Abstract
Background:
An ever-increasing demand is seen for clear aligners and transparent vacuum-formed retainers. They are esthetic and convenient. However, the biomaterials used in these appliances might pose biological safety and biocompatibility threats in terms of their bisphenol-A (BPA) release, cytotoxicity, adverse effects, and estrogenic effects. Due to the controversial results and the lack of any systematic reviews in this regard, we conducted this systematic review.
Materials and Methods:
Web of Science, PubMed, Cochrane, Scopus, and Google Scholar as well as references of the found articles were searched (independently by 3 researchers) up to December 22, 2021, to find studies relevant to the biocompatibility of clear aligners and thermoplastic retainers. The search keywords were a combination of the following (and more): Essix, vacuum-formed aligner, thermoplastic aligner, clear aligner, Invisalign, vacuum-formed retainer, BPA release, monomer release, cytotoxicity, estrogenicity, biocompatibility, chemical properties, and oral epithelial cell. As eligibility criteria, articles in all languages would be included as long as their text could be translated clearly using online translators or by professional translators; all types of publications (article, book, and thesis) would be included if containing relevant studies and information; they should have been on clear liners or thermoplastic retainers; and they should have been on biocompatibility, safety, cytotoxicity, or estrogenicity of clear aligners or thermoplastic retainers. There were no restrictions on the type of study (randomized clinical trials, experimental in vitro studies). Studies focusing merely on the mechanical properties of clear aligners or thermoplastic retainers (without examining their chemical properties) would be excluded. The risk of bias was assessed.
Results:
The risk of bias was rather low. However, the methodologies of the studies were quite different. Overall, 16 articles (1 randomized clinical trial and 15 in vitro studies) were identified. The data for BPA release were reported in four articles (1 clinical trial and 3 in vitro studies). Quantitatively speaking, the amount of released BPA reported by in vitro studies was very low, if not zero. However, the BPA level was very high in the only randomized clinical trial. Many adverse effects were linked to using clear aligners or transparent retainers, including pain and soft-tissue issues such as burning, tingling, sore tongue, lip swelling, blisters, ulceration, dry mouth, periodontal problems, and even systemic problems such as difficulty in breathing. Besides these biological adverse effects, oral dysfunctions and speech difficulties and tooth damage may be associated with clear aligners and should as well be taken into consideration.
Conclusion:
Given the very high levels of BPA leach observed in the only clinical trial and considering other possible dangers of small traces of BPA (even at low doses) and also given the numerous adverse events linked to clear aligners or transparent retainers, it seems that safety of these appliances might be questionable and more clinical studies of biocompatibility are needed in this regard.
Keywords: Adverse effects, aligner, biocompatibility, biomaterials, bisphenol-A release, clear aligners, cytotoxicity, estrogenic effects, orthodontic treatment, retainer, safety, transparent vacuum-formed thermoplastic retainers, vacuumed form
INTRODUCTION
The introduction of transparent thermoplastic materials in orthodontics leads to increase in demand for more esthetically appliances not only for adults[1] but for preadolescents and children.[2]Patients, who prefer “invisible” treatments, have a tendency toward using clear aligner therapy instead of traditional metal brackets[3] and using transparent vacuum-formed retainers instead of Hawley-type retainers.[4]
Clear aligners are plastic-based trays that could correct some dentofacial malocclusions in specific sequences. They must be worn approximately full-time (22 h a day) except during eating and oral cleaning procedures for 2 weeks and be replaced with another one until completing the duration of orthodontic treatment.[5]During the treatment period, aligners are in close contact with the oral environment for a long time and are continually exposed to heat changes, moisture, respiration, bacteria, and salivary enzymes,[6] and also to trauma in oral function like abrasion at the cusp tips or nonfunctional habits like bruxism.[7]
Polyurethane is the basic component used in plastic-based materials that could be affected by changes in the oral environment surrounding plastics.[8]This contact leads to the release of biologically active substances that could be cytotoxic or estrogenic molecules which are able to cause biological reactions and modify gene expression.[9]
One of the materials with estrogenic properties is bisphenol-A (BPA). This compound is starting monomer for the production of epoxy resins and polycarbonates which is found in the structure of clear aligners and retainers.[10,11,12]Intraorally, degradation of these materials could cause leach BPA due to changes in temperature or PH, mechanical wear, and bacterial or salivary enzymatic activity.[13,14,15]Estrogenic interactions are not only potentially harmful effect of BPA but also proliferative stimulation on human cells and toxicity activity were reported.[16,17,18]According to the United State Environmental Protection Agency (EPA) referenced the Food and Drug Administration protocol, safe intake dosage of BPA is 50 m kg/kg/day.[19]However, adverse events have been seen and mentioned with dosages even below this safe level.[20]
Few controversial studies have investigated the toxicity of clear aligners and their extent of BPA leaching. Some of them did not show any estrogenic or cytotoxic effects in treatments.[15,21]In contrast some others reported undesirable events when using aligners.[22]Therefore, the literature is inconsistent in this regard.[23]
Due to the above-mentioned controversies and lack of any systematic reviews in this regard, the aim of this systematic review was to summarize the biocompatibility and safety of clear aligners and transparent vacuum-formed retainers, such as their BPA release, adverse effects, cytotoxicity, and estrogenic effects.
MATERIALS AND METHODS
This systematic review was prepared according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines.
Search strategy
Internet databases and search engines as well as reference lists of relevant articles were searched up until December 22, 2021, to find studies relevant to the biocompatibility of clear aligners and thermoplastic retainers. The Internet databases and search engines in use were Web of Science, PubMed, Cochrane, Scopus, and Google Scholar. The search keywords were related to the biocompatibility of clear aligners and thermoplastic retainers [Table 1]. The search was done by 3 researchers independently.
Table 1.
Combinations of search keywords
| First keyword | Second keyword | Third keyword |
|---|---|---|
| Essix | BPA | Cytotoxicity |
| Vacuum formed aligner | BPA | Estrogenicity |
| Vacuum-formed aligner | BPA | Biocompatibility |
| Thermoplastic aligner | BPA release | Chemical properties |
| Aligner | BPA release | Oral epithelial cell |
| Clear aligner | BPA release | Oral epithelial cells |
| Invisalign | Monomer | |
| Vacuum formed retainer | Monomer release | |
| Vacuum-formed retainer |
BPA: Bisphenol A
Population, intervention, comparator, and outcomes
The population, intervention, comparator, and outcomes items were as follows: The population was any studies (in vivo or in vitro). The intervention was the use of orthodontic clear aligners and transparent vacuum-formed thermoplastic retainers. The comparator was any control group compared with these experimental groups. The outcomes were any BPA release, adverse effects, cytotoxicity, and estrogenic effects of orthodontic clear aligners and transparent vacuum-formed thermoplastic retainers.
Eligibility criteria
The articles published in the literature were screened for the inclusion and exclusion criteria by three authors. This was first done independently, and after that, jointly through discussion. As for eligibility criteria, there was no year limit. Furthermore, articles in all languages would be included as long as their text could be translated clearly (using online translators or by professional translators). All types of publications (article, book, and thesis) would be included if containing relevant studies and information: The included publications should contain research methodology and results, and hence articles such as editorials or letter to editors or such that had only recommendations would be excluded. The included studies should have been on clear liners or thermoplastic retainers. In addition, they should have been on biocompatibility, safety, cytotoxicity, or estrogenicity of clear aligners or thermoplastic retainers. There were no restrictions on the type of study (e.g., randomized clinical trials and experimental in vitro studies). Studies focusing merely on the mechanical properties of clear aligners or thermoplastic retainers (without examining their chemical properties) would be excluded. Reference lists of the excluded studies were read and checked for relevant articles (e.g., references of articles related to the biocompatibility of other types of appliances other than clear aligners or thermoplastic retainers that had been excluded).
