Abstract
Immunotherapy has changed the landscape of contemporary cancer treatment. Different from microsatellite instability-high colorectal cancer (CRC), the microsatellite-stable (MSS) CRC shows little response to immunomonotherapy. Reasonable drug combinations may be a valuable exploration to solve this the dilemma. Here, we report a young patient with refractory metastatic rectal adenocarcinoma at stage IVb who achieved a durable partial response after receiving tislelizumab plus fruquintinib and well-timed local radiotherapy. To date, the patient has a progression-free survival of more than 12 months with obviously declined serum tumor markers, increased peripheral blood effector T cells, alleviated scrotal edema and improved quality of life. This case suggests that an immune checkpoint inhibitor combined with an anti-VEGFR-tyrosine kinase inhibitor and local radiation intervention might be an effective strategy for heavily pretreated metastatic CRC patients with MSS phenotype.
Keywords: Metastatic colorectal cancer (mCRC), microsatellite stability (MSS), tislelizumab, fruquintinib, radiotherapy, quality of life
Introduction
With over 1900,000 new cases and 935,000 deaths each year, colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer-related deaths globally [1]. After years of exploration, the standard first- and second-line treatment for metastatic CRC (mCRC) has been widely agreed among experts globally. While in third line treatment and later settings, only regorafenib, trifluridine/tipiracil (FTD/TPI, TAS-102) and fruquintinib are recommended in the National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO) guidelines, respectively [2-4]. However, the median progression-free survival (mPFS) is merely 2-3 months [5-7]. Therefore, effective combinations or alternatives between agents without overlapping toxicity are urgently needed to be established for mCRC in third-line or above treatment.
In recent decades, immunotherapy has changed the landscape of contemporary cancer treatment. Compared with a satisfactory objective response rate (ORR) in deficient mismatch repair or microsatellite instability-high (dMMR/MSI-H) CRC, the proficient mismatch repair or microsatellite-stable (pMMR/MSS) CRC, which accounts for the majority of phenotypes in these cases, showed little response to programmed death ligand-1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors in the era of precision medicine. Rational combinations of different mechanisms involved in modulating the cancer-immunity cycle should be attempted to break through this barrier.
Herein, we report a heavily pretreated MSS mCRC patient with high tumor burden and poor ECOG PS score who achieved a durable disease control after treatment with tislelizumab plus fruquintinib and radiotherapy. We present the case in accordance with the CARE reporting checklist.
Case presentation
A 44-year-old man was hospitalized for abdominal pain and bloody stools without obvious predisposing causes in August 2019. After colonoscopy and biopsy, he was diagnosed with distal rectal adenocarcinoma that was approximately 15 cm away from the anus. He had a 5-year history of hypertension and a 1-year history of diabetes at diagnosis. At the time, his blood pressure and blood glucose levels were well managed with oral amlodipine besylate and acarbose. In addition, he had been drinking heavily for 30 years and stopped drinking from that day on. The ECOG PS score at that time was 0.
Whole abdominal and pelvic magnetic resonance imaging (MRI) on August 30, 2019 showed that the intestinal wall of the middle and lower rectum was significantly thickened with enhancement, and surrounded by blurred fat space and enlarged adjacent lymph nodes. Several abnormal signal foci in the right lobe of liver and the enlarged lymph nodes in bilateral pelvic wall, retroperitoneal and left inguinal area were radiologically considered as metastases. The TNM stage was cT3N2M1 (stage IVb). Needle aspiration cytology of the left inguinal lymph node was performed on September 3, 2019. It pathologically consisted of poorly differentiated adenocarcinoma with necrosis. The biopsy tissues were further subjected to immunohistochemical (IHC) test and fluorescent polymerase chain reaction (PCR). The results showed that the tumor was pMMR/MSS phenotype with wild-type KRAS, NRAS and BRAF expression. Next generation sequencing was not performed due to economic reasons. The patient received eight cycles of systematic FOLFIRI chemotherapy plus bevacizumab from September 2019 to February 2020. The best response was evaluated as partial response (PR) with significantly shrunken abdominal pelvic and inguinal lymph nodes. Subsequently, the patient continued to receive three cycles of FOLFIRI regimen to consolidate the first-line antitumor effect.
