Abstract
Background:
The TP53 signature that predicts the mutation status of TP53 has been shown to be a prognostic factor and predictor of neoadjuvant chemotherapy (NAC) response.
Objectives:
The current study sought to investigate the utility of the TP53 signature for predicting pathological complete response (pCR) and its prognostic significance among patients with residual disease (RD).
Design:
The study followed a retrospective cohort study design.
Methods:
Patients with T1-3/N0-1 from a cohort of those with HER2-negative breast cancer who received NAC were selected. Ability to predict pCR was evaluated using odds ratio, positive and negative predictive values, sensitivity, and specificity. Prognostic factors in the RD group were explored using the Cox proportional hazards model with distant recurrence-free survival (DRFS). Four independent cohorts were used for validation.
Results:
A total of 333 eligible patients were classified into the TP53 mutant signature (n = 154) and wild-type signature (n = 179). Among the molecular and pathological factors, the TP53 signature had the highest predictive power for pCR. In 4 independent cohorts (n = 151, 85, 104, and 67, respectively), pCR rate in TP53 mutant signature group was significantly higher than that in the wild-type group. Univariate and multivariate analyses on DRFS in the RD group identified the TP53 signature and nodal status as independent prognostic factors, with the former having a better hazard ratio than the latter. After comparing DRFS between 3 groups (pCR, RD/TP53 wild-type signature, and RD/TP53 mutant signature groups), the RD/TP53 mutant signature group showed significantly worse prognosis compared with others. The RD/TP53 wild-type signature group did not exhibit inferior DRFS compared with the pCR group.
Conclusion:
Our results showed that the TP53 mutant signature can predict pCR and that combining pathological response and TP53 mutant signature allows for the identification of subgroups with truly poor prognosis.
Keywords: Breast cancer, neoadjuvant chemotherapy, biomarker, gene expression profile, TP53 gene
Introduction
In 2020, breast cancer had to become the most common type of cancer, accounting for 11.7% (2 261 419 cases) of the total number of cancer cases among men and women and surpassing lung cancer at 11.4% (2 206 771 cases). 1 Therefore, the treatment and prognostic improvement of patients with breast cancer is becoming increasingly important.
Neoadjuvant chemotherapy (NAC) has been widely used to increase the chances of breast-conserving surgery in cases with resectable breast cancer,2,3 with studies suggesting the response to NAC to be a prognostic factor.4,5 Accordingly, patients with pathological complete response (pCR) after NAC have shown favorable prognosis, whereas those without pCR (residual disease, RD) have shown otherwise. Therefore, establishing predictors of pCR may be important for optimizing NAC. Reports have also shown that the addition of postoperative chemotherapy improves prognosis of patients with RD.6-8 As such, clarifying the prognostic factors in patients with RD would allow the selection of the appropriate postoperative treatment.
Several studies have reported that the tumor-suppressor gene TP53 mutation status can be a prognostic factor and predictor of NAC response.9-13 In addition, evidence has shown that the TP53 signature, a gene expression profile that predicts the mutation status of TP53, can also be a predictor of prognosis and NAC response.14-18
The current study therefore investigated the ability of the TP53 signature for predicting pCR, as well as its prognostic significance, among patients with RD.
Materials and Methods
Cohort
A total of 333 patients with T1-3/N0-1 from a cohort (GSE25066) of those with HER2-negative breast cancer (n = 508) who received NAC with taxane and anthracycline were selected. 19 Data on clinicopathological factors, distant recurrence-free survival (DRFS), and pCR were obtained from the database (Gene Expression Omnibus; https://www.ncbi.nlm.nih.gov/geo/). Four independent cohorts (GSE20194, GSE20271, GSE32603, and GSE140494) were used to validate the predictive value of TP53 signature for pCR.20-23 GSE32603 was used to validate the prognostic value of TP53 signature. In GSE20194, GSE20271, and GSE140494, HER2-negative and T1-3/N0-1 cases were included as in GSE25066. In GSE32603, T-category and N-category data were not available for each case; therefore, 104 cases with HER2-negative and pCR data were included. GSE32603 had recurrence-free survival (RFS) data.
Microarray data
Raw or normalized gene expression data were obtained from Gene Expression Omnibus. Raw data were normalized using standard methods with GeneSpringGX version 14.5 (Agilent Technologies, Santa Clara, CA, USA). Among the 33 genes in the TP53 signature gene set, the list of genes that could be obtained in each cohort is shown in Supplemental Table 1.
Diagnostic validity of the TP53 signature using 25 or 27 genes
The diagnostic validity of TP53 signature using the gene sets available in each cohort was examined using microarray data from the original cohort (n = 38). 14 The TP53 signature status was determined according to the methods previously described. 14 The reference TP53 mutant and wild-type signatures were obtained from the previous study (Supplemental Table 1). The TP53 signature status of a case was determined using the correlation coefficient to the reference TP53 signature mutant signature, and if the correlation coefficient was ⩾0, the TP53 signature status of the case was diagnosed as mutant type. For the 38 samples in the original cohort, we compared the TP53 signature status diagnosed using the 33 genes with that of those diagnosed using the 25 and 27 genes that were available in each cohort. The TP53 signature status when using the 25 or 27 genes was in perfect concordance with the TP53 signature status using the 33 genes. Based on these results, the TP53 signature status was determined by the method described above using the TP53 signature gene set available in each cohort.
Ability to predict pCR
The ability to predict pCR was evaluated using odds ratio, positive predictive value (PPV), negative predictive value (NPV), sensitivity, and specificity.
