Abstract
Introduction
Aphasia affects many stroke survivors; therefore, effective treatments are urgently needed. Preliminary clinical findings have suggested an association between contralateral C7–C7 cross nerve transfer and recovery from chronic aphasia. Randomised controlled trials supporting the efficacy of C7 neurotomy (NC7) are lacking. This study will explore the efficacy of NC7 at the intervertebral foramen for improving chronic poststroke aphasia.
Methods and analysis
This study protocol reports a multicentre, randomised, assessor-blinded active-controlled trial. A total of 50 patients with chronic poststroke aphasia for over 1 year and with a aphasia quotient calculated by Western Aphasia Battery Aphasia Quotient (WAB-AQ) score below 93.8 will be recruited. Participants will be randomly assigned to 1 of 2 groups (25 individuals each) to receive NC7 plus intensive speech and language therapy (iSLT), or iSLT alone programme. The primary outcome is the change in Boston Naming Test score from baseline to the first follow-up after NC7 plus 3 weeks of iSLT or iSLT alone. The secondary outcomes include the changes in the WAB-AQ, Communication Activities of Daily Living-3, International Classification of Functioning, Disability and Health (ICF) speech language function, Barthel Index, Stroke Aphasic Depression Questionnaire-hospital version and sensorimotor assessments. The study will also collect functional imaging outcomes of naming and semantic violation tasks through functional MRI and electroencephalogram to evaluate the intervention-induced neuroplasticity.
Ethics and dissemination
This study was approved by the institutional review boards of Huashan Hospital, Fudan University, and all participating institutions. The study findings will be disseminated through peer-reviewed publications and conference presentations.
Trial registration number
ChiCTR2200057180.
Keywords: Stroke, Neurosurgery, Clinical trials
STRENGTHS AND LIMITATIONS OF THIS STUDY
This will be the first multicentre neurosurgery randomised controlled trial aimed at improving language function in patients with chronic poststroke aphasia.
This study will explore the possibility of a new strategy to improve multiple dysfunctions after central nervous system injury, based on peripheral neurosurgery and traditional rehabilitation treatments.
A limitation of this study is that it will be evaluator-blinded rather than double-blinded, and that the experimental group may receive a minor placebo effect.
Introduction
Background and rationale
Aphasia refers to a collection of acquired receptive and expressive language deficits that arise in many neurological diseases or after trauma and is most commonly seen following left hemisphere stroke.1 Globally, more than 10 million new stroke cases are reported each year,2 with at least one-third of these patients experiencing symptoms of aphasia3—one of the most devastating symptoms in stroke survivors.4 5 Aphasia is responsible for substantial costs for individuals with stroke during the acute and chronic phases and is an independent predictor of subsequent functional dependence and death.3 6 The presence of aphasia predicts care and rehabilitation needs7 as well as and the likelihood of failure to return to work.8 Language function in patients with poststroke aphasia recovers spontaneously to varying degrees.9 It is generally recognised that spontaneous recovery in language function reaches a plateau—6–9 months after the first onset of stroke, and further improvements afterwards are few and negligible.10 11 During recovery, both the subtype and severity of aphasia change over time and patients may progress from sensory aphasia to conduction aphasia to naming aphasia and to ‘recovered’.12 Nevertheless, this ‘recovered’ status may also involve a mild, residual impairment that could be detected by a sensitive assessment.13 However, some forms of aphasia persist into the chronic phase in at least half of the patients.14
Although most aphasia therapy studies have enrolled patients with chronic stroke, conceivably, earlier aphasia therapy is also effective, as it has achieved good results after stroke.15 Common aphasia rehabilitation treatments include classic speech-language rehabilitation training, low-frequency electrical stimulation therapy, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS). Many clinical studies16 17 have shown that speech and language therapy (SLT) effectively improves communication, reading, writing and language expression in patients with poststroke aphasia, and high-intensity and long-term mode may have better effects.18 A large-scale randomised controlled trial (RCT) reported that, 3 weeks of intensive SLT (iSLT) showed improvements in patients with chronic poststroke aphasia and significantly enhanced verbal communication among people aged 70 years or younger.17 This beneficial outcome could be maintained for up to 6 months after treatment. Stahl et al19 further determined the optimal daily dosage and total