between April, 2019 and Jan, 2021 | 52–week | ≥18 years and type 2 diabetes, HbA1c 7.0–10.5, metformin alone or combination with an SGLT2 inhibitor for at least 3 months, BMI ≥ 25 kg/m², and stable weight (±5) during the previous3 months. | open label, phase 3 | 122 sites in 13 countries | 200 (56) | 57.2 ± 10.1 |
| 10mg | 360 | 195 (54) | 57.4 ± 9.7 | ||||||
| 15mg | 359 | 194 (54) | 57.5 ± 10.2 | ||||||
| degludec | 360 | 213 (59) | 57.5 ± 10.1 | ||||||
| Prato SD, et al. 2021 (SURPASS–4) | 5mg | 329 | Between Nov, 2018 and April, 2021 | 52–week | ≥18 years with type 2 diabetes, HbA1c 7.5–10.5, three oral glucose–lowering medications either alone or in any combination, BMI ≥ 25 kg/m², and stable weight (≤5) during the previous 3 months. | open–label, phase 3 | 187 sites in 14 countries | 198 (60) | 62.9 ± 8.6 |
| 10mg | 328 | 209 (64) | 63.7 ± 8.7 | ||||||
| 15mg | 338 | 203 (60) | 63.7 ± 8.6 | ||||||
| glargine | 1000 | 636 (64) | 63.8 ± 8.5 | ||||||
| Dahl D, et al. 2022 (SURPASS–5) | 5mg | 116 | Between August, 2019 and January, 2021 | 40–week | adults with type 2 diabetes, HbA1c 7.0–10.5, BMI ≥ 23 kg/m², insulin glargine (>20 IU/d or >0.25 IU/kg/d) with or without metformin (≥1500 mg/d). | phase 3 | 45 sites in in 8 countries | 60 (10) | 62 ± 10 |
| 10mg | 119 | 72 (61) | 60 ± 10 | ||||||
| 15mg | 120 | 65 (54) | 61 ± 10 | ||||||
| placebo | 120 | 66 (55) | 60 ± 10 | ||||||
| Inagaki N, et al. 2022 (SURPASS J–mono) | 5mg | 159 | Between May, 2019 and March, 2021 | 52–week | ≥20 Years with type 2 diabetes, HbA1c 7.0–10.0, BMI ≥23 kg/m², stable weight (±5) during 3 months preceding | phase 3 | Japan | 113 (71.1) | 56.8 ± 10.1 |
| 10mg | 158 | 119 (75.3) | 56.2 ± 10.3 | ||||||
| 15mg | 160 | 132 (82.5) | 56.0 ± 10.7 | ||||||
| dulaglutide | 159 | 117 (73.6) | 57.5 ± 10.2 | ||||||
| Heise T, et al. 2022 | 15 mg | 45 | Between June 28, 2019, and April 8, 2021, | 28-week | 20–74 years, type 2 diabetes for at least 6 months, and were being treated with lifestyle advice and stable doses of metformin, with or without one additional stable dose of another oral anti-hyperglycaemic medicine | phase 1 | 2 sites in Germany | 31 (69.0) | 61.1 ± 7.1 |
| Semaglutide 1 mg | 44 | 34 (77.0) | 63.7 ± 5.9 | ||||||
| Placebo | 28 | 21 (75.0) | 60.4 ± 7.6 | ||||||
| Jastreboff, AM, et al. 2022 (SURMOUNT-1) | 5mg | 630 | Between December 2019 and April 2022 | 72-week | ≥18 years, BMI ≥ 30 kg/m² or BMI ≥ 27 kg/m²or more and at least one weight-related complication. | phase 3 | 119 sites in 9 countries | 204 (32.3) | 45.6±12.7 |
| 10mg | 636 | 209 (32.9) | 44.7±12.4 | ||||||
| 15mg | 630 | 205 (32.5) | 44.9±12.3 | ||||||
| placebo | 643 | 207 (32.2) | 44.4±12.5 |
Quality assessment
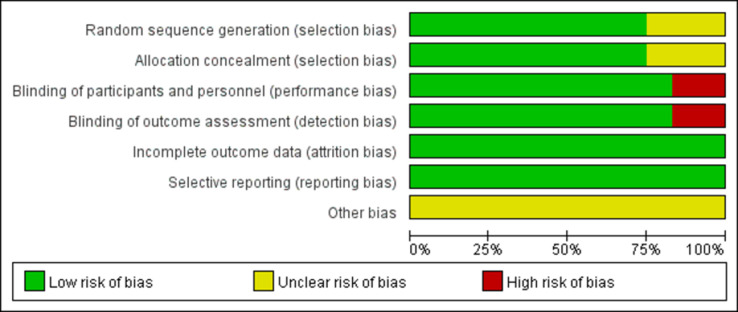
The results of the quality assessment of 10 studies are furnished in Fig 2. Five RCTs described the detailed randomization methods, allocation concealment, blinding of participants and personnel, incomplete outcome data, and other biases. Three RCTs did not have detail randomization methods and allocation concealment. Two RCTs are open-label and have a high bias risk for research. The risks of study design bias was shown in Fig 3.
Fig 2. Graphs of risk of bias for studies.
