Abstract
The onset and widespread dissemination of the severe acute respiratory syndrome coronavirus-2 in late 2019 impacted the world in a way not seen since the 1918 H1N1 pandemic, colloquially known as the Spanish Flu. Much like the Spanish Flu, which was observed to disproportionately impact young adults, it became clear in the early days of the coronavirus disease 2019 (COVID-19) pandemic that certain groups appeared to be at higher risk for severe illness once infected. One such group that immediately came to the forefront and garnered international attention was patients with preexisting cardiovascular disease. Here, we examine the available literature describing the interaction of COVID-19 with a myriad of cardiovascular conditions and diseases, paying particular attention to patients diagnosed with arrythmias, heart failure, and coronary artery disease. We further discuss the association of acute COVID-19 with de novo cardiovascular disease, including myocardial infarction due to coronary thrombosis, myocarditis, and new onset arrhythmias. We will evaluate various biochemical theories to explain these findings, including possible mechanisms of direct myocardial injury caused by the severe acute respiratory syndrome coronavirus-2 virus at the cellular level. Finally, we will discuss the strategies employed by numerous groups and governing bodies within the cardiovascular disease community to address the unprecedented challenges posed to the care of our most vulnerable patients, including heart transplant recipients, end-stage heart failure patients, and patients suffering from acute coronary syndromes, during the early days and height of the COVID-19 pandemic.
Keywords: acute coronary syndrome, arrhythmia, cardiovascular disease, heart failure, pandemic
Coronavirus disease 2019 (COVID-19) caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) resulted in a period of intimidating uncertainty throughout the worldwide medical community.1,2 Following a 3-year period that claimed the lives of 6.5 million people, including nearly 1 million Americans, there is now increased understanding of this unprecedented disease.3 While there were innumerable unanswered questions early on, little time elapsed before a clear association between poor outcomes with COVID-19 in patients with preexisting cardiovascular diseases was established.4–8 Similarly, it quickly became apparent that cases of severe COVID-19 resulted in cardiovascular injury, although the mechanisms for this were largely unclear at the time.5,6,9
Unfortunately, the onset of the pandemic coincided with an increase in the global burden of cardiovascular diseases and cardiovascular mortality, including in high-income countries in which rates were previously declining.10 In 2019 alone, nearly 875 000 deaths attributable to cardiovascular disease occurred in the United States, which represented an ongoing upward trend beginning in 2010.11 In contrast, COVID-19 death forecasts, which were accurately predicted in late 2020 to exceed 1 million in the United States by late 2022,12 remained alarmingly high and resulted in strong public messaging early in the pandemic focused on social distancing with numerous “stay-at-home” orders put into place.13 Therein, a unique challenge of providing necessary cardiovascular care to a fearful population of patients vulnerable to poor outcomes with COVID-19, in the face of limited resources and understanding, became evident.13–15
The aim of this review is to provide a comprehensive discussion of the interaction of COVID-19 and common cardiovascular disorders, examining the most current literature within the contextual vantage point of the challenges brought forth by this historic time.
Coronaviruses and the Cardiovascular System—Overview
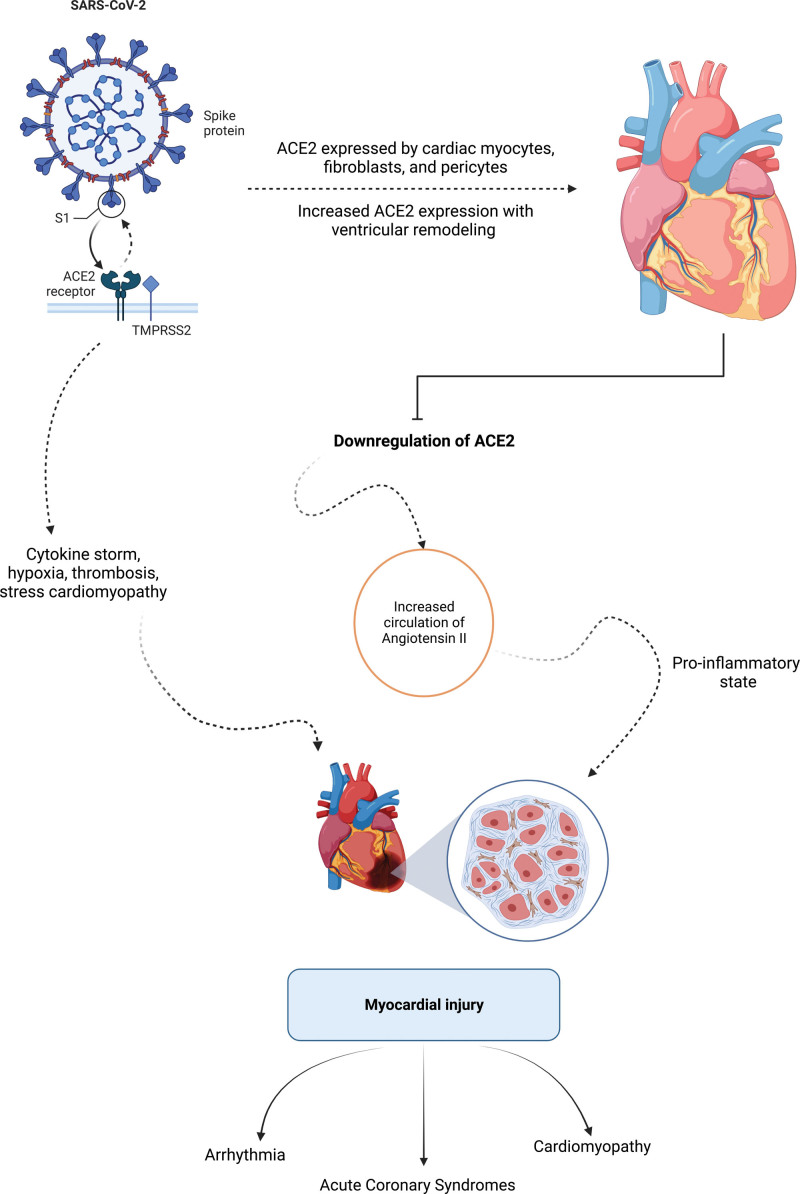
There are 7 identified coronavirus strains known to cause illness in humans, 4 of which account for 15% to 30% of common colds while the other 3 are further classified as causing severe acute respiratory syndrome (SARS).16 SARS-CoV-2, like its predecessor SARS-CoV, the strain responsible for the 2002 pandemic, infects human cells by binding to the host cell receptor ACE2 (angiotensin-converting enzyme 2) via its spike protein; although SARS-CoV-2 does so with higher affinity.17,18 After initial infection with SARS-CoV-2, the virus replicates in nasal and pulmonary epithelial cells, begins to circulate, and is then able to infect more distant cells that express ACE2 and other necessary cell-entry proteins such as proteases and integrin co-receptors.17 Cardiac myocytes, fibroblasts, and pericytes express ACE2 along with the additional necessary cell-entry mediators.19,20 Furthermore, there is increased expression of ACE2 in the setting of ventricular remodeling, a cardioprotective adaptation seen in response to acute and chronic cardiovascular conditions, providing a potential explanation for increased adverse outcomes of COVID-19 in patients with preexisting cardiovascular disease due to potential increased susceptibility to infection.17,18,21 Following entry of SARS-CoV-2 into the host cell, ACE2 is subsequently downregulated, resulting in increased circulation of deleterious angiotensin II, which has been shown to result in cardiac dysfunction (Figure).17,18,22–26
Figure.