Statistical analysis
The 2-sided Fisher exact test and Wilcoxon rank sum test (or Kruskal-Wallis test) were used to analyze patient background. Hazard ratios (HRs) and their confidence intervals (CIs) were calculated using the Cox proportional hazards model. DRFS was estimated using the Kaplan-Meier method, while the log rank test was used for between-group comparisons. A heat map was created using the Multiple Experiment Viewer (http://mev.tm4.org). All statistical analyses were performed using JMP pro, version 16.0.0 (SAS, Cary, NC), with P < .05 indicating statistical significance.
Results
Comparison of the TP53 signature status and patient background
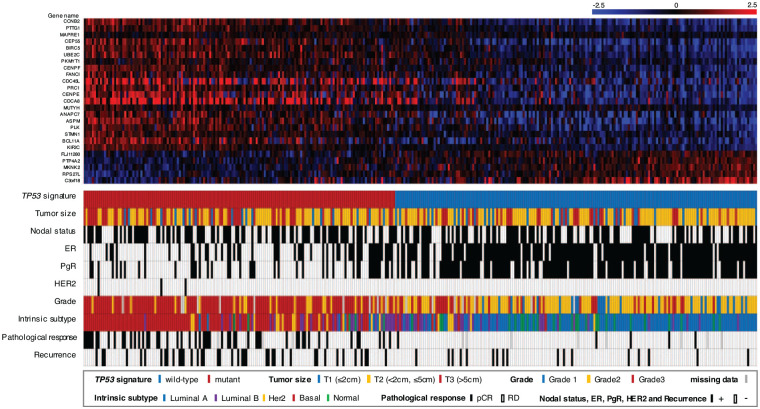
As a result of determining the TP53 signature status of 333 cases in GSE25066, 154 cases had TP53 mutant signature and 179 cases had wild-type signature. The heat map of the TP53 signature, TP53 signature status, clinicopathological background, pCR, and recurrence data are summarized in Figure 1. After comparing patient background between the TP53 mutant signature and wild-type signature groups (Table 1), our finding showed that the former had significantly more cases with a higher AJCC (American Joint Committee on Cancer) stage and T category than the later. The mutant signature group had significantly more cases positive for nodal status and negative for ER and PgR than the wild-type group. Regarding the intrinsic subtype, the mutant group had more basal type cases, while the wild-type group had more Luminal A and Normal type cases. These results were consistent with those presented in a previous report. 16
Figure 1.
Overview of the cohort. Upper heat map showing the TP53 signature. Lower panel shows the TP53 signature status, clinicopathological factors (tumor size, nodal status, ER, PgR, HER2 and grade), intrinsic subtype, pathological response (pCR or RD), and recurrence data. The patients were ordered according to correlation coefficients for the TP53 mutant signature. The color legends are shown in the figure. ER indicates estrogen receptor; HER2, human epidermal growth factor receptor type 2; pCR, pathological complete response; PgR, progesterone receptor; RD, residual disease.
Table 1.
Clinicopathological characteristics stratified according to TP53 signature status.
| Total |
Mutant signature |
Wild-type signature |
P a | ||||
|---|---|---|---|---|---|---|---|
| No. of patients | % | No. of patients | % | No. of patients | % | ||
| Samples | 333 | 154 | 46.3 | 179 | 53.8 | ||
| Age, years (median) | .84 | ||||||
| Median | 49 | 49 | 47.6 | ||||
| Range | 24-75 | 24-72 | 26-75 | ||||
| AJCC stage | .013 | ||||||
| I | 8 | 2.4 | 5 | 3.3 | 3 | 1.7 | |
| IIA | 113 | 33.9 | 39 | 25.3 | 74 | 41.3 | |
| IIB | 140 | 42.0 | 69 | 44.8 | 71 | 39.7 | |
| IIIA | 64 | 19.2 | 38 | 24.7 | 26 | 14.5 | |
| IIIB | 8 | 2.4 | 3 | 2.0 | 5 | 2.8 | |
| T category | .047 | ||||||
| T1 | 48 | 14.4 | 19 | 12.3 | 29 | 16.2 | |
| T2 | 192 | 57.7 | 82 | 53.3 | 110 | 61.5 | |
| T3 | 93 | 27.9 | 53 | 34.4 | 40 | 22.4 | |
| Nodal status | .58 | ||||||
| Positive | 187 | 56.2 | 89 | 57.8 | 98 | 54.8 | |
| Negative | 146 | 43.8 | 65 | 42.2 | 81 | 45.3 | |
| ER | <.0001 | ||||||
| Positive | 205 | 61.6 | 52 | 33.8 | 153 | 85.5 | |
| Negative | 125 | 37.5 | 102 | 66.2 | 23 | 12.9 | |
| NA | 3 | 0.90 | 0 | 0.0 | 3 | 1.7 | |
| PgR | <.0001 | ||||||
| Positive | 181 | 54.4 | 42 | 27.3 | 139 | 77.7 | |
| Negative | 148 | 44.4 | 111 | 72.1 | 37 | 20.7 | |
| NA | 4 | 1.2 | 1 | 0.3 | 3 | 1.7 | |
| HER2 | .10 | ||||||
| Positive | 3 | 0.90 | 3 | 2.0 | 0 | 0.0 | |
| Negative | 320 | 96.1 | 148 | 96.1 | 172 | 96.1 | |
| NA | 10 | 3.0 | 3 | 2.0 | 7 | 3.9 | |
| Grade | <.0001 | ||||||
| 1 | 25 | 7.5 | 0 | 0.0 | 25 | 14.0 | |
| 2 | 132 | 39.6 | 26 | 16.9 | 106 | 59.2 | |
| 3 | 158 | 47.5 | 118 | 76.6 | 40 | 22.4 | |
| NA | 18 | 5.4 | 10 | 6.5 | 8 | 4.5 | |
| Intrinsic subtype | <.0001 | ||||||
| Luminal A | 118 | 35.4 | 7 | 4.6 | 111 | 62.0 | |
| Luminal B | 53 | 15.9 | 24 | 15.6 | 29 | 16.2 | |
| HER2 | 20 | 6.0 | 14 | 9.1 | 6 | 3.4 | |
| Basal | 111 | 33.3 | 104 | 67.5 | 7 | 3.9 | |
| Normal | 31 | 9.3 | 5 | 3.3 | 26 | 14.5 | |
Abbreviations: AJCC, American Joint Committee on Cancer; ER, estrogen receptor; HER2, human epidermal growth factor receptor type 2; NA, not available; PgR, progesterone receptor.
