Abstract
BACKGROUND:
Cardiovascular disease (CVD) may be the main reason for stagnant growth in life expectancy in the US since 2010. The American Heart Association (AHA) recently released updated algorithm for evaluating cardiovascular health (CVH)--life’s essential 8 (LE8) score. We aimed to quantify the associations of CVH levels, estimated by the LE8 score, with life expectancy in a nationally representative sample of US adults.
METHODS:
We included 23,003 non-pregnant, non-institutionalized participants aged 20–79 years who participated in the National Health and Nutrition Examination Survey (NHANES) 2005–2018 and whose mortality was identified through linkage to the National Death Index through December 31, 2019. The overall CVH was evaluated by the LE8 score (range 0–100), as well as the score for each component of diet, physical activity, tobacco/nicotine exposure, sleep duration, body mass index, non-high-density-lipoprotein cholesterol, blood glucose and blood pressure. Life table method was used to estimate life expectancy by levels of the CVH.
RESULTS:
During a median of 7.8 years of follow-up, 1,359 total deaths occurred. The estimated life expectancy at age 50 was 27.3 years (95% CI, 26.1–28.4), 32.9 years (95% CI, 32.3–33.4) and 36.2 years (95% CI, 34.2–38.2) in participants with low (LE8 score<50), moderate (50≤LE8 score<80), and high (LE8 score≥80) CVH, respectively. Equivalently, participants with high CVH had an average 8.9 (95% CI, 6.2–11.5) more years of life expectancy at age 50 compared with those with low CVH. On average, 42.6 % of the gained life expectancy at age 50 from adhering to high CVH was attributable to reduced CVD death. Similarly significant associations of CVH with life expectancy were observed in men and women, respectively. Similarly significant associations of CVH with life expectancy were observed in White participants and Black participants but not in Mexican participants.
CONCLUSIONS:
Adhering to a high CVH, defined as the LE8 score, is related to a considerably increased life expectancy in the US adults, but more research needs to be done in persons of color other than Black (e.g., Hispanic and Asian).
Keywords: Cardiovascular health, CVD mortality, life expectancy
INTRODUCTION
After decades of strong growth, the rise in US life expectancy has stagnated since 20101. A previous study found that a stalled decline in cardiovascular disease (CVD) mortality rate may be responsible for the stalled growth in life expectancy2. CVD accounts for approximately 900,000 deaths in 2019 and is the leading cause of mortality in the US3. To further improve cardiovascular health (CVH) in the general population, and to provide metrics for measuring and monitoring it, the American Heart Association (AHA) recently released the updated algorithm for evaluating CVH--life’s essential 8 (LE8) score4. Compared with the original CVH metrics (Life’s simple 7; LS7), an important update in LE8 is the inclusion of sleep quality (assessed by sleep duration) in the CVH metrics, in addition to the update on the scoring algorithm4.
Life expectancy is one of the most used summary indicators to gauge the overall health of a population. Compared with risk of mortality, the life expectancy estimates are more meaningful metrics for both policy makers and general public because they provide an absolute quantitative assessment. Several previous studies have evaluated the impact of individual CVD risk factors or the number of CVD risk factors on life expectancy5–12. Hasbani et al. also found that adhering to an ideal CVH (evaluated by LS7) was associated with a longer life expectancy as compared with their counterparts with poor CVH13, whereas no study has assessed how well the new LE8 score predict the life expectancy in US adults.
In this study, we assessed the associations of CVH levels, estimated by the LE8 score, with risk of mortality and life expectancy in US adults using data from National Health and Nutrition Examination Survey (NHANES) linked mortality file. Because the differences in life expectancy between sex and race/ethnicity, we also analyzed the associations according to the sex and race/ethnicity.
METHODS
All data and guidance on analytical approaches are publicly and freely available from the US Centers for Disease Control and Prevention’s National Center for Health Statistics and can be accessed at https://www.cdc.gov/nchs/nhanes/index.htm. The study protocols were approved by the institutional review board of the National Center for Health Statistics, and written informed consent was obtained from each participant.
Study design and population
NHANES is a nationally representative, cross-sectional study conducted since 1999 by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention to assess the health and nutritional status of adults and children in the United States. The study design and methods have been described in detail previously (www.cdc.gov/nchs/nhanes/about_nhanes.htm.). We used data from NHANES Linked Mortality File (LMF) which links participants of NHANES with death records in the National Death Index (NDI) dataset through December 31, 2019. Data from 7 cycles (2005–2018) of NHANES-LMF included 36,858 participants aged 20–79 who had available mortality data. The survey protocol was approved by the Research Ethics Review Board of the National Center for Health Statistics. Written informed consent from all participants were obtained by NHANES. A total of 23,003 participants were eligible for this study, after excluding participants who had self-reported CVDs at baseline (n=3,082), who were pregnant or breastfeeding at the time of examination (n=908) and who had missing values on any individual component for CVH, age, sex and race/ethnicity (n=9,865).
Assessments of CVH
The CVH was evaluated by the LE8 score4. The LE8 score contains 8 components: diet, physical activity, tobacco/nicotine exposure, sleep, body mass index (BMI), non-high-density-lipoprotein cholesterol (non-HDL), blood glucose and blood pressure (BP). The Dietary Approaches to Stop Hypertension (DASH) diet score was calculated to evaluate the diet within LE814, by using the mean values of each dietary component collected from 2 nonconsecutive 24-hour dietary recalls at the baseline. The information on physical activity (self-reported minutes of moderate or vigorous physical activity per week), tobacco/nicotine exposure (combustible tobacco use and secondhand smoke exposure), sleep (sleep duration) and medication use were collected by standardized questionnaires. Weight, standing height and BP were measured in mobile examination centers with standard protocols. BMI was calculated as weight in kilograms divided by standing height in meters squared. The average of all available BP measurements at the baseline was used to estimate systolic and diastolic BP (In this study, 97% of participants had three blood pressure measurements). Serum cholesterol was measured enzymatically. Non-HDL cholesterol was calculated by total cholesterol minus HDL cholesterol. Glycated hemoglobin was measured by high-performance liquid chromatography methods. The detailed information and scoring algorithm for each individual CVH metric are provided in the Appendix 1 and previous studies4, 15. The range of each CVH metric was 0–100. The overall CVH was calculated by summing the scores for the 8 metrics and dividing by 8, thus the overall CVH score also ranges from 0–100. We categorized overall CVH into three levels (low: LE8<50, moderate:50≤LE8<80, high: LE8≥80) following the AHA’s recommendations4.
