Key Points
Question
Does the autotaxin inhibitor ziritaxestat improve outcomes, compared with placebo, in patients with idiopathic pulmonary fibrosis who continue to receive standard of care with pirfenidone, nintedanib, or neither standard of care treatment?
Findings
In 2 randomized clinical trials, ISABELA 1 and ISABELA 2, there was no reduction in the 52-week rate of decline for forced vital capacity (a measure of lung function) in the 2 ziritaxestat groups vs placebo, and combined data from both trials showed all-cause mortality rates were numerically higher for ziritaxestat than placebo. Therefore, the trials were stopped early.
Meaning
The autotaxin inhibitor ziritaxestat was ineffective as a treatment for idiopathic pulmonary fibrosis.
Abstract
Importance
There is a major need for effective, well-tolerated treatments for idiopathic pulmonary fibrosis (IPF).
Objective
To assess the efficacy and safety of the autotaxin inhibitor ziritaxestat in patients with IPF.
Design, Setting, and Participants
The 2 identically designed, phase 3, randomized clinical trials, ISABELA 1 and ISABELA 2, were conducted in Africa, Asia-Pacific region, Europe, Latin America, the Middle East, and North America (26 countries). A total of 1306 patients with IPF were randomized (525 patients at 106 sites in ISABELA 1 and 781 patients at 121 sites in ISABELA 2). Enrollment began in November 2018 in both trials and follow-up was completed early due to study termination on April 12, 2021, for ISABELA 1 and on March 30, 2021, for ISABELA 2.
Interventions
Patients were randomized 1:1:1 to receive 600 mg of oral ziritaxestat, 200 mg of ziritaxestat, or placebo once daily in addition to local standard of care (pirfenidone, nintedanib, or neither) for at least 52 weeks.
Main Outcomes and Measures
The primary outcome was the annual rate of decline for forced vital capacity (FVC) at week 52. The key secondary outcomes were disease progression, time to first respiratory-related hospitalization, and change from baseline in St George’s Respiratory Questionnaire total score (range, 0 to 100; higher scores indicate poorer health-related quality of life).
Results
At the time of study termination, 525 patients were randomized in ISABELA 1 and 781 patients in ISABELA 2 (mean age: 70.0 [SD, 7.2] years in ISABELA 1 and 69.8 [SD, 7.1] years in ISABELA 2; male: 82.4% and 81.2%, respectively). The trials were terminated early after an independent data and safety monitoring committee concluded that the benefit to risk profile of ziritaxestat no longer supported their continuation. Ziritaxestat did not improve the annual rate of FVC decline vs placebo in either study. In ISABELA 1, the least-squares mean annual rate of FVC decline was –124.6 mL (95% CI, −178.0 to −71.2 mL) with 600 mg of ziritaxestat vs –147.3 mL (95% CI, −199.8 to −94.7 mL) with placebo (between-group difference, 22.7 mL [95% CI, −52.3 to 97.6 mL]), and –173.9 mL (95% CI, −225.7 to −122.2 mL) with 200 mg of ziritaxestat (between-group difference vs placebo, −26.7 mL [95% CI, −100.5 to 47.1 mL]). In ISABELA 2, the least-squares mean annual rate of FVC decline was –173.8 mL (95% CI, −209.2 to −138.4 mL) with 600 mg of ziritaxestat vs –176.6 mL (95% CI, −211.4 to −141.8 mL) with placebo (between-group difference, 2.8 mL [95% CI, −46.9 to 52.4 mL]) and –174.9 mL (95% CI, −209.5 to −140.2 mL) with 200 mg of ziritaxestat (between-group difference vs placebo, 1.7 mL [95% CI, −47.4 to 50.8 mL]). There was no benefit with ziritaxestat vs placebo for the key secondary outcomes. In ISABELA 1, all-cause mortality was 8.0% with 600 mg of ziritaxestat, 4.6% with 200 mg of ziritaxestat, and 6.3% with placebo; in ISABELA 2, it was 9.3% with 600 mg of ziritaxestat, 8.5% with 200 mg of ziritaxestat, and 4.7% with placebo.
Conclusions and Relevance
Ziritaxestat did not improve clinical outcomes compared with placebo in patients with IPF receiving standard of care treatment with pirfenidone or nintedanib or in those not receiving standard of care treatment.
Trial Registration
ClinicalTrials.gov Identifiers: NCT03711162 and NCT03733444
These 2 randomized clinical trials compare the efficacy and safety of 2 doses of the autotaxin inhibitor ziritaxestat vs placebo in patients with idiopathic pulmonary fibrosis.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease associated with progressive and irreversible fibrosis, dyspnea, lung function decline, and loss of quality of life.1,2 The median survival without treatment is approximately 3 years, with respiratory failure being the most frequent cause of death.3 Even though treatment with pirfenidone or nintedanib slows disease progression, patients continue to experience a loss of lung function and premature death.4 Furthermore, pirfenidone and nintedanib are associated with adverse effects in a substantial proportion of patients, which may lead to treatment discontinuation.5 Thus, there remains a major unmet need for more effective, better tolerated IPF treatments.
Pulmonary fibrosis in patients with IPF is believed to develop when aberrant responses to lung injury, including epithelial apoptosis and fibroblast recruitment, occur.6,7,8 Lysophosphatidic acid is thought to be at least partially responsible for mediating such responses.6,7 Autotaxin, an enzyme involved in the production of lysophosphatidic acid,9 is upregulated in patients with IPF and is therefore a potential target for novel IPF therapies.6,10
Ziritaxestat is a small-molecule, selective autotaxin inhibitor11,12,13,14,15 that showed promising results in a phase 2a study including 23 patients with IPF.16 Ziritaxestat was well tolerated and those treated with ziritaxestat demonstrated a smaller mean change from baseline in forced vital capacity (FVC) at week 12 vs placebo. Furthermore, ziritaxestat reduced the concentration of plasma lysophosphatidic acid, with a maximum reduction from baseline of approximately 90%, confirming target engagement.16
To further evaluate the efficacy and safety of ziritaxestat for the treatment of IPF, 2 identically designed, phase 3, randomized clinical trials, ISABELA 1 and ISABELA 2, were conducted.
Methods
Study Design and Eligibility Criteria
ISABELA 1 and ISABELA 2 were double-blind, placebo-controlled, global randomized clinical trials (the trial protocol appears in Supplement 1, the statistical analysis plan appears in Supplement 2, and eFigure 1 appears in Supplement 3).17 Patients were recruited from pulmonary clinics at 106 sites in 14 countries in ISABELA 1 and at 121 sites in 15 countries in ISABELA 2.
The 2 trials were conducted in accordance with the Declaration of Helsinki and local ethical and legal requirements. The trial protocols were approved by an independent ethics committee or institutional review board for each site or country. All patients provided written informed consent. Enrollment began in November 2018; the 2 trials were terminated early in February 2021.
Eligible men and women were aged 40 years or older who had been diagnosed with IPF within the previous 5 years. Diagnosis of IPF was confirmed through central review of a high-resolution computed tomographic scan of the chest performed within the 12 months prior to screening and lung biopsy (if available). At the time of enrollment, patients were receiving treatment with local standard of care (a stable dose of pirfenidone or nintedanib for at least 2 months prior to screening or neither therapy).
Patients attended 2 screening visits. At visit 1, assessments included inclusion and exclusion criteria, demographics, medical history, alcohol consumption and smoking habits, administration of 50-item St George Respiratory Questionnaire (SGRQ), electrocardiography, oxygen saturation as measured by pulse oximetry (Spo2), spirometry, diffusing capacity of the lung for carbon monoxide, adverse events, prior and concomitant medication, physical examination and vital signs, blood sampling, and the 6-minute walk test. Assessments at the second screening visit were spirometry, Spo2, oxygen titration test for the 6-minute walk test, adverse events, and concomitant medication use. Race was captured in the baseline demographics because IPF disease progression may differ between racial groups. Race was self-reported and based on fixed categories.
Eligible patients had to be able to walk 150 m or farther during the 6-minute walk test at screening visit 1. At visit 2, resting Spo2 had to be 88% or greater with a maximum of 6 L of oxygen/min for the oxygen titration test. During the 6-minute walk test, Spo2 had to be 83% or greater with 6 L of oxygen/min or 88% or greater with 0 L, 2 L, or 4 L of oxygen/min. Additional inclusion criteria included FVC of 45% or greater than predicted of normal, ratio of forced expiratory volume in first second of expiration to FVC of 0.7 or greater, and diffusing capacity of the lung for carbon monoxide corrected for hemoglobin level of 30% or greater than predicted of normal.
