Abstract
MRI is increasingly used as a diagnostic tool for visualising the dentoalveolar complex. A comprehensive review of the current indications and applications of MRI in the dental specialities of orthodontics (I), endodontics (II), prosthodontics (III), periodontics (IV), and oral surgery (V), pediatric dentistry (VI), operative dentistry is still missing and is therefore provided by the present work.
The current literature on dental MRI shows that it is used for cephalometry in orthodontics and dentofacial orthopaedics, detection of dental pulp inflammation, characterisation of periapical and marginal periodontal pathologies of teeth, caries detection, and identification of the inferior alveolar nerve, impacted teeth and dentofacial anatomy for dental implant planning, respectively. Specific protocols regarding the miniature anatomy of the dentofacial complex, the presence of hard tissues, and foreign body restorations are used along with dedicated coils for the improved image quality of the facial skull.
Dental MRI poses a clinically useful radiation-free imaging tool for visualising the dentoalveolar complex across dental specialities when respecting the indications and limitations.
Keywords: Magnetic Resonance Imaging, Dentistry, Diagnostic Imaging
Background
The complex anatomy of the dentomaxillofacial region challenges existing imaging techniques as it consists of a conglomerate of various hard and soft tissues and air- and fluid-filled cavities. Anatomical structures of primary relevance in dentistry include the maxilla, the mandible with the intraosseous course of the inferior alveolar nerve, the teeth, its root canals, the periodontal apparatus, the paranasal sinuses, as well as the nasal and the oral cavities. Metallic, ceramic, and composite foreign materials represent typical structures associated with oral restorations that place particular demands on imaging. X-ray-based techniques like panoramic radiography or cone-beam computed tomography (CBCT) are currently the imaging standard. However, MRI is increasingly used not only for head and neck imaging but also for the dentoalveolar complex. 1,2
Previously, MRI was used in the head and neck region predominantly for the temporomandibular joint, salivary glands and soft tissue pathologies. The advantages of MRI, especially when compared to radiography, are the application of non-ionising radiation and the differentiation of soft tissues. Using specific MRI protocols, bone is displayed for surgical planning in children with craniofacial disorders or patients with bone transplantation in the maxilla and mandible. 3,4 The non-ionising character allows for longitudinal and repeated examinations and imaging in children and young adults who are particularly vulnerable to cumulative risks of ionising radiation. 5–12 Contraindications for MRI must be regarded, especially with high field strengths; however, dental materials and orthodontic braces are not primarily a contraindication concerning MR safety but a source of artefacts that deteriorate image quality. 13,14
Dental practitioners are not provided with information on which indication MRI proves beneficial and have limited access to facilities located in clinics and imaging centres. Nevertheless, efforts have been made to establish MRI in dentistry by introducing specified sequences and equipment for dental imaging with reduced acquisition time and lower costs. The term dental MRI has been adopted; however, it does not refer to a specific imaging sequence or dedicated coil. Dental MRI is a collective term for the aim to focus on specific indications relevant to dentistry by using either standard or adapted imaging protocols as well as standard and dedicated coils for the dentomaxillofacial region. The used MRI systems include various magnetic field strengths. So far, there is no MRI system for the imaging solely of the dentomaxillofacial region. Previous literature review addressed technical specifications and intra- and extraoral coils forMR dental imaging, neurography of the trigemenial nerve and MR imaging of the temporomandibular joint. 15–18
To date, comprehensive knowledge of specifications and image analysis for the dentoalveolar region is missing. The present review aims to comprise information on indicationsfor MRI in the dental specialities of orthodontics (I), endodontics (II), prosthodontics (III), periodontics (IV), and oral surgery (V), paediatric dentistry (VI), operative dentistry.
The current literature on the application of MRI for the dentofacial complex was regarded with a focus on clinical studies and case reports. A systematic search was performed with the focused question “When is MRI used for diagnosis in dental specialties?” on PubMed MEDLINE and Google Scholar databases using MeSH terms and keywords relevant to the focused question. A publication time frame between 2010 and 2022 was selected. An additional hand search was performed in the following journals: Dentomaxillofacial Radiology, European Radiology, Journal of Craniomaxillofacial Surgery, Oral Surgery, Oral Medicine, Oral Pathology, and Oral Radiology.
This narrative review should provide the reader with comprehensive information on the advantages and limitations of MRI in dentistry.
Orthodontics and dentofacial orthopedics
MRI was reported for cephalometry by various authors. 19–27 The requirements are a large acquisition volume to include all relevant landmarks of the skull, the teeth, and the soft tissue profile and a short acquisition time, as the respective patient group might not tolerate long acquisition times. Dental restorations causing image artefacts are not frequent in the young age group; however, orthodontic appliances might be present. Steel (orthodontic) appliances cause artefacts that deteriorate image quality and may be considered a contraindication for MRI. 28,29 The use of contrast agents to show the vascularisation of tissues is redundant in this indication. Figure 1 shows MRI in comparison to CBCT data for the assessment of anatomical landmarks for cephalometry. Table 1 gives an overview of studies on MRI for treatment planning in orthodontics and dentofacial orthopaedics.
Figure 1.
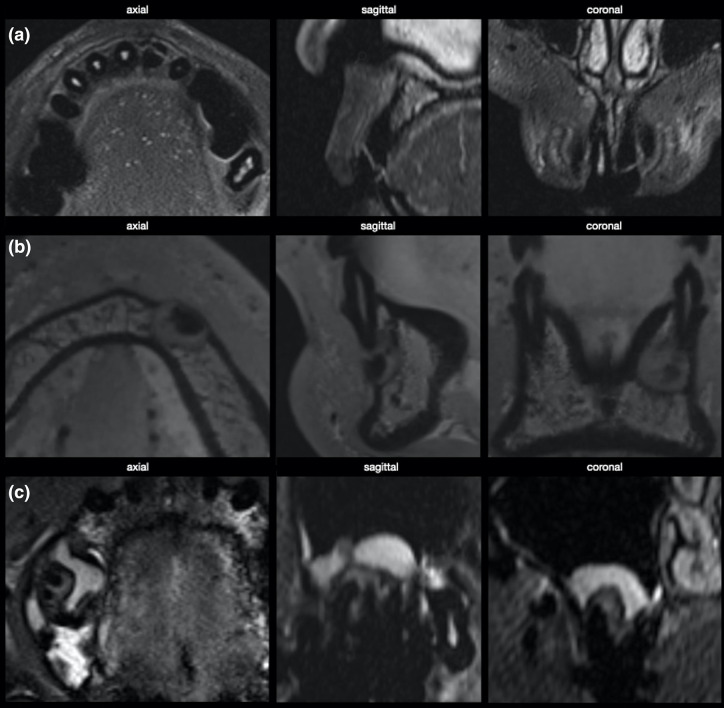
MRI of different indications in endodontics. (a) Horizontal fracture of tooth 21 with loss of MR signal in the necrotic pulp. (a) Apical tumor of tooth 33 with calcified central aspect and surrounding osteolysis. (c) Apical granuloma of tooth 16, accompanied by hyperplasia of the basal sinus membrane.
