Abstract
Objective:
Among untreated adults, functional impairments associated with ADHD are widespread and cumulative, and can include social, educational, and professional impairments, increased risk of accidents and mortality, and reduced quality of life. Here, we review the most prominent functional impairments in adults with ADHD and summarize evidence describing the potential role of medication in improving outcomes.
Method:
Articles related to the search terms “ADHD,” “adult,” and functional impairments were identified through Google Scholar and PubMed and selected for inclusion based on four criteria: strength of evidence, relevance to current challenges in adult ADHD, impact on the field, and recency of the results.
Results:
We identified 179 papers to support the conclusions on the relationship between ADHD and functional impairments, and the impact of pharmacological therapy on functional impairments.
Conclusion:
This narrative review provides evidence that pharmacological treatment can be effective in minimizing not only the symptoms of ADHD, but its functional consequences as well.
Keywords: attention-deficit/hyperactivity disorder, functional impairment, adult ADHD, stimulant, nonstimulant
Introduction
ADHD is a neurodevelopmental disorder with an estimated worldwide prevalence of approximately 5.3% to 7.1% in children and 4.4% to 5.0% in adults (Kessler et al., 2006; Polanczyk et al., 2007; Willcutt, 2012), making it among the most common psychiatric disorders (Alegría et al., 2007; Reynolds et al., 2015; D. J. Stein et al., 2008). Originally thought to remit in adolescence, ADHD is now understood to frequently continue into adulthood (American Psychiatric Association, 2013). Even in the absence of meeting full diagnostic criteria, residual and impairing symptoms consistent with partial remission persist in approximately 65% of cases (Faraone et al., 2006). Symptoms and associated functional impairments have been shown to fluctuate throughout adulthood in approximately 90% of cases (Sibley et al., 2022).
ADHD is characterized by core symptoms of age-inappropriate inattention, impulsivity, and/or hyperactivity (American Psychiatric Association, 2013), the clinical presentation of which typically changes over the lifespan (Biederman et al., 2000; Franke et al., 2018; Turgay et al., 2012; G. Weiss & Hechtman, 1993). While symptoms of inattention often remain relatively stable from childhood to adulthood, symptoms of hyperactivity in adults might instead present as an inability to relax or internal restlessness, and impulsivity may manifest as impatience, inappropriate risk-taking, or emotional lability (Biederman et al., 2000; Franke et al., 2018; Turgay et al., 2012; G. Weiss & Hechtman, 1993). Symptoms of hyperactivity/impulsivity have also been reported to decline with age more rapidly than symptoms of inattention (Biederman et al., 2000), again suggesting a shift in the disorder as individuals age. Consistent with research describing these changes, diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) have been updated to be more inclusive of the signs and presentations of ADHD in adulthood (American Psychiatric Association, 2013; Epstein & Loren, 2013). Despite this change, ADHD is still considered to be an underdiagnosed, undertreated, and often debilitating condition in adults (Ginsberg et al., 2014).
The DSM-5 bases the diagnosis of ADHD predominantly on the presence of the core symptoms and requires that they “interfere with, or reduce the quality of, social, academic, or occupational functioning” (American Psychiatric Association, 2013). This distinction—the presence of symptoms and the requirement that they impair function—underscores the importance of evaluating each of these separate but related constructs. Practically speaking, what likely matters most to individuals and their families is not how many ADHD symptoms they have, but how the disorder impacts their ability to successfully live their lives. Researchers and clinicians have observed that it is often some type of functional impairment, not the presence of ADHD symptoms alone, that drives individuals to seek treatment for their ADHD in the first place (Faraone et al., 2004; M. D. Weiss et al., 2018). Unfortunately, for clinicians, while the DSM-5 provides well-articulated and clearly defined guidelines for assessing the symptoms of ADHD, it provides little guidance for assessing the impact on function.
Few studies have systematically explored the relationships between ADHD symptoms and functional impairment, and, to our knowledge, none have done so in adults. One analysis in pediatric populations using four large-scale studies found that, on average, symptoms of ADHD (number, frequency, and intensity) accounted for less than 10% of the variance in measures of functional impairment, with correlations rarely greater than r = .5 (Gordon et al., 2006). These results are consistent with a recent post-hoc analysis in children and adolescents, that found change-from-baseline scores on the ADHD Rating Scale, Fourth Edition (a scale assessing symptoms) to only moderately correlate (r ~ .6) with scores on the Weiss Functional Impairment Rating Scale—Parent report (a scale assessing functional impairment associated with, but not unique to, ADHD) (Coghill et al., 2019). These data suggest that, at least in pediatric subjects, symptoms and impairment are distinct but not unrelated constructs. The degree to which this relationship is true in adults has yet to be established.
Although not exclusive to ADHD, executive function deficits (EFDs) are a common (30%–73%) (Biederman, Petty et al., 2006; Brown et al., 2009) and persistent impairment in individuals with ADHD (Biederman et al., 2007; Brown, 2009). While EFDs are referenced in the DSM-5 as an associated cognitive problem whose presence supports an ADHD diagnosis (American Psychiatric Association, 2013), recent research has suggested executive dysfunction may be as prevalent in adult ADHD as symptoms of inattention, hyperactivity, and impulsivity (Adler et al., 2017). Executive dysfunction symptoms frequently have broad consequences, impacting an individual in school, work, and home environments. EFDs can manifest as difficulties in time management, planning, organization of one’s environment, and implementing strategies to successfully execute and complete a pre-defined task. Although EFDs have been documented in individuals of all ages with ADHD, the presence of EFDs may be more impairing for adults. Because adults generally live independently and self-sufficiently and are responsible for their own behavior, they must rely extensively on higher-order executive functions in the course of daily activities (e.g., holding a job, avoiding risk-taking or antisocial behavior that may have serious consequences) (Barkley, 1998; Denckla, 1996) . However, the degree to which EFDs or ADHD’s core symptoms relate, or even contribute, to the functional impairments associated with ADHD have yet to be fully elucidated. Nonetheless, EFDs can exacerbate the functional impairments individuals with ADHD already experience.
Although most ADHD research has focused on the disorder in pediatric subjects (i.e., children and adolescents), increasing numbers of studies have been conducted in adults. In this narrative review, we summarize some of the major areas of functional impairment associated specifically with ADHD in adults. A common methodological challenge to interpreting psychiatric research is the distinction between the presence of signs and symptoms associated with a disorder and meeting its full diagnostic criteria. Here, we attempt to quantify such a distinction wherever applicable, though many longitudinal or retrospective studies employ a “lifetime diagnosis” approach, where either a historic or a present diagnosis of ADHD will warrant inclusion. Symptoms of ADHD are often characterized as a continuous measure in survey research, and “high symptomology” may mean either a greater number of symptoms, greater severity of symptoms, or both, frequently in the absence of a formal ADHD diagnosis. We also attempt to distinguish between adults with ADHD or ADHD symptoms, and adults with a history of childhood ADHD, focusing here primarily on adults affected by ADHD or its symptoms in adulthood where data are available.
Methodology
For this narrative review, articles were collected through PubMed and Google Scholar. Search terms included, but were not limited to “ADHD,” “adult,” and the functional impairments described below (“social impairment,” “romantic relationships,” “peer relationships,” “parenting,” “educational achievement,” “occupational impairment,” “accidents and unintentional injuries,” “non-vehicular accidents and injuries,” “driving,” “mortality,” “risky sexual behaviors,” “substance use and abuse,” “criminal activity,” and “sleep”). Articles were selected for these searches based on four criteria: strength of evidence, relevance to current challenges in adult ADHD, impact on the field (historical and contemporary), and recency of the results. Papers had to meet at least one of the four aforementioned criteria, but preferably multiple criteria. However, as this was a narrative review, strict systematic methodology was not utilized. Overall, 208 papers were selected for inclusion in this review with 179 papers cited to support the conclusions on the relationship between ADHD and functional impairments, and the impact of pharmacological therapy on these functional impairments in the main text. Of these 179 main references, 122 are clinical studies whose details are further outlined in Supplemental Tables 1 and 2. The remaining 57 references cited in the main text were not included in the tables as they were meta-analyses (12), reviews or book chapters (34), or were not clinical studies describing a functional impairment or pharmacological therapy (11).
Domains of Functional Impairment
Social Impairment
Romantic Relationships
The effects of ADHD on social interactions is readily apparent in intimate relationships (Supplemental Table 1). Adults with ADHD have fewer and shorter romantic relationships, lower marital satisfaction, greater relationship maladjustment, and higher rates of divorce than adults without ADHD (Eakin et al., 2004; J. J. S. Kooij, 2018; Minde et al., 2003; Murphy & Barkley, 1996). Adults with ADHD appear to have below-average rates of co-habitation and marriage, thought to imply delayed or difficulty meeting culturally normative developmental benchmarks (Minde et al., 2003). Among adults seeking treatment for ADHD, marital problems are among their chief complaints (Dixon, 1995), and “problems with their spouses” is a frequent complaint from adults with ADHD participating in therapy (M. Weiss et al., 2001). Importantly, impairments in romantic relationships appear to persist as individuals age: in a sample of 60 to 94 year-old participants, adults with ADHD were three times more likely than non-ADHD controls to have never married or be divorced (Michielsen et al., 2015). Objective marital problems notwithstanding, adults with ADHD have more negative perceptions of their marriages and family lives than their non-ADHD spouses, although whether this is derived from greater negative affect among ADHD-affected adults or more positive evaluation by the spouses is unclear (Eakin et al., 2004).
While research has demonstrated an association between childhood ADHD and later perpetration of intimate partner violence (IPV) (X. Fang et al., 2010; Wymbs et al., 2012), fewer studies have been conducted on the occurrence of IPV among individuals with ADHD in adulthood. In a longitudinal observation study of adult men and women (N = 347) referred to an outpatient clinic for commission of IPV, adults diagnosed with ADHD scored significantly higher than non-ADHD IPV offenders on ratings of minor physical aggression (p = .004) and minor and severe psychological aggression (p < .001), but not severe physical aggression (p = .08), injury, or sexual coercion (N. Buitelaar et al., 2020). Hierarchical multiple regression analyses demonstrated that ADHD contributed significantly to the frequency of IPV over and above the effects of age, gender, and psychiatric comorbidities such as anxiety or mood disorders, antisocial or paranoid personality disorders, or substance use disorder (N. Buitelaar et al., 2020). Post-hoc analyses from this same population undergoing IPV treatment (i.e., skills training, therapy, pharmacotherapy) revealed a significant association between ADHD symptoms and IPV: as inattentiveness and hyperactivity/impulsivity symptoms decreased, so did physical and psychological aggression (N. J. L. Buitelaar et al., 2021).
A recent study examined rates of IPV perpetration and victimization in men and women (18–45 years) with ADHD that persisted (n = 39) or remitted (n = 56) in adulthood, versus non-ADHD controls (n = 121) (Wymbs et al., 2019). Using self-report data, adults with persistent ADHD reported significantly higher rates of psychological and physical IPV perpetrating and victimization than those whose childhood ADHD remitted or those never diagnosed with ADHD. Interestingly, adults whose childhood ADHD remitted also reported more physical IPV victimization than controls, though to a lesser extent than those with persistent ADHD. Consistent with previous reports (Guendelman et al., 2016; Wymbs et al., 2012), these findings strongly implicate childhood ADHD as a risk factor for adults both perpetrating and being victims of IPV. This analysis also found that gender did not moderate the association between any IPV outcome and ADHD, suggesting men and women with ADHD may be equally at risk of being both perpetrators and victims of psychological and physical IPV.
Peer Relationships
The impact of ADHD on peer relationships in adulthood is widespread: as a whole, adults with ADHD have worse social skills, significantly greater friendship problems, greater difficulty interacting with members of the opposite sex, and are generally lonelier, compared to their non-ADHD counterparts (Harpin et al., 2016; McKee, 2017; Stickley et al., 2017) (Supplemental Table 1). Even relative to other common psychiatric disorders such as anxiety and depression, adults with ADHD reported significantly fewer social contacts, and worse relationship quality with those contacts (Holst & Thorell, 2020). A social experiment using actors to gauge social responses revealed that the behavioral characteristic of ADHD elicited greater rejection from others, evoked negative moods (especially hostility), and ultimately resulted in interpersonal rejection (similar to that which occurs in response to depressive behavior) (Figure 1) (Paulson et al., 2005). Furthermore, the symptoms of ADHD appear to impact social relationships in a “dose-dependent” manner – having more, and more severe, ADHD symptoms is associated with greater difficulty managing interpersonal conflict and providing emotional support in relationships, and an overall reduced relationship quality (McKee, 2017; Sacchetti & Lefler, 2017). Over the long-term, the accumulated negative impact of these social impairments may contribute to significantly increased loneliness reported in adults with ADHD (Stickley et al., 2017).
Figure 1.
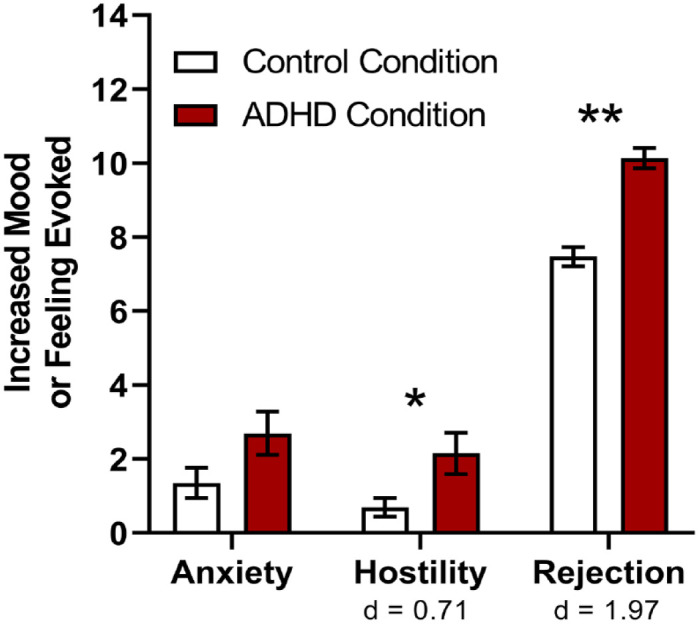
Feelings evoked while watching behaviors characteristic of ADHD. Watching a social interaction in which behaviors characteristic of ADHD were evident (ADHD Condition) invoked more hostility and prompted more rejecting responses than watching an interaction in which no psychopathological behaviors were displayed (Control Condition). Cohen’s d effect sizes were medium and large. Means ± standard error. *p = .012, **p < .01. (Data from Paulson et al., 2005.)
These impairments may be particularly troublesome for young adults transitioning into adulthood through a college or university, a developmental period characterized by psychosocial adjustment, typically paired with a contextual transition into a new environment where many young adults are away from home for the first time (Khalis et al., 2018; Schulenberg et al., 2004). For college students in general, peer relationships and social networks are well known to be crucial support systems during this transitional experience (Buote et al., 2007; Dennis et al., 2005). Not surprisingly, students with ADHD find the transition to a university or college more challenging than their non-ADHD peers, and report more academic problems such as lower grades, lower levels of social skills, and lower self-esteem (Heiligenstein et al., 1999; Shaw-Zirt et al., 2005). Overall, greater ADHD symptomology is associated with worse overall adjustment to social and academic functions in a college or university (Khalis et al., 2018). Conversely, for students with high ADHD symptomology who manage to build peer relationships, greater peer acceptance and reciprocated friendships predicted better university attachment and higher scholastic achievement (Khalis et al., 2018).
Parenting
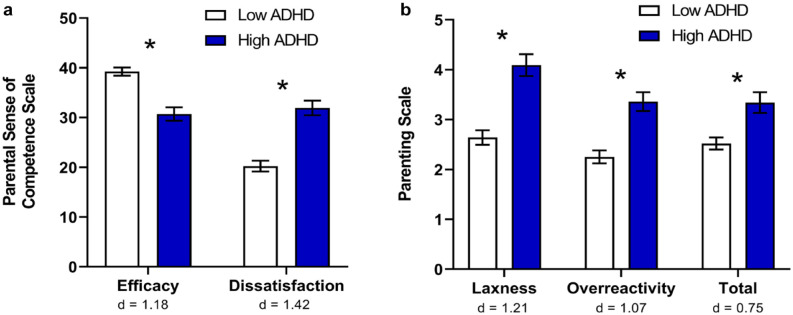
The impact of ADHD in adulthood extends to parenting skills, and also influences the children of parents with ADHD (Supplemental Table 1). Mothers with greater ADHD symptomology have been shown to rate lower on parenting efficacy, involvement, and control, and higher on parenting dissatisfaction, laxness, and over-reactivity, relative to mothers without ADHD (Figure 2) (Banks et al., 2008; Chronis-Tuscano, Raggi et al., 2008). From a qualitative assessment of adults whose partners had ADHD, a common concern was the need to protect children from their ADHD partner’s emotional outbursts (Eakin et al., 2004). One such study assessed the impact of parental ADHD symptoms on interactions with their children (N = 70; 6–10 years old) using self-report, informants, and observation of mother-child interaction (Chronis-Tuscano, Raggi et al., 2008). Mothers with greater ADHD symptomology self-reported lower levels of involvement and positive parenting, and consistent discipline. From researcher observations, maternal ADHD symptoms were negatively associated with positive parenting, and positively associated with negative parenting. These associations were stronger during a structured homework task, relative to a free-play observation period, but nonetheless present during both segments. That the relationship between maternal ADHD symptoms and negative parenting was evident during the unstructured free-play segment suggests that, even when not required to execute a task or place demands on their children, mothers with greater ADHD symptomology are more likely to be negative and critical.
Figure 2.
Parenting variables for mothers with low and high ADHD symptomology. (a) Mothers with greater ADHD symptomology perceived themselves as less effective and felt greater dissatisfaction with their parenting. (b) Mothers with greater ADHD symptomology were more likely to be lax with discipline (e.g., giving in, allowing rules to go unenforced), and more likely to be overreactive (e.g., becoming angry or irritable). Cohen’s d effect sizes ranged from medium to large. *p < .05. (Data from Banks et al., 2008.)
Because an estimated 43% to 57% of parents with ADHD are also raising a child with ADHD (Biederman et al., 1995; Minde et al., 2003), isolating the impact of parental ADHD on children has proven challenging. A 2006 study was among the first to compare a clinically diagnosed (vs. self-reported) sample of mothers with ADHD (n = 30) versus those without (n = 30), while controlling for child ADHD (Murray & Johnston, 2006). This study found that mothers with ADHD demonstrated significantly worse monitoring and knowledge of their child’s behavior, greater parenting inconsistency, and worse problem solving (particularly in terms of the quality of solutions and planning), relative to mothers without ADHD (Cohen’s d effect sizes ranging from 0.72 to 1.82).
