Abstract
Previous studies led to the development of a model of contact-induced enhanced gonococcal invasion of human reproductive cells that utilizes the lutropin receptor (LHr) as both the induction signal for conversion to this enhanced-gonococcal-invasion phenotype (Inv+ GC) and as the specific Inv+ GC uptake mechanism. This model proposes that gonococci express a surface feature that mimics human chorionic gonadotropin (hCG), the cognate ligand for LHr, and that this structure is responsible for the specific and productive interaction of GC with LHr. In this report, we identify a 13-kDa gonococcal protein with immunological similarities to hCG. The antiserum reactivity is specific since interaction with the 13-kDa gonococcal protein can be blocked by the addition of highly purified hCG. This gonococcal “hCG-like” protein, purified from two-dimensional gels and by immunoprecipitation, was determined by N-terminal sequencing to be the ribosomal protein L12. We present evidence that gonococcal L12 is membrane associated and surface exposed in gonococci, as shown by immunoblot analysis of soluble and insoluble gonococcal protein and antibody adsorption studies with fixed GC. Using highly purified recombinant gonococcal L12, we show that preincubation of Inv− GC with micromolar amounts of rL12 leads to a subsequent five- to eightfold increase in invasion of the human endometrial cell line, Hec1B. In addition, nanomolar concentrations of exogenous L12 inhibits gonococcal invasion to approximately 70% of the level in controls. Thus, we propose a novel cellular location for the gonococcal ribosomal protein L12 and concomitant function in LHr-mediated gonococcal invasion of human reproductive cells.
Neisseria gonorrhoeae is the causative agent of gonorrhea, a disease that presents most often as a surface mucosal infection of the genital tract but which can progress into invasive infections of pelvic inflammatory disease (PID) or disseminated gonococcal infection (DGI). It is believed that up to 50% of women with gonococcal infections are asymptomatic, and the lack of treatment enables the progression to complicated gonococcal disease (10). Understanding the process by which gonococci (GC) become invasive would allow the development of therapies which could block this progression of asymptomatic mucosal infections into the serious complicated gonococcal disease patterns. The study of virulence mechanisms in this obligate human pathogen is hampered by the lack of animals models. Using a variety of organ and tissue culture models, many gonococcal features have been identified as factors in gonococcal infection. These include the well-studied roles of lipooligosaccharide, pilin, and opa proteins in the adherence and/or invasion of cellular targets (for recent reviews, see references 17, 36, 40, 43 and 44).
We have previously reported the existence of a contact-inducible enhanced invasion phenotype in N. gonorrhoeae (Inv+ GC) that increases adherence to Hec1B cells, a human endometrial cell line, 2-fold but results in a 5- to 10-fold increase in gonococcal invasion compared to GC grown in tissue culture media alone (Inv− GC) (59). This suggested that changes in the gonococcal surface that occurred following contact with human reproductive cells gave rise to a new adhesin that specifically directs Inv+ GC binding to a host cell uptake process. We have demonstrated that Inv+ GC bind to Hec1B cells by an adhesin not present on Inv− GC and that this novel adherence is completely abolished in the presence of exogenous human chorionic gonadotropin (hCG), a fetal hormone that plays a critical role in the maintenance of uterine function during implantation and pregnancy. In addition, we have found that gonococcal access to the lutropin receptor (LHr), the cognate receptor for hCG, is necessary for the conversion to Inv+ GC (59). Similar studies showed that Inv+ but not Inv− GC invasion is also dependent on LHr expression by target cells.
The putative role of LHr in the fallopian tube is to mediate transcytosis of the fetal hormone hCG into the mother's bloodstream for maintenance of the uterine lining. We proposed that Inv+ GC, via the novel, hCG-inhibitable adhesin, usurps this normal LHr function to achieve transcytosis through the mucosal surface of the fallopian tube. This type of LHr-mediated transcytosis has been observed in endothelial cells from reproductive tissues and is presumed to facilitate the transport of the glycosylated hormones hCG and luteinizing hormone (LH) through the blood vessels for delivery to their target tissues (21). Thus, the gonococcal virulence factors which are induced following contact with LHr may prove to be critical in the progression of gonococcal disease from the surface mucosal infections to the more invasive disease patterns of PID and DGI.
Our model of LHr-mediated gonococcal invasion proposed two roles for this receptor, as both the host cell feature that induces the conversion to Inv+ status and the Inv+ specific uptake mechanism (59). Accordingly, GC must have a constitutively expressed adhesin that interacts with LHr to initiate the phenotypic change to Inv+, an “LHr sensor.” In addition, we propose that the unique Inv+ GC adhesin specifically interacts with LHr in a manner that triggers receptor activation and internalization, a form of hormone mimicry. Immunological techniques, using high-titered antibodies to the native hormone, are commonly used to identify mimetic molecules that share structural and/or functional features. Using this approach, we report on a gonococcal hCG-like molecule that is able, when used as a pretreatment, to enhance Inv− GC invasion of Hec1B cells by five- to eightfold compared to phosphate-buffered saline (PBS)-treated controls. In addition, competitive addition of nanomolar concentrations of the “hCG-like” molecule inhibits invasion by both Inv+ and Inv− GC.
MATERIALS AND METHODS
Bacterial strains.
N. gonorrhoeae F62 was used exclusively. Morphologically transparent, nonpiliated variants were selected on Swanson's medium (60), and frozen stocks of these transparent type IV colonies were maintained and used for all invasion assays. GC were grown in GC broth or solid GC medium base (Difco Laboratories, Detroit, Mich.) with 1% Kellogg's supplement (35) at 37°C in a 5% CO2 incubator.
Tissue culture cells.
The human endometrial cell line Hec1B (HTB113) was purchased from the American Type Culture Collection (Rockville, Md.) and maintained in RPMI 1640 medium containing 2 mM glutamine (Life Technologies, Grand Island, N.Y.) supplemented with 5% fetal bovine serum (FBS) (HyClone Laboratories, Inc., Logan, Utah) and 1 mM sodium pyruvate (Sigma Chemical Co., St. Louis, Mo.) at 37°C in 5% CO2. Cell lines were passaged every 5 days and discarded after approximately 20 passages.
Induction pretreatment.
GC were treated to induce either Inv− or Inv+ phenotypes as previously described (59). Briefly, Inv+ GC were generated by incubating GC on fixed Hec1B cells in complete RPMI for 2 h with rocking at 37°C in a 5% CO2 incubator. After incubation, the monolayers were washed, and the Inv+ GC were separated from Hec1B by vigorous vortexing, followed by brief centrifugation to pellet the Hec1B cells. The Inv+ GC containing supernatant was collected and standardized to a concentration of approximately 8 × 106 GC ml−1 in complete media containing chloramphenicol (1 mg ml−1). Inv− GC were generated by incubating GC in complete RPMI alone at the same relative concentration and under the same incubation conditions as for Inv+ GC. After incubation, the Inv− GC were vigorously vortexed and diluted to the same concentration as described for Inv+ GC. These gonococcal suspensions were added at 0.5 ml per well of a 24-well plate for invasion assays. Input concentrations were determined by obtaining CFU counts for each suspension prepared.
Invasion assays.
GC invasion was determined by use of standard gentamicin resistance assays as previously described (59), using Hec1B cells as the targets at a multiplicity of infection of approximately 10. For the competitive-invasion assays, the denoted amount of recombinant L12 was added immediately prior to the addition of GC. To measure the effects of recombinant L12 (rL12) preincubation on Inv− GC, prepared Inv− GC were incubated with micromolar concentrations of rL12 for 30 min at 37°C 5% CO2 with gentle rocking prior to their use in the invasion assay.
Immunoblot analysis of “hCG-like” gonococcal proteins.