Assessments of covariates
Information on age, sex, race/ethnicity, levels of educational attainment, family income, health insurance were self-reported and collected by standardized questionnaires16. Race/ethnicity was categorized into White, Black, Mexican, other Hispanic, and others (including Asians or multiracial). NHANES oversampled Mexican persons prior to 2007 and oversampled all Hispanic persons from 2007 onward. For combined datasets using survey cycles before and after 2007, the National Center for Health Statistics does not recommend calculating estimates for all Hispanics in cycles prior to 200717, thus we did not combine Mexican and other Hispanic persons in this study. Levels of educational attainment was classified into less than high school degree, high school degree, and some college or above. Ratio of family income to poverty (PIR) was the value of family income divided by official poverty threshold. PIR was classified into <1.3 (low), ≥1.3 and<3.5 (intermediate), and ≥3.5 (high). Health insurance was classified into private insurance, public health insurance only and no health insurance.
Assessments of death
Information on death date of death was identified through linkage to the National Death Index (NDI) through December 31, 2019. Death from CVD was defined as codes I00-I09, I11, I13, I20-I51 and I60-I69 using the International Classification of Diseases, Tenth Revision. The information on ICD-9/ICD10 codes was also derived from the NDI.
The population all-cause mortality rate by single-year ages (50–100 years) in 2019 was derived from the National Center for Health Statistics (by single-year ages and sex or by single-year ages and race, where appropriate)18. We also derived the population CVD mortality rates for 2019 by single-year ages (by single-year ages and sex or by single-year ages and race, where appropriate) ranging from 50 to 84 years from the CDC WONDER database of the US population. Because the database does not provide year-by-year CVD mortality rates over 85 years, we estimated CVD mortality rates in single years of age from 85 to 100 years by extrapolation based on a Poisson regression model with both linear and quadratic terms for the midpoints of single-year age groups minus age of 49.5 years6, 12 (Figure S1).
Statistical analysis
To ensure nationally representative estimates, sampling weights were considered in all analyses to account for oversampling of certain subgroups and complex sample design. Cox proportional hazards models were used to evaluate the association between the levels of CVH and risk of mortality, follow-up time was used as the underlying time metric. Follow-up time was calculated from date of interview or examination until date of death or the end of the study (December 31, 2019). The proportional hazards assumption was tested by Kaplan-Meier method and Schoenfeld residuals method and no violation was found (the range of P-values for the correlation of residuals with ranked failure time was 0.38–0.79). Models were adjusted for following covariates: age (20–34, 35–49, 50–64 and ≥ 65 years old), sex, race/ethnicity, levels of educational attainment, family income and health insurance. Missing data on covariates were coded as a missing indicator category for categorical variables. In sensitivity analysis, we impute missing covariate values with multivariate imputation by chained equations and repeat the analyses. To assess the potential sex or racial/ethnic differences, we performed stratified analyses by sex or race/ethnicity groups (White, Black, and Mexican). To evaluate interactions between CVH and sex or race/ethnicity, multiplicative interaction was assessed by adding interaction terms to the Cox models.
To calculate the life expectancy of participants with different levels of CVH (henceforth “exposure groups”), we used life table method6, 12. We built the life tables starting at age 50 years and ending at age 100 years with the following 3 estimates to calculate the cumulative survival from 50 years onward: (1) age-specific population all-cause mortality rate in 2019 from the National Center for Health Statistics (age- and sex- specific population mortality rate was used when we assessed the association of CVH with life expectancy in men and women; age- and race/ethnicity- specific population mortality rate was used when we assessed the association of CVH with life expectancy in White, Black and Mexican participants)18; (2) adjusted hazard ratios (HRs) of all-cause mortality in each exposure group versus the reference from NHANES; (3) the age-specific population prevalence (categorized in 10-year age groups) of each exposure group from NHANES. The estimated higher survival time (years) due to higher CVH was estimated as difference in the life expectancy at any given age between the reference group and each of the exposure group. Details of the methods used for estimating the difference in expected survival time have been described in Appendix 2. Moreover, we also performed a sensitivity analysis by treating CVH as continuous score (After 50 points of LE8, divide into groups of 10 points: 50-<60, 60-<70, 70-<80, 80-<90, and 90–100). To determine which cause-specific mortality differences were major contributors to the total change in life expectancy, we also estimated the cause-specific contributions to the life expectancy difference between participants with high CVH and those with low CVH with the Arriaga’s decomposition method19, 20. The analytical process consists of two steps: (1) Decomposition by each single age group; (2) Decomposition by cause of death within each single age group The detailed information on the method used for estimating contributions of specific causes of death to the absolute difference in life expectancy has been described in previous studies.
All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc) and R version 3.6.1. Monte Carlo simulation (parametric bootstrapping) with 1000 runs was used to calculate the CIs of the life expectancy estimation with R package boot. All statistical tests were two sided, and we considered P<0.05 to be statistically significant.
RESULTS
Basic characteristics of study participants according to the LE8 score
In the NHANES samples, there were 23,003 adults, representing 148,845,179 US adults. Characteristics of the sample with weighted population numbers are presented in Table 1, 13.3% of participants (representing 19.7 million US adults) had a low CVH (LE8<50); 63.6 % of participants (representing 94.7 million US adults) had a moderate CVH (50≤LE8<80); 23.1 % of participants (representing 34.4 million US adults) had a high CVH (LE8≥80). At baseline, participants with a high CVH were younger, more likely to be women and white, more likely to have a higher level of educational attainment, higher level of family income and a private health insurance (Table 1). Characteristics of participants at baseline by sex or race/ethnicity groups were presented in Table S2 and Table S3, respectively.
Table 1.
