Abstract
Background
Wearable devices have become widespread in research applications, yet evidence on whether they are superior to structured clinic-based assessments is sparse. In this manuscript, we compare traditional, laboratory-based metrics of mobility with a novel accelerometry-based measure of free-living gait cadence for predicting fall rates.
Methods
Using negative binomial regression, we compared traditional in-clinic measures of mobility (6-minute gait cadence, speed, and distance, and 4-m gait speed) with free-living gait cadence from wearable accelerometers in predicting fall rates. Accelerometry data were collected with wrist-worn Actigraphs (GT9X) over 7 days in 432 community-dwelling older adults (aged 77.29 ± 5.46 years, 59.1% men, 80.2% White) participating in the Study to Understand Fall Reduction and Vitamin D in You. Falls were ascertained using monthly calendars, quarterly contacts, and ad hoc telephone reports. Accelerometry-based free-living gait cadence was estimated with the Adaptive Empirical Pattern Transformation algorithm.
Results
Across all participants, free-living cadence was significantly related to fall rates; every 10 steps per minute higher cadence was associated with a 13.2% lower fall rate (p = .036). Clinic-based measures of mobility were not related to falls (p > .05). Among higher-functioning participants (cadence ≥100 steps/minute), every 10 steps per minute higher free-living cadence was associated with a 27.7% lower fall rate (p = .01). In participants with slow baseline gait (gait speed <0.8 m/s), all metrics were significantly associated with fall rates.
Conclusion
Data collected from biosensors in the free-living environment may provide a more sensitive indicator of fall risk than in-clinic tests, especially among higher-functioning older adults who may be more responsive to intervention.
Clinical Trial Registration
Keywords: Fall rates, Remote data collection, Walking, Wearable devices
Falls are a major cause of injuries and functional impairments among older adults (1), often leading to hospitalizations (2), nursing home admissions (3), and substantial morbidity (4). With the prevalence of falls estimated to be approximately 30% per year among older adults (1), it is imperative to identify underlying mechanisms for early risk assessment and prevention. Although multimodal contributors to the risk of falls are still actively investigated, major factors related to physical functioning and fitness have been identified, including daily physical activity (5), balance and posture (6), and gait characteristics (7). Characteristics of physical activity traditionally have been assessed with self-report measures (8) or structured laboratory- and clinic-based tests of fitness capacity (9), but recent technological advancements support objective measurement through wearable devices (10), allowing more detailed investigations into the relationships between characteristics of physical activity (11) and falls (12).
Contemporary physical activity monitors are small, noninvasive devices that can be worn on various body locations and collect data continuously for several days at a time. They provide instantaneous measures of movement for up to 100 observations per second in 3 orthogonal axes and are widely used to quantify volumes and fragmentation (11,13) of daily physical activity and sleep (14). Furthermore, it has been shown in laboratory settings that such detailed data can also be used to estimate kinematic characteristics of movement (15), particularly gait cadence, or the rate of steps taken in the fixed unit of time during walking (16). More recently, accelerometry-based monitoring of gait characteristics has successfully migrated from the laboratory environment to use outside of the research or clinical setting (17), with devices typically worn on the lower back (18), hip (19), thigh (20), or ankle (21). This shift has allowed in-depth assessment of free-living gait speed, cadence, and daily ambulation, and their respective associations with falls risk (22–24). These studies, among others, have fundamentally enhanced epidemiological and clinical research by proving that mobility assessment can be done remotely and that free-living movement contains information related to fall risk that may not be captured in laboratory settings.
Over the past several years, the placement of accelerometers in many epidemiological studies has shifted to the wrist location to facilitate participant compliance and allow 24-hour monitoring of physical activity and sleep on multiple days (13). While establishing a set of comprehensive characteristics of free-living mobility in wrist-worn accelerometry is still under active development, promising analytical methods focused exclusively on gait cadence have begun to surface (25,26). Cadence is proportional to gait speed and can be directly estimated from subsecond level accelerometry data. It is a highly interpretable metric, expressed in steps/minute, that is correlated with gait speed, and translates well into public health and clinical communications.
Estimation of gait cadence using wrist-worn accelerometry collected in an unsupervised, free-living environment is particularly challenging due to noise introduced by movements of the wrist. In addition, when focusing on older individuals, including ones characterized by low mobility and function, sparse walking bouts, and weak signatures of ambulation in the data are to be expected. Although the promise of detailed characterization of the functional spectrum through the variability observed in a large number of measurements taken across the whole day, and over multiple days is undoubtfully attractive, we are unaware of publicly available data-processing methods that could achieve such level of precision for wrist-worn accelerometry in older adults. We, therefore, focus on the central tendency of free-living cadence, previously evaluated in free-living settings (27). To our knowledge, such detailed characterization of gait cadence using wrist-worn accelerometers has not been obtained before in a large sample of older, low-mobility individuals.
We used an open-source pattern recognition algorithm (28) to extract free-living gait cadence from data collected by wrist-worn accelerometers in a large sample of community-dwelling older adults as a part of the Study to Understand Fall Reduction and Vitamin D in You (STURDY) (29). We compared the efficacy of free-living gait cadence to traditional clinic- and laboratory-based measures of mobility, including gait cadence and speed during a standardized 6-minute walk test (30), usual gait speed over a 4-m course measured as part of the Short Physical Performance Battery (SPPB) (9), and total distance traveled during the 6-minute walk in the prediction of fall rates. We hypothesized that free-living, accelerometry-derived gait cadence would better predict falls than traditional laboratory-based assessments of mobility.
Method
Participants
Data were collected as a part of the STURDY, a randomized clinical trial, funded by the National Institute on Aging (29). STURDY was designed to test the effects of 4 doses of vitamin D (200, 1 000, 2 000, and 4 000 IU/day) on the occurrence of falls in older adults at elevated risk for falling. STURDY collected data at 2 centers in Maryland, ProHealth in West Baltimore, and the Comstock Center in Hagerstown, between October 2015 and June 2019. The primary outcome of STURDY was the time to first fall (or death) over 2 years of intervention, and the secondary outcome was gait speed (31). A total of 688 participants (300 women) were enrolled. Inclusion criteria required participants to be at least 70 years of age, have had at least 2 falls or one fall with injury in the year prior to recruitment, or a self-reported fear of falling, and have a serum vitamin D level of 10–29 ng/mL. Participants were followed for up to 2 years across 4 in-person visits scheduled at baseline, 3 months, 12 months, and 24 months.
