Abstract
Background
Pediatric patients with nephrotic syndrome take medications long-term with significant toxicity and complex regimens, yet data on medication adherence are limited.
Methods
In a multicenter observational study of patients with nephrotic syndrome, NEPTUNE (NCT01209000), we surveyed caregivers of patients <19 years old and adolescent patients on medication adherence during longitudinal follow-up beginning in June 2015. Data extraction was in October 2020. We described the proportion of nonadherent patients at first survey. Participant social and economic factors, condition-related factors, therapy-related factors, and patient-related factors were examined for relationships with nonadherence by generalized linear mixed models using the longitudinal data. In exploratory fashion, we assessed the relationship between adherence and subsequent steroid response classification by binary logistic regression and adherence with healthcare utilization by Poisson regression.
Results
A total of 225 participants completed a median of 3 surveys during follow-up (IQR, 2–5), with a total of 743 surveys. Overall, 80 (36%) reported nonadherence with medications. In adjusted analysis, older age (per 1 year; OR 1.08; 95% CI, 1.03 1.12), lower maternal educational level (≥ high school vs. < high school; OR 0.47; 95% CI 0.25 to 0.89), and increased parent and self-identification of medications barriers (per 1 point; OR 1.57; 95% CI, 1.15–2.15) were significantly associated with nonadherence. No relationship between nonadherence and subsequent frequency of healthcare utilization was observed. A trend toward increased subsequent steroid resistance classification was seen with nonadherence, though not statistically significant.
Conclusions
Medication nonadherence is common in pediatric nephrotic syndrome. Investigations into the use of surveys in the clinic setting to identify at-risk patients and ways to support families over time are needed.
Keywords: Nephrotic syndrome, Medication, Adherence, Child, Adolescent
Introduction
Idiopathic nephrotic syndrome (INS) is one of the most common chronic kidney diseases in pediatrics, with an estimated prevalence of 16 per 100,000 children [1]. The standard treatment at time of diagnosis is high-dose corticosteroids for 8–12 weeks to induce disease remission (resolution of proteinuria with urine protein to creatinine ratio (UPCR) < 0.2 mg/mg or < 1+ of protein on urine dipstick for three consecutive days). However, approximately 20% of patients are resistant to the initial corticosteroid therapy (steroid-resistant nephrotic syndrome, SRNS) and 80–90% of patients who respond to treatment (steroid-sensitive nephrotic syndrome, SSNS) will later relapse (return of nephrotic range protein (UPCR ≥2 mg/mg or 3+ protein on urine dipstick) after remission). Moreover, approximately half of the patients relapse frequently (2 relapses in the initial 6 months or 4 relapses in any 12-month period; frequently relapsing nephrotic syndrome, FRNS) or relapse whenever corticosteroids are weaned or shortly after discontinuation (steroid-dependent nephrotic syndrome, SDNS) [2]. Thus, the majority of patients take medications chronically, including repeated courses of corticosteroids for each disease relapse. They may be given second-line immunosuppressive agents (e.g., cyclophosphamide, mycophenolate, calcineurin inhibitors) for maintenance of disease remission. In addition, they are often prescribed antihypertensive agents including angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin II receptor antagonists (ARBs), diuretics, and cholesterol-lowering drugs. For those resistant to treatment, progressive loss of kidney function occurs and additional medications to manage chronic kidney disease (CKD) complications are added [2–6]. Yet, little is known about adherence to therapies and its influence on outcomes in childhood nephrotic syndrome.
According to the World Health Organization, nonadherence to medical instructions is the primary cause of treatment failure in chronic diseases [7]. In pediatric patients, nonadherence has been related to increased hospitalizations, emergency department visits, and healthcare costs [8]. In this analysis, we investigated the prevalence and persistence of medication adherence in a large cohort of children with INS through the multicenter, longitudinal cohort study, the Nephrotic Syndrome Study Network (NEPTUNE, NCT01209000). Potential factors affecting adherence, as outlined by the World Health Organization, including social and economic factors, condition-related factors, therapy-related factors, and patient-related factors were examined for their relationships to medication adherence [7]. Finally, in exploratory fashion, we examined the association between medication adherence and subsequent steroid response classification and frequency of healthcare utilization. Our goals are to understand the determinants of medication adherence and its potential impact on clinical outcomes in childhood nephrotic syndrome.
Methods
Data sources and study population
The NEPTUNE study (NCT01209000) is a multicenter, longitudinal cohort study conducted in the USA and Canada that prospectively collects demographic and clinical information as well as biospecimens on patients with nephrotic syndrome for a follow-up of 36 months. Enrollment began in 2010 and is ongoing. Starting in June 2015, surveys on medication adherence and difficulties with medications were administered to all enrolled participants and prospectively to newly enrolled participants. Full details of the study design have been published elsewhere [9]. The NEPTUNE study included two major groups of pediatric patients: patients <19 years of age who had a kidney biopsy demonstrating focal segmental glomerulosclerosis (FSGS), minimal change disease (MCD), or membranous nephropathy (MN) and who were enrolled within 45 days of biopsy (cohort A—biopsy cohort); and patients with a new clinical diagnosis of INS (nephrotic range proteinuria (urinalysis >2+ protein or random UPCR >2 mg/mg), edema, and serum albumin <3 g/dL) and who were enrolled within 30 days of treatment initiation (cohort B—non-biopsied cohort).
Exclusion criteria for NEPTUNE included prior solid organ or bone marrow transplant, CKD stage 5 treated by dialysis or kidney transplant, secondary nephrotic syndrome (e.g., systemic lupus erythematosus), clinical or histologic evidence of other kidney disease (e.g., Alport syndrome, nail patella syndrome, diabetic nephropathy) or genitourinary malformations with vesicoureteral reflux or renal dysplasia, unwillingness or inability to give informed consent, or institutionalization. The present analysis was performed among pediatric NEPTUNE patients less than 19 years of age in either cohort A or B who have completed at least one survey on adherence to medications. Institutional review board (IRB) approval for this study was obtained via the single IRB of record at the University of Michigan.
