Abstract
Purpose of Review
We reviewed the available literature on mpox in People with HIV (PWH). We highlight special considerations of mpox infection related to epidemiology, clinical presentation, diagnostic and treatment considerations, prevention, and public health messaging in PWH.
Recent Findings
During the 2022 mpox outbreak, PWH were disproportionally impacted worldwide. Recent reports suggest that the disease presentation, management, and prognosis of these patients, especially those with advanced HIV disease, can widely differ from those without HIV-associated immunodeficiency.
Summary
Mpox can often be mild and resolve on its own in PWH with controlled viremia and higher CD4 counts. However, it can be severe, with necrotic skin lesions and protracted healing; anogenital, rectal, and other mucosal lesions; and disseminated organ systems involvement. Higher rates of healthcare utilization are seen in PWH. Supportive, symptomatic care and single or combination mpox-directed antiviral drugs are commonly used in PWH with severe mpox disease. Data from randomized clinical control trials on the efficacy of therapeutic and preventive tools against mpox among PWH are needed to better guide clinical decisions.
Keywords: HIV, AIDS, Mpox, Orthopoxvirus, Vaccina virus, Tecovirimat
Introduction
Mpox (formerly known as monkeypox) is a viral disease caused by the monkeypox virus, a member of the Orthopoxviridae family. These are double-stranded DNA viruses that can infect humans and animals [1]. The variola virus, a well-known member of this family and causative agent of smallpox, was ultimately eradicated in 1980 [2]. Other members of the family include the vaccinia virus, which can cause a smallpox-like disease in humans. A modified vaccinia virus is used in smallpox and the mpox vaccines [3]. Mpox is primarily transmitted through close contact with infected animals or people [4] and was considered endemic to several countries in West and Central Africa [5]. In May 2022, a rapid increase in mpox cases in various non-endemic countries outside of Africa led the World Health Organization to declare the outbreak a Global Public Health Emergency on July 23, 2022 [6]. The United States Department of Health and Human Services followed by declaring mpox a Public Health Emergency on August 4, 2022 [7••].
Unlike endemic mpox, the 2022 mpox outbreak has centered primarily around cisgender men who have sex with men (MSM), and several reports from the USA and Europe suggest that around 40% and up to 90% of cases in some settings occurred in people with HIV (PWH) [8, 9••, 10–15]. During the 2022 outbreak, it was evident that the main route of transmission was through close contact either through sexual activity or direct contact with the virus through saliva or seminal fluid [4, 16]. Overall, the majority of mpox cases have been mild and resolved without treatment; however, severe mpox cases requiring urgent care or emergency department visits, hospitalization, intensive care, and even death have been reported, primarily in immunocompromised individuals including those with advanced, untreated HIV disease [9••, 17].
The objective of this review is to summarize the existing literature and describe the epidemiological features, clinical presentation, treatment considerations, and prevention of mpox in PWH, with a specific focus on those with advanced HIV.
Search Strategy and Selection Criteria
To identify articles on mpox in PWH, we searched PubMed and Scopus using the search terms “monkeypox” OR “mpox” AND “HIV” or “Human Immunodeficiency Virus.” Only articles published in English were considered. The searches ran between 01/01/2000 and 03/8/2023. We supplemented these searches by reviewing citations in identified manuscripts, recent conference abstracts, and our knowledge of the subject.
Epidemiology
Since the beginning of the 2022 mpox outbreak, more than 86,000 laboratory-confirmed cases have been reported globally with 111 deaths involving 110 countries [18••]. Most cases were reported in mid to late August 2022, and as of March 2023, cases have substantially decreased. Most mpox cases were identified through sexual health or other health services and have involved mainly, but not exclusively, MSM with a median age of 34 years (IQR 29–41). The majority of cases have occurred in the USA, Brazil, Spain, France, and other countries in Europe and Latin America [18••]. Globally, up to 48% of individuals with available data are PWH [19]. A recent multi-country cohort of mpox cases among PWH by Mitjà et al. summarized data from 382 adult patients from 19 countries between May 2022 and Jan 2023. Of these cases, 349 (91%) were PWH with CD4 cell counts of less than 350 cells per mm3. In this cohort, 96% of patients were cisgender men, 55% from Latin America, 26% from Europe, 17% from North America, and 2% from Africa. Severe complications, including necrotizing skin lesions, pulmonary involvement, secondary infections, and sepsis, were primarily seen in those with CD4 cell counts less than 100 cells/mm3 [9••]. Overall, 107 (28%) hospitalizations were reported, of whom 27 (25%) died. All deaths occurred in people with CD4 cell counts less than 200 cells/mm3.
As of March 6, 2023, 30,225 cases have been reported in the USA, including 38 deaths. Cohorts among adults with information on HIV status have reported that 38 to 57% of mpox cases had HIV co-infection [7••, 20••, 21]. A report by Kava et al. of US cases from May to October 2022 [21] described how the outbreak mostly affected cisgender MSM who reported male-to-male sexual contact and disproportionally impacted Black and Latino individuals. Curran et al. reported on 1969 people with mpox from May 17 to July 22, 2022, in 8 US jurisdictions. In this cohort, HIV prevalence was 38%. Among PWH, 94% had received HIV care in the preceding 12 months, and 82% had a viral load of fewer than 200 copies/ml. Overall, PWH had higher rates of mpox-related hospitalizations compared to those without HIV, and these rates were highest in PWH who were not virally suppressed on antiretroviral therapy (ART) [20••]. In southern states, the epicenter of the HIV epidemic in the USA, higher incidence of mpox among PWH has been reported. For example, in the state of Georgia, 60–90% of reported mpox cases had HIV co-infection [10, 22••].