Characteristics of participants at baseline by different levels of CVH estimated by the LE8 score.
| Characteristic | Total cohort | Low (LE8<50) |
Moderate (50≤LE8<80) |
High (LE8≥80) |
|---|---|---|---|---|
| Prevalence, % (weighted N) | 149 million | 13.3 (19.7million) | 63.6 (94.7 million) | 23.1 (34.4 million) |
| Number of participants (Sample) | 23,003 | 3,704 | 14,977 | 4,322 |
| Age, years (SE) | 45.7 (0.24) | 50.3 (0.33) | 46.4 (0.25) | 41.9 (0.39) |
| Women, % (weighted N) | 51.8 (77.1 million) | 47.2 (9.3 million) | 49.3 (46.7 million) | 61.4 (21.1 million) |
| Race/ethnicity, % (weighted N) | ||||
| White | 68.8 (102 million) | 65.2 (12.9 million) | 67.9 (64.3 million) | 73.6 (25.3 million) |
| Black | 10.4 (15.5 million) | 16.1 (3.2 million) | 11.0 (10.4 million) | 5.5 (1.9 million) |
| Mexican | 8.3 (12.4 million) | 8.6 (1.7 million) | 8.8 (8.4 million) | 6.7 (2.3 million) |
| Other Hispanic | 5.5 (8.2 million) | 4.9 (1.0 million) | 5.9 (5.5 million) | 4.8 (1.7 million) |
| Others | 6.9 (10.3 million) | 5.2 (1.0 million) | 6.4 (6.1 million) | 9.3 (3.2 million) |
| Educational level, % (weighted N) | ||||
| Less than high school | 13.5 (20.1million) | 25.0 (4.9 million) | 14.1 (13.4 million) | 5.1 (1.8 million) |
| High school | 22.7 (33.8million) | 32.6 (6.4 million) | 24.6 (23.3 million) | 11.8 (4.1 million) |
| Some college or above | 63.8 (94.9million) | 42.3 (8.4 million) | 61.2 (58.0 million) | 83.1 (28.6 million) |
| Family Income, % (weighted N) | ||||
| Low | 17.8 (26.5 million) | 28.3 (5.6 million) | 18.0 (17.0 million) | 11.2 (3.8 million) |
| Intermediate | 32.7 (48.6 million) | 37.2 (7.3 million) | 34.0 (32.2 million) | 26.5 (9.1 million) |
| High | 43.6 (64.9 million) | 28.3 (5.6 million) | 41.8 (39.6 million) | 57.3 (19.7 million) |
| Insurance, % (weighted N) | ||||
| No insurance | 17.7 (26.4 million) | 21.2 (4.2 million) | 18.7 (17.7 million) | 12.9 (4.4 million) |
| Government insurance | 15.5 (23.0 million) | 23.9 (4.7 million) | 15.9 (15.1 million) | 9.4 (3.2 million) |
| Private insurance | 66.6 (99.1 million) | 54.7 (10.8 million) | 65.1 (61.7 million) | 77.4 (26.6 million) |
| AHA Life’s Essential 8 scores (Mean, SE) | ||||
| Mean Total CVH Score (SE) | 67.4 (0.28) | 42.3 (0.15) | 65.4 (0.13) | 87.1 (0.13) |
| Mean DASH diet score (SE) | 46.3 (0.45) | 25.5 (0.58) | 43.3 (0.39) | 66.6 (0.63) |
| Mean physical activity score (SE) | 53.1 (0.76) | 9.3 (0.52) | 48.8 (0.68) | 90.0 (0.52) |
| Mean tobacco/nicotine exposure score (SE) | 70.4 (0.49) | 38.3 (0.96) | 69.4 (0.50) | 91.5 (0.48) |
| Mean sleep health score (SE) | 83.8 (0.28) | 68.2 (0.67) | 83.7 (0.28) | 93.2 (0.30) |
| Mean body mass index score (SE) | 60.9 (0.44) | 34.4 (0.76) | 57.5 (0.41) | 85.3 (0.45) |
| Mean blood lipid score (SE) | 64.5 (0.35) | 43.9 (0.79) | 62.1 (0.36) | 82.8 (0.48) |
| Mean blood glucose score (SE) | 87.5 (0.23) | 68.3 (0.61) | 87.8(0.28) | 97.7 (0.20) |
| Mean blood pressure score (SE) | 72.4 (0.34) | 50.1 (0.65) | 70.6 (0.37) | 90.1 (0.42) |
Data are mean (SE) or percentages (weighted, N). SE, standard error; DASH diet, the Dietary Approaches to Stop Hypertension (DASH) diet score. CVH was categorized into three levels (low: LE8 score <50, moderate:50≤LE8 score <80, high: LE8 score≥80). Missing data on covariates were coded as a missing indicator category for categorical variables.
Association of the LE8 score and risk of mortality
During a median of 7.8 years of follow-up, 1359 total deaths occurred, including 328 deaths from CVD and 1031 deaths from other reasons. The LE8 score was significantly associated with risks of all-cause mortality, either CVD mortality or non-CVD mortality in all participants (Table 2). Compared with participants with low CVH (LE8<50), a high CVH (LE8≥80) was associated with a 61% lower risk of total mortality (HR, 95% CI, 0.39, 0.28–0.53); a 76 % lower risk of CVD mortality (HR, 95% CI, 0.24, 0.12–0.48); and a 55 % lower risk of non-CVD mortality (HR, 95% CI, 0.45, 0.33–0.61); Similar results were observed if multiple imputation was used to impute data for missing covariates (Table S4).
Table 2.
