Abstract
Some Ophiocordyceps species infecting ants are able to manipulate the host behavior. The hosts are manipulated in order to move to location that are advantageous for fungal spore transmission. Ophiocordyceps species that are able to manipulate the ant's behavior are called "zombie-ant fungi". They are widespread within tropical forests worldwide, with relatively few reports from subtropical monsoon evergreen broad-leaf forest. Zombie-ant fungi have been described and reported in different countries worldwide. However, there were a few reports from China. This study proposed six new species of zombie-ant fungi from China based on multi-gene (SSU, LSU, TEF, RPB1 and RPB2) phylogenetic analyses and morphological characteristics. Six novel species of Ophiocordyceps from China were identified as the Ophiocordyceps unilateralis core clade, forming a separate lineage with other species. Six novel species of Ophiocordyceps with hirsutella-like asexual morphs exclusively infecting ants were presented herein, namely, Ophiocordyceps acroasca, Ophiocordyceps bifertilis, Ophiocordyceps subtiliphialida, Ophiocordyceps basiasca, Ophiocordyceps nuozhaduensis and Ophiocordyceps contiispora. Descriptions and illustrations for six taxon were provided. Five of these species were collected from the subtropical monsoon evergreen broad-leaf forest, and one was collected from the rainforest and subtropical monsoon evergreen broad-leaf forest. This work proposes that the same host of Camponotus can be infected by multiple ant pathogenic fungi, while multiple ants of Polyrhachis can be infected by the same pathogenic fungi at the same time. This study contributes towards a better understanding of the evolutionary relationship between hosts and fungi, and provides novel insights into the morphology, distribution, parasitism, and ecology of Ophiocordyceps unilateralis sensu lato. We have provided a method for obtaining living cultures of Ophiocordyceps unilateralis complex species and their asexual morphs based on the living cultures, which is of significant value for further studies of Ophiocordyceps unilateralis complex species in the future.
Keywords: 6 new taxa, Camponotus, Living cultures, Morphology, Multi-gene phylogeny, Ophiocordyceps, Polyrhachis, Taxonomy
INTRODUCTION
Evolutionary relationships between fungi and insects, from parasitism to mutualism, have been widely studied (Suh et al. 2005; Cheek et al. 2020; Haelewaters et al. 2020). Insects are diverse, with more than a million described species (Foottit and Adler 2009), in 29 orders (Misof et al. 2014). The fungal pathogens are able to colonize 19 of 29 orders, resulting in the evolution of extensive diversity of strategies and morphologies, by using the insect body for infection and onward transmission (Araújo and Hughes 2016). Among these insects and fungi strategies, one of the most impressive and sophisticated involved ants and species of fungi within the genus Ophiocordyceps (Andersen et al. 2009). The species of Ophiocordyceps had colonized 13 orders of insects (Crous et al. 2004; Araújo and Hughes 2016), comprised of more than 300 species of entomopathogens (Kepler et al. 2011; Sanjuan et al. 2015; Crous et al. 2016; Araújo et al. 2018; Khonsanit et al. 2018; Araújo and Hughes 2019; Wei et al. 2020; Tang et al. 2022; Xu et al. 2022). The insect hosts orders infected by these fungi included Coleoptera, Diptera, Hemiptera, Hymenoptera, Isoptera, Lepidoptera, Neuroptera, Dragonflies, and Orthoptera (Araújo et al. 2015; Araújo and Hughes 2019). Ants (Hymenoptera) were widely distributed in the arctic to tropical, occupying a wide range of habitats from high canopy to leaf litter; their colonies ranged from a few dozen (Jahyny et al. 2002) to millions of individuals (Currie et al. 2003). In tropical forests, they contributed as much as 50% of animal biomass (Hölldobler et al. 2009). Among the hosts of many entomopathogenic fungi, ants were also the most common host of species within Ophiocordyceps (Evans and Samson 1982; Evans et al. 2011b; Kepler et al. 2011; Luangsa-ard et al. 2011; Kobmoo et al. 2012, 2015; Araújo et al. 2015, 2018, 2020; Sanjuan et al. 2015; Spatafora et al. 2015; Crous et al. 2016; Tasanathai et al. 2019; Wei et al. 2020; Tang et al. 2022; Xu et al. 2022).
Ophiocordyceps was erected by Petch (1931) to accommodate the species of Cordyceps that produce non-disarticulating ascospores. The term as a subgeneric classification was used by Kobayasi, based solely on ascospores morphology, and essentially adopted the diagnosis of Petch (Kobayasi 1941; Petch 1931). Then the subgenera Ophiocordyceps was transferred as subgenus of Cordyceps sensu lato (Mains 1958). The three new families were well-supported in Sung et al. (2007) study, hence their proposition to split them into 3 families (Ophiocordycipitaceae, Clavicipitaceae and Cordycipitaceae). Ophiocordyceps was proposed as a genus of Ophiocordycipitaceae. The classification system of Cordyceps sensu lato was widely accepted (Sung et al. 2007). Ophiocordyceps unilateralis sensu stricto was originally published as Torrubia unilateralis (Tulasne and Tulasne 1865). Torrubia unilateralis was transferred to Ophiocordyceps (Petch 1931). Evans et al. (2018) moved to epitypify O. unilateralis sensu stricto and to clarify its description, providing an interpretive type that was more effective in a biological sense than the illustrations by Tulasne; it was proposed to distinguish O. unilateralis sensu stricto and O. unilateralis sensu lato. Asexual morphs associated with Ophiocordyceps included Hirsutella, Syngliocladium, Stilbella, Paraisaria, Hymenostilbe and Sorosporella (Quandt et al. 2014). Hirsutella, Stilbella, Paraisaria and Hymenostilbe were recorded to be associated with ants. Asexual morphs Hymenostilbe and Hirsutella were commonly found associated with ants (Evans and Samson 1982, 1984; Araújo et al. 2015; Araújo and Hughes 2017).
Members of the O. unilateralis complex were ordinary among the pathogenic fungi on ants (Evans et al. 2011a, 2011b). These fungi could change ant behavior controlling it to leave the nest to die, usually in an exposed position in which they were attached or biting leaves or branches in a "death grip" (Hughes et al. 2011). The manipulative behavior caused by species within O. unilateralis complex has attracted extensive attention (Moore 1995, Thomas et al. 2010, Poulin and Maure 2015, de Bekker et al. 2018, Hafer-Hahmann 2019, Will et al. 2020). However, the mechanism of manipulating host behavior remained unknown (Herbison 2017; Will et al. 2020). Many studies have often used the term O. unilateralis sensu lato for the zombie-ant fungus, including the evolutionary relationship between fungi and hosts, the mechanism of manipulating host behavior, and genomes (Andersen et al. 2009; Hughes et al. 2009; Pontoppidan et al. 2009; Evans et al. 2011a). Regarding the evolutionary relationship between fungi and hosts, Evans et al. (2011b) found that different fungi parasitized different ants; their appearances were very similar but differed in morphological characters. A total of thirty-six species of the O. unilateralis sensu lato have been described. Although this group was estimated to be tens or even hundreds of species worldwide (Evans et al. 2011a), or 580 species discussed by Araújo et al. (Araújo et al. 2018, Araújo and Hughes, unpublished data). There are many species of O. unilateralis sensu lato need further global collections to provide more new taxa to support for exploring the evolutionary relationship between the fungus and its host.
Previous some taxonomic works supported the “one ant-one Ophiocordyceps species” hypothesis (Evans et al. 2011b; Kobmoo et al. 2012; Araújo et al. 2018). They pointed out that host-specific fungal species seemed to be associated to each ant species, leading to the "one ant-one fungus", and the host identity was used as a proxy for fungal identification, such as O. camponoti-atricipis, O. camponoti-balzani, O. camponoti-bispinosi, O. camponoti-chartificis, O. camponoti-femorati, O. camponoti-floridani, O. camponoti-hippocrepidis, O. camponoti-indiani, O. camponoti-leonardi, O. camponoti-melanotici, O. camponoti-nidulantis, O. camponoti-novogranadensis, O. camponoti-renggeri, O. camponoti-rufipedis, O. camponoti-saundersi, O. camponoti-sexguttati, and O. polyrhachis-furca (Evans et al. 2011b; Kobmoo et al. 2012; Araújo et al. 2015, 2018). However, with the deepening of research, different views have emerged, two hosts of the genus Polyrhachis were infected by the ant pathogenic fungus "O. nooreniae" (Crous et al. 2016). Lin et al. (2020) showed that a single species of O. unilateralis sensu lato can infect eight ant species. In addition, Kobmoo et al. (2019) indicated that the ant pathogenic fungus may parasitize the same host based on population genomics study, and constitute further cryptic species, challenging the one ant-one fungus paradigm. The relationship between O. unilateralis sensu lato complex and Formicine ants is still uncertain. Host identification was an important feature to describe and report new taxa. However, in our research, observing hundreds of specimens, we identified that some vital characteristics of the host (such as mouthparts, antennae, legs and abdomens) have been destroyed by pathogenic fungi. Therefore, constructing a host phylogenetic tree using molecular data (COI genes) is of great significance to explore the evolutionary relationship between host and species of O. unilateralis sensu lato.
Ophiocordyceps unilateralis sensu lato has been described and reported in the past two decades. Eighteen species were described from Brazil (Evans and Samson 1982; Evans et al. 2011b; Araújo et al. 2015, 2018), one from Colombia (Araújo et al. 2018), three from the USA (Araújo et al. 2018), one from Ghana (Spatafora et al. 2015), three from Australia (Crous et al. 2016; Araújo et al. 2018), three from Japan (Kepler et al. 2011; Araújo et al. 2018), six from Thailand (Luangsa-ard et al. 2011; Kobmoo et al. 2012, 2015), one from China (Wei et al. 2020). In the past three years, we have also found the species of O. unilateralis sensu lato in Laos and Vietnam (unpublished data). Although multiple taxa of O. unilateralis sensu lato have been described, many questions remain open within the group, such as the evolutionary relationship between host and O. unilateralis sensu lato species, the origins of the group, and the mechanisms that manipulate host behavior. The description and record of the new taxa of O. unilateralis sensu lato is of great importance for the solution of the above problems.
Most species of O. unilateralis sensu lato have been collected from tropical rainforests. There are few or no record of O. unilateralis sensu lato species in the subtropical monsoon evergreen broad-leaf forest. Few species of O. unilateralis sensu lato were reported in China (Wei et al. 2020). The unique geographical location of southwest China is an important area for the diversity of Cordyceps sensu lato. Many species of Ophiocordyceps have been reported from Yunnan province, for example, O. laojunshanensis (Chen et al. 2011), O. lanpingensis (Chen et al. 2013), O. alboperitheciata (Fan et al. 2021), O. pingbianensis (Chen et al. 2021). Our team has spent the past more than two decades investigating and collecting entomopathogenic fungi to describe more new species and to solve taxonomic problems. The six novel species presented herein were collected from Yunnan province in China. Based on morphological and phylogenetic analyses, all species were identified as part of the core clade of O. unilateralis. This study aims to provide additional new taxa that support understanding of the evolutionary relationships between fungi and their hosts, providing novel insights into their living cultures, morphology, ecology, parasitism, and distribution.
MATERIALS AND METHODS
Sampling and isolation
All specimens were collected from Yunnan Province in China in this work. Most specimens were collected from Sun River National Park; some were from Nuozhadu Nature Reserve and Mohan Town, Mengla County. Specimens were noted (e.g., vegetation type, death position, altitude above ground) and photographed in the field, then placed in a sterilized boxes, returned to the laboratory, and stored at 4 °C. Before obtaining axenic cultures, the specimens' fertile region (ascomata) was examined using an Olympus SZ61 stereomicroscope (Olympus Corporation, Tokyo, Japan). Stromata was removed from the head of the ant for morphological observation (sexual and asexual morph). The sclerotium (body of the ant) was immersed in 30% H2O2 for 5–8 min, immersed in 75% ethanol for 1 min, and rinsed five times in sterilized water (the specimens must be complete). After drying on sterilized filter paper, the sclerotium was divided into four segments (the head and abdomen were divided into the same two-part, respectively) and inoculated onto solid medium plates (potato 200 g/L, dextrose 20 g/L, agar 20 g/L, yeast powder 10 g/L and peptone 5 g/L), cultured at 25–28 °C (normal temperature was the best condition). Pure cultures were transplanted to a PDA slant, and stored at 4 °C. The specimens were deposited in the Yunnan Herbal Herbarium (YHH) of Yunnan University. The cultures were stored in Yunnan the Fungal Culture Collection (YFCC) of Yunnan University.
Morphological observations
For sexual morph observation, ascomata were photographed and measured by using an Olympus SZ61 stereomicroscope (Olympus Corporation, Tokyo, Japan). Free-hand or frozen sections of the fruiting structures were mounted in lactophenol cotton blue solution for microscopic study and photomicrography. The frozen sections were used by Freezing Microtome HM525NX (Thermo Fisher Scientific, Massachusetts, America). Micro-morphological characteristics (perithecia, asci, apical caps and ascospores) of fungi were examined using Olympus CX40 and BX53 microscopes. Two methods were used for asexual morphological observations. One was directly observed from stromata, sutures, legs and joints of specimens, and another was observed from the pure culture on solid medium plates. Cultures on solid medium plates were incubated for 30–40 days at 25 °C and photographed using a Canon 750 D camera (Canon Inc., Tokyo, Japan). The solid medium was made 0.5–1 mm thick, then divided into 5 mm long and 5 mm wide. Finally, the medium was placed on the glass slide in the sterile culture dish (there was a glass rod to cushion that it could not be submerged in sterile water). The colony was placed on a solid medium, gently covered the cover slide, added sterile water 3 ml, and placed at 25 °C for 30–40 days. The BX53 microscope and Olympus CX40 were used to examine the asexual characteristics such as conidiophores, conidiogenous cells and conidia. Unfortunately, we were not able to study the germination process in most species because the samples had been previously dried.
DNA extraction, polymerase chain reaction (PCR), and sequencing
Specimens and axenic living cultures were prepared for DNA extraction, and the specimens were treated in the same way as the axenic cultures prior to DNA extraction. Total DNA was extracted using the CTAB method, following the described by Liu et al. (2001). Five genes (SSU, LSU, TEF, RPB1, RPB2) and COI genes were amplified and sequenced. The primer pair NS1 and NS4 were used to amplify a fraction of the nuclear ribosomal small subunit (SSU) (White et al. 1990). The primer pair LR0R (Hopple 1994) and LR5 (Vilgalys and Hester 1990) were used to amplify the nuclear ribosomal large subunit (LSU). The primer pair 2218R and 983F were used to amplify the translation elongation factor 1α (TEF) (Rehner and Buckley 2005). The primer pairs RPB1 and RPB1Cr_oph, fRPB2-7cR and fRPB2-5F, were used to amplify the largest and second largest subunits of RNA polymerase II (RPB1 and RPB2), respectively (Liu et al. 1999; Castlebury et al. 2004; Araújo et al. 2018). The primer pair, LCO1490 and HCO2198 (Hebert et al. 2003) was used to amplify the COI gene. The polymerase chain reaction (PCR) matrix was performed in a final volume of 25 µl, composed of 17.25 µl of sterile water, 2.5 µl of PCR 10 × Buffer (2 mmol/l Mg2+) (Transgen Biotech, Beijing, China), 2 µl of dNTP (2.5 mmol/l), 1 µL of forwarding primers (10 µmol/), 1 µl of reverse primers (10 µmol/l), 0.25 µl of Taq DNA polymerase (Transgen Biotech, Beijing, China), 1 µl of DNA template (500 ng/µl). Amplification reactions were performed in a BIO-RAD T100TM thermal cycler (BIO-RAD Laboratories, Hercules, CA, United States). The PCR program of five genes was conducted as described by Wang et al. (2020), and the COI gene was conducted as described by Hebert et al. (2003). The Beijing Genomics Institute (Chongqing, China) performed the target gene amplification and sequencing.
Phylogenetic analyses
Phylogenetic analyses of fungi
Phylogenetic analyses were based on sequences of five genes (SSU, LSU, TEF, RPB1 and RPB2). Sequences of multiple genes from various species (see Table 1) were retrieved from GenBank and the nucleotide sequences were combined with those generated in our study. Information on specimens and GenBank accession numbers were listed in Table 1. Sequences were aligned using Clustal X (v.2.0) (Larkin et al. 2007), poorly-aligned regions were removed and adjusted manually using MEGA6 (v.6.0) (Tamura et al. 2013). We generated one fungi dataset (SSU, LSU, TEF, RPB1 and RPB2). Modelfinder (Kalyaanamoorthy et al. 2017) was used to select the best-fitting likelihood model for maximum likelihood (ML) analyses, and Bayesian inference (BI) analyses were carried out for the fungi datasets. The Corrected Akaike Information Criterion (AIC) was used to select the model for each gene, and the best-fitting models were provided in Table 3. For ML analyses, tree searches were performed in IQ-tree (v.2.1.3) (Nguyen et al. 2015) based on the best-fit model with 5000 ultrafast bootstraps (Hoang et al. 2017) in a single run. BI analyses were conducted using MrBayes (v.3.2.2) (Ronquist et al. 2012). Four Markov Chain Monte Carlo chains were run, each beginning with a random tree and sampling, one tree every 100 generations of 2000,000 generations, and the first 25% of samples were discarded as burn-in. Each tree was visualized with its maximum-likelihood bootstrap support values (ML-BS) and Bayesian inference posterior probability (BI-PP) in Figtree (v.1.4.3). Adobe Illustrator CS6 was used for editing.
Table 1.
