Abstract
Adherence of erythrocytes infected with Plasmodium falciparum to microvascular endothelial cells (sequestration) is considered to play an important role in parasite virulence and pathogenesis. However, the real importance of sequestration for infection and disease has never been fully assessed. The absence of an appropriate in vivo model for sequestration has been a major barrier. We have examined the rodent malaria parasite Plasmodium chabaudi chabaudi AS in mice as a potential model. Erythrocytes infected with this parasite adhere in vitro to purified CD36, a critical endothelium receptor for binding P. falciparum-infected erythrocytes. P. c. chabaudi-infected erythrocytes adhere in vitro to endothelial cells in a gamma interferon-dependent manner, suggesting the involvement of additional adhesion molecules in the binding process, as is also the case with P. falciparum-infected cells. Furthermore, plasma or sera from infected and hyperimmune mice, respectively, have the ability to block binding of infected erythrocytes to endothelial cells. In vivo, erythrocytes containing mature P. c. chabaudi parasites are sequestered from the peripheral circulation. Sequestration is organ specific, occurring primarily in the liver, although intimate contact between infected erythrocytes and endothelial cells is also observed in the spleen and brain. The results are discussed in the context of the use of this model to study (i) the relationship between endothelial cell activation and the level of sequestration and (ii) the primary function of sequestration in malaria infection.
Withdrawal of erythrocytes infected with mature stages of Plasmodium falciparum from the peripheral circulation occurs through adherence of these cells to the microvascular endothelium in various organs (27). This sequestration may contribute to the pathology observed in cerebral malaria, one of the commonest causes of death due to P. falciparum infection. Many studies have shown an association between massive parasite sequestration in the brain and cerebral malaria (27, 33). Sequestration is also thought to be a mechanism by which a malaria parasite avoids passage through the spleen and destruction by immune mechanisms activated in that organ. However, a real advantage for this mechanism of immune evasion has never been formally proven.
Several molecules on the surfaces of P. falciparum-infected erythrocytes may participate in the cytoadherence properties of these cells. These molecules include Ag332 (20), sequestrin (29), modified band 3 (12), and P. falciparum erythrocyte membrane protein-1 (PfEMP-1) (4, 14, 19, 40). PfEMP-1 has been shown to bind to a number of host receptors and also undergoes antigenic variation (4, 14, 40). Ligands on the surfaces of infected erythrocytes can bind to a number of endothelial surface molecules, including CD36, thrombospondin, ICAM-1, PECAM-1, VCAM-1, chondroitin-4-sulfate, ELAM-1, and P-selectin (3, 5, 9, 30, 31, 36, 37, 41). CD36 is a receptor on host cells that supports adherence of most P. falciparum laboratory lines and isolates from patients.
In some studies of cytoadherence, nonhuman primate models have been used, such as rhesus monkeys infected with Plasmodium coatneyi (2) or Saimiri sciureus infected with P. falciparum (18). However, there are ethical and technical problems associated with the use of these animals. Laboratory mice have a number of advantages over primates for models of malaria, including the availability of congenic animals, a well-studied immune system, the opportunity to measure pathology in groups of animals at all stages of the disease, and the availability of genetic variants. Most in vivo studies of parasite cytoadherence and associated pathology in rodent malaria models have been made using Plasmodium berghei in mice and hamsters (34, 35). However, these models differ from P. falciparum infection in humans because capillaries and venules are obstructed by large mononuclear cells rather than by erythrocytes infected with mature parasite forms.
Sequestration of schizonts (erythrocytes infected with mature parasite stages) has been observed in Plasmodium chabaudi chabaudi-infected mice (10, 16). Antigenic variation at the surface of the infected erythrocyte and sequestration have also been shown to be intimately linked in P. c. chabaudi infection, suggesting that a single molecule is involved in the two phenomena (16), as proposed for PfEMP-1. However, nothing is known about the interaction of P. c. chabaudi-infected erythrocytes with other host cells and whether this interaction is similar to that of P. falciparum-infected erythrocytes in humans.
In this report we show that P. c. chabaudi AS-infected erythrocytes adhere in vitro to CD36. Adherence to endothelial cells in vitro, in a gamma interferon (IFN-γ)-dependent manner, could also be observed. The interaction between P. c. chabaudi AS-infected erythrocytes and the tissues of different organs has been characterized in vivo and indicates that this model shares many features with sequestration in P. falciparum infection. We propose that the use of this model may clarify the relationship between endothelial cell activation and the level of sequestration and elucidate the primary function of sequestration in malaria infection.
MATERIALS AND METHODS
Parasites and infection of experimental animals.
P. c. chabaudi (AS line), originally isolated from a natural host (Thamnomys rutilans) in the Central African Republic, was supplied by D. Walliker (WHO Registry of Standard Strains of Malaria Parasites, University of Edinburgh, Edinburgh, Scotland) as a cloned, recently mosquito-transmitted line (8, 23, 46). Blood stage parasites were collected and maintained as described previously (28). Parasitemia in infected CBA/Ca mice was monitored by light microscopy of air-dried, methanol-fixed, thin tail blood smears stained with 10% Giemsa stain in phosphate buffer, pH 7.4 (15).
Preparation of plasma from P. c. chabaudi AS-infected and normal mice.
A group of mice were infected intraperitoneally with 5 × 104 P. c. chabaudi-parasitized erythrocytes diluted in Kreb's buffered saline containing 0.2% glucose (KGS), and their parasitemias were monitored on thin smears. A sham-infected control group was injected intraperitoneally with KGS alone. On days 11 to 12 postinfection (approximately 1 to 2 days after peak parasitemia), mice from both groups were bled individually into 100 μl of KGS plus 25 U of heparin ml−1 at 4°C. The blood was then centrifuged (2,000 × g at 4°C for 1 to 2 min), and the plasma was removed and snap frozen in liquid nitrogen. Acute-phase plasma (APP) was obtained from the infected mice, and normal plasma (NP) was obtained from the control group. Hyperimmune serum (HIS) was obtained from mice that had been infected at least five times.
Cell culture procedures.
High endothelial cells were isolated from cervical lymph nodes of AO rats and cultured as described previously (1) in RPMI 1640 medium supplemented with NaHCO3, HEPES, sodium pyruvate, penicillin, streptomycin, and monothioglycolate (RPMI 1640 COMP/ABS; Gibco BRL) and 10% fetal calf serum (FCS). Confluent primary cultures were serially passaged, plated at 30 to 50% confluent density every 4 days, and discarded after no more than 20 passages. Cell stocks were stored at −70°C in RPMI 1640 COMP/ABS supplemented with 10% FCS and 10% dimethylsulfoxide.
