Abstract
Patients who have recovered from coronavirus disease 2019 (COVID-19) infection may experience chronic fatigue when exercising, despite no obvious heart or lung abnormalities. The present lack of effective treatments makes managing long COVID a major challenge. One of the underlying mechanisms of long COVID may be mitochondrial dysfunction. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections can alter the mitochondria responsible for energy production in cells. This alteration leads to mitochondrial dysfunction which, in turn, increases oxidative stress. Ultimately, this results in a loss of mitochondrial integrity and cell death. Moreover, viral proteins can bind to mitochondrial complexes, disrupting mitochondrial function and causing the immune cells to over-react. This over-reaction leads to inflammation and potentially long COVID symptoms. It is important to note that the roles of mitochondrial damage and inflammatory responses caused by SARS-CoV-2 in the development of long COVID are still being elucidated. Targeting mitochondrial function may provide promising new clinical approaches for long-COVID patients; however, further studies are needed to evaluate the safety and efficacy of such approaches.
Keywords: SARS-CoV-2, long COVID, pathogen-associated molecular patterns (PAMPs), mitochondrial disorder, inflammatory responses, oxidative phosphorylation, electron transport chain
1. Introduction
The primary symptom of coronavirus disease 2019 (COVID-19) is a respiratory illness [1]. However, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can also cause gastrointestinal symptoms and inflammation, which can affect the lungs and immune response through cytokines in the bloodstream [2]. People with post-COVID-19 illness may experience a variety of symptoms that can persist for weeks, months, or even years after infection [3]. Sometimes, symptoms even disappear and reappear. These symptoms can be difficult to explain and manage. Long COVID has been defined as “signs, symptoms, and conditions that continue or develop after initial SARS-CoV-2 infection” [4]. Clinical evaluations and the assessment of results of routine blood work, chest X-rays, and electrocardiograms may be normal [5]. The most commonly experienced symptoms of post-COVID-19 conditions include an exaggerated hyperventilation response during exercise, tiredness or weakness that interferes with daily life, malaise after exertion, fever, and more [6,7]. The peak oxygen uptake (VO2peak) in patients with long COVID-19 has been found to be significantly lower than normal [8]. In the first few months after infection, due to chronotropic insufficiency and reduced cardiac output, the cardiovascular system can lead to low VO2peak through lower- than-normal cardiac output [8]. Alternatively, peripheral factors such as muscle mass, strength and perfusion, mitochondrial function, or arteriovenous oxygen difference may also contribute to low VO2peak [8]. These symptoms are similar to those reported by patients with myalgic encephalomyelitis/chronic fatigue syndrome and people with genetic mitochondrial diseases [7,9].
Co-localization of the SARS-CoV-2 genome and mitochondria in SARS-CoV-2-infected tissues has been reported [10]. Mitochondria can be extremely vulnerable to physiological and pathological stimuli such as viral infections. The integrity and normal function of mitochondria can be altered during SARS-CoV-2 infection, thereby modulating cellular responses. The replication of SARS-CoV-2 begins with virus-induced double-membrane vesicles (DMV) that originate from the endoplasmic reticulum and eventually integrate to form a complex membrane network [11]. SARS-CoV-2 proteins are known to selectively target organelle compartments such as the endoplasmic reticulum and mitochondria, and they can reside in the host mitochondrial matrix [12]. This results in mitochondrial dysfunction and increased oxidative stress, ultimately leading to the loss of mitochondrial integrity and cell death [13]. These proteins also combine with non-specific and selective channel mitochondrial permeability transition pore (mPTP) complexes on mitochondria, which could alter the mitochondrial morphology and function, disrupt the Ca2+ cycle, inhibit voltage-gated calcium channel (VDCC) activity, and lead to ion homeostasis disorder and mitochondrial dysfunction, thereby disrupting the cellular Ca2+ cycle and cell viability [14].
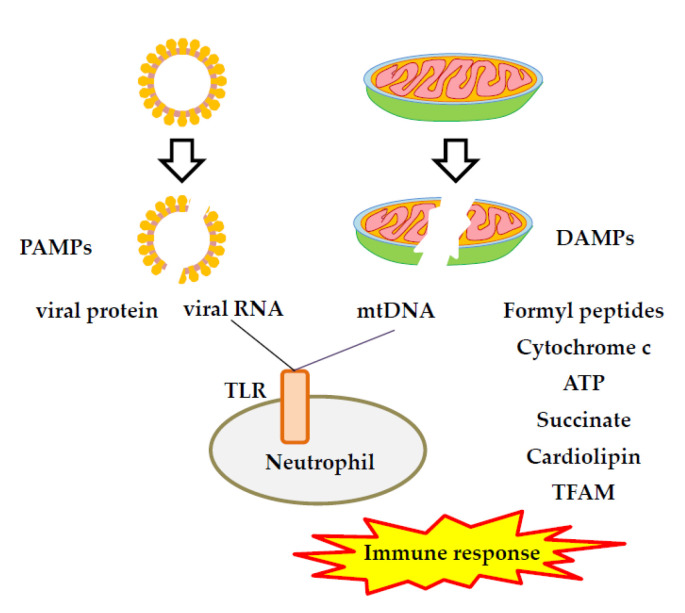
Furthermore, hyperactivation of the NLR family pyrin domain-containing 3 (NLRP3) inflammasome results in hyper-responsiveness in classically activated macrophages when mitochondrial function is compromised, increasing mitochondrial reactive oxygen species (mtROS), and free circulating mitochondrial DNA (mtDNA). Oxidized mtDNA, cardiolipin, and cytochrome c from the damaged mitochondria, when released into the cytoplasm, activate damage-associated molecular patterns (DAMPs), leading to sustained local and systemic inflammatory responses [15,16,17]. This article reviews hypotheses regarding the changes in mitochondrial status under SARS-CoV-2 infection, including mitochondrial redox homeostasis, regulation of inflammation, and antiviral signaling, among others, and discusses why mitochondrial damage may contribute to long COVID.
2. SARS-CoV-2 and Long COVID
2.1. SARS-CoV-2 Non-Structural Proteins
Coronavirus is a single-stranded positive-sense RNA virus containing an envelope. Its genome is structured as 5′-leader-UTR-replicase (open reading frame 1ab)-spike-envelope-membrane-nucleocapsid-3′UTR-polyA tail [18,19]. SARS-CoV-2 virus particles are composed of main structural proteins, such as the spike protein, envelope protein, membrane protein, and nucleocapsid protein. The replicase polyproteins self-cleave the ORF1a and ORF1ab polyproteins of SARS-CoV-2 to form 16 non-structural proteins (NSP1 to NSP16) [20]. These complexes are involved in replication and transcription, playing different roles throughout the coronavirus life cycle (Table 1). For example, NSP16 plays an important role in evading the host immune response and protecting the nascent viral mRNA from degradation [21].
Table 1.
Summary of non-structural proteins of SARS-CoV-2 and their functions.
| Proteins | Length (AA) 1 | Function, Key Features |
|---|---|---|
| NSP1 | 180 | Suppresses host genes and enhances viral RNA expression |
| NSP2 | 638 | For SARS-CoV replication |
| NSP3 | 1945 | Component of the replication/transcription complex |
| NSP4 | 500 | Assembly of cytoplasmic double-membrane vesicles required for viral replication |
| NSP5 | 306 | Cleavage of viral polyproteins |
| NSP6 | 290 | Inhibits the lysosomal autophagy system and stimulates the NLRP3 inflammasome-dependent pyroptotic pathway |
| NSP7 | 83 | Involved in the replication and transcription of SARS |
| NSP8 | 198 | Viral RNA primers co-localize with RdRP to replicate SARS-CoV |
| NSP9 | 113 | Interact with the replication complex |
| NSP10 | 139 | Interact with NSP14 and NSP16 |
| NSP11 | 13 | Unknown |
| NSP12 | 932 | RNA-dependent RNA polymerase |
| NSP13 | 601 | Zinc-binding domain, NTPase, dNTPase. 5′-to-3′ RNA and DNA helicase, RNA 5′-triphosphate |
| NSP14 | 527 | 3′-to-5′ exoribonuclease, zinc-binding domain, and N7-methyltransferase |
| NSP15 | 346 | Uridylate-specific endoribonuclease, homohexamer |
| NSP16 | 298 | Putative ribose-2′-O-methyltransferase |
1 from SARS-CoV-2 reference genome (NC_045512.2). dNTPase, deoxynucleoside triphosphohydrolase; NLRP3, NLR family pyrin domain-containing 3; NSP, non-structural proteins; NTPase, nucleoside-triphosphatase; SARS-CoV, Severe acute respiratory syndrome-associated coronavirus.
In addition to these non-structural protein complexes involved in the replication and transcription of SARS, the open reading frame 3a (ORF3a), ORF3b, ORF3c, ORF3d, ORF6, ORF7a, ORF7b, ORF8, ORF9b, ORF9c, and ORF10 are also involved in the infection mechanism of SARS-CoV-2 (Table 2) [22]. In particular, SARS-CoV-2 lacks ORF8a, compared to SARS-CoV [23,24].
Table 2.
Summary of SARS-CoV-2 open reading frame proteins and their functions.
| Proteins | Length (AA) | Function (Ref) |
|---|---|---|
| ORF3a | 275 | Ion channel [25] Replication and virulence [26] Activating pro-IL-1β gene expression and IL-1β secretion [27] Ultimately activating NFκB signaling and NLRP3 inflammasomes [27] Promoting the cytokine storm [27] Necrotic cell death and lysosomal damage [28] |
| ORF3b | 22 | IFN antagonism [29] |
| ORF3c | 41 | Unknown [30] |
| ORF3d | 57 | Interaction with STOML2 [31] With ORF8, elicits the strongest antibody responses [32] |
| ORF6 | 61 | Localized to the ER, autophagosomes, and lysosomes [33] Blocks STAT transportation from the cytoplasm to the nucleus [34] IFN antagonist [34,35] Inhibits STAT1 nuclear transportation [36] Accumulation of hnRNPA1 in the nucleus [37] |
| ORF7a | 121 | Antagonizes the IFN-I response [38] Hijacks the host ubiquitin system to enhance and antagonize IFN-I responses [39] Decreases antigen-presenting ability and induces expression of pro-inflammatory cytokines [40] |
| ORF7b | 43 | Transmembrane protein localized in the Golgi [41] |
| ORF8 | 121 | Apoptosis [42] Antagonizing the IFN signaling pathway [43] Interacts with MHC-I [24] IFN I antagonist [44] Activates IL-17 signaling pathway and promote proinflammatory factors [45] |
| ORF9b | 97 | Induces autophagy in host cells mediated by ATG5 [46] Tom70 forms a complex with ORF9b to modulate the host immune response by compromising type I IFN synthesis [47] Activation of inflammasome [48] Promotes proteasomal degradation of Drp1 [46] |
| ORF9c | 73 | Protein interaction between ORF9c protein and NFκB-related molecules [31] Interactions with NFκB-related molecules [49] Interferes with IFN signaling [31,50,51] |
| ORF 10 | 38 | Unknown [52] |
ATG5, autophagy-related 5; Drp1, dynein-related protein 1; ER, endoplasmic reticulum; hnRNPA1, heterogeneous nuclear ribonucleoprotein A1; IFN, interferons; IL, interleukin; MHC, major histocompatibility complex; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family pyrin domain-containing 3; ORF, open reading frame; STAT1, signal transducer and activator of transcription 1; STOML2, stomatin-like 2.
