Abstract
Helicobacter pylori infection can induce polymorphonuclear leukocyte (PMNL) infiltration of the gastric mucosa, which characterizes acute chronic gastritis. The mechanisms underlying this process are poorly documented. The lack of an in vitro model has considerably impaired the study of transepithelial migration of PMNL induced by H. pylori. In the present work, we used confluent polarized monolayers of the human intestinal cell line T84 grown on permeable filters to analyze the epithelial PMNL response induced by broth culture filtrates (BCFs) and bacterial suspensions from different strains of H. pylori. We have evaluated the role of the vacuolating cytotoxin VacA and of the cag pathogenicity island (PAI) of H. pylori in PMNL migration via their effects on T84 epithelial cells. We noted no difference in the rates of PMNL transepithelial migration after epithelial preincubation with bacterial suspensions or with BCFs of VacA-negative or VacA-positive H. pylori strains. In contrast, PMNL transepithelial migration was induced after incubation of the T84 cells with cag PAI-positive and cagE-positive H. pylori strains. Finally, PMNL migration was correlated with a basolateral secretion of interleukin-8 by T84 cells, thus creating a subepithelial chemotactic gradient for PMNL. These data provide evidence that the vacuolating cytotoxin VacA is not involved in PMNL transepithelial migration and that the cag PAI, with a pivotal role for the cagE gene, provokes a transcellular signal across T84 monolayers, inducing a subepithelial PMNL response.
Helicobacter pylori has emerged as the causative agent of chronic active gastritis and peptic ulcer disease (28, 36, 46, 58). The inflammatory response observed in active gastritis is characterized by polymorphonuclear leukocyte (PMNL) infiltration into the surface epithelium (14). Secondary to this infection may be the development of chronic atrophic gastritis, gastric metaplasia, mucosa-associated lymphoid tissue lymphoma, and adenocarcinoma of the stomach (28, 46, 58). PMNL play a major role in the pathogenesis of epithelium injury since these cells exert a direct cytotoxic effect on the epithelia by releasing products such as oxidative reagents and elastase (3). Thus, the persistence of a strong local inflammatory response may indirectly result in lesions in cells specialized in acid production and thus may deregulate this production.
Virulence factors of H. pylori are multiple, including urease synthesis, adhesins, vacuolating toxin VacA, and the cag pathogenicity island (PAI) (7, 9, 46). Urease is active at acid pH and hydrolyzes urea generating ammonia, which protects H. pylori (which is not an acidophilic bacterium) by producing a neutral microenvironment around bacteria (15, 49). Recently H. pylori adhesins such as the Lewis b (Leb)-binding adhesin BabA have been identified (24). Adherence at the apical membrane of the epithelium protects the bacteria from the acidity of the gastric lumen. This bacterial Leb-binding phenotype is associated with the presence of the cag PAI among clinical isolates of H. pylori (24). Strains of H. pylori are grouped into two broad families, type I and type II, on the basis of whether or not they express a biologically active VacA and the CagA (cytotoxin-associated gene A) antigen (4, 29, 56). Patients exhibiting the most-severe gastroduodenal diseases (i.e., peptic ulcer and gastric tumors) are most often infected by type I strains, which suggests that CagA and the coexpressed cytotoxin VacA play a critical role in the pathogenicity induced by H. pylori (2, 5, 17).
The C-X-C chemokine family, which includes the PMNL chemoattractant interleukin-8 (IL-8) (48), is a critical factor in the immunopathogenesis of gastritis. High levels of gastric IL-8 are found in patients infected by type I strains of H. pylori (11). Initial studies have shown that inactivation of the genes in the cag PAI reduced IL-8 secretion in epithelial cells (11). Moreover, mutational tests showed that cagA was not needed for induction of IL-8 secretion (11). It was observed earlier that loss of function of the picA gene and the picB/cagE gene close to the right end of the cag PAI abrogates the induction of IL-8 production in gastric epithelial cells (51). Moreover, a recent study has demonstrated that VacA increased the permeability of the epithelium barrier, probably by acting at the tight-junction level (40). In this way, VacA might induce a weakness in transepithelial resistances (TER) and consequently potentialize PMNL migration into the lumen of the stomach.
H. pylori-induced production of IL-8 by epithelial cells has been previously studied by using established gastric epithelial cell cultures (10, 12, 47). However the mechanisms by which H. pylori induced PMNL transepithelial migration have not been previously described. Assays of PMNL migration across the epithelia can only be performed with highly polarized monolayers (i.e., those that develop high TER). In this study we have used human intestinal epithelial cell line T84 to assess PMNL-intestinal epithelial cell interactions as previously reported (21, 30, 41). In the present work, we have examined, using different bacterial strains and broth culture filtrates (BCFs) of H. pylori, how H. pylori-T84 epithelial cell interactions modulate PMNL transepithelial migration. More particularly, we have analyzed (i) whether the cytotoxin VacA modifies the rate of PMNL migration across the epithelium by acting at the tight-junction level and/or by remodeling the epithelial actin cytoskeleton; (ii) whether H. pylori strains which do or do not express the cag PAI, and more specifically cagA or picB/cagE genes, may induce a transepithelial migration of PMNL across T84 monolayers; and (iii) whether a basolateral secretion of IL-8 occurs as a result of the H. pylori-T84 cell interaction and how such an IL-8 secretion contributes to transepithelial migration.
We found that the cytotoxin VacA is not involved in H. pylori-induced PMNL migration across a tight epithelial barrier. Our results also demonstrate that the cag PAI is essential for inducing a basolateral secretion of IL-8 which consecutively contributes to the creation of a PMNL chemotactic gradient on the underlying matrix. Among the genes of the cag PAI, a key role is played by the picB/cagE gene, whereas the cagA gene seems to be irrelevant.
MATERIALS AND METHODS
Bacterial strains.
The different strains of H. pylori used in this study and their phenotypes are summarized in Table 1. We have used the urease+ VacA+ cag+ wild-type H. pylori 60190 strain (ATCC 49503), its isogenic mutants in which vacA (60190:v1) or cagA (60190:M22) or picB/cagE (60190:C−) genes were disrupted by insertional mutagenesis (8, 20, 54, 55), and its urease-negative spontaneous mutant (60190:4) (42) (all these strains have been kindly given by T. L. Cover and M. J. Blaser, Nashville, Tenn.). In addition, we used the wild-type CCUG 17874 (urease+ VacA+ cag+) H. pylori strain (from the culture collection of the University of Goteborg, Goteborg, Sweden) and the wild-type G21 (urease+ VacA− cag mutant) H. pylori strain (kindly given by N. Figura, Siena, Italy) (20, 56).
TABLE 1.
