Abstract
Purpose
The autonomic nervous system may mediate acute apnoea–induced atrial fibrillation (AF). We compared cardiac autonomic function in paroxysmal atrial fibrillation (PAF) patients with and without obstructive sleep apnoea (OSA).
Methods
Case control study of 101 patients with PAF recruited at two tertiary centres. All patients underwent in-laboratory polysomnography. ECG signal demonstrating “steady state” sinus rhythm (i.e. with arrhythmic beats and respiratory events excluded) was included in the analysis. Cardiac autonomic function was assessed via measures of heart rate variability (HRV) and reported by sleep stage (REM vs Non-REM) for patients with and without OSA.
Results
Sixty-five (66.3%) of patients were male, mean age 61.5 ± 11.6 years, mean BMI 27.1 ± 4.3 kg/m2. Global measures of HRV (triangular index, total power) did not differ between PAF patients with and without OSA in either REM or non-REM sleep. Frequency-domain analysis during non-REM sleep in PAF patients with OSA showed increased cardiac parasympathetic modulation (HF-nu: 39.1 ± 15.7 vs 48.0 ± 14.6, p = 0.008) and reduced cardiac sympathetic modulation (LF-nu 54.1 ± 19.7 vs 43.7 ± 18.0, p = 0.012, LF/HF ratio: 2.1 ± 2.0 vs 1.2 ± 1.0, p = 0.007). Results remained significant after adjusting for age, sex, and BMI (adjusted p values 0.024, 0.045 and 0.018 respectively). There were no differences in HRV parameters during REM sleep.
Conclusions
This is the first study of HRV in PAF patients with and without OSA. Our results indicate limited differences in HRV between groups. However, this work suggests a chronic increase in parasympathetic nervous modulation and relative reduction in sympathetic modulation in PAF patients with OSA during steady-state non-REM sleep.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10840-022-01202-3.
Keywords: Heart rate variability, Cardiac autonomic function, Atrial fibrillation, Obstructive sleep apnoea
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with increased risk of stroke and congestive heart failure [1]. Mounting evidence suggests that dysregulation of the cardiac autonomic axis plays an integral role in arrhythmogenesis [2].
OSA is a highly prevalent sleep disorder characterised by upper airway collapse during sleep and is found in up to 63% of AF patients [3]. Attempting to breathe against an obstructed upper airway results in intermittent hypoxia, intra-thoracic pressure swings and activation of the autonomic nervous system; these acute perturbations are thought to trigger and maintain episodes of AF [4, 5], and may lead to long-term atrial remodelling [5, 6].
Understanding the influence of OSA on autonomic function in patients with AF may inform treatment strategies that mitigate pro-arrhythmic autonomic influences. Heart rate variability (HRV) reflects beat-to-beat variation in heartbeat intervals influenced by the combined effects of the sympathetic and parasympathetic nervous system [7]. The study of HRV provides a non-invasive method to assess cardiac autonomic function [7]. We aimed to assess whether in a paroxysmal atrial fibrillation (PAF) cohort the presence of OSA is associated with altered autonomic function. We hypothesised that PAF patients with OSA will show altered HRV parameters indicative of the influence of OSA on cardiac autonomic function.
Methods
Study population
Sequential AF patients were recruited via two centres as part of a prospective diagnostic accuracy study for sleep apnoea in patients with AF [3]. Approval for this trial was obtained from the Northern Sydney Local Health District Human Research Ethics Committee (HREC/16/HAWKE/25) and the North Shore Private Hospital Ethics Committee (approval number 2016–012). The study was performed in accordance with the 1964 Helsinki Declaration and its later amendments. All the patients gave their informed written consent to participate in the study. The trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR): 12,616,001,016,426.
The patients were sequentially recruited via two pathways: emergency department admissions with AF and pulmonary vein isolation waitlists at two tertiary centres between July 2016 and September 2019. All the patients had a history of AF (≥ 2 episodes in the past 12 months) and underwent in-laboratory polysomnography (PSG) to investigate OSA. The patients with a previous known diagnosis of sleep apnoea were excluded.
Data collection
Polysomnography
Polysomnographic recordings were performed and scored by experienced sleep scientists using Compumedics PSG4 V4.1 software (Compumedics, Australia), according to the American Academy of Sleep Medicine (AASM) criteria [8]. An apnoea was defined as complete (≥ 90%) reduction in airflow, lasting ≥ 10 s. A hypopnea was defined as a partial (≥ 30%) reduction in airflow, lasting ≥ 10 s, associated with either an arousal from sleep or an oxygen desaturation of ≥ 3% from baseline. Sleep apnoea was defined as the average number of apnoea and/or hypopneas per hour (apnoea hypopnea index (AHI)) ≥ 5/h. Other standard parameters of OSA severity were generated including ODI (oxygen desaturation index): average number of desaturations per hour > 3% below baseline, and %T < 90: the percent of sleep time with SaO2 < 90%. OSA severity was defined as mild (AHI ≥ 5–14.9/h), moderate (AHI ≥ 15–29.9/h) and severe (AHI ≥ 30/h).
Holter monitor processing
Electrocardiographic (ECG) signals from the polysomnographic recording were processed using Holter software analysis for ectopic beat detection (SpaceLabs Sentinel v11.5.1.12779 and Pathfinder SL version 1.9.2.11104, Snoqualmie, WA 98,065, USA). Three traces were excluded as the ECG quality was insufficient for Holter software analysis. Since heart rate variability (HRV) is ordinarily performed during sinus rhythm, six traces were excluded as patients were in AF for the vast majority (≥ 90%) of the study night. The patients with shorter runs of AF were included, though the periods of AF were excluded from the analysis (three patients, with 1.8, 22.4 and 3.1% of the night spent in AF, respectively).
Heart rate variability analysis
Following Holter analysis, ECG signals were analysed following the guidelines of Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [7]. QRS detection was performed to a resolution of 1 ms (equivalent to a sample rate of 1000 Hz). HRV analysis was performed using a validated algorithm [9] using MATLAB 2017, version 9.2.0.538062 (R2017a), Natick, Massachusetts: The MathWorks Inc. analysis was performed over 2-min epochs averaged across each sleep stage (NREM and REM) of the entire ECG signal. Epochs with mixed sleep stages were excluded, as were periods of arousal, apnoeas, hypopneas, respiratory event-related arousals as well as artefact as per previously published [10–12]. A 15-s interval following obstructive respiratory events was excluded to control for acute post-event autonomic perturbations. Furthermore, periods of cardiac arrhythmia including atrial and ventricular ectopic beats were excluded from the HRV analysis (2.65% of total beats). If the excluded periods of an epoch exceeded 12 s (10% of epoch length), then the complete epoch was excluded. The patients were excluded from further analysis if > 90% of 2-min epochs were excluded on the basis of arrhythmia or sleep apnoea events (3 patients). A total of 89 studies were therefore included in the HRV analysis (Fig. 1).
