Vitamin C protects retinal ganglion cells in animal models of optic nerve injury partially by up-regulating the expression of the neuroprotective signaling molecule SPP1.
Abstract
Glaucoma is a common neurodegenerative disorder characterized by retinal ganglion cell death, astrocyte reactivity in the optic nerve, and vision loss. Currently, lowering the intraocular pressure (IOP) is the first-line treatment, but adjuvant neuroprotective approaches would be welcome. Vitamin C possesses neuroprotective activities that are thought to be related to its properties as a co-factor of enzymes and its antioxidant effects. Here, we show that vitamin C promotes a neuroprotective phenotype and increases gene expression related to neurotropic factors, phagocytosis, and mitochondrial ATP production. This effect is dependent on the up-regulation of secreted phosphoprotein 1 (SPP1) in reactive astrocytes via the transcription factor E2F1. SPP1+ astrocytes in turn promote retinal ganglion cell survival in a mouse model of glaucoma. In addition, oral administration of vitamin C lowers the IOP in mice. This study identifies an additional neuroprotective pathway for vitamin C and suggests a potential therapeutic role of vitamin C in neurodegenerative diseases such as glaucoma.
Introduction
Glaucoma refers to a group of diseases that lead to the degeneration of retinal ganglion cells (RGCs), their axons in the optic nerve, and the concomitant decline in visual function (Quigley, 2011). Positive family history, elevated intraocular pressure (IOP), and especially age are the main risk factors (Tham et al, 2014). The only modifiable of these is the IOP, and all current glaucoma therapies aim at lowering the IOP by pharmacological or surgical means (Garway-Heath et al, 2015). However, this is not effective in all cases and visual field defects can progress even despite apparently well-controlled IOP. Therefore, the search for alternative neuroprotective therapeutic approaches is ongoing. In recent years, several studies have demonstrated that this is possible, at least in animal models. Possible approaches are inhibiting the neuroinflammatory response that accompanies glaucomatous changes in the retina and the optic nerve (Bosco et al, 2008; Cueva Vargas et al, 2016), preventing the entry of monocytes from the bloodstream (Howell et al, 2012; Williams et al, 2019), or interfering with the inflammasome pathway (Krishnan et al, 2016, 2019). Other interventions include the administration of neurotropic factors or antioxidants (Pease et al, 2009; Yang et al, 2016; Pham et al, 2022; Lazaldin et al, 2023), improving mitochondrial function (Ju et al, 2022; Quintero et al, 2022), and preventing the detrimental changes that are common to glaucomatous and age-related degeneration of RGCs (Lu et al, 2020; Xu et al, 2022). In addition to pharmacological treatment, some interventions that fall under the category of lifestyle changes show promise in glaucoma therapy. The benefits of physical exercise for slowing ganglion cell degeneration (and, more generally, neurodegeneration in all parts of the CNS) are well documented (Ong et al, 2018; Chu-Tan et al, 2022; Lopez-Ortiz et al, 2022; Zhang et al, 2022). Dietary intake of vitamins may also have a place in the prevention of neurodegeneration. Vitamin B3 (nicotinamide) has been shown to prevent RGC degeneration in the DBA/2J mouse model of hereditary glaucoma (Williams et al, 2017, 2018), and lower serum levels of nicotinamide are found in human patients with primary open-angle glaucoma (Kouassi Nzoughet et al, 2019). Nicotinamide exerts its effect at least in part by enhancing mitochondrial health and oxidative phosphorylation (Tribble et al, 2021a, 2021b). Other vitamins that may have neuroprotective activity in glaucoma are vitamins A (retinoic acid) and C (ascorbic acid) (Ramdas et al, 2018). This suggests that especially dietary and lifestyle factors that influence mitochondrial function—such as vitamin B3 supplementation or exercise—may be effective adjunct therapies for glaucoma (Williams et al, 2017).
We recently found that the overexpression of the cytokine SPP1 (secreted phosphoprotein 1, osteopontin) in the retina and optic nerve head protects RGCs and rescues visual function in mouse models of aging and glaucoma (Li & Jakobs, 2022). We sought to screen and identify small molecules that increase SPP1 expression as an adjuvant glaucoma treatment. Both vitamins A and C have been described to regulate SPP1 expression in osteoblast and other cell types (Harada et al, 1995; Hadzir et al, 2014; Jeradi & Hammerschmidt, 2016; Nam et al, 2019). In addition, the promoter region of Spp1 contains retinoic acid response elements. Vitamin C, an essential nutrient for humans, fulfills a number of physiological functions, the prevention of scurvy being the most well-known. However, recently vitamin C also was found to play an important role in the development of the nervous system (Tveden-Nyborg, 2021; Coker et al, 2022), synaptogenesis (Moretti & Rodrigues, 2021), and oligodendrocyte differentiation (Guo et al, 2018). At least in animal models of neurodegenerative diseases, vitamin C has shown promise as a neuroprotectant (Kangisser et al, 2021).
The evidence for the use of vitamin C as adjuvant therapy in glaucoma is mixed. A number of studies from the 1960s and 1970s reported IOP-lowering effects of intravenous vitamin C, but these effects are most likely due to creating an osmotic gradient between the blood and the intraocular fluids, and not due to the biochemical activities of the vitamin (Linner, 1969; Fishbein & Goodstein, 1972). As the effect only lasts for 12 h, it is of limited practical use. Whether oral vitamin C supplementation has an IOP-lowering effect in humans is controversial (Yuki et al, 2010; Ramdas et al, 2018; Hysi et al, 2019; Han & Fu, 2022). However, independent of IOP, ascorbic acid may be neuroprotective because of its antioxidant properties (Xu et al, 2014) or the activation of ten–eleven translocation enzymes (TETs) (Blaschke et al, 2013).
Here, we report an additional neuroprotective pathway for vitamin C that involves the cytokine SPP1 and the transcription factor E2F1. We show that vitamin C stimulates the astrocytic expression of the SPP1 and induces a neuroprotective phenotype in astrocytes in vitro and in vivo. In addition, vitamin C lowers the IOP and protects RGCs and visual function in a mouse model of glaucoma.
Results
Vitamin C up-regulates SPP1 expression in astrocytes
We recently reported that the cytokine-like protein SPP1 is highly protective of RGCs and visual function in several models of injury (elevated IOP, optic nerve crush, and aging) (Li & Jakobs, 2022). In search of small molecules that can up-regulate SPP1 expression, we identified vitamins A (retinoic acid) and C that have been described to regulate SPP1 expression in osteoblast and other cell types (Harada et al, 1995; Hadzir et al, 2014; Jeradi & Hammerschmidt, 2016; Nam et al, 2019). In addition, the promoter region of Spp1 contains retinoic acid response elements. In cultures of the optic nerve and retinal astrocytes, 1–100 μM retinoic acid up-regulated SPP1 protein expression in a dose-dependent manner (Figs 1A–C and S1A). Spp1 mRNA expression was increased by more than twofold in response to 100 μM retinoic acid (Fig 1D).
Figure 1. Vitamin C increases SPP1 expression via E2F1 in astrocytes in vitro.
