Abstract
Although tissue homeostasis—the steady state—implies stability, our organs are in a state of continual, large-scale cellular flux. This flux underpins an organ’s ability to homeostatically renew, to non-homeostatically resize upon altered functional demand, and to return to homeostasis after resizing or injury—in other words, to be dynamic. Here, I examine the basic unit of organ-scale cell dynamics: the cellular life cycle of birth, differentiation, and death. Focusing on epithelial organs, I discuss how spatial patterns and temporal kinetics of life cycle stages depend upon lineage organization and tissue architecture. I review how signaling between stages coordinates life cycle dynamics to enforce homeostasis, and I highlight how particular stages are transiently unbalanced to drive organ resizing or repair. Finally, I offer that considering organs as a collective of not cells but rather cell life cycles provides a powerful vantage for deciphering homeostatic and non-homeostatic tissue states.
Keywords: tissue homeostasis, adaptation, stem cells, differentiation, feedback, extrusion
INTRODUCTION
We are multitudes of cells, about 30 trillion of them in total (Sender & Milo 2021). Remarkably, we lose and replace approximately 1.1% of this number each day, a process of cellular turnover O’Brien that replaces the equivalent of our body mass about every 2.4 years (Sender & Milo 2021). Hence, our bodies have been likened to Theseus’ ship, which, due to the ravages of time, eventually must have each of the planks that comprise it replaced as they decay.
The ancient Greeks used the metaphor of Theseus’ ship to debate whether an object retains its identity when its individual components are gradually replaced (Cooper 1997), and the same puzzle would seem to apply to ourselves. Yet, whereas the replacement planks of Theseus’ ship originate from an external source, the replacement cells added to our bodies derive from cells that we already have. Indeed, this insight was the basis of the Cell Theory, the scientific revolution that Rudolf Virchow galvanized in 1858 when he proclaimed “Omnis cellula e cellula” (“All cells from [prior existing] cells”) (Harris 2000, p. 135).
Binary fission events, as these first cell biologists called cell division, were, within a few decades, found to occur even in fully mature tissues (Harris 2000). Virchow’s pupil Guilio Bizzozero (1892) observed that cell divisions in the intestine were restricted to crypts that were not exposed to the intestinal lumen, and he speculated that cells born in the crypts might, over time, move up the villi as a means of replacing lost cells. His speculation may be the earliest recorded notion of cell turnover. This radical notion of turnover met deep skepticism (Leblond 1981), however, and the field of biology would have to wait another half century for a broadly convincing demonstration that cell turnover is continuous and mature organs are in a near-constant state of renewal.
In 1948, Charles Leblond, Catherine Stevens, and Rita Bogoroch (Leblond et al. 1948) effectively performed one of the first unbiased lineage-tracing experiments. By pulse-chase labeling mitotic cells with 3H-thymidine, they demonstrated that, in the small intestine, cells labeled during the pulse phase of the experiment started in the crypts and, during the chase period, were displaced into the villi. This striking finding, together with Leblond & Stevens’ (1948) identification that the tips of villi were cell-extrusion zones, engendered the compelling view that the crypt-villus axis is a continual conveyor belt of cells, with “active cell production due to these mitoses … balanced by an equivalent cell loss, in order to maintain the ‘steady state’ of the tissues” (Leblond & Stevens 1948, p. 357). Similar labeling studies in blood and skin revealed cellular turnover in these tissues (Andreasen & Ottesen 1945, Leblond & Walker 1956), and the possibility that turnover was a widespread feature of mature organs gained acceptance.
Yet biologists underestimated the complexity (and elegance) of turnover as a cellular behavior for a long time. Many thought the mature cells that were lost must be healthy cells accidentally eliminated as a byproduct of physiological activities such as peristalsis (Leblond 1981). Over time, improved electron microscopy techniques revealed the ultrastructure of cells being extruded at villus tips and showed that they were, in fact, infirm and at death’s door (Leblond 1981). Although the connection to apoptosis would not be made for several decades, these findings introduced the concept that mature cells in healthy organs can have a characteristic, finite lifespan, after which they are lost.
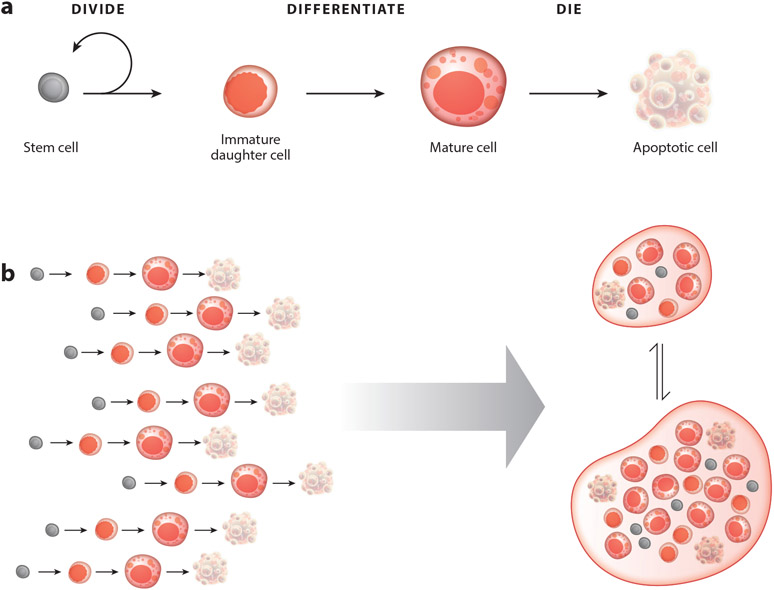
Cell birth and death bookend any cell’s existence, but a key aspect of a cell’s life was still missing: differentiation into a terminal cell fate. That cell differentiation occurred regularly in mature tissues outside of the gonads became starkly evident when McCulloch, Till, and colleagues reported finding adult hematopoietic stem cells in 1963 (Becker et al. 1963, Siminovitch et al. 1963). Their landmark discovery established the existence of a dedicated and relatively undifferentiated blood cell population with the sole purpose of generating new blood cells. A crucial corollary of their discovery was that these new stem cell daughters are born in a nonfunctional state and must acquire their mature function after birth. Shortly after, Leblond and Cheng (Cheng & Leblond 1974) showed in the intestine that dividing cells at the base of crypts serve a similar stem cell function. Advances in digestive physiology helped them to piece together how various types of intestinal cells acquired their specialized functions as they rose up the crypt-villus axis (Cheng & Leblond 1974, Leblond 1981). This gives us the full scope of the cell life cycle in self-renewing adult tissues: birth, via stem cell division of a stem cell, to terminal differentiation to mature physiological function to, finally, death (Figure 1a).
Figure 1.
The cell life cycle, which encompasses (a) birth (via division of a stem cell), terminal differentiation, mature physiological function, and apoptotic death, can be regarded as (b) the basic unit of organ-scale cell dynamics.
The Cell Life Cycle Is the Basic Unit of Organ-Scale Cell Dynamics
It is now widely appreciated that tissue homeostasis is, at its essence, a dynamic equilibrium between cell production and cell loss. In this review, I put forth a more holistic perspective that views tissue homeostasis not as a simple production and loss equilibrium but rather as the integration of individual cell life cycles. Akin to how the cell is the basic unit of organs, the cell life cycle can be viewed as the basic unit of organ-scale cell dynamics (Figure 1b). This holistic view provides explanatory power for the systems-level robustness of tissue homeostasis, because it incorporates three basic features of the cell life cycle. First, at the level of an individual cell, successful progression through each stage of the cell life cycle implies successful execution of earlier stages. Second, a cell’s life cycle stage determines the types of signals that the cell can send and receive, as well as the types of behaviors that the signals can elicit. An illustrative example occurs in the Drosophila intestine, or midgut. In this organ, stem cells generate progeny that express EGFR (epidermal growth factor receptor) at birth but silence it during differentiation (Buchon et al. 2010, Jiang et al. 2009). Hence, the capacity of a cell to be activated by EGF ligands changes with life cycle stage.
The third feature is a crucial, but often overlooked, consequence of the first two: While individual cells communicate and coordinate with cells at other life cycle stages, they are themselves transiting through these stages. The signals that a cell receives help determine the speed of its life cycle transitions—for example, how long it takes a cell to differentiate, or how long it takes to die and be eliminated. When aggregated over many cells, these interconnected signaling circuits control organ-scale properties such as overall cell number and the relative proportions of stem cells, immature cells, and mature cells. Thus, the duration and frequency of each life cycle stage represents a potential regulatory node for tuning the flux of cells through the system in response to changing functional needs or environmental conditions (Gordon 2016).
Can Cell Life Cycles Give Insight into Non-Homeostatic Organ States?
We often regard homeostatic cellular turnover as typical. However, external circumstances such as injury, environmental change, reproductive status, or altered functional demand routinely cause organs to transiently deviate from steady-state equilibrium and drive them into a non-homeostatic state of repair, remodeling, or resizing (O’Brien & Bilder 2013). I suggest that by considering the cell life cycle as the basic unit of organ-scale cell dynamics, we can better understand these non-homeostatic tissue states (Gordon 2016). The paradigmatic example of function-driven resizing is the vertebrate intestine, which grows and shrinks in response to increased or decreased dietary load (Dailey 2014, Drozdowski 2006, Secor et al. 2000). The Drosophila midgut and the mouse skeletal muscle, epidermis, and mammary gland are other well-characterized tissues in which increased or decreased functional demand results in organ growth or shrinkage, respectively (Aragona et al. 2020, Dawson & Visvader 2021, Dekoninck et al. 2020, Fukada 2018,Murach et al. 2018, O’Brien et al. 2011, Seldin et al. 2017).