Because parenting deficits in monitoring, consistency, and problem solving have been strongly associated with negative consequences in child development (Acker & O’Leary, 1996; Ary et al., 1999; Klein et al., 1997), such impairments may have notable downstream implications for children. For those 43% to 57% of parents with ADHD also raising a child with ADHD (Biederman et al., 1995; Minde et al., 2003), parental deficits in monitoring child medication, treatments, etc. may have further deleterious effects on child well-being.
Interestingly, greater maternal inattention has been uniquely and positively associated with inconsistent discipline and lower involvement with their children, while maternal impulsivity was negatively associated with use of positive reinforcement (M. Chen & Johnston, 2007), suggesting the core symptoms of ADHD may be independently associated with altered parenting styles. An analysis of mothers’ ADHD symptoms and their children’s maladaptive social functioning found a significant positive correlation (r ~ .45–.48) (Griggs & Mikami, 2011). From this sample, using hierarchical regression and controlling for ADHD in their children, mothers’ inattentive and hyperactivity/impulsivity symptoms each significantly contributed to greater social impairment in their children. Among children without ADHD, mothers’ inattention symptoms (but not hyperactivity/impulsivity) also predicted their children receiving greater negative evaluations from playgroup peers. While these data do not explore potential behavioral explanations (e.g., an inability to schedule playdates), they do confirm that having a parent (especially a mother) with ADHD symptoms may confer additional maladaptive functional consequences for children, independent of whether or not the child has ADHD.
Educational Achievement
Decades of research have demonstrated the long-term, pervasive, negative educational consequences of childhood ADHD, but less is known of such effects associated with adult ADHD (Supplemental Table 1). Although the true prevalence of ADHD among college students (i.e., typical adult-level education) is unclear, estimates range from 4% to 8% of students (Weyandt & Dupaul, 2008), consistent with the transition from pediatric to adult ADHD prevalence estimates (Kessler et al., 2006; Polanczyk et al., 2007; Willcutt, 2012). College students who self-reported greater ADHD symptomology were less organized, less methodical in their work, had fewer self-control behaviors, and procrastinated significantly more than low-symptom peers (Turnock et al., 1998). Like their peers with learning disabilities, college students with diagnosed ADHD scored significantly worse than controls on measures of motivation, information processing, and self-testing (Reaser et al., 2007). However, college students with ADHD scored lower than college students with learning disabilities on measures of time management, concentration, identifying main ideas, and test strategies (Reaser et al., 2007). Thus, while students with ADHD may share some academic impairments with students who have learning disabilities, they may also be at risk for additional, ADHD-specific challenges.
A search for moderator variables from a meta-analysis found a trend for improved academic functioning with age, though the reason for this is unclear (Frazier et al., 2007). Possible explanations are that this may reflect a true improvement, whereby either academic performance increases as a consequence of improved ADHD symptomology, or functioning increases, perhaps via the development of compensatory strategies (e.g., students learn to triple check their work, seek additional academic support, etc.). Conversely, it may reflect a methodological artifact, where students with ADHD who struggle academically are more likely to drop out, leaving only higher-functioning subjects in the analysis pool (i.e., college). This would be consistent with prospective studies which found increased academic attrition over time for students with ADHD versus those without. In one longitudinal study, fewer than 30% of adults with childhood ADHD completed a 4-year post-secondary degree (compared to over 75% of those without ADHD), and 27% of the ADHD group did not finish any post-high school education (vs. 5% of the controls) (Kuriyan et al., 2013). Interestingly, this study also found that more adults in the ADHD group finished vocational training (19%) and junior/community college (25%) than controls (6% and 12%, respectively), suggesting perhaps a tendency or preference toward alternate education and training environments. As such, it is important to keep in mind that students with ADHD who successfully finish high school and enter college may represent a distinct, exceptionally functional ADHD subpopulation.
Some evidence has suggested symptoms of inattention, and not hyperactivity/impulsivity, may be more impactful on metrics of academic achievement. For instance, a meta-analysis of college students found a small but significant correlation between student inattentiveness scores and performance-based academic probation (Frazier et al., 2007). Regression analysis based on a large Australian sample (N = 3,795) similarly found that adults with greater inattentiveness (but not hyperactivity/impulsivity) had lower educational achievement than those with better attention scores, independent of other factors such as conduct problems (Ebejer et al., 2012).
Occupational Impairment
ADHD in adulthood has been consistently associated with functional impairments relating to work and economic security (Supplemental Table 1). Relative to non-ADHD controls, adults with ADHD are significantly more likely to experience lower rates of job stability, as indicated by higher rates of “chronic employment difficulties,” being disciplined by supervisors, reporting trouble with colleagues, impulsively quitting a job (sometimes even just due to boredom), or being fired by an employer (Barkley et al., 2010; Murphy & Barkley, 1996). Two separate Norwegian studies found that only 22% to 24% of adults with ADHD were in regular work, compared to 79% of non-ADHD controls, or an overall employment rate of 70% to 72% in the population (Gjervan et al., 2012; Halmøy et al., 2009). In a U.S. sample, the difference was less pronounced, but still significant: only 34% of adults with ADHD were employed full time, versus 59% of controls (Biederman & Faraone, 2006).
Employed adults with ADHD have been consistently reported to have reduced work performance (quality and quantity). Work impairments were found to be among the most problematic (relative to interpersonal, life-satisfaction, etc.), even in a sample of well-educated (~85% college-educated or greater) adults with ADHD (Safren et al., 2010). A study of the workforce of a large U.S. manufacturing company found that ADHD was associated with a highly significant 4% to 5% reduction in work performance, as well as significantly greater odds of sickness and other absences and workplace accident injuries (Kessler et al., 2009; Secnik et al., 2005). These trends appear to be global: in a large-scale 2008 epidemiological study across 10 countries, the World Health Organization demonstrated that, relative to non-ADHD controls, workers with ADHD averaged more sick days and more days associated with reduced work quantity and quality per year (de Graaf et al., 2008). Not surprisingly, employers also notice these impairments: despite being blind to their employees’ psychiatric diagnosis, employers rated employees with ADHD as having significantly greater inattentiveness, impairments in punctuality and time management, and overall poorer work performance (Barkley et al., 2010).
ADHD in adulthood is also associated with significantly lower income relative to non-ADHD peers, regardless of the individual’s academic achievement or other personal characteristics (Biederman & Faraone, 2006; Jangmo et al., 2021). A Canadian study of psychosocial function in parents with ADHD incidentally found that, even among adults with relatively high educational status and achievement, those with ADHD had unexpectedly low annual incomes (Minde et al., 2003). While it is unclear if lower incomes were directly caused by increased work impairments or some other mechanism, these data suggest low income may be orthogonal to personal characteristics (e.g., age, marital status, gender) or even educational achievement. Among older adults (60–94 years, many of whom were retired), income was inversely related to ADHD symptomology: adults with greater ADHD symptomology were more likely to be in the lowest income categories (Michielsen et al., 2015).
Work impairments in adults with ADHD appear to be fairly ubiquitous: in a cross-sectional sample of adults (N = 105) with ADHD, 98% were assessed as having at least mild, and 73% at least moderate, work impairments due to their ADHD psychopathology (Safren et al., 2010). Using bivariate correlation analysis, these work impairments were found to be significantly associated with ADHD symptomology, suggesting ADHD psychopathology may play a direct role in these occupational impairments. Because almost 85% of this sample had a college degree or greater (implying a particularly well-educated sample), and all were on medication for their ADHD, the degree and prevalence of impairment may underrepresent the true value in the population at large.
Attempts to more accurately pinpoint which ADHD-related symptoms and deficits are most associated with work and occupational impairments have identified several candidates. Using regression analysis in a large population-based sample (N = 2,092), the core symptoms of ADHD (inattention, hyperactivity, and impulsivity) significantly predicted unemployment and financial stress, even in subjects with few or mild symptoms (Das et al., 2012). In a smaller study in adults with ADHD (N = 149), symptoms of inattentiveness significantly predicted employment status, while hyperactivity/impulsivity symptoms, number of comorbidities, or sociodemographic characteristics did not (Gjervan et al., 2012). A later mediation analysis on this same ADHD population found a stronger-than-anticipated role for inattentiveness (but not hyperactivity/impulsivity) in predicting occupational outcomes (Gjervan et al., 2016). However, this relationship was completely mediated by scales assessing functional impairments due to physical and emotional problems (Gjervan et al., 2016). Taken together, these data suggest that the core symptoms of ADHD, and especially inattention, are likely implicated in the negative occupational outcomes associated with the disorder but may ultimately be mediated by functional impairments in other areas.
Accidents and Unintentional Injuries
Non-Vehicular Accidents and Injuries
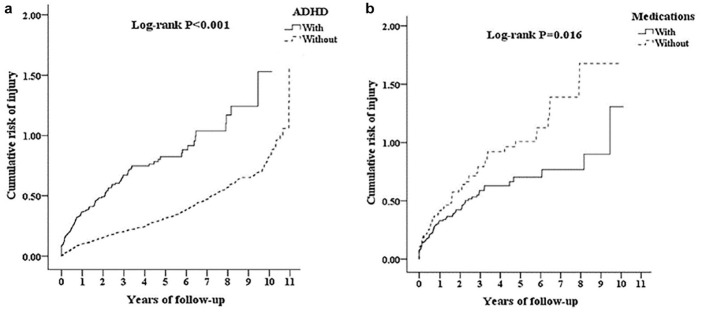
A common way to assess the prevalence and severity of accidents and injuries associated with ADHD is by retrospective analysis of insurance claims databases (Supplemental Table 1). One such analysis from U.S. insurance claims of over 100,000 patients identified 1,308 individuals as having been diagnosed with ADHD at least once in their lifetime (Swensen et al., 2004). Compared to a matched control group, significantly more adults in the ADHD group had an accident claim (38% ADHD vs. 18% controls), and there were significantly more accidents among the ADHD group (accident n = 340) relative to the control group (accident n = 126) (Swensen et al., 2004). From this sample, the likelihood of having an accident was 1.9 times greater (p < .05) for the adult ADHD group than controls, and ADHD was the only significant variable associated with these increased odds (other variables included employment status, comorbidities, age, gender, etc.) (Swensen et al., 2004). A similar analysis from a Taiwanese population found that adults with ADHD had a 143% increased risk of overall injury relative to controls, even after controlling for a variety of confounding factors (Chien et al., 2017). This increased risk appears to be persistent: from this sample, the cumulative risk of injury persisted for the entirety of the 10-year follow-up period (Figure 3a) (Chien et al., 2017).
Figure 3.
Cumulative risk of injury in adults with ADHD with and without medication. (a) The cumulative risk of injury for adults with ADHD (solid line) is significantly greater than for those without (dotted line). (b) Among adults with ADHD, the cumulative risk of injury is significantly greater for those never having received medication for their ADHD (dotted line) than for those who did (solid line). Data are from a Kaplan-Meier analysis and log-rank test. (Picture reprinted with permission from Chien et al., 2017.)
Another large-scale retrospective insurance claims analysis found a higher rate of injury claims among individuals with ADHD (~21%) versus a healthy control group (~16%; p < .0001). Individuals with ADHD also had more months of the year during which they had an injury claim, and more unique injury claims throughout the year, than healthy controls (although these differences were statistically significant, they were numerically small) (Hodgkins et al., 2011). Not surprisingly, the risk for an injury claim was significantly greater in the ADHD group (odds ratio [OR] = 1.32, p < .01, vs. controls). A comparison between adults with ADHD, adults with depression, and non-psychiatric controls found that adults with ADHD were significantly more likely to have injury claims relative to both the control group and individuals with depression.
In another retrospective U.S. claims analysis among all age groups, a lifetime ADHD diagnosis (i.e., diagnosis at any time) significantly increased the risk of a variety of injuries such as joint sprains and strains, open wounds, and limb fractures (Merrill et al., 2009). The rate of severe injuries (e.g., cranial and spinal injuries or fractures) was three times more common among the ADHD group versus the controls and remained significant across all injury categories after controlling for age, sex, and household income. This analysis also found that the rate of injury for ADHD individuals versus non-ADHD controls was greatest among ages 0 to 4 years, followed by ages 20 to 64 years, based on incidents that occurred in the same year as diagnosis of ADHD or after diagnosis with ADHD. These data suggest that, while young children with ADHD may be most at risk of accident injury, adults with the disorder remain highly vulnerable.
Alternatively, several studies have probed data from hospital trauma units to assess whether ADHD increases the risk of accident or injury. Among a Turkish population of hospital-admitted adults (n = 58, ages 18–70) with musculoskeletal trauma, lifetime incidence of ADHD was significantly more prevalent relative to a matched control group (Kaya et al., 2008). When assessed according to trauma severity, adult ADHD was significantly more prevalent among high-energy trauma than low-energy trauma, and more prevalent in individuals with repeated trauma versus those with no previous trauma history. In a similar study examining individuals receiving treatment in German hospital trauma units, adults screening positive for ADHD had significantly greater frequency of accidents in the previous year, and overconfidence about their current accident than the controls (Kittel-Schneider et al., 2019; Wolff et al., 2019). Finally, in an Australian population, significant associations were found between an ADHD diagnosis and injuries, and patients with ADHD had significantly longer hospital stays after controlling for age, gender, socioeconomic status, and cause of injury (Lam, 2002).
Driving
A major area of investigation among ADHD populations is driving-related impairments, which are a frequent source of accidents and injury among adults with ADHD (Supplemental Table 1). Although relative few studies have focused on adult ADHD-associated accidents (relative to those in children), those reporting on the issue have focused largely on driving accidents, suggesting these may be primary sources of injury among adults with ADHD. Typically using self-reports (and less frequently, driving simulations), multiple studies of the driving impairments associated with ADHD revealed that adults with ADHD have significantly more traffic violations (e.g., speeding), license suspensions, vehicular crashes, and related injuries (Barkley, 2004; Barkley et al., 1993; Barkley & Cox, 2007; Barkley et al., 1996, 2002; Jerome et al., 2006, 2006).
Some researchers have incorporated Department of Motor Vehicles records as well as driving simulations to provide a comprehensive, multi-level evaluation. One such study evaluated the driving competencies of young adults (17–30 years) with (n = 25) and without (n = 23) ADHD using structured interviews, behavioral ratings, simulated driving tests, and official motor vehicle records (Barkley et al., 1996). More young adults with ADHD (relative to those without) reported having received a speeding ticket (100% vs. 56%) and at a higher frequency (4.9 vs. 1.3 citations). Relative to adults without ADHD, those with the disorder were also more likely to have been involved in more vehicular crashes as the driver (80% vs. 52%), more total crashes (2.7 vs. 1.6 crashes), and more crashes resulting in injuries (60% vs. 17%). Young adults with ADHD compared to those without ADHD were also more likely to have had their licenses suspended (32% vs. 4%) and were rated by themselves and others as having poorer driving habits. Despite there being no group differences in explicit driving knowledge, in a computer-simulated driving test adults with ADHD had more crashes, scrapes, and erratic steering than adults without ADHD.
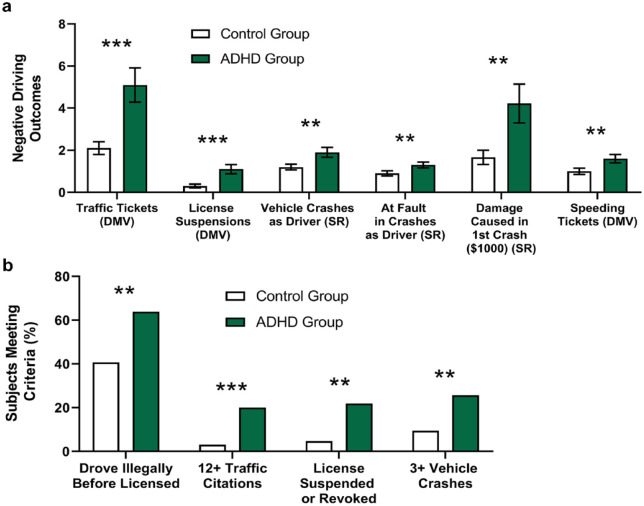
Many of these findings were later replicated in a larger study (Figure 4) (Barkley et al., 2002). On performance tests evaluating inattention (i.e., vigilance), reaction time, and impulsive responding, the ADHD group was substantially less attentive than the control group and showed greater impairment on a reversal learning task (i.e., adjusting behavior in light of new rules). Importantly, subjects with ADHD did not appear to be more impulsive or more impaired on visual discrimination and reaction time components of the task compared to those without ADHD. Instead, these data suggest inattention and difficulties updating rule-governed behavior—both known to be associated with ADHD in children (Barkley, 1997; Corkum & Siegel, 1993)—may be implicated in driving impairments in adults with ADHD.
Figure 4.
Negative driving outcomes. (a) Adults with ADHD had significantly more negative driving outcomes than those without, based on self-report history and official DMV records (means ± standard error). (b) A greater percentage of adults with ADHD met criteria for various negative driving outcomes than the control group, based on self-report history. ** p < .01, *** p ≤ .001. Note. DMV = official Department of Motor Vehicles records; SR = self-report history. (Data from Barkley et al., 2002.)
Another driving simulation study found that, in the absence of alcohol, adults with ADHD had a profile of impairment comparable to that of non-ADHD drivers with blood alcohol levels at the U.S. legal limit (Weafer et al., 2008). When consuming alcohol, adults with ADHD demonstrated additive impairments at each of three alcohol dose levels, faring worse than controls under each condition. These data suggest that, not only are the driving impairments associated with ADHD comparable to those associated with driving under the influence of alcohol, but that the effects of alcohol might compound the consequences of ADHD to considerably impair the driving of adults with ADHD, even at blood alcohol concentrations within the legal limit.
Practically speaking, this increased risk of driving impairments has been associated with greater likelihood of emergency room visits. A large (N = 17,408) longitudinal, population-based Swedish study analyzed the risk of serious driving accidents resulting in a hospital emergency room visit among adults with a history of at least one ADHD diagnosis (Chang, Lichtenstein, D’Onofrio, Sjölander, & Larsson, 2014). Relative to a matched sample with no ADHD history, men and women had greater than twice the risk of serious vehicle accidents, and a ~45% increased rate of serious accidents after controlling for demographic factors, psychiatric diagnoses and medication, and criminal convictions. These data underscore the potentially serious health consequences of ADHD’s functional impairments.