(A) SPP1 immunocytochemical staining in C57BL/6 astrocytes treated with 100 μM vitamin A (retinoic acid) for 24 h. (B) Vitamin A increased SPP1 protein expression in a dose-dependent manner in C57BL/6 astrocytes (n = 14–36 cells from multiple independent wells/group). (C) Dose–response curves showed the effect of vitamin A concentration (Log2Dose) on SPP1 protein expression in C57BL/6 astrocytes (n = 14–36 cells from multiple independent wells/group). (D) Spp1 mRNA was increased by 100 μM vitamin A in C57BL/6 astrocytes (n = 4–6). (E) SPP1 immunostaining in C57BL/6 astrocytes treated with 125 μM vitamin C (ascorbic acid) for 24 h. (F) Vitamin C up-regulated SPP1 protein expression in a dose-dependent manner in C57BL/6 astrocytes (n = 27–123 cells from multiple independent wells/group). (G) Dose–response curves showed the effect of vitamin C concentration (Log10Dose) on SPP1 protein expression in C57BL/6 astrocytes (n = 27–123 cells from multiple independent wells/group). (H) Spp1 mRNA was increased by 125 μM vitamin C in C57BL/6 astrocytes (n = 5). (I) SPP1 immunostaining in C57BL/6 astrocytes treated with 62.5 μM NaAscorbate (sodium ascorbate) for 24 h. (J) NaAscorbate increased SPP1 protein expression in a dose-dependent manner in C57BL/6 astrocytes (n = 11–31 cells from multiple independent wells/group). (K) Dose–response curves showed the effect of NaAscorbate concentration (Log10Dose) on SPP1 protein expression in C57BL/6 astrocytes (n = 11–31 cells from multiple independent wells/group). (L) Spp1 mRNA was up-regulated by 62.5 μM NaAscorbate (n = 6). (M) NaAscorbate pH in different concentrations between 31.25 and 1,000 μM showed that the pH was almost neutral from 7.40 to 7.47. (N) NaAscorbate increased Spp1 and E2f1 mRNA expression, not Runx1 in cultured C57BL/6 astrocytes (n = 6). (O) Increased Spp1 mRNA expression by NaAscorbate was blocked by E2F1 inhibition with HLM006474 (n = 6). (B, D, F, H, J, L, O, N) P-values by a one-way ANOVA (B, F, J), an unpaired two-tailed t test (D, H, L, O), and a two-way ANOVA (N), *P < 0.05, **P < 0.01, and ***P < 0.001. Data are the mean ± SEM.
Source data are available for this figure.
Figure S1. Vitamins A and C increase SPP1 expression in astrocytes in vitro.
(A) SPP1 immunostaining in C57BL/6 astrocytes treated with 1, 25, 50, and 100 μM vitamin A (retinoic acid) for 24 h. (B) SPP1 immunostaining in C57BL/6 astrocytes treated with 31.25, 62.5, 125, and 250 μM vitamin C (ascorbic acid) for 24 h. (C) SPP1 immunostaining in C57BL/6 astrocytes treated with 31.25, 62.5, 125, and 250 μM NaAscorbate for 24 h.
We next tested vitamin C and the sodium salt of vitamin C (sodium ascorbate, NaAscorbate). Vitamin C increased SPP1 protein expression in a dose-dependent manner from 31.25 to 250 μM (Figs 1E–G and S1B). We also found a significant increase in Spp1 mRNA after stimulation with vitamin C compared to control conditions (Fig 1H). As vitamin C is a weak acid, we also tested sodium ascorbate. Sodium ascorbate enhanced SPP1 protein expression in a dose-dependent manner from 31.25 to 250 μM (Figs 1I–K and S1C). There was an increased level of SPP1 mRNA in cultured astrocytes treated with 250 μM sodium ascorbate compared to the vehicle group (Fig 1L). The maximum effect on the increase in SPP1 expression was similar between vitamin C and sodium ascorbate, but the dose for the maximum effect was lower in sodium ascorbate (62.5 μM) than that in vitamin C (125 μM). Sodium ascorbate did not significantly change the pH of the cell culture medium, which was 7.40–7.47 for concentrations between 31.25 and 1,000 μM, excluding the effects of pH on gene transcription in astrocytes (Fig 1M).
Because Spp1 gene expression is regulated by transcription factors RUNX1 and E2F1 in astrocytes (Li & Jakobs, 2022), we further sought to test whether these transcription factors are involved in the up-regulation of Spp1 by vitamin C. Sodium ascorbate up-regulated Spp1 and E2f1, but not Runx1, mRNA expression in cultured astrocytes (Fig 1N). The effect on Spp1 expression was dependent on E2F1, as it was blocked by the E2F1 inhibitor HLM006474 (Fig 1O). Taken together, the results demonstrate that vitamin C up-regulated SPP1 expression via E2F1 in astrocytes.
Vitamin C induces neuroprotective astrocytes via SPP1
We found previously that SPP1 induces a neuroprotective phenotype in astrocytes, including the inhibition of neurotoxic mediators, promotion of synaptogenic, phagocytic, and neurotropic markers, and an up-regulation of oxidative phosphorylation and ATP production (Li & Jakobs, 2022). To further understand the molecular mechanism of vitamin C activity in astrocytes, we used qRT-PCR to test whether vitamin C could also induce neuroprotective astrocytes by regulating these pathways via SPP1. Wild-type astrocytes in culture responded to sodium ascorbate with a moderate up-regulation of genes related to synaptogenesis, oxidative phosphorylation, neurotropic factors, and phagocytosis-related genes, and a down-regulation of toxic factor Il1α, whereas these effects were blocked by Spp1 deletion in astrocytes (Fig 2A).
Figure 2. Vitamin C increases Spp1 expression to promote astrocytic neuroprotection.
(A) Heat map of expression levels of genes related to synaptogenesis, neurotoxic factors, phagocytosis, neurotropic factors, and oxidative phosphorylation in cultured astrocytes treated with vitamin C (n = 4–6). The gene levels were assessed by qRT-PCR. The pairwise comparisons are between vehicle and NaAscorbate in cultured C57BL/6 astrocytes, and between NaAscorbate-treated C57BL/6 and NaAscorbate-treated Spp1 KO astrocytes. The asterisks indicate significance with P < 0.05 in the pairwise comparisons. (B) Effects of 62.5 μM NaAscorbate on gene expression related to astrocyte reactivity in C57BL/6 astrocytes, suggesting NaAscorbate increased “A2” genes. (C) Effects of 62.5 μM NaAscorbate on gene expression related to astrocyte reactivity in Spp1 KO astrocytes, suggesting Spp1 deficiency prevented the NaAscorbate-induced increase in “A2” genes. (A) P-values by a multiple unpaired t test (A), *P < 0.05. Data are the mean ± SEM.
Source data are available for this figure.
A recent publication used sets of marker genes to classify states of reactive astrocytes: a set of pan-reactive genes commonly expressed in reactive astrocytes, a set of “A1” genes that correspond to a neurotoxic astrocyte phenotype, and “A2” genes that characterize predominantly neuroprotective astrocytes (Liddelow et al, 2017). In astrocytes treated with sodium ascorbate, there was a very slight change in A1 neurotoxic markers, but almost all A2 neuroprotective markers were up-regulated (Fig 2B). However, in Spp1 KO astrocytes, the ascorbate-induced increase in A2 neuroprotective genes was absent, except for the Slc10a6 and Cd109 genes that were still up-regulated in the KO mice. This suggests that in astrocytes, vitamin C induces the up-regulation of neuroprotective markers at least partially via the up-regulation of SPP1 (Fig 2C).
Collectively, vitamin C increases the production of neurotropic factors, and leads to an up-regulation of genes related to phagocytosis and oxidative phosphorylation and induces the gene expression of neuroprotective factors in astrocytes in an SPP1-dependent manner.
