Abstract
BACKGROUND:
Previous studies have demonstrated an association between a diagnosis of cancer and the risk of suicide; however, they failed to account for psychiatric care before a cancer diagnosis, which may confound this relationship. The objective of this study was to assess the effect of a cancer diagnosis on the risk of suicide, accounting for prediagnosis psychiatric care utilization.
METHODS:
All adult residents of Ontario, Canada who were diagnosed with cancer (1 of prostate, breast, colorectal, melanoma, lung, bladder, endometrial, thyroid, kidney, or oral cancer) between 1997 and 2014 were identified. Noncancer controls were matched 4:1 based on sociodemographics, including a psychiatric utilization gradient (PUG) score (with 0 indicating none; 1, outpatient; 2, emergency department; and 3, hospital admission). A marginal, cause-specific hazard model was used to assess the effect of cancer on the risk of suicidal death.
RESULTS:
Among 676,470 patients with cancer and 2,152,682 matched noncancer controls, there were 8.2 and 11.4 suicides per 1000 person-years of follow-up, respectively. Patients with cancer had an overall higher risk of suicidal death compared with matched patients without cancer (hazard ratio, 1.34; 95% CI, 1.22–1.48). This effect was pronounced in the first 50 months after cancer diagnosis (hazard ratio, 1.60; 95% CI, 1.42–1.81); patients with cancer did not demonstrate an increased risk thereafter. Among individuals with a PUG score 0 or 1, those with cancer were significantly more likely to die of suicide compared with controls. There was no difference in suicide risk between patients with cancer and controls for those who had a PUG score of 2 or 3.
CONCLUSIONS:
A cancer diagnosis is associated with increased risk of death from suicide compared with the general population even after accounting for precancer diagnosis psychiatric care utilization. The specific factors underlying the observed associations remain to be elucidated.
Keywords: cancer, mental health, psychiatric utilization, psycho-oncology, suicide, suicidal death
INTRODUCTION
Globally, nearly 800,000 people die of suicide every year, accounting for 1.4% of deaths worldwide.1 Suicide deaths are a significant health burden, which is magnified when also considering suicidal ideation and suicide attempts.2,3 Furthermore, approximately 70% of suicides occurring in patients aged >60 years are associated with medical illness, with higher rates among patients who have cancer.4–26 Malignancies that are associated with a particularly high risk of suicidal death include head and neck cancers,4,8 bladder cancer,5,21,27 lung cancer,4,24–26 foregut cancers,4,6,15,19,26 and gynecologic malignancies.11,14
Previous studies have established that suicide rates are higher among patients who have cancer compared with the general population, with specific risk factors (arguably strongest to weakest) including male gender, advanced Cancer Diagnosis and Suicide Risk/Klaassen et al disease, Caucasian race, and unmarried status.5,6,15,21,25,26 Furthermore, a psychiatric comorbidity is a strong risk factor for suicidal death.28–30 However, to our knowledge, no studies to date have assessed the relationship of a cancer diagnosis and the risk of suicide while considering the role of a prior psychiatric diagnosis. Furthermore, the existing literature has not assessed the manner in which suicide risk may change over time after a cancer diagnosis. As such, the objective of this study was to assess the effect of cancer on suicidal death using direct matching to noncancer controls and accounting for precancer diagnosis psychiatric care utilization.
MATERIALS AND METHODS
We conducted a population-based, retrospective, matched cohort study in Ontario, Canada to assess the hypothesis that patients diagnosed with cancer are at increased risk of suicide compared with the general population, even after accounting for precancer diagnosis utilization of psychiatric resources. We identified all adults diagnosed with any 1 of the 10 most prevalent malignancies (prostate, breast, colorectal, melanoma, lung, bladder, endometrial, thyroid, kidney, oral) in Canada between January 1997 and December 2014 and matched controls from the general population using data from the Institute of Clinical Evaluative Sciences (ICES). In Ontario, essential medical care is reimbursed by a single, government-operated health insurance system (Ontario Health Insurance Plan).
This study was designed and conducted according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines31 and the Reporting of Studies Conducted Using Observational Routinely Collected Health Data Statement.32 The University of Toronto Research Ethics Board approved this study.