Clinical Presentation
The incubation period of mpox is approximately 12 days from exposure, and the clinical presentation of mpox can vary from person to person. Before the 2022 outbreak, endemic cases typically presented with a prodrome of fever and other systemic symptoms, followed in a few days by a rash that evolves through macular, papular, vesicular, and pustular stages; the pustules then break open and crust over. These stages of the rash can coexist in different parts of the body at the same time. During the 2022 outbreak, skin lesions have primarily involved the anogenital and oropharyngeal regions [7••, 14]. Other systemic manifestations can include fever, inguinal lymphadenopathy, chills, headache, myalgias, and generalized malaise. Data from the current outbreak suggests that at least 50% of people with mpox did not report the typical prodrome as their first symptom [7••, 12, 23]. Asymptomatic and undetected infection has also been documented [24, 25]. Most people recover from mpox within 2 to 4 weeks, but complications can occur. Oropharyngeal lesions can be complicated by tonsillitis, tonsillar abscesses, or supraglottitis [26]. Severe urethral lesions have resulted in dysuria, hematuria penile edema, paraphimosis, and phimosis [23, 27, 28].
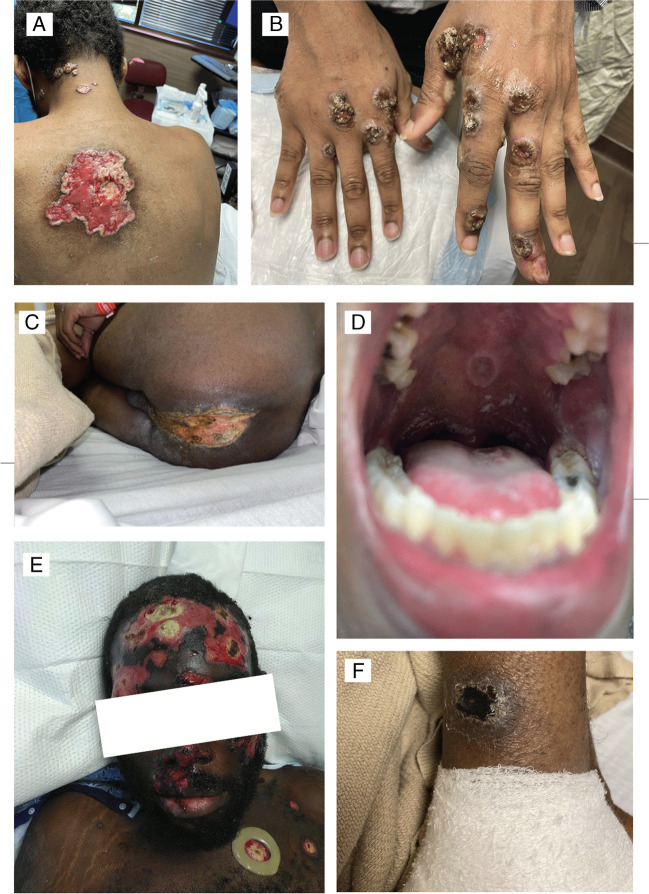
The clinical presentation of mpox in PWH can be more protracted and severe than those without HIV (9••, 17). Patients with CD4 cell counts less than 200 cells/mm3 can present with large ulcerative, coalescent necrotic lesions with protracted healing (> 4 weeks), severe pain, and bacterial or fungal superinfection [9••, 10] (Fig. 1). When compared to those without HIV, PWH are more likely to report anal/rectal pain (34% versus 26%), tenesmus (20% versus 12%), rectal bleeding (19% versus 12%), purulent or bloody stools (15% versus 8%), and proctitis (13% versus 7%) [14, 20••]. Other cohorts [8, 17, 29] from Portugal, the USA, and Spain support the higher frequency of anorectal symptoms in PWH. Involvement of other organ systems has also been reported in people with advanced HIV, including pulmonary nodules, pleural effusions, myopericarditis, gastrointestinal bleeding, colitis, and central nervous system manifestations including encephalitis and transverse myelitis [9••, 15, 17, 30, 31]. Ocular involvement [32] can present as necrotizing blepharoconjunctivitis, keratitis, conjunctival lesions, and vision loss [33, 34]. A fatal case of disseminated mpox complicated with hemophagocytic lymphohistiocytosis was also reported [35].
Fig. 1.
Figure mpox lesions in patients* with HIV. A: Coalescent ulcers. B: Multiple large ulcers pustules in hands. C: Coalescent lesions in the perianal region. D: Single mpox ulcer in the soft palate. E: Large coalescent, necrotic facial and scalp lesions F: Eschar in the lower extremity. Photos by Alexandra Dretler, Jonathan Colasanti, and Valeria D. Cantos *Patients have provided written consent to publish these photographs
Intensive care unit (ICU) admissions and death have been reported among PWH and mpox. Common reasons for ICU admission included sepsis, shock, and severe upper airway lesions or lung involvement requiring endotracheal intubation [9••, 31, 36–40]. In the cohort reported by Mitjà et al., mpox mortality ranged from 15% in PWH with a CD4 cell count below 200 cells/mm3 to 27% among those with CD4 cell counts less than 100 cells/mm3. There were no deaths reported in PWH whose CD4 cell counts were above 200 cells/mm3 [9••]. In addition, among 85 individuals with mpox who started or restarted ART after diagnosis, 21/85 (25%) had suspected mpox immune reconstitution inflammatory syndrome (IRIS). The median days from ART initiation to worsening mpox symptoms and suspected IRIS was 14. Twelve of 21 people (57%) with suspected mpox IRIS died [9••].
The more severe presentation of mpox disease seen in people with advanced HIV disease and decreased CD4 cell counts suggests an important role for CD4 cells in regulating disease severity, including impaired Monkeypox virus mucosal recognition, induction of B-cell response, and viral clearance [41]. More studies are needed to fully understand Monkeypox virus host pathogen interactions in the setting of immune deficiency caused by HIV infection. Some have argued that mpox should be designated an opportunistic infection or AIDS defining disease [9••]. However, others fear labeling mpox as such could spark further stigma towards people with mpox and HIV [42]. Currently, ART is recommended for every person living with HIV [43••], and labeling mpox as an AIDS-defining condition will only serve public health purposes as a mechanism of monitoring the AIDS pandemic.