Vitamin C increases SPP1 expression in RGCs in vivo
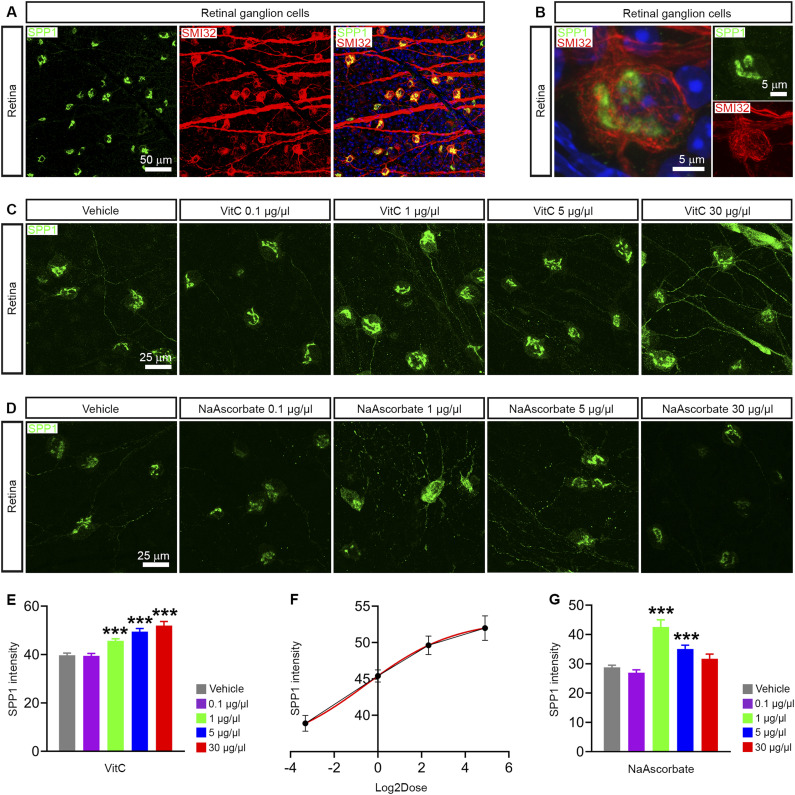
Because vitamin C up-regulated SPP1 in astrocytes in vitro, we then tested whether vitamin C increases SPP1 expression in the retina in vivo. SPP1 was specifically expressed in alpha ganglion cells in the retina, as SPP1 was co-expressed with the marker SMI32 (neurofilament H) that is most prominently expressed in alpha RGCs (Fig 3A and B). Vitamin C up-regulated SPP1 protein expression in the intrinsically SPP1+ alpha ganglion cells in the retina in a dose-dependent manner from 0.1 to 30 μg/μl (Fig 3C, E, and F). We also detected an obvious increase in SPP1 immunoreactivity in dendrites and axons of ganglion cells at high doses (30 μg/μl) of vitamin C (Fig 3C). Similarly, sodium ascorbate also increased SPP1 expression in RGCs, especially at the 1 μg/μl dose (Fig 3D and G). Taken together, the experiments in vivo demonstrated that vitamin C and its salt increased SPP1 expression in ganglion cells in the retina.
Figure 3. Vitamin C increases SPP1 expression in the retina in vivo.
(A) Co-immunostaining of SPP1 and alpha retinal ganglion cell marker SMI32 in the C57BL/6 retina. (B) High-magnification image of SPP1 immunostaining in the alpha retinal ganglion cell of the C57BL/6 retina. (C) SPP1 immunostaining in the C57BL/6 retina treated with 1, 25, 50, and 100 μM vitamin A (retinoic acid) for 24 h. (D) SPP1 immunostaining in the C57BL/6 retina treated with 31.25, 62.5, 125, and 250 μM vitamin C (ascorbic acid) for 24 h. (E) Vitamin C up-regulated SPP1 protein expression in a dose-dependent manner in the C57BL/6 retina (n = 29–103 cells/group). Retinal ganglion cells were from four mice. (F) Dose–response curves showed the effect of vitamin C concentration (Log2Dose) on SPP1 protein expression in the C57BL/6 retina (n = 29–103 cells/group). Retinal ganglion cells were from four mice. (G) NaAscorbate increased SPP1 protein expression in the C57BL/6 retina (n = 26–48 cells/group). Retinal ganglion cells were from three mice. (E, G) P-values by a one-way ANOVA (E, G), ***P < 0.001. Data are the mean ± SEM.
Source data are available for this figure.
Vitamin C promotes astrocytic neuroprotection via the up-regulation of SPP1 in vivo
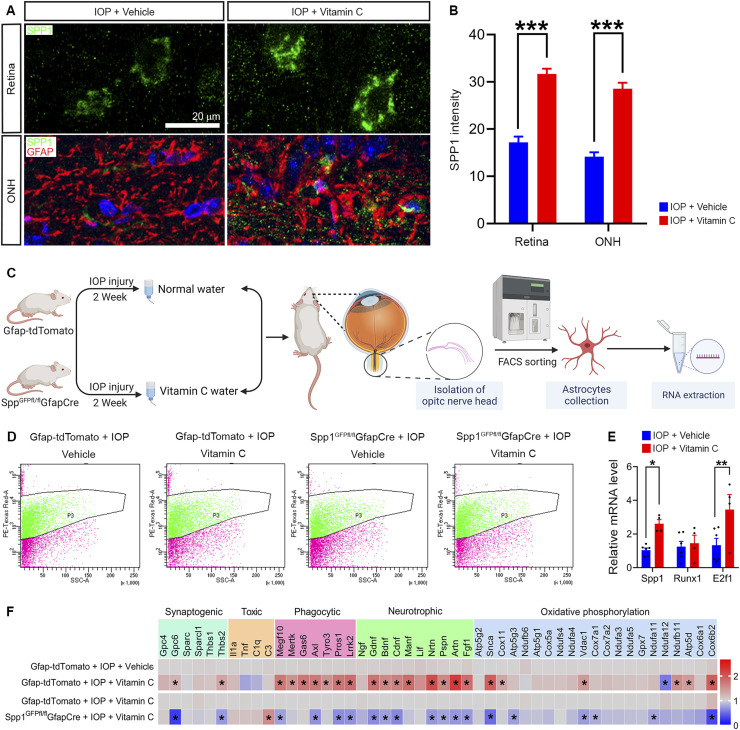
To test the function of vitamin C in glaucoma, we used the microbead occlusion model in 3-mo-old mice to induce unilateral elevated IOP. Because astrocytic SPP1 was found to be protective for RGCs and visual function (Li & Jakobs, 2022), we then verified this effect in vivo by administering vitamin C (1%) to mice in the drinking water for 2 wk. Vitamin C up-regulated SPP1 protein expression in the intrinsically SPP1+ alpha ganglion cells in the retina and astrocytes in the optic nerve in glaucomatous mice in vivo (Fig 4A and B).
Figure 4. Vitamin C increases SPP1 expression to promote astrocytic neuroprotection in vivo.
(A) SPP1 immunostaining in the retina and ONH of glaucomatous mice with oral vitamin C treatment. GFAP was used to label astrocytes. (B) Vitamin C increased SPP1 protein expression in the retina and ONH of glaucomatous mice in vivo (n = 16–38 cells/group). (C) Schematic illustration of tdTomato+ astrocyte sorting by fluorescence-activated cell sorting from ONH of Gfap-tdTomato and Spp1GFPfl/flGfapCre mice treated with oral vitamin C followed by high IOP. (D) tdTomato+ astrocyte sorting from the ONH of Gfap-tdTomato and Spp1GFPfl/flGfapCre mice treated with oral vitamin C followed by high IOP. (E) Vitamin C increased Spp1 and E2f1 mRNA expression, but not Runx1 mRNA in sorted ONH astrocytes of glaucomatous mice (n = 4–6). (F) Heat map of expression levels of genes related to synaptogenesis, neurotoxic factors, phagocytosis, neurotropic factors, and oxidative phosphorylation in ONH astrocytes sorted from Gfap-tdTomato and Spp1GFPfl/flGfapCre mice treated with oral vitamin C followed by high IOP (n = 4–6). These gene levels were assessed by qRT-PCR. The asterisks indicate significance with P < 0.05 in the pairwise comparisons. (B, E, F) P-values by a two-way ANOVA (B, E) or a multiple unpaired t test (F), *P < 0.05, **P < 0.01, and ***P < 0.001. Data are the mean ± SEM.
Source data are available for this figure.
Because vitamin C induced neuroprotective astrocytes in vitro via SPP1, we further investigated the molecular mechanisms of vitamin C in astrocytes in vivo in a mouse glaucoma model. B6.Gfap-cre, a strain expressing Cre recombinase under the control of the astrocyte-specific Gfap promoter, was crossed with a tdTomato reporter strain (Ai14) to get Gfap-tdTomato, in which astrocytes were labeled with a red fluorescent protein. We also used an astrocytic conditional Spp1 knock-out strain, Spp1GFPfl/flGfapCre (Spp1 cKO), generated by crossing Spp1GFPfl/fl with B6.Gfap-cre. In addition to conditional deletion of Spp1, this strain also expresses tdTomato in astrocytes (Li & Jakobs, 2022). The IOP was raised unilaterally in mice that were treated with vitamin C.