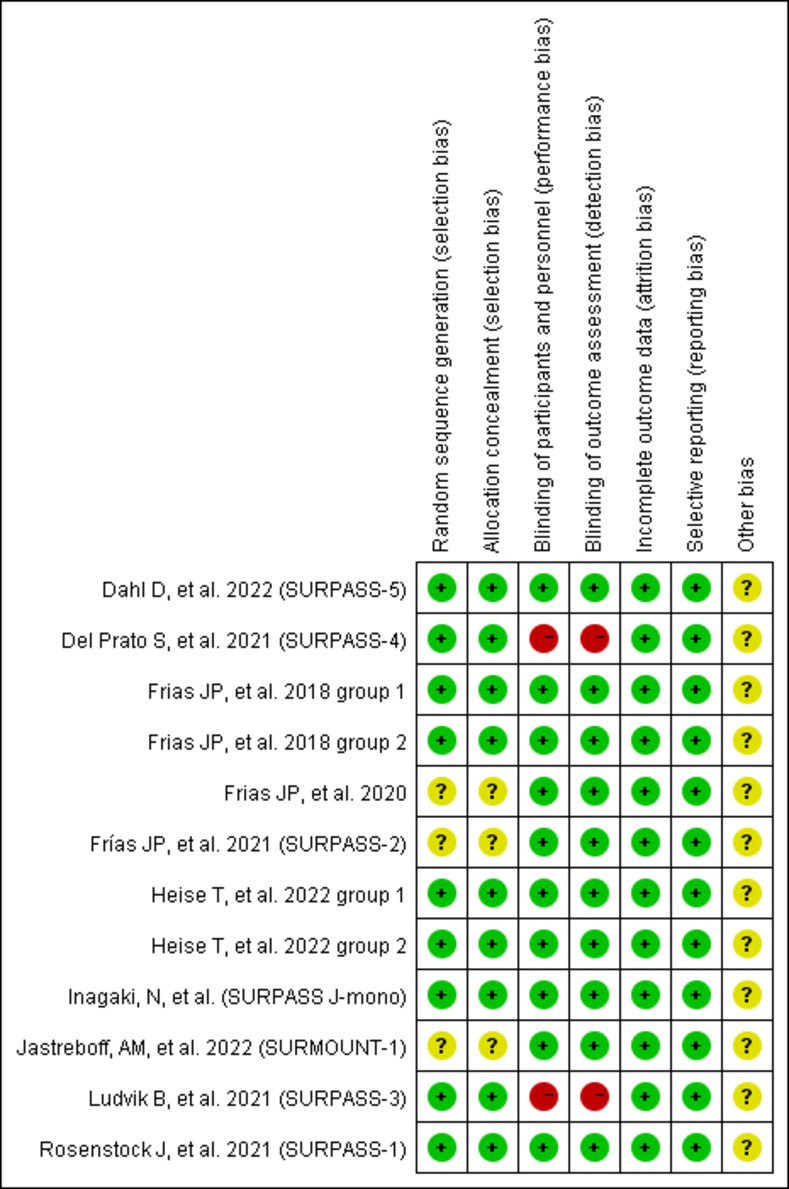
Fig 3. Quality assessment for risk of bias for studies.
Efficacy analysis
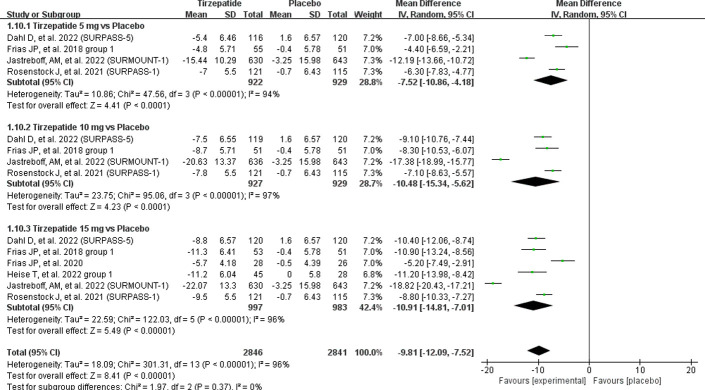
In this meta-analysis, the included 10 RCT studies displayed varying degrees of weight loss efficacy. Over all, meta-analysis showed a significant reduction in body weight in the tirzepatide group versus the placebo group by -9.81 kg (95% CI (-12.09, -7.52). There were three doses investigated compared to the placebo group were affected significantly reduced the body weight of patients [5 mg: MD = -7.52 kg, 95% CI (-10.86, -4.18), P < 0.0001; I2 = 94%; 10 mg: MD = -10.48 kg, 95% CI (-15.34, -5.62), P < 0.0001; I2 = 97%; 15 mg: MD = -10.91 kg, 95% CI (-14.81, -7.01), P < 0.00001; I2 = 96%] (Fig 4). The sensitivity analysis excluding the SURMOUNT-1 [19] trial showed that statistical heterogeneity decreased from 94% to 43%, 97% to 35%, and 96% to 78%, respectively.
Fig 4. Effect of tirzepatide vs placebo on body weight.
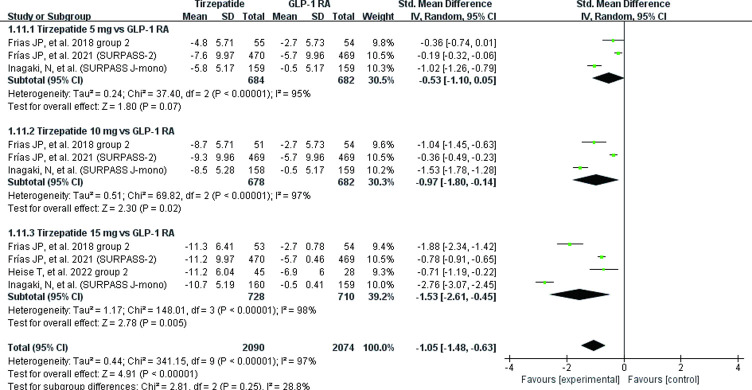
The body weight of patients was significantly reduced 1.05 kg (95% CI (-1.48, -0.63) when compared with GLP-1 RAs group. There were three doses investigated [5 mg: MD = -0.53, 95% CI (-1.10, -0.05), P = 0.07; I2 = 95%; 10 mg: MD = -0.97, 95% CI (-1.80, -0.1), P = 0.02; I2 = 97%; 15 mg: MD = -1.53, 95% CI (-2.61, -0.45), P = 0.005; I2 = 98%] (Fig 5). The sensitivity analysis removing SURPASS J-mono [30] trial showed that statistical heterogeneity decreased from 95% to 0%, 97% to 90%, and 98% to 90%, respectively.
Fig 5. Effect of tirzepatide vs GLP-1 RAs (semaglutide and dulaglutide) on body weight.
