Abstract
Polysaccharides have been successfully used as immunogens for the development of vaccines against bacterial infection; however, there are no oligosaccharide-based vaccines available to date and no previous studies of their processing and presentation. We reported here the intracellular enzymatic processing and antigen presentation of an oligosaccharide-conjugate cancer vaccine prepared from the glycan of Globo-H (GH), a globo-series glycosphingolipid (GSL). This oligosaccharide-conjugate vaccine was shown to elicit antibodies against the glycan moieties of all three globo-series GSLs that are exclusively expressed on many types of cancer and their stem cells. To understand the specificity and origin of cross-reactivity of the antibodies elicited by the vaccine, we found that the vaccine is first processed by fucosidase 1 in the early endosome of dendritic cells to generate a common glycan antigen of the GSLs along with GH for MHC class II presentation. This work represents the first study of oligosaccharide processing and presentation and is expected to facilitate the design and development of glycoconjugate vaccines based on oligosaccharide antigens.
Introduction
Cancer cells often express distinct glycoproteins and glycolipids on the surface with unique glycan structures via aberrant glycosylation.1 These cancer-associated glycoconjugates have been used as markers to distinguish cancer cells from normal cells and as targets for the development of anti-cancer immunotherapy.2−4 Globo-H (GH) (Figure 1), one of the three globo-series glycosphingolipids (GSLs), is an epitope on embryonic stem cells. It generally disappears after differentiation of embryonic stem cells but appears again on at least 15 different types of cancers, like breast, brain, lung, stomach, pancreas, prostate, and liver cancers.5,6 Thus, the oligosaccharide moiety of GH has been a target for the development of cancer immunotherapy. Recent examples include the GH-KLH vaccine with GH glycan conjugated to keyhole limpet hemocyanin (KLH) in combination with QS21 adjuvant which is currently in a global phase 3 trial for the treatment of triple negative breast cancer (NCT03562637), the GH-diphtheria toxin (DT) conjugate vaccine (GH-DT, Scheme 1) with C34 adjuvant (NCT02310464) in phase 2 trials for multiple cancers, and a therapeutic anti-GH antibody (NCT03573544) in phase 2 trials for GH-positive cancers.7
Figure 1.
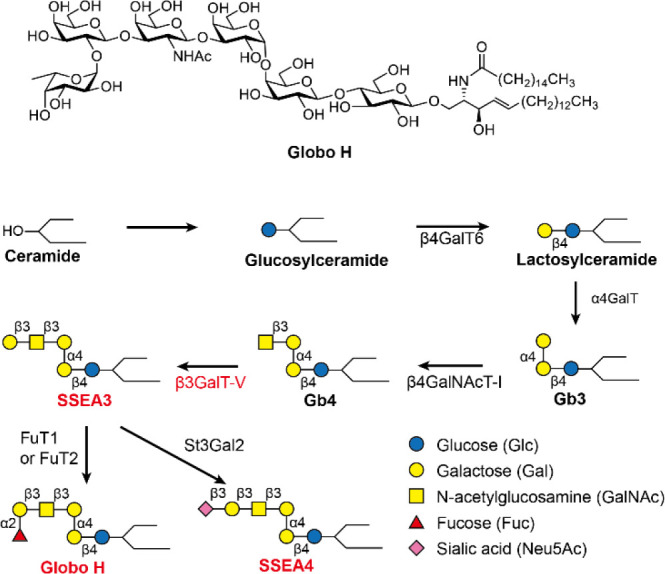
Biosynthesis of the three globo-series GSLs SSEA3, SSEA4, and GH that are exclusively expressed on at least 15 different types of cancer and their stem cells. The galactosyltransferase β3GalT5 is the key enzyme involved in the synthesis of globo-series GLSs, and its expression highly correlates with poor survival of cancer patients. Symbol nomenclature with color code is used for structure presentation. The complete structure of GH is shown.
Scheme 1. GH-Conjugated Vaccine and Oxidative Release of GH Glycan from the Carrier by NaClOa.
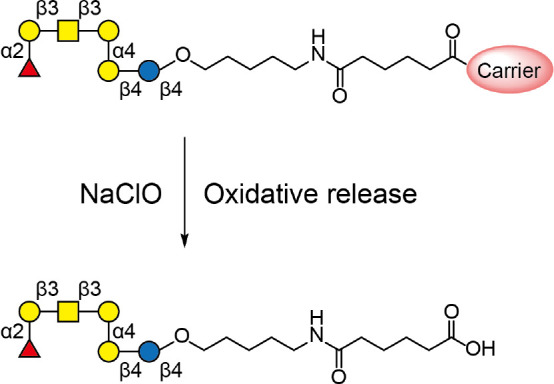
The carrier can be CRM197 (equivalent to DT) or KLH.
In addition to GH, the other two globo-series GSLs, SSEA3 and SSEA4 (Figure 1), are also found exclusively on the cell surface of many cancers5,6,8 and their expression correlates with tumor metastasis and progression.9−12 In the biosynthesis of globo-series GSLs, β1,3-galactosyltransferase V (β3GalT5) is the key enzyme responsible for adding a galactose residue to the normal and non-immunogenic Gb4 to form the cancer-specific SSEA3 and subsequently the other two globo-series GSLs. Overexpression of β3GalT5 was commonly found in cancer patients with high expression of globo-series GSLs.13 The oligosaccharide moieties (glycans with less than seven monosaccharide units) of globo-series GSLs are receiving more and more attention in the development of anti-cancer immunotherapy.7 However, oligosaccharides have not been successfully developed into vaccines as they are generally poor antigens14−17 though polysaccharides from certain pathogens have been successfully developed as preventive vaccines against the pathogens.18−22
In our previous study, the oligosaccharide moiety of GH or SSEA4 conjugated with DT and adjuvanted with C34, an analogue of the glycolipid α-GalCer designed to induce a class-switch and to enhance immune response with a balanced Th1/Th2 ratio, was demonstrated to successfully elicit immune responses against breast cancer in mice.7,23 Interestingly, the GH-DT vaccine elicited antibody responses against the oligosaccharides of all three globo-series GSLs, while SSEA4-DT vaccine induced antibodies against SSEA4 glycan only and SSEA3-DT vaccine induced weak and non-selective immune responses.23 The same phenomenon was observed in human trials with GH-DT and GH-KLH vaccines.21 These results prompted us to speculate that the oligosaccharide-conjugate vaccine is probably more susceptible to glycosidase-mediated processing to generate SSEA3 oligosaccharide, the common epitope of all three globo-series GSLs, thereby eliciting antibody responses against all three globo-series GSLs. In this study, we tested the hypothesis in animal models and investigated the mechanism of antigen processing and presentation of the oligosaccharide moiety of GH-DT vaccine. Since the processing and presentation of oligosaccharide-conjugate vaccines have not been reported, understanding the process of oligosaccharide antigen presentation is crucial for the design and development of cancer vaccines targeting cell-surface oligosaccharides.