2 wk after induction of high IOP, astrocytes from the optic nerve were isolated. We then used fluorescence-activated cell sorting (FACS) to sort optic nerve head SPP1+ astrocytes from Gfap-tdTomato and SPP1- astrocytes from Spp1GFPfl/flGfapCre mice (Fig 4C and D). As in cell culture, vitamin C led to an up-regulation of Spp1 and E2f1 mRNA, but had no effect on Runx1 expression (Fig 4E). Besides, vitamin C strongly increased gene levels associated with phagocytosis and neurotropic factors, and moderately up-regulated gene expression related to oxidative phosphorylation in optic nerve head astrocytes of glaucomatous mice in vivo. These effects induced by vitamin C were blocked in Spp1 cKO mice (Fig 4F).
Vitamin C protects RGCs and rescues vision function in glaucoma
Finally, we asked whether oral supplementation of vitamin C would protect RGCs and visual function in the microbead model of glaucoma. We administered vitamin C (1%) to glaucomatous mice in the drinking water for 2 wk. Age-, strain-, and sex-matched control animals received normal water. 2 wk later, RGC density, pattern ERG, and visual acuity were measured. Vitamin C supplementation in the drinking water improved RGC survival, pattern ERG, and visual acuity in the glaucoma models of C57BL/6 and Spp1 KO mice (Figs 5A–D and S2), but vitamin C also significantly reduced the IOP itself (Fig 5E). This made it difficult to determine to what extent the vitamin C effect was related to its ability to up-regulate SPP1 and to what extent it was related to the effect on IOP.
Figure 5. Vitamin C protects RGCs and vision in a model of glaucoma and ONC.
(A) BRN3A-labeled RGCs in the retina of WT and Spp1−/− mice treated with oral vitamin C followed by high IOP. (B, C, D) Quantification of the BRN3A+ RGC number (B), PERG amplitude (C), and visual acuity (D) in vitamin C–treated WT and Spp1−/− mice with high IOP (n = 8). (E) Traces of IOP in WT and Spp1−/− mice treated with oral vitamin C followed by microbead injection, suggesting vitamin C decreased IOP in glaucomatous mice (n = 8). (F) BRN3A-labeled RGCs in the retina of WT and Spp1−/− mice treated with oral vitamin C followed by ONC. (G) Quantification of the BRN3A+ RGC number in the retina of WT and Spp1−/− mice treated with oral vitamin C followed by optic nerve crush (n = 6). (H) SPP1 immunostaining in the retina and ONH of C57BL/6 mice with oral vitamin C treatment followed by optic nerve crush. GFAP was used to label astrocytes. (I) Vitamin C increased SPP1 protein expression in the retina and ONH of C57BL/6 mice after ONC in vivo (n = 22–27 cells/group). (B, C, D, E, G, I) P-values by a two-way ANOVA (B, C, D, G, I), *P < 0.05, **P < 0.01, and ***P < 0.001; and a two-way ANOVA (E), ***P < 0.001 (IOP + vehicle versus IOP + vitamin C) and ###P < 0.001 (Spp1−/− IOP + vehicle versus Spp1−/− IOP + vitamin C). Data are the mean ± SEM.
Source data are available for this figure.
Figure S2. Representative traces of PERG.
Representative traces of PERG in WT and Spp1−/− mice treated with oral vitamin C followed by high IOP. See also Jakobs (2023) for all traces.
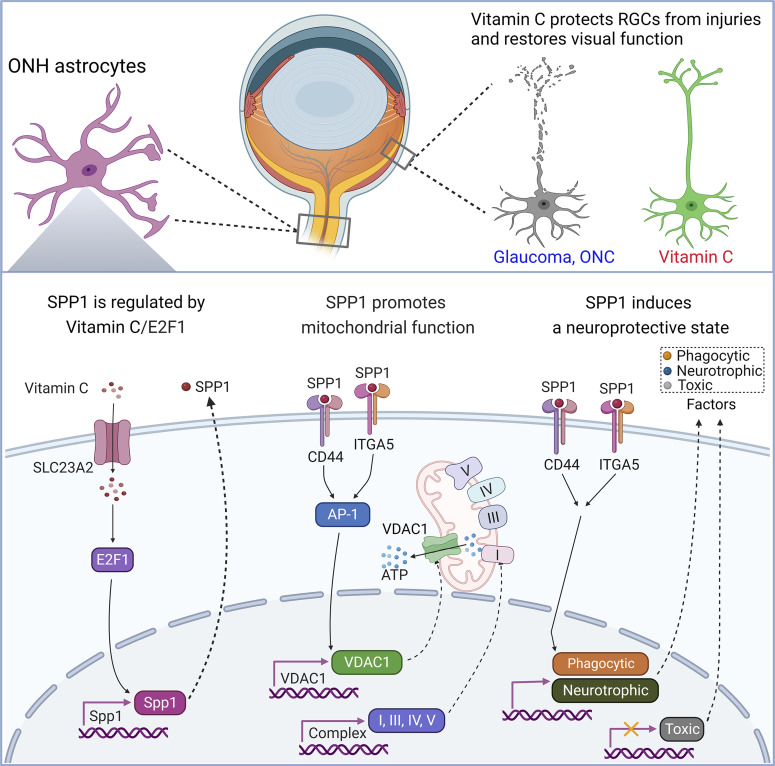
To further investigate whether vitamin C protects RGC survival via SPP1, we used an optic nerve crush model as an IOP-independent model of optic nerve damage. Vitamin C was strongly protective of RGC survival in mice with optic nerve crush. This protection in RGC survival induced by vitamin C was absent in Spp1 KO mice, indicating vitamin C protected RGC survival via SPP1 (Fig 5F and G). We further found vitamin C up-regulated SPP1 protein expression in ganglion cells in the retina and astrocytes in the optic nerve (Fig 5H and I). These results suggest that vitamin C protects RGCs after optic nerve injury partially by up-regulating the expression of the neuroprotective signaling molecule SPP1 (Fig 6).
Figure 6. Proposed model of vitamin C promotion on RGC survival and visual function.
Schematic illustration showing vitamin C up-regulates SPP1 expression in reactive astrocytes via the transcription factor E2F1. Vitamin C increases oxidative phosphorylation and VDAC1 expression through SPP1. Vitamin C promotes phagocytosis and the secretion of neurotropic factors in astrocytes but inhibits the production of neurotoxic and inflammatory mediators through SPP1. Loss of SPP1 increases the vulnerability of retinal ganglion cells to injury. Oral administration of vitamin C protects retinal ganglion cells, lowers the IOP, and preserves visual function in glaucoma and traumatic optic injury.
Discussion
Vitamin C is abundant in the CNS and fulfills several functions in health and disease (Harrison and May, 2009; Moretti & Rodrigues, 2021). Ascorbate is a co-factor for enzymatic reactions, such as the synthesis of norepinephrine by dopamine-β-hydroxylase (May et al, 2013) and collagen synthesis in blood vessel walls (Sotiriou et al, 2002). The anti-oxidative properties of ascorbate have been linked to its neuroprotective properties in ischemia/reperfusion injury (Huang et al, 2001), in lead poisoning (Han et al, 2007), and possibly in Parkinson’s disease (Wagner et al, 1986). Furthermore, ascorbate is directly involved in epigenetic modulation through its interaction with ten–eleven translocation methylcytosine dioxygenases (Blaschke et al, 2013). Here, we show that vitamin C also uses an additional neuroprotective pathway that involves the up-regulation of the cytokine SPP1 in reactive astrocytes.