2.2. Viral PAMPs
When the immune system senses tissue damage, certain molecules are released to trigger an immune response via DAMPs [53]. DAMPs and related pathogen-associated molecular patterns (PAMPs) trigger inflammasome assembly, activate caspase-1 [54], and induce mitochondrial damage [55] (Figure 1). Toll-like receptors (TLRs) are expressed on both innate and non-immune cells. The activation of TLRs plays a dual role [56], including the recognition and elimination of PAMPs in bacteria, viruses, and other pathogens, and the involvement of pathogens in the recognition and elimination of autogenous DAMPs released from dying or lysed cells [57]. Typical PAMPs are nucleic acids, including viral RNA and DNA, but also include surface-exposed glycoproteins, lipoproteins, and various membrane components [57]. The engagement of PAMPs and/or DAMPs can activate pattern recognition receptors (PRRs), such as TLRs or nucleotide-binding oligomerization domain-containing protein 2 (NOD2), resulting in NFκB activation and the activation of gene transcription. It was earlier reported that double-stranded RNA (dsRNA) viral PAMPs are known to activate TLR3 [58], whereas single-stranded RNA (ssRNA) viral PAMPs mainly activate TLR7/8 [59]. Changes in the distribution of charged amino acids in the spike protein of Omicron variants interfere with the recognition of TLRs by PRRs, thereby reducing activation of the NF-κB pathway and related signaling pathways, consequently inhibiting viral replication and systemic immune hyperactivation [60].
Figure 1.
Mitochondrial damage-associated molecular patterns (DAMPs) and related pathogen-associated molecular patterns (PAMPs) in the chronic inflammasome. Inflammation is caused by damage or infection by stimulatory signals, including PAMPs and DAMPs. PAMPs are derived from microbial products such as viral nucleic acids, which trigger inflammation in response to infection. DAMPs originate from host cells or products released by cells in response to signals such as hypoxia, producing sterile inflammatory responses in settings such as myocardial infarction, cancer, autoimmune disease, and atherosclerosis. ATP, adenosine triphosphate; DAMPs, damage-associated molecular patterns; mtDNA, mitochondrial DNA; TFAM, transcription factor A mitochondria; TLR, toll-like receptor.
2.3. Long COVID
“Post-COVID-19 syndrome”, “long COVID-19”, “long-term COVID-19 effects”, “long haulers”, “COVID-19 long tail”, and “persistent COVID-19 symptoms” [61,62,63,64] are all terms used to describe various conditions, such as inflammation, sequelae of organ damage, hospitalization, and social isolation, which persist for a long time after SARS-CoV-2 infection [65]. Long COVID has long-term heterogeneity and complex symptoms [66]. The most common symptoms include chronic fatigue [67]; respiratory manifestations (e.g., cough and shortness of breath) [67]; arrhythmias, palpitations, hypotension, increased heart rate, venous thromboembolic disease, myocarditis, and acute heart failure [68]; and symptoms of neurological and psychiatric related disorders (e.g., peripheral neuropathy, brain fog, anxiety, and depression) [67,69,70]. Diagnostic clusters have shown that long COVID does not have a single phenotype but, instead, a collection of sub-phenotypes subject to different diagnoses and treatments [71].
The mechanism underlying the persistence of long COVID has not yet been identified [72], but it has been speculated that it may be related to abnormal immune responses, virus-specific pathophysiology, inflammatory damage in response to acute infection [73], persistence of the virus in certain tissues [74,75] or exosomes and hypertrophy cells [76,77], SARS-CoV-2 interactions with the host microbiome/virome community, coagulation/coagulation problems, and dysfunctional brainstem/vagal signaling [78]. In addition, the role of SARS-CoV-2 in relation to mitochondrial damage and the subsequent immune response has been recently considered [79,80,81]. For example, the spike protein of SARS-CoV-2 can inhibit the transcription of mitochondrial metabolic genes in the long term, resulting in myocardial fibrosis and myocardial contractile dysfunction [79]. Long COVID neurological symptoms are associated with SARS-CoV-2 proteins and abnormalities in mitochondrial proteins in nerve cells [81].
3. Altered Mitochondrial Function in SARS-CoV-2 Infection
Both SARS-CoV-2-induced and inactivity-induced changes may increase mitochondrial dysfunction and myofibril breakdown, and decrease mitochondrial biogenesis and muscle synthesis [82]. Cells infected with SARS-CoV-2 exhibit abnormally swollen mitochondrial cristae [83]. Mitochondria in SARS-CoV-2-infected cells are markedly thinned, and the mitochondria are translocated and clustered around viral dsRNA containing DMV [84]. NSP4 and ORF9b cause structural changes in mitochondria, as well as the formation of outer membrane macropores and release of mtDNA-laden inner membrane vesicles [85]. In addition, genes related to mitochondria are predominantly downregulated in SARS-CoV-2-infected cells [86]; for example, the expression of mtDNA, antioxidants (e.g., catalase, glutathione synthetase), and mitochondrial respiratory chain proteins (NDUFA9, NADH: ubiquinone oxidoreductase subunit A9; SDHA, succinate dehydrogenase complex flavoprotein subunit A; COX4I1, cytochrome c oxidase subunit 4I1) were significantly reduced in SARS-CoV-2-infected placentas [87].
SARS-CoV ORF7a, ORF8a, and ORF9b are known to be located in the mitochondria, which can inhibit retinoic acid-inducible gene I-mitochondrial antiviral signaling protein (RIG1-MAVS)-dependent interferon signaling, enhance viral replication, and destroy mitochondrial function [88]. Mitochondrial dysfunction is one of the earliest and most prominent neurodegenerative features of SARS-CoV-2-induced neuropathology [89]. In addition, metabolic syndromes such as diabetes, obesity, and cardiovascular and liver diseases —which are related to mitochondria—also lead to susceptibility and adverse outcomes of SARS-CoV-2 infection [90,91,92,93], significantly increasing the mortality rate of SARS-CoV-2 infection [94,95]. Many environmental chemicals, malnutrition, and exacerbated socio-economic stress can also cause mitochondrial damage, which can negatively affect the prognosis of COVID-19 [96]. Furthermore, cardiac mitochondrial disruption after infection, ROS production, and energetic stress lead to mitochondrial alterations and cardiovascular dysfunction in COVID-19 patients, and the incidence of adverse cardiovascular events increases in recovered COVID-19 patients [97].
3.1. Alteration of Mitochondrial Ca2+ Signaling
The host Ca2+ channel affects every process of infection by the virus, which invades the host Ca2+ channel to disrupt homeostasis and benefit its lifecycle at the expense of host survival. When inhibiting the activity of store-operated Ca2+ channels, viral shedding is reduced, thereby reducing the infectivity of many enveloped RNA viruses [98,99]. During viral infection, infectious agents are also known to trigger dysregulation of host cell signaling cascades that affect cellular calcium dynamics [100,101]. This can lead to an imbalance in calcium concentrations, which can have an impact on mitochondrial function. Furthermore, six specific acidic residues (E819, D820, D830, D839, D843, and D848) on the SARS-CoV-2 spike proteins may bind calcium, thereby regulating the ability of the virus to insert into lipid membranes, which, in turn, affects oxidative phosphorylation (OXPHOS) and mitochondrial calcium sequestration [14,102,103].
The endoplasmic reticulum, mitochondria, and plasma membrane are all involved in the coordination of calcium dynamics, and there is evidence that viruses themselves may cause alterations in mitochondrial calcium signaling. ORF3a is known to assemble into dimeric folds and form non-selective Ca2+ permeable cation channels [104]. When ORF3 is expressed, the mitochondria-associated membrane (MAM) formation is increased and cytoplasmic calcium is transported to mitochondria through the endoplasmic reticulum, as ORF3a is a calcium ion transporter [104]. NSP2 and NSP4 interact with the endoplasmic reticulum lipid raft-associated protein 1/2 (ERLIN1/2) complex to regulate Ca2+ signaling from the endoplasmic reticulum to the mitochondria [105]. Furthermore, the calcium influx due to ORF3a activates the switch for calcium-dependent caspases and apoptosis which, in turn, trigger programmed cell death [106]. SARS-CoV-2 proteins significantly disrupt the baseline oscillations of cellular calcium, block L-type calcium channel activity, and cause cellular damage [14]. SARS-CoV-2 proteins alter mPTP, causing it to open and trigger cell death [14]. The mPTP complex components SPG7 matrix AAA peptidase subunit (SPG7) and cyclophilin D are upregulated in cells infected with SARS-CoV-2 [107]. SPG7, peptidylprolyl isomerase F (PPIF), and mitochondrial matrix import factor 23 (MIX23) are known to interact. The knockdown of MIX23 in mitochondria significantly enhanced the ability of mitochondria to sequester calcium. In contrast, an mPTP blocker cyclosporin A is known to restore mitochondrial Ca2+ retention and cell viability after viral infection [14]. ORF3a, ORF9b and 9c, ORF10, and NSP6 of SARS-CoV-2 are associated with mPTP complex proteins involved in mitochondrial dysfunction and cell death [14].
3.2. Alteration in Glycolysis–OXPHOS Equilibrium
Long COVID is a virus-induced chronic and self-perpetuating unresolved state of metabolic imbalance characterized by mitochondrial dysfunction, ROS continuously driving inflammation, and a shift towards glycolysis [108]. The functions of OXPHOS and mPTP can be altered by viruses, resulting in an imbalance of energy and biosynthetic resources or evasion of immune surveillance [109,110]. The NSP4 of SARS-CoV-2 interacts with the mitochondrial chaperone ion peptidase 1 (LONP1), affecting the function of LONP1 to promote mitochondrial protein folding, together with the mitochondrial 70 kDa heat-shock protein (mtHSP70) chaperone system [111]. OXPHOS assembled with an insufficient amount of proteins will have defects, affecting the complex function of OXPHOS. For example, in the bronchoalveolar lavage fluid of patients infected with SARS-CoV-2, the nicotinamide adenine dinucleotide (NADH):ubiquinone oxidoreductase core sub-unit V (NDUFV, complex 1), SDHA (complex 2), COR1 (cytochrome c reductase), ubiquinol–cytochrome c reductase core protein 2 (UQCRC2, complex 3), adenosine triphosphate (ATP) synthase peripheral stalk sub-unit F6 (ATP5PF), ATP synthase F1 sub-unit alpha (ATP5F1A), ATP synthase peripheral stalk sub-unit d (ATP5PD), F-type ATPase A, and inorganic pyrophosphatase 2 (PPA2, ATP synthase) were down-regulated [112]. The OXPHOS of infected patients may not function properly, leading to decreased mitochondrial aerobic respiration and higher oxidative stress. The integrity of the mitochondrial membrane may be compromised after SARS-CoV-2 infection. Complexes II and IV of OXPHOS component proteins were found outside the virus-infected cytoplasm and mitochondrial matrix, indicating that the functions of OXPHOS and the electron transport chain were disrupted [113]. An impaired expression of mitochondrial OXPHOS and antioxidant genes was observed, and the loss of electron transport chain and membrane potential resulted in decreased mitochondrial aerobic respiration and enhanced mtROS [114]. Mitochondrial damage initiates apoptosis under impaired mitochondrial electron transport chain activity, ATP depletion, and increased oxidative stress [115].