Hazard ratios and 95% confidence interval for category of LE8 with the hazard of mortality
| All-cause | CVD mortality | Non-CVD mortality | ||||
|---|---|---|---|---|---|---|
| Cases/Total* | HRs (95% CI) † | Cases/Total* | HRs (95% CI) † | Cases/Total* | HRs (95% CI) † | |
| CVH score | ||||||
| Low (LE8<50) | 422/3,704 | 1 (reference) | 120/3,704 | 1 (reference) | 302/3,704 | 1 (reference) |
| Moderate (50–79) | 834/14,977 | 0.56 (0.47–0.66) | 192/14,977 | 0.41 (0.31–0.55) | 642/14,977 | 0.62 (0.50–0.76) |
| High (≥80) | 103/4,322 | 0.39 (0.28–0.53) | 16/4,322 | 0.24 (0.12–0.48) | 87/4,322 | 0.45 (0.33–0.61) |
Results adjusted for sex, age, race/ethnicity, education, family income and health insurance.
unweighted number of participants;
sampling weights were considered in analyses;
We did not find significant interaction between sex and LE8 on the risk of all-cause mortality, CVD mortality or non-CVD mortality (Table S5). We found significant race/ethnicity differences in the association between levels of CVH and risk of all-cause mortality, between Mexican and other race/ethnicity groups (Table S6). Specifically, similarly significant associations of the levels of CVH with risk of all-cause mortality were observed in White and Black participants, but not in Mexican participants. The HRs of all-cause mortality across the CVH groups were 1.00 (reference), 0.65 (0.40, 1.07) and 1.53 (0.68, 3.44) among Mexican participants; The corresponding HRs were 1.00 (reference), 0.55 (0.44–0.69) and 0.36 (0.25–0.52) in White participants (Mexican vs. White: P-interaction=0.005); The corresponding HRs were 1.00 (reference), 0.68 (0.55–0.85) and 0.38 (0.20–0.71) in Black participants (Mexican vs. Black: P-interaction=0.026). We did not show the results on the interaction between Mexican and other races/ethnicity on the risks of CVD mortality and non-CVD mortality in the manuscript due to limited CVD mortality cases among the ideal CVH groups for Mexican participants (1 case) and Blacks participants (0 case).
Association of the LE8 score with estimated life expectancy
The estimated life expectancy at age 50 was 27.3 years (95% CI, 26.1–28.4), 32.9 years (95% CI, 32.3–33.4) and 36.2 years (95% CI, 34.1–38.2) in participants with low, moderate, and high CVH, estimated by LE8 score, respectively (Figure 1A). Equivalently, the estimated life expectancy at age 50 was on average 8.9 years (95% CI, 6.2–11.5) longer among participants with high CVH compared with those with low CVH (Figure 1B). In the cause-specific decomposition analysis of the life expectancy differences, we observed that the estimated gained life expectancy from adhering to high CVH was mainly attributable to reduced CVD death. In all participants, an average of 42.6 % (3.79/8.9 years) of the gained life expectancy at age 50 from adhering to high CVH was attributable to reduced CVD death (Figure 1C). We also performed a sensitivity analysis by treating CVH as continuous score (per 10 point). Compared with participants with low CVH (LE8<50), the estimated life expectancy at age 50 increased by an average of 2.2 (95% CI, 1.0–3.2) years for every 10-point of LE8 (Table S7).
Figure 1. The estimates of cumulative survival time from 50 years of age onward among participants with different levels of CVH, estimated by the LE8 score, in total population.
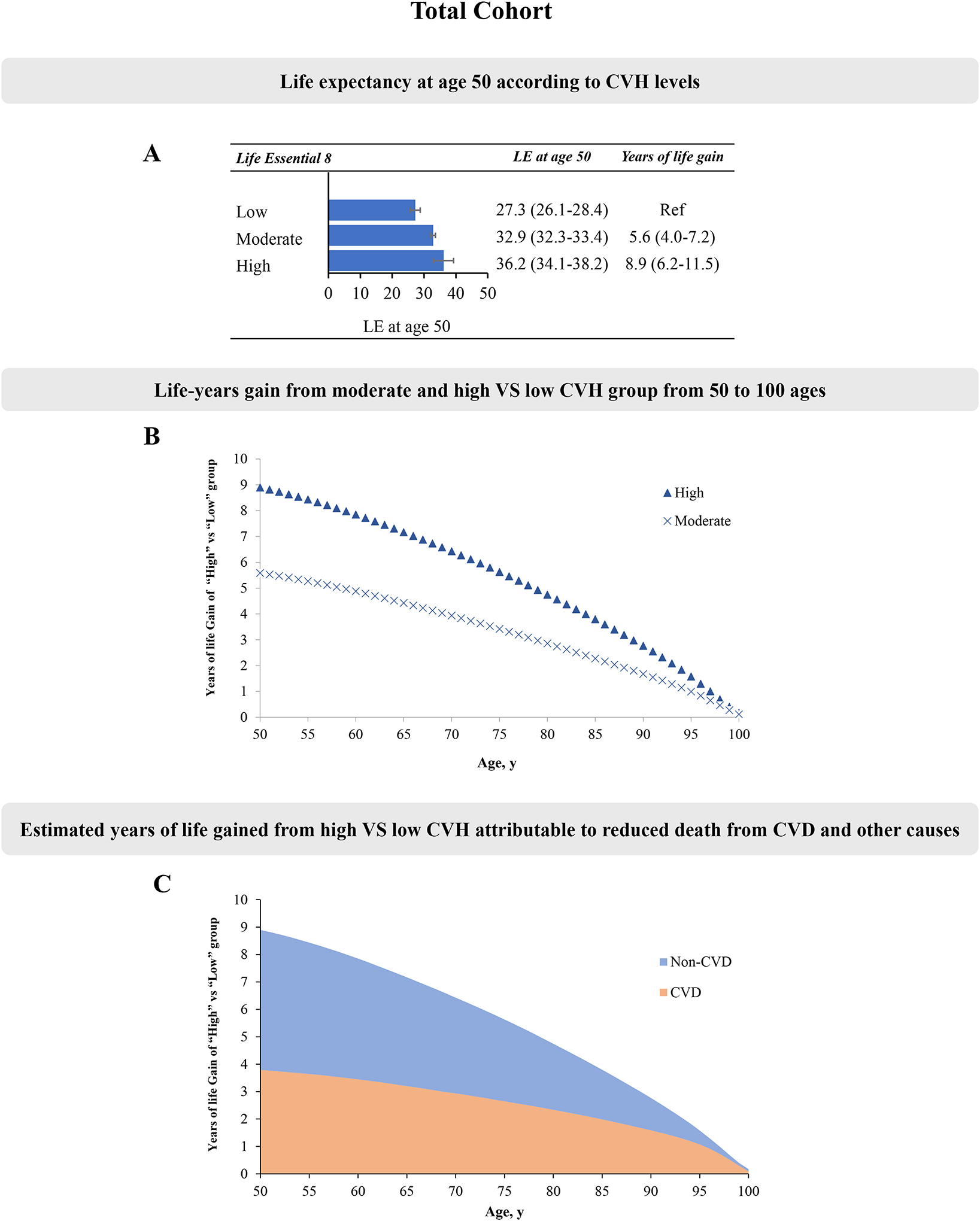
LE, life expectancy. Panel A: Life expectancy at age according to CVH levels; Panel B: Life-years gain from moderate and high versus low CVH group from 50 to 100 ages; Panel C: Estimated years of life gained from high VS low CVH attributable to reduced death from CVD and other causes.