Voucher information, GenBank accession numbers, host and location of the taxa used in this study
| Species | Voucher information | SSU | LSU | TEF | RPB1 | RPB2 | Host | Location |
|---|---|---|---|---|---|---|---|---|
| Hirsutella sp. | NHJ 12525 | EF469125 | EF469078 | EF469063 | EF469092 | EF469111 | Hemiptera | – |
| Hirsutella sp. | OSC 128575 | EF469126 | EF469079 | EF469064 | EF469093 | EF469110 | Hemiptera | – |
| Ophiocordyceps acicularis | ARSEF 5692 | DQ522540 | DQ518754 | DQ522322 | DQ522368 | DQ522418 | Coleoptera | Korea |
| Ophiocordyceps acroasca | YFCC 9049 | ON555837 | ON555918 | ON567757 | ON568677 | ON568130 | Camponotus sp. | China |
| Ophiocordyceps acroasca | YFCC 9019 | ON555838 | ON555919 | ON567758 | ON568678 | ON568131 | Camponotus sp. | China |
| Ophiocordyceps acroasca | YFCC 9017 | ON555839 | ON555920 | ON567759 | ON568679 | ON568132 | Camponotus sp. | China |
| Ophiocordyceps acroasca | YFCC 9018 | ON555840 | ON555921 | ON567760 | ON568680 | ON568133 | Camponotus sp. | China |
| Ophiocordyceps acroasca | YFCC 9016T | ON555841 | ON555922 | ON567761 | ON568681 | ON568134 | Camponotus sp. | China |
| Ophiocordyceps acroasca | YHH 20122 | ON555842 | – | ON567762 | ON568682 | – | Camponotus sp. | China |
| Ophiocordyceps albacongiuae | RC20 | KX713633 | – | KX713670 | – | – | Camponotus sp. | Colombia |
| Ophiocordyceps annullata | CEM 303 | KJ878915 | KJ878881 | KJ878962 | KJ878995 | – | Coleoptera | Japan |
| Ophiocordyceps aphodii | ARSEF 5498 | DQ522541 | DQ518755 | DQ522323 | – | DQ522419 | Coleoptera | – |
| Ophiocordyceps australis | HUA 186097 | KC610786 | KC610765 | KC610735 | KF658662 | – | Hymenoptera | Colombia |
| Ophiocordyceps basiasca | YHH 20191 | ON555828 | ON555910 | ON567748 | ON568672 | ON568121 | Camponotus sp. | China |
| Ophiocordyceps bifertilis | YFCC 9012T | ON555843 | ON555923 | ON567763 | ON568143 | ON568135 | Polyrhachis sp. | China |
| Ophiocordyceps bifertilis | YHH 20162 | ON555844 | – | ON567764 | ON568144 | – | Polyrhachis sp. | China |
| Ophiocordyceps bifertilis | YHH 20163 | ON555845 | ON555924 | ON567765 | ON568145 | ON568136 | Polyrhachis sp. | China |
| Ophiocordyceps bifertilis | YHH 20164 | ON555846 | – | ON567766 | ON568146 | – | Polyrhachis sp. | China |
| Ophiocordyceps bifertilis | YFCC 9048 | ON555847 | ON555925 | ON567767 | ON568147 | ON568137 | Polyrhachis sp. | China |
| Ophiocordyceps bifertilis | YFCC 9013 | ON555848 | ON555926 | ON567768 | ON568148 | ON568138 | Polyrhachis sp. | China |
| Ophiocordyceps blakebarnesii | MISSOU5 | KX713641 | KX713610 | KX713688 | KX713716 | – | Camponotus sp. | USA |
| Ophiocordyceps blakebarnesii | MISSOU4 | KX713642 | KX713609 | KX713685 | KX713715 | – | Camponotus sp. | USA |
| Ophiocordyceps brunneipunctata | OSC 128576 | DQ522542 | DQ518756 | DQ522324 | DQ522369 | DQ522420 | Coleoptera | – |
| Ophiocordyceps buquetii | HMAS_199617 | KJ878940 | KJ878905 | KJ878985 | KJ879020 | – | Hymenoptera | China |
| Ophiocordyceps camponoti-balzani | G143 | KX713658 | KX713595 | KX713690 | KX713705 | – | Camponotus balzani | Brazil |
| Ophiocordyceps camponoti-balzani | G104 | KX713660 | KX713593 | KX713689 | KX713703 | – | Camponotus balzani | Brazil |
| Ophiocordyceps camponoti-bispinosi | OBIS5 | KX713636 | KX713616 | KX713693 | KX713721 | – | Camponotus bispinosus | Brazil |
| Ophiocordyceps camponoti-bispinosi | OBIS4 | KX713637 | KX713615 | KX713692 | KX713720 | – | Camponotus bispinosus | Brazil |
| Ophiocordyceps camponoti-chartificis | MF080 | MK874744 | – | MK863824 | – | – | Camponotus chartifex | Brazil |
| Ophiocordyceps camponoti-femorati | FEMO2 | KX713663 | KX713590 | KX713678 | KX713702 | – | Camponotus femoratus | Brazil |
| Ophiocordyceps camponoti-floridani | Flo4 | KX713662 | KX713591 | – | – | – | Camponotus femoratus | Brazil |
| Ophiocordyceps camponoti-floridani | Flx2 | – | KX713592 | KX713674 | – | – | Camponotus femoratus | Brazil |
| Ophiocordyceps camponoti-hippocrepidis | HIPPOC | KX713655 | KX713597 | KX713673 | KX713707 | – | Camponotus hippocrepis | Brazil |
| Ophiocordyceps camponoti-indiani | INDI2 | KX713654 | KX713598 | – | – | – | Camponotus indianus | Brazil |
| Ophiocordyceps camponoti-leonardi | C27 | – | – | JN819019 | – | – | Camponotus leonardi | Thailand |
| Ophiocordyceps camponoti-leonardi | C25 | – | – | JN819029 | – | – | Camponotus leonardi | Thailand |
| Ophiocordyceps camponoti-nidulantis | NIDUL2 | KX713640 | KX713611 | KX713669 | KX713717 | – | Camponotus nidulans | Brazil |
| Ophiocordyceps camponoti-novogranadensis | Mal63 | KX713648 | KX713603 | – | – | – | Camponotus novogranadensis | Brazil |
| Ophiocordyceps camponoti-novogranadensis | Mal4 | KX713649 | KX713602 | – | – | – | Camponotus novogranadensis | Brazil |
| Ophiocordyceps camponoti-renggeri | RENG2 | KX713632 | – | KX713672 | – | – | Camponotus renggeri | Brazil |
| Ophiocordyceps camponoti-renggeri | ORENG | KX713634 | KX713617 | KX713671 | – | – | Camponotus renggeri | Brazil |
| Ophiocordyceps camponoti-rufipedis | G177 | KX713657 | KX713596 | KX713680 | – | – | Camponotus rufipes | Brazil |
| Ophiocordyceps camponoti-rufipedis | G108 | KX713659 | KX713594 | KX713679 | KX713704 | – | Camponotus rufipes | Brazil |
| Ophiocordyceps camponoti-saundersi | C40 | KJ201519 | – | JN819012 | – | – | Camponotus saundersi | Thailand |
| Ophiocordyceps camponoti-saundersi | Co19 | – | – | JN819018 | – | – | Camponotus saundersi | Thailand |
| Ophiocordyceps citrina | TNSF 18537 | – | KJ878903 | KJ878983 | – | KJ878954 | Hemiptera | Japan |
| Ophiocordyceps clavata | CEM 1762 | KJ878916 | KJ878882 | KJ878963 | KJ878996 | – | Coleoptera | China |
| Ophiocordyceps cochlidiicola | HMAS_199612 | KJ878917 | KJ878884 | KJ878965 | KJ878998 | – | Lepidoptera | China |
| Ophiocordyceps contiispora | YFCC 9025 | ON555829 | ON555911 | ON567749 | ON568139 | ON568122 | Camponotus sp. | China |
| Ophiocordyceps contiispora | YHH 20145 | ON555830 | - | ON567750 | ON568140 | ON568123 | Camponotus sp. | China |
| Ophiocordyceps contiispora | YFCC 9026 | ON555831 | ON555912 | ON567751 | ON568141 | ON568124 | Camponotus sp. | China |
| Ophiocordyceps contiispora | YFCC 9027T | ON555832 | ON555913 | ON567752 | ON568142 | ON568125 | Camponotus sp. | China |
| Ophiocordyceps curculionum | OSC 151910 | KJ878918 | KJ878885 | – | KJ878999 | – | Coleoptera | Guyana |
| Ophiocordyceps daceti | MF01 | – | KX713604 | KX713667 | – | – | Daceton armigerum | Brazil |
| Ophiocordyceps dipterigena | OSC 151911 | KJ878919 | KJ878886 | KJ878966 | KJ879000 | – | Diptera | USA |
| Ophiocordyceps dipterigena | OSC 151912 | KJ878920 | KJ878887 | KJ878967 | KJ879001 | – | Diptera | USA |
| Ophiocordyceps formicarum | TNSF 18565 | KJ878921 | KJ878888 | KJ878968 | KJ879002 | KJ878946 | Hymenoptera | Japan |
| Ophiocordyceps formosana | TNMF 13893 | KJ878908 | – | KJ878956 | KJ878988 | KJ878943 | Coleoptera | Taiwan |
| Ophiocordyceps forquignonii | OSC 151902 | KJ878912 | KJ878876 | – | KJ878991 | KJ878945 | Diptera | France |
| Ophiocordyceps forquignonii | OSC 151908 | KJ878922 | KJ878889 | – | KJ879003 | KJ878947 | Diptera | France |
| Ophiocordyceps ghanensis | Gh41 | KX713656 | – | KX713668 | KX713706 | – | Polyrhachis sp. | Ghana |
| Ophiocordyceps halabalaensis | MY1308T | KM655825 | – | GU797109 | – | – | Camponotus gigus | Thailand |
| Ophiocordyceps halabalaensis | MY5151 | KM655826 | – | GU797110 | – | – | Camponotus gigas | Thailand |
| Ophiocordyceps irangiensis | OSC 128577 | DQ522546 | DQ518760 | DQ522329 | DQ522374 | DQ522427 | Hymenoptera | – |
| Ophiocordyceps irangiensis | OSC 128579 | EF469123 | EF469076 | EF469060 | EF469089 | EF469107 | Hymenoptera | – |
| Ophiocordyceps kimflemingiae | SC30 | KX713629 | KX713622 | KX713699 | KX713727 | – | Camponotus castaneus/americanus | USA |
| Ophiocordyceps kimflemingiae | SC09B | KX713631 | KX713620 | KX713698 | KX713724 | – | Camponotus castaneus/americanus | USA |
| Ophiocordyceps kniphofioides | HUA 186148 | KC610790 | KF658679 | KC610739 | KF658667 | KC610717 | Hymenoptera | Colombia |
| Ophiocordyceps konnoana | EFCC 7295 | EF468958 | – | – | EF468862 | EF468915 | Coleoptera | Korea |
| Ophiocordyceps konnoana | EFCC 7315 | EF468959 | – | EF468753 | EF468861 | EF468916 | Coleoptera | Korea |
| Ophiocordyceps lloydii | OSC 151913 | KJ878924 | KJ878891 | KJ878970 | KJ879004 | KJ878948 | Hymenoptera | Ecuador |
| Ophiocordyceps longissima | TNSF 18448 | KJ878925 | KJ878892 | KJ878971 | KJ879005 | – | Hemiptera | Japan |
| Ophiocordyceps longissima | HMAS_199600 | KJ878926 | – | KJ878972 | KJ879006 | KJ878949 | Hemiptera | China |
| Ophiocordyceps melolonthae | OSC 110993 | DQ522548 | DQ518762 | DQ522331 | DQ522376 | – | Coleoptera | – |
| Ophiocordyceps melolonthae | Ophgrc 679 | – | KC610768 | KC610744 | KF658666 | – | Coleoptera | Colombia |
| Ophiocordyceps monacidis | MF74C | KX713646 | KX713606 | – | – | – | Dolichoderus bispinosus | Bazil |
| Ophiocordyceps monacidis | MF74 | KX713647 | KX713605 | – | KX713712 | – | Dolichoderus bispinosus | Brazil |
| Ophiocordyceps myrmecophila | CEM 1710 | KJ878928 | KJ878894 | KJ878974 | KJ879008 | – | Hymenoptera | China |
| Ophiocordyceps naomipierceae | DAWKSANT | KX713664 | KX713589 | – | KX713701 | – | Polyrhachis cf. robsonii | Australia |
| Ophiocordyceps neovolkiana | OSC 151903 | KJ878930 | KJ878896 | KJ878976 | KJ879010 | – | Coleoptera | Japan |
| Ophiocordyceps nigrella | EFCC 9247 | EF468963 | EF468818 | EF468758 | EF468866 | EF468920 | – | Korea |
| Ophiocordyceps nooreniae | BRIP 55363T | NG065096 | NG059720 | KX673812 | – | KX673809 | Chariomyrma cf. hookeri and Polyrhachis lydiae | Australia |
| Ophiocordyceps nooreniae | BRIP 64868 | KX961142 | – | KX961143 | – | – | Polyrhachis cf. hookeri and Polyrhachis lydiae | Australia |
| Ophiocordyceps nutans | OSC 110994 | DQ522549 | DQ518763 | DQ522333 | DQ522378 | – | Hemiptera | – |
| Ophiocordyceps nuozhaduensis | YHH 20168 | ON555849 | ON555927 | ON567769 | ON568683 | – | Camponotus sp. | China |
| Ophiocordyceps nuozhaduensis | YHH 20169 | ON555850 | ON555928 | ON567770 | ON568684 | – | Camponotus sp. | China |
| Ophiocordyceps odonatae | TNSF 18563 | – | KJ878877 | – | KJ878992 | – | Odonata | Japan |
| Ophiocordyceps odonatae | TNS 27117 | – | KJ878878 | – | – | – | Odonata | Japan |
| Ophiocordyceps oecophyllae | OECO1 | KX713635 | – | – | – | – | Oecophyllas maragdina | Australia |
| Ophiocordyceps ootakii | J14 | KX713651 | – | KX713682 | KX713709 | – | Polyrhachis moesta | Japan |
| Ophiocordyceps ootakii | J13 | KX713652 | KX713600 | KX713681 | KX713708 | – | Polyrhachis moesta | Japan |
| Ophiocordyceps ponerinarum | HUA 186140T | KC610789 | KC610767 | KC610740 | KF658668 | – | Paraponera clavata | Brazil |
| Ophiocordyceps pulvinata | TNS-F 30044T | GU904208 | – | GU904209 | GU904210 | – | Camponotus obscuripes | Japan |
| Ophiocordyceps purpureostromata | TNSF 18430 | KJ878931 | KJ878897 | KJ878977 | KJ879011 | – | Coleoptera | Japan |
| Ophiocordyceps polyrhachis-furcata | P39 | KJ201504 | – | JN819003 | – | – | Polyrhachis furcata | Thailand |
| Ophiocordyceps polyrhachis-furcata | P51 | KJ201505 | – | JN819000 | – | – | Polyrhachis furcata | Thailand |
| Ophiocordyceps ravenelii | OSC 151914 | KJ878932 | – | KJ878978 | KJ879012 | KJ878950 | Coleoptera | USA |
| Ophiocordyceps rhizoidea | NHJ 12529 | EF468969 | EF468824 | EF468765 | EF468872 | EF468922 | Coleoptera | – |
| Ophiocordyceps rhizoidea | NHJ 12522 | EF468970 | EF468825 | EF468764 | EF468873 | EF468923 | Coleoptera | – |
| Ophiocordyceps rami | MY6736T | KM655823 | – | KJ201532 | – | – | Camponotus sp. | Thailand |
| Ophiocordyceps rami | MY6738 | KM655824 | – | KJ201534 | – | – | Camponotus sp. | Thailand |
| Ophiocordyceps satoi | J19 | KX713650 | KX713601 | KX713684 | KX713710 | – | Polyrhachis lamellidens | Japan |
| Ophiocordyceps satoi | J7 | KX713653 | KX713599 | KX713683 | KX713711 | – | Polyrhachis lamellidens | Japan |
| Ophiocordyceps septa | Pur1 | – | – | KJ201528 | – | – | Camponotus sp. | Thailand |
| Ophiocordyceps septa | Pur2 | – | – | KJ201529 | – | – | Camponotus sp. | Thailand |
| Ophiocordyceps septa | C41 | – | – | JN819037 | – | – | Camponotus sp. | Thailand |
| Ophiocordyceps sinensis | EFCC 7287 | EF468971 | EF468827 | EF468767 | EF468874 | EF468924 | Lepidoptera | – |
| Ophiocordyceps sobolifera | KEW 78842 | EF468972 | EF468828 | – | EF468875 | EF468925 | Hemiptera | – |
| Ophiocordyceps sphecocephala | OSC 110998 | DQ522551 | DQ518765 | DQ522336 | DQ522381 | DQ522432 | Hymenoptera | – |
| Ophiocordyceps stylophora | OSC 111000 | DQ522552 | DQ518766 | DQ522337 | DQ522382 | DQ522433 | Coleoptera | – |
| Ophiocordyceps stylophora | OSC 110999 | EF468982 | EF468837 | EF468777 | EF468882 | EF468931 | Coleoptera | – |
| Ophiocordyceps subtiliphialida | YFCC 8815T | ON555833 | ON555914 | ON567753 | ON568673 | ON568126 | Camponotus sp. | China |
| Ophiocordyceps subtiliphialida | YFCC 8814 | ON555834 | ON555915 | ON567754 | ON568674 | ON568127 | Camponotus sp. | China |
| Ophiocordyceps subtiliphialida | YFCC 8816 | ON555835 | ON555916 | ON567755 | ON568675 | ON568128 | Camponotus sp. | China |
| Ophiocordyceps subtiliphialida | YFCC 8817 | ON555836 | ON555917 | ON567756 | ON568676 | ON568129 | Camponotus sp. | China |
| Ophiocordyceps tricentri | CEM 160 | AB027330 | AB027376 | – | – | – | Hemiptera | – |
| Ophiocordyceps tianshanensis | MFLU 19-1207T | MN025409 | MN025407 | MK992784 | – | – | Camponotus japonicus | China |
| Ophiocordyceps tianshanensis | MFLU 19-1208 | MN025410 | MN025408 | MK992785 | – | – | Camponotus japonicus | China |
| Ophiocordyceps unilateralis | VIC 44303 | KX713628 | KX713626 | KX713675 | KX713730 | – | Camponotus sericeiventris | Brazil |
| Ophiocordyceps unilateralis | VIC 44354 | KX713627 | – | KX713676 | KX713731 | – | Camponotus sericeiventris | Brazil |
| Ophiocordyceps yakusimensis | HMAS_199604 | KJ878938 | KJ878902 | – | KJ879018 | KJ878953 | Hemiptera | China |
| Paraisaria amazonica | HUA 186113 | KJ917566 | – | – | KP212903 | KM411980 | Orthoptera | Colombia |
| Paraisaria gracilis | EFCC 8572 | EF468956 | EF468811 | EF468751 | EF468859 | EF468912 | Lepidoptera | – |
| Paraisaria gracilis | EFCC 3101 | EF468955 | EF468810 | EF468750 | EF468858 | EF468913 | Lepidoptera | – |
| Paraisaria heteropoda | OSC 106404 | AY489690 | AY489722 | AY489617 | AY489651 | – | Hemiptera | Australia |
| Tolypocladium inflatum | OSC 71235 | EF469124 | EF469077 | EF469061 | EF469090 | EF469108 | Coleoptera | – |
| Tolypocladium ophioglossoides | CBS 100239 | KJ878910 | KJ878874 | KJ878958 | KJ878990 | KJ878944 | Elaphomyces sp. | – |
TType material. New species were shown in bold
Table 3.
Results of the best-fitting likelihood model for maximum likelihood (ML) and Bayesian inference (BI) for the two datasets
| Gene name | ML | BI |
|---|---|---|
| SSU | TNe + I + G4 | K2P + I + G4 |
| LSU | GTR + F + I + G4 | GTR + F + I + G4 |
| TEF | GTR + F + I + G4 | GTR + F + I + G4 |
| RPB1 | GTR + F + I + G4 | GTR + F + I + G4 |
| RPB2 | TIM + F + I + G4 | GTR + F + I + G4 |
| COI | GTR + F + I + G4 | GTR + F + I + G4 |
Phylogenetic analyses of ants
Phylogenetic analyses were based on COI gene sequences. Sequences of COI gene from various species (see Table 2) were retrieved from GenBank and the nucleotide sequences were combined with those generated in our study. Information on specimens and GenBank accession numbers were listed in Table 2. Sequences were aligned using Clustal X (v.2.0) (Larkin et al. 2007), poorly-aligned regions were removed and adjusted manually using MEGA6 (v.6.0) (Tamura et al. 2013). One host dataset (COI) was generated. Modelfinder (Kalyaanamoorthy et al. 2017) was used to select the best-fitting likelihood model for maximum likelihood (ML) analyses, and Bayesian inference (BI) analyses were carried out for the host datasets. The Corrected Akaike Information Criterion (AIC) was used to select the model for each gene, and the best-fitting models were provided in Table 3. The latter method was consistent with the phylogenetic analyses of fungi.
