Abstract
Background:
Eosinophilic esophagitis (EoE) can co-exist in individuals with food allergy.
Objective:
To evaluate the characteristics of food allergic patients with and without co-existing EoE using a large food allergy patient registry.
Methods:
Data were derived from two Food Allergy Research and Education (FARE) Patient Registry surveys. A series of multivariable regression models were used to evaluate associations between demographic, comorbidity, and food allergy characteristics and the likelihood of reporting EoE.
Results:
Five percent (n=309) of registry participants (n=6,074; ages <1 year->80 years, mean[SD] 20.20[15.37]), reported having EoE. The odds of having EoE were significantly greater in male participants (aOR=1.3, 95%CI: 1.04-1.72) and those with comorbid asthma (aOR=2.0, 95%CI: 1.55-2.49), allergic rhinitis (aOR=1.8, 95%CI: 1.37-2.22), oral allergy syndrome (aOR=2.8, 95%CI: 2.09-3.70), food protein-induced enterocolitis syndrome (aOR=2.5, 95%CI: 1.34-4.84), and hyper-IgE syndrome (aOR=7.6, 95%CI: 2.93-19.92), though not atopic dermatitis (aOR=1.3, 95%CI: 0.99-1.59), when adjusting for demographics (sex, age, race, ethnicity, and geographic location). Those with a greater number of food allergies (aOR=1.3, 95%CI: 1.23-1.32), more frequent food-related allergic reactions (aOR=1.2, 95%CI: 1.11-1.24), previous anaphylaxis (aOR=1.5, 95%CI: 1.15-1.83), and healthcare utilization for food-related allergic reactions (aOR=1.3, 95%CI: 1.01-1.67) – specifically ICU admission (aOR=1.2, 95%CI: 1.07-1.33) – were more likely to have EoE after controlling for demographics. However, no significant difference in ever using epinephrine for food-related allergic reactions was detected.
Conclusion:
These self-reported data showed that co-existing EoE is associated with an increased number of food allergies, food-related allergic reactions per year, and measures of reaction severity, calling attention to the likely increased healthcare needs of food allergic patients with EoE.
Keywords: Eosinophilic esophagitis, food allergy, food allergy registry, food allergic reactions, comorbidities
Introduction
The co-existence of eosinophilic esophagitis (EoE) and immunoglobulin E (IgE)-mediated food allergy is commonly encountered in the clinical setting, though the interplay between these diseases is still incompletely understood. Studies of EoE cohorts indicate that food allergy occurs more frequently in those with EoE1–8, and up to ~25% experience anaphylaxis.2,8 Additionally, EoE appears to occur significantly more often in those with food allergy – with Hill et al reporting 4.7% of food allergic patients in their large database having co-existing EoE versus ~0.05-0.1% in the general population.3,7,9,10
As the field of allergy and immunology has evolved, it has come to recognize ‘food allergy’ as encompassing a wide range of immunologic mechanisms – from the classic IgE-mediated reactions to non-IgE-mediated conditions. As such, EoE could be considered a unique form of food allergy, as it is felt to be food allergen-driven given the frequent success of food elimination diets in achieving disease control.1,11–17 However, the predominant immune mechanism seems to involve a non-IgE-mediated process – supported by the often inability to identify food triggers with IgE-focused testing and the lack of efficacy of omalizumab (an anti-IgE monoclonal antibody) in disease management.13,18–22
Prior studies have evaluated patients with EoE with and without IgE-mediated food allergy with a primary focus on comparison of EoE characteristics.23,24 Pelz et al found that those with EoE and food allergy presented at a younger age and had increased EoE-related symptoms – specifically dysphagia,gagging, cough, and poor appetite – compared to those with EoE without food allergy.24 However, characterization of food allergy in those with EoE is lacking.
Herein, we sought to utilize the Food Allergy Research & Education (FARE) Patient Registry25,26 to evaluate the likelihood of food allergic participants having EoE given specific personal or food allergy characteristics to determine whether there are associations that may impact the future consideration, evaluation, and management of food allergy in those with EoE. We hypothesized that those with a greater number of food allergies, more severe and frequent food-related allergic reactions, and increased healthcare utilization for these reactions – potentially representing a more severe food allergy phenotype – would have greater odds of having EoE.
Methods
Study Methods
Food allergy survey data from 6,139 participants enrolled in the FARE Patient Registry (See Supplemental Methods in Online Repository for registry details) between its launch in May 2017 and data extraction in December 2020 were reviewed. Data were obtained primarily from the FARE Food Allergy History Survey – a 44-item electronic questionnaire eliciting information on demographics; comorbidities; food allergy diagnosis history; specific food allergies (14 major food allergen categories with subcategories when applicable); food-related allergic reaction symptoms, frequency, and treatment; healthcare utilization; and food allergy resolution. Additional reaction severity data was obtained from the FARE Food Allergy Reactions Survey – a 61-item electronic questionnaire focusing on the participant’s most recent food-related allergic reaction (See Online Repository for surveys). All registry participants/enrollees were prompted to complete the FARE Food Allergy History Survey. However, additional surveys, such as the FARE Food Allergy Reactions Survey, were completed at the volition of the participant/enrollee. Consequently, the total number of participants who had completed the FARE Food Allergy Reactions Survey was lower (n=4,676 participants).
Participants with insufficient data or no apparent food allergy (n=65) were excluded (See Supplemental Methods in Online Repository). All participant responses were taken at face value without additional manual curation. Participants were divided into 2 subsets based on self-report of co-existing EoE – those who selected EoE (+EoE) and those who did not select EoE (−EoE) when asked whether they had been diagnosed with any of a list of conditions.
Statistical Analyses
Analyses based on data derived from the FARE Food Allergy History Survey included 6,074 participants (+EoE=309; −EoE=5,765). Only participants who also had completed the FARE Food Allergy Reactions Survey were included in the reaction severity analyses (n=4,075; +EoE=182; −EoE=3,893). Nominal age range values for ages <1 year and >80 years were converted to a representative numeric value (Supplemental Methods in the Online Repository). The geographic locations of the participants are shown in detail in the demographics table (Table I), but only the two main categories of “United States” and “International” were used during model fitting. Similarly, for race and ethnicity, detailed information is provided in the demographics table, but, because of limited sample sizes, the following categories were utilized during model fitting: “Asian”, “Black or African American”, “Multiracial” (representing participants with more than one race category selected, not including “Unknown”), “White”, and “Other or Unknown”. The “Other or Unknown” category included “American Indian or Alaska Native”, “Native Hawaiian or Other Pacific Islander”, “Unknown” and no entry. Overall, the responses “Unknown”, “Prefer not to answer”, and no entry were treated as one category in all statistical analyses. Responses for reaction severity and number of reactions per year were coded as integers for model fitting. Comorbidities or food allergens that were reported by less than 1% of participants in both study groups were excluded from the analyses and noted in footnotes of the respective tables.
Table I.
Demographics of food allergic registry participants with and without co-existing eosinophilic esophagitis
| Demographics | −EoE n=5765 (95%) | +EoE n=309 (5%) | P value1 |
|---|---|---|---|
| Sex | |||
| Female | 3279 (57%) | 160 (52%) | 0.087 |
| Male | 2486 (43%) | 149 (48%) | |
| Current Age at Survey (years) | |||
| Mean (SD) | 19.42 (18.64) | 20.20 (15.37) | 0.39 |
| Range | 0.01-80.00 | 0.5-78.00 | |
| Age at Food Allergy Diagnosis (years) | |||
| Mean (SD)2 | 8.82 (14.77) | 8.13 (13.37) | 0.38 |
| Range | 0.01-76.00 | 0.01-66.00 | |
| Race | |||
| American Indian/Alaska Native | 27 (<1%) | 0 | 0.045 |
| Asian | 221 (4%) | 3 (1%) | |
| Black/African American | 161 (3%) | 8 (3%) | |
| Native Hawaiian/Other Pacific Islander | 8 (<1%) | 0 | |
| White | 4772 (83%) | 271 (88%) | |
| Multiracial | 426 (7%) | 20 (6%) | |
| Unknown/No entry | 150 (2%) | 7 (<1%) | |
| Ethnicity | |||
| Hispanic/Latino | 433 (8%) | 21 (7%) | 0.77 |
| Not Hispanic/Latino | 3861 (67%) | 204 (66%) | |
| Unknown/Prefer not to answer | 1471 (26%) | 84 (1%) | |
| Geographic Location | |||
| United States3 | 5500 (95%) | 300 (97%) | 0.204 |
| Northeast | 1423 (25%) | 69 (23%) | |
| Midwest | 1329 (23%) | 78 (26%) | |
| South | 1662 (29%) | 82 (27%) | |
| West | 1050 (18%) | 69 (23%) | |
| Guam | 1 (<1%) | 0 (<1%) | |
| Puerto Rico | 3 (<1%) | 0 (<1%) | |
| Armed Forces abroad | 1 (<1%) | 1 (<1%) | |
| No state specified | 31 (1%) | 1 (<1%) | |
| Africa | 7 (<1%) | 0 | |
| Asia | 22 (1%) | 1 (<1%) | |
| Australia | 10 (<1%) | 0 | |
| Canada | 96 (2%) | 4 (1 %) | |
| Caribbean Islands | 6 (<1%) | 0 | |
| Central America | 3 (<1%) | 0 | |
| Europe | 85 (2%) | 1 (<1%) | |
| Mexico | 7 (<1%) | 3 (1%) | |
| Middle East | 13 (<1%) | 0 | |
| New Zealand | 2 (<1%) | 0 | |
| South America | 14 (<1%) | 0 | |
EoE: eosinophilic esophagitis; SD: standard deviation
P values obtained by t test for continuous variables and Fisher’s exact test for categorical variables.
