Abstract
Background and Objectives
This study investigates whether subjective memory decline (SMD) in a racially diverse sample of older adults without cognitive impairment at baseline is associated with incident cognitive impairment during a 12-year follow-up period.
Research Design and Methods
With panel data from a national sample (N = 9,244) of cognitively intact Black, White, and Hispanic Americans 65 years or older in 2004, we examine if SMD is associated with the loss of normal cognition by 2016. Cognitive status was assessed every 2 years with a modified version of the Telephone Interview for Cognitive Status to identify the transition from normal cognition to cognitive impairment.
Results
Estimates from Weibull accelerated failure-time models reveal that SMD is associated with earlier incident cognitive impairment (time ratio = 0.96, p < .05). In subsequent models stratified by race-ethnicity, this association was evident among White respondents (time ratio = 0.95, p < .01) but not among Black, U.S.-born Hispanic, or foreign-born Hispanic respondents.
Discussion and Implications
Given that the prognostic validity of SMD differs by race and ethnicity, caution is warranted when using it as a screening or clinical tool in diverse populations.
Keywords: Cognitive function, Disparities, Racial-ethnic differences, Self-reported memory
Background and Objectives
Cognitive impairment is a significant problem in many societies that are experiencing population aging, and subjective memory concerns may be an early indicator of worsening cognitive function or the risk of dementia. Yet, most forms of Alzheimer’s disease and related dementias tend to develop gradually. Thus, it is possible that a person may report worsening memory (or cognition more generally) years before cognitive tests and clinical evaluation can render a definitive diagnosis. Subjective memory decline (SMD) refers to when a person reports that his or her memory function is getting worse regardless of whether there is objective evidence of cognitive decline resulting in impairment (Blazer et al., 1997; Jessen et al., 2020).
Researchers have been intrigued by the prospect of a single measure of subjective (self-reported) memory decline to predict future cognitive decline, but other scholars are critical of the measure because some older adults view memory decline as a natural part of aging, thus underreporting memory problems. Although the correlation between subjective and objective memory is modest in cross-sectional studies, substantial literature shows that SMD is a predictor of objective cognitive decline based on well-established tests of memory, attention, and learning.
Multiple studies reveal that it may take years after SMD (perhaps a decade) to detect objectively measured cognitive decline. The most compelling findings derive from studies with long observation windows, such as 14 (Reisberg et al., 2010) or 18 years (Kaup et al., 2015; Verlinden et al., 2016). Jessen et al. (2020) conclude that subjective cognitive decline (SCD) generally occurs about “10 years before dementia diagnosis” (p. 272), but others assert that the onset of SCD within the past 5 years heightens the risk of preclinical Alzheimer’s disease (Rabin et al., 2015). Indeed, Jorm et al. (2001) examined a sample of persons 70 years or older and found that memory complaints predicted poorer memory performance 3 or 4 years later. The age of the sample is important for interpreting results from these studies. The older the sample, the more likely one may be able to observe that SCD predicts the risk of objectively measured cognitive decline.
Although there is debate regarding the length of time required to observe a link between subjective and objective cognitive decline, the literature has grown rapidly because there is ample evidence that SCD is an early harbinger of mild cognitive impairment and Alzheimer’s disease. Despite its scientific and clinical promise, one serious limitation of this literature is a lack of attention to racial and ethnic variability. We are unaware of any long-term longitudinal study of differences between non-Hispanic Black, non-Hispanic White (hereafter, Black and White), and Hispanic Americans in the relationship between SMD and subsequent objective cognitive decline indicative of impairment.
Related Research and Study Rationale
Research on whether SMD predicts objective cognitive decline in diverse populations is important for two main reasons. First, the percentage of the older-adult population that is Black or Hispanic American will grow considerably between 2018 and 2060—from 9.1 to 12.8 and from 8.4 to 21.0, respectively (Frey, 2018; Vespa et al., 2020). Second, previous research reveals that Black and Hispanic Americans have a notably higher prevalence and incidence of cognitive impairment and dementia than do White Americans (Matthews et al., 2019; Mayeda et al., 2016). Given both population aging and racial-ethnic disparities in the risk of Alzheimer’s disease and related dementias, there is a compelling need to determine whether SMD is useful for predicting an earlier transition to cognitive impairment for Black and Hispanic Americans.
To address this gap, we reviewed evidence from three types of studies related to whether SMD predicts incident cognitive impairment in diverse populations: prevalence, concurrent validity (based on cross-sectional analysis), and prognostic validity (based on longitudinal analysis). First, several studies used data from the Behavioral Risk Factor Surveillance Study (BRFSS) to examine the prevalence of SMD by race and ethnicity. Although each study measures the outcome similarly (confusion or memory loss during the past 12 months), Adams et al. (2013) studied people 60 years or older in 21 states and concluded that Hispanic Americans have the highest prevalence of SMD (16.9%) among six ethnic groups studied. Prevalence among Black Americans was 11.8%, and among White Americans was 12.1%. In contrast, two studies used the BRFSS data for people 45 years or older and showed that Hispanic Americans have a lower prevalence of SMD than Black and White Americans; Taylor et al. (2018) used data from 2015 to 2016 while Gupta (2021) analyzed data from 2015 through 2018. Based on these prevalence studies, it is unclear whether Hispanic Americans are more likely than White Americans to report worsening memory.
Second, there is a large literature on concurrent validity derived from cross-sectional data, but authors may refer to the subjective measure of decline using slightly different terms (SMD, SCD, or memory complaints). Nevertheless, relatively few of these studies systematically examine racial-ethnic differences. We identified two studies giving explicit attention to the relationship between SMD and cognitive performance, either among Black respondents or by comparing Black and White adults. One was restricted to Black older adults in Baltimore (Sims et al., 2011), and a second used a sample of 289 older respondents, including 47 Black adults (Jackson et al., 2017). Both concluded that SMD is not related to concurrent memory performance among Black respondents, but Jackson et al. (2017) reported that SMD was related to memory among White respondents.