Astrocytes react to damage to RGCs or their axons by activating a response that—at least in the early stages of disease—aims at protecting the health of surrounding neurons and their processes (Sun et al, 2017). Identifying astrocyte-derived factors that mediate this protective effect may be useful as therapeutics that act via an IOP-independent mechanism and can be added to the pressure-lowering drugs that currently are the first-line treatment for glaucoma. SPP1 was identified in gene expression studies from the optic nerve and other brain regions (Howell et al, 2011; Zamanian et al, 2012; Qu & Jakobs, 2013). Recently, we reported that its overexpression is highly protective of RGC health and visual function in aging and glaucoma (Li & Jakobs, 2022). The clear neuroprotective effect and the apparent safety even in long-term expression studies make SPP1 as an attractive candidate molecule for neuroprotective therapy in glaucoma. However, as a protein, SPP1 would have to be injected intravitreally. Though intravitreal injection is commonly done in anti-VEGF therapy for age-related macular degeneration (Brown & Regillo, 2007), repeated injections carry a risk of inflammation or damage to the retina (Kiss et al, 2018). AAV-mediated overexpression is feasible but has the potential drawback of not being easily reversible once it is initiated. Small molecules that induce SPP1 expression may therefore be useful. The promoter of the Spp1 gene contains predicted binding sites for retinoic acid receptors and T3 (thyroid hormone) receptors. Retinoic acid does increase SPP1 expression—and T3 would be expected to act similarly—but in both cases, the toxic effects of high doses of vitamin A, or T3 overdoses would limit their use. We therefore mainly concentrated on vitamin C.
In cultured wild-type astrocytes, vitamin C up-regulated SPP1 via the transcription factor E2F1. Vitamin C-treated astrocytes also showed an up-regulation of neuroprotective mediators, genes associated with oxidative phosphorylation, and phagocytosis. These effects were dependent on SPP1, as they were not observed in Spp1 KO astrocytes. In the microbead occlusion model of glaucoma, oral supplementation of vitamin C significantly increased visual function and RGC survival compared with the control group. However, vitamin C also clearly lowered the IOP. As this effect occurred in the wild-type and Spp1 KO mice, it is obviously not mediated by SPP1. It is currently unknown exactly by what mechanisms vitamin C lowers the IOP, but vitamin C affects the trabecular meshwork cells directly, which may improve the aqueous humor outflow facility (Xu et al, 2014).
Though IOP lowering would not be unwelcome in clinical use, in the context of the microbead occlusion model of glaucoma, it acts as a confounding factor as the protection of RGCs and visual function observed in the vitamin C–treated group may be completely due to the IOP-lowering effect. We therefore used optic nerve crush as an IOP-independent model of RGC damage. Optic nerve crush is a much more severe injury than the elevation of IOP and leads to an almost complete loss of RGCs within 2 wk (Berkelaar et al, 1994). Neurotropic factors, including SPP1, can delay, but not completely prevent, RGC loss in this model. Vitamin C was effective in delaying RGC loss in wild-type, but not in Spp1 KO, mice indicating that the neuroprotective effect of vitamin C is at least in part mediated by SPP1.
In the healthy retina and optic nerve, SPP1 is expressed constitutively in ON and OFF alpha ganglion cells, but not in any other type of neurons (Sanes & Masland, 2015). It is only after injury that the astrocytes of the optic nerve (but not those on the retinal surface) and, transiently, microglia express levels of SPP1 that are detectable by immunohistochemistry. Astrocytic SPP1 is a neuroprotective factor (Li & Jakobs, 2022), but the role of SPP1 in alpha ganglion cells is currently unknown. It is tempting to speculate that alpha cells secrete SPP1 in response to injury, either as a signal to glial cells or as a signal to provide direct neuroprotection to surrounding (SPP1-negative) RGCs. Thus, in addition to the direct activity that vitamin C has on RGCs as an antioxidant and a co-factor to enzymes such as TET dioxygenases, a part of the neuroprotection in the ganglion cell layer may be mediated by SPP1.
Most studies of vitamin C in the context of glaucoma have concentrated on the IOP. Interestingly, in human populations, there seems to be no clear-cut effect of vitamin C supplementation on IOP. L-gulono-γ-lactone oxidase catalyzes the last step in the biosynthesis of ascorbic acid. The GULO gene is a pseudogene in humans, other primates, and some other species, which are therefore dependent on a dietary supply of vitamin C. In contrast, mice possess a functional Gulo gene and can synthesize their own vitamin C. In spite of this, supplementing dietary vitamin C is beneficial for mice as demonstrated not only here, but also in other publications that studied parameters such as longevity and neuroprotection. This suggests that mice on standard laboratory chow are probably not optimally supplied with vitamin C. In contrast, vitamin C intake in humans varies with dietary habits. In human subjects who are already optimally supplied, additional intake of vitamin C has no benefit as excess vitamin C is simply excreted via the urine. However, studies that measured serum levels of ascorbate or its metabolites did find negative correlations between serum levels and IOP, suggesting that there is a subpopulation of glaucoma patients who might benefit (Yuki et al, 2010; Hysi et al, 2019).
The recommended daily intake of vitamin C is 90 mg for adult men and 75 mg for adult women (Institute of Medicine (US) Panel on Dietary Antioxidants & Related Compounds, 2000). There is no proven benefit in exceeding these amounts. Though vitamin C is relatively non-toxic, very high (>2 g per day) doses can lead to gastrointestinal disturbances and are not recommended. Vitamin C is therefore certainly not suitable as a stand-alone treatment for glaucoma. However, our data suggest that suboptimal levels of ascorbate would exacerbate RGC loss, and taking care to maintain a sufficient intake of vitamin C would be beneficial for glaucoma patients.
Materials and Methods
Animals
C57BL/6 (000664) and Spp1 KO (B6.129S6(Cg)-Spp1tm1Blh/J, 004936) mice were purchased from the Jackson Laboratory. Spp1 KO mice were deficient for SPP1 expression in all tissues. The B6.Spp1fl-EGFP-stop-tdTomato strain was described in detail in a previous study (Li & Jakobs, 2022) and was used for the conditional deletion of Spp1. B6.Cg-Tg(Gfap-cre)77.6Mvs/2J (024098) mice, which were used for creating the astrocyte-specific deletion, and B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (007914) mice, which were used as a reporter strain Ai14 to create Spp1+ astrocytes expressing a red fluorescent protein. These mice were purchased from the Jackson Laboratory. Male and female mice were used in equal numbers, and all control mice were strictly age- and sex-matched. Mice were housed under a 12-h dark/light cycle at an ambient temperature of 21–22°C and 40–50% humidity, and received water and standard food ad libitum. Mice were anesthetized by i.p. injection of ketamine and xylazine (100 and 10 mg/kg, respectively), supplemented by topical application of 0.5% proparacaine to the ocular surface. After surgery, mice received an s.c. injection of 0.1 mg/kg buprenorphine. At the end of the experimental period, mice were euthanized by CO2 inhalation according to the Guide for the Care and Use of Laboratory Animals of the AAALAC. All animal care and handling procedures were done in accordance with the guidelines of the Association for Research in Vision and Ophthalmology and approved by the Institutional Animal Care and Use Committee at Schepens Eye Research Institute.
Primary astrocyte cultures
Retinas and optic nerves from either C57BL/6 wild-type or Spp1 KO neonatal mice aged 1–3 d were enzymatically digested by 0.25% trypsin and 0.01% DNase at 37°C. The tissue was then triturated by gentle mechanical dissociation with a fire-polished glass Pasteur pipette at room temperature. The suspension was filtered through a 52-μm nylon mesh filter to generate a single-cell suspension.
Cells were plated on poly-lysine–coated coverslips or tissue culture plates in DMEM/F12 with 10% FBS. Cultures were incubated at 37°C, 95% humidity, and 5% CO2 in a medium. The medium was changed by replacing the fresh medium twice a week. After 7–10 d in culture, the plates were shaken at 200 rpm for 18 h on an orbital shaker, and the supernatant was discarded. This removed most of the oligodendrocytes and microglia. The remaining cells were digested using trypsin and passaged. After two rounds of dissociation and reseeding, more than 95% of the remaining cells were positive for the astrocyte marker GFAP.
