SUMMARY
The successful transplantation of stem cells has the potential to transform regenerative medicine approaches and open promising avenues to repair, replace, and regenerate diseased, damaged, or aged tissues. However, pre-/post-transplantation issues of poor cell survival, retention, cell fate regulation, and insufficient integration with host tissues constitute significant challenges. The success of stem cell transplantation depends upon the coordinated sequence of stem cell renewal, specific lineage differentiation, assembly, and maintenance of long-term function. Advances in biomaterials can improve pre-/post-transplantation outcomes by integrating biophysiochemical cues and emulating tissue microenvironments. This review highlights leading biomaterials-based approaches for enhancing stem cell transplantation.
INTRODUCTION
Adult multicellular tissues maintain a healthy tissue state by constantly turning over cells through a careful balance of cell death and cell division (Biteau et al., 2011). However, pathologies due to degenerative diseases, aging, cancers, or idiopathic tissue injuries result in loss-of-functional tissue. Due to the limited ability of the adult tissue to regenerate, with the exception of gut, cornea, skin, and liver, external interventions are needed to restore native tissue and its normal physiological functions (Iismaa et al., 2018; Yun, 2015).
Stem cells are promising interventions because of their ability to self-renew and promote tissue repair and regeneration. The regenerative potential of stem cells and stem cell-derived tissue-specific cells depends on genetics, epigenetics, and their complex extracellular microenvironment, which collectively informs the stem cells’ differentiation pathways (Mahla, 2016; Zakrzewski et al., 2019). However, most studies have demonstrated that stem cell-based therapies provide only modest improvement in tissue function, which could be attributed to pre-/post-transplantation challenges such as low differentiation efficiency and survival, poor localization and retention at the transplant site, and lack of proper tissue integration (Caplan et al., 2019; Cismaru and Cismaru, 2017; Ntege et al., 2020). The lack of appropriate intrinsic and extrinsic biophysiochemical cues are often key contributing factors responsible for the limited success of stem cell transplantation as they regulate cell differentiation, proliferation, protein synthesis, matrix production, and cell survival (Guilak et al., 2009; Wagers, 2012; Xue et al., 2022).
Mimicking the complex in vivo milieu for transplanted stem cells has proved to be challenging for first generation biomaterials, which mainly consisted of inert, biocompatible materials (Hildebrand, 2013; Marin et al., 2020). In recent years, however, biomaterials developed for regenerative therapies have evolved to include more biofunctional capabilities. These biofunctional materials can better mimic the complex physiological microenvironment by providing essential biophysiochemical signals to retain stemness, direct differentiation, promote reprogramming, manipulate genomic and epigenomic traits, or select for functional phenotypes (Cha et al., 2012; Facklam et al., 2020; Mitrousis et al., 2018). In addition, optimal delivery methods and the incorporation of biomolecules in these biofunctional materials can protect stem cells and stem cell-derived tissue-specific cells after transplantation from stress, hypoxia, and immune attack, thus facilitating long-term viability and maintenance.
Biomaterials interact with the stem cells based on the common principle of dynamic reciprocity and tissue-specific tensional homeostasis (Eichinger et al., 2021; Kimura et al., 2020; Stamenović and Smith, 2020; Thorne et al., 2015; Xu et al., 2009). Biomaterials can be modeled to present cell and tissue-specific structural framework and biophysiochemical cues that support proliferation, differentiation, cell fate, and morphogenetic movement. These functional effects are achieved through bidirectional interactions between the regenerating tissue and the surrounding microenvironment based on the underlying phenomenon of dynamic reciprocity. Tensional homeostasis incorporates the viscoelasticity of the biomaterial construct into the overall mechanical properties of the microenvironment. This resulting unified paradigm of biomaterials and stem cells interact to direct tissue regeneration and homeostasis upon transplantation (Eichinger et al., 2021; Kimura et al., 2020; Thorne et al., 2015; Xu et al., 2009).
In this review, biomaterial-based advances to improve the physiological outcome of stem cell transplantation are described. The review does not aim to provide a comprehensive list of all biofunctional materials described in the literature but highlights strategies that employ different biomaterial design paradigms. The ability of biomaterials to provide necessary biophysiochemical signals for stem cells pre-/post-transplantation is also discussed. Emerging theranostic biomaterial approaches in regenerative medicine that can provide both real-time, noninvasive monitoring and tracking capabilities and therapeutic effects to promote tissue regeneration are briefly described. We focus on in vivo studies in the heart, brain, spinal cord, eye, and pancreas, where recent advancements in biomaterial-based approaches have been used to overcome transplantation challenges.
BIOMATERIAL PARADIGMS FOR SUCCESSFUL STEM CELL TRANSPLANTATION
Transplanted stem cells are expected to replace and repair the diseased tissue through cellular regeneration or supporting endogenous repair by inducing key biophysiochemical factors. Hence, long-term survival, retention, integration, and favorable immune regulation are intertwined and remain prerequisites for successful stem cell transplantation. However, pre-/post-transplantation survival of the stem cells remains a significant challenge and substantially limits the treatment’s efficacy. Noticeably, there are several mechanisms contributing to the loss of stem cell grafts, including unwarranted mechanical stress during culture and delivery. Further, cell death due to the absence of sufficient cell adhesive ligands affects cell retention and integration. Oxidative stress, lack of growth factors, and limited vascularization leading to insufficient access to nutrients and oxygen also contribute to the loss of a graft (Hayward et al., 2021; Stokes et al., 2017; Zhao et al., 2019). The success of stem cell transplantation depends on creating a suitable microenvironment that supports long-term stem cell survival and function.
Biomaterial-based approaches have been shown to address many of these aspects to improve the outcome of stem cell transplantation- as the properties of the biomaterial construct can be tuned to coincide with the different phases of tissue regeneration (Figure 1).
Figure 1. Biomaterial-facilitated stem cell transplantation with engineered biophysiochemical traits for tissue regeneration.
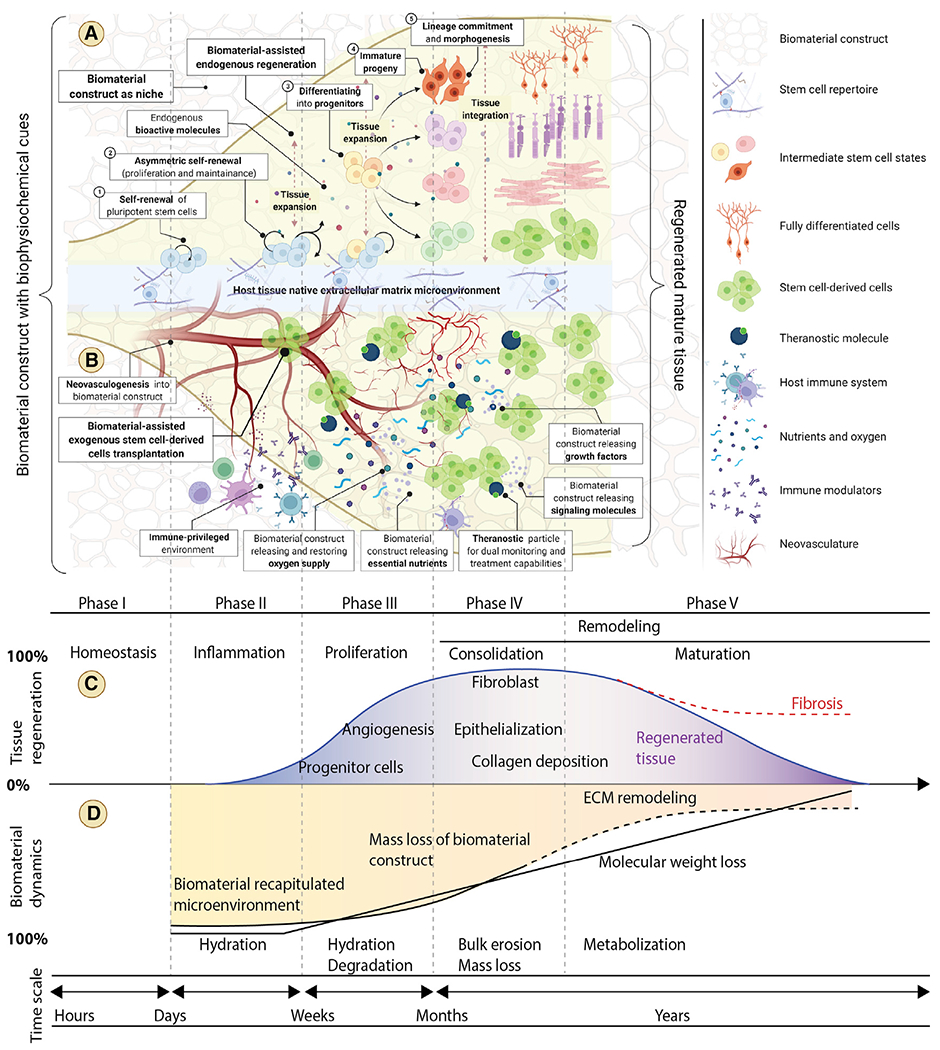
(A) Biomaterial recapitulated microenvironments present essential and complex biophysiochemical cues to retain stemness, direct differentiation, promote reprogramming, manipulate genomic and epigenomic traits, and select for functional phenotypes while dictating stem cell fate during regeneration and repair.
(B) Optimal biomaterial-based methods of stem cell administration by injection or transplantation may improve cell retention and integration with host tissue by allowing for the migration of transplanted and host cells. The intrinsic biomaterial properties (bioinert, bioactive, and biotolerant) and the engineered extrinsic bioactive properties, including biophysical (porosity, pressure, elasticity, force, topography, etc.), biochemical (hormones, cytokines, peptides, growth factors, and immune modulators), and physiochemical (hydrophilicity, temperature, pH, oxygen, nutrients, charge, light, and magnetic field), of the material can protect stem cells after transplantation from stress, hypoxia, starvation, and immune attack, thus facilitating long-term viability and maintenance of the graft.
(C and D) Optimally designed biomaterial constructs should possess dynamic properties that closely align with the different phases of tissue regeneration after implantation. Matching the appropriate timescale of material characteristics including hydration, degradation, bulk erosion, mass loss, and metabolization to regenerative and reparative processes can be beneficial to facilitate tissue regeneration and enable new tissue to overtake functions initially provided by the scaffold while replacing damaged host tissue.
Biomaterial-based stem cell transplantation for improved delivery and retention
Biomaterials that have been utilized for stem cell transplantation are mainly classified into two categories—injectable and implantable biomaterials (Wang et al., 2020; Zhao et al., 2019). Although stem cell transplantation can be minimally invasive with traditional injection-based procedures, it is often difficult to achieve high cell retention and recapitulate the native tissue microenvironment. This is primarily due to a mismatch in the mechanical properties between the injectable material and physiological stiffness (Gattazzo et al., 2014; Hayward et al., 2021; Rozario and DeSimone, 2010). Transplanted cells use specialized proteins to sense and integrate biophysiochemical cues at the molecular, cellular, and tissue levels. Thus, the lack of relevant binding motifs on injectable or implantable biomaterials contribute to the challenge in recapitulating the microenvironment for the cells. With the recent advances in the use of these biomaterials, one can successfully achieve a more hospitable cellular niche. This facilitates the necessary mechanical properties, cell-cell interactions, and biophysiochemical signals that are important for regulating pathways necessary for graft survival (Cha et al., 2012; Perestrelo et al., 2018; Smith and Gerecht, 2016).
Injectable biomaterial-based stem cell transplantation
Injectable biomaterial-based stem cell transplantation is usually carried out using hydrogels due to their potential to recapitulate the microenvironment. They are typically fabricated by physically or chemically cross-linking oligomer precursors. Ionically, cross-linked alginates using divalent calcium ions and self-assembling peptide (SAP) amphiphiles (PAs) are used widely for stem cell delivery (Lee et al., 2019). Stimuli-responsive hydrogels such as thermoresponsive poly(N-isopropylacrylamide (PNIPAAm) (Li et al., 2014), poly(polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN) (Thakur et al., 2016), methyl cellulose (MC), polyethylene glycol (PEG)-poly(lactic-co-glycolic acid) (PLGA-PEG) triblock polymer, pH-sensitive cationic chitosan hydrogel, polyethylenimine (PEI), and zwitterionic poly(2-(methacryloyloxy) ethyl phosphorylcholine) (PMPC) blocks have also been used for stem cell delivery (Zhang et al., 2020). Click chemistry, Diels-Alder reaction, Schiff base reaction, photo-cross-linking, and electrostatic cross-linking are some other methods for cross-linking macromolecules to form hydrogels (Geng et al., 2021; Lee, 2018). Hydrogels can be used as microcarriers (mixed and cross-linked with stem cells), microcapsules (encasing individual cells or cell clusters), or composites of both microcapsules and microcarriers (Fischer et al., 2020; Kupikowska-Stobba and Lewińska, 2020). Microcapsules provide a large surface area for the stem cells to interact with while allowing for better diffusion dynamics of nutrients and waste, whereas microcarriers have interconnected porous structures that facilitate cellular migration, interaction, and integration (Kupikowska-Stobba and Lewińska, 2020; Lee et al., 2021). Mechanical stresses, such as shear and extensional stress, are other significant challenges for injectable stem cell delivery methods using Newtonian fluids. The stem cells experience higher flow resistance near the syringe wall, higher velocity at the center of the syringe, and higher extensional force at the syringe needle interface due to the comparatively smaller needle diameter (Avila et al., 2021; Lee, 2018; Shrestha et al., 2020; Thakur et al., 2016). These mechanical stresses are detrimental to the stem cells, resulting in rapid necrosis and triggering apoptosis that ultimately leads to loss of the graft post-transplant. In a detailed study examining needle gauge, syringe size, flow rate, and vehicle on cell-experienced biomechanical forces, the smallest bore size 32G needle produced significantly higher ejection pressures for all vehicles, and high flow rates with viscous vehicles tended to reduce the viability of injected cells. It was identified that 5-μL/min ejection using a 26G needle increased neuronal differentiation of neural stem cells (NSCs) (Wahlberg et al., 2018). Alginate, hyaluronic acid (HA), and HA MC have shear-thinning properties and exhibit characteristic plug flow that prevent the stem cells from experiencing mechanical stress and improve the retention and viability of retinal stem cells (RSCs), mesenchymal stem cells (MSCs), and adipose stem cells (ASCs) (Aguado et al., 2012; Choi et al., 2020; Vianney et al., 2016). Further, it has been demonstrated that the protective effects from material encapsulation such as alginate are directly due to the mechanical gelation and not the chemistry of the material (Aguado et al., 2012).
Implantable biomaterial-based stem cell transplantation
Implantable biomaterial-based stem cell transplantation is usually invasive but can be a promising strategy due to the ability to better mimic a more complex in vivo cellular microenvironment. Stem cells transplanted onto scaffolds demonstrate the formation of more complex tissue architecture, improvement in cell retention, and better integration with host tissue by allowing the migration of transplanted and host cells (Adu-Berchie and Mooney, 2020; Mitrousis et al., 2018; Stieglitz and Schuettler, 2013). Macroporous scaffolds were successfully used for correcting cranial defects by transplanting MSCs (Liu et al., 2014) and displayed improved osteogenesis and host cell infiltration. Implantable stem cell delivery systems can also be advantageous in preventing anchorage-dependent cell death or anoikis (Mitrousis et al., 2018; Qi et al., 2015; Zhang et al., 2013). The prosurvival anchorage-dependent signals are mediated by the binding of cell surface receptors to the extracellular matrix (ECM) that activates focal adhesion kinase (FAK), phosphoinositide 3-kinase (PI3K), protein kinase B (AKT), and mitogen-activated protein (MAP), although insufficient binding to ECM sites leads to anoikis (Martino et al., 2018; Vachon, 2011). For example, arginylglycylaspartic acid (RGD)-functionalized microporous alginate gels improved cell release by providing more anchoring points for cells to generate traction forces and by inducing differential stress relaxation (Chen et al., 2012). Tissue remodeling is further influenced by the biomaterial degradation behavior and surface topography, which accelerate and provide precise control over morphogenesis and cell functions (Figure 1). PLGA/poly(L-lactic acid) (PLLA) porous scaffolds were investigated as substrates for human embryonic stem cell (hESC) adhesion, differentiation, and capacity to form complex tissue architectures (Li et al., 2016; Serbo and Gerecht, 2013). Semi-interpenetrating polymer networks (sIPNs) poly-NIPAAm-lignocellulose scaffolds were used for short-term pluripotency maintenance, whereas nanofibrillar polyamide matrices showed improvement in self-renewal, morphogenesis, and tissue organization (Dai et al., 2021; Mahou et al., 2017; Masullo et al., 2021).
Biomaterial-based endogenous regeneration
Stem cells are usually expanded and differentiated outside the body, where they are later combined with bioactive factors and biomaterial constructs in vitro. However, exogenous stem cell culture followed by transplantation has several major drawbacks, namely donor tissue morbidity, insufficient robust and reliable differentiation, and immunogenicity (Bowers et al., 2019; Chai and Leong, 2007; Hotaling et al., 2015; Jackson, 2016; Khan and Reddy, 2014). Biomaterial-assisted endogenous tissue regeneration, also called in situ tissue regeneration, is designed to eliminate the need for exogenous stem cell manipulation while improving recruitment, renewal, differentiation, migration, vascularization access, immune compatibility, and tissue integration. This strategy involves the implantation of stem cell-free biomaterials such as polymer scaffolds which have a significant capacity for incorporating nutrients, oxygen, and bioactive molecules that are vital for supporting cellular functions (Bae et al., 2012; Gholipourmalekabadi et al., 2016; Hoganson et al., 2008; Ghavidel Mehr et al., 2014; Yu et al., 2016; Figure 1). The biophysiochemical cues from the scaffolds can trigger chemotaxis and differentiation toward specific cell lineages, whereas topologic features, structure, porosity, stiffness, and degradation behavior can influence tissue organization by altering cell adhesion, infiltration, cell concentration, and vascularization (Badylak, 2015; de Vries et al., 2020; Gattazzo et al., 2014; Jansen et al., 2015). Synchronized scaffold disintegration and endogenous tissue regeneration have a better capacity for load transfer and increased mechanical integrity. Newly regenerated tissue can then assume the functions that were initially provided by the scaffold while replacing damaged host tissue. It has been shown that silk fibroin-based hydrogels can accelerate endogenous bone regeneration by more than 200% compared with untreated controls (Ribeiro et al., 2018). Electroconductive quaternized chitosan-g-polyaniline (QCSP) and benzaldehyde group-functionalized poly(ethylene glycol)-co-poly(glycerol sebacate) (PEGS-FA) hydrogels were shown to be effective in wound repair with higher expressions of vascular endothelial growth factor (VEGF) and transforming growth factor β (TGF-β) (Ertas et al., 2021; Mahou et al., 2017; Xu et al., 2019). HA and PEG microrods have been successfully used to promote cardiac and bone tissue healing while reducing the foreign body reaction (FBR) (Le et al., 2018; Rivera et al., 2021). HA hydrogels with bisphosphonate and dextran with bone morphogenic protein 2 (BMP-2) were reported to be effective in inducing endogenous bone regeneration (Hulsart-Billström et al., 2013). Supermolecular PEG-derivative hydrogels functionalized with ureidopyrimidinone and loaded with hepatocyte growth factor (HGF) and insulin-like growth factor-1 (IGF-1) were used for cardiac tissue regeneration in preclinical studies of chronic myocardial infarction (MI) (Mol et al., 2019; Salimath et al., 2012). Further polynucleotides were also successfully used for endogenous tissue regeneration. In situ chondrogenesis and inhibition of endochondral ossification were achieved using gene-activated scaffolds by activating Sry-related HMG box (SOX) family genes such as SOX-5, SOX-6, and SOX-9 transcription factors (Raftery et al., 2020).
Biomaterials to modulate the host tissue niche
The ability of biomaterials to modulate the host tissue niche is critical to achieving successful stem cell transplantation. Apart from using biomaterials to recapitulate the microenvironment for stem cells, the manipulation of the host tissue niche to create a conducive microenvironment around the ailing tissue is vital. Biomaterial-aided stem cell delivery systems can be engineered to favorably stimulate the host tissue niche by incorporating necessary components such as cytokines, growth factors, mechanical stimuli, vascularization, and immune modulators (Adu-Berchie and Mooney, 2020; Chen et al., 2019; Dziki et al., 2017; Voog and Jones, 2010; Waldeck et al., 2017; Figure 1). To support the in vivo differentiation of stem cells and endogenous differentiation of recruited adult stem cells, a series of appropriate cytokines and growth factors are necessary. These cytokines and growth factors can be incorporated into biomaterial scaffolds to prolong their residence at the stem cell transplantation site (Adu-Berchie and Mooney, 2020; Gschwind et al., 2001; Oyler-Yaniv et al., 2017). The cytokines’ temporal control and release kinetics should be considered based on the desired differentiation stage. Control of the growth factors’ concentration, release behavior, and duration of exposure is necessary to maximize stem cell survival while minimizing potential deleterious consequences. For example, BMP-2 has been used successfully after spinal fusion treatment, but prolonged exposure to high concentrations of BMP-2 may lead to ectopic bone formation and spinal inflammation (Nguyen et al., 2017). Growth factors can be immobilized to the biomaterial by physical blending for rapid release, whereas chemical bonding methods such as protein-protein bonding have been used to achieve long-term release kinetics based on dissociation constants. Glial cell line-derived neurotrophic factor (GDNF) can be blended or covalently immobilized on PLLA nanofiber scaffolds using amine-reactive N-hydroxysuccinimide (NHS)-maleimide chemistry to improve the survival of transplanted stem cells (Chemmarappally et al., 2020; Puhl et al., 2020). MSCs were transplanted on beta-tricalcium phosphate (beta-TCP) scaffolds with epidermal growth factor (EGF), which resulted in a 3-fold increase in survival of MSCs due to the activation of the MAP-kinase pathway (Alvarez et al., 2015). Similarly, stem cells encapsulated in alginate microcapsules with BMP-2 and TGF-β3 together led to effective osteogenesis without neoplastic side effects (Gonzalez-Fernandez et al., 2016). NSCs were transplanted in the spinal cord using PLGA microspheres with dibutyryl cyclic-AMP to improve their differentiation toward neuronal lineage (Kim et al., 2011). To further enhance the outcome of stem cell transplantation, host vasculature plays a vital role as it ensures nonobstructive supplies of oxygen, nutrients, cytokines, and growth factors with a maximum allowable distance of 150–200 μm for their efficient diffusion. Biomaterials’ physiochemical properties such as stiffness, elasticity, degree of cross-linking, along with the incorporation of cell adhesive ligands, growth factor-binding sites, and protease cleavage sites can be modified/controlled independently with high precision for angiogenesis (Fakoya, 2017; Li et al., 2017; Serbo and Gerecht, 2013). The angiogenic growth factor VEGF was immobilized on collagen scaffolds for cardiac repair, which resulted in an increase in blood vessel density and thickness maturation, with parallelly improved recruitment of myofibroblasts resulting in efficient cardiac repair (Miyagi et al., 2011). HA hydrogels used to co-deliver fibronectin and integrins in a stroke injury model led to the generation of mature blood vessels with reduced tortuosity and leakiness due to better ECM deposition followed by pericyte coverage (Erning and Segura, 2020; Li et al., 2017). The transplantation of endothelial cells on aligned fibrin-collagen I scaffolds seeded with primary hepatocytes showed significantly improved vascularization leading to improved hepatic regeneration (Hosseini et al., 2019).
Biomaterials for creating an immune-privileged environment
Biomaterials that can reduce fibrosis and create an immune-privileged environment hold vital importance for improving acute and long-term stem cell transplantation outcomes. Stem cell graft survival and integration with host tissue are affected by contact-dependent blood-mediated reactions, adverse immune reactions, FBRs, and fibrosis. The immune reaction is regulated by T cells and macrophages (Sadtler et al., 2016; Zhang et al., 2021a). T helper (TH) cells recognize the molecular signatures of specific proteins and activate an immune response, whereas macrophages (M) produce toxic compounds to attack foreign bodies. The balance between tissue regeneration and degeneration is well maintained by TH1, TH2, M1, and M2 cells, where TH1 and M1 are associated with a proinflammatory immune response and tissue damage, whereas TH2 and M2 cell types induce anti-inflammatory responses and mediate implant integration and tissue regeneration.
