Abstract
Sarcopenia is the loss of muscle strength, mass, and function, which is often exacerbated by chronic comorbidities including cardiovascular diseases, chronic kidney disease, and cancer. Sarcopenia is associated with faster progression of cardiovascular diseases and higher risk of mortality, falls, and reduced quality of life, particularly among older adults. Although the pathophysiologic mechanisms are complex, the broad underlying cause of sarcopenia includes an imbalance between anabolic and catabolic muscle homeostasis with or without neuronal degeneration. The intrinsic molecular mechanisms of aging, chronic illness, malnutrition, and immobility are associated with the development of sarcopenia. Screening and testing for sarcopenia may be particularly important among those with chronic disease states. Early recognition of sarcopenia is important because it can provide an opportunity for interventions to reverse or delay the progression of muscle disorder, which may ultimately impact cardiovascular outcomes. Relying on body mass index is not useful for screening because many patients will have sarcopenic obesity, a particularly important phenotype among older cardiac patients. In this review, we aimed to: (1) provide a definition of sarcopenia within the context of muscle wasting disorders; (2) summarize the associations between sarcopenia and different cardiovascular diseases; (3) highlight an approach for a diagnostic evaluation; (4) discuss management strategies for sarcopenia; and (5) outline key gaps in knowledge with implications for the future of the field.
Keywords: body mass index, cardiovascular diseases, older adults, sarcopenia
In the United States, the improvement in life expectancy has resulted in a rapid expansion of the older adult population.1 It is projected that 1 in 5 Americans will be >65 years of age by 2030, and those >85 years will account for >20% of the older adult population.1 Older adults are prone to cardiovascular disease (CVD) because the biologic underpinning of aging, including hormonal changes, immunosenescence, impaired autophagy, oxidative stress, and mitochondrial dysfunction, predispose to development of CVD. In addition, management of CVD is complicated by the presence of geriatric syndromes.2,3 A particularly important geriatric syndrome that disproportionally affects older patients with CVD is sarcopenia.2,3 Sarcopenia is the progressive loss of muscle strength, mass, and function, and is associated with increased risk of death, falls, disability, hospitalization, and loss of independence.4
There is a bidirectional association between sarcopenia and CVD.5 Sarcopenia can lead to increased adiposity, insulin resistance, and chronic inflammation, and thus, predispose adults to developing cardiovascular events,6 and the chronic inflammatory state, malnutrition, and decreased physical activity observed in cardiac patients are precursors to a catabolic state, leading to accelerated muscle loss and development of sarcopenia.7 In this review, we aimed to: (1) provide a definition of sarcopenia within the context of muscle wasting disorders; (2) highlight an approach for a diagnostic evaluation; (3) summarize the associations between sarcopenia and different CVDs; (4) discuss management strategies for sarcopenia; and (5) outline key gaps in knowledge with implications for the future of the field.
Sarcopenia and the Wasting Continuum
Definitions
Sarcopenia, Greek for “flesh poverty,” was originally proposed in recognition of a clinical condition of a substantial loss of muscle mass and function observed with aging and results in loss of independence among older adults (Supplemental Material).8 The first operational definition only included low lean mass as a surrogate measure of muscle mass to define sarcopenia.9 Subsequent evidence showed that muscle weakness and impaired mobility are better predictors of mortality and disability than low lean mass alone.10,11
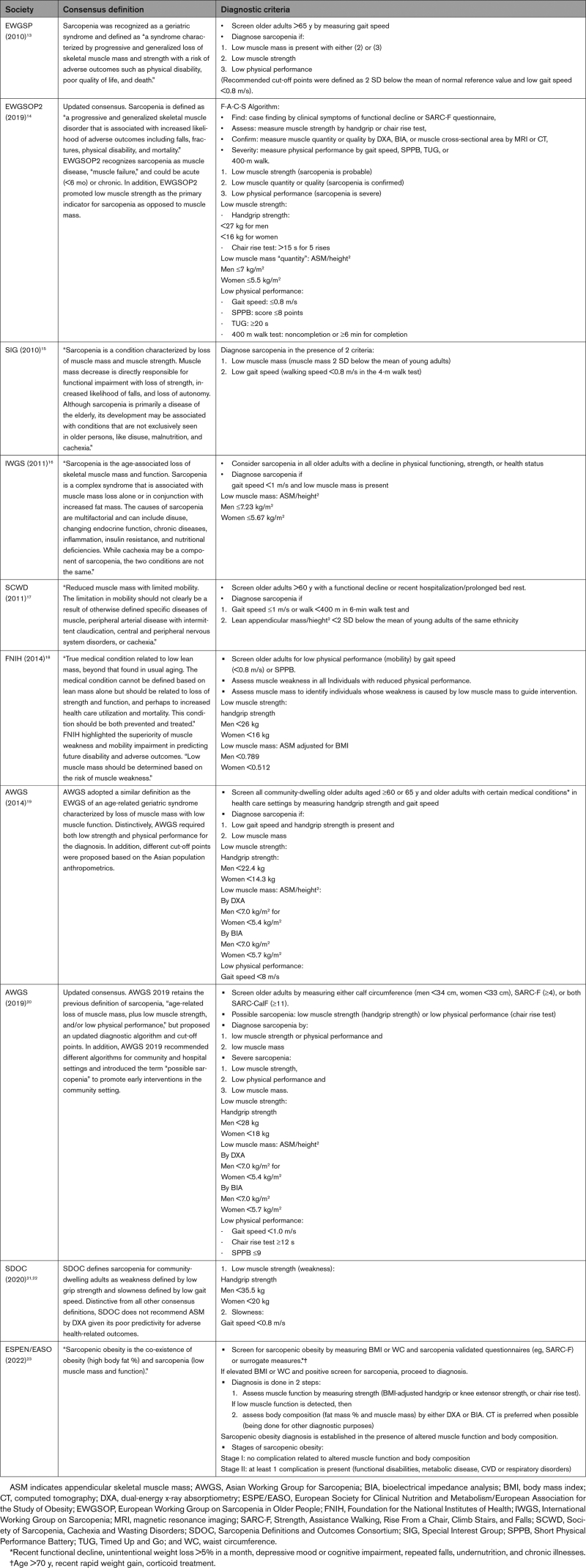
Sarcopenia is a pathologic condition of “muscle failure,” defined as “a progressive loss of muscle strength (dynapenia), mass (quantity), and function (quality), leading to a decline in physical functioning and increasing the risk of disability, fall, and mortality.”12 Although multiple sets of diagnostic criteria for sarcopenia exist, low muscle strength and function are universally agreed upon as key components. The EWGSOP2 (European Working Group on Sarcopenia in Older People 2) and the AWGS (Asian Working Group for Sarcopenia) diagnostic algorithms are the most widely used in clinical practice (Table 1).14,20
Table 1.
Clinical Definitions of Sarcopenia and Sarcopenic Obesity
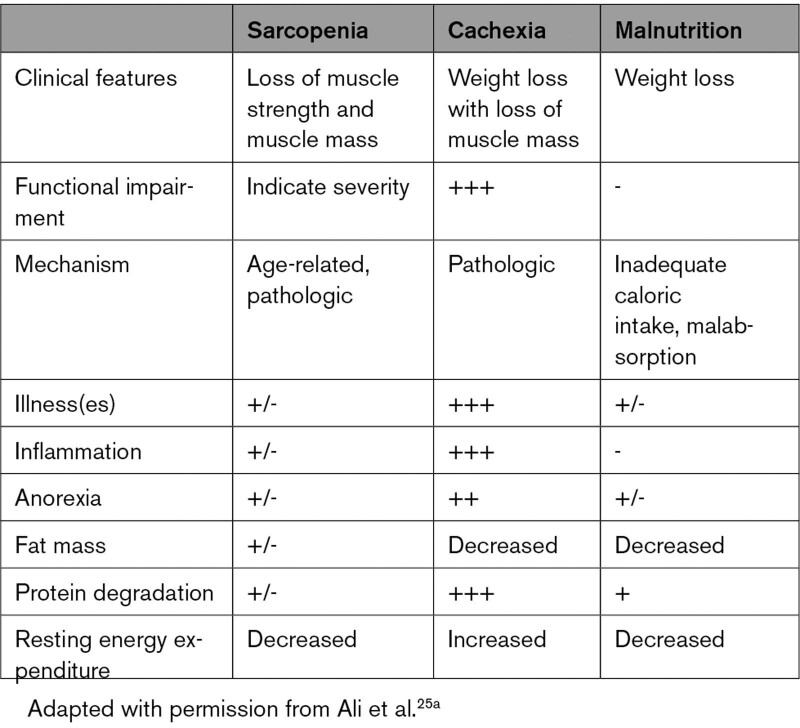
The EWGSOP2 defines sarcopenia as the coexistence of low muscle strength with either low muscle mass or quality; the presence of low physical performance indicates severe sarcopenia, and low muscle strength alone (without low muscle mass or quality) indicates “pre-sarcopenia.” The SDOC (Sarcopenia Definitions and Outcomes Consortium), a collaboration funded by the National Institute on Aging and the Foundation for the National Institutes of Health, defines sarcopenia as “low muscle strength assessed by grip strength and slow gait speed (ie, slowness).”21,24 Sarcopenia is distinct from malnutrition and cachexia, although each exhibits interrelated pathophysiology leading to different magnitudes of “wasting” and susceptibility to cardiovascular events (Table 2; Table S1).25
Table 2.
Differences Among Sarcopenia, Cachexia, and Malnutrition
In this article, we adopted the EWGSOP2 definition of sarcopenia that combines impairments in strength (dynapenia), mass (quantity/structure), and function (quality); thus, we refer to sarcopenia as impairment in mass and function or strength. To indicate a diagnosis of sarcopenia, there must be impairment in any of the following domains, measured as 2 SDs below the mean normal reference value (or low gait speed <0.8 m/s), as follows (Table 1):
Low muscle mass is present (with criterion 2 or 3);
Low muscle strength; or
Low physical performance.
Sarcopenic obesity is a particularly important phenotype of sarcopenia14 because the prevalence of obesity has increased substantially in recent years, now approaching 43% in the general population.26 Sarcopenic obesity is defined as obesity (increased visceral adipose tissue) that concurrently presents with sarcopenia (decline in muscle mass/function).23 Due to lack of consensus on diagnostic criteria, there is substantial variation in the reported prevalence and associated outcomes of sarcopenic obesity.27 Regardless of definition, participants with sarcopenic obesity had the highest incidence of cardiovascular events compared with other forms of muscle disorders.28 The ESPEN (European Society for Clinical Nutrition and Metabolism) and the EASO (European Association for the Study of Obesity) have established an initiative to standardize the diagnostic workup for sarcopenic obesity.23 Sarcopenic obesity may be diagnosed on the basis of independent measures of obesity and sarcopenia.
Pathophysiologic Mechanisms of Sarcopenia
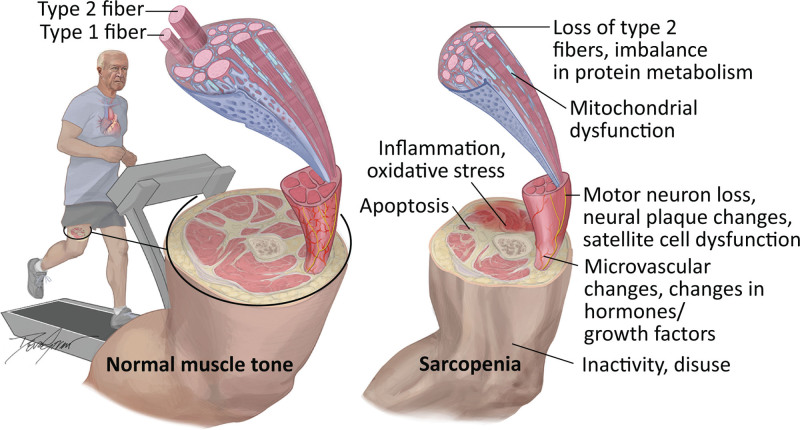
The underlying pathophysiologic mechanism of sarcopenia is complex and involves interactions of multiple physiologic systems (Figure 1).29–35
Figure 1.
Pathophysiologic mechanisms for development of sarcopenia in patients with cardiovascular disease.
Structural Changes of Skeletal Muscle
Human skeletal muscle consists of 2 main fiber types (slow and fast) and is classified into 3 subtypes of myofibers: type I (slow-oxidative and fatigue-resistant in low- intensity prolonged activity), type IIa (fast-oxidative and relatively fatigue-resistant), and type IIb or IIx (fast- glycolytic, functional in rapid and high-intensity movements, and are fatigue-susceptible).36,37 Myofibers are organized in motor units, and each motor unit consists of a myofiber and its alpha motoneuron innervation.38 Satellite cells are the skeletal muscle somatic stem cells responsible for muscle repair and regeneration (myogenesis).39 The disturbances in muscle homeostasis and neuronal degeneration with aging lead to senescence of satellite cells, preferential loss of type II fibers (hypoplasia), and the loss of functional motor units, collectively associated with muscle atrophy and decreased contractile force capacity, leading to muscle weakness and slowness.35,38,40
Myosteatosis
Fat infiltration of skeletal muscles, or myosteatosis, refers to ectopic fat deposition in skeletal muscles that is frequently seen in patients with cardiometabolic disease. The process of fatty infiltration of skeletal muscle is thought to be an independent process from loss of muscle mass and function. Fatty infiltration can take the form of different types of adipose depots within the skeletal muscle structure: (1) intermuscular adipose tissue; (2) intramuscular adipose tissue; and (3) intramyocellular lipids.41 Myosteatosis is associated with frailty and deterioration in muscle mobility and function.42 Excessive adipokines with visceral adiposity accelerates muscle loss because of proinflammatory activity that counters anabolic myokines. Collectively, this leads to a state of chronic inflammation, increasing insulin resistance and muscle breakdown.29,34 It is interesting that fatty infiltration and muscle fibrosis can impair muscle quality without atrophy; in these situations, muscle mass may not actually change.
The muscle–fat imbalance that specifically leads to sarcopenic obesity is, in part, a result of chronic proinflammatory state and metabolic dysregulation from insulin resistance and glucose intolerance. The increased body fat deposition accounts for most gain in body weight, but the total lean muscle mass progressively declines and results in further reductions in basal metabolic rate. With aging, body composition of muscle, bone, and fat changes, with a progressive decline in muscle and bone mass and an increase in total body fat, visceral adiposity, and fatty infiltration of skeletal muscle, liver, and bone marrow.43 These changes make older adults susceptible to myosteatosis and the development of sarcopenic obesity.
