Abstract
Background
Malaria is a public health menace. Resistance to therapeutic armamentarium is impeding its control. Therefore, research targeting the discovery of novel antimalarial drug arsenals is a priority. The important point to begin the search for such drugs is the folkloric medicinal plants. Ripe fruit of Lagenaria siceraria is bored, rinsed with cold water, and one glass is used as a drink early in the morning for the treatment of malaria in Ethiopian folk medicine. In vivo antimalarial efficacy of the plant was not affirmed scientifically, though. Consequently, the present study was conducted to assess the in vivo antiplasmodial effect of Lagenaria siceraria in P. berghei infected mice.
Methods
The fruits were extracted using 80% methanol in water. Acute toxicity test was conducted on the extract. Secondary phytochemicals were assessed. The four day suppressive test was employed in mice infected with P. berghei. Thirty mice were grouped in to five and inoculated with P. berghei. After 3 h, three of the groups received the extract at doses 100, 200 and 400 mg/kg. The remaining groups served as negative (2% Tween80) and positive control (chloroquine). Parasitemia, packed cell volume, weight, temperature and survival time were monitored. SPSS version 22 was used for data analysis.
Results
No toxicity was seen in mice. The crude extract elicited significant suppression (p < 0.05) of the parasite compared to the negative control. The highest parasite suppression (77.37%) was measured at the upper dose. Furthermore, the crude extract significantly (p < 0.05) prevented body weight loss, anemia, reduction in temperature and prolonged the survival time compared to the negative control.
Conclusion
This study asserted that the fruit of Lagenaria siceraria is enriched with in vivo antimalarial activity. Hence, further in depth antimalarial investigations on the plant is strongly recommended.
Keywords: Lagenaria siceraria, In vivo, Mice, Antimalarial, P. berghei
1. Introduction
In spite of the fact that a plethora of therapeutic armamentarium were developed and utilized to tackle malaria, the disease still continues to be a major global public health menace. According to a recent report from World Health Organization (WHO), an estimated 247 million malaria cases were recorded in the year 2021 from the 84 malaria endemic countries. In 2020, the number of cases reported was 241 million whereas it was 227 million in the year 2019 indicating an increase in malaria cases over a time [1]. The highest reported case goes to the WHO African Region which accounted for about 95% of the global cases of malaria. The mortality due to malaria remained more than half a million each year. For instance, the death from malaria in the years 2019, 2020 and 2021 was reported as 568, 000; 625,000; and 619, 000 respectively. The burden of death due to malaria in line with the number of case report is in the WHO African Region which contributes the highest share of mortality from the disease [1].
The sub-Saharan African children under the age of five and pregnant women are the most adversely affected population to the dangers of malaria. In this regard, in 2021, in the WHO African Region alone, 13.3 million (32%) pregnancies were exposed to malaria infection out of 40 million pregnancies. The proposed reason why this group of populations are more vulnerable to the disease is that their immunity is reduced. Malaria during pregnancy can lead to maternal death. Moreover, it induces premature delivery, intrauterine growth retardation, low birth weight, anemia and a risk factor for infant death. Hence, it is recommended that preventive antimalarial therapy with Sulphadoxine-Pyrimethamine had better commenced in this special population in order to reduce the burden of the disease [1,2].
Due to the selection of parasite resistance blunting the effect of the arsenals used in the management of the disease over time, malaria continues to be a life threatening malady. The malaria parasites are currently resistant to most of the antiplasmodial drugs which were previously active. An example to this challenge is Chloroquine which was highly active against Plasmodium falciparum whose activity against this parasite abated. Had been no investigations and discovery of optional drugs, the life of the people of the malaria endemic countries could have been at an extreme risk. The most active antimalarial drugs in the today’s health system are artemisinin which were discovered from Chinese traditional medicinal plant practice by professor Tu [3]. This innovation which has saved millions of life is now at risk of losing its role and leaving the world bare handed [1,4].
Plasmodium falciparum strains partially resistant to the most active drugs, artemisinin, were identified from African countries and South East Asia. Ones these resistant parasites develop full resistance and distributes to different parts of the world, the world loose a weapon and succumb to the deadly malaria. Hence, not to give up, the world has to get prepared taking this alarming sign by searching for novel and better antiplasmodial drugs [4].The main gate to begin such investigation is folk medicinal plants as history indicates where plants were a source for antimalarial drugs such artemesinins and quinine [3]. Lagenaria siceraria is among folk medicinal plants for the treatment of malaria in Ethiopia. Hence, it is a potential investigational plant in the endeavor to discover more effective and safer antimalarial drugs [5,6].
The WHO defines traditional medicine (TM) as the sum of knowledge, skills, and practices based on theories, beliefs, and experiences indigenous to different cultures that are used to maintain health as well as prevent, improve, or treat physical and mental illnesses [7]. The TM practice involves the application of animals, medicinal plants, minerals, and physical techniques such as massaging to disease management. Since antiquity traditional medicinal plants have been used for the management of different ailments including malaria. Because of easy availability, affordability and cultural acceptance, medicinal plants are widely utilized especially in developing world. In Ethiopia, about 80% of the population uses TM to meet their primary health need. However, robust scientific investigations done on this rich flora is limited [[7], [8], [9]].
Lagenaria siceraria is a plant commonly known as a bottle gourd or calabash. The plant has different vernacular names; buqqee hadhaa in Afan Oromo, bottle gourd or calabash in English, and Kwarya in Hausa language [5,10]. It is grown for its fruit (Fig. 1) to primarily use as a water container. Calabash belongs to the family cucurbitaceae. The family cucurbitaceae is highly utilized as food for its plenty of nutrients [10]. For instance, during pregnancy, the demand for nutrients is high [11] and plants in this family such as water melon and pumpkin are important for embryonic development, infant brain and retinal development as they are excellent sources of Omega-3 and Omega-6 fatty acids [12]. This family is also known for its medicinal values. Plants in this family are used in painful menstruation, as anticancer and anthelminthic [13,14].