Table 2.
The COI genes and GenBank accession numbers of the taxa were used in this study
| Species name | Voucher information | GenBank number |
|---|---|---|
| Camponotus americanus | YNH-005 | MZ331828 |
| Camponotus americanus | BKH-019 | MW802204 |
| Camponotus badia | TUCIM:6601 | MF993268 |
| Camponotus badia | TUCIM:6461 | MF993266 |
| Camponotus castaneus | BIOUG03675-H07 | KJ208900 |
| Camponotus castaneus | BIOUG03675-H04 | KJ445248 |
| Camponotus claripes | AECT | JN134855 |
| Camponotus cylindricus | – | EF634204 |
| Camponotus explodens | TUCIM:5080 | MF993254 |
| Camponotus novogranadensis | – | MT904506 |
| Camponotus renggeri | Creng_1_B | KP101600 |
| Camponotus rufipes | BIOUG24424-D11 | OM314604 |
| Camponotus saundersi | – | BK012313 |
| Camponotus saundersi | – | MT904541 |
| Camponotus simulans | AFR-CND-2010-47-F02 | JN270684 |
| Camponotus sp. | CASENT0441197-D01 | GU710187 |
| Camponotus sp. | CASENT0043700-D01 | KF200199 |
| Camponotus sp. | CAMPO014 | MH290634 |
| Camponotus sp. | CASENT0000633-D01 | HM373060 |
| Camponotus sp. | YHH 20122 | OP353539 |
| Camponotus sp. | YHH 20605 | OP353540 |
| Camponotus sp. | YHH 20606 | OP353541 |
| Camponotus sp. | YHH 20607 | OP353542 |
| Camponotus sp. | YHH 20608 | OP353543 |
| Camponotus sp. | YHH 20609 | OP353544 |
| Camponotus sp. | YHH 20610 | OP353545 |
| Camponotus sp. | YHH 20611 | OP353546 |
| Camponotus sp. | YHH 20612 | OP353547 |
| Camponotus sp. | YHH 20168 | OP353548 |
| Camponotus sp. | YHH 20191 | OP353549 |
| Camponotus spanis | G191388 | OM420293 |
| Camponotus sericeiventris | BIOUG13980-G06 | OM558348 |
| Camponotus sericeiventris | BIOUG24738-E05 | OM556713 |
| Camponotus sexguttatus | CASENT0612243 | JF863527 |
| Camponotus vitreus | gvc13410-1L | HM914891 |
| Camponotus vitreus | gvc13412-1L | HM914893 |
| Camponotus wiederkehri | AEKB | JN134865 |
| Dolichoderus bispinosus | – | KU187256 |
| Dolichoderus quadridenticulatus | – | KU187255 |
| Dolichoderus bispinosus | MACN-bar-ins-07510 | MN625067 |
| Daceton armigerum | USNM:ENT:01566820 | MW983875 |
| Oecophylla smaragdina | CSM0633 | KM348012 |
| Oecophylla smaragdina | EM898 | MN619431 |
| Polyrhachis anderseni | ANA42 | KM348248 |
| Polyrhachis ammon | RA0751 | KY939110 |
| Polyrhachis aurea | RA0750 | KM348211 |
| Polyrhachis arnoldi isolate | NDA40 | MK591916 |
| Polyrhachis beccari | FMNH-INS_2842133 | KM348266 |
| Polyrhachis carbonaria | FMNH-INS_2842101 | KM348267 |
| Polyrhachis cf. bismarckensis | FMNH-INS_2842022 | KM348331 |
| Paraponera clavata | YB-BCI150685 | MK769309 |
| Polyrhachis cupreata | CSM1015 | KY939064 |
| Polyrhachis cupreata | CSM0682 | KY939056 |
| Polyrhachis flavibasis | RA0766 | KM348203 |
| Polyrhachis flavibasis | RA0763 | KY939081 |
| Polyrhachis furcata | YB-KHC51412 | MN618329 |
| Polyrhachis gagates | FMNH-INS_2842213 | KM348270 |
| Polyrhachis hookeri | RA0747 | KM348215 |
| Polyrhachis illaudata | FMNH-INS_2842112 | KM348275 |
| Polyrhachis illaudata | FMNH-INS_2842222 | KM348271 |
| Polyrhachis jianghuaensis | GXBL0006 | JQ681069 |
| Polyrhachis latharis | FMNH-INS_2842062 | KM348278 |
| Polyrhachis lamellidens | NSMK-IN-170100347 | OL663445 |
| Polyrhachis lucidula | G160084 | OM420302 |
| Polyrhachis mucronata | RA1154 | KM348338 |
| Polyrhachis mucronata | RA1158 | KM348339 |
| Polyrhachis mucronata | RA1164 | KM348340 |
| Polyrhachis mucronata | CSM0696a | KM348337 |
| Polyrhachis nigropilosa | FMNH-INS_2842045 | KM348284 |
| Polyrhachis noesaensis | FMNH-INS_2842106 | KM348285 |
| Polyrhachis obesior | FMNH-INS_2842054 | KM348286 |
| Polyrhachis ornata | CSM0797 | KM348255 |
| Polyrhachis ornata | CSM0842 | KY939061 |
| Polyrhachis proxima | G191229 | OM420306 |
| Polyrhachis proxima | FMNH-INS_2842042 | KM348289 |
| Polyrhachis proxima | FMNH-INS_2842129 | KM348288 |
| Polyrhachis schistacea | FMNH-INS_2842059 | KM348296 |
| Polyrhachis schistacea | FMNH-INS_2842058 | KM348297 |
| Polyrhachis schistacea | FMNH-INS_2842065 | KM348295 |
| Polyrhachis schistacea | FMNH-INS_2842071 | KM348294 |
| Polyrhachis schistacea | FMNH-INS_2842072 | KM348292 |
| Polyrhachis schlueteri | CASENT | KM348298 |
| Polyrhachis sp. | RA0784 | KM348355 |
| Polyrhachis sp. | FMNH-INS_2842139 | KM348305 |
| Polyrhachis sp. | FMNH-INS_2842198 | KM348309 |
| Polyrhachis sp. | FMNH-INS_2842195 | KM348308 |
| Polyrhachis sp. | FMNH-INS_2842179 | KM348300 |
| Polyrhachis sp. | FMNH-INS_2842190 | KM348304 |
| Polyrhachis sp. | FMNH-INS_2842193 | KM348310 |
| Polyrhachis sp. | FMNH-INS_2842194 | KM348307 |
| Polyrhachis sp. | FMNH-INS_2842074 | KM348226 |
| Polyrhachis sp. | RA736b | KM348229 |
| Polyrhachis sp. | YHH 20162 | OP353532 |
| Polyrhachis sp. | YHH 20163 | OP353533 |
| Polyrhachis sp. | YHH 20164 | OP353534 |
| Polyrhachis sp. | YHH 20601 | OP353535 |
| Polyrhachis sp. | YHH 20602 | OP353536 |
| Polyrhachis sp. | YHH 20603 | OP353537 |
| Polyrhachis sp. | YHH 20604 | OP353538 |
| Polyrhachis turneri | CSM0722 | KY939058 |
| Polyrhachis villipes | FMNH-INS_28421186 | KM348316 |
Boldface: data generated in this study
RESULTS
Phylogenetic analysis of the genus Ophiocordyceps
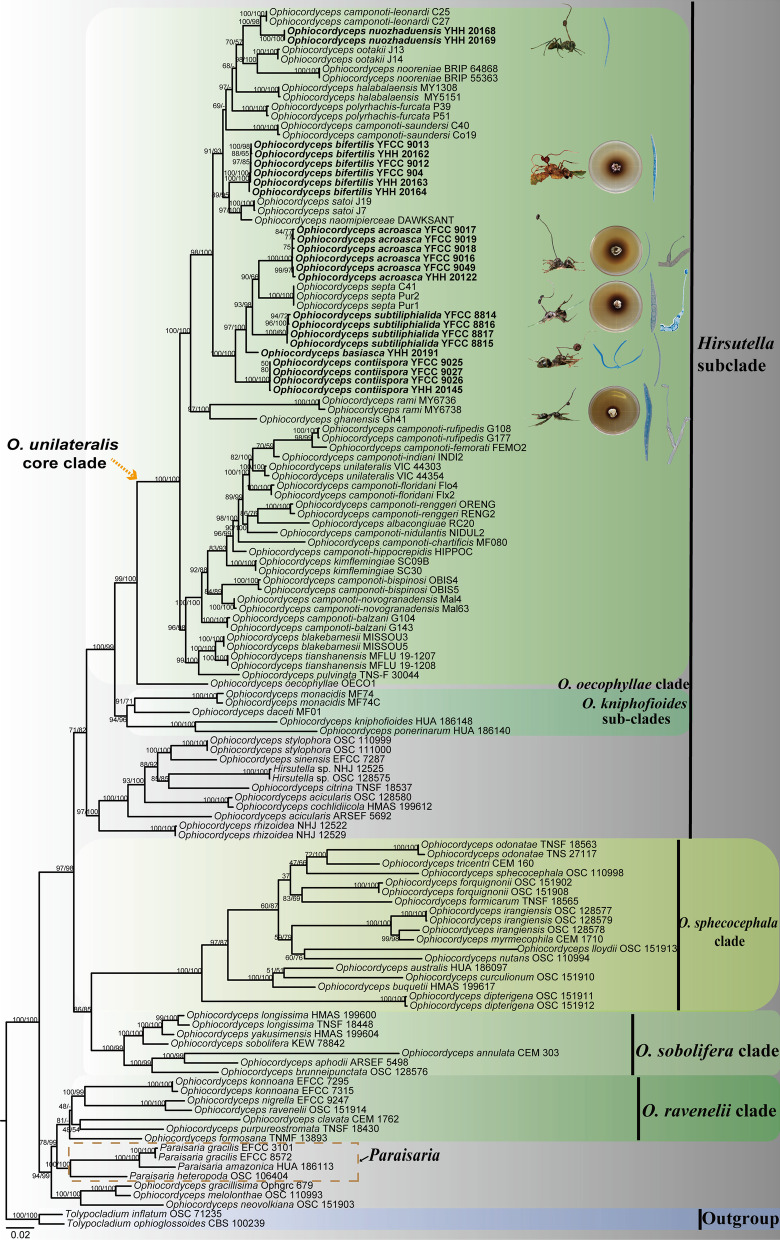
Sequences of 129 samples were used for phylogenetic analysis. Tolypocladium inflatum OSC 71235 and Tolypocladium ophioglossoides CBS 100239 were designated as outgroups. The total length of the concatenated dataset of five genes across the 129 samples was 4785 bp, including 1057 bp for SSU, 952 bp for LSU, 965 bp for TEF, 738 bp for RPB1, and 1073 bp for RPB2. The phylogenetic relationships showed four clades in Ophiocordyceps, including the Hirsutella clade, O. sphecocephala clade, O. sobolifera clade and O. ravenelii clade. Ophiocordyceps unilateralis clade (34 species; BP = 100%, PP = 99%), O. kniphofioides sub-clade (3 species; BP = 94%, PP = 96%) and O. oecophyllae clade (1 species; BP = 99%, PP = 100%) were strongly supported by BI and ML analyses (Fig. 1). All the species collected and described in this work were clustered in the O. unilateralis core clade and clustered into a clade with O. unilateralis sensu lato species reported in Asian African (Ghana, Japan, Thailand) and Oceania (Australia) countries.
Fig. 1.
The phylogenetic tree of Ophiocordyceps and its related genera was inferred from five-gene dataset (SSU, LSU, TEF, RPB1, RPB2) based on Bayesian inference and maximum likelihood analyses. The illustration indicated to characteristics of new species. Tolypocladium inflatum OSC 71235 and Tolypocladium ophioglossoides CBS 100239 were designated as outgroups
Phylogenetic analysis of host ants
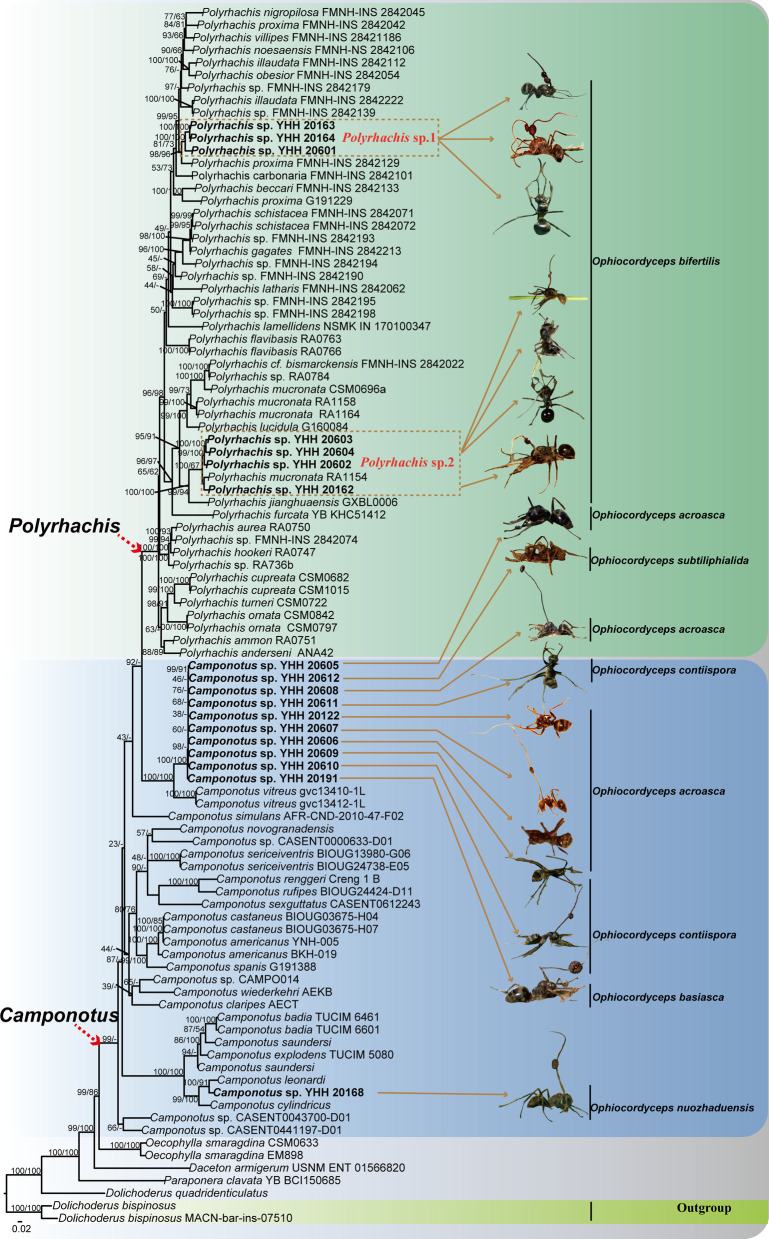
Sequences of 97 specimens were used for phylogenetic analysis. Dolichoderus bispinosus was designated as the outgroup. Phylogenetic relationships have demonstrated that the phylogenetic trees consist of Camponotus, Polyrhachis, Paraponera, Oecophylla and Dolichoderus. Phylogenetic tree showed that O. bifertilis had two ant hosts (Fig. 2), namely, Polyrhachis sp.1 (Polyrhachis sp. YHH 20163, Polyrhachis sp. YHH 20164, Polyrhachis sp. 20601) and Polyrhachis sp.2 (Polyrhachis sp. YHH 20603, Polyrhachis sp. YHH 20604, Polyrhachis sp. YHH 20602, Polyrhachis sp. YHH 20162), with being a higher bootstrap value and posterior probability. Camponotus leonardi was sister to Camponotus sp. based on the host phylogenetic relationships. Their pathogenic fungi, such as O. nuozhaduensis and O. camponoti-leonardi, were also sister species. Notably, the phylogenetic relationships also showed that these ant pathogenic fungi, i.e., O. basiasca, O. contiispora, O. acroasca, O. subtiliphialida, parasitized on the same host Camponotus sp. (Fig. 2).
Fig. 2.
The phylogenetic tree of Polyrhachis and Camponotus including 97 taxa reconstructed using Bayesian inference and maximum likelihood. Each value at a node indicates a Bayesian posterior probability and bootstrap proportions. The Latin name refered to the pathogenic fungus that infected the host ant
TAXONOMY
Ophiocordyceps acroasca Hong Yu bis & D.X. Tang, sp. nov.
Mycobank: MB 844350 (Fig. 3)
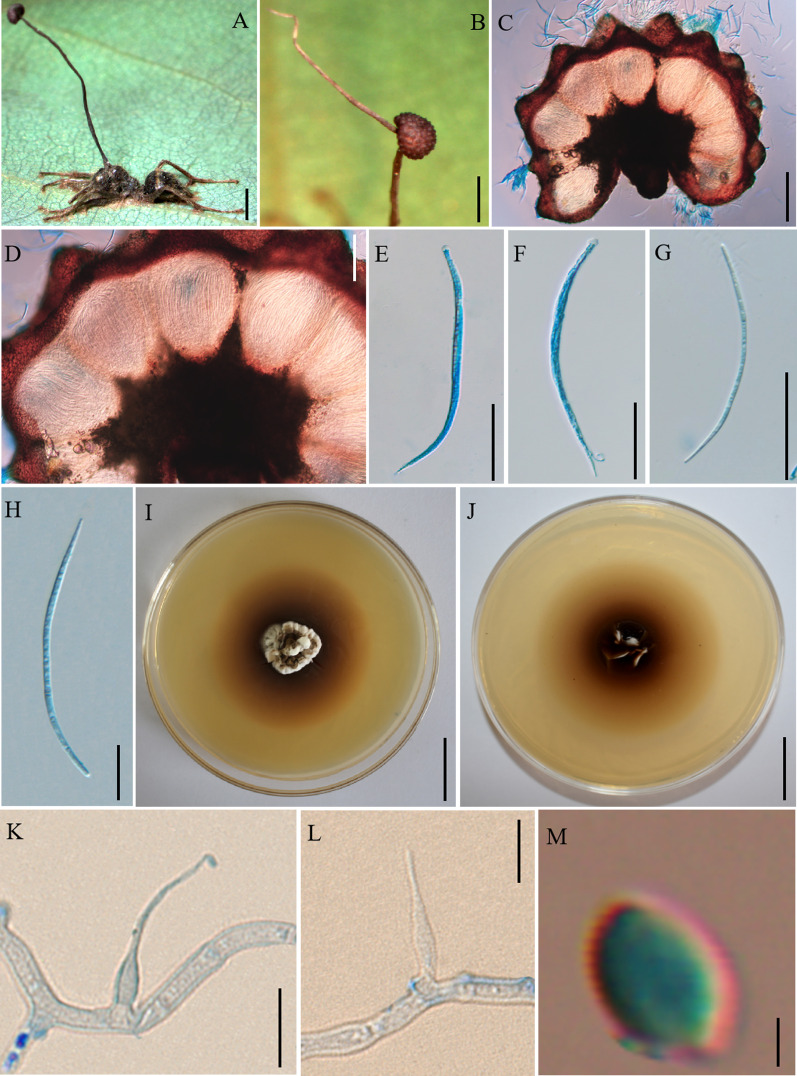
Fig. 3.