Diagnostics Considerations
The diagnosis of mpox is most commonly made by the detection of monkeypox virus by real-time polymerase chain reaction (RT-PCR) from swabs collected directly from the skin lesions. Detection of positive monkeypox virus RT-PCR has also been observed in oropharyngeal, blood, urine, and seminal fluid samples, albeit at lower levels [15, 23, 44, 45]. The positive detection yield strongly decreases between diagnosis time and day 14 after diagnosis [45], and performing multi-site swabs for mpox RT-PCR might lower false negative rates [46]. Lapa et al. reported a case of a PWH who continued to shed monkeypox virus DNA in semen for several weeks after symptom onset. Semen collected during the early phase of the infection (day 6 from initial symptom) may contain replication-competent virus, which could potentially lead to mpox transmission [47]. Persistent disseminated viral replication was documented in a fatal mpox case in a PWH with less than 35 CD4 cells/mm3, where non-variola orthopoxvirus polymerase chain reaction was detected 57 days after the onset of symptoms in skin lesions and autopsy tissue specimens of the brain, bone marrow, and testicles [35].
Given the high rates of HIV co-infection among people with mpox, every patient suspected of having mpox should also be tested for HIV unless previously diagnosed [37, 48••]. If positive, immediate HIV ART should be considered. One study to date described HIV viral load monitoring in 28 PWH and found that HIV-RNA levels did not substantially change during mpox infection. In addition, no significant differences in CD4 cell counts were found before and at the time of mpox diagnosis [49]. Testing for other bacterial sexually transmitted infections (STIs) at the time of mpox suspicion or diagnosis is also important. Cohorts from Germany, the USA, and Spain have shown that up to 50% of individuals with mpox were diagnosed with at least one other STI in the months preceding the infection including herpes, gonorrhea, chlamydia, syphilis, and Mycoplasma genitalium [20••, 23, 50]. Concomitant STIs with mpox have been reported, with syphilis diagnosed in 16.4% of people with mpox and other bacterial STIs in 19% of cases [10, 50]. Furthermore, studies have shown a prevalence of anti-HCV antibodies in 6% of people with mpox [15, 51] and 15.2% among PWH and mpox [51]. It is not yet known how the transmission and pathogenesis of mpox may be altered in the setting of concurrent bacterial STI.
Treatment
All patients with mpox should be offered symptomatic supportive care, including skin wound care (washing lesions with mild soap and water, covering lesions, and silicone-based gels to minimize scarring) [52]. Local anesthetics such as viscous lidocaine, analgesic mouthwash for oral lesions, stool softeners and sitz bath for anorectal lesions, oral antihistamines for pruritus, close monitoring for bacterial superinfection, and systemic pain control are recommended [53]. At present, no Food and Drug Administration (FDA)-approved treatments for human mpox are available. In February 2023, the US Centers for Disease Control and Prevention (CDC) released Interim Clinical Treatment Considerations for Severe Manifestations of mpox [54••], which are more common among PWH, including the use of FDA-approved drugs and biologic agents developed for smallpox preparedness, also known as therapeutic medical countermeasures (MCMs). These MCMs include tecovirimat, brincidofovir, cidofovir, trifluridine ophthalmic solution, and vaccinia immune globulin intravenous (VIGIV).
Tecovirimat
During the 2022 mpox outbreak, the antiviral tecovirimat (TPOXX), currently FDA-approved for treating smallpox, was given an Expanded Access Investigational New Drug (EA-IND) authorization. An accompanying protocol for access is available through CDC [55], primarily for individuals presenting with lesions in sensitive anatomic areas and those at risk for severe disease or severe pain, including PWH. Tecovirimat inhibits the function of a major envelope protein required to produce extracellular virus [56]. It is given two to three times daily and is available orally, taken with a fatty meal, or intravenously, which is prioritized for individuals unable to take oral medications or with impaired absorption. The treatment course is typically 2 weeks, but a longer course (an additional 3–7 days) should be considered for patients with severe or ongoing symptoms despite treatment, and in those individuals with low CD4 cell counts [37, 48••, 57].
Tecovirimat should be initiated in immunocompromised patients with suspected or laboratory diagnosed mpox [48••]. Tecovirimat resistance can occur with a single point mutation [58], and it has been reported in at least four PWH during the 2022 mpox outbreak [9••, 35]. Testing for tecovirimat resistance and pharmacokinetics for public health surveillance purposes is encouraged when any new lesions form after ≥ 7 days of treatment [54••]. Further research will be needed to determine whether PWH are at increased risk of tecovirimat resistance. Given that the effectiveness of Tecovirimat for the treatment of mpox has not been systematically evaluated, CDC strongly encourages enrollment in clinical trials, several of which are ongoing in a variety of settings [54••, 59].
O’Laughlin et al. reported on 549 individuals treated with TPOXX in the USA, of whom 369 had outcome forms [55]. Approximately half of the patients with mpox who received tecovirimat were PWH, although no information related to CD4 cell count or viral load was reported. The median interval from initiation of tecovirimat to subjective improvement was 3 days and did not differ by HIV status. Adverse events were reported for 12 (3.5%) of 340 patients with information on adverse events. These included headache (3), nausea (2), visual disturbance (2), weakness (2), and hospitalization for psychiatric reasons (1). Based on these limited data, tecovirimat appears safe for use among PWH.
Cidofovir and Brincidofovir
Cidofovir and brincidofovir are a different type of antiviral medication that has demonstrated activity against orthopoxviruses in vitro and animal models [60, 61], and act by inhibiting viral replication by selectively inhibiting viral DNA polymerases [62]. One of these drugs can be added (should not be used simultaneously or within 1 week of one another) to tecovirimat treatment for patients with or at risk for severe mpox. They are usually administered once weekly for 2 weeks. Diarrhea and renal toxicity, which can manifest as acute kidney failure, proteinuria, or a Fanconi-type syndrome with proximal tubular dysfunction, are common adverse events associated with cidofovir [63]. Current data on the effectiveness of these agents in PWH are not available, but there is historical experience using cidofovir for other severe viral infections in PWH such as cytomegalovirus [64]. A small, prospective study assessed topical Cidofovir 1% on a compassionate use basis in 12 patients with mpox compared to symptomatic treatment alone. Mpox lesions cleared quicker in the cidofovir-treated group (12 vs 18 days) However, 50% of Cidofovir-treated participants reported local adverse effects including irritation or erosion on and around the site of application [65].