Fig. 1.
Picture of the bottle gourd plant, Lagenaria siceraria, calabash.
The bottle gourd is used as a utensil, container, or a musical instrument. Furthermore, it is used as medicinal remedy. Previous studies on Lagenaria siceraria showed that the plant elicited hepatoprotective, cardioprotective, antihypertensive, diuretic, laxative, stimulant, free radical scavenging, analgesic, anti-inflammatory, antioxidant, antihyperlipidemic, antidiabetic, and memory enhancing properties [13,15,16]. It is also endowed with anthelmintic, antibacterial, and antifungal properties [10,13]. The fruit of the plant has been traditionally used for treatment of malaria in Abay Chomen District, Horo Guduru Wollega Zone, Oromia Region, Ethiopia. Moreover, the ripe fruit of Lagenaria siceraria is bored, rinsed with cold water, and one glass is used as a drink early in the morning for the management of the disease in Ethiopian folk medicine [5,6]. Previous study conducted on the plant also showed that the fruit of Lagenaria siceraria is endowed with in vitro antiplasmodial activity [15,17]. Nevertheless, no scientific investigation was conducted to further affirm its antimalarial activity in animal model. Consequently, based on this traditional claim, the present study evaluated the antimalarial activity of 80% methanolic crude fruit extract of Lagenaria siceraria in Plasmodium berghei infected mice following the peter's four day suppressive test.
2. Materials and methods
2.1. Experimental plant collection
The Lagenaria siceraria branch with attached leaves and fruits was gathered, wrapped in newspaper, and handed to the national herbarium in March 2019, from Abay Chomen District, Horo Guduru Wollega Zone, Oromia Region, Ethiopia for authentication purpose. Identification and authentication of the plant was done at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University, where a voucher specimen was coded (GH 03/2019) and deposited for future reference. In addition, for the purpose of conducting an experimental pharmacological investigation on the plant, the fruit of Lagenaria siceraria was collected from the same location where the folk use it as a traditional antimalarial medicine.
2.2. Crude extract preparation
The plant's harvested fruits were carefully washed with tap water and scrubbed with gauze to eliminate debris. Then, it was chopped with laboratory knife into pieces, and air-dried under shade. The fruit was then ground into a coarse powder using a mortar and pestle (600 g). After that, cold maceration extraction technique was performed as per Habte et al. with slight modification [18]. As per the technique, 300 g of the powdered material was immersed in 1000 ml of 80% methanol in an Erlenmeyer flask. The extraction procedure was aided by the employment of a mechanical shaker (Bibby Scientific Limited Stone Staffo Reshire, UK) running for 72 h at 120 rpm.
Following 72 h, the marc was removed from the distillate, which contained an 80% methanol crude extract. This distillate was then further filtered using Whatman filter paper under suction filtration. The same procedure was repeated twice by adding another fresh solvent into the marc. Rotary evaporator (Buchi Rota vapor R-200, Switzerland) and a lyophilizer were used to remove methanol and concentrate the extract, respectively. Similar procedure was followed for the other 300 g course powder. Eventually, after calculating the percentage yield, the plant extract was kept at −20 °C until use. The following formula was utilized to calculate the percentage yield:
| (1) |
2.3. Parasites and animals for the experimental work
Swiss albino mice, weighing 25–31 g and 6–8 weeks old, were utilized in this investigation. The Ethiopian Public Health Institute (EPHI) was the source for the mice. The experimental work was done at the laboratory setup of the Department of Pharmacy, College of Health Sciences, Mettu University between March and April 2019. Prior to the actual experiment, animals spent a week becoming accustomed to the experimental setting. They were kept in polypropylene cages with a 12-h light/dark cycle. The animals had access to standard pellet diet, and unlimited water. Chloroquine sensitive P. berghei ANKA strain was obtained from EPHI. All procedures in handling of the animals, and the methods utilized in this investigation were in compliance with the generally recognized guideline [19]. Moreover, a letter of ethical clearance with a reference number ‘Pharm 2019/17’ was obtained from the Department of Pharmacy, Research and Technical Review Committee, Mettu University.
2.4. Test for Plant’s acute toxicity
The Organization for Economic Cooperation and Development (OECD) guideline 425 was followed for performing the oral acute toxicity test [20]. First, a single overnight-fasted female mouse was weighed and given a single dosage of the extract (2000 mg/kg) via oral gavage. Food was then denied for an additional 2 h. Following that, the mouse was monitored for 24 h. As neither toxicity signs nor mortality was noticed, four additional female mice received the same dose. They were subsequently monitored for 14 days for any obvious behavioral abnormalities, such as lack of appetite, hair erection, lacrimation, tremors, convulsions, and mortality.
2.5. Antimalarial screening in mice
2.5.1. Animal grouping and dosing
Thirty mice, male in sex, were split into five groups of six in different cages at random. The extract was administered daily for four days to three of the experimental groups at doses of 100 mg/kg, 200 mg/kg, and 400 mg/kg each. The dose was chosen based on the findings of the acute toxicity test and a preliminary investigation carried out on the extract. The remaining two groups served as negative control, provided solvent for reconstitution (Tween 80, 2% v/v), and positive control that received 10 mg/kg of chloroquine phosphate (CQ).
