Abstract
A 37-kDa protein from Borrelia burgdorferi (the agent of Lyme disease) was identified as a target for immune-mediated resolution of Lyme arthritis. Studies in a mouse model have shown that arthritis resolution can be mediated by antibodies (against unknown target antigens) within immune sera from actively infected mice. Immune sera from infected mice were therefore used to screen a B. burgdorferi genomic expression library. A gene was identified whose native product is a putative lipoprotein of approximately 37 kDa, referred to here as arthritis-related protein (Arp). Active and passive immunization of mice with recombinant Arp or Arp antiserum, respectively, did not protect mice from challenge inoculation. However, when Arp antiserum was administered to severe combined immunodeficient (SCID) mice with established infections and with ongoing arthritis and carditis, treatment selectively induced arthritis resolution without affecting the status of carditis or influencing the status of infection, including spirochetemia. The selective arthritis-resolving effect of Arp antiserum mimics the activity of immune serum from immunocompetent mice when such serum is transferred into SCID mice with established infections. The arp gene could not be amplified from unrelated B. burgdorferi isolates but hybridized with those isolates only under very-low-stringency conditions. Arp antiserum reacted against proteins of similar size in a wide range of B. burgdorferi isolates.
Lyme disease in humans, caused by tick-borne Borrelia burgdorferi infection, often presents as arthritis, which undergoes spontaneous resolution with periodic bouts of exacerbation over the course of months or years of persistent infection (32). A mouse model for Lyme disease follows a similar course (6) and has been utilized to show that arthritis resolution is an antibody-mediated event. When sera from actively infected immunocompetent mice that have undergone arthritis resolution (immune sera) are transferred to severe combined immunodeficient (SCID) mice with established infections and with arthritis and carditis, their arthritis resolves, but their carditis remains. Furthermore, immune serum treatment of infected SCID mice does not affect the status of their infection, and the mice remain spirochetemic (7, 8). Although antibody-mediated resolution of arthritis in human Lyme disease patients has not been proven, passively transferred sera from Lyme disease patients have been shown to protect recipient mice against challenge inoculation (22). This observation underscores the importance of humoral immune responses in both human Lyme disease and the mouse model.
Identification of the B. burgdorferi antigens that are targeted by arthritis-resolving antibodies in persistently infected hosts would greatly facilitate an understanding of Lyme disease pathogenesis. We therefore screened a B. burgdorferi strain N40 DNA genomic expression library with sera from actively infected mice and describe here 1 of 46 immunoreactive clones that induces arthritis-resolving antibody responses. Several B. burgdorferi antigens have been shown to induce partial or complete protective immunity against B. burgdorferi challenge, but this is the first report of a specific antigen that selectively modifies the course of Lyme arthritis during persistent infection.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free, 3- to 5-week-old C3H/HeJ (C3H) and C3H/HeSnSmn-scid (C3H-scid) mice were obtained from The Jackson Laboratory, Bar Harbor, Maine.
B. burgdorferi.
All mouse experiments used a low-passage clonal population of the N40 strain of B. burgdorferi (6). For each experiment, a frozen aliquot of B. burgdorferi was expanded at 33°C in BSKII broth (3). Spirochetes were grown to mid-log phase, assessed for viability, and then counted by dark-field microscopy using a bacterial counting chamber. Inocula were diluted to obtain the appropriate dose of spirochetes (depending upon the experiment, detailed below) in 0.1 ml of BSKII broth and then inoculated intradermally above the shoulders. The infection status of mice in all experiments was determined by culture of tissues (blood, spleen, urinary bladder, and inoculation site) in BSKII medium, as described earlier (6). For genetic and antigenic comparison among B. burgdorferi isolates, selected representatives of B. burgdorferi sensu lato were utilized, including B. burgdorferi sensu stricto strains N40 and B31 (closely related northeastern U.S. isolates), Borrelia bissetti 25015 (genetically distinct senso lato species from the same geographic region as N40 and B31), Borrelia afzelii PKo (from Europe), and Borrelia garinii PBi (from Europe). Each of these strains represent clonal populations, derived by repeated (three times) terminal dilution. The genetic identity of these clonal strains has been previously verified (4).
Immune sera and hyperimmune sera.