The following data sets were linked using unique encoded identifiers and analyzed at ICES: the Canadian Institute for Health Information Discharge Abstract Database, which contains records for all acute care hospitalizations33; the Canadian Institute for Health Information National Ambulatory Care Reporting System, which contains records for emergency department visits; the Ontario Mental Health Reporting System, which contains records for psychiatric hospital admissions; the Ontario Health Insurance Plan database, which tracks physician billings and out-of-province providers (physicians, allied health, and hospitals)34; the Ontario Cancer Registry (OCR), a population-based registry estimated to be greater than 95% complete for cancer diagnoses35; the Registered Persons database for demographic information36; and the Office of the Registrar General-Deaths, Vital Statistics database, to identify suicidal death. This database has been validated by coroner-confirmed suicides to capture 95.9% to 98.8% of suicides in Ontario.37 These data were accessed through Data Use Agreement (DUA) 2016–077 and approved by the University of Toronto Institutional Review Board (Protocol reference 34852).
Identification of Patients With Cancer
All residents of Ontario aged ≥18 years with 1 of the aforementioned malignancies during the study interval (1997–2014) were identified in the OCR. Patients were considered eligible if their first cancer diagnosis was 1 of the 10 evaluated. Among 684,147 patients identified, we excluded 7677 for zero or negative follow-up days (incorrectly coded in the database as <0 follow-up days; ie, −5 follow-up days) or cancer stage 0, for a final cohort of 676,470 patients with cancer.
Demographic information collected included age at cancer diagnosis (continuous), sex, socioeconomic status (operationalized as a quintile of the median neighborhood income), geographical region (operationalized by 14 health regions within Ontario), rurality (yes vs no), year of diagnosis (by tertiles), and general comorbidity (Johns Hopkins Aggregate Disease Group [ADG]38), operationalized as low (≤5), intermediate (6–9), and high (≥10). The Johns Hopkins ADG is based on previous health care use and has better discrimination than the Charlson score in comorbidity assessment.39
Psychiatric utilization during the 5 years before cancer diagnosis was operationalized categorically. Specifically, the psychiatric utilization gradient (PUG) score was defined as follows: 0 indicates no psychiatric utilization; 1, outpatient psychiatric care (physician office visits with a diagnosis of depression, schizophrenia, bipolar disorder, etc); 2, emergency department visit for psychiatric care; and 3, hospital admission for psychiatric care. Patients received a PUG score based on their highest level of psychiatric utilization, resulting in mutually exclusive exposure categories: ie, a patient receiving outpatient psychiatric care who subsequently was admitted to the hospital for psychiatric care during the 5-year observation period was assigned a PUG score of 3. Levels of psychiatric utilization used to generate the PUG score were identified using a combination of Ontario Health Insurance Plan outpatient and hospital billing codes.
Identification of the General Population Control Group
For each patient with cancer, we identified 4 noncancer controls from the general population based on a hard match comprising age (±1 year), sex, income quintile, geographic region, PUG score, and ADG comorbidity score. When a suitable match could not be found, fewer than the planned 4 controls were used. We assigned each patient in the control group an index date, which corresponded to the matched patient’s date of cancer diagnosis.
Outcome
The outcome of interest was suicidal death.37 The final day of follow-up was December 31, 2014, because each suicidal death is thoroughly investigated and verified before incorporation into the population data set, thus resulting in a lag time for this variable.
Statistical Analysis
Patients’ demographic and clinical characteristics were compared after stratification by cancer diagnosis. Continuous variables were summarized using medians and interquartile ranges (IQRs), and categorical variables were reported as counts and proportions. Given the large sample size, traditional statistical measures are likely to demonstrate statistically significant differences even when no clinical difference exists. Therefore, we performed between-group comparisons using weighted, standardized differences (the difference in the mean of a variable between 2 groups divided by an estimate of the standard deviation of that variable among both groups).40 A clinically meaningful standardized difference was defined as >0.10.40 To assess the association between cancer diagnosis and suicidal death while accounting for other causes of mortality, we used a marginal cause-specific hazard model.41,42 Robust standard errors were used to account for the nonindependence of matched data. The matching identifier was used as a clustering variable. To assess for residual operational confounding (after matching) of the relationship between cancer diagnosis and suicidal death by baseline characteristics, each of the characteristics was individually added to the model to assess the change in the estimate of the association between cancer diagnosis and suicidal death (conditional cause-specific hazard model).43,44 A change >10% in the hazard ratio (HR) for the primary exposure (cancer diagnosis vs control) was deemed to represent meaningful confounding.45 For the primary analysis, we performed a post hoc, time-specific analysis using the marginal cause-specific hazard model to assess the manner in which suicide risk may change over time after a cancer diagnosis. The following time points in follow-up were used: 1 to 50, 51 to 100, 101 to 150, and >150 months.