Proposed mechanisms of myocardial injury from COVID-19. ACE2 indicates angiotensin-converting enzyme 2; and SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Given this mechanism of cell entry, great concern arose surrounding the safety of continued use of chronic renin angiotensin system inhibitors, such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, as use of these agents has been shown to increase ACE2 expression and presumably increase opportunity for viral cell entry.6,27,28 Meanwhile, others proposed the use of these agents as possible COVID-19 therapeutics to combat the resultant downregulation of ACE2.17,29,30 Numerous studies to evaluate these questions ensued and found no significant differences in all-cause mortality, intensive care unit admissions, or need for mechanical ventilation, among other adverse outcomes in patients receiving these drugs. There was also insufficient evidence for benefit, resulting in recommendations from professional societies to neither stop nor start these agents.31–40
Presence of myocardial injury, as defined by The Fourth Universal Definition of Myocardial Infarction based on elevations in troponin levels, was identified in the earliest COVID-19 patients in Wuhan.4,41–47 This observation was accompanied by the finding that patients with preexisting cardiovascular disease were more likely to develop myocardial injury with COVID-19. Evidence of myocardial injury, irrespective of the presence of prior cardiovascular disease, significantly increased rates of morbidity and mortality.41,48–57 During the interval of 3 years, extensive work has aimed to understand the pathophysiologic basis for this injury, and to extrapolate how injury at the level of the viral particle and endothelial cell or cardiac myocyte begets injury at the level of the cardiovascular organ system as a whole, with particular attention paid to venous and arterial thrombosis, arrhythmias, acute coronary syndromes, myocarditis, and systolic dysfunction.24,25,50,58–68
Arrhythmia
Outside of elevated troponin levels, the most common cardiac manifestation of COVID-19 is arrhythmia, observed in 17% of hospitalized patients and up to 44% of patients admitted to the ICU in early reports from Wuhan.47 Notably, the development of arrhythmia as a complication of infection in an early study was second only to development of acute respiratory distress syndrome.47 A later study assessing incidence of dysrhythmias in hospitalized COVID-19 patients in New York City reported a rate of 7.4%, which again rose substantially to 18.5% when patients with more severe illness requiring mechanical ventilation were assessed.69 Tachyarrhythmias, bradyarrhythmias, atrial arrhythmias, and ventricular arrhythmias have all been described with COVID-19.70–72 Patients who developed arrhythmia unsurprisingly had high rates of cardiovascular comorbidities, despite low incidence of arrhythmia before infection.72 In a large global retrospective analysis, 43% of patients who developed any arrhythmia were critically ill requiring mechanical ventilation, 41% of these critically ill patients survived to hospital discharge, and only 51% of all patients who developed an arrhythmia while hospitalized survived to hospital discharge.72
In patients who develop arrhythmia, atrial fibrillation is the most common.70–81 Moreover, many cases of reported atrial fibrillation in these studies were new onset, and in patients with critical illness, new onset atrial fibrillation was found to occur with an incidence of 14.9%.75,76,82,83 Development of atrial fibrillation has also been shown to be an independent predictor of mortality in hospitalized patients with COVID-19.83,84 In order of incidence following atrial fibrillation (61.5%), the relative proportion of arrhythmias seen in patients with COVID-19 worldwide were the following: bradycardia (12.8%), atrial flutter (10.4%), SVT (9.7%), AV block (8.6%), VT (8.1%), and VF (3.4%).72 Proposed mechanisms of arrhythmogenesis are largely felt to be related to direct myocardial inflammation given the finding of greater degrees of troponin elevation in these patients as well as indirect effects from cytokine storm, hypoxia, and increased circulating angiotensin II resulting in a proinflammatory state (Figure).71,75,77,79,83,85
Similar to the trend seen in much of cardiovascular medicine at the onset of the pandemic, guidance on the management of patients living with preexisting electrical disturbances requiring ongoing electrophysiology care was put forth by the ACC/AHA/HRS in April of 2020. This guidance urged electrophysiology providers to avoid in-person visits, when possible, with a recommendation to perform device monitoring remotely as feasible. The guidance at this time was to postpone all elective procedures and gave clear recommendations on which procedures should be considered urgent versus semiurgent to help facilitate decision-making during this uncertain time.86 Many electrophysiology providers highlighted the challenges they faced providing care to their patients, including the limitations of remote monitoring, the inability to utilize transesophageal echocardiography, and algorithms that were developed for QTc risk management in the setting of hydroxychloroquine use in patients with borderline QTc intervals.87 Updated recommendations from electrophysiology professional societies were made available regularly.88
Heart Failure and Myocarditis
Perhaps one of the most striking observations made early in the pandemic was the marked reduction in acute cardiovascular hospitalizations, many of which were nonelective, including those for heart failure (HF).89–91 In a large academic hospital in Massachusetts, a 5.8% per day reduction in HF hospitalizations was identified throughout the month of March 2020, leading to concerns that this susceptible group of patients was intentionally avoiding necessary cardiovascular care due to the pandemic and public messaging.89 This led to significant concerns that the national burden of HF incidence and undertreatment would rapidly grow due to delays in care.92,93 Meanwhile, data became available revealing that patients with HF were particularly vulnerable with a 5- to 14.5-fold increase in mortality if hospitalized with COVID-19 as opposed to being hospitalized for a HF exacerbation.94,95 Patients with HF hospitalized with COVID-19 in New York City between February and June 2020 had a mortality rate of 40%.96 For patients with chronic HF, several pathophysiologic mechanisms for poor outcomes have been postulated. These include increased susceptibility to thrombosis, decreased cardiopulmonary reserve, baseline systemic inflammation characteristic of chronic HF syndromes, and interactions of COVID-19 with the rennin-angiotensin system.92
Given these findings, a rapid paradigm shift in delivery of chronic HF care took place, with emphasis placed on telehealth and alternate strategies to provide care outside of the inpatient and face-to-face arenas.92 Two and a half years later, longer-term data are now available showing a modest increase in mortality among all hospitalized HF patients during the pandemic compared with prepandemic years regardless of infection status, albeit with a nearly 3-fold higher adjusted likelihood of death for patients with HF hospitalized with COVID-19.97 Encouragingly, additional data are now becoming available showing relative stability in device related markers of HF status, such as thoracic impedance, over a 3-month period during the stay-at-home order in New York City and Minneapolis/Saint Paul.98
An additional high-risk population of interest is patients who have undergone advanced heart failure therapies, namely orthotopic heart transplant (OHT) and left ventricular assist device (LVAD) implantation. Early data with regards to OHT patients showed that most ultimately required inpatient admission with COVID-19 and at least 25% died. The authors highlight the challenges in management of posttransplant immunosuppression in the setting of acute infection.99 Guidance from The International Society of Heart and Lung Transplantation released February 2021 advised that providers consider holding mycophenolate mofetil, mTOR inhibitors, or azathioprine in OHT patients with moderate to severe COVID-19.100 Subsequent larger scale data in this population showed lower overall mortality rates although unchanged in OHT patients requiring hospitalization (24%).101 This data set is further notable for a nearly 20% rate of requiring de novo renal replacement therapy in hospitalized patients, a 7-fold increased mortality risk for OHT patients receiving prednisone along with calcineurin inhibitor and antimetabolite immunosuppressive therapies, and a surprising trend of increased incidence of severe disease in patients who were further out from their transplant (10.9 versus 4.9 years—a finding which may be confounded by age and additional acquired comorbidities).101 Similarly, LVAD patients had high hospitalization (60%) and case fatality (20%) rates with COVID-19.102 A separate single-center analysis, however, showed that LVAD patients had lower case fatality rate than HF and OHT patients. While the authors highlight that most patients, including OHT (86.5%) and HF (69.8%) patients were on therapeutic anticoagulation, all LVAD patients were therapeutically anticoagulated and possibly conferred some benefit from this.103 A few cases are available in the literature of LVAD pump thrombosis in the setting of COVID-19.102,104,105
Acute heart failure has also been described in COVID-19, dating back to the first cases in the United States from Washington state, where a third of patients developed acute cardiomyopathy within a cohort with an exceptionally high mortality rate of 67%.106,107 In patients with severe COVID-19 who died in Wuhan, 49% developed acute heart failure during their course.45 In concordance with this, elevated NT-pro BNP at time of admission, regardless of prior HF status, has been shown to be significantly and independently associated with COVID-19 inpatient mortality in a large nationwide cohort.108
While early hypotheses invoked myocarditis, defined by the presence of inflammatory infiltrate along with nonischemic myocyte necrosis, as the cause of this acute heart failure—the true incidence of COVID-19 myocarditis remains uncertain and debated, but lower than initially reported.23,63,65,68,109–112 A large recent review of postmortem histopathologic data showed a high prevalence of myocardial necrosis and edema without myocarditis, owing to a lack of inflammatory infiltrate.113 However, another up to date systematic review does show the presence of myocarditis, albeit infrequently, in available reported cases.114 Current estimated rates of COVID-19 myocarditis are between 2.4 and 4.1 out of 1000 patients hospitalized for COIVD-19.115
Despite lack of clarity on true incidence of myocarditis, clinicians were, and continue to be, approached with important questions such as return to sport and physical activity with suspected myocardial inflammation following COVID-19. Numerous return to play recommendations have been put forth by international organizations and include recommendations for symptomatic athletes to undergo additional testing before return to activity with laboratory testing (inflammatory markers, troponins), imaging, electrocardiography, and formal cardiology consultation.116
Imaging studies may help to shed some light on the myocardial injury that occurs in these patients.117 In 100 patients with recovered mild to moderate COVID-19 who underwent cardiac MRI, 78 had evidence of cardiac involvement while ongoing myocardial inflammation was present in 60; 3 patients in the study had severe findings by imaging and underwent endomyocardial biopsy revealing active lymphocytic inflammation.118 In order of frequency, imaging abnormalities were characterized by raised myocardial native T1 (representative of diffuse myocardial fibrosis and/or edema; when seen in isolation suggests a healed process with some residual diffuse myocardial damage), raised myocardial native T2 (specific for edema; suggestive of an active inflammatory process when seen along with raised native T1), nonischemic myocardial late gadolinium enhancement (defined anatomically by presence in the epicardium, the midwall, or at insertion points; proposed to be seen in patients with acute or healed myocarditis), pericardial enhancement (representative of regional damage due to myocardial inflammation; frequently seen with associated pericardial effusion attributed to fibrosis and/or edema due to an ongoing active pericarditis), and ischemic pattern late gadolinium enhancement (defined anatomically when present in a subendocardial or transmural pattern).118 In a separate review of cardiac MRI studies involving 199 patients, the authors report myocarditis, diagnosed by Lake Louise criteria, in 40% of studies.119 A review of the echocardiography literature in COVID-19 reveals low rates of LV systolic or diastolic dysfunction, modest rates of RV enlargement and systolic dysfunction, as well as modest rates of strain imaging abnormalities.120 In another study examining echocardiograms performed within the first 24 hours of admission, 68% of studies were abnormal with the most common finding being RV dilation and dysfunction, followed by LV diastolic dysfunction, and then LV systolic dysfunction, which was present in 10% of studies.121 A number of the patients in this study went on to clinically worsen and undergo repeat echocardiography, which predominantly showed deterioration in RV function followed by progressive LV systolic dysfunction.121 RV dysfunction, present in large proportions of hospitalized patients with predictive value for poor outcomes, has been shown by other groups as well.122,123
While it is apparent that there is still no clear consensus, alternate explanations for acute myocardial injury resulting in myocardial dysfunction as identified on imaging studies, with associated poor outcomes, include injury at the level of the endothelial cell with subsequent microvascular and macrovascular thrombosis, stress cardiomyopathy, and toxic effects of angiotensin II.62,109,124–130
Albeit not an effect of COVID-19 and its pursuant syndrome, and therefore not discussed in this review, the discussion of myocarditis as related to COVID-19 vaccination is important and will be reviewed in detail separately within other articles included in this compendium.