−EoE n=5557 and +EoE n=304 for this variable due to missing data.
Regions of the United States as defined by the U.S. Census Bureau.
P value represents comparison of United States versus international participants.
Demographic variables were compared between the −EoE and +EoE subsets using Fisher’s exact test or Student’s t-test. Further, a series of multivariable logistic regression models in several stages were used. In the first stage, all demographic variables in Table I, except age at food allergy diagnosis, were evaluated in a multivariable logistic regression model with EoE as response variable (glm function from R package stats, version 4.1.0) in order to estimate independent associations with the probability of EoE. The category with the largest n was used as respective reference during model fitting. Age at food allergy diagnosis was excluded from all regression models because it was missing for 213 participants. In the second stage, one multivariable model was fitted for each variable of interest. For this, each variable of interest was added individually as main predictor variable to the demographics base model. That way, the associations between EoE and the individual variables were estimated while controlling for sex, age, race, ethnicity, and geographic location. In the last stage, sets of variables of interest were added as main predictors to the demographics base model. The first set included all comorbidities. The second included all 14 major food allergen categories and “Other” food allergen. A free text field was available for “Other” food allergen but these data were not manually curated for the analyses. The results of the last stage as well as the demographics base model were represented as forest plots (R package forestplot, version 2.0.1). Adjusted odds ratios (aORs) with 95% confidence intervals (CIs) were reported for the independent variables modeled.
All statistical tests were performed two-sided, and P<0.05 was considered significant. The P values for the variables of interest from the models in the second stage were adjusted for multiple comparisons within each set of variables represented as one table (i.e. within all comorbidities) using the Benjamini and Hochberg approach to control the false discovery rate (FDR). FDR-adjusted P(Adj.P)<0.05 were considered significant. In these cases, 95% CIs were not adjusted for multiplicity, and the conclusions were based on the adjusted P values and not the CIs.27 All statistical analyses were performed using R software (version 4.1.0), and plotting of the data was done using the ggplot2 R package (version 3.3.5) unless otherwise stated. Mosaic plots were created using the R package ggmosaic (version 0.3.3).
Results
Study Population
A total of 6,074 FARE Patient Registry participants were included in the analyses, ranging in age from <1 year to >80 years with a mean of 19.46 years (standard deviation [SD] 18.49, median 13 years). There was an overall slight female predominance (57%, n=3,439). Geographically, most participants reported residing in the United States (95%, n=5,800) with the remaining 5% (n=274) composed of individuals from international locations (Table I). Race composition was largely White (83%, n=5,043), followed by Multiracial (7%, n=446), Asian (4%, n=224), and Black or African American (3%, n=169). Seven percent (n=454) identified as Hispanic or Latino; 67% (n=4,065) were not Hispanic or Latino; and the remaining 26% (n=1,555) selected “Unknown” or “Prefer not to answer”.
Demographics and Comorbidities of Food Allergic Participants with and without Co-existing EoE
Five percent (n=309) of food allergic participants reported having co-existing EoE (+EoE); the remaining 95% (n=5,765) did not select EoE as a comorbidity (−EoE) (Table I). Current age at time of survey was not significantly different between subsets (+EoE: mean[SD] 20.20 years [15.37], median 15 years; −EoE: mean[SD] 19.42 [18.64], median 13 years; Figure E1) (P=0.39), nor was age at food allergy diagnosis (mean[SD] +EoE 8.13 [13.37], −EoE 8.82 [14.77]) (P=0.38). The subsets also did not differ in sex (P=0.087) – with both having a slight female predominance (+EoE female 52% (n=160), −EoE female 57% (n=3,279)). However, when stratified by whether the enrollee was responding for self or on behalf of another individual, there was a female predominance in the self-responding participants, but a male predominance in the non-self-responding participants (Figure E2). As expected, the reported age was lower for the non-self-respondents (median age=6 years, IQR=9 years) compared to the self-respondents (median age=36 years, IQR=25 years). Furthermore, in the non-self-respondents, a significantly (P=0.026) larger proportion of those with EoE were male (n=124, 70%) compared to those without EoE (n=2,163, 62%). Similarly, in the self-respondents, the proportion of male participants was greater in the +EoE subset (n=25, 19%) than in the −EoE subset (n=318, 14%), though the difference did not reach statistical significance (P=0.13).
To study the odds of reporting EoE when taking all demographic variables into account, a multivariable logistic regression including sex, age, race, ethnicity, and geographic location was used (Figure 1). In this model, male participants were more likely to have EoE than female participants (P=0.025, aOR=1.3, 95% CI: 1.04-1.72), and those who identified as Asian were less likely to report EoE than those who identified as White (P=0.016, aOR=0.24, 95% CI: 0.078-0.77) – both consistent with previous literature.3,7,8,28–34 All further reported aORs were adjusted for these demographic factors (sex, age, race, ethnicity, and geographic location).
Figure 1. Independent associations of demographic characteristics with the odds of reporting co-existing eosinophilic esophagitis (EoE).
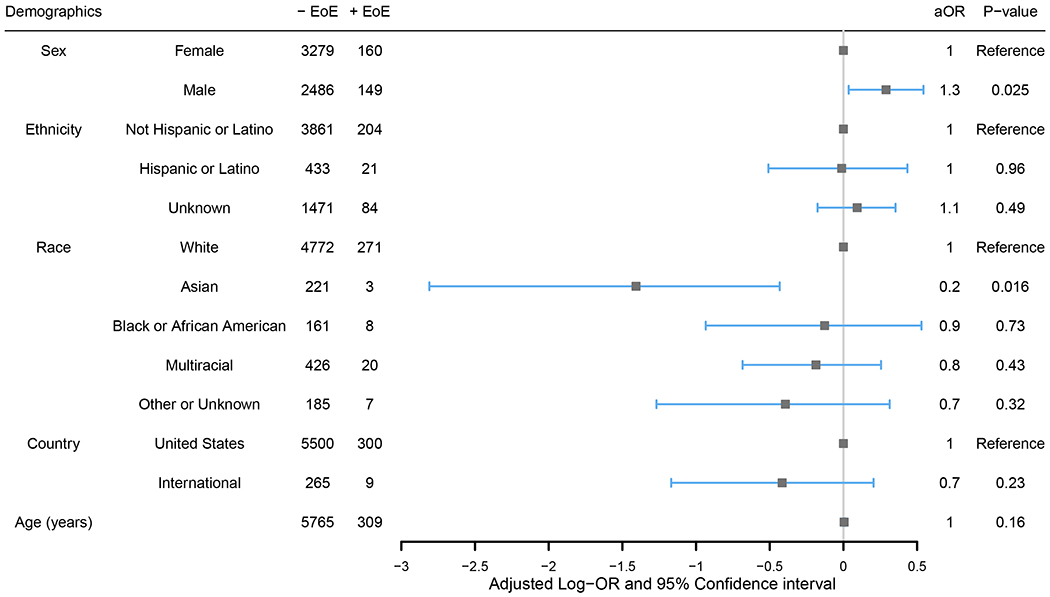
Evaluation was conducted through a multivariable logistic regression including all shown demographic characteristics. Adjusted odds ratios (aOR) are shown. Log-aOR>0 (equivalent to aOR>1) denotes a higher likelihood of reporting EoE.
In analysis of participant personal characteristics, having a close relative (parent or sibling) with history of food allergy was associated with co-existing EoE (P=3.5e-09, aOR=2.1, 95% CI: 1.63-2.66). The odds of having EoE were also higher for participants reporting several allergic/immune-mediated comorbidities, including asthma (Adj.P=3.0e-07, aOR=2.0, 95% CI: 1.55-2.49), allergic rhinitis (Adj.P=3.9e-05, aOR=1.8, 95% CI: 1.37-2.22), oral allergy syndrome (Adj.P=3.8e-11, aOR=2.8, 95% CI: 2.09-3.70), food protein-induced enterocolitis syndrome (FPIES) (Adj.P=0.014, aOR=2.5, 95% CI: 1.34-4.84), and hyper-IgE syndrome (Adj.P=1.7e-04, aOR=7.6, 95% CI: 2.93-19.92) after controlling for sex, age, race, ethnicity, and geographic location (Table II). However, notably, there was no significant difference in comorbid atopic dermatitis (Adj.P=0.099, aOR=1.3, 95% CI: 0.99-1.59). While total numbers were low, it was additionally noted that several non-atopic conditions were associated with having EoE as well, such as arrhythmias and migraines (Table II). Only one of the participants with EoE reported having no comorbidities besides food allergy, while 478 participants without EoE reported no additional comorbidities (Adj.P=0.0046, aOR=0.037, 95% CI: 0.0052-0.26).
Table II.