Biophysiochemical properties such as hydrophilicity, topography, surface coating, surface charge, porosity, encapsulation, biomaterial-protein adsorption, and biomaterial-cellular interaction and mechanics can be tuned to induce a favorable immune response (Kharbikar et al., 2021a). Both immune-evasive and immune-engaging biomaterials have been produced to create immune-privileged environments for stem cell transplantation. For example, rectangular cross-linked polymeric microrod topographies were used successfully to reduce fibrosis and improve cardiac outcomes in an infarct model (Le et al., 2018). Hydrophilic PEG and zwitterionic polymer-decorated biomaterials displayed decreased protein binding, thereby preventing complement activation and immune cell adhesion on the graft. TH2-polarizing cytokines such as IL-4 or anti-inflammatory molecules such as dexamethasone have been incorporated in biomaterials to induce anti-inflammatory M2 macrophage phenotype, reduce fibrosis, and improve tissue integration (Banuelos and Lu, 2016; Ladd et al., 2008; Spellberg and Edwards, 2001). Cell transplantation in combination with chondroitinase ABC (ChABC) demonstrated improvement in the recovery of spinal cord injury (SCI). This strategy inhibited chondroitin sulfate proteoglycans (CSPGs) responsible for glial scar formation while improving the plasticity of adult neuronal cells (Bradbury and Burnside, 2019; Hu et al., 2021; Lee et al., 2010). Polylactic acid (PLA) scaffolds loaded with brain-derived neurotrophic factor (BDNF), a growth factor known to modulate inflammation, were used successfully to bridge transection defects in SCI and demonstrated improved neural rewiring in the spinal cord (Bradbury and Burnside, 2019; Houlton et al., 2019; Tuinstra et al., 2012).
Theranostic biomaterials for stem cell transplantation
Theranostic biomaterials combine prognostic, diagnostic, and monitoring capabilities. They are used to noninvasively monitor transplanted stem cells while predicting pathological anomalies and providing therapeutic effects to promote regeneration and repair in real time (Kharbikar et al., 2021b; Patra et al., 2019). Theranostic capabilities have been incorporated into biomaterials such as implantable scaffolds and injectable hydrogels to noninvasively monitor and evaluate the functional and regenerative outcomes simultaneously in vivo (Kharbikar et al., 2021b; Sajesh et al., 2019). HA and gelatin scaffolds incorporating fluorophores were used successfully to monitor neuronal stem cell proliferation and track scaffold degradation using multispectral near-infrared (NIR) imaging for neural tissue regeneration (Park et al., 2019; Yang et al., 2019a). Silica scaffolds functionalized with calcium phosphate, BMP-2, and integrated with superparamagnetic iron-based metal oxide nanoparticles (SPIONs) coated with gold nanoparticles (NPs) were used to regenerate mineralized dentin tissue and monitor the implant using computer tomography and magnetic resonance imaging (MRI) (Mastrogiacomo et al., 2017; Yang et al., 2019a). The gene-editing system, CRISPR-associated Cas9, was coated onto SPIONs and delivered with guide RNA and donor RNA into cells in vitro, which enabled the real-time monitoring of transfection (Hryhorowicz et al., 2019). In this system, theranostic biomaterials were used to noninvasively monitor viability and quantitatively assess the functions of the transplanted stem cells via reporter genes using bioluminescence imaging. Suicide genes incorporated in the stem cell-laden biomaterial transplants provide an opportunity for therapeutic intervention by inactivating transplanted stem cells if imaging detects any abnormalities or after treatment completion. TGL triple-fusion reporter gene-GFP, firefly luciferase, and herpes simplex virus type 1 thymidine kinase suicide gene were used as part of biomaterial-facilitated stem cell transplantation strategies (Li and Xiang, 2013; Ou et al., 2013).
METHODOLOGIES FOR ENGINEERING BIOMATERIAL TRAITS FOR STEM CELL TRANSPLANTATION
Engineering intrinsic biomaterial properties
Biomaterials used for stem cell transplantation aim to recapitulate aspects of the native microenvironment and can serve as a template to direct tissue regeneration (Ali and Payne, 2021; Liu et al., 2018; Marin et al., 2020; O’Neill et al., 2016; Ratner, 2011, 2015). Important biomaterial considerations include biocompatibility, bioactivity, biodegradability, tunable biophysiochemical properties, and cost. Biocompatibility of the implanted biomaterial must be ensured such that successful integration and appropriate response from host tissue is achieved without risk of adverse side effects. Therefore, sufficient testing and evaluation of the biomaterials must be performed to determine potential toxicity concerns. Reactivity of the material’s chemical constituents, degradation products, reaction by-products, potential unreacted monomers, etc., requires assessment for toxicity battery. Evaluations include cytotoxicity, sensitization, hemocompatibility, pyrogenicity, implantation, genotoxicity, and carcinogenicity among others to assure safety for use in humans (US Food and Drug Administration, 2020). The FDA regulates the standards and toxicity threshold limits that are acceptable for biomaterials/medical devices that come into contact with the human body. Biomaterial biodegradability should be engineered and optimized to facilitate the dynamic regeneration of the tissue (Deshayes and Kasko, 2013). Biomaterial degradation by-products must be nontoxic and ideally be broken down and eliminated via natural metabolic pathways (Marin et al., 2020; Ratner, 2011). It is crucial to consider the proinflammatory mechanisms of biomaterials used in stem cell transplantation as all biomaterials can potentially activate an adverse FBR. As such, biomaterials are usually categorized into three main classes–biotolerant, bioactive, and bioinert. Biotolerant materials are disconnected from host tissues through a fibrous layer; bioactive materials interact with host tissue by means of chemical or topographic interactions, whereas bioinert materials have no direct physical interaction with host tissues (Hildebrand, 2013; Marin et al., 2020).
Another key parameter to consider is the structural design of the scaffold that should provide an appropriate environment for cells to recreate microscopic/macroscopic tissue anisotropies (Crouch et al., 2009; Jell et al., 2009; Kharbikar et al., 2021a). The engineered biomaterial architecture should facilitate cell migration and vascularization while presenting a biological interface with the required ligand density for the adhesion of transplanted and/or newly recruited stem cells. The engineering of biomaterial architecture and mechanical properties are intertwined and essential for tuning precise biomechanics relative to biology as the dynamic forces experienced by the implanted stem cells play a major role in defining cell fate (Gattazzo et al., 2014; Jansen et al., 2015). Finally, issues related to biomaterial manufacturing including fabrication complexity, good manufacturing practice (GMP), manufacturing rate, sterility, and cost-effectiveness should be considered (Abdeen and Saha, 2017; Greenberg-Worisek et al., 2018; Johnson and Procopio, 2019; Sanz-Nogués and O’Brien, 2021; Tarabah, 2015).
Engineering extrinsic biomaterial properties
Engineering biomaterial constructs to recapitulate biophysiochemical microenvironments is a challenging proposition considering the complexity of the native stromal niche, which instructs cellular behavior and steers self-organization toward the desired regeneration (Brassard and Lutolf, 2019; Martino et al., 2018; Prasadh et al., 2020; Shinohara et al., 2017; Voog and Jones, 2010; Zhu et al., 2019). Further, transplanted stem cells on the biomaterial construct receive and generate various biophysiochemical cues by means of intrinsic signals (transcription factors and epigenetic regulations). However, these intrinsic signals may also be informed by extrinsic-engineered biomaterials traits. These extrinsic characteristics actively modulate the native environment that dictates regenerative outcomes. This modulation is in congruence with the timelines for wound healing, biomaterial degradation dynamics, and the state of the transplanted stem cells (Figure 1).
Static and dynamic biophysical properties of the biomaterial constructs can be achieved by modifying various parameters in biomaterial processing conditions such as molecular weight, composition, gelation, cross-linking, etc. (Avila et al., 2021; Kharbikar et al., 2021a; Mitrousis et al., 2018; Qi et al., 2015; Shrestha et al., 2020; Thakur et al., 2016; Willerth and Sakiyama-Elbert, 2019; Wong et al., 2004; Zhang et al., 2013; Zhao et al., 2021). These processing variables can be used to fabricate biomaterial constructs with large ranges of static biomechanical properties that can mimic the rigidity and stiffness of any host tissue under treatment. The desired dynamic stiffness and rigidity can be achieved by using biomaterials that can undergo hydrolytic degradation, which reduces stiffness and rigidity to the appropriate modulus and achieves the required biophysical cues over time. The reduction in rigidity and stiffness in the forward direction is identified as softening (Kapfer et al., 2011; Paul et al., 2018; Sadtler et al., 2016; Salta et al., 2010). Similarly, dynamic stiffness and rigidity in the reverse direction, identified as hardening, can be achieved by means of lazy cross-linking spanning the desired timescale (Carver et al., 2016; Carver and Goldsmith, 2013; Gattazzo et al., 2014; Kiang et al., 2013; Tanaka et al., 2020; Zadpoor, 2017). Dynamic softening and hardening can be combined to achieve reversible biomechanics with bidirectional control over the stiffness and rigidity of biomaterial constructs. The viscoelastic properties of biomaterial constructs further compliment the dynamic biomechanics. These viscoelastic properties can be tweaked by using equilibrium reactions of different strengths such as hydrophobic interactions, electrostatic interactions, and dynamic covalent linkages to achieve tunable stress-strain relaxations that have been known to modulate stem cell behavior (Kharbikar et al., 2021a). Human MSCs were demonstrated to express early tissue-specific lineage differentiation markers when cultured on biomaterial constructs having viscoelastic properties matching the host tissue. For example, neuronal-specific differentiation in human MSCs was observed when the biomaterial construct had a modulus close to that of brain tissue (0.1–1 kPa). Similar observations were made for human MSCs induced into myogenic and osteogenic lineages when cultured on substrates with moduli of muscle (8–17 kPa) and osteoid-like bone (25–40 kPa) (Lee et al., 2016; Li et al., 2021a; Neuss et al., 2011; Pittenger et al., 2019; Sivasubramaniyan et al., 2019; Yoon et al., 2018). Intestinal stem cells (ISCs) showed yes-associated protein (YAP) activation and underwent organogenesis when an initially stiff biomaterial softened upon degradation, which led to a dissipation of stress experienced by the cells (Chen and Guan, 2018; Gjorevski and Ordóñez-Morán, 2017). PNIPAAm-based constructs displayed 2D and 3D volumetric microenvironmental stiffening triggered by physiological temperature (Chen and Guan, 2018; Ma et al., 2018; Rana and de La Hoz Siegler, 2021). Chemical stimuli-triggered protein multimerization was used to create mechanically cyclical biomaterial constructs, where were able to stimulate transcriptional reprogramming in human MSCs. It was found that human MSCs on alginate constructs with rapid stress relaxation showed enhanced spreading, proliferation, and osteogenic differentiation (Foight et al., 2019; Uto et al., 2020).
Static and dynamic biochemical properties can be valuable for introducing specific biochemical factors to the transplant that are required to maintain and stimulate specific biological functions (Iacovacci et al., 2016; Li et al., 2021a; Muncie and Weaver, 2018; Popa and Atanase, 2022). Bioactive proteins, peptides, and small molecules can be chemically or physically tethered throughout or in specific patterns on the biomaterial construct (Bertlein et al., 2017; de Sousa Araújo et al., 2021; Finbloom et al., 2021; Geng et al., 2021; Kharbikar et al., 2021a; Rivera et al., 2021). Biomaterial constructs with dynamic biochemical controls can be designed to achieve biofunctionalization over time. Biochemical decoration of biomaterials can be achieved by using reactive handles which can be exploited by cell-secreted bioactive molecules (Bhardwaj et al., 2022; Chesmel et al., 1995; Quintana et al., 2018). Reversible biofunctionalization or immobilization can be used to recapitulate dynamic bidirectional signaling. Soluble biochemical presentation can also be achieved by modulating the release rate from the biomaterial constructs via restricted diffusion or affinity interactions (Almeida and Bártolo, 2014; Chesmel et al., 1995; Ekdahl et al., 2011; Puleo and Bizios, 2009; Salta et al., 2010; Yu et al., 2011).
Topographic interfacial properties on biomaterial constructs, ranging from nano- to micro-scale, are among some of the critical determinants for modulating stem cell behavior. Engineered spatiotemporal surface topographies include size, shape, length, width, spacing, depth, roughness, wettability, and isotropic/anisotropic geometric arrangements, which can strongly influence stem cell behaviors such as adhesion, alignment, growth, and differentiation (Caldorera-Moore and Peppas, 2009; Primavera et al., 2020; Shapira et al., 2014). The regulatory effects of nanoscale topographic structures are due to their modulation of focal adhesion (FA) formation by the clustering of integrins and other adhesion molecules, which alters cytoskeletal organization (Chen et al., 2014; Cimmino et al., 2018). Topographic cues in the form of pores, grooves, pillars, or pits can be created using a variety of nano-/micro-patterning techniques (Curtis et al., 2001; Kharbikar et al., 2021a, 2015; Kim et al., 2012; Le et al., 2019; Sun et al., 2018; Tsimbouri et al., 2014). The synergistic combinations of multiple nano-/micro-topographies have been used to fabricate complex hierarchical topographic features to mimic biological interfaces at the molecular, cellular, and tissue levels (Liu et al., 2016a; Miao et al., 2016; Zheng et al., 2020a). Hierarchical multiscale nano-/micro-grooves patterned on PLGA constructs demonstrated improved differentiation and adhesion of MSCs (Kim et al., 2019; Miao et al., 2016). Similarly, longitudinal nanogrooves (200 nm) in vivo showed a higher density, renewal, and alignment of neurofilaments for improved regeneration of nerves (Huang et al., 2015; Xue et al., 2021). Additionally, electrical, magnetic, and optical conditioning of stem cells on biomaterial constructs have been explored (Chueng et al., 2016; Du et al., 2017; Gelmi and Schutt, 2021; Hofer and Lutolf, 2021; Höpner et al., 2021; Moysidou et al., 2021; Muzzio et al., 2021; Wang et al., 2019). The combinatorial effects of interfacial topography and pulsatile electric potential on stem cells showed enhanced proliferation and differentiation of cardiac myocytes and cardiac fibroblasts (Bloise et al., 2018; Thavandiran et al., 2013). Electrical potential conditioning on biomaterials has been shown to play a major role in hESC differentiation into conductive tissues such as those from cardiac and neural lineages (Tenreiro et al., 2021).
Biomanufacturing—Top-down and bottom-up
Biomanufacturing or biofabrication for stem cell-based regenerative therapies involves building biomaterial constructs. These biomaterial constructs can recapitulate 3D spatiotemporal native cellular and stromal microenvironments to direct stem cell survival, fate, and functions. Manufacturing of the biomaterial constructs broadly follow two distinct approaches: top-down and bottom-up (Abdeen and Saha, 2017; Ahn et al., 2022; Guzzi and Tibbitt, 2020; Nichol and Khademhosseini, 2009; Rainer et al., 2012; Tiruvannamalai-Annamalai et al., 2014; Zhang et al., 2022).
The top-down approaches use porous scaffold structures with ECM-like architecture that are populated with stem cells and perfused with bioactive molecules. The porosity of the scaffold is expected to allow vasculature integration to ensure nutrient and oxygen supply. The bottom-up approaches use modular engineering to create intricate, microstructural functional building blocks that are then used to create complex tissue (Nichol and Khademhosseini, 2009; Vlahos et al., 2017). Common fabrication methods include solvent casting, gas foaming, particle leaching, phase separation, freeze-drying, bioprinting, soft lithography, photolithography, stereolithography, laser sintering, and additive photo-cross-linking (Babbar et al., 2020; Gill et al., 2015; Kharbikar et al., 2021a, 2015; Montero et al., 2020; Norman and Desai, 2006; Rey and St-Pierre, 2019; Baskapan and Callanan, 2021). Other important methods including encapsulation, directed assembly, self-assembly, microfluidics, and construct-free are reported (Bernards et al., 2012; Cao and Desai, 2020; Desai and Shea, 2017; Ernst et al., 2018; Farina et al., 2019; Finbloom et al., 2021; Kang et al., 2014; Kharbikar et al., 2021a; Mendelsohn and Desai, 2010; Nyitray et al., 2014; Rivera et al., 2021; Schweicher et al., 2014). Some of the aforementioned fabrication methods are reported to be amenable with both top-down and bottom-up approaches.
Biomaterial constructs are fabricated predominantly as scaffolds, microcarriers, microgels, and micro-/macro-encapsulation devices to achieve self-organization upon implantation, regenerate and replace the ailing tissue, and have better scale-up for clinical use (Fischer et al., 2020; Lee et al., 2021; Patel et al., 2021; Shapira et al., 2014; Zhong et al., 2021). The bioreactor is particularly important to realize the potential of biomaterial-facilitated stem cell-based regenerative therapies (DiStefano et al., 2018; Greuel et al., 2019; Mihara et al., 2017; Radisic et al., 2008). The biomaterial scaffold-bioreactor system should be capable of generating spatial gradients of regulatory signals and dynamically changing the microenvironment. This system should also be capable of monitoring cellular behavior and responses in real time. A detailed discussion on the various biomanufacturing approaches is out of the scope of this review.
KEY TRANSLATIONAL DEVELOPMENTS IN BIOMATERIAL-FACILITATED STEM CELL TRANSPLANTATION
The development of biofunctional materials can provide essential insights into the design of optimal environments for stem cells. Knowledge from stem cell-biomaterial interactions and the native biophysiochemical microenvironment can help identify relevant design parameters to achieve better outcomes for stem cell therapies in vivo. We describe recent studies in biomaterials-facilitated stem cell regenerative and reparative therapies for cardiovascular, brain/spinal cord, ophthalmic, and pancreatic tissues. Table 1 highlights key examples of biomaterial-based approaches for stem cell regenerative strategies that have capabilities to promote, improve, and support tissue function.
Table 1.
