Abstract
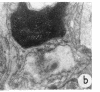
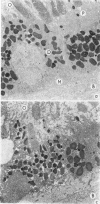
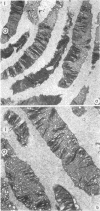
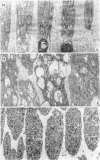
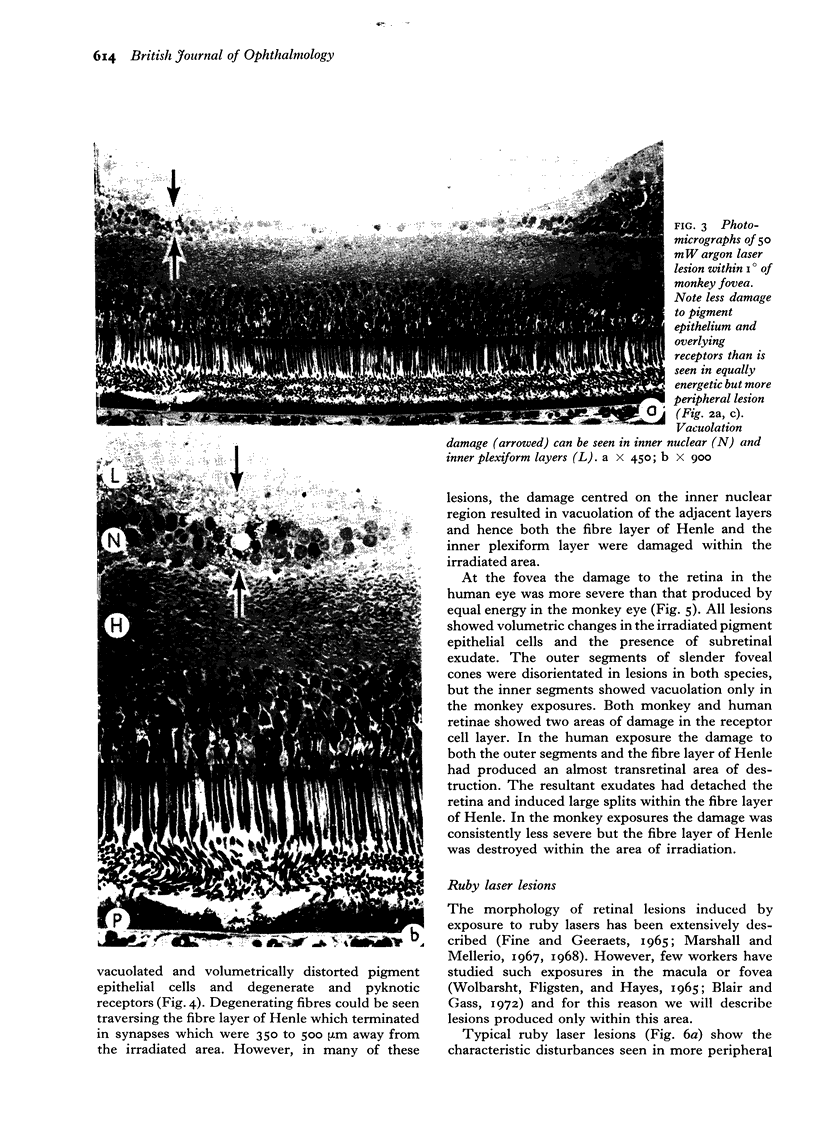
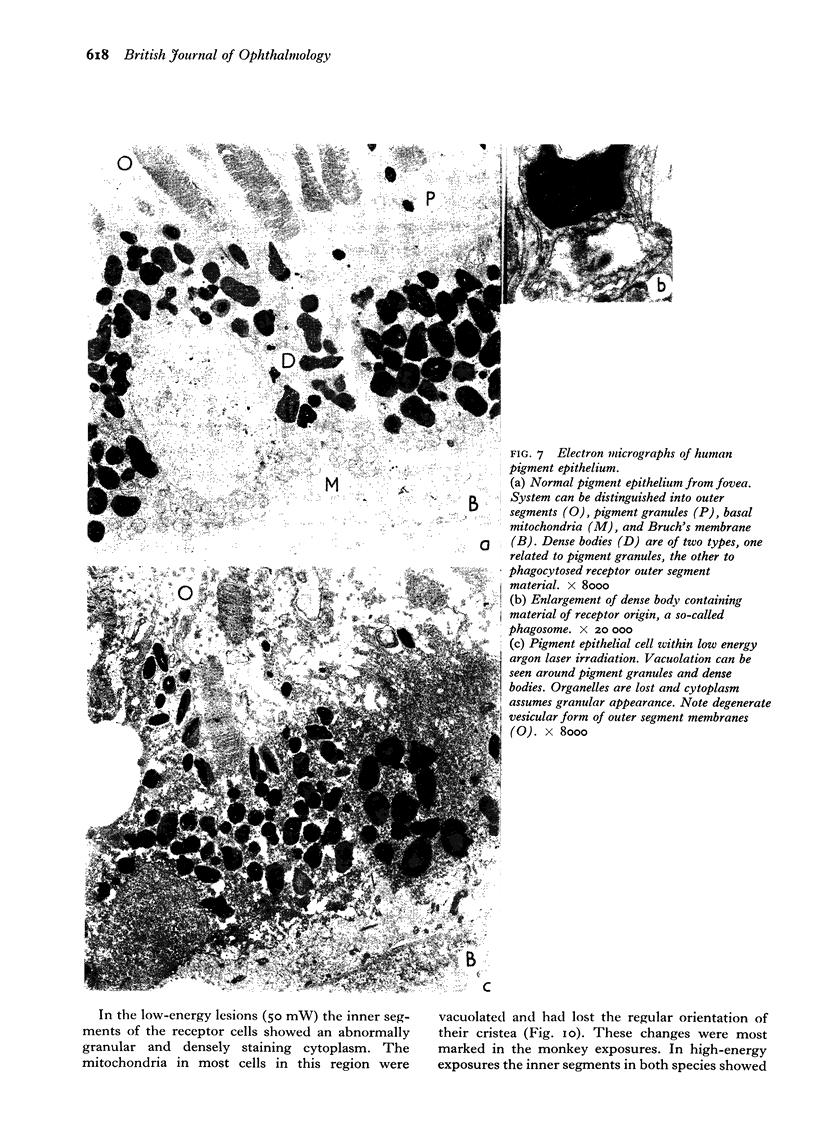
Suprathreshold fundus lesions produced by ruby and argon laser photocoagulation were studied within 24 hours by light and electron microscopy. It was shown that damage was maximal in the outer retina in all ruby laser lesions and extramacular argon laser lesions. In both monkey and human, inner retinal damage occurred independently of outer retinal damage in macular lesions produced by the argon laser. In lesions produced by equal energy, inner retinal damage was more severe in humans than in monkeys. In both species outer retinal damage was less severe in the foveal than the parafoveal region and this disparity was greater in humans than in monkeys. These findings are important to the therapeutic use of argon laser energy for mascular disease. In particular, absortion of energy in the inner retina reduces the energy available in the treatment of subretinal lesions in the foveal area, and causes unwanted neuroretinal damage. The higher sensitivity to argon laser irradiation of the human fovea compared with the monkey fovea, has not been appreciated when defining laser safety limits.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Beatrice E. S., Bedell R. B. Retina: ultrastructural alterations produced by extremely low levels of coherent radiation. Science. 1972 Jul 7;177(4043):58–60. doi: 10.1126/science.177.4043.58. [DOI] [PubMed] [Google Scholar]
- Ansell P. L., Marshall J. The distribution of extra-cellular acid phosphatase in the retinas of retinitis pigmentosa rats. Exp Eye Res. 1974 Sep;19(3):273–279. doi: 10.1016/0014-4835(74)90146-8. [DOI] [PubMed] [Google Scholar]
- Blair C. J., Gass J. D. Photocoagulation of the macula and papillomacular bundle in the human. Arch Ophthalmol. 1972 Aug;88(2):167–171. doi: 10.1001/archopht.1972.01000030169006. [DOI] [PubMed] [Google Scholar]
- Bone R. A., Sparrock J. M. Comparison of macular pigment densities in human eyes. Vision Res. 1971 Oct;11(10):1057–1064. doi: 10.1016/0042-6989(71)90112-x. [DOI] [PubMed] [Google Scholar]