Cell life cycles can also provide insight into how a healthy organ can, over time, exhibit a range of metastable states that differ in terms of organ size and rates of cell turnover (Shingleton 2010). For instance, switching an animal between rich and poor diets causes its intestine to undergo non-homeostatic shrinkage (rich to poor diet) or growth (poor to rich diet), but when the animal is stably maintained on a rich or a poor diet, its intestine remains at a larger or smaller steady-state size (Aldewachi et al. 1975, Altmann 1972, Bonfini et al. 2021, O’Brien et al. 2011, Yilmaz et al. 2012).
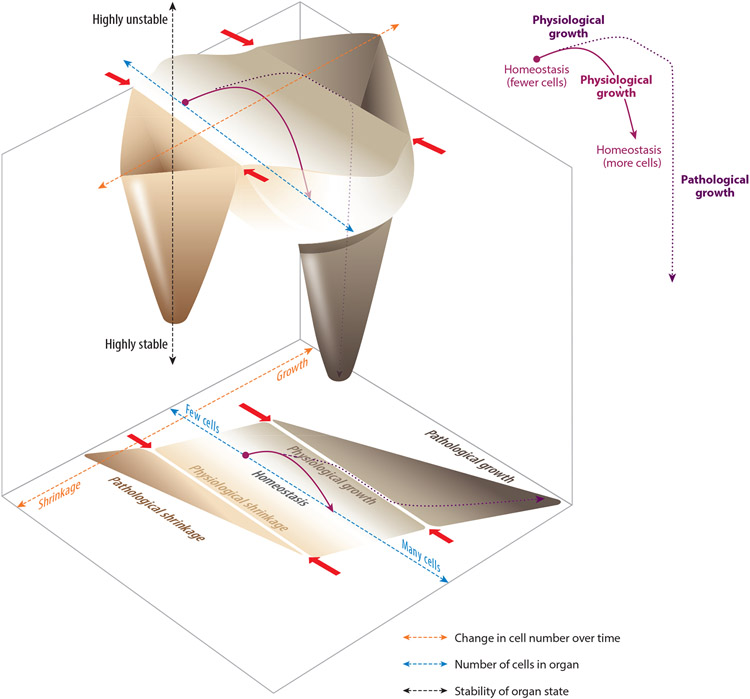
To change size, organs must change the number of total cells, the sizes of individual cells, or both. As a simple thought experiment, let us consider just one variable, cell number, in a generic organ as it grows and shrinks. The relationship between total cell number and rate of change in cell number can be conceptualized as a phase space with regimes of homeostasis, growth, and shrinkage (Figure 2).
Figure 2.
Hypothetical organ state landscapes in two and three dimensions, based on the parameter of cell number. (Bottom) The two-dimensional landscape shows total cell number (blue dashed axis) relative to rate of change in total cell number (orange dashed axis). Within this parameter space, approximate regions are indicated for organ states of homeostasis, physiological growth, pathological growth, physiological shrinkage, and pathological shrinkage. (Top) In the corresponding three-dimensional landscape, homeostasis, pathological growth, and pathological shrinkage are drawn as basins, with the depth of the basin indicating a state’s relative stability (black dashed axis). Physiological growth and physiological shrinkage are represented by the slopes above the floor of the homeostasis basin. Finally, the boundaries between physiological and pathological growth or shrinkage are represented by the ridge tops separating the basins (red arrows). Two hypothetical examples of organ dynamics are illustrated by the purple lines (shown within the landscapes and also isolated to the right). The solid purple line shows a physiological growth trajectory. The organ is initially homeostatic but contains relatively few cells (perhaps, for instance, because it is experiencing low functional demand). In response to environmental change (for instance, an increase in functional demand), the organ undergoes physiological growth and acquires additional cells until it reaches an appropriate, larger size. At this point, the organ ceases growth and returns to homeostasis, but at its new, larger size. By contrast, the dotted purple line shows an organ in which growth becomes pathological. The organ acquires a huge excess of additional cells, crosses the boundary between physiological and pathological growth, and ultimately becomes deeply stuck in a maladaptive state of overgrowth.
I suggest that understanding how organs accomplish these transient departures from homeostasis in such a remarkably coordinated manner and then subsequently settle into a new steady-state size requires that we appreciate how each stage of the cell life cycle is affected and how feedback signals between cells at different life cycle stages are tuned. Although this review focuses on the physiological mechanisms of healthy organs, note that regimes of growth or shrinkage are divided into a physiological realm, which occurs adaptively to optimize organ function, and a pathological realm, which is detrimental to organ function. The latter is characteristic of disease states such as cancer or degeneration. The loss of coordination between stages of the cell life cycle likely plays a major role in causing an organ to venture into these pathological states.
In the following sections, I consider how collective dynamics of the cell life cycle play into the ability of an organ to maintain tissue homeostasis or to transiently depart from homeostasis in response to external events. I examine how temporal and spatial dynamics of each life cycle stage are coordinated at the organ scale, and I explore how these dynamics are altered to shift between states of steady-state turnover, growth, and shrinkage. My examples derive primarily from solid epithelial organs and skeletal muscle because their structured architectures have made our understanding of them the most advanced in terms of the spatial and temporal patterns of the cell life cycle. Finally, I offer that closing the loop on the cell life cycle—i.e., linking the death of one cell to the birth of another—is a parsimonious explanation for how homeostatic tissues can robustly equilibrate division and death at the organ scale.
Ultimately, my goal is to provide a general framework for cell dynamics of adult organs and to delineate a conceptual bridge that links the life cycles of many individual cells to the dynamic state of the organ as a whole.
BIRTH: THE BEGINNING OF THE CELL LIFE CYCLE
That stem cell division is a sort of cellular birth is reflected in the very language we use to describe the event: a so-called mother stem cell divides to produce two daughter cells. One of stem cells’ defining features is their ability to produce daughters with asymmetric fates: one daughter, referred to as self-renewing, remains a stem cell, while the other daughter, referred to as terminal, ultimately differentiates into a cell that specializes to contribute to physiological function. Although it was long believed that every division of a mother stem cell produced one self-renewing and one terminal daughter, we now know that in most adult tissues, such asymmetric fate outcomes occur alongside symmetric renewing outcomes (two stem cell daughters) and symmetric terminal outcomes (two terminal cell daughters) (Klein & Simons 2011, Rulands & Simons 2017). During steady-state turnover, homeostasis is maintained because symmetric renewing divisions occur with equal frequency as do symmetric terminal divisions. Hence, in aggregate, stem cell divisions produce equal numbers of stem daughters and terminal daughters at the organ level (Klein & Simons 2011, Rulands & Simons 2017).
When a stem cell divides, and what cell type its daughters become, are profoundly influenced by the state of the tissue. As the mechanisms that regulate stem cell divisions in states of homeostasis and injury have been discussed in excellent recent reviews (Clevers & Watt 2018, Dekoninck & Blanpain 2019, Ge & Fuchs 2018, Gervais & Bardin 2017, Meizlish et al. 2021, Shivdasani et al. 2021, Wu & Tang 2021), here I focus on how increased stem cell divisions drive adaptive organ growth. I discuss how stem cell populations that are spatially dispersed throughout an organ’s expanse drive growth through different mechanisms compared to stem cell populations that reside in distinct niches. Like adaptive growth, injury repair also requires that stem cells increase their divisions; ultimately, however, repair mechanisms seek to restore the status quo, while growth seeks to change the status quo. At the end of this section, I discuss how these opposite end goals are reflected in various stem cell behaviors during repair versus growth.
Dispersed Stem Cells Drive Growth Through Uniform Expansion
Adaptive growth is a transient state in which the organ shifts from a smaller to a larger steady-state size. Once growth is complete, the enlarged organ may remain at its new size for a long time, so growth must occur in a way that retains the organ’s capacity to self-renew. Some organs in which the stem cell population is uniformly distributed, such as the Drosophila midgut and mouse epidermis, use a strategy of uniform expansion (Aragona et al. 2020, Dekoninck et al. 2020, O’Brien et al. 2011).
Expansion is uniform in two complementary ways. First, the organ expands isometrically, because its stem cell population is dispersed, and stem cells from all regions participate in generating excess terminal daughters. Second, the stem cell population expands proportionally to the organ’s increased size, which keeps the spatial density of stem cells constant as the organ grows. The absolute increase in number of stem cells arises through a transient imbalance of symmetric self-renewing divisions over symmetric terminal divisions during growth. In this manner, the organ ensures it has the right number and distribution of stem cells to sustain healthy renewal once growth is complete.
The adult Drosophila midgut, which is functionally analogous to vertebrate small intestine, grows in response to feeding (Bonfini et al. 2021, Chen et al. 2015, Foronda et al. 2014, O’Brien et al. 2011). Midgut cell number increases by fourfold over a four-day period, driven by rapid, symmetrically skewed stem cell divisions (O’Brien et al. 2011). The trigger for these growth-promoting divisions is Drosophila insulin-like peptide 3 (Dilp3), which is secreted by gut visceral muscle following food ingestion (O’Brien et al. 2011). Dilp3 is received by stem cells, which are individually distributed throughout the gut’s epithelial lining (O’Brien et al. 2011). These stem cells are sensitized to the Dilp3 signal because of elevated expression of the Drosophila insulin receptor, whose translation is boosted by nutrient-activated upregulation of its 5′ mRNA–binding protein Lin-28 (Chen et al. 2015).
Within 3 h of a fly’s initial meal, divisions of the gut’s insulin-activated stem cells skew toward symmetric renewal (Chen et al. 2015). This switch involves cross talk between insulin pathway signaling and the midgut fate determinant Notch, whose activation causes stem cell daughters to become terminal enterocytes. During growth, Notch activation is blunted by the microRNA miR305, whose expression is upregulated by insulin receptor activation (Foronda et al. 2014), and by cytosolic Ca2+, which is elevated by ingested glutamine (Deng et al. 2015). As midguts transition from growth to a new, postgrowth steady-state, midgut Dilp3 does not decrease (O’Brien et al. 2011); this observation raises the question of whether this nutrient-activated stem cell pathway self-attenuates over time or whether it is cancelled by an orthogonal, unidentified stop signal.