We also identified five studies giving explicit attention to the relationship between SMD and cognitive performance among Hispanic adults, but the conclusions were inconsistent. Three studies reported that SMD predicts cognitive performance among Hispanic Americans (Nakhla et al., 2021; Rodríguez et al., 2021; Zlatar et al., 2022); and two studies reported that SMD is not related to cognitive performance (Harwood et al., 1998; Zlatar et al., 2018). Indeed, Zlatar et al. (2018) concluded that “SCD does not accurately reflect concurrent cognitive performance” in a clinical sample of older adults (p. 1198). A different conclusion was reached, however, when studying a multisite probability sample (Hispanic Community Health Study/Study of Latinos): SCD “may be an indicator of concurrent objective condition in diverse middle-aged and older community-dwelling Hispanics/Latinos” (Zlatar et al., 2022, p. 1). None of the five studies, however, examined differences by nativity, which might be related to incident cognitive impairment because U.S.-born and foreign-born Hispanic adults are distinct in many ways, including selective migration, preferred language, and health (Garcia et al., 2017; Hummer & Hayward, 2015).
Third, many studies focusing on the prognostic validity of SMD use longitudinal data and report that among persons free of cognitive impairment, SMD is related to cognitive decline (Jorm et al., 2001; Kaup et al., 2015; Reisberg et al., 2010; van Harten et al., 2018) and dementia (Verlinden et al., 2016). Other studies report that SMD does not predict a subsequent decline in cognitive function (Rickenbach et al., 2015). Unfortunately, most of these longitudinal studies do not address directly whether the relationship between SMD and cognitive decline varies by race-ethnicity.
We identified two studies, however, that systematically examined Black–White variability in the prognostic validity of SMD. Using longitudinal data from adults 65 years or older in North Carolina, Blazer et al. (1997) found that White older adults were more likely than their Black counterparts to report SMD, but that SMD did not predict cognitive decline for White or Black respondents over a follow-up period of 3 years. Black older adults experienced a greater decline in cognition measured objectively, but SMD did not explain the decline. In contrast, Arvanitakis et al. (2018) reported that memory complaints predicted incident cognitive impairment and dementia among Black and White respondents. Thus, the overarching research question that guides our analysis is: Does SMD predict incident cognitive impairment in a diverse sample of older Americans? We are unaware of any study of a nationally representative sample that compares the potential influence of SMD on cognitive impairment among Black, White, and Hispanic Americans.
Our goal is to contribute to the literature by using a diverse national sample and a 12-year follow-up period to detect persons who transition from normal cognition to cognitive impairment. Any decline in cognitive performance may be important, but we focus on when people no longer manifest normal cognition, a common outcome in the literature on SMD (Kaup et al., 2015; Mayeda et al., 2016; Reisberg et al., 2010). The loss of normal cognitive functioning is clinically significant, especially for early therapeutic intervention (Ritchie & Touchon, 2000), and consequential to the older person’s social ties (Perry et al., 2021).
Research Design and Methods
Sample
We analyze data from the Health and Retirement Study (HRS)—a multistage, probability survey of adults aged 50 years and older, with oversampling of Black and Hispanic Americans and Florida residents (Sonnega et al., 2014). We use data beginning from 2004 and successive waves through 2016. The analytic sample comprises respondents meeting the following criteria: (a) participated and had nonzero weights in the 2004 core sample (resulting in a sample of N = 18,701), (b) are at least 65 years of age to receive the extended battery of questions related to cognitive function (N = 10,670), (c) self-identified as either White, Black, U.S.-born Hispanic, or foreign-born Hispanic (N = 10,473), (d) are not missing on cognition in 2004 (N = 9,547), (e) scored 11 or greater on the global cognition measure in 2004, indicating no presence of cognitive impairment or dementia (Langa et al., 2008) at baseline (N = 9,257), and (f) are not missing on any other independent variables, yielding an analytic sample of 9,244 adults 65–102 years of age. Proxy respondents are excluded from the analytic sample.
Dependent Variable
HRS assesses cognitive function using a modified version of the Telephone Interview for Cognitive Status survey, and we use the HRS Imputation of Cognitive Functioning Measures, which provides a cleaned and imputed data set to assess function (McCammon et al., 2019). Global cognition is assessed with a 35-point scale measuring episodic memory, working memory, and orientation (Langa et al., 2008). The variable is based on the following elements: 10-word immediate and delayed recall tests of episodic memory (for a combined score of 0–20); a serial 7s subtraction test of working memory (0–5); and several questions related to orientation (mental status): naming people, naming objects, and counting backward from 20 (0–10). These measures are summed into a composite score ranging from 0 to 35, and “a score of 11 or above was defined as a normal cognitive function” (Langa et al., 2008, p. 136). Scores ≤10 are considered cognitively impaired.
By limiting our sample to those respondents who manifested normal cognitive function at baseline in 2004, we identify those who experienced incident cognitive impairment (coded 1; 0 = normal cognition). We also use the information on the respondent’s age, measured in months, to identify the age of incident cognitive impairment. Respondents who maintained normal cognition remained in the study as part of the risk set as long as they were interviewed. Once respondents experienced the onset of cognitive impairment, they were excluded from the risk set because we studied age at the first manifestation of cognitive impairment.
Independent Variables
Subjective memory decline was measured in 2004 by asking if the respondent’s memory changed during the past 2 years. Respondents were asked, “would you say your memory is better now, about the same, or worse now than it was then?” We dichotomized the variable where 1 = worse, 0 = same or better.
