Abstract
Background:
The role of chronic exposure to ambient air pollutants in increasing COVID-19 fatality is still unclear.
Objectives:
The study aimed to investigate the association between long-term exposure to air pollutants and mortality among 4 million COVID-19 cases in Italy.
Methods:
We obtained individual records of all COVID-19 cases identified in Italy from February 2020 to June 2021. We assigned 2016–2019 mean concentrations of particulate matter (PM) with aerodynamic diameter (), PM with aerodynamic diameter (), and nitrogen dioxide () to each municipality () as estimates of chronic exposures. We applied a principal component analysis (PCA) and a generalized propensity score (GPS) approach to an extensive list of area-level covariates to account for major determinants of the spatial distribution of COVID-19 case–fatality rates. Then, we applied generalized negative binomial models matched on GPS, age, sex, province, and month. As additional analyses, we fit separate models by pandemic periods, age, and sex; we quantified the numbers of COVID-19 deaths attributable to exceedances in annual air pollutant concentrations above predefined thresholds; and we explored associations between air pollution and alternative outcomes of COVID-19 severity, namely hospitalizations or accesses to intensive care units.
Results:
We analyzed 3,995,202 COVID-19 cases, which generated 124,346 deaths. Overall, case–fatality rates increased by 0.7% [95% confidence interval (CI): 0.5%, 0.9%], 0.3% (95% CI: 0.2%, 0.5%), and 0.6% (95% CI: 0.5%, 0.8%) per increment in , , and , respectively. Associations were higher among elderly subjects and during the first (February 2020–June 2020) and the third (December 2020–June 2021) pandemic waves. We estimated COVID-19 deaths were attributable to pollutant levels above the World Health Organization 2021 air quality guidelines.
Discussion:
We found suggestive evidence of an association between long-term exposure to ambient air pollutants with mortality among 4 million COVID-19 cases in Italy. https://doi.org/10.1289/EHP11882
Introduction
The COVID-19 pandemic is one of the most critical public health crises the world has met in the contemporary age: as of 6 February 2023, cases and deaths have occurred worldwide.1 Of them, a total of 25,453,789 confirmed cases of COVID-19 (42,098 cases per 100,000), and 186,833 deaths (309 deaths per 100,000) have been recorded in Italy, ranking it ninth highest in the world in number of cases, and the sixth highest in number of deaths.1
When the COVID-19 pandemic reached Europe, the first and most affected area was northern Italy, incidentally one of the most polluted regions on the continent. Because the same pattern was observed in China, this co-occurrence of a high number of COVID-19 deaths and high levels of atmospheric pollution contributed to generating the hypothesis that the spread of SARS-CoV-2 and the severity of COVID-19 disease might be enhanced by high atmospheric pollution.2–4
However, most epidemiological studies trying to associate long-term air pollution exposure with SARS-CoV-2 incidence or COVID-19 poor prognosis were based on geographical correlations with low spatial resolution and were not designed to elicit possible causal associations.5,6 This approach was taken in several large-scale nationwide studies conducted in the United States, England, and Germany that reported associations between average air pollution levels in the years before the pandemic and COVID-19 disease and case fatality.7–10 These studies were based on large spatial units (city, county, province, state) and accounted for some area-level covariates; however, they did not adequately control for confounding from spatial-temporal patterns of air pollution and COVID-19 health outcomes. As clearly pointed out by Villeneuve and Goldberg, these studies were affected also by several other potential fallacies, including: misclassification and underreporting of incidence and mortality of COVID-19; not accounting properly for the differences of jurisdictions on the pandemic curve; not accounting for physical distancing and other public health interventions; serious problems from clustering of disease; possible issues with spatial-temporal variations in the strains of COVID-19 that may affect sequelae differently; not being able to deal with other determinants of COVID-19 mortality, especially occupation and socioeconomic status; and spatial-temporal assignment of air pollution correlating with socioeconomic status.5
Only a few recent studies have addressed some of these issues. They were large-scale investigations such as the nationwide study in England based on 32,844 small-area units of analysis11; an individual-level study conducted in Mexico City, Mexico12; population-based cohort studies in Catalonia, Spain,13 and Rome, Italy14; a prospective cohort of SARS-CoV-2 cases in Ontario, Canada15; a UK Biobank-based study16; and a statewide, population-based study in California.17
The objectives of the EpiCovAir mortality study, coordinated by the National Institute of Health (ISS) and the National System for the Environmental Protection (ISPRA-SNPA), was to investigate the association between long-term exposure to particulate matter (PM) with aerodynamic diameter (), PM with aerodynamic diameter (), and nitrogen dioxide (), with mortality among the entire population of COVID-19 cases identified in Italy from February 2020 to June 2021. To overcome some of the limitations of previous studies, we developed a fine-scale spatiotemporal machine-learning model for exposure assessment, we adopted a causal modeling framework to account for potential confounding of individual and contextual variables, we considered multiple interaction terms between temporal and spatial components, we compared effect estimates across pandemic waves, and we estimated associations with hospitalizations and accesses to intensive care units as secondary outcomes. Last, we estimated the COVID-19 deaths attributable to annual air pollutant concentrations exceeding the World Health Organization (WHO) air quality guidelines (AQG) or the European Union (EU) limit values.
Methods
COVID-19 Surveillance Data
The national COVID-19 surveillance system is the official source of records of COVID-19 cases in Italy (https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data). This system provides individual records of all subjects who tested positive for SARS-CoV-2 through reverse transcription polymerase chain reaction (starting from 20 February 2020) or by antigen test for SARS-CoV-2 infection confirmed in a regional authorized laboratory or pharmacy (starting from 15 January 2021), for a total of cases registered in Italy up to 16 June 2021. As part of the ISS COVID-19 surveillance system, for each subject information was available on age (at time of COVID-19 diagnosis), sex, municipality of residence (city/town where the patient resided at the time of diagnosis), date of testing, presence of symptoms at onset (distinguished as asymptomatic: no apparent signs or symptoms of disease; paucisymptomatic: general mild symptoms, such as general malaise, low-grade fever, and tiredness but no clear signs of disease; mild: clear signs and symptoms of disease, such as dry cough and shortness of breath, but not severe enough to require hospitalization; severe: clear signs and symptoms of disease, such as respiratory disease, and severe enough to require hospitalization; and critical: clear signs and symptoms of disease and severe enough to require admission to an intensive care unit), hospitalization (whether the patient was admitted following the COVID-19 diagnosis), access to intensive care unit [whether the person was transferred to an intensive care unit (ICU) during the index hospitalization], and vital status at the end of follow-up (death or recovery).18 According to Italian guidelines, based on indications from the WHO, a death was considered related to COVID-19 if it occurred in the presence of a clinical and instrumental picture suggestive of COVID-19, in the absence of a clear cause of death different from COVID-19 (e.g., road accident), and in the absence of a complete clinical recovery from the disease.19 We excluded all records with missing information on age, sex, municipality of residence or area-level covariates [ (see later section titled “Area-Level Contextual Covariates”)]; health care professionals (); and municipalities with cases (), for a total of 3,995,502 cases (124,346 deaths) included in the analysis (Figure S1), equal to 95% of the original population. Health care professionals were excluded because they were considered at much higher risk of being infected by and potentially dying from COVID-19, regardless their environmental exposures.
We had no detailed data on vaccines; however, the vaccination campaign started in Italy only at the end of January 2021. Therefore, there is minimal overlap with our study period.