Chi-square test was used for statistical analysis of patients’ characteristics except for age. Kruskal-Wallis test was used for statistical analysis of patients’ age.
Predictive factors for pCR
After examining the predictive power of molecular and pathological factors (TP53 signature, Grade, Intrinsic subtype, ER, and PR) for pCR, our results found that the TP53 mutant signature had the best odds ratio and PPV (Table 2). The same analysis was performed for ER-positive (n = 198) (Supplemental Table 2) and triple-negative breast cancer (TNBC) (n = 119) subtypes (Supplemental Table 3), with both groups showing that the TP53 mutant signature had the best predictive values for pCR.
Table 2.
Predictive power of molecular and pathological factors for pCR.
| Variable | Pathological response |
P | Odds ratio (95% CI) | PPV (%) | NPV (%) | Sensitivity (%) | Specificity (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| pCR (n = 57) |
RD (n = 263) |
|||||||||
| n | % | n | % | |||||||
| TP53 signature | ||||||||||
| Wild-type signature (n = 171) | 5 | 2.9 | 166 | 97.1 | <.0001 | 1 | 34.9 | 97.1 | 91.2 | 63.1 |
| Mutant signature (n = 149) | 52 | 34.9 | 97 | 65.1 | 17.8 (6.8-46.1) | |||||
| Grade | ||||||||||
| 1-2 (n = 150) | 8 | 5.3 | 142 | 94.7 | <.0001 | 1 | 30.5 | 94.7 | 85.5 | 57.0 |
| 3 (n = 154) | 47 | 30.5 | 107 | 69.5 | 7.8 (3.5-17.2) | |||||
| Intrinsic subtype | ||||||||||
| Luminal A (n = 114) | 3 | 2.6 | 111 | 97.4 | <.0001 | 1 | 26.2 | 97.4 | 94.7 | 42.2 |
| The others (n = 206) | 54 | 26.2 | 152 | 73.8 | 13.1 (4.0-43.1) | |||||
| ER | ||||||||||
| Positive (n = 198) | 19 | 9.6 | 179 | 90.4 | <.0001 | 1 | 31.9 | 90.4 | 66.7 | 68.8 |
| Negative (n = 119) | 38 | 31.9 | 81 | 68.1 | 4.4 (2.4-8.1) | |||||
| PR | ||||||||||
| Positive (n = 176) | 19 | 10.8 | 157 | 89.2 | .0002 | 1 | 27.1 | 89.2 | 66.7 | 60.6 |
| Negative (n = 140) | 38 | 27.1 | 102 | 72.9 | 3.1 (1.7-5.6) | |||||
Abbreviations: CI, confidence interval; ER, estrogen receptor; NPV, negative predictive value; pCR, pathological complete response; PPV, positive predictive value; RD, residual disease.
We validated the predictive power of the TP53 signature for pCR in 4 independent cohorts (Supplemental Table 4). In all cohorts, the pCR rate in the TP53 signature mutant group was significantly higher than that in the wild-type group (23.5%-37.9% vs 5.9%-13.2%, odds ratio 3.8-8.2).
