Abstract
Viral infections are a leading cause of myocarditis and pericarditis worldwide, conditions that frequently coexist. Myocarditis and pericarditis were some of the early comorbidities associated with SARS-CoV-2 infection and COVID-19. Many epidemiologic studies have been conducted since that time concluding that SARS-CoV-2 increased the incidence of myocarditis/pericarditis at least 15× over pre-COVID levels although the condition remains rare. The incidence of myocarditis pre-COVID was reported at 1 to 10 cases/100 000 individuals and with COVID ranging from 150 to 4000 cases/100 000 individuals. Before COVID-19, some vaccines were reported to cause myocarditis and pericarditis in rare cases, but the use of novel mRNA platforms led to a higher number of reported cases than with previous platforms providing new insight into potential pathogenic mechanisms. The incidence of COVID-19 vaccine-associated myocarditis/pericarditis covers a large range depending on the vaccine platform, age, and sex examined. Importantly, the findings highlight that myocarditis occurs predominantly in male patients aged 12 to 40 years regardless of whether the cause was due to a virus-like SARS-CoV-2 or associated with a vaccine—a demographic that has been reported before COVID-19. This review discusses findings from COVID-19 and COVID-19 vaccine-associated myocarditis and pericarditis considering the known symptoms, diagnosis, management, treatment, and pathogenesis of disease that has been gleaned from clinical research and animal models. Sex differences in the immune response to COVID-19 are discussed, and theories for how mRNA vaccines could lead to myocarditis/pericarditis are proposed. Additionally, gaps in our understanding that need further research are raised.
Keywords: COVID-19 vaccines; models, animal; mRNA vaccines; sex characteristics; vaccines
Nearly 3 years have passed since the World Health Organization declared SARS-CoV-2–induced COVID-19 as a pandemic. Some of the early comorbidities reported for COVID-19 were cardiovascular complications including arrhythmias, myocardial infarct, myocarditis, pericarditis, and thromboembolic events. Since that time, many population-based studies have been conducted to examine the incidence or prevalence of myocarditis or pericarditis associated with SARS-CoV-2 infection or COVID-19. Vaccines against SARS-CoV-2 were rapidly developed, including a new mRNA vaccine platform that utilizes mRNA against the dominant antigen of the virus encapsulated in lipid nanoparticles also known as extracellular vesicles (EVs). Soon after the vaccination programs started, case reports describing myocarditis and pericarditis appeared1,2 with data obtained from passive vaccine surveillance programs, hospital data, and from countries with mandatory vaccination programs or integrated health care systems. Over time, many large population-based studies examined the incidence or prevalence of vaccine-associated myocarditis. This review provides a summary of data on the ability of SARS-CoV-2 to infect the heart, the immune response that it generates, animal models of COVID-19 and their relevance to the heart, as well as the epidemiology, symptoms, diagnosis, and management of COVID-19–associated myocarditis and pericarditis including COVID-19 vaccine–associated cases and proposed mechanisms.
SARS-CoV-2 Cardiac Viral Entry
SARS-CoV-2 is a large enveloped RNA virus that shares around 80% sequence homology with SARS-CoV and 50% homology with the Middle Eastern respiratory syndrome coronavirus.3 Importantly, ACE2 (angiotensin-converting enzyme 2) had been identified as the receptor for SARS-CoV4,5 and SARS-CoV-2.6–8 The spike protein of SARS-CoV-2 binds ACE2 and is cleaved by human type II TMPRSS2 (transmembrane serine protease-2) facilitating viral entry into the cytosol.6 TMPRSS2 is also required for SARS-CoV and Middle Eastern respiratory syndrome coronavirus viral entry.9,10 COVID-19 occurs predominantly in men,11–13 which may be explained, at least in part, by a higher expression of ACE2 on male versus female cells.14 Thus, these 3 coronaviruses that cause myocarditis share many similarities in the receptors they use for viral entry.
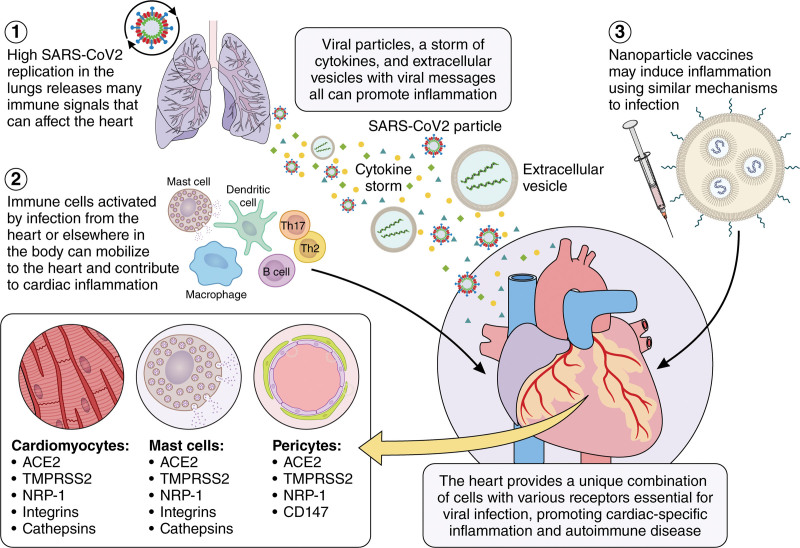
ACE2 expression has been reported for many tissues/organs including the lung (ie, lung type II alveolar cells/AT2, bronchial epithelial cells), brain, kidney, small intestine, colon, and heart.9,14–17 Zou et al16 examined published single-cell RNA sequencing data and found that 7.5% of cells in the heart expressed ACE2. In the heart, ACE2 has been reported to be expressed on cardiomyocytes, pericytes (cells present along the walls of capillaries), and macrophages with lower expression on fibroblasts and endothelial cells.18,19 TMPRSS2 is also expressed on endothelial cells and pericytes.10 The SARS-CoV-2 genome has been detected by polymerase chain reaction (PCR) in cardiac tissues from autopsies of patients with COVID-19,20,21 suggesting the virus can infect the heart. Thus, cardiomyocytes and pericytes express ACE2 and TMPRSS2, as well as other accessory proteins (ie, NRP1 [neuropilin-1 receptor], CD147, integrin α5β1, and cathepsin B/L) needed for viral infection by SARS-CoV-2 (Figure 1; reviewed in the study by Abdi et al22).23–27 In a study examining the prevalence of ACE2 on immune cells, it was found to be expressed primarily on activated tissue macrophages but not on peripheral blood mononuclear cells in healthy people.28 ACE2, TMPRSS2, NRP1, integrins, and cathepsins are also expressed on mast cells (Figure 1),28,29 which can act as antigen-presenting cells (APCs) in addition to their typical activity in promoting T helper (Th) 2 immune responses, remodeling, and fibrosis. Because the level of virus in the heart is thought to be low based on autopsy studies,20,21 it has been questioned whether low SARS-CoV-2 levels in the heart can cause myocarditis.
Figure 1.
Potential mechanisms leading to myocarditis/pericarditis following SARS-CoV-2 infection or vaccination. SARS-CoV-2 initially infects the lungs generating a cytokine storm including TNFα (tumor necrosis factor-alpha), IL (interleukin)-1β, and IL-6 and releasing extracellular vesicles (EVs) that contain virus or virus particles. 2. EVs may traffic through the blood or lymph to the heart where they infect cardiac cells that express the necessary receptors (ACE2 [angiotensin-converting enzyme 2], TMPRSS2 [transmembrane serine protease-2], and NRP1 [neuropilin-1 receptor]) such as cardiomyocytes, pericytes, mast cells, and macrophages. Additionally, resident antigen-presenting cells like mast cells, dendritic cells, and macrophages respond to virus and damaged cardiac tissue by activating an adaptive autoimmune response leading to myocarditis. 3. COVID-19 vaccines may activate resident mast cells or macrophages at the injection site that in susceptible individuals who have cardiac injury may promote an autoimmune response leading to myocarditis. Illustration credit: Sceyence Studios.
SARS-CoV-2–Mediated Cardiac Damage: Potential Mechanisms
Clinicians typically assess myocardial damage (ie, necrosis) by examining serum cardiac troponins.30,31 However, myocarditis often occurs without necrosis so that the absence of elevated troponin does not rule out the presence of myocarditis, even severe myocarditis.32,33 Potentially low SARS-CoV-2 infection may damage cardiomyocytes leading to cardiac myosin release and activation of resident APCs like mast cells and macrophages to recruit inflammation to the heart. Autopsy studies conducted retrospectively to determine the number of myocarditis cases from COVID-19 often have several issues, including requiring the histology to display inflammation and necrosis and not providing or analyzing data according to sex and age. For example, 1 study of 277 autopsy cases from patients with COVID-19 reported myocarditis in 7.2% of cases,34 but the median age of subjects in the study was 75 year-old-men and women while myocarditis predominantly occurs in men under the age of 50 years.
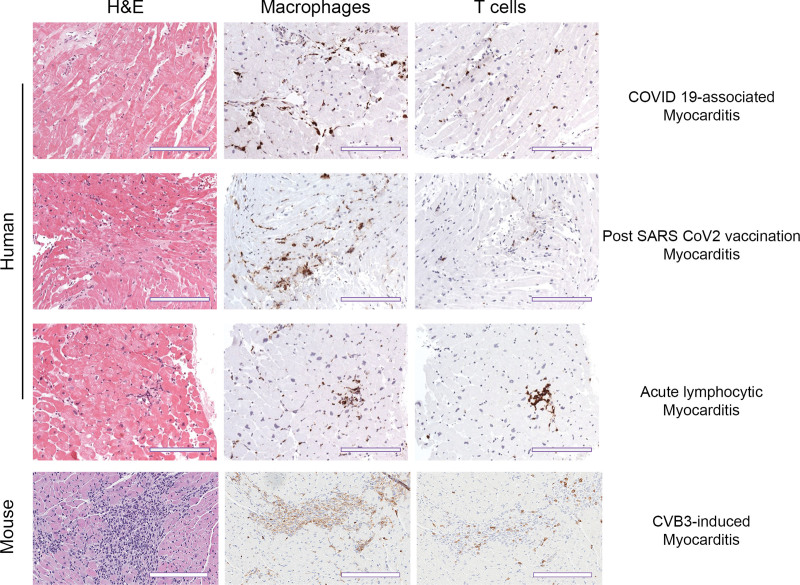
One important question is whether direct infection of cardiac tissues by SARS-CoV-2 can lead to myocarditis or whether other mechanisms such as cytokine storm, indirect infection from EVs, or molecular mimicry are needed. Direct infection with high viral levels is presumed to be the cause of viral myocarditis. However, several animal models of viral myocarditis have low or barely detectable levels of virus in the heart (or use complete Freund’s adjuvant with inactivated Mycobacterium tuberculosis), and these autoimmune models closely resemble the time course and pathogenesis of clinical lymphocytic myocarditis (data shown in review35; Table S1).35–37 A comparison of viral autoimmune myocarditis models to virus- or autoimmune-only models is summarized in Table S1 and reviewed in previous studies.35,37–39 It is important to realize that the dominant immune infiltrate during COVID-19 myocarditis, acute lymphocytic myocarditis, and in autoimmune models of myocarditis are macrophages (50%–80%) with fewer T and B cells (15%; Figure 2), and so the name for this most common form of myocarditis (ie, lymphocytic) is somewhat misleading. Thus, based on findings in autoimmune models of myocarditis, it is not necessary for SARS-CoV-2 to replicate in the heart at a high level to cause myocarditis.
Figure 2.
Similarity in histological staining ratio for macrophages and T cells during COVID-19 myocarditis and vaccination versus pre-COVID myocarditis in humans and mice. Representative immunohistochemistry staining of myocardium in both human and mouse samples. Hematoxylin and eosin (H&E) staining shows inflammatory foci. Species-specific markers for macrophages (CD63+ human, CD11b+ mouse) and T cells (CD3+) show immune cell composition of the inflammatory infiltrate. Human scale bars, 100 µm; mouse scale bars, 200 µm.
Because COVID-19 is associated with cytokine storm,40 it has been proposed that this may lead to myocarditis. However, no animal models of myocarditis exist where administration of proinflammatory cytokines alone induce cardiac inflammation without the use of an adjuvant (ie, active or inactive virus, bacteria, or parasite) and damaged self-tissue. The fundamental question is how inflammation would be directed to the heart unless cardiac damage has occurred or a microbe infects the heart (even at a low level). In this context, elevated circulating cytokines could increase myocarditis as has been shown previously when recombinant TNFα (tumor necrosis factor-alpha), IL (interleukin)-1β, or IL-33 was administered in coxsackievirus B3 (CVB3) virus-only or autoimmune CVB3 animal models.41,42 Similarly, molecular mimicry has been examined for its potential role in virus-induced myocarditis for many years.43,44 Gil-Cruz et al44 found that cross-reactivity between gut bacteria and cardiac myosin-specific T cells promotes myocarditis in the context of a cardiac viral infection. Thus, the simplest explanation for how SARS-CoV-2 infection leads to myocarditis is that damage to the heart from viral infection draws inflammation to the heart and that an autoimmune response to virus and cardiac damage is amplified by the strong circulating proinflammatory milieu in susceptible individuals (ie, young men with more mast cells; Figure 1).