duration of iSLT, reporting no added value from >2 hours of daily SLT within 4 weeks. In addition, non-invasive brain stimulation therapy is widely used in the rehabilitation of various neurological diseases. tDCS uses electrode pads to deliver a weak direct current to specific brain regions, which can affect the function of the cerebral cortex and help improve the accuracy of noun naming in patients with aphasia.20–22 However, sufficient data on the optimal sample size and a strict methodology. Low-frequency rTMS is the regular and repeated application of a pulsed magnetic field that briefly penetrates the skull targeting specific cortical regions; this induces plastic changes in the brain and language function in patients with long-term poststroke aphasia. However, its efficacy remains controversial and warrants further confirmation through large-scale clinical trials.23
We previously developed a surgical procedure for contralateral C7 (CC7) transfer from the non-paralysed to the paralysed side (CC7–C7 cross nerve transfer), after which patients with stroke demonstrated improved motor function and reduced spasticity in the paralysed arm over 12 months.24 To date, more than 1000 patients have undergone this surgery.24 In addition to arm motor recovery, language improvement was frequently self-reported by patients and caregivers during follow-up, and it occurred very rapidly after CC7 treatment. A few days are by far not sufficient for the transferred C7 nerve to regenerate25; therefore, we assumed that the rapid improvement in language function was mediated by the C7 neurotomy (NC7) on the paralysed side (right side), rather than nerve regeneration. During the CC7 operation, we cut the C7 nerve root at the intervertebral foramen to ensure that the C7 nerve on the paralysed side would provide more nerve fibre length.26 The anterior and posterior roots converge into spinal nerves at the intervertebral foramen, and the posterior roots enlarge near the intervertebral foramen to form ganglia, also known as the dorsal root ganglion (DRG).27 28 The exact location of the neurotomy was the transitional junction of the C7 nerve root with the DRG.29 The human C7 nerve contains 80 000 fibres,30 94% of which are sensory fibres emitted by the DRG.31 Hence, neurotomy of the C7 nerve root at the junction with the DRG could block the ascending sensory pathway from the affected limb to the brain. Based on the anatomy of the brain functional areas, we hypothesised that, since the sensorimotor centre is adjacent to the language centre, if the sensorimotor centre is artificially changed by NC7 at the intervertebral foramen, it maybe possible to stimulate the language centre and achieve relevant functional changes. NC7 may also leads to a change in interhemispheric balance, thus affecting the functional neural circuits of language. We designed this trial to evaluate the surgical effect of NC7 at the intervertebral foramen on the underlying neuroplasticity in patients with chronic poststroke aphasia. Meanwhile, the iSLT will be used as the control method to assess the effect of the intensive intervention after 3 weeks and the maintenance of the effect after 6 months in both groups. Neuroimaging methods will be simultaneously used to obtain objective data to test our hypothesis.
Aims of the study
This study will to evaluate the therapeutic efficacy of NC7 at the intervertebral foramen for on language impairment in patients with chronic poststroke aphasia. This paper describes the related study design.
Our objectives are: (1) to evaluate the efficacy of NC7 plus iSLT (3 weeks) compared with iSLT (3 weeks) alone, as well as the safety and long-term stability of NC7 outcomes and (2) to explore the possible central plastic mechanism of improvement after NC7 plus iSLT using functional neuroimaging measurements.
Methods and analysis
Trial design
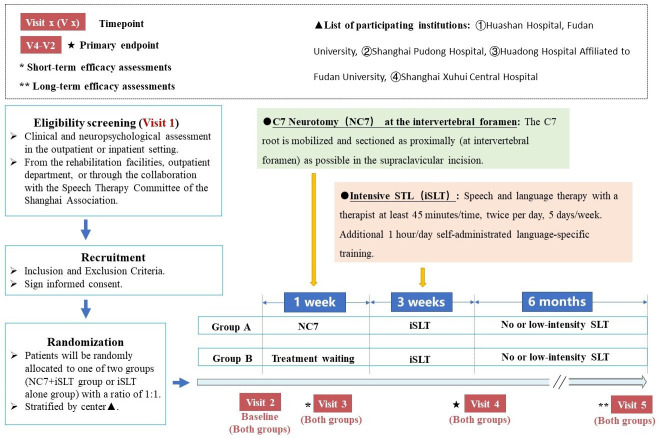
This study will be a multicentre, randomised, assessor-blinded, active-controlled trial. The participants will be randomly allocated to either group (NC7+iSLT group or iSLT alone group) with a ratio of 1:1 at the four participating centres (online supplemental table 1). Patients in group A will be treated with NC7 at the intervertebral foramen combined with 3-week iSLT, and patients in group B will be treated with 3-week iSLT alone. The participants will be recruited from the rehabilitation facilities, outpatient department or through the collaboration with the Speech Therapy Committee of the Shanghai Association. Eligible patients will be invited to participate in this study and will be asked to sign informed consent.
bmjopen-2022-065173supp001.pdf (76.9KB, pdf)
Eligibility criteria
The inclusion criteria are the following:
Aphasia for over 12 months after a single onset of infarction or haemorrhage of the left hemisphere, confirmed by MRI.
Of 40–65 years, male or female sex, right-handed, native Chinese speakers.
Western Aphasia Battery Aphasia Quotient (WAB-AQ) score below 93.8 points.