Subgroup and Sensitivity Analyses
We conducted several preplanned subgroup analyses to identify covariates that modified the effects of risk factors for suicidal death. First, to assess the impact of intensity of psychiatric utilization before cancer diagnosis, a marginal cause-specific hazard model was used to assess the impact of cancer diagnosis stratified within each PUG score cohort. Second, considering that the risk of suicidal death may be affected by disease stage and complete stage information is available in the OCR only since 2007 (TNM staging system according to the American Joint Committee on Cancer Staging Manual, 7th edition; n = 111,620), we stratified patients with cancer according to disease stage. We then examined the relationship between cancer diagnosis and the risk of suicidal death by comparing patients within each stratum versus matched individuals from the general population without cancer (noncancer controls). Third, the risk of suicidal death was individually tested within each individual anatomic malignancy site. All analyses were marginal cause-specific hazard models, clustered for the matching variable. Finally, given the risk of bias because of unmeasured confounders in retrospective analyses, we quantified the prevalence and strength of association necessary for a potential, unmeasured, binary confounder to fully explain the observed differences between patients with cancer and controls using the technique of Lin et al.46 That is, if there was truly no difference in suicidal death, we examined what differential prevalence and strength of association would be necessary for a residual confounder to have produced the observed results.
Statistical significance was set at P < .05 based on 2-tailed comparison. All analyses were performed using SAS version 9.4 (SAS Institute Inc).
RESULTS
We identified 676,470 eligible patients with cancer and 2,152,682 matched noncancer controls. Matching resulted in comparable cohorts (Table 1). Patients with cancer were equally distributed with regard to sex (men, 50.3%), and nearly one-half of the patients had a PUG score of 1 (45.1%) before cancer diagnosis. There were 8.2 suicides per 1000 person-years of follow-up among patients with cancer and 11.4 suicides per 1000 person-years of follow-up for noncancer controls. Among patients with cancer, suicide incidence increased by year tertiles (1997–2002, 0.038%; 2003–2008, 0.082%; 2009–2014, 