For individual components of CVH, higher levels of DASH diet score, physical activity score, tobacco/nicotine exposure score, sleep health score, glucose score and blood pressure score were associated with a longer life expectancy (Table S8). Among these individual components of CVH, tobacco/nicotine exposure score showed the strongest associations with life expectancy. Participants with high tobacco/nicotine exposure score had an average 7.4 (95% CI, 5.7–9.1) more years of life expectancy compared with those with low tobacco/nicotine exposure score. The new component for CVH--high sleep health score was significantly associated with a longer life expectancy at age 50 by 5.0 (95% CI, 3.2–6.7) years for all participants. For BMI score, participants with moderate level had 2.3 (95% CI, 0.6–3.8) more years at age 50 compared with those with low level, whereas no significant differences for those with high level. Participants with high level of blood lipid score had 2.0 (0.5–3.6) less years at age 50 compared with those with low level.
Associations of the LE8 score with estimated life expectancy by sex
Because the differences in life expectancy between sex, we performed stratified analyses by sex. We observed that higher levels of CVH were associated with longer estimated life expectancy in men and women, respectively (Figure 2). In men, participants with high CVH had 8.1 (95% CI, 4.2–12.0) more years of estimated life expectancy at age 50 compared with those with low CVH. The corresponding difference was 9.3 years (95 %, 5.7–12.7) in women (Figure 2). In men, on average, 40.1 % (3.25/8.1 years) of the gained life expectancy at age 50 from adhering to high CVH was attributable to reduced CVD death; for women, the corresponding percentage was 42.9 % (4.00/9.3 years) (Figure 2E and F).
Figure 2. The estimates of cumulative survival time from 50 years of age onward among participants with different levels of CVH, estimated by the LE8 score, in men and women.
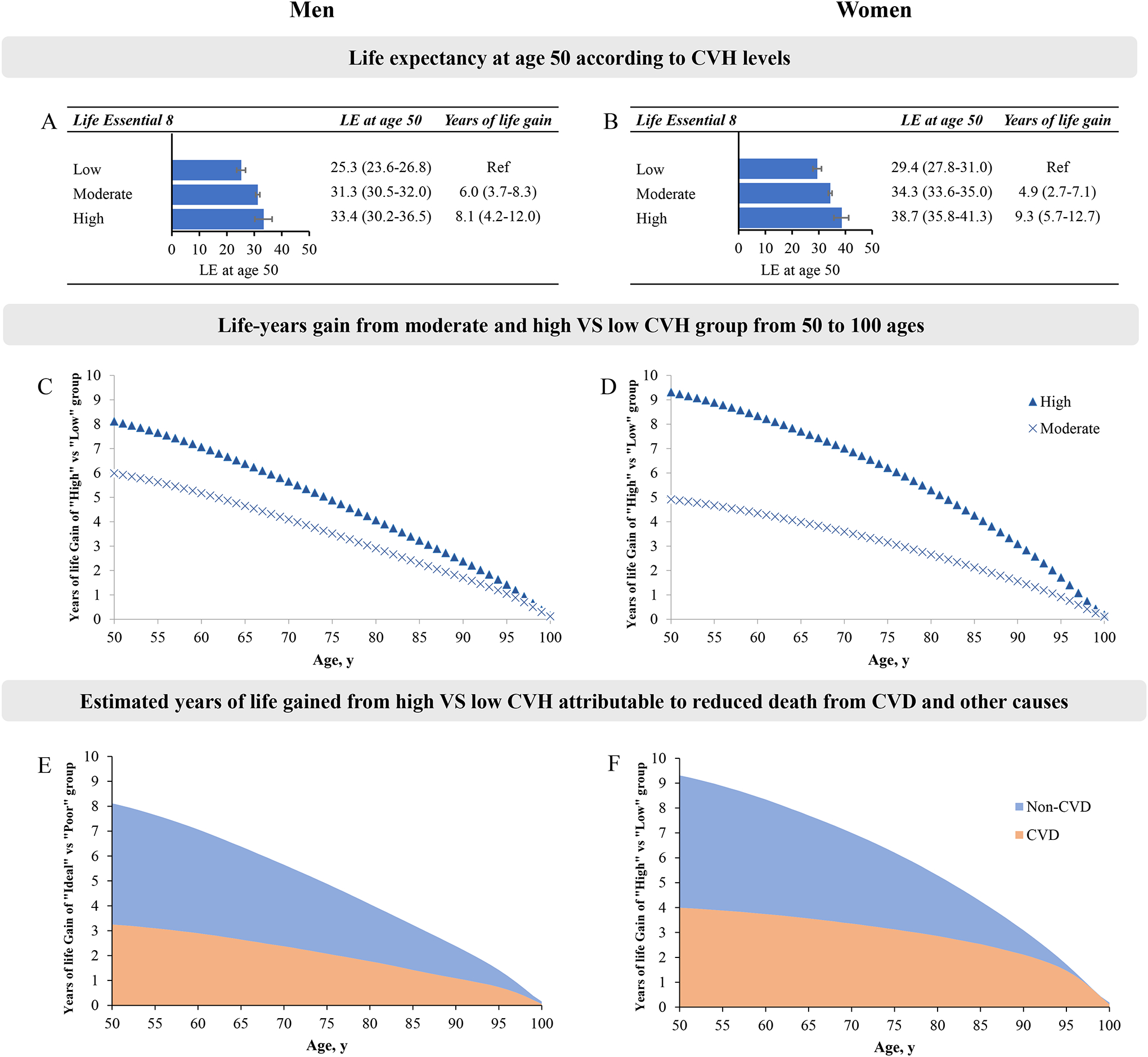
LE, life expectancy. Panels A-B: Life expectancy at age according to CVH levels in men (A) or women (B); Panels C-D: Life-years gain from moderate and high versus low CVH group from 50 to 100 ages in men (C) or women (D); Panels E-F: Estimated years of life gained from high VS low CVH attributable to reduced death from CVD and other causes in men (E) or women (F).