Different strains of H. pylori and their phenotypes used in the present study
| Strain | Phenotype |
|---|---|
| 60190 | VacA+ Cag+ |
| 60190:v1 | VacA− Cag+ |
| CCUG 17874 | VacA+ Cag+ |
| 60190:4 | VacA+ Cag+ urease− |
| 60190:M22 | VacA+ CagA− |
| G21 | VacA− Cag− |
| 60190:C− | VacA+ CagE− |
Preparation of BCFs and bacterial suspensions.
BCFs were prepared as previously described (44–46). Briefly, bacteria were grown in brucella broth (Difco, Detroit, Mich.) supplemented with 1% Vitox (Oxoid, Basingstoke, United Kingdom) and 5% fetal calf serum (Gibco BRL, Paisley, United Kingdom) for 24 to 36 h at 37°C in a thermostatic shaker under microaerophilic conditions. When the bacterial suspensions reached 1.2 units of optical density at 450 nm (corresponding to a bacterial concentration of 5 × 108 CFU/ml), bacteria were removed by centrifugation, and the supernatants were sterilized by passage through a 0.22-μm-pore-size cellulose acetate filter to obtain BCFs. BCFs were used at a dilution of 1:3. The vacuolating power of VacA+ BCFs prepared as described above and diluted 1:3 is equivalent to that exhibited by a final concentration of 0.4 μg of purified VacA/ml (V. Ricci, personal observation).
For the experiments with bacterial suspensions, bacteria were grown on Columbia agar supplemented with 1% Vitox and 10% sheep blood (Oxoid); immediately before the start of the experiments, bacteria were suspended (final concentration: 5 × 108 CFU/ml) in the culture medium used for T84 cells.
Cell culture and electrophysiological studies.
T84 cells (American Type Culture Collection; passages 65 to 90), a human colonic carcinoma cell line, were grown and maintained as confluent monolayers on collagen-coated permeable supports with detailed modifications (30). Monolayers were grown on 0.33-cm2 ring-supported polycarbonate filters (Costar, Cambridge, Mass.) and were utilized 6 to 14 days after being plated. Confluent monolayers on permeable supports were constructed to permit a basolateral-to-apical migration of PMNL (“inverted inserts”) as previously described (22, 30). The cells were then incubated with the BCF from the VacA+ 60190 strain in both the upper and lower reservoirs. To assess currents, transepithelial potentials, and TER, a commercial voltage clamp (Bioengineering Department, University of Iowa) was used as previously described (22, 30). Resistances were monitored during the period of incubation with the BCF from the VacA+ 60190 strain or with the different H. pylori strains used in this study.
Morphological studies. (i) Immunofluorescence and confocal microscopy studies.
After intoxication with the BCF from the VacA+ 60190 strain, T84 cells were washed with phosphate-buffered saline (PBS)–0.4% bovine serum albumin and further incubated with 2 mM acridine orange in Hanks' balanced salt solution (HBSS) for 10 min at room temperature. After several washes with HBSS, the cells were immediately observed and photographed with a laser scanning fluorescence microscope (Leica, DMIRBE, Lyon, France) equipped for epifluorescence.
Fluorescence staining of control and VacA-treated T84 cells grown on permeable filters was performed. Cells were rinsed extensively in HBSS and fixed with 3.7% paraformaldehyde (in PBS; pH 7.4) for 15 min at room temperature and rinsed three times for 5 min each in buffer containing 0.2% gelatin and 0.2% Triton X-100. Cells were then incubated for 30 min in the dark with 500 nM rhodamine-phalloidin diluted in PBS (Sigma). The cells were washed in HBSS, mounted on glass slides in a phenylenediamine-glycerol-PBS medium, and then observed.
(ii) Electron microscopy study.
Inverted T84 monolayers were rinsed extensively in HBSS. Approximately 5 × 107 CFU of the different strains of H. pylori used in this study in 100 μl were gently distributed onto the apical surface and incubated for 4 h at 37°C and pH 7.4. Nonadherent bacteria were removed from the monolayers by extensive washing, and the cells were transferred back into the 24-well tissue culture tray containing HBSS. After removal from the inserts, the T84 monolayers were fixed with 2% freshly prepared paraformaldehyde in 0.1 M sodium cacodylate, pH 7.4, for 1 h at 4°C. Monolayers were rinsed in cacodylate buffer, postfixed in 1% OsO4 for 1 h, dehydrated through graded alcohols, and embedded in epoxy resin. Oriented 1-mm sections were obtained with diamond knives, and multiple areas were thin sectioned. Ultrathin sections were examined on a Jeol 1200 EXII electron microscope. The numbers of adherent bacteria per 50 epithelial cells were determined for random sections.
PMNL transmigration assays.
Human PMNL were isolated from whole blood using a gelatin sedimentation technique as previously described (22).
The physiologically (basolaterally to apically) directed PMNL transepithelial migration assay has been previously described (21). PMNL transmigration experiments were performed at 37°C on 0.33-cm2 inverts. T84 monolayers were washed three times in warm HBSS, and approximately 5 × 107 CFU of the different strains of H. pylori used in this study in 100 μl were gently distributed onto the apical surface and incubated for 4 h at 37°C. Nonadherent bacteria were removed from the monolayers by extensive washing and were then transferred back into the 24-well tissue culture tray containing 1.0 ml of HBSS in each lower reservoir (apical membrane now colonized with H. pylori) and 100 μl in the upper reservoir (basolateral interface). We added 106 PMNL to the inverts. To assess if VacA, by itself, may modify the rate of PMNL transepithelial migration, a set of PMNL transmigration assays was performed in the presence or in the absence of N-formyl-l-methionyl-leucyl-l-phenylalanine (f-MLP; 10−7 M) after T84 cell incubation for 24 h with VacA+ BCF (60190 or CCUG 17874) or VacA− BCF (60190:v1). Control transmigration of PMNL was initiated by adding f-MLP (10−7 M) to the lower reservoir and incubating it for 15 min to allow a transepithelial chemotactic gradient to form prior to the addition of PMNL.
In order to verify that a chemotactic gradient imprinted the subepithelial matrix, an H. pylori T84 epithelial cell-conditioned matrix was prepared by the method previously described (32). Briefly, T84 cell monolayers apically colonized with H. pylori were transferred to fresh 24-well plates containing a solution of 5 × 10−4 M EGTA (Sigma) in Ca2+- and Mg2+-free HBSS with 10 mM HEPES, pH 7.4, at 37°C. T84 cell monolayers were stripped from the collagen-coated polycarbonate filters by bathing the filters in Ca2+- and Mg2+-free EGTA-HBSS for 3 h at 37°C with gentle agitation. Then, the inverts were washed in HBSS and transferred back into the original 24-well tissue culture trays containing 1.0 ml of HBSS buffer in the lower reservoir (apical interface) and 100 μl in the upper reservoir (basolateral interface). Control matrices consisted of those laid down by T84 cell monolayers that had not been colonized with H. pylori. In some experiments neutralizing polyclonal antibodies against human IL-8 (Endogen, Inc., Boston, Mass.) at 60 μg/ml were exposed to the filter matrix after EGTA treatment for 45 min before the addition of PMNL.
