Dear Editor,
We detected a novel heterozygous mutation in the Grb-10 interacting GYF protein-2 (GIGYF2) gene and a short duplication of chromosome 22q11.2 in a person who developed symptoms of psychosis and dementia. Overlaps between the two syndromes are often seen, 1 and understanding the genetic correlates may help better appreciate these syndromes and the related biological mechanisms.
Case Presentation
Ms XZ, a 55-year-old homemaker, presented with a history of gradual change in behavior, perceptual disturbances, and cognitive decline over three years, with rapid progression over the last six months. There was no history of preexisting medical or psychiatric illness, but one sibling had committed suicide. The illness began with suspiciousness, delusion of reference, auditory hallucinations, and unprovoked irritability. She was diagnosed with late-onset psychosis. However, her symptoms did not improve with antipsychotic treatment, and she developed akathisia and tardive dyskinesia. A year later, she developed nihilistic delusions, visual hallucinations, and cognitive impairment. Over the next six months, she became disoriented, misidentified family members, developed apathy and forgetfulness, and required assistance for all activities.
Neurological examination revealed mild tremors in the upper limbs, mask-like face, and decreased expressive movements. She was apathetic and confused; had visuospatial disorientation, anomia, and sundowning; and scored 3/31 on the Hindi Mental Status Examination. 2
Except for mild dyslipidemia, the hemogram, metabolic parameters, electrolytes, thyroid functions, vitamin B12, and folate levels were unremarkable. Investigations for autoimmune encephalitis or common infections of the central nervous system were negative. The electroencephalogram showed fast beta activity, which might be secondary to the use of benzodiazepine medications. Magnetic resonance imaging of the brain showed a mildly dilated ventricular system with a prominence of the Sylvian fissures. The fluorodeoxyglucose-positron emission tomography (FDG-PET) scan revealed a severe decrease in tracer uptake in bilateral parieto-occipital lobes, involving the middle and inferior occipital lobes, cuneus, precuneus region, extending to lingual gyri (Figures S1A and S1B). In occipital lobes, there was distinct sparing of the calcarine cortex and relative sparing of the posterior cingulate gyrus (cingulate island sign; Figures S2A and S2B). The clinical symptoms, features of parkinsonism, sensitivity to antipsychotic drugs, and evidence of atrophy on MRI and PET scans suggested a diagnosis of dementia with Lewy bodies syndrome. 3
The clinical exome (supplementary methods) revealed a duplication of chromosome 22q11.21 region (chr22: 18893888-?_19002019+?dup, HG19), along with a novel heterozygous (predicted pathogenic) mutation in the GIGYF2 gene in chromosome 2 (chr2:233656013C>G, p.Pro380Arg). No repeat expansion for Huntington’s disease; spinocerebellar ataxia types 1, 2, 3, or 12; or the hexanucleotide containing C9orf72 were detected.
Discussion
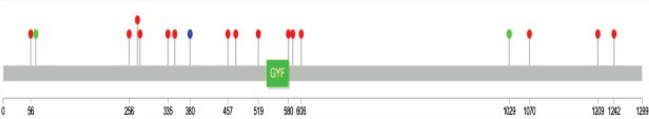
A mutation in the GIGYF2 gene in the PARK11 locus of chromosome 2q 36-37 has been identified in familial Parkinson’s disease (PD) in the European population. 4 Many variants in the gene have been linked to the risk of PD. The variant detected in this patient (p.Pro380Arg) is an ultrarare mutation (not present in gnomAD) and is located in a region of the gene that also hosts many mutations associated with PD (Figure 1). The gene is involved in mRNA processing and is highly intolerant to the loss of function variation (gnomAD, pLI: 1). 5 Variants in the gene are also implicated in autism and schizophrenia in addition to PD and dementia with Lewy bodies (Table S1), and the effects of these on the brain and behavior have been modeled in the zebrafish. 6
Figure 1. Lollipop Plot of GIGYF2 Gene, Showing the Exonic Variants Involved in Parkinson’s Disease (Red) and DLB (Green) from the Previous Studies. The Variant Detected in the Patient Is Depicted in Blue.