Data collection
The data were collected by a minimum of 3 researchers (and in some cases, 4 researchers), first independently and later through discussion. The outcomes of interest were cytotoxicity (cell viability, cell proliferation, cell reaction such as cell mobility and cell inflammatory response and barrier function), estrogenicity, or monomer and BPA leaching. The other variables sought were study design, sample size, technical analysis method, and grouping of interventions.
Risk of bias
Since there is no standard risk-of-bias assessment tool for in vitro studies, the authors borrowed items from other risk-of-bias assessment tools. A rather comprehensive list of items was assembled for investigating the risk of bias, by taking items from relevant renowned risk-of-bias tools for study types other than in vitro studies (since no risk-of-bias assessment tool existed for in vitro studies). All the included studies were screened for risk of bias using a minimum of 3 researchers (and in some cases, 4 researchers), first independently and afterward by discussion.
Data abstraction and synthesis
A panel of three authors examined the studies summarized as tables to be eligible for synthesis. All the units of measurement were converted to the same unit of ppm. There were no missing summary statistics. Sample heterogeneity was assessed using I2 statistics. Possible causes of heterogeneity among study results were assessed using subgroup analysis. Sensitivity analyses were carried out to assess the robustness of the synthesized results.
Statistical analyses
Only four studies had reported the leach of BPA. Since the methodological characteristics of these studies differed greatly and their timings were not matched, it was not possible to aggregate their results into a meta-analysis. Therefore, the only analysis performed was summarization: Their results were summarized and their variances were calculated.
RESULTS
Study characteristics
The initial search yielded 59 relevant articles. According to the selection criteria and after removing duplicates 19 articles were chosen. After removing 3 overlapped studies, 16 papers were found to meet the criterion, among which there were 15 in vitro investigations and 1 randomized control trial [Figure 1 and Table 2].
Figure 1.
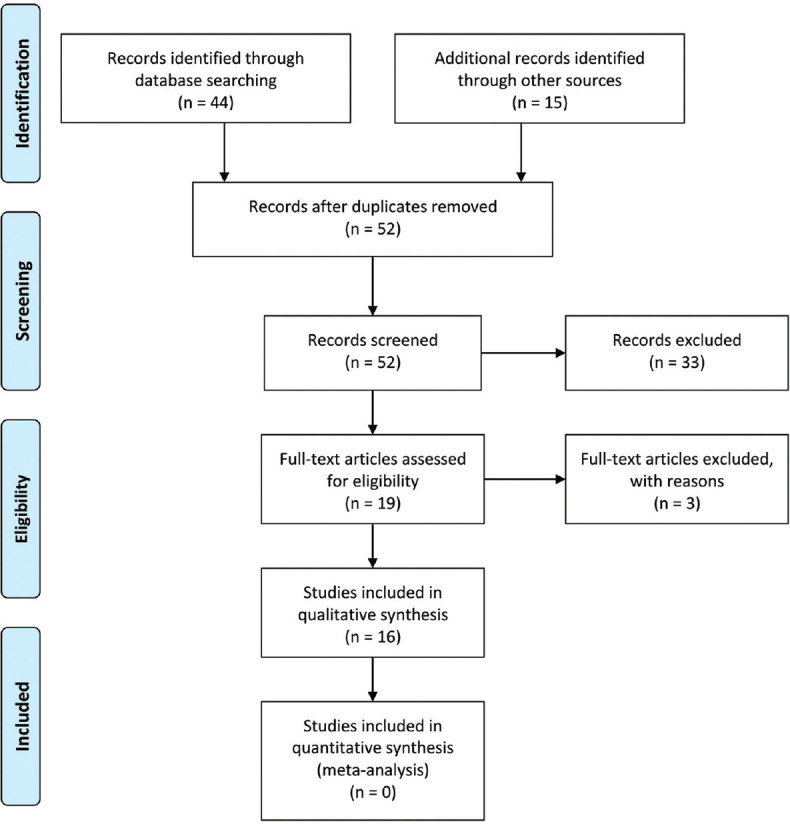
PRISMA flow diagram showing the included studies. PRISMA: Preferred reporting items for systematic reviews and meta-analyses.
Table 2.
All the summarized studies
| Author | Study design | Sample size | Technical analysis method | Groups under comparison | Outcomes |
|---|---|---|---|---|---|
| Schuster etal., (2004) | In vitro | 10 as-received and 12 retrieved aligners | GC-MS | 1. Before placement n=10 2. After retrieval(2weeks) n=12 |
No leaching from the materials.(no residual monomers or oxidative) |
| Eliades etal., (2009) | In vitro | 3 as-received sets of Invisalign(n=6) | 1-MTT assay 2-Estrogenicity assay | 1. Samples of aligner eluents diluted to 5%, 10%, and 20% volume 2. Normal saline solution as the negative control 3. Estradiol and BPA as positive control |
1. No evidence of cytotoxicity on human gingival fibroblast 2. No stimulation on the proliferation of the MCF-7 and no estrogenicity |
| Gracco etal., (2009) | In vitro | 1. One “asreceived” 2. One “asreceived” Invisalign immersed in artificial saliva 3. 10 retrieved Invisalign |
GC-MS | Substances leached from aligners in artificial saliva | No substances released from the aligner in artificial saliva |
| H. MKopperud, etal., (2011) | In vitro | n=5 retainer material cut into circular specimens | GC-MS | 1. One heatcured resin Orthocryl 2. One lightcured(triad VLC) 3. Three thermoplastic materials(Biocryl C, Essix A+, and Essix embrace) |
1. Larger amount of MMA leached from the heat-cured resin 2. Formaldehyde was not detectable in extracts from Biocryl C 3. Minimal leaching was found from the thermoplastic materials |
| Ansari etal., (2014) | In vitro | n=5 retainer material powdered | “Do it yourself test” using potassium permanganate reagent | 1. EVA(1 g) 2. 3A medes(1 g) 3. Ca(1 g) 4. Jaypee(1 g) 5. Ultradent(1 g) 6. Control group |
1. All the products tested leached in variation amount 2. 3A Medes was the first to leach, followed by CA, EVA, Ultradent, and Avac R |
| Kotyka etal., (2014) | In vitro | n=8 retainer material cut into squares | GC-MS | 1. Prethermoformed and thermoformed Biocryl Essix 2. Prethermoformed and thermoformed Biocryl retainer 3. Prethermoformed and thermoformed Dentsply Raintree Essix A 3. Unused and used Invisalign aligner |
1. BPA only leach from thermoformed Biocryl acrylic resin retainer 2. Other materials did not leach 3. Detectable amounts of BPA but were below the reference dose |
| Premaraj etal., (2014) | In vitro | 96 well plates of keratinocytes | 1-MTT assay 2Live/dead flexible stain assay 3-ECIS | 1. Keratinocyte in salinesolution 2. Keratinocyte in salinesolution control(not including particulated Invisalign plastic) 3. Keratinocyte in saliva eluate 4. Keratinocyte in saliva control(not including particulated Invisalign plastic) |
1. Significant increased metabolic inactivity in Saline-solution 2. No significant changes in cell viability in Saliva 3. Salinesolution compromised membrane integrity, reduced cell-to-cell contact and mobility 4. Saliva neutralized or reduced the effects of Invisalign |
| Bradley etal., (2016) | In vitro | Clinically used invisalign appliance (n=5) and as-received aligners of the same brand(n=25) | ATR-FTIR | Chemical composition of the appliances | No important chemical differences in the aging process and molecularcomposition of aligners |
| Afraz Walele1 (2016) | In vitro | n=5 thermoplastic sheet powdered | HPLC | 1. CA 2. Jaypee 3. Ultradent 4. 3A Medes 5. EVA |
1. Jaypee was the least leaching potential 2. The greatest amount of leachingfollowed by EVA, 3AMedes, CA 3. The amounts of leaching varied with the Ultradent |
| Raghavan etal., (2017) | Clinical trial | n=45patients | HPLC | 1. Vacuumformed retainers 2. Hawley retainersheat cure 3. Hawley retainerschemical cure |
