Abstract
We have successfully used the major subunit ClpG of Escherichia coli CS31A fimbriae as an antigenic and immunogenic exposure-delivery vector for various heterologous peptides varying in nature and length. However, the ability of ClpG as a carrier to maintain in vitro and in vivo the native biological properties of passenger peptide has not yet been reported. To address this possibility, we genetically fused peptides containing all or part of the E. coli human heat-stable enterotoxin (STh) sequence to the amino or carboxyl ends of ClpG. Using antibodies to the ClpG and STh portions for detecting the hybrids; AMS (4-acetamido-4′-maleimidylstilbene-2,2′-disulfonate), a potent free thiol-trapping reagent, for determining the redox state of STh in the fusion; and the suckling mouse assay for enterotoxicity, we demonstrated that all ClpG-STh proteins were secreted in vitro and in vivo outside the E. coli cells in a heat-stable active oxidized (disulfide-bonded) form. Indeed, in contrast to many earlier studies, blocking the natural NH2 or COOH extremities of STh had, in all cases, no drastic effect on cell release and toxin activity. Only antigenicity of STh C-terminally extended with ClpG was strongly affected in a conformation-dependent manner. These results suggest that the STh activity was not altered by the chimeric structure, and therefore that, like the natural toxin, STh in the fusion had a spatial structure flexible enough to be compatible with secretion and enterotoxicity (folding and STh receptor recognition). Our study also indicates that disulfide bonds were essential for enterotoxicity but not for release, that spontaneous oxidation by molecular oxygen occurred in vitro in the medium, and that the E. coli cell-bound toxin activity in vivo resulted from an effective export processing of hybrids and not cell lysis. None of the ClpG-STh subunits formed hybrid CS31A-STh fimbriae at the cell surface of E. coli, and a strong decrease in the toxin activity was observed in the absence of CS31A helper proteins. In fact, chimeras translocated across the outer membrane as a free folded monomer once they were guided into the periplasm by the ClpG leader peptide through the CS31A-dependent secretory pathway. In summary, ClpG appears highly attractive as a carrier reporter protein for basic and applied research through the engineering of E. coli for culture supernatant delivery of an active cysteine-containing protein, such as the heat-stable enterotoxin.
The plasmid-encoded heat-stable enterotoxin (STa) produced by enterotoxigenic strains of Escherichia coli is a major cause of diarrheal diseases in infants in developing countries, travelers to areas of endemicity, and domestic livestock (1). STa exerts its toxic effects at the level of the mammalian small intestine, where it causes fluid accumulation by specific binding to the high-affinity transmembrane guanylate cyclase C receptor present on intestinal enterocytes (36). A highly conserved C-terminal sequence including six cysteines that form three intramolecular disulfide bonds (Fig. 1) is required for STa receptor binding (8), full biological activity (14), and heat stability (18). STa falls into two classes. The 18-amino-acid STa designated STp and the 19-amino-acid STa designated STh originated from porcine and human strains, respectively. The nucleotide sequences of genes coding for different STa toxins have been determined elsewhere (19, 38). Both STp and STh are typical extracellular toxins and are synthesized as a Pre-Pro-STa precursor of 72 amino acid residues (29, 31). The Pre region functions as a leader peptide, the Pro region is cleaved in the periplasmic space where the disulfide bonds of STa are formed with the help of DsbA oxidoreductase (43), and the mature folded form of STa passes through the outer membrane. The Pro sequence has been proven to be nonessential for extracellular toxin release (29). Mature STa without the Pro sequence may be able to gain access to the extracellular milieu upon its entry into the E. coli periplasm once guided into this compartment by a heterologous periplasmic leader peptide (35). Conflicting observations (31, 43–45) have been reported for the mechanism of secretion of the toxin from the periplasm to the exterior of the cell, making this mechanism poorly understood. Such disagreement may be explained by the small size of the STa molecule and the escape velocity with which it is released into the extracellular milieu, and thus by the difficulty of detecting and quantifying the intermediates in the different cellular compartments. In addition, STa is poorly antigenic and not immunogenic and reacts unpredictably with conventional protein treatments such as staining, trichloroacetic acid (TCA) precipitation, and electrophoresis (30), thus limiting progress in the study of STa secretion and in vaccine development. For these reasons, a number of efforts have been made to develop genetic fusions between STa and several carrier proteins to facilitate STa detection in secretion and folding studies and to elicit neutralizing and protective antibodies raised against the native three-dimensional structure of STa. These carriers were E. coli heat-labile enterotoxin A subunit (33) or B subunit (3, 9, 20), cholera enterotoxin B subunit (34), E. coli outer membrane protein OmpC (32), E. coli maltose-binding protein (2), a synthetic immunoglobulin G (IgG)-binding fragment derived from Staphylococcus aureus protein A (25), and staphylococcal nuclease A (29, 42). However, in most cases, no hybrid protein with properly folded STa joined covalently to the carrier protein was both extracellularly secreted and fully active. In contrast, in this work, we report that fusions between STa (STh) and the major subunit ClpG of E. coli CS31A fimbriae (16, 17) were secreted outside the cells through the CS31A-dependent pathway as an antigenic heat-stable enterotoxic protein.
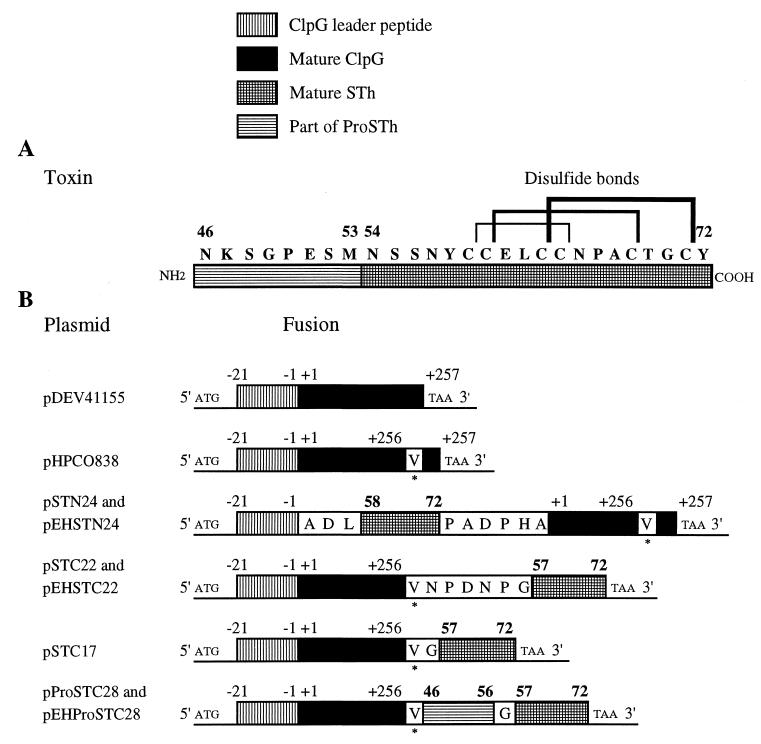
FIG. 1.
Structure of fusion proteins. (A) The STh enterotoxin structure. The STh (STa3) sequence is from the work of Guzman-Verduzco and Kupersztoch (19). Only amino acid residues 46 to 72 of the Pre-Pro-STh precursor are shown in boldface. The six cysteines involved in the three disulfide bonds are indicated. (B) ClpG-STh fusion proteins. An additional valine at the C terminus of ClpG expressed by pHPCO838 did not affect the formation of CS31A fimbriae at the cell surface. Plasmids pEHSTN24 and pSTN24 carry the same fusion, as do pEHProSTC28 and pProSTC28, and pEHSTC22 and pSTC22 (see Materials and Methods). The numbers in lightface above the boxes are the positions of the amino acid residues relative to the signal peptide cleavage site −1/+1 of the ClpG precursor (B), and those in boldface are the positions of the amino acid residues relative to the STh precursor (A and B). Indicated amino acids represent either residues composing the sequence of the linker at the ClpG-STh junction or residues introduced in ClpG by site-directed mutagenesis (marked by an asterisk).
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
E. coli DH5α [F− supE44 Δ(argF-lacZYA)U169 (φ80d lacZΔM15) hsdR17 (rK−mK+) recA1 endA1 gyrA96 (Nalr) thi-1 relA1] (Gibco BRL) was used as the host strain for recombinant proteins. The bovine enterotoxigenic E. coli isolate B41 (O101:K−:H−; K99 or F41) was used as the reference STp enterotoxin-producing strain (38). Bacteria were grown at 37°C in Luria-Bertani (LB) broth or on LB agar supplemented with the following antibiotics: ampicillin, 50 μg/ml; chloramphenicol, 25 μg/ml.
Construction of fusion genes.