Immune sera for screening the genomic expression library were obtained from C3H mice that were infected for 90 days following intradermal inoculation with 102 B. burgdorferi N40 cells. This infective dose has been shown to not induce a detectable antibody response unless the mouse is actively infected, a consideration of importance because active infection induces a different reactivity profile to B. burgdorferi than immunization (associated with high-dose inocula) with the organism (9). To assess serum antibody responses of infected mice against candidate recombinant proteins, groups of five C3H mice were inoculated intradermally with 102 B. burgdorferi N40 cells. Sera were collected from mice at 7, 14, 28, 60, and 90 days after inoculation. Infection of all mice was verified by culture of blood, spleen, urinary bladder, and inoculation site at the 90-day interval. Hyperimmune antisera were generated by subcutaneous immunization of C3H mice with 20 μg of recombinant protein in complete Freund's adjuvant (0.1-ml total volume) and boosted twice at 2-week intervals with 10 μg of protein in incomplete Freund's adjuvant.
Protective immunity.
For challenge immunity experiments, C3H mice were actively immunized, as described above. Prior to challenge of mice, serum antibody reactivity to Arp in the principal group was verified by immunoblotting at serum dilutions of >1:100,000. Immunized mice were challenged intradermally with 103 B. burgdorferi cells. At 2 weeks after challenge, mice were assessed for infection by culture.
Arthritis-resolving immunity.
Arthritis-resolving activity in hyperimmune antiserum was assessed in C3H-scid mice with established infection. C3H-scid mice were inoculated with 104 B. burgdorferi N40 cells, a high dose that assures infection of all mice and induces consistently severe arthritis. Depending upon the experimental design (see Results), mice were treated subcutaneously with 0.3 ml of undiluted recombinant protein-hyperimmune sera at various intervals after infection and then assessed for infection status by culture and disease status by histology.
Genomic expression library, cloning, and expression.
A λ ZAP II B. burgdorferi N40 genomic expression library was provided by R. A. Flavell, Yale University School of Medicine. The λ ZAP II phage contains pBluescript that can be excised and cloned directly with R408 helper phage (Stratagene, La Jolla, Calif.). Phages were incubated with Escherichia coli, protein expression was induced with 10 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and proteins were transferred to nitrocellulose membranes and then incubated with a 1:1,000 dilution of mouse immune serum. After washing, membranes were incubated with a 1:5,000 dilution of alkaline phosphatase-conjugated goat anti-mouse immunoglobulin antibodies (Sigma, St. Louis, Mo.), and bound antibodies were detected by color developed with nitroblue tetrazolium (Stratagene) and 5-bromo-4-chloro-3-indolylphosphate (BCIP; Stratagene). Excision of the pBluescript plasmid from reactive clones was achieved using the R408 helper phage. DNA sequencing was performed at the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale School of Medicine, and both strands were sequenced to ensure accuracy. The DNA sequence was analyzed using the MacVector program (Kodak, New Haven, Conn.).
Among 46 immunoreactive clones, the subject gene (and its product) was selected for evaluation, not only because it was immunoreactive but also because its sequence contained a Leu-X-Y-Cys consensus sequence at amino acids 9 to 12, presumably recognized by signal peptidase II, and a hydrophobic leader sequence. It was therefore likely to be expressed on the surface of B. burgdorferi. Furthermore, its predicted molecular mass of 37 kDa matched a size range on B. burgdorferi lysate immunoblots known to react with early-phase sera from infected mice which contain proven arthritis-resolving activity (5, 8, 10).
Sequence data were submitted to the GenBank Nucleotide Sequence Database (accession number AF050212) and matched the published DNA sequence of a B31 open reading frame (BBF01), with no assigned function, located on linear plasmid (lp) 28-1. There was 100% amino acid identity, with three nucleotide differences at bp 78 (T for N40 in lieu of a C for B31), 282, and 642 (A for N40 in lieu of G for B31) (24). A partial sequence (150 bp shorter at the C terminus due to a premature stop codon resulting from a single extra nucleotide insertion) was discovered by genomic library screening with mouse sera and was published previously (23). Because we can now ascribe function to the full-length gene product, we suggest here the name of arthritis-related protein (Arp).
The arp gene, lacking the sequence encoding for the hydrophobic N-terminal leader region (amino acids 1 to 12), was amplified by PCR from template DNA of the immunoreactive clone by using oligonucleotide primers based on their DNA sequences. The primers corresponded to nucleotides 37 to 73 and 951 to 975 of the gene. Elimination of the signal sequence increased the likelihood that the recombinant protein would be soluble when expressed, as previously described for the purification of OspA (19). The amplified arp gene was cloned in frame with glutathione S-transferase (GST) into pMX, a pGEX-2T vector (Pharmacia, Pistacaway, N.J.) with a modified polylinker (28). The PCR-amplified DNA sequence of arp was confirmed by comparison with the original insert.
