Abstract
Tea tree oil (TTO) is a volatile essential oil obtained by distillation, mainly from the Australian native plant Melaleuca alternifolia (Maiden & Betche) Cheel (Myrtaceae). In this study, a comparative analysis of the chemical constituents of seven tea tree oils (M. alternifolia) and four other Melaleuca spp. oils (M. cajuputi, (MCa), two chemotypes of M. quinquenervia, (MNe and MNi), and M. ericifolia (MRo)) was carried out using gas chromatography–mass spectrometry (GC-MS) and high-performance thin-layer chromatography (HPTLC). Among the seven TTOs, terpinen-4-ol (37.66–44.28%), γ-terpinene (16.42–20.75%), α-terpinene (3.47–12.62%), α-terpineol (3.11–4.66%), and terpinolene (2.75–4.19%) were the most abundant compounds. On the other hand, the most abundant compounds of the other Melaleuca oils varied, such as 1,8-cineole (64.63%) in MCa oil, (E)-nerolidol (48.40%) and linalool (33.30%) in MNe oil, 1,8-cineole (52.20%) in MNi oil, and linalool (38.19%) and 1,8-cineole (27.57%) in MRo oil. HPTLC fingerprinting of Melaleuca oils enabled the discrimination of TTO oils from other Melaleuca spp. oils. Variation was observed in the profile of the Rf values among EOs. The present study shows that HPTLC is one of the best ways to identify and evaluate the quality control in authenticating TTOs, other Melaleuca EOs, or EOs from other species within the Myrtaceae.
Keywords: tea tree oil, cajeput oil, nerolina oil, niaouli oil, rosalina oil, terpinene-4-ol, GC-MS, TLC
1. Introduction
Plant essential oils (EOs), originally used in the perfume and aromatherapy market, have gained widespread acceptance in other industries. Tea tree oil (TTO), for example, an essential oil isolated from Melaleuca alternifolia (Maiden & Betche) Cheel (Myrtaceae) [1], has long been recognized as a safe and effective topical antiseptic in Australia [2,3,4,5]. During World War II, it was considered a necessary commodity for first aid kits and was also used as an insect repellent [6]. Current commercial products that contain TTO include assorted ointments, lotions, shampoos, soaps, toothpastes, and mouthwashes [2,5,7]. Additionally, many EOs have since been evaluated for their antimicrobial [8,9], insect attractant [10,11], repellent [12], or insecticidal properties [13,14,15,16,17]. Isman [18] investigated the potential effect of several EOs as attractants, repellents, and toxicants against insects and other organisms.
Since EOs consist of concentrated plant terpenoids, they have provided an ideal substrate for the development of host-based (kairomone) lures for invasive pests in Florida, including the redbay ambrosia beetle Xyleborus glabratus Eichhoff, the tea shot hole borer Euwallacea perbrevis Schedl [19], and the Mediterranean fruit fly (medfly), Ceratitis capitata (Wiedemann) (Diptera: Tephritidae), which has been a destructive pest in Europe, the Middle East, Australia, Central and South America, and Hawaii, USA [20,21,22]. Behavioral studies conducted at the USDA-ARS in Miami, Florida [23,24], have indicated that tea tree oil (TTO) has a strong short-range attractive effect on sterile male medflies in laboratory bioassays [17,25,26,27,28,29,30,31]. Therefore, it has good potential as an economical new attractant for male medflies, and the identification of the key components of TTO is important for developing lures for pest management.
In recent experiments [27], we used preparative thin-layer chromatography (prep TLC) to separate TTO into five fractions. Bioassays conducted after these separations revealed that two TTO fractions are responsible for the observed attraction in male medflies. Furthermore, TLC-based bioassays played an important role in the isolation of insect kairomones from complex mixtures such as EOs. Although it is not a new technique, TLC continues to be a valuable tool as a preparative technique for a variety of studies in both chemical and biological fields [25,32,33,34,35]. While gas chromatography–mass spectrometry (GC-MS) is exceptional in the identification of unknown chemicals, it works best for trace analysis [36]. In addition, the sample is destroyed in the process of obtaining its fingerprint fragments for proper identification. In cases where a much larger sample is needed or it is required to remain intact for further analysis, TLC proves to be a more suitable method. With the development of high-performance thin-layer chromatography (HPTLC), this separation technique has evolved from a mostly qualitative procedure into a quick and cost-effective quantification method used by the European Pharmacopoeia for the quality control of some EOs [37]. The automated sample application, and the capability of using a universal HPTLC standard mix [38], ensures accurate sample and standard amounts, and provides reproducible quantitative results.
Various Melaleuca species, and even other Myrtaceae, are often confused under the common name ‘tea tree’, (e.g., ‘‘swamp tea tree’’, M. cajuputi; ‘‘paperbark tea tree’’, “broad-leaved tea tree”, or “broad-leaved paper bark”, M. quinquenervia; “black tea tree” or “river tea tree”, M. bracteate; “lemon scented tea tree”, Leptospermum petersonii, etc.). Moreover, kanuka and manuka EOs derived from Kunzea ericoides and Leptospermum scoparium, respectively, are referred to as New Zealand TTOs [2,3,5,6,39,40].
Considering the diversity of compounds present in Melaleuca EOs, and their current and potential applications, including prospective IPM strategies, it is important to study the chemical composition and degree of variability in commercially available Melaleuca EOs. In this study, HPTLC methods were developed for the evaluation of seven TTOs and four different Melaleuca spp. oils selected mainly from open markets in the United States. Separation patterns of various brands of TTO, as well as other Melaleuca oils, were compared to ensure the presence and consistent amount of chemicals attractive for male medflies.
2. Results and Discussion
2.1. Identification of Components in Melaleuca EOs
The identification of the components from the seven M. alternifolia EOs (TTAA, TTAS, TTEG, TTFC, TTNG, TTPT, and TTSAT) and four other Melaleuca EOs (MCa, MNe, MNi, and MRo) (Table 1) was achieved on the GC-MS using a non-polar DB-5 column. One hundred thirty-eight compounds were identified in total among the eleven Melaleuca EOs, accounting for 99.49-99.97% of the total composition (Table 2).
Table 1.
Species, sample codes, and sources of oils used in this study.
| Species | Code | Source |
|---|---|---|
| M. alternifolia (Maiden & Betche) Cheel | TTAA | Aromappeal (Puritan’s Pride, Inc.), Oakdale, NY, USA |
| M. alternifolia (Maiden & Betche) Cheel | TTAS | Apothecary Shoppe, Portland, OR, USA |
| M. alternifolia (Maiden & Betche) Cheel | TTEG | Eden’s Garden, San Clemente, CA, USA |
| M. alternifolia (Maiden & Betche) Cheel | TTFC | Floracopeia, Grass Valley, CA, USA |
| M. alternifolia (Maiden & Betche) Cheel | TTNG | Nature’s Gift, Madison, TN, USA |
| M. alternifolia (Maiden & Betche) Cheel | TTPT | Plant Therapy, Inc, Twin Falls, ID, USA |
| M. alternifolia (Maiden & Betche) Cheel | TTSAT | SAT Group, Kannauj, India |
| M. cajuputi Powell | MCa | Nature’s Gift, Madison, TN, USA |
| M. quinquenervia (Cav.) S.T. Blake | MNe | Nature’s Gift, Madison, TN, USA |
| M. quinquenervia (Cav.) S.T. Blake | MNi | Nature’s Gift, Madison, TN, USA |
| M. ericifolia Sm. | MRo | Nature’s Gift, Madison, TN, USA |
Table 2.
Comparative percentage composition of the Melaleuca EOs.
| # | * RI Exp | ** RILit | Compounds | TTAA | TTAS | TTEG | TTFC | TTNG | TTPT | TTSAT | MCa | MNe | MNi | MRo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 938 | 930 | α-Thujene RI, MS | 0.85 ± 0.10 | 0.77 ± 0.05 | 0.76 ± 0.04 | 0.48 ± 0.02 | 0.97 ± 0.06 | 0.79 ± 0.01 | 0.30 ± 0.10 | 0.77 ± 0.10 | 0.27 ± 0.03 | 1.07 ± 0.28 | 0.17 ± 0.00 |
| 2 | 946 | 939 | α-Pinene RI, MS, Std | 1.89 ± 0.06 | 2.66 ± 0.03 | 2.40 ± 0.41 | 1.67 ± 0.05 | 2.48 ± 0.16 | 1.39 ± 0.02 | 1.29 ± 0.16 | 5.48 ± 0.11 | 0.57 ± 0.12 | 9.75 ± 0.13 | 5.09 ± 0.03 |
| 3 | 954 | 954 | Camphene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.15 ± 0.02 | 0.00 | 0.04 ± 0.02 | 0.28 ± 0.05 |
| 4 | 969 | 960 | Benzaldehyde RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.38 ± 0.04 | 0.09 ± 0.04 | 0.00 |
| 5 | 970 | 975 | Sabinene RI, MS, Std | 0.72 ± 0.03 | 0.05 ± 0.00 | 0.36 ± 0.04 | 0.45 ± 0.06 | 0.94 ± 0.03 | 0.64 ± 0.07 | 0.08 ± 0.02 | 0.18 ± 0.04 | 0.00 | 0.00 | 0.00 |
| 6 | 989 | 979 | β-pinene RI, MS, Std | 0.73 ± 0.01 | 0.88 ± 0.07 | 0.89 ± 0.10 | 0.97 ± 0.16 | 1.06 ± 0.02 | 0.80 ± 0.08 | 0.93 ± 0.01 | 1.11 ± 0.07 | 0.94 ± 0.08 | 2.56 ± 0.33 | 0.64 ± 0.03 |
| 7 | 998 | 990 | Myrcene RI, MS, Std | 0.48 ± 0.06 | 0.58 ± 0.05 | 0.63 ± 0.04 | 0.62 ± 0.12 | 0.77 ± 0.03 | 0.41 ± 0.06 | 0.63 ± 0.02 | 0.65 ± 0.03 | 0.60 ± 0.16 | 0.72 ± 0.20 | 0.61 ± 0.02 |
| 8 | 1009 | 991 | 6-Methyl-5-hepten-2-ol RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.19 ± 0.03 | 0.00 | 0.00 |
| 9 | 1010 | 1002 | α-Phellandrene RI, MS, Std | 0.14 ± 0.07 | 0.54 ± 0.02 | 0.45 ± 0.07 | 0.47 ± 0.10 | 0.53 ± 0.08 | 0.33 ± 0.10 | 0.43 ± 0.13 | 0.50 ± 0.02 | 0.09 ± 0.01 | 0.11 ± 0.03 | 0.03 ± 0.01 |
| 10 | 1012 | 1003 | Pseudolimonene RI, MS | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 11 | 1018 | 1011 | δ-3-Carene RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 ± 0.04 |
| 12 | 1021 | 1014 | 1,4-Cineole RI, MS, Std | 0.00 | 0.00 | 0.04 ± 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 13 | 1022 | 1017 | α-Terpinene RI, MS, Std | 6.14 ± 0.03 | 12.62 ± 0.41 | 9.62 ± 0.14 | 8.35 ± 0.13 | 8.89 ± 0.37 | 7.63 ± 0.19 | 3.47 ± 0.06 | 0.22 ± 0.01 | 0.13 ± 0.03 | 0.00 | 0.17 ± 0.04 |
| 14 | 1030 | 1024 | p-Cymene RI, MS, Std | 5.18 ± 0.55 | 1.66 ± 0.15 | 3.51 ± 0.04 | 3.59 ± 0.27 | 3.22 ± 0.28 | 3.71 ± 0.17 | 11.47 ± 0.19 | 0.79 ± 0.11 | 0.03 ± 0.02 | 0.07 ± 0.02 | 1.21 ± 0.06 |
