Abstract
Background:
Understanding the neurobiology underlying bipolar disorder (BD) vs. major depressive disorder (MDD) is crucial for accurate diagnosis, and for driving the discovery of novel treatments. A promising target is the metabotropic glutamate receptor 5 (mGluR5), a modulator of glutamate transmission associated with synaptic plasticity. We measured mGluR5 availability in individuals with MDD and BD for the first time using positron emission tomography (PET).
Methods:
Individuals with BD (n=17 depressed, BD-dep; n=10 euthymic, BD-euth), MDD (n=17), and 18 healthy controls (HCs) were imaged with [18F]FPEB PET to quantify mGluR5 availability in regions of the prefrontal cortex (PFC) and was compared across groups and assessed in relation to depressive symptoms and cognitive function.
Results:
PFC mGluR5 availability was significantly different across groups (F6,116=2.18, p=0.050). Specifically, mGluR5 was lower in BD vs. MDD and HC groups, with no difference between MDD and HC groups. Further, after dividing the BD group, mGluR5 was lower in both BD-dep and BD-euth groups vs. both MDD and HC groups across ROIs. Interestingly, lower dlPFC mGluR5 was associated with worse depression in MDD (r=−0.67, p=0.005) but not in BD. Significant negative correlations were observed between mGluR5 and working memory in MDD and BD-dep groups.
Conclusion:
This work suggests mGluR5 could be helpful in distinguishing BD and MDD, as a possible treatment target for depression symptoms in MDD and cognitive alterations for both disorders. Further work is needed to confirm differentiating roles for mGluR5 in BD and MDD and to probe modulation of mGluR5 as a preventative/treatment strategy.
Keywords: Bipolar disorder, depression, glutamate, mGluR5, PET, imaging
Introduction
The effective diagnosis and treatment of bipolar disorder (BD) remains one of the most important challenges in psychiatry, in part due to difficulty in differentiating between BD and MDD during depressive episodes. Although episodes of mania/hypomania are the defining diagnostic feature of BD, depression is its most frequent clinical presentation(1). Individuals with BD spend up to three times as much time in the depressed state relative to mania/hypomania(2), and consequently are more likely to present to clinicians in a depressed mood state(3). Further, BD carries the highest rate of suicide mortality among psychiatric disorders (20–30 times the general population)(4, 5), with a majority of suicide attempts occurring when depressive symptoms are present. Despite this, at present there is no accurate way of differentiating a depressive episode in BD from one in major depressive disorder (MDD) and misdiagnosis is common, often resulting in an incorrect treatment path. Indeed, standard antidepressant therapies can have limited efficacy for bipolar depression or potentially increase elevated symptoms. Further, mood stabilizers are not effective for all individuals with BD in treating depressive symptoms(6, 7). The lack of effective, targeted treatments for BD depression is a critically important unmet need. Identifying the distinct neurobiological mechanisms that differentiate BD from MDD, and specifically during depressive episodes, is crucial for a) early and accurate diagnosis, b) developing preventative and risk mitigation strategies and c) identifying targeted, effective and safe treatments.
Glutamate is the primary excitatory neurotransmitter governing the cortico-limbic circuitry that subserves emotional regulation. Accordingly, dysfunction of the glutamate system is thought to play a prominent role in the mood disturbances associated with both BD and MDD. While there are likely shared mechanisms, evidence points to a differential role of glutamate across disorders. A meta-analysis of magnetic resonance spectroscopy (MRS) studies in BD(8) confirmed a pattern of higher levels of glutamate and glutamate+glutamine (Glx) in the cortex of those with BD as compared to those with no psychiatric disorder. However, there is some variability in results likely due to mood state and medication use(8–10), highlighting the importance of taking these factors into account. By contrast, some MRS studies in MDD have generally shown lower levels of glutamate and Glx(11–13), with recent work also showing increases in MDD prefrontal glutamate in response to acute antidepressant treatment (e.g. ketamine, transcranial magnetic stimulation)(14, 15). As in BD, medication use and symptom heterogeneity likely contribute to some variability in findings, and some postmortem and in vivo work has shown higher levels of glutamate in MDD(16–18). However, numerous studies have also shown no alterations in Glx in MDD(19). Indeed, MRS studies across both disorders, while informative, are inconsistent, perhaps highlighting the need for alternative probes such as [18F]FPEB PET to measure glutamate system in-vivo. While direct studies are lacking, it has been hypothesized that distinct patterns of glutamatergic functioning in BD vs. MDD may contribute to differences observed in grey matter volume(20), distinct patterns in functional network organization(21–25) and differences in activation response to emotional-related tasks, converging on prefrontal cortex (PFC) regions across BD and MDD studies(26–28). Indeed, it is known that the PFC is particularly vulnerable to the effects of stress(29). Stress-induced structural changes (including synaptic-loss) seem to be closely related to aberrant glutamatergic functioning, and these inter-related structural and transmitter changes are in turn thought to have downstream effects on function(30). While MRS and structural and functional MRI studies shed some light on potential differentiation in glutamatergic functioning in MDD vs. BD, studies directly comparing structural/functional correlates of the glutamate system are lacking. The metabotropic glutamate receptor 5 (mGluR5) is a G-protein coupled receptor that mediates the neuromodulatory effects of glutamate. It plays a key role in synaptic plasticity and has been implicated in both cognitive function and mood regulation, therefore representing a promising treatment target for psychiatric disorders associated with glutamate dysfunction(31, 32), including BD. Indeed, postmortem work shows lower mGluR5 levels in those with BD(33–35). In line with this, mGluR5 has also been implicated in diurnal variation(36) and sleep-wake regulation(37, 38), in executive function(31, 39–41) and impulsivity(42, 43) – behavioral domains that are particularly relevant to BD. Recent preclinical evidence suggests that mGluR5 regulates susceptibility to chronic social defeat stress through downstream signaling pathways in PFC(44). Further, mGluR5 availability appears to be sensitive to levels of glutamate. In our previous positron emission tomography (PET) study, we demonstrated a downregulation of mGluR5 in response to ketamine administration (likely secondary to a ketamine-induced burst in glutamate release), and an association between MRS Glu and Glx and mGluR5, showing that higher levels of Glu and Glx were associated with lower mGluR5 availability(45).
Despite growing evidence implicating mGluR5 in BD, no published studies have examined mGluR5 availability in vivo in individuals with BD. Likewise, no studies have examined variability in mGluR5 across mood states in BD, and whether a ‘normalization’ occurs in the absence of mania or depression (i.e., in the euthymic state). The present study sought to address these gaps in the literature by examining mGluR5 availability in vivo across BD depression (BD-dep) and BD euthymia (BD-euth), as compared to MDD during an active depressive episode, for the first time. More specifically, using [18]FPEB – a radioligand that binds to mGluR5 – and PET as previously(46, 47), we aimed to determine the utility of mGluR5 as a marker with the potential to differentiate BD from MDD and to help inform the evaluation of novel treatments aimed at targeting mGluR5 in BD and/or MDD. Based on preclinical, postmortem, and MRS literature, we hypothesized that in PFC regions associated with the pathophysiology of both BD and MDD – specifically dorsolateral PFC (dlPFC), ventromedial PFC (vmPFC) and orbitofrontal cortex (OFC) – we would observe lower mGluR5 availability in BD overall relative to MDD and healthy control (HC) individuals; and lower mGluR5 availability in BD-Dep relative to BD-euth, MDD, and HCs. We additionally performed exploratory correlational analyses to examine associations between clinically meaningful measures of mood and cognition with mGluR5 availability in BD.