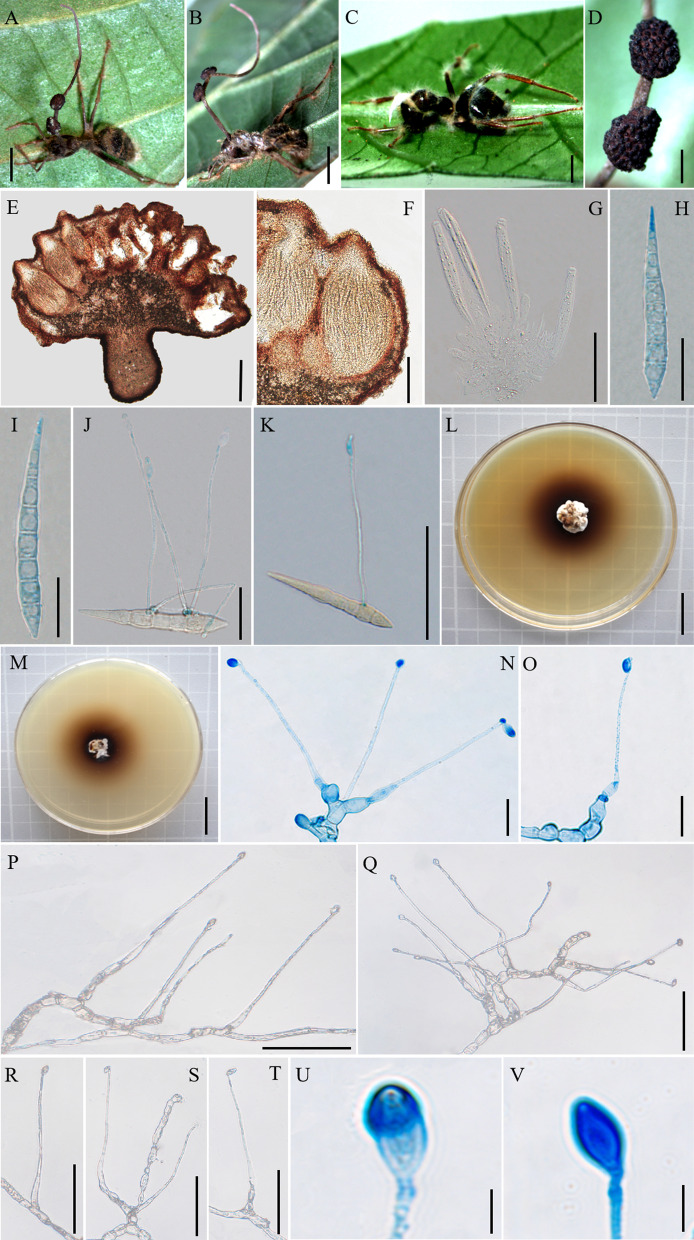
Ophiocordyceps acroasca. A: Infected Camponotus sp. was biting into a leaf of tree seedling. B: The ascoma was produced from the stroma. C, D: Cross-section of the ascoma showing the perithecial arrangement. E, F: Asci. G, H: Ascospores. I, J: Colonies on PDA medium. K, L: Conidiogenous cells and conidia. M: Conidia. Scale bars: A = 3000 µm; B = 2000 µm; C = 200 µm; D = 100 µm; E–G = 50 µm; H = 20 µm; I, J = 2 µm; K, L = 10 µm; M = 2 µm
Etymology: The epithet refered to ascomata of lateral cushions produced from the top of stromata.
Diagnosis: Similar to O. septa in immersed and ostiole perithecia, but O. acroasca differs by ascomata arising from the top of stromata.
Type: China: Yunnan, Puer City, Sun River Natioal Park. Camponotus sp. was infected and bited into a leaf of tree seedling, 22°35′38″ N, 101°6′36″ E, alt. 1452 m, 18 Aug. 2020, Hong Yu bis (YHH 20121 – holotype preserved in the Yunnan Herbal Herbarium; living culture YFCC 9016 – ex-holotype stored in Yunnan Fungal Culture Collection).
Description: Sexual morph: External mycelia produced from the legs and body of the host. Stromata single and curved at the top, produced from dorsal pronotum of the ant, cylindrical, clavate, dark brown at maturity, the top was lighter than other parts of stromata. Fertile regions (ascomata) of lateral cushions produced from the top of stromata, one to two ascomata were found, hemispherical, brown, averaging 3 × 2–3 mm. Perithecia ovoid, immersed to partially erumpent, with short, exposed neck or rounded ostiole, 247–296 × (170–) 176–225 (–238) μm. Asci cylindrical, hyaline, curved, thick, 8-spored, (126–) 131–172 (–180) × 5–8 μm. Ascus caps hemispherical, prominent and small, 3–5 µm high and 4–6 µm wide. Ascospores vermiform, thin-walled, hyaline, 4–5-septate, slightly curved to sinuous, round to slightly tapered at the apex, (76–) 83–108 (–113) × 2–3 µm. Asexual morph: Colonies on PDA slow-growing, 26–27 mm diameter in 60 days at 25 °C, milky white to light brown, hard, with protuberant mycelial at the surface, the pigment produced around colonies, dark brown, reverse light brown to dark brown. Hyphae branched, septate, smooth-walled, hyaline. Hirsutella type-A and Hirsutella type-C produced from colonies, Hirsutella not examined from sutures and joints because the specimens were used to isolated strains. Conidiogenous cells monophialidic, produced from hyphae, smooth, swollen base, cylindrical to lageniform, tapering gradually or abruptly a long neck, slight bending, 17–30 × 1–4 µm. Conidia limoniform, solitary, hyaline, smooth-walled, 2–3 × 1–2 µm.
Germination process: No germination observed because the specimens were dried.
Host: Camponotus sp. (Formicinae)
Habitat: Subtropical monsoon evergreen broad-leaf forest. Infected Camponotus sp. was found biting into a leaf of tree seedling; from 0.5 to 2 m above the ground.
Distribution: China, Yunnan Province, Puer City
Material examined: China: Yunnan, Puer City, Sun River National Park. Infected ants were found biting into a leaf of tree seedling, 22°38′2″ N, 101°6′7″ E, alt. 1468 m, 19 Aug. 2020, Hong Yu bis (living culture YFCC 9017, YFCC 9018, YFCC 9019, YFCC 9049) and 22°34′34″ N, 101°6′24″ E, alt. 1095 m, 23 Aug. 2021, D.X. Tang (YHH 20122).
Notes: Phylogenetic analyses showed that O. acroasca formed a sister lineage with O. septa, and was clustered in the O. unilateralis core clade of Hirsutella, with strong statistical supported by bootstrap proportions (BP = 90%) (Fig. 1). Ophiocordyceps acroasca was similar to O. septa in the behavior of the host biting a leaf, cylindrical or clavate stromata, immersed and ostiole perithecia. However, it differed from O. septa by ascomata of lateral cushion arising from the top of stromata, vermiform ascospores, producing Hirsutella type-A and Hirsutella type-C, cylindrical to lageniform conidiogenous cells, limoniform conidia. In addition, the sizes of perithecia, ascomata, asci, ascospores, phialides, and conidia also differed from O. septa (Table 4).
Table 4.
Comparison of morphological characters and host of Ophiocordyceps unilateralis sensu lato in this study
| Species | Host | Death position | Stromata | Ascomata | Perithecia (μm) | Asci (μm) | Prominent caps | Ascospores (μm) | Septa | Hirsutella asexual morph (μm) | Conidia (μm) | Country | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ophiocordyceps acroasca | Camponotus sp. | Biting leaf | Single | Hemispherical, 3 × 2–3 mm | Ovoid, 247–296 × 176–225 | Cylindrical, 8-spored, 131–172 × 5–8 | Prominent, 3–5 × 4–6 | Vermiform, 83–108 × 2–3 | 4–5 | Hirsutella A-type and Hirsutella C-type, 17–30 × 1–4 | Limoniform, 2–3 × 1–2 | China | This study |
| Ophiocordyceps albacongiuae | Camponotus sp. | Biting epiphites | One or two | Disc-shaped | Flask-shaped, 240–290 × 105–135 | Cylindrical to clavate, 8-spored, 130–160 × 8–11 | Hemispherical, 3–5 × 4–5 | Cylindrical, 80–100 × 5 | 5–6 | – | – | Colombia | Araújo et al. (2018) |
| Ophiocordyceps basiasca | Camponotus sp. | Biting leaf | Single | Spherical, 3 × 2 mm | Flask-shaped or ovoid, 202–242 × 102–149 | Cylindrical, 8-spored, 96–188 × 4–9 | Hemispherical, 3–5 × 4–5 | Vermiform, 89–119 × 2–3 | 4–5 | Hirsutella A-type, 10–23 × 1–5 | Oviform, 1–4 × 1–2 | China | This study |
| Ophiocordyceps bifertilis | Polyrhachis sp.1 and Polyrhachis sp.2 | Biting leaf | Multiple | Disc-shaped or hemispherical, 3 × 2–3 mm | Flask-shaped, 156–211 × 102–129 | Cylindrical, 8-spored, 130–198 × 6–10 | Prominent, 3–5 × 5–6 | Fusiform, 70–94 × 2–4 | 4–5 | Hirsutella A-type, 9–24 × 2–4 | – | China | This study |
| Ophiocordyceps blakebarnesii | Camponotus sp. | Biting inside log | Single | Discshaped to irregular, 1.5 × 1 mm | Flask-shaped, 300–320 × 105–120 | Cylindrical to clavate, 8-spored, 220–250 × 12–14 | – | Cylindrical, 140–160 × 4 | 6–7 | Hirsutella A-type, 75 × 3–4 | Limoniform, 8–9 × 3 | USA | Araújo et al. (2018) |
| Ophiocordyceps contiispora | Camponotus sp. | Biting leaf | Single | Disc-shaped, 0.7–1 mm | Flask-shaped, 158–212 × 69–122 | Cylindrical, 8-spored, 89–130 × 4–9 | Hemispherical or square, 1–3 × 3–5 | Fusiform, 38–48 × 2–4 | No obvious separation | Hirsutella C-type, 57–92 × 1–4 | Olivary or flask-shaped, 4–6 × 1–2 | China | This study |
| Ophiocordyceps camponoti-leonardi | Camponotus leonardi | Biting leaf | Single | – | Fusoid-ellipsoid, 400–430 × 200–230 | Cylindrical, 8-spored, 130–175 × 7–8 | – | Lanceolate, 110–125 × 2–3 | Multiseptate | Hirsutella, 22.5 × 2.0–3.5 | Fusoid 2–4 × 1–2 | Thailand | Kobmoo et al. (2012) |
| Ophiocordyceps camponoti-saundersi | Camponotus saundersi | Biting leaf | Single | – | Fusoid-ellipsoid, 280–320 × 160–180 | Cylindrical, 8-spored, 80–160 × 6–7 | – | Lanceolate, 75–85 × 2–3 | Multiseptate | Hirsutella, 25 × 2–3 | Fusoid, 2–3 × 1–2 | Thailand | Kobmoo et al. (2012) |
| Ophiocordyceps halabalaensis | Camponotus gigas | Biting leaf | Three | – | Fusoid-ellipsoid, 350–420 × 180–210 | Cylindrical, 8-spored, 150–200 × 7–10 | – | Cylindrical, 60–75 × 3–5 | Multiseptate | – | – | Thailand | Luangsa-ard et al. (2011) |
| Ophiocordyceps nooreniae | Polyrhachis cf. hookeri | Biting leaf | – | – | – | – | – | – | Hirsutella A-type, 30–55; Hirsutella C-type, 35–50 × 1.5–8 | Ovoid, 5–6 × 2–3 | Australia | Crous et al. (2016) | |
| Ophiocordyceps nuozhaduensis | Camponotus sp. | Biting leaf | Single | Spherical, 2.4 × 1.6 mm | Flask-shaped, 222–274 × 153–159 | – | Vermiform, 91–126 × 2–5 | 7–13 | Hirsutella A-type, 6–22 × 2–4 | Ellipsoidal or oviform, 2–5 × 2–3 | China | This study | |
| Ophiocordyceps naomipierceae | Polyrhachis cf. robsonii | Biting leaf | – | Hemispherical to irregular, 0.75 × 0.5–0.65 mm | Flask-shaped, 260–320 × 150–200 | Vermiform, cylindrical, 8-spored, 150–180 × 7 | Prominent | Vermiform, 75–105 × 5–6 | 4–6 | Paraisaria-like, 15–35 × 3 |
Conidium, 5–7 × 3 |
Australia | Araújo et al. (2018) |
| Ophiocordyceps ootakii | Polyrhachis sp. | Biting leaf | Single or branched | Fisc-shaped, 1.1 × 0.8 mm | Flask-shaped, 230–260 × 120–150 | Cylindrical to clavate, 8-spored, 130–180 × 8–9 | Prominent | Vermiform, 85–100 × 3 | 5 | Hirsutella A-type, 6–8 × 3–4 | 5 × 3 | Japan | Araújo et al. (2018) |
| Ophiocordyceps polyrhachis-furca | Polyrhachis furca | Biting leaf | Single | – | Fusoid-ellipsoid, 380–400 × 160–180 | Cylindrical, 8-spored, 140–190 × 7–8 µm | – | Lanceolate, 90–100 × 2–3 | Multi-septate | Hirsutella, 30 × 2–3 | Fusoid, 3–5 × 2–3 | Thailand | Kobmoo et al. (2012) |
| Ophiocordyceps rami | Camponotus sp. | Biting leaf | Single | Hemispherical, 2 mm | Fusoid-ellipsoid, 325–500 × 275–300 | Cylindrical, 8-spored, 200–340 × 7–10 | – | Filiform, 200–215 × 2–3 | 7–8 | Hisutella A-type, 9–10 × 3–4; Hisutella C-type, 30 × 3–5 | Cylindrical to narrow fusiform, 3.5–6.5 × 1–2; fusiform to narrowly lemoniform, 9 × 5 | Thailand | Kobmoo et al. (2015) |
| Ophiocordyceps satoi | Polyrhachis lamellidens | Biting twing | Three | 1 × 0.8 mm | Flask-shaped, 230–270 × 120–160 | Cylindrical to clavate, 8-spored, 120–160 × 8–10 | Prominent | Cylindrical, 85–100 × 4 | 5 | Hirsutella A-type, 12 × 7 | – | Japan | Araújo et al. (2018) |
| Ophiocordyceps septa | Camponotus sp. | Biting leaf | Single | Hemispherical, 2 mm | Fusoid-ellipsoid, 280–300 × 100–150 | Cylindrical, 8-spored, 125–165 × 12.5–15 | – | Lanceolate, 45–50 × 6–8 | 7–8 | Hisutella A-type, 25 × 2–3; Hisutella C-type, 50 × 5.5 | Fusiform, 5–6 × 1–2; fusiform to narrowly lemoniform, 9 × 5 | Thailand | Kobmoo et al. (2015) |
| Ophiocordyceps subtiliphialida | Camponotus sp. | Biting leaf | Single | Disc-shaped, 2 × 1.2–1.9 mm | Flask-shaped, 195–296 × 87–161 | Cylindrical, 8-spored, 89–119 × 5–9 | Hemispherical, 2–4 × 5–7 | Lanceolate, 52–72 × 5–8 | 6–7 | Hirsutella C-type, 70–116 × 1–3 | Olivary, 6–10 × 3–6 | China | This study |
| Ophiocordyceps camponoti-atricipis | Camponotus atriceps | Biting leaf | Single | Hemispherical, 1.5 × 0.5–0.8 mm | Flask-shaped, 240–280 × 100–150 | Cylindrical to clavate, 8-spored, hyaline, 110–140 × 6–6.5 | 5 × 5.5 | Vermiform 80–85 × 3 | 5 | Hirsutella A-type, 5–7 × 2–3 | – | Brazil | Araújo et al. (2015) |
| Ophiocordyceps camponoti-balzani | Camponotus balzani | Biting leaf | Single | 1.5 × 1.0 mm | Flask-shaped, 400–450 × 100–150 | Cylindrical, 8-spored, 200–240 × 12–16 | Prominent, 8–10 × 6–8 | Cylindrical, 135–175 × 4.0–5.0 | 14–22 | Hirsutella A-type, Hirsutella C-type 20–25 × 3–4 | Cylindric to fusiform, 12–14 × 2–3 | Brazil | Evans et al. (2011b) |
| Ophiocordyceps camponoti-bispinosi | Camponotus bispinosus | Biting spines | Single | 0.8 × 0.4–0.7 mm | Globose to flask-shaped, 250–290 × 150–170 | Cylindrical to clavate, 8-spored, hyaline, 110–130 × 8–8.5 | 3.5 × 4.5 | Cylindrical, 70–75 × 4.5–5 | 4–5 | Hirsutella A-type, 6 × 2.5–3 | Narrow limoniform, 6–7 × 2 | Brazil | Araújo et al. (2015) |
| Ophiocordyceps camponoti-chartificis | Camponotus chartifex | Biting leaf | Single | Hemispherical, 1.5 × 1 mm | Globose to hemispherical shaped, 200–235 × 135–175 | Cylindrical to clavate, 8-spored, 100–125 × 6 | 6–7 × 3–4 | Vermiform 75–85 × 5 | 9–13 | Hirsutella A-type, 5–6 × 3 | Fusiform to limoniform, 7 × 2.6 | Brazil | Araújo et al. (2018) |
| Ophiocordyceps camponoti-femorati | Camponotus femoratus | Biting leaf/spines | Single | Disc-shaped to hemispherical, 1.2–2.2 × 0.8–1.4 mm | Flask-shaped, 200–230 × 135–165 | Cylindrical to clavate, 8-spored, 110–130 × 8–9 | 6 × 3 | 75–90 × 3 | 5 | Hirsutella A-type, 7–10 × 3–4 | Limoniform, 7–9 × 3 | Brazil | Araújo et al. (2018) |
| Ophiocordyceps camponoti-floridani | Camponotus floridanus | Biting leaf | Single | Disc-shaped | Flask-shaped, 265 × 100 | Cylindrical to clavate, 8-spored, 145 × 9–10 | – | Cylindrical, 75–90 × 4–5 | 5 | Hirsutella A-type, 8–9 × 3–4 | Limoniform, 8–9 × 3 | USA | Araújo et al. (2018) |
| Ophiocordyceps camponoti-hippocrepidis | Camponotus hippocrepis | Biting spines | Single | 2–2.5 × 0.25–0.45 mm | Flask-shaped, 225–250 × 135–165 | Cylindrical to clavate, 8-spored, 115–135 × 7–10 | Prominent, 6–7 × 4 | Cylindrical, 75–85 × 4–5 | 5 | Hirsutella A-type, 8–9 × 4 | Limoniform, 5 × 2 | Brazil | Araújo et al. (2018) |