An emerging mechanism that may also be involved in promoting myocarditis following SARS-CoV-2 infection includes EVs that harbor viral mRNA. Many viruses such as HIV, coxsackievirus, hepatitis B and C, influenza, Epstein-Barr virus, and SARS-CoV-2 highjack cellular and mitochondrial programs to enhance viral replication, package virus into EVs, and use EVs containing virus or viral components to subvert the immune response to obtain a replicative advantage in the host (Figure 1).45–47 This is also true for SARS-CoV-2 RNA, which has been detected in EVs.48–50 Virus-containing EVs could enter the circulation from the lungs or other organs and enter the heart to be taken up by resident APCs like mast cells and macrophages to promote myocarditis (Figure 1). Additionally, it is possible that virus-containing EVs may be taken up by cells that do not express ACE2 using surface ligands on EVs.51
ACE2 as a Modulator of Vascular Function
ACE2 not only functions as a viral receptor for SARS-CoV-2 but also regulates blood pressure.52 When SARS-CoV-2 binds ACE2, it reduces its expression. ACE2 on endothelial cells of the arteries, arterioles, and venules of the heart and kidney determines its ability to regulate vascular function and blood pressure, as reviewed previously.19,53,54 ACE2 is a cell surface metalloenzyme and carboxypeptidase that regulates Ang II (angiotensin II) and Ang 1–7 (angiotensin 1–7). Ang II binds the ATR1 (Ang II receptor 1) receptor leading to release of TNFα and IL-6,55 which is associated with hypertension, diabetes, and heart disease52,56,57—major comorbidities in severe COVID-19.58 ATR1 also increases reactive oxygen species from mitochondria in monocytes/macrophages leading to DNA damage and apoptosis of T cells resulting in endothelial injury and lymphopenia.59,60 This leads to upregulation of complement pathways and TLR (Toll-like receptor) 2, TLR3, and TLR4 leading to elevated IFNs (interferons) and activation of the inflammasome resulting in amplified TNFα, IL-1β, and IL-6 to produce a cytokine storm. 61,62 We have published previously that upregulation of complement and TLRs including TLR4 are key immune pathways that promote myocarditis in the autoimmune-CVB3 animal model (reviewed in the studies by Di Florio et al63 and Fairweather et al64), although we have not examined the role of ACE2/Ang II/ATR1 in this model. However, Tanaka et al65 found that inhibiting Ang II reduced death in a viral-only model of ECMV-induced myocarditis, suggesting that this pathway could be important in viral myocarditis.
COVID-19 Myocarditis and Pericarditis
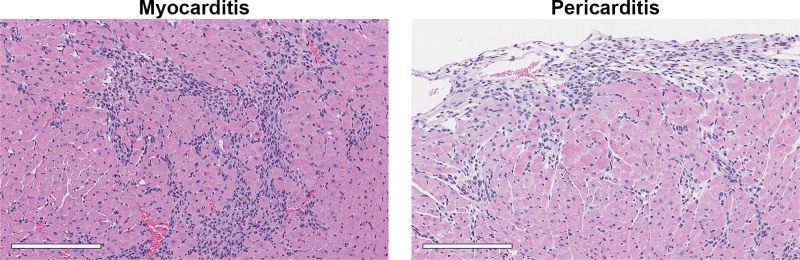
Myocardial damage similar to myocarditis was one of the first complications reported from patients with COVID-19 in Wuhan, China, at the beginning of the pandemic.66,67 Although respiratory complications from the virus were the most commonly reported, it became clear early on that SARS-CoV-2 infection was also leading to adverse cardiac events including ventricular arrhythmias, acute coronary syndromes with obstructive coronary artery disease such as myocardial infarct, thromboembolic syndromes including stroke, acute myocardial damage with elevated troponin levels without evidence of coronary artery disease (ie, myocarditis), and heart failure.66,68,69 In patients with severe COVID-19, elevated biomarkers of cardiac damage that predict heart failure including troponins and NT-proBNP (N-terminal pro-B-type natriuretic peptide) were strongly and independently associated with in-hospital mortality.70–72 Myocarditis is defined as inflammation of the myocardium with or without necrosis and is a leading cause of sudden cardiac death in children and adults worldwide.73,74 Pericarditis is defined as inflammation of the pericardium and in developed countries is primarily caused by viral infections, whereas in developing countries, tuberculosis is a common cause and associated with poor outcomes. Acute myocarditis and pericarditis, termed myopericarditis or perimyocarditis, are often detected together in clinical practice and animal models of myocarditis (Figure 3), and the terms were often used interchangeably in the COVID-19 literature.
Figure 3.
Myocarditis and pericarditis/perimyocarditis in the autoimmune CVB3 model. Male BALB/c mice received 103 plaque-forming units of CVB3 with damaged heart protein on day 0 and myocarditis and pericarditis assessed histologically at day 10 after infection. Hematoxylin and eosin staining. Scale bars, 200 µm.
Myocarditis and pericarditis/myopericarditis from COVID-19 present similarly to other forms of viral myocarditis and pericarditis, with symptoms including fever, cough, chest pain/pressure, dyspnea, palpitations, and syncope.2,31 As for other causes of myocarditis, probable cases of COVID-19 myocarditis are diagnosed as ≥1 new or worsening clinical symptoms, as well as ≥1 of the following: arrhythmias on electrocardiogram, cardiac dysfunction using echocardiography, or cardiac magnetic resonance imaging (cMRI) indicative of myocarditis.31,75 Confirmed diagnosis of myocarditis requires an endomyocardial biopsy (EMB), which was typically not conducted during the pandemic due to heightened concerns for viral transmission to staff.68,76 The diagnosis of myocarditis in patients with COVID-19, in general, relied more heavily on clinical symptoms and the presence of elevated troponins without evidence of coronary artery disease, especially in the United States where EMB is not typically acquired for lymphocytic myocarditis cases.
Management of COVID-19 myocarditis is essentially the same as pre-COVID myocarditis and is based on the expert opinion recommendations by the American College of Cardiology and the European Society of Cardiology guidelines.31,77 There has been some controversy regarding the effect of immunosuppressive therapies such as glucocorticoids for myocarditis, while recent long-term data support its benefits.78,79 Successful application of anti-inflammatory approaches in patients with COVID-19 has been reported in the literature, likely because of the overwhelming proinflammatory and cytokine response observed early after SARS-CoV-2 infection.31,80,81
Epidemiology of COVID-19 Myocarditis/Pericarditis
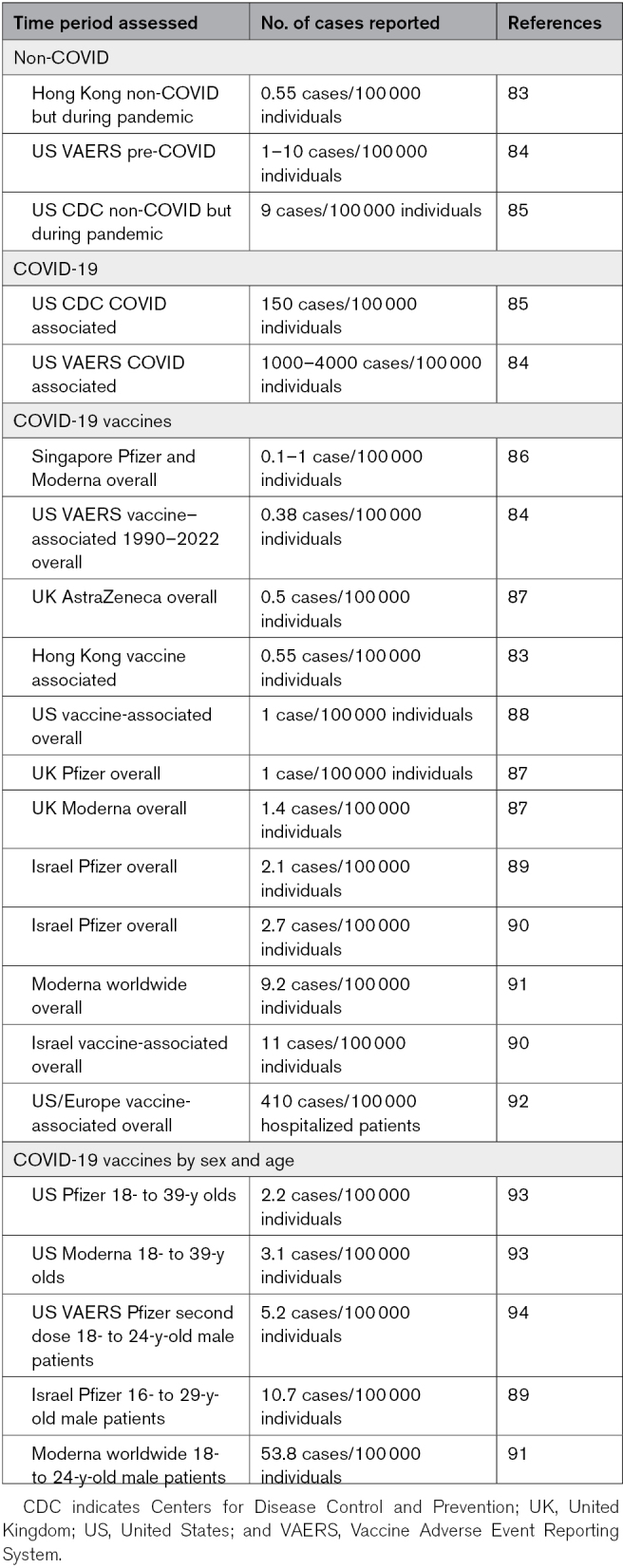
The latest Global Burden of Disease statistics before the COVID-19 pandemic place the worldwide prevalence of myocarditis and cardiomyopathy at 10.2 to 105.6 cases/100 000 individuals.74,82 A recent study estimated pre-COVID myocarditis in the United States at 1 to 10 cases/100 000 individuals (Table 1).84 The incidence of acute pericarditis pre-COVID in a large Finnish registry of all cardiovascular patients (n=670 409) was 3.3 cases/100 000 individuals.95,96 Many epidemiology studies of myocarditis in patients with COVID-19 have been conducted since the pandemic started, with some of the larger studies listed in Table S2. The overall incidence of myocarditis in the United States from SARS-CoV-2 infection has been estimated in a study by the Centers for Disease Control and Prevention at around 150 cases/100 000 versus 9 cases/100 000 individuals in non-COVID cases during the same time period (Table 1).2,85,97 A separate study in the United States and Europe estimated 240 cases/100 000 individuals of definite or probable myocarditis and 410 cases/100 000 individuals for possible myocarditis (Table 1).92 These data indicate around a ≥15-fold increased risk of developing myocarditis from SARS-CoV-2 infection compared with other causes (Table 1).74,84
Table 1.
Summary of Cases of Myocarditis Reported Before and During COVID-19 and Related to Vaccines
SARS-CoV-2 Strains and Myocarditis
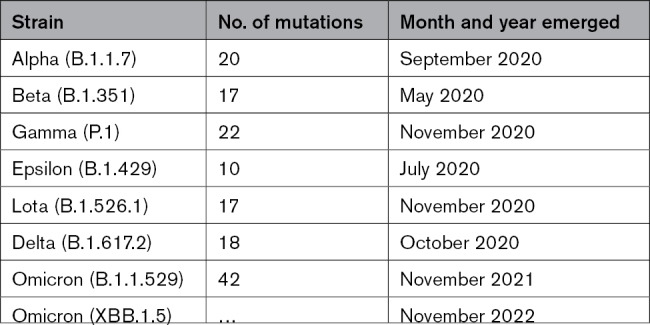
As is characteristic for rapidly replicating small RNA viruses like coxsackievirus and coronaviruses, mutations in key epitopes of the virus allow it to evade the adaptive immune response and promote infectivity depending on the location of the mutation. Table 2 lists the primary SARS-CoV-2 strains that were termed by the World Health Organization as a variant of interest or a variant of concern with the number of their mutations and the approximate date they were identified. According to the Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report, the Alpha variant led to more hospitalization and death than the original SARS-CoV-2 strain.98 Mutations in SARS-CoV-2 that occurred with Delta were found to cause more severe disease in individuals who were not vaccinated than other strains like Alpha.99 Delta remained the dominant strain until Omicron arrived around November 2021. Omicron cases had greater infectivity and the highest hospital admission frequency, but severe illness was lower than Delta and Alpha variants.100,101
Table 2.