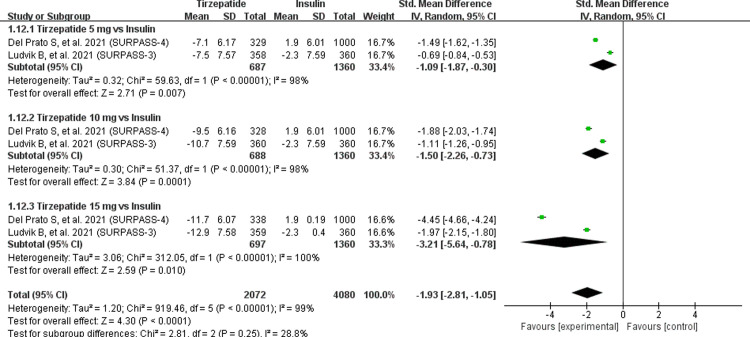
The body weight of patients was significantly decreased 1.93 kg (95% CI (-2.81, -1.05) when compared with insulin group. Three doses were tested [5 mg: MD = -1.09, 95% CI (-1.87, -0.30), P = 0.007; I2 = 98%; 10 mg: MD = -1.50, 95% CI (-2.26, -0.73), P = 0.0001; I2 = 98%; 15 mg: MD = -3.21, 95% CI (-5.64, -0.78), P = 0.01; I2 = 100%] significantly decreased the body weight of patients when compared with insulin group (Fig 6). Initially, the heterogeneities of three tirzepatide doses were observed to be high, but when we removed any one study, the heterogeneities in both groups did not decrease remarkably. Consistently, compared with placebo, GLP-1 RAs and insulin, more participants receiving any of the three tirzepatide doses had reductions in body weight of at least 5%, 10%, or 15% (Table 2).
Fig 6. Effect of tirzepatide vs insulin (insulin degludec and insulin glargine) on body weight.
Table 2. Meta-analysis results for tirzepatide vs placebo, GLP-1 RAs (semaglutide and dulaglutide) and basal insulin (insulin degludec and insulin glargine) for weigh loss.
| Intervention | Comparator | No. of participants with outcome/participants analysed | OR (95% CI) | I2 (%) | P value | |
|---|---|---|---|---|---|---|
| Tirzepatide arm | Comparator arm | |||||
| ≥5% weight loss | ||||||
| Tirzepatide 5 mg | Placebo | 699/922 | 245/929 | 11.93 [9.39, 15.15] | 0 | < 0.00001 |
| GLP-1 RAs | 428/684 | 282/682 | 3.97 [0.97, 16.26] | 95 | 0.06 | |
| Basal insulin | 438/687 | 100/1360 | 22.66 [15.64, 32.84] | 42 | < 0.00001 | |
| Tirzepatide 10 mg | Placebo | 761/927 | 245/929 | 17.33 [13.43, 22.35] | 34 | < 0.00001 |
| GLP-1 RAs | 522/678 | 282/682 | 9.43 [1.58, 56.18] | 97 | 0.01 | |
| Basal insulin | 542/688 | 100/1360 | 48.54 [27.20, 86.65] | 72 | < 0.00001 | |
| Tirzepatide 15 mg | Placebo | 780/924 | 245/929 | 21.41 [16.37, 28.00] | 38 | < 0.00001 |
| GLP-1 RAs | 552/683 | 282/682 | 11.02 [1.66, 73.26] | 97 | 0.01 | |
| Basal insulin | 595/697 | 100/1360 | 75.38 [50.05, 113.54] | 40 | < 0.00001 | |
| ≥10% weight loss | < 0.00001 | |||||
| Tirzepatide 5 mg | Placebo | 502/922 | 214/929 | 15.82 [3.29, 76.10] | 73 | 0.0006 |
| GLP-1 RAs | 169/525 | 118/523 | 1.64 [1.24, 2.16] | 0 | 0.0004 | |
| Basal insulin | 249/687 | 25/1360 | 27.27 [17.59, 42.25] | 42 | < 0.00001 | |
| Tirzepatide 10 mg | Placebo | 613/927 | 214/929 | 33.97 [5.62, 205.33] | 79 | 0.0001 |
| GLP-1 RAs | 240/520 | 118/523 | 2.95 [2.25, 3.86] | 52 | < 0.00001 | |
| Basal insulin | 365/688 | 25/1360 | 54.19 [35.06, 83.75] | 34 | < 0.00001 | |
| Tirzepatide 15 mg | Placebo | 649/924 | 214/929 | 37.79 [7.68, 186.01] | 74 | < 0.00001 |
| GLP-1 RAs | 288/523 | 118/523 | 4.28 [3.27, 5.61] | 0 | < 0.00001 | |
| Basal insulin | 464/697 | 25/1360 | 96.07 [62.15, 148.50] | 15 | < 0.00001 | |
| ≥15% weight loss | ||||||
| Tirzepatide 5 mg | Placebo | 329/922 | 57/929 | 9.95 [7.30, 13.55] | 0 | < 0.00001 |
| GLP-1 RAs | 73/525 | 38/523 | 2.07 [1.37, 3.14] | 0 | 0.0006 | |
| Basal insulin | 89/687 | 5/1360 | 43.49 [16.98, 111.43] | 0 | < 0.00001 | |
| Tirzepatide 10 mg | Placebo | 483/927 | 57/929 | 22.31 [16.25, 30.64] | 0 | < 0.00001 |
| GLP-1 RAs | 124/520 | 38/523 | 3.99 [2.71, 5.88] | 38 | < 0.00001 | |
| Basal insulin | 176/688 | 5/1360 | 95.35 [37.61, 241.73] | 32 | < 0.00001 | |
| Tirzepatide 15 mg | Placebo | 516/924 | 57/929 | 27.14 [19.66, 37.47] | 0 | < 0.00001 |
| GLP-1 RAs | 182/523 | 38/523 | 6.88 [4.72, 10.04] | 0 | < 0.00001 | |
| Basal insulin | 272/697 | 5/1360 | 174.26 [69.74, 435.43] | 33 | < 0.00001 | |
The changes of HbA1c of patients was also collected. When compared with placebo, tirzepatide can significantly reduce the HbA1c of patients [5 mg: MD = -1.55%, 95% CI (-1.72, -1.39), P < 0.00001; I2 = 85%; 10 mg: MD = -1.75%, 95% CI (-1.92, -1.58), P < 0.00001; I2 = 71%; 15 mg: MD = -1.87%, 95% CI (-2.03, -1.70), P < 0.00001; I2 = 86%]. The same results were found in comparing tirzepatide with GLP-1 RAs group and insulin group, the level of HbA1c of all patients was significantly reduced [GLP-1 RAs: 5 mg: MD = -0.51%, 95% CI (-0.62–0.39), P < 0.00001; I2 = 96%; 10 mg: MD = -0.73%, 95% CI (-0.85, -0.62), P < 0.00001; I2 = 96%; 15 mg: MD = -0.89%, 95% CI (-1.00, -0.77), P < 0.00001; I2 = 97%; Insulin: 5 mg: MD = -0.71%, 95% CI (-0.80, -0.63), P < 0.00001; I2 = 81%; 10 mg: MD = -0.94%, 95% CI (-1.03, -0.85), P < 0.00001; I2 = 50%; 15 mg: MD = -1.10%, 95% CI (-1.18, -1.01), P < 0.00001; I2 = 30%].
