Abstract
Objective
To describe the prevalence of multimorbidity and its associations with clinical outcomes across age groups.
Design
Retrospective cohort study using nationwide medical claims data.
Setting
Carried out in Japan between April 2014 and March 2019.
Participants
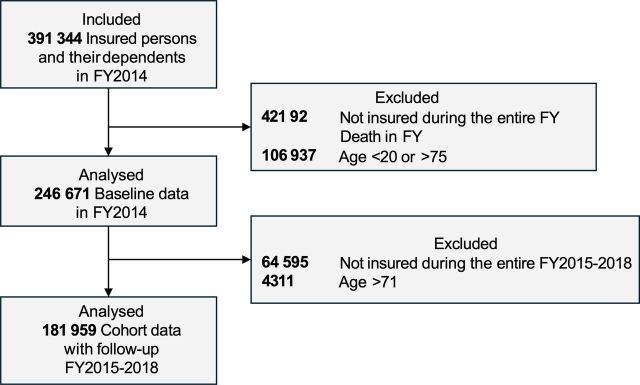
N=246 671 Japanese individuals aged 20–74 enrolled in the health insurance were included into the baseline data set for fiscal year (FY) 2014. Of those, N=181 959 individuals were included into the cohort data set spanning FY2014–FY2018.
Exposures
Multimorbidity was defined as having ≥2 of 15 chronic conditions according to the International Classification of Diseases 10th Revision codes of the Charlson Comorbidity Index.
Primary and secondary outcomes
Primary outcome: the standardised prevalence of multimorbidity across age groups was evaluated using data from FY2014 and extrapolated to the Japanese total population. Secondary outcome: hospitalisation or death events were traced by month using medical claims data and insurer enrolment data. Associations between multimorbidity and 5-year hospitalisation and/or death events across age groups were analysed using a Cox regression model.
Results
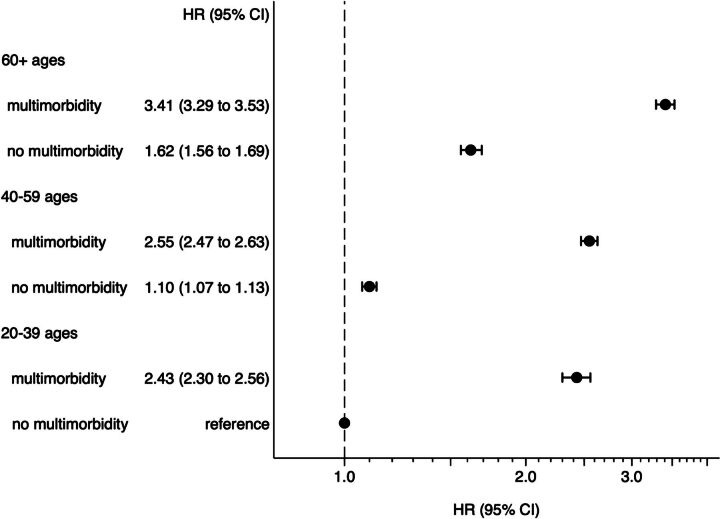
The standardised prevalence rate of multimorbidity in the nationwide Japanese total population was estimated to 26.1%. The prevalence rate with age was increased, approximately 5% (ages 20–29), 10% (30–39), 20% (40–49), 30% (50–59), 50% (60–69) and 60% (70–74). Compared with individuals aged 20–39 without multimorbidity, those with multimorbidity had a higher incidence of clinical events in any age group (HR=2.43 (95% CI 2.30 to 2.56) in ages 20–39, HR=2.55 (95% CI 2.47 to 2.63) in ages 40–59 and HR=3.41 (95% CI 3.23 to 3.53) in ages ≥60). The difference in the incidence of clinical events between multimorbidity and no multimorbidity was larger than that between age groups.
Conclusions
Multimorbidity is already prevalent in the middle-aged generation and is associated with poor clinical outcomes. These findings underscore the significance of multimorbidity and highlight the urgent need for preventive intervention at the public healthcare level.
Keywords: preventive medicine, epidemiology, health economics, public health
Strengths and limitations of this study.
The current study covers a wide age range of individuals from a nationwide general population.
Japan’s high medical insurance coverage rate made it possible to comprehensively identify chronic diseases from receipts.
The longitudinal analysis enabled the examination of clinical outcomes of multiple comorbidities.
The prevalence of multimorbidity may be underestimated because the target population comprised regular employees and their families and might accordingly be healthier than the general population.
