Abstract
Background
Harmful alcohol use is defined as unhealthy alcohol use that results in adverse physical, psychological, social, or societal consequences and is among the leading risk factors for disease, disability and premature mortality globally. The burden of harmful alcohol use is increasing in low‐ and middle‐income countries (LMICs) and there remains a large unmet need for indicated prevention and treatment interventions to reduce harmful alcohol use in these settings. Evidence regarding which interventions are effective and feasible for addressing harmful and other patterns of unhealthy alcohol use in LMICs is limited, which contributes to this gap in services.
Objectives
To assess the efficacy and safety of psychosocial and pharmacologic treatment and indicated prevention interventions compared with control conditions (wait list, placebo, no treatment, standard care, or active control condition) aimed at reducing harmful alcohol use in LMICs.
Search methods
We searched for randomized controlled trials (RCTs) indexed in the Cochrane Drugs and Alcohol Group (CDAG) Specialized Register, the Cochrane Clinical Register of Controlled Trials (CENTRAL) in the Cochrane Library, PubMed, Embase, PsycINFO, CINAHL, and the Latin American and Caribbean Health Sciences Literature (LILACS) through 12 December 2021. We searched clinicaltrials.gov, the World Health Organization International Clinical Trials Registry Platform, Web of Science, and Opengrey database to identify unpublished or ongoing studies. We searched the reference lists of included studies and relevant review articles for eligible studies.
Selection criteria
All RCTs comparing an indicated prevention or treatment intervention (pharmacologic or psychosocial) versus a control condition for people with harmful alcohol use in LMICs were included.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 66 RCTs with 17,626 participants. Sixty‐two of these trials contributed to the meta‐analysis. Sixty‐three studies were conducted in middle‐income countries (MICs), and the remaining three studies were conducted in low‐income countries (LICs). Twenty‐five trials exclusively enrolled participants with alcohol use disorder. The remaining 51 trials enrolled participants with harmful alcohol use, some of which included both cases of alcohol use disorder and people reporting hazardous alcohol use patterns that did not meet criteria for disorder.
Fifty‐two RCTs assessed the efficacy of psychosocial interventions; 27 were brief interventions primarily based on motivational interviewing and were compared to brief advice, information, or assessment only. We are uncertain whether a reduction in harmful alcohol use is attributable to brief interventions given the high levels of heterogeneity among included studies (Studies reporting continuous outcomes: Tau² = 0.15, Q =139.64, df =16, P<.001, I² = 89%, 3913 participants, 17 trials, very low certainty; Studies reporting dichotomous outcomes: Tau²=0.18, Q=58.26, df=3, P<.001, I² =95%, 1349 participants, 4 trials, very low certainty). The other types of psychosocial interventions included a range of therapeutic approaches such as behavioral risk reduction, cognitive‐behavioral therapy, contingency management, rational emotive therapy, and relapse prevention. These interventions were most commonly compared to usual care involving varying combinations of psychoeducation, counseling, and pharmacotherapy. We are uncertain whether a reduction in harmful alcohol use is attributable to psychosocial treatments due to high levels of heterogeneity among included studies (Heterogeneity: Tau² = 1.15; Q = 444.32, df = 11, P<.001; I²=98%, 2106 participants, 12 trials, very low certainty).
Eight trials compared combined pharmacologic and psychosocial interventions with placebo, psychosocial intervention alone, or another pharmacologic treatment. The active pharmacologic study conditions included disulfiram, naltrexone, ondansetron, or topiramate. The psychosocial components of these interventions included counseling, encouragement to attend Alcoholics Anonymous, motivational interviewing, brief cognitive‐behavioral therapy, or other psychotherapy (not specified). Analysis of studies comparing a combined pharmacologic and psychosocial intervention to psychosocial intervention alone found that the combined approach may be associated with a greater reduction in harmful alcohol use (standardized mean difference (standardized mean difference (SMD))=‐0.43, 95% confidence interval (CI): ‐0.61 to ‐0.24; 475 participants; 4 trials; low certainty).
Four trials compared pharmacologic intervention alone with placebo and three with another pharmacotherapy. Drugs assessed were: acamprosate, amitriptyline, baclofen disulfiram, gabapentin, mirtazapine, and naltrexone. None of these trials evaluated the primary clinical outcome of interest, harmful alcohol use.
Thirty‐one trials reported rates of retention in the intervention. Meta‐analyses revealed that rates of retention between study conditions did not differ in any of the comparisons (pharmacologic risk ratio (RR) = 1.13, 95% CI: 0.89 to 1.44, 247 participants, 3 trials, low certainty; pharmacologic in addition to psychosocial intervention: RR = 1.15, 95% CI: 0.95 to 1.40, 363 participants, 3 trials, moderate certainty). Due to high levels of heterogeneity, we did not calculate pooled estimates comparing retention in brief (Heterogeneity: Tau² = 0.00; Q = 172.59, df = 11, P<.001; I2 = 94%; 5380 participants; 12 trials, very low certainty) or other psychosocial interventions (Heterogeneity: Tau² = 0.01; Q = 34.07, df = 8, P<.001; I2 = 77%; 1664 participants; 9 trials, very low certainty). Two pharmacologic trials and three combined pharmacologic and psychosocial trials reported on side effects. These studies found more side effects attributable to amitriptyline relative to mirtazapine, naltrexone and topiramate relative to placebo, yet no differences in side effects between placebo and either acamprosate or ondansetron.
Across all intervention types there was substantial risk of bias. Primary threats to validity included lack of blinding and differential/high rates of attrition.
Authors' conclusions
In LMICs there is low‐certainty evidence supporting the efficacy of combined psychosocial and pharmacologic interventions on reducing harmful alcohol use relative to psychosocial interventions alone. There is insufficient evidence to determine the efficacy of pharmacologic or psychosocial interventions on reducing harmful alcohol use largely due to the substantial heterogeneity in outcomes, comparisons, and interventions that precluded pooling of these data in meta‐analyses. The majority of studies are brief interventions, primarily among men, and using measures that have not been validated in the target population. Confidence in these results is reduced by the risk of bias and significant heterogeneity among studies as well as the heterogeneity of results on different outcome measures within studies. More evidence on the efficacy of pharmacologic interventions, specific types of psychosocial interventions are needed to increase the certainty of these results.
Keywords: Humans, Male, Acamprosate, Alcoholism, Alcoholism/prevention & control, Amitriptyline, Developing Countries, Disulfiram, Mirtazapine, Naltrexone, Ondansetron, Topiramate
Plain language summary
Interventions to reduce harmful alcohol use in low‐ and middle‐income countries
Why is this review important?
Harmful alcohol use is one of the main contributors to the global burden of disease. In low‐ and middle‐income countries, harmful alcohol use is increasing. However, services to prevent and treat harmful alcohol use are limited. One contributor to the lack of available services is the limited information about which intervention approaches are effective in reducing harmful alcohol use and whether these approaches are feasible and acceptable in low‐resource settings. In order to prevent the physical, psychological, and societal burden of harmful alcohol use, it is important that effective interventions are available to reduce alcohol‐related harm.
What is the goal of this review?
The goal of this review is to summarize evidence on whether psychosocial and pharmacologic interventions are able to reduce harmful alcohol use in low‐ and middle‐income countries. We also aim to evaluate the safety of treatments and how many people remain in treatment until its completion.
What does the research say?
We identified 66 randomized controlled trials that evaluated the effect of interventions on reducing harmful alcohol use. Most of these studies assessed psychosocial interventions (n = 52 studies), six assessed pharmacologic interventions alone, and eight assessed combined pharmacologic and psychosocial interventions.
The majority of included trials were funded by government agencies (36 trials) followed by multiple public and private funders (8 trials) or private foundations (5 trials). Seventeen trials did not report the funding source.
We are uncertain whether brief psychosocial interventions and other psychosocial interventions reduce harmful alcohol use. Combined pharmacologic and psychosocial interventions may reduce harmful alcohol use relative to psychosocial interventions combined with placebo, yet the certainty of the evidence has been assessed as low. No studies were found that looked at the effect of pharmacologic interventions alone on harmful alcohol use. We did not find evidence that rates of retention differed between study conditions for any of the intervention types.
Certainty of evidence: The certainty of evidence on the effect of brief and other psychosocial interventions on harmful alcohol use was very low due to lack of masking, differential attrition and missing data, selective outcome reporting, high heterogeneity, and differences in patient populations. The certainty of evidence on the effect of combined pharmacologic and psychosocial interventions on harmful alcohol use was low due to lack of blinding, incomplete outcome data, and heterogeneity in the pharmacologic interventions under investigation. No studies evaluated the effect of pharmacologic interventions alone on harmful alcohol use.
The evidence is current to 12 December 2021.
What are the next steps?
Future research on interventions to reduce harmful alcohol use is needed to address some of the limitations described in this review. Studies evaluating similar interventions across studies and contexts are needed to improve generalizability and understand the necessary characteristics of and conditions for effective intervention. Studies must utilize measurement tools that are valid in the population they are studying. Lastly, studies must be designed to reduce the risk of bias leading to the high degree of uncertainty in the results reported in this review.
Summary of findings
Summary of findings 1. Pharmacologic intervention compared to placebo for individuals with alcohol use disorder.
| Pharmacologic intervention compared to placebo for individuals with alcohol use disorder | |||||
| Patient or population: individuals with alcohol use disorder Setting: Low‐ and middle‐income countries Intervention: Pharmacologic intervention Comparison: placebo | |||||
| Outcomes | № of participants (studies) Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk difference with Pharmacologic intervention | ||||
| Harmful alcohol use | 0 (0 RCTs) | ‐ | not estimable | Study population | |
| 0 per 1,000 | 0 fewer per 1,000 (0 fewer to 0 fewer) | ||||
| Retention | 247 (3 RCTs) | ⊕⊕⊝⊝ Low 1 2 | RR 1.13 (0.89 to 1.44) | Study population | |
| 562 per 1,000 | 73 more per 1,000 (62 fewer to 247 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1 Variability in study conditions (experimental and/or comparison groups)
2 Includes the null, small sample size, and appreciable benefit and harm included in confidence interval
Summary of findings 2. Pharmacologic intervention along with psychosocial intervention compared to psychosocial intervention alone for individuals with alcohol use disorder.
| Pharmacologic intervention along with psychosocial intervention compared to psychosocial intervention alone for individuals with alcohol use disorder | |||||
| Patient or population: individuals with alcohol use disorder Setting: Low‐ and middle‐income countries Intervention: Pharmacologic intervention along with psychosocial intervention Comparison: psychosocial intervention alone | |||||
| Outcomes | № of participants (studies) Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with psychosocial intervention alone | Risk difference with Pharmacologic intervention along with psychosocial intervention | ||||
| Harmful alcohol use (continuous) | 475 (4 RCTs) | ⊕⊕⊝⊝ Low 1 2 | ‐ | The mean harmful alcohol use (continuous) was 0 SD | SMD 0.43 SD lower (0.61 lower to 0.24 lower) |
| Harmful alcohol use (dichotomous) | 102 (1 RCT) | ⊕⊝⊝⊝ Very low 1 3 | RR 0.62 (0.16 to 2.47) | Study population | |
| 96 per 1,000 | 37 fewer per 1,000 (81 fewer to 141 more) | ||||
| Retention | 363 (3 RCTs) | ⊕⊕⊕⊝ Moderate 3 | RR 1.15 (0.95 to 1.40) | Study population | |
| 491 per 1,000 | 74 more per 1,000 (25 fewer to 196 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1 High risk of bias due to limited blinding of participants, personnel, and/or outcome assessors. Most studies reported incomplete outcome data with high or unclear risk of bias
2 Different pharmacologic interventions evaluated across studies for this outcome
3 Includes the null, small sample size, and appreciable benefit and harm included in the confidence interval
Summary of findings 3. Pharmacologic intervention compared to another pharmacologic intervention for individuals with alcohol use disorder.
| Pharmacologic intervention compared to another pharmacologic intervention for individuals with alcohol use disorder | |||||
| Patient or population: individuals with alcohol use disorder Setting: Low‐ and middle‐income countries Intervention: Pharmacologic intervention Comparison: another pharmacologic intervention | |||||
| Outcomes | № of participants (studies) Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with another pharmacologic intervention | Risk difference with Pharmacologic intervention | ||||
| Harmful alcohol use ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ |
| Retention | 190 (2 RCTs) | ⊕⊕⊝⊝ Low 1 | RR 0.95 (0.76 to 1.19) | Study population | |
| 873 per 1,000 | 44 fewer per 1,000 (209 fewer to 166 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1 Differences in comparators
Summary of findings 4. Pharmacologic intervention compared to another pharmacologic intervention in addition to psychosocial intervention for individuals with alcohol use disorder.
| Pharmacologic intervention compared to another pharmacologic intervention in addition to psychosocial intervention for individuals with alcohol use disorder | |||||
| Patient or population: individuals with alcohol use disorder Setting: Low‐ and middle‐income countries Intervention: Pharmacologic intervention Comparison: another pharmacologic intervention in addition to psychosocial intervention | |||||
| Outcomes | № of participants (studies) Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with another pharmacologic intervention in addition to psychosocial intervention | Risk difference with Pharmacologic intervention | ||||
| Harmful alcohol use ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ |
| Retention | 200 (2 RCTs) | ⊕⊕⊝⊝ Low 1 | RR 1.02 (0.96 to 1.08) | Study population | |
| 940 per 1,000 | 19 more per 1,000 (38 fewer to 75 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1 Different comparators
Summary of findings 5. Brief interventions compared to brief advice, information, or assessment only for individuals with hazardous or harmful alcohol use.
| Brief interventions compared to brief advice, information, or assessment only for individuals with hazardous or harmful alcohol use | |||||
| Patient or population: individuals with hazardous or harmful alcohol use Setting: Low‐ and middle‐income countries Intervention: Brief interventions Comparison: brief advice, information, or assessment only | |||||
| Outcomes | № of participants (studies) Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with brief advice, information, or assessment only | Risk difference with Brief interventions | ||||
| Harmful alcohol use (continuous) | 3913 (17 RCTs) | ⊕⊝⊝⊝ Very low 1 2 3 | ‐ | The mean harmful alcohol use (continuous) was 0 SD | see comment |
| Harmful alcohol use (dichotomous) | 1349 (4 RCTs) | ⊕⊝⊝⊝ Very low 1 2 3 | not pooled | Study population | |
| not pooled | not pooled | ||||
| Retention | 5380 (12 RCTs) | ⊕⊝⊝⊝ Very low 1 2 3 | not pooled | Study population | |
| not pooled | not pooled | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1 Lack of blinding of participants, personnel, and/or outcome assessor
2 High levels of heterogeneity
3 Differences in comparison condition and, to a lesser extent, the brief interventions
Summary of findings 6. Other psychosocial intervention compared to usual care, brief intervention, or wait list for individuals with hazardous or harmful alcohol use.
| Other psychosocial intervention compared to usual care, brief intervention, or wait list for individuals with hazardous or harmful alcohol use | |||||
| Patient or population: individuals with hazardous or harmful alcohol use Setting: Low‐ and middle‐income countries Intervention: Other psychosocial intervention Comparison: usual care, brief intervention, or wait list | |||||
| Outcomes | № of participants (studies) Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with usual care, brief intervention, or wait list | Risk difference with Other psychosocial intervention | ||||
| Harmful alcohol use | 2106 (12 RCTs) | ⊕⊝⊝⊝ Very low 1 2 3 | ‐ | The mean harmful alcohol use was 0 | see comment |
| Retention | 1664 (9 RCTs) | ⊕⊝⊝⊝ Very low 1 2 3 4 | not pooled | Study population | |
| not pooled | not pooled | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1 High risk of bias due to lack of blinding participants, personnel, and/or outcome assessors
2 High levels of heterogeneity in estimated effects
3 Variability in experimental and control conditions
4 Appreciable risk and benefit
Background
Description of the condition
Harmful alcohol use, which is broadly defined by the World Health Organization as an unhealthy pattern of alcohol use that "causes detrimental health and social consequences for the drinker, the people around the drinker and society a large, as well as patterns of drinking that are associated with increased risk for adverse health consequences" (WHO 2010), is responsible for more harm to individuals and communities collectively than any other substance type and is among the leading causes of disability and premature mortality worldwide (Murray 2012; Whiteford 2013; Connor 2016). Harmful alcohol use encompasses a spectrum of unhealthy patterns of alcohol use including hazardous drinking, single episodes of harmful alcohol use, harmful drinking patterns, and alcohol use disorder (including alcohol dependence; WHO 2018a; WHO 2010). Individuals are considered to meet criteria for alcohol use disorder when their harmful patterns of alcohol use "lead to clinically significant impairment or distress" (American Psychiatric Association 2013). Definitions and terminology used to describe unhealthy patterns of alcohol use and disorder vary across diagnostic classification systems (American Psychiatric Association 2013; WHO 2018b). However, all definitions include alcohol‐related harms and consequences as a necessary criterion for alcohol use disorder. Thus, for this review, we consider harmful alcohol use to encompass unhealthy patterns of alcohol use that lead to physical, psychological, social, or societal consequences. Other forms of harmful alcohol use that may not meet diagnostic criteria for disorder include hazardous alcohol use, misuse, problem drinking, risky drinking, and heavy episodic/binge‐drinking. These sub‐threshold patterns of harmful alcohol use also contribute substantially to the biologic, psychological, and social harms of alcohol globally despite not meeting clinical criteria for alcohol use disorder (WHO 2014). In this review we focus on interventions that aim to reduce harmful alcohol use either by preventing the transition from sub‐threshold patterns of unhealthy alcohol use to disorder, or the treatment of alcohol use disorder.
Harmful patterns of alcohol use are associated with over 200 other adverse health and social conditions, including liver disease, cancer, cardio‐ and cerebrovascular disease, epilepsy, injury, interpersonal violence, and others (Nutt 2010; Medina‐Mora 2016). Data from the Global Burden of Disease Study found alcohol use to be the leading risk factor for disease and injury among people up to 49 years of age globally, and the leading risk factor in Eastern Europe, Andean Latin America, and southern Africa. Furthermore, alcohol use was responsible for 2,735,511 deaths in 2010, which represents a rise in number and risk factor ranking since the late 1990s (Lim 2012). These challenges disproportionately and increasingly affect low‐ and middle‐income countries (LMICs) where the majority of premature mortality attributable to alcohol use occurs (Medina‐Mora 2016; Ferreira‐Borges 2017). Despite the lower, but increasing prevalence of harmful alcohol use in many LMICs relative to high‐income countries (HICs) in Australia, Europe, and North America, the harm derived from the same levels of alcohol consumption are greater in LMICs due to higher risk drinking patterns and more prevalent and severe alcohol‐related health consequences, such as unintentional injury, in these settings (Anderson 2006; Degenhardt 2008; Medina‐Mora 2016). Certain subgroups also experience an elevated burden related to harmful drinking in LMICs. For example, women in many LMICs experience greater social consequences of harmful drinking relative to women in most HICs given that alcohol use is sometimes seen as socially or culturally inappropriate for women (Medina‐Mora 2001). Furthermore, despite the lower prevalence of alcohol use among women in LMICs, women in some LMICs who do drink, including during high‐risk periods such as pregnancy, engage in more harmful patterns of drinking (Popova 2017). There is a similar pattern for people of low socioeconomic status. Although the prevalence of any drinking tends to be lower among low socioeconomic groups, those who report any alcohol use tend to participate in more harmful patterns of drinking, which increases their vulnerability to alcohol‐related consequences, despite having fewer resources to cope and to access health and social services (Room 2002; Johnson 2019).
Given the biologic, psychological, and social burden attributable to harmful alcohol use and its role as a causal risk factor in an array of prevalent health conditions, identifying effective interventions to reduce harmful alcohol use through treatment and indicated prevention are critically important. Furthermore, examining evaluations of a broad range of interventions in LMICs is important as contextual factors unique to these settings may modify their feasibility, acceptability, and clinical effectiveness.
Description of the intervention
Interventions to reduce harmful alcohol use include pharmacologic interventions for alcohol use disorder, psychosocial interventions for hazardous and harmful alcohol use (i.e. non‐pharmacologic and 12‐step programs), and combined (i.e. pharmacologic and psychosocial) strategies. Guidelines often recommend combined interventions for individuals with alcohol use disorder (Center for Substance Abuse Treatment 2009); however, whether to provide pharmacologic, psychosocial, or combined interventions should be informed by the person's treatment goals (e.g. abstinence, moderation), preferences, and what is locally available and acceptable (Connor 2016; Soyka 2017).
Pharmacologic treatments
Pharmacologic treatments approved and recommended for the treatment of alcohol use disorder and dependence include disulfiram, naltrexone, and acamprosate (Table 7; Kleber 2006; WHO 2011; NICE 2011; Franck 2013; Winslow 2016). Disulfiram is an oral medication administered daily that produces significant unpleasant biologic reactions when alcohol is consumed. This aversive medication is intended to promote abstinence and prevent impulsive drinking via anticipation of its adverse effects (Skinner 2014). Naltrexone, an opioid antagonist, can be administered daily through its oral formulation or monthly via injection. Naltrexone is designed to reduce the euphoric effects of alcohol, thereby attenuating subsequent craving (Rosner 2010a). Acamprosate is intended for maintenance of abstinence among people with alcohol dependence who have undergone detoxification. It is administered orally three times per day and reduces symptoms of protracted abstinence including anxiety, insomnia, dysphoria, and craving (Plosker 2015). Other pharmaceuticals including nalmefene, baclofen, and topiramate are under evaluation by regulatory agencies or early‐stage clinical trials to assess their efficacy in treating alcohol dependence (Minozzi 2018; Franck 2013).
1. Summary of medication assisted treatment approved for harmful alcohol use.
| Medication | Administration | Indication | Intended effect |
| Acamprosate | Oral (3 times daily) | Maintenance of abstinence from alcohol | Reduces symptoms of protracted abstinence (e.g. anxiety, craving) |
| Disulfiram | Oral (once daily) | Promote abstinence from alcohol | Produces adverse effects when alcohol is consumed |
| Naltrexone | Oral (once daily) Injection (once monthly) |
Prevent alcohol craving | Reduces the euphoric effects of alcohol |
Psychosocial interventions
Psychosocial interventions include all non‐pharmacologic interventions. Psychosocial interventions recommended for the indicated prevention and treatment of varying levels of harmful alcohol use severity include psychological treatments, 12‐step facilitation (TSF) programs, and brief interventions (Hamza 2014). Historically, in HICs, mutual‐help organizations, such as Alcoholics Anonymous, were developed out of a lack of available and accessible services in the general population. This was followed by a rise in alcohol treatment centers and providers, which served as the foundation for the development and evaluation of psychological interventions for harmful alcohol use beginning in the mid‐20th century (McCrady 2014). Currently, there is a range of psychological and behavioral interventions available for treatment of harmful alcohol use including cognitive behavioral therapies (CBT), psychodynamic therapies, relapse prevention, contingency management, community reinforcement, TSF, family therapy, and brief interventions (including motivational interviewing). The theoretical orientations underpinning these interventions have followed ideological shifts in clinical psychology beginning with psychoanalytic approaches, which were popularized in the 1940s, to skills‐ and motivation‐based cognitive‐behavioral therapies. These interventions vary in their purported mechanisms of behavior change, delivery format (e.g. individual versus group‐based), duration, frequency, and provider (Kleber 2006). For the purpose of this review we differentiate brief interventions from other psychosocial treatment interventions. Brief interventions may include indicated prevention interventions aimed to prevent to the transition from hazardous alcohol use to harmful alcohol use, including alcohol use disorder, and have also been tested as a treatment strategy. Generally, these interventions apply motivational interviewing techniques, are shorter in duration relative to other psychosocial interventions, and include individuals with sub‐threshold harmful alcohol use (i.e., hazardous alcohol use) with the intention of preventing the onset of alcohol use disorder. We classified the remaining non‐pharmacologic interventions as 'other psychosocial interventions', which often focus on reducing harmful alcohol use using the range of psychological and behavioral modalities described above.
Mutual‐help organizations include traditional 12‐step programs, secular mutual‐help organizations, and other religious mutual‐help organizations. Alcoholics Anonymous, the largest community‐based 12‐step organization for alcohol‐related problems, aims to strengthen emotional, social, and spiritual coping strategies through peer support groups and step‐work (Greenfield 2013). Non‐12‐step mutual‐help organizations include secular groups that focus on non‐spiritual coping strategies for recovery (e.g. SMART (Self‐Management And Recovery Training) Recovery) as well as other religious groups that are not based on the 12‐step framework (e.g. Celebrate Recovery), but do maintain spirituality as a central component of their groups and discussion (Humphreys 2004). These groups have been incorporated into treatment systems through TSF approaches; however, 12‐step groups may also be accessed without engagement through the formal treatment system (Tonigan 2003). The evolution of psychosocial interventions has been well documented in high‐income settings, but little is known about the implementation and utilization of these and other approaches in LMICs (National Institute on Drug Abuse 2000).
Combined pharmacologic and psychosocial interventions
Guidelines for treatment of alcohol use disorder often recommend providing pharmacologic treatment in combination with psychosocial approaches. Combining these intervention modalities is believed to improve compliance with medication, enhance person's willingness to engage in psychosocial treatments and maintain or increase periods of abstinence and promote successful treatment outcomes (Center for Substance Abuse Treatment 2009).
How the intervention might work
In this review we included indicated prevention and treatment interventions to reduce harmful alcohol use. Indicated prevention interventions focus on preventing the transition from hazardous to harmful alcohol use. Treatment interventions aim to increase remission (i.e. abstinence or low‐risk drinking) and reduce harmful alcohol use among individuals with alcohol use disorder. Both indicated prevention and treatment interventions were included because some treatment modalities, particularly brief motivation‐based psychosocial modalities, are used as both indicated prevention and treatment strategies for people with hazardous or harmful alcohol use (NICE 2011). The mechanisms by which these interventions are expected to work vary by type of intervention. Pharmacologic interventions aim to alter the reward system by reducing stimuli that positively reinforce drinking or introducing aversive stimuli that counter‐condition alcohol use (Douaihy 2013). Psychosocial interventions are intended to reduce harmful alcohol use or prevent alcohol use disorder through several mechanisms. Psychosocial treatments for harmful alcohol use (e.g. CBT, contingency management, relapse prevention) are largely predicated on the theory that alcohol use and misuse is reinforced by internal and external cues, psychological and physical responses to alcohol consumption, and lack of adaptive coping skills. Thus, these interventions aim to modify conditioned responses to triggers, reinforce abstinent or moderated drinking behaviors, strengthen adaptive coping skills that reduce harmful alcohol use, or a combination of these (McCrady 2014). Brief motivation‐based interventions aim to increase one's motivation for behavior change through personalized feedback, participant‐directed behavior change and goal‐setting, regardless of the person's readiness to change their alcohol use (Miller 1983; Rollnick 1992). TSF programs encourage participation in self‐ and mutual‐help organizations (e.g. Alcoholics Anonymous), which aim to reduce alcohol use via changes in the composition of one's social network, increases in abstinence self‐efficacy and spirituality, and reductions in negative affect (National Institute on Drug Abuse 2000; Kelly 2012; Humphreys 2014).
Why it is important to do this review
Effective treatment and prevention of harmful alcohol use has significant potential to reduce the burden of disease globally. Populations in LMICs display unique epidemiological patterns of alcohol use and related consequences, socio‐cultural contexts and norms, and health care delivery systems (WHO 2014), all of which have implications for intervention implementation, acceptability, and efficacy. With the disproportionately high burden in LMICs coupled with the lack of evidence regarding effectiveness of treatment and prevention programs in these countries and the large treatment gap (Benegal 2009), a review focused on the treatment and indicated prevention of harmful alcohol use in these settings is needed.
The need for this review is evidenced by the recent increase in the number of published systematic reviews of alcohol interventions for a subset of interventions, populations, or settings that are covered in the current review. These reviews include one systematic review and one meta‐analysis of the efficacy or effectiveness of brief interventions for harmful and hazardous alcohol use in low‐ and/or middle‐income countries (Ghosh 2022;Joseph 2017). These reviews suggest an initial benefit of brief interventions on reducing hazardous and harmful alcohol use that attenuates over time with some heterogeneity explained by the intervention dose and provider type (Ghosh 2022). Both of these reviews noted the heterogeneity among included studies; however, there was mixed agreement on the methodological quality of included studies (Ghosh 2022; Joseph 2017). Two additional reviews examined the effectiveness of psychosocial interventions more generally on hazardous and harmful alcohol use in Sub‐Saharan Africa and LMICs, respectively (Preusse 2020; Sileo 2021). The systematic review and meta‐analysis conducted in Sub‐Saharan Africa identified 19 intervention trials (including randomized and non‐randomized designs) of moderate quality that showed some evidence of the efficacy of psychosocial interventions for promoting the odds of abstinence, but did not identify effects on hazardous or harmful alcohol use or consumption. The authors also noted the substantial heterogeneity that was present across all meta‐analytic models (Sileo 2021). The systematic review that included 21 randomized controlled trials of psychosocial interventions in LMICs reported small effect sizes and clear gaps in coverage of specific populations, such as conflict‐affected populations (Preusse 2020). The current review aims to cover a broader set up interventions (including pharmacologic and combined psychosocial and pharmacologic interventions) and explore a range of factors that explain heterogeneity in the observed effects. This review will add to this growing evidence of the efficacy of psychosocial and pharmacologic interventions for reducing harmful alcohol use in LMICs.
Systematic reviews of population‐level interventions for alcohol use and misuse have been conducted focusing primarily on supply‐side policy strategies (e.g. alcohol prohibition, regulation of alcohol outlets and advertisement, taxation) and have found promising results for reducing alcohol consumption and related harms (Sornpaisarn 2013; Medina‐Mora 2016; Petersen 2016). These types of interventions have great public health impact, but are less likely to be effective for addressing the challenges for individuals with harmful alcohol use. Economic analysis of addictive behaviors has found demand to be less responsive to price increases or supply‐side regulations (i.e. lower price elasticity of demand) in people with a substance use disorder relative to consumers without problematic use (Xu 2011).
Despite the treatment and prevention of harmful alcohol use being recognized as a public health and global mental health priority, there is still a dearth of evidence regarding what is most effective for treating harmful alcohol use and disorder within the unique context of LMICs, which often face limited availability of treatment and services, high levels of adversity, and increasingly severe alcohol use patterns and related consequences (Baingana 2015; Medina‐Mora 2016).
Objectives
To assess the efficacy and safety of psychosocial and pharmacologic treatment and indicated prevention interventions compared with control conditions (wait list, placebo, no treatment, standard care, or active control condition) aimed at reducing harmful alcohol use in low‐ and middle‐income countries (LMICs).
Methods
Criteria for considering studies for this review
Types of studies
This review included randomized controlled trials (RCTs) conducted in LMICs. We excluded observational studies, quasi‐experimental studies, implementation and process evaluation studies (i.e. containing no outcome evaluation), and population‐level interventions.
Types of participants
We included studies that enrolled individuals with harmful alcohol use. Consistent with the definition provided by the World Health Organization (WHO), this includes individuals with patterns of alcohol use that "causes detrimental health and social consequences for the drinker, the people around the drinker and society a large, as well as patterns of drinking that are associated with increased risk for adverse health consequences" (WHO 2010). Therefore, we include participants with a range of unhealthy patterns of alcohol use including hazardous drinking, single episodes of harmful alcohol use, harmful drinking patterns, and alcohol use disorder (including alcohol dependence; WHO 2018a; WHO 2010). We excluded studies enrolling participants with no or low‐risk alcohol use, unless subgroup analyses for the participants with harmful alcohol use at baseline were reported. We included studies of all age groups and applied no restrictions based on demographic characteristics. We defined LMICs using the World Bank Country Lending Group classification of a given country at the time the study was conducted (World Bank 2015). For the 2018 fiscal year, the World Bank defined LMICs as those with a Gross National Income per capita less than USD 12,336 (approximately two‐thirds of countries in the world); however, this cutoff is adjusted each year to account for inflation and changes in exchange rates (World Bank 2017). Studies conducted in countries that were classified as low‐income, lower‐middle‐income, or upper‐middle‐income during the period of study recruitment were included. Historical data on Gross National Income per capita as well as the World Bank cutoffs for classifying countries as low‐, middle‐, or high‐income are available through the World Bank DataBank Development Indicators (World Bank 2021).
Types of interventions
Any type of individual level pharmacologic or psychosocial intervention, or combination therein, focused on treatment or indicated prevention of harmful alcohol use were included. Pharmacologic interventions specifically focused on treating individuals who met criteria for alcohol use disorder; whereas, psychosocial interventions included those meeting criteria for alcohol use disorder or individuals with unhealthy drinking patterns who were at high risk for alcohol use disorder. We did not include policy interventions or universal campaigns (e.g. media awareness programs). We excluded universal and selective prevention programs targeting the prevention of any alcohol use. Indicated prevention programs were included if they focused on preventing the onset of alcohol use disorder among individuals with high levels of risk based on their current drinking patterns.
We included studies with active (i.e. comparative effectiveness) and inactive (e.g. no‐treatment, placebo, standard care, wait‐list) control groups.
Types of outcome measures
Primary outcomes
Reduction in harmful alcohol use. We selected this as the primary outcome given that this outcome has been commonly used in research on alcohol use in LMICs (Ghosh 2022; Joseph 2017; Preusse 2020; Sileo 2021). Harmful alcohol use must have been measured using a self‐report measurement tool.
Retention, defined as number of participants who completed treatment.
Secondary outcomes
Change in alcohol consumption (self‐report or biomarker) compared across studies with similar time scales.
Remission compared across studies with similar time scales. Remission was defined as the number of participants with a prior history of harmful alcohol use who were abstinent, did not relapse, or were classified as low‐risk drinkers at follow‐up (Grella 2013).
Adverse events.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
Published, unpublished, and ongoing studies were identified by searching the following databases from their inception:
Cochrane Drugs and Alcohol Group (CDAG) Specialized Register (most recent);
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2021, issue12);
PubMed (from 1946 to 12 December 2021);
Embase (Ovid) (from January 1974 to 12 December 2021);
PsycINFO (EBSCOhost) (from 1800 to 12 December 2021);
CINAHL (EBSCOhost) (from 1982 to 12 December 2021);
ClinicalTrials.gov (www.clinicaltrials.gov) via the Cochrane Register of Studies (CRS);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch);
Latin American & Caribbean Health Sciences Literature (LILACS) (from 1982 to 12 December 2021).
The search strategies for each database are provided in Appendices 1‐10. Where appropriate, these were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Box 6.4.b.; Higgins 2011).
Searching other resources
We searched the reference lists of all included studies and relevant review articles identified during the screening process for other eligible studies. We searched the Opengrey database to identify unpublished literature that met our eligibility criteria (opengrey.edu). We searched Web of Science to identify studies not available in English or the other databases included in the search strategy, or both.
Data collection and analysis
Selection of studies
Two review authors independently screened titles and abstracts using Covidence (Covidence) with discrepancies resolved through discussion or, if necessary, by a third review author. We excluded duplicates and collated multiple reports of the same trial, so that each trial rather than each report was the unit of analysis in the review. Review authors classified studies as 'yes' if the title and abstract discussed alcohol use, an alcohol treatment or indicated prevention intervention, and LIMICs. Studies were labeled 'maybe' if they mentioned alcohol use, an intervention, and LMICs, or if the study setting was unclear. Studies were marked 'no' if they were not RCTs or intervention studies, if they did not mention alcohol or substance use, or if they were conducted in HICs. We retained all articles classified as 'yes' or 'maybe' for full‐text review. Two review authors independently screened the full text of retained articles based upon the full eligibility criteria. Studies were classified as 'eligible' at this stage if they fit the criteria outlined in the Criteria for considering studies for this review section. Full‐text review screening was similarly completed using Covidence (Covidence).
Data extraction and management
Two review authors independently entered study characteristics and data into piloted and standardized forms. The review authors piloted the data extraction forms on five included studies and any modifications to the forms were made prior to data extraction for the remaining studies. Any discrepancies in data extraction were reviewed and, if necessary, arbitrated by a third review author. We collected and entered the following data from all included studies.
Methods: study design, randomization, allocation concealment procedures, blinding, eligibility criteria, study setting/location, duration of follow‐up, informed consent procedures, and ethical approvals.
Participant characteristics: age group, sex, race/ethnicity or other indicators of ancestry, marital status, baseline harmful alcohol use, co‐occurring disorders, sample size, description of the target population, including whether the intervention targeted certain special populations (e.g. HIV‐infected, women during the perinatal period, etc.).
Interventions: type of intervention (pharmacologic, psychological, brief, mutual‐help, etc.), brief description of intervention, duration of intervention, frequency of intervention, intervention delivery and provider, comparison group.
Outcomes: harmful alcohol use, retention, alcohol consumption, remission, adverse events, measurement tool, summary measures of central tendency/variance, timing of outcome ascertainment.
Results: total number of participants randomized, missing data, post‐treatment mean and variance/standard deviation/standard error for continuous outcomes, group count, and proportions for categorical outcomes.
Country.
Study funding and conflict of interest of study's authors.
Assessment of risk of bias in included studies
Two review authors independently evaluated the included studies for risk of bias according to the parameters recommended by Cochrane (Higgins 2011). The criteria examined for risk of bias were random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessors; incomplete outcome data; and selective outcome reporting. These parameters are described in more detail below.
Random sequence generation referred to the method used to randomize people to study conditions. Adequate random sequence generation that was considered low risk of bias included use of a random number generator or random number table. Improper (e.g. allocation by date) or lack of random sequence generation may introduce selection bias. Thus, we labeled studies with inadequate methods of random sequence generation at high risk of bias. We labeled risk of bias as unclear if the study did not report their method of random sequence generation.
Allocation concealment referred to methods that prevent study personnel from foreseeing participant study assignments. Adequate allocation concealment, which we labeled as having low risk of bias included methods such as central allocation or the use of sequentially numbered opaque, sealed envelopes/drug containers that contained study assignments. Methods with a high risk of bias included those that produced a predictable pattern of allocation (e.g. date of birth) or when the allocation sequence was available to study personnel prior to allocation. We labeled a study as having unclear risk of bias if allocation concealment methods were not described or if it was unclear whether the method would prevent anticipating the allocation of participants. Poor allocation concealment may result in selection bias.
Blinding of participants and personnel referred to preventing the participants and study personnel from being aware of the participant's study condition as a means to reduce performance bias. Study personnel here referred to those that delivered the intervention. Unblinding participants and personnel may have impacted engagement, intervention fidelity, interactions between the participant and personnel, outcome reporting, or a combination of these, all of which may have induced performance bias. Blinding of participants and personnel is very challenging particularly for psychosocial interventions. We classified studies as low risk of bias if the participants and personnel were blinded to study condition. In contrast, we classified studies as high risk of bias if the participants or study personnel, or both, were unblinded. If this information was not included in the manuscript for interventions that could reasonably be blinded, we labeled risk of bias as unclear. Non‐pharmacologic interventions that were impossible to blind due to the nature of the intervention were automatically considered at high risk of bias.
Blinding of outcome assessors referred to preventing the study personnel involved in data collection from being aware of a participant's study condition. Similar to concerns with blinding of participants and other study personnel, unblinded outcome assessors may introduce detection bias, whereby their knowledge of participant study condition may influence how data were recorded, how they asked questions or engaged with the participant. Self‐reported outcomes, such as the primary and many of the secondary outcomes included in this review, were particularly vulnerable to differential reporting arising from the participant's awareness of intervention assignment. The outcomes related to harmful alcohol use included in this review were measured using self‐report instruments typically administered by data collection personnel, which were prone to differential recording based on expectations regarding the effects of participation in a given study condition. We classified studies as low risk of bias if the outcome assessor and participant were blinded to study condition. In contrast, we classified studies as high risk of bias if the outcome assessor or participant was unblinded. If this information was not included in the manuscript, we labeled risk of bias as unclear.
Incomplete outcome data included differential attrition between groups as well as proper handling of missing data. We compared the magnitude and reasons for missingness and attrition between groups. Furthermore, we considered how missing data were handled and whether the researchers conducted an intention‐to‐treat analysis, whereby all participants were analyzed as randomized. Studies with low risk of bias had non‐differential attrition between groups and must have been analyzed according to the intention‐to‐treat principle. Studies with high risk of bias had a large amount of missing data that were poorly handled, differential reasons, and magnitude of attrition between groups, or only employed a per‐protocol analysis. We labeled studies that did not clearly fall into either category as unclear risk of bias.
Reporting bias referred to consistency in the outcomes reported in the methods or study protocol (if available) and those reported in the results. If outcomes were postulated, but not reported in the results, this would be an indication of reporting bias and these studies were classified as high risk of bias. We only classified studies at low risk of bias if a protocol or prior publication was available whereby outcomes were clearly designated a priori and were reported in the included study. Otherwise, we classified studies as unclear risk of bias.
Measures of treatment effect
Continuous data
We assessed the effect of the intervention on harmful alcohol use and other continuous secondary outcomes as differences in changes from baseline to follow‐up between groups. We transformed study‐specific effects to standardized mean differences (SMDs), which allowed us to compare continuous outcomes across studies that applied different outcome measurement tools (Faraone 2008). We also included estimates of variability and uncertainty (e.g. standard deviation (SD), confidence intervals (CIs), range). We used outcome data from the follow‐up closest to the modal post‐randomization time point reported in the included studies for the primary analysis. If we had identified substantial variation in follow‐up time, we planned to conduct analyses at multiple time points.
Dichotomous data
We anticipated that some studies might have applied clinical cutoffs to these continuous measures of harmful alcohol use, consumption, and other secondary outcomes. For dichotomous outcomes, including remission, retention, and dichotomized indicators of harmful alcohol use, we calculated the risk ratio (RR) and associated 95% confidence interval (CI).
Unit of analysis issues
We conducted all analyses at the individual level. We evaluated outcomes from cluster‐RCTs reported at the individual level accounting for clustering in the data using the generic inverse‐variance method.
Studies with more than two groups
If studies included multiple intervention or comparison groups, we first assessed whether more than two study conditions met criteria for types of interventions and comparison groups included in this review. We described all relevant study conditions in the qualitative data synthesis. Conversely, in the quantitative data synthesis, we combined relevant experimental or control conditions to create a single pair‐wise comparison for inclusion in the meta‐analysis.
Dealing with missing data
We contacted authors of studies that had missing information or did not report all results on primary outcomes and important study characteristics. If we did not receive a response within two weeks, we considered the data missing. We excluded studies that were missing data on our primary outcomes from quantitative analyses, but we retained these studies in the qualitative synthesis. We conducted all analyses using intention‐to‐treat; however, we excluded studies with high attrition in a sensitivity analysis to examine the possible effect of missingness on the pooled treatment effect estimate.
Assessment of heterogeneity
We explored clinical, methodologic, and statistical heterogeneity qualitatively and then quantitatively using Cochrane's Q, tau2, and I2 statistical tests. The qualitative evaluation included a comparison of studies with regards to study setting, sampling, eligibility criteria, intervention details, and outcome definitions to identify sources of clinical heterogeneity. We assessed methodologic heterogeneity through comparisons of study procedures (e.g. recruitment, duration of follow‐up), case definition for harmful alcohol use, risk of bias, and measurement methods. We produced forest plots stratified by intervention type to visually inspect variability in the intervention effects of included studies (Sutton 2000). Cochrane's Q, tau2, and I2 statistics quantified statistical heterogeneity. Cochrane's Q tested the null hypothesis that the effect size is equivalent across studies. Statistically significant (P < 0.10) findings suggested that heterogeneity is present. The tau2 statistic described variance in the effect estimate between studies and was used to quantify the distribution of intervention effects. The I2 statistic described the proportion of variability due to statistical heterogeneity regardless of scale or number of included studies. I2 values greater than 50% indicated substantial heterogeneity (Higgins 2003; Borenstein 2009). We did not estimate pooled intervention effects for outcomes with substantial heterogeneity.
Assessment of reporting biases
To minimize reporting biases, we searched and included both published and unpublished literature. We assessed publication bias visually by producing and examining funnel plots. We considered study characteristics that may affect symmetry of the funnel plot (e.g. differences in study quality, true heterogeneity, number of studies).
Data synthesis
We analyzed pharmacologic and psychosocial interventions separately. We further stratified by intervention type and study population to align the intervention comparisons with their appropriate clinical indications. See Summary of findings and assessment of the certainty of evidence.
Qualitative data synthesis
We synthesized results of included studies in a narrative review discussing the effects of each treatment modality on harmful alcohol use. We explored differences in results between studies and discussed how they may relate to study design, study population, measurement methods, etc. We discussed patterns, notable findings, and limitations of the studies and the available literature more broadly.
Quantitative data synthesis
We performed a meta‐analysis with study‐level data using Review Manager 5 (Review Manager 2014). We anticipated that the treatment effects would vary by study setting, population, and other study‐level factors, and therefore used the random‐effects model for all analyses.
Subgroup analysis and investigation of heterogeneity
We did not conduct planned subgroup analyses specified in the study protocol due to the paucity and heterogeneity of study results (seeDifferences between protocol and review).
Sensitivity analysis
We did not conduct planned sensitivity analyses that were specified in the study protocol due to the paucity and heterogeneity of study results (see Differences between protocol and review).
Summary of findings and assessment of the certainty of the evidence
Summary of findings tables
We produced a summary of findings table for each of the following comparisons:
any pharmacologic intervention versus placebo among participants with alcohol use disorder or dependence;
any pharmacologic intervention versus placebo in addition to psychosocial intervention among participants with alcohol use disorder;
any pharmacologic intervention versus another pharmacologic intervention among participants with alcohol use disorder;
any pharmacologic intervention versus another pharmacologic intervention in addition to psychosocial intervention among participants with alcohol use disorder;
brief psychosocial intervention versus brief advice, information, or assessment only among participants with hazardous or harmful alcohol use;
other psychosocial intervention versus usual care or wait list among participants with hazardous or harmful alcohol use.
We included the primary outcomes in the summary of findings tables: harmful alcohol use and retention.
Grading of evidence
We assessed the overall certainty of the evidence for primary and secondary outcomes using the GRADE system. The GRADE Working Group developed a system for grading the quality/certainty of evidence which takes into account issues related to internal validity and external validity, such as directness, consistency, imprecision of results, and publication bias (Schünemann 2013). The summary of findings tables present the main findings of a review in a transparent and simple tabular format. In particular, they provide key information concerning the certainty of evidence, the magnitude of effect of the interventions examined, and the summary of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grades of evidence:
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect;
very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Grading was decreased for the following reasons:
serious (–1) or very serious (–2) study limitation for risk of bias;
serious (–1) or very serious (–2) inconsistency between study results;
some (–1) or major (–2) uncertainty about directness (the correspondence between the population, the intervention, or the outcomes measured in the studies actually found and those under consideration in our systematic review);
serious (–1) or very serious (–2) imprecision of the pooled estimate (–1);
publication bias strongly suspected (–1).
Results
Description of studies
Results of the search
Of the 8740 records identified from searches through December 2021 we identified 309 articles that, based on their title and abstract, were included in the full‐text review. One hundred and three articles, representing 66 unique RCTs, were included in the systematic review. There was a total of 17,626 participants enrolled in the 66 included RCTs (see Characteristics of included studies). We identified six cluster‐randomized trials and the remaining 60 RCTs were individually randomized. We identified 16 ongoing studies that may meet our eligibility criteria and 12 studies (17 articles) that are awaiting classification. See Figure 1 for the PRISMA flow diagram.
1.
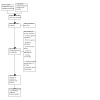
Study flow diagram.
Included studies
See Characteristics of included studies.
Setting
Of the 66 included trials, 34 were conducted in upper‐middle income countries, 27 in lower‐middle‐income countries, three in low‐income countries, and two multi‐site trial conducted in a combination of upper‐middle and lower‐middle income countries. Twelve trials were conducted in Brazil, one in Chile, one in China, 14 in India, one in Iran, four in Kenya, one in Mongolia, one in Nepal, one in Nigeria, one in Poland, three in Russia, five in South Africa, nine in Thailand, one in Turkey, one in Uganda, two in Ukraine, one in Vietnam, three in Zambia, two in Zimbabwe, and two were multi‐site trials that included study sites in Belarus, Brazil, India, Kenya, Mexico, Russia, and Zimbabwe.
Participants
Fifty‐two out of 66 trials recruited participants from health centers including general outpatient and primary care settings, emergency departments, psychiatric/substance use services, and infectious disease clinics. Other recruitment venues included police stations, universities, farms, hotlines or websites, nightclubs and other community settings. Several trials recruited specific vulnerable populations including refugees (n = 1 trial), female sex workers (n = 2 trials), people living with HIV/AIDS (n = 10 trials), and people with tuberculosis (n = 3 trials). Eighteen trials only enrolled males, while only four exclusively enrolled females. Most of the trials that exclusively enrolled females targeted high‐risk subgroups including female sex workers (n = 2 trials) or females at high risk of alcohol‐exposed pregnancy (n = 1 trial). The remaining trial recruited women seeking primary care services. Among those that recruited both males and females, the median percentage of males enrolled was 89.1%. All studies that reported age enrolled a sample with an average age over 18 years. Very few studies explicitly described ethnicity or nativity characteristics of the sample. Sample sizes ranged from 33 in Bolton 2014 to 1400 in Schaub 2021 (Median = 157.5, interquartile range (IQR) = 92.5 to 371).
Harmful alcohol use eligibility criteria varied across studies. Twenty‐five trials recruited participants who met criteria for alcohol use disorder. Thirty‐one trials used cutoffs for hazardous, harmful, or risky drinking, most commonly assessed using the Alcohol Use Disorder Identification Test (AUDIT). Other trials used specific indicators of risky drinking (e.g. extended periods of binge‐drinking, average consumption volume; n = 2 trials), seeking care in the emergency department as a result of alcohol‐related health problems or injury (n = 1 trial), self‐reported alcohol problems (n = 3 trials), partner‐reported alcohol problems (n = 2 trials), enrollment in alcohol treatment services (n = 1 trial), or calling into a support line to stop drinking (n = 2 trials) as a proxy for harmful alcohol use.
Interventions and comparators
The majority of interventions (n = 52 trials, 77.6%) were psychosocial. Brief interventions were the most common type of psychosocial intervention (n = 27 trials, 51.9% of psychosocial trials, 40.3% of all included trials), followed by other psychosocial interventions, which were sometimes delivered in combination with or in comparison to brief intervention elements (n = 25 trials, 48.1% of psychosocial trials, 37.3% of all included trials). Brief interventions ranged from one session to seven sessions and focused primarily on motivational interviewing techniques. Some brief interventions also incorporated elements of cognitive behavioral therapy, risk reduction, relapse prevention, and/or self‐help. Providers were specialist and non‐specialist health workers, including nurses, HIV counselors, general counselors, social workers, psychologists, physicians as well as lay counselors, trained university students, and telephone hotline workers. One brief intervention used a self‐help booklet to supplement the single session delivered by health providers. Comparison conditions for brief interventions included informational controls, brief advice, brief intervention for tobacco use, usual care, a list of available treatment and recovery support services, equal attention nutrition control, assessment only, or a wait‐list control.
Other psychosocial interventions included the following: a transdiagnostic psychotherapy, Common Elements Treatment Approach (CETA) that included cognitive‐behavioral therapy (CBT) and motivational interviewing components; 25‐ to 30‐day inpatient treatment programs; a contingency management program combined with counseling and/or home visits; four to 10 sessions of combined motivational interviewing and cognitive behavioral therapy; 20 group psychotherapy sessions incorporating motivational interviewing, cognitive therapy, coping skills training and relapse prevention supplemented with home visits; seven sessions of relapse prevention within an inpatient detox and treatment setting; eight to 10 sessions of family‐ or individual‐based relapse prevention; 20 sessions of rational emotive psychotherapy; six to eight sessions of CBT; two five to six sessions behavioral interventions; four sessions of alcohol counseling; and a six‐week self‐guided web‐based self‐help program. Comparison conditions for psychosocial treatment interventions included a motivational intervention, Mental Health Gap Action Programme (mhGAP)/enhanced usual care, attention‐matched control, usual care, brief motivation, risk reduction, or wellness intervention; information only, assessments only, and a wait‐list control.
Six trials compared the efficacy of various pharmacologic treatments to other active conditions or placebo on treating alcohol use disorder (9.0% of all included trials). Active pharmacologic study conditions tested acamprosate, baclofen, disulfiram, gabapentin, mirtazapine, and naltrexone. The two trials of gabapentin were placebo‐controlled as was one of the acamprosate trials. The remaining trials compared mirtazapine to amitriptyline, acamprosate to disulfiram, and a three‐arm trial comparing acamprosate, baclofen, and naltrexone. One of the pharmacologic trials encouraged participants to attend Alcoholics Anonymous and, after completion of the 12 weeks of pharmacologic treatment, transitioned participants into 12 weeks of non‐pharmacologic treatment. One of the placebo‐controlled gabapentin trials also included weekly compliance sessions.
Eight trials combined pharmacologic and psychosocial treatments for alcohol use disorder (11.9% of all included trials). The primary objective of seven of these studies was to evaluate the efficacy of a pharmacologic treatment as compared to a placebo or other active pharmacologic treatment. In these trials, the investigators combined the pharmacologic treatments under study with psychosocial interventions. The studies included the following comparisons: 12 weeks of naltrexone with counseling versus placebo with counseling; usual psychosocial care and encouragement to attend Alcoholics Anonymous along with 12 weeks of naltrexone, topiramate, or placebo; naltrexone with brief intervention or placebo with brief intervention, 12 weeks of brief CBT and encouragement to attend Alcoholics Anonymous and either ondansetron or placebo; 12 months of weekly group psychotherapy with either naltrexone or disulfiram; nine months of weekly group psychotherapy with either disulfiram or topiramate; and three to five counseling sessions along with 12 weeks of either topiramate or placebo. The final study combined six months of naltrexone with brief intervention and compared it to either naltrexone alone, brief intervention alone, or placebo.
Outcomes
Sixty‐two of the 66 included trials provided quantitative data that enabled their inclusion in the meta‐analysis. For the primary clinical outcome, harmful alcohol use, 33 trials provided data. Thirty‐five reported harmful alcohol use as a continuous outcome. The primary efficacy analysis examining continuous or dichotomous measures of harmful alcohol use included zero participants enrolled in pharmacologic trials, 5262 participants enrolled in 21 trials of brief interventions, 2106 participants enrolled in 12 trials of psychosocial treatment interventions, and 577 participants enrolled in five combined interventions. All included pharmacologic interventions aimed to reduce harmful alcohol use among individuals with alcohol use disorder, but reported different outcomes. Harmful alcohol use was most commonly measured using standardized tools, such as the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST), or the Alcohol Use Disorder Identification Test (AUDIT/AUDIT‐C), which assess hazardous or harmful alcohol use, or by measuring the different patterns of consumption (frequency, volume, and/or binge‐drinking). The modal follow‐up duration was six months, and thus we selected the outcome data nearest to six months to include in our meta‐analysis for all efficacy outcomes.
The safety outcomes included retention and adverse events. Three placebo‐controlled pharmacologic trials with 247 participants reported on completion of pharmacologic treatment. Similarly, three combined intervention trials including 363 participants reported on completion of treatment. Five pharmacologic and combined trials also reported on adverse events, which were reported as lists of frequencies or proportions of participants in each study condition that experienced adverse events. The four comparative effectiveness pharmacologic trials also reported on retention. Twelve brief psychosocial interventions reported on retention. In these brief psychosocial trials, retention was often defined as receiving the allocated intervention, which was most typically either a single brief motivational interviewing session or brief advice/information. Nine other psychosocial interventions reported retention by study condition, which was operationalized as either receiving or completing the allocated intervention. Ten trials reported on retention in the intervention in the experimental group only. In seven trials it was unclear whether retention was reported in reference to the intervention or the research follow‐up assessments and thus these results were excluded from the meta‐analysis.
Regarding secondary outcomes, 19 trials provided data on alcohol consumption, 19 trials provided data on remission, and five trials provided data on adverse events. The analysis of the efficacy of alcohol interventions on reducing alcohol consumption included 94 participants from one pharmacologic trial, 1779 participants from nine brief intervention trials, 1609 participants from seven psychosocial treatment trials, and 71 participants from one combined intervention trial. Alcohol consumption was measured using the Alcohol Consumption Risky Questionnaire, the ASSIST, the AUDIT, the Timeline Followback, the Form‐90, the Healthy Lifestyle Questionnaire, the Rapid Alcohol Problems Screen, the Severity of Alcohol Dependence Questionnaire, a breathalyzer, or self‐reported consumption. Ten trials also included biomarkers. The analysis of the efficacy of alcohol interventions on increasing remission included 534 participants from five placebo‐controlled pharmacologic trials, 371 participants from four comparative effectiveness pharmacologic trials, 2240 participants from five brief intervention trials, and 1519 participants from seven psychosocial intervention trials. Remission was most often operationalized as a defined period of abstinence and measured using the alcohol consumption measures described above as well as self‐reported consumption. Several studies, particularly brief intervention studies, also included low‐risk or non‐problematic drinking in their definition of remission.
Four trials were not included in the meta‐analysis. One brief psychosocial intervention was not included because it provided insufficient quantitative information. Three other psychosocial interventions were not included in the meta‐analysis because they provided incomplete quantitative data or reported on an outcome that was unable to be combined with meta‐analytic outcomes. The studies that were not included in the meta‐analysis did not appear to differ substantially from the included studies with regard to population or methodological characteristics. Thus, it is unlikely that the exclusion of these studies will yield substantial bias in the meta‐analytic results.
Excluded studies
Two hundred five studies initially selected for full‐text review did not meet our eligibility criteria and were excluded because of the following reasons: wrong study population (n = 52), wrong study setting ( = 48), wrong outcomes (n = 34), wrong study design (n = 24), wrong intervention (n = 10), or wrong comparator (n = 1). See Characteristics of excluded studies.
Studies awaiting classification
We classified 12 studies as awaiting classification, as we did not have the information necessary to evaluate eligibility. Only an abstract or a study protocol for an apparently completed study was available for 11 studies (Balachova 2017; Belokrylov 2018; Clair 2016; Du 2017; Gorny 2019; Jin 2006; Parry 2014; Prasad 2018; Rentala 2020; Staton 2019; Yadav 2019). One cluster‐randomized controlled trial (RCT) of a combined community‐based universal alcohol use prevention intervention and brief intervention for adult males was identified; however, it was unclear whether the reported results were specific to persons with harmful alcohol use (Siriwardhana 2013). Authors for eight studies awaiting classification were contacted between November 2019‐April 2020 (Balachova 2017; Belokrylov 2018; Clair 2016; Parry 2014; Prasad 2018; Siriwardhana 2013; Staton 2019). We were unable to locate the contact information for four studies awaiting classification (Du 2017; Gorny 2019; Jin 2006; Yadav 2019).
Ongoing studies
We classified 167 studies as ongoing. All ongoing studies were randomized controlled studies of interventions to address harmful alcohol use, which are expected to be completed between 2020 and 2024. These studies included a brief counseling intervention for reducing smoking and alcohol use in a hospital setting in Colombia (Almonacid 2020), a couples‐based economic and relationship strengthening intervention for couples who report hazardous alcohol use in Malawi (NCT04906616), an evaluation of a computer‐based alcohol reduction intervention for females living with HIV/AIDS and Hepatitis C virus (HCV) in Russia (Diclemente 2021), ibogaine treatment for alcoholism in Brazil (NCT03380728), a contingency management intervention for people living with HIV/AIDS and tuberculosis in Uganda (Lodi 2021), a mobile based brief intervention for adults in India (Fernandes 2019), a behavioral intervention for adults working in the fishing industry who are living with HIV/AIDS in Uganda (Kiene 2019), a tele‐behavioral health intervention delivered by a psychologist to gay/bisexual males in Romania (Lelutiu‐Weinberger 2019), an evaluation of the impact of a traditional compound used in Chinese medicine on alcohol dependence in China (Liang 2020), a nurse‐delivered motivational interviewing intervention supported by text message boosters in Tanzania (Mmbaga 2021), a motivational interviewing and problem‐solving intervention for adults with HIV and/or diabetes in South Africa (Mutyambizi‐Mafu 2019), a mobile‐based brief intervention in India (Nadkarni 2019a), a brief intervention for older adults with at‐risk drinking in primary care in Brazil (Paula 2021), a comparative effectiveness trial of different combinations of varenicline, nicotine replacement therapy, cystine and placebo for people with HIV/AIDs, heavy drinking and heavy smoking in Russia (NCT02797587), an alcohol use reduction intervention for people co‐infected with HIV and TB in India (Sangle 2021), and a tablet‐based screening, brief intervention, and referral to treatment intervention delivered by community health workers for people with probable alcohol use disorder in Mozambique (NCT03610815).
Risk of bias in included studies
Risk of bias details for individual studies are provided in Characteristics of included studies. Graphical representation of risk of bias in included studies is presented in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The majority of studies (n = 46 trials) described a random sequence generation procedure to randomly allocate study participants or clusters leading to low risk of bias. Sixteen trials did not describe the process of randomization and, while classified as a randomized controlled trial (RCT), this led to unclear risk of bias. Four trials described an inadequate randomization process leading to high risk of bias. Regarding allocation concealment, we considered 35 trials low risk. The remaining 31 trials did not provide sufficient information about the allocation concealment procedures to evaluate risk of bias or described a process that was considered high risk.
Blinding
Few trials reported that both participants and study personnel were blind to study condition, indicating low risk of performance bias. All studies determined to have low risk of performance bias were trials testing a pharmacologic treatment. Five pharmacologic or combined trials did not blind participants and/or personnel leading to high risk of performance bias. All remaining brief and other psychosocial intervention trials were unable to blind participants and personnel given the nature of the intervention, thus leading to high risk of performance bias.
The outcome assessors in three combined pharmacologic and psychosocial trials were blinded to a participant's study condition allocation, leading to low risk of detection bias. Twenty‐five brief and other psychosocial trials reported that the outcome assessor was blinded to study condition, yet primary outcomes were based on self‐report from the unblinded participant resulting in high risk of bias. All remaining brief and other psychosocial intervention trials either did not blind or did not clearly report whether the outcome assessor was blinded to study condition, but were considered as having high risk of bias because of the self‐reported nature of outcome measures and the inability to blind participants.
Six pharmacologic trials did not provide information on whether their outcome assessors were masked leading to unclear risk of detection bias. The remaining three pharmacologic and combined intervention trials did not blind outcome assessors often because these personnel were involved in a participant allocation procedure that precluded blinding and were thus classified as high risk of detection bias.
Incomplete outcome data
Risk of attrition bias was low in 20 trials because researchers reported low or non‐differential attrition and properly handled missing data in an intention‐to‐treat analysis. Nineteen trials had high risk of bias because they reported large amounts of missing data that were poorly handled, differential magnitude or reasons for attrition between study conditions, and/or employed a per protocol analysis. The remaining 27 trials had unclear risk of attrition bias.
Selective reporting
Most studies did not have a study protocol or registration that allowed us to evaluate reporting bias, but did report the results of study outcomes described in the study methods section. Among those that provided information about a priori study outcomes, 11 trials reported outcomes consistent with study protocols, while six trials displayed inconsistencies in the outcomes they proposed and those they reported.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Sixty‐two trials provided quantitative data on the primary and/or secondary outcomes included in this review.
Pharmacologic intervention versus active comparator or placebo among participants with an alcohol use disorder or dependence
Any pharmacologic intervention versus placebo among participants with alcohol use disorder or dependence
Harmful alcohol use
None of the included pharmacologic trials reported on our primary alcohol outcome, harmful alcohol use.
Alcohol consumption
None of the included placebo‐controlled pharmacologic trials reported alcohol consumption as an outcome.
Remission
One included pharmacologic trial with a total of 75 participants reported on remission (Baltieri 2003). Baltieri and colleagues study identified a marginally significant increase in the probability of self‐reported remission attributable to acamprosate as compared to placebo at six months (risk ratio (RR) = 2.13, 95% confidence Interval (CI) 1.00 to 4.52; Analysis 1.1).
1.1. Analysis.