Methods
Participants
Seventeen individuals with MDD currently in an active depressive episode (mean ± SD age = 33.2 ± 12.1yrs; 5 men, 12 women), 17 individuals with BD-dep (age = 38.9 ± 11.9yrs; 6 men, 11 women), 10 individuals with BD-euth (age = 36.6 ± 13.0yrs; 5 men, 5 women), and 18 HC individuals (age = 36.4 ± 12.8yrs; 6 men, 12 women) took part in the study. Participant recruitment methods included flyers, word of mouth, community outreach, and referrals from local clinics/hospitals. The current MDD sample overlaps with the sample in our previously published work where we specifically sought unmedicated individuals(18); however, current pharmacotherapy was not exclusionary for the present study. Demographic, clinical and radiotracer characteristics are shown in Table 1. Importantly, there were no differences between groups in age, male:female ratio, tobacco users, injected tracer mass, injected activity, parent fraction (fP), or the metabolite-corrected input function. Participants underwent physical and neurological examination to rule out major medical or neurological illnesses with potential central nervous system effects. Screening involved electrocardiography, complete blood counts, serum chemistries, thyroid function test, liver function test, urinalysis and urine toxicology screening, and urine pregnancy tests (for women). Diagnosis was confirmed using the Structured Clinical Interview for DSM-5(48). Symptoms of depression were assessed using the Montgomery-Asberg Depression Rating Scale (MADRS)(49). Cognitive domains of working memory and psychomotor speed were assessed using the CogState testing battery (https://www.cogstate.com/), using the Groton maze learning test(50) and detection test(51), respectively. As shown in Table 1, individuals in the MDD and BD-Dep groups were mildly to moderately depressed (as indicated by MADRS score), with no significant difference in depression severity between these groups. Individuals in the BD-euth or HC groups did not report significant depression. There were no significant differences in the proportion of participants taking psychiatric medications across BD-dep, BD-euth, and MDD groups (Table 1). See Table S1 in the Supplement for more information on psychiatric medications across patients.
Table 1.
Demographic and PET parameters for the study population
| HC (n=18) | BD-dep (n=17) | BD-euth (n=10) | MDD (n=17) | Statistical test (F or Chi2, p) | |
|---|---|---|---|---|---|
| Age, years (mean±SD) | 36.4±12.8 | 38.9±11.9 | 36.6±13.0 | 33.2±12.1 | 0.605, 0.615 |
| Sex (%female) | 66.7 | 64.7 | 50.0 | 70.6 | 1.231, 0.746 |
| Tobacco users (%) | 22.2 | 23.5 | 50.0 | 29.4 | 2.790, 0.425 |
| MADRS (mean±SD) | 0.7±1.4** | 19.0±6.1†† | 4.6±3.3** | 21.6±6.4†† | 63.395, <0.001 |
| Psychotropic medication (%taking medication) | NA | 23.5 | 30.0 | 26.7 | 0.139, 0.993 |
| Injected tracer dose, MBq (mean±SD) | 155.9±32.0 | 153.0±46.9 | 164.5±24.3 | 167.5±45.3 | 0.485, 0.694 |
| Injected tracer mass, μg (mean±SD) | 0.48±0.26 | 0.34±0.24 | 0.38±0.18 | 0.49±0.65 | 0.835, 0.443 |
| Plasma free fraction, fP (mean±SD) | 0.045±0.01 | 0.048±0.01 | 0.047±0.01 | 0.048±0.01 | 0.210, 0.889 |
| Input function, 90–120 min. SUV (mean±SD) | 0.178±0.03 | 0.166±0.05 | 0.157±0.03 | 0.150±0.04 | ‡1.301, 0.283 |
punadj<0.01 vs. MDD
punadj<0.01 vs. HC
Main effect of diagnosis, correcting for arterial vs. venous-derived input function
MRI and PET scanning
T1-weighted MRI scans were acquired on a 3T scanner (Prisma; Siemens Medical Systems) to exclude structural abnormality and for coregistration with PET. [18F]FPEB was synthesized onsite as described previously(52). [18F]FPEB was administered intravenously as bolus plus infusion for 120 min, with a Kbol of 190 min(52, 53). Considering previous findings showing circadian variation in mGluR5 availability as measured by PET(54, 55), all injections were performed between 11:25 and 16:21 hrs (average time of 13:18 hrs ±72 min). Based on previous studies showing that equilibrium was reached at 60 min, emission data were acquired 90–120 min after the start of injection on the high-resolution research tomograph (Siemens/CTI), which has intrinsic spatial resolution of ~2.5 mm FWHM. We have previously shown that venous and arterial concentrations are well-matched at equilibrium, allowing venous sampling to be collected for metabolite correction for the majority of participants (n=55, 88.7%) as opposed to the more invasive arterial sampling (2 HC, 3 BD-dep, 1 BD-euth, and 1 MDD)(52). We also showed good test–retest variability (TRV, mean of 12%) of [18F]FPEB binding using this approach(52). Blood samples were acquired at 15, 20, 25, 30, 40, 50, 60, 75, 90, 105, and 120 min post-injection for calculation of a metabolite-corrected input function as validated previously(52). A 6-min transmission scan was obtained for attenuation correction. Head motion was tracked using the Polaris Vicra optical tracking system (Vicra; NDI System). There were no differences in injected dose or mass across groups (all p’s > 0.45; Table 1). Dynamic scan data were reconstructed with corrections for attenuation, normalization, randoms, scatter, dead time, and motion using the ordered subset expectation maximization-based MOLAR algorithm.
Image analysis
PET images were coregistered to each participant’s T1-weighted MRI images using a six-parameter mutual information algorithm (FLIRT; FSL 3.2, Analysis Group, FMRIB), which was then coregistered to the MRI template by nonlinear transformation using Bioimagesuite (version 2.5). Regions of interest (ROIs) were delineated using the Anatomical Automatic Labeling (AAL) atlas(56). The primary ROIs were regions of the PFC – specifically, dlPFC, vmPFC and OFC – selected for their relevance to the pathophysiology of both BD and MDD. Gray matter segmentation was conducted using FSL-FAST, and a gray matter mask was applied to the ROIs. Given the lack of a reference region for mGluR5 targets(57), VT (volume of distribution – ratio of radioligand concentration in ROI to the concentration in plasma at equilibrium) was used as the outcome measure. VT was estimated by the equilibrium analysis method described previously(53) and a venous plasma input function(52).
Statistics
Analysis of variance (ANOVA) with Least Significant Difference (LSD) post hoc tests or Chi2 tests were used to assess differences between demographic and radiotracer characteristics across groups. Group differences in mGluR5 availability ([18F]FPEB VT) were assessed using a multivariate ANOVA (MANOVA) with OFC, vmPFC, and dlPFC VT as the dependent variables, and diagnosis (HC, MDD, BD) as the independent variable. A secondary MANOVA differentiating BD-euth and BD-dep subjects was used to explore the possible effect of mood state on mGluR5 availability. Sphericity of the data was checked by Mauchly’s test and corrected by the Greenhouse – Geisser procedure if violated. Homogeneity of variances was checked by Levene’s test. Effect size was calculated and reported as Cohen’s d (d). Pairwise comparisons were subsequently carried out to determine VT differences between groups; we provide both LSD and Bonferroni post-hoc test results, punadj and padj, respectively. To evaluate associations between mGluR5 availability and clinical and demographic variables, which were normally distributed based on Shapiro-Wilk tests, we computed Pearson’s r. Findings were considered significant at the p < 0.05 level. Power calculations were performed to confirm that our analyses were appropriately powered; additional details and results can be found in the Supplemental Information.