| 15 | 1039 | 1029 | Limonene RI, MS, Std | 1.63 ± 0.23 | 0.30 ± 0.09 | 1.10 ± 0.07 | 1.15 ± 0.02 | 0.87 ± 0.03 | 1.10 ± 0.05 | 0.51 ± 0.19 | 0.10 ± 0.00 | 0.19 ± 0.03 | 2.83 ± 0.18 | 3.03 ± 0.05 |
| 16 | 1039 | 1029 | β-Phellandrene RI, MS, Std | 0.10 ± 0.01 | 0.26 ± 0.01 | 0.86 ± 0.04 | 0.83 ± 0.06 | 0.03 ± 0.00 | 0.44 ± 0.04 | 0.39 ± 0.01 | 0.07 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 |
| 17 | 1040 | 1031 | 1,8-Cineole RI, MS, Std | 2.85 ± 0.38 | 1.17 ± 0.01 | 3.76 ± 0.12 | 3.28 ± 0.07 | 4.79 ± 0.14 | 3.90 ± 0.08 | 4.50 ± 0.09 | 64.63 ± 1.19 | 1.91 ± 0.16 | 52.20 ± 0.28 | 27.57 ± 0.56 |
| 18 | 1049 | 1037 | (Z)-β-Ocimene RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.69 ± 0.10 | 0.00 | 0.04 ± 0.01 |
| 19 | 1062 | 1050 | (E)-β-Ocimene RI, MS, Std | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.74 ± 0.10 | 0.03 ± 0.01 | 0.76 ± 0.08 |
| 20 | 1071 | 1059 | γ-Terpinene RI, MS, Std | 16.42 ± 0.83 | 18.91 ± 0.46 | 19.58 ± 0.10 | 19.60 ± 0.10 | 16.58 ± 0.45 | 20.75 ± 0.20 | 16.61 ± 0.41 | 3.24 ± 0.20 | 0.12 ± 0.05 | 0.57 ± 0.00 | 1.84 ± 0.01 |
| 21 | 1081 | 1070 | cis-Sabinene hydrate RI, MS | 0.00 | 0.01 ± 0.00 | 0.14 ± 0.04 | 0.20 ± 0.09 | 0.29 ± 0.07 | 0.07 ± 0.02 | 0.09 ± 0.01 | 0.01 ± 0.01 | 0.00 | 0.00 | 0.00 |
| 22 | 1084 | 1072 | cis-Linalool oxide RI, MS, Std | 0.00 | 0.00 | 0.30 ± 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 ± 0.00 | 0.00 | 0.13 ± 0.13 |
| 23 | 1098 | 1088 | Terpinolene RI, MS, Std | 2.83 ± 0.05 | 3.61 ± 0.01 | 4.19 ± 0.04 | 3.63 ± 0.21 | 3.39 ± 0.12 | 3.16 ± 0.06 | 2.75 ± 0.04 | 1.04 ± 0.05 | 0.26 ± 0.10 | 0.45 ± 0.07 | 2.14 ± 0.02 |
| 24 | 1107 | 1096 | Linalool RI, MS, Std | 0.07 ± 0.02 | 0.13 ± 0.01 | 0.45 ± 0.15 | 1.12 ± 0.03 | 1.04 ± 0.04 | 0.13 ± 0.06 | 0.22 ± 0.02 | 0.26 ± 0.04 | 33.30± 0.63 | 0.47 ± 0.29 | 38.19 ± 0.06 |
| 25 | 1115 | 1097 | Hotrienol RI, MS | 0.00 | 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.12 ± 0.06 | 0.00 | 0.15 ± 0.02 |
| 26 | 1123 | 1121 | cis-p-Menth-2-en-1-ol | 0.14 ± 0.08 | 0.21 ± 0.06 | 0.21 ± 0.05 | 0.00 | 0.37 ± 0.08 | 0.28 ± 0.01 | 0.36 ± 0.05 | 0.00 | 0.00 | 0.00 | 0.00 |
| 27 | 1125 | 1122 | trans-p-Menth-2-en-1-ol | 0.21 ± 0.01 | 0.18 ± 0.01 | 0.17 ± 0.02 | 0.15 ± 0.02 | 0.31 ± 0.04 | 0.22 ± 0.01 | 0.28 ± 0.04 | 0.00 | 0.00 | 0.00 | 0.00 |
| 28 | 1168 | 1166 | δ-Terpinol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 ± 0.01 | 0.00 | 0.11 ± 0.01 | 0.12 ± 0.01 |
| 29 | 1183 | 1177 | Terpinene-4-ol RI, MS, Std | 44.28 ± 0.90 | 38.62 ± 0.33 | 37.66 ± 0.24 | 40.36 ± 0.53 | 38.42 ± 0.22 | 40.55 ± 0.67 | 39.57 ± 1.37 | 0.71 ± 0.03 | 0.27 ± 0.00 | 0.62 ± 0.01 | 0.79 ± 0.01 |
| 30 | 1188 | 1182 | p-Cymen-8-ol RI, MS | 0.14 ± 0.04 | 0.05 ± 0.02 | 0.12 ± 0.00 | 0.17 ± 0.03 | 0.11 ± 0.01 | 0.10 ± 0.02 | 0.19 ± 0.10 | 0.02 ± 0.01 | 0.00 | 0.00 | 0.10 ± 0.01 |
| 31 | 1198 | 1188 | α-Terpineol RI, MS, Std | 3.74 ± 0.05 | 3.11 ± 0.01 | 3.35 ± 0.07 | 3.61 ± 0.09 | 4.66 ± 0.03 | 3.40 ± 0.04 | 4.20 ± 0.09 | 5.44 ± 0.10 | 0.46 ± 0.01 | 5.08 ± 0.06 | 4.05 ± 0.08 |
| 32 | 1206 | 1196 | cis-Piperitol RI, MS, Std | 0.08 ± 0.02 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.03 ± 0.00 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.13 ± 0.02 | 0.00 | 0.00 | 0.00 | 0.00 |
| 33 | 1218 | 1208 | trans-Piperitol RI, MS | 0.14 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.00 | 0.05 ± 0.00 | 0.08 ± 0.00 | 0.15 ± 0.00 | 0.14 ± 0.06 | 0.00 | 0.00 | 0.00 | 0.00 |
| 34 | 1226 | 1216 | trans-Carveol RI, MS, | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.01 |
| 35 | 1232 | 1221 | cis-Sabinene hydrate acetate RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 ± 0.00 | 0.00 | 0.00 |
| 36 | 1236 | 1225 | Citronellol RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| 37 | 1238 | 1229 | Nerol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 | 0.16 ± 0.01 |
| 38 | 1243 | 1237 | Ascaridole RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 ± 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| 39 | 1245 | 1238 | Neral | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 ± 0.01 |
| 40 | 1253 | 1252 | Geraniol RI, MS, Std | 0.05 ± 0.05 | 0.00 | 0.04 ± 0.03 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.00 | 0.02 ± 0.02 | 0.04 ± 0.02 | 0.19 ± 0.02 | 0.02 ± 0.01 | 0.20 ± 0.00 |
| 41 | 1264 | 1258 | 2-Phenyl ethyl acetate RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 ± 0.02 |
| 42 | 1271 | 1267 | Geranial RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 ± 0.01 |
| 43 | 1273 | 1269 | trans-Ascaridol glycol RI, MS | 0.12 ± 0.02 | 0.01 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.02 | 0.02 ± 0.00 | 0.04 ± 0.01 | 0.06 ± 0.02 | 0.00 | 0.00 | 0.00 | 0.00 |
| 44 | 1291 | 1288 | cis-Ascaridol glycol RI, MS | 0.04 ± 0.02 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.01 | 0.00 | 0.01 ± 0.00 | 0.04 ± 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| 45 | 1295 | 1290 | Thymol RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.01 ± 0.00l | 0.00 | 0.00 | 0.02 ± 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| 46 | 1306 | 1299 | Carvacrol RI, MS, Std | 0.01 ± 0.01 | 0.00 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.00 | 0.00 | 0.02 ± 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| 47 | 1330 | 1324 | Methyl geranate RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 ± 0.03 |
| 48 | 1343 | 1338 | δ-Elemene RI, MS | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 | 0.05 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.02 ± 0.00 |
| 49 | 1355 | 1347 | α-Terpinyl acetate RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.64 ± 0.01 | 0.00 | 1.30 ± 0.02 | 0.00 |
| 50 | 1356 | 1348 | α-Cubebene RI, MS | 0.01 ± 0.01 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.07 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.02 | 0.00 | 0.00 | 0.00 | 0.00 |
| 51 | 1359 | 1359 | Eugenol RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 | 0.00 |
| 52 | 1376 | 1375 | α-Ylangene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 ± 0.02 | 0.00 | 0.00 | 0.00 |
| 53 | 1378 | 1376 | Isoledene RI, MS | 0.06 ± 0.01 | 0.08 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.00 | 0.06 ± 0.00 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.00 | 0.00 | 0.00 | 0.15 ± 0.02 |
| 54 | 1380 | 1376 | α-Copaene RI, MS, Std | 0.10 ± 0.01 | 0.14 ± 0.01 | 0.10 ± 0.01 | 0.07 ± 0.00 | 0.13 ± 0.00 | 0.06 ± 0.02 | 0.13 ± 0.02 | 0.08 ± 0.01 | 0.00 | 0.03 ± 0.00 | 0.02 ± 0.01 |
| 55 | 1382 | 1381 | Geranyl acetate RI, MS, Std | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.02 ± 0.01 |
| 56 | 1395 | 1390 | β-Elemene RI, MS, Std | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.06 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.01 | 0.05 ± 0.03 | 0.00 | 0.00 | 0.32 ± 0.02 |
| 57 | 1403 | 1402 | α-Funebrene RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 ± 0.01 | 0.00 | 0.00 |
| 58 | 1404 | 1403 | Methyl eugenol RI, MS, Std | 0.00 | 0.00 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.00 | 0.00 | 0.02 ± 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 59 | 1409 | 1408 | Isocaryophyllene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.00 | 0.00 |
| 60 | 1410 | 1409 | α-Gurjunene RI, MS | 0.41 ± 0.01 | 0.58 ± 0.01 | 0.36 ± 0.01 | 0.18 ± 0.06 | 0.45 ± 0.01 | 0.35 ± 0.01 | 0.43 ± 0.06 | 0.06 ± 0.03 | 0.00 | 0.07 ± 0.02 | 0.39 ± 0.01 |
| 61 | 1412 | 1411 | α-Cedrene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 ± 0.03 | 0.00 | 0.00 |
| 62 | 1417 | 1416 | β-Maaliene RI, MS | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 | 0.02 ± 0.00 | 0.00 | 0.00 | 0.00 | 0.03 ± 0.01 |
| 63 | 1421 | 1419 | β-Caryophyllene RI, MS, Std | 0.37 ± 0.01 | 0.74 ± 0.02 | 0.29 ± 0.01 | 0.21 ± 0.01 | 0.56 ± 0.02 | 0.28 ± 0.03 | 0.40 ± 0.04 | 3.82 ± 0.12 | 2.40 ± 0.05 | 2.66 ± 0.05 | 0.16 ± 0.01 |
| 64 | 1429 | 1425 | γ-Maaliene RI, MS | 0.06 ± 0.01 | 0.08 ± 0.00 | 0.07 ± 0.00 | 0.05 ± 0.01 | 0.06 ± 0.00 | 0.04 ± 0.00 | 0.06 ± 0.01 | 0.01 ± 0.01 | 0.00 | 0.01 ± 0.01 | 0.10 ± 0.01 |
| 65 | 1433 | 1433 | β-Gurjunene RI, MS | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 | 0.02 ± 0.00 | 0.05 ± 0.01 | 0.00 | 0.00 | 0.10 ± 0.00 |
| 66 | 1435 | 1433 | α-Maaliene RI, MS, Std | 0.07 ± 0.01 | 0.09 ± 0.00 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.00 | 0.05 ± 0.00 | 0.07 ± 0.02 | 0.05 ± 0.01 | 0.00 | 0.00 | 0.12 ± 0.01 |
| 67 | 1439 | 1441 | Aromadendrene RI, MS, Std | 1.51 ± 0.02 | 1.90 ± 0.02 | 1.20 ± 0.03 | 1.25 ± 0.04 | 1.24 ± 0.01 | 1.51 ± 0.19 | 1.27 ± 0.09 | 0.65 ± 0.04 | 0.00 | 0.16 ± 0.08 | 3.38 ± 0.02 |
| 68 | 1443 | 1443 | Selina-5,11-diene RI, MS | 0.16 ± 0.01 | 0.22 ± 0.01 | 0.14 ± 0.01 | 0.08 ± 0.04 | 0.17 ± 0.00 | 0.11 ± 0.01 | 0.15 ± 0.02 | 0.06 ± 0.01 | 0.00 | 0.00 | 0.26 ± 0.01 |
| 69 | 1448 | 1451 | Amorpha-4,11-diene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 ± 0.03 | 0.00 | 0.00 |
| 70 | 1450 | 1453 | trans-Muurola-3.5-diene RI, MS | 0.11 ± 0.01 | 0.19 ± 0.01 | 0.12 ± 0.01 | 0.03 ± 0.00 | 0.31 ± 0.01 | 0.14 ± 0.01 | 0.18 ± 0.03 | 0.02 ± 0.01 | 0.00 | 0.01 ± 0.00 | 0.06 ± 0.01 |
| 71 | 1453 | 1454 | α-Humulene RI, MS, Std | 0.08 ± 0.00 | 0.12 ± 0.00 | 0.07 ± 0.00 | 0.05 ± 0.00 | 0.15 ± 0.02 | 0.07 ± 0.01 | 0.09 ± 0.01 | 1.81 ± 0.08 | 0.40 ± 0.01 | 0.40 ± 0.03 | 0.03 ± 0.00 |
| 72 | 1457 | 1456 | (E)-β-Farnesene RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.93 ± 0.40 | 0.12 ± 0.05 | 0.00 |
| 73 | 1459 | 1460 | Alloaromadendrene RI, MS, Std | 0.67 ± 0.01 | 0.96 ± 0.01 | 0.53 ± 0.02 | 0.47 ± 0.02 | 0.62 ± 0.01 | 0.54 ± 0.01 | 0.62 ± 0.05 | 0.36 ± 0.02 | 0.00 | 0.51 ± 0.05 | 1.41 ± 0.02 |
| 74 | 1464 | 1466 | α-Acoradiene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 | 0.00 |
| 75 | 1467 | 1470 | β-Acoradiene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 ± 0.01 | 0.00 | 0.00 |
| 76 | 1471 | 1475 | 10-epi-β-Acoradiene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 ± 0.01 | 0.00 | 0.00 |