Measurements
Height (cm) and weight (kg) were measured using standard protocols, and age, sex, and race were assessed by questionnaire during the clinic visit. Usual gait speed was measured as a part of the SPPB. Participants were asked to walk at their usual pace over a 4-m course without stopping. The task was performed twice, and the time of each trial was measured using a stopwatch and converted to speed in meters per second (m/s). The faster of the 2 trials was used for analysis. For the 6-minute walk test, participants were instructed to “cover as much ground as possible” on a course marked by 2 cones, spaced 10 m apart. Participants were able to stop and rest during the test or report to the clinic staff that they were unable to finish. Participants did not attempt the 6-minute walk if they had a history of one of the following conditions: heart attack, angioplasty, heart surgery, new or worsening chest pain or pressure, experienced new or worsening symptoms of angina, or experienced new or worsening shortness of breath at rest or low exertion, or had a systolic blood pressure >180 mmHg, diastolic blood pressure >100 mmHg, or resting heart rate >120 bpm. Additionally, participants needing to use crutches or a scooter for daily ambulation were excluded from the 6-minute walk test. The distance covered during the 6-minute walk was recorded in meters and average speed was derived in m/s.
Falls Assessment
A fall was defined, following the World Health Organization characterization, as any fall, slip, or trip in which the participant lost his or her balance and landed on the floor or ground or at a lower level (32). Falls were ascertained using a combination of monthly calendars, scheduled quarterly contacts, and ad hoc telephone reports, with calendars used as a gold standard (33). The field centers provided each participant with a blank falls calendar and a postage-paid envelope with instructions to mail a completed calendar at the end of each month. Participants were instructed to mark at the end of each day (or in the morning of each subsequent day) whether they fell. A standardized follow-up interview was administered to obtain details about when the fall occurred, the circumstances of the fall, and any resulting injuries or treatment. If a fall was marked on a received calendar and the fall had not previously been reported to the center, the participant was called by an interviewer who administered the fall follow-up interview. If a calendar was not received by mail as expected, an interviewer called to inquire about the calendar status and remind the participant to mail the completed calendar. If a previously unreported fall was reported during a missing calendar inquiry call or during one of the routine trimonthly telephone calls, the caller administered the fall follow-up interview (31).
Rates of falls per year were assessed by dividing the number of reported falls by the exact time between baseline and the participant’s most recent clinic visit. Participants with only 3 months of follow-up were excluded from the analysis, under the assumption that such a short exposure may not be representative of the prevalence of falls in the population. Distributions of rates of falls were inspected for outliers to identify potential measurement errors.
Accelerometry Measures
At each clinic visit, participants were equipped with the Actigraph GT9X Link wearable physical activity monitor (Actigraphcorp, Pensacola, FL) collecting at a sampling frequency of 80 Hz (80 observations per second per axis) in 3 orthogonal axes. They were instructed to wear the device continuously for 7 days on the nondominant wrist and remove it only for swimming and bathing lasting longer than 30 minutes. The device was initialized to start collecting the data immediately before the 6-minute walk and to stop after seven 24-hour periods. Upon completion of the data collection period, participants returned the device to the clinical center in preaddressed, prepaid envelopes. Participants who reported using a walker or scooter for daily ambulation were excluded from the accelerometry portion of the study.
Raw data were converted to minute-by-minute epochs of activity counts using Actilife software (version 6.13.3), and 90 minutes of consecutive zero-value observations were labeled as missing due to nonwear (34). Days with more than 144 minutes (10%) of the day missing were labeled as invalid and removed. Data for participants with less than 3 valid days were deemed not representative and not included in the analysis. For periods of missing data on valid days, the average activity counts observed in the same period across other valid days were imputed (35). Mean total daily activity counts (TAC), defined as a sum of activity counts observed on valid days divided by the number of valid days, were used as a metric of physical activity volume. Additionally, raw, subsecond level data were converted from the native GT3X format into comma-separated values to estimate gait cadence.
Estimation of Gait Cadence
The Adaptive Empirical Pattern Transformation (ADEPT) method was used to segment walking strides (a stride is defined as 2 consecutive steps) in the vector magnitude of triaxial, subsecond level accelerometry data. ADEPT is a dictionary-based, statistical pattern recognition algorithm validated for identifying the timing and the duration of strides in high-density time series data collected by wrist-worn accelerometers (28). It is freely available to download in the form of the R-package (36) together with documentation (36), example data (37), and user tutorials (38,39) and has been previously used for the estimation of average free-living cadence (27). The algorithm uses a predefined pattern of stride and detects its repetitions by maximizing the local correlation between the series of time-transformed patterns and measured accelerometry at every time point. The time transformation manipulates the duration of the dictionary pattern, allowing for the detection of free-living strides that are shorter or longer than the baseline dictionary pattern.
First, for each participant, 4 consecutive strides (8 steps) were manually segmented by marking the heel-strike events (20) in the data. For participants who attempted the 6-minute walk, 4 consecutive strides were manually marked in the portion of the data corresponding to the beginning of their walk (first or second lap). In participants who did not attempt the 6-minute walk, 4 consecutive strides were marked in manually identified bouts of walking during the first hour when the accelerometer was worn. The example of a random participant’s walking acceleration data used for manual segmentation is presented in the top panel of Supplementary Figure 1. Manually segmented strides were then evaluated by a second reviewer, not exposed to any of the study data previously. The evaluation included scoring whether the segmented data contained walking (yes, no) and if the manually segmented stride durations were correctly identified (yes, no). The reviewers disagreed in a total of 4 out of 432 cases.
Next, durations of manually segmented strides were normalized by linearly interpolating the data to an angular domain (ranging from 0 at the beginning, to 1 at the end), resulting in strides of equal length. To obtain subject-specific patterns, duration-normalized strides were averaged across 4 consecutive strides. The middle panel of Supplementary Figure 1 represents normalized strides (gray) and the resulting average pattern. Lastly, the average stride pattern was repeated 4 times, producing the final, subject-specific template to be used in the remainder of the ADEPT procedure (Supplementary Figure 1).
For each participant, stride patterns were time-scaled in 12.5-millisecond increments, ranging gradually from 60% to 125% of the average duration of a manually obtained within-subject template. Next, the ADEPT algorithm was used to segment the data into nonoverlapping time intervals consisting of 4 consecutive candidate strides, across all valid days. These intervals were then characterized by the values of cadence, correlation with the stride pattern, and the mean absolute deviation (MAD) of the amplitude. Segments with a correlation of less than 0.7 (interpreted in medical research as “high” (40)) and MAD less than 0.01 g were assumed to represent nonwalking bouts and removed (17) leaving only bouts interpreted as consisting of 4 consecutive strides each and used in further analysis. The Parzen kernel mode estimate of cadence was calculated across all strides detected on valid days and used as a predictor in the statistical analyses.
Study Sample
Of 688 randomized STURDY participants, 24 were not included in the accelerometry measurements and 189 were not followed for at least 12 months, primarily because the trial ended before they reached 12 months of follow-up. An additional 9 participants with more than 10 falls per year and 1 participant with a usual gait speed greater than 1.9 m/s were excluded from further analysis because of potential measurement error. After processing the accelerometry data, 17 participants with less than 3 valid wear-days and 11 participants where neither 6-minute nor free-living walking could be manually identified were excluded. The ADEPT algorithm was unable to identify walking bouts for cadence estimation in 5 participants. The final sample consisted of 432 participants, of whom 394 attempted and 38 did not attempt a 6-minute walk. The corresponding data flow diagram is presented in Supplementary Figure 2.
