Abstract
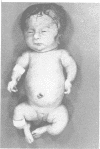
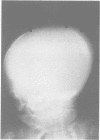
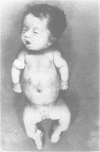
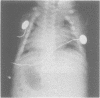
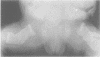
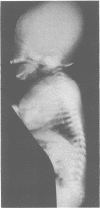
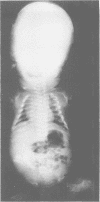
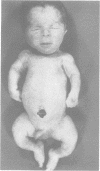
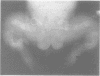
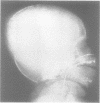
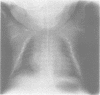
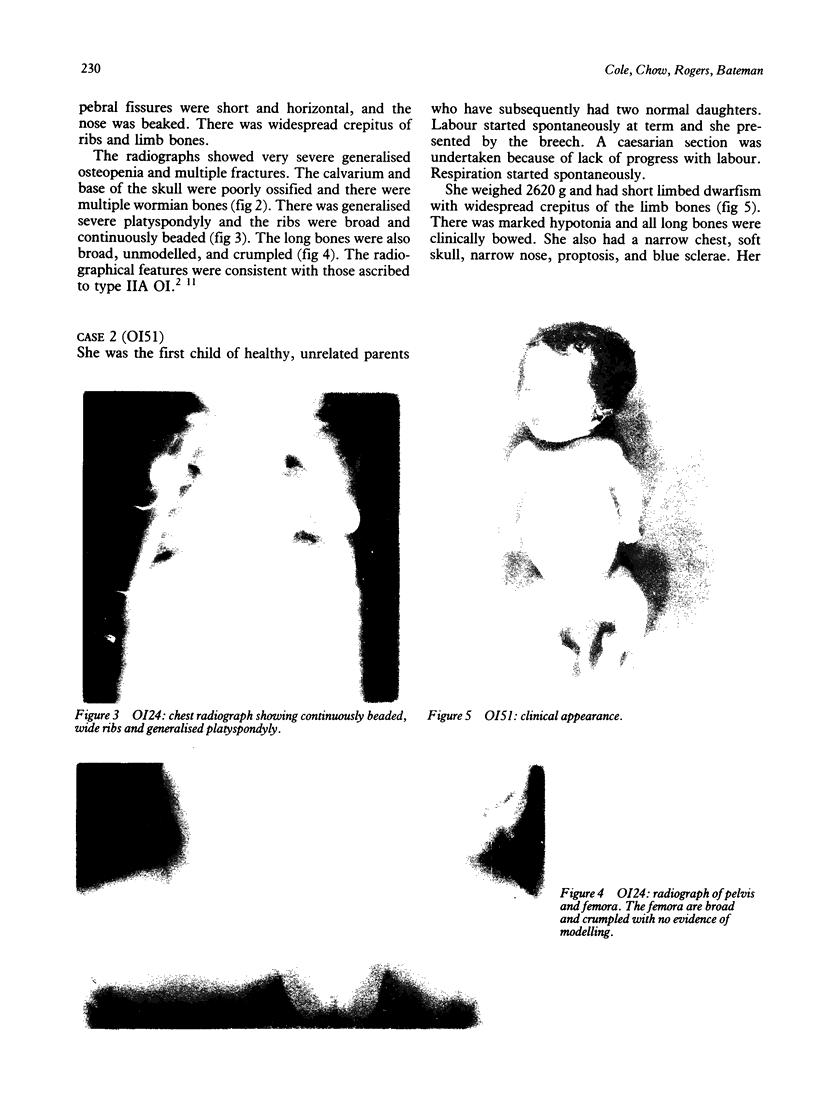
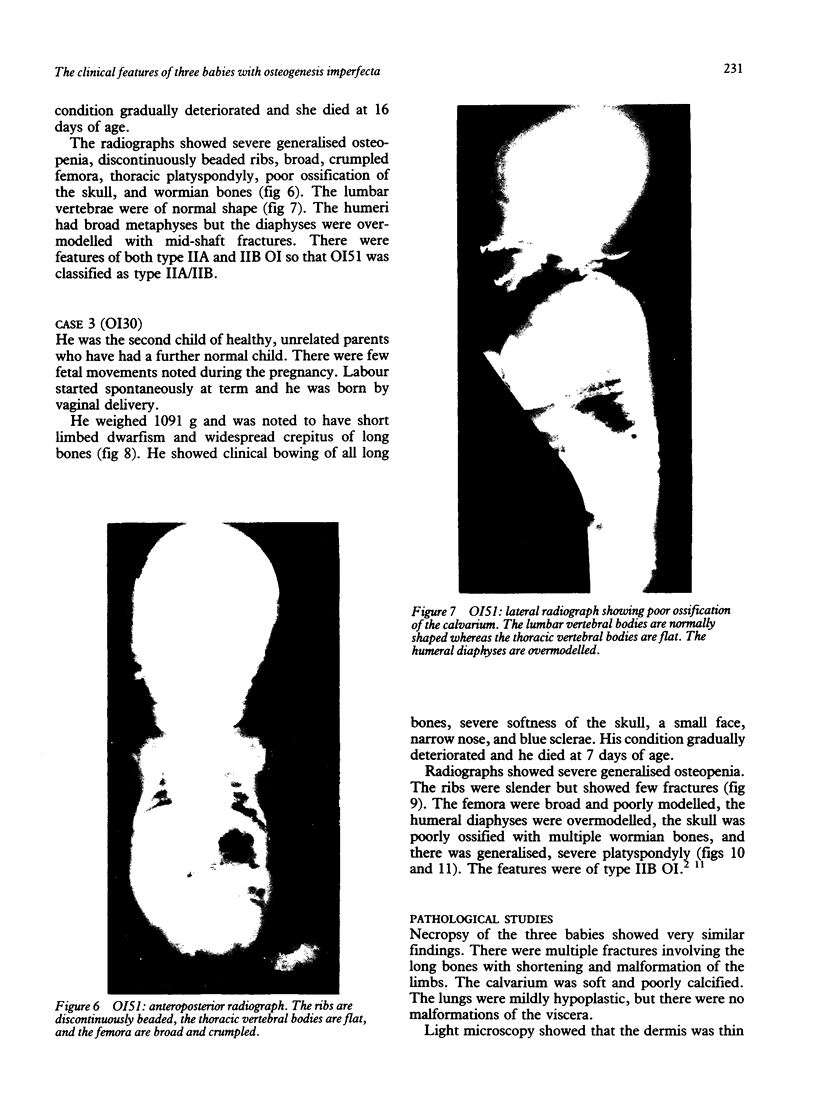
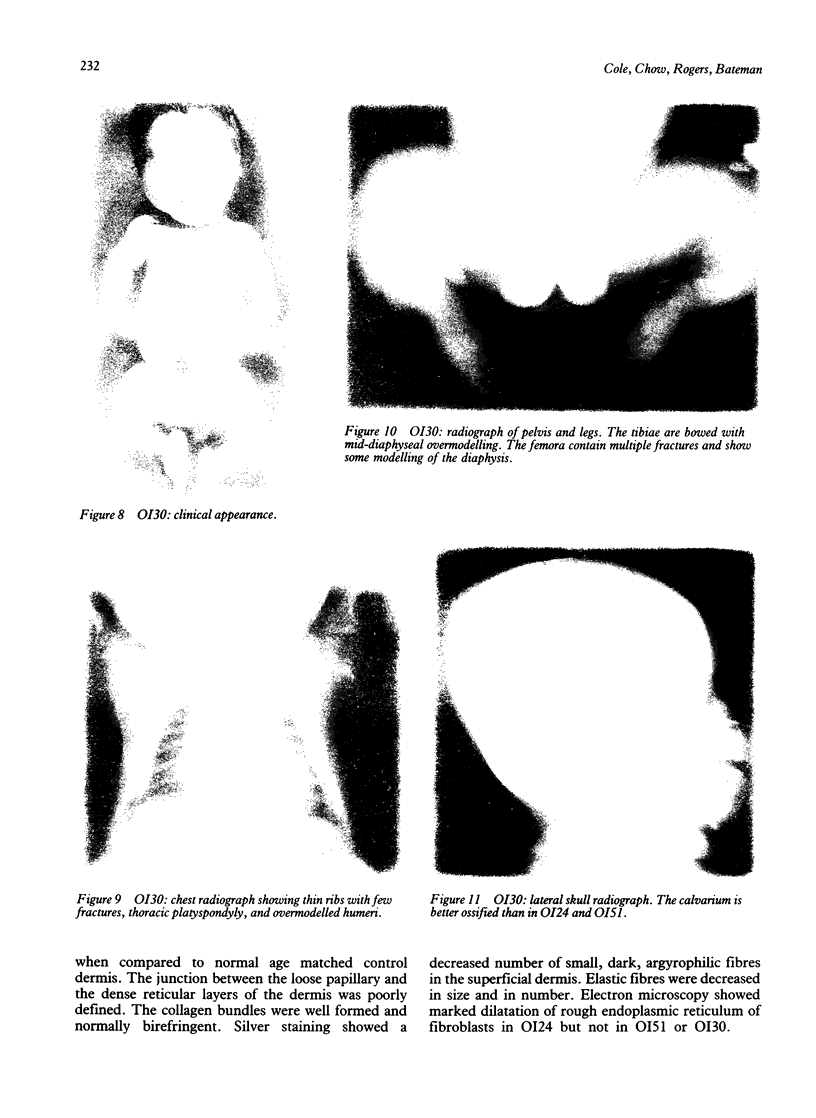
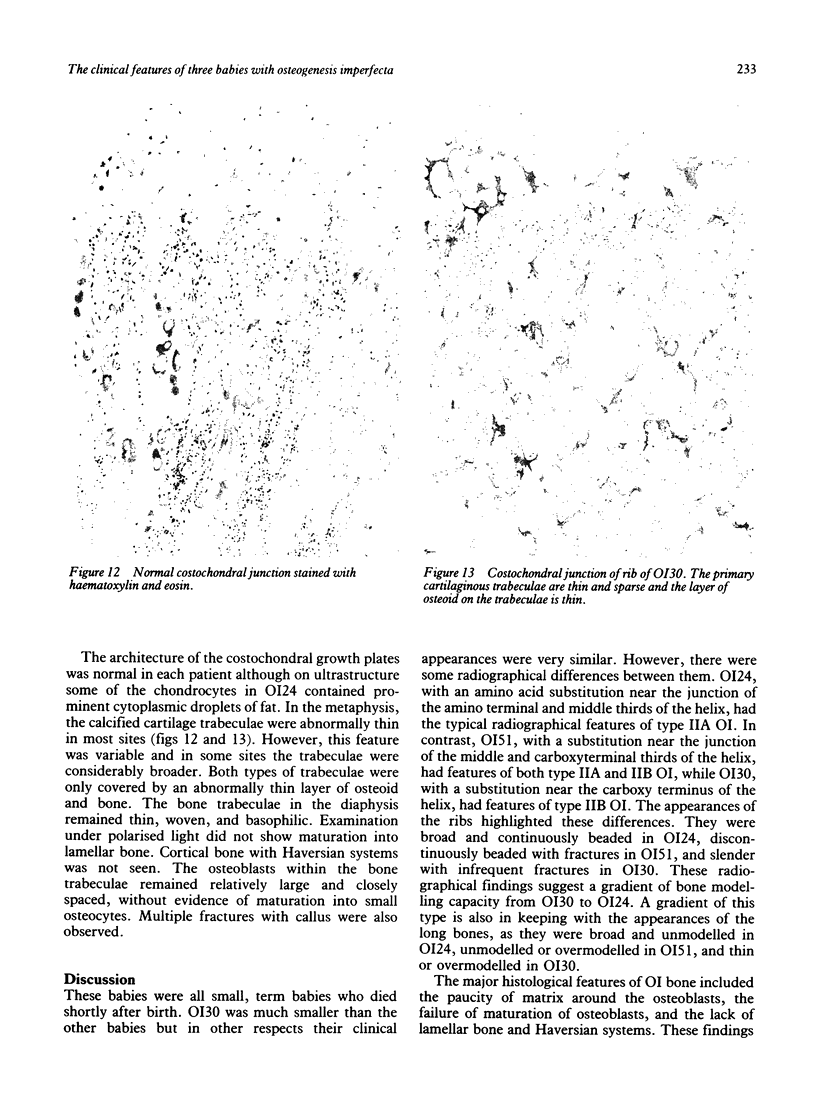
The features of three babies with lethal perinatal osteogenesis imperfecta resulting from the substitution of glycine by arginine in the pro alpha 1(I) chain of type I procollagen were studied. The babies were heterozygous for this substitution at residue 391 in case 1 (0I24), 667 in case 2 (0I51), and 976 in case 3 (0I30). They were all small, term babies who died soon after birth. The ribs were broad and continuously beaded in 0I24, discontinuously beaded in 0I51, and slender with few fractures in 0I30. The overall radiographical classifications were type IIA in 0I24, IIA/IIB in 0I51, and IIB in 0I30. Histological examination confirmed that the long bones were misshapen and porotic. The calcified cartilage trabeculae were covered with an abnormally thin layer of osteoid and the bone trabeculae were thin and basophilic. There was no evidence of lamellar bone or Haversian systems. The osteoblasts remained relatively large and closely spaced. These babies shared many phenotypic features, but differences in the radiographical appearance of the ribs and long bones suggested that there was a gradient of bone modelling capacity from the slender and overmodelled bones in 0I30 to the absence of modelling in 0I24.