Hyperlactation is observed in all COVID-19 deaths [116]. Patients infected with SARS-CoV-2 have altered metabolic pathways that lead to decreased mitochondrial respiration and increased glycolysis, which may worsen the patient’s condition. The SARS-CoV-2 proteins responsible for exacerbation of the disease prompt the cells to switch from an oxidative to a glycolytic phenotype [14]; for example, the spike protein of SARS-CoV-2 decreases basal mitochondrial respiration and ATP production, increases glucose-induced glycolysis, and maximizes the glycolytic transport capacity of the port [117]. This implies that SARS-CoV-2 can take over host mitochondria to facilitate its metabolic pathways [48,118,119,120], resulting in higher lactate and plasma glucose values in SARS-CoV-2-infected patients. When cells were infected with SARS-CoV-2 membrane protein or ORF3a, there was a lower basal mitochondrial oxygen consumption rate (OCR) and a minimal trifluoromethoxy carbonylcyanide phenylhydrazone (FCCP)-induced maximal OCR. NSP6 and NSP7 partially inhibited the maximal OCR in infected cells [14]. Certain SARS-CoV-2 proteins cause cells to switch from an oxidative to a glycolytic phenotype [14]. Hypoxia is induced by lung involvement, favoring glycolysis and lactate accumulation when mitochondrial dysfunction and OXPHOS are reduced. The accumulation of lactate is detrimental to the function of MAVS, and may further reduce the interferon response [121]. Patients infected with SARS-CoV-2 have altered metabolic pathways that lead to decreased mitochondrial respiration and increased glycolysis, which may worsen the patient’s condition. This implies that SARS-CoV-2 can take over the host mitochondria, to obstruct its metabolic pathways. Furthermore, the lactate and plasma glucose values are higher in SARS-CoV-2 infected patients.
3.3. Alteration of Mitochondrial Dynamics
The fusion and fission dynamics of mitochondria remodel the ultrastructure of the membrane [122], allowing for the removal of damaged mitochondria [123]. The mitochondrial morphology is altered or destabilized in the normal physiological division–fusion dynamics of cells, which plays a role in many pathological conditions [124]. For example, inhibiting the mitochondrial fusion-essential protein mitofusin-2 (Mfn2) increased ROS production and increased c-Jun N-terminal kinase (JNK) and nuclear factor kappa-light chain enhancer (NFκB) in activated B cell signaling, resulting in decreased insulin signaling and glucose uptake [125]. Additionally, the aberrant expression of the dynein-related protein 1 (DRP1, mitochondrial fission protein) increased H2O2 production and damaged mitochondria [126].
The ORF9b protein mediates the degradation of the DRP1, leading to the abnormal lengthening of mitochondria [46]. Moreover, infected cells present a down-regulation of genes associated with mitochondrial ribosome synthesis, mitochondrial complex I synthesis, translocase, mitochondrial fission process 1 (MTFP1, mitochondrial fission-promoting protein), and suppressor of cytokine signaling 6 (SOCS6) [86]. The down-regulation of the mechanistic target of rapamycin complex 1 (mTORC1) resulted in decreased expression of MTFP1 and complex I [127,128]. Reduced expression of MTFP1 activates nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and SOCS6, leading to mitochondrial hyper fusion [129].
3.4. Alteration of Autophagy and Apoptosis
Autophagy denotes the removal of unused proteins and damaged mitochondria from cells, but impaired autophagy leads to reduced oxygen consumption and reduced mitochondrial activity. Autophagy can also transform cells into pro-inflammatory generators, and lead to protein clumps that disrupt various processes. Mitochondrial outer membrane permeability (MOMP) is controlled by mitochondria [130], which triggers apoptosis during intracellular DNA damage, oxidative stress, cytosolic Ca2+ overload, endoplasmic reticulum stress, and other perturbations [131]. Viruses deflect mitochondrial function through pro- and anti-apoptotic responses [132]. When pro-apoptotic signals prevail, the outer membrane loses its integrity and re-releases several potentially toxic intermembrane-space (IMS) proteins into the cytoplasm. IMS are driven to release apoptosis-inducing factors in response to DNA damage which, in turn, leads to DNA damage-associated enzyme polymerization (ADP-ribose polymerase, PARP) [133,134,135]. When host cells send out PAMP signals, inflammasomes oligomerize and form receptors, activate caspases to produce cytokines, and induce apoptosis [54]. The inflammasome is activated, leading to the production of pro-inflammatory cytokines (IL-1β and 12) and apoptosis [136]. The permeabilization of mitochondria is critical for apoptosis and regulated necrosis [130,131,137].
ORF6 localizes to the autophagosome and lysosomal membranes [33]. Furthermore, ORF9b induces autophagy in autophagy-related 5 (ATG5)-mediated host cells [46]. ORF3a and ORF8 are involved in apoptosis [42]. NSP4 interacts with B-cell lymphoma 2 (BCL2) antagonist/killer (BAK) to mediate macropore formation ability. ORF9b inhibits the anti-apoptotic member of the BCL2 family protein myeloid leukemia-1 (MCL1) and induces the formation of inner membrane vesicles containing mtDNA. The removal of BAK and/or over-expression of MCL1 significantly reverses SARS-CoV-2-mediated mitochondrial damage. Meanwhile, ORF9b and ORF9c interact with organelles, leading to the inhibition of antiviral responses in infected cells [138].
3.5. Cell Death and mtDNA Release
The activation of MOMP leads to cell death through cytochrome c release, apoptosis protease activating factor-1 (APAF-1)-dependent, and deoxyadenosine triphosphate (dATP)-dependent assembly, followed by caspase activation and the involvement of proinflammatory signaling functions. ORF3a is involved in necrotic cell death and lysosomal damage [28], and influences viral replication and virulence [26]. ORF6 and ORF7a have been shown to display the highest potency for cytotoxicity [33].
SARS-CoV-2 infects mitochondria and forms double-membrane vesicles which, in turn, leads to the release of mtDNA into circulation and the activation of the immune response, resulting in a severe pro-inflammatory state [139]. The mtDNA variation in COVID-19 patients can predict the degree of disease and the risk of developing severe disease [140,141]. After apoptosis, mtDNA is released, further aggravating local and systemic inflammatory responses [142]. Positive correlations between mtDNA levels and pro-inflammatory markers have been determined in the plasma of COVID-19 patients [143,144]. This is now used an indicator of COVID-19 severity or a predictor of mortality. Moreover, when mtDNA is released into the cytosol, it acts as a DAMP, which, in turn, causes a “cytokine storm” and inhibits cellular regulation of excessive inflammatory responses [143].
4. Mitochondrial Redox Status and Inflammatory Response
In individuals with poorly functioning mitochondria, the virus may “tip” the host into a cycle of chronic inflammation. Mitochondria play a vital role in cellular energy production and are a key component of the innate immune response. When the mitochondrial membrane and electron transport chain complex are disrupted, more ROS are generated due to the loss of membrane potential, which can lead to oxidative stress [114] and the activation of pro-inflammatory pathways [145]. While ROS production is critical in immune responses, excess ROS can damage host tissues and exacerbate inflammation [146]. Finally, it can cause damage to tissues or organs, leading to such consequences as muscle disorders [147]. In fact, mtROS —which is higher in SARS-CoV-2-infected cells and monocytes—can enhance inflammatory responses through the up-regulation of IL1-α [112,148]. Furthermore, DAMPs such as oxidized mtDNA can be released into the cytoplasm, which aggravates inflammation by activating the inflammasome [149]. When host cells send out PAMP signals, inflammasomes oligomerize and form receptors, activate caspases to produce cytokines, and induce apoptosis [54].
The ORFs of SARS-CoV-2 are also involved in the inflammatory mechanism of the infection process. ORF3d N-terminal and ORF8 proteins elicit strong antibody responses [32]. ORF9b also activates the inflammasome to evade the immune response and promote viral replication [48]. ORF3b [29], ORF6 [34,35], ORF7a [38], ORF8 [43,44], and ORF9c [31,49,50,51] of SARS-CoV are important interferon-I antagonists, which induce impaired host immune responses [138]. ORF7a hijacks the host ubiquitin system for enhancing the ability to antagonize interferon-I responses [39]. ORF8 interacts with the major histocompatibility complex (MHC-I) [24], activates the IL-17 signaling pathway, and promotes pro-inflammatory cytokines [45]. ORF9c protein interacts with the related proteins of NFκB-related molecules [31] and impairs antigen processing and presentation and complement signaling, and induces IL-6 signaling [49]. ORF7a binds to CD14+ monocytes, leading to a decrease in antigen presentation and inducing the marked expression of pro-inflammatory cytokines [40].
4.1. Mitochondria Induce NLRP-3-Based Inflammatory Response
Pro-inflammatory NLRP-3 signaling activates cytokines, thus generating mitochondrion-related inflammatory cascades [150,151,152]. This is particularly concerning in elderly patients, who experience an increase in the NLRP-3 inflammasome with age [153]. This exacerbates SARS-CoV-2 infection [154]. The mitochondrial phospholipid cardiolipin can directly bind and activate the inflammasome complex NLRP3 [152]. Mitochondrial-generated ROS are involved in the activation of the NLRP3 inflammasome [152]. Stimulation of the NLRP3 inflammasome leads to the activation of caspase-1, which, in turn, leads to the proteolysis of cytokines such as interleukin (IL)-1β and IL-18 [54]. ORF3a activates pro-IL-1β gene expression and IL-1β secretion, which ultimately activates NFκB signaling and the NLRP3 inflammasome, promoting cytokine storm [27].
Viral proteins directly interact with components or regulatory proteins of NLRP3, including the interaction with the NLRP3 receptor via nucleocapsid protein, and the interaction of ORF3a with Caspase 1 and TNF receptor-associated factor 3 (TRAF3) [27,28,155]. In addition to the canonical NLRP3 activation pathways of PAMPs and DAMPs, the envelope proteins ORF3a and ORF8b of SARS-CoV act as NLRP3 agonists [27,156,157] and play a role in the inflammatory pathogenesis [158,159]. In addition, transcription of NFκB-dependent target genes of pro-inflammatory cytokines, such as IL-18 and IL-1b, is increased through nucleocapsid, spike, ORF7a, and OFR3a proteins [28,155,160,161]. ORF3a protein stimulates the NLRP3 cascade in bone marrow macrophages, and induces IL-1β-mediated inflammatory responses [151,162].
4.2. Activation of TLR7 and TLR9 Signaling
During SARS-CoV-2 infection, TLRs first bind their ligands and then recruit adapter proteins such as myeloid differentiation primary response 88 (MyD88) and adapter-inducible interferon-β (TRIF)-containing the TIR domain, which activate downstream immune molecular pathways [163], thus protecting the body from invading antigens. However, excessive activation of TLRs can lead to severe inflammatory responses, resulting in injury and even death [56,164]. Furthermore, when mtROS are increased, it leads to mitochondrial dysfunction and mtDNA leakage, which induces TLR9 and NFκB activation and the release of inflammatory cytokines [165].