Binding of P. c. chabaudi AS-infected erythrocytes to CD36.
The adhesion of infected erythrocytes to soluble recombinant human CD36 (a gift from Chris Newbold, Oxford University) was measured. In these assays, triplicate spots of CD36 (12.5 ng ml−1) or phosphate-buffered saline (PBS) containing 1% (wt/vol) bovine serum albumin (PBS-B) as a control were added to plastic dishes and incubated at 4°C overnight. After this incubation period, the dishes were washed in PBS to remove unbound protein and blocked with PBS-B at 4°C overnight. After three further washes in PBS, 7.5 μl of P. c. chabaudi AS-infected blood at 2% hematocrit was placed over each spot and incubated for 1 h at 37°C. For blood samples containing mature parasites, infected blood containing mainly trophozoites was harvested and the parasites were allowed to develop further by incubation for 2 h in binding medium (RPMI 1640, 25 mM HEPES, 25 μg of gentamycin ml−1, 2 mM glutamine, 2 mM CaCl2, 10% FCS). The subsequent washes, fixation, and Giemsa staining were performed as described above. The results are shown as the number of bound cells per spot.
In vitro cytoadherence assays.
Rat endothelial cells were plated into eight-well chamber slides (Nunc). The cells were seeded at a density of 2 × 104/well and grown for 72 h in the presence or absence of 100 U of IFN-γ ml−1. The culture medium was then replaced with 200 μl of an erythrocyte suspension from a P. c. chabaudi AS infection at 2% hematocrit in culture and binding medium as described above. The slides were incubated at 37°C for 90 min with gentle resuspension of the settled erythrocytes every 10 to 15 min. After incubation, unbound cells were removed by washing the slides three times with PBS, pH 7.2, with gentle rotation (30 rpm). The adherent infected erythrocytes were fixed with 1% glutaraldehyde in PBS for 30 min at 37°C. The slides were stained with 1% Giemsa stain for 30 min, and the number of adherent infected erythrocytes per 100 target cells was determined by light microscopy. To assess the effect of NP, APP, and HIS on adherence, erythrocytes from infected mice were incubated with the respective serum or plasma sample at a concentration of 1:10.
Electron microscopy.
Sets of three P. c. chabaudi AS-infected mice were killed by ether inhalation at days 7, 10, and 13 postinfection, representing prepeak, peak, and postpeak parasitemia time points, respectively. The brains, kidneys, livers, and spleens were immediately removed and placed in ice-cold PBS, sliced (into 1- to 3-mm-thick pieces) with a clean scalpel, and immediately immersion fixed in 2% (vol/vol) glutaraldehyde plus 2.5% paraformaldehyde in 0.1 M sodium cacodylate buffer (SCB), pH 7.2, for 12 to 48 h. Following fixation, the samples were washed in SCB for 15 min, postfixed in 1% osmium tetroxide in SCB for 90 min, and washed again as before. The samples were en bloc stained with 1% aqueous uranyl acetate for 90 min, dehydrated through a graded ethanol series (50% for 5 min, 75% for 10 min, 90% for 10 min, and absolute ethanol, three times for 10 min each), transferred to propylene oxide (two times for 10 min each), and embedded in Araldite CY 212. Sections approximately 60 nm thick were placed on carbon-formvar-coated grids, stained with saturated uranyl acetate in 75% ethanol (30 min), washed in distilled water, and stained in Reynold's lead citrate (5 min). Samples were viewed in a Jeol CX100 electron microscope.
Statistical analyses.
Statistical analyses were performed by Student's t test, with P values of <0.05 considered to be significant.
RESULTS
P. c. chabaudi AS-infected erythrocytes specifically bind CD36.
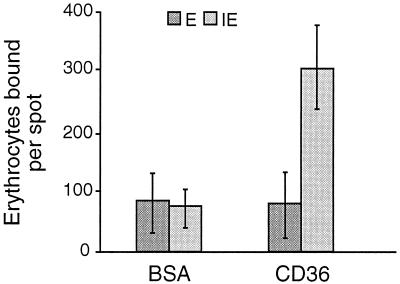
Erythrocytes containing P. c. chabaudi AS bound to CD36 but not to albumin (Fig. 1). Uninfected erythrocytes did not show any specific binding to CD36 (Fig. 1), and the binding of cells infected with ring forms or immature trophozoites was less than that of cells infected with mature trophozoites and schizonts (data not shown).
FIG. 1.
Adhesion of noninfected (E) and P. c. chabaudi AS-infected (IE) erythrocytes to human CD36 in vitro. Binding to bovine serum albumin (BSA) was measured as a control. All data are expressed as the arithmetic means of three independent experiments (±standard deviation).
Cytoadherence of P. c. chabaudi AS-infected erythrocytes to endothelial cells in vitro and the effect of IFN-γ.
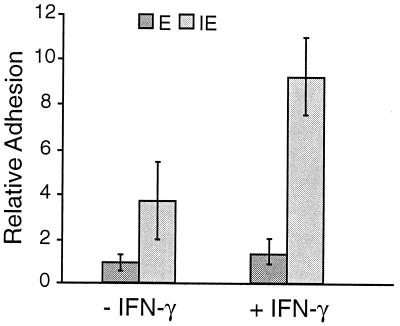
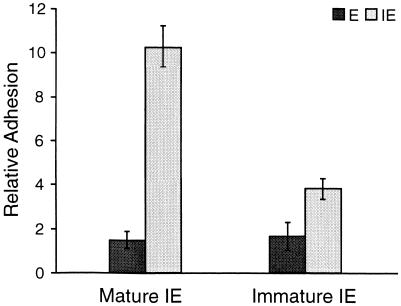
The binding of uninfected and parasite-infected erythrocytes to an endothelial cell line, with or without pretreatment with interferon, was examined. Pretreatment of the rat endothelial cells with IFN-γ upregulates expression of ICAM-1 and VCAM-1 (reference 13 and results not shown) and increases the adhesion of T lymphocytes by two- to threefold (13). The binding of parasite-infected erythrocytes was significantly higher (P < 0.05) than that of uninfected erythrocytes, even to cells that were not pretreated with IFN-γ (Fig. 2). IFN-γ treatment increased the binding of infected erythrocytes but not that of uninfected erythrocytes. The binding of infected erythrocytes containing mature trophozoites and schizonts was significantly higher (P < 0.01) than that of erythrocytes containing immature parasite forms (Fig. 3). For these experiments, the percentages of infected cells in the two populations were approximately the same (27% ± 2% parasitemia in the immature parasite population and 25% ± 3% in the mature parasite population), and the parasites were derived from the same cryopreserved parasite population.