FACS
Astrocytes were purified from the optic nerve head of Gfap-tdTomato and Spp1GFPfl/flGfapCre mice. Briefly, the optic nerve heads were treated with papain (0.6 mg/ml; LS003126; Worthington) and L-cysteine (0.012 mg/ml; C7352; Sigma-Aldrich) for 15 min at 37°C in Ca2+- and Ma2+-free HBSS solution (14185-052; Gibco). After the incubation, tissues were centrifuged at 953g for 5 min and the papain/HBSS solution was removed. Tissues were resuspended in horse serum (10%, 26050-088; Gibco) and DNase I (60 U/ml; D-5025; Sigma-Aldrich) in HBSS and were triturated with a heat-polished Pasteur pipet (TW150-4; World Precision Instruments), and the tissue was completely dissociated. Dissociated cells were centrifuged, resuspended in HBSS, and passed through a 35-μm cell strainer. Astrocytes were identified by tdTomato fluorescence and sorted directly into a collection medium using a BD FACSAria III instrument (BD Biosciences). Astrocytes sorted by FACS were centrifuged at 1,000g for 10 min and were used for RNA extraction.
Electroretinography
Mice were anesthetized, and the pupils were dilated with one drop of 1% tropicamide. Pattern electroretinograms (PERG) were recorded from light-adapted mice on a Celeris small animal testing system (Diagnosys LLC) using a high-contrast horizontal grating (0.05 cycles/degree, reversing in the spatial phase at 1 Hz, 50 cd/m2 mean luminance). Artificial tears (GenTeal) were used to prevent drying and to increase the contact with the recording electrode. An electrode on the contralateral eye served as a reference. A total of 300 complete contrast reversals of pattern ERG were repeated twice in each eye. PERG amplitudes were defined as the difference between the P1 peak and N2. Conventional, light-adapted ERG was recorded directly afterward to ensure that reductions in PERG amplitudes were not due to unrelated ocular disease.
Optomotor response
The visual acuity of mice was measured based on the optomotor reflex using OptoDrum (Striatech GmbH). In the OptoDrum, wake mice were placed on an elevated platform, surrounded by four computer monitors. A camera observed the behavior of the animal from above. The optomotor reflex was triggered with a rotating black-and-white stripe pattern. Tracking behavior was automatically detected and analyzed by OptoDrum software. Stimulus patterns were continuously and automatically adjusted during the experiment until the visual threshold was reached, and the optomotor reflex was not triggered anymore. The highest spatial frequency that still elicited the reflex was recorded as the visual acuity.
Retro-orbital optic nerve crush
Mice were anesthetized, and the intraorbital optic nerve was exposed by dissection of the conjunctiva (Sun et al, 2010). The nerve was crushed for 10 s ∼1 mm distal to the lamina cribrosa using self-closing jeweler’s forceps. 1 wk after surgery, retinas were collected for immunofluorescence to determine the viability of RGCs. Optic nerve heads were used to sort astrocytes by FACS for further qRT-PCR analyses.
Microbead injection and intraocular pressure measurements
The microbead occlusion model was used to achieve intraocular pressure (IOP) elevation according to published protocols (Sappington et al, 2010; Chen et al, 2011). Mice were anesthetized, and a small hole was made in the cornea. Polystyrene microbeads (15 μm diameter; Invitrogen) were injected into the anterior chamber of the right eye through the cornea. Control groups received an injection of sterile saline solution. IOPs were measured every 3 d, at the same time of day, using a rebound tonometer (TonoLab; Icare). The reported IOP for each day consists of an average reading of five measurements from each eye. Mice showing no IOP elevation were omitted from the study.
Tissue preparation
After CO2 euthanasia, the skull was opened, the brain was removed, and eyes and optic nerves were dissected from the surrounding tissue (Jakobs et al, 2005) and immediately fixed in 4% paraformaldehyde overnight. Eyes were hemisected along the ora serrata, and the retinas were removed from the posterior eyecup and whole-mounted on nitrocellulose filters. Optic nerves were left intact and processed for sectioning.
Immunohistochemistry and immunocytochemistry
After euthanasia, whole eyes were dissected and placed in ice-cold 4% paraformaldehyde. Retinas were mounted RGC side-up on nitrocellulose membranes, blocked with 5% goat serum, and stained for 3 d with primary antibodies (Table S1). Optic nerves were cryoprotected in 30% sucrose, embedded in the OCT compound, and sectioned at 12 μm in a Leica cryostat. Sections were incubated with primary antibodies overnight at 4°C. Cultured astrocytes plated on poly-lysine–coated coverslips were fixed with 4% formaldehyde for 10 min. After blocking with 5% goat serum, astrocytes were incubated with anti-SPP1 for 1 h. Primary antibodies were visualized with appropriate secondary antibodies conjugated with Alexa fluorophores (Jackson ImmunoResearch and Molecular Probes). DAPI was used to counterstain nuclei. Samples were then mounted in Vectashield (Vector Laboratories). Immunofluorescent images were collected using a Leica SP8 fluorescence microscope (Leica). SPP1 protein expression in the retina in vivo was quantified by the fluorescence intensities of SPP1 immunostaining in SPP1-positive RGCs. SPP1-negative RGCs were not quantified. SPP1-positive RGCs in the acquired images from parts of the retina were used for quantification, and fluorescence intensities of SPP1 immunostaining were analyzed in the plotted region per cell with ImageJ software. The mean fluorescence of SPP1 immunostaining in all quantified RGCs was recorded and used for comparison.
Table S1. Reagents and Resources. (37KB, docx)
Confocal microscopy
Confocal image stacks were taken on a Leica SP8 confocal microscope (Leica). For RGC counting, images of the whole-mounted retina were obtained as z-stacks through the RGC layer at a 0.5 μm step size, and a z-projection was generated.
cDNA synthesis and quantitative PCR
Because of the small amount of tissue from the optic nerve head, three optic nerve heads were pooled for one sample to sort astrocytes by FACS. One well of cultured astrocytes growing in six-well plates was one sample. Total RNA was isolated from FACS-sorted astrocytes or from cultured astrocytes using RNeasy Plus Micro Kit (QIAGEN). We performed RNA integrity validation and quantification using the Agilent RNA Pico chip analysis in Agilent 2100 Bioanalyzer (Agilent Technologies). Only the RNA samples with an RNA integrity number higher than 7 were used for cDNA synthesis. 10 ng of total RNA purified from astrocytes was reverse-transcribed using the Ovation qRT-PCR system (NuGEN), and the cDNAs were diluted 1:50 as templates to measure transcript levels of candidate genes by quantitative PCR. GAPDH was used as a reference gene as the expression level in the optic nerve head is stable after optic nerve crush (Qu & Jakobs, 2013). Primer sequences are given in Table S1. Gene levels related to synaptogenesis, neurotoxic factors, phagocytosis, neurotropic factors, and oxidative phosphorylation were tested by qRT-PCR. At least five biological replicates were used for each condition, and all samples were run in triplicate with a non-template control on a StepOnePlus qRT-PCR thermocycler (96-Well; Applied Biosystems) and a Roche LightCycler 480 II (384-Well; Roche Diagnostics Corporation).
Oral administration of vitamin C
Mice were given drinking water supplemented with 1% vitamin C (ascorbic acid), as recommended (Massie et al, 1984). The vitamin C–containing water was prepared every day. Vitamin C administration started on the same day when microbeads were injected. Mice were maintained at the stated dose for 2 wk before euthanasia.
Statistical analysis
Statistical significance was performed with GraphPad Prism 8. The data are provided as the mean ± SEM. Data were analyzed using an unpaired two-tailed t test for comparisons of two groups. For comparison of multiple groups, normally distributed data were assessed using a one-way ANOVA with Tukey’s post-test. For bivariate comparisons, a two-way ANOVA with Bonferroni’s post-test was used. Differences were considered statistically significant at P < 0.05. Figures were prepared using Adobe Photoshop CS6. The proposed model (Fig 6) was prepared using BioRender.
Data Availability
Data are available on reasonable request. Pattern ERG traces are available under Jakobs (2023). All data are available from the corresponding author on request.
Supplementary Material
Acknowledgements
This work was supported by the NIH grant R01 EY19703 and the NIH Core Grant for Vision Research P30EY003790, and a Shaffer grant of the Glaucoma Research Foundation.