TLR7 can be activated by ssRNAs rich in guanosine (G) and uridine (U) [166]. Two GU-rich ssRNA sequences in SARS-CoV-2 can activate TLR7 and its downstream MyD88-related pathway in human dendritic cells [167]. TLR7 recognizes SARS-CoV-2 in immune cells, triggering the production of type I interferon and pro-inflammatory cytokines [168,169]. TLR9 can recognize cytosine-guanine (CpG)-rich sequences in viral and mtDNA [170], and CpG in the SARS-CoV-2 envelope protein and ORF10 coding region can be recognized by TLR9 as a ligand [171]. TLR9 promotes inflammation through NFκB signaling and IL-6 production, and it impairs endothelial cell function by reducing endothelial nitric oxide synthase (eNOS) expression [165].
4.3. MAVS Signaling Regulates Inflammatory Responses
Following viral infection, MAVS initiates the innate immune response through the mitochondria. MAVS is mediated by the interaction of translocases with mitochondrial outer membrane proteins (TOM); for example, STING (MITA)—a transmembrane protein present on the endoplasmic reticulum, mitochondria, and mitochondria-associated membranes—contributes to the activation of MAVS [172]. Ring Finger Protein 5 (RNF5) regulates MAVS signaling by ubiquitinating MITA [173].
TOM70 is involved in protein transport for electron transport chain complex assembly in mitochondria [174]. TOM70 interacts with TANK-binding kinase (TBK1) through the heat shock protein 90 (HSP90), in order to bring the signal outside the mitochondria [175], thus activating the interferon response. TOM70 activates TBK1 through HSP90, taking the signal outside the mitochondria to activate interferon [50]. When the interferon regulatory factor (IRF)-3 binds to HSP90, it triggers the MAVS complex, E3 links tumor necrosis factor receptor-associated factors 3 and 6 (TRAF3 and TRAF6) to activate NFκB, and IRF provides antiviral protection [176]. Meanwhile, the membrane protein of SARS-CoV-2 inhibits the aggregation of TRAF3, TBK1, and IRF3 into the MAVS complex [177]. ORF9b inhibits the interaction between TOM70 and HSP90 and forms a complex with TOM70, thus affecting the mitochondrial electron transport chain [51], reducing the host immune response regulated by interferon I [47], and inducing apoptosis [50].
Furthermore, SARS-CoV-2 blocks interferon 1 and interferon 3 by interfering with the MAVS pathway [178]. The membrane protein inhibits the activation of interferon by down-regulating MAVS [177,178]. NSP13 inhibits TBK1 phosphorylation and blocks the downstream of the MAVS pathway [38]. ORF6 blocks the induction of interferon by MDA-5, MAVS, and TBK1 [179]. ORF3 induces ubiquitination of interferon-α receptor subunit 1 (IFNAR1) to down-regulate interferon 1 activity [180].
5. Therapeutic Strategies against COVID-19 Involving Mitochondria
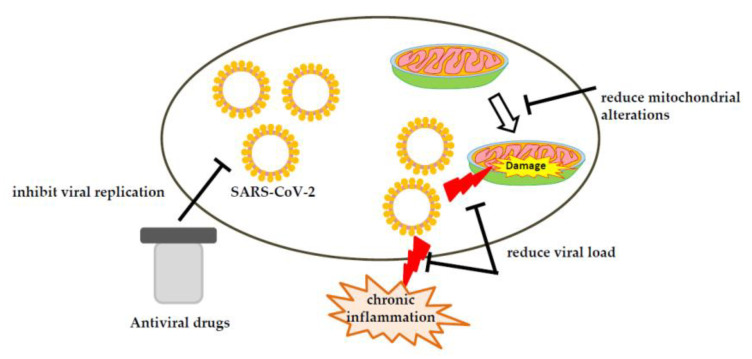
In people with long COVID and chronic fatigue syndrome, reducing the viral load and improving mitochondrial health may be beneficial (Figure 2). First, direct-acting antiviral drugs—especially analogs such as remdesivir—can effectively inhibit viral replication by blocking the activity of viral polymerase [181]. In addition to reducing viral load in cells, they may also reduce sources of chronic inflammation that lead to severe sepsis, multi-organ failure, and mitochondrial damage. However, the recent results of remdesivir treatment showed no benefit for long COVID-19 patients [182,183]. After one year of follow-up, one in six survivors considered recovery from COVID-19 incomplete, and one in four was troubled by fatigue [182]. Another study has reported that one-third of patients experienced fatigue 6 months after COVID-19 [183].
Figure 2.
Using Therapeutic Strategies to Reduce Mitochondrial Disorder.
Generalized antioxidants, such as N-acetylcysteine [184], glutathione [185], and catalase [186], can effectively reduce mitochondrial changes, as they restore and protect mitochondrial function [187], thereby reducing the spread of the virus SARS-CoV-2. Mitochondrial oxidative stress can also be reduced using mitochondrion-targeted catalytic antioxidants such as MnTBAP [188], EUK-8, and EUK-134 [189], as well as Mito-TEMPO and mitoquinol [112,162,190]. In addition, mitochondrial quinone/mitochondrial phenol mesylate (Mito-MES) has been demonstrated to possess significant antiviral activity against SARS-CoV-2 and can reduce excessive inflammation in the host [191]. Effective treatment can also be obtained with mPTP inhibitors such as NIM811 [192,193].
IL-6R and IL-1 receptor blockers are useful in the treatment of cytokine release syndrome [194,195]. For example, the IL-6R-blocking antibody Tocilizumab can be used to treat severe cytokine release syndrome [196,197] and SARS-CoV-2 infection [198]. Likewise, the IL-1 receptor antagonist anakinra significantly improved survival in SARS-CoV-infected mice with over-active NLRP3 inflammasome [195,199,200]. Increased survival and clinical improvement were observed in patients with COVID-19 adult respiratory distress syndrome receiving high-dose intravenous anakinra [201].
In addition, SARS-CoV-2-infected cells mainly rely on glycolysis to meet their energy demands, due to mitochondrial respiratory dysfunction. Inhibition of glycolysis in infected cells has been found to inhibit viral proliferation [107]. Ruthenium red—a mitochondrial calcium uniporter (MCU) inhibitor—also normalizes mitochondrial morphology and function in HIV-infected cells [202]. Other known MCU inhibitors include ruthenium 265, mitoxantrone, and the antibiotic doxycycline [203]. All of these strategies may help to improve mitochondrial health in people with long COVID or chronic fatigue syndrome.
6. Conclusions
Managing long COVID is a major challenge, due to the lack of effective treatments at present. The current understanding of the pathophysiology of long COVID suggests that mitochondrial dysfunction may be one of the underlying mechanisms. Although the exact mechanism by which SARS-CoV-2 induces mitochondrial dysfunction is not fully understood, accumulating evidence has supported such a notion. Therefore, targeting mitochondrial function may represent a new therapeutic approach for long-COVID patients. Several compounds, including antioxidants, mitochondrion-targeting peptides, and modulators of mitochondrial biogenesis, have shown promise for the treatment of mitochondrial dysfunction, in pre-clinical studies. However, further studies are needed to determine the safety and efficacy of mitochondrial-targeted therapies in the context of long COVID.
Author Contributions
Conceptualization, T.-H.C., C.-J.C. and P.-H.H.; writing—original draft preparation, T.-H.C.; writing—review and editing, C.-J.C. and P.-H.H.; visualization, T.-H.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Xie Y., Wang Z., Liao H., Marley G., Wu D., Tang W. Epidemiologic, clinical, and laboratory findings of the COVID-19 in the current pandemic: Systematic review and meta-analysis. BMC Infect. Dis. 2020;20:640. doi: 10.1186/s12879-020-05371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T.H., Hsu M.T., Lee M.Y., Chou C.K. Gastrointestinal Involvement in SARS-CoV-2 Infection. Viruses. 2022;14:1188. doi: 10.3390/v14061188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Kessel S.A.M., Olde Hartman T.C., Lucassen P., van Jaarsveld C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2022;39:159–167. doi: 10.1093/fampra/cmab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services, Office of the Assistant Secretary for Health . National Research Action Plan on Long COVID. Volume 20201 U.S. Department of Health & Human Services; Washington, DC, USA: 2022. [Google Scholar]
- 5.National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases Long COVID or Post-COVID Conditions. [(accessed on 16 December 2022)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html.