Introduction
Ageing societies worldwide face the problem of how to provide adequate and affordable healthcare for a growing number of patients with multiple chronic conditions, termed multimorbidity.1 2 Managing multimorbidity is becoming a global challenge on the clinical and public healthcare level not only in high-income countries, but also in low-income and middle-income countries.3 Many epidemiological studies on multimorbidity have shown its association with age, sociodemographic and socioeconomic factors.4–7 In addition, numerous studies have shown that multiple comorbidities are common in older people.8–11 It has been reported that multimorbid older patients had more than two times as many contacts per year with physicians than those without multimorbidity12 and that the likelihood of being hospitalised was increased by a factor of 5.6 due to multiple comorbidities.8 On the other hand, the accumulation of chronic diseases occurs continuously from middle age. A number of recent studies conducted in various countries have reported that the onset of multimorbidity is shifting toward younger age groups.6 13–15 However, multimorbidity studies tend to focus on older people, and in-depth knowledge on multimorbidity in younger age groups is lacking.
Here, to evaluate the current status of multimorbidity across age groups and examine its association with clinical outcomes, we analysed a large nationwide medical claims cohort. Our findings add to existing knowledge by showing that multimorbidity has a significant impact on health starting from middle age and underscore the need for preventive intervention on the public healthcare level.
Materials and methods
Data source
We used the nationwide medical claims and enrolment data of the Health Insurance Association for Architecture and Civil Engineering companies (HIA2CE), which is one of the largest social insurance associations in Japan. HIA2CE is a comprehensive insurer which includes 1700 companies, from small engineering companies to middle and large construction companies across Japan. This claims database covers a total of 400 000 insured persons, consisting of employees and their dependents.
The insured-based database is used widely and one of the popular real-world data in Japan.16 Japan has maintained a universal health coverage system since 1961. All medical information regarding clinical practice covered by this health insurance is included in the medical claims data, except for self-financed medical care and individuals who receive public assistance. Furthermore, medical facilities have been obliged since 2011 to submit medical claims data as an electronic record. Medical claims data include the names of the diagnosed diseases, the names of medical procedures and the names of prescribed medications, among others. In the present study, we extracted the age, sex, names and International Classification of Diseases 10th Revision (ICD-10) codes of diagnosed diseases, and hospitalisations and deaths from the medical claims data in HIA2CE from fiscal year (FY) 2014 to FY2018 (April 2014 to March 2019). The enrolment data from HIA2CE includes the medical characteristics and in-out information of insured persons as of April 2019.
Research design and study population
We prepared two data sets for analysis. The first was a cross-sectional data set containing baseline data of FY2014, which we used to describe the diagnosed disease prevalence in FY2014. The study population for this baseline data set included individuals aged 20–74 years insured in FY2014 (April 2014 to March 2015). Since HIA2CE is a type of insurance for workers in Japan, the database include only under 75 years individuals. Therefore, the maximum age in this cohort was 74 years. Participants younger than 20 in FY2014 as well as participants who died during FY2014 were excluded (figure 1). The cohort data set contained longitudinal data for a 5-year period, FY2014 to FY2018 (April 2014 to March 2019). The second data set contained participants insured in whole period. We used this cohort data set to conduct Cox regression analysis and calculate HRs for clinical events (figure 1).
Figure 1.
Participant selection flowchart. FY, fiscal year.
Definition of diagnosed diseases and multimorbidity
There are a variety of definitions for chronic conditions in multimorbidity studies.17–19 We used the Charlson Comorbidity Index (CCI) which is a validated tool to assess the diseases associated with a significant risk of clinical events.20 21 The reason, we used CCI, was that we focused on describing the prevalence of each disease and also assessing the association of multimorbidity on hospitalisation or death. The CCI Canadian version has been reported to be applicable to Japanese claims data.22 We therefore defined diagnosed diseases using medical claims data following ICD-10 codes of the CCI Canada version. We merged the conditions ‘diabetes with chronic complication’ and ‘diabetes without chronic complication’ into ‘diabetes mellitus’, and ‘mild liver disease’ and ‘moderate or severe liver disease’ into ‘liver disease’. The following 15 chronic conditions were included: AIDS/HIV, any malignancy (including lymphoma and leukaemia), cerebrovascular disease, chronic pulmonary disease, congestive heart failure, dementia, diabetes mellitus, hemiplegia or paraplegia, metastatic solid tumour, liver disease, myocardial infarction, peptic ulcer disease, renal disease and rheumatological disease. The ICD-10 codes of these diseases are shown in online supplemental eTable 1. Multimorbidity status was defined as the concurrent presence of two or more (≥2) diagnosed diseases among these conditions.23 24 We only used confirmed diagnoses, not including suspected diagnoses, in Japanese claims data.
bmjopen-2022-063216supp001.pdf (147KB, pdf)
Definition of outcome events: hospitalisation or death
We defined two composite outcomes, hospitalisation or death, which occurred during the period from FY2015 to FY2018. Using the medical claims data, both events were traced by month. In Japan, the validity of death event information is reported to be less sensitive if derived from medical claims data only.25 26 Therefore, we also used death information from enrolment data recorded by the insurer: if either contained death information, this was defined as a death event.