Statistical Analysis
The primary analysis involved 394 participants who attempted the 6-minute walk (“main sample”). The race was dichotomized into White (N = 316, 80%) and non-White (N = 78, 20%). Vitamin D randomization group membership was dichotomized into 200 IU and more than 200 IU (ie, 1 000, 2 000, and 4 000 IU). Categorical variables were compared using chi-squared tests and continuous variables using the analysis of variance. Falls per year were compared across age groups using Fisher’s exact test for count data.
Negative binomial regression was used to model rate ratios (RRs) of falls per year with 95% confidence intervals (CIs). The primary predictors were measured at baseline and run in separate models: accelerometry-derived free-living cadence, accelerometry-derived cadence during the 6-minute walk, usual gait speed during the 4-meter walk, 6-minute walk gait speed, and distance covered during the 6-minute walk. All models were adjusted for age, sex, race, weight, height, vitamin D randomization group, and total activity counts per day (TAC). The exposure time for negative binomial regression was defined as the time difference between the baseline visit and the last follow-up visit and expressed in years. Gait speed predictors were multiplied by a factor of 10 so the estimated effects were expressed for each 0.1-unit increment (0.1 m/s). Cadence metrics and the distance covered during the 6-minute walk were divided by a factor of 10 so the effects expressed 10-unit increments (10 steps/minute for cadence and 10 m for the distance covered). Histograms of each primary predictor were visually inspected for outliers and normality.
An α level of 0.05 was used as a threshold for statistical significance. Additional analyses were performed on an expanded sample that included the 394 participants from the main sample and 38 additional participants who had not attempted the 6-minute walk but had template stride patterns estimated manually using free-living data (“expanded sample”; N = 432). Statistical models for the expanded sample were limited to free-living cadence and usual gait speed due to the absence of 6-minute walk data. All expanded sample models were created in the same way as the main sample.
Finally, to determine whether results differed by physical functioning, the same analyses were repeated in the sample stratified by free-living gait cadence and, separately, by usual gait speed (from the 4-m walk). Participants were stratified by free-living gait cadence <100 versus ≥100 steps per minute, as gait cadence of 100 or more steps per minute has been shown to correspond to engaging in moderate-to-vigorous physical activity (41). Analogously, gait speed groups were dichotomized at <0.8 versus ≥0.8 m/s, as gait speeds below this threshold have been associated with risk of frailty and disability (42). All analyses were done using R statistical software (version 3.6.1) with the MASS package (version 7.3-51.4). As the analysis included only planned testing following the main hypothesis focused only on mobility and utilized all metrics collected in the study, there was no adjustment of significance levels for multiple comparisons (43).
Results
The baseline participant demographic and gait characteristics, as well as falls per year during study follow-up, stratified by free-living gait cadence (<100 steps/minute [n = 142] and ≥100 steps/minute [n = 252]) and by usual (4 m) gait speed (<0.8 m/s [n = 131] and ≥0.8 m/s [n = 263]) are presented in Table 1. Participants in the <100 steps per minute group tended to be men, of greater height and weight, and to have lower daily physical activity (TAC) and slower gait speeds across all measures. Table 2 presents the correlations between study covariates. Among all metrics of mobility, gait speed (4-m and 6-minute walk) and distance covered during a 6-minute walk were significantly associated with age, whereas gait cadence (free-living and 6-minute walk) showed no association with age. All metrics of mobility were significantly correlated with one another (p < .05 for all). Additionally, Supplementary Table 1 summarizes baseline characteristics of study participants by age group. Baseline characteristics of metrics of mobility used in the study are represented in Supplementary Table 2.
Table 1.
Baseline Characteristics and Falls Per Year of Study Participants Stratified by Accelerometry-Derived Free-Living Cadence and by Usual Gait Speed
| Stratified by Free-Living Cadence* | |||
|---|---|---|---|
| <100 steps/min (n = 142) | ≥100 steps/min (n = 252) | p | |
| Age (years), mean (SD) | 77.85 (5.92) | 76.98 (5.17) | .131 |
| Male sex, n (%) | 99 (69.7) | 134 (53.2) | .002 |
| Non-White race, n (%) | 35 (24.6) | 43 (17.1) | .093 |
| Height (kg), mean (SD) | 170.43 (9.33) | 166.90 (9.27) | <.001 |
| Weight (kg), mean (SD) | 92.15 (20.18) | 81.79 (15.48) | <.001 |
| Total activity counts (×106), mean (SD) | 1.8684 (0.5422) | 2.0011 (0.5959) | .029 |
| 200 IU vitamin D group, n (%) | 74 (52.1) | 128 (50.8) | .884 |
| Falls per year, median [IQR] | 0.85 [0.00, 1.59] | 0.83 [0.00, 1.50] | .995 |
| Cadence (free-living) [steps/min], mean (SD) | 93.09 (6.58) | 109.54 (6.81) | <.001 |
| 6-minute walk cadence (steps/min), mean (SD)* | 98.82 (10.53) | 112.61 (8.81) | <.001 |
| Usual gait speed (m/s), mean (SD)† | 0.80 (0.22) | 0.96 (0.20) | <.001 |
| 6-minute walk gait speed (m/s), mean (SD) | 0.80 (0.23) | 0.97 (0.18) | <.001 |
| 6-minute walk distance (m), mean (SD) | 285.76 (84.90) | 349.50 (65.47) | <.001 |
| Stratified by Usual Gait Speed† | |||
| <0.8 m/s (n = 131) | ≥0.8 m/s (n = 263) | p | |
| Age (years), mean (SD) | 78.28 (6.05) | 76.80 (5.08) | .011 |
| Male sex, n (%) | 78 (59.5) | 155 (58.9) | .995 |
| Non-White race, n (%) | 41 (31.3) | 37 (14.1) | <.001 |
| Height (kg), mean (SD) | 167.50 (9.30) | 168.51 (9.50) | .316 |
| Weight (kg), mean (SD) | 89.13 (19.80) | 83.72 (16.79) | .005 |
| Total activity counts (×106), mean (SD) | 1.8350 (5.4550) | 2.0121 (5.8854) | .004 |
| 200 IU vitamin D group, n (%) | 61 (46.6) | 141 (53.6) | .226 |
| Falls per year, median [IQR] | 0.95 [0.00, 1.88] | 0.61 [0.00, 1.48] | .207 |
| Free-living cadence (steps/min), mean (SD)* | 99.23 (11.25) | 105.79 (9.19) | <.001 |
| 6-minute walk cadence (steps/min), mean (SD)* | 101.27 (12.34) | 110.82 (9.69) | <.001 |
| Usual gait speed (m/s), mean (SD) | 0.66 (0.10) | 1.02 (0.16) | <.001 |
| 6-minute walk gait speed (m/s), mean (SD) | 0.75 (0.21) | 0.99 (0.17) | <.001 |
| 6-minute walk distance (m), mean (SD) | 267.20 (81.04) | 356.08 (59.15) | <.001 |
Note: SD = standard deviation; IQR = interquartile range.