2.5.2. The four-day suppressive test procedure
The antimalarial activity of the crude fruit extract of Lagenaria siceraria against P. berghei infection that is susceptible to chloroquine was conducted in accordance with the four-day suppressive test outlined by Peter et al. modified and used by Habte et el [18,21,22]. As earlier mentioned, Chloroquine susceptible P. berghei ANKA strain was obtained from EPHI. The parasites were then maintained through weekly serial transmission of blood from infected mice to uninfected ones, which continued until a parasitemia level of 30–37% was reached [23]. By slicing a 0.5–1 mm piece off the mice's tails with scissors and collecting the blood, the donor mice’s parasitemia level was determined. The blood of a donor mouse with a parasitemia level rising to 30–37% was utilized to infect the experimental mice with the parasites.
These donor mice were sacrificed, and blood was drawn by cardiac puncture into a falcon tube that contained 2% trisodium citrate as an anticoagulant. To reduce variability, the blood samples from all the donor mice were combined and diluted in normal saline. Based on the donor mice's parasitemia and the normal mice's RBC count, the dilution was made so that 1 ml of blood contained 5 × 107 infected RBCs [18,23].
Every mouse in each of the mouse groups mentioned under the section ‘parasites and animals for the experimental work’ was coded and intraperitoneally infected on day one (D01) with 0.2 mL of blood carrying around 1 × 107 RBCs infected with P. berghei. After that, treatment began 3 h after the inoculation (D001) and continued every day for the following 4 days (D001-D004). The parameters described below were determined either prior to D001 (D0) or at the end of the fourth day (D4), 96 h from D001, or at both points. The mice were then observed every day for a month for the intent of determining their survival time [18,24,25].
2.5.3. Mice survival time and parasitemia determination
In order to make a thin blood smear, blood samples were taken at D4 from each mouse's tails using frosted slides that are clean and non-greasy. Freshly made 10% Giemsa was used to stain the slides for 15 min. Then after, distilled water was used to clear away the stain. Slides were examined under a microscope with the X100 objective after being given time to air-dry. The percentage of parasitemia (PP) was calculated by determining the number of PRBCs out of RBCs in random microscope fields. Two stained slides were viewed for each mouse. For each slide, three fields with roughly 200–500 cells were counted, and the PP for every mouse involved in this investigation was calculated in the following manner [23,25,26]:
| (2) |
The mean percent of parasitemia suppression (PPS) was determined utilizing the formula given below [18]:
| (3) |
The mean survival time (MST) for all the five groups was individually determined using the formula below [23]:
| (4) |
2.5.4. Changes in mice packed cell volume, temperature, and weight
Each mouse's rectal temperature and body weight were noted just prior to and following treatment on D4. Then, for the mice group in each test, the average changes in percentages of these parameters were computed and analyzed [18,25]. Likewise, the packed cell volume (PCV) measurement was carried out both just prior to and after the administration of the test substance. In order to assess PCV, a sample of blood was taken from each mouse's tail using capillary tubes that are standard heparinized microhaematocrit type. After filling each capillary tube to 75% of its height, using sealing clay, the dry end of the tube was closed. Next, each tube was placed on a micro-hematocrit centrifuge which is made by Centurion Scientific in the UK. The centrifuge was set to spin for a period of 5 min at a rate of eleven thousand revolutions per minute. The PCV was ultimately computed employing hematocrit reader of acceptable standard which is manufactured by Hawksley and Sons in England, using the formula shown below [18]:
| (5) |
2.5.5. Screening for plant phytochemicals
The 80% hydromethanolic crude extract of the fruit of Lagenaria siceraria was subjected to a qualitative and quantitative analysis in accordance with established techniques [27,28]. Glycosides, alkaloids, steroids, saponins, flavonoids, terpenoids, and tannins were among the key plant phytochemicals that were investigated.
2.6. Analysis of the data
The data were organized, then entered into SPSS version 22 for analysis. The MST, average PPS, changes in mean rectal temperature, PCV, and body weight of mice infected with P. berghei were compared between the test substance-received mice groups and the control mice groups, as well as across test substance-received mice groups by employing one-way analysis of variance (ANOVA) accompanied by the Tukey post hoc test. Statistical significance was determined at P-value of less than 0.05 and a confidence interval of 95%.
3. Results
3.1. Yield of the plant extract
The yield obtained from the extraction of the fruits of Lagenaria siceraria grown in the study area using 80% hydromethanolic solution was 114 g (19% yield). The appearance of the substance obtained after concentrating the extract was dry brownish powder.
3.2. Test for Plant’s acute toxicity
Acute toxicity testing revealed no mortality within the initial 24 h and the following 14 days of monitoring. Gross physical and behavioral observations of the mice under investigation showed that the plant did not elicit any obvious symptoms of acute poisoning. Furthermore, no mouse deaths were seen at the tested dose level of 2000 mg/kg.
3.3. The four-day suppressive antimalarial assessment
3.3.1. Effect on mice parasitemia level
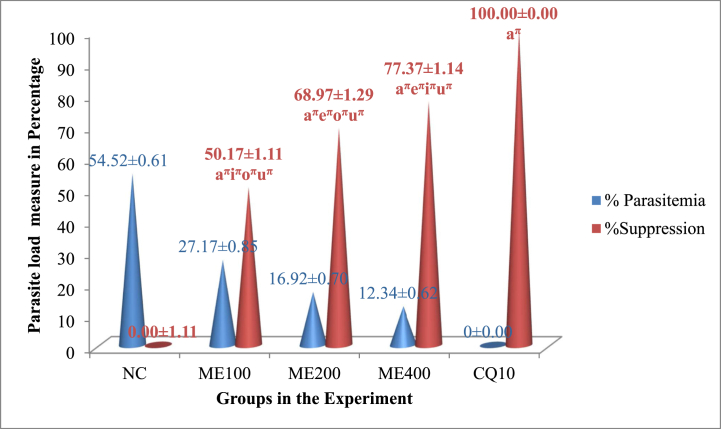
Fig. 2 summarizes the chemosuppressive activity of the extract from the fruits of Lagenaria siceraria. A significant (p < 0.05) statistical variation in the lowering of the load of the parasite between the experimental group and the control group was seen for each dose level of the fruit of the plant extract assessed in the study. In comparison to other doses, the extract at 400 mg/kg/day demonstrated the highest level of suppression of parasitemia (77.37%). However, the crude extract’s effects were inferior to those of the standard medication, which exterminated the parasite to a zero level.