Results
Between group differences in mGluR5 availability
The omnibus MANOVA evaluating mGluR5 availability ([18F]FPEB VT) across HC, MDD and BD groups was significant: F6,114=2.266, p=0.042, d=0.214 (Figure S1). The main group effect was significant for all three regions examined (dlPFC: F2,59=5.778, p=0.005, d=0.328; vmPFC: F2,59=4.237, p=0.019, d=0.252; OFC: F2,59=3.866, p=0.026, d=0.232). Post-hoc indicated significantly lower mGluR5 availability in BD compared to HC in dlPFC (punadj=0.010, padj=0.029), vmPFC (punadj=0.027, padj=0.080) and OFC (punadj=0.040, padj=0.121), and lower than MDD individuals in dlPFC (punadj=0.004, padj=0.013), vmPFC (punadj=0.013, padj=0.038) and OFC (punadj=0.015, padj=0.045). No significant differences were observed between MDD and HC individuals.
Next, we broke down the BD group according to mood state and examined mGluR5 availability across 4 groups (HC, MDD, BD-euth, and BD-dep)(Figure 1). The omnibus F-test was not significant (F9,136.44=1.565, p=0.132, d=0.154), likely due to the fact that BD-euth individuals did not differ in mGluR5 availability from BD-dep group across any ROIs. However, across ROIs, BD-dep individuals had significantly lower mGluR5 availability than the HC group (OFC: punadj=0.043, padj=0.258. vmPFC: punadj=0.031, padj=0.184. dlPFC: punadj=0.015, padj=0.091) and MDD group (OFC: punadj=0.018, padj=0.107. vmPFC: punadj=0.016, padj=0.095. dlPFC: punadj=0.007, padj=0.044). The BD-euth group had significantly lower mGluR5 availability in dlPFC relative to the MDD (punadj=0.037, padj=0.222). Covarying for medication status (Tables S2 and S3) or venous vs. arterial-derived input function (Tables S4 and S5) for each model did not change the results. Data for additional brain regions (i.e., those not included in the primary analyses) and univariate tests for group differences are provided in the SI (Table S6 and S7).
Figure 1.
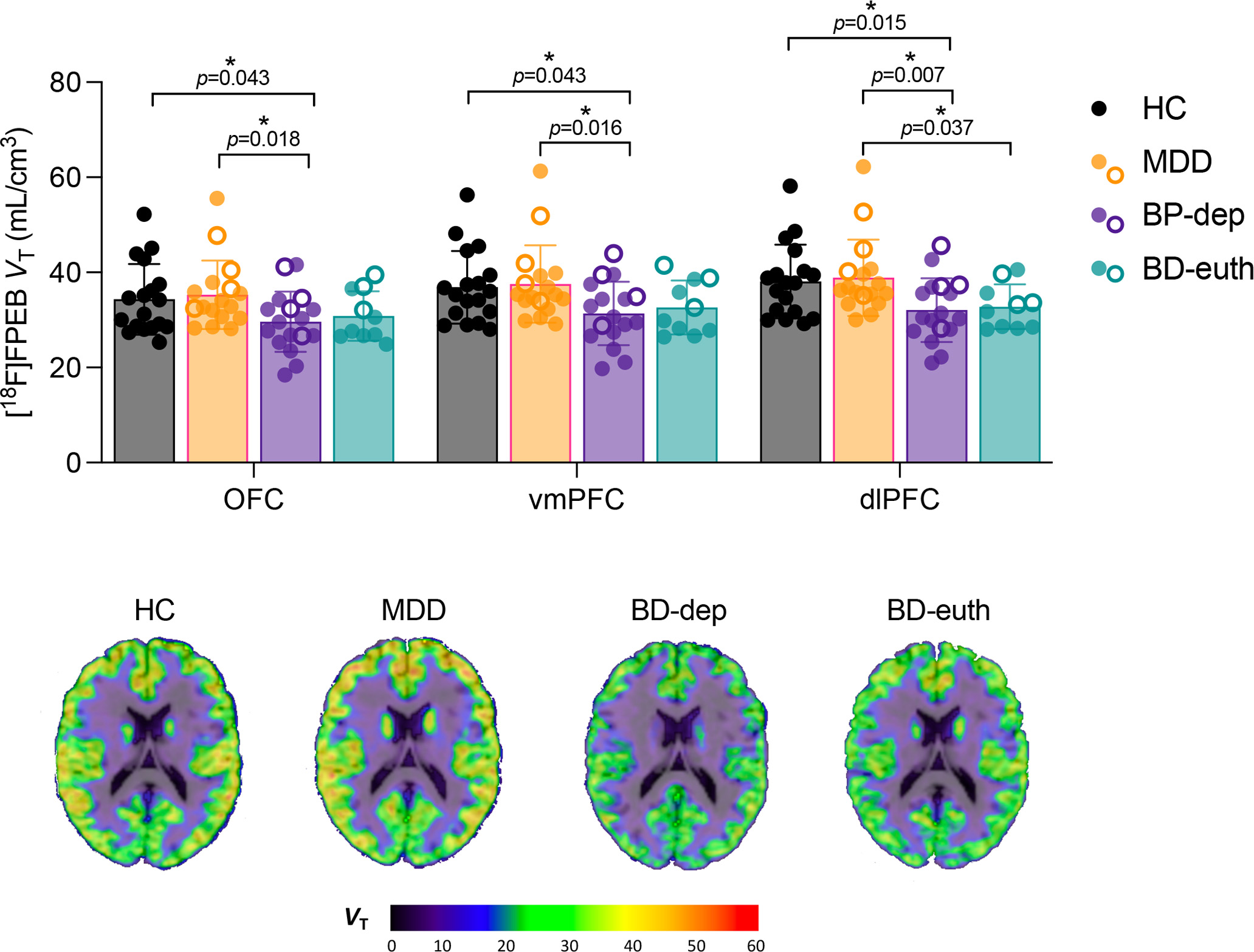
MANOVA of [18F]FPEB VT within PFC subregions across HC (black), MDD (orange), and BD-dep (purple) and BD-euth (teal) groups. Brackets indicate p-values from independent-sample t-tests. Closed circles represent unmedicated subjects, and open circles indicate subjects taking any psychotropic medication (see table S1). Brain images are parametric [18F]FPEB VT images overlaid on structural MRI in template space from representative HC, MDD, BD-dep and BD-euth individuals.
Associations with symptoms
We observed significant negative correlations between symptoms of depression (MADRS) and mGluR5 availability across all ROIs in MDD (all r’s<−0.62, all p’s<0.011) but not in BD group (all r’s <0.20, all p’s >0.35). The correlation remained non-significant when limiting the analysis to BD-dep (all r’s < 0.30, all p’s >0.25) (Figure 2). We observed negative associations between mGluR5 availability and number of errors made during the Groton Maze Learning (GML) test of working memory in both MDD and BD-dep groups (Figure 3, left), such that lower mGluR5 availability was associated with greater working memory difficulties. However, the relationship between mGluR5 availability and psychomotor speed, as measured by the latency to respond during the Detection Test (DET), was significant only in the BD-dep group (Figure 3, right), suggesting a unique associations between psychomotor retardation and lower mGluR5 availability in this clinical population. No significant associations between mGluR5 availability and cognition were observed in the BD-euth group.
