Significance
To address a crucial knowledge deficiency concerning the correlation between fried food consumption and the risk of anxiety and depression, here we revealed that frequent fried food consumption is strongly associated with a higher risk of anxiety and depression. Notably, acrylamide is a representative contaminant in fried foods, thereby further elucidating its toxicological mode of action. We demonstrated that long-term exposure to acrylamide induces anxiety- and depressive-like behaviors via oxidative stress-mediated neuroinflammation, and unravel the underlying mechanism that PPAR signaling pathway mediates acrylamide-induced lipid metabolism disorder in brain. These outcomes are expected to both epidemiologically and mechanistically open an avenue in the significance of reducing fried food consumption for mental health and provide evidence to understand acrylamide-triggered anxiety and depression.
Keywords: fried foods, anxiety and depression, acrylamide, lipid metabolism disturbance, neuroinflammation
Abstract
Western dietary patterns have been unfavorably linked with mental health. However, the long-term effects of habitual fried food consumption on anxiety and depression and underlying mechanisms remain unclear. Our population-based study with 140,728 people revealed that frequent fried food consumption, especially fried potato consumption, is strongly associated with 12% and 7% higher risk of anxiety and depression, respectively. The associations were more pronounced among male and younger consumers. Consistently, long-term exposure to acrylamide, a representative food processing contaminant in fried products, exacerbates scototaxis and thigmotaxis, and further impairs exploration ability and sociality of adult zebrafish, showing anxiety- and depressive-like behaviors. Moreover, treatment with acrylamide significantly down-regulates the gene expression of tjp2a related to the permeability of blood–brain barrier. Multiomics analysis showed that chronic exposure to acrylamide induces cerebral lipid metabolism disturbance and neuroinflammation. PPAR signaling pathway mediates acrylamide-induced lipid metabolism disorder in the brain of zebrafish. Especially, chronic exposure to acrylamide dysregulates sphingolipid and phospholipid metabolism, which plays important roles in the development of anxiety and depression symptoms. In addition, acrylamide promotes lipid peroxidation and oxidation stress, which participate in cerebral neuroinflammation. Acrylamide dramatically increases the markers of lipid peroxidation, including (±)5-HETE, 11(S)-HETE, 5-oxoETE, and up-regulates the expression of proinflammatory lipid mediators such as (±)12-HETE and 14(S)-HDHA, indicating elevated cerebral inflammatory status after chronic exposure to acrylamide. Together, these results both epidemiologically and mechanistically provide strong evidence to unravel the mechanism of acrylamide-triggered anxiety and depression, and highlight the significance of reducing fried food consumption for mental health.
Depression and anxiety are the two most prevalent mental disorders, while anxiety often goes hand-in-hand with depression. The worldwide prevalence of depression and anxiety is climbing with an increase of 27.6% and 25.6%, respectively, due to the COVID-19 pandemic in 2020, and becomes a major cause of the overall global burden of disease (1, 2). Globally, it is estimated that more than 5% of adults suffer from depression (3), which are common but often neglected, influencing the quality of life. Depressive symptoms feature low mood, a loss of enjoyment or interest in activities, slow thinking, or cognitive impairment (4). A combination of genetic, biological, environmental, and psychological factors may be related to the development of depression though the precise etiology still remains unknown (4). Diet has recently been linked with the risk of developing depression and anxiety (5). A Western diet of fried or processed foods, refined grains, sugary products, and beer is associated with a higher risk of depression and anxiety (6). Typically, fried foods are major part of the Western diet, and the consumption of fried foods increases worldwide, especially during the COVID-19 Outbreak (7). A recent longitudinal observational study demonstrated that computer usage time and fried food consumption were lifestyle-related risk factors for mental health problems in Chinese male college students (8), which supported our study regarding the association of fried food consumption with a higher risk of depression and anxiety among the general population.
Frying as a popular cooking method is widely used in fast-food restaurants and home-made meals. Despite the fascinating flavor and texture, the frying process may change the nutrient composition and produce hazardous chemicals. Acrylamide, a major hazardous contaminant of fried foods, is generated from the Maillard reaction especially in carbohydrate-rich foods such as fried potatoes (9). A wide range of populations worldwide from infants to the elderly are exposed to acrylamide from fried foods in their daily lives (10). The means of dietary exposure to acrylamide were 1 and 4 μg/kg·bw/d in general populations and high consumers, respectively, concluding that acrylamide has continuously been a human health concern (11). Acrylamide intake from dietary sources has been identified as a widely concerned risk factor for neurological disorders, obesity, metabolic syndrome, cardiovascular disease (CVD), and depression symptom from epidemiological studies (12–16). In addition, it has been found that acrylamide has multiple toxicities, including neurotoxicity, reproductive toxicity, developmental toxicity, and carcinogenicity from in vivo and in vitro studies (10). Recent cross-sectional study showed that the hemoglobin adduct of acrylamide was associated with increased prevalence of depression among US adults (16), which drives the motivation to address acrylamide and fried foods related risk factors of psychiatric disorders. Despite the extensive public health concern, unfortunately few studies have investigated the potential causation of acrylamide-triggered anxiety and depression.
The neurotoxicity caused by acrylamide has been well recognized (17, 18). Acute exposure to acrylamide may lead to ataxia, skeletal muscle weakness, and numbness of the extremities in both humans and animals (18). Despite acrylamide-induced neurotoxicity in relation to central-peripheral distal axonopathy (19), the molecular initiating event is the disruption of the presynaptic vesicle (17). Notably, the decrease in mood is related to the decrease of monoamine neurotransmitters in the brain, while monoamine depletion is believed to result in anxiety and depression (20). However, how acrylamide exposure is linked to anxiety and depression still remains obscure. To further understand in-depth neurological damage, evidence supported that chronic exposure to acrylamide results in the accumulation of misfolded proteins, neuroinflammation, and neuronal apoptosis, leading to progressive neurodegeneration (21, 22). Moreover, a 2-wk exposure to acrylamide induces acute inflammatory death with hyperlipidemia using human macrophages, dermal cells, and zebrafish models (23). Collectively, we hypothesized that high fried food consumption and related chronic exposure to acrylamide may impact anxiety and depressive-like symptoms via dyslipidemia and inflammation.
To address the above hypothesis, we aimed to unravel the long-term relationship and causation between fried food consumption and the future development of anxiety and depression symptoms. Herein, we reported that i) fried food consumption is associated with a higher risk of anxiety and depression symptoms in humans; ii) chronic exposure to acrylamide exacerbates behavioral scototaxis and thigmotaxis in zebrafish; iii) chronic exposure to acrylamide induces anxiety and depression symptoms by cerebral lipid metabolism disturbance and immune response; and iv) chronic exposure to acrylamide impairs blood–brain barrier and induces neuroinflammation.
Results
Fried Food Consumption Is Associated with the Occurrence of Anxiety and Depression Symptoms.
To assess the associations of fried food consumption with anxiety and depression symptoms, we finally enrolled a total of 140,728 people from the UK Biobank study after excluding the participants who lacked information on diet records, anxiety state, depression state, and/or other mental health conditions (Fig. 1A and SI Appendix, Fig. S1). The baseline characteristics of participants according to fried food consumption are shown in Fig. 1B and SI Appendix, Table S1. A total of 8,294 and 12,735 cases of anxiety and depression symptoms were identified during an average of 11.3 y of follow-up, respectively. Compared with the nonconsumers, those participants consuming more than one serving/d of fried foods were more likely to be male, younger, and active smokers. Besides, they had a higher body mass index, lower household income and education level, lower vitamin supplement use, and higher energy intake. Similar baseline characteristics of participants according to fried white meat and fried potato consumption are shown in SI Appendix, Tables S2 and S3, respectively.
Fig. 1.
Study design and basic characteristics of the study participants. (A) Study design and the associations between fried food and fried potato consumption and the risk of anxiety and depression. (B) Baseline characteristics of participants across fried food consumption in UK biobank (n=140,728). Data are expressed as mean ± SD or numbers with percentages.
Cox proportional hazards regression analyses revealed that fried food consumption was significantly associated with a higher risk of anxiety symptom after adjustment for age and sex. The positive association remained robust after further adjustment for demographic and behavioral covariates (model 2). Finally, fried food consumption was related to a 12% higher risk of anxiety symptom [hazard ratio (HR): 1.12; 95% CI: 1.06 to 1.18; P < 0.001 for trend] after multivariable adjustment (model 3). Consistently, both fried white meat and fried potato consumption were significantly associated with a 4% higher risk of anxiety symptom after full adjustment (P = 0.003 and P = 0.006 for trend, respectively) (SI Appendix, Table S4). As expected, we observed similar results for the positive association of fried food consumption with the risk of depression symptom. Compared with nonconsumers, the multivariable HR of depression risk in the highest category was 1.07 (95% CI: 1.02 to 1.12) for fried foods (P < 0.001 for trend). Furthermore, fried potato consumption was marginally related to a 2% higher risk of depression symptom after multivariable adjustment (P = 0.031 for trend). Fried white meat consumption was related to a higher risk of depression after adjustment for age and sex, but the association became nonsignificant after adjustment for further covariates (SI Appendix, Table S4). In subgroup analyses, we observed a significant interaction of the fried food consumption with anxiety and depression symptoms between male and female (P = 0.003 and P = 0.025 for interaction, respectively) (SI Appendix, Table S5). Both fried food and fried potato consumption were significantly associated with a 7% higher risk of anxiety symptom among male (P = 0.002 and P = 0.025, respectively). Similarly, fried food and fried potato consumption was also related to a 5% higher risk of depression symptom among male (P = 0.007 and P = 0.043, respectively) (SI Appendix, Table S5). Moreover, a significant interaction of the fried food consumption with anxiety and depression symptoms among age was also found (P = 0.002 and P = 0.032 for interaction, respectively) (SI Appendix, Table S6). The associations between fried food consumption with the risk of anxiety and depression symptoms were significant among participants younger than 60 y (SI Appendix, Table S6). In sensitivity analyses, both associations between fried food consumption and anxiety or depression symptom did not change materially after further adjustment for other diseases, including T2D, CVD, or cancer, vitamin and mineral supplementation, aspirin and lipid-lowering medication use, or various food consumption. In addition, these relationships remained significant after further exclusion of participants with incident depression within 2 y or missing covariate data at the baseline (SI Appendix, Tables S7 and S8). Taken together, frequent consumption of fried foods is more likely to be associated with anxiety and depression symptoms among general population, and more pronounced among male and younger consumers.