Race-ethnicity is coded with four binary variables (0,1) because we incorporate nativity status among Hispanic respondents. Numerous studies reveal that nativity is associated with many facets of life, including preferred language, culture, and health. The four categories are White (reference group), Black, U.S.-born Hispanic, and foreign-born Hispanic adults.
Consistent with previous studies, we adjust for variables known to influence cognition in later life (Blazer et al., 1997; Jackson et al., 2017). We use binary variables to differentiate women and men (1 and 0, respectively) as well as respondents who live alone (1, 0 = otherwise). Education is coded as years of schooling (0–17+). We also adjust for baseline cognition because incident cognitive impairment may be more likely for persons scoring closest to the threshold of 10 for cognitive impairment. Given that cognitive complaints often accompany the presentation of symptoms of depression, depressive symptoms are measured with the eight-item version of the Center for Epidemiological Studies Depression Scale (α = 0.84). We use self-rated health as a measure of overall health status ranging from poor to excellent (0–4). Descriptive statistics for the variables are presented in Table 1 for the total sample and by race-ethnicity.
Table 1.
Descriptive Statistics of Respondents, Total Sample and by Race-Ethnicity
| Variables | Range | Total N = 9,244 |
White n = 7,470 |
Black n = 1,108 |
U.S.-born Hispanic n = 299 |
Foreign-born Hispanic n = 367 |
|---|---|---|---|---|---|---|
| Incident cognitive impairment | 0,1 | 0.11 | 0.09 | 0.21a | 0.20b | 0.19c |
| Age of incident cognitive impairment | 65–106 | 81.77 | 82.21 | 79.90a | 79.65b | 80.14c |
| Subjective memory decline | 0,1 | 0.25 | 0.25 | 0.23 | 0.22 | 0.27 |
| Race-ethnicity | ||||||
| White (non-Hispanic; reference) | 0,1 | 0.81 | ― | ― | ― | ― |
| Black (non-Hispanic) | 0,1 | 0.12 | ― | ― | ― | ― |
| U.S.-born Hispanic | 0,1 | 0.03 | ― | ― | ― | ― |
| Foreign-born Hispanic | 0,1 | 0.04 | ||||
| Women | 0.1 | 0.58 | 0.58 | 0.62a | 0.63 | 0.59 |
| Age (baseline) | 65–102 | 74.15 | 74.55 | 72.56a | 72.63b | 71.96c |
| Live alone | 0,1 | 0.28 | 0.29 | 0.31 | 0.18b,d | 0.22c,e |
| Education | 0–17 | 12.18 | 12.70 | 11.07a | 9.55b,d | 7.14c,e,f |
| Cognition (baseline) | 11–35 | 21.95 | 22.63 | 19.05a | 19.55b | 18.81c,f |
| Depressive symptoms | 0–8 | 1.44 | 1.34 | 1.81a | 1.97b | 1.89c |
| Self-rated health | 0–4 | 2.08 | 2.17 | 1.78a | 1.74b | 1.45c,e,f |
Notes: Unweighted means or proportions. Significance at p < .05.
aSignificant difference between Black and White adults.
bSignificant difference between U.S.-born Hispanic and White adults.
cSignificant difference between foreign-born Hispanic and White adults.
dSignificant difference between U.S.-born Hispanic and Black adults.
eSignificant difference between foreign-born Hispanic and Black adults.
fSignificant difference between foreign-born Hispanic and U.S.-born Hispanic adults.
Analytic Plan
We used Weibull accelerated failure-time models to examine if SMD is associated with incident cognitive impairment. We used age as the time metric for modeling to integrate information on the timing of the transition parameterized to the respondent’s age (Lamarca et al., 1998; Thiébaut & Bénichou, 2004). The key inputs for our event history analysis are (a) whether a person experienced incident cognitive impairment (censoring variable) and (b) the age (measured in months) at which respondents first reported scores on the cognition measure that were indicative of incident cognitive impairment, or the age last observed for respondents who maintained normal cognitive functioning (duration variable). Respondents who died during the observation period are included in the analysis because they contribute information to estimate the transition to cognitive impairment prior to death. Out of 9,244 respondents at baseline, 2.62% dropped out for reasons other than death by 2016. Given that over 97% of the respondents known to be alive provided information for the analysis, we used listwise deletion of cases for missing data.
The analysis was comprised of two stages, and all tests were completed in Stata/SE 17. First, we estimated Weibull accelerated failure-time models using the full sample while focusing on SMD, race-ethnicity, and gender then added the full vector of covariates (live alone, education, baseline cognition, depressive symptoms, and self-rated health). Second, we examined differences in the relationship between SMD and incident cognitive impairment across the White, Black, and Hispanic subsamples.
Results
The descriptive statistics in Table 1 reveal that 11% of adults in the total sample transitioned from normal cognitive function to cognitive impairment during the 2004–2016 study period. Compared to White adults, Black, U.S.-born, and foreign-born Hispanic adults were much more likely to experience incident cognitive impairment during the study (proportions of 0.09, 0.21, 0.20, and 0.19, respectively). Also compared to White adults, Black, U.S.-born, and foreign-born Hispanic adults experienced incident cognitive impairment at an earlier age (82.21, 79.90, 79.65, and 80.14 years old, respectively). We found no significant racial-ethnic differences in SMD. About one-quarter of all White, Black, and Hispanic respondents stated their memory was worse in 2004 than it was 2 years earlier. White adults reported more years of education, had higher initial cognition scores, fewer depressive symptoms, and better self-rated health compared to Black and Hispanic adults.