Results
Globo H-DT Vaccine Elicited Antibodies That Recognize All Three Oligosaccharides of Cancer-Associated Globo-Series GSLs
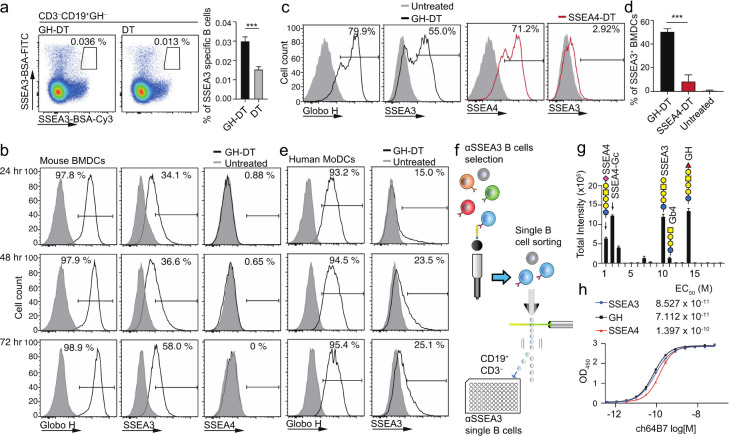
It was found that immunization with GH-DT/C34 in Balb/c mice induced antisera against the oligosaccharides of GH and SSEA3, and to a lesser extent the oligosaccharide of SSEA4, as determined by glycan array analysis (Figure S1a, Table S1 in the Supporting Information).7,23 To understand the specificity of antibodies induced by GH-DT immunization, we first examined the binding of B cells from immunized mice to the glycans of globo-series GSLs. The splenocytes from DT and GH-DT-immunized mice were first stained with B-cell markers and then with the oligosaccharides of GH and SSEA3. The result showed that the splenocytes had higher frequency of GH–SSEA3+ B cells (Figure 2a), indicating that GH-DT elicited specific B cells that specifically recognize the glycan of SSEA3. Apparently, a B cell would present the specific antibody or B-cell receptor on its surface to recognize the oligosaccharide antigen after immunization with an oligosaccharide-conjugate vaccine.
Figure 2.
Oligosaccharide antigen processing and presentation on the cell surface of mouse BMDCs and human MoDCs. (a) B cells targeting SSEA3 glycan (gated on CD3–CD19+GH– cells) were induced 10 days after GH-DT immunization. Statistical significance was shown in the right panel from 5 mice per group. (b) Mouse BMDCs presented GH or SSEA3 glycan, but not SSEA4 glycan, on the cell surface 24, 48, and 72 h after treatment with GH-DT. (c,d) Compared to BMDCs treated with SSEA4-DT, BMDCs treated with GH-DT presented more SSEA3 glycans on the cell surface 48 h after treatment. Statistical significance was shown with 3 mice per group. (e) After treatment with GH-DT, human MoDCs presented GH and SSEA3 glycans on the cell surface at indicated time points. (f) Flow chart for isolation of single B cells that recognize SSEA3 glycan on beads. (g) Binding specificity analysis of monoclonal antibody ch64B7 identified by screen of single B cells from GH-DT-immunized mice using a globo-series glycan array. (h) Binding of ch64B7 to SSEA3, GH, and SSEA4 glycans analyzed by ELISA. Results in (a) right panel, (d), (f), and (g) are mean ± SEM. *** p < 0.001.
We then examined how the anti-SSEA3 glycan antibodies were induced by GH-DT immunization. The antigen-presenting cells (APCs), i.e., the bone marrow-derived dendritic cells (BMDCs), from Balb/c mice were treated with GH-DT for 3 consecutive days, followed by FACS analysis of DCs stained with antibodies against the glycans of GH, SSEA3, and SSEA4. To our surprise, in addition to GH glycan, SSEA3 glycan, but not SSEA4 glycan, was detected on the DC surface at various time points after treatment (Figure 2b). However, BMDCs treated with SSEA4-DT presented much less SSEA3 glycan (Figure 2c,d). This result is consistent with our previous findings that vaccination with GH-DT, but not SSEA4-DT, induced IgG antibodies against GH and SSEA3 glycans in mice.23 Similarly, human monocyte-derived dendritic cells (MoDCs, Figure 2e) and mouse splenic B cells (Figure S1b) also presented GH and SSEA3 glycans on the cell surface after treatment with GH-DT. However, the presentation efficiency of SSEA3 glycan on B cells was lower than that on BMDCs or MoDCs, indicating that GH-DT was mainly processed in DCs to elicit antibody response from B cells (Figure S1b in the Supporting Information).
We next used single B-cell technology to isolate and screen for anti-SSEA3 glycan monoclonal antibodies from the SSEA3 glycan-specific B cells sorted from GH-DT-immunized mice (Figure 2f), and one of the monoclonal antibodies identified (ch64B7) was found to recognize SSEA3, GH, and SSEA4 glycans in a glycan array analysis. The galactose on the non-reducing end of SSEA3 glycan is critical for binding to this antibody as removal of the galactose reduced the binding dramatically. This antibody recognizes SSEA3 glycan with cross-reactivity toward the three globo-series glycans and SSEA4-Gc with the N-glycolyl group as they all contain SSEA3 glycan as a common epitope (Figure 2g). The binding affinities of ch64B7 to SSEA3 and GH glycans were comparable (Kd = 8.527 × 10–11 and 7.112 × 10–11 M, respectively), while the binding to SSEA4 glycan was slightly reduced (Kd = 1.397 × 10–10 M, Figure 2h). This result demonstrated that the anti-SSEA3 glycan antibody induced by GH-DT vaccine cross-reacts with SSEA4 and Globo H glycans. It was also suggested that GH-DT is processed to generate GH glycan and SSEA3 glycan for presentation to elicit two pathways of antibody responses: one is from GH glycan to elicit GH-specific antibody and the other is from SSEA3 glycan to elicit the antibody that recognizes GH, SSEA3, and SSEA4 as all contain the common SSEA3-glycan epitope. Interestingly, our previous study showed that SSEA4-DT vaccination did not induce anti-SSEA3 glycan antibodies, even though SSEA3 glycan is an epitope of SSEA4.23 Therefore, it appears that the GH glycan, but not the SSEA4 glycan, is processed by DCs to remodel the glycan epitope for presentation, and the processing of GH glycan to SSEA3 glycan may be the origin of cross-reactive antibody response.