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A. T., Ramshaw J. A., Chan D., Cole W. G., Bateman J. F. Changes in collagen stability and folding in lethal perinatal osteogenesis imperfecta. The effect of alpha 1 (I)-chain glycine-to-arginine substitutions. Biochem J. 1989 Jul 1;261(1):253–257. doi: 10.1042/bj2610253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Mascara T., Rogers J. G., Cole W. G. Collagen defects in lethal perinatal osteogenesis imperfecta. Biochem J. 1986 Dec 15;240(3):699–708. doi: 10.1042/bj2400699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Walker I. D., Rogers J. G., Cole W. G. Lethal perinatal osteogenesis imperfecta due to the substitution of arginine for glycine at residue 391 of the alpha 1(I) chain of type I collagen. J Biol Chem. 1987 May 25;262(15):7021–7027. [PubMed] [Google Scholar]
- Bateman J. F., Lamande S. R., Dahl H. H., Chan D., Cole W. G. Substitution of arginine for glycine 664 in the collagen alpha 1(I) chain in lethal perinatal osteogenesis imperfecta. Demonstration of the peptide defect by in vitro expression of the mutant cDNA. J Biol Chem. 1988 Aug 25;263(24):11627–11630. [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Cohn D. H., Byers P. H., Steinmann B., Gelinas R. E. Lethal osteogenesis imperfecta resulting from a single nucleotide change in one human pro alpha 1(I) collagen allele. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6045–6047. doi: 10.1073/pnas.83.16.