Event History Analysis
We present estimates from a Weibull accelerated failure-time model for the total sample in Table 2. Model 1 includes SMD only and reveals that persons reporting memory decline were more likely to experience incident cognitive impairment at a younger age during the observation period (β = −0.06). The negative slope reveals that the age of incident cognitive impairment was earlier for persons reporting SMD at baseline, and the time ratio (eβ = 0.95) means that people reporting SMD experienced incident cognitive impairment in 95% of the time as those reporting no SMD (i.e., 5% earlier time to onset). Over a 12-year study, this would translate into more than a half-year earlier for those reporting SMD. After adjusting for demographic information in Model 2, SMD remained significant, but race-ethnic differences are notable. The time ratios reveal that Black, U.S.-born Hispanic, and foreign-born Hispanic adults had roughly a 28% earlier time to onset of incident cognitive impairment compared to Whites (time ratios respectively, 0.71, 0.72, and 0.73). In addition, Model 2 shows that men and women did not differ significantly in the incidence of cognitive impairment.
Table 2.
Weibull Accelerated Failure-Time Models Associated With Incident Cognitive Impairment (N = 9,244)
| Independent variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | Time ratio (eβ) | β | SE | Time ratio (eβ) | β | SE | Time ratio (eβ) | |
| Subjective memory decline | −0.06** | 0.02 | 0.95** | −0.06*** | 0.02 | 0.94*** | −0.04* | 0.02 | 0.96* |
| Black | −0.34*** | 0.02 | 0.71*** | −0.18*** | 0.02 | 0.84*** | |||
| U.S.-born Hispanic | −0.33*** | 0.04 | 0.72*** | −0.14*** | 0.04 | 0.87*** | |||
| Foreign-born Hispanic | −0.32*** | 0.03 | 0.73*** | −0.08 | 0.04 | 0.93 | |||
| Women | -0.03 | 0.02 | 0.97 | −0.06** | 0.02 | 0.94** | |||
| Live alone | 0.11*** | 0.02 | 1.12*** | ||||||
| Education | 0.02*** | 0.00 | 1.02*** | ||||||
| Cognition (baseline) | 0.04*** | 0.00 | 1.04*** | ||||||
| Depressive symptoms | −0.01* | 0.00 | 0.99* | ||||||
| Self-rated health | −0.001 | 0.01 | 1.00 | ||||||
| Constant | 3.65*** | 0.02 | 3.74*** | 0.02 | 2.78*** | 0.05 | |||
| Likelihood ratio χ 2 | 8.43 | 317.95 | 794.78 |
Notes: A negative β reflects earlier onset of incident cognitive impairment (positive, later onset). SE = standard error. Time ratios are exponentiated coefficients and refer to the proportion of time to onset for a 1-unit difference in the predictor.
*p < .05; **p < .01; ***p < .001.
Model 3 includes all covariates, and SMD still predicted incident cognitive impairment with a time ratio of 0.96. After adjusting for covariates in Model 3, the likelihood of incident cognitive impairment remains greater for Black and Hispanic adults compared to White adults, although the size of the effects attenuated, especially for U.S.-born Hispanic adults (from eβ = 0.72 in Model 2 to 0.87 in Model 3). In these fully adjusted models, foreign-born Hispanic adults did not differ significantly from White adults in the likelihood of incident cognitive impairment. In addition, living alone, higher education, and higher cognition at baseline were each associated with slower onset of incident cognitive impairment. Higher levels of depressive symptoms were associated with a slightly earlier transition from normal cognition to cognitive impairment.
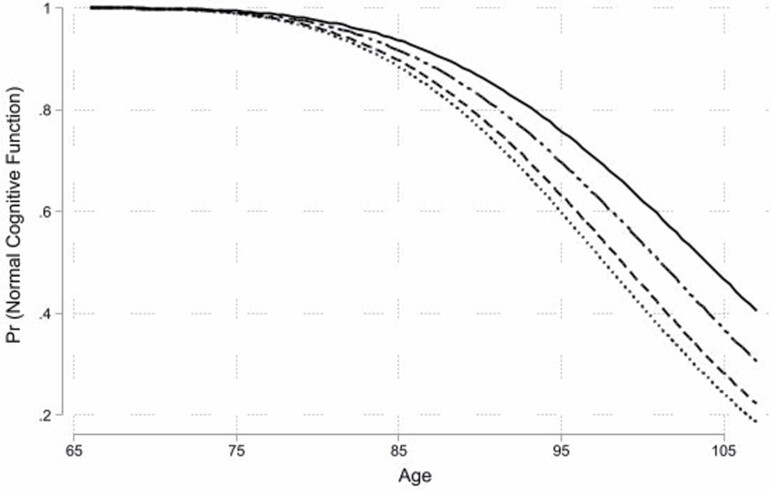
As shown in Table 2, there are striking differences by race-ethnicity in the risk of incident cognitive impairment, and Figure 1 graphically summarizes the predicted probabilities of remaining cognitively intact by age and race-ethnicity. Across the four race-ethnic categories, the predicted probabilities diverge with advancing age, especially after age 85. White and foreign-born Hispanic adults had more favorable profiles than Black and U.S.-born Hispanic adults—and the gap between White and Black adults increased with age.
Figure 1.
Weibull survival curve for normal cognitive function by age and race-ethnicity with all other covariates at their mean for each group.Legend: solid line = White, long-dash dotted line = foreign-born Hispanic, dashed line = U.S.-born Hispanic, dotted line = Black.
Event History Analysis Stratified by Race-Ethnicity
Finally, we turn to event history analysis stratified by race-ethnicity. For each subsample, we present time ratios for the final Weibull accelerated failure-time model with all covariates but also estimated simpler models (parallel to Table 2) by race-ethnicity to inform our interpretation of the results. (To test for differences across samples, we estimated interaction terms between race-ethnicity and SMD, which revealed a better likelihood ratio χ2 for the multiplicative model [p < .01]. We present the subsample results for ease of interpretation.) The results for White adults displayed in Table 3 reveal a time ratio of 0.95, which means that those with SMD experienced incident cognitive impairment in 95% of the time as those without SMD. Subjective memory decline, however, was not associated with the loss of normal cognitive function for Black, U.S.-born Hispanic, and foreign-born Hispanic adults. We reached the same conclusion from simple models with SMD as the only predictor of incident cognitive decline: the relationship was significant for White adults, but not for Black adults or Hispanic adults.