Oligosaccharide Antigen Processing and Presentation of GH-DT Vaccine
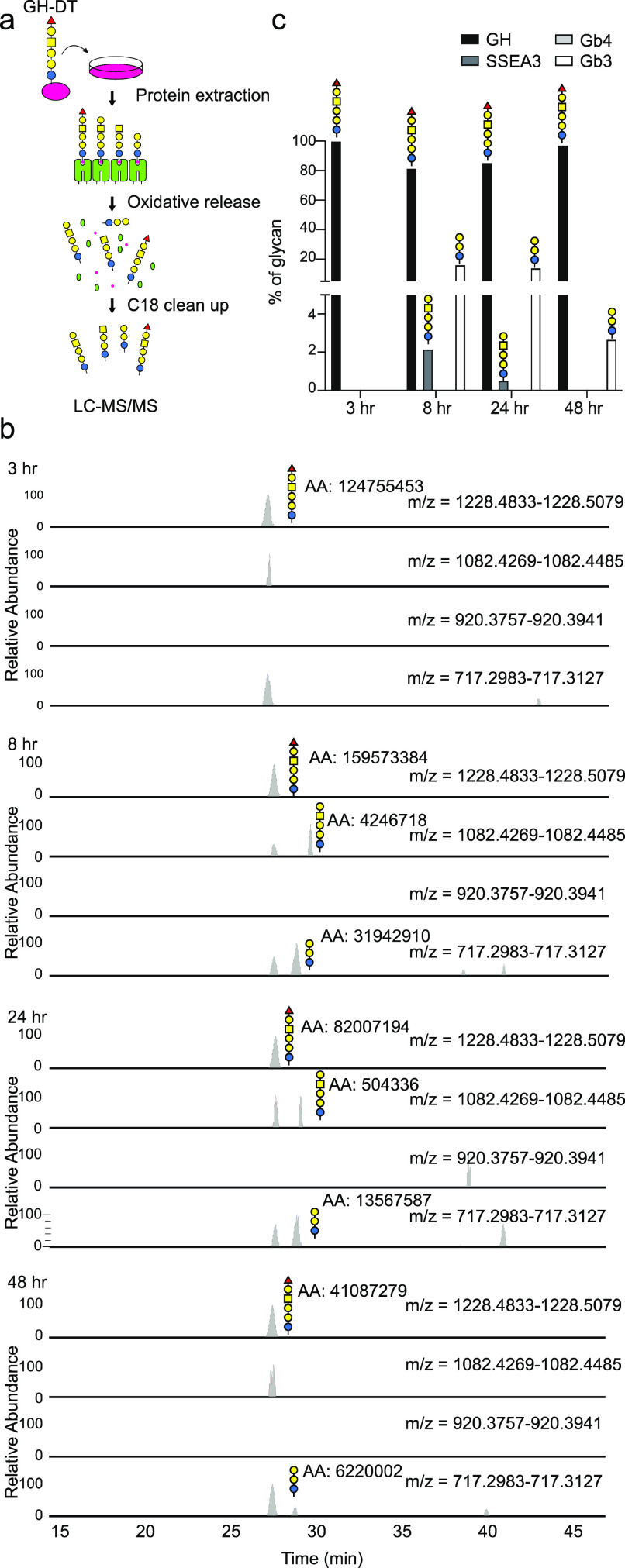
We next examined whether shorter oligosaccharides were generated after processing of GH-DT by BMDCs. Since anti-Gb3 or anti-Gb4 glycan antibody was not available, we used LC–MS/MS to analyze the glycans released from the membrane fraction of cell lysates through NaClO treatment to cleave the amide bond between the linker carboxyl and the side-chain amine of lysine24 (Scheme 1). This oxidative release would not damage GH glycan as there was no degraded glycan detected (Figure S2a in the Supporting Information).
BMDCs were treated with GH-DT and the protein fractions were collected, including the soluble fraction, the membrane fraction, and the detergent insoluble membrane (DIM) fraction of cell lysates at indicated time points for NaClO treatment, followed by LC–MS/MS analysis (Figure 3a). Besides the GH glycan, the Gb3 glycan was detected in all protein fractions 3 h after treatment except in the DIM fraction (Figures 3b and S2b, c in the Supporting Information). SSEA3 glycan was detected exclusively in the DIM fraction after 8 and 24 h at a low level and was further processed to Gb4 and Gb3 glycans by glycosidases (Figure 3c). Gb3 glycan was not further processed in this period and Gb4 and Gb3 glycans are not immunogenic as they are common on normal cells.25,26 This finding is consistent with our previous observation that SSEA3 glycan is a weak immunogen as it is quickly processed to Gb4 and Gb3 glycans. In addition, GH-DT in the culture environment was stable since only around 1% GH glycan was degraded into Gb3 glycan and no SSEA3 glycan was detected (Figure S2d in the Supporting Information). Therefore, SSEA3 and Gb3 glycans were generated from GH enzymatically not from spontaneous degradation.
Figure 3.
Processing of the oligosaccharide of GH-DT to SSEA3, Gb4, and Gb3 glycans in BMDCs. (a) Flow chart of oxidative cleavage and LC–MS/MS analysis of the glycans released from GH-DT-treated BMDCs. (b,c) Digested glycans, including SSEA3 and Gb3 glycans, were detected by LC–MS/MS in the detergent insoluble membrane fraction after treating BMDCs with GH-DT for 8 h. Gb3 glycan was the most abundant glycan among the digested glycans.
The study of glycoconjugate vaccine processing in APCs is limited, especially there was no previous study of oligosaccharide processing and presentation. Though it is known that the capsular polysaccharide (CPS) of group B Streptococcus is presented with peptides derived from the carrier protein in an MHC class II-dependent manner,27,28 and glycopeptides can be presented by both MHC class I and MHC class II,29 the mechanism of oligosaccharide processing and possible remodeling during the presentation process remains unknown.
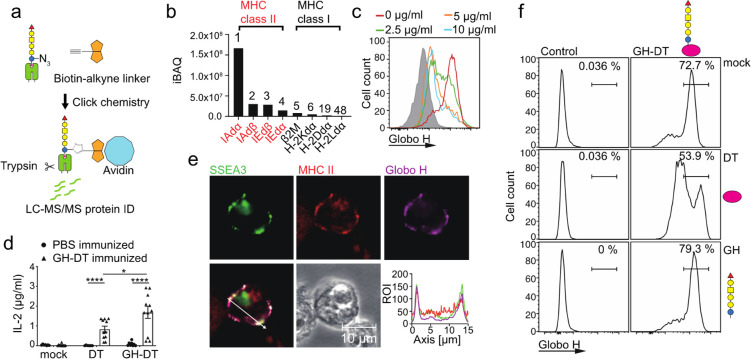
To identify the proteins that mediated the presentation of GH-DT, we introduced a tag to the reducing end glucose of GH glycan to facilitate the identification of interacting proteins. We thought a slight modification of the GH glycan at the reducing end will not affect the processing of terminal sugars. 6-Azido-GH glycan (GHN3) with 6-OH of the reducing end glucose replaced by the azido group was conjugated to DT for treatment with BMDCs followed by click reaction with an alkyne-containing biotin (Scheme 2). The proteins noncovalently bound to GHN3, and its intermediates linked to DT peptides in complex with MHC class II were isolated by avidin beads for LC–MS/MS analysis after biotinylating the N3 group by click reaction (Figure 4a). A total of 1231 proteins were identified and ranked by intensity-based absolute quantification (iBAQ). Ingenuity pathway analysis (IPA) indicated that 194 of the identified proteins were plasma membrane proteins (Table S2 in the Supporting Information), and among them, MHC class II subunits IAdα (Rank 1), IAdβ (Rank 2), IEdβ (Rank 3), and IEdα (Rank 4) were the major ones. All the MHC class I subunits, including β2-microglobulin (B2M) and the three α chains, were also detected in the complex (Figure 4b). These data indicated that MHC class II plays a key role in GH-DT presentation, while MHC class I is a minor player. Besides, most of the proteins in the antigen presentation pathway, including Class II-associated invariant chain peptide (CLIP),30 calreticulin (CALR),31 calnexin (CNX),32 tapasin (TPN),33 protein disulfide isomerase family A member 3 (PDIA3),34 and transporter associated with antigen processing 1 as well as 2 (TAP1 and TAP2),35 were detected (Figure S3a in the Supporting Information). IPA data also revealed that the uptake of GH-DT by BMDCs may be through clathrin-mediated endocytosis (Figure S3b in the Supporting Information) or phagocytosis (Figure S3c in the Supporting Information) since clathrin36 and Rab5, a small GTP-binding protein on phagosomes following phagocytosis,37 and other proteins involved in these two pathways were detected in the GHN3-associated protein complex. It is noted however that this noncovalent pull-down experiment may miss some proteins with weak interaction with the glycans.