SARS-CoV-2 Variant Strains
Zhang et al102 examined cardiovascular complications from 44 patients recovering from the Delta variant versus 25 controls and found that 32% had abnormal findings on cMRI and 20% with evidence of myocarditis. The study had 64% women with a median age of 51 (range, 39–62) years. Myocarditis typically occurs more often in young men aged 16 to 30 years, and the sex ratio for COVID-19 studies is typically observed to be 60% men to 40% women.64,103–105 Thus, if a younger cohort with a more typical sex ratio had been examined, they may have found a higher percentage of possible myocarditis cases. However, multiple studies reported cardiovascular complications from COVID-19 including myocarditis that ranged from 18% to 60% of cases.106–108 Soon after the Omicron variant emerged, case reports of myocarditis appeared.109 A prospective study of 998 patients with COVID-19 that examined cardiovascular outcomes found that traditional biomarkers such as troponins and NT-proBNP predicted mortality regardless of the SARS-CoV-2 strain (ie, Alpha, Beta, Gamma, and Delta),110 but they did not specifically examine myocarditis. Another study examined several strains of SARS-CoV-2 for their ability to infect and kill cultured cardiomyocytes and found that virus replicated to high levels for all strains, but Delta replicated at a higher level, caused more death, worsened function (ie, beating ability), and increased proinflammatory cytokines including IL-1β and IFNs compared with Omicron.111 These findings suggest that Delta may have been more affective at inducing myocarditis than Omicron. In a separate study, investigators found that only coronary artery endothelial cells expressed ACE2, with infection occurring regardless of which variant was examined.112 A recent pediatric study reported that Omicron had the highest admission frequency for poor outcome including death, but severe illness was lower than with Delta and Alpha variants.101 The study included myocarditis as part of the score for worse outcomes but did not examine myocarditis specifically. Thus, myocarditis/pericarditis has been reported as a complication of COVID-19 for all strains of SARS-CoV-2 thus far.
Sex/Gender Differences in COVID-19 Myocarditis and Pericarditis
Myocarditis pre-COVID is known to occur more often in young men under the age of 50 years, with a sex ratio of 2 to 4:1 men to women, while women are more likely to develop myocarditis after menopause, which is reviewed in previous studies.64,113–117 Similar to myocarditis, pre-COVID pericarditis occurs more often in young men under the age of 50 years with a sex ratio of around 2:1.64,95,118 Most studies of COVID-19 report a male dominance of around 60% men to 40% women.12,13 Similarly, myocarditis associated with COVID-19 occurs more often in men than in women (60%–70% men to 30%–40% women).103–105 Two large studies of 3 000 000 and 200 000 patients, respectively, detected no sex difference in whether patients tested PCR positive for SARS-CoV-2, although men had higher rates of hospitalization, intensive care unit admission, and mortality.13,119 This was not the case for all studies. A study of ≈100 000 patients found that men were more often PCR positive for SARS-CoV-2 and had greater mortality than women.120 Proinflammatory cytokines and cardiac biomarkers have been reported to be elevated in men with COVID-19 compared with women including ferritin, CRP (C-reactive protein), IL-6, IL-8, and IL-18.13,121–123 And men have more neutrophils and monocytes, whereas women have more T cells,13,121–123 similar to autoimmune myocarditis.124,125 Thus, cytokines and biomarkers display the same sex differences as clinical myocarditis and animal models before SARS-CoV-2.
Inflammatory Response Associated With COVID-19
Inflammation is a key factor driving cardiac dysfunction in myocarditis. In 1 study, cardiac dysfunction based on echocardiography-derived global longitudinal strain was found in ≈80% of COVID-19 cases that had elevated serum IL-6.126 CD68+ macrophages with fewer T cells are a characteristic finding of immunohistochemistry performed on EMB from patients with COVID-19 myocarditis/pericarditis, and macrophages are the primary infiltrate with fewer T cells in autoimmune models of myocarditis (Figure 2).2,31,127 Thus, the characteristics of myocardial inflammation are similar between COVID-19 myocarditis and CVB3 and autoimmune myocarditis animal models.
cMRI is most often used to diagnose myocarditis using specific sequences that identify myocardial water content and fibrosis. cMRI cannot identify specific cellular components of inflammation and may be less accurate in the early stages of myocarditis because fibrosis typically develops weeks after acute myocarditis.128–130 The accuracy of cMRI depends on the amount of scar tissue present, with men developing more scar tissue and dilated cardiomyopathy (DCM) than women.64,124,131 In support of this hypothesis, myocarditis is often detected using cMRI in patients with COVID-19 1 to 6 months after acute viral infection based on distinct SARS-CoV-2 or COVID-19 symptoms and a positive PCR or antigen test.132 These observations further suggest that many cases of acute myocarditis may be asymptomatic.
The prognosis for viral or idiopathic pericarditis is good, based primarily on the effectiveness of colchicine combined with anti-inflammatories as therapies.118,133 The effectiveness of colchicine as a therapy provides insight into the pathogenesis of pericarditis, which mirrors myocarditis. Colchicine impairs neutrophil adhesion to vascular endothelium and degranulation and blocks activation of the NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome, which is required for cleaving caspase-1 leading to the production of IL-1β and IL-18.134–136 In myocarditis and pericarditis, neutrophil cardiac inflammation occurs before acute myocarditis (around 5–7 days after infection) and is mainly replaced by macrophage/T-cell inflammation during peak disease (7–14 days after infection). Colchicine also increases leukocytic cAMP levels and inhibits IL-1β and TNFα release from macrophages. The TLR4/NLRP3/caspase-1/IL-1β pathway is upregulated in men with myocarditis and is central to both the development of acute myocarditis and the remodeling that leads to DCM, which could explain the increased pericarditis incidence in younger men.
SARS-CoV-2 infection has been documented to strongly activate complement and to activate other innate immune pathways such as TLR4 and the inflammasome, which leads to increased IL-1β and IL-18 levels.137–142 TLR4 signaling is key in driving proinflammatory responses associated with COVID-19 and contributes to an increased Th1-type immune response because IL-18 (and IL-1β) strongly induces IFNs.143–145 Other key innate cytokines that are elevated during COVID-19 include TNFα and IL-6, which is increased by IL-1β.137–142 Tim-3 (T-cell immunoglobulin mucin domain 3) is a receptor that is upregulated on mast cells and macrophages after viral infection that inhibits T-cell responses and is associated with increased IL-10 release from alternatively activated macrophages.124,125,146 This response has been found to be important in CVB3-induced myocarditis in mice. Tim-3 and IL-10 upregulation is also observed in patients with COVID-19.139 This pathway may be responsible for the inhibited T-cell responses that have been reported during COVID-19 in some patients.138,139 COVID-19 is also associated with thromboembolism and clotting, which is driven by a number of factors including complement and mast cell activation.147,148 As is typical for many viruses, IFNs inhibit viral replication and are elevated during COVID-19, which helps drive Th1 and Th17 responses.149,150 Also similar to other viruses, SARS-CoV-2 has developed a number of methods to inhibit the protective IFN response resulting in poorly protective immune responses in some patients with COVID-19.69,150 Mathew et al151 conducted deep immune profiling of T and B cells obtained by flow cytometry from 125 patients with COVID-19 versus healthy controls and identified 3 immune phenotypes associated with worse disease outcome. They observed that COVID-19 results in hyperactivation of innate immune pathways, especially complement and TLR4-related pathways. The prototypical immune response associated with COVID-19 is more severe, but otherwise the immune response to SARS-CoV-2 closely resembles what has already been described for patients with myocarditis and animal models of viral and autoimmune myocarditis.
Immune Response During Murine Autoimmune CVB3 Myocarditis and Experimental Autoimmune Model
Animal models of myocarditis have yielded a wealth of information about the pathogenesis of disease including a number of landmark articles that demonstrate that myocarditis is an autoimmune disease that requires TLR activation by microbes.44,152,153 Immune pathways that are similar between COVID-19 and the pathogenesis of myocarditis in autoimmune animal models (and verified to some extent in patients) are summarized below. All 3 pathways of complement are upregulated in the serum of patients with myocarditis and predict progression to DCM.154 Mice with experimental autoimmune myocarditis (EAM) or autoimmune CVB3 myocarditis also upregulate complement components during the innate immune response and acute myocarditis including C3, CD11b (also known as CR [complement receptor] 3), C3aR, and C5aR.155–157 Elevated expression of C3aR (and CD68+ macrophages) was found in patients with myocarditis compared with those with cardiomyopathy without inflammation.158 The majority of immune cells in the heart during acute myocarditis in EAM, the autoimmune CVB3 myocarditis model, and humans are CD11b+ cells that include neutrophils, macrophages, mast cells, and some dendritic cells.124,146,152,159 Mouse strains that have many mast cells like BALB/c and A/J develop myocarditis that most closely resembles lymphocytic myocarditis that progresses to DCM. Mast cells work in cooperation with macrophages to increase profibrotic inflammation and remodeling that leads to scar and DCM (Figure 4).129,157,158,160
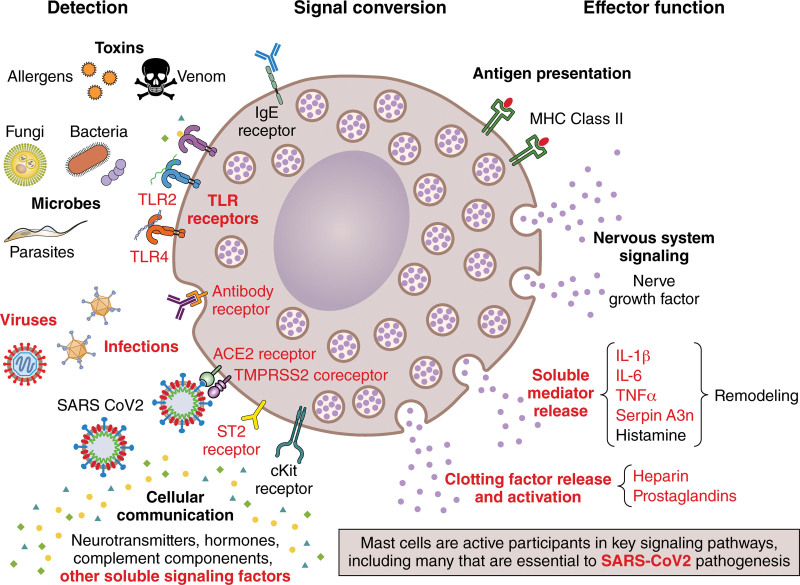
Figure 4.
Mast cell signaling contributes to myocarditis and may contribute to SARS-CoV-2 or vaccine-associated myocarditis. ACE2 indicates angiotensin-converting enzyme 2; cKit, receptor tyrosine kinase; IL, interleukin; MHC, major histocompatibility complex; serpin A3n, serpin family A member 3n (α1-antichymotrypsin); ST2, interleukin-1 receptor-like 1/IL-1RL1; TLR, Toll-like receptor; TMPRSS2, transmembrane protease serine-2; and TNFα, tumor necrosis factor-alpha. Illustration credit: Sceyence Studios.
Another key pathway upregulated in patients, EAM, autoimmune CVB3 myocarditis, and CVB3-only models is the TLR4, caspase-1, and NLRP3 pathways that increase IL-1β and IL-18 levels in the heart.124,146,152,156,161–164 IL-18, originally named IFN-γ–inducing factor, strongly drives Th1 immune responses,143–145 but in BALB/c mice, this produces a mixed Th1/Th2 response that promotes fibrosis and DCM rather than a classical STAT-driven Th1 response.115,146,160,165 Importantly, elevated TLR4 and IL-1β are found on CD11b/CR3-expressing macrophages and mast cells during the innate immune response in the spleen and heart and in the heart during acute myocarditis.124,146 TLR4 was also found to be expressed in the heart of patients with myocarditis and DCM.166,167 IL-1β levels in the heart correlate to the severity of inflammation in male BALB/c mice with autoimmune CVB3 myocarditis.115,129 Additionally, testosterone increases TLR4, caspase-1, and IL-1β levels during CVB3 myocarditis in male mice, which have higher levels of cardiac inflammation.129 Importantly, inhibition of TLR4 and NLRP3 pathways reduces myocarditis in mouse models.161,168 An important regulator of T cells following TLR4 activation is Tim-3, which displays sex differences during CVB3 myocarditis.124,125,146
Type I and II IFNs are a dominant immune response in CVB3 myocarditis models (and EAM) where inhibition of these pathways leads to increased viral replication, pericardial and myocardial inflammation, and DCM.124,128,152,169,170 However, IFNs reduce viral replication and prevent remodeling and fibrosis and thereby progression to DCM.128,169,170 Mast cells are critical for the remodeling and fibrosis that lead to DCM, due to their release of profibrotic cytokines (eg, IL-1β, TGFβ1 [transforming growth factor beta 1], and TNFα), and many enzymes, including Serpin A3n (α1-antichymotrypsin), that are required to activate IL-1β and matrix metalloproteinases that are required for fibrosis (Figure 4).129,171 Mast cells and macrophages work together to drive inflammation and fibrosis.129,158 IL-1β also increases serum and cardiac IL-6 levels that are needed to drive Th17 responses, which also promote fibrosis and progression to DCM and heart failure in patients with myocarditis and in animal models.36,165,172–174 Overall, all of the key immunological features that characterize the immune response to SARS-CoV-2 have previously been reported to play a role in the pathogenesis of EAM and autoimmune CVB3 models of myocarditis.