Table 3.
Weibull Accelerated Failure-Time Model Associated With Incident Cognitive Impairment by Race-Ethnicity
| Independent variables | White | Black | U.S.-born Hispanic | Foreign-born Hispanic |
|---|---|---|---|---|
| Time ratio (eβ) | Time ratio (eβ) | Time ratio (eβ) | Time ratio (eβ) | |
| Subjective memory decline | 0.95** | 0.96 | 1.19 | 1.01 |
| Women | 0.97 | 0.91 | 0.83 | 0.83 |
| Live alone | 1.11*** | 1.13* | 1.49** | 1.05 |
| Education | 1.02*** | 1.02* | 1.02 | 1.02* |
| Cognition (baseline) | 1.03*** | 1.05*** | 1.05*** | 1.04*** |
| Depressive symptoms | 0.99 | 0.98 | 1.01 | 0.98 |
| Self-rated health | 0.99 | 0.99 | 0.98 | 1.16** |
| Constant | 16.09*** | 12.73*** | 11.44*** | 12.60*** |
| N of cases | 7,470 | 1,108 | 299 | 367 |
| Likelihood ratio χ 2 | 334.61 | 105.19 | 45.11 | 47.41 |
Notes: Time ratios are exponentiated coefficients and refer to the proportion of time to onset for a 1-unit difference in the predictor.
*p < .05; **p < .01; ***p < .001.
Note also that across the subsamples, men and women do not manifest significantly different time ratios of incident cognitive impairment after adjusting for the covariates. The effects of education and self-rated health, however, differ across the two categories of Hispanic adults. Foreign-born Hispanic adults with more education and better self-rated health manifested slower onset of incident cognitive impairment (respectively, eβ = 1.02 and eβ = 1.16), but these variables were not related to incident cognitive impairment among U.S.-born Hispanic adults. Living alone was associated with a delayed onset of incident cognitive impairment among White, Black, and U.S.-born Hispanic adults (respectively, eβ = 1.11, eβ = 1.13, and eβ = 1.49), but it was not related to incident cognitive impairment among foreign-born Hispanic adults.
Sensitivity Analyses
We conducted two sensitivity analyses. First, we defined the outcome variables based on the transition from no dementia to dementia (instead of the transition from normal to cognitive impairment). Whereas persons with mild cognitive impairment were in the risk pool, the number of cases was larger (N = 9,419 vs N = 9,244 in the main analysis). When the duration variable was recalculated as the age of incident dementia, the results were very similar (Supplementary Material, Table S1). The only difference in the pattern of relationships was for depressive symptoms; for incident cognitive impairment, it predicted slightly earlier onset, but it was not related to incident dementia. Second, although we incorporated information from respondents who died by 2016 into the analysis, we also tested whether our conclusions might be due to selective survival. We used Heckman’s (1979) sample selection bias model and found that the conclusions were unchanged (Supplementary Material, Table S2).
Discussion and Implications
Our main research question focused on whether SMD predicts incident cognitive impairment in a diverse sample of older Americans. With a nationally representative sample, we found that SMD predicts incident cognitive impairment among White—but not Black or Hispanic—older adults. White older adults who rated their memory worse experienced incident cognitive impairment earlier during the observation period, a finding that is consistent with some studies (Arvanitakis et al., 2018; Jackson et al., 2017) but not others (Blazer et al., 1997). White older adults were not more likely than Black and Hispanic respondents to report SMD, but SMD predicted the loss of cognitive function among White older adults.
Results from this study confirm previous findings that SMD is not related to cognitive decline among Black older adults, either from cross-sectional studies of concurrent validity (Jackson et al., 2017; Sims et al., 2011) or a longitudinal study of prognostic validity (Blazer et al., 1997). We are aware of only one longitudinal study providing evidence of prognostic validity among Black older adults (Arvanitakis et al., 2018), but that is because it uses a distinctive measurement protocol. Although the bulk of the literature relies on a single question asking respondents whether their memory changed during 1 (Gupta, 2021) or 2 years (Rickenbach et al., 2015), Arvanitakis et al. (2018) asked respondents about change over 10 years. The longer time frame is an innovative approach but makes it difficult to compare to prior studies of SMD.
For Hispanic older adults, we found no evidence that SMD predicts incident cognitive impairment, which is consistent with findings by others (Harwood et al., 1998; Zlatar et al., 2018). Evidence that SMD is related to cognitive impairment among Hispanic older adults comes from cross-sectional studies of research volunteers (Nakhla et al., 2021), persons presenting with memory concerns at a primary care clinic (Rodríguez et al., 2021), and a multisite community sample of persons at least 50 years old (Zlatar et al., 2022). The most recent studies conclude that “longitudinal research is necessary” to understand the prognostic validity of SMD (Rodríguez et al., 2021; Zlatar et al., 2022), and our research addressed this call, showing that SMD did not predict incident cognitive impairment among Hispanic adults during more than a decade of observation. Although we considered nativity to address whether the relationship between SMD and incident cognitive impairment varied between U.S.-born and foreign-born Hispanic adults, the relationship was nonsignificant in both categories. We observed, however, that after adjusting for education and health-related variables, there was no difference between White and foreign-born Hispanic adults when predicting incident cognitive impairment, a finding consistent with the healthy-immigrant effect and which may be of interest to scholars studying the Hispanic paradox (Markides & Rote, 2019).