Growth of the mouse epidermis also occurs through rapid, symmetrically biased divisions of distributed stem cells (Aragona et al. 2020, Dekoninck et al. 2020). The epidermis is organized into cellular layers that are stratified by differentiation stage: Terminal, cornified skin cells (keratinocytes) comprise the protective outermost layers, while stem cells are distributed throughout the basal (deepest) layer. Skin stem cells drive uniform epidermal growth during postnatal life, both when the young animal grows to its adult size (Dekoninck et al. 2020) and—strikingly—in response to an inflatable prosthetic implant that delivers exogenous stretch (Aragona et al. 2020). Intriguingly, the tissue deploys the same cellular strategy even though these two types of growth occur over vastly different timescales—60 days for postnatal growth versus 4 days for stretch-induced growth (Aragona et al. 2020, Dekoninck et al. 2020). The stem cell response to stretch involves actomyosin-based mechanosensation, likely via cell adhesion receptors, that leads to the nuclear activation of two well-characterized, force-activated transcription factors, YAP1 (Yes-associated protein 1) and MAL/MKL1 (megakaryoblastic leukemia/myocardin-like 1) (Aragona et al. 2020).
Localized Stem Cells Drive Growth at a Niche-Like Tissue Edge
Other organs do not grow uniformly but instead expand along one particular axis or border by adding cells to an edge. In mammary gland and fish gill, caudal fin, and retina, such edge-directed growth involves a special population of stem cells that resides adjacent to the growing edge (Centanin et al. 2011, Fu et al. 2017, Stolper et al. 2019, Tu & Johnson 2011). During pregnancy, the mammary gland prepares for milk production by forming copious milk-producing acini at the tips of preexisting tubular ducts (Dawson & Visvader 2021). The acinar cells are generated by a distinct population of Lgr5+, Tspan8hi stem cells at the tips of ducts. Unlike other, ductally localized stem cells, acini-forming stem cells are activated to divide by ovarian hormones produced during pregnancy (Fu et al. 2017).
In contrast to the demand-driven, adaptive growth of particular organs, the entire body of a fish grows in concert throughout the animal’s lifespan. Fish gill, retina, and caudal fin each expand radially through growth-specific stem cells found at the outer edge of the existing structure (Centanin et al. 2011, Stolper et al. 2019, Tu & Johnson 2011). These stem cells deposit their terminal daughters in a medial orientation so that the stem cells constantly stay at the growing structure’s edge. At least in the gill, some daughters intriguingly escape differentiation and instead become steady-state stem cells that renew the newly formed tissue region (Stolper et al. 2019). Although these steady-state stem cells are dispersed throughout the gill, akin to stem cells in fly midgut and mammalian skin, they do not contribute to growth. This division of labor between growth-specific and steady-state stem cells may be responsible for the ability of adult fish to grow continuously (Stolper et al. 2019).
Overall, the expanding edges of these organs appear to serve as an anatomic niche for growth-specialized populations of stem cells. It is tempting to speculate that structural and/or molecular signals at tissue edges regulate the identity and proliferation of these populations.
Different Types of Stem Cell Activation Are Used for Growth and Repair
Adaptive growth and injury repair both trigger activated stem cell divisions. Stem cell–driven repair has been thoroughly discussed in excellent recent reviews (Dekoninck & Blanpain 2019, Ge & Fuchs 2018, Meizlish et al. 2021, Shivdasani et al. 2021, Wu & Tang 2021). Here, I highlight illuminating differences and similarities in the dynamics of stem cell activation.
A major difference is the means whereby organs expand the stem cell population. Mathematical modeling argues that the most efficient way to generate a large number of terminal cells is to first add more stem cells, thus enlarging the system’s capacity to produce terminal cells (Itzkovitz et al. 2012). It therefore makes sense that, during growth, stem cell divisions in fly gut and mouse epidermis skew toward symmetric renewing outcomes that generate additional stem cells, as I elaborated in the prior section titled Dispersed Stem Cells Drive Growth Through Uniform Expansion. Yet during injury repair—arguably a tissue state in which new cells are needed desperately—fly gut and mouse epidermal stem cell divisions surprisingly do not skew toward symmetric renewal (Dekoninck et al. 2020, Jin et al. 2017). Instead, they both exhibit the same, balanced fate outcomes as during steady-state turnover (Dekoninck et al. 2020, Jin et al. 2017).
To generate additional stem cells, these injured organs use an alternative strategy: dedifferentiation of terminally committed cells. Notch-activated enteroblasts in the fly gut and Gata6+ sebaceous duct cells in mouse skin reenter the cell cycle to generate additional cells of all lineages (Donati et al. 2017, Tian et al. 2021). Whereas most of this stem-like behavior is facultative, some dedifferentiated cells permanently adopt a stem cell identity and contribute to steady-state turnover in the repaired tissue. Dedifferentiation of mature cells to serve as facultative stem cells also characterizes repair of the small intestine, colon, airway, liver, and prostate (Knopf et al. 2011, Leedham 2020, Nusse et al. 2018, Ohara et al. 2022, Shivdasani et al. 2021, Tata et al. 2013).
Why would tissues use different strategies for growth and repair? Perhaps the larger tissue context offers insight. Adaptive growth is triggered by insufficient functional capacity; widespread dedifferentiation of mature cells during growth, as occurs during repair, would be counterproductive because it would further reduce organ capacity. Injury repair, however, is an acute emergency response in which temporary loss of functional capacity may be an acceptable trade-off.
Another comparison involves specialized populations of stem cells. In the prior section, I discussed growth-specific stem cells that inhabit the expanding edges of some organs. Analogous, repair-specific stem cells are found in organs such as the distal lung and oral mucosa. Akin to growth-specific stem cells, these repair-specific stem cells localize to specific niches. In mouse distal lung, Scgb1a1+, Sftpc+ stem cells (also known as bronchioalveolar stem cells) become activated by damage to regenerate lung alveolar and some airway cells but remain quiescent during steady-state turnover (Liu et al. 2019, Salwig et al. 2019). Similarly, in oral mucosa, Lrig1-enriched stem cells (also known as junctional zone stem cells) activate and regenerate tissue in response to damage but do not participate in steady-state turnover (Byrd et al. 2019).
Like growth-specific stem cells in the mammary gland, which localize to ductal-acinar junctions (Fu et al. 2017), repair-specific stem cells in lung and oral mucosa localize to anatomic junctions that serve as transition zones between the organ’s distinct tissue structures. Bronchioalveolar stem cells in the lung reside at junctions between bronchiolar ducts and alveolar acini (Kim et al. 2005, Liu et al. 2019, Salwig et al. 2019). In oral mucosa, junctional zone stem cells reside at junctions between rugae ridges and interrugae depressions (Byrd et al. 2019). Perhaps transition zones have a distinct physical and/or signaling environment that serves as a selective niche, shielding stem cells from routine renewal signals while transmitting signals for rapid cell production during growth and repair.
DIFFERENTIATION: A LONGER-TERM OPPORTUNITY TO EITHER BOOST OR BUFFER ORGAN-SCALE CELLULAR FLUX
The Case for Considering the Kinetics of Cell Differentiation
Studies of tissue homeostasis have largely focused on stem cell divisions and daughter fate decisions; the next stage, terminal differentiation, is often regarded as a fait accompli in which the cell simply executes its chosen fate program. Rather than a preprogrammed process, however, differentiation is an opportunity for cells to tune intrinsic fate programs in order to accommodate changing tissue-level demands. Cells in most organs require multiple days to terminally differentiate; during this time, the tissue’s needs may change radically. Differentiation thus provides the opportunity for a cell to sense its evolving tissue environment over a longer timescale and, if needed, to adjust its original fate trajectory.
Indeed, differentiating cells are ideally situated to mount a rapid response to changing tissue needs (Hsu et al. 2014, Lander et al. 2009, Tata & Rajagopal 2016). First, they already exist, unlike lagging progeny generated through a stem cell response. Second, they have more phenotypic plasticity compared to fully mature cells, as they are still immature. Finally, any adjustments that differentiating cells make carry less long-term risk to the tissue compared to adjustments made by stem cells, because differentiating cells have limited, if any, ability to propagate.
In this section, I discuss three key variables that can impact a cell’s differentiation trajectory: feedback and feedforward relays between the differentiating cell and cells at other life cycle stages, the temporal pacing of a cell’s differentiation journey, and the physical size that a cell attains as it differentiates. Through collective coordination of these variables, differentiating cells act to expedite or buffer life cycle dynamics at the organ scale. Their linchpin role ensures that an organ’s many asynchronous cell life cycles are harmoniously coordinated to achieve a collective goal, such as maintaining a zero net cellular flux during the steady state.
Differentiating Cells Are in a Dialogue with Both Stem Cells and Mature Cells
Differentiating cells are the ontogenic link between stem cells and fully mature progeny in the cell life cycle. In most organs, they are also in physical contact with these other two populations. These ontogenic and spatial relationships place differentiating cells in a key position to send signals to and receive signals from both stem cells and mature cells.