0.115%). Suicide mortality cumulative incidence did not differ significantly when stratified by cancer and noncancer controls (Fig. 1) (Gray test for equality of cumulative incidence functions; P = .57). Among patients who died of suicide, the median time to suicide was 31.8 months (IQR, 8.9–70.1 months) for patients with cancer compared with 51.0 months (IQR, 22.2–90.5 months) for controls (P < .0001).
TABLE 1.
Baseline Characteristics of Patients Stratified by Cancer Group and Noncancer Control Group
| No. of Patients (%) |
|||
|---|---|---|---|
| Characteristic | With Cancer | Noncancer Controls | Standardized Difference |
| Sample size | 676,470 | 2,152,682 | |
| 0.13 | |||
| Age at diagnosis: Median [IQR], y | 67 [57–75] | 63 [54–73] | 0.13 |
| Sex | 0.00 | ||
| Men | 340,565 (50.3) | 931,084 (43.2) | |
| Women | 335,905 (49.7) | 1,221,598 (56.8) | |
| PUG score | 0.00 | ||
| 0 | 359,597 (53.2) | 1,136,216 (52.8) | |
| 1 | 304,766 (45.1) | 980,569 (45.6) | |
| 2 | 7906 (1.2) | 23,849 (1.1) | |
| 3 | 4201 (0.6) | 12,048 (0.6) | |
| Income quintile | 0.00 | ||
| 1: Lowest | 126,202 (18.9) | 391,460 (18.2) | |
| 2 | 137,253 (20.3) | 431,331 (20.0) | |
| 3 | 133,738 (19.8) | 428,001 (19.9) | |
| 4 | 136,641 (20.2) | 441,357 (20.5) | |
| 5: Highest | 142,636 (21.1) | 460,533 (21.4) | |
| Comorbidity: ADG category | 0.00 | ||
| Low | 211,952 (31.3) | 820,558 (38.1) | |
| Intermediate | 285,094 (42.1) | 886,782 (41.2) | |
| High | 179,424 (26.5) | 445,342 (20.7) | |
| Rurality | 0.00 | ||
| Yes | 100,592 (14.9) | 300,829 (14.0) | |
| Geographic region: Local health integration network | 0.00 | ||
| Erie St Clair | 39,230 (5.8) | 119,999 (5.6) | |
| South West | 56,754 (8.4) | 173,857 (8.1) | |
| Waterloo Wellington | 34,548 (5.1) | 111,955 (5.2) | |
| Hamilton Niagara Haldimand Brant | 83,664 (12.4) | 257,585 (12.0) | |
| Central West | 28,532 (4.2) | 97,523 (4.5) | |
| Mississauga Halton | 47,490 (7.0) | 159,081 (7.4) | |
| Toronto Central | 57,688 (8.5) | 188,110 (8.7) | |
| Central | 74,040 (11.0) | 249,033 (11.6) | |
| Central East | 79,202 (11.7) | 255,541 (11.9) | |
| South East | 32,349 (4.8) | 100,433 (4.7) | |
| Champlain | 63,154 (9.3) | 200,877 (9.3) | |
| North Simcoe Muskoka | 27,066 (4.0) | 80,737 (3.8) | |
| North East | 39,263 (5.8) | 117,325 (5.5) | |
| North West | 13,490 (2.0) | 40,626 (1.9) | |
| Year of diagnosis | 0.08 | ||
| 1997–2002 | 197,412 (29.2) | 551,453 (25.6) | |