Safety analysis
For the safety, meta-analysis showed a significant difference the incidence of any adverse events between tirzepatide group and placebo group [OR = 1.59, 95% CI (1.29, 1.95), P < 0.00001, I² = 53%], GLP-1 RAs group [OR = 1.15, 95% CI (1.00, 1.32), P = 0.05, I² = 0%], and basal insulin [OR = 1.55, 95% CI (1.25, 1.91), P < 0.0001, I² = 69%], respectively. In the sub-analysis, the incidence of any adverse events was lower in the tirzepatide group than in the placebo group and basal insulin. But when compared to GLP-1 RAs, there was no significant difference in the tirzepatide 5 mg [OR = 1.01, 95% CI (0.80, 1.28), P = 0.92] and 10mg [OR = 1.17, 95% CI (0.92, 1.48), P = 0.2] groups (Table 3). Additionally, there was a statistically significant difference in the incidence of adverse events leading to study drug discontinuation between tirzepatide and placebo, between tirzepatide (15mg) and GLP-1 RAs, and between tirzepatide (5mg) and basal insulin. However, no significant statistics were found between tirzepatide and GLP-1 RAs (5mg and 10 mg) or between tirzepatide (10mg and 15 mg) and basal insulin.
Table 3. The results of safety in meta-analysis.
| Intervention | Comparator | Studies (n) | Tirzepatide arm (n) | Comparator arm (n) | I2 (%) | Effect Estimate | P value |
|---|---|---|---|---|---|---|---|
| Any Adverse events | |||||||
| Tirzepatide 5 mg | Placebo | 4 | 718/922 | 647/929 | 0 | 1.55 [1.25, 1.91] | < 0.0001 |
| GLP-1 RAs | 3 | 478/684 | 475/682 | 0 | 1.01 [0.80, 1.28] | 0.92 | |
| Basal insulin | 2 | 451/687 | 872/1360 | 0 | 1.23 [1.01, 1.50] | 0.04 | |
| Tirzepatide 10 mg | Placebo | 4 | 722/927 | 647/929 | 62 | 1.48 [0.98, 2.23] | 0.06 |
| GLP-1 RAs | 3 | 493/678 | 475/682 | 0 | 1.17 [0.92, 1.48] | 0.2 | |
| Basal insulin | 2 | 489/688 | 872/1360 | 69 | 1.58 [1.09, 2.29] | 0.02 | |
| Tirzepatide 15 mg | Placebo | 6 | 780/997 | 682/983 | 71 | 2.06 [1.24, 3.43] | 0.005 |
| GLP-1 RAs | 4 | 553/728 | 518/726 | 0 | 1.28 [1.01, 1.63] | 0.04 | |
| Basal insulin | 2 | 522/697 | 872/1360 | 74 | 1.91 [1.26, 2.89] | 0.002 | |
| Serious adverse events | |||||||
| Tirzepatide 5 mg | Placebo | 4 | 55/922 | 59/929 | 0 | 0.94 [0.64, 1.38] | 0.76 |
| GLP-1 RAs | 3 | 59/684 | 30/682 | 37 | 2.08 [1.32, 3.28] | 0.002 | |
| Basal insulin | 2 | 77/687 | 215/1360 | 71 | 0.94 [0.51, 1.75] | 0.85 | |
| Tirzepatide 10 mg | Placebo | 4 | 62/927 | 59/929 | 0 | 1.06 [0.73, 1.54] | 0.74 |
| GLP-1 RAs | 3 | 53/678 | 30/682 | 0 | 1.87 [1.17, 2.98] | 0.008 | |
| Basal insulin | 2 | 74/688 | 215/1360 | 0 | 0.84 [0.63, 1.13] | 0.24 | |
| Tirzepatide 15 mg | Placebo | 5 | 45/969 | 61/957 | 0 | 0.72 [0.49, 1.07] | 0.11 |
| GLP-1 RAs | 4 | 55/728 | 30/726 | 0 | 1.91 [1.20, 3.01] | 0.006 | |
| Basal insulin | 2 | 67/697 | 215/1360 | 77 | 0.80 [0.39, 1.64] | 0.54 | |
| Adverse event leading to study drug discontinuation | |||||||
| Tirzepatide 5 mg | Placebo | 4 | 43/922 | 25/929 | 0 | 1.77 [1.07, 2.92] | 0.03 |
| GLP-1 RAs | 2 | 33/525 | 25/523 | 0 | 1.34 [0.78, 2.29] | 0.29 | |
| Basal insulin | 2 | 62/687 | 59/1360 | 63 | 3.09 [1.33, 7.18] | 0.009 | |
| Tirzepatide 10 mg | Placebo | 4 | 64/927 | 25/929 | 0 | 2.68 [1.68, 4.30] | < 0.0001 |
| GLP-1 RAs | 2 | 43/520 | 25/523 | 72 | 1.23 [0.30, 5.09] | 0.78 | |
| Basal insulin | 2 | 65/688 | 59/1360 | 89 | 3.46 [0.69, 17.43] | 0.13 | |
| Tirzepatide 15 mg | Placebo | 6 | 74/997 | 29/983 | 48 | 2.57 [1.67, 3.96] | < 0.0001 |
| GLP-1 RAs | 3 | 54/568 | 24/567 | 0 | 2.40 [1.46, 3.95] | 0.0005 | |
| Basal insulin | 2 | 75/697 | 59/1360 | 87 | 4.00 [0.96, 16.79] | 0.06 | |
| Hypoglycemia (blood glucose <70 mg/dL) | |||||||
| Tirzepatide 5 mg | Placebo | 3 | 81/292 | 76/286 | 44 | 1.22 [0.76, 1.96] | 0.4 |
| GLP-1 RAs | 2 | 7/525 | 4/523 | 0 | 1.76 [0.51, 6.13] | 0.37 | |
| Basal insulin | 2 | 142/687 | 811/1360 | 94 | 0.17 [0.06, 0.48] | 0.0008 | |
| Tirzepatide 10 mg | Placebo | 3 | 88/291 | 76/286 | 51 | 1.42 [0.89, 2.27] | 0.14 |
| GLP-1 RAs | 2 | 6/520 | 4/523 | 25 | 1.59 [0.44, 5.73] | 0.48 | |
