Abstract
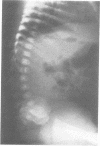
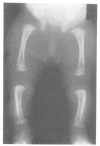
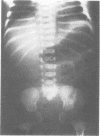
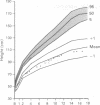
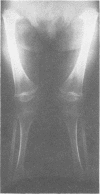
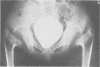
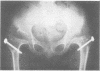
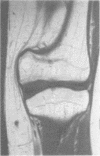
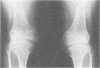
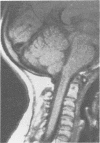
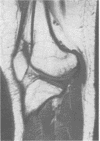
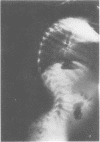
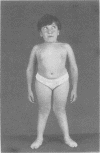
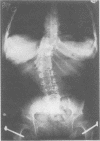
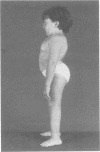
The features of a child with spondyloepiphyseal dysplasia congenita resulting from a mutation in one COL2A1 allele were studied. The child was heterozygous for a G to A transition in exon 48 that resulted in the substitution of glycine 997 by serine in the triple helical domain of alpha 1(II) chains of type II collagen. Her longitudinal growth was close to the mean growth curve for children with this chondrodysplasia. Expression of the mutation by chondrocytes would account for the abnormal growth and development of the bones of the limbs and spine. Early expression of the mutation by epithelial cells and later expression by chondrocytes of the developing craniofacial structures would also account for her complex pattern of craniofacial anomalies. The findings in this study confirm that mutations of exon 48 of the COL2A1 gene, that alter the normal Gly-X-Y triplet structure of the corresponding region of alpha 1(II) chains of type II collagen, produce the spondyloepiphyseal dysplasia congenita phenotype.