*Free-living cadence and 6-minute walk cadence were derived from accelerometry data.
†Usual gait speed was measured over a 4-m distance as part of the Short Physical Performance Battery.
Table 2.
Correlation Between Study Covariates
| Age (years) | Weight (kg) | Height (kg) | Total Activity Counts (× 106) | Falls Per Year | Free-Living Cadence* (steps/min) | 6-Minute Walk Cadence (steps/min) | Usual Gait Speed† (m/s) | 6-Minute Walk Gait Speed (m/s) | |
|---|---|---|---|---|---|---|---|---|---|
| Weight (kg) | −0.21* | ||||||||
| Height (kg) | −0.06 | 0.50* | |||||||
| Total activity counts (× 106) | −0.22* | −0.19* | −0.21* | ||||||
| Falls per year | 0.10* | −0.04 | −0.04 | −0.02 | |||||
| Free-living cadence* (steps/min) | −0.08 | −0.35* | −0.21* | 0.19* | −0.07 | ||||
| 6-minute walk cadence (steps/min) | −0.05 | −0.26* | −0.18* | 0.18* | −0.04 | 0.76* | |||
| Usual gait speed† (m/s) | −0.14* | −0.12* | 0.10* | 0.17* | −0.06 | 0.37* | 0.47* | ||
| 6-minute walk gait speed (m/s) | −0.21* | −0.19* | 0.17* | 0.19* | −0.08 | 0.49* | 0.66* | 0.69* | |
| 6-minute walk distance (m) | −0.21* | −0.20* | 0.17* | 0.20* | −0.08 | 0.48* | 0.65* | 0.69* | 0.99* |
*Free-living cadence and 6-minute walk cadence were derived from accelerometry data.
†Usual gait speed was measured over a 4-m distance as part of the Short Physical Performance Battery.
*p < .05.
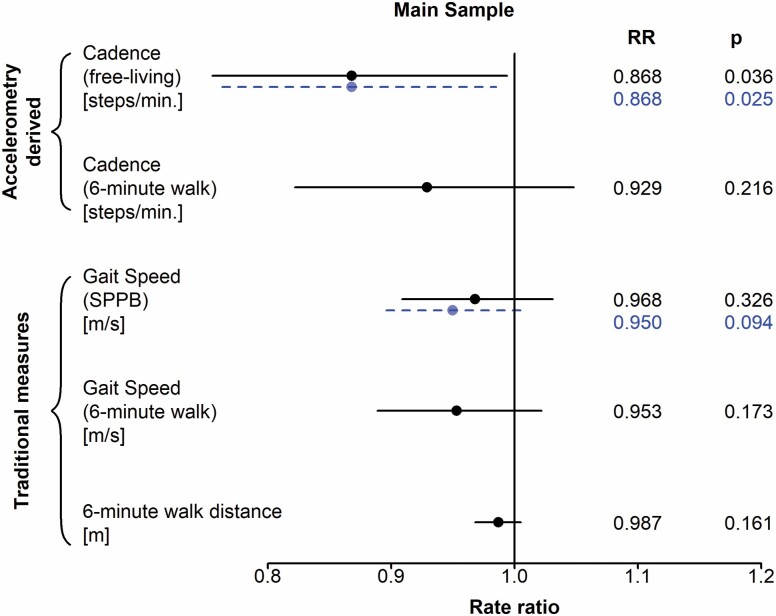
Based on adjusted negative binomial regression models incorporating each baseline gait predictor separately, only free-living gait cadence derived from accelerometry showed a significant association with falls per year, in the main sample (N = 394, Table 3). Specifically, for each 10 steps per minute faster gait cadence, the rate of falls was 13.2% lower over follow-up (RR = 0.868, CI = 0.755, 0.994; p = .036). Laboratory-based gait metrics, namely, 6-min gait cadence (RR = 0.929, CI = 0.822, 1.048; p = .216), usual 4-m gait speed (RR = 0.968, CI = 0.909, 1.031; p = .326), 6-minute gait speed (RR = 0.953, CI = 0.889, 1.022; p = .173), and 6-minute walk distance (RR = 0.987, CI = 0.968, 1.005; p = . 161) were not significantly associated with rates of falls. After expanding the sample to include the 38 participants who did not attempt the 6-minute walk (N = 432), the associations with free-living gait cadence and fall rates (RR = 0.868; CI = 0.763, 0.985; p = .025) and 4-m gait speed and fall rates (RR = 0.950; CI = 0.896, 1.010; p = .094) remained essentially unchanged. Results of all regression models are presented in the forest plot in Figure 1.
Table 3.