Negative feedback from differentiating cells to stem cells regulates the rate of stem cell divisions in multiple organs. The organ-scale dynamics of this feedback have been mapped in the adult zebrafish brain at single-cell resolution in real time (Dray et al. 2021). In the fish brain, stem cells and terminally committed daughter cells form a single layer in the brain pallium. Terminal daughters express a membrane-bound Delta ligand, which activates Notch receptors on the surface of stem cells that they physically contact (Dray et al. 2021). Notch activation causes these stem cells to enter quiescence, an effect that persists for 9–12 days after the terminal daughter’s birth (Dray et al. 2021). Negative feedback also occurs in the fly midgut, where E-cadherin-mediated adhesion between stem cells and differentiating daughters (enteroblasts) acts to inhibit additional stem cell divisions (Choi et al. 2011). As in the fish brain, stem cells and terminal daughters in the fly gut are dispersed within a single cellular layer. Mathematical modeling suggests that such short-range, long-duration feedback within a single layer of dispersed stem and daughter cells can explain how stem cell divisions are spatially distributed in a nonrandom pattern throughout such organs over time (Dray et al. 2021).
Differentiating cells can also be on the receiving end of negative feedback. In mouse olfactory epithelium, differentiating cells are inhibited by fully mature cells, which prevent transit-amplifying divisions of the differentiating cells by secreting Gdf11 (growth differentiation factor 11) to activate differentiating cells’ TGFβR1 (Gokoffski et al. 2011, Lander et al. 2009).
Differentiating cells can also participate in feedforward circuits (Tata & Rajagopal 2016). In mouse airway, differentiating daughter cells require a feedforward signal from stem cells for their continued existence. Intriguingly, this stem cell signal is Jagged 2, another Notch ligand which, like Delta, is membrane bound. In airway, Jagged 2 on stem cells binds to the Notch receptor on differentiating daughter cells, providing the daughters with a crucial survival signal (Pardo-Saganta et al. 2015). The common use of Notch in both feedback circuits (e.g., fish brain) and feedforward circuits (e.g., mouse airway) emphasizes the central importance of short-range signaling between adjacent cells and highlights the versatility of the actual molecular pathways.
In all these preceding examples (fish brain, fly gut, and mouse olfactory and airway epithelia), different stages of the cell life cycle intermingle with each other in a single layer of cells. This single-layered organization contrasts with skin epidermis, which has multiple stratified cell layers. Each layer corresponds to a stage of a skin cell’s life cycle: epidermal stem cells and their newborn daughter cells reside in the basal layer, and cells in progressively later stages of differentiation reside in progressively more superficial layers. This stage-specific layering gives rise to an interesting variation of short-range feedback from differentiating cells to stem cells (Mesa et al. 2018). When a daughter cell commits to terminal differentiation by exiting the basal layer and moving to the layer above, this event triggers a stem cell to divide immediately adjacent to the (now-departed) differentiating cell (Mesa et al. 2018). Thus, birth of a new daughter is spatially and temporally coupled to differentiation of an older daughter through the latter’s departure from the basal layer. The feedback signals that achieve this coordination are unknown at present, but the spatial proximity of the daughter cell’s basal exit and subsequent stem cell division implies that the feedback in skin acts over single-cell distances, akin to feedback in organs with a single cellular layer.
The Kinetics of Cell Differentiation Are the Primary Driver of Adaptive Resizing of the Rodent Intestine
A compelling example of how differentiating cells throttle organ-scale cellular flux emerged from early investigations of diet-driven intestinal resizing in rodents. When rats are deprived of food, the speed at which cells differentiate slows down (Altmann 1972). Furthermore, differentiating cells—not stem cells—are the first population to slow their divisions, the first population to shrink in number, and, over time, the population that is most sharply winnowed (Altmann 1972). After seven days of starvation, the numbers of differentiating cells in the rat duodenum decreased by 40%, whereas the numbers of stem cells decreased by only 14% (Altmann 1972). Altogether, these dramatic changes to differentiation behaviors account for most of the starved organ’s decreased cell number and slower turnover (Yilmaz et al. 2012).
Differentiating cells are also the first intestinal cells to respond when starved rats are refed. They reactivate transit-amplifying divisions within 30 min of refeeding, whereas stem cells reactivate after 8 h of refeeding (Aldewachi et al. 1975). Also, in this 30-min timeframe, differentiation speed dramatically accelerates (Aldewachi et al. 1975). When cell divisions are blocked with methotrexate during refeeding, differentiation speed still accelerates (Aldewachi et al. 1975); this finding demonstrates that the accelerated differentiation is a direct response to refeeding, not an indirect consequence of increased divisions. In fact, stem cells in these intestines, prevented from generating new daughters, end up differentiating themselves, with consequent decimation of the organ’s stem cell pool (Aldewachi et al. 1975). These findings indicate that differentiating cells are largely responsible for adaptive intestinal growth, akin to their role in shrinkage.
A key takeaway from these pioneering studies is that the absolute time required by differentiating cells to become fully mature is tuned over an impressively large range to steer organ-scale cell dynamics. By accelerating or slowing the speed at which they differentiate, cells exert two related effects. First, they directly modulate cellular flux by controlling the progression of cells through the life cycle. Second, they indirectly modulate the strength of feedback and feedforward signaling with other life cycle stages. This second effect arises because (a) the duration of a cell’s differentiation stage establishes the duration of its signaling, and (b) the speed of differentiation can, if out of sync with other life cycle stages, change the organ’s proportion of differentiating cells, with a commensurate change in their collective signaling strength.
Differentiation-Associated Cell Growth Dynamically Complements Cell Divisions to Control Organ Size
In many organs, including the mammalian airway, intestine, skeletal muscle, mammary gland, and prostate and the Drosophila midgut, stem cells are smaller than their mature progeny. Cell growth thus becomes a basic feature of terminal differentiation. In the fly gut, the volume of stem cell daughters increases by 50–100-fold as cells terminally differentiate (L.A. Koyama, P. Moreno-Roman, A. Galenza & L.E. O’Brien, unpublished observations). Because organ size is determined by both the overall number of cells and the sizes of individual cells, one might expect that changes in organ size would involve not only changes in cell number, as I discussed in the preceding section, but also changes in cell size.
Indeed, during diet-driven resizing of the fly gut, the growth of single differentiating cells is the dominant factor in changing overall organ size (Bonfini et al. 2021). Switching flies from a nutrient-poor to a nutrient-rich diet not only causes gut stem cells to generate more daughters through insulin receptor activation (Bonfini et al. 2021, O’Brien et al. 2011), but also causes terminal daughters to grow bigger through Tor pathway activation (Amcheslavsky et al. 2011, Bonfini et al. 2021, Kapuria et al. 2012). When stem cell divisions are blocked, for instance by cell-specific depletion of the insulin receptor, terminal cells grow bigger still, such that the organ ultimately achieves the same size as control guts in which stem cells can mount nutrient-activated divisions (Bonfini et al. 2021). By contrast, when terminal cell growth is inhibited, for example by cell-specific depletion of Tor, stem cells cannot make enough additional cells to fully compensate, and organ growth is consequently stunted (Bonfini et al. 2021). [In the fly gut, stem cells account for virtually all new cell production (Boumard & Bardin 2021).] These findings demonstrate that differentiating cells dynamically modulate their size in response to organ-scale needs, and that, at least in these experimental conditions, the capacity of differentiating cells to grow eclipses the capacity of stem cells to divide.
Some evidence suggests that this complementary balance between cell number and cell size persists even when the fly gut is at a steady state. A comparison of steady-state guts from 153 wild-caught Drosophila isolines revealed marked, strain-characteristic variation in both the mitotic indices of stem cells and the sizes of terminal cell nuclei (a proxy for cell size) (Tamamouna et al. 2020). Intriguingly, these two variables exhibit strong inverse correlation across the 153 isolines (Tamamouna et al. 2020), which suggests that some complementary balancing of cell number and cell size may occur in all tissue states.
Genome-wide association study analysis of these isolines led to the tumor necrosis factor α (TNFα) ligand Eiger as one possible regulator (Tamamouna et al. 2020). Eiger is secreted by stem cells (Doupé et al. 2018), while its receptor Grindelwald is expressed by mature cells and, at lower levels, by stem cells and new daughters (Li et al. 2022). Depleting Eiger leads to decreased stem cell divisions and larger terminal cell nuclei; overexpressing Eiger has the opposite effects (Tamamouna et al. 2020). It is tempting to speculate that Grindelwald on terminal cells modulates the growth-controlling Tor pathway (Amcheslavsky et al. 2011, Bonfini et al. 2021, Kapuria et al. 2012). Such a feedforward mechanism from stem cells to differentiating cells might direct an appropriate amount of terminal cell growth in accordance with local concentrations of stem cell–derived Eiger.
This Eiger signaling pathway still leaves open the mystery of exactly how differentiating cells sense what their appropriate size should be in order to keep cell number and cell size homeostatically balanced at the organ scale. Here, one tempting possibility is suggested by budding yeast and Xenopus embryos, in which growth-dependent progression through cell cycle checkpoints is gated by size-sensing mechanisms that effectively measure the ratio of protein biomass to DNA content (Xie et al. 2022). Perhaps the fly gut uses a multicellular version of size sensing in which the ratio of protein biomass to DNA content is calculated not at the single-cell level but at the whole-organ level. Since the growth of differentiating gut cells is tightly coupled to DNA endoreplication (Bailey et al. 2021, Øvrebø & Edgar 2018), a multicellular size-sensing mechanism could be used to regulate endocycling and thus the growth of individual cells. Guts might achieve a constant ratio of protein to DNA by either generating more cells with lower ploidy or fewer cells with higher ploidy, with genetic variation influencing a preference for one or the other. Furthermore, the precise value of this ratio would vary depending on diet and other environmental inputs to set different sizes for a single gut over time. While hypothetical at present, such a mechanism is intuitively appealing because it might generally apply to any of the wide array of organs that contain polyploid cells, such as the worm intestine, fly abdominal epithelium, fish heart, and mammalian liver (Xie et al. 2022, Bailey et al. 2021).