Acute Coronary Syndromes and Cardiac Catheterization Laboratory Considerations
Similar to the observations made with HF hospitalizations at the onset of the pandemic, hospitalizations for chest pain and acute coronary syndromes (ACS) fell precipitously at the onset of the pandemic—including a marked reduction in ST-segment elevation myocardial infarction (STEMI) activations and primary percutaneous coronary intervention (PCI) volumes.89,131–133 Furthermore, a significant fall in emergency transfers for acute cardiovascular conditions, including STEMI, was identified in Cleveland in March through May of 2020.134 Despite this, interventional cardiologists prepared to adapt to a changing landscape in the delivery of cardiac catheterization laboratory (CCL) care through guidance put forth by professional societies on how to approach STEMI, non-ST-segment elevation myocardial infarction, and elective interventions.135,136 These were updated regularly and, in April 2020, guidance was released indicating that primary PCI remained the standard of care for STEMI; as well as additional work flows to assist in navigation of situations such as receiving STEMI patients from referral hospitals and equivocal STEMI diagnoses.137 Another adaptation to providing ACS care in the midst of the pandemic included consideration for utilization of risk scores for STEMI triage to identify patients who could be candidates for early discharge and non-ICU postprocedural care.138 Early discussions regarding reintroduction of care were also put forward.14 A thorough overview of best practices for CCL operations and periprocedural care in the face of pandemic has recently been made available.139
While these preparations were underway across the United States, early data became available from areas across the world that provided insight into the impact of COVID-19 on ACS presentations. During the initial COVID-19 surge in Northern Italy, a review of CCL activations over a month-long period highlighted that nearly 40% of typical STEMI presentations had no culprit lesion identified on angiography.140 Given this unprecedented time, the North American COVID-19 Myocardial Infarction (NACMI) registry was created to assist in providing further guidance. Initial findings from the registry, involving data points from 1185 patients, of which 230 were confirmed to have COVID-19 and 495 were under investigation (and ultimately found to be negative), were made available in April 2021.141 Again, a high incidence (23%) of CCL activations with no culprit lesion identified was observed in COVID-19 patients along with atypical presenting symptomatology.141 This unique observation of STEMI without a culprit lesion on coronary angiography occurred more frequently in women (33% versus 18%).142 In an effort to mitigate COVID-19 exposure risk and potentially unnecessary procedural risks, as well as data supporting diagnostic utility of coronary CT angiography in the acute setting, society recommendations included consideration of coronary CT angiography in place of angiography in select patients.137,143
Additional findings from the NACMI registry included high rates of cardiogenic shock, excess morbidity and mortality, and lower rates of invasive angiography in this group of predominantly ethnic minority patients.141 Data from European registries showed similar findings including prolonged door to balloon times, significantly increased rates of in-hospital mortality, and suboptimal postprocedural TIMI flow in STEMI patients with active COVID-19 undergoing primary PCI.144 Results from the International COVID-ACS registry were again consistent with the aforementioned registry data emphasizing increased in-hospital mortality, cardiogenic shock, and prolonged door to balloon times.145,146 Using the NACMI registry, a weighted integer risk score was developed using readily available clinical data at time of STEMI presentation in patients with COVID-19 (respiratory rate >35, prePCI shock, hypoxia, age >55, infiltrates on chest x-ray, creatinine >1.5, diabetes, subjective dyspnea) to accurately predict in-hospital mortality.147 Updated data from the NACMI registry showed that with the onset of vaccination in 2021, there was a reduction of in-hospital mortality, prePCI shock, and pulmonary manifestations, with an overall encouraging trend towards prepandemic STEMI outcomes in patients who present with STEMI and COVID-19.148
Immune system stimulation resulting in a hyperinflammatory state resulting in plaque rupture events as well as upregulation of procoagulants and platelet activation resulting in microvascular and coronary thrombosis is one of the proposed mechanisms for STEMI in COVID-19.149 Interestingly, STEMI patients with COVID-19 were found to require higher doses of heparin intraprocedurally and had overall higher thrombus burdens requiring more aspiration thrombectomy use.150 This is in line with the robust body of literature now available investigating the magnitude of the prothrombotic effects of COVID-19, and potential utility of anticoagulant treatments, highlighting these phenomena as a primary pathophysiologic mechanism for many of the cardiovascular clinical presentations encountered.151–159
Outside of ACS, life-prolonging and symptom-ameliorating procedures performed in the CCL, namely those performed for treatment of structural heart disease, required adaptation to the pandemic.160 Again, professional society guidance on the triage of patients undergoing evaluation for or pending transcatheter valve therapies was put forth and of great utility to the interventional cardiology community.161 Recommendations included navigating deferred procedure monitoring via telehealth, utilization of moderate procedural sedation, postprocedural care modifications to minimize ICU bed needs, and indications for preprocedural PCI before transcatheter aortic valve replacement.161 Similar to the 55% reduction in PCI volume observed early in the pandemic, with many patients experiencing STEMI electing to stay-at-home rather than risk exposure to the virus, there was a 64% reduction in transcatheter aortic valve replacement volume felt to be due to an increase in preprocedure deaths while awaiting intervention.162–165 In a 3-month period in early 2020, over 45 000 fewer cardiovascular procedures were performed as compared to prior years in England alone, which highlighted the far-reaching implications of the pandemic on all procedures performed in the CCL.166
When severe, the result of the aforementioned cardiovascular manifestations of COVID-19 is cardiogenic shock. Thankfully, this appeared to occur infrequently, but resulted in astonishingly high mortality rates when present.167,168 In the context of STEMI, presentation with cardiogenic shock was increased with COVID-19 and delayed presentations resulted in an increase in mechanical complications and consequential shock physiology.15,169 The approach to in-hospital resuscitation in critically ill COVID-19 patients, particularly those who suffered an in-hospital cardiac arrest with an observed survival rate of less than 6%, was scrutinized.170,171 The need for mechanical circulatory support in COVID-19 STEMI patients who developed cardiogenic shock was also associated with excessive mortality rates.133,172 The decision to pursue these aggressive interventions in this population should be highly selective and offered after consideration by a multidisciplinary team.172–174
Disparities in Cardiovascular Care During the Pandemic
While it was well understood that significant disparities existed in the delivery of cardiovascular care to certain racial, ethnic, and socioeconomic groups prior the pandemic, clear trends emerged early and persisted showing disproportionate cardiovascular morbidity and mortality during the pandemic, particularly among communities of color and historically marginalized groups.175,176 The higher prevalence of both cardiovascular disease, as well as cardiovascular disease risk factors, in nonwhite populations has been well characterized. This, however, does not sufficiently explain why certain nonwhite populations (specifically Black, Hispanic, and Asians), experienced a 20% increase in cardiovascular death, while White individuals experienced only a 2% increase.177,178 Disparities in treatment and care were noted both in the inpatient and outpatient settings. An institutional review from the United Kingdom demonstrated that nonwhite patients admitted with acute coronary syndromes during the pandemic had significantly longer times to reperfusion and were referred less frequently for invasive testing than matched white patients during the same time period.179 In the outpatient setting, emphasis on a patient’s age, race, and other social determinants with regard to their participation in the evolving world of telemedicine was scrutinized. A large single-center study from New England showed that older patients for whom English was not their primary language, patients from lower socioeconomic backgrounds, and Black patients were all less likely to be reached by providers via telemedicine platforms.180 This is especially striking when considering the sharp rise in use and reliance on telemedicine observed during late 2019 through 2020.181 The notable differences in the delivery of cardiovascular care, as well as the burden of cardiovascular morbidity and mortality as pertinent to social and racial constructs in the era of COVID-19, warrants further investigation.