Table 1.
MRI for treatment planning in orthodontics and dentofacial orthopaedics. Technical information is given in Supplementary Table 1.
| Authors | Study design/subjects | Research question | Findings |
|---|---|---|---|
| Eley et al. 2013 19 | 8 patients | Comparison of cephalometric measurements on MRI and LCR; ease of landmark identification |
|
| Heil et al. 2017 20 | 20 participants, 13.95 ± 5.34 y (8-26) | Comparison of cephalometric measurements in MRI and LCR |
|
| Juerchott, Freudelsperger, Zingler et al. 2019 21 | 16 participants (23.3 ± 7.5 y) | Reliability of landmark identification in MRI |
|
| Juerchott, Saleem, Hilgenfeld et al. 2018 22 | Three volunteers | Accuracy and reproducibility of cephalometric measurements on MRI |
|
| Juerchott, Freudelsperger, Weber et al. 2019 23 | 12 participants (26 ± 6.6 y) | Comparison of cephalometric measurements in MRI and CBCT |
|
| Maspero et al. 2019 24 | 18 participants (37.8 ± 10.2 y) | Comparison of cephalometric measurements in MRI and CBCT |
|
| Jency et al. 2019 25 | 11 participants (18–30 y) | Comparison of cephalometric measurements in MRI and LCR |
|
| Grandoch et al. 2019 26 | 12 participants (44 ± 16.2 y) | Identification of anatomical landmarks in MRI and CBCT |
|
| Kupka et al. 2022 27 | 10 participants 13 y (2-16) | Accuracy of cephalometric measurements in black bone MRI |
|
CBCT, cone-beam CT; ICC, intraclass correlation coefficient; LCR, lateral cephalometric radiography.
Endodontics and paediatric dentistry
Recent studies on MRI in endodontic indications reached high image resolutions of around 0.7 mm3 30–32 (Table 2). The vascularisation of the dental pulp and differentiation between a healthy and inflamed pulp were displayed without contrast agents. 30,31 However, signal enhancement in the dental pulp using contrast agents was discussed as a potentially valuable diagnostic tool and used more recently as a measurement for the healthy and inflamed pulp by Juerchott et al. 33 Hyperperfusion of the pulp correlated with a high signal, and T2-values related to a presumed inflammation adjacent to caries were mapped using incremental echo times. 31 The degree of perfusion of the dental pulp correlated with the signal in MRI, and terminated perfusion and pulp necrosis show no signal. 30,31,34
Table 2.
MRI for the display of the dental pulp. Technical information is given in Supplementary Table 2.
| Authors | Study design/subjects | Research question | Findings |
|---|---|---|---|
| Assaf et al. 2015 30 | Seven participant (8–17 y), 12 teeth | Visualisation and measurement of revitalisation of the dental pulp after dental trauma using MRI; comparison of signal intensity of trauma affected and non-affected teeth |
|
| Cankar et al. 2020 31 | 12 participants (34.4 + −7.3 y), 72 teeth | Quantification of dental pulp signal in teeth with caries; correlation between signal and extent of caries lesion |
|
| Juerchott et al. 2021 33 | 70 participants (three cohorts: 27.5 ± 3.1, 42.2 ± 11.6, 44.1 ± 14.6 y), 1585 teeth | Investigation of PCE patterns in dMRI in healthy teeth |
|
| Tesfai et al. 2022 32 | Five participants | Comparison of intraoral coil with conventional head and surface coils and CBCT in terms of SNR and visibility |
|
CBCT, cone-beam CT; PCE, pulpal contrast enhancement; SNR, signal-to-noise-ratio.
MRI may detect periapical inflammation at early stages due to oedema and subsequent signal enhancement, even without demineralisation or bone resorption. 34 In the case of periapical granulomas or cysts, a signal hyperintensity appears in MRI, contrary to radiolucency in CBCT. 35 Several image characteristics in MRI, including signal intensity, signal homogeneity, margins, low-intensity outline, and contrast distribution pattern, were established on the existing data to differentiate between cysts and granuloma. 36 The comparison of MRI and CBCT implies an overestimation of lesions in MRI. 37 MRI detects regions with oedema that are not visible in CBCT; therefore, MRI’s more accurate representation is hypothesised. 38–40 The referenced studies mostly did not use contrast agents to display periapical lesions. 35,37,38,40 Juerchott et al applied a contrast agent and assessed no predictable characterisation with non-contrast agent T1-sequences, whereas contrast-enhanced T1- and T2-sequences yielded differentiation of peripheral and central parts of each lesion. 39 Figure 1 presents three different indications for MRI in endodontics with a tooth fracture and pulp necrosis, an apical tumor in region 43 and an apical granuloma and sinus membrane swelling in region 16. Table 3 gives an overview of MRI studies on the periapical region.
Table 3.
Studies on the use of MRI for the display of the periapical region. Technical information is given in Supplementary Table 3.
| Authors | Study design/subjects | Research question | Findings |
|---|---|---|---|
| Geibel et al. 2015 37 | 19 participants (43 +- 13 y), 34 teeth | Applicability of MRI for the assessment of periapical lesions and individual comparison of MRI and CBCT findings |
|
| Geibel et al. 2017 38 | 13 participants (41 +- 27 y), 15 teeth | Assessment of periapical lesions and characterisation of lesions with MRI using different contrast weightings; correlation with histopathology |
|
| Juerchott et al. 2018 39 | 11 participants (mean 39.5 y, range 21–60 y), 11 teeth | Assessment and characterisation of periapical lesions with MRI using different contrast weightings and contrast agent, correlation with histopathology |
|
| Lizio et al. 2018 36 | 34 patients | Diagnostic reliability and accuracy of MRI for periapical lesions, correlation with histopathology |
|
| Pigg et al. 2014 40 | 20 patients (mean 52, range 34–65 y) | Assessment of signal changes in MRI in patients with atypical odontalgia and correlation of MRI and CBCT |
|
| Cassetta et al. 2012 35 | 10 patients (mean age: 38.8 y, range 21–63 y) | Assessment of MRI for intraosseous pathological findings, characterisation of MRI findings and correlation to histopathology |
|
CBCT, cone beam CT; 3D CISS, three-dimensional constructive interference in steady state.
Prosthodontics: caries detection
Caries is delineatedin MRI with a hyperintense signal due to its porous character and the infiltration of liquid. 41 Due to the lack of a gold-standard for measuring caries lesions, the congruence of its presentation in MRI and its actual size has not been studied. One study addressed the use of MRI for caries diagnosis (Table 4).
Table 4.
Studies on the use of MRI for the display of caries lesions. Technical information is given in Supplementary Table 4.
| Authors | Study design/subjects | Research question | Findings |
|---|---|---|---|
| Bracher et al. 2013 41 | 40 participants (161 lesions) | Is UTE MRI clinically applicable for the identification of caries lesions? |
|
TSE, turbo spin echo; UTE, ultrashort echo-time.