Regression Model Rate Ratio Results for Each Cadence and Gait Predictor of Falls Per Year
| Main Sample* (N = 394) | Expanded Sample† (N = 432) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted‡ | Unadjusted | Adjusted‡ | |||||
| Cadence (free-living) | 0.897 | 0.868 | 0.883 | 0.868 | ||||
| 95% CI (p value) | 0.789, 1.016 (.078) | 0.755, 0.994 (.036*) | 0.784, 0.992 (.031*) | 0.763, 0.985 (.025*) | ||||
| Cadence (6-minute walk) | 0.945 | 0.929 | — | — | ||||
| 95% CI (p value) | 0.843, 1.058 (.310) | 0.822, 1.048 (.216) | ||||||
| Usual gait speed (SPPB) | 0.972 | 0.968 | 0.958 | 0.95 | ||||
| 95% CI (p value) | 0.918, 1.029 (.334) | 0.909, 1.031 (.326) | 0.908, 1.010 (.120) | 0.896, 1.007 (.094) | ||||
| Gait speed (6-minute walk) | 0.958 | 0.953 | — | — | ||||
| 95% CI (p value) | 0.903, 1.016 (.153) | 0.889, 1.022 (.173) | ||||||
| 6-minute walk distance | 0.988 | 0.987 | — | — | ||||
| 95% CI (p value) | 0.973, 1.004 (.152) | 0.968, 1.005 (.161) | ||||||
| Cadence (free-living)
≥100 steps/min (N = 252) |
Cadence (free-living)
<100 steps/min (N = 142) |
Cadence (free-living)
≥100 steps/min (N = 278) |
Cadence (free-living)
<100 steps/min (N = 154) |
|||||
| Unadjusted | Adjusted‡ | Unadjusted | Adjusted‡ | Unadjusted | Adjusted‡ | Unadjusted | Adjusted‡ | |
| Cadence (free-living) | 0.738 | 0.723 | 0.867 | 0.795 | 0.756 | 0.746 | 0.877 | 0.833 |
| 95% CI (p value) | 0.577, 0.945 (.013*) | 0.559, 0.933 (.010*) | 0.625, 1.167 (.367) | 0.562, 1.096 (.179) | 0.602, 0.948 (.014*) | 0.590, 0.943 (.013*) | 0.639, 1.169 (.383) | 0.595, 1.137 (.257) |
| Cadence (6-minute walk) | 0.820 | 0.799 | 1.081 | 1.045 | — | — | — | — |
| 95% CI (p value) | 0.689, 0.975 (.033*) | 0.666, 0.958 (.018*) | 0.882, 1.322 (.457) | 0.845, 1.291 (.685) | ||||
| Usual gait speed (SPPB) | 0.954 | 0.953 | 0.993 | 0.996 | 0.945 | 0.934 | 0.981 | 0.985 |
| 95% CI (p value) | 0.882, 1.032 (.251) | 0.875, 1.037 (.273) | 0.905, 1.091 (.894) | 0.895, 1.110 (.946) | 0.878, 1.016 (.134) | 0.864, 1.010 (.094) | 0.898, 1.072 (.684) | 0.889, 1.093 (.781) |
| Gait speed (6-minute walk) | 0.926 | 0.933 | 0.988 | 0.981 | — | — | — | — |
| 95% CI (p value) | 0.850, 1.008 (.090) | 0.843, 1.032 (.186) | 0.899, 1.085 (.803) | 0.880, 1.093 (.728) | ||||
| 6-minute walk distance | 0.979 | 0.980 | 0.997 | 0.994 | — | — | — | — |
| 95% CI (p value) | 0.956, 1.002 (.084) | 0.953, 1.008 (.167) | 0.972, 1.022 (.818) | 0.965, 1.023 (.686) | ||||
| Gait Speed (SPPB)
≥0.8 m/s (N = 263) |
Gait Speed (SPPB)
<0.8 m/s (N = 131) |
Gait Speed (SPPB)
≥0.8 m/s (N = 285) |
Gait Speed (SPPB)
<0.8 m/s (N = 147) |
|||||
| Unadjusted | Adjusted‡ | Unadjusted | Adjusted‡ | Unadjusted | Adjusted‡ | Unadjusted | Adjusted‡ | |
| Cadence (free-living) | 0.896 | 0.937 | 0.915 | 0.807 | 0.862 | 0.926 | 0.931 | 0.846 |
| 95% CI (p value) | 0.746, 1.075 (.218) | 0.765, 1.148 (.507) | 0.755, 1.102 (.337) | 0.663, 0.975 (.022*) | 0.723, 1.028 (.084) | 0.763, 1.123 (.408) | 0.786, 1.099 (.392) | 0.709, 1.004 (.050*) |
| Cadence (6-minute walk) | 0.992 | 1.062 | 0.905 | 0.777 | — | — | — | — |
| 95% CI (p value) | 0.831, 1.185 (.920) | 0.876, 1.290 (.499) | 0.757, 1.079 (.242) | 0.651, 0.924 (.003*) | ||||
| Usual gait speed (SPPB) | 0.999 | 0.993 | 0.915 | 0.812 | 0.981 | 0.972 | 0.901 | 0.829 |
| 95% CI (p value) | 0.903, 1.107 (.983) | 0.894, 1.104 (.897) | 0.755, 1.102 (.376) | 0.661, 0.993 (.047*) | 0.890, 1.082 (.695) | 0.880, 1.075 (.582) | 0.758, 1.066 (.247) | 0.695, 0.986 (.040*) |
| Gait speed (6-minute walk) | 0.990 | 0.996 | 0.921 | 0.873 | — | — | — | — |
| 95% CI (p value) | 0.899, 1.090 (.843) | 0.894, 1.111 (.948) | 0.829, 1.022 (.102) | 0.778, 0.977 (.015*) | ||||
| 6-minute walk distance | 0.996 | 0.998 | 0.980 | 0.965 | — | — | — | — |
| 95% CI (p value) | 0.970, 1.023 (.794) | 0.968, 1.028 (.883) | 0.953, 1.008 (.128) | 0.935, 0.994 (.014*) |
Note: SPPB = Short Physical Performance Battery; CI = confidence interval
*Main sample includes only participants who attempted the 6-minute walk.
†Expanded sample includes participants who did not attempt the 6-minute walk but who had valid accelerometry data.
‡Models adjusted for age, sex, weight, height, race, vitamin D randomization group, and total activity counts.
*p < .05.
Figure 1.
Forest plot depicting rates of falls by separate characteristics of gait cadence, speed, and distance. The solid line represents the main sample consisting only of participants who attempted the 6-minute walk (N = 394). The dashed line represents the expanded sample consisting of all participants with valid accelerometry measurements (N = 432). SPPB = Short Physical Performance Battery.
Among participants with low function, defined by baseline 4-m gait speed <0.8 m/s (N = 131), all laboratory and free-living gait-related predictors were significantly associated with fall rates. For every 10 steps per minute faster free-living gait cadence, fall rates were 19.3% lower (RR = 0.807, CI = 0.663, 0.975, p = .022) over follow-up. For 6-minute gait cadence, each 10 steps per minute faster cadence was related to a 22.3% lower fall rate (RR = 0.777, CI = 0.651, 0.924, p = .003). Faster 4-m gait speed was associated with a 18.8% lower fall rate (RR = 0.812, CI = 0.661, 0.993, and p = .047,), and faster 6-minute gait speed was associated with a 12.7% lower fall rate (RR = 0.873, CI = 0.778, 0.977, and p = .015). For every 10 m greater 6-minute walk distance, fall rates were 3.5% lower (RR = 0.965, CI = 0.935, 0.994, p = .014). Results in the expanded sample remained similar (N = 147); the RR for free-living gait cadence was 0.846 (CI = 0.709, 1.004; p = .050), and the RR for 4-m gait speed was 0.829 (CI = 0.695, 0.986; p = .040). The above results are presented in the forest plot in Supplementary Figure 3.
In participants with higher function, defined by free-living cadence ≥100 steps per minute (N = 252), each 10 steps per minute faster free-living gait cadence was associated with a 27.7% lower rates of falls (RR = 0.723, CI = 0.559, 0.932; p = .010). Findings in the expanded sample (N = 278), which included participants who did not attempt the 6-minute walk, were similar, with 25.4% lower fall rates per each 10 steps per minute faster free-living cadence (RR = 0.746, CI = 0.590, 0.943; p = .013). Additionally, each 10 steps per minute faster 6-minute walk cadence was significantly associated with 20.1% lower fall rates (RR = 0.799, CI = 0.666, 0.958; p = .018). Results of all regression models for participants with free-living cadence ≥100 steps per minute are presented in the forest plot in Supplementary Figure 4. All study results from unadjusted and adjusted models are presented in Table 3.