Whole-cell gonococcal lysates were prepared in 1% sodium dodecyl sulfate (SDS). The proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) using 13 or 15% polyacrylamide gels under reducing conditions and blotted onto a nitrocellulose 0.2-μm (pore-size) transfer membrane (Schleicher & Schuell, Inc., Keene, N.H.) using a wet tank electroblotter according to the manufacturer's directions (Hoefer/Amersham Pharmacia Biotech, Inc., Piscataway, N.J.). Proteins with immunological similarity to hCG were identified by their specific reactivity in blots probed with a polyclonal rabbit α-hCG serum (1:1,000) as opposed to those probed with pooled normal rabbit serum (1:500), both from ICN (ICN Biomedical, Costa Mesa, Calif.), followed by horseradish peroxidase-conjugated anti-rabbit antibody (Amersham) at 1:5,000. Bands were visualized by standard enhanced chemiluminescence protocols (Amersham). In Fig. 1, the blots were probed with the immunoglobulin G (IgG) fraction of either the α-hCG or normal rabbit serum at 10 μg ml−1. The IgG fractions were purified from the sera using staphylococcal proteins A and G conjugated to beads (SPA- and SPG-beads [SPA/G-beads]) according to the manufacturer's directions (Pierce, Rockford, Ill.).
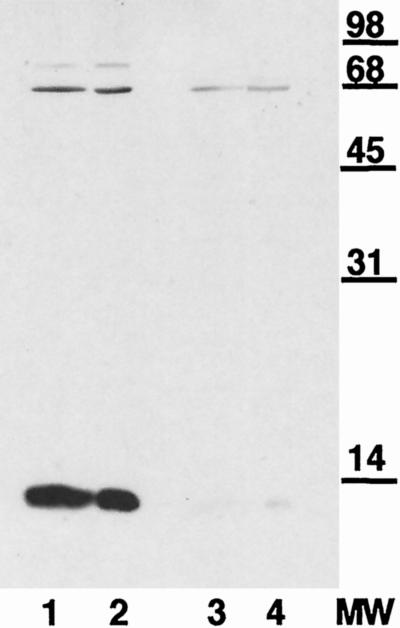
FIG. 1.
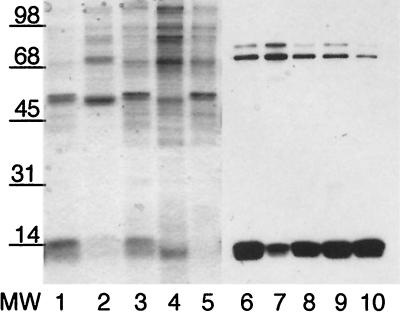
Immunoblot of whole-cell gonococcal lysates separated by SDS-PAGE on 15% polyacrylamide gels from Inv− GC (lanes 1 and 3) or Inv+ GC (lanes 2 and 4) probed with rabbit α-hCG antiserum (lanes 1 and 2) or normal rabbit serum (lanes 3 and 4) at 10 μg ml−1. This demonstrates that a 13-kDa gonococcal protein shares immunological similarities with hCG.
Immunoprecipitation.
Gonococci for immunoprecipitation were metabolically labeled for 2 h with 15 μCi of Expre35S35S ml−1, a mixture of [35S]methionine and [35S]cysteine (New England Nuclear, Boston, Mass.) while grown in a 50:50 mix of RPMI and RPMI deficient in both methionine and cysteine and supplemented with 1 mM pyruvate and 5% FBS. Whole-cell lysates were made of these 35S-labeled GC in a 10 mM Tris buffer containing 1% Triton X-100, 10 mM EDTA, and 0.1% SDS. The lysates were precleared by incubation with normal rabbit serum (pooled) overnight at 4°C and removal of all immune complexes with SPA-beads according to the manufacturer's directions (Pierce). Then, either α-hCG or normal rabbit serum was added to aliquots of the precleared gonococcal lysates, which were incubated overnight at 4°C with rocking, and the specific immune complexed material was collected with SPA-beads. Following extensive washing in lysis buffer, the immunoprecipitated material was removed from the SPA/G-beads by boiling the samples in 2× SDS–PAGE loading buffer. The mixtures were centrifuged to pellet the beads, and the clarified samples were analyzed by SDS-PAGE on a 13% polyacrylamide gel under reducing conditions. These gels were transferred onto nitrocellulose or polyvinylidene difluoride (PVDF) membranes (Schleicher & Schuell, Inc.) according to standard procedures using a wet-tank electroblotter (Hoefer). Samples on nitrocellulose were used for autoradiography, followed by immunoblotting as previously described. The appropriate protein band on PVDF was determined by Western blot analysis of a duplicate lane, and the N-terminal sequence was determined by the Micro Peptide Protein Sequencing Core Facility, University of Rochester, Rochester, N.Y.
Bacterial cell fractionation.
Gonococcal proteins from both Inv+ and Inv− GC were separated into soluble and membrane fractions. GC were induced to the desired phenotype as previously described (59). Following induction, GC were washed two times in PBS plus Ca and Mg and once in 50 mM Tris (pH 8) and then resuspended in 50 mM Tris (pH 8) with 20% sucrose. Freshly prepared lysozyme (1 mg ml−1 in 0.1 mM EDTA) was added, and the mixture was incubated for 30 min on ice. Cell lysis was achieved by adding a 5× volume of ice-cold 10 mM Tris (pH 8), followed by sonication. The suspension was centrifuged for 10 min at 1,000 × g to remove intact cells. The supernatant was centrifuged for 1 h at 3,000 × g to pellet the membrane without removing ribosomal complexes from the supernatant. Following a brief wash in 10 mM Tris, the membrane pellet was dissolved in 0.1% SDS. The soluble proteins in the supernatant were concentrated by precipitating with a 2× volume of ice-cold ethanol followed by incubation overnight at 4°C. The precipitated proteins from the soluble fraction were collected by centrifugation at 30,000 × g for 1 h at 4°C and redissolved in 0.1% SDS. Equal amounts (5 μg) of protein preparations were added to duplicate lanes for SDS-PAGE and Western analysis.
L12 overexpression and purification.
The entire putative gonococcal L12 reading frame was amplified by PCR from F62 genomic DNA by using primers designed from the 5′ end (5′-TAAAATggatccCTATTACTAAAGAAGACATTTTGG-3′; contains a BamHI site) and the 3′ end (5′-TAAGAAgaattcAATTATTTGATTTCGACTTTAGCG-3′; contains an EcoRI site) of the L12 coding region. The 5′ primer was designed to eliminate the initial methionine, since N-terminal sequencing of the native L12 started with alanine, the second amino acid in the putative reading frame. Following PCR (55°C annealing, 72°C extension, 35 cycles), the amplification product was digested with BamHI and EcoRI, ligated to a similarly digested pGEX-2T expression vector (Pharmacia), and transformed first into Escherichia coli XL-1 Blue and then into the E. coli expression strain BL21(DE3). The reading frame and sequence of the expression construct in the resulting plasmid, pGCL12, were verified by DNA sequencing using primers directed to the pGEX vector (5′ sequencing primer, 5′-GGGCTGGCAAGCCACGTTTGGTG-3′; 3′ sequencing primer, 5′-CCGGGAGCTGCATGTGTCAGAGG-3′). Expression of the recombinant fusion protein was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM) to a mid-log culture of BL21 containing pGCL12. Following an additional 3 to 5 h of growth, the bacteria were collected by centrifugation and frozen. Bacteria were lysed with B-Per (Pierce) containing the serine protease inhibitor Pefabloc SC (Boehringer Mannheim) at 0.4 mM and then separated into soluble and membrane fractions according to the manufacturer's directions. The recombinant glutathione S-transferase (GST)-GCL12 fusion protein was isolated from the soluble fraction by bulk adsorption to glutathione-Sepharose beads. The beads were washed, and the native gonococcal L12 molecule was cleaved from the fusion protein with thrombin (Sigma) according to the manufacturer's directions (Pharmacia). Protein concentration and purity was determined by the BCA method (Pierce) and SDS-PAGE.
In E. coli, the ribosomal protein L7 is produced by acetylation of the N-terminal serine of L12. We believe that the recombinant gonococcal L12 is not modified to L7 for two reasons. First, the recombinant is produced by a fusion of GST with the N terminus of L12; thus, the N terminus is not available for modification within the E. coli host. When the recombinant protein is cleaved from GST, all other E. coli proteins have been previously removed by washing. Second, the N-terminal amino acid in the gonococcal L12 is alanine, not serine as found in E. coli, and therefore would not be suitable substrate for the E. coli L12 acetylating enzyme (61).