1. Significant BPA levels in saliva for all groups. 2. The highest levels: Hawley retainers fabricated by chemical cure 3. The lowest levels: Hawley retainers fabricated by heat cure |
| Al Naghbi etal., (2018) | In vitro | n=6 sets of Vivera retainers(3 as-received and 3 retrieved) | Estrogenicity assays | 1. Asreceived(n=6) 2. Retrieved(n=6) 3. bEstradiol(βE2) was used as positive control(solutions, at concentrations: 5%, 10%, and 20%) |
1. No significant MCD7 proliferation cells. 2. Bestradiol induced a potent stimulation of MCF-7 cells proliferation 3. No effect was observed on MDAMB231 cells |
| Martina etal., (2019) | In vitro | n=4 different brands of aligners | MTT assay | 1. Duran, Biolon, Zendura, SmartTreck 2. DMEM medium was negative control for 100%cell viability 3. Para rubber instead used as a positive control |
1. 4 materials show slight cytotoxicity: Biolon(highest cytotoxicity level on HGFs) followed by Zendura, SmartTreck and finally Duran 2. Thermoformed showed the highest cytotoxicity level |
| Fayyaz Ahmad etal., (2020) | In vitro | n=91 wells of mice fibroblasts(84 as case and 7 as control) | MTT assay | 1. Dental LT 2. EGuard 3. Smart Track Invisalign 4. 7 wells served as control |
1. All materials exhibited slight cytotoxicity 2. Significantly increasing in cell viability from day 1 to 7 3. The higher cytotoxicity: EGuard clear then dental LT and the least cytotoxicity by Smartrack Invisalign 4. Astatistically significant difference in cell viability between Invisalign and Dental LT and Invisalign and E-Guard 5. No significant difference in cell viability between dental LT and E-Guard |
| Nemec etal., (2020) | In vitro | n=69 sets of aligners (cutout disks of 6 mm in diameter) | 1-SEM 2Livecell movie analyzer 3-MTT Assay 4Live/dead stain assay 5-Quantitative real-time PCR | 1. Oral epithelial cells grown on inner surface of SmartTrack 2. Oral epithelial cells were grown on outer surface of SmartTrack 3. Oral epithelial cells grown on tissue culture plastic(control group) |
1. The proliferation/viability of cells growing on aligners was significantly lower 2. Rare occurrence of dead cells on aligners 3. Increasing gene expression level of all inflammatory markers in cells grown on aligners 4. Increasing gene expression levels of the proteins involved in barrier function(Conclusion: Aligner’s material exhibits no cytotoxic effect on oral epithelial cells, but alters their behavior and the expression of proteins involved in the inflammatory response and barrier function |
| El idrissi etal., (2020) | In vitro | n=10 as-received aligners n=10 retrieved aligners | HPLC | 1. Before placement=n=10 2. After retrieval(2weeks) n=10 In artificial saliva |
Chromatograms of bisphenol samples in new and aged aligners did not show traces of bisphenol for up to 8weeks |
| Katras etal., (2021) | In vitro | n=37 glass vials containing each of three types of aligners in 3 different media | HPLC/tandem mass spectrometry (LCMS/MS) | 1. Smile direct club aligner in artificial saliva, artificial gastric fluid, and ethanol on T0, T1, T2, T6, T10, and T20 2. Invisalign aligner in artificial saliva, artificial gastric fluid, and ethanol on T0, T1, T2, T6, T10, and T20 3. Essix Ace aligner in artificial saliva, artificial gastric fluid, and ethanol on T0, T1, T2, T6, T10, and T20 |
1. No significant difference in BPA concentration between the 3 types of aligners in the 3 media 2. The majority of BPA release occurred during the first 24 h 3. BPA released was below the established safety levels for adult patients |
PCR: Polymerase chain reaction; GC-MS: Gas chromatography-mass spectrometry; MTT: 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; ECIS: Electric cell-substrate impedance sensing; ATR-FTIR: Attenuated total reflectance Fourier transform infrared spectroscopy; HPLC: High-performance liquid chromatography; SEM: Scanning electron microscopy; LC-MS: Liquid chromatography with tandem mass spectrometry; BPA: Bisphenol-A; VLC: Visible light-cured; MCF: Michigan cancer foundation; MMA: Methyl methacrylate; MCD: Methyl-β cyclodextrin; HGFs: Human gingival fibroblast; MDA-MB-231 cells: M.D. Anderson - Metastatic Breast 231 cells
In vitro studies
In this review, 15 in vitro research were assessed.[4,6,7,8,9,15,21,22,24,25,26,27,28,29,30]3-[4]5-dimethythiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) assay was used in five research.[15,22,24,26,27]MTT is a colorimetric assay for assessing cell metabolic activity. In four out of five studies that used MTT assay, clear aligner materials were found to be slightly toxic, and cell proliferation and viability were lowered as a result of the clear aligner's action.[22,24,26,27]One of them showed no toxicity effect on human gingival fibroblast[15] [Table 2].
In order for evaluating cell proliferation due to estrogenicity, two research employed the estrogenicity assay.[9,15]The estrogenicity assay involved two cell lines and was based on estrogen receptors: An estrogen-sensitive (Michigan cancer foundation-7) and an estrogen-insensitive (MDAMB-231 human breast adenocarcinoma) were used to exclude the possibility of the decrease in proliferation of cells, drugs, and materials. Both studies found that aligners have no estrogenic effects.[9,15]Gas chromatography-mass spectrometry (GC-MS) was used in three articles to measure leaching from aligner materials.[6,7,21]Both of them were unable to confirm the release of monomers, implying that the chemical substance is stable.[6,7]The third study, which analyzed BPA released rate, indicated that BPA was slightly leached. Nevertheless, a low BPA dose could be due to medical reasons could be cause medical disorders, that should be decreased[21] [Table 2].
Clinical trial
This review took one clinical trial into account. The first research examined BPA level in 45 patients’ saliva before and after using aligners (vacuum-formed, heat cure, and chemical cure). BPA level was found after the placement of three types of aligners.
The results expressed the chemical cured with the highest amount of BPA whereas the heat cured with the lowest amount[23] [Table 2].
Risk of bias
The overall risk of bias was low in almost all studies [Table 3].
Table 3.