Another gene (p37) and its product were also identified and cloned by screening the genomic expression library (GenBank accession number AF035553). The p37 gene is located on linear plasmid 36 (24), and its amino acid sequence suggests that it, like Arp, may be a lipoprotein. P37 protein, devoid of its leader sequence, was generated in an identical fashion as for Arp.
Recombinant protein.
E. coli DH5α cells containing recombinant plasmids were grown to an optical density at 600 nm (OD600) of 0.5 (ca. 3 h), and the recombinant GST fusion proteins were induced with IPTG at a final concentration of 1 mM (2 h). Bacterial cells were centrifuged at 4,000 rpm for 20 min, and pellets were washed with phosphate-buffered saline (PBS) and then dissolved in a 1:10 volume of PBS with 1% Triton X-100. The mixtures were sonicated and centrifuged at 10,000 rpm. Coomassie blue-stained polyacrylamide gels showed that GST-recombinant fusion proteins were soluble. The supernatants containing GST-recombinant fusion proteins were loaded onto glutathione-Sepharose 4B columns (Pharmacia), and then 25 U of thrombin was added to the columns and incubated at room temperature for 2 h to remove the GST partner. The Arp and P37 recombinant proteins were eluted and collected from columns, free of their GST fusion partner, and dialyzed against PBS three times (31).
Enzyme-linked immunosorbent assay (ELISA) and immunoblotting.
A total of 100 μl of 1 μg of recombinant Arp or GST in carbonate coating buffer (pH 9.6) per ml was plated in 96-well plates (Becton Dickinson Labware, Franklin Lakes, N.J.) overnight at 4°C and then washed with PBS with 0.1 M Tween 20 (PBST) four times. Then, 150 μl of 1% bovine serum albumin in PBS was added to each well and incubated for 30 min at room temperature. Duplicate samples of each mouse serum pool, including uninfected normal mouse serum as a control, were serially diluted three times from an initial dilution of 1:100 in PBST, added to the plates at 4°C overnight, and then washed four times with PBST. Anti-mouse immunoglobulin G (IgG) linked with peroxidase (Sigma) at 1:5,000 was added to the plates at room temperature for another 2 h. Plates were washed with PBST four times, and 1 mg of p-nitrophenyl phosphate per ml was added to the plates. Plates were incubated at room temperature for 30 min and read on a Kinetic Microplate Reader (Sunnyvale, Calif.) at OD405 values subtracted from background reactivity against normal mouse serum. For immunoblots, Arp, GST, or B. burgdorferi lysates were resolved in sodium dodecyl sulfate (SDS)–12% polyacrylamide gels by electrophoresis and then transferred to nitrocellulose membranes, which were cut into strips. The strips were probed with test mouse sera. The secondary antibody was alkaline phosphatase-conjugated goat anti-mouse IgG (Stratagene).
Histology.
Rear legs and hearts were fixed in neutral buffered formalin (pH 7.2), processed by routine histologic technique, stained with hematoxylin and eosin, and blindly examined without knowledge of experiment design or treatment group. Arthritis prevalence was assessed by microscopic examination of the tibiotarsal joints from both rear legs of each mouse, and carditis prevalence was assessed by microscopic examination of the heart base (aorta and surrounding tissue) for evidence of inflammation. Arthritis severity was scored on a scale of 0 (negative) to 3 (severe), as described earlier (7, 8).
Genomic DNA purification.
Spirochetes were grown to 107 spirochetes/ml in a total volume of 100 ml and then harvested by centrifugation at 3,500 rpm (900 × g) for 20 min. Pellets were washed with 1 ml of ice-cold PBS and centrifuged at 10,000 rpm for 2 min. Supernatants were discarded, and pellets were dissolved in 1 ml of digestion buffer (100 mM NaCl, 10 mM Tris [pH 8], 25 mM EDTA, 0.5% SDS, 0.1 ml of proteinase K per ml) at 50°C overnight. Then, 1 ml of phenol-chloroform-isoamyl alcohol (25:24:1) was added to the mixtures and vortexed for 20 s. The mixtures were centrifuged at 14,500 rpm for 5 min, and the aqueous phase was collected. A total of 100 μl of 3.5 M sodium acetate and 2 ml of 100% ethanol was added to the aqueous phase. The mixtures were frozen at −70°C for at least 2 h, and then DNA was ethanol precipitated, air dried, and dissolved in double-distilled water.