Fig. 1.
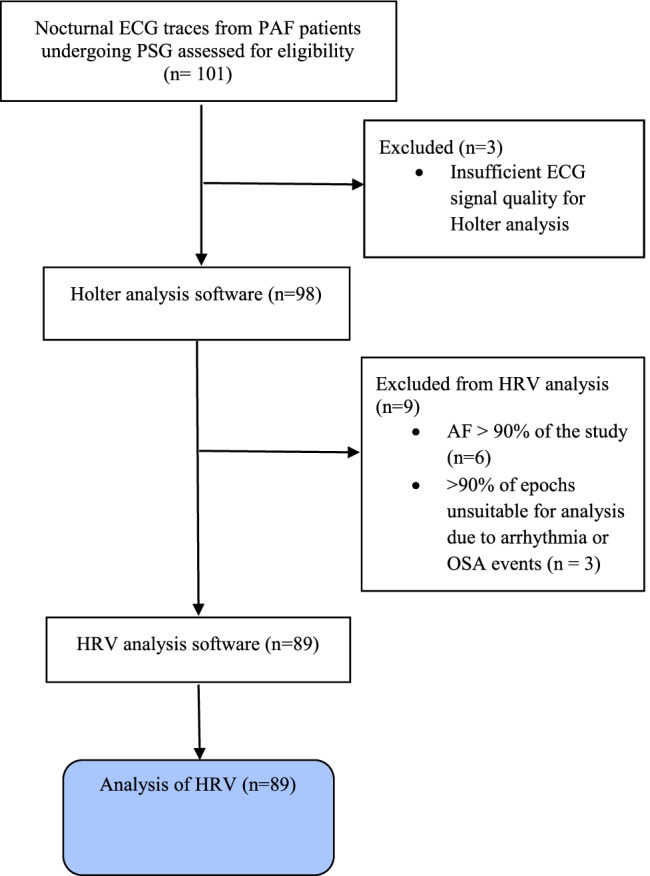
Study flowchart
Time and frequency-domain measures of HRV
Time-domain measures of HRV provide the simplest of method to evaluate variations in heart rate. These include (a) HRV triangular index (HRVi) indicative of overall HRV, (b) RMSSD which is the root mean square of successive differences in NN intervals and (c) pNN50, the proportion of successive NN intervals which differ by more than 50 ms. Both RMSSD and pNN50 are short-term measures and are estimates of high frequency variations in heart rate; these measures reflect parasympathetic modulation of the heart [7, 13].
Frequency-domain measures of HRV provide information on sympathetic and parasympathetic modulation of beat-to-beat fluctuations in the heart rate. An analysis was performed using a Lomb periodogram method, and the spectral bands for HRV were investigated in the range of 0–0.4 Hz: very low frequency (VLF) at 0 to 0.04 Hz, low frequency (LF) at 0.04–0.15 Hz and high frequency (HF) power at 0.15 to 0.4 Hz [7]. LF and HF were also expressed in normalised units, LF-nu and HF-nu, respectively [7]. The HF component of HRV, synchronous with respiration, is considered a strong marker of parasympathetic modulation [7]. The interpretation of the LF component of HRV is more controversial; however, when expressed in normalised units (nu), it is a marker of sympathetic modulation. We have applied this methodology in evaluating the level of sympathetic and parasympathetic modulation at the atrial site [7]. A summary of time and frequency domain–HRV variables is provided in Table 1.
Table 1.
Summary of time and frequency domain–HRV parameters
| HRV parameter (units) | Description |
|---|---|
| Time domain | |
| NN interval (ms) | Interval between R wave peaks for two normal successive beats |
| Average NN interval (ms) | Average time interval between normal successive R waves |
| RMSSD (ms) | Square root of the mean squared differences of successive NN intervals |
| pNN50 (%) | Proportion of NN intervals which differ by more than 50 ms |
| Triangular index (nu) | Integral of the density of the RR interval histogram divided by its height, a measure of total HRV |
| Frequency domain | |
| High frequency (ms2) | Power in the 0.15–0.40-Hz band |
| High frequency–normalised units (%) | HF power divided by power ≥ 0.04 Hz |
| Low frequency (ms2) | Power in the 0.04–0.15-Hz band |
| Low Frequency–normalised units (%) | LF power divided by power ≥ 0.04 Hz |
| Very low frequency (ms2) | Power less than 0.04 Hz |
| LF/HF ratio (nu) | Low frequency power divided by high frequency power |
| Total power | Power from 0 to Nyquist frequency |
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 27.0, Armonk, NY: IBM Corp. HRV parameters which were not normally distributed were natural-log transformed to normalise their skewed distributions. Data are presented as the mean + / − standard deviation (SD), or as mean (interquartile range (IQR)) for non-normally distributed data. HRV parameters were compared using t-tests (continuous variables) or chi-square tests (categorical variables) as appropriate. Analysis of covariance (ANCOVA) was performed to adjust for age, sex and BMI. Correlations between HRV parameters and standard measures of OSA severity (AHI, ODI and T < 90%) were performed. This was an exploratory analysis of the comparison of multiple HRV parameters reflecting different aspects of ANS modulation; therefore, we did not adjust the significance level for multiple comparisons. We considered a p value of < 0.05 statistically significant.
Results
Patient characteristics
A total of 101 AF patients underwent overnight polysomnography. The patients with OSA demonstrated a higher BMI and were predominantly male compared to the no-OSA group. They also had increased hypertension but reduced thyroid disease and peripheral vascular disease. There were no significant differences in other cardiovascular comorbidities including diabetes, ischaemic heart disease (IHD), congestive cardiac failure (CCF) and cerebrovascular disease (CVD), although the OSA group did have a lower ejection fraction (55.5 ± 9.7 vs 59.6 ± 1.1%, p = 0.027). The OSA group also had an increased left atrial area (25.5 ± 5.3 vs 22.3 ± 4.5 cm2, p = 0.032) and increased proportion of “high burden AF”, defined as > 12 self-reported episodes in the last 12 months. CHA2DS2-Vasc scores were not significantly different between the groups (1.4 ± 1.2 vs 1.7 ± 1.4, p = 0.270). Neither were there significant differences in anti-arrhythmic medications between groups. Although nine patients were excluded from the final HRV analysis, excluded patients were not significantly different from the group in terms of key baseline characteristics including age, sex and BMI. Baseline characteristics are presented in Table 2.