Muscle Homeostasis and Anabolic Resistance
Muscle homeostasis is maintained through a balance between the anabolic and catabolic molecular signaling. The anabolic pathway is stimulated by the upregulation of serine/threonine kinase Akt/mTOR (mammalian target of rapamycin) cascade, and the catabolic pathway is regulated by Fox-O (Forkhead O), NF (nuclear factor)-κB/ubiquitin proteasome, caspases cascade, and myostatin pathway.44 Myostatin is a skeletal muscle myokine; it downregulates Akt/mTOR and decreases the number of satellite cells, inhibiting muscle formation and repair.44,45 Dysregulation in muscle homeostasis leads to muscle atrophy or hypertrophy, depending on which pathway predominates. Anabolic stimuli of skeletal muscle, such as muscle contraction (exercise), essential amino acids (eg, leucine), and anabolic hormones, such as testosterone, insulin, and IGF-1 (insulin-like growth factor-1), work by upregulating the Akt/mTOR pathway, inhibiting myostatin and downregulating Fox-O, stimulating muscle protein synthesis, and inhibiting protein breakdown. Muscle atrophy is likely to develop when muscle protein breakdown exceeds muscle protein synthesis capacity, resulting in a net negative protein balance.45
The response of skeletal muscle to anabolic stimuli, particularly to essential amino acids, is blunted with aging, a phenomenon called “anabolic resistance.”46 The attenuated effect of dietary essential amino acids in stimulating muscle protein synthesis is likely a result of a diminished expression of Akt/mTOR signaling.46,47 Anabolic resistance is proposed to be related to age-associated vascular changes, which diminish muscular perfusion, subsequently impairing nutrients and oxygen delivery.48 Cross-sectional studies have shown that low lean muscle mass and sarcopenia are associated with arterial stiffness and arteriolosclerosis.49–51 Although the exact causal mechanism of the association between arterial dysfunction and the decline in muscle mass and function remains to be further elucidated, many theories have been suggested.51,52 Chronic inflammation, oxidative stress, insulin resistance, and impaired blood flow, from both endothelial dysfunction and calcification of skeletal muscle vasculature, have all been highlighted as possibly contributory.52 Insulin is well known for its anabolic action by promoting skeletal muscle protein uptake53; however, insulin also has a pivotal role in redistributing blood flow from nonnutritive to nutritive capillaries and activates endothelial nitric oxide in precapillary muscle arterioles, increasing the capillary surface area for nutrient exchange.48 With older age, muscle and vasculature become less sensitive to insulin, leading to diminished insulin-mediated microvascular blood flow, thus decreasing the delivery of amino acids.54
Inflammation and Mitochondrial Dysfunction
Together, immunosenescence, the accumulation of senescent cells, and increased visceral adiposity induce a state of low-grade chronic systemic inflammation characterized by increased levels of proinflammatory cytokines in the absence of infection (“inflammaging”).55,56 Proinflammatory cytokines such as CRP (C-reactive protein), IL-1 (interleukin 1), IL-6 (interleukin 6), and TNF-α (tumor necrosis factor-alpha) are key factors in inducing cell degradation by mechanism of skeletal muscle mitochondrial dysfunction, leading to increased production of reactive oxygen species that causes activation of the ubiquitin proteosome cascade and increasing muscle proteolysis.34,56 In addition, IL-6 induces insulin resistance, which hinders the activation of the Akt/mTOR pathway and impedes muscle protein synthesis.55 Individuals with low appendicular skeletal muscle were found to have significantly higher levels of IL-6 and CRP.57 “Inflammaging” is associated with increased risk for multiple chronic conditions, including heart failure (HF), atherosclerotic CVD, frailty, and poor health outcomes.58
The role of inflammation is most enhanced among patients with HF. In these patients, sarcopenia often begins early, with the activation of gastrometabolic, musculoskeletal, inflammatory, neurohormonal, sympathetic, and oxidative factors.59 These known HF factors interact in a complex manner to induce sarcopenia through the upregulation of muscle atrophy via the ubiquitin-proteasome system and the autophagy-lysosome system.60 In addition, proinflammatory factors such as TNF-α, IL-6, and IL-1 are elevated in patients with chronic HF.61 Skeletal muscle wasting is also thought to be heightened secondary to mitochondrial dysfunction from reactive oxygen species62 and significant upregulation of myostatin released from both peripheral myocytes and cardiomyocytes.63 In adults with HF, there is also a decline in anabolic hormones that exacerbates the naturally reduced levels observed with aging,64 including testosterone,65 ghrelin,66 IGF-1, and growth hormone.67 These cellular processes, when coupled with interstitial edema of the gut that cause early satiety, anorexia, and malabsorption, are associated not only with an enhanced catabolic state, but also with an independent decrease in muscle synthesis and mass.68
Neuronal Pathway
The age-related degenerative changes of the nervous system are believed to be a contributor to the development of sarcopenia. Muscle strength and power are determined by contractile force and velocity. Impairment in the integrity of the neuromuscular system plays a significant role in the decline of muscle strength and power, with consequent muscle weakness (dynapenia).31,32,69 The mechanism by which the nervous system specifically causes sarcopenia is not fully understood,32 but axonal degeneration, neuronal hypoexitability, and loss of α-motoneurons (particularly large motor-neuron innervating fast-twitch motor units) lead to dysregulation in the denervation–reinnervation cycle of motor neurons. In turn, “sick motoneurons” are associated with impairments in contractile velocity, muscle synergy, and, subsequently, muscle weakness.31,32,38,39 In healthy adults, muscle mass and strength (and relatedly, performance) peak by the third decade of life.70 As individuals approach their fourth decade, muscle mass and strength decline, with reductions in muscle strength by an average of ≈8% to 10% per decade. By 80 years of age, most adults have lost nearly 30% of their peak muscle mass and 50% of their peak muscle strength.71–73 Naturally, the rate of decline is influenced by comorbid conditions as well as genetic, behavioral, and environmental factors.35,47,74 Taken together, these lead to impaired muscle quality, diminished muscle remodeling, muscle atrophy, and loss of muscle fibers or motor neuronal units, which, in turn, translate into the development and progression of sarcopenia.45,55,75,76
Clinical Contributors to Sarcopenia
Sarcopenia is conceptualized as an age-related, multifaceted condition involving biological, environmental, socioeconomic, and genetic factors that collectively contribute to the loss of muscle mass and function. The interaction of multiple domains at the individual level can either counteract or hasten the effect of aging on muscle loss.74 Multiple factors such as malnutrition, prolonged immobility, and chronic systemic inflammatory state caused by malignancy, chronic diseases such as HF, coronary disease, diabetes, and end-organ failure have been recognized to influence the rate of age-related muscle decline.
The degree of muscle loss is exacerbated by a sedentary lifestyle, prolonged bed rest, smoking and alcohol intake, malnutrition, and anorexia of aging. Midlife obesity has been found to be associated with an increased incidence of sarcopenia later in life.77 Lutski et al found that among 337 men who were followed for 19.9 years, more than half of the participants (54.3%) who were obese (body mass index [BMI], ≥30.0 kg/m2) at baseline developed sarcopenia later in life (adjusted odds ratio, 5.31 [95% CI, 2.50–11.27]) compared with 24.8% of participants who had normal weight (BMI, 18.5 to 24.9 kg/m2).77 In another cohort of older men with a mean age of 68.7 ± 5.5 years and a mean follow-up of ≈11 years, sarcopenic obesity (obesity measured by waist circumference and sarcopenia by mid-upper-arm circumference) had the highest rate of all-cause mortality, even after adjusting for lifestyle and inflammatory markers.78 This may indicate that lifestyle factors partly explain the increased cardiovascular risk, and also indicate that behavioral interventions that target lifestyle could modify cardiometabolic risks among adults with sarcopenic obesity.
Sarcopenia is also particularly common in adults with kidney disease, especially among those with end-stage renal disease on hemodialysis. Several studies have documented that a loss of muscle mass and performance is a consistent feature in patients with chronic kidney disease, which is regarded as a model of “accelerated aging.”79 In one study that evaluated dialysis patients, quadriceps muscle weakness was observed in more than two-thirds of participants, and those with malnourishment exhibited slowing of muscle relaxation compared with healthy controls.80 The primary mechanism for muscle weakness is thought to be muscle atrophy, which is present even after adjusting for age, sex, and physical activity.81 Malnourished patients on dialysis had significantly smaller type IIb fiber areas compared with well-nourished patients.80
Sarcopenia is also frequently observed among older people with cancer. Decreased oral intake associated with chemotherapies, malnutrition, progressive protein catabolism, chronic inflammation, and increased metabolic demand, and physical inactivity are frequently observed in cancer patients. The presence of sarcopenia is a poor prognostic marker in different types of cancers and can influence the response to chemotherapy and radiation therapy, leading to higher adverse events and treatment interruption.82,83
Implication of Sarcopenia on CVD
Sarcopenia and HF
Sarcopenia is common among patients with HF with reduced ejection fraction and HF with preserved ejection fraction. The prevalence of sarcopenia ranges from 34% to 66% (Table S2), and it is highest among those hospitalized for acute decompensated HF (≈66%).84 Sarcopenia is an independent risk factor for prolonged and recurrent hospitalizations and increased risk for mortality.85,86 As a result of muscle wasting and myopathy, there is a substantial decline in physical function, capability, and performance. Exercise intolerance is a hallmark of HF with reduced ejection fraction and HF with preserved ejection fraction resulting from congestion and low cardiac output87; sarcopenia and muscle apoptosis further exacerbate these impairments,87 with contributions to symptoms such as shortness of breath and fatigue. Indeed, the SICA-HF trial (Studies Investigating Co-morbidities Aggravating Heart Failure) aimed to compare the effect of changes in body composition (sarcopenia and cachexia) in symptomatic patients with HF with reduced ejection fraction or patients with HF with preserved ejection fraction and a left atrial diameter >50%. There was a lower peak VO2 (maximal oxygen consumption, VO2 max) in patients with HF and sarcopenia compared with those without sarcopenia.88 Sarcopenia also contributes to the risk of falls among adults with HF, which can be significant as a result of pharmacological therapy for HF such as loop diuretics, osteoporosis secondary to decreased calcium reuptake, and poor vitamin D synthesis from being functionally homebound.88 Table S2 summarizes the studies that have examined the diagnosis, prognosis, pathophysiology, and treatment of sarcopenia in patients with HF.
Sarcopenia and Atherosclerotic CVD
Sarcopenia and coronary artery disease (CAD) share similar underlying biological mechanisms, namely, low-grade chronic systemic inflammation. Accordingly, sarcopenia is common among adults with CAD; many studies have shown that sarcopenia (defined by AWGS) affects 1 out of 8 community-dwelling adults with CAD, and 1 out of 4 hospitalized patients with CAD (Table S3).89 This is important because low skeletal muscle mass in older patients with CAD is associated with increased cardiovascular mortality, major adverse cardiovascular events, myocardial infarction, and low exercise capacity.90,91 Sarcopenia may also be a risk factor for CAD; previous work has shown that low skeletal muscle mass among asymptomatic community-dwelling older adults is associated with subclinical atherosclerosis, increased coronary artery calcium score, arterial stiffness, and carotid arterial wall thickening.92–94
Sarcopenia and Peripheral Arterial Disease
Patients with peripheral arterial disease (PAD) are at an increased risk for muscle mass disorders such as sarcopenia, particularly those with chronic limb ischemia. Among those with lower extremity vascular disease, sarcopenia is observed in up to 35% of the patients.95 The typical age-related pathophysiologic changes to muscle occur prematurely in patients with PAD; this includes oxidative stress, inflammation, mitochondrial dysfunction, impaired signaling pathways, and ischemia-reperfusion injury.96 As a result, reductions in the number and size of type II muscle fibers that occur in PAD patients lead to muscle weakness, functional impairment, and abnormal muscle histology. Sarcopenia is also a poor prognostic marker in patients with PAD. Prospective evaluations found a strong association between skeletal muscle weakness with long-term mortality, major adverse cardiovascular events, and limb events in patients with PAD.97,98
Sarcopenia and Transcatheter Aortic Valve Replacement
The prevalence of sarcopenia in patients undergoing aortic valve replacement ranges between 21% and 70.2%, depending on the instrument used, degree of impairment, and type of valve replacement.99–102 Pre–transcatheter aortic valve replacement sarcopenia is associated with longer hospital lengths of stay,101,103,104 higher resource use,105 in-hospital adverse outcomes,105,106 disability,107 discharge to a skilled nursing facility,107 readmission,108 and higher 30/90-day and long-term mortality109 (Table S4). The transcatheter aortic valve replacement procedure has substantially improved survival for severe aortic stenosis, but 50% of survivors have sarcopenia, and many of these participants report poor HR-QOL (health-related quality of life) and a decline in their overall functional status during follow-up. A study of 13 351 older adults found that 39% either died or had a poor or declining quality of life 1 year after transcatheter aortic valve replacement.110 The authors attributed the decline in quality of life and poor outcomes to sarcopenia, frailty, disability, unintentional weight loss, and inability to perform activities of daily living.110 Sarcopenia and these geriatric syndromes are intimately connected to prognosis, and there is an unmet need to address these age-associated risks among older patients.