- Campbell C. J., Rittler M. C., Noyori K. S., Swope C. H., Koester C. J. The threshold of the retina to damage by laser energy. Arch Ophthalmol. 1966 Sep;76(3):437–442. doi: 10.1001/archopht.1966.03850010439026. [DOI] [PubMed] [Google Scholar]
- DINGLE J. T. Studies on the mode of action of excess of vitamin A. 3. Release of a bound protease by the action of vitamin A. Biochem J. 1961 Jun;79:509–512. doi: 10.1042/bj0790509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine B. S., Geeraets W. J. Observations on early pathologic effects of photic injury to the rabbit retina. Acta Ophthalmol (Copenh) 1965;43(5):684–691. doi: 10.1111/j.1755-3768.1965.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Frisch G. D., Beatrice E. S., Holsen R. C. Comparative study of argon and ruby retinal damage thresholds. Invest Ophthalmol. 1971 Nov;10(11):911–919. [PubMed] [Google Scholar]
- Frisch G. D., Shawaluk P. D., Adams D. O. Remote nerve fibre bundle alterations in the retina as caused by argon laser photocoagulation. Nature. 1974 Mar 29;248(447):433–435. doi: 10.1038/248433a0. [DOI] [PubMed] [Google Scholar]
- Gass J. D. Photocoagulation of macular lesions. Trans Am Acad Ophthalmol Otolaryngol. 1971 May-Jun;75(3):580–608. [PubMed] [Google Scholar]
- Ham W. T., Jr, Geeraets W. J., Mueller H. A., Williams R. C., Clarke A. M., Cleary S. F. Retinal burn thresholds for the helium-neon laser in the rhesus monkey. Arch Ophthalmol. 1970 Dec;84(6):797–809. doi: 10.1001/archopht.1970.00990040799021. [DOI] [PubMed] [Google Scholar]
- Hogan M. J. Role of the retinal pigment epithelium in macular disease. Trans Am Acad Ophthalmol Otolaryngol. 1972 Jan-Feb;76(1):64–80. [PubMed] [Google Scholar]
- Kuwabara T., Gorn R. A. Retinal damage by visible light. An electron microscopic study. Arch Ophthalmol. 1968 Jan;79(1):69–78. doi: 10.1001/archopht.1968.03850040071019. [DOI] [PubMed] [Google Scholar]
- Lappin P. W., Coogan P. S. Relative sensitivity of various areas of the retina to laser radiation. Arch Ophthalmol. 1970 Sep;84(3):350–354. doi: 10.1001/archopht.1970.00990040352015. [DOI] [PubMed] [Google Scholar]
- Leibowitz H. M., Peacock G. R., Friedman E. The retinal pigment epithelium. Radiation thresholds associated with the Q-switched ruby laser. Arch Ophthalmol. 1969 Sep;82(3):332–338. doi: 10.1001/archopht.1969.00990020334007. [DOI] [PubMed] [Google Scholar]
- Marshall J., Mellerio H. J. Histology of retinal lesions produced with Q-switched lasers. Exp Eye Res. 1968 Apr;7(2):225–230. doi: 10.1016/s0014-4835(68)80071-5. [DOI] [PubMed] [Google Scholar]