Antibody adsorption was performed using gonococci that had been fixed in 1% paraformaldehyde. To prevent autolysis, an enzymatic process, GC were chilled by suspension in ice-cold buffer, and all subsequent steps were performed at 0°C. Type IV F62 were washed three times in ice-cold D-PBS plus Ca and Mg and incubated for 1 h on ice in 1% paraformaldehyde (Sigma) with occasional gentle mixing, followed by several more washes with D-PBS plus Ca and Mg to remove all of the paraformaldehyde. Microscopic examination of the fixed GC revealed only intact bacteria, indicating that no detectable lysis occurred during the fixation process. The fixed GC were tightly pelleted (16,000 × g for 5 min), resuspended in antiserum, and incubated on ice for 1 h with occasional mixing. After centrifugation, the antiserum was collected and incubated with a second fixed gonococcal pellet for 1 h on ice. Following the second incubation, the sample was centrifuged, and the adsorbed antiserum was collected.
RESULTS
Immunological identification of a gonococcal hCG-like protein.
Our previous studies have found that GC have both constitutive and contact-induced adhesins which mediate binding to Hec1B cells in a manner that can be inhibited by exogenous hCG (59). This datum implies that GC have at least one and possibly more surface features that are structurally similar to hCG. One approach that has been successfully used to identify bacterial hCG-like molecules employed high-titered polyclonal antiserum to hCG in a variety of immunochemical assays, including immunocytolabeling and Western analysis (1–3). Using a commercially available rabbit anti-hCG (pooled), we were able to identify a gonococcal protein from whole-cell lysates of approximately 13 kDa that reacted strongly with the α-hCG serum, used at 10 μg of IgG ml−1. This interaction appears to be specific since commercially available normal rabbit serum (pooled), also used at 10 μg of IgG ml−1, showed no reaction with gonococcal proteins in this size range (Fig. 1). This immunoreactive protein was found in both Inv+ and Inv− GC in apparently equivalents amounts.
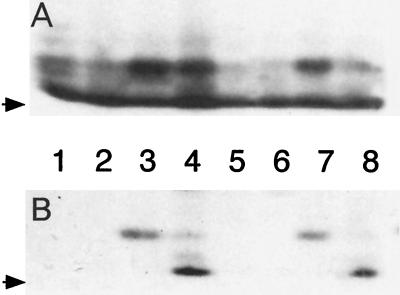
To confirm the specificity of this antibody reaction, immunoprecipitation of whole-cell gonococcal lysates was performed in the presence or absence of exogenous hCG. According to routine protocols, 35S-labeled GC lysates of both Inv+ and Inv− GC were made and precleared of nonspecific reactions with rabbit immunoglobulins, followed by incubation with α-hCG antibodies in the presence or the absence of hCG. The gonococcal proteins precipitated with α-hCG antiserum were separated by SDS-PAGE, transferred to nitrocellulose, and analyzed by autoradiography and immunoblotting. The results from Inv+ GC clearly showed that a 13-kDa protein was immunoprecipitated by α-hCG antiserum and that this reaction was blocked by the addition of exogenous hCG (Fig. 2A). The results from Inv− GC samples were not so clear in that the addition of hCG did not block, though it did lessen, the immunoprecipitation of the 13-kDa hCG-like protein, as seen by autoradiography (Fig. 2A, lanes 3 and 4). Western analysis of this autoradiographic blot shows that hCG is capable of specifically inhibiting the immunoprecipitation of the 13-kDa gonococcal hCG-like protein (Fig. 2B, lanes 3 and 4).
FIG. 2.
Autoradiography (A) and immunoblot (B) analysis of 35S-labeled gonococcal proteins from whole-cell lysates of either Inv− GC (lanes 1 to 4) or Inv+ GC (lanes 5 to 8). Whole-cell gonococcal lysates were immunoprecipitated with either normal rabbit serum (lanes 1, 2, 5, and 6) or rabbit α-hCG antiserum (lanes 3, 4, 7, and 8) in the presence (lanes 2, 4, 6, and 8) or absence (lanes 1, 3, 5, and 7) of hCG. (A) Autoradiography of SDS–13% PAGE of the immunoprecipitates. (B) Immunoblot of the blot from panel A, probed with rabbit α-hCG antiserum (1:1,000 dilution). The arrows denote the dye front of the gel. Immunoreactive material observed at the dye front in lanes 4 and 8 is from the hCG preparation. These data show that the 13-kDa gonococcal protein, identified in both Inv+ and Inv− GC by the polyclonal rabbit α-hCG antiserum, is due to reactivity with antibodies specific for hCG.
Before continuing with extensive analysis of the gonococcal hCG-like protein, we wanted some indication that this protein could be involved in gonococcal adherence and/or invasion. To participate in gonococcus-host cell interactions, the hCG-like molecule must be exposed on the surface of the GC. To address this question, we tested the ability of fixed GC to adsorb from the polyclonal α-hCG sera those antibodies that reacted with the gonococcal hCG-like protein. GC that were fixed in 1% paraformaldehyde were able to remove all reactivity against the 13-kDa protein (Fig. 3). In addition, antibody eluted from fixed GC with 0.1 M glycine (pH 2.5) was capable of reacting with the 13-kDa protein by immunoblot (data not shown). This is strong evidence that the 13-kDa protein is truly surface exposed since the GC were washed multiple times, using low-speed centrifugation to avoid trapping soluble proteins in the cell pellet prior to fixation. Various dilutions of the adsorbed α-hCG antiserum were tested in the immunoblot analysis to control for any unaccounted dilution during the adsorption process.
FIG. 3.
Immunoblot analysis of whole-cell gonococcal lysate probed with rabbit α-hCG antiserum (lanes 4 to 6) or α-hCG antiserum adsorbed with fixed GC (lanes 1 to 3). Gonococcal proteins from a whole-cell lysate were separated by SDS–15% PAGE. The gonococcal proteins were probed with various dilutions of α-hCG antiserum adsorbed with paraformaldehyde-fixed GC (lane 1, 1:250; lane 2, 1:500; lane 3, 1:1,000) or nonadsorbed α-hCG antiserum (lane 4, 1:500; lane 5, 1:1,000; lane 6, 1:2,000). The ability of fixed GC to adsorb all reactivity to the 13-kDa gonococcal protein implies that this protein is surface exposed on intact GC.
Purification and sequencing of the gonococcal hCG-like protein.
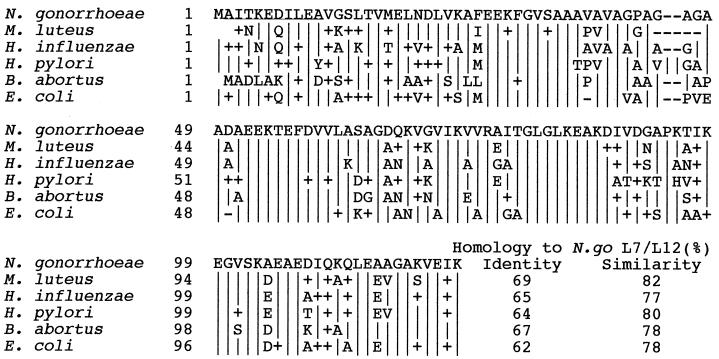
Gonococcal proteins immunoprecipitated with α-hCG antibodies were separated by SDS-PAGE (15%), transferred onto PVDF membranes, and Coomassie blue stained, and the appropriate band was submitted for N-terminal sequencing (Micro Peptide Protein Sequencing Core Facility, University of Rochester, Rochester, N.Y.). The identity of the hCG-like gonococcal molecule was confirmed by N-terminal sequencing of a 13-kDa protein from a blot of a two-dimensional gel analysis of Inv− GC whole-cell lysate that reacted with α-hCG antiserum (data not shown). The 10 amino acids derived from these sequence analyses (AITKEDILEA) were used to search the N. gonorrhoeae genome (B. A. Roe, S. P. Lin, L. Song, X. Yuan, S. Clifton, T. Ducey, L. Lewis, and D. W. Dyer, Gonococcal Genome Sequencing Project, http://www/genome.ou.edu/gono.html). The only high-probability match (E < 0.005) was a perfect match with the 10-amino-acid search sequence and identified an open reading frame (ORF) on contig 130 (8/8/99). A comparison search of gene banks (5; National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/) found that this ORF has significant homology (>62% identity, >75% similarity) to the ribosomal protein L7/L12 (RplL) from many different prokaryotes (Fig. 4). The putative gonococcal L7/L12 reading frame encodes a protein of 123 amino acids, with an expected mass of 12.5 kDa. This is in good agreement with the apparent size of the hCG-like gonococcal protein found by SDS-PAGE.