Periodontics
Four clinical studies used MRI and contrast agents to display marginal periodontal structures (Table 5). 42–45 Ruetters et al measured the marginal attachment in MRI and periapical radiographs using a contrast agent. Juerchott et al reported molar teeth' bone support and furcation involvement in CBCT and MRI. 41,42 A clinical study using a dedicated surface coil for dental imaging assessed palatal mucosa thickness at several teeth using MRI. 44 Probst et al investigated the correlation between bone oedema depicted in MRI images and clinical findings in patients with generalised periodontitis. 45
Table 5.
MRI for indications in periodontics. Technical information is given in Supplementary Table 5.
| Authors | Study design/subjects | Research question | Findings |
|---|---|---|---|
| Ruetters et al. 2018 42 | 5 patients (21 teeth) | Agreement of measurements of the periodontal bone support in periapical radiographs and MRI |
|
| Juerchott et al. 2020 43 | 22 patients | Comparison of CBCT and MRI for the assessment of periodontal bone support in molar teeth (furcation involvement) |
|
| Hilgenfeld et al. 2018 44 | 5 volunteers | Reliability of MRI measurements of the thickness of the palatal mucosa |
|
| Probst et al. 2021 45 | 42 patients (28–79 y, mean 56 ± 14.6), 34 healthy control (21–32 y, mean 23 ± 1.9) | Correlation of MRI findings and clinical findings in patients with generalised periodontitis |
|
CBCT, cone-beam CT; ICC, intraclass correlation coefficient.
Oral surgery
MRI has been proposed for several indications in oral surgery. Existing studies recommended MRI to show impacted teeth (Table 6). Out of five studies, only one study compared MRI to panoramic imaging. 48 None of the studies included CBCT compared to MRI. 46–48 The largest group comprised 59 patients, in which impacted teeth were displayed within 5 min using surface coils. 47 None of the studies reported isotropic image resolution. For the detailed assessment of the status of the eruption of impacted teeth, a high in-plane resolution of 0.3 mm with a greater slice thickness (2 mm) was recommended over an in-plane resolution of 0.6 mm with a slice thickness of 1 mm. 49 A slice thickness of 4 mm did not allow adequate assessment of the status of the eruption of third molars. 50 In a small number of patients, imaging was not successful due to orthodontic braces and movement artefacts. None of the studies used contrast agents to delineate impacted teeth with MRI.
Table 6.
MRI for the display of impacted teeth. Technical information is given in Supplementary Table 6.
| Authors | Study design/subjects | Research question | Findings |
|---|---|---|---|
| Tymofiyeva et al. 2013 46 | 16 patients; mean age 10.8 y, range 8–15 y | Feasibility of imaging impacted teeth in children with MRI |
|
| Tymofiyeva et al. 2009 47 | 59 patients | Assessment of position and angulation of impacted teeth in children and adults with MRI |
|
| Kirnbauer et al. 2018 48 | 28 patients | Assessment of the position of third molars and expected surgical complexity of their removal in PAN and MRI |
|
| De Tobel et al. 2019 49 | 11 volunteers | Comparison of multiple MRI protocols for the assessment of apical closure of third molars |
|
| Kindler et al. 2018 50 | 1915 volunteers | Correlation of eruption status of third molars assessed using MRI and clinical measurement of periodontal apparatus of second molars |
|
3D CISS, three-dimensional constructive interference in steady state.
Depiction of the inferior alveolar nerve
Present studies used various protocols to differentiate the inferior alveolar nerve from surrounding tissues, assess the accuracy of its representation, and evaluate the occurrence of imaging artefacts due to dental restorations or implants. 51–56 Image acquisition times were between 4 and 6:30 min, and image resolution was higher than 1 mm3. Limiting the field of view achieved an isotropic image resolution of 0.5 mm3. 52
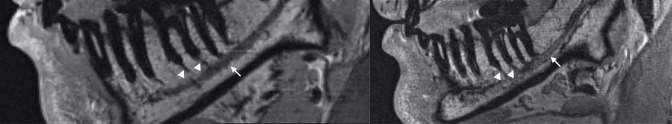
Most studies applied standard head- and neck coils to image the complete mandible and the inferior alveolar nerve on both sides and did not use contrast agents (Table 7). Figure 2 shows a sagittal view of the mandible with the inferior alveolar nerve and Rami dentales.
Table 7.
MRI to display the course of the inferior alveolar nerve. Technical information is given in Supplementary Table 7.
| Authors | Study design/subjects | Research questions | Findings |
|---|---|---|---|
| Chau et al. 2012 51 | 11 participants | Comparison of the detection of the IAN by different examiners on CBCT and MRI |
|
| Kreutner et al. 2017 52 | 7 participants | Comparison of two MRI protocols for the accuracy and reproducibility of the detection of the IAN by different examiners; accuracy of segmentation of IAN |
|
| Probst et al. 2017 53 | 7 participants | Assessment of artefact size in MRI using different sequences and display of IAN |
|
| Deepho et al. 2017 55 | 49 patients | Comparison of detection of IAN in fusion images MRI/CT and CT images as assessed by different examiners |
|
| Beck et al. 2021 57 | 53 patients | Comparison of detection of IAN and third molars in MRI and CT/CBCT by different examiners |
|
| Al/Haj Husain et al. 2021 56 | 19 patients (30.5 ± 13 y) | Evaluation of intraosseus position of IAN using MRI (3D-DESS) |
|
CBCT, cone-beam CT; 3D VIBE, three-dimensional volumetric interpolated breath-hold examination; IAC, internal auditory canal; IAN, inferior alveolar nerve; SEMAC, slice-encoding for metal artefact correction.
Figure 2.
Display of inferior alveolar nerve (white arrows) with Rami dentales (white triangles) in region 36.
Implant planning
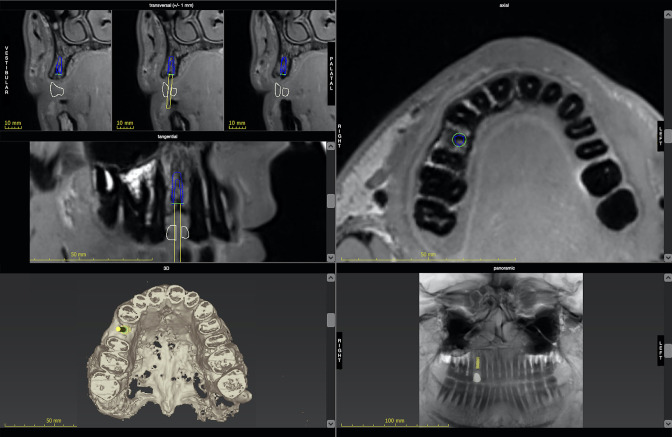
One case series and one pilot study reported software-based dental implant planning and fully guided implant placement using drill guides, 58,59 and another study reported partially guided MRI based implant surgery. 60 The comparison of CBCT and MRI for virtual implant planning was performed without the actual guided implant placement. 61 A further recent study confirmed a high interrater agreement for MRI-based implant planning and high agreement for MRI and CBCT for this indication. 62 Imaging protocols featured an isotropic image resolution of 0.5 mm3 without the application of contrast agent, useful for software-based planning and individual inspection of implant sites. 60 The deviations between planned and realised implant position based on MRI were comparable to the protocol based on CBCT. 59 Results for dental implant planning based on MRI are shown in Table 8. Figure 3presents an example for dental implant planning in region 14 with the display of the prospective implant in transversal, sagittal, axial and panoramic reconstructions.
