Abstract
Chimeric antigen receptor (CAR) T-cells are an emerging therapy for the treatment of relapsed/refractory B-cell malignancies. While CD19 CAR-T cells have been FDA-approved, CAR T-cells targeting CD22, as well as dual-targeting CD19/CD22 CAR T-cells, are currently being evaluated in clinical trials. This systematic review and meta-analysis aimed to evaluate the efficacy and safety of CD22-targeting CAR T-cell therapies. We searched MEDLINE, EMBASE, Web of Science, and the Cochrane Central Register of Controlled Trials from inception to March 3rd 2022 for full-length articles and conference abstracts of clinical trials employing CD22-targeting CAR T-cells in acute lymphocytic leukemia (ALL) and non-Hodgkin’s lymphoma (NHL). The primary outcome was best complete response (bCR). A DerSimonian and Laird random-effects model with arcsine transformation was used to pool outcome proportions. From 1068 references screened, 100 were included, representing 30 early phase studies with 637 patients, investigating CD22 or CD19/CD22 CAR T-cells. CD22 CAR T-cells had a bCR of 68% [95% CI, 53-81%] in ALL (n= 116), and 64% [95% CI, 46-81%] in NHL (n= 28) with 74% and 96% of patients having received anti-CD19 CAR T-cells previously in ALL and NHL studies respectively. CD19/CD22 CAR T-cells had a bCR rate of 90% [95% CI, 84-95%] in ALL (n= 297) and 47% [95% CI, 34-61%] in NHL (n= 137). The estimated incidence of total and severe (grade ≥3) CRS were 87% [95% CI, 80-92%] and 6% [95% CI, 3-9%] respectively. ICANS and severe ICANS had an estimated incidence of 16% [95% CI, 9-25%] and 3% [95% CI, 1-5%] respectively. Early phase trials of CD22 and CD19/CD22 CAR T-cells show high remission rates in ALL and NHL. Severe CRS or ICANS were (1)rare and dual-targeting did not increase toxicity. Variability in CAR construct, dose, and patient factors amongst studies limits comparisons, with long-term outcomes yet to be reported.
Systematic review registration
https://www.crd.york.ac.uk/prospero, identifier CRD42020193027.
Keywords: CAR T-cell, CD22, B-cell malignancies, efficacy, safety, systematic review & meta-analysis
Introduction
The treatment of relapsed/refractory (R/R) B-cell malignancies remains a challenge. Among patients with B-cell acute lymphoblastic leukemia (B-ALL) who have failed standard induction chemotherapy, only 45% achieve complete remission with salvage chemotherapy, and one-year survival is only 26% (1). Likewise, in diffuse large B-cell lymphoma (DLBCL), patients with refractory disease have a CR rate of 7% with salvage chemotherapy and a one-year survival of 28% (2). The emergence of chimeric antigen receptor (CAR) T-cell immunotherapy, in which T-cells are genetically engineered to express CARs targeting specific tumor-associated antigens, has significantly changed the treatment of these R/R diseases. CD19 CAR T-cells, the most well-established B-cell antigen target, demonstrated promising responses in clinical trials, with CR rates of 70-90% in B-ALL and 50% in certain Non-Hodgkin’s lymphoma (NHL) patients (3–6). Today, there are four FDA-approved CD19 CAR T-cell therapies approved for use in NHL (axi-cel, tisa-cel, liso-cel, and brexu-cel), with brexu-cel now also approved for use in patients under 25 with ALL (6–14).
Despite these impressive results, around 30% of patients fail to respond to CD19 CAR T-cells, and 36-57% of patients who achieve CR relapse within one year (5, 11, 15). In particular, a subset of relapses are associated with loss of CD19 expression or escape splice variants on malignant cells, with prior studies finding 16-68% of relapses to be CD19-negative (11, 15, 16). Alternate CAR targets for B-cell malignancies are now being explored, including CD22, CD20, CD79b and BAFF-R (17–23). Among these, CD22 has been the focus of a large number of clinical trials in recent years, both as a single target and as part of dual-targeting CD19/CD22 CAR T-cells. One theoretical advantage of dual-targeting is the prevention of antigen-negative relapse.
The recent influx of CD22 and CD19/CD22 CAR T-cell therapies entering clinical trials warrants a systematic review to evaluate their efficacy and to assess the risk of adverse events that are common in CAR T-cell therapies, such as cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS). There is limited synthesis of clinical trial findings, with the majority of prior systematic reviews focusing on CD19 CAR T-cells (24–26). Grigor et al. and Yu et al. conducted broader reviews of all CAR T-cells, but only included studies up to late 2017 and early 2018, respectively, and as such both only include one CD22 CAR T-cell trial (5, 27). Li et al. is the only systematic review we identified that focused on CD22 and/or CD19/CD22 CAR T-cell therapies, however, it only included ten studies (28). We conducted a preliminary scan and found that a significant amount of CD22 CAR T-cell clinical trial data is currently published only in the form of conference abstracts, which were not included in the review by Li et al. As such, a meta-analysis including data from abstracts will provide a more comprehensive review of current findings.
We conducted a systematic review and meta-analysis of CAR T-cells targeting CD22, alone or in combination with other antigen targets, to evaluate their efficacy and safety in the treatment of patients with B-cell malignancies.
Methods
Registration
This systematic review and meta-analysis was conducted in accordance with the PRISMA guidelines (details in Supplementary Materials ) (29). The protocol was prospectively registered in PROSPERO (CRD42020193027), and the full protocol is published in a peer-reviewed journal (30).
Eligibility criteria, data sources and search strategy
Interventional studies, with or without a comparator, on CD22 CAR T-cell therapy in patients with B-cell malignancies were eligible for inclusion. This included studies investigating multi-target therapies, such as multi-targeted CD19 and CD22 CAR T-cells (CD19/CD22 CAR T-cell therapy). Only studies that reported the primary outcome of interest, complete response (CR), were included. Full-length articles, conference abstracts, letters and case reports were considered, while reviews, editorials, and commentaries were excluded. Studies for which an associated clinical trial could not be identified (using a clinical trial registration number) were excluded to avoid double-counting participants.
MEDLINE, EMBASE, Web of Science, and the Cochrane Central Register of Controlled Trials were searched from inception to March 3 2022. Additionally, the conference proceedings of the American Society of Hematology, American Society of Clinical Oncology, and European Hematology Association were searched manually. Bibliographies of all included studies were also searched. The search strategy was created in collaboration with an experienced health science librarian. No language restrictions were applied. The full search strategy can be found in the Supplementary Materials . In addition to the search of study reports, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) were searched to catalogue any relevant registered clinical trials.
Study selection
Search results were uploaded to Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Title and abstract screening, full-text screening, data extraction and risk of bias assessment were conducted by two reviewers in duplicate (N.J.F and K.A). Disagreements were resolved by discussion or a third reviewer (K.A.H. and H.A.). Within included reports, multiple reports of the same study were identified and grouped by associated clinical trial number. For each study, the most recent full-length article was used as the primary report for data extraction, with any other reports cross-referenced for Supplemental Information . If no full-length article existed for a given study, the most recent conference abstract or case report was used as the primary report.
Data items and extraction
A piloted form on Covidence was used for data extraction. Publication, study, patient and intervention characteristics as well as manufacturing, efficacy, and safety outcomes were extracted. Health Related Quality of Life (HRQoL) or Patient-Reported Outcomes (PRO) were also sought.
The primary outcome extracted for meta-analysis was best CR rate (bCR), defined as the proportion of patients reported to have achieved CR at any point during follow-up; one-month CR and three-month CR rate were also extracted. Secondary efficacy outcomes included overall response, relapse rate, and time-to-event data (overall survival and progression-free survival). Safety outcomes included reported incidence of adverse events (CRS, ICANS, graft-versus-host disease, infection, and other reported adverse events) and 30-day mortality rate. Full details of these and other data items are available in the previously published protocol (30). Study quality was assessed using a modified Institute of Health Economics (IHE) risk of bias tool (31).
Data synthesis and analysis
Meta-analysis was deemed appropriate for bCR, CRS, and ICANS; other outcomes are synthesized narratively as there was significant variation in reporting by included studies. Meta-analyses were conducted using R statistical software (v.4.2.2). Binary outcomes are presented as proportions with 95% confidence intervals (CI). A random effects model (DerSimonia and Laird) was employed to pool proportions using an arcsine-based transformation (metaprop function from R package metafor). Given the prevalence of low and high event rates in our data, an arcsine transformation was deemed more appropriate than a logit transformation (32). Cochrane I2 statistic is used to assess statistical heterogeneity between summary data.
Subgroup analysis was undertaken using a meta-regression technique (metareg function from the R package metafor). Pre-specified subgroups of interest were malignancy type, single vs. dual targeting therapy, age group, and previous therapy (previous transplant, previous CD19 CAR T-cell therapy, previous non-CAR-T cell immunotherapy). A sensitivity analysis was performed by removing data from conference abstracts and evaluating the effect on the results. An alternative funnel plot (study size vs arcsine transformed outcome proportion) was used to assess publication bias (33). The GRADE approach was used to evaluate confidence in treatment effects (34).