| Ophiocordyceps camponoti-indiani | Camponotus indianus | Biting leaf | Multiple | Hemispherical | Ovoid to flask-shaped, 230–310 × 120–175 | Cylindrical, 8-spored, 170 × 8.5 | Prominent, 4.5 × 5 | Cylindrical, 75 × 4.5 | 5 | Hirsutella A-type, 7.5 × 3.5; Hirsutella C-type | – | Brazil | Araújo et al. (2015) |
| Ophiocordyceps camponoti-melanotici | Camponotus melanoticus | Biting leaf | Single | 1.3 × 0.8 mm | Flask-shaped, 400–450 × 100–150 | 8-spored, 200–275 × 12–16 | 8–10 × 6–8 | Cylindrical, 170–210 × 4–5 | 27–35 | Hirsutella A-type | – | Brazil | Evans et al. (2011b) |
| Ophiocordyceps camponoti-nidulantis | Camponotus niduland | Biting saplings | Single | Disc-shaped to hemispherical, 1.5 × 1 mm | Flask-shaped, 200–240 × 100–150 | Vermiform to clavate, 8-spored, 110–145 × 6–8 | 4 × 6 | Vermiform, 90–105 × 3–4 | 5 | Hirsutella A-type; Hirsutella C-type, 70–120 × 4–6 | Limoniform, 8 × 3 | Brazil | Araújo et al. (2015) |
| Ophiocordyceps camponoti-novogranadensis | Camponotus novogranadensis | Biting epiphites | Single | 0.8–1.0 × 0.5–0.6 µm | 225–250 × 125–155 | Cylindrical, 8-spored, 95–120 × 9–10 | Prominent, 5–6 × 3–4 | Filiform, 75–95 × 2.5–3.5 | 5–10 | Hirsutella A-type; Hirsutella B-type, 80–100 × 35–40 | Narrowly clavate to obclavate, 10–12 × 1.5–2.0 | Brazil | Evans et al. (2011b) |
| Ophiocordyceps camponoti-renggeri | Camponotus renggeri | Biting leaf/moss | Single | Hemispherical to globose, 1–1.5 × 0.8–1 mm | Flask-shaped, 220–250 × 100–165 | Cylindrical, 8-spored, 130–145 × 8–10 | Prominent, 7–8 × 3 | Vermiform 90–120 × 4 | 5–8 | Hirsutella C-type, 40–60 × 3–5 | – | Brazil | Araújo et al. (2018) |
| Ophiocordyceps camponoti-rufipedis | Camponotus rufipes | Biting leaf | Single | Discshaped to hemisphaerical, 1 × 0.5 mm | Flask-shaped, 175–260 × 100–130 | Cylindrical to clavate, 8-spored, 120–160 × 8–10 | Prominent, 4.0–5.5 × 3.0–4.5 | Vermiform, 80–95 × 2–3 | 4–7 | Hirsutella A-type, 10 × 2 | Fusiform to narrowly limoniform, 5 × 1.5 | Brazil | Evans et al. (2011b) |
| Ophiocordyceps camponoti-sexguttati | Camponotus sexguttatus | Biting leaf | Single | Disc-shaped, 1 × 1 mm | Flask-shaped, 225–230 × 135 | Cylindrical, 8-spored, 150–160 × 8–9 | Prominent, 6 × 3 | Cylindrical, 120–140 × 3 | 7 | Hirsutella A-type, 5–8 × 3–4 | Limoniform, 5 × 2 | Brazil | Araújo et al. (2018) |
| Ophiocordyceps kimflemingiae | Camponotus castaneus | Biting twig | Single | Disc-shaped, 1.5–2 × 1.3 mm | Flask-shaped, 250–275 × 120–160 | Cylindrical to clavate, 8-spored, 120–150 × 10–11 | Prominent | Cylindrical, 80–90 × 5 | 5–6 | Hirsutella A-type; Hirsutella C-type | – | USA | Araújo et al. (2018) |
| Ophiocordyceps oecophyllae | Oecophylla smaragdina | Biting leaf | – | – | – | – | – | – | – | 30–50 × 3–4 | Ovoid to cylindrical, 5.5–10 × 1.5–3 | Australia | Araújo et al. (2018) |
| Ophiocordyceps monacidis | Dolichoderus bispinosus | Base of trunk | Single | – | – | – | – | – | – | – | – | Brazil | Araújo et al. (2018) |
| Ophiocordyceps daceti | Daceton armigerum | Leaf (not biting) | Single | – | – | – | – | – | – | Hirsutella, 16–18 × 4 | Cylindrical, 7–10 × 3 | Brazil | Araújo et al. (2018) |
| Ophiocordyceps kniphofioides | Cephalotes atratus | Base of trunk | Single | 5–6 × 0.7–1 mm | Ovoid to lageniformia, 170–250 × 110–140 | Narrow cylindrical, 140–200 × 6–12 | – | Filiform, 110–150 × 1.5–3 | 3–5 | Hirsutella A-type, 10–16 × 0.6–4; Hirsutella B-type | Narrowly clavate, 7–9 × 1.5–2.5; ovoid to cylindrical, 8–12 × 4–5 | Brazil | Evans and Samson (1982) |
| Ophiocordyceps ponerinarum | Paraponera clavata | Base of trunk | – | 8–14 × 0.8–1 mm | Flask-shaped, 210–320 × 140–190 | – | – | – | – | Hirsutella A-type, 10–14 × 1.8–2.5 | Clavate, 7–9 × 1.8–3 | Brazil | Evans and Samson (1982) |
| Ophiocordyceps pulvinata | Camponotus obscuripes | Clinging to twigs | Single | – | 400–600 × 150–250 | Clavate, 8-spored, 220–300 × 9–19 | 4–5.4 × 6–9 | Filiform, 160–220 × 3–5 | – | – | – | Japan | Kepler et al. (2011) |
| Ophiocordyceps tianshanensis | Camponotus japonicus | The bark of a dilapidated (not biting) | – | Disc-shaped, 1.1–1.6 × 0.5–1.1 mm | Flask-shaped, 220–260 × 100–140 | – | – | – | – | Hirsutella A-type, 8–9 × 2.5–3.5 | Fusiform to obpyriform, 6–9.2 × 2.2–3 | China | Wei et al. (2020) |
| Ophiocordyceps unilateralis | Camponotus sericeiventris | Biting leaf | Single | – | Flask-shaped, 200–250 × 140–160 | Cylindrical, 8-spored, 95–125 × 6–8 | 5–6 × 4–5 | Filiform, 75–85 × 2–2.5 | 4–5 | Hirsutella A-type, 10–12 × 3–3.5; Hirsutella B-type, 14–16 × 2.5–3 | Limoniform, 6.5–8 × 2–2.5; cylindrical-fusoid, 8–11 × 2.5–3 | Brazil | Evans et al. (2018) |
New species are shown in bold
Ophiocordyceps bifertilis Hong Yu bis & D.X. Tang, sp. nov.
Mycobank: MB 844351 (Fig. 4)
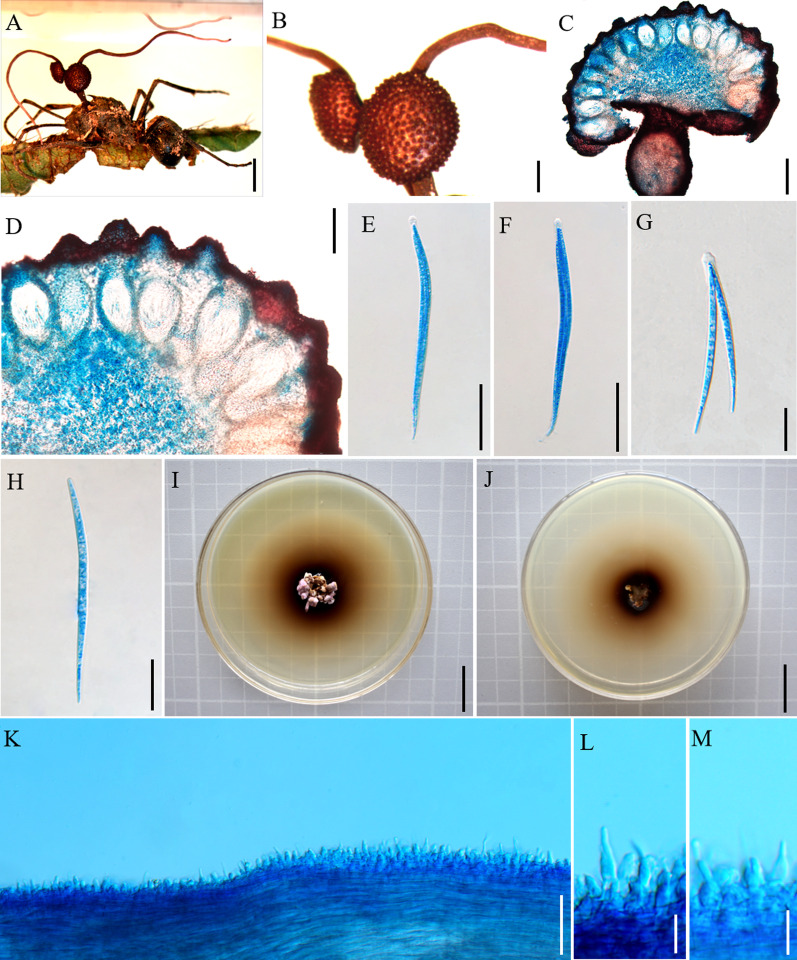
Fig. 4.
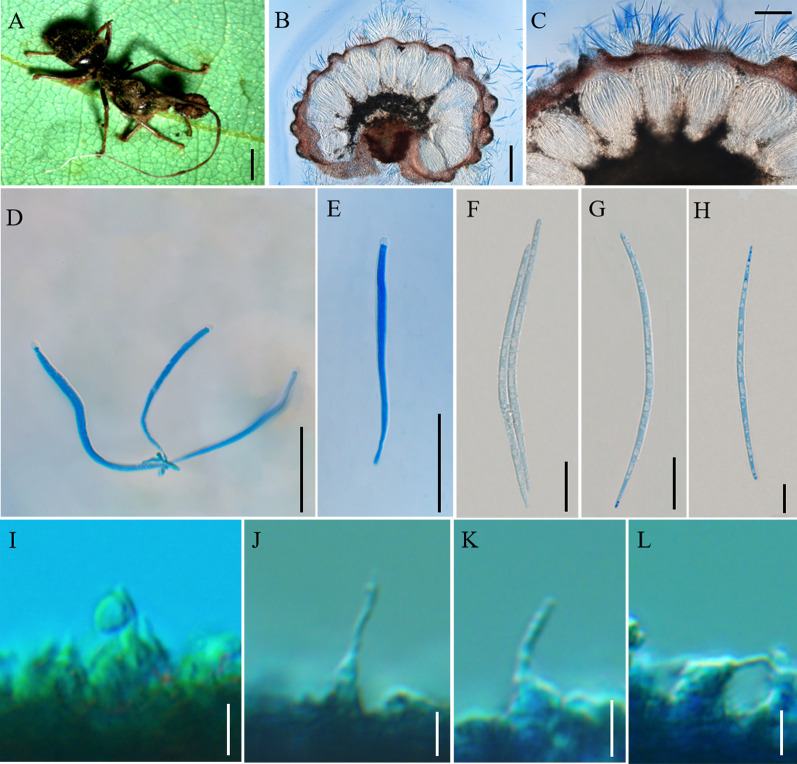
Ophiocordyceps bifertilis. A: Infected Polyrhachis sp.1was biting a leaf of Pteridophyta. B: Two ascomata plates attached to stromata. C, D: Cross-section of the ascoma showing the perithecial arrangement. E, F: Asci. G, H: Ascospores. I, J: Colonies on PDA medium. K, L: Phialides. Scale bars: A = 4000 µm; B = 1000 µm; C = 200 µm; D = 100 µm; E, F = 50 µm; G, H = 20 µm; I, J = 2 cm; K–M = 10 µm
Etymology: The epithet refered to two fertile regions produced from stromata.
Diagnosis: Ophiocordyceps bifertilis similar to O. satoi regarding the production of multiple stalks, but O. bifertilis differed by stromata branching, with only two ascomata.
Type: China: Yunnan, Puer City, Sun River National Park. An adult Polyrhachis sp. was hanging upside down on the underside of the leaves, 2°20′24″ N, 101°6′43″ E, alt. 1487 m, 18 August 2020, Hong Yu bis (YHH 20160 – holotype preserved in the Yunnan Herbal Herbarium; living culture YFCC 9012 – ex-holotype stored in Yunnan Fungal Culture Collection).
Description: Sexual morph: External mycelia scarce, produced from sutures and joints. One to multiple stromata at the head of the ant, few branching, curved, cylindrical, clavate, dark brown. Ascomata of lateral cushions produced from stromata, two ascomata were observed, disc-shaped or hemispherical, brown, averaging 3 × 2–3 mm. Perithecia flask-shaped, immersed to partially erumpent, with short, exposed neck or rounded ostiole, (149–) 156–211 (–236) × (91–) 102–129 (–134) μm. Asci cylindrical, hyaline, 8-spored, (123–) 130–198 (–211) × 6–10 μm. Ascus caps were hemispherical, prominent, 3–5 µm high, and 5–6 µm wide. Ascospores fusiform, hyaline, 4–5-septate, round to tapered at the apex, 70–94 (–96) × 2–4 µm. Asexual morph: Colonies on PDA grows slowly, 19–20 mm diameter in 120 days at 25 °C, light purple to light brown, hard, with protuberant mycelia at the edge, reverse light brown to dark brown, pigment light brown to dark brown. Hirsutella type-A was present along stromata; Hirsutella was not observed from the sutures and joints. Phialides lageniform, smooth, swollen base, tapering abruptly a neck, short, 9–24 (–29) × 2–4 µm. Conidia were not observed.
Germination process: No germination observed because the specimens were dried.
Host: Polyrhachis sp.1 and Polyrhachis sp.2 (Formicinae)
Habitat: Subtropical monsoon evergreen broad-leaf forest. Infected Polyrhachis sp.1 was found biting into a leaf of Pteridophyta, and Polyrhachis sp.2 biting into a leaf of Gramineae, always at lower heights, ranging from 0.5 to 1.5 m.
Distribution: China, Yunnan Province, Puer City
Material examined: China: Yunnan, Puer City, Sun River National Park. Adult Polyrhachis sp.1 and Polyrhachis sp.2 were hanging upside down on the underside of the leaves of Pteridophyta and Gramineae, 22°35′50″ N, 101°6′39″ E, alt. 1529 m, 19 Aug. 2020, Hong Yu bis (living culture YFCC 9013, YFCC 9048) and 22°35′51″ N, 101°6′40″ E, alt. 1532 m, 23 Aug. 2021, D.X. Tang (YHH 20162, YHH 20163, YHH 20164).
Notes: Phylogenetic analyses revealed that O. bifertilis formed a sister lineage with O. satoi and O. naomipierceae, was clustered in the O. unilateralis core clade of Hirsutella, with statistical support from BI posterior probabilities (PP = 95%) and ML bootstrap proportions (BP = 89%) (Fig. 1). Ophiocordyceps bifertilis was similar to O. satoi and O. naomipierceae in the behavior of the host Polyrhachis infected and biting a leaf. In addition, it was also similar to O. satoi in clavate stromata, flask-shaped perithecia, Hirsutella type-A, lageniform phialides. However, it differed from O. satoi by branching stromata, fusiform ascospores. Moreover, the sizes of phialides also differed from O. satoi and O. naomipierceae (Table 4).
Ophiocordyceps subtiliphialida Hong Yu bis & D.X. Tang, sp. nov.
Mycobank: MB 844352 (Fig. 5)
Fig. 5.
Ophiocordyceps subtiliphialida. A, C: Camponotus sp. was infected and bited into a leaf of a sapling. D: Fertile structure produced from the stroma; E–F: Cross-section of the ascoma showing the perithecial arrangement; G: Asci; H, I: Ascospores. J, K: Ascospore with long capilliconidia. L, M: Colonies on PDA medium. N–V: Conidiogenous cells and conidia. Scale bars: A, B = 0.4 cm; C = 0.2 cm; D = 0.1 cm; E = 200 µm; F = 100 µm; G = 50 µm; H–J = 20 µm; K = 50 µm; L, M = 2 cm; N, O = 20 µm; P–T = 50 µm; U, V = 5 µm
Etymology: The epithet refered to the phialides slender than related species.
Diagnosis: Similar to O. contiispora in phialides monophialidic or rarely polyphialidic, but phialides of O. subtiliphialida (70–116 × 1–3 µm) was slender than O. contiispora (57–92 × 1–4 µm).
Type: China: Yunnan, Puer City, Sun River National Park. Camponotus sp. was infected and bited into a leaf of tree seedling, 22°34′34″ N, 101°6′24″ E, alt. 1420 m, 18 Aug. 2020, Hong Yu bis (YHH 20139 – holotype preserved in the Yunnan Herbal Herbarium; living culture YFCC 8815 – ex-holotype stored in Yunnan Fungal Culture Collection).
Description: Sexual morph: External mycelia produced from the sutures and joints of the ant. Stromata single, produced from dorsal pronotum of the ant, cylindrical, clavate, brown at maturity. Fertile part of lateral cushions produced from stromata, 1–2, disc-shaped, brown, averaging 2 × 1.2–1.9 mm. Perithecia flask-shaped, immersed to partially erumpent, with short, exposed ostiole, (195–) 199–296 (–303) × (87–) 97–161 (–168) μm. Asci cylindrical, hyaline, short and wide, 8-spored, 89–119 × 5–9 μm. Ascus caps hemispherical, 2–4 µm high, 5–7 µm wide. Ascospores lanceolate, hyaline, 6–7-septate, slightly curved, round to tapered at the apex, 52–72 × 5–7 (–8) µm. Asexual morph: Colonies grows slowly on PDA medium, 19–20 mm diameter in 60 days at 25 °C, milky white to light brown, raising cottony-shaped mycelia density at the edge, protuberant mycelia light brow at the centrum, reverse light brown to dark brown. Hyphae immersed in the medium, milky white, branched, septate, smooth-walled, hyaline. Hirsutella type-C only. Conidiophores rare, cylindrical, produced from the hyphae, septate, short and wide. Phialides monophialidic or rarely polyphialidic, forming on side hyphae or the conidiophores, smooth, slight swollen base, lageniform, septate, tapering gradually a slender neck, slight bending, 70–116 (–124) × 1–3 µm. Conidia olivary, solitary, hyaline, smooth-walled, 6–10 × 3–6 µm.
Germination process: Ascospores germinating in 72 h to produce 1–4, long and narrow capilliconidiophore, (44–) 58–79 μm long, 0.8–1.9 μm wide, bearing a single capilliconidium, averaging (6–) 7–9 × 2–3 μm.
Host: Camponotus sp. (Formicinae).
Habitat: Subtropical monsoon evergreen broad-leaf forest. Infected Camponotus sp. was found biting into a leaf of a sapling. Died in the lower position, collected from 0.5 to 1 m.
Distribution: China, Yunnan Province, Puer City.
Material examined: China: Yunnan, Puer City, Sun River National Park. Infected Camponotus sp. was found biting into a leaf of a sapling, 22°35′51″ N, 101°6′40″ E, alt. 1430 m, 19 Aug. 2020, Hong Yu bis (living culture YFCC 8814, YFCC 8816, YFCC 8817).