Insights on the Pathogenesis of Myocarditis From SARS-CoV-2 Models
An engineered heart tissue model of COVID-19 found that SARS-CoV-2 infection of the heart tissue led to contractile issues, sarcomere disassembly, TNFα cytokine production, macrophage infiltration, and cell death, mimicking viral myocarditis.27 A number of animal models have been developed to examine the pathogenesis of SARS-CoV-2 with an emphasis on understanding the effect on the lungs, but several studies also examined the heart. The primary animal models of COVID-19 from SARS-CoV-2 infection include the golden hamster, ferret, nonhuman primates, and mouse models (reviewed in the studies by Munoz-Fontela et al175 and Chu et al176). Although mouse models have the most information available about their biology and many research tools, the ACE2 receptor is significantly different between mice and humans; so mouse models most often use a humanized ACE2 or lung passage to overcome this obstacle.177–179 Investigators found viral replication in a number of organs with the highest expression in the lung and brain and increased serum cytokines including IFNγ; however, the mouse background used in these models was C57BL/6, which responds to antigens with elevated Th1-type immune responses because they have few mast cells.160,177,178 In a BALB/c mouse model (high mast cells) where the virus was passaged through the lung 6× to increase viral tropism for the lung, SARS-CoV-2 was detected in the heart at days 3 and 5 after infection, and disease was worse in old (9 months old) versus young (6 weeks old) mice with elevated IL-1β and IL-6.179 But they did not describe whether inflammation was found in the heart using this model. In another model of COVID-19 using BALB/c mice, TNFα, IL-1β, and IL-6 were increased in the lung during peak disease, but investigators did not describe the response in the heart.180 In a humanized transgenic mouse model, ACE2 was expressed in the heart and virus replication detected, but they did not observe cardiac inflammation.181 Most of these articles did not describe whether they examined male or female mice/cells while significant differences in myocarditis occur by sex in patients and animal models as already described.
SARS-CoV-2 infection of male Syrian hamsters caused viral infection and induced inflammation in the heart according to quantitative real-time PCR and immunohistochemistry (individually positive cells), but myocardial foci were not described.182 They also found increased TNFα and IL-1β in the heart and perivascular fibrosis, which in our experience typically indicates perivascular mast cell degranulation.157,160,182,183 They found increased CD15+ cells (a marker of myeloid cells such as granulocytes, neutrophils, eosinophils, mast cells, and macrophages), CD68+ macrophages, and CD4 and CD8 T cells in the heart in SARS-CoV-2–infected hamsters, but they did not specifically examine mast cells.182 This immune response (ie, dominant macrophages with fewer T cells) is typical of EAM, autoimmune CVB3 myocarditis, and human myocarditis in males (Figure 2).182,184,185 Male Syrian hamsters also develop worse myocarditis compared with females.186 In a separate study using female Syrian hamsters, cardiomyocyte hypertrophy (at days 4 and 35 after infection) and cardiac fibrosis and diastolic dysfunction were observed at day 35 after SARS-CoV-2 infection using echocardiography.187 This time course is the same as is observed with the autoimmune CVB3 and murine cytomegalovirus models of myocarditis and EAM (Table S1).35,124,170,188 However, they did not show a change in LV end diastolic dimension or end LV systolic dimension indicative of DCM; however, this may be because they examined females rather than males. Few females progress to DCM in humans or autoimmune/viral myocarditis animal models.64,124
Although mast cells are typically associated with IgE-mediated allergic responses, they have a wide array of roles including as APCs that respond to infections (Figure 4).171 Although resident mast cells are found in tissues such as the heart in small numbers, they are highly potent with local and far-ranging effects that influence the immune, hormone (including sex hormones), and central/peripheral nervous systems (Figure 4).171 We showed that mast cells are the first APCs to respond to CVB3 within 15 minutes of intraperitoneal infection during autoimmune CVB3 myocarditis, leading to upregulation of TLR4, and that this response leads to rapid increases in TNF, IL-1β, IL-6, and IFNγ in many organs including the heart.124,146,160 The critical role of mast cells as APCs during viral infections and in promoting myocarditis and pericarditis highlights their importance in the pathogenesis of disease. The activation of mast cells via ACE2, TMPRSS2, and NRP1 associated with COVID-19 is likely to be a crucial factor in promoting myocarditis/pericarditis following SARS-COV-2 infection (Figures 1 and 4).
COVID-19 Vaccine–Associated Myocarditis and Pericarditis
Not long after COVID-19 vaccination began in the general population, case reports appeared identifying myocarditis and pericarditis as a side effect of vaccination, especially after the second dose. Since that time, many large, population-based epidemiology studies have been conducted around the world that report myocarditis/pericarditis after vaccination (Table S3; reviewed in the study by Heidecker et al2). Many COVID-19 vaccines have been developed using various platforms and with several names for the same vaccine (summarized in Table S4). The reported incidence of myocarditis or pericarditis varies widely depending on the vaccine type and how many doses were administered, with the highest levels reported for the Moderna mRNA vaccine, with an overall incidence of ≈10/100 000 and around 50/100 000 in men under 40 years of age (Table 1).91 All reports agree that the greatest risk of developing myocarditis occurs after the second vaccine dose in young men aged 12 to 39 years. Ages past 50 years had few reports of vaccine-associated myocarditis, similar to pre- and COVID-19–associated myocarditis. It is difficult to compare these incidence figures to prepandemic cases as previous reports did not typically report myocarditis by sex and age (or race).
A comprehensive study of all cases of myocarditis, pericarditis, or myopericarditis from vaccines passively reported in the United States to the Vaccine Adverse Events System from January 1, 1990, to July 20, 2021, identified 1841 definitive, probable, or possible cases out of 1 048 575 individuals.84 They found that 67.9% of myocarditis or pericarditis cases were related to mRNA vaccines. Smallpox vaccines were next most common followed by other vaccine platforms. Over this time, 80.5% of cases of myocarditis were male and 83.5% aged 12 to 40 years, while 71.2% of pericarditis cases were male and 58.7% aged 12 to 40 years. Of the cases, 38.1% were reported for ages 12 to 20 and <5% for those over 60 years; 60.1% were reported after the second dose regardless of the vaccine platform. The study found 0.38 cases/100 000 individuals for COVID-19 vaccines in the United States compared with 1000 to 4000 cases/100 000 individuals for COVID-19 (Table 1).84 The highest number of cases were reported for men under 30 years of age, but it is important to realize that only around 50% of individuals in the United States in this age group were vaccinated during this time. Additionally, studies in the United States using the passive reporting system Vaccine Adverse Events System report a lower incidence of myocarditis/pericarditis than population-based studies from countries with integrated health care systems or a requirement for vaccination (Table 1; Table S3).
Myocarditis has been reported as a rare adverse event for other vaccines before the COVID-19 pandemic, mainly smallpox vaccines.189,190 Studies indicate that the highest risk for myocarditis from vaccination are the new mRNA vaccines (eg, Moderna and Pfizer), especially for Moderna (Table 1; Table S3). The mRNA vaccines against COVID-19 contain modified mRNA that encodes the viral spike glycoprotein of SARS-CoV-2 encapsulated by lipid nanoparticles or EVs (Figure 1). Importantly, the mRNA vaccines do not contain live or heat-inactivated virus. Other COVID-19 vaccine platforms associated with myocarditis/pericarditis include adenovirus-vector and attenuated live virus vaccines (Tables S3 and S4).88
Signs and symptoms of COVID-19 vaccine–associated myocarditis include shortness of breath, chest pain or pressure, palpitations, malaise, or fatigue, similar to other forms of myocarditis.2 Signs may include elevated serum biomarkers including troponins and potentially elevated CRP (especially if pericarditis is present), arrhythmias, and symptoms of heart failure. Electrocardiogram changes are typically subtle and nonspecific and may include mild diffuse ST-segment changes, PQ-segment depressions, nonspecific ST-segment changes, sinus tachycardia, and supraventricular or rarely ventricular arrhythmias.2 In 1 study from Israel, 81% of patients presented with chest pain, 2% with palpitations, 6% with dyspnea, 9% with fever, and 20% with pericardial effusion.89 In this study, troponin T was required to be elevated in all patients as part of the diagnostic criteria for myocarditis. Seventy-nine percent presented with abnormal electrocardiogram, while the left ventricular ejection fraction was normal in 71% of patients; the majority of patients presented with mild to moderate cardiac dysfunction.89 A study of 40 hospitals located in Washington, Oregon, Montana, and California of over 2 million people distinguished between patients with myocarditis or pericarditis without myocarditis (ie, not perimyocarditis) following the COVID-19 vaccination.88 They found that 80% of myocarditis cases occurred after the second dose of one of the RNA vaccines (Pfizer and Moderna) versus 60% of pericarditis cases occurred after a single dose or with Ad26.COV2.S (Johnson and Johnson) vaccine, and 75% were men. Symptom onset after vaccination was early for myocarditis (median, 3–11 days), whereas for pericarditis symptoms, the median was 20 days after vaccination. Myocarditis occurred primarily in young men under 40 years of age, while pericarditis occurred primarily in men over 50 years of age. Ninety-five percent of patients who developed myocarditis were White compared with 84% of pericarditis patients. Ninety-five percent of patients with myocarditis were admitted to the hospital for 3 days with 10% in the intensive care unit compared with only 35% of pericarditis patients admitted to the hospital and 3% in intensive care. Seventy-five percent of patients with myocarditis received NSAIDs versus 49% with pericarditis. Similar percentages of patients received colchicine as a therapy. Forty percent of patients with myocarditis were treated for heart failure versus 14% with pericarditis.88 Treatment for vaccine-associated myocarditis is summarized in a recent European Society of Cardiology Consensus Statement.2
Overall, most cases of myocarditis associated with vaccines have been reported to be mild and of short duration. Most patients are hospitalized only to monitor for arrhythmias and heart failure, rather than for severe signs and symptoms. Cases of vaccine-related myocarditis are similar to cases of lymphocytic myocarditis attributed to viral and autoimmune myocarditis, which are also mild, with normal left ventricular ejection fraction and a moderately quick recovery. Because most of the vaccine cases appear mild, evaluation of the heart using cMRI or EMB is typically not conducted. This is likely also true for many mild cases of non–vaccine-related myocarditis. Most cases of vaccine-induced myocarditis fall into the clinically suspected or probable cases diagnostic categories.75 Several studies included only cases with elevated troponins, but as discussed earlier, elevated troponins should not be required for a diagnosis of myocarditis as they are unreliable biomarkers for myocarditis and may select only more severe cases.
Mechanisms for COVID-19 Vaccine–Induced Myocarditis/Pericarditis
A number of mechanisms have been hypothesized for how vaccines, and mRNA vaccines in particular, could cause myocarditis including molecular mimicry between the spike protein of SARS-CoV-2 and cardiac myosin, cytokine storm from the immune response to the vaccine, and bystander activation—all long-standing hypotheses for how viruses could cause myocarditis.2,31,191–193 mRNA vaccines mount an immune response directed against the spike protein of SARS-CoV-2 leading to the development of spike protein–specific IgG antibodies that bind ACE2 and prevent binding by the virus to ACE2. Modifications to the spike protein are intended to reduce the innate immune response by inhibiting proinflammatory cytokines, while at the same time, the lipid nanoparticle vehicle/EV for the mRNA acts as an adjuvant to enhance the immune response.194–196 mRNA vaccines have been found to produce symptoms associated with myocarditis within 3 to 11 days after the second vaccine dose and to produce a mixed infiltrate (macrophages and lymphocytes) in EMB (Figure 2), which is the typical time course of inflammation based on histology from viral and autoimmune myocarditis in patients and animal models (eg, lymphocytic, giant cell myocarditis, and CVB3 myocarditis).2,126,197 The fact that rare cases of myocarditis and pericarditis that are reported following vaccination with mRNA vaccines predominantly occur in the same demographic (men aged 12–30 years) with a similar cardiac immune infiltrate as pre-COVID and COVID myocarditis suggests a similar pathogenic mechanism (Figure 1). Especially because myocarditis is always rare, no matter the cause. Most evidence from translational animal models indicates that a microbial infection or antigen stimulation of TLRs is needed in the context of damaged heart protein to cause myocarditis, and so common and ubiquitous infections such as coxsackievirus, influenza, and SARS-CoV-2 are not likely to cause myocarditis on their own, otherwise the incidence of myocarditis would be far, far higher. Animal models suggest that autoimmunity is important.