Conclusions
As outlined throughout this review, the cardiovascular community has faced distinct and profound challenges throughout the COVID-19 pandemic. From an elevated risk of COVID-19 related complications in patients with preexisting cardiovascular disease, cardiovascular manifestations of COVID-19, and restrictions placed on the delivery of life-saving care, cardiologists across the globe have been called on to adapt quickly and diligently.182 Cardiovascular professional societies responded quickly with frequently updated guidance and registries were formed to facilitate navigating this challenging time for the sake of our patients’ health.183 A primary challenge, from which the community is still recovering, involves the omissions and delays of care along with their downstream consequences for both acute and chronic cardiovascular conditions.184 Without doubt, there were deep psychological and social impacts of the pandemic both affecting cardiovascular healthcare workers and their patients, with those most at risk suffering the greatest.15,185
Through these challenges, important lessons have been learned. Examples of these include the impact of public messaging on behaviors, the outcomes associated with these risk-aversion behaviors, strategies to tailor public messaging to mitigate associated poor outcomes due to these behaviors, and earlier recognition and intervention when unintended consequences of public health efforts arise.13,14,186,187 Alleviation of the impacts the pandemic has caused on the cardiovascular scientific community will be of utmost importance moving forward.188
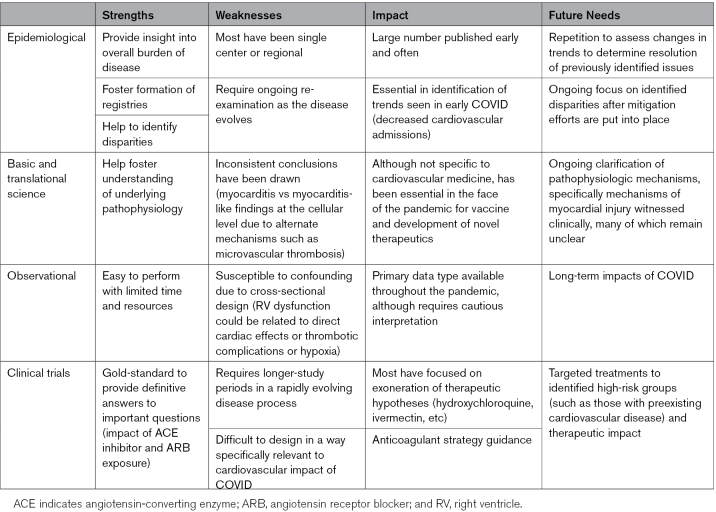
As new data begin to emerge highlighting long-term impacts of COVID-19 infection on cardiovascular health, particularly increased incidence of nearly all cardiovascular disorders with prior history of COVID-19, the cardiovascular community must remain prepared and noncomplacent to tackle novel challenges that will continue to surface.189,190 As should be evident through this review, a major limitation of our current understanding of cardiovascular manifestations of COVID-19 is the lack of high-quality research for clinicians to turn to and overall substandard for the level of evidence that the cardiovascular community has grown to expect (Table). The majority of the available literature, which has rapidly grown to an immeasurable collection, is largely observational and fraught with methodologic flaws, as highlighted by lack of consistency with frequent contradictory findings. Significant confounding cannot be excluded in many of these available studies, which is an inherent limitation to their cross-sectional design. While we appreciate that this work is an essential hypothesis-generating step in scientific discovery, follow-up studies investigating these hypotheses in rigorous scientific manner is necessary moving forward before conclusions can be made. A major focus moving forward should be on the production and dissemination of this high-quality reproducible evidence to guide our understanding and clinical practice in the face of this evolving pandemic.
Table.
Available Research by Study Type
Article Information
Sources of Funding
The authors received no financial support for the research, authorship, and publication of this article.
Disclosures
The authors whose names are listed above certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or nonfinancial interest in the subject matter or materials discussed in this article.
Nonstandard Abbreviations and Acronyms
- ACE2
- angiotensin-converting enzyme 2
- ACS
- acute coronary syndrome
- CCL
- cardiac catheterization laboratory
- HF
- heart failure
- LVAD
- left ventricular assist device
- OHT
- orthotopic heart transplant
- PCI
- percutaneous coronary intervention
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- STEMI
- ST-segment elevation myocardial infarction
For Sources of Funding and Disclosures, see page 1266.
Contributor Information
Peter K. Boulos, Email: peter.boulos@cuanschutz.edu.
Scott V. Freeman, Email: Scott.V.Freeman@ucdenver.edu.
Timothy D. Henry, Email: tim.henry@thechristhospital.com.
Ehtisham Mahmud, Email: emahmud@health.ucsd.edu.
References
- 1.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, et al. ; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. ; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. COVID data tracker. US department of health and human services, CDC. Accessed November 12, 2022. https://covid.cdc.gov/covid-data-tracker.
- 4.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 7.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. ; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. COVID-19 forecasts: deaths. NCIRD: division of viral diseases. Accessed November 12, 2022. https://www.cdc.gov/coronavirus/2019-ncov/science/forecasting/forecasting-us.html.
- 13.Cannata A, Bromage DI, McDonagh TA. The collateral cardiovascular damage of COVID-19: only history will reveal the depth of the iceberg. Eur Heart J. 2021;42:1524–1527. doi: 10.1093/eurheartj/ehab097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood DA, Mahmud E, Thourani VH, Sathananthan J, Virani A, Poppas A, Harrington RA, Dearani JA, Swaminathan M, Russo AM, et al. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: from the north American society leadership. J Am Coll Cardiol. 2020;75:3177–3183. doi: 10.1016/j.jacc.2020.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry TD, Kereiakes DJ. The direct and indirect effects of the COVID-19 pandemic on cardiovascular disease throughout the world. Eur Heart J. 2022;43:1154–1156. doi: 10.1093/eurheartj/ehab782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286 [DOI] [PubMed] [Google Scholar]
- 17.Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV, 3rd, Kwon DH, Singh T, Tilton JC, Tsai EJ, et al. COVID-19 and cardiovascular disease: from bench to bedside. Circ Res. 2021;128:1214–1236. doi: 10.1161/CIRCRESAHA.121.317997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das M, Bristow MR, Chung MK. The essential vulnerability of human cardiac myocytes to SARS-CoV-2. JACC Basic Transl Sci. 2021;6:346–349. doi: 10.1016/j.jacbts.2021.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bristow MR, Zisman LS, Altman NL, Gilbert EM, Lowes BD, Minobe WA, Slavov D, Schwisow JA, Rodriguez EM, Carroll IA, et al. Dynamic regulation of SARS-Cov-2 binding and cell entry mechanisms in remodeled human ventricular myocardium. JACC Basic Transl Sci. 2020;5:871–883. doi: 10.1016/j.jacbts.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman NL, Berning AA, Saxon CE, Adamek KE, Wagner JA, Slavov D, Quaife RA, Gill EA, Minobe WA, Jonas ER, et al. Myocardial injury and altered gene expression associated with SARS-CoV-2 infection or mRNA vaccination. JACC Basic Transl Sci. 2022;8:124–137. doi: 10.1016/j.jacbts.2022.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey AL, Dmytrenko O, Greenberg L, Bredemeyer AL, Ma P, Liu J, Penna V, Winkler ES, Sviben S, Brooks E, et al. SARS-CoV-2 infects human engineered heart tissues and models COVID-19 myocarditis. JACC Basic Transl Sci. 2021;6:331–345. doi: 10.1016/j.jacbts.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Prete A, Conway F, Della Rocca DG, Biondi-Zoccai G, De Felice F, Musto C, Piciche M, Martuscelli E, Natale A, Versaci F. COVID-19, acute myocardial injury, and infarction. Card Electrophysiol Clin. 2022;14:29–39. doi: 10.1016/j.ccep.2021.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomon SD, Metra M. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22:957–966. doi: 10.1002/ejhf.1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katwa LC, Mendoza C, Clements M. CVD and COVID-19: emerging roles of cardiac fibroblasts and myofibroblasts. Cells. 2022;11:1316. doi: 10.3390/cells11081316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sankrityayan H, Kale A, Sharma N, Anders HJ, Gaikwad AB. Evidence for use or disuse of renin-angiotensin system modulators in patients having COVID-19 with an underlying cardiorenal disorder. J Cardiovasc Pharmacol Ther. 2020;25:299–306. doi: 10.1177/1074248420921720 [DOI] [PubMed] [Google Scholar]
- 29.Duarte M, Pelorosso F, Nicolosi LN, Salgado MV, Vetulli H, Aquieri A, Azzato F, Castro M, Coyle J, Davolos I, et al. Telmisartan for treatment of Covid-19 patients: an open multicenter randomized clinical trial. EClinicalMedicine. 2021;37:100962. doi: 10.1016/j.eclinm.2021.100962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gnanenthiran SR, Borghi C, Burger D, Caramelli B, Charchar F, Chirinos JA, Cohen JB, Cremer A, Di Tanna GL, Duvignaud A, et al. ; COVID-METARASI Consortium. Renin-angiotensin system inhibitors in patients with COVID-19: a meta-analysis of randomized controlled trials led by the international society of hypertension. J Am Heart Assoc. 2022;11:e026143. doi: 10.1161/JAHA.122.026143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence?. JAMA. 2020;323:1769–1770. doi: 10.1001/jama.2020.4812 [DOI] [PubMed] [Google Scholar]