Medication adherence and reported barriers survey measures
Two surveys, the Medication Adherence Survey (MAS) and the Medication Barriers Scale (MBS), were administered to study participants taking medications at each of the study visits beginning in June 2015. Among participants already enrolled in NEPTUNE (predominately cohort A participants), the surveys began at any point during study participation. Among patients enrolled since June 2015 (cohort B participants and newly enrolled cohort A participants), the surveys began at study enrollment and follow-up visits at 6 weeks, 4, 8, 12, 16, 26, 30, and 36 months, and yearly thereafter. The caregiver completed the surveys for patients <11 years of age. For patients 11 to 18 years, either the caregiver or the adolescent participant completed the surveys. In 73 instances, both the caregiver and the adolescent patient completed the MAS surveys. In these instances, we compared the results and found weak inter-rater reliability of adherence reporting between the adolescent self-reporting versus caregiver proxy-reporting (kappa, 0.39), with more nonadherence reported in self-reports (Supplemental Table 1). There were 36 instances when both the caregiver and the adolescent patient completed the MBS surveys. The inter-rater reliability was moderate for the MBS surveys (intra-class correlation coefficients 0.41–0.77, Supplemental Table 2). Therefore, whenever self-reports were available, we used the self-report results over proxy reports.
The MAS is a 3-item measure of extent of nonadherence adapted from a validated survey by Voils et al. [10]. Using a five-point scale (1 = strongly disagree, 5 = strongly agree), participants were asked to endorse the following 3 items referencing the previous 7 days: (1) I/my child took all my/his/her kidney disease medication, (2) I/my child missed or skipped at least one dose of my/his/her kidney disease medication, and (3) I/my child was not able to take all of my/his/her kidney medication. A patient was considered nonadherent at the time of survey if they provided an answer of 1–3 for question 1 or 3–5 for questions 2 or 3.
To examine patient perceptions of medications and relationships with medication adherence, an important component of patient-related factors [7], we used the validated MBS [11]. The MBS contained 17 questions scored between 1 to 5 (1 = strongly disagree, 5 = strongly agree) pertaining to domains of (a) disease frustration/adolescent issues (8 questions), (b) regimen adaptation/cognitive issues (6 questions), and (c) ingestion issues (3 questions). The MBS was designed to help providers determine the most prominent issues that may be interfering with adherence. Example issues within each domain include: (a) “tired of living with a medical condition” and “doesn’t like what the medication dose to his/her appearance,” (b) “hard to stick to a fixed medication schedule,” and (c) “does not like how the medicine tastes.” Reported MBS scores for the total survey (composite score) and by each barrier domain were described by median and interquartile ranges (IQRs).
Other potential factors affecting adherence
We collected social and economic measures at time of NEPTUNE enrollment, including self-reported annual household income, maternal and paternal educational level, insurance status at enrollment, and primary language spoken at home (English or other). Demographic characteristics fall into several categories (condition-related, patient-related, and social and economic factors) and they included sex, age at survey, race, and ethnicity. Condition-related characteristics included duration of disease at time of survey, steroid response classification, renal histology, estimated glomerular filtration rate (eGFR) by the Bedside Schwartz Equation [12] at enrollment, UPCR at enrollment, and serum albumin at enrollment. Therapy-related factors included number and type of treatments for INS received at time of survey: corticosteroids, non-steroid immunosuppressants, and ACEIs/ARBs. Patient-related factors, as described above, included MBS scoring for the domains of disease frustration/adolescent issues, regimen adaptation/cognitive issues, ingestion issues, and composite scoring. Data extraction was performed on October 12, 2020. Patient characteristics were described by frequencies and percentages for categorical variables and medians and interquartile ranges (IQRs) for continuous variables.
Analysis
Medication adherence and associated factors
We described the proportion of participants with self-reported nonadherence to medications at the first MAS survey. Chi-square and Kruskall-Wallis tests were used to examine the differences in participant characteristics between the groups in this cross-sectional analysis using the first completed survey, including time since initial INS diagnosis. We also performed chi-square and Kruskall-Wallis tests to examine differences between participants who completed MAS surveys versus those who did not.
Generalized linear mixed models with binary distribution and logit link with random slopes and intercepts were used to examine associations between condition-related, patient-related, social and economic, and therapy-related factors and medication adherence during longitudinal follow-up with repeated MAS surveys. Age at time of survey, duration of disease, steroid response classification, UPCR, eGFR, serum albumin, number of medications, and MBS composite and domain scores at each time the MAS surveys were completed were collected and treated as time-varying covariates. Static variables included sex, race, Hispanic ethnicity, histology, and maternal education level. Interaction with age at time of survey, increasing over time of study follow-up, was tested to evaluate potential varying effects over follow-up time. Backwards selection process was used for the final adjusted model. All univariate predictors significant at α = 0.20 were included in the backwards selection process. Variables were sequentially removed in descending value of p-value until all remaining variables were significant at p < 0.05.
Medication adherence and steroid response and frequency of healthcare utilization
In exploratory fashion, we assessed the relationship between parent or patient-reported medication adherence at each surveyed instance and clinical outcomes in the follow-up period until the next study visit. That is, medication adherence reported via the MAS survey at visit N was examined for associations with the number of hospitalizations and emergency department (ED) visits, the number of outpatient nephrology visits, and steroid response between visit = N and visit N + 1.
Poisson regression was used to examine the association between medication adherence and the number of hospitalizations and ED visits and the number of outpatient nephrology clinic visits (i.e., count data). Medication adherence and association with subsequent steroid response classification (steroid-sensitive and infrequently relapsing, IRNS; FRNS, or SDNS; SRNS) was assessed by binary logistic regression (i.e., the odds of having SRNS vs. SSNS and FRNS/SDNS vs. IRNS based on medication adherence). Significance level was set at p < 0.05.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Cohort
From June 2015 to October 2020, 225 NEPTUNE participants <19 years old completed the MAS at least once, which represented 86% of the total enrolled pediatric patients in NEPTUNE within this time period. Of the 225 participants, 74 (33%) were enrolled within 30 days following diagnosis. Median follow-up was 9.7 months (IQR, 1.0–25.4). Figure 1 shows the derivation of the study cohort. The surveyed participant characteristics at the time of first survey, grouped by self- or proxy-reported medication adherence, are presented in Table 1. We compared participant characteristics between those who completed at least one MAS survey (n = 225) and those who did not complete any MAS surveys (n = 25) and found that those who did not complete any MAS surveys had greater missingness for the variables ethnicity and maternal educational level (Supplemental Table 3).