Results
Results of search
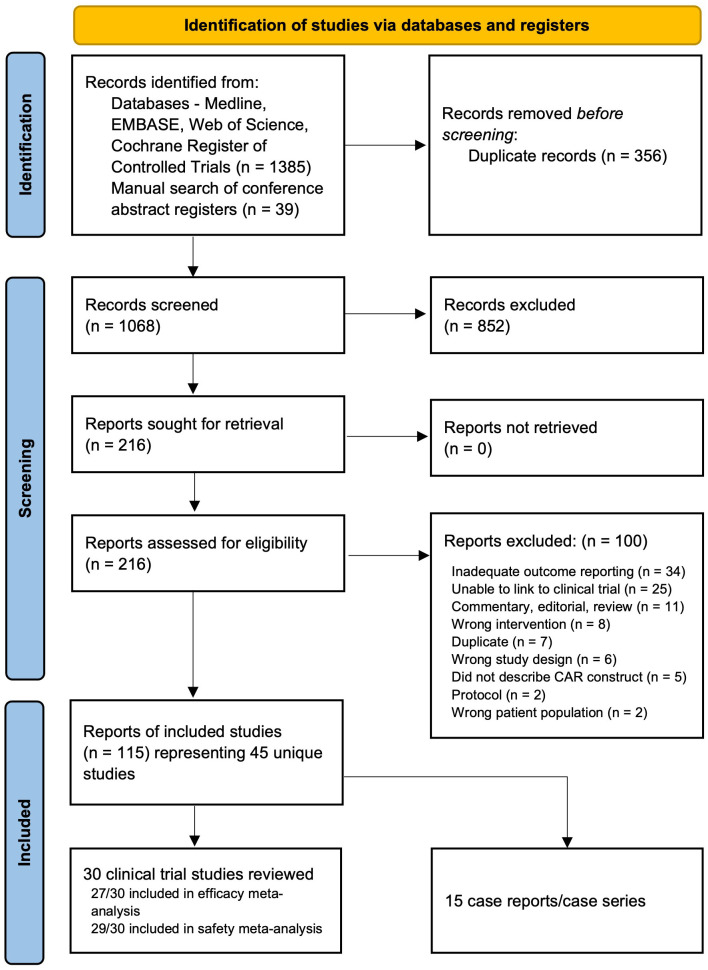
From 1068 unique references, 115 references were included in our review, representing 45 unique studies ( Figure 1 ) (35–79). Among these, 29 were eligible for meta-analysis (27 studies with a total of 578 patients for efficacy analysis and 29 studies with a total of 637 patients for safety analysis); and 15 were treated as case reports/series. Of note Liu 2021A was excluded from both the response and safety meta-analyses due to heterogeneity in study design and insufficient safety data, however is summarized narratively and in our tabular data synthesis. Details of the studies included in the meta-analysis are presented in Table 1 . All studies were early-phase single-arm clinical trials. A full list of included references grouped by study can be found in Supplementary Materials . Outcomes from case reports and case series are summarized separately in Supplementary Table 1 .
Figure 1.
PRISMA flow diagram of references identified, screened, excluded (with reasons) and included.
Table 1.
Study Characteristics of Included Clinical Trials.
| Study ID | Clinical Trial No. | Country | Phase | Publication Type | Malignancy | Antigen Target |
|---|---|---|---|---|---|---|
| Annesley 2021 | NCT03330691 | USA | 1 | Abstract | ALL | CD19/CD22 |
| Baird 2021 | NCT04088890 | USA | 1 | Letter | LBCL | CD22 |
| Cao 2021 | ChiCTR-OPN-16009847 | China | 0 | Full Report | NHL | CD19/CD22 |
| Cordoba 2021 | NCT03289455 | UK | 1 | Full Report | ALL | CD19/CD22 |
| Dai 2020 | NCT03185494 | China | 1 | Full Report | ALL | CD19/CD22 |
| Frey 2021 | NCT03620058 | USA | 1 | Abstract | ALL | CD19/CD22 |
| Gardner 2020 | NCT03330691 | USA, Canada | 1 | Abstract | ALL | CD19/CD22 |
| Hu 2021 | NCT04227015 | China | 1 | Full Report | ALL | CD19/CD22 |
| Liu 2021 A | ChiCTR-ONC-17013648 | China | 1 | Full Report | ALL | CD19/CD22 |
| Liu 2021 B | NCT03614858 | China | 1/2 | Abstract | ALL | CD19/CD22 |
| Liu 2022 | ChiCTR1800014457 | China | 1 | Full Report | NHL | CD19/CD22/ CD20 |
| Pan 2019 | ChiCTR-OIC-17013523 | China | 1 | Full Report | ALL | CD22 |
| Pan 2020 | ChiCTR-OIB-17013670 | China | 1 | Letter | ALL | CD19/CD22 |
| Ramakrishnan 2020 | NCT03287817 | UK, USA | 1 | Abstract | DLBCL | CD19/CD22 |
| Schultz 2018 | NCT03241940 | USA | 1 | Abstract | ALL | CD19/CD22 |
| Shah 2020 | NCT02315612 | USA | 1 | Full Report | ALL | CD22 |
| Shalabi 2020 | NCT03448393 | USA | 1 | Abstract | ALL | CD19/CD22 |
| Singh 2021† | NCT02650414 and NCT02588456 | USA | 1 | Full Report | ALL | CD22 |
| Speigel 2021 | NCT03233854 | USA | 1 | Full Report | ALL, LBCL | CD19/CD22 |
| Summers 2021†† | NCT03244306 (V1) and NCT04571138 (V2) | USA | 1 | Abstract | ALL | CD22 |
| Tan 2021 | ChiCTR2000028793 | China | 1 | Letter | ALL | CD22 |
| Wang 2020 | ChiCTR-OPN-16008526 | China | 1 | Full Report | ALL, NHL | CD19/CD22 |
| Wang 2021 | ChiCTR2000032211 | China | 0 | Full Report | ALL | CD19/CD22 |
| Wei 2021 | ChiCTR1800015575 | China | 1 | Full Report | ALL, NHL | CD19/CD22 |
| Yang 2018 | NCT03312205 | China | 1 | Abstract | ALL | CD19/CD22 |
| Yang 2020 | NCT04129099 | China | 1 | Abstract | ALL | CD19/CD22 |
| Yang 2019 | NCT03825731 | China | 1 | Abstract | ALL | CD19/CD22 |
| Zhang 2021 A | NCT03196830 | China | 2 | Full Report | NHL | CD19/CD22 |
| Zhang 2021 B | NCT04539444 | China | 2 | Abstract | NHL | CD19/CD22 |
| Zhu 2021 | ChiCTR1800019298 | China | 1 | Full Report | ALL, DLBCL | CD22 |
ALL, acute lymphoblastic leukemia; NHL, Non-Hodgkin’s Lymphoma; LBCL, large B-cell lymphoma; DLBCL, diffuse large B-cell lymphoma. †Singh 2021 reports combined results of an adult and pediatric study investigating the same intervention. ††Summers 2021 reports results of an initial (V1) and optimized (V2) CAR construct.
Patient characteristics
The majority of studies examined R/R B-ALL patients as the population of interest, however a significant subset of studies examined R/R NHL. Among the seven CD22 CAR T-cell studies, five included only B-ALL patients, one included B-NHL, and one examined both B-ALL and B-NHL. Among the 23 studies examining CD19/CD22 CAR T-cells, 15 included only B-ALL patients, 5 included B-NHL patients, and 3 included both B-ALL and B-NHL patients. Patient demographics, disease status, and prior therapies are presented in Table 2 . Among CD22 CAR T-cell studies that provided information on prior CAR T-cell therapy (5 out of 7 studies), the majority of patients had received prior CD19 CAR T-cells (108/137, 79%). This includes Zhu 2021 who included only patients who had relapsed post-CD19 CAR T-cells. In comparison, prior CD19 CAR T-cell therapy was less commonly reported in CD19/CD22 CAR T-cell studies, with only 11% of patients (20/176) having had prior CD19 CAR-T cell therapy among 11 studies, with the remaining 12 studies not providing information on prior CAR T-cell therapies. Many patients had received other prior therapies, including hematopoietic stem cell transplant (HCT) and targeted immunotherapies such as blinatumomab and inotuzumab.
Table 2.
Intervention Characteristics of Included Clinical Trials.
| Study ID | Method of co-targeting | sCFV domain | Costimulatory Domain | T-cell source | CAR T-cell Dose in cells/kg (except where marked * indicating non-weight based dose) |
|---|---|---|---|---|---|
| CD22 | |||||
| Pan 2019 | N/A | YK-CD22 | 41BB | Auto/allo† | Median 5 x105, range 0.2-34.7 |
| Shah 2020 | N/A | m971 | 41BB | Auto | DL1: 3x105, DL2: 1x106, DL3: 3x106 |
| Singh 2021 | N/A | m971 (long linker) | 41BB | Auto | Patients <50 kg: 1–10 x 106 cells/kg. Patients >50 kg: 5.0 x 108 cells total* in fractioned adaptive dosing scheme. |
| Summers 2021 | N/A | m971 | 41BB | Auto | V1 DL1: 1 x 106, DL2: 3 x 106
V2: 2 x 105 |
| Tan 2021 | N/A | FH80 | 41BB | Auto | Median: 1.2 x 106, range 0.68-9.4 |
| Zhu 2021 | N/A | NR | 41BB | Auto | 2.0 x 106 |
| Baird 2021 | N/A | m971 | 41BB | Auto | DL1: 1 x 106 (n = 12), DL2: 3 x 106 (n = 9) |
| CD19/CD22 | |||||
| Annesley 2021 | Co-transduction | NR | CD19: 41BB CD22: 41BB |
Auto | DL1: 0.5 x 106 (n = 3) DL2: 1 x 106 (n = 3) DL3: 3 x 106 (n =6) |
| Cordoba 2021 | Bicistronic vector | Humanized | CD19: OX40 CD22: 41BB |
Auto | DL1: 1x106 (n = 2) DL2: 3x106 (n = 5) DL3: 5x106 (n = 5) |
| Dai 2020 | Bivalent CAR | CD19: FMC63 CD22: m971 |
41BB | Auto | Mean: 2.28 x106 (range: 1.7-3) |
| Frey 2021 | Co-administration | Humanized | CD19: 41BB CD22: 41BB |
Auto | Planned CD19 dose: 2.0 x 106
Planned CD22 dose: 2.0 x 106 With fractionated adaptive dosing scheme. |
| Gardner 2020 | Co-transduction | CD19: FMC63 CD22: m971 |
CD19: 41BB CD22: 41BB |
Auto | DL1: 1x106
DL2: 3x106 |
| Hu 2021 | Bivalent CAR | CD19: FMC63 CD22: m971 |
41BB | Allo (UCART)†† | DL1: 1 x 106
DL2: 3 x 106 |
| Liu 2021 A | Sequential infusion (months) | CD19: FMC63 CD22: Human phage library |
CD19: 41BB CD22: 41BB |
Auto | Median CD19: 1.0 x 105 (range 0.486 - 5.0) Median CD22: 2.0 x 105 (range 0.32 - 5.0) |
| Liu 2021 B | Group 1: Tandem Group 2: Sequential |
NR | NR | NR | NR |
| Pan 2020 | Sequential infusion (months) | CD19: FMC63 CD22: YK-CD22 |
CD19: 4-1BB CD22: 4-1BB |
Auto | CD19: 10 x 105 (range 3.3 - 42.8) CD22: 10 x 105 (range, 0.25 - 47.4) |
| Schultz 2018 | Bivalent CAR | CD19: FMC63 CD22: m971 |
41BB | Auto | DL1: 1 x 106
Subsequent doses not yet reported |
| Shalabi 2020 | Bivalent CAR | CD19: FMC63 CD22: m971 |
41BB | Auto | DL1: 3 x 105
DL2: 1 x 106 DL3: 3 x 106 |
| Speigel 2021 | Bivalent CAR | CD19: FMC63 CD22: m971 |
41BB | Auto | DL1: 1 x 106
DL2: 3 x 106 |
| Wang 2020 | Sequential infusion (days) | NR | CD19: CD28, 4-1BB CD22: CD28, 4-1BB |
Auto | ALL: CD19: 2.5 x 106, CD22: 2.5 x 106
NHL: CD19: 5 x 106, CD22: 5x 106 |
| Wang 2021 | Co-administration | NR | 41BB | Auto | Mean CD19: 3.975 x 106 (range 3-6) Mean CD22: 3.125 x 106 (range 2-4) |