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N. N., Ala-Kokko L., Knowlton R. G., Jimenez S. A., Weaver E. J., Maguire J. I., Tasman W., Prockop D. J. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6624–6627. doi: 10.1073/pnas.88.15.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Kokko L., Baldwin C. T., Moskowitz R. W., Prockop D. J. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY D. K. The normal cervical spine in infants and children. Radiology. 1952 Nov;59(5):712–719. doi: 10.1148/59.5.712. [DOI] [PubMed] [Google Scholar]
- Bailey A. J. Structure, function and ageing of the collagens of the eye. Eye (Lond) 1987;1(Pt 2):175–183. doi: 10.1038/eye.1987.34. [DOI] [PubMed] [Google Scholar]
- Bruggeman L. A., Xie H. X., Brown K. S., Yamada Y. Developmental regulation for collagen II gene expression in transgenic mice. Teratology. 1991 Aug;44(2):203–208. doi: 10.1002/tera.1420440208. [DOI] [PubMed] [Google Scholar]
- Carey J. C., Fineman R. M., Ziter F. A. The Robin sequence as a consequence of malformation, dysplasia, and neuromuscular syndromes. J Pediatr. 1982 Nov;101(5):858–864. doi: 10.1016/s0022-3476(82)80348-x. [DOI] [PubMed] [Google Scholar]
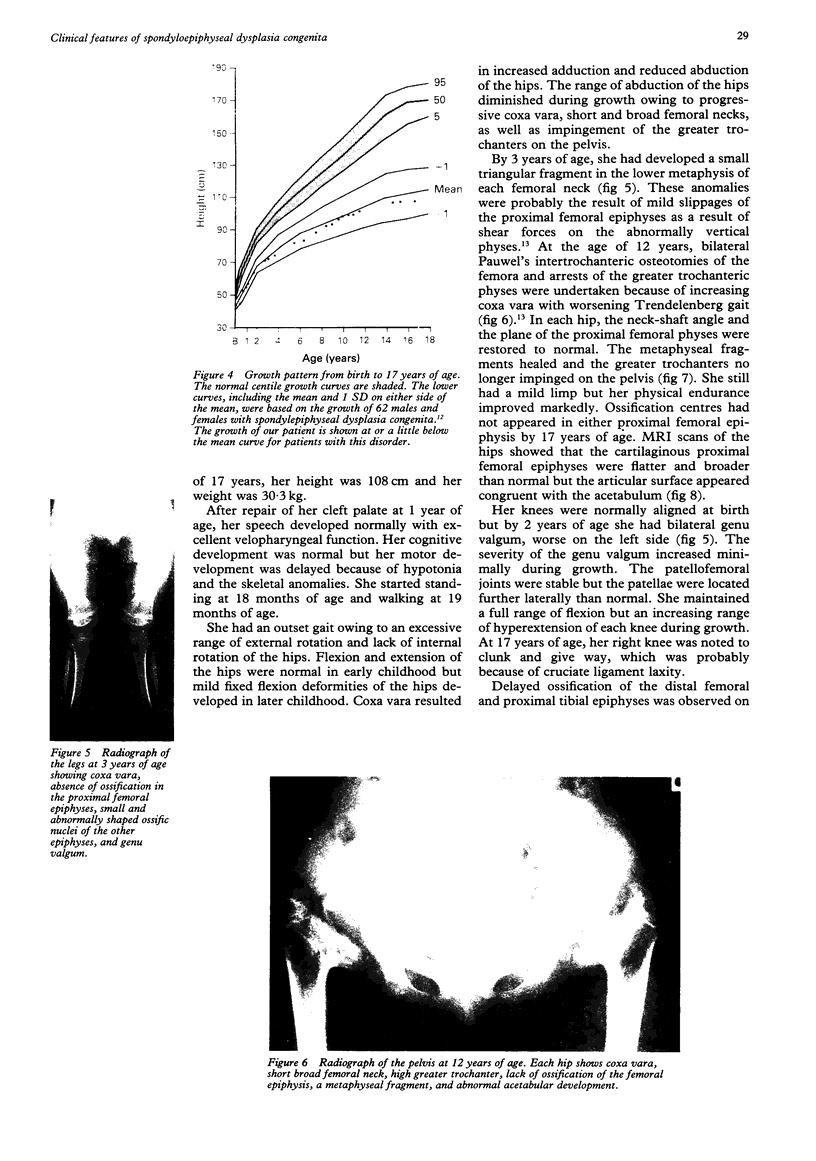
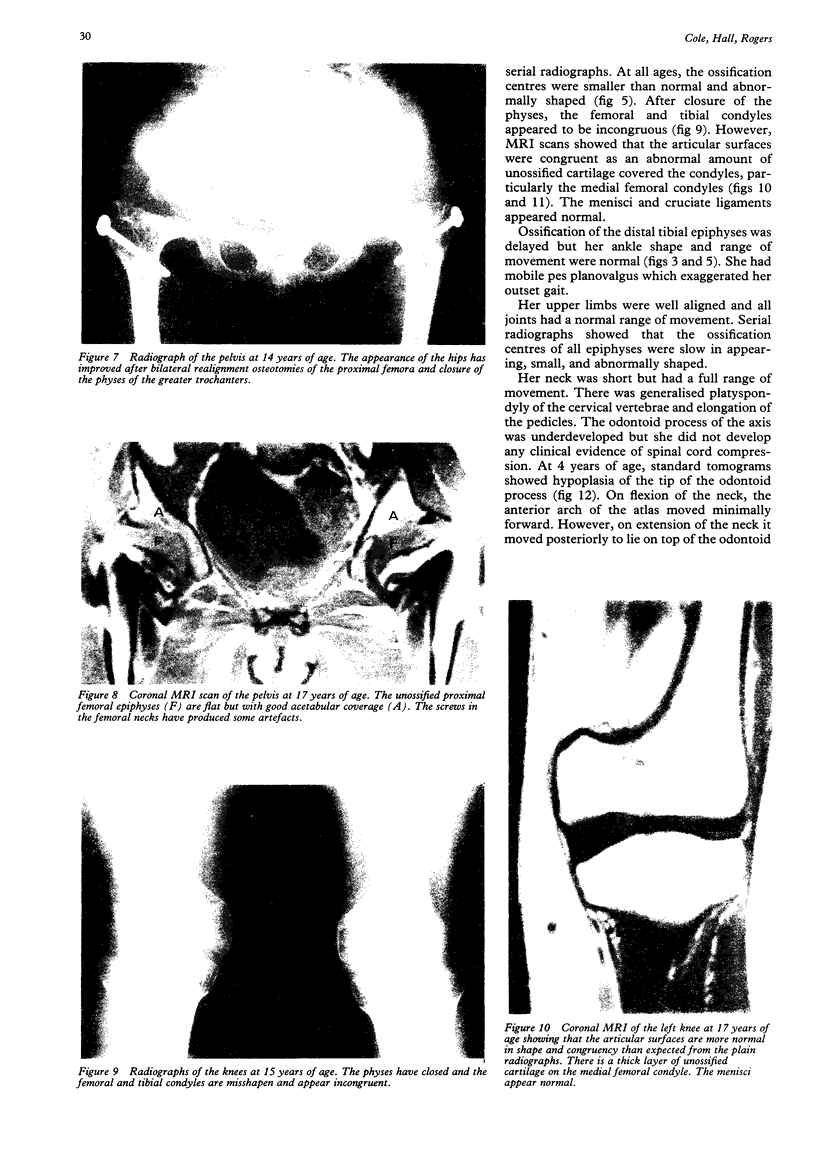
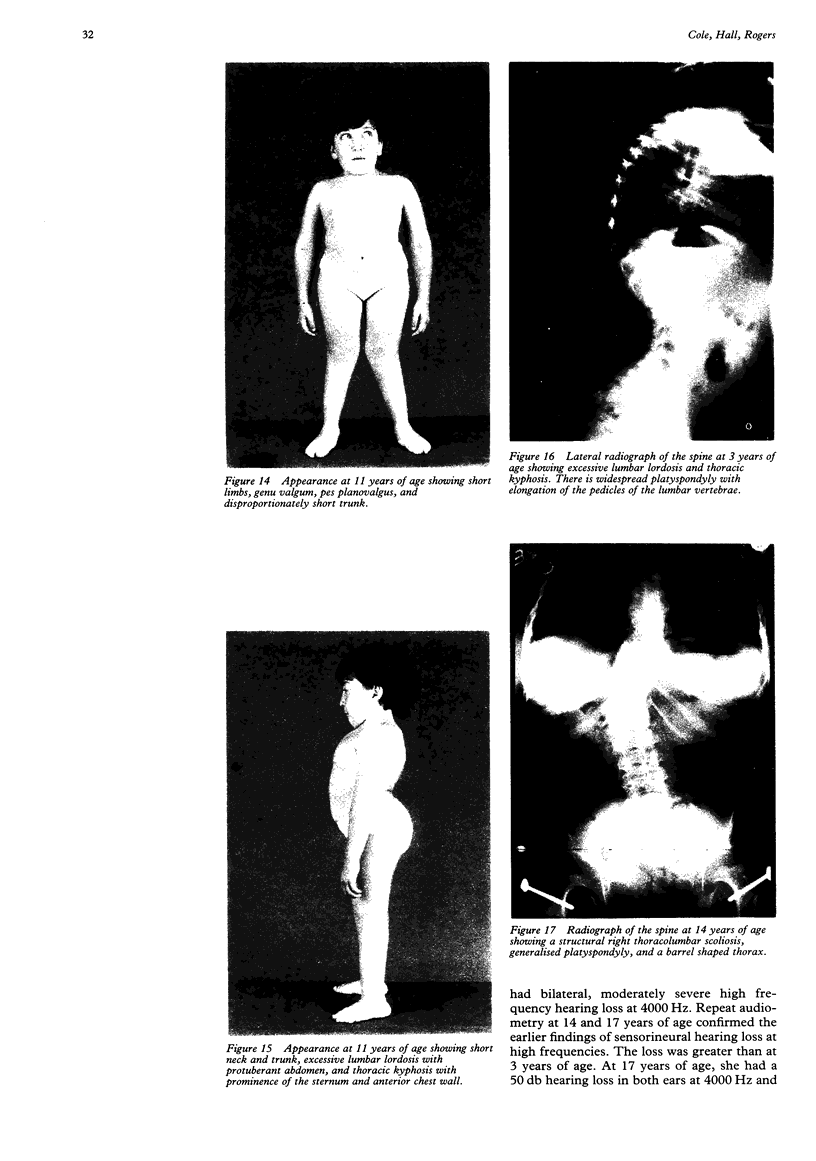
- Chan D., Cole W. G. Low basal transcription of genes for tissue-specific collagens by fibroblasts and lymphoblastoid cells. Application to the characterization of a glycine 997 to serine substitution in alpha 1(II) collagen chains of a patient with spondyloepiphyseal dysplasia. J Biol Chem. 1991 Jul 5;266(19):12487–12494. [PubMed] [Google Scholar]