Scheme 2. Isolation and Identification of the Proteins That Interact with Peptide-Linked GH-Glycan-N3, SSEA3-Glycan-N3, and Intermediates Generated from GHN3-DT-Treated BMDCs for Click Reaction with an Alkynyl Biotin Linker.
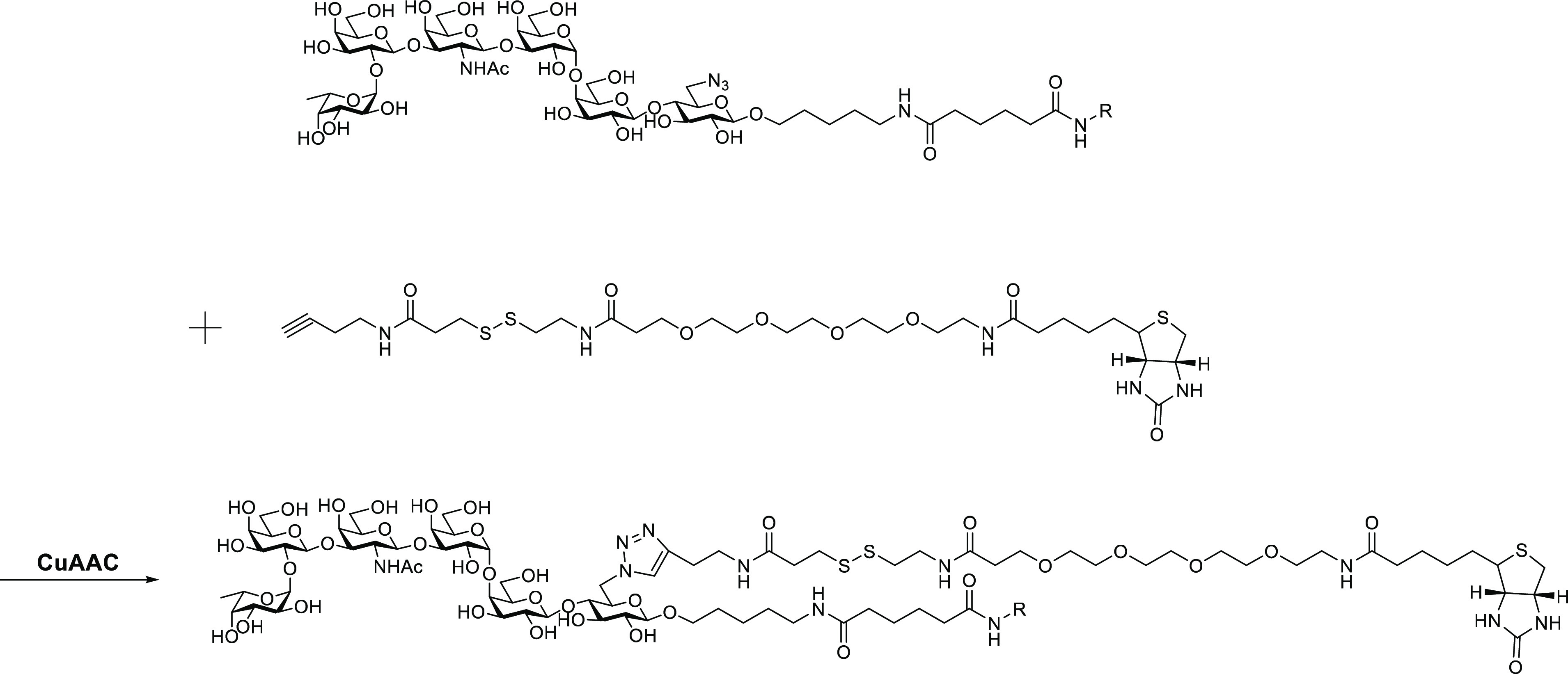
Figure 4.
GH and SSEA3 glycans were presented through MHC class II on BMDCs treated with GHN3-DT. (a) Flow chart for isolation and identification of proteins interacting with GHN3 glycan and intermediates during antigen presentation in GHN3-DT-treated BMDCs. The proteins interacting with GHN3 and its intermediates in complex with MHC class II were isolated for LC–MS/MS analysis. The azido groups of the complex were first biotinylated with click reaction followed by treatment with avidin beads to pull down the interacting proteins in the complex. (b) Among the 1231 identified proteins, 194 proteins were membrane proteins and MHC class II was the most dominant hit. All of MHC class I subunits were also identified. (c) Addition of anti-MHC class II antibody reduced the presentation of GH glycan on BMDCs 48 h after GH-DT treatment. (d) Compared to DT, GH-DT induced more IL-2 production from CD4+ T cells isolated from GH-DT-immunized mice. (e) GH and SSEA3 glycans were colocalized with MHC class II on the surface of GH-DT-treated BMDCs. Cells were harvested and stained 24 h after GH-DT treatment. Shown is the distribution of SSEA3 glycan (green), MHC class II (red), and GH glycan (purple) indicated by fluorescence intensities across the section with a white line. (f) Effect of DT (1 mg/mL) or GH glycan (1 mg/mL) on antigen presentation of GH-DT-treated BMDCs. Results in (d) are mean ± SEM (n = 11). * p < 0.05, **** p < 0.0001.
Globo H-DT Vaccine Is Processed in the Early Endosome of Dendritic Cells by Fucosidase 1 to SSEA3 Glycan for MHC Class II Presentation To Elicit Immune Responses
To study the relationship between GH-DT presentation and MHC class II, an anti-MHC class II antibody was added to GH-DT-treated BMDCs, and it was found that the level of MHC class II (Figure S4 in the Supporting Information) and antigen presentation on BMDCs were reduced (Figure 4c), probably due to endocytosis induced by the antibody binding to MHC class II that interfered with MHC class II-antigen complexation.38 BMDCs were then treated with GH-DT or DT and cocultured with CD4+ T cells isolated from the spleen of GH-DT-immunized mice to understand the role of MHC class II in CD4+ T-cell activation. Compared with DT-treated BMDCs, GH-DT-treated BMDCs induced a significantly higher level of IL-2 production by CD4+ T cells (Figure 4d). Confocal microscopy imaging analysis further revealed that GH glycan was colocalized with MHC class II on BMDCs 24 h after treatment with GH-DT (Figure 4e). Since oligosaccharide antigens could be presented by DCs through the c-type lectin DC-SIGN,39 the presentation pathway of GH-DT was investigated. Addition of DT to GH-DT-treated BMDCs reduced the presentation of GH glycan from GH-DT, while GH glycan alone could not be presented nor interfere with the presentation of GH glycan from GH-DT. These results indicated that the presentation of GH-DT depends on the protein carrier not the GH glycan (Figure 4f).