Fig. 1.
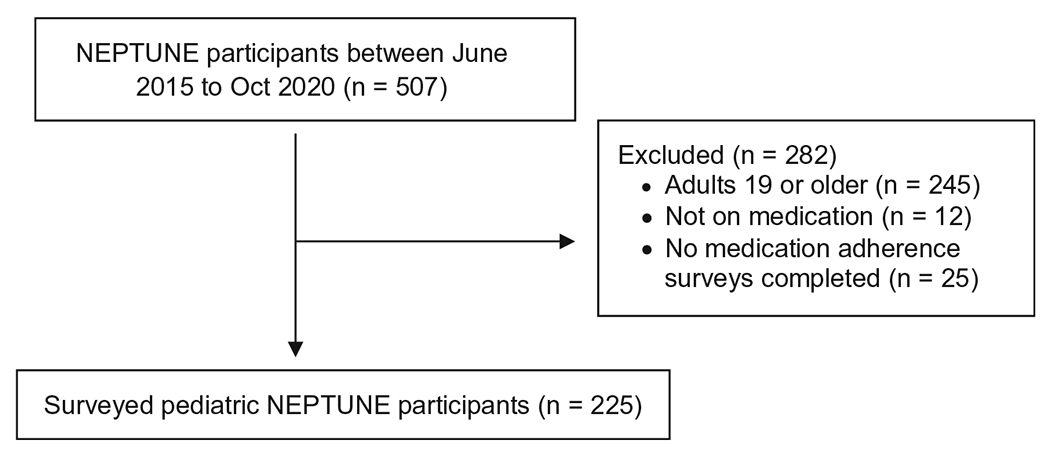
Pediatric NEPTUNE patients analyzed in current study of medication adherence
Table 1.
Demographic, socioeconomic, and clinical characteristics comparison of NEPTUNE pediatric patients surveyed between 2015 and 2020 for medication adherence
| All children (n = 225) | Adherent (n = 145) | Nonadherent (n = 80) | p | |
|---|---|---|---|---|
| MAS raw score, median (IQR) | 3.0 (3.0, 7.0) | 3.0 (3.0, 3.0) | 7.0 (7.0, 10.0) | < .0001 |
| Age at time of diagnosis (y), median (IQR) | 4 (2, 10) | 4 (2, 9) | 6 (3, 11) | 0.09 |
| Age at time of survey (y), median (IQR) | 7 (3, 13) | 5 (3, 11) | 10 (6, 16) | < .001 |
| Age at time of survey (y), n (%) | < .01 | |||
| 0–5 | 93 (41) | 73 (50) | 20 (25) | |
| 6–11 | 66 (29) | 37 (26) | 29 (36) | |
| 12–17 | 53 (24) | 30 (21) | 23 (29) | |
| 18 + a | 13 (6) | 5 (3) | 8 (10) | |
| Missing | 0 (0) | 0 (0) | 0 (0) | |
| Sex, n (%) | 0.88 | |||
| Male | 125 (56) | 80 (55) | 45 (56) | |
| Female | 100 (44) | 65 (45) | 35 (44) | |
| Missing | 0 (0) | 0 (0) | 0 (0) | |
| Race, n (%) | 0.17 | |||
| Black | 67 (30) | 37 (26) | 30 (38) | |
| White | 110 (49) | 74 (51) | 36 (45) | |
| Other | 35 (16) | 25 (17) | 10 (13) | |
| Unknown/missing | 13 (6) | 9 (6) | 4 (5) | |
| Hispanic, n (%) | 0.07 | |||
| Hispanic | 42 (19) | 21 (14) | 21 (26) | |
| Non-Hispanic | 178 (79) | 121 (83) | 57 (71) | |
| Unknown/missing | 5 (2) | 3 (2) | 2 (3) | |
| Duration of disease at time of survey (m), n (%) | 0.0001 | |||
| 0–3 months | 119 (53) | 92 (63) | 27 (34) | |
| > 3–12 months | 17 (8) | 13 (9) | 4 (5) | |
| > 12–24 months | 13 (6) | 7 (5) | 6 (8) | |
| > 24 months | 68 (30) | 31 (21) | 37 (46) | |
| Unknown/missing | 8 (4) | 2 (1) | 6 (8) | |
| Duration of disease at time of survey (m), median (IQR) | 2 (1, 35) | 1 (0, 16) | 24 (1, 51) | < .0001 |
| Steroid response classification, n (%) | 0.06 | |||
| Responsive, infrequently relapses | 40 (18) | 30 (21) | 10 (13) | |
| Relapsing, steroid-dependent or frequently relapsing | 69 (31) | 39 (27) | 30 (38) | |
| Resistant | 33 (15) | 25 (17) | 8 (10) | |
| Unknown/not yet classified | 83 (37) | 51 (35) | 32 (40) | |
| Histology, n (%) | < .0001 | |||
| Focal segmental glomerulosclerosis | 46 (20) | 26 (18) | 20 (25) | |
| Minimal Change | 78 (35) | 37 (26) | 41 (51) | |
| Not biopsied | 101 (45) | 82 (57) | 19 (24) | |
| UPCR (mg/mg) at enrollment, median (IQR) | 7.3 (2.2, 13.9) | 8.9 (2.8, 15.6) | 5.5 (1.9, 10.0) | 0.04 |
| UPCR (mg/mg) at enrollment, n (%) | < .01 | |||
| < 0.3 | 26 (12) | 21 (14) | 5 (6) | |
| 0.3–1.9 | 24 (11) | 9 (6) | 15 (19) | |
| ≥ 2.0 | 166 (74) | 108 (74) | 58 (73) | |
| Missing | 9 (4) | 7 (5) | 2 (3) | |
| UPCR (mg/mg) at time of survey, median (IQR) | 0.5 (0.1, 5.3) | 0.3 (0.1, 5.2) | 1.1 (0.0, 5.6) | 0.86 |
| UPCR (mg/mg) at time of survey, n (%) | 0.40 | |||
| < 0.3 | 93 (41) | 65 (45) | 28 (35) | |
| 0.3–1.9 | 28 (12) | 16 (11) | 12 (15) | |
| ≥ 2.0 | 76 (34) | 48 (33) | 28 (35) | |
| Missing | 28 (12) | 16 (11) | 12 (15) | |
| eGFR (ml/min/1.73 m2)b at enrollment, median (IQR) | 104 (89, 129) | 106 (90, 131) | 100 (87, 124) | 0.25 |
| eGFR (ml/min/1.73 m2)b at enrollment, n (%) | 0.57 | |||
| > 90 | 163 (72) | 106 (73) | 57 (71) | |
| 60–90 | 50 (22) | 31 (21) | 19 (24) | |
| 30–60 | 7 (3) | 4 (3) | 3 (4) | |
| < 30 | 1 (0) | 0 (0) | 1 (1) | |
| Missing | 4 (2) | 4 (3) | 0 (0) | |
| eGFR (ml/min/1.73 m2) b at time of survey, median (IQR) | 99 (82, 127) | 99 (83, 128) | 99 (77, 125) | 0.42 |
| eGFR (ml/min/1.73 m2) b at time of survey, n (%) | 0.42 | |||
| > 90 | 135 (60) | 90 (62) | 45 (56) | |
| 60–90 | 60 (27) | 39 (27) | 21 (26) | |
| 30–60 | 9 (4) | 6 (4) | 3 (4) | |
| < 30 | 4 (2) | 1 (1) | 3 (4) | |
| Missing | 17 (8) | 9 (6) | 8 (10) | |
| Serum albumin (g/dL) at enrollment, median (IQR) | 2.7 (2.0, 3.6) | 2.8 (2.3, 3.4) | 2.7 (1.8, 3.8) | 0.56 |