Figure 2.
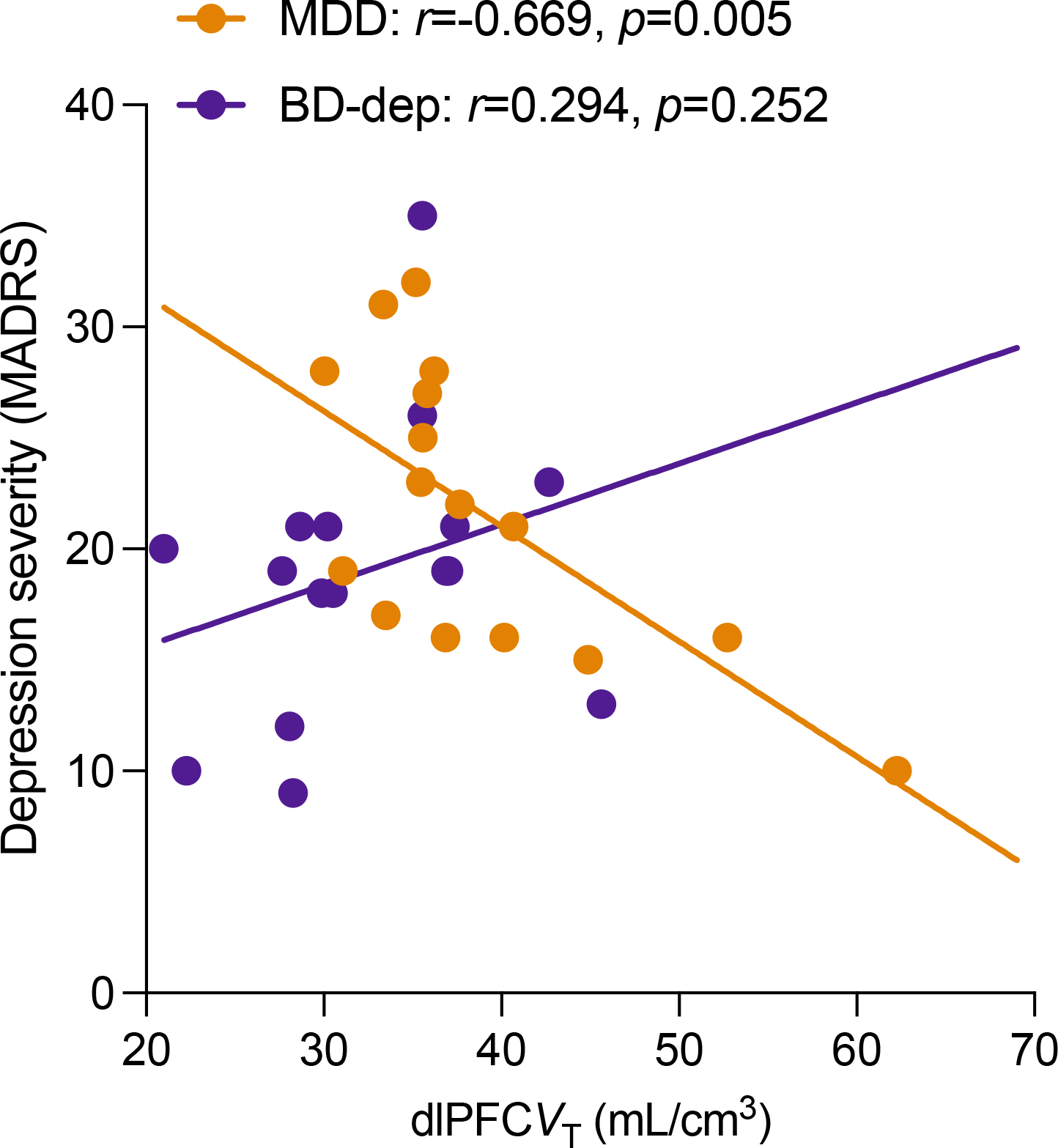
Correlation between dlPFC mGluR5 availability and depression severity (MADRS) in the MDD (orange) and BD-dep groups (purple).
Figure 3.
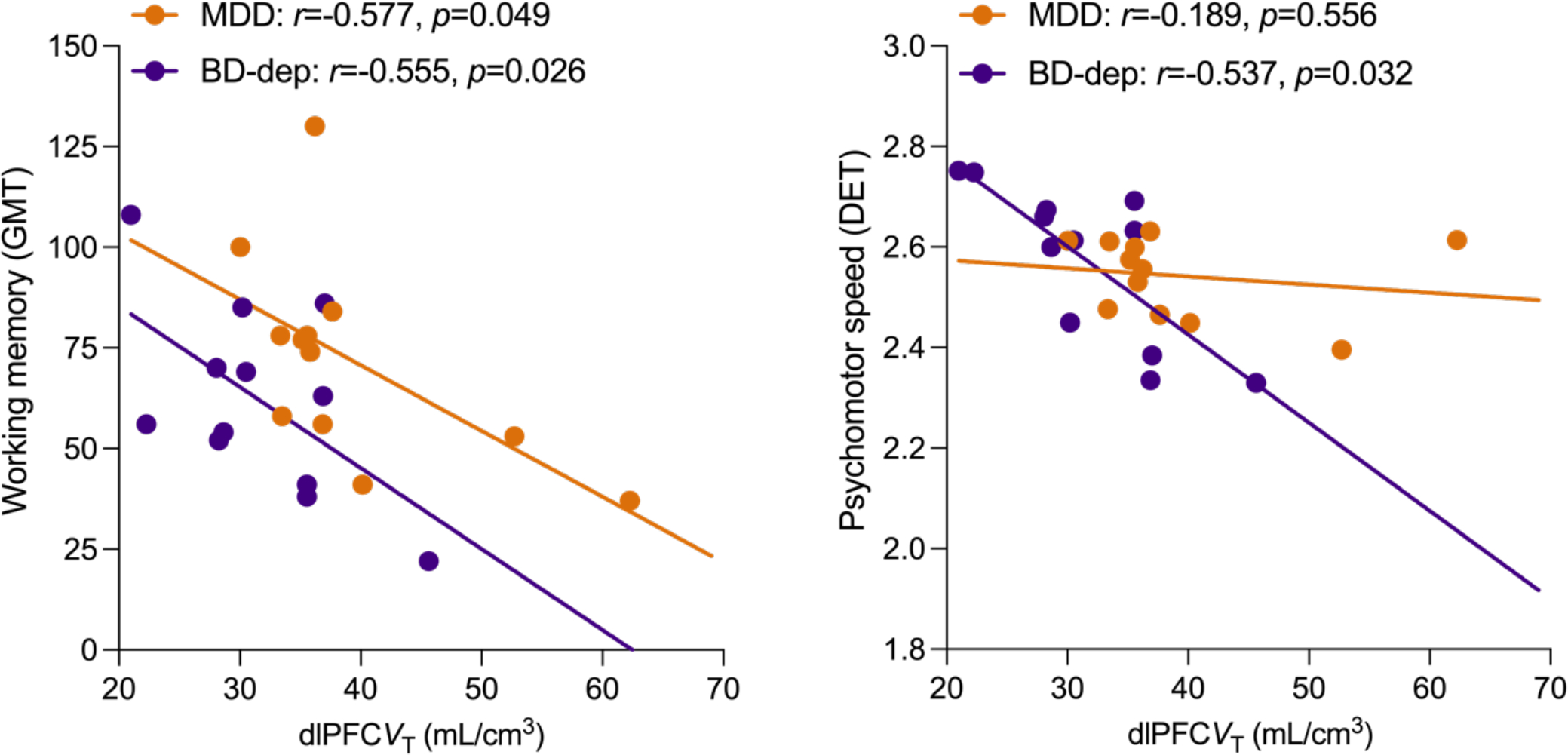
Correlations between dlPFC mGluR5 availability and working memory (Groton Maze Learning Test, GMT) [left] and psychomotor speed (Detection Test, DET) [right] in subjects with MDD (orange) and BD-dep (purple).