Table 2.
Baseline characteristics of AF patients with and without OSA (AHI ≥ 5/h)
| Characteristic | N (%) or mean ± SD | |||
|---|---|---|---|---|
| Mean (SD) or n (%) n = 98 | AHI < 5/h n = 36 | AHI ≥ 5/h n = 62 | p value | |
| General demographics | ||||
| Recruitment stream: ER | 54 (55.1) | 19 (52.8) | 35 (56.5) | 0.834 |
| Age (years) | 61.5 ± 11.6 | 58.9 ± 12.5 | 63.0 ± 10.8 | 0.098 |
| Male | 65 (66.3) | 19 (52.8) | 46 (74.2) | 0.045 |
| Ethnicity: Caucasian | 91 (92.9) | 34 (94.4) | 57 (91.9) | 0.384 |
| Phenotypic characteristics | ||||
| BMI (kg/m2) | 27.1 ± 4.3 | 24.7 ± 3.5 | 28.5 ± 4.2 | < 0.001 |
| Neck circumference (cm) | 40.0 ± 4.9 | 38.8 ± 4.7 | 40.7 ± 4.9 | 0.065 |
| Modified Mallampati score | 2.7 ± 0.9 | 2.4 ± 0.9 | 2.8 ± 0.8 | 0.028 |
| Co-morbidities/AF risk factors (n = 99) | ||||
| Alcohol excess (≥ 10 SD/week), n = 105 | 21 (21.4) | 12 (33.3) | 11 (17.8) | 0.136 |
| Thyroid disease | 16 (16.3) | 11 (30.6) | 5 (8.1) | 0.009 |
| Family history of AF | 29 (29.6) | 13 (36.1) | 16 (25.8) | 0.203 |
| Mod-severe MS/prosthetic heart valve | 3 (3.0) | 2 (2.9) | 1 (3.1) | 0.865 |
| Hypertension | 38 (38.8) | 8 (22.2) | 31 (50.0) | 0.010 |
| Diabetes | 4 (4.1) | 2 (5.6) | 3 (4.8) | 0.876 |
| IHD | 4 (4.1) | 1 (2.8) | 3 (8.3) | 0.619 |
| CCF | 18 (18.4) | 5 (13.8) | 13 (21.0) | 0.431 |
| Cerebrovascular disease | 2 (2.0) | 0 (0) | 2 (3.2) | 0.530 |
| Peripheral vascular disease | 3 (3.1) | 3 (8.3) | 0 (0) | 0.047 |
| CHA2DS2-Vasc score | 1.6 ± 1.3 | 1.4 ± 1.2 | 1.7 ± 1.4 | 0.270 |
| Anti-coagulant therapy | 78 (79.6) | 30 (83.3) | 49 (79.0) | 0.792 |
| AF characteristics | ||||
| Paroxysmal | 90 (91.8) | 36 (100.0) | 57 (91.9) | 0.080 |
| High burden (≥ 10 episodes AF last 12 M) | 31 (31.6) | 8 (22.2) | 23 (37.1) | 0.039 |
| Prior PVI (number) | 0.4 ± 0.6 | 0.33 ± 0.05 | 0.40 ± 0.64 | 0.570 |
| Prior cardioversion (number) | 1.0 ± 1.5 | 0.64 ± 1.18 | 1.16 ± 1.57 | 0.086 |
| Anti-arrhythmic therapy | ||||
| Prescribed anti-arrhythmic medication? | 78 (79.6) | 30 (83.3) | 50 (80.6) | 0.794 |
| Number of anti-arrhythmic medications | 1.05 (0.6) | 1.06 (0.63) | 1.06 (0.67) | 0.948 |
| Class 1 anti-arrhythmic | 29 (29.6) | 11 (30.6) | 18 (29.0) | 0.873 |
| Class 2 anti-arrhythmic | 30 (30.6) | 11 (30.6) | 19 (30.6) | 0.993 |
| Class 3 anti-arrhythmic | 28 (28.6) | 9 (0.25) | 19 (30.6) | 0.646 |
| Class 4 anti-arrhythmic | 9 (9.2) | 3 (8.3) | 6 (9.7) | 0.824 |
| Class 5 anti-arrhythmic | 3 (3.1) | 1 (2.8) | 2 (3.2) | 0.901 |
| Echocardiographic parameters (n = 74) | ||||
| Cardiac ejection fraction (%) | 57.1 ± 8.7 | 59.6 ± 1.1 | 55.5 ± 9.7 | 0.027 |
| Left atrial diameter (cm) (n = 53) | 4.1 ± 6.3 | 3.9 ± 0.5 | 4.2 ± 0.7 | 0.105 |
| Left atrial area (cm2) (n = 46) | 24.2 ± 5.2 | 22.3 ± 4.5 | 25.5 ± 5.3 | 0.032 |
| OSA symptoms | ||||
| ESS | 6.2 ± 3.4 | 5.7 ± 3.8 | 6.4 ± 3.1 | 0.349 |
| Self-reported snoring | 64 (65.3) | 18 (50.0) | 46 (74.2) | 0.027 |
| Sleep parameters from PSG | ||||
| AHI (/h) | 13.8 ± 15.9 | 1.7 ± 1.4 | 20.8 ± 16.3 | < 0.001 |
| ODI (/h) | 7.4 ± 10.9 | 0.8 ± 1.5 | 11.2 ± 12.2 | < 0.001 |
| CAI (/h) | 0.6 ± 1.5 | 0.1 ± 1.2 | 0.8 ± 1.8 | 0.018 |
| AI (/h) | 2.4 ± 5.3 | 0.2 ± 0.3 | 3.7 ± 6.3 | < 0.001 |
| HI (/h) | 11.4 ± 13.2 | 1.5 ± 1.4 | 17.1 ± 13.8 | < 0.001 |
| Presence of arrhythmia on PSG | ||||
| AF (% of total beats) | 6.2 ± 23.4 | 2.9 ± 16.6 | 8.1 ± 26.4 | 0.283 |
| SVEB (% of total beats) | 0.6 ± 1.6 | 0.9 ± 2.0 | 0.5 ± 1.4 | 0.136 |
| VEB (% of total beats) | 0.5 ± 1.7 | 0.5 ± 1.4 | 0.4 ± 1.8 | 0.826 |