A recent study provides a glimpse at a possible mechanism for vaccine-associated myocarditis. Thurner et al12 found that patients with biopsy-confirmed myocarditis following COVID-19 vaccination had elevated levels of antibodies directed against IL-1RA, which is part of the TLR4/IL-1R signaling family. They found that patients with elevated IL-1RA antibodies had higher levels of cardiac inflammation, CRP, and troponin.12 As described earlier, the TLR4/IL-1R signaling pathway that produces IL-1β is upregulated on mast cells and macrophages in males and is key in initiating myocarditis/pericarditis in animal models. Since ACE2/TMPRSS2/NRP1 receptors are found on mast cells, they may be directly activated at the site of vaccination and possibly at distant sites, such as the heart, at the time of vaccination. We see this occur in the autoimmune CVB3 model.160 Additionally, mast cells drive Th2-type immune responses that increase Th2 responses, antibody levels, and autoantibody levels, which are important in the development of autoimmune myocarditis. All autoimmune animal models require 2 signals: one from self and another from an adjuvant. Possibly both the mRNA against the SARS-CoV-2 spike protein and the lipid nanoparticle vehicle could provide the adjuvant effect needed to promote myocarditis following vaccination with an mRNA vaccine.35,51,198
Conclusions, Gaps, and Future Directions
Myocarditis and pericarditis associated with COVID-19 in the United States increased around 15× compared with pre-COVID levels. In adults, myocarditis/pericarditis occurs predominantly in men under the age of 50 years regardless of the cause, with sudden cardiac death from myocarditis occurring predominantly in young men under 30 years of age. This demographic is also reported for myocarditis and pericarditis associated with COVID-19 and COVID-19 vaccination, providing insight into how live viruses or virus antigens may cause myocarditis. Animal models of viral and autoimmune myocarditis have provided valuable translational information about the pathogenesis of myocarditis and suggest that pathogens/adjuvants (ie, virus, bacteria, parasite, and vaccine) can serve as an adjuvant trigger in the context of an autoimmune response. Thus, a reason why myocarditis could be so rare, regardless of cause, is because it is an autoimmune disease with susceptibility determined by sex, race/ethnicity, presence of mast cells (their presence determines genetic predisposition to lymphocytic myocarditis that progresses to DCM in animal models), pathogen antigen (activating TLRs), and damaged heart tissue, which must be presented to APC at the same time in order to develop autoimmune disease (Figure 1). Data from autoimmune animal models also indicate that low levels of viral replication in the heart may be sufficient to induce autoimmune disease if other susceptibility factors are present.
Several gaps exist that need further investigation. Because myocarditis occurs primarily in young men under the age of 50 years regardless of cause, data on myocarditis including autopsy studies should be reported according to sex, age, and race (myocarditis in the United States primarily occurs in White people). Currently, there is no standard method of reporting cases and incidence. Additionally, researchers should indicate whether necrosis was present histologically for autopsy studies and EMBs and not exclude samples if it is absent. The selection of potential myocarditis patients for research studies should not be restricted to those with elevated troponins as this biomarker is an unreliable indicator of myocarditis, especially for milder cases. The presence of SARS-CoV-2 in EVs of patients with COVID-19 suggests that mRNA vaccine platforms that resemble EVs could activate the immune response similar to natural EVs containing virus leading to myocarditis/pericarditis. Future investigation should explore the mechanism for how an immune response that is activated by EVs containing mRNA could be directed to the heart.
Article Information
Acknowledgments
We thank Presley Giresi and Brandy Edenfield for help with mouse immunohistochemistry staining.
Sources of Funding
This work was supported by the National Institutes of Health (NIH) R01 HL164520, R21 AI145356, R21 AI152318, and R21 AI154927 to D. Fairweather; American Heart Association 20TPA35490415 to D. Fairweather; NIH grant TL1 TR002380 to D.J. Beetler, D.N. Di Florio, and D. Fairweather; R01 HL135165 to L.T. Cooper; the For Elyse Foundation to D. Fairweather; and Mayo Clinic Center for Regenerative Medicine to D. Fairweather.
Disclosures
D. Fairweather is on the advisory board of Cytokinetics. B. Heidecker is an inventor on patents that use RNA for diagnosis of myocarditis. L.T. Cooper has served as a consultant for myocarditis to Bristol Meyers Squibb, CardiolRx, Kiniksa, and Moderna. He has equity ownership in Stromal Therapeutics, Inc. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACE2
- angiotensin-converting enzyme 2
- Ang II
- angiotensin II
- Ang 1–7
- angiotensin 1–7
- APC
- antigen-presenting cell
- ATR1
- angiotensin II receptor 1
- cMRI
- cardiac magnetic resonance imaging
- CR
- complement receptor
- CRP
- C-reactive protein
- CVB3
- coxsackievirus B3
- DCM
- dilated cardiomyopathy
- EAM
- experimental autoimmune myocarditis
- EMB
- endomyocardial biopsy
- EV
- extracellular vesicle
- IFN
- interferon
- IL
- interleukin
- NLRP3
- nucleotide-binding domain, leucine- rich–containing family, pyrin domain–containing-3
- NRP1
- neuropilin-1 receptor
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- PCR
- polymerase chain reaction
- TGF
- transforming growth factor
- Th
- T helper
- Tim-3
- T-cell immunoglobulin mucin domain 3
- TLR
- toll-like receptor
- TMPRSS2
- transmembrane serine protease-2
- TNFα
- tumor necrosis factor-alpha
D. Fairweather and D.J. Beetler contributed equally.
For Sources of Funding and Disclosures, see page 1314.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.123.321878.
References
- 1.Shay DK, Shimabukuro TT, DeStefano F. Myocarditis occurring after immunization with mRNA-based COVID-19 vaccines. JAMA Cardiol. 2021;6:1115–1117. doi: 10.1001/jamacardio.2021.2821 [DOI] [PubMed] [Google Scholar]
- 2.Heidecker B, Dagan N, Balicer R, Eriksson U, Rosano G, Coats A, Tschöpe C, Kelle S, Poland GA, Frustaci A, et al. Myocarditis following COVID-19 vaccine: incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC working group on myocardial and pericardial diseases. Eur J Heart Fail. 2022;24:2000–2018. doi: 10.1002/ejhf.2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, et al. SARS-VoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5 [DOI] [PubMed] [Google Scholar]
- 8.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theoharides TC. Potential association of mast cells with coronavirus disease 2019. Ann Allergy Asthma Immunol. 2021;126:217–218. doi: 10.1016/j.anai.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, et al. ; ISARIC4C investigators. Features of 20133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurner L, Kessel C, Fadle N, Regitz E, Seidel F, Kindermann I, Lohse S, Kos I, Tschöpe C, Kheiroddin P, et al. IL-1RA antibodies in myocarditis after SARS-CoV-2 vaccination. N Engl J Med. 2022;387:1524–1527. doi: 10.1056/NEJMc2205667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scully EP, Schumock G, Fu M, Massaccesi G, Muschelli J, Betz J, Klein EY, West NE, Robinson M, Garibaldi BT, et al. Sex and gender differences in testing, hospital admission, clinical presentation, and drivers of severe outcomes from COVID-19. Open Forum Infect Dis. 2021;8:ofab448. doi: 10.1093/ofid/ofab448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fodoulian L, Tuberosa J, Rossier D, Boillat M, Kan C, Pauli V, Egervari K, Lobrinus JA, Landis BN, Carleton A, et al. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. iScience. 2020;23:101839. doi: 10.1016/j.isci.2020.101839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-NCOV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Li Z, Cui X, Xiao J, Zhan J, et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010–1018. doi: 10.1136/gutjnl-2020-320953 [Google Scholar]
- 18.Hikmet F, Mear L, Edvinsson A, Micke P, Uhlen M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16:e9610. doi: 10.15252/msb.20209610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devaux CA, Camoin-Jau L. An update on angiotensin-converting enzyme 2 structure/functions, polymorphism, and duplicitous nature in the pathophysiology of coronavirus disease 2019: implications for vascular and coagulation disease associated with severe acute respiratory syndrome coronavirus infection. Front Microbiol. 2022;13:1042200. doi: 10.3389/fmicb.2022.1042200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindner D, Fitzek A, Brauninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss H-P, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poloni TE, Moretti M, Medici V, Turturici E, Belli G, Cavriani E, Visonà SD, Rossi M, Fantini V, Ferrari RR, et al. COVID-19 pathology in the lung, kidney, heart and brain: the different roles of T-cells, macrophages, and microthrombosis. Cells. 2022;11:3124. doi: 10.3390/cells11193124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdi A, AlOtaiby S, Badarin FA, Khraibi A, Hamdan H, Nader M. Interaction of SARS-CoV-2 with cardiomyocytes: insight into the underlying molecular mechanisms of cardiac injury and pharmacotherapy. Biomed Pharmacother. 2022;146:112518. doi: 10.1016/j.biopha.2021.112518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bojkova D, Wagner JUG, Shumliakivska M, Aslan GS, Saleem U, Hansen A, Luxán G, Günther S, Pham MD, Krishnan J, et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res. 2020;116:2207–2215. doi: 10.1093/cvr/cvaa267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson EL, Alkass K, Bergmann O, Maguire JJ, Roderick HL, Davenport AP. Genes encoding ACE2, TMPRSS2 and related proteins mediating SARS-CoV-2 viral entry are upregulated with age in human cardiomyocytes. J Mol Cell Cardiol. 2020;147:88–91. doi: 10.1016/j.yjmcc.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Chen T, Zhou Y. Mediators of SARS-CoV-2 entry are preferentially enriched in cardiomyocytes. Hereditas. 2021;158:4. doi: 10.1186/s41065-020-00168-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Petitjean SJL, Koehler M, Zhang Q, Dumitru AC, Chen W, Derclaye S, Vincent SP, Soumillion P, Alsteens D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat Commun. 2020;11:4541. doi: 10.1038/s41467-020-18319-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey AL, Dmytrenko O, Greenberg L, Bredemeyer AL, Ma P, Liu J, Penna V, Winkler ES, Sviben S, Brooks E, et al. SARS-CoV-2 infects human engineered heart tissues and models COVID-19 myocarditis. JACC Basic Transl Sci. 2021;6:331–345. doi: 10.1016/j.jacbts.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song X, Hu W, Yu H, Zhao L, Zhao Y, Zhao X, Xue HH, Zhao Y. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytometry A. 2020;103:136–145. doi: 10.1002/cyto.a.24285 [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovell JP, Cihakova D, Gilotra NA. COVID-19 and myocarditis: review of clinical presentations, pathogenesis and management. Heart Int. 2022;16:20–27. doi: 10.17925/HI.2022.16.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilotra NA, Minkove N, Bennett MK, Tedford RJ, Steenbergen C, Judge DP, Halushka MK, Russell SD. Lack of relationship between serum cardiac troponin I level and giant cell myocarditis diagnosis and outcomes. J Card Fail. 2016;22:583–585. doi: 10.1016/j.cardfail.2015.12.022 [DOI] [PubMed] [Google Scholar]
- 33.Berg J, Kottwitz J, Baltensperger N, Kissel CK, Lovrinovic M, Mehra T, Scherff F, Schmied C, Templin C, Lüscher TF, et al. Cardiac magnetic resonance imaging in myocarditis reveals persistent disease activity despite normalization of cardiac enzymes and inflammatory parameters at 3-month follow-up. Circ Heart Fail. 2017;10:e004262. doi: 10.1161/CIRCHEARTFAILURE.117.004262 [DOI] [PubMed] [Google Scholar]