Imaging reexamination on June 2020 showed that the patient’s right retroperitoneal, right pelvic wall and left inguinal lymph nodes were enlarged compared with those in March 2020. A disease progression (PD) was evaluated. Then, from June 2020 to September 2020, the patient received helical tomotherapy (TOMO, 44.2 Gy/17 F for rectal and inguinal lymph nodes and 51 Gy/17 F for retroperitoneal, para-iliac and obturator lymph nodes) combined with concurrent S-1 and followed by two cycles of second-line mFOLFOX6 chemotherapy. The reexamination of whole abdominal and pelvic computed tomography (CT) showed that the lesions in the radiotherapy area were generally reduced and reached PR again, but the liver metastases were larger than before, so PD was finally considered.
The patient then received two cycles of CAPEOX plus regorafenib from November 2020, after which traditional Chinese medicine (TCM) and regorafenib maintainance was administrated. In March 2021, the effect was evaluated as stable disease (SD) without obvious progression. However, MRI reexamination in May 2021 demonstrated a tumor in the distal rectum with thickened intestinal wall accompanied by increased and enlarged lymph nodes bilaterally in the pelvic wall, as well as inguinal, retroperitoneal and hilar regions. The low-density mass in the right lobe of the liver was enlarged and some new scattered micronodules appeared in both lungs. So, PD was evaluated again. The patient was recommended to rechallenge chemotherapy plus bevacizumab, as he previously responded well to this regimen, but the patient declined. In September 2021, the patient had further disease deterioration in both lungs and liver metastases with suspected invasions of the right portal vein and the posterior wall of the bladder. Local radiofrequency ablation (RFA) of liver metastases was performed to relieve the hepatic discomfort.
Soon after, the patient began to suffer scrotal edema. With the degree of edema gradually being aggravated, the patient’s daily life was seriously affected. He had a weight loss of 25 kilograms compared to his pre-illness weight and the total Nutritional Risk Screening 2002 (NRS2002) score was 3. He had severe insomnia and fatigue, moderate constipation, mild nausea, vomiting, abdomen pain, diarrhea but no sclera icteric. The ECOG PS score was 2. To determine the next treatment option, we had a multidisciplinary discussion. After informed consent was obtained, the patient was treated with seven cycles of tislelizumab (200 mg, ivgtt, q3w) plus fruquintinib (5 mg, po, 3 weeks on/1 week off, q4w) from October 2021 to May 2022. Meanwhile, from January 17, 2022 to February 28, 2022, the patient received intensity modulated radiation therapy (IMRT, 43.7 Gy/23 F for right lobe liver metastases and 50.6 Gy/23 F for portal vein tumor thrombus). After imaging evaluation, the patient achieved a best response of PR, accompanied by partial disappeared or regressive micro-metastases in both lungs, fused and necrotic liver lesions, shrunken lymph nodes in the hilar, retroperitoneal and bilateral inguinal areas, marked palliative scrotal edema, alleviated rectal lesion and clear surrounding structures (Figure 1A and 1B).
Figure 1.

Representative images of the patient over the course of therapy. A. CT imaging showed that the multiple micro-metastases in both lungs remained basically stable with partial lesions disappearance or regression, and no new metastasis occurred. B. MRI imaging showed that the necrotic liver lesions, shrunken lymph nodes in hilar, retroperitoneal and bilateral inguinal, marked palliatory scrotal edema, alleviated rectal lesion and cleaner surrounding structures after four cycles of treatment with tislelizumab in combination with fruquintinib. The top images are those before treatment, and the bottom images are those after treatment. The red arrows indicate the main lesions.
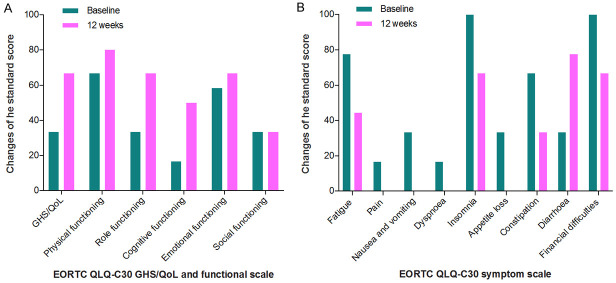
We monitored the dynamic changes of serum tumor markers and observed a significant reduction in CEA from 360.14 ng/mL to 88.14 ng/mL and in CA199 from 237.93 U/mL to 135.69 U/mL after two cycles of treatment (Figure 2A). Peripheral blood lymphocyte analyses also revealed a sustained increase in total lymphocytes, CD4+ and CD8+ effector T cells, and NK cells (Figure 2B). During the course of treatment, we regularly conducted a quality of life questionnaire, and the results showed substantial improvements in health status, role and cognitive functioning and clinical symptoms such as fatigue, nausea and vomiting, insomnia, appetite loss and constipation (Figure 3A and 3B). In terms of safety, the patient only developed adverse events (AEs) of grade 1 hand-foot skin reaction, hoarseness, hypothyroidism, fatigue, and appetite loss, grade 2 diarrhea and grade 1-2 myelosuppression after radiotherapy, which were quickly recovered after appropriate hormonal, antibiotic, granulocyte-colony stimulating factor and nutritional support therapies. No unexpected drug-related toxicity or treatment-related death occurred. Due to the rebound of the COVID-19 epidemic and the inconvenience of transportation caused by regional control, the patient chose to continue the treatment at a local hospital. Through telephone follow-up, we learned that the patient’s last examination was in September 2022, and the imaging tests indicated that his disease was still stable. The timeline of the whole clinical treatment course was shown in Figure 4.