Exclusion criteria included presence of immunosuppressive conditions (eg, HIV and those that were congenital, acquired, or induced by medication); having a positive serological result for hepatitis B or hepatitis C; having a malignancy within the past 5 years (except for carcinoma in situ of the uterine cervix, basal carcinoma of the skin that showed no evidence of recurrence after treatment, prostate cancer that was medically managed with active surveillance or watchful waiting, squamous cell carcinoma of the skin if fully resected, and ductal carcinoma in situ); use of certain medications (eg, strong inducers or inhibitors of CYP3A4 and potent inducers or inhibitors of P-glycoprotein); presence of an acute IPF exacerbation within 6 months; having a lower respiratory tract infection requiring antibiotics within 4 weeks; presence of severe pulmonary hypertension; underwent a lung volume reduction surgery or lung transplant; presence of interstitial lung disease associated with known primary diseases (such as sarcoidosis or amyloidosis), exposures (such as radiation), or medications (such as amiodarone); presence of unstable cardiovascular, pulmonary (other than IPF), or other disease within 6 months; having a creatinine clearance less than 30 mL/min; having a hemoglobin level less than 10 g/dL; presence of alcohol or substance miuse; being pregnant; or having an abnormal liver function test result.
Patients were randomized at visit 3. After randomization, patients were assessed at weeks 2, 4, 8, 12, 18, 26, 34, 42, and 52. Spirometry assessments were performed at each of these visits and then every 12 weeks; blood samples for pharmacokinetic and pharmacodynamic assessments were collected at weeks 12, 26, 34, and 52 and then every 24 weeks. If patients could not attend study visits due to the COVID-19 pandemic, appointments were conducted virtually or via phone calls.
In both studies, patients were randomized 1:1:1 to receive 600 mg of oral ziritaxestat, 200 mg of ziritaxestat, or matching placebo (Figure 1 and Figure 2) once daily in addition to local standard of care with pirfenidone, nintedanib, or neither treatment until the last patient completed 52 weeks to allow the collection of long-term efficacy and safety data. Initiation of pirfenidone or nintedanib or switching standard of care during the trials was allowed. Treatment was allocated to each patient using a centralized electronic system (interactive web-based response system) with permuted block sizes of 9. Both patients and study personnel were blinded to treatment allocation.
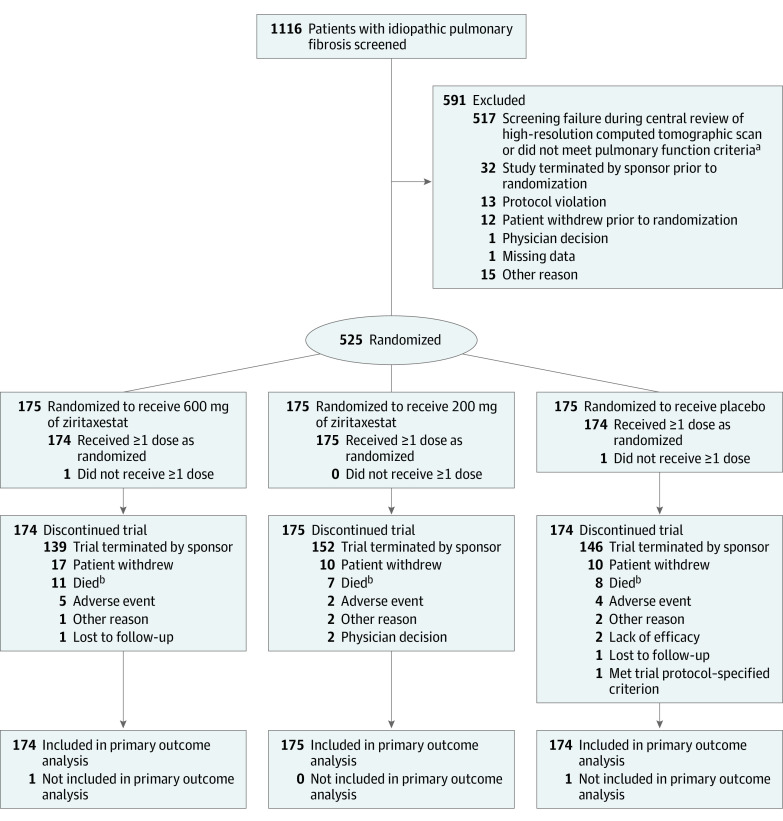
Figure 1. Screening, Exclusions, Randomization, and Flow of Patients in the ISABELA 1 Trial.
At the time of trial termination, enrollment was ongoing in the ISABELA 1 trial and had completed in the ISABELA 2 trial.
aNot meeting pulmonary function criteria was defined as a forced vital capacity (FVC) of 45% or greater than predicted of normal, a ratio of forced expiratory volume in first second of expiration to FVC of 0.7 or greater, and a diffusing capacity for carbon monoxide corrected for hemoglobin level of 30% or greater than predicted of normal.
bThe discrepancy between the number of deaths reported in this Figure and in the Results section is because of incomplete data cleaning due to the early termination of the trials. The total number who died was 14 in the 600 mg of ziritaxestat group; 8 in the 200 mg of ziritaxestat group; and 11 in the placebo group.
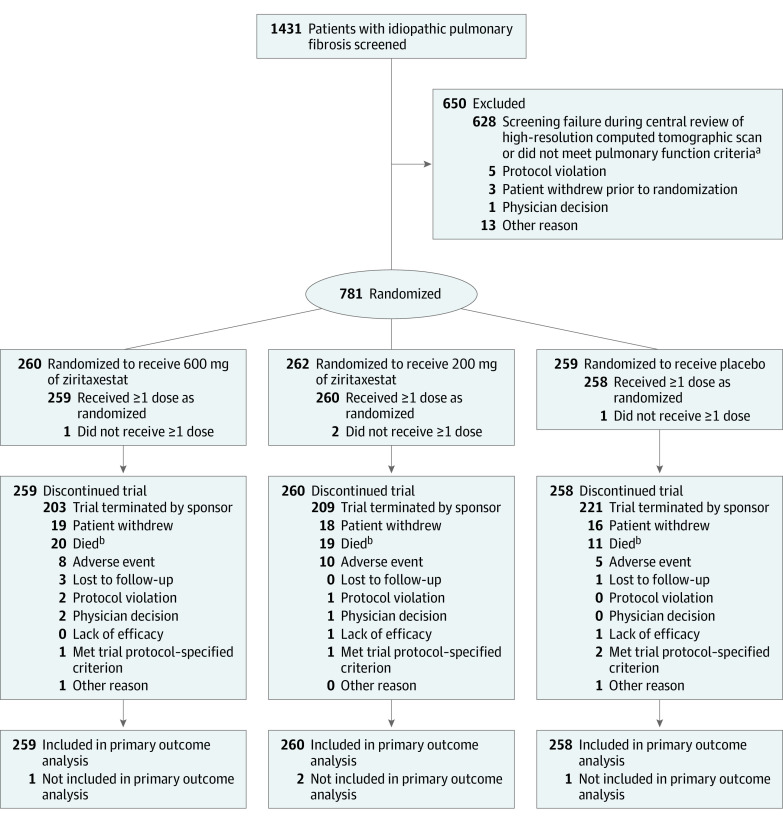
Figure 2. Screening, Exclusions, Randomization, and Flow of Patients in the ISABELA 2 Trial.
At the time of trial termination, enrollment was ongoing in the ISABELA 1 trial and had completed in the ISABELA 2 trial.
aNot meeting pulmonary function criteria was defined as a forced vital capacity (FVC) of 45% or greater than predicted of normal, a ratio of forced expiratory volume in first second of expiration to FVC of 0.7 or greater, and a diffusing capacity for carbon monoxide corrected for hemoglobin level of 30% or greater than predicted of normal.
bThe discrepancy between the number of deaths reported in this Figure and in the Results section is because of incomplete data cleaning due to the early termination of the trials. The total number who died was 24 in the 600 mg of ziritaxestat group; 22 in the 200 mg of ziritaxestat group; and 12 in the placebo group.