Can older people sense a meaningful decline in memory long before it is detected as cognitive impairment? The evidence is clear that SMD predicts incident cognitive impairment among White older adults but not among Black or Hispanic older adults. What we have not yet answered definitively is why this pattern exists, and there is a need for research to probe how people perceive the earliest symptoms of memory decline (Kim et al., 2022). We considered three potential explanations that merit further investigation. First, with data from the Harmonized Cognitive Assessment Protocol project, Jang et al. (2022) offer evidence that Black and Hispanic adults interpret their cognitive function as better than objectively measured, but that White adults are more negative when interpreting their cognitive function, which raises concern of item bias by race-ethnicity (Jones, 2003). Instead of investigating SMD, Jang et al. capitalized on a rating of current cognitive status to examine the concurrent validity of positive ratings versus negative ratings. They concluded that there are older people from each racial-ethnic category for whom their ratings of cognition are discordant with the objective measures: White older adults are prone to falsely negative perceptions while Black and Hispanic older adults are prone to falsely positive ratings. For Black older adults, this is consistent with recent research showing that they generally appraise stressors as less upsetting than do White older adults (Brown et al., 2020). Thus, being somewhat more negative in evaluating their cognitive status may manifest in SMD holding more prognostic validity for White people. Modest concern about memory decline may be helpful; high concern may inadvertently exacerbate the problem.
Second, cognitive decline is inherently relative to an earlier timepoint, and people’s assessments of change may be related to where they started, leading to a dissociation between the perception of change and risk of incident impairment. For instance, a person who began the study with a low cognitive score may not perceive much change over time, even though that person is close to crossing the threshold for cognitive impairment. Whereas Black and Hispanic older adults generally have lower baseline scores on cognitive tests than White older adults, they may not perceive much change. To address this concern, we adjusted our models for initial cognitive function, but the main conclusions remained with or without accounting for initial levels of cognitive functioning. Thus, it might not be just the initial level of cognition during this study, but a lifetime of Black and Hispanic adults interpreting their cognitive function as better than objectively measured (Jang et al., 2022).
Third, lack of access to high-quality care and potential discrimination in medical care may predispose Black and Hispanic people to be less trusting than White people when seeking professional help. Distrust, in turn, may delay help-seeking until the concerns become more serious, which may lead to the underdiagnosis of cognitive problems and dementia in minority populations (Gianattasio et al., 2019; Zhu et al., 2021).
We also acknowledge several limitations of this research. First, we used a limited number of control variables, which may influence our conclusions. For instance, if an older adult has someone who provides support for limitations in instrumental activities of daily living, such as paying bills and shopping, the recipient may be less aware of his or her cognitive decline. Second, it is possible that insufficient statistical power limits our ability to detect whether SMD influences incident cognitive impairment among the Black and Hispanic subsamples. Third, a family history of dementia may predispose people toward greater concern about cognitive decline, but HRS does not provide information on family history of dementia or Alzheimer’s disease.
Policy Implications
Given that about one third of the U.S. population expresses fear of getting Alzheimer’s disease (Metlife Foundation, 2011), many people are concerned about whether early indications of memory problems may be a harbinger of eventual cognitive impairment. Although our study found that SMD predicts incident cognitive impairment among White older adults, we did not uncover evidence that SMD predicts incident cognitive impairment among Black or Hispanic adults. Thus, we recommend caution in the use of SMD as an early marker of impending cognitive decline in diverse populations, especially because it may be years (perhaps a decade) before the loss of normal cognitive function. We also welcome future quantitative, qualitative, or mixed method research to empirically assess whether Black and Hispanic older adults may underreport memory problems because they are more likely to view memory decline as a natural part of aging.
Supplementary Material
Acknowledgments
Data used in this article were available from the Health and Retirement Study (https://hrs.isr.umich.edu/about). Imputation for the measures of cognitive functioning were available from the Institute for Social Research, University of Michigan (McCammon et al., 2019, see reference list). The study reported in this manuscript was not preregistered.
Contributor Information
Kenneth F Ferraro, Department of Sociology, Purdue University, West Lafayette, Indiana, USA; Center on Aging and the Life Course, Purdue University, West Lafayette, Indiana, USA.
Madison R Sauerteig-Rolston, Department of Sociology, Purdue University, West Lafayette, Indiana, USA; Center on Aging and the Life Course, Purdue University, West Lafayette, Indiana, USA.
Lisa L Barnes, Department of Neurological Sciences at Rush University Medical Center, Chicago, Illinois, USA; Rush Alzheimer’s Disease Center at Rush University Medical Center, Chicago, Illinois, USA.
Elliot Friedman, Center on Aging and the Life Course, Purdue University, West Lafayette, Indiana, USA; Department of Human Development and Family Studies, Purdue University, West Lafayette, Indiana, USA.
Laura P Sands, Center for Gerontology, Virginia Tech, Blacksburg, Virginia, USA.
Patricia A Thomas, Department of Sociology, Purdue University, West Lafayette, Indiana, USA; Center on Aging and the Life Course, Purdue University, West Lafayette, Indiana, USA.
Funding
Support for this research was provided by grants from the National Institute on Aging (AG043544 to K. F. Ferraro; AG068388 to K. F. Ferraro).
Conflict of Interest
None declared.