Estimation of diagnosed disease prevalence to a nationwide scale
Diagnosed disease prevalence from baseline data was standardised to the nationwide Japanese total population. We calculated prevalence rates according to groups by 5-year age brackets and sex. Then, we estimated the prevalence rates standardised to Japanese total population (age-sex standardised prevalence rate), using the number from the vital statistics 2014 in Japan.27
Association of multimorbidity with outcome by age group
To examine the association of multimorbidity with outcome by age group, we performed Cox regression analysis adjusted by sex using cohort data from four consecutive years (FY2015 to FY2018). The independent and additive effect of multimorbidity and ageing, we defined combined categories according to three age groups representing ‘young’, ‘middle’ and ‘old’ ages (20–39, 40–59 and ≥60, respectively) and the binary status of multimorbidity, with the reference set as no multimorbidity individuals aged 20–39. This model was able to show HR for ageing alone (eg, HR for 40–59 ages without multimorbidity vs 20–39 ages without multimorbidity) and complex of ageing and morbidity (eg, HR for 40–59 ages with multimorbidity vs 20–39 ages without multimorbidity).
Statistical analysis
Cox regression was conducted for the association of multimorbidity with outcome by age group. Our hypothesis was ageing and multimorbidity or these combination leads to worsen clinical events. Therefore, we defined six groups which were a combination of three categories of generation and multimorbidity, and we estimated HR in each group in reference of young (aged 20–39) without multimorbidity. Regarding this model, we interpreted both the independent impact of generation and multimorbidity on outcomes and the impact of multimorbidity in each generation. Results were considered statistically significant at a two-sided p value of less than 0.05. All analyses were conducted using Stata software V.15.1 (StataCorp).
Patient and public involvement
Patients or the public were not involved in this research. However, the results of this study will be disseminated to the public through various means including published papers and presentations.
Results
Study participants
We analysed n=246 671 individuals in the baseline data set in FY2014 (table 1) and n=181 959 individuals in the cohort data set FY2014–FY2018. Because the follow-up was 4 years, the cohort data set was slightly smaller than the baseline data set, especially as a number of young individuals aged 20–24 and older individuals aged >60 dropped out. This may be due to raising children or early retirement, and explains the higher proportion of men in the cohort data set. Mean age and comorbidity numbers among CCI diseases were mostly comparable between the two data sets, although the prevalence differed for diabetes mellitus, cerebrovascular disease and chronic pulmonary disease (online supplemental eTable 2). In the cohort data set, differences in disease prevalence between genders were observed. Notably, men had a higher prevalence of diabetes mellitus (p=0.001) whereas women had a higher prevalence of chronic pulmonary disease (p=0.002).
Table 1.
Prevalence of diagnosed diseases in the baseline data in FY2014
| Overall | Baseline data in FY2014 | |||||
| Men | Women | |||||
| N=2 46 671 | % | N=1 44 237 | % | N=1 02 434 | % | |
| Men | 144 237 | 58.5 | – | – | – | – |
| Age (mean, SD) | 45.0 | 12.9 | 44.6 | 12.9 | 45.7 | 12.8 |
| 20–24 | 18 524 | 7.5 | 11 315 | 7.8 | 7209 | 7.0 |
| 25–29 | 17 251 | 7.0 | 12 014 | 8.3 | 5237 | 5.1 |
| 30–34 | 18 093 | 7.3 | 11 104 | 7.7 | 6989 | 6.8 |
| 35–39 | 23 878 | 9.7 | 13 278 | 9.2 | 10 600 | 0.3 |