Sarcopenia and Cardiac Surgery
An increasing number of older adults are undergoing cardiac surgery for various cardiovascular conditions. Frailty, malnutrition, and sarcopenia are common in older patients undergoing cardiac surgery.111 Similar to other conditions, sarcopenia is associated with postoperative adverse events and long-term mortality in patients undergoing cardiac surgery.106,112,113 Age, comorbidities, and malnutrition in association with underlying cardiovascular disorders cause muscle wasting, immobility, and decline in physical function before, during, and after hospitalizations (Table S5).111 During the period before surgery, reduced mobilization leads to reduced muscle strength, function, and reduced respiratory muscle performance.114 This loss of muscle mass and strength is further exacerbated by prolonged bed rest, greater length of intensive care unit stay, and inadequate nutrition, and causes significant functional limitations that may persist for years after discharge.111 Rehabilitation interventions to maintain or recover function are traditionally performed during the hospitalization postoperatively and after discharge. Preoperative rehabilitation interventions, also termed prehabilitation, have garnered interest. Prehabilitation encompasses nutrition, exercise, respiratory and cardiocirculatory training, psychological interventions, and optimization of drug therapies. Prehabilitation is safe in most patients and may be associated with a reduction in length of stay and postoperative complications after a major surgery.111,114
Approach to Diagnosis
Screening
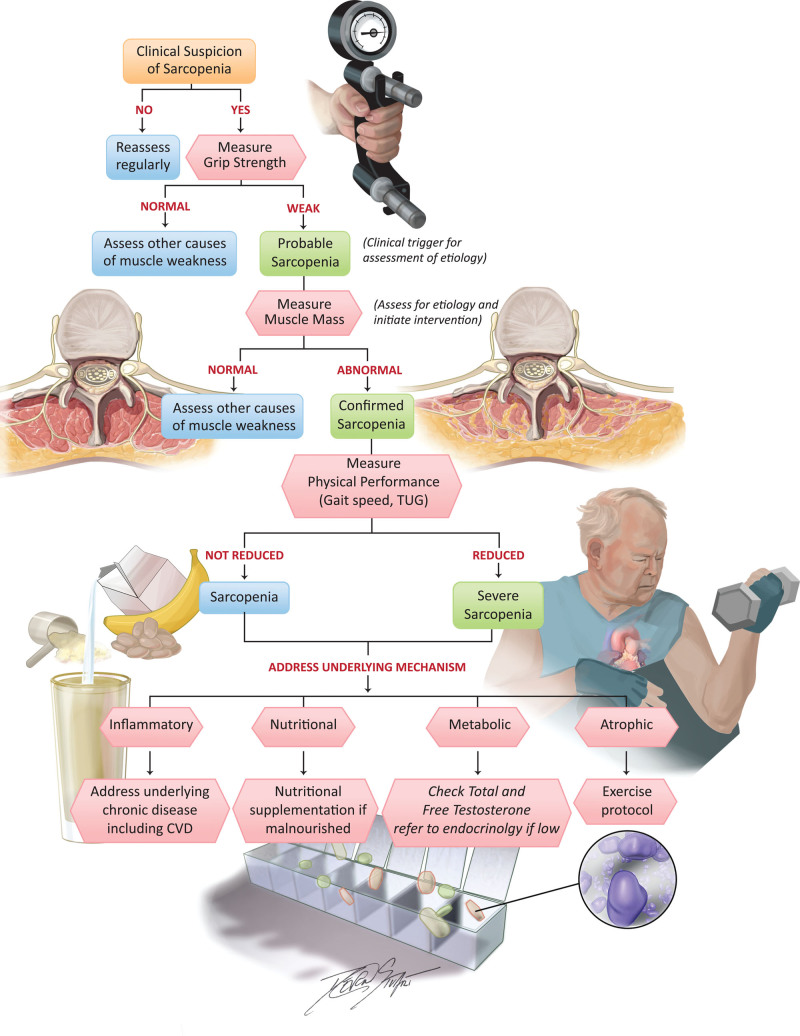
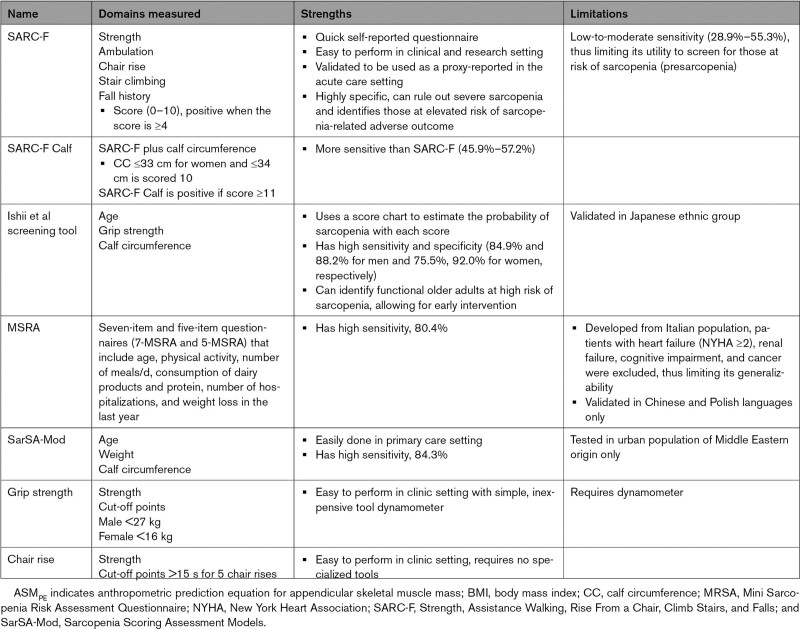
Screening for early signs and stages of sarcopenia is important because therapeutic interventions are likely to be most effective before sarcopenia has reached advanced stages. There are myriad clinical screening tools that can be used to identify low muscle function and surrogate measures for low muscle mass (Figure 2). There is no current consensus on the best screening tool for clinical practice. The strengths and limitations of each screening tool are listed in Table 3. Of note, the SARC-F (Strength, Assistance Walking, Rise from a Chair, Climb Stairs, and Falls) questionnaire and Ishii equation can be used as screening methods for sarcopenia in either the ambulatory or hospital setting.115 If the screening tool suggests probable sarcopenia, a confirmatory test should follow (see section “Confirmatory Testing”).
Figure 2.
Algorithm providing a screening and diagnostic approach for sarcopenia and potential therapeutic interventions associated with improvement in patients with cardiovascular disease. CVD indicates cardiovascular disease; and TUG, Timed Up and Go.
Table 3.
Screening Tools for Sarcopenia
Symptoms that should trigger formal screening for sarcopenia include falls, subjective weakness, slow walking speed, difficulty rising from seated position, weight loss, and difficulties with activities of daily living. It may be reasonable to concurrently screen for malnutrition as well because malnutrition commonly coexists with sarcopenia.
It is important to note that BMI should not be used to screen for sarcopenia among older adults in whom sarcopenic obesity is common. Although BMI could be a good screening tool for younger individuals,116 changes in body muscle and fat composition with aging are more complex, and patients with sarcopenia may appear well nourished on the basis of body habitus. Thus, BMI is less predictive.117,118 Older adults could maintain the same BMI by increasing their body fat and visceral adiposity and decreasing muscle mass, important body composition changes that increase the risk of CVD and mortality. Tracking sarcopenia during weight gain or weight loss is accordingly complex. For example, with successful intended weight loss from treatment of cardiometabolic disease, there is a natural loss of muscle quantity, but it can be difficult to assess whether the degree of muscle loss is appropriate or inappropriate (ie, signs of worsening sarcopenia). For those in whom sarcopenic obesity is present, it may be worthwhile to evaluate waist circumference and waist-to-hip ratio, which are well-known surrogate markers for visceral adiposity and can potentially predict CVD events119 and risk of death across all BMI categories.120
Confirmatory Testing
Multiple instruments can be used to confirm the presence of sarcopenia, although diagnostic criteria vary in terms of cut points (summarized in Table 1).14,20,21,24 Confirmatory testing instruments can be classified as: (1) imaging and body composition assessment tools121; (2) physical performance; and (3) laboratory tests.
Imaging Modalities and Body Composition Measurement Tools
Both computed tomography (CT) and magnetic resonance imaging are considered the “gold standard” instruments to quantify muscle mass because they can provide direct visualization of cross-sectional area or volume, which can then be converted into mathematical estimates of overall body muscle mass.122 In a study that compared estimates of muscle mass from cadaveric sections with CT and MRI, both modalities provided accurate assessment of adipose tissue–free skeletal mass, interstitial adipose tissue, and subcutaneous adipose tissue. CT can also provide a measure of muscle density (the radiodensity of the muscle in Hounsfield units); low muscle density is thought to be a measure of poor muscle quality and is related to declines in function and mortality in older adults.123 CT imaging at the third lumbar vertebra has been used as a surrogate measure for whole-body muscle mass, which correlates with prognosis in multiple cardiovascular populations. The measurement at the level of the third lumbar is also useful to detect patients with sarcopenic obesity. The quantification of skeletal muscle at the third lumbar can be done by MRI as well, but midthigh measurement is also a good predictor of whole-body skeletal mass. Use of these modalities in the clinical setting is limited by their cost and feasibility. In addition, diagnostic cut-off points for sarcopenia in the clinical setting have not been uniformly established.14
Muscle ultrasound has been used in clinical research, and it holds promise as a tool to assess for sarcopenia, especially given that it can be conducted at the bedside with low cost.124 Muscle ultrasound provides measures for muscle mass and muscle quality and correlates well with CT, MRI, and dual-energy x-ray absorptiometry (DXA).14,125 To date, its use in the clinical setting is limited because it requires trained personnel and a standardized protocol.
The DXA scan provides measures of body composition but not muscle quality. It is the most commonly used instrument in research and clinical setting to measure appendicular skeletal muscle and appendicular muscle mass index/height2. Although DXA scan is relatively inexpensive and fast, and the diagnostic cut-off points are validated, it has drawbacks. First, it only measures lean mass, which is only one component of muscle mass.121 Second, hydration status can influence the diagnostic accuracy of the test because it has a poor ability to distinguish extracellular fluid from muscle mass; this can lead to overestimation of muscle mass/quantity among individuals with excess fluid such as those with HF. Finally, DXA is only weakly associated with measures of performance and function.126 For that reason, DXA may not be the best measure to assess function decline in older patients.127
The bioelectrical impedance analysis estimates lean mass based on whole-body electrical conductivity. Bioelectrical impedance analysis is an inexpensive instrument that can be performed at bedside or in the office. Similar to DXA, hydration status can influence its accuracy.128
Laboratory Biomarkers
Cardiovascular clinicians are familiar with measuring cardiac biomarkers to diagnose cardiovascular conditions (eg, natriuretic peptides for HF and cardiac specific troponins for acute myocardial infarction).129 Unfortunately, to date, there is no single circulating biomarker that is diagnostic of sarcopenia, although there is some promise for the future.
For the assessment of muscle mass, the D3-creatine (D3-Cr) dilution method is a promising strategy with high accuracy. The assay measures the quantity of deuterated creatinine from a single spot urine analysis performed 48 to 96 hours after administering a D3-Cr tracer orally. The D3-Cr obtains a steady state in skeletal muscle ≈48 hours after ingestion. There is a fairly constant creatine skeletal muscle turnover each day so that the amount of D3-Cr in the urine is proportional to total body muscle mass.130,131 D3-Cr correlates well to MRI-determined whole body muscle mass (r=0.87) and is potentially more accurate than DXA.132,133
A simpler alternative to D3-Cr for circulating markers of muscle mass assessment has been proposed, called the sarcopenia index, which is represented by (serum creatinine/serum cystatin C) × 100. Initially proposed by Kim et al, the index was found in ambulatory adults to associate strongly with DXA and also correlated with muscle mass in critically ill patients estimated by abdominal CT.133,134 The sarcopenia index was shown to be prognostic of intermediate-term death in previously hospitalized older adults and major adverse cardiovascular event in older adults with coronary disease.135,136 The sarcopenia index leverages the concept that creatine is nearly exclusively produced by muscle, but cystatin C is produced by all nucleated cells. A caveat is that although cystatin C production is thought to be steady-state, factors such as hyperthyroidism, malignancy, and some forms of inflammation can alter cystatin C levels.137
Physical Performance Tests
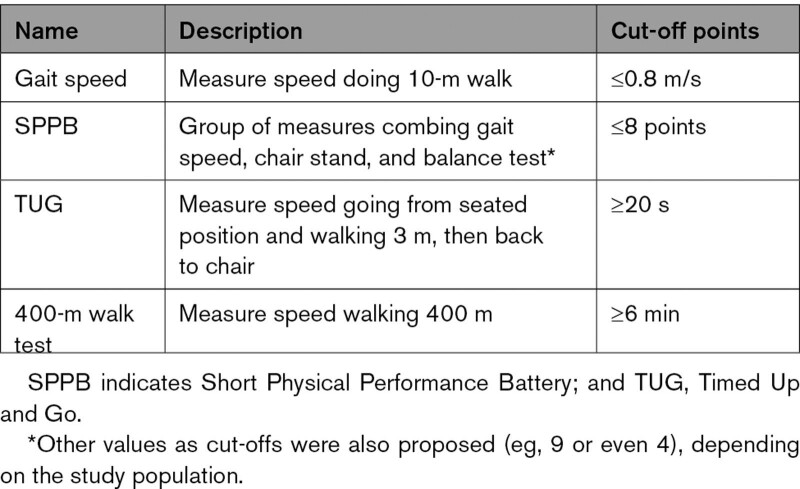
Physical performance testing has been used for assessing the severity of sarcopenia and for its prognostication (Figure 2). The EWGSOP recommends using physical performance/function to assess the severity of sarcopenia, and the SDOC recommends using physical performance/function to predict clinical outcomes. Table 4 summarizes instruments that can be used for physical performance tests, although performance of these tests is likely influenced by myriad factors, including concurrent comorbid conditions and other geriatric syndromes such as frailty.
Table 4.
Physical Performance Testing to Assess for Severity of Sarcopenia
Treatment
Sarcopenia is a modifiable condition that could revert to a normal state with early intervention (Figure 2). Such interventions may be similarly important for those with presarcopenia, which is also modifiable; for example, in a study, participants with probable sarcopenia were found to have equal likelihood of either reverting to normal or progressing into sarcopenia at 5-year follow-up (10.7% versus 10.3%).138 There are also several management strategies currently under investigation, including resistance exercise, nutritional supplements, and hormonal or pharmacotherapies (Table S6).
Treatment of Comorbid Conditions
The first step to treating sarcopenia is to identify and address underlying contributing factors. For example, treating and optimizing metabolic health and addressing obesity through exercise and caloric intake may reduce myosteatosis.139 Treating co-occurring CVD that contributes to inflammation is similarly important. For example, treating HF with guideline-directed medical therapy can forestall disease progression and potentially suppress the inflammatory processes known to contribute to adverse changes in muscle pathophysiology that drives sarcopenia.
Resistance Exercise Training
An important factor in the development of sarcopenia is the lack of physical activity followed by anabolic resistance. Therefore, the strongest evidence currently available for the treatment and prevention of sarcopenia is derived from studies on exercise intervention programs, such as resistance and aerobic training, that have been shown to increase muscle mass, strength, and physical performance. Progressive resistance training programs can be used as a successful intervention for the management of sarcopenia. In these programs, participants exercise their muscles against increasing external force for at least 2 to 3 times a week for a duration of 8 to 12 weeks. The amount of resistance and duration of the session increase gradually over time depending on each individual’s capability and progress. In healthy older adults, improvement in muscle strength can be achieved with as few as one resistance exercise session per week.140 Liang et al noted that among sarcopenic older adults 80 to 99 years of age, combined resistance and balance exercise twice weekly during 12 weeks accomplished a significant improvement in performance of activities of daily living, with a 10% absolute risk reduction in the number of falls compared with resistance exercise alone.141
Muscle hypertrophy occurs when protein synthesis in the muscle exceeds protein breakdown. Older patients performing resistance exercise show a characteristic increase in muscle protein synthesis without an increase in total body protein breakdown. Some evidence indicates a significant increase in the size of both type 1 and type 2 muscle fibers after both progressive resistance training and a prolonged exercise program consisting of resistance and high-intensity interval training, which could explain the improved muscle strength and performance.142,143 Roth et al demonstrated that resistance strength training significantly increases the proportion of active satellite cells for both older adults, 65 to 75 years of age, and young individuals, 20 to 30 years of age. However, older women had the most augmented improvement.144 Although increases in myofiber size explain some observed clinical strength gains with resistance training programs, most gains are derived from increased recruitment of motor units and other neural adaptations.