APOPTOTIC ELIMINATION: A TIME TO BREAK DOWN, A TIME TO CAST AWAY
The Cell Life Cycle Ends Through Apoptosis
Ninety percent of cell elimination occurs through apoptosis, including approximately 33 billion cells each day in an adult human (Morioka et al. 2019). Apoptotic cells in epithelial organs are primarily eliminated through cell extrusion, a coordinated process in which the dying cell and its immediate neighbors push and pull together to squeeze the dying cell out of the tissue and into the luminal space for clearance (Ohsawa et al. 2018). [The major exceptions to this general rule are skin epidermal keratinocytes, which undergo a unique form of cell death, corneoptosis, that is simultaneously the last step in their differentiation because it prepares their corpses to function as the skin’s outermost protective layer (Lippens et al. 2005, Matsui et al. 2021).] Work in cell culture and in developing tissues has revealed the cytoskeletal dynamics of cell extrusion (Franco et al. 2019, Gu et al. 2011, Levayer et al. 2016, Rosenblatt et al. 2001, Santacreu et al. 2021, Villars & Levayer 2020). Extrusion mechanics are likely similar in adult tissues (Bullen et al. 2006; Wang et al. 2011, 2016; Watson et al. 2009), so I do not review them here.
Yet despite many insights into how apoptotic cells are eliminated, the factors that control which, when, and, in many tissues, exactly where this final life cycle stage transpires remain stubbornly elusive. This refractoriness is because apoptotic cells, once they are cleared, leave no trace in the tissue. Hence, standard approaches to label and monitor cells in vivo, such as the lineage-tracing techniques that revolutionized our understanding of cell birth and differentiation, are unable to report on cell elimination.
Thus, much of our current knowledge comes from two organs in which apoptotic cells can predictably be caught in the act: the intestine, where cells die in extrusion zones at villus tips, and the mammary gland, in which massive cell death drives organ shrinkage when milk production ceases upon weaning (Dawson & Visvader 2021). These organs reveal that apoptotic cell death, like birth and differentiation, is dynamically modulated to drive organ-scale changes in cell number in response to changing functional demands.
Altered Rates of Cell Extrusion Help Drive the Resizing of Epithelial Organs
In the 1920s—decades before the first adult stem cells were identified and the reality of continual cell turnover was accepted—researchers noticed that rat intestines exhibited large numbers of extruding cells at the tips of their villi within hours after the animals had been deprived of food (Jackson 1925, Sun 1927). Subsequent time courses (in which experimenters heroically harvested extruded cells ex vivo by single-villus lavage) showed that this dramatic response was an initial spike (Clarke 1975). With continued starvation, the frequency of extruding cells gradually dropped until it settled at a new, steady-state frequency just one-quarter of that in fed intestines (Clarke 1975). It is likely that this initial surge of extruding cells makes a major contribution to the reduction in cell number that characterizes shrinkage, that the reduced frequency of extrusions over time reflects the organ-scale transition from a fed size to a starved size, and that the eventual plateau in extrusion frequency marks the attainment of the organ’s steady-state size when it is starved.
Intestinal extrusions also respond dramatically to refeeding. Within 30 min of a starved animal’s first meal, extrusion frequencies grind to a near halt—dropping to just one-half of their already low, starved steady-state level (Clarke 1975). By stopping virtually all extrusions, the starved intestine retains as many mature cells as possible to immediately process newly arriving food until cell division and differentiation, lagging stages of the cell life cycle, can catch up. After 4 h of refeeding, extrusion frequencies begin to rise, reaching fed steady-state frequency within one day (Clarke 1975). Overall, the extrusion response to dietary manipulation demonstrates how the rate of cell elimination is both highly sensitive to functional load and tunable across more than an order of magnitude under physiological conditions.
Factors other than dietary load also can influence extrusion rates. In fly midgut and mouse airway, for instance, cells enter apoptosis and extrude to correct for overshooting in the wake of injury repair (Loudhaief et al. 2017, Tadokoro et al. 2016). Overshooting arises when stem cells make more cells than needed to replace damaged cells; the organ’s total cell number immediately after repair exceeds that before injury (Loudhaief et al. 2017, Tadokoro et al. 2016). After a few days, however, cell number is restored to its prior range, because the excess cells enter apoptosis and are extruded (Loudhaief et al. 2017, Tadokoro et al. 2016).
Must a cell enter apoptosis to be eliminated? Here, a particularly compelling demonstration comes from mouse intestines, in which researchers blocked apoptosis through genetic manipulation (Ghazavi et al. 2022). These intestines compensate by extruding nonapoptotic cells at the same rate that normal intestines extrude apoptotic cells, such that overall intestinal size and cell number are unaltered (Ghazavi et al. 2022). Exactly which nonapoptotic cells are selected for extrusion is an intriguing, unanswered question. Nonetheless, this facile switch to nonapoptotic extrusions, perhaps a consequence of cellular crowding in the apoptosis-blocked intestines (Eisenhoffer et al. 2012, Marinari et al. 2012), suggests that tight control of cell elimination is critically important.
A Twist of Fate for Cell Elimination in the Mammary Gland: Some Milk-Producing Cells Become Phagocytes and Consume Their Dying Siblings
The mammary gland response to reduced functional demand is particularly extreme. Within 24 h of rodent pup weaning, a massive wave of apoptosis sweeps through the gland’s milk-producing alveoli, culling these now-superfluous structures (Dawson & Visvader 2021). [Intriguingly, a small population of alveolar cells escapes this apoptotic wave and will serve as facultative stem cells to help generate new alveoli in subsequent pregnancies (Tao et al. 2015, Wagner et al. 2002).]
This rapid, efficient restoration of the gland to its prepregnancy size (a process known as involution) creates a new challenge: clearing the organ of its consequent glut of cell corpses. Alveoli are blind-ended sacs at the ends of long tubular ducts. Unlike alveoli, ducts are largely maintained during shrinkage (Dawson & Visvader 2021). In contrast to cell corpses in the intestine, which are extruded into the gut lumen and eventually discharged from the body through peristalsis, cell corpses in the mammary gland cannot easily be discharged because they clog the thin mammary ducts (Akhtar et al. 2016), which are the gland’s sole means of egress. The clogging results in inflammation, which prevents the gland from regrowing during subsequent pregnancies (Akhtar et al. 2016).
Phagocytes solve this challenge, voraciously engulfing and digesting cell corpses to clear the involuting organ. Some of these phagocytes are professionals: immune system macrophages that have taken up residence in the mammary gland and proliferate during lactation (Dawson et al. 2020). Strikingly, however, many are amateurs recruited from the mammary gland’s own ranks (Akhtar et al. 2016, Monks et al. 2008). During shrinkage, large numbers of previously milk-producing acinar cells postpone apoptosis and instead become phagocytes that work to clear their apoptotic neighbors before dying themselves (Akhtar et al. 2016, Monks et al. 2008). This interesting twist of fate (Monks & Henson 2009) can be viewed as a form of positive feedback between stages of cell elimination and cell differentiation, in which dying cells spur their sibling neighbors to transiently acquire a new fate that accelerates overall clearance of the organ.
Cell Elimination as Cell Competition: How Does Organ State Select Winners and Losers?
What criteria target a cell for elimination, and how do these criteria change when organ state changes? The phenomenon of cell competition provides a conceptual framework to parse these questions (Baker 2020, Pellettieri & Alvarado 2007). Cell competition occurs in diverse multicellular contexts throughout metazoans; in brief, cells in a particular community compare their relative levels of fitness to designate so-called losers and winners. Losers are driven to enter apoptosis, while winners remain and continue to function. Three main mechanisms of cell competition have been described: cell surface receptors, in which outcomes are determined through direct interactions between cells; imbalanced death and survival signals that select against particular cells; and mechanical forces that, due to tissue geometry or cell-intrinsic mechanical properties, drive extrusions of certain cells (Baker 2020).
Competition-based designation of loser status likely targets the loser cells, which are presumably less fit than the winner cells, for apoptotic elimination (Pellettieri & Alvarado 2007). Indeed, experiments that manipulate known fitness factors provide proof of principle that cell competition can drive apoptotic cell elimination in the Drosophila midgut and mouse esophagus (Colom et al. 2020, Kolahgar et al. 2015, Suijkerbuijk et al. 2016). Furthermore, also in the fly gut, corrective extrusions in the wake of postrepair overshooting require Hippo/YAP pathway activation (Loudhaief et al. 2017), which is known in other contexts to mediate mechanosensitive cell competition (Baker 2020). At present, however, the cellular and molecular bases for the fitness comparisons that designate winners and losers within mature cell populations in normal adult tissues await identification.
CLOSING THE LOOP ON THE CELL LIFE CYCLE: FROM THE DEATH OF ONE CELL TO THE BIRTH OF ANOTHER
Every limit is a beginning as well as an ending.
—George Eliot, Middlemarch (1871)
Feedback from Cell Elimination to Cell Birth Links One Life Cycle to the Next
Throughout this review, I have examined how cells at various stages of the life cycle communicate to control life cycle dynamics within an organ. Feedback signaling from cell elimination that instructs stem cell division is one such type of communication. This particular type is unique, however, because it is the only mechanism that links the death of one cell to the birth of a new one. Indeed, tissue homeostasis can be viewed as closing the loop on the cell life cycle at the scale of an organ.
How, then, do dying cells generate feedback signals that generate new cell life cycles for the organ’s particular needs, and how tightly are these signals calibrated? A priori, the feedback signals must be either secreted, such as a diffusible factor, or else propagated from cell to cell through direct contact, such as adhesion-transduced mechanical force. I suggest that the strength of these signals is modulated by the dynamic spatial relationship between dying cells and stem cells within an organ’s particular architecture. In this section, I consider two categories of spatial relationships between dying cells and stem cells, dispersed (fly midgut) and distant (mouse intestine), and I speculate on how each gives rise to a distinct form of calibrated feedback that closes the cell life cycle loop during steady-state turnover.