Long-Term Exposure to Acrylamide Induces Anxiety-Like Behavior by Exacerbating both Scototaxis and Thigmotaxis of Zebrafish.
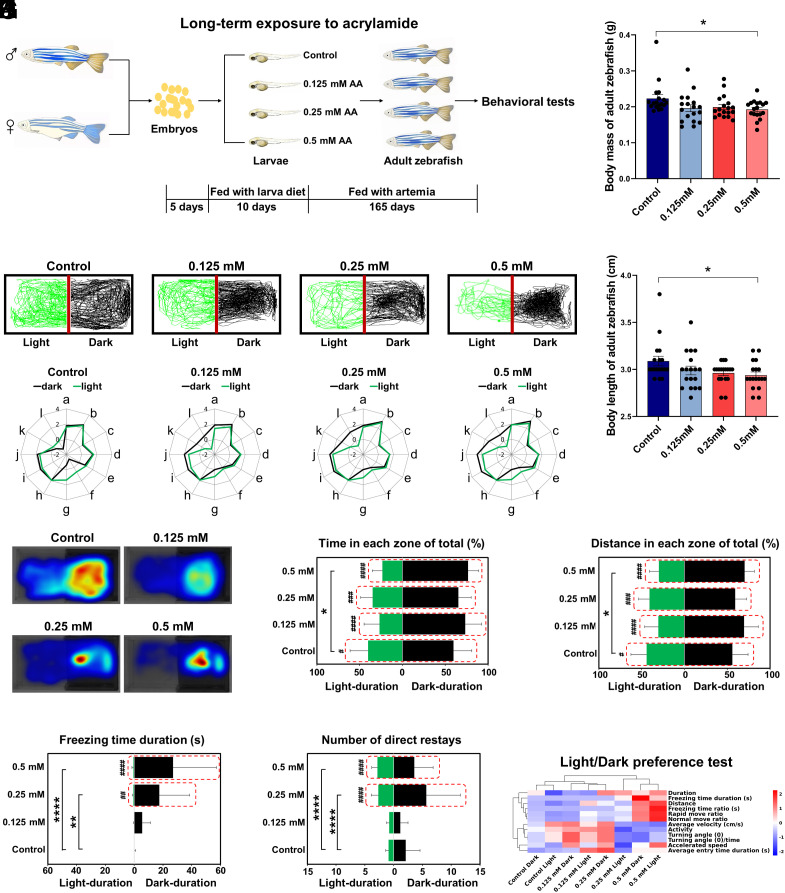
In our human study, the causation and mechanism of action linking dietary exposure to acrylamide, a typical processing contaminant in fried foods, with anxiety and depression symptoms remain unclear. We then established a zebrafish model (AB strain) chronically exposed to acrylamide to investigate how acrylamide induces anxiety and depression (Fig. 2A). Chronic exposure to acrylamide (0.5 mM) for 180 d caused significant decreases in body mass and body length of male zebrafish, respectively (all P < 0.05; Fig. 2 B and C). Notably, chronic exposure to acrylamide within low concentration levels (0.125, 0.25, and 0.5 mM) remarkably forced the scototaxis of adult zebrafish by observing their swimming trajectories and hence induced anxiety-like behavior in light/dark preference test (Fig. 2D), showing that spending more time in the dark zone indicates a higher anxiety level of zebrafish (24). We further evaluated the dark dwelling, an index of anxiety-like behavior, and its significance levels, wherein the parameters of distance, duration, latency, and traversing times were closely related to the dark dwelling in the dark zone. Representative radar maps and visualized heatmaps showed significant changes in swimming trajectories of zebrafish in the high-exposure group (0.5 mM acrylamide) compared with those in the control group (Fig. 2 E and F). For detailed parameters of behavioral trajectories, long-term exposure to 0.5 mM acrylamide significantly elevated both swimming time and distance ratios in the dark zone while suppressed those in the light zone (Fig. 2 G and H). Furthermore, we found the freezing time duration of zebrafish in the dark zone exhibited a dose-dependent increasing trend in acrylamide exposure groups; however, the trend in the light zone did not seem significant (Fig. 2I). To further determine the anxiety state of zebrafish, we counted traversing times between light and dark zones in all the groups. The histogram results showed that a 180-d aquatic exposure to acrylamide at moderate and high concentrations (0.25 and 0.5 mM) significantly elevated traversing times of zebrafish (Fig. 2J). We recorded the light/dark preference test to visualize the behavioral alteration between the control and each acrylamide-treated group (Movie S1). The movie shows that zebrafish with acrylamide treatment spent more time in the dark zone and traveled more times between light and dark zones, indicating a higher anxiety level, compared with those in the control group. To assemble individual findings from the test, hierarchical clustering analysis concluded that long-term treatment with acrylamide significantly increased the scototaxis of zebrafish (Fig. 2K).
Fig. 2.
Behavioral profiles of zebrafish by the long-term exposure to acrylamide (AA) in the light/dark preference test. (A) Schematic of the zebrafish study by the long-term exposure to acrylamide. (B and C) Effects of long-term exposure to acrylamide for 180 d on body mass and length of zebrafish, respectively. (D) Representative swimming trajectories of zebrafish in the control group and three acrylamide exposure groups (0 mM wide type, 0.125 mM, 0.25 mM, and 0.5 mM). The left side is the light chamber, and the right side is the dark chamber. (E) Radar chart of 12 behavioral parameters of zebrafish in different groups (0 mM wide type, 0.125 mM, 0.25 mM, and 0.5 mM). a, duration; b, distance; c, average velocity (cm/s); d, accelerated speed; e, average entry time duration (s); f, turning angle (°); g, turning angle (°)/time; h, activity; i, rapid move ratio; j, normal move ratio; k, freezing time ratio (s); l, freezing time duration (s). (F) Heatmap visualization of zebrafish trajectories in the light/dark preference test. (G) Duration time spent in the light or dark zone of total time (%). (H) Distance traveled in the light or dark zone of total distance (%). (I) Freezing duration time (s) in the light or dark zone. (J) Traversing times between the light and dark zones. (K) Hierarchical clustering of zebrafish in the light/dark preference test. All the histograms were present with mean ± SEM, while all behavioral parameter data were analyzed by the two-way ANOVA followed by multiple comparisons. The level of significance was defined as *P < 0.05, **P < 0.01, ****P < 0.0001; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 (* indicates significance between different groups and # indicates significance between different regions within the same group).
Similarly, the novel tank diving test was alternatively used to assess anxiety-like behaviors in zebrafish, which prefer to stay near the bottom of the tank when feeling anxious (25). In the current diving test, representative radar maps and visualized heatmaps exhibited dose-dependent changes of diving for the bottom in swimming trajectories of zebrafish in the exposure groups compared to those in the control group, indicating that chronic exposure to acrylamide notably exacerbates the thigmotaxis by decreasing the swimming time spent in the top part and increasing the time in the bottom part (SI Appendix, Fig. S2 A–C). Besides, the parameters of distance, duration, latency, and traversing times were more inclined to the thigmotaxis in the bottom zone in all the acrylamide treatment groups (SI Appendix, Fig. S2 D–G), the results of which were also supported by hierarchical clustering analysis (SI Appendix, Fig. S2H) and recorded through videotapes (Movie S2). The movie displays that zebrafish in the acrylamide exposure groups preferred to stay near the bottom of the tank, indicating that acrylamide exposure exacerbates thigmotaxis and thus increases the risk of anxiety-like symptom in zebrafish. Collectively, findings from light/dark preference and novel tank diving tests indicated that long-term exposure to acrylamide alters the cognitive development by exacerbating both scototaxis and thigmotaxis and thus increases the risk of anxiety-like symptom in zebrafish.
Long-Term Exposure to Acrylamide Induces Anxiety- and Depressive-Like Behaviors by Reducing Exploration Ability and Impairing Sociality of Zebrafish.
When zebrafish are relocated to a new environment, they seek protection by diving and staying at the safety home, until they feel safe enough to explore the unfamiliar environment (26). The swimming trajectories showed that zebrafish in the control group can swiftly explore and adapt to the novel environment, but chronic exposure to acrylamide reduces the ability to adapt to the unfamiliar environment (Fig. 3A). Visualized heatmaps showed significant changes in swimming trajectories of zebrafish in the acrylamide exposure groups compared with those in the control group (Fig. 3B). Furthermore, we found the swimming time and distance ratios in Zone 1 exhibited a dose-dependent decreasing trend in acrylamide exposure groups. Chronic exposure to acrylamide (0.5 mM) significantly decreased both swimming time and distance in Zone 1 and increased those in Zone 2 (Fig. 3 C and D). We recorded the novel object exploration test to visualize the behavioral alteration between the control and each acrylamide-treated group (Movie S3). The movie displays that zebrafish in the control group could swiftly explore and adapt to the novel environment, but chronic exposure to acrylamide reduced the ability to adapt to the unfamiliar environment, which indicated that acrylamide induces anxiety- and depressive-like behaviors by reducing exploration ability of zebrafish.
Fig. 3.