Randomization was stratified by local standard of care for IPF (nintedanib, pirfenidone, or neither treatment), and recruitment could be restricted in 1 or more strata, while the other strata continued to recruit to maintain a balanced proportion of patients between the 3 strata. Clinical study personnel monitored the amount of study drug dispensed to a patient and the amount returned to assess treatment adherence. For doses taken at home, the intake was reported using patient diary cards. The study drug was to be discontinued if aspartate transaminase or alanine transaminase levels exceeded 8 times the upper limit of normal or were 3 times or greater than the upper limit of normal with signs of severe liver damage.
Primary and Secondary Outcomes
The primary outcome was the annual rate of decline for FVC at week 52. The key secondary outcomes were disease progression (a composite outcome of first occurrence of ≥10% absolute decline in percent predicted FVC or all-cause mortality at week 52); time to first respiratory-related hospitalization; and change from baseline in SGRQ total score at week 52 (score range, 0-100; higher scores indicate poorer health-related quality of life).
Other secondary outcomes included time to respiratory-related mortality, time to all-cause mortality or respiratory-related hospitalization, time to first acute IPF exacerbation, change from baseline in FVC, distance during the 6-minute walk test, and safety and tolerability over time until the end of the trials (eMethods in Supplement 3).
Data were reviewed by an independent data and safety monitoring committee and clinical endpoint adjudication committee. The independent data and safety monitoring committee assessed potential safety risks and examined unblinded FVC data to assess the effect of treatment on lung function so a recommendation could be made to the trial sponsor regarding continuation of the trials. The independent data and safety monitoring committee regularly reviewed the data every 3 to 4 months. In addition, an interim futility analysis was planned once at least 25% of patients from the 2 trials combined had completed 52 weeks of treatment. However, the trials were terminated after the sixth regular data review before the interim futility analysis occurred. The clinical endpoint adjudication committee adjudicated major events, including exacerbations, hospitalizations, and deaths based on blinded data.
Pharmacokinetic and Pharmacodynamic Assessments
Pharmacokinetic assessments included the plasma concentration of ziritaxestat, pirfenidone, and nintedanib. Target engagement (autotaxin inhibition) was determined by plasma concentration of lysophosphatidic acid C18:2. Modeling was performed to determine relationships between treatment exposure and response (eMethods in Supplement 3).18
Statistical Analyses
The full analysis set was used for the primary outcome and the safety analyses and comprised all randomized patients who received at least 1 dose of study drug. Assuming 600 mg of ziritaxestat had an effect on FVC of 80 mL or greater vs placebo, a sample size of 250 patients per group would provide 80% power to show a significant effect. Therefore, each trial was expected to enroll 750 patients (250 in the 600 mg of ziritaxestat group, 250 in the 200 mg of ziritaxestat group, and 250 in the placebo group).
For the primary outcome, a random coefficient regression model (linear slope model19) was used that included sex, age, height, and stratification factor as covariates and a random slope and intercept. A protocol-defined sensitivity analysis was performed to assess the annual rate of FVC decline at the end of the trials using all data. A post hoc sensitivity analysis was performed to assess FVC decline at the end of the trials using all data according to treatment strata.
Logistic regression analysis was used to assess the proportion of patients with disease progression at week 52. Time to event data are presented as Kaplan-Meier estimates. A Cox proportional hazards model was used to estimate and test hazard ratios (HRs) for each dose of ziritaxestat vs placebo. A mixed-effect model was applied to the SGRQ total score (eMethods in Supplement 3).
Statistical testing was at a global 2-sided 5% α level. To account for multiple testing due to 2 doses of ziritaxestat being compared with a placebo, a Bonferroni approach was used with higher priority given to the high-dose group. The primary outcome was tested using an α level of 4% for the comparison of 600 mg of ziritaxestat vs placebo and an α level of 1% for the comparison of 200 mg of ziritaxestat vs placebo. The rate of decline model, which assumes a linear trend over time, was applied to all available data. Therefore, imputation of missing data was not performed. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Study Termination
The 2 trials were terminated early after a planned review of pooled unblinded data by the independent data and safety monitoring committee that concluded there were safety concerns regarding increased mortality in the 600 mg of ziritaxestat group along with a lack of efficacy in all treatment groups. Therefore, the benefit to risk profile of ziritaxestat no longer supported the continuation of the trials. At that time, 1116 patients had been screened in the ISABELA 1 trial and 1431 patients in the ISABELA 2 trial.
Patient Disposition and Baseline Characteristics
In the ISABELA 1 trial, 525 patients were randomized and 523 received at least 1 dose of their assigned study drug (Figure 1). In the ISABELA 2 trial, 781 patients were randomized and 777 received at least 1 dose of their assigned study drug (Figure 2). After the premature termination of the trials, all patients discontinued use of the study drug. At the time of trial termination, enrollment was ongoing in the ISABELA 1 trial and had completed in the ISABELA 2 trial.
The median duration between the high-resolution computed tomographic scan and trial enrollment was 51 days in the ISABELA 1 trial (41 days in the 600 mg of ziritaxestat group, 58 days in the 200 mg of ziritaxestat group, and 60 days in the placebo group) and was 50 days in the ISABELA 2 trial (64 days in the 600 mg of ziritaxestat group, 50 days in the 200 mg of ziritaxestat group, and 43 days in the placebo group). In the ISABELA 1 trial, 20 lung biopsies were reviewed in the 600 mg of ziritaxestat group, 27 in the 200 mg of ziritaxestat group, and 19 in the placebo group. In the ISABELA 2 trial, 28 lung biopsies were reviewed in the 600 mg of ziritaxestat group, 33 in the 200 mg of ziritaxestat group, and 28 in the placebo group.
In each trial, baseline demographics and disease characteristics were similar in the 600 mg of ziritaxestat group, in the 200 mg of ziritaxestat group, and in the placebo group (Table 1). There was a greater proportion of Asian patients in the ISABELA 2 trial than in the ISABELA 1 trial (28.1% vs 5.5%, respectively) owing to the geographic location of sites. The mean baseline FVC was slightly higher in the ISABELA 1 trial than in the ISABELA 2 trial (2921.1 mL vs 2764.7 mL, respectively) as well as the percent predicted FVC (79.3% vs 77.3%). A higher proportion of patients in the ISABELA 2 trial than in the ISABELA 1 trial were former smokers (71.4% vs 61.6%, respectively; Table 1).
Table 1. Baseline Patient Demographics and Disease Characteristics for the ISABELA 1 and ISABELA 2 Trials.