Mortality
That ADHD affects not just quality of life, but lifespan itself has received very little attention or research until recently (Supplemental Table 1). Given the increase in accidents and injuries, it is not surprising that ADHD is also associated with lower life expectancy and elevated mortality rates, particularly deaths due to unnatural causes such as accidents (Dalsgaard et al., 2015; London & Landes, 2016). Using data from longitudinal studies combined with estimate-of-life-expectancy scores, a childhood history of ADHD was associated with 8.4 fewer years of life (and 9.5 fewer years of healthy life) relative to controls without ADHD; if ADHD persisted into adulthood, life expectancy decreased even further by 12.7 years and healthy life by 11.1 years (Barkley & Fischer, 2019). In a meta-review comparison among a variety of psychiatric disorders, the risk of all-cause mortality associated with childhood ADHD was relatively low (1.9 vs. 5.9 in anorexia nervosa, or 2.6 from heavy smoking, included as a non-psychiatric reference), but is nonetheless increased (i.e., almost doubled) relative to the general population (Chesney et al., 2014). While comorbid disorders such as conduct disorder, oppositional defiant disorder, and substance use disorder increased the mortality rate ratio (MRR) even more, ADHD remained associated with additional mortality even after adjusting for these factors (Dalsgaard et al., 2015).
Several large-scale studies found that individuals with ADHD had a significantly increased risk of premature death and overwhelmingly died from unnatural causes (81.6%; adjusted hazard ratio [HR] = 6.48; vs. HR of dying from natural causes = 2.47), primarily from unintentional injury (35.8%; HR = 1.30), suicide (31.4%; HR = 2.09), and even homicide (HR = 2.09) (V. C. Chen et al., 2019; Sun et al., 2019). A global survey of suicidal behavior among psychiatric disorders across 21 countries (N = 108,664) found that suicidal behaviors in respondents with ADHD were increased in both developing nations (ideation OR = 2.2, attempt OR = 2.8) and developed nations (ideation OR = 1.7, attempt OR = 1.9) (all p values < .05) (Nock et al., 2009), further emphasizing the global impact of ADHD.
Girls and women with ADHD appear to have a higher MRR than boys and men (3.01 for females vs. 1.93 for males). Although the difference in female and male MRR did not reach statistical significance (p = .091), it may be of clinical importance in light of sex differences in impairment, diagnosis, and treatment (Dalsgaard et al., 2015). Although the cause of this difference is unclear, as a potential explanation the authors posit that because girls and women are less likely to be diagnosed with ADHD, those who reach the symptom and impairment threshold for referral may be among the most severely ill and impaired, thereby perhaps more at risk of premature death.
These mortality data suggest that the consequences of ADHD are not just cognitive or behavioral, but a major health concern that confers increased risk for early death from both natural and unnatural causes. Although the precise reasons for this increase in mortality are unknown, it is likely the result of multiple factors. Firstly, ADHD is associated with various adverse medical conditions, such as obesity and eating pathologies, type II diabetes, coronary heart disease, upper respiratory infections, and asthma (reviewed in Barkley, 2020) which would directly impact all-cause mortality rates. Beyond these first-order factors, behavioral disinhibition (a component of executive function) was found to explain more than 30% of the variance in life expectancy among individuals with childhood and/or persistent ADHD (Barkley & Fischer, 2019). As previously discussed, EFDs are a common and persistent impairment among individuals with ADHD of all ages. These EFDs and associated disinhibition may play a role in greater engagement in adverse activities associated with increased mortality (e.g., greater risk taking, lower rates of self-care) (Barkley, 2020).
Regardless of the etiology, several studies have demonstrated that early diagnosis (and likely treatment) significantly reduce the risk of mortality (Dalsgaard et al., 2015; Sun et al., 2019), while individuals diagnosed in adulthood have a greater risk of death than those diagnosed in childhood or adolescence (Dalsgaard et al., 2015). Specifically, the all-cause mortality risks significantly increased with the age at first ADHD diagnosis (HRs: ≤12 years old = 1.50; 13–17 years old = 2.69; ≥18 years old = 10.34), suggesting that early diagnosis (and subsequent treatment) may be particularly influential in lowering the risk of death (Sun et al., 2019). Because death is (by many measures) the ultimate functional impairment, these data alone present a compelling case for greater ADHD screening, diagnosis, and treatment, when clinically indicated.
Risky Sexual Behaviors
Adults with a history of ADHD in childhood or adolescence have been reported to engage in a variety of risky sexual behaviors (RSB) (e.g., inconsistent condom use, alcohol use before sex, number of sex partners, having an HIV-positive sex partner), more so than their non-ADHD peers (Supplemental Table 1). For instance, adults with a childhood history of ADHD tend to have intercourse at an earlier age, are less reliable in their use of birth control, have more sexual partners, a higher rate of sexually transmitted diseases (STDs), and higher rates of pregnancy (including early pregnancy, i.e., before age 20 [Akil et al., 2018; Meinzer et al., 2020]) (Barkley et al., 2006; Flory et al., 2006). Although fewer studies have been conducted in adults with current ADHD, the available evidence suggests similar sexual risk-taking. For instance, from a large longitudinal sample of adolescents and young adults diagnosed with ADHD (N = 17,898, vs. 71,592 controls), the ADHD group was more likely to develop an STD than the comparison group, even after controlling for demographic data, psychiatric comorbidities, and ADHD medications (M.-H. Chen et al., 2018).
A smaller study of young adults (ages 19–39, N = 120) used self-reporting to evaluate ADHD symptomology and RSB (Spiegel & Pollak, 2019). Even when controlling for overall level of sexual behavior, ADHD symptoms were positively correlated with RSB. In this study, researchers also inquired as to participants’ perceived benefits and risk of RSBs, and, consistent with research on broader risk-taking associated with ADHD (Shoham et al., 2016, 2021), found that ADHD symptomology positively correlated with participants’ perceived benefit, but not the perceived risk (Spiegel & Pollak, 2019). Mediation analysis demonstrated an indirect pathway, where ADHD symptoms predicted greater perceived benefit of RSB, which subsequently predicted an increased probability of engaging in such behaviors.
Although most studies have been conducted predominantly on men, these trends also appear to be true for women. From a longitudinal study, women 18 to 30 years old (N = 462) were assessed for ADHD symptomology and RSB (Hosain et al., 2012). Women who engaged in RSBs had significantly higher ADHD symptom scores than those who didn’t, and correlations between the RSB scores and ADHD symptoms scores were highly significant (all p’s < .0001). Another longitudinal study identified a clear connection between childhood ADHD and later unplanned pregnancy, where ~43% of girls with ADHD eventually developed an unplanned pregnancy, versus only ~11% of girls without ADHD (E. B. Owens et al., 2017), independent of sociodemographic factors, IQ, or psychiatric comorbidities (E. B. Owens & Hinshaw, 2020; E. B. Owens et al., 2017). Somewhat unexpectedly, this association did not appear to depend on whether the ADHD symptoms persisted into adulthood (E. B. Owens & Hinshaw, 2020; E. B. Owens et al., 2017), suggesting that childhood ADHD may have some long-term, persistent functional consequences in adulthood.
Substance Use and Abuse
It has been well established that a childhood ADHD diagnosis confers a significantly greater risk for later substance use and/or substance use disorder (SUD) (Supplemental Table 1). Indeed, several meta-analyses have confirmed significant and reliable prospective associations between childhood ADHD and future SUD for nicotine, alcohol, cannabis, cocaine, psychoactive substances, and general non-alcohol SUD (Charach et al., 2011; Lee et al., 2011). Further, SUD patients with a lifetime ADHD diagnosis developed SUD at a younger age, transitioned more easily from casual use to addiction, had lower SUD remission rates, and experienced more substance-related hospitalizations relative to SUD patients without ADHD (Arias et al., 2008; Molina et al., 2018). Importantly, the increased risk of substance use becomes increasingly clear in early adulthood: by college age, an ADHD diagnosis has been associated with significantly increased rates of tobacco, alcohol, and drug use on several outcome measures, including higher likelihood of using such substances, using them more frequently, and engaging in more risky (e.g., binge) consumption (Rooney et al., 2012).
These trends were recently replicated in a long-term study of a large, diverse sample of subjects with (N = 547) and without (N = 258) ADHD, who were followed from ages 10 to 25 (Molina et al., 2018). In adulthood, marijuana and cigarette use was more prevalent in the ADHD group. Participants in the ADHD group also engaged in substance use (alcohol, cigarettes, marijuana, and illicit drugs) at an earlier age, and their use escalated more quickly. A later analysis of these same data found that the relationship between peer use and personal alcohol/marijuana use in adolescence was weaker in the ADHD group versus controls (Kennedy et al., 2019). While substance use and experimentation typically wanes among developmentally normative young adults as they exit adolescence, this was not the case in the ADHD group in which heavy drinking in adolescence predicted increased drinking in young adulthood versus decreased use among controls (Kennedy et al., 2019). Although causative explanations were not tested in this study, the authors suggest that for young adults with ADHD, substance use may be only minimally governed by peer and social influence, consistent with reports that adults with ADHD may have difficulty with behavioral and social norms (see Social Impairment, above).
A recent prospective study (N = 807) probed tobacco, alcohol, and marijuana use by age 24 among young men and women with and without ADHD (Elkins et al., 2020). This study classified participants’ ADHD as either remitted (i.e., diagnosed in childhood, but remitted by age 17), persistent (i.e., continuing from childhood into adulthood), or late-onset (i.e., symptoms not identified until age 17 or later), and compared each to non-ADHD controls. By age 24, both persistent and late-onset ADHD groups had comparably high substance abuse rates relative to the ADHD-remitters group, and all ADHD groups had higher rates of substance abuse than the non-ADHD controls. For instance, more persistent (32.5%) and late-onset (40.9%) ADHD participants had a marijuana use disorder relative to the non-ADHD group (12.7%), with a similarly low rate among remitters (17.9%). The increased risk (OR, relative to the non-ADHD group) was significant among persistent (2.64) and late-onset (4.12) ADHD participants, but not remitters (1.16). Not surprisingly, adults whose ADHD had remitted had lower risk for many substance use outcomes than those whose ADHD persisted, suggesting the presence of ADHD is associated with a greater risk of substance use problems versus having only a childhood history of ADHD (Elkins et al., 2020). Somewhat unexpectedly, this study also found that women were at a higher risk for two-thirds of the substance outcomes analyzed. For instance, women with persistent ADHD were over five times more likely to develop tobacco use disorder than women without ADHD, while men did not differ significantly from controls on this measure (increased SUD risk among girls and women has been further described in other studies, e.g., Dalsgaard et al., 2014). The same trend was true for daily smoking and alcohol binge drinking. Because more boys are diagnosed with ADHD than girls, and most ADHD studies have historically been conducted in boys, this might likely indicate an under-studied and under-treated population. Ultimately, this research indicates that the presence or emergence of ADHD in early adulthood increased the risk of substance use problems, a risk to which young women may be particularly vulnerable.
Criminal Activity
A variety of studies have found ADHD to be associated with higher rates of criminal activity, prosecutions, and arrests (Supplemental Table 1). Criminal justice researchers using meta-analysis revealed a strong association (p < .01) between measures of ADHD and criminality, suggesting ADHD may be an important risk factor for crime and delinquency (Pratt et al., 2002). In a U.S. community sample of 1,001 adults, those self-reporting an ADHD diagnosis were more likely to have been arrested (37%, vs. 18% of controls, p ≤ .001) (Biederman, Faraone, Spencer, et al., 2006). Similarly, in a multi-country European study, significantly more adults with ADHD (14%) reported having been prosecuted for criminal acts in adulthood versus controls (6%) (Karlsdotter et al., 2016).
Within a psychiatric sample, ADHD is more likely to be associated with criminal activity than other disorders. One such study compared young adults (18–45 years) with ADHD (n = 50; 30 assessed as also having EFDs) and non-ADHD clinical controls diagnosed with other psychiatric disorders (n = 45, including mood, anxiety, bipolar, and personality disorders) (Holst & Thorell, 2020). Relative to the clinical controls, more participants with ADHD had been arrested by the police (ADHD = 54%, controls = 27%) and had reported engaging in more criminal acts (Cohen’s d = 0.80). When comparing participants from the ADHD group with and without EFDs, those with EFDs reported more criminal acts (d = 0.60), and more arrests (63%, vs. 40% in the non-EFD group). These data highlight again the impact of EFDs in exacerbating the functional impairments of ADHD.
Not surprisingly, ADHD is over-represented in correctional facilities, with prison prevalence estimates thought to be between 25% and 40% (Ginsberg et al., 2010; S. Young et al., 2009, 2015) versus ~5% in the general adult population (Kessler et al., 2006; Polanczyk et al., 2007; Willcutt, 2012). A meta-analysis of data from 15 countries found that, relative to the general population, 30% of incarcerated youth and 26% of incarcerated adults had ADHD (S. Young et al., 2015). Interestingly, this meta-analysis found no significant differences on rates of ADHD among incarcerated men and women (30% vs. 26%, respectively). Practically speaking, because prisons are environments that demand conformity and carrying out orders from correctional officers, there is reason to believe that prison inmates with undiagnosed and/or untreated ADHD might be at a disadvantage for successfully navigating incarceration. Specifically, the inattention, hyperactivity, and impulsive psychopathology associated with ADHD may be misinterpreted as further delinquency and insubordination, and ADHD-associated impairments in learning and social function may further impede efforts at successful coping and rehabilitation. Indeed, an analysis from a Scottish prison population found that ADHD symptomology had a significant impact on the number and severity of critical incidents, particularly verbal and physical aggression, even after controlling for antisocial personality disorder (S. Young et al., 2009). Unfortunately, most incarcerated adults with ADHD participating in research studies report that they have never previously received an ADHD diagnosis (Ginsberg et al., 2010; S. Young et al., 2016), suggesting that participation in these studies might be the first recognition of their disorder.
ADHD is also associated with increased recidivism (Langevin & Curnoe, 2011). A recent study demonstrated that offenders with ADHD have been shown to reoffend not only more frequently, but also more quickly than those without ADHD (6 vs. 25 months, respectively), even when controlling for risk factors such as antisocial personality disorder (Philipp-Wiegmann et al., 2018). Conversely, one longitudinal study found no direct link between childhood ADHD and repeat criminality: although ADHD was six times more prevalent in a population of German male prisoners (relative to the general population), survival analysis did not identify ADHD as a predictor of recidivism (Grieger & Hosser, 2012). However, this analysis did find that offenders diagnosed with ADHD reoffended more quickly after release than those without ADHD (Grieger & Hosser, 2012). Ultimately, although numerous studies point to a role for ADHD in increased recidivism, the issue is sufficiently complex as to warrant greater investigation, particularly to parse out the role of common comorbidities such as substance use disorder, conduct disorder, and antisocial personality traits (Knecht et al., 2015; Langevin & Curnoe, 2011).
Sleep
Various sleep disturbances (insomnia, etc.) are well documented among children and increasingly, in adults with ADHD (Supplemental Table 1). Earlier versions of the DSM even included sleep disturbances (e.g., “moves excessively during sleep”) in the diagnostic criteria for ADHD (American Psychiatric Association, 1980), but such criteria were later removed. As with many outcomes in ADHD research, surveys, clinician interviews, and self-reporting are among the most feasible methods for identifying sleep problems. However, some studies also incorporate neurophysiological measures of sleep quality (e.g., via polysomnography) to provide an objective measure of sleep outcomes. In either method, it is important to isolate the association of ADHD versus comorbidities on sleep problems (e.g., depression, which is independently associated with greater sleep disturbances). Further, because stimulant pharmacotherapies are well known to disturb sleep due to their psychostimulant properties (Cortese et al., 2013), studies probing the association between ADHD and sleep problems should consider whether participants are medicated at the time of study.
A variety of survey-based studies have found that the core symptoms of ADHD are clearly associated with a variety of sleep disturbances, differing only in whether the core symptoms are distinctly associated with various sleep components (Gau et al., 2007; Mahajan et al., 2010). For example, in a small questionnaire-based study of 22 non-medicated adults with ADHD, hyperactivity/impulsivity symptoms were significantly associated with a variety of sleep disturbances, such as lower overall sleep quality, shorter sleep duration, and decreased sleep efficiency (Mahajan et al., 2010). Although total ADHD symptoms and inattention symptoms were also associated with several sleep outcomes, these were not significant after correcting for multiple comparisons. A broader and larger cross-sectional survey study of young adults (N = 2,284) found that inattention symptoms, but not hyperactivity/impulsivity symptoms, were associated with greater sleep need and greater difference between sleep need and sleep duration (Gau et al., 2007). Conversely, hyperactivity/impulsivity, but not inattention symptoms were associated with decreased sleep duration. Other studies have found no such distinction, reporting instead that inattention and hyperactivity/impulsivity symptoms were each independently associated with generally poor sleep quality (Gregory et al., 2017).
Because sleep habits and hygiene are at least in part behavioral (i.e., habitually-driven and subject to learned experiences), adults whose ADHD began in childhood may develop poor sleep hygiene habits or negative associations with their bedtimes and bedrooms that persist into long-term sleep problems, even if their ADHD remits in adulthood. A recent longitudinal study sought to untangle this issue by comparing four groups (N = 2,232): subjects with childhood ADHD which persisted into adulthood (age 18 or later), childhood ADHD which remitted by adulthood, subjects whose ADHD first emerged in adulthood, and a control group with no history of ADHD (Gregory et al., 2017). The authors found that participants with ADHD in adulthood had worse sleep quality than the control group, and this impaired sleep quality did not differ between participants whose childhood ADHD persisted into adulthood and those for whom it emerged only after age 18 (Figure 5). Further, participants whose childhood ADHD remitted in adulthood had no more sleep problems than the control group. These findings were true even after excluding participants taking ADHD medication at the time of the survey and remained after controlling for a wide range of comorbid psychiatric conditions (e.g., depression, anxiety). These data suggest that concurrent (not childhood) ADHD symptoms are associated with adult sleep quality, and that recovery from ADHD-associated sleep problems in childhood may be possible.
Figure 5.