| Basal insulin | 2 | 156/688 | 811/1360 | 72 | 0.22 [0.15, 0.34] | < 0.00001 | |
| Tirzepatide 15 mg | Placebo | 5 | 92/367 | 81/340 | 24 | 1.25 [0.81, 1.93] | 0.3 |
| GLP-1 RAs | 4 | 17/728 | 5/726 | 0 | 3.29 [1.25, 8.69] | 0.02 | |
| Basal insulin | 2 | 178/697 | 811/1360 | 86 | 0.25 [0.14, 0.46] | < 0.00001 | |
| Nausea | |||||||
| Tirzepatide 5 mg | Placebo | 4 | 195/922 | 74/929 | 0 | 3.15 [2.34, 4.17] | < 0.00001 |
| GLP-1 RAs | 3 | 112/684 | 112/682 | 35 | 1.00 [0.65, 1.56] | 0.99 | |
| Basal insulin | 2 | 80/687 | 29/1360 | 0 | 6.38 [4.02, 10.11] | < 0.00001 | |
| Tirzepatide 10 mg | Placebo | 4 | 260/927 | 74/929 | 0 | 4.59 [3.46, 6.07] | < 0.00001 |
| GLP-1 RAs | 3 | 132/678 | 112/682 | 77 | 1.31 [0.62, 2.75] | 0.48 | |
| Basal insulin | 2 | 134/688 | 29/1360 | 58 | 11.02 [5.21, 23.31] | < 0.00001 | |
| Tirzepatide 15 mg | Placebo | 6 | 281/997 | 83/983 | 53 | 4.19 [2.37, 7.39] | < 0.00001 |
| GLP-1 RAs | 4 | 168/728 | 125/726 | 54 | 1.51 [0.94, 2.44] | 0.09 | |
| Basal insulin | 2 | 161/697 | 29/1360 | 0 | 14.34 [9.30, 22.10] | < 0.00001 | |
| Diarrhea | |||||||
| Tirzepatide 5 mg | Placebo | 4 | 153/922 | 70/929 | 39 | 2.13 [1.30, 3.48] | 0.003 |
| GLP-1 RAs | 3 | 96/684 | 74/682 | 63 | 1.34 [0.97, 1.85] | 0.08 | |
| Basal insulin | 2 | 96/687 | 58/1360 | 0 | 3.63 [2.53, 5.19] | < 0.00001 | |
| Tirzepatide 10 mg | Placebo | 4 | 180/927 | 70/929 | 59 | 2.59 [1.41, 4.76] | 0.002 |
| GLP-1 RAs | 3 | 104/678 | 74/682 | 0 | 1.50 [1.09, 2.06] | 0.01 | |
| Basal insulin | 2 | 125/688 | 58/1360 | 0 | 5.20 [3.69, 7.32] | < 0.00001 | |
| Tirzepatide 15 mg | Placebo | 6 | 214/997 | 78/983 | 50 | 2.65 [1.58, 4.44] | 0.0002 |
| GLP-1 RAs | 4 | 104/728 | 87/726 | 0 | 1.23 [0.90, 1.67] | 0.19 | |
| Basal insulin | 2 | 130/697 | 58/1360 | 0 | 5.47 [3.90, 7.68] | < 0.00001 | |
| Vomiting | |||||||
| Tirzepatide 5 mg | Placebo | 4 | 59/922 | 17/929 | 50 | 3.67 [2.13, 6.33] | < 0.00001 |
| GLP-1 RAs | 3 | 42/684 | 46/682 | 79 | 1.16 [0.25, 5.34] | 0.85 | |
| Basal insulin | 2 | 37/687 | 20/1360 | 0 | 3.94 [2.19, 7.09] | < 0.00001 | |
| Tirzepatide 10 mg | Placebo | 4 | 77/927 | 17/929 | 60 | 4.86 [2.86, 8.27] | < 0.00001 |
| GLP-1 RAs | 3 | 52/678 | 46/682 | 35 | 1.25 [0.60, 2.59] | 0.55 | |
| Basal insulin | 2 | 61/688 | 20/1360 | 0 | 6.77 [3.91, 11.71] | < 0.00001 | |
| Tirzepatide 15 mg | Placebo | 6 | 102/997 | 19/983 | 37 | 5.76 [3.51, 9.45] | < 0.00001 |
| GLP-1 RAs | 4 | 76/728 | 51/726 | 69 | 1.75 [0.66, 4.61] | 0.26 | |
| Basal insulin | 2 | 65/697 | 20/1360 | 0 | 7.13 [4.15, 12.26] | < 0.00001 | |
| Decreased appetite | |||||||
| Tirzepatide 5 mg | Placebo | 4 | 83/922 | 25/929 | 0 | 3.60 [2.28, 5.68] | < 0.00001 |
| GLP-1 RAs | 3 | 68/684 | 35/682 | 54 | 2.39 [1.15, 4.96] | 0.02 | |
| Basal insulin | 2 | 51/687 | 7/1360 | 0 | 15.83 [6.93, 36.18] | < 0.00001 | |
| Tirzepatide 10 mg | Placebo | 4 | 109/927 | 25/929 | 1 | 4.85 [3.11, 7.56] | < 0.00001 |
| GLP-1 RAs | 3 | 68/678 | 35/682 | 64 | 2.58 [1.11, 5.99] | 0.03 | |
| Basal insulin | 2 | 73/688 | 7/1360 | 0 | 22.72 [9.99, 51.68] | < 0.00001 | |
| Tirzepatide 15 mg | Placebo | 6 | 126/997 | 32/983 | 25 | 4.27 [2.84, 6.43] | < 0.00001 |
| GLP-1 RAs | 4 | 114/728 | 66/726 | 79 | 2.18 [0.86, 5.50] | 0.1 | |
| Basal insulin | 2 | 78/697 | 7/1360 | 0 | 23.59 [10.38, 53.61] | < 0.00001 | |
| Injection site reactions | |||||||
| Tirzepatide 5 mg | Placebo | 4 | 29/922 | 5/929 | 0 | 4.78 [1.83, 12.49] | 0.001 |
| GLP-1 RAs | 3 | 14/684 | 19/682 | 80 | 0.78 [0.09, 6.65] | 0.82 | |
| Basal insulin | 2 | 2/687 | 10/1360 | 0 | 0.31 [0.07, 1.40] | 0.13 | |
| Tirzepatide 10 mg | Placebo | 4 | 47/927 | 5/929 | 36 | 6.18 [1.80, 21.17] | 0.004 |
| GLP-1 RAs | 3 | 22/678 | 19/682 | 80 | 1.29 [0.20, 8.15] | 0.79 | |
| Basal insulin | 2 | 8/688 | 10/1360 | 0 | 1.13 [0.44, 2.94] | 0.8 | |
| Tirzepatide 15 mg | Placebo | 5 | 53/969 | 17/957 | 81 | 2.78 [0.45, 17.40] | 0.27 |
| GLP-1 RAs | 4 | 34/728 | 31/726 | 83 | 0.68 [0.07, 6.36] | 0.74 | |
| Basal insulin | 2 | 9/697 | 10/1360 | 0 | 1.19 [0.46, 3.06] | 0.72 | |
| Nasopharyngitis | |||||||