- Cheah K. S., Lau E. T., Au P. K., Tam P. P. Expression of the mouse alpha 1(II) collagen gene is not restricted to cartilage during development. Development. 1991 Apr;111(4):945–953. doi: 10.1242/dev.111.4.945. [DOI] [PubMed] [Google Scholar]
- Clague R. B., Firth S. A., Holt P. J., Skingle J., Greenbury C. L., Webley M. Serum antibodies to type II collagen in rheumatoid arthritis: comparison of 6 immunological methods and clinical features. Ann Rheum Dis. 1983 Oct;42(5):537–544. doi: 10.1136/ard.42.5.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole W. G., Chow C. W., Rogers J. G., Bateman J. F. The clinical features of three babies with osteogenesis imperfecta resulting from the substitution of glycine by arginine in the pro alpha 1(I) chain of type I procollagen. J Med Genet. 1990 Apr;27(4):228–235. doi: 10.1136/jmg.27.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Weis M. A., Moskowitz R. W. Cartilage expression of a type II collagen mutation in an inherited form of osteoarthritis associated with a mild chondrodysplasia. J Clin Invest. 1991 Jan;87(1):357–361. doi: 10.1172/JCI114994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. W. Palate development. Development. 1988;103 (Suppl):41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Francomano C. A., Liberfarb R. M., Hirose T., Maumenee I. H., Streeten E. A., Meyers D. A., Pyeritz R. E. The Stickler syndrome: evidence for close linkage to the structural gene for type II collagen. Genomics. 1987 Dec;1(4):293–296. doi: 10.1016/0888-7543(87)90027-9. [DOI] [PubMed] [Google Scholar]