Comparison 1: Any pharmacologic intervention vs. placebo among participants with alcohol use disorder or dependence, Outcome 1: Remission
Retention
Three included pharmacologic trials with 247 participants reported on retention in treatment (Baltieri 2003; Chompookham 2018; Furieri 2007). None of these studies identified a significant difference in the likelihood of retention comparing acamprosate or gabapentin to placebo (RR = 1.13, 95% CI 0.89 to 1.44; Heterogeneity: Tau² = 0.02; Q = 3.67, df = 2 (P = 0.16); I² = 46%; low‐certainty evidence; Analysis 1.2; Table 1).
1.2. Analysis.

Comparison 1: Any pharmacologic intervention vs. placebo among participants with alcohol use disorder or dependence, Outcome 2: Retention
Adverse events
One included pharmacologic trials with 75 participants reported on adverse events across study conditions. Baltieri 2003 reported the proportion of each study condition that experienced specific side effects including diarrhea, pruritis, polyuria, and headache. Although no statistical examination comparing reported side effects was conducted, the proportion of the sample that experienced headaches and pruritis in the acamprosate group was more than double the proportion of the placebo group that reported these same side effects (Analysis 1.3).
1.3. Analysis.
Comparison 1: Any pharmacologic intervention vs. placebo among participants with alcohol use disorder or dependence, Outcome 3: Adverse events
| Adverse events | ||
| Study | Heading 1 | Heading 2 |
| Baltieri 2003 | Experimental: 13% diarrhea, 10% pruritis, 7% polyuria, 10% headache | Control: 11% diarrhea, 4% pruritis, 11% polyuria, 4% headache |
Any pharmacologic intervention placebo given in addition to psychosocial intervention among participants with alcohol use disorder
Harmful alcohol use
Five included combined pharmacologic and psychosocial intervention trials with 577 participants reported on harmful alcohol use. Four of these trials reported harmful alcohol use on a continuous scale (Baltieri 2008; Castro 2009; Likhitsathian 2013; Shin 2013) and one reported harmful alcohol use as a dichotomous outcome (Correa Filho 2013). All five of these trials evaluated harmful alcohol use using self‐reported consumption between 3‐12 months post‐randomization and did not report the use of standardized and validated assessment tools. We identified a significant reduction in harmful alcohol use attributable to the combined intervention among studies that reported harmful alcohol use continuously (standardized mean difference (SMD) = ‐0.43, 95% CI: ‐0.61 to ‐0.24; n =4 trials, 475 participants; Heterogeneity: Tau² = 0.00; Q = 2.32, df = 3,P = 0.51; I² = 0%; low‐certainty evidence; Analysis 2.1). The study that examined the presence of harmful alcohol use, defined as heavy drinking, at follow‐up did not find a significant difference between participants who were allocated to received odanestron with brief cognitive‐behavioral therapy and encouragement to attend Alcoholics Anonymous as compared to individuals who only received brief cognitive‐behavioral therapy and encouragement to attend Alcoholics Anonymous (RR = 0.62, 95% CI: 0.16 to 2.47; n = 1 trial, 102 participants ; I2 = 0%; very low‐certainty evidence; Analysis 2.2; Table 2).
2.1. Analysis.

Comparison 2: Any pharmacologic intervention vs. placebo given in addition to psychosocial intervention among participants with alcohol use disorder, Outcome 1: Harmful alcohol use (continuous)
2.2. Analysis.

Comparison 2: Any pharmacologic intervention vs. placebo given in addition to psychosocial intervention among participants with alcohol use disorder, Outcome 2: Harmful alcohol use (dichotomous)
Alcohol consumption
One included combined intervention with 71 participants comparing naltrexone with a brief intervention to a placebo with brief intervention control condition examined alcohol consumption as an outcome (Castro 2009). This intervention did not find a significant effect of naltrexone with a brief intervention on reducing alcohol consumption relative to the brief intervention alone at four months (SMD = 0.34, 95% CI: ‐0.13 to 0.81; n=1, trial, 71 participants; Analysis 2.3).
2.3. Analysis.

Comparison 2: Any pharmacologic intervention vs. placebo given in addition to psychosocial intervention among participants with alcohol use disorder, Outcome 3: Alcohol consumption
Remission
Four included combined intervention trials with 462 participants reported on remission from alcohol use disorder. These trials operationalized remission as abstinence (Baltieri 2008; Correa Filho 2013), non‐relapse (Likhitsathian 2013), or did not provide a definition of remission (Ahmadi 2004). We identified an 19% increase in the probability of remission attributable to the combined pharmacologic and psychosocial intervention relative to the psychosocial intervention and placebo condition (RR=1.19, 95% CI: 1.01 to 1.40; n = 4 trials, 462 participants; Heterogeneity: Tau² = 0.01; Q = 3.66, df = 3, P = 0.30; I² = 18%; Analysis 2.4).
2.4. Analysis.

Comparison 2: Any pharmacologic intervention vs. placebo given in addition to psychosocial intervention among participants with alcohol use disorder, Outcome 4: Remission
Retention
Three included combined interventions with 363 participants did not find evidence of differences in study conditions comparing a pharmacologic and psychosocial intervention to the psychosocial intervention with placebo (RR = 1.15, 95% CI 0.95 to 1.40; n = 3 trials, 363 participants; Heterogeneity: Tau² = 0.00; Q = 1.05, df = 2, P = 0.59; I2 = 0%; moderate‐certainty evidence; Analysis 2.5; Baltieri 2008; Correa Filho 2013; Likhitsathian 2013).
2.5. Analysis.

Comparison 2: Any pharmacologic intervention vs. placebo given in addition to psychosocial intervention among participants with alcohol use disorder, Outcome 5: Retention
Adverse events
Three included combined intervention trials with 324 participants reported on adverse events across study conditions. The studies reported participant self‐reported side effects as a proportion of each side effect experienced across study conditions (Likhitsathian 2013), the number of each side effect experienced across study conditions (Ahmadi 2004), and the proportion of participants in each study condition who reported experiencing any side effects (Correa Filho 2013).
Any pharmacologic intervention versus another pharmacologic intervention among participants with alcohol use disorder
Harmful alcohol use
The two included studies that compared active pharmacologic interventions did not report on our primary alcohol outcome, harmful alcohol use.
Alcohol consumption
De Sousa and colleagues reported on time to first alcohol intake and relapse. Unlike other studies reported on alcohol consumption, they did not compare the quantity, frequency, or other patterns of alcohol consumption. This study found that relapse occurred significantly sooner among participants randomized to acamprosate as compared to disulfiram (de Sousa 2005).
Remission
Two studies with 171 participants comparing active pharmacologic interventions reported on alcohol consumption. These studies found evidence of a reduced likelihood of remission among participants who were allocated to receive acamprosate as compared to another active pharmacologic treatment (baclofen, disulfiram, or naltrexone; RR = 0.58, 95% CI 0.42 to 0.79; n = 2 trials, 171 participants; Heterogeneity: Tau² = 0.01; Q = 1.18, df = 1, P = 0.28; I2 = 15%; Analysis 3.1; de Sousa 2005; Kumar 2020).
3.1. Analysis.

Comparison 3: Any pharmacologic intervention vs. another pharmacologic intervention among participants with alcohol use disorder, Outcome 1: Remission
Retention
Two pharmacologic comparative effectiveness studies with 190 participants included in this review did not identify significant differences in retention between participants randomized to receive acamprosate as compared to another active pharmacologic treatment (baclofen, disulfiram, naltrexone; low‐certainty evidence; Analysis 3.2; Table 3).
3.2. Analysis.

Comparison 3: Any pharmacologic intervention vs. another pharmacologic intervention among participants with alcohol use disorder, Outcome 2: Retention
Any pharmacologic intervention versus another pharmacologic intervention in addition to psychosocial intervention among participants with alcohol use disorder
Harmful alcohol use
Neither of the two comparative effectiveness studies of active pharmacologic treatments delivered alongside a psychosocial intervention reported on the primary alcohol outcome, harmful alcohol use.
Alcohol consumption
Neither of the studies comparing two pharmacologic interventions both delivered alongside a psychosocial intervention reported on alcohol consumption.
Remission
The two studies with 200 participants that reported comparative effectiveness studies of pharmacologic interventions delivered alongside a psychosocial intervention compared disulfiram to either topiramate or naltrexone. Both studies reported a significantly greater probability of remission, which was defined as abstinence, among participants randomized to receive disulfiram (RR = 1.78, 95% CI: 1.40 to 2.25; n = 2 trials, 200 participants; Heterogeneity: Tau² = 0.01, Q = 1.31, df = 1, P = 0.25, I2 = 23%; Analysis 4.1; Table 4).
4.1. Analysis.

Comparison 4: Any pharmacologic intervention vs. another pharmacologic intervention in addition to psychosocial intervention among participants with alcohol use disorder, Outcome 1: Remission
Retention
Neither of the included studies identified a difference in rates of retention between disulfiram and either topiramate or naltrexone (RR = 1.02, 95% CI: 0.96 to 1.08; n =2 trials, 200 participants; Heterogeneity: Tau² = 0.00, Q=0.11, df = 1, P = 0.74, I2 = 0%; low‐certainty evidence; Analysis 4.2).
4.2. Analysis.

Comparison 4: Any pharmacologic intervention vs. another pharmacologic intervention in addition to psychosocial intervention among participants with alcohol use disorder, Outcome 2: Retention
Pharmacologic intervention among participants with hazardous or harmful alcohol use
No studies have been retrieved for this comparison. All studies evaluating a pharmacologic intervention enrolled only individuals who met criteria for alcohol use disorder or dependence.
Psychosocial intervention versus brief advice or information, assessment only/wait‐list or usual care among participants with hazardous or harmful alcohol use
Brief psychosocial intervention versus brief advice, information, or assessment only among participants with hazardous or harmful alcohol use
The certainty of evidence was very low for all outcomes evaluated in brief intervention trials due to high risk of bias, clinical heterogeneity specifically with respect to the study populations, and statistical heterogeneity (Table 5).
Harmful alcohol use
Twenty‐one included brief intervention trials with 5262 participants reported on harmful alcohol use. Seventeen of these trials reported harmful alcohol use on a continuous scale (Assanangkornchai 2015; Cherpitel 2009; Dutra Ponce 2021; Huis In't Veld 2019; Kamal 2020; Noknoy 2010; Pal 2007; Peltzer 2013; Pengpid 2013a; Pengpid 2013b; Pengpid 2015; Poblete 2017; Segatto 2011; Sheikh 2017; Simao 2008; Wandera 2017; Xiaolu 2017), while four trials reported harmful alcohol use as a dichotomous outcome (Allen 2011; Baldin 2018; L'Engle 2014; Rendall‐Mkosi 2013). We identified substantial heterogeneity that precluded estimating a pooled effect of brief interventions (Heterogeneity among studies reporting continuous outcomes: Tau²=0.15, Q=139.64, df=16, P <.001, I² = 89%, n=17 trials; very low‐certainty evidence; Analysis 5.1; Heterogeneity among studies reporting dichotomous outcomes: Tau² = 0.18, Q = 58.26, df = 3, P <.001, I² =95%, n =4 trials,; very low‐certainty evidence; Analysis 5.2). Most of the brief intervention trials included in this meta‐analysis used the Alcohol Use Disorder Identification Test (AUDIT), Alcohol, Smoking and Substance Involvement Screening Test (ASSIST), or the Rutgers Alcohol Problem Index (RAPI) to assess harmful alcohol use between 3‐12 months post‐randomization.
5.1. Analysis.

Comparison 5: Brief psychosocial intervention vs. brief advice, information, or assessment only among participants with hazardous or harmful alcohol use, Outcome 1: Harmful alcohol use (continuous)
5.2. Analysis.

Comparison 5: Brief psychosocial intervention vs. brief advice, information, or assessment only among participants with hazardous or harmful alcohol use, Outcome 2: Harmful alcohol use (dichotomous)
Alcohol consumption
Nine included brief intervention trials with 1779 participants reported on alcohol consumption. Alcohol consumption was operationalized differently across studies: frequency of alcohol use (Simao 2008), number of drinking days (Cherpitel 2009; Pal 2007; Segatto 2011), drinks per drinking day (Noknoy 2010), alcohol drinking volume/units (Dutra Ponce 2021; Pengpid 2015; Thapinta 2017), patterns of alcohol consumption (Segatto 2011), or using another measurement tool (Polshkova 2016). Alcohol consumption was measured using the Rapid Alcohol Problems Screen (RAPS4), Alcohol, Smoking and Substance Involvement Screening Test (ASSIST), Addiction Severity Index (ASI), the Alcohol Use Disorder Identification Test (AUDIT), Rutgers Alcohol Problem Index (RAPI), Alcohol Consumption Questionnaire (ACQ), Timeline Followback (TLFB), or self‐reported consumption. We identified substantial heterogeneity that precluded estimating a reliable pooled effect of brief interventions on alcohol consumption (Heterogeneity: Tau² = 0.48; Q = 171.34, df = 8, P <.001, I² = 95%; n =9 trials; Analysis 5.3).
5.3. Analysis.

Comparison 5: Brief psychosocial intervention vs. brief advice, information, or assessment only among participants with hazardous or harmful alcohol use, Outcome 3: Alcohol consumption
Remission
Five included brief intervention trials with 2240 participants reported on remission from harmful alcohol use (Allen 2011; Huis In't Veld 2019; L'Engle 2014; Pengpid 2015; Signor 2013). All trials operationalized remission as abstinence and/or low‐risk drinking. We did not estimate a pooled effect of brief interventions on remission due to high levels of heterogeneity (Heterogeneity: Tau² = 0.11; Q = 51.45, df = 4, P <.001; I² = 92%; n=5 trials; Analysis 5.4).
5.4. Analysis.

Comparison 5: Brief psychosocial intervention vs. brief advice, information, or assessment only among participants with hazardous or harmful alcohol use, Outcome 4: Remission
Retention
Twelve brief psychosocial intervention trials with 5380 participants reported on retention (Assanangkornchai 2015; Constant 2021; Dutra Ponce 2021; Harder 2019; Huis In't Veld 2019; Kamal 2020; L'Engle 2014; Pengpid 2013b; Pengpid 2015; Poblete 2017; Thapinta 2017; Wandera 2017). Most of these studies did not identify a significant difference in completion of a brief intervention; however we did not estimate a pooled estimate due to high heterogeneity (Heterogeneity: Tau² = 0.00; Q = 172.59, df = 11, P <.001; I2 = 94%; n = 12 trials; very low‐certainty evidence; Analysis 5.5) . The control condition was typically a single session of brief advice or information.
5.5. Analysis.

Comparison 5: Brief psychosocial intervention vs. brief advice, information, or assessment only among participants with hazardous or harmful alcohol use, Outcome 5: Retention
Other psychosocial intervention versus usual care or wait list among participants with hazardous or harmful alcohol use
Harmful alcohol use
Twelve included psychosocial treatment trials with 2106 participants reported on harmful alcohol use. All of these trials reported harmful alcohol use on a continuous scale (Bolton 2014; Jordans 2019; Kane 2022; Madhombiro 2019; Madhombiro 2020; Moraes 2010; Murray 2020; Nadkarni 2017; Nadkarni 2019; Omeje 2018; Schaub 2021; Witte 2011). We did not estimate a pooled effect of psychosocial treatment interventions due to high statistical and clinical heterogeneity (Heterogeneity: Tau² = 1.15; Q = 444.32, df = 11, P <.001; I² = 98%; n = 12 trials; very low‐certainty evidence; Analysis 6.1; Table 6). Most of these trials used the Alcohol Use Disorder Identification Test (AUDIT) to assess harmful alcohol use between three and six months post‐randomization.
6.1. Analysis.

Comparison 6: Other psychosocial intervention vs. usual care or wait list among participants with hazardous or harmful alcohol use, Outcome 1: Harmful alcohol use
Alcohol consumption
Seven included psychosocial treatment trials with 1609 participants reported on alcohol consumption. Alcohol consumption was reported using variable metrics including grams of alcohol consumed (Nattala 2010), number of drinks in the past week (Schaub 2021), percent/number of drinking days (Hartmann 2020; Ng 2020; Papas 2011; Papas 2020), or using a standardized measure assessing alcohol consumption (e.g., Severity of Alcohol Dependence Questionnaire, SADQ; Satyanarayana 2016). We did not estimate a pooled effect of psychosocial treatment interventions due to high statistical and clinical heterogeneity (Heterogeneity: Tau² = 0.07; Q = 25.68, df = 6, P <.001; I² = 77%; n = 7 trials; Analysis 6.2). The certainty of this evidence was reduced due to the clinical heterogeneity between studies including the variable study populations, interventions, and implementation approaches.
6.2. Analysis.

Comparison 6: Other psychosocial intervention vs. usual care or wait list among participants with hazardous or harmful alcohol use, Outcome 2: Alcohol consumption
Remission
Seven included psychosocial treatment trials with 1519 participants reported on remission from harmful alcohol use. Remission was operationalized as reduction to low‐risk drinking levels (Jordans 2019; Nadkarni 2017; Nadkarni 2019; Schaub 2021) or abstinence (Jirapramukpitak 2020; Moraes 2010; Ng 2020). We identified a 44% increase in the probability of remission attributable to psychosocial treatment interventions (RR = 1.44, 95% CI: 1.19 to 1.76; Heterogeneity: Tau² = 0.03; Chi² = 110.25, df = 6, P = 0.11; I² = 41%; n =7 trials; Analysis 6.3). There was minimal statistical and limited clinical heterogeneity as three of the four studies evaluated the same intervention, Counseling for Alcohol Problems (Jordans 2019; Nadkarni 2017; Nadkarni 2019).
6.3. Analysis.

Comparison 6: Other psychosocial intervention vs. usual care or wait list among participants with hazardous or harmful alcohol use, Outcome 3: Remission
Retention
Nine other psychosocial treatment trials with 1664 participants reported on retention in the intervention (Hartmann 2020; Jirapramukpitak 2020; Jordans 2019; Kane 2022; Moraes 2010; Ng 2020; Omeje 2018; Papas 2011; Samet 2015). We did not estimate a pooled estimate due to high heterogeneity (Heterogeneity: Tau² = 0.01; Q = 34.07, df = 8, P <.001; I2 = 77%; n = 9 trials; very low‐certainty evidence; Analysis 6.4). Individual studies varied between displaying a greater likelihood of retention in home visits and psychotherapy relative to psychotherapy alone (Moraes 2010), but lower likelihood of retention in motivational interviewing and/or cognitive behavioral therapy relative to usual care (Jordans 2019; Papas 2011). The remaining studies showed no difference in the likelihood of retention when comparing a contingency management intervention, rational emotive therapy, combined cognitive behavioral therapy and motivational interviewing, relapse prevention, or behavioral intervention to monitoring or an inactive control condition (Hartmann 2020; Jirapramukpitak 2020; Kane 2022; Ng 2020; Omeje 2018; Samet 2015).
6.4. Analysis.

Comparison 6: Other psychosocial intervention vs. usual care or wait list among participants with hazardous or harmful alcohol use, Outcome 4: Retention
Discussion
Summary of main results
We identified 66 randomized controlled trials (RCTs) evaluating indicated prevention and treatment interventions to reduce harmful alcohol use in low‐ and middle‐income countries (LMICs). However, the heterogeneity across these included studies in their intervention and comparison conditions, study outcomes, and other features precluded pooling of their quantitative estimates within this review.
Most of these studies were conducted in middle‐income countries (MICs), with very few conducted in low‐income countries (LICs). The majority of these studies evaluated brief psychosocial interventions providing elements of motivational interviewing for individuals with hazardous, harmful, or high‐risk alcohol use. Among the other psychosocial interventions, we identified many different types of interventions with the most common type being treatments for alcohol use disorder that combine motivational enhancement, cognitive‐behavioral therapy, and relapse prevention approaches. Combined and pharmacologic intervention trials were few and primarily conducted in Brazil or India. Adult males made up the majority of participants in these trials. Other target populations included persons living with HIV/AIDS, tuberculosis, or co‐occurring common mental disorder (i.e. depression or post‐traumatic stress disorder (PTSD)). TheAlcohol Use Disorder Identification Test (AUDIT), Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) and Timeline Followback were the most commonly used outcome measures; however, most studies did not report that these had been validated in their target population. Many studies did not use standardized assessment tools to measure alcohol‐related outcomes. Furthermore, the outcomes in these studies differed from common intervention outcomes in trials conducted in high‐income settings. For example, in this review, several studies considered abstinence or low‐risk and non‐problematic drinking as positive treatment outcomes, which we defined as 'remission'. This is in contrast to much of the alcohol intervention research conducted in high‐income settings, for which abstinence goals have been the convention and often neglect moderation or low‐risk drinking as a successful treatment outcome (Mann 2017). This contrast may indicate differences in acceptable and relevant treatment goals among studies conducted in LMICs relative to high‐income countries (HICs).
We are uncertain whether reductions in harmful alcohol use are attributable to brief psychosocial and other psychosocial interventions when compared primarily with brief advice/information or usual care, respectively, in LMICs approximately six months post‐study enrolment as the certainty of evidence is very low and heterogeneity is substantial. Combined (psychosocial and pharmacologic) intervention may be effective in reducing harmful alcohol use. Despite the inclusion of a large number of trials, we were unable to estimate pooled effect sizes for brief or other psychosocial interventions due to the substantial clinical, methodological, and statistical heterogeneity among the included studies. The certainty of evidence for psychosocial interventions was further reduced due to the risk of performance and detection bias.
Alcohol consumption and remission, the secondary alcohol‐related outcomes, displayed mixed findings across types of interventions with high heterogeneity across studies. Overall, we did not observe an effect of pharmacologic interventions on alcohol consumption. Across all intervention types, the certainty of findings related to alcohol consumption was very low primarily due to high heterogeneity and risk of bias. Other psychosocial interventions and combined pharmacologic and psychosocial interventions increased the probability of remission in the studies that were included in the meta‐analysis.
Overall completeness and applicability of evidence
We identified a wide range of interventions including pharmacologic, psychosocial, and combined pharmacologic and psychosocial interventions. In many of these studies, investigators encouraged participants in both study conditions to attend 12‐step or other mutual help groups, but we did not identify any trials specifically evaluating the efficacy of 12‐step facilitation in LMICs, which has been shown to be a cost‐effective strategy for maintaining reductions in harmful alcohol use (Kelly 2020), a particularly important consideration in LMICs. The pharmacologic trials included the three medications indicated for the treatment of alcohol use disorder: acamprosate, disulfiram and naltrexone (see Table 7). The psychosocial interventions represented a wide range of therapeutic approaches including many strategies recommended and considered evidence‐based by the World Health Organization including brief motivational interventions, cognitive behavioral therapy (CBT), and behavioral therapy. Notably, the certainty of evidence for these interventions is low. The World Health Organization recommends Social Network therapy and Contingency Management as evidence‐based interventions to reduce harmful alcohol use(WHO 2015); however, we only identified two trials evaluating a contingency management intervention and few interventions that integrated family or other members of one's social network.
Most included studies reported on primary or secondary alcohol‐related outcomes. The pharmacologic and combined intervention studies reported measures of alcohol consumption and remission while the psychosocial intervention studies were more likely to report either harmful alcohol use or alcohol consumption. Very little data were available on adverse events and side effects of pharmacologic and combined interventions.
The high clinical and statistical heterogeneity among studies limits the inferences of this review. The psychosocial intervention studies, in particular, displayed substantial heterogeneity in their study populations by often applying broad eligibility criteria ranging from risky alcohol use through alcohol use disorder. In the next update of this review we will explore in greater depth the components of the interventions, characteristics of the intervention implementation strategy, details of the study population, and methodological features that explain this heterogeneity in order to make stronger conclusions about the efficacy of pharmacologic, psychosocial, and combined interventions in LMICs under different conditions.
Quality of the evidence
Although RCTs are the gold standard for internal validity of intervention evaluations, we identified substantial risk of bias among the included studies, particularly with respect to risk of information bias attributed to lack of blinding of personnel (i.e. performance bias) and outcome assessors (i.e. detection bias). Limitations in study reporting and the absence of many trial registrations and/or protocols resulted in unclear risk of bias across many domains. These challenges resulted in high levels of uncertainty and poor overall quality of the evidence described in this review, a finding that was consistent between our risk of bias assessment and grading of trials.
Risk of selection bias was lowest across the included domains primarily due to the restriction of this review to RCTs. Despite this study design eligibility criterion, many trials did not report their randomization and/or allocation procedures leading to unclear risk of bias. Among studies that reported methods for random sequence generation and allocation concealment, almost all employed high‐quality designs that would protect against emmigrative selection bias. Emigrative selection bias may have been introduced through attrition, which was moderate to high in several included studies. Most trials reported an intention‐to‐treat analysis and made conservative assumptions about participants who dropped out of the trial. However, 27 included studies did not provide enough information about loss to follow‐up or how missing data were handled, making it difficult to evaluate the risk of attrition bias in these trials. We were unable to determine risk of reporting bias in the majority of included trials due to the lack of a published protocol, trial registration, or transparent reporting of a priori hypotheses and outcomes. Transparency and consistency in reporting on RCTs, including study registration protocols, standard reporting of participant flow using CONSORT diagrams, and detailed reporting of statistical analysis, including how missing data were handled, is needed to clarify the quality of RCTs on alcohol interventions in LMICs.
The largest weakness in the quality of evidence of included studies related to lack of blinding. Blinding of participants and personnel only occurred in pharmacologic and combined interventions where placebos that were identical to the active medication or sham could be administered. In psychosocial interventions it is not possible to maintain participants' and therapists' blinding to study condition allocation. Many pharmacologic, psychosocial and combined interventions were able to blind outcome assessors to study condition allocation. Despite the challenges in blinding participants and personnel, trial investigators should aim to blind outcome assessors to mitigate some of the risk of information bias in these studies and incorporate outcome measures other than self‐report to reduce the risk of bias introduced by unblinded participants.
In addition to the risk of bias in included trials, we also identified high level of heterogeneity among studies as well as among outcomes. These findings suggest inconsistent effects of interventions on alcohol‐related outcomes both between‐ and within‐trials. There were notable inconsistencies between the interventions themselves, including among brief interventions. Although most brief interventions were based on motivational interviewing, the number of sessions ranged from one to seven sessions and other aspects of the implementation of the brief intervention varied. Some of this variability was explained by the type of measures used, many of which were not validated or standardized, and almost all of which relied on self‐report exclusively. Measurement of harmful alcohol use across settings is challenged by different types of alcohol consumed, different patterns of alcohol use, and different consequences of alcohol use. Most alcohol measures rely on the use of standard drink equivalents to quantify the volume of alcohol consumed; however, in many contexts it is difficult to operationalize the alcohol content by volume and to account for the different size and contents of alcohol beverages consumed. Future research validating standard drink equivalents and assessments of the quantity of alcohol consumed across contexts is urgently needed to understand how cross‐culturally valid these measures are. Interestingly, we found differences in the effect of intervention by the alcohol‐related outcomes. For example, we found significant effects of brief interventions on harmful alcohol use, but no effect on alcohol consumption. This may be partially attributable to the different studies included in these meta‐analyses, but it does highlight the importance of consistency in outcome definitions as well as measurement tools, and the need for objective alcohol measurements in clinical trials.
In general, the results of this review provide the largest synthesis of experimental research on interventions to reduce harmful alcohol use in LMICs and identified benefits of interventions on reducing harmful alcohol use. However, in order to make reliable and credible recommendations further exploration of the sources of heterogeneity in the observed effects and future RCTs that address the identified weaknesses of the existing literature are needed.
The observed clinical and statistical heterogeneity and limitations in study designs reduce the certainty of our findings and point toward opportunities for improving the evidence on the efficacy of alcohol interventions in LMIC settings. Improving the certainty of evidence on alcohol interventions is needed, particularly as the prevalence of alcohol use and alcohol use disorder increases in LMICs (WHO 2018a; Walls 2020) and harmful alcohol use is increasingly becoming a critical public health and development priority (Collin 2016). This can be done by harmonizing outcome definitions and assessment tools, validating measures of harmful alcohol use in diverse settings, reducing the risk of selection and information bias, and evaluating interventions that are comparable with respect to their components, length, and comparison condition.
Potential biases in the review process
Although we attempted to mitigate potential sources of meta‐bias in the review process, the results from must be interpreted in light of several potential limitations. We attempted to conduct a comprehensive search of the literature, including unpublished literature, and without date or language restrictions; however, potential publication bias may have precluded our ability to include eligible trials in this review. Examination of funnel plots for our primary outcome, harmful alcohol use, revealed the potential for publication bias among psychosocial interventions (Figure 4). We did not find evidence for publication bias among brief intervention studies (Figure 5), or combined intervention trials (Figure 6).
4.

Funnel plot of comparison: 8 Other psychosocial intervention vs. usual care or wait list among participants with hazardous or harmful alcohol use, outcome: 8.1 Harmful alcohol use.
5.

Funnel plot of comparison: 7 Brief psychosocial intervention vs. brief advice, information, or assessment only among participants with hazardous or harmful alcohol use, outcome: 7.1 Harmful alcohol use (continuous).
6.