Author Contributions
S Li: conceptualization, data curation, formal analysis, investigation, visualization, methodology, and writing—review and editing.
TC Jakobs: conceptualization, data curation, formal analysis, supervision, funding acquisition, investigation, methodology, project administration, and writing—original draft, review, and editing.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ (1994) Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci 14: 4368–4374. 10.1523/JNEUROSCI.14-07-04368.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke K, Ebata KT, Karimi MM, Zepeda-Martinez JA, Goyal P, Mahapatra S, Tam A, Laird DJ, Hirst M, Rao A, et al. (2013) Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 500: 222–226. 10.1038/nature12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML (2008) Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci 49: 1437–1446. 10.1167/iovs.07-1337 [DOI] [PubMed] [Google Scholar]
- Brown DM, Regillo CD (2007) Anti-VEGF agents in the treatment of neovascular age-related macular degeneration: Applying clinical trial results to the treatment of everyday patients. Am J Ophthalmol 144: 627–637.e2. 10.1016/j.ajo.2007.06.039 [DOI] [PubMed] [Google Scholar]
- Chen H, Wei X, Cho KS, Chen G, Sappington R, Calkins DJ, Chen DF (2011) Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Invest Ophthalmol Vis Sci 52: 36–44. 10.1167/iovs.09-5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu-Tan JA, Kirkby M, Natoli R (2022) Running to save sight: The effects of exercise on retinal health and function. Clin Exp Ophthalmol 50: 74–90. 10.1111/ceo.14023 [DOI] [PubMed] [Google Scholar]
- Coker SJ, Smith-Diaz CC, Dyson RM, Vissers MCM, Berry MJ (2022) The epigenetic role of vitamin C in neurodevelopment. Int J Mol Sci 23: 1208. 10.3390/ijms23031208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva Vargas JL, Belforte N, Di Polo A (2016) The glial cell modulator ibudilast attenuates neuroinflammation and enhances retinal ganglion cell viability in glaucoma through protein kinase A signaling. Neurobiol Dis 93: 156–171. 10.1016/j.nbd.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Fishbein SL, Goodstein S (1972) The pressure lowering effect of ascorbic acid. Ann Ophthalmol 4: 487–491. [PubMed] [Google Scholar]
- Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, Azuara-Blanco A, Bourne RR, Broadway DC, Cunliffe IA, et al. (2015) Latanoprost for open-angle glaucoma (UKGTS): A randomised, multicentre, placebo-controlled trial. Lancet 385: 1295–1304. 10.1016/s0140-6736(14)62111-5 [DOI] [PubMed] [Google Scholar]
- Guo YE, Suo N, Cui X, Yuan Q, Xie X (2018) Vitamin C promotes oligodendrocytes generation and remyelination. Glia 66: 1302–1316. 10.1002/glia.23306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadzir SN, Ibrahim SN, Abdul Wahab RM, Zainol Abidin IZ, Senafi S, Ariffin ZZ, Abdul Razak M, Zainal Ariffin SH (2014) Ascorbic acid induces osteoblast differentiation of human suspension mononuclear cells. Cytotherapy 16: 674–682. 10.1016/j.jcyt.2013.07.013 [DOI] [PubMed] [Google Scholar]
- Han FF, Fu XX (2022) Vitamin intake and glaucoma risk: A systematic review and meta-analysis. J Fr Ophtalmol 45: 519–528. 10.1016/j.jfo.2021.10.010 [DOI] [PubMed] [Google Scholar]
- Han JM, Chang BJ, Li TZ, Choe NH, Quan FS, Jang BJ, Cho IH, Hong HN, Lee JH (2007) Protective effects of ascorbic acid against lead-induced apoptotic neurodegeneration in the developing rat hippocampus in vivo. Brain Res 1185: 68–74. 10.1016/j.brainres.2007.09.044 [DOI] [PubMed] [Google Scholar]
- Harada H, Miki R, Masushige S, Kato S (1995) Gene expression of retinoic acid receptors, retinoid-X receptors, and cellular retinol-binding protein I in bone and its regulation by vitamin A. Endocrinology 136: 5329–5335. 10.1210/endo.136.12.7588278 [DOI] [PubMed] [Google Scholar]
- Harrison FE, May JM (2009) Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic Biol Med 46: 719–730. 10.1016/j.freeradbiomed.2008.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Macalinao DG, Sousa GL, Walden M, Soto I, Kneeland SC, Barbay JM, King BL, Marchant JK, Hibbs M, et al. (2011) Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest 121: 1429–1444. 10.1172/jci44646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, MacNicoll KH, Barbay JM, Porciatti V, et al. (2012) Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest 122: 1246–1261. 10.1172/jci61135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, et al. (2001) Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc Natl Acad Sci U S A 98: 11720–11724. 10.1073/pnas.171325998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysi PG, Khawaja AP, Menni C, Tamraz B, Wareham N, Khaw KT, Foster PJ, Benet LZ, Spector TD, Hammond CJ (2019) Ascorbic acid metabolites are involved in intraocular pressure control in the general population. Redox Biol 20: 349–353. 10.1016/j.redox.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds (2000) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington (DC): National Academies Press (US). Copyright 2000 by the National Academy of Sciences. All rights reserved. [PubMed] [Google Scholar]
- Jakobs TC (2023) Vitamin C and SPP1 in glaucoma. Harv Dataverse 10.7910/DVN/WTEOGO [DOI] [Google Scholar]
- Jakobs TC, Libby RT, Ben Y, John SW, Masland RH (2005) Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol 171: 313–325. 10.1083/jcb.200506099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeradi S, Hammerschmidt M (2016) Retinoic acid-induced premature osteoblast-to-preosteocyte transitioning has multiple effects on calvarial development. Development 143: 1205–1216. 10.1242/dev.129189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju WK, Perkins GA, Kim KY, Bastola T, Choi WY, Choi SH (2022) Glaucomatous optic neuropathy: Mitochondrial dynamics, dysfunction and protection in retinal ganglion cells. Prog Retin Eye Res in press: 101136. 10.1016/j.preteyeres.2022.101136 [DOI] [PubMed] [Google Scholar]
- Kangisser L, Tan E, Bellomo R, Deane AM, Plummer MP (2021) Neuroprotective properties of vitamin C: A scoping review of pre-clinical and clinical studies. J Neurotrauma 38: 2194–2205. 10.1089/neu.2020.7443 [DOI] [PubMed] [Google Scholar]
- Kiss S, Dugel PU, Khanani AM, Broder MS, Chang E, Sun GH, Turpcu A (2018) Endophthalmitis rates among patients receiving intravitreal anti-VEGF injections: A USA claims analysis. Clin Ophthalmol 12: 1625–1635. 10.2147/opth.s169143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouassi Nzoughet J, Chao de la Barca JM, Guehlouz K, Leruez S, Coulbault L, Allouche S, Bocca C, Muller J, Amati-Bonneau P, Gohier P, et al. (2019) Nicotinamide deficiency in primary open-angle glaucoma. Invest Ophthalmol Vis Sci 60: 2509–2514. 10.1167/iovs.19-27099 [DOI] [PubMed] [Google Scholar]
- Krishnan A, Fei F, Jones A, Busto P, Marshak-Rothstein A, Ksander BR, Gregory-Ksander M (2016) Overexpression of soluble fas ligand following adeno-associated virus gene therapy prevents retinal ganglion cell death in chronic and acute murine models of glaucoma. J Immunol 197: 4626–4638. 10.4049/jimmunol.1601488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Kocab AJ, Zacks DN, Marshak-Rothstein A, Gregory-Ksander M (2019) A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J Neuroinflammation 16: 184. 10.1186/s12974-019-1576-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaldin MAM, Iezhitsa I, Agarwal R, Agarwal P, Ismail NM (2023) Neuroprotective effects of exogenous brain-derived neurotrophic factor on amyloid-beta 1-40-induced retinal degeneration. Neural Regen Res 18: 382–388. 10.4103/1673-5374.346546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jakobs TC (2022) Secreted phosphoprotein 1 slows neurodegeneration and rescues visual function in mouse models of aging and glaucoma. Cell Rep 41: 111880. 10.1016/j.celrep.2022.111880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541: 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linner E (2009) The pressure lowering effect of ascorbic acid in ocular hypertension. Acta Ophthalmologica 47: 685–689. 10.1111/j.1755-3768.1969.tb08156.x [DOI] [PubMed] [Google Scholar]