Figure 2.
Dynamic changes of serum tumor markers (A) and peripheral blood lymphocytes (B) in this patient over the course of therapy. CA125, carbohydrate antigen 125; CA199, carbohydrate antigen 199; CAPEOX, capecitabine/oxaliplatin chemotherapy; CEA, carcinoembryonic antigen; FOLFIRI, folinic acid/5-fluorouracil/irinotecan chemotherapy; FOLFOX, folinic acid/5-fluorouracil/oxaliplatin chemotherapy; IMRT, intensity modulated radiation therapy; L, line of therapy; PD, progressive disease; RFA, radiofrequency ablation; TOMO, tomotherapy.
Figure 3.
Changes in standard scores on GHS/QoL and functional scales (A) and symptom scales (B) from baseline to week 12. Higher GHS/QoL and functional subscale scores represent better health status and functioning, whereas higher symptom subscale scores indicate more severe symptoms and worse health. EORTC QLQ-C30: European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30; GHS/QoL: general health status/quality of life.
Figure 4.

Timeline of the clinical course. AEs, adverse events; CT, computed tomography; CAPEOX, capecitabine/oxaliplatin chemotherapy; FOLFIRI, folinic acid/5-fluorouracil/irinotecan chemotherapy; mFOLFOX, modified-folinic acid/5-fluorouracil/oxaliplatin chemotherapy; IMRT, intensity modulated radiation therapy; MRI, magnetic resonance imaging; PD, progressive disease; PR, partial response; RFA, radiofrequency ablation; SD, stable disease; TCM, traditional Chinese medicine; TOMO, tomotherapy.
Discussion
This is the first report demonstrating a young MSS mCRC patient with significant scrotal edema who achieved a durable disease control under a salvage-line treatment with tislelizumab plus fruquintinib and radiotherapy even after prior regorafenib-based treatment failure.
In this decade, the great breakthroughs made in the treatment of multiple solid tumors with immune checkpoint inhibitors (ICIs) have significantly improved cancer patients’ clinical outcomes especially for those patients with highly immunogenic tumors. Based on the results of the KEYNOTE-177 study and a series of other trials, ICIs including pembrolizumab, nivolumab and envafolimab, currently, have been preferentially recommended in translational treatment or palliative care for unresectable or metastatic MSI-H/dMMR solid tumors, including CRC [8-10]. However, due to the immunosuppressive tumor microenvironment, anti-PD-1/PD-L1 monotherapy has not been recommended in any guidelines for advanced CRC patients with MSS phenotype.
Accumulating evidence showed that rational combinations between PD-1/PD-L1 antibodies and chemotherapy, radiotherapy, chemoradiotherapy, targeted therapy, other immune checkpoint inhibitors, or metabolic modulators can achieve higher response in solid tumors [11-17].
Anti-angiogenic targeted therapy as an important part of the whole-process management of mCRC had been reported to reduce angiogenesis, normalize disordered vasculature, increase tumor perfusion and oxygenation, and re-engineer the tumor immunosuppressive microenvironment towards a more immunosupportive profile by enhancing the release of chemokines, increasing the activation of effector T cells, decreasing the maturation and accumulation of myeloid-derived suppressor cells, and polarizing tumor-associated macrophages to M1-like phenotype [18]. Clinical data indicated a potential synergistic effect of ICIs combined with anti-angiogenetic tyrosine kinase inhibitors (TKIs) in MSS mCRC patients who failed to respond to prior standard treatments. In the REGONIVO study, regorafenib plus nivolumab for MSS advanced CRC patients who had failed multiple chemotherapies yielded an ORR of 36% and a mPFS of 7.9 months [19]. The REGOTORI study, published in the 2020 ESMO meeting, showed an ORR of 15.2%, a mPFS of 2.1 months and a mOS of 15.5 months in MSS mCRC patients who had received second or later lines chemotherapy [20]. In the LEAP-005 study presented at the 2021 ASCO meeting, pembrolizumab combined with lenvatinib for MSS mCRC patients in the third-line setting resulted an ORR of 22% and a DCR of 47% with the mPFS of 2.3 months and mOS of 7.5 months [21].