Fig. 2.
The parasite load of mice treated with the 80% methanolic crude fruit extract of Lagenaria siceraria following infection with P. berghei. Measured values are expressed as mean ± SEM (mice number in each group = 6). NC, negative control, 2% tween 80; ME, 80% methanol extract; CQ, positive control, chloroquine base; a, versus NC, 2% tween 80,10 ml/kg; e, versus 100 mg/kg; i, versus 200 mg/kg; o, versus 400 mg/kg; u, versus CQ10; πp<0.05. The numbers adjacent to the letters on the horizontal axis (10, 100, 200,400) denote the dose in mg/kg.
3.3.2. Effect on survival time
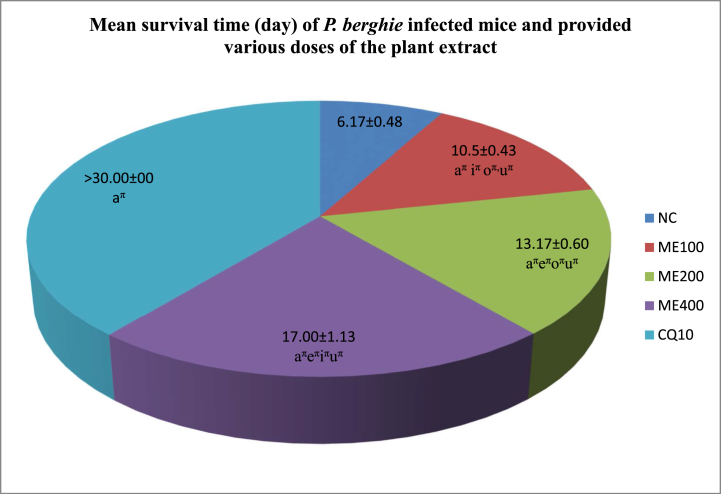
After the mice were observed for 30 days, the mean survival time was calculated and summarized in Fig. 3. The maximum dose administered demonstrated the longest average duration of survival (17.00 days). On the contrary, those that received CQ treatment survived for the full 30-day observation period.
Fig. 3.
The length of survival time in days of P. berghie infected mice and provided various doses of the 80% methanolic crude fruit extract of Lagenaria siceraria. Measured values are expressed as mean ± SEM (mice number in each group = 6). NC, negative control, 2% tween 80; ME, 80% methanol extract; CQ, positive control, chloroquine base; a, versus NC, 2% tween 80,10 ml/kg; e, versus 100 mg/kg; i, versus 200 mg/kg; o, versus 400 mg/kg; u, versus CQ10; πp<0.05. The numbers adjacent to the letters in the legends (10, 100, 200,400) denote the dose in mg/kg. The mice were under investigation for 30-days observation period.
3.3.3. Impact on mice rectal temperature and body weight
Three of the doses of the extract of the plant tested significantly (p < 0.05) prevented body weight loss in comparison to the group that received only the vehicle. Additionally, each dose level of the extract of the plant was effective in significantly inhibiting body temperatures from fall because of the infecting parasite in comparison to the group which received the reconstituting vehicle. This defense against weight loss and a reduction in rectal temperature was dose-dependent. As a result, the highest dose administered, 400 mg/kg/day (Table 1), demonstrated the maximum prevention of loss of weight and a fall in temperature.
Table 1.
Rectal temperature and body weight changes of P. berghei infected mice, and treated with 80% methanolic crude fruit extract of Lagenaria siceraria.
| Group | Body Weight (g) |
Rectal Temperature (°C) |
||||
|---|---|---|---|---|---|---|
| D0 | D4 | Change (%) | D0 | D4 | Change (%) | |
| NC | 28.45 ± 0.81 | 24.342 ± 0.47 | −14.26 ± 1.64 | 36.63 ± 0.24 | 34.28 ± 0.25 | −6.44 ± 0.19 |
| ME100 | 28.79 ± 0.61 | 26.06 ± 0.66 | −9.49 ± 0.52aπiπoπuπ | 36.48 ± 0.31 | 35.09 ± 0.40 | −3.82 ± 0.33aπiπoπzπ |
| ME200 | 29.17 ± 0.68 | 27.73 ± 0.65 | −1.02 ± 0.28aπeπoπuπ | 37.05 ± 0.33 | 36.12 ± 0.31 | −2.51 ± 0.10aπeπoπzπ |
| ME400 | 28.91 ± 0.62 | 28.62 ± 0.68 | 2.6644 ± 0.31aπeπiπuπ | 36.90 ± 0.62 | 36.50 ± 0.28 | −1.08 ± 0.20aπeπiπoπ |
| CQ10 | 28.80 ± 0.81 | 29.568 ± 0.83 | 0.30 ± 0.05aπ | 36.98 ± 0.20 | 37.21 ± 0.18 | 0.67 ± 0.19aπ |
Measured values are expressed as mean ± SEM (mice number in each group = 6). D0 = pre-treatment value on day 0, D4 = post-treatment value on day four, NC = negative control, ME = crude fruit extract of 80% methanol, CQ = chloroquine base. a, versus NC; e, versus 100 mg/kg; i, versus 200 mg/kg; o, versus 400 mg/kg; u, versus CQ10; πp<0.05. The numbers adjacent to the letters in the first column (10,100,200,400) denote the dose in mg/kg.