Plasmids pEH524 (26), pDSPH524 (6), and pDEV41155 (10) were previously constructed. pEH524 and its derivative pDSPH524 have a pSC101 replicon, while pDEV41155 contains a pColE1 replicon. The pEH524-determined clp gene cluster contains seven structural genes encoding all the secretory proteins required for CS31A biogenesis (26), including the major 257-amino-acid subunit ClpG encoded by the clpG gene (17) and several minor proteins involved in the cell surface assembly of the CS31A polymer. Plasmid pDSPH524 is pEH524 with clpG deleted, and plasmid pDEV41155 is pBluescript (pSK+; Stratagene) with clpG only. All constructions specific to this work are shown in Fig. 1. Plasmid pHPCO838 was constructed from pDEV41155 by in vitro site-directed mutagenesis using mutagenic and selection primers. The former primer (5′-CGTGGCAGTAACTTATGTTAACTAATTGGCTTGA-3′) created a unique HpaI site and added a valine residue between the penultimate tyrosine and the last C-terminal residue (asparagine) of ClpG. The latter primer (5′-CGCAGGAAAGAAGATCTGAGCAAAAGGCG-3′) mutated the single AflIII restriction site of the pSK+ plasmid vector into a single BglII site. An additional valine at the C terminus of ClpG expressed by pHPCO838 did not affect the formation of CS31A fimbriae at the cell surface. Plasmid pSTN24 was engineered from pHPCO838 as follows: a synthetic double-stranded DNA (the 5′→3′ single coding strand was CGGCAGATCTGTACTG CTGTGAACTTTGTTGTAATCCTGCCTGTACAGGATGTTACCCTGCAG ATCCTCATG) encoding the last 15 amino acids of mature STh (mSTh) followed by the hexapeptide PADPHA and containing SphI-flanked ends was inserted in frame in the correct orientation into the SphI site of pHPCO838. Plasmid pSTC22 was constructed by cloning a synthetic DNA duplex (the 5′→3′ single coding strand was AACCCTGATAACCCCGGGAACTACTGCTGTGAACTTTGTTGTAATCCTGCCTGTACAGGATGTTACTAA) encoding the heptapeptide VNPDNPG followed by the last 16 amino acids of mSTh and containing HpaI and SmaI sites, between the HpaI and XbaI sites of pHPCO838. We constructed pSTC17 by deleting the HpaI/SmaI fragment from pSTC22, resulting in the addition of only two amino acid residues (VG) between ClpG and STh. Plasmid pProSTC28 was made by inserting a blunt-ended double-stranded DNA fragment (the 5′→3′ single coding strand was AACAAAAGTGGTCCTGAATCGATGAATTCTAGC) encoding amino acid residues 46 to 53 (NKSGPESM) of the Pro-STa peptide plus the first three residues (NSS) of mSTh between the HpaI and SmaI sites of pSTC22.
Plasmids pEHSTN24 and pEHSTC22 consisted of pEH524 in which the ClpG-encoding SwaI-HpaI fragment was replaced by the hybrid-encoding EcoRV-Ecl136II fragment from pSTN24 and pSTC22, respectively. Plasmid pEHProSTC28 was pEH524 in which the ClpG-encoding MunI-HpaI fragment was replaced by the hybrid-encoding MunI-Ecl136II fragment from pProSTC28. Therefore, pEHSTN24 and pSTN24 carry the same fusion, as do pEHProSTC28 and pProSTC28, and pEHSTC22 and pSTC22. Because many attempts to subclone the fusion gene from pSTC17 into the clp operon failed, we trans complemented pSTC17 with pDSPH524 to allow hybrid CS31A formation. Synthetic oligonucleotides were purified by reverse-phase chromatography (Eurogentec, Seraing, Belgium), and gene fusions were checked by sequencing.
Production of fusion proteins.
LB broth preculture (1 or 2 ml) containing exponentially growing cells was poured onto LB plates which were incubated overnight at 37°C in a humid atmosphere with the agar surface facing up. Bacteria were carefully harvested by being scraped from the agar surface, and the final suspension volume was made up to 2 ml with phosphate-buffered saline (PBS). After centrifugation at 12,000 × g for 10 min, the resulting supernatants were designated the solid culture supernatant fractions. Cell pellets were suspended and washed in PBS and resuspended in PBS in a final volume of 2 ml. This suspension was then divided into two equal parts. One part was used as the whole-cell fraction. The other part was sonicated and centrifuged, and the resulting supernatant was referred to as the cell sonicate fraction. Supernatants from LB broth cultures were obtained after centrifugation and used as the liquid culture supernatant fractions. The bacterial enumeration with data expressed in CFU per milliliter was done by spreading out dilutions of PBS-suspended cells on MacConkey lactose agar medium containing the appropriate antibiotic and incubating them overnight at 37°C.
Competitive ELISA.
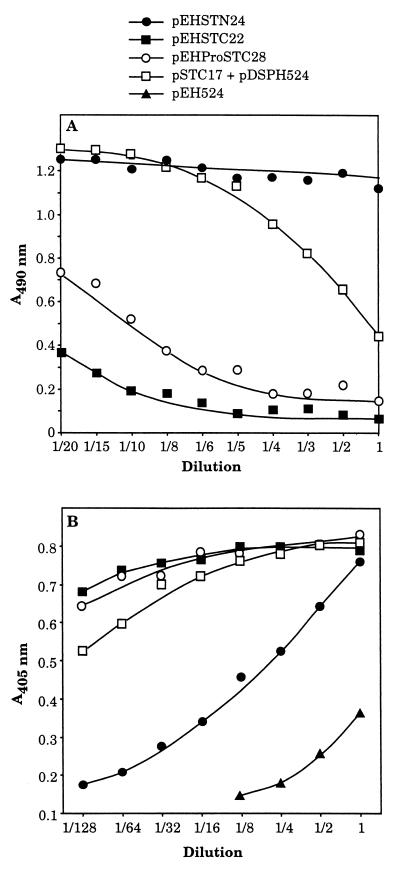
Competitive enzyme-linked immunosorbent assay (ELISA) using the commercially available assay kit for E. coli STa (COLI ST EIA) produced by Denka Seiken Co., Ltd., Tokyo, Japan (37), was performed to detect fusion proteins in culture supernatants. The reactivity of the STa moiety of hybrids was determined by using a supplied STa-monospecific horseradish peroxidase (HRP)-conjugated antibody and ELISA plates coated with synthetic STa peptide. After wells were washed once with the supplied buffer, 200 μl of various dilutions of the supernatant to be tested and 10 μl of conjugated monoclonal antibody were added. Following incubation for 90 min at room temperature, the wells were washed five times with buffer. Enzyme substrate solution (100 μl), prepared by adding an H2O2 solution to o-phenylenediamine, was added, and the plate was left in the dark at room temperature for 30 min. The reaction was stopped by addition of 100 μl of 1.5 N H2SO4, and the absorbance at 490 nm (A490) was measured using a Dynatech MR5000 microplate reader. A positive sample was identified by inhibition of binding of HRP-conjugated monoclonal antibodies to the well, as demonstrated by a decrease in A490 (Fig. 2). The positive samples were defined as giving an A490 of <0.2 (as this is a competitive assay), and the negative samples were defined as giving an A490 of ≥1.2.
FIG. 2.
Detection of fusion proteins. (A) Culture supernatants were assayed for the presence of STh in the hybrid by competitive ELISA, consisting of the addition of various dilutions of the supernatant and of STa-monospecific HRP-conjugated antibodies to plates coated with synthetic STa. A positive sample was identified by binding inhibition of STa-specific HRP-conjugated monoclonal antibody to the immobilized STa as monitored by a decrease in A490. A sample giving an A490 of ≥1.2 contained no STh. (B) Double-antibody sandwich ELISA consisted of the addition of serially twofold-diluted supernatant sample to plates coated with the STa-monospecific 11C antibody followed by binding of rabbit anti-ClpG antibodies. Antibody binding was detected by the measurement of A405 using HRP-labeled goat anti-rabbit IgG. The double-sandwich ELISA was performed to ensure probing of the entire hybrid proteins, thus overcoming the risk of detection of intact STh free of ClpG.
Double-antibody sandwich ELISA.
Microtiter plates (Immulon 2; Dynatech) were coated by overnight incubation at 4°C with 100 μl of the STa-specific monoclonal antibody 11C (42) diluted 1:250 in 50 mM carbonate buffer (pH 9.6). After removal of buffer, plates were incubated overnight at 4°C with blocking buffer (PBS–2% dry milk–1% fetal calf serum) and washed twice with PBS–0.05% Tween 20 and once with PBS. Supernatants were serially plated in twofold dilutions (100 μl per well) in antibody buffer (PBS–0.2% dry milk–0.5% fetal calf serum) and incubated for 2 h at 37°C. After washing twice with PBS–0.05% Tween 20 and once with PBS, 100 μl of 1:500-dilution rabbit anti-ClpG antiserum (16) was dispensed in each well and the plates were incubated for 90 min at 37°C. After washing, 100 μl of 1:1,000-dilution goat anti-rabbit HRP-conjugated IgG was added and the plates were incubated for 2 h at 37°C. After washing, 100 μl of 2 nM H2O2 and 10 mg of ABTS [2-2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt] (Sigma) substrate in 100 ml of phosphate-citrate buffer (80 mM citric acid, 125 mM Na2HPO4) were added and the plates were incubated for 20 min in the dark at room temperature. The A405 was read on a Dynatech MR5000 reader.