- 33.Williams B, Zhang Y. Hypertension, renin-angiotensin-aldosterone system inhibition, and COVID-19. Lancet. 2020;395:1671–1673. doi: 10.1016/S0140-6736(20)31131-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hippisley-Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, Rowan K, Aveyard P, Pavord ID, Watkinson PJ. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106:1503–1511. doi: 10.1136/heartjnl-2020-317393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen JB, Hanff TC, William P, Sweitzer N, Rosado-Santander NR, Medina C, Rodriguez-Mori JE, Renna N, Chang TI, Corrales-Medina V, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopes RD, Macedo AVS, de Barros ESPGM, Moll-Bernardes RJ, Dos Santos TM, Mazza L, Feldman A, D’Andrea Saba Arruda G, de Albuquerque DC, Camiletti AS, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, Carmona-Rubio AE, Jacob M, Procop GW, Harrington S, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalra A, Hawkins ES, Nowacki AS, Jain V, Milinovich A, Saef J, Thomas G, Gebreselassie SK, Karnik SS, Jehi L, et al. Angiotensin-converting enzyme inhibitors versus angiotensin II receptor blockers: a comparison of outcomes in patients with COVID-19. Circ Cardiovasc Qual Outcomes. 2020;13:e007115. doi: 10.1161/CIRCOUTCOMES.120.007115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 43.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandoval Y, Januzzi JL, Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, et al. ; Mount Sinai COVID Informatics Center. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siddiqi HK, Weber B, Zhou G, Regan J, Fajnzylber J, Coxen K, Corry H, Yu XG, DiCarli M, Li JZ, et al. Increased prevalence of myocardial injury in patients with SARS-CoV-2 viremia. Am J Med. 2021;134:542–546. doi: 10.1016/j.amjmed.2020.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, Huang H, Luo YC, Zhou X, Liu ZY, et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106:1154–1159. doi: 10.1136/heartjnl-2020-317007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jafari-Oori M, Moradian ST, Ebadi A, Jafari M, Dehi M. Incidence of cardiac complications following COVID-19 infection: an umbrella meta-analysis study. Heart Lung. 2022;52:136–145. doi: 10.1016/j.hrtlng.2022.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chehab O, El Zein S, Kanj A, Moghrabi A, Sebastian J, Halboni A, Alkassis S, El-Hor N, Briasoulis A, Lieberman R, et al. SARS-CoV-2 viral load and myocardial injury: independent and incremental predictors of adverse outcome. Mayo Clin Proc Innov Qual Outcomes. 2021;5:891–897. doi: 10.1016/j.mayocpiqo.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5:751–753. doi: 10.1001/jamacardio.2020.1105 [DOI] [PubMed] [Google Scholar]
- 55.Raad M, Dabbagh M, Gorgis S, Yan J, Chehab O, Dagher C, Jamoor K, Hussein IH, Cook B, Van Harn M, et al. Cardiac injury patterns and inpatient outcomes among patients admitted with COVID-19. Am J Cardiol. 2020;133:154–161. doi: 10.1016/j.amjcard.2020.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Guan B, Su T, Liu W, Chen M, Bin Waleed K, Guan X, Gary T, Zhu Z. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106:1142–1147. doi: 10.1136/heartjnl-2020-317062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohammad KO, Rodriguez JBC, Urey MA. Coronavirus disease 2019 and the cardiologist. Curr Opin Cardiol. 2022;37:335–342. doi: 10.1097/HCO.0000000000000958 [DOI] [PubMed] [Google Scholar]
- 58.Atri D, Siddiqi HK, Lang JP, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lang JP, Wang X, Moura FA, Siddiqi HK, Morrow DA, Bohula EA. A current review of COVID-19 for the cardiovascular specialist. Am Heart J. 2020;226:29–44. doi: 10.1016/j.ahj.2020.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaffe AS, Cleland JGF, Katus HA. Myocardial injury in severe COVID-19 infection. Eur Heart J. 2020;41:2080–2082. doi: 10.1093/eurheartj/ehaa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henning RJ. Cardiovascular complications of COVID-19 severe acute respiratory syndrome. Am J Cardiovasc Dis. 2022;12:170–191. PMID: 36147783 [PMC free article] [PubMed] [Google Scholar]
- 63.Imazio M, Klingel K, Kindermann I, Brucato A, De Rosa FG, Adler Y, De Ferrari GM. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis?. Heart. 2020;106:1127–1131. doi: 10.1136/heartjnl-2020-317186 [DOI] [PubMed] [Google Scholar]
- 64.Yang Y, Wei Z, Xiong C, Qian H. Direct mechanisms of SARS-CoV-2-induced cardiomyocyte damage: an update. Virol J. 2022;19:108. doi: 10.1186/s12985-022-01833-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nuzzi V, Del Mestre E, Degrassi A, Bromage DI, Manca P, Piper S, Artico J, Gentile P, Scott PA, Chiatto M, et al. Cardiovascular damage in COVID-19: what we know two years later. Curr Cardiol Rep. 2022;24:1085–1091. doi: 10.1007/s11886-022-01730-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang WT, Toh HS, Liao CT, Yu WL. Cardiac involvement of COVID-19: a comprehensive review. Am J Med Sci. 2021;361:14–22. doi: 10.1016/j.amjms.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132–1141. doi: 10.1136/heartjnl-2020-317056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siripanthong B, Asatryan B, Hanff TC, Chatha SR, Khanji MY, Ricci F, Muser D, Ferrari VA, Nazarian S, Santangeli P, et al. The pathogenesis and long-term consequences of COVID-19 cardiac injury. JACC Basic Transl Sci. 2022;7:294–308. doi: 10.1016/j.jacbts.2021.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR, Jr, Nahid M, Ringel JB, et al. Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gopinathannair R, Merchant FM, Lakkireddy DR, Etheridge SP, Feigofsky S, Han JK, Kabra R, Natale A, Poe S, Saha SA, et al. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020;59:329–336. doi: 10.1007/s10840-020-00789-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varney JA, Dong VS, Tsao T, Sabir MS, Rivera AT, Ghula S, Moriles KE, Cherukuri ML, Fazal R, Azevedo CB, et al. COVID-19 and arrhythmia: an overview. J Cardiol. 2022;79:468–475. doi: 10.1016/j.jjcc.2021.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coromilas EJ, Kochav S, Goldenthal I, Biviano A, Garan H, Goldbarg S, Kim JH, Yeo I, Tracy C, Ayanian S, et al. Worldwide survey of COVID-19-associated arrhythmias. Circ Arrhythm Electrophysiol. 2021;14:e009458. doi: 10.1161/CIRCEP.120.009458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhatla A, Mayer MM, Adusumalli S, Hyman MC, Oh E, Tierney A, Moss J, Chahal AA, Anesi G, Denduluri S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colon CM, Barrios JG, Chiles JW, McElwee SK, Russell DW, Maddox WR, Kay GN. Atrial arrhythmias in COVID-19 patients. JACC Clin Electrophysiol. 2020;6:1189–1190. doi: 10.1016/j.jacep.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peltzer B, Manocha KK, Ying X, Kirzner J, Ip JE, Thomas G, Liu CF, Markowitz SM, Lerman BB, Safford MM, et al. Arrhythmic complications of patients hospitalized with COVID-19: incidence, risk factors, and outcomes. Circ Arrhythm Electrophysiol. 2020;13:e009121. doi: 10.1161/CIRCEP.120.009121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ergun B, Ergan B, Sozmen MK, Kucuk M, Yakar MN, Comert B, Gokmen AN, Yaka E. New-onset atrial fibrillation in critically ill patients with coronavirus disease 2019 (COVID-19). J Arrhythm. 2021;37:1196–1204. doi: 10.1002/joa3.12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Musikantow DR, Turagam MK, Sartori S, Chu E, Kawamura I, Shivamurthy P, Bokhari M, Oates C, Zhang C, Pumill C, et al. Atrial fibrillation in patients hospitalized with COVID-19: incidence, predictors, outcomes, and comparison to influenza. JACC Clin Electrophysiol. 2021;7:1120–1130. doi: 10.1016/j.jacep.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia-Zamora S, Lee S, Haseeb S, Bazoukis G, Tse G, Alvarez-Garcia J, Gul EE, Cinier G, Alexander B, Martins Pinto-Filho M, et al. Arrhythmias and electrocardiographic findings in Coronavirus disease 2019: a systematic review and meta-analysis. Pacing Clin Electrophysiol. 2021;44:1062–1074. doi: 10.1111/pace.14247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magnocavallo M, Vetta G, Della Rocca DG, Gianni C, Mohanty S, Bassiouny M, Di Lullo L, Del Prete A, Cirone D, Lavalle C, et al. Prevalence, management, and outcome of atrial fibrillation and other supraventricular arrhythmias in COVID-19 patients. Card Electrophysiol Clin. 2022;14:1–9. doi: 10.1016/j.ccep.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Romero J, Gabr M, Diaz JC, Purkayastha S, Gamero MT, Reynbakh O, Matias J, Alviz I, Velasco A, Della Rocca DG, et al. Electrocardiographic features of patients with COVID-19: an updated review. Card Electrophysiol Clin. 2022;14:63–70. doi: 10.1016/j.ccep.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saha SA, Russo AM, Chung MK, Deering TF, Lakkireddy D, Gopinathannair R. COVID-19 and cardiac arrhythmias: a contemporary review. Curr Treat Options Cardiovasc Med. 2022;24:87–107. doi: 10.1007/s11936-022-00964-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wetterslev M, Jacobsen PK, Hassager C, Jons C, Risum N, Pehrson S, Bastiansen A, Andreasen AS, Tjelle Kristiansen K, Bestle MH, et al. Cardiac arrhythmias in critically ill patients with coronavirus disease 2019: a retrospective population-based cohort study. Acta Anaesthesiol Scand. 2021;65:770–777. doi: 10.1111/aas.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mountantonakis SE, Saleh M, Fishbein J, Gandomi A, Lesser M, Chelico J, Gabriels J, Qiu M, Epstein LM, Northwell C-RC. Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm. 2021;18:501–507. doi: 10.1016/j.hrthm.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Z, Shao W, Zhang J, Ma J, Huang S, Yu P, Zhu W, Liu X. Prevalence of atrial fibrillation and associated mortality among hospitalized patients with COVID-19: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:720129. doi: 10.3389/fcvm.2021.720129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhan Y, Yue H, Liang W, Wu Z. Effects of COVID-19 on arrhythmia. J Cardiovasc Dev Dis. 2022;9:292. doi: 10.3390/jcdd9090292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lakkireddy DR, Chung MK, Gopinathannair R, Patton KK, Gluckman TJ, Turagam M, Cheung JW, Patel P, Sotomonte J, Lampert R, et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the heart rhythm society COVID-19 task force; electrophysiology section of the American college of cardiology; and the electrocardiography and arrhythmias committee of the council on clinical cardiology, American heart association. Heart Rhythm. 