| Serum albumin (g/dL) at enrollment, n (%) | 0.13 | |||
| ≤ 2.5 | 55 (24) | 28 (19) | 27 (34) | |
| > 2.5 | 78 (35) | 50 (34) | 28 (35) | |
| Missing | 92 (41) | 67 (46) | 25 (31) | |
| Serum albumin (g/dL) at time of survey, median (IQR) | 3.4 (2.5, 4.2) | 3.2 (2.6, 4.1) | 3.6 (2.2, 4.4) | 0.49 |
| Serum albumin (g/dL) at time of survey, n (%) | 0.33 | |||
| ≤ 2.5 | 37 (16) | 21 (14) | 16 (20) | |
| > 2.5 | 105 (47) | 69 (48) | 36 (45) | |
| Missing | 83 (37) | 55 (38) | 28 (35) | |
| Number of therapies, median (IQR) | 2 (1, 3) | 2 (1, 2) | 2 (1, 3) | 0.20 |
| Therapies at time of survey, n (%)c | < .0001 | |||
| ACEi/ARB only | 23 (10) | 11 (8) | 12 (15) | |
| CST only | 121 (54) | 95 (66) | 26 (33) | |
| ACEi/ARBs + CST | 20 (9) | 12 (8) | 8 (10) | |
| Non-CST IST | 59 (26) | 26 (18) | 33 (41) | |
| Other | 2 (1) | 1 (1) | 1 (1) | |
| Therapies at time of survey, n (%)d | ||||
| CST | 172 (76) | 119 (82) | 53 (66) | < .01 |
| CNI | 47 (21) | 19 (13) | 28 (35) | < .001 |
| MMF | 15 (7) | 11 (8) | 4 (5) | 0.46 |
| Rituximab | 2 (1) | 0 (0) | 2 (3) | 0.13 |
| CTX | 0 (0) | 0 (0) | 0 (0) | N/A |
| ACEi/ARB | 63 (28) | 31 (21) | 32 (40) | < .01 |
| Primary language, n (%) | 0.46 | |||
| English | 208 (92) | 136 (94) | 72 (90) | |
| Other | 16 (7) | 9 (6) | 7 (9) | |
| Unknown/missing | 1 (0) | 0 (0) | 1 (1) | |
| Insurance status, n (%) | 1.00 | |||
| Insured | 223 (99) | 143 (99) | 80 (100) | |
| Uninsured | 1 (0) | 1 (1) | 0 (0) | |
| Unknown/missing | 1 (0) | 1 (1) | 0 (0) | |
| Maternal educational level, n (%) | 0.04 | |||
| < High school | 41 (18) | 18 (12) | 23 (29) | |
| High school | 59 (26) | 42 (29) | 17 (21) | |
| Some college | 39 (17) | 23 (16) | 16 (20) | |
| College | 47 (21) | 33 (23) | 14 (18) | |
| Graduate school | 23 (10) | 16 (11) | 7 (9) | |
| Unknown/missing | 16 (7) | 13 (9) | 3 (4) | |
| Reported annual household income, n (%) | 0.33 | |||
| < $40,000 | 72 (32) | 40 (28) | 32 (40) | |
| $40,000–$79,999 | 37 (16) | 22 (15) | 15 (19) | |
| ≥ $80,000 | 54 (24) | 37 (26) | 17 (21) | |
| Unknown/missing | 62 (28) | 46 (32) | 16 (20) | |
| Medication Barriers Scale | ||||
| Composite | 2.0 (1.2, 2.6) | 1.9 (1.4, 2.6) | 2.1 (1.2, 2.6) | 0.81 |
| Disease frustration/adolescent issues | 2.3 (1.3, 2.9) | 2.0 (1.3, 2.9) | 2.4 (1.3, 3.0) | 0.59 |
| Regimen adaption/cognitive issues | 1.5 (1.0, 2.3) | 1.3 (1.0, 2.3) | 1.8 (1.0, 2.0) | 0.08 |
| Ingestion issues | 2.0 (1.2, 2.6) | 2.0 (1.4, 2.6) | 1.8 (1.2, 2.3) | 0.08 |
| Missing | 76 (34) | 46 (32) | 30 (38) |
Participants were < 18 years old at enrollment
Estimated GFR is calculated from serum creatinine at enrollment using the Beside Schwartz formula [12]
Categories are mutually exclusive
Categories are non-mutually exclusive
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CST, corticosteroid; eGFR, estimated glomerular filtration rate; IQR, interquartile range; IST, immunosuppressive therapy; m, month; MAS, Medication Adherence Survey; UPCR, urine protein to creatinine ratio; y, year
The surveyed participants are diverse in terms of demographic, socioeconomic, and clinical characteristics. Thirty percent of the participants were adolescents at time of first survey, and Black and Hispanic patients make up 28% and 19% of the cohort, respectively. More than half of the participants (53%) were first surveyed within 3 months following diagnosis, and for 37% of total cohort, the steroid response classification was not yet determined. The cohort also included those with long-standing INS > 12 months (37%) at time of first survey. Fifty-five percent of enrolled patients were biopsied, representing cohort A patients with enrollment beginning in 2010. Patients were treated with various agents for INS. The cohort contained patients from across socioeconomic strata by maternal education and household income.