Aerobic exercise, such as swimming, walking, and jogging, has long been associated with improved cardiovascular fitness and physical performance. With aerobic exercise, the large muscle groups move in a rhythmic manner for a sustained period of time that increases the energy expenditure.145 It has been shown to increase the muscle fiber cross-sectional area, but it is less likely to cause muscle hypertrophy.146 After aerobic exercise alone, mitochondrial volume and enzyme activity increase, indicating that muscle quality improves regardless of age.147 In patients with both HF and sarcopenia, aerobic activity is associated with reduced hospitalizations and mortality, which is thought to be a result of reductions in skeletal muscle inflammatory markers, isoform of nitric oxide synthase, and myostatin, and an increase in the skeletal muscle cross-sectional area.148
Cardiac Rehabilitation
Cardiac rehabilitation (CR) is a multidisciplinary secondary prevention program designed for patients after a cardiovascular event (eg, myocardial infarction, percutaneous or surgical revascularization, and other cardiac surgical procedures) to promote cardiovascular health through education, risk factors management, exercise training, and psychosocial health. Although CR has not been specifically studied as a therapeutic option for sarcopenia, it has the potential to target risk factors that contribute to the progression of muscle wasting disorders such as sedentariness, malnutrition, and polypharmacy.149,150 The tailored exercise training that is part of CR programs improves muscle strength, performance, and nutritional assessment, and counseling can identify and address malnutrition. In a retrospective study of 322 Japanese older adults with CVD attending a comprehensive CR program that included exercise training (aerobic, resistance, and balance) and nutritional intervention, sarcopenia was present in 28% of participants, and those with sarcopenia tended to be female and of older age.151 Participants both with and without sarcopenia observed a significant improvement in muscle strength and gait speed. Posttraining muscle strength of those with sarcopenia was similar to the pretraining muscle strength of those without sarcopenia.151 This suggests that comprehensive CR programs have the potential to reverse or delay the progression of sarcopenia.
Nutritional Intervention
Protein and Amino Acids
Diet may be an important intervention to mitigate the negative effects of sarcopenia. The role of dietary interventions such as protein, antioxidants, creatine, fatty acids, and vitamin D has been evaluated. In brief, protein supplementation and amino acids appear to be the most promising dietary supplementation (Figure 2). Incorporating protein concentrates (eg, whey) or branched-chain amino acids, such as leucine, into the diet can increase the rate of mixed skeletal muscle protein synthesis.44 The Food and Nutrition Board of the National Academy of Sciences of the United States recommends 0.8 g/kg/d of protein for all adults to prevent muscle mass loss. Despite this recommendation, >38% of older men and 41% of older women do not meet this recommended daily allowance.152 The European Society for Clinical Nutrition and Metabolism recommends that for healthy older people, the diet should provide at least 1.0 to 1.2 g protein/kg body weight per day.153 For older people who are malnourished or at risk for malnourishment due to acute or chronic illnesses, the diet should provide at least 1.2 to 1.5 g protein/kg body weight per day, and even higher intake for those with severe chronic illnesses or injuries. The society also recommends coupling a high-protein diet with daily physical activity (resistance and aerobic exercise) for all older individuals.153
Combined Resistance Exercise Training and Protein Supplementation
The effects of combining a moderate- to high -rotein diet and resistance exercise remain an area of active investigation. An increase in muscle protein synthesis without concurrent change in muscle protein breakdown after amino acid ingestion can happen in both young and older individuals.154 A diet that is inadequate in proteins or amino acids may further facilitate muscle protein loss in older adults by blunting protein synthesis and promoting subsequent protein catabolism.155
Consuming a diet that is higher in protein than the recommended daily allowance but within the Acceptable Macronutrient Distribution Range may have health benefits. Coupling a high-protein diet with resistance exercise can result in an increase in muscle strength and greater muscle mass preservation, mainly when consumed in a state of negative energy balance. Further, the beneficial effect on muscle protein synthetic response is greater if protein intake is distributed across meals in a balanced manner.156 The type of protein can influence digestion and absorption. For instance, whey and soy, a milk protein, are frequently described as a “fast” protein because they rapidly release amino acids when digested, as opposed to casein, another milk protein, which is considered a “slow” protein. In a study by Tang et al, consumption of whey or soy protein in young men with resistance exercise resulted in greater muscle protein synthesis compared with casein.157
Iglay et al158 conducted a study in which 36 men and women >50 years of age participated in 12 weeks of resistance training in conjunction with a lower protein (0.9 g protein.kg-1.d-1) or higher protein (1.2 g protein.kg-1.d-1) intake. Although all outcome measures showed overall improvement (eg, increased total-body protein mass, increased strength, and decreased fat mass), no differences were observed between the low-protein and high-protein groups. Furthermore, Andrews et al159 proposed that daily protein consumption (1.35 versus 0.72 protein.kg-1.d-1) has no additional effect on lean mass gains associated with resistance training. Variability in protein consumption was not associated with variability in muscle hypertrophy among both groups. Further studies to investigate which protein supplement type is most effective in a clinically meaningful improvement in muscle function are needed.
Testosterone Replacement
The level of testosterone gradually declines with aging. At 60 years of age, nearly 20% of men have testosterone levels in the hypogonadal range, and by 80 years of age, >50% of men have low testosterone.160 Although higher testosterone is linked to increased muscle mass and decreased percentage of total body fat in older men, studies on testosterone treatment in older men are controversial, and outcomes have varied by the dosage, subject, and method of administering testosterone (Table S6).161 Several studies have found that oral testosterone supplementation was associated with increased lean body mass, knee extension, improved chest press strength and stair-climbing power, and decreased fat mass.162–164 Other studies reported no difference in muscle strength, performance, and activity with testosterone supplementation.165 Given mixed results, testosterone therapy cannot be recommended as a routine therapy for sarcopenia, especially given the potentially increased risks of testosterone therapy in certain subpopulations with CVD.
Selective Androgen Receptor Modulators
GTx-024 (enobosarm) is an agent that has shown a dose-dependent increase in total lean body mass and improvement in physical performance such as the stair-climb test.166,167 Although enobosarm and other selective androgen receptor modulators demonstrated improvement in lean muscle mass, the improvement in physical function has not been consistent. In addition, acute liver damage was reported with the use of selective androgen receptor modulators as a major adverse event.168 These drugs are not yet available, and further studies are needed to establish their efficacy and safety in patients with sarcopenia.
Angiotensin-Converting Enzyme Inhibitors
For many years, angiotensin-converting enzyme inhibitors (ACE-Is) have been used to treat hypertension and HF. The use of ACE-Is is associated with improved skeletal muscle function. ACE-I is one of the cornerstone therapies in HF, and its effect on sarcopenia could simply relate to treating the underlying syndrome. However, ACE-Is could also produce their beneficial effects through improving endothelial function, anti-inflammatory effects, and angiogenesis, thus enhancing skeletal muscle blood flow. ACE-I can potentially increase IGF-1 levels and improve mitochondrial function, reducing muscle catabolism.169 Therefore, there is a biologic rationale for the potential of ACE-Is to delay the development or progression of sarcopenia.170,171 A previous observational study showed that continuous use of ACE-I for 3 years was associated with a lower rate of decline in muscle strength and walking speed among hypertensive older women when compared with intermittent use of ACE-Is or other antihypertensive medications.172
Gaps in Knowledge
There is an unprecedented need to standardize the definitions of sarcopenia (and clinical concepts related to muscle mass, quality, and function). The tools used to diagnose sarcopenia in practice need further investigation, with a focus on reliable and reproducible diagnostic instruments including the use of standardized cut points for imaging and biomarkers that evaluate skeletal muscles, especially in the setting of obesity (ie, for sarcopenic obesity).
Given that sarcopenia often occurs in the setting of end-organ damage/failure, chronic inflammation, malnutrition, and prolonged immobility, biomarkers that integrate multi “omics” could be helpful for detection and longitudinal assessments of sarcopenia. A working group from the TAME trial (Targeting Aging With Metformin) has accordingly recommended the following: IL-6, TNF-α receptor I or II, CRP, GDF15, insulin, IGF-1, cystatin C, NT-proBNP (N-terminal pro-B-type natriuretic peptide), and hemoglobin A1c, but also noted a paucity of evidence for biomarkers in human studies reflecting processes of aging.173 Examining mitochondrial-specific proteins, which may play a role in the pathophysiology of sarcopenia, could also be a fruitful area for further investigation.174 Future work using omics-based “big data” approaches could identify combinations of biomarkers strongly associated with muscle mass and function, prognosis, and potentially treatment response.175
Studying clinical variability of muscle quality and function among different ethnic groups at risk for or living with CVD is necessary and may provide mechanistic insights on development and progression of sarcopenia among the most vulnerable populations. Moreover, we need rigorous studies of interventions that can prevent or forestall development or progression. This should include an evaluation of structured resistance exercise programs with or without nutritional supplementation. Further evaluation of how best to leverage the infrastructure of cardiac rehabilitation to meet the needs of adults with CVD is similarly necessary. Finally, research is warranted to examine the efficacy and safety of pharmacotherapeutic strategies targeting key substrates of sarcopenia, with attention paid to key myosteatosis, which is particularly important in the development of sarcopenic obesity.
Conclusions
Sarcopenia, defined as the loss of muscle strength, mass, and function, is common in cardiac patients and has a bidirectional relationship with CVD. It is important to note that sarcopenia is associated with a range of adverse outcomes, including mortality, falls, and impaired health-related quality of life. Sarcopenia may be modifiable, especially during its earlier stages. Accordingly, screening for sarcopenia to facilitate its early recognition is important because early intervention can potentially prevent or forestall progression of sarcopenia. Interventions such as resistance training and nutritional supplementation are promising strategies to address sarcopenia, although future work is needed in the area of skeletal muscle therapeutics.
Article Information
Acknowledgments
The authors acknowledge Drs Todd Brown, professor of medicine and epidemiology at Johns Hopkins University, and Adrian Dobs, professor of medicine and oncology and director of Johns Hopkins Clinical Research Network, for their insights on the management algorithm for patients with sarcopenia. We also acknowledge Devon Stuart for her assistance with medical illustration.
Sources of Funding
Dr Damluji receives research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging (P30-AG021334) and a mentored patient-oriented research career development award from the National Heart, Lung, and Blood Institute (K23-HL153771-01). Dr Alexander receives research funding from the National Institute of Aging (grant No. U19AG065188). Dr Rich receives support from the National Institutes of Health (R01 AG060499 and R01 AG078153). Dr Goyal receives research funding from the American Heart Association (grant No. 20CDA35310455) and the National Institute on Aging (grant No. K76AG064428). Dr Forman receives research funding from the National Institute of Aging (grant Nos. R01 AG058883, R01 AG060499, U19AG065188, R01 AG073633, R01 AG077179, and P30AG024827) and VA RR&D (1I21RX004409 and HSR&D1 I01 HX003518; and PCORI IHS-2021C3-24147). Dr Cawthon received research funding (grants AG066671, CA246695, and AG070804) and is a consultant to BioAge Labs for work outside of this article.
Disclosures
None.
Supplemental Material
Supplemental Material
Tables S1–S6
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACE-I
- angiotensin-converting enzyme inhibitor
- AWGS
- Asian Working Group for Sarcopenia
- BMI
- body mass index
- CAD
- coronary artery disease
- CR
- cardiac rehabilitation
- CT
- computed tomography
- CVD
- cardiovascular disease
- D3-Cr
- D3-creatine
- DXA
- dual-energy x-ray absorptiometry
- EWGSOP
- European Working Group on Sarcopenia in Older People
- HF
- heart failure
- MRI
- magnetic resonance imaging
- PAD
- peripheral arterial disease
A.A. Damluji and M. Alfaraidhy contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.123.064071.
For Sources of Funding and Disclosures, see pages 1547–1548.
Circulation is available at www.ahajournals.org/journal/circ.
Contributor Information
Maha Alfaraidhy, Email: malfaraidhy@gmail.com.
Noora AlHajri, Email: Nalhajri007@gmail.com.
Namit N. Rohant, Email: dr.rohant@gmail.com.
Manish Kumar, Email: mkumar@uchc.edu.
Christina Al Malouf, Email: Christina.AlMalouf@mountsinai.org.
Samira Bahrainy, Email: samira11@uw.edu.
Min Ji Kwak, Email: min.ji.kwak@uth.tmc.edu.
Wayne B. Batchelor, Email: Batch002@gmail.com.
Daniel E. Forman, Email: formand@pitt.edu.
Michael W. Rich, Email: mrich@wustl.edu.
James Kirkpatrick, Email: kirkpatj@cardiology.washington.edu.
Ashok Krishnaswami, Email: ashok.krishnaswami@kp.org.
Karen P. Alexander, Email: karen.alexander@duke.edu.
Gary Gerstenblith, Email: gblith@jhmi.edu.
Peggy Cawthon, Email: PCawthon@sfcc-cpmc.net.
Christopher R. deFilippi, Email: Christopher.Defilippi@Inova.org.
Parag Goyal, Email: pag9051@med.cornell.edu.
