Abstract
Introduction: Chronic exposure to methamphetamine (Meth) results in permanent central nervous system damage and learning and memory dysfunction. This study aimed at investigating the therapeutic effects of bone marrow mesenchymal stem cells (BMMSCs) on cognitive impairments in Meth addicted rats and comparing intravenous (IV) delivery with intranasal (IN) delivery of BMMSCs.
Methods: Adult Wistar rats were randomly divided into 6 groups; Control; Meth-addicted; IV-BMMSC (Meth administered and received IV BMMSCs); IN-BMMSC (Meth administered and received IN BMMSCs); IV-PBS (Meth administered and received IV Phosphate-buffered saline (PBS); IN-PBS (Meth administered and received IN PBS). BMMSCs were isolated, expanded in vitro, immunophenotyped, labeled, and administered to BMMSCs-treated groups (2 × 106 cells). The therapeutic effect of BMMSCs was measured using Morris water maze and Shuttle Box. Moreover, relapse-reduction was evaluated by conditioning place preference after 2 weeks following BMMSCs administration. The expression of brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF) in rat hippocampus was assessed using immunohistochemistry method.
Results: Administration of BMMSCs caused a significant improvement in the learning and memory functions of Meth-addicted rats and reduced the relapse (P<0.01). In behavioral tests, comparison of IV and IN BMMSC-treated groups did not show any significant difference. Administration of BMMSCs improved the protein level of BDNF and GDNF in the hippocampus, as well as causing behavioral improvement (P<0.001).
Conclusion: BMMSC administration might be a helpful and feasible method to treat Meth-induced brain injuries in rats and to reduce relapse. BMMSCs were significantly higher in IV-treated group compared to the IN route. Moreover, the expression of BDNF and GDNF was higher in IN-treated rats compared with IV treated group.
Keywords: Addiction, Methamphetamine, Cognition, Intranasal, Intravenous, Mesenchymal stem cell
Introduction
Based on the United Nations' report in 2017 on drugs and crime, amphetamine-related disorders have a significant share in the worldwide diseases attributed to drug abuse, after the ones due to the misuse of opioids. Evidence suggests that out of all amphetamines, methamphetamine (Meth) causes the greatest global health concern. Meth abuse is increasing and a growing population of Meth users wish to be treated.1 Neurocognitive disorders such as learning and memory dysfunctions, low information processing speed, and frontal lobe malfunction are associated with Meth use.2 Several magnetic resonance imaging (MRI) studies have shown that amphetamine has serious and specific neuropathological effects on the consumers in comparison with other drugs. No ventricular and cortical volume loss was observed in drug addicts’ brain, but psychostimulant specifically had some effects on the hippocampus, prefrontal, and medial temporal lobe.3-5
The most important brain circuits and structures involved by drugs include the reward circuits (nucleus accumbens and ventral tegmental area), memory and learning (hippocampus and amygdala), motivation (orbitofrontal cortex), and executive functions (prefrontal cortex).6 Meth induces changes in dopaminergic, serotonergic, and noradrenergic systems via the stimulated release of monoamines and inhibits reuptake.7 Following the release of dopamine (DA), dopaquinone redox-cycling is initiated and subsequently oxygen-based radicals such as superoxide radicals are formed. Glutamate-induced excitotoxicity and activation of glutamate receptors are accompanied by Meth neurotoxicity. Glutamate toxicity is dependent, in part, on the production of nitric oxide (NO). In addition to their roles in the damage of monoaminergic terminals, oxygen-based radicals and NO appear to be involved in Meth-related microglial reactions and reducing neurotrophic factors.8
Moreover, in adults, drugs via various mechanisms can regulate the proliferation, differentiation, and survival of neural stem cells, which significantly affect their neurogenesis. In this regard, the hippocampus can be investigated since it plays a pivotal role in different aspects of addictive processes.9 A drug-induced hippocampal neuroadaptive mechanism with effects similar to those of opiates and psychostimulants is recently introduced, which can decrease neurogenesis in the subgranular zone (SGZ).10 Taken altogether, Meth greatly influences brain structure and function in different manners. Recently, it has been revealed that over 40% of formerly Meth-dependent subjects experience neurocognitive impairments after a long period of quitting the Meth.11 To date, no FDA-approved medications are available for Meth dependence.12
Stem cell-based regenerative therapy is a therapeutic approach for incurable brain diseases. Animal model studies of Parkinson’s, Huntington, and Alzheimer’s diseases have indicated that stem cell therapies can improve behavioral deficits. Several studies have described the positive outcomes of treatment with bone marrow-derived mesenchymal stem cells (BMMSCs) in animal models of neural damages.13
It is almost unknown MSC through which mechanism affects the nervous system, but there are some areas of research. The suggested mechanisms of action are as follows: (A) neuroprotection, (B) neurogenesis, and (C) synaptogenesis. The MSC via autocrine and paracrine mechanisms can combat neural tissue damages and apoptosis; such mechanisms are activated by the release of neurotrophins, inhibition of neuron apoptosis and microglial activation, induction of microglia phenotype switch, and inhibition of the proliferation astrocytes, and oxidative stress molecules. Some evidence shows that endogenous neurogenesis is induced by MSC; in addition, in vitro studies have revealed the differentiation of MSCs into functional neurons. Synaptic plasticity of neurons, both existing and newly formed ones, is increased following the MSC transplantation. To sustain synaptic plasticity, the production of tissue plasminogen activator should be stimulated and synaptophysin expression has to be increased. Other suggested mechanisms for the MSC effect are synaptic detachment and astroglia decrease, and brain-derived neurotrophic factor (BDNF) release.14,15
The delivery routes of stem cells to the brain are different. The intravenous (IV) route is the most widely applied method because of its effectiveness, low side effects, and simple application technique. The new method of delivery of cells to the brain is the intranasal (IN) route. Direct transplantation of stem cells to the brain is an invasive method and may cause serious brain lesions, but IN route is a non-invasive method.16,17 In this study we attempted to deliver BMMSC to Meth addicted rats and to evaluate their therapeutic effects on hippocampus-dependent learning and memory impairment and relapse. In the next part, we aimed to compare IN route with IV delivery of BMMSC to see their effects on brain recovery.
Materials and Methods
Animals
Adult male Wistar rats (mean weight, 200 g) were used in this study to develop an inhalation self-administration animal model of addiction (Royan Institute, Tehran, Iran). The animals were stored under a 12-hour light-dark cycle and 21 ± 3°C temperature with water and diet freely accessible. This research was conducted according to the guidelines of the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Drugs and materials
Methamphetamine hydrochloride (Sigma-Aldrich; Merck Millipore, M8750, USA) solution was provided in distilled water (DW). BMMSCs isolation, culture, and labeling were performed using the Dulbecco’s modified Eagle medium (DMEM; Gibco, 31600-083, UK) supplemented with 10% fetal bovine serum (FBS; Invitrogen), phosphate-buffered saline (PBS Tablets; Merck, 6500-OP, Germany), Trypsin-EDTA solution (Merck, 4010-OP, Germany), and 5´-bromo-2´-deoxyuridine (BrdU, B5002, Sigma-Aldrich). Fluorescent-labeled antibodies targeting the BMMSCs surface markers were CD73 (BioLegend Cat. No. 127202, CA, USA), CD90 (BioLegend, Cat. No. 105201, CA, USA), CD44 (BioLegend, Cat. No. 338802, CA, USA), and CD45 (BioLegend, Cat. No. 103101 CA, USA). Immunohistochemical staining was carried out using the anti-BrdU antibody (Alexa Fluor® 488 Goat anti-mouse IgG, BioLegend, USA), anti-BDNF antibody (PA1-18371, Thermo Fisher Scientific, USA), anti-GDNF (glial-derived neurotrophic factor) (PA1-1837159, 1:200, Thermo Fisher Scientific, USA), and the secondary antibody FITC-anti-rabbit IgG (ab97022, 1:200, Abcam, UK).