Vaccinia Immune Globulin Intravenous
Vaccinia immune globulin intravenous (VIGIV) is composed of concentrated antibodies pooled from people who have been vaccinated with the smallpox vaccine [66]. This treatment may offer some protection against other viruses within the orthopoxvirus family, including mpox. During the 2022 MPXV outbreak, VIGIV has been recommended for individuals who cannot mount a substantial immune response to aid on viral clearance, such as those with advanced HIV. Additional doses may be considered in cases where new lesions appear several days after the initial dose, when a large percentage of the body surface area is affected, or when there is persistent immunocompromised [54••].
Miller et al. reported on 57 patients from August 10 to October 2022 with severe mpox, of which 47 had HIV [37]. Mpox-directed therapy included Tecovirimat: oral in 53, intravenous in 37, intravenous vaccinia immune globulin in 29, and Cidofovir in 13 patients. Among PWH, the majority had CD4 cell counts below 50, and only 4/47 received antiretroviral therapy before being diagnosed with mpox. In the Mitjà et al. cohort, 62 (16%) of 382 individuals received tecovirimat (five received both oral and intravenous doses) and seven (2%) received cidofovir or brincidofovir. All people receiving mpox-specific antiviral therapy were treated in Europe or the USA, except two who received tecovirimat in Brazil. No information on outcome based on treatment option was provided in these reports.
Antiretroviral Treatment
As none of the MCMs used for mpox are virucidal, optimization of the immune system is likely critical to clear the infection. As such, early ART initiation, continuation, or resumption is key in treating PWH with mpox, regardless of CD4 cell count. Clinicians should be aware of potential drug interactions between tecovirimat and rilpivirine (including combination with cabotegravir), doravirine, and maraviroc, through induction of CYP3A4, although the guidelines advise against dose adjustments [43••, 48••]. Experts also advise that drug interactions should not preclude using tecovirimat and or initiating antiretrovirals when indicated. Similarly, HIV pre-exposure prophylaxis (PrEP) should be continued in individuals already taking it, and referral to PrEP should be prioritized in HIV-negative people with mpox [48••, 67].
Prevention
The JYNNEOS vaccine, manufactured by Bavarian Nordic, is a live, nonreplicating modified vaccinia Ankara virus. It is the only vaccine being used in the USA during the current outbreak for mpox prevention [68, 69] and is the preferred vaccine for people with HIV [48••]. The replication-competent vaccinia virus vaccine, ACAM 2000 (replaced Dryvax in 2008), is contraindicated for people with HIV due to the risk of severe adverse effects in immunocompromised persons [70]. JYNNEOS can also be administered as post-exposure prophylaxis (PEP) for people who have had mpox exposure. When used for PEP, it should be administered to asymptomatic contacts ideally within 4 days but up to 14 days after exposure. The CDC currently also recommends JYNNEOS vaccination for prevention in groups at high risk for mpox exposure or at risk for severe disease, including PWH [69].
JYNNEOS is a 2-dose, subcutaneous (0.5 mL per dose) vaccine administered 28 days apart. The FDA issued an EUA on August 9, 2022 to authorize the intradermal administration (0.1 mL per dose) in an effort to increase the number of persons who could be vaccinated with the available supply based on an immunogenicity study from 2015 [71]. Efficacy of the JYNNEOS vaccine against smallpox and mpox is estimated to be more than 80% [72–74], but these efficacy data are limited and extrapolated from immunogenicity studies. Real-world mpox ecologic data showed that mpox incidence was 9.6 higher among unvaccinated individuals compared to those who had received 2 vaccine doses and 7.4 times higher among persons who had received one dose of the vaccine suggesting high vaccine efficacy, with no preliminary difference between subcutaneous and intradermal administration routes [75]. Similarly, a retrospective study from London [76] of 10,068 individuals who received at least one dose of JYNNEOS vaccine showed that only 15 (0.15%) developed mpox post-vaccination. These individuals presented with mpox symptoms on average 4 days (IQR 3–9) days after vaccination, suggesting mpox exposure prior to receipt of vaccination. Wolff et al. estimated a vaccine efficacy of 86% in their cohort of 2054 eligible males of whom 1037 (50%) were vaccinated and completed at least 90 days of follow-up [77]. Clinical trials are underway assessing vaccine efficacy, including a study evaluating immunogenicity of dose reduction strategies. Of note, an apparent case of mpox reinfection was reported by Golden et al. in a non-immunosuppressed man who completed two doses of subcutaneous JYNNEOS after an initial mpox diagnosis and presented with genital ulcerative disease 4 months after vaccination [78].
Long-term effectiveness of smallpox vaccines in the general population and in people living with HIV remains unknown, particularly in people with low CD4 cell counts. Titanji et al. reported a cohort of 298 US military personnel with laboratory confirmed mpox. Of all individuals, 208 had received either the Dryvax or ACAM2000 smallpox vaccination including 29% PWH. Vaccine effectiveness estimates were 66% for Dryvax and 72% for ACAM 2000. Median time from receiving a smallpox vaccination to mpox diagnosis was 13 years, suggesting long-term effectiveness of these vaccines against mpox. No subgroup analysis based on HIV-status or CD4 cell count has been reported [79].
An immunological study by Agrati et al. reported on 17 patients with mpox, of which 7 were PWH. The immunological signature (early expansion of activated effector CD4 and CD8 T cells) after mpox infection persisted over time and suggested that it might not be necessary to vaccinate people who have already had natural mpox infection [80].
During the 2022 mpox outbreak in the USA, vaccine deployment was challenged by supply and demand issues, along with vaccination rate inequities based on HIV status. Cline et al. reported on mpox cases and vaccination from New York Safety Hospitals. Despite the marked association of HIV with mpox, only 3% of male patients with HIV received the vaccine in this cohort [81]. Racial and ethnic inequities in vaccination rates have also been observed. Despite the disproportional impact of mpox and HIV in Black and Latino groups, as of March 7 2023, White, Latino, and Black individuals accounted for 48%, 20%, and 11% respectively, of the 1,201,210 vaccine doses administered nationally [82].