| Wei 2021 | Bivalent CAR | CD19: FMC63 CD22: human phage library |
41BB | Auto | BCL: Median 6.3 x 106 (range 4.9-9.4) ALL: 4.85 x 106 (range 1.04-7.02) |
| Yang 2018 | Co-transduction | NR | CD19: CD28, 4-1BB CD22: CD28, 4-1BB |
Auto | Median CD19: 2 x 105, range 0.9-5 Median CD22: 0.5 x 105, range 0.4-12 |
| Yang 2020 | Bivalent CAR | NR | 41BB | Auto | DL1: 6.0 x 104 (n = 2) DL2: 1.0-1.5 x 105 (n=7) DL3: 2.25 x 105 (n=1) |
| Yang 2019 | Bivalent CAR | NR | 41BB | Auto | DL1: 2.5-5 x 105(n = 4) DL2: 1-2.5 x 106 (n = 7) DL3: 3-5 x 106 (n = 5) |
| Cao 2021 | Sequential infusion (days) following HSCT | Murine | CD19: CD28, 41BB CD22: CD28, 41BB |
Auto | Median CD19: 4.1 x 106 (range 1.8 - 10) Median CD22: 4.0 x 106 (range 1.0 - 10) |
| Liu 2022§ | Sequential infusion (months) - CD22 only given after CD19 failure | CD22: Human phage library CD19: FMC63/human phage library |
CD19: 41BB CD22: 41BB |
Auto | Median CD19: 2.0 x 106 (range 0.11 - 3.0) Median CD22: 2.0 x 106 (range 0.17 - 4.13) Median CD20: 1.29 x 106 (range 0.44 - 2.17) |
| Ramakrishnan 2020 | Bicistronic vector | NR | CD19: OX40 CD22: 41BB |
Auto | DL1: 50 x 106 CAR T-cells total* DL2: 150 x 106 CAR T-cells total* DL3: 450 x 106 CAR T-cells total* |
| Zhang 2021 A | Bivalent CAR | CD19: FMC63 CD22: m971 |
41BB | Auto | Median: 8.258 x 108 CAR T-cells total* (range 3.690 x 10^8 to 3.285 x 109) |
| Zhang 2021 B* | Sequential infusion (days) | NR | CD19: 41BB CD22: 41BB |
NR | 0.5 - 2 x 107 |
Co-transduction: Simultaneously transducing T-cells with two separate vectors. Bivalent CAR: A single CAR molecule that has two specificity domains. Bicistronic vector: transduction of a single vector that expresses two CARs. Sequential infusion: infusion of one CAR-T cell product followed by a different CAR-T cell product, either on successive days (days) or delayed (months). DL, dose level. Fractionated adaptive dosing scheme: dose is given in fractions over 3 days, and subsequent doses held if develop adverse events after first dose. †Pan 2019: allogeneic cells were allowed in patients with previous transplant. ††Hu 2021 used CRISPR-Cas9 engineered universal CAR T-cells (donor-derived). §Liu 2022 only gave CD22 after patients failed CD19, therefore not all patients received CD22. *In Zhang 2021, intervention included anti-PD-1 antibody administered after sequential infusion of CD19 and CD22 CAR-T cells.
N/A, not applicable; NR, not reported.
Intervention characteristics
There were seven studies of CAR T-cell therapy solely targeting CD22 (CD22 CAR T-cells), and 23 studies investigated CAR T-cell therapies targeting both CD19 and CD22 (CD19/CD22 CAR T-cells). This includes Liu 2022, in which select patients also received CD20 CAR T-cells if CD19 and CD22 CAR T-cells failed. Various dual-targeting methods were used, including bivalent CAR molecules, bicistronic vectors, co-administration, and sequential infusion of two CAR T-cell populations. Details are presented in Table 3 . Cao 2021 was unique in that it involved sequential CD19/CD22 CAR T-cell infusion after all participants had received autologous HCT. Ramakrishnan 2020 and Zhang 2021B both combined CAR T-cells with anti-PD1 antibody therapy. Hu 2021 was unique because it utilized a universal donor-derived CAR T-cell product in which CRISPR/Cas9 was used to disrupt the TRAC region and CD52 gene of CAR T-cells to minimize host CAR T-cell rejection and to allow for anti-CD52-mediated targeted depletion of autologous T-cells.
Table 3.
Characteristics of Treated Patients.
| Study ID | Malignancy | N | Sex (% M) | Median age (range) | Prior CD19 CAR T-cells | Other prior therapy | |
|---|---|---|---|---|---|---|---|
| CD22 | |||||||
| Pan 2019 | ALL | 34 | 59% | 10 (1-55) | 31/34 | Allo-HCT: 13/34 | |
| Shah 2020 | ALL | 58 | NR | 17.5 (4.4-30.6) | 36/58 | CD22 CAR T cells: 5/58 Inotuzumab: 14/58 CD19-targeted therapy: 51/58 Blinatumomab: 23/58 HCT: 39/58 |
|
| Singh 2021 | ALL | 8 | NR | NR; 3 adults, 5 pediatrics | 5/8 | Prior allo-HSCT: 3/8 Blinatumomab: 3/8 |
|
| Summers 2021 | V1 | ALL | 4 | NR | NR | NR | NR |
| V2 | ALL | 3 | NR | NR | 3/3 | NR | |
| Tan 2021 | ALL | 8 | 25% | 9 (5-16) | NR | Prior CD19 and CD22 directed therapies: 8/8 Prior allo-HSCT: 4/8 |
|
| Zhu 2021 | ALL | 6 | 50% | 39.5 (25-58) | 13/13 | NR | |
| DLBCL | 7 | 71% | 56 (16-70) | ||||
| Baird 2021 | LBCL | 21 | 62% | 64 (36-79) | 20/21 | Allo-SCT: 6/21 | |
| CD19/CD22 | |||||||
| Annesley 2021 | ALL | 11 | NR | NR | 4/11 | CD19 or CD22 targeted therapies: 11/12 enrolled pts | |
| Cordoba 2021 | ALL | 15 | 73% | 8 (4-16) | 1/15 | Allo-SCT: 7/15 | |
| Dai 2020 | ALL | 6 | 67% | 23.5 (17-44) | NR | NR | |
| Frey 2021 | ALL | 13 | NR | 46 (28-71) | 2/13 | Blinatumomab: 8/13 Inotuzumab: 8/13 Prior allogeneic SCT: 10/13 |
|
| Gardner 2020 | ALL | 27 | NR | NR | NR | CD19 or CD22 targeted therapies: 13/27 | |
| Hu 2021 | ALL | 6 | 33% | 49 (26-56) | NR | NR | |
| Liu 2021 A | ALL | 27 | 52% | 21 (1.6–55) | NR | All had relapsed post allo-HCT | |
| Liu 2021 B | Tandem | ALL | 49 | NR | NR | NR | NR |
| Sequential | ALL | 13 | NR | NR | NR | ||
| Pan 2020 | ALL | 20 | 65% | 6 (1-16) | NR | NR | |
| Schultz 2018 | ALL | 4 | NR | NR (2-17) | NR | NR | |
| Shalabi 2020 | ALL | 11 | NR | 21 (5-28) | 5/11 | NR | |
| Speigel 2021 | ALL | 17 | 71% | 47 (26-68) | 1/17 | Allo-SCT: 12/17 | |
| LBCL | 21 | 67% | 69 (25-78) | NR | Auto-SCT: 4/21 | ||
| Wang 2020 | ALL | 51 | 63% | 27 (9-62) | NR | Allo-HCT: 9/51 Auto-HCT: 3/51 |
|
| NHL | 38 | 58% | 47 (17-11) | NR | Auto-HCT: 6/38 | ||
| Wang 2021 | TCF3-HLF+ALL | 4 | 100% | 6.8 (2.9-14.7) | NR | NR | |
| Wei 2021 | ALL | 15 | 47% | 27 (16-65) | 0/15 | Prior HSCT: 1/15 | |
| NHL | 16 | 50% | 52.5 (23-68) | 0/16 | Auto-HSCT: 1/16 | ||
| Yang 2018 | ALL | 15 | 73% | 19 (4-45) | NR | NR | |
| Yang 2020 | ALL | 10 | 50% | 11.5 (3-48)† | 3/10 | Allo-HSCT: 1/10 | |
| Yang 2019 | ALL | 16 | 59% | 8 (4-45) | 4/17 | NR | |
| Cao 2021 | Aggressive NHL | 42 | 57% | 41 (24-61) | NR | No prior HSCT | |
| Liu 2022 | Burkitt | 23 | NR | 8 (2-12) | NR | NR | |
| Ramakrishnan 2020 | DLBCL | 19 | NR | 57 (28-71) | 0/19 | No prior CD19 or CD22-directed therapies or allo-HCT | |
| Zhang 2021 A | NHL | 32 | 59% | NR | 0/32 | Prior HSCT: 4/32 | |
| Zhang 2021 B | NHL | 11 | NR | NR | NR | NR | |
NR, not reported.
Thirteen of 30 studies were dose escalation trials, but only six identified a recommended expansion phase dose. Annesley 2021, Gardner 2020, and Spiegel 2020 all identified the recommended dose of CD19/CD22 CAR T-cells to be 3x106 CAR T-cells/kg for ALL patients (and LBCL patients in Spiegel 2020), with no dose-limiting toxicities identified. Spiegel 2020 commented that they did not pursue higher doses due to toxicity concerns at higher doses seen in other clinical trials. Conversely, Cordoba 2021 treated ALL patients with up to 5x106 CD19/CD22 CAR T-cells cells/kg. No dose-limiting toxicities were identified, and while CAR T-cell persistence was identified as an issue, higher doses were not pursued due to a lack of correlation between higher dose and persistence. Among CD22 CAR T-cell studies, Baird 2021 examined two dose levels of 1x106 and 3x106 CAR T-cells/kg for LCBL patients, however 1x106 was the maximum tolerated dose. Shah 2020 also initially used 1x106 CD22 CAR T-cells/kg in their expansion phase to treat ALL patients, but the dose was de-escalated to 3x105 CAR T-cells/kg following increased toxicity with the institution of a CD4/CD8 selection procedure during manufacturing. In all studies except Ramakrishnan 2020, CAR T-cells were dosed by weight. A number of studies also used a fractionated adaptive dosing scheme, in which the target dose was given in split infusions, with subsequent infusions being held if toxicity developed.