Experimental protocol
The present study used a model of Meth self-administration through inhalation (a device made with Noavaran Sanaye Amouzeshi, Mashhad, Iran) in rats. First, the animal should receive training to initiate self-administration; so, after 24-hour food deprivation, the animals were put in the apparatus 4 h/day. Using a task in which rats following a period of exploratory behavior in the cage and pressing the levers, learned how to get food. This period varied from 1-5 days among laboratory animals. Pressing the passive lever had no programmed consequences. Upon active lever pressing to obtain reward (food), rats received an illumination of the cue red lamp above; then the rats conditioned with a few mistakes and some successes, learned to press the active lever for the reward. Then, during the drug addiction period by seeking behavior, this lever was used to receive the needed drug. After the completion of the rat training for self-administration, the animal was put in the apparatus only for getting the drug. In this model, drug addiction first started with low doses (1 mg/1 cc) of Meth, and the amount of drug used by each animal during the time it was placed in the apparatus, showed an increase through self-administration dosage.
After the training period of self-administration, all animals of addicted groups were placed in the self-administration device to self-administer Meth through inhalation (15 min/day). Active lever presses resulted in an infusion and dropping of Meth at a dose of 0.05 mg/kg in a volume of 50 µL (0.05 mL) over a period of 2 seconds. In the second week of addiction, rats self-administered Meth through inhalation (0.1 mg/kg/50 μL) by each paddling. The maximum drug usage was set on 1 cc daily. Therefore, rats self-administered Meth through inhalation in the first week of addiction at 1mg/cc (5 mg/kg), and in the second week at 2 mg/cc (10 mg/kg).18 During 2 weeks, the addicted groups were placed, 15 minutes each day, in a rodents’ self-administration modeling apparatus for the voluntary Meth intake. To determine plasma concentrations of Meth after 2 weeks of addiction, 0.3 cc blood was drained from the tail vein and the gas chromatography–mass spectrometry (GC-MS) was used to measure the Meth level. The mean serum concentration of Meth in rats was 1.92 ± 0.3 ng/mL.19
Rats were randomly divided into 6 groups as follows:
Addicted group; The animals in this group (n = 7) received 14 days of inhaled Meth followed by 14 days of drug abstinence.
Treatment group 1 (IV-BMMSC); The animals in this group (n = 8) received 14 days of inhaled Meth and IV administration of BMMSCs.
Treatment group 2 (IN-BMMSC); The animals in this group (n = 6) received 14 days of inhaled Meth and IN administration of BMMSCs.
Vehicle group 1 (IV-PBS); The animals in this group (n = 4) received 14 days of inhaled Meth and IV administration of PBS.
Vehicle group 2 (IN-PBS); The animals in this group (n = 4) received 14 days of inhaled Meth and IN administration of PBS.
Control group of untreated intact animals (n=8).
BMMSCs-treated rats received an equal dose of stem cells and PBS- treated rats received the same volume of PBS, 3 days after ending of addiction period. Moreover, the learning and memory functions of rats were tested in the Morris water maze, and shuttle box and relapse reduction was evaluated by conditioning place preference (CPP) after 2 weeks following the BMMSCs administration.
Behavioral tests
Morris water maze test
This test was conducted following the self-administration of Meth and 14 days after BMMSCs administration in a round pool (diameter, 150 cm; height, 50 cm) with four equal quadrants. By adding warm water to the pool, its temperature was kept at 25 ± 0.5°C. The task involved finding an invisible platform 2 cm below the water (height, 35 cm; diameter, 10 cm) at the center of zone I. Each day, four trials were repeated in different quadrants for four consecutive days; the platform position was not changed on the training and test days.
The animals were randomly placed at the starting point of the pool in each trial, with their heads facing the wall based on the software selection. The animals continued swimming until climbing the platform; the maximum duration of the trial was one minute. In case of failure to find the platform in this time frame, it was guided towards the platform and remained there for 10 seconds. An overhead video camera was used to determine the required time for reaching the hidden platform (escape latency); the data were analyzed with a video tracking program. The average escape latency for four trials was measured as the final result for statistical analysis. To perform the probe test, 24 hours after the final training session, the platform was removed from the pool, and the rat was given 60 seconds to swim freely; the spent time and distance moved in each zone were measured and analyzed statistically.20
Shuttle box test
The passive avoidance learning and memory test was performed after Meth self-administration through inhalation and 14 days after BMMSCs administration, using an apparatus known as a shuttle box. It consists of a box divided into two similar compartments (20 × 30 × 20 cm); a guillotine door between them (7 × 6 cm) could be lifted manually. The two compartments consisted of transparent and dark walls, respectively. The dark compartment floor was made of stainless steel bars (diameter, 2 mm). An isolated stimulator was used to deliver 50 Hz electric shocks (3 s, 1.5 mA) to the grid floor of the dark compartment.
Prior to the experiment, the animal was placed in the apparatus for at least 60 minutes. After the animal stayed in the transparent compartment for 10 seconds, the guillotine door was opened to facilitate its entrance to the dark compartment. The latency to enter this compartment was recorded, and animals with a latency time > 300 seconds were eliminated. The door was closed when the animal entered the next chamber with all four limbs, followed by immediate shocks to the grid floor. The animal was then removed from the apparatus within 20 seconds and placed in its cage temporarily. The animal was placed in the light compartment one day after training, and the door was opened after 10 seconds. In the absence of electric shocks, step-through latency (STL) was calculated to represent passive avoidance memory.21
Conditioned place preference (CPP)
A three-chamber apparatus was used for conditioning. The walls of the middle compartment (neutral; 15 × 30 × 40 cm) were white; also, two doors led to two conditioning compartments (30 × 30 × 40 cm). The walls of the left compartment had vertical black-and-white stripes, and the right compartment had black walls. The spent time in every compartment was monitored with a sensor in the floors of the chambers, recorded, and showed in the control panel. A 70% camphorated ethanol solution was used to clean the apparatus after each trial.
The procedure for place conditioning consisted of three phases (habituation, induction, and test). First, the rats were familiarized with the apparatus for 15 minutes on day one. Then, by lifting the removable wall, the animal was allowed to move between the compartments. The induction phase consisted of six 45-minute sessions, which were held twice a day (days 2-4) within a six-hour interval. The removable wall, which was placed along the seam, separated the two compartments, and the groups were limited to one compartment.
The groups inhaled Meth and were restricted to only one compartment for 45 minutes. Similarly, they were confined to the other compartment for 45 minutes after DW administration. The order of drug administration and treatment compartment were counterbalanced for either group. One day after the final session of conditioning, the test session was held on day five; every animal was only examined once. The wall was lifted so that the animals could enter the compartments on the 15-minute extinction test; the spent time in the Meth- and DW-paired compartments was documented. To measure the conditioning scores (mean ± SEM), the spent time in the Meth-paired compartment was subtracted from the spent time in the DW-paired compartment.22 Fourteen days after BMMSCs administration, the CPP test was repeated to evaluate the relapse.