Eighty participants (36%) reported nonadherence with medications on the first survey. Among those with newly diagnosed disease, 92/119 (77%) were adherent with medications, versus just 31/68 (46%) of those with disease duration >24 months. Those who reported medication nonadherence were older, more likely to have longer disease duration, more likely to have undergone a kidney biopsy, and more likely to be on a second-line immunosuppressive agent and ACEIs/ARBs instead of corticosteroid alone compared to those who were adherent.
One hundred and forty-nine participants (66%) completed the MBS at the same time as the MAS survey. Scores in the disease frustration/adolescent issues category were the highest, indicating stronger identification with issues in this domain (e.g., “tired of living with a medical condition” and “doesn’t like what the medication dose to his/her appearance”), followed by ingestion and regimen adaptation/cognitive issues (e.g., “does not like how the medicine tastes”).
Associated factors
The participants completed a median of 3 MAS surveys during follow-up (IQR, 2–5), with a total of 743 MAS surveys returned during the analysis period. Participant characteristics, both static and time-varying, at the time of the surveys are tabulated in Supplemental Table 4. In 367 (49%) of the MAS-surveyed instances, the MBS survey was completed at the same time. We compared participant characteristics at time of MAS survey between those who did and did not complete an MBS survey (at the same time as the MAS survey) and did not detect obvious differences to suggest that we should pursue imputation (Supplemental Table 5).
Unadjusted analysis using all 743 surveys (Table 2) identified older age, longer duration of disease, biopsied MCD pathology compared to non-biopsied participants, and higher scores on the MBS survey (both composite and within each domain) to be associated with medication nonadherence. There is a significant interaction between Hispanic ethnicity and age, with increased medication nonadherence in unadjusted analysis. In the final adjusted analysis, older age (per 1 year; OR 1.08; 95% CI, 1.03–1.12), lower maternal educational level (high school or above vs. less than high school; OR 0.47; 95% CI, 0.25–0.89), and increased composite MBS score (per 1 point; OR 1.57; 95% CI, 1.15–2.15) were significantly associated with medication nonadherence.
Table 2.
Results of generalized non-linear mixed effects models examining associated factors of medication nonadherence among pediatric patients with nephrotic syndrome (n = 225 patients, n = 743 observations)
| Main effects |
Interactions with age |
|||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Unadjusted | ||||
| Age at survey (per 1y)a | 1.08 (1.04–1.12) | < 0.001 | ||
| Female | 0.84 (0.39–1.85) | 0.67 | 1.02 (0.94–1.1) | 0.68 |
| Race | ||||
| Black vs. White | 1.62 (0.65–4) | 0.30 | 0.98 (0.9–1.06) | 0.58 |
| Other vs. White | 1.2 (0.35–4.09) | 0.77 | 0.96 (0.86–1.08) | 0.51 |
| Hispanic | 0.64 (0.24–1.72) | 0.37 | 1.12 (1.01–1.25) | 0.03 |
| Duration of disease at survey (per 1y)a | 1.11 (1.03–1.2) | 0.01 | ||
| Treatment response at surveya | ||||
| IRNS vs. SRNS | 0.53 (0.28–1) | 0.05 | ||
| FRNS/SDNS vs. SRNS | 0.82 (0.49–1.36) | 0.44 | ||
| Unknown/missing vs. SRNS | 0.97 (0.54–1.73) | 0.92 | ||
| Histology | ||||
| FSGS vs. not biopsied | 0.63 (0.14–2.8) | 0.54 | 1.05 (0.91–1.2) | 0.51 |
| MCD vs. not biopsied | 2.68 (1.05–6.85) | 0.04 | 0.91 (0.81–1.03) | 0.13 |
| UPCR at survey (per log)a | 1.07 (0.99–1.16) | 0.09 | ||
| eGFR at survey (per 30 ml/min/1.73 m2)a | 0.94 (0.84–1.05) | 0.28 | ||
| Serum albumin at survey (per g/dL)a | 1.01 (0.82–1.25) | 0.90 | ||
| Number of medications (per med)a | 1.06 (0.95–1.18) | 0.31 | ||
| Primary English vs. other | 0.66 (0.15–2.88) | 0.58 | 0.97 (0.84 to 1.13) | 0.73 |
| Maternal education level | ||||
| ≥ High school vs. < high school | 0.89 (0.35–2.26) | 0.81 | 0.96 (0.87 to 1.05) | 0.38 |
| Medication Barriers Scale (per 1 scale)a | 0.89 (0.35–2.26) | 0.81 | 0.96 (0.87 to 1.05) | 0.38 |
| Composite | 1.52 (1.12–2.06) | 0.01 | ||
| Disease frustration/adolescent issues | 1.45 (1.11–1.88) | 0.01 | ||
| Ingestion | 1.31 (1–1.73) | 0.05 | ||
| Regimen adaptation/cognitive Issues | 1.44 (1.1–1.89) | 0.01 | ||
| Adjusted | ||||
| Age at survey (per 1y) | 1.08 (1.03–1.13) | 0.001 | ||
| Maternal education level | ||||
| ≥ High school vs. < high school | 0.46 (0.24–0.86) | 0.02 | ||
| Medication Barriers Scale (per 1 scale) | ||||
| Composite | 1.56 (1.14–2.13) | 0.01 | ||
Time-varying covariate
95% CI, 95% confidence interval; eGFR, estimated glomerular filtration rate; FRNS, frequently relapsing nephrotic syndrome; FSGS, focal segmental glomerulosclerosis; IQR, interquartile range; IRNS, infrequently relapsing nephrotic syndrome; IST, immunosuppressive therapy; m, month; MCD, minimal change disease; OR, odds ratio; SDNS, steroid-dependent nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome; SRNS, steroid-resistant nephrotic syndrome; UPCR, urine protein to creatinine ratio; y, year
Medication adherence and steroid response and frequency of healthcare utilization
We explored the relationship between adherence and subsequent steroid response classification and healthcare utilization. Results are shown in Table 3. Nonadherence with medication was associated with a 59% increase in odds of developing SRNS vs. SSNS (OR 1.59; 95% CI, 0.93–2.71); however, results were not statistically significant (p = 0.09). No statistically significant associations between adherence and subsequent frequency of healthcare utilization were seen.