Discussion
To our knowledge, this is the first study to examine in vivo mGluR5 availability in BD, both within and across mood states, in comparison to MDD. Consistent with study hypotheses, we observed significantly lower mGluR5 availability in BD (across depressed and euthymic mood states) relative to both MDD-dep and HC. In keeping with our previous work(47, 58), no differences in mGluR5 availability were observed between MDD and HC. However, lower mGluR5 availability was associated with more severe depression in MDD. Exploratory correlations with cognitive function indicated that lower mGluR5 availability was associated with poorer working memory in MDD and with worse psychomotor speed in BD-Dep.
These findings suggest that lower mGluR5 availability in BD-dep and persists in euthymia and may, therefore, contribute to vulnerability of the disorder, particularly its depressive states. Further work assessing mGluR5 availability in mania/hypomania is needed to confirm whether mGluR5 is also associated with vulnerability to elevated mood states of BD. Possible explanations for mGluR5 findings warrant consideration. One possibility is that dysregulation of mGluR5 in BD - through internalization and/or reduced number of receptors - could potentially help to explain observed disturbances in synaptic plasticity in BD. Indeed, mGluR5 is known to play an important role in modulating synaptic plasticity throughout the brain(59–63), and a growing body of evidence suggests disturbances in synaptic density in BD, including MRI studies demonstrating lower cortical gray matter volume and thickness - largely in regions of the PFC- relative to HC, with findings in similar regions in MDD although the magnitude and extent of the findings may differ(64–76). Although there is regional overlap in synaptic/structural abnormalities across BD and MDD, the underlying substrate for these changes – potentially the involvement of mGluR5 – could be differentiating. A second possibility is that the presence of excess glutamate in the extracellular space in BD, possibly as a result of a putative imbalance in inhibitory/excitatory outputs(77), affects mGluR5 availability. The primary pathophysiological mechanisms underlying BD and/or direct effects of mood episodes or the stress associated with them could be associated with adverse impacts on GABA, glutamate and glial systems and disruption of glutamate/GABA function, culminating in excess glutamate and subsequent excitotoxicity(78, 79). Excess glutamate in the extracellular space could in turn lead to internalization of mGluR5. Support for this is provided by our own MRS/PET work showing that higher levels of Glu and Glx are associated with lower mGluR5 availability in MDD(45). We also showed that administration of ketamine is associated with a downregulation of mGluR5, likely secondary to a ketamine-induced increase in glutamate in the extracellular space(80, 81). Alternatively, abnormalities in the expression or function of mGluR5 could reflect a genetic risk for BD. Indeed, there is evidence of involvement of glutamate-related genes in risk for mood disorders(82). That we observed lower mGluR5 availability in both BD-dep and BD-euth (i.e., across mood states), suggests that mGluR5 could act as a trait marker in BD. It is possible that dysfunction in mGluR5 represents an early marker of BD that could aid in the discovery and initiation of preventative strategies, as well as accurate diagnosis. Additional work is needed to better understand the observed differences in mGluR5 in BD – whether they should be interpreted as inferring risk, a consequence of exposure to episodes or stress, and/or related to other factors such as medication use.
Importantly, in addition to group differences in mGluR5 availability, we observed significant relationships between mGluR5 and several clinically meaningful indices of mood and cognitive function in both BD and MDD. In the BD-Dep group, we observed moderate negative associations between mGluR5 and several indices of cognitive functioning (working memory, psychomotor speed). These findings are particularly notable given increasing evidence suggesting that the neurocognitive difficulties associated with BD not only persist during euthymia(83–90) but are associated with impaired functioning, quality of life and prognosis(91–93). Despite this, current treatments for BD largely fail to address cognitive dysfunction. Promisingly, preclinical literature suggests that positive allosteric modulation of mGluR5 has pro-cognitive effects; the potential for mGluR5 directed treatments to affect cognitive dysfunction in BD, therefore, should excite additional interest in potential use in BD(31). Consistent with previous work(47, 58), though no absolute differences in mGluR5 availability were observed in MDD relative to HC, we observed moderate negative associations between depressed mood and mGluR5 availability across ROIs in MDD, such that lower mGluR5 was associated with higher depression severity. Given that we previously showed that lower mGluR5 was associated with higher Glx(13), the observed association could represent an acute stress-induced increase in extracellular glutamate. mGluR5 could therefore represent a state marker of depression in MDD, perhaps as a consequence of acute stress(30). This is in contrast to BD, where we see lower mGluR5 across both BD-dep and BD-euth, and no clinical correlations, suggesting that mGluR5 could perhaps represent a trait marker in BD. Further work is needed to clarify potential roles of mGluR5 as a state marker in MDD and trait marker in BD. Including MDD-euthymic (i.e., in remission) in future investigations would help answer this question. mGluR5 availability was also associated with working memory in MDD, adding to the evidence base implicating mGluR5 as a substrate and potential treatment target for cognitive dysfunction in mood disorders(31).
A number of limitations of the present study warrant mention. First, our sample size in the BD-euth subgroup, was limited. Comparatively small sample size limits statistical power, and likely contributed to some of the observed non-significant findings. Larger studies confirming and extending the present results are needed. Second, our sample did not include a sub-group in the manic mood state, which limits our ability to determine whether mGluR5 may increase vulnerability to elevated, in addition to depressed, episodes of BD. Third, across clinical groups, between 22 and 30 percent of our sample reported using some type of psychotropic medication (see SI for full medication lists). Inclusion of medicated individuals, while arguably providing greater ecological validity, introduces a potential confound given potential effects on individual neurochemistry. Although in the current study we did not observe effects of psychotropic medications on mGluR5 availability, we lacked the statistical power to assess medications, and specific medication subclasses, in a systematic fashion, and future investigation of the potential influence of past or present medication use on mGluR5 availability in both BD and MDD populations is certainly warranted. Finally, we limited our mGluR5 analysis to subregions of the PFC. Therefore, we cannot comment on possible contribution of regions outside beyond our a priori selected ROIs. Certainly, other region, particularly amygdala and hippocampus, have been implicated in the pathobiology of BD and MDD(32, 64, 65, 67), and will be important to include in future investigations. Finally, measuring both mGluR5 and Glx (ie. Using PET and MRS) would have been ideal to more completely understand the role of glutamate function across MDD and BD. Further work should consider combining imaging modalities.