- Garofalo S., Vuorio E., Metsaranta M., Rosati R., Toman D., Vaughan J., Lozano G., Mayne R., Ellard J., Horton W. Reduced amounts of cartilage collagen fibrils and growth plate anomalies in transgenic mice harboring a glycine-to-cysteine mutation in the mouse type II procollagen alpha 1-chain gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9648–9652. doi: 10.1073/pnas.88.21.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey M., Hollister D. W. Type II achondrogenesis-hypochondrogenesis: identification of abnormal type II collagen. Am J Hum Genet. 1988 Dec;43(6):904–913. [PMC free article] [PubMed] [Google Scholar]
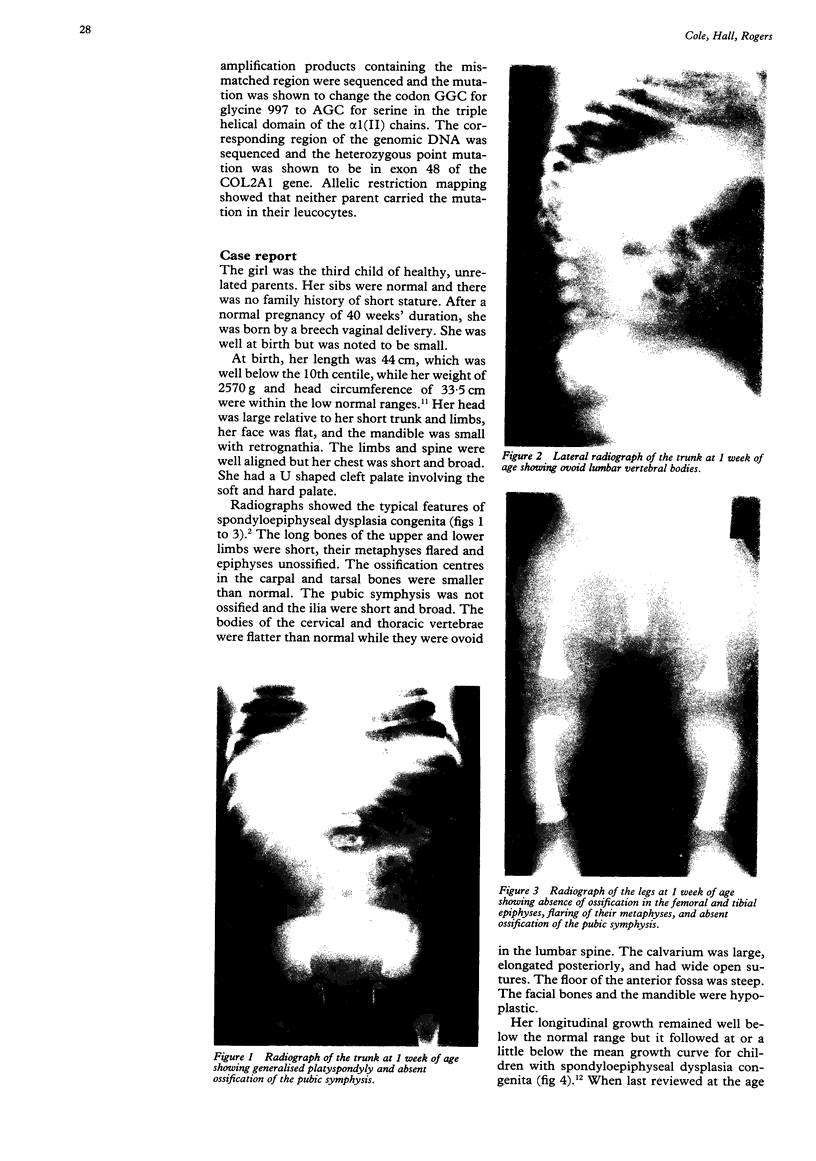
- Horton W. A., Hall J. G., Scott C. I., Pyeritz R. E., Rimoin D. L. Growth curves for height for diastrophic dysplasia, spondyloepiphyseal dysplasia congenita, and pseudoachondroplasia. Am J Dis Child. 1982 Apr;136(4):316–319. doi: 10.1001/archpedi.1982.03970400034010. [DOI] [PubMed] [Google Scholar]
- Kitchen W. H., Robinson H. P., Dickinson A. J. Revised intrauterine growth curves for an Australian hospital population. Aust Paediatr J. 1983 Sep;19(3):157–161. doi: 10.1111/j.1440-1754.1983.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Lee B., Vissing H., Ramirez F., Rogers D., Rimoin D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science. 1989 May 26;244(4907):978–980. doi: 10.1126/science.2543071. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Rosenberg L., Hollister D., Murray L., Rimoin D. Kniest dysplasia is characterized by an apparent abnormal processing of the C-propeptide of type II cartilage collagen resulting in imperfect fibril assembly. J Clin Invest. 