| 77 | 1473 | 1476 | trans-Cadina-1(6),4-diene RI, MS | 0.36 ± 0.01 | 0.55 ± 0.02 | 0.36 ± 0.01 | 0.20 ± 0.01 | 0.45 ± 0.02 | 0.42 ± 0.02 | 0.45 ± 0.05 | 0.00 | 0.02 ± 0.01 | 0.00 | 0.00 |
| 78 | 1474 | 1477 | γ-Gurjunene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.35 ± 0.01 |
| 79 | 1476 | 1479 | γ-Muurolene RI, MS | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.01 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.05 ± 0.01 | 0.45 ± 0.03 | 0.00 | 0.07 ± 0.01 | 0.08 ± 0.01 |
| 80 | 1480 | 1480 | ar-Curcumene RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 ± 0.04 | 0.00 | 0.00 |
| 81 | 1481 | 1482 | γ-Curcumene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 ± 0.02 | 0.00 | 0.00 |
| 82 | 1483 | 1484 | α-Amorphene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.30 ± 0.01 | 0.00 | 0.00 | 0.00 |
| 83 | 1484 | 1485 | Germacrene D RI, MS | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.18 ± 0.02 | 0.00 |
| 84 | 1486 | 1490 | β-Selinene RI, MS | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.00 | 0.05 ± 0.01 | 0.08 ± 0.02 | 1.28 ± 0.05 | 0.00 | 0.20 ± 0.01 | 0.23 ± 0.01 |
| 85 | 1488 | 1490 | Alloaromadendr-9-ene RI, MS | 0.11 ± 0.01 | 0.14 ± 0.01 | 0.07 ± 0.04 | 0.08 ± 0.01 | 0.11 ± 0.00 | 0.07 ± 0.01 | 0.11 ± 0.02 | 0.00 | 0.00 | 0.18 ± 0.00 | 0.29 ± 0.01 |
| 86 | 1491 | 1492 | δ-Selinene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.35 ± 0.01 | 0.00 | 0.00 | 0.00 |
| 87 | 1492 | 1493 | cis-β-Guaiene RI, MS | 0.19 ± 0.01 | 0.28 ± 0.01 | 0.18 ± 0.01 | 0.11 ± 0.01 | 0.31 ± 0.01 | 0.16 ± 0.01 | 0.21 ± 0.03 | 0.11 ± 0.01 | 0.00 | 0.01 ± 0.01 | 0.16 ± 0.01 |
| 88 | 1494 | 1496 | Ledene RI, MS | 1.14 ± 0.01 | 1.62 ± 0.02 | 0.86 ± 0.03 | 0.47 ± 0.03 | 1.06 ± 0.01 | 1.38 ± 0.05 | 1.69 ± 0.05 | 0.19 ± 0.03 | 0.16 ± 0.06 | 1.76 ± 0.17 | 0.98 ± 0.02 |
| 89 | 1495 | 1498 | α-Selinene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.12 ± 0.08 | 0.00 | 0.00 | 0.00 |
| 90 | 1496 | 1499 | (Z,E)-α-Farnesene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.64 ± 0.14 | 0.00 | 0.00 |
| 91 | 1497 | 1500 | Bicyclogermacrene RI, MS | 0.91 ± 0.01 | 0.96 ± 0.06 | 0.67 ± 0.02 | 0.31 ± 0.01 | 0.72 ± 0.02 | 0.91 ± 0.04 | 0.60 ± 0.03 | 0.00 | 0.00 | 0.02 ± 0.01 | 0.62 ± 0.02 |
| 92 | 1498 | 1500 | α-Muurolene RI, MS | 0.17 ± 0.01 | 0.20 ± 0.00 | 0.14 ± 0.00 | 0.11 ± 0.01 | 0.22 ± 0.00 | 0.16 ± 0.01 | 0.20 ± 0.02 | 0.06 ± 0.01 | 0.00 | 0.09 ± 0.01 | 0.03 ± 0.00 |
| 93 | 1501 | 1501 | Epizonarene RI, MS | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.19 ± 0.01 | 0.00 | 0.00 | 0.00 |
| 94 | 1503 | 1502 | trans-β-Guaiene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 ± 0.07 | 0.00 | 0.00 |
| 95 | 1505 | 1505 | (Z)-α-Bisabolene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.23 ± 0.03 | 0.00 | 0.00 |
| 96 | 1506 | 1505 | (E,E)-α-Farnesene RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.67 ± 0.38 | 0.11 ± 0.04 | 0.00 |
| 97 | 1508 | 1512 | δ-Amorphene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.19 ± 0.01 |
| 98 | 1512 | 1513 | γ-Cadinene RI, MS | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.07 ± 0.01 | 0.00 | 0.25 ± 0.02 | 0.06 ± 0.01 |
| 99 | 1514 | 1515 | (Z)-γ-Bisabolene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.21 ± 0.05 | 0.00 | 0.00 |
| 100 | 1517 | 1522 | 7-epi-α-Selinene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 ± 0.01 | 0.00 | 0.00 | 0.06 ± 0.01 |
| 101 | 1518 | 1522 | trans-Calamene RI, MS | 0.07 ± 0.06 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 102 | 1521 | 1523 | δ-Cadinene RI, MS | 1.35 ± 0.01 | 1.71 ± 0.02 | 1.22 ± 0.04 | 1.06 ± 0.04 | 1.32 ± 0.02 | 1.78 ± 0.14 | 1.79 ± 0.09 | 0.23 ± 0.03 | 0.13 ± 0.04 | 0.35 ± 0.01 | 0.17 ± 0.03 |
| 103 | 1527 | 1529 | Zonarene RI, MS | 0.75 ± 0.02 | 0.58 ± 0.04 | 0.33 ± 0.02 | 0.30 ± 0.01 | 0.37 ± 0.02 | 0.54 ± 0.04 | 0.48 ± 0.06 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.00 | 0.03 ± 0.02 |
| 104 | 1529 | 1531 | (E)-γ-Bisabolene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.19 ± 0.05 | 0.00 | 0.00 |
| 105 | 1531 | 1534 | trans-Cadina-1,4-diene | 0.22 ± 0.01 | 0.28 ± 0.01 | 0.19 ± 0.01 | 0.14 ± 0.01 | 0.29 ± 0.01 | 0.24 ± 0.01 | 0.27 ± 0.02 | 0.00 | 0.00 | 0.00 | 0.00 |
| 106 | 1535 | 1538 | α-Cadinene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 ± 0.03 | 0.00 | 0.00 | 0.01 ± 0.00 |
| 107 | 1540 | 1545 | α-Calacorene RI, MS | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.00 | 0.01 ± 0.00 | 0.04 ± 0.03 | 0.00 | 0.00 | 0.00 | 0.03 ± 0.03 |
| 108 | 1542 | 1546 | Selina-3,7(11)-diene RI, MS | 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 | 0.16 ± 0.02 | 0.00 | 0.00 | 0.00 |
| 109 | 1548 | 1547 | (E)-α-Bisabolene RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.41 ± 0.11 | 0.00 | 0.00 |
| 110 | 1559 | 1561 | Germacrene B RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 ± 0.01 | 0.00 | 0.00 | 0.00 |
| 111 | 1560 | 1563 | (E)-Nerolidol RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 48.40 ± 1.21 | 6.89 ± 0.44 | 0.00 |
| 112 | 1562 | 1567 | Maaliol RI, MS | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.13 ± 0.01 | 0.03 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 113 | 1563 | 1568 | Palustrol RI, MS | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.19 ± 0.02 | 0.04 ± 0.00 | 0.02 ± 0.01 | 0.08 ± 0.02 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.09 ± 0.01 |
| 114 | 1572 | 1578 | Spathulenol RI, MS | 0.12 ± 0.02 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.14 ± 0.01 | 0.05 ± 0.00 | 0.03 ± 0.01 | 0.10 ± 0.05 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.25 ± 0.01 |
| 115 | 1578 | 1583 | Caryophyllene oxide RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.00 | 0.20 ± 0.05 | 0.00 | 0.00 |
| 116 | 1585 | 1590 | Globulol RI, MS, Std | 0.40 ± 0.02 | 0.43 ± 0.02 | 0.35 ± 0.02 | 0.94 ± 0.04 | 0.25 ± 0.01 | 0.22 ± 0.01 | 0.23 ± 0.02 | 0.38 ± 0.03 | 0.31 ± 0.04 | 0.35 ± 0.01 | 0.59 ± 0.02 |
| 117 | 1586 | 1592 | Viridiflorol RI, MS, Std | 0.19 ± 0.01 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.41 ± 0.02 | 0.10 ± 0.00 | 0.11 ± 0.01 | 0.16 ± 0.03 | 0.40 ± 0.02 | 0.07 ± 0.01 | 6.23 ± 0.17 | 0.15 ± 0.01 |
| 118 | 1588 | 1595 | Cubeban-11-ol RI, MS | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.13 ± 0.01 | 0.35 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.12 ± 0.02 | 0.00 | 0.00 | 0.00 | 0.12 ± 0.01 |
| 119 | 1594 | 1600 | Rosiflorol RI, MS | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.29 ± 0.01 | 0.06 ± 0.00 | 0.04 ± 0.01 | 0.10 ± 0.02 | 0.00 | 0.00 | 0.00 | 0.10 ± 0.01 |
| 120 | 1597 | 1600 | Guaiol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.09 ± 0.01 | 0.11 ± 0.01 |
| 121 | 1599 | 1602 | Ledol RI, MS | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.02 | 0.01 ± 0.00 | 0.05 ± 0.01 | 0.01 ± 0.00 | 0.72 ± 0.02 | 0.03 ± 0.00 |
| 122 | 1609 | 1607 | 5-epi-7-epi-α-Eudesmol RI, MS | 0.11 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.30 ± 0.02 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.08 ± 0.02 | 0.00 | 0.00 | 0.00 | 0.14 ± 0.01 |
| 123 | 1611 | 1608 | Humulene epoxide II RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.00 |
| 124 | 1619 | 1619 | 1,10-di-epi-Cubenol RI, MS | 0.20 ± 0.01 | 0.22 ± 0.01 | 0.19 ± 0.01 | 0.40 ± 0.02 | 0.14 ± 0.00 | 0.14 ± 0.01 | 0.22 ± 0.04 | 0.00 | 0.00 | 0.02 ± 0.01 | 0.00 |
| 125 | 1626 | 1623 | 10-epi-γ-Eudesmol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 ± 0.01 | 0.00 | 0.00 | 0.00 |
| 126 | 1630 | 1628 | 1-epi-Cubenol RI, MS | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.17 ± 0.01 | 0.07 ± 0.00 | 0.06 ± 0.01 | 0.15 ± 0.01 | 0.00 | 0.00 | 0.06 ± 0.03 | 0.01 ± 0.00 |
| 127 | 1632 | 1631 | Muurola-4,10(14)-dien-1β-ol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.00 | 0.00 |
| 128 | 1633 | 1632 | γ-Eudesmol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 ± 0.01 | 0.00 | 0.00 | 0.00 |
| 129 | 1639 | 1640 | T-Cadinol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 | 0.00 | 0.00 |
| 130 | 1643 | 1646 | α-Muurolol RI, MS | 0.06 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.07 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.01 | 0.00 | 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 |
| 131 | 1645 | 1646 | Cubenol RI, MS | 0.00 | 0.01 ± 0.00 | 0.00 | 0.02 ± 0.01 | 0.00 | 0.00 | 0.02 ± 0.00 | 0.00 | 0.00 | 0.06 ± 0.01 | 0.00 |
| 132 | 1649 | 1650 | β-Eudesmol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 ± 0.01 | 0.00 | 0.00 | 0.00 |
| 133 | 1652 | 1653 | α-Eudesmol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 ± 0.01 | 0.00 | 0.01 ± 0.00 | 0.00 |
| 134 | 1668 | 1671 | Bulnesol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 | 0.01 ± 0.00 | 0.00 |
| 135 | 1679 | 1684 | epi-α-Bisabolol RI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 ± 0.00 | 0.00 | 0.00 |
| 136 | 1681 | 1685 | α-Bisabolol RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 ± 0.01 | 0.00 | 0.00 |
| 137 | 1710 | 1715 | (E,Z)-Farnesol RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.01 | 0.00 | 0.00 |
| 138 | 1720 | 1723 | (Z,E)-Farnesol RI, MS, Std | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 ± 0.01 | 0.00 | 0.00 |
| Total | 99.70 ± 0.07 | 99.85 ± 0.04 | 99.65 ± 0.00 | 99.84 ± 0.03 | 99.87 ± 0.01 | 99.97 ± 0.02 | 99.73 ± 0.26 | 99.49 ± 0.37 | 99.72 ± 0.11 | 99.79 ± 0.13 | 99.53 ± 0.05 |
* RIexp: Retention indices (RIs) calculated from the current study; ** RIlit: RI from the literature [41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. Identification method: RI: retention index; MS: computer matching of the mass spectra libraries and comparison with the literature data; Std: standards compounds were purchased. TTO and samples and their corresponding major components are represented in blue. Other Melaleuca oils and their respective major components are highlighted in gray.