Notes: Phylogenetic analyses showed that the four samples of the O. subtiliphialida group together with high statistical support (PP = 60%; BP = 100%), were clustered within the O. unilateralis core clade of Southeast Asian countries (Fig. 1). It was similar to O. septa, O. acroasca and O. basiasca in swollen and lageniform base. However, it differed from O. septa, O. acroasca and O. basiasca by lanceolate ascospores, rare conidiophores, monophialidic or rarely polyphialidic phialides, tapering a narrow and slender neck, olivary conidia.
Ophiocordyceps basiasca Hong Yu bis & D.X. Tang, sp. nov.
Mycobank: MB 844353 (Fig. 6)
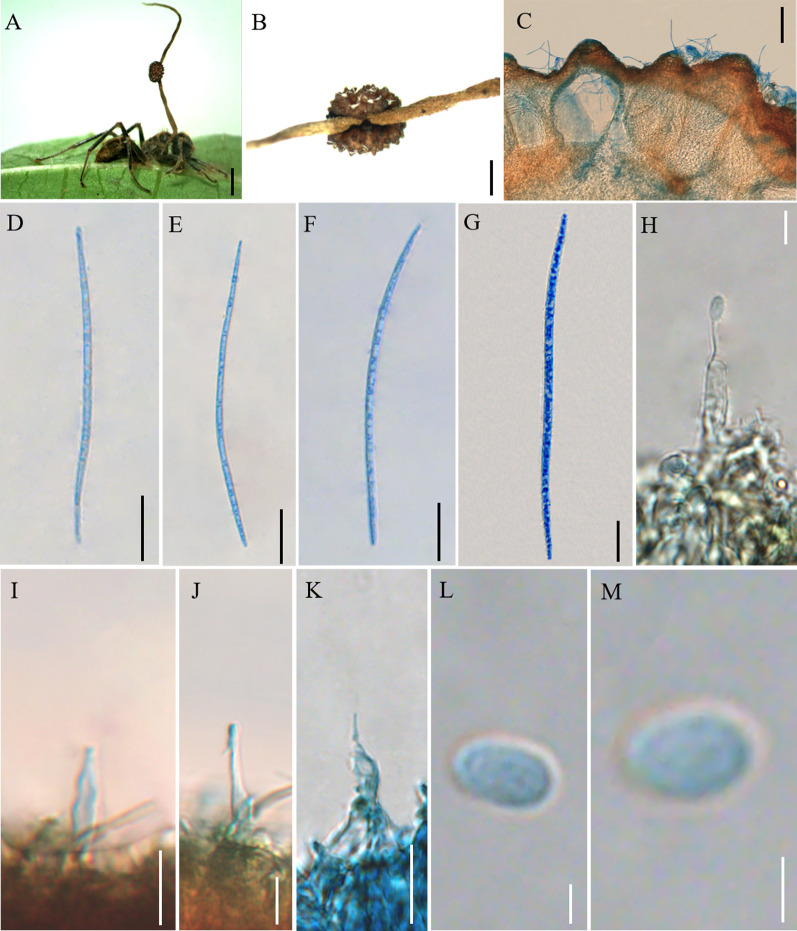
Fig. 6.
Ophiocordyceps basiasca. A: Infected Camponotus sp. was biting into a leaf of tree sapling. B, C: Cross-section of the ascoma showing the perithecial arrangement. D, E: Asci. F–H: Ascospores. I–L: Phialides and conidia. Scale bars: A = 2000 µm; B = 200 µm; C = 100 µm; D, E = 50 µm; F–H = 20 µm; I–L = 5 µm
Etymology: The epithet refered to ascomata of lateral cushions produced from the basal of stromata.
Diagnosis: Similar to O. contiispora in conidia olivary, however, ascospores vermiform of O. basiasca was differed to O. contiispora (fusiform).
Type: China: Yunnan, Puer City, Sun River National Park. Camponotus sp. was infected and bited the middle vein of a leaf of tree seedling, 22°38′2″ N, 101°6′7″ E, alt. 1468 m, 19 Aug. 2020, Hong Yu bis (YHH 20190 – holotype preserved in the Yunnan Herbal Herbarium).
Description: Sexual morph: External mycelia produced from the sutures and joints, one stroma at the head of the ant, curved at the top, cylindrical, clavate, the base of stromata were dark brown, pale white at the top. Ascomata of lateral cushions produced from the basal of stromata, one ascoma was observed, spherical, brown, averaging 3 × 2 mm. Perithecia flask-shaped or ovoid, immersed to partially erumpent, with short, exposed neck or rounded ostiole, (195–) 202–242 (–248) × (92–) 102–149 μm. Asci cylindrical, hyaline, 8-spored, 96–188 (–212) × 4–9 (–10) μm. Ascus caps hemispherical, 3–5 µm high, 4–5 µm wide. Ascospores vermiform, hyaline, 4–5-septate, round to slightly tapered at the apex, 89–119 (–122) × 2–3 µm. Asexual morph: Hirsutella type-A only. Phialides lageniform, smooth, swollen base, tapering abruptly a neck, short, (8–) 10–23 (–26) × 1–5 µm. Conidia oviform, hyaline, smooth-walled, 1–4 × 1–2 µm.
Germination process: No ascospores examined from dried specimens.
Host: Camponotus sp. (Formicinae)
Habitat: Subtropical monsoon evergreen broad-leaf forest. Camponotus sp. was infected and bited into a leaf of tree seedling. It was collected from 1.5 m above the ground.
Distribution: China, Yunnan Province, Puer City
Material examined: China: Yunnan, Puer City, Sun River National Park. Infected ants were found biting into a leaf of tree seedling, 22°38′2″ N, 101°6′7″ E, alt. 1468 m, 19 August 2020, Hong Yu bis (YHH 20191).
Notes: Phylogenetic analyses showed that O. basiasca formed a separate clade in the O. unilateralis core clade; it was closed to O. subtiliphialida and O. contiispora, with statistical supported from BI posterior probabilities (PP = 100%) and ML bootstrap proportions (BP = 97%) (Fig. 1). Ophiocordyceps basiasca was similar to O. subtiliphialida and O. contiispora in lageniform phialides, olivary conidia. However, it differed from O. subtiliphialida and O. contiispora by vermiform ascospores, Hirsutella type-A.
Ophiocordyceps nuozhaduensis Hong Yu bis & D.X. Tang, sp. nov.
Mycobank: MB 844354 (Fig. 7)
Fig. 7.
Ophiocordyceps nuozhaduensis. A: Camponotus sp. was infected and bited into a leaf of tree sapling. B: The ascoma was produced from the stroma. C: Cross-section of the ascoma showing the perithecial arrangement. D–G: Ascospores. H: Conidiogenous cells and conidia. I–K: Phialides. L, M: Conidia. Scale bars: A = 3000 µm; B = 1000 µm; C = 100 µm; D–G = 20 µm; H = 5 µm; I–K = 10 µm; L, M = 2 µm
Etymology: The epithet refered to the locality (Nuozhadu) where the holotype was collected.
Diagnosis: Similar to O. camponoti-leonardi in perithecia rounded ostiole, but O. nuozhaduensis differs by ellipsoidal or oviform conidia, smaller flask-shaped perithecia (215–285 × 128–172 μm).
Type: China: Yunnan, Puer City, Nuozhadu Nature Reserve. Camponotus sp. was infected and bited into a leaf of tree sapling, 22°38′27″ N, 100°29′53″ E, alt. 1107 m, 24 Aug. 2021, Hong Yu bis (YHH 20167 – holotype preserved in the Yunnan Herbal Herbarium).
Description: Sexual morph: External mycelia produced from sutures and joints of the ant. One stroma at the head of the ant, curved at the top, cylindrical, clavate, and dark brown at maturity. Fertile regions of lateral cushions produced from the middle of stromata, one ascoma was observed, spherical, brown, averaging 2.4 × 1.6 mm. Perithecia flask-shaped, immersed to partially erumpent, with short, exposed neck or rounded ostiole, (215–) 222–274 (–285) × (128–) 153–159 (–172) μm. Asci were not observed. Ascospores vermiform, hyaline, 7–13-septate, round to slightly tapered at the apex, 91–126 (–132) × 2–5 µm. Asexual morph: Hirsutella type-A present on the stroma and the legs. Phialides cylindrical or lageniform, smooth, swollen base, tapering abruptly a neck, short, 6–22 (–22) × 2–4 µm. Conidia ellipsoidal or oviform, 2–5 × 2–3 µm.
Germination process: No germination examined because the specimens were dried.
Host: Camponotus sp. (Formicinae)
Habitat: Subtropical monsoon evergreen broad-leaf forest. Camponotus sp. was infected and bited into a leaf of tree sapling. Always at lower heights, collected from 25 to 50 cm above the ground.
Distribution: China, Yunnan Province, Puer City.
Material examined: China: Yunnan, Puer City, Nuozhadu Nature Reserve. Infected ants were found biting a leaf of tree seedling, 22°38′27″ N, 100°29′53″ E, alt. 1107 m, 24 Aug. 2021, Hong Yu bis (YHH 20168, YHH 20169).
Notes: Phylogenetically, this species was closed to O. camponoti-leonardi, was clustered in the O. unilateralis core clade, with high statistical supported by BI (PP = 98%) and ML (BP = 100%) (Fig. 1). It was similar to sister O. camponoti-leonardi in rounded ostiole perithecia. However, it differed from O. camponoti-leonardi in vermiform ascospores, ellipsoidal or oviform conidia.
Ophiocordyceps contiispora Hong Yu bis & D.X. Tang, sp. nov.
Mycobank: MB 844355 (Fig. 8)
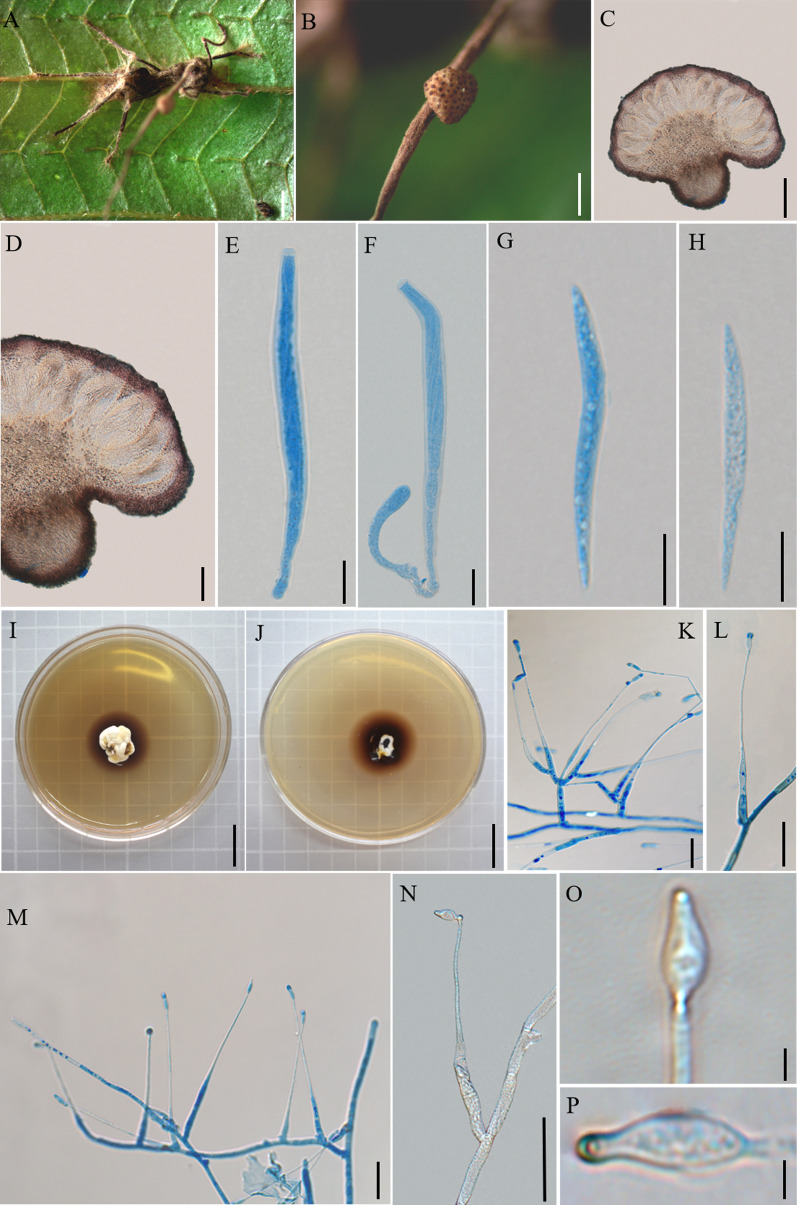
Fig. 8.
Ophiocordyceps contiispora. A: Infected Camponotus sp. was biting into a leaf of epiphytes. B: Close-up of the ascoma. C, D: Cross-section of the ascoma showing the perithecial arrangement. E, F: Asci. G, H: Ascospores. I, J: Colonies on PDA medium. K: Conidiophores and phialides. L–N: Conidiogenous cells and conidia. O, P: Conidia. Scale bars: A = 1000 µm; B = 500 µm; C = 200 µm; D = 100 µm; E, F = 20 µm; G, H = 10 µm; I, J = 2 cm; K–N = 20 µm; O, P = 2 µm
Etymology: The epithet refered to the top of conidia having a protuberance like a spear.
Diagnosis: Similar to O. subtiliphialida in the top of conidia has a protuberance, but the protuberance of O. contiispora was more prominent and the width of conidia was smaller (4–6 × 1–2 μm) than O. subtiliphialida (6–10 × 3–6 μm).
Type: China: Yunnan, Mengla County, Mohan Town, Xinming Village. Camponotus sp. was infected and bited into a leaf of epiphytes, 21°9′35″ N, 101°45′49″ E, alt. 1173 m, 2 Oct. 2019, Hong Yu bis (YHH 20144 – holotype preserved in the Yunnan Herbal Herbarium; living culture YFCC 9027 – ex-holotype stored in Yunnan Fungal Culture Collection).
Description: Sexual morph: External mycelia produced dense from the joints, covering the host body, sparsely when touching the substrate. Stromata single, produced from dorsal pronotum of the ant, cylindrical, clavate, brown at maturity. Fertile part of lateral cushions produced from stromata, one ascoma was observed, disc-shaped, brown, averaging 1.3–1.8 × 1–1.5 mm. Perithecia flask-shaped, immersed to partially erumpent, with short, exposed ostiole, (146–) 158–212 (–224) × 69–122 μm. Asci cylindrical, hyaline, curved, 8-spored, (74–) 89–130 (–134) × 4–9 μm. Ascus caps hemispherical or square, small, 1–3 µm high, 3–5 µm wide. Ascospores fusiform, hyaline, no obvious separation, occasionally curved, round to slightly tapered at the apex, (29–) 38–48 (–62) × 2–4 µm. Asexual morph: Colonies on PDA medium slow-growing, 28–30 mm diameter in 30 days at 25 °C, milky white to light brown, raising cottony-shaped mycelia density, protuberant mycelia at the centrum, reverse light brown to dark brown. Hyphae immersed in the medium, milky white, branched, septate, smooth-walled, hyaline. Hirsutella type-C only. Conidiophores rare, cylindrical, produced from the hyphae, septate, short, 11–12 × 3–4 µm. Conidiogenous cells monophialidic or rarely polyphialidic, forming on side hyphae or conidiophores, smooth, swollen base, lageniform, tapering gradually a long neck, straight, (42–) 57–92 (–97) × 1–4 µm. Conidia olivary or flask-shaped, hyaline, the top of conidia has a protuberance like a spear, smooth-walled, 4–6 × 1–2 µm.
Germination process: No germination observed from dried specimens.
Host: Camponotus sp. (Formicinae)
Habitat: Rainforest and subtropical monsoon evergreen broad-leaf forest. Camponotus sp. was infected and bited into a leaf of epiphytes. Dying in an elevated position, collected from 1 to 2 m above the ground.
Distribution: China, Yunnan Province, Puer City and Jinghong City.
Material examined: China: Yunnan, Mengla County, Mohan Town, Xinming Village. Camponotus sp. was infected and bited into a leaf of epiphytes, 22°21′20″ N, 101°69′01″ E, alt. 865 m, 3 Oct. 2019, D.X. Tang (YHH 20145; living culture YFCC 9026). Other specimens were collected from China, Yunnan Province, Puer City, Sun River National Park. Infected ants were found biting into a leaf of tree sapling, 22°38′2″ N, 101°6′7″ E, alt. 1468 m, 19 Aug. 2020, Hong Yu bis (living culture YFCC 9025).
Notes: Ophiocordyceps contiispora was phylogenetically sister to O. basiasca with high statistical supported by BP = 100% and PP = 100%. It was similar to O. basiasca in flask-shaped perithecia, cylindrical asci, lageniform phialides. However, it differed from O. basiasca by fusiform ascospores, producing Hirsutella type-C.
Discussion
Many phylogenetic classifications have been undertaken for O. unilateralis sensu lato (Evans et al. 2011a; kobmoo et al. 2012, 2015; Araújo et al. 2018; Wei et al. 2020), these groups have been continuously supplemented and improved based on morphology, molecular phylogeny and ecology. This study focused on the phylogenetic investigation of O. unilateralis sensu lato species collected from Yunnan Province, China. The phylogenetic tree showed that six new species were clustered in the O. unilateralis core clade of Hirsutella (Fig. 1). Four species (O. contiispora, O. basiasca, O. subtiliphialida, and O. acroasca) were formed a sister lineage with O. septa. In addition, O. bifertilis formed a sister lineage with O. satoi and O. naomipierceae, and O. nuozhaduensis also formed a sister lineage with O. camponoti-leonardi. The phylogenetic framework was consistent with previous studies (Kobmoo et al. 2012; Crous et al. 2016; Araújo et al. 2018; Wei et al. 2020). However, some species were lower support for topologies, including O. camponoti-leonardi, O. polyrhachis-furcata, O. nuozhaduensis, O. ootakii and O. nooreniae. The reason might be that a few genes were used for O. camponoti-leonardi and O. polyrhachis-furcata. Both phylogenetic analysis and morphological characters supported that the six fungi were distinctive in the core clade of O. unilateralis. Six new species were proposed to be located in the O. unilateralis core clade of the Hirsutella clade within Ophiocordyceps.
The O. unilateralis complex was composed of O. unilateralis core clade, O. oecophyllae clade, O. kniphofioides sub-clades (Araújo et al. 2018). Many species were described in the O. unilateralis core clade, with most of the hosts being Camponotus and Polyrhachis. Species within the O. unilateralis core clade shared many macro-morphological characteristics that made them easily recognized in this study, such as stromata, ascomata, type of host, and location of host attachment. Ophiocordyceps unilateralis complex species commonly bitten and attached leaves, spines, epiphites, saplings, moss, twing in a "death grip" (Evans et al. 2011b, 2018; Hughes et al. 2011; Kepler et al. 2011; Luangsa-ard et al. 2011; Kobmoo et al. 2012, 2015; Araújo et al. 2015, 2018; Crous et al. 2016), with dying in an elevated position, from 0.25 to 2 m or higher above the ground. Fewer O. unilateralis complex species did not have biting and grasping behavior, such as O. tianshanensis (Wei et al. 2020). These species, i.e., O. acroasca, O. basiasca, O. bifertilis, O. contiispora, O. nuozhaduensis, and O. subtiliphialida, died by biting onto the middle vein of a sapling, Pteridophyta, Gramineae, and epiphytes in death position, and at an elevated position, from 0.25 to 2 m above the ground, with results being consistent with previous work (Andersen et al. 2009; Araújo et al. 2018). Species of the O. unilateralis complex have been investigated at the same site in this survey for two years. It was found that the height at which the host died on vegetation from the ground appeared to be affected by climate, such as rainfall, humidity, temperature, etc.. The death position of species in the O. unilateralis complex was 0.5 to 1 m or higher above the ground in the first year, while these species died 0.25 m or less above the ground in the second year. This adaption might occupy a niche and provide for effective spores dispersal (Andersen et al. 2009; Hughes et al. 2011).