| 2003–2008 | 226,975 (33.6) | 701,828 (32.6) | |
| 2009–2014 | 252,083 (37.3) | 899,401 (41.8) | |
Abbreviations: ADG, Aggregated Diagnosis Group; IQR, interquartile range; PUG, psychiatric utilization gradient.
Figure 1.
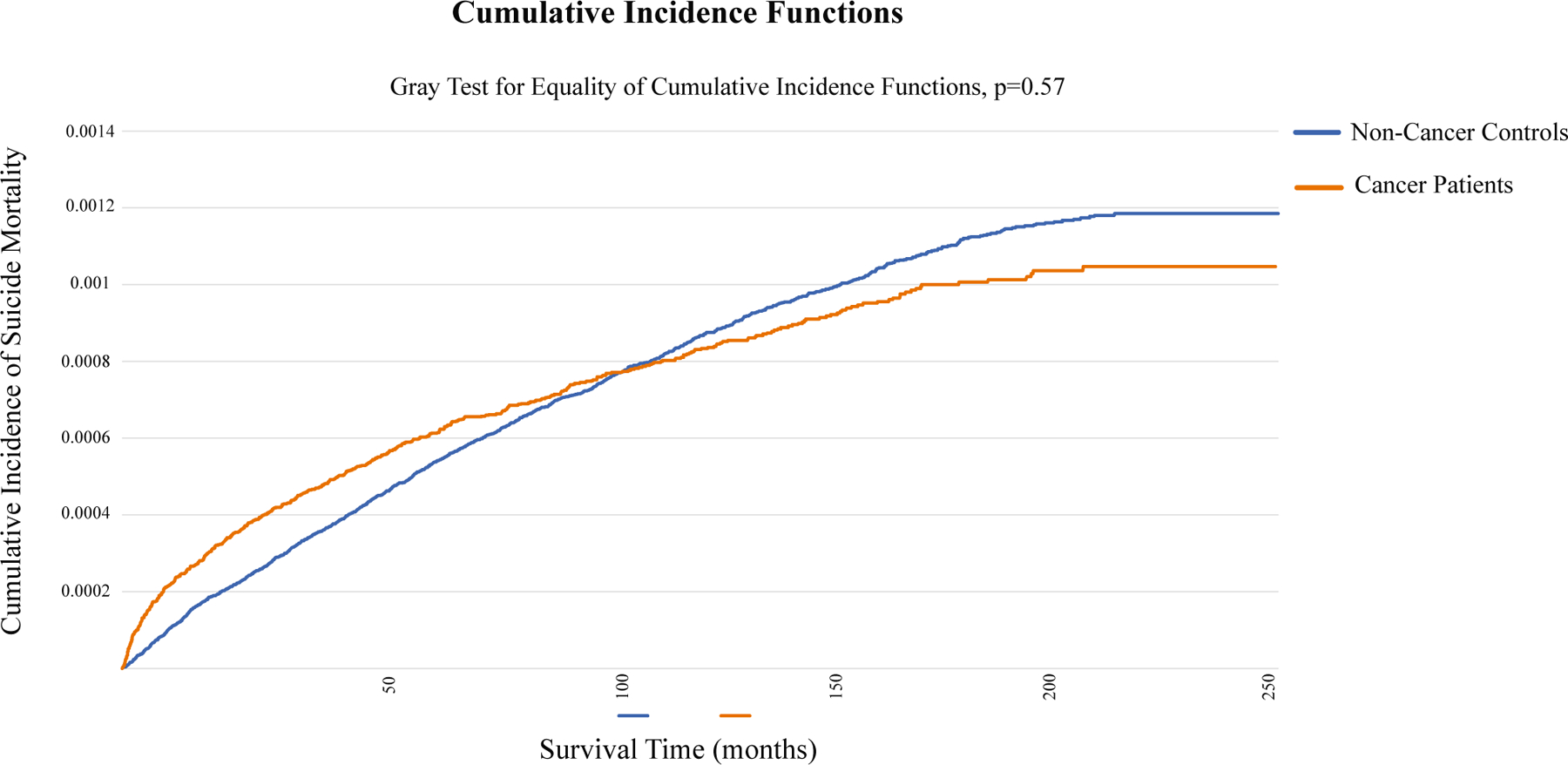
This is a cumulative incidence function plot of suicidal death stratified by patients with cancer and noncancer controls.
Primary Outcome Analysis
The 1-year, 2-year, 5-year, and 10-year suicide-specific survival probabilities for patients with cancer andnoncancer controls stratified by PUG score are listed in Supporting Table 1. In competing-risk survival analyses accounting for the correlation of matched data, patients with cancer were more likely to die of suicide than those without cancer (HR, 1.34; 95% CI, 1.22–1.48). When each demographic characteristic was included in a bivariate model, there was no evidence of operational confounding after matching (Table 2). Considering that the cumulative incidence of suicide mortality did not differ significantly between patients with and without cancer (Fig. 1), we performed a post hoc, time-specific analysis to further assess this relationship. Because the curves crossed at approximately 100 months’ survival time, the time intervals selected were 1 to 50, 51 to 100, 101 to 150, and >150 months of survival time. We found that cancer significantly increased suicide risk by 60% (HR, 1.60; 95% CI, 1.42–1.81) in the first 50 months of survival time, whereas, with additional time, this effect was temporized (Supporting Table 2).
TABLE 2.
Estimates of the Association Between Cancer Diagnosis and Suicidal Death in Bivariate Models to Identify Potential Operational Confounders
| Patients With Cancer vs Noncancer Controls |
||
|---|---|---|
| Potential Confounder | HR | 95% CI |
| Primary analysis | 1.34 | 1.22–1.48 |
| Age | 1.34 | 1.22–1.48 |
| Sex | 1.25 | 1.13–1.37 |
| PUG score | 1.35 | 1.22–1.48 |
| Income quintile | 1.35 | 1.23–1.48 |
| ADG comorbidity score | 1.31 | 1.19–1.44 |
| Rurality | 1.34 | 1.22–1.47 |
| Geographic region | 1.34 | 1.22–1.48 |
| Year of diagnosis | 1.32 | 1.20–1.45 |
Abbreviations: ADG, Aggregated Diagnosis Group; HR, hazard ratio; PUG, psychiatric utilization gradient.
Subgroup Analyses
Stratified by PUG score, a significant association between cancer diagnosis and suicidal death was identified among individuals with PUG scores of 0 or 1 but not those with PUG scores of 2 or 3 (Fig. 2).
Figure 2.