Western immunoblotting.
Samples were mixed with an equal volume of 2× Laemmli buffer, boiled for 5 min, applied to a sodium dodecyl sulfate–15% polyacrylamide gel, and semidry electrotransferred onto 0.2-μm nitrocellulose paper (Bio-Rad). Blots were blocked and washed with 1% bovine serum albumin–0.1% Tween 20 in Tris-buffered saline (TBS) and incubated overnight with primary antibodies, including ClpG-specific rabbit antiserum (16) and the STa-monospecific antibodies 11C (40) and 20C1 (7). Filters were then developed with secondary goat anti-rabbit or anti-mouse HRP-conjugated IgG and with H2O2–α-chloronaphthol as a substrate. In another procedure for development of Western blots, a 1:1,000 dilution of biotinylated IgG (Sigma) in TBS–0.05% Tween 20 was used as secondary antibody. After incubation for 1 h and washing, membranes were again incubated for 30 min with a 1:100 dilution of HRP-conjugated streptavidin (250 μg/ml; Sigma) in TBS–0.05% Tween 20 and developed with H2O2–α-chloronaphthol.
Assay for enterotoxin activity.
Enterotoxin activity of supernatant fractions was examined in the suckling mouse assay as described previously (15). Three-day-old Swiss OF1 suckling mice were separated from their mothers immediately before use and randomly divided into groups. A 0.1-ml aliquot of each sample was directly delivered to the stomach of infant mice using a flexible plastic tube. Three hours later, the entire intestine from each mouse was removed and weighed, and the ratio of the gut weight to the remaining carcass weight (G/C ratio) was calculated. The mean G/C ratio and standard error of the mean of several separated assays were determined for each mouse batch. A G/C ratio of ≥0.090 corresponded to unambiguous accumulation of fluid in the gut lumen. One mouse unit (MU) was defined as the enterotoxin activity corresponding to a minimum effective dose that gave a positive response. In our study, the minimum effective dose of STa necessary to produce an activity of 1 MU was 8 ng, as determined by using pure toxin STp (Calbiochem).
Redox state analysis of hybrid toxin.
Redox states of extracellular fusion proteins were assessed as previously described (21). Culture supernatants were incubated on ice for 1 h with final 5% TCA. As indicated, when necessary, 100 mM dithiothreitol (DTT) was added, and the mixture was incubated for 10 min at 37°C before the TCA precipitation step to completely reduce extracellular proteins. The precipitates were centrifuged at 16,000 × g for 15 min at 4°C, and the supernatants were discarded. The pellets were then washed with cold acetone and after centrifugation were air dried. The precipitates were resuspended in 0.1 mM 1% SDS–50 mM Tris-HCl (pH 8.0)–1 mM EDTA containing 10 mM AMS (4-acetamido-4′-maleimidylstilbene-2,2′-disulfonate) (Molecular Probes, Inc.). Proteins were separated by nonreducing SDS–12% polyacrylamide gel electrophoresis (PAGE), transferred to a nitrocellulose membrane, and probed with anti-ClpG or anti-STa antibodies.
Quantitation of free thiols.
Quantitation of free thiols in supernatants was determined by using the Thiol and Sulfide Quantitation kit from Molecular Probes as recommended by the suppliers. In this sensitive spectrophotometric assay, thiols reduce an inactive disulfide derivative of papain (papain-S-SCH3), stoichiometrically releasing the active enzyme (papain-SH). The reactivated papain catalyzes the hydrolysis of the chromogenic substrate, N-benzoyl-l-arginine–p-nitroanilide (l-BAPNA), resulting in an amplified spectrophotometric signal proportional to the initial amount of thiol. The activity of the enzyme is evaluated by measuring the A410 of the p-nitroaniline chromophore released from l-BAPNA. The thiol concentration in the samples was read at A410 from the standard curve generated in the papain-S-SHC3-based assay using an 0.1 mM l-cysteine working solution as a thiol standard. This solution was previously calibrated by the Ellman assay (13,600 M−1 cm−1 is the molar extinction at 412 nm of 5-thio-2-nitrobenzoate generated from Ellman's reagent in reacting with the free thiol of the l-cysteine).
Cell surface detection of fusion proteins.
The CS31A heteropolymer, a class 3-related fimbria (24), mediates E. coli and Klebsiella pneumoniae adhesion to the human intestinal cell line Intestine-407 (12) through the receptor-binding domain of the ClpG protein (13). Therefore, formation of CS31A-STh fimbriae at the cell surface of recombinant E. coli DH5α strains was examined by adhesion assay on monolayers of Intestine-407 cells and by electron microscopy after immunogold labeling with primary rabbit anti-ClpG antibodies and secondary goat anti-rabbit colloidal gold-labeled IgG (Sigma). The adherence and immunolabeling assays were performed as previously described by Di Martino et al. (13) and Der Vartanian et al. (10), respectively. Strains DH5α(pEH524) and DH5α(pDSPH524) were used as positive and negative controls, respectively.
RESULTS
Fusion proteins are extracellularly secreted.
Four distinct ClpG-STh fusion proteins with a foreign insert of 17 to 28 amino acids in length were obtained (Fig. 1). Hybrids in supernatants were detected by competitive and double-antibody sandwich ELISAs using microtiter plates precoated with synthetic STa (Fig. 2A) and STa-monospecific antibody 11C (Fig. 2B), respectively. Supernatants of strains harboring pEHSTC22, pEHProSTC28, or, to a lesser extent, pSTC17+pDSPH524 reacted with 11C (Fig. 2). In contrast, the supernatant of DH5α(pEHSTN24) retained no (Fig. 2A) or little (Fig. 2B) affinity for 11C. In the two ELISAs, maximum expression of antigenicity was obtained with hybrid STh from DH5α(pEHSTC22) and DH5α(pEHProSTC28).
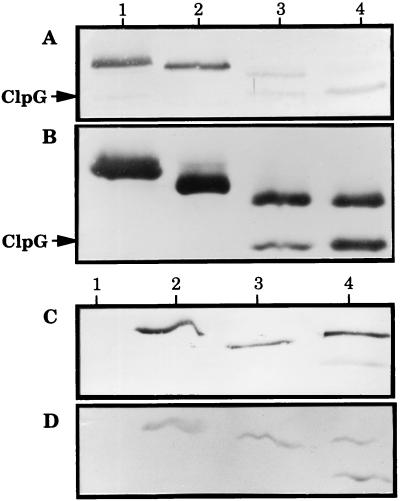
Secretion of hybrids was also analyzed by Western immunoblotting using ClpG-specific antiserum (Fig. 3A and B) and two distinct STa-monospecific antibodies (Fig. 3C and D). Immunoblots of the liquid (Fig. 3A) and solid (Fig. 3B) culture supernatant fractions showed identical electrophoretic profiles. The yield of proteins in supernatants from plate-grown cultures (5 × 1010 to 2 × 1011 CFU/ml) greatly exceeded that from broth cultures (1 × 109 to 2 × 109 CFU/ml), explaining why samples from solid cultures were preferentially used throughout this study. Hybrids encoded by pEHSTN24 and pEHSTC22 (Fig. 3B, lanes 1 and 2) were visualized as a single protein band migrating more slowly than native ClpG. By contrast, those specified by pEHProSTC28 and pDSPH524+pSTC17 (Fig. 3B, lanes 3 and 4) migrated as two protein bands, the lower running like ClpG. A chimera from pEHSTN24 reacted with anti-ClpG (Fig. 3B, lane 1) but not with anti-STa antibodies (Fig. 3C and D, lane 1), indicating, together with the competitive ELISA data (Fig. 2), a very low antigenicity due to the fusion rather than a loss of the STh moiety from the hybrid. In contrast (Fig. 3C and D), the single protein from pEHSTC22 (lane 2) and the upper proteins from pEHProSTC28 (lane 3) and pDSPH524+pSTC17 (lane 4) reacted with anti-STa antibodies. Therefore, the single proteins from pEHSTN24 and pEHSTC22, and the upper proteins from pEHProSTC28 and pDSPH524+pSTC17, represented the full-length hybrid molecules. The degradation product from pEHProSTC28 reacted only with anti-ClpG (Fig. 3A and B, lane 3), while the one from pDSPH524+pSTC17 was additionally detected with 11C and 20C1 (Fig. 3, lane 4), suggesting that some hybrids from pEHProSTC28 and pDSPH524+pSTC17 were C-terminally and N-terminally cleaved, respectively.