Funnel plot of comparison: 2 Any pharmacologic intervention vs. placebo given in addition to psychosocial intervention among participants with alcohol use disorder, outcome: 2.1 Harmful alcohol use (continuous).
Other sources of information bias may have been introduced during the review and data extraction process. It is possible that the accuracy of the risk of bias assessment and data extraction was inaccurate or incomplete. To mitigate this risk we had two independent review authors screen for potentially relevant studies at each phase of the screening process (title abstract, full text) with any discrepancies reviewed by at least two review authors. Furthermore, the quality assessment and data extraction process was completed by two independent review authors with a similar consensus process to improve the reliability and quality of the data.
Agreements and disagreements with other studies or reviews
Findings from this review are consistent with previous Cochrane systematic reviews examining the efficacy or effectiveness of brief interventions in primary care populations (Kaner 2018), brief interventions for heavy drinkers admitted to the hospital (McQueen 2011), brief interventions delivered to people with alcohol use disorders by non‐specialists in LMICs (van Ginneken 2013), and acamprosate for persons with alcohol dependence (Rosner 2010b). The systematic reviews of brief interventions similarly found mixed findings regarding the efficacy of these interventions that differed by outcome, low‐ to moderate‐quality evidence, high heterogeneity between included studies, yet similar effect sizes. With the exception of the review conducted by van Ginneken and colleagues (van Ginneken 2013), the majority of systematic reviews have primarily focused on high‐income countries (HICs).
Reviews of alcohol interventions focused on LMICs are also consistent with our study findings. The Cochrane Review (van Ginneken 2013) identified two randomized controlled trials of brief interventions that were delivered by non‐specialist health workers to people with alcohol use disorder in LMICs. Similar to our review, the study investigators identified a small, but statistically significant reduction in alcohol consumption attributable to the brief intervention; however, the quality of the evidence was low (van Ginneken 2013). Both of the studies included in their review were also included in the current systematic review (Noknoy 2010; Papas 2011). Other reviews have identified a dearth of research on alcohol interventions in specific populations in LMICs (Giusto 2018; Greene 2019a; Perngparn 2008). Another review conducted in 2007 identified 11 alcohol treatment and prevention intervention studies in LMICs. All harmful alcohol use indicated prevention and treatment randomized trials reported in this review were also included in the present review. Similarly, the investigators acknowledged the under‐representation of studies on interventions for harmful alcohol use relative to other mental, neurological and substance use disorders in LMICs (Patel 2007). Since this review was registered, four systematic reviews have been published on the efficacy or effectiveness of either brief interventions or other psychosocial interventions in low‐ and/or middle‐income countries (Ghosh 2022; Joseph 2017; Preusse 2020; Sileo 2021). These reviews display substantial overlap with the current review in terms of included articles; however, some were specifically focused on studies conducted in sub‐Saharan Africa (Sileo 2021) or middle‐income countries (Joseph 2017). Furthermore, one of the reviews also included non‐randomized studies (Sileo 2021). Reviews that included a meta‐analysis similarly identified high levels of heterogeneity. Each of these reviews concluded that brief and other psychosocial interventions show promise for reducing hazardous and/or harmful alcohol use, but they acknowledged the high levels of uncertainty and small effect sizes.
Authors' conclusions
Implications for practice.
We identified very low‐ to moderate‐certainty evidence supporting the efficacy of psychosocial and combined (pharmacologic and psychosocial) interventions for reducing harmful alcohol use in low‐ and middle‐income countries (LMICs) when compared with brief advice/information, usual care, or psychosocial intervention and placebo, respectively. Studies differed in terms of the target population, measurement of primary outcomes, content and delivery of the intervention, control condition, and observed results. The number of completed trials included in this review suggests that implementing pharmacologic and psychosocial interventions to reduce harmful alcohol use is feasible and has the potential to reduce the burden of harmful alcohol use in LMICs.
Implications for research.
The quality of evidence resulting from 66 trials included in this review was variable and highlights the need for improving the methods used to evaluate these intervention strategies in several specific areas. First, improved measurement of alcohol‐related outcomes is needed. This relates to both the validation and utilization of standardized measurement tools evaluating alcohol consumption, harmful alcohol use, and remission. The Alcohol Use Disorder Identification Test (AUDIT) and Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) have been validated in a variety of settings as screening tools for hazardous alcohol use; however, their utility in intervention evaluations (e.g. their sensitivity to change over time) has been less frequently examined and the AUDIT was found to produce smaller intervention effect sizes relative to other measures of harmful alcohol use in subgroup analyses. Studies reporting alcohol consumption should similarly utilize standard measures intended to aid in recall of drinking behaviors (e.g. Timeline Followback). Second, future studies should evaluate interventions that are comparable to previous trials in terms of content and delivery. The psychosocial treatment interventions included in this review, in particular, displayed substantial heterogeneity in the content and delivery of the intervention. Future efforts to evaluate comparable psychosocial interventions are needed. Third, we found evidence of heterogeneity in the effects of brief intervention being explained by the type of comparison condition with some evidence suggesting limited benefit of psychosocial interventions relative to simple active controls (e.g. providing information or brief advice). Future research should employ attention‐matched controls and conduct mediation analyses to examine whether the purported active ingredients of psychosocial interventions are explaining observed alcohol‐related outcomes. Fourth, we observed significant variation in the alcohol eligibility criteria used to enroll participants in intervention trials and how certain terms describing patterns of alcohol use and clinical definitions related to disorder, particularly in psychosocial interventions. Furthermore, many of these studies included a broad range of alcohol‐related conditions from risky drinking to alcohol dependence. Future intervention trials should specify the population and alcohol‐related condition they aim to target and align these eligibility criteria with recommended guidelines for these interventions. Fifth, efforts to reduce risk of information bias and emigrative selection bias are needed to improve the quality and certainty of the available evidence. Blinding of outcome assessors is critical to maximizing the internal validity of these intervention studies, particularly when reliant on self‐reported alcohol outcomes. Most studies reported using an intention‐to‐treat analysis to protect against attrition and emigrative selection bias, but other methods, such as multiple imputation, should be applied to further reduce the risk of attrition bias resulting from non‐random, differential missingness.
History
Protocol first published: Issue 6, 2019
Notes
None reported
Acknowledgements
We would like to acknowledge Rashelle J Musci (Assistant Professor, Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, US), Pamela J Surkan (Associate Professor, Department of International Health, Johns Hopkins Bloomberg School of Public Health), and Andrea Wirtz (Assistant Scientist, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health) for their review of the original protocol. We would also like to acknowledge the contribution of the peer reviewers: Jan Klimas and Tara Carney.
Appendices
Appendix 1. Cochrane CENTRAL search strategy
#1 MeSH descriptor: [Drinking Behavior] explode all trees
#2 MeSH descriptor: [Alcohol Drinking] explode all trees
#3 MeSH descriptor: [Binge Drinking] explode all trees
#4 MeSH descriptor: [Alcohol‐Related Disorders] explode all trees
#5 MeSH descriptor: [Alcoholism] explode all trees
#6 ("hazardous alcohol use" or "alcohol misuse" or "alcohol use" or alcohol or "alcohol consumption" or "heavy drinking" or "binge drinking" or "alcohol use disorder" or "alcohol abuse" or "alcohol dependence" or alcoholism or alcoholic or drinking):ti,ab
#7 (#1 or #2 or #3 or #4 or #5 or #6)
#8 (intervention or program or therapy or treatment or prevention or rehabilitation or "brief intervention" or counseling or detoxification or "medical management" or withdrawal or support or "mutual help" or "self‐help" or "twelve step" or 12‐step or disulfiram or antabuse or naltrexone or revia or acamprosate or campral):ti,ab
#9 (Africa or Asia or Caribbean or "West Indies" or "South America" or "Latin America" or "Central America"):ti,ab
#10 (Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or "Burkina Faso" or "Burkina Fasso" or "Upper Volta" or Burundi or Urundi or Cambodia or "Khmer Republic" or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or "Cape Verde" or "Central African Republic" or Chad or Chile or China or Colombia or Comoros or "Comoro Islands" or Comores or Mayotte or Congo or Zaire or "Costa Rica" or "Cote d'Ivoire" or "Ivory Coast" or Croatia or Cuba or Cyprus or Czechoslovakia or "Czech Republic" or Slovakia or "Slovak Republic"):ti,ab
#11 (Djibouti or "French Somaliland" or Dominica or "Dominican Republic" or "East Timor" or "East Timur" or "Timor Leste" or Ecuador or Egypt or "United Arab Republic" or "El Salvador" or Eritrea or Estonia or Ethiopia or Fiji or Gabon or "Gabonese Republic" or Gambia or Gaza or Georgia or Georgian or Ghana or "Gold Coast" or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or "Isle of Man" or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or "Kyrgyz Republic" or Kirghiz or Kirgizstan or "Lao PDR" or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania):ti,ab
#12 (Macedonia or Madagascar or "Malagasy Republic" or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or "Marshall Islands" or Mauritania or Mauritius or "Agalega Islands" or Mexico or Micronesia or "Middle East" or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or "Netherlands Antilles" or "New Caledonia" or Nicaragua or Niger or Nigeria or "Northern Mariana Islands" or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or "Puerto Rico"):ti,ab
#13 (Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or "Saint Kitts" or "St Kitts" or Nevis or "Saint Lucia" or "St Lucia" or "Saint Vincent" or "St Vincent" or Grenadines or Samoa or "Samoan Islands" or "Navigator Island" or "Navigator Islands" or "Sao Tome" or "Saudi Arabia" or Senegal or Serbia or Montenegro or Seychelles or "Sierra Leone" or Slovenia or "Sri Lanka" or Ceylon or "Solomon Islands" or Somalia or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or "Togolese Republic" or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or "Soviet Union" or "Union of Soviet Socialist Republics" or Uzbekistan or Uzbek or Vanuatu or "New Hebrides" or Venezuela or Vietnam or "Viet Nam" or "West Bank" or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia):ti,ab
#14 (developing or less* next developed or "under developed" or underdeveloped or "middle income" or low* next income or underserved or "under served" or deprived or poor*) next (countr* or nation* or population* or world):ti,ab
#15 ((developing or less* next developed or "under developed" or underdeveloped or "middle income" or low* next income) next (economy or economies)):ti,ab
#16 (low* next (gdp or gnp or "gross domestic" or "gross national")):ti,ab
#17 (low near/3 middle near/3 countr*):ti,ab
#18 (lmic or lmics or "third world" or "lami country" or "lami countries"):ti,ab
#19 ("transitional country" or "transitional countries"):ti,ab
#20 (#9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19)
#21 (#7 AND #8 AND #20)
Appendix 2. Cochrane Drugs and Alcohol Group (CDAG) Specialized Register
#1 (Africa or Asia or Caribbean or "West Indies" or "South America" or "Latin America" or "Central America"):ti,ab,kw,ky,emt,mh,mc AND INREGISTER #2 (Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or "Burkina Faso" or "Burkina Fasso" or "Upper Volta" or Burundi or Urundi or Cambodia or "Khmer Republic" or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or "Cape Verde" or "Central African Republic" or Chad or Chile or China or Colombia or Comoros or "Comoro Islands" or Comores or Mayotte or Congo or Zaire or "Costa Rica" or "Cote d'Ivoire" or "Ivory Coast" or Croatia or Cuba or Cyprus or Czechoslovakia or "Czech Republic" or Slovakia or "Slovak Republic"):ti,ab,kw,ky,emt,mh,mc AND INREGISTER #3 (Djibouti or "French Somaliland" or Dominica or "Dominican Republic" or "East Timor" or "East Timur" or "Timor Leste" or Ecuador or Egypt or "United Arab Republic" or "El Salvador" or Eritrea or Estonia or Ethiopia or Fiji or Gabon or "Gabonese Republic" or Gambia or Gaza or Georgia or Georgian or Ghana or "Gold Coast" or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or "Isle of Man" or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or "Kyrgyz Republic" or Kirghiz or Kirgizstan or "Lao PDR" or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania):ti,ab,kw,ky,emt,mh,mc AND INREGISTER #4 (Macedonia or Madagascar or "Malagasy Republic" or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or "Marshall Islands" or Mauritania or Mauritius or "Agalega Islands" or Mexico or Micronesia or "Middle East" or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or "Netherlands Antilles" or "New Caledonia" or Nicaragua or Niger or Nigeria or "Northern Mariana Islands" or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or "Puerto Rico"):ti,ab,kw,ky,emt,mh,mc AND INREGISTER #5 (Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or "Saint Kitts" or "St Kitts" or Nevis or "Saint Lucia" or "St Lucia" or "Saint Vincent" or "St Vincent" or Grenadines or Samoa or "Samoan Islands" or "Navigator Island" or "Navigator Islands" or "Sao Tome" or "Saudi Arabia" or Senegal or Serbia or Montenegro or Seychelles or "Sierra Leone" or Slovenia or "Sri Lanka" or Ceylon or "Solomon Islands" or Somalia or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or "Togolese Republic" or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or "Soviet Union" or "Union of Soviet Socialist Republics" or Uzbekistan or Uzbek or Vanuatu or "New Hebrides" or Venezuela or Vietnam or "Viet Nam" or "West Bank" or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia):ti,ab,kw,ky,emt,mh,mc AND INREGISTER #6 ((developing or "less* developed" or "under developed" or underdeveloped or "middle income" or "low* income" or underserved or "under served" or deprived or poor*) NEAR (countr* or nation* or population* or world)):ti,ab,kw,ky,emt,mh,mc AND INREGISTER #7 (low NEAR3 middle NEAR3 countr*):ti,ab,kw,ky,emt,mh,mc AND INREGISTER #8 (low* NEXT (GDP or GNP or "grossdomestic" or "gross national")):ti,ab,kw,ky,emt,mh,mc AND INREGISTER #9 (LMIC or LMICs or "third world" or "LAMI country" or "LAMI countries"):ti,ab,kw,ky,emt,mh,mc AND INREGISTER #10 ("transitionalcountry" or "transitional countries"):ti,ab,kw,ky,emt,mh,mc AND INREGISTER #11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #12 MESH DESCRIPTOR Alcohol‐Related Disorders EXPLODE ALL AND INREGISTER #13 MESH DESCRIPTOR Alcohol Drinking EXPLODE ALL AND INREGISTER #14 (alcohol NEAR3 (drink* OR intoxicat* OR abus*)):ti,ab,kw,ky AND INREGISTER #15 (alcohol NEAR3 (misus* OR risk* OR consum*)):ti,ab,kw,ky AND INREGISTER #16 (alcohol NEAR3 (withdraw* OR detox*)):ti,ab,kw,ky AND INREGISTER #17 (alcohol NEAR3 (treat* OR therap* OR intervention)):ti,ab,kw,ky AND INREGISTER #18 (alcohol NEAR3 (excess* or reduc* or cessation)):ti,ab,kw,ky AND INREGISTER #19 (drink* NEAR3 excess):ti,ab,kw,ky AND INREGISTER #20 (drink* NEAR3 (heavy OR heavily)):ti,ab,kw,ky AND INREGISTER #21 (drink* NEAR3 (binge or harmful or problem*)):ti,ab,kw,ky AND INREGISTER #22 (alcohol*):XDI AND INREGISTER #23 (alcohol*):XIN AND INREGISTER #24 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 #25 #24 AND #11
Appendix 3. PubMed search strategy
Developing Countries[Mesh:noexp] OR Africa[Mesh:noexp] OR Africa, Northern[Mesh:noexp] OR Africa South of the Sahara[Mesh:noexp] OR Africa, Central[Mesh:noexp] OR Africa, Eastern[Mesh:noexp] OR Africa, Southern[Mesh:noexp] OR Africa, Western[Mesh:noexp] OR Asia[Mesh:noexp] OR Asia, Central[Mesh:noexp] OR Asia, Southeastern[Mesh:noexp] OR Asia, Western[Mesh:noexp] OR Caribbean Region[Mesh:noexp] OR West Indies[Mesh:noexp] OR South America[Mesh:noexp] OR Latin America[Mesh:noexp] OR Central America[Mesh:noexp] OR Afghanistan[Mesh:noexp] OR Albania[Mesh:noexp] OR Algeria[Mesh:noexp] OR American Samoa[Mesh:noexp] OR Angola[Mesh:noexp] OR "Antigua and Barbuda"[Mesh:noexp] OR Argentina[Mesh:noexp] OR Armenia[Mesh:noexp] OR Azerbaijan[Mesh:noexp] OR Bahrain[Mesh:noexp] OR Bangladesh[Mesh:noexp] OR Barbados[Mesh:noexp] OR Benin[Mesh:noexp] OR Byelarus[Mesh:noexp] OR Belize[Mesh:noexp] OR Bhutan[Mesh:noexp] OR Bolivia[Mesh:noexp] OR Bosnia‐Herzegovina[Mesh:noexp] OR Botswana[Mesh:noexp] OR Brazil[Mesh:noexp] OR Bulgaria[Mesh:noexp] OR Burkina Faso[Mesh:noexp] OR Burundi[Mesh:noexp] OR Cambodia[Mesh:noexp] OR Cameroon[Mesh:noexp] OR Cape Verde[Mesh:noexp] OR Central African Republic[Mesh:noexp] OR Chad[Mesh:noexp] OR Chile[Mesh:noexp] OR China[Mesh:noexp] OR Colombia[Mesh:noexp] OR Comoros[Mesh:noexp] OR Congo[Mesh:noexp] OR Costa Rica[Mesh:noexp] OR Cote d'Ivoire[Mesh:noexp] OR Croatia[Mesh:noexp] OR Cuba[Mesh:noexp] OR Cyprus[Mesh:noexp] OR Czechoslovakia[Mesh:noexp] OR Czech Republic[Mesh:noexp] OR Slovakia[Mesh:noexp] OR Djibouti[Mesh:noexp] OR "Democratic Republic of the Congo"[Mesh:noexp] OR Dominica[Mesh:noexp] OR Dominican Republic[Mesh:noexp] OR East Timor[Mesh:noexp] OR Ecuador[Mesh:noexp] OR Egypt[Mesh:noexp] OR El Salvador[Mesh:noexp] OR Eritrea[Mesh:noexp] OR Estonia[Mesh:noexp] OR Ethiopia[Mesh:noexp] OR Fiji[Mesh:noexp] OR Gabon[Mesh:noexp] OR Gambia[Mesh:noexp] OR "Georgia (Republic)"[Mesh:noexp] OR Ghana[Mesh:noexp] OR Greece[Mesh:noexp] OR Grenada[Mesh:noexp] OR Guatemala[Mesh:noexp] OR Guinea[Mesh:noexp] OR Guinea‐Bissau[Mesh:noexp] OR Guam[Mesh:noexp] OR Guyana[Mesh:noexp] OR Haiti[Mesh:noexp] OR Honduras[Mesh:noexp] OR Hungary[Mesh:noexp] OR India[Mesh:noexp] OR Indonesia[Mesh:noexp] OR Iran[Mesh:noexp] OR Iraq[Mesh:noexp] OR Jamaica[Mesh:noexp] OR Jordan[Mesh:noexp] OR Kazakhstan[Mesh:noexp] OR Kenya[Mesh:noexp] OR Korea[Mesh:noexp] OR Kosovo[Mesh:noexp] OR Kyrgyzstan[Mesh:noexp] OR Laos[Mesh:noexp] OR Latvia[Mesh:noexp] OR Lebanon[Mesh:noexp] OR Lesotho[Mesh:noexp] OR Liberia[Mesh:noexp] OR Libya[Mesh:noexp] OR Lithuania[Mesh:noexp] OR Macedonia[Mesh:noexp] OR Madagascar[Mesh:noexp] OR Malaysia[Mesh:noexp] OR Malawi[Mesh:noexp] OR Mali[Mesh:noexp] OR Malta[Mesh:noexp] OR Mauritania[Mesh:noexp] OR Mauritius[Mesh:noexp] OR Mexico[Mesh:noexp] OR Micronesia[Mesh:noexp] OR Middle East[Mesh:noexp] OR Moldova[Mesh:noexp] OR Mongolia[Mesh:noexp] OR Montenegro[Mesh:noexp] OR Morocco[Mesh:noexp] OR Mozambique[Mesh:noexp] OR Myanmar[Mesh:noexp] OR Namibia[Mesh:noexp] OR Nepal[Mesh:noexp] OR Netherlands Antilles[Mesh:noexp] OR New Caledonia[Mesh:noexp] OR Nicaragua[Mesh:noexp] OR Niger[Mesh:noexp] OR Nigeria[Mesh:noexp] OR Oman[Mesh:noexp] OR Pakistan[Mesh:noexp] OR Palau[Mesh:noexp] OR Panama[Mesh:noexp] OR Papua New Guinea[Mesh:noexp] OR Paraguay[Mesh:noexp] OR Peru[Mesh:noexp] OR Philippines[Mesh:noexp] OR Poland[Mesh:noexp] OR Portugal[Mesh:noexp] OR Puerto Rico[Mesh:noexp] OR Romania[Mesh:noexp] OR Russia[Mesh:noexp] OR "Russia (Pre‐1917)"[Mesh:noexp] OR Rwanda[Mesh:noexp] OR "Saint Kitts and Nevis"[Mesh:noexp] OR Saint Lucia[Mesh:noexp] OR "Saint Vincent and the Grenadines"[Mesh:noexp] OR Samoa[Mesh:noexp] OR Saudi Arabia[Mesh:noexp] OR Senegal[Mesh:noexp] OR Serbia[Mesh:noexp] OR Montenegro[Mesh:noexp] OR Seychelles[Mesh:noexp] OR Sierra Leone[Mesh:noexp] OR Slovenia[Mesh:noexp] OR Sri Lanka[Mesh:noexp] OR Somalia[Mesh:noexp] OR South Africa[Mesh:noexp] OR Sudan[Mesh:noexp] OR Suriname[Mesh:noexp] OR Swaziland[Mesh:noexp] OR Syria[Mesh:noexp] OR Tajikistan[Mesh:noexp] OR Tanzania[Mesh:noexp] OR Thailand[Mesh:noexp] OR Togo[Mesh:noexp] OR Tonga[Mesh:noexp] OR "Trinidad and Tobago"[Mesh:noexp] OR Tunisia[Mesh:noexp] OR Turkey[Mesh:noexp] OR Turkmenistan[Mesh:noexp] OR Uganda[Mesh:noexp] OR Ukraine[Mesh:noexp] OR Uruguay[Mesh:noexp] OR USSR[Mesh:noexp] OR Uzbekistan[Mesh:noexp] OR Vanuatu[Mesh:noexp] OR Venezuela[Mesh:noexp] OR Vietnam[Mesh:noexp] OR Yemen[Mesh:noexp] OR Yugoslavia[Mesh:noexp] OR Zambia[Mesh:noexp] OR Zimbabwe[Mesh:noexp]
Macedonia[tw] OR Madagascar[tw] OR Malagasy Republic[tw] OR Malaysia[tw] OR Malaya[tw] OR Malay[tw] OR Sabah[tw] OR Sarawak[tw] OR Malawi[tw] OR Nyasaland[tw] OR Mali[tw] OR Malta[tw] OR Marshall Islands[tw] OR Mauritania[tw] OR Mauritius[tw] OR Agalega Islands[tw] OR Mexico[tw] OR Micronesia[tw] OR Middle East[tw] OR Moldova[tw] OR Moldovia[tw] OR Moldovian[tw] OR Mongolia[tw] OR Montenegro[tw] OR Morocco[tw] OR Ifni[tw] OR Mozambique[tw] OR Myanmar[tw] OR Myanma[tw] OR Burma[tw] OR Namibia[tw] OR Nepal[tw] OR Netherlands Antilles[tw] OR New Caledonia[tw] OR Nicaragua[tw] OR Niger[tw] OR Nigeria[tw] OR Northern Mariana Islands[tw] OR Oman[tw] OR Muscat[tw] OR Pakistan[tw] OR Palau[tw] OR Palestine[tw] OR Panama[tw] OR Paraguay[tw] OR Peru[tw] OR Philippines[tw] OR Philipines[tw] OR Phillipines[tw] OR Phillippines[tw] OR Poland[tw] OR Portugal[tw] OR Puerto Rico[tw] OR Romania[tw] OR Rumania[tw] OR Roumania[tw] OR Russia[tw] OR Russian[tw] OR Rwanda[tw] OR Ruanda[tw] OR Saint Kitts[tw] OR St Kitts[tw] OR Nevis[tw] OR Saint Lucia[tw] OR St Lucia[tw] OR Saint Vincent[tw] OR St Vincent[tw] OR Grenadines[tw] OR Samoa[tw] OR Samoan Islands[tw] OR Navigator Island[tw] OR Navigator Islands[tw] OR Sao Tome[tw] OR Saudi Arabia[tw] OR Senegal[tw] OR Serbia[tw] OR Montenegro[tw] OR Seychelles[tw] OR Sierra Leone[tw] OR Slovenia[tw] OR Sri Lanka[tw] OR Ceylon[tw] OR Solomon Islands[tw] OR Somalia[tw] OR Sudan[tw] OR Suriname[tw] OR Surinam[tw] OR Swaziland[tw] OR Syria[tw] OR Tajikistan[tw] OR Tadzhikistan[tw] OR Tadjikistan[tw] OR Tadzhik[tw] OR Tanzania[tw] OR Thailand[tw] OR Togo[tw] OR Togolese Republic[tw] OR Tonga[tw] OR Trinidad[tw] OR Tobago[tw] OR Tunisia[tw] OR Turkey[tw] OR Turkmenistan[tw] OR Turkmen[tw] OR Uganda[tw] OR Ukraine[tw] OR Uruguay[tw] OR USSR[tw] OR Soviet Union[tw] OR Union of Soviet Socialist Republics[tw] OR Uzbekistan[tw] OR Uzbek OR Vanuatu[tw] OR New Hebrides[tw] OR Venezuela[tw] OR Vietnam[tw] OR Viet Nam[tw] OR West Bank[tw] OR Yemen[tw] OR Yugoslavia[tw] OR Zambia[tw] OR Zimbabwe[tw] OR Rhodesia[tw]
Africa[tw] OR Asia[tw] OR Caribbean[tw] OR West Indies[tw] OR South America[tw] OR Latin America[tw] OR Central America[tw] OR Afghanistan[tw] OR Albania[tw] OR Algeria[tw] OR Angola[tw] OR Antigua[tw] OR Barbuda[tw] OR Argentina[tw] OR Armenia[tw] OR Armenian[tw] OR Aruba[tw] OR Azerbaijan[tw] OR Bahrain[tw] OR Bangladesh[tw] OR Barbados[tw] OR Benin[tw] OR Byelarus[tw] OR Byelorussian[tw] OR Belarus[tw] OR Belorussian[tw] OR Belorussia[tw] OR Belize[tw] OR Bhutan[tw] OR Bolivia[tw] OR Bosnia[tw] OR Herzegovina[tw] OR Hercegovina[tw] OR Botswana[tw] OR Brasil[tw] OR Brazil[tw] OR Bulgaria[tw] OR Burkina Faso[tw] OR Burkina Fasso[tw] OR Upper Volta[tw] OR Burundi[tw] OR Urundi[tw] OR Cambodia[tw] OR Khmer Republic[tw] OR Kampuchea[tw] OR Cameroon[tw] OR Cameroons[tw] OR Cameron[tw] OR Camerons[tw] OR Cape Verde[tw] OR Central African Republic[tw] OR Chad[tw] OR Chile[tw] OR China[tw] OR Colombia[tw] OR Comoros[tw] OR Comoro Islands[tw] OR Comores[tw] OR Mayotte[tw] OR Congo[tw] OR Zaire[tw] OR Costa Rica[tw] OR Cote d'Ivoire[tw] OR Ivory Coast[tw] OR Croatia[tw] OR Cuba[tw] OR Cyprus[tw] OR Czechoslovakia[tw] OR Czech Republic[tw] OR Slovakia[tw] OR Slovak Republic[tw] OR Djibouti[tw] OR French Somaliland[tw] OR Dominica[tw] OR Dominican Republic[tw] OR East Timor[tw] OR East Timur[tw] OR Timor Leste[tw] OR Ecuador[tw] OR Egypt[tw] OR United Arab Republic[tw] OR El Salvador[tw] OR Eritrea[tw] OR Estonia[tw] OR Ethiopia[tw] OR Fiji[tw] OR Gabon[tw] OR Gabonese Republic[tw] OR Gambia[tw] OR Gaza[tw] OR Georgia Republic[tw] OR Georgian Republic[tw] OR Ghana[tw] OR Gold Coast[tw] OR Greece[tw] OR Grenada[tw] OR Guatemala[tw] OR Guinea[tw] OR Guam[tw] OR Guiana[tw] OR Guyana[tw] OR Haiti[tw] OR Honduras[tw] OR Hungary[tw] OR India[tw] OR Maldives[tw] OR Indonesia[tw] OR Iran[tw] OR Iraq[tw] OR Isle of Man[tw] OR Jamaica[tw] OR Jordan[tw] OR Kazakhstan[tw] OR Kazakh[tw] OR Kenya[tw] OR Kiribati[tw] OR Korea[tw] OR Kosovo[tw] OR Kyrgyzstan[tw] OR Kirghizia[tw] OR Kyrgyz Republic[tw] OR Kirghiz[tw] OR Kirgizstan[tw] OR "Lao PDR"[tw] OR Laos[tw] OR Latvia[tw] OR Lebanon[tw] OR Lesotho[tw] OR Basutoland[tw] OR Liberia[tw] OR Libya[tw] OR Lithuania[tw]
"developing country"[tw] OR "developing countries"[tw] OR "developing nation"[tw] OR "developing nations"[tw] OR "developing population"[tw] OR "developing populations"[tw] OR "developing world"[tw] OR "less developed country"[tw] OR "less developed countries"[tw] OR "less developed nation"[tw] OR "less developed nations"[tw] OR "less developed population"[tw] OR "less developed populations"[tw] OR "less developed world"[tw] OR "lesser developed country"[tw] OR "lesser developed countries"[tw] OR "lesser developed nation"[tw] OR "lesser developed nations"[tw] OR "lesser developed population"[tw] OR "lesser developed populations"[tw] OR "lesser developed world"[tw] OR "under developed country"[tw] OR "under developed countries"[tw] OR "under developed nation"[tw] OR "under developed nations"[tw] OR "under developed population"[tw] OR "under developed populations"[tw] OR "under developed world"[tw] OR "underdeveloped country"[tw] OR "underdeveloped countries"[tw] OR "underdeveloped nation"[tw] OR "underdeveloped nations"[tw] OR "underdeveloped population"[tw] OR "underdeveloped populations"[tw] OR "underdeveloped world"[tw] OR "middle income country"[tw] OR "middle income countries"[tw] OR "middle income nation"[tw] OR "middle income nations"[tw] OR "middle income population"[tw] OR "middle income populations"[tw] OR "low income country"[tw] OR "low income countries"[tw] OR "low income nation"[tw] OR "low income nations"[tw] OR "low income population"[tw] OR "low income populations"[tw] OR "lower income country"[tw] OR "lower income countries"[tw] OR "lower income nation"[tw] OR "lower income nations"[tw] OR "lower income population"[tw] OR "lower income populations"[tw] OR "underserved country"[tw] OR "underserved countries"[tw] OR "underserved nation"[tw] OR "underserved nations"[tw] OR "underserved population"[tw] OR "underserved populations"[tw] OR "underserved world"[tw] OR "under served country"[tw] OR "under served countries"[tw] OR "under served nation"[tw] OR "under served nations"[tw] OR "under served population"[tw] OR "under served populations"[tw] OR "under served world"[tw] OR "deprived country"[tw] OR "deprived countries"[tw] OR "deprived nation"[tw] OR "deprived nations"[tw] OR "deprived population"[tw] OR "deprived populations"[tw] OR "deprived world"[tw] OR "poor country"[tw] OR "poor countries"[tw] OR "poor nation"[tw] OR "poor nations"[tw] OR "poor population"[tw] OR "poor populations"[tw] OR "poor world"[tw] OR "poorer country"[tw] OR "poorer countries"[tw] OR "poorer nation"[tw] OR "poorer nations"[tw] OR "poorer population"[tw] OR "poorer populations"[tw] OR "poorer world"[tw] OR "developing economy"[tw] OR "developing economies"[tw] OR "less developed economy"[tw] OR "less developed economies"[tw] OR "lesser developed economy"[tw] OR "lesser developed economies"[tw] OR "under developed economy"[tw] OR "under developed economies"[tw] OR "underdeveloped economy"[tw] OR "underdeveloped economies"[tw] OR "middle income economy"[tw] OR "middle income economies"[tw] OR "low income economy"[tw] OR "low income economies"[tw] OR "lower income economy"[tw] OR "lower income economies"[tw] OR "low gdp"[tw] OR "low gnp"[tw] OR "low gross domestic"[tw] OR "low gross national"[tw] OR "lower gdp"[tw] OR "lower gnp"[tw] OR "lower gross domestic"[tw] OR "lower gross national"[tw] OR lmic[tw] OR lmics[tw] OR "third world"[tw] OR "lami country"[tw] OR "lami countries"[tw] OR "transitional country"[tw] OR "transitional countries"[tw]
#1 OR #2 OR #3 OR #4
alcohol‐related disorders[MeSH]
Alcohol Drinking[MeSH]
((alcohol*[Title/Abstract]) AND (drink*[Title/Abstract] OR intoxicat*[Title/Abstract] OR use*[Title/Abstract] OR abus*[Title/Abstract] OR misus*[Title/Abstract] OR risk*[Title/Abstract] OR consum*[Title/Abstract] OR withdraw*[Title/Abstract] OR detox*[Title/Abstract] OR treat*[Title/Abstract] OR therap*[Title/Abstract] OR excess*[Title/Abstract] OR reduc*[Title/Abstract] OR cessation[Title/Abstract] OR intervention*[Title/Abstract]))))
alcoholic*[tiab]
#6 OR #7 OR #8 OR #9
randomized controlled trial [pt]
controlled clinical trial [pt]
random* [tiab]
placebo [tiab]
drug therapy [MeSH]
trial [tiab]
groups [tiab]
#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17
animals [mh] NOT human [mh]
#18 NOT #19
#5 AND #10 AND #20
Appendix 4. Embase search strategy
(‘hazardous alcohol use’:ab,ti OR ‘alcohol misuse’:ab,ti OR ‘alcohol use’:ab,ti OR alcohol:ab,ti OR ‘alcohol consumption’:ab,ti OR ‘heavy drinking’:ab,ti OR ‘binge drinking’:ab,ti OR ‘alcohol use disorder’:ab,ti OR ‘alcohol abuse’:ab,ti OR ‘alcohol dependence’:ab,ti OR alcoholism:ab,ti OR alcoholic:ab,ti OR drinking:ab,ti OR ‘Drinking behavior’/exp OR ‘Alcohol Abstinence’/exp OR ‘Alcohol Drinking’ OR ‘Binge Drinking’/exp OR ‘Alcohol Abuse’/exp OR ‘Underage Drinking’/exp OR ‘Alcohol Intoxication’/exp OR Alcoholism/exp) AND (Random* or Factorial* or Crossover* or (cross NEXT/1 over*) or Placebo* or (Doubl* NEXT/1 blind*) or (singl* NEXT/1 blind*) or Assign* or Allocate* or Volunteer* or 'Crossover Procedure'/exp or 'double blind procedure'/exp or 'randomized controlled trial'/exp or 'single blind procedure'/exp) AND (intervention:ab,ti OR program:ab,ti OR therapy:ab,ti OR treatment:ab,ti OR prevention:ab,ti OR rehabilitation:ab,ti OR ‘brief intervention’:ab,ti OR counseling:ab,ti OR detoxification:ab,ti OR “medical management”:ab,ti OR withdrawal:ab,ti OR support:ab,ti OR ‘mutual help’:ab,ti OR ‘self‐help’:ab,ti OR ‘twelve step’:ab,ti OR ‘12‐step’:ab,ti OR disulfiram:ab,ti OR antabuse:ab,ti OR naltrexone:ab,ti OR revia:ab,ti OR acamprosate:ab,ti OR campral:ab,ti OR disulfiram/exp OR naltrexone/exp OR acamprosate/exp) AND ('developing country':ti,ab OR 'developing countries':ti,ab OR 'developing nation':ti,ab OR 'developing nations':ti,ab OR 'developing population':ti,ab OR 'developing populations':ti,ab OR 'developing world':ti,ab OR 'less developed country':ti,ab OR 'less developed countries':ti,ab OR 'less developed nation':ti,ab OR 'less developed nations':ti,ab OR 'less developed population':ti,ab OR 'less developed populations':ti,ab OR 'less developed world':ti,ab OR 'lesser developed country':ti,ab OR 'lesser developed countries':ti,ab OR 'lesser developed nation':ti,ab OR 'lesser developed nations':ti,ab OR 'lesser developed population':ti,ab OR 'lesser developed populations':ti,ab OR 'lesser developed world':ti,ab OR 'under developed country':ti,ab OR 'under developed countries':ti,ab OR 'under developed nation':ti,ab OR 'under developed nations':ti,ab OR 'under developed population':ti,ab OR 'under developed populations':ti,ab OR 'under developed world':ti,ab OR 'underdeveloped country':ti,ab OR 'underdeveloped countries':ti,ab OR 'underdeveloped nation':ti,ab OR 'underdeveloped nations':ti,ab OR 'underdeveloped population':ti,ab OR 'underdeveloped populations':ti,ab OR 'underdeveloped world':ti,ab OR 'middle income country':ti,ab OR 'middle income countries':ti,ab OR 'middle income nation':ti,ab OR 'middle income nations':ti,ab OR 'middle income population':ti,ab OR 'middle income populations':ti,ab OR 'low income country':ti,ab OR 'low income countries':ti,ab OR 'low income nation':ti,ab OR 'low income nations':ti,ab OR 'low income population':ti,ab OR 'low income populations':ti,ab OR 'lower income country':ti,ab OR 'lower income countries':ti,ab OR 'lower income nation':ti,ab OR 'lower income nations':ti,ab OR 'lower income population':ti,ab OR 'lower income populations':ti,ab OR 'underserved country':ti,ab OR 'underserved countries':ti,ab OR 'underserved nation':ti,ab OR 'underserved nations':ti,ab OR 'underserved population':ti,ab OR 'underserved populations':ti,ab OR 'underserved world':ti,ab OR 'under served country':ti,ab OR 'under served countries':ti,ab OR 'under served nation':ti,ab OR 'under served nations':ti,ab OR 'under served population':ti,ab OR 'under served populations':ti,ab OR 'under served world':ti,ab OR 'deprived country':ti,ab OR 'deprived countries':ti,ab OR 'deprived nation':ti,ab OR 'deprived nations':ti,ab OR 'deprived population':ti,ab OR 'deprived populations':ti,ab OR 'deprived world':ti,ab OR 'poor country':ti,ab OR 'poor countries':ti,ab OR 'poor nation':ti,ab OR 'poor nations':ti,ab OR 'poor population':ti,ab OR 'poor populations':ti,ab OR 'poor world':ti,ab OR 'poorer country':ti,ab OR 'poorer countries':ti,ab OR 'poorer nation':ti,ab OR 'poorer nations':ti,ab OR 'poorer population':ti,ab OR 'poorer populations':ti,ab OR 'poorer world':ti,ab OR 'developing economy':ti,ab OR 'developing economies':ti,ab OR 'less developed economy':ti,ab OR 'less developed economies':ti,ab OR 'lesser developed economy':ti,ab OR 'lesser developed economies':ti,ab OR 'under developed economy':ti,ab OR 'under developed economies':ti,ab OR 'underdeveloped economy':ti,ab OR 'underdeveloped economies':ti,ab OR 'middle income economy':ti,ab OR 'middle income economies':ti,ab OR 'low income economy':ti,ab OR 'low income economies':ti,ab OR 'lower income economy':ti,ab OR 'lower income economies':ti,ab OR 'low gdp':ti,ab OR 'low gnp':ti,ab OR 'low gross domestic':ti,ab OR 'low gross national':ti,ab OR 'lower gdp':ti,ab OR 'lower gnp':ti,ab OR 'lower gross domestic':ti,ab OR 'lower gross national':ti,ab OR lmic:ti,ab OR lmics:ti,ab OR 'third world':ti,ab OR 'lami country':ti,ab OR 'lami countries':ti,ab OR 'transitional country':ti,ab OR 'transitional countries':ti,ab OR africa:ti,ab OR asia:ti,ab OR caribbean:ti,ab OR 'west indies':ti,ab OR 'south america':ti,ab OR 'latin america':ti,ab OR 'central america':ti,ab OR 'atlantic islands':ti,ab OR 'commonwealth of independent states':ti,ab OR 'pacific islands':ti,ab OR 'indian ocean islands':ti,ab OR 'eastern europe':ti,ab OR afghanistan:ti,ab OR albania:ti,ab OR algeria:ti,ab OR angola:ti,ab OR antigua:ti,ab OR barbuda:ti,ab OR argentina:ti,ab OR armenia:ti,ab OR armenian:ti,ab OR aruba:ti,ab OR azerbaijan:ti,ab OR bahrain:ti,ab OR bangladesh:ti,ab OR barbados:ti,ab OR benin:ti,ab OR byelarus:ti,ab OR byelorussian:ti,ab OR belarus:ti,ab OR belorussian:ti,ab OR belorussia:ti,ab OR belize:ti,ab OR bhutan:ti,ab OR bolivia:ti,ab OR bosnia:ti,ab OR herzegovina:ti,ab OR hercegovina:ti,ab OR botswana:ti,ab OR brasil:ti,ab OR brazil:ti,ab OR bulgaria:ti,ab OR 'burkina faso':ti,ab OR 'burkina fasso':ti,ab OR 'upper volta':ti,ab OR burundi:ti,ab OR urundi:ti,ab OR cambodia:ti,ab OR 'khmer republic':ti,ab OR kampuchea:ti,ab OR cameroon:ti,ab OR cameroons:ti,ab OR cameron:ti,ab OR camerons:ti,ab OR cameroun:ti,ab OR 'cape verde':ti,ab OR 'central african republic':ti,ab OR chad:ti,ab OR chile:ti,ab OR china:ti,ab OR colombia:ti,ab OR comoros:ti,ab OR 'comoro islands':ti,ab OR comores:ti,ab OR mayotte:ti,ab OR congo:ti,ab OR zaire:ti,ab OR 'costa rica':ti,ab OR 'cote divoire':ti,ab OR 'ivory coast':ti,ab OR croatia:ti,ab OR cuba:ti,ab OR cyprus:ti,ab OR czechoslovakia:ti,ab OR 'czech republic':ti,ab OR slovakia:ti,ab OR 'slovak republic':ti,ab OR djibouti:ti,ab OR 'french somaliland':ti,ab OR dominica:ti,ab OR 'dominican republic':ti,ab OR 'east timor':ti,ab OR 'east timur':ti,ab OR 'timor leste':ti,ab OR ecuador:ti,ab OR egypt:ti,ab OR 'united arab republic':ti,ab OR 'el salvador':ti,ab OR eritrea:ti,ab OR estonia:ti,ab OR ethiopia:ti,ab OR fiji:ti,ab OR gabon:ti,ab OR 'gabonese republic':ti,ab OR gambia:ti,ab OR gaza:ti,ab OR 'georgia republic':ti,ab OR 'georgian republic':ti,ab OR ghana:ti,ab OR 'gold coast':ti,ab OR greece:ti,ab OR grenada:ti,ab OR guatemala:ti,ab OR guinea:ti,ab OR guam:ti,ab OR guiana:ti,ab OR guyana:ti,ab OR haiti:ti,ab OR honduras:ti,ab OR hungary:ti,ab OR india:ti,ab OR maldives:ti,ab OR indonesia:ti,ab OR iran:ti,ab OR iraq:ti,ab OR 'isle of man':ti,ab OR jamaica:ti,ab OR jordan:ti,ab OR kazakhstan:ti,ab OR kazakh:ti,ab OR kenya:ti,ab OR kiribati:ti,ab OR korea:ti,ab OR kosovo:ti,ab OR kyrgyzstan:ti,ab OR kirghizia:ti,ab OR 'kyrgyz republic':ti,ab OR kirghiz:ti,ab OR kirgizstan:ti,ab OR 'lao pdr':ti,ab OR laos:ti,ab OR latvia:ti,ab OR lebanon:ti,ab OR lesotho:ti,ab OR basutoland:ti,ab OR liberia:ti,ab OR libya:ti,ab OR lithuania:ti,ab OR macedonia:ti,ab OR madagascar:ti,ab OR 'malagasy republic':ti,ab OR malaysia:ti,ab OR malaya:ti,ab OR malay:ti,ab OR sabah:ti,ab OR sarawak:ti,ab OR malawi:ti,ab OR nyasaland:ti,ab OR mali:ti,ab OR malta:ti,ab OR 'marshall islands':ti,ab OR mauritania:ti,ab OR mauritius:ti,ab OR 'agalega islands':ti,ab OR 'melanesia':ti,ab OR mexico:ti,ab OR micronesia:ti,ab OR 'middle east':ti,ab OR moldova:ti,ab OR moldovia:ti,ab OR moldovian:ti,ab OR mongolia:ti,ab OR morocco:ti,ab OR mozambique:ti,ab OR myanmar:ti,ab OR myanma:ti,ab OR burma:ti,ab OR namibia:ti,ab OR nepal:ti,ab OR 'netherlands antilles':ti,ab OR 'new caledonia':ti,ab OR nicaragua:ti,ab OR niger:ti,ab OR nigeria:ti,ab OR 'northern mariana islands':ti,ab OR oman:ti,ab OR muscat:ti,ab OR pakistan:ti,ab OR palau:ti,ab OR palestine:ti,ab OR panama:ti,ab OR paraguay:ti,ab OR peru:ti,ab OR philippines:ti,ab OR philipines:ti,ab OR phillipines:ti,ab OR phillippines:ti,ab OR poland:ti,ab OR portugal:ti,ab OR 'puerto rico':ti,ab OR romania:ti,ab OR rumania:ti,ab OR roumania:ti,ab OR russia:ti,ab OR russian:ti,ab OR rwanda:ti,ab OR ruanda:ti,ab OR 'saint kitts':ti,ab OR 'st kitts':ti,ab OR nevis:ti,ab OR 'saint lucia':ti,ab OR 'st lucia':ti,ab OR 'saint vincent':ti,ab OR 'st vincent':ti,ab OR grenadines:ti,ab OR samoa:ti,ab OR 'samoan islands':ti,ab OR 'navigator island':ti,ab OR 'navigator islands':ti,ab OR 'sao tome':ti,ab OR 'saudi arabia':ti,ab OR senegal:ti,ab OR serbia:ti,ab OR montenegro:ti,ab OR seychelles:ti,ab OR 'sierra leone':ti,ab OR slovenia:ti,ab OR 'sri lanka':ti,ab OR ceylon:ti,ab OR 'solomon islands':ti,ab OR somalia:ti,ab OR sudan:ti,ab OR suriname:ti,ab OR surinam:ti,ab OR swaziland:ti,ab OR syria:ti,ab OR syrian:ti,ab OR tajikistan:ti,ab OR tadzhikistan:ti,ab OR tadjikistan:ti,ab OR tadzhik:ti,ab OR tanzania:ti,ab OR thailand:ti,ab OR togo:ti,ab OR 'togolese republic':ti,ab OR tonga:ti,ab OR trinidad:ti,ab OR tobago:ti,ab OR tunisia:ti,ab OR turkey:ti,ab OR turkmenistan:ti,ab OR turkmen:ti,ab OR tuvalu:ti,ab OR uganda:ti,ab OR ukraine:ti,ab OR uruguay:ti,ab OR ussr:ti,ab OR 'soviet union':ti,ab OR 'union of soviet socialist republics':ti,ab OR uzbekistan:ti,ab OR uzbek OR vanuatu:ti,ab OR 'new hebrides':ti,ab OR venezuela:ti,ab OR vietnam:ti,ab OR 'viet nam':ti,ab OR 'west bank':ti,ab OR yemen:ti,ab OR yugoslavia:ti,ab OR zambia:ti,ab OR zimbabwe:ti,ab OR rhodesia:ti,ab OR 'developing country'/exp OR 'africa'/de OR 'africa south of the sahara'/de OR 'central africa'/de OR 'asia'/de OR 'southeast asia'/de OR 'caribbean'/de OR 'caribbean islands'/de OR 'south america'/de OR 'south and central america'/de OR 'atlantic islands'/de OR 'ussr'/de OR 'pacific islands'/de OR 'indian ocean'/de OR 'eastern europe'/de OR 'afghanistan'/exp OR 'albania'/exp OR 'algeria'/exp OR 'american samoa'/exp OR 'angola'/exp OR 'antigua and barbuda'/exp OR 'argentina'/exp OR 'armenia'/exp OR 'azerbaijan'/exp OR 'bahrain'/exp OR 'baltic states'/exp OR 'bangladesh'/exp OR 'barbados'/exp OR 'benin'/exp OR 'belarus'/exp OR 'belize'/exp OR 'bhutan'/exp OR 'bolivia'/exp OR 'bosnia and herzegovina'/exp OR 'botswana'/exp OR 'brazil'/exp OR 'bulgaria'/exp OR 'burkina faso'/exp OR 'burundi'/exp OR 'cambodia'/exp OR 'cameroon'/exp OR 'cape verde' OR 'central african republic'/exp OR 'chad'/exp OR 'chile'/exp OR 'china'/exp OR 'colombia'/exp OR 'comoros'/exp OR 'congo'/exp OR 'costa rica'/exp OR 'cote divoire' OR 'croatia'/exp OR 'cuba'/exp OR 'cyprus'/exp OR 'czechoslovakia'/exp OR 'czech republic'/exp OR 'slovakia'/exp OR 'djibouti'/exp OR 'democratic republic congo'/exp OR 'north korea'/exp OR 'dominica'/exp OR 'dominican republic'/exp OR 'dominican (dominican republic)'/exp OR 'timor leste'/exp OR 'ecuador'/exp OR 'egypt'/exp OR 'el salvador'/exp OR 'eritrea'/exp OR 'estonia'/exp OR 'ethiopia'/exp OR 'equatorial guinea'/exp OR 'fiji'/exp OR 'french guiana'/exp OR 'gabon'/exp OR 'gambia'/exp OR 'georgia (republic)'/exp OR 'ghana'/exp OR 'greece'/exp OR 'grenada'/exp OR 'guatemala'/exp OR 'guinea'/exp OR 'guinea bissau'/exp OR 'guam'/exp OR 'guyana'/exp OR 'haiti'/exp OR 'honduras'/exp OR 'hungary'/exp OR 'samoa'/exp OR 'india'/exp OR 'indonesia'/exp OR 'iran'/exp OR 'iraq'/exp OR 'jamaica'/exp OR 'jordan'/exp OR 'kazakhstan'/exp OR 'kenya'/exp OR 'south korea'/exp OR 'kyrgyzstan'/exp OR 'laos'/exp OR 'latvia'/exp OR 'lebanon'/exp OR 'lesotho'/exp OR 'liberia'/exp OR 'libyan arab jamahiriya'/exp OR 'lithuania'/exp OR 'macedonia (republic)'/exp OR 'madagascar'/exp OR 'malawi'/exp OR 'malaysia'/exp OR 'mali'/exp OR 'malta'/exp OR 'mauritania'/exp OR 'mauritius'/exp OR 'melanesia'/exp OR 'mexico'/exp OR 'federated states of micronesia'/exp OR 'middle east'/de OR 'moldova'/exp OR 'mongolia'/exp OR 'morocco'/exp OR 'mozambique'/exp OR 'myanmar'/exp OR 'namibia'/exp OR 'nepal'/exp OR 'netherlands antilles'/exp OR 'new caledonia'/exp OR 'nicaragua'/exp OR 'niger'/exp OR 'nigeria'/exp OR 'oman'/exp OR 'pakistan'/exp OR 'palau'/exp OR 'panama'/exp OR 'papua new guinea'/exp OR 'paraguay'/exp OR 'peru'/exp OR 'philippines'/exp OR 'poland'/exp OR 'portugal'/exp OR 'puerto rico'/exp OR 'romania'/exp OR 'russian federation'/exp OR 'rwanda'/exp OR 'saint kitts and nevis'/exp OR 'saint lucia'/exp OR 'saint vincent and the grenadines'/exp OR 'saudi arabia'/exp OR 'senegal'/exp OR 'serbia'/exp OR 'montenegro (republic)'/exp OR 'seychelles'/exp OR 'sierra leone'/exp OR 'slovenia'/exp OR 'sri lanka'/exp OR 'somalia'/exp OR 'south africa'/exp OR 'sudan'/exp OR 'suriname'/exp OR 'swaziland'/exp OR 'syrian arab republic'/exp OR 'tajikistan'/exp OR 'tanzania'/exp OR 'thailand'/exp OR 'togo'/exp OR 'tonga'/exp OR 'trinidad and tobago'/exp OR 'tunisia'/exp OR 'turkey (republic)'/exp OR 'turkmenistan'/exp OR 'uganda'/exp OR 'ukraine'/exp OR 'uruguay'/exp OR 'uzbekistan'/exp OR 'vanuatu'/exp OR 'venezuela'/exp OR 'viet nam'/exp OR 'yemen'/exp OR 'yugoslavia'/exp OR 'yugoslavia (pre‐1992)' OR 'zambia'/exp OR 'zimbabwe'/exp)
Appendix 5. PsycINFO search strategy
((TW “hazardous alcohol use” OR TW “alcohol misuse” OR TW “alcohol use” OR TW alcohol OR TW “alcohol consumption” OR TW “heavy drinking” OR TW “binge drinking” OR TW “alcohol use disorder” OR TW “alcohol abuse” OR TW “alcohol dependence” OR TW alcoholism OR TW alcoholic OR TW drinking OR DE “Drinking Behavior” OR DE Sobriety OR DE “Alcohol Drinking Patterns” OR DE “Binge Drinking” OR DE “Underage Drinking” OR DE “Alcohol Intoxication” OR DE Alcoholism) AND (SU.EXACT("Treatment Effectiveness Evaluation") OR SU.EXACT.EXPLODE("Treatment Outcomes") OR SU.EXACT("Placebo") OR SU.EXACT("Followup Studies") OR placebo* OR random* OR "comparative stud*" OR clinical NEAR/3 trial* OR research NEAR/3 design OR evaluat* NEAR/3 stud* OR prospectiv* NEAR/3 stud* OR (singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)) AND (TW "developing country" OR TW "developing countries" OR TW "developing nation" OR TW "developing nations" OR TW "developing population" OR TW "developing populations" OR TW "developing world" OR TW "less developed country" OR TW "less developed countries" OR TW "less developed nation" OR TW "less developed nations" OR TW "less developed population" OR TW "less developed populations" OR TW "less developed world" OR TW "lesser developed country" OR TW "lesser developed countries" OR TW "lesser developed nation" OR TW "lesser developed nations" OR TW "lesser developed population" OR TW "lesser developed populations" OR TW "lesser developed world" OR TW "under developed country" OR TW "under developed countries" OR TW "under developed nation" OR TW "under developed nations" OR TW "under developed population" OR TW "under developed populations" OR TW "under developed world" OR TW "underdeveloped country" OR TW "underdeveloped countries" OR TW "underdeveloped nation" OR TW "underdeveloped nations" OR TW "underdeveloped population" OR TW "underdeveloped populations" OR TW "underdeveloped world" OR TW "middle income country" OR TW "middle income countries" OR TW "middle income nation" OR TW "middle income nations" OR TW "middle income population" OR TW "middle income populations" OR TW "low income country" OR TW "low income countries" OR TW "low income nation" OR TW "low income nations" OR TW "low income population" OR TW "low income populations" OR TW "lower income country" OR TW "lower income countries" OR TW "lower income nation" OR TW "lower income nations" OR TW "lower income population" OR TW "lower income populations" OR TW "underserved country" OR TW "underserved countries" OR TW "underserved nation" OR TW "underserved nations" OR TW "underserved population" OR TW "underserved populations" OR TW "underserved world" OR TW "under served country" OR TW "under served countries" OR TW "under served nation" OR TW "under served nations" OR TW "under served population" OR TW "under served populations" OR TW "under served world" OR TW "deprived country" OR TW "deprived countries" OR TW "deprived nation" OR TW "deprived nations" OR TW "deprived population" OR TW "deprived populations" OR TW "deprived world" OR TW "poor country" OR TW "poor countries" OR TW "poor nation" OR TW "poor nations" OR TW "poor population" OR TW "poor populations" OR TW "poor world" OR TW "poorer country" OR TW "poorer countries" OR TW "poorer nation" OR TW "poorer nations" OR TW "poorer population" OR TW "poorer populations" OR TW "poorer world" OR TW "developing economy" OR TW "developing economies" OR TW "less developed economy" OR TW "less developed economies" OR TW "lesser developed economy" OR TW "lesser developed economies" OR TW "under developed economy" OR TW "under developed economies" OR TW "underdeveloped economy" OR TW "underdeveloped economies" OR TW "middle income economy" OR TW "middle income economies" OR TW "low income economy" OR TW "low income economies" OR TW "lower income economy" OR TW "lower income economies" OR TW TW "low gdp" OR TW "low gnp" OR TW "low gross domestic" OR TW "low gross national" OR TW "lower gdp" OR TW "lower gnp" OR TW "lower gross domestic" OR TW "lower gross national" OR TW lmic OR TW lmics OR TW "third world" OR TW "lami country" OR TW "lami countries" OR TW "transitional country" OR TW "transitional countries" OR TW Africa OR TW Asia OR TW Caribbean OR TW “West Indies” OR TW “South America” OR TW “Latin America” OR TW “Central America” OR TW "Atlantic Islands" OR TW "Commonwealth of Independent States" OR TW "Pacific Islands" OR TW "Indian Ocean Islands" OR TW "Eastern Europe" OR TW Afghanistan OR TW Albania OR TW Algeria OR TW Angola OR TW Antigua OR TW Barbuda OR TW Argentina OR TW Armenia OR TW Armenian OR TW Aruba OR TW Azerbaijan OR TW Bahrain OR TW Bangladesh OR TW Barbados OR TW Benin OR TW Byelarus OR TW Byelorussian OR TW Belarus OR TW Belorussian OR TW Belorussia OR TW Belize OR TW Bhutan OR TW Bolivia OR TW Bosnia OR TW Herzegovina OR TW Hercegovina OR TW Botswana OR TW Brasil OR TW Brazil OR TW Bulgaria OR TW “Burkina Faso” OR TW “Burkina Fasso” OR TW “Upper Volta” OR TW Burundi OR TW Urundi OR TW Cambodia OR TW “Khmer Republic” OR TW Kampuchea OR TW Cameroon OR TW Cameroons OR TW Cameron OR TW Camerons OR TW Cameroun OR TW “Cape Verde” OR TW “Central African Republic” OR TW Chad OR TW Chile OR TW China OR TW Colombia OR TW Comoros OR TW “Comoro Islands” OR TW Comores OR TW Mayotte OR TW Congo OR TW Zaire OR TW “Costa Rica” OR TW “Cote d'Ivoire” OR TW “Ivory Coast” OR TW Croatia OR TW Cuba OR TW Cyprus OR TW Czechoslovakia OR TW Czech Republic OR TW Slovakia OR TW “Slovak Republic” OR TW Djibouti OR TW “French Somaliland” OR TW Dominica OR TW “Dominican Republic” OR TW “East Timor” OR TW “East Timur” OR TW “Timor Leste” OR TW “Ecuador” OR TW Egypt OR TW “United Arab Republic” OR TW “El Salvador” OR TW Eritrea OR TW Estonia OR TW Ethiopia OR TW Fiji OR TW Gabon OR TW “Gabonese Republic” OR TW Gambia OR TW Gaza OR TW “Georgia Republic” OR TW “Georgian Republic” OR TW Ghana OR TW “Gold Coast” OR TW Greece OR TW Grenada OR TW Guatemala OR TW Guinea OR TW Guam OR TW Guiana OR TW Guyana OR TW Haiti OR TW Honduras OR TW Hungary OR TW India OR TW Maldives OR TW Indonesia OR TW Iran OR TW Iraq OR TW “Isle of Man” OR TW Jamaica OR TW Jordan OR TW Kazakhstan OR TW Kazakh OR TW Kenya OR TW Kiribati OR TW Korea OR TW Kosovo OR TW Kyrgyzstan OR TW Kirghizia OR TW “Kyrgyz Republic” OR TW Kirghiz OR TW Kirgizstan OR TW "Lao PDR" OR TW Laos OR TW Latvia OR TW Lebanon OR TW Lesotho OR TW Basutoland OR TW Liberia OR TW Libya OR TW Lithuania OR TW Macedonia OR TW Madagascar OR TW “Malagasy Republic” OR TW Malaysia OR TW Malaya OR TW Malay OR TW Sabah OR TW Sarawak OR TW Malawi OR TW Nyasaland OR TW Mali OR TW Malta OR TW “Marshall Islands” OR TW Mauritania OR TW Mauritius OR TW “Agalega Islands” OR TW Melanesia OR TW Mexico OR TW Micronesia OR TW “Middle East” OR TW Moldova OR TW Moldovia OR TW Moldovian OR TW Mongolia OR TW Montenegro OR TW Morocco OR TW Ifni OR TW Mozambique OR TW Myanmar OR TW Myanma OR TW Burma OR TW Namibia OR TW Nepal OR TW “Netherlands Antilles” OR TW “New Caledonia” OR TW Nicaragua OR TW Niger OR TW Nigeria OR TW “Northern Mariana Islands” OR TW Oman OR TW Muscat OR TW Pakistan OR TW Palau OR TW Palestine OR TW Panama OR TW Paraguay OR TW Peru OR TW Philippines OR TW Philipines OR TW Phillipines OR TW Phillippines OR TW Poland OR TW Portugal OR TW “Puerto Rico” OR TW Romania OR TW Rumania OR TW Roumania OR TW Russia OR TW Russian OR TW Rwanda OR TW Ruanda OR TW “Saint Kitts” OR TW “St Kitts” OR TW Nevis OR TW “Saint Lucia” OR TW “St Lucia” OR TW “Saint Vincent” OR TW “St Vincent” OR TW Grenadines OR TW Samoa OR TW “Samoan Islands” OR TW “Navigator Island” OR TW “Navigator Islands” OR TW “Sao Tome” OR TW “Saudi Arabia” OR TW Senegal OR TW Serbia OR TW Montenegro OR TW Seychelles OR TW “Sierra Leone” OR TW Slovenia OR TW “Sri Lanka” OR TW Ceylon OR TW “Solomon Islands” OR TW Somalia OR TW Sudan OR TW Suriname OR TW Surinam OR TW Swaziland OR TW Syria OR TW Syrian OR TW Tajikistan OR TW Tadzhikistan OR TW Tadjikistan OR TW Tadzhik OR TW Tanzania OR TW Thailand OR TW Togo OR TW “Togolese Republic” OR TW Tonga OR TW Trinidad OR TW Tobago OR TW Tunisia OR TW Turkey OR TW Turkmenistan OR TW Turkmen OR TW Tuvalu OR TW Uganda OR TW Ukraine OR TW Uruguay OR TW USSR OR TW “Soviet Union” OR TW “Union of Soviet Socialist Republics” OR TW Uzbekistan OR TW Uzbek OR TW Vanuatu OR TW “New Hebrides” OR TW Venezuela OR TW Vietnam OR TW “Viet Nam” OR TW “West Bank” OR TW Yemen OR TW Yugoslavia OR TW Zambia OR TW Zimbabwe OR TW Rhodesia OR DE Developing Countries))
Appendix 6. CINAHL search strategy
((TI (“hazardous alcohol use” OR “alcohol misuse” OR “alcohol use” OR alcohol OR “alcohol consumption” OR “heavy drinking” OR “binge drinking” OR “alcohol use disorder” OR “alcohol abuse” OR “alcohol dependence” OR alcoholism OR alcoholic OR drinking) OR AB (“hazardous alcohol use” OR “alcohol misuse” OR “alcohol use” OR alcohol OR “alcohol consumption” OR “heavy drinking” OR “binge drinking” OR “alcohol use disorder” OR “alcohol abuse” OR “alcohol dependence” OR alcoholism OR alcoholic OR drinking)) OR MH “Drinking behavior” OR MH “Alcohol Drinking” OR MH “Binge Drinking” OR MH “Alcohol‐Related Disorders” OR MH “Alcoholic Intoxication” OR MH Alcoholism) AND (TI (intervention OR program OR therapy OR treatment OR prevention OR rehabilitation OR “brief intervention” OR counseling OR detoxification OR “medical management” OR withdrawal OR support OR “mutual help” OR “self‐help” OR “twelve step” OR “12‐step” OR disulfiram OR antabuse OR naltrexone OR revia OR acamprosate OR campral) OR AB (intervention OR program OR therapy OR treatment OR prevention OR rehabilitation OR “brief intervention” OR counseling OR detoxification OR “medical management” OR withdrawal OR support OR “mutual help” OR “self‐help” OR “twelve step” OR “12‐step” OR disulfiram OR antabuse OR naltrexone OR revia OR acamprosate OR campral)) AND (TX "developing country" TX "developing countries" OR TX "developing nation" OR TX "developing nations" OR TX "developing population" OR TX "developing populations" OR TX "developing world" OR TX "less developed country" OR TX "less developed countries" OR TX "less developed nation" OR TX "less developed nations" OR TX "less developed population" OR TX "less developed populations" OR TX "less developed world" OR TX "lesser developed country" OR TX "lesser developed countries" OR TX "lesser developed nation" OR TX "lesser developed nations" OR TX "lesser developed population" OR TX "lesser developed populations" OR TX "lesser developed world" OR TX "under developed country" OR TX "under developed countries" OR TX "under developed nation" OR TX "under developed nations" OR TX "under developed population" OR TX "under developed populations" OR TX "under developed world" OR TX "underdeveloped country" OR TX "underdeveloped countries" OR TX "underdeveloped nation" OR TX "underdeveloped nations" OR TX "underdeveloped population" OR TX "underdeveloped populations" OR TX "underdeveloped world" OR TX "middle income country" OR TX "middle income countries" OR TX "middle income nation" OR TX "middle income nations" OR TX "middle income population" OR TX "middle income populations" OR TX "low income country" OR TX "low income countries" OR TX "low income nation" OR TX "low income nations" OR TX "low income population" OR TX "low income populations" OR TX "lower income country" OR TX "lower income countries" OR TX "lower income nation" OR TX "lower income nations" OR TX "lower income population" OR TX "lower income populations" OR TX "underserved country" OR TX "underserved countries" OR TX "underserved nation" OR TX "underserved nations" OR TX "underserved population" OR TX "underserved populations" OR TX "underserved world" OR TX "under served country" OR TX "under served countries" OR TX "under served nation" OR TX "under served nations" OR TX "under served population" OR TX "under served populations" OR TX "under served world" OR TX "deprived country" OR TX "deprived countries" OR TX "deprived nation" OR TX "deprived nations" OR TX "deprived population" OR TX "deprived populations" OR TX "deprived world" OR TX "poor country" OR TX "poor countries" OR TX "poor nation" OR TX "poor nations" OR TX "poor population" OR TX "poor populations" OR TX "poor world" OR TX "poorer country" OR TX "poorer countries" OR TX "poorer nation" OR TX "poorer nations" OR TX "poorer population" OR TX "poorer populations" OR TX "poorer world" OR TX "developing economy" OR TX "developing economies" OR TX "less developed economy" OR TX "less developed economies" OR TX "lesser developed economy" OR TX "lesser developed economies" OR TX "under developed economy" OR TX "under developed economies" OR TX "underdeveloped economy" OR TX "underdeveloped economies" OR TX "middle income economy" OR TX "middle income economies" OR TX "low income economy" OR TX "low income economies" OR TX "lower income economy" OR TX "lower income economies" OR TX "low gdp" OR TX "low gnp" OR TX "low gross domestic" OR TX "low gross national" OR TX "lower gdp" OR TX "lower gnp" OR TX "lower gross domestic" OR TX "lower gross national" OR TX lmic OR TX lmics OR TX "third world" OR TX "lami country" OR TX "lami countries" OR TX "transitional country" OR TX "transitional countries" OR TX Africa OR TX Asia OR TX Caribbean OR TX West Indies OR TX South America OR TX Latin America OR TX Central America OR TX "Atlantic Islands" OR TX "Commonwealth of Independent States" OR TX "Pacific Islands" OR TX "Indian Ocean Islands" OR TX "Eastern Europe" OR TX Afghanistan OR TX Albania OR TX Algeria OR TX Angola OR TX Antigua OR TX Barbuda OR TX Argentina OR TX Armenia OR TX Armenian OR TX Aruba OR TX Azerbaijan OR TX Bahrain OR TX Bangladesh OR TX Barbados OR TX Benin OR TX Byelarus OR TX Byelorussian OR TX Belarus OR TX Belorussian OR TX Belorussia OR TX Belize OR TX Bhutan OR TX Bolivia OR TX Bosnia OR TX Herzegovina OR TX Hercegovina OR TX Botswana OR TX Brasil OR TX Brazil OR TX Bulgaria OR TX Burkina Faso OR TX Burkina Fasso OR TX Upper Volta OR TX Burundi OR TX Urundi OR TX Cambodia OR TX Khmer Republic OR TX Kampuchea OR TX Cameroon OR TX Cameroons OR TX Cameron OR TX Camerons OR TX Cameroun OR TX Cape Verde OR TX Central African Republic OR TX Chad OR TX Chile OR TX China OR TX Colombia OR TX Comoros OR TX Comoro Islands OR TX Comores OR TX Mayotte OR TX Congo OR TX Zaire OR TX Costa Rica OR TX Cote d'Ivoire OR TX Ivory Coast OR TX Croatia OR TX Cuba OR TX Cyprus OR TX Czechoslovakia OR TX Czech Republic OR TX Slovakia OR TX Slovak Republic OR TX Djibouti OR TX French Somaliland OR TX Dominica OR TX Dominican Republic OR TX East Timor OR TX East Timur OR TX Timor Leste OR TX Ecuador OR TX Egypt OR TX United Arab Republic OR TX El Salvador OR TX Eritrea OR TX Estonia OR TX Ethiopia OR TX Fiji OR TX Gabon OR TX Gabonese Republic OR TX Gambia OR TX Gaza OR TX Georgia Republic OR TX Georgian Republic OR TX Ghana OR TX Gold Coast OR TX Greece OR TX Grenada OR TX Guatemala OR TX Guinea OR TX Guam OR TX Guiana OR TX Guyana OR TX Haiti OR TX Honduras OR TX Hungary OR TX India OR TX Maldives OR TX Indonesia OR TX Iran OR TX Iraq OR TX Isle of Man OR TX Jamaica OR TX Jordan OR TX Kazakhstan OR TX Kazakh OR TX Kenya OR TX Kiribati OR TX Korea OR TX Kosovo OR TX Kyrgyzstan OR TX Kirghizia OR TX Kyrgyz Republic OR TX Kirghiz OR TX Kirgizstan OR TX "Lao PDR" OR TX Laos OR TX Latvia OR TX Lebanon OR TX Lesotho OR TX Basutoland OR TX Liberia OR TX Libya OR TX Lithuania OR TX Macedonia OR TX Madagascar OR TX Malagasy Republic OR TX Malaysia OR TX Malaya OR TX Malay OR TX Sabah OR TX Sarawak OR TX Malawi OR TX Nyasaland OR TX Mali OR TX Malta OR TX Marshall Islands OR TX Mauritania OR TX Mauritius OR TX Agalega Islands OR TX "Melanesia" OR TX Mexico OR TX Micronesia OR TX Middle East OR TX Moldova OR TX Moldovia OR TX Moldovian OR TX Mongolia OR TX Montenegro OR TX Morocco OR TX Ifni OR TX Mozambique OR TX Myanmar OR TX Myanma OR TX Burma OR TX Namibia OR TX Nepal OR TX Netherlands Antilles OR TX New Caledonia OR TX Nicaragua OR TX Niger OR TX Nigeria OR TX Northern Mariana Islands OR TX Oman OR TX Muscat OR TX Pakistan OR TX Palau OR TX Palestine OR TX Panama OR TX Paraguay OR TX Peru OR TX Philippines OR TX Philipines OR TX Phillipines OR TX Phillippines OR TX Poland OR TX Portugal OR TX Puerto Rico OR TX Romania OR TX Rumania OR TX Roumania OR TX Russia OR TX Russian OR TX Rwanda OR TX Ruanda OR TX Saint Kitts OR TX St Kitts OR TX Nevis OR TX Saint Lucia OR TX St Lucia OR TX Saint Vincent OR TX St Vincent OR TX Grenadines OR TX Samoa OR TX Samoan Islands OR TX Navigator Island OR TX Navigator Islands OR TX Sao Tome OR TX Saudi Arabia OR TX Senegal OR TX Serbia OR TX Montenegro OR TX Seychelles OR TX Sierra Leone OR TX Slovenia OR TX Sri Lanka OR TX Ceylon OR TX Solomon Islands OR TX Somalia OR TX Sudan OR TX Suriname OR TX Surinam OR TX Swaziland OR TX Syria OR TX Syrian OR TX Tajikistan OR TX Tadzhikistan OR TX Tadjikistan OR TX Tadzhik OR TX Tanzania OR TX Thailand OR TX Togo OR TX Togolese Republic OR TX Tonga OR TX Trinidad OR TX Tobago OR TX Tunisia OR TX Turkey OR TX Turkmenistan OR TX Turkmen OR TX Tuvalu OR TX Uganda OR TX Ukraine OR TX Uruguay OR TX USSR OR TX Soviet Union OR TX Union of Soviet Socialist Republics OR TX Uzbekistan OR TX Uzbek OR TX Vanuatu OR TX New Hebrides OR TX Venezuela OR TX Vietnam OR TX Viet Nam OR TX West Bank OR TX Yemen OR TX Yugoslavia OR TX Zambia OR TX Zimbabwe OR TX Rhodesia OR (MH "Developing Countries") OR (MH "Africa") OR (MH "Africa South of the Sahara") OR (MH "Africa, Western") OR (MH "Africa, Southern") OR (MH "Africa, Northern") OR (MH "Africa, Eastern") OR (MH "Africa, Central") OR (MH "Asia") OR (MH "Asia, Southeastern") OR (MH "Asia, Central") OR (MH "Asia, Western") OR (MH "West Indies") OR (MH "South America") OR (MH "Latin America") OR (MH "Central America") OR (MH "Atlantic Islands") OR (MH "Commonwealth of Independent States") OR (MH "Pacific Islands") OR (MH "Indian Ocean Islands") OR (MH "Europe, Eastern") OR (MH "Middle East") OR (MH "Afghanistan") OR (MH "Albania") OR (MH "Algeria") OR (MH "American Samoa") OR (MH "Angola") OR (MH "Democratic Republic of the Congo") OR (MH "Antigua") OR (MH "Argentina") OR (MH "Armenia") OR (MH "Azerbaijan") OR (MH "Bahrain") OR (MH "Baltic States") OR (MH "Bangladesh") OR (MH "Barbados") OR (MH "Benin") OR (MH "Central African Republic") OR (MH "Byelarus") OR (MH "Dominican Republic") OR (MH "Georgia (Republic)") OR (MH "Czech Republic") OR (MH "Macedonia (Republic)") OR (MH "Guinea") OR (MH "USSR") OR (MH "Slovakia") OR (MH "Belize") OR (MH "Bhutan") OR (MH "Bolivia") OR (MH "Bosnia‐Herzegovina") OR (MH "Botswana") OR (MH "Brazil") OR (MH "Bulgaria") OR (MH "Burkina Faso") OR (MH "Cote d'Ivoire") OR (MH "Burundi") OR (MH "Cambodia") OR (MH "Cameroon") OR (MH "Cape Verde") OR (MH "Chad") OR (MH "Chile") OR (MH "China") OR (MH "Myanmar") OR (MH "Mongolia") OR (MH "Colombia") OR (MH "Congo") OR (MH "Costa Rica") OR (MH "Croatia") OR (MH "Mediterranean Islands") OR (MH "Czechoslovakia") OR (MH "Djibouti") OR (MH "North Korea") OR (MH "Dominica") OR (MH "East Timor") OR (MH "Papua New Guinea") OR (MH "Ecuador") OR (MH "Egypt") OR (MH "El Salvador") OR (MH "Eritrea") OR (MH "Estonia") OR (MH "Ethiopia") OR (MH "Equatorial Guinea") OR (MH "Guinea‐Bissau") OR (MH "Melanesia") OR (MH "French Guiana") OR (MH "Gabon") OR (MH "Gambia") OR (MH "Ghana") OR (MH "Greece") OR (MH "Guatemala") OR (MH "Guam") OR (MH "Guyana") OR (MH "Haiti") OR (MH "Honduras") OR (MH "Hungary") OR (MH "Independent State of Samoa") OR (MH "India") OR (MH "Indonesia") OR (MH "Iran") OR (MH "Iraq") OR (MH "Jamaica") OR (MH "Jordan") OR (MH "Kazakhstan") OR (MH "Kenya") OR (MH "Korea") OR (MH "Kyrgyzstan") OR (MH "Laos") OR (MH "Latvia") OR (MH "Lebanon") OR (MH "Lesotho") OR (MH "Liberia") OR (MH "Libya") OR (MH "Lithuania") OR (MH "Madagascar") OR (MH "Malawi") OR (MH "Malaysia") OR (MH "Mali") OR (MH "Mauritania") OR (MH "Indian Ocean Islands") OR (MH "Mexico") OR (MH "Micronesia") OR (MH "Moldova") OR (MH "Morocco") OR (MH "Mozambique") OR (MH "Namibia") OR (MH "Nepal") OR (MH "Netherlands Antilles") OR (MH "Nicaragua") OR (MH "Niger") OR (MH "Nigeria") OR (MH "Oman") OR (MH "Pakistan") OR (MH "Panama") OR (MH "Paraguay") OR (MH "Peru") OR (MH "Philippines") OR (MH "Poland") OR (MH "Portugal") OR (MH "Puerto Rico") OR (MH "South Korea") OR (MH "Romania") OR (MH "Russia") OR (MH "Rwanda") OR (MH "Samoa") OR (MH "Saudi Arabia") OR (MH "Senegal") OR (MH "Sierra Leone") OR (MH "Slovenia") OR (MH "Sri Lanka") OR (MH "Somalia") OR (MH "South Africa") OR (MH "Sudan") OR (MH "Suriname") OR (MH "Swaziland") OR (MH "Syria") OR (MH "Tajikistan") OR (MH "Tanzania") OR (MH "Thailand") OR (MH "Togo") OR (MH "Polynesia") OR (MH "Trinidad and Tobago") OR (MH "Tunisia") OR (MH "Turkey") OR (MH "Turkmenistan") OR (MH "Uganda") OR (MH "Ukraine") OR (MH "Uruguay") OR (MH "Uzbekistan") OR (MH "Venezuela") OR (MH "Vietnam") OR (MH "Yemen") OR (MH "Yugoslavia") OR (MH "Zambia") OR (MH "Zimbabwe"))