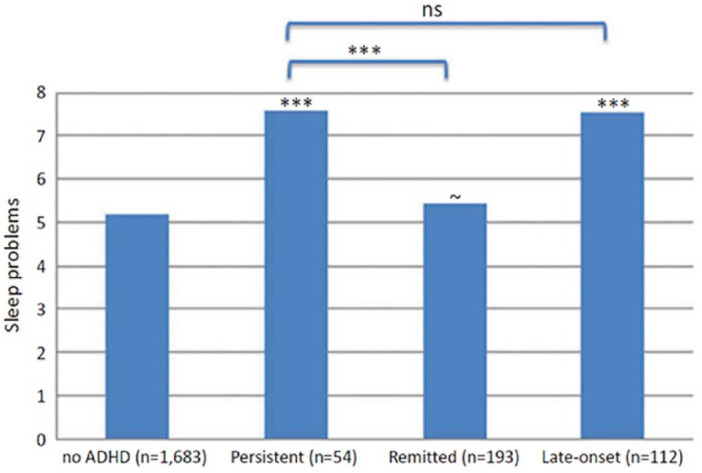
Sleep problems in adults with persistent, remitted, or late-onset ADHD. Sleep problems were greatest among participants whose childhood ADHD persisted into adulthood and those with adult-onset ADHD, versus those whose childhood ADHD remitted in adulthood or those never diagnosed with ADHD at all. Asterisks directly above the bars are for comparisons with the “no ADHD” group and asterisks above the brackets are for comparisons between the groups indicated. ~ p < .10, *** p < .001. Note. ns = not significant. (Reprinted with permission from Gregory et al., 2017.)
Although well studied, the nature of the relationship between ADHD and sleep remains largely unclear. For instance, the directionality of the link between ADHD and sleep disturbances has yet to be elucidated. It is also well known that poor sleep can result in symptoms that mimic those of ADHD (Dahl, 1996; J. A. Owens, 2005), and that improving certain sleep-related problems (e.g., improving nighttime disordered breathing) has been shown to reduce ADHD symptoms (reviewed in Sedky et al., 2014). Although a causal relationship seems plausible, it is also possible that this relationship is due to shared neuroanatomical and/or functional overlap of the brain regions involved in attentional processes, and arousal and sleep regulation, though more research is necessary to fully describe this mechanism (J. Owens et al., 2013).
Impact of Pharmacological Treatment
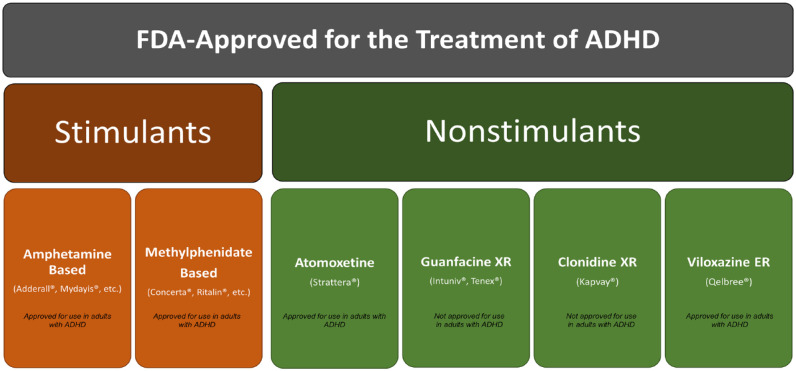
Because most studies demonstrating the efficacy of U.S. Food and Drug Administration (FDA)-approved stimulant (e.g., amphetamines, methylphenidate) and nonstimulant (atomoxetine, guanfacine extended-release, clonidine extended-release, and viloxazine extended-release) medications (Figure 6) to treat ADHD have been conducted in pediatric subjects, the body of knowledge regarding their safety and efficacy in adults is less robust (Supplemental Table 2). Available studies of ADHD medications in adults largely replicate the success demonstrated in children, with adults responding well to many treatments. However, adults appear to have a slightly lower therapeutic response rate (~60%) and demonstrate greater variability across studies (response rates ranging from 25% to 78%), though this may be due to a variety of methodological differences (Wilens et al., 2011). Further, although many clinical trials report the success of pharmacotherapy in treating core symptoms of ADHD, fewer studies have been conducted on outcomes of functional impairment. Although they are assumed to be closely related, exactly how and to what degree core symptoms of ADHD and functional impairments are linked remains unclear, though correlations between the two in pediatric subjects have been shown to be generally modest (typically, r < .5) (Gordon et al., 2006). Nonetheless, a growing body of evidence suggests that pharmacotherapy is likely to significantly mitigate ADHD-associated functional impairments in adults (Coghill et al., 2017).
Figure 6.
FDA-approved treatments for ADHD. FDA-approved treatments for ADHD include stimulants and nonstimulants. Stimulants include amphetamine- or methylphenidate-based products, of which there are a variety of formulations available. Nonstimulants include atomoxetine, guanfacine extended-release (guanfacine XR), clonidine extended-release (clonidine XR), and viloxazine extended-release (viloxazine ER). Guanfacine XR and clonidine XR have not been approved to treat ADHD in adults; a randomized, placebo-controlled phase 3 trial of viloxazine ER in adults with ADHD has recently been completed (NCT04016779), with an open-label extension underway (Nasser et al., 2022). Note. FDA = Food and Drug Administration.
Social Impairment and Relationships
A combination double-blind trial followed by a 12-month open-label trial of methylphenidate found large, significant improvements in social functioning as assessed by informant reports and structured interview using the Weissman Social Adjustment Scale (Wender et al., 2011) (Supplemental Table 2). The percent of participants who scored moderately impaired or worse in their overall social adjustment dropped from 89% at baseline to 9% and 16% at 6 and 12 months of treatment, respectively. These reductions were seen in all subscales: Social Leisure dropped from 63% to 21% and 14%, Extended Family dropped from 40% to 12% and 9%, and Marital dropped from 55% to 21% and 15%. In all areas except Economic Functioning, the average overall score improved 37%, which falls between mild maladjustment and good functioning. The authors noted a variety of qualitative improvements: spouse informants reported that subjects made fewer conversational interruptions, their moods were more stable, and children were treated more calmly and consistently by the parent with ADHD (Wender et al., 2011).
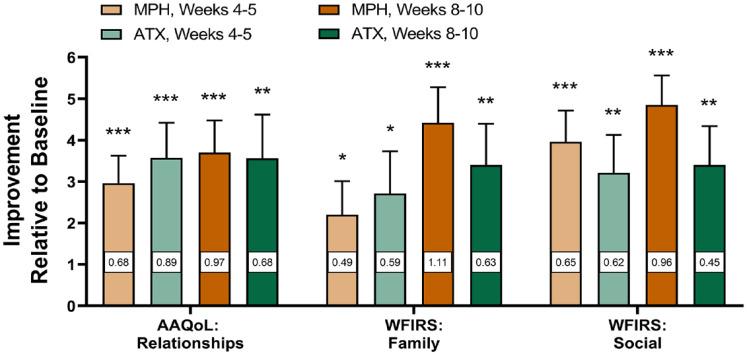
A head-to-head, randomized, open-label study compared immediate-release (IR) methylphenidate versus atomoxetine in 63 adults with ADHD in Taiwan (Ni et al., 2017). Although both drugs significantly improved the core ADHD symptoms by midpoint (Weeks 4–5, relative to baseline), atomoxetine reduced hyperactivity/impulsivity symptoms more than IR methylphenidate, although this difference was not significant by the last visit (Weeks 8–10) (Figure 7). Both drugs significantly improved scores on the Relationship items of the Adult ADHD Quality of Life Scale by midpoint, and by the last visit this improvement was greater for IR methylphenidate than for atomoxetine (p = .01). Similarly, both drugs significantly improved Family and Social items on the Weiss Functional Impairment Rating Scale–Self-Report at both time points. Importantly, IR methylphenidate was administered thrice daily (vs. once daily atomoxetine), which may have resulted in missed doses, particularly in the first few weeks of treatment, possibly explaining the greater improvement among atomoxetine-treated subjects by midpoint. Nonetheless, these data demonstrate that both stimulants and nonstimulants can significantly improve relationships and social functioning in adults with ADHD according to standardized, self-reported clinical assessments (Ni et al., 2017).
Figure 7.
Improvement on social and relationship items after stimulant and nonstimulant treatment. Treatment with instant-release methylphenidate (MPH) and atomoxetine (ATX) significantly improved social and relationship items on the Adult ADHD Quality of Life Scale (AAQoL) and Weiss Functional Impairment Rating Scale–Self-Report (WFIRS) in ADHD within 4 to 5 weeks of treatment. Cohen’s d effect sizes (white boxes within bars) were medium to large. Means ± standard error. * p < .05, ** p < .01, *** p < .001, relative to baseline. (Data from Ni et al., 2017.)
Parenting
A small, double-blind, placebo-controlled study of mothers with ADHD (N = 23) found that treatment with osmotic release oral system (OROS) methylphenidate significantly improved measures of parenting (Chronis-Tuscano, Seymour et al., 2008) (Supplemental Table 2). Specifically, stimulant treatment resulted in significant reductions in self-reported inconsistent discipline and corporal punishment, and improved maternal involvement, monitoring/supervision, and consistency in discipline. Cohen’s d effect sizes were medium to large, ranging from 0.42 to 0.71.
These findings were later replicated in both mothers and fathers with ADHD treated with the stimulant pro-drug lisdexamfetamine (Waxmonsky et al., 2014). Using a combination within- and between-subjects design and observer-rated laboratory tasks, this study showed that treating parental ADHD improved parents’ capacity to appropriately adapt their demands on their children to the individual task at hand. Parents receiving active treatment also made 2 to 4 times more praising statements than parents on placebo. Not only did parental behavior improve, but significant, demonstrable reductions in children’s inappropriate behaviors were noted when parents were receiving treatment (vs. placebo) (Waxmonsky et al., 2014). Moreover, these improvements occurred even at the start of treatment, when only limited changes in parental behavior were observed. The authors suggest that even minor reductions in parental ADHD symptoms may improve their children’s behavior, which may in turn increase parental praise, responsiveness, and productive parenting techniques resulting a positive feedback loop. If true, these data suggest even incremental improvements in parental ADHD could have a meaningful impact on children.
Educational Achievement
Considering that the bulk of one’s education occurs during childhood and adolescence; it is not surprising that most studies examining the impact of pharmacotherapy on academic achievement have been conducted in pediatric populations (Supplemental Table 2). A meta-analysis of 43 studies (pooled N = 2,110 children) found that drug treatment (particularly with methylphenidate and amphetamines) modestly improved the amount of schoolwork completed by ~15% and increased the amount of class time spent “on task” by ~14% (Prasad et al., 2013). Other measures of academic performance (e.g., number of test questions answered, accuracy of work completed) did not reliably improve with medication. Only two of the 43 studies assessed the effects of atomoxetine, and neither demonstrated reliable improvements on any measure of academic performance. Another meta-analysis (pooled N = 8,721 children) found that long-term medication use was associated with improved standardized test scores and grades, although the effect size was small and the educational relevance was questionable (Langberg & Becker, 2012).
Although there are few, if any, studies examining the impact of medication on academic performance in college, students believe that medications help. In one instance, more than 90% of interviewed students with ADHD said their medication helped them academically, although this was not supported by analysis of their educational outcomes (Advokat et al., 2011). Another study of nonmedical stimulant use among college students found that ~75% to 90% of respondents believed the medications helped them concentrate better, study longer, feel less restless, and keep better track of assignments (Rabiner et al., 2009).
In light of studies showing only modest improvements in academic performance, some researchers have advocated targeting the practical, functional skills associated with educational success such as attentiveness/organizational skills, emotional regulation, explicit academic skills, and accessing external supports (Fleming & McMahon, 2012). Because college success is typically built on years of success in school, it follows that college students with ADHD are more likely to lack these basic skills and would benefit from explicit interventions. Although ADHD medications may not directly improve academic achievement, they are likely to improve the skills necessary to succeed in college (e.g., attentiveness), which would subsequently allow the individual to perform at their best academically.
Occupational Impairments
A large cross-sectional study (N = 414) found a direct association between treating childhood ADHD and being in work as an adult (Halmøy et al., 2009) (Supplemental Table 2). Specifically, adults with ADHD who were presently receiving or had a childhood history of receiving pharmacotherapy for ADHD were significantly less likely to be out of work (relative to those never having received treatment). For those having received treatment in childhood, this was true even when controlling for a variety of comorbidities. Similarly, the age of first stimulant treatment was negatively correlated with being in work, where beginning treatment at a later age was associated with lower levels of employment (Gjervan et al., 2012). These data underscore the benefit of early diagnosis and treatment in promoting a productive occupational trajectory (Halmøy et al., 2009).
In the previously described head-to-head comparison between IR methylphenidate and atomoxetine, both drugs significantly improved scores on the Work items of the Weiss Functional Impairment Rating Scale at 5 weeks (both p’s < .01, d’s = 0.6) (Ni et al., 2017). By Week 10, IR methylphenidate (mean score, −7.41, p < .001 relative to baseline, d = 1.13) had improved scores on this item more than atomoxetine (mean score, −4.19, p < .01 relative to baseline, d = 0.84), though this difference was not statistically significant.
A novel methylphenidate formulation (PRC-063) was assessed among adults with ADHD in a dose-optimized, double-blind, placebo-controlled crossover study (Wigal et al., 2020). After dose-optimization, subjects were tested on two separate days (one on active drug, one on placebo) in a simulated workplace environment (i.e., a controlled environment with structured activities provided throughout the day, designed to provoke behaviors associated with ADHD in such a way as to yield quantifiable outcomes). Here, methylphenidate significantly improved scores over placebo within 1 hr of dosing through the last time point (16 hr), suggesting both a rapid onset of effect and sustained benefits in a simulated work environment.
Accidents, Unintentional Injuries, and Driving
The benefits of pharmacological treatment for ADHD on the occurrence of unintentional injuries have been well established in children and adolescents (Ruiz-Goikoetxea et al., 2018), and now a large body of evidence suggests the same might be true in adults (Supplemental Table 2). ADHD medication use has been associated with significant decreases in a wide variety of unintentional injuries, including dislocations, sprains and strains, intracranial and internal injuries, superficial injuries and contusions, head injuries, and traumatic brain injuries, relative to un-medicated individuals with ADHD (Figure 3b) (Chien et al., 2017; Liou et al., 2018). Among the most-documented benefits of ADHD pharmacotherapy is a reduction in driving accidents. In a large longitudinal Swedish study (N = 17,408), the rate of serious traffic accidents (resulting in emergency department visits) was 58% lower in adult men when medicated for their ADHD, relative to periods of their life during which they were not medicated (Chang, Lichtenstein, D’Onofrio, Sjölander, & Larsson, 2014). This study estimated that at least 41% of the accidents could be attributable to non-treatment, suggesting they could have been avoided with medication treatment (Chang, Lichtenstein, D’Onofrio, Sjölander, & Larsson, 2014).
Pharmacological treatments for ADHD appear to also improve driving performance, often after only a few weeks of treatment. For instance, an early study on this issue used a within- and between-subjects driving simulator paradigm (Biederman et al., 2012). Here, adults with ADHD (N = 61, 18–26 years) were randomized to 6 weeks of lisdexamfetamine or placebo. At end of study, treatment with lisdexamfetamine significantly improved reaction time and lowered the number of simulated driving collisions relative to placebo. Overwhelmingly, reports about the efficacy of ADHD medications reference stimulant medications, presumably due to their widespread use; stimulants not only predate the FDA-approval of nonstimulant treatment options, but greatly outnumber nonstimulant options as well.
Although to our knowledge no studies directly compare the efficacy of stimulants versus nonstimulants on driving performance, a 2014 systematic review of medicated ADHD patients evaluated reports of six stimulants and one nonstimulant (Gobbo & Louzã, 2014). Although the studies were methodologically highly variable, it was clear that therapeutic stimulant use improved driving performance in ADHD patients, particularly in young adults. Not surprisingly, although both osmotic release oral system (OROS) methylphenidate and instant-release methylphenidate (MPH-IR) produced similar improvements during the day, MPH-IR lost its efficacy in the evening. Studies with atomoxetine, the only nonstimulant evaluated, reported mixed results. Perhaps the most interesting result from this review is the potential for a rebound effect from extended-release stimulants: while extended-release mixed amphetamine salts improved driving performance during the day, it worsened driving in the evening. Because no direct comparisons between stimulants or between stimulants and nonstimulants exist for these measures, any comparisons must be interpreted with consideration to the methodological differences among studies.
The potential for pharmacotherapy to improve accidents and injuries is of great consequence. Not only is unintentional injury a global public health concern, but the majority of injuries in available studies are related to traffic incidents (Chandran et al., 2010). Specifically, the World Health Organization estimates that almost four million people die each year from unintentional injuries, with 33% of these due to road traffic incidents (Chandran et al., 2010). Due to increasing numbers of vehicles on the road, and increasing complexity and sharing of roadways, this number is expected to increase over time. Because these injuries are more common among young, healthy individuals, the number of years lived with an injury-induced disability can be substantial. On an individual level, driving facilitates a wide variety of other functional domains (e.g., work, family, social engagements) for many adults. Thus, any disorder with the potential to adversely impact driving or otherwise restrict this privilege would be expected to have downstream consequences. Since ADHD is a treatable condition, clinicians should strongly consider the functional safety benefits of treatment for their patients who may be at risk of injury or driving impairments.
Mortality
Whether treatment can reduce mortality among individuals with ADHD requires further study, as most published reports have examined whether ADHD medications increase mortality from a perspective of drug safety (Supplemental Table 2). Because deaths are infrequent events, studies that have examined whether medication can decrease mortality often come from observational studies (both prospective and retrospective) in which large samples with long-term observation periods can allow adequate numbers of rare events to be evaluated. Regardless of the methodology used, the study of mortality among psychiatric disorders is complex, and a well-designed analysis should take into account the presence of comorbid disorders, the timing of symptom/impairment onset which may drive individuals to seek medical help, and medication adherence issues among other factors. Nonetheless, some trends in the data have emerged.
A recent population-based cohort study examined all-cause mortality rates among children and adolescents with ADHD after treatment with methylphenidate (N = 68,096) and compared these results to matched controls (V. C. Chen et al., 2020). Even after adjusting for potential confounds, methylphenidate use was still associated with a significantly lower risk of mortality, particularly among boys aged 4 to 11. Consistent with a protective effect of treatment, a longer interval between ADHD diagnosis and starting methylphenidate treatment was associated with higher mortality rates (adjusted HR = 1.05, p = .009), and longer treatment duration was associated with lower mortality (adjusted HR = 0.83, p = .031). Whether and how these results might be generalized to nonstimulants or adult populations is unclear.