This study represented the first direct in vivo comparison of mGuR5 availability in BD both relative to MDD and across mood states of BD (depressed and euthymic). Our findings implicate an association between mGluR5 and depressive symptoms in MDD, and as a potentially promising treatment target for clinically meaningful measures of cognitive function in BD. Moreover, based on our results, mGluR5 may show promise as a trait marker for BD, which could aid early differential diagnosis from MDD, particularly during depression. Further, it could represent a target for stabilizing glutamate function/synaptic plasticity in BD. Indeed, given the putative pro-resilient effects of mGluR5 mediated pathways(44, 94), targeting mGluR5 could in turn improve mood and cognitive outcomes in BD. While initial findings are promising, further work is needed to confirm the nature of the observed findings (in terms of trait vs. state), to evaluate the effect of glutamate/mGluR5 modulation in BD relative to MDD, and to examine mGluR5 availability in BD individuals during manic/hypomanic mood states.
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Chemical Compound, radiotracer | 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile, [18F]FPEB | In house. DOI:10.1016/j.apradiso.2014.09.006 | ||
| Software | FSL v3.2 | Analysis Group, FMRIB, Oxford, UK | RRID:SCR_002823 | |
| Software | Bioimage Suite v2.5 | Neuroimageing Tools & Resources Collaboratory | RRID:SCR_002986 | |
| Software | IBM SPSS Statistics v28.0.0.0 | IBM Corporation | RRID:SCR_002865 |
Acknowlegements
This work was funded by the Dana Foundation (Esterlis), The Brain & Behavior Research Foundation (NARSAD, Esterlis), and National Institutes of Health (R01MH104459, Esterlis; T32MH014276-47, Asch; R03 MH118609 and UL1 TR001863, Holmes).
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. (2002): The long-term natural history of the weekly symptomatic status of bipolar I disorder. Archives of general psychiatry. 59:530–537. [DOI] [PubMed] [Google Scholar]
- 2.Kupka R, Altshuler L, Nolen W, Suppes T, Luckenbaugh D, Leverich G, et al. (2007): Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar disorders. 9:531–535. [DOI] [PubMed] [Google Scholar]
- 3.Hirschfeld RM, Cass AR, Holt DC, Carlson CA (2005): Screening for bipolar disorder in patients treated for depression in a family medicine clinic. The Journal of the American board of family practice. 18:233–239. [DOI] [PubMed] [Google Scholar]
- 4.Schmaal L, Harmelen Av, Chatzi V, Lippard E, Toenders Y, Averill L, et al. (2019): Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Molecular psychiatry. 25:408–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pompili M, Gonda X, Serafini G, Innamorati M, Sher L, Amore M, et al. (2013): Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar disorders. 15:457–490. [DOI] [PubMed] [Google Scholar]
- 6.Go Goodwin, Psychopharmacology CGotBAf (2009): Evidence-based guidelines for treating bipolar disorder: revised second edition—recommendations from the British Association for Psychopharmacology. Journal of Psychopharmacology. 23:346–388. [DOI] [PubMed] [Google Scholar]
- 7.Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, et al. (2013): Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar disorders. 15:1–44. [DOI] [PubMed] [Google Scholar]
- 8.Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN (2012): Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar disorders. 14:478–487. [DOI] [PubMed] [Google Scholar]
- 9.Scotti-Muzzi E, Umla-Runge K, Soeiro-de-Souza MG (2021): Anterior cingulate cortex neurometabolites in bipolar disorder are influenced by mood state and medication: A meta-analysis of (1)H-MRS studies. Eur Neuropsychopharmacol. 47:62–73. [DOI] [PubMed] [Google Scholar]
- 10.Szulc A, Wiedlocha M, Waszkiewicz N, Galińska-Skok B, Marcinowicz P, Gierus J, et al. (2018): Proton magnetic resonance spectroscopy changes after lithium treatment. Systematic review. Psychiatry Res Neuroimaging. 273:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Yildiz-Yesiloglu A, Ankerst DP (2006): Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Research: Neuroimaging. 147:1–25. [DOI] [PubMed] [Google Scholar]
- 12.Luykx J, Laban K, Van Den Heuvel M, Boks M, Mandl R, Kahn R, et al. (2012): Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of 1H-MRS findings. Neuroscience & Biobehavioral Reviews. 36:198–205. [DOI] [PubMed] [Google Scholar]
- 13.Abdallah CG, Hannestad J, Mason GF, Holmes SE, DellaGioia N, Sanacora G, et al. (2017): Metabotropic glutamate receptor 5 and glutamate involvement in major depressive disorder: a multimodal imaging study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfrey KE, Muthukumaraswamy SD, Stinear CM, Hoeh N (2021): Effect of rTMS on GABA and glutamate levels in treatment-resistant depression: An MR spectroscopy study. Psychiatry Research: Neuroimaging. 317:111377. [DOI] [PubMed] [Google Scholar]
- 15.Abdallah CG, De Feyter HM, Averill LA, Jiang L, Averill CL, Chowdhury GM, et al. (2018): The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology. 43:2154–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanacora G, Gueorguieva R, Epperson CN, Wu Y-T, Appel M, Rothman DL, et al. (2004): Subtype-Specific Alterations of γ-Aminobutyric Acid and Glutamatein Patients With Major Depression. Archives of general psychiatry. 61:705–713. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto K, Sawa A, Iyo M (2007): Increased levels of glutamate in brains from patients with mood disorders. Biological psychiatry. 62:1310–1316. [DOI] [PubMed] [Google Scholar]
- 18.Abdallah C, Mason G, DellaGioia N, Sanacora G, Jiang L, Matuskey D, et al. (2017): mGluR5 and glutamate involvement in MDD: a multimodal imaging study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truong V, Cheng PZ, Lee H-C, Lane TJ, Hsu T-Y, Duncan NW (2021): Occipital gamma-aminobutyric acid and glutamate-glutamine alterations in major depressive disorder: An mrs study and meta-analysis. Psychiatry Research: Neuroimaging. 308:111238. [DOI] [PubMed] [Google Scholar]
- 20.Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, et al. (2011): Structural Neuroimaging Studies in Major Depressive Disorder: Meta-analysis and Comparison With Bipolar Disorder. Archives of general psychiatry. 68:675–690. [DOI] [PubMed] [Google Scholar]
- 21.Syan SK, Smith M, Frey BN, Remtulla R, Kapczinski F, Hall GB, et al. (2018): Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review. Journal of Psychiatry and Neuroscience. 43:298–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Wang J, Jia Y, Zhong S, Niu M, Sun Y, et al. (2017): Shared and specific intrinsic functional connectivity patterns in unmedicated bipolar disorder and major depressive disorder. Scientific Reports. 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doucet GE, Bassett DS, Yao N, Glahn DC, Frangou S (2017): The role of intrinsic brain functional connectivity in vulnerability and resilience to bipolar disorder. American Journal of Psychiatry. 174:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I (2015): Resting-state functional connectivity in major depressive disorder: a review. Neuroscience & Biobehavioral Reviews. 56:330–344. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015): Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blond BN, Fredericks CA, Blumberg HP (2012): Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala–anterior paralimbic neural system. Bipolar disorders. 14:340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumberg HP, Leung H-C, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, et al. (2003): A functional magnetic resonance imaging study of bipolar disorder: state-and trait-related dysfunction in ventral prefrontal cortices. Archives of general psychiatry. 60:601–609. [DOI] [PubMed] [Google Scholar]