Associations of the LE8 score with estimated life expectancy by race/ethnicity groups
We also performed stratified analyses by race/ethnicity groups (Figure 3). We observed that higher levels of CVH were associated with longer estimated life expectancy in White and Black participants. In White participants, participants with high CVH had 9.6 (95% CI, 6.4–12.7) more years of estimated life expectancy at age 50 compared with those with low CVH. The corresponding difference was 9.9 years (95 %, 3.1–15.9) in Black participants (Figure 3). In White participants, on average, 39.7 % (3.81/9.6 years) of the gained life expectancy at age 50 from adhering to high CVH was attributable to reduced CVD death (Figure S2). Since there were no CVD death in the high CVH group in Black participants, we did not perform the cause-specific decomposition analysis of the life expectancy differences in Black participants. We did not find significant differences in life expectancy among Mexican participants with different levels of CVH (Figure S3).
Figure 3. The estimates of cumulative survival time from 50 years of age onward among participants with different levels of CVH, estimated by the LE8 score, in White and Black participants.
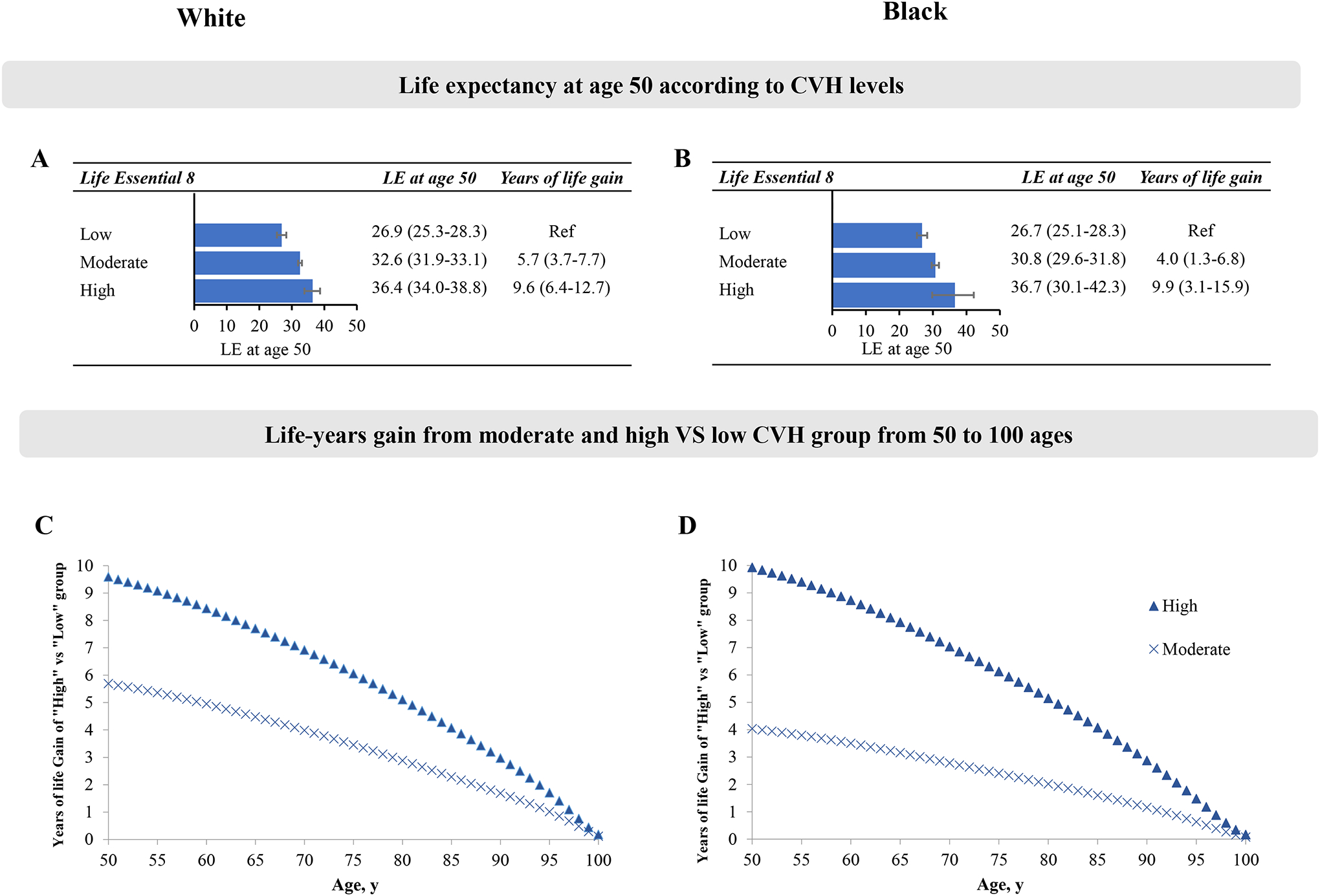
LE, life expectancy. Panels A-B: Life expectancy at age according to CVH levels in White (A) or Black (B) participants; Panels C-D: Life-years gain from moderate and high versus low CVH group from 50 to 100 ages in White (A) or Black (B) participants.