Both a large Taiwanese study and U.S. claims data analysis (N > 3 million participants with ADHD) found that ADHD medication was associated with significantly lower risk of suicidal attempts (Chang et al., 2020; Liang et al., 2018). In the Taiwanese study, the decrease in risk was proportional to length of prescribed treatment: individuals prescribed 1 to 90 days of methylphenidate had a nonsignificant HR of 0.86 (relative to no prescription), while those prescribed 91 to 180 days of methylphenidate had a significantly reduced HR of 0.41 (p = .025), and those prescribed more than 180 days had an even larger HR reduction of 0.28 (p < .0001) (Liang et al., 2018).
A similarly large population-based study of methylphenidate found that the risk of suicide attempts was significantly increased during the 90 days prior to beginning methylphenidate treatment and the first 90 days after initiating treatment (Man et al., 2017). After this initial 90-day period, the risk returned to baseline levels (risk ratio = 1.35, 95% confidence intervals [CI] = [0.77, 2.38]). The authors posit that the increased risk of suicide attempts before treatment may reflect the emergence of symptoms and impairments, subsequently driving individuals to seek medical intervention. However, suicidal thoughts could have also prompted individuals to seek evaluation for psychiatric disorders, resulting in diagnosis of ADHD and subsequent medical intervention. Because the 90 days before and after beginning treatment did not significantly differ in their risk ratios (0.78, 95% CI = [0.26, 2.35]), the authors emphasize that these results do not suggest causality between treatment and suicide attempts.
The FDA has required a black box suicide warning for atomoxetine since 2005, and now for the nonstimulant viloxazine ER which was recently approved for children (6–17 years) and adults. For atomoxetine, this labeling resulted from a modest increase in suicidal ideation (0.4% over and above placebo) and one suicide attempt in children and adolescents reported in a meta-analysis of 14 studies (N = 2,200) (Strattera [Atomoxetine] Prescribing Information, 2017). Because all events occurred within the first month of treatment with atomoxetine, it is unclear whether the risk of suicidal ideation in pediatric patients extends to long-term use. Notably, a similar analysis among studies in adults receiving atomoxetine revealed no such increased risk of suicide events (i.e., ideation, attempts, completions) (Strattera [Atomoxetine] Prescribing Information, 2017). In the 15 years since, numerous analyses have failed to find any evidence of increased suicide events associated with atomoxetine (Bangs et al., 2014; Chang et al., 2020; Linden et al., 2016), and the risk has been reported to be comparable to that after treatment with methylphenidate (Bushe & Savill, 2013). Contemporary controlled studies comparing the risk of suicide with use of nonstimulants compared to stimulants are needed. Nonetheless, because the ADHD disorder is associated with an increased risk of suicide events compared to the general population, the FDA’s warning should be considered in light of the risk(s) associated with foregoing treatment.
Risky Sexual Behaviors (RSB)
Few studies have directly examined the impact of ADHD medication on RSB in adults; however ADHD medication has been associated with a lower probability of RSBs in adolescents (Suppelemental Table 2). A retrospective Medicaid claims data analysis (N = 145,264) found that ADHD medication significantly reduced the probability of a teenager with ADHD contracting an STD or becoming pregnant (Chorniy & Kitashima, 2016), though this study did not compare risk reduction among different medications. Similarly, a recent 2021 study found that long-term ADHD medication use among adolescents was associated with a lower risk of early pregnancy (i.e., pregnancy before the age of 20; HR = 0.69) (Hua et al., 2021).
Two recent studies have examined this issue among the broader pool of adolescents and young adults. A 2019 study examined RSB in young men (N = 31, ages 15–25 years) with diagnosed ADHD and comorbid alcohol use disorder after 6-month, open-label treatment with methylphenidate (Kumar et al., 2019). ADHD scores (particularly the hyperactivity/impulsivity subscale) were significantly correlated with RSB (Spearmen’s ρ = 0.34, p < .05), and RSB was significantly reduced at both 3 months (p = .02) and 6 months (p = .004) relative to baseline. Further, ADHD total scores significantly predicted the reduction in RSB (odds ratio = 1.26, p = .01), but not in alcohol-use severity or in perceived stress (Kumar et al., 2019), suggesting the improvement was unique to RSBs.
Similarly, using a large Taiwanese sample of adolescents and young adults diagnosed with ADHD (N = 17,898 vs. 71,592 controls), individuals with ADHD were more likely to develop a sexually transmitted infection (STI) than the comparison group, even after controlling for demographic data, psychiatric comorbidities, and ADHD medications (M.-H. Chen et al., 2018). In this study, the use of both methylphenidate and atomoxetine were evaluated in terms of short-term (1 month to 1 year) and long-term (greater than 1 year) use (though results were not stratified by drug type). Both short- and long-term use of ADHD medications were significantly associated with a lower risk of subsequent STIs (HRs of 0.70 and 0.59, respectively). Interestingly, when stratified by sex, these results (both short- and long-term use) were only significant for males but not females. Further, when stratified by age, the decreased risk of STI was only significant for adolescents (<18 years) after long-term use, and not significant for young adults (18–29 years) after either short- or long-term use. These data suggest that, while medication is likely to decrease the risk of STI, the results may vary based on demographic variables such as age and sex. Due to the paucity of data available in adults, further research is warranted.
Substance Use and Abuse
Most ADHD medications (and certainly all those before FDA approval of atomoxetine in 2002) are stimulants, which carry the risk of misuse, abuse, and diversion (Clemow & Walker, 2014) (Supplemental Table 2). In individuals with ADHD and substance use disorder (SUD), further risks include the potential for exacerbated cardiovascular effects if stimulant medications were to be combined with illicit drugs as well as the potential to promote drug craving or relapse/worsening of SUD (as described in numerous animal models, e.g., Farrell et al., 2018). As a result, clinicians have been historically hesitant to use stimulant therapies in the treatment of individuals with ADHD and SUD (or those at risk of SUD) (Schubiner et al., 1995).
Early clinical approaches thus sought to first attain sobriety, then treat the patient’s ADHD—an approach met with limited success. Among the earliest reports to contradict this guidance was a 1995 report of three case studies describing the successful treatment of SUD in adult ADHD patients, in which all three patients responded to psychostimulant treatment and remained abstinent for several years (Schubiner et al., 1995). Since then, multiple studies have demonstrated that long-acting stimulant and nonstimulant medications for ADHD do not generally cause or exacerbate SUDs (Schubiner et al., 2002; Wilens et al., 2008). In fact, ADHD medications can often, though not always (Schubiner et al., 2002), lower the risk of developing a SUD (Biederman, 2003; Quinn et al., 2017) and even help aid recovery (Levin et al., 2015; Schubiner et al., 1995).
The lack of correlation between ADHD medication and SUDs have been validated in large-scale retrospective analyses. A 4-year analysis of a Swedish national register (N = 38,753, ages 8–46 at the start of the study) found that treating ADHD with medication was not only not associated with increased rates of substance abuse events (e.g., substance-related hospitalizations), it appeared to significantly lower the rate by ~30% (Chang, Lichtenstein, Halldner, et al., 2014). This study also found that the rate of substance abuse decreased the longer medication was prescribed. Remarkably similar results were found in a later U.S. health care claims analysis of almost 3 million patients with ADHD (Quinn et al., 2017). Here, the authors found a significant 30% to 35% decline of substance use-related incidents during periods of active pharmacological ADHD treatment, and similarly no increased risk of SUD-related problems.
Stimulant medications have been found to be generally safe in healthy populations, but do carry increased cardiovascular safety risks in some predisposed patients, even in those with minor elevations in heart rate and blood pressure (Sinha et al., 2016). A systematic review of 10 population-based observational studies found no association between stimulant use and cardiovascular safety concerns among children and adolescents, but did find such an association in two of the three adult studies evaluated (Westover & Halm, 2012). Several studies have explicitly examined the safety of stimulant medications and atomoxetine in combination with commonly-abused substances such as cocaine, and found modest cardiovascular effects, though generally not clinically significant or unexpected (Collins et al., 2006; Rush et al., 2009; Stoops et al., 2008). However, outside the controlled environment of a clinical trial, how these medications might interact with illicit drugs of unknown quantities or purity, or in combination with multiple drugs, remains unclear and obviously is not recommended.
A 2020 consensus statement (55 experts, 17 countries) on adolescents with ADHD and SUD found that all agreed on the risk of abuse, misuse, and diversion as well as the increased cardiovascular risk among those seeking treatment with psychostimulants (Özgen et al., 2020). Interestingly, no consensus was reached on whether sobriety or abstinence was required prior to starting psychostimulant therapy, suggesting the need for greater research in this area. For clinicians who maintain this concern, nonstimulant ADHD pharmacotherapy may prove a suitable alternative. Indeed, atomoxetine has a lower abuse liability than psychostimulants (Jasinski et al., 2008), and has been shown to reduce the number of heavy alcohol drinking days by 26% (vs. placebo) in a 12-week study (Wilens et al., 2008).
Although not FDA-approved for ADHD in adults, guanfacine extended-release has also been shown to reduce cocaine and alcohol craving in cocaine-dependent women (Fox et al., 2014), and there has been a growing interest in its application to cocaine addiction (Lustberg & Weinshenker, 2020; Milivojevic et al., 2017). Similarly, the novel nonstimulant viloxazine extended-release, currently approved to treat ADHD in children (≥6 years) and adults, has not demonstrated any potential for abuse in non-human primate models using the original, instant-release formulation (Yanagita et al., 1980). Although the abuse liability of viloxazine extended-release has not been directly tested in humans, the original formulation of viloxazine has a 30+ year history of clinical use in Europe, with no indication of abuse potential (Findling et al., 2021).
Criminal Activity
A recent study demonstrated that offenders with ADHD have been shown to reoffend not only more frequently, but more quickly than those without ADHD (6 vs. 25 months, respectively) even when controlling for risk factors such as antisocial personality disorder (Philipp-Wiegmann et al., 2018) (Supplemental Table 2). Interestingly, only current (and not lifetime) ADHD impacted the number of further offenses, although both current and a history of ADHD did significantly increase the number of re-incarcerations. These data reiterate the disadvantages associated with current symptoms and impairments, versus simply having a history (i.e., childhood) of ADHD. Presumably, then, if current ADHD symptoms and impairments could be controlled, a positive downstream effect on criminal behavior might follow.
While it is clear that ADHD medications can reduce ADHD symptoms in incarcerated adults, whether the benefits translate into reduced criminality is methodologically difficult to assess. A small, year-long study of 30 prison inmates found that stimulant treatment clearly improved ADHD symptoms, and the number of reported critical incidents during the trial were clearly lower than the previous year, prior to the trial (Ginsberg et al., 2012). However, due to methodological constraints, these data were not analyzed via inferential statistics. Some of the most convincing data to support the efficacy of treatment to reduce criminal activities comes from a large Swedish registry of 25,656 adults with ADHD, followed over 3 years (Lichtenstein et al., 2012). During periods of medication, criminality dropped 32% for men and 41% for women. This reduction did not differ significantly between stimulants and nonstimulants and appeared to be unique to ADHD medications, as there was no association between criminality rates and the use of selective serotonin reuptake inhibitors (Figure 8).
Figure 8.
Associations between medication usage and criminal conviction among men with ADHD. Using data from the Swedish National Patient Register, both stimulant and nonstimulant ADHD medications significantly reduced the likelihood of any criminal conviction among men with ADHD, whereas selective serotonin reuptake inhibitor (SSRI) medication did not (a). The use of ADHD medication was associated with significantly decreased rates of crime as assessed by multiple outcomes (b). Hazard ratio (below each red circle) ± 95% confidence intervals. Confidence intervals which include 1.0 are not significantly different from the comparison (here, relative to no medication). (Data from Lichtenstein et al., 2012.).
A 2011 U.K. consensus statement on ADHD within the criminal justice system provides practical guidelines on managing this population (S. J. Young et al., 2011). It asserts that treating ADHD within this population will not only help control the inattentiveness, physical restlessness, impulsivity, and mood lability associated with the disorder, but will also facilitate better engagement with available rehabilitation programs. It also recommends that treating and controlling ADHD in this population may lead to subsequent, downstream improvements in common comorbid disorders (e.g., substance abuse, anxiety).
Sleep
Understanding the relationship between sleep and ADHD is particularly complicated by the prolific use of psychostimulant medication as the first-line therapy for ADHD (Supplemental Table 2). It is well established that psychostimulants promote central nervous system arousal, thereby promoting wakefulness and insomnia (Wood et al., 2014). Paradoxically, they have also been reported to result in improved sleep in some children and adults with ADHD, possibly via a “calming effect” (J. J. Kooij et al., 2001; Sobanski et al., 2008; M. A. Stein et al., 2012). For instance, a polysomnographic study compared adults with ADHD before and after treatment with methylphenidate over ≥26 days (Sobanski et al., 2008). At the end-of-study assessment, treatment resulted in significantly decreased latency to fall asleep, increased sleep efficiency, and improved subjective feelings about sleep, but no change in other measures such as rapid eye movement (REM), sleep parameters, or number of awakenings. Importantly, this study did not evaluate whether the administered dose also improved other symptoms or impairments associated with ADHD.
While fewer studies have examined the impact of nonstimulant ADHD medications on sleep parameters, a direct comparison of atomoxetine and methylphenidate in children found better sleep with atomoxetine treatment and worse sleep with methylphenidate (Sangal et al., 2006). A 2017 review of the relationship between ADHD treatment and insomnia reported the rate of insomnia to be 40% to 45% in a trial of mixed amphetamine salts (vs. 13% placebo), 11% to 31% in a trial of extended-release methylphenidate (vs. 4% placebo), and 15% and 8% after atomoxetine among poor and non-poor cytochrome P450 2D6 metabolizers, respectively (Wynchank et al., 2017). In a placebo-controlled trial, insomnia was noted as a treatment-emergent adverse reaction to atomoxetine among 20.8% of adults receiving active treatment (vs. 8.7% placebo) (Michelson et al., 2003).
In summary, untreated ADHD is associated with poor sleep quality which may be improved with medication, and nonstimulant treatments are associated with lower rates of insomnia than stimulants. Therefore, researchers should use caution and account for the potential impact of medications to exacerbate sleep difficulties when reporting sleep parameters among adults with ADHD. Clinicians, in turn, should consider baseline and medication-induced sleep problems when considering their patients’ treatment plans.
Conclusions
In summary, ADHD not only persists into adulthood, but is associated with a wide swath of functional impairments, many of which are distinct from those faced by pediatric populations. Unlike children with ADHD who are reliant on caregivers, adults living independently and self-sufficiently may be more vulnerable to the functional downstream consequences of inattention, hyperactivity, and impulsivity. Not surprisingly, the core symptoms and functional impairments characterizing adult ADHD are also associated with decreased optimism, decreased life satisfaction, and overall reduced quality of life across family, social, and occupational domains (Biederman, Faraone, Spencer, et al., 2006). Although loneliness does not appear to be a major concern in pediatric populations with ADHD (Diamantopoulou et al., 2005; Houghton et al., 2015), this is not the case in adulthood. Adults with ADHD experience significantly increased loneliness (Stickley et al., 2017) particularly among older populations (60–94 years old) (Michielsen et al., 2015), suggesting this impairment may be unique to adults with the disorder.
Several studies have also reported that undiagnosed adults presenting with ADHD signs and symptoms have significantly lower quality of life across multiple domains relative to those diagnosed with ADHD (Able et al., 2007; Pawaskar et al., 2020), suggesting that simply recognizing the disorder may significantly improve quality of life and well-being. A recent review found that treatment with both stimulant and nonstimulant ADHD medications significantly improves symptoms, functional impairment, and health-related quality of life among adults with ADHD (Coghill et al., 2017). The wealth of literature on functional impairments associated with ADHD presented in this review underscores very clearly the need for recognition and treatment of ADHD. This stands in sharp contrast to the occasional public discourse positing an inherent advantage to ADHD in adulthood. The published literature offers no compelling evidence that the ADHD disease state confers measurable benefits at any point across the lifespan of the disease. Because it has been suggested that the functional and quality of life consequences of ADHD might be cumulative over the lifespan (Brod et al., 2012), greater emphasis on its early diagnosis and treatment might result in significant improvements across multiple domains for individuals with ADHD.
Supplemental Material
Supplemental material, sj-docx-1-jad-10.1177_10870547231158572 for Functional Impairments Associated With ADHD in Adulthood and the Impact of Pharmacological Treatment by Alisa R. Kosheleff, Oren Mason, Rakesh Jain, Jennifer Koch and Jonathan Rubin in Journal of Attention Disorders
Supplemental material, sj-docx-2-jad-10.1177_10870547231158572 for Functional Impairments Associated With ADHD in Adulthood and the Impact of Pharmacological Treatment by Alisa R. Kosheleff, Oren Mason, Rakesh Jain, Jennifer Koch and Jonathan Rubin in Journal of Attention Disorders
Author Biographies
Alisa R. Kosheleff, PhD, is a Senior Manager in Medical Affairs.
Oren Mason, MD, is a physician at Attention MD and a clinical associate instructor at Wayne State University School of Medicine’s Grand Rapids Medical Education Consortium.
Rakesh Jain, MD, MPH, is a physician and clinical professor in the Department of Psychiatry at Texas Tech University School of Medicine - Permian Basin.
Jennifer Koch, PhD, is a Manager of Medical Publications at Supernus Pharmaceuticals, Inc.
Jonathan Rubin, MD, is the Chief Medical Officer and Senior Vice President, Research & Development, at Supernus Pharmaceuticals, Inc.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Alisa Kosheleff is a former employee of Supernus Pharmaceuticals, Inc. Jennifer Koch and Jonathan Rubin are current employees of Supernus Pharmaceuticals, Inc. Oren Mason has received speaking or consulting funds from Eisai, Ironshore Pharmaceuticals, Arbor Pharmaceuticals, and Supernus Pharmaceuticals. Rakesh Jain has received research support from, served as a consultant or speaker for, or served on an advisory board for Abbvie, Allergan, Acadia, Alfasigma, Alkermes, Axsome, Cingulate Therapeutics, Corium, Eisai, Evidera, Impel, Intra-Cellular Therapies, Ironshore Pharmaceuticals, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Osmotica, Otsuka, Pamlab, Pfizer, Sage Therapeutics, Shire, Sunovion, Supernus Pharmaceuticals, Takeda, Teva, and Tris Pharmaceuticals.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Supernus Pharmaceuticals, Inc.
ORCID iD: Alisa R. Kosheleff  https://orcid.org/0000-0002-5738-7221
https://orcid.org/0000-0002-5738-7221
Supplemental Material: Supplemental material for this article is available online.