- 28.Tang Y, Kong L, Wu F, Womer F, Jiang W, Cao Y, et al. (2013): Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychological medicine. 43:1921–1927. [DOI] [PubMed] [Google Scholar]
- 29.Arnsten AF (2009): Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popoli M, Yan Z, McEwen BS, Sanacora G (2012): The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 13:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krystal JH, Mathew SJ, D’Souza DC, Garakani A, Gunduz-Bruce H, Charney DS (2010): Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 24:669–693. [DOI] [PubMed] [Google Scholar]
- 32.Esterlis I, Holmes SE, Sharma P, Krystal JH, DeLorenzo C (2018): Metabotropic glutamatergic receptor 5 and stress disorders: Knowledge gained from receptor imaging studies. Biological psychiatry. 84:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fatemi S, Folsom T, Rooney R, Thuras P (2013): mRNA and protein expression for novel GABAA receptors θ and ρ2 are altered in schizophrenia and mood disorders; relevance to FMRP-mGluR5 signaling pathway. Translational psychiatry. 3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fatemi S, Folsom T (2014): Existence of monomer and dimer forms of mGluR5, under reducing conditions in studies of postmortem brain in various psychiatric disorders. Schizophr Res. 158:270–271. [DOI] [PubMed] [Google Scholar]
- 35.Folsom T, Thuras P, Fatemi S (2015): Protein expression of targets of the FMRP regulon is altered in brains of subjects with schizophrenia and mood disorders. Schizophr Res. 165:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLorenzo C, Gallezot J-D, Gardus J, Yang J, Planeta B, Nabulsi N, et al. (2017): In vivo variation in same-day estimates of metabotropic glutamate receptor subtype 5 binding using [11C] ABP688 and [18F] FPEB. Journal of Cerebral Blood Flow & Metabolism. 37:2716–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hefti K, Holst SC, Sovago J, Bachmann V, Buck A, Ametamey SM, et al. (2013): Increased metabotropic glutamate receptor subtype 5 availability in human brain after one night without sleep. Biological psychiatry. 73:161–168. [DOI] [PubMed] [Google Scholar]
- 38.Terbeck S, Akkus F, Chesterman LP, Hasler G (2015): The role of metabotropic glutamate receptor 5 in the pathogenesis of mood disorders and addiction: combining preclinical evidence with human Positron Emission Tomography (PET) studies. Frontiers in neuroscience. 9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cleva RM, Olive MF (2011): Positive allosteric modulators of type 5 metabotropic glutamate receptors (mGluR5) and their therapeutic potential for the treatment of CNS disorders. Molecules. 16:2097–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ménard C, Quirion R (2012): Successful cognitive aging in rats: a role for mGluR5 glutamate receptors, homer 1 proteins and downstream signaling pathways. PLoS One. 7:e28666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahnaou A, Langlois X, Steckler T, Bartolome-Nebreda J, Drinkenburg W (2015): Negative versus positive allosteric modulation of metabotropic glutamate receptors (mGluR5): indices for potential pro-cognitive drug properties based on EEG network oscillations and sleep-wake organization in rats. Psychopharmacology. 232:1107–1122. [DOI] [PubMed] [Google Scholar]
- 42.Milella MS, Marengo L, Larcher K, Fotros A, Dagher A, Rosa-Neto P, et al. (2014): Limbic system mGluR5 availability in cocaine dependent subjects: A high-resolution PET [11C]ABP688 study. NeuroImage. 98:195–202. [DOI] [PubMed] [Google Scholar]
- 43.Milella M, Marengo L, Larcher K, Fotros A, Dagher A, Rosa-Neto P, et al. (2014): Limbic system mGluR5 availability in cocaine dependent subjects: a high-resolution PET [11 C] ABP688 study. Neuroimage. 98:195–202. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Kang S, Choi T-Y, Chang K-A, Koo JW (2022): Metabotropic Glutamate Receptor 5 in Amygdala Target Neurons Regulates Susceptibility to Chronic Social Stress. Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- 45.Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, et al. (2018): Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11C] ABP688 and PET imaging study in depression. Molecular psychiatry. 23:824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmes SE, Girgenti MJ, Davis MT, Pietrzak RH, DellaGioia N, Nabulsi N, et al. (2017): Altered metabotropic glutamate receptor 5 markers in PTSD: In vivo and postmortem evidence. Proceedings of the National Academy of Sciences. 114:8390–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis MT, Hillmer A, Holmes SE, Pietrzak RH, DellaGioia N, Nabulsi N, et al. (2019): In vivo evidence for dysregulation of mGluR5 as a biomarker of suicidal ideation. Proceedings of the National Academy of Sciences. 116:11490–11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.First M, Williams J, Karg R, Spitzer R (2015): Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association.1–94. [Google Scholar]
- 49.Montgomery SA, Åsberg M (1979): A new depression scale designed to be sensitive to change. The British journal of psychiatry. 134:382–389. [DOI] [PubMed] [Google Scholar]
- 50.Pietrzak RH, Maruff P, Mayes LC, Roman SA, Sosa JA, Snyder PJ (2008): An examination of the construct validity and factor structure of the Groton Maze Learning Test, a new measure of spatial working memory, learning efficiency, and error monitoring. Archives of Clinical Neuropsychology. 23:433–445. [DOI] [PubMed] [Google Scholar]
- 51.Darby D, Maruff P, Collie A, McStephen M (2002): Mild cognitive impairment can be detected by multiple assessments in a single day. Neurology. 59:1042–1046. [DOI] [PubMed] [Google Scholar]
- 52.Park E, Sullivan JM, Planeta B, Gallezot J-D, Lim K, Lin S-F, et al. (2015): Test–retest reproducibility of the metabotropic glutamate receptor 5 ligand [18F] FPEB with bolus plus constant infusion in humans. European journal of nuclear medicine and molecular imaging. 42:1530–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan JM, Lim K, Labaree D, Lin S-f, McCarthy TJ, Seibyl JP, et al. (2013): Kinetic analysis of the metabotropic glutamate subtype 5 tracer [18F] FPEB in bolus and bolus-plus-constant-infusion studies in humans. Journal of Cerebral Blood Flow & Metabolism. 33:532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elmenhorst D, Mertens K, Kroll T, Oskamp A, Ermert J, Elmenhorst E, et al. (2016): Circadian variation of metabotropic glutamate receptor 5 availability in the rat brain. J Sleep Res Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.DeLorenzo C, Gallezot J-D, Gardus J, Yang J, Planeta B, Nabulsi N, et al. (2016): In vivo variation in same-day estimates of metabotropic glutamate receptor subtype 5 binding using [11C]ABP688 and [18F]FPEB. Journal of Cerebral Blood Flow & Metabolism. 37:2716–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rolls ET, Huang C-C, Lin C-P, Feng J, Joliot M (2020): Automated anatomical labelling atlas 3. Neuroimage. 206:116189. [DOI] [PubMed] [Google Scholar]
- 57.Patel S, Hamill TG, Connolly B, Jagoda E, Li W, Gibson RE (2007): Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F] F-PEB. Nuclear medicine and biology. 34:1009–1017. [DOI] [PubMed] [Google Scholar]
- 58.Abdallah C (Under Review): mGluR5 and glutamate involvement in MDD: a multimodal imaging study. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elmenhorst D, Mertens K, Kroll T, Oskamp A, Ermert J, Elmenhorst EM, et al. (2016): Circadian variation of metabotropic glutamate receptor 5 availability in the rat brain. J Sleep Res. [DOI] [PubMed] [Google Scholar]