Bone marrow mesenchymal stem cells (BMMSCs)
Isolation, culture, and identification of BMMSCs
To harvest BMMSCs, male Wistar rats, weighing 70-80 g (2-3 weeks old; Royan Laboratory), were used. In short, the femoral and tibial bones were used for extracting BMMSCs. After suspending the cells in DMEM medium containing 10% FBS, they were incubated at 37°C in 5% CO2; every three days, the medium was changed. As soon as the cells reached 80-90% confluence, the primary cultures were passaged at a ratio of 1:2. For cell characterization, BMMSCs were harvested after three passages and exposed to flowcytometric assessment for mesenchymal markers, CD73+, CD90+, CD44+, and CD45+. During 30 minutes, fluorescent antibodies were used to incubate 1×106 cells at 37°C, which were then rinsed with PBS in triplicate. Afterward, a flowcytometer (Becton Dickinson, NJ, USA) was used to examine the cells.23,24
BMMSCs labeling and administration
After three passages, BMMSCs were harvested and prelabeled with 3 μg/mL of BrdU in 5% CO2 for 72 hours at 37°C before injection. Afterward, the cells were digested using 0.25% trypsin for five minutes to prepare single cell suspension; they were then rinsed with 0.1 M PBS. After removing 30 µL of the cell sample, it was mixed with 0.4% trypan blue stain (30 µL). The cells were tallied by a hemacytometer and a counter using a phase-contrast microscope. In the IV-BMMSC group, a BMMSC suspension (2×106/mL), which was dissolved in 0.5 mL of PBS, was slowly injected within one minute to the tail vein. The IV-PBS group received 0.5 mL of PBS via the tail vein.25
The animals of the IN-BMMSC group remained anesthetized throughout the IN cell administration, typically 1 hour from the onset of anesthesia. The rats received intraperitoneal (IP) ketamine and xylazine (100 and 10 mg/kg, respectively). In the supine position, the head angle was fixed at 70° or 90° to the stereotaxic device. BMMSCs (2×106/mL) were suspended in 24 µL of total fluid volume PBS and were dropped into both nostrils and allowed to snort. The drop volume was 6 µL. The IN-PBS group received the same volume of PBS intranasally.26
A thermostatically controlled warm pad beneath the body was used to maintain the body temperature at 36-37°C. Following BMMSCs administration, the animals were placed in their individual cages. Immunosuppressants were used for no animals. The animals received IV and IN BMMSCs (or PBS) in two days and allowed 14 days after cell administration27; the behavioral tests were then carried out as earlier described.
Tissue preparation and BrdU immunohistochemistry
After the behavioral tests, 1 mL/kg of 10% chloral hydrate was used to anesthetize the rats. Perfusion was done through the left cardiac ventricle using almost 200-250 mL of 4% paraformaldehyde in 0.1-PBS (after perfusion using 100-150 mL of 0.9% saline). The animals’ brains were fixed in 4% paraformaldehyde and paraffin-embedded for immunohistochemistry. Coronal sections (thickness, 5 μm) were cut from every block (including hippocampus) by a sliding microtome.
To determine MSC-derived cells, immunohistochemical staining was carried out. Briefly, a standard paraffin block of the brain was used. After cutting the sections (100-μm interval; thickness, 5 μm), they were examined by light and fluorescent microscopes. The number of BrdU-reactive cells was measured for detecting the transplanted MSCs distribution. The sections were added to citrate buffer in an oven for 2 hours at 65°C following deparaffinization. After washing the sections with PBS twice, incubation was performed at 37°C in 2 mol/L HCl for half an hour. Using 0.5 % Triton X-100, the sections were rinsed again for 20 minutes and treated overnight using an anti-BrdU antibody at 4°C. The sections were washed in PBS, followed by incubation in FITC conjugated antibody.28 The mounted slides were observed with an immunofluorescence microscope (Olympus Corporation, Japan).
Cell counting
A blinded observer examined high-power images for each animal. It was counting the stained cells, considering 10 sections per animal (five rats per group). Images were analyzed using the automatic cell counting method with ImageJ software.
BDNF and GDNF immunohistochemistry
The BDNF expression was examined in the hippocampus via immunohistochemistry. The brain sections were microwaved for 15 minutes in 0.01-M citrate buffer to identify the expression of immune-like BDNF proteins. They were then incubated at 37°C for 45 minutes in a blocking solution (10% normal goat serum and 3% Triton X-100 in PBS), followed by overnight incubation in a polyclonal anti-BDNF antibody in PBS. After washing the sections in PBS (3×5 minutes), they were incubated in FITC anti-rabbit IgG in PBS at room temperature for 2 hours.
For staining the nucleus, the tissue was incubated with propidium iodide (1 mg/ML) for 15 minutes. The BDNF protein expression was analyzed by fluorescence microscopy (Olympus Corporation, Japan). A blinded investigator measured the positive cell count on five random fields using the automatic cell counting method with Image J software. The same method was used to evaluate GDNF. The primary antibody was a polyclonal anti-GDNF, and the secondary antibody was FITC-anti-rabbit IgG.29
Statistical analysis
All data were plotted and analyzed using GraphPad Prism 7 software (Prism for Windows, version 5.0, GraphPad Software Inc., San Diego, CA, USA) and expressed as mean ± SEM. For data analysis, paired or unpaired t test, one-way ANOVA, and Wilcoxon test were applied as appropriate. P value below 0.05 was regarded as statistically significant.
Results
Isolation and characterization of BMMSCs
The BMMSCs were collected from the rats and isolated using conventional procedures. The isolated BMMSCs were either spindle or triangular. The distribution of cells (mostly spindle) was uniform after culture for three passages, covering the bottom every three to four days (Fig. 1A). The flowcytometric analysis was performed to identify the surface markers; the high expression of CD90 (99.9%), CD44 (99.9%), and CD73 (99.8%) was reported, while CD45 showed almost no expression (1.25%) (Fig. 1B). These findings are consistent with the characteristics of BMMSC surface markers, suggesting the depletion of hematopoietic stem cells during subcultivation. Therefore, the used cells in our study were considered to be BMMSCs.
Fig. 1.
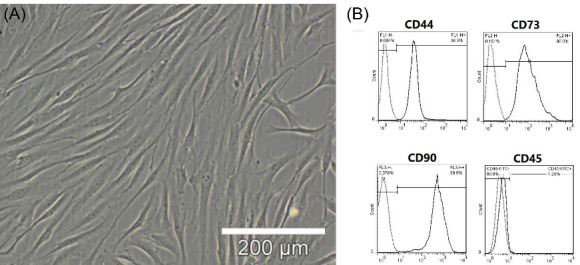
BMMSC Isolation and Identification. (A), BMMSCs (passage 3) were formed as densely packed spindle-shaped cells. The cells were isolated from two- to three-week-old rats (70-80 g). (B) Flowcytometric analysis was used to determine the cell surface markers of BMMSCs (passage3); high CD90 (99.9%), CD44 (99.9%), and CD73 (99.8%) expression was observed, whereas almost no CD45 expression was detected (1.25%).