REFERENCES
- 1.He W, Sengupta M, Velkoff V, DeBarros K. In; 65+ in the United States: 2005, Current Population Reports. 2005:P23–209. Accessed April 28, 2023. https://www.census.gov/content/dam/Census/library/publications/2005/demo/p23-209.pdf
- 2.Damluji AA, Forman DE, van Diepen S, Alexander KP, Page RL, Hummel SL, Menon V, Katz JN, Albert NM, Afilalo J, et al. ; American Heart Association Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Older adults in the cardiac intensive care unit: factoring geriatric syndromes in the management, prognosis, and process of care: a scientific statement from the American Heart Association. Circulation. 2020;141:e6–e32. doi: 10.1161/CIR.0000000000000741 [DOI] [PubMed] [Google Scholar]
- 3.Damluji AA, Forman DE, Wang TY, Chikwe J, Kunadian V, Rich MW, Young BA, Page RL, DeVon HA, Alexander KP; American Heart Association Cardiovascular Disease in Older Populations Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Lifestyle and Cardiometabolic Health. Management of acute coronary syndrome in the older adult population: a scientific statement from the American Heart Association. Circulation. 2023;147:e32–e62. doi: 10.1161/CIR.0000000000001112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudart C, Zaaria M, Pasleau F, Reginster J-Y, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12:e0169548. doi: 10.1371/journal.pone.0169548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao K, Cao LF, Ma WZ, Gao YJ, Luo MS, Zhu J, Li T, Zhou D. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. EClinicalMedicine. 2022;44:101264. doi: 10.1016/j.eclinm.2021.101264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He N, Zhang Y, Zhang L, Zhang S, Ye H. Relationship between sarcopenia and cardiovascular diseases in the elderly: an overview. Front Cardiovasc Med. 2021;8:743710. doi: 10.3389/fcvm.2021.743710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki K-I, Kakuma T, Sasaki M, Ishizaki Y, Fukami A, Enomoto M, Adachi H, Matsuse H, Shiba N, Ueno T, et al. The prevalence of sarcopenia and subtypes in cardiovascular diseases, and a new diagnostic approach. J Cardiol. 2020;76:266–272. doi: 10.1016/j.jjcc.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520 [DOI] [PubMed] [Google Scholar]
- 10.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985). 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003 [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72 [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Rodriguez D, Marco E, Cruz-Jentoft AJ. Defining sarcopenia: some caveats and challenges. Curr Opin Clin Nutr Metab Care. 2020;23:127–132. doi: 10.1097/MCO.0000000000000621 [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel J-P, Rolland Y, Schneider SM, et al. ; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, et al. ; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 16.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Stewart Coats AJ, Cummings SR, Evans WJ, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS, Chou M-Y, Chen L-Y, Hsu P-S, Krairit O, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 20.Chen LK, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 21.Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, Magaziner JM, Newman AB, Kiel DP, Cooper C, et al. Sarcopenia definition: the position statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc. 2020;68:1410–1418. doi: 10.1111/jgs.16372 [DOI] [PubMed] [Google Scholar]
- 22.Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP, Santanasto AJ, Ensrud KE, Xue QL, Shardell M, et al. Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc. 2020;68:1429–1437. doi: 10.1111/jgs.16517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar D, Maria Batsis A, Bauer M, Juergen Boirie Y, Cruz-Jentoft J, Alfonso Dicker D, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. 2022;1:15. doi: 10.1159/000521241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirk B, Zanker J, Bani Hassan E, Bird S, Brennan-Olsen S, Duque G. Sarcopenia Definitions and Outcomes Consortium (SDOC) criteria are strongly associated with malnutrition, depression, falls, and fractures in high-risk older persons. J Am Med Dir Assoc. 2021;22:741–745. doi: 10.1016/j.jamda.2020.06.050 [DOI] [PubMed] [Google Scholar]
- 25.Severin R, Berner PM, Miller KL, Mey J. The crossroads of aging: an intersection of malnutrition, frailty, and sarcopenia. Topics Geriatr Rehab. 2019;35:79–87. doi: 10.1097/tgr.0000000000000218 [Google Scholar]
- 25a.Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology. 2014;60:294–305.doi: 10.1159/000356760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fryar CD, C M, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. 2020. Accessed April 28, 2023. https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/overweight-obesity-adults-H.pdf
- 27.Stephen WC, Janssen I. Sarcopenic-obesity and cardiovascular disease risk in the elderly. J Nutr Health Aging. 2009;13:460–466. doi: 10.1007/s12603-009-0084-z [DOI] [PubMed] [Google Scholar]
- 28.Farmer RE, Mathur R, Schmidt AF, Bhaskaran K, Fatemifar G, Eastwood SV, Finan C, Denaxas S, Smeeth L, Chaturvedi N. Associations between measures of sarcopenic obesity and risk of cardiovascular disease and mortality: a cohort study and Mendelian randomization analysis using the UK Biobank. J Am Heart Assoc. 2019;8:e011638. doi: 10.1161/JAHA.118.011638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abete I, Konieczna J, Zulet MA, Galmés-Panades AM, Ibero-Baraibar I, Babio N, Estruch R, Vidal J, Toledo E, Razquin C, et al. ; PREDIMED-PLUS Investigators. Association of lifestyle factors and inflammation with sarcopenic obesity: data from the PREDIMED-Plus trial. J Cachexia Sarcopenia Muscle. 2019;10:974–984. doi: 10.1002/jcsm.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark BC, Manini TM. What is dynapenia? Nutrition. 2012;28:495–503. doi: 10.1016/j.nut.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark BC. Neuromuscular changes with aging and sarcopenia. J Frailty Aging. 2019;8:7–9. doi: 10.14283/jfa.2018.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark BC, Carson RG. Sarcopenia and neuroscience: learning to communicate. J Gerontol A Biol Sci Med Sci. 2021;76:1882–1890. doi: 10.1093/gerona/glab098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukunishi S, Asai A, Yokohama K, Nishiguchi S, Higuchi K. Pathophysiology and mechanisms of primary sarcopenia (review). Int J Mol Med. 2021;48:156. doi: 10.3892/ijmm.2021.4989 [DOI] [PubMed] [Google Scholar]
- 34.Bilski J, Pierzchalski P, Szczepanik M, Bonior J, Zoladz JA. Multifactorial mechanism of sarcopenia and sarcopenic obesity. Role of physical exercise, microbiota and myokines. Cells. 2022;11:160. doi: 10.3390/cells11010160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018;47:123–132. doi: 10.1016/j.arr.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cretoiu D, Pavelescu L, Duica F, Radu M, Suciu N, Cretoiu SM. Myofibers. In: Advances in Experimental Medicine and Biology. Springer Singapore, 2018:23–46. [DOI] [PubMed] [Google Scholar]
- 37.Schiaffino S, Reggiani C, Murgia M. Fiber type diversity in skeletal muscle explored by mass spectrometry-based single fiber proteomics. Histol Histopathol. 2020;35:239–246. doi: 10.14670/HH-18-170 [DOI] [PubMed] [Google Scholar]
- 38.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Aging-related loss of muscle mass and function. Physiol Rev. 2019;99:427–511. doi: 10.1152/physrev.00061.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csete ME. Basic science of frailty-biological mechanisms of age-related sarcopenia. Anesth Analg. 2021;132:293–304. doi: 10.1213/ANE.0000000000005096 [DOI] [PubMed] [Google Scholar]
- 41.Correa-de-Araujo R, Addison O, Miljkovic I, Goodpaster BH, Bergman BC, Clark RV, Elena JW, Esser KA, Ferrucci L, Harris-Love MO, et al. Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Front Physiol. 2020;11:963. doi: 10.3389/fphys.2020.00963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, Newman AB, Simonsick EM, Studenski SA, Nicklas BJ, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97:552–560. doi: 10.3945/ajcn.112.047860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2006;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x [DOI] [PubMed] [Google Scholar]
- 44.Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology. 2014;60:294–305. doi: 10.1159/000356760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dao T, Green AE, Kim YA, Bae SJ, Ha KT, Gariani K, Lee MR, Menzies KJ, Ryu D. Sarcopenia and muscle aging: a brief overview. Endocrinol Metab (Seoul). 2020;35:716–732. doi: 10.3803/EnM.2020.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje [DOI] [PubMed] [Google Scholar]
- 47.Lee EJ, Neppl RL. Influence of age on skeletal muscle hypertrophy and atrophy signaling: established paradigms and unexpected links. Genes (Basel). 2021;12:688. doi: 10.3390/genes12050688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banks NF, Rogers EM, Church DD, Ferrando AA, Jenkins NDM. The contributory role of vascular health in age-related anabolic resistance. J Cachexia Sarcopenia Muscle. 2022;13:114–127. doi: 10.1002/jcsm.12898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohara M, Kohara K, Tabara Y, Ochi M, Nagai T, Igase M, Miki T. Sarcopenic obesity and arterial stiffness, pressure wave reflection and central pulse pressure: the J-SHIPP study. Int J Cardiol. 2014;174:214–217. doi: 10.1016/j.ijcard.2014.03.194 [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Guo Q, Feng B-L, Wang C-Y, Han P-P, Hu J, Sun X-D, Zeng W-F, Zheng Z-X, Li H-S, et al. A cross-sectional study of the association between arterial stiffness and sarcopenia in Chinese community-dwelling elderly using the Asian Working Group for Sarcopenia Criteria. J Nutr Health Aging. 2019;23:195–201. doi: 10.1007/s12603-018-1147-9 [DOI] [PubMed] [Google Scholar]
- 51.Tap L, Kirkham FA, Mattace-Raso F, Joly L, Rajkumar C, Benetos A. Unraveling the links underlying arterial stiffness, bone demineralization, and muscle loss. Hypertension. 2020;76:629–639. doi: 10.1161/HYPERTENSIONAHA.120.15184 [DOI] [PubMed] [Google Scholar]
- 52.Jeon YK, Shin MJ, Saini SK, Custodero C, Aggarwal M, Anton SD, Leeuwenburgh C, Mankowski RT. Vascular dysfunction as a potential culprit of sarcopenia. Exp Gerontol. 2021;145:111220. doi: 10.1016/j.exger.2020.111220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Timmerman KL, Volpi E. Endothelial function and the regulation of muscle protein anabolism in older adults. Nutr Metab Cardiovasc Dis. 2013;23:S44–S50. doi: 10.1016/j.numecd.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 56.Nishikawa H, Fukunishi S, Asai A, Yokohama K, Nishiguchi S, Higuchi K. Pathophysiology and mechanisms of primary sarcopenia (review). Int J Mol Med. 2021;48:156. doi: 10.3892/ijmm.2021.4989 [DOI] [PubMed] [Google Scholar]
- 57.Buchmann N, Fielitz J, Spira D, König M, Norman K, Pawelec G, Goldeck D, Demuth I, Steinhagen-Thiessen E. Muscle mass and inflammation in older adults: impact of the metabolic syndrome. Gerontology. 2022;68:989–998. doi: 10.1159/000520096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin J, Lu X, Qian Z, Xu W, Zhou X. New insights into the pathogenesis and treatment of sarcopenia in chronic heart failure. Theranostics. 2019;9:4019–4029. doi: 10.7150/thno.33000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45:2121–2129. doi: 10.1016/j.biocel.2013.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Haehling S, Schefold JC, Lainscak M, Doehner W, Anker SD. Inflammatory biomarkers in heart failure revisited: much more than innocent bystanders. Heart Fail Clin. 2009;5:549–560. doi: 10.1016/j.hfc.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 62.Bouzid MA, Filaire E, McCall A, Fabre C. Radical oxygen species, exercise and aging: an update. Sports Med. 2015;45:1245–1261. doi: 10.1007/s40279-015-0348-1 [DOI] [PubMed] [Google Scholar]
- 63.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98:9306–9311. doi: 10.1073/pnas.151270098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bossone E, Arcopinto M, Iacoviello M, Triggiani V, Cacciatore F, Maiello C, Limongelli G, Masarone D, Perticone F, Sciacqua A, et al. ; TOSCA Investigators. Multiple hormonal and metabolic deficiency syndrome in chronic heart failure: rationale, design, and demographic characteristics of the T.O.S.CA. Registry. Intern Emerg Med. 2018;13:661–671. doi: 10.1007/s11739-018-1844-8 [DOI] [PubMed] [Google Scholar]
- 65.Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, Casaburi R, Bhasin S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–1485. doi: 10.1210/jc.2002-021231 [DOI] [PubMed] [Google Scholar]
- 66.Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, et al. Ghrelin. Mol Metab. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Onder G, Liperoti R, Russo A, Soldato M, Capoluongo E, Volpato S, Cesari M, Ameglio F, Bernabei R, Landi F. Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol Endocrinol Metab. 2006;291:E829–E834. doi: 10.1152/ajpendo.00138.2006 [DOI] [PubMed] [Google Scholar]
- 68.I͘lhan B, Bahat G, Erdog˘an T, Kiliç C, Karan MA. Anorexia is independently associated with decreased muscle mass and strength in community dwelling older adults. J Nutr Health Aging. 2019;23:202–206. doi: 10.1007/s12603-018-1119-0 [DOI] [PubMed] [Google Scholar]
- 69.Russ DW, Gregg-Cornell K, Conaway MJ, Clark BC. Evolving concepts on the age-related changes in “muscle quality.” J Cachexia Sarcopenia Muscle. 2012;3:95–109. doi: 10.1007/s13539-011-0054-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, Der G, Gale CR, Inskip HM, Jagger C, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9:e113637. doi: 10.1371/journal.pone.0113637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83:1581–1587. doi: 10.1152/jappl.1997.83.5.1581 [DOI] [PubMed] [Google Scholar]
- 72.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059 [DOI] [PubMed] [Google Scholar]
- 73.Evans WJ, Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50A:11–16. doi: 10.1093/gerona/50A.Special_Issue.11 [DOI] [PubMed] [Google Scholar]