FIG. 3.
Immunoblot analysis of fusion proteins produced by E. coli DH5α harboring the indicated plasmid. Lanes: 1, pEHSTN24; 2, pEHSTC22; 3, pEHProSTC28; 4, pSTC17+pDSPH524. Recombinants were cultured at 37°C in LB liquid (A) or LB agar medium (B to D). (A) Supernatants were from broth cultures containing 1 × 109 to 2 × 109 CFU/ml. (B) Supernatants were from plate-gown cultures containing 5 × 1010 to 2 × 1011 CFU/ml. Supernatant samples were boiled in Laemmli buffer with β-ME and loaded onto 0.1% SDS–15% polyacrylamide gels. After electrophoresis, proteins were electrotransferred to nitrocellulose membranes and incubated either with ClpG-specific antiserum (A and B) or with the STa-specific monoclonal antibody 11C (C) or 20C1 (D). HRP-labeled goat anti-rabbit IgG (A and B) or biotin-labeled goat anti-mouse IgG in concert with HRP-conjugated streptavidin (C and D) was used as secondary antibody. The arrows at left point to the position of ClpG as assessed by the simultaneous migration of ClpG-containing solid culture supernatant from E. coli DH5α(pEH524) (data not shown).
Fusion proteins retain full heat-stable enterotoxin activity.
STh activity of the supernatant and of the whole-cell fractions was determined in vivo by means of a suckling mouse assay and compared with that of the natural STp toxin produced by the bovine E. coli B41 strain (Table 1). The measure of the minimal effective dose allowing expression of enterotoxicity in MU was determined as based on Fig. 4. The mean G/C ratio values and the score values varied, respectively, between 0.109 and 0.157 and between 75 and 100%, whatever the source of the fusion proteins (supernatant or whole cell) tested in both solid and liquid media (Table 1). Thus, in all cases, results showed positive enterotoxicity. Specific toxin activity in supernatants (expressed in MU per 1010 CFU) was 27- to 65-fold higher in liquid culture conditions than in solid culture conditions, probably because of better oxygenation. Activity of the supernatant of recombinant E. coli DH5α strains was 0.7- to 2.5-fold higher than that of the E. coli B41 control strain, and enterotoxicity of the whole cells reached about 5 to 112% of that of B41. Overall, the supernatant and whole-cell fractions of DH5α(pEHProSTC28) appeared as the most highly active samples, whereas those of DH5α(pSTC17+pDSPH524) were the least active. No enterotoxin activity was detected in cell sonicate fractions (data not shown), suggesting that they contained no or little properly folded STh. All solid culture supernatant fractions retained suckling mouse activity even after heat treatment at up to 95°C for 15 min (Table 2). Unexpectedly, hybrid STh expressed by pSTC17 seemed be more heat stable than the pure native toxin STp.
TABLE 1.
Enterotoxicity of STh chimerasa
| E. coli strain with or without plasmid | Solid medium
|
Liquid medium (supernatants)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Supernatants
|
Whole cells
|
||||||||
| G/C ratio | Score (%) | MU/1010 CFUb | G/C ratio | Score (%) | MU/1010 CFU | G/C ratio | Score (%) | MU/1010 CFU | |
| B41c | 0.120 | 7/7 (100) | 7.5 | 0.130 | 5/5 (100) | 22.2 | 0.138 | 14/15 (93) | 329 |
| DH5α | |||||||||
| pEH524d | 0.059 | 0/5 (0) | NDe | 0.076 | 0/4 (0) | ND | 0.054 | 0/4 (0) | ND |
| pEHSTN24 | 0.124 | 8/8 (100) | 8.8 | 0.145 | 7/7 (100) | 14.7 | 0.138 | 8/8 (100) | 575 |
| pEHSTC22 | 0.152 | 8/8 (100) | 8.4 | 0.140 | 8/8 (100) | 5.9 | 0.134 | 8/9 (89) | 379 |
| pEHProSTC28 | 0.145 | 9/9 (100) | 18.5 | 0.152 | 3/3 (100) | 25.0 | 0.127 | 6/8 (75) | 513 |
| pSTC17+pDSPH524 | 0.157 | 9/9 (100) | 6.4 | 0.128 | 3/3 (100) | 1.1 | 0.109 | 3/4 (75) | 221 |
Enterotoxicity of supernatant and whole-cell fractions from plate (solid medium)- and broth (liquid medium)-grown cultures was tested by the in vivo suckling mouse assay. G/C ratio values of ≥0.090 are considered positive. Score, number of positive mice/total number of mice. MU, enterotoxin activity of the minimal effective dose giving a G/C ratio of 0.090. For supernatant fractions, minimal effective dose is deduced from the toxin titer expressed as the highest dilution giving a G/C ratio of 0.090. For whole-cell fractions, minimal effective dose is the minimal number of live recombinant cells giving a G/C ratio of 0.090. Titers and minimal effective dose values are calculated as shown in Fig. 4.
Specific activity values represent the ratio of STh activity level (MU per milliliter) to bacterial density (CFU per milliliter).
Strain used as a positive reference control.
Plasmid used as a negative control.
ND, not detectable because no activity was found in the undiluted sample.
FIG. 4.
Determination of minimal effective dose of the whole-cell (A) and culture supernatant (B) fractions. Only data obtained under solid culture conditions are presented as an example for minimal effective dose calculation. After centrifugation of overnight cultures, supernatants were separated from the cell pellets which were washed and suspended with PBS buffer (2 ml). Before centrifugation and after suspension in PBS, bacteria were enumerated on MacConkey lactose agar medium. Various dilutions of the supernatant and whole-cell preparations were tested in the suckling mouse assay. The enterotoxin titer was expressed as the highest dilution that gave a G/C ratio of 0.090 corresponding to an enterotoxin activity of 1 MU. Each datum point is the average of G/C ratios, which were plotted semilogarithmically versus bacterial densities (A) or supernatant dilution values (B), and the point at which the curve crossed the line equal to a G/C ratio of 0.090 (dotted line) was defined as the density (A) or the dilution (B) giving an enterotoxin activity of 1 MU. The points of the data with whole cells are 108.60, 108.82, 109.20, and 109.96 CFU, for DH5α(pEHProSTC28), DH5α(pEHSTN24), DH5α(pEHSTC22), and DH5α(pSTC17+pDSPH524), respectively.
TABLE 2.
Heat stability of STh chimerasa
| Toxic sample (2 MU) | Enterotoxicityb
|
||||
|---|---|---|---|---|---|
| Unheated control | Heated for 15 min
|
||||
| 90°C | 95°C | 100°C | 105°C | ||
| STp (control)c | 0.151 (100) | 0.144 (100) | 0.067 (25) | 0.081 (25) | NT |
| pEHSTN24 | 0.141 (100) | 0.105 (62) | 0.094 (50) | 0.079 (25) | 0.061 (0) |
| pEHSTC22 | 0.127 (100) | 0.099 (57) | 0.089 (50) | 0.078 (25) | 0.067 (0) |
| pEHProSTC28 | 0.154 (100) | 0.107 (62) | 0.095 (50) | 0.076 (20) | NT |
| pSTC17 + pDSPH524 | 0.134 (100) | NT | 0.143 (100) | 0.102 (100) | 0.104 (66) |
The solid culture supernatant fractions of E. coli DH5α strains with the indicated plasmids were prepared, their titers were adjusted to 2 MU, and they were tested in the suckling mouse assay (5 to 10 mice per sample).
As described in Table 1, footnote a. Values are G/C ratios; the values in parentheses are scores expressed as percentages. NT, not tested.
Pure native STp toxin (Calbiochem) was adjusted in titer to 2 MU and used as a positive reference control.
Enterotoxicity, but not secretion, is affected by reducing conditions, and disulfide bonds are able to reform spontaneously with air oxidation.