Intentional efforts to expand vaccination in disproportionately affected populations, including minorities of color, were described during the 2022 mpox outbreak. A report by Millman et al. highlighted successful efforts by the Georgia Department of Public Health to enhance vaccine equity among Black MSM by requesting additional allocation of vaccine, partnering with community-based organizations, and distributing in locations and outreach events catering to this community. Ultimately, more than half the doses of vaccines reached communities of color (48% Black and 8% Hispanic) [22••].
Health Promotion and Messaging
Mpox messaging should be accurate and transparent, and efforts should prioritize communities disproportionately impacted by the disease. Stigma, especially in already marginalized communities, can result in individual disengagement in healthcare, limiting access to testing, treatment, and prevention [16]. This is particularly relevant for PWH who are disproportionately affected by social inequities and have historically suffered from multi-faceted stigma related to their status and sexual orientation.
During the 2022 mpox outbreak, federal agencies and local Health Departments focused their communication efforts on harm reduction and mitigation strategies. Reports by Hubach and Delaney included hundreds or participants recruited through convenience sampling or dating apps and showed that close to half of the participants reported a change in their sexual behaviors due to the mpox outbreak, including limiting the number of sexual partners, abstaining from sex, and or avoiding crowded venues (83, 84). Although Public Health interventions including scale-up of vaccination, messaging, and individual behavior modification may have contributed to the overall decline in mpox cases worldwide, it remains unclear to what extent each of these contributed to reductions in new diagnoses. More research is needed to understand the impact of these interventions and prevent future outbreaks.
Finally, clinical encounters related to mpox, whether for diagnosis and treatment or vaccination, represent an opportunity to conduct a holistic sexual health screening and include co-testing for other STI including gonorrhea, chlamydia, syphilis, herpes, HCV, and HIV. Clinicians should initiate conversations regarding HIV pre-exposure prophylaxis for those testing negative for HIV and offer rapid entry to HIV care for those who test positive [67]. Clinicians are uniquely positioned to be advocates and leaders in stigma prevention. Providing a sex-positive and judgment-free clinical environment would allow patients to disclose sexual practices and inform targeted education and mitigation strategies. Public Health and institutional messaging also need to be transparent and free from sexual and gender identity bias to facilitate informed decision making, including behavioral modification and reduced exposure.
Conclusion
The 2022 mpox outbreak has disproportionately impacted cisgender MSM and PWH from racial and ethnic minorities. Mpox in PWH with low CD4 counts often results in severe skin and mucosal disease, dissemination to distant organ systems, high healthcare utilization, and death. Intensive supportive care and direct antiviral medication are recommended for PWH presenting with mpox, based on animal-based and observational data. While clinical trials are ongoing, declining cases globally could reduce participant enrollment and limit timely data on effective treatments and preventive options against mpox. Collaborations between government and private funders, public health agencies, academic institutions, and community-based organizations are crucial for prompt deployment of multi-level strategies to halt ongoing disease transmission and implement research studies to inform future public health responses. The lessons learned from the 2022 mpox outbreak highlight the importance of intentional targeted interventions and destigmatizing education efforts to effectively reach at-risk populations.
Declarations
Conflict of Interest
Drs. Saldana, Aldred, and Cantos declare that they have no conflict of interest. Dr. Kelley reports grants from Moderna, grants from ViiV, grants from Novavax, grants from Humanigen, outside the submitted work;
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Elsayed S, Bondy L, Hanage WP. Monkeypox virus infections in humans. Clin Microbiol Rev. 2022;35(4):e0009222. doi: 10.1128/cmr.00092-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambo TA. Eradication of smallpox. N Engl J Med. 1981;305(4):224. doi: 10.1056/NEJM198107233050415. [DOI] [PubMed] [Google Scholar]
- 3.Volz A, Sutter G. Modified vaccinia virus Ankara: history, value in basic research, and current perspectives for vaccine development. Adv Virus Res. 2017;97:187–243. doi: 10.1016/bs.aivir.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan D, Nazareth J, Sze S, Martin CA, Decker J, Fletcher E, et al. Transmission of Mpox: a narrative review of environmental, viral, host and population factors in relation to the 2022 international outbreak. J Med Virol. 2023. [DOI] [PMC free article] [PubMed]
- 5.Ogoina D, Iroezindu M, James HI, Oladokun R, Yinka-Ogunleye A, Wakama P, et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 6.Nuzzo JB, Borio LL, Gostin LO. The WHO declaration of monkeypox as a global public health emergency. JAMA. 2022;328(7):615–617. doi: 10.1001/jama.2022.12513. [DOI] [PubMed] [Google Scholar]
- 7.Philpott D, Hughes CM, Alroy KA, Kerins JL, Pavlick J, Asbel L, et al. Epidemiologic and clinical characteristics of monkeypox cases—United States, May 17-July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(32):1018–1022. doi: 10.15585/mmwr.mm7132e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caria J, Pinto R, Leal E, Almeida V, Cristóvão G, Gonçalves AC, et al. Clinical and epidemiological features of hospitalized and ambulatory patients with human monkeypox infection: a retrospective observational study in Portugal. Infect Dis Rep. 2022;14(6):810–823. doi: 10.3390/idr14060083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.•• Mitjà O, Alemany A, Marks M, Lezama Mora JI, Rodríguez-Aldama JC, Torres Silva MS, et al. Mpox in people with advanced HIV infection: a global case series. Lancet. 2023. This article reports on clinical outcomes of mpox among people with HIV, particularly among those with low CD4 cell counts, indication severe disease and higher mortality. [DOI] [PubMed]
- 10.Aldred B KC, Colasanti J, Marconi V, Sheth A, Nguyen M, Scott JY, Kandiah S, Kalapila A, Gromer D, Aldredge A, Szabo B, Hall B, Cantos VD. . Characteristics of the 2022 Mpox outbreak in a southeastern US city [abstract]. Presented at: CROI 2023.