FIG. 2.
Adhesion of noninfected (E) and P. c. chabaudi AS-infected (IE) erythrocytes to rat endothelial cells and the effect of interferon. Endothelial cells were treated with 100 U of IFN-γ ml−1 (+IFN-γ) or left untreated (−IFN-γ) and then mixed with P. c. chabaudi AS-parasitized erythrocytes containing mature parasites (24% ± 2% parasitemia). Relative adhesion is the number of bound erythrocytes per 100 endothelial cells. The error bars indicate standard deviations.
FIG. 3.
Adhesion of P. c. chabaudi AS-infected erythrocytes to rat endothelial cells depends on parasite age. Endothelial cells were treated with 100 U of IFN-γ ml−1 and then exposed to an erythrocyte suspension containing erythrocytes (IE) infected with mature parasites (25% ± 3% parasitemia) or immature parasites (27% ± 2% parasitemia), and uninfected erythrocytes (E). Relative adhesion is the number of bound erythrocytes per 100 endothelial cells. The error bars indicate standard deviations.
Treatment of infected erythrocytes with APP or HIS reduces their binding to endothelial cells.
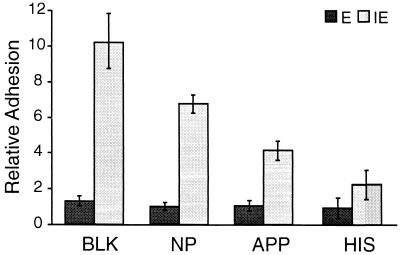
Antibody in sera from immune individuals inhibits the in vitro binding of P. falciparum-infected erythrocytes to host cells. Therefore, the effects of different serum and plasma samples on the adhesion of P. c. chabaudi AS-infected cells to interferon-treated endothelial cells was investigated. The adhesion of parasite-infected erythrocytes (containing mature trophozoites and schizonts) to endothelial cells was significantly decreased by preincubation with HIS (P < 0.01) and APP (P < 0.05) but not NP (Fig. 4). These treatments had no effect on the overall marginal binding of uninfected erythrocytes.
FIG. 4.
Antibodies induced by natural infection reduce adhesion of infected erythrocytes to rat endothelial cells. Endothelial cells were treated with 100 U of IFN-γ ml−1. An erythrocyte suspension containing erythrocytes (IE) infected with mature parasites (24% ± 2% parasitemia) and noninfected erythrocytes (E) was incubated with KGS (BLK), NP, APP, or HIS and then added to the endothelial cells in vitro. Relative adhesion is the number of bound erythrocytes per 100 endothelial cells. The error bars indicate standard deviations.
Sequestration of infected erythrocytes in different organs during P. c. chabaudi AS infection of CBA/Ca mice.
For transmission electron microscopy studies, samples were obtained from brain, kidney, liver, and spleen at days 7, 10, and 13 postinfection with parasites at the schizont stage and compared with samples from either uninfected mice or mice with ring stage parasites.
(i) Brain.
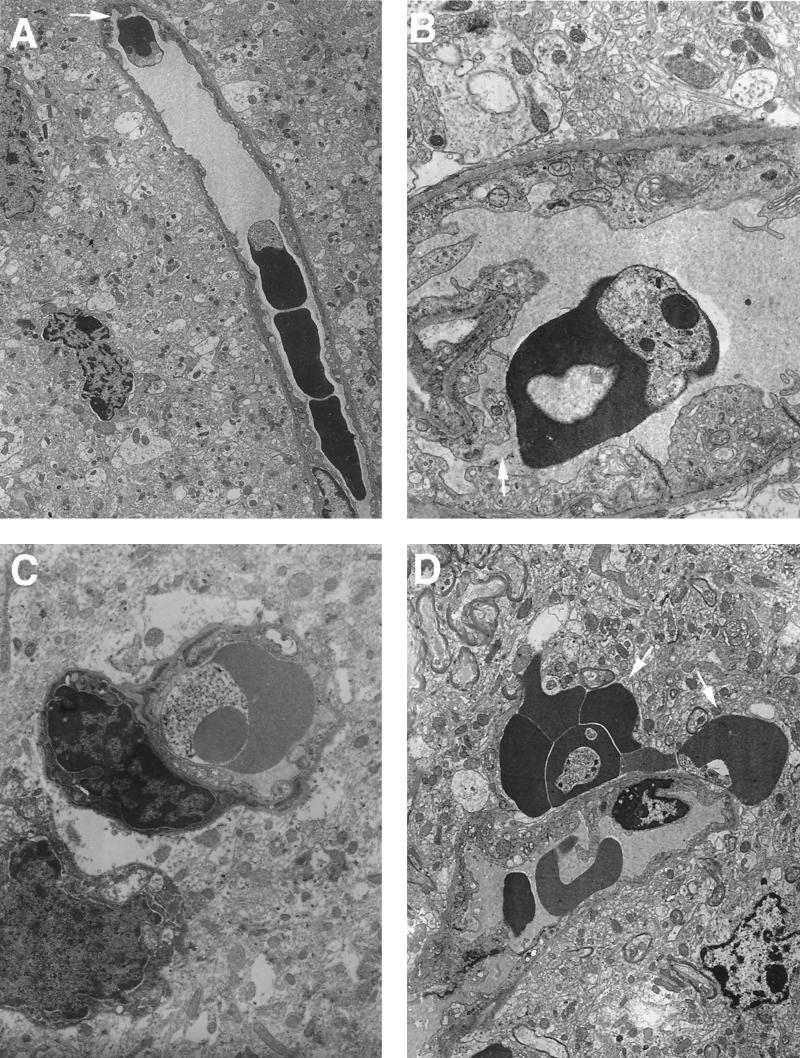
At days 7 and 13 postinfection, only low numbers of infected erythrocytes were detectable in the blood vessels of the brain. At day 10 postinfection, infected erythrocytes were detectable in significant numbers, and at this time parasites in the brain looked healthy, as judged by the appearance of their cytoplasm, organelles, and membrane integrity. Infected erythrocytes without any intimate contact with endothelial cells were a common observation (Fig. 5A). However, in small vessels without occlusion, endothelial cell processes were frequently seen in contact with infected erythrocytes (Fig. 5A and B). Swelling of the endothelium was also frequently observed in the brains of infected mice (Fig. 5B, C, and D). In contrast, in uninfected mice, this feature was never observed (n = 3) (results not shown). Another very common and striking finding was the presence of groups of extravascular erythrocytes, although some of these erythrocytes appeared uninfected in the plane of section (Fig. 5D).