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C. D., Nielsen K. B., Prockop D. J. A lethal variant of osteogenesis imperfecta has a single base mutation that substitutes cysteine for glycine 904 of the alpha 1(I) chain of type I procollagen. The asymptomatic mother has an unidentified mutation producing an overmodified and unstable type I procollagen. J Clin Invest. 1989 Feb;83(2):574–584. doi: 10.1172/JCI113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen W. H., Robinson H. P., Dickinson A. J. Revised intrauterine growth curves for an Australian hospital population. Aust Paediatr J. 1983 Sep;19(3):157–161. doi: 10.1111/j.1440-1754.1983.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Lamande S. R., Dahl H. H., Cole W. G., Bateman J. F. Characterization of point mutations in the collagen COL1A1 and COL1A2 genes causing lethal perinatal osteogenesis imperfecta. J Biol Chem. 1989 Sep 25;264(27):15809–15812. [PubMed] [Google Scholar]
- Sillence D. O., Barlow K. K., Garber A. P., Hall J. G., Rimoin D. L. Osteogenesis imperfecta type II delineation of the phenotype with reference to genetic heterogeneity. Am J Med Genet. 1984 Feb;17(2):407–423. doi: 10.1002/ajmg.1320170204. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Bateman J., Choi T., Mascara T., Cole W., Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988 Mar 10;332(6160):131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- Starman B. J., Eyre D., Charbonneau H., Harrylock M., Weis M. A., Weiss L., Graham J. M., Jr, Byers P. H. Osteogenesis imperfecta. The position of substitution for glycine by cysteine in the triple helical domain of the pro alpha 1(I) chains of type I collagen determines the clinical phenotype. J Clin Invest. 