References
- Adams, M. L., Deokar, A. J., Anderson, L. A., & Edwards, V. J. (2013). Self-reported increased confusion or memory loss and associated functional difficulties among adults aged ≥60 years—21 states, 2011. MMWR. Morbidity and Mortality Weekly Report, 62(18), 345. doi: 10.1016/j.jalz.2013.05.1759 [DOI] [PubMed] [Google Scholar]
- Arvanitakis, Z., Leurgans, S. E., Fleischman, D. A., Schneider, J. A., Rajan, K. B., Pruzin, J. J., Shah, R. C., Evans, D. A., Barnes, L. L., & Bennett, D. A. (2018). Memory complaints, dementia, and neuropathology in older blacks and whites. Annals of Neurology, 83(4), 718–729. doi: 10.1002/ana.25189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer, D. G., Hays, J. C., Fillenbaum, G. G., & Gold, D. T. (1997). Memory complaint as a predictor of cognitive decline: A comparison of African American and White elders. Journal of Aging and Health, 9(2), 171–1 84. doi: 10.1177/089826439700900202 [DOI] [PubMed] [Google Scholar]
- Brown, L. L., Mitchell, U. A., & Ailshire, J. A. (2020). Disentangling the stress process: Race/ethnic differences in the exposure and appraisal of chronic stressors among older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(3), 650–660. doi: 10.1093/geroni/igx004.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey, W. H.(2018). Diversity explosion: How new racial demographics are remaking America. Brookings Institution Press. doi: 10.1080/01944363.2015.1030932 [DOI] [Google Scholar]
- Garcia, M. A., Downer, B., Crowe, M., & Markides, K. S. (2017). Aging and disability among Hispanics in the United States: Current knowledge and future directions. Innovation in Aging, 1(2), 1–11. doi: 10.1093/geroni/igx020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianattasio, K. Z., Prather, C., Glymour, M. M., Ciarleglio, A., & Power, M. C. (2019). Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimer’s and Dementia, 5, 891–898. doi: 10.1016/j.trci.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S. (2021). Racial and ethnic disparities in subjective cognitive decline: A closer look, United States, 2015–2018. BMC Public Health, 21(1), 1–12. doi: 10.1186/s12889-021-11068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harten, A. C., Mielke, M. M., Swenson-Dravis, D. M., Hagen, C. E., Edwards, K. K., Roberts, R. O., Geda, Y. E., Knopman, D. S., & Petersen, R. C. (2018). Subjective cognitive decline and risk of MCI: The Mayo Clinic Study of Aging. Neurology, 91(4), e300–e312. doi: 10.1212/wnl.0000000000005863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood, D., Barker, W., Ownby, R., & Duara, R. (1998). Memory complaints in the elderly: A comparative analysis of informant and subject reports among Hispanics and White non-Hispanics. Clinical Gerontologist, 18(3), 56–60. doi: 10.1212/wnl.52.3.551 [DOI] [Google Scholar]
- Heckman, J. J. (1979). Sample selection bias as a specification error. Econometrica, 47(1), 153–161. doi: 10.2307/1912352 [DOI] [Google Scholar]
- Hummer, R. A., & Hayward, M. D. (2015). Hispanic older adult health and longevity in the United States: Current patterns and concerns for the future. Daedalus, 144(2), 20–30. doi: 10.1162/DAED_a_00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, J. D., Rentz, D. M., Aghjayan, S. L., Buckley, R. F., Meneide, T. F., Sperling, R. A., & Amariglio, R. E. (2017). Subjective cognitive concerns are associated with objective memory performance in Caucasian but not African-American persons. Age and Ageing, 46(6), 988–993. doi: 10.1093/ageing/afx077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, Y., Haley, W. E., Choi, E. Y., & Franco, Y. (2022). Racial/ethnic differences in correspondence between subjective cognitive ratings and cognitive impairment. American Journal of Geriatric Psychiatry, 30(5), 627–635. doi: 10.1016/j.jagp.2021.10.015 [DOI] [PubMed] [Google Scholar]
- Jessen, F., Amariglio, R. E., Buckley, R. F., van der Flier, W. M., Han, Y., Molinuevo, J. L., Rabin, L., Rentz, D. M., Rodriguez-Gomez, O., Saykin, A. J, Sikkes, S. A. M., Smart, C. M., Wolfsgruber, S., & Wagner, M. (2020). The characterisation of subjective cognitive decline. The Lancet Neurology, 19(3), 271–278. doi: 10.1016/s1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, R. N. (2003). Racial bias in the assessment of cognitive functioning of older adults. Aging and Mental Health, 7(2), 83–102. doi: 10.1080/1360786031000045872 [DOI] [PubMed] [Google Scholar]
- Jorm, A. F., Christensen, H., Korten, A. E., Jacomb, P. A., & Henderson, A. S. (2001). Memory complaints as a precursor of memory impairment in older people: A longitudinal analysis over 7–8 years. Psychological Medicine, 31(3), 441–449. doi: 10.1017/s0033291701003245 [DOI] [PubMed] [Google Scholar]
- Kaup, A. R., Nettiksimmons, J., LeBlanc, E. S., & Yaffe, K. (2015). Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology, 85(21), 1852–1858. doi: 10.1212/wnl.0000000000002153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H., Sereika, S. M., Albert, S. M., Bender, C. M., & Lingler, J. H. (2022). Do perceptions of cognitive changes matter in self-management behaviors among persons with mild cognitive impairment? The Gerontologist, 62(4), 577–588. doi: 10.1093/geront/gnab129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarca, R., Alonso, J., Gomez, G., & Muñoz, A. (1998). Left-truncated data with age as time scale: An alternative for survival analysis in the elderly population. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 53(5), M337–M343. doi: 10.1093/gerona/53a.5.m337 [DOI] [PubMed] [Google Scholar]
- Langa, K. M., Larson, E. B., Karlawish, J. H., Cutler, D. M., Kabeto, M. U., Kim, S. Y., & Rosen, A. B. (2008). Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimer’s and Dementia, 4(2), 134–144. doi: 10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markides, K. S., & Rote, S. (2019). The healthy immigrant effect and aging in the United States and other western countries. The Gerontologist, 59(2), 205–214. doi: 10.1093/geront/gny136 [DOI] [PubMed] [Google Scholar]