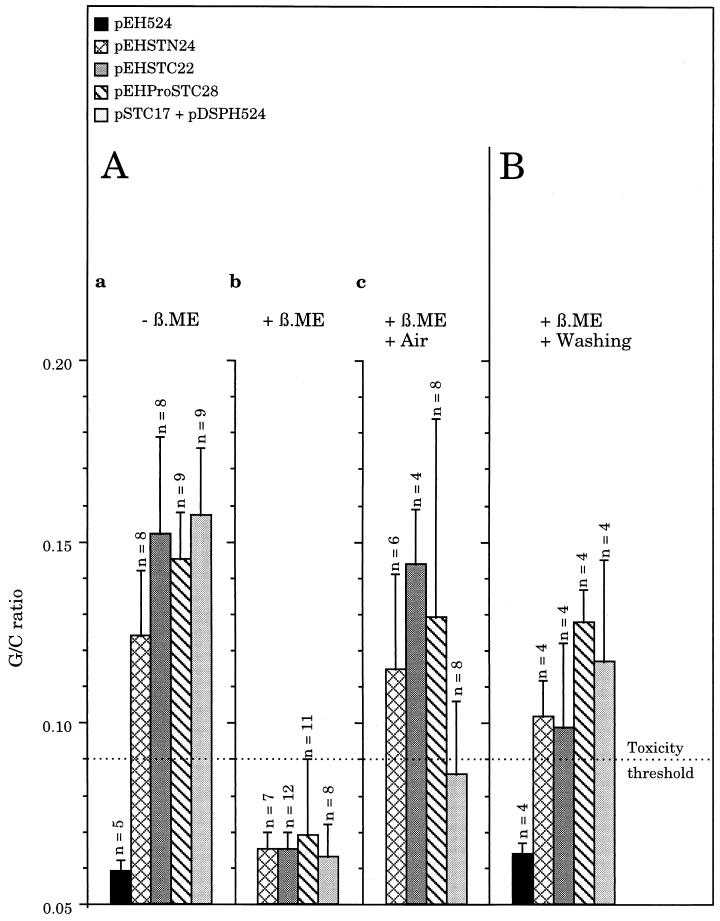
Strains were grown with 5 mM β-mercaptoethanol (β-ME) and the biological properties of STh hybrids after β-ME reduction were checked (Fig. 5). Supernatants showed an overall defect in enterotoxicity only when recombinant strains were cultured in the presence of β-ME (Fig. 5A, a and b). Reduced hybrids were extracellularly released in vitro since supernatants from β-ME-grown cultures partially recovered activity after they had been left overnight in contact with air oxygen (Fig. 5A, c). To verify the oxidizing effect of air on β-ME-inactivated STh, we estimated the degree of air oxidation by quantifying the reduced protein products in the supernatants of β-ME-treated strains and by testing the activity of these supernatants after exposure to air for 18 and 42 h (Table 3). As a function of time, increasing activity was correlated with decreasing free thiol concentration in samples and therefore with increasing oxidation. Furthermore, live recombinant bacteria previously cultured with β-ME and then washed with PBS secreted hybrids capable of suckling mouse activity (Fig. 5B).
FIG. 5.
Effect of reducing culture conditions on the suckling mouse activity of the supernatant (A) and whole-cell (B) fractions. LB broth-grown cells (2 ml) were poured onto LB agar medium containing (A, b and c, and B) or not (A, a) 5 mM (β-ME) and spread over the agar surface before overnight incubation at 37°C in a humid atmosphere with the agar surface facing up. Bacteria were then harvested by being scraped from the agar surface. After centrifugation, all of the supernatants were separated from cells (A), and each of them was then halved. One half was tested as it was (a and b), and the other was kept overnight at room temperature in contact with air before use (c). The corresponding β-ME-treated cell pellets were washed and resuspended in PBS, thus constituting the β-ME-cleared whole-cell fractions (B). Bars are the means ± standard errors of 2 determinations using 4 to 12 mice per sample as indicated above the bars. The dotted line equal to a G/C ratio of 0.090 represents the toxicity threshold above which the samples are considered positive.
TABLE 3.
Spontaneous air oxidationa
| Plasmid | Value at time:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
t0
|
t18
|
t42
|
|||||||
| Thiolb (μM) | Activityc
|
Thiol (μM) | Activity
|
Oxidationd | Thiol (μM) | Oxidation (%) | |||
| G/C ratio | Score (%) | G/C ratio | Score (%) | ||||||
| pEHSTN24 | 4.25 | 0.065 | 0/7 (0) | 2.35 | 0.115 | 5/6 (83) | 45 | 0.89 | 79 |
| pEHSTC22 | 9.01 | 0.065 | 0/12 (0) | 2.55 | 0.144 | 4/4 (100) | 72 | 1.33 | 85 |
| pEHProSTC28 | 11.38 | 0.069 | 1/11 (9) | 3.04 | 0.129 | 5/8 (62) | 73 | 2.83 | 75 |
| pSTC17 + pDSPH524 | 11.24 | 0.063 | 0/8 (0) | 6.10 | 0.086 | 2/8 (25) | 46 | 1.49 | 87 |
LB broth-grown E. coli DH5α cells harboring the indicated plasmids, as a preculture (1 or 2 ml), were spread onto LB agar medium containing 5 mM β-ME, and the plates were then incubated overnight at 37°C in a humid atmosphere with the agar surface facing up. Bacteria were harvested by scraping, and the final suspension volume was made up to 2 ml with PBS. After centrifugation at 12,000 × g for 10 min, each resulting supernatant fraction was divided into three parts equal in volume. One part was tested immediately (t0), and the two others were kept in contact with air for 18 h (t)18) or 42 h (t42) at room temperature.
Quantitation of free thiols in samples was determined by reading the A410 from a standard curve generated in the papain-based assay as described in Materials and Methods. This assay measures both the amount of protein cysteine and any remaining β-ME in the culture medium. Therefore, to estimate only the oxidation state of the Clp-STh proteins, data (micromolar concentrations of thiols) were obtained by subtracting the values corresponding to the extracts with hybrids from those corresponding to the extracts without hybrids (from pEH524 expressing ClpG and not STh).
As described in Table 1, footnote a.
Degree of oxidation was estimated as based on the comparison between the thiol concentration at t18 or t42 and that at t0.
STh in the fusions is in an oxidized state.
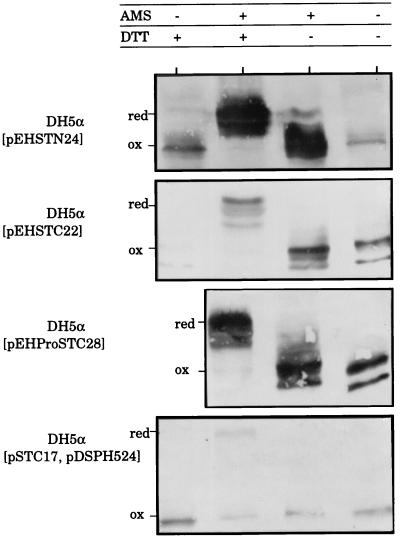
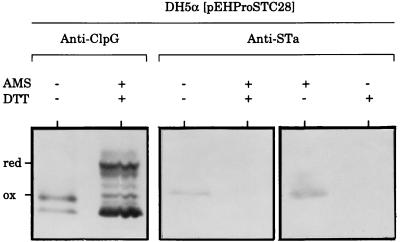
We were able to achieve very clear separation between the oxidized and reduced forms of ClpG-STh hybrids by examining the electrophoretic mobility of samples treated or not with DTT and AMS, which is a potent thiol-blocking reagent (Fig. 6). Reduced and oxidized forms of chimeras from supernatant samples were not distinguishable on immunoblots when they were treated only with either DTT or AMS. In contrast, hybrids treated with both DTT and AMS showed an upward change in position. This indicates that the shift of the band position was due to chemical binding of AMS to the sulfhydryl groups generated by the reduction of oxidized cysteines by DTT. Consequently, extracellular hybrids are in an oxidized state. As shown in Fig. 7, in which only the supernatant of DH5α(pEHProSTC28) is presented as an example, unoxidized full-length hybrids reacted with anti-ClpG serum (lane +AMS +DTT) but not with anti-STa antibodies (lanes +AMS +DTT and −AMS +DTT). In contrast, the oxidized form reacted with both antibodies (lane −AMS −DTT for anti-ClpG and lanes −AMS −DTT and +AMS −DTT for anti-STa). Given that ClpG contains no cysteine residue, it is therefore clear that STh in the fusions is disulfide bonded.
FIG. 6.
Redox state of extracellular hybrids. LB broth-gown cells (2 ml) were poured onto LB agar medium and spread over the agar surface before overnight incubation at 37°C in a humid atmosphere with the agar surface facing up. Bacteria were then harvested by being scraped from the agar surface, separated from supernatants by centrifugation, and discarded. Supernatants were first reduced (+) or not (−) with 100 mM DTT at 37°C for 10 min, precipitated with TCA, washed with acetone, and then diluted in 1% SDS–1 mM EDTA–50 mM Tris-HCl (pH 8.0), containing (+) or not (−) 10 mM AMS. Samples were subjected to nonreducing SDS–12% PAGE, electrotransfer, and blotting with anti-ClpG antiserum. AMS is a potent thiol-blocking reagent highly soluble in aqueous solutions that blocks irreversibly free cysteines by producing thioesters (21). The reduced and oxidized forms can be separated by the charge difference due to AMS which increases the apparent molecular mass by 490 Da. Oxidized forms are resistant to reaction with AMS and migrate as a lower-molecular-weight protein band than do reduced forms. The position labeled “red” refers to reduced STh bound to AMS, while the position labeled “ox” designates either oxidized STh treated or not with AMS or unoxidized STh uncoupled to AMS.