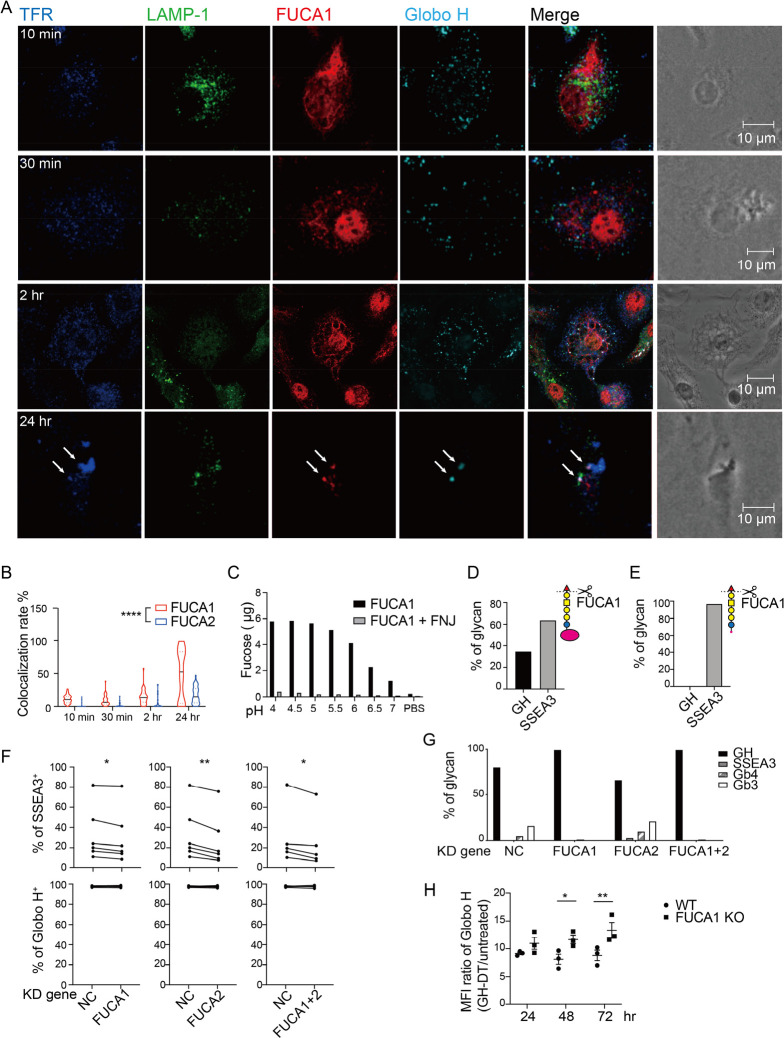
The presentation of CPSs depends on the structure of polysaccharides. CPS from type 3 Streptococcus pneumoniae is presented by DCs, while CPS from Neisseria meningitidis is not due to its rapid degradation in endolysosome through oxidative depolymerization and acidic hydrolysis.40 Since the non-reducing end of GH glycan is a fucose residue and the fucosidase activity in the lysosome of DCs is relatively high,41 the processing of GH glycan may be initiated by enzymatic removal of the terminal fucose. Therefore, it is likely that after being processed, the glycan epitopes presented on APCs could be changed and thus elicit different antibodies. In addition, after GH-DT treatment, splenic B cells presented less SSEA3 glycan as compared with BMDCs. So, we performed glycosidase gene expression microarray analysis to compare the glycan-related gene expression between splenic B cells and BMDCs 48 h after GH-DT treatment, and we found that BMDCs expressed higher levels of glycosidase-related genes after treatment with GH-DT, including two fucosidases, FUCA1 and FUCA2 (Figure S5a, Table S3 in the Supporting Information). To further understand the mechanism of GH glycan processing in DCs, we investigated the location of fucosidases and found that FUCA1, not FUCA2, was colocalized with GH glycan in BMDCs 2 h after GH-DT treatment (Figures 5a and S5b in the Supporting Information). Interestingly, we also found that GH glycan and FUCA1 were colocalized with the early endosome marker, transferrin receptor,42 24 h after GH-DT treatment, suggesting that GH glycan was digested prior to carrier protein (Figure 5b).
Figure 5.
GH-DT is processed by FUCA1 to SSEA3 glycan in BMDCs. (a) GH glycan was colocalized with FUCA1 in BMDCs within 24 h after treatment with GH-DT. TFR and LAMP-1 represent transferrin receptor and lysosomal-associated membrane protein 1, respectively. Arrows indicate early endosomes containing both FUCA1 and GH glycan. (b) The colocalization rate between GH glycan and FUCA1 was higher than that between GH glycan and FUCA2. 45 cells per group were counted. (c) Recombinant FUCA1 digested GH glycan with the highest activity at pH = 4.5. Addition of FUCA1 inhibitor, fuconojirimycin (FNJ), inhibited the digestion. (d) Oxidative release of glycan and LC–MS/MS analysis showed that FUCA1 partially hydrolyzed GH glycan on GH-DT and (e) FUCA1 completely hydrolyzed GH glycan on GH-peptides in vitro. (f) The presentation of SSEA3 glycan was reduced in FUCA1 or/and FUCA2 knockdown BMDCs compared to the negative control (NC) BMDCs. Data were analyzed by paired t tests. (g) Oxidative release of glycans and LC–MS/MS analysis showed that the level of digested glycans was reduced in FUCA1 knockdown BMDCs. (h) BMDCs with knockdown of FUCA1 presented more GH glycans on the cell surface as compared to WT BMDCs. Results in (h) are mean ± SEM (n = 3). * p < 0.05, ** p < 0.01, **** p < 0.0001.
We then expressed and purified both fucosidases from 293T cells to examine their activities against GH glycan. Recombinant FUCA1 hydrolyzed GH glycan with high activity at pH 4.5 to 5 in vitro (Figure 5c), while recombinant FUCA2 had no activity on GH glycan. To further study the enzymatic process, GH-DT was incubated with FUCA1 first and then digested by chymotrypsin or digested by chymotrypsin first and then treated with FUCA1, followed by LC–MS/MS analysis. The result showed that FUCA1 could digest 63.9% of the GH glycan on GH-DT (Figure 5d) and all the GH glycan of chymotrypsin-treated GH-DT (Figure 5e) at pH 5.5. These results further support that GH glycan is digested by FUCA1 before DT is processed into peptides in DCs.
Next, we knocked down the expression of these two fucosidases by siRNA in BMDCs (Figure S5c, S5d in the Supporting Information) to examine whether the presentation of SSEA3 glycan was altered. After knocking down the expression of FUCA1 or/and FUCA2 in BMDCs, the presentation of SSEA3 glycan was significantly reduced (Figure 5f). Knockdown of FUCA1 reduced GH glycan degradation as determined by the oxidative release of glycans and LC–MS/MS analysis. Compared to the negative control, FUCA1 or FUCA1/FUCA2 double knockdown cells showed a reduced level of Gb4 and Gb3 glycans. However, knockdown of FUCA2 did not reduce GH degradation (Figures 5g and S5e–h in the Supporting Information). These studies suggest that FUCA1 is responsible for the processing of GH glycan to SSEA3 glycan in DCs. Finally, BMDCs derived from FUCA1 knockout mice presented more GH glycans, as compared with normal BMDCs (Figures 5h and S6 in the Supporting Information).