Southern blotting.
Genomic DNA was purified from B. burgdorferi strains N40, B31, 25015, PKo, and PBi. A total of 16 μg of DNA from each strain was digested with EcoRI and run on 1% agarose gels. DNA was transferred to Hybond-N+ nylon filters (Amersham Pharmacia, Piscataway, N.J.) and hybridized with full-length arp DNA as the probe using ECL System (Amersham Pharmacia) as recommended by the manufacturer. Hybridization was performed at either low stringency (37°C overnight, followed by a primary wash at 42°C) or moderate stringency (42°C overnight, followed by a primary wash at 55°C).
RESULTS
Serum antibody reactivity to Arp.
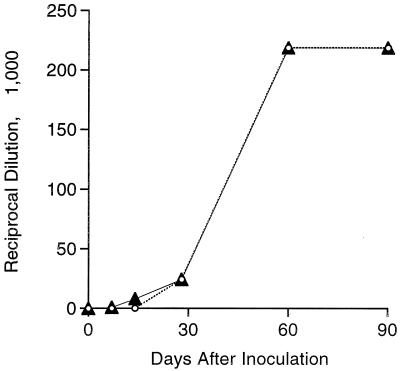
Immune sera from actively infected mice were verified to contain IgG antibody reactivity to recombinant Arp antigen by ELISA as early as 7 days after infection, with rising titers through 90 days of active infection (Fig. 1). Thus, native Arp was immunologically recognized during early infection and elicited a strong antibody response, confirming that Arp is a major immunogen during the early phase of infection with B. burgdorferi.
FIG. 1.
Anti-Arp (open circles) compared to anti-B. burgdorferi (triangles) IgG ELISA titers (expressed as reciprocal serum dilutions) in sera from C3H mice at intervals after intradermal inoculation with B. burgdorferi. Nearly identical curves were obtained with the same sera tested against both antigens, indicating that active infection stimulated a strong immune response to native Arp that can be measured with the recombinant protein as antigen.
Assessment of protective immunity induced by Arp immunization.
Because immune sera from persistently infected mice have been shown to contain protective antibodies (5, 8), we sought to determine whether immunization with recombinant Arp would protect mice against challenge with of B. burgdorferi. The results indicated that mice could not be protected by either active or passive immunization. Groups of five mice were actively immunized with recombinant Arp or GST (controls), antibody titers were verified, and then mice were challenged with B. burgdorferi N40. At 2 weeks after challenge, all mice in both treatment groups were infected, demonstrating no protective effect. A confirmatory experiment, in which groups of five C3H mice were passively immunized with 0.1 ml of Arp- or GST-hyperimmune sera and then challenged with B. burgdorferi, also revealed no evidence of protection.
Assessment of arthritis-resolving activity in Arp-antiserum.
Because sera from persistently infected mice have been shown to contain arthritis-resolving antibodies (7, 8), we next sought to determine if Arp-antiserum would induce arthritis resolution in infected C3H-scid mice with progressive arthritis. Groups of four C3H-scid mice were inoculated with B. burgdorferi N40. At 6 and 10 days after inoculation, mice were treated subcutaneously with 0.3 ml of either Arp- or GST-hyperimmune antisera. A third group of mice was passively immunized with hyperimmune antiserum against an irrelevant but similar-molecular-weight B. burgdorferi protein (P37), which we have found to have no protective or arthritis-resolving activity. Mice were assessed for infection by culture and for disease by histology at 14 days after inoculation.
Arp-antiserum significantly reduced both tibiotarsal arthritis prevalence and severity compared with P37- and GST-hyperimmune antisera (Table 1). All mice in all three groups were culture positive, including blood (spirochetemia). Remarkably, although there was a significant reduction in both the prevalence and severity of arthritis compared to controls, antiserum treatment had no effect upon carditis. The experiment was repeated, using groups of four C3H-scid mice treated with Arp- or GST-hyperimmune antisera. There was arthritis attenuation in Arp-antiserum-treated mice (mean prevalence ± standard deviation (SD), 1.3 ± 0.5; mean severity ± SD, 0.8 ± 0.5) compared to GST-antiserum-treated controls (mean prevalence, 2.0; mean severity ± SD, 1.5 ± 0). As before, all mice remained culture positive and spirochetemic, and all mice had active carditis.