| Tirzepatide 5 mg | Placebo | 3 | 28/292 | 39/289 | 0 | 0.69 [0.41, 1.16] | 0.16 |
| GLP-1 RAs | 2 | 32/214 | 32/213 | 23 | 1.00 [0.58, 1.70] | 0.99 | |
| Basal insulin | 2 | 21/687 | 87/1360 | 0 | 0.47 [0.28, 0.77] | 0.003 | |
| Tirzepatide 10 mg | Placebo | 3 | 18/291 | 39/289 | 0 | 0.42 [0.24, 0.76] | 0.004 |
| GLP-1 RAs | 2 | 27/209 | 32/213 | 31 | 0.83 [0.48, 1.45] | 0.52 | |
| Basal insulin | 2 | 30/688 | 87/1360 | 0 | 0.69 [0.45, 1.07] | 0.1 | |
| Tirzepatide 15 mg | Placebo | 4 | 33/339 | 42/317 | 0 | 0.70 [0.43, 1.14] | 0.15 |
| GLP-1 RAs | 3 | 32/258 | 40/257 | 0 | 0.76 [0.46, 1.26] | 0.29 | |
| Basal insulin | 2 | 31/697 | 87/1360 | 0 | 0.70 [0.45, 1.07] | 0.1 | |
GLP-1 Ras: semaglutide and dulaglutide; Basal insulin: insulin degludec and insulin glargine.
In this study, there was no significant difference in the incidence of serious adverse events between tirzepatide and placebo, and basal insulin, but significant difference to GLP-1 RAs. Hypoglycemia is the major SAEs, hypoglycemia definition as blood glucose < 70 mg/dL. Across all trials, the hypoglycemia risk of tirzepatide did not differ compared with placebo and GLP-1 RAs, and was lower with tirzepatide compared with basal insulin.
After consuming tirzepatide, most of the patients experienced diarrhea, nausea, vomiting, decreased appetite, constipation, injection site reactions, and nasopharyngitis. Compared with basal insulin and placebo, more frequent gastrointestinal adverse events occurred, including diarrhea, nausea, vomiting, decreased appetite, and constipation in all tirzepatide groups. When compared with the GLP-1 RAs, the tirzepatide group showed a similar risk of nausea, diarrhea, vomiting, and constipation. While tirzepatide 5 mg and 10 mg were also associated with a higher incidence of decreased appetite. Meanwhile, there were no statistically significant differences in the incidence of injection site reactions between tirzepatide and GLP-1 RAs, and basal insulin. When compared with placebo, the incidence of injection site reactions was lower in tirzepatide (5 mg and 10 mg), but no significant difference in tirzepatide (15 mg). Besides, there was no significant difference in the incidence of nasopharyngitis was noticed between tirzepatide and placebo, GLP-1 RAs, and basal insulin.
Discussions
Tirzepatide as the first dual GIP and GLP-1 RA drug, which shown effects on hypoglycemia, body weight and cardiovascular indicators in previous studies [31–33]. Its effect on body weight could make it useful as a weight loss drug. Thus, in this meta-analysis, a systematic review to assess the weight loss efficacy and safety of tirzepatide is conducted. Based on our findings, both doses of tirzepatide (5 mg, 10 mg and 15 mg) were more effective than other drugs in reducing body weight.
With the increase of obese people, the drugs for obesity treatment has been on the rise, and most of the drugs currently used to treat obesity started out as treatments for diabetes [34]. Based on our findings, both doses of tirzepatide were more effective in reducing bodyweight compared with other drugs. In this study, we included 10 RCT (12 reports) and compared the weight loss effects of tirzepatide with placebo, insulin, and GLP-1 RAs. The results showed that tirzepatide could reduce the weight of T2DM and obese patients. This is the same as the previous study showed [32, 35]. At the same time, the network meta-analysis results of Alkhezi et al. also showed that compared with liragrutide and semaglutide, tirzepatide had better weight reduction in non-diabetes patients, and the safety results were similar without difference [36]. Furthermore, some studies found that meaningful weight loss is achieve 5 ~ 10% [37]. In our study, tirzepatide were more effective than other drugs in reducing bodyweight of 5%, 10%, or 15%. More importantly, compared with placebo, GLP-1 RAs and insulin, tirzepatide can significantly reduce their HbA1c level of patients, and the results were same with other studies [32, 38].