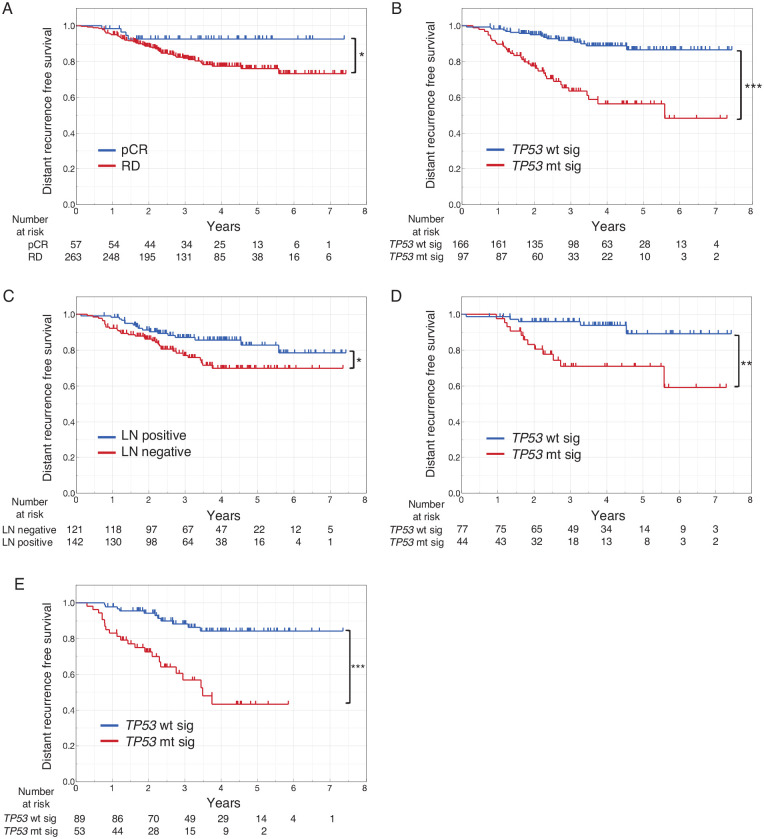
Prognostic factors in the RD group
As is well known, pCR was a strong prognostic factor in this cohort, with the RD group having a significantly poorer prognosis than the pCR group (Figure 2A). Given that only 4 cases had a distant recurrence in the pCR group, determining the prognostic factors therein was difficult. To explore the prognostic factors in the RD group (n = 263) in GSE25066, univariate and multivariate analyses for DRFS was performed using the Cox proportional hazards model (Table 3). Accordingly, univariate analysis showed that nodal status, ER, PgR, grade, intrinsic subtype, and TP53 signature status were significantly associated with DRFS. Meanwhile, multivariate analysis identified nodal status and TP53 signature status as independent prognostic factors. Interestingly, however, the TP53 signature had a larger HR compared with nodal status, suggesting that the former had greater prognostic power than the latter. Figure 2B and C details the DRFS according to the TP53 signature status and nodal status in the RD group, respectively. Moreover, the TP53 signature was identified as a prognostic factor in both node-negative and node-positive patients with RD (Figure 2D and E).
Figure 2.
DRFS stratified according to clinicopathological factors and TP53 signature. (A) DRFS stratified according to pathological response in all patients. (B) DRFS stratified according to the TP53 signature in patients with RD. (C) DRFS stratified according to nodal status in patients with RD. (D) DRFS stratified according to the TP53 signature in node-negative group of patients with RD. (E) DRFS stratified according to the TP53 signature in node-positive group of patients with RD. DRFS indicates distant recurrence-free survival; LN, lymph node; pCR, pathological complete response; RD, residual disease; TP53 mt sig, TP53 mutant signature; TP53 wt sig, TP53 wild-type signature.
*P < .05; **P < .01; ***P < .001.
Table 3.
Univariate and multivariate analyses (Cox proportional hazard model) for DRFS in patients with RD.
| Variable | Patients with RD |
||||||
|---|---|---|---|---|---|---|---|
| n | Univariate |
Multivariate |
|||||
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | |||||||
| >50 | 120 | 1.00 | – | — | |||
| ⩽50 | 143 | 1.14 | 0.65-2.00 | .64 | |||
| T category | |||||||
| 1-2 | 193 | 1.00 | – | – | |||
| 3 | 70 | 1.48 | 0.82-2.65 | .19 | |||
| Nodal status | |||||||
| Negative | 121 | 1.00 | – | – | 1.00 | – | – |
| Positive | 142 | 1.90 | 1.06-3.42 | .032 | 2.13 | 1.12-4.03 | .020 |
| ER | |||||||
| Positive | 179 | 1.00 | – | – | 1.00 | – | – |
| Negative | 81 | 3.34 | 1.91-5.83 | <.0001 | 1.57 | 0.71-3.45 | .26 |
| PgR | |||||||
| Positive | 157 | 1.00 | – | – | 1.00 | – | – |
| Negative | 102 | 3.01 | 1.70-5.34 | .0002 | 1.16 | 0.52-2.58 | .71 |
| Grade | |||||||
| 1-2 | 142 | 1.00 | – | – | 1.00 | – | – |
| 3 | 107 | 2.14 | 1.18-3.88 | .013 | 0.80 | 0.39-1.63 | .54 |
| Intrinsic subtype | |||||||
| Luminal A | 111 | 1.00 | – | – | 1.00 | – | – |
| The others | 152 | 4.77 | 2.38-9.55 | <.0001 | 1.81 | 0.68-4.82 | .24 |
| TP53 signature | |||||||
| Wild-type signature | 166 | 1.00 | – | – | 1.00 | – | – |
| Mutant signature | 97 | 4.68 | 2.58-8.50 | <.0001 | 2.50 | 1.09-5.74 | .030 |
Abbreviations: CI, confidence interval; DRFS, distant recurrence-free survival; ER, estrogen receptor; HR, hazard ratio; PgR, progesterone receptor; RD, residual disease.