Behavioral profiles of zebrafish by the long-term exposure to acrylamide in the novel object exploration test and the social preference test. (A) Representative swimming trajectories of zebrafish in the control group and three acrylamide exposure groups (0 mM wide type, 0.125 mM, 0.25 mM, and 0.5 mM). A novel object for zebrafish was placed in the left part (Zone 1) and the right part was Zone 2. (B) Heatmap visualization of zebrafish trajectories in the novel object exploration test. (C) Duration time spent in Zone 1 or Zone 2 of total time (%). (D) Distance traveled in Zone 1 or Zone 2 of total distance (%). (E) Representative swimming trajectories of zebrafish in different groups (0 mM wide type, 0.125 mM, 0.25 mM, and 0.5 mM). (F) Radar chart of 12 behavioral parameters of zebrafish in different groups (0 mM wide type, 0.125 mM, 0.25 mM, and 0.5 mM). a, duration; b, distance; c, average velocity (cm/s); d, accelerated speed; e, average entry time duration (s); f, turning angle (°); g, turning angle (°)/time; h, activity; i, rapid move ratio; j, normal move ratio; k, freezing time ratio (s); l, freezing time duration (s). (G) Heatmap visualization of zebrafish trajectories in the social preference test. (H) Duration time spent in the left or right chamber of total time (%). (I) Distance traveled in the left or right chamber of total distance (%). (J) Traversing times between the left and right chambers. (K) Numbers of crossing the middle line. (L) Hierarchical clustering of zebrafish in the social preference test. All the histograms were present with mean ± SEM, while all behavioral parameter data were analyzed by the two-way ANOVA followed by multiple comparisons or the one-way ANOVA followed by the Turkey post hoc test. The level of significance was defined as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 (* indicates significance between different groups and # indicates significance between different regions within the same group).
Moreover, the social preference test was used to assess sociality of zebrafish. Since the zebrafish are a group preference species, they frequently swim by forming a school and swim closely to one another (27). In the current social preference test, representative radar maps and visualized heatmaps exhibited significant changes of preference in swimming trajectories of zebrafish in acrylamide exposure groups compared to those in the control group, indicating that chronic exposure to acrylamide remarkably impairs the sociality of zebrafish (Fig. 3 E–G). For detailed parameters of behavioral trajectories, chronic exposure to acrylamide (0.5 mM) significantly increased both swimming time and distance ratios in the left zone and decreased those in the right zone (Fig. 3 H and I). Notably, chronic exposure to acrylamide (0.5 mM) significantly elevated traversing times and number of crossing the middle line (Fig. 3 J and K). Besides, the above outcomes were supported by hierarchical clustering analysis (Fig. 3L) and recorded videos (Movie S4). The movie shows that zebrafish in the acrylamide exposure groups could be hardly to swim closely to the fish school, while zebrafish in the control group frequently swam closely, indicating that chronic exposure to acrylamide remarkably impairs the sociality and thus increases the risk of anxiety- and depressive-like symptoms in zebrafish. Taken together, findings from novel object exploration test and social preference test indicated that chronic exposure to acrylamide for 180 d reduces exploration ability and impairs sociality of zebrafish and thus increases the risk of anxiety- and depressive-like symptoms in zebrafish.
Chronic Exposure to Acrylamide Induces Cerebral Lipid Metabolism Disturbance and Immune Response.
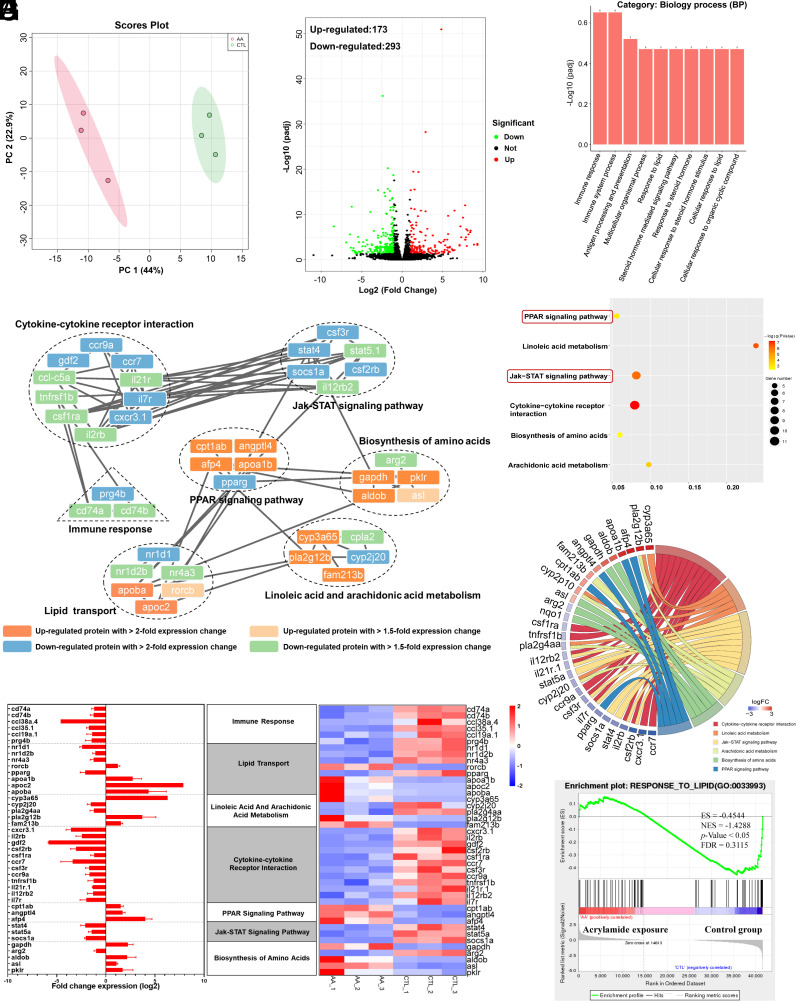
To elucidate the underlying mechanism of acrylamide-induced abnormal behavior and growth retardation, we next conducted a transcriptomic analysis in brain of zebrafish after chronic exposure to acrylamide. The statistics for transcriptome information from the six RNA-seq libraries are shown in SI Appendix, Table S9. The principal component analysis (PCA) indicated that the six samples were significantly distinguished into two groups (Fig. 4A). There were 466 differentially expressed genes (DEGs) in the acrylamide (0.5 mM) treatment group compared with the control group, including 173 up-regulated and 293 down-regulated genes (Fig. 4B). Furthermore, the heatmaps of hierarchical cluster analysis of DEGs and Pearson’s correlation between samples showed a similar relationship among all the six samples sequenced (SI Appendix, Fig. S3 A and B). Based on these DEGs, we then investigated gene ontology (GO) database to explore the association of these DEGs with biology process (BP), and finally filtered 10 GO terms enriched in BP (Fig. 4C). The results indicated that the most terms in BP were immune response, immune system process, antigen processing and presentation, multicellular organismal process, and response to lipid. Furthermore, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was used for the enrichment analysis of metabolic pathways, and the results indicated that six KEGG pathways were significantly enriched by chronic exposure to acrylamide (Fig. 4D and SI Appendix, Table S10), which involved in arachidonic acid metabolism, biosynthesis of amino acids, cytokine–cytokine receptor interaction, JAK-STAT signaling pathway, linoleic acid metabolism, and PPAR signaling pathway. Subsequently, in the protein–protein network, the interactions between the products of gene expression related to immune response and lipid metabolism were most obvious (Fig. 4E). We also found cytokine–cytokine receptor interactions, PPAR signaling, and JAK-STAT signaling pathway-related gene expression product interactions (Fig. 4F). The changes in upregulation and downregulation of gene expression are shown in histograms and heatmaps (Fig. 4G and SI Appendix, Table S11). Moreover, we performed pathway enrichment analysis by employing gene set enrichment analysis (GSEA) algorithm to identify biological pathways affected by chronic exposure to acrylamide. In this study, GSEA revealed significant inhibition of many pathways associated with lipid biosynthetic and immune system process (SI Appendix, Fig. S3 C–F), and significant activation of DNA replication and repair-related pathways (SI Appendix, Fig. S3 G–L). Notably, a significant enrichment of pathway associated with response to lipid was inhibited (Fig. 4H). Collectively, these findings suggested chronic exposure to acrylamide induce cerebral lipid metabolism disturbance mediated by PPAR signaling pathway, and elicit immune response.
Fig. 4.
Chronic exposure to acrylamide induces lipid perturbation and inflammatory response based on DEGs analysis. (A) The control and acrylamide-treated groups were completely distinguished by different colors in PCA plots. (B) Volcano map based on DEGs [padj < 0.05 and absolute Log2 FC (fold change) ≥ 1]. (C) Enrichment analysis of GO function based on DEGs. (D) Dotplot of the enriched KEGG pathway for DEGs in the brain of zebrafish due to acrylamide exposure. (E) Interaction network of differentially expressed proteins in major pathways with significant changes due to acrylamide exposure. (F) Relationship between DEGs and KEGG pathways. (G) Visual analysis of DEGs in major pathways. A horizontal bar graph of gene expression (Left) and a heat map of gene expression (Right). (H) GSEA results showing response to lipid-related pathways with significant changes in the brain of zebrafish after exposure to acrylamide for 180 d.
Cerebral Lipid Metabolism Disturbance Links to Acrylamide-Induced Anxiety and Depression Symptoms.