TABLE 1.
Arthritis resolution in B. burgdorferi-infected SCID mice treated with Arp-hyperimmune antiserum compared to infected SCID mice treated with P37 (a non-arthritis-resolving antigen)- or GST (control)-antiseraa
| Antiserum group | Culture (no. positive/ total no.)
|
Tibiotarsal arthritis (mean no. [±SD])
|
Carditis prevalence (no. positive/ total no.) | ||
|---|---|---|---|---|---|
| Blood | Bladder | Prevalence | Severity | ||
| GST (control) | 4/4 | 3/3 | 2.0 ± 0 | 1.8 ± 0.6 | 4/4 |
| P37 (control) | 4/4 | 4/4 | 2.0 ± 0 | 1.3 ± 0.5 | 4/4 |
| Arp | 4/4 | 3/3 | 0.5 ± 0.6b | 0.3 ± 0.3c | 4/4 |
Groups of four C3H-scid mice were infected with B. burgdorferi N40 for 6 days and then treated with 0.3 ml of Arp-, P37-, or GST-antisera on days 6 and 10. The infection status (culture) is indicated. The tibiotarsal arthritis prevalence (among both tibiotarsi) and arthritis severity (mean score of both tibiotarsi) are summarized as means ± SDs. The carditis prevalence is also indicated. Denominators less than 4 are due to bacterial contamination of cultures.
P < 0.01 (chi-square test).
P < 0.01 (unpaired Student's t test).
To further confirm the arthritis-resolving effects of Arp-antiserum, we next infected C3H-scid mice, as above, and then commenced Arp- or GST-antiserum treatment on days 12, 18, and 24. This experiment differed from the previous experiments in that the C3H-scid mice were allowed to be infected longer (12 versus 6 days), thereby allowing more severe arthritis to develop, and then treating the mice with three doses (rather than two) of antisera and examining them for arthritis at a later interval (28 versus 14 days). As expected, mice treated with Arp-antiserum had less-severe arthritis compared to GST-antiserum-treated control mice (Table 2). As before, infection status, including spirochetemia, and carditis were not affected by treatment. Arthritis prevalence was not affected, since residual inflammation remained (and was scored positive, albeit less severe) in these mice with more advanced disease.
TABLE 2.
Arthritis resolution in B. burgdorferi-infected SCID mice treated with Arp-hyperimmune antiserum compared to infected SCID mice treated with GST-antiseruma
| Antiserum group | Culture (no. positive/ total no.)
|
Arthritis (mean no. [±SD])
|
Carditis prevalence (no. positive/ total no.) | ||
|---|---|---|---|---|---|
| Blood | Bladder | Prevalence | Severity | ||
| GST (control) | 5/5 | 5/5 | 2.0 ± 0 | 2.9 ± 0.3 | 5/5 |
| Arp | 5/5 | 5/5 | 1.8 ± 0.5 | 1.1 ± 0.5b | 5/5 |
C3H-scid mice were infected with B. burgdorferi N40 for 12 days, an interval at which arthritis and carditis become well established, and then treated with 0.3 ml of Arp- or GST-antiserum on days 12, 18, and 24. Infection status (culture) is indicated. The arthritis prevalence (among both tibiotarsi) and arthritis severity (mean of both tibiotarsi) are summarized as means ± SDs. The carditis prevalence is also indicated.
P < 0.001 (unpaired Student's t test).
Arp among B. burgdorferi sensu lato strains.
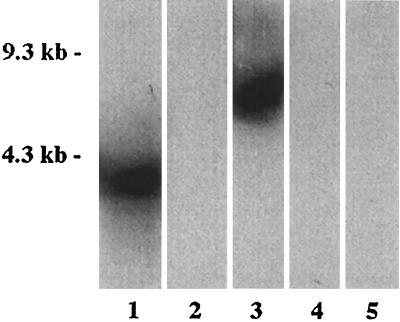
Because B. burgdorferi belongs to a large genospecies complex, we next sought to determine if Arp was conserved or unique among a broad array of B. burgdorferi senso lato species, including strains N40, B31, 25015, PKo, and PBi. We first attempted to amplify the arp gene from target DNA of each B. burgdorferi strain by using the primers corresponding to nucleotides 37 to 73 and 951 to 975 described above. A product was amplified from N40 and B31 but not from the other strains. We next performed Southern blottings, in which genomic DNA was transferred to nylon filters and then blotted with N40 arp DNA as a probe, using relatively moderately stringent conditions (42°C overnight, followed by a primary wash at 55°C). Single bands of different sizes were detected from strains N40 and B31 but not from strains 25015, PKo, or PBi (Fig. 2). We next attempted to hybridize arp DNA with target DNA from these strains, using very-low-stringency conditions (37°C overnight, followed by a primary wash at 42°C). Under these very-low-stringency conditions, arp DNA hybridized with all strains. These results suggested that strains N40 and B31 possess single copies of arp genes, in keeping with published B31 genome sequence data. The results also suggest that homologous genes among B. burgdorferi sensu lato strains are distantly related.