Response data
27 of 30 studies were included in meta-analysis of bCR rates. Cao 2018, Liu 2021A, Liu 2022, and a subset of Summers 2021 were excluded in accordance with our prespecified protocol. Cao 2021 included patients who were in CR at the start of therapy. Liu 2021A and Liu 2022 had a complicated study design involving sequential rounds of CAR T-cells where some patients only received CD19 CAR T-cells and not CD22 CAR-T cells, so efficacy outcomes were not comparable to the other included studies. Summers 2021 included two CAR T-cell products (V1 and V2), with a focus on the latter improved product, and did not clearly report CR among patients receiving V1. Out of 23 studies that included participants with B-ALL, 21 reported minimal residual disease (MRD) status.
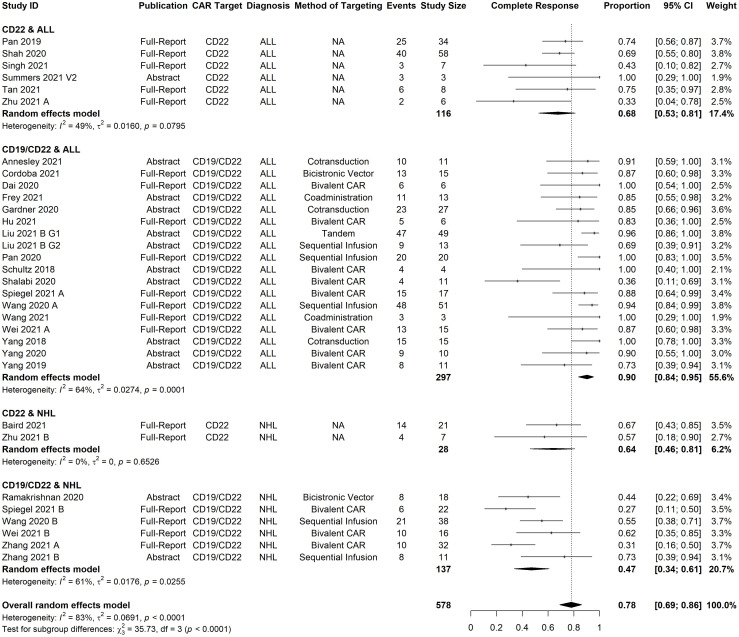
The all-study model of bCR rate had significant heterogeneity (I2 = 83%, p-value <0·0001). Through meta-regression with our pre-specified subgroups of interest, diagnosis (ALL vs. NHL) was identified as a significant predictor of bCR. ( Supplemental Table 2 ). The residual heterogeneity was reduced to moderate (I2 = 66%) by grouping studies according to both CAR target (CD22 vs CD19/CD22) and diagnosis (ALL vs. NHL). Therefore, we present the meta-analysis of bCR in these subgroups ( Figure 2 ).
Figure 2.
Forest plot of best complete response rate organized by malignancy type and antigen target. Pooled estimates, represented by the black diamond, were calculated for each subgroup and overall weighted effect.
CD22 CAR T-cell therapies had an estimated CR of 68% [95% CI, 53-81%] in B-ALL patients (n=116), and 64% [95% CI, 46-81%] in NHL patients (n= 28). CD19/CD22 CAR T-cell therapies had an estimated CR rate of 90% [95% CI, 84-95%] in B-ALL patients (n= 297) and 47% [95% CI, 34-61%] in NHL patients (n= 137). The cumulative percentage of CRs which were MRD negative, among B-ALL studies which reported these data, was 93% (199/213) for CD19/CD22 CAR T-cells and 86% (68/79) for CD22 CAR T-cells.
Data for time-to-event outcomes, follow-up, relapse, and antigen status are presented in Table 4 . Length of follow-up was reported in 20 studies, with reported median length ranging from 2-27.3 months. The methods of reporting relapse and survival were inconsistent with variable length of follow-up, limiting any direct comparisons. In general, relapse was common in these studies, with relapses being observed over a year after CR. Among CD22 studies that had at least 6 months median follow-up, the relapse rate range ranged from 17-69%, with Shah et al., 2020 having the longest median follow-up of 24 months and 49 out of 71 (69%) patients who achieved CR eventually relapsing. Among CD19/CD22 studies with at least 6 months of median follow-up, relapse rate ranged from 17-69%, with most of these studies having >40% relapse rates.
Table 4.
Survival and Relapse Data from Included Studies.
| Study ID | Disease | Months of follow up (median, range) | Relapse Rate (% of CR) |
Overall survival data | Event-free survival |
|---|---|---|---|---|---|
| CD22 CAR T- cell Studies | |||||
| Pan 2019 | ALL | 3.2 (0-14.5) | 6/26 (23%) | NR | 1-year LFS rate (among those who achieved CR): 58.1% |
| Shah 2020 | ALL | 24* | 49/71 (69%) | Median OS: 13.4 months (95% CI 7.7-20.3) |
Median EFS: 3.2 months (95% CI 1.4-5.5) Median RFS (restricted to those in CR): 13.4 months (95% CI 7.7-20.3) |
| Singh 2021 | ALL | IR | 4/4 (100%) | NR | NR |
| Summers 2021 v2 | ALL | NR | 0/3 (0%) | NR | NR |
| Tan 2021 | ALL | 6 (2-11) | 2/7 (29%) | IR | IR |
| Zhu 2021 | ALL | IR | 0/2 (0%) | 6-month OS of pts who did not receive HSCT: 20.5% | 6 month PFS of patients who did not receive HSCT: 20.0% |
| DLBCL | 0/4 (0%) | 6-month OS of pts who did not receive HSCT: 67.07% | 6 month PFS of patients who did not receive HSCT: 66.7% | ||
| Baird 2021 | LBCL | 7.3** (1.2-21.3) | 3/18 (17%) | NR | NR |
| CD19/CD22 CAR T-cell Studies | |||||
| Cordoba 2021 | ALL | 14 (2-28) | 9/13 (69%) | 6 month OS rate: 80% | 6 and 12-month EFS rate: 48%, 32% 6, 12-month molecular-free PFS rate: 38%, 23% |
| Dai 2020 | ALL | 8.5 (4-12) | 3/6 (50%) | NR | NR |
| Frey 2021 | ALL | 6.2*** (0.2 - 25) | 1/11 (9%) | 6-month OS rate: 85% | NR |
| Gardner 2020 | ALL | NR | 4/23 (17%) | NR | NR |
| Hu 2021 | ALL | 4.3 (2-8) | 1/5 (20%) | NR | NR |
| Liu 2021 A | ALL | 19.7 (5.6-27.3) | After CD19: 3/26 After CD22: 5/19 |
ITT analysis of all 27 patients: 12-month OS rate: 84.0% (95% CI 70.7-99.8) |
ITT analysis of all 27 patients: 12-month EFS rate: 65.2% (95% CI, 47.8 to 88.9) |
| Liu 2021 B | ALL | NR | NR | Tandem: 6-month OS rate: 90.0% | Tandem: 6-month LFS: 76.2% |
| Sequential: 6-month OS rate: 88.9% | Sequential: 6-month LFS: 88.9% | ||||
| Pan 2020 | ALL | 27.3 (9.8 to 36) | 8/20 (40%) | 2-year OS rate: 80.9% (95%CI 61.2-100.0%) | 2-year LFS: 60% (95%CI, 38.5-81.5%) |
| Shalabi 2020 | ALL | 3.3 (1-8.5) | 2/8 (25%) | NR | NR |
| Speigel 2021 | ALL | 9.3 (95% CI 7.2-NE) | 10/17 (59%) | Median OS: 11.8 mo (95%CI 5.5-NE) | Median PFS: 5.8 months (95% CI 2.6–NE) |
| LBCL | 10 (95%CI 8.7 - 21.5) | 8/13 (62%) | Median OS: 22.5 mo (95%CI 8.3–NE). | Median PFS: 3.2 months (95% CI 1.2–5.5) | |
| Wang 2020 | ALL | 16.7 (1.3-33.3) | 24/49 (49%) | Median OS: 31 months (95% CI 10.6-NR) |
Median PFS: 13.6 months (95% CI, 6.5-NR); 1-year PFS rate: 52.9% (95% CI 38.5-65.5) |
| NHL | 14.4 (0.4-27.4) | NR | Median OS: 18 months (95% CI 6.1-NR) |
Median PFS: 9.9 months (95% CI 3.3-NR); 1-year PFS rate: 50.0% (95% CI, 33.4-64.5) |
|
| Wang 2021 | ALL | Incomplete reporting | 2/4 (50%) | Incomplete reporting, only patient-level data | |
| Wei 2021 | ALL | NR | 3/4 (75%) of pts that did not proceed to HSCT | Median OS: 652 days (95% CI 390-905 days) | Median PFS: 90 days (95% CI 41-139 days) |
| BCL | Median 397 days, range NR | 7/14 (50%) | Median OS not reached 1-year OS rate: 77.3% 2-year OS rate: 77.3% |
Median PFS: 246 days 1-year PFS rate: 40.2% 2-year PFS rate: 40.2% |
|
| Yang 2018 | ALL | 4.4 (0.8-13.1) | 2/15 (13%) | NR | NR |
| Yang 2020 | ALL | 3.3 (0.5-7) | 2/9 (22%) | Incomplete reporting, only patient-level data | |
| Yang 2019 | ALL | 2 (0.2-4.6) | 0/8 (0%) | NR | NR |
| Cao 2021 | NHL | 24.3 (4.9 - 49.2) | N/A**** | NR | NR |
| Liu 2022 | NHL | 17 (15-23) | After CD19: 6/15 (40%) After CD22: 0/13 (0%) |
NR | 18-month PFS: 78% (95% CI 55-90%) |
| Zhang 2021 A | NHL | 8.7 (3-NR) | 10/23 (43%) | Median OS not reached OS rate 69.1% at 6 months OS rate 63.3% at 12 months |
Median PFS: 6.8 months PFS rate 51.4% at 6 months PFS rate 40.0% at 12 months |
| Zhang 2021 B | NHL | 5.8 (3-NR) | 0/8 (0%) | 6-month OS rate: 100% | 6-month PFS rate: 80.8% |
LFS, leukemia-free survival; PFS, progression-free survival. Incomplete reporting includes studies that only reported follow-up for a subset of patients, or that include survival data in graphical form but do not provide numerical data points. *Shah et al. report “median potential follow-up” of 24 months. **Baird 2021 only reports median follow-up for patients in CR. ***Frey 2021 only reports median-follow-up for living patients. ****Cao 2021 included patients who were in CR at the start of remission, therefore is excluded from data involving CR rate.