Risk of bias assessed for different studies
| Author, year | Was there adequate randomization? | Were baseline conditions similar for different groups? (according to brands or being as-received or retrieved aligners) | Were baseline conditions similar for different groups? | Were experimental procedures similar for different groups? | Were operators blinded of grouping? | Were outcome data complete without missing? | Were all measured outcomes adequately reported? | Were there any reports of outcomes that were not adequately explained in methods? | Any other inconsistencyor source of bias |
|---|---|---|---|---|---|---|---|---|---|
| Schusteretal., (2004) | Yes | No | ? | Yes | Yes | Yes | Yes | No | No |
| Eliadesetal., (2009) | Yes | Yes | ? | No | ? | Yes | Yes | No | No |
| Graccoetal., (2009) | Yes | No | ? | No | ? | Yes | Yes | No | No |
| Kopperud(2011) | Yes | No | No | Yes | No | Yes | Yes | No | No |
| Ansarietal.,(2014) | ?a | No | ? | Yes | ? | Yes | Yes | Yes | No |
| Kotykaetal.,(2014) | Yes | No | ? | Yes | ? | Yes | Yes | No | No |
| Premarajetal.,(2014) | ? | Yes | ? | No | ? | Yes | Yes | No | No |
| Bradleyetal.,(2015) | ? | No | ? | Yes | ? | Yes | Yes | No | No |
| Afraz Walele(2016) | ? | No | ? | Yes | Yes | Yes | Yes | No | No |
| Raghavanetal.,(2017) | Yes | No | ? | No | ? | Yes | Yes | No | No |
| Al Naghbietal.,(2018) | Yes | No | ? | No | ? | Yes | Yes | No | No |
| Martinaetal.,(2019) | ? | No | ? | Yes | Yes | Yes | Yes | No | No |
| Fayyaz Ahmadetal.,(2020) | ? | No | ? | Yes | ? | Yes | Yes | No | No |
| Nemecetal.,(2020) | ? | No | ? | No | ? | Yes | Yes | No | No |
| El idrissietal.,(2020) | Yes | No | ? | Yes | Yes | Yes | Yes | No | No |
| Katras(2021) | Yes | No | ? | Yes | Yes | Yes | Yes | No | No |
aNot explicitly stated, but it appears to be this way from the explanations. ?: Not explicitly given
Adverse events
Bisphenol-A release
Only 4 studies had reported BPA release [Table 4]. In vitro studies mostly showed very low amounts of BPA. However, the clinical trial showed much higher levels of BPA.
Table 4.
Summary of bisphenol-A release at different time points
| Study | n | Groups | Time 1 | Time 2 | Time 3 | Time 4 | Time 5 | Time 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||||
| Mean | Variance | Mean | Variance | Mean | Variance | Mean | Variance | Mean | Variance | Mean | Variance | |||
| Kotykaetal., (2014) | n=8 retainer material cut into squares | 1-Prethermoformed and thermoformed Biocryl Essix | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 2-Prethermoformed Biocryl retainer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| 3-Thermoformed Biocryl retainer | 760 | 846,400 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| 4-Prethermoformed and thermoformed dentsply raintree Essix A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| 5-Unused and used Invisalign aligner | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Raghavanetal., (2017) | n=45 patients | 1-Vacuum-formed retainers | 0.01 | 0.0001 | 1203.36 | 127042.3 | 2384.2 | 3229712 | 203.96 | 7584.668 | ||||
| 2-Hawley retainersheat cure | 0.06 | 0.0016 | 0.91 | 0.6561 | 0.45 | 0.0064 | 0.67 | 1.9881 | ||||||
| 3-Hawley retainerschemical cure | 0.09 | 0.0036 | 60.31 | 650.25 | 3.63 | 0.25 | 9.34 | 5.6169 | ||||||
| El Idrissi Ietal., (2020) | n=10 new aligners | 110 new “SCHEU Dental Clear Aligner®”aligners | 0 | 0 | For up to 8 weeks | |||||||||
| 10 used aligners | 2-10 used aligners from the same supplier after stay in the mouth of 15days | 0 | 0 | For up to 8 weeks | ||||||||||
| Katrasetal., (2021) | n=37 | 1-Smile direct Club aligner in artificial gastric fluid on T0, T1, T2, T6, T10, T20 | 1.53 | 0.3844 | 2.82 | 0.3364 | 0.7 | 0.0196 | 3.11 | 0.7225 | 2.4 | 0.2116 | 2.02 | 0.7569 |
| 2-Smile direct Club aligner in artificial saliva on T0, T1, T2, T6, T10, T20 | 1.4 | 0.0441 | 3.02 | 0.81 | 4.02 | 0.16 | 3.72 | 0.4225 | 3.82 | 0.2116 | 5 | 5.5696 | ||
| 3-Essix ace aligner in artificial gastric fluid on T0, T1, T2, T6, T10, T20 | 1.53 | 3844 | 3.54 | 0.2704 | 3.13 | 0.0625 | 2.73 | 0.6889 | 2.65 | 0.0576 | 1.4 | 0.1089 | ||
| 4Essix ace aligner in artificial saliva on T0, T1, T2, T6, T10, T20 | 1.4 | 0.0441 | 3.26 | 6.9696 | 3.37 | 11.9716 | 4.5 | 11.8336 | 6.3 | 22.5625 | 6.18 | 13.9876 | ||
| 5-Invisalign aligner in artificial gastric fluid on T0, T1, T2, T6, T10, T20 | 1.53 | 0.3844 | 2.39 | 0.09 | 2.66 | 0.0324 | 1.99 | 0.0484 | 2.49 | 0.4096 | 1.9 | 1.4641 | ||
| 6-Invisalign aligner in artificial saliva on T0, T1, T2, T6, T10, T20 | 1.4 | 0.0441 | 3.53 | 3.4596 | 3.16 | 1.7956 | 3.49 | 1.7689 | 0.58 | 0.0049 | 0.51 | 0.1296 | ||
| 7-Smile direct Club aligner in ethanol on T0, T1, T2, T6, T10, T20 | 1.01 | 0.0625 | 1.75 | 0.04 | - | - | - | -0 | 1.9 | 0.0169 | 1.8 | 0.1444 | ||
| 8-Essix Ace aligner in ethanol on T0, T1, T10 and T20 | 1.01 | 0.0625 | 1.52 | 0.2916 | - | - | - | - | 1.87 | 0.1024 | 0.47 | 0.0529 | ||
| 9-Invisalign aligner in ethanol on T0, T1, T10 and T20 | 1.01 | 0.0625 | 7.89 | 17.9776 | - | - | - | - | 9.18 | 22.09 | 3.89 | 6.8644 | ||
Pain and soft tissue problems
Ten of all articles examined pain and soft-tissue problems such as burning, tingling, sore tongue, swelling of lip, blister, ulceration, and dry mouth. All of them confirmed these side effects during wearing clear aligner[31,32,33,34,35,36,37,38,39,40] [Table 5].
Table 5.