Transmigration of PMNL was assayed by quantification of the azurophil granule marker myeloperoxidase (MPO) as described previously (23).
IL-8 assay.
In order to characterize the IL-8 secretion in the matrices and the lower reservoirs, experiments were performed with the conditioned matrix prepared as described above. The matrices and the lower reservoirs were assayed in triplicate for IL-8 by enzyme-linked immunosorbent assay (ELISA). The ELISA was carried out using a mouse monoclonal antibody to IL-8 and a phosphatase-conjugated goat anti-IL-8 polyclonal antibody (Sandoz Pharmaceutical, Rueil-Malmaison, France).
Data analysis.
Resistance time courses were compared by two-factor analysis of variance. MPO assays were compared by Student's t test. Values are expressed as the means ± standard errors of the means (SEM) of a number of experiments.
RESULTS
Incubation of confluent T84 monolayers with VacA does not modify the epithelial actin cytoskeleton and epithelial permeability.
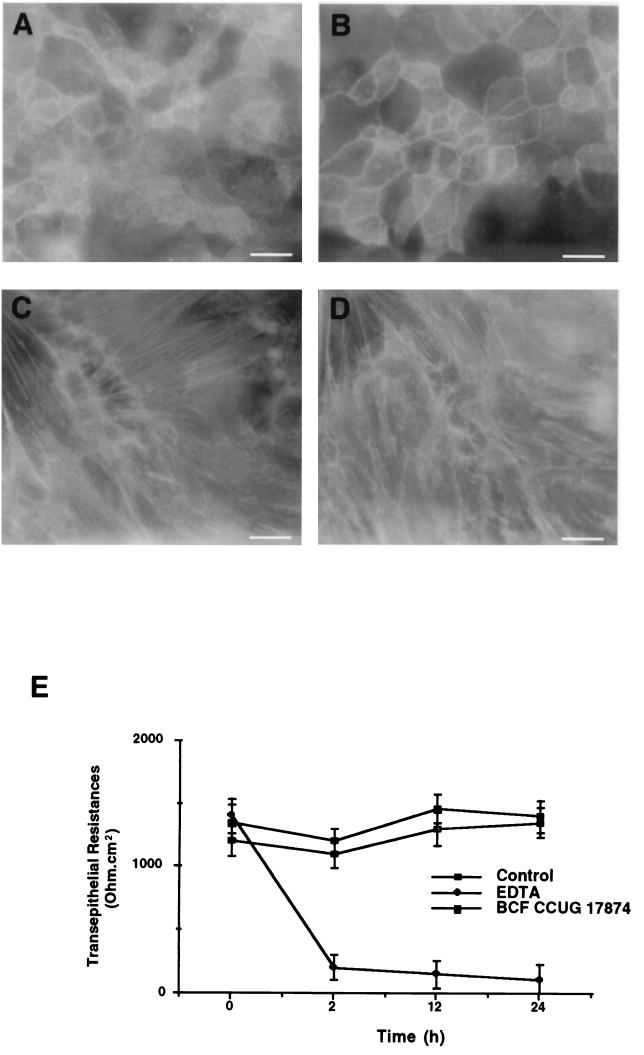
We first verified that the T84 cell line was sensitive to the vacuolating effect of VacA. T84 cells treated with VacA+ BCF 24 h after plating on filters showed, after acridine orange incorporation, large intracellular vacuoles in the cytosol, predominantly in perinuclear spaces (not shown). Moreover these vacuoles showed an intense staining using polyclonal anti-rab7 and anti-VacA antibodies (not shown). No vacuoles and staining were noted at cell confluency. In order to analyze the consequences of VacA intoxication for the epithelial actin cytoskeleton network, fluorescein isothiocyanate-phalloidin labeling was performed at different times following cell plating on filters. One week after plating, we failed to detect any modification of the F-actin network in T84 cells treated with VacA+ BCF by comparison with control cells (not shown). We concluded that VacA intoxication of confluent monolayers did not affect F-actin distribution in treated and control cells (Fig. 1). The perijunctional ring of actin at the tight-junction level (Fig. 1A and B) and prominent stress fibers observed in the basolateral compartment (Fig. 1C and D) in VacA-treated cells were similar to those in control cells.
FIG. 1.
(A to D) Effect of the vacuolating cytotoxin VacA (BCF from the VacA+ 60190 strain) on the actin cytoskeleton of confluent T84 cell monolayers. Cells were stained by fluorescein isothiocyanate-phalloidin and observed by epifluorescence. VacA does not modify the perijunctional F-actin rings in control cells (A) and VacA-treated T84 monolayers (B) and does not alter the basolateral actin filament network of the T84 epithelial monolayers in control cells (C) and VacA-treated T84 monolayers (D). Bars, 20 μm. (E) Measurements of TER in T84 cell monolayers after 2, 12, and 24 h of incubation with either the BCF from the VacA+ 60190 strain or 2 × 10−3 M EDTA. Note the lack of effect of VacA (solid squares) on TER during the course of incubation compared to data for control cells (open squares). As expected, monolayers responded to EDTA (solid diamond) by decreasing junctional resistances. Results are means ± SEM for six experiments.
To verify whether the permeability of polarized T84 cell monolayers could be altered by VacA intoxication, we monitored the TER during 24 h of incubation in the presence or absence of BCF from the VacA+ 60190 strain. TER were measured at the onset of VacA addition and after 2, 12, and 24 h. As shown in Fig. 1E, VacA did not significantly alter the barrier function of epithelial monolayers, since a TER of 1,400 ± 140 Ω · cm2 was found in VacA-treated T84 cells versus 1,350 ± 120 Ω · cm2 in untreated T84 monolayers after 24 h.
Incubation of T84 monolayers with VacA does not alter the rate of PMNL transmigration.