- 34.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR. From infection to autoimmunity. J Autoimmun. 2001;16:175–186. doi: 10.1006/jaut.2000.0492 [DOI] [PubMed] [Google Scholar]
- 36.Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, Fairweather DL, Stoner JA, Cox CJ, Cunningham MW. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight. 2016;1:e85851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poli V BK, Fairweather D. Autoimmune myocarditis: animal models. Caforio A, ed. In: Myocarditis: Pathogenesis, Diagnosis and Treatment. Springer Nature; 2020:111–128. [Google Scholar]
- 38.Fairweather D, Stafford KA, Sung YK. Update on coxsackievirus B3 myocarditis. Curr Opin Rheumatol. 2012;24:401–407. doi: 10.1097/BOR.0b013e328353372d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pankuweit S KK. Viral myocarditis: From experimental models to diagnosis in patients. Caforio A, ed. In: Myocarditis: Pathogenesis, diagnosis and treatment. Springer Nature; 2020:91–110. [Google Scholar]
- 40.Ryabkova VA, Churilov LP, Shoenfeld Y. Influenza infection, SARS, MERS and COVID-19: cytokine storm - the common denominator and the lessons to be learned. Clin Immunol. 2021;223:108652. doi: 10.1016/j.clim.2020.108652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane JR, Neumann DA, Lafond-Walker A, Herskowitz A, Rose NR. Role of IL-1 and tumor necrosis factor in coxsackie virus-induced autoimmune myocarditis. J Immunol. 1993;151:1682–1690. [PubMed] [Google Scholar]
- 42.Abston ED, Coronado MJ, Bucek A, Bedja D, Shin J, Kim JB, Kim E, Gabrielson KL, Georgakopoulos D, Mitzner W, et al. Th2 regulation of viral myocarditis in mice: different roles for TLR3 versus TRIF in progression to chronic disease. Clin Dev Immunol. 2012;2012:129486. doi: 10.1155/2012/129486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massilamany C, Huber SA, Cunningham MW, Reddy J. Relevance of molecular mimicry in the mediation of infectious myocarditis. J Cardiovasc Transl Res. 2014;7:165–171. doi: 10.1007/s12265-013-9519-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gil-Cruz C, Perez-Shibayama C, De Martin A, Ronchi F, van der Borght K, Niederer R, Onder L, Lütge M, Novkovic M, Nindl V, et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science. 2019;366:881–886. doi: 10.1126/science.aav3487 [DOI] [PubMed] [Google Scholar]
- 45.Robinson SM, Tsueng G, Sin J, Mangale V, Rahawi S, McIntyre LL, Williams W, Kha N, Cruz C, Hancock BM, et al. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog. 2014;10:e1004045. doi: 10.1371/journal.ppat.1004045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sin J, McIntyre L, Stotland A, Feuer R, Gottlieb RA. Coxsackievirus B escapes the infected cell in ejected mitophagosomes. J Virol. 2017;91:e01347–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodrigues M, Fan J, Lyon C, Wan M, Hu Y. Role of extracellular vesicles in viral and bacterial infections: pathogenesis, diagnostics, and therapeutics. Theranostics. 2018;8:2709–2721. doi: 10.7150/thno.20576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clough E, Inigo J, Chandra D, Chaves L, Reynolds JL, Aalinkeel R, Schwartz SA, Khmaladze A, Mahajan SD. Mitochondrial dynamics in SARS-CoV-2 spike protein treated human microglia: implications for neuro-COVID. J Neuroimmune Pharmacol. 2021;16:770–784. doi: 10.1007/s11481-021-10015-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Wu Y, Meng X, Wang Z, Younis M, Liu Y, Wang P, Huang X. SARS-CoV-2 membrane protein causes the mitochondrial apoptosis and pulmonary edema via targeting bok. Cell Death Differ. 2022;29:1395–1408. doi: 10.1038/s41418-022-00928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faizan MI, Chaudhuri R, Sagar S, Albogami S, Chaudhary N, Azmi I, Akhtar A, Ali SM, Kumar R, Iqbal J, et al. NSP4 and ORF9B of SARS-CoV-2 induce pro-inflammatory mitochondrial DNA release in inner membrane-derived vesicles. Cells. 2022;11:2969. doi: 10.3390/cells11192969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beetler DJ, Di Florio DN, Bruno KA, Ikezu T, March KL, Cooper LT, Jr, Wolfram J, Fairweather D. Extracellular vesicles as personalized medicine. Mol Aspects Med. 2022;91:101155. doi: 10.1016/j.mam.2022.101155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786 [DOI] [PubMed] [Google Scholar]
- 53.Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-mas receptor axis. Hypertens Res. 2009;32:533–536. doi: 10.1038/hr.2009.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beyerstedt S, Casaro EB, Rangel EB. Covid-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905–919. doi: 10.1007/s10096-020-04138-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eguchi S, Kawai T, Scalia R, Rizzo V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension. 2018;71:804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tikellis C, Pickering R, Tsorotes D, Du XJ, Kiriazis H, Nguyen-Huu TP, Head GA, Cooper ME, Thomas MC. Interaction of diabetes and ACE2 in the pathogenesis of cardiovascular disease in experimental diabetes. Clin Sci (Lond). 2012;123:519–529. doi: 10.1042/CS20110668 [DOI] [PubMed] [Google Scholar]
- 57.Sahara M, Ikutomi M, Morita T, Minami Y, Nakajima T, Hirata Y, Nagai R, Sata M. Deletion of angiotensin-converting enzyme 2 promotes the development of atherosclerosis and arterial neointima formation. Cardiovasc Res. 2014;101:236–246. doi: 10.1093/cvr/cvt245 [DOI] [PubMed] [Google Scholar]
- 58.Kim L, Garg S, O’Halloran A, Whitaker M, Pham H, Anderson EJ, Armistead I, Bennett NM, Billing L, Como-Sabetti K, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the us coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clin Infect Dis. 2021;72:e206–e214. doi: 10.1093/cid/ciaa1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nabah YN, Mateo T, Estelles R, Mata M, Zagorski J, Sarau H, Cortijo J, Morcillo EJ, Jose PJ, Sanz M-J. Angiotensin II induces neutrophil accumulation in vivo through generation and release of cxc chemokines. Circulation. 2004;110:3581–3586. doi: 10.1161/01.CIR.0000148824.93600.F3 [DOI] [PubMed] [Google Scholar]
- 60.Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal. 2013;19:1085–1094. doi: 10.1089/ars.2012.4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han Y, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ Res. 1999;84:695–703. doi: 10.1161/01.res.84.6.695 [DOI] [PubMed] [Google Scholar]
- 62.Ruiz-Ortega M, Lorenzo O, Suzuki Y, Ruperez M, Egido J. Proinflammatory actions of angiotensins. Curr Opin Nephrol Hypertens. 2001;10:321–329. doi: 10.1097/00041552-200105000-00005 [DOI] [PubMed] [Google Scholar]
- 63.Di Florio DN, Sin J, Coronado MJ, Atwal PS, Fairweather D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020;31:101482. doi: 10.1016/j.redox.2020.101482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fairweather D, Beetler DJ, Musigk N, Heidecker B, Lyle MA, Cooper LT, Jr, Bruno KA. Sex and gender differences in myocarditis and dilated cardiomyopathy: an update. Front Cardiovasc Med. 2023;10:1129348. doi: 10.3389/fcvm.2023.1129348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka A, Matsumori A, Wang W, Sasayama S. An angiotensin II receptor antagonist reduces myocardial damage in an animal model of myocarditis. Circulation. 1994;90:2051–2055. doi: 10.1161/01.cir.90.4.2051 [DOI] [PubMed] [Google Scholar]
- 66.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hendren NS, Drazner MH, Bozkurt B, Cooper LT, Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng P, Ke Z, Ying B, Qiao B, Yuan L. The diagnostic and prognostic role of myocardial injury biomarkers in hospitalized patients with COVID-19. Clin Chim Acta. 2020;510:186–190. doi: 10.1016/j.cca.2020.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Donnell C, Ashland MD, Vasti EC, Lu Y, Chang AY, Wang P, Daniels LB, de Lemos JA, Morrow DA, Rodriguez F, et al. N-terminal pro-B-type natriuretic peptide as a biomarker for the severity and outcomes with COVID-19 in a nationwide hospitalized cohort. J Am Heart Assoc. 2021;10:e022913. doi: 10.1161/JAHA.121.022913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Falco R, Vargas M, Palma D, Savoia M, Miscioscia A, Pinchera B, Vano M, Servillo G, Gentile I, Fortunato G, et al. B-type natriuretic peptides and high-sensitive troponin I as COVID-19 survival factors: which one is the best performer? J Clin Med. 2021;10:2726. doi: 10.3390/jcm10122726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ, Jr, Olsen EG, Schoen FJ. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 74.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. ; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Welt FGP, Shah PB, Aronow HD, Bortnick AE, Henry TD, Sherwood MW, Young MN, Davidson LJ, Kadavath S, Mahmud E, et al. ; American College of Cardiology’s Interventional Council and the Society for Cardiovascular Angiography and Interventions. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from the ACC’s Interventional Council and SCAI. J Am Coll Cardiol. 2020;75:2372–2375. doi: 10.1016/j.jacc.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anwar HK, Qudsia U. Pathology and therapeutics of COVID-19: a review. Int J Medical Students. 2020;8:4–11. [Google Scholar]
- 78.Chimenti C, Russo MA, Frustaci A. Immunosuppressive therapy in virus-negative inflammatory cardiomyopathy: 20-year follow-up of the TIMIC trial. Eur Heart J. 2022;43:3463–3473. doi: 10.1093/eurheartj/ehac348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brala D, Thevathasan T, Grahl S, Barrow S, Violano M, Bergs H, Golpour A, Suwalski P, Poller W, Skurk C, et al. Application of magnetocardiography to screen for inflammatory cardiomyopathy and monitor treatment response. J Am Heart Assoc. 2023;12:e027619. doi: 10.1161/JAHA.122.027619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sawalha K, Abozenah M, Kadado AJ, Battisha A, Al-Akchar M, Salerno C, Hernandez-Montfort J, Islam AM. Systematic review of COVID-19 related myocarditis: insights on management and outcome. Cardiovasc Revasc Med. 2021;23:107–113. doi: 10.1016/j.carrev.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamarullah W, Nurcahyani, Mary Josephine C, Bill Multazam R, Ghaezany Nawing A, Dharma S. Corticosteroid therapy in management of myocarditis associated with COVID-19; a systematic review of current evidence. Arch Acad Emerg Med. 2021;9:e32. doi: 10.22037/aaem.v9i1.1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang K, Cheng X, Qu N, Song H, Luo Y, Ye T, Xu Q, Tian H, Kan C, Hou N. Global burden of cardiomyopathy and myocarditis in the older adults from 1990 to 2019. Front Public Health. 2022;10:1018385. doi: 10.3389/fpubh.2022.1018385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chou OHI, Zhou J, Lee TTL, Kot T, Lee S, Wai AKC, Wong WT, Zhang Q, Cheng SH, Liu T, et al. Comparisons of the risk of myopericarditis between COVID-19 patients and individuals receiving COVID-19 vaccines: a population-based study. Clin Res Cardiol. 2022;111:1098–1103. doi: 10.1007/s00392-022-02007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rafaniello C, Gaio M, Zinzi A, Sullo MG, Liguori V, Ferraro M, Petronzelli F, Felicetti P, Marchione P, Marra AR, et al. Disentangling a thorny issue: myocarditis and pericarditis post COVID-19 and following mRNA COVID-19 vaccines. Pharmaceuticals (Basel). 2022;15:525. doi: 10.3390/ph15050525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boehmer TK, Kompaniyets L, Lavery AM, Hsu J, Ko JY, Yusuf H, Romano SD, Gundlapalli AV, Oster ME, Harris AM. Association between COVID-19 and myocarditis using hospital-based administrative data - United States, March 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singapore Health Sciences Authority. HSA’s safety update #9 COVID-19 vaccine. 2021; https://www.hsa.gov.sg/docs/default-source/hprg-vcb/safety-update-on-covid19-vaccines/hsa-safety-update-no-13-on-covid-19-vaccines-(31-august-2022).pdf.
- 87.GOV.UK. Archived - coronavirus vaccine - summary of yellow card reporting. 2023.