3.3.4. Impact of the extract on mice packed cell volume
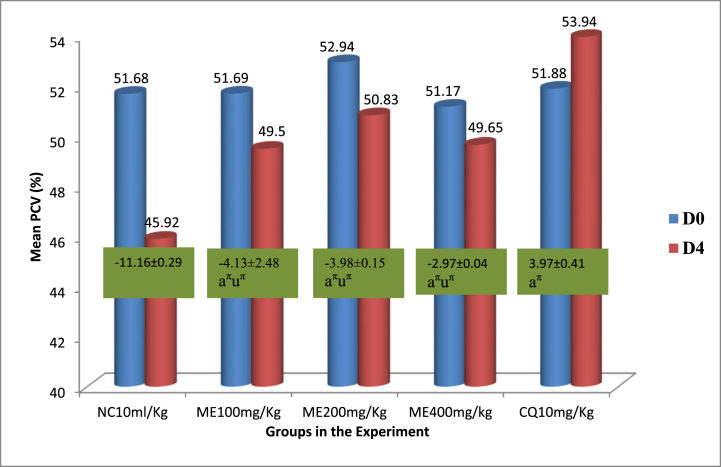
The group of mice in the extract-treated group showed a statistically significant (p < 0.05) defense against PCV decline which improved as dose increased when compared to the vehicle-treated mice. Moreover, the extract's maximum dose (400 mg/kg/day) demonstrated the greatest protection against PCV decline. However, extract’s protection from plasmodium driven PCV lowering was inferior to the standard medication, 10 mg/kg dosing of CQ (Fig. 4).
Fig. 4.
Packed cell volume change of P. berghei infected mice and provided various doses of the 80% methanolic crude fruit extract of Lagenaria siceraria. Measured values are expressed as mean ± SEM (mice number in each group = 6). D0, pre-treatment value on day 0; D4, post-treatment value on day four; CN, negative control, 2% tween 80; ME, 80% methanol extract; CQ, positive control, chloroquine base; PCV, packed cell volume; a, versus CN, 2% tween 80,10 ml/kg; e, versus 100 mg/kg; i, versus 200 mg/kg; o, versus 400 mg/kg; u, versus CQ10; πp<0.05. The numbers displayed across the graphs within rectangles represent the percentage difference in mean PCV between D0 and D4. The numbers adjacent to the letters in the horizontal axis (10, 100, 200,400) denote the dose in mg/kg.
3.3.5. Screening for the plant phytochemicals
The major secondary phytochemical constituents found in the 80% hydromethanolic extract of the fruit of Lagenaria siceraria grown in the study area were saponins, glycosides, terpenoids, alkaloids and flavonoids. On the other hand, the plant extract devoid of tannins and steroids. The most abundant secondary phytochemicals assessed in the plant extract were alkaloids whereas terpenoids were at the bottom of the other constituents (Table 2).
Table 2.
Assessment of the secondary phytoconstituents of the 80% methanolic crude fruit extract of Lagenaria siceraria.
| Phytochemicals | Test result | Content (%) |
|---|---|---|
| Alkaloids | + | 15.13 |
| Tannins | – | – |
| Saponins | + | 8.51 |
| Flavonoids | + | 3.24 |
| Terpenoids | + | 2.63 |
| Steroids | – | – |
| Glycosides | + | 4.07 |
-, absence of the phytoconstituent; +, presence of the phytoconstituent.
4. Discussion
After certain time of appropriately treating the disease, the anti-malarial drugs have been losing their effectiveness overtime. This is due to the selection of resistance by the malaria parasites. Plasmodium falciparum is now resistant to the previously effective drugs such as Chloroquine and Sulfadoxine-Pyremethamine. Even, resistance of this most deleterious parasite type to the currently declared most effective medication, artemisinin, is reported in Africa and South East Asia. These lead to continues search for new drugs which are active against the resistant parasites. In the discovery of antimalarial dugs, the role of medicinal plants is immense. Essential antiplasmodial drugs such as Quinine from cinchona species and Artemisinin from Artemesia species are derived from medicinal plants. Hence, medicinal plants are the area of investigation for better antimalarial drug development [1,26].
Despite utilization of medicinal plants by more than 80% of the communities of malaria endemic countries to address their most basic health need, yet a lot pharmacological investigation is expected to establish their safety, efficacy and scientific standardization. Previous medicinal plants which were eventually standardized and used in the present health facilities passed through robust preclinical and clinical investigations. The preclinical studies direct investigational compounds affirming their safety and efficacy to the tests involving human participants. Furthermore, investigational medicinal plants need to pass through in vitro and in vivo studies before they are produced by industrial pharmacies and prescribed and dispensed to the patients. Lagenaria siceraria is among the medicinal plants that are used for the management of malaria in Ethiopian folk medicine. The fruit of Lagenaria siceraria is bored, rinsed with cold water, and one glass is used as a drink early in the morning to treat malaria in Ethiopian folk medicine. The study on the plant also showed the plant's in vitro antipalasmodial activity. However, no any further in vivo investigation was done on the plant despite this traditional claim and in vitro activity. Hence, the present study investigated the plant's antimalarial effect in a rodent malaria model, employing the four day suppressive test towards taking the investigations on the plant to the next level. The four day suppressive test is standard test that has been most widely utilized for antimalarial screening [5,26].
As per our investigation, the extract of the fruit of Lagenaria siceraria is active in suppressing the parasites. Because, in four day suppressive test, a mean parasitemia level of less than or equal to 90% to that of mock treated control animals usually indicates that the test compound is active in standard screening studies. Furthermore, a potential and effective antimalarial substance should result in parasite suppression of at least 30%. In our investigation, the plant extract showed a minimum chemosuppression of 50.17% at lowest dose tested. Therefore, the plant possesses a promising chemosuppression activity. Literature rates a compound's antimalarial activity as very good, moderate or good based on doses that result in a growth inhibition percentage of ≥50%. As per to this grading, the assignment is as follows: very good (≤100 mg/kg/day), good (250-100 mg/kg/day), and moderate (500-250 mg/kg/day) [18,23]. As a result, Lagenaria siceraria's fruit has very good antimalarial properties.