Table 8.
MRI for dental implant planning. Technical information is given in Supplementary Table 8.
| Authors | Study design/subjects | Research question | Findings |
|---|---|---|---|
| Flügge et al. 2020 58 | 5 patients | Feasibility of dental implant planning using CAD/CAM processes based on MRI |
|
| Hilgenfeld et al. 2020 62 | 30 patients | Accuracy and reliability of dental implant planning based on MRI, comparison to dental implant planning based on CBCT |
|
| Probst et al. 2020 59 | 12 patients | Feasibility of dental implant planning using CAD/CAM processes based on MRI, comparison of planned and actual implant positions |
|
| Schwindling et al. 2021 60 | 27 patients, 41 implants | Accuracy of PIGS based on MRI |
|
| Grandoch et al. 2021 61 | 16 patients, 22 implants | Comparison of MRI and CBCT-based dental implant planning |
|
CBCT, cone-beam CT; PIGS, partially guided dental implant surgery.
Figure 3.
Dental implant planning based on MRI with transversal, tangential and axial cross-sections, panoramic reconstruction, and 3D rendering. Note the missing dental surfaces in the 3D image based on grey values that are reversed in comparison to CBCT. 3D, three-dimensional; CBCT, cone-beam CT.
Discussion
MRI is vastly used to display the dentoalveolar process across various indications and specialties in dentistry. In orthodontics and dentofacial orthopedics, specific MR surface coils may be used for cephalometry prior to and after orthodontic treatment and is not useful during treatment with fixed appliances. 19,26 A large image volume including facial bones and the soft tissue profile is acquired within 5–7 min using specific surface coils for the facial skull. 20–23 High reliability of landmark measurements on MRI in different head positions, high intra- and interrater reliability, and equivalence of MRI and CBCT for cephalometry were reported with different protocols. 20–23 When lower image resolution (larger voxel sizes) was reported, the identification of landmarks was inferior to CBCT. 19,24–26 Landmarks on the anterior teeth may not be visible when the lips are not in contact during imaging; however, none of the articles elaborated on this detail. The radiologist should be trained in interpreting MRI to perform accurate landmark definition. 24 The routine use of MRI for cephalometry is limited by the availability of MRI systems, specific hardware and imaging protocols, and trained radiologists for these findings.
In endodontics, a small image volume of one to three teeth, including the periapical area and a high image resolution showing the delicate and ramified anatomy of the pulp are required. Specific intraoral coils enable a high image resolution of around 0.3 mm3 for two to three teeth and might be particularly interesting for endodontic indications; however, they have not been used in this specific field. 63,64 Studies published until 2007 have two major constraints. Image resolution was low (<1.0 mm3), and a contrast agent was used to observe signal intensity in the dental pulp. 65,66
In vivo studies focused on the age-related perfusion and the detection of pulp vitality and reperfusion after tooth replantation and transplantation, respectively. 30,65,66 High signal intensity was correlated with a perfused, vital pulp and no signal with pulp necrosis. As a differential diagnosis, a hyperintense signal indicates an inflammation. Recently, one article on the characterisation of pulp signal in MRI in the presence of caries lesions was published. 31
In summary, the degree of perfusion of the dental pulp correlates with the signal in MRI. 65,66 Physiological perfusion shows MR signal enhancement after administration of contrast agent. 63 The signal enhancement correlates with the perfusion level; however, the interpretation is difficult due to missing reference data. Further in vivo studies are therefore required for image characterisation of an inflammation.
Periapical lesions include granuloma, radicular cysts, or other tumorous processes. Whereas granuloma may completely recede after root canal treatment, cysts or tumours must be surgically resected. Periapical lesions are diagnosed with good diagnostic accuracy using panoramic or intraoral radiographs when the demineralisation is extended in cancellous bone or has reached the buccal and oral cortical bone plate. 67,68 At an earlier stage, periapical lesions might be present; however, not accessible to routine radiographic imaging. As an alternative to two-dimensional radiography, CBCT may be used for a three-dimensional assessment of periapical demineralisation. The character of the tissue or lesion substituting for bone, may not be identified with CBCT unless it contains mineralised parts that are displayed radiographically. Furthermore, due to cost and radiation exposure, CBCT is not routinely performed to detect a periapical focus.
MRI allows for a more detailed characterisation of periapical lesions. 37,38 For differentiation of a granuloma and radicular cysts, either contrast-enhanced T 1- or T 2 weighted images have been advocated. 39 Several authors could verify diagnoses in MR images with histopathological analysis and reported the high correlation of findings. 35,36,38,39
Caries is diagnosed clinically and radiographically using the periapical radiograph or bitewing technique. Clinical diagnosis may not deliver information on the full extent of a carious lesion. Imaging of caries requires a high image resolution in a relatively small image volume. Bitewing or periapical radiographs are prone to overlaying structures; however, they deliver a high image resolution. MRI could complement routine radiographic imaging due to its property to account for inflammatory processes. An inflammation of the pulp in correlation to a carious lesion could be demonstrated. A high image resolution was only fulfiled in one study using a self-built intraoral coil that is not commercially available. 69 The costly hardware requirements for MRI and the lack of a proven and applicable protocol to display caries restrict its use in this indication.
The marginal bone level and its pathological recession are observed for the diagnosis of periodontal diseases. Findings are mainly collected clinically; however, panoramic radiographs substantiate the diagnosis. In specific cases of attachment loss, CBCT may be used to display defect configuration. 70 A large acquisition volume covering the maxilla and mandible is useful for image diagnosis. Not only anatomical structures but also the inflammatory status of the tissue is regarded. MRI could, therefore, be a valuable tool for the diagnosis of periodontal disease.
The visibility of periodontal structures and their dimensionally accurate delineation has been shown in cadaver porcine mandibles. 67 Clinical studies have shown that inflammatory periodontal disease might be detected using a contrast agent, and measurements of periodontal defects may be performed in MRI. 42,43 The comparability of clinical measurements and tomographic imaging, MRI and CBCT, is lacking, as the cemento-enamel-junction as a clinical landmark for measurement of attachment loss is not shown with MRI. 42
To display impacted teeth, the inferior alveolar nerve, and allow for dental implant planning, the coverage of a large image volume, including the complete maxilla and mandible, is required. For surgical planning, multiplanar reconstruction centering on the region of interest is commonly used and requires an isotropic image resolution.