N/A, not applicable; NR, not reported.
Among CD22 CAR T-cell trials, Pan 2019 reported predominantly antigen-positive relapse while Shah 2020 reported predominantly antigen loss or diminished site density at relapse; Baird 2021 and Tan 2020 had small sample sizes with few relapses but reported 1/3 and 2/2 relapses involved CD22 loss or downregulation, respectively. Among CD19/CD22 CAR T-cell trials, the majority of relapses with reported antigen status were CD19+/CD22+ (42/50, 84%); among antigen-negative relapses, a common pattern observed was CD19-negative malignant cells with diminished CD22 site density (CD19-/CD22dim) ( Supplementary Materials , p3).
In-vivo CAR T-cell expansion data was reported by 24 out of 30 studies. Both Wei 2021 and Hu 2021 found that patients who achieved CR had higher peak levels of CAR T-cells than non-responders. Long-term persistence of CAR T-cells was variable with limited reporting, and persistence ranged from 42 days to 10 months. Cao 2021 found that patients with progressive disease had no detectable CAR T-cells at three months, while most patients in CR did have detectable CAR T-cells at three months. Details of expansion and persistence data can be found in Supplementary Materials (page 3, Supplementary Table 5 ).
Data on manufacturing outcomes demonstrated no major challenges in CAR T-cell manufacturing, with bivalent CARs having comparable mean transduction efficacy to monovalent CARs. Details are presented in Supplementary Materials (page 3, Supplementary Table 6 ).
There was inadequate patient level data to perform subgroup analysis by age group, prior HSCT, prior CD19 CAR T-cell therapy, or other prior immunotherapies.
Safety data
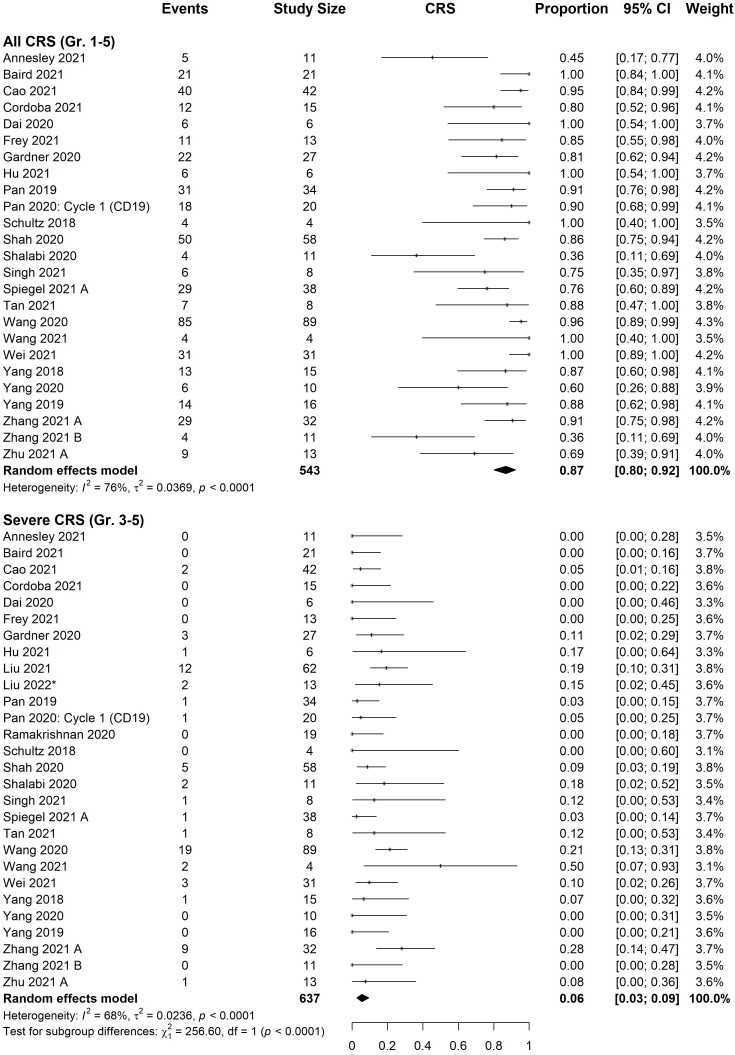
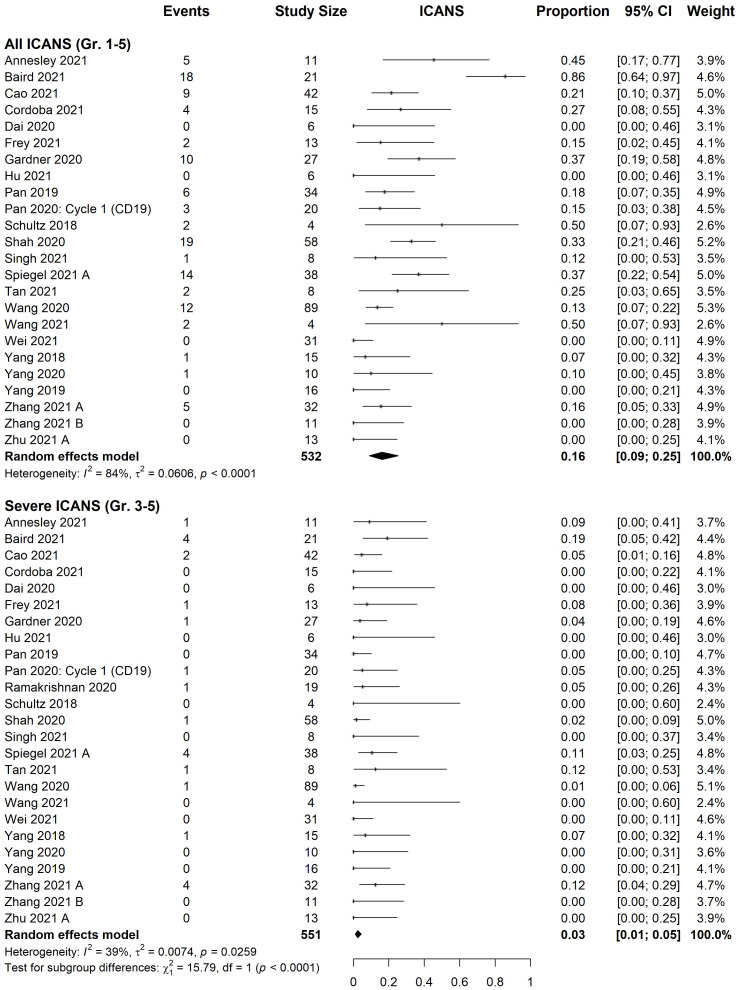
All studies provided information on CRS and ICANS. The estimated incidence of total and severe (grade ≥3) CRS were 87% [95% CI, 80%-92%] and 6% [95% CI, 3%-9%], respectively ( Figure 3 ). Estimated ICANS and severe ICANS incidence were 16% [95% CI, 9-25%] and 3% [95% CI, 1-5%], respectively ( Figure 4 ). Meta-regression revealed no significant difference in the incidence of adverse events (total or severe) between those treated with CD22 versus CD19/CD22 CAR T-cells ( Supplementary Table 3 ). There was no significant difference in the incidence of CRS or ICANS (total or severe) between B-ALL and NHL patients.
Figure 3.
Forest plot of cytokine release syndrome rate organized by severity. Pooled estimate of effect, black diamond, was calculated for both all grades (1-5) and severe grades (3-5). Pan 2020: cycle 1 (CD19) was excluded from analysis, although rates did not significantly differ from cycle 2 (18/20 all grades, 1/20 severe).
Figure 4.
Forest plot of immune effector cell-associated neurotoxicity syndrome rate organized by severity. Pooled estimate of effect, black diamond, was calculated for both all grades (1-5) and severe grades (3-5). Pan 2020: cycle 1 (CD19) was excluded from analysis, although rates did not significantly differ from cycle 2 (3/20 all grades, 1/20 severe).
All-cause 30-day mortality was available for 18 studies (n=457). The estimated incidence from these studies was 1% [95% CI, 0-3%] ( Supplementary Figure 1 ). Pan 2019 and Frey 2021 were outliers with a 30-day mortality of 12% (4/34) and 15% (2/13), respectively. In Pan 2019, two patients died of infection-related causes, and two patients who had previously received transplantation had death attributable to a combination of CRS and graft reaction. In Frey 2021, one patient died of grade 4 ICANS and sepsis, and one died from a rapidly progressive disease.
Shah 2020 was the only study to report and characterize hemophagocytic histiocytosis (HLH)-like toxicities. In a retrospective analysis of 59 patients, they found that 21/52 (40%) of patients who developed CRS also developed HLH. The onset of HLH was delayed (median onset 14 days), and in 11/21 patients HLH developed after CRS was already resolving. HLH was effectively treated with corticosteroids and anakinra, however one patient died secondary to bacterial sepsis prior to HLH resolution. All other patients fully recovered.
Bridging to hematopoietic cell transplant
Nineteen of the 30 studies commented on patients undergoing hematopoietic cell transplant (HCT) after achieving CR following CAR T-cell therapy. Among these, 99/292 (34%) of reported patients who achieved CR proceeding to HCT. Of note, this excludes Cao 2021, in which all patients received HCT prior to, rather than following, CAR T-cell therapy.
There was limited reporting of comparative survival in transplanted vs. non-transplanted groups. Shah 2020 found allogeneic HCT to be positively associated with relapse-free survival and event-free survival based on a time-covariate analysis. Similarly, Pan 2019 noted that at the observation endpoint, 8/11 CR patients who received transplant were relapse free (2 died of treatment-related mortality, 1 relapsed) while only 3/7 CR patients who had no further treatment were relapse free. In contrast, subgroup analysis of B-ALL patients by Wang 2020 found that transplant was not associated with a survival benefit.
Among studies examining HCT prior to CAR T-cell therapy, Cao 2021 showed significant efficacy of sequential HCT + CD19/CD22 CAR T-cells, with 2-year PFS of 83%. In Wang 2020, among the subset of patients that had a history of relapse post-HCT, 22/23 were able to achieve CR with CD19/CD22 CAR T-cell therapy, with 1-year PFS of 59.2%.