Severity score assessed using the Boston Diagnostic Aphasia Examination (BDAE) test of level 1 and above.
Good compliance and ability to cooperate with language rehabilitation training.
Ability to understand fully and agree with the doctor’s treatment plan and sign the informed consent.
Criteria 3 and 4 will to be confirmed through the diagnostic evaluations of two attending specialists.
Exclusion criteria are the following:
Any surgical contraindication, determined by a qualified anaesthesiologist or clinician.
History of aphasia before the last onset of a stroke.
Serious, untreated mental illness.
Aphasia due to neurodegenerative diseases or traumatic brain injury.
Contraindications for electroencephalogram (EEG) and MRI evaluation.
Inability to complete the assessments and rehabilitation required per study design.
Severe motor speech disorder and hearing impairment.
Having received intensive poststroke rehabilitation therapy 4 weeks before recruitment.
Interventions
NC7 at the intervertebral foramen
A 6 cm long longitudinal incision will be made along the medial border of the sternocleidomastoid muscle on the right side after the cervical plexus, under local/general anaesthesia (depending on the patient’s preference and the anaesthesiologist’s risk assessment). The structure will be carefully separated layer-by-layer and the brachial plexus will be identified by marking the C7 nerve with a vessel loop. The C7 root will be mobilised and sectioned proximally at the intervertebral foramen. Considering that some patients have limb hemiplegia, CC7 surgery may be required to improve limb function by the end of this trial. Therefore, we will fix the severed C7 root to the fascia, at the junction of the scapulohyoid and sternocleidomastoid muscles, with a silk thread; this will facilitate retrieval and anastomosis with the CC7 root during later CC7 surgical intervention.26
iSLT rehabilitation
Base on previous studies, we formulated a 3-week iSLT plan. SLT will be performed by a therapist for at least 45 min, twice daily, 5 days a week. The intervention will also involve an additional 1 hour/day of self-administrated language-specific training. The patients will receive rehabilitation treatment at different centres from qualified rehabilitation therapists.
Study setting
This study will begin in July 2022, and the participants will be recruited between July 2022 and July 2023. The study is expected to be completed in July 2024. Fifty patients diagnosed with chronic aphasia and hemiplegia after stroke will be recruited. The treatment and visit plans are shown in figure 1. Patients will be selected on their first visit according to the eligibility criteria. Eligible patients will be randomly assigned to one of the two groups at different centres. The patients in group A will receive NC7 after baseline assignment and the first assessment on day 3 (+1). The iSLT treatment for group A will starts 1 week after NC7 surgery. Meanwhile, the patients in group B will be awaiting the therapy programme, and receive a short-term efficacy assessment after 3 (+1) days. These patients will undergo the same iSLT programme as patients in group A. Three weeks after the iSLT programme, all patients will undergo the second follow-up (3 weeks±3 days after iSLT commencement). All patients will undergo long-term evaluation 6 months after iSLT commencement. The schedules for enrolment, interventions and assessments are presented in table 1.
Figure 1.
Trial design in detail and patient flow chart. SLT, speech and language therapy.
Table 1.
Timeline of enrolment, intervention and assessment schedule
| NC7+iSLT (group A: experimental group) | |||||
| Time point | Visit 1 (eligibility screening) |
Visit 2 (baseline) |
Visit 3 (3 days+1 day post-NC7) |
Visit 4 (3 weeks±3 days post-iSLT onset) |
Visit 5 (24 weeks±7 days post-iSLT onset) |
| Informed consent | √ | ||||
| Inclusion and exclusion criteria scrutiny | √ | ||||
| Demographic information | √ | ||||
| General physical examination | √ | ||||
| Primary outcome | √ | √ | √ | √ | |
| Secondary outcomes | √ | √ | √ | √ | |
| Safety outcomes | √ | √ | √ | √ | |
| Brain plasticity evaluation | √ | √ | √ | ||
| iSLT alone (group B: control group) | |||||
| Time point | Visit 1 (eligibility screening) |
Visit 2 (baseline) |
Visit 3 (3 days+1 day, waiting periods onset) |
Visit 4 (3 weeks±3 days post-iSLT onset) |
Visit 5 (24 weeks±7 days post-iSLT onset) |
| Informed consent | √ | ||||
| Inclusion and exclusion criteria scurtiny | √ | ||||
| Demographic information | √ | ||||
| General physical examination | √ | ||||
| Primary outcome | √ | √ | √ | √ | |
| Secondary outcomes | √ | √ | √ | √ | |
| Safety assessment | √ | √ | √ | √ | |
| Brain plasticity evaluation | √ | √ | √ | ||
iSLT, intensive speech and language therapy; NC7, C7 neurotomy.;
Participant timeline
Table 1 shows the overall study timeline including enrolment, intervention and assessment schedule.