Adjusted1 odds of eosinophilic esophagitis for food allergic registry participants with specific comorbidities
| Comorbidities2 | −EoE n=5765 | +EoE n=309 | Adjusted OR (95% CI) | FDR-adjusted P value |
|---|---|---|---|---|
| Allergic/Immune-Mediated Conditions | ||||
| Allergic rhinitis | 2235 (39%) | 161 (52%) | 1.8 (1.37, 2.22) | 3.9e-05 |
| Asthma | 2603 (45%) | 192 (62%) | 2.0 (1.55, 2.49) | 3.0e-07 |
| Atopic dermatitis | 2751 (48%) | 163 (53%) | 1.3 (0.99, 1.59) | 0.099 |
| Bee sting allergy | 271 (5%) | 17 (6%) | 1.2 (0.68, 1.96) | 0.73 |
| Contact dermatitis | 785 (14%) | 55 (18%) | 1.4 (1.00, 1.84) | 0.092 |
| Drug allergy | 1163 (20%) | 78 (25%) | 1.4 (1.01, 1.81) | 0.09 |
| Food protein-induced enterocolitis syndrome | 85 (1%) | 11 (4%) | 2.5 (1.34, 4.84) | 0.014 |
| Hyper-IgE syndrome | 16 (<1%) | 6 (2%) | 7.6 (2.93, 19.92) | 1.7e-04 |
| Latex allergy | 375 (7%) | 28 (9%) | 1.5 (0.95, 2.21) | 0.12 |
| Mast cell disorder | 61 (1%) | 3 (1%) | 0.91 (0.28, 2.93) | 0.93 |
| Oral allergy syndrome | 582 (10%) | 72 (23%) | 2.8 (2.09, 3.70) | 3.8e-11 |
| Cardiovascular Conditions | ||||
| Arrhythmia | 135 (2%) | 17 (6%) | 2.4 (1.41, 4.14) | 0.0052 |
| Heart defects | 96 (2%) | 6 (2%) | 1.2 (0.51, 2.72) | 0.79 |
| High blood pressure | 305 (5%) | 18 (6%) | 0.99 (0.58, 1.71) | 0.98 |
| Hypertension | 152 (3%) | 10 (3%) | 1.1 (0.57, 2.25) | 0.79 |
| Endocrinologic Conditions | ||||
| Thyroid disease | 291 (5%) | 16 (5%) | 0.98 (0.56, 1.70) | 0.96 |
| Type 2 diabetes mellitus | 90 (2%) | 1 (<1%) | 0.17 (0.02, 1.25) | 0.12 |
| Gastrointestinal Conditions | ||||
| Celiac disease | 139 (2%) | 12 (4%) | 1.6 (0.88, 2.98) | 0.17 |
| Gluten sensitivity | 369 (6%) | 40 (13%) | 2.3 (1.62, 3.36) | 3.9e-05 |
| Heartburn | 1024 (18%) | 129 (42%) | 3.8 (2.92, 4.88) | 9.3e-23 |
| Inflammatory bowel disease | 62 (1%) | 10 (3%) | 3.0 (1.49, 5.88) | 0.0071 |
| Irritable bowel syndrome | 436 (8%) | 29 (9%) | 1.3 (0.83, 1.94) | 0.37 |
| Lactose intolerance | 531 (9%) | 40 (13%) | 1.5 (1.06, 2.17) | 0.053 |
| Neuropsychiatric Conditions | ||||
| Attention deficit hyperactivity Disorder | 427 (7%) | 34 (11%) | 1.5 (1.01, 2.12) | 0.090 |
| Autism | 94 (2%) | 10 (3%) | 1.9 (0.98, 3.71) | 0.099 |
| Migraines | 686 (12%) | 51 (17%) | 1.6 (1.10, 2.19) | 0.030 |
| Oncologic/Rheumatologic/Musculoskeletal Conditions | ||||
| Cancer | 114 (2%) | 3 (1%) | 0.41 (0.13, 1.34) | 0.19 |
| Connective tissue disorder | 72 (1%) | 10 (3%) | 2.6 (1.32, 5.15) | 0.017 |
| Rheumatoid arthritis | 81 (1%) | 6 (2%) | 1.4 (0.58, 3.21) | 0.61 |
| Osteoarthritis | 215 (4%) | 11 (4%) | 0.85 (0.44, 1.64) | 0.75 |
| Other comorbidity | 525 (9%) | 42 (14%) | 1.6 (1.10, 2.17) | 0.030 |
| No comorbidities | 478 (8%) | 1 (<1%) | 0.037 (0.0052, 0.26) | 0.0046 |
CI: confidence interval; EoE: eosinophilic esophagitis; FDR: false discovery rate; OR: odds ratio
Adjusted for sex, age, race, ethnicity, and geographic location in multivariable logistic regression models.
“Histamine toxicity”, “Stroke”, “Heart disease”, and “Type 1 diabetes mellitus” excluded due to <1% of participants reporting these comorbidities in both subsets.
To identify comorbidities that were independently associated with reporting EoE, a multivariable logistic regression analysis including demographic variables and all comorbidities was performed (Figure 2). Several of the comorbidities that were found to be associated with having EoE while not controlling for the other comorbidities also resulted in higher odds of EoE in this assessment. Specifically, participants that reported asthma (P=0.0012, aOR=1.5, 95% CI: 1.18-1.95), hyper-IgE syndrome (P=0.0075, aOR=4.5, 95% CI: 1.49-13.40), oral allergy syndrome (P=6.7e-06, aOR=2.0, 95% CI: 1.49-2.76), arrhythmia (P=0.038, aOR=1.9, 95% CI: 1.03-3.40), gluten sensitivity (P=0.0041, aOR=1.8, 95% CI: 1.21-2.73), or heartburn (P=6.3e-16, aOR=3.0, 95% CI: 2.32-3.99) were more likely to report EoE after adjustment for the other comorbidities and demographic variables.
Figure 2. Independent associations between comorbidities and reported EoE.
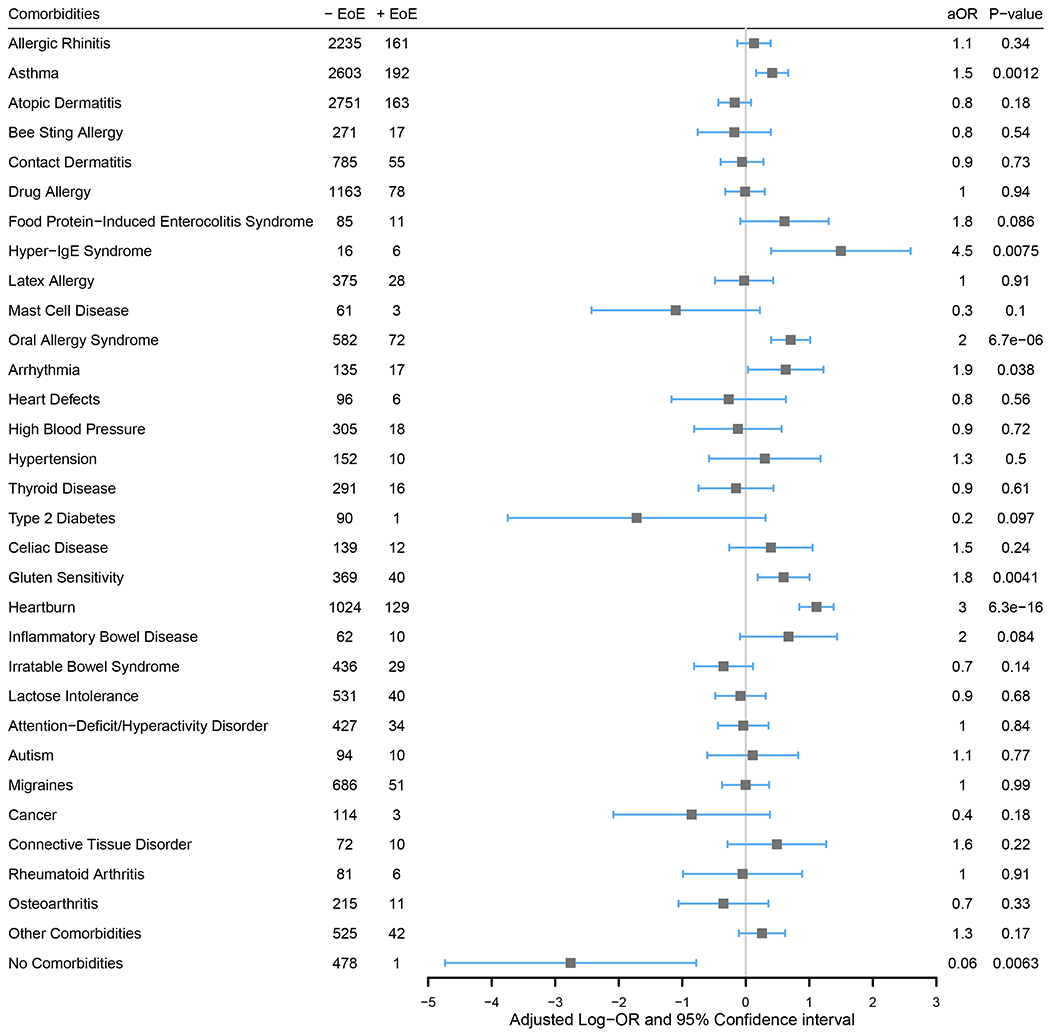
An adjusted OR (aOR) above 1 (equivalent to an adjusted log-OR above 0; gray line) denotes a higher odds of reporting EoE. One multivariable logistic regression model with all 32 shown comorbidity categories was fit while also controlling for sex, age, race, ethnicity, and geographic location.