- Lopez-Ortiz S, Lista S, Valenzuela PL, Pinto-Fraga J, Carmona R, Caraci F, Caruso G, Toschi N, Emanuele E, Gabelle A, et al. (2022) Effects of physical activity and exercise interventions on alzheimer’s disease: An umbrella review of existing meta-analyses. J Neurol 270: 711–725. 10.1007/s00415-022-11454-8 [DOI] [PubMed] [Google Scholar]
- Lu Y, Brommer B, Tian X, Krishnan A, Meer M, Wang C, Vera DL, Zeng Q, Yu D, Bonkowski MS, et al. (2020) Reprogramming to recover youthful epigenetic information and restore vision. Nature 588: 124–129. 10.1038/s41586-020-2975-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Doherty TJ (1984) Dietary vitamin C improves the survival of mice. Gerontology 30: 371–375. 10.1159/000212659 [DOI] [PubMed] [Google Scholar]
- May JM, Qu ZC, Nazarewicz R, Dikalov S (2013) Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res Bull 90: 35–42. 10.1016/j.brainresbull.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti M, Rodrigues ALS (2021) Functional role of ascorbic acid in the central nervous system: A focus on neurogenic and synaptogenic processes. Nutr Neurosci 25: 2431–2441. 10.1080/1028415x.2021.1956848 [DOI] [PubMed] [Google Scholar]
- Nam SM, Seo JS, Nahm SS, Chang BJ (2019) Effects of ascorbic acid on osteopontin expression and axonal myelination in the developing cerebellum of lead-exposed rat pups. Int J Environ Res Public Health 16: 983. 10.3390/ijerph16060983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SR, Crowston JG, Loprinzi PD, Ramulu PY (2018) Physical activity, visual impairment, and eye disease. Eye (Lond) 32: 1296–1303. 10.1038/s41433-018-0081-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease ME, Zack DJ, Berlinicke C, Bloom K, Cone F, Wang Y, Klein RL, Hauswirth WW, Quigley HA (2009) Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci 50: 2194–2200. 10.1167/iovs.08-3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham JH, Johnson GA, Rangan RS, Amankwa CE, Acharya S, Stankowska DL (2022) Neuroprotection of rodent and human retinal ganglion cells in vitro/ex vivo by the hybrid small molecule SA-2. Cells 11: 3741. 10.3390/cells11233741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Jakobs TC (2013) The time course of gene expression during reactive gliosis in the optic nerve. PLoS One 8: e67094. 10.1371/journal.pone.0067094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA (2011) Glaucoma. Lancet 377: 1367–1377. 10.1016/s0140-6736(10)61423-7 [DOI] [PubMed] [Google Scholar]
- Quintero H, Shiga Y, Belforte N, Alarcon-Martinez L, El Hajji S, Villafranca-Baughman D, Dotigny F, Di Polo A (2022) Restoration of mitochondria axonal transport by adaptor Disc1 supplementation prevents neurodegeneration and rescues visual function. Cell Rep 40: 111324. 10.1016/j.celrep.2022.111324 [DOI] [PubMed] [Google Scholar]
- Ramdas WD, Schouten J, Webers CAB (2018) The effect of vitamins on glaucoma: A systematic review and meta-analysis. Nutrients 10: 359. 10.3390/nu10030359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Masland RH (2015) The types of retinal ganglion cells: Current status and implications for neuronal classification. Annu Rev Neurosci 38: 221–246. 10.1146/annurev-neuro-071714-034120 [DOI] [PubMed] [Google Scholar]
- Sappington RM, Carlson BJ, Crish SD, Calkins DJ (2010) The microbead occlusion model: A paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci 51: 207–216. 10.1167/iovs.09-3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, et al. (2002) Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med 8: 514–517. 10.1038/0502-514 [DOI] [PubMed] [Google Scholar]
- Sun D, Lye-Barthel M, Masland RH, Jakobs TC (2010) Structural remodeling of fibrous astrocytes after axonal injury. J Neurosci 30: 14008–14019. 10.1523/jneurosci.3605-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Moore S, Jakobs TC (2017) Optic nerve astrocyte reactivity protects function in experimental glaucoma and other nerve injuries. J Exp Med 214: 1411–1430. 10.1084/jem.20160412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121: 2081–2090. 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Tribble JR, Hui F, Joe M, Bell K, Chrysostomou V, Crowston JG, Williams PA (2021. a) Targeting diet and exercise for neuroprotection and neurorecovery in glaucoma. Cells 10: 295. 10.3390/cells10020295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble JR, Otmani A, Sun S, Ellis SA, Cimaglia G, Vohra R, Joe M, Lardner E, Venkataraman AP, Dominguez-Vicent A, et al. (2021. b) Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol 43: 101988. 10.1016/j.redox.2021.101988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveden-Nyborg P (2021) Vitamin C deficiency in the young brain-findings from experimental animal models. Nutrients 13: 1685. 10.3390/nu13051685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Carelli RM, Jarvis MF (1986) Ascorbic acid reduces the dopamine depletion induced by methamphetamine and the 1-methyl-4-phenyl pyridinium ion. Neuropharmacology 25: 559–561. 10.1016/0028-3908(86)90184-x [DOI] [PubMed] [Google Scholar]
- Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, Smithies O, John SW (2017) Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355: 756–760. 10.1126/science.aal0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Harder JM, Cardozo BH, Foxworth NE, John SWM (2018) Nicotinamide treatment robustly protects from inherited mouse glaucoma. Communicative Integr Biol 11: e1356956. 10.1080/19420889.2017.1356956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Braine CE, Kizhatil K, Foxworth NE, Tolman NG, Harder JM, Scott RA, Sousa GL, Panitch A, Howell GR, et al. (2019) Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma. Mol Neurodegeneration 14: 6. 10.1186/s13024-018-0303-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Lin Y, Porter K, Liton PB (2014) Ascorbic acid modulation of iron homeostasis and lysosomal function in trabecular meshwork cells. J Ocul Pharmacol Ther 30: 246–253. 10.1089/jop.2013.0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Rydz C, Nguyen Huu VA, Rocha L, Palomino La Torre C, Lee I, Cho W, Jabari M, Donello J, Lyon DC, et al. (2022) Stress induced aging in mouse eye. Aging Cell 21: e13737. 10.1111/acel.13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hondur G, Tezel G (2016) Antioxidant treatment limits neuroinflammation in experimental glaucoma. Invest Ophthalmol Vis Sci 57: 2344–2354. 10.1167/iovs.16-19153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K, Murat D, Kimura I, Ohtake Y, Tsubota K (2010) Reduced-serum vitamin C and increased uric acid levels in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol 248: 243–248. 10.1007/s00417-009-1183-6 [DOI] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32: 6391–6410. 10.1523/jneurosci.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhen K, Su Q, Chen Y, Lv Y, Yu L (2022) The effect of aerobic exercise on cognitive function in people with alzheimer[R8S2Q1M7]s disease: A systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health 19: 15700. 10.3390/ijerph192315700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Source Data for Figure 1LSA-2023-01976_SdataF1.xlsx (31.7KB, xlsx)
Source Data for Figure 2LSA-2023-01976_SdataF2.xlsx (22.9KB, xlsx)
Source Data for Figure 3LSA-2023-01976_SdataF3.xlsx (19.4KB, xlsx)
Source Data for Figure 4LSA-2023-01976_SdataF4.xlsx (20.8KB, xlsx)
Source Data for Figure 5LSA-2023-01976_SdataF5.xlsx (16.6KB, xlsx)
Table S1. Reagents and Resources. (37KB, docx)
Data Availability Statement
Data are available on reasonable request. Pattern ERG traces are available under Jakobs (2023). All data are available from the corresponding author on request.