Tracking of BMMSCs after transplantation
After prelabeling BMMSCs with BrdU, 2×106 cells were injected intravenously or intranasally to the treatment groups. Fluorescent microscopy was used to observe the cells in the hippocampus. Based on microscopic images, a clear nuclear and faint cytoplasmic green fluorescence was observed in the BrdU-labeled BMMSCs. A large number of scattered BrdU-positive cells was detected in the BMMSC-transplanted group 14 days post-injection (Fig. 2). In the IV-treated group, the number of BMMSCs was significantly higher than the IN group (P< 0.001).
Fig. 2.
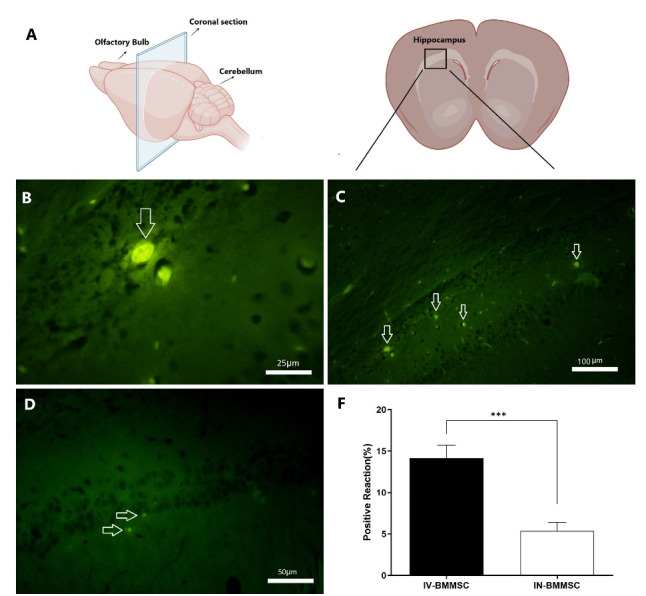
The location of BMMSCs after transplantation. (A), Schematic diagrams showing the rat brain regions examined (Retrieved from https://app.biorender.com/biorender-templates);(B), The BrdU-labeled BMMSCs exhibited clear nuclear and faint cytoplasmic green fluorescence. In comparison with the IV BMMSCs group, many BrdU-labeled cells were detected in the hippocampus of the BMMSC-treated group 14 days post-transplantation. (C), IV BMMSCs group; (D), IN BMMSCs group; (E), BMMSCs were significantly higher in IV-BMMSC group than in the IN-BMMSC (*** P <0.001).
Effects of BMMSCs on learning and memory of Meth-addicted rats
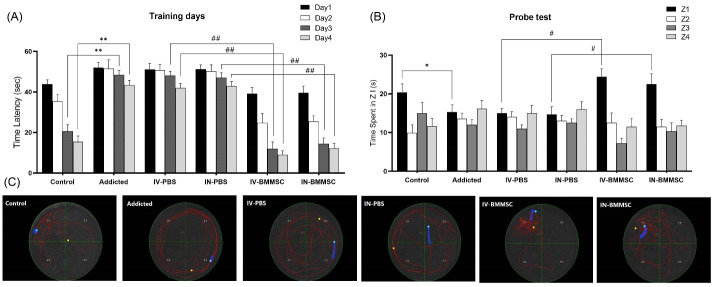
In rodents, Meth self-administration results in learning and memory disorders due to the hippocampus vulnerability to Meth-induced neurotoxicity. In the present study, Wistar rats (n = 29) received Meth self-administration via vapor inhalation (first week 1 mg/cc and second week 2 mg/cc). Treatment group 1 (IV-BMMSC) received IV BMMSCs after Meth self-administration. Treatment group 2 (IN-BMMSC), after Meth self-administration through inhalation received IN BMMSCs. Two PBS-treated groups received IV and IN PBS after Meth self-administration through inhalation. The Meth-addicted group remained in abstinence for 2 weeks. Two weeks after the BMMSCs administration, learning and memory recovery were assessed by Morris water maze. Learning is assessed through four consecutive training days and memory is tested by the spent time in the previous zone of a hidden platform when the platform is removed in the probe trial.30
In the Morris water maze, escape latency significantly increased after 14 days of Meth self-administration (16.2 ± 2.13, n = 7, P< 0.01) indicating reduced learning performance in comparison with the control group (11.63 ± 2.03, n=8) on training days. The distance moved to reach the hidden platform decreased in the BMMSC-treated groups (IV-BMMSC: 11.50±2.19, n = 7; IN-BMMSC: 11.83 ± 1.3, n=6), decreased in comparison with PBS-treated groups (IV-PBS: 15.65 ± 2.49, n = 4; IN-PBS: 16.3 ± 2.56, n = 4, P< 0.01) (Fig. 3). According to the results of one-way ANOVA, the escape latency was significantly different between the groups (F5, 15 = 23.38, P< 0.001).
Fig. 3.
Effect of BMMSCs on memory and learning impairment of rats induced by Meth. (A), Morris water maze in training days showing the time latency of rats was significantly prolonged after self-administration of Meth. Mean ± SEM, **P < 0.01 versus Meth-addicted group. ## P < 0.01 versus PBS-treated groups. (B), Morris water maze probe test indicating a longer spent time in zone I (previous location of hidden platform) among BMMSC-treated rats, compared to PBS-treated rats. Mean ± SEM. *P < 0.05 versus Meth-addicted group. # P < 0.05 versus PBS-treated groups. (C), Typical trajectories in the Morris water maze probe test. Previous location of hidden platform was zone I. (Control (n=8); IV-PBS (n=4); IN-PBS (n=4); Addicted (n=7); IV-BMMSC (n=8); IN-BMMSC (n=6).
It was observed that the spent time in zone I (previous location of the hidden platform) of Morris water maze performance was different between the addicted, PBS, and other groups (Control, IV-BMMSC, and IN-BMMSC) (P< 0.05). The results of ANOVA indicated a significant difference in the escape latency (F5, 15 = 0.01359, P< 0.05) between the groups. Therefore, administration of BMMSCs may improve Meth-induced learning and memory disorders. Comparison of IV and IN BMMSC-treated groups did not show any significant difference between them.
Effects of BMMSCs on the passive avoidance memory of meth-addicted rats
The shuttle box test was performed to measure passive avoidance memory and compare it between the groups. According to the shuttle box test protocol, on the first day of habituation, training phase, and the second day, the test was performed. The time spent entering the dark chamber was reported as STL. The results showed that passive avoidance memory in the BMMSCs-administered groups (IV-BMMSC and IN-BMMSC) on the day of the test was significantly better than that on the day of training. A significant difference was found in STL on the days of training and testing between the IV-BMMSC and IN-BMMSC groups and the control group with the Meth-addicted group (P< 0.001); no significant difference was found between the IV and IN treatment groups. In addition, the Meth-addicted and PBS-treated groups were not significantly different (Fig. 4).
Fig. 4.