| ISABELA 1 trial | ISABELA 2 trial | |||||
|---|---|---|---|---|---|---|
| 600 mg of Ziritaxestat (n = 174) | 200 mg of Ziritaxestat (n = 175) | Placebo (n = 174) | 600 mg of Ziritaxestat (n = 259) | 200 mg of Ziritaxestat (n = 260) | Placebo (n = 258) | |
| Age, mean (SD), y | 69.4 (7.2) | 70.0 (6.7) | 70.6 (7.7) | 69.2 (7.2) | 69.7 (7.3) | 70.6 (6.6) |
| Sex, No. (%) | ||||||
| Male | 142 (81.6) | 143 (81.7) | 146 (83.9) | 209 (80.7) | 213 (81.9) | 209 (81.0) |
| Female | 32 (18.4) | 32 (18.3) | 28 (16.1) | 50 (19.3) | 47 (18.1) | 49 (19.0) |
| Race, No. (%) | (n = 174) | (n = 175) | (n = 174) | (n = 251) | (n = 252) | (n = 252) |
| American Indian or Alaska Native | 10 (5.7) | 6 (3.4) | 6 (3.4) | 2 (0.8) | 1 (0.4) | 2 (0.8) |
| Asian | 11 (6.3) | 9 (5.1) | 9 (5.2) | 72 (28.7) | 72 (28.6) | 68 (27.0) |
| Black or African American | 1 (0.6) | 0 | 0 | 2 (0.8) | 0 | 1 (0.4) |
| Multiple races | 0 | 0 | 0 | 0 | 1 (0.4) | 2 (0.8) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 1 (0.4) | 0 | 1 (0.4) |
| White | 152 (87.4) | 160 (91.4) | 159 (91.4) | 174 (69.3) | 178 (70.6) | 178 (70.6) |
| Geographic region, No. (%) | ||||||
| Asia-Pacific | 24 (13.8) | 29 (16.6) | 22 (12.6) | 67 (25.9) | 76 (29.2) | 71 (27.5) |
| Europe, the Middle East, and Africa | 73 (42.0) | 70 (40.0) | 77 (44.3) | 100 (38.6) | 90 (34.6) | 86 (33.3) |
| Latin America | 35 (20.1) | 21 (12.0) | 22 (12.6) | 30 (11.6) | 33 (12.7) | 27 (10.5) |
| North America | 42 (24.1) | 55 (31.4) | 53 (30.5) | 62 (23.9) | 61 (23.5) | 74 (28.7) |
| Former smoker, No. (%) | 99 (56.9) | 110 (62.9) | 113 (64.9) | 177 (68.3) | 195 (75.0) | 183 (70.9) |
| Current smoker, No. (%) | 5 (2.9) | 2 (1.1) | 0 | 5 (1.9) | 2 (0.8) | 5 (1.9) |
| Body mass index, mean (SD)a | 27.6 (4.0) | 28.3 (3.9) | 27.9 (3.8) | 26.7 (3.9) | 27.3 (3.7) | 27.1 (4.3) |
| Oxygen saturation at rest, mean (SD), % | 95.8 (2.1) | 95.8 (2.0) | 95.7 (1.9) | 95.6 (2.2) | 95.5 (2.0) | 95.7 (2.1) |
| Requires oxygen therapy at rest, No. (%) | 11 (6.3) | 7 (4.0) | 9 (5.2) | 16 (6.2) | 10 (3.8) | 13 (5.0) |
| Duration of idiopathic pulmonary fibrosis | ||||||
| Mean (SD), y | 2.3 (1.4) | 2.3 (1.4) [n = 174] | 2.3 (1.4) | 2.2 (1.5) | 2.3 (1.5) | 2.2 (1.5) [n = 257] |
| Median (range), y | 2.1 (0.1-5.8) | 2.1 (0.1-5.5) [n = 174] | 2.0 (0.1-6.6) | 2.0 (0.1-11.8) | 2.1 (0.1-6.3) | 1.9 (0.1-5.8) [n = 257] |
| FVC, mean (SD), mL | 2947.0 (820.8) | 2873.2 (815.8) | 2943.3 (738.7) | 2775.7 (823.2) | 2768.6 (701.9) | 2749.5 (785.1) [n = 256] |
| Predicted FVC, mean (SD), %b | 80.4 (17.5) | 77.9 (18.1) | 79.7 (15.9) | 76.5 (16.8) | 77.7 (15.5) | 77.6 (17.2) [n = 256] |
| Ratio of FEV1 to FVC, mean (SD), % | 82.0 (5.9) | 82.4 (5.6) | 82.3 (4.7) | 82.9 (5.8) | 82.8 (5.7) | 82.7 (6.0) [n = 256] |
| Total score for SGRQ, mean (SD), %c | 34.1 (18.5) [n = 173] | 35.9 (19.1) | 35.2 (17.9) [n = 173] | 36.9 (20.2) [n = 258] | 36.6 (20.3) [n = 258] | 34.9 (19.2) [n = 257] |
| Distance on 6-minute walk test, mean (SD), md | 416.3 (101.6) [n = 173] | 403.7 (105.1) | 400.8 (119.1) | 408.3 (110.9) | 395.7 (133.9) [n = 257] | 400.4 (105.4) [n = 257] |
| Predicted DLco corrected for hemoglobin, mean (SD), % | 56.4 (18.9) | 54.0 (16.3) | 53.1 (16.3) | 54.3 (19.6) [n = 258] | 53.1 (17.5) [n = 259] | 57.1 (21.7) [n = 257] |
| Concomitant use of PPIs, No. (%) | 91 (52.3) | 90 (51.4) | 86 (49.4) | 133 (51.4) | 131 (50.4) | 139 (53.9) |
| Receiving standard of care, No. (%)e | ||||||
| Pirfenidone | 69 (39.7) | 70 (40.0) | 69 (39.7) | 84 (32.4) | 85 (32.7) | 83 (32.2) |
| Nintedanib | 60 (34.5) | 61 (34.9) | 60 (34.5) | 89 (34.4) | 91 (35.0) | 91 (35.3) |
| Neither treatment | 45 (25.9) | 44 (25.1) | 45 (25.9) | 86 (33.2) | 84 (32.3) | 84 (32.6) |
Abbreviations: DLco, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in first second of expiration; FVC, forced vital capacity; PPIs, proton pump inhibitors; SGRQ, St George Respiratory Questionnaire.
Calculated as weight in kilograms divided by height in meters squared.
Calculated using the 2012 Global Lung Function Initiative Equations.
Scores range from 0 to 100; a higher score indicates poorer health-related quality of life.
An average distance is 659 m in healthy individuals aged 55 to 75 years.
The doses appear in eTable 3 in Supplement 3.20
At enrollment in the ISABELA 1 trial, 39.8% of patients were taking pirfenidone, 34.6% were taking nintedanib, and 25.6% were not taking either drug. At enrollment in the ISABELA 2 trial, 32.4% of patients were taking pirfenidone, 34.9% were taking nintedanib, and 32.7% were not taking either drug (Table 1). The mean dose of pirfenidone at enrollment was 2216 mg/d in the ISABELA 1 trial (2184 mg/d in the 600 mg of ziritaxestat group, 2165 mg/d in the 200 mg of ziritaxestat group, and 2300 mg/d in the placebo group). The mean dose of pirfenidone at enrollment was 2083 mg/d in the ISABELA 2 trial (2020 mg/d in the 600 mg of ziritaxestat group, 2031 mg/d in the 200 mg of ziritaxestat group, and 2201 mg/d in the placebo group). The mean dose of nintedanib at enrollment was 283 mg/d in the ISABELA 1 trial (293 mg/d in the 600 mg of ziritaxestat group, 278 mg/d in the 200 mg of ziritaxestat group, and 278 mg/d in the placebo group). The mean dose of nintedanib at enrollment was 267 mg/d in the ISABELA 2 trial (262 mg/d in the 600 mg of ziritaxestat group, 270 mg/d in the 200 mg of ziritaxestat group, and 270 mg/d in the placebo group).
Of patients not taking either pirfenidone or nintedanib at the time of enrollment, 79.1% had never been treated with either therapy in the ISABELA 1 trial and 68.1% in the ISABELA 2 trial. The mean duration of IPF was shorter in those not taking either pirfenidone or nintedanib (1.8 years in both ISABELA 1 and ISABELA 2) than in those taking either pirfenidone (2.4 years in ISABELA 1 and 2.6 years in ISABELA 2) or nintedanib (2.4 years in both ISABELA 1 and ISABELA 2) (eTable 1 in Supplement 3). The percent predicted FVC was slightly lower in patients taking either pirfenidone or nintedanib (range, 75.0%-77.4%) than in those not taking either drug (85.6% in ISABELA 1 and 79.9% in ISABELA 2) (eTable 1 in Supplement 3).
Treatment Duration and Exposure
Treatment exposures were longer in the ISABELA 2 trial than in the ISABELA 1 trial (eTable 2 in Supplement 3), reflecting that the patients were followed up for longer on average in ISABELA 2. Treatment adherence was high in all treatment groups (the median was 100% in each treatment group in both trials; eTable 2 in Supplement 3). The dose of nintedanib was reduced or interrupted in a greater proportion of patients in the 600 mg of ziritaxestat group than in the 200 mg of ziritaxestat group or the placebo group (Table 2). In the 600 mg of ziritaxestat group, the proportion of patients who underwent dose reductions or interruptions of pirfenidone or nintedanib was greater with nintedanib than pirfenidone (Table 2).