The TTOs were characterized by a high amount of terpinen-4-ol (37.66–44.28%), followed by γ-terpinene (16.42–20.75%), α-terpinene (3.47–12.62%), α-terpineol (3.11–4.66%), terpinolene (2.75–4.19%), p-cymene (1.66–11.47%), α-pinene (1.29-2.66%), aromadendrene (1.20–1.90%), 1,8-cineole (1.17–4.79%), ledene (0.47–1.69%), and limonene (0.30–1.63%).
The GC-MS data indicated that the components of the other Melaleuca EOs varied notably from each other. The MCA oil contained 1,8-cineole (64.43%) as a principal component, followed by α-pinene (5.48), α-terpineol (5.44%), β-caryophyllene (3.82%), γ-terpinene (3.24%), α-humulene (1.81%), β-selinene (1.28%), α-selinene (1.12%), β-pinene (1.11%), and terpinolene (1.04%). The most abundant constituents identified in the MNe oil were largely (E)-nerolidol (48.40%) and linalool (33.30%), whereas 1,8-cineole (52.20%), α-pinene (9.75%), (E)-nerolidol (6.89%), viridiflorol (6.23%), α-terpineol (5.08%), limonene (2.83%), β-caryophyllene (2.66%), β-pinene (2.56%), and ledene (1.76%) were identified as the main constitutes in the MNi oil. Linalool (38.19%) and 1,8-cineole (27.57%) were the main constituents of the MRo oil.
The International Standard, ISO 4730, requires terpinen-4-ol chemotype to be present in commercial TTO production [58]. ISO standards allow terpinen-4-ol between 30 and 48%, along with γ-terpinene (10–28%), α-terpinene (5–13%), 1,8-cineole (<0.01–15%), α-terpineol (1.5–8%), p-cymene (0.5–8%), α-pinene (1–6%), and terpinolene (1.5–5%), and containing a mixture of minor terpenoids with sabinene (<0.01–3.5%), aromadendrene (<0.01–3.0%), δ-cadinene (<0.01–3.0%), ledene (viridiflorene, <0.01–3.0%), limonene (0.5–1.5%), globulol (<0.01–1.0), and viridiflorol (<0.01–1.0%) [ISO]. Our GC-MS analysis revealed that all the TTO samples fitted into the terpinen-4-ol chemotype (37.7–44.3%) (Table 2). The therapeutic use of TTO is attributed to the concentration of terpinen-4-ol and 1,8-cineole (eucalyptol), yet 1,8-cineole has been reported to cause skin and mucous membranes irritation [59,60,61]. Therefore, low concentrations of 1,8-cineole are preferred to maximize the therapeutic use of TTO, and it is critical to distinguish between TTOs and other commercially available Melaleuca oils.
M. cajuputi oil has three chemotypes. Chemotype 1 contains a high concentration (50–70%) of 1,8-cineole, while chemotype 2 contains a lower concentration of 1,8-cineole (31%), and chemotype 3 contains no 1,8-cineole. M. cajuputi subsp. cajuputi is the main source of cajuput oil, which does contain 1,8-cineole [61]. Our M. cajuputi (MCa) oil from Thailand was found to be dominated by a 1,8-cineole-rich chemotype (Table 2).
M. quinquenervia can be a source of 1,8-cineole-rich essential oil [61]. Four chemotypes were reported for M. quinquenervia [62]; the cineole chemotype (1) contains 1,8-cineole (55.0–65.0%), α-pinene (7.0–12.0%), limonene (6.0–12.0%), α-terpineol (4.0-10.0%), β-pinene (1.5–4.5%), viridiflorol (1.0–3.5%), β-caryophyllene (0.01–2.0%), and myrcene (0.01–2.0%), and is called niaouli oil; the linalool chemotype (2) contains (E)-nerolidol (61.1%), linalool (23.9%), 1,8-cineole (2.6%), α-pinene (1.9%), terpinene-4-ol (1.8%), viridiflorol (1.6%), and β-caryophyllene (1.1%), and is called nerolina oil; the nerolidol chemotype (3) contains (E)-nerolidol (75.7–92.5%), β-caryophyllene (0.5–8.7%), 1,8-cineole (0.01–6.6%), caryophyllene oxide (0.1–6.1%), α-pinene + α-thujene (0–4.5%), δ-cadinol (0–2.5%), viridiflorol (0.1–1.7%), and α-terpineol + viridiflorene (ledene) (0-1.5%), and is also called niaouli oil; and the viridiflorol chemotype (4) contains viridiflorol (40.0–45.0%), 1,8-cineole (30.0–35.0%), (E)-nerolidol (3.0–6.0%), and ledol (0.01–4.0%), and is called niaouli oil, too. Our M. quinquenervia oil (MNi) from Madagascar represents chemotype 1, and the oil (MNe) from Australia represents chemotype 2 (Table 2).
M. ericifolia is from native Australian plants and is known as the lavender tea tree or rosalina oil. The major compounds of rosalina oil were identified as linalool (35.0–55.0%), 1,8-cineole (18.0–26.0%), and α-pinene (5.0–12.0%) [61,62]. Our rosalina oil (MRo) from Australia contained a high abundance of linalool and 1,8-cineole, followed by α-pinene (Table 2). Consequently, TTOs can be distinguished from other Melaleuca oils by the high amount of terpinene-4-ol and low amounts of 1,8-cineole and linalool, and the absence of (E)-nerolidol.
TTO and other Melaleuca EOs were subjected to PCA and HCA in order to identify which constituents (detected at ≥0.5%) are different among the TTOs and the four other Melaleuca species. Principle components are reflected by eigenvalues. Table 3 shows that the seven components with eigenvalues greater than one account for 96.35% of the total variance. According to the rules of PCA, the highest eigenvalues, F1 (14.92) and F2 (8.60), were selected and then subjected to the PCA analysis. The Bartlett′s sphericity test carried out on the correlation matrix shows a calculated x2 = 1519.98, greater than the critical value x2 = 52.19 with 37 degrees of freedom (p < 0.0001), thus proving that PCA can achieve a significant reduction in the dimensionality of the original data set.
Table 3.
Eigenvalues and percentage of variability and cumulative.
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
|---|---|---|---|---|---|---|---|
| Eigenvalue | 14.92 | 8.60 | 4.80 | 3.85 | 1.97 | 1.31 | 1.17 |
| Variability (%) | 39.26 | 22.62 | 12.62 | 10.13 | 5.20 | 3.45 | 3.07 |
| Cumulative (%) | 39.26 | 61.88 | 74.50 | 84.63 | 89.83 | 93.27 | 96.35 |
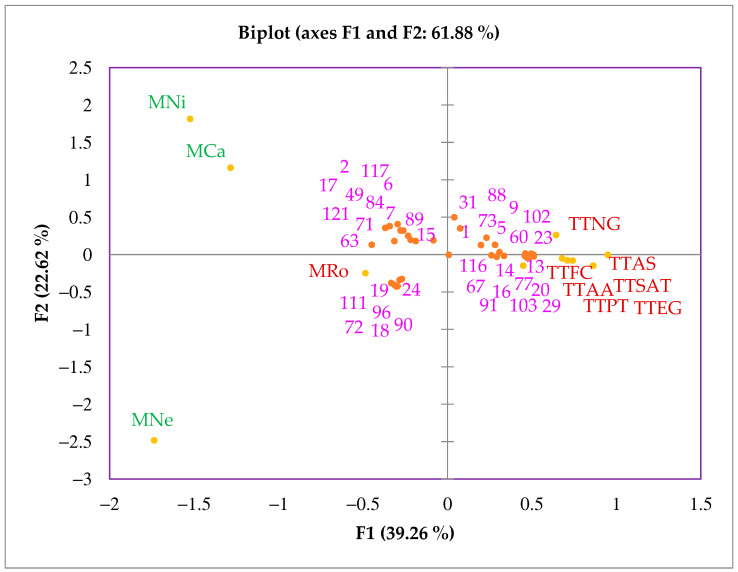
The PCA plot established according to the first two PCA axes is shown in Figure 1. Principal component 1 (F1, 39.26%) was strongly loaded for TTOs, with negative scores in MCa, MNe, MNi, and MRo. Principal component 2 (F2, 22.62%) demonstrated strong scores in MCa, MNe, MNi, and low correlations with TTOs and MRo, adding up to 61.88% of the variance in eleven Melaleuca EOs.
Figure 1.
PCA biplot of TTO and other Melaleuca EOs based on their chemical composition. EO sample codes are listed according to the code reported in Table 1. The nomenclature of volatile compounds is listed in Table 2. Same color highlights similarity in chemical composition based on statistical analysis (See Section 3.5).
PCA provides a simple way to visualize similarities among different samples; a short range between the samples means a small or very little difference, and a long distance means a strong difference. Factor loadings and squared cosine (cos2) indicate the importance of components representing the individual components for a given principal component (Table 4). The cos2 similarity always tends to be 1, showing a high linear correlation relationship of the variables with the components. The highest cos2 values were in F1: γ-terpinene (0.902), terpinene-4-ol (0.900), terpinolene (0.886), δ-cadinene (0.844), trans-cadina-1(6),4-diene (0.828), bicyclogermacrene (0.798), α-gurjunene (0.798), zonarene (0.762), and α-terpinene (0.724). Similarly, the highest cos2 value for F2 was a-terpineol (0.858). When the relationship between the factor loadings and their percentage contributions to the matrix was analyzed, it could be concluded that α-thujene, α-pinene, β-pinene, 1,8-cineole, linalool, α-terpinyl acetate, α-terpineol, β-caryophyllene, α-humulene, (E)-β-farnesene, (E)-nerolidol, viridiflorol, and ledol had a negative relationship with the TTOs. Higher percentages of compounds then appear to be of interest for the selection of quality assessment of tea tree M. alternifolia EOs.
Table 4.