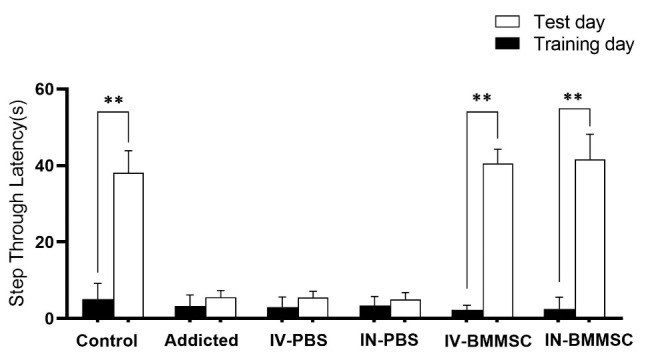
Effect of BMMSCs on passive avoidance memory impairment of Meth in rats. The latency was the time to cross from the brightly compartment (shock-free zone) to the darkened compartment (shock zone). Twenty-four hours following electric foot- shock delivery in the darkened compartment (training), rats were tested. The average latency increased significantly in the BMMSCs-administered groups. There was a significant improvement in passive avoidance memory performance in the BMMSCs-treated groups. Data shown are Means ± SEM, **P<0.01 vs Training day. (Control (n=8); IV-PBS (n=4); IN-PBS (n=4); Addicted (n=7); IV-BMMSC (n=8); IN-BMMSC (n=6).
Effects of BMMSCs on the relapse of Meth-addicted rats
The aversive or rewarding effects of drugs are assessed via CPP. The longer time spent in the drug-paired context shows that the context has incentive value, suggestive of the drug’s rewarding features. After conditioning in rodents, CPP remains robust for weeks and shows high resistance to extinction.31
The animals in all groups received Meth inhalation or DW over three days of conditioning as described earlier. Fig. 5 presents Meth self-administration for place conditioning in rats (P< 0.001). Animals receiving DW twice a day (six sessions) showed no preference. Differences in the time spent in the drug-paired chamber were significant after BMMSCs administration between CPP 1 and CPP 2 (P< 0.01). Whilst, the Meth-addicted and PBS-treated groups were not significantly different.
Fig. 5.
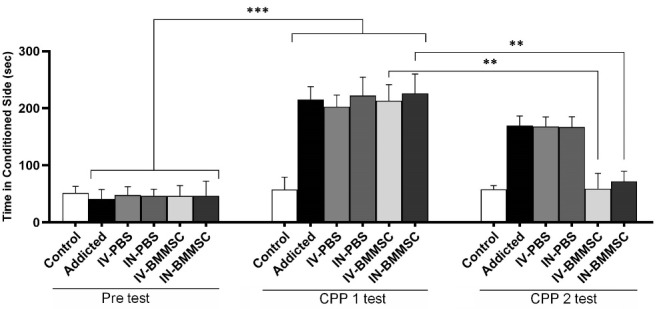
Conditioned place preference induced by inhalation of Meth in rats and reduced relapse after BMMSCs administration. Difference in time spent in the drug-paired chamber between CPP1 and CPP2 tests revealed a significant effect of BMMSCs administration. Data represent group mean ± SEM. ** P<0.01, *** P<0.001. (Control (n=8); IV-PBS (n=4); IN-PBS (n=4); Addicted (n=7); IV-BMMSC (n=8); IN-BMMSC (n=6).
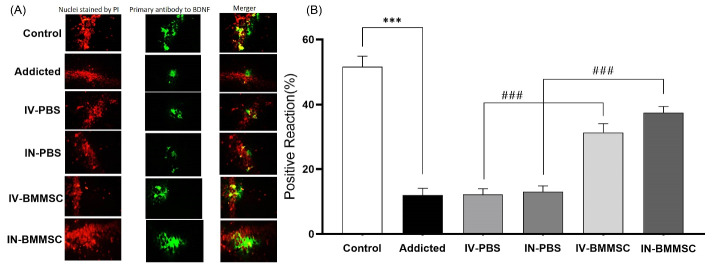
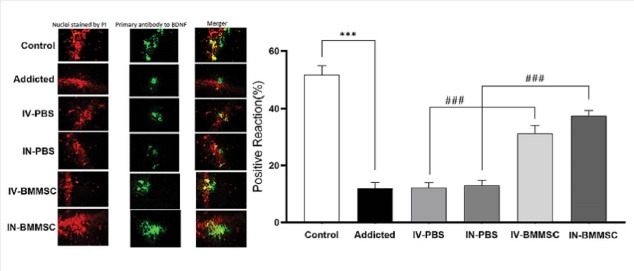
Effects of BMMSCs on BDNF expression in the hippocampus of Rats
BDNF, which is distributed in the hippocampus, contributes to neuron survival and neuronal functions, including learning and memory. The effect of BMMSC administration on the hippocampal BDNF expression was examined in this study. After BMMSC administration, BDNF immunoreactivity increased in the neurons of the hippocampus. Based on the one-way ANOVA, the groups were significantly different regarding BDNF of the hippocampus (F3, 27 = 253.5, P< 0.001). According to the quantitative analysis, the BDNF-positive reaction was higher in the hippocampus of BMMSC-treated rats, compared to the PBS-treated groups (P< 0.001) (Fig. 6). Therefore, BMMSCs transplantation may improve BDNF expression in the hippocampus. Moreover, the expression of BDNF was higher in the IN-BMMSC group in comparison with the IV-BMMMSC group; however, the difference was not significant (Fig. 6). Moreover, the Meth-addicted and PBS-treated groups were not significantly different.
Fig. 6.
Distribution of immunoreaction products of BDNF in the hippocampus. BDNF protein was detected by Immunoflurecent staining in the hippocampus 2 weeks after the BMMSCs administration. (A) Nuclei staining by DAPI and primary antibody to BDNF and merge of them from each group (magnification, ×400). (B), The percentage of positive reaction in each group. BMMSCs-administered rats and control groups compared with the Meth-addicted group. ***P < 0.001 versus Meth-addicted group. ### P < 0.001 versus PBS-treated groups. Data are presented as the Mean ± SEM; Scale bar: 20μm; BDNF: brain-derived neurotrophic factor; (Control (n=8); IV-PBS (n=4); IN-PBS (n=4); Addicted (n=7); IV-BMMSC (n=8); IN-BMMSC (n=6).
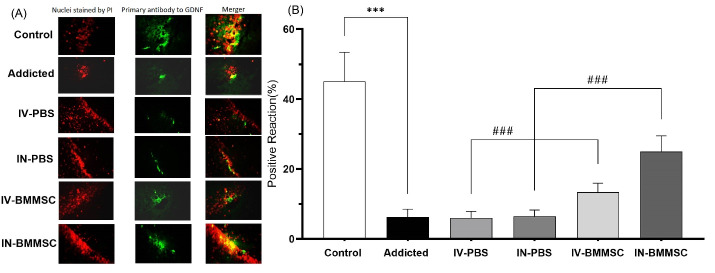
Effects of BMMSCs on the expression of GDNF in the hippocampus of rats
Following injury, the survival of dopaminergic neurons is promoted by the GDNF in the adult brain, which is involved in motor neuron development and survival, hippocampal synaptogenesis, and the development of sympathetic and sensory neurons.32,33 Strong evidence suggests GDNF as a negative regulator of some drug seeking behaviors, behavioral and biochemical adaptations, and relapse to psychostimulants. The effect of BMMSC administration on GDNF expression was examined in the hippocampus. Following BMMSC administration, the immunoreactivity of GDNF increased in hippocampal neurons. One-way ANOVA showed a significant difference in GDNF of the hippocampus between the groups (F3, 27 = 67.57, P<0.001).
According to the quantitative analysis, the GDNF-positive reaction was much higher in the hippocampus of BMMSC-treated rats, compared to the PBS-treated groups (P< 0.001) (Fig. 7). Therefore, GDNF expression may increase in the hippocampus as a result of BMMSCs transplantation. Comparisons between the BMMSCs-treated groups showed that hippocampal GDNF in IN-treated rats was significantly higher than that of the IV-treated group (P< 0.001); the Meth-addicted and PBS-treated groups were not significantly different.