Air Pollution Data
Chronic exposure to ambient air pollution was assigned to the municipality of residence of each COVID-19 case based on a previously developed spatiotemporal exposure model that predicted mean concentrations of , , and for each square kilometer of the Italian territory during 2016–2019.20 Specifically, we collected daily concentrations of the air pollutants from monitoring stations in Italy and trained a machine-learning model, the random forest, using spatiotemporal (dispersion models, satellite-based aerosol optical depth, air temperature, and other meteorological parameters from Copernicus, vegetation indices), as well as spatial (elevation, road network, land cover, population density administrative regions, light-at-night) predictors. The models were carefully cross-validated by partitioning the monitors into training and testing sets. Finally, the model output was extrapolated to all grid cells of Italy and all days in 2016–2019.20 From these estimates, we derived 2016–2019 mean concentrations at each municipality () by averaging the daily values of all the grid cells intersecting the municipality with weights proportional to the population residing in each cell (population-weighted exposures). We could not go back beyond 2016 (because estimates from the spatiotemporal model were available only for the latest period), but we assume that the spatial distribution of 2016–2019 population-average pollutants adequately captured the chronic exposure of the study population.
Area-Level Contextual Covariates
We collected data on 54 municipality-level variables classified into five main domains aimed at describing the most relevant determinants of the spatial distribution of COVID-19 cases and deaths (Table S1):
Municipality characteristics: a set of 12 variables related to municipality code, region and province, area size, elevation, altimetric zone, coastal/island location, urbanization degree, and geographic coordinates
Population: five variables related to population size (years 2011 and 2019), population classes, population density and percentage of population above 65 y old
-
Mobility: a set of 13 variables including:
Attraction Index: ratio between movements of individuals who work or study in the municipality, and total individuals in the area
Self-containment Index: ratio between individuals who work or study in the municipality, and total movements in the area
Numbers of flights and passengers during 2019 and 2020
Movements in, out, and total: number of individuals who moved outbound or inbound (and total) of the municipality for work or study reasons
Code of Local Work System (Sistema Locale del Lavoro, SLL): a composite index developed by the Italian Institute of Statistics to characterize connections among municipalities
Presence (yes/no) and number of rail stations
Number of airports within of the municipality centroid.
Socioeconomic and health status: a set of 10 variables, including income, number of enterprises per 1,000 inhabitants, composite socioeconomic position index,21 cause-specific hospitalization and mortality rates
Health care offer: a set of 14 variables, including, for each municipality, numbers of hospitals, nursing homes or emergency rooms; numbers of beds for different types of wards; distances between the municipality centroid and the closest facility, by type of facilities; and average number of workers in healthcare residences.
These variables were then synthesized in 12 principal components (PCs), as described in Bauleo et al.22 and summarized in the next section.
Principal Component Analysis (PCA)
A PCA was performed for each of the five domains separately. The goal of the PCA was to reduce the large number of initial correlated variables into a smaller number of components by preserving most of their informative content. This process occurred through a linear transformation of the original standardized variables into new ones that were orthogonal (i.e., independent) and sorted in decreasing order of variance. The reduction of complexity was achieved by retaining only the components with eigenvalues , for a total number of final components equal to 12, distributed across the five domains.22
Statistical Analysis
The propensity score is the conditional probability of being exposed, given the observed covariates.23 Originally developed for binary treatments, it has been recently generalized to continuous exposures, hence the term “generalized propensity score” (GPS).24 In the continuous case, the GPS represents the conditional likelihood of being exposed to the observed exposure level given the covariates. Applied to our study, GPS represents the conditional likelihood, at the municipality level, of exposure to the observed level of air pollution, given observed area-level covariates. We adopted different formulations of GPS corresponding to different sets of covariates. In the main approach, we used, as the only covariates, the four PCs pertaining the two domains of a) socioeconomic and health status, and b) health care offer. This choice was motivated by the rationale that only these two domains are plausibly related to the spatial distribution of fatal events among COVID-19 cases, whereas the other three domains (municipality characteristics, population, mobility) are responsible for the geographical distribution of SARS-CoV-2 cases (incidence).
The GPS model assumes the following formula:
| (1) |
where represents the long-term average air pollution (, , or , in turn) in municipality , is the model intercept, … are regression coefficients estimated for principal components . is the first principal component of the domain “socioeconomic and health status” and refers to socioeconomic conditions of the municipality; is the second principal component of the same domain and refers to overall population health (annual mortality and morbidity rates for cardiovascular and respiratory diseases); and are the first and second principal components of the domain “health care offer,” and capture, respectively, availability (presence, number of beds, etc.) and accessibility (distance) to health care facilities.
Once the previous model was defined, we built the actual GPS as follows:
| (2) |
where represents our main GPS in municipality i, defined as a multinormal covariate of the original exposure values, centered in the fitted values of the previous model, with SD equal to the SD of the residuals.
In sensitivity analysis 1, we defined the GPS based on the entire set of 12 PCs for the 5 domains, whereas in sensitivity analysis 2 we used as covariates for GPS estimation the 9 original area-level covariates describing the 2 domains of socioeconomic and health status and health care offer (rather than the corresponding 4 PCs) (see Supplemental Material, Figures S5–S7 and Excel Tables S3–S5).
The association between long-term exposure to air pollutants and case–fatality rates was estimated with negative binomial regression models. First, we aggregated COVID-19 cases (denominators) and deaths (numerators) by municipality, year, month, age (5-y classes), and sex. Second, we fit negative binomial regression models with the number of deaths as the outcome variable, the number of cases as the offset term, the air pollutant as the exposure, and with increasing level of confounding adjustment, as detailed below:
where GLM identifies generalized linear models, and represent, respectively, the count of deaths (numerator) and the number of COVID-19 cases (denominator) in municipality i for each age-sex-year-month stratum; is the air pollution average (, or , in turn) of municipality i; M1 represents the crude (e.g., unadjusted) model.
M2 further adjusted for province of the case (categorical variable of 110 provinces in total): The aim was to control for all (known and unknown) covariates varying from province to province but fixed in time.
M3 further adjusts for all interactions between categorical variables of year and month (i.e., pandemic phase) and province: The aim was to control for all (known and unknown) covariates varying from province to province differently over time.
M4 further adjusts for all interactions between time trends, province, and age classes (5-y groups): The aim was to control for all covariates varying from province to province differently over time and by age groups of COVID-19 cases and deaths.
M5 further adjusts for all interactions between time trends, province, age class, and sex.
M6 represents our “main” model. In addition to accounting for all the above interactions, it also accounts for GPS distribution by matching on ventiles (i.e., quantiles that partition GPS in 20 equal-sized groups) of the GPS. The aim of this model was to restrict the inference on comparisons among municipalities belonging to the same province, at the same pandemic stage, with same age and sex distribution, and with approximately the same values of the GPS (matching on ventiles).
Although Models 1–6 represent nested models with increasing degrees of confounding adjustment, models 7–13 below represent alternative ways to adjust for the confounding role of area-level covariates:
M7 adjusts for GPS as a linear term in the model (instead of matching on ventiles).
M8 directly adjusts for the four PCs defining the main GPS.
M9 adjusts for GPS by using inverse probability weights.
M10 matches by percentiles (instead of ventiles) of the main GPS.
M11 matches by deciles (instead of ventiles) of the main GPS.
M12 matches by ventiles of the GPS described above in sensitivity analysis 1.
M13 matches by ventiles of the GPS described above in sensitivity analysis 2.