Discussion
Our findings suggest that free-living metrics of gait cadence are more sensitive indicators of future falls than traditional in-lab measures of gait speed and walking endurance, particularly among those who are higher functioning. Gait speed is a proven prognostic indicator of disability and death in older adults, but associations with falls have been inconsistent (22,44). Additionally, we show that a highly interpretable metric of gait cadence can be estimated from wrist-worn accelerometers even in low-mobility older adults and successfully used as a predictor of future falls. This reinforces findings of previously published works of others that utilized trunk-worn accelerometers and multidimensional prediction (23) and classification models (24) to assess the risk of fall. However, our approach in contrast to these works uses a single and intuitive gait characteristic that makes obtained results more interpretable and easier for future translation into practical clinical settings. To this end, the application of wearable accelerometers may provide heightened opportunities to detect subclinical changes in functional status over and above traditional measures at higher levels of function, when opportunities to intervene may be most effective.
Across all metrics of mobility, only free-living gait cadence was significantly associated with rates of future falls in the nonstratified sample. On average, 10 steps per minute higher baseline free-living cadence was associated with a 13.2% lower rate of falls per year. Presented findings were independent of daily physical activity (TAC) and demographic characteristics. Furthermore, this association was maintained in the expanded sample that included participants who did not attempt the 6-minute walk due to safety concerns. Analyses performed in participants with free-living gait cadence ≥100 steps per minute strengthened these findings by showing much larger effects, with a 27.7% lower fall rate for every 10 steps per minute higher baseline cadence in the main sample, and a 25.4% lower rate in the expanded sample. This speaks to the importance of objective measurements that better capture mobility in the participant’s natural environment.
Although laboratory-based metrics of cadence and gait speed were not significantly related to fall rates in the nonstratified analyses, similar and expected trends were present across all analyses, generally indicating that higher physical function and mobility are protective in the context of falls. Though free-living gait characteristics and their relation to health require further investigation, it is possible to speculate that measures obtained in the natural environment better represent unbiased, therefore, normal, conditions of individuals across a wide functional spectrum. Conversely, traditional in-lab measurements may suffer from the bias introduced by the experiment itself. Such biases may include both over- and underperformance generated by “white coat syndrome,” as well as conditions that are free from tripping hazards, uneven terrain, or other distractions. Free-living collection of data over multiple days appears to alleviate this bias by enabling researchers to capture characteristics of gait in the participant’s natural environment across a large number of observations. Additionally, traditional lab testing is limited by time and space constraints making some mobility and performance tests impossible to administer. For example, a 4-m walk was used in the STURDY trial to assess the usual gait speed, instead of a more precise 10-m walk due to the space limitation of one of the study clinics. In contrast, wearables can collect ecological data for days, weeks, or even months, providing a more comprehensive representation of physical function across a variety of contexts at the same time reducing the effort of clinical staff and overcoming space limitations.
Indeed, differences between in-clinic and free-living mobility metrics have been reported (45), including studies in older, community-dwelling adults (17,45,46) further justifying efforts to better understand the contrast between both controlled and objective measurements in aging and health research. While traditional in-clinic and novel accelerometry-based metrics of mobility are not independent of one another, in the current study, free-living measurements performed better in the context of falls and show great potential for remote mobility assessment in the growing number of scenarios when access to in-person health care is limited.
Among participants with free-living cadence ≥100 steps per minute, 6-minute walk cadence was associated with fall rates, with a 20.1% lower rate for every 10 steps per minute faster cadence. In the same subsample, results for 4-m and 6-minute gait speeds and 6-minute distance remained statistically insignificant. Furthermore, we observed a significant negative correlation between age and gait speed, but not cadence (Table 2). As gait speed is a function of both step length and cadence (16), these findings suggest that cadence may be more informative in the context of falls and functional status than step length. In contrast, among older adults with slow gait (4-m gait speed <0.8 m/s), all study metrics showed statistically significant relations to fall rates. These findings indicate that among low-functioning, low-mobility individuals, traditional in-lab metrics perform as well as their free-living counterparts, suggesting that persons with low function perform at similar levels despite varying contexts and environments, particularly once gait speed reaches a critically slow level. Moreover, it might explain a lack of consensus across studies on gait speed and falls. Finally, these findings hint that free-living gait metrics may be more appropriate for discerning fall risk among higher-functioning individuals (cadence ≥100 steps/minute), with the potential for improved early detection of functional decline. As gait cadence and speed are just moderately correlated (Table 2), simultaneous measurements of both would be ideal for determining individual contributions of these metrics to overall fall risk. Although gait cadence is a characteristic directly reflected in the accelerometry signals (28), the gait speed is not and can only be approximated from the data (25,26). As methodological research in accelerometry advances, new methods and algorithms for estimation of gait speed will appear giving hope for future research focused on mobility of older adults. As of today, however, gait speed cannot be reliably estimated from wrist accelerometer data collected in free-living, especially in older, low-mobility individuals.
Yearly fall rates were no different across high- and low-mobility groups (Table 1), but participants in the high-mobility groups were more active (higher TAC) and tended to have better 6-minute walk distances, suggesting better fitness. Collectively, these results hint at the previously established (21,47) presence of a U-shaped relationship between mobility and falls where higher-functioning individuals may be more likely to fall due to a higher likelihood of engaging in more intense/outdoor activities. Analogously, low-mobility individuals are at risk of falling due to lower functioning status but do not generate the same exposure risk because of more restricted movement profiles. Although beyond the scope of the current analyses, more research is needed to normalize the association between falls and gait metrics with respect to the amount of time spent active and the intensity of daily activities. Albeit the link between accelerometry-derived characteristics of gait and falls has been previously demonstrated (23,24), to the best of our knowledge, such detailed analyses have never been performed in as large a sample, nor in an older, at-risk population, using data collected by wrist-worn devices.
At its core, the ADEPT algorithm estimates gait cadence by searching for similarities between patterns of in-lab walking and free-living data. Therefore, the free-living results may be biased toward in-clinic gait characteristics. As this assumption is difficult to validate, due to the lack of gold standard measurements of free-living walking, we corroborate our results based on (a) the agreement with previously published in-lab values of cadence in older adults (48–50), (b) the correlation between free-living and in-clinic estimates (Table 2), and (c) independent manual validation of walking patterns by a second reviewer. While not without limitations, we believe that this concordance provides additional confidence in the novel, remote, accelerometry-based characteristics introduced in this manuscript and warrants further research in this area.
As results were obtained in a sample of at-risk older adults, an analogous analytical approach applied to a more representative sample is necessary to fully understand the potential and utility of free-living gait features in the assessment of functional status and the risk of falls. Future, in-depth analysis of these relations should also involve distinguishing between the types of falls in both circumstances (eg, indoor/outdoor) and consequences (eg, injurious/noninjurious) frames (3). Additionally, we recognize that, for further research, more attention should be given to adults with impaired gait and those who use walking aids to better delineate associations between free-living mobility. Finally, the ADEPT algorithm requires manual input for each individual, significantly increasing data-processing time. As of today, scientific research with wearables can still be viewed as challenging due to their novelty, complexity, and size of collected data.