Fig. 7.
Distribution of immunoreaction products of GDNF in the hippocampus. GDNF protein was detected by Immunofluorescent in the hippocampus 2 weeks after the BMMSCs administration. (A) Nuclei staining by DAPI and primary antibody to GDNF and merge of them from each group (magnification, ×400). (B), The percentage of positive reaction in each group. ***P < 0.001 versus Meth-addicted group. ### P < 0.001 versus PBS-treated groups. Data are presented as the Mean ± SEM; Scale bar: 20 μm; GDNF, glia cell line derived neurotrophic factor. (C; Control group (n=8); PBS1, IV-PBS administration group (n=4); PBS2, IV-PBS administration group (n=4); A, Addicted group (n=7); T1, IV-BMMSCs administration group (n=8); T2, IN-BMMSC administration group (n=6).
Discussion
This study aimed to determine the effects of BMMSCs administration on learning and memory impairment due to Meth consumption and to compare IV and IN routes of administration. The major findings obtained from the present research are as follows: (1) Meth induced learning and memory impairment; (2) BMMSCs administration to the rats improved Meth-induced learning and memory impairment; (3) BMMSCs administration to the Meth addicted rats decreased relapse; (4) BMMSCs administration improved the protein level of BDNF and GDNF in the hippocampus. (5) BMMSCs in the hippocampus were higher in the IV-treated group compared to the IN route. (6) The expressions of BDNF and GDNF were higher in IN-treated rats compared with IV-treated group.
In this study, Morris water maze was used to assess spatial navigation learning and memory, which is hippocampus-dependent learning and one of the major regulators of hippocampal neurogenesis.34 In our study, learning and memory deficits significantly improved via BMMSCs administration in animals exposed to inhaled Meth, preventing Meth-induced hippocampal damage. The observed improvement is consistent with previous studies using stem cells in other memory disorders due to Alzheimer's disease or alcohol abuse and its related complications. In a study by Yang et al, reduction of oxidative damage and trophic factor production by transplanted BMMSCs were suggested as the cause of functional benefit reported in BMMSC-grafted alcohol-associated dementia rats.35 Moreover, Shirasaka et al reported an increase in the GABAergic interneuron count and synaptic protein density in the hippocampus, anterior cingulate cortex, and amygdala, followed by the reversal of memory impairment due to the intravenous administration of neural stem cells in a fetal alcohol spectrum disorder model.36
In stem cell transplantation, the route of cellular delivery is of great significance.37 In many studies, BMMSCs have been directly injected in a lesion of tissues via an invasive route, such as intracerebroventricular or intracerebral parenchyma. This route of cell administration is invasive and requires special equipment and surgery. IV injection of stem cells is the most commonly used method. Now the researchers prefer to choose IN BMMSCs administration as an alternative non-invasive model of cell delivery.38,39 The administered stem cells were observed in the subarachnoid space, olfactory bulb, thalamus, cerebral cortex, hippocampus, subventricular zone, and damaged brain area post IN route.40 Stem cells have been detected in the brain one-hour after cell administration via the IN route. This fast delivery of stem cells recommended the potential route from the nose to the brain that can circumvent the blood-brain barrier and give access to CNS.41 Stem cell delivery into the CNS with IV administration, a fraction of injected stem cells may be trapped in other organs than the CNS. Secondary malignancies can develop because of the systemic injection of stem cells.42,43 Meth-induced pathophysiological changes and neuroinflammation due to neurodegenerative disorders (multiple sclerosis, Alzheimer‘s disease, etc) disrupt BBB function by induction of oxidative stress in brain endothelial cells.44,45 This is a probable reason for higher detected stem cells in IV administration. Therefore, a concern was raised about stem cell tumorigenicity in the IV route. Donega et al showed that IN administration of MSC was safe and did not induce any lesions in the brain or any peripheral organ in the long term.46
The probable routes of stem cell migration from the nose to the brain tissue are cervical lymph nodes, trigeminal nerve, olfactory bulb, and vascular pathway through IN cell administration.16
Based on the results, transplanted stem cells might differentiate into neurons in the hippocampus. The hippocampal region seems to play a major role in encoding and retrieving learning and memory. Although we did not aim to examine the mechanisms of memory improvement, we can suggest several mechanisms which may be involved.47 One mechanism is the capacity of these cells to add to the pool of functioning neurons and integrate with the neighboring cells. Administered mesenchymal stem cells detected in the hippocampal circuitry and cognitive recovery of animals in behavioral tests may be a reflection of the integration of new neurons into the existing circuitry.48 One possible explanation for the reduced relapse is that administered stem cells, which produce new neurons in hippocampal networks, are likely to be selected for encoding new memories. On the other hand, the new neurons create new memories that are still not recorded addiction in it.49 The present findings showed that Meth induced significant CPP in an unbiased setup, which is in agreement with previous research.50
Moreover, due to the increased survival and activity of the existing neurons, these stem cells may have therapeutic applications. BMMSCs secrete different trophic factors, which might be important for tissue regeneration. The neurotrophic factors, BDNF, and GDNF, are considered essential for neuron growth, survival, and differentiation. These factors are also involved in learning and memory, synaptic plasticity, and the function and survival of adult neurons.51 In our experiment, BDNF and GDNF, as critical neurotrophic factors in BMMSCs which play important protective roles in the brain, were evaluated via both IV and IN routes. Our findings showed that GDNF significantly increased following IN BMMSCs administration. Gliogenesis is more common in the adult mammalian brain than neurogenesis.52
BDNF may have neuroprotective functions against drug neurotoxicity.53,54 CPP is described as a contextual learning task, and hippocampal BDNF is known to be involved in contextual. BDNF may also contribute to addiction-related neuroplasticity, and increased brain BDNF may be associated with addiction.55 Based on several studies, the plasma levels of BDNF increase in addicts; also, the results showed that the serum levels declined with withdrawal. However, in other studies, the serum BDNF level during withdrawal was lower in Meth addicts, compared to the controls. The drug type, the route of consumption, the gender, or even the developmental period and time are contributing factors in these conflicting conclusions.56 Both GDNF and BDNF contribute to synaptic and structural plasticity, as well as changes in drug-induced synaptic plasticity, which may result in drug-taking behaviors. Koskela et al in their review concluded that the role of BDNF or GDNF in drug-seeking behaviors is related to the type of drug, addiction phase, and the timing of GDNF/BDNF treatment in relation to drug administration.57 Moreover, elevated plasma levels of BDNF in Meth users may prevent neural damage58 and cognitive impairments,59 and GDNF may have neuroprotective effects against toxicity induced by drug abuse.60,61
Research Highlights
What is the current knowledge?
√ To date, no FDA-approved medications are available for Meth dependence and relapse.
What is new here?
√ BMMSCs administration improved cognitive function of Meth-addicted rats.
√ BMMSCs administration reduced relapse in Meth-addicted rats.
√ BMMSCs in brain were significantly higher in IV BMMSCs-treated rats.
√ Expression levels of BDNF and GDNF were higher in intranasal BMMSCs-treated rats.