FIG. 2.
Southern blots (enhanced chemiluminescence) representing hybridization of B. burgdorferi N40 arp DNA with EcoRI-digested genomic DNA from B. burgdorferi N40 (lane 1), B. bissetti 25015 (lane 2), B. burgdorferi B31 (lane 3), B. afzelii PKo (lane 4), and B. garinii PBi (lane 5).
To further evaluate Arp among B. burgdorferi sensu lato strains, we performed immunoblots on N40, B31, 25015, PKo, and PBi lysates that were transferred to nitrocellulose filters and probed with Arp antiserum. Reactivity against 37- to 38-kDa proteins was detected among all B. burgdorferi strains (Fig. 3). These results suggest that arp genes were different on the DNA level but that all strains shared at least some common antigenic epitopes of similarly sized proteins.
FIG. 3.
Immunoblots (alkaline phosphatase) representing reactivity of B. burgdorferi N40-Arp antiserum against lysates of B. burgdorferi N40 (lane 1), B. bissetti 25015 (lane 2), B. burgdorferi B31 (lane 3), B. afzelii PKo (lane 4), and B. garinii PBi. Antiserum recognized antigens of approximately the same molecular mass in all B. burgdorferi sensu lato strains.
DISCUSSION
We describe here a 37-kDa arthritis-related protein (Arp) that elicits a strong antibody response during early infection with B. burgdorferi and also is capable of generating arthritis-resolving antibody upon immunization of mice with the recombinant protein. It appears that humans (and mice) infected with B. burgdorferi develop antibody to one or more 37-kDa antigens on B. burgdorferi lysates, as determined by immunoblotting (1, 18, 25) during early infection. Genomic expression library screening with immune serum from patients or mice has resulted in the identification of at least two previously described 37-kDa proteins that are reactive with immune sera, including FlaA, an outer sheath protein of the periplasmic flagella (25), and P37, a lipoprotein that is preferentially expressed in vivo (21). We report here two additional immunoreactive 37-kDa lipoproteins, one of which we have designated Arp. These findings reinforce the need to name genes and gene products based upon function rather than molecular weight to avoid confusion.
The gene sequence of arp matched the sequence of a B31 open reading frame located on lp28-1 (24). A partial sequence (150 bp shorter at the C terminus due to a premature stop codon resulting from a single extra nucleotide insertion) was discovered by genomic library screening with mouse sera and was published previously (23). The partial gene product was named ErpT, but the designation of either ErpT or Arp as belonging to the E- or F-related protein (Erp) paralogous family may be inappropriate. First, Arp (or ErpT) does not share the highly conserved upsteam homology region characteristic of the Erp family. Second, the arp gene is located on lp28-1 and not on cp32/18, which is typical for the Erp family. The only similarity between Arp and members of the Erp family is within the leader sequence (2, 11, 33, 36). This suggests a remote evolutionary relatedness of Arp to Erps, but Arp clearly falls outside of the characteristics of the Erp family as most recently defined (2, 11). For these reasons and because we can now ascribe function to the full-length gene product, we suggest the name of arthritis-related protein (Arp).
It is notable that in a previous study on the truncated (ErpT) form of Arp, active immunization with the ErpT recombinant protein failed to induce protective or arthritis-resolving immunity in mice (23). Comparison of these findings with the current study is valid, since one of the authors (S. W. B.) performed the arthritis evaluation in both studies. However, in the previous study on ErpT, mice were hyperimmunized with the truncated recombinant protein and found to be fully susceptible to challenge infection and developed arthritis to the same degree as control mice. Although the earlier study did not assess arthritis by passive immunization, active immunization should have abrogated the development of arthritis in the mice. It remains to be determined if the arthritis-resolving epitopes of Arp are indeed located in the carboxy terminus of the protein.