Adverse events occurred in different studies
| Author | Difficulty breathing (%) | Sore throat(%) | Swollen throat | Swollen tongue | Hives and itchiness | Anaphylaxis (%) | Swollen lips | Feeling of throat closing/tight airway/airway obstruction/laryngospasm | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nedwed and Miethke[31] | - | - | - | - | - | - | - | - | |||
| Shalishetal.[32] | - | - | - | - | - | - | - | - | |||
| Fujiyamaetal.[33] | - | - | - | - | - | - | - | - | |||
| Thavarajah and Thennukonda[34] | 54 events(30.86) | 66 events (37.71) | Swollen throat was not reported | - | - | 20 events (11.43) | - | - | |||
| Bräscheretal.[35] | - | - | - | - | - | - | - | - | |||
| Allareddyetal.[36] | 56 events | 35 events | 34 events | 31 events | 31 events | 30 events | 27 events | 24 events | |||
| Awosikaetal.[37] | - | - | - | - | Acute urticaria on the neck and face | - | - | - | |||
| Alajmi[38] | - | - | - | - | - | - | - | - | |||
| Lietal.[43] | - | - | - | - | - | - | - | - | |||
| AntonioZancajoetal.[39] | - | - | - | - | - | - | - | - | |||
| Madariagaetal.[41] | - | - | - | - | - | - | - | - | |||
| Wuetal.[42] | - | - | - | - | - | - | - | - | |||
| Notaetal.[40] | - | - | - | - | - | - | - | - | |||
|
| |||||||||||
| Author | Gastrointestinal issues(stomach upset, diarrhea, and vomiting)(%) | Neuromuscular issues(muscle cramps, spasm, and pain)(%) | Fever(%) | Cardiac-related issues(%) | Dry mouth | Headaches (%) | Swelling of eyes | Blisters or sores of lips | Fatigue | Burning/tingling/sore tongue | |
|
| |||||||||||
| Nedwed and Miethke[31] | - | - | - | - | - | - | - | - | - | Mild in 24% andstrong in 6% | |
| Shalishetal.[32] | - | - | - | - | - | - | - | - | - | - | |
| Fujiyamaetal.[33] | - | - | - | - | - | - | - | - | - | - | |
| Thavarajah and Thennukonda[34] | 11 events(6.29) | 13 events (7.43) | 3 events (1.71) | 12 events (6.86) | - | 10 events (5.71) | - | 39 events (22.29%) | - | 38 events (21.71%) | |
| Bräscheretal.[35] | - | - | - | - | - | - | - | - | - | Irritation of tongue | |
| Allareddyetal.[36] | 56 events | 31 events | 31 events | 27 events | 11 events | 10 events | 9 events | 9 events | 8 events | 7 events | |
| Awosikaetal.[37] | - | - | - | - | - | - | Preorbital swelling in a case report | Burning/stinging of the lips and oral mucosa in a case report | - | - | |
| Alajmi[38] | - | - | - | - | - | - | - | - | Assessing daily routine in respect to malaise or fatigue | - | |
| Lietal.[43] | - | - | - | - | - | - | - | - | - | - | |
| AntonioZancajoetal.[39] | - | - | - | - | - | - | - | - | - | - | |
| Madariagaetal.[41] | - | - | - | - | - | - | - | - | - | - | |
| Wuetal.[42] | - | - | - | - | - | - | - | - | - | - | |
| Notaetal.[40] | - | Muscular pain on palpation | - | - | - | - | - | - | - | - | |
|
| |||||||||||
| Author | Chest pain | Cough (%) | Nausea (%) | Difficulty swallowing | Abnormalities of palate (%) | Abnormalities of face (%) | Abnormalities of cheek (%) | Unfavorable attrition,loss of veneers and chipping of teeth (%) | |||
|
| |||||||||||
| Nedwed and Miethke[31] | - | - | - | - | - | - | - | - | |||
| Shalishetal.[32] | - | - | - | - | - | - | - | - | |||
| Fujiyamaetal.[33] | - | - | - | - | - | - | - | - | |||
| Thavarajah and Thennukonda[34] | - | 13 events (7.43) | 12 events (6.86) | - | 5 events (2.86) | 20 events (11.43) | 10 events (5.71) | 8 events (4.6) | |||
| Bräscheretal.[35] | - | - | - | - | - | - | - | - | |||
| Allareddyetal.[36] | 19 events | 19 events | 18 events | 12 events | - | - | - | - | |||
| Awosikaetal.[37] | - | - | - | - | - | - | - | - | |||
| Alajmi[38] | - | - | - | - | - | - | - | - | |||
| Lietal.[43] | - | - | - | - | - | - | - | - | |||
| AntonioZancajoetal.[39] | - | - | - | - | - | - | - | - | |||
| Madariagaetal.[41] | - | - | - | - | - | - | - | - | |||
| Wuetal.[42] | - | - | - | - | - | - | - | - | |||
| Notaetal.[40] | - | - | - | - | - | - | - | - | |||
|
| |||||||||||
| Author | Blisters or ulcerations on tongue | Swelling of gums (%) | Angioedema | Occlusion issues (%) | Dental decay (%) | Periodontal issues leading to loss of teeth (%) | Oral pain | Mucosal irritation and ulceration | Root resorption | Bleeding index, plaque index, probing depth | Oral dysfunction, (difficulties in speech, swallowing, and opening of the mouth) |
|
| |||||||||||
| Nedwed and Miethke[31] | - | - | - | - | - | - | Mild pain | - | - | - | Avoided speaking during the initial phase |
| Shalishetal.[32] | - | - | - | - | - | - | Severe pain in first day | - | - | - | Oral dysfunction significantly decreased over time |
| Fujiyamaetal.[33] | - | - | - | - | - | - | Pain of deformation of attachment and tray and nonsmoothed margins was measured | - | - | - | - |
| Thavarajah and Thennukonda[34] | 11 events of mouth ulcers (6.29%) | 22 events (12.57) | 4 events (2.29%) | 4 events (2.3) | 3 events (1.7) | 5 events (2.9) | - | - | - | - | - |
| Bräscheretal.[35] | - | - | - | - | - | - | Pain during meals | Irritation of buccal mucosa | - | - | - |
| Allareddyetal.[36] | 6 events | 5 events | - | - | - | - | - | - | - | - | - |
|
| |||||||||||
| Author | Blisters or ulcerations on tongue | Swelling of gums (%) | Angioedema | Occlusion issues (%) | Dental decay (%) | Periodontal issues leading to loss of teeth (%) | Oral pain | Mucosal irritation and ulceration | Root resorption | Bleeding index, plaque index, probing depth | Oral dysfunction, (difficulties in speech, swallowing, and opening of the mouth) |
|
| |||||||||||
| Awosikaetal.[37] | Ulcerations of the oral mucosa in a case report | - | Was seen in a case report | - | - | - | - | - | - | - | - |
| Alajmi[38] | - | - | - | - | - | - | Reported experienced pain | Reported more mucosal ulcerations | - | - | Significantlymore difficulty in speech |
| Lietal.[43] | - | - | - | - | - | - | - | - | Apical root resorption was measured by CBCT | - | - |
| AntonioZancajoetal.[39] | - | - | - | - | - | - | Pain and its relationship with the oral quality of life was analysed | - | - | - | - |
| Madariagaetal.[41] | - | - | - | - | - | - | - | - | - | Increased periodontal parameters | - |
| Wuetal.[42] | - | - | - | - | - | - | - | - | - | Increased periodontal parameters | - |
| Notaetal.[40] | - | - | - | - | - | - | - | - | - | - | - |
CBCT: Cone-beam computed tomography
Periodontal problems
Only three out of thirteen articles have examined the periodontal effect of clear aligner which demonstrated the increase in periodontal problems.[34,41,42]Thavarajah and Thennukonda[34] mentioned periodontal effects leading to the loss of teeth (2.9%) [Table 5].[34]
Speech difficulty and oral dysfunctions
Nedwed and Miethke. and Alajmi et al. noticed speech difficulty during clear aligner therapy.[31,38]Shalish et al. indicated oral dysfunction which decreased over time [Table 5].[32]
Systemic problems
Four articles have investigated systemic complications of clear aligner.[32,34,36,37,38]Thavarajah and Thennukonda[34] and Allareddy et al.[36] analyzed several signs and symptoms of the systemic issues. The evidence showed that the difficulty in breathing is the most common of the systemic problems [Table 5].[34,36]
Tooth and restoration problems
Li et al.[43] expressed that apical root resorption is one side effect of clear aligner therapy. Thavarajah and Thennukonda[34] revealed tooth attrition, fail of veneers, and chipping of teeth (4.6%) in orthodontic patients who used clear aligners [Table 5].[34]
DISCUSSION
The increasing demand for the application of thermoplastic clear aligners in both active orthodontic treatment and retention phase indicates the necessity of a comprehensive assessment of this popular and new treatment approach. Fluctuations in oral cavity PH, humidity, pressure, and temperature in addition to the enzymatic function of bacteria and saliva lead to mechanical and chemical transformation of these thermoplastic materials which may result in leaching of unreacted components including BPA.[16,44,45,46,47]Ryokawa et al. evaluated aligners in a stimulated intraoral environment and revealed that these materials absorbed the highest amount of water after 24 and 336 h among all tested materials; and during the study, they did not saturate completely which showed the probability of monomer leaching from the aligners.[48]BPA has been supposed to have a number of estrogenic or cytotoxic effects including disruption of beta-pancreatic cells’ physiologic activity (insulin tolerance) and increased risk of prostate cancer and breast cancer.[49,50]Several publications confirmed adverse effects of BPA even at doses lower than the safe standard dose of 50 μg/Kg weight.[20,51,52,53]Patients usually use each aligner for 22 h every day approximately for 2 weeks; aligners wrap around the teeth and contact approximately a third of the gingiva; hence, their safety toward oral cavity cells including keratinocytes and fibroblasts is of great concern. A number of studies have proposed concerns about BPA release from dental adhesives[54,55] and other orthodontic materials[6,56,57] but evidence on safety grounds of clear aligners is inconsistent and not sufficient.