- Matthews, K. A., Xu, W., Gaglioti, A. H., Holt, J. B., Croft, J. B., Mack, D., & McGuire, L. C. (2019). Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s and Dementia, 15(1), 17–24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda, E. R., Glymour, M. M., Quesenberry, C. P., & Whitmer, R. A. (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s and Dementia, 12(3), 216–224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCammon, R. J., Fisher, G. G., Hassan H., Faul, J. D., Rodgers, W. L., & Weir, D. R. (2019). Health and retirement study imputation of cognitive functioning measures: 1992–2016 (Version 1.0). Survey Research Center, University of Michigan. https://hrspubs.sites.uofmhosting.net/sites/default/files/biblio/COGIMP9216_dd.pdf [Google Scholar]
- Metlife Foundation. (2011, February). What America thinks: MetLife Foundation Alzheimer’s survey. https://www.metlife.com/content/dam/microsites/about/corporate-profile/alzheimers-2011.pdf
- Nakhla, M. Z., Cohen, L., Salmon, D. P., Smirnov, D. S., Marquine, M. J., Moore, A. A., Schiehser, D. M., & Zlatar, Z. Z. (2021). Self-reported subjective cognitive decline is associated with global cognition in a community sample of Latinos/as/x living in the United States. Journal of Clinical and Experimental Neuropsychology, 43(7), 663–676. doi: 10.1080/13803395.2021.1989381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, B. L., McConnell, W. R., Peng, S., Roth, A., Coleman, M., Manchella, M., Roessler, M., Francis, H., Sheean, H., & Apostolova, L. (2021). Social networks and cognitive function: An evaluation of social bridging and bonding mechanisms. The Gerontologist. Advance online publication. doi: 10.1093/geront/gnab112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin, L. A., Smart, C. M., Crane, P. K., Amariglio, R. E., Berman, L. M., Boada, M., Buckley, R. F., Chételat, G., Dubois, B., Ellis, K. A., Gifford, K. A., Jefferson, A. L., Jessen, F., Katz, M. J., Lipton, R. B., Luck, T., Maruff, P., Mielke, M. M., Molinuevo, J. L., & Sikkes, S. A. (2015). Subjective cognitive decline in older adults: An overview of self-report measures used across 19 international research studies. Journal of Alzheimer’s Disease, 48(s1), S63–S86. doi: 10.1016/j.jalz.2015.07.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg, B., Shulman, M. B., Torossian, C., Leng, L., & Zhu, W. (2010). Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer’s and Dementia, 6(1), 11–24. doi: 10.1016/j.jalz.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenbach, E. H., Agrigoroaei, S., & Lachman, M. E. (2015). Awareness of memory ability and change: (In)accuracy of memory self-assessments in relation to performance. Population Ageing, 8(1–2), 71–99. doi: 10.1007/s12062-014-9108-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, K., & Touchon, J. (2000). Mild cognitive impairment: Conceptual basis and current nosological status. The Lancet, 355(9199), 225–228. doi: 10.1016/s0140-6736(99)06155-3 [DOI] [PubMed] [Google Scholar]
- Rodríguez, D., Ayers, E., Weiss, E. F., & Verghese, J. (2021). Cross-cultural comparisons of subjective cognitive complaints in a diverse primary care population. Journal of Alzheimer’s Disease, 81(2), 545–555. doi: 10.3233/jad-201399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims, R. C., Whitfield, K. E., Ayotte, B. J., Gamaldo, A. A., Edwards, C. L., & Allaire, J. C. (2011). Subjective memory in older African Americans. Experimental Aging Research, 37(2), 220–240. doi: 10.1080/0361073x.2011.555640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnega, A., Faul, J. D., Ofstedal, M. B., Langa, K. M., Phillips, J. W., & Weir, D. R. (2014). Cohort profile: The health and retirement study (HRS). International Journal of Epidemiology, 43(2), 576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, C. A., Bouldin, E. D., & McGuire, L. C. (2018). Subjective cognitive decline among adults aged ≥45 years—United States, 2015–2016. Morbidity and Mortality Weekly Report, 67(27), 753. doi: 10.1002/gps.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiébaut, A. C., & Bénichou, J. (2004). Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: A simulation study. Statistics in Medicine, 23(24), 3803–3820. doi: 10.1002/sim.2098 [DOI] [PubMed] [Google Scholar]
- Verlinden, V. J., van der Geest, J. N., de Bruijn, R. F., Hofman, A., Koudstaal, P. J., & Ikram, M. A. (2016). Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimer’s and Dementia, 12(2), 144–153. doi: 10.1016/j.jalz.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Vespa, J., Armstrong, D. M., & Medina, L. (2020). Demographic turning points for the United States: Population projections for 2020 to 2060. US Department of Commerce, Economics and Statistics Administration, US Census Bureau. doi: 10.3886/icpsr08863.v2 [DOI] [Google Scholar]
- Zhu, Y., Chen, Y., Crimmins, E. M., & Zissimopoulos, J. M. (2021). Sex, race, and age differences in prevalence of dementia in Medicare claims and survey data. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(3), 596–606. doi: 10.1093/geronb/gbaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatar, Z. Z., Muniz, M. C., Espinoza, S. G., Gratianne, R., Gollan, T. H., Galasko, D., & Salmon, D. P. (2018). Subjective cognitive decline, objective cognition, and depression in older Hispanics screened for memory impairment. Journal of Alzheimer’s Disease, 63(3), 949–956. doi: 10.3233/jad-170865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatar, Z. Z., Tarraf, W., González, K. A., Vásquez, P. M., Marquine, M. J., Lipton, R. B., Gallo, L. C., Khambaty, T., Zeng, D., Youngblood, M. E., Estrella, M. L., Isasi, C. R., Daviglus, M., & González, H. M. (2022). Subjective cognitive decline and objective cognition among diverse U.S. Hispanics/Latinos: Results from the Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA). Alzheimer’s and Dementia, 18(1), 43–52. doi: 10.1002/alz.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.