Appendix 7. LILACS search strategy
((“hazardous alcohol use” OR “alcohol misuse” OR “alcohol use” OR alcohol OR “alcohol consumption” OR “heavy drinking” OR “binge drinking” OR “alcohol use disorder” OR “alcohol abuse” OR “alcohol dependence” OR alcoholism OR alcoholic OR drinking OR “Drinking behavior” OR “Alcohol Abstinence” OR “Alcohol Drinking” OR “Binge Drinking” OR “Underage Drinking” OR “Alcohol‐Related Disorders” OR “Alcohol Intoxication” OR Alcoholism) AND (intervention OR program OR therapy OR treatment OR prevention OR rehabilitation OR “brief intervention” OR counseling OR detoxification OR “medical management” OR withdrawal OR support OR “mutual help” OR “self‐help” OR “twelve step” OR “12‐step” OR disulfiram OR antabuse OR naltrexone OR revia OR acamprosate OR campral) AND (randomized OR randomised OR trial) AND ("developing country" OR "developing countries" OR "developing nation" OR "developing nations" OR "developing population" OR "developing populations" OR "developing world" OR "less developed country" OR "less developed countries" OR "less developed nation" OR "less developed nations" OR "less developed population" OR "less developed populations" OR "less developed world" OR "lesser developed country" OR "lesser developed countries" OR "lesser developed nation" OR "lesser developed nations" OR "lesser developed population" OR "lesser developed populations" OR "lesser developed world" OR "under developed country" OR "under developed countries" OR "under developed nation" OR "under developed nations" OR "under developed population" OR "under developed populations" OR "under developed world" OR "underdeveloped country" OR "underdeveloped countries" OR "underdeveloped nation" OR "underdeveloped nations" OR "underdeveloped population" OR "underdeveloped populations" OR "underdeveloped world" OR "middle income country" OR "middle income countries" OR "middle income nation" OR "middle income nations" OR "middle income population" OR "middle income populations" OR "low income country" OR "low income countries" OR "low income nation" OR "low income nations" OR "low income population" OR "low income populations" OR "lower income country" OR "lower income countries" OR "lower income nation" OR "lower income nations" OR "lower income population" OR "lower income populations" OR "underserved country" OR "underserved countries" OR "underserved nation" OR "underserved nations" OR "underserved population" OR "underserved populations" OR "underserved world" OR "under served country" OR "under served countries" OR "under served nation" OR "under served nations" OR "under served population" OR "under served populations" OR "under served world" OR "deprived country" OR "deprived countries" OR "deprived nation" OR "deprived nations" OR "deprived population" OR "deprived populations" OR "deprived world" OR "poor country" OR "poor countries" OR "poor nation" OR "poor nations" OR "poor population" OR "poor populations" OR "poor world" OR "poorer country" OR "poorer countries" OR "poorer nation" OR "poorer nations" OR "poorer population" OR "poorer populations" OR "poorer world" OR "developing economy" OR "developing economies" OR "less developed economy" OR "less developed economies" OR "lesser developed economy" OR "lesser developed economies" OR "under developed economy" OR "under developed economies" OR "underdeveloped economy" OR "underdeveloped economies" OR "middle income economy" OR "middle income economies" OR "low income economy" OR "low income economies" OR "lower income economy" OR "lower income economies" OR "low gdp" OR "low gnp" OR "low gross domestic" OR "low gross national" OR "lower gdp" OR "lower gnp" OR "lower gross domestic" OR "lower gross national" OR lmic OR lmics OR "third world" OR "lami country" OR "lami countries" OR "transitional country" OR "transitional countries" OR Africa OR Asia OR Caribbean OR West Indies OR South America OR Latin America OR Central America OR "Atlantic Islands" OR "Commonwealth of Independent States" OR "Pacific Islands" OR "Indian Ocean Islands" OR "Eastern Europe" OR Afghanistan OR Albania OR Algeria OR Angola OR Antigua OR Barbuda OR Argentina OR Armenia OR Armenian OR Aruba OR Azerbaijan OR Bahrain OR Bangladesh OR Barbados OR Benin OR Byelarus OR Byelorussian OR Belarus OR Belorussian OR Belorussia OR Belize OR Bhutan OR Bolivia OR Bosnia OR Herzegovina OR Hercegovina OR Botswana OR Brasil OR Brazil OR Bulgaria OR Burkina Faso OR Burkina Fasso OR Upper Volta OR Burundi OR Urundi OR Cambodia OR Khmer Republic OR Kampuchea OR Cameroon OR Cameroons OR Cameron OR Camerons OR Cameroun OR Cape Verde OR Central African Republic OR Chad OR Chile OR China OR Colombia OR Comoros OR Comoro Islands OR Comores OR Mayotte OR Congo OR Zaire OR Costa Rica OR Cote d'Ivoire OR Ivory Coast OR Croatia OR Cuba OR Cyprus OR Czechoslovakia OR Czech Republic OR Slovakia OR Slovak Republic OR Djibouti OR French Somaliland OR Dominica OR Dominican Republic OR East Timor OR East Timur OR Timor Leste OR Ecuador OR Egypt OR United Arab Republic OR El Salvador OR Eritrea OR Estonia OR Ethiopia OR Fiji OR Gabon OR Gabonese Republic OR Gambia OR Gaza OR Georgia Republic OR Georgian Republic OR Ghana OR Gold Coast OR Greece OR Grenada OR Guatemala OR Guinea OR Guam OR Guiana OR Guyana OR Haiti OR Honduras OR Hungary OR India OR Maldives OR Indonesia OR Iran OR Iraq OR Isle of Man OR Jamaica OR Jordan OR Kazakhstan OR Kazakh OR Kenya OR Kiribati OR Korea OR Kosovo OR Kyrgyzstan OR Kirghizia OR Kyrgyz Republic OR Kirghiz OR Kirgizstan OR "Lao PDR" OR Laos OR Latvia OR Lebanon OR Lesotho OR Basutoland OR Liberia OR Libya OR Lithuania OR Macedonia OR Madagascar OR Malagasy Republic OR Malaysia OR Malaya OR Malay OR Sabah OR Sarawak OR Malawi OR Nyasaland OR Mali OR Malta OR Marshall Islands OR Mauritania OR Mauritius OR Agalega Islands OR "Melanesia" OR Mexico OR Micronesia OR Middle East OR Moldova OR Moldovia OR Moldovian OR Mongolia OR Montenegro OR Morocco OR Ifni OR Mozambique OR Myanmar OR Myanma OR Burma OR Namibia OR Nepal OR Netherlands Antilles OR New Caledonia OR Nicaragua OR Niger OR Nigeria OR Northern Mariana Islands OR Oman OR Muscat OR Pakistan OR Palau OR Palestine OR Panama OR Paraguay OR Peru OR Philippines OR Philipines OR Phillipines OR Phillippines OR Poland OR Portugal OR Puerto Rico OR Romania OR Rumania OR Roumania OR Russia OR Russian OR Rwanda OR Ruanda OR Saint Kitts OR St Kitts OR Nevis OR Saint Lucia OR St Lucia OR Saint Vincent OR St Vincent OR Grenadines OR Samoa OR Samoan Islands OR Navigator Island OR Navigator Islands OR Sao Tome OR Saudi Arabia OR Senegal OR Serbia OR Montenegro OR Seychelles OR Sierra Leone OR Slovenia OR Sri Lanka OR Ceylon OR Solomon Islands OR Somalia OR Sudan OR Suriname OR Surinam OR Swaziland OR Syria OR Syrian OR Tajikistan OR Tadzhikistan OR Tadjikistan OR Tadzhik OR Tanzania OR Thailand OR Togo OR Togolese Republic OR Tonga OR Trinidad OR Tobago OR Tunisia OR Turkey OR Turkmenistan OR Turkmen OR Tuvalu OR Uganda OR Ukraine OR Uruguay OR USSR OR Soviet Union OR Union of Soviet Socialist Republics OR Uzbekistan OR Uzbek OR Vanuatu OR New Hebrides OR Venezuela OR Vietnam OR Viet Nam OR West Bank OR Yemen OR Yugoslavia OR Zambia OR Zimbabwe OR Rhodesia OR Developing Countries OR Africa OR Africa, Northern OR Africa South of the Sahara OR Africa, Central OR Africa, Eastern OR Africa, Southern OR Africa, Western OR Asia OR Asia, Central OR Asia, Southeastern OR Asia, Western OR Caribbean Region OR West Indies OR South America OR Latin America OR Central America OR "Atlantic Islands" OR "Commonwealth of Independent States" OR "Pacific Islands" OR "Indian Ocean Islands" OR "Europe, Eastern" OR Afghanistan OR Albania OR Algeria OR American Samoa OR Angola OR "Antigua and Barbuda" OR Argentina OR Armenia OR Azerbaijan OR Bahrain OR "Baltic States" OR Bangladesh OR Barbados OR Benin OR "Republic of Belarus" OR Belize OR Bhutan OR Bolivia OR Bosnia‐Herzegovina OR Botswana OR Brazil OR Bulgaria OR Burkina Faso OR Burundi OR Cambodia OR Cameroon OR Cape Verde OR Central African Republic OR Chad OR Chile OR China OR Colombia OR Comoros OR Congo OR Costa Rica OR Cote d'Ivoire OR Croatia OR Cuba OR Cyprus OR Czechoslovakia OR Czech Republic OR Slovakia OR Djibouti OR "Democratic Republic of the Congo" OR "Democratic People's Republic of Korea" OR Dominica OR Dominican Republic OR East Timor OR Ecuador OR Egypt OR El Salvador OR Eritrea OR Estonia OR Ethiopia OR "Equatorial Guinea" OR Fiji OR "French Guiana" OR Gabon OR Gambia OR "Georgia (Republic)" OR Ghana OR Greece OR Grenada OR Guatemala OR Guinea OR Guinea‐Bissau OR Guam OR Guyana OR Haiti OR Honduras OR Hungary OR "Independent State of Samoa" OR India OR Indonesia OR Iran OR Iraq OR Jamaica OR Jordan OR Kazakhstan OR Kenya OR Korea OR Kyrgyzstan OR Laos OR Latvia OR Lebanon OR Lesotho OR Liberia OR Libya OR Lithuania OR "Macedonia (Republic)" OR Madagascar OR Malawi OR Malaysia OR Mali OR Malta OR Mauritania OR Mauritius OR "Melanesia" OR Mexico OR Micronesia OR Middle East OR Moldova OR Mongolia OR Montenegro OR Morocco OR Mozambique OR Myanmar OR Namibia OR Nepal OR Netherlands Antilles OR New Caledonia OR Nicaragua OR Niger OR Nigeria OR Oman OR Pakistan OR Palau OR Panama OR Papua New Guinea OR Paraguay OR Peru OR Philippines OR Poland OR Portugal OR Puerto Rico OR "Republic of Korea" OR Romania OR Russia OR "Russia (Pre‐1917)" OR Rwanda OR "Saint Kitts and Nevis" OR Saint Lucia OR "Saint Vincent and the Grenadines" OR Samoa OR Saudi Arabia OR Senegal OR Serbia OR Montenegro OR Seychelles OR Sierra Leone OR Slovenia OR Sri Lanka OR Somalia OR South Africa OR Sudan OR Suriname OR Swaziland OR Syria OR Tajikistan OR Tanzania OR Thailand OR Togo OR Tonga OR "Trinidad and Tobago" OR Tunisia OR Turkey OR Turkmenistan OR Uganda OR Ukraine OR Uruguay OR USSR OR Uzbekistan OR Vanuatu OR Venezuela OR Vietnam OR Yemen OR Yugoslavia OR Zambia OR Zimbabwe))
Appendix 8. World Health Organization International Clinical Trials Registry search strategy
(alcohol) AND (randomized OR randomised) AND (treatment OR prevention OR intervention)
Filters: Index – LILACS< WPRIM, IMEMR, IMSEAR, WHOLIS; Type of study – Controlled clinical trial; Limits – Humans; Language ‐ English
Appendix 9. Opengrey search strategy
(alcohol) AND (Africa OR Asia OR Caribbean OR “West Indies” OR “South America” OR “Latin America” OR “Central America” OR "Atlantic Islands" OR "Commonwealth of Independent States" OR "Pacific Islands" OR "Indian Ocean Islands" OR "Eastern Europe”)
Appendix 10. clinicaltrials.gov search strategy
Condition/Disease: alcohol
Study Type: Interventional Studies
Country: (Africa OR Asia OR Caribbean OR “South America” OR “Latin America” OR “Central America” OR "Pacific Islands" OR "Eastern Europe”)
Data and analyses
Comparison 1. Any pharmacologic intervention vs. placebo among participants with alcohol use disorder or dependence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Remission | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [1.00, 4.52] |
| 1.2 Retention | 3 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.89, 1.44] |
| 1.3 Adverse events | 0 | Other data | No numeric data |
Comparison 2. Any pharmacologic intervention vs. placebo given in addition to psychosocial intervention among participants with alcohol use disorder.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Harmful alcohol use (continuous) | 4 | 475 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.61, ‐0.24] |
| 2.2 Harmful alcohol use (dichotomous) | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.16, 2.47] |
| 2.3 Alcohol consumption | 1 | 71 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.13, 0.81] |
| 2.4 Remission | 4 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.01, 1.40] |
| 2.5 Retention | 3 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.95, 1.40] |
| 2.6 Adverse events | 0 | Other data | No numeric data |
2.6. Analysis.
Comparison 2: Any pharmacologic intervention vs. placebo given in addition to psychosocial intervention among participants with alcohol use disorder, Outcome 6: Adverse events
| Adverse events | ||
| Study | Heading 1 | Heading 2 |
| Ahmadi 2004 | Experimental (n=58): 20 nausea, 13 lethargy, 5 nightmares, 7 dizziness, 10 insomnia, 14 headache, 14 anxiety | Control (n=58): 9 nausea, 0 nighmares, 1 dizziness, 4 insomnia, 6 headache, 10 anxiety |
| Correa Filho 2013 | Experimental (n=50): 26 reported side effects (52%) | Control (n=52): 26 reported side effects (50%) |
| Likhitsathian 2013 | Experimental (n=53): 3.8% headache, 1.9% fatigue, 1.9% somnolence, 0% back pain, 0% tremor, 0% dizziness, 0% numbness, 45.3% parasthesia, 15.1% taste perversion, 17.0% poor appetite, 17.0% impaired concentration, 20.7% pruritus, 1.9% psychomotor retardation, 1.9% joint pain, 0% nervousness, 0% tongue numbness, 0% flushed face | Control (n=53): 0% headache, 1.9% fatigue, 5.7% somnolence, 1.9% back pain, 3.8% tremor, 1.9% dizziness, 1.9% numbness, 17.0% parasthesia, 5.7% taste perversion, 9.4% poor appetite, 11.3% impaired concentration, 15.1% pruritus, 0% psychomotor retardation, 0% joint pain, 1.9% nervousness, 3.8% tongue numbness, 1.9% flushed face |
Comparison 3. Any pharmacologic intervention vs. another pharmacologic intervention among participants with alcohol use disorder.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Remission | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.42, 0.79] |
| 3.2 Retention | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.19] |
| 3.3 Adverse events | 0 | Other data | No numeric data |
3.3. Analysis.
Comparison 3: Any pharmacologic intervention vs. another pharmacologic intervention among participants with alcohol use disorder, Outcome 3: Adverse events
| Adverse events | ||
| Study | Heading 1 | Heading 2 |
| Altintoprak 2008 | Experimental: 11 side effects reported | Control: 28 side effects reported |
Comparison 4. Any pharmacologic intervention vs. another pharmacologic intervention in addition to psychosocial intervention among participants with alcohol use disorder.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Remission | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.40, 2.25] |
| 4.2 Retention | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.96, 1.08] |
Comparison 5. Brief psychosocial intervention vs. brief advice, information, or assessment only among participants with hazardous or harmful alcohol use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Harmful alcohol use (continuous) | 17 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2 Harmful alcohol use (dichotomous) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.3 Alcohol consumption | 9 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4 Remission | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.5 Retention | 12 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Other psychosocial intervention vs. usual care or wait list among participants with hazardous or harmful alcohol use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Harmful alcohol use | 12 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.2 Alcohol consumption | 7 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.3 Remission | 7 | 1519 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.19, 1.76] |
| 6.4 Retention | 9 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmadi 2004.
| Study characteristics | ||
| Methods |
Study design: Rrandomized controlled trial Country: Iran Setting: outpatient clinic Eligibility criteria: included were adult males with a current diagnosis of alcohol dependence and between 3‐30 days abstinent prior to enrollment. Excluded were individuals with other substance use disorders; current use of opioids, neuroleptics, or disulfiram; bilirubin level and alanine aminotransferase greater than five times normal levels. Duration of follow‐up: 36 weeks Informed consent: all participants provided informed consent Ethical approvals: not listed |
|
| Participants |
Sample size: 116 (58 intervention, 58 control) Description of the target population: males in outpatient treatment for alcohol use disorder Age: 42.97 years (SD = 9.17) Sex: 100% male Race/Ethnicity: Not listed Marital status: 87.07% married, 12.93% single Harmful alcohol use (baseline): not listed Co‐occurring disorders: not listed |
|
| Interventions |
Type: pharmacologic + non‐pharmacologic Description: naltrexone and alcohol counseling (consequences and relapse prevention) Duration and frequency: 50 mg/day of naltrexone and 12 weekly 30‐minute counseling sessions Delivery and provider: not listed Comparison group: placebo and alcohol counseling (consequences and relapse prevention) |
|
| Outcomes |
Primary outcome(s): abstinence/remission, relapse Primary outcome measurement tool(s): Timeline follow‐back Secondary outcome(s) :Retention in Treatment,Adverse events Secondary outcome measurement tool(s): not listed Time points: 4, 8, 12, 16, 20, 24, 28, 32, 36 weeks |
|
| Notes |
Study funding and conflicts of interest: none listed Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were allocated randomly to receive either naltrexone 50mg/day or an identical placebo capsule for 36 weeks." Pg. 35 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"Patients and research staff were blind to the capsule prescribed" Pg. 35 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"Patients and research staff were blind to the capsule prescribed" Pg. 35 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Allen 2011.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Russia Setting: health facility Eligibility criteria: included were males who reported weekly consumption of 250 mL or more of ethanol over the past year as well as extended periods of binge drinking (i.e., Zapoi) in the last year, using drinking surrogates (i.e. non‐beverage alcohol) in the last year, excessive drunkenness, and/or going to sleep clothed due to being drunk twice or more per week on average over the past year. Excluded were males who refused to have a baseline health check or refused at baseline to be re‐interviewed for follow‐up. Duration of follow‐up: 3‐months Informed consent: men randomized to intervention received an information sheet and provided consent for participating in the intervention and follow‐up. Men randomized to control provided general consent to be followed up Ethical approvals: not listed |
|
| Participants |
Sample size: 441 (221 intervention, 220 control) Description of the target population: working age men with hazardous alcohol use Age: 30‐59 years Sex: 100% male Race/Ethnicity: not listed Marital status: not listed Harmful alcohol use (baseline): 71.6% self‐reported hazardous alcohol use; 93.9% proxy‐reported hazardous alcohol use Co‐occurring disorders: not listed |
|
| Interventions |
Type: non‐pharmacologic Description: brief motivational interviewing Duration and frequency: 2 required and 2 optional sessions Delivery and provider: narcologist (specialists in treating alcohol and other drug use disorders), psychiatrists, social workers, school psychologists Comparison group: general health promotion and feedback sent by mail after general health check |
|
| Outcomes |
Primary outcome(s): hazardous and harmful drinking Primary outcome measurement tool(s): Defined as 1+ occurrences of zapoi in the past month, use of alcohol surrogates in the past month, hangover on average twice or more per week in past month, or 250 mL or more of ethanol in the past week Secondary outcome(s): Aalcohol consumption, Treatment compliance Secondary outcome measurement tool(s): average consumption ‐ average weekly consumption of ethanol in past month >250 mL; Treatment compliance ‐ Both MI sessions before 3 months follow‐up Time points: 3‐months |
|
| Notes |
Study funding and conflicts of interest: Wellcome Trust Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Data collected at the baseline re‐interview were sent to the randomisation service in London, allowing participants to be allocated in a 1:1 ratio to MI intervention or no MI. Minimisation criteria (age, surrogate use in past year, and living alone status) were used to ensure a reasonable balance of confounding factors. An online randomisation program was used to generate the random allocation." Pg. 3 |
| Allocation concealment (selection bias) | Low risk | Quote:"At the end of the health check, the doctor undertaking the physical examination opened a sealed envelope containing the allocation." Pg. 3 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention Quote:"Telling the control group about the alcohol‐specific MI intervention and the alcohol‐specific outcomes, would have sensitised the control group to our primary research interest and thereby in itself may have altered behaviour. This could have also diluted any effect of the MI intervention" Pg. 3 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:"Follow up at 3 months was very high (97% of those having a health check), and similar in the two trial arms (183 in the intervention and 187 in the control)." Pg. 5 |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Altintoprak 2008.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Turkey Setting: hospital specialized addiction unit Eligibility criteria: individuals who were 18‐65 years of age, met criteria for alcohol dependence and depressive disorder, were experiencing a current depressive episode, but had no serious physical illness. Female participants also needed to be adequately protected against pregnancy. Excluded were individuals with another major psychiatric disorder, a history of psychiatric problems other than depressive disorder, the presence of organic brain disease, a history of hypersensitivity to mirtazapine or amitriptyline, pregnant or breastfeeding, other substance (non‐alcohol, tobacco, or caffeine) use disorder, and having consumed alcohol during the study. Duration of follow‐up: 56 days Informed consent: all participants provided written informed consent Ethical approvals: all procedures were approved by Ege University Ethics Committee |
|
| Participants |
Sample size: 36 (20 mirtazapine, 16 amitriptyline) Description of target population: patients seeking primary healthcare with alcohol dependence and depression Age: 44.0 years (range 18‐65) Sex: 8.3% female Race/Ethnicity: not reported Marital status: 63.9% married Harmful alcohol use (baseline): Michigant Alcoholism Screening Test (MAST): 38.9 (SD=6.1) Co‐occurring disorders: 100% major depressive disorder and current depressive episode |
|
| Interventions |
Type: pharmacologic Description: mirtazapine following alcohol detoxification with diazepam taper and vitamin B Duration and frequency: started with 15 mg/day. On the third day patients were increased to 30 mg/day. At the end of the first week dose was increased to 45 mg to 60 mg/day if severity of symptoms persisted Delivery and provider: clinician delivered capsules consumed orally Comparison group: amitriptyline (50 mg/day increased to 100 mg/day on third day and 125‐150 mg/day at end of first week if severity of symptoms persisted) following detoxification with diazepam taper and vitamin B |
|
| Outcomes |
Primary outcome(s): alcohol craving Primary outcome measurement tool(s): alcohol craving questionnaire developed by study investigators Secondary outcome(s): State anxiety, trait anxiety, Depression Secondary outcome measurement tool(s): Spielberger State‐Trait Anxiety Inventory (STAI); Hamilton Depression Rating Scale (HDRS) Time points: 0, 7, 14, 28, 42, 56 days |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the end of the alcohol detoxification treatment with diazepam (approximately 10–14 days), the patients with HDRS scores 14 were included in the study and they were randomly allocated to mirtazapine or amitriptyline treatment groups." Pg. 315 |
| Allocation concealment (selection bias) | Unclear risk | Drugs were administered in identical looking opaque capsules, but unclear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both the clinicians and patients were blind to the treatment." Pg. 315 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Initially, 97 patients were enrolled in the study but after the detoxification period there were only 44 patients meeting the criteria for current major depressive disorder. Eight of the patients dropped out (three of the patients dropped out due to adverse effects of drugs, one of them dropped out due to an another psychiatric disorder emerged during the study, two patients dropped out due to alcohol consumption in the treatment setting, and two patients decided not to remain in the study). Thirty‐six patients completed the study. Dropouts were not included in the analysis due to missing data." Pgs. 315‐316 |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Assanangkornchai 2015.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Thailand Setting: District hospitals and sub‐district health centers in urban and rural communities Eligibility criteria: included were outpatients aged 16‐65 years living within 20 kilometers from the health facility with moderate risk of substance problems, able to communicate in Thai or Jawi, able to participate in an interview and intervention session for 30 minutes, and were willing to participate in a 6‐month follow‐up study. Excluded were outpatients who exhibited aggressive behavior, were known to have been involved in serious criminal activity with risk of imprisonment during the subsequent 6 months, had severe cognitive impairment, or used only tobacco. Duration of follow‐up: 6 months Informed consent: written consent was obtained from participants directly Ethical approvals: trial procedures were approved by the Ethics Committee (EC) for Research in Human Subjects, Faculty of Medicine, Prince of Songkla University. |
|
| Participants |
Sample size: 82 with moderate alcohol problems (out of 236 total with substance use problems); 47 intervention, 35 control Description of the target population: outpatients with substance use problems Age: not reported for alcohol group Sex: not reported for alcohol group Race/Ethnicity: not reported Marital status: not reported Harmful alcohol use (baseline): average ASSIST score of 18.1 (SD‐3.6) in brief intervention and 15.6 (SD = 3.9) in simple advice control group Co‐occurring disorders: other substance use problems |
|
| Interventions |
Type: Non‐pharmacologic Description: ASSIST‐linked brief intervention Duration and frequency: 1 session up to 15 minutes (8.8 minutes on average) Delivery and provider: study investigator/researcher Comparison group: smple advice |
|
| Outcomes |
Primary outcome(s): alcohol use severity/involvement Primary outcome measurement tool(s): ASSIST‐Substance Specific Involvement Score (ASSIST‐SSIS) Secondary outcome(s): Total Substance Involvement Score, Number of converted cases to low‐risk category Secondary outcome measurement tool(s): Time points: 0‐, 3‐, 6‐months |
|
| Notes |
Study funding and conflicts of interest: Integrated Community Management for Substance Abuse Problems, Thai Promotion Foundation; National Science and Technology Development Agency, Ministry of Science and Technology, Thailand Linked study records: Trial protocol: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362718; Assanangkornchai 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation list based on a permutation block was prepared prior to the data collection phase and a randomisation number was kept in an opaque envelope for each patient." Pg. 76 |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation list based on a permutation block was prepared prior to the data collection phase and a randomisation number was kept in an opaque envelope for each patient." Pg. 76 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The main limitation is that the same interviewers conducted the baseline, three‐ and six‐month interviews and thus the trial was not fully blinded. However, the ASSIST questions are structured, the patient’s answers mainly fit into fixed‐response categories, and the treatment groups were not revealed to the interviewers at the time of follow‐up. After three to six months it is also unlikely that the interviewers remembered the patients, who prior to the study were unknown to them and whom they had seen only briefly, or the treatment groups to which they had been allocated. For these reasons, any response bias resulting from incomplete blinding is unlikely to be high. Nonetheless, our results should be considered as those of two parallel groups rather than those of a single‐blind trial." Pg. 79 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The attrition rates were high in both arms of the study – 40% and 35% for BI and SA at six months, respectively – but this difference was not significant; nor was there a significant difference between trial completers and non‐completers in baseline characteristics and ASSIST scores." Pg. 79 |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported in the study as described in the trial protocol (https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362718). |
Baldin 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Brazil Setting: web‐based; Recruitment through night clubs Eligibility criteria:included were adults who were seeking entry to a nightclub, completed a screening assessment where they indicated that they had consumed alcohol in the past year and were identified as problem drinkers (AUDIT > = 8). Duration of follow‐up: 6‐months Informed consent: not reported Ethical approvals: not reported |
|
| Participants |
Sample size: 465 (224 web‐based intervention, 241 assessment only) Description of the target population: nightclub patrons with problem drinking (AUDIT> = 8) Age: 24.7 years (SD = 6.0) Sex: 35.5% female Race/Ethnicity: not reported Marital status: not reported Harmful alcohol use (baseline): AUDIT 12.6 (SD=4.1, Range = 8‐33) Co‐occurring disorders:nNot reported |
|
| Interventions |
Type: Non‐pharmacologic Description: web‐based intervention including personalized normative feedback about alcohol use Duration and frequency: 1 online session Delivery and provider: web‐based Comparison group: assessment only |
|
| Outcomes |
Primary outcome(s): binge drinking Primary outcome measurement tool(s): AUDIT Secondary outcome(s): lack of control over drinking behavior, hazardous alcohol use Secondary outcome measurement tool(s): AUDIT Time points: 6‐months |
|
| Notes |
Study funding and conflicts of interest: Sao Paulo Research Foundation (FAPESP) Linked study records: Sanchez 2018 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation in the groups was randomized and stratified, performed on the website itself, using a stratified permuted block randomization algorithm, considering the following categories for each of the three randomization strata: 1) sex ( female, male), 2) age group (18–24, 25–34, 35+), and 3) pattern of alcohol consumption (AUDIT classification in four categories, that is, 0–7, 8–14, 15–19, 20–40 points)." Pg. 4 |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation in the groups was randomized and stratified, performed on the website itself, using a stratified permuted block randomization algorithm, considering the following categories for each of the three randomization strata: 1) sex ( female, male), 2) age group (18–24, 25–34, 35+), and 3) pattern of alcohol consumption (AUDIT classification in four categories, that is, 0–7, 8–14, 15–19, 20–40 points)." Pg. 4 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "At the six‐month follow‐up, we obtained the response of 79 (50.6%) participants from the intervention group and 77 (49.4%) participants from the control group. Table 2 shows the attrition results of the sociodemographic data, AUDIT score at baseline, and allocation group among these 156 respondents versus nonresponders. There was no statistically significant difference between participants who answered the questionnaire after six months versus nonresponders." Pg. 6 |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Baltieri 2003.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Brazil Setting: outpatient alcohol treatment center Eligibility criteria: included were males 18‐60 years of age who weighted more than 60 kg with alcohol dependence and enrolled in outpatient alcohol treatment. Excluded were individuals prescribed medications or with serious co‐occurring physical diseases or psychiatric disorders that may require specific pharmacological treatment. Duration of follow‐up: 6 months Informed consent: all participants provided informed consent Ethical approvals: not reported |
|
| Participants |
Sample size: 75 (40 intervention, 35 control) Description of the target population: males in outpatient treatment with alcohol dependence Age: 44.2 years (SD = 8.28) Sex: 100% male Race/Ethnicity: not reported Marital status: not reported Harmful alcohol use (baseline): average daily alcohol intake was 370.1 (SD = 164.91) g/day in intervention and 348.5 (SD = 132.46) g/day for placebo Co‐occurring disorders: Not reported |
|
| Interventions |
Type: pharmacologic Description: Acamprosate and encouragement to attend Alcoholics Anonymous Duration and frequency: 1.998 mg Acamprosate per day for 12 weeks Delivery and provider: not reported Comparison group: placebo and encouragement to attend Alcoholics Anonymous |
|
| Outcomes |
Primary outcome(s): abstinence Primary outcome measurement tool(s): self‐reported quantity and frequency of alcohol consumption; verified using GGT test Secondary outcome(s): Alcohol consumption, Relapse, Retention, Side effects, Secondary outcome measurement tool(s): self‐reported quantity and frequency of alcohol consumption Time points: 1, 2, 3, 4, 6, 8, 12, 20, and 24 weeks |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: Baltieri 2004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Following a 1‐week detoxification period, the patients were randomly divided in two groups" Pg. 137 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The possibility of unblinding as a result of differential adverse events in this study seems remote, but particular testing for blinding was not performed." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Ten patients who were receiving acamprosate and seven patients who were receiving placebo dropped out (X2=0.27, 1 df, p=0.61). The reasons for dropping out were unwillingness to continue the treatment (two patients of the acamprosate group and two of the placebo group); 'protocol violation', which was defined as the use of other psychopharmacological drugs during the study (one patient of the acamprosate group and one of the placebo group); and unavailability for follow‐up (seven patients of the acamprosate group and four of the placebo group). The patients who were withdrawn from treatment were included in the ITT." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Baltieri 2008.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Brazil Setting:outpatient alcohol treatment center Eligibility criteria: included were males age 18‐60 years with a diagnosis of alcohol dependence, a history of problems associated with alcohol misuse for two or more years, and admitted to an outpatient treatment center for alcohol use disorder. Excluded were individuals with serious clinical co‐occurring diseases, previous treatment with naltrexone or topiramate within 6 months of randomization, other drug dependence (excluding nicotine), co‐occurring psychiatric disorders that may require pharmacological treatment, inability to provide informed consent, and history of developmental disorders. Duration of follow‐up: 3‐months Informed consent: all participants provided written informed consent Ethical approvals: approved by the Ethics Committee of the Clinical Hospital of the University of Sao Paulo, Brazil |
|
| Participants |
Sample size: 155 (52 topiramate, 49 naltrexone, 54 placebo) Description of the target population: Males in outpatient treatment for alcohol use disorder Age: 44.3 years (SD=8.4) Sex: 100% male Race/Ethnicity: 71.0% White, 7.1% Black, 21.9% Mixed race Marital status: 51.6% Married, 16.8% Single, 31.6% Separated/Widowed Harmful alcohol use (baseline): Average quantity of alcohol used per day was 301g (SD=174) and mean dependence scores indicated moderate to severe alcohol dependence (SAD=29, SD=8.5). Co‐occurring disorders: Average depression scores (HAM‐D) indicated mild depression |
|
| Interventions |
Type: Pharmacologic + non‐pharmacologic Description: A) Naltrexone, standard cognitive behavioral therapy, relapse prevention, encouragement to attend Alcoholics Anonymous, and 1 week of detoxification prior to initiating naltrexone; B) Topiramate, standard cognitive behavioral therapy, relapse prevention, encouragement to attend Alcoholics Anonymous, and 1 week of detoxification prior to initiating topiramate Duration and frequency: A) 50 mg/day naltrexone + 1 placebo capsule per day; B) Up to 200 mg/day (escalated dose) of topiramate twice per day Delivery and provider: medications provided to participants weekly at which time they also received standard non‐pharmacologic treatment for 12 weeks Comparison group: 2 placebo capsules per day, standard cognitive behavioral therapy, relapse prevention, encouragement to attend Alcoholics Anonymous, 1 week of detoxification prior to initiating placebo |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): self‐reported quantity and frequency of alcohol consumption Secondary outcome(s): relapse, abstinence, heavy drinking, side effects, retention Secondary outcome measurement tool(s): Self‐reported alcohol consumption, self‐reported side effects Time points: 1, 2, 3, 4, 6, 8, 10, and 12‐weeks |
|
| Notes |
Study funding and conflicts of interest: Fundacao de Amparo a Pesquisa do Estado de Sao Paulo/The State of Sao Paulo Research Foundation Linked study records: Baltieri 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following the 1‐week detoxification period, the patients were assigned randomly to one of the three medication conditions through a random number list." Pg. 2037 |
| Allocation concealment (selection bias) | Low risk | Quote: "Once a week, they received an envelope with two packages of seven capsules." Pg. 2037 "The packages containing the capsules were delivered by two blinded research assistants who also assessed patient outcomes throughout the study." Pg. 2038 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "At each appointment, all patients received standardized brief cognitive behavioural interventions from their doctors who were blind to medication conditions." Pg. 2038 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The packages containing the capsules were delivered by two blinded research assistants who also assessed patient outcomes throughout the study." Pg. 2038 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Differences between conditions in overall dropout rates approached significance and were statistically significant within the lost‐to‐follow‐up category with a significant difference between topiramate and placebo in post‐hoc analysis." Pg. 2039 |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Bolton 2014.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Thailand Setting: Community‐based; Refugee camp Eligibility criteria: included were Burmese refugees who witnessed or experienced a traumatic event, had moderate to severe depression and/or post‐traumatic stress symptoms based on locally validated measures, and reported harmful alcohol use. Excluded were individuals with active psychosis. Duration of follow‐up: 4‐months Informed consent: all eligible participants provided informed consent Ethical approvals: approved by the Institutional Review Board at Johns Hopkins University, the Johns Hopkins Bloomberg School of Public Health, and a local ethics committee in Mae Sot, Thailand composed of five Burmese members from NGOs and community‐based organizations and led by a local physician |
|
| Participants |
Sample size: 33 (18 intervention, 15 control) Description of the target population: refugees with psychological distress and harmful alcohol use Age: not reported Sex: not reported Race/Ethnicity: 100% Burmese Marital status: not reported Harmful alcohol use (baseline): average AUDIT item score (range 0‐4) was 1.73 (95% CI: 1.43, 2.03) in intervention and 1.69 (95% CI: 1.38, 2.00) in control Co‐occurring disorders: all participants had moderate to severe depression or post‐traumatic stress symptoms |
|
| Interventions |
Type: non‐pharmacologic Description: a transdiagnostic psychological intervention, the Common Elements Treatment Approach (CETA), including a screening and brief intervention component with motivational interviewing techniques Duration and frequency: weekly 1‐hour sessions; Number of sessions varied between 7‐13 (average 9.7 sessions) Delivery and provider: trained lay counselors who were staff at local service organizations Comparison group: safety check phone calls |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): Alcohol Use Disorder Identification Test (AUDIT) Secondary outcome(s): Depression, Post‐traumatic stress symptoms, Anxiety, Functional impairment, Aggression Secondary outcome measurement tool(s): Hopkins Symptom Checklist‐25, Harvard Trauma Questionnaire, locally developed Functioning measure, 12‐item Aggression Questionnaire Time points: 4 months |
|
| Notes |
Study funding and conflicts of interest: USAID Victims of Torture Fund Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each counselor then assigned participants the next available ID number from a block of 20 sequential participant ID numbers per counselor randomly allocated to intervention or wait‐list control (WLC) status. The project site director generated these random numbers using STATA" Pg. 7 |
| Allocation concealment (selection bias) | Low risk | Quote: "Counselors opened a pre‐sealed envelope (corresponding to the ID number) containing assignment to immediate treatment or wait‐list." Pg. 7 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "non‐blinding of service providers and participants" Pg. 1 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Outcomes were assessed by interviewers blinded to participant allocation" Pg. 1 Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Loss to follow‐up was higher than previous counseling intervention trials in low‐resource settings. Higher losses are likely due to the high population mobility characteristic of migrant and displaced populations, rather than to problems with the intervention, since losses were greater among WLCs (23.6%) than in the intervention group (18.7%). It is quite likely that the missing data have affected our outcome estimates, including the effect sizes, although the size and direction of the change is not known." Pg. 13. Analysis was ITT (see Figure 2) |
| Selective reporting (reporting bias) | Low risk | Quote: "trial registration occurred before an outcome data were collected" (Pg. 3) Registered outcomes and procedures described in protocol are consistent with the paper. |
Castro 2009.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Brazil Setting: Substance use outpatient treatment clinic Eligibility criteria: included were adults 18‐65 years who met DSM‐IV criteria for alcohol dependence, were seeking care at a substance use treatment center, resided in Sao Paulo, and reported not drinking alcohol between 5‐30 days prior to enrollment. Excluded were people with mental disorders requiring psychiatric hospitalization or pharmacological treatment, health conditions that were contraindicated for naltrexone, liver problems, use of other drugs in the past 30 days, and previous use of disulfiram, naltrexone, or acamprosate. Duration of follow‐up: 12 weeks Informed consent: all participants provided informed consent Ethical approvals: study procedures approved by ethical review committee at Sao Paulo Hospital |
|
| Participants |
Sample size: 71 (35 naltrexone, 36 placebo) Description of the target population: patients seeking care for alcohol dependence in substance use clinic Age: not reported Sex: not reported Race/Ethnicity: not reported Marital Status: not reported Harmful alcohol use (baseline): not reported Co‐occurring disorders: not reported |
|
| Interventions |
Type: Pharmacologic + non‐pharmacologic Description: Naltrexone and brief intervention Duration and frequency: 50 mg of naltrexone daily for 12 weeks + brief intervention Delivery and provider: not reported Comparison group: lacebo daily + brief intervention |
|
| Outcomes |
Primary outcome(s): alcohol consumption (number of days) Primary outcome measurement tool(s): not reported Secondary outcome(s): moderate alcohol consumption, heavy drinking days Secondary outcome measurement tool(s): not reported Time points: 12 weeks |
|
| Notes |
Study funding and conflicts of interest: Foundation of Research for the State of Sao Paulo (FAPESP) Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly divided into two groups using a list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Cherpitel 2009.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Poland Setting: emergency department Eligibility criteria: included were adult (18+ years) patients at the emergency department who were not presently being treated for an alcohol‐related problem, reported unhealthy alcohol use (i.e. screened positive for alcohol dependence in the past 3 months, reported 11 or more drinks per week (males)/6 or more drinks per week (females), or 4+ drinks per drinking day (males)/3 or more drinks per drinking day (females) in the past year), were willing to give informed consent and be randomized, and were willing to provide contact information for at least two individuals who would always know the participant's whereabouts. Excluded were people with a severe health condition or high level of intoxication or inability to locate the patient in the emergency department. Duration of follow‐up: 12‐months Informed consent: all participants provided verbal consent for screening and written consent for full assessment and brief intervention Ethical approvals: not reported |
|
| Participants |
Sample size: 446 (147 screening only, 152 assessment only, 147 brief intervention) Description of the target population: patients admitted to the emergency department who screen positive for alcohol dependence and heavy drinking Age: 38.0% <30 years Sex: 84.5% male Race/Ethnicity: not reported Marital status: not reported Harmful alcohol use (baseline): 39.0% screened positive for alcohol dependence (RAPS4), 87.0% met criteria for at risk drinking, and averaged 2.7 drinking days per week, 6.0 drinks per drinking day, and 9.6 maximum drinks on one occasion during the last month Co‐occurring disorders: not reported |
|
| Interventions |
Type: Non‐pharmacologic Description: brief intervention consisted of screening, assessment of drinking behaviors, and motivational interviewing. Motivational interviewing included personalized feedback, discussion of norms, evaluating pros and cons of drinking behaviors, readiness to change, and developing a plan for behavior change. All participants received a list of Alcoholics Anonymous groups and specialized services for alcohol treatment and counseling Duration and frequency: one 15‐ to 20‐ minute session Delivery and provider: nurse trained in SBIRT (screening, brief intervention, and referral to treatment) Comparison group: A) assessment only: Screening, assessment of drinking behaviors (breathalyzer, recent and general drinking behaviors, and related consequences) and a list of Alcoholics Anonymous groups and specialized services for alcohol treatment and counseling administered by study staff; B) Screening only: Screening with RAPS4 for alcohol dependence, three questions about at‐risk drinking, and a list of Alcoholics Anonymous groups and specialized services for alcohol treatment and counseling administered by study staff |
|
| Outcomes |
Primary outcome(s): at‐risk drinking Primary outcome measurement tool(s): timeline followback, Short Inventory of Problems (SIPS+6) (based on number of drinking days per week, number of drinks per drinking day, maximum number of drinks on occasion, and negative consequences of drinking) Secondary outcome(s): acohol consumption patterns, negative consequences of drinking Secondary outcome measurement tool(s): timeline followback, Short Inventory of Problems (SIPS+6) Time points: 3‐ and 12‐months |
|
| Notes |
Study funding and conflicts of interest: National Institute on Alcohol Abuse and Alcoholism (R21AA016081) Linked study records: Cherpitel 2010, Korcha 2010, Korcha 2012, Cherpitel 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After agreeing to participate and providing a signed informed consent, patients were then randomized using a two‐stage process, whereby they were first randomized to either the screen‐only or assessment condition by the study interviewer (generally college students or new graduates) who drew an envelope with the condition assignment. The envelope of those receiving an assessment contained a second envelope, which was opened by the interviewer following assessment to determine whether or not the patient was assigned to the intervention condition." Pg. 984 |
| Allocation concealment (selection bias) | Low risk | Quote: "After agreeing to participate and providing a signed informed consent, patients were then randomized using a two‐stage process, whereby they were first randomized to either the screen‐only or assessment condition by the study interviewer (generally college students or new graduates) who drew an envelope with the condition assignment. The envelope of those receiving an assessment contained a second envelope, which was opened by the interviewer following assessment to determine whether or not the patient was assigned to the intervention condition." Pg. 984 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Those in the assessment and intervention conditions were recontacted by telephone and reassessed at 3 months by an interviewer blinded to group status" Pg. 986 Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Follow‐up rates at 12‐months for those followed‐up at 3 months were 63% for the screened condition (n=92), 65% for the assessment condition (n=99) and 59% for the intervention condition (n=87). Reasons for non‐follow‐ups were not significant across the three conditions, with 24% due to refusals, 56% to failure to locate the patient and the remained to 9 patients who had died, 15 who had moved away, 2 in prison and 2 in the hospital" Pg. 3, Cherpitel 2010 "Of the 147 patients randomized to the intervention condition two refused the intervention but are analyzed in the intervention condition as intention to treat" Pg. 984 |
| Selective reporting (reporting bias) | High risk | Quote: "Because of the significance differences between the assessment and intervention conditions at baseline and because the literature suggests that brief intervention is most efficacious for those drinking at a nondependent level, subsequent analyses were restricted to those who reported an average of not more than six drinks per day at baseline" Pg. 986 |
Chompookham 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Thailand Setting: inpatient alcohol treatment unit Eligibility criteria: included were inpatients with a current diagnosis of alcohol dependence. Excluded were individuals who had major psychiatric disorders, were receiving other medications not in the study protocol, had a history of using substances other than tobacco, had a medical disease (including liver disease), had a history of alcohol withdrawal seizures or delirium, had moderate to severe alcohol withdrawal symptoms, had cognitive impairment, had a history of allergy to gabapentin, or who were pregnant or breastfeeding. Duration of follow‐up: 24 weeks Informed consent: all participants provided written informed consent Ethical approvals: study procedures were approved by the PMNIDAT Research Ethics Committee |
|
| Participants |
Sample size: 112 (56 gabapentin, 56 placebo) Description of the target population: Patients with alcohol dependence who are being discharged from inpatient alcohol treatment Age: 42.8 years Sex: 8.7% female Race/Ethnicity: not reported Marital status: 54.8% married Harmful alcohol use (baseline): daily consumption = 330.3 g; 77.9% reported everyday drinking Co‐occurring disorders: Not reported |
|
| Interventions |
Type: pharmacologic Description: Gabapentin following herbal medication and detoxification Duration and frequency: at discharge from alcohol treatment patients received 300 mg/day for 1 week, which then increased to 600 mg and 900 mg/day if they continued drinking alcohol as reported in follow‐up sessions Delivery and provider: oral capsule provided by a pharmacist Comparison group: placebo following herbal medication and detoxification |
|
| Outcomes |
Primary outcome(s): aalcohol consumption Primary outcome measurement tool(s): timeline followback, GGT Secondary outcome(s): Heavy drinking, Adverse events Secondary outcome measurement tool(s): Timeline followback Time points: 1, 2, 4, 8, 12, 20, and 24 weeks |
|
| Notes |
Study funding and conflicts of interest: Princess Mother National Institute on Drug Treatment; Center for Alcohol Studies, Thailand; Fogarty International Center (National Institutes of Health) Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to the gabapentin treatment group (n = 56) or the placebo group (n = 56) by means of block randomization with a block size of four." Pg. 36 |
| Allocation concealment (selection bias) | Low risk | Quote: "Identical capsules of gabapentin and placebo were prepared by a pharmacist who was also in charge of the block randomization." Pg. 36 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients and investigators, i.e., physicians and research nurses, were blinded to the identity of the treatment assignment." Pg. 36 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Twenty subjects in the gabapentin group and fourteen subjects in the placebo group completed the study regimen at the end of week 12, as shown in Fig. 1. The reasons for dropout in the gabapentin group were fever (n = 1) and loss to follow‐up (n = 35). The reasons for dropout in the placebo group were allergic reaction to another medication (trazodone, n = 1), pneumonia (n = 1), and loss to follow‐up (n = 40)." Pg. 36 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Constant 2021.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Brazil Setting: telephone counseling call center Eligibility criteria: adults who self‐reported alcohol use and the desire to stop drinking Duration of follow‐up: 6 months Informed consent: participants provided informed consent Ethical approvals: study procedures were approved by the Committee of Research Ethics at the Federal University of Healthcare Sciences of Porto Alegre ‐ UFCSPA. |
|
| Participants |
Sample size: 1400 (687 ntervention, 713 control) Description of the target population: adults seeking support from a call center to stop drinking Age: 32.9 years (SD = 10.3) Sex: 78% male Race/Ethnicity: not reported Marital status: not reported Harmful alcohol use (baseline): 88% met DSM‐5 criteria for alcohol use disorder Co‐occurring disorders: 11% of participants reported only alcohol consumption, 10.4% reported combined alcohol and cocaine use, 11% reported combined alcohol and tobacco use, 67.6% reported combined alcohol with other illegal drug use |
|
| Interventions |
Type: non‐pharmacologic Description: brief motivational intervention including information and counseling on alcohol and drugs by phone Duration and frequency: 7 telephone contacts (40 minutes each) Delivery and provider: telephone counselors who received training and supervision Comparison group:iInformation and counseling on drugs by phone (7 telephone contacts, 40 minutes each) |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): not reported Secondary outcome(s): Motivation to change behavior; Coping Secondary outcome measurement tool(s): Contemplation ladder; Coping Behaviors Inventory Time points: 1, 2, and 7 days; 1, 2, 3, and 6 months |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The inclusion in each group followed random sampling using a random number technique (Microsoft software application)." Pg. 2 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of providers and participants not possible given the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of attrition (>50%) |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
Correa Filho 2013.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Brazil Setting: 0utpatient alcohol treatment center Eligibility criteria:iIncluded were males ages 18‐60 years with an ICD‐10 diagnosis of alcohol dependence and enrolled in the outpatient alcohol treatment program. Excluded were individuals with significant medical disease that might have interfered with the evaluation of the study medication or presence of a safety concern, positive screen for other types of substance misuse except nicotine, clinically significant psychiatric illness, previous treatment with ondansetron within 6 months of randomization, current use of disulfiram, naltrexone or acamprosate, current use of any psychotropic medication, inability to give full informed consent, and clinical history of developmental disorder Duration of follow‐up: 3‐months Informed consent: all participants provided written informed consent Ethical approvals: The Ethics Committee of the Clinical Hospital of the University of Sao Paulo |
|
| Participants |
Sample size: 52 (50 Odansetron, 52 Placebo) Description of the target population: Males in outpatient treatment for alcohol dependence Age: 42.9 years Sex: 100% male Race/Ethnicity: 33.3% White, 21.6% Black, 45.1% Mixed Marital status: 60.0% married, 15.8% single, 24.2% separated/widowed Harmful alcohol use (baseline): mean AUDIT Score = 29.1 Co‐occurring disorders: mean Hamilton Depression Rating Scale Score = 9.0 |
|
| Interventions |
Type: pharmacologic + non‐pharmacologic Description: Odansetron, brief cognitive behavioral interventions, encouragement to attend Alcoholics Anonymous Duration and frequency: Two 8 mg capsules Odansetron per day for 12 weeks + 12 weekly appointments with cognitive behavioral intervention and encouragement to attend Alcoholics Anonymous Delivery and provider: trained research assistants Comparison group: two placebo capsules per day for 12 weeks + 12 weekly brief cognitive behavioral intervention sessions including encouragement to attend Alcoholics Anonymous |
|
| Outcomes |
Primary outcome(s): abstinence, heavy drinking Primary outcome measurement tool(s): Timeline followback, Hepatic lab indices, Collateral (family) reporting Secondary outcome(s): mean drinks per day, side effects, retention, depression Secondary outcome measurement tool(s): Timeline followback, UKU Side Effect Rating Scale, Obsessive‐Compulsive Drinking Scale Time points: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12‐weeks (Depression, Obsessive‐Compulsive Drinking assessed at weeks 1, 6, and 12) |
|
| Notes |
Study funding and conflicts of interest: The State of Sao Paulo Research Foudation (FAPESP) Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were randomly divided into 2 groups through computer‐generated random numbers" Pg. 2046 |
| Allocation concealment (selection bias) | Low risk | Quote: "Once a week the patients received an envelope with 2 packages containing 7 capsules. One package was designated for morning dosing and the other for night‐time." Pg. 2046 "All capsules in each treatment group were identical in appearance and size and had been manufactured and distributed by the Pharmace Sector" Pg. 2046 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The codes referent to the medications used were revealed to the researchers only after all patients had completed the study. Only 2 pharmacists from the pharmacy sector at the Psychiatric Institute of the Clinical Hospital of the University of Sao Paulo knew what medication corresponded to which specific code. The packages containing the capsules were distributed to patients by 2 trained research assistants, who had also been blinded to the study and who assessed the outcome of each patient throughout the study period." Pg. 2046 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The codes referent to the medications used were revealed to the researchers only after all patients had completed the study. Only 2 pharmacists from the pharmacy sector at the Psychiatric Institute of the Clinical Hospital of the University of Sao Paulo knew what medication corresponded to which specific code. The packages containing the capsules were distributed to patients by 2 trained research assistants, who had also been blinded to the study and who assessed the outcome of each patient throughout the study period." Pg. 2046 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Reasons for dropout were classified as refusal to continue (2%), protocol violation (26%) or lost to follow‐up (72%). Dropout rates among participants randomized to placebo were 57.7% and 42% among those randomized to ondansetron. The overall difference between both groups was not significant" Pg. 2047 "As half of our sample discontinued the treatment, we first used GEE to analyze the data of those who completed it. Little's test was used to verify the missing‐data mechanism. Subsequently, we performed a multiple‐imputation analysis with Markov Chain Monte Carlo approaches and then used GEE in SPSS‐18. Maintaining the original variability of the missing data was achieved by creating imputed values based on variables associated with the causes of missing data." Pg. 2046. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Daengthoen 2014.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Thailand Setting: inpatient psychiatric department Eligibility criteria:iIncluded were men with alcohol dependence who had undergone detox, were able to attend 28 days of inpatient rehabilitation, and voluntarily agreed to participate. Excluded were individuals with serious mental or physical health problems. Duration of follow‐up: 6‐months Informed consent: participants provided written informed consent Ethical approvals: approved by the Ethics Review Committee for Research INvolving Human Research Subjects, Phramongkutklao hospital and the local committee |
|
| Participants |
Sample size: 100 (47 intervention, 53 usual care) Description of the target population: men with alcohol dependence in inpatient treatment Age: 46 years (SD = 8.9, Range = 24‐68) Sex: 100% male Race/Ethnicity: not reported Marital status: not reported Harmful alcohol use (baseline): 59% almost daily drinkers, 19% daily drinkers Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: Inpatient treatment following detoxification based in cognitive behavioral therapy and 12‐step. Five components include the Buddhist 12‐step process, cognitive behavioral therapy, health education, family education, and relaxation therapy. Duration and frequency: 28 days involving 4 hours of therapy per day (5 days/week) Delivery and provider: group therapy sessions delivered by professional staff, non‐professional staff, and people in recovery Comparison group: detoxification, health education, and brief counseling (14 days) |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): self‐reported; Instrument not reported Secondary outcome(s): Quality of life, Self‐efficacy, Readiness to Change Secondary outcome measurement tool(s): WHOQOL‐BREF‐THAI, 5‐point self‐efficacy item, URICA Time points: 1, 3, 6‐months |
|
| Notes |
Study funding and conflicts of interest: Integrated Management of Alcohol Intervention Program (I‐MAP) Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "One hundred alcohol‐dependent patients were randomized by using a computer program in order to assign them into the PMK model group and the usual care group." Pg. 82 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The intent‐to‐treat analysis was applied" Pg. 83 Quote: "In all, 90% of participants completed all two groups and 90.5% (usual care group), 89.4% (PMK model group), respectively completed the 6‐month follow‐up" Pg. 83 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