To further link the disturbance of lipid metabolism to acrylamide-induced anxiety and depression, we conducted a widely targeted quantitative lipidomics analysis of brain samples of zebrafish in the acrylamide exposure group compared with those in the control group using LC-ESI-MS/MS. To validate the reliability of the current lipidomics analysis, we tested the robustness of instrumentation and data variability. The R-value of quality control samples using Pearson’s correlation analysis was nearly 1, indicating good operating condition of instrumental facility (SI Appendix, Fig. S4A). The PCA model was built to evaluate the analytical ability of lipid datasets, and the results of PCA score plots exhibited the unambiguous separation between acrylamide exposure (0.5 mM) group and the control group (SI Appendix, Fig. S4B). A total of 1,980 lipid substances were identified based on accurately matching masses and molecular structures against a self-compiled database (Metware database, Wuhan, China). They were classified into six lipid categories, including 1,041 glycerophospholipids (GPs), 267 sphingolipids (SPs), 545 glycerolipids (GLs), 22 sterol lipids (STs), 104 fatty acyls (FAs), and 1 prenol lipid (PL). Furthermore, all lipids were grouped into 29 lipid subclasses, including 66 phosphatidic acids (PAs), 333 phosphatidylethanolamines (PEs), 65 hexosylceramides (HexCers), 4 lyso-phosphatidylinositols (LPIs), 85 phosphatidylinositols (PIs), 3 phosphatidyl methanols (PMeOHs), 5 sphingols (SPHs), 8 lyso-phosphatidic acids (LPAs), 8 lyso-phosphatidylserines (LPSs), 16 eicosanoids, 42 carnitines (CARs), 6 bis-methyl phosphatidy lserines (BMPs), 384 triglycerides (TGs), 35 lyso-phosphatidylethanolamines (LPEs), 28 monoglycerides (MGs), 133 diglycerides (DGs), 46 free fatty acids (FFAs), 18 cholesterol esters (CEs), 61 lyso-phosphatidylcholines (LPCs), 5 lyso-phosphatidylglycerols (LPGs), 3 bile acids (BAs), 1 cholesterol (Cho), 37 sphingomyelins (SMs), 18 N-acyl-lyso-phosphatidylethanolamines (LNAPEs), 160 ceramides (Cers), 117 phosphatidylserines (PSs), 105 phosphatidylglycerols (PGs), 1 coenzyme Q (CoQ), and 187 phosphatidylcholines (PCs) (SI Appendix, Fig. S4 C and D). Furthermore, the orthogonal partial least squares discrimination analysis (OPLS-DA) score plots revealed a clear separation of lipid profiles between the acrylamide treatment (0.5 mM) group and the control group (SI Appendix, Fig. S5A). Also, the resulting R2X, R2Y, and Q2 of 200 random permutations test model validation were 0.508, 0.937, and 0.708, respectively, indicating that the OPLS-DA model was not overfitting and was reliable to screen differential lipids (SI Appendix, Fig. S4E). Variable importance in the projection (VIP) and fold change (FC) values for each lipid were calculated, and the higher the VIP value, the more the contribution of the lipid to the OPLS-DA model construction. To screen and identify differential metabolites, the criteria for selecting significant lipids were VIP > 1, P < 0.05, and |log2 FC| ≥ 1, compared with the control group. We found that 406 lipids were significantly up-regulated, and 11 lipids were significantly down-regulated in the acrylamide-treated group (SI Appendix, Fig. S5B). Over half of the differential lipids were GLs, including 368 TGs, 2 DGs, and 3 MGs. The GPs containing 2 BMPs, 1 LNAPE, 3 LPCs, 1 LPI, 1 LPS, 4 PEs, 1 PI, and 5 PSs were also found. Furthermore, the FFAs were identified and composed of 1 eicosanoid and 4 FFAs. In addition, the SPs with 14 Cers, 3 HexCer, and 1 SM, and STs with 3 CEs were screened. The finding of these differential lipids revealed that chronic exposure to acrylamide remarkably disturbs internal relationship of conversion between different lipid substances, we then formulated a correlation matrix using Pearson’s correlation coefficients for these six categories and 29 subclasses, and revealed overall strong correlations (SI Appendix, Fig. S4 F–I). Correlation analyses revealed that a strong positive correlation between LPC and PC, indicating that acrylamide induces an inflammatory storm in brain of zebrafish. LPC is mainly derived from the turnover of PC in the circulation by phospholipase A2 (PLA2) (28). LPC increases the production of proinflammatory cytokines, induces oxidative stress, and promotes apoptosis, which can aggregate inflammation and promote the development of neurodegenerative diseases (29). Low levels of PE and high levels of LPE result in accelerating the progression of Alzheimer's disease (AD) related to inflammatory response (30). A strong inverse correlation between LPE and PE suggested that acrylamide exposure may induce inflammatory response and cognitive impairment. Also, a positive correlation between SM and Cer indicated that both lipids may be at play during brain injury and neurological diseases, since the accumulation of sphingomyelin increases membrane fluidity and impairs neural differentiation (31). Therefore, the mutual transformation among GPs and SPs may be associated with anxiety and depression symptoms of zebrafish. Importantly, we found significant changes of TG in both categories and subclasses based on random forest analysis (SI Appendix, Fig. S5 C and D). We then subsequently explored the correlation between TG and other categories and subclasses, respectively. GL exhibited a positive correlation with FA, ST, PL, and SP (SI Appendix, Fig. S4G), while TG showed a positive correlation with DG but a significantly inverse correlation with PI (SI Appendix, Fig. S4I). Furthermore, we summarized the top 20 significantly changed lipids, while TG (18:2_18:2_22:1) was the most significantly changed when specific to lipid molecules in TGs (SI Appendix, Fig. S5D). The amount accumulation and the profile alteration for TGs were connected with disturbance of lipid metabolism. To determine the pathways by which acrylamide disturbs cerebral lipid metabolism, the KEGG database was used to analyze the metabolic pathway with the 417 significantly changed lipids. As a result, we enriched eight significant metabolic pathways, including insulin resistance, lipid and atherosclerosis, cholesterol metabolism, regulation of lipolysis in adipocytes, vitamin digestion and absorption, thermogenesis, fat digestion and absorption, and glycerolipid metabolism (all P < 0.05; SI Appendix, Fig. S5E). The heatmap of hierarchical cluster analysis showed that lipid molecules in TGs were up-regulated in the acrylamide-treated group compared to the control group (SI Appendix, Fig. S5F), which was consistent with previous results (SI Appendix, Fig. S5D). The findings were also in agreement with the quantified results of selected lipids in the TGs, including TG (18:2_18:2_22:1), TG (18:0_17:1_18:1), TG (19:0_18:1_22:6), TG (18:1_24:1_18:2), TG (12:0_18:2_18:3), and TG (17:1_18:1_22:2) (SI Appendix, Fig. S5G). Combined with the selected significantly changed lipids and metabolic pathways, we focused on the cholesterol metabolism since the increase in TGs was closely related to cholesterol metabolism. Consistently, the cerebral histopathological examinations showed no signs of lipid deposition in the control group but increased lipid droplets infiltrating into brain cells in the acrylamide exposure group (SI Appendix, Fig. S5H). Given that the disorder of lipid metabolism is related to anxiety and depression emotions (32), these findings outlined that acrylamide-induced anxiety and depression symptoms may be ascribed to the disorders of cerebral lipid metabolism.
Chronic Exposure to Acrylamide Dysregulates Sphingolipid and Phospholipid Metabolism.
To further characterize the changes in metabolic pathways of lipids, we found that the sphingolipid and phospholipid metabolism was significantly dysregulated in acrylamide-treated group compared with the control group (SI Appendix, Fig. S6A). Specifically, the lipid signature molecules in this metabolic pathway, PS (18:0_16:1), PS (16:0_20:5), PI (16:1_22:3), LPI (16:0/0:0), and LNAPE (22:6/N-20:1), were significantly down-regulated; while Cer (d18:1/15:0), HexCer (d16:1/22:1), and SM (d18:1/20:1) were significantly up-regulated (all P < 0.05, SI Appendix, Fig. S6 A and B). Notably, PS (18:0_16:1) was the most decreased lipid among these signature lipid molecules (SI Appendix, Fig. S5D), suggesting chronic exposure to acrylamide perturbs the central nervous system (CNS) since PS is involved in the multiple cerebral functions, such as antiinflammation, neurotransmission, and synaptic refinement (33). Meanwhile, chronic exposure to acrylamide significantly up-regulated the levels of ceramides, which advance mitochondrial membrane permeability and facilitate the release of apoptogenic factors. These factors subsequently trigger the caspase cascade that constitutes the intrinsic pathway of apoptosis (34). In addition, the accumulation of SM (d18:1/20:1) may promote cell proliferation and neurogenesis in order to compensate for neuronal cell death in brain (35). In brief, perturbations in sphingolipid and phospholipid metabolism may play important roles in acrylamide-induced CNS damage and the anxiety and depression symptoms.
We next highlighted differential lipids interactions and identified strong interactions between TG (12:0_14:0_18:2), 2-arachidonylglycerol, and 5-KETE (5-oxoETE) (SI Appendix, Fig. S6C). In accordance with results from quantitative lipidomics, the level of TG (12:0_14:0_18:2) significantly increased in the acrylamide-treated group compared with the control group (P < 0.001); long-term exposure to acrylamide significantly reduced the level of 2-arachidonylglycerol, which involves in arachidonic acid metabolism (P = 0.016); while the level of 5-KETE (5-oxoETE), one of lipid preoxidation products, was marginally enhanced by the chronic exposure to acrylamide (P = 0.096) (SI Appendix, Fig. S6D). Briefly, the results unveiled that chronic exposure to acrylamide disorders cholesterol metabolism and arachidonic acid metabolism, and elevates lipid peroxidation levels.
Chronic Exposure to Acrylamide Impairs Blood–Brain Barrier and Induces Neuroinflammation.