2020;17:e233–e241. doi: 10.1016/j.hrthm.2020.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berman JP, Abrams MP, Kushnir A, Rubin GA, Ehlert F, Biviano A, Morrow JP, Dizon J, Wan EY, Yarmohammadi H, et al. Cardiac electrophysiology consultative experience at the epicenter of the COVID-19 pandemic in the United States. Indian Pacing Electrophysiol J. 2020;20:250–256. doi: 10.1016/j.ipej.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varma N, Marrouche NF, Aguinaga L, Albert CM, Arbelo E, Choi JI, Chung MK, Conte G, Dagher L, Epstein LM, et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. Europace. 2021;23:313. doi: 10.1093/europace/euaa187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhatt AS, Moscone A, McElrath EE, Varshney AS, Claggett BL, Bhatt DL, Januzzi JL, Butler J, Adler DS, Solomon SD, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76:280–288. doi: 10.1016/j.jacc.2020.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hall ME, Vaduganathan M, Khan MS, Papadimitriou L, Long RC, Hernandez GA, Moore CK, Lennep BW, McMullan MR, Butler J. Reductions in heart failure hospitalizations during the COVID-19 pandemic. J Card Fail. 2020;26:462–463. doi: 10.1016/j.cardfail.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cox ZL, Lai P, Lindenfeld J. Decreases in acute heart failure hospitalizations during COVID-19. Eur J Heart Fail. 2020;22:1045–1046. doi: 10.1002/ejhf.1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeFilippis EM, Reza N, Donald E, Givertz MM, Lindenfeld J, Jessup M. Considerations for heart failure care during the COVID-19 pandemic. JACC Heart Fail. 2020;8:681–691. doi: 10.1016/j.jchf.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barghash MH, Pinney SP. Heart failure in the COVID-19 pandemic: where has all New York’s congestion gone?. J Card Fail. 2020;26:477–478. doi: 10.1016/j.cardfail.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chatrath N, Kaza N, Pabari PA, Fox K, Mayet J, Barton C, Cole GD, Plymen CM. The effect of concomitant COVID-19 infection on outcomes in patients hospitalized with heart failure. ESC Heart Fail. 2020;7:4443–4447. doi: 10.1002/ehf2.13059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhatt AS, Jering KS, Vaduganathan M, Claggett BL, Cunningham JW, Rosenthal N, Signorovitch J, Thune JJ, Vardeny O, Solomon SD. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021;9:65–73. doi: 10.1016/j.jchf.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, Contreras J, Mitter SS, LaRocca G, Tlachi P, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keshvani N, Mehta A, Alger HM, Rutan C, Williams J, Zhang S, Young R, Alhanti B, Chiswell K, Greene SJ, et al. Heart failure quality of care and in-hospital outcomes during the COVID-19 pandemic: findings from the get with the guidelines-heart failure registry. Eur J Heart Fail. 2022;24:1117–1128. doi: 10.1002/ejhf.2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu Y, Jones PW, Caraballo C, Mahajan S, Massey DS, Ahmed R, Bader EM, Krumholz HM. Cardiac status among heart failure patients with implantable cardioverter defibrillators before, during, and after COVID-19 lockdown. J Card Fail. 2022;28:1372–1374. doi: 10.1016/j.cardfail.2022.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Latif F, Farr MA, Clerkin KJ, Habal MV, Takeda K, Naka Y, Restaino S, Sayer G, Uriel N. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020;5:1165–1169. doi: 10.1001/jamacardio.2020.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fida N. Management of heart failure, durable left ventricular assist device, and heart transplant patients in the COVID-19 era. Methodist Debakey Cardiovasc J. 2021;17:63–72. doi: 10.14797/mdcvj.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Genuardi MV, Moss N, Najjar SS, Houston BA, Shore S, Vorovich E, Atluri P, Molina M, Chambers S, Sharkoski T, et al. Coronavirus disease 2019 in heart transplant recipients: risk factors, immunosuppression, and outcomes. J Heart Lung Transplant. 2021;40:926–935. doi: 10.1016/j.healun.2021.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Birati EY, Najjar SS, Tedford RJ, Houston BA, Shore S, Vorovich E, Atluri P, Urgo K, Molina M, Chambers S, et al. Characteristics and outcomes of COVID-19 in patients on left ventricular assist device support. Circ Heart Fail. 2021;14:e007957. doi: 10.1161/CIRCHEARTFAILURE.120.007957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.George S, Cunningham LC, Nelson DP, Horstmanshof DA, Long JW, El Banayosy AM. Outcomes of COVID-19 in heart failure, LVAD, and heart transplant patients in an advanced heart failure practice. Am Heart J Plus. 2022;24:100223. doi: 10.1016/j.ahjo.2022.100223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maharaj V, Steiner M, Boyle B, Kazmirczak F, Markowitz J, Alexy T, Shaffer A, John R, Martin CM, Cogswell R, et al. Rapidly progressive left ventricular assist device outflow graft thrombosis associated with COVID-19 infection. Circ Heart Fail. 2021;14:e008334. doi: 10.1161/CIRCHEARTFAILURE.121.008334 [DOI] [PubMed] [Google Scholar]
- 105.Wadiwala IJ, Garg P, Alomari M, Elawady MS, Alamouti-Fard E, Raavi L, Mateen N, Khan F, Hussain MWA, Pham SM, et al. Accelerated LVAD pump thrombosis in COVID-19 patient: Case report and mini review. J Card Surg. 2022;37:5313–5319. doi: 10.1111/jocs.17097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rey JR, Caro-Codon J, Rosillo SO, Iniesta AM, Castrejon-Castrejon S, Marco-Clement I, Martin-Polo L, Merino-Argos C, Rodriguez-Sotelo L, Garcia-Veas JM, et al. ; CARD-COVID Investigators. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail. 2020;22:2205–2215. doi: 10.1002/ejhf.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Donnell C, Ashland MD, Vasti EC, Lu Y, Chang AY, Wang P, Daniels LB, de Lemos JA, Morrow DA, Rodriguez F, et al. N-terminal pro-B-type natriuretic peptide as a biomarker for the severity and outcomes with COVID-19 in a nationwide hospitalized cohort. J Am Heart Assoc. 2021;10:e022913. doi: 10.1161/JAHA.121.022913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kawakami R, Sakamoto A, Kawai K, Gianatti A, Pellegrini D, Nasr A, Kutys B, Guo L, Cornelissen A, Mori M, et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77:314–325. doi: 10.1016/j.jacc.2020.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ozieranski K, Tyminska A, Jonik S, Marcolongo R, Baritussio A, Grabowski M, Filipiak KJ, Opolski G, Caforio ALP. Clinically suspected myocarditis in the course of severe acute respiratory syndrome novel coronavirus-2 infection: fact or fiction?. J Card Fail. 2021;27:92–96. doi: 10.1016/j.cardfail.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schaller T, Hirschbuhl K, Burkhardt K, Braun G, Trepel M, Markl B, Claus R. Postmortem examination of patients with COVID-19. JAMA. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caforio ALP, Baritussio A, Basso C, Marcolongo R. Clinically suspected and biopsy-proven myocarditis temporally associated with SARS-CoV-2 infection. Annu Rev Med. 2022;73:149–166. doi: 10.1146/annurev-med-042220-023859 [DOI] [PubMed] [Google Scholar]
- 113.Almamlouk R, Kashour T, Obeidat S, Bois MC, Maleszewski JJ, Omrani OA, Tleyjeh R, Berbari E, Chakhachiro Z, Zein-Sabatto B, et al. ; Cardiac Autopsy in COVID-19 Study Group. COVID-19-Associated cardiac pathology at the postmortem evaluation: a collaborative systematic review. Clin Microbiol Infect. 2022;28:1066–1075. doi: 10.1016/j.cmi.2022.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Torge D, Bernardi S, Arcangeli M, Bianchi S. Histopathological features of SARS-CoV-2 in extrapulmonary organ infection: a systematic review of literature. Pathogens. 2022;11:867. doi: 10.3390/pathogens11080867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ammirati E, Lupi L, Palazzini M, Hendren NS, Grodin JL, Cannistraci CV, Schmidt M, Hekimian G, Peretto G, Bochaton T, et al. Prevalence, characteristics, and outcomes of COVID-19-associated acute myocarditis. Circulation. 2022;145:1123–1139. doi: 10.1161/CIRCULATIONAHA.121.056817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McKinney J, Connelly KA, Dorian P, Fournier A, Goodman JM, Grubic N, Isserow S, Moulson N, Philippon F, Pipe A, et al. COVID-19-myocarditis and return to play: reflections and recommendations from a Canadian working group. Can J Cardiol. 2021;37:1165–1174. doi: 10.1016/j.cjca.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petersen SE, Friedrich MG, Leiner T, Elias MD, Ferreira VM, Fenski M, Flamm SD, Fogel M, Garg R, Halushka MK, et al. Cardiovascular magnetic resonance for patients with COVID-19. JACC Cardiovasc Imaging. 2022;15:685–699. doi: 10.1016/j.jcmg.2021.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ojha V, Verma M, Pandey NN, Mani A, Malhi AS, Kumar S, Jagia P, Roy A, Sharma S. Cardiac magnetic resonance imaging in coronavirus disease 2019 (COVID-19): a systematic review of cardiac magnetic resonance imaging findings in 199 patients. J Thorac Imaging. 2021;36:73–83. doi: 10.1097/RTI.0000000000000574 [DOI] [PubMed] [Google Scholar]