FIG. 5.
Electron micrographs of brain sections from P. c. chabaudi AS-infected mice. Mice were killed at a parasitemia of approximately 45% and at the time of maximal parasite withdrawal from the peripheral blood. Magnifications, ×6,500 (A), ×20,750 (B), ×16,500 (C) and ×8,250 (D). (A and B) Cross-sectional view of small vessels where there is no occlusion but there are some signs of endothelium proliferation; endothelial processes can be seen in contact with infected erythrocytes (arrows). (C) Cross-sectional view of an infected erythrocyte inside a small capillary which shows swelling of the endothelium. (D) Section showing erythrocytes outside a vessel wall (arrows).
(ii) Kidney.
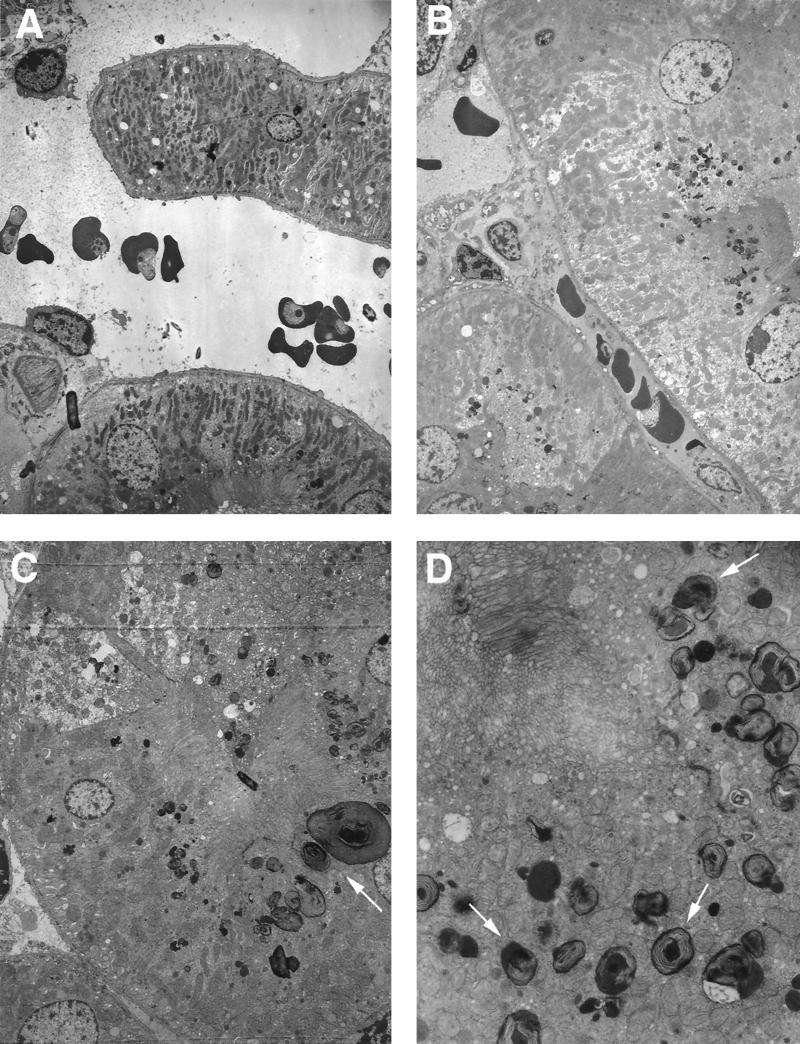
No major differences between the kidneys of infected and noninfected mice were evident at day 7 postinfection. At day 10, parasites in the kidney appeared healthy (Fig. 6A). The behavior of infected and noninfected erythrocytes appeared to be similar in glomerular capillaries and proximal and distal tubule regions. Adhesion of infected cells to endothelial cells was not observed (Fig. 6A and B). Around the brush borders of proximal tubules, endocytosed deposits and lysosomes were observed (Fig. 6C and D). This process occurred on a massive scale, with large numbers of lysosomes in a very noticeable size range, up to 2.0 μm in diameter. The appearance of the contents of these very complex lysosomes bears a striking resemblance to hemoglobin, especially in the large ones. Smaller lysosomes tended to have a more obvious membranous content. These lysosomes had almost completely disappeared by day 13 postinfection (results not shown).
FIG. 6.
Electron micrographs of kidney sections from P. c. chabaudi AS-infected mice. Mice were killed at a parasitemia of approximately 45% and at the time of maximal parasite withdrawal from the peripheral blood. Magnifications, ×4,000 (A), ×8,250 (B), ×5,000 (C) and ×16,500 (D). (A and B) Cross-sectional views of kidney vessels where infected and uninfected erythrocytes do not show any intimate contact with the endothelial cells. (C and D) Cross-sectional view of proximal kidney tubules showing the presence of large numbers of lysosomes (arrows).
(iii) Liver.