- 74.Gustafsson T, Sarcopenia UB. What is the origin of this aging-induced disorder? Front Genet. 2021;12:688526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tournadre A, Vial G, Capel F, Soubrier M, Boirie Y. Sarcopenia. Joint Bone Spine. 2019;86:309–314. doi: 10.1016/j.jbspin.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 76.Kim JW, Kim R, Choi H, Lee SJ, Bae GU. Understanding of sarcopenia: from definition to therapeutic strategies. Arch Pharm Res. 2021;44:876–889. doi: 10.1007/s12272-021-01349-z [DOI] [PubMed] [Google Scholar]
- 77.Lutski M, Weinstein G, Tanne D, Goldbourt U. Overweight, obesity, and late-life sarcopenia among men with cardiovascular disease, Israel. Prev Chronic Dis. 2020;17:E164. doi: 10.5888/pcd17.200167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc. 2014;62:253–260. doi: 10.1111/jgs.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stenvinkel P, Larsson TE. Chronic kidney disease: a clinical model of premature aging. Am J Kidney Dis. 2013;62:339–351. doi: 10.1053/j.ajkd.2012.11.051 [DOI] [PubMed] [Google Scholar]
- 80.Fahal IH, Bell GM, Bone JM, Edwards RH. Physiological abnormalities of skeletal muscle in dialysis patients. Nephrol Dial Transplant. 1997;12:119–127. doi: 10.1093/ndt/12.1.119 [DOI] [PubMed] [Google Scholar]
- 81.Johansen KL, Shubert T, Doyle J, Soher B, Sakkas GK, Kent-Braun JA. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63:291–297. doi: 10.1046/j.1523-1755.2003.00704.x [DOI] [PubMed] [Google Scholar]
- 82.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030 [DOI] [PubMed] [Google Scholar]
- 83.Anjanappa M, Corden M, Green A, Roberts D, Hoskin P, McWilliam A, Choudhury A. Sarcopenia in cancer: risking more than muscle loss. Tech Innov Patient Support Radiat Oncol. 2020;16:50–57. doi: 10.1016/j.tipsro.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Zhang J, Ni W, Yuan X, Zhang H, Li P, Xu J, Zhao Z. Sarcopenia in heart failure: a systematic review and meta-analysis. ESC Heart Fail. 2021;8:1007–1017. doi: 10.1002/ehf2.13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Attaway A, Bellar A, Dieye F, Wajda D, Welch N, Dasarathy S. Clinical impact of compound sarcopenia in hospitalized older adult patients with heart failure. J Am Geriatr Soc. 2021;69:1815–1825. doi: 10.1111/jgs.17108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Onoue Y, Izumiya Y, Hanatani S, Tanaka T, Yamamura S, Kimura Y, Araki S, Sakamoto K, Tsujita K, Yamamoto E, et al. A simple sarcopenia screening test predicts future adverse events in patients with heart failure. Int J Cardiol. 2016;215:301–306. doi: 10.1016/j.ijcard.2016.04.128 [DOI] [PubMed] [Google Scholar]
- 87.Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, Garten R, Rodriguez-Miguelez P, Guazzi M, Lavie CJ, et al. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2209–2225. doi: 10.1016/j.jacc.2019.01.072 [DOI] [PubMed] [Google Scholar]
- 88.Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, Loncar G, Springer J, Doehner W, Lainscak M, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-Morbidities Aggravating Heart Failure (SICA-HF). Eur J Heart Fail. 2018;20:1580–1587. doi: 10.1002/ejhf.1304 [DOI] [PubMed] [Google Scholar]
- 89.Zhang N, Zhu WL, Liu XH, Chen W, Zhu ML, Kang L, Tian R. Prevalence and prognostic implications of sarcopenia in older patients with coronary heart disease. J Geriatr Cardiol. 2019;16:756–763. doi: 10.11909/j.issn.1671-5411.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang DO, Park SY, Choi BG, Na JO, Choi CU, Kim EJ, Rha SW, Park CG, Hong SJ, Seo HS. Prognostic impact of low skeletal muscle mass on major adverse cardiovascular events in coronary artery disease: a propensity score-matched analysis of a single center all-comer cohort. J Clin Med. 2019;8:712. doi: 10.3390/jcm8050712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okamura H, Kimura N, Mieno M Yuri K Yamaguchi A. Preoperative sarcopenia is associated with late mortality after off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg. 2020;58:121–129. doi: 10.1093/ejcts/ezz378 [DOI] [PubMed] [Google Scholar]
- 92.Ko BJ, Chang Y, Jung HS, Yun KE, Kim CW, Park HS, Chung EC, Shin H, Ryu S. Relationship between low relative muscle mass and coronary artery calcification in healthy adults. Arterioscler Thromb Vasc Biol. 2016;36:1016–1021. doi: 10.1161/ATVBAHA.116.307156 [DOI] [PubMed] [Google Scholar]
- 93.Jun JE, Choi MS, Park SW, Kim G, Jin SM, Kim K, Hwang YC, Ahn KJ, Chung HY, Jeong IK, et al. Low skeletal muscle mass is associated with the presence, incidence, and progression of coronary artery calcification. Can J Cardiol. 2021;37:1480–1488. doi: 10.1016/j.cjca.2021.04.002 [DOI] [PubMed] [Google Scholar]
- 94.Xia MF, Chen LY, Wu L, Ma H, Li XM, Li Q, Aleteng Q, Hu Y, He WY, Gao J, et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: a cross-sectional study. Clin Nutr. 2021;40:571–580. doi: 10.1016/j.clnu.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 95.Pizzimenti M, Meyer A, Charles A-L, Giannini M, Chakfé N, Lejay A, Geny B. Sarcopenia and peripheral arterial disease: a systematic review. J Cachexia Sarcopenia Muscle. 2020;11:866–886. doi: 10.1002/jcsm.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pottecher J, Adamopoulos C, Lejay A, Bouitbir J, Charles AL, Meyer A, Singer M, Wolff V, Diemunsch P, Laverny G, et al. Diabetes worsens skeletal muscle mitochondrial function, oxidative stress, and apoptosis after lower-limb ischemia-reperfusion: implication of the RISK and SAFE pathways? Front Physiol. 2018;9:579. doi: 10.3389/fphys.2018.00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taniguchi R, Deguchi J, Hashimoto T, Sato O. Sarcopenia as a possible negative predictor of limb salvage in patients with chronic limb-threatening ischemia. Ann Vasc Dis. 2019;12:194–199. doi: 10.3400/avd.oa.18-00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shimazoe H, Mii S, Koyanagi Y, Ishida M. Impact of low activity of daily living on the prognosis of patients with critical limb ischemia and sarcopenia. Ann Vasc Surg. 2019;61:156–164. doi: 10.1016/j.avsg.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 99.Bertschi D, Kiss CM, Schoenenberger AW, Stuck AE, Kressig RW. Sarcopenia in patients undergoing transcatheter aortic valve implantation (TAVI): a systematic review of the literature. J Nutr Health Aging. 2021;25:64–70. doi: 10.1007/s12603-020-1448-7 [DOI] [PubMed] [Google Scholar]
- 100.Mok M, Allende R, Leipsic J, Altisent OA, Del Trigo M, Campelo-Parada F, DeLarochellière R, Dumont E, Doyle D, Côté M, et al. Prognostic value of fat mass and skeletal muscle mass determined by computed tomography in patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2016;117:828–833. doi: 10.1016/j.amjcard.2015.12.015 [DOI] [PubMed] [Google Scholar]
- 101.Nemec U, Heidinger B, Sokas C, Chu L, Eisenberg RL. Diagnosing sarcopenia on thoracic computed tomography: quantitative assessment of skeletal muscle mass in patients undergoing transcatheter aortic valve replacement. Acad Radiol. 2017;24:1154–1161. doi: 10.1016/j.acra.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 102.Tokuda T, Yamamoto M, Kagase A, Koyama Y, Otsuka T, Tada N, Naganuma T, Araki M, Yamanaka F, Shirai S, et al. ; OCEAN-TAVI Investigators. Importance of combined assessment of skeletal muscle mass and density by computed tomography in predicting clinical outcomes after transcatheter aortic valve replacement. Int J Cardiovasc Imaging. 2020;36:929–938. doi: 10.1007/s10554-020-01776-x [DOI] [PubMed] [Google Scholar]
- 103.Damluji AA, Rodriguez G, Noel T, Davis L, Dahya V, Tehrani B, Epps K, Sherwood M, Sarin E, Walston J, et al. Sarcopenia and health-related quality of life in older adults after transcatheter aortic valve replacement. Am Heart J. 2020;224:171–181. doi: 10.1016/j.ahj.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tzeng YH, Wei J, Tsao TP, Lee YT, Lee KC, Liou HR, Sung HJ, Huang KC, Hsiung MC, Yin WH. Computed tomography-determined muscle quality rather than muscle quantity is a better determinant of prolonged hospital length of stay in patients undergoing transcatheter aortic valve implantation. Acad Radiol. 2020;27:381–388. doi: 10.1016/j.acra.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 105.Garg L, Agrawal S, Pew T, Hanzel GS, Abbas AE, Gallagher MJ, Shannon FL, Hanson ID. Psoas muscle area as a predictor of outcomes in transcatheter aortic valve implantation. Am J Cardiol. 2017;119:457–460. doi: 10.1016/j.amjcard.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 106.Lee SA, Jang IY, Park SY, Kim KW, Park DW, Kim HJ, Kim JB, Jung SH, Choo SJ, Chung CH, et al. Benefit of sarcopenia screening in older patients undergoing surgical aortic valve replacement. Ann Thorac Surg. 2021;113:2018–2026. doi: 10.1016/j.athoracsur.2021.06.067 [DOI] [PubMed] [Google Scholar]
- 107.Mamane S, Mullie L, Lok Ok Choo W, Piazza N, Martucci G, Morais JA, Kim DH, Lauck S, Webb JG, Afilalo J; FRAILTY-AVR Investigators. Sarcopenia in older adults undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;74:3178–3180. doi: 10.1016/j.jacc.2019.10.030 [DOI] [PubMed] [Google Scholar]
- 108.Sakuyama A, Saitoh M, Iwai K, Kon K, Hori K, Nagayama M. Psoas muscle volume and attenuation are better predictors than muscle area for hospital readmission in older patients after transcatheter aortic valve implantation. Phys Ther Res. 2021;24:128–135. doi: 10.1298/ptr.E10079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Furzan A, Quraishi SA, Brovman E, Weintraub A, Connors A, Allen D, Patel PA, Cobey FC. Skeletal muscle characteristics may inform preprocedural risk stratification in transcatheter aortic valve replacement patients. J Cardiothorac Vasc Anesth. 2021;35:2618–2625. doi: 10.1053/j.jvca.2020.12.024 [DOI] [PubMed] [Google Scholar]
- 110.Arnold SV, Cohen DJ, Dai D, Jones PG, Li F, Thomas L, Baron SJ, Frankel NZ, Strong S, Matsouaka RA, et al. Predicting quality of life at 1 year after transcatheter aortic valve replacement in a real-world population. Circ Cardiovasc Qual Outcomes. 2018;11:e004693. doi: 10.1161/CIRCOUTCOMES.118.004693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hill A, Arora RC, Engelman DT, Stoppe C. Preoperative treatment of malnutrition and sarcopenia in cardiac surgery: new frontiers. Crit Care Clin. 2020;36:593–616. doi: 10.1016/j.ccc.2020.06.002 [DOI] [PubMed] [Google Scholar]
- 112.Okamura H, Kimura N Tanno K Mieno M Matsumoto H Yamaguchi A Adachi H. The impact of preoperative sarcopenia, defined based on psoas muscle area, on long-term outcomes of heart valve surgery. J Thorac Cardiovasc Surg. 2019;157:1071–1079.e3. doi: 10.1016/j.jtcvs.2018.06.098 [DOI] [PubMed] [Google Scholar]
- 113.Hawkins RB, Mehaffey JH, Charles EJ, Kern JA, Lim DS, Teman NR, Ailawadi G. Psoas muscle size predicts risk-adjusted outcomes after surgical aortic valve replacement. Ann Thorac Surg. 2018;106:39–45. doi: 10.1016/j.athoracsur.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hulzebos EH, van Meeteren NL. Making the elderly fit for surgery. Br J Surg. 2016;103:e12–e15. doi: 10.1002/bjs.10033 [DOI] [PubMed] [Google Scholar]
- 115.Alsadany MA, Sanad HT, Elbanouby MH, Ali S. Detecting a valid screening method for sarcopenia in acute care setting. J Frailty Sarcopenia Falls. 2021;6:111–118. doi: 10.22540/JFSF-06-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Knowles R, Carter J, Jebb SA, Bennett D, Lewington S, Piernas C. Associations of skeletal muscle mass and fat mass with incident cardiovascular disease and all-cause mortality: a prospective cohort study of UK Biobank participants. J Am Heart Assoc. 2021;10:e019337. doi: 10.1161/jaha.120.019337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes. 2016;40:761–767. doi: 10.1038/ijo.2015.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wannamethee SG, Atkins JL. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc. 2015;74:405–412. doi: 10.1017/s002966511500169x [DOI] [PubMed] [Google Scholar]
- 119.Sharma S Batsis JA, Coutinho T Somers VK Hodge DO Carter RE Sochor O Kragelund C Kanaya AM Zeller M et al. Normal-weight central obesity and mortality risk in older adults with coronary artery disease. Mayo Clin Proc. 2016;91:343–351. doi: 10.1016/j.mayocp.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 120.de Hollander EL, Bemelmans WJ, Boshuizen HC, Friedrich N, Wallaschofski H, Guallar-Castillón P, Walter S, Zillikens MC, Rosengren A, Lissner L, et al. ; WC Elderly Collaborators. The association between waist circumference and risk of mortality considering body mass index in 65- to 74-year-olds: a meta-analysis of 29 cohorts involving more than 58 000 elderly persons. Int J Epidemiol. 2012;41:805–817. doi: 10.1093/ije/dys008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chianca V, Albano D, Messina C, Gitto S, Ruffo G, Guarino S, Del Grande F, Sconfienza LM. Sarcopenia: imaging assessment and clinical application. Abdom Radiol (NY). 2022;47:3205–3216. doi: 10.1007/s00261-021-03294-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.1151152/jappl.1151998.11585 [DOI] [PubMed] [Google Scholar]
- 123.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 124.Perkisas S, Baudry S, Bauer J, Beckwée D, De Cock AM, Hobbelen H, Jager-Wittenaar H, Kasiukiewicz A, Landi F, Marco E, et al. Application of ultrasound for muscle assessment in sarcopenia: towards standardized measurements. Eur Geriatr Med. 2018;9:739–757. doi: 10.1007/s41999-018-0104-9 [DOI] [PubMed] [Google Scholar]
- 125.Abe T, Loenneke JP, Young KC, Thiebaud RS, Nahar VK, Hollaway KM, Stover CD, Ford MA, Bass MA, Loftin M. Validity of ultrasound prediction equations for total and regional muscularity in middle-aged and older men and women. Ultrasound Med Biol. 2015;41:557–564. doi: 10.1016/j.ultrasmedbio.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 126.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. doi: 10.1093/gerona/glr010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D(3) -creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:14–21. doi: 10.1002/jcsm.12390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sergi G, De Rui M, Stubbs B, Veronese N, Manzato E. Measurement of lean body mass using bioelectrical impedance analysis: a consideration of the pros and cons. Aging Clin Exp Res. 2017;29:591–597. doi: 10.1007/s40520-016-0622-6 [DOI] [PubMed] [Google Scholar]