FIG. 4.
Comparisons of the deduced amino acid sequences of L7/L12 from several bacterial species using the CLUSTAL W multiple sequence alignment algorithm (62). Amino acids identical to those of the N. gonorrhoeae sequence are denoted by “|”, nonidentical but similar amino acids are denoted by “+”, and dashes represent gaps.
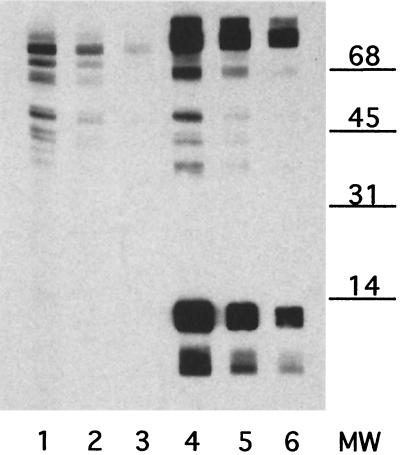
The identification of this gonococcal hCG-like protein as a ribosomal protein raised a question concerning the location of this protein in gonococci. The expression of most ribosomal proteins is tightly regulated, with very little found outside the ribosomal complex. To test whether gonococcal L7/L12 could be found associated with the outer membrane, whole-cell gonococcal lysates were carefully fractionated into soluble and membrane proteins. Since the ribosomal complex can be pelleted by centrifugation at >100,000 × g, the membrane fraction was prepared at a centrifugation force no greater than 30,000 × g, and the membrane pellet was carefully washed to limit any contamination of the preparation with soluble proteins. The membrane and soluble protein fractions were analyzed by immunoblot for the presence of the immunoreactive 13-kDa hCG-like protein (Fig. 5). The 13-kDa hCG-like gonococcal protein is readily apparent in all preparations. This shows that gonococcal protein L7/L12 is present in significant quantities at sites other than in the ribosome.
FIG. 5.
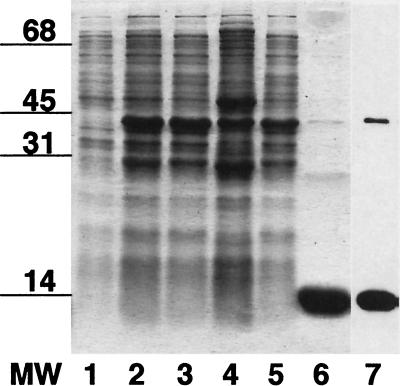
SDS-PAGE and immunoblot analysis of soluble and membrane gonococcal proteins. Duplicate samples of gonococcal proteins (5 μg/lane) were separated by SDS–15% PAGE and stained with Coomassie blue (lanes 1 to 5) or transferred to nitrocellulose and probed with rabbit α-hCG antiserum (lanes 6 to 10). Samples are gonococcal whole-cell lysate (lanes 1 and 6), Inv− GC soluble proteins (lanes 2 and 7) and membrane proteins (lanes 3 and 8), and Inv+ GC soluble proteins (lanes 4 and 9) and membrane proteins (lanes 5 and 10). The 13-kDa gonococcal protein recognized by rabbit α-hCG antiserum is found in both the soluble and the membrane protein fractions from both Inv+ and Inv− GC. This shows that gonococcal L7/L12 is present in both membrane-associated and ribosomal (soluble) locations and supports the previous finding that L7/L12 is expressed on the gonococcal surface (Fig. 3).
Since there is no animal model for gonococcal infection and since L7/L12 is an essential gene, we measured the effects of L12 in a well-characterized in vitro invasion system using Hec1B cells (15, 29, 58, 59). If L7/L12 is expressed on the gonococcal surface and functions as an adhesin or invasin, then the addition of exogenous L12 should competitively inhibit gonococcal invasion.
Purified gonococcal L12 was prepared using the pGEX expression system. The recombinant gonococcal L12 was cleaved from the GST fusion protein by thrombin and subjected to SDS-PAGE analysis to assess purity and test for its immunoreactivity with α-hCG antiserum. The native rL12 is >95% pure and retains the ability to react with α-hCG antiserum in a manner similar to that of the native protein (Fig. 6). In addition, purified rL12, immobilized on nitrocellulose, was able to adsorb all reactivity to the gonococcal 13-kDa protein from the α-hCG antiserum (data not shown). These data suggest that the recombinant gonococcal L12 is a suitable analog for use in invasion competition studies.
FIG. 6.
SDS-PAGE and immunoblot analysis of the purification of recombinant gonococcal L12 from E. coli BL21 transformed with pGCL12. The Coomassie blue stain of the SDS-PAGE shows: lane 1, whole-cell lysate before IPTG induction; lane 2, whole-cell lysate following IPTG induction; lane 3, soluble proteins following IPTG induction; lane 4, insoluble proteins following IPTG induction; lane 5, nonadsorbed proteins from glutathione-affinity adsorption; lane 6, recombinant gonococcal L12 following thrombin cleavage of GST-GC L12 fusion protein adsorbed onto glutathione-beads. Lane 7 is an immunoblot of a duplicate of lane 6, probed with rabbit α-hCG antiserum. The recombinant gonococcal L12 preparation was judged to be >95% pure.
Effects of rL12 on gonococcus-host cell interactions.
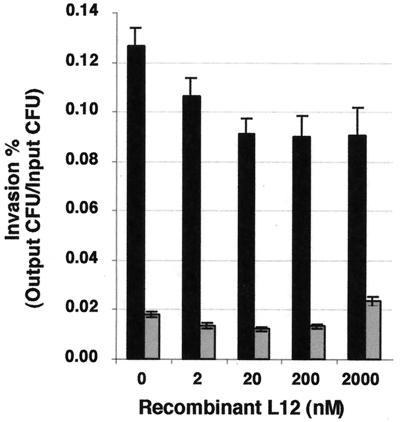
Competitive-invasion assays were performed in triplicate with both Inv+ and Inv− GC in the presence of either buffer (no L12 control) or increasing concentrations of rL12. Invasion was measured using gentamicin resistance assays and is presented as a percentage (gentamicin-resistant CFU/input CFU). The results are cumulative data from more than six experiments using three different preparations of gonococcal L12 (Fig. 7). We determined that Inv+ GC invasion was inhibited at all concentrations of rL12 tested, while inhibition of Inv− GC invasion was found at 2 to 20 nM concentrations of rL12 with P < 0.005 (one-tailed Student's t test). However, the degree of inhibition, while quite reproducible, was only to 70% of the controls. Competitive-invasion assays using 1 to 2 μM concentrations of rL12 did not inhibit Inv− GC invasion and in some cases actually increased invasion levels relative to Inv− GC controls. No such changes were observed with Inv+ GC.
FIG. 7.
Effect of exogenous gonococcal rL12 on GC invasion of Hec1B cells. Various amounts of rL12 were added to Hec1B cells simultaneously with Inv+ GC (black bars) or Inv− GC (gray bars). Invasion is expressed as the percentage of gentamicin-resistant CFU/input CFU. The results are averages of data from triplicate wells from over six experiments. The inhibition of Inv+ GC invasion observed at all concentrations of rL12 and with Inv− GC for rL12 concentrations between 2 and 200 nM is significantly different from the no-rL12 controls (P ≤ 0.005; one-tailed paired Student's t test). At 2,000 nM of rL12, the invasion level of Inv− GC is significantly enhanced over the no-rL12 controls (P < 0.001; one-tailed paired Student's t test). The error bars denote the standard error of the mean.