Table 3.
Medication nonadherence and subsequent steroid response classification and healthcare utilization
| Hospitalizations and ED visitsa |
Outpatient nephrology visitsa |
SRNS vs. SSNSb |
FRNS/SDNS vs. IRNSb |
|||||
|---|---|---|---|---|---|---|---|---|
| Estimate (IRR), (95% CI) | p | Estimate (IRR), (95% CI) | p | Estimate (OR), (95% CI) | p | Estimate (OR), (95% CI) | p | |
| Nonadherent vs. adherent | 1.06 (0.75, 1.51) | 0.74 | 0.91 (0.72, 1.15) | 0.44 | 1.59 (0.93, 2.71) | 0.09 | 1.21 (0.66, 2.20) | 0.54 |
Poisson regression analysis
Binary logistic regression analysis
95% CI, 95% confidence interval; ED, emergency department; FRNS, frequently relapsing nephrotic syndrome; IRNS, infrequently relapsing nephrotic syndrome; IRR, incident rate-ratio; OR, odds ratio; SDNS, steroid-dependent nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome; SRNS, steroid-resistant nephrotic syndrome
Discussion
In this analysis of 225 pediatric patients surveyed between June 2015 and October 2020, we describe the scope of medication nonadherence, associated factors, and potential impact on clinical outcomes in childhood nephrotic syndrome. Overall, a substantial percentage of patients reported nonadherence with medications (36%), and nonadherence appears to be associated with older age, lower maternal education level, and increased perceived medication barriers. Our exploratory analysis suggested that medication nonadherence may increase the likelihood of being classified as steroid-resistant, though this observation was not statistically significant.
Childhood nephrotic syndrome is one of the leading causes of pediatric CKD [13]. Pediatric CKD patients with glomerular diseases, including FSGS, have a 1.5-fold greater number of medications compared to pediatric patients without glomerular disease, yet there are limited data on medication adherence in this vulnerable group [14]. A single-center study in the USA found that 41% of children with INS were deemed to be nonadherent with medications by their treating physician in the first 3 years following diagnosis [4], a prevalence similar to our cohort. In a randomized trial of a pharmacist-led intervention program to improve medication adherence among adults with nephrotic syndrome, participants in the control group with routine care had declining adherence over 6-months follow-up, from 36.7% having high-adherence by self-report at baseline to 16.6% with high-adherence at 6-months follow-up [15].
In our analysis, adherence is also lower among patients with longer disease duration, with multivariable analysis using the longitudinal follow-up data indicating that this was likely due to older patient age. Adolescent patients with INS had the lowest parent- and self-reported medication adherence compared with younger children. The increased risk of medication nonadherence among adolescents is similarly observed among pediatric CKD patients and kidney transplant recipients [14, 16, 17]. This highlights the importance of ongoing monitoring and support for adolescents who may have had long-standing INS diagnosed during toddler years and who face unique social and psychological challenges, including shifting dependence on caregivers for disease management [18]. Importantly, we also found that lower maternal education level was associated with higher risk of nonadherence. This is consistent with pediatric kidney transplant studies that have consistently shown family sociodemographic factors to influence medication adherence among adolescents [19]. There is a need to understand how to support the family unit as they face the management tasks of INS and evolving developmental needs of children.
Higher MBS scores, indicating parent- or self-identified barriers to taking medications, were significantly associated with medication nonadherence in our pediatric INS cohort. This finding has practical implications in that short surveys on parent or self-reported barriers to medications may be used to identify INS patients less likely to take medications consistently, and offers the opportunity to proactively support these patients. Therapeutic options such as long-acting intramuscular triamcinolone acetonide or intravenous rituximab which requires infrequent dosing may be considered to help achieve disease control for patients who are unable to take oral medications and suffer from disease complications [20]. We did not observe differences in adherence by therapeutic agent, likely because of the very small numbers of patients in each category of non-corticosteroid immunosuppressant. Another limitation is that the MBS scale was not completed in 51% of the surveyed MAS instances, which likely reduced the strength of the associations between the MBS scale and medication nonadherence.
Important questions remain on the clinical impact of medication nonadherence in INS. In our study, we did not find statistically significant associations between medication adherence and steroid response classification and healthcare utilization. This may have been impacted by indication bias: providers adjusting drug therapies or management and follow-up in response to patient adherence and thereby affecting clinical outcomes. Though NEPTUNE survey results were not made available to the participants’ providers, the study is observational and providers may make assessments on a patient’s adherence based on clinical encounters outside of the study. We furthermore relied on caregiver and self-reports of medication adherence as opposed to direct or objective measures of adherence (e.g., drug concentration in body fluids, electronic medication packaging devices), which may be less sensitive and specific for detecting nonadherence [21]. Specifically, 25 participants did not complete MAS surveys despite being on medications. Though the MAS survey has sound psychometric properties [22], biases inherent in subjective reporting may have resulted in an underestimation of the prevalence of nonadherence and affected the validity of our analyses. Our analysis was also limited by relatively short follow-up (median 9 months) for the outcomes of interest. We did observe a 59% increase in odds of developing SRNS vs. SSNS (OR 1.59; 95% CI, 0.93–2.71). Though this did not meet the threshold for statistical significance, there may have been a modest, but clinically important, relationship between adherence and treatment response undetected in this relatively small sample. For reference, this sample had 80% power to detect an OR of 2.93, and if the true effect is less than 2.93, then this sample would be underpowered by conventional standards. Determining the role that medication nonadherence plays in INS clinical outcomes is important, as physicians, caregivers, and patients decide on optimal therapy that not only has the best efficacy-toxicity profile, but can also be used reliably long-term in this chronic disease.