Findings from most of the experimental in vitro studies support the idea that there is no significant estrogenic or cytotoxic capacity of thermoplastic materials, either for as-received or retrieved appliances. Absolutely, the unique environment of the oral cavity such as unpredictable mastication stresses and fluctuating PH and temperature besides the protective and neutralizing effects of saliva which was supported by Premaraj et al.'s study[22] cannot be simulated perfectly in any in vitro experimental study. In the oral cavity, mastication and attrition induced by the consumption of acidic beverages and enzymatic functions might result in clearer aligner abrasion and attrition which lead to more particle release. This is more pronounced in areas such as cusp tips which are exposed to the pressure of antagonist's teeth during mastication. Perhaps, this is why the only clinical trial in this regard showed much greater levels of BPA leach compared to the in vitro studies.[23]
It is supposed that particulate forms of polymers might induce higher biological action relative to bulk materials due to the increased surface-to-volume ratio which tends to increased reactivity with the surrounding environment.[6]Therefore, studies such as Ansari et al.,[25]Walele et al.,[4] and Premaraj et al.,[22] which considered particulate or powdered forms of aligner materials may be more reflective of the real effect of clear aligners in the oral cavity.
Renewal of aligners in 2-week intervals may expose the patient to another cycle of abrasion and this cumulative cycle may induce the results different from the in vitro studies which consider just a limited range of time. Indeed, studies considering the serial application of new aligners with 2-week intervals in longer periods which can mimic the orthodontic treatment are more reliable and thus recommended. In addition, the oral environment is a physically dynamic media and the gradual force of moving teeth on the clear aligner during orthodontic treatment can be another source of difference between experimental and in vivo studies. Considering all the above-mentioned reasons, the obtained data from these studies should be interpreted with caution.
Currently, a broad spectrum of cytotoxicity and cell viability assays is applied in the fields of toxicology and pharmacology. The choice of the precise assay is crucial in the assessment of obtained results. MTT assay is one of the most commonly used colorimetric assays to evaluate cytotoxicity or cell viability. This assay reveals cell viability through the determination of mitochondrial function by measuring the activity of mitochondrial enzymes such as succinate dehydrogenase. In this assay, MTT is reduced to a purple formazan and light absorbance at a specific wavelength can then quantify this product. This assay is highly reproducible, safe, and more precise than dye exclusion methods; however, experimental studies demonstrated that it tends to produce to false-positive results for viability which induce underestimation of the cytotoxic effect of tested materials.[58,59]MTT formazan is insoluble in water, and it forms purple needle-shaped crystals in the cells. Therefore, before measuring the absorbance, an organic solvent such as dimethyl sulfoxide or isopropanol is required to solubilize the crystals.[58,59]In addition, the cytotoxicity of MTT formazan makes it difficult to remove cell culture media from the plate wells due to floating cells with MTT formazan needles, giving significant well-to-well error. Additional control experiments should be conducted to reduce false-positive or false-negative results that caused by background interference due to the inclusion of particles. This interference could lead to an overestimation of the cell viability.[58,59]Besides, damaged mitochondria may be still able to reduce MTT to formazan crystals.[60,61,62]Loveland revealed that cells with inactivated mitochondria were also able to produce formazan crystals as well as cells with active mitochondria.[63]In the reviewed studies, Martina et al.[26] and Ahamed et al.[24] considered this assay as the only method for the measurement of cell viability and both of the studies revealed no cytotoxicity but this result is prone to all mentioned shortcomings of this assay and should be interpreted with caution. In addition, in their studies, Nemec et al.[27] and Premaraj et al.[22] had considered methods other than MTT and the reduced metabolic function of oral epithelial cells which were exposed to clear aligners and decrease in cell proliferation was demonstrated noting that no cell cytotoxicity was reported. A different result may be expected if these studies were conducted in oral environment conditions.
Another common method for cell viability measurement is the GC-MS analysis. This assay is consisted of a storage condition in the ethanol-water solution which is more aggressive than the oral environment and may not reveal the real behavior of clear aligners. Gracco et al. stated that the environment in this analysis may underestimate the chemical stability of the aligners.[7]Despite the aggressive condition of this assay and underestimated chemical stability, no leaching was revealed in Schuster et al.[6] and Gracco et al.[7] study while the leaching was under the reference dose for daily intake in Kotyk and Wiltshire.[21] study.
In some studies, only a single assay was conducted. Considering all mentioned shortcomings of different assays, it is obvious that a single assay is not sufficient to reflect the precise results. Hence, to increase the reliability of obtained results, more than one assay is recommended to be used in in vitro studies for cytotoxicity determination. Materials of aligners like polyurethane affect the expression of many factors involved in the epithelial barrier function and local inflammatory response.[27]The role of these factors in any of the side effects of aligners’ therapy has still to be established in clinical studies.
Although according to the EPA, the safe dose of BPA for daily intake is supposed to be 50 μg/kg,[19,64,65] there is evidence which show the human seminoma cell proliferation at low doses of BPA; if the low dose of BPA can cross the placenta, it can interfere with the fetal germ cell proliferation and differentiation.[66]In male mice, vom Saal et al. asserted that doses of 0.02, 0.2, and 2.0 Ng/g/day increased adult prostate weight, whereas a 200 Ng/g dose decreased adult prostate weight in male offspring.[52]Their study revealed that a small increase in BPA may change prostate cell differentiation, resulting in a permanent increase in prostatic androgen receptors and prostate size.[52]Hence, BPA leaching even in negligible amounts may presume adverse effects and is not definitely safe.
CONCLUSION
There were considerable variations among the methodologies of the available studies. Still, it seems that quantitatively speaking, the amount of leached BPA reported by in vitro studies is very low, if not zero. However, given the very high levels of BPA leach observed in the only clinical trial and considering other possible dangers of small traces of BPA (even at low doses), as well as noting the frequent adverse events associated with clear aligners and transparent vacuum-formed retainers, it seems that their potential biocompatibility issues should be taken seriously, and therefore, more clinical trials should be conducted to assess the leached BPA amounts and other hazard indicators (such as cytotoxicity) in the oral environment. Such potential effects of BPA even at low doses might describe in part why even despite the observed low amounts of released BPA, many adverse effects were linked to using clear aligners, including pain and soft-tissue issues such as burning, tingling, sore tongue, swelling of lip, blister, ulceration, dry mouth, periodontal problems, and even systemic problems such as difficulty in breathing. Besides these biological adverse effects, oral dysfunctions, and speech difficulties and tooth damages may be associated with clear aligners and should as well be taken into consideration.