Here, we report our experience on the case of a young MSS mCRC patient who relapsed with multiple standard therapies. After treatment with fruquintinib plus tislelizumab and well-timed IMRT, the patient achieved a durable disease control with a PFS of more than 12 months. The combination strategy of fruquintinib and tislelizumab was selected mainly based on the following research basis. Fruquintinib is a highly selective anti-VEGFR-TKI that has been approved for third-line or later treatment for mCRC in China with a mPFS of 3.7 months and a mOS of 9.3 months [7]. Compared with other recommended third-line drugs, fruquintinib was more effective and demonstrated a better safety profile in patients with poor ECOG PS scores [22]. Even in patients who developed disease progression or intolerance after prior treatment with regorafenib or TAS-102, sequential administration of fruquintinib remained in effect according to the international multicenter phase 3 FRESCO-2 study [23]. In line with the findings, our case seems to validate the effectiveness of this sequential treatment. Tislelizumab is an anti-PD-1 antibody with higher affinity and specificity and lower off-target rates. It was engineered to minimize binding to Fcγ receptors (FcγR) on macrophages to greatly reduce antibody dependent phagocytosis, a potential mechanism of T-cell clearance and resistance to anti-PD-1 therapy [24]. Radiotherapy as an immunomodulator can promote tumor neoantigens release, increase T-cell infiltration and induce immunogenic cell death, thereby enhancing immunotherapy response in solid tumors, including MSS CRC [12].
In brief, the cooperative effect between fruquintinib and tislelizumab and localized radiation intervention was confirmed in this case. In the course of treatment, we witnessed the obviously relieved tumor burden in the young man in the aspects of alleviated scrotal edema, shrunken or necrotic metastases, reduced serum tumor markers, increased effector T cells, ameliorative fatigue, insomnia, appetite loss and constipation, and improved health status and body function without unexpected drug-related toxicity. Therefore, this combination strategy may be an implementable clinical practice with satisfactory efficacy and controllable safety for heavily pretreated MMS mCRC patients.
However, many questions remain to be considered. Indeed, there are still a lot of patients who have developed primary or secondary resistance to the novel therapeutic strategy and another challenge that cannot be ignored is the identification of a reliable set of biomarkers (except for serum tumor markers and/or cell-free DNA) that can be used to detect anti-tumor efficacy, screen the most appropriate patients and ultimately guide clinical decisions making. Sometimes, the discrepancy between the clinical symptoms and imaging findings is also a growing issue in the era of immunotherapy, requiring serious thinking and deeper collaboration between clinicians and radiologists. In short, further studies with larger samples and different combinations or sequences are required to improve the outcomes in mCRC patients with MSS status.
Conclusion
We report a novel case of a young MSS mCRC patient with a massive scrotal edema and poor ECOG PS score who achieved a durable disease control and gained a PFS of more than 12 months after tislelizumab combined with fruquintinib and radiotherapy. This case indicates that some MSS mCRC patients may also respond well to anti-PD-1 based therapy after failure of prior systemic therapy. As effective immunomodulators, anti-angiogenesis and radiation intervention may be important co-factors to ensure the synergistic effect of combined immunotherapy for the salvage-line treatment in MSS mCRC.
Acknowledgements
The authors thank the patient and his caregivers for allowing us to publish his clinical case. This work was supported by the National Natural Science Foundation of China (No. 82102954).
Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this case report.