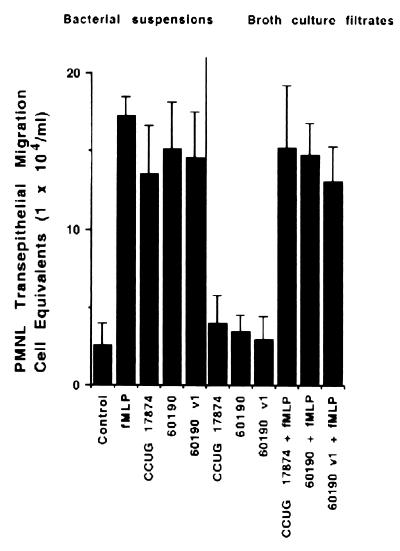
Attachment of 60190:v1 (VacA− cag+), 60190 (VacA+ cag+), and CCUG 17874 (VacA+ cag+) H. pylori strains to the apical membranes of T84 cells similarly induced, after incubation for 4 h, the basolateral-to-apical migration of PMNL ([14.6 ± 2.9] × 104, [15.1 ± 3] × 104, and [13.5 ± 3.1] × 104 PMNL cell equivalents per monolayer for 60190:v1, 60190, and CCUG 17874 strains, respectively) (Fig. 2). Incubation of the T84 cell monolayers for 24 h with the VacA+ BCFs (CCUG 17874 or 60190) and the VacA− BCF (60190:v1) did not significantly modify the transepithelial migration of PMNL in comparison to a migration obtained in control cells ([4 ± 1.8] × 104, [3.5 ± 1] × 104, [2.9 ± 1.5] × 104, and [2.6 ± 1.2] × 104 PMNL cell equivalents per monolayer for CCUG 17874, 60190, and 60190:v1 strains and control cells, respectively) (Fig. 2). In the latter conditions no significant differences were noted, with or without incubation with BCFs, in the transepithelial migration of PMNL induced by f-MLP (10−7 M) ([15.2 ± 4] × 104, [14.8 ± 2] × 104, [13.1 ± 2.2] × 104, and [17.2 ± 1.3] × 104 PMNL cell equivalents per monolayer for CCUG 17874, 60190, and 60190:v1 strains and control T84 cells, respectively) (Fig. 2).
FIG. 2.
VacA does not modify the rate of PMNL transmigration across the T84 cell monolayers. T84 monolayers were incubated for 4 h with bacterial suspensions or for 24 h with BCFs from different H. pylori strains. Shown is an MPO assay indicating total numbers of PMNL (PMNL-associated monolayers plus PMNL that transmigrated to the lower reservoirs) after a 2-h transmigration, induced by f-MLP (10−7 M) or not induced. Results are means ± SEM for 6 to 12 monolayers.
Wild-type 60190, 60190:M22, and 60190:C−, but not G21, H. pylori strains adhered to the apical membrane of the T84 epithelial cells.
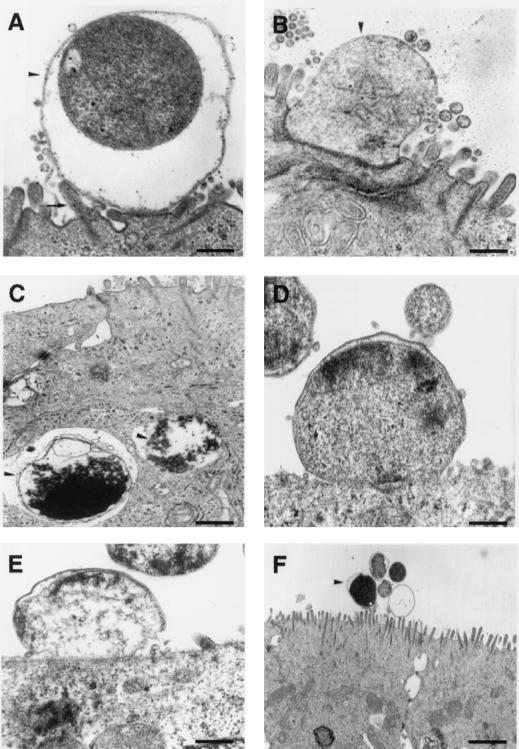
The 60190 (VacA+ cag+) H. pylori strain added to the apical side of confluent T84 monolayers became intimately associated with the brush border. More specifically, adherence pedestals were observed (Fig. 3A). In some preparations the bacterial membrane was closely apposed to the epithelial cell membrane and the brush border architecture disappeared at the contact site (Fig. 3B). Some bacteria were observed in deep invaginations. Exceptionally, engulfment features were observed, and these resulted in the formation of intracellular vacuoles containing bacteria (Fig. 3C). However internalization of H. pylori remained very rare. Similarly, 60190:M22 (VacA+ CagA−) (Fig. 3D) and 60190:C− (VacA+ cagE) (Fig. 3E) H. pylori strains showed features of adherence with the epithelial cell membrane. In contrast the G21 (VacA− cag mutant) strain was not adherent or was loosely adherent to the brush border. No pedestal formation or brush border alteration at the contact site was observed (Fig. 3F). By counting the bacteria adherent to the T84 monolayers (i.e., the number of adherent bacteria observed per 50 epithelial cells), we confirmed that only wild-type 60190, 60190:M22, and 60190:C−, not G21, H. pylori strains were adherent to the epithelial cells (221 ± 18, 191 ± 23, 189 ± 16, and 23 ± 9 adherent bacteria per 50 epithelial cells for wild-type 60190, 60190:M22, 60190:C−, and G21 H. pylori strains, respectively; P < 0.01). In all conditions, T84 cells did not show any nuclear or cytoplasmic apoptotic features such as nuclear condensation or fragmentation and intracytoplasmic vacuoles.
FIG. 3.
The effects of H. pylori strain interactions with polarized T84 monolayers on apical membrane structure. (A to C) H. pylori strain 60190. (A) A bacterium (arrowhead) is associated with the brush border, with adherence pedestal formation (arrow). (B) The bacterial membrane (arrowhead) is closely apposed to the epithelial cell membrane, and the brush border architecture disappears at the contact site. (C) Some bacteria are exceptionally internalized (arrowheads). (D and E) 60190:M22 (D) and 60190:C− (E) H. pylori strains are adherent to the brush border. (F) G21 H. pylori strain. No adherence pedestal or association of bacteria (arrowhead) with the epithelial membrane is noted. Bars, 0.5 μm (A to E) and 3 μm (F).
The cag PAI and more particularly the picB/cagE gene of H. pylori is essential for inducing a transepithelial migration of PMNL across T84 monolayers.