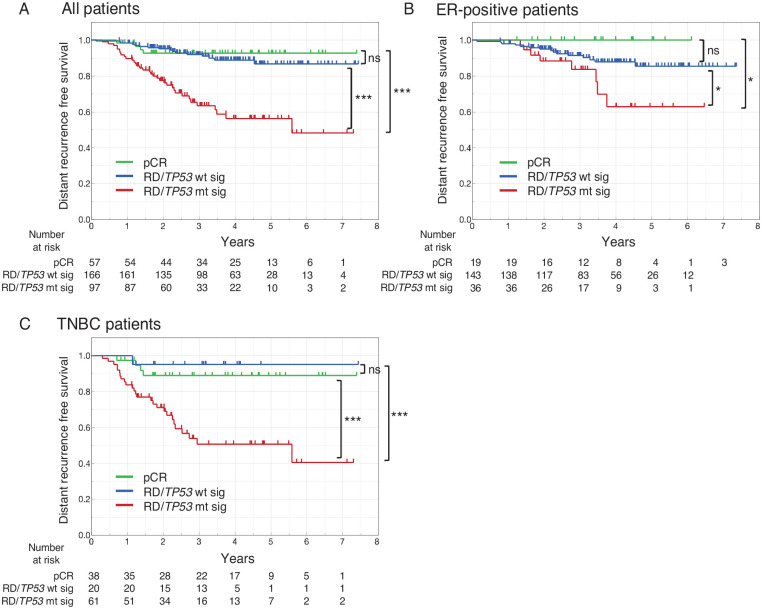
Prognostic significance of combining pathological response and TP53 signature
Given our results showing that the TP53 signature was a prognostic factor in the RD group, we compared DRFS among 3 groups: the pCR, RD/TP53 mutant signature, and RD/TP53 wild-type signature groups in GSE25066 (Figure 3A). Accordingly, our results showed no significant difference in DRFS between the pCR and RD/TP53 wild-type signature groups, while the RD/TP53 mutant signature group had significantly worse DRFS than the other 2 groups, a result also observed in the ER-positive subtype (Figure 3B) and TNBC subtype (Figure 3C).
Figure 3.
DRFS stratified by 3 groups, pCR, RD/TP53 wt sig, and RD/TP53 mt sig. Patients were classified into 3 groups: pCR, RD and TP53 wild-type signature (RD/TP53 wt sig), and RD and TP53 mutant signature (RD/TP53 mt sig), after which DRFS was estimated in all patients (A), those with ER-positive subtype (B), and those with TNBC subtype (C). DRFS indicates distant recurrence-free survival; pCR, pathological complete response; RD, residual disease; TNBC, triple-negative breast cancer; TP53 mt sig, TP53 mutant signature; TP53 wt sig, TP53 wild-type signature.
*P < .05; ***P < .001.
To validate these findings, we compared RFS among pCR, RD/TP53 mutant signature, and RD/TP53 wild-type signature using GSE32603, for which RFS data were available (Supplemental Figure 1). Similar to the results of GSE25066, our results showed no significant difference in RFS between the pCR group and RD/TP53 wild-type signature group, and the RD/TP53 mutant signature had significantly worse RFS than the other 2 groups in the entire eligible cases (Supplemental Figure 1a). In the ER-positive subtype, there was no significant difference in RFS between the pCR group and RD/TP53 mutant signature group, but the latter showed a trend toward poor prognosis (Supplemental Figure 1b). In the TNBC subtype, there was no significant difference in RFS between RD/TP53 wild-type signature group and the RD/TP53 mutant signature group, but the latter tended to have a worse prognosis (Supplemental Figure 1c). RFS of the RD/TP53 mutant signature group was significantly worse than that of the pCR group.
The aforementioned findings showed that the TP53 wild-type signature group had a favorable prognosis even in patients with RD and that the combination of TP53 signature and pathological response to NAC (pCR/RD) can be used to identify patients with very poor prognosis (patients with RD/TP53 mutant signature).
Discussion
The TP53 signature consists of genes differentially expressed between TP53 wild-type and mutant breast cancers by microarray analysis. The TP53 signature does not include p53 target genes, such as CDKN1A (cording p21), MDM2, and BAX, which are transcriptionally activated by wild-type p53. 24 On the contrary, among the 24 genes upregulated in the TP53 mutant signature group, 5 genes (CCNB2, PLK, PTTG1, BIRC5, and STMN1) that are known to be transcriptionally repressed directly or indirectly by wild-type p53 were contained (Supplemental Table 1).25,26 The known p53 target genes, such as CDKN1A, are transactivated only when p53 is overexpressed in response to various types of genotoxic stresses. As p53 overexpression is not always observed in the TP53 wild-type breast cancers, it is likely that no known p53 target genes transactivated by wild-type p53 were included in the TP53 signature.
The TP53 signature has been reported to be a prognostic factor in several studies of breast cancer.14,16,27 Moreover, Oshima et al 15 reported that the TP53 signature can be used as a predictor of NAC response. Using a cohort of 72 patients with breast cancer receiving paclitaxel followed by 5-FU/epirubicin/cyclophosphamide, Oshima et al 15 showed that the TP53 signature mutant-like group had significantly better pCR than the wild-type-like group. Furthermore, the TP53 signature was superior to direct TP53 gene sequencing and p53 protein immunohistochemistry in predicting pCR. Lehmann et al 17 previously verified the prognostic predictability of 351 reported gene expression profiles in a meta-analysis based on 31 breast cancer cohorts. They found that the TP53 signature was a robust prognostic factor and was better than well-known gene expression profiles such as the OnctypeDX and Mammaprint. Furthermore, Lehmann et al 17 also verified that the TP53 signature was a predictor of therapeutic response in their meta-analysis. Thus, the aforementioned findings confirm that TP53 signature is not only a prognostic factor but also a predictor of NAC response.
The present study used a cohort of patients with HER2-negative breast cancer who received NAC to examine the significance of the TP53 signature in predicting the therapeutic effects of NAC and determine whether it could be a prognostic factor for patients with RD who have a poor prognosis. In all included patients (n = 333), the TP53 signature was a better predictor of pCR than grade, intrinsic subtype, ER, and PgR (Table 2). Furthermore, its ability to predict pCR was better than that of grade and intrinsic subtype in the ER-positive and TNBC subtypes (Supplemental Table 2 and Supplemental Table 3). Moreover, the TP53 signature was significantly associated with pCR in 4 cohorts independent of GSE25066, and the TP53 signature was validated as a predictor of pCR in multiple cohorts.