Catalogue of registered clinical trials
Through our search of clinical trial databases, we catalogued 99 registered clinical trials investigating CAR T-cells that target CD22 alone (29%) or in combination with other antigen targets (63%). 34 of the 62 (54%) multi-targeting trials did not specify the multi-targeting approach used. 82 (83%) and 55 (56%) trial registries did not report the costimulatory domain(s) or T-cell source (allogeneic vs. autologous), respectively. Results have been reported for 36/99 (36%) of the registered trials so far, with three of these studies ineligible for inclusion in this review due to inadequate or absent reporting of clinical outcomes. Of note, we excluded 25 published reports which for which the corresponding clinical trial could not be identified and 33 publications that reported pooled outcomes of multiple clinical trials or CAR T-cell therapies, in which data on CD22-targeted therapies specifically could not be extracted ( Supplementary Materials ).
Risk of bias
The risk of bias for all domains are presented in Supplemental Figure 2 and summarized in Supplemental Figure 3 . The majority of studies were single-center and did not provide estimates of random variability. No studies reported having blinded outcome assessors. The modified funnel plot appeared symmetrical, suggesting there was no publication bias ( Supplementary Figure 4 ). The sensitivity analysis for publication type showed that removing data from conference abstracts did not substantially alter the estimates of CR ( Supplementary Figure 5 ).
The evidence was assessed as low quality using the GRADE approach ( Supplementary Table 4 ). While estimates were fairly consistent across studies, all studies were single-arm interventional studies with serious risks of bias.
Discussion
We provide a narrative synthesis and meta-analysis of 30 early-phase single-arm studies representing 637 patients. There was a strong signal of efficacy with an estimated CR for CD22 CAR T-cells in B-ALL of 68% [95% CI, 54-77%], and 64% in NHL [95% CI, 46-81%]. Further, dual-targeting CD19/CD22 CAR T-cells had an estimated CR of 90% [95% CI, 84-98%] and 47% [95% CI, 34-61%] in B-ALL and NHL patients respectively. There was also an acceptable safety profile for both CD22 and CD19/CD22 CAR T-cells in R/R B-cell malignancies, with estimated rates of severe CRS and ICANS of just 6% [95% CI, 3%-9%] and 3% [95% CI, 1-5%].
Estimated CR rates in ALL patients were significantly higher with CD19/CD22 CAR T-cell therapy compared to single-target CD22 CAR T-cells. In contrast, the difference in bCR rates in NHL patients treated with CD19/CD22 CAR T-cells versus CD22 CAR T-cells was not statistically significant, however these groups did have a smaller sample size. It should be noted that a greater proportion of patients in the single-target CD22 CAR T-cell studies had failed CD19 CAR T-cells, and were receiving CD22 as a second-line CAR-T cell therapy. Thus, the lower CR rates seen among CD22 studies compared to dual target studies in ALL may be the result of selecting patients that were more refractory to treatment; in this case, substantial CR rates despite previous CAR T-cell failure point towards the value of CD22 CAR T-cells as a treatment option.
Prior meta-analyses of CD19 CAR T-cells reported estimated CR rates of 77% [95% CI, 63-87%] and 80% [95% CI, 76-85%] among ALL patients, which are similar to our estimated CR rates for CD22 CAR T-cells, but slightly lower than the CR rates estimated for CD19/CD22 CAR-T cells in ALL in our study.4,8 Among NHL patients, meta-analyses of CD19 CAR T-cells reported CR rates of 48% [95%CI: 42–54%] and 44% [95% CI: 34-55%], similar to our estimated CR for both CD22 CAR T-cells and CD19/CD22 CAR T-cells in NHL patients (26). This may indicate that dual therapy is more effective in ALL but not NHL patients, although direct comparison is not possible due to differing methodologies between meta-analyses, and given that prior meta-analyses included earlier generations of CAR T-cells that had lower efficacy. Among CD19/CD22 CAR T-cell therapies, a number of multi-targeting strategies were employed but given the small number of trials per group no comparison of efficacy could be made.
We saw no indication that dual-target CD19/CD22 therapies have a higher incidence of adverse events compared to single-target CD22 CAR T-cells. Compared to prior CD19 CAR T-cell meta-analyses, both CD22 and CD19/CD22 CAR T-cells had lower estimated rates of severe CRS and ICANS but higher rates of total CRS (5, 24). Notably, a retrospective study that compared CD19 and CD19/CD22 CAR T-cells from two clinical trials also showed that CD19 CAR T-cells actually had a statistically significant increased risk of severe CRS compared to CD19/CD22 CAR T-cells (80). These differences may be due to the inherent biology of the CARs or could be explained by variability in reporting guidelines, patients, dosing regimens, or improvement in treatment protocols. Overall 30-day mortality for all CD22 CAR T-cells was comparable to that of CD19 CAR T-cells and HCT (5, 81). In contrast to CD19 CAR T-cell studies, the incidence of adverse events was relatively homogenous across studies in our review (5).
Among studies that report long-term data, it appears that relapse within a year is common, and thus durability of response remains a challenge. However, there is inadequate data to determine whether relapse rates are significantly different to that of other CAR T-cell therapies, particularly given that many prior CD19 CAR T-cell studies used first-generation CARs, limiting the comparison to the current second-generation CD22 CARs. Among dual-target studies, relapses were often CD19+/CD22+, indicating that mechanisms other than antigen loss, including poor CAR T-cell persistence, may impact long-term outcomes. Long-term data are needed to determine whether dual-targeting improves relapse-free survival compared to single-targeting. Regardless, our study showed that CD22 CAR T-cells present another line of therapy for patients who relapse post-CAR T-cell therapy with CD19-negative disease.
Strengths of this review include our broad and methodical search strategy, which included both electronic searches of multiple databases and manual searching of conference abstracts. Sensitivity analysis confirmed that conference abstracts, although they had limited data on secondary outcomes, did not affect the heterogeneity of our meta-analysis, making them a valuable inclusion to our analysis. To further our confidence that all relevant research had been identified we searched clinical trial registries and catalogued our results.
Overall, many clinical trial registry entries in ClinicalTrials.gov or the WHO ICTRP poorly describe their respective intervention. Basic characteristics such as CAR target, structure, dosing regimen, and population criteria are often incomplete or missing. CAR T-cells are highly modular therapies and pre-clinical studies have shown that simple modifications (e.g. switching co-stimulatory domain, altering linker length) can have drastic effects on function (82–84). We strongly advocate for authors to follow a more robust and transparent approach to CAR T-cell trial registration, which should at a minimum provide the interventional and patient characteristics described in our protocol (30).
This review has a number of important limitations which should be recognized. Firstly, all studies are early-phase, single-arm, interventional trials, with a significant risk of bias. Concurrently, many outcomes of interest were not provided by the majority of studies. Nonetheless, there was sufficient data on CR rates and adverse events to achieve the primary aim of this review, which was to evaluate the efficacy and safety of CD22 CAR T-cell therapies in B-cell malignancies.
This systematic review of CD22 CAR-T cell clinical trials observed a strong signal of efficacy and safety of both single (CD22) and dual-target (CD19/CD22) CAR T-cells in patients with R/R B-cell malignancies. CD22 appears to be a viable antigen target and may be an option for those who relapse after CD19 CAR T-cell therapy. However, many early-phase interventional studies are still ongoing and have not yet published results, while others have reported preliminary results without having reached the maximum tolerated dose. The long-term efficacy of these therapies at their optimal therapeutic level thus has yet to be seen. Durability of response appears to remain a key limitation, as with other CAR T-cell therapies. Future trials are needed to determine the comparative efficacy of these therapies and identify strategies to improve the durability of response.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
NF and KA (contributed equally): Literature search, compiled and verified the data, performed the statistical analysis and drafted manuscript. NK, HA and KH: Conceptualization, initial design, and supervision. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to acknowledge Risa Schorr who helped design and run the search strategy.
Funding Statement
This project was funded in part by a grant from Biotherapeutics for Cancer Treatment (BioCanRx), a Canadian Network of Centers of Excellence (FY20/ES15). NF’s salary was supported by a summer studentship award from BioCanRx (Ref # FY20/SS10), payments made to HA affiliated institution. KA contributed as part of the FLEX Course at the UBC Medical Undergraduate Program.