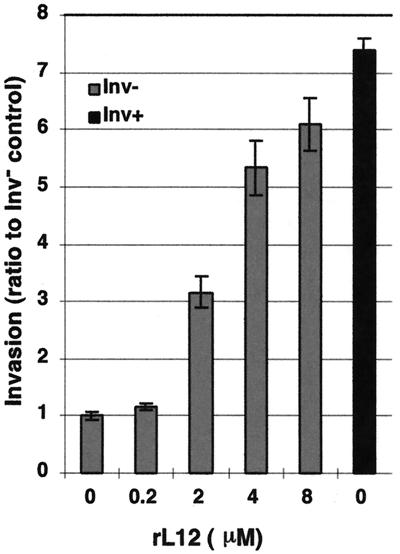
To further investigate the effects of higher levels of rL12 on Inv− GC invasion, we preincubated Inv− GC with micromolar concentrations of rL12 for 30 min prior to their use in standard invasion assays. The results are presented as a ratio to the invasion level observed with PBS-pretreated Inv− GC (Fig. 8). These assays showed that micromolar concentrations of rL12 greatly enhanced Inv− GC invasion levels, with amounts approaching the invasiveness observed with Inv+ GC (Fig. 8). This is conclusive evidence of the potential for L12 to mediate gonococcal invasion of human reproductive cells.
FIG. 8.
Effect of gonococcal rL12 pretreatment on GC invasion of Hec1B cells. Various amounts of rL12 were added to Inv− GC (gray bars) for 30 min prior to their use in the gentamicin-resistant invasion assay. Invasion is expressed as the ratio to the Inv− GC pretreated with PBS alone. The results are averages of data from triplicate wells. Highly significant increases in invasion were observed for all concentrations of rL12 of ≥2 μM. Inv+ GC invasion ratios from the same experiments are also shown (black bars). The error bars denote the standard error of the mean.
Preliminary studies on the effect of rL12 on gonococcal adherence to Hec1B cells, using the same parameters as in the invasion assays, produced no statistically significant effects. The results are similar in pattern to those observed in competitive-invasion assays, in that nanomolar concentrations of rL12 slightly decrease Inv− GC adherence, by <10%, while micromolar concentrations slightly enhanced adherence, but again only by a fraction of that observed by invasion (data not shown). This indicates that the rL12 effects on invasion are not due to overall changes in gonococcal adherence to target cells and suggests that rL12 inhibits gonococcal interactions with a specific uptake mechanism. These results were not surprising since GC have many potent adherence mechanisms, and blocking adherence to a low-density host cell receptor, such as LHr, would be difficult to ascertain against such a high background. These data parallel our previous findings in which minor increases in Inv+ GC adherence (2) resulted in a significantly larger increase in invasion (five to ten times) compared to Inv− GC (59).
DISCUSSION
Gonococcal invasion of human host cells is a multifactorial process. Much work has been done regarding the roles of lipo-oligosaccharide, opa, and pilin in gonococcal infection using a variety of in vitro systems. However, since GC are obligate human pathogens, the true in vivo role of these various factors is difficult to ascertain. The human male volunteer studies have allowed some evaluation of virulence factors crucial for initial colonization and have confirmed the high degree of antigenic and phase variation that occurs during gonococcal infections (31, 57). However, appropriate ethical restraints limit the testing of gonococcal factors related to the development of invasive gonococcal disease, such as DGI and PID, both of which occur disproportionately in women.
We have demonstrated two roles for LHr in gonococcal invasion (59). This suggests that GC might make structural homologues to hCG. Many bacteria are known to make hCG-like molecules (1–3, 7, 27, 40, 54). These molecules were identified by immunological techniques using a variety of antibodies, including those to the intact hCG heterodimer, to the individual α and β chains, and to specific regions of the β chain of hCG. Some microbes have also been found to produce proteins that bind hCG. These microbial hCG-like receptors are able to bind their cognate ligands with Kd in the range of 10−8 to 10−11 M (12–14). In the case of Stenotrophomonas maltophilia, this hCG-like ligand and receptor system is capable of regulating growth and cell morphology in a dose-dependent manner (11). This implies that these bacterial hCG-like homologues are functional and not simply a curious anomaly. In addition, it has been reported that Candida albicans, a human pathogen, uses its hCG-like molecule to regulate germ tube formation, a known virulence factor (14). Thus, the existence of microbial hCG-like molecules and their function in virulence has precedence.
Using a similar immunological approach, we identified a gonococcal hCG-like molecule with a molecular mass of approximately 13-kDa (Fig. 1) from both Inv− and Inv+ GC. We immunoprecipitated a 13-kDa gonococcal protein with rabbit α-hCG antiserum, and this immunoprecipitation could be inhibited by the addition of highly purified hCG (Fig. 2A). This implies that the antibodies reacting with the 13-kDa gonococcal protein are of high affinity and that the reaction is due to antibodies specific for hCG epitopes. While hCG was able to inhibit the immunoprecipitation of the 13-kDa protein from both Inv+ and Inv− GC, the degree of inhibition appeared to be greater with Inv+ (Fig. 2A, lanes 7 and 8) than with Inv− GC whole-cell lysates (Fig. 2A, lanes 3 and 4). However, the Inv− protein immunoprecipitated in the presence of hCG did not appear to react as strongly by Western blot analysis as the autoradiographic data would imply (Fig. 2, lane 4).
A possible explanation for this observation is that Inv− GC produce a protein of approximately 13 kDa that binds to hCG-like molecules and was coprecipitated with hCG. Since it was not immunoprecipitated due to its reaction with antibodies, it would not react with α-hCG by Western analysis. In addition, the binding of a gonococcal protein to hCG could limit the ability of hCG to bind antibody. If the antisera was not saturated with hCG, it would be available to immunoprecipitate some of the 13-kDa gonococcal hCG-like protein, giving rise to the significantly different level of reaction observed in Fig. 2B, lanes 4 and 8.
The identification of the 13-kDa gonococcal protein that reacts strongly with α-hCG antiserum as the ribosomal protein L7/L12 is very convincing. Comparison of the N-terminal sequence of the gonococcal hCG-like protein isolated by immunoprecipitation and from two-dimensional gel analysis with N. gonorrhoeae genome sequence data (Roe et al., Gonococcal Genome Sequencing Project) by tBLASTp (4) demonstrated only one match, which had 100% identity. The predicted amino acid sequence of the identified ORF found significant homology to other known bacterial L7/L12. In addition, the sequence immediately upstream of the putative gonococcal L7/L12 gene has homology to the ribosomal protein L10 (RplJ), while the downstream sequence has homology to the beta subunit of DNA-directed RNA polymerase (RpoB), a genetic organization that is found in other prokaryotes (48, 52, 71). While the identification of this protein as the gonococcal L7/L12 is relatively straightforward, the putative role of this molecule as a virulence factor on the surface of GC is more problematic, especially with regard to the tight regulation of expression observed with most ribosomal proteins.
The rplL gene directs the formation of a protein core which is identified as L12. This protein can also undergo an acetylation, whose product is called L7. These two ribosomal proteins are unique in several ways, including their acidic nature and their 4:1 ratio within the ribosomal complex. The regulation of L7/L12 expression is partially controlled at the level of translation by attenuation. While this operon is attenuated by L10 alone in vitro, it has been shown that the L10-L7/L12 complex is needed to inhibit translation in vitro. The need for the L10-L7/L12 complex to inhibit translation suggests that a protein which binds L7/L12 with a greater affinity that L10 might disrupt translational control by attenuation.