1989 Oct;84(4):1206–1214. doi: 10.1172/JCI114286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. M., Young I. D., Hall C. M., Pembrey M. E. Recurrence risks and prognosis in severe sporadic osteogenesis imperfecta. J Med Genet. 1987 Jul;24(7):390–405. doi: 10.1136/jmg.24.7.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel B. E., Doelz R., Kadler K. E., Hojima Y., Engel J., Prockop D. J. A substitution of cysteine for glycine 748 of the alpha 1 chain produces a kink at this site in the procollagen I molecule and an altered N-proteinase cleavage site over 225 nm away. J Biol Chem. 1988 Dec 15;263(35):19249–19255. [PubMed] [Google Scholar]
- Wenstrup R. J., Cohn D. H., Cohen T., Byers P. H. Arginine for glycine substitution in the triple-helical domain of the products of one alpha 2(I) collagen allele (COL1A2) produces the osteogenesis imperfecta type IV phenotype. J Biol Chem. 1988 Jun 5;263(16):7734–7740. [PubMed] [Google Scholar]
- de Vries W. N., de Wet W. J. The molecular defect in an autosomal dominant form of osteogenesis imperfecta. Synthesis of type I procollagen containing cysteine in the triple-helical domain of pro-alpha 1(I) chains. J Biol Chem. 1986 Jul 5;261(19):9056–9064. [PubMed] [Google Scholar]
- van der Harten H. J., Brons J. T., Dijkstra P. F., Meijer C. J., van Geijn H. P., Arts N. F., Niermeijer M. F. Perinatal lethal osteogenesis imperfecta: radiologic and pathologic evaluation of seven prenatally diagnosed cases. Pediatr Pathol. 1988;8(3):233–252. doi: 10.3109/15513818809042968. [DOI] [PubMed] [Google Scholar]