1988 Feb;81(2):579–589. doi: 10.1172/JCI113356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky N. B., Savage J. E., Cefaratti L. K., Yoo T. J. Electron-microscopic localization of type II, IX, and V collagen in the organ of Corti of the gerbil. Cell Tissue Res. 1992 Mar;267(3):413–418. doi: 10.1007/BF00319363. [DOI] [PubMed] [Google Scholar]
- Spranger J. Bone dysplasia 'families'. Pathol Immunopathol Res. 1988;7(1-2):76–80. doi: 10.1159/000157098. [DOI] [PubMed] [Google Scholar]
- Tiller G. E., Rimoin D. L., Murray L. W., Cohn D. H. Tandem duplication within a type II collagen gene (COL2A1) exon in an individual with spondyloepiphyseal dysplasia. Proc Natl Acad Sci U S A. 1990 May;87(10):3889–3893. doi: 10.1073/pnas.87.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg P., Khillan J. S., Prockop D. J., Helminen H., Kontusaari S., Ala-Kokko L. Expression of a partially deleted gene of human type II procollagen (COL2A1) in transgenic mice produces a chondrodysplasia. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7640–7644. doi: 10.1073/pnas.88.17.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Godfrey M., Hollister D. W. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989 Nov 5;264(31):18265–18267. [PubMed] [Google Scholar]
- Wood A., Ashhurst D. E., Corbett A., Thorogood P. The transient expression of type II collagen at tissue interfaces during mammalian craniofacial development. Development. 1991 Apr;111(4):955–968. doi: 10.1242/dev.111.4.955. [DOI] [PubMed] [Google Scholar]
- Yoo T. J., Floyd R., Ishibe T., Shea J. J., Bowman C. Immunologic testing of certain ear diseases. Am J Otol. 1985 Jan;6(1):96–101. [PubMed] [Google Scholar]