| 40–44 | 39 721 | 16.1 | 21 640 | 15.0 | 18 081 | 17.7 |
| 45–49 | 40 908 | 16.6 | 24 191 | 16.8 | 16 717 | 16.3 |
| 50–54 | 29 466 | 11.9 | 17 577 | 12.2 | 11 889 | 11.6 |
| 55–59 | 20 149 | 8.2 | 11 343 | 7.9 | 8806 | 8.6 |
| 60–64 | 21 278 | 8.6 | 12 706 | 8.8 | 8572 | 8.4 |
| 65–69 | 11 931 | 4.8 | 6768 | 4.7 | 5163 | 5.0 |
| 70–74 | 5472 | 2.2 | 2301 | 1.6 | 3171 | 3.1 |
| AIDS/HIV | 96 | 0.0 | 62 | 0.0 | 34 | 0.0 |
| Any malignancy* | 12 047 | 4.9 | 5611 | 3.9 | 6436 | 6.3 |
| Cerebrovascular disease | 10 866 | 4.4 | 6510 | 4.5 | 4356 | 4.3 |
| Chronic pulmonary disease | 43 216 | 17.5 | 22 484 | 15.6 | 20 732 | 20.2 |
| Congestive heart failure | 8497 | 3.4 | 5515 | 3.8 | 2982 | 2.9 |
| Dementia | 447 | 0.2 | 210 | 0.1 | 237 | 0.2 |
| Diabetes mellitus | 27 344 | 11.1 | 17 881 | 12.4 | 9463 | 9.2 |
| Hemiplegia or paraplegia | 813 | 0.3 | 533 | 0.4 | 280 | 0.3 |
| Liver disease | 27 127 | 11.0 | 16 954 | 11.8 | 10 173 | 9.9 |
| Metastatic solid tumour | 2532 | 1.0 | 1263 | 0.9 | 1269 | 1.2 |
| Myocardial infarction | 1628 | 0.7 | 1325 | 0.9 | 303 | 0.3 |
| Peptic ulcer disease | 26 047 | 10.6 | 14 511 | 10.1 | 11 536 | 11.3 |
| Peripheral vascular disease | 10 407 | 4.2 | 5723 | 4.0 | 4684 | 4.6 |
| Renal disease | 2573 | 1.0 | 1751 | 1.2 | 822 | 0.8 |
| Rheumatological disease | 4146 | 1.7 | 1397 | 1.0 | 2749 | 2.7 |
| ≥1 disease among top 5 | 71 880 | 29.1 | 40 833 | 28.3 | 31 047 | 30.3 |
| Disease no. among CCI | ||||||
| No disease | 171 140 | 69.4 | 101 857 | 70.6 | 69 283 | 67.6 |
| 1 disease | 22 947 | 9.3 | 12 032 | 8.3 | 10 915 | 10.7 |
| 2 diseases | 17 120 | 6.9 | 8994 | 6.2 | 8126 | 7.9 |
| 3 diseases | 12 822 | 5.2 | 7273 | 5.0 | 5549 | 5.4 |
| 4 diseases | 9588 | 3.9 | 5874 | 4.1 | 3714 | 3.6 |
| ≥5 diseases | 13 054 | 5.3 | 8207 | 5.7 | 4847 | 4.7 |
| Multimorbidity (≥2 diseases among CCI) | 52 584 | 21.3 | 30 348 | 21.0 | 22 236 | 21.7 |
Values are numbers (%) unless otherwise stated.
*Any malignancy includes leukaemia and lymphoma.
CCI, Charlson Comorbidity Index; FY, fiscal year.
Estimated prevalence of multimorbidity in the Japanese total population
The prevalence of diagnosed diseases in FY2014 was applied to the vital statistics of the Japanese population in 2014. The standardised prevalence of multimorbidity was estimated to 26.1% (26.1% in men, 26.0% in women) in the Japanese total population (online supplemental eTable 3A). The prevalence rate with age was increased, that is, approximately 5% (25–24 (3.9%), 25–29 (7.7%)), 10% (30–34 (9.7%), 35–39 (12.5%)), 20% (40–44 (14.6%), 45–49 (19.0%)), 30% (50–54 (25.9%), 55–59 (33.2%)), 50% (60–64 (40.7%), 65–69 (49.9%)) and 60% (70–74) (figure 2A). Details of the prevalence of diseases as well as the below results are shown in online supplemental eTable 3B. Figure 2B shows the types of diseases and their prevalence across age groups. The top five diseases across the age groups ‘young’ (20–39), ‘middle-aged (40–59) and ‘old’ (60–74) in order of prevalence were ‘young’: chronic pulmonary disease, peptic ulcer disease, liver disease, diabetes mellitus and any malignancy; ‘middle-aged’: chronic pulmonary disease, diabetes mellitus, liver disease, peptic ulcer disease and any malignancy; and ‘old’: diabetes mellitus, chronic pulmonary disease, liver disease, peptic ulcer disease and cerebrovascular disease. Notably, diabetes mellitus moved up across the age groups from ranking fourth to first. In figure 2C, disease prevalence is shown in comparison to disease prevalence in the 40–44 age group. After the age of 40–44, the top five accelerating diseases were dementia, cerebrovascular disease, peripheral vascular disease, metastatic tumour and congestive heart failure.
Figure 2.