In conclusion, medication nonadherence is common in INS. Adolescent patients, patients in families with lower maternal education, and those with parent- and patient-reported barriers to taking medications are at increased risk for nonadherence. Additional research on the clinical impact of nonadherence and the development and testing of screening and support strategies are needed for this group of patients with chronic kidney disease.
Supplementary Material
Acknowledgements
We are indebted to the patients and families who graciously participated in this research study. We would also like to thank all members of the NEPTUNE consortium listed below:
Members of the Nephrotic Syndrome Study Network (NEPTUNE) NEPTUNE Enrolling Centers
Cleveland Clinic, Cleveland, OH: K Dell*, J Sedor**, M Schachere#, J Negrey#
Children’s Hospital, Los Angeles, CA: K Lemley*, B Silesky#
Children’s Mercy Hospital, Kansas City, MO: T Srivastava , A Garrett#
Cohen Children’s Hospital, New Hyde Park, NY: C Sethna*, O Bullaro #
Columbia University, New York, NY: P Canetta*, A Pradhan#
Emory University, Atlanta, GA: L Greenbaum*, C Wang**, C Kang#
Harbor-University of California Los Angeles Medical Center: S Adler*, J LaPage#
John H Stroger Jr. Hospital of Cook County, Chicago, IL: A Athavale*, M Itteera
Johns Hopkins Medicine, Baltimore, MD: M Atkinson*, T Dell#
Mayo Clinic, Rochester, MN: F Fervenza*, M Hogan**, J Lieske*, V Chernitskiy#
Montefiore Medical Center, Bronx, NY: F Kaskel*, M Ross*, P Flynn#
NIDDK Intramural, Bethesda MD: J Kopp*, J Blake#
New York University Medical Center, New York, NY: H Trachtman*, O Zhdanova**, F Modersitzki#, S Vento#
Stanford University, Stanford, CA: R Lafayette*, K Mehta#
Temple University, Philadelphia, PA: C Gadegbeku*, S Quinn-Boyle#
University Health Network, Toronto: M Hladunewich**, H Reich**, P Ling#, M Romano#
University of Miami, Miami, FL: A Fornoni*, C Bidot#
University of Michigan, Ann Arbor, MI: M Kretzler*, D Gipson*, A Williams#, C Klida#
University of North Carolina, Chapel Hill, NC: V Derebail*, K Gibson*, E Cole#, J Ormond-Foster#
University of Pennsylvania, Philadelphia, PA: L Holzman*, K Meyers**, K Kallem#, A Swenson#
University of Texas Southwestern, Dallas, TX: K Sambandam*, Z Wang#, M Rogers#
University of Washington, Seattle, WA: A Jefferson*, S Hingorani**, K Tuttle**§, M Bray #, E Pao#, A Cooper#§
Wake Forest University Baptist Health, Winston-Salem, NC: JJ Lin*, Stefanie Baker#
Data Analysis and Coordinating Center: M Kretzler*, L Barisoni**, J Bixler, H Desmond, S Eddy, D Fermin, C Gadegbeku**, B Gillespie**, D Gipson**, L Holzman**, V Kurtz, M Larkina, S Li, S Li, CC Lienczewski, J Liu, T Mainieri, L Mariani**, M Sampson**, J Sedor**, A Smith, A Williams, J Zee.
Digital Pathology Committee: Carmen Avila-Casado (University Health Network, Toronto), Serena Bagnasco (Johns Hopkins University), Joseph Gaut (Washington University in St Louis), Stephen Hewitt (National Cancer Institute), Jeff Hodgin (University of Michigan), Kevin Lemley (Children’s Hospital of Los Angeles), Laura Mariani (University of Michigan), Matthew Palmer (University of Pennsylvania), Avi Rosenberg (Johns Hopkins University), Virginie Royal (University of Montreal), David Thomas (University of Miami), Jarcy Zee (University of Pennsylvania). Co-Chairs: Laura Barisoni (Duke University) and Cynthia Nast (Cedar Sinai).
*Principal Investigator, **Co-investigator, #Study Coordinator
§Providence Medical Research Center, Spokane, WA
Funding
The Nephrotic Syndrome Study Network Consortium (NEPTUNE), U54-DK-083912, is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through a collaboration between the Office of Rare Diseases Research, National Center for Advancing Translational Sciences and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK). Additional funding and/or programmatic support for this project has also been provided by the University of Michigan, the NephCure Kidney International and the Halpin Foundation. Chia-shi Wang is supported by the NIH-NIDDK under Award Number K23DK118189. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Jonathan Troost was supported in part by the National Center for Advancing Translational Sciences (NCATS) for the Michigan Institute for Clinical and Health Research (UL1TR002240).
Conflict of interest/Competing interest
Tarak Srivastava has received research funding from Bristol-Myers-Squibb, Retrophin, Mallinckrodt, and Alexion, and has consulted for Alnylam. Larry A. Greenbaum receives research support from Reata Pharmaceuticals and Vertex. He also serves as a consultant for Travere. Howard Trachtman has consultancy agreements through NYU Grossman School of Medicine with Travere Therapeurtics and Goldfinch Bio. He is a consultant to Otsuka and Chemocentryx as member of study data monitoring committees. Debbie S Gipson receives research support from Goldfinch Bio, Novartis, Travere, and Reata. She is a scientific advisor through the University of Michigan with Vertex and AstraZeneca. Kimberly Reidy is a site investigator for Advicienne- and Travere-sponsored clinical trials. Katherine Dell is a site investigator for Travere and AMGEN- sponsored clinical trials and has a consultancy agreement with Sanofi Genzyme.