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:329–359. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 3.Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:1139–1167. doi: 10.6004/jnccn.2022.0051. [DOI] [PubMed] [Google Scholar]
- 4.Yuan Y, Wang X, Chen G, Wang Y, Sheng W, Li X, Zhou A, Zhang Z, Li G, Cai S, Xu R, Li J, Zhang S. Updates in version 2019 of CSCO guidelines for colorectal cancer from version 2018. Chin J Cancer Res. 2019;31:423–425. doi: 10.21147/j.issn.1000-9604.2019.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 6.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. 2018;319:2486–2496. doi: 10.1001/jama.2018.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fourchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zhong WY, Fogelman D, Marinello P, Andre T KEYNOTE-177 Investigators. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659–670. doi: 10.1016/S1470-2045(22)00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, Garcia-Alfonso P, Neyns B, Luppi G, Cardin DB, Dragovich T, Shah U, Abdullaev S, Gricar J, Ledeine JM, Overman MJ, Lonardi S. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II checkmate 142 study. J. Clin. Oncol. 2022;40:161–170. doi: 10.1200/JCO.21.01015. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Deng Y, Zhang W, Zhou AP, Guo W, Yang J, Yuan Y, Zhu L, Qin S, Xiang S, Lu H, Gong J, Xu T, Liu D, Shen L. Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors. J Hematol Oncol. 2021;14:95. doi: 10.1186/s13045-021-01095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A, Rodriguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 12.Parikh AR, Szabolcs A, Allen JN, Clark JW, Wo JY, Raabe M, Thel H, Hoyos D, Mehta A, Arshad S, Lieb DJ, Drapek LC, Blaszkowsky LS, Giantonio BJ, Weekes CD, Zhu AX, Goyal L, Nipp RD, Dubois JS, Van Seventer EE, Foreman BE, Matlack LE, Ly L, Meurer JA, Hacohen N, Ryan DP, Yeap BY, Corcoran RB, Greenbaum BD, Ting DT, Hong TS. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer. 2021;2:1124–1135. doi: 10.1038/s43018-021-00269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeno J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Ozguroglu M PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 14.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 15.Ramalingam SS, Thara E, Awad MM, Dowlati A, Haque B, Stinchcombe TE, Dy GK, Spigel DR, Lu S, Iyer Singh N, Tang Y, Teslenko I, Iannotti N. JASPER: phase 2 trial of first-line niraparib plus pembrolizumab in patients with advanced non-small cell lung cancer. Cancer. 2022;128:65–74. doi: 10.1002/cncr.33885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutierrez E, Rutkowski P, Gogas HJ, Lao CD, De Menezes JJ, Dalle S, Arance A, Grob JJ, Srivastava S, Abaskharoun M, Hamilton M, Keidel S, Simonsen KL, Sobiesk AM, Li B, Hodi FS, Long GV RELATIVITY-047 Investigators. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, Rotin D, Anafi L, Avivi C, Melnichenko J, Steinberg-Silman Y, Mamtani R, Harati H, Asher N, Shapira-Frommer R, Brosh-Nissimov T, Eshet Y, Ben-Simon S, Ziv O, Khan MAW, Amit M, Ajami NJ, Barshack I, Schachter J, Wargo JA, Koren O, Markel G, Boursi B. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603) J. Clin. Oncol. 2020;38:2053–2061. doi: 10.1200/JCO.19.03296. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, Luo HY, Li JB, Wang FH, Qiu MZ, Lv ZD, Wang DS, Li YH, Zhang DS, Xu RH. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2:100383. doi: 10.1016/j.xcrm.2021.100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Roca C, Yanez E, Im SA, Alvarez EC, Senellart H, Doherty M, García-Corbacho J, Lopez JS, Basu B, Maurice-Dror C, Gill SS, Ghori R, Kubiak P, Jin F, Norwood KG, Chung HC. LEAP-005: a phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors-results from the colorectal cancer cohort. J. Clin. Oncol. 2021;39:94. [Google Scholar]
- 22.Li J, Cai Y, Deng Y. Selection of oral therapeutics in China for the treatment of colorectal cancer. Curr Treat Options Oncol. 2021;22:55. doi: 10.1007/s11864-021-00852-1. [DOI] [PubMed] [Google Scholar]
- 23.Dasari A, Sobrero A, Yao J, Yoshino T, Schelman W, Yang Z, Chien C, Kania M, Tabernero J, Eng C. FRESCO-2: a global phase III study investigating the efficacy and safety of fruquintinib in metastatic colorectal cancer. Future Oncol. 2021;17:3151–3162. doi: 10.2217/fon-2021-0202. [DOI] [PubMed] [Google Scholar]
- 24.Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, Zhang Y, Zhou X, Wang Z, Wang Y, Shi Y, Bai H, Liu N, Yang X, Cui X, Cao Y, Liu Q, Song J, Li Y, Tang Z, Guo M, Wang L, Li K. The binding of an anti-PD-1 antibody to FcgammaRIota has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67:1079–1090. doi: 10.1007/s00262-018-2160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]