FIG. 7.
STh in the fusion is disulfide bonded. The oxidation state of the fusion protein present in the overnight solid culture supernatant of DH5α(pEHProSTC28) was determined as described in the legend to Fig. 6, except that Western blots were additionally probed with anti-STa antibodies consisting of a mixture of the monoclonal antibodies 11C and 20C1.
CS31A export pathway, but not CS31A biogenesis, is required for extracellular secretion.
The presence of CS31A-STh hybrid fimbriae at the cell surface of recombinant bacteria was determined by the CS31A-mediated adhesion assay on monolayers of the Intestine-407 cell line and by electron microscopy after immunogold labeling using anti-ClpG antibodies. All recombinants gave negative results in the two assays (data not shown). The ability of hybrid subunits to polymerize into a chimeric CS31A fimbrial structure was also investigated by immunoblot analysis using PAGE under nondenaturing conditions (data not shown). The fusion proteins never appeared as a ladder of oligomeric bands of regularly increasing molecular mass, constituting the polymeric form of native CS31A (11). All these data confirm a lack of cell surface assembly of CS31A chimeras.
To gauge the importance of the CS31A export pathway in ClpG-STh protein release, we used plasmids carrying the clpG-STh fusion gene (Fig. 1) but not the CS31A helper genes encoding the secretory proteins required for full CS31A biogenesis. The specific activity of hybrids expressed by these plasmids was then measured (Table 4). In all experiments performed in liquid and solid culture conditions, only supernatants and whole cells of DH5α(pProSTC28) unequivocally displayed secretion and enterotoxicity. Nevertheless, this strain had toxin activity at least one-fifth the level seen in Table 1, in which all four chimeras are shown to have enterotoxin activity. In addition, no chimeric toxin from pProSTC28 was detected in immunoblots using anti-ClpG and anti-STa antibodies (data not shown), suggesting that processing of native toxin occurred. These findings underline the stimulating effect of the CS31A export machinery on the secretion of fusion proteins.
TABLE 4.
Influence of the clp helper genes on enterotoxicity of STh chimerasa
| Plasmid | Solid medium
|
Liquid medium (supernatants)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Supernatants
|
Whole cells
|
|||||||
| G/C ratio | Score (%) | MU/1010 CFU | G/C ratio | Score (%) | G/C ratio | Score (%) | MU/1010 CFU | |
| pHPCO838b | 0.063 | 0/11 (0) | NDc | 0.086 | 1/8 (12) | ND | ND | ND |
| pSTN24 | 0.099 | 12/18 (67) | 2.0 | 0.078 | 0/11 (0) | 0.070 | 0/8 (0) | ND |
| pSTC22 | 0.078 | 6/20 (30) | ND | 0.084 | 3/11 (27) | 0.053 | 0/9 (0) | ND |
| pProSTC28 | 0.133 | 13/16 (81) | 3.6 | 0.103 | 7/11 (64) | 0.110 | 7/7 (100) | 112 |
| pSTC17 | 0.060 | 0/19 (0) | ND | 0.077 | 0/11 (0) | 0.050 | 0/8 (0) | ND |
Enterotoxicity of supernatant and whole-cell fractions from plate (solid medium) and broth (liquid medium) cultures of recombinant E. coli strains deficient in clp-encoded CS31A helper proteins was tested as indicated in Table 1, footnote a.
Plasmid used as a negative control.
ND, not detectable because no activity was found in the undiluted sample.
DISCUSSION
Recently, ClpG, the major subunit of E. coli CS31A fimbriae, has been shown to accept various virus epitopes without affecting CS31A formation (6, 10, 11, 27). In this study, we exploited ClpG as a provider of a signal peptide and an extracellular export carrier for the active cysteine-containing passenger peptide, the small human heat-stable enterotoxin STh of E. coli. We obtained four distinct ClpG-STh proteins after having fused various STh-encoding DNA sequences to the 5′ or 3′ extremity of the clpG gene. All were secreted in vitro in the culture supernatants with a heat-stable enterotoxin activity comparable to that of the natural STa toxin, indicating that they retained a three-dimensional structure close to that of native STa, which must be flexible since it is able to tolerate ClpG at its N- or C-terminal end. Our results indicate a much higher specific activity of toxin from broth cultures than of that from plate-grown cultures (Table 1). We speculate that the higher specific activity in broth is due to better oxygenation, and thus to better oxidation of cysteine-containing proteins, although protein yields are higher from plate-grown cells (Fig. 3) and the protein is fully oxidized (Fig. 6). Nevertheless, the fact that protein from solid culture is fully oxidized does not necessarily mean that it is properly folded in an active form. One can imagine that plate-grown cultures generate more chimeric STh species with incorrectly formed disulfide bonds not compatible with enterotoxin activity compared with broth cultures, in which increased oxygenation enhanced the rapid formation of the correct disulfide bond. It is here shown that disulfide bonds of STh can be formed spontaneously in the presence of molecular oxygen. It is also known that, for aerobically growing cells, the air oxygen may be the ultimate source of the oxidizing power and that the oxidizing equivalent for the correct formation of protein disulfide bonds in vivo appears to be provided by oxygen through the respiratory chain (22, 23). In contrast to cell sonicates, live recombinant bacteria induced fluid accumulation in the mouse intestine, suggesting that toxin activity in vivo resulted from an effective export processing of hybrids and not cell lysis. These findings show that chimeras were processed in vitro and in vivo in such a way that carriage by ClpG and the gastrointestinal environment did not alter STh activity. Thus, hybrids might be directed into the E. coli periplasm by the ClpG leader peptide, and the structure of the chimeras attaining the periplasm was suitable for translocation across the outer membrane in vitro and in vivo. However, it cannot be excluded that some turnover of the chimera molecules may occur during secretion, leading to the loss of intact STh moieties, and that processing of these hybrids to release free STh may take place in the mouse assay both for the cell-free samples and for the cell-bound activity. If true, cleavage between ClpG and STh domains should occur whatever the nature of the joint peptide and the fusion protein, which is unlikely. Indeed, only two of the four fusion proteins generated truncated derivative products in culture supernatants, indicating differences in conformation-dependent protease sensitivity of hybrids. Moreover, the extracellular hybrid STh was shown to be completely oxidized and, therefore, disulfide bonded, dismissing the idea that STh folds only if it is released from fusion proteins.
Supernatants of bacteria cultured in the presence of β-ME displayed no activity which was recovered after prolonged exposure to air, while the corresponding whole E. coli cells expressed enterotoxin activity after being cleared of β-ME, thus confirming that, as hypothesized above, activity in vivo resulted from an effective export processing of hybrids. Taken together, these results provide evidence that disulfide bond formation in STh was essential for toxin activity but not for outer membrane translocation and that bonds can be formed in vitro in the medium by spontaneous air oxidation. Thus, folding of chimeric STh can occur in the extracellular milieu, as suggested by Rasheed et al. (31) and Yang et al. (45) for the natural toxin.
Like the natural STa peptide (40), hybrid STh reacted poorly with STa-monospecific antibodies only when in an unoxidized form and exhibited very low antigenicity only when C-terminally blocked with ClpG. These results suggest that, in agreement with those of Sanchez et al. (34), the free carboxyl end in the toxin is required for antibody recognition and, above all, that the overall shape of the STh in the hybrid is important for proper anti-STa antibody binding and, therefore, that likely anti-STa antibodies recognized conformational epitopes on chimeric STh. Clements (9) emphasized the importance of including an appropriate linker between the two domains of the LT-B (B subunit of heat-labile enterotoxin)–STa fusion for antigenicity of the hybrid proteins and showed that maximum antigenicity was obtained with a 7-amino-acid proline-containing linker. In this study, among the fusions with STh N-terminally extended with ClpG, those with a linker of at least 7 amino acids with one or two proline residues at the ClpG-STh junction were more antigenic. In addition, the presence of linkers with two proline residues seemed to protect chimeras against proteolytic cleavage, as deduced from immunoblot analysis. On the other hand, the hybrid containing a linker with only two amino acids was poorly antigenic and enterotoxic but, however, exhibited more heat stability, suggesting that the biological properties of STh can be modified by altering the stereochemistry of the molecule by means of rearrangement of the disulfide bonds.