AF atrial fibrillation, AHI apnoea hypopnea index, BMI body mass index, CAI central apnoea index, CCF congestive cardiac failure, ER emergency room, ESS Epworth Sleepiness Scale, IHD ischaemic heart disease, MS mitral stenosis, ODI oxygen desaturation index, PVI pulmonary vein isolation procedure waitlist, SD standard drinks, SVE supraventricular ectopic beats, VE ventricular ectopic beats
Atrial fibrillation characteristics
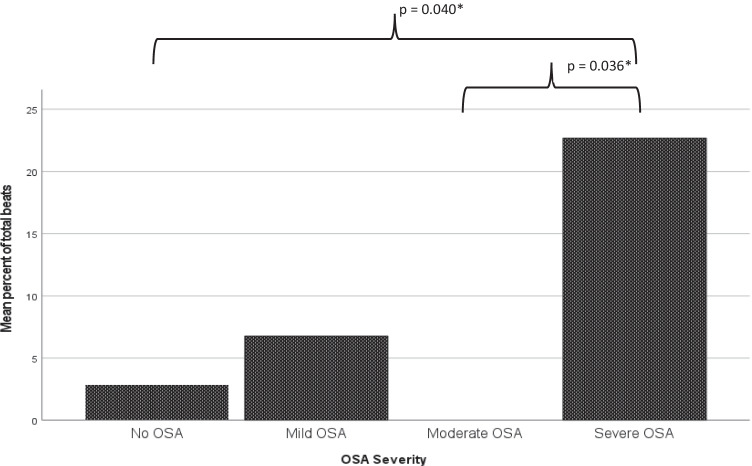
There were no significant differences in the presence of arrhythmia (AF beats, ventricular ectopic beats (VEBs) and supraventricular ectopic beats (SVBs)) between AF patients with and without OSA using a cut-off AHI of 5/h (see Table 2). On subgroup analysis, however, PAF patients with severe OSA (AHI ≥ 30/h) had more AF beats and ventricular ectopic beats (VEBs) than those without severe OSA (22.7 ± 42.8% vs 3.7 ± 17.9%, p = 0.006, 1.7 ± 3.8 vs 0.3 ± 0.9%, p = 0.004, respectively). Similarly, ANOVA for OSA severity groups showed that PAF patients with severe OSA had a higher % AF beats than patients with no OSA or moderate OSA (mean difference 19.8 ± 7.3%, p = 0.040; 22.7 ± 8.3%, p = 0.036 respectively, see Fig. 2) and that PAF patients with severe OSA had a higher %VEBs than patients with mild or moderate OSA (mean difference 1.6 ± 0.5%, p = 0.019; 1.6 ± 0.6%, p = 0.035, respectively). There were no significant differences in SVEs across OSA severity groups.
Fig. 2.
AF beats as a percent of total beats by OSA severity. Patients with no OSA (AHI ≤ 5/h, n = 36) had 2.9 ± 16.6% AF beats; patients with mild OSA (AHI 5–14.9/h, n = 31) had 6.7 ± 23.6% AF beats; patients with moderate OSA (AHI 15–29.9/h, n = 18) had 0.0 ± 0.0% AF beats, and those with severe OSA (AHI ≥ 30/h, n = 13) had 22.7 ± 42.8% AF beats
Heart rate variability in PAF patients with and without OSA
In non-REM sleep, time-domain measures of HRV did not differ between OSA and no-OSA groups (Table 3). However, we saw selective differences in frequency-domain measures (Table 4). Specifically, PAF patients with OSA showed increased parasympathetic modulation (HF-nu: 48.0 ± 14.6 vs 39.1 ± 15.7, p = 0.01) and reduced sympathetic modulation (LF-nu 43.7 ± 18.0 vs 54.1 ± 19.7, p = 0.01). Consistent with these results, the LF/HF ratio showed a relative decrease in parasympathetic modulation (1.2 ± 1.0 vs 2.1 ± 2.0, p = 0.007) (Table 4). Furthermore, these results remained significant after adjusting for age, sex and BMI.
Table 3.
HRV time–domain parameters by OSA status in a cohort with atrial fibrillation
| All patients | No OSA AHI < 5/h | OSA AHI > 5/h | p value | p value (adjusted for age, sex, BMI) | |
|---|---|---|---|---|---|
| n = 89 | n = 35 | n = 54 | |||
| Average NN interval (ms) | |||||
| Non-REM | 1080.2 ± 162.9 | 1062.0 ± 133.9 | 1091.9 ± 179.4 | 0.401 | 0.475 |
| REM | 1067.2 ± 166.4 | 1041.2 ± 136.7 | 1084.3 ± 182.5 | 0.243 | 0.492 |
| †RMSSD (ms) | |||||
| Non-REM | 28.7 (23.1) | 27.3 (20.7) | 30.9 (23.6) | 0.447 | 0.570 |
| REM | 26.7 (22.7) | 20.4 (20.8) | 25.3 (23.5) | 0.376 | 0.374 |
| †pNN50 (%) | |||||
| Non-REM | 5.5 (14.7) | 4.5 (12.2) | 6.2 (15.9) | 0.063 | 0.061 |
| REM | 2.8 (12.3) | 1.9 (11.1) | 3.3 (17.2) | 0.772 | 0.631 |
| Triangular index (nu) | |||||
| Non-REM | 10.8 ± 4.3 | 10.7 ± 4.4 | 10.8 ± 4.4 | 0.975 | 0.612 |
| REM | 11.7 ± 4.6 | 11.7 ± 4.6 | 11.6 ± 4.7 | 0.885 | 0.541 |
Data are presented as mean ± SD or median (IQR). Natural log-transformed data are indicated by †
Non-REM non-rapid eye movement, REM rapid eye movement, average NN interval average of N wave to N wave variation, RMSSD square root of the mean squared differences of successive NN intervals, pNN50 percentage of successive NN intervals that differ by more than 50 ms, triangular index: integral of the density of the RR interval histogram divided by its height
Table 4.