- 6.Twomey R., DeMars J., Franklin K., Culos-Reed S.N., Weatherald J., Wrightson J.G. Chronic Fatigue and Postexertional Malaise in People Living with Long COVID: An Observational Study. Phys. Ther. 2022;102:pzac005. doi: 10.1093/ptj/pzac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh I., Joseph P., Heerdt P.M., Cullinan M., Lutchmansingh D.D., Gulati M., Possick J.D., Systrom D.M., Waxman A.B. Persistent Exertional Intolerance After COVID-19: Insights from Invasive Cardiopulmonary Exercise Testing. Chest. 2022;161:54–63. doi: 10.1016/j.chest.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwendinger F., Knaier R., Radtke T., Schmidt-Trucksass A. Low Cardiorespiratory Fitness Post-COVID-19: A Narrative Review. Sports Med. 2023;53:51–74. doi: 10.1007/s40279-022-01751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornstein S.R., Voit-Bak K., Donate T., Rodionov R.N., Gainetdinov R.R., Tselmin S., Kanczkowski W., Muller G.M., Achleitner M., Wang J., et al. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis? Mol. Psychiatry. 2022;27:34–37. doi: 10.1038/s41380-021-01148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabanella F., Barbato C., Corbi N., Fiore M., Petrella C., de Vincentiis M., Greco A., Ferraguti G., Corsi A., Ralli M., et al. Exploring Mitochondrial Localization of SARS-CoV-2 RNA by Padlock Assay: A Pilot Study in Human Placenta. Int. J. Mol. Sci. 2022;23:2100. doi: 10.3390/ijms23042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlman S., Netland J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu K.E., Fazal F.M., Parker K.R., Zou J., Chang H.Y. RNA-GPS Predicts SARS-CoV-2 RNA Residency to Host Mitochondria and Nucleolus. Cell Syst. 2020;11:102–108.e3. doi: 10.1016/j.cels.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y., Zhou Z., Min W. Mitochondria, Oxidative Stress and Innate Immunity. Front. Physiol. 2018;9:1487. doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran K., Maity S., Muthukumar A.R., Kandala S., Tomar D., Abd El-Aziz T.M., Allen C., Sun Y., Venkatesan M., Madaris T.R., et al. SARS-CoV-2 infection enhances mitochondrial PTP complex activity to perturb cardiac energetics. iScience. 2022;25:103722. doi: 10.1016/j.isci.2021.103722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dela Cruz C.S., Kang M.J. Mitochondrial dysfunction and damage associated molecular patterns (DAMPs) in chronic inflammatory diseases. Mitochondrion. 2018;41:37–44. doi: 10.1016/j.mito.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grazioli S., Pugin J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front. Immunol. 2018;9:832. doi: 10.3389/fimmu.2018.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchi S., Guilbaud E., Tait S.W.G., Yamazaki T., Galluzzi L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023;23:159–173. doi: 10.1038/s41577-022-00760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subissi L., Posthuma C.C., Collet A., Zevenhoven-Dobbe J.C., Gorbalenya A.E., Decroly E., Snijder E.J., Canard B., Imbert I. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc. Natl. Acad. Sci. USA. 2014;111:E3900–E3909. doi: 10.1073/pnas.1323705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brian D.A., Baric R.S. Coronavirus Replication and Reverse Genetics. Volume 287. Springer; Berlin/Heidelberg, Germany: 2005. Coronavirus genome structure and replication; pp. 1–30. Current Topics in Microbiology and Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L.J., Chen T.H. NSP16 2′-O-MTase in Coronavirus Pathogenesis: Possible Prevention and Treatments Strategies. Viruses. 2021;13:538. doi: 10.3390/v13040538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kesheh M.M., Hosseini P., Soltani S., Zandi M. An overview on the seven pathogenic human coronaviruses. Rev. Med. Virol. 2022;32:e2282. doi: 10.1002/rmv.2282. [DOI] [PubMed] [Google Scholar]
- 23.Farrag M.A., Amer H.M., Bhat R., Hamed M.E., Aziz I.M., Mubarak A., Dawoud T.M., Almalki S.G., Alghofaili F., Alnemare A.K., et al. SARS-CoV-2: An Overview of Virus Genetics, Transmission, and Immunopathogenesis. Int. J. Environ. Res. Public Health. 2021;18:6312. doi: 10.3390/ijerph18126312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Chen Y., Li Y., Huang F., Luo B., Yuan Y., Xia B., Ma X., Yang T., Yu F., et al. The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-Iota. Proc. Natl. Acad. Sci. USA. 2021;118:e2024202118. doi: 10.1073/pnas.2024202118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azad G.K., Khan P.K. Variations in Orf3a protein of SARS-CoV-2 alter its structure and function. Biochem. Biophys. Rep. 2021;26:100933. doi: 10.1016/j.bbrep.2021.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castano-Rodriguez C., Honrubia J.M., Gutierrez-Alvarez J., DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeno J.M., Regla-Nava J.A., Fernandez-Delgado R., Verdia-Baguena C., Queralt-Martin M., et al. Role of Severe Acute Respiratory Syndrome Coronavirus Viroporins E, 3a, and 8a in Replication and Pathogenesis. mBio. 2018;9:e02325-17. doi: 10.1128/mBio.02325-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siu K.L., Yuen K.S., Castano-Rodriguez C., Ye Z.W., Yeung M.L., Fung S.Y., Yuan S., Chan C.P., Yuen K.Y., Enjuanes L., et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue Y., Nabar N.R., Shi C.S., Kamenyeva O., Xiao X., Hwang I.Y., Wang M., Kehrl J.H. SARS-Coronavirus Open Reading Frame-3a drives multimodal necrotic cell death. Cell Death Dis. 2018;9:904. doi: 10.1038/s41419-018-0917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konno Y., Kimura I., Uriu K., Fukushi M., Irie T., Koyanagi Y., Sauter D., Gifford R.J., Consortium U.-C., Nakagawa S., et al. SARS-CoV-2 ORF3b Is a Potent Interferon Antagonist Whose Activity Is Increased by a Naturally Occurring Elongation Variant. Cell Rep. 2020;32:108185. doi: 10.1016/j.celrep.2020.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finkel Y., Mizrahi O., Nachshon A., Weingarten-Gabbay S., Morgenstern D., Yahalom-Ronen Y., Tamir H., Achdout H., Stein D., Israeli O., et al. The coding capacity of SARS-CoV-2. Nature. 2021;589:125–130. doi: 10.1038/s41586-020-2739-1. [DOI] [PubMed] [Google Scholar]
- 31.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hachim A., Kavian N., Cohen C.A., Chin A.W.H., Chu D.K.W., Mok C.K.P., Tsang O.T.Y., Yeung Y.C., Perera R., Poon L.L.M., et al. ORF8 and ORF3b antibodies are accurate serological markers of early and late SARS-CoV-2 infection. Nat. Immunol. 2020;21:1293–1301. doi: 10.1038/s41590-020-0773-7. [DOI] [PubMed] [Google Scholar]
- 33.Lee J.G., Huang W., Lee H., van de Leemput J., Kane M.A., Han Z. Characterization of SARS-CoV-2 proteins reveals Orf6 pathogenicity, subcellular localization, host interactions and attenuation by Selinexor. Cell Biosci. 2021;11:58. doi: 10.1186/s13578-021-00568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miorin L., Kehrer T., Sanchez-Aparicio M.T., Zhang K., Cohen P., Patel R.S., Cupic A., Makio T., Mei M., Moreno E., et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc. Natl. Acad. Sci. USA. 2020;117:28344–28354. doi: 10.1073/pnas.2016650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopecky-Bromberg S.A., Martinez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J.Y., Liao C.H., Wang Q., Tan Y.J., Luo R., Qiu Y., Ge X.Y. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res. 2020;286:198074. doi: 10.1016/j.virusres.2020.198074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato K., Ikliptikawati D.K., Kobayashi A., Kondo H., Lim K., Hazawa M., Wong R.W. Overexpression of SARS-CoV-2 protein ORF6 dislocates RAE1 and NUP98 from the nuclear pore complex. Biochem. Biophys. Res. Commun. 2021;536:59–66. doi: 10.1016/j.bbrc.2020.11.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia H., Cao Z., Xie X., Zhang X., Chen J.Y., Wang H., Menachery V.D., Rajsbaum R., Shi P.Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020;33:108234. doi: 10.1016/j.celrep.2020.108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Z., Xia H., Rajsbaum R., Xia X., Wang H., Shi P.Y. Ubiquitination of SARS-CoV-2 ORF7a promotes antagonism of interferon response. Cell. Mol. Immunol. 2021;18:746–748. doi: 10.1038/s41423-020-00603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z., Huang C., Zhou Z., Huang Z., Su L., Kang S., Chen X., Chen Q., He S., Rong X., et al. Structural insight reveals SARS-CoV-2 ORF7a as an immunomodulating factor for human CD14(+) monocytes. iScience. 2021;24:102187. doi: 10.1016/j.isci.2021.102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu D.X., Fung T.S., Chong K.K., Shukla A., Hilgenfeld R. Accessory proteins of SARS-CoV and other coronaviruses. Antiviral Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C.Y., Ping Y.H., Lee H.C., Chen K.H., Lee Y.M., Chan Y.J., Lien T.C., Jap T.S., Lin C.H., Kao L.S., et al. Open reading frame 8a of the human severe acute respiratory syndrome coronavirus not only promotes viral replication but also induces apoptosis. J. Infect. Dis. 2007;196:405–415. doi: 10.1086/519166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong H.H., Fung T.S., Fang S., Huang M., Le M.T., Liu D.X. Accessory proteins 8b and 8ab of severe acute respiratory syndrome coronavirus suppress the interferon signaling pathway by mediating ubiquitin-dependent rapid degradation of interferon regulatory factor 3. Virology. 2018;515:165–175. doi: 10.1016/j.virol.2017.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin X., Fu B., Yin S., Li Z., Liu H., Zhang H., Xing N., Wang Y., Xue W., Xiong Y., et al. ORF8 contributes to cytokine storm during SARS-CoV-2 infection by activating IL-17 pathway. iScience. 2021;24:102293. doi: 10.1016/j.isci.2021.102293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi C.S., Qi H.Y., Boularan C., Huang N.N., Abu-Asab M., Shelhamer J.H., Kehrl J.H. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 2014;193:3080–3089. doi: 10.4049/jimmunol.1303196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreimendahl S., Rassow J. The Mitochondrial Outer Membrane Protein Tom70-Mediator in Protein Traffic, Membrane Contact Sites and Innate Immunity. Int. J. Mol. Sci. 2020;21:7262. doi: 10.3390/ijms21197262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh K.K., Chaubey G., Chen J.Y., Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol. Cell. Physiol. 2020;319:C258–C267. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dominguez Andres A., Feng Y., Campos A.R., Yin J., Yang C.C., James B., Murad R., Kim H., Deshpande A.J., Gordon D.E., et al. SARS-CoV-2 ORF9c Is a Membrane-Associated Protein that Suppresses Antiviral Responses in Cells. bioRxiv. 2020 doi: 10.1101/2020.08.18.256776. [DOI] [Google Scholar]