Conclusion
We showed that IV- and IN-administration of BMMSCs may recover learning and memory impairment induced in self-administered Meth-addicted rats and reduced relapse. This new therapeutic approach has the potential to revolutionize the treatment of the addiction in the new century. BMMSCs were significantly higher in the IV-treated group than in the IN route. Moreover, the expression levels of BDNF and GDNF were higher in IN-treated rats compared with the IV-treated group. The results of this study showed that BMMSCs, when administered intranasally, are effectively absorbed from the nasal mucosa, so that this route may be attractive, avoiding the discomfort of an intravenous or any other invasive injection. Future studies are recommended assessing the quality of MSCs through long-term memory performance and cell survival.
Acknowledgments
The current research was extracted from a Ph.D. thesis on addiction and the authors gratefully acknowledge the Deputy for Research of Shahroud University of Medical Sciences. The authors also would like to acknowledge Dr. Mehdi Khaksari for suggestions in this study.
Funding sources
This paper is based on the Ph.D. thesis on addiction studies of Shahroud University of Medical Sciences and funded by this university (Project Code 9519).
Ethical statement
The local Ethics Committee affiliated with Shahroud University of Medical Sciences approved this study (Registration code of Ethics: IR.SHMU.REC.1395.42).
Competing interests
The authors have no conflict of interests to declare.
References
- 1. Hurst T. World Drug Report. The Encyclopedia of Women and Crime. 2019; 1-2.
- 2. Potvin S, Pelletier J, Grot S, Hébert C, Barr A, Lecomte T. Cognitive deficits in individuals with methamphetamine use disorder: A meta-analysis. Addict Behav 2018.80: 154-60. 10.1016/j.addbeh.2018.01.021. [DOI] [PubMed]
- 3.Wearne TA, Cornish JL. Inhibitory regulation of the prefrontal cortex following behavioral sensitization to amphetamine and/or methamphetamine psychostimulants: A review of GABAergic mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 2019;95:109681. doi: 10.1016/j.pnpbp.2019.109681. [DOI] [PubMed] [Google Scholar]
- 4.Golsorkhdan SA, Boroujeni ME, Aliaghaei A, Abdollahifar MA, Ramezanpour A, Nejatbakhsh R, et al. Methamphetamine administration impairs behavior, memory and underlying signaling pathways in the hippocampus. Behav Brain Res. 2020;379:112300. doi: 10.1016/j.bbr.2019.112300. [DOI] [PubMed] [Google Scholar]
- 5.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addict. 2007;102:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moszczynska A. Neurobiology and Clinical Manifestations of Methamphetamine Neurotoxicity. Psychiatr Times. 2016;33:16. [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Wang Y, Li Q, Zhong Y, Chen L, Du Y, et al. The main molecular mechanisms underlying methamphetamine-induced neurotoxicity and implications for pharmacological treatment. Front Mol Neurosci. 2018;11:186. doi: 10.1097/TP.0000000000000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takashima Y, Mandyam CD. The role of hippocampal adult neurogenesis in methamphetamine addiction. Brain Plast. 2018;3:157–68. doi: 10.3233/BPL-170058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teuchert-Noodt G, Dawirs R, Hildebrandt K. Adult treatment with methamphetamine transiently decreases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm. 2000;107:133–43. doi: 10.1007/s007020050012. [DOI] [PubMed] [Google Scholar]
- 11.Cadet JL, Gold M. Methamphetamine-induced psychosis: who says all drug use is reversible. Curr Psychiatry. 2018;16:15–20. [Google Scholar]
- 12.Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10:305–14. doi: 10.1080/17512433.2017.1268916. [DOI] [PubMed] [Google Scholar]
- 13.Mukai T, Tojo A, Nagamura-Inoue T. Mesenchymal stromal cells as a potential therapeutic for neurological disorders. Regen Ther. 2018;9:32–7. doi: 10.1016/j.reth.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsieh J-Y, Wang H-W, Chang S-J, Liao K-H, Lee I-H, Lin W-S, et al. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PloS One 2013; 8. 10.1371/journal.pone.0072604. [DOI] [PMC free article] [PubMed]
- 15.Badyra B, Sułkowski M, Milczarek O, Majka M. Mesenchymal stem cells as a multimodal treatment for nervous system diseases. Stem Cells Transl Med. 2020;9:1174–89. doi: 10.1002/sctm.19-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y, Zhu J, Xu G, Liu X. Intranasal delivery of stem cells to the brain. Expert Opin Drug Deliv. 2011;8:623–32. doi: 10.1517/17425247.2011.566267. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Bonamici N, Dey M, Lesniak MS, Balyasnikova IV. Intranasal delivery of stem cell-based therapies for the treatment of brain malignancies. Expert Opin Drug Deliv. 2018;15:163–72. doi: 10.1080/17425247.2018.1378642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahidi S, Komaki A, Sadeghian R, Asl SS. Different doses of methamphetamine alter long-term potentiation, level of BDNF and neuronal apoptosis in the hippocampus of reinstated rats. J Physiol Sci. 2019;69:409–19. doi: 10.1007/s12576-019-00660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafaiee R, Ahmadiankia N, Mousavi SA, Rezaeian L, Niroumand Sarvandani M, Shekari A, et al. Inhalant Self-Administration of Methamphetamine: The Most Similar Model to Human Methamphetamine Addiction. Iran J Psychiatry Behav Sci. 2019;13:e90561. doi: 10.5812/ijpbs.90561. [DOI] [Google Scholar]
- 20. Au - Nunez J. Morris Water Maze Experiment. JoVE 2008; e897. 10.3791/897. [DOI] [PMC free article] [PubMed]
- 21.Seifhosseini S, Jahanshahi M, Moghimi A, Aazami N-S. The effect of scopolamine on avoidance memory and hippocampal neurons in male Wistar rats. Basic Clin Neurosci. 2011;3:9–15. [Google Scholar]
- 22.Zarrindast MR, Karami M, Sepehri H, Sahraei H. Influence of nitric oxide on morphine-induced conditioned place preference in the rat central amygdala. Eur J Pharmacol. 2002;453:81–9. doi: 10.1016/S0014-2999(02)02328-2. [DOI] [PubMed] [Google Scholar]
- 23. Chaudhary J, Rath P. A simple method for isolation, propagation, characterization, and differentiation of adult mouse bone marrow-derived multipotent mesenchymal stem cells. J Cell Sci Ther 2017; 8. 10.4172/2157-7013.1000261. [DOI]
- 24.Yusop N, Battersby P, Alraies A, Sloan AJ, Moseley R, Waddington RJ. Isolation and Characterisation of Mesenchymal Stem Cells from Rat Bone Marrow and the Endosteal Niche: A Comparative Study. Stem Cells Int. 2018;2018:6869128. doi: 10.1155/2018/6869128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S-P, Wang Z-H, Peng D-Y, Li S-M, Wang H, Wang X-H. Therapeutic effect of mesenchymal stem cells in rats with intracerebral hemorrhage: Reduced apoptosis and enhanced neuroprotection. Mol Med Rep. 2012;6:848–54. doi: 10.3892/mmr.2012.997. [DOI] [PubMed] [Google Scholar]
- 26.Nijboer CH, Kooijman E, Van Velthoven CT, Van Tilborg E, Tiebosch IA, Eijkelkamp N, et al. Intranasal stem cell treatment as a novel therapy for subarachnoid hemorrhage. Stem Cells Dev. 2018;27:313–25. doi: 10.1089/scd.2017.0148. [DOI] [PubMed] [Google Scholar]