de Sousa 2004.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: Outpatient hospital Eligibility criteria: included were patients between 18‐65 years of age who met criteria for alcohol dependence and had a stable family environment. Excluded were patients with other substance use and dependence (except nicotine dependence), any comorbid psychiatric disorder that met DSM‐IV criteria (excluding nicotine dependence), any medical condition that would interfere with treatment compliance and is a contraindication for the drugs used int he study, liver function three times higher or greater than normal values, and previous treatment with naltrexone or disulfiram. Duration of follow‐up: 12 months Informed consent: all participants provided written informed consent Ethical approvals: not reported |
|
| Participants |
Sample size: 100 (50 naltrexone, 50 disulfiram) Description of the target population: patients who had completed detox for alcohol dependence in a hospital setting Age: 44.4 years Sex: 100% male Race/Ethnicity: not reported Marital status: 95% married Harmful alcohol use (baseline): severity of alcohol dependence scale mean score=28.5; Addiction severity index mean score=0.71; Composite craving measure mean score=51.5; Mean days of drinking in last 6 months = 87; Typical number of drinks per day = 12.4; Serum GGT=107.5; Serum ALT = 82.5; Serum AST = 65.6; Days between last drink and start of study = 15.5 Co‐occurring disorders: not reported |
|
| Interventions |
Type: pharmacologic + non‐pharmacologic Description: Naltrexone + group psychotherapy Duration and frequency: 50 mg daily dose of Naltrexone, weekly group psychotherapy Delivery and provider: oral administration Comparison group: 250 mg daily of Disulfiram with weekly group psychotherapy |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): self‐reported, serum GGT Secondary outcome(s): relapse, abstinence, retention Secondary outcome measurement tool(s): self‐reported Time points: 12‐months |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The list for randomization was provided by a qualified statistician. Patients were allocated by the clinic staff according to a serial wise number on the list" Pg. 528 |
| Allocation concealment (selection bias) | Low risk | Quote: "The list for randomization was provided by a qualified statistician. Patients were allocated by the clinic staff according to a serialcwise number on the list" Pg. 528 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was an open study and the investigators were not blinded. At the start of the study the investigator was not aware as to the type of treatment that would be more effective. But as the study progressed, there was a better outcome noted with disulfiram that may have resulted in the investigators making more effort to ensure better compliance in that group. This could have, hypothetically, introduced a bias. However, there was also greater improvement seen in the disulfiram group in terms of serum GGT; this provides an objective corroboration of the self‐report made by the patient and investigator‐rated measures" Pg. 530 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "To improve the consistency and independence of the ratings, the final outcomes were rated by a psychologist independent of the study. However, because she was on the staff of the clinic, she was not necessarily blinded to the treatment group in all cases." Pg. 529 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All outcome analyses were conducted under the principle of intention‐to‐treat. Drop‐outs were considered as those who relapsed." Pg. 529 "During the study, one patient dropped out in the naltrexone group due to irregular attendance whereas two dropped out of the disulfiram group ‐ one due to side‐effects and one due to stopping of medication" Pg. 529 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
de Sousa 2005.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: private psychiatric hospital detoxification service Eligibility criteria: included were adults 18‐65 years who met criteria for alcohol dependence and had a stable family environment so the family could ensure treatment compliance and provide follow‐up information. excluded were individuals with other substance use disorders (excluding nicotine dependence), a comorbid psychiatric disorder, any medication condition that would interfere with the intervention, poor liver function, or having been previously treated with disulfiram and/or acamprosate. Duration of follow‐up: 8 months Informed consent: all participants provided written informed consent Ethical approvals: not reported |
|
| Participants |
Sample size: 100 (50 acamprosate, 50 disulfiram) Description of the target population: Patients in detoxification for alcohol dependence Age: 42.2 years Sex: not reported Race/Ethnicity: not reported Marital status: 95% married Harmful alcohol use (baseline): SADS = 26, ASI = 0.73, Days of drinking in last 6 months = 85, Typical number of drinks per day = 11, Days of abstinence = 19.5 Co‐occurring disorders: not reported |
|
| Interventions |
Type: pharmacologic Description: Acamprosate. Weekly group psychotherapy was available, but not prescribed Duration and frequency: 666 mg three times per day Delivery and provider: self‐administered, monitored by family Comparison group: 250 mg disulfiram self‐administered once per day and monitored by the family. Weekly group psychotherapy was also available, but not prescribed |
|
| Outcomes |
Primary outcome(s): alcohol consumption (days to first alcohol intake) Primary outcome measurement tool(s): not reported Secondary outcome(s): days to first relapse, number of drinks taken, Days of abstinence, craving severity, serum GGT, retention Secondary outcome measurement tool(s): craving measure, GGT Time points: 8 months |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The list for randomisation was provided by a qualified statistician." Pg. 545 |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment was allocated by the clinic’s staff according to the serial number on the list." Pg. 545 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were also told that the drug given to them would be chosen at random but they would know which drug they were receiving." Pg. 546 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "To improve the consistency and independence of the ratings the final outcomes were rated by a psychologist independent of the study. Since she was on the staff of the clinic, she was not blinded to the treatment group in all cases." Pg. 546 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "During the study, three dropped out of the ACP group due to irregular attendance while four dropped out of the DSF group—three due to side effects (neuropathy) and one due to stoppage of medication." Pg. 546 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
de Sousa 2008.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: outpatient hospital Eligibility criteria: included were individuals who completed detoxification and met criteria for alcohol dependence between ages 18‐65 years and had a stable family environment. Excluded were individuals with the presence of other substance use disorders (excluding nicotine dependence), the presence of any comorbid psychiatric disorder, any medical condition present that would interfere with treatment compliance or be a contraindication to the drugs in the study, any routine liver function tests more than three times greater than the normal value, or previous treatment with any topiramate or disulfiram. Duration of follow‐up: 9 months Informed consent: all participants provided written informed consent Ethical approvals: not reported |
|
| Participants |
Sample size: 100 (50 disulfiram, 50 topiramate) Description of the target population: patients who had completed detox for alcohol dependence in a hospital setting Age: 43.3 years Sex: 100% male Race/Ethnicity: not listed Marital status: 98% married Harmful alcohol use (baseline): Average Substance Abuse Dependence Scale score = 27, Average Addiction Severity Index score=0.71, Average craving score=54.5, Number of drinks per day = 10, Serum GGT = 113.5, Serum ALT = 70, Serum AST = 67.5, Days of abstinence = 19.5 Co‐occurring disorders: Not reported |
|
| Interventions |
Type: pharmacologic + non‐pharmacologic Description: Disulfiram + group psychotherapy Duration and frequency: 250 mg of disulfiram once per day + weekly group psychotherapy Delivery and provider: disulfiram administered orally and psychotherapy provided by clinic staff Comparison group: 50 mg of topiramate orally administered three times per day + weekly group psychotherapy |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): Self‐reported Secondary outcome(s): Relapse, abstinence, Craving, GGT, Retention, Side effects Secondary outcome measurement tool(s): self‐reported Time points: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36‐weeks |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a qualified statistician. Treatment was allocated by the clinic's staff according to the serial number on the list" Pg. 461 |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by a qualified statistician. Treatment was allocated by the clinic's staff according to the serial number on the list" Pg. 461 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Open, nonblinded, randomized study" Pg. 461 "Patients were also told that the drugs given to them would be chosen at random but that they would know what drug they were receiving" Pg. 461 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This was an open study and the investigators were not blinded" Pg. 463 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Dutra Ponce 2021.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Brazil Setting: primary healthcare Eligibility criteria: adult women seeking primary care with hazardous or harmful alcohol use (assessed using the AUDIT) Duration of follow‐up: 3 months Informed consent: all participants provided written informed consent. Ethical approvals:study procedures were approved by an IRB. Institution is not specified. |
|
| Participants |
Sample size: 44 (21 intervention, 23 control) Description of the target population: women in primary care with hazardous or harmful alcohol use Age: not reported Sex: 100% female Race/Ethnicity: not reported Marital status: Not reported Harmful alcohol use (baseline): AUDIT = 12.2 (SD = 2.6) Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: brief intervention based on the FRAMES and incorporating gender issues and norms related to alcohol consumption Duration and frequency: 1 session (20‐30 minutes) Delivery and provider: nurse Comparison group: Five minutes of brief advice on alcohol consumption |
|
| Outcomes |
Primary outcome(s): Hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): frequency of alcohol use; Amount of alcohol use Secondary outcome measurement tool(s): 2 items to assess frequency of alcohol use Time points: 0, 1, 3 months |
|
| Notes |
Study funding and conflicts of interest:nNot reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization process involved generating a numerical list (www.randomization.com) allocating participants who met the inclusion criteria to two groups: the Control Group (CG) or the Intervention Group (IG)." Pg. 738 |
| Allocation concealment (selection bias) | Low risk | Quote: "Based on the numerical list, numbered and sealed envelopes containing cards with letters assigning the participants to either the CG or IG were prepared. The envelopes were prepared by someone not involved in the research." Pg. 738 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind providers or participants given the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High levels of loss to follow‐up (>50%) |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
Furieri 2007.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Brazil Setting: outpatient substance use treatment center Eligibility criteria: included were individuals 18‐65 years of age who had consumed at least an average of 35 drinks per week during the last year and at least 35 drinks per week during the past 90 days, were abstinent from alcohol for no longer than 14 days prior to baseline, met criteria (DSM‐IV) for alcohol dependence, had normal serum liver transaminases, had plasma gamma‐glutamyl transferase (GGT) levels less than 900 UL, and were able to read, write and speak in Portuguese. Excluded were people with dementia, people with severe withdrawal signs or symptoms, people who met diagnostic criteria for other substance intoxication or withdrawal, people with unstable mental or medical disorders other than alcohol dependence (except nicotine or caffeine), people who had convulsions or delirium tremens during abstinence from alcohol, people who used pharmacologic agents to reduce the convulsive threshold or alter alcohol withdrawal/craving during the past 30 days, and people who had previous history of drug hypersensitivity or adverse reactions to gabapentin, diazepam, or other benzodiazepines/haloperidol. Duration of follow‐up: 1 month Informed consent: all participants provided written informed consent Ethical approvals: Brazilian Institutional Review Board at the Federal University of Espirito Santo Health Science Center |
|
| Participants |
Sample size: 60 (30 gabapentin, 30 placebo) Description of the target population: males with alcohol dependence in outpatient treatment who had undergone detox Age: 44.27 years Sex: 100% male Race/Ethnicity: not reported Marital status: 36.67% married Harmful alcohol use (baseline): average age of onset = 16.22 years, Drinking years = 27.82 years, Drinks per day in the last 90 days = 33.57 days, Days of abstinence before baseline = 8.42 days, 60% attended the emergency department because of alcohol use at least once, 51.67% received prior treatment for alcohol dependence Co‐occurring disorders: not reported |
|
| Interventions |
Type: pharmacologic Description: gabapentin Duration and frequency:u up to 600 mg/day for 4 weeks Delivery and provider: oral Comparison group: 2 placebo tablets per day for 4 weeks |
|
| Outcomes |
Primary outcome(s): gamma glutamyltransferase (GGT) Primary outcome measurement tool(s): GGT test Secondary outcome(s): not reported Secondary outcome measurement tool(s): not reported Time points: 0, 28 days |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization code was held by a research supervisor, to be broken only in case of emergency. It is important to note that the code was not broken until the study was completed" Pg. 1692. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Gamma‐glutamyl transferase gamma‐glutamyl transferase"Subjects and psychiatrist were blind to the treatment condition" Pg. 1692 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Ganavadiya 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: inpatient substance use treatment center Eligibility criteria: included were patients admitted to the addiction treatment center with addiction to tobacco and alcohol, having a high level of tobacco dependence (Fagerstrom scale>5), and agreed to stay as inpatients in the treatment center for 25‐30 days. Excluded were participants with addiction only to alcohol and/or other substances and not tobacco, who were undergoing pharmacological interventions during the study period, who had known drug hypersensitivities, patients with epilepsy, pregnant, lactation, serious physical comorbidities, or low levels of dependence. Duration of follow‐up: 2 months Informed consent: not reported Ethical approvals: not reported |
|
| Participants |
Sample size: 83 (27 reading‐writing therapy, 28 games‐narrative therapy, 28 motivational intervention only) Description of the target population: patients in inpatient treatment with alcohol and tobacco dependence Age: 32.2 years (SD=9.12) Sex: 100% male Race/Ethnicity: not reported Marital status: not reported Harmful alcohol use (baseline): practice: 2.89 (SD = 1.56) Co‐occurring disorders: 100% tobacco dependence |
|
| Interventions |
Type: non‐pharmacologic Description: A) Reading‐writing therapy + motivational intervention: use of self‐help materials to motivate and guide behavior change. In each group session participants were given autobiographies of previous patients who had successful substance use treatment outcomes to read. In writing therapy, participants were given a diary and pen. They were asked to express their experience with substance use, adverse consequences, and potential benefits of reducing their substance use in these diaries; B) Games‐narrative therapy + motivational intervention: Cognitive, behavioral and motivational approaches to promote interaction between patients and divert participant's attention to rigorous activity to mask craving and withdrawal symptoms. The games included short mind games and puzzles. The narrative/story therapy consisted of sharing stories related to their personal life and addiction, including the relationship between their substance use and personal life circumstances. Delivery and frequency: A) not reported, group‐based; B) 2 hours per day for 25‐30 days of inpatient treatment, group or individual sessions Duration and provider: A) trained counselors Comparison group: motivational interviewing consisting of group counseling, individual counseling, and Anonymous meetings over the course of 25‐30 days of inpatient treatment; Provider was a trained counselor |
|
| Outcomes |
Primary outcome(s): alcohol practice Primary outcome measurement tool(s): Knowledge, Attitudes, and Practices related to alcohol Secondary outcome(s): none Secondary outcome measurement tool(s): N/A Time points: 1, 2 months |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The randomization and group allocation were done on a quota basis in the de‐addiction center by the center in charge. The first five eligible participants admitted to the de‐addiction center were allotted to Group A, and then, the next five eligible participants to Group B and Group C, respectively. This group allocation strategy was followed as it was convenient to administer the intervention. The group code was entered on the assessment form of the participant following randomization and the information on the actual intervention offered to different groups was concealed from the investigator to ensure blinding in the study." Pg. 3 |
| Allocation concealment (selection bias) | High risk | Quote: "The randomization and group allocation were done on a quota basis in the de‐addiction center by the center in charge. The first five eligible participants admitted to the de‐addiction center were allotted to Group A, and then, the next five eligible participants to Group B and Group C, respectively. This group allocation strategy was followed as it was convenient to administer the intervention. The group code was entered on the assessment form of the participant following randomization and the information on the actual intervention offered to different groups was concealed from the investigator to ensure blinding in the study." Pg. 3 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention Quote: "The randomization and group allocation were done on a quota basis in the de‐addiction center by the center in charge. The first five eligible participants admitted to the de‐addiction center were allotted to Group A, and then, the next five eligible participants to Group B and Group C, respectively. This group allocation strategy was followed as it was convenient to administer the intervention. The group code was entered on the assessment form of the participant following randomization and the information on the actual intervention offered to different groups was concealed from the investigator to ensure blinding in the study." Pg. 3. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Go 2020.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Vietnam Setting: HIV clinics Eligibility criteria: adults living with HIV who are receiving antiretroviral therapy, report hazardous alcohol use, and plan to reside in the study area for 24 months were included. Participants reporting high risk of alcohol withdrawal were excluded. Duration of follow‐up: 12 months Informed consent: all participants provided written informed consent Ethical approvals: study procedures were approved by institutional review boards at the University of North Carolina Gillings School of Global Public Health, the Johns Hopkins Bloomberg School of Public Health, and the Thai Nguyen Center for Preventive Medicine |
|
| Participants |
Sample size: 440 (147 MET+CBT, 147 MET only, 146 usual care) Description of the target population: people in treatment for HIV and reporting hazardous alcohol use Age: 40.2 years (SD = 5.8) Sex: 96.8% male Race/Ethnicity: not reported Marital status: 72.3% married Harmful alcohol use (baseline): median AUDIT score = 12 (IQR = 9, 16) Co‐occurring disorders: 100% HIV positive; 19.5% mild depression; 5.7% moderate or more severe depression; 3.4% anxiety |
|
| Interventions |
Type: non‐pharmacologic Description: 1) Brief intervention; 2) Brief intervention and cognitive behavioral therapy Duration and frequency: Brief intervention was administered through 2 face‐to‐face sessions and 2 telephone booster sessions. Cognitive behavioral therapy was delivered in 6 face‐to‐face sessions and 3 optional group sessions. Delivery and provider: paraprofessional counselors Comparison group: standard recommendation from HIV clinican to decrease alcohol use and referral to harm reduction services |
|
| Outcomes |
Primary outcome(s): per cent days abstinent, viral suppression Primary outcome measurement tool(s): Timeline followback Secondary outcome(s): number of drinks per drinking day; number of heavy drinking days Secondary outcome measurement tool(s): Timeline followback, Phosphatidylethanol (PEth) blood test Time points: 0, 3, 6, 12 months |
|
| Notes |
Study funding and conflicts of interest: National Institute on Drug Abuse (R01DA037440) and the University of North Carolina at Chapel Hill Center for Aids Research, funded by the National Institutes of Health (P30AI050410) Linked study records: Blackburn 2021 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization and Masking: Participants were randomly assigned to the combined intervention, the brief intervention, or the SOC group at a ratio of 1:1:1. To achieve this, a randomization schedule was generated in SAS version 9.4 (SAS Institute) using permuted‐block randomization with a block size of 3, such that each block contained a random assignment to each of the 3 groups. Participants randomly assigned within 1 triplet block all belonged to the same ART clinic." Pg. 3 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Masking in the field was not feasible due to the nature of the intervention; however, analysts and principal investigators were masked to study assignment until the analyses for this paper were complete." Pg. 3 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Masking in the field was not feasible due to the nature of the intervention; however, analysts and principal investigators were masked to study assignment until the analyses for this paper were complete." Pg. 3 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Excluding participants who died, 405 of 435 participants (93.1%) completed the 3‐month follow‐up, 410 of 432 (94.9%) completed the 6‐month follow‐up, and 390 of 425 (91.8%) completed the 12‐month follow‐up. The most common reasons for missed visits at 12 months were incarceration and relocation. By the 12‐month visit, 390 of 440 participants (88.6%) provided abstinence and viral load outcomes." Pg. 5 |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
Harder 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Kenya Setting: primary health center Eligibility criteria: included were adults in primary health centers who screened positive for alcohol problems Duration of follow‐up: 6 months Informed consent: all participants provided informed consent Ethical approvals: Kenya Medical Research Institute, University of Vermont |
|
| Participants |
Sample size: 300 (92 in‐person motivational interviewing, 104 mobile motivational interviewing, 104 wait‐list control) Description of the target population: adults in primary care with alcohol problems Age: 38 (11.7) Sex: 22% female Race/Ethnicity: not reported Marital status: 60% married Harmful alcohol use (baseline): not reported Co‐occurring disorders: 47% in comprehensive care for HIV/AIDS |
|
| Interventions |
Type: non‐pharmacologic Description: A) Motivational interviewing to encourage motivation for change in alcohol use delivered via mobile phone; B) In‐person single motivational interviewing session Duration and frequency: single 30‐minute session Delivery and provider: elivered by a trained clinician (nurse, clinical psychologist, medical doctor) by A) mobile phone or B) In‐person Comparison group: wait‐list control |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): AUDIT‐C Secondary outcome(s): None Secondary outcome measurement tool(s): N/A Time points: 0, 1, 6 months |
|
| Notes |
Study funding and conflicts of interest: National Institutes of Health, Fogarty International Center (R21TW009786) Linked study records: Harder 2018 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote "The experimental intervention, mobile MI, was prioritized in the randomization each day by the clinicians with the first eligible participant randomized to mobile MI (n = 104), followed by waiting‐list (n = 104) and third to in‐person MI (n = 92)." Pg. 4 |
| Allocation concealment (selection bias) | High risk | Quote "The experimental intervention, mobile MI, was prioritized in the randomization each day by the clinicians with the first eligible participant randomized to mobile MI (n = 104), followed by waiting‐list (n = 104) and third to in‐person MI (n = 92)." Pg. 4 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No masking (NCT03573167) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No masking (NCT03573167) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote "An intention‐to‐treat (ITT) approach was used, and missing AUDIT‐C data at 1 and 6 months due to lost to follow‐up were filled in with baseline AUDIT‐C scores" Pg. 5 |
| Selective reporting (reporting bias) | Low risk | Primary outcomes align with those reported in the trial registration (NCT03573167) |
Hartmann 2020.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: community‐based organization providing self‐help groups Eligibility criteria: included were married couples where the female partner was between 18‐40 years of age, reports that her male partner has a drinking problem, reports ever experiencing intimate partner violence by her current partner, and both partners speak Kannada. Excluded were couples if the male partner had severe alcohol dependence, the female partner reported six or more occurrences of severe physical or sexual intimate partner violence in the past 6 months, or at least one member of the couple feared that the intervention may increase violence. Duration of follow‐up: 4 months Informed consent: both the male and female partner provided informed consent Ethical approvals: study procedures were approved by the St. John's Medical College Hospital |
|
| Participants |
Sample size: 67 couples (24 Counseling + Incentives, 22 Incentives, 21 Breathalyzer only) Description of the target population: couples engaged with community‐based organizations who report intimate partner violence and male partner alcohol problems Age: Females: median age 29 years; Males: median age 35 years Sex: 100% couples included 1 female and 1 male partner Race/Ethnicity: not reported Marital status: 100% married; 53% married for 10 or more years Harmful alcohol use (baseline): 95% male partners drink at least 1 day/week in the past month; 28% male partners drink every day, 40% drink a few times per week Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: A) Incentives and Counseling: Twice‐daily reminders to complete breathalyzer screen for 4 weeks and offered a monetary incentive for each negative BrAC score (i.e., contingency management) + behavioral couples therapy covering topics such as alcohol use and communication; B) Incentives only Duration and frequency: A) Incentive + Counseling: Breathalyzer 2x per day (random timing) for 1 month + 4 weekly counseling sessions lasting 1 hour per session; B) Incentive: Breathalyzer 2x per day (random timing) for 1 month; C) Control: Breathalyzer every other day (random timing) for 1 month Delivery and provider: counseling was delivered by a lay counselor with prior social work experience; Breathalyzers were self‐administered by the male partner Comparison group: completing a breathalyzer screen every other day without any monetary incentive beyond participation fee |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): Soberlink Breathalyzer (Breath Alcohol Content; BrAC) Secondary outcome(s): intimate partner violence Secondary outcome measurement tool(s): Indian Family Violence and Control Scale Time points: 0, 1, 4 months |
|
| Notes |
Study funding and conflicts of interest: none Linked study records: Hartmann 2018 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Participants will be randomized at the couple‐level prior to the start of the intervention by a statisticians using a random number generator" (Hartmann 2018) |
| Allocation concealment (selection bias) | Low risk | Quote "Their randomization assignment will be revealed during an orientation session using a sealed envelope with their study ID" (Hartmann 2018) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote "Attrition was relatively equal across arms... Once enrolled in the study, retention was high, with 95% of couples continuing through the end of the intervention and 90% completing the 4‐month follow‐up survey" Pgs. 10‐11 |
| Selective reporting (reporting bias) | High risk | Not all outcomes described in study protocol (Hartmann 2018) are reported in the final publication |
Huis In't Veld 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: South Africa Setting: outpatient clinics Eligibility criteria: included were patients with HIV‐1 who were 18+ years of age, visiting HIV outpatient treatment clinics, and met criteria for hazardous or harmful alcohol use. Excluded were patients with mental impairment, pregnant women, or those who were already undergoing treatment for unhealthy alcohol use. Duration of follow‐up: 12‐months Informed consent: all participants provided informed consent Ethical approvals: all procedures were approved by the Medunsa Research and Ethics Committee and the Tshwane Research Committee |
|
| Participants |
Sample size: 560 (267 brief intervention, 293 health education leaflet) Description of the target population: HIV‐positive outpatients Age: 36 (range: 31‐42) Sex: 46.1% female Race/Ethnicity: not reported Marital Status: 28.4% married Harmful alcohol use (baseline): 11.94 AUDIT Co‐occurring disorders: 100% HIV‐positive |
|
| Interventions |
Type: non‐pharmacologic Description: WHO Brief intervention including personalized feedback on AUDIT results, a health education leaflet with information about reducing alcohol use, and advice with brief counseling on reducing excessive drinking Duration and frequency: 1 session lasting an average of 40 minutes Delivery and provider: research assistants (4 nurses) Comparison group: health education leaflet with information on responsible drinking and drinking limits provided by a research assistant/nurse. The single session lasted 35 minutes on average |
|
| Outcomes |
Primary outcome(s):hazardous alcohol use, harmful alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): abstinence/low risk alcohol use Secondary outcomes measurement tool(s): AUDIT Time points: 3, 12‐months |
|
| Notes |
Study funding and conflicts of interest: Foundation for Alcohol Research (ABMRF), the Directorate General for Development Cooperation through the Flemish Interuniversity Council (VLIR‐UOS) Linked study records: Huis In't Veld 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomization was done by the trial coordinator (not involved in inclusion of patients) by concealed, centrally allocated computer‐generated random numbers and made available for the research assistants in envelopes." Pg. 3 |
| Allocation concealment (selection bias) | Low risk | Quote "Randomization was done by the trial coordinator (not involved in inclusion of patients) by concealed, centrally allocated computer‐generated random numbers and made available for the research assistants in envelopes." Pg. 3 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote "A limitation of the study was that there was no blinding, neither for study nurses nor for statisticians" Pg. 13 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote "A limitation of the study was that there was no blinding, neither for study nurses nor for statisticians, and the lack of a biomarker to assess the effect of the intervention. Blinding was intended, as described in the original study protocol, with nurses switching from one clinic to the other after inclusion. However, the inclusion period was longer than intended, giving an overlap of inclusion and follow up points (whereby the nurses recognized the patients and the group they were randomized into). Also, patients did not show up for follow up themselves, but the nurses had to recognise them in the waiting room and ask if they wanted to come for a follow up interview." Pg. 13 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Reported outcomes align with those reported in study protocol (Huis In't Veld 2012) |
Jirapramukpitak 2020.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Thailand Setting: community/home visits Eligibility criteria: adults with alcohol dependence who have not engaged in alcohol treatment in the past 3 months Duration of follow‐up: 16 weeks Informed consent: all participants provided informed consent Ethical approval: study procedures were approved by the ethics committee of Thammasat University |
|
| Participants |
Sample size: 161 (80 intervention, 81 control) Description of the target population: People with alcohol dependence in the community Age: 50.1 years (SD = 11.5) Sex: 75.2% male Race/Ethnicity: not reported Marital status: 59.6% married Harmful alcohol use (baseline): 41% had a positive breathalyzer sample Co‐occurring disorders: 3.7% had a comorbid psychiatric illness |
|
| Interventions |
Type: non‐pharmacologic Description: home visits with contingency management (2 doses: high value prizes, low value prizes) Duration and frequency: 40 home visits (10‐15 minutes each) over 12 weeks Delivery and provider: Village health volunteers Comparison group: 40 home visits focused on giving advice on alcohol use |
|
| Outcomes |
Primary outcome(s): abstinence Primary outcome measurement tool(s): breathalyzer Secondary outcome(s): current psychological distress Secondary outcome measurement tool(s): Addiction Severity Index Time points: 2, 3, 8, 12, 16 weeks |
|
| Notes |
Study funding and conflicts of interest: Thai Health Promotion Foundation Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "The unit of randomization was the individual participant. Random‐ ization of participants to different arms was carried out at the Coor‐ dinating Centre in the Mental Health Clinic of Thammasat University Hospital using a standard randomization table (Pocock, 1983). Strat‐ ified randomization procedures were followed to randomly assign participants to HV, CM‐L and CM‐H arms in a 2:1:1 ratio. The variables on which groups were stratified included: age (greater than 50), gender and current psychological distress." Pg. 173 |
| Allocation concealment (selection bias) | Unclear risk | Quote "In order to keep both the HV and CM groups of similar size, random allocation was done in blocks of 4." Pg. 173 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not able to mask providers or participants given nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition and levels of missing data |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
Jordans 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Nepal Setting: community‐ and facility‐based primary care Eligibility criteria: included were adults with alcohol use disorder. Excluded were individuals that were pregnant, needed urgent medical treatment, were diagnosed with psychosis or epilepsy, or were unable to communicate clearly. Duration of follow‐up: 12‐months Informed consent: all participants provided informed consent Ethical approvals: study procedures were approved by the Nepal Health Research Council (NHRC), the University of Cape Town, and the World Health Organization |
|
| Participants |
Sample size: 162 (80 Counseling for Alcohol Problems, 82 mhGAP/treatment as usual) Description of the target population: adults with alcohol problems in the community Age: 16‐30: 13.0%; 30‐50: 59.9%, >50: 26.5% Sex: 16.0% female Race/Ethnicity: not reported Marital status: 93.8% married Harmful alcohol use (baseline): AUDIT median between 26‐27 Co‐occurring disorders: PHQ‐9 median between 7.5‐9 |
|
| Interventions |
Type: non‐pharmacologic Description: counseling for alcohol problems: a motivational interviewing‐based intervention consisting of personal assessment, developing cognitive‐behavioral skills, and relapse prevention/management Duration and frequency: 4 individual sessions delivered weekly Delivery and provider: community‐based counselors Comparison group: treatment as usual (mhGAP), which includes assessment, psychoeducation, pharmacologic management, and referral |
|
| Outcomes |
Primary outcome(s): Hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): disability, low‐risk drinking Secondary outcome measurement tool(s): WHODAS, AUDIT Time points: 3, 12‐months |
|
| Notes |
Study funding and conflicts of interest: UK Department of International Development, National Institutes of Health Linked study records: ISRCTN Protocol ISRCTN72875710 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After each participant completed their baseline assessments for the cohort study (without imposing a randomisation constraint), randomisation was done by the research coordinator in Kathmandu (N.P.L.) by using computer‐generated random numbers (in SPSS Version 22 for Windows). A list of numbers (1–400) was randomised so that each number corresponded to either the treatment or control group." Pg. 486 |
| Allocation concealment (selection bias) | Low risk | Quote:"The ID code of each new eligible participant was sent to the research coordinator, who then matched it to the next number on the list." Pg. 486 "For those allocated to the treatment condition, the ID code was send to the study field coordinator and clinical supervisor so that they could connect these respondents to research assistant and community counsellors, respectively." Pg. 486 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"participants could not be blinded" Pg. 487 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Research assessors were blinded to participants’ assignment to study arms" Pg. 487 Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:"In the AUD trial, participants retained in and lost to follow‐up from the study differed in terms of religion and education." Pg. 488 "All outcome analyses were on an intention‐to‐treat basis with use of multiple imputation for those with missing outcome data. Continuous variables were imputed using Poisson models, whereas binary variables were imputed using logistic regression. Sensitivity analyses were conducted by running the same analyses among all participants with available data, without imputation." Pg. 488 |
| Selective reporting (reporting bias) | Low risk | Article reports on primary outcomes from study protocol |
Kamal 2020.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: university Eligibility criteria: students were included if they screened positive for hazardous alcohol use. Students with harmful alcohol use or displaying severe withdrawal symptoms were excluded. Duration of follow‐up: 3 months Informed consent:all participants provided informed consent Ethical approvals: study procedures were approved by the PGIMER Institute Ethics Committee |
|
| Participants |
Sample size: 130 (64 intervention, 66 control) Description of the target population: university students with hazardous alcohol use Age: 19 years Sex: 80.8% male Race/Ethnicity: not reported Marital status: not reported Harmful alcohol use (baseline): mean AUDIT score is 9.53 (SD=2.0) in intervention group and 9.6 (SD = 2.1) in control group Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: Brief intervention based on the FRAMES model involving personalized feedback, advice, menu of options, empathy, self‐efficacy, pros/cons, intent/reason to quit, brainstorm alternatives and means to quit, and goal setting. Duration and frequency: one session (15‐20 minutes) Delivery and provider: trained nurse Comparison group: Brief advice involving AUDIT feedback (1 session) |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): binge drinking, transition from high to low‐risk alcohol use Secondary outcome measurement tool(s): AUDIT Time points: 0, 3 months |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "One of the investigators (DK) had generated a random sequence by the Statistical Package for Social Sciences (16.0). The nature of intervention was decided on the odd and even numbers. The sequen‐ tially numbered opaque sealed envelopes were used for allocation concealment. The envelopes would be opened by DK immediately before the intervention and she would telephonically inform the nurse, KK, about the type of the intervention." Pg. 286 |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequentially numbered opaque sealed envelopes were used for allocation concealment." ‐ Pg. 286 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind the providers and participants given the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "we used the self‐administered paper version of the AUDIT, for initial screening as well as for follow‐up. Therefore, the participants them‐ selves were outcome assessors. Hence, the study could be considered as double‐blind, because although the nurse could know the group of each subject, the AUDIT form was completed by the subject, not the nurse." Pg. 286 Outcome assessment based on self report by unblinded participant |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Data were analyzed by using Statistical Package for Social Sciences (IBM SPSS Statistics 16.0). Last observation carried forward method was used to replace missing values (loss to follow‐up)." Pg. 286 |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
Kane 2022.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Zambia Setting: HIV clinics Eligibility criteria: adults living with HIV who were receiving HIV treatment services in Lusaka with current hazardous (moderate‐severe) alcohol use and either depression, post‐traumatic stress/anxiety, and/or other drug use problems. Duration of follow‐up: 6 months Informed consent: all participants provided informed consent Ethical approvals: ethical approval obtained from the institutional review boards at Johns Hopkins Bloomberg School of Public Health, Columbia University Medical Center, University of Zambia Research Ethics Committee, and the National Health Research Authority in Zambia. |
|
| Participants |
Sample size: 160 (82 intervention, 78 control) Description of the target population: people in treatment for HIV with unhealthy alcohol use Age: 40.2 years (SD = 9.3) Sex: 56% male Race/Ethnicity: not reported Marital status: 62% currently married Harmful alcohol use (baseline): average AUDIT score at baseline was 21.7 (95% CI: 20.0 to 23.5) in brief intervention + CETA and 21.5 (95% CI: 19.7 to 23.3) in brief intervention alone Co‐occurring disorders: all participants with moderate risk alcohol use had co‐occurring depression, post‐traumatic stress symptoms, anxiety, and/or other drug use problems. |
|
| Interventions |
Type: non‐pharmacologic Description: Brief intervention + Common Elements Treatment Approach (CETA): Brief intervention is based on motivational interviewing skills and cognitive behavioral therapy lasting 30‐40 minutes for a single session. CETA is a transdiagnostic, modular psychotherapy based on cognitive behavioral elements lasting multiple sessions. Duration and frequency: 1 session of brief intervention and 6‐12 CETA sessions Delivery and provider: HIV peer educators Comparison group: Brief intervention alone |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): depression, post‐traumatic stress disorder, recent substance use Secondary outcome measurement tool(s): Center for Epidemiological Studies‐Depression, CES‐D (Depression); Harvard Trauma Questionnaire, HTQ (Post‐traumatic stress symptoms); Alcohol, Smoking, and Substance Involvement Screening Test, ASSIST (Substance use) Time points: 6 months |
|
| Notes |
Study funding and conflicts of interest: National Institute on Alcohol Abuse and Alcoholism (R34AA027200) Linked study records: |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomized 1:1 to either BI alone or BI plus CETA using blocks of N = 20 stratified by clinic and sex. A statistician not otherwise associated with the study generated four randomization lists: one each for males and females at each of the two clinics. The purpose of having separate lists by clinic and gender was to facilitate approxi‐ mately equal numbers of BI alone and BI + CETA assign‐ ments within each clinic and within both male and female participants. Within each list, the randomization assignments were in random order in blocks of 20 such that 10 were BI alone and 10 were BI + CETA." Pg. 3 |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization assignments were contained within sealed envelopes organized in sequential order in locked cabinets in each of the HIV clin‐ ics. Envelopes for males and females were kept separately." Pg. 3 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study counselors, clinical team, study coordinator, and participants were not blinded." Pg. 3 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "ACASI masked the outcomes assessment and data analysis was also conducted blind." Pg. 3 Outcome assessment based on self report by unblinded participant |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Moderate attrition, but consistent across study conditions |
| Selective reporting (reporting bias) | Low risk | Outcomes align with trial registration |
Kumar 2020.
| Study characteristics | ||
| Methods |
Study design: randomized controlled cross‐over trial Country: India Setting: 0utpatient alcohol treatment Eligibility criteria: individuals with alcohol dependence seeking outpatient treatment and reporting no other substance use Duration of follow‐up: 6 months Informed consent: all participants provided written informed consent Ethical approvals: ethical review obtained. Institution not specified. |
|
| Participants |
Sample size: 90 (30 naltrexone, 30 acamprosate, 30 baclofen) Description of the target population: Males in outpatient alcohol treatment Age: not reported Sex: 100% male Race/Ethnicity: not reported Marital status: >85% Harmful alcohol use (baseline): mean duration of alcohol intake was 18.58 years (SD = 10.62) in baclofen group, 15.43 years (SD = 8.76) in acamprosate group, and 15.75 years (SD = 10 years) in naltrexone group. Co‐occurring disorders: not described |
|
| Interventions |
Type: pharmacologic Description (Group 1): Baclofen Duration and frequency Group 1): started with 20 mg/day and increased to an average of 38.11 mg/day Description (Group 2): Acamprosate Duration and frequency Group 2): started with 333 mg/day and increased to an average of 1021.2 mg/day Description (Group 3): Naltrexone Duration and frequency Group 3): started with 25 mg/day and increased to 50 mg/day Delivery and provider: not described |
|
| Outcomes |
Primary outcome(s): relapse Primary outcome measurement tool(s): Obsessive Compulsive Drinking Scale Secondary outcome(s): risk of relapse Secondary outcome measurement tool(s): Advance Warning of Relapse (AWARE) Questionnaire Time points: 6 months |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of the participants was done using a computerized random table." Pg. 2 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Our study was not blinded" Pg. 6 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Our study was not blinded" Pg. 6 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout not equal across conditions (Table 5) |
| Selective reporting (reporting bias) | Unclear risk | Not described |
L'Engle 2014.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Kenya Setting: HIV drop‐in centers Eligibility criteria: included were females who have exchanged sex for gifts/money in the past 6 months, were at least 18 years old, planning to live in Mombasa for the next 12 months, were registered with the HIV drop‐in centers, were moderate risk drinkers (7‐19 AUDIT scores), and had laboratory confirmed negative results for gonorrhea, chlamydia, and trichomoniasis at enrollment. HIV status did not affect enrollment eligibility. Duration of follow‐up: 12‐months Informed consent: all participants provided written informed consent Ethical approvals: Kenyatta National Hospital Ethical Research Committee and FHI 360 Protection of Human Subjects Committee |
|
| Participants |
Sample size: 818 (410 intervention, 408 control) Description of the target population: female sex workers Age: 27.5 years (SD = 6.6, Range = 18‐54) Sex: 100% female Race/Ethnicity: not reported Marital status: 3.4% married Harmful alcohol use (baseline): average AUDIT Score=13.6 (SD=3.6, Range=7‐19); 65% hazardous drinkers; 35% Harmful drinkers Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: motivational interviewing based on the WHO brief intervention for alcohol use. Intervention contained elements of stages of change and social cognitive health behavior change theories, motivational interviewing, goal‐setting, increasing self‐efficacy, provision of positive feedback and encouragement for change, and review of AUDIT screening results. Duration and frequency: 6 monthly 20‐minute sessions Delivery and provider: nurse counselors Comparison group: Equal‐attention nutrition intervention including assessment of women's nutritional status, addressing nutritional needs for women, children, and other key groups delivered by nurse counselors (6 monthly 20‐minute sessions) |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): self‐reported frequency of drinking (Note: AUDIT administered, but not used because not sensitive to change) Secondary outcome(s): binge drinking, binge drinking before sex with clients, binge drinking before sex with nonpaying partners, sexual violence from clients in past 30 days, sexual violence from nonpaying partners in past 30 days, Secondary outcome measurement tool(s): self‐reported (Likert scale) Time points: 6, 12‐months |
|
| Notes |
Study funding and conflicts of interest: Public Health Evaluation (PHE) component of the President's Emergency Plan for AIDS Relief (PEPFAR; PHE #KE09.0235). Funding was provided through the US Agency for International Development (USAID) under the terms of AID‐623‐A‐11‐00007. Linked study records: Parcesepe 2016; NCT101756469 (clinicaltrials.gov) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random permuted blocks method was used to randomly assign women to the brief intervention for alcohol use or an equal‐attention nutrition intervention control group in a 1:1 ration, separately for each [Drop‐In Center]. A statistician not otherwise involved in the study generated the randomization sequences using the random function RANUNI in SAS" Pg. 447 |
| Allocation concealment (selection bias) | Low risk | Quote: "A statistician not otherwise involved in the study generated the randomization sequences using the random function RANUNI in SAS and produced written assignments sealed in individual tamper‐evidence opaque envelopes. The envelopes were fully protected until the site coordinator confirmed the prospective participant's eligibility, obtained written informed consent, and collected all baseline data." Pg. 447 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Given the nature of the intervention, study participants and site staff could not be masked to treatment allocation" Pg. 447 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "...study investigators and analysts were masked until data handling and analysis decisions were finalized" Pg. 447 Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analysis of primary and secondary outcomes was by intention to treat. All participants who attended at least 1 scheduled visit were included in the analysis. All end point definitions and key data handling and analysis decisions were specified before unblinding" Pg. 448 "Completion rates were high with 752 participants (92%) completing at least 1 follow‐up data collection visit and were comparable between groups" Pg. 449 |
| Selective reporting (reporting bias) | High risk | Did not report primary outcome, AUDIT |
Likhitsathian 2013.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Thailand Setting: residential treatment center Eligibility criteria: included were individuals 18 ‐ 60 years of age with alcohol dependence and more than 1 week of 35 or more standard drinks (men) or 28 or more standard drinks (women) in the four weeks prior to admission, an AUDIT score of 8 or greater, mild or no alcohol withdrawal, likely to be discharged within 14 days, BMI at least 18 kg/m2, and an intention to decrease or stop drinking. Excluded were individuals with previous or current cognitive disorder, schizophrenia and other psychotic disorders, bipolar disorder, antisocial personality disorder, or other substance dependence (besides nicotine and caffeine) during the six months prior to enrollment. Excluded were also individuals who were being treated with antipsychotics, mood stabilizers, anticonvulsants, opioid analgesics, systemic steroids, carbonic anhydrase inhibitors, hydrochlorothiazide, metformin, pioglitazone, disulfiram, at risk of suicide during the four weeks prior to enrollment, having a physical illness including narrow angle glaucoma, renal impairment, and epilepsy, unstable medical conditions, pregnant or breastfeeding, or receiving medication for fourteen days or longer while in inpatient service. Duration of follow‐up: 12 weeks Informed consent: all participants provided informed consent Ethical approvals: Ethics Committee for Human Research/Institutional Review Board at each site approved the study |
|
| Participants |
Sample size: 106 (53 topiramate, 53 placebo) Description of the target population: patients in residential treatment for alcohol dependence Age: 41.5 years (SD = 8.9) Sex: 100% male Race/Ethnicity: not reported Marital status: 47.2% married Harmful alcohol use (baseline): mean drinks per day: 15.4 (SD = 11.8) Co‐occurring disorders: major depression (3.8%) |
|
| Interventions |
Type: Pharmacologic + Non‐pharmacologic Description: topiramate and residential treatment involving detoxification, motivational enhancement therapy, and family counseling Duration and frequency: 50 mg capsule of topiramate titrated on a dose escalation to 100mg by week 3 followed by adjustments between 100‐300 mg until week 12 and then tapered from weeks 12‐14. 3‐5 sessions of counseling Delivery and provider: trained psychologist, mental health nurses, physicians Comparison group: placebo and residential treatment involving detoxification, motivational enhancement therapy, and family counseling |
|
| Outcomes |
Primary outcome(s): heavy drinking Primary outcome measurement tool(s): Timeline followback Secondary outcome(s): alcohol craving, relapse, drinking days, drinks per day, drinks per drinking day, GGT, health‐related quality of life, side effects Secondary outcome measurement tool(s): Timeline followback, Craving Likert scale, GGT biomarker, Medical Outcomes Study Short Form 36, Side effects Likert scale Time points: 0, 4, 8 and 12‐weeks |
|
| Notes |
Study funding and conflicts of interest: Integrated Management of Alcohol Intervention Program (I‐MAP), ThaiHealth Promotion Foundation Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was balanced using permuted blocks of six. Random allocation sequences were generated by the computer." Pg. 441 |
| Allocation concealment (selection bias) | Low risk | Quote: "A random number indicating intervention or control treatment was kept in an opaque and sealed envelope. The envelope was opened after the baseline assessment" Pg. 441 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The participants, care providers, and those assessing outcomes were blinded to the assigned treatment" Pg. 441 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The participants, care providers, and those assessing outcomes were blinded to the assigned treatment" Pg. 441 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "number of dropouts was high in both groups" Pg. 445 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Madhombiro 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Zimbabwe Setting: tertiary HIV clinic Eligibility criteria: included were adults (18+ years) with problem drinking (AUDIT 6+ for females, 7+ for males) receiving HIV treatment. Excluded were individuals with cognitive impairment Informed consent: all participants provided informed consent Ethical approvals: Health Research Ethics Committee of the Stellenbosch University, the Medical Research Council of Zimbabwe, the Harare Hospital Ethics Committee |
|
| Participants |
Sample size: 40 (20 motivational interviewing + CBT, 20 brief advice) Description of the target population: people living with HIV/AIDS Age: 39.5 (SD = 9.6) Sex: 42.5% female Race/Ethnicity: not reported Marital status: 52.5% married Harmful alcohol use (baseline): AUDIT=15.45 Co‐occurring disorders: 100% HIV‐positive |
|
| Interventions |
Type: non‐pharmacologic Description: motivational interviewing + cognitive behavioral therapy: Personalized feedback, change talk, readiness to change, goal setting, dealing with cravings/cues, thinking errors, dealing with stress, and developing drinking refusal skills Duration and frequency: four sessions ranging from 30‐60 minutes each Delivery and provider: registered general nurses Comparison group: Brief advice (mhGAP) including an assessment of alcohol use history, brief advice on harmful alcohol use, and referral for probable alcohol dependence over a single session lasting approximately 60 minutes with a registered general nurse |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): disability, Quality of life Secondary outcome measurement tool(s): WHODAS, WHOQOL Time points: 0, 3 months |
|
| Notes |
Study funding and conflicts of interest: South African Research Chairs Initiative in PTSD, Department of Science and Technology; National Research Foundation; Partnership for Alcohol and AIDS Intervention Research, Stellenbosch University Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomization schedule was used to assign participants to the two interventions." Pg. 3 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The retention rate was 77% at 3 months with no statistically significant group difference" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Madhombiro 2020.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomized controlled trial Country: Zimbabwe Setting: HIV clinics Eligibility criteria: adults living with HIV on combination antiretroviral therapy with hazardous or harmful alcohol use and without cognitive impairment. Duration of follow‐up: 6 months Informed consent: all participants will provide informed consent (specified in study protocol) Ethical approvals: study procedures were approved by the Medical Research Council of Zimbabwe, Stellenbosch University Human Research Ethics Committee, the Harare Hospital Ethics Committee, and the Ministry of Health Permanent Secretary of Zimbabwe. |
|
| Participants |
Sample size: 234 (108 intervention, 126 control) Description of the target population: Adult HIV‐positive individuals on HAART in HIV care clinics in Zimbabwe with hazardous alcohol use Age: 43 years Sex: 79% male Race/Ethnicity: not reported Marital status: 62% married Harmful alcohol use (baseline): mean AUDIT score was 14.9 (SD = 6.3) in intervention group and 14.7 (SD = 6.2) in control group Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: motivational interviewing and cognitive behavioral therapy Duration and frequency: up to 10 sessions lasting 45‐60 minutes each and integrated into usual care Delivery and provider: registered general nurse Comparison group: mhGAP Intervention Guide Alcohol Module including personalized feedback and psychoeducation |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): viral load, CD4, functionality, quality of life Secondary outcome measurement tool(s): blood samples were collected to measure viral load and CD4 count, WHODAS, WHOQoL HIV Time points: 0, 6 months |
|
| Notes |
Study funding and conflicts of interest: Fogary International Center, National Institute of Nursing Research, National Institute of Mnetal Health, National Institute of Dental and Craniofacial Research, National Institute of Neurological Disorders and STroke, National Heart, Lung and Blood Institute, African Academy of Sciences, New Partnership for Africa's Development Planning and Coordinating Agency, Wellcome Trust, King's College London Partnership Fund Linked study records: Madhombiro 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a computer‐generated randomization schedule to allocate clusters to MI‐CBT and EUC arms in a 1:1 ratio." Pg. 2 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Second, participants were not and could not be blinded to the allocation which was a limitation of our study." Pg. 8 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "However, the outcome assessors were blinded to the arm of the facility." Pg. 8 Outcome assessment based on self report by unblinded participant |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Third, our study had differential loss to follow‐up (which was, however, not statisti‐ cally significant) with more participants lost in the control arm, which is a limitation. The loss to follow‐up was greater in the EUC arm than in the MI‐CBT arm. The effect of the MI‐CBT intervention on reduction in alcohol use and in viral load was greater than the effect of the EUC intervention, although this was only significant at the 0.05 level for alcohol use and was non‐significant in the case of viral load. If the follow‐up had been more equal, we might have been able to demonstrate a stronger effect of the intervention on alcohol use and even on viral load." Pg. 8 |
| Selective reporting (reporting bias) | Low risk | Study outcomes align with those reported in study protocol (Madhombiro 2017) |
Moraes 2010.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Brazil Setting: outpatient alcohol clinic and home‐based visits Eligibility criteria: included were persons between 20‐60 years who had been enrolled for outpatient treatment in the alcohol unit of the Alcohol and Drugs Research Unit (UNIAD). Excluded were patients who were abstinent within the 30 days prior to the first interview. Duration of follow‐up: 2.5 months Informed consent: all participants provided written informed consent Ethical approvals: Ethics Research Committee of the Federal University of Sao Paulo |
|
| Participants |
Sample size: 120 (62 intervention, 58 control) Description of the target population: patients in outpatient treatment for alcohol use disorder Age: 43 years Sex: 10% female Race/Ethnicity: 76.7% Caucasian, 19.2% Black, 4.2% Mixed Marital status: 41.7% married Harmful alcohol use (baseline): 2.5% abstinent in past month, 0.8% mild dependence, 14.2% intermediate dependence, 85% severe dependence Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: home visits involving motivational interviewing and standard clinic‐based care (outpatient detox, psychiatric evaluation, group sessions including relapse prevention and motivational enhancement therapy) Duration and frequency: 20 group sessions in outpatient treatment, 4 home visits at the beginning of treatment and in 7‐day intervals thereafter over 3 months Delivery and provider: interdisciplinary team of psychiatrists, nurses, psychologists, social workers, and psychology trainees Comparison group: outpatient detox by a nurse, a psychiatric evaluation, and 20 group sessions including relapse prevention and motivational enhancement techniques |