Factor loadings, contributions (%), and squared cosine (cos2) values. The positive important contributions are highlighted in blue, and the negative important contributions are highlighted in pink. The highest cos2 values are highlighted in green.
| Factor Loadings and Contributions (%) |
Squared Cosines (cos2) |
||||
|---|---|---|---|---|---|
| # | Compounds | F1 | F2 | F1 | F2 |
| 1 | α−Thujene | 0.138 (0.129) | −0.649 (4.945) | 0.019 | 0.425 |
| 2 | α−Pinene | −0.546 (2.024) | −0.759 (6.695) | 0.302 | 0.575 |
| 5 | Sabinene | 0.563 (2.191) | −0.055 (0.042) | 0.327 | 0.004 |
| 6 | β-Pinene | −0.517 (1.799) | −0.601 (4.208) | 0.268 | 0.362 |
| 7 | Myrcene | −0.341 (0.824) | −0.317 (1.311) | 0.123 | 0.113 |
| 9 | α-Phellandrene | 0.525 (1.832) | −0.237 (0.681) | 0.273 | 0.059 |
| 13 | α-Terpinene | 0.853 (4.851) | 0.033 (0.010) | 0.724 | 0.001 |
| 14 | p-Cymene | 0.621 (2.579) | 0.033 (0.011) | 0.385 | 0.001 |
| 15 | Limonene | −0.157 (0.163) | −0.357 (1.474) | 0.024 | 0.127 |
| 16 | β-Phellandrene | 0.539 (1.963) | 0.052 (0.036) | 0.293 | 0.003 |
| 17 | 1,8-Cineole | −0.685 (3.144) | −0.667 (5.139) | 0.469 | 0.442 |
| 18 | (Z)-β-Ocimene | −0.561 (2.106) | 0.794 (7.323) | 0.314 | 0.629 |
| 19 | (E)-β-Ocimene | −0.527 (1.866) | 0.628 (4.594) | 0.278 | 0.395 |
| 20 | γ-Terpinene | 0.947 (6.044) | 0.013 (0.002) | 0.902 | 0.000 |
| 23 | Terpinolene | 0.943 (5.940) | −0.001 (0.000) | 0.886 | 0.000 |
| 24 | Linalool | −0.502 (1.693) | 0.605 (4.263) | 0.253 | 0.366 |
| 29 | Terpinene-4-ol | 0.948 (6.034) | 0.047 (0.024) | 0.900 | 0.002 |
| 31 | α-Terpineol | 0.074 (0.037) | −0.923 (9.987) | 0.006 | 0.858 |
| 49 | α-Terpinyl acetate | −0.637 (2.725) | −0.708 (5.793) | 0.407 | 0.498 |
| 60 | α-Gurjunene | 0.862 (4.918) | −0.023 (0.008) | 0.734 | 0.001 |
| 63 | β-Caryophyllene | −0.833 (4.665) | −0.247 (0.703) | 0.696 | 0.060 |
| 67 | Aromadendrene | 0.483 (1.556) | 0.015 (0.003) | 0.232 | 0.000 |
| 71 | α-Humulene | −0.586 (2.293) | −0.338 (1.318) | 0.342 | 0.113 |
| 72 | (E)-β-Farnesene | −0.582 (2.268) | 0.753 (6.598) | 0.338 | 0.567 |
| 73 | Alloaromadendrene | 0.373 (0.904) | −0.237 (0.654) | 0.135 | 0.056 |
| 77 | trans-Cadina-1(6),4-diene | 0.911 (5.546) | 0.050 (0.026) | 0.828 | 0.002 |
| 84 | β-Selinene | −0.434 (1.261) | −0.469 (2.534) | 0.188 | 0.218 |
| 88 | Ledene | 0.431 (1.232) | −0.422 (2.043) | 0.184 | 0.176 |
| 89 | α-Selinene | −0.407 (1.106) | −0.369 (1.568) | 0.165 | 0.135 |
| 90 | (Z,E)-α-Farnesene | −0.549 (2.022) | 0.786 (7.179) | 0.302 | 0.617 |
| 91 | Bicyclogermacrene | 0.894 (5.351) | 0.067 (0.053) | 0.798 | 0.005 |
| 96 | (E,E)-α-Farnesene | −0.584 (2.283) | 0.751 (6.563) | 0.341 | 0.564 |
| 102 | δ-Cadinene | 0.917 (5.655) | −0.024 (0.005) | 0.844 | 0.000 |
| 103 | Zonarene | 0.873 (5.105) | 0.073 (0.065) | 0.762 | 0.006 |
| 111 | (E)-Nerolidol | −0.620 (2.580) | 0.707 (5.811) | 0.385 | 0.499 |
| 116 | Globulol | 0.016 (0.001) | 0.005 (0.000) | 0.000 | 0.000 |
| 117 | Viridiflorol | −0.486 (1.593) | −0.602 (4.188) | 0.238 | 0.360 |
| 121 | Ledol | −0.506 (1.718) | −0.599 (4.143) | 0.256 | 0.356 |
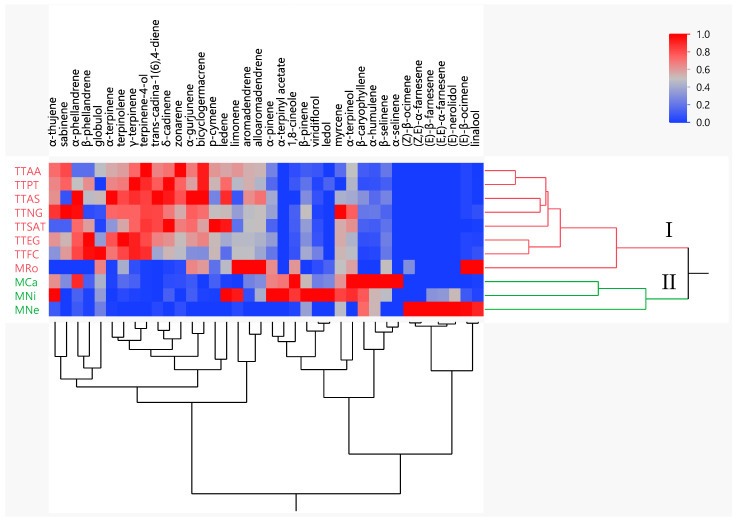
HCA classified Melaleuca EOs in two main groups (Figure 2): group I clustered samples with high contents of terpinene-4-ol, γ-terpinene, α-terpinene, and terpinolene. Although MRo did not belong to M. alternifolia (TTOs), it was grouped in cluster I due to its high levels of interfering compounds such as limonene, aromadendrene, and alloaromadendrene. The Euclidean distance between TTAA and TTPT was 1.74, and between TTAA and MRo it was 7.51. Group II clustered samples MCa, MNi, and MNe, which presented high contents of 1,8-cineole, linalool, and (E)-nerolidol, with intermediate values of α-pinene, α-terpineol, and β-caryophyllene, and lower contents of terpinene-4-ol, γ-terpinene, terpineol, and α-terpinene, indicating that the TTOs contained significantly higher concentrations of terpinen-4-ol when compared to the other Melaleuca EOs.
Figure 2.
Two-way dendrogram of the hierarchical cluster analysis (HCA) performed on the chemical composition of the seven tea tree (M. alternifolia) EOs and four other Melaleuca EOs. Sample codes refer to Table 1. The color box indicated the abundance of each compound. Red represents high density and blue represents low density.
2.2. HPTLC Analysis of Melaleuca EOs
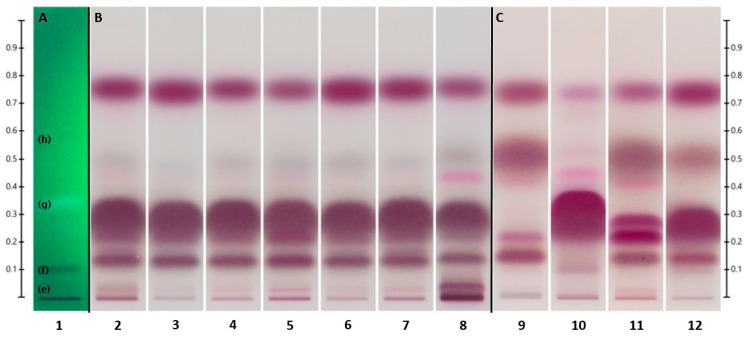
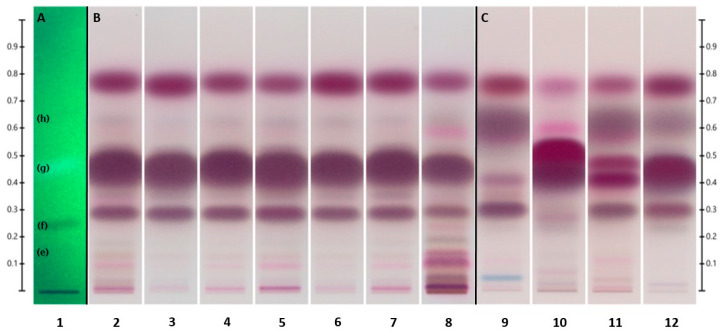
The less-polar components of the UHM, labeled (e) through (h) according to Do et al. [38], separated well under the selected HPTLC development conditions, as shown in Figure 3A. This indicated that the initial method involving Hex/EtOAc 90:10 (v/v) as the solvent system was appropriate for the separation of non-polar components of interest in our samples. However, the Rf of some Melaleuca oil components appear to exceed that of the highest UHM component. For future purposes, an additional component with higher Rf may need to be added to the UHM to better encompass the range of our samples.
Figure 3.
Separation of the UHM under UV254 (A) (see Table 5), tea tree oils (B), and other Melaleuca oils (C) (see Table 1) under visible light, developed with Hex/EtOAc 90:10 (v/v) on Silica gel 60 F254.
In general, the HPTLC results complemented the findings obtained from the GC-MS analysis. The oils of M. alternifolia (TTOs) (Figure 3B), despite their wide variety of sources, exhibited a very similar separation pattern dominated by terpinen-4-ol as the primary component. When developed with Hex/EtOAc 90:10 (v/v), this main constituent of TTO appeared at an Rf value of 0.278 ± 0.067. A group of mono- and sesquiterpenes, including α-and β-pinene (Rf = 0.767 ± 0.031) and (+)-aromadendrene (Rf = 0.738 ± 0.062), was showcased as the second most prominent band, with a collective Rf of 0.747 ± 0.065. Included in this group were ledene and δ-cadinene, identified by GC-MS. This band was followed in intensity by α-terpineol (Rf = 0.147 ± 0.052) and 1,8-cineole (Rf = 0.504 ± 0.081), respectively.
In cases where oil components were merged, as observed by the overlapping or blending of colors, such components exhibited a shift in Rf values compared to those of the corresponding reference standards. This may be caused by interactions due to large amounts of components competing for the limited silica surface area. The Rf of the merged oil constituents, as well as the presence of additional constituents, was established by GC-MS.
The other four Melaleuca EOs (Figure 3C) displayed a diverse pattern compared to each other, as well as in comparison to the TTOs. 1,8-cineole could be observed as the most characteristic component in MCa (track 9) and MNi (track 11), merged with small amounts of α-terpinyl acetate (Rf = 0.539 ± 0.050) and limonene (Rf = 0.486 ± 0.047). In contrast with MCa and MNi, cineole was less prominent in MRo (track 12) and almost insignificant in MNe (track 10). No terpinyl acetate was observed in either MNe or MRo, yet limonene was still present in MRo.
The second most noticeable band of MCa was a group of mono- and sesquiterpenes merged at Rf = 0.743 ± 0.052. These were identified by GC-MS as α- and β-pinene, β-caryophyllene (Rf = 0.727 ± 0.059), α-humulene (Rf = 0.727 ± 0.062), and α-phellandrene (Rf = 0.759 ± 0.031). Also identified by GC-MS in small amounts were α- and β-selinene. Two other chemicals present at a lower intensity, yet highly identifiable on MCa, were α-terpineol and linalool (Rf = 0.243 ± 0.056).
The two most prominent bands in MNe, combined near 0.3 Rf, were identified as nerolidol (Rf = 0.279 ± 0.056) and linalool, respectively. They were followed in intensity by the mono- and sesquiterpene band near 0.75 Rf, comprised of β-caryophyllene, β-pinene, β-ocimene (Rf = 0.747 ± 0.047), and farnesene (Rf = 0.737 ± 0.060), as identified by GC-MS. Small, yet still characteristic of MNe, was geraniol at Rf = 0.124 ± 0.067. A bright-pink band at Rf = 0.432 ± 0.045 was recognized as caryophyllene oxide by isolating the component on a TLC preparation plate (Section 3.4.2) and confirmed by GC-MS analysis against its reference standard and available GC-MS libraries. Caryophyllene oxide appeared to be a byproduct of β-caryophyllene since it was present in both TLC and TIC reference standard chromatograms.
The MNi oil was characterized by a large amount of 1,8-cineole. As with MCa, this large band was mixed with a small amount of terpinyl acetate and limonene. The second-largest band was composed of mono- and sesquiterpenes, confirmed by GC-MS as α- and β-pinene, β-caryophyllene, and ledene. Other main components were nerolidol, α-terpineol, and viridiflorol (Rf = 0.213 ± 0.021).
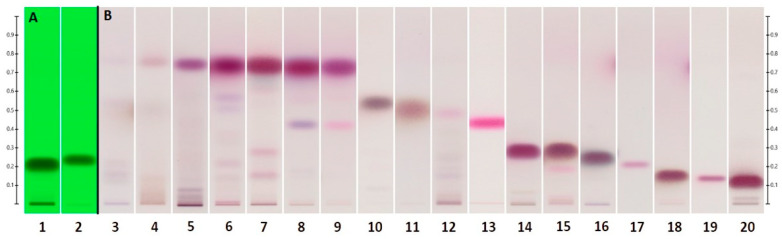
In MRo, the signature component was linalool, with a small amount of terpinen-4-ol merging into it. Mono- and sesquiterpenes composed the second-strongest band near 0.76 Rf, followed by cineole as the third characteristic band. Constituents of the second band were recognized as α-pinene, aromadendrene, alloaromadendrene, and ledene by GC-MS. Merged into a fourth band was α-terpineol, followed by globulol a bit below, at Rf = 0.137 ± 0.022. System suitability as well as Rf values for the major components were established by SSTs (Figure 4A) and reference standards (Figure 4B), analyzed under the same HPTLC conditions.
Figure 4.
SSTs (A) are shown under UV254 light. Reference standards (B) (see Table 6) are shown under visible light. Developed with Hex/EtOAc 90:10 (v/v) on Silica gel 60 F254.