The Geometry of Feedback Is an Organ-Scale Property: Short-Range Point Source Versus Long-Range Propagation
Both dying cells and stem cells are singly dispersed throughout the expanse of numerous organs including the fly gut and mammalian airway. In the fly gut, blocking apoptosis results in a compensatory decrease in stem cell divisions (Liang et al. 2017). This feedback involves signaling from dying cells to stem cells: An apoptotic cell emits epidermal growth factor (EGF) ligands through caspase-triggered induction of their obligate protease, Rhomboid (Liang et al. 2017). These EGF ligands bind EGF receptors on stem cells and promote stem cell division (Liang et al. 2017).
Crucially, the feedback signal is both weak [the signaling range of a single cell’s EGF signal has a radius of ~25 μm (Liang et al. 2017)] and transient [the lapsed time between apoptotic initiation and extrusion is from ~3 to 6 h (Martin et al. 2018, Amcheslavsky et al. 2020)]. I suggest that this diffusible signal acts as a rapidly fading point source that reaches only a small number of stem cells—the mean steady-state spacing between stem cells is ~15 μm (X. Du, J.L. Martin & L.E. O’Brien, unpublished observations)—activating, on average, the division of just one stem cell under steady-state conditions (Liang et al. 2017). In this scenario, stochastic deviations from the production of exactly one replacement cell would self-correct over time, because the production of too many or not enough cells would alter the spacing of stem cells and, as a consequence, alter the probability that a stem cell will be in range of future EGF signals (Liang et al. 2017).
In contrast, feedback geometry in mammalian small intestine dictates a radically different strategy from the diffusible, point-source feedback used by the fly gut, because it occurs over a distance of millimeters rather than microns. In humans, for instance, ~1 mm separates stem cells in the crypt base from apoptotic cells at the villus tip. And yet feedback signals demonstrably operate across this expanse, as illustrated by the finding that altered rates of extrusion at the villus are compensated by altered rates of cell division in the crypts (Hermiston et al. 1996). Recent work provides evidence that this long-range feedback involves the propagation of mechanical tension within the intestinal epithelial sheet. The extrusion of mature cells from villus tips causes neighbor cells to migrate toward the extrusion zone to take their place (Krndija et al. 2019). Because intestinal epithelial cells are physically coupled by strong cell–cell adhesions, the directed movement of these tip cells propagates throughout the epithelial sheet, ultimately pulling new stem cell daughters out of the crypt to enter the villus (Pérez-González et al. 2021). Given that mechano-adhesive forces have been shown to activate epithelial cell divisions in numerous contexts (Benham-Pyle et al. 2015, Gudipaty et al. 2017, He et al. 2018, Ladoux & Mège 2017), it is tempting to speculate that stem cells in the crypt sense these pulling forces and divide as a consequence. In this scenario, changes in extrusion rates would change the rates of tip-directed sheet migration, thus changing the magnitude of pulling forces experienced by stem cells and tuning their rate of division.
TAKEAWAYS AND OPEN QUESTIONS
Cell Life Cycles and Organ States
In mature, stem cell–based organs, individual cells continually transit through life cycle stages of birth, differentiation, and death.
Akin to how cells are the fundamental unit of organs, cell life cycles are the fundamental unit of organ-scale cell dynamics.
Broadly, organ-scale cell dynamics are either homeostatic or non-homeostatic. Homeostatic dynamics include steady-state turnover, when cell birth and cell elimination are in equilibrium at the organ scale and organ size remains constant over time, while non-homeostatic dynamics include adaptive growth and shrinkage. These are physiological responses to altered functional demand or environmental conditions in which organs transiently depart from homeostasis and adaptively grow or shrink to alter their functional capacity.
The basis of concerted organ-scale cell dynamics is the collective coordination of each cell life cycle stage with the other life cycle stages. This coordination can involve tuning a stage’s spatial distribution, temporal frequency, or temporal duration relative to other stages.
Cell Birth
The frequency of cell birth increases when organs undergo adaptive growth. The spatial pattern of cell birth determines the geometry of organ expansion: Broadly dispersed stem cell divisions drive isometric organ expansion. In contrast, when stem cell divisions occur at a niche-like tissue edge, the organ expands along a single axis parallel to this edge.
Like growth, injury repair is characterized by increased frequency of stem cell division. Yet growth- and injury-activated divisions differ in terms of their fate outcomes and spatial distributions. These align with the distinct organ-scale objectives of growth (expanding functional capacity) and repair (restoring tissue integrity).
How stem cell subpopulations become specialized for particular organ states (homeostasis, growth, and repair) and the interconvertibility of these subpopulations over time remain largely unknown.
Differentiation
Differentiating cells are the intermediate link between stem cells and fully mature progeny in the cell life cycle; furthermore, they are typically in physical contact with these other two populations. This linchpin position makes differentiation an ideal nexus for sending and receiving signals that coordinate life cycle stages.
During steady-state turnover, differentiating cells’ feedback and feedforward signals typically act over short distances (single cell lengths) yet long timescales (hours to days). These spatial-temporal dynamics help ensure that life cycle transitions (e.g., division and differentiation) are evenly distributed throughout the organ’s expanse.
During adaptive growth and shrinkage, in contrast, differentiating cells respond rapidly and acutely to signals that signify altered functional demand. In many cases, the differentiating cells’ response is the dominant contributor to changes in organ size.
Polyploidization of differentiating cells can be a major determinant of overall organ size. An individual cell’s ploidy, which can vary according to environmental and genetic factors, correlates tightly with its cytoplasmic volume, and some evidence suggests that overall organ size involves coordinated control of cell size (via ploidy) in addition to cell number. Such coordination might be achieved by a size-sensing mechanism that maintains a constant ratio of DNA to protein biomass at the organ scale, but the nature of such a hypothetical mechanism is unknown at present.
Apoptotic Elimination
Apoptotic elimination, the final stage of the cell life cycle, is also the least understood. This intractability is in part because eliminated cells leave no trace in the tissue and hence cannot be recorded via conventional lineage tracing or other retrospective approaches.
A sharp increase in apoptotic elimination drives organ shrinkage, with eliminated cells extruded into the lumen in most tubular epithelial organs. Yet remarkable variations exist. In mammary ducts, which are too thin to eliminate large quantities of extruded cells during involution, some cells transdifferentiate into phagocytes that clear their apoptotic siblings before themselves dying.
A major unresolved mystery is how specific cells are selected for apoptotic elimination during steady-state organ turnover. Proof-of-principle experiments have implicated cell competition, but the physiological basis of winner and loser cell designations is unknown.
Closing the Loop
Tissue homeostasis can be viewed as closing the loop on the cell life cycle at the scale of an organ.
Apoptotic cells destined for elimination send signals that instruct stem cells to divide. This relay links the death of one cell to the birth of another, which effectively closes the loop on the cell life cycle.
The dynamics of this feedback depend on how the stages of cell birth and elimination are spatially distributed. Short-range, limited duration signals are used when both stages are broadly dispersed and intermingle. Long-range, prolonged duration signals are used when cell birth and elimination occur in distant tissue regions.
How the kinetics of these feedback signals are calibrated for organ-scale homeostasis is unknown.