- 120.Hong GH, Hays AG, Gilotra NA. The evolving role of echocardiography during the coronavirus disease 2019 pandemic. Heart Int. 2022;16:28–36. doi: 10.17925/HI.2022.16.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, Gal Oz A, Rothschild E, Baruch G, Peri Y, et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S, Lerakis S, Narula J. Right ventricular dilation in hospitalized patients with COVID-19 Infection. JACC Cardiovasc Imaging. 2020;13:2459–2461. doi: 10.1016/j.jcmg.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bieber S, Kraechan A, Hellmuth JC, Muenchhoff M, Scherer C, Schroeder I, Irlbeck M, Kaeaeb S, Massberg S, Hausleiter J, et al. Left and right ventricular dysfunction in patients with COVID-19-associated myocardial injury. Infection. 2021;49:491–500. doi: 10.1007/s15010-020-01572-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peng X, Wang Y, Xi X, Jia Y, Tian J, Yu B, Tian J. Promising therapy for heart failure in patients with severe COVID-19: calming the cytokine storm. Cardiovasc Drugs Ther. 2021;35:231–247. doi: 10.1007/s10557-020-07120-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Louis DW, Saad M, Vijayakumar S, Ilyas S, Kokkirala A, Aronow HD. The cardiovascular manifestations of COVID-19. Cardiol Clin. 2022;40:277–285. doi: 10.1016/j.ccl.2022.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shah RM, Shah M, Shah S, Li A, Jauhar S. Takotsubo syndrome and COVID-19: associations and implications. Curr Probl Cardiol. 2021;46:100763. doi: 10.1016/j.cpcardiol.2020.100763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tsao CW, Strom JB, Chang JD, Manning WJ. COVID-19-associated stress (Takotsubo) cardiomyopathy. Circ Cardiovasc Imaging. 2020;13:e011222. doi: 10.1161/CIRCIMAGING.120.011222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A, Nasr A, Kutys R, Guo L, Cornelissen A, et al. Microthrombi as a major cause of cardiac injury in COVID-19: A Pathologic Study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828 [DOI] [PubMed] [Google Scholar]
- 129.Bois MC, Boire NA, Layman AJ, Aubry MC, Alexander MP, Roden AC, Hagen CE, Quinton RA, Larsen C, Erben Y, et al. COVID-19-associated nonocclusive fibrin microthrombi in the heart. Circulation. 2021;143:230–243. doi: 10.1161/CIRCULATIONAHA.120.050754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jabri A, Kalra A, Kumar A, Alameh A, Adroja S, Bashir H, Nowacki AS, Shah R, Khubber S, Kanaa NA, et al. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2014780. doi: 10.1001/jamanetworkopen.2020.14780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the united states during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garcia S, Stanberry L, Schmidt C, Sharkey S, Megaly M, Albaghdadi MS, Meraj PM, Garberich R, Jaffer FA, Stefanescu Schmidt AC, et al. Impact of COVID-19 pandemic on STEMI care: an expanded analysis from the United States. Catheter Cardiovasc Interv. 2021;98:217–222. doi: 10.1002/ccd.29154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guddeti RR, Yildiz M, Nayak KR, Alraies MC, Davidson L, Henry TD, Garcia S. Impact of COVID-19 on acute myocardial infarction care. Cardiol Clin. 2022;40:345–353. doi: 10.1016/j.ccl.2022.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khot UN, Reimer AP, Brown A, Hustey FM, Hussain MS, Kapadia SR, Svensson LG. Impact of COVID-19 pandemic on critical care transfers for ST-segment-elevation myocardial infarction, stroke, and aortic emergencies. Circ Cardiovasc Qual Outcomes. 2020;13:e006938. doi: 10.1161/CIRCOUTCOMES.120.006938 [DOI] [PubMed] [Google Scholar]
- 135.Welt FGP, Shah PB, Aronow HD, Bortnick AE, Henry TD, Sherwood MW, Young MN, Davidson LJ, Kadavath S, Mahmud E, et al. ; American College of Cardiology’s Interventional Council and the Society for Cardiovascular Angiography and Interventions. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from the ACC’s interventional council and SCAI. J Am Coll Cardiol. 2020;75:2372–2375. doi: 10.1016/j.jacc.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mahmud E. SCAI initiatives during the COVID-19 pandemic. Catheter Cardiovasc Interv. 2020;96:995–996. doi: 10.1002/ccd.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mahmud E, Dauerman HL, Welt FGP, Messenger JC, Rao SV, Grines C, Mattu A, Kirtane AJ, Jauhar R, Meraj P, et al. Management of acute myocardial infarction during the COVID-19 pandemic: a position statement from the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP). J Am Coll Cardiol. 2020;76:1375–1384. doi: 10.1016/j.jacc.2020.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lopez JJ, Ebinger JE, Allen S, Yildiz M, Henry TD. Adapting STEMI care for the COVID-19 pandemic: the case for low-risk STEMI triage and early discharge. Catheter Cardiovasc Interv. 2021;97:847–849. doi: 10.1002/ccd.28993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nayak KR, Maves RC, Henry TD. Management principles for the cardiac catheterization laboratory during the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic. Interv Cardiol Clin. 2022;11:325–338. doi: 10.1016/j.iccl.2022.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, Metra M, Curello S, Maffeo D, Pero G, et al. ST-Elevation myocardial infarction in patients With COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garcia S, Dehghani P, Grines C, Davidson L, Nayak KR, Saw J, Waksman R, Blair J, Akshay B, Garberich R, et al. ; Society for Cardiac Angiography and Interventions, the Canadian Association of Interventional Cardiology, and the American College of Cardiology Interventional Council. Initial findings from the North American COVID-19 myocardial infarction registry. J Am Coll Cardiol. 2021;77:1994–2003. doi: 10.1016/j.jacc.2021.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Quesada O, Van Hon L, Yildiz M, Madan M, Sanina C, Davidson L, Htun WW, Saw J, Garcia S, Dehghani P, et al. Sex Differences in clinical characteristics, management strategies, and outcomes of STEMI with COVID-19: NACMI registry. J Soc Cardiovasc Angiogr Interv. 2022;1:100360. doi: 10.1016/j.jscai.2022.100360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Onnis C, Muscogiuri G, Paolo Bassareo P, Cau R, Mannelli L, Cadeddu C, Suri JS, Cerrone G, Gerosa C, Sironi S, et al. Non-invasive coronary imaging in patients with COVID-19: a narrative review. Eur J Radiol. 2022;149:110188. doi: 10.1016/j.ejrad.2022.110188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.De Luca G, Silverio A, Verdoia M, Siudak Z, Tokarek T, Kite TA, Gershlick AH, Rodriguez-Leor O, Cid-Alvarez B, Jones DA, et al. Angiographic and clinical outcome of SARS-CoV-2 positive patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: a collaborative, individual patient data meta-analysis of six registry-based studies. Eur J Intern Med. 2022;105:69–76. doi: 10.1016/j.ejim.2022.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kite TA, Ludman PF, Gale CP, Wu J, Caixeta A, Mansourati J, Sabate M, Jimenez-Quevedo P, Candilio L, Sadeghipour P, et al. ; International COVID-ACS Registry Investigators. International prospective registry of acute coronary syndromes in patients with COVID-19. J Am Coll Cardiol. 2021;77:2466–2476. doi: 10.1016/j.jacc.2021.03.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kite TA, Pallikadavath S, Gale CP, Curzen N, Ladwiniec A. The direct and indirect effects of COVID-19 on acute coronary syndromes. Cardiol Clin. 2022;40:309–320. doi: 10.1016/j.ccl.2022.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dehghani P, Schmidt CW, Garcia S, Okeson B, Grines CL, Singh A, Patel RAG, Wiley J, Htun WW, Nayak KR, et al. North American COVID-19 myocardial infarction (NACMI) risk score for prediction of in-hospital mortality. J Soc Cardiovasc Angiogr Interv. 2022;1:100404. doi: 10.1016/j.jscai.2022.100404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Garcia S, Dehghani P, Stanberry L, Grines C, Patel RAG, Nayak KR, Singh A, Htun WW, Kabour A, Ghasemzadeh N, et al. Trends in clinical presentation, management, and outcomes of STEMI in patients with COVID-19. J Am Coll Cardiol. 2022;79:2236–2244. doi: 10.1016/j.jacc.2022.03.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ghasemzadeh N, Kim N, Amlani S, Madan M, Shavadia JS, Chong AY, Bagherli A, Bagai A, Saw J, Singh J, et al. A Review of ST-elevation myocardial infarction in patients with COVID-19. Cardiol Clin. 2022;40:321–328. doi: 10.1016/j.ccl.2022.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Choudry FA, Hamshere SM, Rathod KS, Akhtar MM, Archbold RA, Guttmann OP, Woldman S, Jain AK, Knight CJ, Baumbach A, et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2020;76:1168–1176. doi: 10.1016/j.jacc.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bohula EA, Berg DD, Lopes MS, Connors JM, Babar I, Barnett CF, Chaudhry SP, Chopra A, Ginete W, Ieong MH, et al. ; COVID-PACT Investigators. Anticoagulation and antiplatelet therapy for prevention of venous and arterial thrombotic events in critically Ill patients with COVID-19: COVID-PACT. Circulation. 2022;146:1344–1356. doi: 10.1161/CIRCULATIONAHA.122.061533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng NM, Chan YC, Cheng SW. COVID-19 related thrombosis: a mini-review. Phlebology. 2022;37:326–337. doi: 10.1177/02683555211052170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kankaria R, Sanina C, Gabr M, Wiley J, Bortnick AE. Extracardiac Prothrombotic effects of COVID-19. Cardiol Clin. 2022;40:337–344. doi: 10.1016/j.ccl.2022.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Libby P, Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lowenstein CJ, Solomon SD. Severe COVID-19 is a microvascular disease. Circulation. 