In addition, from ANOVA results, the plant extract showed a significant mean parasitemia suppression compared to a group that received the negative control substance. The higher the dose of the extract administered, the higher the parasitemia suppression attained. When compared with previous antimalarial screening studies, the parasitemia suppression in our work is superior to the study done on the leaf latex of Aloe weloensis [29], Eucalyptus globulus [24], Dalbergia katangensis [30], Schinus molle [23], Leaf Latex of Aloe melanacantha [25], Hypoestes forskalei [31] and Globba malaccensis [32]. The chemosuppression is comparable to the study conducted on Theobroma cacao [33], and Alstonia boonei and Carica papaya [34]. On the other hand, the inhibition of parasitemia is inferior to the work done on Allium ursinum [35], Chromolaena odorata [36] and Capsicum frutescens [18].
Previous studies conducted on the plant indicated that the plant is endowed with free radical scavenging, anti-inflammatory and antioxidant eliciting phytoconstituents such as terpenoids and flavonoids. These active moieties could be contributing to the chemosuppressive effect of the plant [15,16,37]. Furthermore, the plant contains huge amount of alkaloids as mentioned under the section phytochemical screening. The alkaloids have well established antimalarial activity as evidenced by drugs such as quinine, alkaloidal drug derived from cinchona bark. Hence, the plentifully localized alkaloids in the extract of the plant under investigation may account for its inhibitory effect on the plasmodium parasite. The parasite suppression induced by the plant material may be due to an indirectly strengthened immune system. It might also be from the inhibition of the other pathways and targets that have not yet been completely understood. Moreover, the targets identified in the earlier works related to malaria pathogenesis and the plasmodial parasite life cycle could be responsible with either a distinct or similar mode of action [18].
The other parameters measured in plant extract malaria screening in vivo model includes change in body weight, temperature, packed cell volume and survival time of the mice. In this regard, an ideal plant extract is expected to prevent weight loss, reduction in PCV and inhibit reduction in body temperature occurring because of parasite load in the mice and prolong the survival time [18]. The plant extract significantly averted the weight loss, reduction in PCV and inhibited reduction in body temperature in comparison to the negative control. In all the three parameters, the inhibition demonstrated by the plant crude extract was not 100% despite statistically significant difference from the negative control. The reason could be attributed to recrudesce, low dose of the extract, or compounds may be short acting due to enhanced metabolism of the responsible phytoconstituents which lead to the extract’s failure to fully remove the plasmodial parasite [23]. When compared with the previous antimalarial screening studies, the inhibition of reduction of the mentioned parameters in our work is higher than the study done on Senna alata and Piliostigma reticulatum [38,39]. On the contrary, the inhibition of reduction of the mentioned parameters is inferior to the work done on Osyris quadripartite [40] and Spondias pinnata [41]. The antimalarial herbal remedy should improve the mice's duration of survival. At all the doses tested, the extract of the plant significantly improved the mice's duration of survival comparing with the negative control. As the parasites were not completely cleared from the mice, the maximum mean survival of 30 days or more was not attained in extract treated mice. On the contrary, CQ cleared the parasites completely from the mice, the mice survived for more than or equal to 30 days. The mean survival time is comparable with the antimalarial investigation conducted on the Saccharum ofcinarum [42], but slightly lower than the work done on Senna alata [38].
The test for the acute toxicity showed that the plant caused no visible signs of acute toxicity as evidenced by the gross behavioral and physical observation of the experimental mice. Furthermore, according to OECD guideline No. 425, the plant extract's median lethal dose (LD50) may exceed 2000 mg/kg [20]. Furthermore, this finding could in part augment the claimed safe use of the plant as a malaria remedy by the Ethiopian local people.
5. Limitations of the present investigation
The study was conducted on the strain of the parasite which is sensitive to Chloroquine. Hence, the plant's ability to halt the burden of the resistant strains remains for future studies. This is due the lack of resistant parasites infecting mice in our vicinity which is similar to the other previous studies done in a resource limited countries of the world. The other constraint was lack of methods and/or reagents such as gas chromatography, mass spectrometry, high performance liquid chromatography, nuclear magnetic resonance and related techniques which are important for the characterization of each molecule responsible for the antiplasmodial activity of the plant. However, most of the previous works aimed to assert the plants' antimalarial activity followed similar fashions to that of ours. Hence, as the plant has antiplasmodial activity and potential antimalarial could be isolated from it, isolation of those compounds is highly recommended in the future study.
6. Conclusion
The findings from our experimental investigation asserted that the fruit of Lagenaria siceraria is enriched with in vivo antiplasmodial parasite activity. The highest parasitemia inhibition of 77.37% attained by the maximum dose tested in this study perhaps due to the abundant phytoconstituents in this dose. Hence, the plant’s use as folk medicine to combat malaria by Ethiopian traditional healers is found to be logical. Based on this experimental work, the fruit of Lagenaria siceraria might potentially be a source to discover safer and more efficient anti-malarial medications. Further in depth antimalarial investigations on the plant involving isolation and characterization of each molecule is highly recommended.
Author contribution statement
Getu Habte: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sanbato Tamiru; Kedir Eyasu: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
No additional information is available for this paper.