In disagreement with many studies (see the Introduction), we showed that blocking the natural amino or carboxy terminus of STh with a heterologous carrier can be permissive for secretion, folding, and enterotoxicity. Some of these studies (19, 44) concluded that, for successful mobilization through the membranes and for full biological activity, the STa domain has to contain the natural 18 or 19 amino acids not N- or C-terminally blocked by other residues. These discrepancies lead us to suspect the CS31A export pathway of being involved in the transport of ClpG-STh chimeras across the outer membrane. To address this possibility, we examined the abilities of hybrid ClpG subunits to form CS31A-STh fimbriae at the cell surface of the CS31A helper protein-proficient cells (clp+) and to be secreted by the CS31A helper protein-deficient cells (clp mutant). Surprisingly, none of these chimeras was cell surface assembled into a fimbrial structure on clp+ strains, clearly proving that ClpG in the fusion adopted a conformation incompatible with anchorage, assembly, and elongation of fimbriae. These data definitely rule out the idea that presentation of hybrid subunits at the cell surface of bacteria as repeating units along the fimbrial structure constitutes an essential feature for the exit of the hybrid to the extracellular milieu. On the other hand, out of four recombinant constructs devoid of the clpG gene, only pProSTC28 significantly expressed secretable active ClpG-STh chimeras in clp mutant strains. The possibility that processing of native toxin from pProSTC28 happened is supported by the findings that (i) this chimera possesses part of the naturally cleavable Pro-STh region; (ii) in contrast to other ClpG-STh fusions, only hybrids from pEHProSTC28 were probably C-terminally cleaved, as based on immunoblot analysis; and (iii) no protein was detected in supernatants from plate- and broth-grown cultures when using anti-ClpG and anti-STa antibodies in immunoblotting experiments. In summary, secretion of STh chimeras, as free monomers, follows the CS31A-dependent pathway by using ClpG as an extracellular export carrier probably capable of interacting directly with one or several CS31A-specific minor proteins, especially with the CS31A chaperone ClpE (5), for effective outer membrane translocation. However, since most of the fusion constructs involve fusions at the C terminus of the fimbrial subunit, ClpG, it appeared surprising that they could be secreted by the CS31A fimbrial secretion machinery. Indeed, mainly based on pilus Pap studies (39), the current model of interaction of fimbrial subunits with the periplasmic chaperone would predict that these fusions would not interact well with the chaperones and thus be subject to degradation in the periplasm. However, the C-terminal part of ClpG has not been deleted and none of the amino acids composing this part has been changed. Therefore, we believe that the binding chaperone site on the ClpG chimera is still accessible and that the linker between the ClpG and STh domains maintains this accessibility by improving the flexibility of ClpG at the fusion junction, thus minimizing the disturbing effect of the fusion on the native C-terminal ClpG and N-terminal STh conformations. Other possibilities are that ClpG in the fusion may be completely or partially protected by the STh structure against proteolytic degradation in the periplasm and that other regions of ClpG may be involved in chaperone binding, as suggested for K88, a CS31A-related fimbria (4).
Conflicting observations have been reported for the mechanism of secretion of the toxin from the periplasm to the outside of the E. coli cell, making this mechanism poorly understood. Some authors (43, 44) found that the Pro-STa region is cleaved in the periplasmic space where the mature STa is correctly folded, while others (31, 45) hypothesized that Pro-STa can exit to the extracellular milieu and that STa is disulfide bonded outside the cell. On the other hand, an important aspect of vaccine development is the possibility that effective neutralization and protection against STa require the production of antibodies to STa antigen in the fusion protein that may recognize native STa and that are directed against epitopes associated with toxicity. Our unpublished preliminary studies indicate that all of the four toxic ClpG-STh fusions could induce high titers of anti-STh serum antibodies, among which some neutralized native STa toxin activity. By contrast, a nontoxic ClpG-STh mutant induced no anti-STh antibodies. Therefore, although the ClpG-STh fusion proteins could be processed differently from the natural STa, we think that these fusions may be helpful for studying STa action, secretion, and folding and for contributing to vaccine development by taking advantage of the easy detection of STh hybrids using ClpG as an immunogenic marker carrier protein. One can also imagine using ClpG as a carrier for a nonimmunogenic, functionally active, full-length native cysteine-containing protein of medical interest such as, for an example, human chorionic gonadotropin or gonadotropin releasing hormone. Active immunization of women against human chorionic gonadotropin and that of domestic animals against gonadotropin releasing hormone have been considered as cost-effective promising options for immunocontraception (28, 41).
ACKNOWLEDGMENTS
This project was supported by the Conseil Régional Auvergne (Clermont-Ferrand, France) and the Institut National de la Recherche Agronomique (Paris, France).
We are grateful for donation of mouse anti-STa monoclonal antibodies by R. A. Gianella (20C1) and T. Takeda (11C). P. Di Martino and A. Darfeuille-Michaud are gratefully acknowledged for help in regard to the adherence to Intestine-407 cells. We thank M. Chavarot, C. De Martrin, A. Garrivier, B. Jaffeux, and G. Vert for technical assistance and S. Dutilloy for secretarial assistance.
REFERENCE
- 1.Acheson D W K. Enterotoxins in acute infective diarrhoea. J Infect. 1992;24:225–245. doi: 10.1016/s0163-4453(05)80028-3. [DOI] [PubMed] [Google Scholar]
- 2.Aitken R, Hirst T R. Development of an immunoassay using recombinant maltose-binding protein-STa fusions for quantitating antibody responses against STa, the heat-stable enterotoxin of Escherichia coli. J Clin Microbiol. 1992;30:732–734. doi: 10.1128/jcm.30.3.732-734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aitken R, Hirst T R. Recombinant enterotoxins as vaccines against Escherichia coli-mediated diarrhoea. Vaccine. 1993;11:227–233. doi: 10.1016/0264-410x(93)90022-p. [DOI] [PubMed] [Google Scholar]
- 4.Bakker D, Vader C E M, Roosendaal B, Mooi F R, Oudega B, de Graaf F K. Structure and function of periplasmic chaperone-like proteins involved in the biosynthesis of K88 and K99 fimbriae in enterotoxigenic Escherichia coli. Mol Microbiol. 1991;5:875–886. doi: 10.1111/j.1365-2958.1991.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 5.Bertin Y, Girardeau J P, Der Vartanian M, Martin C. The ClpE protein involved in biogenesis of the CS31A capsule-like antigen is a member of a periplasmic chaperone family in Gram-negative bacteria. FEMS Microbiol Lett. 1993;108:59–68. doi: 10.1016/0378-1097(93)90488-n. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet F, Martin C, Girardeau J-P, Méchin M-C, Der Vartanian M, Laude H, Contrepois M. CS31A capsule-like antigen as an exposure vector for heterologous antigenic determinants. Infect Immun. 1994;62:2553–2561. doi: 10.1128/iai.62.6.2553-2561.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandwein H, Deutsch A, Thompson M, Giannella R. Production of neutralizing monoclonal antibodies to Escherichia coli heat-stable enterotoxin. Infect Immun. 1985;47:242–246. doi: 10.1128/iai.47.1.242-246.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpick B W, Gariépy J. Structural characterization of functionally important regions of the Escherichia coli heat-stable enterotoxin STIb. Biochemistry. 1991;30:4803–4809. doi: 10.1021/bi00233a023. [DOI] [PubMed] [Google Scholar]