The ant manipulation behavior of the O. unilateralis complex occurred only in host-specific species, especially those entomopathogenic fungi that were parasitic on the ants of Camponotus (Evans et al. 2011b; de Bekker et al. 2014; Araújo et al. 2018; Sakolrak et al. 2018). Crous et al. (2016) reported that O. nooreniae infected two host species of Polyrhachis (Polyrhachis cf. hookeri and Polyrhachis lydiae). Our survey also found that a fungus infected multiple hosts of Polyrhachis, and behavior manipulation of the ant almost tended to be consistent, such as the host Polyrhachis sp.1 and Polyrhachis sp.2 were infected by O. bifertilis. The majority of ant pathogenic fungi parasitic on the host of the genus Polyrhachis were reported from Southeast Asia, and some species found in Australia (Crous et al. 2016; Araújo et al. 2018). Pathogenic fungi infecting Polyrhachis ants, such as O. bifertilis, often induced the host to bite onto the main vein of a Pteridophyta leaf. It was similar to O. naomipierceae, O. ootakii, O. polyrhachis-furca, O. nooreniae and O. satoi in phylogeny, habitat, biting and attachment behaviour. The phylogenetic tree of the host ants also indicated that they were closely related species (Fig. 2). This evidence showed that the pathogenic fungi and the host ants were closely related in genetic evolution, and that the diversity of the host might affect the diversity of the pathogenic fungus. Polyrhachis was the second most species-rich genus in Formicinae, currently comprising 706 valid species (http://antcat.org/2022). Polyrhachis originated in Southeast Asia, and dispersed out of Southeast Asia to Australia (Mezger and Moreau 2015). They were widely distributed, ranging from tropical regions in Africa and Asia to Australia and a few Pacific islands. The highest species richness and diversity were in China and Australia. Currently, at least five pathogenic fungi of the host Polyrhachis had been reported in Australia and Southeast Asia. There might exist many pathogenic fungi hosted by Polyrhachis ants to be discovered worldwide, especially in China, Southeast Asia, and Austrilia.
Parasite manipulation of host behavior was an active research topics in various fields (Evans et al. 2011b, 2018; Hughes et al. 2011; Kepler et al. 2011; Luangsa-ard et al. 2011; Kobmoo et al. 2012, 2015; Araújo et al. 2015, 2018; Crous et al. 2016; de Bekker et al. 2018; Will et al. 2020). Multiple reports indicated that manipulation of ant behavior was host-specific (de Bekker et al. 2014, 2018). Host-specific fungal species seemed to be associated with each ant species, leading to the "one ant, one fungus", and the host identity used as criteria for fungal species identification (Evans et al. 2011b; Kobmoo et al. 2012; Araújo et al. 2015, 2018). Population genomics also supported the host-specificity in ant pathogenic fungi by Kobmoo et al. (2019). In this work, the result suggested that multiple ant pathogenic fungi, including O. acroasca, O. basiasca, O. contiispora, O. subtiliphialida (Fig. 2), infecting the same host Camponotus sp.. Interestingly, Kobmoo et al. (2019) revealed that genetic clusters in ant pathogenic fungi sharing the same host. This study supports previous studies that the same host of Camponotus can be infected by different ant pathogenic fungi, while the ant pathogenic fungi of Polyrhachis can infect multiple hosts at the same time. It is not known that an ant fungus infects multiple hosts of the genus Camponotus at the same time. Ophiocordyceps unilateralis complex species were composed of distinct evolutionary species leads to a global diversity of the ant pathogenic fungi (Kobmoo et al. 2012, 2015; Araújo et al. 2015, 2018; Crous et al. 2016). Camponotus was the most species-rich genus in Formicinae, currently comprising 1087 valid species (http://antcat.org/2022). Camponotus ants were distributed in the terrestrial environment worldwide. However, up to now, less than 30 pathogenic fungi have been reported to parasitize Camponotus ants, and some ant pathogenic fungi tend to between sharing the same microhabitat and niche overlap, which might lead to a diversity of the ant pathogenic fungi.
In recent decades, a large amount of research has been conducted to discuss how many fungi exist in the world (Weir and Hammond 1997; Hawksworth 2001; Hawksworth and Lücking 2017). Through the continuous efforts of scientists, the original estimate of 1.5 million (Hawksworth 2001) fungi has changed to 2.2 to 3.8 million fungi (Hawksworth and Lücking 2017). However, relatively few studies have discussed the number of entomopathogenic fungi worldwide. One significant study on host-specificity by Weir and Hammond (1997) in relation to insects was that on the Laboulbeniales on beetles. These studies suggest a beetle (Coleoptera): fungus ratio of 1.68–2: 1. Araújo and Hughes (2019) research shows that zombie-ant fungal lineage likely arose from an ancestor that infected beetle (Coleoptera) larvae. At present, it has been reported that seven genera in the family Formicidae were infected by the O. unilateralis complex species, including Camponotus, Cephalotes, Daceton, Dolichoderus, Oecophylla, Paraponera and Polyrhachis (Evans and Sampson 1982, Kepler et al. 2011, Evans et al. 2011b, Kobmoo et al. 2012, Luangsa ard et al. 2011, Araújo et al. 2015, Kobmoo et al. 2015, Crous et al. 2016, Araújo et al. 2018, Evans et al. al. 2018, Wei et al. 2020). There were 2046 valid species in seven genera of Formicidae (excluding valid subspecies) (https://antcat.org/2022). If all entomopathogenic fungi accord with the beetle (Coleoptera): fungus ratio of 1.68–2: 1 by Weir and Hammond (1997), then there may be 1217–1023 species of entomopathogenic fungi in the world that can infect ants of seven genera in Formicidae, including the O. unilateralis complex.
Morphological characters were diverse for O. unilateralis sensu lato species. Most of the morphological features of O. unilateralis sensu lato species included cylindrical and clavate stromata that arose from the dorsal pronotum of the host, at least one ascoma that grew from lateral cushions of stromata. Some species produced multiple stromata, such as O. camponoti-indiani, O. halabalaensis, O. satoi (Araújo et al. 2015, 2018). Similar results were obtained in this study, the species, O. bifertilis, two stromata produced from the head of Polyrhachis sp.1 and Polyrhachis sp.2, resulting in two ascomata from stromata. Ascomata of O. unilateralis sensu lato were usually characterized by hemispherical, disc-shaped, spherical, one to multiple. All species in this group produced ascospores that were not disarticulate into part spores, and the shape includes vermiform, cylindrical, lanceolate, and fusiform. These shapes might to better dispersal for their spores.
Most species formed an asexual morph characterized by Hirsutella type-A phialides, tapering to a long neck and bearing a single conidium at their apices. There were also two types of asexual morphs, i.e., Hirsutella type-B and Hirsutella type-C. Most species produced phialides along stromata, legs and joints. The phialides of these species, such as O. basiasca (Hirsutella type-A), O. bifertilis (Hirsutella type-A), O. nuozhaduensis (Hirsutella type-A), were also observed from stromata, legs and joints. However, their phialides were shorter than O. acroasca (Hirsutella type-A and Hirsutella type-C), O. subtiliphialida (Hirsutella type-C) and O. contiispora (Hirsutella type-C) (Table 4). The phialides of O. acroasca, O. subtiliphialida, O. contiispora were produced from pure culture. This structure, rarely polyphialidic and conidiophores, were observed in the species of O. subtiliphialida, O. contiispora. Ophiocordyceps subtiliphialida and O. contiispora were only observed in Hirsutella type-C, and Hirsutella A-type was not been observed. The same result was also reported in Araújo et al. (2018). Unfortunately, the phialides produced from pure cultures and specimens were not compared, as specimens were used to isolate strains, or were dried, or made into permanent specimens. Conidia were diverse in O. acroasca (limoniform), O. basiasca (oviform), O. nuozhaduensis (ellipsoidal or oviform), O. subtiliphialida (olivary) and O. contiispora (olivary or flask-shaped) (Table 4). In addition, characteristics of the living cultures were introduced in the present work more than in previous studies (Kobmoo et al. 2012; Crous et al. 2016; Araújo et al. 2018; Wei et al. 2020). They were slow-growing, hard, light brown to dark brown in color, and produced pigment. This work has provided a method (see materials and methods for details) for obtaining living cultures of O. unilateralis complex species and asexual morph based on pure culture, which is of real value for further studies of O. unilateralis complex species in the future.
Conclusions
Six zombie-ant fungi were described from Yunnan Province, China. These novel species of Ophiocordyceps with hirsutella-like asexual morphs exclusively infecting ants were well supported based on molecular phylogenetic data and morphological evidence. This work proposes that the same host of Camponotus can be infected by multiple ant pathogenic fungi, while multiple species of Polyrhachis can be infected by the same pathogenic fungi at the same time. This study provides six new taxa support to explore the evolutionary relationship between the host and the fungus, and provides novel insights into the morphology, parasitism, distribution and ecology of O. unilateralis sensu lato within Ophiocordyceps. It has provided a method to obtain living cultures of the O. unilateralis complex and asexual morphs based on pure culture, which is of great value for further future studies of zombie-ant fungi.
Key to Ophiocordyceps unilateralis complex species worldwide
1a. On host Camponotus…………………………………………………………………………………………………………...2
1b. On host Cephalotes…………………………………………………………………………………….Ophiocordyceps kniphofioides
1c. On host Daceton………………… …………………………………………………………………..Ophiocordyceps daceti
1d. On host Dolichoderus………………………………………………………………………………..Ophiocordyceps monacidis
1e. On host Oecophylla…………………………………………………………………………………..Ophiocordyceps oecophyllae
1f. On host Paraponera…………………………………………………………………………………..Ophiocordyceps ponerinarum
1 g. On host Polyrhachis………………………………………...............................................................................................14
2a. The host without a biting behavior…………………………………………………………………...Ophiocordyceps tianshanensis
2b. The host with biting behavior………………………………………...................................................................................3
3a. Death position of the host was biting leaf………………………………….................................................................4
3b. Death position of the host was biting twing………………………………..............................................................12
4a. Ascospores not obvious separation………………............................................................................Ophiocordyceps contiispora
4b. Ascospores obvious separation………………………………………..................................................................................5
5a. The widest ascospore was not more than 3 μm……………………………………..........................................................6
5b. The widest ascospore was more than 3 μm…………………………………................................................................7
6a. Ascospores 83–108 × 2–3 μm, perithecia 247–296 × 176–225 μm, asci 131–172 × 5–8 μm………...Ophiocordyceps acroasca
6b. Ascospores 89–119 × 2–3 μm, perithecia 202–242 × 102–149 μm, asci 96–188 × 4–9 μm………….Ophiocordyceps basiasca
6c. Ascospores 110–125 × 2–3 μm, perithecia 400–430 × 200–230 μm, asci 130–175 × 7–8 μm……….Ophiocordyceps camponoti-leonardi
6d. Ascospores 75–85 × 2–3 μm, perithecia 280–320 × 160–180 μm, asci 80–160 × 6–7 μm…………...Ophiocordyceps camponoti-saundersi
6e. Ascospores 200–215 × 2–3 μm, perithecia 325–500 × 275–300 μm, asci 200–340 × 7–10 μm……...Ophiocordyceps rami
6f. Ascospores 80–85 × 3 μm, perithecia 240–280 × 100–150 μm, asci 110–140 × 6–6.5 μm…………...Ophiocordyceps camponoti-atricipis
6g. Ascospores 75–90 × 3 μm, perithecia 200–230 × 135–165 μm, asci 110–130 × 8–9 μm…………….Ophiocordyceps camponoti-femorati
6h. Ascospores 80–95 × 2–3 μm, perithecia 175–260 × 100–130 μm, asci 120–160 × 8–10 μm………..Ophiocordyceps camponoti-rufipedis
6i. Ascospores 120–140 × 3 μm, perithecia 225–230 × 135 μm, asci 150–160 × 8–9 μm……………….Ophiocordyceps camponoti-sexguttati
6j. Ascospores 75–85 × 2–2.5 μm, perithecia 200–250 × 140–160 μm, asci 95–125 × 6–8 μm………....Ophiocordyceps unilateralis
7a. Ascospores vermiform………………………………………...........................................................................................8
7b. Ascospores cylindrical…………………………………..............................................................................................10
7c. Ascospores filiform………………...................................................................................................Ophiocordyceps camponoti-novogranadensis
7d. Ascospores lanceolate…………………………………………...............................................................................................11
8a. Ascospores the longest was not more than 85 μm………………......................................................Ophiocordyceps camponoti-chartificis
8b. Ascospores the longest was more than 85 μm………………………………............................................................9
9a. Ascospores 91–126 × 2–5 μm, perithecia 222–274 × 153–159 μm……………….............................Ophiocordyceps nuozhaduensis
9b. Ascospores 90–105 × 3–4 μm, perithecia 200–240 × 100–150 μm……………….............................Ophiocordyceps camponoti-nidulantis
9c. Ascospores 90–120 × 4 μm, perithecia 220–250 × 100–165 μm………………..................................Ophiocordyceps camponoti-renggeri
10a. Ascospores 80–100 × 5 μm, distributed in Colombia………………................................................Ophiocordyceps albacongiuae
10b. Ascospores 60–75 × 3–5 μm, distributed in Thailand………………................................................Ophiocordyceps halabalaensis
10c. Ascospores 135–175 × 4–5 μm, distributed in Brazil……………….................................................Ophiocordyceps camponoti-balzani
10d. Ascospores 70–75 × 4.5–5 μm, distributed in Brazil………………..................................................Ophiocordyceps camponoti-bispinosi
10e. Ascospores 75–90 × 4–5 μm, distributed in USA……………….......................................................Ophiocordyceps camponoti-floridani
10f. Ascospores 75–85 × 4–5 μm, distributed in Brazi…………………...................................................Ophiocordyceps camponoti-hippocrepidis
10g. Ascospores 75 × 4.5 μm, distributed in Brazil………………............................................................Ophiocordyceps camponoti-indiani
10h. Ascospores 170–210 × 4–5 μm, distributed in Brazil…………………..............................................Ophiocordyceps camponoti-melanotici
11a. Ascospores 45–50 × 6–8 μm, distributed in Thailand…………………..............................................Ophiocordyceps septa
11b. Ascospores 52–72 × 5–8 μm, distributed in China………………......................................................Ophiocordyceps subtiliphialida
12a. Ascospores cylindrical………………………………………………................................................................................................13
12b. Ascospores filiform………………....................................................................................................Ophiocordyceps pulvinata
13a. Two types of Hirsutella asexual morph………………......................................................................Ophiocordyceps kimflemingiae
13b. One types of Hirsutella asexual morph………………......................................................................Ophiocordyceps blakebarnesii
14a. Biting leaf……………………………………..................................................................................................................15
14b. Biting twing………………...............................................................................................................Ophiocordyceps satoi
15a. Paraisaria-like phialides………………............................................................................................Ophiocordyceps naomipierceae
15b. Hirsutella-like phialides………………………………….............................................................................................16
16a. Two types of Hirsutella asexual morph……………….......................................................................Ophiocordyceps nooreniae
16b. One types of Hirsutella asexual morph…………………………….......................................................................17
17a. Phialides 9–24 × 2–4 μm, distributed in China………………............................................................Ophiocordyceps bifertilis
17b. Phialides 6–8 × 3–4 μm, distributed in Japan………………..............................................................Ophiocordyceps ootakii
17c. Phialides 30 × 2–3 μm, distributed in Thailand………………...........................................................Ophiocordyceps polyrhachis-furca
Acknowledgements
We thank Jing Zhao for providing important support to the phylogenetic analysis in this work. We thank Tahir Khan for providing English editing.
Abbreviations
- BI
Bayesian inference
- BP
Bayesian posterior probability
- bp
Base pair
- CTAB
Cetyl-trimethyl-ammonium bromide
- DNA
Deoxyribonucleic acid
- ML
Maximum likelihood
- SSU
The nuclear ribosomal small subunit
- LSU
The nuclear ribosomal large subunit
- PCR
Polymerase chain reaction
- PP
Posterior probabilities
- PDA
Potato dextrose agar
- RPB1
The largest subunits of RNA polymerase II
- RPB2
The second largest subunits of RNA polymerase II
- TEF
The translation elongation factor 1α
Author contributions
D-XT, W-QZ, GY, and HY collected samples. D-XT and W-QZ isolated cultures and performed DNA isolation and PCR amplification. OH, Y-BW, YW, Q-YD, and TS analyzed data. D-XT wrote the original draft. HY reviewed and edited the draft. All authors read and approved the final manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (31870017, 32060007).
Availability of data and materials
All sequence data generated for this work can be accessed via GenBank: https://www.ncbi.nlm.nih.gov/genbank/. All alignments for phylogenetic analyses were deposited in TreeBASE (http://www.treebase.org; the following links were available: http://purl.org/phylo/treebase/phylows/study/TB2:S29994?x-access-code=8e258e97fca38d4f834975a2fefb47a1&format=html.)
Declarations
Ethics approval and consent to participate
Not applicable.