In each of these models, the air pollutant was added preliminary with a linear term, and associations are expressed as the percent increase of fatality rate (%IR), and corresponding 95% confidence intervals (CI), per unit increment of exposure, by transforming the regression coefficient with the following formula:
| (3) |
We performed a number of additional analyses. First, we defined three pandemic waves in agreement with indications from the Italian Institute of Health, as: first (20 February 2020–31 May 2020), second (15 September 2020–15 December 2020) and third (16 December 2020–15 June 2021), and we fit separate models by pandemic waves. Second, we fit separate models by age class, sex, presence of symptoms at onset (asymptomatic vs. all the others), and geographical area (Po valley), to identify population subgroups or areas potentially more vulnerable to the adverse effects of chronic exposure to air pollution. Third, we modeled air pollutants with natural splines with three degrees of freedom to describe the shape of the exposure–response functions. Fourth, we ran two-pollutant models, where pairs of air pollutants ( and , and ) were entered simultaneously in the regression model. Fifth, we quantified the numbers of COVID-19 deaths attributable to exceedances in annual air pollutant concentrations above predefined thresholds corresponding to WHO AQG or EU limit values, as described in the next section. Sixth, we investigated the association between air pollution and hospitalizations or accesses to ICUs among COVID-19 cases. Seventh, we included health care professionals in the analyses to test the robustness of the main results to their inclusion/exclusion.
Attributable Cases
The associations estimated above from our main model 6 (either single-pollutant or two-pollutant) were used to quantify the numbers of deaths attributable to exceedances in PM and above predefined thresholds.
For the single-pollutant case, we applied the following formula:
| (4) |
where:
quantifies the total number of deaths attributable to concentrations of air pollutant exceeding the threshold (, 20, 25, 30, 35, for ; , 10, 15, 20, or for ; and , 20, 30, or for ).
is the count of deaths among COVID-19 cases in the municipality i.
is the regression coefficient representing the log(relative risk) of death per unit increment in exposure. For the computation of attributable cases, we have used the resulting from the base model M6, where exposure was modeled with a linear term.
For the two-pollutant case, we applied the following formula:
| (5) |
where:
quantifies the total number of deaths attributable to concentrations of air pollutant exceeding the threshold 1 and, at the same time, air pollutant exceeding the threshold t2;
is the count of deaths among COVID-19 cases in the municipality i;
is the regression coefficient representing the log(relative risk) of death per unit increment in exposure E, and is the regression coefficient representing the log(relative risk) of death per unit increment in exposure F. In this case, for the computation of attributable cases, we used the and resulting from the base model M6 where both pollutants were modeled simultaneously (two-pollutant model) with linear terms.
All statistical analyses have been performed with the R statistical software (version 4.1.2; R Development Core Team). We excluded observations with missing data from the analyses, which amounted to 54,269 (1.3%) of the eligible population. All maps have been produced with ArcGIS software (ESRI ArcGIS Desktop: Release 10; Environmental Systems Research Institute), using the shapefile of year 2019 municipalities released by ISTAT as base layer.
Results
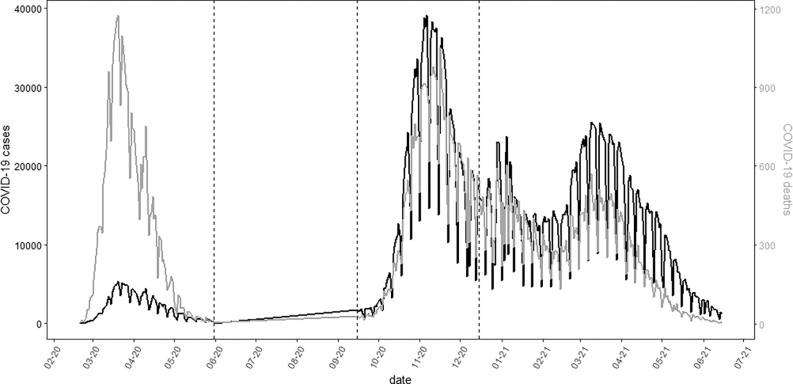
We analyzed data on 3,995,202 COVID-19 cases and 124,346 deaths (Table 1 and Figure 1; Excel Table S1). Most of the cases were diagnosed in the second and third pandemic waves (38% and 56%, respectively), although mortality was much higher in the first period (176/1,000 in the first wave, against 27/1,000 and 21/1,000 in the second and third, respectively). Cases were mostly diagnosed among young and adult subjects (79%, 0–64 y old), whereas mortality increased exponentially with age (72%, y old), with no major differences by sex. COVID-19 fatality rates were higher in symptomatic cases than among asymptomatic subjects (45 vs. 13/1,000). Finally, we did not detect differential susceptibility by socioeconomic status, with case–fatality rates homogeneous across categories of the deprivation index (Table 1).
Table 1.
Descriptive statistics of the study population: distribution of the covariates and air pollutant concentrations among COVID-19 cases and among deceased subjects. Italy, 20 February 2020–15 June 2021 ( COVID-19 cases with nonmissing data).
| Cases [ (%)] | Deaths [ (%)] | Fatality rate (per 1,000) | |
|---|---|---|---|
| Original population | 4,038,677 | 125,238 | 31 |
| Population (without missing) | 3,995,202 (100.0) | 124,346 (100.0) | 31 |
| Wave of the COVID-19 pandemic | |||
| 1st: 20 February 2020–31 May 2020 | 201,210 (5.0) | 35,440 (28.5) | 176 |
| 2nd: 15 September 2020–15 December 2020 | 1,534,950 (38.4) | 41,620 (33.5) | 27 |
| 3rd: 16 December 2020–15 June 2021 | 2,259,042 (56.5) | 47,286 (38.0) | 21 |
| Covariates | |||
| Age (y) | |||
| 0–64 | 3,173,243 (79.4) | 11,879 (9.6) | 4 |
| 65–74 | 369,907 (9.3) | 23,164 (18.6) | 63 |
| 75–84 | 282,527 (7.1) | 44,914 (36.1) | 159 |
| 169,525 (4.2) | 44,389 (35.7) | 262 | |
| Sex (females) | 2,021,052 (50.6) | 54,060 (43.5) | 27 |
| Clinical state at onset | |||
| Asymptomatic | 1,740,258 (43.6) | 23,252 (18.7) | 13 |
| Symptomatic | 2,254,944 (56.4) | 101,094 (81.3) | 45 |
| Socioeconomic deprivation index (quintiles) | |||
| Lowest | 1,164,100 (29.1) | 37,922 (30.5) | 33 |
| Low | 784,627 (19.6) | 25,420 (20.4) | 32 |
| Medium | 790,333 (19.8) | 24,023 (19.3) | 30 |
| High | 718,853 (18.0) | 22,016 (17.7) | 31 |
| Highest | 537,289 (13.4) | 14,965 (12.0) | 28 |
| Exposures [mean (IQR)] | |||
| () | 17.1 (8.5) | 17.8 (9.0) | — |
| () | 25.6 (8.9) | 26.1 (9.9) | — |
| () | 23.1 (11.9) | 23.6 (12.0) | — |
Note: —, fatality rates for continuous covariates cannot be computed; IQR, interquartile range; , nitrogen dioxide; PM, particulate matter; , particulate matter with aerodynamic diameter micrometers; , particulate matter with aerodynamic diameter micrometers.
Figure 1.
Time trends of COVID-19 cases (black) and deaths (light gray) between February 2020 and 15 June 2021 in Italy. Relevant data in Excel Table S1. Dashed lines delimit different pandemic waves: first (20 February 2020–31 May 2020), second (15 September 2020–15 December 2020) and third (16 December 2020–15 June 2021).