For the safety, a significant difference the incidence of any adverse events between tirzepatide group and placebo/GLP-1 RAs/basal insulin. This is contrary to the results of a study by Bhagavathula et al. the results shown that no significant difference the incidence of any adverse events [32]. In addition, no significant difference in the incidence of SAEs between tirzepatide and placebo, and basal insulin, but significant difference to GLP-1 RAs. Across all trials, the risk of hypoglycemia with tirzepatide did not differ compared to placebo and GLP-1 RAs, but was lower with tirzepatide than with basal insulin. In the same results has been found in the study by Karagiannis et al. that incidence of serious adverse events did not differ between any of the tirzepatide doses and any comparator [35].
Gastrointestinal adverse events were the most common adverse events in all groups. In this study, the incidence of gastrointestinal adverse events, including diarrhea, nausea, vomiting, and decreased appetite, and the incidence of diarrhea, nausea, vomiting, and constipation were similar when comparing tirzepatide with GLP-1 RAs. However, in comparison with placebo or basal insulin, tirzepatide increased the odds of diarrhea, nausea, vomiting, decreased appetite, and constipation. The results were the same as this study [35]. The clinical trials of SURPASS reported on the gastrointestinal system, and nausea, diarrhea and vomiting were the most common AEs [17]. The results from other studies found that GLP-1 receptor agonists are often accompanied by nausea, emesis, and undesired anorexia. Importantly, the hypophagic and emetic effects of GLP-1 receptor agonists are caused by activation of central GLP-1 receptors [39]. Gastrointestinal side effects of high-dose GLP-1 RAs and co-agonists occurred in 30% ~ 70% of patients, mostly arising within the first 2 weeks of the first dose, being mild or moderate in severity, and transient [40]. A study found the incidence of gastrointestinal bleeding occurred most frequently 0 ~ 4 weeks after the first dose and was higher for the 15 mg tirzepatide group [41].
In addition, all the included studies were RCT, and some studies have a high bias risk for research because they did not have detailed randomization methods and allocation concealment or open-label. Of these, this may affect the positive outcomes. Moreover, this study could have a higher effect on weight loss due to participants of the SURMOUNT-1 study not having T2DM and the SURPASS J-Combo study with only Japanese populations. Sensitive analysis results showed that statistical heterogeneity was decreased after removing the SURMOUNT-1 study and the SURPASS J-Combo study, but the statistical effect did not change.
There are limitations in the current study. First, as all the RCTs involved the pharmaceutical industry, the positive outcomes should be interpreted cautiously [42, 43]. Second, only one study focused on the obesity patients in all 10 RCTs (12 reports), and RCTs have generally carried out in selected populations of T2DM or obesity patients. More research is suggested in the available guidance. Fortunately, 20 additional trials [17, 44, 45] aiming to investigate the efficacy of tirzepatide in the clinical setting of T2DM or obesity are ongoing. We can obtain more data to analyze after these trials are completed in the near future.
Conclusion
To sum up, this meta-analysis indicated that tirzepatide could significantly decrease body weight in T2DM and obesity patients, and it is a potential therapeutic regimen for weight-loss, but we need to be vigilant about its gastrointestinal reaction.
Supporting information
(XLSX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Gadde K.M., et al., Obesity: Pathophysiology and Management. J Am Coll Cardiol, 2018. 71(1): 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caballero B., Humans against Obesity: Who Will Win? Adv Nutr, 2019. 10(suppl_1): S4–s9. doi: 10.1093/advances/nmy055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto C.M., Heartbeat: weight loss interventions in patients with cardiovascular disease. Heart, 2021. 107(19): 1521–1523. doi: 10.1136/heartjnl-2021-320238 [DOI] [PubMed] [Google Scholar]
- 4.Yannakoulia M. and Panagiotakos D., Weight loss through lifestyle changes: impact in the primary prevention of cardiovascular diseases. Heart, 2021. 107(17): 1429–1434. doi: 10.1136/heartjnl-2019-316376 [DOI] [PubMed] [Google Scholar]
- 5.Cosentino F., et al., 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J, 2020. 41(2): 255–323. doi: 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 6.Joseph J.J., et al., Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation, 2022. 145(9): e722–e759. doi: 10.1161/CIR.0000000000001040 [DOI] [PubMed] [Google Scholar]
- 7.Jepsen M.M. and Christensen M.B., Emerging glucagon-like peptide 1 receptor agonists for the treatment of obesity. Expert Opin Emerg Drugs, 2021. 26(3): 231–243. doi: 10.1080/14728214.2021.1947240 [DOI] [PubMed] [Google Scholar]
- 8.Williams D.M., et al., Glucagon-like Peptide-1 Receptor Analogues for the Treatment of Obesity. touchREV Endocrinol, 2022. 18(1): 43–48. doi: 10.17925/EE.2022.18.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baggio L.L. and Drucker D.J., Biology of incretins: GLP-1 and GIP. Gastroenterology, 2007. 132(6): 2131–57. doi: 10.1053/j.gastro.2007.03.054 [DOI] [PubMed] [Google Scholar]
- 10.Mathiesen D.S., et al., The Effects of Dual GLP-1/GIP Receptor Agonism on Glucagon Secretion-A Review. Int J Mol Sci, 2019. 20(17). doi: 10.3390/ijms20174092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Montes D.E.O.A., Pellitero S., and Puig-Domingo M., Obesity and GLP-1. Minerva Endocrinol (Torino), 2021. 46(2): 168–176. doi: 10.23736/S2724-6507.20.03369-6 [DOI] [PubMed] [Google Scholar]
- 12.Chadda K.R., Cheng T.S., and Ong K.K., GLP-1 agonists for obesity and type 2 diabetes in children: Systematic review and meta-analysis. Obes Rev, 2021. 22(6): e13177. doi: 10.1111/obr.13177 [DOI] [PubMed] [Google Scholar]
- 13.Nauck M.A., et al., GLP-1 receptor agonists in the treatment of type 2 diabetes—state-of-the-art. Mol Metab, 2021. 46: 101102. doi: 10.1016/j.molmet.2020.101102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willard F.S., et al., Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight, 2020. 5(17). doi: 10.1172/jci.insight.140532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Administration, U.S.F.a.D., FDA Approves Novel, Dual-Targeted Treatment for Type 2 Diabetes. https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes, 2018. 20(9): 2113–2120.