Financial support and sponsorship
The study was self-funded by the authors and their institutions.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Ziuchkovski JP, Fields HW, Johnston WM, Lindsey DT. Assessment of perceived orthodontic appliance attractiveness. Am J Orthod Dentofacial Orthop. 2008;133:S68–78. doi: 10.1016/j.ajodo.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Walton DK, Fields HW, Johnston WM, Rosenstiel SF, Firestone AR, Christensen JC. Orthodontic appliance preferences of children and adolescents. Am J Orthod Dentofacial Orthop. 2010;138:12.e1–12. doi: 10.1016/j.ajodo.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Wong BH. Invisalign A to Z. Am J Orthod Dentofacial Orthop. 2002;121:540–1. doi: 10.1067/mod.2002.123036. [DOI] [PubMed] [Google Scholar]
- 4.Walele SP, Chaudhari A, Patil C, Yaragamblimath P, Survase R. Leaching from thermoplastic sheets-A quantitative assessment. Int J Contemp Medical Research. 2016;3:1518–21. [Google Scholar]
- 5.Zheng M, Liu R, Ni Z, Yu Z. Efficiency, effectiveness and treatment stability of clear aligners: A systematic review and meta-analysis. Orthod Craniofac Res. 2017;20:127–33. doi: 10.1111/ocr.12177. [DOI] [PubMed] [Google Scholar]
- 6.Schuster S, Eliades G, Zinelis S, Eliades T, Bradley TG. Structural conformation and leaching from in vitro aged and retrieved Invisalign appliances. Am J Orthod Dentofacial Orthop. 2004;126:725–8. doi: 10.1016/j.ajodo.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Gracco A, Mazzoli A, Favoni O, Conti C, Ferraris P, Tosi G, et al. Short-term chemical and physical changes in invisalign appliances. Aust Orthod J. 2009;25:34–40. [PubMed] [Google Scholar]
- 8.Bradley TG, Teske L, Eliades G, Zinelis S, Eliades T. Do the mechanical and chemical properties of Invisalign TM appliances change after use.A retrieval analysis? Eur J Orthod. 2016;38:27–31. doi: 10.1093/ejo/cjv003. [DOI] [PubMed] [Google Scholar]
- 9.Al Naqbi SR, Pratsinis H, Kletsas D, Eliades T, Athanasiou AE. In vitro assessment of cytotoxicity and estrogenicity of Vivera® retainers. J Contemp Dent Pract. 2018;19:1163–8. [PubMed] [Google Scholar]
- 10.Quesada I, Fuentes E, Viso-León MC, Soria B, Ripoll C, Nadal A. Low doses of the endocrine disruptor Bisphenol-A and the native hormone 17beta-estradiol rapidly activate transcription factor CREB. FASEB J. 2002;16:1671–3. doi: 10.1096/fj.02-0313fje. [DOI] [PubMed] [Google Scholar]
- 11.Azarpazhooh A, Main PA. Pit and fissure sealants in the prevention of dental caries in children and adolescents: A systematic review. J Can Dent Assoc. 2008;74:171–7. [PubMed] [Google Scholar]
- 12.Zampeli D, Papagiannoulis L, Eliades G, Pratsinis H, Kletsas D, Eliades T. In vitro estrogenicity of dental resin sealants. Pediatr Dent. 2012;34:312–6. [PubMed] [Google Scholar]
- 13.Staples CA, Dorn PB, Klecka GM, O’Block ST, Harris LR. A review of the environmental fate, effects, and exposures of Bisphenol A. Chemosphere. 1998;36:2149–73. doi: 10.1016/s0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- 14.Prokop Z, Hanková L, Jeřábek K. Bisphenol A synthesis – Modeling of industrial reactor and catalyst deactivation. React Funct Polym. 2004;60:77–83. [Google Scholar]
- 15.Eliades T, Pratsinis H, Athanasiou AE, Eliades G, Kletsas D. Cytotoxicity and estrogenicity of Invisalign appliances. Am J Orthod Dentofacial Orthop. 2009;136:100–3. doi: 10.1016/j.ajodo.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Olea N, Pulgar R, Pérez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, et al. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaido KW, Leonard LS, Lovell S, Gould JC, Babaï D, Portier CJ, et al. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharmacol. 1997;143:205–12. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- 18.Pupo M, Pisano A, Lappano R, Santolla MF, De Francesco EM, Abonante S, et al. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ Health Perspect. 2012;120:1177–82. doi: 10.1289/ehp.1104526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleisch AF, Sheffield PE, Chinn C, Edelstein BL, Landrigan PJ. Bisphenol A and related compounds in dental materials. Pediatrics. 2010;126:760–8. doi: 10.1542/peds.2009-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekizawa J. Low-dose effects of Bisphenol A: A serious threat to human health? J Toxicol Sci. 2008;33:389–403. doi: 10.2131/jts.33.389. [DOI] [PubMed] [Google Scholar]
- 21.Kotyk MW, Wiltshire WA. An investigation into Bisphenol-A leaching from orthodontic materials. Angle Orthod. 2014;84:516–20. doi: 10.2319/081413-600.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Premaraj T, Simet S, Beatty M, Premaraj S. Oral epithelial cell reaction after exposure to invisalign plastic material. Am J Orthod Dentofacial Orthop. 2014;145:64–71. doi: 10.1016/j.ajodo.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Raghavan AS, Pottipalli Sathyanarayana H, Kailasam V, Padmanabhan S. Comparative evaluation of salivary Bisphenol A levels in patients wearing vacuum-formed and Hawley retainers: An in-vivo study. Am J Orthod Dentofacial Orthop. 2017;151:471–6. doi: 10.1016/j.ajodo.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Ahamed SF, Kumar SM, Kanna A. Cytotoxic evaluation of directly 3D printed aligners and invisalign. Eur J Mol Clin Med. 2020;7:1129–40. [Google Scholar]
- 25.Ansari S, Chaudhari A, Patil C, Pullori S. Simple method of testing polymer leaching from thermoplastic sheets used for clear aligner. APOS Trends Orthod. 2014;4:66. [Google Scholar]
- 26.Martina S, Rongo R, Bucci R, Razionale AV, Valletta R, D’Antò V. In vitro cytotoxicity of different thermoplastic materials for clear aligners. Angle Orthod. 2019;89:942–5. doi: 10.2319/091718-674.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemec M, Bartholomaeus HM, H Bertl M, Behm C, Ali Shokoohi-Tabrizi H, Jonke E, et al. Behaviour of human oral epithelial cells grown on Invisalign(®) SmartTrack(®) material. Materials (Basel) 2020;13:5311. doi: 10.3390/ma13235311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Idrissi I, Bouchafra H, Zaoui F, Cheikh A, Faouzi MA, Bahije L. Assessment of Bisphenol A release by orthodontic aligners: In vitro study. Integr J Med Sci. 2020;7:1–5. [Google Scholar]
- 29.Katras S, Ma D, Al Dayeh A, Tipton D. Bisphenol A release from orthodontic clear aligners: An in-vitro study. Recent Prog Mater. 2021;3:1. [Google Scholar]
- 30.Kopperud HM, Kleven IS, Wellendorf H. Identification and quantification of leachable substances from polymer-based orthodontic base-plate materials. Eur J Orthod. 2011;33:26–31. doi: 10.1093/ejo/cjq020. [DOI] [PubMed] [Google Scholar]
- 31.Nedwed V, Miethke RR. Motivation, acceptance and problems of invisalign patients. J Orofac Orthop. 2005;66:162–73. doi: 10.1007/s00056-005-0429-0. [DOI] [PubMed] [Google Scholar]
- 32.Shalish M, Cooper-Kazaz R, Ivgi I, Canetti L, Tsur B, Bachar E, et al. Adult patients’ adjustability to orthodontic appliances.Part I: A comparison between Labial, Lingual, and Invisalign™. Eur J Orthod. 2012;34:724–30. doi: 10.1093/ejo/cjr086. [DOI] [PubMed] [Google Scholar]
- 33.Fujiyama K, Honjo T, Suzuki M, Matsuoka S, Deguchi T. Analysis of pain level in cases treated with Invisalign aligner: Comparison with fixed edgewise appliance therapy. Prog Orthod. 2014;15:64. doi: 10.1186/s40510-014-0064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thavarajah R, Thennukonda RA. Analysis of adverse events with use of orthodontic sequential aligners as reported in the manufacturer and user facility device experience database. Indian J Dent Res. 2015;26:582–7. doi: 10.4103/0970-9290.176919. [DOI] [PubMed] [Google Scholar]