DISCUSSION
In a national representative cohort of US adults, we found that the newly released LE8 metrics, an indicator of CVH, was significantly related to life expectancy. We observed that participants who had a high CVH was associated with a longer life expectancy at age 50 by 8.9 years, as compared with those with low CVH. In addition, we observed that more than 40% estimated gained life expectancy was attributable to reduced death from CVD. In 2019, the life expectancy for US adults at age of 50 was 31.8 years. In this study, we observed that the life expectancies were 36.2 years for participants with high CVH, whereas the corresponding life expectancies were only 27.3 years in participants with low CVH, respectively.
To our knowledge, this is the first study to investigate the associations of the new metric for evaluating CVH--LE8 score with life expectancy in US adults. Several previous studies have investigated the association of individual CVD risk factor or the combination of CVD risk factors with life expectancy in the US population5–12. Stamler et al observed that adhering to a low risk-factor profile (the low risk-factor profile was defined as concurrently meeting serum cholesterol level<200mg/dL, blood pressure ≤120/80 mm Hg and no current smoking) was associated with a longer life expectancy by 6.0 and 5.7 years for middle-aged men and women in the 1990s, compared with those without a low risk-factor profile, respectively5. Li et al observed that adherence to 5 low-risk lifestyle factors was associated with a longer life expectancy at age 50 by 12.2 and 14.0 years for men and women in 2014, respectively, as compared with participants who adopted zero low-risk lifestyle factor6. It is difficult to directly compare our results with previous studies because the use of life tables for different years and the differences in characteristics of study participants, follow-up years and definition of each CVD risk factor. One of strengths of the CVH metrics is that it considers not only healthy lifestyle factors but also CVD related biomarkers21. Moreover, compared with the original CVH metrics (LS7), the use of the new scoring system and the inclusion of sleep factors allow the new CVH metrics (LE8) to provide a more comprehensive and detailed assessment of CVH status at both population and individual levels4, 15.
Moreover, in the cause-specific decomposition analysis of the life expectancy differences, we found that more than 40% of the increased life expectancy at age 50 from adhering to high CVH could be explained by the reduced CVD death. These results indicate that a better CVH reduces not only the burden of CVD but also the burden of other chronic diseases. Several previous studies have shown that a better CVH, which was evaluated by the original CVH metrics (Life’s simple 7), was significantly associated with lower risks of other chronic diseases, such as diabetes, cancer and dementia22–24. Future studies are needed to evaluate the associations of the new CVH metrics (LE8) with risk of other diseases.
In analyses by racial/ethnic groups, we found similar associations of levels of CVH with estimated life expectancy in White and Black participants, but not in Mexican participants, which is the largest Hispanic origin group in the US. These results should be interpreted with caution due to the relatively smaller sample size for Mexican group compared with other racial/ethnic groups in this study (Among Mexican participants, comparing with low CVH group, the statistical power was only 0.54 for high CVH group, the corresponding statistical power was 1.00 and 0.99 among White and Black participants, respectively). Possible reasons for the observed racial/ethnic difference include the differences in the prevalence of high CVH metrics, mortality rates and life expectancy across racial/ethnic groups15, 18. Moreover, substantial evidence has shown that some psychological factors are also closely related to the risk of CVD25, 26. Intriguingly, high levels of social support, familism and optimism are considered important parts of Hispanic culture. Therefore, a good psychological status may buffer the adverse effects of low CVH on the mortality risk and life expectancy14. By contrast, the Northern Manhattan Study found the associations between CVH (defined as Life’s simple 7) and risk of mortality were similar between elderly Caribbean Hispanic participants and other racial/ethnic groups living in the same community27. The inconsistent results may be partly due to the differences in the Hispanic origin, age distribution, and study design. Further studies with larger sample size are needed to investigate the association of CVH with health outcome in Hispanic participants.
The strengths of our study include the use of nationally representative data, the new LE8 score for evaluating the relations of CVH with both mortality and life expectancy, and the stratified analyses by racial/ethnic groups. This study also has several limitations. First, the lifestyle factors, such as diet and sleep duration, were self-reported in NHANES, the measurement errors are inevitable. Second, because information on the CVH metrics was only available at baseline, we did not consider potential changes of CVH during the follow-up period. Notably, the new scoring algorithm gives LE8 score powerful ability to detect intra-individual variation in CVH4, further studies are needed to evaluate the association of change in CVH and health outcomes. Third, for analyses in different racial/ethnic groups, only White, Black and Mexican participants were included in our analyses due to the limited sample size for the other racial/ethnic groups (e.g., Asian). Fourth, we did not include information on e-cigarettes when calculating the LE8 score, as this information was only available after 2013, which would lead to a slight overestimation of the LE8 score.
In conclusion, our study indicates that adhering to a high CVH, defined as the LE8 score, is related to a considerably increased life expectancy, but more research needs to be done in persons of color other than Black (e.g., Hispanic and Asian). Our findings lend support to the validity and importance of the newly released LE8 metrics in assessment and monitoring CVH in the general populations.
Supplementary Material
Clinical Perspective.
What Is New?
We observed that the life expectancies at age 50 were 36.2 years for participants with high CVH, whereas the corresponding life expectancies were only 27.3 years in participants with low CVH, respectively.
Equivalently, participants with high CVH could gain 8.9 years of life expectancy at age 50 compared with those with low CVH.
Moreover, we found that more than 40% of the increased life expectancy at age 50 from adhering to ideal CVH could be explained by the reduced CVD death.
What Are the Clinical Implications?
Our findings lend support to the validity and importance of the newly released LE8 metrics in assessment and monitoring cardiovascular health in the general populations.
Improving CVH may extend life expectancy.
Acknowledgements:
Dr. Qi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Qi, Ma, Wang; Acquisition, analysis, or interpretation of data: Qi, Ma, Wang; Critical revision of the manuscript for important intellectual content: All authors; Drafting of the manuscript: Qi, Ma; Statistical analysis: Ma and Wang.
Sources of Funding:
The study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616).