References
- Able S. L., Johnston J. A., Adler L. A., Swindle R. W. (2007). Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychological Medicine, 37(1), 97–107. 10.1017/S0033291706008713 [DOI] [PubMed] [Google Scholar]
- Acker M. M., O’Leary S. G. (1996). Inconsistency of mothers’ feedback and toddlers’ misbehavior and negative affect. Journal of Abnormal Child Psychology, 24(6), 703–714. [DOI] [PubMed] [Google Scholar]
- Adler L. A., Faraone S. V., Spencer T. J., Berglund P., Alperin S., Kessler R. C. (2017). The structure of adult ADHD. International Journal of Methods in Psychiatric Research, 26(1), e1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advokat C., Lane S. M., Luo C. (2011). College students with and without ADHD: Comparison of self-report of medication usage, study habits, and academic achievement. Journal of Attention Disorders, 15(8), 656–666. [DOI] [PubMed] [Google Scholar]
- Akil H., Gordon J., Hen R., Javitch J., Mayberg H., McEwen B., Meaney M. J., Nestler E. J. (2018). Treatment resistant depression: A multi-scale, systems biology approach. Neuroscience and Biobehavioral Reviews, 84, 272–288. 10.1016/j.neubiorev.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegría M., Mulvaney-Day N., Torres M., Polo A., Cao Z., Canino G. (2007). Prevalence of psychiatric disorders across Latino subgroups in the United States. American Journal of Public Health, 97(1), 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (1980). Diagnostic and statistical manual of mental disorders (3rd ed.). American Psychiatric Publishing. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing. [Google Scholar]
- Arias A. J., Gelernter J., Chan G., Weiss R. D., Brady K. T., Farrer L., Kranzler H. R. (2008). Correlates of co-occurring ADHD in drug-dependent subjects: Prevalence and features of substance dependence and psychiatric disorders. Addictive Behaviors, 33(9), 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ary D. V., Duncan T. E., Biglan A., Metzler C. W., Noell J. W., Smolkowski K. (1999). Development of adolescent problem behavior. Journal of Abnormal Child Psychology, 27(2), 141–150. [DOI] [PubMed] [Google Scholar]
- Bangs M. E., Wietecha L. A., Wang S., Buchanan A. S., Kelsey D. K. (2014). Meta-analysis of suicide-related behavior or ideation in child, adolescent, and adult patients treated with atomoxetine. Journal of Child and Adolescent Psychopharmacology, 24(8), 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks T., Ninowski J. E., Mash E. J., Semple D. L. (2008). Parenting behavior and cognitions in a community sample of mothers with and without symptoms of attention-deficit/hyperactivity disorder. Journal of Child and Family Studies, 17(1), 28–43. [Google Scholar]
- Barkley R. A. (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65–94. [DOI] [PubMed] [Google Scholar]
- Barkley R. A. (1998). Developmental course, adult outcome, and clinic-referred ADHD adults. In Barkley R. A. (Ed.), Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (pp. 186–224). Guildford. [Google Scholar]
- Barkley R. A. (2004). Driving impairments in teens and adults with attention-deficit/hyperactivity disorder. The Psychiatric clinics of North America, 27(2), 233–260. [DOI] [PubMed] [Google Scholar]
- Barkley R. A. (2020). ADHD adversely impacts health, mortality risk, and estimated life expectancy by adulthood. The ADHD Report, 28(4), 1–4. [Google Scholar]
- Barkley R. A., Cox D. (2007). A review of driving risks and impairments associated with attention-deficit/hyperactivity disorder and the effects of stimulant medication on driving performance. Journal of Safety Research, 38(1), 113–128. 10.1016/j.jsr.2006.09.004 [DOI] [PubMed] [Google Scholar]
- Barkley R. A., Fischer M. (2019). Hyperactive child syndrome and estimated life expectancy at young adult follow-up: The role of ADHD persistence and other potential predictors. Journal of Attention Disorders, 23(9), 907–923. [DOI] [PubMed] [Google Scholar]
- Barkley R. A., Fischer M., Smallish L., Fletcher K. (2006). Young adult outcome of hyperactive children: adaptive functioning in major life activities. Journal of the American Academy of Child and Adolescent Psychiatry, 45(2), 192–202. [DOI] [PubMed] [Google Scholar]
- Barkley R. A., Guevremont D. C., Anastopoulos A. D., DuPaul G. J., Shelton T. L. (1993). Driving-related risks and outcomes of attention deficit hyperactivity disorder in adolescents and young adults: A 3- to 5-year follow-up survey. Pediatrics, 92(2), 212–218. [PubMed] [Google Scholar]
- Barkley R. A., Murphy K. R., Dupaul G. J., Bush T. (2002). Driving in young adults with attention deficit hyperactivity disorder: Knowledge, performance, adverse outcomes, and the role of executive functioning. Journal of the International Neuropsychological Society, 8(5), 655–672. [DOI] [PubMed] [Google Scholar]
- Barkley R. A., Murphy K. R., Fischer M. (2010). ADHD in adults: What the science says. Guilford Press. [Google Scholar]
- Barkley R. A., Murphy K. R., Kwasnik D. (1996). Motor vehicle driving competencies and risks in teens and young adults with attention deficit hyperactivity disorder. Pediatrics, 98(6), 1089–1095. [PubMed] [Google Scholar]
- Biederman J. (2003). Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: Findings from a longitudinal follow-up of youths with and without ADHD. Journal of Clinical Psychiatry, 64, 3–8. [PubMed] [Google Scholar]
- Biederman J., Faraone S. V. (2006). The effects of attention-Deficit/hyperactivity disorder on employment and household income. Medgenmed: Medscape General Medicine, 8(3), 12–28. [PMC free article] [PubMed] [Google Scholar]
- Biederman J., Faraone S. V., Mick E., Spencer T., Wilens T., Kiely K., Guite J., Ablon J. S., Reed E., Warburton R. (1995). High risk for attention deficit hyperactivity disorder among children of parents with childhood onset of the disorder: AA pilot study. American Journal of Psychiatry, 152, 431–435. [DOI] [PubMed] [Google Scholar]
- Biederman J., Faraone S. V., Spencer T. J., Mick E., Monuteaux M. C., Aleardi M. (2006). Functional impairments in adults with self-reports of diagnosed ADHD: A controlled study of 1001 adults in the community. Journal of Clinical Psychiatry, 67(4), 524–540. [DOI] [PubMed] [Google Scholar]
- Biederman J., Fried R., Hammerness P., Surman C., Mehler B., Petty C. R., Faraone S. V., Miller C., Bourgeois M., Meller B., Godfrey K. M., Reimer B. (2012). The effects of lisdexamfetamine dimesylate on the driving performance of young adults with ADHD: A randomized, double-blind, placebo-controlled study using a validated driving simulator paradigm. Journal of Psychiatric Research, 46(4), 484–491. 10.1016/j.jpsychires.2012.01.007 [DOI] [PubMed] [Google Scholar]
- Biederman J., Mick E., Faraone S. V. (2000). Age-dependent decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. American Journal of Psychiatry, 157(5), 816–818. [DOI] [PubMed] [Google Scholar]
- Biederman J., Petty C., Fried R., Fontanella J., Doyle A. E., Seidman L. J., Faraone S. V. (2006). Impact of psychometrically defined deficits of executive functioning in adults with attention deficit hyperactivity disorder. American Journal of Psychiatry, 163(10), 1730–1738. [DOI] [PubMed] [Google Scholar]
- Biederman J., Petty C. R., Fried R., Doyle A. E., Spencer T., Seidman L. J., Gross L., Poetzl K., Faraone S. V. (2007). Stability of executive function deficits into young adult years: A prospective longitudinal follow-up study of grown up males with ADHD. Acta Psychiatrica Scandinavica, 116(2), 129–136. [DOI] [PubMed] [Google Scholar]
- Brod M., Schmitt E., Goodwin M., Hodgkins P., Niebler G. (2012). ADHD burden of illness in older adults: AA life course perspective. Quality of Life Research, 21(5), 795–799. [DOI] [PubMed] [Google Scholar]
- Brown T. E. (2009). ADD/ADHD and impaired executive function in clinical practice. Current Attention Disorders Reports, 1(1), 37–41. [Google Scholar]
- Brown T. E., Reichel P. C., Quinlan D. M. (2009). Executive function impairments in high IQ adults with ADHD. Journal of Attention Disorders, 13(2), 161–167. [DOI] [PubMed] [Google Scholar]
- Buitelaar N., Posthumus J., Bijlenga D., Ferdinand R., Buitelaar J. (2020). Type and severity of intimate partner violence in offenders with and without ADHD. International Journal of Forensic Mental Health, 19(2), 142–151. [Google Scholar]
- Buitelaar N. J. L., Posthumus J. A., Bijlenga D., Buitelaar J. K. (2021). The impact of ADHD treatment on intimate partner violence in a forensic psychiatry setting. Journal of Attention Disorders, 25(7), 1021–1031. [DOI] [PubMed] [Google Scholar]
- Buote V. M., Pancer S. M., Pratt M. W., Adams G., Birnie-Lefcovitch S., Polivy J., Wintre M. G. (2007). The importance of friends: Friendship and adjustment among 1st-year university students. Journal of Adolescent Research, 22(6), 665–689. [Google Scholar]
- Bushe C. J., Savill N. C. (2013). Suicide related events and attention deficit hyperactivity disorder treatments in children and adolescents: A meta-analysis of atomoxetine and methylphenidate comparator clinical trials. Child and Adolescent Psychiatry and Mental Health, 7(1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran A., Hyder A. A., Peek-Asa C. (2010). The global burden of unintentional injuries and an agenda for progress. Epidemiologic Reviews, 32(1), 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z., Lichtenstein P., D’Onofrio B. M., Sjölander A., Larsson H. (2014). Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: A population-based study. JAMA Psychiatry, 71(3), 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z., Lichtenstein P., Halldner L., D’Onofrio B., Serlachius E., Fazel S., Långström N., Larsson H. (2014). Stimulant ADHD medication and risk for substance abuse. Journal of Child Psychology and Psychiatry, 55(8), 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z., Quinn P. D., O’Reilly L., Sjölander A., Hur K., Gibbons R., Larsson H., D’Onofrio B. M. (2020). Medication for attention-deficit/hyperactivity disorder and risk for suicide attempts. Biological Psychiatry, 88(6), 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charach A., Yeung E., Climans T., Lillie E. (2011). Childhood attention-deficit/hyperactivity disorder and future substance use disorders: Comparative meta-analyses. Journal of the American Academy of Child and Adolescent Psychiatry, 50(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Chen M., Johnston C. (2007). Maternal inattention and impulsivity and parenting behaviors. Journal of Clinical Child & Adolescent Psychology, 36(3), 455–468. [DOI] [PubMed] [Google Scholar]
- Chen M.-H., Hsu J.-W., Huang K.-L., Bai Y.-M., Ko N.-Y., Su T.-P., Li C.-T., Lin W.-C., Tsai S.-J., Pan T.-L., Chang W. H., Chen T. J. (2018). Sexually transmitted infection among adolescents and young adults with attention-deficit/hyperactivity disorder: A nationwide longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry, 57(1), 48–53. [DOI] [PubMed] [Google Scholar]
- Chen V. C., Chan H.-L., Wu S.-I., Lee M., Lu M.-L., Liang H.-Y., Dewey M. E., Stewart R., Lee C. T. (2019). Attention-deficit/hyperactivity disorder and mortality risk in Taiwan. JAMA Network Open, 2(8), e198714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen V. C., Chan H.-L., Wu S.-I., Lu M.-L., Dewey M. E., Stewart R., Lee C. T. (2020). Methylphenidate and mortality in children with attention-deficit hyperactivity disorder: Population-based cohort study. The British Journal of Psychiatry.Advance online publication. 10.1192/bjp.2020.129. [DOI] [PubMed]
- Chesney E., Goodwin G. M., Fazel S. (2014). Risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry, 13(2), 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien W. C., Chung C. H., Lin F. H., Yeh C. B., Huang S. Y., Lu R. B., Chang H. A., Kao Y. C., Chiang W. S., Chou Y. C., Tsao C. H., Wu Y. F., Tzeng N. S. (2017). The risk of injury in adults with attention-deficit hyperactivity disorder: A nationwide, matched-cohort, population-based study in Taiwan. Research in Developmental Disabilities, 65, 57–73. [DOI] [PubMed] [Google Scholar]
- Chorniy A., Kitashima L. (2016). Sex, drugs, and A DHD: The effects of ADHD pharmacological treatment on teens’ risky behaviors. Labour Economics, 43, 87–105. [Google Scholar]
- Chronis-Tuscano A., Raggi V. L., Clarke T. L., Rooney M. E., Diaz Y., Pian J. (2008). Associations between maternal attention-deficit/hyperactivity disorder symptoms and parenting. Journal of Abnormal Child Psychology, 36(8), 1237–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemow D. B., Walker D. J. (2014). The potential for misuse and abuse of medications in ADHD: A review. Postgraduate Medicine, 126(5), 64–81. 10.3810/pgm.2014.09.2801 [DOI] [PubMed] [Google Scholar]
- Coghill D. R., Banaschewski T., Soutullo C., Cottingham M. G., Zuddas A. (2017). Systematic review of quality of life and functional outcomes in randomized placebo-controlled studies of medications for attention-deficit/hyperactivity disorder. European Child & Adolescent Psychiatry, 26(11), 1283–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill D. R., Joseph A., Sikirica V., Kosinski M., Bliss C., Huss M. (2019). Correlations between clinical trial outcomes based on symptoms, functional impairments, and quality of life in children and adolescents with ADHD. Journal of Attention Disorders, 23(13), 1578–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. L., Levin F. R., Foltin R. W., Kleber H. D., Evans S. M. (2006). Response to cocaine, alone and in combination with methylphenidate, in cocaine abusers with ADHD. Drug and Alcohol Dependence, 82(2), 158–167. [DOI] [PubMed] [Google Scholar]
- Corkum P. V., Siegel L. S. (1993). Is the continuous performance task a valuable research tool for use with children with attention-deficit-hyperactivity disorder? Journal of Child Psychology and Psychiatry, 34(7), 1217–1239. [DOI] [PubMed] [Google Scholar]
- Cortese S., Holtmann M., Banaschewski T., Buitelaar J., Coghill D., Danckaerts M., Dittmann R. W., Graham J., Taylor E., Sergeant J. (2013). Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. Journal of Child Psychology and Psychiatry, 54(3), 227–246. [DOI] [PubMed] [Google Scholar]
- Dahl R. E. (1996). The impact of inadequate sleep on children’s daytime cognitive function. Seminars in Pediatric Neurology, 3, 44–50. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S., Mortensen P. B., Frydenberg M., Thomsen P. H. (2014). ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood - a naturalistic long-term follow-up study. Addictive Behaviors, 39(1), 325–328. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S., Østergaard S. D., Leckman J. F., Mortensen P. B., Pedersen M. G. (2015). Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: A nationwide cohort study. Lancet, 385(9983), 2190–2196. [DOI] [PubMed] [Google Scholar]
- Das D., Cherbuin N., Butterworth P., Anstey K. J., Easteal S. (2012). A population-based study of attention deficit/hyperactivity disorder symptoms and associated impairment in middle-aged adults. PLoS One, 7(2), e31500. 10.1371/journal.pone.0031500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf R., Kessler R. C., Fayyad J., Ten Have M., Alonso J., Angermeyer M., Borges G., Demyttenaere K., Gasquet I., de Girolamo G., Haro J. M., Jin R., Karam G., Ormel J., Posada-Villa J. (2008). The prevalence and effects of adult attention-deficit/hyperactivity disorder (ADHD) on the performance of workers: Results from the WHO World Mental Health Survey Initiative. Occupational and Environmental Medicine, 65(12), 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denckla M. B. (1996). A theory and model of executive function: A neuropsychological perspective. In Lyon G. R., Krasnegor N. A. (Eds.), Attention, memory, and executive function (pp. 263–278). Paul H. Brookes. [Google Scholar]
- Dennis J. M., Phinney J. S., Chuateco L. I. (2005). The role of motivation, parental support, and peer support in the academic success of ethnic minority first-generation college students. Journal of College Student Development, 46(3), 223–236. [Google Scholar]
- Diamantopoulou S., Henricsson L., Rydell A.-M. (2005). ADHD symptoms and peer relations of children in a community sample: Examining associated problems, self-perceptions, and gender differences. International Journal of Behavioral Development, 29(5), 388–398. [Google Scholar]
- Dixon E. B. (1995). Impact of adult ADD on the family. In Nadeau K. G. (Ed.), A comprehensive guide to attention deficit disorder in adults: Research, diagnosis, and treatment (pp. 236–259). Brunner/Mazel, Inc. [Google Scholar]
- Eakin L., Minde K., Hechtman L., Ochs E., Krane E., Bouffard R., Greenfield B., Looper K. (2004). The marital and family functioning of adults with ADHD and their spouses. Journal of Attention Disorders, 8(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Ebejer J. L., Medland S. E., van der Werf J., Gondro C., Henders A. K., Lynskey M., Martin N. G., Duffy D. L. (2012). Attention deficit hyperactivity disorder in Australian adults: Prevalence, persistence, conduct problems and disadvantage. PLoS ONE, 7, e47404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins I. J., Saunders G. R., Malone S. M., Wilson S., McGue M., Iacono W. G. (2020). Differential implications of persistent, remitted, and late-onset ADHD symptoms for substance abuse in women and men: A twin study from ages 11 to 24. Drug and Alcohol Dependence, 212, 107947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J. N., Loren R. E. (2013). Changes in the definition of ADHD in DSM-5: Subtle but important. Neuropsychiatry, 3(5), 455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X., Massetti G. M., Ouyang L., Grosse S. D., Mercy J. A. (2010). Attention-deficit/hyperactivity disorder, conduct disorder, and young adult intimate partner violence. Archives of General Psychiatry, 67(11), 1179–1186. [DOI] [PubMed] [Google Scholar]
- Faraone S. V., Biederman J., Mick E. (2006). The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychological Medicine, 36(2), 159–165. 10.1017/S003329170500471X [DOI] [PubMed] [Google Scholar]