Specific Food Allergies
The four most frequently reported major food allergen categories were the same for both +EoE and −EoE subsets – peanut, tree nuts, egg, and milk (Figure 3, Table E1). In individual multivariable logistic regression models for each of the major food allergen categories with adjustment for demographic variables, participants who reported the respective food allergy for 13 of the 14 major food allergens had higher odds of having EoE (Adj.P<0.05; Figure 3, Table E1). Peanut was the only major food allergen which showed no significant association with EoE (Adj.P=0.38, aOR=1.1, 95% CI: 0.87-1.47). See Table E1 for reported allergies by food allergen subcategories with aORs and 95% CIs. When comparing number of reported allergies to the 14 major food allergen categories or “Other” food allergen, participants with a greater number of food allergies were more likely to report EoE (P=7.5e-37, aOR=1.3, 95% CI: 1.23-1.32) (Figure 4).
Figure 3. Percentage reporting allergies to the major food allergen categories.
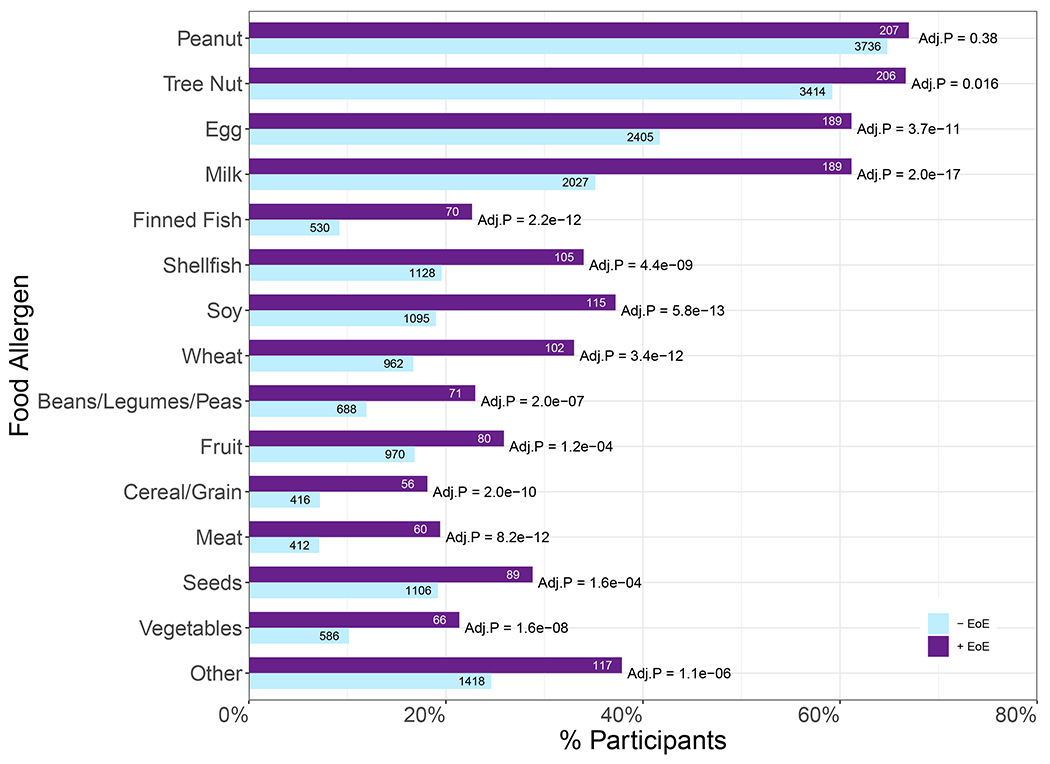
Percentage of participants in the −EoE (n=5,765) and +EoE (n=309) subsets that reported allergy to the major food allergen categories or “Other” food allergen. For each food allergen category, a multivariable logistic regression model was fitted adjusting for demographic data (sex, age, race, ethnicity, and geographic location). FDR-adjusted P-values are shown.
Figure 4. Number of major food allergies.
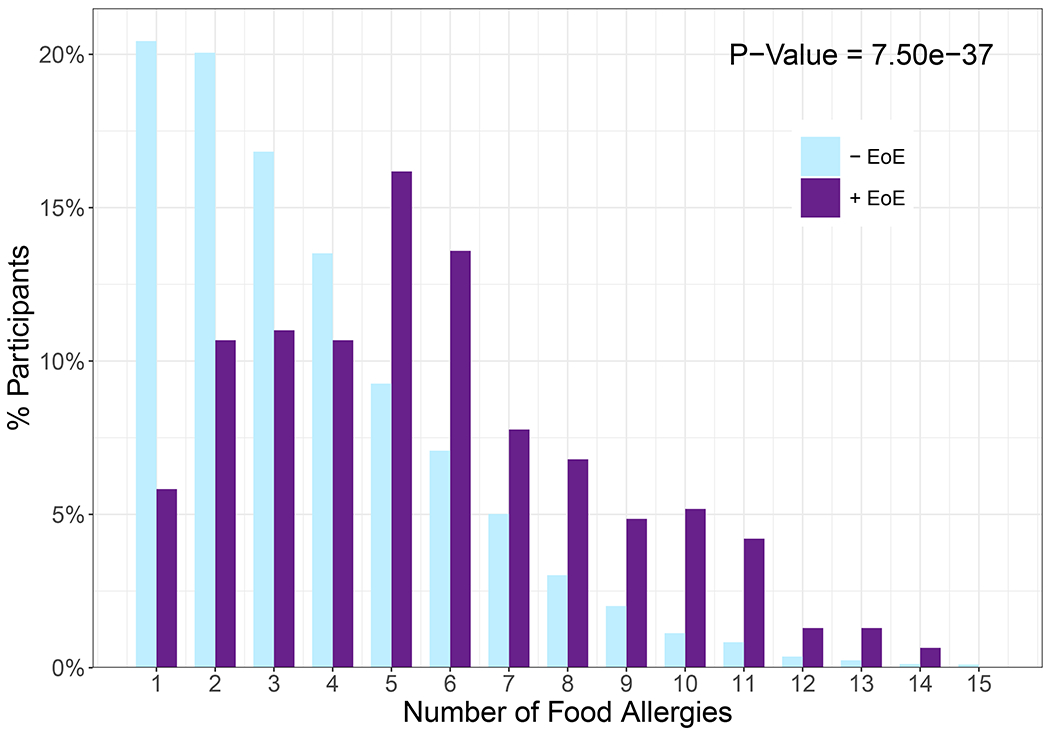
The percentage of each study population reporting n number of allergies to the major food allergen categories or “Other”. The mean number of food allergies for the −EoE and +EoE subsets is 3.62 and 5.57 respectively (aOR=1.3, 95% CI: 1.23-1.32). Significance and odds ratio determined by multivariable logistic regression with adjustment for demographics.
Similar to the comorbidity analysis, a multivariable logistic regression evaluation of co-existing EoE was conducted including these food allergen categories and demographic variables as predictors in order to estimate independent associations of the specific food allergies with reported EoE (Figure 5). The odds of EoE were significantly higher in those who reported an allergy to milk (P= 2.1e-06, aOR=2.0, 95% CI: 1.49-2.62), finned fish (P=0.0048, aOR=1.6, 95% CI: 1.16-2.29), soy (P=0.021, aOR=1.4, 95% CI: 1.05-1.88), meat (P=0.0087, aOR=1.6, 95% CI: 1.12-2.22) and “Other” foods (P=1.2e-04, aOR=1.6, 95% CI: 1.28-2.12) compared to those who did not report these respective food allergies.
Figure 5. Independent associations between food allergen categories and reported EoE.
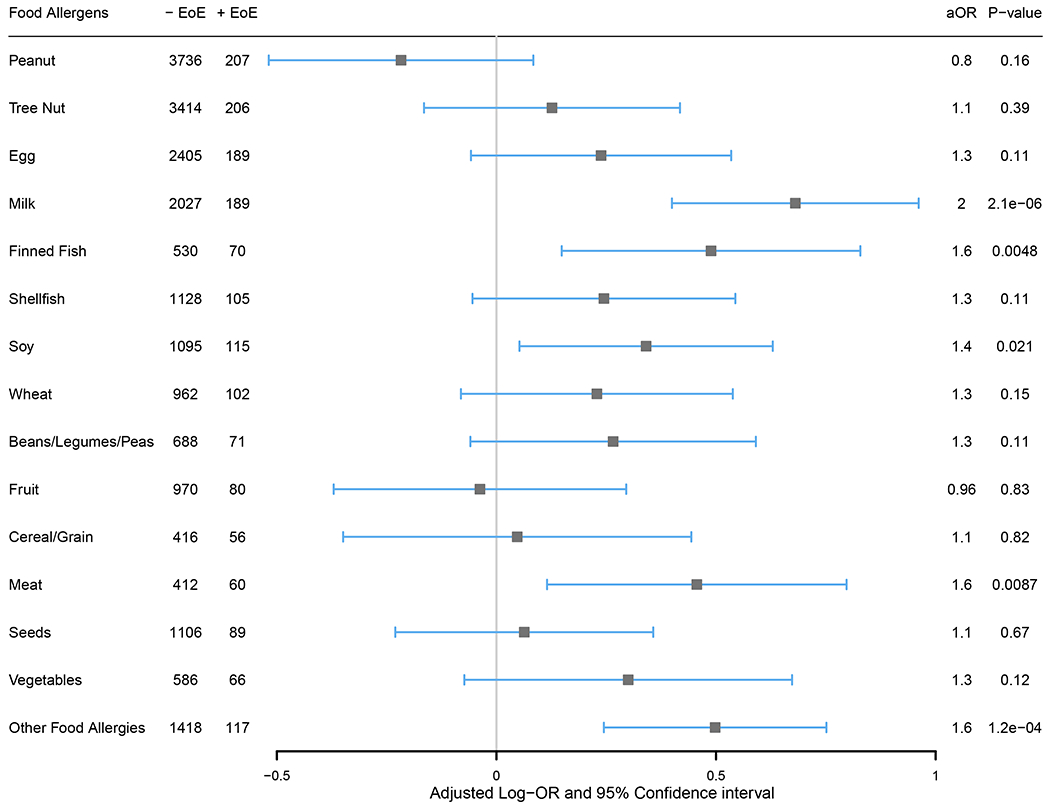
An adjusted OR above 1 (equivalent to an adjusted log-OR above 0; gray line) denotes a higher likelihood of reporting EoE. One multivariable logistic regression model with all 14 major food allergen categories and “Other” was fit while also controlling for demographic variables.