- 11.Núñez I, García-Grimshaw M, Ceballos-Liceaga SE, Toledo-Salinas C, Carbajal-Sandoval G, Sosa-Laso L, et al. Epidemiological and clinical characteristics of patients with human monkeypox infection in Mexico: a nationwide observational study. Lancet Reg Health Am. 2023;17:100392. doi: 10.1016/j.lana.2022.100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann C, Jessen H, Boesecke C. Monkeypox in Germany. Dtsch Arztebl Int. 2022;119(33–34):551–557. doi: 10.3238/arztebl.m2022.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P, Li J, Ayada I, Avan A, Zheng Q, Peppelenbosch MP, et al. Clinical features, antiviral treatment and patient outcomes: a systematic review and comparative analysis of the previous and the 2022 mpox outbreaks. J Infect Dis. 2023. [DOI] [PMC free article] [PubMed]
- 14.Liu Q, Fu L, Wang B, Sun Y, Wu X, Peng X, et al. Clinical characteristics of human Mpox (monkeypox) in 2022: a systematic review and meta-analysis. Pathogens. 2023;12(1). [DOI] [PMC free article] [PubMed]
- 15.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 16.Allan-Blitz LT, Gandhi M, Adamson P, Park I, Bolan G, Klausner JD. A position statement on Mpox as a sexually transmitted disease. Clin Infect Dis. 2022. [DOI] [PMC free article] [PubMed]
- 17.Chastain DB, Motoa G, Ortiz-Martínez Y, Gharamti AE, Henao-Martínez AF. Characteristics and clinical manifestations of monkeypox among people with and without HIV in the United States: a retrospective cohort. Aids. 2022. [DOI] [PubMed]
- 18.•• CDC. 2022 outbreak cases and data. 2023. https://www.cdc.gov/poxvirus/mpox/response/2022/index.html [last accessed 3/13/2023]. Updated source of latest epidemiology worldwide and in the U.S.
- 19.2022–23 Mpox Outbreak: Global Trends. Geneva: World Health Organization, 2023. Available online:https://worldhealthorg.shinyapps.io/mpx_global/ (last cited: [3/08/202]).
- 20.•• Curran KG, Eberly K, Russell OO, Snyder RE, Phillips EK, Tang EC, et al. HIV and sexually transmitted infections among persons with monkeypox—eight U.S. jurisdictions, May 17-July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(36):1141–7. Reports of coinfection with HIV and other STI among individuals with mpox, along with chracterization depending on HIV status. [DOI] [PMC free article] [PubMed]
- 21.Kava CM, Rohraff DM, Wallace B, Mendoza-Alonzo JL, Currie DW, Munsey AE, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17-October 6, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(45):1449–1456. doi: 10.15585/mmwr.mm7145a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millman AJ, Denson DJ, Allen ML, Malone JA, Daskalakis DC, Durrence D, et al. A health equity approach for implementation of JYNNEOS vaccination at large, community-based LGBTQIA+ events—Georgia, August 27-September 5, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(43):1382–1883. doi: 10.15585/mmwr.mm7143e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann C, Jessen H, Wyen C, Grunwald S, Noe S, Teichmann J, et al. Clinical characteristics of monkeypox virus infections among men with and without HIV: a large outbreak cohort in Germany. HIV Med. 2022. [DOI] [PubMed]
- 24.Abbasi J. Reports of asymptomatic monkeypox suggest that, at the very least, some infections go unnoticed. JAMA. 2022;328(11):1023–1025. doi: 10.1001/jama.2022.15426. [DOI] [PubMed] [Google Scholar]
- 25.Waddell CJ FT, Prasad N, et al. Possible undetected Mpox infection among persons accessing homeless services and staying in encampments—San Francisco, California, October–November 2022. MMWR Morb Mortal Wkly Rep 2023;72:227–231. 10.15585/mmwr.mm7209a3. [DOI] [PMC free article] [PubMed]
- 26.Amos D, Collins J, Walker DT. Monkeypox presenting as supraglottitis in an immunocompromised patient. BMJ Case Rep. 2023;16(2). [DOI] [PMC free article] [PubMed]
- 27.Patel ABJ, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DA Thompson GR, Neumeister SM, Arutyunova AM, Cohen SH. Phimosis as a complication of resolved Mpox. Clin Infect Dis. 2023;76(1):178–179. doi: 10.1093/cid/ciac677. [DOI] [PubMed] [Google Scholar]
- 29.Vivancos-Gallego MJ, Sánchez-Conde M, Rodríguez-Domínguez M, Fernandez-Gonzalez P, Martínez-García L, Garcia-Mouronte E, et al. Human monkeypox in people with HIV: transmission, clinical features, and outcome. Open Forum Infect Dis. 2022;9(11):ofac557. doi: 10.1093/ofid/ofac557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contag CA, Karan A, Studemeister L, Bansil R, Fong I, Srinivasan K, et al. Case report: monkeypox—not just a rash. Am J Trop Med Hyg. 2023. [DOI] [PMC free article] [PubMed]
- 31.Fink DL, Callaby H, Luintel A, Beynon W, Bond H, Lim EY, et al. Clinical features and management of individuals admitted to hospital with monkeypox and associated complications across the UK: a retrospective cohort study. Lancet Infect Dis. 2022. [DOI] [PubMed]
- 32.Perzia B, Theotoka D, Li K, Moss E, Matesva M, Gill M, et al. Treatment of ocular-involving monkeypox virus with topical trifluridine and oral tecovirimat in the 2022 monkeypox virus outbreak. Am J Ophthalmol Case Rep. 2023;29:101779. doi: 10.1016/j.ajoc.2022.101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasquez-Perez A, Magan T, Volpe G, Osborne SF, McFaul K, Vahdani K. Necrotizingblepharoconjunctivitis and keratitis in human monkeypox. JAMA Ophthalmol. 2023. [DOI] [PMC free article] [PubMed]
- 34.Cash-Goldwasser S LS, McCormick DW, et al. CDC monkeypox clinical escalations team. Ocular monkeypox—United States, July–September 2022. MMWR Morb Mortal Wkly Rep 2022;71:1343–7. 10.15585/mmwr.mm7142e1. [DOI] [PMC free article] [PubMed]
- 35.Alarcón J, Kim M, Terashita D, Davar K, Garrigues JM, Guccione JP, et al. An Mpox-related death in the United States. N Engl J Med. 2023. [DOI] [PMC free article] [PubMed]