Key translationally relevant studies in biomaterial-facilitated stem cell-based regenerative therapies
| Targets | Technologies/platforms | Engineered biomaterials and cells | Merits and outcomes | References |
|---|---|---|---|---|
| Cardiovascular system | four-dimensional (4D) cardiac patch | photocurable GelMA/PEGDA inks with tricultured hiPSC-CMs, hMSCs, and hECs | recapitulated the architectural and biological features of the native myocardial tissue and provided anisotropic mechanical adaption that improved cardiomyocyte maturation, vascularization, and engraftment in models of myocardial infarction | (Cui et al., 2020) |
| electronically stable conductive patch (CP)-based scaffold | polyaniline (PANI) doped with phytic acid chelated on the surface of a chitosan film tested on myocardium | provided a robust conductive system that could be interfaced with electroresponsive cardiac tissue without inducing proarrhythmogenic activities | (Mawad et al., 2016) | |
| myocardial extracellular matrix (ECM) | ventricular porcine myocardium-derived ECM and endogenous recruitment of stem cells | increased cardiac muscle, improved contractility, enhanced cardiac function, prevented negative left ventricular remodeling, and increased cardiac regeneration by recruiting stem cells after myocardial infarction | (Seif-Naraghi et al., 2013) | |
| cardiac cell-integrated microneedle patch | polyvinyl alcohol (PVA) with cardiac stromal cells | robustly reduced myocardial apoptosis, promoted angiomyogenesis in the peri-infarct area, and thus encouraged regeneration, improved retention, and enhanced engraftment, morphology, and cardiac output | (Tang et al., 2018) | |
| therapeutic replenishable epicardial reservoir (Therepi) | polyurethane (TPU) polymer device encapsulated with cardiac progenitors and macromolecules | ensured continuous and on-demand access to the bioactive molecules, improved retention, regeneration, and provided functional benefits in ejection fraction, stroke work, and fractional shortening | (Whyte et al., 2018) | |
| perfusable multifunctional epicardial device (PerMed) | poly(glycerol sebacate) and poly(ε-caprolactone) (PCL) for endogenous repair | improved ventricular function, displayed targeted and sustained release of growth factors, and enhanced efficacy of cardiac repair | (Huang et al., 2021) | |
| polypyrrole-loaded cardiogel | precardiogel (pCG–decellularized heart) cross-linked with polypyrrole for cardiac progenitor delivery | improved mechanical properties, enhanced electrical conductivity, decreased fibrotic tissue, increased retention, and enhanced vasculature and regeneration | (Parchehbaf-Kashani et al., 2021) | |
| multivascular network hydrogels | poly(ethylene glycol) diacrylate (PEGDA), GelMA | intravascular and multivascular design was achieved using photopolymerization of the hydrogel and demonstrated successful vessel generation, blood flow, and gas exchange | (Grigoryan et al., 2019) | |
| acellular, artificial cardiac patch | decellularized porcine myocardial extracellular matrix scaffold with synthetic cardiac stromal cells (PLGA microparticles loaded with cardiac stromal cell factors) | maintained potency after long-term cryopreservation and reduced scarring, encouraged angiomyogenesis, and improved cardiac function in rodent models of acute myocardial infarction | (Huang et al., 2020) | |
| injectable mesoporous silica nanoparticles (MSNs)/miRNA hydrogel | aldehyde-capped poly(ethylene glycol) (PEG) hydrogel matrix (Gel@MSN/miR-21-5p) | promoted anti-inflammatory and proangiogenic effects and effectively reduced infarct size in a porcine model of myocardial infarction | (Li et al., 2021b) | |
| Central nervous system | 3D microtopographic scaffolds | tyrosine-derived polycarbonate pDTEc with human-induced pluripotent stem cell (hiPSC)-derived neurons | enhanced subtype-specific neuronal reprogramming, transplantation, survival, and integration in a rodent model; potential to reprogram iPSCs to other specific subtypes | (Carlson et al., 2016) |
| hybrid synthetic matrix-assisted and rapidly templated (SMART) neurospheres | manganese dioxide (MnO2) graphene oxide (GO) nanosheets with hiPSC-neural stem cells (NSCs) and laminin | supported high survival rates, controlled differentiation, and functional recovery in a SCI rodent model; represents a substantial development in material-facilitated 3D cell culture systems | (Rathnam et al., 2021) | |
| designer injectable gels | shear-thinning hydrogel for injectable encapsulation and long-term delivery (SHIELD) hydrogels made from C7 protein, 8-arm PEG polymer modified with proline-rich peptides, and PNIPAAm for Schwann cell transplants | increased Schwann cell survival and retention, significantly improved spatial distribution within endogenous tissue, reduced cystic cavitation and neuronal loss, and substantially increased forelimb strength and coordination | (Marquardt et al., 2020) | |
| glycomaterial implants | acellular-engineered chondroitin sulfate (eCS) matrix with brain-derived neurotrophic factor (BDNF) and fibroblast growth factor 2 (FGF-2) | accelerated cellular repair and gross motor function recovery, enhanced volumetric vascularization, activity-regulated cytoskeleton (Arc) protein expression, and perilesional sensorimotor connectivity in chronic severe TBI | (Latchoumane et al., 2021) | |
| Wharton’s Jelly | scaffolds derived from human platelet lysate and human plasma fibrinogen with thrombin as a cross-linker and encapsulated with human mesenchymal stem cells (hMSCs) | demonstrated high survivability, stable proliferation rate, migration out of the hydrogel, upregulated expression of neurotrophic factors, cytokines, and neural markers, and increased expression of neural differentiation markers | (Lech et al., 2020) | |
| Elastic ECM | thin polyacrylamide substrates (PA), ECM with myelinating glia | demonstrated inhibited branching and differentiation of oligodendrocytes (OLs) on rigid, lesion-like matrices whereas Schwann cells (SCs) developed normally in both soft and stiffer matrices to promote healing and regeneration in both CNS and PNS | (Urbanski et al., 2016) | |
| HYDROSAP hydrogels | self-assembling peptides (SAPs) hydrogels with human neural stem cell (hNSC) | decreased astrogliosis and immune response, increased neuronal markers, improved hNSC engraftment, enhanced behavioral recovery, and formation of 3D functional neuronal networks | (Marchini et al., 2019) | |
| brain stiffness-mimicking gel | tilapia collagen gel with hiPSCs-derived dorsal cortical neurons | demonstrated lineage commitment to the terminal neural subtype, improved neurogenesis and neural function, and enhanced production of dorsal cortical neurons | (Iwashita et al., 2019) | |
| thermosensitive hydrogels combined growth factors | acellular spinal cord scaffold with bFGF and heparin-poloxamer (HP) for endogenous regeneration | efficient inhibition of glial scars and improved functional recovery via regeneration of nerve axons and the differentiation of neural stem cells in the SCI | (Xu et al., 2016) | |
| photoresponsive neuroprotective protein hydrogel | His6-tagged recombinant protein, SpyTag-ELP-CarHC-ELP-SpyTag (ACA), metal ions, and adenosylco-balamin with hMSCs and leukemia inhibitory factors (LIFs) | showed excellent injectability, photodegradability, facile encapsulation and delivery of cells and proteins, prolonged cellular signaling, and enhanced axon regeneration | (Jiang et al., 2020) | |
| multichannel polymer scaffold | PLGA scaffolds with activated Schwann cells and MSCs | exhibited significant recovery of nerve function, enhanced differentiation into neuron-like cells, good colocalization with host neurons, and formation of robust bundles of regenerated fibers | (Yang et al., 2017) | |
| bioactive scaffolds with enhanced supramolecular motion | library of IKVAV peptide amphiphiles with different sequences of amino acids V, A, and G (IKVAV PA1 to PA8) for endogenous regeneration | intensified molecular motions within scaffold fibrils enhanced vascular growth, axonal regeneration, myelination, survival of motor neurons, and functional recovery with reduced gliosis | (Álvarez et al., 2021) | |
| growth facilitators | diblock copolypeptide hydrogel K180L20 with FGF-2, EGF, GNDF for endogenous regeneration | regrew full spinal segment beyond lesion centers into neural tissue with terminal-like contacts and displaying synaptic markers, improved electrophysiological conduction, and reinstated developmentally essential mechanisms to facilitate axon growth | (Anderson et al., 2018) | |
| 3D scalable culture system | PNIPAAm-PEG hydrogel with pluripotent stem cells from human oligodendrocyte precursors | generated oligodendrocyte precursor cells (OPCs) in 3D culture without enrichment that displayed excellent engraftment, migration, and maturation into myelinating oligodendrocytes in vivo | (Rodrigues et al., 2017) | |
| Ocular system | 3D micro and ultra-fine matrix | porcine urinary bladder matrix (UBM) with a complex mixture of intracellular and extracellular proteins | UBM particulate substantially reduced corneal haze and promoted proregenerative environments by stimulating type 2 immune response that led to improved wound healing and vision restoration | (Wang et al., 2021a) |
| retinal cell sheets | hESC-derived retinal pigment epithelial (RPE) cells sheets on human amniotic membrane | rescued photoreceptor cells and improved visual acuity in models of retinal degeneration | (M’Barek et al., 2017) | |
| biosynthetic cornea | recombinant human collagen type III (RHCIII) | successful integration of the biosynthetic cornea that remained avascular without the use of long-term immunosuppression, restoration of the tear film, regeneration of nerves, and improvement in vision | (Fagerholm et al., 2010) | |
| polarized RPE polymer matrix | adult human RPE stem cells on polyethylene terephthalate (PET) | human RPE monolayer remained polarized and survived on PET carriers in the subretinal space | (Stanzel et al., 2014) | |
| rotating-wall vessel bioreactors | retinal organoids derived from iPSC, ESCs cultured on a poly(2-hydroxyethyl methacrylate) (polyHEMA)-coated substrate | improved bioprocess for organoid growth and differentiation in the rotating-wall vessel (RWV) bioreactors was observed | (DiStefano et al., 2018) | |
| ultrathin micromolded 3D scaffolds | poly(glycerol sebacate) scaffold with retinal organoids generated from hPSCs | microfabricated scaffolds patterned with high-density photoreceptors produced a multicellular photoreceptor layer for outer retinal reconstruction | (Lee et al., 2021) | |
| retinal pigment epithelium patch | PLGA scaffolds with iPSC-derived RPE | improved integration and functionality of RPE; promising alternative autologous therapy for dry and wet AMD | (Sharma et al., 2019a) | |
| self-organizing human retinal tissue | hESC differentiation to neural retina (NR), GSK3, and FGFR inhibitors | NR-RPE boundary tissue self-organizes a niche for ciliary margin stem cells and expands NR peripherally via de novo progenitor generation | (Kuwahara et al., 2015) | |
| substrate with matching corneal biomechanics | type-I collagen substrates with limbal epithelial stem cell (LESC) | Collagenase-treated burned surface of the cornea restores its appropriate mechanical properties and supports growth of undifferentiated LESCs by YAP suppression | (Gouveia et al., 2019) | |
| rhodopsin genomic loci DNA nanoparticles | polyethylene glycol-substituted polylysine (CK30PEG) conjugated with TAT peptide, rhodopsin genomic loci DNA | gDNA vectors resulted in long-term increased levels of transgene expression and helped rescue retinal degeneration | (Zheng et al., 2020b) | |
| bioprinted construct | gelatin methacryloyl with conjunctival stem cells (CjSCs) | demonstrated injectable delivery of CjSC microtissue to treat of ocular surface diseases | (Zhong et al., 2021) | |
| Scaffold for thick sheet of retinal cells | Scaffold composed of gelatin type A, chondroitin sulfate, and hyaluronic acid with hESCs | successfully simulated the extracellular matrix of the neurosensory retina and supported differentiation into retinal cell types | (Singh et al., 2018) | |
| dual synthetic corneal tissue | synthetic Bowman’s membrane (sBM) and synthetic stromal layer (sSL) for endogenous repair | supported rapid re-epithelialization, maintained corneal transparency, improved mechanical strength, and enabled host/implant integration | (Wang et al., 2020) | |
| full-thickness artificial cornea | acellular porcine cornea matrix (APCM) with limbal epithelial cell-like (LEC-like) cells and corneal endothelial cell-like (CEC-like) cells | successful construction of a full-thickness cornea substitute with good host integration and transparency | (Zhang et al., 2017) | |
| pancreatic tissues | organoid microphysiological system | machined fluidic chips from optically clear PMMA and PFA membrane with islets isolated from rodents | demonstrated dynamic in vitro microenvironment for the preservation of primary organoid function | (Patel et al., 2021) |
| electrogenetic macro-encapsulation device | bioelectronic encapsulation device with electrosensitive designer cells (Electroβ cells) | demonstrated wireless electrical stimulation of vesicular insulin release to attenuate postprandial hyperglycemia | (Krawczyk et al., 2021), | |
| rapid oxygenation of cell encapsulation SONIC scaffold | poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) with islets isolated from rodents | biomimetic scaffold with internal continuous air channels enhanced O2 diffusivity by 10,000-fold and thus survival of transplanted graft | (Wang et al., 2021b) | |
| convection-enhanced macro-encapsulation device (ceMED) | poly(methyl methacrylate) (PMMA), PTFE membranes, and HF modified polyethersulfone with stem cell-derived beta-cells | 3D geometry of ceMED maximized cell loading, improved GSIS and nutrient exchange due to convection, enhanced cell viability, and rapid reduction of hyperglycemia | (Yang et al., 2021) | |
| fluorocapsules | 19F MRI detectable perfluoro-15-crown-5-ether (PFC) and Ba2+-gelled alginate microcapsules with luciferase-expressing mouse βTC6 insulinoma cells | demonstrated the use of 19F MRI signal as a predictive imaging surrogate biomarker for monitoring failure of encapsulated islet cell therapy | (Arifin et al., 2019) | |
| cell-particle hybrids polymeric microspheres | PLGA and FK506 (Tacrolimus) immune suppressant with islets isolated from rodents | demonstrated a method for local immunomodulation with higher efficacy and safety; the platform can be applied for cell tracking and combinatorial deliveries of therapeutic entities | (Nguyen et al., 2019) | |
| exosome loaded immunomodulatory biomaterials (AlgXO) | UPLVG alginate and exosomes with umbilical cord-derived mesenchymal stem cells (UC-MSCs) and rodent islets | successfully attenuated the local immune microenvironment by suppressing proinflammatory macrophages partly by interfering with NF-κB pathway | (Mohammadi et al., 2021) | |
| amino acid augmented macro-encapsulation device | polycaprolactone, alanine, and glutamine with stem cell-derived beta-cells | enhanced viability of encapsulated beta-cells in nutrient-limited conditions | (Chendke et al., 2019) | |
| ready-to-use cryopreserved pancreatic islets | trehalose, MitoQ, and DMSO with rodent islets | demonstrated an improved cryopreservation method to increase the on-demand availability of islets for transplantation | (Dolezalova et al., 2021) | |
| graphene-Dex bioscaffolds | graphene, nickel foam, and PMMA with AD-MSCs and rodent islets | graphene bioscaffold functionalized for local immunomodulation by Dex together with AD-MSC significantly improved the survival and function of transplanted islets | (Razavi et al., 2021) | |
| zwitterionic polyurethane (ZPU) nanoporous device | 3-(Butylbis(2-hydroxyethyl) ammonio) propane-1-sulfonate (SB-Diol) and Polyurethanes with rodent islets | electrospun ZPU device lowered FBR when implanted in immunocompetent animals and showed better scalability and retrievability | (Liu et al., 2021) | |
| lotus-root-shaped cell-encapsulated constructs (LENCON) | microfluidic multicoaxial encapsulation device, laminin, and sodium hyaluronate with human stem cell-derived pancreatic beta-cells (hSC-βs) | demonstrated scalability, retrievability, and maintained the functionality of beta-cells in immunocompetent animals | (Ozawa et al., 2021) | |
| cellulose-based scaffolds | carboxymethyl cellulose (CMC) cryogels with INS1E beta-cells | prompted beta-cells to generate clusters and create specific ranges of pseudoislets; these scaffolds can control the organization and function of insulin-producing beta-cells | (Velasco-Mallorquí et al., 2021) | |
| extracellular matrix/alginate hydrogels | pancreatic acellular matrix and pECM/alginate hydrogel with iPSC-derived beta-cells | provided an ideal biomimetic microenvironment, improved differentiation efficiency, promoted insulin secretion, and increased expression of insulin-related genes | (Wang et al., 2021c) | |
| retrievable macro-encapsulation device | PCTE membrane and PDMS chips and zwitterionic monomers (CBMA and SBMA) with rodent islets | synthetic polymer coating prevented fibrosis for improved long-term function of the device in the absence of immunosuppression and demonstrated retrievability | (Bose et al., 2020) | |
| theranostic silencing nanoparticles | double-stranded siRNAs targeting baboon caspase-3, dextran-coated iron-oxide magnetic nanoparticles | reduced insulin requirements in animals transplanted with a marginal number of labeled islets and demonstrated a novel strategy to minimize the number of donor islets required | (Pomposelli et al., 2020) | |
| gene modification and microscaffold encapsulation | gelatin with MSCs engineered with Exendin-4 (MSC-Ex-4), a glucagon-like peptide-1 (GLP-1) | augmented insulin sensitivity and suppressed senescence and apoptosis of pancreatic beta-cells | (Zhang et al., 2021b) |
GelMA/PEGDA, gelatin methacrylamine (GelMA)-poly(ethylene glycol) diacrylate (PEGDA); hiPSC-CMs, human-induced pluripotent stem cell-derived cardiomyocytes; hMSCs, human mesenchymal stem cells; hECs, human endothelial cells; ECM, extracellular matrix; PLGA, poly(lactic-co-glycolic acid); miRNA, microRNA; hiPSCs, human-induced pluripotent stem cells; bFGF, basic fibroblast growth factor; MSCs, mesenchymal stem cells; IKVAV, laminin-derived functional peptide; FGF-2, fibroblast growth factor 2; EGF, epidermal growth factor; GDNF, glial cell line-derived neurotrophic factor; PNIPAAm-PEG, poly(N-isopropylacrylamide)-co-poly(ethylene glycol); hESCs, human embryonic stem cells; iPSC, induced pluripotent stem cells; ESCs, embryonic stem cells; GSK3, glycogen synthase kinase 3; FGFRs, fibroblast growth factor receptors; TAT, transactivator of transcription; PMMA, poly(methyl methacrylate); PFAs, perfluoroalkoxy alkanes; SONIC, speedy oxygenation network for islet constructs; PTFE, polytetrafluoroethylene; UPLVG, high guluronate low viscosity alginate; MitoQ, mitochondria-targeted ubiquinone; DMSO, dimethylsulfoxide; AD-MSCs, adipose-derived mesenchymal stem cells; PCTE, polycarbonate track etched; PDMS, polydimethylsiloxane; CBMA, 3-[[2-(methacryloyloxy)ethyl]dimethylammonio]propionate; and SBMA, sulfobetaine methacrylate.
Cardiovascular regeneration
Cardiovascular diseases (CVDs) account for about 31% of annual morbidity and mortality worldwide (Roth et al., 2020). Due to the poor prognosis of current pharmacological and surgical interventions, as well as the limited regenerative potential of mature cardiomyocytes, stem cell transplantations hold great promise to regenerate and restore cardiovascular tissue function. However, stem cell-based clinical trials have shown limited functional recovery of the myocardium and vasculature mainly due to low survival and retention of transplanted stem cells (Banerjee et al., 2018). The emerging biomaterial-facilitated stem cell transplantation methods are poised to improve the overall outcomes of stem cell therapy.
Both biochemical and biophysical attributes of the biomaterial play important roles in facilitating the efficacy of stem cell transplantation for cardiovascular purposes. Notably, the abilities to recapitulate appropriate architecture in the native cardiac microenvironment as well as bestow mechanical properties that can withstand the contractile mechanisms of the heart are imperative. By providing a 3D structural scaffold for the transplanted cells, not only is cell retention in the target site greatly increased, but the ability to provide key physical cues to aid in stem cell differentiation into functional myocytes can be achieved (Segers and Lee, 2011). Mechanical stiffness, nanotopographic architecture, physical stretch, and anisotropic patterns have all been shown to guide the differentiation of stem cells with success (Mohindra and Desai, 2021; Segers and Lee, 2011). Proteins, growth factors, genes, and microRNA (miRNA) have all also been used to modulate the biochemical microenvironment to one that is more amenable to cardiac repair (Li et al., 2009; Padin-Iruegas et al., 2009; Yang et al., 2019b).
To repair the damaged postinfarct myocardium and prevent maladaptive left ventricular (LV) remodeling, a dynamic, multicellular 4D hydrogel-based cardiac construct was developed. Beam-scanning stereolithography printing was used to fabricate a physiologically adaptable design that mimicked spatiotemporal architecture and relevant biophysiochemical properties (Figure 2A1). A triculture of human-induced pluripotent stem cell (hiPSC) cardiomyocytes (CMs), human mesenchymal stromal cells, and human endothelial cells (hECs) in the bioink consisting of gelatin methacrylate (GelMA) and PEG diacrylate (PEGDA) was used to print the 4D cardiac tissue construct with anisotropic nonlinear microstructure to imitate epicardial fibers and the surrounding vascular network. In vivo evaluation in a rodent model exhibited high levels of cardiomyocyte maturation, engraftment, and vascularization with excellent functional contraction-relaxation and electrophysiological behavior (Cui et al., 2020; Figures 2A2–2A6). Another approach was developed to address the drawbacks of traditional injectable cellular cardiomyoplasty. A porcine myocardial ECM-derived, nonthrombogenic injectable scaffold, which could be delivered using minimally invasive catheter procedures, was developed for cardiac repair post-MI. Post-transplant analysis showed minimal negative LV remodeling, reduced infarct fibrosis, and increased cardiac muscle. Infarcted pigs that were treated with percutaneous transendocardial injections showcased favorable outcomes as echocardiography indicated significant improvement in cardiac functions, ventricular volumes, and global wall motion scores post-treatment (Huang et al., 2020).
Figure 2. Biomaterial-facilitated stem cell-based regenerative therapies for cardiovascular applications.
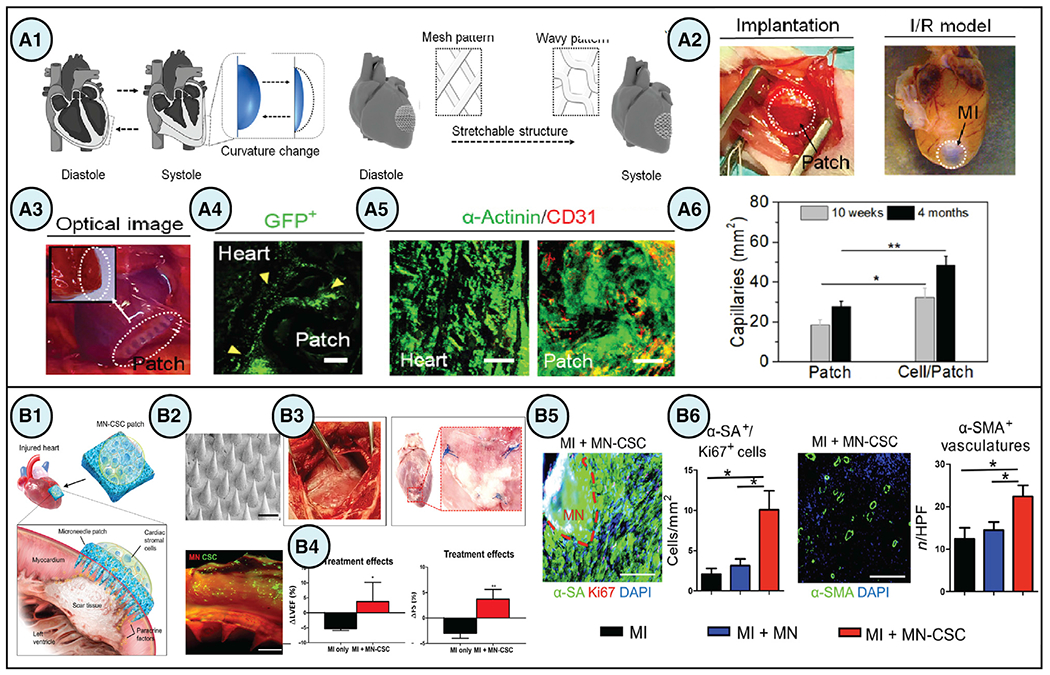
(A) (A1) An engineered design of a 4D biomaterial patch with enhanced biomechanical properties using stretchable architecture to accommodate changes in cardiac tissue curvature during diastole and systole. (A2–A4) In vivo implantation of the 4D patches in rodent models of ischemia reperfusion MI demonstrated high engraftment of cardiomyocytes on the patch at week 3. Scale bars, 100 μm. (A5) Immunostaining of α-actinin (green) and human-specific CD31 (red) showed cellularization of the patch after 4 months. Scale bars, 50 μm. (A6) Quantification of von Willebrand factor staining depicted increased vascularization of the patch from 10 weeks to 4 months. Data are presented as means ± SD, *p < 0.05 and **p < 0.01 (Cui et al., 2020).
(B) (B1) Microneedle (MN) patches integrated with cardiac stromal cells (CSCs) is a promising strategy for cardiac regeneration after MI. (B2) DiO-labeling of CSCS (green) demonstrated successful incorporation of the cells onto the MN patch (red). Scale bars, 500 μm. (B3 and B4) Treatment with MN patches in porcine models of acute MI improved ejection fraction and fractional shortening after 48 h. Data are presented as means ± SD, *p < 0.05 and **p < 0.01. (B5 and B6) immunostaining demonstrated an increased presence of proliferating cardiomyocytes and vasculature in post-MI rat hearts treated with MN-CSCs. Data are presented as means ± SD, *p < 0.05. Scale bars, 200 μm (Tang et al., 2018). Figures reproduced with permission.
The clinical translation of cardiac regenerative therapies has been hampered by delivery challenges such as poor stem cell retention at the transplant site, short half-life of biologics, and adverse off-target effects due to systemic delivery. To improve overall regenerative outcomes of stem cell transplantations, a multimodal thermoplastic polyurethane (TPU) epicardial device called Therepi was developed. The Therepi device encapsulated stem cells as well as small and large molecules and enabled their sustained and repeated administration directly to the epicardium. The repeated localized administration of cardiac progenitors and macromolecules using the epicardial reservoir enhanced ejection fraction, fractional shortening, and stroke work (Whyte et al., 2018). With clinical safety and efficacy in mind, a next-generation fluid-driven refillable pouch for minimally invasive cell delivery to the heart was developed. This design eliminated the need for more invasive open-chest surgery and enabled opportunities for repeat dosing. These pouches consisted of a cover membrane, a semipermeable membrane, and a compressible solid skeletal structure that allowed for facile delivery to the heart via two small incisions. Upon pericardial implantation in rodent MI models, pouches that were refilled with MSCs yielded much more favorable therapeutic effects, including smaller infarct size, greater infarct wall thickness, and increased viable cardiac tissue (Mei et al., 2021). Another unique technology that was developed was based on a microneedle (MN) patch integrated with cardiac stromal cells (CSCs) to further improve stem cell retention and integration. Polyvinyl alcohol (PVA) polymeric MNs were fabricated using micromolding and applied to create conduits between host myocardium and therapeutic CSCs. This allowed CSCs to secrete regenerative paracrine factors into the injured myocardium and promote repair while the transplanted patch received nutrients from the heart via the same MN conduits (Figures 2B1 and 2B2). The evaluation of the MN-CSC patch in the rat MI model showed significant augmentation of cardiac function, cardiomyogenesis, angiogenesis, and a reduction of scar tissue (Tang et al., 2018; Figures 2B3–2B6). Alternatively, to improve angiogenesis and reduce immune response, an injectable porous aldehyde-capped PEG hydrogel matrix containing mesoporous silica nanoparticles (MSNs) encapsulating miRNA-21 was developed. The injectable hydrogel matrix facilitated the delivery of acidic pH stimuli-responsive miRNA-21 to treat post-MI tissue. The MSN/miRNA-21 complex demonstrated the successful remodeling of the local infarcted myocardium microenvironment by inhibiting M1 macrophage polarization into an inflammatory phenotype. This biomaterial technology rescued cardiomyocytes, promoted neovascularization, and effectively reduced infarct size (Li et al., 2021b).
Central nervous system regeneration
Central nervous system (CNS) degenerative disorders are difficult to cure due to the inherently limited capacity for neuroregeneration and inflammatory microenvironment at the site of disease or injury (GBD 2017 US Neurological Disorders Collaborators et al., 2021). Stem cell transplants for treating CNS injuries, and diseases have been limited due to poor viability and retention, inefficient integration, low neural plasticity, and uncontrolled differentiation of transplanted stem cells, which is further aggravated by the proinflammatory microenvironment (Badyra et al., 2020; He et al., 2020). Biomaterial-facilitated stem cell transplants could successfully treat neurological disorders by generating functional neural tissue and rebuilding damaged neural circuits.
The use of biomaterials to deliver trophic factors and provide physical cues to transplanted cells is imperative for successful cell-based therapies for neural repair. Diffusion-based protein delivery and protein immobilization are some important strategies used to achieve appropriate spatiotemporal signals in sustained and/or localized manners (Bruggeman et al., 2019). Hydrogel co-delivery of factors such as GDNF and BDNF has been shown to increase dopaminergic cell survival and improve differentiation of hESC-derived cortical progenitors and vascularization in animal models (Moriarty et al., 2019; Nisbet et al., 2018). Similarly, the incorporation of ECM molecules such as laminin can yield enhanced neuronal survival, adhesion, and differentiation (Somaa et al., 2017). Biomaterial architecture can be modulated to provide appropriate fiber alignment, width, and interfiber distance. This design enabled optimal neural cell adhesion, provided axon support, and modulated stiffness to better match the mechanical properties of the brain (Nisbet et al., 2009). Reports have also demonstrated that the co-delivery of cells with hydrogels can promote the survival and function of cells while reducing host inflammation (Zhong et al., 2010).