ACKNOWLEDGMENTS
I thank Erin Sanders, Adam Gries, Deborah Gordon, Jon-Michael Knapp, Shicong Xie, Claudia Vasquez, and members of the O’Brien lab for insightful discussions, and Jon-Michael Knapp for assistance in editing the manuscript. Research in the O’Brien lab is supported by US National Institutes of Health grants GM141885, DK128485, and OD028273 to L.E.O., and by Chan Zuckerberg Biohub grant DAF2020-217689 to L.E.O. L.E.O. is a Chan Zuckerberg Biohub Investigator.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Akhtar N, Li W, Mironov A, Streuli CH. 2016. Rac1 controls both the secretory function of the mammary gland and its remodeling for successive gestations. Dev. Cell 38(5):522–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldewachi HS, Wright NA, Appleton DR, Watson AJ. 1975. The effect of starvation and refeeding on cell population kinetics in the rat small bowel mucosa. J. Anat 119(Part 1):105–21 [PMC free article] [PubMed] [Google Scholar]
- Altmann GG. 1972. Influence of starvation and refeeding on mucosal size and epithelial renewal in the rat small intestine. Am. J. Anat 133(4):391–400 [DOI] [PubMed] [Google Scholar]
- Amcheslavsky A, Ito N, Jiang J, Ip YT. 2011. Tuberous sclerosis complex and Myc coordinate the growth and division of Drosophila intestinal stem cells. J. Cell Biol 193(4):695–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A, Lindblad JL, Bergmann A. 2020. Transiently “undead” enterocytes mediate homeostatic tissue turnover in the adult Drosophila midgut. Cell Rep. 33(8):108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen E, Ottesen J. 1945. Studies on the lymphocyte production. Investigations on the nucleic acid turnover in the lymphoid organs. Acta Physiol. Scand 10(3–4):258–70 [Google Scholar]
- Aragona M, Sifrim A, Malfait M, Song Y, Van Herck J, et al. 2020. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 584(7820):268–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EC, Kobielski S, Park J, Losick VP. 2021. Polyploidy in tissue repair and regeneration. Cold Spring Harb. Perspect. Biol 13:a040881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE. 2020. Emerging mechanisms of cell competition. Nat. Rev. Genet 21(11):683–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AJ, McCulloch EA, Till JE. 1963. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197(4866):452–54 [DOI] [PubMed] [Google Scholar]
- Benham-Pyle BW, Pruitt BL, Nelson WJ. 2015. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science 348(6238):1024–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzozero G 1892. Ueber die schlauchförmigen Drüsen des Magendarmkanals und die Beziehungen ihres Epithels zu dem Oberflächenepithel der Schleimhaut [On the tubular glands of the gastrointestinal tract and the relation of their epithelium to the surface epithelium of the mucous membrane]. Arch. Für Mikrosk. Anat 40(1):325–75 [Google Scholar]
- Bonfini A, Dobson AJ, Duneau D, Revah J, Liu X, et al. 2021. Multiscale analysis reveals that diet-dependent midgut plasticity emerges from alterations in both stem cell niche coupling and enterocyte size. eLife 10:e64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumard B, Bardin AJ. 2021. An amuse-bouche of stem cell regulation: underlying principles and mechanisms from adult Drosophila intestinal stem cells. Curr. Opin. Cell Biol 73:58–68 [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, Lemaitre B. 2010. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen TF, Forrest S, Campbell F, Dodson AR, Hershman MJ, et al. 2006. Characterization of epithelial cell shedding from human small intestine. Lab. Invest 86(10):1052–63 [DOI] [PubMed] [Google Scholar]
- Byrd KM, Piehl NC, Patel JH, Huh WJ, Sequeira I, et al. 2019. Heterogeneity within stratified epithelial stem cell populations maintains the oral mucosa in response to physiological stress. Cell Stem Cell 25(6):814–29.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanin L, Hoeckendorf B, Wittbrodt J. 2011. Fate restriction and multipotency in retinal stem cells. Cell Stem Cell 9(6):553–62 [DOI] [PubMed] [Google Scholar]
- Chen C-H, Luhur A, Sokol N. 2015. Lin-28 promotes symmetric stem cell division and drives adaptive growth in the adult Drosophila intestine. Development 142(20):3478–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Leblond CP. 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am. J. Anat 141(4):537–61 [DOI] [PubMed] [Google Scholar]
- Choi N-H, Lucchetta E, Ohlstein B. 2011. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. PNAS 108(46):18702–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RM. 1975. The time-course of changes in mucosal architecture and epithelial cell production and cell shedding in the small intestine of the rat fed after fasting. J. Anat 120(Part 2):321–27 [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Watt FM. 2018. Defining adult stem cells by function, not by phenotype. Annu. Rev. Biochem 87:1015–27 [DOI] [PubMed] [Google Scholar]
- Colom B, Alcolea MP, Piedrafita G, Hall MWJ, Wabik A, et al. 2020. Spatial competition shapes the dynamic mutational landscape of normal esophageal epithelium. Nat. Genet 52(6):604–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JM. 1997. Plato: Complete Works. Indianapolis, IN: Hackett Publ. Co. [Google Scholar]
- Dailey MJ. 2014. Nutrient-induced intestinal adaption and its effect in obesity. Physiol. Behav 136:74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson CA, Pal B, Vaillant F, Gandolfo LC, Liu Z, et al. 2020. Tissue-resident ductal macrophages survey the mammary epithelium and facilitate tissue remodelling. Nat. Cell Biol 22(5):546–58 [DOI] [PubMed] [Google Scholar]
- Dawson CA, Visvader JE. 2021. The cellular organization of the mammary gland: insights from microscopy. J. Mammary Gland Biol. Neoplasia 26(1):71–85 [DOI] [PubMed] [Google Scholar]
- Dekoninck S, Blanpain C. 2019. Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol 21(1):18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekoninck S, Hannezo E, Sifrim A, Miroshnikova YA, Aragona M, et al. 2020. Defining the design principles of skin epidermis postnatal growth. Cell 181(3):604–20.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Gerencser AA, Jasper H. 2015. Signal integration by Ca2+ regulates intestinal stem-cell activity. Nature 528(7581):212–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati G, Rognoni E, Hiratsuka T, Liakath-Ali K, Hoste E, et al. 2017. Wounding induces dedifferentiation of epidermal Gata6+ cells and acquisition of stem cell properties. Nat. Cell Biol 19(6):603–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupé DP, Marshall OJ, Dayton H, Brand AH, Perrimon N. 2018. Drosophila intestinal stem and progenitor cells are major sources and regulators of homeostatic niche signals. PNAS 115(48):12218–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray N, Mancini L, Binshtok U, Cheysson F, Supatto W, et al. 2021. Dynamic spatiotemporal coordination of neural stem cell fate decisions occurs through local feedback in the adult vertebrate brain. Cell Stem Cell 28(8):1457–72.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdowski. 2006. Intestinal mucosal adaptation. World J. Gastroenterol 12(29):4614–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhoffer GT,Loftus PD, Yoshigi M, Otsuna H, Chien C-B, et al. 2012. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484(7395):546–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foronda D, Weng R, Verma P, Chen Y-W, Cohen SM. 2014. Coordination of insulin and Notch pathway activities by microRNA miR-305 mediates adaptive homeostasis in the intestinal stem cells of the Drosophila gut. Genes Dev. 28(21):2421–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco JJ, Atieh Y, Bryan CD, Kwan KM, Eisenhoffer GT. 2019. Cellular crowding influences extrusion and proliferation to facilitate epithelial tissue repair. Mol. Biol. Cell 30(16):1890–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu NY, Rios AC, Pal B, Law CW, Jamieson P, et al. 2017. Identification of quiescent and spatially restricted mammary stem cells that are hormone responsive. Nat. Cell Biol 19(3):164–76 [DOI] [PubMed] [Google Scholar]
- Fukada S 2018. The roles of muscle stem cells in muscle injury, atrophy and hypertrophy. J. Biochem 163(5):353–58 [DOI] [PubMed] [Google Scholar]
- Ge Y, Fuchs E. 2018. Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat. Rev. Genet 19(5):311–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais L, Bardin AJ. 2017. Tissue homeostasis and aging: new insight from the fly intestine. Curr. Opin. Cell Biol 48:97–105 [DOI] [PubMed] [Google Scholar]
- Ghazavi F, Huysentruyt J, De Coninck J, Kourula S, Martens S, et al. 2022. Executioner caspases 3 and 7 are dispensable for intestinal epithelium turnover and homeostasis at steady state. PNAS 119(6):e2024508119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokoffski KK, Wu H-H, Beites CL, Kim J, Kim EJ, et al. 2011. Activin and GDF11 collaborate in feedback control of neuroepithelial stem cell proliferation and fate. Development 138(19):4131–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM. 2016. The evolution of the algorithms for collective behavior. Cell Sys. 3(6):514–20 [DOI] [PubMed] [Google Scholar]
- Gu Y, Forostyan T, Sabbadini R, Rosenblatt J. 2011. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J. Cell Biol 193(4):667–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, et al. 2017. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 543(7643):118–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. 2000. The Birth of the Cell. New Haven, CT: Yale Univ. Press [Google Scholar]
- He L, Si G, Huang J, Samuel ADT, Perrimon N. 2018. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature 555(7694):103–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston ML, Wong MH, Gordon JI. 1996. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev. 10(8):985–96 [DOI] [PubMed] [Google Scholar]
- Hsu Y-C, Li L, Fuchs E. 2014. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 157(4):935–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzkovitz S, Blat IC, Jacks T, Clevers H, van Oudenaarden A. 2012. Optimality in the development of intestinal crypts. Cell 148(3):608–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CM. 1925. The Effects of Inanition and Malnutrition Upon Growth and Structure. Philadelphia: P. Blakiston’s Son & Co. [Google Scholar]
- Jiang H, Edgar BA. 2009. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136(3):483–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Patel PH, Kohlmaier A, Pavlovic B, Zhang C, Edgar BA. 2017. Intestinal stem cell pool regulation in Drosophila. Stem Cell Rep. 8(6):1479–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuria S, Karpac J, Biteau B, Hwangbo D, Jasper H. 2012. Notch-mediated suppression of TSC2 expression regulates cell differentiation in the Drosophila intestinal stem cell lineage. PLOS Genet. 8(11):e1003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CFB, Jackson EL, Woolfenden AE, Lawrence S, Babar I, et al. 2005. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121(6):823–35 [DOI] [PubMed] [Google Scholar]
- Klein AM, Simons BD. 2011. Universal patterns of stem cell fate in cycling adult tissues. Development 138(15):3103–11 [DOI] [PubMed] [Google Scholar]
- Knopf F, Hammond C, Chekuru A, Kurth T, Hans S, et al. 2011. Bone regenerates via dedifferentiation of osteoblasts in the zebrafish fin. Dev. Cell 20(5):713–24 [DOI] [PubMed] [Google Scholar]