Besides FUCA1, other glycosidases may be involved in the degradation of GH derivatives, including β-galactosidase for SSEA3, hexosaminidase for Gb4, α-galactosidase for Gb3, and β-galactosidase for lactose. According to the result of gene expression microarrays and RT-qPCR, all these glycosidases expressed at much higher levels in BMDCs than in B cells (Table S3, Figure S7a in the Supporting Information). In addition, our proteomics analysis showed that more β-galactosidase and hexosaminidase are associated with GH glycan as compared to α-galactosidase (Figure S7b in the Supporting Information). The accumulation of Gb3 glycan in BMDCs treated with GH-DT may result from the low activity of α-galactosidase to process Gb3 glycan. Also, there was no lactose detected in BMDCs treated with GH-DT as lactose derived from Gb3 glycan may be digested quickly due to the high β-galactosidase activity.
Discussion
The globo-series GSLs, including GH, SSEA3, and SSEA4, are exclusively expressed on many tumor cells and their stem cells, and they have little or no expression on normal tissues. As such, globo-series GSLs are promising targets for the development of cancer immunotherapy. However, oligosaccharides are difficult to induce proper immune responses and produce sufficient anti-tumor antibody titers. By conjugating an oligosaccharide antigen to a carrier protein and combining the conjugate with an adjuvant, the oligosaccharide-conjugate vaccine can induce significant T-dependent responses. Our previous study showed that a cancer vaccine with GH glycan conjugated to DT and administrated with C34 adjuvant induced better immune responses with higher IgG titer against GH glycan than GH-KLH/QS21 did.23 In addition to vaccine development, the potential of globo-series GSLs as targets for cancer therapy is further supported by the finding that SSEA3 is a marker of cancer stem cells13 and that the monoclonal antibodies against SSEA4 and GH glycans have significant anti-cancer efficacy.9 After vaccination in mice with GH-DT, the glycoconjugate vaccine is processed by DCs to present GH and SSEA3 glycans on the cell surface through MHC class II complexation. Moreover, the presentation is carrier protein-dependent because unconjugated GH glycan cannot be presented or interfere with the presentation of GH glycan from GH-DT. Although oxidative depolymerization and acidic hydrolysis have been shown to cause the degradation of carbohydrate antigen,40 the processing of oligosaccharide antigen by glycohydrolases as demonstrated in this study can alter the structure of antigen and change the specificity of immune responses. Therefore, understanding the pathway of oligosaccharide processing in immune cells is essential for the development of oligosaccharide-based vaccines.
FUCA1 is important for the breakdown of complex glycans on glycolipids or glycoproteins. FUCA1 deficiency may cause accumulation of fucosylated glycoconjugates in tissues and result in fucosidosis, a lysosomal storage disease.43 FUCA1 is able to cleave terminal fucose with α1,2 linkage to expose the galactose residue or with α1,3, α1,4, or α1,6 linkage to expose the N-acetylglucosamine residue on glycoproteins and glycolipids.44 Although FUCA1 is expressed in DCs,45,46 its role in antigen processing and presentation in DCs is unknown. In this study, we have demonstrated that FUCA1 can trim the oligosaccharide of GH-DT vaccine and result in the presentation of modified glycans on DCs.
Protein antigen is usually broken down in lysosome (pH 4.5) by lysosomal proteases, especially cathepsins.47 However, the colocalization of GH glycan and FUCA1 in early endosome in DCs treated with GH-DT and the fucose-cleavage activity of recombinant FUCA1 toward GH glycan at pH 5.5 indicated that the processing of GH glycan occurs before the carrier protein. It was reported that B cells may recognize antigen-pulsed DCs to promote B cell–DC interaction.48 We therefore suspect that GH-DT-primed DCs may directly activate B cells to recognize SSEA3 and promote their production of antibody, though the underlying mechanistic pathways remained to be examined.
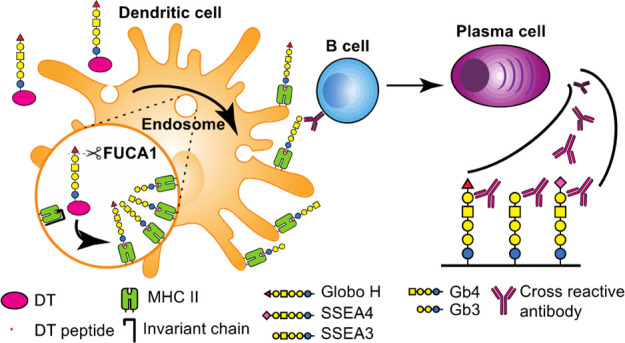
In summary, we have demonstrated in this study that the GH-DT vaccine is processed in DCs to generate GH and SSEA3 glycans for presentation. The antibody induced from GH-glycan presentation recognizes GH and the antibody induced from SSEA3 glycan recognizes SSEA3 and cross-reacts with GH and SSEA4 as both also contain the SSEA3 glycan as the common epitope (Figure 6). The processed glycans are mainly presented by MHC class II and to a lesser extent by MHC class I on DCs to elicit the cross-reactive immune responses. However, SSEA3 glycan is not a good antigen for vaccine design as it will be quickly processed to the truncated glycans which are commonly found on normal cells and thus elicit very weak and non-selective immune responses. On the other hand, SSEA4 glycan is slowly processed and elicits mainly SSEA4-specific immune response. As demonstrated in this study, the oligosaccharide antigen of GH-DT vaccine is first processed by FUCA1 in DCs to generate SSEA3 glycan prior to carrier protein degradation and that the immune cells can tune the properties of the oligosaccharide-based vaccine and edit the immune responses. As an example, one may introduce a fluorine group to the fucose residue of GH to avoid the processing of fucosidase and thus elicit more GH-specific immune responses. We believe that a better understanding of oligosaccharide-conjugate in the immune system will facilitate the future development of oligosaccharide-based vaccines with desired antigen presentation and antibody responses against specific cell-surface oligosaccharides.
Figure 6.
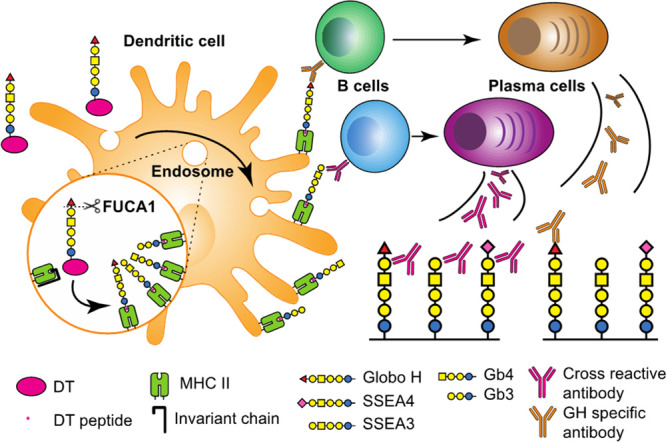
GH-DT was processed by FUCA1 in DCs to generate GH and SSEA3 glycans for presentation. The antibody induced from GH glycan presentation recognizes GH and the antibody induced from SSEA3 glycan recognizes SSEA3 and cross-reacts with GH and SSEA4 as both also contain the SSEA3-glycan epitope.