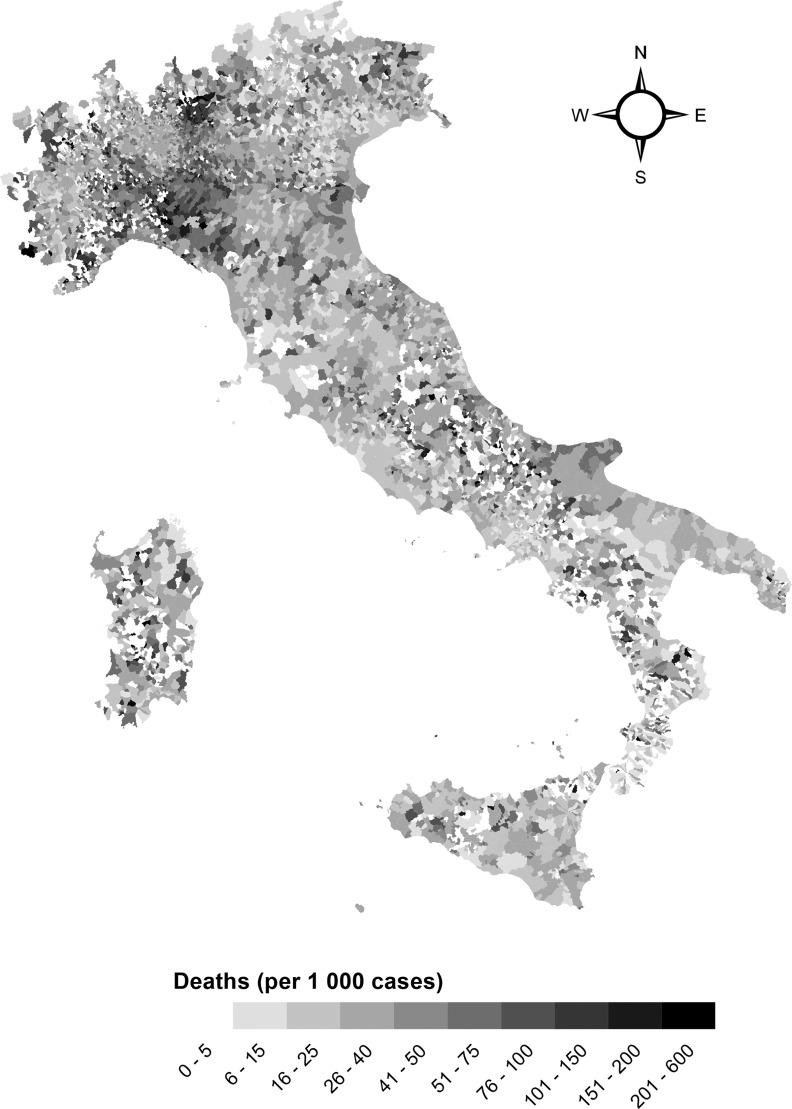
The spatial distribution of deaths shows much higher fatality rates in northern Italy (Figure 2), mostly driven by deaths in the first wave, whereas distributions were more homogeneous in the second and third periods (Figure S2). Similarly, municipality-specific rates of hospitalization and access to ICUs showed higher values in northern Italy (Figure S3).
Figure 2.
Map of COVID-19 case–fatality rates by municipality, Italy 20 February 2020–31 May 2020 and 15 September 2020–15 June 2021. Municipalities with fewer than three cases are in white.
The spatial distribution of air pollutants concentrations shows much higher exposures in northern Italy and specifically in the Po valley region, incidentally the same geographical area where the SARS-CoV-2 outbreak initially started (Figure S4).
The results of the association between chronic exposure to air pollutants and case–fatality are presented in Table 2: In our main model (model 6, adjusted for multiple interactions between year-month, province, age class, sex, and ventiles of the main GPS), increments in , , and were associated with increases in case–fatality rates of 0.7% (95% CI: 0.5%, 0.9%), 0.3% (95% CI: 0.2%, 0.5%), and 0.6% (95% CI: 0.5%, 0.8%), respectively. Effect estimates substantially dropped with increasing degree of confounding adjustment, especially once spatial patterns (model 2 for and ) and age-specific distributions (model 4 for all pollutants) were accounted for. Further adjustment for GPS by matching (model 6, the main model) slightly reduced the effect estimates for PM in comparison with model 5, whereas it did not change associations with . For all pollutants, results were robust to alternative ways of adjusting for the main GPS (models 7–11), as well as to adjustment for the two alternative GPS (models 12–13). In addition, results were robust to inclusion/exclusion of health care professionals (Table S2).
Table 2.
Effect of air pollutants on mortality, main approach, and sensitivity analyses: percent increase in mortality risk (%IR), and 95% CI per increment in air pollutants. Italy, 20 February 2020–15 June 2021 ( COVID-19 cases, deaths).
| Model | Description | %IR (95% CI) | %IR (95% CI) | %IR (95% CI) |
|---|---|---|---|---|
| Increasing adjustment levels | ||||
| M1 | Crude | 3.2 (3.0, 3.4) | 2.3 (2.1, 2.4) | 2.4 (2.2, 2.5) |
| M2 | 1.7 (1.3, 2.1) | 1.4 (1.1, 1.7) | 2.6 (2.4, 2.8) | |
| M3 | 1.5 (1.1, 1.9) | 1.2 (0.5, 1.5) | 2.2 (2.1, 2.4) | |
| M4 | 0.9 (0.6, 1.2) | 0.7 (0.5, 0.9) | 0.5 (0.4, 0.6) | |
| M5 | 1.0 (0.7, 1.2) | 0.7 (0.5, 0.9) | 0.5 (0.4, 0.7) | |
| M6 | Main model: (matched on ventiles) | 0.7 (0.5, 0.9) | 0.3 (0.2, 0.5) | 0.6 (0.5, 0.8) |
| Sensitivity models | ||||
| M7 | (linear term) | 1.0 (0.8, 1.3) | 0.7 (0.5, 0.9) | 0.5 (0.4, 0.7) |
| M8 | added as covariates | 1.3 (1.0, 1.6) | 1.0 (0.8, 1.2) | 0.8 (0.6, 0.9) |
| M9 | (inverse weights) | 1.3 (1.0, 1.5) | 1.0 (0.8, 1.1) | 0.7 (0.5, 0.8) |
| M10 | (matched on percentiles) | 1.0 (0.7, 1.3) | 0.6 (0.4, 0.7) | 0.4 (0.3, 0.6) |
| M11 | (matched on deciles) | 0.6 (0.3, 0.8) | 0.6 (0.4, 0.7) | 0.7 (0.6, 0.9) |
| M12 | GPS #1 | 1.3 (1.1, 1.5) | 0.6 (0.5, 0.8) | 1.0 (0.9, 1.1) |
| M13 | GPS #2 | 1.0 (0.8, 1.2) | 0.8 (0.7, 0.9) | 0.9 (0.8, 1.0) |
Note: CI, confidence interval; GPS, generalized propensity score; IR, increase of risk; M, model; nitrogen dioxide; , particulate matter with aerodynamic diameter micrometers; , particulate matter with aerodynamic diameter micrometers.
Similar results are presented for rates of hospitalization (Table S3) and access to ICUs (Table S4): We estimated increases in hospitalization rates of 0.9% (95% CI: 0.7%, 1.1%), 0.6% (95% CI: 0.5%, 0.8%), and 0.7% (95% CI: 0.6%, 0.8%), per increment in , , and , respectively. Corresponding increases in rates of access to ICUs were 1.6% (95% CI: 1.3%, 1.9%), 1.5% (95% CI: 1.3%, 1.7%), and 1.0% (95% CI: 0.8%, 1.1%). For both outcomes, results were largely robust to alternative models of confounding adjustment.