In addition, it has been reported that a significant amount of L7/L12 exists in a nonribosomal pool in E. coli (44, 45). The nonribosomal L7/L12 molecules shared identical amino acid composition and electrophoretic characteristics and were immunologically identical to ribosomal L7/L12 (45). However, these nonribosomal L7/L12 molecules were not able to interact with protein-stripped ribosomes, showed different gel filtration elutions, and presented a different trypsin peptide map than that observed with ribosomal L7/L12. The authors of these studies did not determine a function for this nonribosomal pool but postulate that the differences might be due to modification of nonribosomal L7/L12 to inactivate excess L7/L12 or to convert it into a form with other biological activities (44, 45). If these processes occur in N. gonorrhoeae, it could serve as the source of L7/L12 for surface expression.
The proposed model of LHr-mediated gonococcal invasion requires that both the “LHr sensor” and the contact-induced adhesin must be surface expressed to interact with LHr on host target cells. Thus, prior to the identification of L7/L12 as a gonococcal hCG-like protein, studies were done to confirm the surface-exposed nature of this 13-kDa protein that reacted with α-hCG antiserum. We showed that fixed GC can completely remove the antiserum reactivity to the 13-kDa protein (Fig. 3). Following the identification of this 13-kDa protein as L7/L12, these experiments were repeated, with extensive washing of GC prior to fixation to ensure removal of proteins that might be nonspecifically adsorbed onto the gonococcal surface during growth. In addition, both Inv− and Inv+ GC were tested for their ability to adsorb the reactivity to L7/L12. Both phenotypes were capable of removing the anti-L7/L12 activity, and antibody with L7/L12 reactivity could be eluted from these fixed GC.
This ability of intact GC to adsorb anti-hCG antibodies is consistent with previous studies on prokaryote hCG-like molecules which used immunocytochemical protocols to demonstrate production of hCG-like material on the surface of bacteria (1–3). In addition, studies have determined that this hCG-like material was associated with the bacterial membranes as well as in the soluble protein fraction (19). While it is difficult to obtain adequate amounts of Inv+ GC for cell fractionation studies, we were able to separate Inv+ and Inv− GC proteins into soluble and membrane fractions. Care was taken to ensure that contamination of the membrane fraction with ribosomes was minimized by using a relatively low-speed spin (30,000 × g) to avoid the pelleting of the ribosomal complexes and washing the membrane pellet prior to dissolving the proteins in 1% SDS for electrophoresis and immunoblotting. The results (Fig. 5) demonstrate that the 13-kDa, α-hCG-reactive gonococcal protein was found in the soluble (lanes 2 and 4) and membrane (lanes 3 and 5) fractions from both Inv+ and Inv− GC and that the reactive 13-kDa band in the membrane fraction was comparable to that observed in a whole-cell gonococcal lysate (lane 1). Taken together, these data from antibody adsorption and cell fractionation studies are strong evidence that the L7/L12 protein is surface exposed in N. gonorrhoeae.
The final test of L7/L12 surface exposure and functionality was to determine the effect of rL12 on gonococcal invasion. Preincubation of Inv− GC with micromolar concentrations of rL12 greatly increased invasion of Hec1B cells, to the levels observed with Inv+ GC (Fig. 8). This clearly demonstrated the ability of L12 to serve as an invasin for Hec1B cells. If L7/L12 is not naturally present as a functional invasin on the gonococcal surface, it is very unlikely that the addition of exogenous L12 would have any inhibitory effect on GC-host cell interactions. In competitive-invasion assays we showed that nanomolar concentrations of L7/L12 decreased the gonococcal invasion of both Inv+ and Inv− GC in a statistically significant manner (P < 0.005; one-tailed paired Student's t test) (Fig. 7). The ability of such a low concentration of L12 to significantly affect invasion implies a physiologically relevant function for L12 in this process. However, invasion was only suppressed to approximately 70% of the level of controls.
We believe that the most likely explanation for these observations is that L12 interacts with both Hec1B via LHr and a gonococcal surface feature, with different affinities, and that the invasion outcome depends on L12 concentration. At low concentrations, rL12 interaction with LHr inhibits binding by the LHr sensor, blocking any low level of conversion to Inv+ status. At higher concentrations, we propose that the binding of rL12 to the gonococcal surface is enhanced, as seen by the ability of rL12 pretreatment to significantly increase gonococcal invasion in a dose-dependent manner. Once the amount of rL12 on the gonococcal surface reaches a critical density, its relative affinity overcomes the inhibition posed by the soluble rL12, and uptake occurs. Whether this difference in apparent affinities is due to the multimeric presentation of rL12 on the gonococcal surface or to conformational differences of the membrane-associated rL12 compared to the soluble form is unknown at this time. It is known that L12 readily forms dimers and tetramers and is a relatively flexible protein with an identified hinge region. It is feasible that rL12 binds to Inv− GC by way of its native surface-exposed L7/L12.
Another strong possibility is that surface-exposed L7/L12 protein is chemically modified in vivo, which enhances its affinity to LHr compared to the nonmodified recombinant protein. We believe this is the reason for the limited effect of rL12 on Inv+ GC invasion. The L7/L12 molecule is known to undergo multiple types of chemical modifications, including acetylation at the N terminus to generate L7, phosphorylation (31), and acylation. In Brucella abortus, the acylated L12 is the essential epitope for the delayed-type hypersensitivity response to the Brucella vaccine (6). This modification is especially interesting with regard to its potential to enhance L7/L12 association with membranes.
We have demonstrated that the conversion from Inv− to Inv+ involves the expression of a novel adhesin (59). It is feasible that this new adhesin is generated by chemical modification of a preexisting Inv− GC protein, the surface-expressed L12, and that this modification enhances the affinity of the Inv+ adhesin for LHr. We believe that only the Inv+ adhesin is capable of triggering receptor activation. This would, in many ways, parallel what is known of LHr interactions with its cognate ligands (reviewed in reference 55). It has been shown that receptor occupation alone is insufficient for activation, since deglycosylated hCG binds to LHr with high affinity and yet is incapable of receptor activation (53, 69). It would be intriguing to find that gonococcal L7/L12, in an unmodified form, binds to LHr but only activates the receptor if it is modified by glycosylation.
The remaining question is how does L7/L12 get to the gonococcal surface? The gonococcus is autolytic, as seen in many naturally competent bacteria. Autolysis has been described as a gonococcal virulence feature, one used as a means of generating the vast diversity of pilin types expressed in vivo (34, 56). Thus, it is possible that L7/L12 is adsorbed from lysed neighbors. A similar process, called altruistic autolysis, has been proposed for Helicobacter pylori to explain the surface localization of the cytoplasmic proteins urease and HspB (21). In Streptococcus pneumoniae, autolysis is the proposed mechanism by which the cytoplasmic toxin, pneumolysin (32), is released in vivo (37, 50). In addition, insertionally inactivated autolysin mutants have reduced virulence in animal models of pneumococcal disease (8). We have shown that GC have contact-induced behavior that enhances invasion of target cells, which has similarities to the behavior of pathogens that express the contact-induced type III secretion system. While protein sequence comparisons to the gonococcal genome have revealed few homologues to any of the type III proteins involved in the secretory apparatus, and only to PilQ, which has a role in pili expression, it is still possible that neisseria contains relatively unique proteins that serve the same function. It is also possible that an hCG-like binding protein may bind to the hCG-like determinants on L7/L12 and direct the expression of L7/L12 to the bacterial surface. The finding of nominally intracellular proteins that serve as surface-exposed virulence factors could aid in the elucidation of these different processes in N. gonorrhoeae. Understanding this process may allow development of therapies that block the conversion of GC to the enhanced invasion phenotype.
In summary, we have demonstrated that the gonococcal L7/L12 protein has immunologic similarities to hCG. This protein is surface exposed on GC, so it is available to interact with LHr-expressing cells, as found in the reproductive tract of women. We have purified recombinant gonococcal L12 and shown that preincubation of Inv− GC with micromolar concentrations resulted in their phenotypic conversion to Inv+ GC. This proved the potential of L12 to serve as an invasin. In addition, we showed that both Inv+ and Inv− gonococcal invasion of human endometrial cells can be inhibited to a statistically significant degree by nanomolar concentrations of recombinant gonococcal L12. This ability of hormone-like concentrations to influence invasion of Hec1B cells suggests this protein has a physiologically relevant role in this process. These findings may help in the understanding of basic virulence processes in N. gonorrhoeae, with special regard to the development of DGI and PID in women.