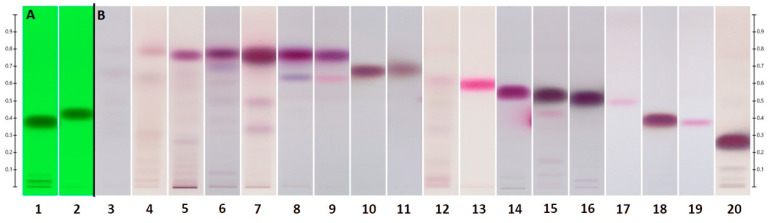
An enhanced separation of more polar constituents in the oils (lower Rf range) was obtained when developed with Hex/EtOAc at a ratio of 80:20 (v/v). Figure 5A emphasizes an increased distance among UHM constituents. Additionally, target oil components that merged at low Rf values under the previous solvent system were now appearing mid-range and better separated.
Figure 5.
Separation of the UHM under UV254 (A) (see Table 5), tea tree oils (B), and other Melaleuca oils (C) (see Table 1) under visible light, developed with Hex/EtOAc 80:20 (v/v) on Silica gel 60 F254.
The most significant improvement with a more polar solvent system was an increased separation of terpinen-4-ol (Rf = 0.532 ± 0.049) and α-terpineol (Rf = 0.385 ± 0.054) in TTOs (Figure 5B). With the other Melaleuca oils (Figure 5C), where the differences among samples relied more on the polar region, a greater distinction could be made among nerolidol (Rf = 0.549 ± 0.052), linalool (Rf = 0.514 ± 0.055), viridiflorol (Rf = 0.494 ± 0.027), and terpinen-4-ol (Rf = 0.532 ± 0.049), as well as between α-terpineol (Rf = 0.385 ± 0.054), globulol (Rf = 0.376 ± 0.021), and geraniol (Rf = 0.263 ± 0.052). These differences in Rf were more evident at lower concentrations, as demonstrated by the SSTs (Figure 6A) and the individual reference standards (Figure 6B) analyzed under exact HPTLC developing conditions.
Figure 6.
SSTs (A) are shown under UV254 light. Reference standards (B) (see Table 6) are shown under visible light. Developed with Hex/EtOAc 80:20 (v/v) on Silica gel 60 F254.
3. Materials and Methods
3.1. Sample Selection and Preparation
TTOs from M. alternifolia were selected based on their previously established biological activity as a potential attractant for the male Mediterranean fruit fly [23,24,26,27]. Essential oils from other Melaleuca species were also included for the purpose of comparison (Table 1). Each sample was diluted to 20% of its original purity using methylene chloride, ACS Reagent, CAS# 75-09-2 (J.T. Baker-Avantor, Center Valley, PA, USA). If necessary, concentration and application volume were adjusted for optimum HPTLC separation.
3.2. Standard Selection and Preparation
A universal HPTLC calibration mix (UHM) (Sigma-Aldrich, St. Louis, MO, USA) was used as a reference standard for HPTLC separations. It contains eight different compounds diluted in methanol at ready-to-use concentrations [38], four of which were observed under our HPTLC conditions using a UV254 light source (Table 5).
Table 5.
List of UHM * components used in this study.
| Label | Name | CAS# |
|---|---|---|
| (e) | Phthalamide | 85-41–6 |
| (f) | 9-Hydroxyfluorene | 1689-64-1 |
| (g) | Thioxanthen-9-one | 492-22-8 |
| (h) | 2-(2H-Benzotriazol-2-yl)-4-(1,1,3,3-tetramethylbutyl) phenol | 3147-75-9 |
* UHM components visible under the established analytical conditions.
Stock solutions of isoeugenol, CAS# 97-54-1, and isoeugenyl acetate, CAS# 93-29-8 (Sigma-Aldrich, St. Louis, MO, USA), were prepared in methylene chloride at a concentration of 100 µL/mL and 100 µg/mL, respectively. A 20 µL/mL (21.6 mg/mL; δ = 1.08 g/mL) methylene chloride dilution of isoeugenol, as well as a 20 mg/mL dilution of isoeugenyl acetate, were prepared from their corresponding stock and used as system suitability standards (SST1 and SST2).
A series of reference standards (Table 6) were obtained from Sigma-Aldrich (St. Louis, MO, USA), and were prepared and analyzed under the same conditions as the EOs to confirm the Rf values of the oil components on HPTLC.
Table 6.
System suitability standards (SSTs) and reference standards for Melaleuca EOs.
| Track | Name | CAS# | Track | Name | CAS# |
|---|---|---|---|---|---|
| 1 | Isoeugenol | 97-54-1 | 11 | 1,8-Cineole | 470-82-6 |
| 2 | Isoeugenyl acetate | 93-29-8 | 12 | (S)-(−)-Limonene | 5989-54-8 |
| 3 | (1R)-(+)-α-Pinene | 7785-70-8 | 13 | (−)-Caryophyllene oxide | 1139-30-6 |
| 4 | α-Phellandrene | 99-83-2 | 14 | Nerolidol | 7212-44-4 |
| 5 | Ocimene mix | 13877-91-3 | 15 | (−)-Terpinen-4-ol | 20126-76-5 |
| 6 | (+)-Aromadendrene | 489-39-4 | 16 | (−)-Linalool | 1126-91-0 |
| 7 | Farnesene mix | 502-61-4 | 17 | Viridiflorol | 0552-02-03 |
| 8 | α-Humulene | 6753-98-6 | 18 | (−)-α-Terpineol | 10482-56-1 |
| 9 | β-Caryophyllene | 87-44-5 | 19 | (−)-Globulol | 489-41-8 |
| 10 | α-Terpinyl acetate | 80-26-2 | 20 | Geraniol | 106-24-1 |
3.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
Melaleuca EO samples were analyzed on an Agilent 7890B GC coupled with a 5977B mass selective detector (GC-MS) (Agilent Technologies, Santa Clara, CA, USA). A DB-5 column (30 m × 0.25 mm inner diameter with 0.25 μm film thickness) was used with an electron ionization source set at 70 eV. The temperatures of the ion source and quadrupole were 230 °C and 150 °C, respectively. The mass spectrometry transmission line was 250 °C. Injector and detector temperatures were kept at 220 °C and 230 °C, respectively. The oven temperature program was set at 60 °C for 1.3 min and increased to 246 °C at 3 °C/min. A constant helium flow of 1.3 mL/min was applied [41]. The selected mass range was m/z 35 to 450 Da and scan rate was 2.8 scans/s. Mass Hunter B.07.06 software (Agilent Technologies) was used for data acquisition and processing. One μL of diluted samples was injected into the GC–MS on splitless mode.
Linear retention indices (RIs) were calculated using the van Den Dool and Kratz [42] equation in relation to a homologous series of n-alkanes (C9–C21). Compound identification was achieved by comparison of their corresponding mass spectra and RIs to those reported in a mass spectral library developed at the USDA-ARS-SHRS laboratory with authentic compounds and with the commercial libraries MassFinder [43], Adams Library [41], Flavours and Fragrances of Natural and Synthetic Compounds 3 (FFNSC-3) [44], Wiley 12/NIST 2020 [45], and an in-house library “SHRS Essential Oil Constituents-DB-5 Column”. Retention indices were also verified with data reported in the specific literature [46,47,48,49,50,51,52,53,54] and internet sources [55,56,57]. Each oil was analyzed in triplicate. Relative percentages were directly obtained from peak total ion current (TIC) areas. All these standards were purchased from the following sources: α-pinene (CAS# 80-56-8), camphene (CAS# 79-92-5), benzaldehyde (CAS# 100-52-7), sabinene (CAS# 3387-41-5), β-pinene (CAS# 127-91-3), myrcene (CAS# 123-35-3), 6-methyl-5-hepten-2-ol (CAS# 1569-60-4), α-phellandrene (CAS# 99-792-5), δ-3-carene (CAS# 13466-78-9), 1,4-cineole (CAS# 470-67-7), α-terpinene (CAS# 99-86-5), p-cymene (CAS# 99-87-6), limonene (CAS# 5989-27-5), 1,8-cineole (CAS# 470-82-6), ocimene mixture (CAS# 13877-91-3), γ-terpinene (CAS# 99-85-4), linalool oxide (CAS# 60047-17-8), terpinolene (CAS# 586-62-9), linalool (CAS# 78-70-6), terpinen-4-ol (CAS# 20126-76-5), α-terpineol (CAS# 10482-56-1), citronellol (CAS# 106-22-9), geraniol (CAS# 106-24-1), thymol (CAS# 89-83-8), carvacrol (CAS# 499-75-2), α-terpinyl acetate (CAS# 80-26-2), eugenol (CAS# 97-53-0), geranyl acetate (CAS# 105-87-3), methyl eugenol (CAS# 93-15-2), β-caryophyllene (CAS# 87-44-5), aromadendrene (CAS# 489-39-4), α-humulene (CAS# 6753-98-6), (E)-β-farnesene (CAS# 18797-84-8), farnesene, mixture of isomers (product number W383902), alloaromadendrene (CAS# 25246-27-9), nerolidol (CAS# 7212-44-4), caryophyllene oxide (CAS# 1139-30-6), globulol (CAS# 489-41-8), viridiflorol (CAS# 552-02-3), α-bisabolol (Cas# 23089-26-2), farnesol mixture (CAS# 4602-84-0) from Sigma-Aldrich, St. Louis, MO, USA; β-phellandrene (CAS# 555-10-2) from Toronto Research Chemicals (Toronto, ON, Canada); α-copaene (CAS# 3856-25-5) and β-elemene (CAS# 515-13-9) from Fluka Chemical Co., Buchs, SG, Switzerland); and (+)-ar-curcumene (CAS# 4176-06-1) from BOC Sciences Shirley, NY, USA.
3.4. Thin-Layer Chromatography Analysis
3.4.1. Automated High-Performance Thin-Layer Chromatography (HPTLC) Analysis
Chromatography was performed using a CAMAG HPTLC system equipped with VisionCATS 3.1 software (CAMAG, Muttenz, Switzerland). Initial conditions were set following the established HPTLC/TLC protocol for essential oils [37,63]. An HPTLC Silica gel 60 F254 glass-backed plate, 20 × 10 cm (Supelco Merck KGaA, Darmstadt, Germany, operating as Millipore-Sigma in St. Louis, MO, USA), was activated by heat using a TLC Plate Heater III (CAMAG, Muttenz, Switzerland) for 10 min. at 65 °C prior to analysis. Toluene/ethyl acetate 93:7 (v/v) was used as the mobile phase. However, previous bioassays (P.E.K. unpublished data) had indicated that sterile male medflies were repelled by toluene residue, prompting the search for an alternative mobile phase. Hexane was selected due to its similar polarity to toluene.
Melaleuca EO constituents appear in different amounts and cover a relatively wide polarity range when separated by TLC. Tabanca et al. [27] used hexane/acetone 90:10 (v/v) and obtained a good separation that produced two TTO fractions attractive to sterile male medflies, yet these fractions still contained a mixture of chemicals. Further separation was necessary to identify possible individual attractants. Various ratios of hexane/ethyl acetate were then attempted in preliminary experiments and it was decided that two separate solvent combinations provided improved resolution of the fractions of interest.
For the separation of monoterpenes and other non-polar oil constituents, a solution of 45 mL hexane (Hex), Certified ACS, CAS# 92112-69-1 (Fisher Chemical, Thermo Fisher Scientific, Waltham, MA, USA), and 5 mL ethyl acetate (EtOAc), HPLC grade, ≥99.7%, CAS#141-78-6 (Sigma-Aldrich, St. Louis, MO, USA), was prepared for a ratio of 90:10 (v/v). To favor the separation of more polar compounds, 40 mL Hex and 10 mL EtOAc were mixed for an 80:20 (v/v) ratio.
An aliquot of each oil sample was dispensed into a 1.5 mL screw-cap vial, covered with TFE/SIL septum cap (J.G. Finneran Associates, Inc., Vineland, NJ, USA), and placed into an Automatic TLC Sampler (ATS4) (CAMAG, Muttenz, Switzerland). An activated silica gel plate was placed in its corresponding holder. Samples were applied as thin bands (8 mm long, 8 mm from the bottom edge of the plate) using a 25 µL Hamilton syringe with spray application needle and nozzle. Syringe and needle were automatically rinsed 5 times with methanol, ACS grade, CAS# 67-56-1 (Supelco Merck KGaA, Darmstadt, Germany, as EMD Millipore Corporation, Burlington, MA, USA), between samples.