Acknowledgments
We thank Chien-Hung Chen for glycopeptide liquid chromatography MS/MS analysis, the National RNAi Core Facility at Academia Sinica for RNAi preparation, and the Animal Center at GRC for immunization study. This research was supported by Academia Sinica, NIH (AI130227), and NSF (CHE-1954031).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c02003.
General methods; GH-DT vaccine processing in mouse splenic B cells; oxidative release of globo-series glycan analyzed by LC-MS/MS; identification of proteins interacting with GHN3 and intermediates; FACS analysis of MHC II on BMDCs; GH processing initiated by FUCA1 in DCs; knockout of FUCA1 in BMDCs presented more GH on the cell surface as compared to WT BMDCs; glycosidase gene expression or association with GH glycan; glycan structure on the glycan array slide; plasma membrane proteins associated with GH; and fold increase of glycosidase-related gene expression in BMDCs as compared to B cells (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Zhang S.; Cordon-Cardo C.; Zhang H. S.; Reuter V. E.; Adluri S.; Hamilton W. B.; Lloyd K. O.; Livingston P. O. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int. J. Cancer 1997, 73, 42–49. . [DOI] [PubMed] [Google Scholar]
- Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol. 2001, 491, 369–402. 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- Buskas T.; Thompson P.; Boons G. J. Immunotherapy for cancer: synthetic carbohydrate-based vaccines. Chem. Commun. 2009, 36, 5335–5349. 10.1039/b908664c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidijahanabad Z.; Huang X. Recent advances in tumor associated carbohydrate antigen based chimeric antigen receptor T cells and bispecific antibodies for anti-cancer immunotherapy. Semin. Immunol. 2020, 47, 101390 10.1016/j.smim.2020.101390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y. W.; Wang P. Y.; Yeh S. C.; Chuang P. K.; Li S. T.; Wu C. Y.; Khoo K. H.; Hsiao M.; Hsu T. L.; Wong C. H. Stage-specific embryonic antigen-4 as a potential therapeutic target in glioblastoma multiforme and other cancers. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 2482–2487. 10.1073/pnas.1400283111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. W.; Lee C. H.; Lee P.; Lin J.; Hsu C. W.; Hung J. T.; Lin J. J.; Yu J. C.; Shao L. E.; Yu J.; Wong C. H.; Yu A. L. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 11667–11672. 10.1073/pnas.0804979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danishefsky S. J.; Shue Y. K.; Chang M. N.; Wong C. H. Development of Globo-H cancer vaccine. Acc. Chem. Res. 2015, 48, 643–652. 10.1021/ar5004187. [DOI] [PubMed] [Google Scholar]
- Wang C. C.; Huang Y. L.; Ren C. T.; Lin C. W.; Hung J. T.; Yu J. C.; Yu A. L.; Wu C. Y.; Wong C. H. Glycan microarray of Globo H and related structures for quantitative analysis of breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 11661–11666. 10.1073/pnas.0804923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang P. K.; Hsiao M.; Hsu T. L.; Chang C. F.; Wu C. Y.; Chen B. R.; Huang H. W.; Liao K. S.; Chen C. C.; Chen C. L.; Yang S. M.; Kuo C. W.; Chen P.; Chiu P. T.; Chen I. J.; Lai J. S.; Yu C. T.; Wong C. H. Signaling pathway of globo-series glycosphingolipids and beta1,3-galactosyltransferase V (beta3GalT5) in breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 3518–3523. 10.1073/pnas.1816946116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran P.; Baca Y.; Xiu J.; Zhang J.; Battaglin F.; Arai H.; Goldberg R. M.; Weinberg B. A.; Lou E.; Hall M. J.; Khushman M.; Sohal D.; Soni S.; Wang J.; Zhang W.; Millstein J.; Korn W. M.; Lenz H.-J. Globo H expression in metastatic colorectal cancer (CRC). J. Clin. Oncol. 2021, 39, 3527–3527. 10.1200/JCO.2021.39.15_suppl.3527.34559549 [DOI] [Google Scholar]
- Zhang W.; Ding M.-L.; Zhang J.-N.; Qiu J.-R.; Shen Y.-H.; Ding X.-Y.; Deng L.-F.; Zhang W.-B.; Zhu J. mTORC1 Maintains the Tumorigenicity of SSEA-4+ High-Grade Osteosarcoma. Sci. Rep. 2015, 5, 9604. 10.1038/srep09604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia A.; Petrova E.; Tomiuk S.; Bissels U.; Déas O.; Saini M.; Zickgraf F. M.; Wagner S.; Spaich S.; Sütterlin M.; Schneeweiss A.; Reitberger M.; Rüberg S.; Gerstmayer B.; Agorku D.; Knöbel S.; Terranegra A.; Falleni M.; Soldati L.; Sprick M. R.; Trumpp A.; Judde J.-G.; Bosio A.; Cairo S.; Hardt O. The sialyl-glycolipid stage-specific embryonic antigen 4 marks a subpopulation of chemotherapy-resistant breast cancer cells with mesenchymal features. Breast Cancer Res. 2015, 17, 146–146. 10.1186/s13058-015-0652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S. K.; Chuang P. K.; Huang H. W.; Hwang-Verslues W. W.; Cho C. H.; Yang W. B.; Shen C. N.; Hsiao M.; Hsu T. L.; Chang C. F.; Wong C. H. Stage-specific embryonic antigen-3 (SSEA-3) and beta3GalT5 are cancer specific and significant markers for breast cancer stem cells. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, 960–965. 10.1073/pnas.1522602113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub A. Immunology of bacterial polysaccharide antigens. Carbohydr. Res. 2003, 338, 2539–2547. 10.1016/j.carres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Jaurigue J. A.; Seeberger P. H. Parasite Carbohydrate Vaccines. Front. Cell. Infect. Microbiol. 2017, 7, 248. 10.3389/fcimb.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci F. Y.; Kasper D. L. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 2010, 28, 107–130. 10.1146/annurev-immunol-030409-101159. [DOI] [PubMed] [Google Scholar]
- Guttormsen H. K.; Wetzler L. M.; Finberg R. W.; Kasper D. L. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect. Immun. 1998, 66, 2026–2032. 10.1128/IAI.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R.; Barrera O.; Sutton A.; Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980, 152, 361–376. 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C.; van Rossum F.; Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect. Immun. 1982, 37, 15–22. 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R.; Paoletti L. C.; Rodewald A. K.; Michon F.; DiFabio J.; Jennings H. J.; Kasper D. L. Stimulation of protective antibodies against type Ia and Ib group B streptococci by a type Ia polysaccharide-tetanus toxoid conjugate vaccine. Infect. Immun. 1993, 61, 4760–4766. 10.1128/iai.61.11.4760-4766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivatare S. S.; Shivatare V. S.; Wong C. H. Glycoconjugates: Synthesis, Functional Studies, and Therapeutic Developments. Chem. Rev. 2022, 122, 15603–15671. 