The development of a 3D cell assembly method called synthetic matrix-assisted and rapidly templated (SMART) assembly has paved the way for the potential treatment of SCI and traumatic brain injury (TBI). SMART assembly uses a 2D manganese dioxide nanosheet for the rapid assembly of hiPSC-derived NSCs (hiPSC-NSCs) into hybrid 3D neurospheres (Figures 3A1 and 3A2). This strategy demonstrated efficient in vivo survival, spatiotemporal distribution, differentiation, and functional recovery in rodent SCI models (Figures 3A3 and 3A4). SMART neurospheres were used to deliver Notch inhibitors N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT). Imaging studies demonstrated the successful downregulation of Notch signaling pathways associated with gliogenesis. This resulted in the mitigation of local inflammation while enhancing neurogenesis and axonal elongation at the CNS disease/injury sites. It also enabled in vivo tracking of drug delivery using MRI (Rathnam et al., 2021). Another 3D micro-scale biomaterial-aided stem cell transplantation technology was developed to ameliorate neurodegenerative dysfunction and CNS injuries by in situ reprogramming neurons. A tunable 3D microtopographic electrospun poly(desaminotyrosyl tyrosine ethyl ester carbonate) (pDTEc) polymer scaffold with “thin” and “thick” dual fiber topography demonstrated successful in situ neuronal reprogramming of iPSCs when grafted into organotypic hippocampal brain slices. The injectable micro-scale fibrous scaffolds were used as transplantation vehicles and demonstrated neurite outgrowth, survival, and electrical activity after transplantation (Carlson et al., 2016). Another biomaterial-based strategy to enhance the efficacy of cell therapy in SCI is hydrogel-assisted transplantation of patient-derived Schwann cells (SCs). A thixotropic physically cross-linked engineered recombinant protein (C7) and a thermoresponsive multiarm, PEG-PNIPAAm copolymer conjugated with proline-rich peptides (P) hydrogel, known as shear-thinning hydrogel for injectable encapsulation and long-term delivery (SHIELD), was developed (Figures 3B1 and 3B2). Its physical properties were designed to mimic neural tissue stiffnesses at the SCI lesion. The SHIELD showed excellent spatial distribution of SCs post-transplantation while reducing cystic cavitation and neuronal loss in endogenous tissue. It also showed a substantial increase in forelimb strength and coordination in the cervical contusion rodent model (Marquardt et al., 2020; Figures 3B3 and 3B4). Another biomaterial platform was developed to coordinate large-scale chronic structural and functional repair of the brain after severe TBI. Chondroitin sulfate-engineered (eCS) matrices loaded with neurotrophic factors fibroblast growth factor 2 (FGF-2) and BDNF were implanted into the intracortical region after TBI and stroke. These biomaterial constructs proved successful in achieving complex structural and functional repair of brain tissue by promoting chronic neurogenesis and neuroplasticity. It enhanced proliferation of endogenous NSCs and neurotrophic factor expression and thus effectively mitigated significant volume loss and improved vascular density and reach-to-grasp function recovery after TBI (Latchoumane et al., 2021). It also advanced our understanding of biomaterials, dynamics of cell-microenvironment interactions, and their effect on stemness, self-renewal, lineage commitment, cell physiology, and metabolism. A biomaterial-facilitated multicellular stem cell transplantation platform was developed to improve axonal regeneration. A multichannel PLGA scaffold was used to cotransplant activated SCs and bone marrow-derived MSCs in a transection gap in a SCI rodent model. This strategy subsequently exhibited significant neurogenesis and recovery of motor function with robust bundles of nerve fibers with mature myelin sheaths and normal electrophysiology (Yang et al., 2017).
Figure 3. Biomaterial-facilitated stem cell-based regenerative therapies for central nervous system applications.
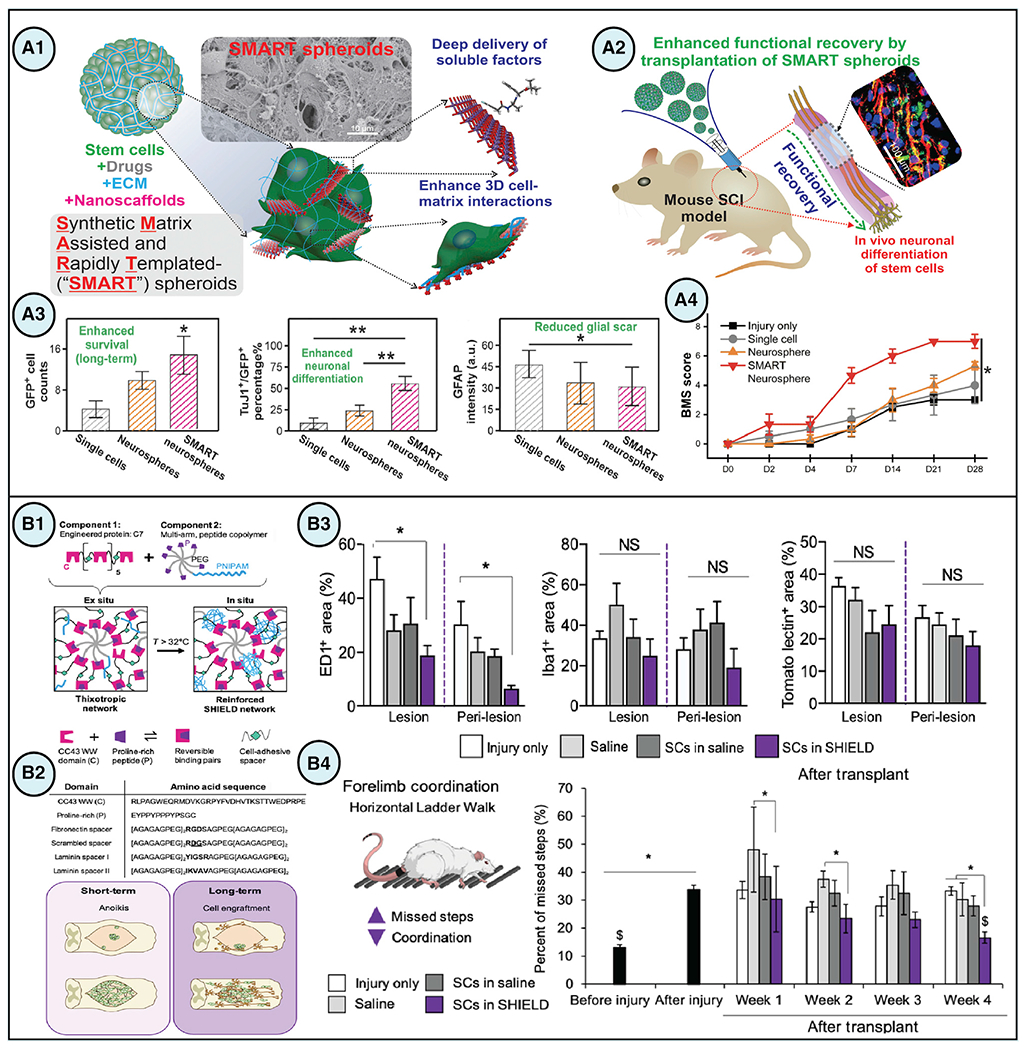
(A) (A1 and A2) SMART spheroids were developed to improve cell-cell and cell-matrix interactions and achieve controlled drug release to enhance in vivo neuronal differentiation of transplanted stem cells, thereby leading to functional recovery in models of SCI. (A3) Injection of SMART neurospheres (spheroids assembled from NSCs) achieved long-term stem cell survival and neuronal differentiation along with reduced glial scar and functional recovery 1 month postinjection. Data are presented as means ± SEM, *p < 0.05 and **p < 0.01. (A4) Treatment with SMART neurospheres resulted in faster recovery rates at 1 month based on the Basso mouse scale (BMS) scoring. Data are presented as means ± SEM, *p < 0.05 (Rathnam et al., 2021).
(B) (B1 and B2) SHIELD, an injectable shear-thinning hydrogel, was designed to improve cell survival and engraftment after transplantation by incorporating celladhesive ligands and employing self-healing and thixotropic characteristics. (B3) immunostaining quantification of the lesion and perilesion regions in spinal cord sections revealed a significant reduction of the pan-macrophage marker ED1 in animals treated with Schwann cells (SCs) in SHIELD compared with injury only controls, whereas no significant differences were observed between the groups for Iba1, microglia marker, or Tomato lectin, vasculature marker. Data are presented as means ± SEM, *p < 0.05. (B4) Forelimb coordination significantly increased in SHIELD-delivered SCs-treated animals after 4 weeks as measured by a decrease in the percentage of missed steps with the horizontal ladder walk test. Data are presented as means ± SEM, *p < 0.05 and $p = 0.970 comparison between before injury and 4-week SCs in SHIELD (Marquardt et al., 2020). Figures reproduced with permission.
In another significant development, a multifunctional hydrogel was engineered to promote efficient maturation of NSCs and neural regeneration to treat SCI. A synthetic bioabsorbable SAP hydrogel called hNSC-HYDROSAP was designed to support human NSC (hNSC) differentiation in 3D serum-free conditions. This biomaterial construct facilitated hNSC distribution, survival, induction of electrically active neuronal phenotype, and formation of entangled neuronal networks. hNSC-HYDROSAP was shown to improve behavioral recovery and reduce glial scar formation in a SCI rodent model (Marchini et al., 2019). Finally, another study used a photoresponsive thixotropic self-healing injectable hydrogel for delivering neuroprotective proteins for axonal regeneration. Photoreceptor (PR) His6-CarHC proteins were assembled into a macroscopic photoresponsive Zn2+-coordinated hydrogel system. The oligomeriation was achieved using metal/His6-tag interactions in combination with adenosylcobalamin (AdoB12). This biomaterial formulation, which was designed to release neuroprotective leukemia inhibitory factor (LIF), resulted in enhanced neuronal survival and axon regeneration in vivo in rodents (Jiang et al., 2020).
Ocular regeneration
Disease, injuries, or aging can cause pathological changes in specific tissues in the human eye resulting in vision deterioration or loss. Examples include age-related macular degeneration (AMD), corneal scarring, glaucoma, and hereditary dystrophies. Over 285 million people suffer from visual impairment, of which 13.7% are blind and 86.3% are suffering from progressive vision loss (He et al., 2020). The advent of stem cell transplants such as limbal epithelial stem cells (LESCs), ESCs, MSCs, and iPSCs has created new avenues to repair regenerate, stabilize, and enhance the function of the anterior and posterior segments of the eye (Mead et al., 2015). However, the effectiveness of traditional stem cell transplants has been hampered by issues such as low viability, poor retention, hyperproliferation, hypoxia, and fibrosis (Caras et al., 2021; Rama et al., 2010).
For degenerative ocular disorders, stem cell-based therapies in combination with biomaterial and bioactive molecules are being developed to address some of the challenges associated with stem cell transplants. Biomaterial strategies can provide valuable biophysiochemical cues to better mimic the native physiological properties of ocular tissue, such as the Bruch’s membrane, stroma, and retina (Nair et al., 2021). Scaffolds made from ECM proteins such as collagen I with a nanofibrous configuration similar to that of native Bruch’s membrane have supported RPE cell attachment and morphology (Warnke et al., 2013). Surface topography also has a significant impact on cell behavior, including alignment, proliferation, and protein expression (Mahdavi et al., 2020). Combinations of biochemical cues with material scaffolds increased human retinal progenitor cell adhesion, reduced hyperproliferation, and induced differentiation to PR phenotypes (Lawley et al., 2015). However, a drawback of natural scaffolds is their poor mechanical strength and fast degradation rate. Synthetic biomaterials can overcome these limitations and, being more inert in nature, may prove beneficial in reducing potential immunogenic response upon implantation (Christiansen et al., 2012). As biomaterial-facilitated stem cell transplantation remains an active area of research, several biomaterials in combination with stem cells have been investigated with mixed success. Examples include the transplantation of retinal pigment epithelium (RPE) using collagen, PLGA, PLLA, gelatin, or Bruch’s membrane to treat AMD or the use of amniotic membranes seeded with LESCs to treat corneal damage (Jemni-Damer et al., 2020; Williams et al., 2018). In this section, we discuss the recent developments in biofunctional materials for the regeneration and repair of ocular tissues.
The avascular environment of corneal tissue limits its regenerative potential. Proinflammatory cascade in the injured cornea further aggravates injury by inducing stromal apoptosis and overproduction of ECM by myofibroblasts, leading to fibrosis. The fibrotic response is mediated by cytokines such as interleukin 1 (IL-1), tumor necrosis factor alpha (TNF-α), and TGF-β and recruitment of neutrophils, macrophages, and lymphocytes that promotes inflammation. Although taking advantage of the immune modulatory capability of the ECM, a micro- and ultra-fine porcine urinary bladder matrix (UBM) scaffold was developed. The UBM matrix scaffold successfully promoted an antiinflammatory type 2 immune response by recruiting CD4+ TH2 helper T cells. This resulted in increased production of IL-4 and reduced the differentiation of corneal stromal cells into alpha-smooth muscle actin-positive (αSMA+) myofibroblasts. Thus, UBM created a proregenerative and reparative microenvironment that led to corneal regeneration, reduced corneal hazing, and diminished scarring in the rodent corneal wound model (Wang et al., 2021a). Another approach is focused on the regeneration of RPE for treating degenerative retinal diseases such as retinitis pigmentosa (RP). hESC-derived RPE cell sheets were developed and transplanted on a human amniotic membrane (hAM)-based scaffold (Figures 4A1 and 4A2). The transplantation of hESC-derived RPE cell sheets on the hAM scaffold improved visual acuity, retinal electrophysiology, morphology, and PR viability in a rodent model (Figures 4A3–4A5). In addition, hESC-RPE-hAM scaffolds revived the damaged Bruch’s membrane of the choroid, a common place of injury in AMD, thus opening an avenue to treat AMD, Bietti’s corneoretinal dystrophy (BCD), and other ocular degenerative diseases (M’Barek et al., 2017).
Figure 4. Biomaterial-facilitated stem cell-based regenerative therapies for ocular tissues.
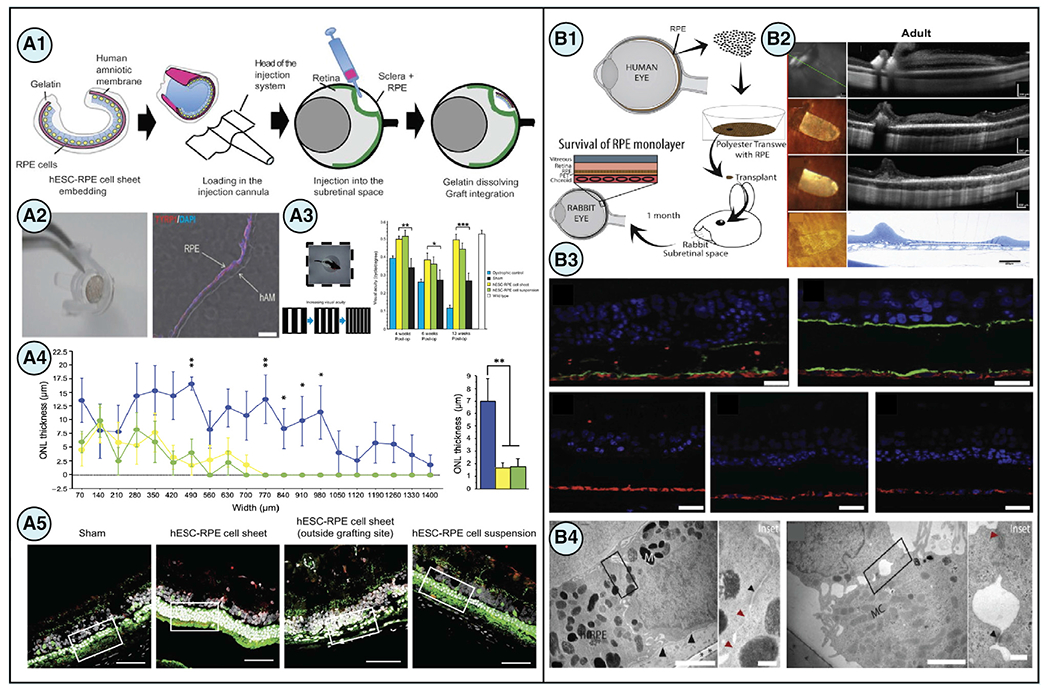
(A) (A1) A grafting strategy devised to introduce tissue-engineered human embryonic stem cell-derived retinal pigment-epithelial (hESC-RPE) cell sheets to the subretinal space of the eye via injection while maintaining polarity of the hESC-RPE cell sheet. (A2) Immunostaining of the tissue construct demonstrated the organization of hESC-RPE cells in a monolayer (TYRP1 = red, DAPI/nuclei = blue). Scale bars, 50 μm. (A3) An optokinetic test determined that treatment with transplanted hESC-RPE cell sheets significantly improved visual acuity compared with sham at various time points post-transplantation (4, 6, and 13 weeks). Data are presented as means ± SEM, *p < 0.05, **p < 0.01, and ***p < 0.001. (A4) Outer nuclear layer (ONL) thickness was increased in rats treated with hESC-RPE cell sheets. Data are presented as means ± SEM, *p < 0.05 and **p < 0.01. (A5) Histological analysis confirmed that more photoreceptor cell nuclei were preserved after transplantation in rat eyes with hESC-RPE cell sheets compared with hESC-RPE cell suspensions. Scale bars, 50 μm (M’Barek et al., 2017).
(B) (B1) Human RPE stem cell-derived RPE monolayers grown on PET membranes were being evaluated for their potential as a cell-replacement therapy for age-related macular degeneration. (B2) After 1 week, retinal tissue loss was observed over the implant center but remained stable for the duration of later time points, pointing to a future challenge that remains for hRPE xenografts. Scale bars, 200 μm (rows 1–3) and 250 μm (row 4). (B3) Immunostaining for human-specific marker SC121 (red) confirmed survival of the human RPE monolayer subretinally for 1 month, although costaining of SC121 with apical membrane markers MCT1 and ezrin (top left and right, respectively) (green) confirmed that the RPE was still polarized. The absence of Ki67, phosphohistone H3, and caspase-3 (bottom, from left to right, respectively) indicated that neither proliferation nor apoptosis was occurring. (B4) TEM imaging revealed polarized fetal and adult hRPE cells on the PET carriers. Scale bars, 2 μm. Inset scale bars, 0.2 μm (Stanzel et al., 2014). Figures reproduced with permission.
Yet another key development is biomimetic and biosynthetic corneas as alternatives to donor corneas. The biosynthetic cornea was developed using recombinant human collagen that was cross-linked using ethyl(dimethylaminopropyl) carbodiimide N-hydroxysuccinimide (EDC-NHS) coupling followed by molding. The biosynthetic corneas were implanted in human patients with distorted corneas and were monitored for 2 years. The implanted biosynthetic corneas were stably anchored by the recruitment of stromal cells at the implant interface in patients without rejection, and no peripheral or central vascularization was observed. This strategy demonstrated tear film-aided oxygen and nutrient supplementation and reduced observed infection. Successful re-epithelization and nerve restoration were observed in the biosynthetic cornea, regaining its sensitivity to mechanical stimulation, which is essential to protect the eye from injury. The biosynthetic cornea enabled the endogenous regenerative repair of resected corneal tissue without the use of donor human corneal tissue (Fagerholm et al., 2010).
Significant developments are also underway for retinal tissue replacements. Adult human RPE stem cell (hRPESC)-derived RPE were grown and polarized on porous polyethylene terephthalate (PET) polymeric substrates to achieve architecture similar to that of native RPE (Figure 4B1). These constructs were successfully transplanted subretinally and improved neural retinal health and polarity of hRPESC-derived RPE. Moreover, it also prevented uncontrolled cellular proliferation and did not induce an immune reaction (Figures 4B2–4B4). Such approaches pave the way for new biomaterial-assisted stem cell transplants to treat AMD (Stanzel et al., 2014). For example, a 3D poly(glycerol sebacate) (PGS) biomaterial scaffold was developed to treat severe PR degeneration and later stages of inherited retinal disorder (IRD). The 3D PGS scaffold with mechanical properties that match the retina was fabricated by polymer micromolding and was used to transplant human PSC-derived PRs. Using this approach, a multilayer, multicellular high-density PR replacement was achieved in a rodent model (Lee et al., 2021). Similarly, PLGA scaffolds loaded with clinical-grade iPSC-RPE cells were developed for the treatment of dry and wet AMD. The PLGA scaffold was loaded with autologous iPSC-derived RPE and was subretinally transplanted. It led to significant improvement in integration and functionality of RPE in rodent and porcine AMD models (Salas et al., 2021). A critical biomaterial-based endogenous gene modification technology was developed to treat retinal degeneration, especially RP. CK30PEG-TAT gDNA NPs with a full-length genomic form of rhodopsin genes (gRho) with all endogenous regulatory elements including an endogenous promoter, enhancers, suppressors, and introns were transduced into primary retinal cells. It showed successful structural and functional rescue of PRs in rhodopsin knockout (RKO) mice (Zheng et al., 2020b). The biomaterial microtissue intervention was developed to remedy ocular surface conjunctival disorders (OSCDs), which severely affect vision. One approach integrated conjunctival stem cell (CjSC) expansion strategies with digital light processing (DLP)-bioprinted gelatin methacryloyl injectable scaffolds. The bioprinted CjSC-hydrogel microtissue, delivered to bulbar conjunctival epithelium, enhanced viability, renewal, and differentiation of the CjSCs into conjunctival goblet cells. It further demonstrated marked potential as a platform for the treatment of diseases such as ocular cicatricial pemphigoid, Stevens-Johnson syndrome, and toxic epidermal necrolysis (Zhong et al., 2021). Scaffold containing stem cell-derived exosomes have also been used for tissue regeneration, including ocular tissues. Stem cell-derived exosomes-loaded onto thermosensitive hydrogels were used for the treatment and regeneration of the corneal epithelium and stroma. The sustained release of iPSC-MSC-derived exosomes containing miR-432-5p was incorporated into thermosensitive chitosan-based hydrogels (CHI hydrogels) containing corneal stromal stem cells to modulate collagen synthesis. This biomaterial construct acted by suppressing translocation-associated membrane protein 2 (TRAM2) to avert the deposition of ECM. This multipronged approach diminished scar tissue formation and accelerated corneal healing (Tang et al., 2022).
Beta-cell regeneration
The pancreas consists of two parts: exocrine and endocrine. Pancreatitis and pancreatic cancers affect the exocrine pancreas, whereas diabetes mellitus (DM) and neuroendocrine cancers affect the endocrine pancreas. The exocrine pancreas possesses excellent intrinsic regenerative capacity, whereas, in contrast, adult endocrine islets have limited regenerative capacity, resulting in substantial beta-cell loss, particularly in autoimmune type 1 diabetes (T1D). Diabetes affects more than 422 million people worldwide and can lead to life-threatening microvascular, macrovascular, and neurological disorders (Lin et al., 2020; Mobasseri et al., 2020). Stem cell-derived beta-cell transplantation is one promising approach for the restoration of endocrine tissue function and the treatment of T1D. However, the lack of a suitable native microenvironment, robust vasculature, and destruction of cell-cell/cell-ECM interactions leads to nutritional deficiency, hypoxia, adverse immune reaction, and fibrosis. These challenges have resulted in the limited wide-spread application of stem cell-based therapies for diabetes (Desai and Tang, 2018; Kerper et al., 2021; Sneddon et al., 2018).
Over the years, multiple biomaterial-facilitated stem cell transplantation approaches have been developed to address these challenges, including micro-/macro-encapsulation immunoprotective devices, prevascularized devices, 3D scaffolds, and oxygen-releasing biomaterials. Through these biomaterial strategies, there are opportunities to provide biophysiochemical signals to improve cellular viability, protect against host immune reactions, and enable sufficient transfer of nutrients and oxygen. Biomaterial co-delivery of small molecules, cytokines, chemokines, and immunomodulatory molecules may prove helpful in extending cell survival, preserving cell function, and minimizing immune response (Chendke et al., 2019; Coronel et al., 2020; Liu et al., 2016b). Surface topographic modulation of pancreatic cell function via micropatterned collagen sheets that mimicked the microstructural architecture of pancreatic tissue improved islet-like cluster organization and insulin secretion levels in cells (Seo et al., 2020). Importantly, the appropriate selection of inert biomaterials, size-scale used, surface modification, and porosity have all been found to minimize host immune response to the implanted device. Minimizing pore size prevented undesirable antibody and immune cell interactions with the encapsulated cells and affected macrophage elongation and phenotype transition to prohealing phenotypes (Tylek et al., 2020). Here, we discuss recent developments in biomaterial-assisted stem cell transplantation technologies for beta-cell replacement.