|
| Outcomes |
Primary outcome(s): abstinence Primary outcome measurement tool(s): self‐reported Secondary outcome(s): retention, alcohol consumption, change stage, level of change readiness, quality of life, mental health, AST, ALT, GGT Secondary outcome measurement tool(s): URICA, SOCRATES, ASI, SF‐36, SRQ, AST/ALT/GGT biomarkers Time points: 0, 5, 10 weeks |
|
| Notes |
Study funding and conflicts of interest: The State of Sao Paulo Research Foundation (FAPESP Grant No. 04/15062‐2) Linked study records: Moraes 2010b |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After being detoxified, all patients were randomly distributed by using a random‐number table. In order to avoid selection bias, the person responsible for distributing the patients did not take part in the study." Pg. 70 |
| Allocation concealment (selection bias) | Unclear risk | Quote: "In order to avoid selection bias, the person responsible for distributing the patients did not take part in the study." Pg. 70 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Murray 2020.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Zambia Setting: community Eligibility criteria: included were couples living in 1 of the 3 study neighborhoods in Lusaka, who spoke English, Bemba, or Nyanja, were each 18 years or older, the female partner reporting at least moderate past year physical or sexual intimate partner violence perpetrated by her male partner, the male partner had hazardous alcohol use (male‐ or female‐partner reported. Excluded were couples with a recent suicide attempt or ideation with a specific intent, plan, or self‐harm, a diagnosed psychotic disorder, severe developmental disorder, or currently on an unstable psychiatric drug regimen. Duration of follow‐up: 12 months Informed consent: all participants provided oral informed consent Ethical approvals: Johns Hopkins Bloomberg School of Public Health Institutional Review Board; University of Zambia Biomedical Ethics Review Committee |
|
| Participants |
Sample size: 248 couples (123 intervention, 125 control) Description of target population: couples with IPV and male partner hazardous alcohol use in low‐income communities Age: 66% of females and 49% of males were 35 years or younger Sex: 100% heterosexual couples (50% female, 50% male) Race/Ethnicity: not reported Marital status: 37% married couples Harmful alcohol use (baseline): male average self‐reported AUDIT score = 14.7; Male average partner‐reported AUDIT score=20.6; Female average self‐reported AUDIT score=10.7; Female average partner‐reported AUDIT=9.4 Co‐occurring disorders: 41.0% of females and 27.0% of males living with HIV/AIDS; 67.5% of females and 57.0% of males living with a physical disability |
|
| Interventions |
Type: non‐pharmacologic Description: Common Elements Treatment Approach (CETA) a transdiagnostic, modular cognitive‐behavioral psychotherapy include brief intervention for alcohol misuse. Modules include engagement, psychoeducation, safety, alcohol and other substance use reduction, cognitive coping and restructuring, problem solving, behavioral activation, relaxation, and exposure. The substance use module involves risk reduction and relapse prevention components as well as spousal support related to recovery from alcohol and other drug use problems. Duration and frequency: approximately 6 months between baseline and post‐treatment assessment Delivery and provider: individually‐delivered by sex‐matched lay counselors Comparison group: enhanced usual care including referrals to community‐based services and regular safety phone of home visit check‐ins |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): intimate partner violence Secondary outcome measurement tool(s): Severity of Violence Against Women Scale (SVAWS) Time points: 0‐months, approx 6‐months, 12‐months |
|
| Notes |
Study funding and conflicts of interest: UK Department for International Development, South Africa Medical Research Council Linked study records: clinicaltrials.gov NCT03966885 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Microsoft Excel random number generator was used to create the random sequence." Pg. 5 |
| Allocation concealment (selection bias) | Low risk | Quote: "The ID numbers were allocated to the next available slot in the randomization list" Pg. 6 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Due to the nature of the CETA intervention and TAU‐Plus control condition, study participants and counselors were not masked" Pg. 6 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Research assistants who conducted outcome assessments were masked to the participant’s
treatment assignment, as was the data analyst" Pg. 6 Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Primary analyses were intent‐to‐treat and included all enrolled participants. Multiple imputation with chained equations was used to address missing data" Pg. 8 |
| Selective reporting (reporting bias) | Unclear risk | Quote: "The primary trial paper reports on the IPV outcomes... and alcohol outcomes. The study also captured a wide range of secondary outcomes from adult and child participants that will be reported in subsequent papers" Pg. 8 |
Nadkarni 2017.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: primary care Eligibility criteria: included were men aged above 18 years, living in Goa, without a comorbid physical or mental illness requiring emergency treatment, and scoring 12‐19 on the AUDIT. Excluded were patients who needed emergency medical treatment or inpatient admission, who were unable to communicate clearly, and who were intoxicated at the time of screening. Duration of follow‐up: 3 months Informed consent: all participants provided written informed consent Ethical approvals: Institutional Review Boards (IRB) of Sangath, the London School of Hygiene & Tropical Medicine, and the Health Ministry Screening Committee of the Indian Council of Medical Research |
|
| Participants |
Sample size: 378 (188 intervention, 190 control) Description of the target population: patients in primary health care Age: 42.0 years Sex: 100% male Race/Ethnicity: not reported Marital status: 79.6% married Harmful alcohol use (baseline): AUDIT = 14.9 Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: Counseling for Alcohol Problems (CAP) incorporated motivational interviewing, assessment and personalized feedback, family engagement, drinking refusal skills, skills to address drinking urges, problem‐solving skills, handling difficult emotions, and relapse prevention and management Duration and frequency: 1 to 4 30‐45 minute sessions Delivery and provider:lLay counselor Comparison group: provided mhGAP treatment guidelines and AUDIT scores to patient's primary health care provider |
|
| Outcomes |
Primary outcome(s): remission (AUDIT<8), Past 2‐week alcohol consumption Primary outcome measurement tool(s): Alcohol Use Disorder Identification Test (AUDIT), Timeline Followback Secondary outcome(s): alcohol‐related consequences, drinking days, percentage days abstinent, percent days heavy drinking, disability, number of suicide attempts, intimate partner violence perpetration, Secondary outcome measurement tool(s): Timeline Followback, Short Inventory of Problems, World Health Organization Disability Schedule (WHODAS) II, Time points: 3, 12‐months |
|
| Notes |
Study funding and conflicts of interest: Wellcome Trust Senior Research Fellowship grant (091834) Linked study records: Nadkarni 2015 (different sample), Nadkarni 2017b, Patel 2014, Singla 2019 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation list in randomly sized blocks (four to six), stratified by PHC, was generated by a statistician independent of the trial. The randomisation code was concealed and allocated by trained health assistants based at the primary health centres at the individual level after completion of the baseline assessments using sequential numbered opaque sealed envelopes." Pg. 188 |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation code was concealed and allocated by trained health assistants based at the primary health centres at the individual level after completion of the baseline assessments using sequential numbered opaque sealed envelopes. Pg. 188 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention Quote: "Physicians providing enhanced usual care (EUC) were masked to allocation status, as were the independent assessors who did the outcome assessments, and these people had no contact with the PHCs or other team members. All authors, apart from the data manager (BB), were masked until the trial results were unmasked. Instances of unmasking of outcome assessors in the CAP group will be summarised on the basis of overall prevalence and the exact point during the interview that the interviewer was unmasked" Pg. 188 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "Physicians providing enhanced usual care (EUC) were masked to allocation status, as were the independent assessors who did the outcome assessments, and these people had no contact with the PHCs or other team members." Pg. 188 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analyses were on an intention‐to‐treat basis, with multiple imputation for missing outcome data assuming data were missing at random, assuming predictive mean matching for positively skewed outcomes." Pg. 189 |
| Selective reporting (reporting bias) | Low risk | Outcomes align with those reported in study protocol (Patel 2014) |
Nadkarni 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: primary care Eligibility criteria: males 18‐65 years of age residing in primary health center catchment area and intending to stay at the same address for 12+ months, able to communicate clearly, not presenting with an emergency medical condition, and meeting criteria for probable dependence (AUDIT>=20). Duration of follow‐up: 12‐months Informed consent: all participants provided informed consent Ethical approvals: study procedures were approved by the London School of Hygiene and Tropical Medicine, Sangath, and the Indian Council for Medical Research |
|
| Participants |
Sample size: 135 (69 intervention, 66 control) Description of the target population: patients in primary care with probable alcohol dependence Age: 41.5 years Sex: 100% male Race/Ethnicity: not reported Marital status: 77.8% married Harmful alcohol use (baseline): AUDIT average=24.3 Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: counseling for Alcohol Problems: a manualized intervention including personalized feedback, developing cognitive and behavioral skills, and managing relapses. Patients are referred to detoxification services as needed Duration and frequency: four 30‐45 minute sessions delivered weekly or biweekly Delivery and provider: lay counselors Comparison group: enhanced usual care (mhGAP) which included assessment results provided to primary care physicians and determine treatment or referral plan (1 consultation session). |
|
| Outcomes |
Primary outcome(s): remission (AUDIT<8), Alcohol consumption Primary outcome measurement tool(s): AUDIT, Timeline Followback Secondary outcome(s): percent days abstinent, Percent days heavy drinking, Depression, Alcohol‐related consequences, Disability, Remission, Abstinence, Suicidal behavior, IPV perpetration Secondary outcome measurement tool(s): Timeline Followback, Short Inventory of Problems, Patient Health Questionnaire‐9, World Health Organization Disability Assessment Schedule Time points: 3‐, 12‐months |
|
| Notes |
Study funding and conflicts of interest: Directorate of Health Services of the Government of Goa; Wellcome Trust Senior Research Fellowship Linked study records: ISRCTN76465238 https://doi.org/10.1186/ISRCTN76465238 Program for effective mental health interventions in under‐resourced health systems |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomization list in randomly sized blocks (two to four), stratified by PHC, was generated by a statistician independent of the trial." Pg. 1193 |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization code was concealed and consenting participants were randomized at the individual level by trained health assistants based at the primary health centres in a 1:1 allocation scheme to either of two intervention arms [enhanced usual care (EUC) or EUC plus CAP] after completion of the baseline assessments, using sequentially numbered opaque sealed envelopes." Pg. 1193 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention "Physicians providing EUC were masked to allocation status, as were the independent assessors who performed the outcome assessments, and these people had no contact with the PHCs or other team members. All authors, apart from the data manager (B.B.), were masked." Pg. 1194 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "Physicians providing EUC were masked to allocation status, as were the independent assessors who performed the outcome assessments, and these people had no contact with the PHCs or other team members." Pg. 1194 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Given the highly skewed distribution of ethanol consumption and small sample size in this trial, multiple imputation (MI) was problematic. Hence, considering recent methodological developments which indicate that for trials with one primary endpoint, analyses which adjust for factors associated with missingness are equivalent to MI, to handle missing data we followed the analyses strategy of adjusting for baseline variables associated with drop‐out." Pg. 1195 |
| Selective reporting (reporting bias) | Low risk | Outcomes match those reported in study protocol (ISRCTN76465238 https://doi.org/10.1186/ISRCTN76465238) |
Nattala 2010.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: substance use treatment facility (inpatient and outpatient) Eligibility criteria: included were individuals 20‐60 years of age with a diagnosis of alcohol dependence, at least one family member in the age range of 20‐60 years who had lived with the participant for a minimum of 3 months before assessment and was currently staying with that person, the ability to converse, read, and write in the regional language/English, and recommended inpatient treatment by the treating psychiatrist. Excluded were patients with comorbid medical or psychiatric diagnoses, including multiple substance use (excluding nicotine) disorders. Duration of follow‐up: 6‐months Informed consent: all participants and family members provided written informed consent Ethical approvals: NIMHANS Institutional Ethics Committee |
|
| Participants |
Sample size: 87 (30 dyadic relapse prevention, 30 individual relapse prevention, 27 control) Description of the target population: patients seeking inpatient or outpatient treatment for alcohol problems Age: 39 years (SD=8) Sex: 100% male Race/Ethnicity: not reported Marital status: 86% married Harmful alcohol use (baseline): past month consumption was 7917 g, on average (SD = 1753) Co‐occurring disorders: 16% Antisocial personality disorder |
|
| Interventions |
Type: non‐pharmacologic Description: A) Dyadic relapse prevention sessions that included the family member. The sessions focused on identifying triggers, creating a plan when confronted with triggers, role playing, drink refusal skills, daily schedules and budgeting, problem‐solving techniques, the role of the family in relapse prevention, and the patient's discharge plan with homework for patient and his family member. B) Individual relapse prevention sessions focused on identifying triggers, creating a plan when confronted with triggers, role playing, drink refusal skills, daily schedules, budgeting, and problem‐solving techniques. Duration and frequency: 8‐10 sessions occurring 2‐3 times per week; 1 hour per session Delivery and provider: Psychiatric nurse Comparison group: three weeks of usual care involving detox, long‐term medication management for withdrawal and craving, and psychosocial interventions (psychoeducation, managing triggers, refusal skills, positive lifestyle changes) |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): Form 90 Secondary outcome(s): family problems, occupational problems, financial problems, relapse, caregiver stress Secondary outcome measurement tool(s): Brief Addiction Rating Scale, Form 90 Time points: 6‐months |
|
| Notes |
Study funding and conflicts of interest: Fogarty International Center ICOHRTA Training Program in Behavioral Disorders Grant TW05811‐08 Linked study records: Nattala 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was followed by random assignment to one of three groups: (a) individual relapse prevention (IRP; 30 participants), (b) dyadic relapse prevention (DRP; 30 participants and their respective family members), and (c) TAU (30 participants)." Pg. 582 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Three participants from TAU were not included in the analyses because they were not available for follow‐up after discharge." Pg. 584 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Ng 2020.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: inpatient alcohol clinic Eligibility criteria: included were men ages 18‐60 years with a diagnosis of alcohol dependence, had the ability to converse, read, and write in the regional language or English, and were recommended to inpatient management by the treating psychiatrist. Duration of follow‐up: 12 weeks Informed consent: all participants provided written informed consent Ethical approvals: ethical approval obtained, but institution not specified. |
|
| Participants |
Sample size: 60 (30 intervention, 30 control) Description of the target population: Males in inpatient alcohol treatment Age: 36 years Sex: 100% male Race/Ethnicity: not listed Marital Status: 76.7% married Harmful alcohol use (baseline): no difference in number of drinking days in past 30 days between intervention and control group (28.8 drinks, SD = 3.07 in intervention; 29.76 drinks, SD = 0.97 in control) Co‐occurring disorders: not listed |
|
| Interventions |
Type: non‐pharmacologic Description: group relapse prevention Duration and frequency: 7 sessions over 3 week period; 60‐90 minutes per session Delivery and provider: group sessions delivered by a certified nurse Comparison: usual care involving detoxification and combined pharmacologic and non‐pharmacologic intervention |
|
| Outcomes |
Outcomes: number of drinking days Primary outcome measurement tool(s): Form 90‐AQ Secondary outcomes: alcohol craving Secondary outcome measurement tool(s): Penn alcohol craving scale Time points: 1, 2, 3 months |
|
| Notes |
Study funding and conflicts of interest: Department of Social Work and Social Administration, University of Hong Kong Linked study records: Not listed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using computer‐generated random sequence numbers, 97 subjects were assigned to either of the BMS and treatment‐as‐usual (TAU) groups. At the end of 3 months of follow‐up, each group constituted of 30 subjects (see Figure 1)." Pg. 278 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Baseline and follow‐up assessment data were collected by a registered nurse who was trained on the assessment but was blind to participants' group status (BMS/TAU)." Pg. 278 Outcome assessment based on self report by unblinded participant |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High levels of attrition |
| Selective reporting (reporting bias) | Unclear risk | Not described |
Noknoy 2010.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Thailand Setting: primary healthcare Eligibility criteria: Included were primary care patients who scored 8 or higher on the AUDIT. Excluded were alcohol‐dependent patients diagnosed by a physician, patients with a history of liver disease, patients with a history of regular alcohol drinking starting early in the morning, recent consumption of high amounts per day (>120 g for men, >80 g for women), patients with neurological disease or psychiatric disorders, pregnant women, and patients less than 18 or over 65 years of age. Duration of follow‐up: 6‐months Informed consent: all participants provided written informed consent Ethical approvals: Royal Thai Army Medical Department Ethics Review Committee, Phramongkutklao Army Hospital and College of Medicine |
|
| Participants |
Sample size: 117 (59 intervention, 58 control) Description of the target population: patients seeking primary healthcare Age: 37.0 years Sex: 8.5% female Race/Ethnicity: not reported Marital status: 75.2% married Harmful alcohol use (baseline): AUDIT mean = 17.4 Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: motivational enhancement therapy including evaluation of patient's motivation to change their drinking behavior, empathic counseling, feedback, support according to their stage of change, and relapse prevention Duration and frequency: three 15‐minute sessions (baseline, 2‐weeks, 6‐weeks) Delivery and provider: nurse Comparison group: assessment of drinking and other health‐related behaviors by a nurse |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): health survey questionnaire, GGT Secondary outcome(s): binge drinking, drunkeness, hazardous drinking Secondary outcome measurement tool(s): healthy survey questionnaire, GGT Time points: 6‐weeks, 3‐months, 6‐months |
|
| Notes |
Study funding and conflicts of interest: Thai Health Promotion Foundation Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of subjects to the intervention and control groups was carried out from the Coordinating Centre in Phramongkutklao Hospital in Bangkok using a standard randomization table" Pg. 264 |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization codes were distributed to each PCU in sealed envelopes" Pg. 265 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention Quote: "In order to minimize the intervention effect of the research procedures, the subjects randomized into the control condition were told that the trial focused on health behaviours, which included questions on smoking, exercise, eating behaviour, weight and alcohol use. The study interviewers at follow‐up visits were not aware of the assignment allocation of the study participants." Pg. 265 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention Quote: "The study interviewers at follow‐up visits were not aware of the assignment allocation of the study participants." Pg. 265 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Used last observation carried forward to construct intention‐to‐treat analyses (e.g. if baseline data only were available, these were subsequently imputed as outcomes, thus assuming no change)" Pg. 265 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Omeje 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Nigeria Setting: community (intervention venue ‐ secondary schools) Eligibility criteria: included were community‐dwelling HIV‐infected patients with alcohol use disorder symptoms and residents of the Southeastern state of Nigeria Informed consent: all participants provided informed consent Ethical approvals: study procedures were approved by the University of Nigeria |
|
| Participants |
Sample size: 124 (61 rational emotive therapy, 63 control) Description of the target population: community‐dwelling people living with HIV/AIDS Age: 33.76 (SD=2.16) Sex: 31.45% female Race/Ethnicity: not reported Marital status: 79.9% single Harmful alcohol use (baseline): AUD scale = 41.91 Co‐occurring disorders: 100% HIV‐positive |
|
| Interventions |
Type: non‐pharmacologic Description: Rational Emotive Health Therapy including discussion of problematic beliefs and training in cognitive, behavioral and emotive techniques. Behavioral techniques included training in skills to cope with alcohol use symptoms, managing craving, and preparing a daily schedule for distraction. Emotive techniques include humor, alcohol‐related poems, and satirical songs related to alcohol use to generate feelings that could help to change negative thoughts related to alcohol Duration and frequency: 20 sessions lasting 50 minutes each, twice per week for 10 weeks Delivery and provider: counselors and psychologists Comparison group: waitl‐ist control |
|
| Outcomes |
Primary outcome(s): alcohol use disorder symptoms Primary outcome measurement tool(s): 12‐item Alcohol Use Disorder scale developed by researchers; Alcohol‐related Irrational Beliefs Scale (AIBS) Secondary outcome(s): none Secondary outcome measurement tool(s): N/A Time points: 0, 10, 12 weeks |
|
| Notes |
Study funding and conflicts of interest: Otusum Consult, Nigeria Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The first 124 persons presenting with a severe level of AUD and alcohol‐related irrational beliefs were selected and randomly assigned to the treatment and control groups using randomization software." Pg. 3 |
| Allocation concealment (selection bias) | Low risk | Quote: "All participants were asked to pick an envelope from a container to eliminate selection bias. Each of the envelopes contained 1 card labeled either “T” (for allocation to the treatment group) or “C” (for allocation to the control group). The labels were based on a computer‐generated random list (Random Allocation Software version 1.0)" Pg. 4 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "To avoid selection and expectation bias, research assistants and data analysts were not exposed to group assignment sequences." Pg. 4 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Pal 2007.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: India Setting: community Eligibility criteria: included were individuals 20‐45 years of age with an AUDIT score between 8‐24 and who gave consent while not demonstrating significant medical or psychiatric illness Duration of follow‐up: 3‐months Informed consent: all participants provided informed consent Ethical approvals: not reported |
|
| Participants |
Sample size: 90 (45 intervention, 45 control) Description of the target population: males with alcohol problems who have not sought treatment Age: 29.7 years (SD = 9.89) Sex: 100% male Race/Ethnicity: not reported Marital status: 67.7% married Harmful alcohol use (baseline): AUDIT between 8‐24 Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: motivational interviewing including personalized feedback related to drinking consequences, personal responsibility to change, alternatives to drinking, self‐efficacy, optimism, evaluation of high‐risk situations, and coping Duration and frequency: Two 45‐minute sessions delivered 3‐5 days apart Delivery and provider: research staff Comparison group: 0ne 5‐minute session of simple advice involving empathic expression of concern based on drinking consequences with advice to cut down or stop drinking |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): Addiction Severity Index (ASI) Secondary outcome(s): alcohol‐related problems, Addiction severity, Quality of life Secondary outcome measurement tool(s): Addiction Severity Index (ASI), WHO Quality of Life (WHOQOL)‐BREF Time points: 0, 1, 3‐months |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The first case was then allocated to the intervention arm by the toss of a coin and subsequent cases were allocated alternatively to BI or SA. The first case was allocated to BI if the coin came up ‘heads’, or to SA if it was ‘tails’." " Pg. 329 |
| Allocation concealment (selection bias) | High risk | Quote: "The first case was then allocated to the intervention arm by the toss of a coin and subsequent cases were allocated alternatively to BI or SA. The first case was allocated to BI if the coin came up ‘heads’, or to SA if it was ‘tails’." Pg. 329 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention Quote: "Interviewer 2 (Deepak Yadav) carried out the intervention for all the subjects after which they were followed up at 1 month and 3 months, and assessments were done by interviewer 1 who was unaware of the intervention allocation of the subjects." Pg. 329 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | AUDIT and CAGE outcome scores were not reported |
Papas 2011.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Kenya Setting: HIV outpatient clinic Eligibility criteria: Included were adults 18+ years of age enrolled in a HIV outpatient clinic with hazardous alcohol use or binge drinking (AUDIT‐C>=3, 6+ drinks per occasion monthly or more frequently), alcohol use in the past 30 days, eligible for antiretroviral treatment or initiated in the past year, speaks Kiswahili, living within one hour from the clinic with no plans to move away during the study period, and available during weekly group time. Excluded were individuals with active psychosis or suicidality, who attended an existing alcohol peer support group in the past year, or had participated int he study's group cognitive behavioral therapy pre‐pilot development study. Duration of follow‐up: 4.5 months Informed consent: All participants provided written informed consent Ethical approvals: Approved by IRBs at all participated universities ‐ Brown University, Moi University, Indiana University, Yale University, Syracuse University |
|
| Participants |
Sample size: 75 (42 intervention, 33 control) Description of the target population: Outpatients living with HIV Age: 37.07 years (SD=8.04) Sex: Not reported Race/Ethnicity: Not reported Marital status: 57.33% married Harmful alcohol use (baseline): 9.99 days of alcohol use in the past month (SD=7.89); 5.68 drinks per drinking day (SD=4.01) Co‐occurring disorders: HIV/AIDS |
|
| Interventions |
Type: Non‐pharmacologic Description: Cognitive behavioral therapy (abstinence‐focused) Duration and frequency: 6 weekly 90‐minute gender‐specific group sessions Delivery and provider: Counselor with no prior experience with cognitive behavioral therapy and minimal to no counseling experience Comparison group: Usual care involving routine medical care, option to attend peer‐led HIV support group, assessment |
|
| Outcomes |
Primary outcome(s): Alcohol consumption (percent drinking days) Primary outcome measurement tool(s): Timeline Followback Secondary outcome(s): Drinks per drinking day Secondary outcome measurement tool(s): Timeline Followback Time points: 1.5, 2.5, 3.5, 4.5‐months post‐treatment |
|
| Notes |
Study funding and conflicts of interest: National Institute on Alcohol Abuse and Alcoholism (R21AA016884), a grant to the USAID‐AMPATH Partnership from the United States Agency for International Development as part of the President's Emergency Plan for Aid Relief, and by the Nationa Institute on Drug Abuse (P50DA09241) Linked study records: Papas 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A stratified simple randomization procedure was used to form gender‐ stratified cohorts. Within gender‐based cohorts, participants were randomly assigned until a minimum was achieved of 7 CBT and 5 usual care participants, thereby creating some waiting time. A group of 7 was required for CBT to enhance participation, while fewer were required for the individual usual care condition to minimize waiting time before treatment initiation. Each participant was randomized after she or he drew from a jar a paper with the name of the condition. The papers were prepared by study administrators to conceal the name of the condition during the drawing, which was supervised by staff." Pg. 4 |
| Allocation concealment (selection bias) | Low risk | "A stratified simple randomization procedure was used to form gender‐ stratified cohorts. Within gender‐based cohorts, participants were randomly assigned until a minimum was achieved of 7 CBT and 5 usual care participants, thereby creating some waiting time. A group of 7 was required for CBT to enhance participation, while fewer were required for the individual usual care condition to minimize waiting time before treatment initiation. Each participant was randomized after she or he drew from a jar a paper with the name of the condition. The papers were prepared by study administrators to conceal the name of the condition during the drawing, which was supervised by staff." Pg. 4 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Nonblinded research assistants both recruited and interviewed participants; none delivered study interventions" Pg. 4 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Papas 2020.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Kenya Setting: HIV outpatient clinic Eligibility criteria: included were adults enrolled in AMPATH's HIV outpatient program who met criteria for hazardous drinking or binge drinking, reported alcohol use in the past 30 days, spoke Swahili, lived within one hour of the clinic, and were available for weekly intervention sessions Duration of follow‐up: 9‐months post‐ntervention (10.5 months from baseline) Informed consent: Written informed consent was obtained by all participants Ethical approvals: IRBs at all affiliated universities reviewed and approved study procedures |
|
| Participants |
Sample size: 614 (312 intervention, 302 control) Description of the target population: HIV‐positive patients reporting hazardous alcohol use Age: 38.9 years (SD = 8.0) Sex: 28.5% male Race/Ethnicity: not reported Marital status: 52.6% married Harmful alcohol use (baseline): average AUDIT = 19.4 (SD = 7.4) Co‐occurring disorders: HIV/AIDS |
|
| Interventions |
Type: non‐pharmacologic Description: 6‐session group cognitive‐behavioral therapy Duration and frequency: 6 weekly sessions Delivery and provider: group‐based cognitive‐behavioral therapy delivered by trained paraprofessionals Comparison group: time‐and attention‐matched group‐based healthy lifestyles education control |
|
| Outcomes |
Primary outcome(s): percent days drinking, drinks per drinking day Primary outcome measurement tool(s): Timeline Followback Secondary outcome(s): alcohol withdrawal symptoms Secondary outcome measurement tool(s): CIWA‐Ar Time points: Baseline; Intervention period ‐ 1, 2, 3, 4, 5, 6 weeks; Follow‐up period ‐ 2.5, 4.5, 6.5, 8.5, 10.5 months |
|
| Notes |
Study funding and conflicts of interest: NIAAA (R01AA020805; K0516928); USAID‐AMPATH Partnership Linked study records: Papas 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A stratified block randomization procedures with random block sizes of two and four was generated by an analyst to balance gender and antiretroviral (ARV) use (yes/no) across the two intervention conditions. Equal allocation was intended between conditions." Pg. 12 |
| Allocation concealment (selection bias) | Low risk | Quote: "Individual assignment based on block was printed in sealed envelopes which were handed to the RA by the study coordinator after each enrolment." Pg. 12 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for thsi type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Non‐blinded research assistants both recruited and interviewed participants." Pg. 13 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We assumed that missing occurred at random (MAR), which would provide valid inferences for the mixed modeling approach. We conducted sensitivity analyses for MAR, assuming that CBT and HL interventions had no effect, so missing outcomes among lost‐to‐follow‐up participants were the same as baseline scores." Pgs. 24‐15 Similar number of lost‐to‐follow‐up across groups (Pg. 24) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Peltzer 2013.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomized controlled trial Country: South Africa Setting: primary care clinics Eligibility criteria: included were clinics with a high tuberculosis caseload in 3 study districts. Adult (18+ years) patients with active tuberculosis and retreatment patients, males and females who visited the primary health care facility and scored 8 (men) or 7 (women) on the AUDIT after screening were included in the study. Excluded were people less than 18 years of age. Duration of follow‐up: 6‐months Informed consent: informed consent was provided by all patients; consent of the clinics was not described Ethical approvals: Human Sciences Research Council Research Ethics Committee |
|
| Participants |
Sample size: 40 clinics (20 intervention, 20 control); 1196 patients (741 intervention, 455 control) Description of the target population: patients in primary care with active tuberculosis Age: 36.7 years Sex: 25.7% female Race/Ethnicity: not reported Marital status: not reported Harmful alcohol use (baseline): average AUDIT = 15.7; 73.2% had AUDIT scores between 7‐19, 26.8% had AUDIT scores between 20‐40 Co‐occurring disorders: 54.2% HIV positive, All had active tuberculosis |
|
| Interventions |
Type: non‐pharmacologic Description: Brief counseling included assessment, treatment as usual, and counseling to identify any alcohol‐related problems, psychoeducation, personalized feedback, information on the relationship between alcohol use and tuberculosis treatment, problem solving, keeping a drinking diary, identifying a helper, planning for follow‐up counseling, and other motivation‐behavioral skills Duration and frequency: Two 15‐30 minute sessions within 1 month of each other Delivery and provider: Trained lay HIV counselors and nurses Comparison group: Health education on responsible drinking, assessment, and treatment as usual for 1 session provided by counselors and nurses |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): alcohol dependence, heavy episodic drinking Secondary outcome measurement tool(s): AUDIT Time points: 0‐, 3‐, and 6‐months |
|
| Notes |
Study funding and conflicts of interest: Department of Health in South Africa (TNDOH: 21/2010‐2011) Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was conducted using a secure remote randomization service. Within each district in each of the three provinces the 14 public primary health clinics with the highest TB caseloads were randomly assigned to the treatment and control arms. A secure remote randomisation service carried out randomisation." Pg. 3 |
| Allocation concealment (selection bias) | Low risk | Quote: "A secure remote randomisation service carried out randomisation. Twenty one allocations were initially generated for each of the possible factorial combinations of clinic type, TB case load, and intervention" Pg. 3 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Participants (clinic staff members and TB patients) were not blinded to their intervention or control status" Pg. 4 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention Quote: "However, to protect against information biases in the reporting of alcohol use and TB treatment adherence behaviour, the data collection team who assessed the outcomes were blinded to the clinic’s status as intervention or control group." Pg. 4 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "As illustrated in Figure 1, response rates were higher in the intervention than in the control group at both follow‐ups. At the 3‐month follow‐up, response rates for the control and intervention were 39% and 54%, respectively, and at 6 months, the control and intervention group response rates were 59% and 79%, respectively. In the control group 41% did not complete the last follow‐up survey (i.e., the dropout rate was 41%); in the intervention group, 21% did not complete the last follow‐up survey. The main difference between groups was in the dropout rate (percentage of participants that did not complete the last follow‐up survey): 21% in the intervention group versus 41% in the control group. Attrition analyses were conducted to check for differential attrition by examining the condition by dropout interactions at baseline. Dropout was significantly related to the condition (P < 0.001) and poverty (P = 0.003), indicating a relationship between these variables and dropout status depending on condition: 21% in the intervention group versus 41% in the control group dropped out and the poverty index was 9.9 in the intervention versus 8.9 in the control group. Dropout was not related to gender (P = 0.308), age (P = 0.078), education (P = 0.866), AUDIT score (P = 0.958), AUDIT (7–40) (P = 0.516), AUDIT (7–19) (P = 0.952), AUDIT (20–40) (P = 0.918), heavy episodic drinking (P = 0.192), daily or almost daily tobacco use (P = 0.929), HIV status (P = 0.488), and TB new or retreatment patient (P = 0.082)." Pg. 7 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Pengpid 2013a.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: South Africa Setting: university Eligibility criteria: included were university studies 18 years of age and older who visited public recruitment venues at the university campus and who were risky drinkers (AUDIT>=8). Excluded were students who were pregnant or already in alcohol treatment. Duration of follow‐up: 12‐months Informed consent: all participants provided informed consent Ethical approvals: Medunsa Research and Ethics Committee |
|
| Participants |
Sample size: 152 (81 intervention 71 control) Description of the target population: university students with risky drinking Age: 21.9 years (SD = 3.5) Sex: 12.7% female Race/Ethnicity: not reported Marital status: not reported Harmful alcohol use (baseline): AUDIT=16.2 (SD=7.1) Co‐occurring disorders: 23.8% PTSD, 12.6% Depression |
|
| Interventions |
Type: non‐pharmacologic Description: Brief intervention including personalized feedback, health education, simple advice, brief counseling about reducing excessive drinking informed by the Information‐Motivation‐Behavioral Skills Model Duration and frequency: one 20‐minute session Delivery and provider: Nurse counselor Comparison group: one health education session focused on responsible drinking and feedback on alcohol screening delivered by a nurse counselor |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): heavy episodic drinking, PTSD symptoms, Depressive symptoms, High risk alcohol use, Alcohol dependence, Secondary outcome measurement tool(s): AUDIT, 7‐item PTSD screener, CES‐D Time points: 0, 6, 12‐months |
|
| Notes |
Study funding and conflicts of interest: Directorate General for Development Corporation (DGDC) through the Flemish Interuniversity Council (VLIR‐UOS) Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After baseline assessment, each student was randomized to either a control or a brief intervention group. Students were randomized using sequentially numbered opaque sealed envelopes prepared according to a computer‐generated (prepared using Stata version 10) randomization allocation sequence. This was carried out separately by an off‐side data management group. After randomization interventionists were instructed to implement the brief intervention." Pg. 2045 |
| Allocation concealment (selection bias) | Low risk | Quote: "Students were randomized using sequentially numbered opaque sealed envelopes prepared according to a computer‐generated (prepared using Stata version 10) randomization allocation sequence." Pg. 2045 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Research assistant nurses and university students were not blind to their intervention." Pg. 2046 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention Quote: "However, to protect against information biases in the reporting of alcohol use behaviour, the data collection team who assessed the outcomes were blind to the client’s status as intervention arm." Pg. 2046 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The extent of the missing component was 3.3% at 12 months. The single follow‐up measurement and the extent of the expected dropout was considered for the statistical method that was used to provide an unbiased estimate of the intervention effect under the principle of intention to treat." Pg. 2049 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Pengpid 2013b.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: South Africa Setting: Hospital outpatient setting Eligibility criteria: included were outpatients 18 years and above without mental impairment and reporting hazardous alcohol use (AUDIT = 8‐19 for men, AUDIT = 7‐19 for women). Duration of follow‐up: 12‐months Informed consent: all participants provided consent Ethical approvals: Meduna Research and Ethics Committee & Dr. George Mukhari Hospital |
|
| Participants |
Sample size: 392 (196 intervention, 196 control) Description of the target population: hospital outpatients Age: 35.6 years Sex: 17.6% female Race/Ethnicity: not reported Marital status: 32.4% married Harmful alcohol use (baseline): Average AUDIT = 12 (SD = 3.5); AUDIT 7‐15 (81.8%); AUDIT 16‐19 (18.4%) Co‐occurring disorders: not reported |
|
| Interventions |
Type: non‐pharmacologic Description: Brief intervention on alcohol risk reduction including personalized feedback, health education leaflet, simple advice, brief counseling about reducing excessive drinking informed by the Information‐Motivation‐Behavioral Skills Model Duration and frequency: one 20‐minute session Delivery and provider: nurse counselor Comparison group: health education leaflet on responsible drinking |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): heavy episodic drinking, Harmful alcohol use Secondary outcome measurement tool(s): AUDIT Time points: 0, 6, 12‐months |
|
| Notes |
Study funding and conflicts of interest: Directorate General for Development Cooperation (DGDC) through the Flemish Interuniversity Council (VLIR‐UOS) Linked study records: Pengpid 2012; PACTR201110000319392 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After baseline assessment, each patient was randomized to either a control or a brief intervention group. Patients were randomized using sequentially numbered opaque sealed envelopes prepared according to a computer‐generated randomization allocation sequence. Block randomization using randomly varying block sizes (prepared using Stata version 10) ensured equal numbers of patients were recruited into each group" Pg. 2 |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized using sequentially numbered opaque sealed envelopes prepared according to a computer‐generated randomization allocation sequence." Pg. 2 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Hospital staff members and outpatients were not blind to their intervention." Pg. 2 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "However, to protect against information biases in the reporting of alcohol use behaviour, the data collection team who assessed the outcomes were blind to the client’s status as intervention arm." Pg. 2 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "As illustrated in Figure 1, response rates were higher in the second compared to the first follow‐up. At the 6‐month follow‐up, response rates for the control and intervention were 56% and 66%, respectively, and at 12 months, the control and intervention group response rates were 71% and 73%, respectively. In the control group 29% did not complete the last follow‐up survey (i.e., the dropout rate was 29%); in the intervention group, 27% did not complete the last follow‐up survey. Attrition analyses were conducted to check for differential attrition by examining the condition by dropout inter‐ actions at baseline. The dropout was not significantly related to the condition" Pgs. 4‐5 |
| Selective reporting (reporting bias) | High risk | Study protocol (Pengpid 2012) reports the SF‐12 (Health status) as an outcome measure, which is not reported in Pengpid 2013 |
Pengpid 2015.
| Study characteristics | ||
| Methods |
Study design: cluster ‐ controlled trial Country: Thailand Setting: outpatient hospital setting Eligibility criteria: Included were patients between 18‐60 years of age, able to participate at 3‐ and 6‐month follow‐up, able to give contract details for 2‐3 other people, having a permanent address, without pending incarceration in next 3 months, absence of cognitive impairment or severe behavior problems, not intoxicated or going through withdrawal, not currently in alcohol or nicotine treatment, and with moderate risk of alcohol and tobacco problems. Excluded were individuals with low or high risk of alcohol problems, or who had frequently injected drugs in the past 3 months. Duration of follow‐up: 6‐months Informed consent: all participants provided informed consent Ethical approvals: Mahidol University Research and Ethics Committee |
|
| Participants |
Sample size: 620 (215 joint alcohol and tobacco brief intervention, 199 tobacco only brief intervention, 206 control alcohol only brief intervention) Description of the target population: patients seeking care in outpatient hospital department with moderate risk for problematic tobacco and alcohol use Age: 33.7 years Sex: 2.3% female Race/Ethnicity: not reported Marital status: 79.4% married Harmful alcohol use (baseline): Alcohol ASSIST = 16.8 Co‐occurring disorders: Tobacco ASSIST = 20.1 |
|
| Interventions |
Type: non‐pharmacologic Description: ASSIST‐linked brief intervention for heavy drinking smokers with feedback, discussion on the relationship between drinking and smoking, the potential effects of alcohol on smoking cessation, an emphasis on personal responsibility for behavior change, advice to avoid or minimize drinking during smoking cessation, strategies to carry out behavior change, use of empathy, and encouragement of self‐efficacy Duration and frequency: 3 sessions within a 3‐week period Delivery and provider: trained counselors Comparison group: 1) Three sessions of ASSIST‐linked intervention focusing on alcohol use, 2) Three sessions of ASSIST‐linked intervention focusing on tobacco use |
|
| Outcomes |
Primary outcome(s): hazardous alcohol use Primary outcome measurement tool(s): ASSIST Alcohol Scores Secondary outcome(s): alcohol consumption, non‐problematic/low‐risk drinking (remission) Secondary outcome measurement tool(s): ASSIST, Timeline Followback Time points: 0‐, 3‐, 6‐months |
|
| Notes |
Study funding and conflicts of interest: The Thai Health Promotion Foundation Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was conducted using a secure remote randomization service. The 4 out of 8 district hospitals were randomly assigned to the treatment (co‐joint intervention) and control arms (alcohol only or tobacco only interventions). Patients moderately at risk for conjoint alcohol and tobacco use were randomized to either the treatment or control groups." Pg. 3 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants (clinic staff members and patients) were not blind to their intervention status." Pg. 3 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "However, to protect against information biases in the reporting of substance use, the data collection team who assessed the outcomes were blind to the hospital’s status as intervention." Pg. 3 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Researchers used an intent‐to‐treat analysis by including all participants who completed baseline assessments and who were randomized to intervention conditions." Pg. 5 "Listwise deletion (i.e., using complete cases only) in the context of generalized estimating equations (GEE) was used with missing data. The extent of the missing component was 27.4 % at six months." Pg. 5 High levels and poor handling (complete case analysis) of missing data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Poblete 2017.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Chile Setting: emergency departments, primary care, and police stations Eligibility criteria: Included were individuals between 19‐55 years of age with moderate alcohol and drug use risk. Excluded were individuals at high risk for any substance use (except tobacco), individuals who had received a similar intervention for alcohol or drugs in the last 3 months, individuals with cognitive or communication impairments, individuals in treatment for mental disorders, individuals who had been charged with a crime, and pregnant women. Duration of follow‐up: 3‐months Informed consent: all participants provided informed consent Ethical approvals: Pontifica Universidad Catolica de Chile ethics committee |
|
| Participants |
Sample size: 423 (211 intervention, 212 control) Description of the target population: individuals in emergency departments, primary care, or police stations Age: not reported for moderate alcohol risk subgroup Sex: not reported for moderate alcohol risk subgroup Race/Ethnicity: not reported for moderate alcohol risk subgroup Marital status: not reported for moderate alcohol risk subgroup Harmful alcohol use (baseline): not reported for moderate alcohol risk subgroup Co‐occurring disorders: not reported for moderate alcohol risk subgroup |
|
| Interventions |
Type: non‐pharmacologic Description: ASSIST‐linked brief intervention and self‐help guide including feedback and information, skills training, and motivation enhancement to modify alcohol use Duration and frequency: 1 session lasting 17.6 minutes on average Delivery and provider: social workers and psychologists Comparison group: single session with a healthcare professional lasting 11.9 minutes, on average, where the individual is presented with a pamphlet containing information related to substance use risk and harm |
|
| Outcomes |
Primary outcome(s): alcohol risk Primary outcome measurement tool(s): ASSIST Secondary outcome(s): none Secondary outcome measurement tool(s): none Time points: 0‐, 3‐months |
|
| Notes |
Study funding and conflicts of interest: Study was funded by SENDA Linked study records:https://clinicaltrials.gov/ct2/show/nct01573416 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was stratified using the ASSIST‐ specific substance involvement score: for alcohol, from 11 to 15 and 16 to 20; and for the other substances, from 4 to 12 and 13 to 20. Random‐sequence was generated using SAS version 9.1 for Windows (SAS Institute Inc., Cary, NC, USA) with a 1: 1 allocation, using random block sizes of 50." Pg. 1463 |
| Allocation concealment (selection bias) | Low risk | Quote: "Each site received the sequence in sealed boxes with sealed opaque envelopes. The allocation was performed by the administrative staff and, when this was not possible, by the professional who administered the ASSIST." Pg. 1463 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Due to the nature of the intervention, it was not possible to blind the participants or the providers of the BI." Pg. 1465 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "Finally, it was important that our design included a blinded outcomes assessment which attempted to eliminate any bias in assessing the effects of BI." Pg. 1468 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Analysis was undertaken for every sample following set protocol (62% of the original sample completed follow up) and intention‐to‐treat (ITT). For the ITT analysis it was assumed that participants who did not complete follow‐up (n = 309) remained with the same scores at entry (last observation carried forward)." Pg. 1465 High levels of attrition/missing data |
| Selective reporting (reporting bias) | Low risk | Primary outcome aligns with what is reported in clinicaltrials.gov registration |
Polshkova 2016.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Country: Ukraine Setting: hospitals and universities Eligibility criteria: included were adults 18‐25 who were presenting to the hospital or students in the University who speak and write Ukrainian or Russian and reported and AUDIT‐C score >5. Excluded were individuals with psychosis, lacking competence for consent, having a history of suicide attempts/thoughts, or women who were pregnant. Duration of follow‐up: 3‐months Informed consent: all participants provided informed consent Ethical approvals: not reported |
|
| Participants |
Sample size: 120 (60 brief intervention, 60 control) Description of the target population: Young adults seeking health services or enrolled in University Age: 21.3 years (SD = 2.5) Sex: 45% female Race/Ethnicity: Not reported Marital status: 62.5% married or living with partner Harmful alcohol use (baseline): AUDIT‐C=5.6 (SD = 0.8) Co‐occurring disorders: average depression (PHQ‐9) Score = 8.2 (SD = 3.7), Average (GAD‐7) anxiety score = 12.6 (SD = 3.2) |
|
| Interventions |
Type: non‐pharmacologic Description: motivational interviewing including exploring motivation to change their drinking, reviewing consequences and medical concerns related to drinking, goal‐setting, developing a plan, identifying barriers to change, and providing continued support Duration and frequency: one 5‐minute in‐person session followed by a 10‐15 minute booster session Delivery and provider: psychologists/psychiatrists Comparison group: not reported |
|
| Outcomes |
Primary outcome(s): alcohol consumption Primary outcome measurement tool(s): AUDIT‐C Secondary outcome(s): Alcohol consequences, Abstinent days, Other drug use, Depression, Anxiety, Physical Aggression, Verbal aggression, Quality of life Secondary outcome measurement tool(s): Rutgers Alcohol Problem Index (RAPI), Quality of Life Scale (QLS), Patient Health Questionnaire (PHQ‐9), Generalized Anxiety disorder (GAD‐7), Drug Abuse Screening Test (DAST‐10), Buss Perry Aggression Questionnaire (BPAQ) Time points: 0, 3‐months |
|
| Notes |
Study funding and conflicts of interest: not reported Linked study records: Voloshyna 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was made in a flip a coin way using a list in which every other person was assigned to the BI condition." Pg. 3 |
| Allocation concealment (selection bias) | High risk | Quote: "every other person was assigned to the BI condition" Pg. 3 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Still, it may be that participants in the BI condition, who could not be blind to condition assignment, underreported because they did not believe their data would be confidential, that is, kept private from their clinicians." Pg. 7 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Participants self‐administered a computerized follow‐up assessment after 3 months, either during a return visit at the clinic or school, or online from home." Pg. 3 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Follow‐up rates were 100%, and occurred after 3 months" Pg. 4 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Rendall‐Mkosi 2013.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: South Africa Setting: Primary care and farms in rural communities Eligibility criteria: Included were women age 18‐44 years who were not pregnant, engaged in risky drinking, were using ineffective or no forms of contraception, had not undergone sterilization or hysterectomy, had vaginal sex in the past 3 months, and resided within a 25‐km radius of the main town. Duration of follow‐up: 12‐months Informed consent: All participants provided informed consent Ethical approvals: Faculty of Health Sciences Research Ethics Committees of the Universities of Pretoria and Cape Town |
|
| Participants |
Sample size: 165 (82 intervention, 83 control) Description of the target population: Rural women at risk for alcohol‐exposed pregnancy Age: 29.8 years Sex: 100% female Race/Ethnicity: 98.8% coloured Marital status: Not reported Harmful alcohol use (baseline): AUDIT=19.88, 50% were possible cases of alcohol dependence Co‐occurring disorders: Not reported |
|
| Interventions |
Type: Non‐pharmacologic Description: Motivational interviewing including an informational pamphlet on fetal alcohol syndrome prevention, a women's health handbook, assessing readiness to change, confidence in enacting behavior change, development of behavior change plan, implementation of behavior change plan, reinforce after‐care plan Duration and frequency: 5 sessions Delivery and provider: Trained lay counselor Comparison group: Assessment and informational pamphlet on fetal alcohol syndrome and a women's health handbook |
|
| Outcomes |
Primary outcome(s): At risk for alcohol‐exposed pregnancy Primary outcome measurement tool(s): Locally developed questionnaire Secondary outcome(s): Risky drinking Secondary outcome measurement tool(s): Quantity/frequency questions Time points: 3‐, 12‐months |
|
| Notes |
Study funding and conflicts of interest: Centers for Disease Control and Prevention (CDC) Cooperative Agreement Number 1 U01 DD00044 Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women who qualified were allocated randomly to the MI, life‐skills or control group. A system of sealed envelopes to indicate random group allocation had been prepared in advance based on computerized individual randomization. The life‐skills arm of the study was stopped after recruitment reached approximately 30 women in each group, because of poor adherence to the life‐skills intervention and practical difficulty with implementation precluded continuing with recruitment." Pg. 726 |
| Allocation concealment (selection bias) | Low risk | "A system of sealed envelopes to indicate random group allocation had been prepared in advance" Pg. 726 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Blinding of the fieldworkers regarding the group allocation of the participants was difficult in this small rural community setting. However, fieldworkers were different from the counsellors and trained specifically to administer the questionnaire, and did not conduct the counselling." Pg. 727 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not avilable |
Samet 2015.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Russia Setting: HIV and alcohol use treatment centers (inpatient and outpatient) Eligibility criteria: Included were individuals 18+ years of age, HIV‐positive, report of anal or vaginal sex without a condom in the past 6 months, provision of contact information of two persons, a stable home address within 150 km of St. Petersburg, fluency in Russian, possession of a home or cell telephone, ability to provide informed consent, and report of at‐risk drinking levels in past 30‐days (>14 drinks per week or >4 drinks on a single occasion for men, >7 drinks per week or >3 drinks on a single occasion for women). Five months into the study, inclusion criteria were expanded to include participants with at least one binge drinking day in the past 6 months. Excluded were individuals with cognitive impairment, acute illness precluding participation, pending legal issues that could lead to incarceration, or ongoing efforts to conceive. Duration of follow‐up: 12‐months Informed consent: All participants provided written informed consent Ethical approvals: Boston Medical Center, First St. Petersburg Pavlov State Medical University |
|
| Participants |
Sample size: 700 (350 intervention, 350 control) Description of the target population: HIV‐positive individuals with at‐risk drinking Age: 30.1 years (SD=5.2) Sex: 40.7% female Race/Ethnicity: Not reported Marital status: 35.9% married/living with a partner Harmful alcohol use (baseline): 63.7% alcohol dependent, 81.4% with heavy alcohol use Co‐occurring disorders: 100% HIV‐positive |
|
| Interventions |
Type: Non‐pharmacologic Description: HIV and alcohol risk reduction intervention including discussion about disclosure of HIV serostatus and sexual risk behaviors, including alcohol use Duration and frequency: 5 sessions (2 individual, 3 group sessions) Delivery and provider: Peer interventionists, mixed gender groups Comparison group: General health intervention (5 sessions ‐ 2 individual, 3 group) focused on stress reduction, social support, and nutrition delivered by peer interventionists |
|
| Outcomes |
Primary outcome(s): Alcohol consumption (average number of drinks per drinking day) Primary outcome measurement tool(s): Timeline Followback Secondary outcome(s): Heavy drinking days Secondary outcome measurement tool(s): Timeline Followback Time points: 0‐, 6‐, and 12‐months |
|
| Notes |