Randomisation and blinding
This study’s stratified block randomisation process will be performed using an interactive web response system; the stratified factor will be the centre: Huashan Hospital, Fudan University; Shanghai Pudong Hospital; Huadong Hospital affiliated to Fudan University or Shanghai Xuhui Central Hospital. Because the intervention in this study includes surgery at the neck, the assessor-blinded method will be applied at the outcome evaluation stage. All patients will be required to wear a cervical collar to cover their neck during each evaluation, and the evaluation process which will be videotaped. A third-party independent team consisting of two trained evaluators will conduct the language function final scoring based on the videos.
Outcome measures
The primary outcome is the change in the total score of the Boston Naming Test (BNT-60) scale of groups A and B, from baseline (visit 2) to postintervention (visit 4). The BNT is a classic measurement tool for evaluating language function; BNT-60 is the international version. The BNT scale shows high concurrent validity with other standard naming ability assessment tools and is particularly suitable for the postacute/chronic phase after stroke aphasia. In this study, we used validated Chinese version of the BNT.32 33
Secondary outcomes include aphasia quotient, daily communication (using the Communication Activities of Daily Living-Third Edition (CADL-3) score), activities of daily living (ADL) (using the Barthel Index), speech language function assessment (using the International Classification of Functioning, Disability and Health (ICF)34 speech language function assessment), poststroke depression assessment (using the Stroke Aphasic Depression Questionnaire-hospital version) and surgical safety-related outcomes. The assessments performed to collect data on primary, secondary and surgical safety outcomes are listed in table 2.
Table 2.
Assessments for primary, secondary and safety outcome data collection
| Variable | Measure |
| Primary outcome | |
| Naming ability | Change in Boston Naming Test (BNT) score from baseline (visit 2) to postintervention (visit 4, after 3 weeks of iSLT). The BNT scale is a performance-based measure commonly used to assess the visual confrontation naming ability among adults with aphasia. Participants are shown pictures of common objects and asked to name each stimulus item within 20 s. The score rang is 0–60; higher scores mean better outcomes in naming ability. |
| Secondary outcome | |
| Aphasia quotient | Change in Western Aphasia Battery (WAB) score compared with baseline. The WAB scale is a weighted average of all subtest scores relating to spoken language. It consists of the sum of all subtest scores from the first four parts of the WAB (spontaneous speech, auditory verbal comprehension, repetition, and naming and word finding), recording the total average score and SD. The score range is 0–100; higher scores indicate better performance. |
| Daily communication | Change in the Communication Activities of Daily Living-Third Edition (CADL-3) compared with baseline. The CADL-3 scale contains 50 items assessing the functional communication skills of adults with neurogenic communication disorders in seven areas. The participants receive a score of 0, 1 or 2 for each item; higher scores reflect better communicative success. |
| Speech language function assessment | Change in International Classification of Functioning, Disability and Health (ICF) speech language function assessment compared with baseline. The aphasia-adapted ICF speech language function assessment will be used for self-evaluation of communication functions, participation and activity. The score rand is −2 to +2; higher scores mean better outcome in quality of life. |
| Activities of daily living | Change in Barthel Index score compared with baseline. |
| Poststroke depression assessment | Change in Stroke Aphasic Depression Questionnaire-hospital version compared with baseline. |
| Surgical safety outcomes | |
| Muscle strength | Change in Medical Research Council grading system score compared with baseline. |
| Spasticity | Change in the Modified Ashworth Scale score compared with baseline. |
| Range of motion | Change in range of motion of the main joints of the upper limbs score compared with baseline. |
| Sensory function assessment | Change in the tactile sensory threshold and 2-point discrimination score compared with baseline. |
iSLT, intensive speech and language therapy.
Brain plasticity evaluation
Explorative evaluations included brain functional plasticity detection using functional MRI (fMRI) and EEG. Resting-state, task-designed functional and structural MRI using a GE 3.0 T MRI scanner (MR750) will be collected at baseline, and at V2, V4 and V5 follow-ups. In the task-designed MRI and EEG evaluations, picture naming tasks and semantic prediction tasks will be used to assess patient recovery and the related central plasticity mechanism.
Adverse events
Patient safety will be monitored at each study visit. Patients will receive detailed information regarding who to contact in case of an adverse event. The investigators will record all descriptions of adverse events during each visit. In this clinical trial, severe adverse events (SAEs) will be considered death, life-threatening severe deterioration of health requiring inpatient hospitalisation or prolongation of current hospitalisation, and persistent or significant disability/incapacity requiring intervention to prevent permanent impairment or damage. Patients with SAEs will be withdrawn from the clinical trial, as it would be unsafe to continue the trial procedure. If an SAE occurs, investigators will take immediate treatment measures to ensure patient the safety and report to the institutional review board and relevant competent authorities within 24 hours.