The patient group for indications in oral surgery includes all ages; however, dental restorations causing image artefacts are more prevalent in patients intended for dental implant therapy and may impair the accurate transfer of planning to the surgical site. 71 Depiction of the inferior alveolar nerve in case of iatrogenic injury often includes artefact-causing foreign materials such as dental implants. Imaging of impacted teeth may as well be impaired by dental restorations or orthodontic braces in younger age groups.
Impacted teeth are imaged pre-operatively to identify their location and neighbouring anatomical structures. Three-dimensional imaging is advised in cases of impaction and proximity to relevant anatomical structures, e.g. vessels, nerves, and neighbouring periodontal ligaments around dental roots, especially the intraosseous course of the inferior alveolar nerve. MRI was considered suitable for the visualisation of impacted third molars. To date, there is no study comparing CBCT as the clinical standard for pre-operative tomographic imaging with MRI. 46–48,56
Several studies suggest that MRI might be more adequate for imaging the inferior alveolar nerve compared to CBCT of the mandibular canal. 51,53,55,57 The intraosseous nerve course is diagnosed pre-operatively in surgical procedures of the mandible and post-operatively if complications and paresthesia have occurred. 54 Radiographic imaging cannot depict the nerve, only indirectly in cases of intraosseous course, e.g. the mandibular canal. In case of its indistinct delineation in radiographic images, MRI may serve as an alternative as it directly depicts nerve and accompanying vessels.
Diagnostic imaging for dental implant planning is performed to assess bone dimensions in the planned implant region. Dental implant planning is performed virtually using dedicated software. Therefore, image resolution and format should regard the use of dental implant planning software. The first report of MRI for dental implant planning was already in the 1990s. 72 Until 2010, MRI for dental implant planning featured low resolution and long acquisition time, and images were viewed but not used with dental implant planning software. 72–74
The available reports performed dental implant planning with MRI and compared it to the clinical standard of CBCT 58–62 (Table 4). One clinical study reported the implant position’s accuracy. 59 Systematic clinical studies on the use of MRI for dental implant planning and the accuracy of its transfer using guided implant surgery are not yet available.
Conclusions
In summary, MRI is applicable to a broad spectrum of indications in dentomaxillofacial imaging as an alternative to conventional radiography. Using specific surface coils for dental imaging or otherwise designed surface coils has helped achieve high image resolution within acceptable acquisition times.The image resolution of MRI is comparable with CBCT for in-plane resolution. However, for an isotropic image resolution, 0.4 mm3 is currently the threshold value.
Shorter acquisition times and specific hardware for dental imaging have furthermore helped to reduce the occurrence of motion artefacts and enabled the use of MRI in clinical practice.
The future of dental MRI in clinical application is challenged by its limited availability and high cost. Therefore, technical developments for short scanning times using simple and inexpensive equipment that sustain the demands for dental imaging are required.
Key points
MRI is used for imaging in dentistry for various indications.
Specific hardware and imaging protocols are used for MR imaging of the dentomaxillofacial region to achieve optimal image quality.
Significant limitations of dental MRI are image artefacts caused by dental restorations and the restricted availability of MRI systems.
Footnotes
Funding: Open Access funding enabled and organized by Projekt DEAL.
Authors’ contributions: TF and KN analyzed data and designed the manuscript. TF, CG, UL, JH, SN, MH, and KN contributed to the writing, reviewing, and editing of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Tabea Flügge, Email: tabea.fluegge@charite.de.
Christian Gross, Email: christian.gross@uniklinik-freiburg.de.
Ute Ludwig, Email: Ute.Ludwig@uniklinik-freiburg.de.
Johanna Schmitz, Email: johanna.schmitz@charite.de.
Susanne Nahles, Email: susanne.nahles@charite.de.
Max Heiland, Email: max.heiland@charite.de.
Katja Nelson, Email: katja.nelson@uniklinik-freiburg.de.
REFERENCES
- 1. Niraj LK, Patthi B, Singla A, Gupta R, Ali I, Dhama K, et al. Mri in dentistry- a future towards radiation free imaging-systematic review. J Clin Diagn Res 2016; 10: ZE14–19. doi: 10.7860/JCDR/2016/19435.8658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakamura T. Dental MRI: a road beyond CBCT. Eur Radiol 2020; 30: 6389–91. doi: 10.1007/s00330-020-07321-7 [DOI] [PubMed] [Google Scholar]
- 3. Lu A, Gorny KR, Ho ML. Zero te MRI for craniofacial bone imaging. AJNR Am J Neuroradiol 2019; 40: 1562–66. doi: 10.3174/ajnr.A6175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flügge T, Ludwig U, Amrein P, Kernen F, Vach K, Maier J, et al. Mri for the display of autologous onlay bone grafts during early healing-an experimental study. Dentomaxillofac Radiol 2021; 50: 20200068. doi: 10.1259/dmfr.20200068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stratis A, Zhang G, Jacobs R, Bogaerts R, Bosmans H. The growing concern of radiation dose in paediatric dental and maxillofacial CBCT: an easy guide for daily practice. Eur Radiol 2019; 29: 7009–18. doi: 10.1007/s00330-019-06287-5 [DOI] [PubMed] [Google Scholar]
- 6. Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012; 380: 499–505. doi: 10.1016/S0140-6736(12)60815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang WY, Muo CH, Lin CY, Jen YM, Yang MH, Lin JC, et al. Paediatric head CT scan and subsequent risk of malignancy and benign brain tumour: a nation-wide population-based cohort study. Br J Cancer 2014; 110: 2354–60. doi: 10.1038/bjc.2014.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krille L, Dreger S, Schindel R, Albrecht T, Asmussen M, Barkhausen J, et al. Risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: results from a German cohort study. Radiat Environ Biophys 2015; 54: 1–12. doi: 10.1007/s00411-014-0580-3 [DOI] [PubMed] [Google Scholar]