As is already widely known, patients who achieve pCR after NAC have a good prognosis, whereas those with RD show the opposite.4,5 Given that the TP53 signature can be used as a prognostic factor, we hypothesized that it could be used to identify patients with good and poor prognosis among those with RD, who is considered to have a poor prognosis. Accordingly, the results of univariate and multivariate analyses for DRFS in the RD group showed that nodal status and TP53 signature were independent prognostic factors (Table 3). Moreover, the pCR and RD/TP53 wild-type signature groups had comparable DRFS, while even patients with RD who had the TP53 wild-type signature exhibited a better prognosis. On the contrary, among patients with RD, those with the TP53 mutant signature had significantly worse DRFS than those with pCR and RD/TP53 wild-type signature. These results were validated in an independent cohort (GSE32603) (Supplemental Figure 1a). There was no significant difference in RFS between the pCR group (n = 6) and RD/TP53 mutant signature group (n = 19) in the ER-positive subtype and between RD/TP53 wild-type signature (n = 4) and RD/TP53 mutant signature group (n = 19) in the TNBC subtype, but these might be due to the small sample size. In agreement with the results of GSE25066, we found that the RD/TP53 mutant signature group tended to have a worse prognosis than the pCR group and RD/TP53 wild-type signature group in both ER-positive and TNBC subtypes. Ungerleider et al 12 reported that the addition of hormone therapy to chemotherapy improved the survival of patients with TP53 wild-type tumors, but not patients with TP53 mutant tumors by sequence, because hormone therapy could eradicate arrested/senescent cells by chemotherapy. Their results suggested that in the ER-positive subtype, the RD/TP53 wild-type signature group had improved prognosis because of the postoperative hormone therapy, as ER-positive patients of GSE25066 cohort did not receive hormone therapy 19 (Figure 3B). For the TNBC subtype, they reported that patients with TP53 wild-type tumors have a worse prognosis than those with TP53 mutant tumors because TNBC patients do not receive hormone therapy. Our results in TNBC subtype (Figure 3C) seem to differ from their results, but this difference could be explained by the fact that our analysis only included the RD group after NAC in the TP53 signature stratified analysis. Most of the cases in the pCR group are TP53 mt signature, and the impact of excluding favorable prognosis cases (pCR group) on the prognosis is greater in the TP53 mt signature group than in the TP53 wild-type signature group.
These results showed that the TP53 signature is not only a predictor of treatment response to NAC but also a more accurate prognostic predictor when combined with RD. In other words, patients with the TP53 wild-type signature have a poor response to NAC while having a good prognosis in both cases of pCR and RD. On the contrary, patients with TP53 mutant signature have a good response to NAC and a good prognosis in the case of pCR, but a poor prognosis in the case of RD. Recent studies have reported that the addition of postoperative adjuvant therapy can improve the prognosis of patients who do not achieve pCR after NAC.6-8 Considering this evidence together with our findings, we can infer that patients with the TP53 mutant signature would benefit from additional postoperative treatment among patients with RD. On the contrary, even among patients with RD after NAC, the prognosis of those with the TP53 wild-type signature group was similar to those with pCR, suggesting minimal benefit of additional postoperative chemotherapy. Although validation studies are needed, the current study suggests that the TP53 signature can not only predict pCR following NAC but also provide useful information when considering indications for postoperative adjuvant chemotherapy in patients with RD.
The current study has several limitations worth noting. First, TP53 gene mutations could not be evaluated because of the lack of data of TP53 gene mutations in the cohort used in this study. However, the predictability of TP53 mutations by TP53 signature has been well documented in previous reports.14,15,27 Second, this was a retrospective study that used a database cohort. Third, given our cohort of patients with HER2-negative breast cancer, we were unable to examine the significance of the TP53 signature in HER2-positive cases. Finally, prognostic data were only available for DRFS or RFS, while the significance of the TP53 signature for overall survival had not been investigated. However, the results presented herein showed that the TP53 signature can predict pCR, with the combination of pathological response (pCR or RD) and TP53 signature being able to accurately identify subgroups with poor prognosis using multiple cohorts.
Previous prospective clinical trials on NAC for T1c-3N0-1M0/T1-3N1M0 breast cancer have been conducted in Japan.28-30 The present study was conducted on patients with T1-3/N0-1M0 breast cancer who have similar clinical characteristics to the patients in these trials. Currently, we are developing a simple diagnostic method for the TP53 signature and are conducting a retrospective study to validate the predictive value of the TP53 signature for pCR in a prospective clinical trial cohort who had received NAC (UMIN000037505).
Conclusion
We clarified that TP53 signature is not only a predictive factor of pCR but also a prognostic factor of RD, and we hope that the TP53 signature would be able to provide useful information that would contribute toward the optimization of perioperative chemotherapy.