- 60.Lepannetier S, Gualdani R, Tempesta S, Schakman O, Seghers F, Kreis A, et al. (2018): Activation of TRPC1 channel by metabotropic glutamate receptor mGluR5 modulates synaptic plasticity and spatial working memory. Front Cell Neurosci.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manahan-Vaughan D, Braunewell K-H (2005): The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cerebral cortex. 15:1703–1713. [DOI] [PubMed] [Google Scholar]
- 62.Purgert CA, Izumi Y, Jong Y-JI, Kumar V, Zorumski CF, O’Malley KL (2014): Intracellular mGluR5 can mediate synaptic plasticity in the hippocampus. Journal of Neuroscience. 34:4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terbeck S, Akkus F, Chesterman LP, Hasler G (2015): The role of metabotropic glutamate receptor 5 in the pathogenesis of mood disorders and addiction: combining preclinical evidence with human Positron Emission Tomography (PET) studies. Front Neurosci. 9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Liu P, Zhang A, Yang C, Liu S, Wang J, et al. (2021): Specific Gray Matter Volume Changes of the Brain in Unipolar and Bipolar Depression. Front Hum Neurosci. 14:592419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jabbi M, Weber W, Welge J, Nery F, Tallman M, Gable A, et al. (2020): Frontolimbic brain volume abnormalities in bipolar disorder with suicide attempts. Psychiatry Res. 294:113516. [DOI] [PubMed] [Google Scholar]
- 66.Cho I, Goghari V (2020): The relationship between maintenance and manipulation components of working memory and prefrontal and parietal brain regions in bipolar disorder. J Affect Disord. 264:519–526. [DOI] [PubMed] [Google Scholar]
- 67.Abé C, Liberg B, Song J, Petrovic BS, Ekman C, Sellgren C, et al. (2020): Longitudinal Cortical Thickness Changes in Bipolar Disorder and the Relationship to Genetic Risk, Mania, and Lithium Use. Biol Psychiatry. 87:271–281. [DOI] [PubMed] [Google Scholar]
- 68.Niu M, Wang Y, Jia Y, Wang J, Zhong S, Lin J, et al. Common and Specific Abnormalities in Cortical Thickness in Patients with Major Depressive and Bipolar Disorders. EBioMedicine. 16:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abé C, Ekman C, Sellgren C, Petrovic P, Ingvar M, Landén M (2016): Cortical thickness, volume and surface area in patients with bipolar disorder types I and II. J Psychiatry Neurosci. 41:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oertel-Knöchel V, Reuter J, Reinke B, Marbach K, Feddern R, Alves G, et al. Association between age of disease-onset, cognitive performance and cortical thickness in bipolar disorders. J Affect Disord. 174:627–635. [DOI] [PubMed] [Google Scholar]
- 71.Lan M, Chhetry B, Oquendo M, Sublette M, Sullivan G, Mann J, et al. (2014): Cortical thickness differences between bipolar depression and major depressive disorder. Bipolar disorders. 16:378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Najt P, Wang F, Spencer L, Johnston J, Lippard EC, Pittman B, et al. (2016): Anterior Cortical Development During Adolescence in Bipolar Disorder. Biol Psychiatry. 79:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F, Kalmar JH, Womer FY, Edmiston E, Chepenik L, Chen R, et al. (2011): Olfactocentric paralimbic cortex morphology in adolescents with bipolar disorder. Brain. 124:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalmar J, Wang F, Spencer L, Edmiston E, Lacadie C, Martin A, et al. (2009): Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J Int Neuropsychol Soc. 15:476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blumberg H, Krystal J, Bansal R, Martin A, Dziura J, Durkin K, et al. (2006): Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 59:611–618. [DOI] [PubMed] [Google Scholar]
- 76.Winter NR, Leenings R, Ernsting J, Sarink K, Fisch L, Emden D, et al. (2022): Quantifying Deviations of Brain Structure and Function in Major Depressive Disorder Across Neuroimaging Modalities. JAMA Psychiatry. 79:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ketter TA, Wang PW (2003): The emerging differential roles of GABAergic and antiglutamatergic agents in bipolar disorders. Journal of Clinical Psychiatry. 64:15–20. [PubMed] [Google Scholar]
- 78.Popoli M, Yan Z, McEwen BS, Sanacora G (2012): The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature Reviews Neuroscience. 13:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duman RS, Sanacora G, Krystal JH (2019): Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 102:75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, et al. (2018): Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11 C] ABP688 and PET imaging study in depression. Molecular psychiatry. 23:824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holmes SE, Gallezot J-D, Davis MT, DellaGioia N, Matuskey D, Nabulsi N, et al. (2019): Measuring the effects of ketamine on mGluR5 using [18F]FPEB and PET. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism.271678X19886316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Sousa RT, Loch AA, Carvalho AF, Brunoni AR, Haddad MR, Henter ID, et al. (2017): Genetic Studies on the Tripartite Glutamate Synapse in the Pathophysiology and Therapeutics of Mood Disorders. Neuropsychopharmacology. 42:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Normala I, Abdul H, Azlin B, Ruzyanei NN, Hazli Z, Shah S (2010): Executive function and attention span in euthymic patients with bipolar 1 disorder. Med J Malaysia. 65:199–203. [PubMed] [Google Scholar]
- 84.Mur M, Portella M, Martínez-Arán A, Pifarré J, Vieta E (2008): Long-term stability of cognitive impairment in bipolar disorder: a 2-year follow-up study of lithium-treated euthymic bipolar patients. J Clin Psychiatry. 69:712–719. [PubMed] [Google Scholar]
- 85.Torres I, Boudreau V, Yatham L (2007): Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand Suppl.17–26. [DOI] [PubMed] [Google Scholar]
- 86.Thompson J, Gallagher P, Hughes J, Watson S, Gray J, Ferrier I, et al. (2005): Neurocognitive impairment in euthymic patients with bipolar affective disorder. Br J Psychiatry. 186:32–40. [DOI] [PubMed] [Google Scholar]
- 87.Frye M, Gitlin M, Altshuler L (2004): Unmet needs in bipolar depression. Depress Anxiety. 19:199–208. [DOI] [PubMed] [Google Scholar]
- 88.Torrisi S, Moody T, Vizueta N, Thomason M, Monti M, Townsend J, et al. (2013): Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar disorders. 15:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Townsend J, Altshuler L (2012): Emotion processing and regulation in bipolar disorder: a review. Bipolar disorders. 14:326–339. [DOI] [PubMed] [Google Scholar]
- 90.Kerr N, Scott J, Phillips M (2005): Patterns of attentional deficits and emotional bias in bipolar and major depressive disorder. Br J Clin Psychol. 44:343–356. [DOI] [PubMed] [Google Scholar]
- 91.Baune B, Li X, Beblo T (2013): Short- and long-term relationships between neurocognitive performance and general function in bipolar disorder. J Clin Exp Neuropsychol. 35:759–774. Review. [DOI] [PubMed] [Google Scholar]
- 92.Daniel B, Montali A, Gerra M, Innamorati M, Girardi P, Pompili M, et al. (2013): Cognitive impairment and its associations with the path of illness in affective disorders: a comparison between patients with bipolar and unipolar depression in remission. J Psychiatr Pract. 19:275–287. [DOI] [PubMed] [Google Scholar]
- 93.Keilp J, Gorlyn M, Russell M, Oquendo M, Burke A, Harkavy-Friedman J, et al. (2013): Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med. 43:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Z, Ma H, Long S, Zhang Y (2022): Metabotropic Glutamate Receptor 5: A Potential Molecular Switch and Beyond. Biological Psychiatry. 92:98–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