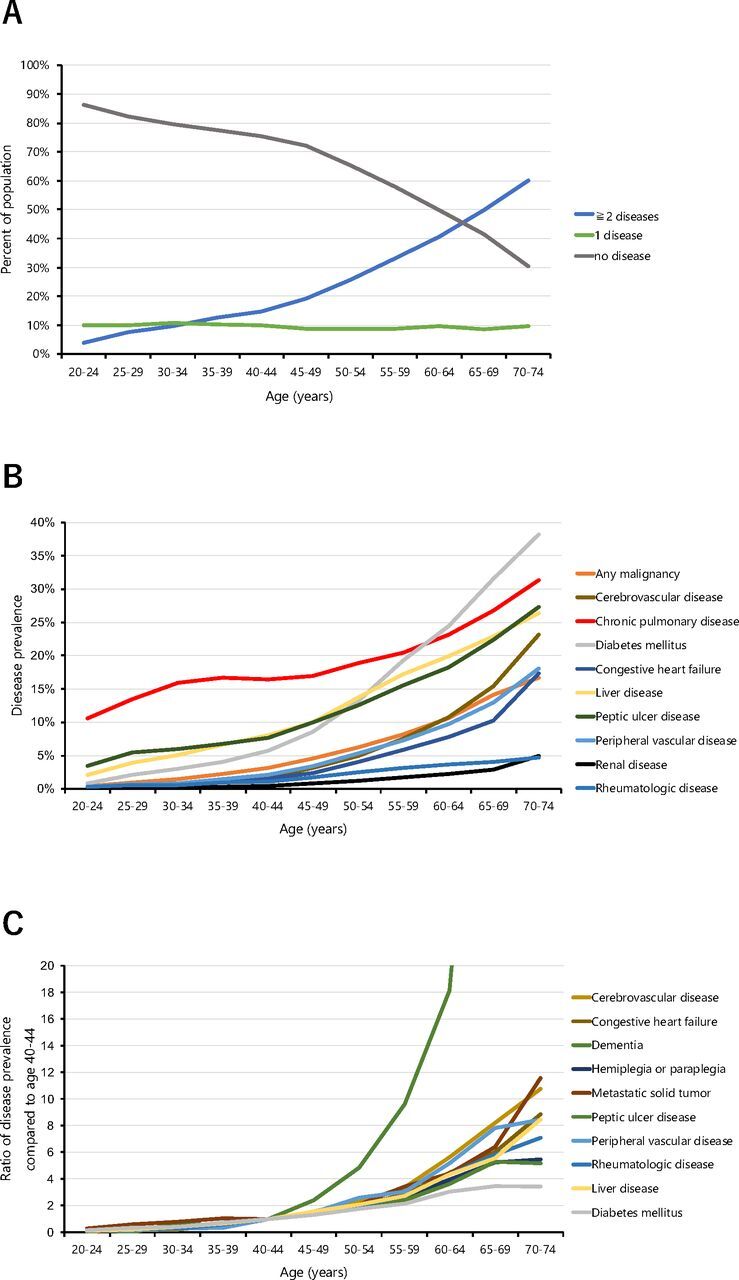
Multimorbidity across age groups in the Japanese total population aged 20–74. (A) Percentage of the population having 0 to ≥5 chronic diseases by age group. (B) Prevalence of the top 10 chronic diseases by age group. (C) The top 10 chronic diseases with the steepest increase after age 40–44 years.
The association of multimorbidity with outcome by age group.
The composite outcomes occurred 17.2% (death 0.8%, hospitalisation 16.9%) in the follow-up period (online supplemental eTable 2). Cox regression analysis showed that young individuals aged 20–39 with multimorbidity had a higher HR compared with the same age group without multimorbidity (HR=2.43 (95% CI 2.30 to 2.56)). Further, HRs increased across age groups (HR=2.55 (95% CI 2.47 to 2.63) ages 40–59; HR=3.41 (95% CI 3.23 to 3.53) ages ≥60) (figure 3). The impact of multimorbidity on outcome exceeded that of ageing (HR=1.62 (95% CI 1.56 to 1.69) ages ≥60 and HR=1.10 (95% CI 1.07 to 1.13) ages 40–59 without multimorbidity) (figure 3). That was to say, even in aged 20–39 with multimorbidity has a risk more than ages≥60 without multimorbidity.
Figure 3.
Hazard ratios of no multimorbidity (0–1 disease) versus multimorbidity (≥2 diagnosed diseases) in three age groups in a 5-year cohort of n=181 959 Japanese aged 20–71. Cox regression analysis.
We also assessed HRs for non-multimorbid and multimorbid women and men separately and found that women had a lower HR than men in the 20–39 age group but a higher HR than men in the ≥60 age group (online supplemental eTable 4).