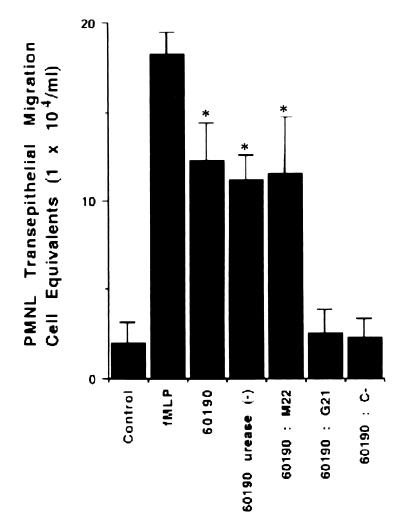
Since an important infiltration of PMNL seems to occur in vivo in response to cag+ strain H. pylori infection, we investigated whether this phenomenon could be modeled in vitro. Before starting the transmigration assays, we verified that the TER of T84 cells incubated for 4 h with the different H. pylori strains used in this study was not affected (not shown). As shown in Fig. 4, attachment of the 60190 (VacA+ cag+) H. pylori strain to the apical membrane of T84 cells induced a basolateral-to-apical migration of PMNL. PMNL transmigration induced by the 60190 H. pylori strain was 62% of that which occurred in response to f-MLP ([18.2 ± 1.3] × 104 and [12.3 ± 2.1] × 104 PMNL cell equivalents per monolayer for f-MLP and strain 60190, respectively). To determine whether the number of PMNL that transmigrate a monolayer is dependent on the cag PAI or on cagA and/or cagE genes, transmigration assays were performed with T84 monolayers incubated with bacterial suspensions from G21 (VacA− cag mutant), 60190:M22 (VacA+ cagA), and 60190:C−(VacA+ cagE) strains. As shown in Fig. 4, G21 and 60190:C− strains failed to induce transmigration of PMNL across the T84 monolayer, whereas the 60190:M22 strain induced a transmigration of PMNL similar to that obtained with the 60190 strain ([2.5 ± 1.4] × 104, [2.3 ± 1.1] × 104, and [11.2 ± 1.4] × 104 PMNL cell equivalents per monolayer for G21, 60190:C−, and 60190:M22 strains, respectively; P < 0.01). Finally, no significant difference in the numbers of transmigrated PMNL between the wild-type 60190 strain and its urease-negative mutant was observed ([12.3 ± 2.1] × 104 versus [11.2 ± 1.4] × 104 PMNL cell equivalents per monolayer, respectively) (Fig. 4).
FIG. 4.
PMNL transmigration across the T84 cell monolayers is dependent on cag PAI expression. Epithelial cells were incubated for 4 h with bacterial suspensions from different H. pylori strains. The MPO assay indicates total numbers of PMNL (PMNL-associated monolayers plus PMNL that migrated to the lower reservoirs) after a 2-h transmigration. Data are pooled from 6 to 12 individual monolayers for each condition, and results are means ± standard errors. ∗, P < 0.01.
Colonization of the T84 apical membrane with cag+ H. pylori strains imprints a directional chemotactic signal on the underlying matrix.
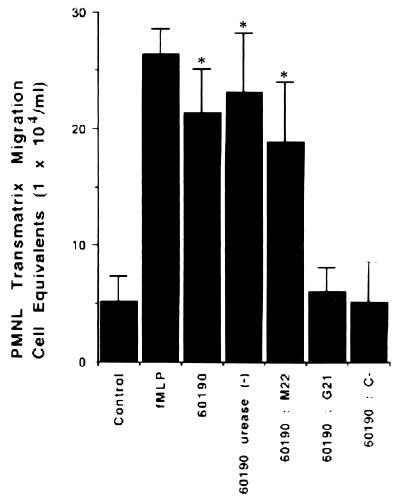
In contrast to control matrices, matrices isolated from T84 monolayers apically colonized with the 60190 (VacA+ cag+) H. pylori strain were imprinted by signals which supported spontaneous PMNL migration responses ([21.4 ± 3.8] × 104 and [5.2 ± 2.1] × 104 PMNL cell equivalents per monolayer for strain 60190 and the control, respectively; P < 0.01) (Fig. 5). Similarly, PMNL migration induced by the 60190:M22 (VacA+ CagA−) H. pylori strain was 79% as effective as that which occurred in response to f-MLP ([18.8 ± 5.2] × 104 and [26.5 ± 2.1] × 104 PMNL cell equivalents per monolayer for the 60190:M22 strain and f-MLP, respectively) (Fig. 5). In contrast, the number of PMNL crossing the matrices was decreased to the basal level in matrices conditioned by the G21 (VacA− cag mutant) and 60190:C− (VacA+ cagE) H. pylori strains ([6.1 ± 2] × 104 and [5.2 ± 3.4] × 104 PMNL cell equivalents per monolayer for G21 and 60190:C−, respectively) (Fig. 5). Finally, no significant difference in the numbers of PMNL that migrated across the matrices between wild-type strain 60190 and its urease-negative mutant was observed ([21.4 ± 3.8] × 104 and [23.2 ± 5] × 104 PMNL cell equivalents per monolayer for wild-type strain 60190 and the 60190 urease-negative mutant, respectively) (Fig. 5).
FIG. 5.
Colonization of the apical membrane with cag+ H. pylori strains imprints a directional chemotactic signal on the underlying matrix. Epithelial cells were incubated for 4 h with bacterial suspensions from different H. pylori strains. The MPO assay indicates total numbers of PMNL (PMNL-associated matrices plus PMNL that migrated to the lower reservoirs) after a 2-h transmigration. Data are pooled from 6 to 12 individual monolayers for each condition, and results are means ± standard errors. ∗, P < 0.01.
IL-8 is the chemotactic factor basolaterally secreted by the T84 cells which induces migration across the matrix.
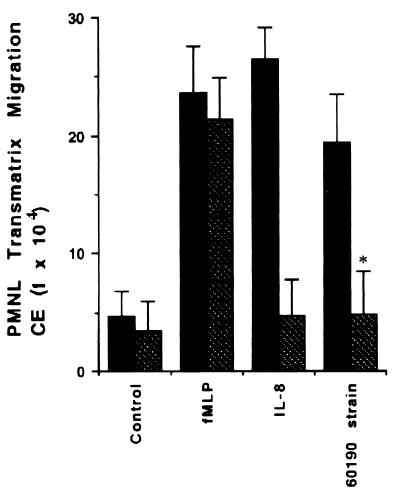
We next showed that the 60190 (VacA+ cag+), 60190:v1 (VacA− cag+), 60190:4 (VacA+ cag+ urease−), CCUG 17874 (VacA+ cag+), and 60190:M22 (VacA+ CagA−) H. pylori strains induced T84 cell-polarized monolayers to secrete significantly more IL-8 than was secreted by control T84 cells (4.67 ± 1, 4.5 ± 1.2, 4.7 ± 1.5, 4.1 ± 1, 4.21 ± 1.3, and 0.10 ± 0.11 ng/ml secreted into the basolateral compartment for strains 60190, 60190:v1, 60190:4, CCUG 17874, and 60190:M22 strain and control T84 cells, respectively; P < 0.01) (Table 2). In contrast, IL-8 production by T84 cells incubated with the G21 (VacA− cag mutant) and 60190:C− (VacA+ cagE) H. pylori strains was greatly reduced (0.5 ± 0.2 and 0.3 ± 0.1 ng/ml secreted into the basolateral compartment for the G21 and 60190:C− strains, respectively) (Table 2). We confirmed that the spontaneous chemotaxis of PMNL across imprinted matrices induced by strain 60190 of H. pylori was directed by IL-8 by using neutralizing polyclonal antibodies to IL-8. As shown in Fig. 6, neutralizing antibodies to IL-8 effectively reduced PMNL transmatrix migration across H. pylori-conditioned matrices ([19.45 ± 4] × 104 versus [4.81 ± 3.7] × 104 cell equivalents for the H. pylori 60190-conditioned T84 cell matrix in the presence and the absence of 60 μg of neutralizing polyclonal IL-8 antibodies/ml; P < 0.01). The IL-8 antibody had no effect on PMNL migration across control matrices driven by f-MLP (10−7 M). By contrast, the IL-8 antibody ablated PMNL migration across control matrices driven by exogenous IL-8 (100 ng/ml; Genentech) gradients (Fig. 6).