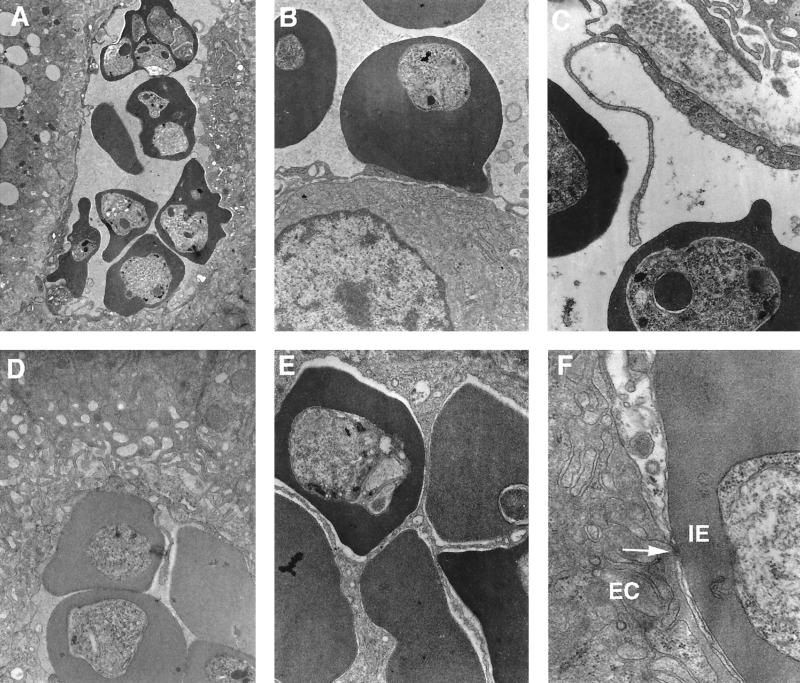
The most common observation in the liver was that of infected erythrocytes densely packed together within large sinusoids (Fig. 7A). Endothelial cell processes were often observed projecting into the lumens of blood vessels (Fig. 7C), and they were frequently observed in direct contact with infected erythrocytes (Fig. 7D). Swelling of the endothelium was a very common feature at day 10 (Fig. 7A and D). A much higher parasitemia was observed in liver sections (75% ± 4%) than in the peripheral circulation (30% ± 3%). These parasites appeared undamaged, and infected erythrocytes were frequently observed adhering to the endothelial linings of large vessels. The interaction between infected erythrocytes and endothelial cells was frequently manifested by points of adhesion with a very close association (<20 nm) (Fig. 7F). In a progressive and dynamic manner, endothelial cells consistently appeared to develop a highly folded surface, creating a series of “pockets” enclosing infected erythrocytes (Fig. 7B through E). In fact, many plane sections appeared to suggest a complete enclosure of infected erythrocytes by hepatic endothelium (Fig. 7E). However, complete enclosure could result in a totally blocked sinusoid, and anoxia and cell death would rapidly occur, an event that was never observed. As in the spleen (see below), but to a lesser extent, macrophages containing hemoglobin were observed. By day 13 postinfection, fewer infected erythrocytes were detectable in the liver and endothelial cells had returned to normal size and appearance.
FIG. 7.
Electron micrographs of liver sections from P. c. chabaudi AS-infected mice. Mice were killed at a parasitemia of approximately 45% and at the time of maximal parasite withdrawal from the peripheral blood. Magnifications, ×2,250 (A), ×24,500 (B and D), ×39,200 (C and E), and ×63,700 (F). (A) Cross-sectional view of a vessel with a much higher proportion of infected erythrocytes than in the peripheral circulation. (B) Very close contact between infected erythrocytes and endothelial cells. (C and D) Endothelial processes maintaining contact with infected erythrocytes. (E) Endothelial proliferation involving several infected erythrocytes. (F) Infected erythrocyte (IE) without knobs in contact with an endothelial cell (EC) showing a point of adhesion (arrow).
(iv) Spleen.
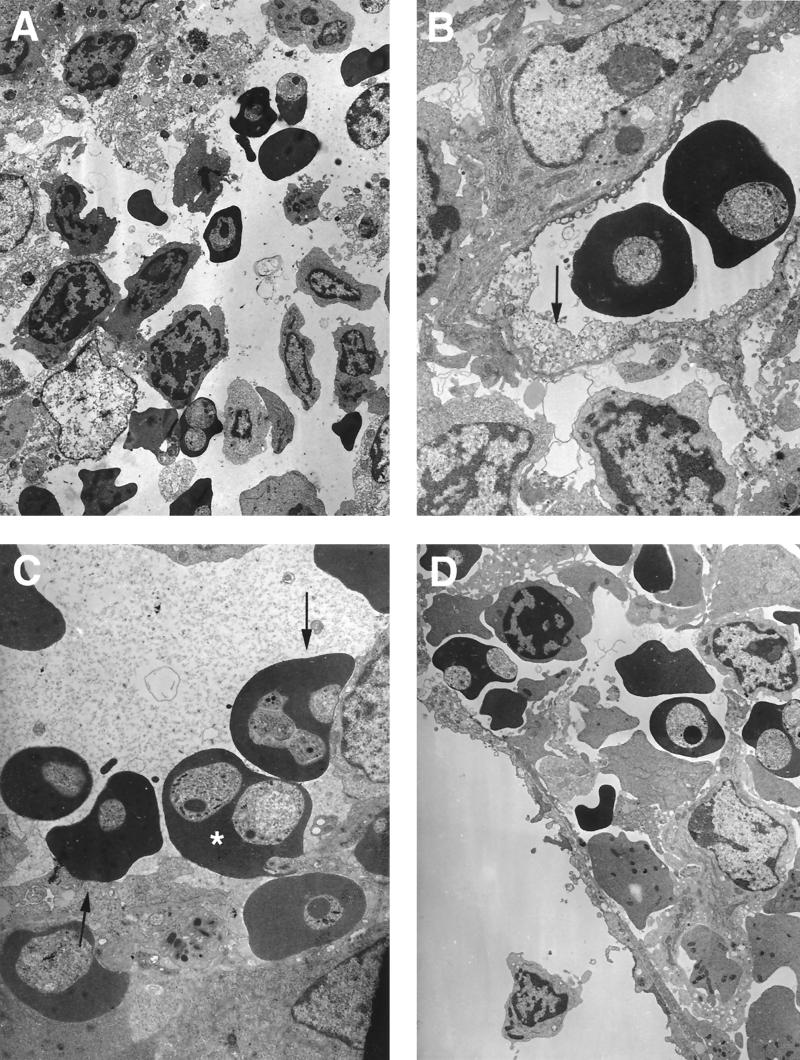
At day 7 postinfection, no major differences in infected-cell adherence were observed in the spleens of infected and uninfected mice. By day 10, a higher parasitemia (50% ± 2%) was measured in the spleen than in the peripheral circulation (30% ± 3%) (results not shown). It is possible that infected cells may be present in the spleen as the result of clearance of some infected cells from the circulation. Thus, it is known that in splenic cords erythrocytes can enter and mingle with splenic macrophages (Fig. 8A and D), and some vessels nearby do not show any infected erythrocytes adhering to endothelium (Fig. 8D). However, in some other vessels intimate contact between infected erythrocytes and endothelial cells was frequently observed (Fig. 8C). Signs of endothelial fragility, such as swelling and cell damage, were very commonly observed (Fig. 8B). Macrophages containing hemoglobin were very common, not only at day 10 but also at day 13 postinfection.