- Marshall J. Thermal and mechanical mechanisms in laser damage to the retina. Invest Ophthalmol. 1970 Feb;9(2):97–115. [PubMed] [Google Scholar]
- Poole A. R., Howell J. I., Lucy J. A. Lysolecithin and cell fusion. Nature. 1970 Aug 22;227(5260):810–814. doi: 10.1038/227810a0. [DOI] [PubMed] [Google Scholar]
- RUDDOCK K. H. EVIDENCE FOR MACULAR PIGMENTATION FROM COLOUR MATCHING DATA. Vision Res. 1963 Dec;61:417–429. doi: 10.1016/0042-6989(63)90093-2. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H. Phagocytosis by pigment epithelium of human retinal cones. Nature. 1974 Nov 22;252(5481):305–307. doi: 10.1038/252305a0. [DOI] [PubMed] [Google Scholar]
- TORMEY J. M. DIFFERENCES IN MEMBRANE CONFIGURATION BETWEEN OSMIUM TETROXIDE-FIXED AND GLUTARALDEHYDE-FIXED CILIARY EPITHELIUM. J Cell Biol. 1964 Dec;23:658–664. doi: 10.1083/jcb.23.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso M. O., Wallow I. H., Powell J. O. Differential susceptibility of rod and cone cells to argon laser. Arch Ophthalmol. 1973 Mar;89(3):228–234. doi: 10.1001/archopht.1973.01000040230014. [DOI] [PubMed] [Google Scholar]
- VOS J. J. A theory of retinal burns. Bull Math Biophys. 1962 Jun;24:115–128. doi: 10.1007/BF02477421. [DOI] [PubMed] [Google Scholar]
- Wallow I. H., Lund O. E., Gabel V. P., Birngruber R., Hillenkamp F. A comparison of retinal argon laser lesions in man and in cynomolgus monkey. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974 Jan 22;189(3):159–164. doi: 10.1007/BF00414777. [DOI] [PubMed] [Google Scholar]
- Watzke R. C., Moore R. T. Ruby laser photocoagulation of the papillomacular bundle. An experimental study. Arch Ophthalmol. 1972 Jun;87(6):684–687. doi: 10.1001/archopht.1972.01000020686014. [DOI] [PubMed] [Google Scholar]
- Wolbarsht M. L., Fligsten K. E., Hayes J. R. Retina: pathology of neodymium and ruby laser burns. Science. 1965 Dec 10;150(3702):1453–1454. doi: 10.1126/science.150.3702.1453. [DOI] [PubMed] [Google Scholar]
- Young R. W., Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969 Aug;42(2):392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W. Shedding of discs from rod outer segments in the rhesus monkey. J Ultrastruct Res. 1971 Jan;34(1):190–203. doi: 10.1016/s0022-5320(71)90014-1. [DOI] [PubMed] [Google Scholar]