In summary, gait cadence observed in the free-living environment over an extended time provides substantial insights into the risk of falls. This is particularly true among older adults who are low functioning, but whose mobility has not reached critically low thresholds, and thus, may be more responsive to the benefits of intervention and rehabilitation. The novel metrics utilized in this manuscript are freely available through open-source software (28,36), paving the way for others to use more advanced analytical tools in health applications. Therefore, future research focused on validation of the estimates of free-living cadence in clinical and research settings is warranted.
Supplementary Material
Acknowledgments
We are thankful to the participants and the field center staff for their contributions to the STURDY trial.
Contributor Information
Jacek K Urbanek, Division of Geriatric Medicine and Gerontology, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA; Center on Aging and Health, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
David L Roth, Division of Geriatric Medicine and Gerontology, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA; Center on Aging and Health, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Marta Karas, Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Amal A Wanigatunga, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Christine M Mitchell, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, Maryland, USA.
Stephen P Juraschek, Harvard Medical School/Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Yurun Cai, Department of Health and Community Systems, University of Pittsburgh School of Nursing, Pittsburgh, PA.
Lawrence J Appel, Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, Maryland, USA; Division of General Internal Medicine, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Jennifer A Schrack, Center on Aging and Health, Johns Hopkins School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Funding
This work was supported by the National Institutes of Health (grant numbers U01AG047837, K01AG048765 to J.A.S., T32DK007732 to S.P.J., K23HL135273 to S.P.J.) with support from the Office of Dietary Supplements; the Mid-Atlantic Nutrition Obesity Research Center (P30DK072488); and the Johns Hopkins Institute for Clinical and Translation Research (UL1TR003098). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
None declared.
Author Contributions
J.K.U. and J.A.S. designed and conceptualized the analysis. J.K.U. performed the accelerometry data processing and statistical analyses, assembled figures, tables, and wrote the manuscript. J.A.S. designed and supervised accelerometry data collection. M.K. provided technical support for the ADEPT algorithm. C.M.M. provided technical support for data management. L.J.A. served as a principal investigator for the STURDY trial. D.L.R., M.K., A.A.W., C.M.M., S.P.J., Y.C., L.J.A., and J.A.S. provided a critical review and edits to the manuscript and contributed to the interpretation of the results.
Data Availability
Deidentified participant data and data dictionary will be available at https://archive.data.jhu.edu/ starting 1 year after the publication of the main results article (31), contingent upon institutional review board approval.
References
- 1. Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(16):1696–1704. doi: 10.1001/jama.2018.3097 [DOI] [PubMed] [Google Scholar]
- 2. Alexander BH, Rivara FP, Wolf ME. The cost and frequency of hospitalization for fall-related injuries in older adults. Am J Public Health. 1992;82(7):1020–1023. doi: 10.2105/ajph.82.7.1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelsey JL, Procter-Gray E, Berry SD, et al. Reevaluating the implications of recurrent falls in older adults: location changes the inference. J Am Geriatr Soc. 2012;60(3):517–524. doi: 10.1111/j.1532-5415.2011.03834.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu G, Baker SP. Recent increases in fatal and non-fatal injury among people aged 65 years and over in the USA. Inj Prev. 2010;16(1):26–30. doi: 10.1136/ip.2009.023481 [DOI] [PubMed] [Google Scholar]
- 5. Wijlhuizen GJ, de Jong R, Hopman-Rock M. Older persons afraid of falling reduce physical activity to prevent outdoor falls. Prev Med. 2007;44(3):260–264. doi: 10.1016/j.ypmed.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 6. Zhou J, Habtemariam D, Iloputaife I, Lipsitz LA, Manor B. The complexity of standing postural sway associates with future falls in community-dwelling older adults: the MOBILIZE Boston Study. Sci Rep. 2017;7(1):2924. doi: 10.1038/s41598-017-03422-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delbaere K, Sturnieks DL, Crombez G, Lord SR. Concern about falls elicits changes in gait parameters in conditions of postural threat in older people. J Gerontol A Biol Sci Med Sci. 2009;64(2):237–242. doi: 10.1093/gerona/gln014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010 [DOI] [PubMed] [Google Scholar]
- 9. Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 10. Troiano RP, McClain JJ, Brychta RJ, Chen KY. Evolution of accelerometer methods for physical activity research. Br J Sports Med. 2014;48(13):1019–1023. doi: 10.1136/bjsports-2014-093546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wanigatunga AA, Di J, Zipunnikov V, et al. Association of total daily physical activity and fragmented physical activity with mortality in older adults. JAMA Netw Open. 2019;2(10):e1912352. doi: 10.1001/jamanetworkopen.2019.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jefferis BJ, Iliffe S, Kendrick D, et al. How are falls and fear of falling associated with objectively measured physical activity in a cohort of community-dwelling older men? BMC Geriatr. 2014;14:114. doi: 10.1186/1471-2318-14-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu F, Wanigatunga AA, Schrack JA. Assessment of Physical Activity in Adults Using Wrist Accelerometers. Epidemiol Rev. 2022;43(1):65-93. doi: 10.1093/epirev/mxab004. PMID: 34215874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alfini AJ, Schrack JA, Urbanek JK, et al. Associations of actigraphic sleep parameters with fatigability in older adults. J Gerontol A Biol Sci Med Sci. 2020;75(9):e95–e102. doi: 10.1093/gerona/glaa137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang CC, Hsu YL. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors (Basel). 2010;10(8):7772–7788. doi: 10.3390/s100807772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lord SR, Lloyd DG, Li SK. Sensori-motor function, gait patterns and falls in community-dwelling women. Age Ageing. 1996;25(4):292–299. doi: 10.1093/ageing/25.4.292 [DOI] [PubMed] [Google Scholar]
- 17. Urbanek JK, Zipunnikov V, Harris T, Crainiceanu C, Harezlak J, Glynn NW. Validation of gait characteristics extracted from raw accelerometry during walking against measures of physical function, mobility, fatigability, and fitness. J Gerontol A Biol Sci Med Sci. 2018;73(5):676–681. doi: 10.1093/gerona/glx174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hausdorff JM, Lowenthal J, Herman T, Gruendlinger L, Peretz C, Giladi N. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease: effects of RAS on gait variability in PD. Eur J Neurosci. 2007;26(8):2369–2375. doi: 10.1111/j.1460-9568.2007.05810.x [DOI] [PubMed] [Google Scholar]