- 9. Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013; 346: f2360. doi: 10.1136/bmj.f2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pauwels R, Cockmartin L, Ivanauskaité D, Urbonienė A, Gavala S, Donta C, et al. Estimating cancer risk from dental cone-beam CT exposures based on skin dosimetry. Phys Med Biol 2014; 59: 3877–91. doi: 10.1088/0031-9155/59/14/3877 [DOI] [PubMed] [Google Scholar]
- 11. Aanenson JW, Till JE, Grogan HA. Understanding and communicating radiation dose and risk from cone beam computed tomography in dentistry. J Prosthet Dent 2018; 120: 353–60: S0022-3913(18)30083-0. doi: 10.1016/j.prosdent.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 12. Yeh JK, Chen CH. Estimated radiation risk of cancer from dental cone-beam computed tomography imaging in orthodontics patients. BMC Oral Health 2018; 18(1): 131. doi: 10.1186/s12903-018-0592-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dill T. Contraindications to magnetic resonance imaging: non-invasive imaging. Heart 2008; 94: 943–48. doi: 10.1136/hrt.2007.125039 [DOI] [PubMed] [Google Scholar]
- 14. Nordbeck P, Ertl G, Ritter O. Magnetic resonance imaging safety in pacemaker and implantable cardioverter defibrillator patients: how far have we come? Eur Heart J 2015; 36: 1505–11. doi: 10.1093/eurheartj/ehv086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demirturk Kocasarac H, Geha H, Gaalaas LR, Nixdorf DR. Mri for dental applications. Dent Clin North Am 2018; 62: 467–80: S0011-8532(18)30021-1. doi: 10.1016/j.cden.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 16. Van der Cruyssen F, Croonenborghs T-M, Renton T, Hermans R, Politis C, Jacobs R, et al. Magnetic resonance neurography of the head and neck: state of the art, anatomy, pathology and future perspectives. Br J Radiol 2021; 94(1119): 20200798. doi: 10.1259/bjr.20200798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van der Cruyssen F, Peeters F, Croonenborghs T-M, Fransen J, Renton T, Politis C, et al. A systematic review on diagnostic test accuracy of magnetic resonance neurography versus clinical neurosensory assessment for post-traumatic trigeminal neuropathy in patients reporting neurosensory disturbance. Dentomaxillofac Radiol 2021; 50(1): 20200103. doi: 10.1259/dmfr.20200103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Orhan K, Seki U, Rozylo-Kalinowska I. Diagnostic accuracy of magnetic resonance imaging and clinical signs of temporomandibular joint disorders: a 10-year research update review. Oral Radiol 2017; 33: 81–91. doi: 10.1007/s11282-017-0278-8 [DOI] [Google Scholar]
- 19. Eley KA, Watt-Smith SR, Golding SJ. “ black bone ” MRI: a potential non-ionizing method for three-dimensional cephalometric analysis -- a preliminary feasibility study. Dentomaxillofac Radiol 2013; 42(10): 20130236. doi: 10.1259/dmfr.20130236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heil A, Lazo Gonzalez E, Hilgenfeld T, Kickingereder P, Bendszus M, Heiland S, et al. Lateral cephalometric analysis for treatment planning in orthodontics based on MRI compared with radiographs: a feasibility study in children and adolescents. PLoS One 2017; 12(3): e0174524. doi: 10.1371/journal.pone.0174524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juerchott A, Freudlsperger C, Zingler S, Saleem MA, Jende JME, Lux CJ, et al. In vivo reliability of 3D cephalometric landmark determination on magnetic resonance imaging: a feasibility study. Clin Oral Investig 2020; 24: 1339–49. doi: 10.1007/s00784-019-03015-7 [DOI] [PubMed] [Google Scholar]
- 22. Juerchott A, Saleem MA, Hilgenfeld T, Freudlsperger C, Zingler S, Lux CJ, et al. 3D cephalometric analysis using magnetic resonance imaging: validation of accuracy and reproducibility. Sci Rep 2018; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Juerchott A, Freudlsperger C, Weber D, Jende JME, Saleem MA, Zingler S, et al. In vivo comparison of MRI- and CBCT-based 3D cephalometric analysis: beginning of a non-ionizing diagnostic era in craniomaxillofacial imaging? Eur Radiol 2020; 30: 1488–97. doi: 10.1007/s00330-019-06540-x [DOI] [PubMed] [Google Scholar]
- 24. Maspero C, Abate A, Bellincioni F, Cavagnetto D, Lanteri V, Costa A, Farronato M(2019) Comparison of a tridimensional cephalometric analysis performed on 3T-MRI compared with CBCT: a pilot study in adults. Prog Orthod 20(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jency AA, Krithika CL, Kannan A (2019) Perfomance of black bone mr imaging as a cephalometric tool in orthodontics. Int J Sci Res 8(9):1-5. [Google Scholar]
- 26. Grandoch A, Nestmann F, Kreppel M, Buller J, Borggrefe J, Zirk M, et al. Comparison of MRI with dedicated head and neck signal amplification coil and cone beam computed tomography: MRI is a useful tool in diagnostics of cranio-facial growth disorders. J Craniomaxillofac Surg 2019; 47: 1827–33: S1010-5182(19)30116-7. doi: 10.1016/j.jcms.2019.07.023 [DOI] [PubMed] [Google Scholar]
- 27. Kupka MJ, Aguet J, Wagner MM, Callaghan FM, Goudy SL, Abramowicz S, et al. Preliminary experience with black bone magnetic resonance imaging for morphometry of the mandible and visualisation of the facial skeleton. Pediatr Radiol 2022; 52: 951–58. doi: 10.1007/s00247-021-05257-8 [DOI] [PubMed] [Google Scholar]
- 28. Blankenstein F, Truong BT, Thomas A, Thieme N, Zachriat C. Predictability of magnetic susceptibility artifacts from metallic orthodontic appliances in magnetic resonance imaging. J Orofac Orthop 2015; 76: 14–29. doi: 10.1007/s00056-014-0258-0 [DOI] [PubMed] [Google Scholar]
- 29. Shalish M, Dykstein N, Friedlander-Barenboim S, Ben-David E, Gomori JM, Chaushu S. Influence of common fixed retainers on the diagnostic quality of cranial magnetic resonance images. Am J Orthod Dentofacial Orthop 2015; 147: 604–9: S0889-5406(14)01152-4. doi: 10.1016/j.ajodo.2014.11.022 [DOI] [PubMed] [Google Scholar]
- 30. Assaf AT, Zrnc TA, Remus CC, Khokale A, Habermann CR, Schulze D, et al. Early detection of pulp necrosis and dental vitality after traumatic dental injuries in children and adolescents by 3-tesla magnetic resonance imaging. J Craniomaxillofac Surg 2015; 43: 1088–93: S1010-5182(15)00175-4. doi: 10.1016/j.jcms.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 31. Cankar K, Vidmar J, Nemeth L, Serša I. T2 mapping as a tool for assessment of dental pulp response to caries progression: an in vivo MRI study. Caries Res 2020; 54: 24–35. doi: 10.1159/000501901 [DOI] [PubMed] [Google Scholar]