Study funding and conflicts of interest: National Institute on Alcohol Abuse and Alcoholism (R01AA016159, U24AA0207708, U24AA020779, K24AA015674) Linked study records: Tsui 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were assigned to either the intervention or control group using stratified‐blocked randomization. The randomization procedure was stratified by gender, injection drug use, and recruitment site (main site [Botkin] vs. all other). Within each stratum, random block sizes of 4, 6, or 8 were used to ensure a comparable number of participants in each treatment group. Randomization was equally likely between the intervention and control groups (i.e., a 1:1 allocation ratio was used)." Pg. 6 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The research participants could not be blinded due to the nature of the intervention." Pg. 6 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "RAs conducting interview assessments were blinded to the individual’s randomization status." Pg. 6 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "For the primary efficacy analyses only the observed data were used, imputation of missing data was not performed. However subsequent sensitivity analyses were performed using multiple imputation to account for missing follow‐up data for the primary outcome. Baseline variables used in the imputation were age, gender, education, CD4 cell count, injection drug use, randomization group, marital status, multiple sex partners, unprotected sex episodes and baseline" Pg. 7 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Satyanarayana 2016.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: India Setting: Inpatient psychiatric services Eligibility criteria: Included were males 21+ years of age admitted to inpatient treatment for alcohol dependence, currently married, having at least 1 child below 16 years of age, screening positive for IPV perpetration in the past 6 months, and reporting that their wife was their primary caregiver. Excluded were patients with comorbid psychiatric diagnoses Duration of follow‐up: 3‐months Informed consent: All participants with their wives provided written informed consent Ethical approvals: St. John's Medical College and Hospital (SJMCH) |
|
| Participants |
Sample size: 177 (88 intervention, 89 control) Description of the target population: Male inpatients with alcohol dependence who have perpetrated IPV in the past 6 months Age: 38 years (SD=6.3) Sex: 100% male Race/Ethnicity: Not reported Marital status: 100% married Harmful alcohol use (baseline): Average severity of alcohol dependence questionnaire (SADQ) score=28.1; Wives reports of husband's drinking categorized 67.5% as heavy drinkers, 69.0% as very severe drinkers, 18.5% as moderately severe drinkers, 1.5% as less severe drinkers, and 11.0% as not having a drinking problem Co‐occurring disorders: Not reported |
|
| Interventions |
Type: Non‐pharmacologic Description: Integrated cognitive behavioral intervention involving discussion about the relationship between alcohol and IPV, triggers for alcohol use and IPV, consequences and prevention of IPV, relaxation, anger management, assertiveness training, and cognitive restructuring Duration and frequency: Eight 45‐60 minute sessions Delivery and provider: Master's level clinical psychologist Comparison group: Routine home‐based care including pharmacotherapy and 1 session of psychoeducation about alcohol dependence, symptom manifestations, possible treatment options, preventing relapse, and adherence to treatment delivered by a master's level clinical psychologist |
|
| Outcomes |
Primary outcome(s): Alcohol consumption Primary outcome measurement tool(s): Severity of Alcohol Dependence Questionnaire (SADQ) Secondary outcome(s): Intimate partner violence perpetration (severity) Secondary outcome measurement tool(s): Index of Spouse Abuse (ISA; reported by wife) Time points: 0, 1, 3‐months |
|
| Notes |
Study funding and conflicts of interest: Indian Council of Medical Research (ICMR #5/11/6/2010‐SBR) Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random numbers were used for this purpose and the random allotment of each participant was available in sealed envelopes which were opened by the study coordinator following baseline assessment." Pg. 30 |
| Allocation concealment (selection bias) | Low risk | "Computer generated random numbers were used for this purpose and the random allotment of each participant was available in sealed envelopes which were opened by the study coordinator following baseline assessment." Pg. 30 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "Follow‐up assessment was carried out 1 month (n = 163; 88 in ICBI and 75 in TAU) and 3 months (n = 156; 87 in ICBI and 69 in TAU) after the intervention on relevant outcome variables by a research assistant who was blind to the randomization" Pg. 30 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Schaub 2021.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Belarus, Brazil, India, Mexico Setting: Online Eligibility criteria: Adults (18‐75 years) who reside in one of the participating countries, screen positive for hazardous or harmful alcohol use, and have weekly internet access were included. Individuals were excluded if they reported current use of opioids, inhalants, cocaine/crack, amphetamine/amphetamine‐like stimulants, or sedatives over the past month. Individuals were also excluded if they reported cannabis or synthetic cannabinoid use for more than four days in the past month. Duration of follow‐up: 6 months Informed consent: Online informed consent document was submitted by all participants prior to baseline Ethical approvals: Study procedures were approved by the WHO Ethics Review Committee and four ethics committees in the participating countries. |
|
| Participants |
Sample size: 1400 (687 intervention, 713 control) Description of the target population: Adults with hazardous alcohol use in Belarus, Brazil, India, and Mexico Age: 37.6 years (SD=10.5) Sex: 70.1% male Race/Ethnicity: Not reported Marital status: Not reported Harmful alcohol use (baseline): Mean AUDIT=23.0 (SD=7.7); Average number of standard drinks in past week=43.7 (SD=41.4) Co‐occurring disorders: Not reported |
|
| Interventions |
Type: Non‐pharmacologic Description: Web‐based self‐help program that aimed to help people reduce or discontinue their alcohol use. The Alcohol e‐Health program encouraged people to think about their drinking habits, decide whether the change their behaviors and set goals, take action toward their goals and monitor progress, and manage relapses. Duration and frequency: 6 weeks Delivery and provider: Self‐guided Comparison: Referred to a website with information about alcohol use, wait list control |
|
| Outcomes |
Primary outcome(s): Hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): Alcohol consumption, alcohol‐free days, cessation of harmful/hazardous drinking Secondary outcome measurement tool(s): Single question asking about alcohol use each day in the past week; AUDIT Time points: 0, 6 months |
|
| Notes |
Study funding and conflicts of interest: World Health Organization; Associacao Fundo de Incentivo a Pesquisa, CNPq, CAPES, and the Sao Paulo Research Foundation (FAPESP) Linked study records: Formigoni 2018; Schaub 2018 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized, by a computer, to either the Alcohol e‐Health program or control group, in a 1:1 ratio in each country" |
| Allocation concealment (selection bias) | Unclear risk | "This nonblinded assignment was registered automatically in the background database" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "This nonblinded assignment was registered automatically in the background database" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "This nonblinded assignment was registered automatically in the background database" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High levels of attrition (>50%) |
| Selective reporting (reporting bias) | Low risk | Outcomes are consistent with study protocol |
Segatto 2011.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Brazil Setting: Emergency department Eligibility criteria: Included were adolescents (16‐25 years) who were seeking care in the emergency department and had consumed alcohol within the past 6 hours. Excluded were individuals without a permanent address within the Sao Paulo, who couldn't complete the interview due to a severe physical condition, having a psychotic disorder or mental/cognitive difficulties, were under arrest, or were receiving substance use treatment. Duration of follow‐up: 3‐months Informed consent: All participants provided written informed consent Ethical approvals: Universidade Federal de Sao Paulo (UNIFESP), Universidade Federal de Uberlandia (UBU) Ethics Committee |
|
| Participants |
Sample size: 175 (87 intervention, 88 control) Description of the target population: Adolescents/young adults seeking care in the emergency department for alcohol‐related problems Age: 21.8 years (SD=2.6) Sex: 9.7% female Race/Ethnicity: 71.4% Caucasian Marital status: 27.4% married Harmful alcohol use (baseline): Days of use: 10.4 (SD=18.8); Alcohol‐related problems (RAPI) score: 1.00 (SD=0.84); 36.6% met criteria for alcohol dependence Co‐occurring disorders: Not reported |
|
| Interventions |
Type: Non‐pharmacologic Description: Motivational interviewing focused on developing skills and arguments in favor of behavior change, offering information and guidance, removing barriers, encouraging reflection, establishing a plan for change, an educational brochure, and following up on plan development by telephone Duration and frequency: One 45‐minute session followed by telephone contact at 1‐ and 3‐months Delivery and provider: Senior psychologist Comparison group: One 5‐minute session delivered by a junior research psychologist (post‐graduate or master's level) involving an educational brochure with guidance on alcohol consumption and tips to reduce or avoid alcohol‐related problems followed by telephone contact at 1‐ and 3‐months |
|
| Outcomes |
Primary outcome(s): Days of alcohol use Primary outcome measurement tool(s): Alcohol consumption questionnaire (ACQ) Secondary outcome(s): Days of light, moderate, and heavy alcohol use, Alcohol‐related problems Secondary outcome measurement tool(s): Rutgers Alcohol Problem Index (RAPI), Alcohol Consumption Risk QUestionnaire (ACRQ) Time points: 0‐ and 3‐months |
|
| Notes |
Study funding and conflicts of interest: None Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible subjects were randomized at baseline to one of two groups: motivational interviewing group (MI) plus educational brochure group (EB) or EB alone. A lottery system was employed and it was performed by ER personnel not linked to the clinical trial in order to avoid selection bias" Pg. 227 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention "Patients were blinded to the intervention applied." Pg. 228. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Analyses were performed to subjects who completed the process ("completers")." Pg. 228 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Sheikh 2017.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Zambia Setting: Hospital Eligibility criteria: Included were individuals who met DSM‐IV‐TR criteria for alcohol dependence and were admitted to a hospital with primary alcohol problems Duration of follow‐up: 8‐weeks Informed consent: All participants provided consent Ethical approvals: Biomedical Research Ethics Committee of the University of Zambia |
|
| Participants |
Sample size: 114 (58 intervention, 56 control) Description of the target population: Patients seeking treatment for alcohol problems at a hospital Age: Mean=32 years, Range=18‐65 years Sex: 3.5% female Race/Ethnicity: Not reported Marital status: 30% married Harmful alcohol use (baseline): All scored >15 on the AUDIT Co‐occurring disorders: Not reported |
|
| Interventions |
Type: Non‐pharmacologic Description: Family‐based brief relapse prevention intervention from mhGAP with involvement from a close family member following detoxification with diazepam and vitamin supplementation. The brief relapse prevention intervention included discussion of alcohol‐related problems, attitudes toward alcohol and the problems it has caused, actively educating and involving the family, and self‐help groups. Duration and frequency: One 20‐minute interview with the patient and family member Delivery and provider: Psychosocial counselor Comparison group: Detoxification only with diazepam and vitamin supplementation |
|
| Outcomes |
Primary outcome(s): Hazardous alcohol use Primary outcome measurement tool(s): AUDIT‐C and questions about drinking habits Secondary outcome(s): Time to first drink Secondary outcome measurement tool(s): AUDIT‐C and questions about drinking habits Time points: 0‐, 8‐weeks |
|
| Notes |
Study funding and conflicts of interest: Not reported Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization and statistical analyses were performed using sealed envelopes containing patient assignment devised from the randomization facility of the statistics program, Statistical Package for the Social Sciences (SPSS), version 20." Pg. 114 |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes containing patient assignment" Pg. 114 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All 114 consecutive admissions with primary alcohol problems agreed to take part, and follow‐up information was obtained for all participants at 8 weeks (refusal rate 0%; follow‐up rate 100%)." Pg. 115 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Shin 2013.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Russia Setting: Tuberculosis treatment clinic; Inpatient and outpatient hospital setting Eligibility criteria: Included were patients with tuberculosis (TB) who were registered for TB therapy and initiated treatment in one of the three study sites along with a diagnosis of alcohol abuse or dependence. Excluded were individuals under 18 years of age, patients who had liver function tests three times above the upper limit of normal range, patients who reported opioid use in the past month or returned positive urine screens for opioids, patients who were pregnant or breastfeeding, and patients with any other co‐occurring medical or psychiatric condition that would affect their ability to participate. Duration of follow‐up: 6‐months Informed consent: Not reported Ethical approvals: Partners Healthcare IRB and the State Research Center Virology and Biotechnology, Novosibirsk Region and the Siberian State Medical University of the Gederal Agency for Healthcare and Social Development IRB |
|
| Participants |
Sample size: 196 (53 brief counseling, 47 naltrexone, 45 brief counseling + naltrexone, 51 treatment as usual) Description of the target population: Patients in treatment for tuberculosis Age: 40.9 years (SD=11.2) Sex: 17.9% female Race/Ethnicity: Not reported Marital status: 37.8% married/cohabitating Harmful alcohol use (baseline): AUDIT=18.3 (SD=7.6); 63% met criteria for alcohol dependence Co‐occurring disorders: Tuberculosis |
|
| Interventions |
Type: Non‐pharmacologic; Pharmacologic; Non‐pharmacologic+pharmacologic Description: A) Brief counseling: an adapted version of NIAAAs Helping Patients with Alcohol Health Practitioner's Guide involving motivational interviewing embedded within treatment as usual; B) Naltrexone: Naltrexone along with brief adherence counseling embedded within treatment as usual; C) Combined brief counseling and naltrexone (study conditions A+B) Duration and frequency: Brief counseling involved six 10‐15 minute sessions delivered monthly. Naltrexone was prescribed as 50 mg/day for six months and six 5‐10 minute monthly adherence counseling sessions. The combined brief counseling and naltrexone condition involved six 15‐25 minute sessions focused on brief counseling and naltrexone adherence counseling. Delivery and provider: Tuberculosis physicians administered the brief counseling. Naltrexone was administered by TB clinic staff Comparison group: Treatment as usual included psychotherapy, disulfiram, and placebo implants administered by a narcologist/addiction specialist |
|
| Outcomes |
Primary outcome(s): Abstinence Primary outcome measurement tool(s): Timeline Followback Secondary outcome(s): Heavy drinking days Secondary outcome measurement tool(s): Timeline Followback Time points: 6‐months |
|
| Notes |
Study funding and conflicts of interest: National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse (R01AA016318, K24DA019855) Linked study records: clinicaltrials.gov NCT00675961 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Non‐blinded randomization assignment was generated by computer by the study team." Pg. 3 |
| Allocation concealment (selection bias) | High risk | "Non‐blinded randomization assignment was generated by computer by the study team." Pg. 3 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Non‐blinded randomization assignment was generated by computer by the study team." Pg. 3 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "We performed analysis based on "intention‐to‐treat". To address missing data on alcohol endpoints, log‐linear regression analyses were performed for multivariable analysis on datasets multiply imputed using Markov Chain Monte Carlo methods" Pg. 5 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Signor 2013.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Brazil Setting: Telephone‐based intervention Eligibility criteria: Included were self‐reported alcohol users from Brazil who had consumed alcohol in the past month and who were seeking help for alcohol use problems through a hotline or a health provider. Excluded were individuals who were unable to answer interview questions due to cognitive difficulties or the effects of alcohol/drugs and those who could not be contacted for a follow‐up interview. Duration of follow‐up: 6‐months Informed consent: Not reported Ethical approvals: Universidade Federal de Ciencias de Saude de Porto Alegre (UFCSPA) Ethics Committee |
|
| Participants |
Sample size: 637 (293 intervention, 344 control) Description of the target population: Help‐seeking individuals in general population Age: 27 years (Range 21‐37) Sex: 29% female Race/Ethnicity: Not reported Marital status: 30% married or cohabitating Harmful alcohol use (baseline): 84% met criteria for dependence Co‐occurring disorders: Not reported |
|
| Interventions |
Type: Non‐pharmacologic Description: Brief motivational intervention including self‐help material sent by mail, a 20‐minute brief motivational interview session by phone along with a 10‐minute assessment, and 7 follow‐up calls over a 6‐month period Duration and frequency: One 30‐minute session and 7 follow‐up calls at 1 day, 3 days, 7 days, 1 month, 2 months, 3 months and 6 months Delivery and provider: Trained university students from health programs Comparison group: Necessary advice provided by phone from trained university students from health programs and self‐help material sent by mail |
|
| Outcomes |
Primary outcome(s): Abstinence Primary outcome measurement tool(s): Not reported Secondary outcome(s): Not reported Secondary outcome measurement tool(s): N/A Time points: 6‐months |
|
| Notes |
Study funding and conflicts of interest: Brazilian National Secretariat on Drug Policies (Secretaria Nacional Antidrogas, SENAD), Associacao Mario Tannhauser de Ensino Pesquisa e Assistencia (AMTEPA), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Coordenacao de Aperfeicoamento de Pessoal d Nivel Superior Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group allocation was performed using a Microsoft H software application that applied a random number technique." Pg. 255 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "Counselors were blinded to the collection of follow‐up data by a random assignment that used scripts to guide the telephone interviews." Pg. 256 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Based on continuous abstinence time and the ITT principle, in which dropouts are considered people who relapse, a Cox proportional hazards model detected a significant difference between the BMI and control groups" Pg. 256 "The study attrition rate was 76.9% (490 participants dropped out)." Pg. 256 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Simao 2008.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Brazil Setting: University Eligibility criteria: Included were students identified as at‐risk drinkers (AUDIT>=8 and 5+ harmful alcohol‐related consequences). Excluded were students from the areas of Humanities and Exact Sciences and participants with drug dependence (not including tobacco dependence and occasional use of marijuana). Duration of follow‐up: 24‐months Informed consent: All participants provided written informed consent Ethical approvals: Medical School of Botucatu‐Sao Paulo State University (UNESP) |
|
| Participants |
Sample size: 266 (145 intervention, 121 control) Description of the target population: University students who are at‐risk drinkers Age: 19.6 years (SD=1.8) Sex: 43.6% female Race/Ethnicity: Not reported Marital status: 100% single Harmful alcohol use (baseline): AUDIT=9.7 (SD=3.6), ADS=5.7 (SD=3.9), RAPI=7.3 (SD=5.9), Drinks per weekend=4.2 (SD=2.2), Frequency of drinking per week=2.6 (SD=1.0), Drinks on occasion=3.4 (SD=1.3) Co‐occurring disorders: Not reported |
|
| Interventions |
Type: Non‐pharmacologic Description: Brief Alcohol Sreening and Intervention for College Students (BASIC) involving motivational interviewing and harm reduction. Specific components were review of drinking patterns and comparable norms, discussion of alcohol‐related problems, belief about alcohol's effects, and suggestions to reduce alcohol consumption Duration and frequency: Not reported Delivery and provider: Social assistants specialized in mental health, clinical psychology, or psychiatry Comparison group: No intervention |
|
| Outcomes |
Primary outcome(s): Hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): Drinks per drinking day, Frequency of drinking, Number of drinks in past 30 days, Number of drinks per weekend, Hazardous alcohol use, Alcohol‐related consequences Secondary outcome measurement tool(s): Rutgers Alcohol Problem Inventory (RAPI), Brief Drinker Profile (BDP), Alcohol Dependence Scale (ADS) Time points: 0, 12, 24 months |
|
| Notes |
Study funding and conflicts of interest: Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP, 00/03583‐7) Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Thapinta 2017.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Thailand Setting: Hospital‐based primary care Eligibility criteria: Included were patients seeking primary care between 18‐60 years of age with alcohol dependence, mild depression, and normal communication, literacy, and intelligence. Excluded were individuals receiving anti‐depressant medication, alcohol medication, or cognitive behavioral therapy. Excluded were also individuals with a current diagnosis of psychosis by a psychiatrist or any physical illness that required inpatient care. Duration of follow‐up: 6‐months Informed consent: All participants provided written informed consent Ethical approvals: Chiang Mai University Ethics Committee |
|
| Participants |
Sample size: 350 (175 intervention, 175 control) Description of the target population: Patient seeking primary care with mild depression and alcohol dependence Age: 39.0 years Sex: 11.8% female Race/Ethnicity: Not reported Marital status: Not reported Harmful alcohol use (baseline): Age at first drink: 18.4 years Co‐occurring disorders: Mild depression (100%; PHQ‐9=5‐8) |
|
| Interventions |
Type: Non‐pharmacologic Description: Cognitive behavioral therapy (CBT) self help booklet Duration and frequency: Usual care with CBT booklet containing 5 components including instructions on how to use the book, explanations and examples of negative automatic thoughts, psychoeducation, the CBT model, and exercises to help patients practice identifying negative automatic thoughts, evaluate negative automatic thoughts, respond to them, and engage in problem solving where negative automatic thoughts were not distortions of observable reality Delivery and provider: 1 session with a nurse + self‐directed activities guided by the book; Patient could contact nurse throughout study Comparison group: Usual care including self‐care for depression, a brief explanation about depression (causes, signs/symptoms, counseling) and medical treatment for physical symptoms delivered by a primary healthcare worker |
|
| Outcomes |
Primary outcome(s): Alcohol consumption Primary outcome measurement tool(s): Timeline Followback Secondary outcome(s): Depression Secondary outcome measurement tool(s): Patient Health Questionnaire (PHQ‐9) Time points: 0‐months, 1‐week, 1‐month, 3‐months, 6‐months |
|
| Notes |
Study funding and conflicts of interest: Thai Health Promotion Foundation and Integrated Management for Alcohol Intervention Program (I‐MAP) Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "simple random sampling was used to assign participants into either (i) CBT‐SHB with usual care or (ii) usual care alone (n = 175)" Pg. 965 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "This was single‐blind randomized controlled trial that com‐ pared the CBT‐SHB and usual care with usual care alone. Outcome assessors were blind to the assignment of participants to the study groups to minimize bias in collecting data." Pg. 965 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "Outcome assessors were blind to the assignment of participants to the study groups to minimize bias in collecting data." Pg. 965 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Dropout of subjects in both groups, is the limitation of this study. Although dropout may impact the number of subjects that we calculated and cannot meet power analysis minimum, the size was still good and we found statistically significant results." Pg. 969 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Thomas 2017.
| Study characteristics | ||
| Methods |
Study design: Cluster randomized controlled trial Country: India Setting: Chennai City zones where TB investigations and treatment are offered through the Revised National Tuberculosis Control Programme (RNTCP) Eligibility criteria: Included were zones with TB investigations and treatment offered through the RNTCP in Chennai. Participants were included if they were new TB patients, 18 years and older who were registered for an anti‐tuberculosis treatment. Duration of follow‐up: 6‐months Informed consent: All participants provided written informed consent Ethical approvals: Study procedures were approved by the National Institute for Research in Tuberculosis, the Indian Council of Medical Research, and Chennai Corporation |
|
| Participants |
Sample size: 298 (113 alcohol counseling, 185 usual care) Description of the target population: Patients with tuberculosis Age: 44 years Sex: Not reported Race/Ethnicity: Not reported Marital status: 72% married Harmful alcohol use (baseline): 37% hazardous alcohol use, 20% harmful use, 33% dependence Co‐occurring disorders: 76% were new cases of tuberculosis, 24% were retreatment cases |
|
| Interventions |
Type: Non‐pharmacologic Description: Alcohol counseling including discussion of the relationship between alcohol and tuberculosis, and the effect of alcohol on physical health, family and society. Duration and frequency: Four 45‐60 minute sessions at 0, 2, 4, and 6 months Delivery and provider: Trained lay counselor Comparison group: Usual care for tuberculosis without alcohol counseling |
|
| Outcomes |
Primary outcome(s): Alcohol consumption Primary outcome measurement tool(s): AUDIT Secondary outcome(s): Adherence to anti‐tuberculosis treatment Secondary outcome measurement tool(s): Regular adherence, irregular adherence, or lost to follow‐up Time points: 0 and 6 months |
|
| Notes |
Study funding and conflicts of interest: UNAIDS Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "This was a pilot, two‐arm, parallel‐cluster randomised trial carried out in four zones (TB units) where TB investigations and treatment are offered through the Revised National Tuberculosis Control Programme (RNTCP). The four zones were divided into two strata, two of which were high‐prevalence and two were low‐prevalence zones. Within each stratum, one zone was allocated to the intervention arm and the other to the control arm. Here the zones were the units of randomisation (clusters)" Pg. 948 |
| Allocation concealment (selection bias) | High risk | "For administrative purposes, Chennai City is divided into 10 corporation zones and 155 divisions. This was a pilot, two‐arm, parallel‐cluster randomised trial carried out in four zones (TB units) where TB investigations and treatment are offered through the Revised National Tuberculosis Control Programme (RNTCP). The four zones were divided into two strata, two of which were high‐prevalence and two were low‐prevalence zones. Within each stratum, one zone was allocated to the intervention arm and the other to the control arm. Here the zones were the units of randomisation (clusters)" Pg. 948 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Information on overall treatment adherence was available for 104 (92%) intervention group participants and for 173 (94%) control group participants." Pg. 950 |
| Selective reporting (reporting bias) | High risk | Does not provide quantitative results by alcohol risk level. |
Vlasova 2011.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Ukraine Setting: Alcohol and drug treatment center Eligibility criteria: Included were new admissions 19 years and above to the treatment center with a primary diagnosis of alcohol dependence and willing to participate in 4 months of an outpatient treatment program following completion of 30+ days of inpatient treatment. Excluded were people with severe psychiatric disorder or organic brain syndrome preventing their ability to provide informed consent Duration of follow‐up: 4 months Informed consent: All participants provided written informed consent Ethical approvals: University of Alabama Birmingham Instutional Review Board, Vinnitsya National Medical University‐Pirogov Ethics Board |
|
| Participants |
Sample size: 68 (34 intervention, 34 control) Description of the target population: Patients admitted to inpatient treatment for alcohol use disorder Age: 37.2 years (SD=11.8) Sex: 14.7% female Race/Ethnicity: Not reported Marital status: Not reported Harmful alcohol use (baseline): 100% reported past 30‐day alcohol use and met criteria for alcohol dependence Co‐occurring disorders: Not reported |
|
| Interventions |
Type: Non‐pharmacologic Description: Inpatient treatment followed by intensive outpatient treatment including individual sessions, 12‐step facilitation, weekly aftercare, and sponsors group Duration and frequency: 30+ day inpatient medical treatment, 2 months of 12‐step oriented day treatment (8 hours per day), 1 month of aftercare (2 hours per week), and 1 month of sponsors group (2 hours per week) Delivery and provider: Physicians, social workers, psychologists, nurses Comparison group: Usual care (inpatient treatment) involving medical detoxification, acute medical care, primary medical care, and some counseling and social services for 30 days provided by physicians, social workers, psychologists, and nurses |
|
| Outcomes |
Primary outcome(s): Alcohol consumption (days) Primary outcome measurement tool(s): Form 90 Secondary outcome(s): Drinks per day, Heavy drinking days, Alcohol‐related consequences, Mental health, Depression Secondary outcome measurement tool(s): Form 90, Drinker Inventory of Consequences, Short Form‐36 Version 2 Health Survey, Beck Depression Inventory II Time points: 2, 4 months |
|
| Notes |
Study funding and conflicts of interest: National Institute of Health and Fogarty International Center Linked study records: Vlasova 2005 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were recruited from interested alcohol‐dependent patients admitted to the RND early in treatment. They were assessed and randomly assigned to 1 of the 2 intervention groups." Pg. 8 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Wandera 2017.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Uganda Setting: Hospital‐based infectious disease clinic Eligibility criteria: Included were adult participants receiving HIV/AIDS care in an infectious disease clinic who reported an AUDIT‐C score of 3 or higher. Excluded were participants who were very sick or pregnant. Duration of follow‐up: 6 months Informed consent: All participants provided written informed consent Ethical approvals: Infectious Disease Institute, Makerere University School of Public Health Research and Ethics Committee; Uganda National Council of Science and Technology |
|
| Participants |
Sample size: 337 (167 brief intervention, 170 control) Description of the target population: HIV‐positive patients in an infectious disease clinic Age: Median=39 (IQR=32, 46) Sex: 34.4% female Race/Ethnicity: Not reported Marital status: 55.4% married/cohabitating Harmful alcohol use (baseline): AUDIT‐C=6.6 (SD=2.3); AUDIT=11.3 (SD=6.2); Number of drinking days in past month=9.1 (SD=8.9); Number of US standard drink equivalents on a typical drinking day Median=4.8 (IQR=4,7) Co‐occurring disorders: 100% HIV‐positive; Depression: 26.7% (CESD>=10) |
|
| Interventions |
Type: Non‐pharmacologic Description: Brief intervention including standardized prevention intervention with advice and brief counseling based on motivational interviewing (reflective listening, goal setting) Duration and frequency: 10‐30 minutes of information/advice + 20‐30 minutes of brief counseling Delivery and provider: Counselor with a bachelor's degree and 5+ years of clinical experience in HIV clinical counseling strategies Comparison group: Brief information and advice related to opportunistic infections, nutrition, medication adherence, encouraging HIV disclosure to sexual partners, preventing STIs, safer sexual behaviors, and avoiding alcohol and substance use for 10‐30 minutes by a bachelor's‐level counselor with 5+ years of clinical experience in HIV clinical counseling |
|
| Outcomes |
Primary outcome(s): Hazardous alcohol use Primary outcome measurement tool(s): AUDIT‐C, AUDIT Secondary outcome(s): Number of drinks per drinking day, Median drinking days Secondary outcome measurement tool(s): Timeline Followback Time points: 0, 3, 6 months |
|
| Notes |
Study funding and conflicts of interest: Wellcome Trust: Training of Health Researchers into Vocational Excellence in East Africa (THRIVE ) Consortium grant no. 087540; National Institute of Health K24AA022586 Linked study records: clinicaltrials.gov: NCT01802736 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was based on a computer‐ generated random numbers using permuted blocks of 6, 8, and 10 for an overall total of 15 blocks and assigned to the 2 arms randomly in a 1:1 ratio." Pg. 277 |
| Allocation concealment (selection bias) | Low risk | "Participants were randomized using previously prepared, consecutively numbered, opaque, sealed envelopes. Inside the envelopes was a written paper marked with the treatment arm assignment. Treatment allocation was done by the study nurse who provided assessment interview by opening the next sequentially numbered sealed opaque envelope to reveal the participant’s study arm." Pg. 277 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "To examine the robustness of our primary findings to miss‐ ing data or categorical outcome variable, a set of 2 sensitivity analyses were conducted. The primary linear mixed model was rerun after multiple imputation of missing data. Because we had nonmonotone missingness, we used the Markov chain Monte Carlo method to create 20 imputed data sets that were further analyzed as the primary outcome analysis and their results combined using the PROC MIanalyze function in SAS." Pg. 279 "Statistical analyses were carried out using SAS V.9.1.3 (SAS institute) based on intention‐to‐treat principle, and a P value <.05 was considered statistically significant" Pg. 278 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
WHO Brief Intervention Study Group 1996.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Country: Kenya, Mexico, Russia, Zimbabwe Setting: Hospital wards, emergency departments, primary care clinics, teachers college, or health screening agency Eligibility criteria: Included were heavy (more than 50g/day for men or 32 g/day for women and drinking to intoxication) and frequent adult (18‐70 years) drinkers who were at risk of accidents, arrests, poor job performance, or other social problems. Excluded were people with a prior history of serious mental illness, liver damage, or alcohol dependence. The study also excluded pregnant women, people who had been warned by a doctor or other professional to refrain completely from alcohol, people with past or recent history of morning drinking, people with recent consumption of extremely high amounts of alcohol, social/residential instability, and people who were too impaired to qualify for this level of treatment. Duration of follow‐up: 20 months Informed consent: Not reported Ethical approvals: All sites followed appropriate ethical review procedures recommended by the World Health Organization |
|
| Participants |
Sample size: 681 Description of the target population: People in health and education settings Age: 35.9 years (female), 36.9 years (males) Sex: 36% female Race/Ethnicity: Not reported Marital status: Not reported Harmful alcohol use (baseline): Not reported Co‐occurring disorders: Not reported |
|
| Interventions |
Type: Non‐pharmacologic Description: Brief counseling involving a health interview, counseling about drinking, and an informational pamphlet. In Mexico and Russia, participants received extended counseling Duration and frequency: One 20‐minute health interview + 15 minutes of counseling (and, in Mexico and Russia, 3 follow‐up visits) Delivery and provider: Health advisors (nurses, doctors, psychologists, and other professionals) Comparison group: 1) Simple advice including a single 20‐minute health interview followed by 5 minutes of brief advice about alcohol use and abstinence, the alcohol content of local drinks, and drinking limits/goals delivered by a health advisor; 2) No treatment involved a single 20 minute health interview delivered by a health advisor |
|
| Outcomes |
Primary outcome(s): Alcohol consumption Primary outcome measurement tool(s): Not reported Secondary outcome(s): Intensity of drinking Secondary outcome measurement tool(s): Not reported Time points: 9 months |
|
| Notes |
Study funding and conflicts of interest: Division of Mental Health at the World Health Organization; Australian National Campaign Against Drug Abuse; US National Institute on Alcohol Abuse and Alcoholism; Mexican National Council of Science and Technology; Russian National Research Center for Alcoholism and Drug Addiction; United Kingdom Alcohol Education Research Council; Zimbabwe Ministry of Health Linked study records: Ivanets 1991, Babor 1994 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "an intention to treat sample consisting of both appropriately and inappropriately randomized subjects was used in conducting all analyses." Pg. 953 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "an intention to treat sample consisting of both appropriately and inappropriately randomized subjects was used in conducting all analyses." Pg. 953 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Witte 2011.
| Study characteristics | ||
| Methods |
Study design: Cluster randomized controlled trial Country: Mongolia Setting: HIV/STI risk reduction center Eligibility criteria: Included were women 18 years or older, currently enrolled in the National AIDS Foundation program, had engaged in sexual intercourse in the past 90‐days in exchange for money/alcohol/other goods, engaged in unprotected sex in the past 90‐days with a paying sexual partner, and met criteria for harmful alcohol use (AUDIT >=8). Excluded were women with severe cognitive or psychiatric impairment that would interfere with her ability to provide consent or complete assessments. Duration of follow‐up: 6‐months Informed consent: Participants provided informed consent Ethical approvals: Approved annually by the Institutional Review Boards at Columbia University and the National University of Mongolia in Ulaanbaatar |
|
| Participants |
Sample size: 166 (58 intervention, 49 risk reduction control, 59 wellness control) Description of the target population: Women with harmful alcohol use who have recently engaged in sex work and are enrolled in an HIV/STI risk reduction program Age: 9.6% under 25 years of age Sex: 100% female Race/Ethnicity: Not reported Marital status: Not reported Harmful alcohol use (baseline): Mean AUDIT score: 30.8 Co‐occurring disorders: 1.2% were HIV‐positive, 19.5% reported STI symptoms in the past year |
|
| Interventions |
Type: Non‐pharmacologic Description: HIV/STI risk reduction + motivational interviewing Duration and frequency: 4 group HIV/STI risk reduction sessions and 2 motivational interviewing sessions, 90‐minutes per session Delivery and provider: Female facilitators Comparison group: A) 4 group HIV/STI risk reduction sessions (90 minutes/session); B) 4 weekly wellness knowledge and skill sessions |
|
| Outcomes |
Primary outcome(s): Harmful alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): Number of unprotected vaginal sexual acts with paying partners, Proportion of unprotected vaginal sexual acts with paying partners Secondary outcome measurement tool(s): Adapted Timeline Follow‐back Time points: 2‐weeks, 3‐months, 6‐months |
|
| Notes |
Study funding and conflicts of interest: National Institute of Alcohol and Alcohol Abuse (R21AA016286) Linked study records: Carlson 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random number generated was used to randomly assign a new group of participants" Pg. 1786 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "For the first two groups, the PI disclosed the group condition to the study team so that they could prepare for the session ahead of time." Pg. 1787 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention "All assessment staff was blinded to participant intervention assignment." Pg. 1787 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential loss to follow‐up between groups (Figure 1; Pg. 1787) "All women were considered as randomized, and therefore (based on an intention to treat approach) were considered part of the study." Pgs. 1786‐1787 "Analysis of data from participants missing due to follow‐up (approximately 25% at each time point) indicated that young, unmarried women and women with higher income were more likely to be missing in the surveys after intervention. To account for missing data, the pretreatment attributes of age, income, and marital status were included in the model." Pg. 1789 "As in may HIV prevention trials where time passes between randomization and the first session, this strategy yielded considerable attrition, which could have potentially biased the treatment effect estimates." Pg. 1787 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Xiaolu 2017.
| Study characteristics | ||
| Methods |
Study design: Cluster randomized controlled trial Country: China Setting: Outpatient hospital clinics Eligibility criteria: Included were patients who were residents of Wenchuan county, 18+ years of age, capable of communicating, not in alcohol treatment, not pregnant, without mental illness, and reported an AUDIT score >= 7. Duration of follow‐up: 3 months Informed consent: All participants provided written informed consent Ethical approvals: Shanghai Mental Health Center ethics review board |
|
| Participants |
Sample size: 239 (118 intervention, 121 control) Description of the target population: Participants seeking outpatient hospital‐based care in a post‐earthquake setting Age: 51.57 years (SD=12.81) Sex: 0.84% female Race/Ethnicity: Not reported Marital status: 92.47% married Harmful alcohol use (baseline): AUDIT=19.41 (SD=4.55) Co‐occurring disorders: High levels of anxiety and depressive symptoms |
|
| Interventions |
Type: Non‐pharmacologic Description: Motivational interviewing and general health education Duration and frequency: One 15‐30 minutes brief intervention Delivery and provider: Hospital staff who received training in brief interventions Comparison group: General health education |
|
| Outcomes |
Primary outcome(s): Hazardous alcohol use Primary outcome measurement tool(s): AUDIT Secondary outcome(s): Anxiety, Depression Secondary outcome measurement tool(s): Self‐Rating Anxiety Scale (SAS), Self‐Rating Depression Scale (SDS) Time points: 0, 3 months |
|
| Notes |
Study funding and conflicts of interest: China‐WHO cooperation project (CHN‐12‐MNH‐003426); National Nature Science Foundation (U1502228); Shanghai Municipal Health and Family Planning Commission Joint Research Project (2014YJB0002) Linked study records: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "From 18 village hospitals in Beichuan county, 9 hospitals were randomly selected as intervention hospitals with the remaining 9 as control hospitals." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel cannot be blinded for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes reported by participant not blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
ASSIST: Alcohol, Smoking and Substance Involvement Screening Test; ASSIST:; AUDIT: Alcohol Use Disorder Identification Test; CETA: Common Elements Treatment Approach; DSM IV: Diagnostic and Statistical Manual of Mental Disorders, fourth edition; ICD‐10:International Statistical Classification of Diseases 10th edition;; ITT: intention‐to‐treat; LMICs: low‐ and middle‐income countries ; mhGAP: Mental Health Gap Action Programme; GGT: MI: myocardial infarction; PEth: Phosphatidylethanol testing; RCT: randomized controlled trial; SD: standard deviation STAI: State‐Trait Anxiety Inventory.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aira 2013 | Wrong population ‐ study included some non‐drinkers and non‐problematic drinkers. There was no subgroup analysis that included only people with unhealthy alcohol use |
| Burnhams 2015 | Wrong population ‐ study included some non‐drinkers and non‐problematic drinkers. There was no subgroup analysis that included only people with unhealthy alcohol use |
| Chaudhury 2016 | Wrong population ‐ majority of sample were non‐drinkers or non‐problematic drinkers |
| Dawson 2012 | Wrong intervention ‐ universal community education program |
| Holla 2020 | Wrong intervention ‐ transcranial direct current stimulation (tDCS) |
| Kalichman 2007 | Wrong population ‐ majority of sample were non‐problematic drinkers |
| Kalichman 2008 | Wrong population and outcomes ‐ not all participants met criteria for hazardous alcohol use. The authors did conduct a moderation analysis by heavy drinking, but the primary outcomes were related to high‐risk sexual behaviors as opposed to alcohol use. |
| Kalichman 2011 | Wrong population ‐ study included non‐drinkers. There is a moderation analysis that evaluates the effect of intervention on sexual risk outcomes for light and heavy drinkers, but this analysis is not available for alcohol outcomes. |
| Mertens 2014 | Wrong population ‐ included participants with non‐alcohol substance use and did not include a subgroup analysis of participants with problematic alcohol use |
| Myers 2016 | Wrong population ‐ included participants with non‐alcohol substance use and did not include a subgroup analysis of participants with problematic alcohol use |
| Rongkavilit 2013 | Wrong population ‐ included non‐drinkers |
| Rotheram‐Borus 2015 | Wrong population ‐ included non‐drinkers and non‐problematic drinkers |
| Sorsdahl 2015 | Wrong population ‐ included participants with non‐alcohol substance use and did not include a subgroup analysis of participants with problematic alcohol use |
| Thapinta 2014 | Wrong intervention ‐ intervention was aimed at treating depression, not alcohol use disorder |
| Ward 2015 | Wrong population ‐ included participants with non‐alcohol substance use and did not include a subgroup analysis of participants with problematic alcohol use |
| Wechsberg 2006 | Wrong population ‐ eligibility criteria focused on cocaine, not alcohol use |
| Wechsberg 2008 | Wrong population ‐ included participants with non‐alcohol substance use and did not include a subgroup analysis of participants with problematic alcohol use |
| Wechsberg 2016 | Wrong population ‐ inclusion criteria included men reporting that they frequented shebeens and drank alcohol, not necessarily that they displayed problematic alcohol use |
Characteristics of studies awaiting classification [ordered by study ID]
Balachova 2017.
| Methods | Cluster randomized controlled trial |
| Participants | 767 non‐pregnant women at risk for an alcohol‐exposed pregnancy in OB/GYN clinics in Russia |
| Interventions | Two face‐to‐face 5‐minute sessions incorporated into routine clinical OB/GYN care visits |
| Outcomes | Alcohol consumption, alcohol patterns (latent transition analysis) |
| Notes | Conference abstract only; Author also published abstracts in 2012, 2013, 2014, and 2015 that appear to report on same trial; Authors contacted for full report on November 7, 2019 Linked study records (Balachova 2012, Balachova 2013, Balachova 2014, Balachova 2015) |
Belokrylov 2018.
| Methods | Randomized controlled trial |
| Participants | 220 inpatients with alcohol dependence in Russia |
| Interventions | Psychoanalysis two times per week for one month (90‐minute sessions) vs. psycho‐evacuation sessions with elements of behavioral therapy |
| Outcomes | Alcohol dependence symptoms, Quality of life, remission |
| Notes | Abstract only; Emailed author on April 9, 2020 |
Clair 2016.
| Methods | Randomized controlled trial |
| Participants | Adults with moderate or high‐risk drinking seeking care at public or private healthcare clinics in Kenya |
| Interventions | Online training for community health workers in ASSIST‐linked brief intervention |
| Outcomes | Alcohol consumption, binge drinking, alcohol‐related stigma, depression, suicidality, quality of life, risky sexual behaviors |
| Notes | Abstract only; NCT02388243 ; Emailed author on April 9, 2020 |
Du 2017.
| Methods | TBD |
| Participants | 1336 clients from 18 Beichuan count village hospitals in Sichuan, China |
| Interventions | 15‐30 minute brief intervention + general health education vs. general health education alone |
| Outcomes | Unhealthy alcohol use, substance use knowledge, depression, anxiety, well‐being |
| Notes | Conference abstract only; Unable to locate author's contact information |
Gorny 2019.
| Methods | TBD |
| Participants | 908 patients in primary care in Russia |
| Interventions | Advice by a narcologist vs. preventive counseling vs. no counseling |
| Outcomes | Alcohol consumption |
| Notes | Abstract only; Unable to locate author's contact information |
Jin 2006.
| Methods | Randomized controlled trial |
| Participants | 35 male inpatients with alcohol dependence in China |
| Interventions | Aversion therapy with electro‐acupuncture |
| Outcomes | Alcohol dependence, anxiety |
| Notes | Abstract only; Unable to locate author's contact information |
Parry 2014.
| Methods | Randomized controlled trial |
| Participants | Patients with hazardous alcohol use who are also living with HIV and receiving antiretroviral therapy |
| Interventions | Motivational Interviewing + Problem Solving Therapy vs. Equal‐Attention Wellness Intervention vs. Treatment As Usual |
| Outcomes | Alcohol consumption, Harmful/hazardous alcohol use, Drinking biomarkers |
| Notes | Study protocol; Parry 2019; Authors contacted for trial results on November 17, 2019 |
Prasad 2018.
| Methods | Randomized controlled trial |
| Participants | 70 male hospital workers with moderate and high risk alcohol use |
| Interventions | WHO ASSIST‐linked brief intervention |
| Outcomes | ASSIST, WHOQOL‐BREF, RCQ, Motivation to Seek Treatment |
| Notes | Abstract only; Contacted the author on April 9, 2020; Manuscript in preparation |
Rentala 2020.
| Methods | Randomized controlled trial |
| Participants | Individuals with alcohol dependence |
| Interventions | Integrative Body Mind Spirit intervention |
| Outcomes | Relapse, quantity of drinking, craving, number of drinking days |
| Notes |
Siriwardhana 2013.
| Methods | Cluster randomized controlled trial |
| Participants | Universal intervention: Community members Brief intervention: Males 18‐80 years of age |
| Interventions | Universal community alcohol education program vs. no intervention Brief intervention vs. no intervention |
| Outcomes | Hazardous alcohol use, alcohol consumption, type of alcohol consumed |
| Notes | Unclear if the alcohol outcomes are specific to men who had alcohol problems and received the brief intervention or if this includes all men 18‐80 years of age in study communities Contacted the author on November 17, 2019 |
Staton 2019.
| Methods | Randomized controlled trial |
| Participants | 75 patients in the emergency department and reported ingesting alcohol recently or an AUDIT score >=8 |
| Interventions | Brief negotiational intervention |
| Outcomes | Retention, Alcohol outcomes TBD |
| Notes | Conference abstract only; clinicaltrials.gov: NCT02828267; Contacted author on April 9, 2020 |
Yadav 2019.
| Methods | Randomized controlled trial |
| Participants | 80 inpatients with alcohol dependence |
| Interventions | Breathing‐relaxation training (4 days) |
| Outcomes | AUDIT, WHO Wellbeing Index |
| Notes | Abstract only; Unable to locate author's contact information |
ASSIST: Alcohol, Smoking and Substance Involvement Screening Test; AUDIT: Alcohol Use Disorder Identification Test
Characteristics of ongoing studies [ordered by study ID]
Almonacid 2020.
| Study name | EFICO |
| Methods | Randomized controlled trial |
| Participants | Adults scheduled for surgery who are current smokers or risky alcohol drinkers |
| Interventions | Brief counseling |
| Outcomes | Behavior change; Risky drinking; Smoking |
| Starting date | 2018 |
| Contact information | |
| Notes | ClinicalTrials.gov Identifier: NCT03521622 |
Diclemente 2021.
| Study name | Computer‐based alcohol reduction intervention |
| Methods | Randomized controlled trial |
| Participants | Females ages 21‐45 years receiving HIV medical care (including antiretroviral therapy) with HCV infection and recent alcohol use |
| Interventions | Computer‐based alcohol reduction intervention including cognitive behavioral therapy modules vs. usual care |
| Outcomes | Alcohol use (EtG), PEth, HIV viral load, CD4 cell count, liver damage (FibroTest), liver stiffness |
| Starting date | January 3, 2018 |
| Contact information | Ralph DiClemente |
| Notes | Study expected to end in 2020 |
Fernandes 2019.
| Study name | Alcohol Use Disorders ‐ Mobile Based Brief Intervention Treatment (AMBIT) |
| Methods | Randomized controlled trial |
| Participants | Adults in educational institutions and male adults in workplaces/primary health centers who have a mobile phone and are hazardous drinkers (India) |
| Interventions | Mobile‐based intervention involving messages and calls 2‐3 times per week over 8 weeks. Messages cover topics related to self‐awareness, self‐reflection, motivational messages, safe drinking, alcohol reduction, drinking management, risk management, craving management, drinking alternatives, health education, personalized feedback and information, help‐seeking resources, and goal setting vs. face‐to‐face mhGAP single‐session brief intervention vs. active control involving an educational leaflet |
| Outcomes | Frequency of drinking (TLFB), quantity of drinking, heavy drinking, intensity of drinking |
| Starting date | September 17, 2019 |
| Contact information | Danielle Fernandes |
| Notes | Study expected to end April 30, 2020 |
Kiene 2019.
| Study name | KISOBOKA |
| Methods | Randomized controlled trial |
| Participants | HIV‐positive adults working in the fishing industry who have been on antiretroviral therapy for at least 6 months, have an HIV viral load greater than 200 or less than 90% adherence in the past 2 weeks, consume 5 or more drinks per occasion two or more times per month, have a mobile phone, and are not planning on moving outside of the study area (Wakiso District, Uganda) within the next 6 months |
| Interventions | KISOBOKA intervention (combination of behavioral components ‐ alcohol screening, financial literacy, goal‐setting, alcohol counseling, HIV care engagement ‐ and structural components ‐ changing the mode of work payment from cash to mobile money) vs. screening and referral |
| Outcomes | Frequency of heavy/binge drinking, HIV care engagement, ART adherence, PEth, HIV viral load |
| Starting date | September 2019 |
| Contact information | Susan M. Kiene |
| Notes | Study expected to end July 2021 |
Lelutiu‐Weinberger 2019.
| Study name | Comunica |
| Methods | Randomized cross‐over study |
| Participants | Gay/bisexual males 16+ years in Romania who reported 3 or more acts of condomless anal sex with an HIV‐positive or status‐unknown partner in the prior 3 months, 15 or more days of heavy drinking the past 3 months, own a mobile device, and are HIV‐negative |
| Interventions | Comunica: a 60‐minute live chat session delivered by a trained psychologist on a mobile platform that focuses on sexual health, motivation to change their HIV risk behaviors and alcohol use, and behavioral skills necessary for reducing their risk vs. education attention control |
| Outcomes | Sexual behavior, alcohol use (TLFB), hazardous alcohol use (AUDIT), depression, anxiety, HIV knowledge, alcohol effects, readiness for change, motivation to use condoms, safer‐sex self‐efficacy, confidence in reducing alcohol use, rejection sensitivity, sexual orientation concealment, assertiveness, sexual minority identity, LGBT victimization, perceived social support |
| Starting date | May 13, 2019 |
| Contact information | Corina Lelutiu‐Weinberger; John Pachankis |
| Notes | Study expected to end July 2024 |
Liang 2020.
| Study name | ASF in the treatment of patients with alcohol dependence |
| Methods | Randomized placebo‐controlled trial |
| Participants | Adults (18‐65 years) diagnosed with alcohol dependence without obvious withdrawal symptoms |
| Interventions | ASF (a compound of traditional Chinese medicine) |
| Outcomes | Alcohol consumption, abstinence |
| Starting date | |
| Contact information | |
| Notes |
Lodi 2021.
| Study name | Drinkers' Intervention to Prevent Tuberculosis (DIPT Study) |
| Methods | Randomized controlled trial |
| Participants | HIV‐positive adults prescribed antiretroviral therapy for at least 6 months, a positive tuberculin skin test, AST and ALT less than twice the upper limit of normal, no history of active TB or TB treatment/preventive therapy who report heavy alcohol use and live within 2 hours from the study site in Uganda |
| Interventions | Escalating economic incentives (EtG tests) vs. Escalating incentives (IsoScreen tests) vs. Escalating incentives (EtG + IsoScreen tests) vs. not intervention |
| Outcomes | Non‐heavy drinking (AUDIT‐C + PEth), isoniazid treatment adherence |
| Starting date | April 16, 2018 |
| Contact information | Nneka Emenyonu |
| Notes | Study expected to end February 2022 |
Mmbaga 2021.
| Study name | PRACT |
| Methods | Adaptive randomized trial |
| Participants | Adults seeking care at an emergency department for acute injury who are not clinically intoxicated and have either disclosed alcohol use prior to the injury, screened positive for hazardous or harmful alcohol use, or test positive for alcohol consumption on a breathalyzer. |
| Interventions | Variations of a brief intervention involving a one‐time nurse‐led motivational interview (MI) session in the emergency department vs. one MI session with weekly standard text boosters vs. one MIsession with weekly personalized boosters |
| Outcomes | Binge drinking, frequency of alcohol use, quantity of alcohol use, alcohol‐related harms, alcohol use disorder, depression |
| Starting date | 2020 |
| Contact information | |
| Notes |
Mutyambizi‐Mafu 2019.
| Study name | Project MIND |
| Methods | Cluster‐randomized controlled trial |
| Participants | Adults with hazardous alcohol use and receiving treatment for diabetes or HIV, but not currently receiving treatment for alcohol use |
| Interventions | Motivational interviewing (MI) and problem solving therapy (PST) delivered in 3 sessions over 6 weeks with 1 optional booster session |
| Outcomes | Psychosocial functioning, Depression, Hazardous alcohol use (AUDIT) |
| Starting date | 2017 |
| Contact information | Ms. Vimbayi Mutyambizi‐Mafunda |
| Notes | Study expected to end in 2020 |
Nadkarni 2019a.
| Study name | AMBIT |
| Methods | Randomized controlled trial |
| Participants | Adults in educational institutions, workplaces, and primary health centers who report hazardous drinking and own a mobile phone |
| Interventions | Mobile‐based brief intervention (AMBIT) |
| Outcomes | Percent days abstinent, quantity of alcohol use, percent days heavy drinking, intensity of drinking |
| Starting date | 2019 |
| Contact information | |
| Notes | Clinical Trials identifier: NCT04078360 |
NCT02797587.
| Study name | St PETER HIV |
| Methods | Randomized controlled trial |
| Participants | HIV‐positive adults with heavy drinking, heavy smoking, a stable resident and telephone number, and interested in cutting down/quitting tobacco or alcohol |
| Interventions | A) Varenicline + Nicotine Replacement Therapy Placebo; B) Varenicline Placebo + Nicotine Replacement Therapy; C) Cytisine + Nicotine Replacement Therapy Placebo; D) Cystine Placebo + Nicotine Replacement Therapy |
| Outcomes | Heavy drinking days, alcohol craving, smoking cessation, coronary heart disease risk, mortality risk, cigarettes per day, hsCRP levels, IL‐6 levels |
| Starting date | July 19, 2017 |
| Contact information | Jeffery Samet |
| Notes | None |
NCT03380728.
| Study name | Ibogaine in the treatment of alcoholism |
| Methods | Randomized controlled trial |
| Participants | Adults with alcoholism and at least two previous failed treatments for alcoholism in Brazil |
| Interventions | Ibogaine hydrochloride (240 mg on day 1, placebo on day 4 & 7) vs. Ibogaine hydrochloride (240 mg on day 1, 320 mg on day 4, placebo on day 7) vs. Ibogaine hydrochloride (240 mg on day 1, 320 mg on day 4, 400 mg on day 7) |
| Outcomes | Days abstinent, subjective effects, biomarkers, cardiovascular effects |
| Starting date | August 2020 |
| Contact information | Rafael dos Santos |
| Notes | Study expected to end December 2022 |
NCT03610815.
| Study name | I‐STAR |
| Methods | Cluster randomized controlled trial |
| Participants | Community health workers in Mozambique |
| Interventions | Mobile Screening, Brief Intervention, and Referral to Treatment (SBIRT) vs. in‐person SBIRT |
| Outcomes | Reach, AUDIT, cost |
| Starting date | December 2, 2019 |
| Contact information | Milton L. Wainberg |
| Notes | Study expected to end June 30, 2024 |
NCT04906616.
| Study name | An economic and relationship‐strengthening intervention for HIV‐affected couples who drink alcohol in Malawi |
| Methods | Randomized controlled trial |
| Participants | Married partners with at least one partner who screens positive on the AUDIT‐C and is currently on ARTs |
| Interventions | Mlambe intervention: couples‐based intervention to reduce drinking and improve economic and HIV outcomes |
| Outcomes | Enrollment rate, retention rate, satisfaction, intervention completion, survey completion, alcohol use, viral suppression, ART adherence |
| Starting date | 2021 |
| Contact information | |
| Notes | Clinical Trials identifier: NCT04906616 |
Paula 2021.
| Study name | BIO |
| Methods | Randomized controlled trial |
| Participants | 60+ year old individuals registered at primary care centers and identified as at‐risk drinkers based on AUDIT score |
| Interventions | Brief intervention for older adults that involves personalized feedback, information, brief advice, and a booklet |
| Outcomes | At‐risk drinking, alcohol consumption, cognitive performance |
| Starting date | |
| Contact information | |
| Notes |
Sangle 2021.
| Study name | HATHI |
| Methods | Randomized controlled trial |
| Participants | Adults with active TB and hazardous alcohol use |
| Interventions | Counseling for Alcohol Problems |
| Outcomes | Drinking days, heavy drinking days, drinks per drinking day, PEth levels, TB outcomes |
| Starting date | 2020 |
| Contact information | |
| Notes | WHO International Clinical Trials Registry Identifier: CTRI/2020/03/024141 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; AUDIT: Alcohol Use Disorder Identification Test; PEth: Phosphatidylethanol testing