- Faraone S. V., Spencer T. J., Montano C. B., Biederman J. (2004). Attention-deficit/hyperactivity disorder in adults: AA survey of practice in psychiatry and primary care practice in psychiatry and primary carundefined. Archive of Internal Medicine, 164(11), 1221–1226. [DOI] [PubMed] [Google Scholar]
- Farrell M. R., Schoch H., Mahler S. V. (2018). Modeling cocaine relapse in rodents: Behavioral considerations and circuit mechanisms. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 87, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling R. L., Candler S. A., Nasser A. F., Schwabe S., Yu C., Garcia-Olivares J., O’Neal W., Newcorn J. H. (2021). Viloxazine in the management of CNS disorders: A historical overview and current status. CNS Drugs, 35, 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A. P., McMahon R. J. (2012). Developmental context and treatment principles for ADHD among college students. Clinical Child and Family Psychology Review, 15(4), 303–329. [DOI] [PubMed] [Google Scholar]
- Flory K., Molina B. S., Pelham We, Jr, Gnagy E, Smith B. (2006). Childhood ADHD predicts risky sexual behavior in young adulthood. Journal of Clinical Child & Adolescent Psychology, 35(4), 571–577. [DOI] [PubMed] [Google Scholar]
- Fox H. C., Morgan P. T., Sinha R. (2014). Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology, 39(6), 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B., Michelini G., Asherson P., Banaschewski T., Bilbow A., Buitelaar J. K., Cormand B., Faraone S. V., Ginsberg Y., Haavik J., Kuntsi J., Larsson H., Lesch K. P., Ramos-Quiroga J. A., Réthelyi J. M., Ribases M., Reif A. (2018). Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. European Neuropsychopharmacology, 28(10), 1059–1088. 10.1016/j.euroneuro.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier T. W., Youngstrom E. A., Glutting J. J., Watkins M. W. (2007). ADHD and achievement: Meta-analysis of the child, adolescent, and adult literatures and a concomitant study with college students. Journal of Learning Disabilities, 40(1), 49–65. [DOI] [PubMed] [Google Scholar]
- Gau S. S. F., Kessler R. C., Tseng W. L., Wu Y. Y., Chiu Y. N., Yeh C. B., Hwu H. G. (2007). Association between sleep problems and symptoms of attention-deficit/hyperactivity disorder in young adults. Sleep, 30(2), 195–201. [DOI] [PubMed] [Google Scholar]
- Ginsberg Y., Hirvikoski T., Grann M., Lindefors N. (2012). Long-term functional outcome in adult prison inmates with ADHD receiving OROS-methylphenidate. European Archives of Psychiatry and Clinical Neuroscience, 262(8), 705–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg Y., Hirvikoski T., Lindefors N. (2010). Attention deficit hyperactivity disorder (ADHD) among longer-term prison inmates is a prevalent, persistent and disabling disorder. BMC Psychiatry, 10(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg Y., Quintero J., Anand E., Casillas M., Upadhyaya H. P. (2014). Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: A review of the literature. The Primary Care Companion For CNS Disorders, 16(3), 13r01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjervan B., Hjemdal O., Nordahl H. M. (2016). Functional impairment mediates the relationship between adult ADHD inattentiveness and occupational outcome. Journal of Attention Disorders, 20(6), 510–518. 10.1177/1087054712474689 [DOI] [PubMed] [Google Scholar]
- Gjervan B., Torgersen T., Nordahl H. M., Rasmussen K. (2012). Functional impairment and occupational outcome in adults with ADHD. Journal of Attention Disorders, 16(7), 544–552. 10.1177/1087054711413074 [DOI] [PubMed] [Google Scholar]
- Gobbo M. A., Louzã M. R. (2014). Influence of stimulant and non-stimulant drug treatment on driving performance in patients with attention deficit hyperactivity disorder: AA systematic review. European Neuropsychopharmacology, 24(9), 1425–1443. [DOI] [PubMed] [Google Scholar]
- Gordon M., Antshel K., Faraone S., Barkley R., Lewandowski L., Hudziak J. J., Biederman J., Cunningham C. (2006). Symptoms versus impairment: The case for respecting DSM-IV’s criterion D. Journal of Attention Disorders, 9(3), 465–475. [DOI] [PubMed] [Google Scholar]
- Gregory A. M., Agnew-Blais J. C., Matthews T., Moffitt T. E., Arseneault L. (2017). ADHD and sleep quality: Longitudinal analyses from childhood to early adulthood in a twin cohort. Journal of Clinical Child & Adolescent Psychology, 46(2), 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger L., Hosser D. (2012). Attention deficit hyperactivity disorder does not predict criminal recidivism in young adult offenders: Results from a prospective study. International Journal of Law and Psychiatry, 35(1), 27–34. [DOI] [PubMed] [Google Scholar]
- Griggs M. S., Mikami A. Y. (2011). The role of maternal and child ADHD symptoms in shaping interpersonal relationships. Journal of Abnormal Child Psychology, 39(3), 437–449. [DOI] [PubMed] [Google Scholar]
- Guendelman M. D., Ahmad S., Meza J. I., Owens E. B., Hinshaw S. P. (2016). Childhood attention-deficit/hyperactivity disorder predicts intimate partner victimization in young women. Journal of Abnormal Child Psychology, 44(1), 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmøy A., Fasmer O. B., Gillberg C., Haavik J. (2009). Occupational outcome in adult ADHD: Impact of symptom profile, comorbid psychiatric problems, and treatment: A cross-sectional study of 414 clinically diagnosed adult ADHD patients. Journal of Attention Disorders, 13(2), 175–187. [DOI] [PubMed] [Google Scholar]
- Harpin V., Mazzone L., Raynaud J. P., Kahle J., Hodgkins P. (2016). Long-term outcomes of ADHD: A systematic review of self-esteem and social function. Journal of Attention Disorders, 20(4), 295–305. 10.1177/1087054713486516 [DOI] [PubMed] [Google Scholar]
- Heiligenstein E., Guenther G., Levy A., Savino F., Fulwiler J. (1999). Psychological and academic functioning in college students with attention deficit hyperactivity disorder. Journal of American College Health, 47(4), 181–185. 10.1080/07448489909595644 [DOI] [PubMed] [Google Scholar]
- Hodgkins P., Montejano L., Sasané R., Huse D. (2011). Risk of injury associated with attention-deficit/hyperactivity disorder in adults enrolled in employer-sponsored health plans: A retrospective analysis. The Primary Care Companion For CNS Disorders, 13(2), 21m03047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst Y., Thorell L. B. (2020). Functional impairments among adults with ADHD: A comparison with adults with other psychiatric disorders and links to executive deficits. Applied Neuropsychology. Adult, 27, 243–255. [DOI] [PubMed] [Google Scholar]
- Hosain G. M., Berenson A. B., Tennen H., Bauer L. O., Wu Z. H. (2012). Attention deficit hyperactivity symptoms and risky sexual behavior in young adult women. Journal of Women’s Health, 21(4), 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton S., Roost E., Carroll A., Brandtman M. (2015). Loneliness in children and adolescents with and without attention-deficit/hyperactivity disorder. Journal of Psychopathology and Behavioral Assessment, 37(1), 27–37. [Google Scholar]
- Hua M.-H., Huang K.-L., Hsu J.-W., Bai Y.-M., Su T.-P., Tsai S.-J., Li C.-T., Lin W.-C., Chen T.-J., Chen M.-H. (2021). Early pregnancy risk among adolescents with ADHD: A nationwide longitudinal study. Journal of Attention Disorders, 25, 1199–1206. [DOI] [PubMed] [Google Scholar]
- Jangmo A., Kuja-Halkola R., Pérez-Vigil A., Almqvist C., Bulik C. M., D’Onofrio B., Lichtenstein P., Ahnemark E., Werner-Kiechle T., Larsson H. (2021). Attention-deficit/hyperactivity disorder and occupational outcomes: The role of educational attainment, comorbid developmental disorders, and intellectual disability. PLoS One, 16(3), e0247724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski D. R., Faries D. E., Moore R. J., Schuh L. M., Allen A. J. (2008). Abuse liability assessment of atomoxetine in a drug-abusing population. Drug and Alcohol Dependence, 95(1-2), 140–146. [DOI] [PubMed] [Google Scholar]
- Jerome L., Habinski L., Segal A. (2006). Attention-deficit/hyperactivity disorder (ADHD) and driving risk: A review of the literature and a methodological critique. Current Psychiatry Reports, 8(5), 416–426. [DOI] [PubMed] [Google Scholar]
- Jerome L., Segal A., Habinski L. (2006). What we know about ADHD and driving risk: A literature review, meta-analysis and critique. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 15(3), 105–125. [PMC free article] [PubMed] [Google Scholar]
- Karlsdotter K., Bushe C., Hakkaart L., Sobanski E., Kan C. C., Lebrec J., Kraemer S., Dieteren N. A., Deberdt W. (2016). Burden of illness and health care resource utilization in adult psychiatric outpatients with attention-deficit/hyperactivity disorder in Europe. Current Medical Research and Opinion, 32(9), 1547–1556. 10.1080/03007995.2016.1189892 [DOI] [PubMed] [Google Scholar]
- Kaya A., Taner Y., Guclu B., Taner E., Kaya Y., Bahcivan H. G., Benli I. T. (2008). Trauma and adult attention deficit hyperactivity disorder. Journal of International Medical Research, 36(1), 9–16. [DOI] [PubMed] [Google Scholar]
- Kennedy T. M., Howard A. L., Mitchell J. T., Hoza B., Arnold L. E., Hechtman L. T., Swanson J. M., Stehli A., Molina B. S. G. (2019). Adult substance use as a function of growth in peer use across adolescence and young adulthood in the context of ADHD: Findings from the MTA. Addictive Behaviors, 99, 106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. C., Adler L., Barkley R., Biederman J., Conners C. K., Demler O., Faraone S. V., Greenhill L. L., Howes M. J., Secnik K., Spencer T., Ustun T. B., Walters E. E., Zaslavsky A. M. (2006). The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. American Journal of Psychiatry, 163(4), 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. C., Lane M., Stang P. E., Van Brunt D. L. (2009). The prevalence and workplace costs of adult attention deficit hyperactivity disorder in a large manufacturing firm. Psychological Medicine, 39(1), 137–147. [DOI] [PubMed] [Google Scholar]
- Khalis A., Mikami A. Y., Hudec K. L. (2018). Positive peer relationships facilitate adjustment in the transition to university for emerging adults with ADHD symptoms. Emerging Adulthood, 6(4), 243–254. [Google Scholar]
- Kittel-Schneider S., Wolff S., Queiser K., Wessendorf L., Meier A. M., Verdenhalven M., Brunkhorst-Kanaan N., Grimm O., McNeill R., Grabow S., Reimertz C., Nau C., Klos M., Reif A. (2019). Prevalence of ADHD in accident victims: Results of the PRADA study. Journal of Clinical Medicine, 8(10), 1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Forehand R., Armistead L., Long P. (1997). Delinquency during the transition to early adulthood: Family and parenting predictors from early adolescence. Adolescence, 32(125), 61–80. [PubMed] [Google Scholar]
- Knecht C., de Alvaro R., Martinez-Raga J., Balanza-Martinez V. (2015). Attention-deficit hyperactivity disorder (ADHD), substance use disorders, and criminality: A difficult problem with complex solutions. International Journal of Adolescent Medicine and Health, 27(2), 163–175. [DOI] [PubMed] [Google Scholar]
- Kooij J. J., Middelkoop H. A., van Gils K., Buitelaar J. K. (2001). The effect of stimulants on nocturnal motor activity and sleep quality in adults with ADHD: An open-label case-control study. Journal of Clinical Psychiatry, 62(12), 952–956. [DOI] [PubMed] [Google Scholar]
- Kooij J. J. S. (2018). Attention-deficit hyperactivity disorder (ADHD), intimate relationships and sexuality. In Jannini E. A., Siracusano A. (Eds.), Sexual dysfunctions in mentally ill patients (pp. 75–82). Springer International Publishing. [Google Scholar]
- Kumar S., Devendran Y., Ns M., Thejas J. A. (2019). Correlates and treatment outcome of risky sexual behaviour in young males with ADHD and comorbid alcohol use disorder: A prospective study. Journal of Psychosexual Health, 1(1), 62–69. [Google Scholar]
- Kuriyan A. B., Pelham W. E., Molina B. S., Waschbusch D. A., Gnagy E. M., Sibley M. H., Babinski D. E., Walther C., Cheong J., Yu J. (2013). Young adult educational and vocational outcomes of children diagnosed with ADHD. Journal of Abnormal Child Psychology, 41(1), 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam L. T. (2002). Attention deficit disorder and hospitalization due to injury among older adolescents in New South Wales, Australia. Journal of Attention Disorders, 6(2), 77–82. [DOI] [PubMed] [Google Scholar]
- Langberg J. M., Becker S. P. (2012). Does long-term medication use improve the academic outcomes of youth with attention-deficit/hyperactivity disorder? Clinical Child and Family Psychology Review, 15(3), 215–233. [DOI] [PubMed] [Google Scholar]
- Langevin R., Curnoe S. (2011). Psychopathy, ADHD, and brain dysfunction as predictors of lifetime recidivism among sex offenders. International Journal of Offender Therapy and Comparative Criminology, 55(1), 5–26. [DOI] [PubMed] [Google Scholar]
- Lee S. S., Humphreys K. L., Flory K., Liu R., Glass K. (2011). Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review, 31(3), 328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin F. R., Mariani J. J., Specker S., Mooney M., Mahony A., Brooks D. J., Babb D., Bai Y., Eberly L. E., Nunes E. V., Grabowski J. (2015). Extended-release mixed amphetamine salts vs placebo for comorbid adult attention-deficit/hyperactivity disorder and cocaine use disorder: A randomized clinical trial. JAMA Psychiatry, 72(6), 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S. H., Yang Y.-H., Kuo T.-Y., Liao Y.-T., Lin T.-C., Lee Y., McIntyre R. S., Kelsen B. A., Wang T.-N., Chen V. C. (2018). Suicide risk reduction in youths with attention-deficit/hyperactivity disorder prescribed methylphenidate: A Taiwan nationwide population-based cohort study. Research in Developmental Disabilities, 72, 96–105. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P., Halldner L., Zetterqvist J., Sjölander A., Serlachius E., Fazel S., Långström N., Larsson H. (2012). Medication for attention deficit-hyperactivity disorder and criminality. New England Journal of Medicine, 367(21), 2006–2014. 10.1056/NEJMoa1203241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden S., Bussing R., Kubilis P., Gerhard T., Segal R., Shuster J. J., Winterstein A. G. (2016). Risk of suicidal events with atomoxetine compared to stimulant treatment: A cohort study. Pediatrics, 137(5), e20153199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou Y.-J., Wei H.-T., Chen M.-H., Hsu J.-W., Huang K.-L., Bai Y.-M., Su T.-P., Li C.-T., Yang A. C., Tsai S.-J., Lin W. C., Chen T. J. (2018). Risk of traumatic brain injury among children, adolescents, and young adults with attention-deficit hyperactivity disorder in Taiwan. Journal of Adolescent Health, 63(2), 233–238. [DOI] [PubMed] [Google Scholar]
- London A. S., Landes S. D. (2016). Attention deficit hyperactivity disorder and adult mortality. Preventive Medicine, 90, 8–10. [DOI] [PubMed] [Google Scholar]
- Lustberg D., Weinshenker D. (2020). Stressing the potential of guanfacine as a treatment for cocaine use disorder. Neuropsychopharmacology, 45(9), 1407–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan N., Hong N., Wigal T. L., Gehricke J.-G. (2010). Hyperactive-impulsive symptoms associated with self-reported sleep quality in nonmedicated adults with ADHD. Journal of Attention Disorders, 14(2), 132–137. [DOI] [PubMed] [Google Scholar]
- Man K. K. C., Coghill D., Chan E. W., Lau W. C. Y., Hollis C., Liddle E., Banaschewski T., McCarthy S., Neubert A., Sayal K., Ip P., Schuemie M. J., Sturkenboom M. C. J. M., Sonuga-Barke E., Buitelaar J., Carucci S., Zuddas A., Kovshoff H., Garas P., , . . . Wong I. C. K. (2017). Association of risk of suicide attempts with methylphenidate treatment. JAMA Psychiatry, 74(10), 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee T. E. (2017). Peer relationships in undergraduates with ADHD symptomatology: Selection and quality of friendships. Journal of Attention Disorders, 21(12), 1020–1029. [DOI] [PubMed] [Google Scholar]
- Meinzer M. C., LeMoine K. A., Howard A. L., Stehli A., Arnold L. E., Hechtman L., Hinshaw S. P., Molina B. S. G., Murray D. W., Sibley M. H., Swanson J. M., Tamm L., Chronis-Tuscano A. (2020). Childhood ADHD and involvement in early pregnancy: Mechanisms of risk. Journal of Attention Disorders, 24(14), 1955–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill R. M., Lyon J. L., Baker R. K., Gren L. H. (2009). Attention deficit hyperactivity disorder and increased risk of injury. Advances in Medical Sciences, 54(1), 20–26. [DOI] [PubMed] [Google Scholar]
- Michelson D., Adler L., Spencer T., Reimherr F. W., West S. A., Allen A. J., Kelsey D., Wernicke J., Dietrich A., Milton D. (2003). Atomoxetine in adults with ADHD: Two randomized, placebo-controlled studies. Biological Psychiatry, 53(2), 112–120. [DOI] [PubMed] [Google Scholar]
- Michielsen M., Comijs H. C., Aartsen M. J., Semeijn E. J., Beekman A. T. F., Deeg D. J. H., Kooij J. J. S. (2015). The relationships between ADHD and social functioning and participation in older adults in a population-based study. Journal of Attention Disorders, 19(5), 368–379. [DOI] [PubMed] [Google Scholar]
- Milivojevic V., Sinha R., Fox H. (2017). Patient responses to guanfacine in cocaine addiction. In Preedy V. R. (Ed.), The Neuroscience of cocaine (pp. 679–688). Elsevier. [Google Scholar]
- Minde K., Eakin L., Hechtman L., Ochs E., Bouffard R., Greenfield B., Looper K. (2003). The psychosocial functioning of children and spouses of adults with ADHD. Journal of Child Psychology and Psychiatry, 44(4), 637–646. [DOI] [PubMed] [Google Scholar]
- Molina B. S. G., Howard A. L., Swanson J. M., Stehli A., Mitchell J. T., Kennedy T. M., Epstein J. N., Arnold L. E., Hechtman L., Vitiello B., Hoza B. (2018). Substance use through adolescence into early adulthood after childhood-diagnosed ADHD: Findings from the MTA longitudinal study. Journal of Child Psychology and Psychiatry, 59(6), 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Barkley R. A. (1996). Attention deficit hyperactivity disorder adults: Comorbidities and adaptive impairments. Comprehensive Psychiatry, 37(6), 393–401. [DOI] [PubMed] [Google Scholar]