Macro-encapsulation devices (MEDs) for islet transplantation are well established for creating physical immune barriers to block the attack of immune cells on transplanted islets. Despite this, encapsulation systems have inherent challenges such as limited and crowded cell-loading capacity, slow response dynamics for glucose sensing, and poor insulin release due to reliance on diffusion kinetics. To overcome this challenge, convective nutrient transport has been incorporated in the MEDs, and traditional planar geometry was changed to a 3D polymeric capsule geometry to build a convection-enhanced MED (ceMED). This design change helped increase loading capacity multifold, enhance cell viability, and improve glucose equilibration. The transplantation of beta-cells using ceMEDs in immunocompetent diabetic rodent models demonstrated vasculature-independent improvement in glucose-stimulated insulin response, diabetic correction, and reduced FBR (Yang et al., 2021). To further address challenges relating to limited nutrient access for encapsulated stem cells, an alternative approach was devised by incorporating internal nutrient reservoirs inside the MEDs. The MEDs were designed to incorporate internal, zero-order monolithic alanine and glutamine compartments that were fabricated in polycaprolactone (PCL) polymer. The incorporation of the amino acid reservoirs enabled the supply of amino acids to the encapsulated islets and enhanced the viability of insulin-producing beta-like cells in nutrient-limiting conditions in poorly vascularized subcutaneous space (Chendke et al., 2019).
Several biomaterial-based technologies have been developed to modulate the local immune system for transplanted stem cell-derived beta-cells. For example, immunosuppressive hybrid alginate exosomes (umbilical cord MSC-derived XO) microcapsules (AlgXO) were synthesized and used for islet encapsulation. The XO released from the AlgXO capsules successfully attenuated the local immune microenvironment by suppressing proinflammatory macrophages by interfering with the NF-κB pathway. Successful long-term xenotransplantation of islets encapsulated in AlgXO in an immunocompetent T1D rodent model was achieved with lower inflammatory response and enhanced functional performance (Mohammadi et al., 2021). In an important development for achieving xenotransplantation, a multilayer, nanothin microencapsulation approach was successfully demonstrated. Neonatal porcine-derived islets were microencapsulated in nanothin multilayers of an antioxidant tannic acid and poly(N-vinylpyrrolidone) (PVPON) that maintained normoglycemia while reducing proinflammatory innate immune response in a rodent model (Barra et al., 2021).
Another technological development interfaced biological components with electronic systems to build an electrogenetic cellular insulin release system (egCIRS) (Figures 5A1 and 5A2). The bioelectronic interface between stem cell-derived beta-cells and an electronic device allowed for direct control over insulin release to restore euglycemia. The egCIRS exploited the electrogenetic interoperability between cellular metabolism and electronics to trigger controlled vesicular insulin release. This was achieved by electrically modulating membrane polarization, causing ectopic expression of calcium and potassium channels on the beta-cells (Electroβ cells). The engineered Electroβ cells encapsulated in the device demonstrated the potential of wireless electrical stimulation of vesicular insulin release to attenuate postprandial hyperglycemia in a T1D rodent model comparable with that of transplanted human islets (Krawczyk et al., 2020; Figures 5A3 and 5A4).
Figure 5. Biomaterial-facilitated stem cell-based regenerative therapies for pancreatic tissues.
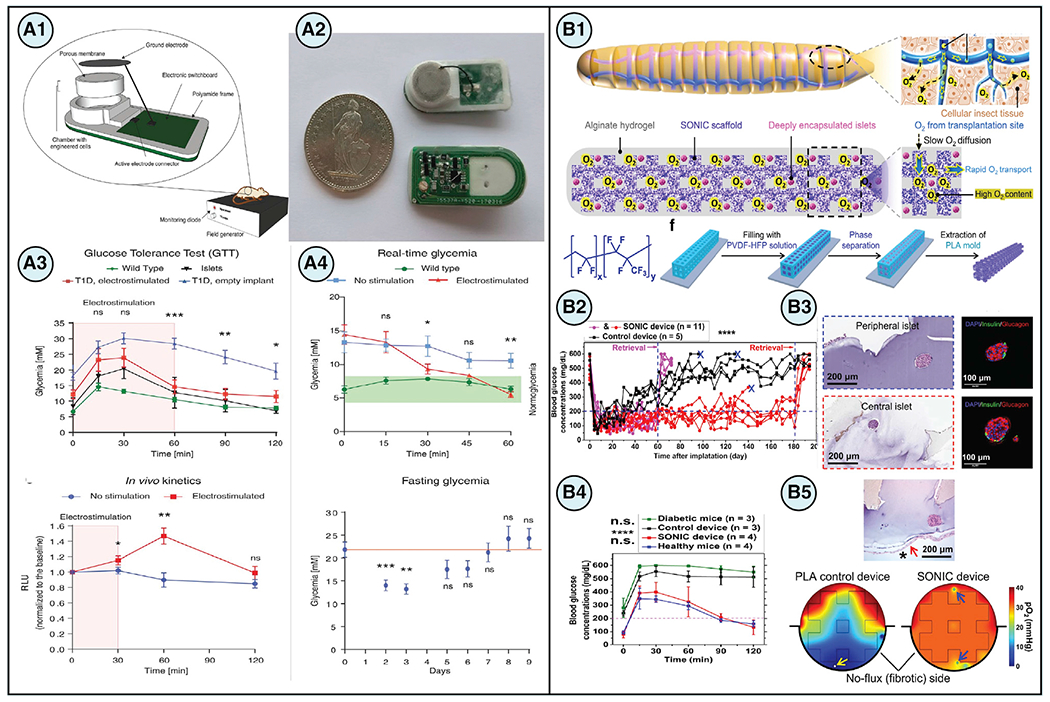
(A) (A1 and A2) A strategy employing wireless electrical stimulation of engineered electrosensitive beta-cells (Electroβ cells) housed inside of a bioelectronic device enabled electrogenic control of insulin release from cells that could be used for type 1 diabetes therapy. The bioelectronic implant was placed subcutaneously in a mouse, whereas a field generator provided the necessary wireless energy transmission. (A3) Electroβ cells re-established postprandial glucose metabolism and achieved fast vesicular secretion after electrostimulation in insulin-deficient type 1 diabetic mice. Data are presented as means ± SEM, *p < 0.05, **p < 0.01, and ***p < 0.001. (A4) Moreover, it was found that blood glucose levels could be quickly restored to normoglycemia after electrostimulation and that glycemia could be controlled over long periods of time without experiencing hypoglycemia. Data are presented as means ± SEM, *p < 0.05, **p < 0.01, and ***p < 0.001 (Krawczyk et al., 2020).
(B) (B1) A novel biomimetic scaffold design called SONIC utilized continuous air channels to improve oxygen diffusivity within cell encapsulation systems and was inspired by the tracheal systems in mealworms. (B2) Diabetic C57BL/6 mice implanted with SONIC devices with rat islets demonstrated long-term controlled blood glucose readings spanning 6 months until device retrieval, where blood glucose levels returned to hyperglycemia. Individual device data are presented, ****p < 0.0001. (B3) Histological evaluation and immunostaining of insulin (green) and glucagon (red) confirmed islet viability and function from cells in retrieved devices on day 60. Scale bars, 200 μm (left) and 100 μm (right). (B4) Intraperitoneal glucose tolerance tests were conducted on day 180 postimplantation, and the results showed animals treated with the SONIC device had glycemic profiles similar to that of healthy mice, with blood glucose levels returning to normoglycemia within 2 h. Data are presented as means ± SD, ****p < 0.0001 (diabetic mice versus SONIC device-treated mice, diabetic mice versus healthy mice, control device-treated mice versus SONIC device-treated mice, and control device-treated mice versus healthy mice). (B5) Histology confirmed that islets near regions of fibrosis remained healthy and corroborated findings from computational simulations of fibrosis where control devices were found to be hypoxic with high levels of islet necrosis, whereas SONIC devices were sufficiently oxygenated throughout the implant. Scale bars, 200 μm (Wang et al., 2021b). Figures reproduced with permission.
To ensure adequate oxygenation for encapsulated beta-cells, approaches have been developed to also address the limited passive diffusion of oxygen (O2) due to placement in poorly vascularized microenvironments under ischemic conditions. A poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) polymer was used to fabricate an air-filled scaffold called speedy oxygenation network for islet constructs (SONIC), which mimicked the natural gas-phase tracheal O2 delivery system of mealworms (Figure 5B1). The SONIC scaffold system design was shown to overcome the distance limitation for O2 diffusion with 10,000-fold higher O2 diffusivity than that of hydrogels. It demonstrated therapeutic efficacy and islet survival in diabetic immunocompetent rodent models (Wang et al., 2021b; Figures 5B2–5B5).
CONCLUSIONS AND OUTLOOK
The importance of biomaterials in the context of regenerative medicine is becoming increasingly evident. Biomaterials can be customized to provide biophysical and biochemical cues that are needed for regeneration. They can also be used to further our understanding of cell-microenvironment interactions and their effects on stemness, self-renewal, lineage commitment, cell physiology, and metabolism (Abdeen and Saha, 2017; Chai and Leong, 2007). In other words, biomaterials can be used to create a better home for stem cells.
Although current strategies have primarily focused on introducing a single biomaterial component, combinatorial biomaterial strategies with synergistic effects could lead to improved outcomes for stem cell transplantation. Multifunctional biomaterials with state-dependent cellular behavior should be designed to regulate coordinated sequences of stem cell renewal, differentiation, and functional performance, among other behaviors (Brassard and Lutolf, 2019; Guilak et al., 2009; Li et al., 2021c; Perestrelo et al., 2018; Sharma et al., 2019b; Vunjak-Novakovic and Scadden, 2011; Xia and Izpisua Belmonte, 2019). Developing long-term and functional, multicellular biomaterial constructs that can be effectively integrated into an immunocompetent host is still a huge challenge. Although strategies have emerged recently that concurrently regulate two to three facets of the regenerative responses, more work is needed to modulate intracellular (growth, function) and extracellular (immunogenicity, mechanics) factors. Combinatorial biomaterials are the next frontier in creating more efficacious regenerative therapies.
Regulatory pathways are an additional challenge facing the utilization of biomaterial-engineered stem cell transplants as viable regenerative therapies. Devices, drugs, and biological products are all governed by different regulations within the United States. Therefore, biologic/device combination products such as stem cell/biomaterial strategies require special regulatory consideration to ensure that both constituent parts and the combination product are found to have sufficient quality, safety, and efficacy. The FDA’s Office of Combination Products (OCP) is responsible for assigning a primary agency center that will take the lead for the review and regulation of a specified combination product (George, 2019; US Food and Drug Administration, 2006). Assignment of the product’s lead center (i.e., Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, and Center for Drug Evaluation and Research) is determined based on the evaluation of which constituent serves the primary mode of action (PMOA) of the final combination product. The PMOA is defined as “the single mode of action of a combination product that provides the most important therapeutic action of the combination product” (eCFR, 2017). As appropriate, the lead center may often collaborate with other agencies to evaluate the information provided for regulatory submission (George, 2019; US Food and Drug Administration, 2006; US Government, 2022). Given the sheer breadth of possible combination products and that devices and biologics are typically developed and manufactured in accordance with different regulations, it is understandable that there is no gold-standard development approach and regulatory guidance that can be accurately applied to all combination products. Hence, existing guidance needs to be adapted to fully address the regulatory demands of each unique combination product. Although biomaterial-engineered stem cell strategies may require significant regulatory considerations, several innovative programs such as the breakthrough therapy and regenerative medicine advanced therapy designation in the United States, the PRIME initiative in EU, and the Sakigake designation in Japan are being developed to enable patient access to experimental regenerative medicines (Cogle et al., 2003; Prestwich et al., 2012; Qiu et al., 2020). Finally, any cell-based strategy must consider the issues of accessibility and affordability for widescale clinical translation. Overcoming these limitations promises to revolutionize and transform regenerative medicine to address our critical need for alternatives to allogeneic organ transplantation.
ACKNOWLEDGMENTS
B.N.K. and P.M. contributed to this work and T.A.D. provided direction and edited the manuscript. This work was supported, in part, by grants from JDRF, CIRM (U.S., DISC2-09559), and NIH (U.S., T32GM008155 and R01HL137209). B.N.K. is supported by a grant from the Diabetes Research Connection (U.S., A135548). P.M. is supported by the NIH (U.S., R01HL137209) and the American Heart Association (U.S., 18PRE34030271).
Footnotes
DECLARATION OF INTERESTS
The authors declare the following competing financial interest(s): T.A.D. is a scientific founder of Encellin, a cell therapy device company, and she is listed as an inventor of a macro-encapsulation technology (US Patent #10,865,378) described in this paper.
REFERENCES
- Abdeen AA, and Saha K (2017). Manufacturing cell therapies using engineered biomaterials. Trends Biotechnol. 35, 971–982. 10.1016/J.TIBTECH.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu-Berchie K, and Mooney DJ (2020). Biomaterials as local niches for immunomodulation. Acc. Chem. Res 53, 1749–1760. 10.1021/ACS.ACCOUNTS.0C00341. [DOI] [PubMed] [Google Scholar]
- Aguado BA, Mulyasasmita W, Su J, Lampe KJ, and Heilshorn SC (2012). Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 18, 806–815. 10.1089/ten.TEA.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn CB, Lee J-H, Kim JH, Kim TH, Jun H-S, Son KH, and Lee JW (2022). Development of a 3D subcutaneous construct containing insulin-producing beta-cells using bioprinting. Bio-Des. Manuf 5, 265–276. 10.1007/S42242-021-00178-9/FIGURES/8. [DOI] [Google Scholar]
- Ali M, and Payne SL (2021). Biomaterial-based cell delivery strategies to promote liver regeneration. Biomater. Res 25, 5. 10.1186/S40824-021-00206-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida HA, and Bártolo PJ (2014). Design of tissue engineering scaffolds based on hyperbolic surfaces: structural numerical evaluation. Med. Eng. Phys 35, 1033–1040. 10.1016/J.MEDENGPHY.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Alvarez LM, Rivera JJ, Stockdale L, Saini S, Lee RT, and Griffith LG (2015). Tethering of epidermal growth factor (EGF) to beta tricalcium phosphate (βTCP) via fusion to a high affinity, multimeric βTCP-binding peptide: effects on human multipotent stromal cells/connective tissue progenitors. PLoS One 10. e0129600. 10.1371/JOURNAL.PONE.0129600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez Z, Kolberg-Edelbrock AN, Sasselli IR, Ortega JA, Qiu R, Syrgiannis Z, Mirau PA, Chen F, Chin SM, Weigand S, et al. (2021). Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science 374, 848–856. 10.1126/science.abh3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, O’Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, et al. (2018). Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400. 10.1038/s41586-018-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arifin DR, Kulkarni M, Kadayakkara D, and Bulte JWM (2019). Fluorocapsules allow in vivo monitoring of the mechanical stability of encapsulated islet cell transplants. Biomaterials 221, 119410. 10.1016/j.biomaterials.2019.119410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila R, Wu Y, Rogers JA, Huang Y, Rogers JA, and Huang Y (2021). A mechanics model for injectable microsystems in drug delivery. J. Mech. Phys. Solids 156, 104622. 10.1016/JJMPS.2021.104622. [DOI] [Google Scholar]
- Babbar A, Jain V, Gupta D, Singh S, Prakash C, and Pruncu C (2020). Biomaterials and fabrication methods of scaffolds for tissue engineering applications. Materials Horizons: from Nature to Nanomaterials (Springer; ), pp. 167–186. 10.1007/978-981-15-5424-7_8. [DOI] [Google Scholar]
- Badylak SF (2015). Host Response to Biomaterials: The Impact of Host Response on Biomaterial Selection (Elsevier; ). [Google Scholar]
- Badyra B, Sułkowski M, Milczarek O, and Majka M (2020). Mesenchymal stem cells as a multimodal treatment for nervous system diseases. Stem Cells Transl. Med 9, 1174–1189. 10.1002/SCTM.19-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H, Puranik AS, Gauvin R, Edalat F, Carrillo-Conde B, Peppas NA, and Khademhosseini A (2012). Building vascular networks. Sci. Transl. Med 4, 160ps23. 10.1126/scitranslmed.3003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee MN, Bolli R, and Hare JM (2018). Clinical studies of cell therapy in cardiovascular medicine: recent developments and future directions. Circ. Res 123, 266–287. 10.1161/CIRCRESAHA.118.311217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuelos J, and Lu NZ (2016). A gradient of glucocorticoid sensitivity among helper T cell cytokines. Cytokine Growth Factor Rev. 31, 27–35. 10.1016/J.CYTOGFR.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra JM, Kozlovskaya V, Kepple JD, Seeberger KL, Kuppan P, Hunter CS, Korbutt GS, Kharlampieva E, and Tse HM (2021). Xenotransplantation of tannic acid-encapsulated neonatal porcine islets decreases proinflammatory innate immune responses. Xenotransplantation 28. e12706. 10.1111/XEN.12706. [DOI] [PubMed] [Google Scholar]
- Bernards DA, Lance KD, Ciaccio NA, and Desai TA (2012). Nanostructured thin film polymer devices for constant-rate protein delivery. Nano Lett. 12, 5355–5361. 10.1021/nl302747y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertlein S, Brown G, Lim KS, Jungst T, Boeck T, Blunk T, Tessmar J, Hooper GJ, Woodfield TBF, and Groll J (2017). Thiolene Clickable gelatin: a platform bioink for multiple 3D biofabrication technologies. Adv. Mater 29. 10.1002/adma.201703404. [DOI] [PubMed] [Google Scholar]
- Bhardwaj G, Vakani M, Srivastava A, Rawal K, Kalathil A, and Gupta S (2022). Influence of metabolically compromised Adipose derived stem cell secretome on islet differentiation and functionality. Exp. Cell Res 410, 112970. 10.1016/J.YEXCR.2021.112970. [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, and Jasper H (2011). Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell 9, 402–411. 10.1016/J.STEM.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloise N, Petecchia L, Ceccarelli G, Fassina L, Usai C, Bertoglio F, Balli M, Vassalli M, Cusella De Angelis MGC, Gavazzo P, et al. (2018). The effect of pulsed electromagnetic field exposure on osteoinduction of human mesenchymal stem cells cultured on Nano-TiO2 surfaces. PLoS One 13, e0199046. 10.1371/JOURNAL.PONE.0199046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Volpatti LR, Thiono D, Yesilyurt V, McGladrigan C, Tang Y, Facklam A, Wang A, Jhunjhunwala S, Veiseh O, et al. (2020). A retrievable implant for the long-term encapsulation and survival of therapeutic xenogeneic cells. Nat. Biomed. Eng 4, 814–826. 10.1038/s41551-020-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers DT, Song W, Wang LH, and Ma M (2019). Engineering the vasculature for islet transplantation. Acta Biomater. 95, 131–151. 10.1016/j.actbio.2019.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, and Burnside ER (2019). Moving beyond the glial scar for spinal cord repair. Nat. Commun 10, 3879. 10.1038/s41467-019-11707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassard JA, and Lutolf MP (2019). Engineering stem cell self-organization to build better organoids. Cell Stem Cell 24, 860–876. 10.1016/J.STEM.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Bruggeman KF, Moriarty N, Dowd E, Nisbet DR, and Parish CL (2019). Harnessing stem cells and biomaterials to promote neural repair. Br. J. Pharmacol 176, 355–368. 10.1111/BPH.14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldorera-Moore M, and Peppas NA (2009). Micro- and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv. Drug Deliv. Rev 61, 1391–1401. 10.1016/j.addr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, and Desai TA (2020). TiO 2-based nanotopographical cues attenuate the Restenotic phenotype in primary human vascular endothelial and smooth muscle cells. ACS Biomater. Sci. Eng 6, 923–932. 10.1021/ACSBIOMATERIALS.9B01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, Bedi S, Toledano-Furman NE, Triolo F, Kamhieh-Milz J, et al. (2019). Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front. Immunol 10, 1645. 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras IW, Collins LR, and Creasey AA (2021). A stem cell journey in ophthalmology: from the bench to the clinic. Stem Cells Transl. Med 10, 1581–1587. 10.1002/SCTM.21-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AL, Bennett NK, Francis NL, Halikere A, Clarke S, Moore JC, Hart RP, Paradiso K, Wernig M, Kohn J, et al. (2016). Generation and transplantation of reprogrammed human neurons in the brain using 3D micro-topographic scaffolds. Nat. Commun 7, 1–10. 10.1038/ncomms10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver W, Esch AM, Fowlkes V, and Goldsmith EC (2016). The biomechanical environment and impact on tissue fibrosis. In The Immune Response to Implanted Materials and Devices: the Impact ofthe Immune System on the Success of an Implant (Springer International Publishing; ), pp. 169–188. 10.1007/978-3-319-45433-7_9. [DOI] [Google Scholar]
- Carver W, and Goldsmith EC (2013). Regulation of tissue fibrosis by the biomechanical environment. BioMed Res. Int 2013, 101979. 10.1155/2013/101979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha C, Liechty WB, Khademhosseini A, and Peppas NA (2012). Designing biomaterials to direct stem cell fate. ACS Nano 6, 9353–9358. 10.1021/NN304773B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai C, and Leong KW (2007). Biomaterials approach to expand and direct differentiation of stem cells. Mol. Ther 15, 467–480. 10.1038/SJ.MT.6300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemmarappally JM, Pegram HCN, Abeywickrama N, Fornari E, Hargreaves AJ, de Girolamo LA, and Stevens B (2020). A co-culture nanofibre scaffold model of neural cell degeneration in relevance to Parkinson’s disease. Sci. Rep 10, 2767. 10.1038/s41598-020-59310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, and Guan KL (2018). Colonic epithelium rejuvenation through YAP/TAZ. EMBO J. 37, 164–166. 10.15252/EMBJ.201798618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kim DH, and Lim CT (2019). Special Issue: biomaterials for cell Mechanobiology. ACS Biomater. Sci. Eng 5, 3685–3687. 10.1021/acsbiomaterials.9b01123. [DOI] [PubMed] [Google Scholar]
- Chen W, Shao Y, Li X, Zhao G, and Fu J (2014). Nanotopographical surfaces for stem cell fate control: engineering mechanobiology from the bottom. Nano Today 9, 759–784. 10.1016/J.NANTOD.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhou H, Weir MD, Bao C, and Xu HHK (2012). Umbilical cord stem cells released from alginate-fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement for bone regeneration. Acta Biomater. 8, 2297–2306. 10.1016/J.ACTBIO.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendke GS, Faleo G, Juang C, Parent A.v., Bernards DA, Hebrok M, Tang Q, and Desai TA (2019). Supporting survival of transplanted stem-cell-derived insulin-producing cells in an encapsulation device augmented with controlled release of amino acids. Adv. Biosyst 3, 1900086. 10.1002/adbi.201900086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesmel KD, Clark CC, Brighton CT, and Black J (1995). Cellular responses to chemical and morphologic aspects of biomaterial surfaces. II. The biosynthetic and migratory response of bone cell populations. J. Biomed. Mater. Res 29, 1101–1110. 10.1002/jbm.820290910. [DOI] [PubMed] [Google Scholar]