Differences between protocol and review
The following changes were made to the methods from what was originally proposed in the published protocol. First, we modified the terminology used to differentiate the different forms of psychosocial interventions. In this review we stratified psychosocial interventions as "brief psychosocial interventions” if that is how they were described by study authors and “other psychosocial interventions” to describe the remaining psychosocial interventions. This decision was done to improve clarity and the distinction between these psychosocial intervention approaches. The majority of brief interventions were indicated prevention approaches intended to reduce harmful alcohol use among those with sub‐threshold or mild alcohol use disorder. In contrast, psychosocial treatments were primarily aimed to treat alcohol use disorder. However, it is important to note that some brief interventions also enrolled individuals meeting criteria for alcohol use disorder and may also be considered a treatment strategy. Second, instead of separating the continuous and binary meta‐analyses for our primary outcome, harmful alcohol use, we combined these outcomes to quantify the pooled standardized mean difference using the generic inverse‐variance method. Third, we conducted a Grade analysis for both primary and secondary outcomes. Fourth, we did not conduct the planned sensitivity and subgroup analyses due the paucity of results and the significant clinical and methodological heterogeneity that precluded pooled estimates for the overall meta‐analyses.
Contributions of authors
MCG wrote the first draft of the protocol and review.
WAT supervised protocol development with input from RMJ and JCK.
MCG, MA, JM, MS, KL, AG and TM completed article screening and data extraction
All review authors provided revisions to the manuscript.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute on Drug Abuse, USA
MCG is supported by the National Institute on Drug Abuse (T32DA007292; PI: R. M. Johnson)
-
National Institute of Mental Health, USA
MCG is supported by the National Institute of Mental Health (T32MH096724; PI: M. L. Wainberg)
Declarations of interest
GMC: none.
KJ: none.
AM: none.
MCJ: none.
NT: none.
WM: none.
JRM: none.
TWA: none.
New
References
References to studies included in this review
Ahmadi 2004 {published data only}
- Ahmadi J, Babaeebeigi M, Maany I, Porter J, Mohagheghzadeh M, Ahmadi N, et al. Naltrexone for alcohol-dependent patients. Irish Journal of Medical Science 2004;173(1):34-7. [DOI] [PubMed] [Google Scholar]
Allen 2011 {published data only}ISRCTN82405938
- Allen E, Polikina O, Saburova L, McCambridge J, Elbourne D, Pakriev S, et al. The efficacy of a brief intervention in reducing hazardous drinking in working age men in Russia: the HIM (Health for Izhevsk men) individually randomised parallel group exploratory trial. Trials 2011;12:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altintoprak 2008 {published data only}
- Altintoprak AE, Zorlu N, Cokunol H, Akdeniz F, Kitapcioglu G. Effectiveness and tolerability of mirtazapine and amitriptyline in alcoholic patients with co-morbid depressive disorder: a randomized, double-blind study. Human Psychopharmacology 2008;23(4):313-9. [DOI] [PubMed] [Google Scholar]
Assanangkornchai 2015 {published data only}
- Assanangkornchai S, Nima P, McNeil EB, Edwards JG. Comparative trial of the WHO ASSIST-linked brief intervention and simple advice for substance abuse in primary care. Asian Journal of Psychiatry 2015;18:75-80. [DOI] [PubMed] [Google Scholar]
- Assanangkornchai S, Nima P. Screening and brief intervention for harmful alcohol and drug use in primary care and community settings in Thailand. Alcoholism: Clinical and Experimental Research 2012;36:24A. [Google Scholar]
Baldin 2018 {published data only}
- Baldin YC, Sanudo A, Sanchez ZM. Effectiveness of a web-based intervention in reducing binge drinking among nightclub patrons. Revista de Saúde Pública 2018;52:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez ZM, Sanudo A. Web-based alcohol intervention for nightclub patrons: opposite effects according to baseline alcohol use disorder classification. Substance Abuse 2019;39(3):361-70. [DOI] [PubMed] [Google Scholar]
Baltieri 2003 {published data only}
- Baltieri DA, Andrade AG. Acamprosate in alcohol dependence: a randomized controlled efficacy study in a standard clinical setting. Journal of Studies on Alcohol and Drugs 2004;65(1):136-9. [DOI] [PubMed] [Google Scholar]
- Baltieri DA, Andrade AG. Efficacy of acamprosate in the treatment of alcohol-dependent outpatients. Revista Brasileira de Psiquiatria 2003;25(3):156-9. [DOI] [PubMed] [Google Scholar]
Baltieri 2008 {published data only}
- Baltieri DA, Daro FR, Ribeiro PL, Andrade AG. Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction 2008;103(12):2035-44. [DOI] [PubMed] [Google Scholar]
- Baltieri DA, Daro FR, Ribeiro PL, Andrade AG. Effects of topiramate on tobacco use among male alcohol-dependent outpatients. Drug and Alcohol Dependence 2009;105(1-2):33-41. [DOI] [PubMed] [Google Scholar]
Bolton 2014 {published data only}
- Bolton P, Lee C, Haroz EE, Dorsey S, Robinson C, Ugueto AM, et al. A transdiagnostic community-based mental health treatment for comorbid disorders: development and outcomes of a randomized controlled trial among Burmese refugees in Thailand. PLOS Medicine 2014;11(11):e1001757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castro 2009 {published data only}
- Castro LA, Laranjeira R. A double blind, randomized and placebo-controlled clinical trial with naltrexone and brief intervention in outpatient treatment of alcohol dependence. Jornal Brasileiro de Psiquiatria 2009;58(2):79-85. [Google Scholar]
Cherpitel 2009 {published data only}
- Cherpitel CJ, Korcha RA, Moskalewicz J, Swiatkiewicz G, Ye Y, Bond J. Screening, brief intervention, and referral to treatment (SBIRT): 12-month outcomes of a randomized controlled clinical trial in a Polish emergency department. Alcoholism, Clinical and Experimental Research 2010;34(11):1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherpitel CJ, Moskalewicz J, Swiatkiewicz G, Ye Y, Bond J. Screening, brief intervention, and referral to treatment (SBIRT) in a Polish emergency department: three-month outcomes of a randomized, controlled clinical trial. Journal of Studies on Alcohol and Drugs 2009;70(6):982-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherpitel CJ, Ye Y, Moskalewicz J, Swiatkiewicz G. Does brief intervention work for heavy episodic drinking? A comparison of emergency department patients in two cultures. Alkoholizm i Narkomania 2015;28(3):145-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korcha RA, Cherpitel CJ, Bond JC, Ye Y, Moskalewicz J, Swiatkiewicz G. The relationship of readiness to change, drinking outcomes, and negative consequences in a polish emergency department. Alcoholism: Clinical and Experimental Research 2010;34(6):228A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korcha RA, Cherpitel CJ, Moskalewicz J, Swiatkiewicz G, Bond J, Ye Y. Readiness to change, drinking, and negative consequences among Polish SBIRT patients. Addictive Behaviors 2012;37(3):287-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chompookham 2018 {published data only}
- Chompookham P, Rukngan W, Nilaban S, Suwanmajo S, Yoosom P, Kalayasiri R. A randomized trial of low-dose gabapentin for post hospitalization relapse prevention in a Thai clinical sample of alcohol dependence. Psychiatry Research 2018;270:34-40. [DOI] [PubMed] [Google Scholar]
Constant 2021 {published data only}
- Constant HM, Ferigolo M, Barros HM, Moret-Tatay C. A clinical trial on a brief motivational intervention in reducing alcohol consumption under a telehealth supportive counseling. Psychiatry Research 2021;303:114608. [DOI] [PubMed] [Google Scholar]
Correa Filho 2013 {published data only}
- Correa Filho JM, Baltieri DA. A pilot study of full-dose ondansetron to treat heavy-drinking men withdrawing from alcohol in Brazil. Addictive Behaviors 2013;38:2044-51. [DOI] [PubMed] [Google Scholar]
Daengthoen 2014 {published data only}
- Daengthoen L, Saengcharnchai P, Yingwiwattanapong J, Perngparn U. Effects of the Phramongkutklao model on alcohol-dependent patient: a randomized controlled trial. Journal of Substance Use 2014;19(1-2):81-8. [Google Scholar]
de Sousa 2004 {published data only}
- Sousa A, Sousa A. A one-year pragmatic trial of naltrexone vs disulfiram in the treatment of alcohol dependence. Alcohol and Alcoholism 2004;39(6):528-31. [DOI] [PubMed] [Google Scholar]
de Sousa 2005 {published data only}
- Sousa A. An open randomized study comparing disulfiram and acamprosate in the treatment of alcohol dependence. Alcohol and Alcoholism 2005;40(6):545-8. [DOI] [PubMed] [Google Scholar]
de Sousa 2008 {published data only}
- de Sousa 2008. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. Journal of Substance Abuse Treatment 2008;34(4):460-3. [DOI] [PubMed] [Google Scholar]
Dutra Ponce 2021 {published data only}
- Dutra Ponce T, Leon EG, Vargas D. Reducing women's alcohol use: a brief intervention pilot study at a primary health care service in Brazil. Women & Health 2021;61(8):737-44. [DOI] [PubMed] [Google Scholar]
Furieri 2007 {published data only}
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2007;68(11):1691-700. [DOI] [PubMed] [Google Scholar]
Ganavadiya 2018 {published data only}
- Ganavadiya R, Chandrashekar BR, Singh P, Gupta R, Rana PT, Jain S. Knowledge, attitude, and practice among tobacco and alcohol addicts before and after psychological intervention in a de-addiction center at Madhya Pradesh, India. Indian Psychiatry Journal 2018;27(1):27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Go 2020 {published data only}
- Blackburn NA, Go VF, Bui Q, Hutton H, Tampi RP, Sripaipan T, et al. The cost-effectiveness of adapting and implementing a brief intervention to target frequent alcohol use among persons with HIV in Vietnam. AIDS and Behavior 2021;25(7):2108-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go VF, Hutton HE, Ha TV, Chander G, Latkin CA, Mai NV, et al. Effect of 2 Integrated Interventions on alcohol abstinence and viral suppression among Vietnamese adults with hazardous alcohol use and HIV: a randomized clinical trial. JAMA Network Open 2020;3(9):e2017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harder 2019 {published data only}
- Harder V. Mobile phone-based motivational interviewing in Kenya. clinicaltrials.gov/ct2/show/NCT03573167 2018. [NCT03573167]
- Harder VS, Musau AM, Musyimi CW, Ndetei DM, Mutiso VN. A randomized clinical trial of mobile phone motivational interviewing for alcohol use problems in Kenya. Addiction 2020;115(6):1050-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann 2020 {published data only}
- Hartmann M, Datta S, Browne EN, Appiah P, Banay R, Caetano V, et al. A combined behavioral economics and cognitive behavioral therapy intervention to reduce alcohol use and intimate partner violence among couples in Bengaluru, India: results of a pilot study. Journal of Interpersonal Violence 2020;36(23-24):Np12456-np12480. [DOI] [PubMed] [Google Scholar]
- Hartmann MA, Datta S, Banay RF, Caetano V, Floreak R, Appaiah P, et al. Designing a pilot study protocol to test a male alcohol use and intimate partner violence reduction intervention in India: Beautiful Home. Frontiers in Public Health 2018;6:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huis In't Veld 2019 {published data only}
- Huis In't Veld D, Ensoy-Musoro C, Pengpid S, Peltzer K, Colebunders R. The efficacy of a brief intervention to reduce alcohol use in persons with HIV in South Africa, a randomized clinical trial. PLOS One 2019;14(8):e0220799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huis In't Veld D, Skaal L, Peltzer K, Colebunders R, Ndimande JV, Pengpid S. The efficacy of a brief intervention to reduce alcohol misuse in patients with HIV in South Africa: study protocol for a randomized controlled trial. Trials 2012;13:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jirapramukpitak 2020 {published data only}
- Jirapramukpitak T, Pattanaseri K, Chua KC, Takizawa P. Home-based contingency management delivered by community health workers to improve alcohol abstinence: a randomized control trial. Alcohol and Alcoholism 2020;55(2):171-8. [DOI] [PubMed] [Google Scholar]
Jordans 2019 {published data only}
- Jordans MJD, Luitel NP, Garman E, Kohrt BA, Rathod SD, Shrestha P, et al. Effectiveness of psychological treatments for depression and alcohol use disorder delivered by community-based counsellors: two pragmatic randomised controlled trials within primary healthcare in Nepal. British Journal of Psychiatry 2019;215(2):485-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamal 2020 {published data only}
- Kamal K, Sunita S, Karobi D, Abhishek G. Nurse-delivered screening and brief intervention among college students with hazardous alcohol use: a double-blind randomized clinical trial from India. Alcohol and Alcoholism 2020;55(3):284-90. [DOI] [PubMed] [Google Scholar]
Kane 2022 {published data only}
- Kane JC, Sharma A, Murray LK, Chander G, Kanguya T, Lasater ME, et al. Common Elements Treatment Approach (CETA) for unhealthy alcohol use among persons with HIV in Zambia: Study protocol of the ZCAP randomized controlled trial. Addictive Behaviors Reports 2020;12:100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JC, Sharma A, Murray LK, Chander G, Kanguya T, Skavenski S, et al. Efcacy of the Common Elements Treatment Approach (CETA) for unhealthy alcohol use among adults with HIV in Zambia: results from a pilot randomized controlled trial. AIDS and Behavior 2022;26(2):523-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JC. Zambia Common Elements Treatment Approach Pilot Study (ZCAP). clinicaltrials.gov 2019. [NCT03966885]
Kumar 2020 {published data only}
- Kumar A, Sharma A, Bansal P, Bahetra M, Gill H, Kumar R. A comparative study on the safety and efficacy of naltrexone versus baclofen versus acamprosate in the management of alcohol dependence. Indian Journal of Psychiatry 2020;62(6):650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Engle 2014 {published data only}
- L'Engle KL, Mwarogo P, Kingola N, Sinkele W, Weiner DH. A randomized controlled trial of a brief intervention to reduce alcohol use among female sex workers in Mombasa, Kenya. Journal of Acquired Immune Deficiency Syndromes 2014;67(4):446-53. [DOI] [PubMed] [Google Scholar]
- Parcesepe AM, L'Engle KL, Martin SL, Green S, Sinkele W, Suchindran C, et al. The impact of an alcohol harm reduction intervention on interpersonal violence and engagement in sex work among female sex workers in Mombasa, Kenya: results from a randomized controlled trial. Drug and Alcohol Dependence 2016;161:21-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Likhitsathian 2013 {published data only}
- Likhitsathian S, Uttawichai K, Booncharoen H, Wittayanookulluk A, Angkurawaranon C, Srisurapanont M. Topiramate treatment for alcoholic outpatients recently receiving residential treatment programs: a 12-week, randomized, placebo-controlled trial. Drug and Alcohol Dependence 2013;133(2):440-6. [DOI] [PubMed] [Google Scholar]
Madhombiro 2019 {published data only}
- Madhombiro M, Dube B, Dube M, Zunza M, Chibanda D, Rusakaniko S, et al. Intervention for alcohol use disorders at an HIV care clinic in Harare: a pilot and feasibility study. Addiction Science & Clinical Practice 2019;14(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madhombiro 2020 {published data only}
- Madhombiro M, Dube-Marimbe B, Dube M, Chibanda D, Zunza M, Rusakaniko S, et al. A cluster randomised controlled trial protocol of an adapted intervention for alcohol use disorders in people living with HIV and AIDS: Impact on alcohol use, general functional ability, quality of life and adherence to HAART. BMC Psychiatry 2017;17(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhombiro M, Kidd M, Dube B, Dube M, Mutsvuke W, Muronzie T, et al. Effectiveness of a psychological intervention delivered by general nurses for alcohol use disorders in people living with HIV in Zimbabwe: a cluster randomized controlled trial. Journal of the International AIDS Society 2020;23(12):e25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moraes 2010 {published data only}
- Moraes E, Campos GM, Figlie NB, Laranjeira R, Ferraz MB. Cost-effectiveness of home visits in the outpatient treatment of patients with alcohol dependence. European Addiction Research 2010;16(2):69-77. [DOI] [PubMed] [Google Scholar]
- Moraes E, Campos GM, Figlie NB, Ferraz MB, Laranjeira R. Home visits in the outpatient treatment of individuals dependent on alcohol: randomized clinical trial. Addictive Disorders & Their Treatment 2010;9(1):18-31. [Google Scholar]
Murray 2020 {published data only}
- Kane JC, Glass N, Bolton PA, Mayeya J, Paul R Mwenge M, et al. Two-year treatment effects of the common elements treatment approach (CETA) for reducing intimate partner violence and unhealthy alcohol use in Zambia. Global Mental Health 2021;8:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JC, Skavenski Van Wyk S, Murray SM, Bolton P, Melendez F, et al. Testing the effectiveness of a transdiagnostic treatment approach in reducing violence and alcohol abuse among families in Zambia: study protocol of the Violence and Alcohol Treatment (VATU) trial. Global Mental Health 2017;4:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JC, Van Wyk S, Kavenski S, Murray SM, Melendez F, Munthali S, et al. Effectiveness of a transdiagnostic treatment for alcohol misuse and intimate partner violence in Zambia: Design and implementation of a randomized trial. Alcoholism: Clinical and Experimental Research 2018;42:257A. [Google Scholar]
- Murray LK, Kane JC, Glass N, Skavenski van Wyk S, Melendez F, Paul R, et al. Effectiveness of the Common Elements Treatment Approach (CETA) in reducing intimate partner violence and hazardous alcohol use in Zambia (VATU): a randomized controlled trial. PLOS Medicine 2020;17(4):e1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadkarni 2017 {published data only (unpublished sought but not used)}ISRCTN76465238
- Nadkarni A, Velleman R, Dabholkar H, Shinde S, Bhat B, McCambridge J, et al. The systematic development and pilot randomized evaluation of counselling for alcohol problems, a lay counselor-delivered psychological treatment for harmful drinking in primary care in India: the PREMIUM study. Alcoholism: Clinical and Experimental Research 2015;39(3):522-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni A, Weiss HA, Weobong B, McDaid D, Singla DR, Park AL, et al. Sustained effectiveness and cost-effectiveness of counselling for alcohol problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial. PLOS Medicine 2017;14(9):e1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni A, Weobong B, Weiss HA, McCambridge J, Bhat B, Katti B, et al. Counselling for Alcohol Problems (CAP), a lay counsellor-delivered brief psychological treatment for harmful drinking in men, in primary care in India: a randomised controlled trial. Lancet 2017;389(10065):186-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Weobong B, Nadkarni A, Weiss HA, Anand A, Naik S, et al. The effectiveness and cost-effectiveness of lay counsellor-delivered psychological treatments for harmful and dependent drinking and moderate to severe depression in primary care in India: PREMIUM study protocol for randomized controlled trials. Trials 2014;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla DR, Hollon SD, Velleman R, Weobong B, Nadkarni A, Fairburn CG, et al. Temporal pathways of change in two randomized controlled trials for depression and harmful drinking on Goa, India. Psychol Medicine 2019;50:68-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadkarni 2019 {published data only}
- Nadkarni A, Weiss HA, Velleman R, McCambridge J, McDaid D, Park AL, et al. Feasibility, acceptability and cost-effectiveness of a brief, lay counsellor-delivered psychological treatment for men with alcohol dependence in primary care: an exploratory randomized controlled trial. Addiction 2019;114(7):1192-203. [ISRCTN76465238] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synowski J, Weiss HA, Velleman R, Patel V, Nadkarni A. A lay-counsellor delivered brief psychological treatment for men with comorbid alcohol use disorder and depression in primary care: secondary analysis of data from a randomized controlled trial. Drug and Alcohol Dependence 2021;227:108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nattala 2010 {published data only}
- Nattala P, Leung KS, Nagarajaiah, Murthy P. Family member involvement in relapse prevention improves alcohol dependence outcomes: a prospective study at an addiction treatment facility in India. Journal of Studies on Alcohol and Drugs 2010;71(4):581-7. [DOI] [PubMed] [Google Scholar]
- Nattala P, Nagarajaiah A, Murthy P. Effects of individuals vs dyadic relapse prevention on stress levels of caregivers of alcohol-dependent subjects: correlates of caregiver stress. In: Proceedings of the 71st Annual Scientific Meeting of the College on Problems of Drug Dependence. Vol. 109. 2009.
Ng 2020 {published data only}
- Ng SM, Rentala S, Chan CLW, Nayak RB. Nurse-led body-mind-spirit based relapse prevention intervention for people with diagnosis of alcohol use disorder at a mental health care setting, India: a pilot study. Journal of Addictions Nursing 2020;31(4):276-86. [DOI] [PubMed] [Google Scholar]
Noknoy 2010 {published data only}
- Noknoy S, Rangsin R, Saengcharnchai P, Tantibhaedhyangkul U, McCambridge J. RCT of effectiveness of motivational enhancement therapy delivered by nurses for hazardous drinkers in primary care units in Thailand. Alcohol and Alcoholism 2010;45(3):263-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Omeje 2018 {published data only}
- Omeje JC, Otu MS, Aneke AO, Adikwu VO, Nwaubani OO, Chigbu EF, et al. Effect of rational emotive health therapy on alcohol use among community-dwelling, HIV-positive patients. Medicine 2018;97(35):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pal 2007 {published data only}
- Pal HR, Yadav D, Mehta S, Mohan I. A comparison of brief intervention versus simple advice for alcohol use disorders in a North India community-based sample followed for 3 months. Alcohol and Alcoholism 2007;42(4):328-32. [DOI] [PubMed] [Google Scholar]
Papas 2011 {published data only (unpublished sought but not used)}
- Papas RK, Ayuku DO, Sidle JE, Ballidawa JB, Omolo OE, Songole R, et al. Pilot results from a Stage 1 cognitive behavioral trial to reduce alcohol use among HIV-infected Kenyans. In: Alcoholism: Clinical and Experimental Research. Vol. 33. 2009:197A.
- Papas RK, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM, et al. Treatment outcomes of a stage 1 cognitive-behavioral trial to reduce alcohol use among human immunodeficiency virus-infected out-patients in western Kenya. Addiction 2011;106(12):2156-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papas 2020 {published data only (unpublished sought but not used)}
- Papas R, Gakinya B, Mwaniki M, Lee H, Keyer A, Martino S, et al. A randomized clinical trial of a group cognitive-behavioral therapy to reduce alcohol use among human immunodeficiency virus-infected out-patients in western Kenya. Addiction 2020;epub:1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas RK, Gakinya BN, Mwaniki MM, Lee H, Keter AK, Klein DA, et al. Sucessful treatment outcomes from a stage 2 randomized clinical trial of CBT to reduce alcohol use among HIV-infected outpatients in Western Kenya. Alcoholism: Clinical and Experimental Research 2017;41:253A. [Google Scholar]
Peltzer 2013 {published data only}
- Peltzer K, Naidoo P, Louw J, Matseke G, Zuma K, McHunu G, et al. Screening and brief interventions for hazardous and harmful alcohol use among patients with active tuberculosis attending primary public care clinics and South Africa: results from a cluster randomized controlled trial. BMC Public Health 2013;13:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pengpid 2013a {published data only}
- Pengpid S, Peltzer K, Van der Heever H, Skaal L. Screening and brief interventions for hazardous and harmful alcohol use among university students in South Africa: Results from a randomized controlled trial. International Journal of Environmental Research and Public Health 2013;10(5):2043-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pengpid 2013b {published data only}
- Pengpid S, Peltzer K, Skaal L, Van der Heever H. Screening and brief interventions for hazardous and harmful alcohol use among hospital outpatients in South Africa: results from a randomized controlled trial. BMC Public Health 2013;13:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengpid S, Peltzer K, Skaal L, Heever H, Van Hal G. Screening and brief intervention for alcohol problems in Dr George Mukhari Hospital out-patients in Gauteng, South Africa: a single-blinded randomized controlled trial protocol. BMC Public Health 2012;12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pengpid 2015 {published data only}
- Pengpid S, Peltzer K, Puckpinyo A, Viripiromgool S, Thamma-Aphiphol K, Suthisukhon K, et al. Screening and concurrent brief intervention of conjoint hazardous or harmful alcohol and tobacco use in hospital out-patients in Thailand: a randomized controlled trial. Substance Abuse Treatment, Prevention, and Policy 2015;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poblete 2017 {published data only}
- Poblete F, Barticevic NA, Zuzulich MS, Portilla R, Castillo-Carniglia A, Sapag JC, et al. A randomized controlled trial of a brief intervention for alcohol and drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in primary health care in Chile. Addiction 2017;112(8):1462-9. [DOI] [PubMed] [Google Scholar]
- Poblete F. Effectiveness of a brief intervention for substances consumption linked to the alcohol, smoking and substance involvement screening test (ASSIST): A randomized control trial in Chilean primary care. clinicaltrials.gov/ct2/show/NCT01573416 2012. [NCT01573416]
Polshkova 2016 {published data only}
- Polshkova S, Voloshyna D, Cunningham RM, Zucker RA, Walton MA. Prevention of alcohol and other drug use using motivational interviewing among young adults in Ukraine. Psychosomatic Medicine and General Practice 2016;1(1):e010111. [Google Scholar]
- Voloshyna D, Walton MA, Zucker RA, Cunningham RM, Polshkova S. Prevention of alcohol and other drug use using motivational interviewing among youth in the Ukraine. Alcoholism: Clinical and Experimental Research 2016;40:71A. [Google Scholar]
Rendall‐Mkosi 2013 {published data only}
- Rendall-Mkosi K, Morojele N, London L, Moodley S, Singh C, Girdler-Brown B. A randomized controlled trial of motivational interviewing to prevent risk for an alcohol-exposed pregnancy in the Western Cape, South Africa. Addiction 2013;108(4):725-32. [DOI] [PubMed] [Google Scholar]
Samet 2015 {published data only}
- Samet JH, Raj A, Cheng DM, Blokhina E, Bridden C, Chaisson CE, et al. HERMITAGE--a randomized controlled trial to reduce sexually transmitted infections and HIV risk behaviors among HIV-infected Russian drinkers. Addiction 2015;110(1):80-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Cheng DM, Coleman SM, Lira MC, Blokhina E, Bridden C, et al. Pain is associated with risky drinking over time among HIV-infected persons in St. Petersburg, Russia. Drug and Alcohol Dependence 2014;144:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Satyanarayana 2016 {published data only}
- Satyanarayana VA, Nattala P, Selvam S, Pradeep J, Hebbani S, Hegde S, et al. Integrated cognitive behavioral intervention reduces intimate partner violence among alcohol dependent men, and improves mental health outcomes in their spouses: a clinic based randomized controlled trial from South India. Journal of Substance Abuse Treatment 2016;64:29-34. [DOI] [PubMed] [Google Scholar]
Schaub 2021 {published data only (unpublished sought but not used)}
- Formigoni M, Andrade ALM. Partial results of a web-based self-help program to reduce alcohol use in adults with harmful, hazardous or suggestive of dependence drinking patterns. Alcoholism: Clinical and Experimental Research 2018;42:204A. [Google Scholar]
- Schaub MP, Tiburcio M, Martinez N, Ambekar A, Balhara YP, Wenger A, et al. Alcohol e-Help: study protocol for a web-based self-help program to reduce alcohol use in adults with drinking patterns considered harmful, hazardous or suggestive of dependence in middle-income countries. Addiction 2018;113(2):346-52. [DOI] [PubMed] [Google Scholar]
- Schaub MP, Tiburcio M, Martinez-Velez N, Ambekar A, Bhad R, Wenger A, et al. The effectiveness of a web-based self-help program to reduce alcohol use among adults with drinking patterns considered harmful, hazardous, or suggestive of dependence in four low- and middle-income countries: randomized controlled trial. Journal of Medical Internet Research 2021;23(8):e21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Segatto 2011 {published data only}
- Segatto ML, Andreoni S, Sousa e Silva R, Diehl A, Pinsky I. Brief motivational interview and educational brochure in emergency room settings for adolescents and young adults with alcohol-related problems: a randomized single-blind clinical trial. Revista Brasileira de Psiquiatria 2011;33(3):225-33. [DOI] [PubMed] [Google Scholar]
Sheikh 2017 {published data only}
- Sheikh WA, Paul R, Banda H, Agath K, Luty J. Impact of brief relapse prevention intervention in patients with alcohol dependence in Zambia. Journal of Substance Use 2017;22(1):113-7. [Google Scholar]
Shin 2013 {published data only}
- Shin S, Livchits V, Connery HS, Shields A, Yanov S, Yanova G, et al. Effectiveness of alcohol treatment interventions integrated into routine tuberculosis care in Tomsk, Russia. Addiction 2013;108(8):1387-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Signor 2013 {published data only}
- Signor L, Pierozan PS, Ferigolo M, Fernandes S, Moreira TC, Mazoni CG, et al. Efficacy of the telephone-based brief motivational intervention for alcohol problems in Brazil. Revista Brasileira de Psiquiatria 2013;35(3):254-61. [DOI] [PubMed] [Google Scholar]
Simao 2008 {published data only}
- Simao MO, Kerr-Correa F, Smaira SI, Trinca LA, Floripes TM, Dalben I, et al. Prevention of "risky" drinking among students at a Brazilian university. Alcohol and Alcoholism 2008;43(4):470-6. [DOI] [PubMed] [Google Scholar]
Thapinta 2017 {published data only}
- Thapinta D, Skulphan S, Kitsumban V, Longchoopol C. Cognitive behavior therapy self-help booklet to decrease depression and alcohol use among people with alcohol dependence in Thailand. Issues in Mental Health Nursing 2017;38(11):964-70. [DOI] [PubMed] [Google Scholar]
Thomas 2017 {published data only}
- Thomas B, Watson B, Senthil EK, Deepalakshmi A, Balaji G, Chandra S, et al. Alcohol intervention strategy among tuberculosis patients: a pilot study from South India. International Journal of Tuberculosis and Lung Disease 2017;21(8):947-52. [DOI] [PubMed] [Google Scholar]
Vlasova 2011 {published data only}
- Vlasova N, Schumacher J, Dumchev K, Kim Y. STEPS outpatient program demonstrates efficacy in reducing alcohol use in Ukraine. In: Proceedings of the 67th annual scientific meeting of the College on Problems of Drug Dependence. 2005.
- Vlasova N, Schumacher JE, Oryschhuk O, Dumchev KV, Slobodyanyuk P, Moroz VM, et al. STEPS outpatient program improves alcohol treatment outcomes in Ukrainian regional narcologic dispensary. Addictive Disorders & Their Treatment 2011;10(1):6-13. [Google Scholar]
Wandera 2017 {published data only}
- Wandera B, Tumwesigye NM, Nankabirwa JI, Mafigiri DK, Parkes-Ratanshi RM, Kapiga S, et al. Efficacy of a single, brief alcohol reduction intervention among men and women living with HIV/AIDS and using alcohol in Kampala, Uganda: a randomized trial. Journal of the International Association of Providers of AIDS Care 2017;16(3):276-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO Brief Intervention Study Group 1996 {published data only}
- Babor TF, Grant M, Acuda W, Burns FH, Campillo C, Del Boca FK, et al. A randomized clinical trial of brief interventions in primary health care: summary of a WHO project. Addiction 1994;89(6):657-60. [DOI] [PubMed] [Google Scholar]
- Ivanets NN, Lukomskaya MI. Evaluation of early intervention strategies used in primary health care: a report on the World Health Organization (WHO) Project on Identification and Treatment of Persons with Harmful Alcohol Consumption. Alcohol and Alcoholism 1991;1:489-91. [PubMed] [Google Scholar]
- WHO Brief Intervention Study Group. A Cross-National trial of Brief Interventions with Heavy Drinkers. American Journal of Public Health 1996;86(7):948-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witte 2011 {published data only}
- Carlson CE, Chen J, Chang M, Batsukh A, Toivgoo A, Riedel M, et al. Reducing intimate and paying partner violence against women who exchange sex in Mongolia: results from a randomized clinical trial. Journal of Interpersonal Violence 2012;27(10):1911-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte SS, Altantsetseg B, Aira T, Riedel M, Chen J, Potocnik K, et al. Reducing sexual HIV/STI risk and harmful alcohol use among female sex workers in Mongolia: a randomized clinical trial. AIDS and Behavior 2011;15(8):1785-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiaolu 2017 {published data only}
- Xiaolu R, Wenwen W, Ali R, Xu L, Hong W, Min Z, et al. Feasibility of studying a brief intervention to help Chinese villagers with problem alcohol use after an earthquake. Alcohol and Alcoholism 2017;52(4):472-6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aira 2013 {published data only}
- Aira T, Wang W, Riedel M, Witte S. Reducing risk behaviors linked to noncommunicable diseases in Mongolia: a randomized controlled trial. American Journal of Public Health 2013;103(9):1666-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burnhams 2015 {published data only}
- Burnhams NH, London L, Laubscher R, Nel E, Parry C. Results of a cluster randomised controlled trial to reduce risky use of alcohol, alcohol-related HIV risks and improve help-seeking behaviour among safety and security employees in the Western Cape, South Africa. Substance Abuse Treatment, Prevention, and Policy 2015;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaudhury 2016 {published data only}
- Chaudhury S, Brown FL, Kirk CM, Mukunzi S, Nyirandagijimana B, Mukandanga J, et al. Exploring the potential of a family-based prevention intervention to reduce alcohol use and violence within HIV-affected families in Rwanda. AIDS Care 2016;28:118-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawson 2012 {published data only}
- Dawson AH, Siriwardhana P, Abeyasinge R. A randomized controlled trial of a community based alcohol education program in changing the drinking pattern in rural Sri Lanka. Clinical Toxicology 2012;50(4):350. [Google Scholar]
Holla 2020 {published data only}
- Holla B, Biswal J, Ramesh V, Shivakumar V, Bharath RD, Benegal V, et al. Effect of prefrontal tDCS on resting brain fMRI graph measures in Alcohol Use Disorders: A randomized, double-blind, sham-controlled study. Progress in Neuropsychopharmacology and Biological Psychiatry 2020;102:109950. [DOI] [PubMed] [Google Scholar]
Kalichman 2007 {published data only}
- Kalichman SC, Simbayi LC, Vermaak R, Cain D, Jooste S, Peltzer K. HIV/AIDS risk reduction counseling for alcohol using sexually transmitted infections clinic patients in Cape Town, South Africa. Journal of Acquired Immune Deficiency Syndromes 2007;44(5):594-600. [DOI] [PubMed] [Google Scholar]
Kalichman 2008 {published data only}
- Kalichman SC, Simbayi LC, Vermaak R, Cain D, Smith G, Mthebu J, et al. Randomized trial of a community-based alcohol-related HIV risk-reduction intervention for men and women in Cape Town South Africa. Annals of Behavioral Medicine 2008;36(3):270-9. [DOI] [PubMed] [Google Scholar]
Kalichman 2011 {published data only}
- Kalichman SC, Cain D, Eaton L, Jooste S, Simbayi LC. Randomized clinical trial of brief risk reduction counseling for sexually transmitted infection patients in Cape Town, South Africa. American Journal of Public Health 2011;101(9):e9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mertens 2014 {published data only}
- Mertens JR, Ward CL, Bresick GF, Broder T, Weisner CM. Effectiveness of nurse-practitioner-delivered brief motivational intervention for young adult alcohol and drug use in primary care in South Africa. Alcohol and Alcoholism 2014;49(4):430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2016 {published data only}
- Myers B, Westhuizen C, Naledi T, Stein DJ, Sorsdahl K. Readiness to change is a predictor of reduced substance use involvement: findings from a randomized controlled trial of patients attending South African emergency departments. BMC Psychiatry 2016;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rongkavilit 2013 {published data only}
- Rongkavilit C, Naar-King S, Wang B, Panthong A, Bunupuradah T, Parsons JT, et al. Motivational interviewing targeting risk behaviors for youth living with HIV in Thailand. AIDS and Behavior 2013;17(6):2063-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotheram‐Borus 2015 {published data only}
- Rotheram-Borus MJ, Tomlinson M, le Roux I, Stein JA. Alcohol use, partner violence, and depression: a cluster randomized controlled trial among urban South African mothers over 3 years. American Journal of Preventive Medicine 2015;49(5):715-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorsdahl 2015 {published data only}
- Sorsdahl K, Stein DJ, Corrigall J, Cuijpers P, Smits N, Naledi T, et al. The efficacy of a blended motivational interviewing and problem solving therapy intervention to reduce substance use among patients presenting for emergency services in South Africa: a randomized controlled trial. Substance Abuse Treatment, Prevention, and Policy 2015;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thapinta 2014 {published data only}
- Thapinta D, Skulphan S, Kittrattanapaiboon. Brief cognitive behavioral therapy for depression among patients with alcohol dependence in Thailand. Issues in Mental Health Nursing 2014;35(9):689-93. [DOI] [PubMed] [Google Scholar]
Ward 2015 {published data only}
- Ward CL, Mertens JR, Bresick GF, Little F, Weisner CM. Screening and brief intervention for substance misuse: does it reduce aggression and HIV-related risk behaviours? Alcohol and Alcoholism 2015;50(3):302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wechsberg 2006 {published data only}
- Wechsberg WM, Luseno WK, Lam WKK, Parry CDH, Morojele NK. Substance use, sexual risk, and violence: HIV prevention intervention with sex workers in pretoria. AIDS and Behavior 2006;10(2):131-7. [DOI] [PubMed] [Google Scholar]
Wechsberg 2008 {published data only}
- Wechsberg WM, Luseno WK, Karg RS, Young S, Rodman N, Myers B, et al. Alcohol, cannabis, and methamphetamine use and other risk behaviours among Black and Coloured South African women: a small randomized trial in the Western Cape. International Journal of Drug Policy 2008;19(2):130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wechsberg 2016 {published data only}
- Wechsberg WM, Zule WA, El-Bassel N, Doherty IA, Minnis AM, Novak SD, et al. The male factor: Outcomes from a cluster randomized field experiment with a couples-based HIV prevention intervention in a South African township. Drug and Alcohol Dependence 2016;161:307-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Balachova 2017 {published data only (unpublished sought but not used)}
- Balachova T, Bonner B, Chaffin M, Isurina G, Tsvetkova L, Volkova E, et al. Dual-focused brief physician intervention to reduce the risk for alcohol-exposed pregnancies: a randomized controlled trial. Alcoholism: Clinical and Experimental Research 2013;37:50A. [Google Scholar]
- Balachova T, Bonner B, Chaffin M, Isurina GL, Tsvetkova LA, Volkova EN. A randomized controlled trial of a dual-focused brief physician intervention in obgyn clinics in Russia. Alcoholism: Clinical and Experimental Research 2012;36:23A. [Google Scholar]
- Balachova T, Chaffin M, Isurina GI, Tsvetkova LA, Volkova EN, Agrawal S, et al. Efficacy of a brief intervention at women's clinics in Russia. Alcoholism: Clinical and Experimental Research 2015;39:284A. [Google Scholar]
- Balachova TB, Chaffin, MJ, Bonner BL, Isurina GL, Tsvetkova LA, Volkova EN. Randomized cluster trial of a brief fasd prevention intervention for Russian women in OB/GYN care. Alcoholism: Clinical and Experimental Research 2017;41:336A. [Google Scholar]
- Balachova TN, Chaffin MJ, Isurina GL, Tsvetkova LA, Volkova EN, Agrawal S, et al. A brief intervention to reduce the risk for an alcohol-exposed pregnancy: outcomes of a randomized clinical trial in Russia. Alcoholism: Clinical and Experimental Research 2014;38:343A. [Google Scholar]
Belokrylov 2018 {published data only}
- Belokrylov I, Bryukhin A, Karnozov V. Experience in the application of group psychoanalytic psychotherapy in the clinic of addictive pathology. European Psychiatry 2018;48:S544-5. [Google Scholar]
Clair 2016 {published data only (unpublished sought but not used)}
- Clair V, Mutiso V, Musau A, Frank E, Ndetei D. Online learning improves substance use care in Kenya: randomized control trial results and implications. Annals of Global Health 2016;82(3):320-1. [NCT02388243] [Google Scholar]
Du 2017 {published data only}
- Du J, Ruan X, Zhao M. Brief intervention helped individuals with problem alcohol use who experienced the Wenchuan earthquake in Sichuan, China. Journal of Neuroimmune Pharmacology 2017;12(2):S100. [Google Scholar]
Gorny 2019 {published data only}
- Gorny BE, Kalinina AM, Dolgova SV, Nabiullina GA, Paliy IA, Bunova AS. Organizational and functional technology for screening and preventive counseling of patients with harmful alcohol consumption and the results of testing the technology in primary health care. Profilakticheskaya Meditsina 2019;22(3):14-19. [Google Scholar]
Jin 2006 {published data only}
- Jin M, Li J, Yang YC. Evaluation of the clinical effect of electro-acupuncture aversion therapy of alcohol-dependence. Chinese Journal of Clinical Rehabilitation 2006;10(47):18-20. [Google Scholar]
Parry 2014 {published data only (unpublished sought but not used)}
- Parry C, Shuper P, Londani BM, Myers B, Nkosi S, Manda S, et al. Drinking among patients on antiretroviral therapy in South Africa: gender differences and outcomes of a clinical trial. Alcoholism: Clinical and Experimental Research 2019;43:266A. [Google Scholar]
- Parry CD, Morojele NK, Myers BJ, Kekwaletswe CT, Manda SO, Sorsdahl K, et al. Efficacy of an alcohol-focused intervention for improving adherence to antiretroviral therapy (ART) and HIV treatment outcomes - a randomised controlled trial protocol. BMC Infectious Diseases 2014;14:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prasad 2018 {published data only}
- Prasad S, Basu D, Mattoo SK, Subodh BN. Brief intervention to reduce substance use in Class C male hospital employees: a randomised controlled trial. Indian Journal of Psychiatry 2018;60(5):S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rentala 2020 {published data only}
- Rentala S, Ng SM, Chan CLW, Bevoor P, Nayak RB, Desai M. Effect of holistic relapse prevention intervention among individuals with alcohol dependence: a prospective study at a mental health care setting in India. Journal of Ethnicity in Substance Abuse 2020;21(1):687-707. [DOI] [PubMed] [Google Scholar]
Siriwardhana 2013 {published data only}
- Siriwardhana P, Dawson AH, Abeyasinge R. Acceptability and effect of a community-based alcohol education program in rural Sri Lanka. Alcohol and Alcoholism 2013;48(2):250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Staton 2019 {published data only}
- Staton C, Vissoci JRN, Swann M, Hirshon JM, Bartlett J, Mmbaga B. Feasibility of a pragmatic randomized adaptive clinical trial of a brief intervention for alcohol use in Moshi, Tanzania. Alcoholism: Clinical and Experimental Research 2019;43:80A. [Google Scholar]
Yadav 2019 {published data only}
- Yadav P. Follow up study of effects of breathing technique and relaxation program on male patients of Alcohol Dependence Syndrome. Indian Journal of Psychiatry 2019;61(9):S591. [Google Scholar]
References to ongoing studies
Almonacid 2020 {published data only}
- Almonacid I, Olaya L, Cuevas V, Castillo JS, Becerra N, Delgado J, et al. Effectiveness of brief counseling in a hospital setting for smoking cessation and risky alcohol drinking reduction: randomized clinical trial protocol. Revista Colombiana de Psycquiatria 2020;20:30085-8. [Google Scholar]
Diclemente 2021 {published data only}
- DiClemente RJ, Brown JL, Capasso A, Revzina N, Sales JM, Boeva E, et al. Computer-based alcohol reduction intervention for alcohol-using HIV/HCV coinfected Russian women in clinical care: study protocol for a randomized controlled trial. Trials 2021;22:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diclemente R. Computer-based intervention for alcohol-using HIV/HCV+ women. clinicaltrials.gov. [NCT03362476]
Fernandes 2019 {published data only}
- Fernandes D. Alcohol use disorders - mobile based brief intervention treatment (AMBIT): a pilot RCT. clinicaltrials.gov 2019. [NCT04078360]
Kiene 2019 {published data only}
- Kiene SM. Development of an intervention to reduce heavy drinking and improve HIV care engagement among fisherfolk in Uganda. clinicaltrials.gov. [NCT03919695]
Lelutiu‐Weinberger 2019 {published data only}
- Lelutiu-Weinberger C, Pachankis JE. Building mobile HIV prevention and mental health support in low-resource settings. clinicaltrials.gov 2019. [NCT03912753]
Liang 2020 {published data only}
- Liang X, Hu X, Zhang X Fu H. ASF (a compound of traditional chinese medicine) in the treatment of patients with alcohol dependence: study protocol of a randomized, double-blinded, placebo-controlled clinical trial. Medicine 2020;99(5):e23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lodi 2021 {published data only}
- Emenyonu N, Marson K. Drinkers' intervention to prevent tuberculosis (DIPT Study). clinicaltrials.gov. [NCT03492216] [DOI] [PMC free article] [PubMed]
- Lodi S, Emenyonu NI, Marson K, Kwarisiima D, Fatch R, McDonell MG, et al. The Drinkers’ Intervention to Prevent Tuberculosis (DIPT) trial among heavy drinkers living with HIV in Uganda: study protocol of a 2×2 factorial trial. Trials 2021;22:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mmbaga 2021 {published data only}
- Mmbaga BT, Staton CA. PRACT: A pragmatic randomized adaptive clinical trial to investigate controlling alcohol related harms in a low-income setting: Emergency department brief interventions in Tanzania. ClinicalTrials.gov 2021.
Mutyambizi‐Mafu 2019 {published data only (unpublished sought but not used)}
- Mutyambizi-Mafunda V, Myers B, Sorsdahl K, Lund C, Naledi T, Cleary S. Integrating a brief mental health intervention into primary care services for patients with HIV and diabetes in South Africa: study protocol for a trial-based economic evaluation. BMJ Open 2019;9(5):e026973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadkarni 2019a {published data only}
- Nadkarni A. Alcohol use disorders- Mobile based brief intervention treatment (AMBIT): a pilot RCT. ClinicalTrials.gov 2019.
NCT02797587 {published data only}
- NCT02797587. Studying partial-agonists for ethanol and tobacco elimination in Russians with HIV (St PETER HIV). clinicaltrials.gov/ct2/show/record/NCT02797587 (first received 8th June 2016). [NCT02797587]
NCT03380728 {published data only}
- NCT03380728. Ibogaine in the treatment of alcoholism: a randomized, double-blind, placebo-controlled, escalating dose, phase 2 trial. clinicaltrials.gov/ct2/show/record/NCT03380728 (first received 15th December 2017). [NCT03380728]
NCT03610815 {published data only}
- NCT03610815. Community I-STAR Mozambique: Community implementation of SBIRT using technology for alcohol use reduction in Mozambique. clinicaltrials.gov/ct2/show/NCT03610815 (first received 24thJune 2018). [NCT03610815]
NCT04906616 {published data only}
- NCT04906616. An economic and relationship-strengthening for HIV-affected couples who drink alcohol in Malawi. clinicaltrials.gov/ct2/show/NCT04906616 2021.
Paula 2021 {published data only}
- Ferri CP, Paula TC. Evaluation of an intervention to reduce alcohol consumption among older adults. WHO International Clinical Trial Registry Platform 2020.
- Paula TC, Chagas C, Noto AR, Formigoni MLO, Pereira TV, Ferri CP. Brief interventions for older adults (BIO) delivered by non-specialist community health workers to reduce at-risk drinking in primary care: a study protocol for a randomised controlled trial. BMJ Open 2021;11:e043918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sangle 2021 {published data only}
- Sangle S. Alcohol reduction intervention study for TB and HIV patients in India. WHO International Clinical Trials Registry Platform 2021.
Additional references
American Psychiatric Association 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington (VA): American Psychiatric Association, 2013. [Google Scholar]
Anderson 2006
- Anderson P. Global use of alcohol, drugs and tobacco. Drug and Alcohol Review 2006;25:489-502. [DOI] [PubMed] [Google Scholar]
Baingana 2015
- Baingana F, Al'Absi M, Becker AE, Pringle B. Global research challenges and opportunities for mental health and substance-use disorders. Nature 2015;527:S172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benegal 2009
- Benegal V, Chand PK, Obot IS. Packages of care for alcohol use disorders in low- and middle-income countries. PLOS Medicine 2009;6(10):e1000170. [DOI: 10.1371/journal.pmed.1000170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Identifying and Quantifying Heterogeneity. West Sussex (UK): John Wiley & Sons, Ltd, 2009. [Google Scholar]
Center for Substance Abuse Treatment 2009
- Center for Substance Abuse Treatment. Incorporating alcohol pharmacotherapies into medical practice,2009. Treatment Improvement Protocol (TIP) Series 49. HHS Publication No. (SMA) 09-4380. adaiclearinghouse.org/downloads/TIP-49-Incorporating-Alcohol-Pharmacotherapies-into-Medical-Practice-45.pdf (accessed prior to 22 May 2019).
Collin 2016
- Collin J, Caswell S. Alcohol and the sustainable development goals. Lancet 2016;387(10038):2582-3. [DOI] [PubMed] [Google Scholar]
Connor 2016
- Connor JP, Haber PS, Hall WD. Alcohol use disorders. Lancet 2016;387:988-98. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed December 21, 2021. Available at covidence.org.
Degenhardt 2008
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO world mental health surveys. PLOS Medicine 2008;5:1053-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Douaihy 2013
- Douaihy AB, Kelly TM, Sullivan C. Medications for substance use disorders. Social Work in Public Health 2013;28(3-4):264-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faraone 2008
- Faraone SV. Interpreting estimates of treatment effects: implications for managed care. Physical Therapy 2008;33(12):700-11. [PMC free article] [PubMed] [Google Scholar]
Ferreira‐Borges 2017
- Ferreira-Borges C, Parry CD, Babor TF. Harmful use of alcohol: a shadow over Sub-Saharan Africa in need of workable solutions. International Journal of Environmental Research and Public Health 2017;14(346):E346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franck 2013
- Franck J, Jayaram-Lindstrom N. Pharmacotherapy for alcohol dependence: status of current treatments. Current Opinion in Neurobiology 2013;23:696-9. [DOI] [PubMed] [Google Scholar]
Ghosh 2022
- Ghosh A, Singh P, Das N, Pandi PM, Das S, Sarkar S. Efficacy of brief intervention for harmful and hazardous alcohol use: a systematic review and meta-analysis of studies from low middle-income countries. Addiction 2022;117(3):545-58. [DOI] [PubMed] [Google Scholar]
Giusto 2018
- Giusto A, Puffer E. A systematic review of interventions targeting men's alcohol use and family relationships in low- and middle-income countries. Global Mental Health 2018;5:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greene 2019a
- Greene MC, Ventevogel P, Kane JC. Substance use services for refugees. Bulletin of the World Health Organization 2019;97:246-246A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenfield 2013
- Greenfield BL, Tonigan JS. The general alcoholics anonymous tools of recovery: the adoption of 12-step practices and beliefs. Psychology of Addictive Behaviors 2013;27(3):553-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grella 2013
- Grella CE, Stein JA. Remission from substance dependence: differences between individuals in a general population longitudinal survey who do and do not seek help. Drug and Alcohol Dependence 2013;133(1):146-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamza 2014
- Hamza DM, Silverstone PH. A systematic review of treatment modalities for alcohol use disorder. Journal of Substance Abuse & Alcoholism 2014;2(3):1023. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Greene S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Humphreys 2004
- Humphreys K. Circles of Recovery: Self-help Organizations for Addictions. Cambridge (UK): Cambridge University Press, 2004. [Google Scholar]
Humphreys 2014
- Humphreys K, Blodgett JC, Wagner TH. Estimating the efficacy of Alcoholics Anonymous without self-selection bias: an instrumental variables re-analysis of randomized clinical trials. Alcoholism: Clinical and Experimental Research 2014;38(11):2688-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2019
- Johnson RM, Linton S, Reisner SL, Martins S, Mortensen BP, Eaton WW. Adult mental disorders in association with socioeconomic position, race/ethnicity, and sexual & gender minority (SGM) status. In: Eaton WW, Fallin MD, editors(s). Public Mental Health. Oxford (UK): Oxford University Press, 2019. [Google Scholar]
Joseph 2017
- Joseph J, Basu D. Efficacy of brief interventions in reducing hazardous or harmful alcohol use in middle-income countries: systematic review of randomized controlled trials. Alcohol and Alcoholism 2017;52(1):56-64. [DOI] [PubMed] [Google Scholar]
Kaner 2018
- Kaner EF, Beyer FR, Muirhead C, Campbell F, Pienaar ED, Bertholet N, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD004148. [DOI: 10.1002/14651858.CD004148.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2012
- Kelly JF, Hoeppner B, Stout RL, Pagano M. Determining the relative importance of the mechanisms of behavior change within Alcoholics Anonymous: a multiple mediator analysis. Addiction 2012;107(2):289-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2020
- Kelly JF, Humphreys K, Ferri M. Alcoholics Anonymous and other 12-step programs for alcohol use disorder. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD012880. [DOI: 10.1002/14651858.CD012880.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleber 2006
- Kleber HD, Weiss RD, Anton RF, George TP, Greenfield SF, Kostein TR, et al. Practice guidelines for the treatment of patients with substance use disorders, 2006. psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/substanceuse.pdf (accessed prior to 22 May 2019).
Lim 2012
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mann 2017
- Mann K, Aubin HJ, Witkiewitz K. Reducing drinking in alcohol dependence treatment: What is the evidence? European Addiction Research 2017;23(5):219-30. [DOI] [PubMed] [Google Scholar]
McCrady 2014
- McCrady BS, Owens MD, Borders AZ, Brovko JM. Psychosocial approaches to alcohol use disorders since 1940: a review. Journal of Studies on Alcohol and Drugs 2014;75 Suppl 17:68-78. [DOI] [PubMed] [Google Scholar]
McQueen 2011
- McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database of Systematic Reviews 2011, Issue 8. Art. No: CD005191. [DOI: 10.1002/14651858.CD005191.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Medina‐Mora 2001
- Medina-Mora ME. Women and alcohol in developing countries. Salud Mental 2001;24(2):3-10. [Google Scholar]
Medina‐Mora 2016
- Medina-Mora ME, Monteiro M, Room R, Rehm J, Jernigan D, Sanchez-Moreno D, et al. Alcohol use and alcohol use disorders. In: Patel V, Chisholm D, Dua T, Laxminarayan R, Medina-Mora ME, editors(s). Mental, Neurological, and Substance Use Disorders: Disease Control Priorities. 3rd edition. Vol. 4. Washington (DC): World Bank Publication, 2016:127-44. [Google Scholar]
Miller 1983
- Miller WR. Motivational interviewing with problem drinkers. Behavioural Psychotherapy 1983;11:147-72. [Google Scholar]
Minozzi 2018
- Minozzi S, Saulle R, Rosner S. Baclofen for alcohol use disorder. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD012557. [DOI: 10.1002/14651858.CD012557.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2012
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2197-23. [DOI] [PubMed] [Google Scholar]
National Institute on Drug Abuse 2000
- National Institute on Drug Abuse. Principles of drug addiction treatment: a research-based guide, 2000. www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition (accessed prior to 22 May 2019).
NICE 2011
- National Collaborating Centre for Mental Health (UK). Alcohol-use disorders: diagnosis, assessment and management of harmful drinking and alcohol dependence. NICE Clinical Guidelines, No. 115, 2011. www.nice.org.uk/guidance/cg115 (accessed prior to 22 May 2019).
Nutt 2010
- Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet 2010;376(9752):1558-65. [DOI] [PubMed] [Google Scholar]
Patel 2007
- Patel V, Araya R, Chatterjee S, Chisholm D, Cohen A, De Silva M, et al. Treatment and prevention of mental disorders in low- and middle-income countries. Lancet 2007;370(9591):991-1005. [DOI] [PubMed] [Google Scholar]
Perngparn 2008
- Perngparn U, Assanangkornchai S, Pilley C, Aramrattana A. Drug and alcohol services in middle-income countries. Current Opinion in Psychiatry 2008;21(3):229-233. [DOI] [PubMed] [Google Scholar]
Petersen 2016
- Petersen I, Evans-Lacko S, Semrau M, Barry MM, Chisholm D, Gronholm P, et al. Promotion, prevention and protection: interventions at the population- and community-levels for mental, neurological and substance use disorders in low- and middle-income countries. International Journal of Mental Health Systems 2016;10:30. [DOI: 10.1186/s13033-016-0060-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plosker 2015
- Plosker GL. Acamprosate: a review of its use in alcohol dependence. Drugs 2015;75(11):1255-68. [DOI] [PubMed] [Google Scholar]
Popova 2017
- Popova S, Lange S, Probst C, Gmel G, Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Global Health 2017;5(3):e290-9. [DOI] [PubMed] [Google Scholar]
Preusse 2020
- Preusse M, Neuner F, Ertl V. Effectiveness of psychosocial interventions targeting hazardous and harmful alcohol use and alcohol-related symptoms in low- and mIddle-income countries: a systematic review. Frontiers in Psychiatry 2020;11:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rollnick 1992
- Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. Journal of Mental Health 1992;1(1):25-37. [Google Scholar]
Room 2002
- Room R, Jernigan D, Carlini B, Gureje O, Makela K, Marshall M, et al. Alcohol in Developing Societies: a Public Health Approach. Helsinki (Finland): Finnish Foundation for Alcohol Studies, World Health Organization, 2002. [Google Scholar]
Rosner 2010a
- Rosner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD001867. [DOI: 10.1002/14651858.CD001867.pub3] [DOI] [PubMed] [Google Scholar]
Rosner 2010b
- Rosner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database of Systematic Reviews 2010, Issue 9. Art. No: CD004332. [DOI: 10.1002/14651858.CD004332.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brozek J, Guyatt G, Oxman A, editor(s), the GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations, 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 22 May 2019).
Sileo 2021
- Sileo KM, Miller AP, Wagman JA, Kiene SM. Psychosocial interventions for reducing alcohol consumption in sub-Saharan African settings: a systematic review and meta-analysis. Addiction 2021;116(3):457-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 2014
- Skinner MD, Lahmek P, Pham H, Aubin HJ. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLOS One 2014;9(2):e87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sornpaisarn 2013
- Sornpaisarn BK, Shield K, Cohen J, Schwartz R, Rehm J. Elasticity of alcohol consumption, alcohol-related harms, and drinking initiation in low- and middle-income countries: a systematic review and meta-analysis. International Journal of Alcohol and Drug Research 2013;2(1):45-58. [Google Scholar]
Soyka 2017
- Soyka M, Kranzler HR, Hesselbrock V, Kasper S, Mutschler J, Moller HJ, et al. Guidelines for biological treatment of substance use and related disorders, part 1: alcoholism. World Journal of Biological Psychiatry 2017;18(2):86-119. [DOI] [PubMed] [Google Scholar]
Sutton 2000
- Sutton AJ, Abrams KR, Jones DR, Sheldon TQ, Song F. Assessing Between Study Heterogeneity. Methods for Meta-analysis in Medical Research. West Sussex (UK): John Wiley & Sons, Ltd, 2000. [Google Scholar]
Tonigan 2003
- Tonigan J, Connors G, Miller W. Participation and involvement in Alcoholics Anonymous. In: Babor TF, Del Boca FK, editors(s). Treatment Matching in Alcoholism. Cambridge (UK): Cambridge University Press, 2003:184-204. [Google Scholar]
van Ginneken 2013
- Ginneken N, Tharyan P, Lewin S, Rao GN, Meera SM, Pian J, et al. Non-specialist health worker interventions for the care of mental, neurological and substance-abuse disorders in low- and middle-income countries. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD009149. [DOI: 10.1002/14651858.CD009149.pub2] [DOI] [PubMed] [Google Scholar]
Walls 2020
- Walls H, Cook S, Matzopoulos R, London L. Advancing alcohol research in low-income and middle-income countries: a global alochol environment framework. BMJ Global Health 2020;5:e001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiteford 2013
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013;382(9904):1575-86. [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Global strategy to reduce the harmful use of alcohol. https://www.who.int/substance_abuse/alcstratenglishfinal.pdf.
WHO 2011
- World Health Organization. mhGAP intervention guide for mental, neurological and substance use disorders in non-specialized health settings, 2011. www.who.int/mental_health/publications/mhGAP_intervention_guide/en/ (accessed prior to 22 May 2019).
WHO 2014
- World Health Organization. Global status report on alcohol and health, 2014. www.who.int/substance_abuse/publications/global_alcohol_report/msb_gsr_2014_1.pdf (accessed prior to 22 May 2019).
WHO 2015
- World Health Organization. Psychosocial interventions for management of alcohol dependence: Evidence review. https://www.who.int/mental_health/mhgap/evidence/resource/alcohol_q5.pdf?ua=1 2015.
WHO 2018a
- World Health Organization. Global status report on alcohol and health, 2018. www.who.int/substance_abuse/publications/global_alcohol_report/en/ (access prior to 22 May 2019).
WHO 2018b
- World Health Organization. ICD-11 for mortality and morbidity statistics (ICD-11 MMS), 2018. icd.who.int/browse11/l-m/en (accessed prior to 22 May 2019).
Winslow 2016
- Winslow BT, Onysko M, Hebert M. Medications for alcohol use disorder. American Family Physician Journal 2016;93(6):457-65. [PubMed] [Google Scholar]
World Bank 2015
- World Bank. Country and lending groups, 2015. data.worldbank.org/about/country-and-lending-groups (accessed prior to 22 May 2019).
World Bank 2017
- World Bank. World Bank country and lending groups, 2017. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed prior to 22 May 2019).
World Bank 2021
- World Bank. DataBank World Development Indicators. databank.worldbank.org 2021.
Xu 2011
- Xu X, Chaloupka FJ. The effects of prices on alcohol use and its consequences. Alcohol Research and Health 2011;34(2):236-45. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Greene 2019b
- Greene MC, Kane J, Johnson RJM, Tol WA. Psychosocial and pharmacologic interventions to reduce harmful alcohol use in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD013350. [DOI: 10.1002/14651858.CD013350] [DOI] [PMC free article] [PubMed] [Google Scholar]