Table 2. Treatment-Emergent Adverse Events (TEAEs) and Dose Reductions, Interruptions, or Discontinuations.
| Events, No. (%)a | ||||||
|---|---|---|---|---|---|---|
| ISABELA 1 trial | ISABELA 2 trial | |||||
| 600 mg of Ziritaxestat (n = 174) |
200 mg of Ziritaxestat (n = 175) |
Placebo (n = 174) |
600 mg of Ziritaxestat (n = 259) |
200 mg of Ziritaxestat (n = 260) |
Placebo (n = 258) |
|
| TEAEs | ||||||
| ≥1 TEAE | 137 (78.7) | 148 (84.6) | 147 (84.5) | 210 (81.1) | 223 (85.8) | 195 (75.6) |
| Serious TEAE | 38 (21.8) | 38 (21.7) | 36 (20.7) | 64 (24.7) | 63 (24.2) | 42 (16.3) |
| Severity of worst TEAE | ||||||
| Died | 9 (5.2) | 6 (3.4) | 8 (4.6) | 22 (8.5) | 20 (7.7) | 10 (3.9) |
| Life-threatening | 3 (1.7) | 5 (2.9) | 4 (2.3) | 5 (1.9) | 1 (0.4) | 4 (1.6) |
| Severe | 29 (16.7) | 35 (20.0) | 26 (14.9) | 38 (14.7) | 44 (16.9) | 33 (12.8) |
| Moderate | 82 (47.1) | 69 (39.4) | 80 (46.0) | 111 (42.9) | 108 (41.5) | 108 (41.9) |
| Mild | 14 (8.0) | 33 (18.9) | 29 (16.7) | 34 (13.1) | 50 (19.2) | 40 (15.5) |
| TEAE related to treatment | ||||||
| Study drug | 60 (34.5) | 53 (30.3) | 53 (30.5) | 96 (37.1) | 80 (30.8) | 70 (27.1) |
| Pirfenidone | 22 (12.6) | 28 (16.0) | 26 (14.9) | 19 (7.3) | 34 (13.1) | 30 (11.6) |
| Nintedanib | 50 (28.7) | 39 (22.3) | 26 (14.9) | 80 (30.9) | 54 (20.8) | 42 (16.3) |
| Dose reductions, interruptions, or discontinuations | ||||||
| Study drug | ||||||
| Permanently stopped | 18 (10.3) | 10 (5.7) | 13 (7.5) | 29 (11.2) | 27 (10.4) | 18 (7.0) |
| Interrupted | 30 (17.2) | 29 (16.6) | 23 (13.2) | 57 (22.0) | 37 (14.2) | 33 (12.8) |
| Reduced | 9 (5.2) | 6 (3.4) | 3 (1.7) | 30 (11.6) | 12 (4.6) | 9 (3.5) |
| Pirfenidone | ||||||
| Permanently stopped | 3 (1.7) | 2 (1.1) | 4 (2.3) | 8 (3.1) | 3 (1.2) | 8 (3.1) |
| Interrupted | 4 (2.3) | 4 (2.3) | 5 (2.9) | 8 (3.1) | 9 (3.5) | 5 (1.9) |
| Reduced | 1 (0.6) | 3 (1.7) | 3 (1.7) | 5 (1.9) | 6 (2.3) | 2 (0.8) |
| Nintedanib | ||||||
| Permanently stopped | 4 (2.3) | 6 (3.4) | 0 | 10 (3.9) | 3 (1.2) | 2 (0.8) |
| Interrupted | 13 (7.5) | 10 (5.7) | 10 (5.7) | 25 (9.7) | 19 (7.3) | 14 (5.4) |
| Reduced | 7 (4.0) | 3 (1.7) | 6 (3.4) | 24 (9.3) | 8 (3.1) | 10 (3.9) |
Abbreviation: TEAE, treatment-emergent adverse event.
The events occurred during the trial, on or after the start of study drug intake, and within 30 days after the last dose of study drug until the end of the trial.
Diarrhea was the most common treatment-emergent adverse event (TEAE) leading to dose reductions for nintedanib (ISABELA 1: 6.7% in the 600 mg of ziritaxestat group, 4.9% in the 200 mg of ziritaxestat group, and 3.3% in the placebo group; ISABELA 2: 22.5%, 6.6%, and 8.8%, respectively) or dose interruptions (ISABELA 1: 10.0% in the 600 mg of ziritaxestat group, 8.2% in the 200 mg of ziritaxestat group, and 6.7% in the placebo group; ISABELA 2: 22.5%, 13.2%, and 8.8%, respectively). The mean daily doses of pirfenidone and nintedanib appear in eTable 3 in Supplement 3. During the ISABELA 1 trial, 5.0% of patients switched from nintedanib to pirfenidone and 5.9% of patients switched in the ISABELA 2 trial, whereas 1.9% switched from pirfenidone to nintedanib in the ISABELA 1 trial and 1.6% in the ISABELA 2 trial. In the ISABELA 1 trial, 11.9% of patients who were assigned ziritaxestat alone (ie, no additional use of pirfenidone or nintedanib) initiated treatment with pirfenidone or nintedanib (standard of care) during the trial; and in the ISABELA 2 trial, 9.1% of patients initiated standard of care treatment (eTable 4 in Supplement 3).
Primary Outcome: Annual Rate of Decline for FVC
Ziritaxestat did not improve the annual rate of decline for FVC vs placebo (Table 3). In the ISABELA 1 trial, the least-squares mean annual rate of decline for FVC at week 52 was –124.6 mL (95% CI, –178.0 to –71.2 mL) with 600 mg of ziritaxestat, –173.9 mL (95% CI, –225.7 to –122.2 mL) with 200 mg of ziritaxestat, and –147.3 mL (95% CI, –199.8 to –94.7 mL) with placebo. In the ISABELA 2 trial, the least-squares mean annual rate of FVC decline at week 52 was –173.8 mL (95% CI, –209.2 to –138.4 mL) with 600 mg of ziritaxestat, –174.9 mL (95% CI, –209.5 to –140.2 mL) with 200 mg of ziritaxestat, and –176.6 mL (95% CI, –211.4 to –141.8 mL) with placebo. Pooled data from both trials appear in Table 3.
Table 3. Primary Outcome of Annual Rate of Decline for Forced Vital Capacity (FVC) at Week 52 and at the End of the ISABELA 1 and ISABELA 2 Trials.
| Annual rate of decline for FVC | 600 mg of Ziritaxestat | 200 mg of Ziritaxestat | Placebo | Between-group difference (95% CI), mLa | ||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | Least-squares mean (95% CI), mL |
No. of patients | Least-squares mean (95% CI), mL |
No. of patients | Least-squares mean (95% CI), mL |
600 mg of Ziritaxestat vs placebo |
200 mg of Ziritaxestat vs placebo |
|
| At 52 wk | ||||||||
| ISABELA 1 | 174 | −124.6 (−178.0 to −71.2) | 175 | −173.9 (−225.7 to −122.2) | 174 | −147.3 (−199.8 to −94.7) | 22.7 (−52.3 to 97.6) | −26.7 (−100.5 to 47.1) |
| ISABELA 2 | 259 | −173.8 (−209.2 to −138.4) | 260 | −174.9 (−209.5 to −140.2) | 258 | −176.6 (−211.4 to −141.8) | 2.8 (−46.9 to 52.4) | 1.7 (−47.4 to 50.8) |
| Pooled results (ISABELA 1 and 2) | 428 | −156.5 (−186.2 to −126.7) | 431 | −173.7 (−202.7 to −144.7) | 422 | −165.7 (−194.9 to −136.4) | 9.2 (−32.5 to 50.9) | −8.0 (−49.2 to 33.1) |
| At end of the trials (ISABELA 1 and 2) | ||||||||
| Pooled results | 428 | −161.0 (−187.4 to −134.5) | 431 | −174.4 (−200.0 to −148.7) | 422 | −169.0 (−195.0 to −143.0) | 8.0 (−29.0 to 45.1) | −5.4 (−41.9 to 31.1) |
| Pirfenidone | 151 | −194.3 (−238.2 to −150.3) | 154 | −217.9 (−259.5 to −176.3) | 149 | −189.0 (−231.6 to −146.3) | −5.3 (−66.6 to 56.0) | −29.0 (−88.6 to 30.6) |
| Nintedanib | 146 | −132.4 (−176.2 to −88.6) | 151 | −132.2 (−175.2 to −89.2) | 147 | −163.1 (−205.7 to −120.5) | 30.7 (−30.4 to 91.9) | 30.9 (−29.7 to 91.5) |
| Neither treatment | 131 | −155.1 (−204.8 to −105.3) | 126 | −168.4 (−217.5 to −119.3) | 126 | −149.1 (−199.5 to −98.6) | −6.0 (−76.9 to 64.9) | −19.3 (−89.7 to 51.1) |
A positive difference in values indicates that patients taking ziritaxestat deteriorate less than those taking placebo.