Data collection and management
Data will be collected using an electronic data-capture system. The data administrator will be responsible for data management and revision. After the data will be checked and the database is confirmed to be correct, they will be locked and submitted for statistical analysis. The original functional neuroimaging dataset containing patient-identifying information will be presented as a disk, while the data anonymised equivalent will be saved and analysed.
Analyses
Sample size
As mentioned, the primary outcome of this study will be the change in naming score from baseline to postintervention. According to the literature,16 19 the naming score can be improved after intensive speech therapy and verified after non-invasive brain stimulation therapy.21 22 In the preprint data, which is a phase-I cohort study35 including patients with poststroke aphasia who received NC7 plus iSLT, the patient naming score evaluated by BNT was increased by 11.2 points on average, while that of the patients who received iSLT alone increased by 5.7 points on average from our literature review.36 According to results of our phase-I study, the SD was 6.2. With a two-sided significance level of 0.05, a sample size of 40 patients (20 per group) provided 80% statistical power to demonstrate the difference in the change in BNT scores compared with baseline. Considering a drop-out rate of 20%, 50 patients (25 per group) will recruit.
Statistical analysis
Analyses of the primary endpoints will be performed on the full analysis set, which will include all patients randomly allocated to the study treatment. The per-protocol set will include all patients who will have completed the study without major protocol deviations. Safety will be evaluated in all patients receiving study treatment and analysed using descriptive statistics.
Categorical data will be presented as frequencies and percentages, and continuous data as mean (SD) or median (IQR). Analysis of covariance will be used to conduct between-group comparisons of changes from baseline in primary and secondary outcomes. The baseline and centre values will be covariates. Generalised estimating equation models will be used to analyse the longitudinal data between the groups. Subgroup analyses will include study centre and type, aetiology and severity of aphasia. Sensitivity analysis will be performed on missing data for the primary endpoint. All hypothesis tests will be are two sided, and statistical significance will be considered at p<0.05.
Ethics and dissemination
Ethical approval for this trial was granted by the Institutional Review Board of Huashan Hospital, Fudan University, and by the institutional review boards of all participating institutions (online supplemental table 1). All patients will sign informed prior to enrolment. Patients may withdraw from the study at any time. Important protocol modifications will be communicated to the relevant members of the research team. All procedures will be performed in accordance with the principles of the Declaration of Helsinki. The study findings will be disseminated through peer-reviewed publications and conference presentations.
Patient and public involvement
Patients and the public were not involved in the design and conception of this study. The study results will be disseminated to the public upon completion of the trial and individual test results will be provided to patients upon request.
Discussion
Owing to the high morbidity and heavy disease burden of stroke in China,37 there is an urgent need for effective treatments of chronic poststroke aphasia. This current manuscript describes the methodology of a trial designed to evaluate the effects of NC7 on language-impairment symptoms in patients with chronic poststroke aphasia. This study bears major importance because it could provide evidence for the validity of a novel therapeutic strategy for improving language function while attenuating stroke-related dysfunctions.
This study focuses on the evaluation of postoperative language function. Language function can be assessed using naming tests and communication ability assessments, for instance. Patients with aphasia who receive iSLT or non-invasive peripheral stimulation can exhibit improvements in naming ability38 and social communication17; however, the effect sizes are usually modest. In a previous study, we found that the naming ability of patients after CC7 significantly improved, and many other researchers have also used the suitable correct spontaneous naming scores as the only BNT-related index for evaluating language function.39 40 In this study, we will use the BNT scale as an evaluation index for language function as the primary outcome and the WAB, CADL Communication Scale, ADL Scale and Post-Stroke Depression Scale scores as secondary indicators of language repetition, listening comprehension, communication ability, daily life and psychological status. The ICF speech and language function assessment can detect the degree of changes in voice intonation, oral motor ability, articulation intelligibility and oral expression to exclude the possible reduction of spasticity after neurotomy for the interference with study results.
CC7 was first invented by Gu et al41–43 to treat limb dysfunction after brachial plexus injury, and the follow-up work showed neuroplastic changes between the hemispheres after surgery.44 45 Based on this theoretical perspective, Xu et al originally proposed the scientific viewpoint that ‘One hemisphere controls both limbs’ and expanded the development of the ‘CC7 nerve transfer’ to ‘CC7–C7 cross nerve transfer’ for treating central hemiplegia.46–48 Our previous investigations suggested the possible correlation between CC7 and the improvement of chronic aphasia, an effect occurring in the early postoperative period.35 Thus, the possibility of aphasia recovery through NC7 at the intervertebral foramen caught our attention. In this study, we measured the muscle strength, joint range of motion, upper limb Modified Ashworth Scale score and sensory assessment as safety indicators to evaluate the effects of NC7 on the right side. If NC7 does improve language function without physical dysfunction, it will provide an entirely novel perspective for the treatment of chronic poststroke aphasia. However, the mechanisms underlying NC7 efficacy in chronic aphasia are not fully understood. We believe that this clinical improvement in language function is related to NC7 stimulating neuroplasticity. This requires more objective functional imaging evidence. Several recent studies have reviewed the mechanisms of underlying the recovery from aphasia. In some cases, the therapy mechanisms49 50 are evidenced by changes in task-related brain activations or changes in functional connectivity within functional networks.51 52 Here, we will use fMRI and EEG methods in relation to naming ability and semantic prediction to investigate the neural and physiological states induced by changes in language function after NC7.