- Choi UY, Joshi HP, Payne S, Kim KT, Kyung JW, Choi H, Cooke MJ, Kwon SY, Roh EJ, Sohn S, et al. (2020). An injectable hyaluronan-methylcellulose (HAMC) hydrogel combined with Wharton’s jelly-derived mesenchymal stromal cells (WJ-MSCs) promotes degenerative disc repair. Int. J. Mol. Sci 21, 1–20. 10.3390/IJMS21197391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen AT, Tao SL, Smith M, Wnek GE, Prause JU, Young MJ, Klassen H, Kaplan HJ, la Cour M, and Kiilgaard JF (2012). Subretinal implantation of electrospun, short nanowire, and smooth polY(ε-caprolactone) scaffolds to the subretinal space of porcine eyes. Stem Cells Int. 2012, 454295. 10.1155/2012/454295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueng SD, Yang L, Zhang Y, and Lee KB (2016). Multidimensional nanomaterials for the control of stem cell fate. Nano Converg. 3, 23. 10.1186/S40580-016-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino C, Rossano L, Netti PA, and Ventre M (2018). Spatio-temporal control of cell adhesion: toward programmable platforms to manipulate cell functions and fate. Front. Bioeng. Biotechnol 6, 190. 10.3389/FBIOE.2018.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismaru A, and Cismaru G (2017). Optimal delivery strategy for stem cell therapy in patients with ischemic heart disease. In Stem Cells in Clinical Practice and Tissue Engineering (IntechOpen; ). 10.5772/INTECHOPEN.69537. [DOI] [Google Scholar]
- Cogle CR, Guthrie SM, Sanders RC, Allen WL, Scott EW, and Petersen BE (2003). An overview of stem cell research and regulatory issues. Mayo Clinic Proc. 78, 993–1003. 10.4065/78.8.993. [DOI] [PubMed] [Google Scholar]
- Coronel MM, Martin KE, Hunckler MD, Barber G, O’Neill EB, Medina JD, Opri E, McClain CA, Batra L, Weaver JD, et al. (2020). Immunotherapy via PD-L1-presenting biomaterials leads to long-term islet graft survival. Sci. Adv 6. eaba5573. 10.1126/sciadv.aba5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch AS, Miller D, Luebke KJ, and Hu W (2009). Correlation of anisotropic cell behaviors with topographic aspect ratio. Biomaterials 30, 1560–1567. 10.1016/j.biomaterials.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Cui H, Liu C, Esworthy T, Huang Y, Yu ZX, Zhou X, San H, Lee SJ, Hann SY, Boehm M, et al. (2020). 4D physiologically adaptable cardiac patch: a 4-month in vivo study for the treatment of myocardial infarction. Sci. Adv 6. eabb5067. 10.1126/sciadv.abb5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis ASG, Casey B, Gallagher JO, Pasqui D, Wood MA, and Wilkinson CDW (2001). Substratum nanotopography and the adhesion of biological cells. Are symmetry or regularity of nanotopography important? Biophys. Chem 94, 275–283. 10.1016/S0301-4622(01)00247-2. [DOI] [PubMed] [Google Scholar]
- Dai Z, Yang X, Wu F, Wang L, Xiang K, Li P, Lv Q, Tang J, Dohlman A, Dai L, et al. (2021). Living fabrication of functional semi-interpenetrating polymeric materials. Nat. Commun 12, 3422. 10.1038/s41467-021-23812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Araújo E, Domingues Stocco T, Fernandes de Sousa G, Afewerki S, Marciano FR, Alexandre Finzi Corat M, Michelle Machado de Paula M, Ferreira Cândido Lima Verde T, Cristina Moreira Silva M, and Oliveira Lobo A (2021). Oxygen-generating microparticles in chondrocytesladen hydrogels by facile and versatile click chemistry strategy. Colloids Surf. B Biointerfaces 205, 111850. 10.1016/j.colsurfb.2021.111850. [DOI] [PubMed] [Google Scholar]
- de Vries R, Stell A, Mohammed S, Hermanns C, Martinez AH, Jetten M, and van Apeldoorn A (2020). Bioengineering, biomaterials, and β-cell replacement therapy. In Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas (Academic Press; ). 10.1016/b978-0-12-814831-0.00033-6. [DOI] [Google Scholar]
- Desai T, and Shea LD (2017). Advances in islet encapsulation technologies. Nat. Rev. Drug Discov 16, 338–350. 10.1038/nrd.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai TA, and Tang Q (2018). Islet encapsulation therapy—racing towards the finish line? Nat. Rev. Endocrinol 14, 630–632. 10.1038/s41574-018-0100-7. [DOI] [PubMed] [Google Scholar]
- Deshayes S, and Kasko AM (2013). Polymeric biomaterials with engineered degradation. J. Polym. Sci. Part A: Polym. Chem 51, 3531–3566. 10.1002/pola.26765. [DOI] [Google Scholar]
- DiStefano T, Chen HY, Panebianco C, Kaya KD, Brooks MJ, Gieser L, Morgan NY, Pohida T, and Swaroop A (2018). Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Rep. 10, 300–313. 10.1016/j.stemcr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezalova N, Gruszczyk A, Barkan K, Gamble JA, Galvin S, Moreth T, O’Holleran K, Mahbubani KT, Higgins JA, Gribble FM, et al. (2021). Accelerating cryoprotectant diffusion kinetics improves cryopreservation of pancreatic islets. Sci. Rep 11, 10418. 10.1038/s41598-021-89853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du V, Luciani N, Richard S, Mary G, Gay C, Mazuel F, Reffay M, Menasché P, Agbulut O, and Wilhelm C (2017). A 3D magnetic tissue stretcher for remote mechanical control of embryonic stem cell differentiation. Nat. Commun 8, 400. 10.1038/s41467-017-00543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziki JL, Huleihel L, Scarritt ME, and Badylak SF (2017). Extracellular matrix bioscaffolds as immunomodulatory biomaterials. Tissue Eng. Part A 23, 1152–1159. 10.1089/ten.TEA.2016.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger JF, Haeusel LJ, Paukner D, Aydin RC, Humphrey JD, and Cyron CJ (2021). Mechanical homeostasis in tissue equivalents: a review. Biomech. Model. Mechanobiol 20, 833–850. 10.1007/s10237-021-01433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl KN, Lambris JD, Elwing H, Ricklin D, Nilsson PH, Teramura Y, Nicholls IA, and Nilsson B (2011). Innate immunity activation on biomaterial surfaces: a mechanistic model and coping strategies. Adv. Drug Deliv. Rev 63, 1042–1050. 10.1016/j.addr.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erning K, and Segura T (2020). Materials to promote recovery After stroke. Current opinion in biomedical engineering 14, 9. 10.1016/J.C0BME.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst AU, Wang L-H, and Ma M (2018). Islet encapsulation. J. Mater. Chem. B 6, 6705–6722. 10.1039/C8TB02020E. [DOI] [PubMed] [Google Scholar]
- Ertas YN, Sadat Vaziri A, Abedi-Dorcheh K, Kazemi-Aghdam F, Sohrabinejad M, Tutar R, Rastegar-Adib F, Ashammakhi N, Ertas YN, Vaziri AS, et al. (2021). Ian situ tissue engineering: a new dimension. In Engineering Materials for Stem Cell Regeneration (Springer; ), pp. 325–350. 10.1007/978-981-16-4420-7_13. [DOI] [Google Scholar]
- Facklam AL, Volpatti LR, and Anderson DG (2020). Biomaterials for personalized cell therapy. Adv. Mater 32. e1902005. 10.1002/ADMA.201902005. [DOI] [PubMed] [Google Scholar]
- Fagerholm P, Lagali NS, Merrett K, Jackson WB, Munger R, Liu Y, Polarek JW, Söderqvist M, and Griffith M (2010). A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci. Transl. Med 2. 46ra61. 10.1126/sci-translmed.3001022. [DOI] [PubMed] [Google Scholar]
- Fakoya AOJ (2017). New delivery systems of stem cells for vascular regeneration in ischemia. Front. Cardiovasc. Med 4, 7. 10.3389/FCVM.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Alexander JF, Thekkedath U, Ferrari M, and Grattoni A (2019). Cell encapsulation: overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Deliv. Rev 139, 92–115. 10.1016/j.addr.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Finbloom JA, Raghavan P, Kharbikar BN, Yu MA, and Desai TA (2021). Polyelectrolyte nanocomplex formation combined with electrostatic self-assembly enables the co-delivery of synergistic antimicrobials to treat bacterial biofilms. Preprint at bioRxiv. 10.1101/2021.11.22.469570. [DOI]
- Fischer A, Lilienthal S, Vázquez-González M, Fadeev M, Sohn YS, Nechushtai R, and Willner I (2020). Triggered release of loads from microcapsule-in-microcapsule hydrogel microcarriers: en-route to an “artificial Pancreas”. J. Am. Chem. Soc 142, 4223–4234. 10.1021/jacs.9b11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foight GW, Wang Z, Wei CT, Jr Greisen P, Warner KM, Cunningham-Bryant D, Park K, Brunette TJ, Sheffler W, Baker D, and Maly DJ (2019). Multi-input chemical control of protein dimerization for programming graded cellular responses. Nat. Biotechnol 37, 1209–1216. 10.1038/S41587-019-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattazzo F, Urciuolo A, and Bonaldo P (2014). Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 1840, 2506–2519. 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, Alahdab F, Amit AML, Bärnighausen TW, Beghi E, Beheshti M, Chavan PP, Criqui MH, et al. (2021). Burden of neurological disorders across the US From 1990–2017: a global burden of disease study. JAMA Neurol. 78, 165–176. 10.1001/JAMANEUROL.2020.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmi A, and Schutt CE (2021). Stimuli-responsive biomaterials: scaffolds for stem cell control. Adv. Healthc. Mater 10. e2001125. 10.1002/ADHM.202001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Z, Shin JJ, Xi Y, and Hawker CJ (2021). Click chemistry strategies for the accelerated synthesis of functional macromolecules. J. Polym. Sci 59, 963–1042. 10.1002/P0L.20210126. [DOI] [Google Scholar]
- George B (2019). Regulation of combination products—evolving landscape. In Encyclopedia of Tissue Engineering and Regenerative Medicine (Elsevier; ), pp. 207–213. 10.1016/B978-0-12-801238-3.65582-4. [DOI] [Google Scholar]
- Ghavidel Mehr NG, Li X, Ariganello MB, Hoemann CD, and Favis BD (2014). Poly(ε-caprolactone) scaffolds of highly controlled porosity and interconnectivity derived from co-continuous polymer blends: model bead and cell infiltration behavior. J. Mater. Sci. Mater. Med 25, 2083–2093. 10.1007/s10856-014-5256-7. [DOI] [PubMed] [Google Scholar]
- Gholipourmalekabadi M, Zhao S, Harrison BS, Mozafari M, and Seifalian AM (2016). Oxygen-generating biomaterials: a new, viable paradigm for tissue engineering? Trends Biotechnol. 34, 1010–1021. 10.1016/j.tibtech.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Gill AA, Ortega Í, Kelly S, and Claeyssens F (2015). Towards the fabrication of artificial 3D microdevices for neural cell networks. Biomed. Microdevices 17, 27. 10.1007/s10544-015-9929-x. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, and Ordóñez-Morán P (2017). Intestinal stem cell niche insights gathered from both in vivo and novel in vitro models. Stem Cells Int. 2017, 8387297. 10.1155/2017/8387297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez T, Tierney EG, Cunniffe GM, O’Brien FJ, and Kelly DJ (2016). Gene delivery of TGF-β3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng. Part A 22, 776–787. 10.1089/ten.TEA.2015.0576. [DOI] [PubMed] [Google Scholar]
- Gouveia RM, Lepert G, Gupta S, Mohan RR, Paterson C, and Connon CJ (2019). Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat. Commun 10, 1496. 10.1038/s41467-019-09331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg-Worisek AJ, Runge BK, Solyntjes SA, St Helene-Kraft J, Glass SL, Waletzki BE, Herrick JL, Miller AL, Yaszemski MJ, Winde-bank AJ, and Wang H (2018). Establishing a current good manufacturing practice Facility for Biomaterials and Biomolecules in an Academic Medical Center. Tissue Eng. Part B Rev 24, 493–498. 10.1089/ten.TEB.2018.0114. [DOI] [PubMed] [Google Scholar]
- Greuel S, Hanci G, Böhme M, Miki T, Schubert F, Sittinger M, Mandenius CF, Zeilinger K, and Freyer N (2019). Effect of inoculum density on human-induced pluripotent stem cell expansion in 3D bioreactors. Cell Prolif. 52. e12604. 10.1111/cpr.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, et al. (2019). Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464. 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwind A, Zwick E, Prenzel N, Leserer M, and Ullrich A (2001). Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 20, 1594–1600. 10.1038/sj.onc.1204192. [DOI] [PubMed] [Google Scholar]
- Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, and Chen CS (2009). Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17–26. 10.1016/J.STEM.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzi EA, and Tibbitt MW (2020). Additive manufacturing of precision biomaterials. Adv. Mater 32. e1901994. 10.1002/adma.201901994. [DOI] [PubMed] [Google Scholar]
- Hayward MK, Muncie JM, and Weaver VM (2021). Tissue mechanics in stem cell fate, development, and cancer. Dev. Cell 56, 1833–1847. 10.1016/J.DEVCEL.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JQ, Sussman ES, and Steinberg GK (2020). Revisiting stem cell-based clinical trials for ischemic stroke. Front. Aging Neurosci 12, 464. 10.3389/FNAGI.2020.575990/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Nie A, Pei J, Ji Z, Jia J, Liu H, Wan P, Ji M, Zhang C, Zhu Y, et al. (2020). Prevalence and causes of visual impairment in population more than 50 years old: the Shaanxi Eye Study. Medicine 99. e20109. 10.1097/MD.0000000000020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand HF (2013). Biomaterials—a history of 7000 years. Bionanomaterials 14, 119–133. 10.1515/bnm-2013-0014. [DOI] [Google Scholar]
- Hofer M, and Lutolf MP (2021). Engineering organoids. Nat. Rev. Mater 6, 402–420. 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoganson DM, Pryor HI, and Vacanti JP (2008). Tissue engineering and organ structure: a vascularized approach to liver and lung. Pediatr. Res 63, 520–526. 10.1203/01.pdr.0000305879.38476.0c. [DOI] [PubMed] [Google Scholar]
- Höpner SS, Raykova A, Radpour R, Amrein MA, Koller D, Baerlocher GM, Riether C, and Ochsenbein AF (2021). LIGHT/LTβR signaling regulates self-renewal and differentiation of hematopoietic and leukemia stem cells. Nat. Commun 12, 1065. 10.1038/s41467-021-21317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini V, Maroufi NF, Saghati S, Asadi N, Darabi M, Ahmad SNS, Hosseinkhani H, and Rahbarghazi R (2019). Current progress in hepatic tissue regeneration by tissue engineering. J. Transl. Med 17, 383. 10.1186/S12967-019-02137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotaling NA, Tang L, Irvine DJ, and Babensee JE (2015). Biomaterial strategies for immunomodulation. Annu. Rev. Biomed. Eng 17, 317–349. 10.1146/annurev-bioeng-071813-104814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlton J, Abumaria N, Hinkley SFR, and Clarkson AN (2019). Therapeutic potential of neurotrophins for repair after brain injury: a helping hand from biomaterials. Front. Genet 10, 790. 10.3389/FNINS.2019.00790/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryhorowicz M, Grześkowiak B, Mazurkiewicz N, Śledziński P, Lipiński D, and Słomski R (2019). Improved delivery of CRISPR/Cas9 system using magnetic nanoparticles into porcine fibroblast. Mol. Biotechnol 61, 173–180. 10.1007/S12033-018-0145-9. [DOI] [PubMed] [Google Scholar]
- Hu J, Rodemer W, Zhang G, Jin LQ, Li S, and Selzer ME (2021). Chondroitinase ABC promotes axon regeneration and reduces retrograde apoptosis signaling in lamprey. Front. Cell Dev. Biol 9, 617. 10.3389/FCELL.2021.653638/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ouyang Y, Niu H, He N, Ke Q, Jin X, Li D, Fang J, Liu W, Fan C, and Lin T (2015). Nerve guidance conduits from aligned nanofibers: improvement of nerve regeneration through longitudinal nanogrooves on a fiber surface. ACS Appl. Mater. Interfaces 7, 7189–7196. 10.1021/am509227t. [DOI] [PubMed] [Google Scholar]
- Huang K, Ozpinar EW, Su T, Tang J, Shen D, Qiao L, Hu S, Li Z, Liang H, Mathews K, et al. (2020). An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med 12, 9683. 10.1126/scitranslmed.aat9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Lei D, Yang Q, Yang Y, Jiang C, Shi H, Qian B, Long Q, Chen W, Chen Y, et al. (2021). A perfusable, multifunctional epicardial device improves cardiac function and tissue repair. Nat. Med 27, 480–490. 10.1038/S41591-021-01279-9. [DOI] [PubMed] [Google Scholar]
- Hulsart-Billström G, Yuen PK, Marsell R, Hilborn J, Larsson S, and Ossipov D (2013). Bisphosphonate-linked hyaluronic acid hydrogel sequesters and enzymatically releases active bone morphogenetic protein-2 for induction of osteogenic differentiation. Biomacromolecules 14, 3055–3063. 10.1021/BM400639E. [DOI] [PubMed] [Google Scholar]
- Iacovacci V, Ricotti L, Menciassi A, and Dario P (2016). The bioartificial pancreas (BAP): biological, chemical and engineering challenges. Biochem. Pharmacol 100, 12–27. 10.1016/j.bcp.2015.08.107. [DOI] [PubMed] [Google Scholar]
- Iismaa SE, Kaidonis X, Nicks AM, Bogush N, Kikuchi K, Naqvi N, Harvey RP, Husain A, and Graham RM (2018). Comparative regenerative mechanisms across different mammalian tissues. npj Regen. Med 3, 6. 10.1038/s41536-018-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita M, Ohta H, Fujisawa T, Cho M, Ikeya M, Kidoaki S, and Kosodo Y (2019). Brain-stiffness-mimicking tilapia collagen gel promotes the induction of dorsal cortical neurons from human pluripotent stem cells. Sci. Rep 9, 3068. 10.1038/s41598-018-38395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JD (2016). Immunology: host responses to biomaterials. In In Situ Tissue Regeneration: Host Cell Recruitment and Biomaterial Design (Elsevier; ). 10.1016/B978-0-12-802225-2.00003-9. [DOI] [Google Scholar]
- Jansen KA, Donato DM, Balcioglu HE, Schmidt T, Danen EHJ, and Koenderink GH (2015). A guide to mechanobiology: where biology and physics meet. Biochim. Biophys. Acta 1853, 3043–3052. 10.1016/j.bbamcr.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Jell G, Minelli C, and Stevens MM (2009). Biomaterial-related approaches: surface structuring. In Fundamentals of Tissue Engineering and Regenerative Medicine (Springer; ), pp. 469–484. 10.1007/978-3-540-77755-7_35. [DOI] [Google Scholar]
- Jemni-Damer N, Guedan-Duran A, Fuentes-Andion M, Serrano-Bengoechea N, Alfageme-Lopez N, Armada-Maresca F, Guinea G.v., Perez-Rigueiro J, Rojo F, Gonzalez-Nieto D, et al. (2020). Biotechnology and biomaterial-based therapeutic strategies for age-related macular degeneration. Part II: Cell and tissue engineering therapies. Front. Bioeng. Biotechnol 8, 1419. 10.3389/FBIOE.2020.588014/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Liu X, Yang C, Yang Z, Luo J, Kou S, Liu K, and Sun F (2020). Injectable, photoresponsive hydrogels for delivering neuroprotective proteins enabled by metal-directed protein assembly. Sci. Adv 6. eabc4824. 10.1126/sciadv.abc4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AR, and Procopio AT (2019). Low cost additive manufacturing of microneedle masters. 3D Print. Med 5, 2. 10.1186/S41205-019-0039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang AR, Park JS, Ju J, Jeong GS, and Lee SH (2014). Cell encapsulation via microtechnologies. Biomaterials 35, 2651–2663. 10.1016/j.biomaterials.2013.12.073. [DOI] [PubMed] [Google Scholar]
- Kapfer SC, Hyde ST, Mecke K, Arns CH, and Schröder-Turk GE (2011). Minimal surface scaffold designs for tissue engineering. Biomaterials 32, 6875–6882. 10.1016/j.biomaterials.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Kerper N, Ashe S, and Hebrok M (2021). Pancreatic β-cell development and regeneration. Cold Spring Harbor Perspect. Biol a040741. 10.1101/CSHPERSPECT.A040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan TA, and Reddy ST (2014). Immunological principles regulating immunomodulation with biomaterials. Acta Biomater. 10, 1720–1727. 10.1016/j.actbio.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Kharbikar BN, Chendke GS, and Desai TA (2021a). Modulating the foreign body response of implants for diabetes treatment. Adv. Drug Deliv. Rev 174, 87–113. 10.1016/j.addr.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbikar BN, Kumar SH, Kr S, and Srivastava R (2015). Hollow silicon microneedle array based trans-epidermal antiemetic patch for efficient management of chemotherapy induced nausea and vomiting. In SPIE Micro+Nano Materials, Devices, and Systems, Eggleton BJ and Palomba S, eds., p. 96682W. 10.1117/12.2207407. [DOI] [Google Scholar]
- Kharbikar BN, Zhong JX, and Cuylear DL (2021b). Theranostic biomaterials for tissue engineering. Curr Opin Biomed Eng. 100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JD, Wen JH, del Álamo JC, and Engler AJ (2013). Dynamic and reversible surface topography influences cell morphology. J. Biomed. Mater. Res. A 101, 2313–2321. 10.1002/jbm.a.34543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Provenzano PP, Smith CL, and Levchenko A (2012). Matrix nanotopography as a regulator of cell function. J. Cell Biol 197, 351–360. 10.1083/jcb.201108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Zahir T, Tator CH, and Shoichet MS (2011). Effects of dibutyryl cyclic-AMP on survival and neuronal differentiation of neural stem/progenitor cells transplanted into spinal cord injured rats. PLoS One 6. e21744. 10.1371/JOURNAL.PONE.0021744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Lee EJ, Wu Y, Kang YG, and Shin JW (2019). The combined effects of hierarchical scaffolds and mechanical stimuli on ex vivo expansion of haematopoietic stem/progenitor cells. Artif. Cells Nanomed. Biotechnol 47, 586–593. 10.1080/21691401.2019.1573180. [DOI] [PubMed] [Google Scholar]
- Kimura S, Tsuchiya A, Ogawa M, Ono M, Suda N, Sekimoto K, Takeo M, and Tsuji T (2020). Tissue-scale tensional homeostasis in skin regulates structure and physiological function. Commun. Biol 3, 637. 10.1038/s42003-020-01365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk K, Xue S, Buchmann P, Charpin-El-Hamri G, Saxena P, Hussherr MD, Shao J, Ye H, Xie M, and Fussenegger M (2020). Electrogenetic cellular insulin release for real-time glycemic control in type 1 diabetic mice. Science 368, 993–1001. 10.1126/science.aau7187. [DOI] [PubMed] [Google Scholar]
- Kupikowska-Stobba B, and Lewińska D (2020). Polymer microcapsules and microbeads as cell carriers for in vivo biomedical applications. Biomater. Sci 8, 1536–1574. 10.1039/C9BM01337G. [DOI] [PubMed] [Google Scholar]
- Kuwahara A, Ozone C, Nakano T, Saito K, Eiraku M, and Sasai Y (2015). Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat. Commun 6, 6286. 10.1038/ncomms7286. [DOI] [PubMed] [Google Scholar]
- Ladd J, Zhang Z, Chen S, Hower JC, and Jiang S (2008). Zwitterionic polymers exhibiting high resistance to nonspecific protein adsorption from human serum and plasma. Biomacromolecules 9, 1357–1361. 10.1021/bm701301s. [DOI] [PubMed] [Google Scholar]
- Latchoumane C.F.v., Betancur MI, Simchick GA, Sun MK, Forghani R, Lenear CE, Ahmed A, MohanKumar R, Balaji N, Mason HD, et al. (2021). Engineered glycomaterial implants orchestrate large-scale functional repair of brain tissue chronically after severe traumatic brain injury. Sci. Adv 7. eabe0207. 10.1126/SCIADV.ABE0207/SUPPL_FILE/ABE0207_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley E, Baranov P, and Young M (2015). Hybrid vitronectin-mimicking polycaprolactone scaffolds for human retinal progenitor cell differentiation and transplantation. J. Biomater. Appl 29, 894–902. 10.1177/0885328214547751. [DOI] [PubMed] [Google Scholar]