Footnotes
Ethics approval Institutional review board (IRB) approval for this study was obtained via the single IRB of record at the University of Michigan.
Consent to participate Consent for study participation was obtained at NEPTUNE enrollment from the legal guardians of the minor participants. Assent was obtained where applicable according to single and local IRB guidelines.
Data availability
Datasets generated and/or analyzed during the current study are available in the NEPTUNE data repository. Interested researchers can contact NEPTUNE at neptune-study.org/ancillary studies for data access.
References
- 1.Eddy AA, Symons JM (2003) Nephrotic syndrome in childhood. Lancet 362:629–639 [DOI] [PubMed] [Google Scholar]
- 2.Lombel RM, Gipson DS, Hodson EM, Kidney Disease: Improving Global Outcomes (2013) Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol 28:415–426 [DOI] [PubMed] [Google Scholar]
- 3.Banh TH, Hussain-Shamsy N, Patel V, Vasilevska-Ristovska J, Borges K, Sibbald C, Lipszyc D, Brooke J, Geary D, Langlois V, Reddon M, Pearl R, Levin L, Piekut M, Licht CP, Radhakrishnan S, Aitken-Menezes K, Harvey E, Hebert D, Piscione TD, Parekh RS (2016) Ethnic differences in incidence and outcomes of childhood nephrotic syndrome. Clin J Am Soc Nephrol 11:1760–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang CS, Yan J, Palmer R, Bost J, Wolf MF, Greenbaum LA (2017) Childhood nephrotic syndrome management and outcome: a single center retrospective analysis. Int J Nephrol 2017:2029583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombel RM, Hodson EM, Gipson DS, Kidney Disease: Improving Global Outcomes (2013) Treatment of steroid-resistant nephrotic syndrome in children: new guidelines from KDIGO. Pediatr Nephrol 28:409–414 [DOI] [PubMed] [Google Scholar]
- 6.Ashoor IF, Mansfield SA, O’Shaughnessy MM, Parekh RS, Zee J, Vasylyeva TL, Kogon AJ, Sethna CB, Glenn DA, Chishti AS, Weaver DJ, Helmuth ME, Fernandez HE, Rheault MN, CureGN Consortium (2019) Prevalence of cardiovascular disease risk factors in childhood glomerular diseases. J Am Heart Assoc 8:e012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization (2003) Adherence to long-term therapies: evidence for action. Sabaté E (ed). https://apps.who.int/iris/handle/10665/42682 [Google Scholar]
- 8.McGrady ME, Hommel KA (2013) Medication adherence and health care utilization in pediatric chronic illness: a systematic review. Pediatrics 132:730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, Sampson MG, Kopp JB, Lemley KV, Nelson PJ, Lienczewski CC, Adler SG, Appel GB, Cattran DC, Choi MJ, Contreras G, Dell KM, Fervenza FC, Gibson KL, Greenbaum LA, Hernandez JD, Hewitt SM, Hingorani SR, Hladunewich M, Hogan MC, Hogan SL, Kaskel FJ, Lieske JC, Meyers KE, Nachman PH, Nast CC, Neu AM, Reich HN, Sedor JR, Sethna CB, Trachtman H, Tuttle KR, Zhdanova O, Zilleruelo GE, Kretzler M (2013) Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83:749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voils CI, Maciejewski ML, Hoyle RH, Reeve BB, Gallagher P, Bryson CL, Yancy WS Jr (2012) Initial validation of a self-report measure of the extent of and reasons for medication nonadherence. Med Care 50:1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simons LE, Blount RL (2007) Identifying barriers to medication adherence in adolescent transplant recipients. J Pediatr Psychol 32: 831–844 [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United States Renal Data System (2020) USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: https://adr.usrds.org/2020 [Google Scholar]
- 14.Blydt-Hansen TD, Pierce CB, Cai Y, Samsonov D, Massengill S, Moxey-Mims M, Warady BA, Furth SL (2014) Medication treatment complexity and adherence in children with CKD. Clin J Am Soc Nephrol 9:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin D, Guo Q, Geng X, Song Y, Song J, Wang S, Li X, Duan J (2020) The effect of inpatient pharmaceutical care on nephrotic syndrome patients after discharge: a randomized controlled trial. Int J Clin Pharm 42:617–624 [DOI] [PubMed] [Google Scholar]
- 16.Hsu C-N, Huang S-H, Tain Y-L(2019) Adherence to long-term use of renin-angiotensin II-aldosterone system inhibitors in children with chronic kidney disease. BMC Pediatr 19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN (2010) Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant 14:603–613 [DOI] [PubMed] [Google Scholar]
- 18.Tong A, Morton R, Howard K, Craig JC (2009) Adolescent experiences following organ transplantation: a systematic review of qualitative studies. J Pediatr 155:542–549 [DOI] [PubMed] [Google Scholar]
- 19.Nevins TE, Nickerson PW, Dew MA (2017) Understanding medication nonadherence after kidney transplant. J Am Soc Nephrol 28: 2290–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulinski T, Carlier-Legris A, Schlecht D, Ranchin B, Cochat P (2005) Triamcinolone acetonide: a new management of noncompliance in nephrotic children. Pediatr Nephrol 20:759–762 [DOI] [PubMed] [Google Scholar]
- 21.Lam WY, Fresco P (2015) Medication adherence measures: an overview. Biomed Res Int 2015:217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan YH, Weng SD, Loh DHF, Phang JK, Oo LJY, Blalock DV, Chew EH, Yap KZ, Tan CYK, Yoon S, Fong W, Ostbye T, Low LL, Bosworth HB, Thumboo J (2020) Measurement properties of existing patient-reported outcome measures on medication adherence: systematic review. J Med Internet Res 22:e19179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated and/or analyzed during the current study are available in the NEPTUNE data repository. Interested researchers can contact NEPTUNE at neptune-study.org/ancillary studies for data access.