We next evaluated the gene expression alternations of tight junctions (TJs) and adherens junctions (AJs) to understand how acrylamide impairs the blood–brain barrier (BBB) function for disturbing lipid metabolism. Here, acrylamide exposure down-regulated gene expression of both TJs and AJs. However, only tjp2a was significantly down-regulated (P < 0.01) (SI Appendix, Fig. S7A), suggesting acrylamide may impair TJs, which are present between the cerebral endothelial cells and form a diffusion barrier selectively preventing most blood-borne substances from entering the brain (36). The downregulation of AJs did not seem significant (SI Appendix, Fig. S7A), although AJs are a prerequisite for the assembly of TJs that allow epithelial cells to establish polarity with different proteins and lipids in the apical and basal plasma membranes (37). These alterations are associated with increased permeability of the BBB, but the leaky BBB due to exposure to acrylamide may facilitate neuroinflammation and contribute to the development of anxiety and depression symptoms. Given the impaired integrity of BBB, we further observed that acrylamide induced the damage of brain tissue by progressively aggravating the infiltration of inflammatory cells with the increase in exposure to acrylamide from 0.125 mM to 0.5 mM (SI Appendix, Fig. S7B). Taken both disturbance of lipid metabolism and neuroinflammation into consideration, we next focused on the changes in lipid mediators especially proinflammatory mediators involved in the oxidative stress by the exposure to acrylamide. As shown in SI Appendix, Fig. S7C, 16 different oxylipins belonging to arachidonic, linoleic acid, docosahexaenoic acid, and eicosapentaenoic acid pathways were detected and quantified in brain of zebrafish, while most of oxylipins were up-regulated in the acrylamide-treated group compared with the control group. Particularly, 5-oxo-eicosatetraenoic acid (5-oxoETE), 5-hydroxyeicosatetraenoic acid [(±)5-HETE], and 11-hydroxyeicosatetraenoic acid [11(S)-HETE] as markers of lipid peroxidation (38) were dramatically elevated (SI Appendix, Fig. S7D). In addition, we also observed the upregulation of two proinflammatory lipid mediators, 12-hydroxyeicosatetraenoic acid [(±)12-HETE] and 14-hydroxydocosahexaenoic acid [14(S)-HDHA] (39), indicating elevated cerebral inflammatory response in the acrylamide-treated group compared with the control group (SI Appendix, Fig. S7D). Moreover, exposure to acrylamide promoted both leukotoxin [9,10-epoxy-12Z-octadecenoate (9,10-EpOME)] and isoleukotoxin [12,13-epoxy-9Z-octadecenoic acid (12,13-EpOME)] (SI Appendix, Fig. S7D), supporting the evidence of acrylamide-induced proinflammatory effect and oxidation stress. In general, 9,10-EpOME and 12,13-EpOME can influence physiological functions via targeting PPARγ receptors (40), and the diols in particular exhibit cytotoxic, immunological, and prooxidative effects (41). Collectively, chronic exposure to acrylamide promotes inflammation and oxidation stress, which participate cerebral neuroinflammation.
To further investigate the role of neurotransmitters, we found the concentrations of γ-aminobutyric acid (GABA) and glutamine (Gln) significantly increased in the acrylamide-treated group compared with the control group (all P < 0.01; SI Appendix, Fig. S7E), suggesting that typical neurotransmitter metabolism be disrupted in brain of acrylamide-exposed zebrafish. Disturbance of the GABA system and glutamatergic system is implicated in the pathophysiology of many neuropsychiatric disorders, including anxiety and depression (42, 43). We speculated that brain shifts in neurotransmitter metabolism may facilitate anxiety and depression symptoms in acrylamide-exposed zebrafish. Furthermore, GSEA verified a significant enrichment of biological GO terms associated with immune response after exposure to 0.5 mM acrylamide (SI Appendix, Fig. S7F), which supported the findings of acrylamide-induced oxidative stress and neuroinflammation, and thus elicited cerebral immune response. Together, chronic exposure to acrylamide induces BBB damage by down-regulating TJs and AJs exacerbates oxidative stress and neuroinflammation due to elevated oxylipins, and disturbs GABA and glutamatergic system.
Chronic Exposure to Acrylamide Induces Anxiety and Depression Symptoms via Disturbing Lipid Metabolism and Inflammatory Response.
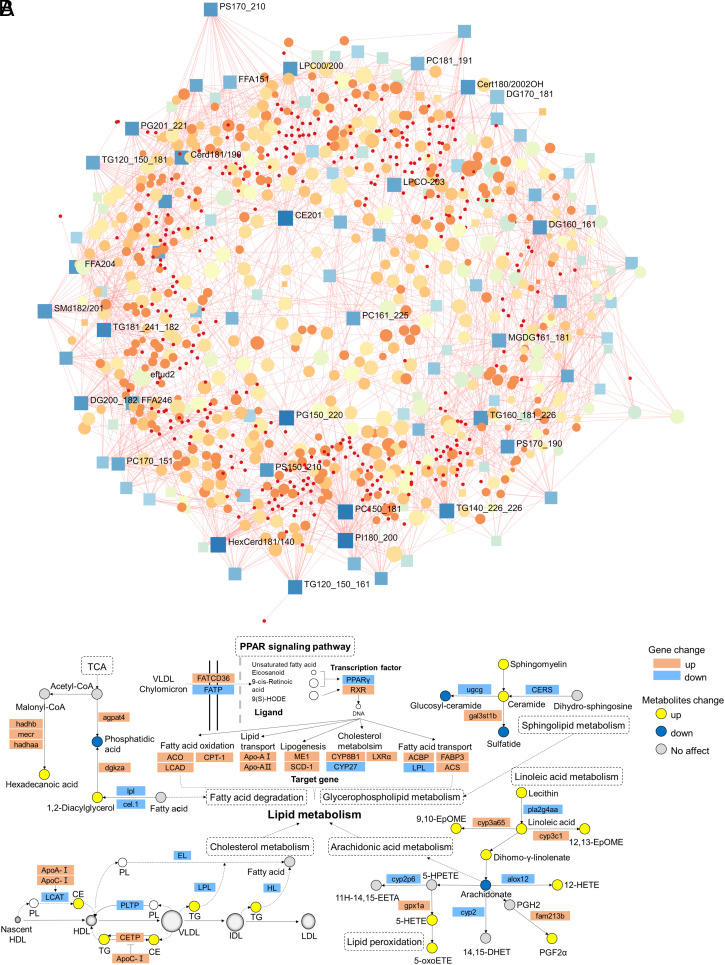
We further investigated the underlying mechanism of lipid metabolism dysfunction caused by acrylamide exposure in brain of zebrafish. The integration of lipidomics and transcriptomics enhances the comprehension of underlying biological processes and allows grasping the mechanism of action for exposure to toxic substances at a system level (44). Currently, pathway-based omics data integration using lipidomics and transcriptomics benefits for the discovery of gene-metabolite relationship at the branch points in the network defined by KEGG database. Here, we evaluated and identified the datasets of statistically significant affected genes and lipid metabolites, indicating close interactions between these significant genes and lipids (Fig. 5A). Meanwhile, the enriched KEGG pathways from transcriptomic profiles showed the exposure to acrylamide perturbs the PPAR signaling pathway, JAK-STAT signaling pathway, lipid transport, biosynthesis of amino acids, and linoleic acid and arachidonic acid metabolism. To verify these findings from transcriptomic profiles, we applied qRT-PCR to examine the relative gene expressions along the target pathways (SI Appendix, Fig. S8 and Table S13). Furthermore, we conducted the pathway-based lipidomics-transcriptomics integration analysis to examine the effect of acrylamide exposure on the PPAR signaling pathway and lipid metabolism (Fig. 5B). The enriched KEGG pathway from transcriptomic profiles showed acrylamide exposure perturbed the PPAR signaling pathway (Fig. 4D), in which unsaturated fatty acid, eicosanoid, and other ligands are transported into the cell, activating the transcription factor expression, including downregulating peroxisome proliferator-activated receptor gamma (PPARγ) and up-regulating RXR gene expression. The transcription factor of PPARγ is a ligand-activated receptor that regulates lipid metabolism and suppresses neuronal inflammation (45, 46). PPARγ plays an important role in mediating lipid metabolism disorder by regulating the gene expression related to cholesterol metabolism, lipogenesis, fatty acid oxidation, fatty acid transport, and lipid transport. Meanwhile, we recognized significant changes in the relative expressions of target genes related to fatty acid oxidation, lipid transport, lipogenesis, cholesterol metabolism, and fatty acid transport. Importantly, the perturbation of PPAR signaling pathway mediates the disorder of downstream lipid metabolism, including cholesterol metabolism and arachidonic acid metabolism. Cerebral cholesterol homeostasis plays an important role in neurological disorders (47). In detail, the upregulation of ApoC-I inhibits the interconversion of TG and CE, while the down-regulation of LCAT improves the conversion from PL to CE, and the downregulation of LPL and HL genes promotes the conversion from TG to fatty acids. Acrylamide exposure disrupts cholesterol homeostasis and promotes fatty acid oxidation, which is correlated with the enhancement of oxidative stress in brain. On the other hand, the downregulation of arachidonate in arachidonic acid metabolism plays a key role in inflammation, while arachidonic acid is oxygenated and further transformed into a variety of products which mediate or modulate inflammatory reactions (48). The arachidonic acid metabolism is closely related to linoleic acid metabolism and lipid peroxidation. Lipid peroxidation products induce oxidative stress and cause proinflammatory effects, therefore, partly explain acrylamide-induced anxiety- and depressive-like behaviors. Notably, we found elevated level of ceramide and reduced level of sulfatide by the exposure to acrylamide. Along with its metabolic precursor galactosylceramide, sulfatide and galactosylceramide constitute the predominant lipid components that ensure the normal structural and functional attributes of myelin sheath (49). We speculated that chronic exposure to acrylamide may damage the normal structural and functional attributes of myelin sheath in brain, which is considered as a contributing factor to anxiety and depression symptoms. In addition, the products of TCA cycle participate in regulating the levels of phosphatidic acid, 1,2-diacylglycerol, and hexadecanoic acid, indicating that energy metabolism in brain may also be perturbed by the exposure to acrylamide. In addition, energy metabolism is closely related to mitochondria, the cell organelles, which use energy sources to generate ATP (50). Mitochondria possess a plethora of cellular functions, such as regulating lipid metabolism, ion homeostasis, and neurotransmitter levels (51). Thus, alterations in energy metabolism could also affect lipid metabolism. In summary, lipid metabolism and inflammatory response play a causal role in the anxiety- and depressive-symptoms induced by chronic exposure to acrylamide. Disrupted lipid metabolism is intertwined with lipid peroxidation and oxidative stress, and ultimately damages myelin sheath and BBB.