- 32. Tesfai AS, Vollmer A, Özen AC, Braig M, Semper-Hogg W, Altenburger MJ, et al. Inductively coupled intraoral flexible coil for increased visibility of dental root canals in magnetic resonance imaging. Invest Radiol 2022; 57: 163–70. doi: 10.1097/RLI.0000000000000826 [DOI] [PubMed] [Google Scholar]
- 33. Juerchott A, Jelinek C, Kronsteiner D, Jende JME, Kurz FT, Bendszus M, et al. Quantitative assessment of contrast‐enhancement patterns of the healthy dental pulp by magnetic resonance imaging: a prospective in vivo study . Int Endodontic J 2022; 55: 252–62. doi: 10.1111/iej.13662 [DOI] [PubMed] [Google Scholar]
- 34. Assaf AT, Zrnc TA, Remus CC, Schönfeld M, Habermann CR, Riecke B, et al. Evaluation of four different optimized magnetic-resonance-imaging sequences for visualization of dental and maxillo-mandibular structures at 3 T. J Craniomaxillofac Surg 2014; 42: 1356–63: S1010-5182(14)00106-1. doi: 10.1016/j.jcms.2014.03.026 [DOI] [PubMed] [Google Scholar]
- 35. Cassetta M, Di Carlo S, Pranno N, Stagnitti A, Pompa V, Pompa G. The use of high resolution magnetic resonance on 3.0-T system in the diagnosis and surgical planning of intraosseous lesions of the jaws: preliminary results of a retrospective study. Eur Rev Med Pharmacol Sci 2012; 16: 2021–28. [PubMed] [Google Scholar]
- 36. Lizio G, Salizzoni E, Coe M, Gatto MR, Asioli S, Balbi T, et al. Differential diagnosis between a granuloma and radicular cyst: effectiveness of magnetic resonance imaging. Int Endod J 2018; 51: 1077–87. doi: 10.1111/iej.12933 [DOI] [PubMed] [Google Scholar]
- 37. Geibel MA, Schreiber ES, Bracher AK, Hell E, Ulrici J, Sailer LK, et al. Assessment of apical periodontitis by MRI: a feasibility study. Rofo 2015; 187: 269–75. doi: 10.1055/s-0034-1385808 [DOI] [PubMed] [Google Scholar]
- 38. Geibel M-A, Schreiber E, Bracher A-K, Hell E, Ulrici J, Sailer L-K, et al. Characterisation of apical bone lesions: comparison of MRI and CBCT with histological findings-a case series. Eur J Oral Implantol 2017; 10: 197–211. [PubMed] [Google Scholar]
- 39. Juerchott A, Pfefferle T, Flechtenmacher C, Mente J, Bendszus M, Heiland S, et al. Differentiation of periapical granulomas and cysts by using dental MRI: a pilot study. Int J Oral Sci 2018; 10: 17: 17. doi: 10.1038/s41368-018-0017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pigg M, List T, Abul-Kasim K, Maly P, Petersson A. A comparative analysis of magnetic resonance imaging and radiographic examinations of patients with atypical odontalgia. J Oral Facial Pain Headache 2014; 28: 233–42. doi: 10.11607/ofph.1230 [DOI] [PubMed] [Google Scholar]
- 41. Bracher AK, Hofmann C, Bornstedt A, Boujraf S, Hell E, Ulrici J, et al. Feasibility of ultra-short echo time (Ute) magnetic resonance imaging for identification of carious lesions. Magn Reson Med 2011; 66: 538–45. doi: 10.1002/mrm.22828 [DOI] [PubMed] [Google Scholar]
- 42. Ruetters M, Juerchott A, El Sayed N, Heiland S, Bendszus M, Kim TS. Dental magnetic resonance imaging for periodontal indication-a new approach of imaging residual periodontal bone support. Acta Odontol Scand 2019; 77: 49–54. doi: 10.1080/00016357.2018.1499959 [DOI] [PubMed] [Google Scholar]
- 43. Juerchott A, Sohani M, Schwindling FS, Jende JME, Kurz FT, Rammelsberg P, et al. In vivo accuracy of dental magnetic resonance imaging in assessing maxillary molar furcation involvement: a feasibility study in humans. J Clin Periodontol 2020; 47: 809–15. doi: 10.1111/jcpe.13299 [DOI] [PubMed] [Google Scholar]
- 44. Hilgenfeld T, Kästel T, Heil A, Rammelsberg P, Heiland S, Bendszus M, et al. High-Resolution dental magnetic resonance imaging for planning palatal graft surgery-a clinical pilot study. J Clin Periodontol 2018; 45: 462–70. doi: 10.1111/jcpe.12870 [DOI] [PubMed] [Google Scholar]
- 45. Probst M, Burian E, Robl T, Weidlich D, Karampinos D, Brunner T, et al. Magnetic resonance imaging as a diagnostic tool for periodontal disease: a prospective study with correlation to standard clinical findings-is there added value? J Clin Periodontol 2021; 48: 929–48. doi: 10.1111/jcpe.13458 [DOI] [PubMed] [Google Scholar]
- 46. Tymofiyeva O, Proff PC, Rottner K, Düring M, Jakob PM, Richter EJ. Diagnosis of dental abnormalities in children using 3-dimensional magnetic resonance imaging. J Oral Maxillofac Surg 2013; 71: 1159–69: S0278-2391(13)00205-X. doi: 10.1016/j.joms.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 47. Tymofiyeva O, Rottner K, Jakob PM, Richter EJ, Proff P. Three-Dimensional localization of impacted teeth using magnetic resonance imaging. Clin Oral Investig 2010; 14: 169–76. doi: 10.1007/s00784-009-0277-1 [DOI] [PubMed] [Google Scholar]
- 48. Kirnbauer B, Jakse N, Rugani P, Schwaiger M, Magyar M. Assessment of impacted and partially impacted lower third molars with panoramic radiography compared to MRI-a proof of principle study. Dentomaxillofac Radiol 2018; 47(4): 20170371. doi: 10.1259/dmfr.20170371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. De Tobel J, Parmentier GIL, Phlypo I, Descamps B, Neyt S, Van De Velde WL, et al. Magnetic resonance imaging of third molars in forensic age estimation: comparison of the Ghent and graz protocols focusing on apical closure. Int J Legal Med 2019; 133: 583–92. doi: 10.1007/s00414-018-1905-6 [DOI] [PubMed] [Google Scholar]
- 50. Kindler S, Holtfreter B, Koppe T, Mksoud M, Lucas C, Seebauer C, et al. Third molars and periodontal damage of second molars in the general population. J Clin Periodontol 2018; 45: 1365–74. doi: 10.1111/jcpe.13008 [DOI] [PubMed] [Google Scholar]
- 51. Chau A. Comparison between the use of magnetic resonance imaging and conebeam computed tomography for mandibular nerve identification. Clin Oral Implants Res 2012; 23: 253–56. doi: 10.1111/j.1600-0501.2011.02188.x [DOI] [PubMed] [Google Scholar]
- 52. Kreutner J, Hopfgartner A, Weber D, Boldt J, Rottner K, Richter E, et al. High isotropic resolution magnetic resonance imaging of the mandibular canal at 1.5 T: a comparison of gradient and spin echo sequences. Dentomaxillofac Radiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Probst M, Richter V, Weitz J, Kirschke JS, Ganter C, Troeltzsch M, et al. Magnetic resonance imaging of the inferior alveolar nerve with special regard to metal artifact reduction. J Craniomaxillofac Surg 2017; 45: 558–69: S1010-5182(17)30020-3. doi: 10.1016/j.jcms.2017.01.009 [DOI] [PubMed] [Google Scholar]