Conflict of interest
KH serves on advisory board for Kite/Gilead, Cellgene/BMS, Novartis, Janssen and receives payments for Jazz Pharmaceutical educational programs. KH has also received research funding from Janssen. NK serves on advisory board for Kite/Gilead, Cellgene/BMS, and Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1178403/full#supplementary-material
References
- 1. Gokbuget N, Dombret H, Ribera JM, Fielding AK, Advani A, Bassan R, et al. International reference analysis of outcomes in adults with b-precursor ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica (2016) 101(12):1524–33. doi: 10.3324/haematol.2016.144311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large b-cell lymphoma: results from the international SCHOLAR-1 study. Blood (2017) 130(16):1800–8. doi: 10.1182/blood-2017-03-769620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mullard A. FDA Approves first CAR T therapy. Nat Rev Drug Discov (2017) 16(10):669–9. doi: 10.1038/nrd.2017.196 [DOI] [PubMed] [Google Scholar]
- 4. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med (2014) 371(16):1507–17. doi: 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grigor EJM, Fergusson D, Kekre N, Montroy J, Atkins H, Seftel MD, et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: a systematic review and meta-analysis. Transfus Med Rev (2019) 33(2):98–110. doi: 10.1016/j.tmrv.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 6. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med (2020) 382(14):1331–42. doi: 10.1056/NEJMoa1914347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sengsayadeth S, Savani BN, Oluwole O, Dholaria B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. eJHaem (2022) 3(S1):6–10. doi: 10.1002/jha2.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomasik J, Jasiński M, Basak GW. Next generations of CAR-T cells - new therapeutic opportunities in hematology? Front Immunol (2022) 13:1034707. doi: 10.3389/fimmu.2022.1034707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory Large b-cell lymphoma. N Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse Large b-cell lymphoma. N Engl J Med (2019) 380(1):45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 11. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N Engl J Med (2018) 378(5):439–48. doi: 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large b-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet Lond Engl (2020) 396(10254):839–52. doi: 10.1016/S0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]
- 13. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for Large b-cell lymphoma. N Engl J Med (2022) 386(7):640–54. doi: 10.1056/NEJMoa2116133 [DOI] [PubMed] [Google Scholar]
- 14. Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult b-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet Lond Engl (2021) 10299):491–502. doi: 10.1016/S0140-6736(21)01222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med (2018) 378(5):449–59. doi: 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grover P, Veilleux O, Tian L, Sun R, Previtera M, Curran E, et al. Chimeric antigen receptor T-cell therapy in adults with b-cell acute lymphoblastic leukemia. Blood Adv (2022) 6(5):1608–18. doi: 10.1182/bloodadvances.2020003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, et al. Anti-CD22–chimeric antigen receptors targeting b-cell precursor acute lymphoblastic leukemia. Blood (2013) 121(7):1165–74. doi: 10.1182/blood-2012-06-438002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qin H, Dong Z, Wang X, Cheng WA, Wen F, Xue W, et al. CAR T cells targeting BAFF-r can overcome CD19 antigen loss in b cell malignancies. Sci Transl Med (2019) 11(511):eaaw9414. doi: 10.1126/scitranslmed.aaw9414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed b cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med (2020) 26(10):1569–75. doi: 10.1038/s41591-020-1081-3 [DOI] [PubMed] [Google Scholar]
- 20. Ormhøj M, Scarfò I, Cabral ML, Bailey SR, Lorrey SJ, Bouffard AA, et al. Chimeric antigen receptor T cells targeting CD79b show efficacy in lymphoma with or without cotargeting CD19. Clin Cancer Res Off J Am Assoc Cancer Res (2019) 25(23):7046–57. doi: 10.1158/1078-0432.CCR-19-1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang X, Zhu L, Zhang H, Chen S, Xiao Y. CAR-T cell therapy in hematological malignancies: current opportunities and challenges. Front Immunol (2022) 13:927153. doi: 10.3389/fimmu.2022.927153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qualls D, Salles G. Optimizing CAR T cell therapy in lymphoma. Hematol Oncol (2021) 39(S1):104–12. doi: 10.1002/hon.2844 [DOI] [PubMed] [Google Scholar]
- 23. Wong DP, Roy NK, Zhang K, Anukanth A, Asthana A, Shirkey-Son NJ, et al. A BAFF ligand-based CAR-T cell targeting three receptors and multiple b cell cancers. Nat Commun (2022) 13(1):217. doi: 10.1038/s41467-021-27853-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anagnostou T, Riaz IB, Hashmi SK, Murad MH, Kenderian SS. Anti-CD19 chimeric antigen receptor T-cell therapy in acute lymphocytic leukaemia: a systematic review and meta-analysis. Lancet Haematol (2020) 7(11):e816–26. doi: 10.1016/S2352-3026(20)30277-5 [DOI] [PubMed] [Google Scholar]
- 25. Cao JX, Gao WJ, You J, Wu LH, Liu JL, Wang ZX. The efficacy of anti-CD19 chimeric antigen receptor T cells for b-cell malignancies. Cytotherapy (2019) 21(7):769–81. doi: 10.1016/j.jcyt.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 26. Ying Z, Song Y, Zhu J. Effectiveness and safety of anti-CD19 chimeric antigen receptor-T cell immunotherapy in patients with Relapsed/Refractory Large b-cell lymphoma: a systematic review and meta-analysis. Front Pharmacol (2022) 13:834113. doi: 10.3389/fphar.2022.834113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu WL, Hua ZC. Chimeric antigen receptor T-cell (CAR T) therapy for hematologic and solid malignancies: efficacy and safety–a systematic review with meta-analysis. Cancers (2019) 11(1):47. doi: 10.3390/cancers11010047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li L, Wang L, Liu Q, Wu Z, Zhang Y, Xia R. Efficacy and safety of CD22-specific and CD19/CD22-bispecific CAR-T cell therapy in patients with hematologic malignancies: a systematic review and meta-analysis. Front Oncol (2022) 12:954345. doi: 10.3389/fonc.2022.954345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adeel K, Fergusson NJ, Shorr R, Atkins H, Hay KA. Efficacy and safety of CD22 chimeric antigen receptor (CAR) T cell therapy in patients with b cell malignancies: a protocol for a systematic review and meta-analysis. Syst Rev (2021) 10(1):35. doi: 10.1186/s13643-021-01588-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grigor EJM, Fergusson DA, Haggar F, Kekre N, Atkins H, Shorr R, et al. Efficacy and safety of chimeric antigen receptor T-cell (CAR-T) therapy in patients with haematological and solid malignancies: protocol for a systematic review and meta-analysis. BMJ Open (2017) 7(12). doi: 10.1136/bmjopen-2017-019321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rousseau MJ, Evans JC. Key statistical assumptions and methods in one-arm meta-analyses with binary endpoints and low event rates, including a real-life example in the area of endoscopic colonic stenting. Cogent Med (2017) 4(1):1334318. doi: 10.1080/2331205X.2017.1334318 [DOI] [Google Scholar]
- 33. Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol (2014) 67(8):897–903. doi: 10.1016/j.jclinepi.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 34. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Annesley C, Summers C, Pulsipher MA, Skiles JL, Li AM, Vatsayan A, et al. SCRI-CAR19x22v2 T cell product demonstrates bispecific activity in b-ALL. Blood (2021) 138(Supplement 1):470. doi: 10.1182/blood-2021-148881 [DOI] [Google Scholar]
- 36. Baird JH, Frank MJ, Craig J, Patel S, Spiegel JY, Sahaf B, et al. CD22-directed CAR T-cell therapy induces complete remissions in CD19-directed CAR–refractory large b-cell lymphoma. Blood (2021) 137(17):2321–5. doi: 10.1182/blood.2020009432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cao Y, Xiao Y, Wang N, Wang G, Huang L, Hong Z, et al. CD19/CD22 chimeric antigen receptor T cell cocktail therapy following autologous transplantation in patients with Relapsed/Refractory aggressive b cell lymphomas. Transplant Cell Ther (2021) 27(11):910.e1–910.e11. doi: 10.1016/j.jtct.2021.08.012 [DOI] [PubMed] [Google Scholar]
- 38. Cordoba S, Onuoha S, Thomas S, Pignataro DS, Hough R, Ghorashian S, et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory b cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med (2021) 27(10):1797–805. doi: 10.1038/s41591-021-01497-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory b cell acute lymphoblastic leukemia. J Hematol OncolJ Hematol Oncol (2020) 13(1):30. doi: 10.1186/s13045-020-00856-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frey NV, Gill S, Hwang WT, Luger SM, Martin ME, McCurdy SR, et al. CART22-65s Co-administered with huCART19 in adult patients with relapsed or refractory ALL. Blood (2021) 138(Supplement 1):469. doi: 10.1182/blood-2021-153955 [DOI] [Google Scholar]
- 41. Gardner RA, Annesley C, Wilson A, Summers C, Prabha N, Wu V, et al. Efficacy of SCRI-CAR19x22 T cell product in b-ALL and persistence of anti-CD22 activity. J Clin Oncol (2020) 38(15_suppl):3035–5. doi: 10.1200/JCO.2020.38.15_suppl.3035 [DOI] [Google Scholar]
- 42. Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, et al. CRISPR/Cas9-engineered universal CD19/CD22 dual-targeted CAR-T cell therapy for Relapsed/Refractory b-cell acute lymphoblastic leukemia. Clin Cancer Res (2021) 27(10):2764–72. doi: 10.1158/1078-0432.CCR-20-3863 [DOI] [PubMed] [Google Scholar]
- 43. Liu S, Deng B, Yin Z, Lin Y, An L, Liu D, et al. Combination of CD19 and CD22 CAR-T cell therapy in relapsed b-cell acute lymphoblastic leukemia after allogeneic transplantation. Am J Hematol (2021) 96(6):671–9. doi: 10.1002/ajh.26160 [DOI] [PubMed] [Google Scholar]
- 44. Liu S, Zhang X, Dai H, Cui Q, Cui W, Yin J, et al. Tandem CD19/CD22 dual targets CAR-T cells therapy obtains superior CR rate than single CD19 CAR-T cells infusion as well as sequential CD19 and CD22 CAR-T cells infusion for Relapsed/Refractory b-cell acute lymphoblastic leukemia patients. Blood (2021) 138(Supplement 1):1755. doi: 10.1182/blood-2021-152927 [DOI] [Google Scholar]
- 45. Liu Y, Deng B, Hu B, Zhang W, Zhu Q, Liu Y, et al. Sequential different b-cell antigen–targeted CAR T-cell therapy for pediatric refractory/relapsed burkitt lymphoma. Blood Adv (2022) 6(3):717–30. doi: 10.1182/bloodadvances.2021004557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z, et al. CD22 CAR T-cell therapy in refractory or relapsed b acute lymphoblastic leukemia. Leukemia (2019) 33(12):2854–66. doi: 10.1038/s41375-019-0488-7 [DOI] [PubMed] [Google Scholar]