- 88.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326:1210–1212. doi: 10.1001/jama.2021.13443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, Hernán MA, Lipsitch M, Kohane I, Netzer D, et al. Safety of the BNT162B2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Straus W, Urdaneta V, Esposito DB, Mansi JA, Rodriguez CS, Burton P, Vega JM. Analysis of myocarditis among 252 million mRNA-1273 recipients worldwide. Clin Infect Dis. 2022;76:e544–e552. doi: 10.1093/cid/ciac446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ammirati E, Lupi L, Palazzini M, Hendren NS, Grodin JL, Cannistraci CV, Schmidt M, Hekimian G, Peretto G, Bochaton T, et al. Prevalence, characteristics, and outcomes of COVID-19-associated acute myocarditis. Circulation. 2022;145:1123–1139. doi: 10.1161/CIRCULATIONAHA.121.056817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goddard K, Lewis N, Fireman B, Weintraub E, Shimabukuro T, Zerbo O, Boyce TG, Oster ME, Hanson KE, Donahue JG, et al. Risk of myocarditis and pericarditis following BNT162B2 and mRNA-1273 COVID-19 vaccination. Vaccine. 2022;40:5153–5159. doi: 10.1016/j.vaccine.2022.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, Edwards K, Soslow JH, Dendy JM, Schlaudecker E, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kyto V, Sipila J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601–1606. doi: 10.1161/CIRCULATIONAHA.114.010376 [DOI] [PubMed] [Google Scholar]
- 96.Jain A. BK, Matsumori A, Cooper LT, Jr, Yamani MH, Fairweather D. Myocardits and pericarditis. Kenakin T, ed. In: Comprehensive Pharmacology. Elsevier; 2022:413–431. [Google Scholar]
- 97.Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19:75–77. doi: 10.1038/s41569-021-00662-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paul P, France AM, Aoki Y, Batra D, Biggerstaff M, Dugan V, Galloway S, Hall AJ, Johansson MA, Kondor RJ, et al. Genomic surveillance for SARS-CoV-2 variants circulating in the United States, December 2020-May 2021. MMWR Morb Mortal Wkly Rep. 2021;70:846–850. doi: 10.15585/mmwr.mm7023a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Twohig KA, Nyberg T, Zaidi A, Thelwall S, Sinnathamby MA, Aliabadi S, Seaman SR, Harris RJ, Hope R, Lopez-Bernal J, et al. ; COVID-19 Genomics UK (COG-UK) consortium. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22:35–42. doi: 10.1016/S1473-3099(21)00475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, Hinsley W, Bernal JL, Kall M, Bhatt S, et al. ; COVID-19 Genomics UK (COG-UK) consortium. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bahl A, Mielke N, Johnson S, Desai A, Qu L. Severe COVID-19 outcomes in pediatrics: an observational cohort analysis comparing alpha, delta, and omicron variants. Lancet Reg Health Am. 2023;18:100405. doi: 10.1016/j.lana.2022.100405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang L, Wei X, Wang H, Jiang R, Tan Z, Ouyang J, Li X, Lei C, Liu H, Liu J. Cardiac involvement in patients recovering from Delta variant of COVID-19: a prospective multi-parametric MRI study. ESC Heart Fail. 2022;9:2576–2584. doi: 10.1002/ehf2.13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bemtgen X, Kaier K, Rilinger J, Rottmann F, Supady A, von Zur Muhlen C, Westermann D, Wengenmayer T, Staudacher DL. Myocarditis mortality with and without COVID-19: insights from a national registry. Clin Res Cardiol. 2022;Dec 24: 1–7. doi: 10.1007/s00392-022-02141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davis MG, Bobba A, Chourasia P, Gangu K, Shuja H, Dandachi D, Farooq A, Avula SR, Shekhar R, Sheikh AB. COVID-19 associated myocarditis clinical outcomes among hospitalized patients in the United States: a propensity matched analysis of national inpatient sample. Viruses. 2022;14:2791. doi: 10.3390/v14122791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pommier T, Benzenine E, Bernard C, Mariet AS, Bejot Y, Giroud M, Morgant M-C, Steinmetz E, Guenancia C, Bouchot O, et al. Trends of myocarditis and endocarditis cases before, during, and after the first complete COVID-19-related lockdown in 2020 in France. Biomedicines. 2022;10:1231. doi: 10.3390/biomedicines10061231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ng MY, Ferreira VM, Leung ST, Yin Lee JC, Ho-Tung Fong A, To Liu RW, Man Chan JW, Wu AKL, Lung KC, Crean AM, et al. Patients recovered from COVID-19 show ongoing subclinical myocarditis as revealed by cardiac magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2476–2478. doi: 10.1016/j.jcmg.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, Patel R, Chacko L, Brown JT, Coyle C, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weckbach LT, Curta A, Bieber S, Kraechan A, Brado J, Hellmuth JC, Muenchhoff M, Scherer C, Schroeder I, Irlbeck M, et al. Myocardial inflammation and dysfunction in COVID-19-associated myocardial injury. Circ Cardiovasc Imaging. 2021;14:e012220. doi: 10.1161/CIRCIMAGING.120.011713 [DOI] [PubMed] [Google Scholar]
- 109.Fishman B, Goitein O, Berkovitch A, Rahav G, Matetzky S. First report of myocarditis in two patients with COVID-19 omicron variant: case report. Eur Heart J Case Rep. 2022;6:ytac407. doi: 10.1093/ehjcr/ytac407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lionte C, Sorodoc V, Haliga RE, Bologa C, Ceasovschih A, Petris OR, Elena Coman A, Stoica A, Sirbu O, Puha G, et al. Inflammatory and cardiac biomarkers in relation with post-acute COVID-19 and mortality: what we know after successive pandemic waves. Diagnostics (Basel). 2022;12:1373. doi: 10.3390/diagnostics12061373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nchioua R, Diofano F, Noettger S, von Maltitz P, Stenger S, Zech F, Münch J, Sparrer KMJ, Just S, Kirchhoff F. Strong attenuation of SARS-CoV-2 omicron BA.1 and increased replication of the BA.5 subvariant in human cardiomyocytes. Signal Transduct Target Ther. 2022;7:395. doi: 10.1038/s41392-022-01256-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wagner JUG, Bojkova D, Shumliakivska M, Luxan G, Nicin L, Aslan GS, Milting H, Kandler JD, Dendorfer A, Heumueller AW, et al. Increased susceptibility of human endothelial cells to infections by SARS-CoV-2 variants. Basic Res Cardiol. 2021;116:42. doi: 10.1007/s00395-021-00882-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fairweather D, Cooper LT, Jr, Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol. 2013;38:7–46. doi: 10.1016/j.cpcardiol.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kyto V, Sipila J, Rautava P. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart. 2013;99:1681–1684. doi: 10.1136/heartjnl-2013-304449 [DOI] [PubMed] [Google Scholar]
- 115.Coronado MJ, Bruno KA, Blauwet LA, Tschope C, Cunningham MW, Pankuweit S, van Linthout S, Jeon E-S, McNamara DM, Krejčí J, et al. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. J Am Heart Assoc. 2019;8:e008968. doi: 10.1161/JAHA.118.008968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ozieranski K, Tyminska A, Kruk M, Kon B, Skwarek A, Opolski G, Grabowski M. Occurrence, trends, management and outcomes of patients hospitalized with clinically suspected myocarditis-ten-year perspectives from the MYO-PL nationwide database. J Clin Med. 2021;10:4672. doi: 10.3390/jcm10204672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 118.Imazio M, Brucato A, Cemin R, Ferrua S, Maggiolini S, Beqaraj F, Demarie D, Forno D, Ferro S, Maestroni S, et al. ; ICAP Investigators. A randomized trial of colchicine for acute pericarditis. N Engl J Med. 2013;369:1522–1528. doi: 10.1056/NEJMoa1208536 [DOI] [PubMed] [Google Scholar]
- 119.Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ICU admission. Nat Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vahidy FS, Pan AP, Ahnstedt H, Munshi Y, Choi HA, Tiruneh Y, Nasir K, Kash BA, Andrieni JD, McCullough LD. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross-sectional analysis from a diverse US metropolitan area. PLoS One. 2021;16:e0245556. doi: 10.1371/journal.pone.0245556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, Silva J, Mao T, Oh JE, Tokuyama M, et al. ; Yale IMPACT Research Team. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lau ES, McNeill JN, Paniagua SM, Liu EE, Wang JK, Bassett IV, Selvaggi CA, Lubitz SA, Foulkes AS, Ho JE. Sex differences in inflammatory markers in patients hospitalized with COVID-19 infection: insights from the MGH COVID-19 patient registry. PLoS One. 2021;16:e0250774. doi: 10.1371/journal.pone.0250774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, Rose NR, Fairweather D. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710 [DOI] [PubMed] [Google Scholar]
- 125.Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, Fairweather DL. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. 2009;23:649–657. doi: 10.1016/j.bbi.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Minhas AS, Gilotra NA, Goerlich E, Metkus T, Garibaldi BT, Sharma G, Bavaro N, Phillip S, Michos ED, Hays AG. Myocardial work efficiency, a novel measure of myocardial dysfunction, is reduced in COVID-19 patients and associated with in-hospital mortality. Front Cardiovasc Med. 2021;8:667721. doi: 10.3389/fcvm.2021.667721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJ, Njoku DB, Rose NR. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Coronado MJ, Brandt JE, Kim E, Bucek A, Bedja D, Abston ED, Shin J, Gabrielson KL, Mitzner W, Fairweather DL. Testosterone and interleukin-1beta increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. Am J Physiol Heart Circ Physiol. 2012;302:H1726–H1736. doi: 10.1152/ajpheart.00783.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A, Mazzanti A, McMurray J, et al. Dilated cardiomyopathy. Nat Rev Dis Primers. 2019;5:32. doi: 10.1038/s41572-019-0084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cocker MS, Abdel-Aty H, Strohm O, Friedrich MG. Age and gender effects on the extent of myocardial involvement in acute myocarditis: a cardiovascular magnetic resonance study. Heart. 2009;95:1925–1930. doi: 10.1136/hrt.2008.164061 [DOI] [PubMed] [Google Scholar]
- 132.Blagova OV, Kogan EA, Lutokhina YA, Kukleva AD, Ainetdinova DH, Novosadov VM, Rud RS, Zaitsev AY, Zaidenov VA, Kupriyanova AG, et al. Subacute and chronic post-COVID myoendocarditis: clinical presentation, role of coronavirus persistence and autoimmune mechanisms. Kardiologiia. 2021;61:11–27. doi: 10.18087/cardio.2021.6.n1659 [DOI] [PubMed] [Google Scholar]
- 133.Imazio M, Bobbio M, Cecchi E, Demarie D, Demichelis B, Pomari F, Moratti M, Gaschino G, Giammaria M, Ghisio A, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the colchicine for acute pericarditis (COPE) trial. Circulation. 2005;112:2012–2016. doi: 10.1161/CIRCULATIONAHA.105.542738 [DOI] [PubMed] [Google Scholar]
- 134.Bhattacharyya B, Panda D, Gupta S, Banerjee M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med Res Rev. 2008;28:155–183. doi: 10.1002/med.20097 [DOI] [PubMed] [Google Scholar]
- 135.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 136.Deftereos S, Giannopoulos G, Papoutsidakis N, Panagopoulou V, Kossyvakis C, Raisakis K, Cleman MW, Stefanadis C. Colchicine and the heart: pushing the envelope. J Am Coll Cardiol. 2013;62:1817–1825. doi: 10.1016/j.jacc.2013.08.726 [DOI] [PubMed] [Google Scholar]
- 137.Carvelli J, Demaria O, Vely F, Batista L, Chouaki Benmansour N, Fares J, Carpentier S, Thibult M-L, Morel A, Remark R, et al. ; Explore COVID-19 IPH group. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020;588:146–150. doi: 10.1038/s41586-020-2600-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huber S, Massri M, Grasse M, Fleischer V, Kellnerova S, Harpf V, Knabl L, Knabl L, Heiner T, Kummann M, et al. Systemic inflammation and complement activation parameters predict clinical outcome of severe SARS-CoV-2 infections. Viruses. 2021;13:2376. doi: 10.3390/v13122376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shahbazi F, Karami M, Mirzaei M, Mohammadi Y. Survival rates and prognostic factors in patients with coronavirus disease 2019: a registry-based retrospective cohort study. J Res Health Sci. 2021;21:e00515. doi: 10.34172/jrhs.2021.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Toldo S, Bussani R, Nuzzi V, Bonaventura A, Mauro AG, Cannata A, Pillappa R, Sinagra G, Nana-Sinkam P, Sime P, et al. Inflammasome formation in the lungs of patients with fatal COVID-19. Inflamm Res. 2021;70:7–10. doi: 10.1007/s00011-020-01413-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Amin S, Aktar S, Rahman MM, Chowdhury MMH. NLRP3 inflammasome activation in COVID-19: an interlink between risk factors and disease severity. Microbes Infect. 2022;24:104913. doi: 10.1016/j.micinf.2021.104913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ren X, Wen W, Fan X, Hou W, Su B, Cai P, Li J, Liu Y, Tang F, Zhang F, et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184:1895–1913.e19. doi: 10.1016/j.cell.2021.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hunter CA, Chizzonite R, Remington JS. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 144.Cai G, Kastelein R, Hunter CA. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect Immun. 2000;68:6932–6938. doi: 10.1128/IAI.68.12.6932-6938.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Clark JT, Weizman OE, Aldridge DL, Shallberg LA, Eberhard J, Lanzar Z, Wasche D, Huck JD, Zhou T, Ring AM, et al. IL-18bp mediates the balance between protective and pathological immune responses to Toxoplasma gondii. Cell Rep. 2023;42:112147. doi: 10.1016/j.celrep.2023.112147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Frisancho-Kiss S, Nyland JF, Davis SE, Frisancho JA, Barrett MA, Rose NR, Fairweather DL. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta1 signaling and IFN-gamma increase inflammation in males independent from STAT4. Brain Res. 2006;1126:139–147. doi: 10.1016/j.brainres.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 147.Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, Davila J, DeSancho MT, Diuguid D, Griffin DO, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gao LN, Li Q, Xie JQ, Yang WX, You CG. Immunological analysis and differential genes screening of venous thromboembolism. Hereditas. 2021;158:2. doi: 10.1186/s41065-020-00166-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zheng Y, Zhuang MW, Han L, Zhang J, Nan ML, Zhan P, Kang D, Liu X, Gao C, Wang P-H. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct Target Ther. 2020;5:299. doi: 10.1038/s41392-020-00438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu Y, Zhang Z, Song J, Qian W, Gu X, Yang C, Shen N, Xue F, Tang Y. SARS-CoV-2-encoded miRNAs inhibit host type I interferon pathway and mediate allelic differential expression of susceptible gene. Front Immunol. 2021;12:767726. doi: 10.3389/fimmu.2021.767726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, Alanio C, Kuri-Cervantes L, Pampena MB, D’Andrea K, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369:eabc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M, Penninger JM. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9:1484–1490. doi: 10.1038/nm960 [DOI] [PubMed] [Google Scholar]