Supplemental Material
Supplemental material, sj-pptx-5-bcb-10.1177_11782234231167655 for TP53 Signature Can Predict Pathological Response From Neoadjuvant Chemotherapy and Is a Prognostic Factor in Patients With Residual Disease by Shin Takahashi, Keiju Sasaki and Chikashi Ishioka in Breast Cancer: Basic and Clinical Research
Supplemental material, sj-xlsx-1-bcb-10.1177_11782234231167655 for TP53 Signature Can Predict Pathological Response From Neoadjuvant Chemotherapy and Is a Prognostic Factor in Patients With Residual Disease by Shin Takahashi, Keiju Sasaki and Chikashi Ishioka in Breast Cancer: Basic and Clinical Research
Supplemental material, sj-xlsx-2-bcb-10.1177_11782234231167655 for TP53 Signature Can Predict Pathological Response From Neoadjuvant Chemotherapy and Is a Prognostic Factor in Patients With Residual Disease by Shin Takahashi, Keiju Sasaki and Chikashi Ishioka in Breast Cancer: Basic and Clinical Research
Supplemental material, sj-xlsx-3-bcb-10.1177_11782234231167655 for TP53 Signature Can Predict Pathological Response From Neoadjuvant Chemotherapy and Is a Prognostic Factor in Patients With Residual Disease by Shin Takahashi, Keiju Sasaki and Chikashi Ishioka in Breast Cancer: Basic and Clinical Research
Supplemental material, sj-xlsx-4-bcb-10.1177_11782234231167655 for TP53 Signature Can Predict Pathological Response From Neoadjuvant Chemotherapy and Is a Prognostic Factor in Patients With Residual Disease by Shin Takahashi, Keiju Sasaki and Chikashi Ishioka in Breast Cancer: Basic and Clinical Research
Acknowledgments
None.
Declarations
Ethics Approval and Consent to Participate: This study was designed in compliance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects. This study protocol was reviewed and approved by the Ethics Committee at the Graduate School of Medicine, Tohoku University, approval number 2021-1-1070. As all cohort data are already available in public databases, it was decided that the consent from patients for this study is not required by the Ethics Committee at the Graduate School of Medicine, Tohoku University.
Consent for Publication: Not applicable. As all cohort data used in this study were obtained from public databases, it was not possible to obtain consent for publication from participants.
Author Contributions: Shin Takahashi: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Resources; Validation; Visualization; Writing – original draft.
Keiju Sasaki: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – review & editing.
Chikashi Ishioka: Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Grants-in-Aid from the JSPS KAKENHI (24701000) and the Research funds for preventive medicine from Miyagi Health Care Association (no grant number).
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Takahashi reports personal fees from Taiho, Chugai, Asahikasei, Bayer, Japan Blood Products Organization, Medicon, Termo, Sanofi, Nippon-kayaku, Takeda, and Yakult; grants and personal fees from Merckbiopharma; and grants from Ono outside the submitted work. In addition, Dr Takahashi has a patent (JP4370409B2) issued. Dr Sasaki has no conflicts of interest. Prof. Ishioka reports grants and personal fees from Merck biopharma, Asahi Kasei, Sanofi, Takeda, Eisai, and Chugai; personal fees from Eli Lilly, Mundipharma, Teijin, Konica Minolta, Pfizer, and Mochida; and grants from Riken Genesis, MSD, and Linical outside the submitted work. In addition, Prof. Ishioka has a patent (JP4370409B2) issued.
Availability of Data and Materials: Publicly available datasets were used in this study. These can be found in the Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/, reference number GSE25066, GSE20194, GSE20271, GSE32603, and GSE140494.
Supplemental Material: Supplemental material for this article is available online.
References
- 1.International Agency for Research on Cancer (IARC) WHOW. Breast cancer, cancer fact sheets. Cancer Today. https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf. Published 2020.
- 2.Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;2007:Cd005002. doi: 10.1002/14651858.CD005002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27-39. doi: 10.1016/s1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project protocols B-18 and B-27. J Clin Oncol. 2008;26:778-785. doi: 10.1200/jco.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796-1804. doi: 10.1200/jco.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 6.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 7.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617-628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 8.Toi M, Imoto S, Ishida T, et al. Adjuvant S-1 plus endocrine therapy for oestrogen receptor-positive, HER2-negative, primary breast cancer: a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:74-84. doi: 10.1016/s1470-2045(20)30534-9. [DOI] [PubMed] [Google Scholar]
- 9.Bertheau P, Plassa F, Espié M, et al. Effect of mutated TP53 on response of advanced breast cancers to high-dose chemotherapy. Lancet (London, England). 2002;360:852-854. doi: 10.1016/s0140-6736(02)09969-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Xu Y, Chen J, et al. TP53 mutations are associated with higher rates of pathologic complete response to anthracycline/cyclophosphamide-based neoadjuvant chemotherapy in operable primary breast cancer. Int J Cancer. 2016;138:489-496. doi: 10.1002/ijc.29715. [DOI] [PubMed] [Google Scholar]