- Le LV, Mkrtschjan MA, Russell B, and Desai TA (2019). Hang on tight: reprogramming the cell with microstructural cues. Biomed. Microdevices 21, 1–17. 10.1007/s10544-019-0394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le LV, Mohindra P, Fang Q, Sievers RE, Mkrtschjan MA, Solis C, Safranek CW, Russell B, Lee RJ, and Desai TA (2018). Injectable hyaluronic acid based microrods provide local micromechanical and biochemical cues to attenuate cardiac fibrosis after myocardial infarction. Biomaterials 169, 1–21. 10.1016/J.BIOMATERIALS.2018.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech W, Sarnowska A, Kuczynska Z, Dabrowski F, Figiel-Dabrowska A, Domanska-Janik K, Buzanska L, and Zychowicz M (2020). Biomimetic microenvironmental preconditioning enhance neuroprotective properties of human mesenchymal stem cells derived from Wharton’s jelly (WJ-MSCs). Sci. Rep 10, 16946. 10.1038/s41598-020-74066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, and Bellamkonda RV (2010). Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 107, 3340–3345. 10.1073/PNAS.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IK, Ludwig AL, Joseph Phillips M, Lee J, Xie R, Sajdak BS, Jager LD, Gong S, Gamm DM, and Ma Z (2021). Ultrathin micromolded 3D scaffolds for high-density photoreceptor layer reconstruction. Sci. Adv 7, eabf0344. 10.1126/SCIADV.ABF0344/SUPPL_FILE/ABF0344_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Abdeen AA, Tang X, Saif TA, and Kilian KA (2016). Matrix directed adipogenesis and neurogenesis of mesenchymal stem cells derived from adipose tissue and bone marrow. Acta Biomater. 42, 46–55. 10.1016/J.ACTBIO.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH (2018). Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res 22, 1–14. 10.1186/S40824-018-0138-6/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Trinh THT, Yoo M, Shin J, Lee H, Kim J, Hwang E, Lim YB, and Ryou C (2019). Self-assembling peptides and their application in the treatment of diseases. Int. J. Mol. Sci 20, 5850. 10.3390/IJMS20235850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Ma J, Khoo TS, Abdullah N, Noordin Kahar NNFNM, Abdul Hamid ZA, and Mustapha M (2021). Polysaccharide-based hydrogels for microencapsulation of stem cells in regenerative medicine. Front. Bioeng. Biotechnol 9, 947. 10.3389/FBIOE.2021.735090/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ouyang L, Armstrong JPK, and Stevens MM (2021c). Advances in the fabrication of biomaterials for gradient tissue engineering. Trends Biotechnol 39, 150–164. 10.1016/JTIBTECH.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Li CW, Pan WT, Ju JC, and Wang GJ (2016). An endothelial cultured condition medium embedded porous PLGA scaffold for the enhancement of mouse embryonic stem cell differentiation. Biomed. Mater 11, 025015. 10.1088/1748-6041/11/2/025015. [DOI] [PubMed] [Google Scholar]
- Li L, Okada H, Takemura G, Kosai KI, Kanamori H, Esaki M, Takahashi T, Goto K, Tsujimoto A, Maruyama R, et al. (2009). Postinfarction gene therapy with adenoviral vector expressing decorin mitigates cardiac remodeling and dysfunction. Am. J. Physiol. Heart Circ. Physiol 297, 1504–1513. 10.1152/AJPHEART.00194.2009/ASSET/IMAGES/LARGE/ZH40100990540007.JPEG. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen X, Jin R, Chen L, Dang M, Cao H, Dong Y, Cai B, Bai G, Justin Gooding J, et al. (2021b). Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv 7, 6740–6764. 10.1126/SCIADV.ABD6740/SUPPL_FILE/ABD6740_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu Y, Zhang Y, Yao B, Enhejirigala B, Li Z, Song W, Wang Y, Duan X, Yuan X, et al. (2021a). Biophysical and biochemical cues of biomaterials guide mesenchymal stem cell behaviors. Front. Cell Dev. Biol 9, 397. 10.3389/FCELL.2021.640388/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Nih LR, Bachman H, Fei P, Li Y, Nam E, Dimatteo R, Carmichael ST, Barker TH, and Segura T (2017). Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat. Mater 16, 953–961. 10.1038/NMAT4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, and Xiang AP (2013). Safeguarding clinical translation of pluripotent stem cells with suicide genes. Organogenesis 9, 34–39. 10.4161/ORG.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhou J, Liu Z, Chen J, Lü S, Sun H, Li J, Lin Q, Yang B, Duan C, et al. (2014). A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials 35, 5679–5688. 10.1016/J.BIOMATERIALS.2014.03.067. [DOI] [PubMed] [Google Scholar]
- Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, and Shan PF (2020). Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci. Rep 10, 14790. 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wang X, Chiu A, Liu W, Fuchs S, Wang B, Wang LH, Flanders J, Zhang Y, Wang K, et al. (2021). A zwitterionic polyurethane nanoporous device with low foreign-body response for islet encapsulation. Adv. Mater 33. e2102852. 10.1002/ADMA.202102852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Schackel T, Weidner N, and Puttagunta R (2018). Biomaterial-supported cell transplantation treatments for spinal cord injury: challenges and perspectives. Front. Cell. Neurosci 11, 430. 10.3389/FNCEL.2017.00430/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang P, Chen W, Weir MD, Bao C, and Xu HHK (2014). Human embryonic stem cells and macroporous calcium phosphate construct for bone regeneration in cranial defects in rats. Acta Biomater 10, 4484–4493. 10.1016/J.ACTBIO.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M’Barek K. ben, Habeler W, Plancheron A, Jarraya M, Regent F, Terray A, Yang Y, Chatrousse L, Domingues S, Masson Y, et al. (2017). Human ESC-derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci. Transl. Med 9, 7471. 10.1126/scitranslmed.aai7471. [DOI] [PubMed] [Google Scholar]
- Mahdavi SS, Abdekhodaie MJ, Mashayekhan S, Baradaran-Rafii A, and Djalilian AR (2020). Bioengineering approaches for corneal regenerative medicine. Tissue Eng. Regen. Med 17, 567–593. 10.1007/S13770-020-00262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahla RS (2016). Stem cells applications in regenerative medicine and Disease Therapeutics. Int. J. Cell Biol 2016, 6940283. 10.1155/2016/6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahou R, Zhang DKY, Vlahos AE, and Sefton MV (2017). Injectable and inherently vascularizing semi-interpenetrating polymer network for delivering cells to the subcutaneous space. Biomaterials 131, 27–35. 10.1016/J.BI0MATERIALS.2017.03.032. [DOI] [PubMed] [Google Scholar]
- Marchini A, Raspa A, Pugliese R, Abd El Malek MA, Pastori V, Lecchi M, Vescovi AL, and Gelain F (2019). Multifunctionalized hydrogels foster hNSC maturation in 3D cultures and neural regeneration in spinal cord injuries. Proc. Natl. Acad. Sci. USA 116, 7483–7492. 10.1073/PNAS.1818392116/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin E, Boschetto F, and Pezzotti G (2020). Biomaterials and biocompatibility: an historical overview. J. Biomed. Mater. Res. A 108, 1617–1633. 10.1002/JBMA36930. [DOI] [PubMed] [Google Scholar]
- Marquardt LM, Doulames VM, Wang AT, Dubbin K, Suhar RA, Kratochvil MJ, Medress ZA, Plant GW, and Heilshorn SC (2020). Designer, injectable gels to prevent transplanted Schwann cell loss during spinal cord injury therapy. Sci. Adv 6. eaaz1039. 10.1126/sciadv.aaz1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino F, Perestrelo AR, Vinarský V, Pagliari S, and Forte G (2018). Cellular mechanotransduction: from tension to function. Front. Physiol 9, 824. 10.3389/fphys.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiacomo S, Güvener N, Dou W, Alghamdi HS, Camargo WA, Cremers JGO, Borm PJA, Heerschap A, Oosterwijk E, Jansen JA, et al. (2017). A theranostic dental pulp capping agent with improved MRI and CT contrast and biological properties. Acta Biomater. 62, 340–351. 10.1016/J.ACTBI0.2017.08.018. [DOI] [PubMed] [Google Scholar]
- Masullo U, Cavallo A, Greco MR, Reshkin SJ, Mastrodonato M, Gallo N, Salvatore L, Verri T, Sannino A, Cardone RA, and Madaghiele M (2021). Semi-interpenetrating polymer network cryogels based on poly(ethylene glycol) diacrylate and collagen as potential off-the-shelf platforms for cancer cell research. J. Biomed. Mater. Res. B Appl. Biomater 109, 1313–1326. 10.1002/JBM.B.34792. [DOI] [PubMed] [Google Scholar]
- Mawad D, Mansfield C, Lauto A, Perbellini F, Nelson GW, Tonkin J, Bello SO, Carrad DJ, Micolich AP, Mahat MM, et al. (2016). A conducting polymer with enhanced electronic stability applied in cardiac models. Sci. Adv 2. e1601007. 10.1126/sciadv.1601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Berry M, Logan A, Scott RAH, Leadbeater W, and Scheven AA (2015). Stem cell treatment of degenerative eye disease. Stem Cell Res 14, 243–257. 10.1016/J.SCR.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei X, Zhu D, Li J, Huang K, Hu S, Li Z, de Juan Abad BL, and Cheng K (2021).A fluid-powered refillable origami heart pouch for minimally invasive delivery of cell therapies in rats and pigs. Med 2, 1253–1268.e4. 10.1016/J.MEDJ.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn A, and Desai T (2010). Inorganic nanoporous membranes for immunoisolated cell-based drug delivery. Adv. Exp. Med. Biol 670, 104–125. 10.1007/978-1-4419-5786-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao S, Zhu W, Castro NJ, Leng J, and Zhang LG (2016). Four-dimensional printing hierarchy scaffolds with highly biocompatible smart polymers for tissue engineering applications. Tissue Eng. Part C Methods 22, 952–963. 10.1089/ten.tec.2015.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara Y, Matsuura K, Sakamoto Y, Okano T, Kokudo N, and Shimizu T (2017). Production of pancreatic progenitor cells from human induced pluripotent stem cells using a three-dimensional suspension bioreactor system. J. Tissue Eng. Regen. Med 11, 3193–3201. 10.1002/term.2228. [DOI] [PubMed] [Google Scholar]
- Mitrousis N, Fokina A, and Shoichet MS (2018). Biomaterials for cell transplantation. Nat. Rev. Mater 3, 441–456. 10.1038/s41578-018-0057-0. [DOI] [Google Scholar]
- Miyagi Y, Chiu LLY, Cimini M, Weisel RD, Radisic M, and Li RK (2011). Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials 32, 1280–1290. 10.1016/J.BIOMATERIALS.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard HH, and Ghojazadeh M (2020). Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot. Perspect 10, 98–115. 10.34172/HPP.2020.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi MR, Rodriguez SM, Luong JC, Li S, Cao R, Alshetaiwi H, Lau H, Davtyan H, Jones MB, Jafari M, et al. (2021). Exosome loaded immunomodulatory biomaterials alleviate local immune response in immunocompetent diabetic mice post islet xenotransplantation. Commun. Biol 4, 685. 10.1038/s42003-021-02229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohindra P, and Desai TA (2021). Micro- and nanoscale biophysical cues for cardiovascular disease therapy. Nanomedicine 34, 102365. 10.1016/J.NANO.2021.102365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol EA, Lei Z, Roefs MT, Bakker MH, Goumans MJ, Doevendans PA, Dankers PYW, Vader P, and Sluijter JPG (2019). Injectable supramolecular Ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv. Healthc. Mater 8. e1900847. 10.1002/ADHM.201900847. [DOI] [PubMed] [Google Scholar]
- Montero P, Flandes-Iparraguirre M, Musquiz S, Pérez Araluce M, Plano D, Sanmartín C, Orive G, Gavira JJ, Prosper F, and Mazo MM (2020). Cells, materials, and fabrication processes for cardiac tissue engineering. Front. Bioeng. Biotechnol 8, 955. 10.3389/fbioe.2020.00955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty N, Cabré S, Alamilla V, Pandit A, and Dowd E (2019). Encapsulation of young donor age dopaminergic grafts in a GDNF-loaded collagen hydrogel further increases their survival, reinnervation, and functional efficacy after intrastriatal transplantation in hemi-Parkinsonian rats. Eur. J. Neurosci 49, 487–496. 10.1111/EJN.14090. [DOI] [PubMed] [Google Scholar]
- Moysidou CM, Barberio C, and Owens RM (2021). Advances in engineering human tissue models. Front. Bioeng. Biotechnol 8, 1566. 10.3389/FBIOE.2020.620962/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncie JM, and Weaver VM (2018). The physical and biochemical properties of the extracellular matrix regulate cell fate. Curr. Top. Dev. Biol 130, 1–37. 10.1016/BS.CTDB.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzio N, Moya S, and Romero G (2021). Multifunctional scaffolds and synergistic strategies in tissue engineering and regenerative medicine. Pharmaceutics 13, 792. 10.3390/PHARMACEUTICS13060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair DSR, Seiler MJ, Patel KH, Thomas V, Camarillo JCM, Humayun MS, and Thomas BB (2021). Tissue engineering strategies for retina regeneration. Appl. Sci. (Basel) 11, 2154. 10.3390/APP11052154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuss S, Denecke B, Gan L, Lin Q, Bovi M, Apel C, Wöltje M, Dhanasingh A, Salber J, Kniichel R, and Zenke M (2011).Transcriptome analysis of MSC and MSC-derived osteoblasts on Resomer® LT706 and PCL: impact of biomaterial substrate on osteogenic differentiation. PLoS One 6. e23195. 10.1371/JOURNAL.PONE.0023195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Pham TT, Nguyen HT, Nepal MR, Phung CD, You Z, Katila N, Pun NT, Jeong TC, Choi DY, et al. (2019). Engineering “cell-particle hybrids” of pancreatic islets and bioadhesive FK506-loaded polymeric microspheres for local immunomodulation in xenogeneic islet transplantation. Biomaterials 221, 119415. 10.1016/j.biomaterials.2019.119415. [DOI] [PubMed] [Google Scholar]
- Nguyen V, Meyers CA, Yan N, Agarwal S, Levi B, and James AW (2017). BMP-2-induced bone formation and neural inflammation. J. Orthop 14, 252–256. 10.1016/JJOR.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol JW, and Khademhosseini A (2009). Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter 5, 1312–1319. 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet DR, Rodda AE, Horne MK, Forsythe JS, and Finkelstein DI (2009). Neurite infiltration and cellular response to electrospun polycaprolactone scaffolds implanted into the brain. Biomaterials 30, 4573–4580. 10.1016/J.BIOMATERIALS.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Nisbet DR, Wang TY, Bruggeman KF, Niclis JC, Somaa FA, Penna V, Hunt CPJ, Wang Y, Kauhausen JA, Williams RJ, et al. (2018). Shear containment of BDNF within molecular hydrogels promotes human stem cell engraftment and postinfarction remodeling in stroke. Adv. Biosyst 2, 1800113. 10.1002/ADBI.201800113. [DOI] [Google Scholar]
- Norman JJ, and Desai TA (2006). Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann. Biomed. Eng 34, 89–101. 10.1007/s10439-005-9005-4. [DOI] [PubMed] [Google Scholar]
- Ntege EH, Sunami H, and Shimizu Y (2020). Advances in regenerative therapy: a review of the literature and future directions. Regen. Ther 14, 136–153. 10.1016/J.RETH.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyitray CE, Chavez MG, and Desai TA (2014). Compliant 3D microenvironment improves β-cell cluster insulin expression through mechanosensing and β-catenin signaling. Tissue Eng. Part A 20, 1888–1895. 10.1089/ten.TEA.2013.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill HS, Gallagher LB, O’Sullivan J, Whyte W, Curley C, Dolan E, Hameed A, O’Dwyer J, Payne C, O’Reilly D, et al. (2016). Biomaterial-enhanced cell and drug delivery: lessons learned in the cardiac field and future perspectives. Adv. Mater 28, 5648–5661. [DOI] [PubMed] [Google Scholar]
- Ou W, Li P, and Reiser J (2013). Targeting of herpes simplex virus 1 thymidine kinase gene sequences into the OCT4 locus of human induced pluripotent stem cells. PLoS One 8. e81131. 10.1371/JOURNAL.PONE.0081131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler-Yaniv A, Oyler-Yaniv J, Whitlock BM, Liu Z, Germain RN, Huse M, Altan-Bonnet G, and Krichevsky O (2017).A tunable diffusion-consumption mechanism of cytokine propagation enables plasticity in cell-to-cell communication in the immune system. Immunity 46, 609–620. 10.1016/j.immuni.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa F, Nagata S, Oda H, Yabe SG, Okochi H, and Takeuchi S (2021). Lotus-root-shaped cell-encapsulated construct as a retrieval graft for long-term transplantation of human iPSC-derived β-cells. iScience 24, 102309. 10.1016/j.isci.2021.102309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padin-Iruegas ME, Misao Y, Davis ME, Segers VFM, Esposito G, Tokunou T, Urbanek K, Hosoda T, Rota M, Anversa P, et al. (2009). Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation 120, 876–887. 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchehbaf-Kashani M, Ansari H, Mahmoudi E, Barekat M, Sepantafar M, Rajabi S, and Pahlavan S (2021). Heart repair induced by cardiac progenitor cell delivery within polypyrrole-loaded Cardiogel post-ischemia. ACS Appl. Bio Mater 4, 4849–4861. 10.1021/acsabm.1c00133. [DOI] [PubMed] [Google Scholar]
- Park GK, Kim SH, Kim K, Das P, Kim BG, Kashiwagi S, Choi HS, and Hwang NS (2019). Dual-channel fluorescence imaging of hydrogel degradation and tissue regeneration in the brain. Theranostics 9, 4255–4264. 10.7150/THNO.35606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SN, Ishahak M, Chaimov D, Velraj A, LaShoto D, Hagan DW, Buchwald P, Phelps EA, Agarwal A, and Stabler CL (2021). Organoid microphysiological system preserves pancreatic islet function within 3D matrix. Sci. Adv 7, 5515–5527. 10.1126/sciadv.aba5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra HK, Azharuddin M, Islam MM, Papapavlou G, Deb S, Osterrieth J, Zhu GH, Romu T, Dhara AK, Jafari MJ, et al. (2019). Rational nano-toolbox with theranostic potential for medicated pro-regenerative corneal implants. Adv. Funct. Mater 29, 1903760. 10.1002/adfm.201903760. [DOI] [Google Scholar]
- Paul CD, Hruska A, Staunton JR, Burr HA, Jiang N, Tanner K, and Kim J (2018). Decoupling cellular response to topography and stiffness in three dimensions. Food, Pharmaceutical and Bioengineering Division 2018—Core Programming Area at the 2018 AIChE Annual Meeting 2, 617–618. [Google Scholar]
- Perestrelo T, Correia M, Ramalho-Santos J, and Wirtz D (2018). Metabolic and mechanical cues regulating pluripotent stem cell fate. Trends Cell Biol. 28, 1014–1029. 10.1016/JTCB.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, and Caplan AI (2019). Mesenchymal stem cell perspective: cell biology to clinical progress. npj Regen. Med 4, 22. 10.1038/S41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomposelli T, Wang P, Takeuchi K, Miyake K, Ariyoshi Y, Watanabe H, Chen X, Shimizu A, Robertson N, Yamada K, and Moore A (2020). Protection of pancreatic islets using theranostic silencing nanoparticles in a baboon model of islet transplantation. Diabetes 69, 2414–2422. 10.2337/db20-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa M, and Atanase LI (2022). Biological macromolecules for drug delivery in tissue engineering. J. Biol. Macromol 393–418. 10.1016/B978-0-323-85759-8.00017-8. [DOI] [Google Scholar]
- Prasadh S, Ratheesh V, and Wong R (2020). Impact of biomaterial mechanics on cellular and molecular responses. In Handbook of biomaterials biocompatibility (Elsevier; ), pp. 85–109. 10.1016/b978-0-08-102967-1.00006-2. [DOI] [Google Scholar]
- Prestwich GD, Bhatia S, Breuer CK, Dahl SLM, Mason C, McFarland R, McQuillan DJ, Sackner-Bernstein J, Schox J, Tente WE, and Trounson A (2012). What is the greatest regulatory challenge in the translation of biomaterials to the clinic? Sci. Transl. Med 4, 160–174. 10.1126/SCITRANSLMED.3004915/ASSET/F3E619A5-BAE7-41BE-B88F-963904926805/ASSETS/GRAPHIC/4160CM14-F3.JPEG. [DOI] [PubMed] [Google Scholar]
- Primavera R, Kevadiya BD, Swaminathan G, Wilson RJ, de Pascale A, Decuzzi P, and Thakor AS (2020). Emerging nano- and micro-technologies used in the treatment of type-1 diabetes. Nanomaterials (Basel) 10, 789. 10.3390/nano10040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl DL, Funnell JL, Nelson DW, Gottipati MK, and Gilbert RJ (2020). Electrospun fiber scaffolds for engineering glial cell behavior to promote neural regeneration. Bioengineering (Basel) 8, 4. 10.3390/BIOENGI-NEERING8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puleo DA, and Bizios R (2009). Biological Interactions on Materials Surfaces: Understanding and Controlling Protein, Cell, and Tissue Responses (Springer; ). 10.1007/978-0-387-98161-1. [DOI] [Google Scholar]
- Qi C, Yan X, Huang C, Melerzanov A, and Du Y (2015). Biomaterials as carrier, barrier and reactor for cell-based regenerative medicine. Protein Cell 6, 638–653. 10.1007/s13238-015-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu T, Hanna E, Dabbous M, Borislav B, and Toumi M (2020). Regenerative medicine regulatory policies: a systematic review and international comparison. Health Policy 124, 701–713. 10.1016/J.HEALTHPOL.2020.05.004. [DOI] [PubMed] [Google Scholar]
- Quintana J, Stinchcomb A, Kostyo J, Robichaud B, Plunk M, and Kane R (2018). Chemical strategies for improving islet transplant outcomes. OBM Transplant. 2, 1. 10.21926/obm.transplant.1804036. [DOI] [Google Scholar]
- Radisic M, Marsano A, Maidhof R, Wang Y, and Vunjak-Novakovic G (2008). Cardiac tissue engineering using perfusion bioreactor systems. Nat. Protoc 3, 719–738. 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery RM, Gonzalez Vazquez AG, Chen G, and O’Brien FJ (2020). Activation of the SOX-5, SOX-6, and SOX-9 trio of transcription factors using a gene-activated scaffold stimulates mesenchymal stromal cell chondrogenesis and inhibits endochondral ossification. Adv. Healthc. Mater 9. e1901827. 10.1002/ADHM.201901827. [DOI] [PubMed] [Google Scholar]
- Rainer A, Giannitelli SM, Accoto D, de Porcellinis S, Guglielmelli E, and Trombetta M (2012). Load-adaptive scaffold architecturing: a bioinspired approach to the design of porous additively manufactured scaffolds with optimized mechanical properties. Ann. Biomed. Eng 40, 966–975. 10.1007/S10439-011-0465-4. [DOI] [PubMed] [Google Scholar]
- Rama P, Matuska S, Paganoni G, Spinelli A, de Luca M, and Pellegrini G (2010). Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med 363, 147–155. 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- Rana MM, and de La Hoz Siegler H (2021). Tuning the properties of PNIPAm-based hydrogel scaffolds for cartilage tissue engineering. Polymers 13, 3154. 10.3390/POLYM13183154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C, Yang L, Castro-Pedrido S, Luo J, Cai L, and Lee KB (2021). Hybrid SMART spheroids to enhance stem cell therapy for CNS injuries. Sci. Adv 7. eabj2281. 10.1126/sciadv.abj2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner BD (2011). The biocompatibility manifesto: biocompatibility for the twenty-first century. J. Cardiovasc. Transl. Res 4, 523–527. 10.1007/s12265-011-9287-x. [DOI] [PubMed] [Google Scholar]
- Ratner BD (2015). The Biocompatibility of Implant Materials, Host Response to Biomaterials: The Impact of Host Response on Biomaterial Selection (Elsevier Inc.). 10.1016/B978-0-12-800196-7.00003-7. [DOI] [Google Scholar]
- Razavi M, Wang J, and Thakor AS (2021). Localized drug delivery graphene bioscaffolds for cotransplantation of islets and mesenchymal stem cells. Sci. Adv 7. eabf9221. 10.1126/SCIADV.ABF9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey DFV, and St-Pierre JP (2019). Fabrication techniques of tissue engineering scaffolds. In Handbook of Tissue Engineering Scaffolds: Volume One (Elsevier; ), pp. 109–125. 10.1016/B978-0-08-102563-5.00006-X. [DOI] [Google Scholar]
- Ribeiro VP, Pina S, Oliveira JM, and Reis RL (2018). Silk fibroin-based hydrogels and scaffolds for osteochondral repair and regeneration. Adv. Exp. Med. Biol 1058, 305–325. 10.1007/978-3-319-76711-6_14. [DOI] [PubMed] [Google Scholar]
- Rivera KO, Cuylear DL, Duke V, O’Hara KM, Kharbikar BN, Kryger AN, Miclau T, Bahney CS, and Desai TA (2021). Localized delivery of β-NGF via injectable microrods accelerates endochondral fracture repair. Preprint at bioRxiv. 10.1101/2021.11.16.468864. [DOI] [PMC free article] [PubMed]