- 47. Pan J, Zuo S, Deng B, Xu X, Li C, Zheng Q, et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r b-ALL. Blood (2020) 135(5):387–91. doi: 10.1182/blood.2019003293 [DOI] [PubMed] [Google Scholar]
- 48. Ramakrishnan A, Marzolini M, Osborne W, Tholouli E, Bachier C, McSweeney P, et al. Phase 1 Alexander study of AUTO3 the first bicistronic chimeric antigen receptor (CAR) targeting CD19 and CD22 with pembrolizumab in patients with Relapsed/Refractory diffuse Large b cell lymphoma. 25th Congr Eur Hematol Assoc Hemasphere (2020) 4(S1):80. doi: 10.1097/HS9.0000000000000404 [DOI] [Google Scholar]
- 49. Schultz LM, Davis KL, Baggott C, Chaudry C, Marcy AC, Mavroukakis S, et al. Phase 1 study of CD19/CD22 bispecific chimeric antigen receptor (CAR) therapy in children and young adults with b cell acute lymphoblastic leukemia (ALL). Blood (2018) 132(Supplement 1):898–8. doi: 10.1182/blood-2018-99-117445 [DOI] [Google Scholar]
- 50. Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol (2020) 38(17):1938–50. doi: 10.1200/JCO.19.03279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shalabi H, Yates B, Shahani S, Qin H, HIghfill SL, Panch S, et al. Abstract CT051: safety and efficacy of CD19/CD22 CAR T cells in children and young adults with relapsed/refractory ALL. Cancer Res (2020) 80(16_Supplement):CT051. doi: 10.1158/1538-7445.AM2020-CT051 [DOI] [Google Scholar]
- 52. Singh N, Frey NV, Engels B, Barrett DM, Shestova O, Ravikumar P, et al. Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells. Nat Med (2021) 27(5):842–50. doi: 10.1038/s41591-021-01326-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory b cell malignancies: a phase 1 trial. Nat Med (2021) 27(8):1419–31. doi: 10.1038/s41591-021-01436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Summers C, Baxter B, Annesley C, Yokoyama J, Rhea S, Huang W, et al. CD22 CAR optimization for improved in-human activity following inadequate CD22 CAR activity in phase 1 clinical trial PLAT-04. Blood (2021) 138(Supplement 1):403. doi: 10.1182/blood-2021-147928 [DOI] [Google Scholar]
- 55. Tan Y, Cai H, Li C, Deng B, Song W, Ling Z, et al. A novel full-human CD22-CAR T cell therapy with potent activity against CD22low b-ALL. Blood Cancer J (2021) 11(4):1–6. doi: 10.1038/s41408-021-00465-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang N, Hu X, Cao W, Li C, Xiao Y, Cao Y, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed b-cell malignancies. Blood (2020) 135(1):17–27. doi: 10.1182/blood.2019000017 [DOI] [PubMed] [Google Scholar]
- 57. Wang T, Wan X, Yang F, Shi W, Liu R, Ding L, et al. Successful treatment of TCF3-HLF–positive childhood b-ALL with chimeric antigen receptor T-cell therapy. Clin Lymphoma Myeloma Leuk (2021) 21(6):386–92. doi: 10.1016/j.clml.2021.01.014 [DOI] [PubMed] [Google Scholar]
- 58. Wei G, Zhang Y, Zhao H, Wang Y, Liu Y, Liang B, et al. CD19/CD22 dual-targeted CAR T-cell therapy for Relapsed/Refractory aggressive b-cell lymphoma: a safety and efficacy study. Cancer Immunol Res (2021) 9(9):1061–70. doi: 10.1158/2326-6066.CIR-20-0675 [DOI] [PubMed] [Google Scholar]
- 59. Yang J, Li J, Zhang X, LV F, Guo X, Wang Q, et al. A feasibility and safety study of CD19 and CD22 chimeric antigen receptors-modified T cell cocktail for therapy of b cell acute lymphoblastic leukemia. Blood (2018) 132(Supplement 1):277. doi: 10.1182/blood-2018-99-114415 29764839 [DOI] [Google Scholar]
- 60. Yang J, Jiang P, Zhang X, Zhu X, Dong Q, He J, et al. Anti-CD19/CD22 dual CAR-T therapy for refractory and relapsed b-cell acute lymphoblastic leukemia. Blood (2019) 134(Supplement_1):284. doi: 10.1182/blood-2019-126429 [DOI] [Google Scholar]
- 61. Yang J, Jiang P, Zhang X, Li J, Wu Y, Xu L, et al. Successful 24-hours manufacture of anti-CD19/CD22 dual chimeric antigen receptor (CAR) T cell therapy for b-cell acute lymphoblastic leukemia (B-ALL). Blood (2020) 136(Supplement 1):2–3. doi: 10.1182/blood-2020-136866 [DOI] [Google Scholar]
- 62. Zhang Y, Li J, Zong X, Zhou J, Jia S, Geng H, et al. Chimeric antigen receptor T cell targeting to CD19 and CD22 combined with anti-PD-1 antibody induce high response in patients with relapsed or refractory b-cell non-Hodgkin lymphoma. Blood (2021) 138(Supplement 1):1730. doi: 10.1182/blood-2021-146293 [DOI] [Google Scholar]
- 63. Zhang Y, Li J, Lou X, Chen X, Yu Z, Kang L, et al. A prospective investigation of bispecific CD19/22 CAR T cell therapy in patients with relapsed or refractory b cell non-Hodgkin lymphoma. Front Oncol (2021) 11:664421. doi: 10.3389/fonc.2021.664421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu H, Deng H, Mu J, Lyu C, Jiang Y, Deng Q. Anti-CD22 CAR-T cell therapy as a salvage treatment in b cell malignancies refractory or relapsed after anti-CD19 CAR-T therapy. ONCOTARGETS Ther (2021) 14:4023–37. doi: 10.2147/OTT.S312904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fu XH, Wang Y, Wang HJ, Wei SN, Xu YX, Xing HY, et al. CD19 antigen loss after treatment of bispecific T-cell engager and effective response to salvage bispecific CAR-T therapy in b cell acute lymphoblastic leukemia: a case report and literature review. Zhonghua Xue Ye Xue Za Zhi Zhonghua Xueyexue Zazhi. (2020) 41(4):282–6. doi: 10.3760/cma.j.issn.0253-2727.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hua J, Qian W, Wu X, Zhou L, Yu L, Chen S, et al. Sequential infusion of anti-CD22 and anti-CD19 chimeric antigen receptor T cells for a pediatric ph-like b-ALL patient that relapsed after CART-cell and haplo-HSCT therapy: a case report and review of literature. OncoTargets Ther (2020) 13:2311–7. doi: 10.2147/OTT.S235882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jain N, Roboz GJ, Konopleva M, Liu H, Schiller GJ, Jabbour EJ, et al. Preliminary results from the Flu/Cy/Alemtuzumab arm of the phase I BALLI-01 trial of UCART22, an anti-CD22 allogeneic CAR-T cell product, in adult patients with relapsed or refractory (R/R) CD22+ b-cell acute lymphoblastic leukemia (B-ALL). Blood (2021) 138(Supplement 1):1746. doi: 10.1182/blood-2021-150779 [DOI] [Google Scholar]
- 68. Jiao C, Zvonkov E, Lai X, Zhang R, Liu Y, Qin Y, et al. 4SCAR2.0: a multi-CAR-T therapy regimen for the treatment of relapsed/refractory b cell lymphomas. Blood Cancer J (2021) 11(3):59. doi: 10.1038/s41408-021-00455-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jin A, Feng J, Wei G, Wu W, Yang L, Xu H, et al. CD19/CD22 chimeric antigen receptor T-cell therapy for refractory acute b-cell lymphoblastic leukemia with FLT3-ITD mutations. Bone Marrow Transplant (2020) 55(4):717–21. doi: 10.1038/s41409-020-0807-7 [DOI] [PubMed] [Google Scholar]
- 70. Lai X, Gu X, Tsao ST, Zhang Q, Liu YC, Yaxian J, et al. Double CD19/CD22 chimeric antigen receptor-modified T cells for the treatment of stage IV relapsed and refractory follicular lymphoma. Blood (2017) 130(Supplement 1):5154. doi: 10.1182/blood.V130.Suppl_1.5154.5154 [DOI] [Google Scholar]
- 71. Li C, Zhang C, Chen X, Zhang Y, Chen J, Kang L, et al. Relative depletion of soluble interleukin 6 receptors abolished the development of cytokine release syndrome after CART19/22 and lenalidomide treatment for lymphoma. Blood (2019) 134(Supplement_1):5313. doi: 10.1182/blood-2019-126821 [DOI] [Google Scholar]
- 72. Li N, Wang SA, Lin P, Jabbour E, Thompson P, Chen Z, et al. Relapsed b-acute lymphoblastic leukemia with aberrant myeloperoxidase expression following CAR T-cell therapy: a diagnostic challenge. Am J Hematol (2019) 94(9):1049–51. doi: 10.1002/ajh.25478 [DOI] [PubMed] [Google Scholar]
- 73. Liang Z, Cui J, Chang AH, Yu J, Hu Y, Huang H. Successful treatment of relapsed acute b-cell lymphoblastic leukemia with CD20/CD22 bispecific chimeric antigen receptor T-cell therapy. Regener Ther (2020) 15:281–4. doi: 10.1016/j.reth.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meijing L, Cui R, Mu J, Yuan J j, Mou N, Yang Z x, et al. A refractory b-ALL patient relapsed with leukemia cells expressing CD19 and anti-CD19 CAR gene 14 days after CR from anti-CD19 CAR T-cell therapy. Blood (2019) 134(Supplement_1):5124. doi: 10.1182/blood-2019-126398 [DOI] [Google Scholar]
- 75. Sun Y, Su Y, Wang Y, Liu N, Li Y, Chen J, et al. CD19 CAR-T cells with membrane-bound IL-15 for b-cell acute lymphoblastic leukemia after failure of CD19 and CD22 CAR-T cells: case report. Front Immunol (2021) 12:728962. doi: 10.3389/fimmu.2021.728962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wei S, Gu R, Xu Y, Liu X, Xing Y, Gong X, et al. Adjuvant ruxolitinib therapy relieves steroid-refractory cytokine-release syndrome without impairing chimeric antigen receptor-modified T-cell function. Immunotherapy (2020) 12(14):1047–52. doi: 10.2217/imt-2020-0116 [DOI] [PubMed] [Google Scholar]
- 77. Yan N, Wang N, Zhang P, Wang G, Mao X, Peng D, et al. Case report: successful chimeric antigen receptor T cell therapy in haploidentical-allogeneic stem cell transplant patients with post-transplant lymphoproliferative disorder. Front Oncol (2021) 11:709370. doi: 10.3389/fonc.2021.709370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang Wq, Hu B, Jin L, Yang J, Du J, Wang S, et al. Chimeric antigen receptor T-cells (car-T) for refractory and relapsed burkitt’s lymphoma: early response in pediatric patients. Hematol Oncol (2019) 37(S2):59–60. doi: 10.1002/hon.28_2629 [DOI] [Google Scholar]
- 79. Zi FM, Ye LL, Zheng JF, Cheng J, Wang QM. Using JAK inhibitor to treat cytokine release syndrome developed after chimeric antigen receptor T cell therapy for patients with refractory acute lymphoblastic leukemia: a case report. Med (Baltimore) (2021) 100(19):e25786. doi: 10.1097/MD.0000000000025786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang Y, Yang Y, Hong R, Zhao H, Wei G, Wu W, et al. A retrospective comparison of CD19 single and CD19/CD22 bispecific targeted chimeric antigen receptor T cell therapy in patients with relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J (2020) 10(10):1–3. doi: 10.1038/s41408-020-00371-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Styczyński J, Tridello G, Koster L, Iacobelli S, van Biezen A, van der Werf S, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant (2020) 55(1):126–36. doi: 10.1038/s41409-019-0624-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Singh N, Frey NV, Engels B, Barrett DM, Shestova O, Ravikumar P, et al. Single chain variable fragment linker length regulates CAR biology and T cell efficacy. Blood (2019) 134(Supplement_1):247–7. doi: 10.1182/blood-2019-131024 [DOI] [Google Scholar]
- 83. Qin H, Ramakrishna S, Nguyen S, Fountaine TJ, Ponduri A, Stetler-Stevenson M, et al. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol Ther Oncolytics (2018) 11:127–37. doi: 10.1016/j.omto.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med (2015) 21(6):581–90. doi: 10.1038/nm.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.