Food Allergy Reaction History & Healthcare Utilization
Participants with more frequent food-related allergic reactions per year were more likely to report having EoE than those with less frequent reactions after controlling for demographic variables (Figure 6a, P=3.4e-08, aOR=1.2, 95% CI: 1.11-1.24). Conversely, those without EoE were more likely to report never having had a prior food-related allergic reaction. There was no significant difference in the likelihood of having EoE based on self-reported subjective severity of most recent food-related allergic reaction (Figure 6b, P=0.15, aOR=0.78, 95% CI: 0.55-1.10). However, those who reported ever having experienced anaphylaxis (Figure 6c, P=0.0015, aOR=1.5, 95% CI: 1.15-1.83) or utilizing acute healthcare services (urgent care, emergency department, hospital, or intensive care unit (ICU)) for food-related allergic reactions (Figure 6d, P=0.043, aOR=1.3, 95% CI: 1.01-1.67) – and specifically the ICU (Figure 6e, P=0.014, aOR=1.7, 95% CI: 1.09-2.64) – had significantly higher likelihood of having EoE.
Figure 6. Food-related allergic reaction severity by subset.
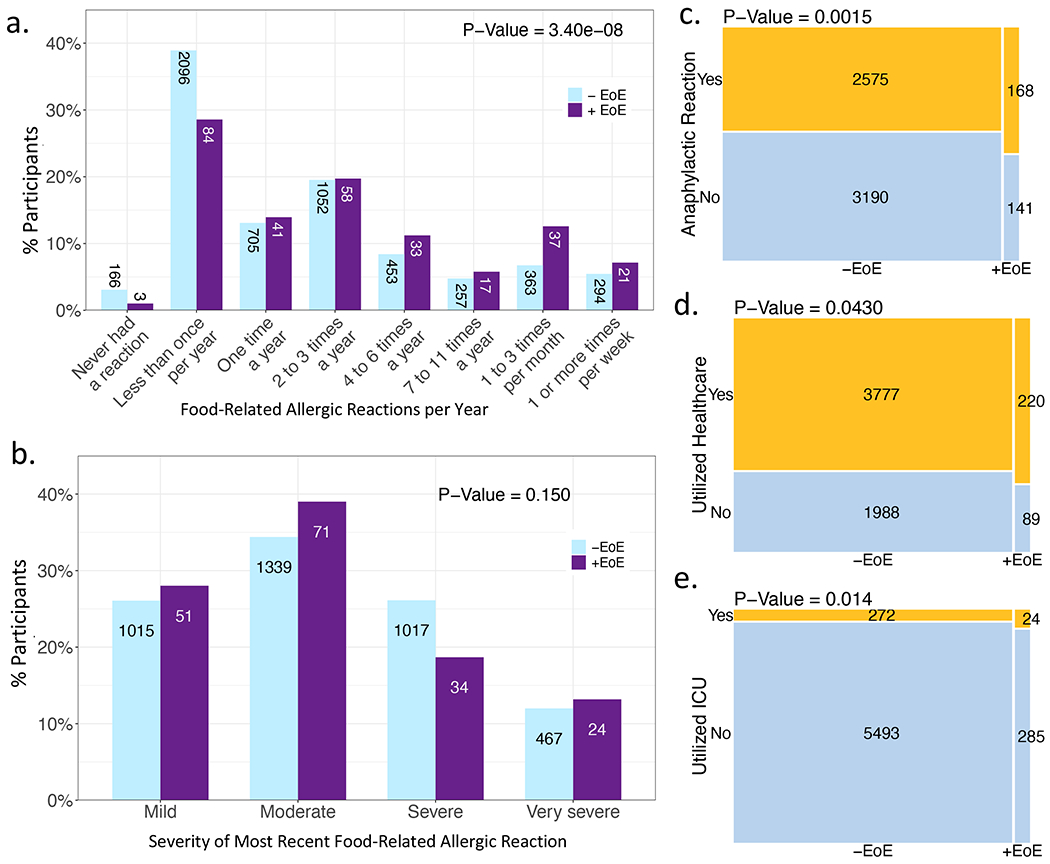
(a) Reaction frequency. (b) Severity of most recent reaction. Mosaic plots of (c) those with/without anaphylaxis, (d) those who have/have not utilized healthcare (urgent care, emergency department, hospitalization, or intensive care unit (ICU)) for food-related allergic reactions, and (e) those who have/have not required ICU admission for food-related allergic reactions. P-values adjusted for demographics.
When considering food-related allergic reactions, participants that reported systemic symptoms within 2 hours of eating the food(s), including gastrointestinal, autonomic, and motor involvement, were significantly more likely to have co-existing EoE, whereas those without EoE were more likely to report cutaneous symptoms (Table III). Those with EoE were also more likely to report respiratory symptoms, though this did not reach statistical significance. There was no significant difference between the two subsets in ever using intramuscular epinephrine (Adj.P=0.73, aOR=0.95, 95% CI: 0.70-1.28) or intravenous epinephrine (Adj.P=0.10, aOR=1.4, 95% CI: 0.95-1.99) for food-related allergic reaction management, though participants with EoE were more likely to report use of H1-antagonists (Adj.P=0.027, aOR=1.5, 95% CI: 1.07-2.04), H2-antagonists (Adj.p=5.0e-07, aOR=2.1, 95% CI: 1.61-2.78), oral corticosteroids (Adj.P=4.2e-04, aOR=1.6, 95% CI: 1.25-2.05), bronchodilators (Adj.P=1.9e-06, aOR=1.8, 95% CI: 1.45-2.33), and oxygen therapy (Adj.P=0.0084, aOR=1.8, 95% CI: 1.20-2.77) for reaction management when controlling for demographic variables.
Table III.