- 50.Gordon D.E., Hiatt J., Bouhaddou M., Rezelj V.V., Ulferts S., Braberg H., Jureka A.S., Obernier K., Guo J.Z., Batra J., et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020;370:eabe9403. doi: 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang H.W., Zhang H.N., Meng Q.F., Xie J., Li Y., Chen H., Zheng Y.X., Wang X.N., Qi H., Zhang J., et al. SARS-CoV-2 Orf9b suppresses type I interferon responses by targeting TOM70. Cell. Mol. Immunol. 2020;17:998–1000. doi: 10.1038/s41423-020-0514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pancer K., Milewska A., Owczarek K., Dabrowska A., Kowalski M., Labaj P.P., Branicki W., Sanak M., Pyrc K. The SARS-CoV-2 ORF10 is not essential in vitro or in vivo in humans. PLoS Pathog. 2020;16:e1008959. doi: 10.1371/journal.ppat.1008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seong S.Y., Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 54.Broz P., Dixit V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 55.Yu J., Nagasu H., Murakami T., Hoang H., Broderick L., Hoffman H.M., Horng T. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc. Natl. Acad. Sci. USA. 2014;111:15514–15519. doi: 10.1073/pnas.1414859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng M., Karki R., Williams E.P., Yang D., Fitzpatrick E., Vogel P., Jonsson C.B., Kanneganti T.D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021;22:829–838. doi: 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kircheis R., Planz O. The Role of Toll-like Receptors (TLRs) and Their Related Signaling Pathways in Viral Infection and Inflammation. Int. J. Mol. Sci. 2023;24:6701. doi: 10.3390/ijms24076701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bell J.K., Askins J., Hall P.R., Davies D.R., Segal D.M. The dsRNA binding site of human Toll-like receptor 3. Proc. Natl. Acad. Sci. USA. 2006;103:8792–8797. doi: 10.1073/pnas.0603245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z., Ohto U., Shibata T., Krayukhina E., Taoka M., Yamauchi Y., Tanji H., Isobe T., Uchiyama S., Miyake K., et al. Structural Analysis Reveals that Toll-like Receptor 7 Is a Dual Receptor for Guanosine and Single-Stranded RNA. Immunity. 2016;45:737–748. doi: 10.1016/j.immuni.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Kircheis R., Planz O. Could a Lower Toll-like Receptor (TLR) and NF-kappaB Activation Due to a Changed Charge Distribution in the Spike Protein Be the Reason for the Lower Pathogenicity of Omicron? Int. J. Mol. Sci. 2022;23:5966. doi: 10.3390/ijms23115966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmad M.S., Shaik R.A., Ahmad R.K., Yusuf M., Khan M., Almutairi A.B., Alghuyaythat W.K.Z., Almutairi S.B. “LONG COVID”: An insight. Eur. Rev. Med. Pharmacol. Sci. 2021;25:5561–5577. doi: 10.26355/eurrev_202109_26669. [DOI] [PubMed] [Google Scholar]
- 62.Doykov I., Hallqvist J., Gilmour K.C., Grandjean L., Mills K., Heywood W.E. ’The long tail of COVID-19’—The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Research. 2020;9:1349. doi: 10.12688/f1000research.27287.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- 64.Nabavi N. Long covid: How to define it and how to manage it. BMJ. 2020;370:m3489. doi: 10.1136/bmj.m3489. [DOI] [PubMed] [Google Scholar]
- 65.Garg P., Arora U., Kumar A., Wig N. The "post-COVID" syndrome: How deep is the damage? J. Med. Virol. 2021;93:673–674. doi: 10.1002/jmv.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Visco V., Vitale C., Rispoli A., Izzo C., Virtuoso N., Ferruzzi G.J., Santopietro M., Melfi A., Rusciano M.R., Maglio A., et al. Post-COVID-19 Syndrome: Involvement and Interactions between Respiratory, Cardiovascular and Nervous Systems. J. Clin. Med. 2022;11:524. doi: 10.3390/jcm11030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chudzik M., Babicki M., Kapusta J., Kaluzinska-Kolat Z., Kolat D., Jankowski P., Mastalerz-Migas A. Long-COVID Clinical Features and Risk Factors: A Retrospective Analysis of Patients from the STOP-COVID Registry of the PoLoCOV Study. Viruses. 2022;14:1755. doi: 10.3390/v14081755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leng A., Shah M., Ahmad S.A., Premraj L., Wildi K., Li Bassi G., Pardo C.A., Choi A., Cho S.M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells. 2023;12:816. doi: 10.3390/cells12050816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Budzynska N., Morys J. Anxiety and Depression Levels and Coping Strategies among Polish Healthcare Workers during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health. 2023;20:3319. doi: 10.3390/ijerph20043319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfaff E.R., Madlock-Brown C., Baratta J.M., Bhatia A., Davis H., Girvin A., Hill E., Kelly E., Kostka K., Loomba J., et al. Coding long COVID: Characterizing a new disease through an ICD-10 lens. BMC Med. 2023;21:58. doi: 10.1186/s12916-023-02737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lledo G.M., Sellares J., Brotons C., Sans M., Anton J.D., Blanco J., Bassat Q., Sarukhan A., Miro J.M., de Sanjose S., et al. Post-acute COVID-19 syndrome: A new tsunami requiring a universal case definition. Clin. Microbiol. Infect. 2022;28:315–318. doi: 10.1016/j.cmi.2021.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., Cho A., Jankovic M., Schaefer-Babajew D., Oliveira T.Y., et al. Evolution of Antibody Immunity to SARS-CoV-2. bioRxiv. 2021 doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Melo G.D., Lazarini F., Levallois S., Hautefort C., Michel V., Larrous F., Verillaud B., Aparicio C., Wagner S., Gheusi G., et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021;13:eabf8396. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weinstock L.B., Brook J.B., Walters A.S., Goris A., Afrin L.B., Molderings G.J. Mast cell activation symptoms are prevalent in Long-COVID. Int. J. Infect. Dis. 2021;112:217–226. doi: 10.1016/j.ijid.2021.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Afrin L.B., Weinstock L.B., Molderings G.J. COVID-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020;100:327–332. doi: 10.1016/j.ijid.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Proal A.D., VanElzakker M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021;12:698169. doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao X., Nguyen V., Tsai J., Gao C., Tian Y., Zhang Y., Carver W., Kiaris H., Cui T., Tan W. The SARS-CoV-2 Spike protein induces long-term transcriptional perturbations of mitochondrial metabolic genes, causes cardiac fibrosis, and reduces myocardial contractile in obese mice. bioRxiv. 2023 doi: 10.1101/2023.01.05.522853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Motta C.S., Torices S., da Rosa B.G., Marcos A.C., Alvarez-Rosa L., Siqueira M., Moreno-Rodriguez T., Matos A.D.R., Caetano B.C., Martins J., et al. Human Brain Microvascular Endothelial Cells Exposure to SARS-CoV-2 Leads to Inflammatory Activation through NF-kappaB Non-Canonical Pathway and Mitochondrial Remodeling. Viruses. 2023;15:745. doi: 10.3390/v15030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peluso M.J., Deeks S.G., Mustapic M., Kapogiannis D., Henrich T.J., Lu S., Goldberg S.A., Hoh R., Chen J.Y., Martinez E.O., et al. SARS-CoV-2 and Mitochondrial Proteins in Neural-Derived Exosomes of COVID-19. Ann. Neurol. 2022;91:772–781. doi: 10.1002/ana.26350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Piotrowicz K., Gasowski J., Michel J.P., Veronese N. Post-COVID-19 acute sarcopenia: Physiopathology and management. Aging Clin. Exp. Res. 2021;33:2887–2898. doi: 10.1007/s40520-021-01942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nardacci R., Colavita F., Castilletti C., Lapa D., Matusali G., Meschi S., Del Nonno F., Colombo D., Capobianchi M.R., Zumla A., et al. Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis. Cell Death Dis. 2021;12:263. doi: 10.1038/s41419-021-03527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cortese M., Lee J.Y., Cerikan B., Neufeldt C.J., Oorschot V.M.J., Kohrer S., Hennies J., Schieber N.L., Ronchi P., Mizzon G., et al. Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies. Cell Host Microbe. 2020;28:853–866.e5. doi: 10.1016/j.chom.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Faizan M.I., Chaudhuri R., Sagar S., Albogami S., Chaudhary N., Azmi I., Akhtar A., Ali S.M., Kumar R., Iqbal J., et al. NSP4 and ORF9b of SARS-CoV-2 Induce Pro-Inflammatory Mitochondrial DNA Release in Inner Membrane-Derived Vesicles. Cells. 2022;11:2969. doi: 10.3390/cells11192969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh K., Chen Y.C., Hassanzadeh S., Han K., Judy J.T., Seifuddin F., Tunc I., Sack M.N., Pirooznia M. Network Analysis and Transcriptome Profiling Identify Autophagic and Mitochondrial Dysfunctions in SARS-CoV-2 Infection. Front. Genet. 2021;12:599261. doi: 10.3389/fgene.2021.599261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mando C., Savasi V.M., Anelli G.M., Corti S., Serati A., Lisso F., Tasca C., Novielli C., Cetin I. Mitochondrial and Oxidative Unbalance in Placentas from Mothers with SARS-CoV-2 Infection. Antioxidants. 2021;10:1517. doi: 10.3390/antiox10101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hartsell E.M., Gillespie M.N., Langley R.J. Does acute and persistent metabolic dysregulation in COVID-19 point to novel biomarkers and future therapeutic strategies? Eur. Respir. J. 2022;59:2102417. doi: 10.1183/13993003.02417-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pliss A., Kuzmin A.N., Prasad P.N., Mahajan S.D. Mitochondrial Dysfunction: A Prelude to Neuropathogenesis of SARS-CoV-2. ACS Chem. Neurosci. 2022;13:308–312. doi: 10.1021/acschemneuro.1c00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., Lei F., Wang H., Xie J., Wang W., et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068–1077 e1063. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garreta E., Prado P., Stanifer M.L., Monteil V., Marco A., Ullate-Agote A., Moya-Rull D., Vilas-Zornoza A., Tarantino C., Romero J.P., et al. A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells. Cell Metab. 2022;34:857–873 e859. doi: 10.1016/j.cmet.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S., Southern W.N., Mantzoros C.S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holder K., Reddy P.H. The COVID-19 Effect on the Immune System and Mitochondrial Dynamics in Diabetes, Obesity, and Dementia. Neuroscientist. 2021;27:331–339. doi: 10.1177/1073858420960443. [DOI] [PubMed] [Google Scholar]
- 94.Mavrogiannaki A.N., Migdalis I.N. Nonalcoholic Fatty liver disease, diabetes mellitus and cardiovascular disease: Newer data. Int. J. Endocrinol. 2013;2013:450639. doi: 10.1155/2013/450639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Costa F.F., Rosario W.R., Ribeiro Farias A.C., de Souza R.G., Duarte Gondim R.S., Barroso W.A. Metabolic syndrome and COVID-19: An update on the associated comorbidities and proposed therapies. Diabetes Metab. Syndr. 2020;14:809–814. doi: 10.1016/j.dsx.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moreno Fernandez-Ayala D.J., Navas P., Lopez-Lluch G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp. Gerontol. 2020;142:111147. doi: 10.1016/j.exger.2020.111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang X., Ismail N.I., Rahman A., Xu D., Chan R.W.Y., Ong S.G., Ong S.B. Long COVID-19 and the Heart: Is Cardiac Mitochondria the Missing Link? Antioxid. Redox Signal. 2023;38:599–618. doi: 10.1089/ars.2022.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen X., Cao R., Zhong W. Host Calcium Channels and Pumps in Viral Infections. Cells. 2019;9:94. doi: 10.3390/cells9010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han Z., Madara J.J., Herbert A., Prugar L.I., Ruthel G., Lu J., Liu Y., Liu W., Liu X., Wrobel J.E., et al. Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention. PLoS Pathog. 2015;11:e1005220. doi: 10.1371/journal.ppat.1005220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qu Y., Sun Y., Yang Z., Ding C. Calcium Ions Signaling: Targets for Attack and Utilization by Viruses. Front. Microbiol. 2022;13:889374. doi: 10.3389/fmicb.2022.889374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saurav S., Tanwar J., Ahuja K., Motiani R.K. Dysregulation of host cell calcium signaling during viral infections: Emerging paradigm with high clinical relevance. Mol. Asp. Med. 2021;81:101004. doi: 10.1016/j.mam.2021.101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lai A.L., Freed J.H. SARS-CoV-2 Fusion Peptide has a Greater Membrane Perturbating Effect than SARS-CoV with Highly Specific Dependence on Ca(2) J. Mol. Biol. 2021;433:166946. doi: 10.1016/j.jmb.2021.166946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Straus M.R., Tang T., Lai A.L., Flegel A., Bidon M., Freed J.H., Daniel S., Whittaker G.R. Ca(2+) Ions Promote Fusion of Middle East Respiratory Syndrome Coronavirus with Host Cells and Increase Infectivity. J. Virol. 2020;94:e00426-20. doi: 10.1128/JVI.00426-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kern D.M., Sorum B., Mali S.S., Hoel C.M., Sridharan S., Remis J.P., Toso D.B., Kotecha A., Bautista D.M., Brohawn S.G. Cryo-EM structure of SARS-CoV-2 ORF3a in lipid nanodiscs. Nat. Struct. Mol. Biol. 2021;28:573–582. doi: 10.1038/s41594-021-00619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davies J.P., Almasy K.M., McDonald E.F., Plate L. Comparative Multiplexed Interactomics of SARS-CoV-2 and Homologous Coronavirus Nonstructural Proteins Identifies Unique and Shared Host-Cell Dependencies. ACS Infect. Dis. 2020;6:3174–3189. doi: 10.1021/acsinfecdis.0c00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ren Y., Shu T., Wu D., Mu J., Wang C., Huang M., Han Y., Zhang X.Y., Zhou W., Qiu Y., et al. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell. Mol. Immunol. 2020;17:881–883. doi: 10.1038/s41423-020-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., Cinatl J., Munch C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PubMed] [Google Scholar]