The associations between air pollutants and case–fatality were highest in the first and third pandemic waves (PM only), increased substantially with age (all pollutants), were similar between men and women, and were similar among subjects with or without symptoms at onset of COVID-19 disease. Finally, associations in the Po valley were similar to, if not smaller than, those estimated in the rest of the country (Table 3). Associations by individual-level characteristics specific for each pandemic wave are reported in Table S5.
Table 3.
Effect of air pollutants on mortality by pandemic wave, individual-level covariates, and geographical area: percent increase in mortality risk (%IR), and 95% CI, per increment in air pollutants. Italy, 20 February 2020–15 June 2021 ( COVID-19 cases, deaths).
| %IR (95% CI) | %IR (95% CI) | %IR (95% CI) | ||
|---|---|---|---|---|
| All | 3,995,202 | 0.7 (0.5, 0.9) | 0.3 (0.2, 0.5) | 0.6 (0.5, 0.8) |
| Wave | ||||
| 1st | 201,210 | 1.1 (0.5, 1.6) | 0.7 (0.4, 1.0) | 0.7 (0.4, 1.0) |
| 2nd | 1,534,950 | 0.1 (, 0.5) | 0.1 (, 0.4) | 0.6 (0.4, 0.8) |
| 3rd | 2,259,042 | 0.9 (0.6, 1.3) | 0.3 (0.0, 0.5) | 0.7 (0.5, 0.8) |
| Age (y) | ||||
| 0–64 | 3,173,243 | (, ) | (, ) | 0.3 (0.1, 0.5) |
| 65–74 | 369,907 | (, 0.5) | 0.0 (, 0.5) | 0.3 (0.0, 0.7) |
| 75–84 | 282,527 | 1.0 (0.3, 1.6) | 0.5 (0.0, 0.9) | 0.9 (0.5, 1.2) |
| 169,525 | 1.7 (1.0, 2.5) | 0.8 (0.4, 1.3) | 0.8 (0.4, 1.1) | |
| Sex | ||||
| Female | 2,021,052 | 0.9 (0.5, 1.2) | 0.4 (0.2, 0.6) | 0.6 (0.4, 0.7) |
| Male | 1,974,150 | 0.6 (0.2, 0.9) | 0.3 (0.1, 0.5) | 0.7 (0.5, 0.9) |
| Clinical state at onset | ||||
| Symptomatic | 2,254,944 | 0.9 (0.6, 1.1) | 0.4 (0.3, 0.6) | 0.6 (0.4, 0.7) |
| Geographical area | ||||
| Po Valley | 1,888,148 | 0.5 (0.2, 0.8) | 0.2 (0.1, 0.4) | 0.3 (0.1, 0.4) |
Note: Results of main model 6, adjusted for interactions between year and month, province, age classes, sex and ventiles of the generalized propensity score. Pandemic waves are defined as first: 20 February 2020–31 May 2020; second: 15 September 2020–15 December 2020; third: 16 December 2020–15/06/2021. CI, confidence interval; IR, increase of risk; , nitrogen dioxide; , particulate matter with aerodynamic diameter micrometers; , particulate matter with aerodynamic diameter micrometers.
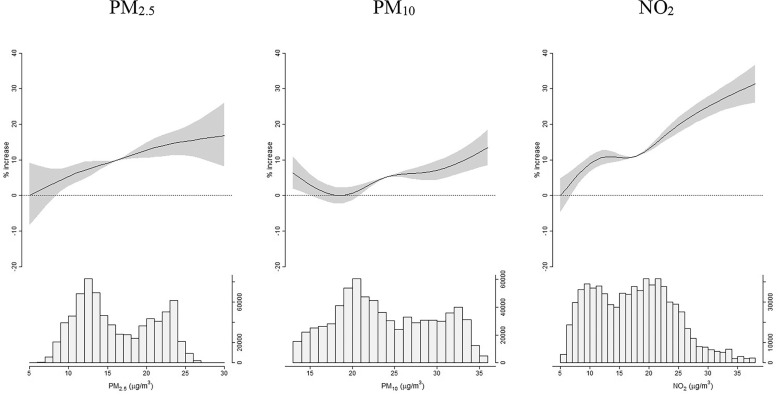
The association between and COVID-19 fatality was robust to PM adjustment, whereas associations with or became null after adjustment for (Table S6). The exposure–response functions displayed in Figure 3 and in Excel Table S2 are consistent with linear associations, with case–fatality rates increasing significantly already at very low and concentrations.
Figure 3.
Exposure–response functions: percentage increase in case–fatality risk (%IR), and 95% confidence intervals (95% CI), per increasing levels of air pollutants, from natural spline models. Italy, 20 February 2020–15 June 2021 ( COVID-19 cases, deaths). Relevant data in Excel Table S2. Y axes of the top graphs display percentage increases of risk, x axes of the top graphs report air pollutants concentrations. Bottom graphs show histograms of air pollutants’ distributions. Results from the main model, adjusted for interaction terms between month, province, age, sex, and ventiles of the generalized propensity score.
Finally, we estimated 10,514 (95% CI: 7,007; 13,902), 4,582 (95% CI: 2,512; 6,607) and 10,155 (95% CI: 8,295; 11,973) deaths among COVID-19 cases exposed to annual concentrations of , , or exceeding the WHO 2021 AQG of 5, 15, and , respectively (Tables 4 and 5). Corresponding estimates from two-pollutant models are: 9,163 (95% CI: 394; 17,182) for and simultaneously exceeding the WHO 2021 AQG values (Table 4) and 7,430 (95% CI: 326; 14,008) for and simultaneously above the WHO 2021 AQG values (Table 5), demonstrating large overlap of COVID-19 deaths due to exceedances of the three air pollutants.
Table 4.
Deaths attributable to and concentrations above predefined thresholdsa: results from both single-pollutant and two-pollutant models. Each cell reports attributable cases and 95% confidence interval. Italy, 20 February 2020–15 June 2021 ( COVID-19 cases, deaths).
| thresholds | thresholds | Single pollutant : Deaths (95% CI) | |||
|---|---|---|---|---|---|
| 10: Deaths (95% CI) | 20: Deaths (95% CI) | 30: Deaths (95% CI) | 40: Deaths (95% CI) | ||
| 5 | 9,163 (394; 17,182) | 2,121 (; 7,616) | 186 (; 2,187) | (; 512) | 10,514 (7,007; 13,902) |
| 10 | 10,762 (4,338; 16,728) | 3,278 (; 7,304) | 612 (; 2,072) | (; 481) | 6,513 (4,321; 8,650) |
| 15 | 9,235 (5,391; 12,838) | 3,598 (999; 6,065) | 917 (; 1,830) | 100 (; 450) | 3,080 (2,036; 4,105) |
| 20 | 6,963 (4,907; 8,908) | 3,349 (2,015; 4,628) | 1,053 (522; 1,565) | 213 (2, 418) | 1,023 (673; 1,370) |
| 25 | 594 (462, 720) | 319 (242, 394) | 100 (76, 124) | 17 (13, 21) | 14 (9, 19) |
| Single pollutant | 10,155 (8,295; 11,973) | 4,187 (3,410; 4,951) | 1,136 (924; 1,346) | 239 (194, 285) | — |
Note: Results of main model 6, adjusted for interactions between year and month, province, age classes, sex, and ventiles of the generalized propensity score. —, no data; AQG, air quality guidelines; CI, confidence interval; , nitrogen dioxide; , particulate matter with aerodynamic diameter micrometers; , particulate matter with aerodynamic diameter micrometers; WHO, World Health Organization.