Abbreviations and Acronyms
- ANOVA
Analysis of Variance
- CQ
Chloroquine
- EPHI
Ethiopian Public Health Institute
- MST
Mean Survival Time
- OECD
Organization for Economic Cooperation and Development
- PCV
Packed Cell Volume
- PP
Percentage Parasitemia
- PPS
Percentage Parasitemia Suppression
- SEM
Standard Error of the Mean
- SPSS
Statistical Package for Social Sciences
- TM
Traditional Medicine
- WHO
World Health Organization
References
- 1.World Health Organization . World Health Organization; 2022. World Malaria Report; p. 238. [Google Scholar]
- 2.Takem E.N., D’Alessandro U. Malaria in pregnancy. Mediterr. J. Hematol. Infect. Dis. 2013;5:15. doi: 10.4084/MJHID.2013.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su X., Miller L.H. Physiol. Behav. 2016;176:100–106. doi: 10.1007/s11427-015-4948-7. [DOI] [Google Scholar]
- 4.World Health Organization . World Health Organization; 2022. Strategy to Respond to Antimalarial Drug Resistance in Africa. [Google Scholar]
- 5.Mulugeta K. Diversity, knowledge and use of medicinal plants in Abay Chomen District, Horo Guduru Wollega Zone, Oromia Region of Ethiopia. J. Med. Plants Res. 2017;11:480–500. doi: 10.5897/jmpr2016.6274. [DOI] [Google Scholar]
- 6.Nigussie G., Wale M. Medicinal plants used in traditional treatment of malaria in Ethiopia: a review of ethnomedicine, anti-malarial and toxicity studies. Malar. J. 2022;21:262. doi: 10.1186/s12936-022-04264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . World Health Organization; 2013. WHO Traditional Medicine Strategy. [Google Scholar]
- 8.Deribe K., Amberbir A. Original article A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop. J. Health Dev. 2014;20 doi: 10.4314/ejhd.v20i2.10023. [DOI] [Google Scholar]
- 9.Dkhil M.A., Al-Quraishy S., Al-Shaebi E.M., Abdel-Gaber R., Thagfan F.A., Qasem M.A.A. Medicinal plants as a fight against murine blood-stage malaria. Saudi J. Biol. Sci. 2021;28:1723–1738. doi: 10.1016/j.sjbs.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashari O., Osesua B., Danjumma B., Udeme A. Antibacterial activity and phytochemical screening of Calabash seed oil on Clinical isolates from skin. Int. J. Adv. Acad. Res. Sci. Eng. 2018;4:1–17. [Google Scholar]
- 11.Gezimu W., Bekele F., Habte G. Pregnant mothers' knowledge, attitude, practice and its predictors towards nutrition in public hospitals of Southern Ethiopia: a multicenter cross-sectional study. SAGE Open Med. 2022;10:1–10. doi: 10.1177/20503121221085843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhardallou S.B., Elawad A.M., Khairi N.A., Gobouri A.A., Dhahawi H.O. A review on Omega-3 and Omega-6 essential fatty acids: uses, benefits and their availability in pumpkins (Cucurbita maxima) seed and desert dates (Balanites aegyptiaca) seed kernel oils. Pak. J. Biol. Sci. 2014;17:1195–1208. doi: 10.3923/pjbs.2014.1195.1208. [DOI] [PubMed] [Google Scholar]
- 13.Shrivastava A., Shikha R. Cucurbitaceae: a ethnomedicinally important vegetable family. J. Med. Plants Stud. 2013;1:16–20. [Google Scholar]
- 14.Mukherjee P.K., Singha S., Kar A., Chanda J., Banerjee S., Dasgupta B., Haldar P.K., Sharma N. Therapeutic importance of Cucurbitaceae: a medicinally important family. J. Ethnopharmacol. 2022;282:1–27. doi: 10.1016/j.jep.2021.114599. [DOI] [PubMed] [Google Scholar]
- 15.Saeed M., Khan M.S., Amir K., Bi J.B., Asif M., Madni A., Kamboh A.A., Manzoor Z., Younas U. Lagenaria siceraria fruit : a review of its phytochemistry , pharmacology , and promising traditional uses. Front. Nutr. 2022;9:1–15. doi: 10.3389/fnut.2022.927361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman A., Rahman A. Bottle Gourd (Lagenaria siceraria) A vegetable for good health. Nat. Product. Radiance. 2003;2:249–250. [Google Scholar]
- 17.Kalpana V.N., Alarjani K.M., Rajeswari V.D. Enhancing malaria control using Lagenaria siceraria and its mediated zinc oxide nanoparticles against the vector Anopheles stephensi and its parasite Plasmodium falciparum. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-77854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habte G., Assefa S. In vivo antimalarial activity of Crude fruit extract of Capsicum frutescens var. Minima (Solanaceae) against plasmodium berghei -infected mice. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/1320952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Academy of Sciences . eighth ed. National Academy Press; Washington, DC: 2011. National Academy of Sciences Guide for the Care and Use of Laboratory Animals. [DOI] [Google Scholar]
- 20.OECD . OECD Guidelines for the Testing of Chemicals; 2008. OECD Guideline 425: Acute Oral Toxicity—Up-And-Down Procedure. [Google Scholar]
- 21.Peters W., Portus J.H., Robinson B.L. The chemotherapy of rodent malaria, XXII. Ann. Trop. Med. Parasitol. 1975;69:155–171. doi: 10.1080/00034983.1975.11686997. [DOI] [PubMed] [Google Scholar]
- 22.Knight D.J., Peters W. Annals of tropical medicine & parasitology the antimalarial activity of N- benzyloxydihydrotriazines. Ann. Trop. Med. Parasitol. 2017;4983 doi: 10.1080/00034983.1980.11687360. [DOI] [PubMed] [Google Scholar]