Adjusted1 odds of eosinophilic esophagitis for food allergic registry participants based on reported reaction symptoms for food-related allergic reactions
| Reaction Symptoms | −EoE n=5765 | +EoE n=309 | Adjusted OR (95% CI) | FDR-adjusted P value |
|---|---|---|---|---|
| Skin | 5060 (88%) | 230 (74%) | 0.41 (0.31, 0.54) | 3.8e-09 |
| Hives | 3768 (65%) | 148 (48%) | 0.47 (0.37 0.59) | 6.9e-09 |
| Itching | 3420 (59%) | 160 (52%) | 0.75 (0.60, 0.95) | 0.041 |
| Flushing | 2103 (36%) | 95 (31%) | 0.76 (0.59, 0.97) | 0.062 |
| Swelling | 2143 (37%) | 106 (34%) | 0.89 (0.70, 1.13) | 0.42 |
| Rash | 2797 (49%) | 122 (39%) | 0.70 (0.56, 0.89) | 0.012 |
| Red, itchy, or watery eyes | 1938 (34%) | 105 (34%) | 1.0 (0.79, 1.29) | 0.93 |
| Other skin manifestations | 282 (5%) | 17 (6%) | 1.1 (0.69, 1.90) | 0.64 |
| Respiratory | 3789 (66%) | 222 (72%) | 1.3 (1.04, 1.74) | 0.056 |
| Chest tightening | 1318 (23%) | 94 (30%) | 1.5 (1.16, 1.96) | 0.0065 |
| Chest pain | 407 (7%) | 53 (17%) | 2.8 (2.05, 3.89) | 3.8e-09 |
| Coughing | 1966 (34%) | 124 (40%) | 1.3 (1.03, 1.65) | 0.057 |
| Hoarse voice | 1117 (19%) | 81 (26%) | 1.5 (1.13, 1.94) | 0.012 |
| Nasal congestion/runny nose | 1948 (34%) | 119 (39%) | 1.2 (0.96, 1.54) | 0.18 |
| Sneezing | 923 (16%) | 66 (21%) | 1.4 (1.09, 1.91) | 0.027 |
| Trouble breathing | 1858 (32%) | 112 (36%) | 1.2 (0.94, 1.53) | 0.21 |
| Wheezing | 1565 (27%) | 92 (30%) | 1.1 (0.88, 1.45) | 0.44 |
| Other respiratory manifestations | 196 (3%) | 8 (3%) | 0.73 (0.36, 1.50) | 0.47 |
| Gastrointestinal | 4359 (76%) | 274 (89%) | 2.6 (1.81, 3.73) | 1.9e-06 |
| Bloating | 776 (13%) | 65 (21%) | 1.9 (1.37, 2.53) | 4.4e-04 |
| Bloody stools | 92 (2%) | 6 (2%) | 1.2 (0.52, 2.77) | 0.71 |
| Constipation | 290 (5%) | 34 (11%) | 2.4 (1.66, 3.56) | 3.8e-05 |
| Diarrhea | 1381 (24%) | 85 (28%) | 1.2 (0.93, 1.58) | 0.22 |
| Difficulty swallowing | 1016 (18%) | 117 (38%) | 3.0 (2.36, 3.89) | 1.7e-16 |
| Itchy throat/ears | 1910 (33%) | 134 (43%) | 1.6 (1.25, 2.01) | 8.2e-04 |
| Nausea | 1710 (30%) | 129 (42%) | 1.7 (1.36, 2.18) | 4.1e-05 |
| Odd taste | 489 (8%) | 30 (10%) | 1.2 (0.79, 1.74) | 0.50 |
| Reflux | 511 (9%) | 84 (27%) | 4.1 (3.13, 5.45) | 1.3e-21 |
| Stomach pain/cramps | 1770 (31%) | 140 (45%) | 2.0 (1.54, 2.47) | 3.7e-07 |
| Tingling mouth | 1557 (27%) | 106 (34%) | 1.4 (1.12, 1.84) | 0.012 |
| Tongue swelling/throat tightness | 1511 (26%) | 108 (35%) | 1.6 (1.22, 2.02) | 0.0019 |
| Vomiting | 2054 (36%) | 122 (39%) | 1.2 (0.95, 1.52) | 0.21 |
| Other gastrointestinal manifestations | 182 (3%) | 21 (7%) | 2.2 (1.37, 3.51) | 0.0042 |
| Cardiovascular | 1723 (30%) | 101 (33%) | 1.2 (0.90, 1.49) | 0.37 |
| Cardiac arrest | 17 (<1%) | 4 (1%) | 4.9 (1.61, 14.86) | 0.014 |
| Chest pain | 245 (4%) | 33 (11%) | 2.8 (1.91, 4.18) | 1.9e-06 |
| Irregular heart rate | 285 (5%) | 22 (7%) | 1.5 (0.94, 2.37) | 0.15 |
| Lightheadedness/dizziness | 967 (17%) | 61 (20%) | 1.2 (0.92, 1.68) | 0.22 |
| Low blood pressure | 471 (8%) | 31 (10%) | 1.2 (0.85, 1.83) | 0.37 |
| Rapid heartbeat | 813 (14%) | 46 (15%) | 1.1 (0.77, 1.49) | 0.71 |
| Slow heartbeat | 83 (1%) | 9 (3%) | 2.1 (1.04, 4.23) | 0.072 |
| Turning blue | 193 (3%) | 12 (4%) | 1.2 (0.66, 2.17) | 0.61 |
| Weak pulse | 205 (4%) | 11 (4%) | 0.99 (0.53, 1.85) | 0.98 |
| Other cardiovascular manifestations | 100 (2%) | 3 (1%) | 0.53 (0.17, 1.69) | 0.39 |
| Emotional | 4085 (71%) | 244 (79%) | 1.6 (1.19, 2.10) | 0.0061 |
| Anxiety | 2705 (47%) | 185 (60%) | 1.7 (1.36, 2.2) | 4.7e-05 |
| Confusion | 754 (13%) | 49 (16%) | 1.3 (0.92, 1.73) | 0.22 |
| Depression | 421 (7%) | 26 (8%) | 1.2 (0.78, 1.81) | 0.50 |
| Fatigue | 1256 (22%) | 83 (27%) | 1.3 (1.03, 1.74) | 0.064 |
| Headache | 815 (14%) | 67 (22%) | 1.8 (1.30, 2.36) | 9.5e-04 |
| Irritability | 1386 (24%) | 86 (28%) | 1.3 (0.97, 1.62) | 0.15 |
| Impending doom | 1012 (18%) | 72 (23%) | 1.5 (1.09, 1.92) | 0.025 |
| Panic | 1527 (26%) | 94 (30%) | 1.2 (0.96, 1.59) | 0.16 |
| Sleep disturbance | 618 (11%) | 45 (15%) | 1.5 (1.04, 2.04) | 0.057 |
| Withdrawal | 771 (13%) | 65 (21%) | 1.8 (1.33, 2.37) | 5.7e-04 |
| Other emotional manifestations | 248 (4%) | 10 (3%) | 0.73 (0.38, 1.39) | 0.42 |
| Autonomic | 1365 (24%) | 89 (29%) | 1.4 (1.05, 1.77) | 0.049 |
| Abnormal sweating | 532 (9%) | 34 (11%) | 1.2 (0.83, 1.78) | 0.41 |
| Dry skin | 486 (8%) | 35 (11%) | 1.5 (1.03, 2.14) | 0.07 |
| Dehydration | 348 (6%) | 20 (6%) | 1.1 (0.69, 1.78) | 0.71 |
| Fainting | 281 (5%) | 22 (7%) | 1.5 (0.98, 2.43) | 0.11 |
| Sexual dysfunction | 33 (1%) | 4 (1%) | 2.3 (0.79, 6.61) | 0.20 |
| Urinary dysfunction | 74 (1%) | 5 (2%) | 1.3 (0.52, 3.29) | 0.61 |
| Uterine contractions | 34 (1%) | 4 (1%) | 2.3 (0.80, 6.52) | 0.21 |
| Weight-loss | 177 (3%) | 20 (6%) | 2.3 (1.42, 3.71) | 0.0030 |
| Other autonomic manifestations | 96 (2%) | 8 (3%) | 1.6 (0.74, 3.25) | 0.34 |
| Motor | 391 (7%) | 34 (11%) | 1.8 (1.25, 2.68) | 0.0065 |
| Arm weakness | 178 (3%) | 11 (4%) | 1.2 (0.65, 2.29) | 0.61 |
| Clawing of toes | 48 (1%) | 4 (1%) | 1.7 (0.60, 4.77) | 0.41 |
| Leg weakness | 237 (4%) | 22 (7%) | 1.9 (1.21, 3.07) | 0.014 |
| Muscle wasting | 47 (1%) | 11 (4%) | 4.9 (2.49, 9.72) | 3.5e-05 |
| Other motor manifestations | 90 (2%) | 3 (1%) | 0.61 (0.19, 1.96) | 0.48 |
CI: confidence interval; EoE: eosinophilic esophagitis; FDR: false discovery rate; OR: odds ratio
Adjusted for sex, age, race, ethnicity, and geographic location in multivariable logistic regression models.
Food Allergy Resolution
Thirty-four percent (n=104) of participants with EoE reported outgrowing any food allergy compared to 29% (n=1,682) of those without EoE, though this difference was not statistically significant (Table E2).
Discussion
Herein, we evaluated the impact of co-existing EoE in a large cohort of individuals with self-reported food allergy. The proportion of participants with reported co-existing EoE (5%) in this registry sample is consistent with previously published food allergy literature.3 Notably, this study suggests that those with a greater number of food allergies, increased food-related allergic reaction frequency, and increased measures reflective of reaction severity, including history of systemic symptoms and healthcare utilization for food-related allergic reactions, have greater odds of EoE. Increased use of medications like bronchodilators and oxygen therapy for reaction management in those with EoE further supports this. Additionally, the observed increase in reported ICU admission for food-related allergic reactions in those with EoE may signify more severe systemic disease in this population. However, there was no significant increase in ever using epinephrine for food-related allergic reactions in those with EoE, which may reflect the potential limitation of patient-recall in such self-reported survey data. These findings, in combination with the limitations of the data, warrant further research attention.
The increased likelihood of EoE in participants with a greater number of food allergies could be influenced by the potential higher rate of detected sensitization in those with EoE due to greater likelihood of empiric food allergy panels obtained or inclusion of EoE triggers in reported allergies. However, our finding is consistent with a previous report of increased risk of EoE in those with more than one food allergy from a large study with data verified by manual chart review.3 Of note, our participants both with and without EoE reported allergies to the same four foods most frequently (peanut, tree nut, egg, and milk) – reflective of the most common food allergies in the general food allergy population – the top two of which are less common EoE triggers, potentially lending support to the data pertaining to true food allergy in this subset.1,3,12,35,36
The association between co-existing EoE and increased food-related allergic reaction frequency and severity measures may be a reflection of a truly more severe food allergy phenotype – potentially influenced and compounded by the shared Th2 pathology.11,15,17,18,21,37 However, it is also possible that participants/enrollees recalled reaction episodes and healthcare utilization for food-related EoE exacerbations rather than food-related allergic reactions. While the reaction symptom data does suggest an increased rate of gastrointestinal and potentially related symptoms (i.e. chest pain/tightening) in those with EoE, which could reflect EoE exacerbations, the increased rate of other systemic symptoms would be less consistent with an EoE exacerbation. It is of note that several of the increased systemic symptoms are more subjective in nature and some symptoms, such as chest pain, which was included in the survey as a respiratory or cardiovascular symptom, could also represent a gastrointestinal manifestation. Additionally, some symptoms included in the original survey, such as weight loss and muscle wasting, would not be considered manifestations of acute allergic reactions but were retained in the interest of including all possible survey responses.