Discussion
In this study, we analysed nationwide medical claims data for 15 chronic diseases in a large cohort of the general population of Japan. As key findings, standardised prevalence rates for multimorbidity were estimated to 26.1% for men and 26.0% for women. Further, age group-specific prevalence rates for multimorbidity ranged from 3.9% (20–24 years) to 14.6% (40–44 years) and 60.1% (70–74 years), showing an accelerating increase after age 40. Importantly, significant differences in the clinical outcomes of multimorbidity versus no multimorbidity were already present in young and middle-aged individuals.
The present study drew individuals covering a wide age range from a nationwide general population. This allowed us to examine the burden of multiple comorbidities in young, middle-aged and old age groups in the real world. In addition, because Japan has a high medical insurance coverage rate, it was possible to comprehensively identify chronic diseases from receipts. Further, longitudinal analysis enabled us to examine the clinical outcomes of multiple comorbidities. With regard to limitations, the target population comprised regular employees and their families and might accordingly be healthier than the general population. Also, we defined multimorbidity by disease list included in CCI most likely to lead to death, hence, we were not able to consider other diseases associated with health-related quality of life loss. CCI originally includes comorbidities that have a strong impact on mortality, not quality of life and well-being. The presence of mental or psychosomatic disorders, which have been shown to be increasing, particularly in individuals already suffering from other chronic diseases,28 younger people29 and people with a low socioeconomic status.30 Such diseases often remain undiagnosed or under-reported in health records.31 Also, we collected diseases which were occurred during the year (FY2014). Therefore, patients who were untreated, undiagnosed or discontinued treatment cannot be picked up. These limitations likely contributed to an underestimation of multimorbidity in our cohort. Further, because we did not manually verify the presence of disease using the physician’s medical records data or medication information, disease names extracted from the medical claims data might be incorrect in some cases. In particular, Japanese physicians sometimes change the name of the disease in the medical record to the ‘correct’ disease name for the medication they wish to prescribe, a practice called ‘disease name for claims data’.
Because of differences in data sources and study populations, direct comparison of population-based prevalence rates between studies is not straightforward. Nonetheless, the standardised prevalence rates for multimorbidity as reported in the present study—26.1% for men and 26.0% for women—are similar to those reported by recent studies in other high-income countries, such as the USA (25% in men, 25% in women),32 England (24.4% in men, 30% in women),30 Canada (24.3% whole population)5 and Denmark (19.3% in men, 23.7% in women).6 Also recently some Asian countries reported similar prevalence, Iran (13.4% in men, 25.0% in women),33 India and Bangladesh (53.7%–56.5% in both genders, over aged 60).34 Many previous studies on multimorbidity focused on the older generation, aged 65 and up, because of the larger number of chronic diseases in this age group and the increasing number of people entering it. However, our present data show that already approximately 10% of 30–34, 19% of 45–49 and 33% of 55–59 years old have ≥2 chronic diseases. Further, 1% of 30–34, 4% of 45–49 and 9% of 55–59 years old have ≥5 chronic diseases. These results show that multimorbidity is already prominent in the middle-aged population. Recent studies reported similar or slightly higher prevalence rates for ≥2 chronic diseases in an American (8% of 30, 20% of 45 and 37% of 55 years old)35 and a Canadian (10.6% of 18–44, 27.4% of 45–54 and 46.6% of 55–64 years old)5 population, although these two studies also included mental diseases and osteoporosis, which our present study did not. Our present study shows that, among 15 chronic diseases, the top five diseases in the 55–64 age group are chronic pulmonary disease (20.4%–23.1%), diabetes mellitus (19.3%–24.5%), liver disease (17.2%–19.9%), peptic ulcer disease (15.6%–18.2%) and any malignancy (8.2%–10.7%). With regard to diabetes mellitus, the prevalence in the present study is similar to that previously reported in an American population (15%–30% in individuals aged 55–65)35 but higher than that in a Canadian population (16.6% in individuals aged 55–64).5 The prevalence of chronic pulmonary disease in our present 55–65 years old was almost two times as high as those seen for the combined prevalence of asthma and chronic obstructive pulmonary disease (COPD) in an American population aged 55–65 years (2–3%)35 and in a Canadian population aged 55–64 years (3.3%).5 This difference might have arisen due to our inclusion of various other pulmonary diseases besides asthma and COPD. Regarding liver disease, the prevalence seen for 55–65 years old in the present study was comparable to that seen in an adult population in Northern Italy36 and in an adult population in Korea,37 although this comparison requires care since the types of liver disease in these studies and the age groups included vary.
Analysis of clinical outcomes using Cox regression revealed that the presence of multimorbidity increased HRs in all age groups, including young individuals. In addition, the comparison of the increased HRs resulting from multimorbidity versus no multimorbidity showed that the impact of multimorbidity exceeds that of increasing age. These results indicate that multimorbidity places a burden on all age groups.