HRV frequency–domain parameters by OSA status in a cohort with atrial fibrillation
| All patients | No OSA AHI < 5/h | OSA AHI > 5/h | p value | p value (adjusted for age, sex, BMI) | |
|---|---|---|---|---|---|
| n = 89 | n = 35 | n = 54 | |||
| †High frequency (ms2) | |||||
| Non-REM | 333.3 (437.8) | 238.8 (913.9) | 403.5 (723.7) | 0.643 | 0.730 |
| REM | 223.3 (437.8) | 206.6 (344.1) | 239.8 (531.1) | 0.530 | 0.426 |
| High frequency–normalised units (%) | |||||
| Non-REM | 44.5 ± 15.6 | 39.1 ± 15.7 | 48.0 ± 14.6 | 0.008 | 0.024 |
| REM | 37.4 ± 17.2 | 32.6 ± 16.0 | 40.5 ± 17.4 | 0.036 | 0.143 |
| †Low frequency (ms2) | |||||
| Non-REM | 336.1 (863.9) | 339.6 (1155.7) | 327.6 (760.1) | 0.447 | 0.559 |
| REM | 302.9 (817.5) | 358.5 (1103.7) | 298.8 (831.0) | 0.533 | 0.904 |
| Low frequency–normalised units (%) | |||||
| Non-REM | 47.8 ± 19.2 | 54.1 ± 19.7 | 43.7 ± 18.0 | 0.012 | 0.045 |
| REM | 55.2 ± 22.1 | 60.4 ± 20.8 | 51.8 ± 22.5 | 0.076 | 0.228 |
| †Very low frequency (ms2) | |||||
| Non-REM | 470.0 (860.0) | 603.8 (1055.1) | 445.2 (605.6) | 0.135 | 0.148 |
| REM | 865.4 (1322.0) | 954.9 (1548.9) | 826.5 (1307.6) | 0.417 | 0.663 |
| LF/HF ratio (nu) | |||||
| Non-REM | 1.5 ± 1.5 | 2.1 ± 2.0 | 1.2 ± 1.0 | 0.007 | 0.018 |
| REM | 2.3 ± 2.2 | 2.9 ± 2.6 | 2.0 ± 1.9 | 0.063 | 0.108 |
| †Total power | |||||
| Non-REM | 1352.7 (2078.9) | 1183.2 (1514.3) | 1109.0 (1573.3) | 0.526 | 0.987 |
| REM | 1128.2 (1498.7) | 1439.0 (2360.6) | 1318.0 (2137.8) | 0.666 | 0.988 |
Data are presented as mean (SD) or median (IQR). Natural log-transformed data are indicated by †. High frequency: power in the 0.15–0.40 Hz band; high frequency–normalised units: HF power divided by power ≥ 0.04 Hz; low frequency: power in the 0.04–0.15 Hz band; low frequency–normalised units: LF power divided by power ≥ 0.04 Hz; very low frequency: power less than 0.04 Hz; LF/HF, low frequency/high frequency ratio: low frequency power divided by high frequency power; total power: power from 0 to Nyquist frequency
In REM sleep, time-domain measures of HRV did not differ between OSA and no-OSA groups (AHI ≥ or < 5/h) (Table 3) in PAF patients. PAF patients with OSA showed increased parasympathetic modulation (HF-nu 32.6 ± 16.0 vs 40.5 ± 17.4, p = 0.036); however, significance was not maintained after adjusting for age, sex and BMI (Table 4). We extended the analysis to include OSA at different severity levels (AHI < or > 15/h, AHI < or > 30/h), although the above changes were not significant in these groups (see Tables S3, S4, S5 and S6 in the supplemental material).
HRV correlations with markers of OSA
We examined correlations between HRV parameters and AHI, as well as other markers of OSA severity, including those that reflect hypoxic burden (ODI and %T < 90). In REM sleep, there was a weak negative correlation between LF-nu and all markers of OSA severity (AHI, ODI and %T < 90). Correlation analysis of other HRV (time and frequency) measures with OSA severity metrics were not significant (Supplementary material, Table S1 and S2).
In non-REM sleep, we saw a weak negative correlation between average NN interval and ODI as well as %T < 90. Correlations of other time and frequency HRV measures with markers of OSA severity (AHI, ODI and T < 90%) were not significant. (Supplementary material, Tables S1 and S2).
Discussion
To our knowledge, this is the first study to compare HRV parameters in PAF patients with and without OSA. We found some evidence that PAF patients with OSA showed increased cardiac parasympathetic modulation (HF-nu) and blunted cardiac sympathetic modulation (LF-nu and LF/HF ratio) compared to PAF patients without OSA. The pathophysiological mechanism behind this finding needs further investigation but may provide future avenues for anti-arrhythmic therapeutic research. That these findings were limited to non-REM sleep is not surprising, given that REM sleep is a time of cardiovascular instability which may potentially mask differences in HRV between the groups.
Overall HRV (HRVi and total power) did not differ between PAF patients with and without OSA. Reduced overall HRV reflects a less adaptable ANS and is a strong independent predictor of mortality, in particular after myocardial infarction [14–22] and congestive heart failure [23–27]. Similarly, studies in AF patients show an association between depressed overall HRV and adverse outcome [28–33]. According to the Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology, a triangular index < 15 indicates a severely depressed sinus node activation. In our entire cohort of AF patients, the triangular index was, perhaps not surprisingly, well below this critical number (during non-REM and REM sleep), although it was similar between PAF patients with and without OSA.
Experimental studies indicate changes in the ANS and play a critical role in facilitating arrhythmic events and that concomitant modifiable risk factors such as OSA may further trigger AF [5]. Our short-term HRV analysis indicates there were no differences in overall HRV in PAF patients with and without OSA in any of the sleep stages. These results are in line with our recent systematic review that revealed nocturnal short-term measures of overall HRV were similar between patients with and without OSA [34] and therefore may extend to patient populations with PAF.
OSA events are well known to precipitate acute autonomic responses. For example, the initial apnoeic period is characterised by vagally-driven bradycardia, followed by a sympathetically-driven surge in heart rate and blood pressure with an accompanying arousal at the conclusion of the apnoeic event [35]. In this study, we deliberately excluded OSA events and the immediate post-apnoeic period (15 s) from the analysis in order to exclude the acute autonomic perturbations that accompany these events. This was done in order to compare chronic autonomic changes between the groups during a period of “steady state” sinus rhythm. Accordingly, we used short-term measures of HRV with a 2-min epoch. This was designed to maximise the availability of steady state ECG available for analysis, due to the frequency of excluded arrhythmic and respiratory events.