TABLE 2.
IL-8 secretion in the matrices and the lower reservoirs after incubation of T84 monolayers with different H. pylori strains
| Strain | IL-8 concn (ng/ml)a |
|---|---|
| 0190 | 4.67* |
| 60190:v1 | 4.5* |
| CCUG 17874 | 4.1* |
| 60190:4 | 4.7* |
| 60190:M22 | 4.22* |
| G21 | 0.5 |
| 60190:C− | 0.3 |
| None (T84 alone) | 0.10 |
∗, P < 0.01.
FIG. 6.
The effect of neutralizing anti-IL-8 antibodies on PMNL migration across T84-derived matrices. The MPO assay indicates total numbers of PMNL (PMNL-associated matrices plus PMNL that migrated to the lower reservoirs) after a 2-h transmigration. Solid bars, absence of IL-8 antibody; hatched bars, presence of IL-8 antibody. Data are pooled from 6 to 12 individual monolayers for each condition, and results are means ± standard errors. ∗, P < 0.01. CE, cell equivalents.
DISCUSSION
Our data present evidence that T84 monolayers grown on permeable filters represent a model of a polarized epithelium in which transepithelial migration of PMNL induced by H. pylori infection can be studied experimentally. To our knowledge, this is the first model allowing the migration of PMNL across an epithelium after apical colonization by H. pylori. The T84 cell line has previously been used extensively to study the transepithelial migration of PMNL and to analyze the modifications of TER under different conditions (21, 22, 41). We have selected this model because human gastric epithelial cell lines available and primary cultures of human gastric epithelial cells organize leaky epithelial monolayers or do not form confluent monolayers, conditions which are not well suited for the study of PMNL transepithelial migration. Previous work has used rabbit gastric epithelial cells to study PMNL migration (18). All these models fail to develop high TER.
To validate our model, we first verified that T84 cells were sensitive to VacA. VacA produced by H. pylori induces large vacuoles in the T84 epithelial cell line. Vacuolated cells were only observed in nonconfluent monolayers. Similarly, other epithelial cell lines, such as AGS, HeLa, MDCK, and MKN 28, demonstrate cell vacuolation after VacA intoxication only when they are cultivated at subconfluence (13, 40, 43). Previous studies have shown that the vacuoles in HeLa cells caused by VacA are enriched in rab7, a small GTP-binding protein typically associated with late endosomes, and that overexpression of a rab7 dominant-negative mutant prevents vacuolation caused by VacA (35, 39). We verified that the vacuoles induced by VacA in T84 cells originated from the late endosomal compartment since these vacuoles were stained by anti-rab7 antibodies. In our system, T84 cell vacuolation induced by VacA intoxication was not observed after incubation of both apical and basolateral sides of confluent T84 cell monolayers grown on permeable filters.
In our experiments the BCF from the VacA+ 60190 strain of H. pylori did not decrease the TER of T84 monolayers. Matysiak-Budnik et al. have shown similar integrity of the TER using HT29-19A intestinal cells (31). In contrast, another study has shown an increase in the permeability of polarized T84 cell monolayers due to VacA (40). These discrepancies with our results might be explained by the higher resistances achieved in the study by Papini et al. (40). In the present work we provide evidence that VacA does not influence the transepithelial migration of PMNL, since the rate of cell migration was induced to the same extent after incubation of T84 cells with BCFs or bacterial suspensions from both VacA-producing and non-VacA-producing strains. These data confirmed indirectly that the tight junctions are not altered by the VacA toxin. A recent study has shown that VacA inhibited actin stress fiber formation in RGM1 cells derived from gastric mucosa of the rat (38). This effect was especially marked after 48 h of incubation of RGM1 cells with VacA (38). In our work we did not observe alterations of the F-actin network at the perijunctional and at the basolateral cytoplasmic compartment levels after VacA intoxication of the monolayers. These divergent results may be explained by the different cell lines used and by the fact that T84 cells were grown on filters and not on coverslips. Moreover the incubation of T84 cells with VacA was only for up to 24 h.
Several works have shown that H. pylori infection is associated with an increase in the level of production of the C-X-C chemokine IL-8 in gastric epithelial cell lines (47, 52). Several pieces of evidence have demonstrated both in vitro and in vivo that increased IL-8 production is associated with the presence of type I strains of H. pylori. In particular cag+ H. pylori strains are able to induce IL-8 synthesis and secretion in the AGS epithelial cell line (47). Previous works have demonstrated the ability of cag+ H. pylori strains to activate the transcription factor NF-κB (27, 51). This activation was correlated with the ability to induce IL-8 transcription (27, 51). IL-8 transcription is also dependent on tyrosine kinase activation (50, 53). IL-8 is a chemokine factor which induces PMNL chemotaxis and activation (48). In parallel, it has been shown that H. pylori infection in vivo is associated with increased gastric mucosal levels of IL-8 (57). These increased levels are correlated with infection with specific H. pylori strains that possess the cag PAI (51). Moreover cag+ H. pylori-associated gastritis is characterized by a dense infiltration of PMNL in the mucosa (57). Using the T84 model, we have shown in vitro that the cag+ H. pylori strains, and more particularly those that possess the picB/cagE gene, can trigger a PMNL transepithelial migration which is correlated with an epithelial basolateral secretion of IL-8. As changes in the TER modify the rate of PMNL transmigration, we have verified that H. pylori infection did not alter these resistances. Terres et al. have shown that sonicates of H. pylori or whole bacteria can induce a significant decrease of TER in T84 monolayers (53). One possible explanation for this discrepancy might be that the number of bacteria and the area of the filters used in that study were different from those used in our work. In the present work, we have observed a paracellular location of the bacteria and a TER decrease when a high concentration of H. pylori was incubated at the apical side of the T84 monolayers (not shown).
Our ultrastructural study confirmed that H. pylori is internalized only exceptionally in the epithelial cells and is a noninvasive bacterium (25, 37). We have observed these features in very few cells. Previous ultrastructural studies have demonstrated that H. pylori can exceptionally penetrate into human gastric cells without causing any detectable damage to the cell membrane (16, 26). Attachment of cag+ H. pylori to cultured T84 cells induced transmigration of PMNL. However, we present here evidence that intimate attachment of H. pylori is not sufficient to induce this migration since the adherent 60190:C− strain failed to induce PMNL transepithelial migration. Although it has been previously demonstrated that H. pylori adhesion to T84 cells is increased at acidic pH (6), we used a neutral pH for incubating the bacteria to preserve the epithelial cell metabolism and the TER values.