Similar results were observed in the sensitivity analysis that included all data until the end of each trial; when analyzed by standard of care treatment, the annual rate of decline for FVC was greatest in those taking pirfenidone (Table 3). In each trial, the P value for interaction was <.001 for study treatment, standard of care treatment, and time, indicating a significant interaction. For the primary outcome of annual rate of decline for FVC, 19 patients (1%) from the full analysis set (n = 1281) were excluded from the linear slope model analysis due to missing FVC data after baseline. At week 52, FVC data were missing for 92 of 646 patients (14%).
Key Secondary Outcomes
At week 52, disease progression was similar across treatment groups both when data from the ISABELA 1 trial and the ISABELA 2 trial were pooled and when each trial was assessed separately (eFigure 2 in Supplement 3).
For time to first respiratory-related hospitalization, the outcomes were worse in the ziritaxestat groups vs the placebo groups in both trials (eFigure 3 in Supplement 3). The reasons for hospitalization appear in eTable 5 in Supplement 3. The change from baseline in SGRQ total score at week 52 was similar across treatment groups in each trial (eTable 6 in Supplement 3).
Other Secondary Outcomes
For the following outcomes, worse outcomes were observed with ziritaxestat vs placebo: time to respiratory-related mortality, time to all-cause mortality or respiratory-related hospitalization, and time to first acute IPF exacerbation (eFigures 4-6 in Supplement 3).
The pooled study data showed observed mean change from baseline in FVC was similar across treatment groups at week 52 (eFigure 7 in Supplement 3). The changes from baseline in FVC by standard of care treatment appear in eFigures 8-9 in Supplement 3.
The distance on the 6-minute walk test decreased from baseline to week 52 in all treatment groups in both trials (eTable 6 in Supplement 3).
All-Cause Mortality
The mortality rate until the end of the trials was numerically higher with 600 mg of ziritaxestat vs placebo in the ISABELA 1 trial and with both doses of ziritaxestat vs placebo in the ISABELA 2 trial (Table 4). The pooled data showed all-cause mortality was 8.9% with 600 mg of ziritaxestat and 7.0% with 200 mg of ziritaxestat vs 5.5% with placebo (HR, 1.8 [95% CI, 1.1 to 3.0] for 600 mg of ziritaxestat vs placebo and HR, 1.3 [95% CI, 0.8 to 2.3] for 200 mg of ziritaxestat vs placebo; Table 4).
Table 4. Risk of All-Cause Mortality With Ziritaxestat vs Placebo at the End of the ISABELA 1 and ISABELA 2 Trials.
| All-cause mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 600 mg of Ziritaxestat (n = 428) | 200 mg of Ziritaxestat (n = 431) | Placebo (n = 422) | 600 mg of Ziritaxestat vs placebo | 200 mg of Ziritaxestat vs placebo | ||||||
| No. of events (%) | IR/100 patient-years of exposure | No. of events (%) | IR/100 patient-years of exposure | No. of events (%) | IR/100 patient-years of exposure | Between-group difference in IR (95% CI) |
Hazard ratio (95% CI) | Between-group difference in IR (95% CI) |
Hazard ratio (95% CI) | |
| ISABELA 1 | 14 (8.0) | 8.1 | 8 (4.6) | 4.3 | 11 (6.3) | 6.3 | 1.8 (−4.3 to 8.0) | 1.4 (0.6 to 3.1) | −1.9 (−7.5 to 3.6) | 0.7 (0.3 to 1.8) |
| ISABELA 2 | 24 (9.3) | 8.9 | 22 (8.5) | 8.1 | 12 (4.7) | 4.4 | 4.5 (0 to 9.1) | 2.1 (1.1 to 4.3) | 3.7 (−0.7 to 8.1) | 1.9 (0.9 to 3.8) |
| Pooled results (ISABELA 1 and 2) | 38 (8.9) | 8.6 | 30 (7.0) | 6.6 | 23 (5.5) | 5.1 | 3.5 (−0.1 to 7.1) | 1.8 (1.1 to 3.0) | 1.5 (−1.9 to 4.8) | 1.3 (0.8 to 2.3) |
Abbreviation: IR, incidence rate.
Respiratory-related deaths were the primary cause of mortality (adjudicated events that took place during the trials) and occurred in 3.6% of patients in the 600 mg of ziritaxestat group, 3.5% in the 200 mg of ziritaxestat group, and 1.8% in the placebo group in the ISABELA 1 trial and in 5.8%, 4.2%, and 1.6% of patients, respectively, in the ISABELA 2 trial (eTable 7 in Supplement 3).
TEAEs
In the ISABELA 1 trial, 78.7% of patients in the 600 mg of ziritaxestat group had 1 or more TEAE, 84.6% in the 200 mg of ziritaxestat group, and 84.5% in the placebo group (Table 2). In the ISABELA 2 trial, 81.1% of patients in the 600 mg of ziritaxestat group had 1 or more TEAE, 85.8% in the 200 mg of ziritaxestat group, and 75.6% in the placebo group. In the ISABELA 1 trial, serious TEAEs occurred in 21.8% of patients in the 600 mg of ziritaxestat group, 21.7% in the 200 mg of ziritaxestat group, and 20.7% in the placebo group (Table 2). In the ISABELA 2 trial, serious TEAEs occurred in 24.7% of patients in the 600 mg of ziritaxestat group, 24.2% in the 200 mg of ziritaxestat group, and 16.3% in the placebo group. The data on TEAEs by standard of care treatment appear in eTable 8 in Supplement 3. The TEAEs with an incidence rate of 5% or greater in at least 1 treatment group appear in eTable 9 in Supplement 3. The most common TEAEs were gastrointestinal disorders.
In the ISABELA 1 trial, the TEAEs leading to death occurred in 5.2% of patients in the 600 mg of ziritaxestat group, 3.4% in the 200 mg of ziritaxestat group, and 4.6% in the placebo group (Table 2). In the ISABELA 2 trial, the TEAEs leading to death occurred in 8.5% of patients in the 600 mg of ziritaxestat group, 7.7% in the 200 mg of ziritaxestat group, and 3.9% in the placebo group.
In the 600 mg of ziritaxestat group, IPF was the most common TEAE leading to death in both trials (n = 4 in ISABELA 1 and n = 5 in ISABELA 2). In the 200 mg of ziritaxestat group, COVID-19 pneumonia was the most common TEAE leading to death in ISABELA 1 (n = 2) and COVID-19 was the most common TEAE leading to death in ISABELA 2 (n = 4) (eTable 10 in Supplement 3).
The causes of death as provided by the investigators appear in eTable 11 in Supplement 3. Overall, COVID-19–related TEAEs occurred in 3.8% of patients (eTable 12 in Supplement 3). Adjudicated deaths due to COVID-19 occurred in 0% of patients in the 600 mg of ziritaxestat group, 1.8% in the 200 mg of ziritaxestat group, and 0.6% in the placebo group in the ISABELA 1 trial and 0.8%, 1.9%, and 0%, respectively, in the ISABELA 2 trial. Data on the number of missing study visits because of COVID-19 appears in eTable 13 in Supplement 3.
Pharmacokinetics and Target Engagement
Pharmacokinetic parameters of ziritaxestat appear in eTable 14 in Supplement 3. A mixture model, which accounted for 2 responder subtypes, showed that in more than 70% of the patients receiving active therapy, lysophosphatidic acid level decreased from baseline (eFigure 10 in Supplement 3). In the remaining patients, which was a smaller cohort, there was an increase in lysophosphatidic acid level after treatment that was not attributable to any difference in exposure to ziritaxestat.
Discussion
In the ISABELA 1 and ISABELA 2 trials, ziritaxestat did not lead to a reduction in the annual rate of decline for FVC vs placebo; thus, the primary outcome was not met. Ziritaxestat also failed to show benefit in any of the secondary efficacy outcomes (time to first respiratory-related hospitalization, time to first respiratory-related mortality, time to first all-cause mortality or respiratory-related hospitalization, time to first acute IPF exacerbation, SGRQ total score, or distance on 6-minute walk test). All-cause mortality data showed a higher proportion of deaths with those taking 600 mg of ziritaxestat than with placebo in ISABELA 1, and a higher proportion of deaths with each ziritaxestat dose than with placebo in ISABELA 2.
Ziritaxestat did not reduce FVC decline compared with placebo, unlike in the prior phase 2a study,16 although the latter included a limited number of patients. As in the phase 2a study, decreases in lysophosphatidic acid were observed after ziritaxestat dosing; therefore, lack of target engagement is not considered the reason for the absence of effect on the clinical outcomes. It is unknown why the positive results of the prior phase 2a study were not replicated in the ISBAELA trials, but the limitations associated with early phase trials such as small sample sizes, short duration, and limited use of standard of care therapies may be contributing factors.