- 129.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 130.Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D3-creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:14–21. doi: 10.1002/jcsm.12390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;S1071-9164(21)00050-6. doi: 10.1016/j.cardfail.2021.01.022 [DOI] [PubMed] [Google Scholar]
- 132.Clark RV, Walker AC, O’Connor-Semmes RL, Leonard MS, Miller RR, Stimpson SA, Turner SM, Ravussin E, Cefalu WT, Hellerstein MK. Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol. 2014;116:1605–1613. doi: 10.1152/japplphysiol.00045.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cawthon P, Orwoll E, Peters K, Ensrud K, Cauley J, Kado D, Stefanick M, Shikany J, Strotmeyer E, Glynn N. Osteoporotic G fractures in men study research, strong relation between muscle mass determined by D3-creatine dilution, physical performance and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2018;74:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim SW, Jung HW, Kim CH, Kim KI, Chin HJ, Lee H. A new equation to estimate muscle mass from creatinine and cystatin C. PLoS One. 2016;11:e0148495. doi: 10.1371/journal.pone.0148495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang T, Zhuo Y, Xie L, Wang H, Yang M. Sarcopenia index based on serum creatinine and cystatin C is associated with 3-year mortality in hospitalized older patients. Sci Rep. 2020;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee HS, Park KW, Kang J, Ki Y-J, Chang M, Han J-K, Yang H-M, Kang H-J, Koo B-K, Kim H-S. Sarcopenia index as a predictor of clinical outcomes in older patients with coronary artery disease. J Clin Med. 2020;9:3121. doi: 10.3390/jcm9103121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Amado CA, Ruiz de Infante MM. Sarcopenia index: more than an marker of muscle mass. Clin Nutr. 2019;38:1479. doi: 10.1016/j.clnu.2019.02.043 [DOI] [PubMed] [Google Scholar]
- 138.Trevisan C, Vetrano DL, Calvani R, Picca A, Welmer AK. Twelve-year sarcopenia trajectories in older adults: results from a population-based study. J Cachexia Sarcopenia Muscle. 2022;13:254–263. doi: 10.1002/jcsm.12875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985). 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taaffe DR, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47:1208–1214. doi: 10.1111/j.1532-5415.1999.tb05201.x [DOI] [PubMed] [Google Scholar]
- 141.Liang Y Wang R, Jiang J Tan L Yang M. A randomized controlled trial of resistance and balance exercise for sarcopenic patients aged 80-99 years. Sci Rep. 2020;10:18756. doi: 10.1038/s41598-020-75872-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Snijders T, Nederveen JP Bell KE Lau SW Mazara N Kumbhare DA Phillips SM Parise G. Prolonged exercise training improves the acute type II muscle fibre satellite cell response in healthy older men. J Physiol. 2019;597:105–119. doi: 10.1113/JP276260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moro T, Brightwell CR, Volpi E, Rasmussen BB, Fry CS. Resistance exercise training promotes fiber type-specific myonuclear adaptations in older adults. J Appl Physiol (1985). 2020;128:795–804. doi: 10.1152/japplphysiol.00723.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2001;56:B240–B247. doi: 10.1093/gerona/56.6.b240 [DOI] [PubMed] [Google Scholar]
- 145.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- 146.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286:E92–101. doi: 10.1152/ajpendo.00366.2003 [DOI] [PubMed] [Google Scholar]
- 147.Burton LA, Sumukadas D. Optimal management of sarcopenia. Clin Interv Aging. 2010;5:217–228. doi: 10.2147/cia.s11473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gielen S, Adams V, Möbius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9 [DOI] [PubMed] [Google Scholar]
- 149.Alfaraidhy MA, Regan C, Forman DE. Cardiac rehabilitation for older adults: current evidence and future potential. Expert Rev Cardiovasc Ther. 2022;20:13–34. doi: 10.1080/14779072.2022.2035722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lutz AH, Forman DE. Cardiac rehabilitation in older adults: apropos yet significantly underutilized. Prog Cardiovasc Dis. 2022;70:94–101. doi: 10.1016/j.pcad.2022.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harada H, Kai H, Niiyama H, Nishiyama Y, Katoh A, Yoshida N, Fukumoto Y, Ikeda H. Effectiveness of cardiac rehabilitation for prevention and treatment of sarcopenia in patients with cardiovascular disease - a retrospective cross-sectional analysis. J Nutr Health Aging. 2017;21:449–456. doi: 10.1007/s12603-016-0743-9 [DOI] [PubMed] [Google Scholar]
- 152.Delmonico MJ, Beck DT. The current understanding of sarcopenia. Am J Lifestyle Med. 2017;11:167–181. doi: 10.1177/1559827615594343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznariç Z, Nair KS, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929–936. doi: 10.1016/j.clnu.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132:S32193219s–S3219S3224. doi: 10.1093/jn/131.10.3219s [DOI] [PubMed] [Google Scholar]
- 155.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003 [DOI] [PubMed] [Google Scholar]
- 156.Stokes T, Hector AJ, Morton RW, McGlory C, Phillips SM. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients. 2018;10:180180. doi: 10.3390/nu10020180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (1985). 2009;107:987–992. doi: 10.1152/japplphysiol.00076.2009 [DOI] [PubMed] [Google Scholar]
- 158.Iglay HB, Thyfault JP, Apolzan JW, Campbell WW. Resistance training and dietary protein: effects on glucose tolerance and contents of skeletal muscle insulin signaling proteins in older persons. Am J Clin Nutr. 2007;85:1005–1013. doi: 10.1093/ajcn/85.4.1005 [DOI] [PubMed] [Google Scholar]
- 159.Andrews RD, MacLean DA, Riechman SE. Protein intake for skeletal muscle hypertrophy with resistance training in seniors. Int J Sport Nutr Exerc Metab. 2006;16:362–372. doi: 10.1123/ijsnem.16.4.362 [DOI] [PubMed] [Google Scholar]
- 160.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shin MJ, Jeon YK, Kim IJ. Testosterone and sarcopenia. World J Mens Health. 2018;36:192–198. doi: 10.5534/wjmh.180001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA, Wu FC. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. doi: 10.1210/jc.2009-1251 [DOI] [PubMed] [Google Scholar]
- 163.Storer TW, Basaria S, Traustadottir T, Harman SM, Pencina K, Li Z, Travison TG, Miciek R, Tsitouras P, Hally K, et al. Effects of testosterone supplementation for 3 years on muscle performance and physical function in older men. J Clin Endocrinol Metab. 2017;102:583–593. doi: 10.1210/jc.2016-2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Travison TG, Basaria S, Storer TW, Jette AM, Miciek R, Farwell WR, Choong K, Lakshman K, Mazer NA, Coviello AD, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66:1090–1099. doi: 10.1093/gerona/glr100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Barnouin Y, Armamento-Villareal R, Celli A, Jiang B, Paudyal A, Nambi V, Bryant MS, Marcelli M, Garcia JM, Qualls C, et al. Testosterone replacement therapy added to intensive lifestyle intervention in older men with obesity and hypogonadism. J Clin Endocrinol Metab. 2021;106:e1096–e1110. doi: 10.1210/clinem/dgaa917 [DOI] [PubMed] [Google Scholar]
- 166.Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, Morton RA, Steiner MS. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–161. doi: 10.1007/s13539-011-0034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fonseca G, Dworatzek E, Ebner N, Von Haehling S. Selective androgen receptor modulators (SARMs) as pharmacological treatment for muscle wasting in ongoing clinical trials. Expert Opin Investig Drugs. 2020;29:881–891. doi: 10.1080/13543784.2020.1777275 [DOI] [PubMed] [Google Scholar]
- 168.Bedi H, Hammond C, Sanders D, Yang HM, Yoshida EM. Drug-induced liver injury from enobosarm (Ostarine), a selective androgen receptor modulator. ACG Case Rep J. 2021;8:e00518. doi: 10.14309/crj.0000000000000518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Springer J, von Haehling S. ACE inhibitors and sarcopenia: covering all the BASEs?. Drugs Aging. 2016;33:839–840. doi: 10.1007/s40266-016-0417-7 [DOI] [PubMed] [Google Scholar]
- 170.Giovannini S, Cesari M Marzetti E Leeuwenburgh C Maggio M Pahor M. Effects of ACE-inhibition on IGF-1 and IGFBP-3 concentrations in older adults with high cardiovascular risk profile. J Nutr Health Aging. 2010;14:457–460. doi: 10.1007/s12603-010-0036-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Maggio M, Ceda GP, Lauretani F, Pahor M, Bandinelli S, Najjar SS, Ling SM, Basaria S, Ruggiero C, Valenti G, et al. Relation of angiotensin-converting enzyme inhibitor treatment to insulin-like growth factor-1 serum levels in subjects >65 years of age (the InCHIANTI study). Am J Cardiol. 2006;97:1525–1529. doi: 10.1016/j.amjcard.2005.11.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Onder G, Penninx BW, Balkrishnan R, Fried LP, Chaves PH, Williamson J, Carter C, Di Bari M, Guralnik JM, Pahor M. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930. doi: 10.1016/s0140-6736(02)08024-8 [DOI] [PubMed] [Google Scholar]
- 173.Justice JN, Ferrucci L, Newman AB, Aroda VR, Bahnson JL, Divers J, Espeland MA, Marcovina S, Pollak MN, Kritchevsky SB, et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. Geroscience. 2018;40:419–436. doi: 10.1007/s11357-018-0042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Picca A, Beli R, Calvani R, Coelho-Júnior HJ, Landi F, Bernabei R, Bucci C, Guerra F, Marzetti E. Older adults with physical frailty and sarcopenia show increased levels of circulating small extracellular vesicles with a specific mitochondrial signature. Cells. 2020;9:973. doi: 10.3390/cells9040973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Calvani R, Picca A, Marini F, Biancolillo A, Gervasoni J, Persichilli S, Primiano A, Coelho-Junior HJ, Cesari M, Bossola M. Identification of biomarkers for physical frailty and sarcopenia through a new multi-marker approach: results from the BIOSPHERE study. GeroScience. 2021;43:727–740. doi: 10.1007/s11357-020-00197-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Critchley M. The neurology of old age. Lancet. 1931;217:11191221–11191127. doi: 10.1016/s0140-6736(00)90705-0 [Google Scholar]
- 177.Shock NW. Physiologic aspects of aging. J Am Diet Assoc. 1970;56:491–496. [PubMed] [Google Scholar]
- 178.Tzankoff SP, Norris AH. Effect of muscle mass decrease on age-related BMR changes. JAppl Physiol Respir Environ Exerc Physiol. 1977;43:1001–1006. doi: 10.1152/jappl.1977.43.6.1001 [DOI] [PubMed] [Google Scholar]
- 179.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x [DOI] [PubMed] [Google Scholar]
- 180.Freeman LM, Roubenoff R. The nutrition implications of cardiac cachexia. Nutr Rev. 1994;52:340–347. doi: 10.1111/j.1753-4887.1994.tb01358.x [DOI] [PubMed] [Google Scholar]
- 181.Anker SD, Ponikowski P Varney S Chua TP Clark AL Webb-Peploe KM Harrington D Kox WJ Poole-Wilson PA Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/s0140-6736(96)07015-8 [DOI] [PubMed] [Google Scholar]
- 182.Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–1083. doi: 10.1016/s0140-6736(03)12892-9 [DOI] [PubMed] [Google Scholar]
- 183.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 184.Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. 2013;34:512–519. doi: 10.1093/eurheartj/ehs381 [DOI] [PubMed] [Google Scholar]
- 185.Steinbeck L, Ebner N, Valentova M, Bekfani T, Elsner S, Dahinden P, Hettwer S, Scherbakov N, Schefold JC, Sandek A, et al. Detection of muscle wasting in patients with chronic heart failure using C-terminal agrin fragment: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur J Heart Fail. 2015;17:1283–1293. doi: 10.1002/ejhf.400 [DOI] [PubMed] [Google Scholar]
- 186.Ishii S, Tanaka T Shibasaki K Ouchi Y Kikutani T Higashiguchi T Obuchi SP Ishikawa-Takata K Hirano H Kawai H et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014;14:93–101. doi: 10.1111/ggi.12197 [DOI] [PubMed] [Google Scholar]
- 187.Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V, Schefold JC, et al. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol. 2016;222:41–46. doi: 10.1016/j.ijcard.2016.07.135 [DOI] [PubMed] [Google Scholar]
- 188.Hajahmadi M, Shemshadi S, Khalilipur E, Amin A, Taghavi S, Maleki M, Malek H, Naderi N. Muscle wasting in young patients with dilated cardiomyopathy. J Cachexia Sarcopenia Muscle. 2017;8:542–548. doi: 10.1002/jcsm.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tsuchida K, Fujihara Y, Hiroki J, Hakamata T, Sakai R, Nishida K, Sudo K, Tanaka K, Hosaka Y, Takahashi K, et al. Significance of sarcopenia evaluation in acute decompensated heart failure. Int Heart J. 2018;59:143–148. doi: 10.1536/ihj.17-057 [DOI] [PubMed] [Google Scholar]
- 190.Nozaki Y, Yamaji M, Nishiguchi S, Fukutani N, Tashiro Y, Shirooka H, Hirata H, Yamaguchi M, Tasaka S, Matsubara K, et al. Sarcopenia predicts adverse outcomes in an elderly outpatient population with New York Heart Association class II–IV heart failure: a prospective cohort study. Aging Med Healthcare. 2019;10:53–61. doi: 10.33879/AMH.2019.1809 [Google Scholar]
- 191.Fonseca GWPD, Santos MRD, Souza FR, Costa MJAD, Haehling SV, Takayama L, Pereira RMR, Negrão CE, Anker SD, Alves MJNN. Sympatho-vagal imbalance is associated with sarcopenia in male patients with heart failure. Arq Bras Cardiol. 2019;112:739–746. doi: 10.5935/abc.20190061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Canteri AL, Gusmon LB, Zanini AC, Nagano FE, Rabito EI, Petterle RR, Jonasson TH, Boguszewski CL, Borba VZC. Sarcopenia in heart failure with reduced ejection fraction. Am J Cardiovasc Dis. 2019;9:116–126. [PMC free article] [PubMed] [Google Scholar]