The cag PAI is a 40-kb DNA segment of H. pylori containing at least 31 genes (5, 7). In the cag PAI, cagA (which encodes an immunodominant protein), picA, and picB/cagE genes have been extensively studied (1). Our in vitro study confirmed that the cagE gene, but not the cagA gene, is essential to induce both IL-8 secretion by T84 cells and PMNL transepithelial migration. In parallel, we have demonstrated that IL-8 secretion in H. pylori-infected T84 cells is basolaterally directed, leading to the formation of a chemokine gradient in the underlying matrix. It has been previously shown that noninvasive mutants of Salmonella enterica serovar Typhimurium (phoPc, hilA, and invA mutants), which harbor critical invasion deficiencies, do not induce PMNL transmigration (33). Moreover, a recent study has demonstrated that S. enterica serovar Typhimurium invasion is not sufficient to induce IL-8 secretion and to promote PMNL transmigration, suggesting that some specific interactions between the apical surface of the T84 cells and S. enterica serovar Typhimurium are required to allow a transepithelial signaling pathway (19). Here, we show that the noninvasive bacterium H. pylori is able to induce IL-8 secretion in the basolateral side for PMNL activation. Further work is needed to determine whether or not the ultimate step of the PMNL migration across the epithelial barrier is directed by the apical epithelial release of a soluble factor, as is observed for infection of the T84 cells by S. enterica serovar Typhimurium (34).
In conclusion, using an in vitro model, we have shown that the transepithelial migration induced by H. pylori-infected T84 cells is not related to the production of VacA cytotoxin. This PMNL migration is essentially dependent on cagE expression in the cag PAI of H. pylori. However, the possibility that the same effect might be achieved by some other genes in the cag PAI cannot be ruled out. Finally, the cag PAI, and more particularly cagE, induces an IL-8 gradient at the basolateral side, allowing migration of PMNL through the epithelial barrier.
ACKNOWLEDGMENTS
We acknowledge Mireille Mari, Dominique Sadoulet, Anne Doye, Aurore Grima, Christine Ordonnez, and Bernard Ferrua for their excellent technical assistance.
These studies were supported by the Fondation pour la Recherche Medicale (to V. Hofman). V. Ricci was a visiting scientist (Poste Vert INSERM) from the University of Pavia Medical School, Pavia, Italy.
REFERENCES
- 1.Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek S E, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–54. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 2.Atherton J C, Peek R M, Jr, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 3.Baik S C, Youn H S, Chung M H, Lee W K, Cho M J, Ko G H, Park C K, Kasai H, Rhee K H. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 1996;56:1279–1282. [PubMed] [Google Scholar]
- 4.Blaser M J. Ecology of Helicobacter pylori in the human stomach. J Clin Investig. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corthesy-Theulaz I, Porta N, Pringault E, Racine L, Bogdanova A, Kraehenbuhl J P, Blum A L, Michetti P. Adhesion of Helicobacter pylori to polarized T84 human intestinal cell monolayers is pH dependent. Infect Immun. 1996;64:3827–3832. doi: 10.1128/iai.64.9.3827-3832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covacci A, Telford J L, del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 8.Cover T L, Tummuru M K R, Cao P, Thompson S A, Blaser M J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 9.Cover T L. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996;20:241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree J E, Farmery S M, Lindley I J D, Figura N, Peichl P, Tompkins D S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994;47:945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtree J E, Covacci A, Farmery S M, Xiang Z, Tompkins D S, Perry S, Lindley I J D, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with cagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe S E, Alvarez L, Sherman P M, Jin Y, Dytoc M, Hunt R H, Patel J, Muller M J, Renst P B. Expression of interleukin-8 and CD54 by human gastric epithelium after H. pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 13.De Bernard M, Moschioni M, Papini E, Telford J, Rappuoli R, Montecucco C. Cell vacuolization induced by Helicobacter pylori VacA toxin: cell line sensitivity and quantitative estimation. Toxicol Lett. 1998;99:109–115. doi: 10.1016/s0378-4274(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 14.Dixon M F, Genta R M, Yardley J H, Correa P. Classification and grading of gastritis. The updated Sydney sytem. Am J Surg Pathol. 1996;20:1161–1183. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Shoura S M. Helicobacter pylori: I. Ultrastructural sequences of adherence, attachment, and penetration into the gastric mucosa. Ultrastruct Pathol. 1995;19:323–333. doi: 10.3109/01913129509064237. [DOI] [PubMed] [Google Scholar]
- 17.Figura N, Guglielmetti P, Rossolini A, Barberi A, Cusi G, Musmanno R, Russi M, Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989;27:225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiwara Y, Arakawa T, Fukuda T, Sasaki E, Nakagawa K, Fujiwara K, Higuchi K, Kobayashi K, Tarnawski A. Interleukin-8 stimulates leukocyte migration across a monolayer of cultured rabbit gastric epithelial cells. Effect associated with the impairment of gastric epithelial barrier function. Dig Dis Sci. 1997;42:1210–1215. doi: 10.1023/a:1018850006714. [DOI] [PubMed] [Google Scholar]
- 19.Gewirtz A T, Siber A M, Madara J L, McCormick B A. Orchestration of PMNL movement by intestinal epithelial cells in response to Salmonella typhimurium can be uncoupled from bacterial internalization. Infect Immun. 1999;67:608–617. doi: 10.1128/iai.67.2.608-617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghiara P, Marchetti M, Blaser M J, Tummuru M K R, Cover T L, Segal E D, Tompkins L S, Rappuoli R. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect Immun. 1995;63:4154–4160. doi: 10.1128/iai.63.10.4154-4160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofman P, D'Andrea L, Carnes D, Colgan S P, Madara J L. The actin cytoskeleton of intestinal epithelial cells selectively constrains lumen to tissue directed migration of PMNLs. Am J Physiol. 1996;271:312–320. doi: 10.1152/ajpcell.1996.271.1.C312. [DOI] [PubMed] [Google Scholar]
- 22.Hofman P, Selva E, D'Andrea L, Guérin S, Le Negrate G, Rossi B, Auberger P. CD10 inhibitors increase F-Met-Leu-Phe-induced PMNL transmigration. J Leukoc Biol. 1998;63:312–320. doi: 10.1002/jlb.63.3.312. [DOI] [PubMed] [Google Scholar]
- 23.Hofman P, Flatau G, Selva E, Gauthier M, Le Negrate G, Fiorentini C, Rossi B, Boquet P. Escherichia coli necrotizing factor 1 (CNF1) effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect Immun. 1998;66:2494–2500. doi: 10.1128/iai.66.6.2494-2500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilver D, Arnqvist A, Ögren J, Frick I M, Kersulyte D, Incecik E T, Berg D E, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 25.Janas B, Czkwianianc E, Bak-Romanisyn L, Bartel H, Tosik D, Planeta-Malecka I. Electron microscopic study of association between coccoid forms of Helicobacter pylori and gastric epithelial cells. Am J Gastroenterol. 1995;90:1829–1833. [PubMed] [Google Scholar]