Adherence to national and international regulations
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andersen SB, Gerritsma S, Yusah KM, Mayntz D, Hywel-Jones NL, Billen J, Boomsma JJ, Hughes DP. The life of a dead ant: the expression of an adaptive extended phenotype. Am Nurserym. 2009;174:424–433. doi: 10.1086/603640. [DOI] [PubMed] [Google Scholar]
- Araújo JPM, Evans HC, Geiser DM, Mackay WP, Hughes DP. Unravelling the diversity behind the Ophiocordyceps unilateralis (Ophiocordycipitaceae) complex: Three new species of zombie-ant fungi from the Brazilian Amazon. Phytotaxa. 2015;220:224–238. doi: 10.11646/phytotaxa.220.3.2. [DOI] [Google Scholar]
- Araújo JPM, Hughes DP. Diversity of entomopathogenic fungi: which groups conquered the insect body? Adv Genet. 2016;94:1–39. doi: 10.1016/bs.adgen.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Araújo JPM, Hughes DP. The fungal spore: myrmecophilous Ophiocordyceps as a case study. In: Dighton J, White JM, editors. The fungal community: its organization and role in the ecosystem. USA: CRC Press; 2017. [Google Scholar]
- Araújo JPM, Evans HC, Kepler R, Hughes DP. Zombie-ant fungi across continents: 15 new species and new combinations within Ophiocordyceps I. Myrmecophilous hirsutelloid species. Stud Mycol. 2018 doi: 10.1016/j.simyco.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo JPM, Hughes DP. Zombie-ant fungi emerged from non-manipulating, beetle-infecting ancestors. Curr Biol. 2019 doi: 10.1016/j.cub.2019.09.004. [DOI] [PubMed] [Google Scholar]
- Araújo JPM, Evans HC, Fernandese IO, Ishler MJ, Hughes DP. Zombie-ant fungi cross continents: II. Myrmecophilous hymenostilboid species and a novel zombie lineage. Mycologia. 2020 doi: 10.1080/00275514.2020.1822093. [DOI] [PubMed] [Google Scholar]
- Mezger D, Moreau CS. Out of South-East Asia: phylogeny and biogeography of the spiny ant genus Polyrhachis Smith (Hymenoptera: Formicidae) Syst Entomol. 2015;41:369–378. doi: 10.1111/syen.12163. [DOI] [Google Scholar]
- Castlebury LA, Rossman AY, Sung GH, Hyten AS, Spatafora JW. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol Res. 2004;108:864–872. doi: 10.1017/S0953756204000607. [DOI] [PubMed] [Google Scholar]
- Currie CR, Wong B, Stuart AE, Schultz TR, Rehner SA, Mueller UG, Sung GH, Spatafora JW, Straus NA. Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science. 2003;299:386–388. doi: 10.1126/science.1078155. [DOI] [PubMed] [Google Scholar]
- Cheek M, Nic Lughadha E, Kirk P, Lindon H, Carretero J, Looney B, Douglas B, Haelewaters D, Gaya E, Llewellyn T, Ainsworth AM, Gafforov Y, Hyde K, Crous P, Hughes M, Walker BE, Forzza RC, Wong KM, Niskanen T. New scientific discoveries: plants and fungi. Pants, People, Planet. 2020;2:371–388. doi: 10.1002/ppp3.10148. [DOI] [Google Scholar]
- Chen JY, Cao YQ, Yang DR, Li MH. A new species of Ophiocordyceps (Clavicipitaceae, Ascomycota) from southwestern China. Mycotaxon. 2011;115:1–4. doi: 10.5248/115.1. [DOI] [Google Scholar]
- Chen ZH, Dai YD, Yu H, Yang K, Yang ZL, Yuan F, Zeng WB. Systematic analyses of Ophiocordyceps lanpingensis sp. nov. a new species of Ophiocordyceps in China. Microbiol Res. 2013;168:525–532. doi: 10.1016/j.micres.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Chen SQ, Wang Y, Zhu KF, Yu H. Mitogenomics, Phylogeny and morphology reveal Ophiocordyceps pingbianensis sp. Nov., an entomopathogenic fungus from China. Life. 2021;11:686. doi: 10.3390/life11070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. MycoBank: an online initiative to launch mycology into the 21st century. Stud Mycol. 2004;50:19–22. [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, Hardy GESTJ, Crane C, Barrett S, Cano-Lira JF, Le Roux JJ, Thangavel R, Guarro J, Stchigel AM, Martín MP, Alfredo DS, Barber PA, Barreto RW, Baseia IG, Cano-Canals J, Cheewangkoon R, Ferreira RJ, Gené J, Lechat C, Moreno G, Roets F, Shivas RG, Sousa JO, Tan YP, Wiederhold NP, Abell SE, Accioly T, Albizu JL, Alves JL, Antoniolli ZI, Aplin N, Araújo J, Arzanlou M, Bezerra JDP, Bouchara JP, Carlavilla JR, Castillo A, Castroagudín VL, Ceresini PC, Claridge GF, Coelho G, Coimbra VRM, Costa LA, da Cunha KC, da Silva SS, Daniel R, de Beer ZW, Dueñas M, Edwards J, Enwistle P, Fiuza PO, Fournier J, García D, Gibertoni TB, Giraud S, Guevara-Suárez M, Gusmão LFP, Haituk S, Heykoop M, Hirooka Y, Hofmann TA, Houbraken J, Hughes DP, Kautmanová I, Koppel O, Koukol O, Larsson E, Latha KPD, Lee DH, Lisboa DO, Lisboa WS, López-Villalba Á, Maciel JLN, Manimohan P, Manjón JL, Marincowitz S, Marney TS, Meijer M, Miller AN, Olariaga I, Paiva LM, Piepenbring M, Poveda-Molero JC, Raj KNA, Raja HA, Rougeron A, Salcedo I, Samadi R, Santos TAB, Scarlett K, Seifert KA, Shuttleworth LA, Silva GA, Silva M, Siqueira JPZ, Souza-Motta CM, Stephenson SL, Sutton DA, Tamakeaw N, Telleria MT, Valenzuela-Lopez N, Viljoen A, Visagie CM, Vizzini A, Wartchow F, Wingfield BD, Yurchenko E, Zamora JC, Groenewald JZ. Fungal Planet description sheets:469–557. Persoonia. 2016;37:218–403. doi: 10.3767/003158516X694499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bekker C, Quevillon LE, Smith PB, Fleming KR, Ghosh D, Patterson AD, Hughes DP. Species-specific ant brain manipulation by a specialized fungal parasite. BMC Evolut Biol. 2014 doi: 10.1186/s12862-014-0166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bekker C, Will I, Das B, Adams RMM. The ants (Hymenoptera: Formicidae) and their parasites: Effects of parasitic manipulations and host responses on ant behavioral ecology. Myrmecol News. 2018;28:1–24. doi: 10.25849/myrmecol.news_028:001. [DOI] [Google Scholar]
- Evans HC, Samson RA. Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems. I. The Cephalotes (Myrmicinae) complex. Trans Br Mycol Soc. 1982;79:431–453. doi: 10.1016/S0007-1536(82)80037-5. [DOI] [Google Scholar]
- Evans HC, Samson RA. Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems. II. The Camponotus (Formicinae) complex. Trans Br Mycol Soc. 1984;82:127–150. doi: 10.1016/S0007-1536(84)80219-3. [DOI] [Google Scholar]
- Evans HC, Elliot SL, Hughes DP. Ophiocordyceps unilateralis: a keystone species for unraveling ecosystem functioning and biodiversity of fungi in tropical forests? Commun Integr Biol. 2011;4:598–602. doi: 10.4161/cib.4.5.16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HC, Elliot SL, Hughes DP. Hidden diversity behind the zombie-ant fungus Ophiocordyceps unilateralis: four new species described from carpenter ants in Minas Gerais, Brazil. PLoS ONE. 2011;6:e17024. doi: 10.1371/journal.pone.0017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HC, Araújo JPM, Halfeld VR, Hughes DP. Epitypification and re-description of the zombie-ant fungus, Ophiocordyceps unilateralis (Ophiocordycipitaceae) Fungal Syst Evol. 2018;1:13–22. doi: 10.3114/fuse.2018.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Wang YB, Zhang GD, Tang DX, Yu H. Multigene phylogeny and morphology of Ophiocordyceps alboperitheciat sp. nov., a new entomopathogenic fungus attacking lepidopteran larva from Yunnan, China. Mycobiology. 2021;49:133–141. doi: 10.1080/12298093.2021.1903130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foottit RG, Adler PH. Insect biodiversity. Science and Society. Oxford, UK: Wiley Blackwell; 2009. [Google Scholar]
- Haelewaters D, Blackwell M, Pfister DH (2020) Laboulbeniomycetes: intimate fungal associates of arthropods. Ann Rev Entomol. 10.1146/annurev-ento-013020-013553 [DOI] [PubMed]
- Hafer-Hahmann N (2019) Experimental evolution of parasitic host manipulation. Proce R Soc B Biol Sci. 10.1098/rspb.2018.2413 [DOI] [PMC free article] [PubMed]
- Hawksworth DL. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res. 2001;105:1422–1432. doi: 10.1017/s0953756201004725. [DOI] [Google Scholar]
- Hawksworth DL, Lücking R (2017) Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol Spectrum 5:FUNK-0052-2016. 10.1128/microbiolspec.FUNK-0052-2016 [DOI] [PubMed]
- Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proc R Soc b Biol Sci. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison REH. Lessons in mind control: trends in research on the molecular mechanisms behind parasite-host behavioral manipulation. Front Ecol Evol. 2017;5:102. doi: 10.3389/fevo.2017.00102. [DOI] [Google Scholar]
- Hopple JS (1994) Phylogenetic investigations in the genus coprinus based on morphological and molecular characters. Ph.D. Dissertation, Duke University, Durham, NC, USA
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 2017;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DP, Evans HC, Hywel-jones N, Boomsma JJ, Armitage SAO. Novel fungal disease in complex leaf-cutting ant societies. Ecol Entomol. 2009;34:214–220. doi: 10.1111/j.1365-2311.2008.01066.x. [DOI] [Google Scholar]
- Hughes DP, Andersen SB, Hywel-Jones NL, Himaman W, Billen J, Boomsma JJ. Behavioral mechanisms and morphological symptoms of zombie ants dying from fungal infection. BMC Ecol. 2011;11:13. doi: 10.1186/1472-6785-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO, Nelson MC. The superorganism: the beauty, elegance, and strangeness of insect societies. New York, USA: Norton & Company; 2009. [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepler RM, Kaitsu Y, Tanaka E, Shimano S, Spatafora JW. Ophiocordyceps pulvinata sp. nov., a pathogen of ants with a reduced stroma. Mycoscience. 2011;52:39–47. doi: 10.1007/s10267-010-0072-5. [DOI] [Google Scholar]
- Khonsanit A, Luangsa-ard JJ, Thanakitpipattana D, Kobmoo N, Piasai O (2018) Cryptic species within Ophiocordyceps myrmecophila complex on formicine ants from Thailand. Mycol Progress. 10.1007/s11557-018-1412-7
- Kobayasi Y. The genus Cordyceps and its allies. Sci Rep. 1941;5:53–260.. [Google Scholar]
- Kobmoo N, Mongkolsamrit S, Tasathai K, Thanakitpipattana D, Luangsa-ard JJ. Molecular phylogenies reveal host-specific divergence of Ophiocordyceps unilateralis sensu lato following its host ants. Mol Ecol. 2012;21:3022–3031. doi: 10.1111/j.1365-294x.2012.05574.x. [DOI] [PubMed] [Google Scholar]
- Kobmoo N, Mongkolsamrit S, Wutikhun T, Tasanathai K, Khonsanit A, Thanakitpipattana D, Luangsa-ard JJ. New species of Ophiocordyceps unilateralis, an ubiquitous pathogen of ants from Thailand. Fungal Biol. 2015;119:44–52. doi: 10.1016/j.funbio.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Kobmoo N, Mongkolsamrita S, Arnamnarta N, Luangsa-ard JJ, Giraud T. Population genomics revealed cryptic species within host-specific zombieant fungi (Ophiocordyceps unilateralis) Mol Phylogenetics Evolut. 2019;140:106580. doi: 10.1016/j.ympev.2019.106580. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Liu ZY, Liang ZQ, Whalley AJS, Yao YJ, Liu AY. Cordyceps brittlebankisoides, a new pathogen of grubs and its anamorph, Metarhizium anisopliae var. majus. J Invertebr Pathol. 2001;78:178–182. doi: 10.1006/jipa.2001.5039. [DOI] [PubMed] [Google Scholar]
- Lin WJ, Lee YI, Liu SL, Lin CC, Chung TY, Chou JY. Evaluating the tradeofs of a generalist parasitoid fungus, Ophiocordyceps unilateralis, on diferent sympatric ant hosts. Sci Rep. 2020;10:6428. doi: 10.1038/s41598-020-63400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luangsa-ard JJ, Ridkaew R, Tasanathai K, Thanakitpipattana D, Hywel-Jones N. Ophiocordyceps halabalaensis: a new species of Ophiocordyceps pathogenic to Camponotus gigas in Hala Bala Wildlife Sanctuary, Southern Thailand. Fungal Biol. 2011;115:608–614. doi: 10.1016/j.funbio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Mains EB. North American entomogenous species of Cordyceps. Mycologia. 1958;50:169–222. doi: 10.1080/00275514.1958.12024722. [DOI] [Google Scholar]
- Misof B, Liu S, Meusemann K, Peters RS, Donath A, Mayer C, Frandsen PB, Ware J, Flouri T, Beutel RG, Niehuis O, Petersen M, Izquierdo-Carrasco F, Wappler T, Rust J, Aberer AJ, Aspöck U, Aspöck H, Bartel D, Blanke A, Berger S, Böhm A, Buckley TR, Calcott B, Chen JQ, Friedrich F, Fukui M, Fujita M, Greve C, Grobe P, Gu SC, Huang Y, Jermiin LS, Kawahara AY, Krogmann L, Kubiak M, Lanfear R, Letsch H, Li YY, Li ZY, Li JG, Lu HR, Machida R, Mashimo Y, Kapli P, McKenna DD, Meng GL, Nakagaki Y, Navarrete-Heredia JL, Ott M, Ou YX, Pass G, Podsiadlowski L, Pohl H, von Reumont BM, Schütte K, Sekiya K, Shimizu S, Slipinski A, Stamatakis A, Song WH, Su X, Szucsich NU, Tan M, Tan XM, Tang M, Tang JB, Timelthaler G, Tomizuka S, Trautwein M, Tong XL, Uchifune T, Walzl MG, Wiegmann BM, Wilbrandt J, Wipfler B, Wong TKF, Wu Q, Wu GX, Xie YL, Yang SZ, Yang Q, Yeates DK, Yoshizawa K, Zhang Q, Zhang R, Zhang WW, Zhang YH, Zhao J, Zhou CG, Zhou LL, Ziesmann T, Zou SJ, Li YG, Xu X, Zhang Y, Yang HM, Wang J, Wang J, Kjer KM, Zhou X. Phylogenomics resolves the timing and pattern of insect evolution. Science. 2014;346:763–767. doi: 10.1126/science.1257570. [DOI] [PubMed] [Google Scholar]
- Moore J. The behavior of parazitized animals. Bioscience. 1995;45:89–96.. doi: 10.2307/1312610. [DOI] [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petch T. Notes on entomogenous fungi. Trans Br Mycol Soc. 1931;16:55–75. doi: 10.1016/S0007-1536(31)80006-3. [DOI] [Google Scholar]
- Pontoppidan MB, Himaman W, Hywel-Jones NL, Boomsma JJ, Hughes DP. Graveyards on the move: the spatio-temporal distribution of dead Ophiocordyceps-infected ants. PLoS ONE. 2009;4:e4835. doi: 10.1371/journal.pone.0004835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin R, Maure F. Host manipulation by parasites: a look back before moving forward. Trends Parasitol. 2015;31:563–570. doi: 10.1016/j.pt.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Quandt CA, Kepler RM, Gams W, Araújo JPM, Ban S, Evans HC, Hughes D, Humber R, Hywel-Jones NL, Li ZZ, Luangsa-ard JJ, Rehner SA, Sanjuan T, Sato H, Shrestha B, Sung GH, Yao YJ, Zare R, Spatafora JW. Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus. 2014;5:121–134. doi: 10.5598/imafungus.2014.05.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehner SA, Buckley E. A beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakolrak B, Blatrix R, Sangwanit U, Kobmoo N (2018) Experimental infection of the ant Polyrhachis furcata with Ophiocordyceps reveals specificity of behavioural manipulation. Fungal Ecol 33:122–124. 10.1016/j.funeco.2018.03.001
- Sanjuan TI, Fanco-Molano AE, Kepler RM, Spatafora JW, Tabima J, Vasco-Palacios AM, Restrepo S. Five new species of entomopathogenic fungi from the Amazon and evolution of neotropical Ophiocordyceps. Fungal Biol. 2015;119:901–916. doi: 10.1016/j.funbio.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Suh SO, McHugh JV, Pollock DD, Blackwell M. The beetle gut: a hyper diverse source of novel yeasts. Mycol Res. 2005;109:261–265. doi: 10.1017/S0953756205002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatafora JW, Quandt CA, Kepler RM, Sung GH, Shrestha B, Hywel-Jones NL, Luangsa-ard JJ. New 1F1N Species Combinations in Ophiocordycipitaceae (Hypocreales) IMA Fungus. 2015;6:357–362. doi: 10.5598/imafungus.2015.06.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung GH, Hywel-Jones N, Sung JM, Luangsa-ard JJ, Shrestha B, Spatafora JW. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DX, Zhu JY, Luo LJ, Hou DH, Wang ZQ, Yang SD, Yu H. Ophiocordyceps ovatospora sp. Nov. (Ophiocordycipitaceae, Hypocreales), pathogenic on termites from China. Phytotaxa. 2022;574:105–117. doi: 10.11646/phytotaxa.574.1.8. [DOI] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasanathai K, Noisripoom W, Chaitika T, Khonsanit A, Hasin S, Luangsa-ard J. Phylogenetic and morphological classification of Ophiocordyceps species on termites from Thailand. MycoKeys. 2019;56:101–129. doi: 10.3897/mycokeys.56.37636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F, Poulin R, Brodeur J. Host manipulation by parasites: a multidimensional phenomenon. Oikos. 2010;119:1217–1223. doi: 10.1111/j.1600-0706.2009.18077.x. [DOI] [Google Scholar]
- Tulasne LR, Tulasne C (1865) Selecta fungorum carpologia III. Paris Museum 1–221.
- Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YB, Wang Y, Fan Q, Duan DE, Zhang GD, Dai RQ, Dai YD, Zeng WB, Chen ZH, Li DD, Tang DX, Xu ZH, Sun T, Nguyen TT, Tran NL, Dao VM, Zhang CM, Huang LD, Liu YJ, Zhang XM, Yang DR, Sanjuan T, Liu XZ, Yang ZL, Yu H. Multigene phylogeny of the family Cordycipitaceae (Hypocreales): new taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Divers. 2020;103:1–46. doi: 10.1007/s13225-020-00457-3. [DOI] [Google Scholar]
- Wei DP, Wanasinghe DN, Hyde KD, Mortimer PE, Xu JC, To-Anun C, Yu FM, Zha LS. Ophiocordyceps tianshanensis sp. Nov. on ants from Tianshan mountains, PR China. Phytotaxa. 2020;464:277–292. doi: 10.11646/phytotaxa.464.4.2. [DOI] [Google Scholar]
- Weir A, Hammond PM. Laboulbeniales on beetles : host utilization patterns and species richness of the parasites. Biodivers Conserv. 1997;6:701–719. doi: 10.1023/A:1018318320019. [DOI] [Google Scholar]
- White TJ, Bruns S, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guide Methods Appl. 1990 doi: 10.1016/b978-0-12-372180-8.50042-1. [DOI] [Google Scholar]
- Will I, Das B, Trinh T, Brachmann A, Ohm RA, de Bekker C. Genetic underpinnings of host manipulation by Ophiocordyceps as revealed by comparative transcriptomics. G3-Genes Genomes Genet. 2020;10:2275–2296. doi: 10.1101/2020.01.03.893917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZH, Tran NL, Wang Y, Zhang GD, Dao VM, Nguyen TT, Wang YB, Yu H. Phylogeny and morphology of Ophiocordyceps puluongensis sp. Nov. (Ophiocordycipitaceae, Hypocreales), a new fungal pathogen on termites from Vietnam. J Invertebr Pathol. 2022;192:107771. doi: 10.1016/j.jip.2022.107771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequence data generated for this work can be accessed via GenBank: https://www.ncbi.nlm.nih.gov/genbank/. All alignments for phylogenetic analyses were deposited in TreeBASE (http://www.treebase.org; the following links were available: http://purl.org/phylo/treebase/phylows/study/TB2:S29994?x-access-code=8e258e97fca38d4f834975a2fefb47a1&format=html.)