Lung function in patients receiving standard of care therapies was expected to decline at a slower rate than in untreated patients; however, this was not the case in the 2 ISABELA trials. The extent of FVC decline in the ISABELA trials differed between type of standard of care therapy and was greatest in patients taking pirfenidone. Even though suboptimal pirfenidone dosing was observed in some patients, it was not overly frequent, and is not thought to explain the apparent worsening of lung function. In addition, phase 1 data show ziritaxestat does not affect pirfenidone concentration. Therefore, the reason for this finding remains unclear.
Unlike for pirfenidone, phase 1 studies show ziritaxestat increases plasma levels of nintedanib. An increase in nintedanib levels could have led to the higher proportion of dose reductions, dose interruptions, and nondiarrheal TEAEs observed with nintedanib vs pirfenidone in the 600 mg of ziritaxestat group in the ISABELA trials. However, the dose of nintedanib administered has been shown to predict risk of diarrhea (this was the most frequent TEAE in the 600 mg of ziritaxestat group) better than plasma exposure.21 In the prior phase 2a study,16 ziritaxestat was administered as monotherapy and treatment with nintedanib was prohibited, reducing the probability of dose reductions or treatment interruptions due to interactions between drugs.
As stated, the independent data and safety monitoring committee’s recommendation to terminate the ISABELA trials was based on both a lack of efficacy and a perceived increased mortality risk. Pooled data from both ISABELA trials showed an all-cause mortality rate of 8.9% with 600 mg of ziritaxestat, 7.0% with 200 mg of ziritaxestat, and 5.5% with placebo over a study duration of longer than 100 weeks. A greater proportion of patient deaths occurred with 600 mg of ziritaxestat than with placebo in ISABELA 1, and there was a greater proportion of patient deaths with both ziritaxestat doses vs placebo in ISABELA 2. At the time of trial termination, the number of patients enrolled and the number of patient-years of study drug exposure were greater in ISABELA 2 than in ISABELA 1. Compared with ISABELA 1, there was a greater proportion of Asian patients in ISABELA 2. Whether this contributed to the greater proportion of deaths (including COVID-19–related deaths) in ISABELA 2 requires greater understanding of regional and racial differences in patients with IPF.
The COVID-19 pandemic, which arose after the trials were initiated, had some effect on study conduct and resulted in many clinic-based visits being missed by patients. Study safety was ensured by permitting telephone visits in place of scheduled clinic visits and performance of blood safety assessments in local laboratories. The proportion of COVID-19–related deaths was low. When these COVID-19–related deaths occurred, they disproportionally affected patients in the ziritaxestat treatment groups. A limited proportion of patients had missing spirometry data (1% had no FVC data after the baseline assessment), which arguably did not affect the analyses, and in addition, a mixed model for repeated measures was used, which mitigates the effect of some missing data.
Despite the regulatory requirement, testing new IPF medications when the patient is taking a standard of care therapy is challenging because lung function is likely to decline at a slower rate than in untreated patients; however, as noted, this was not the case in the ISABELA trials. Furthermore, variability among individual patients may be amplified when testing a new therapy if the patient is taking a standard of care therapy, which can make data interpretation challenging.
Although the design of the ISABELA trials is not considered to have contributed to the negative findings, possible considerations for future IPF studies (which may increase the likelihood of identifying treatment effects) include using adaptive designs with a bayesian approach and using biomarker-based enrichment strategies with prognostic biomarkers of early or more rapid disease progression.22 Knowledge of patients’ prior change in lung function would also allow better understanding of the rate of decline after the introduction of therapy. Even though the ISABELA trials failed, they demonstrate the potential value of observing data for 52 weeks or longer and provide information regarding the utility of different clinical outcomes. In addition, information collected during the trials, such as the faster than anticipated rate of decline for FVC in the patients taking standard of care therapy, adverse events associated with standard of care, and treatment patterns (eg, the proportion of patients switching or initiating standard of care therapy during the trials), may help inform the design of future IPF studies.
Further investigation is needed to determine why the ISABELA trials failed. This may be determined by ongoing studies of other autotaxin inhibitors with different pharmacological characteristics to those of ziritaxestat (such as BBT-87723) or lysophosphatidic acid receptor antagonists (such as BMS-98627824). Of note, the lysophosphatidic acid receptor antagonist BMS-986020 was discontinued due to hepatobiliary toxicity; however, this was found to be unrelated to lysophosphatidic acid antagonism25,26 and, indeed, no such safety issues were identified in the ISABELA trials.
Limitations
There are several limitations to the 2 trials. First, the early termination of the trials is considered a possible limitation because it may have reduced the ability to adequately interpret the effect of treatment on the primary outcome. Second, enrollment in the ISABELA 1 trial was not complete so fewer patients entered the trial than planned and the outcomes beyond week 52 were not captured for all patients. Third, there were missing data or unattended study visits due to the COVID-19 pandemic. Fourth, the COVID-19 pandemic also may have influenced trial participation, and therefore the patient population may not reflect those participating in prior IPF trials. Fifth, the ISABELA trials were not powered to assess true differences among standard of care treatments (nintedanib vs pirfenidone vs no standard of care treatment).
Conclusions
Ziritaxestat did not improve clinical outcomes compared with placebo in patients with IPF receiving standard of care treatment with pirfenidone or nintedanib or in those not receiving standard of care treatment.
Trial protocol
Statistical analysis plan
eFigure 1. Number of patients in each country participating in ISABELA 1 and 2
eFigure 2. Time to disease progression (composite endpoint of first occurrence of ≥10% absolute decline in percent predicted FVC or all-cause mortality) in ISABELA 1 (a), ISABELA 2 (b) and ISABELA 1 and 2 combined (c)
eFigure 3. Time to first adjudicated respiratory-related hospitalization for ISABELA 1 (a), ISABELA 2 (b) and ISABELA 1 and 2 combined (c)
eFigure 4. Time to respiratory-related mortality in ISABELA 1 (a) and ISABELA 2 (b) (adjudicated)
eFigure 5. Time to all-cause mortality or respiratory-related hospitalization (adjudicated) for ISABELA 1 (a), ISABELA 2 (b) and ISABELA 1 and 2 combined (c)
eFigure 6. Time to first acute IPF exacerbation (adjudicated) in ISABELA 1 (a) and ISABELA 2 (b)
eFigure 7. Mean change from baseline in FVC over time for ISABELA 1 and 2 combined
eFigure 8. Change from baseline in FVC in the pirfenidone (a), nintedanib (b) and neither (c) stratum of ISABELA 1
eFigure 9. Change from baseline in FVC in the pirfenidone (a), nintedanib (b) and neither (c) stratum of ISABELA 2
eFigure 10. Two responder phenotypes in the population of ISABELA 1 (a), ISABELA 2 (b) and ISABELA 1 and 2 combined (c)
eMethods
List of investigators who randomized patients to the studies
eTable 1. Baseline demographics and disease characteristics by strata
eTable 2. Duration of treatment and exposure (until the end of the studies)
eTable 3. Doses of standard of care during the study period
eTable 4. Number of patients who switched standard of care (until the end of the studies)
eTable 5. Primary cause of adjudicated all-cause hospitalization
eTable 6. Change from baseline at Week 52 in efficacy endpoints
eTable 7. Adjudicated all-cause mortality events in ISABELA 1 and 2
eTable 8. Treatment-emergent adverse events and dose reductions/interruptions/discontinuations according to stratum in ISABELA 1 and 2