- Kolahgar G, Suijkerbuijk SJE, Kucinski I, Poirier EZ, Mansour S, et al. 2015. Cell competition modifies adult stem cell and tissue population dynamics in a JAK-STAT-dependent manner. Dev. Cell 34(3):297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krndija D, Marjou FE, Guirao B, Richon S, Leroy O, et al. 2019. Active cell migration is critical for steady-state epithelial turnover in the gut. Science 365(6454):705–10 [DOI] [PubMed] [Google Scholar]
- Ladoux B, Mège R-M. 2017. Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol 18(12):743–57 [DOI] [PubMed] [Google Scholar]
- Lander AD, Gokoffski KK, Wan FYM, Nie Q, Calof AL. 2009. Cell lineages and the logic of proliferative control. PLOS Biol. 7(1):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CP. 1981. The life history of cells in renewing systems. Am. J. Anat 160(2):114–58 [DOI] [PubMed] [Google Scholar]
- Leblond CP, Stevens CE. 1948. The constant renewal of the intestinal epithelium in the albino rat. Anat. Rec 100(3):357–77 [DOI] [PubMed] [Google Scholar]
- Leblond CP, Stevens CE, Bogoroch R. 1948. Histological localization of newly-formed desoxyribonucleic acid. Science 108(2811):531–33 [DOI] [PubMed] [Google Scholar]
- Leblond CP, Walker BE. 1956. Renewal of cell populations. Physiol. Rev 36(2):255–76 [DOI] [PubMed] [Google Scholar]
- Leedham SJ. 2020. Reserving the right to change the intestinal stem cell model. Cell Stem Cell 26(3):301–2 [DOI] [PubMed] [Google Scholar]
- Levayer R, Dupont C, Moreno E. 2016. Tissue crowding induces caspase-dependent competition for space. Curr. Biol 26(5):670–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Janssens J, De Waegeneer M, Kolluru SS, Kristofer D, et al. 2022. Fly Cell Atlas: a single-nucleus transcriptomic atlas of the adult fruit fly. Science 375(6584):eabk2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Balachandra S, Ngo S, O’Brien LE. 2017. Feedback regulation of steady-state epithelial turnover and organ size. Nature 548(7669):588–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippens S, Denecker G, Ovaere P, Vandenabeele P, Declercq W. 2005. Death penalty for keratinocytes: apoptosis versus cornification. Cell Death Differ. 12(2):1497–508 [DOI] [PubMed] [Google Scholar]
- Liu Q, Liu K, Cui G, Huang X, Yao S, et al. 2019. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat. Genet 51(4):728–38 [DOI] [PubMed] [Google Scholar]
- Loudhaief R, Brun-Barale A, Benguettat O, Nawrot-Esposito M-P, Pauron D, et al. 2017. Apoptosis restores cellular density by eliminating a physiologically or genetically induced excess of enterocytes in the Drosophila midgut. Development 144(5):808–19 [DOI] [PubMed] [Google Scholar]
- Marinari E, Mehonic A, Curran S, Gale J, Duke T, Baum B. 2012. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484(7395):542–45 [DOI] [PubMed] [Google Scholar]
- Martin JL, Sanders EN, Moreno-Roman P, Jaramillo Koyama LA, Balachandra S, et al. 2018. Long-term live imaging of the Drosophila adult midgut reveals real-time dynamics of division, differentiation and loss. eLife 7:e36248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Kadono-Maekubo N, Suzuki Y, Furuichi Y, Shiraga K, et al. 2021. A unique mode of keratinocyte death requires intracellular acidification. PNAS 118(17):e2020722118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meizlish ML, Franklin RA, Zhou X, Medzhitov R. 2021. Tissue homeostasis and inflammation. Annu. Rev. Immunol 39(1):557–81 [DOI] [PubMed] [Google Scholar]
- Mesa KR, Kawaguchi K, Cockburn K, Gonzalez D, Boucher J, et al. 2018. Homeostatic epidermal stem cell self-renewal is driven by local differentiation. Cell Stem Cell 23(5):677–86.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks J, Henson PM. 2009. Differentiation of the mammary epithelial cell during involution: implications for breast cancer. J. Mammary Gland Biol. Neoplasia 14(2):159–70 [DOI] [PubMed] [Google Scholar]
- Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. 2008. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol. Reprod 78(4):586–94 [DOI] [PubMed] [Google Scholar]
- Morioka S, Maueröder C, Ravichandran KS. 2019. Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity 50(5):1149–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach KA, Fry CS, Kirby TJ, Jackson JR, Lee JD, et al. 2018. Starring or supporting role? Satellite cells and skeletal muscle fiber size regulation. Physiology 33(1):26–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse YM, Savage AK, Marangoni P, Rosendahl-Huber AKM, Landman TA, et al. 2018. Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature 559(7712):109–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LE, Bilder D. 2013. Beyond the niche: tissue-level coordination of stem cell dynamics. Annu. Rev. Cell Dev. Biol 29:107–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LE, Soliman SS, Li X, Bilder D. 2011. Altered modes of stem cell division drive adaptive intestinal growth. Cell 147(3):603–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara TE, Colonna M, Stappenbeck TS. 2022. Adaptive differentiation promotes intestinal villus recovery. Dev. Cell 57(2):166–79.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa S, Vaughen J, Igaki T. 2018. Cell extrusion: a stress-responsive force for good or evil in epithelial homeostasis. Dev. Cell 44(3):284–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øvrebø JI, Edgar BA. 2018. Polyploidy in tissue homeostasis and regeneration. Development 145(14):dev156034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Saganta A, Tata PR, Law BM, Saez B, Chow RD-W, et al. 2015. Parent stem cells can serve as niches for their daughter cells. Nature 523(7562):597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellettieri J, Alvarado AS. 2007. Cell turnover and adult tissue homeostasis: from humans to planarians. Annu. Rev. Genet 41:83–105 [DOI] [PubMed] [Google Scholar]
- Pérez-González C, Ceada G, Greco F, Matejčić M, Gómez-González M, et al. 2021. Mechanical compart-mentalization of the intestinal organoid enables crypt folding and collective cell migration. Nat. Cell Biol 23(7):745–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J, Raff MC, Cramer LP. 2001. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol 11(23):1847–57 [DOI] [PubMed] [Google Scholar]
- Rulands S, Simons BD. 2017. Emergence and universality in the regulation of stem cell fate. Curr. Opin. Syst. Biol 5:57–62 [Google Scholar]
- Salwig I, Spitznagel B, Vazquez-Armendariz AI, Khalooghi K, Guenther S, et al. 2019. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 38(12):e102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacreu BJ, Romero DJ, Pescio LG, Tarallo E, Sterin-Speziale NB, Favale NO. 2021. Apoptotic cell extrusion depends on single-cell synthesis of sphingosine-1-phosphate by sphingosine kinase 2. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1866(4):158888. [DOI] [PubMed] [Google Scholar]
- Secor SM, Whang EE, Lane JS, Ashley SW, Diamond J. 2000. Luminal and systemic signals trigger intestinal adaptation in the juvenile python. Am. J. Physiol. Gastrointest. Liver Physiol 279(6):G1177–87 [DOI] [PubMed] [Google Scholar]
- Seldin L, Le Guelte A, Macara IG. 2017. Epithelial plasticity in the mammary gland. Curr. Opin. Cell Biol 49:59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Milo R. 2021. The distribution of cellular turnover in the human body. Nat. Med 27(1):45–48 [DOI] [PubMed] [Google Scholar]
- Shingleton AW. 2010. The regulation of organ size in Drosophila. Organogenesis 6(2):76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani RA, Clevers H, de Sauvage FJ. 2021. Tissue regeneration: reserve or reverse? Science 371(6531):784–86 [DOI] [PubMed] [Google Scholar]
- Siminovitch L, McCulloch EA, Till JE. 1963. The distribution of colony-forming cells among spleen colonies. J. Cell. Comp. Physiol 62(3):327–36 [DOI] [PubMed] [Google Scholar]
- Stolper J, Ambrosio EM, Danciu D-P, Buono L, Elliott DA, et al. 2019. Stem cell topography splits growth and homeostatic functions in the fish gill. eLife 8:e43747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suijkerbuijk SJE, Kolahgar G, Kucinski I, Piddini E. 2016. Cell competition drives the growth of intestinal adenomas in Drosophila. Curr. Biol 26(4):428–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TP. 1927. Histophysiological study of the epithelial changes in the small intestine of the albino mouse after starvation and refeeding. Anat. Rec 34(5):341–49 [Google Scholar]
- Tadokoro T, Gao X, Hong CC, Hotten D, Hogan BLM. 2016. BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Development 143(5):764–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamouna V, Panagi M, Theophanous A, Demosthenous M, Michail M, et al. 2020. Evidence of two types of balance between stem cell mitosis and enterocyte nucleus growth in the Drosophila midgut. Development 147(11):dev189472. [DOI] [PubMed] [Google Scholar]
- Tao L, van Bragt MPA, Li Z. 2015. A long-lived luminal subpopulation enriched with alveolar progenitors serves as cellular origin of heterogeneous mammary tumors. Stem Cell Rep. 5(1):60–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, et al. 2013. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503(7475):218–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Rajagopal J. 2016. Regulatory circuits and bi-directional signaling between stem cells and their progeny. Cell Stem Cell 19(6):686–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian A, Morejon V, Kohoutek S, Huang Y-C, Deng W-M, Jiang J. 2021. Re-entry into mitosis and regeneration of intestinal stem cells through enteroblast dedifferentiation in Drosophila midguts. bioRxiv 469515. 10.1101/2021.11.22.469515 [DOI] [Google Scholar]
- Tu S, Johnson SL. 2011. Fate restriction in the growing and regenerating zebrafish fin. Dev. Cell 20(5):725–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villars A, Levayer R. 2020. Cell extrusion: crowd pushing and sticky neighbours. Curr. Biol 30(4):R168–71 [DOI] [PubMed] [Google Scholar]
- Wagner K-U, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. 2002. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development 129(6):1377–86 [DOI] [PubMed] [Google Scholar]
- Wang F, Wang F, Zou Z, Liu D, Wang J, Su Y. 2011. Active deformation of apoptotic intestinal epithelial cells with adhesion-restricted polarity contributes to apoptotic clearance. Lab. Invest 91(3):462–71 [DOI] [PubMed] [Google Scholar]
- Wang Y, George SP, Roy S, Pham E, Esmaeilniakooshkghazi A, Khurana S. 2016. Both the anti- and proapoptotic functions of villin regulate cell turnover and intestinal homeostasis. Sci. Rep 6:35491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AJM, Duckworth CA, Guan Y, Montrose MH. 2009. Mechanisms of epithelial cell shedding in the mammalian intestine and maintenance of barrier function. Ann. N.Y. Acad. Sci 1165(1):135–42 [DOI] [PubMed] [Google Scholar]
- Wu H, Tang N. 2021. Stem cells in pulmonary alveolar regeneration. Development 148(2):dev193458. [DOI] [PubMed] [Google Scholar]
- Xie S, Swaffer M, Skotheim JM. 2022. Eukaryotic cell size control and its relation to biosynthesis and senescence. Annu. Rev. Cell Dev. Biol 38:291–319 [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, et al. 2012. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486(7404):490–95 [DOI] [PMC free article] [PubMed] [Google Scholar]