Analysis of erpT mRNA in selected tissues of infected mice suggested that erpT (and therefore Arp) was expressed by spirochetes in the joints, heart, and spleen but not by spirochetes in skin (23). However, in the present study, the disease-resolving activity of Arp antiserum was selective for joints, without an effect on heart disease. This may seem in conflict with the observation that ErpT (Arp) is also expressed in the heart, but it is important to note that whether or not the antigenic targets are the same for immune-mediated carditis resolution, carditis resolution is not effectively mediated by antibody compared with arthritis (7, 8). Clearly, quantitative kinetic studies are needed to examine these issues and are under way.
It may seem incongruous that antiserum to a single B. burgdorferi protein (Arp) can selectively induce arthritis resolution without invoking protective immunity, altering infection status (including spirochemia) or influencing the status of carditis, but this, in fact, is the expected result and validates our findings with immune sera from infected mice. When immune sera from actively infected mice (containing undefined antibody) are passively transferred to naive mice, very small quantities of such sera will protect the mice against high-dose challenge (5, 8). We believe that the protective activity in immune sera is likely to be due to antibody against decorin-binding protein A (DbpA) (20, 26, 27). Active and passive immunization with DbpA elicits protective immunity but does not alter infection or affect arthritis or carditis in actively infected mice (20). When immune sera are transferred to C3H-scid mice with established infections and with existing joint and heart disease, immune sera induce arthritis resolution, but mice continue to be spirochetemic, and their carditis remains unaffected by serum treatment (7, 8). Our current data, which identify Arp as the target for selective arthritis-resolving antibody, and other studies, which identify DbpA as a target for protective antibody, lend credence to the hypothesis that protective immunity, arthritis-resolving immunity, and carditis-resolving immunity, which all evolve in actively infected immunocompetent mice, are separate phenomena that may involve different B. burgdorferi target antigens or immune responses. Indirect evidence is also available suggesting that arthritis-resolving activity in sera from mice infected with different B. burgdorferi sensu lato strains may be strain specific (4), thus confirming our current findings of Arp antigenic cross-reactivity among strains but distant relatedness of the genes. It remains to be determined if Arp is the only antigen responsible for the arthritis-resolving activity in the immune serum of actively infected mice.
It is now certain that B. burgdorferi is a very dynamic organism which up- and downregulates different genes in different environments. For example, OspA is abundantly expressed by B. burgdorferi within the midgut of flat (resting) ticks but is repressed upon onset of feeding and entry into the mammalian host, whereas OspC is upregulated during tick feeding and in vivo (15–17, 30). Other proteins are selectively expressed in the mammalian host, including the Erp paralogue family, fibronectin-binding protein, DbpA/B, and Arp (based upon ErpT findings). Some of these gene products appear to be upregulated at different times during infection or within the context of different tissues (12–14, 21, 23, 29, 33–35, 37, 38).
Arp is the first B. burgdorferi gene product to be identified that elicits a selective Lyme disease-resolving immune response during persistent infection of the host, thereby mimicking the biological behavior of immune sera from infected mice. A notable exception is a report that described the treatment of SCID mice, infected with B. burgdorferi ZS7 (a European isolate), with antiserum to outer surface protein C (OspC). These mice were cured of infection by such treatment (39), suggesting that ZS7 may constitutively express OspC during infection, thereby making it uniquely vulnerable to OspC antibody. OspC active and passive immunization of mice challenged or infected with B. burgdorferi N40 had neither protective, arthritis-resolving, nor curative effects (8, 11). The selective arthritis-resolving effects that we have demonstrated with Arp-antiserum precisely fit the effect of passively transferred immune serum, thereby validating the biological significance of Arp in Lyme disease.
ACKNOWLEDGMENTS
This work was supported by grants AI26815 and AI45253 from the National Institutes of Health and a gift from SmithKline Beecham Biologicals.