Fig. 5.
Chronic exposure to acrylamide disturbed cholesterol metabolism and arachidonic acid metabolism via the PPAR signaling pathway. (A) Network of the DEGs and lipid metabolites in zebrafish with exposure to acrylamide. Squares represent lipid metabolites and circles represent genes. (B) Key pathways (PPAR signaling pathway, cholesterol metabolism, arachidonic acid metabolism, lipid peroxidation, fatty acid degradation, sphingolipid metabolism, and linoleic acid metabolism) in the integration of transcriptomics and lipidomics. The bars are shown as mean ± SD. Statistical differences were determined using an unpaired Student's t test. AA, the acrylamide-treated (0.5 mM) group; CTL, the control group.
Discussion
There is mounting evidence demonstrating the importance of dietary factors in the development and progression of metal disorders such as anxiety and depression (52, 53). Previous studies have revealed high-fat diet, inadequate tryptophan and dietary protein, high intake of sugar and refined carbohydrates, and “unhealthy” dietary patterns were associated with higher levels of anxiety (52). Fried foods as a favorite component of daily diet worldwide is a major risk factor for the progression of various chronic diseases and health problems, including overweight/obesity, hypertension, T2D, and CVD (54, 55). Additionally, several contributions supported the occurrence of anxiety and depression have been linked to these metabolic disorders (56, 57). Unfortunately, the relationship between fried food consumption and anxiety and depression symptoms and underlying mechanisms have been unclear prior to the current study. Here, we revealed that frequent fried food consumption especially fried potato consumption is strongly associated with a higher risk of anxiety and depression symptoms among general population while males are the susceptible group (Fig. 1 and SI Appendix, Tables S4–S6). Previous research showed that females tend to suffer more from mental health problems than males (58, 59). This study emphasized frequent consumption of fried foods is more likely to be associated with anxiety and depression symptoms among male consumers. Our result is consistent with a recent longitudinal observational study, which demonstrated that computer usage time and fried food consumption were lifestyle-related risk factors for mental health problems in male college students (8). This sex-dependent effects may be related to differences in lifestyle behaviors and gonadal hormones with mental health status between males and females (59, 60). The causal relationship may be ascribed to the harmful effect of chemical contaminants in fried foods (61), while acrylamide as a typical hazardous Maillard reaction product is widely present in heat-processed carbohydrate-rich food, especially in fried potatoes (9). Recently, a population-based cross-sectional study revealed that acrylamide exposure is associated with an increased risk of depressive symptoms (16). Consistently, our study found chronic acrylamide exposure also induces anxiety and depression symptoms in zebrafish model within a 180-d exposure period because 180 dpf is the human-like prime of life for zebrafish (62–64). Furthermore, acrylamide exposure could induce cognitive and social behavioral deficits.
We found that chronic acrylamide exposure remarkably disturbs the behavioral profiles of zebrafish, alters the preference for light/dark, reduces the exploration ability of zebrafish in the novel tank and novel object exploration tests, and affects the group preference of zebrafish (Figs. 2 and 3 and SI Appendix, Fig. S2). The behavioral phenomics alterations indicated that life-cycle exposure to acrylamide can inactivate the neurobehavior of zebrafish, and increase the risk of anxiety, reduce the ability to adapt to the unfamiliar environment, and alter the instinctual behavior of zebrafish preference for the group. To unravel intrinsic mechanism, acrylamide may lead to neurotoxic effects in zebrafish, like the dysregulation of genes related to microtubules and presynaptic vesicle alteration (65). Although previous study evidenced acrylamide-treated zebrafish exhibited “depressive-like” phenotype comorbid with anxiety behavior due to acute neurotoxicity (18), we first showed that chronic acrylamide exposure exhibits anxiety symptoms and social disability, and weakens adaptability to the new environment. As shown in SI Appendix, Fig. S7E, chronic exposure to acrylamide interferes with the endogenous level of monoamine neurotransmitters in zebrafish brain. Monoamine neurotransmitters play a key role in regulating neurobehavioral function (66–68). Consistent with the current results, the excessive Gln concentration and the dysfunction of the GABA signaling system in the brain can lead to depression (69, 70). Here, we showed that chronic acrylamide exposure significantly elevates the cerebral levels of GABA and Gln in brain and aggravates the neurobehavioral dysfunction in zebrafish.
To further understand the molecular mechanism underlying chronic acrylamide-induced abnormal behavioral profiles, our GO and KEGG analyses at the transcriptomic level indicated that chronic acrylamide exposure may lead to gene expression changes in PPAR signaling pathway, lipid metabolism, and immune response (Fig. 4D). PPARs are a family of ligand-regulated nuclear receptors, including PPAR-α, PPAR-β, and PPAR-γ. Moreover, the PPAR signaling pathway is mainly involved in lipid metabolism. Membrane-associated proteins such as fatty translocase (FAT/CD36) and fatty acid transport protein (FATP) facilitate the transmembrane transfer of fatty acids (FAs), which are subsequently bound by cellular fatty acid-binding proteins (FABPs). After that, FAs complete the lipid metabolic process by mediating signal transduction and nuclear transcription by PPARs (71). The upregulation of FATCD36 (cd36) and downregulation of FATP (slc27a4) expression indicated that acrylamide disturbs the transmembrane transport of FAs in zebrafish brain (Fig. 5B). The influenced PPAR signaling pathway may participate in the acrylamide-induced lipid metabolism perturbations and behavior defects.
The integration of transcriptomics and lipidomics indicated that the influenced PPAR signaling pathway may mediate downstream lipid metabolism, including cholesterol metabolism, arachidonic acid metabolism, and sphingolipid metabolism (Fig. 5B). Lipid metabolism ensures the operation of normal physiological functions, which is of great significance to everyday life activities (72). Previous studies have also shown that acrylamide perturbs lipoprotein metabolism to result in the exacerbation of atherosclerosis (23). In addition, cholesterol homeostasis is vital for brain function, while cholesterol metabolism dysfunction is tightly associated with anxiety and depression behaviors (73). In the current study, we observed acrylamide induces cholesterol metabolism perturbation, suggesting that metabolic disorders may contribute to the occurrence of anxiety and depression behaviors. Alternatively, chronic exposure to acrylamide also interferes with arachidonic acid metabolism by significantly reducing the level of 2-arachidonoylglycerol compared with that in the control group (SI Appendix, Fig. S6D). Metabolites derived from arachidonic acid metabolism have been implicated in immune surveillance, inflammatory response, glucose metabolism, and lipid metabolism (74). Changes in behavior and brain integrity may be associated with disturbed neurotransmission and arachidonic acid metabolism (75), while cerebral metabolic disorder of arachidonic acid metabolism contributes to depressive- and anxiety-like behaviors (76). In concordance with some previous findings, exposure to acrylamide up-regulates ceramide, a toxic metabolite in sphingolipid metabolism, while depression and anxiety symptoms positively associate for ceramide concentrations (77, 78). Therefore, we provide an avenue to understand the cause of anxiety- and depressive-like behaviors from a long-term acrylamide exposure by inhibiting phosphatidylinositol lipid signaling and inducing cholesterol metabolism and arachidonic acid metabolism disorders.
Numerous studies demonstrated that acrylamide induces oxidative stress and lipid peroxidation via reactive oxygen species (ROS) production (79, 80). Free radicals, which are produced from the peroxidation of polyunsaturated fatty acids, damage cellular components notably cell membranes and induce protein oxidation (81). Based on quantified lipidomics and metabolic pathway analysis, we found that acrylamide-treated zebrafish display a higher level of lipid peroxidation in the brain than the nontreated control group. Excessive lipid oxidation alters the physical properties of cellular membranes and leads to neuronal damage and neuronal cell death via the covalent modification of proteins and nucleic acids (82). Moreover, lipid peroxidation products may be potential biomarkers for oxidative stress status in vivo (83). Thus, oxidative stress induces many downstream biological events, such as the activation of JAK/STAT pathway, and mediates the inflammatory response. Previous reports indicated that acrylamide exposure potentially increases the risk of inflammation, for example, by increasing levels of proinflammatory cytokines such as TNF-α, IL-1β, and iNOS (84). Furthermore, oxidative stress and inflammation are also the major influencing factors for metabolic disorders (85, 86). Therefore, we infer that ROS is also one of the major contributors for acrylamide-induced chronic damage to nervous system. In addition, the BBB is a complex active and passive structure surrounding cerebral microvessels and protects the brain against harmful endogenous and exogenous substances (87). Previous studies demonstrated that acrylamide crosses and disturbs the BBB, and exerts its neurotoxic effect in the CNS (88). Meanwhile, BBB dysfunction depends on ROS-mediated disruption of AJ and TJ proteins in zebrafish. TJs, major components of the barrier, tie the endothelial cells that line the walls of these capillaries together around their borders (37). In our study, the gene expressions of AJs and TJs vary degrees of reduction, indicating that chronic of acrylamide exposure induce bad permeability of BBB due to TJ breakage (SI Appendix, Fig. S7A). Altogether, these results suggest that excessive lipid oxidation-mediated oxidative stress and inflammation by the activation of JAK/STAT pathway may promote brain metabolic disorders and impact the permeability of BBB due to TJ breakage in zebrafish with acrylamide exposure.