The HPTLC plate was developed in an Automatic Developing Chamber (ADC2) (CAMAG, Muttenz, Switzerland). The chamber containing a saturation pad was saturated for 20 min with 25 mL of the selected mobile phase. To remove as much moisture as possible, the system was also activated for 10 min with a saturated magnesium chloride aqueous solution prepared from magnesium chloride hexahydrate, (MgCl2.6H2O), CAS# 7791-18-6 (Sigma-Aldrich, St. Louis, MO, USA). Development was automatically started and stopped once the solvent front reached a preset height of 85 mm. After development, the plate was allowed to dry for 1 min at room temperature in the fume hood.
A vanillin/sulfuric acid derivatizing reagent was prepared following Wagner and Bladt [63], by adding 0.4 g of vanillin Reagent Plus, 99%, CAS# 121-33-5 (Sigma-Aldrich, St. Louis, MO, USA), to 100 mL of 190 proof ethanol, USP, CAS# 64-17-5 (Decon Labs, Inc., King of Prussia, PA, USA). The ethanolic solution was kept refrigerated until use. Concentrated sulfuric acid, Certified ACS Plus, CAS# 7664-93-9 (Fisher Chemical, Thermo Fisher Scientific, Waltham, MA, USA), was added shortly before use at a proportion of 20 µL acid per mL of vanillin solution.
Automatic derivatization of the plate to generate color occurred inside a Derivatizer chamber (CAMAG, Muttenz, Switzerland) with 2 mL of vanillin/H2SO4 reagent. The derivatizing reagent was applied by spraying through a yellow nozzle at spray level 3. Colors were observed after heating the plate for 1.5 to 3.0 min at 100 °C on a CAMAG Plate Heater III, depending on color intensity. Images of the plate were taken at various stages of the process using a Visualizer 2 (CAMAG, Muttenz, Switzerland) with a 16 mm lens under RT White, UV254, and UV366 light. The retention factors (Rf) values were calculated by VisionCATS software version 3.1. Profiles and comparisons were also generated using VisionCATS.
3.4.2. Automated Preparative Thin-Layer Chromatography Analysis
Preparative TLC was used to isolate unknown bands for identification in cases where a component was not readily identified, and a standard could not be easily referenced for confirmation. An HPTLC Silica gel 60 F254 glass-backed plate, 20 × 10 cm (Supelco Merck KGaA, Darmstadt, Germany, operating as Millipore-Sigma in St. Louis, MO, US), was used to separate and collect a reasonable amount of the unknown chemical for further identification. The plate was initially activated by heating at 100 °C for 15 min on a CAMAG TLC Plate Heater III.
Sample application was conducted in a CAMAG ATS4 autosampler, where 2 µL oil was applied in 20 consecutive bands, 8 mm long each, making a solid horizontal line 8 mm from the bottom edge of the plate. Once the sample was applied, the plate was developed in a previously saturated CAMAG ADC2 chamber. A total of 35 mL mobile phase was used, 25 mL for saturation and 10 for development. Development stopped when solvent front reached 85 mm.
In the case of nerolina oil, a bright pink band at Rf = 0.451 ± 0.022 (40–45 mm from the bottom) was our target chemical. A 5 × 10 cm strip was cut out of the developed plate and was sprayed with vanillin reagent in a CAMAG Derivatizer. The bright pink band on the derivatized strip provided the measurements of the area to scrape to obtain our unknown from the remaining (non-derivatized) portion of the plate. Scraped silica containing our compound of interest was extracted with 1 mL methylene chloride and filtered through a 0.2 µm Whatman AUTOVIAL™ 5 syringeless filter (Global Life Sciences Solutions USA LLC–Cytiva, Marlborough, MA, USA) for GC-MS analysis.
3.5. Statistical Analysis
Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were applied to TTOs and other Melaleuca EOs and their chemical constituents, using the XLSTAT 2021 (Addinsoft, New York, NY, USA) for PCA and JMP (JMP® Pro 17.0.0, SAS Institute Inc. Cary, NC, USA) for HCA. Both PCA and HCA were performed on the means of those volatile constituents higher than 0.5%; the covariance data matrix was 38 × 11 (418 data). Pearson’s correlation model was used for PCA, Euclidean distance for measure, and Ward’s method for HCA analysis.
4. Conclusions
The results of this study demonstrate that HPTLC serves as a quick and effective analytical technique for the screening of selected Melaleuca oils. Its automated steps eliminate most human error and provide better reproducibility. A wide variety of samples may be analyzed by combining the most suitable mobile and stationary phases for the target analytes. This allows for the selection of less toxic solvents, such as hexane instead of toluene, while maintaining comparable retention factors to those in the Pharmacopeia. It also provides a fast detection tool for more polar additives or contaminants that may not be detected under GC-MS conditions. Samples can be applied as a long, narrow band, allowing multiple samples to be simultaneously analyzed and a cleaner separation of individual components.
An advantage over other analytical techniques is that multiple samples and standards may be analyzed at the same time and under true identical conditions using HPTLC. Moreover, the development process is nondestructive, which allows samples to be scraped and extracted from the plate for further studies. For this purpose, a template may be created from a prior plate derivatized with color reagent.
On the other hand, there are some disadvantages to this procedure. In the case of highly volatile constituents, there is a high probability of evaporation during the process. In addition, some oil components do not react with the derivatizing reagent and therefore do not emit a visible color. While some may be seen under UV light, others may not be visible at all. Another complicating factor is that compounds found in trace amounts may fall under the detection limit of the HPTLC instrument. A more complex mixture of coeluting chemicals also represents a challenge. Two or more developments may be required for better separation of these target constituents.
New studies are currently in process, involving two-dimensional and multigradient developments to address the above-mentioned challenges. Future work prospects include the addition of a densitometry module and a TLC-MS interface to achieve a more accurate quantification and precise recovery of individual oil components for further analysis. With so many favorable features and few obstacles, this technique proved to be an efficient and reliable screening tool for the selected Melaleuca oils.
Acknowledgments
The authors are grateful to Elena Q. Schnell (USDA-ARS, Miami, FL, USA) and Wayne S. Montgomery (USDA-ARS, Miami, FL, USA) for their technical assistance with this work, and to Kevin R. Cloonan (USDA-ARS, Miami, FL, USA), Xiangbing Yang (USDA-ARS, Miami, FL, USA), and three anonymous referees for providing reviews of a previous version of this manuscript.
Author Contributions
Conceptualization, A.V., N.T. and P.E.K.; methodology, A.V. and N.T.; software, A.V. and N.T.; validation, A.V., N.T. and P.E.K.; formal analysis, A.V. and N.T.; investigation, A.V., N.T. and P.E.K.; data curation, A.V. and N.T; writing—original draft preparation, A.V. and N.T.; writing—review and editing, A.V., N.T. and P.E.K.; visualization, A.V.; supervision, N.T. and P.E.K.; project administration, P.E.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest. Mention of a proprietary product does not constitute an endorsement by the USDA-ARS. USDA is an equal opportunity provider and employer.
Sample Availability
Not applicable.
Funding Statement
This study was supported by appropriated funds from the United States Department of Agriculture, Agricultural Research Service (USDA-ARS) (Project Number: 6038-22000-007-00D), and an appointment (AV) to the ARS Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE), an interagency agreement between the U.S. Department of Energy (DOE) and the USDA, managed under DOE contract # DE-SC0014664.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.The Plant List. [(accessed on 22 November 2022)]. Available online: https://www.theplantlist.org/
- 2.Bejar E. Botanical Adulterants Prevention Bulletin. ABC-AHP-NCNPR Botanical Adulterants Prevention Program; Austin, TX, USA: 2017. Adulteration of Tea Tree Oil (Melaleuca alternifolia and M. linariifolia) pp. 1–8. [Google Scholar]
- 3.Gafner S., Dowell A. Botanical Adulterants Prevention Bulletin. ABC-AHP-NCNPR Botanical Adulterants Prevention Program; Austin, TX, USA: 2018. Tea Tree Oil Laboratory Guidance Document; pp. 1–13. [Google Scholar]
- 4.Hammer K.A., Carson C.F., Riley T.V. In-vitro Activity of Essential Oils, In Particular Melaleuca alternifolia (tea tree) Oil and Tea Tree Oil Products, Against Candida spp. J. Antimicrob. Chemother. 1998;42:591–595. doi: 10.1093/jac/42.5.591. [DOI] [PubMed] [Google Scholar]
- 5.Carson C.F., Hammer K.A., Riley T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lis-Balchin M., Hart S.L., Deans S.G. Pharmacological and Antimicrobial Studies on Different Tea-Tree Oils (Melaleuca alternifolia, Leptospermum scoparium or Manuka and Kunzea ericoides or Kanuka), Originating in Australia and New Zealand. Phytother. Res. 2000;14:623–629. doi: 10.1002/1099-1573(200012)14:8<623::AID-PTR763>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Kairey L., Agnew T., Bowles E.J., Barkla B.J., Wardle J., Lauche R. Efficacy and Safety of Melaleuca alternifolia (Tea Tree) Oil for Human Health—A Systematic Review of Randomized Controlled Trials. Front. Pharmacol. 2023;24:1116077. doi: 10.3389/fphar.2023.1116077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chouhan S., Sharma K., Guleria S. Antimicrobial Activity of Some Essential Oils—Present Status and Future perspectives. Medicines. 2017;4:58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Man A., Santacroce L., Jacob R., Mare A., Man L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens. 2019;8:15. doi: 10.3390/pathogens8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendra P.E., Montgomery W.S., Niogret J., Tabanca N., Owens D., Epsky N.D. Utility of Essential Oils for Development of Host-Based Lures for Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), Vector of laurel Wilt. Open Chem. 2018;16:393–400. doi: 10.1515/chem-2018-0045. [DOI] [Google Scholar]
- 11.Niogret J., Gill M.A., Espinosa H.R., Kendra P.E., Epsky N.D. Attraction and Electroantennogram Responses of Male Mediterranean Fruit Fly (Diptera: Tephritidae) to Six Plant Essential Oils. J. Entomol. Zool. Stud. 2017;5:958–964. [Google Scholar]
- 12.Tabanca N., Bernier U.R., Agramonte N.M., Tsikolia M., Bloomquist J.R. Discovery of Repellents from Natural Products. Curr. Org. Chem. 2016;20:2690–2702. doi: 10.2174/1385272820666160421151503. [DOI] [Google Scholar]
- 13.Kurtca M., Tumen I., Keskin H., Tabanca N., Yang X., Dermirci B., Kendra P.E. Chemical Composition of Essential Oils from Leaves and Fruits of Juniperus foetidissima and Their Attractancy and Toxicity to Two Economically Important Tephritid Fruit Fly Species, Ceratitis capitata and Anastrepha suspensa. Molecules. 2021;26:7054. doi: 10.3390/molecules26247504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson B.J., Tabanca N., Kirimer N., Demirci B., Baser K.H.C., Khan I.A., Spiers J.M., Wedge D.E. Insecticidal Activity of 23 Essential Oils and Their Major Compounds Against Adult Lipaphis pseudobrassicae (Davis) (Aphididae: Homoptera) Pest Manag. Sci. 2005;61:1122–1128. doi: 10.1002/ps.1100. [DOI] [PubMed] [Google Scholar]
- 15.Sakhanokho H.F., Sampson B.J., Tabanca N., Wedge D.E., Demirci B., Baser K.H.C., Bernier U.R., Tsikolia M., Agramonte N.M., Becnel J.J., et al. Chemical Composition, Antifungal and Insecticidal Activities of Hedychium Essential Oils. Molecules. 2013;18:4308–4327. doi: 10.3390/molecules18044308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali A., Murphy C.C., Demirci B., Wedge D.E., Sampson B.J., Khan I.A., Baser K.H.C., Tabanca N. Insecticidal and Biting Deterrent Activity of Rose-Scented Geranium (Pelargonium spp.) Essential Oils and Individual Compounds Against Stephanitis pyrioides and Aedes aegypti. Pest Manag. Sci. 2013;69:1385–1392. doi: 10.1002/ps.3518. [DOI] [PubMed] [Google Scholar]
- 17.Luu-Dam N.A., Tabanca N., Estep A.S., Nguyen D.H., Kendra P.E. Insecticidal and Attractant Activities of Magnolia citrata Leaf Essential Oil against Two Major Pests from Diptera: Aedes aegypti (Culicidae) and Ceratitis capitata (Tephritidae) Molecules. 2021;26:2311. doi: 10.3390/molecules26082311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isman M.B. Plant Essential Oils for Pest and Disease Management. Crop Prot. 2000;19:603–608. doi: 10.1016/S0261-2194(00)00079-X. [DOI] [Google Scholar]
- 19.Kendra P.E., Tabanca N., Montgomery W.S., Niogret J., Owens D., Carrillo D. Essential Oils as Lures for Invasive Ambrosia Beetles. In: Baser K.H.C., Buchbauer G., editors. Handbook of Essential Oils: Science, Technology, and Applications. 3rd ed. CRC Press; Boca Raton, FL, USA: 2020. pp. 495–513. [DOI] [Google Scholar]
- 20.Jones O.T. Ceratitis capitata. In: Robinson A.S., Hooper G., editors. Fruit Flies: Their Biology, Natural Enemies and Control. 1st ed. Volume 3A. Elsevier; Amsterdam, The Netherlands: 1989. pp. 179–183. [Google Scholar]
- 21.Silva J.G., Meixner M.D., McPheron B.A., Steck G.J., Sheppard W.S. Recent Mediterranean Fruit Fly (Diptera: Tephritidae) Infestations in Florida—A Genetic Perspective. J. Econ. Entomol. 2003;96:1711–1718. doi: 10.1603/0022-0493-96.6.1711. [DOI] [PubMed] [Google Scholar]
- 22.University of Florida—Institute of Food and Agricultural Science Featured Creatures, Common Name: Mediterranean Fruit Fly. [(accessed on 2 November 2022)]. Available online: https://entnemdept.ufl.edu/creatures/fruit/mediterranean_fruit_fly.htm.