In conclusion, this will be the first RCT to evaluate the effect of surgery in patients with chronic poststroke aphasia for whom no effective treatment is available. If found to be efficient, this strategy can be implemented regularly because of its ease of application and low cost. Moreover, larger trials should be extended to other diseases with central nerve injuries to assess the positive effects of this strategy on language and other functions. Once our hypothesis is confirmed, this trial will provide important evidence to support the use of NC7 at the intervertebral foramen as a novel treatment approach for chronic aphasia. A limitation of this study is that it is not double-blind but evaluator-blind, and the experimental group may experience a minor placebo effect. Nevertheless, a secondary endpoint assessment at 6 months postintervention is scheduled to be conducted to offset the short-term postsurgery placebo effect. At that time, the patient’s placebo effect due to invasive interventions will be greatly reduced.
Supplementary Material
Acknowledgments
We would like to thank all the study participants, our colleagues at Huashan Hospital, Fudan University and all the participating institutions.
Footnotes
Contributors: WX is the principal investigator of this study and refined the protocol. TL and JF wrote the manuscript and contributed to the design of the study. ML the medical statistician for the study, contributed to the statistical design and ethical approval. WC contributed to brain plasticity evaluation design and guidance. YG participated in the concept and design of the study. RH, XM, WQ, YZ, XC and LD have revised the protocol critically for multicentre intellectual content. All authors read and approved the final manuscript.
Funding: This work was supported by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-007), Chinese Academy of Medical Sciences Special Project of Clinical and Translational Medicine research cultivating program (2021-I2M-C&T-B-100), National Natural Science Foundation of China (81830063, 82021002) and Shanghai Clinical Research Center for Aging and Medicine (19MC1910500).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Stefaniak JD, Halai AD, Lambon Ralph MA. The neural and neurocomputational bases of recovery from post-stroke aphasia. Nat Rev Neurol 2020;16:43–55. 10.1038/s41582-019-0282-1 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation 2017;135:e146–603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelter ST, Gostynski M, Papa S, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis [Stroke; a journal of cerebral circulation 2006;37(6):1379-84]. Stroke 2006;37:1379–84. 10.1161/01.STR.0000221815.64093.8c [DOI] [PubMed] [Google Scholar]
- 4.Hilari K. The impact of stroke: are people with aphasia different to those without? Disabil Rehabil 2011;33:211–8. 10.3109/09638288.2010.508829 [DOI] [PubMed] [Google Scholar]
- 5.Pollock A, St George B, Fenton M, et al. Top ten research priorities relating to life after stroke. Lancet Neurol 2012;11:209. 10.1016/S1474-4422(12)70029-7 [DOI] [PubMed] [Google Scholar]
- 6.Flowers HL, Skoretz SA, Silver FL, et al. Poststroke aphasia frequency, recovery, and outcomes: a systematic review and meta-analysis. Arch Phys Med Rehabil 2016;97:2188–201. 10.1016/j.apmr.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Bonilha HS, Simpson AN, Ellis C, et al. The one-year attributable cost of post-stroke dysphagia. Dysphagia 2014;29:545–52. 10.1007/s00455-014-9543-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doucet T, Muller F, Verdun-Esquer C, et al. Returning to work after a stroke: a retrospective study at the physical and rehabilitation medicine center La tour de gassies. Ann Phys Rehabil Med 2012;55:112–27. 10.1016/j.rehab.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 9.Yagata SA, Yen M, McCarron A, et al. Rapid recovery from aphasia after infarction of Wernicke’s area. Aphasiology 2017;31:951–80. 10.1080/02687038.2016.1225276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkana O, Frost R, Kramer U, et al. Cerebral language reorganization in the chronic stage of recovery: a longitudinal fMRI study. Cortex 2013;49:71–81. 10.1016/j.cortex.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 11.Pedersen PM, Jørgensen HS, Nakayama H, et al. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol 1995;38:659–66. 10.1002/ana.410380416 [DOI] [PubMed] [Google Scholar]