- 36.Manoharan A, Braz BX, McBride A, Hernandez S, Balfour M, Quiroz T, et al. Severe monkeypox with superimposed bacterial infection in an immunocompetent patient: a case report. IDCases. 2022;30:e01626. doi: 10.1016/j.idcr.2022.e01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller MJ, Cash-Goldwasser S, Marx GE, Schrodt CA, Kimball A, Padgett K, et al. Severe monkeypox in hospitalized patients—United States, August 10-October 10, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(44):1412–1417. doi: 10.15585/mmwr.mm7144e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfäfflin F, Wendisch D, Scherer R, Jürgens L, Godzick-Njomgang G, Tranter E, et al. Monkeypox in-patients with severe anal pain. Infection. 2022. [DOI] [PMC free article] [PubMed]
- 39.Viguier C, de Kermel T, Boumaza X, Benmedjahed NS, Izopet J, Pasquier C, et al. A severe monkeypox infection in a patient with an advanced HIV infection treated with tecovirimat: clinical and virological outcome. Int J Infect Dis. 2022;125:135–137. doi: 10.1016/j.ijid.2022.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong L, Gonzales-Zamora JA, Beauchamps L, Henry Z, Lichtenberger P. Clinical presentation of Monkeypox among people living with HIV in South Florida: a case series. Infez Med. 2022;30(4):610–618. doi: 10.53854/liim-3004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lum F-M, Torres-Ruesta A, Tay MZ, Lin RTP, Lye DC, Rénia L, et al. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat Rev Immunol. 2022;22(10):597–613. doi: 10.1038/s41577-022-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Núñez I, Valdés-Ferrer SI. Fulminant mpox as an AIDS-defining condition: useful or stigmatising? Lancet. 2023. [DOI] [PubMed]
- 43.Gandhi RT, Bedimo R, Hoy JF, Landovitz RJ, Smith DM, Eaton EF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society—USA Panel. JAMA. 2023;329(1):63–84. doi: 10.1001/jama.2022.22246. [DOI] [PubMed] [Google Scholar]
- 44.León-Figueroa DA, Barboza JJ, Saldaña-Cumpa HM, Moreno-Ramos E, Bonilla-Aldana DK, Valladares-Garrido MJ, et al. Detection of monkeypox virus according to the collection site of samples from confirmed cases: a systematic review. Trop Med Infect Dis. 2022;8(1). [DOI] [PMC free article] [PubMed]
- 45.Palich R, Burrel S, Monsel G, Nouchi A, Bleibtreu A, Seang S, et al. Viral loads in clinical samples of men with monkeypox virus infection: a French case series. Lancet Infect Dis. 2023;23(1):74–80. doi: 10.1016/S1473-3099(22)00586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moretti M, Heymans B, Yin N, Kaur S, Libois A, Quoilin S, et al. Diagnostic approach to monkeypox outbreak, a case-control study. Int J STD Aids. 2023;9564624231152789. [DOI] [PubMed]
- 47.Lapa DCF, Mazzotta V, Matusali G, Pinnetti C, Meschi S, Gagliardini R, Colavita F, Mondi A, Minosse C, Scorzolini L, Cicalini S, Maffongelli G, Specchiarello E, Camici M, Bettini A, Baldini F, Francalancia M, Mizzoni K, Garbuglia AR, Nicastri E, Girardi E, Antinori A, Vaia F, Maggi F, INMI Monkeypox Study Group Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect Dis. 2022;22(9):1267–1269. doi: 10.1016/S1473-3099(22)00513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Shea J, Filardo TD, Morris SB, Weiser J, Petersen B, Brooks JT. Interim guidance for prevention and treatment of monkeypox in persons with HIV infection—United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71(32):1023–1028. doi: 10.15585/mmwr.mm7132e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raccagni AR, Mileto D, Galli L, Bruzzesi E, Canetti D, Rizzo A, et al. HIV viral load monitoring during monkeypox virus infection among PLWH. Aids. 2023. [DOI] [PubMed]
- 50.Maldonado-Barrueco A, Sanz-González C, Gutiérrez-Arroyo A, Grandioso-Vas D, Roces-Álvarez P, Sendagorta-Cudos E, et al. Sexually transmitted infections and clinical features in monkeypox (mpox) patients in Madrid. Spain Travel Med Infect Dis. 2023;52:102544. doi: 10.1016/j.tmaid.2023.102544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perazzo H, Silva MST, Coutinho C, Peixoto EM, da Costa Cruz Silva S, Cardoso SW, et al. Monkeypox outbreak as an opportunity to identify new cases of HCV infection in limited resource settings. J Viral Hepat. 2023;30(1):83–5. doi: 10.1111/jvh.13771. [DOI] [PubMed] [Google Scholar]
- 52.Association AD. Monkeypox CARING FOR THE SKIN. 2022.
- 53.CDC. Clinical Considerations for Pain Management of Mpox. 2022.
- 54.Rao AK, Schrodt CA, Minhaj FS, Waltenburg MA, Cash-Goldwasser S, Yu Y, et al. Interim clinical treatment considerations for severe manifestations of Mpox—United States, February 2023. MMWR Morb Mortal Wkly Rep. 2023;72(9):232–243. doi: 10.15585/mmwr.mm7209a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Laughlin K, Tobolowsky FA, Elmor R, Overton R, O'Connor SM, Damon IK, et al. Clinical use of Tecovirimat (Tpoxx) for treatment of monkeypox under an investigational new drug protocol—United States, May-August 2022. MMWR Morb Mortal Wkly Rep. 2022;71(37):1190–1195. doi: 10.15585/mmwr.mm7137e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeLaurentis CE, Kiser J, Zucker J. New perspectives on antimicrobial agents: Tecovirimat for treatment of human monkeypox virus. Antimicrob Agents Chemother. 2022;66(12):e0122622. doi: 10.1128/aac.01226-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.David C. Philpott RAB, Paul J. Weidle, Kathryn G. Curran, John T. Brooks, George Khalil, Lauren Barrineau-Vejjajiva, Amanda Feldpausch, Jessica Pavlick, Pascale Wortley, Jesse O’Shea. CD4count <350 cells/mm3 increases risk of hospitalization with MPOX in PWH. Presented at: CROI 2023. 2023.
- 58.Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J Virol. 2005;79(20):13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.ClinicalTrials.gov. Study of tecovirimat for human monkeypox virus (STOMP): NCT05534984. 2022.