- 108.Nunn A.V.W., Guy G.W., Brysch W., Bell J.D. Understanding Long COVID.; Mitochondrial Health and Adaptation-Old Pathways, New Problems. Biomedicines. 2022;10:3113. doi: 10.3390/biomedicines10123113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qu C., Zhang S., Wang W., Li M., Wang Y., van der Heijde-Mulder M., Shokrollahi E., Hakim M.S., Raat N.J.H., Peppelenbosch M.P., et al. Mitochondrial electron transport chain complex III sustains hepatitis E virus replication and represents an antiviral target. FASEB J. 2019;33:1008–1019. doi: 10.1096/fj.201800620R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh D., Agusti A., Anzueto A., Barnes P.J., Bourbeau J., Celli B.R., Criner G.J., Frith P., Halpin D.M.G., Han M., et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019;53:1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 111.Shin C.S., Meng S., Garbis S.D., Moradian A., Taylor R.W., Sweredoski M.J., Lomenick B., Chan D.C. LONP1 and mtHSP70 cooperate to promote mitochondrial protein folding. Nat. Commun. 2021;12:265. doi: 10.1038/s41467-020-20597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Codo A.C., Davanzo G.G., Monteiro L.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C.A.O., Crunfli F., et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1alpha/Glycolysis-Dependent Axis. Cell Metab. 2020;32:498–499. doi: 10.1016/j.cmet.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Soria-Castro E., Soto M.E., Guarner-Lans V., Rojas G., Perezpena-Diazconti M., Criales-Vera S.A., Manzano Pech L., Perez-Torres I. The kidnapping of mitochondrial function associated with the SARS-CoV-2 infection. Histol. Histopathol. 2021;36:947–965. doi: 10.14670/HH-18-354. [DOI] [PubMed] [Google Scholar]
- 114.Starkov A.A., Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 115.Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 116.Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J., Shen B., Gong Z. Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lei Y., Zhang J., Schiavon C.R., He M., Chen L., Shen H., Zhang Y., Yin Q., Cho Y., Andrade L., et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021;128:1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shang C., Liu Z., Zhu Y., Lu J., Ge C., Zhang C., Li N., Jin N., Li Y., Tian M., et al. SARS-CoV-2 Causes Mitochondrial Dysfunction and Mitophagy Impairment. Front. Microbiol. 2021;12:780768. doi: 10.3389/fmicb.2021.780768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andrade Silva M., da Silva A., do Amaral M.A., Fragas M.G., Camara N.O.S. Metabolic Alterations in SARS-CoV-2 Infection and Its Implication in Kidney Dysfunction. Front. Physiol. 2021;12:624698. doi: 10.3389/fphys.2021.624698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moolamalla S.T.R., Balasubramanian R., Chauhan R., Priyakumar U.D., Vinod P.K. Host metabolic reprogramming in response to SARS-CoV-2 infection: A systems biology approach. Microb. Pathog. 2021;158:105114. doi: 10.1016/j.micpath.2021.105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang W., Wang G., Xu Z.G., Tu H., Hu F., Dai J., Chang Y., Chen Y., Lu Y., Zeng H., et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell. 2019;178:176–189.e15. doi: 10.1016/j.cell.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giacomello M., Pyakurel A., Glytsou C., Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020;21:204–224. doi: 10.1038/s41580-020-0210-7. [DOI] [PubMed] [Google Scholar]
- 123.Terman A., Kurz T., Navratil M., Arriaga E.A., Brunk U.T. Mitochondrial turnover and aging of long-lived postmitotic cells: The mitochondrial-lysosomal axis theory of aging. Antioxid. Redox Signal. 2010;12:503–535. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De R., Sarkar S., Mazumder S., Debsharma S., Siddiqui A.A., Saha S.J., Banerjee C., Nag S., Saha D., Pramanik S., et al. Macrophage migration inhibitory factor regulates mitochondrial dynamics and cell growth of human cancer cell lines through CD74-NF-kappaB signaling. J. Biol. Chem. 2018;293:19740–19760. doi: 10.1074/jbc.RA118.003935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bach D., Pich S., Soriano F.X., Vega N., Baumgartner B., Oriola J., Daugaard J.R., Lloberas J., Camps M., Zierath J.R., et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J. Biol. Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 126.Smith M.E., Tippetts T.S., Brassfield E.S., Tucker B.J., Ockey A., Swensen A.C., Anthonymuthu T.S., Washburn T.D., Kane D.A., Prince J.T., et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochem. J. 2013;456:427–439. doi: 10.1042/BJ20130807. [DOI] [PubMed] [Google Scholar]
- 127.Morita M., Prudent J., Basu K., Goyon V., Katsumura S., Hulea L., Pearl D., Siddiqui N., Strack S., McGuirk S., et al. mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1. Mol. Cell. 2017;67:922–935.e5. doi: 10.1016/j.molcel.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 128.Morita M., Gravel S.P., Hulea L., Larsson O., Pollak M., St-Pierre J., Topisirovic I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle. 2015;14:473–480. doi: 10.4161/15384101.2014.991572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zemirli N., Pourcelot M., Ambroise G., Hatchi E., Vazquez A., Arnoult D. Mitochondrial hyperfusion promotes NF-kappaB activation via the mitochondrial E3 ligase MULAN. FEBS J. 2014;281:3095–3112. doi: 10.1111/febs.12846. [DOI] [PubMed] [Google Scholar]
- 130.Galluzzi L., Vitale I., Abrams J.M., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., Dawson T.M., Dawson V.L., El-Deiry W.S., Fulda S., et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kroemer G., Galluzzi L., Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 132.Madeddu E., Maniga B., Zaffanello M., Fanos V., Marcialis A. The SARS-CoV2 and mitochondria: The impact on cell fate. Acta Biomed. 2022;93:e2022199. doi: 10.23750/abm.v93i2.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao G., Xing J., Xiao X., Liou A.K., Gao Y., Yin X.M., Clark R.S., Graham S.H., Chen J. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J. Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y., Kim N.S., Haince J.F., Kang H.C., David K.K., Andrabi S.A., Poirier G.G., Dawson V.L., Dawson T.M. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos) Sci. Signal. 2011;4:ra20. doi: 10.1126/scisignal.2000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y., Kim N.S., Li X., Greer P.A., Koehler R.C., Dawson V.L., Dawson T.M. Calpain activation is not required for AIF translocation in PARP-1-dependent cell death (parthanatos) J. Neurochem. 2009;110:687–696. doi: 10.1111/j.1471-4159.2009.06167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kesavardhana S., Kanneganti T.D. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int. Immunol. 2017;29:201–210. doi: 10.1093/intimm/dxx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 138.Redondo N., Zaldivar-Lopez S., Garrido J.J., Montoya M. SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns. Front. Immunol. 2021;12:708264. doi: 10.3389/fimmu.2021.708264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Valdes-Aguayo J.J., Garza-Veloz I., Badillo-Almaraz J.I., Bernal-Silva S., Martinez-Vazquez M.C., Juarez-Alcala V., Vargas-Rodriguez J.R., Gaeta-Velasco M.L., Gonzalez-Fuentes C., Avila-Carrasco L., et al. Mitochondria and Mitochondrial DNA: Key Elements in the Pathogenesis and Exacerbation of the Inflammatory State Caused by COVID-19. Medicina. 2021;57:928. doi: 10.3390/medicina57090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dirican E., Savrun S.T., Aydin I.E., Gulbay G., Karaman U. Analysis of mitochondrial DNA cytochrome-b (CYB) and ATPase-6 gene mutations in COVID-19 patients. J. Med. Virol. 2022;94:3138–3146. doi: 10.1002/jmv.27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu Y., Wang X.H., Li X.H., Song L.Y., Yu S.L., Fang Z.C., Liu Y.Q., Yuan L.Y., Peng C.Y., Zhang S.Y., et al. Common mtDNA variations at C5178a and A249d/T6392C/G10310A decrease the risk of severe COVID-19 in a Han Chinese population from Central China. Mil. Med. Res. 2021;8:57. doi: 10.1186/s40779-021-00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Riley J.S., Tait S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020;21:e49799. doi: 10.15252/embr.201949799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scozzi D., Cano M., Ma L., Zhou D., Zhu J.H., O’Halloran J.A., Goss C., Rauseo A.M., Liu Z., Sahu S.K., et al. Circulating mitochondrial DNA is an early indicator of severe illness and mortality from COVID-19. JCI Insight. 2021;6:143299. doi: 10.1172/jci.insight.143299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Andargie T.E., Tsuji N., Seifuddin F., Jang M.K., Yuen P.S., Kong H., Tunc I., Singh K., Charya A., Wilkins K., et al. Cell-free DNA maps COVID-19 tissue injury and risk of death and can cause tissue injury. JCI Insight. 2021;6:147610. doi: 10.1172/jci.insight.147610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xie J.H., Li Y.Y., Jin J. The essential functions of mitochondrial dynamics in immune cells. Cell. Mol. Immunol. 2020;17:712–721. doi: 10.1038/s41423-020-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dickson K.B., Zhou J. Role of reactive oxygen species and iron in host defense against infection. Front. Biosci. Landmark Ed. 2020;25:1600–1616. doi: 10.2741/4869. [DOI] [PubMed] [Google Scholar]
- 147.Chen T.H., Koh K.Y., Lin K.M., Chou C.K. Mitochondrial Dysfunction as an Underlying Cause of Skeletal Muscle Disorders. Int. J. Mol. Sci. 2022;23:12926. doi: 10.3390/ijms232112926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miller B., Silverstein A., Flores M., Cao K., Kumagai H., Mehta H.H., Yen K., Kim S.J., Cohen P. Host mitochondrial transcriptome response to SARS-CoV-2 in multiple cell models and clinical samples. Sci. Rep. 2021;11:3. doi: 10.1038/s41598-020-79552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu Q., Zhang D., Hu D., Zhou X., Zhou Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 2018;103:115–124. doi: 10.1016/j.molimm.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 150.Burtscher J., Cappellano G., Omori A., Koshiba T., Millet G.P. Mitochondria: In the Cross Fire of SARS-CoV-2 and Immunity. iScience. 2020;23:101631. doi: 10.1016/j.isci.2020.101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He Y., Hara H., Nunez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 153.Volt H., Garcia J.A., Doerrier C., Diaz-Casado M.E., Guerra-Librero A., Lopez L.C., Escames G., Tresguerres J.A., Acuna-Castroviejo D. Same molecule but different expression: Aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 2016;60:193–205. doi: 10.1111/jpi.12303. [DOI] [PubMed] [Google Scholar]