Additionally, particular anti-arrhythmic medications including Flecainide (class 1c) and β-blockers (class 11) are known to impact HRV through their effect on the ANS. For example, Flecainide has been shown to reduce HRV time–domain parameters [36]. In our study, the use of anti-arrhythmic medications in each individual class was not significantly different between the two groups, though the dosage and administration times were not measured. Furthermore, certain co-morbidities including acute myocardial infarction, diabetic neuropathy, heart transplantation and tetraplegia are known to significantly alter the function of the autonomic nervous system and hence HRV[36]. In our study, we corrected for the effect of age, sex and BMI. Most measured co-morbidities were not significantly different between groups, with the exception of hypertension, thyroid disease and peripheral vascular disease (see Table 2). Little is known about the influence of these particular conditions on HRV. However, one study demonstrated an increase in time domain and frequency domain–HRV parameters in AF patients with hypertension compared to patients with hypertension alone [37].
Several physiological studies demonstrated the importance of the autonomic nervous system in mediating sleep apnoea–induced AF. For example, vagal activation during the intra-thoracic pressure changes caused by acute apnoeic events shortens the atrial effective refractory period, thus increasing AF inducibility [38]. In a dog-model, Ghias et al. showed that after ablation of cardiac parasympathetic innervation, there was a significant decrease in apnoea-induced AF. This also occurred with sympatho-vagal blockade [39]. Similarly, Linz et al. showed in a pig model that the application of negative tracheal pressure induced AF via a shortening of the atrial refractory period and that this effect was negated by parasympathetic deactivation, either in the form of atropine administration or vagotomy [38]. During an acute obstructive apnoea, the profound vagal activation followed by combined sympathetic activation is thought to trigger and maintain AF. Experimental studies in chronic intermitted hypoxia show AF vulnerability and depends principally on parasympathetic activation; furthermore, parasympathetic activation has been identified as the major pro-arrhythmogenic mechanism in the rodent model [40]. Our data are line with this body of work, where PAF patients with OSA show increased parasympathetic modulation compared to PAF patients without OSA.
In addition to a major parasympathetic component, the sympathetic nervous system is also likely to contribute to AF promotion. However, in our study of PAF patients, we did not see elevated sympathetic modulation. Rather, our results suggest a blunted cardiac sympathetic modulation in PAF patients with OSA compared to PAF patients without OSA. This is somewhat surprising given that elevated sympathetic activity is well documented in OSA [34] and chronic intermittent hypoxia [41, 42]. However, one study in rats exposed to chronic intermittent hypoxia demonstrated elevated AF vulnerability that was accompanied by an elevated cholinergic response and damped beta-adrenergic response of the atrial myocardium [40]. It is possible that sympathetic activation maybe less important compared to parasympathetic activation in promoting AF due to elevated spatial dispersion of atrial refractoriness during parasympathetic activation [43]. Furthermore, the blunted sympathetic modulation in PAF patients with OSA in our study maybe associated with a ceiling effect driven by higher intrinsic adrenergic tone [40].
Arrhythmia analysis
The study methodology provided an opportunity to compare the presence of nocturnal arrhythmia between AF patients with and without OSA, although this was not a primary aim of the study. On patient recall at interview, the patients in the OSA group reported a higher incidence of “high burden” AF, defined as ≥ 10 episodes in the past 12 months (8/36 patients (22%) vs 23/62 patients (37.1%), p = 0.039. On the sleep study night, there was no significant difference in % AF beats between the OSA and no-OSA groups, although the trend towards increased %AF beats in the OSA group was noted (2.9 ± 16.6 vs 8.1 ± 26.4%, p = 0.283. On subgroup analysis, however, the patients with severe OSA (AHI > 30/h) had more AF beats and more ventricular ectopic beats, but not supraventricular ectopic beats on nocturnal polysomnography. Similarly, PAF patients with severe OSA had a higher % AF beats than patients with no OSA or moderate OSA (mean difference 19.8 ± 7.3%, p = 0.040; 22.7 ± 8.3%, p = 0.036, respectively, Fig. 2). These findings of higher AF burden according to OSA severity are consistent with the findings of Mehra et al. [44], showing that nocturnal arrhythmia including AF and VEBs were more common in patients with severe sleep-disordered breathing, also using a cut-off of AHI > 30/h. To our knowledge, our study is the first to replicate this finding in a cohort of patients with PAF with and without OSA.
Limitations
Although this study provides some novel insights into the HRV profile of those with OSA and PAF, there are limitations to the study. Twenty-four hour Holter recording is ideal for HRV analysis, accounting for both diurnal and nocturnal variability [7]. For this study, HRV parameters were derived from nocturnal polysomnograpy and thus are subject to all the usual autonomic perturbations of sleep, which may explain the selective differences seen across time and frequency-domain measures. Furthermore, we were unable to control for differences in undiagnosed conduction disturbances between the two groups, including, for example, the presence of sinus nodal disease which has a high prevalence in AF patients [45]. However, we analysed only periods of sinus rhythm in order to minimise the contribution of AV nodal dysfunction. Our study contained some patients who had undergone previous PVI: patients had, on average, undergone 0.4 ± 0.6 previous PVI procedures. Since PVI may cause neuronal damage to the intrinsic cardiac nervous system [46], caution must be used when extrapolating the results to other groups. Although we excluded periods of arrhythmia, it is possible that autonomic disturbances related to the arrhythmia may have preceded or persisted beyond these events. It is also possible that HRV parameters may have been impacted by autonomic disruptions from acute obstructive respiratory events in the OSA group. We attempted to allow for this by excluding ECG trace during and immediately following sleep apnoea events from the analysis; however, we excluded a post-event period of 15 s, and it is possible autonomic disturbance may persist beyond this interval. In addition, some sub-criterion respiratory events are likely to have remained in the analysis.
Clinical implications
There is mounting evidence that the perturbations during OSA have a profound influence on the myocardium [6]. Atrial remodelling leading to changes to the electrical conduction and ANS activation is thought to trigger and maintain AF [6]. Our work shows altered autonomic function in PAF patients with co-morbid OSA which we believe supports previous observations that progression of AF is promoted by the presence of modifiable risk factors such as OSA [5, 6]. Treatment of OSA may modulate autonomic function and protect the atrial myocardium from pro-arryhthmic autonomic influences from OSA. Therefore, future studies should look to replicate our findings in a larger cohort and determine the effect of OSA therapy on modulation of the ANS and whether indeed such interventions may mitigate arrhythmogenesis in PAF.