- 23.Habte G., Nedi T., Assefa S. Antimalarial activity of aqueous and 80% methanol Crude seed extracts and solvent fractions of Schinus molle Linnaeus (Anacardiaceae) in plasmodium berghei -infected mice. J. Trop. Med. 2020;2020 doi: 10.1155/2020/9473250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayalew M. Evaluation of the antimalarial activity of Crude extract of Eucalyptus globulus Labill . Leaf against plasmodium berghei in mice. Evid. Based Complement. Alternat. Med. 2021;2021 doi: 10.1155/2021/7068999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebremariam G.K., Desta H.K., Teklehaimanot T.T., Girmay T.G. In vivo antimalarial activity of leaf latex of Aloe melanacantha against plasmodium berghei infected mice. J. Trop. Med. 2021;2021 doi: 10.1155/2021/6690725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fidock D.A., Rosenthal P.J., Croft S.L., Brun R., Nwaka S., Einstein A. Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. Drug Discov. 2004;3 doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 27.Ezeonu C.S., Ejikeme C.M. Qualitative and quantitative determination of phytochemical contents of indigenous Nigerian softwoods. New J. Sci. 2016;2016:1–9. doi: 10.1155/2016/5601327. [DOI] [Google Scholar]
- 28.Tiwari, Kumar B., Kaur M., Kaur G., Kaur H. Department, phytochemical screening and extraction: a Review. Int. Pharm. Sci. 2011:1. [Google Scholar]
- 29.Teka T., Awgichew T., Kassahun H. Antimalarial activity of the leaf latex of Aloe weloensis (aloaceae) against plasmodium berghei in mice. J. Trop. Med. 2020;2020 doi: 10.1155/2020/1397043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valentin Bashige Chiribagula, Salvius Bakari Amuri, Joseph Kahumba Byanga, Philippe Okusa Ndjolo, Jean-Baptiste Lumbu Simbi. Antiplasmodial, antioxidant and toxicological study of leaves extracts of Dalbergia katangensis Lecheneaud (Fabaceae) from Eastern DR Congo. GSC Adv. Res. Rev. 2020;4:34–45. doi: 10.30574/gscarr.2020.4.2.0066. [DOI] [Google Scholar]
- 31.Misganaw D., Amare G.G., Mengistu G. Chemo suppressive and curative potential of hypoestes forskalei against plasmodium berghei: evidence for in vivo antimalarial activity. J. Exp. Pharmacol. 2020;12:313–323. doi: 10.2147/JEP.S262026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaniad P., Techarang T., Phuwajaroanpong A., Horata N., Septama A.W., Punsawad C. 2022. Exploring Potential Antimalarial Candidate from Medicinal Plants of Kheaw Hom Remedy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komlaga G., Forkuo A.D., Suleman N., Nkrumah D., Nketia R., Bekoe S.O. Antimalarial property and acute toxicity of the leaves of theobroma cacao L., evidence-based complement. Alternative Med. 2021;2021 doi: 10.1155/2021/2852442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atanu F.O., Idih F.M., Nwonuma C.O., Hetta H.F., Alamery S., El-Saber Batiha G. Evaluation of antimalarial potential of extracts from Alstonia boonei and Carica papaya in plasmodium berghei -infected mice, evidence-based complement. Alternative Med. 2021;2021 doi: 10.1155/2021/2599191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khanabadi F., Badirzadeh A., Kalantari-Hesari A., Akbariqomi M., Torkashvand H., Didehdar M., Elmi T. In vivo anti-malarial activity and toxicity of Allium ursinum (Wild Garlic) hydroalcoholic extract. J. Appl. Biotechnol. Rep. 2022;9:781–789. doi: 10.30491/JABR.2022.344903.1538. [DOI] [Google Scholar]
- 36.Nworgu C.O., Elele K., Adikwu E. Toxicity and antiplasmodial assessments of Chromolaena odorata leaf extract on plasmodium berghei -infected mice. J. Infectious Diseases Epidemiol. 2022;8 doi: 10.23937/2474-3658/1510276. [DOI] [Google Scholar]
- 37.Adedapo A., Adewuyi T., Sofidiya M. Phytochemistry, anti-inflammatory and analgesic activities of the aqueous leaf extract of lagenaria breviflora (Cucurbitaceae) in laboratory animals. Rev. Biol. Trop. 2013;61:281–290. doi: 10.15517/rbt.v61i1.11127. [DOI] [PubMed] [Google Scholar]
- 38.Atanu F.O., Rotimi D., Ilesanmi O.B., Al Malki J.S., Batiha G.E., Idakwoji P.A. Hydroethanolic extracts of Senna alata leaves possess antimalarial effects and reverses haematological and biochemical pertubation in plasmodium berghei -infected mice. J. Evid. Based Integr. Med. 2022:1–7. doi: 10.1177/2515690X221116407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdulkadir S.S., Jatau A.I., Abdussalam U.S., Bichi L.A. In vivo antiplasmodial activity of the methanol leaf extract of Piliostigma reticulatum (Dc.) Hochst (Fabaceae) Bull. Natl. Res. Cent. 2022 doi: 10.1186/s42269-022-00910-0. [DOI] [Google Scholar]
- 40.Kemal T., Feyisa K., Bisrat D., Asres K. In vivo antimalarial activity of the leaf extract of Osyris quadripartita Salzm . ex Decne and its major Compound. J. Trop. Med. 2022;2022 doi: 10.1155/2022/3391216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prapaporn C., Phuwajaroanpong A., Plirat W., Techarang T. In vivo assessment of the antimalarial activity and acute oral toxicity of an ethanolic seed extract of Spondias pinnata (L. f.) Kurz. BMC Complement. Med. Ther. 2022;9:1–13. doi: 10.1186/s12906-022-03546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okokon J.E., Mobley R., Edem U.A., Bassey A.I. In vitro and in vivo antimalarial activity and chemical profiling of sugarcane leaves. Sci. Rep. 2022:1–13. doi: 10.1038/s41598-022-14391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.