WHO 2021 AQG levels: for , for ; WHO 2005 AQG levels: for , for ; EU (European Union) air quality standards: for , for .
Table 5.
Deaths attributable to and concentrations above predefined thresholdsa: results from both single-pollutant and two-pollutant models. Each cell reports attributable cases and 95% CI. Italy, 20 February 2020–15 June 2021 ( COVID-19 cases, deaths).
| thresholds | thresholds | Single pollutant : Deaths | |||
|---|---|---|---|---|---|
| 10: Deaths (95% CI) | 20: Deaths (95% CI) | 30: Deaths (95% CI) | 40: Deaths (95% CI) | ||
| 15 | 7,430 (326; 14,008) | 1,404 (; 5,798) | (; 1,573) | (, 331) | 4,582 (2,512; 6,607) |
| 20 | 8,588 (3,324; 13,512) | 2,513 (; 5,749) | 370 (; 1,570) | (; 333) | 2,699 (1,476; 3,903) |
| 25 | 7,303 (3,999; 10,410) | 3,021 (858; 5,084) | 730 (; 1,534) | 38 (; 335) | 1,267 (691; 1,837) |
| 30 | 4,878 (3,176; 6,484) | 2,494 (1,401; 3,541) | 838 (373; 1,287) | 152 (; 336) | 393 (214, 571) |
| 35 | 1,073 (555; 1,544) | 683 (250; 1,083) | 338 (40; 618) | 46 (; 236) | 231 (126; 333) |
| 40 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Single pollutant | 10,155 (8,295; 11,973) | 4,187 (3,410; 4,951) | 1,136 (924; 1,346) | 239 (194, 285) | — |
Note: Results of main model 6, adjusted for interactions between year and month, province, age classes, sex and ventiles of the generalized propensity score. —, no data; AQG, airquality guidelines; CI, confidence interval; , nitrogen dioxide; , particulate matter with aerodynamic diameter micrometers; , particulate matter with aerodynamic diameter micrometers; WHO, World Health Organization.
WHO 2021 AQG levels: for , for ; WHO 2005 AQG levels: for , for ; EU (European Union) air quality standards: for , for .
Discussion
We found statistically significant associations between long-term exposure to air pollution and mortality, hospital admissions, and access to intensive care units in a large national study of 4 million COVID-19 cases documented in Italy in three epidemic waves from February 2020 to June 2021. The associations with mortality were robust to alternative choices of confounding adjustment, were stronger among elderly subjects, did not differ by sex or by presence of symptoms, and were higher during the first and the third pandemic waves. We estimated (8%) deaths attributable to exceedances in annual air pollutant concentrations above the WHO 2021 AQG.
Bozack et al. analyzed patient-level data from seven New York City hospitals and found that higher residential exposures to concentrations were associated with an increased risk of mortality and ICU admission [ (95% ) and (95% ) per increase in , respectively].25 The associations estimated by Bozack et al. are consistent with those found in some large-scale ecological analyses. For example, an analysis of COVID-19 mortality across 3,143 U.S. counties found that a increase in exposure was associated with an 8% increase in COVID-19 mortality rate.7 When the effect of ecological bias is minimized by exploiting the variability of the exposure at high geographical resolution and increasing degree of confounding adjustment, the effect estimates are closer to our results: A nationwide cross-sectional study in England estimated small but statistically significant associations between long-term exposure to or with COVID-19 mortality, with percentage increased (95% CI: 0.2, 1.2), and 1.4% (95% CI: 2.1, 5.1), per increment, respectively, after adjusting for confounding and spatial autocorrelation.11 However, the hospital setting and ecological design of these studies limit causal interpretation and do not allow an optimal comparison with findings on our population sample. Our study results are similar, in strength and direction, to those found in a cohort of 151,105 confirmed SARS-CoV-2 infections in Ontario, Canada.15 Chen et al. estimated, for each interquartile range increase in exposure to (), odds ratios of 1.06 (95% CI: 1.01, 1.12), 1.09 (95% CI: 0.98, 1.21) and 1.00 (95% CI: 0.90, 1.11) for hospital admission, ICU admission, and death, respectively, whereas smaller estimates were observed for .15
English et al., using individual-level patient data and highly localized exposure estimates in SARS-CoV-2 cases and 49,691 COVID-19 deaths that occurred in California, found a 3.8% increased mortality risk per when comorbidity conditions were considered.17 A study from Mexico City using individual-level data showed that the risk of dying from COVID-19 increased by 0.77% per increase in 2000–2018 average concentration, after adjustment for individual- and municipality-level covariates.12
A meta-analysis estimated positive comparable associations between COVID-19 mortality and increases in (, 95% CI: 1.01, 1.06) and (, 95% CI: 1.02, 1.07), from studies that adequately adjusted for the confounding effects of population density and air temperature, whereas no association was found with other air pollutants, like nitrogen oxides (), ozone (), or .26 A prospective, individual-level cohort study (COVICAT) conducted in Catalonia, Spain, also found significant associations between residential air pollution concentrations and both hospitalizations from COVID-19 disease and self-reported symptoms, with adjusted RRs of 1.00 (95% CI: 1.00, 1.02) and 1.09 (95% CI: 1.02, 1.16) per increases in and , respectively, and associations being stronger for more severe forms of the disease.13
Our results showed that, using the exposure–response coefficients estimated in our main models, (8%) deaths were attributable to exceedances in annual air pollutant concentrations above the WHO 2021 AQG. The hypothesized links between air pollution and COVID-19 make the public health consequences of the pandemic more critical, because ambient air pollution is the seventh global risk factor for mortality, responsible for deaths worldwide in 2019 (12% of the overall global burden).27
In our study, long-term exposure to air pollution shows a weaker effect on COVID-19 fatality rate during the second wave when compared with the first and the third wave. We have no definitive explanation for this finding, but we can speculate that in the first wave the most affected areas were the northern regions (with also higher air pollution levels), and the surveillance system possibly detected mainly severe cases, because only symptomatic people could be tested for SARS-CoV-2 infection; the second wave affected the Italian regions in a more homogeneous way, but most fragile cases, for which the long-term effect of air pollution was a priori more plausible, had already been affected in the previous wave; in the third wave, the dominant strain became the Delta variant, making the effect of air pollution closer to what was observed in the first wave. Furthermore, we cannot exclude a possible role of air temperature in the differential effects of air pollution across waves, as suggested in a recent study.28
A large body of evidence has accumulated over the past several years, demonstrating that air pollution affects almost all organ systems29,30 and causes a broad variety of effects, spanning from asthma symptoms and exacerbation to illness and death from ischemic heart disease, lung cancer, COPD, lower-respiratory infections, stroke, type 2 diabetes, and adverse birth outcomes.31,32
The global spread of the COVID-19 outbreak contributed to a renewed attention to the adverse effects of air pollution for three main reasons: a) PM has been hypothesized to be a carrier for the SARS-CoV-2 virus and therefore able to increase the contagion2,3; b) long-term exposure to and has been associated with overexpression of ACE-2 receptors, to which the SARS-CoV-2 spike protein binds, increasing the virus susceptibility and the severity of COVID-19 disease33,34; c) air pollution–related chronic health conditions, such as diabetes, cardiovascular disease, and chronic obstructive pulmonary disease (COPD), have also been associated with increased vulnerability to COVID-19.35–38 Concerning the last point, long-term exposure to air pollution can worsen the prognosis of COVID-19 by increasing the risk of chronic diseases associated with COVID-19, both by directly suppressing or influencing early immune responses to SARS-CoV-2 infection and by altering the host’s immunity toward respiratory infections, and these mechanisms have been shown to be biologically plausible.39–41 In addition, researchers have found that many preexisting chronic comorbidities, such as diabetes, cardiovascular disease, cancer, and kidney diseases, are also important risk factors for more severe COVID-19.42,43
This study has several strengths. First, to the best of our knowledge, it is the only study ever conducted in Italy with individual records on the entire population of COVID-19 cases. We were able to analyze data on 4 million cases diagnosed between February 2020 and June 2021, with individual-level information on sociodemographic characteristics and clinical state at onset. Such data were complemented by an extensive list of contextual variables on municipality topography, population density, mobility, socioeconomic and health status, and access to health care resources. This information allowed strict control for all major individual and area-level determinants of COVID-19 severity in the epidemiological analyses. Second, we adopted a causal modeling framework, the GPS, to adjust for the potential confounding of contextual covariates. We still refrain from considering our association estimates as causal; however, we believe that our methodology, paired with the inclusion in the statistical models of multiple interaction terms between temporal and spatial components as well as the extensive list of sensitivity models, provides suggestive evidence of a plausible causal link between chronic exposure to air pollution and COVID-19 poor prognosis. This belief is further supported by the consistent associations we found with alternative outcomes, such as rates of hospitalization and access to ICUs. Finally, we were able to characterize long-term exposure to different air pollutants on the basis of a sophisticated machine-learning model trained on a large set of spatial and spatiotemporal predictors.