Conclusions
This is the first study to compare sympatho-vagal balance, assessed by HRV, in PAF patients with and without OSA. Our results indicate limited differences in HRV between groups. However, we found some evidence of increased parasympathetic modulation and decreased sympathetic modulation in the OSA group. Altered autonomic function in this group may promote arrhythmogenesis and impair antiarrhythmic therapy. Elucidating influence of OSA on autonomic function in patients with AF may inform treatment strategies that mitigate pro-arrhythmic autonomic influences. Future studies should look to replicate our findings in a larger cohort and determine the effect of OSA therapy on modulation of the ANS.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions AMM received research grants from Ramsay Research and Teaching Fund, Australia and ResMed Foundation/Sleep Health Foundation Research Entry Scholarship/Royal Australasian College of Physicians.
Declarations
Ethical approval
Approval for this trial was obtained from the Northern Sydney Local Health District Human Research Ethics Committee (HREC/16/HAWKE/25) and the North Shore Private Hospital Ethics Committee (approval number 2016–012). The study was performed in accordance with the 1964 Helsinki Declaration and its later amendments. The trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR): 12616001016426.
Informed consent
All the patients gave their informed written consent to participate in the study.
Competing interests
AMM reports in-kind support from Resmed Pty Ltd, Australia and Somnomed Australia.
PAC has an appointment to an endowed academic Chair at the University of Sydney that was created from ResMed funding. He has received research support from ResMed, SomnoMed, Zephyr Sleep Technologies and Bayer. He is a consultant/adviser to Zephyr Sleep Technologies, ResMed, SomnoMed and Signifier Medical Technologies. He has a pecuniary interest in SomnoMed related to a previous role in R&D (2004).
KS reports in-kind support from SomnoMed for a previous investigator-initiated research study. PdeC holds an endowed academic chair at the University of Sydney, established through funding from ResMed and has received research support from ResMed, SpaceLabs and Somnomed. HUD has received funding from the ResMed Foundation.
Footnotes
Anna Mary Mohammadieh and Hasthi U. Dissanayake are joint first authors.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hämmerle P, Eick C, Blum S, Schlageter V, Bauer A, Rizas KD, et al. Heart rate variability triangular index as a predictor of cardiovascular mortality in patients with atrial fibrillation. J Am Heart Assoc. 2020;9(15):e016075. doi: 10.1161/JAHA.120.016075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna P, Buch E, Stavrakis S, Meyer C, Tompkins JD, Ardell JL, et al. Neuroscientific therapies for atrial fibrillation. Cardiovasc Res. 2021;117(7):1732–1745. doi: 10.1093/cvr/cvab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammadieh AM, Sutherland K, Kanagaratnam LB, Whalley DW, Gillett MJ, Cistulli PA. Clinical screening tools for obstructive sleep apnea in a population with atrial fibrillation: a diagnostic accuracy trial. J Clin Sleep Med. 2021;17(5):1015. doi: 10.5664/jcsm.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850. doi: 10.1161/CIRCULATIONAHA.117.029400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linz D, Elliott AD, Hohl M, Malik V, Schotten U, Dobrev D, et al. Role of autonomic nervous system in atrial fibrillation. Int J Cardiol. 2019;287:181–188. doi: 10.1016/j.ijcard.2018.11.091. [DOI] [PubMed] [Google Scholar]
- 6.Linz D, McEvoy RD, Cowie MR, Somers VK, Nattel S, Levy P, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532–540. doi: 10.1001/jamacardio.2018.0095. [DOI] [PubMed] [Google Scholar]
- 7.Electrophysiology TFotESoCtNASoP Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043–65. [PubMed] [Google Scholar]
- 8.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wessel N, Voss A, Malberg H, Ziehmann C, Voss HU, Schirdewan A, et al. Nonlinear analysis of complex phenomena in cardiological data. Herzschrittmacher Elektrophysiol. 2000;11(3):159–173. [Google Scholar]
- 10.Jurysta F, Kempenaers C, Lanquart J-P, Noseda A, Van De Borne P, Linkowski P. Long-term CPAP treatment partially improves the link between cardiac vagal influence and delta sleep. BMC Pulm Med. 2013;13(1):1–11. doi: 10.1186/1471-2466-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palma J-A, Urrestarazu E, Lopez-Azcarate J, Alegre M, Fernandez S, Artieda J, et al. Increased sympathetic and decreased parasympathetic cardiac tone in patients with sleep related alveolar hypoventilation. Sleep. 2013;36(6):933–940. doi: 10.5665/sleep.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palma J-A, Iriarte J, Fernandez S, Alegre M, Valencia M, Artieda J, et al. Long-term continuous positive airway pressure therapy improves cardiac autonomic tone during sleep in patients with obstructive sleep apnea. Clin Auton Res. 2015;25(4):225–232. doi: 10.1007/s10286-015-0297-7. [DOI] [PubMed] [Google Scholar]
- 13.DeGiorgio CM, Miller P, Meymandi S, Chin A, Epps J, Gordon S, et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav. 2010;19(1):78–81. doi: 10.1016/j.yebeh.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 15.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ, Investigators A. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. The Lancet. 1998;351(9101):478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 16.Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 17.Odemuyiwa O, Malik M, Farrell T, Bashir Y, Poloniecki J, Camm J. Comparison of the predictive characteristics of heart rate variability index and left ventricular ejection fraction for all-cause mortality, arrhythmic events and sudden death after acute myocardial infarction. Am J Cardiol. 1991;68(5):434–439. doi: 10.1016/0002-9149(91)90774-f. [DOI] [PubMed] [Google Scholar]
- 18.Martin GJ, Magid NM, Myers G, Barnett PS, Schaad JW, Weiss JS, et al. Heart rate variability and sudden death secondary to coronary artery disease during ambulatory electrocardiographic monitoring. Am J Cardiol. 1987;60(1):86–89. doi: 10.1016/0002-9149(87)90990-8. [DOI] [PubMed] [Google Scholar]