- 12.Laska AC, Hellblom A, Murray V, et al. Aphasia in acute stroke and relation to outcome. J Intern Med 2001;249:413–22. 10.1046/j.1365-2796.2001.00812.x [DOI] [PubMed] [Google Scholar]
- 13.El Hachioui H, Visch-Brink EG, de Lau LML, et al. Screening tests for aphasia in patients with stroke: a systematic review. J Neurol 2017;264:211–20. 10.1007/s00415-016-8170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wade DT, Hewer RL, David RM, et al. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry 1986;49:11–6. 10.1136/jnnp.49.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fridriksson J, Hillis AE. Current approaches to the treatment of post-stroke aphasia. J Stroke 2021;23:183–201. 10.5853/jos.2020.05015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brady MC, Kelly H, Godwin J, et al. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev 2016;2016:CD000425. 10.1002/14651858.CD000425.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breitenstein C, Grewe T, Flöel A, et al. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet 2017;389:1528–38. 10.1016/S0140-6736(17)30067-3 [DOI] [PubMed] [Google Scholar]
- 18.Doppelbauer L, Mohr B, Dreyer FR, et al. Long-Term stability of short-term intensive language-action therapy in chronic aphasia: a 1-2 year follow-up study. Neurorehabil Neural Repair 2021;35:861–70. 10.1177/15459683211029235 [DOI] [PubMed] [Google Scholar]
- 19.Stahl B, Mohr B, Büscher V, et al. Efficacy of intensive aphasia therapy in patients with chronic stroke: a randomised controlled trial. J Neurol Neurosurg Psychiatry 2018;89:586–92. 10.1136/jnnp-2017-315962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving aphasia in adults with aphasia after stroke. Cochrane Database Syst Rev 2019;5:CD009760. 10.1002/14651858.CD009760.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fridriksson J, Rorden C, Elm J, et al. Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: a randomized clinical trial. JAMA Neurol 2018;75:1470–6. 10.1001/jamaneurol.2018.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meinzer M, Darkow R, Lindenberg R, et al. Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain 2016;139(Pt 4):1152–63. 10.1093/brain/aww002 [DOI] [PubMed] [Google Scholar]
- 23.Heikkinen PH, Pulvermüller F, Mäkelä JP, et al. Combining rTMS with intensive language-action therapy in chronic aphasia: a randomized controlled trial. Front Neurosci 2018;12:1036. 10.3389/fnins.2018.01036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng J, Li T, Lv M, et al. Reconstruction of paralyzed arm function in patients with hemiplegia through contralateral seventh cervical nerve cross transfer: a multicenter study and real-world practice guidance. EClinicalMedicine 2022;43:101258. 10.1016/j.eclinm.2021.101258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maas MB, Lev MH, Ay H, et al. The prognosis for aphasia in stroke. J Stroke Cerebrovasc Dis 2012;21:350–7. 10.1016/j.jstrokecerebrovasdis.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen-Dong X. n.d. Surgical technique of Xu’s CC7 procedure "contralateral C7 to C7 cross nerve transfer through a trans longus colli, prespinal route for treating spastic arm. Operative Neurosurgery;2020. [DOI] [PubMed] [Google Scholar]
- 27.Leijnse JN, D’Herde K. Revisiting the segmental organization of the human spinal cord. J Anat 2016;229:384–93. 10.1111/joa.12493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi K, Ninomiya T. Morphological changes of dorsal root ganglion cells in the process-forming period. Prog Neurobiol 1987;29:393–410. 10.1016/0301-0082(87)90020-7 [DOI] [PubMed] [Google Scholar]
- 29.Zhong L-Y, Wang A-P, Hong L, et al. Microanatomy of the brachial plexus roots and its clinical significance. Surg Radiol Anat 2017;39:601–10. 10.1007/s00276-016-1784-9 [DOI] [PubMed] [Google Scholar]
- 30.Gesslbauer B, Hruby LA, Roche AD, et al. Axonal components of nerves innervating the human arm. Ann Neurol 2017;82:396–408. 10.1002/ana.25018 [DOI] [PubMed] [Google Scholar]
- 31.Mille-Hamard L, Bauchet L, Baillet-Derbin C, et al. Estimation of the number and size of female adult rat C4, C5 and C6 dorsal root ganglia (DRG) neurons. Somatosensory & Motor Research 1999;16:223–8. 10.1080/08990229970474 [DOI] [PubMed] [Google Scholar]
- 32.Cheung RW, Cheung MC, Chan AS. Confrontation naming in Chinese patients with left, right or bilateral brain damage. J Int Neuropsychol Soc 2004;10:46–53. 10.1017/S1355617704101069 [DOI] [PubMed] [Google Scholar]