- 19. Kamada M, Shiroma EJ, Harris TB, Lee IM. Comparison of physical activity assessed using hip- and wrist-worn accelerometers. Gait Posture. 2016;44:23–28. doi: 10.1016/j.gaitpost.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Del Din S, Godfrey A, Rochester L. Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and Parkinson’s disease: toward clinical and at home use. IEEE J Biomed Health Inform. 2016;20(3):838–847. doi: 10.1109/JBHI.2015.2419317 [DOI] [PubMed] [Google Scholar]
- 21. Ramulu PY, Mihailovic A, West SK, Friedman DS, Gitlin LN. What is a falls risk factor? Factors associated with falls per time or per step in individuals with glaucoma. J Am Geriatr Soc. 2019;67(1):87–92. doi: 10.1111/jgs.15609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rispens SM, van Schooten KS, Pijnappels M, Daffertshofer A, Beek PJ, van Dieën JH. Identification of fall risk predictors in daily life measurements: gait characteristics’ reliability and association with self-reported fall history. Neurorehabil Neural Repair. 2015;29(1):54–61. doi: 10.1177/1545968314532031 [DOI] [PubMed] [Google Scholar]
- 23. van Schooten KS, Pijnappels M, Rispens SM, et al. Daily-life gait quality as predictor of falls in older people: a 1-year prospective cohort study. PLoS One. 2016;11(7):e0158623. doi: 10.1371/journal.pone.0158623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weiss A, Brozgol M, Dorfman M, et al. Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-day accelerometer recordings. Neurorehabil Neural Repair. 2013;27(8):742–752. doi: 10.1177/1545968313491004 [DOI] [PubMed] [Google Scholar]
- 25. Fasel B, Duc C, Dadashi F, et al. A wrist sensor and algorithm to determine instantaneous walking cadence and speed in daily life walking. Med Biol Eng Comput. 2017;55(10):1773–1785. doi: 10.1007/s11517-017-1621-2 [DOI] [PubMed] [Google Scholar]
- 26. Soltani A, Dejnabadi H, Savary M, Aminian K. Real-world gait speed estimation using wrist sensor: a personalized approach. IEEE J Biomed Health Inform. 2020;24(3):658–668. doi: 10.1109/JBHI.2019.2914940 [DOI] [PubMed] [Google Scholar]
- 27. Karas M, Urbanek JK, Illiano VP, et al. Estimation of free-living walking cadence from wrist-worn sensor accelerometry data and its association with SF-36 quality of life scores. Physiol Measurement. 2021;42(6). doi: 10.1088/1361-6579/ac067b. PMID: 34049292. [DOI] [PubMed] [Google Scholar]
- 28. Marta K, Marcin S, William F, Jaroslaw H, Ciprian MC, Jacek KU. Adaptive empirical pattern transformation (ADEPT) with application to walking stride segmentation. Biostatistics. 2019;22(2):331–347. 10.1093/biostatistics/kxz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michos ED, Mitchell CM, Miller ER3rd, et al. ; STURDY Collaborative Research Group . Rationale and design of the Study To Understand Fall Reduction and Vitamin D in You (STURDY): a randomized clinical trial of Vitamin D supplement doses for the prevention of falls in older adults. Contemp Clin Trials. 2018;73:111–122. doi: 10.1016/j.cct.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Enright PL. The six-minute walk test. Respir Care. 2003;48(8):783-785. PMID: 12890299 [PubMed] [Google Scholar]
- 31. Appel LJ, Michos ED, Mitchell CM, et al. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults : A Response-Adaptive, Randomized Clinical Trial. Ann Intern Med. 2021;174(2):145-156. doi: 10.7326/M20-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization, ed. WHO Global Report on Falls Prevention in Older Age. World Health Organization; 2008. [Google Scholar]
- 33. Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;2012(9):CD007146. doi: 10.1002/14651858.CD007146.pub3. PMID: 22972103; PMCID: PMC8095069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–364. doi: 10.1249/MSS.0b013e3181ed61a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69(8):973–979. doi: 10.1093/gerona/glt199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karas M, Urbanek JK, Crainiceanu C. ADEPT: Adaptive Empirical Pattern Transformation. R Package Version 1.1.2.; 2019. Accessed March 10, 2021. https://cran.r-project.org/web/packages/adept/index.html
- 37. Karas M, Urbanek JK, Harezlak J, Fadel WF. ADEPT data: Accelerometry Data Sets. R Package Version 1.0.1.; 2019. Accessed March 10, 2021. https://cran.r-project.org/web/packages/adeptdata/index.html
- 38. Karas M, Crainiceanu C, Urbanek JK. Introduction to ADEPT package. June 2019. Accessed March 10, 2021. https://cran.r-project.org/web/packages/adept/vignettes/adept-intro.html
- 39. Karas M, Crainiceanu C, Urbanek JK. Walking strides segmentation with ADEPT. June 2019. Accessed March 10, 2021. https://martakarass.github.io/adept/articles/adept-strides-segmentation.html
- 40. Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69-71. PMID: 23638278; PMCID: PMC3576830. [PMC free article] [PubMed] [Google Scholar]
- 41. Tudor-Locke C, Han H, Aguiar EJ, et al. How fast is fast enough? Walking cadence (steps/min) as a practical estimate of intensity in adults: a narrative review. Br J Sports Med. 2018;52(12):776–788. doi: 10.1136/bjsports-2017-097628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perera S, Patel KV, Rosano C, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci. 2016;71(1):63–71. doi: 10.1093/gerona/glv126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43-46. PMID: 2081237. [PubMed] [Google Scholar]
- 44. Callisaya ML, Blizzard L, Schmidt MD, et al. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age Ageing. 2011;40(4):481–487. doi: 10.1093/ageing/afr055 [DOI] [PubMed] [Google Scholar]
- 45. Warmerdam E, Hausdorff JM, Atrsaei A, et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020;19(5):462–470. doi: 10.1016/S1474-4422(19)30397-7 [DOI] [PubMed] [Google Scholar]
- 46. Atrsaei A, Corrà MF, Dadashi F, et al. Gait speed in clinical and daily living assessments in Parkinson’s disease patients: performance versus capacity. NPJ Parkinsons Dis. 2021;7(1):24. doi: 10.1038/s41531-021-00171-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Quach L, Galica AM, Jones RN, et al. The nonlinear relationship between gait speed and falls: the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. J Am Geriatr Soc. 2011;59(6):1069–1073. doi: 10.1111/j.1532-5415.2011.03408.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait Posture. 2011;34(1):111–118. doi: 10.1016/j.gaitpost.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kressig RW, Gregor RJ, Oliver A, et al. Temporal and spatial features of gait in older adults transitioning to frailty. Gait Posture. 2004;20(1):30–35. doi: 10.1016/S0966-6362(03)00089-4 [DOI] [PubMed] [Google Scholar]
- 50. Jerome GJ, Ko SU, Kauffman D, Studenski SA, Ferrucci L, Simonsick EM. Gait characteristics associated with walking speed decline in older adults: results from the Baltimore Longitudinal Study of Aging. Arch Gerontol Geriatr. 2015;60(2):239–243. doi: 10.1016/j.archger.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data and data dictionary will be available at https://archive.data.jhu.edu/ starting 1 year after the publication of the main results article (31), contingent upon institutional review board approval.