- 193.Kono Y, Izawa H, Aoyagi Y, Ishikawa A, Sugiura T, Mori E, Ueda S, Fujiwara W, Hayashi M, Saitoh E. The difference in determinant factor of six-minute walking distance between sarcopenic and non-sarcopenic elderly patients with heart failure. J Cardiol. 2020;75:42–46. doi: 10.1016/j.jjcc.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 194.Fonseca GWPD, Dos Santos MR, de Souza FR, Takayama L, Rodrigues Pereira RM, Negrão CE, Alves MNN. Discriminating sarcopenia in overweight/obese male patients with heart failure: the influence of body mass index. ESC Heart Fail. 2020;7:84–91. doi: 10.1002/ehf2.12545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eschalier R, Massoullié G Boirie Y Blanquet M Mulliez A Tartière PL Anker S D’Agrosa Boiteux MC Richard R Jean F et al. Sarcopenia in patients after an episode of acute decompensated heart failure: an underdiagnosed problem with serious impact. Clin Nutr. 2021;40:4490–4499. doi: 10.1016/j.clnu.2020.12.033 [DOI] [PubMed] [Google Scholar]
- 196.Kang DO, Park SY, Choi BG, Na JO, Choi CU, Kim EJ, Rha SW, Park CG, Hong SJ, Seo HS. Prognostic impact of low skeletal muscle mass on major adverse cardiovascular events in coronary artery disease: a propensity score-matched analysis of a single center all-comer cohort. J Clin Med. 2019;8:712. doi: 10.3390/jcm8050712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lee HS, Park KW, Kang J, Ki YJ, Chang M, Han JK, Yang HM, Kang HJ, Koo BK, Kim HS. Sarcopenia index as a predictor of clinical outcomes in older patients with coronary artery disease. J Clin Med. 2020;9:3121. doi: 10.3390/jcm9103121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Campos AM, Moura FA, Santos SN, Freitas WM, Sposito AC, Brasilia Study on Healthy Aging and Brasilia Heart Study. Sarcopenia, but not excess weight or increased caloric intake, is associated with coronary subclinical atherosclerosis in the very elderly. Atherosclerosis. 2017;258:138–144. doi: 10.1016/j.atherosclerosis.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 199.Lin GM, Li YH, Lai CP, Lin CL, Wang JH. The obesity-mortality paradox in elderly patients with angiographic coronary artery disease: a report from the ET-CHD registry. Acta Cardiol. 2015;70:479–486. doi: 10.1080/ac.70.4.3096897 [DOI] [PubMed] [Google Scholar]
- 200.Diercks DB, Roe MT, Mulgund J, Pollack CV, Kirk JD, Gibler WB, Ohman EM, Smith SC, Boden WE, Peterson ED. The obesity paradox in non-ST-segment elevation acute coronary syndromes: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J. 2006;152:140–148. doi: 10.1016/j.ahj.2005.09.024 [DOI] [PubMed] [Google Scholar]
- 201.Goel K, Gulati R, Reeder GS, Lennon RJ, Lewis BR, Behfar A, Sandhu GS, Rihal CS, Singh M. Low body mass index, serum creatinine, and cause of death in patients undergoing percutaneous coronary intervention. J Am Heart Assoc. 2016;5:e003633. doi: 10.1161/JAHA.116.003633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Uchida S, Kamiya K, Hamazaki N, Matsuzawa R, Nozaki K, Ichikawa T, Suzuki Y, Nakamura T, Yamashita M, Kariya H, et al. Association between sarcopenia and atherosclerosis in elderly patients with ischemic heart disease. Heart Vessels. 2020;35:769–775. doi: 10.1007/s00380-020-01554-8 [DOI] [PubMed] [Google Scholar]
- 203.Leistner DM, Bazara S, Münch C, Steiner J, Erbay A, Siegrist PT, Skurk C, Lauten A, Müller-Werdan U, Landmesser U, et al. Association of the body mass index with outcomes in elderly patients (≥80 years) undergoing percutaneous coronary intervention. Int J Cardiol. 2019;292:73–77. doi: 10.1016/j.ijcard.2019.06.044 [DOI] [PubMed] [Google Scholar]
- 204.Li Z, Tong X Ma Y Bao T Yue J. Relationship between low skeletal muscle mass and arteriosclerosis in Western China: a cross-sectional study. Front Cardiovasc Med. 2021;8:735262. doi: 10.3389/fcvm.2021.735262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nichols S, O’Doherty AF, Taylor C, Clark AL, Carroll S, Ingle L. Low skeletal muscle mass is associated with low aerobic capacity and increased mortality risk in patients with coronary heart disease - a CARE CR study. Clin Physiol Funct Imaging. 2019;39:93–102. doi: 10.1111/cpf.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu HM, Zhang Q, Shen WD, Li BY, Lv WQ, Xiao HM, Deng HW. Sarcopenia-related traits and coronary artery disease: a bi-directional Mendelian randomization study. Aging (Albany NY). 2020;12:3340–3353. doi: 10.18632/aging.102815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang YP, Yao SH, Liu D, Shen T, Zhao W, Gao W, Xu SL. [Relationships between percentage of skeletal muscle mass and cardiorespiratory fitness in elderly patients with coronary heart disease]. Zhonghua Yi Xue Za Zhi. 2018;98:831–836. doi: 10.3760/cma.j.issn.0376-2491.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 208.Gallone G, Depaoli A, D’Ascenzo F, Tore D, Allois L, Bruno F, Casale M, Atzeni F, De Lio G, Bocchino PP, et al. Impact of computed-tomography defined sarcopenia on outcomes of older adults undergoing transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr. 2021;16:207–214. doi: 10.1016/j.jcct.2021.12.001 [DOI] [PubMed] [Google Scholar]
- 209.Dahya V, Xiao J, Prado CM, Burroughs P, McGee D, Silva AC, Hurt JE, Mohamed SG, Noel T, Batchelor W. Computed tomography-derived skeletal muscle index: a novel predictor of frailty and hospital length of stay after transcatheter aortic valve replacement. Am Heart J. 2016;182:21–27. doi: 10.1016/j.ahj.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 210.Heidari B, Al-Hijji MA, Moynagh MR, Takahashi N, Welle G, Eleid M, Singh M, Gulati R, Rihal CS, Lerman A. Transcatheter aortic valve replacement outcomes in patients with sarcopaenia. EuroIntervention. 2019;15:671–677. doi: 10.4244/EIJ-D-19-00110 [DOI] [PubMed] [Google Scholar]
- 211.Kleczynski P, Tokarek T, Dziewierz A, Sorysz D, Bagienski M, Rzeszutko L, Dudek D. Usefulness of psoas muscle area and volume and frailty scoring to predict outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2018;122:135–140. doi: 10.1016/j.amjcard.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 212.Krishnan A, Suarez-Pierre A, Zhou X, Lin CT, Fraser CD, 3rd, Crawford TC, Hsu J, Hasan RK, Resar J, Chacko M, et al. Comparing frailty markers in predicting poor outcomes after transcatheter aortic valve replacement. Innovations (Phila).2019;14:43–54. doi: 10.1177/1556984519827698 [DOI] [PubMed] [Google Scholar]
- 213.Lee SA, Jang IY, Park SY, Kim KW, Park DW, Kim HJ, Kim JB, Jung SH, Choo SJ, Chung CH, et al. Benefit of sarcopenia screening in older patients undergoing surgical aortic valve replacement. Ann Thorac Surg. 2021;113:2018–2026. doi: 10.1016/j.athoracsur.2021.06.067 [DOI] [PubMed] [Google Scholar]
- 214.Luetkens JA, Faron A, Geissler HL, Al-Kassou B, Shamekhi J, Stundl A, Sprinkart AM, Meyer C, Fimmers R, Treede H, et al. Opportunistic computed tomography imaging for the assessment of fatty muscle fraction predicts outcome in patients undergoing transcatheter aortic valve replacement. Circulation. 2020;141:234–236. doi: 10.1161/CIRCULATIONAHA.119.042927 [DOI] [PubMed] [Google Scholar]
- 215.Saji M, Lim DS, Ragosta M, LaPar DJ, Downs E, Ghanta RK, Kern JA, Dent JM, Ailawadi G. Usefulness of psoas muscle area to predict mortality in patients undergoing transcatheter aortic valve replacement. Am J Cardiol. 2016;118:251–257. doi: 10.1016/j.amjcard.2016.04.043 [DOI] [PubMed] [Google Scholar]
- 216.Uchida Y, Ishii H, Tanaka A, Yonekawa J, Satake A, Makino Y, Suzuki W, Kurobe M, Mizutani K, Mizutani Y, et al. Impact of skeletal muscle mass on clinical outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Cardiovasc Interv Ther. 2021;36:514–522. doi: 10.1007/s12928-020-00725-8 [DOI] [PubMed] [Google Scholar]
- 217.van Mourik MS, Janmaat YC van Kesteren F Vendrik J Planken RN Henstra MJ Velu JF Vlastra W Zwinderman AH Koch KT et al. CT determined psoas muscle area predicts mortality in women undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2019;93:E248–E254. doi: 10.1002/ccd.27823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Walpot J, Van Herck P Collas V Bossaerts L Vandendriessche T Van De Heyning CM Heidbuchel H Rodrigus I Bosmans J. Computed tomography measured psoas muscle attenuation predicts mortality after transcatheter aortic valve implantation. J Cardiovasc Med (Hagerstown). 2022;23:60–68. doi: 10.2459/JCM.0000000000001234 [DOI] [PubMed] [Google Scholar]
- 219.Yoon YH, Ko Y, Kim KW, Kang DY, Ahn JM, Ko E, Park H, Cho SC, Kim HJ, Kim JB, et al. Prognostic value of baseline sarcopenia on 1-year mortality in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2021;139:79–86. doi: 10.1016/j.amjcard.2020.10.039 [DOI] [PubMed] [Google Scholar]
- 220.Iwasaki Y, Shiotsuka J, Kawarai Lefor A, Sanui M. The psoas muscle index is associated with prognosis in elderly patients undergoing cardiovascular surgery. Anesth Pain Med. 2021;11:e118608. doi: 10.5812/aapm.118608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kiriya Y, Toshiaki N, Shibasaki I, Ogata K, Ogawa H, Takei Y, Tezuka M, Seki M, Kato T, Lefor AK, et al. Sarcopenia assessed by the quantity and quality of skeletal muscle is a prognostic factor for patients undergoing cardiac surgery. Surg Today. 2020;50:895–904. doi: 10.1007/s00595-020-01977-w [DOI] [PubMed] [Google Scholar]
- 222.Yamashita M, Kamiya K Matsunaga A Kitamura T Hamazaki N Matsuzawa R Nozaki K Ichikawa T Nakamura T Yamamoto S et al. Preoperative skeletal muscle density is associated with postoperative mortality in patients with cardiovascular disease. Interact Cardiovasc Thorac Surg. 2020;30:515–522. doi: 10.1093/icvts/ivz307 [DOI] [PubMed] [Google Scholar]
- 223.Yamashita M, Kamiya K Matsunaga A Kitamura T Hamazaki N Matsuzawa R Nozaki K Tanaka S Nakamura T Maekawa E et al. Prognostic value of sarcopenic obesity estimated by computed tomography in patients with cardiovascular disease and undergoing surgery. J Cardiol. 2019;74:273–278. doi: 10.1016/j.jjcc.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 224.Wittmann F, Schloglhofer T, Riebandt J, Schaefer AK, Wiedemann D, Tschernko E, Beitzke D, Loewe C, Laufer G, Zimpfer D. Psoas muscle area predicts mortality after left ventricular assist device implantation. Life (Basel). 2021;11:922. doi: 10.3390/life11090922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Thurston B, Pena GN Howell S Cowled P Fitridge R. Low total psoas area as scored in the clinic setting independently predicts midterm mortality after endovascular aneurysm repair in male patients. J Vasc Surg. 2018;67:460–467. doi: 10.1016/j.jvs.2017.06.085 [DOI] [PubMed] [Google Scholar]
- 226.Teng CH, Chen SY, Wei YC, Hsu RB, Chi NH, Wang SS, Chen YS, Chen CC. Effects of sarcopenia on functional improvement over the first year after cardiac surgery: a cohort study. Eur J Cardiovasc Nurs. 2019;18:309–317. doi: 10.1177/1474515118822964 [DOI] [PubMed] [Google Scholar]
- 227.Morimoto Y, Matsuo T, Yano Y, Fukushima T, Eishi K, Kozu R. Impact of sarcopenia on the progress of cardiac rehabilitation and discharge destination after cardiovascular surgery. J Phys Ther Sci. 2021;33:213–221. doi: 10.1589/jpts.33.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lim MH, Lee CH, Ju MH, Je HG. Impact of sarcopenia on outcomes of minimally invasive cardiac surgery. Semin Thorac Cardiovasc Surg. 2021; 35:77–85. doi:10.1053/j.semtcvs.2021.11.005 [DOI] [PubMed] [Google Scholar]
- 229.Yuenyongchaiwat K, Kulchanarat C, Satdhabudha O. Sarcopenia in open heart surgery patients: a cohort study. Heliyon. 2020;6:e05759. doi: 10.1016/j.heliyon.2020.e05759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bellomo RG, Iodice P, Maffulli N, Maghradze T, Coco V, Saggini R. Muscle strength and balance training in sarcopenic elderly: a pilot study with randomized controlled trial. Eur J Inflammation. 2013;11:193–201. doi: 10.1177/1721727x1301100118 [Google Scholar]
- 231.Murphy CH, Flanagan EM, De Vito G, Susta D, Mitchelson KAJ, de Marco Castro E, Senden JMG, Goessens JPB, Miklosz A, Chabowski A, et al. Does supplementation with leucine-enriched protein alone and in combination with fish-oil-derived n-3 PUFA affect muscle mass, strength, physical performance, and muscle protein synthesis in well-nourished older adults? A randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2021;113:1411–1427. doi: 10.1093/ajcn/nqaa449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Martinez-Arnau FM, Fonfria-Vivas R, Buigues C, Castillo Y, Molina P, Hoogland AJ, van Doesburg F, Pruimboom L, Fernandez-Garrido J, Cauli O. Effects of leucine administration in sarcopenia: a randomized and placebo-controlled clinical trial. Nutrients. 2020;12:932. doi: 10.3390/nu12040932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Alemán-Mateo H, Macías L, Esparza-Romero J, Astiazaran-García H, Blancas AL. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderlymen: evidence from a randomized clinical trial using a protein-rich food. Clin IntervAging. 2012;7:225–234. doi: 10.2147/CIA.S32356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gray-Donald K, St-Arnaud-McKenzie D, Gaudreau P, Morais JA, Shatenstein B, Payette H. Protein intake protects against weight loss in healthy community-dwelling older adults. J Nutr. 2014;144:321–326. doi: 10.3945/jn.113.184705 [DOI] [PubMed] [Google Scholar]
- 235.Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, McMurdo ME, Mets T, Seal C, Wijers SL, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015;16:740–747. doi: 10.1016/j.jamda.2015.05.021 [DOI] [PubMed] [Google Scholar]
- 236.Maltais ML, Ladouceur JP, Dionne IJ. The effect of resistance training and different sources of postexercise protein supplementation on muscle mass and physical capacity in sarcopenic elderly men. J Strength Cond Res. 2016;30:1680–1687. doi: 10.1519/JSC.0000000000001255 [DOI] [PubMed] [Google Scholar]
- 237.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501 [DOI] [PubMed] [Google Scholar]
- 238.Schellenbaum GD, Smith NL, Heckbert SR, Lumley T, Rea TD, Furberg CD, Lyles MF, Psaty BM. Weight loss, muscle strength, and angiotensin-converting enzyme inhibitors in older adults with congestive heart failure or hypertension. J Am Geriatr Soc. 2005;53:1996–2000. doi: 10.1111/j.1532-5415.2005.53568.x [DOI] [PubMed] [Google Scholar]