- 16.Aroda V.R., et al., Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1–7 trials. Diabetes Metab, 2019. 45(5): 409–418. doi: 10.1016/j.diabet.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 17.Min T. and Bain S.C., The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther, 2021. 12(1): 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sattar N., et al., Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med, 2022. 28(3): 591–598. doi: 10.1038/s41591-022-01707-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jastreboff A.M., et al., Tirzepatide Once Weekly for the Treatment of Obesity. New England Journal of Medicine, 2022. [DOI] [PubMed] [Google Scholar]
- 20.Zeng J., et al., The effect of soy intervention on insulin-like growth factor 1 levels: A meta-analysis of clinical trials. Phytother Res, 2020. 34(7): 1570–1577. doi: 10.1002/ptr.6630 [DOI] [PubMed] [Google Scholar]
- 21.Frías J.P., et al., Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet, 2018. 392(10160): 2180–2193. doi: 10.1016/S0140-6736(18)32260-8 [DOI] [PubMed] [Google Scholar]
- 22.Frias J.P., et al., Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes Metab, 2020. 22(6): 938–946. doi: 10.1111/dom.13979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenstock J., et al., Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet, 2021. 398(10295): 143–155. doi: 10.1016/S0140-6736(21)01324-6 [DOI] [PubMed] [Google Scholar]
- 24.Dahl D., et al., Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. Jama, 2022. 327(6): 534–545. doi: 10.1001/jama.2022.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heise T., et al., Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol, 2022. doi: 10.1016/S2213-8587(22)00085-7 [DOI] [PubMed] [Google Scholar]
- 26.Ludvik B., et al., Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet, 2021. 398(10300): 583–598. doi: 10.1016/S0140-6736(21)01443-4 [DOI] [PubMed] [Google Scholar]
- 27.Del Prato S., et al., Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet, 2021. 398(10313): 1811–1824. doi: 10.1016/S0140-6736(21)02188-7 [DOI] [PubMed] [Google Scholar]
- 28.Frías J.P., et al., Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med, 2021. 385(6): 503–515. doi: 10.1056/NEJMoa2107519 [DOI] [PubMed] [Google Scholar]
- 29.Inagaki N., et al., Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol, 2022. 10(9): 623–633. doi: 10.1016/S2213-8587(22)00188-7 [DOI] [PubMed] [Google Scholar]
- 30.ClinicalTrials.gov, A Study of Tirzepatide (LY3298176) Compared to Dulaglutide in Participants With Type 2 Diabetes (SURPASS J-mono). https://clinicaltrials.gov/ct2/show/NCT03861052, 2021.
- 31.Bastin M. and Andreelli F., Dual GIP–GLP1-receptor agonists in the treatment of type 2 diabetes: A short review on emerging data and therapeutic potential. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 2019. 12: 1973–1985. doi: 10.2147/DMSO.S191438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhagavathula A.S., Vidyasagar K., and Tesfaye W., Efficacy and Safety of Tirzepatide in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Phase II/III Trials. Pharmaceuticals (Basel), 2021. 14(10). doi: 10.3390/ph14100991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conlon J.M., F.P.M. O’Harte, and Flatt P.R., Dual-agonist incretin peptides from fish with potential for obesity-related Type 2 diabetes therapy–A review. Peptides, 2022. 147. doi: 10.1016/j.peptides.2021.170706 [DOI] [PubMed] [Google Scholar]
- 34.Apovian C.M., et al., Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab, 2015. 100(2): 342–62. doi: 10.1210/jc.2014-3415 [DOI] [PubMed] [Google Scholar]
- 35.Karagiannis T., et al., Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia, 2022. doi: 10.1007/s00125-022-05715-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alkhezi O.S., et al., Comparative effectiveness of glucagon-like peptide-1 receptor agonists for the management of obesity in adults without diabetes: A network meta-analysis of randomized clinical trials. Obes Rev, 2023. 24(3): e13543. doi: 10.1111/obr.13543 [DOI] [PubMed] [Google Scholar]
- 37.Bays H.E., et al., Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan R., et al., Efficacy and safety of tirzepatide in patients with type 2 diabetes mellitus: A bayesian network meta-analysis. Front Pharmacol, 2022. 13: 998816. doi: 10.3389/fphar.2022.998816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borner T., et al., Glucagon-like peptide-1 in diabetes care: Can glycaemic control be achieved without nausea and vomiting? Br J Pharmacol, 2022. 179(4): 542–556. doi: 10.1111/bph.15647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Block C.E.M., et al., Efficacy and safety of high-dose glucagon-like peptide-1, glucagon-like peptide-1/glucose-dependent insulinotropic peptide, and glucagon-like peptide-1/glucagon receptor agonists in type 2 diabetes. Diabetes Obes Metab, 2022. 24(5): 788–805. doi: 10.1111/dom.14640 [DOI] [PubMed] [Google Scholar]
- 41.Kadowaki T., et al., Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol, 2022. 10(9): 634–644. doi: 10.1016/S2213-8587(22)00187-5 [DOI] [PubMed] [Google Scholar]
- 42.Fundytus A., et al., Industry Funding of Oncology Randomised Controlled Trials: Implications for Design, Results and Interpretation. Clin Oncol (R Coll Radiol), 2022. 34(1): 28–35. doi: 10.1016/j.clon.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 43.Janiaud P., Cristea I.A., and Ioannidis J.P.A., Industry-funded versus non-profit-funded critical care research: a meta-epidemiological overview. Intensive Care Med, 2018. 44(10): 1613–1627. doi: 10.1007/s00134-018-5325-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaneko S., Tirzepatide: A Novel, Once-weekly Dual GIP and GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes. touchREV Endocrinol, 2022. 18(1): 10–19. doi: 10.17925/EE.2022.18.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frías J.P., Tirzepatide: a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) dual agonist in development for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab, 2020. 15(6): 379–394. doi: 10.1080/17446651.2020.1830759 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.