- 33.Chen T-B, Lin C-Y, Lin K-N, et al. Culture qualitatively but not quantitatively influences performance in the Boston naming test in a Chinese-speaking population. Dement Geriatr Cogn Dis Extra 2014;4:86–94. 10.1159/000360695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pike C, Kritzinger A, Pillay B. Social participation in working-age adults with aphasia: an updated systematic review. Top Stroke Rehabil 2017;24:627–39. 10.1080/10749357.2017.1366012 [DOI] [PubMed] [Google Scholar]
- 35.Feng J, Ma X, Hu R, et al. Improvement of language function after C7 neurotomy at the intervertebral foramen in patients with chronic post-stroke aphasia: a phase I cohort study. Surgery [Preprint]. 10.1101/2023.03.22.23287523 [DOI]
- 36.The REhabilitation and recovery of peopLE with Aphasia after StrokE (RELEASE) Collaborators, Brady MC, Ali M, et al. Dosage, intensity, and frequency of language therapy for aphasia: a systematic review–based, individual participant data network meta-analysis. Stroke 2022;53:956–67. 10.1161/STROKEAHA.121.035216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. The Lancet 2019;394:1145–58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridriksson J, den Ouden D-B, Hillis AE, et al. Anatomy of aphasia revisited. Brain 2018;141:848–62. 10.1093/brain/awx363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi A, Burkhart C, Dell-Kuster S, et al. Serum anticholinergic activity and postoperative cognitive dysfunction in elderly patients. Anesth Analg 2014;119:947–55. 10.1213/ANE.0000000000000390 [DOI] [PubMed] [Google Scholar]
- 40.Nousia A, Siokas V, Aretouli E, et al. Beneficial effect of multidomain cognitive training on the neuropsychological performance of patients with early-stage Alzheimer’s disease. Neural Plast 2018;2018:2845176. 10.1155/2018/2845176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu Y-D, Zhang G-M, Chen D-S, et al. Seventh cervical nerve root transfer from the contralateral healthy side for treatment of brachial plexus root avulsion. Journal of Hand Surgery 1992;17:518–21. 10.1016/S0266-7681(05)80235-9 [DOI] [PubMed] [Google Scholar]
- 42.Gu Y, Xu J, Chen L, et al. Long term outcome of contralateral C7 transfer: a report of 32 cases. Chin Med J (Engl) 2002;115:866–8. [PubMed] [Google Scholar]
- 43.Gu Y. Contralateral C7 root transfer over the last 20 years in China. Chin Med J (Engl) 2007;120:1123–6. [PubMed] [Google Scholar]
- 44.Hua X-Y, Liu B, Qiu Y-Q, et al. Long-Term ongoing cortical remodeling after contralateral C-7 nerve transfer. J Neurosurg 2013;118:725–9. 10.3171/2012.12.JNS12207 [DOI] [PubMed] [Google Scholar]
- 45.Jiang S, Li Z-Y, Hua X-Y, et al. Reorganization in motor cortex after brachial plexus avulsion injury and repair with the contralateral C7 root transfer in rats. Microsurgery 2010;30:314–20. 10.1002/micr.20747 [DOI] [PubMed] [Google Scholar]
- 46.Zheng M-X, Hua X-Y, Feng J-T, et al. Trial of contralateral seventh cervical nerve transfer for spastic arm paralysis. N Engl J Med 2018;378:22–34. 10.1056/NEJMoa1615208 [DOI] [PubMed] [Google Scholar]
- 47.Xu W-D, Hua X-Y, Zheng M-X, et al. Contralateral C7 nerve root transfer in treatment of cerebral palsy in a child: case report. Microsurgery 2011;31:404–8. 10.1002/micr.20877 [DOI] [PubMed] [Google Scholar]
- 48.Hua X-Y, Qiu Y-Q, Li T, et al. Contralateral peripheral neurotization for hemiplegic upper extremity after central neurologic injury. Neurosurgery 2015;76:187–95. 10.1227/NEU.0000000000000590 [DOI] [PubMed] [Google Scholar]
- 49.Nardo D, Holland R, Leff AP, et al. Less is more: neural mechanisms underlying anomia treatment in chronic aphasic patients. Brain 2017;140:3039–54. 10.1093/brain/awx234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crinion JT, Leff AP. Using functional imaging to understand therapeutic effects in poststroke aphasia. Curr Opin Neurol 2015;28:330–7. 10.1097/WCO.0000000000000217 [DOI] [PubMed] [Google Scholar]
- 51.Kiran S, Meier EL, Johnson JP. Neuroplasticity in aphasia: a proposed framework of language recovery. J Speech Lang Hear Res 2019;62:3973–85. 10.1044/2019_JSLHR-L-RSNP-19-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartwigsen G, Saur D. Neuroimaging of stroke recovery from aphasia – insights into plasticity of the human language network. NeuroImage 2019;190:14–31. 10.1016/j.neuroimage.2017.11.056 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065173supp001.pdf (76.9KB, pdf)