2020;142:1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nappi F, Nappi P, Gambardella I, Avtaar Singh SS. Thromboembolic disease and cardiac thrombotic complication in COVID-19: a systematic review. Metabolites. 2022;12:889. doi: 10.3390/metabo12100889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schroder AS, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khialani B, MacCarthy P. Transcatheter management of severe aortic stenosis during the COVID-19 pandemic. Heart. 2020;106:1183–1190. doi: 10.1136/heartjnl-2020-317221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shah PB, Welt FGP, Mahmud E, Phillips A, Kleiman NS, Young MN, Sherwood M, Batchelor W, Wang DD, Davidson L, et al. ; American College of Cardiology and the Society for Cardiovascular Angiography and Interventions. Triage considerations for patients referred for structural heart disease intervention during the COVID-19 pandemic: an ACC/SCAI position statement. JACC Cardiovasc Interv. 2020;13:1484–1488. doi: 10.1016/j.jcin.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saad M, Kennedy KF, Imran H, Louis DW, Shippey E, Poppas A, Wood KE, Abbott JD, Aronow HD. Association between COVID-19 diagnosis and in-hospital mortality in patients hospitalized with ST-segment elevation myocardial infarction. JAMA. 2021;326:1940–1952. doi: 10.1001/jama.2021.18890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yong CM, Ang L, Welt FGP, Gummidipundi S, Henry TD, Pinto DS, Cox D, Wang P, Asch S, Mahmud E, et al. ; Society for Cardiovascular Angiography and Interventions (SCAI) and the American College of Cardiology (ACC) Interventional Council. Cardiac procedural deferral during the coronavirus (COVID-19) pandemic. Catheter Cardiovasc Interv. 2020;96:1080–1086. doi: 10.1002/ccd.29262 [DOI] [PubMed] [Google Scholar]
- 164.Bharmal M, DiGrande K, Patel A, Shavelle DM, Bosson N. Impact of coronavirus disease 2019 pandemic on cardiac arrest and emergency care. Cardiol Clin. 2022;40:355–364. doi: 10.1016/j.ccl.2022.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rashid Hons M, Gale Hons CP, Curzen Hons N, Ludman Hons P, De Belder Hons M, Timmis Hons A, Mohamed Hons MO, Luscher Hons TF, Hains Hons J, Wu J, et al. Impact of coronavirus disease 2019 pandemic on the incidence and management of out-of-hospital cardiac arrest in patients presenting with acute myocardial infarction in England. J Am Heart Assoc. 2020;9:e018379. doi: 10.1161/JAHA.120.018379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mohamed MO, Banerjee A, Clarke S, de Belder M, Patwala A, Goodwin AT, Kwok CS, Rashid M, Gale CP, Curzen N, et al. Impact of COVID-19 on cardiac procedure activity in England and associated 30-day mortality. Eur Heart J Qual Care Clin Outcomes. 2021;7:247–256. doi: 10.1093/ehjqcco/qcaa079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Varshney AS, Omar WA, Goodrich EL, Bhatt AS, Wolley AE, Gong J, Senman BC, Silva D, Levangie MW, Berg DD, et al. Epidemiology of cardiogenic shock in hospitalized adults with COVID-19: a report from the American heart association COVID-19 cardiovascular disease registry. Circ Heart Fail. 2021;14:e008477. doi: 10.1161/CIRCHEARTFAILURE.121.008477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hollenberg SM, Safi L, Parrillo JE, Fata M, Klinkhammer B, Gayed N, Glotzer T, Go RC, Gourna-Paleoudis E, Landers D, et al. Hemodynamic profiles of shock in patients with COVID-19. Am J Cardiol. 2021;153:135–139. doi: 10.1016/j.amjcard.2021.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Damluji AA, Gangasani NR, Grines CL. Mechanical complication of acute myocardial infarction secondary to COVID-19 disease. Cardiol Clin. 2022;40:365–373. doi: 10.1016/j.ccl.2022.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gorder K, Henry TD. Coding the COVID patient: is it futile?. Catheter Cardiovasc Interv. 2022;99:9–10. doi: 10.1002/ccd.30035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mir T, Sattar Y, Ahmad J, Ullah W, Shanah L, Alraies MC, Qureshi WT. Outcomes of in-hospital cardiac arrest in COVID-19 patients: a proportional prevalence meta-analysis. Catheter Cardiovasc Interv. 2022;99:1–8. doi: 10.1002/ccd.29525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gorder K, Young W, Kapur NK, Henry TD, Garcia S, Guddeti RR, Smith TD. Mechanical circulatory support in COVID-19. Cardiol Clin. 2022;40:329–335. doi: 10.1016/j.ccl.2022.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chow J, Alhussaini A, Calvillo-Arguelles O, Billia F, Luk A. Cardiovascular collapse in COVID-19 infection: the role of Venoarterial Extracorporeal Membrane Oxygenation (VA-ECMO). CJC Open. 2020;2:273–277. doi: 10.1016/j.cjco.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ranard LS, Fried JA, Abdalla M, Anstey DE, Givens RC, Kumaraiah D, Kodali SK, Takeda K, Karmpaliotis D, Rabbani LE, et al. Approach to acute cardiovascular complications in COVID-19 infection. Circ Heart Fail. 2020;13:e007220. doi: 10.1161/CIRCHEARTFAILURE.120.007220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Youmans QR, Hastings-Spaine L, Princewill O, Shobayo T, Okwuosa IS. Disparities in cardiovascular care: past, present, and solutions. Cleve Clin J Med. 2019;86:621–632. doi: 10.3949/ccjm.86a.18088 [DOI] [PubMed] [Google Scholar]
- 176.Janus SE, Makhlouf M, Chahine N, Motairek I, Al-Kindi SG. Examining disparities and excess cardiovascular mortality before and during the COVID-19 pandemic. Mayo Clin Proc. 2022;97:2206–2214. doi: 10.1016/j.mayocp.2022.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Javed Z, Haisum Maqsood M, Yahya T, Amin Z, Acquah I, Valero-Elizondo J, Andrieni J, Dubey P, Jackson RK, Daffin MA, et al. Race, racism, and cardiovascular health: applying a social determinants of health framework to racial/ethnic disparities in cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2022;15:e007917. doi: 10.1161/CIRCOUTCOMES.121.007917 [DOI] [PubMed] [Google Scholar]
- 178.Wadhera RK, Figueroa JF, Rodriguez F, Liu M, Tian W, Kazi DS, Song Y, Yeh RW, Joynt Maddox KE. Racial and ethnic disparities in heart and cerebrovascular disease deaths during the COVID-19 pandemic in the United States. Circulation. 2021;143:2346–2354. doi: 10.1161/CIRCULATIONAHA.121.054378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rashid M, Timmis A, Kinnaird T, Curzen N, Zaman A, Shoaib A, Mohamed MO, de Belder MA, Deanfield J, Martin GP, et al. Racial differences in management and outcomes of acute myocardial infarction during COVID-19 pandemic. Heart. 2021;107:734–740. doi: 10.1136/heartjnl-2020-318356 [DOI] [PubMed] [Google Scholar]
- 180.Wang X, Hidrue MK, Del Carmen MG, Weiner RB, Wasfy JH. Sociodemographic disparities in outpatient cardiology telemedicine during the COVID-19 pandemic. Circ Cardiovasc Qual Outcomes. 2021;14:e007813. doi: 10.1161/CIRCOUTCOMES.121.007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Koonin LM, Hoots B, Tsang CA, Leroy Z, Farris K, Jolly T, Antall P, McCabe B, Zelis CBR, Tong I, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1595–1599. doi: 10.15585/mmwr.mm6943a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Henry TD, Garcia S. COVID-19 pandemic: direct and indirect cardiovascular effects. Cardiol Clin. 2022;40:xiii–xiv. doi: 10.1016/j.ccl.2022.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Henry TD, Garcia S. Resilience in the face of adversity: the cardiology community comes together. J Am Coll Cardiol. 2021;77:2477–2479. doi: 10.1016/j.jacc.2021.03.315 [DOI] [PubMed] [Google Scholar]
- 184.Raisi-Estabragh Z, Mamas MA. Cardiovascular health care implications of the COVID-19 pandemic. Cardiol Clin. 2022;40:389–396. doi: 10.1016/j.ccl.2022.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nadarajah R, Wu J, Hurdus B, Asma S, Bhatt DL, Biondi-Zoccai G, Mehta LS, Ram CVS, Ribeiro ALP, Van Spall HGC, et al. The collateral damage of COVID-19 to cardiovascular services: a meta-analysis. Eur Heart J. 2022;43:3164–3178. doi: 10.1093/eurheartj/ehac227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Butt JH, Fosbol EL, Gerds TA, Andersson C, Kragholm K, Biering-Sorensen T, Andersen J, Phelps M, Andersen MP, Gislason G, et al. All-cause mortality and location of death in patients with established cardiovascular disease before, during, and after the COVID-19 lockdown: a Danish nationwide cohort study. Eur Heart J. 2021;42:1516–1523. doi: 10.1093/eurheartj/ehab028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Czeisler ME, Tynan MA, Howard ME, Honeycutt S, Fulmer EB, Kidder DP, Robbins R, Barger LK, Facer-Childs ER, Baldwin G, et al. Public attitudes, behaviors, and beliefs related to COVID-19, stay-at-home orders, nonessential business closures, and public health guidance - United States, New York City, and Los Angeles, May 5-12, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:751–758. doi: 10.15585/mmwr.mm6924e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McNally EM, Elkind MSV, Benjamin IJ, Chung MK, Dillon GH, Hernandez AF, Ibeh C, Lloyd-Jones DM, McCullough LD, Wold LE, et al. Impact of the COVID-19 pandemic on cardiovascular science: anticipating problems and potential solutions: a presidential advisory from the American heart association. Circulation. 2021;144:e461–e471. doi: 10.1161/CIR.0000000000001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Abbasi J. The COVID heart-one year after SARS-CoV-2 infection, patients have an array of increased cardiovascular risks. JAMA. 2022;327:1113–1114. doi: 10.1001/jama.2022.2411 [DOI] [PubMed] [Google Scholar]
- 190.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]