REFERENCES
- 1.AgueroRosenfeld M E, Nowakowski J, Bittker S, Cooper D, Nadelman R B, Wormser G P. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol. 1996;34:1–9. doi: 10.1128/jcm.34.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins D R, Caimano M J, Yang X, Cerna F, Norgard M V, Radolf J D. Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect Immun. 1999;67:1526–1532. doi: 10.1128/iai.67.3.1526-1532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold S W. Specificity of infection immunity among Borrelia burgdorferi sensu lato species. Infect Immun. 1999;67:36–42. doi: 10.1128/iai.67.1.36-42.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold S W, deSouza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:951–971. [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold S W, deSouza M, Feng S. Serum-mediated resolution of Lyme arthritis in mice. Lab Investig. 1996;74:57–67. [PubMed] [Google Scholar]
- 8.Barthold S W, Feng S, Bockenstedt L K, Fikrig E, Feen K. Protective and arthritis-resolving activity in serum from mice infected with Borrelia burgdorferi. Clin Infect Dis. 1997;25:S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 9.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bockenstedt L K, Hodzic E, Feng S, Bourell K W, deSilva A, Montgomery R, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caimano M J, Yang X, Papova T G, Clawson M L, Akins D R, Norgard M V, Radolf J D. Molecular and evolutionary characterization of the cp32/18 family of supercoiled plasmids in Borrelia burgdorferi 297. Infect Immun. 2000;68:1574–1586. doi: 10.1128/iai.68.3.1574-1586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassatt D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champion C I, Blanco D R, Skare J T, Haake D A, Giladi M, Foley D, Miller J N, Lovett M A. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das S, Barthold S W, Giles S S, Montgomery R R, Telford III S R, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 gene expression in ticks and the mammalian host. J Clin Investig. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeSilva A M, Fikrig E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Investig. 1997;99:377–379. doi: 10.1172/JCI119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeSilva A, Fikrig E, Hodzic E, Telford III S R, Barthold S W. Immune evasion by tick-borne and host-adapted Borrelia burgdorferi. J Infect Dis. 1998;177:395–400. doi: 10.1086/514200. [DOI] [PubMed] [Google Scholar]
- 17.DeSilva A, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 19.Dunn J J, Lade B N, Barbour A G. Outer surface protein A (OspA) from the Lyme disease spirochete, Borrelia burgdorferi: high level expression and purification of a soluble recombinant form of OspA. Protein Expr Purif. 1990;1:159–168. doi: 10.1016/1046-5928(90)90011-m. [DOI] [PubMed] [Google Scholar]
- 20.Feng S, Hodzic E, Stevenson B, Barthold S W. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fikrig E, Barthold S W, Sun W, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 22.Fikrig E, Bockenstedt L K, Barthold S W, Chen M, Tao H, Ali-Salaam P, Telford III S R, Flavell R A. Sera from patients with chronic Lyme disease protect mice from Lyme borreliosis. J Infect Dis. 1994;169:568–574. doi: 10.1093/infdis/169.3.568. [DOI] [PubMed] [Google Scholar]
- 23.Fikrig E, Chen M, Barthold S W, Anguita J, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol Microbiol. 1999;31:281–290. doi: 10.1046/j.1365-2958.1999.01171.x. [DOI] [PubMed] [Google Scholar]
- 24.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 25.Gilmore R D, Jr, Murphree R L, James A M, Sullivan S A, Johnson B J B. The Borrelia burgdorferi 37-kilodalton immunoblot band (P37) used in serodiagnosis of early Lyme disease is a flaA gene product. J Clin Microbiol. 1999;37:548–552. doi: 10.1128/jcm.37.3.548-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagman K E, Lahdenne P, Papova T G, Porcella S F, Akins D R, Radolf J D, Norgard M V. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanson M S, Cassatt D R, Guo B P, Patel N K, McCarthy M P, Dorward D W, Hook M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam T T, Nguyen T K, Montgomery R R, Kantor F S, Fikrig E, Flavell R A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Probert W S, Johnson B J. Identification of a 47-kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 30.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of outer surface protein on Borrelia burgdorferi during tick-feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sears J E, Fikrig E, Nakagawa T Y, Deponte K, Marcantonio N, Kantor F S, Flavell R A. Molecular mapping of OspA-mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 1991;147:1995–2001. [PubMed] [Google Scholar]
- 32.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 33.Stevenson B, Bono J L, Schwan T G, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung S Y, McDowell J V, Carlyon J A, Marconi R T. Mutation and recombination in the upstream homology box-flanked ospE-related genes of the Lyme disease spirochetes result in the development of new antigenic variants during infection. Infect Immun. 2000;68:1319–1327. doi: 10.1128/iai.68.3.1319-1327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J-R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:1–20. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 39.Zhong W, Stehle T, Museteanu C, Siebers A, Gern L, Kramer M, Wallich R, Simon M M. Therapeutic passive vaccination against chronic Lyme disease in mice. Proc Natl Acad Sci USA. 1997;94:12533–12538. doi: 10.1073/pnas.94.23.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]