- 19.Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt G, Brugada P, et al. Task force on sudden cardiac death of the European Society of Cardiology. Eur Heart J. 2001;22(16):1374–1450. doi: 10.1053/euhj.2001.2824. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz PJ, La Rovere MT. ATRAMI: a mark in the quest for the prognostic value of autonomic markers. Eur Heart J. 1998;19(11):1593–1595. doi: 10.1053/euhj.1998.1292. [DOI] [PubMed] [Google Scholar]
- 21.Malik M, Farrell T, Cripps T, Camm AJ. Heart rate variability in relation to prognosis after myocardial infarction: selection of optimal processing techniques. Eur Heart J. 1989;10(12):1060–1074. doi: 10.1093/oxfordjournals.eurheartj.a059428. [DOI] [PubMed] [Google Scholar]
- 22.Myers GA, Martin GJ, Magid NM, Barnett PS, Schaad JW, Weiss JS, et al. Power spectral analysis of heart rate variability in sudden cardiac death: comparison to other methods. IEEE Trans Biomed Eng. 1986;12:1149–1156. doi: 10.1109/TBME.1986.325694. [DOI] [PubMed] [Google Scholar]
- 23.Wijbenga JAM, Balk A, Meij SH, Simoons ML, Malik M. Heart rate variability index in congestive heart failure: relation to clinical variables and prognosis. Eur Heart J. 1998;19(11):1719–1724. doi: 10.1053/euhj.1998.1148. [DOI] [PubMed] [Google Scholar]
- 24.Binder T, Frey B, Porenta G, Heinz G, Wutte M, Kreiner G, et al. Prognostic value of heart rate variability in patients awaiting cardiac transplantation. Pacing Clin Electrophysiol. 1992;15(11):2215–2220. doi: 10.1111/j.1540-8159.1992.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 25.Brouwer J, van Veldhuisen DJ, Man In't Veld AJ, Haaksma J, Dijk WA, Visser KR, et al. Prognostic value of heart rate variability during long-term follow-up in patients with mild to moderate heart failure. J Am Coll Cardiol 1996;28(5):1183–9. [DOI] [PubMed]
- 26.Ponikowski P, Anker SD, Chua TP, Szelemej R, Piepoli M, Adamopoulos S, et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79(12):1645–1650. doi: 10.1016/s0002-9149(97)00215-4. [DOI] [PubMed] [Google Scholar]
- 27.Yi G, Goldman JH, Keeling PJ, Reardon M, McKenna WJ, Malik M. Heart rate variability in idiopathic dilated cardiomyopathy: relation to disease severity and prognosis. Heart. 1997;77(2):108–114. doi: 10.1136/hrt.77.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hämmerle P, Eick C, Blum S, Schlageter V, Bauer A, Rizas KD, et al. Heart rate variability triangular index as a predictor of cardiovascular mortality in patients with atrial fibrillation. J Am Heart Assoc. 2020;9(15):e016075. doi: 10.1161/JAHA.120.016075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frey B, Heinz G, Binder T, Wutte M, Schneider B, Schmidinger H, et al. Diurnal variation of ventricular response to atrial fibrillation in patients with advanced heart failure. Am Heart J. 1995;129(1):58–65. doi: 10.1016/0002-8703(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 30.Stein KM, Borer JS, Hochreiter C, Devereux RB, Kligfield P. Variability of the ventricular response in atrial fibrillation and prognosis in chronic nonischemic mitral reurgitation. Am J Cardiol. 1994;74(9):906–911. doi: 10.1016/0002-9149(94)90584-3. [DOI] [PubMed] [Google Scholar]
- 31.Yamada A, Hayano J, Sakata S, Okada A, Mukai S, Ohte N, et al. Reduced ventricular response irregularity is associated with increased mortality in patients with chronic atrial fibrillation. Circulation. 2000;102(3):300–306. doi: 10.1161/01.cir.102.3.300. [DOI] [PubMed] [Google Scholar]
- 32.Cygankiewicz I, Corino V, Vazquez R, Bayes-Genis A, Mainardi L, Zareba W, et al. Reduced irregularity of ventricular response during atrial fibrillation and long-term outcome in patients with heart failure. Am J Cardiol. 2015;116(7):1071–1075. doi: 10.1016/j.amjcard.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 33.Platonov PG, Holmqvist F. Atrial fibrillatory rate and irregularity of ventricular response as predictors of clinical outcome in patients with atrial fibrillation. J Electrocardiol. 2011;44(6):673–677. doi: 10.1016/j.jelectrocard.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Dissanayake HU, Bin YS, Ucak S, de Chazal P, Sutherland K, Cistulli PA. Association between autonomic function and obstructive sleep apnea: a systematic review. Sleep Med Rev. 2021;57:101470. doi: 10.1016/j.smrv.2021.101470. [DOI] [PubMed] [Google Scholar]
- 35.Smith RP, Veale D, Pépin JL, Lévy PA. Obstructive sleep apnoea and the autonomic nervous system. Sleep Med Rev. 1998;2(2):69–92. doi: 10.1016/s1087-0792(98)90001-6. [DOI] [PubMed] [Google Scholar]
- 36.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–81. [PubMed]
- 37.Khan AA, Junejo RT, Thomas GN, Fisher JP, Lip GYH. Heart rate variability in patients with atrial fibrillation and hypertension. Eur J Clin Invest. 2021;51(1):e13361. doi: 10.1111/eci.13361. [DOI] [PubMed] [Google Scholar]
- 38.Linz D, Schotten U, Neuberger HR, Bohm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011;8(9):1436–1443. doi: 10.1016/j.hrthm.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 39.Ghias M, Scherlag BJ, Lu Z, Niu G, Moers A, Jackman WM, et al. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol. 2009;54(22):2075–2083. doi: 10.1016/j.jacc.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Bober SL, Ciriello J, Jones DL. Atrial arrhythmias and autonomic dysfunction in rats exposed to chronic intermittent hypoxia. Am J Physiol-Heart Circ Physiol. 2018;314(6):H1160–H1168. doi: 10.1152/ajpheart.00173.2017. [DOI] [PubMed] [Google Scholar]
- 41.Zoccal DB, Bonagamba LGH, Oliveira FRT, Antunes-Rodrigues J, Machado BH. Increased sympathetic activity in rats submitted to chronic intermittent hypoxia. Exp Physiol. 2007;92(1):79–85. doi: 10.1113/expphysiol.2006.035501. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Martin MC, Vega-Agapito V, Prieto-Lloret J, Agapito MT, Castaneda J, Gonzalez C. Effects of intermittent hypoxia on blood gases plasma catecholamine and blood pressure. Arterial Chemoreceptors: Springer; 2009. pp. 319–328. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol-Heart Circ Physiol. 1997;273(2):H805–H816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 44.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.John RM, Kumar S. Sinus node and atrial arrhythmias. Circulation. 2016;133(19):1892–1900. doi: 10.1161/CIRCULATIONAHA.116.018011. [DOI] [PubMed] [Google Scholar]
- 46.Scherschel K, Hedenus K, Jungen C, Lemoine MD, Rübsamen N, Veldkamp MW, et al. Cardiac glial cells release neurotrophic S100B upon catheter-based treatment of atrial fibrillation. Sci Transl Med. 2019;11(493). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.