Taken together, we reveal that fried food consumption is a dietary risk factor for increasing the risk of anxiety and depression in humans especially males, which is mainly ascribed to the generation of acrylamide during frying process. The current study further demonstrates that long-term exposure to acrylamide induces oxidative stress and inflammation that may promote cerebral metabolic disorders and cause anxiety and depression behaviors of zebrafish. Chronic acrylamide exposure disorders sphingolipid and phospholipid metabolism, cholesterol metabolism, and arachidonic acid metabolism, and elevates lipid peroxidation levels. The signaling pathways of JAK/STAT and PPAR mediate acrylamide-induced changes in lipid metabolism in the brain of zebrafish. Our findings propose an understanding that long-term exposure to acrylamide impairs blood-brain barrier and exacerbates neuroinflammation and thus disturbs behavioral profiles of adult zebrafish. Among the study, our findings highlight the key role of phosphatidylinositol lipid signaling, cholesterol metabolism, and arachidonic acid metabolism in acrylamide-induced anxiety- and depressive-like behavior, and unravel an in-depth understanding of the causative link and underlying mechanisms of the detrimental role of fried food consumption on anxiety and depression symptoms. In this sense, we provide insights into the adverse effects of fried foods and acrylamide as a typical food processing contaminant on mental health from humans to zebrafish.
Our study has some limitations that should be addressed in the future research. Although a large sample size from UK Biobank was used in the current human study, further multiple population-based studies including different ethnic groups with various classes of ages would be more representative. Additionally, longitudinal and longer term follow-up studies would be necessary to better understand the strength and verify our conclusions. Finally, despite the zebrafish models have widely been used to observe anxiety- and depressive-like behaviors in the aquatic environment, they cannot be directly compared to human anxiety and depression evaluation and brain morphology. Future studies are also needed to evaluate the adverse effects of fried foods on anxiety and depression symptoms in a population-based intervention trial.
Materials and Methods
A total of 140,728 participants from the UK Biobank were eligible and included in the main analysis for the association of fried food consumption with incident anxiety/depression symptoms in our human study. The UK Biobank had ethical approval granted by the North West Multi-Centre Research Ethics Committee (reference number 06/MRE09/65), and all participants were required to provide written informed consent. Zebrafish were exposed to acrylamide (0, 0.125, 0.25, and 0.5 mM) from 2 hpf to 180 dpf. Then, the light/dark preference test, novel tank diving test, novel object exploration test, and social preference test were conducted to evaluate the anxiety and depression symptoms of zebrafish. Brain samples were carefully collected for further analysis, including HE and oil red staining, transcriptomics, qRT-PCR, and lipidomics analysis. Animal experiments were conducted according to the protocols approved by the Ethics Committee of Institutional Animal Care and Use Committee (IACUC) of Zhejiang University (Hangzhou, China) (Approval no. ZJU20210140). For a detailed description of materials and methods, see SI Appendix, Materials and Methods.
Supplementary Material
Appendix 01 (PDF)
Light/dark preference test. Related to figure 2. The movie shows that zebrafish with acrylamide treatment spend more time in the dark zone and travel more times between light and dark zone, indicating a higher anxiety level of zebrafish, compared to the control group.
Novel tank diving test. Related to figure S2. The movie displays that zebrafish in the acrylamide exposure groups prefer to stay near the bottom of the tank, indicating that acrylamide exposure exacerbates thigmotaxis and thus increases the risk of anxiety-like symptom in zebrafish.
Novel object exploration test. Related to figure 3. The movie displays that zebrafish in the control group can swiftly explore and adapt to the novel environment, but chronic exposure to acrylamide reduces the ability to adapt to the unfamiliar environment. This indicates that acrylamide induces anxiety- and depressive-like behaviors by reducing exploration ability of zebrafish.
Social preference test. Related to figure 3. The movie shows that zebrafish in the acrylamide exposure groups can hardly to swim closely to the fish school, while zebrafish in the control group frequently swam closely to the fish school. This indicates that chronic exposure to acrylamide remarkably impairs the sociality of zebrafish and thus increases the risk of anxiety- and depressive-like symptoms in zebrafish.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant no. 21976156) and the China National Program for Support of Top-notch Young Professionals. We also thank members of the National Institutes of Health Research Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King's College London and the participants who enrolled in the UK Biobank. This research has been conducted using the UK Biobank Resource under Application No. 47365. We also thank for the support of experimental conditions in the zebrafish platform of Zhejiang Provincial Key Laboratory of Drug Prevention and Control Technology Research.
Author contributions
J.J. and Y.Z. designed research; A.W., X.W., P.Z., W.J., B.W., Y.W., Z.X., J.W., and W.Y. performed research; Y.A., X.L., Y.H., and J.Y. contributed new reagents/analytic tools; X.W., Y.A., X.L., Y.T., and L.Z. analyzed data; Y.Z. conceived the study, supervised experiments, and secured funding; and A.W. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Charlson F., et al. , New WHO prevalence estimates of mental disorders in conflict settings: A systematic review and meta-analysis. Lancet 394, 240–248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santomauro D. F., et al. , Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398, 1700–1712 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, Fact Sheets: Depression (World Health Organization, 2021). [Google Scholar]
- 4.Institute for Health Metrics and Evaluation (IHME). Global Health DataExchange (GHDx). http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b.
- 5.Firth J., Gangwisch J. E., Borisini A., Wootton R. E., Mayer E. A., Food and mood: How do diet and nutrition affect mental wellbeing? BMJ 369, m2382 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacka F. N., et al. , Association of Western and traditional diets with depression and anxiety in women. Am. J. Psychiatry 167, 305–311 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Bennett G., Young E., Butler I., Coe S., The impact of lockdown during the COVID-19 outbreak on dietary habits in various population groups: A scoping review. Front. Nutr. 8, 626432 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang B. W., et al. , Lifestyle-related risk factors correlated with mental health problems: A longitudinal observational study among 686 male college students in Chongqing, China. Front. Public Health 10, 1040410 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mottram D. S., Wedzicha B. L., Dodson A. T., Food chemistry: Acrylamide is formed in the Maillard reaction. Nature 419, 448–449 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Koszucka A., Nowak A., Nowak I., Motyl I., Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit. Rev. Food Sci. Nutr. 60, 1677–1692 (2020). [DOI] [PubMed] [Google Scholar]
- 11.FAO/WHO Expert Committee on Food Additives, Safety evaluation of certain contaminants in food: Seventy-second meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), (World Health Organization, 2011), vol. 799. [Google Scholar]
- 12.Calleman C. J., Bergmark E., Stern L. G., Costa L. G., A nonlinear dosimetric model for hemoglobin adduct formation by the neurotoxic agent acrylamide and its genotoxic metabolite glycidamide. Environ. Health Perspect. 99, 221–223 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang M., Zhuang P., Jiao J., Wang J., Zhang Y., Association of acrylamide hemoglobin biomarkers with obesity, abdominal obesity and overweight in general US population: NHANES 2003–2006. Sci. Total Environ. 631–632, 589–596 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Wan X., et al. , Associations of hemoglobin adducts of acrylamide and glycidamide with prevalent metabolic syndrome in a nationwide population-based study. J. Agric. Food Chem. 70, 8755–8766 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., et al. , Exposure to acrylamide and the risk of cardiovascular diseases in the National Health and Nutrition Examination Survey 2003–2006. Environ. Int. 117, 154–163 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Li Z., Sun J., Zhang D., Association between acrylamide hemoglobin adduct levels and depressive symptoms in US adults: NHANES 2013–2016. J. Agric. Food Chem. 69, 13762–13771 (2021). [DOI] [PubMed] [Google Scholar]
- 17.LoPachin R. M., Gavin T., Molecular mechanism of acrylamide neurotoxicity: Lessons learned from organic chemistry. Environ. Health Perspect. 120, 1650–1657 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faria M., et al. , Acrylamide acute neurotoxicity in adult zebrafish. Sci. Rep. 8, 7918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LoPachin R. M., Balaban C. D., Ross J. F., Acrylamide axonopathy revisited. Toxicol. Appl. Pharmacol. 188, 135–153 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Ruhe H. G., Mason N. S., Schene A. H., Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol. Psychiatry 12, 331–359 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Erkekoglu P., Baydar T., Acrylamide neurotoxicity. Nutr. Neurosci. 17, 49–57 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Izumi Y., Fujii C., O’Dell K. A., Zorumski C. F., Acrylamide inhibits long-term potentiation and learning involving microglia and pro-inflammatory signaling. Sci. Rep. 12, 12429 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S.-M., et al. , Modified lipoproteins by acrylamide showed more atherogenic properties and exposure of acrylamide induces acute hyperlipidemia and fatty liver changes in zebrafish. Cardiovasc. Toxicol. 15, 300–308 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Serra E. L., Medalha C. C., Mattioli R., Natural preference of zebrafish (Danio rerio) for a dark environment. J. Med. Biol. Res. 32, 1551–1553 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Kysil E. V., et al. , Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish 14, 197–208 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Egan R. J., et al. , Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 205, 38–44 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaser R., Gerlai R., Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behav. Res. Methods 38, 456–469 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Law S.-H., et al. , An updated review of lysophosphatidylcholine metabolism in human diseases. Int. J. Mol. Sci. 20, 1149 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu P., et al. , The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 247, 117443 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Llano D. A., Devanarayan V., Alzheimer’s disease neuroimaging initiative, serum phosphatidylethanolamine and lysophosphatidylethanolamine levels differentiate alzheimer’s disease from controls and predict progression from mild cognitive impairment. J. Alzheimer’s Dis. 80, 311–319 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Fan W., et al. , SIRT1 regulates sphingolipid metabolism and neural differentiation of mouse embryonic stem cells through c-Myc- SMPDL3B. Elife 10, e67452 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., et al. , Negative correlation between cerebrospinal fluid FGF21 levels and BDI scores in male Chinese subjects. Psychiatry Res. 252, 111–113 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Ma X., et al. , Phosphatidylserine, inflammation, and central nervous system diseases. Front. Aging Neurosci. 