FIG. 8.
Electron micrographs of spleen sections from P. c. chabaudi AS-infected mice. Mice were killed at a parasitemia of approximately 45% and at the time of maximal parasite withdrawal from the peripheral blood. Magnifications, ×5,000 (A), ×12,500 (B), ×12,500 (C), and ×6,500 (D). (A) Cross-sectional view of a splenic cord where erythrocytes mix with other cells. (B) Cross-sectional view of a vessel where infected erythrocytes do not show any contact with endothelial cells, which show signs of fragility (arrow). (C and D) Cross-sectional views of infected erythrocytes adhering to endothelial cells (arrows) of a vessel, showing migration of an infected erythrocyte through the vessel wall (∗) (C) and presence of infected and uninfected erythrocytes free in the surrounding tissue (D).
DISCUSSION
Sequestration of infected erythrocytes occurs in P. falciparum infections in humans. Furthermore, sequestration in the brain has been considered to be the main cause of cerebral malaria, one of the most life-threatening pathological consequences of acute P. falciparum infection in humans. P. falciparum is the only human malaria parasite to sequester, and the reason(s) for this fact remains unclear. A complete understanding of the interaction between host endothelial cells and infected erythrocytes will be an essential step for development of therapeutics against this target. The rodent models that have been used extensively to study cerebral malaria and sequestration have been P. berghei ANKA in mice (26) and P. berghei in hamsters (34, 35). These models exhibit some properties of P. falciparum-induced human cerebral malaria, such as vascular plugging and microhemorrhages. However, the mechanism underlying the observed pathology is distinct from that observed in P. falciparum infections, as lymphocytes and monocytes instead of infected erythrocytes are the main cell types sequestered in the venules in these rodent models. Infection of mice with the lethal (17XL) line of Plasmodium yoelii has been shown to cause blockage of brain capillaries, associated with cytoadherence of infected erythrocytes to the endothelium of postcapillary venules in the brain (24). Since P. yoelii 17XL infection results in little, if any, accumulation of monocytes or macrophages in the brain, this is arguably a more suitable model for cerebral malaria than P. berghei ANKA. However, 17XL is a lethal parasite producing an overwhelming parasitemia and cannot be used as a model to study the importance and role of sequestration in the course of a less virulent malaria infection.
In young adult male CBA/Ca mice, P. c. chabaudi AS causes an acute (biphasic) blood stage infection usually lasting only 3 to 4 weeks, and peak parasitemia rarely exceeds 30 to 40%. Host immunity quickly reduces the parasitemia to subpatent levels followed by full resolution of the infection, sometimes after a period of low-level chronic infection. The pattern of infection of CBA/Ca mice with this parasite line is intermediate between that in C57BL/6 and BALB/c mouse strains, which are slightly more and slightly less resistant, respectively (22). Although there is no evidence to suggest that sequestration during P. c. chabaudi AS infection causes cerebral malaria, other potentially lethal pathological consequences of sequestration can be studied. Recent studies from this laboratory have identified specific protective antibody responses directed to the infected erythrocyte surface (28), which may contribute to initial parasite clearance. It was important to study sequestration in this model and determine whether the antibody induced by infection could block or inhibit binding to endothelial cells.
The present study describes for the first time the capacity of erythrocytes infected with a rodent malaria parasite (P. c. chabaudi AS) to adhere specifically to purified human CD36, the major host receptor for binding P. falciparum-infected erythrocytes. Human, murine, and rat CD36s show over 90% identity at the amino acid level, and all three support avid cytoadherence of P. falciparum-infected erythrocytes (39). Using an adhesion assay with rat endothelial cells that bind mouse lymphocytes, we also show that mouse erythrocytes infected with mature parasites bind to receptors on these cells which can be up-regulated by treatment with IFN-γ, suggesting the involvement of other adhesion molecules in the binding process. Pretreatment of the endothelial cells with IFN-γ induces high-level expression of several adhesion molecules, such as ICAM-1 and VCAM-1 (reference 13 and data not shown). Furthermore, preincubation of P. c. chabaudi AS-infected erythrocytes with purified CD36 results in only partial reduction of the IFN-γ-induced binding (results not shown), reinforcing the idea that other molecules are also involved in adherence. In P. falciparum, PfEMP-1 on the surface of the infected erythrocyte mediates adhesion to the endothelium and is targeted by variant-specific antibodies. Antibodies against such P. falciparum variant-specific antigens may play an important role in immunity to malaria (7). Potentially protective antibody is induced during acute P. c. chabaudi AS infection and specifically recognizes parasite line-specific antigens on the surfaces of infected erythrocytes (28). In this report, we show that this antibody blocks the binding of P. c. chabaudi AS-infected erythrocytes to endothelial cells. This inhibition of adherence is due to specific antibody binding to the surface of the infected erythrocyte, which, at the concentration used in these experiments, is parasite line and species specific (28).
The adhesive properties of P. c. chabaudi AS-infected erythrocytes share many features with those of P. falciparum-infected erythrocytes. The ultrastructural studies of different organs during P. c. chabaudi AS infection showed a very close interaction between infected erythrocytes and endothelial cells of blood vessel walls. Erythrocytes infected with mature P. falciparum parasites have morphologically distinct surface modifications (knobs) that facilitate and strengthen interactions between infected-cell and endothelial cell receptors (11). In contrast, P. c. chabaudi-infected cells do not have typical knobs at their surfaces, although points of adhesion between infected erythrocytes and endothelial cells were observed. Although direct comparisons have not been made, the absence of knobs in P. c. chabaudi AS might account for the lower level of adherence we observe (approximately 10 times lower) compared to similar results obtained with P. falciparum (e.g., reference 41).