- 35.Bräscher AK, Zuran D, Feldmann RE Jr , Benrath J.Patient survey on Invisalign(®) treatment comparing [corrected] the SmartTrack(®) material to the previously used [corrected] aligner material. J Orofac Orthop. 2016;77:432–8. doi: 10.1007/s00056-016-0051-3. [DOI] [PubMed] [Google Scholar]
- 36.Allareddy V, Nalliah R, Lee MK, Rampa S, Allareddy V. Adverse clinical events reported during invisalign treatment: Analysis of the MAUDE database. Am J Orthod Dentofacial Orthop. 2017;152:706–10. doi: 10.1016/j.ajodo.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Awosika O, Kao S, Rengifo-Pardo M, Ehrlich A. Angioedema, stomatitis, and urticaria caused by contact allergy to invisalign. Dermatitis. 2017;28:323–4. doi: 10.1097/DER.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 38.Alajmi S, Shaban A, Al-Azemi R. Comparison of short-term oral impacts experienced by patients treated with invisalign or conventional fixed orthodontic appliances. Med Princ Pract. 2020;29:382–8. doi: 10.1159/000505459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonio-Zancajo L, Montero J, Albaladejo A, Oteo-Calatayud MD, Alvarado-Lorenzo A. Pain and oral-health-related quality of life in orthodontic patients during initial therapy with conventional, low-friction, and lingual brackets and aligners (invisalign): A prospective clinical study. J Clin Med. 2020;9:2088. doi: 10.3390/jcm9072088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nota A, Caruso S, Ehsani S, Ferrazzano GF, Gatto R, Tecco S. Short-term effect of orthodontic treatment with clear aligners on pain and sEMG Activity of Masticatory Muscles. Medicina (Kaunas) 2021;57:178. doi: 10.3390/medicina57020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madariaga AC, Bucci R, Rongo R, Simeon V, D’Antò V, Valletta R. Impact of fixed orthodontic appliance and clear aligners on the periodontal health: A prospective clinical study. Dent J (Basel) 2020;8:4. doi: 10.3390/dj8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Y, Cao L, Cong J. The periodontal status of removable appliances versus fixed appliances: A comparative meta-analysis. Medicine (Baltimore) 2020;99:e23165. doi: 10.1097/MD.0000000000023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Deng S, Mei L, Li Z, Zhang X, Yang C, et al. Prevalence and severity of apical root resorption during orthodontic treatment with clear aligners and fixed appliances: A cone beam computed tomography study. Prog Orthod. 2020;21:1. doi: 10.1186/s40510-019-0301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Hiyasat AS, Darmani H, Elbetieha AM. Leached components from dental composites and their effects on fertility of female mice. Eur J Oral Sci. 2004;112:267–72. doi: 10.1111/j.1600-0722.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 45.Darmani H, Al-Hiyasat AS, Milhem MM. Cytotoxicity of dental composites and their leached components. Quintessence Int. 2007;38:789–95. [PubMed] [Google Scholar]
- 46.Gioka C, Eliades T, Zinelis S, Pratsinis H, Athanasiou AE, Eliades G, et al. Characterization and in vitro estrogenicity of orthodontic adhesive particulates produced by simulated debonding. Dent Mater. 2009;25:376–82. doi: 10.1016/j.dental.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Kingman A, Hyman J, Masten SA, Jayaram B, Smith C, Eichmiller F, et al. Bisphenol A and other compounds in human saliva and urine associated with the placement of composite restorations. J Am Dent Assoc. 2012;143:1292–302. doi: 10.14219/jada.archive.2012.0090. [DOI] [PubMed] [Google Scholar]
- 48.Ryokawa H, Miyazaki Y, Fujishima A, Miyazaki T, Maki K. The mechanical properties of dental thermoplastic materials in a simulated intraoral environment. Orthod Waves. 2006;65:64–72. [Google Scholar]
- 49.Soto AM, Murai JT, Siiteri PK, Sonnenschein C. Control of cell proliferation: Evidence for negative control on estrogen-sensitive T47D human breast cancer cells. Cancer Res. 1986;46:2271–5. [PubMed] [Google Scholar]
- 50.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci U S A. 2005;102:7014–9. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of Bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–33. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A. 1997;94:2056–61. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of Bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 54.Eliades T, Voutsa D, Sifakakis I, Makou M, Katsaros C. Release of bisphenol-A from a light-cured adhesive bonded to lingual fixed retainers. Am J Orthod Dentofacial Orthop. 2011;139:192–5. doi: 10.1016/j.ajodo.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 55.Kang YG, Kim JY, Kim J, Won PJ, Nam JH. Release of bisphenol A from resin composite used to bond orthodontic lingual retainers. Am J Orthod Dentofacial Orthop. 2011;140:779–89. doi: 10.1016/j.ajodo.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki K, Ishikawa K, Sugiyama K, Furuta H, Nishimura F. Content and release of Bisphenol A from polycarbonate dental products. Dent Mater J. 2000;19:389–95. doi: 10.4012/dmj.19.389. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe M, Hase T, Imai Y. Change in the bisphenol A content in a polycarbonate orthodontic bracket and its leaching characteristics in water. Dent Mater J. 2001;20:353–8. doi: 10.4012/dmj.20.353. [DOI] [PubMed] [Google Scholar]
- 58.Karakaş D, Ari F, Ulukaya E. The MTT viability assay yields strikingly false-positive viabilities although the cells are killed by some plant extracts. Turk J Biol. 2017;41:919–25. doi: 10.3906/biy-1703-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aslantürk ÖS. Genotoxicity-A Predictable Risk to Our Actual World. Vol. 2. London, United Kingdom: IntechOpen; 2018. In vitro cytotoxicity and cell viability assays: Principles, advantages, and disadvantages; pp. 64–80. [Google Scholar]
- 60.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 61.Pagé M, Bejaoui N, Cinq-Mars B, Lemieux P. Optimization of the tetrazolium-based colorimetric assay for the measurement of cell number and cytotoxicity. Int J Immunopharmacol. 1988;10:785–93. doi: 10.1016/0192-0561(88)90001-x. [DOI] [PubMed] [Google Scholar]
- 62.Sieuwerts AM, Klijn JG, Peters HA, Foekens JA. The MTT tetrazolium salt assay scrutinized: How to use this assay reliably to measure metabolic activity of cell cultures in vitro for the assessment of growth characteristics, IC50-values and cell survival. Eur J Clin Chem Clin Biochem. 1995;33:813–23. doi: 10.1515/cclm.1995.33.11.813. [DOI] [PubMed] [Google Scholar]
- 63.Loveland BE, Johns TG, Mackay IR, Vaillant F, Wang ZX, Hertzog PJ. Validation of the MTT dye assay for enumeration of cells in proliferative and antiproliferative assays. Biochem Int. 1992;27:501–10. [PubMed] [Google Scholar]
- 64.Alonso-Magdalena P, Ropero AB, Soriano S, Quesada I, Nadal A. Bisphenol-A: A new diabetogenic factor? Hormones (Athens) 2010;9:118–26. doi: 10.1007/BF03401277. [DOI] [PubMed] [Google Scholar]
- 65.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bouskine A, Nebout M, Brücker-Davis F, Benahmed M, Fenichel P. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect. 2009;117:1053–8. doi: 10.1289/ehp.0800367. [DOI] [PMC free article] [PubMed] [Google Scholar]