Of note, there were 169 (3%) participants who reported having food allergy but no previous history of food-related allergic reaction. These participants could have conceivably had food allergy testing in the absence of reaction history (i.e. severe atopic dermatitis in children) that was suggestive of significantly high likelihood of reaction and could have consequently been diagnosed with food allergy, though the survey did not elicit this data.
After adjusting for age, race, ethnicity, and geographic location, male participants were 1.3 times as likely as females to report co-existing EoE. In the stratification by self-respondent versus non-self-respondent data, the noted male predominance in the non-self-respondent group and female predominance in the self-respondents are likely a function of age, as non-self-respondents likely represent minors, supported by their lower median age. This pattern of male predominance in children and female predominance in adults has been reflected in the food allergy literature.3,4,38 Thus, the distribution of sex in the dataset followed that expected for food allergy, rather than EoE even within the EoE subset – likely reflective of the biased focus of the survey on those with reported food allergy. There has also been a well-established response bias in survey data with females being more likely to respond,39,40 which may have contributed to these distributions, reflected in the self- and non-self-respondents.
The EoE literature demonstrates a strong White predominance, ranging from 62 to 94%.3,8,9,24,33 with 88% White in the EoE subset, the racial composition of this cohort is consistent with previously reported rates. Additionally, the proportion of those with EoE identifying as Hispanic or Latino (7%) is also reflective of prior published findings of 5-11%.24,33
Finding strong association with atopic diseases in those with EoE – specifically the high rates of comorbid asthma (62%), allergic rhinitis (52%), and atopic dermatitis (53%) – is consistent with previous EoE cohorts.1,2,7,8,24,37 The significantly greater odds of EoE in those with asthma and allergic rhinitis though not atopic dermatitis are not surprising given the high rate of comorbid atopic dermatitis in those with food allergy in general.41,42 This strong association provides support for use of more systemic targeting of the shared Th2 pathology in EoE management.11,15,17,18,21,37 It is notable that genetic susceptibility to EoE involves the interplay of genetic variants in atopy-associated genes, as well as EoE-specific genes, which likely contributes to the co-enrichment of both diseases.43
While previous EoE studies have focused primarily on comorbid asthma, allergic rhinitis, and atopic dermatitis, our study also presents novel comparison of the likelihood of EoE in those with other comorbidities – both atopic and non-atopic – notably with higher odds of EoE in those with FPIES, oral allergy syndrome, hyper-IgE syndrome (though very small n and potentially skewed by self-report), inflammatory bowel disease, gluten sensitivity, arrhythmias, connective tissue disorder, and migraines. Previous literature has reported associations between EoE and oral allergy syndrome,17,44–46 hyper-IgE syndrome,47,48 inflammatory bowel disease,49–52 connective tissue disorders,53,54 mixed findings with FPIES,55,56 and wheat (gluten) as a known trigger of EoE,1,3,12,35,36 though an association specifically between EoE and arrhythmias or migraines has not been described. Of note, despite the association with gluten sensitivity, there was no significant association with reported celiac disease, which has been noted in previous literature.57–59 The reasoning for this is unclear, although this could potentially reflect dietary wheat elimination in these participants.
The conclusions and generalizability of our findings are limited by the nature of the study’s cross-sectional design and reliance on unvalidated self-reported data. The study is subject to recall bias and selection bias inherent to voluntary registry enrollment and completion of the surveys. Additionally, the survey did not elicit data regarding oral food challenges or biomarkers for food allergy diagnosis, esophagoduodenoscopy for EoE diagnosis, or chronic treatments, which could have implications on the study data, particularly for those with EoE. Likewise, there was no information on disease activity of EoE or the other atopic comorbidities. Furthermore, given the interplay between EoE, food allergy, and other atopic comorbidities, such as asthma, it is possible that the measures of severity could be skewed by respondent inclusion of EoE exacerbations in recall, and that the combination of atopic disorders could be contributing to the observed results.
Overall, this study supports the conclusion that food allergic patients with co-existing EoE have an increased number of food allergies, food-related allergic reactions, and some measures reflective of increased reaction severity, which could suggest a more severe food allergy phenotype. This information can be utilized by clinicians to inform their evaluation and management of patients with EoE, tailor their counseling of patients/families, anticipate potential increased healthcare needs, and ultimately optimize the medical care for this unique patient population.
Supplementary Material
Highlights Box.
What is already known about this topic?
Eosinophilic esophagitis (EoE) and food allergy are important comorbidities with current literature reporting co-existing food allergy in up to 70% of those with EoE.
What does this article add to our knowledge?
Through novel comparison of self-reported food allergy characteristics in registry participants with and without EoE, this study suggests that co-existing EoE is associated with increased food allergies, food-related allergic reactions, and measures of reaction severity.
How does this study impact current management guidelines?
The presence of co-existing EoE should be considered in food allergic patients, especially those with a severe food allergy phenotype; and for those with known co-existing EoE, clinicians should anticipate potential increased healthcare needs.
Conflicts of Interest:
J.T.S. is a consultant for Shire/Takeda and has received research funding from Knopp Biosciences. A.L.D received research funding from the National Center for Advancing Translational Sciences. M.E.R. is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celldex, Nextstone One, Bristol Myers Squibb, Astra Zeneca, Ellodi Pharma, GlaxoSmith Kline, Regeneron/Sanofi, Revolo Biotherapeutics, and Guidepoint and has an equity interest in the first seven listed, and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust) and UpToDate. M.E.R. is an inventor of patents owned by Cincinnati Children’s Hospital. S.A. received research funding from the NIH. All other authors indicate no conflict of interest.
Funding:
This work was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number: UH2AI145837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported in part by the Campaign Urging Research for Eosinophilic Disorder (CURED) and by funds from the Food Allergy Research and Education, Inc (FARE).
Abbreviations
- Adj.P
false discovery rate-adjusted P value
- aOR
adjusted odds ratio
- CI
confidence interval
- EoE
eosinophilic esophagitis
- FARE
Food Allergy Research & Education
- FDR
false discovery rate
- FPIES
food protein-induced enterocolitis syndrome
- ICU
intensive care unit
- IgE
immunoglobulin E
- IQR
interquartile range
- OR
odds ratio
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130. [DOI] [PubMed] [Google Scholar]
- 2.Sugnanam KKN, Collins JT, Smith PK, Connor F, Lewindon P, Cleghom G, et al. Dichotomy of food and inhalant allergen sensitization in eosinophilic esophagitis. Allergy Eur J Allergy Clin Immunol. 2007;62. [DOI] [PubMed] [Google Scholar]
- 3.Hill DA, Dudley JW, Spergel JM. The Prevalence of Eosinophilic Esophagitis in Pediatric Patients with IgE-Mediated Food Allergy. J Allergy Clin Immunol Pract. 2017;5:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw open. 2019;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loh W, Tang MLK. The epidemiology of food allergy in the global context. Int J Environ Res Public Health. 2018;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141. [DOI] [PubMed] [Google Scholar]
- 7.Hruz P Epidemiology of eosinophilic esophagitis. Dig Dis. 2014;32:40–7. [DOI] [PubMed] [Google Scholar]
- 8.Assa’ad AH, Putnam PE, Collins MH, Akers RM, Jameson SC, Kirby CL, et al. Pediatric patients with eosinophilic esophagitis: An 8-year follow-up. J Allergy Clin Immunol. 2007;119:731–8. [DOI] [PubMed] [Google Scholar]
- 9.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HP, Vance RB, Shaheen NJ, Dellon ES. Su1132 Prevalence of Endoscopic Findings in Eosinophilic Esophagitis: A Meta-Analysis. Gastroenterology. 2012;142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin SK, Sabharwal G, Ghaffari G. A review of the evidence linking eosinophilic esophagitis and food allergy. Allergy Asthma Proc. 2015;36:26–33. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JM, Li RC, McGowan EC. The role of food allergy in eosinophilic esophagitis. J Asthma Allergy. 2020;13:679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chehade M, Aceves SS. Food allergy and eosinophilic esophagitis. Curr Opin Allergy Clin Immunol. 2010;10:231–7. [DOI] [PubMed] [Google Scholar]
- 14.Chevalley L, Schoepfer A. Evolution of pediatric eosinophilic esophagitis over 10 years. Swiss Med Wkly. 2019;149. [Google Scholar]