eTable 9. Treatment-emergent adverse events with a ≥5% incidence in at least one treatment group in ISABELA 1 and 2
eTable 10. Treatment-emergent adverse events leading to death in ISABELA 1 and 2
eTable 11. Summary of adverse events leading to deaths
eTable 12. Treatment-emergent adverse events related to COVID-19 infections and suspected infections in ISABELA 1 and 2
eTable 13. Impact of COVID-19 on study visits
eTable 14. Model-derived ziritaxestat pharmacokinetic parameters in ISABELA 1 and 2
eReferences
Nonauthor collaborators
Data sharing statement
References
- 1.Martinez FJ, Collard HR, Pardo A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. [DOI] [PubMed] [Google Scholar]
- 2.Kreuter M, Swigris J, Pittrow D, et al. The clinical course of idiopathic pulmonary fibrosis and its association to quality of life over time. Respir Res. 2019;20(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431-440. [DOI] [PubMed] [Google Scholar]
- 4.Sharif R. Overview of idiopathic pulmonary fibrosis (IPF) and evidence-based guidelines. Am J Manag Care. 2017;23(11)(suppl):S176-S182. [PubMed] [Google Scholar]
- 5.Galli JA, Pandya A, Vega-Olivo M, et al. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice. Respirology. 2017;22(6):1171-1178. [DOI] [PubMed] [Google Scholar]
- 6.Oikonomou N, Mouratis MA, Tzouvelekis A, et al. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47(5):566-574. [DOI] [PubMed] [Google Scholar]
- 7.Funke M, Zhao Z, Xu Y, et al. The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury. Am J Respir Cell Mol Biol. 2012;46(3):355-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sgalla G, Iovene B, Calvello M, et al. Idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraljić K, Jelić D, Žiher D, et al. Benzoxaboroles-novel autotaxin inhibitors. Molecules. 2019;24(19):3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tager AM, LaCamera P, Shea BS, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14(1):45-54. [DOI] [PubMed] [Google Scholar]
- 11.Van Der Aar EM, Fagard L, Desrivot J, et al. Favorable human safety, pharmacokinetics and pharmacodynamics of the autotaxin inhibitor GLPG1690, a potential new treatment in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:A2701. [Google Scholar]
- 12.Desroy N, Housseman C, Bock X, et al. Discovery of 2-[[2-ethyl-6-[4-[2-(3-hydroxyazetidin-1-yl)-2-oxoethyl]piperazin-1-yl]-8-methylimidazo[1,2-a]pyridin-3-yl]methylamino]-4-(4-fluorophenyl)thiazole-5-carbonitrile (GLPG1690), a first-in-class autotaxin inhibitor undergoing clinical evaluation for the treatment of idiopathic pulmonary fibrosis. J Med Chem. 2017;60(9):3580-3590. [DOI] [PubMed] [Google Scholar]
- 13.Coornaert B, Duys I, Van Der Schueren J, et al. Autotaxin inhibitor GLPG1690 affects TGFβ-induced production of the pro-fibrotic mediators CTGF, IL-6 and ET-1 in fibroblasts. Am J Respir Crit Care Med. 2017;195:A2404. [Google Scholar]
- 14.Ongenaert M, Dupont S, Blanqué R, et al. Strong reversal of the lung fibrosis disease signature by autotaxin inhibitor GLPG1690 in a mouse model for IPF. Eur Respir J. 2016;48(suppl 60):OA4540. doi: 10.1183/13993003.congress-2016.OA4540 [DOI] [Google Scholar]
- 15.Heckmann B, Blanque R, Triballeau N, et al. Autotaxin inhibitors in IPF. Am J Respir Crit Care Med. 2019;199:A2585. [Google Scholar]
- 16.Maher TM, van der Aar EM, Van de Steen O, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA). Lancet Respir Med. 2018;6(8):627-635. [DOI] [PubMed] [Google Scholar]
- 17.Maher TM, Kreuter M, Lederer DJ, et al. Rationale, design and objectives of two phase III, randomised, placebo-controlled studies of GLPG1690, a novel autotaxin inhibitor, in idiopathic pulmonary fibrosis (ISABELA 1 and 2). BMJ Open Respir Res. 2019;6(1):e000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taneja A, Desrivot J, Diderichsen PM, et al. Population pharmacokinetic and pharmacodynamic analysis of GLPG1690, an autotaxin inhibitor, in healthy volunteers and patients with idiopathic pulmonary fibrosis. Clin Pharmacokinet. 2019;58(9):1175-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molenberghs GV, Verbeke G. Linear Mixed Models for Longitudinal Data. Springer; 2000. [Google Scholar]
- 20.Camarri B, Eastwood PR, Cecins NM, et al. Six minute walk distance in healthy subjects aged 55-75 years. Respir Med. 2006;100(4):658-665. [DOI] [PubMed] [Google Scholar]
- 21.Schmid U, Weber B, Sarr C, Freiwald M. Exposure-safety analyses of nintedanib in patients with chronic fibrosing interstitial lung disease. BMC Pulm Med. 2021;21(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan F, Stewart I, Howard L, et al. The Its Not JUST Idiopathic pulmonary fibrosis Study (INJUSTIS). BMJ Open Respir Res. 2019;6(1):e000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee G, Kang S, Ryou J-H, et al. BBT-877, a potent autotaxin inhibitor in clinical development to treat idiopathic pulmonary fibrosis. Eur Respir J. 2019;54:PA1293. doi: 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2577 [DOI] [Google Scholar]
- 24.Corte TJ, Lancaster L, Swigris JJ, et al. Phase 2 trial design of BMS-986278, a lysophosphatidic acid receptor 1 (LPA1) antagonist, in patients with idiopathic pulmonary fibrosis (IPF) or progressive fibrotic interstitial lung disease (PF-ILD). BMJ Open Respir Res. 2021;8(1):e001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill MW, Murphy BJ, Cheng PTW, et al. Mechanism of hepatobiliary toxicity of the LPA1 antagonist BMS-986020 developed to treat idiopathic pulmonary fibrosis. Toxicol Appl Pharmacol. 2022;438:115885. [DOI] [PubMed] [Google Scholar]
- 26.Zulfikar S, Mulholland S, Adamali H, Barratt SL. Inhibitors of the autotaxin-lysophosphatidic acid axis and their potential in the treatment of interstitial lung disease. Clin Pharmacol. 2020;12:97-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eFigure 1. Number of patients in each country participating in ISABELA 1 and 2
eFigure 2. Time to disease progression (composite endpoint of first occurrence of ≥10% absolute decline in percent predicted FVC or all-cause mortality) in ISABELA 1 (a), ISABELA 2 (b) and ISABELA 1 and 2 combined (c)
eFigure 3. Time to first adjudicated respiratory-related hospitalization for ISABELA 1 (a), ISABELA 2 (b) and ISABELA 1 and 2 combined (c)
eFigure 4. Time to respiratory-related mortality in ISABELA 1 (a) and ISABELA 2 (b) (adjudicated)
eFigure 5. Time to all-cause mortality or respiratory-related hospitalization (adjudicated) for ISABELA 1 (a), ISABELA 2 (b) and ISABELA 1 and 2 combined (c)
eFigure 6. Time to first acute IPF exacerbation (adjudicated) in ISABELA 1 (a) and ISABELA 2 (b)
eFigure 7. Mean change from baseline in FVC over time for ISABELA 1 and 2 combined
eFigure 8. Change from baseline in FVC in the pirfenidone (a), nintedanib (b) and neither (c) stratum of ISABELA 1
eFigure 9. Change from baseline in FVC in the pirfenidone (a), nintedanib (b) and neither (c) stratum of ISABELA 2
eFigure 10. Two responder phenotypes in the population of ISABELA 1 (a), ISABELA 2 (b) and ISABELA 1 and 2 combined (c)
eMethods
List of investigators who randomized patients to the studies
eTable 1. Baseline demographics and disease characteristics by strata
eTable 2. Duration of treatment and exposure (until the end of the studies)
eTable 3. Doses of standard of care during the study period
eTable 4. Number of patients who switched standard of care (until the end of the studies)
eTable 5. Primary cause of adjudicated all-cause hospitalization
eTable 6. Change from baseline at Week 52 in efficacy endpoints
eTable 7. Adjudicated all-cause mortality events in ISABELA 1 and 2
eTable 8. Treatment-emergent adverse events and dose reductions/interruptions/discontinuations according to stratum in ISABELA 1 and 2
eTable 9. Treatment-emergent adverse events with a ≥5% incidence in at least one treatment group in ISABELA 1 and 2
eTable 10. Treatment-emergent adverse events leading to death in ISABELA 1 and 2
eTable 11. Summary of adverse events leading to deaths
eTable 12. Treatment-emergent adverse events related to COVID-19 infections and suspected infections in ISABELA 1 and 2
eTable 13. Impact of COVID-19 on study visits
eTable 14. Model-derived ziritaxestat pharmacokinetic parameters in ISABELA 1 and 2
eReferences
Nonauthor collaborators
Data sharing statement