- 154.Zhou Z., Zhang M., Wang Y., Zheng F., Huang Y., Huang K., Yu Q., Cai C., Chen D., Tian Y., et al. Clinical characteristics of older and younger patients infected with SARS-CoV-2. Aging. 2020;12:11296–11305. doi: 10.18632/aging.103535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pan P., Shen M., Yu Z., Ge W., Chen K., Tian M., Xiao F., Wang Z., Wang J., Jia Y., et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021;12:4664. doi: 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shi C.S., Nabar N.R., Huang N.N., Kehrl J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nieto-Torres J.L., DeDiego M.L., Verdia-Baguena C., Jimenez-Guardeno J.M., Regla-Nava J.A., Fernandez-Delgado R., Castano-Rodriguez C., Alcaraz A., Torres J., Aguilella V.M., et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10:e1004077. doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fung S.Y., Yuen K.S., Ye Z.W., Chan C.P., Jin D.Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: Lessons from other pathogenic viruses. Emerg. Microbes Infect. 2020;9:558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Astuti I., Ysrafil Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Albornoz E.A., Amarilla A.A., Modhiran N., Parker S., Li X.X., Wijesundara D.K., Aguado J., Zamora A.P., McMillan C.L.D., Liang B., et al. SARS-CoV-2 drives NLRP3 inflammasome activation in human microglia through spike protein. Mol. Psychiatry. 2022 doi: 10.1038/s41380-022-01831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Su C.M., Wang L., Yoo D. Activation of NF-kappaB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep. 2021;11:13464. doi: 10.1038/s41598-021-92941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kasuga Y., Zhu B., Jang K.J., Yoo J.S. Innate immune sensing of coronavirus and viral evasion strategies. Exp. Mol. Med. 2021;53:723–736. doi: 10.1038/s12276-021-00602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ebermeyer T., Cognasse F., Berthelot P., Mismetti P., Garraud O., Hamzeh-Cognasse H. Platelet Innate Immune Receptors and TLRs: A Double-Edged Sword. Int. J. Mol. Sci. 2021;22:7894. doi: 10.3390/ijms22157894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Costa T.J., Potje S.R., Fraga-Silva T.F.C., da Silva-Neto J.A., Barros P.R., Rodrigues D., Machado M.R., Martins R.B., Santos-Eichler R.A., Benatti M.N., et al. Mitochondrial DNA and TLR9 activation contribute to SARS-CoV-2-induced endothelial cell damage. Vascul. Pharmacol. 2022;142:106946. doi: 10.1016/j.vph.2021.106946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li Y., Chen M., Cao H., Zhu Y., Zheng J., Zhou H. Extraordinary GU-rich single-strand RNA identified from SARS coronavirus contributes an excessive innate immune response. Microbes Infect. 2013;15:88–95. doi: 10.1016/j.micinf.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Salvi V., Nguyen H.O., Sozio F., Schioppa T., Gaudenzi C., Laffranchi M., Scapini P., Passari M., Barbazza I., Tiberio L., et al. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight. 2021;6:150542. doi: 10.1172/jci.insight.150542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bortolotti D., Gentili V., Rizzo S., Schiuma G., Beltrami S., Strazzabosco G., Fernandez M., Caccuri F., Caruso A., Rizzo R. TLR3 and TLR7 RNA Sensor Activation during SARS-CoV-2 Infection. Microorganisms. 2021;9:1820. doi: 10.3390/microorganisms9091820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cervantes-Barragan L., Zust R., Weber F., Spiegel M., Lang K.S., Akira S., Thiel V., Ludewig B. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Digard P., Lee H.M., Sharp C., Grey F., Gaunt E. Intra-genome variability in the dinucleotide composition of SARS-CoV-2. Virus Evol. 2020;6:veaa057. doi: 10.1093/ve/veaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Smith J.A. STING, the Endoplasmic Reticulum, and Mitochondria: Is Three a Crowd or a Conversation? Front. Immunol. 2020;11:611347. doi: 10.3389/fimmu.2020.611347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhong B., Zhang L., Lei C., Li Y., Mao A.P., Yang Y., Wang Y.Y., Zhang X.L., Shu H.B. The ubiquitin ligase RNF5 regulates antiviral responses by mediating degradation of the adaptor protein MITA. Immunity. 2009;30:397–407. doi: 10.1016/j.immuni.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 174.Wei X., Du M., Xie J., Luo T., Zhou Y., Zhang K., Li J., Chen D., Xu P., Jia M., et al. Mutations in TOMM70 lead to multi-OXPHOS deficiencies and cause severe anemia, lactic acidosis, and developmental delay. J. Hum. Genet. 2020;65:231–240. doi: 10.1038/s10038-019-0714-1. [DOI] [PubMed] [Google Scholar]
- 175.Liu X.Y., Wei B., Shi H.X., Shan Y.F., Wang C. Tom70 mediates activation of interferon regulatory factor 3 on mitochondria. Cell Res. 2010;20:994–1011. doi: 10.1038/cr.2010.103. [DOI] [PubMed] [Google Scholar]
- 176.Elesela S., Lukacs N.W. Role of Mitochondria in Viral Infections. Life. 2021;11:232. doi: 10.3390/life11030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fu Y.Z., Wang S.Y., Zheng Z.Q., Yi H., Li W.W., Xu Z.S., Wang Y.Y. SARS-CoV-2 membrane glycoprotein M antagonizes the MAVS-mediated innate antiviral response. Cell. Mol. Immunol. 2021;18:613–620. doi: 10.1038/s41423-020-00571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Han L., Zhuang M.W., Deng J., Zheng Y., Zhang J., Nan M.L., Zhang X.J., Gao C., Wang P.H. SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5-MAVS, TLR3-TRIF, and cGAS-STING signaling pathways. J. Med. Virol. 2021;93:5376–5389. doi: 10.1002/jmv.27050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yuen C.K., Lam J.Y., Wong W.M., Mak L.F., Wang X., Chu H., Cai J.P., Jin D.Y., To K.K., Chan J.F., et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 2020;9:1418–1428. doi: 10.1080/22221751.2020.1780953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Minakshi R., Padhan K., Rani M., Khan N., Ahmad F., Jameel S. The SARS Coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor. PLoS ONE. 2009;4:e8342. doi: 10.1371/journal.pone.0008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kokic G., Hillen H.S., Tegunov D., Dienemann C., Seitz F., Schmitzova J., Farnung L., Siewert A., Hobartner C., Cramer P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021;12:279. doi: 10.1038/s41467-020-20542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nevalainen O.P.O., Horstia S., Laakkonen S., Rutanen J., Mustonen J.M.J., Kalliala I.E.J., Ansakorpi H., Kreivi H.R., Kuutti P., Paajanen J., et al. Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial. Nat. Commun. 2022;13:6152. doi: 10.1038/s41467-022-33825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Boglione L., Meli G., Poletti F., Rostagno R., Moglia R., Cantone M., Esposito M., Scianguetta C., Domenicale B., Di Pasquale F., et al. Risk factors and incidence of long-COVID syndrome in hospitalized patients: Does remdesivir have a protective effect? QJM Int. J. Med. 2022;114:865–871. doi: 10.1093/qjmed/hcab297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Geiler J., Michaelis M., Naczk P., Leutz A., Langer K., Doerr H.W., Cinatl J., Jr. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem. Pharmacol. 2010;79:413–420. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 185.Cai J., Chen Y., Seth S., Furukawa S., Compans R.W., Jones D.P. Inhibition of influenza infection by glutathione. Free Radic. Biol. Med. 2003;34:928–936. doi: 10.1016/S0891-5849(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 186.Shi X., Shi Z., Huang H., Zhu H., Zhou P., Zhu H., Ju D. Ability of recombinant human catalase to suppress inflammation of the murine lung induced by influenza A. Inflammation. 2014;37:809–817. doi: 10.1007/s10753-013-9800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Soto M.E., Guarner-Lans V., Soria-Castro E., Manzano Pech L., Perez-Torres I. Is Antioxidant Therapy a Useful Complementary Measure for Covid-19 Treatment? An Algorithm for Its Application. Medicina. 2020;56:386. doi: 10.3390/medicina56080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Melov S., Schneider J.A., Day B.J., Hinerfeld D., Coskun P., Mirra S.S., Crapo J.D., Wallace D.C. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat. Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- 189.Melov S., Doctrow S.R., Schneider J.A., Haberson J., Patel M., Coskun P.E., Huffman K., Wallace D.C., Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J. Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.De R., Mazumder S., Sarkar S., Debsharma S., Siddiqui A.A., Saha S.J., Banerjee C., Nag S., Saha D., Bandyopadhyay U. Acute mental stress induces mitochondrial bioenergetic crisis and hyper-fission along with aberrant mitophagy in the gut mucosa in rodent model of stress-related mucosal disease. Free Radic. Biol. Med. 2017;113:424–438. doi: 10.1016/j.freeradbiomed.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 191.Petcherski A., Sharma M., Satta S., Daskou M., Vasilopoulos H., Hugo C., Ritou E., Dillon B.J., Fung E., Garcia G., et al. Mitoquinone mesylate targets SARS-CoV-2 infection in preclinical models. bioRxiv. 2022 doi: 10.1101/2022.02.22.481100. [DOI] [Google Scholar]
- 192.Dittmar M., Lee J.S., Whig K., Segrist E., Li M., Kamalia B., Castellana L., Ayyanathan K., Cardenas-Diaz F.L., Morrisey E.E., et al. Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-Cov-2. Cell Rep. 2021;35:108959. doi: 10.1016/j.celrep.2021.108959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Toth E., Maleth J., Zavogyan N., Fanczal J., Grassalkovich A., Erdos R., Pallagi P., Horvath G., Tretter L., Balint E.R., et al. Novel mitochondrial transition pore inhibitor N-methyl-4-isoleucine cyclosporin is a new therapeutic option in acute pancreatitis. J. Physiol. 2019;597:5879–5898. doi: 10.1113/JP278517. [DOI] [PubMed] [Google Scholar]
- 194.Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A., Cron R.Q., Opal S.M. Interleukin-1 Receptor Blockade Is Associated with Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit. Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M., Sanvito F., Ponzoni M., Doglioni C., Cristofori P., et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 196.Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A., Przepiorka D., Farrell A.T., Pazdur R. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kotch C., Barrett D., Teachey D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev. Clin. Immunol. 2019;15:813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Hlh Across Speciality Collaboration U.K. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Giavridis T., van der Stegen S.J.C., Eyquem J., Hamieh M., Piersigilli A., Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vijay R., Fehr A.R., Janowski A.M., Athmer J., Wheeler D.L., Grunewald M., Sompallae R., Kurup S.P., Meyerholz D.K., Sutterwala F.S., et al. Virus-induced inflammasome activation is suppressed by prostaglandin D(2)/DP1 signaling. Proc. Natl. Acad. Sci. USA. 2017;114:E5444–E5453. doi: 10.1073/pnas.1704099114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C., Castiglioni B., Tassan Din C., Boffini N., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Piccoli C., Scrima R., Quarato G., D’Aprile A., Ripoli M., Lecce L., Boffoli D., Moradpour D., Capitanio N. Hepatitis C virus protein expression causes calcium-mediated mitochondrial bioenergetic dysfunction and nitro-oxidative stress. Hepatology. 2007;46:58–65. doi: 10.1002/hep.21679. [DOI] [PubMed] [Google Scholar]
- 203.Woods J.J., Wilson J.J. Inhibitors of the mitochondrial calcium uniporter for the treatment of disease. Curr. Opin. Chem. Biol. 2020;55:9–18. doi: 10.1016/j.cbpa.2019.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.