- Rodrigues GMC, Gaj T, Adil MM, Wahba J, Rao AT, Lorbeer FK, Kulkarni RU, Diogo MM, Cabral JMS, Miller EW, et al. (2017). Defined and scalable differentiation of human oligodendrocyte precursors from pluripotent stem cells in a 3D culture system. Stem Cell Rep. 8, 1770–1783. 10.1016/J.STEMCR.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton A, Benjamin EJ, Benziger CP, et al. (2020). Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol 76, 2982–3021. 10.1016/JJACC.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozario T, and DeSimone DW (2010). The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol 341, 126–140. 10.1016/J.YDBIO.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadtler K, Singh A, Wolf MT, Wang X, Pardoll DM, and Elisseeff JH (2016). Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat. Rev. Mater 1, 1–17. 10.1038/natrevmats.2016.40. [DOI] [Google Scholar]
- Sajesh KM, Ashokan A, Gowd GS, Sivanarayanan TB, Unni AKK, Nair S.V.v., and Koyakutty M (2019). Magnetic 3D scaffold: A theranostic tool for tissue regeneration and non-invasive imaging in vivo. Nanomedicine 18, 179–188. 10.1016/j.nano.2019.02.022. [DOI] [PubMed] [Google Scholar]
- Salas A, Duarri A, Fontrodona L, Ramírez DM, Badia A, Isla-Magrané H, Ferreira-de-Souza B, Zapata MÁ, Raya Á, Veiga A, and García-Arumí J (2021). Cell therapy with hiPSC-derived RPE cells and RPCs prevents visual function loss in a rat model of retinal degeneration. Mol. Ther. Methods Clin. Dev 20, 688–702. 10.1016/j.omtm.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimath AS, Phelps EA, Boopathy AV, Che PL, Brown M, Garcia AJ, and Davis ME (2012). Dual delivery of hepatocyte and vascular endothelial growth factors via a protease-degradable hydrogel improves cardiac function in rats. PLoS One 7. e50980. 10.1371/JOURNAL.PONE.0050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salta M, Wharton JA, Stoodley P, Dennington SP, Goodes LR, Werwinski S, Mart U, Wood RJK, and Stokes KR (2010). Designing biomimetic antifouling surfaces. Philos. Trans. A Math. Phys. Eng. Sci 368, 4729–4754. 10.1098/rsta.2010.0195. [DOI] [PubMed] [Google Scholar]
- Sanz-Nogués C, and O’Brien T (2021). Current good manufacturing practice considerations for mesenchymal stromal cells as therapeutic agents. Biomater. Biosyst 2, 100018. 10.1016/J.BBIOSY.2021.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweicher J, Nyitray C, and Desai TA (2014). Membranes to achieve immunoprotection of transplanted islets. Front. Biosci 19, 49–76. 10.2741/4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers VFM, and Lee RT (2011). Biomaterials to enhance stem cell function in the heart. Circ. Res 109, 910–922. 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, et al. (2013). Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl. Med 5. 173ra25. 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Son J, and Park JK (2020). Controlled 3D co-culture of beta cells and endothelial cells in a micropatterned collagen sheet for reproducible construction of an improved pancreatic pseudo-tissue. APL Bioeng. 4, 046103. 10.1063/5.0023873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbo JV, and Gerecht S (2013). Vascular tissue engineering: biodegradable scaffold platforms to promote angiogenesis. Stem Cell Res. Ther 4, 1–8. 10.1186/SCRT156/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira A, Kim DH, and Dvir T (2014). Advanced micro- and nanofabrication technologies for tissue engineering. Biofabrication 6, 020301. 10.1088/1758-5082/6/2/020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Khristov V, Rising A, Jha BS, Dejene R, Hotaling N, Li Y, Stoddard J, Stankewicz C, Wan Q, et al. (2019a). Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Transl. Med 11, 5580. 10.1126/sci-translmed.aat5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Mujawar MA, and Kaushik A (2019b). State-of-art functional biomaterials for tissue engineering. Front. Mater 6, 172. 10.3389/fmats.2019.00172. [DOI] [Google Scholar]
- Shinohara M, Komori K, Fujii T, and Sakai Y (2017). Enhanced self-organization of size-controlled hepatocyte aggregates on oxygen permeable honeycomb microwell sheets. Biomed. Phys. Eng. Express 3. 045016. 10.1088/2057-1976/aa7c3d. [DOI] [Google Scholar]
- Shrestha P, Regmi S, and Jeong JH (2020). Injectable hydrogels for islet transplantation: a concise review. J. Pharm. Investig 50, 29–45. 10.1007/s40005-019-00433-3. [DOI] [Google Scholar]
- Singh D, Wang SB, Xia T, Tainsh L, Ghiassi-Nejad M, Xu T, Peng S, Adelman RA, and Rizzolo LJ (2018). A biodegradable scaffold enhances differentiation of embryonic stem cells into a thick sheet of retinal cells. Biomaterials 154, 158–168. 10.1016/J.BIOMATERIALS.2017.10.052. [DOI] [PubMed] [Google Scholar]
- Sivasubramaniyan K, Koevoet WJLM, Hakimiyan AA, Sande M, Farrell E, Hoogduijn MJ, Verhaar JAN, Chubinskaya S, Bühring HJ, and van Osch GJVM (2019). Cell-surface markers identify tissue resident multipotential stem/stromal cell subsets in synovial intimal and sub-intimal compartments with distinct chondrogenic properties. Osteoarthr. Cartil 27, 1831–1840. 10.1016/JJOCA.2019.08.006. [DOI] [PubMed] [Google Scholar]
- Smith Q, and Gerecht S (2016). Stem cell fate is a touchy subject. Cell Stem Cell 19, 289–290. 10.1016/j.stem.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Sneddon JB, Tang Q, Stock P, Bluestone JA, Roy S, Desai T, and Hebrok M (2018). Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell 22, 810–823. 10.1016/j.stem.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somaa FA, Wang TY, Niclis JC, Bruggeman KF, Kauhausen JA, Guo H, McDougall S, Williams RJ, Nisbet DR, Thompson LH, and Parish CL (2017). Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep. 20, 1964–1977. 10.1016/j.celrep.2017.07.069. [DOI] [PubMed] [Google Scholar]
- Spellberg B, and Edwards JE (2001). Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis 32, 76–102. 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- Baskapan B, and Callanan A (2021). Electrospinning fabrication methods to incorporate laminin in polycaprolactone for kidney tissue engineering. Tissue Eng. Regen. Med 19, 73–82. 10.1007/S13770-021-00398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenović D, and Smith ML (2020). Tensional homeostasis at different length scales. Soft Matter 16, 6946–6963. 10.1039/D0SM00763C. [DOI] [PubMed] [Google Scholar]
- Stanzel B.v., Liu Z, Somboonthanakij S, Wongsawad W, Brinken R, Eter N, Corneo B, Holz FG, Temple S, Stern JH, and Blenkinsop TA (2014). Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Rep. 2, 64–77. 10.1016/J.STEMCR.2013.11.005/ATTACH-MENT/851B902D-B78D-4CCE-9CBF-AE3D33EA4A86/MMC6.MP4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz T, and Schuettler M (2013). Material-tissue interfaces in implantable systems. In Implantable Sensor Systems for Medical Applications (Elsevier; ). 10.1533/9780857096289.1.39. [DOI] [Google Scholar]
- Stokes RA, Cheng K, Lalwani A, Swarbrick MM, Thomas HE, Loudovaris T, Kay TW, Hawthorne WJ, O’Connell PJ, and Gunton JE (2017). Transplantation sites for human and murine islets. Diabetologia 60, 1961–1971. 10.1007/s00125-017-4362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Hourwitz MJ, Baker EM, Schmidt BUS, Losert W, and Fourkas JT (2018). Replication of biocompatible, nanotopographic surfaces. Sci. Rep 8, 564. 10.1038/s41598-017-19008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Nakahata M, Linke P, and Kaufmann S (2020). Stimuli-responsive hydrogels as a model of the dynamic cellular microenvironment. Polym. J 52, 861–870. 10.1038/s41428-020-0353-6. [DOI] [Google Scholar]
- Tang J, Wang J, Huang K, Ye Y, Su T, Qiao L, Hensley MT, Caranasos TG, Zhang J, Gu Z, and Cheng K (2018). Cardiac cell–integrated microneedle patch for treating myocardial infarction. Sci. Adv 4. eaat9365. 10.1126/sciadv.aat9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Lu B, He J, Chen X, Fu Q, Han H, Luo C, Yin H, Qin Z, Lyu D, et al. (2022). Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 280, 121320. 10.1016/J.BIOMATERIALS.2021.121320. [DOI] [PubMed] [Google Scholar]
- Tarabah F (2015). Good manufacturing practice (GMP) for biomaterials and medical devices in the EU and the USA. In Regulatory Affairs for Biomaterials and Medical Devices (Elsevier; ), pp. 115–143. 10.1533/9780857099204.115. [DOI] [Google Scholar]
- Tenreiro MF, Louro AF, Alves PM, and Serra M (2021). Next generation of heart regenerative therapies: progress and promise of cardiac tissue engineering. npj Regen. Med 6, 30. 10.1038/s41536-021-00140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A, Jaiswal MK, Peak CW, Carrow JK, Gentry J, Dolatshahi-Pirouz A, and Gaharwar AK (2016). Injectable shear-thinning nanoengineered hydrogels for stem cell delivery. Nanoscale 8, 12362–12372. 10.1039/C6NR02299E. [DOI] [PubMed] [Google Scholar]
- Thavandiran N, Nunes SS, Xiao Y, and Radisic M (2013). Topological and electrical control of cardiac differentiation and assembly. Stem Cell Res. Ther 4, 14. 10.1186/SCRT162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne JT, Segal TR, Chang S, Jorge S, Segars JH, and Leppert PC (2015). Dynamic reciprocity Between cells and their microenvironment in reproduction. Biol. Reprod 92, 25. 10.1095/BIOLREPROD.114.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruvannamalai-Annamalai R, Armant DR, and Matthew HWT (2014). A glycosaminoglycan based, modular tissue scaffold system for rapid assembly of perfusable, high cell density, engineered tissues. PLoS One 9. e84287. 10.1371/JOURNAL.PONE.0084287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimbouri P, Gadegaard N, Burgess K, White K, Reynolds P, Herzyk P, Oreffo R, and Dalby MJ (2014). Nanotopographical effects on mesenchymal stem cell morphology and phenotype. J. Cell. Biochem 115, 380–390. 10.1002/jcb.24673. [DOI] [PubMed] [Google Scholar]
- Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, Ko SY, Margul DJ, Bartels AK, Boehler RM, et al. (2012). Bridges delivering neurotrophin encoding lentivirus enhance regeneration following spinal cord injury. Biomaterials 33, 1618–1626. 10.1016/J.BIO-MATERIALS.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylek T, Blum C, Hrynevich A, Schlegelmilch K, Schilling T, Dalton PD, and Groll J (2020). Precisely defined fiber scaffolds with 40-μm porosity induce elongation driven M2-like polarization of human macrophages. Biofabrication 12, 025007. 10.1088/1758-5090/AB5F4E. [DOI] [PubMed] [Google Scholar]
- Urbanski MM, Kingsbury L, Moussouros D, Kassim I, Mehjabeen S, Paknejad N, and Melendez-Vasquez CV (2016). Myelinating glia differentiation is regulated by extracellular matrix elasticity. Sci. Rep 6, 1–12. 10.1038/srep33751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration (2006). Guidance for Industry and FDA Staff: Early Development Considerations for Innovative Combination Products. [Google Scholar]
- US Food and Drug Administration (2020). Use of International Standard ISO 10993-1, Biological evaluation of medical devices-Part 1: Evaluation and testing within a risk management process. [Google Scholar]
- US Government (2022). Code of Federal Regulations Title 21. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-3/subpart-A/section-3.2.
- Uto K, Arakawa CK, and Deforest CA (2020). Next-generation biomaterials for culture and manipulation of stem cells. Cold Spring Harbor Perspect. Biol 12, a035691. 10.1101/CSHPERSPECT.A035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon PH (2011). Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. J. Signal Transduct 2011, 738137. 10.1155/2011/738137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Mallorquí F, Rodríguez-Comas J, and Ramón-Azcón J (2021). Cellulose-based scaffolds enhance pseudoislets formation and functionality. Biofabrication 13, 34075893. 10.1088/1758-5090/ac00c3. [DOI] [PubMed] [Google Scholar]
- Vianney D, Roger T, Brian B, Michael C, Derek VDK, and Molly S (2016). Bioengineered hyaluronan-based hydrogels for retinal cell delivery. Front. Bioeng. Biotechnol 4, 1031–1045. 10.3389/conf.FBIOE.2016.01.00844. [DOI] [Google Scholar]
- Vlahos AE, Cober N, and Sefton MV (2017). Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc. Natl. Acad. Sci. USA 114, 9337–9342. 10.1073/pnas.1619216114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voog J, and Jones DL (2010). Stem cells and the niche: a dynamic duo. Cell Stem Cell 6, 103–115. 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, and Scadden DT (2011). Biomimetic platforms for human stem cell research. Cell Stem Cell 8, 252–261. 10.1016/J.STEM.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ (2012). The stem cell niche in regenerative medicine. Cell Stem Cell 10, 362–369. 10.1016/J.STEM.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Wahlberg B, Ghuman H, Liu JR, and Modo M (2018). Ex vivo biomechanical characterization of syringe-needle ejections for intracerebral cell delivery. Sci. Rep 8, 9194. 10.1038/S41598-018-27568-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck HM, Guerra AD, and Kao WJ (2017). Extracellular matrix: inspired biomaterials. Comprehensive Biomaterials II (Elsevier; ), pp. 132–146. 10.1016/B978-0-12-803581-8.10147-X. [DOI] [Google Scholar]
- Wang X, Chung L, Hooks J, Maestas DR, Lebid A, Andorko JI, Huleihel L, Chin AF, Wolf M, Remlinger NT, et al. (2021a). Type 2 immunity induced by bladder extracellular matrix enhances corneal wound healing. Sci. Adv 7, 2635–2651. 10.1126/sciadv.abe2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Ernst AU, An D, Datta AK, Epel B, Kotecha M, and Ma M (2021b). A bioinspired scaffold for rapid oxygenation of cell encapsulation systems. Nat. Commun 12, 5846. 10.1038/s41467-021-26126-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Huang D, Ren J, Li R, Feng Z, Guan C, Bao B, Cai B, Ling J, and Zhou C (2019). Optogenetic control of mesenchymal cell fate towards precise bone regeneration. Theranostics 9, 8196–8205. 10.7150/THNO.36455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Majumdar S, Soiberman U, Webb JN, Chung L, Scarcelli G, and Elisseeff JH (2020). Multifunctional synthetic Bowman’s membrane-stromal biomimetic for corneal reconstruction. Biomaterials 241, 119880. 10.1016/J.BIOMATERIALS.2020.119880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhu Y, Huang Y, Zhu J, Zhu B, Zhao Y, Lu Y, Wang Z, and Guo Y (2021c). Pancreatic extracellular matrix/alginate hydrogels provide a supportive microenvironment for insulin-producing cells. ACS Biomater. Sci. Eng 7, 3793–3805. 10.1021/ACSBIOMATERIALS.1C00269. [DOI] [PubMed] [Google Scholar]
- Warnke PH, Alamein M, Skabo S, Stephens S, Bourke R, Heiner P, and Liu Q (2013). Primordium ofan artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater. 9, 9414–9422. 10.1016/J.ACTBIO.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Whyte W, Roche ET, Varela CE, Mendez K, Islam S, O’Neill H, Weafer F, Shirazi RN, Weaver JC, Vasilyev NV, et al. (2018). Sustained release of targeted cardiac therapy with a replenishable implanted epicardial reservoir. Nat. Biomed. Eng 2, 416–428. 10.1038/s41551-018-0247-5. [DOI] [PubMed] [Google Scholar]
- Willerth SM, and Sakiyama-Elbert SE (2019). Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. Stem Journal 1, 1–25. 10.3233/STJ-180001. [DOI] [PubMed] [Google Scholar]
- Williams R, Lace R, Kennedy S, Doherty K, and Levis H (2018). Biomaterials for regenerative medicine approaches for the anterior segment of the eye. Adv. Healthc. Mater 7. e1701328. 10.1002/ADHM.201701328. [DOI] [PubMed] [Google Scholar]
- Wong JY, Leach JB, and Brown XQ (2004). Balance of chemistry, topography, and mechanics at the cell-biomaterial interface: issues and challenges for assessing the role of substrate mechanics on cell response. Surf. Sci 570, 119–133. 10.1016/j.susc.2004.06.186. [DOI] [Google Scholar]
- Xia Y, and Izpisua Belmonte JC (2019). Design approaches for generating organ constructs. Cell Stem Cell 24, 877–894. 10.1016/j.stem.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Xu HL, Tian FR, Lu CT, Xu J, Fan ZL, Yang JJ, Chen PP, Huang YD, Xiao J, and Zhao YZ (2016). Thermo-sensitive hydrogels combined with decellularised matrix deliver bFGF for the functional recovery of rats after a spinal cord injury. Sci. Rep 6, 38332. 10.1038/srep38332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu Y, and Hsu SH (2019). Hydrogels based on Schiff base linkages for biomedical applications. Molecules 24, 3005. 10.3390/MOLECULES24163005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Boudreau A, and Bissell MJ (2009). Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 28, 167–176. 10.1007/S10555-008-9178-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Shi W, Kong Y, Kuss M, and Duan B (2021). Anisotropic scaffolds for peripheral nerve and spinal cord regeneration. Bioact. Mater 6, 4141–4160. 10.1016/J.BIOACTMAT.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Baig R, and Dong Y (2022). Recent advances of biomaterials in stem cell therapies. Nanotechnology 33, 132515. 10.1088/1361-6528/ac4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Qin X, Wang H, Zhao X, Liu Y, Wo HT, Liu C, Nishiga M, Chen H, Ge J, et al. (2019b). An in vivo miRNA delivery system for restoring infarcted myocardium. ACS Nano 13, 9880–9894. 10.1021/ACSNAN0.9B03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EZ, Zhang GW, Xu JG, Chen S, Wang H, Cao LL, Liang B, and Lian XF (2017). Multichannel polymer scaffold seeded with activated Schwann cells and bone mesenchymal stem cells improves axonal regeneration and functional recovery after rat spinal cord injury. Acta Pharmacol. Sinica 38, 623–637. 10.1038/aps.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Park GK, McDonald EJ, and Choi HS (2019a). Targeted near-infrared fluorescence imaging for regenerative medicine. Tissue Eng. Regen. Med 16, 433–442. 10.1007/S13770-019-00219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, O’Cearbhaill ED, Liu SS, Zhou A, Chitnis GD, Hamilos AE, Xu J, Verma MKS, Giraldo JA, Kudo Y, et al. (2021).A therapeutic convection-enhanced macroencapsulation device for enhancing β cell viability and insulin secretion. Proc. Natl. Acad. Sci. USA 118. e2101258118. 10.1073/pnas.2101258118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JK, Kang ML, Park JH, Lee KM, Shin YM, Lee JW, Kim HO, and Sung HJ (2018). Direct control of stem cell behavior using biomaterials and genetic factors. Stem Cells Int. 2018, 8642989. 10.1155/2018/8642989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Zhang Y, Wang H, Brash J, and Chen H (2011). Anti-fouling bioactive surfaces. Acta Biomater. 7, 1550–1557. 10.1016/j.actbio.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wu RX, Yin Y, and Chen FM (2016). Directing immunomodulation using biomaterials for endogenous regeneration. J. Mater. Chem. B 4, 569–584. 10.1039/c5tb02199e. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang W, Jiang G, Mei X, Wang Z, Wang K, and Cui J (2016a). Study on hierarchical structured PDMS for surface super-hydrophobicity using imprinting with ultrafast laser structured models. Appl. Surf. Sci 364, 528–538. 10.1016/j.apsusc.2015.12.190. [DOI] [Google Scholar]
- Liu JMH, Zhang J, Zhang X, Hlavaty KA, Ricci CF, Leonard JN, Shea LD, and Gower RM (2016b). Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets. Biomaterials 80, 11–19. 10.1016/J.BI0MATERIALS.2015.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Lin M, Huang G, Li Y, Wang S, Bai G, Lu TJ, Xu F, et al. (2018). 3D spatiotemporal mechanical microenvironment: a hydrogel-based platform for guiding stem cell fate. Adv. Mater 30, 1705911. 10.1002/ADMA.201705911. [DOI] [PubMed] [Google Scholar]
- Yun MH (2015). Changes in regenerative capacity through lifespan. Int. J. Mol. Sci 16, 25392–25432. 10.3390/IJMS161025392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadpoor AA (2017). Biomaterials and tissue biomechanics: a match made in heaven? Materials (Basel) 10, 528. 10.3390/ma10050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewski W, Dobrzyński M, Szymonowicz M, and Rybak Z (2019). Stem cells: past, present, and future. Stem Cell Res. Ther 10, 1–22. 10.1186/S13287-019-1165-5/FIGURES/8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Du L, Sun P, Shen L, Zhu J, Pang K, and Wu X (2017). Construction of tissue-engineered full-thickness cornea substitute using limbal epithelial cell-like and corneal endothelial cell-like cells derived from human embryonic stem cells. Biomaterials 124, 180–194. 10.1016/J.BI0MATERIALS.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang M, Qin C, Wang Y, Hu X, Ma J, Zhuang H, Xue J, Wan L, Chang J, Zou W, and Wu C (2022). 3D printing of tree-like scaffolds for innervated bone regeneration. Addit. Manuf 54, 102721. 10.1016/J.ADDMA.2022.102721. [DOI] [Google Scholar]
- Zhang B, Su Y, Zhou J, Zheng Y, and Zhu D (2021a). Toward a better regeneration through implant-mediated immunomodulation: harnessing the immune responses. Adv. Sci. (Weinh) 8. e2100446. 10.1002/ADVS.202100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wu D, Zhao X, Pakvasa M, Tucker AB, Luo H, Qin KH, Hu DA, Wang EJ, Li AJ, et al. (2020). Stem cell-friendly scaffold biomaterials: applications for bone tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol 8, 1449. 10.3389/FBI0E.2020.598607/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao S, Liang K, Wu Z, Yan X, Liu W, Li J, Wu B, and Du Y (2021b). Exendin-4 gene modification and microscaffold encapsulation promote self-persistence and antidiabetic activity of MSCs. Sci. Adv 7, eabi4379. 10.1126/sciadv.abi4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Gupte MJ, and Ma PX (2013). Biomaterials and stem cells for tissue engineering. Expert Opin. Biol. Ther 13, 527–540. 10.1517/14712598.2013.756468. 10.1517/14712598.2013.756468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Cui K, and Li Z (2019). The role of biomaterials in stem cell-based regenerative medicine. Future Med. Chem 11, 1777–1790. 10.4155/fmc-2018-0347. [DOI] [PubMed] [Google Scholar]
- Zhao X, Li Q, Guo Z, and Li Z (2021). Constructing a cell microenvironment with biomaterial scaffolds for stem cell therapy. Stem Cell Res. Ther 12, 1–13. 10.1186/S13287-021-02650-W/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Mitra RN, Weiss ER, and Han Z (2020b). Rhodopsin genomic loci DNA nanoparticles improve expression and rescue of retinal degeneration in a model for retinitis pigmentosa. Mol. Ther 28, 523–535. 10.1016/j.ymthe.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Tian Y, Gao Q, Yu Y, Xia X, Feng Z, Dong F, Wu X, and Sui L (2020a). Hierarchical Micro-Nano topography promotes cell adhesion and osteogenic differentiation via integrin α2-PI3K-AKT signaling axis. Front. Bioeng. Biotechnol 8, 463. 10.3389/fbioe.2020.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Chan A, Morad L, Kornblum HI, Fan G, and Carmichael ST (2010). Hydrogel matrix to support stem cell survival After brain transplantation in stroke. Neurorehab. Neural Repair 24, 636–644. 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Deng X, Wang P, Yu C, Kiratitanaporn W, Wu X, Schimelman J, Tang M, Balayan A, Yao E, et al. (2021). Rapid bioprinting of conjunctival stem cell micro-constructs for subconjunctival ocular injection. Biomaterials 267, 120462. 10.1016/J.BI0MATERIALS.2020.120462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Li W, Dong X, Yuan X, Midgley AC, Chang H, Wang Y, Wang H, Wang K, Ma PX, et al. (2019). In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun 10, 4620. 10.1038/s41467-019-12545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