Host endothelial cells show many alterations during P. c. chabaudi AS infection, such as swelling and proliferation or elongation. These two phenomena seem to be independent. Proliferation or elongation was only observed when infected erythrocytes were in the vicinity. In this case, endothelial cells formed processes that could surround infected erythrocytes and thus restrict blood flow in the region. Whether this is induced by the presence of the infected erythrocyte is unknown. On the other hand, swelling was a generalized feature of the endothelium in the liver, spleen, and brain, even when only noninfected erythrocytes were present in the plane of section. This observation cannot merely be a fixation artifact, since other tissues in the same section or endothelium in corresponding tissue samples from noninfected mice, fixed in the same way, did not show any signs of fragility. Both phenomena have been described in P. falciparum infections in humans (33), but their significance is still unknown. Cytokines, such as IFN-γ and tumor necrosis factor alpha, have important regulatory roles in local vascular proliferation (17, 38), and these cytokines, which are known to be induced by both P. chabaudi and P. falciparum infection (21, 25, 32), might be responsible.
In human infection with P. falciparum, only a small proportion of individuals develop cerebral malaria, in spite of the fact that mature parasites are rarely seen in peripheral blood. This fact strongly supports the idea that in human infection the sites of sequestration vary between individuals or may reflect the binding of infected erythrocytes to different endothelial cell receptors. The factors which determine in which organs sequestration is likely to occur are still unknown. It has been proposed that up-regulated expression of adhesion receptors in the brain may determine that sequestration of some P. falciparum-infected cells occurs in this site (6). Recently, it was shown that in humans infected with P. falciparum, widespread endothelial activation is a feature of the disease in both nonfatal and fatal malaria (43, 44). Our results show that in P. c. chabaudi AS infection, sequestration occurs mainly in the liver, but some sequestration was also observed in the spleens and brains of infected mice. Examination of endothelial cell phenotype and function in individual tissues is necessary, and the P. c. chabaudi AS model may prove very useful in determining the relationship between endothelial cell activation and levels of sequestration.
Intimate contact between infected erythrocytes and endothelial cells was also observed in some brain vessels. Furthermore, some leakage of erythrocytes into the extravascular space was evident. Although endothelium rupture was not apparent, the blood-brain barrier appeared to be compromised in infected mice by day 10 postinfection. Punctiform hemorrhages of erythrocytes surrounding a ruptured cerebral vessel are common in P. falciparum cerebral malaria (42).
There are no overt clinical signs of cerebral malaria in P. c. chabaudi AS-infected mice, so this host-parasite combination is not a direct rodent model for human cerebral malaria. However, our observations suggest this model may be useful to study some aspects of sequestration, such as the identities and levels of expression of adhesion molecules that might determine the site of sequestration as well as a possible role in natural infection and immune evasion. Many Plasmodium species do not sequester during natural infection, and therefore the premise that avoidance of passage through the spleen is the primary advantage of sequestration should be reexamined. Nonsequestering clones of P. c. chabaudi show no difference in parasite clearance rate compared to sequestering clones (16), although they do produce lower parasitemias. These observations suggest that sequestration has evolved primarily not to avoid splenic clearance but to modulate the host immune response. Recent observations support this possibility, for example, the case of P. falciparum lines expressing PfEMP-1 that inhibits the activation and antigen presentation of dendritic cells (45). Furthermore, there are strong indications that active signaling can occur between Plasmodium and its hosts. Thus, passage of cloned organisms through splenectomized animals may result in the appearance of parasites that no longer sequester. The sequestering phenotype can reappear by passage of the nonsequestering parasites into intact animals (16). A proper understanding of the mechanism and role of sequestration in Plasmodium infections will be crucial in the development of new therapies against malaria. The use of an in vivo model such as P. c. chabaudi AS in mice will help to resolve many of the outstanding questions.
ACKNOWLEDGMENTS
We thank Lawrie Bannister for critical reading of the manuscript, Ann Ager for advice and for supplying the rat endothelial cells, and Chris Newbold for supplying purified CD36.
M.M.M. was supported by the PRAXIS XXI Program (BD2665/94), Portugal.
REFERENCES
- 1.Ager A. Isolation and culture of high endothelial cells from rat lymph nodes. J Cell Sci. 1987;87:133–144. doi: 10.1242/jcs.87.1.133. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa M, Brown A, Smith C D, Tegoshi T, Howard R J, Hasler T H, Ito Y, Perry G, Collins W E, Webster K. A primate model for human cerebral malaria: Plasmodium coatneyi-infected rhesus monkeys. Am J Trop Med Hyg. 1992;46:391–397. doi: 10.4269/ajtmh.1992.46.391. [DOI] [PubMed] [Google Scholar]
- 3.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M, Ingravallo P. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Investig. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baruch D I, Gormely J A, Ma C, Howard R J, Pasloske B L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule-1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 6.Berendt A R, Turner G D H, Newbold C I. Cerebral malaria: the sequestration hypothesis. Parasitol Today. 1994;10:412–414. doi: 10.1016/0169-4758(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 7.Bull P C, Lowe B S, Kortok M, Molyneux C S, Newbold C I, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter R. Studies on enzyme variation in the murine malaria parasites Plasmodium berghei, P. yoelii, P. vinckei and P. chabaudi by starch gel electrophoresis. Parasitology. 1978;76:241–267. doi: 10.1017/s0031182000048137. [DOI] [PubMed] [Google Scholar]
- 9.Cooke B M, Berendt A R, Craig A G, MacGregor J, Newbold C I, Nash G B. Rolling and stationary cytoadhesion of red blood cells parasitized by Plasmodium falciparum: separate roles for ICAM-1, CD36 and thrombospondin. Br J Haematol. 1994;87:162–170. doi: 10.1111/j.1365-2141.1994.tb04887.x. [DOI] [PubMed] [Google Scholar]