We are currently investigating the nature of surface-expressed L12, with special regard to possible chemical modification. We are also determining what other gonococcal proteins are associated with L12, which associations are involved in surface expression, and whether Inv+ and Inv− GC differ in these associations.
ACKNOWLEDGMENTS
Highly purified hCG (NICHD CR 127) was kindly provided by the National Hormone and Pituitary Agency of the National Institute of Diabetes and Digestive and Kidney Diseases.
This work was supported by Public Health Service grants RO1 AI33973 and RO1-AI11709 from the National Institutes of Health to V.L.C. In addition, J.M.S. was supported in part by NIH grant T32 AI07362.
REFERENCES
- 1.Acevedo H F, Campbell-Acevedo E, Kloos W E. Expression of human choriogonadotropin-like material in coagulase-negative Staphylococcus species. Infect Immun. 1985;50:860–868. doi: 10.1128/iai.50.3.860-868.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acevedo H F, Pardo M, Campbell-Acevedo E, Domingue G J. Human choriogonadotropin-like material in bacteria of different species: electron microscopy and immunocytochemical studies with monoclonal and polyclonal antibodies. J Gen Microbiol. 1987;133:783–791. doi: 10.1099/00221287-133-3-783. [DOI] [PubMed] [Google Scholar]
- 3.Affronti L F, DeBlaker D F. Immunological detection of hCG-like substances in aerobic bacteria of both tumour and non-tumor origin. Microbios. 1986;48:173–182. [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Altschul S F, Madden T L, Schaffer A A, Zhang J, Shang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachrach G, Banal M, Fishman Y, Bercovier H. Delayed-type hypersensitivity activity of the Brucella L7/L12 ribosomal protein depends on posttranslational modification. Infect Immun. 1997;65:267–271. doi: 10.1128/iai.65.1.267-271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backus B T, Affrontri L F. Tumor-associated bacteria capable of producing a human choriogonadotropin-like substance. Infect Immun. 1981;32:1211–1215. doi: 10.1128/iai.32.3.1211-1215.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry A, Paton J C, Hansman D. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb Pathog. 1992;12:87–93. doi: 10.1016/0882-4010(92)90111-z. [DOI] [PubMed] [Google Scholar]
- 9.Bessen D, Gotschlich E C. Interactions of gonococci with HeLa cells: Attachment, detachment, replication, penetration, and the role of protein II. Infect Immun. 1986;54:154–160. doi: 10.1128/iai.54.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks G F, Donegan E A. Gonococcal infection. Vol. 5. London, England: Edward Arnold Publishers, Ltd.; 1985. [Google Scholar]
- 11.Carrell D T, Hammond M E, Odell W D. Evidence for an autocrine/paracrine function of chorionic gonadotropin in Xanthomonas maltophilia. Endocrinology. 1993;132:1085–1089. doi: 10.1210/endo.132.3.7679968. [DOI] [PubMed] [Google Scholar]
- 12.Carrell D T, Odell W D. A bacterial binding site which binds human chorionic gonadotropin but not human luteinizing hormone. Endocrine Res. 1992;18:51–58. doi: 10.3109/07435809209035928. [DOI] [PubMed] [Google Scholar]
- 13.Carrell D T, Woods M E, Griffin J, Odell W D. Identification of a CG/LH binding site in two strains of Mycobacterium vaccae. Endocrine Res. 1997;23:59–67. doi: 10.1080/07435809709031842. [DOI] [PubMed] [Google Scholar]
- 14.Caticha O, Odell W D. Characterization and purification of the chorionic gonadotropin-like protein binding site in Candida albicans. Endocrine Res. 1994;20:1–19. doi: 10.1080/07435809409035852. [DOI] [PubMed] [Google Scholar]
- 15.Chen J C-R, Bavoil P, Clark V L. Enhancement of the invasive ability of Neisseria gonorrhoeae by contact with Hec1B, an adenocarcinoma endometrial cell line. Mol Microbiol. 1991;5:1531–1538. doi: 10.1111/j.1365-2958.1991.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, Gotschlich E C. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper M D, McGraw P A, Melly M A. Localization of gonococcal lipopolysaccharide and its relationship to toxic damage in fallopian tube mucosa. Infect Immun. 1986;51:425–430. doi: 10.1128/iai.51.2.425-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehio C, Gray-Owen S D, Meyer T F. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 1998;6:489–495. doi: 10.1016/s0966-842x(98)01365-1. [DOI] [PubMed] [Google Scholar]
- 19.Domingue G J, Acevedo H F, Powell J E, Stevens V C. Antibodies to bacterial vaccines demonstrating specificity for human choriogonadotropin (hCG) and immunochemical detection of hCG-like factor in subcellular bacterial fractions. Infect Immun. 1986;53:95–98. doi: 10.1128/iai.53.1.95-98.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duensing T D, van Putten J P M. Vitronectin binds to the gonococcal adhesin OpaA through a glycosaminoglycan molecular bridge. Biochem J. 1998;334:133–139. doi: 10.1042/bj3340133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn B E, Vakil N B, Schneider B G, Miller M M, Zitzer J B, Peutz T, Phadnis S H. Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies. Infect Immun. 1997;65:1181–1188. doi: 10.1128/iai.65.4.1181-1188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda R. Autogenous regulation of the synthesis of ribosomal proteins, L10 and L7/L12, in Escherichia coli. Mol Gen Genet. 1980;178:482–486. doi: 10.1007/BF00270505. [DOI] [PubMed] [Google Scholar]
- 23.Grassme H U C, Ireand R M, van Putten J P M. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect Immun. 1996;64:1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray-Owen S D, Lorenzen D R, Haude A, Meyer T F, Dehio C. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol Microbiol. 1997;26:971–980. doi: 10.1046/j.1365-2958.1997.6342006.x. [DOI] [PubMed] [Google Scholar]
- 25.Gregg C R, Melly M A, McGee Z A. Gonococcal lipopolysaccharide: a toxin for human fallopian tube mucosa. Am J Obstet Gynecol. 1980;138:981–984. doi: 10.1016/0002-9378(80)91092-3. [DOI] [PubMed] [Google Scholar]
- 26.Griffiss J M, Lammel C J, Wang J, Dekker N P, Brooks G F. Neisseria gonorrhoeae coordinately uses pili and opa to activate Hec-1-B cell microvilli, which causes engulfment of the gonococci. Infect Immun. 1999;67:3469–3480. doi: 10.1128/iai.67.7.3469-3480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grover S, Woodward S R, Odell W D. Complete sequence of the gene encoding a chorionic gonadotropin-like protein from Xanthomonas maltophilia. Gene. 1995;156:75–78. doi: 10.1016/0378-1119(95)00056-c. [DOI] [PubMed] [Google Scholar]
- 28.Gudkov A T. The L7/L12 ribosomal domain of the ribosome: structural and functional studies. FEBS Lett. 1997;407:253–256. doi: 10.1016/s0014-5793(97)00361-x. [DOI] [PubMed] [Google Scholar]
- 29.Ilver D, Kallstrom H, Normark S, Jonsson A-B. Transcellular passage of Neisseria gonorrhoeae involves pilus phase variation. Infect Immun. 1998;66:469–473. doi: 10.1128/iai.66.2.469-473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Issenger O G, Traut R R. Selective phosphorylation from GTP of proteins L7 and L12 of E. coli 50S ribosomes by a protein kinase from rabbit reticulocytes. Biochem Biophys Res Commun. 1974;59:829–836. doi: 10.1016/s0006-291x(74)80054-9. [DOI] [PubMed] [Google Scholar]
- 31.Jerse A E, Cohen M S, Drown P M, Whicker L G, Isbey S F, Seifert H S, Cannon J G. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson M K. Cellular location of pneumolysin. FEMS Microbiol Lett. 1977;2:243–245. [Google Scholar]