The five most prevalent diseases (diabetes mellitus, chronic pulmonary disease, liver disease, peptic ulcer disease and any malignancy) in the present study are lifestyle-related diseases that develop slowly over time. This trend should be greeted with alarm. We trust that this study raises awareness of the potential health risks and burden associated with the early onset of multimorbidity in young and middle-aged, the period when one is busy working and raising children. Future studies should investigate the specific lifestyle factors associated with an elevated risk of multimorbidity in the Japanese working population. Ultimately, public healthcare policies should be aimed at efforts to reverse the trend toward early multimorbidity onset.
In conclusion, the present study confirmed the prevalence of multimorbidity by including in the denominator those who did not have the receipt of medical claims and to estimate the prevalence of multimorbidity in the general population. Furthermore, we revealed that the impact of multimorbidity is already clinically significant in middle-aged Japanese, with elevated adverse events such as hospitalisation or death. In addition, the risk posed by multimorbidity exceeds that of ageing in all age groups. These results underscore the need to undertake healthcare intervention against the onset of multimorbidity before middle-aged, and not to leave it as a problem for geriatricians.
Supplementary Material
Acknowledgments
The authors are grateful to HIA2CE for providing data for the present study.
Footnotes
Contributors: Concept and design: YS, SF. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: YS, SF. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: YS, SF. Obtained funding: YS. Administrative, technical or material support: YS, SF. Study supervision: SF, AI, TN. YS as the guarantor had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors gave final approval and agreed to be accountable for all aspects of work.
Funding: This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 19K19458 (YS).
Disclaimer: The sponsor had no role in the design of the study; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Institutional Review Board (IRB) of Kyoto University (approval number: R0817). The IRB waived informed consent for this observational study.
References
- 1. Bleich SN, Sherrod C, Chiang A, et al. Systematic review of programs treating high-need and high-cost people with multiple chronic diseases or disabilities in the United States, 2008-2014. Prev Chronic Dis 2015;12:E197. 10.5888/pcd12.150275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hajat C, Stein E. The global burden of multiple chronic conditions: a narrative review. Prev Med Rep 2018;12:284–93. 10.1016/j.pmedr.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prathapan S, Fernando GVMC, Matthias AT, et al. The rising complexity and burden of multimorbidity in a middle-income country. PLoS One 2020;15:e0243614. 10.1371/journal.pone.0243614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Low LL, Kwan YH, Ko MSM, et al. Epidemiologic characteristics of multimorbidity and sociodemographic factors associated with multimorbidity in a rapidly aging Asian country. JAMA Netw Open 2019;2:e1915245. 10.1001/jamanetworkopen.2019.15245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pefoyo AJK, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health 2015;15:415. 10.1186/s12889-015-1733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schiøtz ML, Stockmarr A, Høst D, et al. Social disparities in the prevalence of multimorbidity-a register-based population study. BMC Public Health 2017;17:422. 10.1186/s12889-017-4314-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sum G, Ishida M, Koh GC-H, et al. Implications of multimorbidity on healthcare utilisation and work productivity by socioeconomic groups: cross-sectional analyses of Australia and Japan. PLoS One 2020;15:e0232281. 10.1371/journal.pone.0232281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bähler C, Huber CA, Brüngger B, et al. Multimorbidity, health care utilization and costs in an elderly community-dwelling population: a claims data based observational study. BMC Health Serv Res 2015;15:23. 10.1186/s12913-015-0698-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu R-H, Hsiao F-Y, Chen L-J, et al. Increasing age- and gender-specific burden and complexity of multimorbidity in Taiwan, 2003-2013: a cross-sectional study based on nationwide claims data. BMJ Open 2019;9:e028333. 10.1136/bmjopen-2018-028333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lenzi J, Avaldi VM, Rucci P, et al. Burden of multimorbidity in relation to age, gender and immigrant status: a cross-sectional study based on administrative data. BMJ Open 2016;6:e012812. 10.1136/bmjopen-2016-012812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Picco L, Achilla E, Abdin E, et al. Economic burden of multimorbidity among older adults: impact on healthcare and societal costs. BMC Health Serv Res 2016;16:173. 10.1186/s12913-016-1421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van den Bussche H, Schön G, Kolonko T, et al. Patterns of ambulatory medical care utilization in elderly patients with special reference to chronic diseases and multimorbidity-results from a claims data based observational study in Germany. BMC Geriatr 2011;11:54. 