- 10.Cox J, Semoff S, Hommel M. Plasmodium chabaudi: a rodent malaria model for in-vivo and in-vitro cytoadherence of malaria parasites in the absence of knobs. Parasite Immunol. 1987;9:543–561. doi: 10.1111/j.1365-3024.1987.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 11.Crabb B S, Cooke B M, Reeder J C, Waller R F, Caruana S R, Davern K M, Wickham M E, Brown G V, Coppel R L, Cowman A F. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89:287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 12.Crandall I, Collins W E, Gysin J, Sherman I W. Synthetic peptides based on motifs present in human band 3 protein inhibit cytoadherence/sequestration of the malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1993;90:4703–4707. doi: 10.1073/pnas.90.10.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faveeuw C, Di Mauro M E, Price A A, Ager A. Roles of alpha(4) integrins/VCAM-1 and LFA-1/ICAM-1 in the binding and transendothelial migration of T lymphocytes and T lymphoblasts across high endothelial venules. Int Immunol. 2000;12:241–251. doi: 10.1093/intimm/12.3.241. [DOI] [PubMed] [Google Scholar]
- 14.Gardner J P, Pinches R A, Roberts D J, Newbold C I. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnham P C C. Malaria parasites and other haemosporidia. Oxford, United Kingdom: Blackwell Scientific Publications; 1966. [Google Scholar]
- 16.Gilks C F, Walliker D, Newbold C I. Relationships between sequestration, antigenic variation and chronic parasitism in Plasmodium chabaudi chabaudi—a rodent malaria model. Parasite Immunol. 1990;12:45–64. doi: 10.1111/j.1365-3024.1990.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 17.Giraudo E, Primo L, Audero E, Gerber H P, Koolwijk P, Soker S, Klagsbrun M, Ferrara N, Bussolino F. Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem. 1998;273:22128–22135. doi: 10.1074/jbc.273.34.22128. [DOI] [PubMed] [Google Scholar]
- 18.Gysin J, Aikawa M, Tourneur N, Tegoshi T. Experimental Plasmodium falciparum cerebral malaria in the squirrel monkey Saimiri sciureus. Exp Parasitol. 1992;75:390–398. doi: 10.1016/0014-4894(92)90252-6. [DOI] [PubMed] [Google Scholar]
- 19.Howard R J, Gilladoga A D. Molecular studies related to the pathogenesis of cerebral malaria. Blood. 1989;74:2603–2618. [PubMed] [Google Scholar]
- 20.Iqbal J, Perlmann P, Berzins K. Plasmodium falciparum: analysis of the cytoadherence inhibition of the human monoclonal antibody 33G2 and of antibodies reactive with antigen Pf332. Exp Parasitol. 1993;77:79–87. doi: 10.1006/expr.1993.1063. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs P, Radzioch D, Stevenson M M. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarra W. Studies on the induction and expression of protective immunity in rodent malaria: Plasmodium berghei and P. c. chabaudi infections of inbred mice. Ph.D. thesis. London, United Kingdom: Brunel University; 1982. [Google Scholar]
- 23.Jarra W, Brown K N. Protective immunity to malaria: studies with cloned lines of Plasmodium chabaudi and P. berghei in CBA/Ca mice. I. The effectiveness and inter- and intra-species specificity of immunity induced by infection. Parasite Immunol. 1985;7:595–606. doi: 10.1111/j.1365-3024.1985.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaul D K, Nagel R L, Llena J F, Shear H L. Cerebral malaria in mice: demonstration of cytoadherence of infected red blood cells and microrheologic correlates. Am J Trop Med Hyg. 1994;50:512–521. doi: 10.4269/ajtmh.1994.50.512. [DOI] [PubMed] [Google Scholar]
- 25.Kern P, Hemmer C J, Van Damme J, Gruss H J, Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989;87:139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- 26.Mackey L J, Hochmann A, June C H, Contreras C E, Lambert P H. Immunopathological aspects of Plasmodium berghei infection in five strains of mice. II. Immunopathology of cerebral and other tissue lesions during the infection. Clin Exp Immunol. 1980;42:412–420. [PMC free article] [PubMed] [Google Scholar]
- 27.MacPherson G G, Warrell M J, White N J, Looareesuwan S, Warrell D A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 28.Mota M M, Brown K N, Holder A A, Jarra W. Acute Plasmodium chabaudi chabaudi malaria infection induces antibodies which bind to the surface of parasitized erythrocytes and promote their phagocytosis by macrophages in vitro. Infect Immun. 1998;66:4080–4086. doi: 10.1128/iai.66.9.4080-4086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ockenhouse C F, Klotz F W, Tandon N N, Jamieson G A. Sequestrin, a CD36 recognition protein on Plasmodium falciparum malaria-infected erythrocytes identified by anti-idiotype antibodies. Proc Natl Acad Sci USA. 1991;88:3175–3179. doi: 10.1073/pnas.88.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ockenhouse C F, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan K E, Thway Y, Win K, Aikawa M, Lobb R R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- 32.Phillips R S. Effector mechanisms against asexual erythrocytic stages of Plasmodium. Immunol Lett. 1994;41:109–114. doi: 10.1016/0165-2478(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 33.Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991;44:168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- 34.Rest J R. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76:410–415. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- 35.Rest J R. Pathogenesis of cerebral malaria in golden hamsters and inbred mice. Contrib Microbiol Immunol. 1983;7:139–146. [PubMed] [Google Scholar]
- 36.Roberts D D, Sherwood J A, Spitalnik S L, Panton L J, Howard R J, Dixit V M, Frazier W A, Miller L H, Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985;318:64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- 37.Rogerson S J, Chaiyaroj S C, Ng K, Reeder J C, Brown G V. Chondroitin sulphate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saegusa Y, Ziff M, Welkovich L, Cavender D. Effect of inflammatory cytokines on human endothelial cell proliferation. J Cell Physiol. 1990;142:488–495. doi: 10.1002/jcp.1041420307. [DOI] [PubMed] [Google Scholar]
- 39.Serghides L, Crandall I, Hull E, Kain K C. The Plasmodium falciparum-CD36 interaction is modified by a single amino acid substitution in CD36. Blood. 1998;92:1814–1819. [PubMed] [Google Scholar]
- 40.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L H. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. PECAM/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 42.Turner G. Cerebral malaria. Brain Pathol. 1997;7:569–582. doi: 10.1111/j.1750-3639.1997.tb01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner G D, Morrison H, Jones M, Davis T M, Looareesuwan S, Buley I D, Gatter K C, Newbold C I, Pukritayakamee S, Nagachinta B. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 44.Turner G D, Ly V C, Nguyen T H, Tran T H, Nguyen H P, Bethell D, Wyllie S, Louwrier K, Fox S B, Gatter K C, Day N P, Tran T H, White N J, Berendt A R. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol. 1998;152:1477–1487. [PMC free article] [PubMed] [Google Scholar]
- 45.Urban B C, Ferguson D J P, Pain A, Willcox N, Plebanski M, Austyn J M, Roberts D J. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 46.Walliker D. The genetic basis of diversity in malaria parasites. Adv Parasitol. 1983;22:217–259. doi: 10.1016/s0065-308x(08)60463-7. [DOI] [PubMed] [Google Scholar]