- 54. Wanner L, Ludwig U, Hövener J-B, Nelson K, Flügge T. Magnetic resonance imaging-a diagnostic tool for postoperative evaluation of dental implants: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol 2018; 125: e103–7: S2212-4403(18)30041-5. doi: 10.1016/j.oooo.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 55. Deepho C, Watanabe H, Kotaki S, Sakamoto J, Sumi Y, Kurabayashi T. Utility of fusion volumetric images from computed tomography and magnetic resonance imaging for localizing the mandibular canal. Dentomaxillofac Radiol 2017; 46: 20160383: 20160383. doi: 10.1259/dmfr.20160383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Al-Haj Husain A, Stadlinger B, Winklhofer S, Müller M, Piccirelli M, Valdec S. Mandibular third molar surgery: intraosseous localization of the inferior alveolar nerve using 3D double-echo steady-state MRI (3D-DESS). Diagnostics (Basel) 2021; 11(7): 1245. doi: 10.3390/diagnostics11071245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beck F, Austermann S, Bertl K, Ulm C, Lettner S, Toelly A, et al. Is MRI a viable alternative to CT/CBCT to identify the course of the inferior alveolar nerve in relation to the roots of the third molars? Clin Oral Investig 2021; 25: 3861–71. doi: 10.1007/s00784-020-03716-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Flügge T, Ludwig U, Hövener JB, Kohal R, Wismeijer D, Nelson K. Virtual implant planning and fully guided implant surgery using magnetic resonance imaging-proof of principle. Clin Oral Implants Res 2020; 31: 575–83. doi: 10.1111/clr.13592 [DOI] [PubMed] [Google Scholar]
- 59. Probst FA, Schweiger J, Stumbaum MJ, Karampinos D, Burian E, Probst M. Magnetic resonance imaging based computer-guided dental implant surgery-A clinical pilot study. Clin Implant Dent Relat Res 2020; 22: 612–21. doi: 10.1111/cid.12939 [DOI] [PubMed] [Google Scholar]
- 60. Schwindling FS, Juerchott A, Boehm S, Rues S, Kronsteiner D, Heiland S, et al. Three-Dimensional accuracy of partially guided implant surgery based on dental magnetic resonance imaging. Clin Oral Implants Res 2021; 32: 1218–27. doi: 10.1111/clr.13819 [DOI] [PubMed] [Google Scholar]
- 61. Grandoch A, Peterke N, Hokamp NG, Zöller JE, Lichenstein T, Neugebauer J. 1.5 T MRI with a dedicated dental signal-amplification coil as noninvasive, radiation-free alternative to CBCT in presurgical implant planning procedures. Int J Oral Maxillofac Implants 2021; 36: 1211–18. doi: 10.11607/jomi.8103 [DOI] [PubMed] [Google Scholar]
- 62. Hilgenfeld T, Juerchott A, Jende JME, Rammelsberg P, Heiland S, Bendszus M, et al. Use of dental MRI for radiation-free guided dental implant planning: a prospective, in vivo study of accuracy and reliability. Eur Radiol 2020; 30: 6392–6401. doi: 10.1007/s00330-020-07262-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Flügge T, Hövener JB, Ludwig U, Eisenbeiss AK, Spittau B, Hennig J, et al. Magnetic resonance imaging of intraoral hard and soft tissues using an intraoral coil and flash sequences. Eur Radiol 2016; 26: 4616–23. doi: 10.1007/s00330-016-4254-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ludwig U, Eisenbeiss A-K, Scheifele C, Nelson K, Bock M, Hennig J, et al. Dental MRI using wireless intraoral coils. Sci Rep 2016; 6: 23301: 23301. doi: 10.1038/srep23301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kress B, Buhl Y, Anders L, Stippich C, Palm F, Bähren W, et al. Quantitative analysis of MRI signal intensity as a tool for evaluating tooth pulp vitality. Dentomaxillofac Radiol 2004; 33: 241–44. doi: 10.1259/dmfr/33063878 [DOI] [PubMed] [Google Scholar]
- 66. Kress B, Buhl Y, Hähnel S, Eggers G, Sartor K, Schmitter M. Age- and tooth-related pulp cavity signal intensity changes in healthy teeth: a comparative magnetic resonance imaging analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: 134–37. doi: 10.1016/j.tripleo.2006.04.007 [DOI] [PubMed] [Google Scholar]
- 67. Briseño Marroquin B, Willershausen-Zönchen B, Pistorius A, Göller M. The reliability of apical X-ray pictures in the diagnosis of mandibular bone lesions. A review of the literature and in-vitro study. Schweiz Monatsschr Zahnmed 1995; 105: 1142–48. [PubMed] [Google Scholar]
- 68. Leonardi Dutra K, Haas L, Porporatti AL, Flores-Mir C, Nascimento Santos J, Mezzomo LA, et al. Diagnostic accuracy of cone-beam computed tomography and conventional radiography on apical periodontitis: a systematic review and meta-analysis. J Endod 2016; 42: 356–64: S0099-2399(15)01145-0. doi: 10.1016/j.joen.2015.12.015 [DOI] [PubMed] [Google Scholar]
- 69. Tymofiyeva O, Boldt J, Rottner K, Schmid F, Richter EJ, Jakob PM. High-Resolution 3D magnetic resonance imaging and quantification of carious lesions and dental pulp in vivo. MAGMA 2009; 22: 365–74. doi: 10.1007/s10334-009-0188-9 [DOI] [PubMed] [Google Scholar]
- 70. Woelber JP, Fleiner J, Rau J, Ratka-Krüger P, Hannig C. Accuracy and usefulness of CBCT in periodontology: a systematic review of the literature. Int J Periodontics Restorative Dent 2018; 38: 289–97. doi: 10.11607/prd.2751 [DOI] [PubMed] [Google Scholar]
- 71. Flügge T, Derksen W, Te Poel J, Hassan B, Nelson K, Wismeijer D. Registration of cone beam computed tomography data and intraoral surface scans-a prerequisite for guided implant surgery with CAD/CAM drilling guides. Clin Oral Implants Res 2017; 28: 1113–18. doi: 10.1111/clr.12925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zabalegui J, Gil JA, Zabalegui B. Magnetic resonance imaging as an adjunctive diagnostic aid in patient selection for endosseous implants: preliminary study. Int J Oral Maxillofac Implants 1990; 5: 283–87. [PubMed] [Google Scholar]
- 73. Gray CF, Redpath TW, Smith FW. Pre-Surgical dental implant assessment by magnetic resonance imaging. J Oral Implantol 1996; 22: 147–53. [PubMed] [Google Scholar]
- 74. Pompa V, Galasso S, Cassetta M, Pompa G, De Angelis F, Di Carlo S. A comparative study of magnetic resonance (Mr) and computed tomography (CT) in the pre-implant evaluation. Ann Stomatol (Roma) 2010; 1: 33–38. [PMC free article] [PubMed] [Google Scholar]