- 15.McGowan EC, Platts-Mills TAE, Wilson JM. Food allergy, eosinophilic esophagitis, and the enigma of IgG4. Ann Allergy, Asthma Immunol. 2019;122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexandropoulou K, Wong T. Eosinophilic oesophagitis and food allergy. Med (United Kingdom). 2019;47:286–91. [Google Scholar]
- 17.Spergel J, Aceves SS. Allergic components of eosinophilic esophagitis. Vol. 142, Journal of Allergy and Clinical Immunology. Mosby Inc; 2018. p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuta GT, Katzka DA. Eosinophilic Esophagitis. Ingelfinger JR, editor. N Engl J Med. 2015;373:1640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. Vol. 119, Journal of Allergy and Clinical Immunology. 2007. [DOI] [PubMed] [Google Scholar]
- 20.Rocha R, Vitor AB, Trindade E, Lima R, Tavares M, Lopes J, et al. Omalizumab in the treatment of eosinophilic esophagitis and food allergy. Eur J Pediatr. 2011;170. [DOI] [PubMed] [Google Scholar]
- 21.Otani IM, Nadeau KC. Biologic Therapies for Immunoglobulin E–mediated Food Allergy and Eosinophilic Esophagitis. Immunol Allergy Clin North Am. 2017;37:369–96. [DOI] [PubMed] [Google Scholar]
- 22.Simon D, Cianferoni A, Spergel JM, Aceves S, Holbreich M, Venter C, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Vol. 71, Allergy: European Journal of Allergy and Clinical Immunology. 2016. [DOI] [PubMed] [Google Scholar]
- 23.Pelz BJ, Wechsler JB, Krier-Burris R, Wershil B, Kagalwalla AF, Bryce P. The Relationship of Eosinophilic Esophagitis and Food Allergy: Evaluating the Spectrum of Eosinophilic Esophagitis. J Allergy Clin Immunol. 2015;135:AB43. [Google Scholar]
- 24.Pelz BJ, Wechsler JB, Amsden K, Johnson K, Singh AM, Wershil BK, et al. IgE-associated food allergy alters the presentation of paediatric eosinophilic esophagitis. Clin Exp Allergy. 2016;46:1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta RS, Sehgal S, Brown DA, Das R, Fierstein JL, Casale TB, et al. Characterizing Biphasic Food-Related Allergic Reactions Through a US Food Allergy Patient Registry. J Allergy Clin Immunol Pract. 2021;9. [DOI] [PubMed] [Google Scholar]
- 26.Raimundo K, Schuldt R, Gupta S, Rajput Y, Wang R, Bulson A, et al. P101 Characteristics of Patients with Single Versus Multiple Food Allergies from the FARE Patient Registry. Ann Allergy, Asthma Immunol. 2021;127. [Google Scholar]
- 27.Vickerstaff V, Omar RZ, Ambler G. Methods to adjust for multiple comparisons in the analysis and sample size calculation of randomised controlled trials with multiple primary outcomes. BMC Med Res Methodol. 2019;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishimura N, Shimura S, Jiao D, Mikami H, Okimoto E, Uno G, et al. Clinical features of eosinophilic esophagitis: Differences between Asian and Western populations. J Gastroenterol Hepatol. 2015;30. [DOI] [PubMed] [Google Scholar]
- 29.Genta RM, Spechler SJ. Low prevalence of eosinophilic esophagitis in hispanics and asians in the united states. Gastroenterology. 2014;146. [Google Scholar]
- 30.Mansoor E, Cooper GS. The 2010–2015 Prevalence of Eosinophilic Esophagitis in the USA: A Population-Based Study. Dig Dis Sci. 2016;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma P, Aguilar R, Abi Nader M, Siddiqui S, Baniya R, Masoud A. Women With Eosinophilic Esophagitis (EoE) Have Increased Comorbidities Whereas Men With EoE Develop More Complications. Am J Gastroenterol. 2017;112. [Google Scholar]
- 32.Biedermann L, Holbreich M, Atkins D, Chehade M, Dellon ES, Furuta GT, et al. Food-induced immediate response of the esophagus—A newly identified syndrome in patients with eosinophilic esophagitis. Allergy Eur J Allergy Clin Immunol. 2021;76:339–47. [DOI] [PubMed] [Google Scholar]
- 33.Anderson J, Moonie S, Hogan MB, Scherr R, Allenback G. Eosinophilic esophagitis: comorbidities and atopic disease in Nevada. Dis Esophagus. 2021;33:1–6. [DOI] [PubMed] [Google Scholar]
- 34.Weiler T, Mikhail I, Singal A, Sharma H. Racial Differences in the Clinical Presentation of Pediatric Eosinophilic Esophagitis. J Allergy Clin Immunol Pract. 2014;2:320–5. [DOI] [PubMed] [Google Scholar]
- 35.Henderson CJ, Abonia JP, King EC, Putnam PE, Collins MH, Franciosi JP, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2012;129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagalwalla AF, Shah A, Li BUK, Sentongo TA, Ritz S, Manuel-Rubio M, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53. [DOI] [PubMed] [Google Scholar]
- 37.Brown-Whitehorn TF, Spergel JM. The link between allergies and eosinophilic esophagitis: Implications for management strategies. Expert Rev Clin Immunol. 2010;6:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afify SM, Pali-Schöll I. Adverse reactions to food: The female dominance - A secondary publication and update. World Allergy Organ J. 2017;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cull WL, O’Connor KG, Sharp S, Tang SFS. Response rates and response bias for 50 surveys of pediatricians. Vol. 40, Health Services Research. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith WG. Does gender influence online survey participation? A record-linkage analysis of university faculty online survey response behavior. Eric Ed501717. 2008; [Google Scholar]
- 41.Tsakok T, Marrs T, Mohsin M, Baron S, Du Toit G, Till S, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol. 2016;137. [DOI] [PubMed] [Google Scholar]
- 42.Samady W, Warren C, Kohli S, Jain R, Bilaver L, Mancini AJ, et al. The prevalence of atopic dermatitis in children with food allergy. Ann Allergy, Asthma Immunol. 2019;122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin LJ, He H, Collins MH, Abonia JP, Biagini Myers JM, Eby M, et al. Eosinophilic esophagitis (EoE) genetic susceptibility is mediated by synergistic interactions between EoE-specific and general atopic disease loci. J Allergy Clin Immunol. 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leigh LY, Spergel JM. An in-depth characterization of a large cohort of adult patients with eosinophilic esophagitis. Ann Allergy, Asthma Immunol. 2019;122. [DOI] [PubMed] [Google Scholar]
- 45.Irahara M, Nomura I, Takeuchi I, Yamamoto-Hanada K, Shimizu H, Fukuie T, et al. Pediatric patient with eosinophilic esophagitis and pollen-food allergy syndrome. Asia Pac Allergy. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Letner D, Farris A, Khalili H, Garber J. Pollen-food allergy syndrome is a common allergic comorbidity in adults with eosinophilic esophagitis. Dis Esophagus. 2018;31. [DOI] [PubMed] [Google Scholar]
- 47.Dixit C, Thatayatikom A, Pappa H, Knutsen AP. Treatment of severe atopic dermatitis and eosinophilic esophagitis with dupilumab in a 14-year-old boy with autosomal dominant hyper-IgE syndrome. J Allergy Clin Immunol Pract. 2021;9. [DOI] [PubMed] [Google Scholar]
- 48.Arora M, Bagi P, Strongin A, Heimall J, Zhao X, Lawrence MG, et al. Gastrointestinal Manifestations of STAT3-Deficient Hyper-IgE Syndrome. J Clin Immunol. 2017;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Limketkai BN, Shah SC, Hirano I, Bellaguarda E, Colombel JF. Epidemiology and implications of concurrent diagnosis of eosinophilic oesophagitis and IBD based on a prospective population-based analysis. Gut 2019;68. [DOI] [PubMed] [Google Scholar]
- 50.Sonnenberg A, Turner KO, Genta RM. Comorbid Occurrence of Eosinophilic Esophagitis and Inflammatory Bowel Disease. Vol. 19, Clinical Gastroenterology and Hepatology. 2021. [DOI] [PubMed] [Google Scholar]
- 51.Fan YC, Steele D, Kochar B, Arsene D, Long MD, Dellon ES. Increased Prevalence of Esophageal Eosinophilia in Patients with Inflammatory Bowel Disease. Inflamm Intest Dis. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore H, Wechsler J, Frost C, Whiteside E, Baldassano R, Markowitz J, et al. Comorbid Diagnosis of Eosinophilic Esophagitis and Inflammatory Bowel Disease in the Pediatric Population. J Pediatr Gastroenterol Nutr. 2021;72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abonia JP, Wen T, Stucke EM, Grotjan T, Griffith MS, Kemme KA, et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. 2013;132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lecouffe-Desprets M, Groh M, Bour B, Le Jeunne C, Puéchal X. Eosinophilic gastrointestinal disorders associated with autoimmune connective tissue disease. Vol. 83, Joint Bone Spine. 2016. [DOI] [PubMed] [Google Scholar]
- 55.Cianferoni A, Warren CM, Brown-Whitehorn T, Schultz-Matney F, Nowak-Wegrzyn A, Gupta RS. Eosinophilic esophagitis and allergic comorbidities in a US-population-based study. Vol. 75, Allergy: European Journal of Allergy and Clinical Immunology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cianferoni A Food protein-induced enterocolitis syndrome epidemiology. Vol. 126, Annals of Allergy, Asthma and Immunology. 2021. [DOI] [PubMed] [Google Scholar]
- 57.Capucilli P, Cianferoni A, Grundmeier RW, Spergel JM. Comparison of Comorbid Diagnoses in Children with and without Eosinophilic Esophagitis in a Large Population. Vol. 121, Annals of Allergy, Asthma and Immunology. 2018. [DOI] [PubMed] [Google Scholar]
- 58.Jensen ET, Eluri S, Lebwohl B, Genta RM, Dellon ES. Increased Risk of Esophageal Eosinophilia and Eosinophilic Esophagitis in Patients With Active Celiac Disease on Biopsy. Vol. 13, Clinical Gastroenterology and Hepatology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mansoor E, Abou Saleh M, Cooper G. The Epidemiology of Celiac Disease in Eosinophilic Gastroenteritis and Eosinophilic Colitis in the United States From 2012 to 2017: Results From a Population-Based National Study: 2017 Fellows-in-Training Award (Small Intestine Category): 1173. Vol 112, American Journal of Gastroenterology. 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