Several limitations should also be acknowledged. First, individual residence was assessed at the municipality level; therefore, we had to aggregate cases and deaths and adopt an ecological study design. This approach has been criticized as prone to residual confounding, as opposed to individual-level prospective longitudinal studies.5,6 However, the availability of individual-level data on age, sex, and clinical state at onset allowed a further stratification for such variables. In addition, our analysis of COVID-19 cases (rather than the general population) eliminated the potential confounding role of unmeasurable determinants of SARS-CoV-2 spread (person-to-person contacts, fine-scale mobility, etc.), allowing us to focus our study hypothesis on COVID-19 poor prognosis. A second limit of our database is the inherent difficulty of the surveillance system to intercept asymptomatic cases, especially at the early stages of the pandemic. Even though information on the symptomatic state at onset was available for most cases, it is likely that many infected individuals, especially those with no or mild symptoms, were not included in the analysis. Therefore, our results are not representative of the total truly infected population. Third, we lacked information on the quality of care received by each hospitalized case. The quality of care could be, in principle, a strong determinant of prognosis, regardless of the severity of the disease, with potential differences over space and time. However, it is unlikely for this factor to be related with the spatial distribution of air pollution, once time trends of case–fatality rates by province have been accounted for in the models. A fourth limitation is related to the definition of GPS from the principal components: Because only a few components were selected from the original list of contextual covariates, the GPS was ultimately estimated based on four variables only, with limited ability to capture the complex relationship between area-level characteristics and COVID-19 case–fatality. However, application of alternative GPSs or adjustment for individual covariates did not alter the main findings. Fifth, despite the fact that the surveillance system also collected data on preexisting diseases, these were largely unavailable ( missing, data not shown), preventing their use in the epidemiological analyses. However, they are not expected to bias the studied association because they might act as mediators, rather than confounders, of the air pollution–COVID-19 fatality association. Finally, some of the deficiencies underscored by Villeneuve and Goldberg in most of the early epidemiological studies on air pollution and COVID-19 could not be entirely addressed in our paper, namely potential misclassification and underreporting of incidence and mortality of COVID-19; lack of adjustment for physical distancing and other public health interventions; problems from clustering of disease or deaths (such as cases occurring in nursing homes) leading to potential spatial autocorrelation in COVID-19 cases; and potential residual confounding from poor adjustment of other individual-level determinants of COVID-19 mortality, especially occupation and socioeconomic status. However, we believe that our strategy of adjusting for multiple interaction terms between spatial (provinces), temporal (months), individual-level (age and sex) and area-level (ventiles of GPS) covariates may have minimized the potential residual confounding from these factors.
Despite the different strengths of this study, the highlighted limitations suggest that studies of air pollution and COVID-19 require a multidisciplinary approach that include models borrowed from infectious disease epidemiology combined with environmental epidemiology study designs. Further research should confirm our findings and provide a better understanding of the possible mechanisms linking air pollution to COVID-19 severity. We estimated a significant association between long-term exposure to air pollution and mortality among 4 million cases, and we quantified on the order of 10,000 the number of COVID-19 deaths attributable to annual exposures above the WHO 2021 AQG thresholds.44 These findings provide additional support for the broad public health benefits of reducing levels of outdoor air pollution in Italy.
Supplementary Material
Acknowledgments
This study was carried out in the framework of the EpiCovAir project, coordinated by the National Institute of Health (ISS) and the National System for the Environmental Protection (ISPRA-SNPA), with the collaboration of the Italian Environmental Health Network (RIAS). This research received funding from “Bando ISS Ricerca Indipendente 2020-2022”
References
- 1.WHO (World Health Organization). WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ [accessed 6 February 2023].
- 2.Frontera A, Martin C, Vlachos K, Sgubin G. 2020. Regional air pollution persistence links to COVID-19 infection zoning. J Infect 81(2):318–356, PMID: , 10.1016/j.jinf.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martelletti L, Martelletti P. 2020. Air pollution and the novel Covid-19 disease: a putative disease risk factor. SN Compr Clin Med 2(4):383–387, PMID: , 10.1007/s42399-020-00274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conticini E, Frediani F, Caro D. 2020. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ Pollut 261:114465, PMID: , 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villeneuve PJ, Goldberg MS. 2020. Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ Health Perspect 128(9):95001, PMID: , 10.1289/EHP7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicole W. 2020. Air of uncertainty: can we study pollution and COVID-19 in the midst of a pandemic? Environ Health Perspect 128(11):114005, PMID: , 10.1289/EHP8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Nethery RC, Sabath MB, Braun D, Dominici F. 2020. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci Adv 6(45):eabd4049, PMID: , 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang D, Shi L, Zhao J, Liu P, Sarnat JA, Gao S, et al. 2020. Urban air pollution may enhance COVID-19 case-fatality and mortality rates in the United States. Innovation (Camb) 1(3):100047, PMID: , 10.1016/j.xinn.2020.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travaglio M, Yu Y, Popovic R, Selley L, Leal NS, Martins LM. 2021. Links between air pollution and COVID-19 in England. Environ Pollut 268(pt A):115859, PMID: , 10.1016/j.envpol.2020.115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prinz AL, Richter DJ. 2022. Long-term exposure to fine particulate matter air pollution: an ecological study of its effect on COVID-19 cases and fatality in Germany. Environ Res 204(pt A):111948, PMID: , 10.1016/j.envres.2021.111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konstantinoudis G, Padellini T, Bennett J, Davies B, Ezzati M, Blangiardo M. 2021. Long-term exposure to air-pollution and COVID-19 mortality in England: a hierarchical spatial analysis. Environ Int 146:106316, PMID: , 10.1016/j.envint.2020.106316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Feldman A, Heres D, Marquez-Padilla F. 2021. Air pollution exposure and COVID-19: a look at mortality in Mexico City using individual-level data. Sci Total Environ 756:143929, PMID: , 10.1016/j.scitotenv.2020.143929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kogevinas M, Castaño-Vinyals G, Karachaliou M, Espinosa A, de Cid R, Garcia-Aymerich J, et al. 2021. Ambient air pollution in relation to SARS-CoV-2 infection, antibody response, and COVID-19 disease: a cohort study in Catalonia, Spain (COVICAT study). Environ Health Perspect 129(11):117003, PMID: , 10.1289/EHP9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobile F, Michelozzi P, Ancona C, Cappai G, Cesaroni G, Davoli M, et al. 2022. Air pollution, SARS-CoV-2 incidence and COVID-19 mortality in rome - a longitudinal study. Eur Respir J 60(3):2200589, PMID: , 10.1183/13993003.00589-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Wang J, Kwong J, Kim J, van Donkelaar A, Martin RV, et al. 2022. Association between long-term exposure to ambient air pollution and COVID-19 severity: a prospective cohort study. CMAJ 194(20):E693–E700, PMID: , 10.1503/cmaj.220068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheridan C, Klompmaker J, Cummins S, James P, Fecht D, Roscoe C. 2022. Associations of air pollution with COVID-19 positivity, hospitalisations, and mortality: observational evidence from UK Biobank. Environ Pollut 308:119686, PMID: , 10.1016/j.envpol.2022.119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.English PB, Von Behren J, Balmes JR, Boscardin J, Carpenter C, Goldberg DE, et al. 2022. Association between long-term exposure to particulate air pollution with SARS-CoV-2 infections and COVID-19 deaths in California, U.S.A. Environ Adv 9:100270, PMID: , 10.1016/j.envadv.2022.100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riccardo F, Ajelli M, Andrianou XD, Bella A, Del Manso M, Fabiani M, et al. 2020. Epidemiological characteristics of COVID-19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 March 2020. Euro Surveill 25(49):2000790, PMID: , 10.2807/1560-7917.ES.2020.25.49.2000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. International Guidelines for Certification and Classification (Coding) of COVID-19 as Cause of Death. https://www.who.int/publications/m/item/international-guidelines-for-certification-and-classification-(coding)-of-covid-19-as-cause-of-death [accessed 3 May 2023].