10.1021/acs.chemrev.1c01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Zhou Z.; Tang S.; Guo Z. Carbohydrate-monophosphoryl lipid a conjugates are fully synthetic self-adjuvanting cancer vaccines eliciting robust immune responses in the mouse. ACS Chem. Biol. 2012, 7, 235–240. 10.1021/cb200358r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. L.; Hung J. T.; Cheung S. K.; Lee H. Y.; Chu K. C.; Li S. T.; Lin Y. C.; Ren C. T.; Cheng T. J.; Hsu T. L.; Yu A. L.; Wu C. Y.; Wong C. H. Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 2517–2522. 10.1073/pnas.1222649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.; Ju H.; Lasanajak Y.; Kudelka M. R.; Smith D. F.; Cummings R. D. Oxidative release of natural glycans for functional glycomics. Nat. Methods 2016, 13, 528–534. 10.1038/nmeth.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T.; Kiyokawa N.; Katagiri Y. U.; Taguchi T.; Suzuki T.; Sekino T.; Sato N.; Ohmi K.; Nakajima H.; Takeda T.; Fujimoto J. Globotriaosyl ceramide (CD77/Gb3) in the glycolipid-enriched membrane domain participates in B-cell receptor-mediated apoptosis by regulating lyn kinase activity in human B cells. Exp. Hematol. 2000, 28, 1260–1268. 10.1016/S0301-472X(00)00538-5. [DOI] [PubMed] [Google Scholar]
- Bieri J.; Ros C. Globoside Is Dispensable for Parvovirus B19 Entry but Essential at a Postentry Step for Productive Infection. J. Virol. 2019, 93, e00972-19 10.1128/JVI.00972-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci F. Y.; Li X.; Tsuji M.; Kasper D. L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z.; Schreiber J. R. Antigen processing of glycoconjugate vaccines; the polysaccharide portion of the pneumococcal CRM(197) conjugate vaccine co-localizes with MHC II on the antigen processing cell surface. Vaccine 2009, 27, 3137–3144. 10.1016/j.vaccine.2009.03.064. [DOI] [PubMed] [Google Scholar]
- Sun L.; Middleton D. R.; Wantuch P. L.; Ozdilek A.; Avci F. Y. Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology 2016, 26, 1029–1040. 10.1093/glycob/cww062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riberdy J. M.; Newcomb J. R.; Surman M. J.; Barbosa J. A.; Cresswell P. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature 1992, 360, 474–477. 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- Gao B.; Adhikari R.; Howarth M.; Nakamura K.; Gold M. C.; Hill A. B.; Knee R.; Michalak M.; Elliott T. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity 2002, 16, 99–109. 10.1016/S1074-7613(01)00260-6. [DOI] [PubMed] [Google Scholar]
- Diedrich G.; Bangia N.; Pan M.; Cresswell P. A role for calnexin in the assembly of the MHC class I loading complex in the endoplasmic reticulum. J. Immunol. 2001, 166, 1703–1709. 10.4049/jimmunol.166.3.1703. [DOI] [PubMed] [Google Scholar]
- Sadasivan B.; Lehner P. J.; Ortmann B.; Spies T.; Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity 1996, 5, 103–114. 10.1016/S1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- Garbi N.; Tanaka S.; Momburg F.; Hammerling G. J. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat. Immunol. 2006, 7, 93–102. 10.1038/ni1288. [DOI] [PubMed] [Google Scholar]
- Suh W. K.; Cohen-Doyle M. F.; Fruh K.; Wang K.; Peterson P. A.; Williams D. B. Interaction of MHC class I molecules with the transporter associated with antigen processing. Science 1994, 264, 1322–1326. 10.1126/science.8191286. [DOI] [PubMed] [Google Scholar]
- Mettlen M.; Chen P. H.; Srinivasan S.; Danuser G.; Schmid S. L. Regulation of Clathrin-Mediated Endocytosis. Annu. Rev. Biochem. 2018, 87, 871–896. 10.1146/annurev-biochem-062917-012644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels A. M.; Trost M.; Beyaert R.; Hoffmann E. Patterns, Receptors, and Signals: Regulation of Phagosome Maturation. Trends Immunol. 2017, 38, 407–422. 10.1016/j.it.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki K.; Hiraki Y.; Onishi H.; Satoh Y.; Roche P. A.; Tanaka S.; Furuta K. Ligation of MHC Class II Induces PKC-Dependent Clathrin-Mediated Endocytosis of MHC Class II. Cell 2020, 9, 1810. 10.3390/cells9081810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista F. D.; Harwood N. E. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 2009, 9, 15–27. 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- Sun X.; Stefanetti G.; Berti F.; Kasper D. L. Polysaccharide structure dictates mechanism of adaptive immune response to glycoconjugate vaccines. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 193–198. 10.1073/pnas.1816401115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamarre L.; Pack M.; Chang H.; Mellman I.; Trombetta E. S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 2005, 307, 1630–1634. 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R.; McGraw T. E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004, 5, 121–132. 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Michalski J. C.; Klein A. Glycoprotein lysosomal storage disorders: alpha- and beta-mannosidosis, fucosidosis and alpha-N-acetylgalactosaminidase deficiency. Biochim. Biophys. Acta 1999, 1455, 69–84. 10.1016/S0925-4439(99)00077-0. [DOI] [PubMed] [Google Scholar]
- Johnson S. W.; Alhadeff J. A. Mammalian alpha-L-fucosidases. Comp. Biochem. Physiol. B 1991, 99, 479–488. 10.1016/0305-0491(91)90327-A. [DOI] [PubMed] [Google Scholar]
- Robbins S. H.; Walzer T.; Dembele D.; Thibault C.; Defays A.; Bessou G.; Xu H.; Vivier E.; Sellars M.; Pierre P.; Sharp F. R.; Chan S.; Kastner P.; Dalod M. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008, 9, R17. 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worah K.; Mathan T. S. M.; Vu Manh T. P.; Keerthikumar S.; Schreibelt G.; Tel J.; Duiveman-de Boer T.; Skold A. E.; van Spriel A. B.; de Vries I. J. M.; Huynen M. A.; Wessels H. J.; Gloerich J.; Dalod M.; Lasonder E.; Figdor C. G.; Buschow S. I. Proteomics of Human Dendritic Cell Subsets Reveals Subset-Specific Surface Markers and Differential Inflammasome Function. Cell Rep. 2016, 16, 2953–2966. 10.1016/j.celrep.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurley N.; Mellman I. Monocyte-derived dendritic cells exhibit increased levels of lysosomal proteolysis as compared to other human dendritic cell populations. PLoS One 2010, 5, e11949 10.1371/journal.pone.0011949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenoff M.; Egen J. G.; Koo L. Y.; Laugier J. P.; Brau F.; Glaichenhaus N.; Germain R. N. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006, 25, 989–1001. 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.