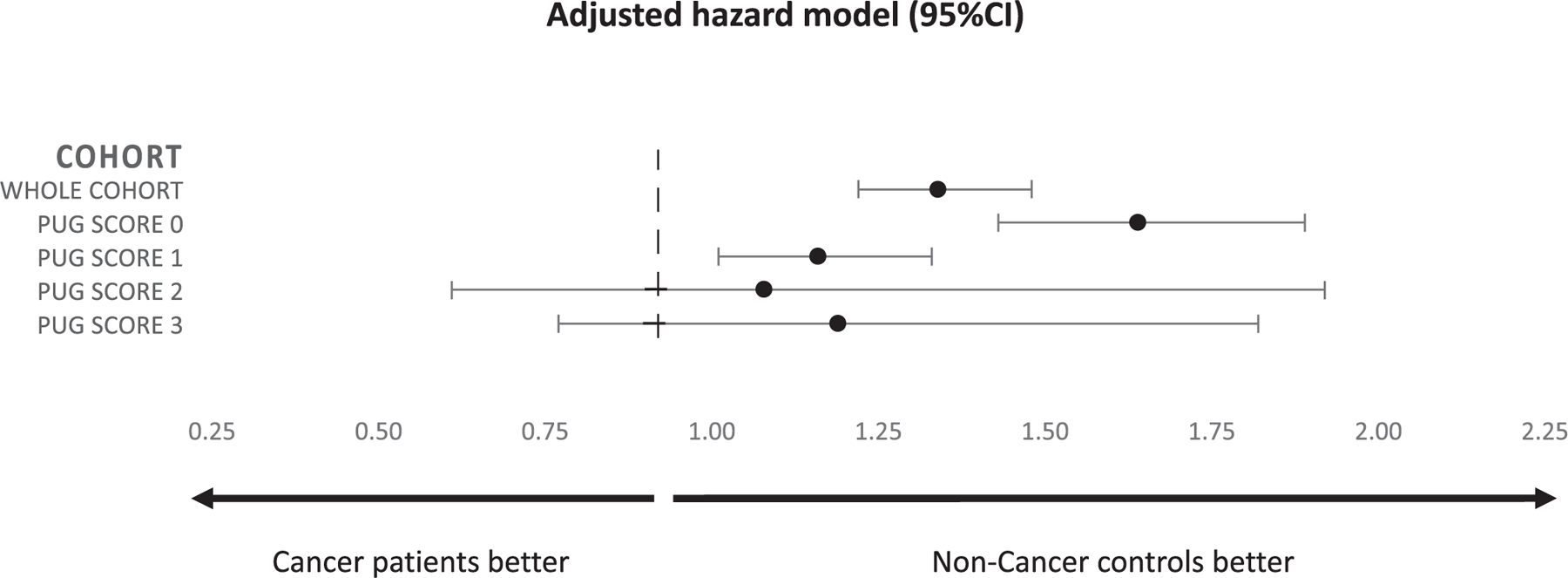
This forest plot demonstrates the association between cancer diagnosis and suicidal death stratified by prior psychiatric care utilization (PUG) score.
Among patients with cancer who had stage data available and their respective controls (n = 457,924), those with cancer were at increased risk of suicidal death compared with noncancer controls (HR, 1.59; 95% CI, 1.26–2.01). Increasing stage was associated with an increased risk of suicidal death compared with stage I (stage II: HR, 1.91 [95% CI, 1.09–3.34]; stage III: HR, 1.48 [95% CI, 0.66–3.03]; stage IV: HR, 5.38 [95% CI, 3.16–9.17]). In analyses stratified by stage, a significant association between cancer diagnosis and suicidal death was only seen in patients who had stage IV disease compared with their noncancer controls (Supporting Table 3).
Examining each anatomic malignancy site, patients with lung cancer (HR, 2.49; 95% CI, 1.98–3.13), colorectal cancer (HR, 1.58; 95% CI, 1.27–1.96), bladder cancer (HR, 1.73; 95% CI; 1.14–2.62), and oral cancer (HR, 2.55; 95% CI, 1.59–4.12) were at significantly higher risk of suicidal death compared with noncancer controls (Table 3). We failed to demonstrate a statistically increased risk in patients with prostate, breast, melanoma, endometrial, and kidney cancer.
TABLE 3.