14, 975176 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colombini M., Ceramide channels and mitochondrial outer membrane permeability. J. Bioenerg. Biomembr. 49, 57–64 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Hunter M., Demarais N. J., Faull R. L. M., Grey A. C., Curtis M. A., Subventricular zone lipidomic architecture loss in Huntington’s disease. J. Neurochem. 146, 613–630 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Ballabh P., Braun A., Nedergaard M., The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 16, 1–13 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Alahmari A., Blood-Brain barrier overview: Structural and functional correlation. Neural Plast. 2021, 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickens C. A., Sordillo L. M., Zhang C., Fenton J. I., Obesity is positively associated with arachidonic acid-derived 5- and 11-hydroxyeicosatetraenoic acid (HETE). Metabolism 70, 177–191 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Hartling I., et al. , Quantitative profiling of inflammatory and pro-resolving lipid mediators in human adolescents and mouse plasma using UHPLC-MS/MS. Clin. Chem. Lab. Med. 59, 1811–1823 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Lecka-Czernik B., et al. , Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 143, 2376–2384 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Sisemore M. F., et al. , Cellular characterization of leukotoxin diol-induced mitochondrial dysfunction. Arch. Biochem. Biophys. 392, 32–37 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Cryan J. F., Kaupmann K., Don’t worry "B" happy!: A role for GABA(B) receptors in anxiety and depression. Trends Pharmacol. Sci. 26, 36–43 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Sanacora G., Treccani G., Popoli M., Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu J., Gong Z. Y., Bae S., Ecotoxicogenomic analysis of zebrafish embryos exposed to triclosan and mixture triclosan and methyl triclosan using suppression subtractive hybridization and next-generation sequencing. J. Hazard. Mater. 414, 125450 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Grimaldi B., et al. , PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 12, 509–520 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo W., et al. , Bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate affects lipid metabolism in zebrafish larvae via DNA methylation modification. Environ. Sci. Technol. 54, 355–363 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Feng X., Lin Y.-L., Wei L.-N., Behavioral stress reduces RIP140 expression in astrocyte and increases brain lipid accumulation. Brain Behav. Immun. 46, 270–279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuelsson B., Arachidonic acid metabolism: Role in inflammation. Z. Rheumatol. 1, 3–6 (1991). [PubMed] [Google Scholar]
- 49.Coetzee T., et al. , Myelination in the absence of galactocerebroside and sulfatide: Normal structure with abnormal function and regional instability. Cell 86, 209–219 (1996). [DOI] [PubMed] [Google Scholar]
- 50.Hebert-Chatelain E., et al. , A cannabinoid link between mitochondria and memory. Nature 539, 555–559 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Ülgen D. H., Ruigrok S. R., Sandi C., Powering the social brain: Mitochondria in social behaviour. Curr. Opin. Neurobiol. 79, 102675 (2023). [DOI] [PubMed] [Google Scholar]
- 52.Aucoin M., et al. , Diet and anxiety: A scoping review. Nutrients 13, 4418 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Y., et al. , Role of dietary factors in the prevention and treatment for depression: An umbrella review of meta-analyses of prospective studies. Transl. Psychiatry 11, 478 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin P., et al. , Fried-food consumption and risk of overweight/obesity, type 2 diabetes mellitus, and hypertension in adults: a meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 7, 1–12 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Qin P., et al. , Fried-food consumption and risk of cardiovascular disease and all-cause mortality: a meta-analysis of observational studies. Heart 107, 1567–1575 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Hidese S., Asano S., Saito K., Sasayama D., Kunugi H., Association of depression with body mass index classification, metabolic disease, and lifestyle: A web-based survey involving 11,876 Japanese people. J. Psychiatr. Res. 102, 23–28 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Ogrodnik M., et al. , Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 29, 1061–1077 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salk R. H., Hyde J. S., Abramson L. Y., Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 143, 783–822 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varì R., et al. , Gender-related differences in lifestyle may affect health status. Ann. Ist. Super. Sanita 52, 158–166 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Franceschini A., Fattore L., Gender-specific approach in psychiatric diseases: Because sex matters. Eur. J. Pharmacol. 896, 173895 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Davis K. E., Prasad C., Vijayagopal P., Juma S., Imrhan V., Advanced glycation end products, inflammation, and chronic metabolic diseases: Links in a chain? Crit. Rev. Food Sci. Nutr. 56, 989–998 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Gerhard G. S., et al. , Life spans and senescent phenotypes in two strains of Zebrafish (Danio rerio). Exp. Gerontol. 37, 1055–1068 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Yang P., Yamaki M., Kuwabara S., Kajiwara R., Itoh M., A newly developed feeder and oxygen measurement system reveals the effects of aging and obesity on the metabolic rate of zebrafish. Exp. Gerontol. 127, 110720 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Westerfield M., The Zebrafish Book: A Guide for the Laboratory use of Zebrafish (Danio rerio) (University of Oregon Press, ed. 4, 1995). [Google Scholar]
- 65.Park J. S., et al. , Developmental and neurotoxicity of acrylamide to zebrafish. Int. J. Mol. Sci. 22, 3518 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheggi S., et al. , PPARα modulation of mesolimbic dopamine transmission rescues depression-related behaviors. Neuropharmacology 110, 251–259 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Dong G., Li X., Han G., Du L., Li M., Zebrafish neuro-behavioral profiles altered by acesulfame (ACE) within the range of “no observed effect concentrations (NOECs)”. Chemosphere 243, 125431 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Zhang S. H., et al. , Reversal of reserpine-induced depression and cognitive disorder in zebrafish by sertraline and Traditional Chinese Medicine (TCM). Behav. Brain Funct. 14, 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiba S., et al. , Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 39, 112–119 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Croarkin P. E., Levinson A. J., Daskalakis Z. J., Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci. Biobehav. Rev. 35, 818–825 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Owada Y., Fatty acid binding protein: Localization and functional significance in the brain. Tohoku J. Exp. Med. 214, 213–220 (2008). [DOI] [PubMed] [Google Scholar]
- 72.Ren B., et al. , Enantioselective bioaccumulation and toxicity of the novel chiral antifungal agrochemical penthiopyrad in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 228, 113010 (2021). [DOI] [PubMed] [Google Scholar]
- 73.Li D. F., Zhang J., Liu Q., Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 45, 401–414 (2022). [DOI] [PubMed] [Google Scholar]
- 74.Tallima H., Ridi R. E., Arachidonic acid: Physiological roles and potential health benefits - A review. J. Adv. Res. 11, 33–41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramadan E., et al. , Transient postnatal fluoxetine leads to decreased brain arachidonic acid metabolism and cytochrome P450 4A in adult mice. Prostaglandins Leukot. Essent. Fatty Acids 90, 191–197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ansorge M. S., Zhou M. M., Lira A., Hen R., Gingrich J. A., Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306, 879–881 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Zoicas I., et al. , Ceramides affect alcohol consumption and depressive-like and anxiety-like behavior in a brain region- and ceramide species-specific way in male mice. Addict. Biol. 25, e12847 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Kornhuber J., et al. , The role of ceramide in major depressive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 259, S199–S204 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Yang L., et al. , Acrylamide induces abnormal mtDNA expression by causing mitochondrial ROS accumulation, biogenesis, and dynamics disorders. J. Agric. Food Chem. 69, 7765–7776 (2021). [DOI] [PubMed] [Google Scholar]
- 80.Pan X., et al. , Melatonin attenuates oxidative damage induced by acrylamide in vitro and in vivo. Oxid. Med. Cell. Longev. 2015, 703709 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LoPachin R. M., Barber D. S., Gavin T., Molecular mechanisms of the conjugated alpha, beta-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol. Sci. 104, 235–249 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaschler M. M., Stockwell B. R., Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 482, 419–425 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niki E., Lipid peroxidation products as oxidative stress biomarkers. Biofactors 34, 171–180 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Guo J., Cao X., Hu X., Li S., Wang J., The anti-apoptotic, antioxidant and anti-inflammatory effects of curcumin on acrylamide-induced neurotoxicity in rats. BMC Pharmacol. Toxicol. 21, 62 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rani V., Deep G., Singh R. K., Palle K., Yadav U. C. S., Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 148, 183–193 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Hotamisligil G. S., Inflammation and metabolic disorders. Nature 444, 860–867 (2006). [DOI] [PubMed] [Google Scholar]
- 87.Carpentier A., et al. , Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 8, 343re2 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Tabeshpour J., Mehri S., Abnous K., Hosseinzadeh H., Role of oxidative stress, MAPKinase and apoptosis pathways in the protective effects of thymoquinone against acrylamide-induced central nervous system toxicity in rat. Neurochem. Res. 45, 254–267 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Light/dark preference test. Related to figure 2. The movie shows that zebrafish with acrylamide treatment spend more time in the dark zone and travel more times between light and dark zone, indicating a higher anxiety level of zebrafish, compared to the control group.
Novel tank diving test. Related to figure S2. The movie displays that zebrafish in the acrylamide exposure groups prefer to stay near the bottom of the tank, indicating that acrylamide exposure exacerbates thigmotaxis and thus increases the risk of anxiety-like symptom in zebrafish.
Novel object exploration test. Related to figure 3. The movie displays that zebrafish in the control group can swiftly explore and adapt to the novel environment, but chronic exposure to acrylamide reduces the ability to adapt to the unfamiliar environment. This indicates that acrylamide induces anxiety- and depressive-like behaviors by reducing exploration ability of zebrafish.
Social preference test. Related to figure 3. The movie shows that zebrafish in the acrylamide exposure groups can hardly to swim closely to the fish school, while zebrafish in the control group frequently swam closely to the fish school. This indicates that chronic exposure to acrylamide remarkably impairs the sociality of zebrafish and thus increases the risk of anxiety- and depressive-like symptoms in zebrafish.
Data Availability Statement
All study data are included in the article and/or SI Appendix.