- 20.Stafoggia M, Cattani G, Ancona C, Ranzi A. 2020. Exposure assessment of air pollution in Italy 2016-2019 for future studies on air pollution and COVID-19. Epidemiol Prev 44(5–6 suppl 2):161–168. [In Italian], PMID: , 10.19191/EP20.5-6.S2.115. [DOI] [PubMed] [Google Scholar]
- 21.Rosano A, Pacelli B, Zengarini N, Costa G, Cislaghi C, Caranci N. 2020. Update and review of the 2011 Italian deprivation index calculated at the census section level. Epidemiol Prev 44(2–3):162–170. [In Italian], PMID: , 10.19191/EP20.2-3.P162.039. [DOI] [PubMed] [Google Scholar]
- 22.Bauleo L, Giannini S, Ranzi A, Nobile F, Stafoggia M, Ancona C, et al. 2022. A methodological approach to use contextual factors for epidemiological studies on chronic exposure to air pollution and COVID-19 in Italy. Int J Environ Res Public Health 19(5):2859, PMID: , 10.3390/ijerph19052859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbaum PR, Rubin DB. 1983. The central role of the propensity score in observational studies for causal effects. Biometrika 70(1):41–55, 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 24.Hirano K, Imbens GW. 2004. The propensity score with continuous treatments. In: Applied Bayesian Modeling and Causal Inference from Incomplete-Data Perspectives. 1st ed. Gelman A, Meng XL, eds. Hoboken, NJ: John Wiley & Sons, Inc., 73–84. [Google Scholar]
- 25.Bozack A, Pierre S, DeFelice N, Colicino E, Jack D, Chillrud SN, et al. 2022. Long-term air pollution exposure and COVID-19 mortality: a patient-level analysis from New York City. Am J Respir Crit Care Med 205(6):651–662, PMID: , 10.1164/rccm.202104-0845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zang ST, Luan J, Li L, Yu HX, Wu QJ, Chang Q, et al. 2022. Ambient air pollution and COVID-19 risk: evidence from 35 observational studies. Environ Res 204(pt B):112065, PMID: , 10.1016/j.envres.2021.112065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GBD 2019 Risk Factors Collaborators. 2020. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258):1223–1249, PMID: , 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia E, Marian B, Chen Z, Li K, Lurmann F, Gilliland F, et al. 2022. Long-term air pollution and COVID-19 mortality rates in California: findings from the spring/summer and winter surges of COVID-19. Environ Pollut 292(pt B):118396, PMID: , 10.1016/j.envpol.2021.118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurston GD, Kipen H, Annesi-Maesano I, Balmes J, Brook RD, Cromar K, et al. 2017. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J 49:1600419, PMID: , 10.1183/13993003.00419-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, et al. 2019. Air pollution and noncommunicable diseases: a review by the forum of international respiratory societies’ environmental committee, part 1: the damaging effects of air pollution. Chest 155(2):409–416, PMID: , 10.1016/j.chest.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Hoek G. 2020. Long-term exposure to PM and all-cause and cause-specific mortality: a systematic review and meta-analysis. Environ Int 143:105974, PMID: , 10.1016/j.envint.2020.105974. [DOI] [PubMed] [Google Scholar]
- 32.Huangfu P, Atkinson R. 2020. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: a systematic review and meta-analysis. Environ Int 144:105998, PMID: , 10.1016/j.envint.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paital B, Agrawal PK. 2021. Air pollution by NO2 and PM2.5 explains COVID-19 infection severity by overexpression of angiotensin-converting enzyme 2 in respiratory cells: a review. Environ Chem Lett 19(1):25–42, PMID: , 10.1007/s10311-020-01091-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodby B, Arnold MM, Valacchi G. 2021. SARS-CoV-2 infection, COVID-19 pathogenesis, and exposure to air pollution: what is the connection? Ann NY Acad Sci 1486(1):15–38, PMID: , 10.1111/nyas.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Health Effects Institute. 2020. State of Global Air 2020. A Special Report on Global Exposure to Air Pollution and Its Health Impacts. Boston, MA: Health Effects Institute. [Google Scholar]
- 36.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. 2020. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55(5):2000547, PMID: , 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen ZJ, Hoffmann B, Morawska L, Adams M, Furman E, Yorgancioglu A, et al. 2021. Air pollution and COVID-19: clearing the air and charting a post-pandemic course: a joint workshop report of ERS, ISEE, HEI and WHO. Eur Respir J 58:2101063, PMID: , 10.1183/13993003.01063-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. 2020. Prevalence of comorbidities and its effects in patients infected with SARS CoV2: a systematic review and meta-analysis. Int J Infect Dis 94:91–95, PMID: , 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Z, McGoogan JM. 2020. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323(13):1239–1242, PMID: , 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 40.Ciencewicki J, Jaspers I. 2007. Air pollution and respiratory viral infection. Inhal Toxicol 19(14):1135–1146, PMID: , 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- 41.Bourdrel T, Annesi-Maesano I, Alahmad B, Maesano CN, Bind MA. 2021. The impact of outdoor air pollution on COVID-19: a review of evidence from in vitro, animal, and human studies. Eur Respir Rev 30(159):200242, PMID: , 10.1183/16000617.0242-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazucanti CH, Egan JM. 2020. SARS-CoV-2 disease severity and diabetes: why the connection and what is to be done? Immun Ageing 17:21, PMID: , 10.1186/s12979-020-00192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. 2020. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One 15(8):e0238215, PMID: , 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO. 2021. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. https://apps.who.int/iris/handle/10665/345329 [accessed 6 February 2023]. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.