- 26.Kazi J L. Ultrastructural study of Helicobacter pylori: associated gastritis. J Pathol. 1990;161:65–70. doi: 10.1002/path.1711610111. [DOI] [PubMed] [Google Scholar]
- 27.Keates S, Hitti Y S, Upton M, Kelly C P. Helicobacter pylori activate NFκB in gastric epithelial cells. Gastroenterology. 1997;113:1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 28.Labigne A, de Reuse H. Determinants of Helicobacter pylori pathogenicity. Infect Agents Dis. 1996;5:191–202. [PubMed] [Google Scholar]
- 29.Logan R P H, Berg D E. Genetic diversity of Helicobacter pylori. Lancet. 1996;348:1462–1463. doi: 10.1016/s0140-6736(05)65885-0. [DOI] [PubMed] [Google Scholar]
- 30.Madara J L, Colgan S, Nusrat A, Delp C, Parkos C. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing PMNL-epithelial monolayers. J Tissue Cult Methods. 1992;14:209–216. [Google Scholar]
- 31.Matysiak-Budnik T, Terpend K, Alain S, Sanson le Pors M J, Desjeux J F, Megraud F, Heyman M. Helicobacter pylori alters exogenous antigen absorption and processing in a digestive tract epithelial cell line model. Infect Immun. 1998;66:5785–5791. doi: 10.1128/iai.66.12.5785-5791.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormick B A, Hofman P M, Kim J, Carnes D, Miller S, Madara J L. Surface binding of Salmonella typhimurium to intestinal epithelia: imprinting of pathways directing PMNL movement on the underlying matrix. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick B A, Miller S, Carnes D, Madara J L. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun. 1995;63:2302–2309. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick B A, Parkos C A, Colgan S P, Madara J L. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- 35.Molinari M, Galli C, Norais N, Telford J L, Rappuoli R, Luzio J P, Montecucco C. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J Biol Chem. 1997;272:25339–25344. doi: 10.1074/jbc.272.40.25339. [DOI] [PubMed] [Google Scholar]
- 36.NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 37.Noach L A, Rolf T M, Tytgat G N J. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994;47:699–704. doi: 10.1136/jcp.47.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pai R, Cover T L, Tarnawski A S. Helicobacter pylori vacuolating cytotoxin (VacA) disorganizes the cytoskeletal architecture of gastric epithelial cells. Biochem Biophys Res Commun. 1999;262:245–250. doi: 10.1006/bbrc.1999.1194. [DOI] [PubMed] [Google Scholar]
- 39.Papini E, Barbara S, Bucci C, de Bernard M, Telford J L, Manetti R, Rappuoli R, Zerial M, Montecucco C. The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J. 1997;16:15–24. doi: 10.1093/emboj/16.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papini E, Satin B, Norais N, de Bernard M, Telford J R, Rappuoli R, Montecucco C. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J Clin Investig. 1998;102:813–820. doi: 10.1172/JCI2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkos C A, Delp C, Arnaout M A, Madara J L. PMNL migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Investig. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pères-Pères G I, Olivares A S, Cover T L, Blaser M J. Characteristics of Helicobacter pylori variants selected for urease deficiency. Infect Immun. 1992;60:3658–3663. doi: 10.1128/iai.60.9.3658-3663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricci V, Sommi P, Fiocca R, Figura N, Romano M, Ivey K J, Solcia E, Ventura U. Cytotoxicity of Helicobacter pylori on human gastric epithelial cells in vitro: role of cytotoxin(s) and ammonia. Eur J Gastroenterol Hepatol. 1993;5:687–694. [Google Scholar]
- 44.Ricci V, Ciacci C, Zarrilli R, Sommi P, Tummuru M K R, Del Vecchio Blanco C, Bruni C B, Cover T L, Blaser M J, Romano M. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect Immun. 1996;64:2829–2833. doi: 10.1128/iai.64.7.2829-2833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricci V, Sommi P, Fiocca R, Romano M, Solcia E, Ventura U. Helicobacter pylori vacuolating toxin accumulates within the endosomal-vacuolar compartment of cultured gastric cells and potentiates the vacuolating activity of ammonia. J Pathol. 1997;183:453–459. doi: 10.1002/(SICI)1096-9896(199712)183:4<453::AID-PATH950>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Ricci V, Zarrilli R, Sommi P, Romano M. Mechanisms of Helicobacter pylori-induced damage to gastric mucosa. J Dig Protect. 1999;1:9–20. [Google Scholar]
- 47.Rieder G, Hatz R A, Moran A P, Walz A, Stolte A, Enders G. Role of adherence in interleukin-8 induction in Helicobacter pylori associated gastritis. Infect Immun. 1997;65:3622–3630. doi: 10.1128/iai.65.9.3622-3630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rollins B J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 49.Scott D, Weeks D, Melchers K, Sachs G. The life and death of Helicobacter pylori. Gut. 1998;43(Suppl. 1):S56–S60. doi: 10.1136/gut.43.2008.s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segal E D, Lange C, Covacci A, Tomkins L S, Falkow S. Induction of host signal transduction pathway by Helicobacter pylori. Proc Natl Acad Sci USA. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma S A, Tummuru M K, Blaser M J, Kerr L D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-κB in gastric epithelial cells. J Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 52.Shude D L, Kersulyte D, Lindley I J D, Neelam B, Berg D E, Crabtree J E. Multiple genes in the left half of the cag pathogenicity island of Helicobacter pylori are required for tyrosine kinase-dependent transcription of interleukin-8 in gastric epithelial cells. Infect Immun. 1999;67:3893–3899. doi: 10.1128/iai.67.8.3893-3899.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terres A M, Hopkins J M, Murphy A, Moran A, Baird A W, Kelleher D. Helicobacter pylori disrupts epithelial barrier function in a process inhibited by protein kinase C activators. Infect Immun. 1998;66:2943–2950. doi: 10.1128/iai.66.6.2943-2950.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tummuru M K R, Cover T L, Blaser M J. Mutation of the cytotoxin-associated cagA gene does not affect the vacuolating cytotoxin activity of Helicobacter pylori. Infect Immun. 1994;62:2609–2613. doi: 10.1128/iai.62.6.2609-2613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tummuru M K R, Sharma S A, Blaser M J. Helicobacter pylori picB, a homolog of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 56.Xiang Z, Censini S, Bayeli P F, Telford J L, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating toxin. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut. 1998;42:609–617. doi: 10.1136/gut.42.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zarrilli R, Ricci V, Romano M. Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage. Cell Microbiol. 1999;1:93–99. doi: 10.1046/j.1462-5822.1999.00018.x. [DOI] [PubMed] [Google Scholar]