- 11.Chen MB, Zhu YQ, Xu JY, et al. Value of TP53 status for predicting response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. PLoS ONE. 2012;7:e39655. doi: 10.1371/journal.pone.0039655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ungerleider NA, Rao SG, Shahbandi A, et al. Breast cancer survival predicted by TP53 mutation status differs markedly depending on treatment. Breast Cancer Res. 2018;20:115. doi: 10.1186/s13058-018-1044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahbandi A, Nguyen HD, Jackson JG.TP53 mutations and outcomes in breast cancer: reading beyond the headlines. Trends Cancer. 2020;6:98-110. doi: 10.1016/j.trecan.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi S, Moriya T, Ishida T, et al. Prediction of breast cancer prognosis by gene expression profile of TP53 status. Cancer Sci. 2008;99:324-332. doi: 10.1111/j.1349-7006.2007.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshima K, Naoi Y, Kishi K, et al. Gene expression signature of TP53 but not its mutation status predicts response to sequential paclitaxel and 5-FU/epirubicin/cyclophosphamide in human breast cancer. Cancer Lett. 2011;307:149-157. doi: 10.1016/j.canlet.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi S, Takahashi S, Mogushi K, et al. Molecular and clinical features of the TP53 signature gene expression profile in early-stage breast cancer. Oncotarget. 2018;9:14193-14206. doi: 10.18632/oncotarget.24447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann BD, Ding Y, Viox DJ, et al. Evaluation of public cancer datasets and signatures identifies TP53 mutant signatures with robust prognostic and predictive value. BMC Cancer. 2015;15:179. doi: 10.1186/s12885-015-1102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. 2012;132:1049-1062. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatzis C, Pusztai L, Valero V, et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA. 2011;305:1873-1881. doi: 10.1001/jama.2011.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi L, Campbell G, Jones WD, et al. The MicroArray Quality Control (MAQC)-II study of common practices for the development and validation of microarray-based predictive models. Nat Biotechnol. 2010;28:827-838. doi: 10.1038/nbt.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen K, Song N, Kim Y, et al. A systematic evaluation of multi-gene predictors for the pathological response of breast cancer patients to chemotherapy. PLoS ONE. 2012;7:e49529. doi: 10.1371/journal.pone.0049529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magbanua MJ, Wolf DM, Yau C, et al. Serial expression analysis of breast tumors during neoadjuvant chemotherapy reveals changes in cell cycle and immune pathways associated with recurrence and response. Breast Cancer Res. 2015;17:73. doi: 10.1186/s13058-015-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edlund K, Madjar K, Lebrecht A, et al. Gene expression-based prediction of neoadjuvant chemotherapy response in early breast cancer: results of the prospective multicenter EXPRESSION trial. Clin Cancer Res. 2021;27:2148-2158. doi: 10.1158/1078-0432.Ccr-20-2662. [DOI] [PubMed] [Google Scholar]
- 24.el-Deiry WS.Regulation of p53 downstream genes. Semin Cancer Biol. 1998;8:345-357. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- 25.Kho PS, Wang Z, Zhuang L, et al. p53-regulated transcriptional program associated with genotoxic stress-induced apoptosis. J Biol Chem. 2004;279:21183-21192. doi: 10.1074/jbc.M311912200. [DOI] [PubMed] [Google Scholar]
- 26.Lipski R, Lippincott DJ, Durden BC, et al. p53 Dimers associate with a head-to-tail response element to repress cyclin B transcription. PLoS ONE. 2012;7:e42615. doi: 10.1371/journal.pone.0042615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uji K, Naoi Y, Kagara N, et al. Significance of TP53 mutations determined by next-generation “deep” sequencing in prognosis of estrogen receptor-positive breast cancer. Cancer Lett. 2014;342:19-26. doi: 10.1016/j.canlet.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Toi M, Nakamura S, Kuroi K, et al. Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res Treat. 2008;110:531-539. doi: 10.1007/s10549-007-9744-z. [DOI] [PubMed] [Google Scholar]
- 29.Iwata H, Sato N, Masuda N, et al. Docetaxel followed by fluorouracil/epirubicin/cyclophosphamide as neoadjuvant chemotherapy for patients with primary breast cancer. Jpn J Clin Oncol. 2011;41:867-875. doi: 10.1093/jjco/hyr081. [DOI] [PubMed] [Google Scholar]
- 30.Ohno S, Chow LW, Sato N, et al. Randomized trial of preoperative docetaxel with or without capecitabine after 4 cycles of 5-fluorouracil-epirubicin-cyclophosphamide (FEC) in early-stage breast cancer: exploratory analyses identify Ki67 as a predictive biomarker for response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013;142:69-80. doi: 10.1007/s10549-013-2691-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pptx-5-bcb-10.1177_11782234231167655 for TP53 Signature Can Predict Pathological Response From Neoadjuvant Chemotherapy and Is a Prognostic Factor in Patients With Residual Disease by Shin Takahashi, Keiju Sasaki and Chikashi Ishioka in Breast Cancer: Basic and Clinical Research
Supplemental material, sj-xlsx-1-bcb-10.1177_11782234231167655 for TP53 Signature Can Predict Pathological Response From Neoadjuvant Chemotherapy and Is a Prognostic Factor in Patients With Residual Disease by Shin Takahashi, Keiju Sasaki and Chikashi Ishioka in Breast Cancer: Basic and Clinical Research
Supplemental material, sj-xlsx-2-bcb-10.1177_11782234231167655 for TP53 Signature Can Predict Pathological Response From Neoadjuvant Chemotherapy and Is a Prognostic Factor in Patients With Residual Disease by Shin Takahashi, Keiju Sasaki and Chikashi Ishioka in Breast Cancer: Basic and Clinical Research
Supplemental material, sj-xlsx-3-bcb-10.1177_11782234231167655 for TP53 Signature Can Predict Pathological Response From Neoadjuvant Chemotherapy and Is a Prognostic Factor in Patients With Residual Disease by Shin Takahashi, Keiju Sasaki and Chikashi Ishioka in Breast Cancer: Basic and Clinical Research
Supplemental material, sj-xlsx-4-bcb-10.1177_11782234231167655 for TP53 Signature Can Predict Pathological Response From Neoadjuvant Chemotherapy and Is a Prognostic Factor in Patients With Residual Disease by Shin Takahashi, Keiju Sasaki and Chikashi Ishioka in Breast Cancer: Basic and Clinical Research