- 153.Lv H, Havari E, Pinto S, Gottumukkala RV, Cornivelli L, Raddassi K, Matsui T, Rosenzweig A, Bronson RT, Smith R, et al. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest. 2011;121:1561–1573. doi: 10.1172/JCI44583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cooper LT, Jr, Onuma OK, Sagar S, Oberg AL, Mahoney DW, Asmann YW, Liu P. Genomic and proteomic analysis of myocarditis and dilated cardiomyopathy. Heart Fail Clin. 2010;6:75–85. doi: 10.1016/j.hfc.2009.08.012 [DOI] [PubMed] [Google Scholar]
- 155.Kaya Z, Afanasyeva M, Wang Y, Dohmen KM, Schlichting J, Tretter T, Fairweather D, Holers VM, Rose NR. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat Immunol. 2001;2:739–745. doi: 10.1038/90686 [DOI] [PubMed] [Google Scholar]
- 156.Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster HP, Kopf M. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107:320–325. doi: 10.1161/01.cir.0000043802.38699.66 [DOI] [PubMed] [Google Scholar]
- 157.Fairweather D, Frisancho-Kiss S, Njoku DB, Nyland JF, Kaya Z, Yusung SA, Davis SE, Frisancho JA, Barrett MA, Rose NR. Complement receptor 1 and 2 deficiency increases coxsackievirus B3-induced myocarditis, dilated cardiomyopathy, and heart failure by increasing macrophages, IL-1beta, and immune complex deposition in the heart. J Immunol. 2006;176:3516–3524. doi: 10.4049/jimmunol.176.6.3516 [DOI] [PubMed] [Google Scholar]
- 158.Mueller KAL, Patzelt J, Sauter M, Maier P, Gekeler S, Klingel K, Kandolf R, Seizer P, Gawaz M, Geisler T, et al. Myocardial expression of the anaphylatoxin receptor C3AR is associated with cardiac inflammation and prognosis in patients with non-ischaemic heart failure. ESC Heart Fail. 2018;5:846–857. doi: 10.1002/ehf2.12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fairweather D, Coronado MJ, Garton AE, Dziedzic JL, Bucek A, Cooper LT, Jr, Brandt JE, Alikhan FS, Wang H, Endres CJ, et al. Sex differences in translocator protein 18 kDa (TSPO) in the heart: implications for imaging myocardial inflammation. J Cardiovasc Transl Res. 2014;7:192–202. doi: 10.1007/s12265-013-9538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fairweather D, Frisancho-Kiss S, Gatewood S, Njoku D, Steele R, Barrett M, Rose NR. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004;37:131–145. doi: 10.1080/0891693042000196200 [DOI] [PubMed] [Google Scholar]
- 161.Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, Rose NR. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J Immunol. 2003;170:4731–4737. doi: 10.4049/jimmunol.170.9.4731 [DOI] [PubMed] [Google Scholar]
- 162.Roberts BJ, Dragon JA, Moussawi M, Huber SA. Sex-specific signaling through Toll-like receptors 2 and 4 contributes to survival outcome of coxsackievirus B3 infection in C57BL/6 mice. Biol Sex Differ. 2012;3:25. doi: 10.1186/2042-6410-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tschope C, Muller I, Xia Y, Savvatis K, Pappritz K, Pinkert S, Lassner D, Heimesaat MM, Spillmann F, Miteva K, et al. Nod2 (nucleotide-binding oligomerization domain 2) is a major pathogenic mediator of coxsackievirus B3-induced myocarditis. Circ Heart Fail. 2017;10:e003870. doi: 10.1161/CIRCHEARTFAILURE.117.003870 [DOI] [PubMed] [Google Scholar]
- 164.Fallach R, Shainberg A, Avlas O, Fainblut M, Chepurko Y, Porat E, Hochhauser E. Cardiomyocyte Toll-like receptor 4 is involved in heart dysfunction following septic shock or myocardial ischemia. J Mol Cell Cardiol. 2010;48:1236–1244. doi: 10.1016/j.yjmcc.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 165.Eriksson U, Kurrer MO, Sonderegger I, Iezzi G, Tafuri A, Hunziker L, Suzuki S, Bachmaier K, Bingisser RM, Penninger JM, et al. Activation of dendritic cells through the interleukin 1 receptor 1 is critical for the induction of autoimmune myocarditis. J Exp Med. 2003;197:323–331. doi: 10.1084/jem.20021788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Satoh M, Nakamura M, Akatsu T, Iwasaka J, Shimoda Y, Segawa I, Hiramori K. Expression of Toll-like receptor 4 is associated with enteroviral replication in human myocarditis. Clin Sci (Lond). 2003;104:577–584. doi: 10.1042/CS20020263 [DOI] [PubMed] [Google Scholar]
- 167.Satoh M, Nakamura M, Akatsu T, Shimoda Y, Segawa I, Hiramori K. Toll-like receptor 4 is expressed with enteroviral replication in myocardium from patients with dilated cardiomyopathy. Lab Invest. 2004;84:173–181. doi: 10.1038/labinvest.3700031 [DOI] [PubMed] [Google Scholar]
- 168.Pappritz K, Lin J, El-Shafeey M, Fechner H, Kuhl U, Alogna A, Spillmann F, Elsanhoury A, Schulz R, Tschöpe C, et al. Colchicine prevents disease progression in viral myocarditis via modulating the NLRP3 inflammasome in the cardiosplenic axis. ESC Heart Fail. 2022;9:925–941. doi: 10.1002/ehf2.13845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Steele RA, Gatewood SJL, Rose NR. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol. 2005;174:261–269. doi: 10.4049/jimmunol.174.1.261 [DOI] [PubMed] [Google Scholar]
- 170.Afanasyeva M, Georgakopoulos D, Fairweather D, Caturegli P, Kass DA, Rose NR. Novel model of constrictive pericarditis associated with autoimmune heart disease in interferon-gamma-knockout mice. Circulation. 2004;110:2910–2917. doi: 10.1161/01.CIR.0000147538.92263.3A [DOI] [PubMed] [Google Scholar]
- 171.Legere SA, Haidl ID, Legare JF, Marshall JS. Mast cells in cardiac fibrosis: new insights suggest opportunities for intervention. Front Immunol. 2019;10:580. doi: 10.3389/fimmu.2019.00580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, Gabrielson K, Iwakura Y, Rose NR, Cihakova D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. 2010;106:1646–1655. doi: 10.1161/CIRCRESAHA.109.213157 [DOI] [PubMed] [Google Scholar]
- 173.Blanco-Dominguez R, Sanchez-Diaz R, de la Fuente H, Jimenez-Borreguero LJ, Matesanz-Marin A, Relano M, Jiménez-Alejandre R, Linillos-Pradillo B, Tsilingiri K, Martín-Mariscal ML, et al. A novel circulating microRNA for the detection of acute myocarditis. N Engl J Med. 2021;384:2014–2027. doi: 10.1056/NEJMoa2003608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Heidecker B, Kittleson MM, Kasper EK, Wittstein IS, Champion HC, Russell SD, Hruban RH, Rodriguez ER, Baughman KL, Hare JM. Transcriptomic biomarkers for the accurate diagnosis of myocarditis. Circulation. 2011;123:1174–1184. doi: 10.1161/CIRCULATIONAHA.110.002857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Munoz-Fontela C, Dowling WE, Funnell SGP, Gsell PS, Riveros-Balta AX, Albrecht RA, Andersen H, Baric RS, Carroll MW, Cavaleri M, et al. Animal models for COVID-19. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chu H, Chan JF, Yuen KY. Animal models in SARS-CoV-2 research. Nat Methods. 2022;19:392–394. doi: 10.1038/s41592-022-01447-w [DOI] [PubMed] [Google Scholar]
- 177.Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y [DOI] [PubMed] [Google Scholar]
- 178.Sun SH, Chen Q, Gu HJ, Yang G, Wang YX, Huang XY, Liu S-S, Zhang NN, Li XF, Xiong R, et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28:124–133.e4. doi: 10.1016/j.chom.2020.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gu H, Chen Q, Yang G, He L, Fan H, Deng YQ, Wang Y, Teng Y, Zhao Z, Cui Y, et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020;369:1603–1607. doi: 10.1126/science.abc4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dinnon KH, 3rd, Leist SR, Schafer A, Edwards CE, Martinez DR, Montgomery SA, West A, Yount BL, Hou YJ, Adams LE, et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020;586:560–566. doi: 10.1038/s41586-020-2708-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J, Fox JM, Chen RE, Earnest JT, Keeler SP, et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21:1327–1335. doi: 10.1038/s41590-020-0778-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Viveiros A, Noyce RS, Gheblawi M, Colombo D, Bilawchuk LM, Clemente-Casares X, Marchant DJ, Kassiri Z, Del Nonno F, Evans DH, et al. SARS-CoV-2 infection downregulates myocardial ACE2 and potentiates cardiac inflammation in humans and hamsters. Am J Physiol Heart Circ Physiol. 2022;323:H1262–H1269. doi: 10.1152/ajpheart.00578.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bruno KA, Mathews JE, Yang AL, Frisancho JA, Scott AJ, Greyner HD, Molina FA, Greenaway MS, Cooper GM, Bucek A, et al. BPA alters estrogen receptor expression in the heart after viral infection activating cardiac mast cells and T cells leading to perimyocarditis and fibrosis. Front Endocrinol (Lausanne). 2019;10:598. doi: 10.3389/fendo.2019.00598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hou X, Chen G, Bracamonte-Baran W, Choi HS, Diny NL, Sung J, Hughes D, Won T, Wood MK, Talor MV, et al. The cardiac microenvironment instructs divergent monocyte fates and functions in myocarditis. Cell Rep. 2019;28:172–189.e7. doi: 10.1016/j.celrep.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Barin JG, Baldeviano GC, Talor MV, Wu L, Ong S, Quader F, Chen P, Zheng D, Caturegli P, Rose NR, et al. Macrophages participate in IL-17-mediated inflammation. Eur J Immunol. 2012;42:726–736. doi: 10.1002/eji.201141737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yuan S, Ye ZW, Liang R, Tang K, Zhang AJ, Lu G, Ong CP, Man Poon VK, Chan CC, Mok BW, et al. Pathogenicity, transmissibility, and fitness of SARS-CoV-2 Omicron in Syrian hamsters. Science. 2022;377:428–433. doi: 10.1126/science.abn8939 [DOI] [PubMed] [Google Scholar]
- 187.Daems M, Liesenborghs L, Boudewijns R, Simmonds SJ, Kraisin S, Van Wauwe J, Cuijpers I, Raman J, Geuens N, Buyten TV, et al. SARS-CoV-2 infection causes prolonged cardiomyocyte swelling and inhibition of HIF1alpha translocation in an animal model COVID-19. Front Cardiovasc Med. 2022;9:964512. doi: 10.3389/fcvm.2022.964512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lenzo JC, Fairweather D, Cull V, Shellam GR, James Lawson CM. Characterisation of murine cytomegalovirus myocarditis: cellular infiltration of the heart and virus persistence. J Mol Cell Cardiol. 2002;34:629–640. doi: 10.1006/jmcc.2002.2003 [DOI] [PubMed] [Google Scholar]
- 189.Eckart RE, Love SS, Atwood JE, Arness MK, Cassimatis DC, Campbell CL, Boyd SY, Murphy JG, Swerdlow DL, Collins LC, et al. ; Department of Defense Smallpox Vaccination Clinical Evaluation Team. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44:201–205. doi: 10.1016/j.jacc.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 190.Engler RJ, Nelson MR, Collins LC, Jr, Spooner C, Hemann BA, Gibbs BT, Atwood JE, Howard RS, Chang AS, Cruser DL, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10:e0118283. doi: 10.1371/journal.pone.0118283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Root-Bernstein R, Fairweather D. Complexities in the relationship between infection and autoimmunity. Curr Allergy Asthma Rep. 2014;14:407. doi: 10.1007/s11882-013-0407-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Root-Bernstein R, Fairweather D. Unresolved issues in theories of autoimmune disease using myocarditis as a framework. J Theor Biol. 2015;375:101–123. doi: 10.1016/j.jtbi.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 195.Alameh MG, Tombacz I, Bettini E, Lederer K, Sittplangkoon C, Wilmore JR, Gaudette BT, Soliman OY, Pine M, Hicks P, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021;54:2877–2892.e7. doi: 10.1016/j.immuni.2021.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nagasaka T, Koitabashi N, Ishibashi Y, Aihara K, Takama N, Ohyama Y, Yokoyama T, Kaneko Y. Acute myocarditis associated with COVID-19 vaccination: a case report. J Cardiol Cases. 2022;25:285–288. doi: 10.1016/j.jccase.2021.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Afanasyeva M, Georgakopoulos D, Belardi DF, Bedja D, Fairweather D, Wang Y, Kaya Z, Gabrielson KL, Rodriguez ER, Caturegli P, et al. Impaired up-regulation of CD25 on CD4+ T cells in IFN-gamma knockout mice is associated with progression of myocarditis to heart failure. Proc Natl Acad Sci USA. 2005;102:180–185. doi: 10.1073/pnas.0408241102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.