- Murray C., Johnston C. (2006). Parenting in mothers with and without attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology, 115(1), 52–61. [DOI] [PubMed] [Google Scholar]
- Nasser A., Hull J. T., Chaturvedi S. A., Liranso T., Odebo O., Kosheleff A. R., Fry N., Cutler A. J., Rubin J., Schwabe S., Childress A. (2022). A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs, 36(8), 897–915. 10.1007/s40263-022-00938-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H.-C., Lin Y.-J., Gau S. S., Huang H.-C., Yang L.-K. (2017). An open-label, randomized trial of methylphenidate and atomoxetine treatment in adults with ADHD. Journal of Attention Disorders, 21(1), 27–39. [DOI] [PubMed] [Google Scholar]
- Nock M. K., Hwang I., Sampson N., Kessler R. C., Angermeyer M., Beautrais A., Borges G., Bromet E., Bruffaerts R., de Girolamo G., de Graaf R., Florescu S., Gureje O., Haro J. M., Hu C., Huang Y., Karam E. G., Kawakami N., Kovess V., . . . Williams D. R. (2009). Cross-national analysis of the associations among mental disorders and suicidal behavior: Findings from the WHO World Mental Health Surveys. PLoS Medicine, 6(8), e1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens E. B., Hinshaw S. P. (2020). Adolescent mediators of unplanned pregnancy among women with and without childhood ADHD. Journal of Clinical Child & Adolescent Psychology, 49(2), 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens E. B., Zalecki C., Gillette P., Hinshaw S. P. (2017). Girls with childhood ADHD as adults: Cross-domain outcomes by diagnostic persistence. Journal of Consulting and Clinical Psychology, 85(7), 723–736. 10.1037/ccp0000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J., Gruber R., Brown T., Corkum P., Cortese S., O’Brien L., Stein M., Weiss M. (2013). Future research directions in sleep and ADHD: Report of a consensus working group. Journal of Attention Disorders, 17(7), 550–564. [DOI] [PubMed] [Google Scholar]
- Owens J. A. (2005). The ADHD and sleep conundrum: A review. Journal of Developmental & Behavioral Pediatrics, 26(4), 312–322. [DOI] [PubMed] [Google Scholar]
- Özgen H., Spijkerman R., Noack M., Holtmann M., Schellekens A. S., Van De Glind G., Banaschewski T., Barta C., Begeman A., Casas M. (2020). International consensus statement for the screening, diagnosis, and treatment of adolescents with concurrent attention-deficit/hyperactivity disorder and substance use disorder. European Addiction Research, 26(4-5), 223–232. [DOI] [PubMed] [Google Scholar]
- Paulson J. F., Buermeyer C., Nelson-Gray R. O. (2005). Social rejection and ADHD in young adults: An analogue experiment. Journal of Attention Disorders, 8(3), 127–135. [DOI] [PubMed] [Google Scholar]
- Pawaskar M., Fridman M., Grebla R., Madhoo M. (2020). Comparison of quality of life, productivity, functioning and self-esteem in adults diagnosed with ADHD and with symptomatic ADHD. Journal of Attention Disorders, 24(1), 136–144. 10.1177/1087054719841129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp-Wiegmann F., Rösler M., Clasen O., Zinnow T., Retz-Junginger P., Retz W. (2018). ADHD modulates the course of delinquency: A 15-year follow-up study of young incarcerated man. European Archives of Psychiatry and Clinical Neuroscience, 268(4), 391–399. [DOI] [PubMed] [Google Scholar]
- Polanczyk G., de Lima M. S., Horta B. L., Biederman J., Rohde L. A. (2007). The worldwide prevalence of ADHD: A systematic review and Metaregression analysis. American Journal of Psychiatry, 164, 942–948. [DOI] [PubMed] [Google Scholar]
- Prasad V., Brogan E., Mulvaney C., Grainge M., Stanton W., Sayal K. (2013). How effective are drug treatments for children with ADHD at improving on-task behaviour and academic achievement in the school classroom? A systematic review and meta-analysis. European Child & Adolescent Psychiatry, 22(4), 203–216. [DOI] [PubMed] [Google Scholar]
- Pratt T. C., Cullen F. T., Blevins K. R., Daigle L., Unnever J. D. (2002). The relationship of attention deficit hyperactivity disorder to crime and delinquency: A meta-analysis. International Journal of Police Science and Management, 4(4), 344–360. [Google Scholar]
- Quinn P. D., Chang Z., Hur K., Gibbons R. D., Lahey B. B., Rickert M. E., Sjölander A., Lichtenstein P., Larsson H., D’Onofrio B. M. (2017). ADHD medication and substance-related problems. American Journal of Psychiatry, 174(9), 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner D. L., Anastopoulos A. D., Costello E. J., Hoyle R. H., McCabe S. E., Swartzwelder H. S. (2009). Motives and perceived consequences of nonmedical ADHD medication use by college students: Are students treating themselves for attention problems? Journal of Attention Disorders, 13(3), 259–270. [DOI] [PubMed] [Google Scholar]
- Reaser A., Prevatt F., Petscher Y., Proctor B. (2007). The learning and study strategies of college students with ADHD. Psychology in the Schools, 44(6), 627–638. [Google Scholar]
- Reynolds K., Pietrzak R. H., El-Gabalawy R., Mackenzie C. S., Sareen J. (2015). Prevalence of psychiatric disorders in US older adults: Findings from a nationally representative survey. World Psychiatry, 14(1), 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney M., Chronis-Tuscano A., Yoon Y. (2012). Substance use in college students with ADHD. Journal of Attention Disorders, 16(3), 221–234. [DOI] [PubMed] [Google Scholar]
- Ruiz-Goikoetxea M., Cortese S., Aznarez-Sanado M., Magallón S., Zallo N. A., Luis E. O., de Castro-Manglano P., Soutullo C., Arrondo G. (2018). Risk of unintentional injuries in children and adolescents with ADHD and the impact of ADHD medications: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 84, 63–71. [DOI] [PubMed] [Google Scholar]
- Rush C. R., Stoops W. W., Hays L. R. (2009). Cocaine effects during D-amphetamine maintenance: A human laboratory analysis of safety, tolerability and efficacy. Drug and Alcohol Dependence, 99(1-3), 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti G. M., Lefler E. K. (2017). ADHD symptomology and social functioning in college students. Journal of Attention Disorders, 21(12), 1009–1019. [DOI] [PubMed] [Google Scholar]
- Safren S. A., Sprich S. E., Cooper-Vince C., Knouse L. E., Lerner J. A. (2010). Life impairments in adults with medication-treated ADHD. Journal of Attention Disorders, 13(5), 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangal R. B., Owens J., Allen A. J., Sutton V., Schuh K., Kelsey D. (2006). Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep, 29(12), 1573–1585. [DOI] [PubMed] [Google Scholar]
- Schubiner H., Saules K. K., Arfken C. L., Johanson C.-E., Schuster C. R., Lockhart N., Edwards A., Donlin J., Pihlgren E. (2002). Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Experimental and Clinical Psychopharmacology, 10(3), 286–294. [DOI] [PubMed] [Google Scholar]
- Schubiner H., Tzelepis A., Isaacson J. H., Warbasse L. H., 3rd, Zacharek M., Musial J. (1995). The dual diagnosis of attention-deficit/hyperactivity disorder and substance abuse: Case reports and literature review. Journal of Clinical Psychiatry, 56, 146–150. [PubMed] [Google Scholar]
- Schulenberg J. E., Sameroff A. J., Cicchetti D. (2004). The transition to adulthood as a critical juncture in the course of psychopathology and mental health. Development and Psychopathology, 16(4), 799–806. [DOI] [PubMed] [Google Scholar]
- Secnik K., Swensen A., Lage M. J. (2005). Comorbidities and costs of adult patients diagnosed with attention-deficit hyperactivity disorder. PharmacoEconomics, 23(1), 93–102. [DOI] [PubMed] [Google Scholar]
- Sedky K., Bennett D. S., Carvalho K. S. (2014). Attention deficit hyperactivity disorder and sleep disordered breathing in pediatric populations: A meta-analysis. Sleep Medicine Reviews, 18(4), 349–356. [DOI] [PubMed] [Google Scholar]
- Shaw-Zirt B., Popali-Lehane L., Chaplin W., Bergman A. (2005). Adjustment, social skills, and self-esteem in college students with symptoms of ADHD. Journal of Attention Disorders, 8(3), 109–120. [DOI] [PubMed] [Google Scholar]
- Shoham R., Sonuga-Barke E., Yaniv I., Pollak Y. (2021). What drives risky behavior in ADHD: Insensitivity to its risk or fascination with its potential benefits? Journal of Attention Disorders, 25, 1988–2002. [DOI] [PubMed] [Google Scholar]
- Shoham R., Sonuga-Barke E. J. S., Aloni H., Yaniv I., Pollak Y. (2016). ADHD-associated risk taking is linked to exaggerated views of the benefits of positive outcomes. Scientific Reports, 6(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley M. H., Arnold L. E., Swanson J. M., Hechtman L. T., Kennedy T. M., Owens E., Molina B. S. G., Jensen P. S., Hinshaw S. P., Roy A., Chronis-Tuscano A., Newcorn J. H., Rohde L. A. (2022). Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. American Journal of Psychiatry, 179, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A., Lewis O., Kumar R., Yeruva S. L., Curry B. H. (2016). Adult ADHD medications and their cardiovascular implications. Case Reports in Cardiology, 2016, 2343691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobanski E., Schredl M., Kettler N., Alm B. (2008). Sleep in adults with attention deficit hyperactivity disorder (ADHD) before and during treatment with methylphenidate: A controlled polysomnographic study. Sleep, 31(3), 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel T., Pollak Y. (2019). Corrigendum: Attention deficit/hyperactivity disorder and increased engagement in sexual risk-taking behavior: The role of benefit perception. Frontiers in Psychology, 10, 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. J., Seedat S., Herman A., Moomal H., Heeringa S. G., Kessler R. C., Williams D. R. (2008). Lifetime prevalence of psychiatric disorders in South Africa. The British Journal of Psychiatry, 192(2), 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M. A., Weiss M., Hlavaty L. (2012). ADHD treatments, sleep, and sleep problems: Complex associations. Neurotherapeutics, 9(3), 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickley A., Koyanagi A., Takahashi H., Ruchkin V., Kamio Y. (2017). Attention-deficit/hyperactivity disorder symptoms and loneliness among adults in the general population. Research in Developmental Disabilities, 62, 115–123. [DOI] [PubMed] [Google Scholar]
- Stoops W. W., Blackburn J. W., Hudson D. A., Hays L. R., Rush C. R. (2008). Safety, tolerability and subject-rated effects of acute intranasal cocaine administration during atomoxetine maintenance. Drug and Alcohol Dependence, 92(1-3), 282–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strattera [Atomoxetine] Prescribing Information . (2017). Eli Lilly and Company, LLC. [Google Scholar]
- Sun S., Kuja-Halkola R., Faraone S. V., D’Onofrio B. M., Dalsgaard S., Chang Z., Larsson H. (2019). Association of psychiatric comorbidity with the risk of premature death among children and adults with attention-deficit/hyperactivity disorder. JAMA Psychiatry, 76(11), 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen A., Birnbaum H. G., Ben Hamadi R., Greenberg P., Cremieux P.-Y., Secnik K. (2004). Incidence and costs of accidents among attention-deficit/hyperactivity disorder patients. Journal of Adolescent Health, 35(4), 346.e1–e346.9. [PubMed] [Google Scholar]
- Turgay A., Goodman D. W., Asherson P., Lasser R. A., Babcock T. F., Pucci M. L., Barkley R.; ADHD Transition Phase Model Working Group. (2012). Lifespan persistence of ADHD: The life transition model and its application. Journal of Clinical Psychiatry, 73(2), 192–201. 10.4088/JCP.10m06628 [DOI] [PubMed] [Google Scholar]
- Turnock P., Rosen L. A., Kaminski P. L. (1998). Differences in academic coping strategies of college students who self-report high and low symptoms of attention deficit hyperactivity disorder. Journal of College Student Development, 39(5), 484–493. [Google Scholar]
- Waxmonsky J. G., Waschbusch D. A., Babinski D. E., Humphrey H. H., Alfonso A., Crum K. I., Bernstein M., Slavec J., Augustus J. N., Pelham W. E. (2014). Does pharmacological treatment of ADHD in adults enhance parenting performance? Results of a double-blind randomized trial. CNS Drugs, 28(7), 665–677. [DOI] [PubMed] [Google Scholar]
- Weafer J., Camarillo D., Fillmore M. T., Milich R., Marczinski C. A. (2008). Simulated driving performance of adults with ADHD: Comparisons with alcohol intoxication. Experimental and Clinical Psychopharmacology, 16(3), 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G., Hechtman L. T. (1993). Hyperactive children grown up: ADHD in children, adolescents, and adults (2nd ed.). Guilford Press. [Google Scholar]
- Weiss M., Hechtman L. T., Weiss G. (2001). ADHD in adulthood: A guide to current theory, diagnosis, and treatment. The Johns Hopkins University Press. [Google Scholar]
- Weiss M. D., McBride N. M., Craig S., Jensen P. (2018). Conceptual review of measuring functional impairment: Findings from the Weiss Functional Impairment Rating Scale. Evidence-Based Mental Health, 21(4), 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender P. H., Reimherr F. W., Marchant B. K., Sanford M. E., Czajkowski L. A., Tomb D. A. (2011). A one year trial of methylphenidate in the treatment of ADHD. Journal of Attention Disorders, 15(1), 36–45. [DOI] [PubMed] [Google Scholar]
- Westover A. N., Halm E. A. (2012). Do prescription stimulants increase the risk of adverse cardiovascular events?: A systematic review. BMC Cardiovascular Disorders, 12(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyandt L. L., Dupaul G. J. (2008). ADHD in college students: Developmental Findings. Developmental Disabilities Research Reviews, 14(4), 311–319. [DOI] [PubMed] [Google Scholar]
- Wigal S. B., Wigal T., Childress A., Donnelly G. A. E., Reiz J. L. (2020). The time course of effect of multilayer-release methylphenidate hydrochloride capsules: A randomized, double-blind study of adults with ADHD in a simulated adult workplace environment. Journal of Attention Disorders, 24(3), 373–383. [DOI] [PubMed] [Google Scholar]
- Wilens T. E., Adler L. A., Weiss M. D., Michelson D., Ramsey J. L., Moore R. J., Renard D., Brady K. T., Trzepacz P. T., Schuh L. M., Ahrbecker L. M., Levine L. R. (2008). Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug and Alcohol Dependence, 96(1-2), 145–154. [DOI] [PubMed] [Google Scholar]
- Wilens T. E., Morrison N. R., Prince J. (2011). An update on the pharmacotherapy of attention-deficit/hyperactivity disorder in adults. Expert Review of Neurotherapeutics, 11(10), 1443–1465. 10.1586/ern.11.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt E. G. (2012). The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics, 9(3), 490–499. 10.1007/s13311-012-0135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S., Queiser K., Wessendorf L., Meier A. M., Verdenhalven M., Grimm O., Reimertz C., Nau C., Klos M., Reif A., Kittel-Schneider S. (2019). Accident patterns in trauma surgery patients with and without self-reported ADHD. Journal of Neural Transmission, 126(9), 1163–1173. [DOI] [PubMed] [Google Scholar]
- Wood S., Sage J. R., Shuman T., Anagnostaras S. G. (2014). Psychostimulants and cognition: A continuum of behavioral and cognitive activation. Pharmacological Reviews, 66(1), 193–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymbs B. T., Molina B., Pelham W., Cheong J., Gnagy E., Belendiuk K., Walther C., Babinski D., Waschbusch D. (2012). Risk of intimate partner violence among young adult males with childhood ADHD. Journal of Attention Disorders, 16(5), 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymbs B. T., Dawson A. E., Egan T. E., Sacchetti G. M. (2019). Rates of intimate partner violence perpetration and victimization among adults with ADHD. Journal of Attention Disorders, 23(9), 949–958. [DOI] [PubMed] [Google Scholar]
- Wynchank D., Bijlenga D., Beekman A. T., Kooij J. J. S., Penninx B. W. (2017). Adult attention-deficit/hyperactivity disorder (ADHD) and insomnia: An update of the literature. Current Psychiatry Reports, 19(12), 98. [DOI] [PubMed] [Google Scholar]
- Yanagita T., Wakasa Y., Kiyohara H. (1980). Drug dependence potential of viloxazine hydrochloride tested in rhesus monkeys. Pharmacology Biochemistry and Behavior, 12(1), 155–161. [DOI] [PubMed] [Google Scholar]
- Young S., González R. A., Mutch L., Mallet-Lambert I., O’Rourke L., Hickey N., Asherson P., Gudjonsson G. H. (2016). Diagnostic accuracy of a brief screening tool for attention deficit hyperactivity disorder in UK prison inmates. Psychological Medicine, 46(7), 1449–1458. [DOI] [PubMed] [Google Scholar]
- Young S., Gudjonsson G. H., Wells J., Asherson P., Theobald D., Oliver B., Scott C., Mooney A. (2009). Attention deficit hyperactivity disorder and critical incidents in a Scottish prison population. Personality and Individual Differences, 46(3), 265–269. [Google Scholar]
- Young S. J., Adamou M., Bolea B., Gudjonsson G., Müller U., Pitts M., Thome J., Asherson P. (2011). The identification and management of ADHD offenders within the criminal justice system: A consensus statement from the UK adult ADHD network and criminal justice agencies. BMC Psychiatry, 11(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Moss D., Sedgwick O., Fridman M., Hodgkins P. (2015). A meta-analysis of the prevalence of attention deficit hyperactivity disorder in incarcerated populations. Psychological Medicine, 45(2), 247–258. 10.1017/S0033291714000762 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jad-10.1177_10870547231158572 for Functional Impairments Associated With ADHD in Adulthood and the Impact of Pharmacological Treatment by Alisa R. Kosheleff, Oren Mason, Rakesh Jain, Jennifer Koch and Jonathan Rubin in Journal of Attention Disorders
Supplemental material, sj-docx-2-jad-10.1177_10870547231158572 for Functional Impairments Associated With ADHD in Adulthood and the Impact of Pharmacological Treatment by Alisa R. Kosheleff, Oren Mason, Rakesh Jain, Jennifer Koch and Jonathan Rubin in Journal of Attention Disorders