- 9.Clements J D. Construction of a nontoxic fusion peptide for immunization against Escherichia coli strains that produce heat-labile and heat-stable enterotoxins. Infect Immun. 1990;58:1159–1166. doi: 10.1128/iai.58.5.1159-1166.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Der Vartanian M, Méchin M-C, Jaffeus B, Bertin Y, Félix I, Gaillard-Martinie B. Permissible peptide insertions surrounding the signal peptide-mature protein junction of the ClpG prepilin: CS31A fimbriae of Escherichia coli as carriers of foreign sequences. Gene. 1994;148:23–32. doi: 10.1016/0378-1119(94)90229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Der Vartanian M, Girardeau J-P, Martin C, Rousset E, Chavarot M, Laude H, Contrepois M. An Escherichia coli CS31A fibrillum chimera capable of inducing memory antibodies in outbred mice following booster immunization with the entero-pathogenic coronavirus transmissible gastroenteritis virus. Vaccine. 1997;15:111–120. doi: 10.1016/S0264-410X(96)00172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Martino P, Bertin Y, Girardeau J-P, Livrelli V, Joly B, Darfeuille-Michaud A. Molecular characterization and adhesive properties of CF29K, an adhesin of Klebsiella pneumoniae strains involved in nosocomial infections. Infect Immun. 1995;63:4336–4344. doi: 10.1128/iai.63.11.4336-4344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Martino P, Girardeau J-P, Der Vartanian M, Joly B, Darfeuille-Michaud A. The central variable V2 region of the CS31A major subunit is involved in the receptor-binding domain. Infect Immun. 1997;65:609–616. doi: 10.1128/iai.65.2.609-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gariépy J, Judd A K, Schoolnick G K. Importance of disulfide bonds in the structure and activity of Escherichia coli enterotoxins ST1b. Proc Natl Acad Sci USA. 1987;84:8907–8911. doi: 10.1073/pnas.84.24.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannella R A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976;14:95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girardeau J-P, Der Vartanian M, Ollier J L, Contrepois M. CS31A, a new K88-related fimbrial antigen on bovine enterotoxigenic and septicemic Escherichia coli strains. Infect Immun. 1988;56:2180–2188. doi: 10.1128/iai.56.8.2180-2188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girardeau J-P, Bertin Y, Martin C, Der Vartanian M, Boeuf C. Sequence analysis of the clpG gene, which codes for surface antigen CS31A subunit: evidence of an evolutionary relationship between CS31A, K88, and F41 subunit genes. J Bacteriol. 1991;173:7673–7683. doi: 10.1128/jb.173.23.7673-7683.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg R N, Guerrant R L. E. coli heat-stable enterotoxin. Pharmacol Ther. 1981;13:507–531. doi: 10.1016/0163-7258(81)90027-9. [DOI] [PubMed] [Google Scholar]
- 19.Guzman-Verduzco L-M, Kupersztoch Y M. Rectification of two Escherichia coli heat-stable enterotoxin allele sequences and lack of biological effect of changing the carboxy-terminal tyrosine to histidine. Infect Immun. 1989;57:645–648. doi: 10.1128/iai.57.2.645-648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman-Verduzco L-M, Kupersztoch Y M. Export and processing analysis of a fusion between the extracellular heat-stable enterotoxin and the periplasmic B subunit of the heat-labile enterotoxin in Escherichia coli. Mol Microbiol. 1990;4:253–264. doi: 10.1111/j.1365-2958.1990.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 21.Joly J C, Leung W S, Swartz J R. Overexpression of Escherichia coli oxidoreductases increases recombinant insulin-like growth factor-I accumulation. Proc Natl Acad Sci USA. 1998;95:2773–2777. doi: 10.1073/pnas.95.6.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi T, Kishigami S, Sone M, Inokuchi H, Mogi T, Ito K. Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc Natl Acad Sci USA. 1997;94:11857–11862. doi: 10.1073/pnas.94.22.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi T, Ito K. Respiratory chain strongly oxidizes the CXXC motif of DsbB in the Escherichia coli disulfide bond formation pathway. EMBO J. 1999;18:1192–1198. doi: 10.1093/emboj/18.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Low D, Braaten B, Van Der Woude M. Fimbriae. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 146–157. [Google Scholar]
- 25.Löwenadler B, Lake M, Elmblad A, Holmgren E, Holmgren J, Karlström A, Svennerholm A-M. A recombinant Escherichia coli heat-stable enterotoxin (STa) fusion protein eliciting anti-STa neutralizing antibodies. FEMS Microbiol Lett. 1991;82:271–278. doi: 10.1016/0378-1097(91)90273-d. [DOI] [PubMed] [Google Scholar]
- 26.Martin C, Bœuf C, Bousquet F. Escherichia coli CS31A fimbriae: molecular cloning, expression and homology with the K88 determinant. Microb Pathog. 1991;10:429–442. doi: 10.1016/0882-4010(91)90108-m. [DOI] [PubMed] [Google Scholar]
- 27.Méchin M-C, Der Vartanian M, Martin C. The major subunit ClpG of Escherichia coli CS31A fibrillae as an expression vector for different combinations of two TGEV coronavirus epitopes. Gene. 1996;179:211–218. doi: 10.1016/S0378-1119(96)00348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukhopadhyay A. Reversible protection of disulfide bonds followed by oxidative folding render recombinant hCGβ highly immunogenic. Vaccine. 2000;18:1802–1810. doi: 10.1016/s0264-410x(99)00482-x. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto K, Takahara M. Synthesis of Escherichia coli heat-stable enterotoxin STp as a pre-pro form and role of the pro sequence in secretion. J Bacteriol. 1990;172:5260–5265. doi: 10.1128/jb.172.9.5260-5265.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasheed J K, Guzman-Verduzco L-M, Kupersztoch Y M. Hyperproduction of heat-stable enterotoxin (STA4) of Escherichia coli and analysis of the unusual electrophoretic behavior of reduced and alkylated forms of STAs. Microb Pathog. 1988;5:333–343. doi: 10.1016/0882-4010(88)90034-4. [DOI] [PubMed] [Google Scholar]
- 31.Rasheed J K, Guzman-Verduzco L-M, Kupersztoch Y M. Two precursors of the heat-stable enterotoxin of Escherichia coli: evidence of extracellular processing. Mol Microbiol. 1990;4:265–273. doi: 10.1111/j.1365-2958.1990.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 32.Saarilahti H T, Palva E T, Holmgren J, Sanchez J. Fusion of genes encoding Escherichia coli heat-stable enterotoxin and outer membrane protein OmpC. Infect Immun. 1989;57:3663–3665. doi: 10.1128/iai.57.11.3663-3665.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez J, Hirst T R, Uhlin B E. Hybrid enterotoxin LTA::STa proteins and their protection from degradation by in vivo association with B-subunits of Escherichia coli heat-labile enterotoxin. Gene. 1998;64:265–275. doi: 10.1016/0378-1119(88)90341-1. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez J, Johansson S, Löwenadler B, Svennerholm A-M, Holmgren J. Recombinant cholera toxin B subunit and gene fusion proteins for oral vaccination. Res Microbiol. 1990;141:971–979. doi: 10.1016/0923-2508(90)90137-f. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez J, Solorzano R M, Holmgren J. Extracellular secretion of STa heat-stable enterotoxin by Escherichia coli after fusion to a heterologous leader peptide. FEBS Lett. 1993;330:265–269. doi: 10.1016/0014-5793(93)80885-x. [DOI] [PubMed] [Google Scholar]
- 36.Schulz S, Green C K, Yuen P S T, Garbers D L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990;63:941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 37.Scotland S M, Willshaw G A, Said B, Smith H R, Rowe B. Identification of Escherichia coli that produces heat-stable enterotoxin STA by a commercially available enzyme-linked immunoassay and comparison of the assay with infant mouse and DNA probe tests. J Clin Microbiol. 1989;27:1697–1699. doi: 10.1128/jcm.27.7.1697-1699.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.So M, McCarthy B J. Nucleotide sequence of the bacterial transposon Tn1681 encoding a heat-stable (ST) toxin and its identification in enterotoxigenic Escherichia coli strains. Proc Natl Acad Sci USA. 1980;77:4011–4015. doi: 10.1073/pnas.77.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soto G E, Dodson K W, Ogg D, Liu C, Heuser J, Knight S, Kihlberg J, Jones C H, Hultgren S J. Periplasmic chaperone recognition motif of subunits mediates quaternary interactions in the pilus. EMBO J. 1998;17:6155–6167. doi: 10.1093/emboj/17.21.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda T, Nair G B, Suzuki K, Xiao Zhe H, Yokoo Y, De Mol P, Hemelhof W, Butzler J P, Takeda Y, Shimonishi Y. Epitope mapping and characterization of antigenic determinants of heat-stable enterotoxin (STh) of enterotoxigenic Escherichia coli by using monoclonal antibodies. Infect Immun. 1993;61:289–294. doi: 10.1128/iai.61.1.289-294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van der Zee A, Noordegraaf C V, van den Bosch H, Gielen J, Bergmans H, Hoekstra W, van Die I. P-fimbriae of Escherichia coli as carriers for gonadotropin releasing hormone: development of a recombinant contraceptive vaccine. Vaccine. 1995;13:753–758. doi: 10.1016/0264-410x(94)00039-p. [DOI] [PubMed] [Google Scholar]
- 42.Yamanaka H, Fuke Y, Hitotsubashi S, Fujii Y, Okamoto K. Functional properties of pro region of Escherichia coli heat-stable enterotoxin. Microbiol Immunol. 1993;37:195–205. doi: 10.1111/j.1348-0421.1993.tb03200.x. [DOI] [PubMed] [Google Scholar]
- 43.Yamanaka H, Kameyama M, Baba T, Fujii Y, Okamoto K. Maturation pathway of Escherichia coli heat-stable enterotoxin I: requirement of DsbA for disulfide bond formation. J Bacteriol. 1994;176:2906–2913. doi: 10.1128/jb.176.10.2906-2913.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamanaka H, Nomura T, Fujii Y, Okamoto K. Extracellular secretion of Escherichia coli heat-stable enterotoxin I across the outer membrane. J Bacteriol. 1997;179:3383–3390. doi: 10.1128/jb.179.11.3383-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y, Gao Z, Guzman-Verduzco L-M, Tachias K, Kuperztoch Y M. Secretion of the STA3 heat-stable enterotoxin of Escherichia coli: extracellular delivery of pro-STA is accomplished by either pro or STA. Mol Microbiol. 1992;6:3521–3529. doi: 10.1111/j.1365-2958.1992.tb01787.x. [DOI] [PubMed] [Google Scholar]