- 60.Hutson CL, Kondas AV, Mauldin MR, Doty JB, Grossi IM, Morgan CN, et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, Brincidofovir, in a lethal monkeypox virus animal model. MSphere. 2021;6(1). [DOI] [PMC free article] [PubMed]
- 61.Stittelaar KJ, Neyts J, Naesens L, van Amerongen G, van Lavieren RF, Holý A, et al. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439(7077):745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- 62.Polis MA, Spooner KM, Baird BF, Manischewitz JF, Jaffe HS, Fisher PE, et al. Anticytomegaloviral activity and safety of cidofovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob Agents Chemother. 1995;39(4):882–886. doi: 10.1128/AAC.39.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vandercam B, Moreau M, Goffin E, Marot JC, Cosyns JP, Jadoul M. Cidofovir-induced end-stage renal failure. Clin Infect Dis. 1999;29(4):948–949. doi: 10.1086/520475. [DOI] [PubMed] [Google Scholar]
- 64.Studies of Ocular Complications of AIDS Research Group The AIDS Clinical Trials Group The ganciclovir implant plus oral ganciclovir versus parenteral cidofovir for the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome: The Ganciclovir Cidofovir Cytomegalovirus Retinitis Trial. Am J Ophthalmol. 2001;131(4):457–67. doi: 10.1016/S0002-9394(01)00840-6. [DOI] [PubMed] [Google Scholar]
- 65.Sobral-Costas TG, Escudero-Tornero R, Servera-Negre G, Bernardino JI, Arroyo AG, Díaz-Menéndez M, et al. Human monkeypox outbreak: epidemiological data and therapeutic potential of topical cidofovir in a prospective cohort study. J Am Acad Dermatol. 2022. [DOI] [PMC free article] [PubMed]
- 66.Wittek R. Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. Int J Infect Dis. 2006;10(3):193–201. doi: 10.1016/j.ijid.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Mussini C, Guaraldi G, Orkin C. Monkeypox vaccination—an opportunity for HIV prevention. Lancet HIV. 2022;9(11):e741–e742. doi: 10.1016/S2352-3018(22)00292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.FDA. FDA approves first live, a non-replicating vaccine to prevent smallpox and monkeypox.
- 69.CDC, Mpox—Vaccination. 2023.
- 70.CDC. Interimclinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. Mpox outbreak. 2022.
- 71.Frey SE, Wald A, Edupuganti S, Jackson LA, Stapleton JT, El Sahly H, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33(39):5225–5234. doi: 10.1016/j.vaccine.2015.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greenberg RN, Hay CM, Stapleton JT, Marbury TC, Wagner E, Kreitmeir E, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia Ankara smallpox vaccine (MVA-BN®) in 56–80-year-old subjects. PLoS ONE. 2016;11(6):e0157335. doi: 10.1371/journal.pone.0157335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Overton ET, Stapleton J, Frank I, Hassler S, Goepfert PA, Barker D, et al. Safety and immunogenicity of modified vaccinia Ankara-Bavarian Nordic smallpox vaccine in Vaccinia-naive and experienced human immunodeficiency virus-infected individuals: an open-label, controlled clinical phase II trial. Open Forum Infect Dis. 2015;2(2):ofv040. doi: 10.1093/ofid/ofv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pittman PR, Hahn M, Lee HS, Koca C, Samy N, Schmidt D, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381(20):1897–1908. doi: 10.1056/NEJMoa1817307. [DOI] [PubMed] [Google Scholar]
- 75.Payne AB, Ray LC, Cole MM, Canning M, Houck K, Shah HJ, et al. Reduced risk for Mpox after receipt of 1 or 2 doses of JYNNEOS vaccine compared with risk among unvaccinated persons—43 U.S. jurisdictions, July 31-October 1, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(49):1560–4. [DOI] [PMC free article] [PubMed]
- 76.Agunbiade S, Burton F, Muirhead J, Whitlock GG, Girometti N. Clinical characteristics of mpox infection in individuals who received a first dose of modified vaccinia Ankara immunisation. Sex Transm Infect. 2023. [DOI] [PMC free article] [PubMed]
- 77.Wolff Sagy Y, Zucker R, Hammerman A, Markovits H, Arieh NG, Abu Ahmad W, et al. Real-world effectiveness of a single dose of mpox vaccine in males. Nat Med. 2023;1–5. [DOI] [PMC free article] [PubMed]
- 78.Golden J, Harryman L, Crofts M, Muir P, Donati M, Gillett S, et al. Case of apparent mpox reinfection. Sex Transm Infect. 2023. [DOI] [PMC free article] [PubMed]
- 79.Titanji BK et al. Effectiveness of smallpox vaccination to prevent mpox in military personnel. Conference on Retroviruses and Opportunistic Infections, Seattle, abstract 207, 2023. [DOI] [PMC free article] [PubMed]
- 80.Agrati C, Cossarizza A, Mazzotta V, Grassi G, Casetti R, De Biasi S, et al. Immunological signature in human cases of monkeypox infection in 2022 outbreak: an observational study. Lancet Infect Dis. 2022. [DOI] [PMC free article] [PubMed]
- 81.Cline A, Marmon S. Demographics and disease associations of patients with monkeypox and recipients of monkeypox vaccine from safety net hospitals in New York City: a cross-sectional study. J Am Acad Dermatol. 2022. [DOI] [PMC free article] [PubMed]
- 82.CDC, Mpox vaccine administration in the U.S, https://www.cdc.gov/poxvirus/mpox/response/2022/vaccines_data.html [accesed 3/12/2023].
- 83.Delaney KP, Sanchez T, Hannah M, Edwards OW, Carpino T, Agnew-Brune C, et al. Strategies adopted by gay, bisexual, and other men who have sex with men to prevent monkeypox virus transmission—United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71(35):1126–1130. doi: 10.15585/mmwr.mm7135e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hubach RD, Owens C. Findings on the monkeypox exposure mitigation strategies employed by men who have sex with men and transgender women in the United States. Arch Sex Behav. 2022;51(8):3653–3658. doi: 10.1007/s10508-022-02423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]