- 27.Babaei P, Soltani Tehrani B, Alizadeh A. Transplanted bone marrow mesenchymal stem cells improve memory in rat models of Alzheimer's disease. Stem Cells Int. 2012;2012:369417. doi: 10.1155/2012/369417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eminaga S, Teekakirikul P, Seidman CE, Seidman JG. Detection of Cell Proliferation Markers by Immunofluorescence Staining and Microscopy Imaging in Paraffin-Embedded Tissue Sections. Curr Protoc Mol Biol 2016; 115: 14.25.1-14.25.14. 10.1002/cpmb.13. [DOI] [PMC free article] [PubMed]
- 29.Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J Neurosci. 2001;21:581–9. doi: 10.1523/JNEUROSCI.21-02-00581.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 31.Napier TC, Herrold AA, De Wit H. Using conditioned place preference to identify relapse prevention medications. Neurosci Biobehav Rev. 2013;37:2081–6. doi: 10.1016/j.neubiorev.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledda F, Paratcha G, Sandoval-Guzmán T, Ibánez CF. GDNF and GFRα1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat Neurosci. 2007;10:293. doi: 10.1038/nn1855. [DOI] [PubMed] [Google Scholar]
- 33.Baloh RH, Enomoto H, Johnson EM, Milbrandt J. The GDNF family ligands and receptors — implications for neural development. Curr Opin Neurobiol. 2000;10:103–10. doi: 10.1016/S0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 34.Garthe A, Kempermann G. An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front Neurosci. 2013;7:63. doi: 10.3389/fnins.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H-Y, Wu X-M, Liu Y, He D. Transplantation of bone marrow mesenchymal stem cells promotes learning and memory functional recovery and reduces hippocampal damage in rats with alcohol-associated dementia. Transplantation. 2015;99:492–9. doi: 10.1097/TP.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 36.Shirasaka T, Hashimoto E, Ukai W, Yoshinaga T, Ishii T, Tateno M, et al. Stem cell therapy: social recognition recovery in a FASD model. Transl Psychiatry. 2012;2:e188. doi: 10.1038/tp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanelidis AJ, Premer C, Lopez J, Balkan W, Hare JM. Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction: a meta-analysis of preclinical studies and clinical trials. Circ Res. 2017;120:1139–50. doi: 10.1161/CIRCRESAHA.116.309819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y-h, Feng L, Zhang G-X, Ma C-g. Intranasal delivery of stem cells as therapy for central nervous system disease. Exp Mol Pathol. 2015;98:145–51. doi: 10.1016/j.yexmp.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 39. Horton KC. Mesenchymal stem cell delivery, survival and migration in a non-invasive system for central nervous system therapeutics [dissertation]. California State University, Sacramento; 2015.
- 40. Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci 2017. 10.1016/j.lfs.2017.12.025. [DOI] [PubMed]
- 41.Pardeshi CV, Belgamwar VS. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood–brain barrier: an excellent platform for brain targeting. Expert Opin Drug Deliv. 2013;10:957–72. doi: 10.1517/17425247.2013.790887. [DOI] [PubMed] [Google Scholar]
- 42.Danylesko I, Shimoni A. Second malignancies after hematopoietic stem cell transplantation. Curr Treat Options Oncol. 2018;19:1–17. doi: 10.1007/s11864-018-0528-y. [DOI] [PubMed] [Google Scholar]
- 43.Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21:1045–56. doi: 10.1089/hum.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss N, Miller F, Cazaubon S, Couraud P-O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–57. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez SH, Potula R, Fan S, Eidem T, Papugani A, Reichenbach N, et al. Methamphetamine disrupts blood–brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:1933–45. doi: 10.1038/jcbfm.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donega V, Nijboer CH, van Velthoven CTJ, Youssef SA, de Bruin A, van Bel F, et al. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr Res. 2015;78:520–6. doi: 10.1038/pr.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rafaiee R, Ahmadiankia N. Derived Mesenchymal Stem Cells in Addiction Related Hippocampal Damages. Int J Mol Cell Med. 2018;7:69–79. doi: 10.22088/ijmcm.bums.7.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo Furno D, Mannino G, Giuffrida R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J Cell Physiol. 2018;233:3982–99. doi: 10.1002/jcp.26192. [DOI] [PubMed] [Google Scholar]
- 49.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Risca HI, Zuarth-Gonzalez JD, Baker LE. Conditioned place preference following concurrent treatment with 3, 4-methylenedioxypyrovalerone (MDPV) and methamphetamine in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2020;198:173032. doi: 10.1016/j.pbb.2020.173032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther. 2016;7:1–14. doi: 10.1186/s13287-016-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rusznák Z, Henskens W, Schofield E, Kim WS, Fu Y. Adult neurogenesis and gliogenesis: possible mechanisms for neurorestoration. Exp Neurobiol. 2016;25:103–12. doi: 10.5607/en.2016.25.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burford K. The effects of BDNF Knockdown on Neuroinflammation in the Male and Female rat brain [Thesis]. University of Tennessee; 2019.
- 54.Ostrova I, Avrushchenko MS. Expression of brain-derived neurotrophic factor (BDNF) increases the resistance of neurons to death in the postresuscitation period. Gen Reanimatol. 2015;11:45–53. doi: 10.1016/j.jneuroim.2015.06.013. [DOI] [Google Scholar]
- 55.Alvandi MS, Bourmpoula M, Homberg JR, Fathollahi Y. Association of contextual cues with morphine reward increases neural and synaptic plasticity in the ventral hippocampus of rats. Addict Biol. 2017;22:1883–94. doi: 10.1111/adb.12547. [DOI] [PubMed] [Google Scholar]
- 56. Geoffroy H, Noble F. Chapter Seventeen - BDNF During Withdrawal. In: Litwack G, editor. Vitamins and Hormones: Academic Press; 2017. p. 475-96. [DOI] [PubMed]
- 57.Koskela M, Bäck S, Võikar V, Richie CT, Domanskyi A, Harvey BK, et al. Update of neurotrophic factors in neurobiology of addiction and future directions. Neurobiol Dis. 2017;97:189–200. doi: 10.1016/j.nbd.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hajheidari S, Sameni HR, Bandegi AR, Miladi-gorji H. Effects of prolonged abstinence from METH on the hippocampal BDNF levels, neuronal numbers and apoptosis in methamphetamine-sensitized rats. Neurosci Lett. 2017;645:80–5. doi: 10.1016/j.neulet.2017.02.051. [DOI] [PubMed] [Google Scholar]
- 59.Cheng M, Liu Q, Wang Y, Hao Y, Jing P, Jiao S, et al. MMP-9-BDNF pathway is implicated in cognitive impairment of male individuals with methamphetamine addiction during early withdrawal. Behav Brain Res. 2019;366:29–35. doi: 10.1016/j.bbr.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 60.Liran M, Rahamim N, Ron D, Barak S. Growth factors and alcohol use disorder. Cold Spring Harb Perspect Med. 2020;10:a039271. doi: 10.1101/cshperspect.a039271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valvassori SS, Mariot E, Varela RB, Bavaresco DV, Dal-Pont GC, Ferreira CL, et al. The role of neurotrophic factors in manic-, anxious-and depressive-like behaviors induced by amphetamine sensitization: Implications to the animal model of bipolar disorder. J Affect Disord. 2019;245:1106–13. doi: 10.1016/j.jad.2018.10.370. [DOI] [PubMed] [Google Scholar]