The relationship between duplication of the 22q11 region and psychosis or dementia is relatively ambiguous. Duplications are detected in 1 in 700 individuals with developmental disorders, often inherited in an autosomal dominant manner (OMIM:608363), with concomitant intellectual disability, facial dysmorphism, stunted growth, Attention deficit hyperactivity disorder, autism,7, 8 and severe psychoses. 9 There is no history of any developmental deficits or intellectual disability in this patient, and the physical examination had not revealed any dysmorphic features. However, the identified duplication of a short region involves three genes (DGCR6, PRODH, and DGCR5) often implicated in the risk for schizophrenia, as suggested in 22q11del syndrome.
Both variations detected in the person may have a bearing on her clinical condition. Each of these variants by themselves may be insufficient to explain the clinical syndrome. The simultaneous occurrence of two rare variants could be the reason for this unusual emergence of both psychotic symptoms and dementia in middle age. This was much younger than the usual age at the onset of dementia but much older than a typical onset of schizophrenia. Co-occurrence of multiple risk variants can happen in individuals in a stochastic manner or by de novo genetic alterations, as was reported in a person with chromosome 22q11 deletion, who also had an expanded Huntington’s disease allele and complex symptomatology involving features of both. 10 We cannot rule out the possibility that rare exonic variants in unidentified risk genes or noncoding variants that are not explored as part of clinical exome have a role in this complex phenotype.
Even though a direct genotype–phenotype relationship cannot be made in the absence of functional studies, this case report illustrates that greater use of genomic information may help us identify “risk” genes and pathways, which, although rare, may help us understand the neurobiology of these syndromes better.
Supplemental Material
Supplemental material for this article is available online.
Acknowledgments
The authors acknowledge Strand Life Sciences, Bengaluru, Karnataka, India, for their support with the Next Generation Sequencing and analysis. The authors also thank Professor Carrie Bearden from the Departments of Psychiatry and Biobehavioral Sciences and Psychology, Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles, for her suggestions and encouragement.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: RKN and AV are funded by the accelerator program for discovery in brain disorders using stem cells (ADBS; jointly funded by the Department of Biotechnology, Government of India, and the Pratiksha trust).
Statement of Informed Consent and Ethical Approval: Necessary ethical clearances and informed consent was received and obtained respectively before initiating the study.
References
- 1.Mukku SSR, Nadella RK, Sivakumar PT, et al. Is late onset schizophrenia a forerunner of Frontotemporal dementia? A case series. Schizophr Res, 2021; 228: 56–57. [DOI] [PubMed] [Google Scholar]
- 2.Ganguli M, Ratcliff G, Chandra V, et al. A Hindi version of the MMSE: The development of a cognitive screening instrument for a largely illiterate rural elderly population in india. Int J Geriatr Psychiatry, 1995; 10: 367–377. [Google Scholar]
- 3.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology, 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Sun Q-Y, Yu R-H, et al. The contribution of GIGYF2 to Parkinson’s disease: A meta-analysis. Neurol Sci, 2015; 36: 2073–2079. [DOI] [PubMed] [Google Scholar]
- 5.Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature, 2020; 581: 434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thyme SB, Pieper LM, Li EH, et al. Phenotypic landscape of schizophrenia-associated genes defines candidates and their shared functions. Cell, 2019; 177: 478–491.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderoni S, Ricca I, Balboni G, et al. Evaluation of chromosome microarray analysis in a large cohort of females with autism spectrum disorders: A single center Italian study. JPM, 2020; 10: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeffding LK, Trabjerg BB, Olsen L, et al. Risk of psychiatric disorders among individuals with the 22q11.2 deletion or duplication: A Danish nationwide, register-based study. JAMA Psychiatry, 2017; 74: 282. [DOI] [PubMed] [Google Scholar]
- 9.Amelsvoort T van, Denayer A, Boermans J, et al. Psychotic disorder associated with 22q11.2 duplication syndrome. Psychiatry Res, 2016; 236: 206–207. [DOI] [PubMed] [Google Scholar]
- 10.Farrell M, Lichtenstein M, Crowley JJ, et al. Developmental delay, treatment-resistant psychosis, and early-onset dementia in a man with 22q11 deletion syndrome and Huntington’s disease. AJP, 2018; 175: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for this article is available online.