- 23.Shelly T.E., Epsky N.D. Exposure to Tea Tree Oil Enhances the Mating Success of Male Mediterranean Fruit Flies (Diptera: Tephritidae) Fla. Entomol. 2015;98:1127–1133. doi: 10.1653/024.098.0417. [DOI] [Google Scholar]
- 24.Epsky N.D., Niogret J. Short Range Attraction of Ceratitis capitata (Diptera: Tephritidae) Sterile Males to Six Commercially Available Plant Essential Oils. Nat. Volatiles Essent. Oils. 2017;4:1–7. [Google Scholar]
- 25.Tabanca N., Wedge D.E., Li X., Gao Z., Ozek T., Bernier U.R., Epsky N.D., Baser K.H.C., Ozek G. Biological Evaluation, Overpressured Layer Chromatography (OPLC) Separation and Isolation of a New Acetylenic Derivative Compound from Prangos platychlaena ssp. platychlaena Fruit Essential Oils. J. Planar. Chromatogr. Mod. TLC. 2018;31:61–71. doi: 10.1556/1006.2018.31.1.8. [DOI] [Google Scholar]
- 26.Tabanca N., Masi M., Epsky N.D., Nocera P., Cimmino A., Kendra P.E., Niogret J., Evidente A. Laboratory Evaluation of Natural and Synthetic Aromatic Compounds as Potential Attractants for Male Mediterranean Fruit Fly, Ceratitis capitata. Molecules. 2019;24:2409. doi: 10.3390/molecules24132409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabanca N., Niogret J., Kendra P.K., Epsky N.D. TLC-Based Bioassay to Isolate Kairomones from Tea Tree Essential Oil That Attract Male Mediterranean Fruit Flies, Ceratitis capitata (Wiedemann) Biomolecules. 2020;10:683. doi: 10.3390/biom10050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabanca N., Nalbantsoy A., Kendra P.E., Demirci F., Demirci B. Chemical Characterization and Biological Activity of Mastic Gum Essential Oils from Pistacia lentiscus var. chia from Turkey. . Molecules. 2020;25:2136. doi: 10.3390/molecules25092136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blythe E.K., Tabanca N., Demirci B., Kendra P.E. Chemical Composition of Essential Oil from Tetradenia riparia and Its Attractant Activity for Mediterranean Fruit Fly, Ceratitis capitata. Nat. Prod. Commun. 2020;15:1–6. doi: 10.1177/1934578X20953955. [DOI] [Google Scholar]
- 30.Stappen I., Wanner J., Tabanca N., Bernier U.R., Kendra P.E. Blue Tansy Oil: Chemical Composition, Repellent Activity Against Aedes aegypti and Attractant Activity for Ceratitis capitata. Nat. Prod. Commun. 2021;16:1–8. doi: 10.1177/1934578X21990194. [DOI] [Google Scholar]
- 31.Yusufoglu H.S., Alqarni M.H., Salkini M.A., Tabanca N., Demirci B., Kendra P.E. Chemical Composition of Essential Oils of Pulicaria Species Growing in Saudi Arabia and Activity for Mediterranean Fruit Fly, Ceratitis capitata. Phytochem. Lett. 2021;46:51–55. doi: 10.1016/j.phytol.2021.08.021. [DOI] [Google Scholar]
- 32.Lata E., Fulczyk A., Ott P.G., Kowalska T., Sajewicz M., Moricz A.M. Thin-Layer Chromatographic Quantification of Magnolol and Honokiol in Dietary Supplements and Selected Biological Properties of These Preparations. J. Chromatogr. A. 2020;1625:461230. doi: 10.1016/j.chroma.2020.461230. [DOI] [PubMed] [Google Scholar]
- 33.Wang X., Habib E., Leon F., Radwan M.M., Tabanca N., Gao J., Wedge D.E., Cutler S.J. Antifungal Metabolites from the Roots of Diospyros virginiana by Overpressure Layer Chromatography. Chem. Biodivers. 2011;8:2331–2340. doi: 10.1002/cbdv.201000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perera W.H., Frommenwiler D.A., Sharaf M.H.M., Reich E. An Improved High-Performance Thin-Layer Chromatographic Method to Unambiguously Assess Ginkgo biloba Leaf Finished Products. J. Planar. Chromatogr. Mod. TLC. 2022;34:559–560. doi: 10.1007/s00764-021-00146-0. [DOI] [Google Scholar]
- 35.Baglyas M., Ott P.G., Garadi Z., Glavnik V., Beni S., Vovk I., Moricz A.M. High-Performance Thin-Layer Chromatography—Antibacterial Assay First Reveals Bioactive Clerodane Diterpenes in Giant Goldenrod (Solidago gigantea Ait.) J. Chromatogr. A. 2022;1677:463308. doi: 10.1016/j.chroma.2022.463308. [DOI] [PubMed] [Google Scholar]
- 36.Trovato E., Micalizzi G., Dugo P., Utczas M., Mondello L. Use of Linear Retention Indices in GC-MS Libraries for Essential Oil Analysis. In: Baser K.H.C., Buchbauer G., editors. Handbook of Essential Oils: Science, Technology, and Applications. 3rd ed. CRC Press; Boca Raton, FL, USA: 2020. pp. 229–251. [Google Scholar]
- 37.Do T.K.T., Trettin I., De Vaumas R., Cañigueral S., Valder C., Reich E. Proposal for a Standardized Method for The Identification of Essential Oils by HPTLC. Pharmeuropa Bio Sci. Notes. 2021;2021:157–166. [PubMed] [Google Scholar]
- 38.Do T.K.T., Schmid M., Phanse M., Charegaonkar A., Sprecher H., Obkircher M., Reich E. Development of The First Universal Mixture for Use in System Suitability Tests for High-Performance Thin Layer Chromatography. J. Chromatogr. A. 2021;1638:461830. doi: 10.1016/j.chroma.2020.461830. [DOI] [PubMed] [Google Scholar]
- 39.Yasin M., Younis A., Javed T., Akram A., Ahsan M., Shabbir R., Ali M.M., Tahir A., El-Ballat E.M., Sheteiwy M.S., et al. River Tea Tree Oil: Composition, Antimicrobial and Antioxidant Activities, and Potential Applications in Agriculture. Plants. 2021;10:2105. doi: 10.3390/plants10102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabir S., Arshad M., Zahara K., Tabassum S., Chaudhari S.K. Pharmacological Attributes and Nutritional Benefits of Tea Tree Oil. Int. J. Biol. Sci. 2014;5:80–91. [Google Scholar]
- 41.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corp; Carol Stream, IL, USA: 2017. pp. 1–804. [Google Scholar]
- 42.van Den Dool H., Kratz P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 43.MassFinder . MassFinder Software. Dr. Hochmuth Scientific Consulting; Hamburg, Germany: 2004. Version 3. [Google Scholar]
- 44.FFNSC3 Flavors and Fragrances of Natural and Synthetic Compounds. 2015.
- 45.Wiley Science Solution, Wiley Registry 12th Edition/NIST 2020 Mass Spectral Library, Solutions. Scientific Instrument Services Inc.; Ringoes, NJ, USA: 2020. [Google Scholar]
- 46.Fitsiou I., Tzakou O., Hancianu M., Poiata A. Volatile Constituents and Antimicrobial Activity of Tilia tomentosa Moench and Tilia cordata Miller Oils. J. Essent. Oil Res. 2007;19:183–185. doi: 10.1080/10412905.2007.9699255. [DOI] [Google Scholar]
- 47.Al-Kaf A.G., Crouch R.A., Denkert A., Porzel A., Al-Hawshabi O.S.S., Ali N.A.A., Setzer W.N. Chemical Composition and Biological Activity of Essential Oil of Chenopodium ambrosioides from Yemen. Am. J. Essent. Oil. Nat. Prod. 2016;4:20–22. [Google Scholar]
- 48.Olawore N.O., Usman L.A., Ogunwande I.A., Adeleke K.A. Constituents of Rhizome Essential Oils of Two Types of Cyperus articulatus L. Grown in Nigeria. J. Essent. Oil Res. 2006;18:604–606. doi: 10.1080/10412905.2006.9699179. [DOI] [Google Scholar]
- 49.Garneau F.X., Collin G.J., Jean F.I., Gagnon H., Arze J.B.L. Essential Oils from Bolivia. XII. Asteraceae: Ophryosporus piquerioides (D.C.) Benth. ex Baker. J. Essent. Oil Res. 2013;25:388–393. doi: 10.1080/10412905.2013.827478. [DOI] [Google Scholar]
- 50.Huong L.T., Hung N.H., Dai D.N., Tai T.A., Hien V.T., Satyal P., Setzer. W.N. Chemical Compositions and Mosquito Larvicidal Activities of Essential Oils from Piper Species Growing Wild in Central Vietnam. Molecules. 2019;24:3871. doi: 10.3390/molecules24213871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowalski R., Wierciński J., Mardarowicz M. Essential Oil in Leaves and Inflorescences of Silphium integrifolium Michx. J. Essent. Oil Res. 2005;17:220–222. doi: 10.1080/10412905.2005.9698881. [DOI] [Google Scholar]
- 52.Lazarević J., Radulović N., Palić R., Zlatković B. Chemical Composition of the Essential Oil of Doronicum austriacum Jacq. subsp. giganteum (Griseb.) Stoj. et Stef. (Compositae) From Serbia. J. Essent. Oil Res. 2009;21:507–510. doi: 10.1080/10412905.2009.9700230. [DOI] [Google Scholar]
- 53.Upadhyaya K., Dixit V.K., Padalia R.C., Matgela C.S. Chemical Composition of the Essential Oil of Caryopteris grata Benth. J. Essent. Oil Res. 2009;21:69–70. doi: 10.1080/10412905.2009.9700113. [DOI] [Google Scholar]
- 54.Babushok V.I., Linstrom P.J., Zenkevich I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data. 2011;40:043101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
- 55.The Pherobase Database of Pheromones and Semiochemicals. [(accessed on 7 January 2023)]. Available online: https://www.pherobase.com.
- 56.PubChem. [(accessed on 17 January 2023)]; Available online: https://pubchem.ncbi.nlm.nih.gov.
- 57.Acree T.E., Arn H. The Flavornet. [(accessed on 2 February 2023)]. Available online: https://www.flavornet.org.
- 58.Oil of Melaleuca, Terpinen-4-ol Type (Tea Tree Oil) ISO; Geneva, Switzerland: 2004. [Google Scholar]
- 59.Barbosa L.C.A., Silva C.J., Teixeira R.R., Meira R.M.S.A., Pinheiro A.L. Chemistry and Biological Activities of Essential Oils from Melaleuca L. Species. Agric. Conspec. Sci. 2013;78:11–23. [Google Scholar]
- 60.Tisserand R., Young R. Essential Oil Safety. 2nd ed. Churchill Livingston Elsevier; London, UK: 2014. pp. 224, 364–265, 404. [Google Scholar]
- 61.Marinkovic J., Markovic T., Nikolic B., Ciric A., Mitic-Culafic D., Dukanovic S., Krstic A., Pavlica D., Vlajic T., Markovic D. Biocompatibility and Antibacterial Potential of the Cinnamomum camphora cineoliferum (L.) J. Presl. and Melaleuca ericifolia Sm. Essential Oils against Facultative and Obligate Endodontic Anaerobes. J. Essent. Oil-Bear. Plants. 2022;25:111–125. doi: 10.1080/0972060X.2022.2040386. [DOI] [Google Scholar]
- 62.Zhao Q., Bowles J.E., Zhang H.Y. Antioxidant Activities of Eleven Australian Essential Oils. Nat. Prod. Commun. 2008;3:837–842. doi: 10.1177/1934578X0800300531. [DOI] [Google Scholar]
- 63.Wagner H., Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. 2nd ed. Springer; Berlin, Germany: 2001. p. 364. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the authors upon request.