10.1186/1471-2318-11-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katikireddi SV, Skivington K, Leyland AH, et al. The contribution of risk factors to socioeconomic inequalities in multimorbidity across the lifecourse: a longitudinal analysis of the twenty-07 cohort. BMC Med 2017;15:152. 10.1186/s12916-017-0913-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kone AP, Mondor L, Maxwell C, et al. Rising burden of multimorbidity and related socio-demographic factors: a repeated cross-sectional study of Ontarians. Can J Public Health 2021;112:737–47. 10.17269/s41997-021-00474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singer L, Green M, Rowe F, et al. Trends in multimorbidity, complex multimorbidity and multiple functional limitations in the ageing population of England, 2002-2015. J Comorb 2019;9:2235042X19872030. 10.1177/2235042X19872030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hiramatsu K, Barrett A, Miyata Y, et al. Current status, challenges, and future perspectives of real-world data and real-world evidence in Japan. Drugs Real World Outcomes 2021;8:459–80. 10.1007/s40801-021-00266-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee ES, Koh HL, Ho EQ-Y, et al. Systematic review on the instruments used for measuring the association of the level of multimorbidity and clinically important outcomes. BMJ Open 2021;11:e041219. 10.1136/bmjopen-2020-041219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 19. Jürisson M, Pisarev H, Uusküla A, et al. Prevalence of chronic conditions and multimorbidity in Estonia: a population-based cross-sectional study. BMJ Open 2021;11:e049045. 10.1136/bmjopen-2021-049045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 21. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge Abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 22. Sundararajan V, Quan H, Halfon P, et al. Cross-National comparative performance of three versions of the ICD-10 Charlson index. Med Care 2007;45:1210–5. 10.1097/MLR.0b013e3181484347 [DOI] [PubMed] [Google Scholar]
- 23. Johnston MC, Crilly M, Black C, et al. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health 2019;29:182–9. 10.1093/eurpub/cky098 [DOI] [PubMed] [Google Scholar]
- 24. Le Reste JY, Nabbe P, Manceau B, et al. The European general practice research network presents a comprehensive definition of multimorbidity in family medicine and long term care, following a systematic review of relevant literature. J Am Med Dir Assoc 2013;14:319–25. 10.1016/j.jamda.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 25. Ooba N, Setoguchi S, Ando T, et al. Claims-based definition of death in Japanese claims database: validity and implications. PLoS One 2013;8:e66116. 10.1371/journal.pone.0066116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakai M, Ohtera S, Iwao T, et al. Validation of claims data to identify death among aged persons utilizing enrollment data from health insurance unions. Environ Health Prev Med 2019;24:63. 10.1186/s12199-019-0819-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Statistics Bureau of Japan . Preliminary count of the Japanese population. 2014. Available: http://www.stat.go.jp/data/jinsui/index.html
- 28. Aoki T, Yamamoto Y, Shimizu S, et al. Physical multimorbidity patterns and depressive symptoms: a nationwide cross-sectional study in Japan. Fam Med Community Health 2020;8:e000234. 10.1136/fmch-2019-000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry 2007;29:409–16. 10.1016/j.genhosppsych.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 30. Cassell A, Edwards D, Harshfield A, et al. The epidemiology of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2018;68:e245–51. 10.3399/bjgp18X695465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Violán C, Foguet-Boreu Q, Hermosilla-Pérez E, et al. Comparison of the information provided by electronic health records data and a population health survey to estimate prevalence of selected health conditions and multimorbidity. BMC Public Health 2013;13:251. 10.1186/1471-2458-13-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. St Sauver JL, Boyd CM, Grossardt BR, et al. Risk of developing multimorbidity across all ages in an historical cohort study: differences by sex and ethnicity. BMJ Open 2015;5:e006413. 10.1136/bmjopen-2014-006413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alimohammadian M, Majidi A, Yaseri M, et al. Multimorbidity as an important issue among women: results of a gender difference investigation in a large population-based cross-sectional study in West Asia. BMJ Open 2017;7:e013548. 10.1136/bmjopen-2016-013548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pati S, Swain S, Hussain MA, et al. Prevalence and outcomes of multimorbidity in South Asia: a systematic review. BMJ Open 2015;5:e007235. 10.1136/bmjopen-2014-007235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and race/ethnicity. Mayo Clin Proc 2014;89:1336–49. 10.1016/j.mayocp.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bellentani S, Tiribelli C, Saccoccio G, et al. Prevalence of chronic liver disease in the general population of northern Italy: the dionysos study. Hepatology 1994;20:1442–9. 10.1002/hep.1840200611 [DOI] [PubMed] [Google Scholar]
- 37. Park SH, Plank LD, Suk KT, et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998-2017. Clin Mol Hepatol 2020;26:209–15. 10.3350/cmh.2019.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063216supp001.pdf (147KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available.