Non-standard Abbreviations and Acronyms
- CVD
Cardiovascular disease
- CVH
Cardiovascular health
- AHA
American Heart Association
- NHANES
National Health and Nutrition Examination Survey
- LMF
Linked Mortality File
- NDI
National Death Index
- LE8
Life’s essential 8
- LS7
Life’s simple 7
- NCHS
National Center for Health Statistics
- BMI
body mass index
- Non-HDL
non-high-density-lipoprotein cholesterol
- BP
blood glucose and blood pressure
- PIR
family income to poverty
Footnotes
Disclosures: None
Supplemental Materials
References
- 1.Ho JY, Hendi AS. Recent trends in life expectancy across high income countries: retrospective observational study. BMJ. 2018;362:k2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta NK, Abrams LR, Myrskylä M. US life expectancy stalls due to cardiovascular disease, not drug deaths. Proc Natl Acad Sci U S A. 2020;117:6998–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind M, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Allen NB, Anderson C, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, Rosamond W, American Heart Association. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation. 2022;146:e18–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, Dyer AR, Liu K, Greenland P. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–2018. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Pan A, Wang DD, Liu X, Dhana K, Franco OH, Kaptoge S, Di Angelantonio E, Stampfer M, Willett WC, Hu FB. Impact of Healthy Lifestyle Factors on Life Expectancies in the US Population. Circulation. 2018;138:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke R, Emberson J, Fletcher A, Breeze E, Marmot M, Shipley MJ. Life expectancy in relation to cardiovascular risk factors: 38 year follow-up of 19,000 men in the Whitehall study. BMJ. 2009;339:b3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. [DOI] [PubMed] [Google Scholar]
- 9.Bjørnelv G, Halsteinli V, Kulseng BE, Sonntag D, Ødegaard RA. Modeling Obesity in Norway (The MOON Study): A Decision-Analytic Approach-Prevalence, Costs, and Years of Life Lost. Med Decis Making. 2021;41:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li K, Hüsing A, Kaaks R. Lifestyle risk factors and residual life expectancy at age 40: a German cohort study. BMC Med. 2014;12:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chudasama YV, Khunti KK, Zaccardi F, Rowlands AV, Yates T, Gillies CL, Davies MJ, Dhalwani NN. Physical activity, multimorbidity, and life expectancy: a UK Biobank longitudinal study. BMC Med. 2019;17:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, O’Keeffe LM, Gao P, Wood AM, Burgess S, Freitag DF, Pennells L, Peters SA, Hart CL, Håheim LL, Gillum RF, Nordestgaard BG, Psaty BM, Yeap BB, Knuiman MW, Nietert PJ, Kauhanen J, Salonen JT, Kuller LH, Simons LA, van der Schouw YT, Barrett-Connor E, Selmer R, Crespo CJ, Rodriguez B, Verschuren WM, Salomaa V, Svärdsudd K, van der Harst P, Björkelund C, Wilhelmsen L, Wallace RB, Brenner H, Amouyel P, Barr EL, Iso H, Onat A, Trevisan M, Sr DRB, Cooper C, Kavousi M, Welin L, Roussel R, Hu FB, Sato S, Davidson KW, Howard BV, Leening MJ, Leening M, Rosengren A, Dörr M, Deeg DJ, Kiechl S, Stehouwer CD, Nissinen A, Giampaoli S, Donfrancesco C, Kromhout D, Price JF, Peters A, Meade TW, Casiglia E, Lawlor DA, Gallacher J, Nagel D, Franco OH, Assmann G, Dagenais GR, Jukema JW, Sundström J, Woodward M, Brunner EJ, Khaw KT, Wareham NJ, Whitsel EA, Njølstad I, Hedblad B, Wassertheil-Smoller S, Engström G, Rosamond WD, Selvin E, Sattar N, Thompson SG, Danesh J. Association of Cardiometabolic Multimorbidity With Mortality. JAMA. 2015;314:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasbani NR, Ligthart S, Brown MR, Heath AS, Bebo A, Ashley KE, Boerwinkle E, Morrison AC, Folsom AR, Aguilar D, de Vries PS. American Heart Association’s Life’s Simple 7: Lifestyle Recommendations, Polygenic Risk, and Lifetime Risk of Coronary Heart Disease. Circulation. 2022;145:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Piña IL, Ramirez SM, Rodriguez B, Sims M. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd-Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, Foraker RE, Black T, Grandner MA, Allen NB, Anderson C, Lavretsky H, Perak AM. Status of Cardiovascular Health in US Adults and Children Using the American Heart Association’s New “Life’s Essential 8” Metrics: Prevalence Estimates From the National Health and Nutrition Examination Survey (NHANES), 2013 Through 2018. Circulation. 2022;146:822–835. [DOI] [PubMed] [Google Scholar]
- 16.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1. 2013:1–37. [PubMed] [Google Scholar]
- 17.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2. 2013:1–24. [PubMed] [Google Scholar]
- 18.Arias E, Xu J. United States Life Tables, 2019. Natl Vital Stat Rep. 2022;70:1–59. [PubMed] [Google Scholar]
- 19.Arriaga EE. Measuring and explaining the change in life expectancies. Demography. 1984;21:83–96. [PubMed] [Google Scholar]
- 20.Auger N, Feuillet P, Martel S, Lo E, Barry AD, Harper S. Mortality inequality in populations with equal life expectancy: Arriaga’s decomposition method in SAS, Stata, and Excel. Ann Epidemiol. 2014;24:575–580, 580.e1. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabia S, Fayosse A, Dumurgier J, Schnitzler A, Empana JP, Ebmeier KP, Dugravot A, Kivimäki M, Singh-Manoux A. Association of ideal cardiovascular health at age 50 with incidence of dementia: 25 year follow-up of Whitehall II cohort study. BMJ. 2019;366:l4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fretts AM, Howard BV, McKnight B, Duncan GE, Beresford SA, Mete M, Zhang Y, Siscovick DS. Life’s Simple 7 and incidence of diabetes among American Indians: the Strong Heart Family Study. Diabetes Care. 2014;37:2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. [DOI] [PubMed] [Google Scholar]
- 26.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. [DOI] [PubMed] [Google Scholar]
- 27.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woloshin S, Schwartz LM, Welch HG. The risk of death by age, sex, and smoking status in the United States: putting health risks in context. J Natl Cancer Inst. 2008;100:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang CL. Life table and mortality analysis. Life table and mortality analysis.. 1978. pp.xiv + 399 pp. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