- 33.Jonsson A-B, Ilver D, Falk P, Pepose J, Normark S. Sequence changes in the pilus subunit lead to tropism variation of Neisseria gonorrhoeae to human tissue. Mol Microbiol. 1994;13:403–416. doi: 10.1111/j.1365-2958.1994.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson A B, Nyberg G, Normark S. Phase variation of gonococci pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellogg D S, Peacock W L J, Deacon W E, Brown L, Pirkle C I. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kupsch E-M, Knepper B, Kuroki T, Heuer I, Meyer T F. Variable Opa outer membrane proteins account for the cell tropism displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez R, Sanchez-Puelles J M, Garcia E, Garcia J L, Ronda C, Garcia P. Isolation, characterization and physiological properties of an autolytic-deficient mutant of Streptococcus pneumoniae. Mol Gen Genet. 1986;204:237–242. doi: 10.1007/BF00425504. [DOI] [PubMed] [Google Scholar]
- 38.Makino S-I, van Putten J P M, Meyer T F. Phase variation of the Opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J. 1991;10:1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandrell R E, Apicella M A. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology. 1993;187:382–402. doi: 10.1016/S0171-2985(11)80352-9. [DOI] [PubMed] [Google Scholar]
- 40.Maruo T, Cohen H, Segal S J, Koide S S. Production of choriogonadotropin-like factor by a microorganism. Proc Natl Acad Sci USA. 1979;76:6622–6626. doi: 10.1073/pnas.76.12.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGee Z A, Johnson A P, Robinson D T- Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981;143:413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- 42.Merz A J, Ridenbery D B, Arvidson C G, So M. Traversal of a polarized epithelium by pathogenic neisseriae: facilitation by type IV pili and maintenance of epithelial barrier function. Mol Med. 1996;2:745–754. [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer T F. Pathogenic neisseriae—interplay between pro- and eukaryotic worlds. Folia Microbiol. 1998;43:311–319. doi: 10.1007/BF02818617. [DOI] [PubMed] [Google Scholar]
- 44.Morrissey J J, Cupp L E, Weissbach H, Brot N. Synthesis of ribosomal proteins L7-L12 in relaxed and stringent strains of Escherichia coli. J Biol Chem. 1976;251:5516–5521. [PubMed] [Google Scholar]
- 45.Morrissey J J, Weissbach H, Brot N. The identification and characterization of proteins similar to L7, L12 in ribosome-free extracts of Escherichia coli. Biochem Biophys Res Commun. 1965;65:293–302. doi: 10.1016/s0006-291x(75)80092-1. [DOI] [PubMed] [Google Scholar]
- 46.Nassif X, Pujol C, Morand P, Eugene E. Interactions of pathogenic Neisseria with host cells. Is it possible to assemble the puzzle? Mol Microbiol. 1999;32:1124–1132. doi: 10.1046/j.1365-2958.1999.01416.x. [DOI] [PubMed] [Google Scholar]
- 47.Nassif X, So M. Interaction of pathogenic neisseriae with nonphagocytic cells. Clin Microbiol Rev. 1995;8:376–388. doi: 10.1128/cmr.8.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nomura M, Morgan E A, Jaskunas S R. Genetics of bacterial ribosomes. Annu Rev Genet. 1977;11:297–347. doi: 10.1146/annurev.ge.11.120177.001501. [DOI] [PubMed] [Google Scholar]
- 49.Paruchuri D K, Seifert S S, Ajioka R S, Karlsson K-A, So M. Identification and characterization of a Neisseria gonorrhoeae gene encoding a glycolipid-binding adhesin. Proc Natl Acad Sci USA. 1990;87:333–337. doi: 10.1073/pnas.87.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paton J C, Andrew P W, Boulnois G J, Mitchell T J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993;47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 51.Porse B T, Garrett R A. Ribosomal mechanics, antibiotics and GTP hydrolysis. Cell. 1999;97:423–426. doi: 10.1016/s0092-8674(00)80751-5. [DOI] [PubMed] [Google Scholar]
- 52.Post L E, Strychatz G D, Nomura M, Lewis H, Dennis P P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit b in Escherichia coli. Proc Natl Acad Sci USA. 1979;76:1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rebois R V, Fishman P H. Deglycosylated human chorionic gonadotropin. J Biol Chem. 1983;258:12775–12778. [PubMed] [Google Scholar]
- 54.Richert N D, Ryan R J. Specific gonadtropin binding to Pseudomonas maltophilia. Proc Natl Acad Sci USA. 1977;74:878–882. doi: 10.1073/pnas.74.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segaloff D L, Ascoli M. The lutropin/choriogonadotropin receptor … 4 years later. Endocrine Rev. 1993;14:324–348. doi: 10.1210/edrv-14-3-324. [DOI] [PubMed] [Google Scholar]
- 56.Seifert H S, Ajioka R S, Marchal C, Sparling P F, So M. DNA transformation leads to pilin antigenic variation in Neisseria gonorrhoeae. Nature. 1988;336:392–395. doi: 10.1038/336392a0. [DOI] [PubMed] [Google Scholar]
- 57.Seifert H S, Wright C J, Jerse A E, Cohen M S, Cannon J G. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Investig. 1994;93:2744–2749. doi: 10.1172/JCI117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw J H, Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988;56:1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spence J M, Chen J C-R, Clark V L. A proposed role for the lutropin receptor in contact-inducible gonococcal invasion of Hec1B cells. Infect Immun. 1997;65:3736–3742. doi: 10.1128/iai.65.9.3736-3742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swanson J. Studies on gonococcal infection XII. Colony color and opacity variants of gonococci. Infect Immun. 1978;19:320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka S, Matsushita Y, Yoshikawa A, Isono K. Cloning and molecular characterization of the gene rimL which encodes an enzyme acetylating ribosomal protein L12 of Escherichia coli K-12. Mol Gen Genet. 1989;217:289–293. doi: 10.1007/BF02464895. [DOI] [PubMed] [Google Scholar]
- 62.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Putten J P M, Duensing T D, Carlson J. Gonococcal invasion of epithelial cells driven by P.IA, a bacterial ion channel with GTP binding properties. J Exp Med. 1998;188:941–952. doi: 10.1084/jem.188.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Putten J P M, Grassme H U C, Robertson B D, Schwan E T. Function of lipopolysaccharide in the invasion of Neisseria gonorrhoeae into human mucosal cells. Prog Clin Biol Res. 1995;392:49–58. [PubMed] [Google Scholar]
- 65.van Putten J P M, Paul S M. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry in to human mucosal cells. EMBO J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Virji M, Makepeace K, Ferguson D J P, Watt S M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 67.Waldbeser L S, Ajioka R S, Mera A J, Puaol D, Lin L, Thomas M, So M. The opaH locus of Neisseria gonorrhoeae MS11A is involved in epithelial cell invasion. Mol Microbiol. 1994;13:919–928. doi: 10.1111/j.1365-2958.1994.tb00483.x. [DOI] [PubMed] [Google Scholar]
- 68.Weel J F L, Hopman C T P, van Putten J P M. In situ expression and localization of Neisseria gonorrhoeae opacity proteins in infected epithelial cells: apparent role of Opa proteins in cellular invasion. J Exp Med. 1991;173:1395–1405. doi: 10.1084/jem.173.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson C A, Leigh A J, Chapman A J. Gonadotrophin glycosylation and function. J Endocrinol. 1990;125:3–14. doi: 10.1677/joe.0.1250003. [DOI] [PubMed] [Google Scholar]
- 70.Yates J L, Dean D, Strycharz W A, Nomura M. E. coli ribosomal protein L19 inhibits translation of L10 and L7/L12 mRNAs by acting at a single site. Nature. 1981;294:190–192. doi: 10.1038/294190a0. [DOI] [PubMed] [Google Scholar]
- 71.Yura T, Ishihama A. Genetics of bacterial RNA polymerases. Annu Rev Genet. 1979;13:59–97. doi: 10.1146/annurev.ge.13.120179.000423. [DOI] [PubMed] [Google Scholar]