Association Between Cancer Diagnosis and Suicidal Death Stratified by Anatomic Cancer Site
| No. of Patients |
||||
|---|---|---|---|---|
| Anatomic Site | With Cancer | Noncancer Controls | Total No. of Suicidal Events | With Cancer vs Noncancer Controls: HR (95% CI) |
| Prostate | 137,734 | 376,885 | 633 | 1.07 (0.90–1.27) |
| Breast | 131,648 | 486,734 | 259 | 1.15 (0.85–1.54) |
| Lung | 123,053 | 359,222 | 421 | 2.49 (1.98–3.13) |
| Colorectal | 119,241 | 364,599 | 410 | 1.58 (1.27–1.96) |
| Melanoma | 41,710 | 137,555 | 148 | 1.06 (0.71–1.57) |
| Thyroid | 30,555 | 115,580 | 100 | 0.42 (0.21–0.84) |
| Bladder | 29,897 | 77,883 | 99 | 1.73 (1.14–2.62) |
| Endometrial | 28,349 | 104,374 | 42 | 0.89 (0.39–2.01) |
| Kidney | 23,503 | 73,619 | 104 | 1.26 (0.79–2.02) |
| Oral | 10,780 | 35,188 | 74 | 2.55 (1.59–4.12) |
Abbreviation: HR, hazard ratio.
Finally, to assess potential residual confounding, we conducted a sensitivity analysis to quantify the magnitude of effect necessary for a confounder to account for the observed effect of cancer compared with noncancer controls on suicidal death across a wide spectrum of confounder prevalence. A hypothetical confounder present at 20% in patients with cancer would have to have an HR of 3.5 if it was present in 40% of patients without noncancer (HR, 2.5 if present in 50%, 2.0 if present in 60%, 1.7 if present in 70%, etc) to nullify the impact of cancer on suicidal death (Supporting Fig. 1). As such, any hypothetical residual confounder would have to be both strongly associated with suicidal death (HRs in excess of 2.0) and have highly differential prevalence to annul the observed effect.
DISCUSSION
In this population-based, matched cohort study accounting for prediagnosis psychiatric care utilization, patients with prevalent malignancies had a significantly increased risk of suicidal death, particularly within the first 50 months after diagnosis. This effect persisted when accounting for oncologic stage and was driven primarily by patients who had minimal prediagnosis psychiatric morbidity and those with advanced malignancies.
Previous population-level analyses, particularly those using the Surveillance, Epidemiology, and End Results database,4,5,7,8,11,12,14,15,19–27 have shown that patients across several disease sites have an increased risk of suicidal death. However, these analyses are unable to account for psychiatric comorbidity, an important confounder. With the well established predilection for suicide among psychiatric patients and the poor cancer outcomes experienced by psychiatric patients,42,47,48 psychiatric health service utilization, as a surrogate for severity of mental illness, is important to account for when investigating mental health outcomes in patients with cancer. Our finding of a differential effect of cancer diagnosis on the risk of suicidal death based on the prediagnosis PUG score highlights the importance of using such a framework.
Although noncancer controls had a greater number of suicides per 1000 person-years of follow-up (11.4 vs 8.2 per 1000 person-years of follow-up) compared with patients who had cancer, the median time to suicide was much shorter for patients who had cancer compared with noncancer controls (median, 31.8 vs 51.0 months). As such, we found an increased risk of suicide among patients who had cancer compared with noncancer controls using a time-to-event analysis (HR, 1.34), with prediagnosis (within 5 years) psychiatric care utilization included in the matching criteria, suggesting that, even when accounting for previous psychiatric comorbidities, a cancer diagnosis portends an increased risk of suicidal death. Up until approximately 100 months, patients with cancer have an increased cumulative incidence of suicide mortality compared with noncancer controls, at which point the curves cross and patients with cancer are at less risk (Fig. 1). By using time-specific analyses to further assess this relationship, we found that patients with cancer were particularly at risk for suicidal death (HR, 1.60; 95% CI, 1.42–1.81) in the first 50 months after diagnosis. We found no evidence of increased risk thereafter. A recently published Surveillance, Epidemiology, and End Results study also found that patients with cancer were especially at risk of suicide within 12 months of diagnosis compared with the general population, particularly for those who had pancreatic and lung cancer.26 We hypothesize that, during those first 50 months, the burden of a cancer diagnosis, treatment, surveillance, etc, places patients with cancer at increased risk of suicide. After this time point, one could surmise that patients with cancer are at no additional risk because either 1) they are out of the phase of increased suicidal risk secondary to cancer-related effects, 2) they have died of cancer, or 3) they have undergone psychologic adjustment as a result of surviving cancer for a prolonged time such that they have acquired resilience/protection, which may protect against subsequent suicide.
Stratified according to the severity of prediagnosis psychiatric utilization, a significant association between cancer diagnosis and suicidal mortality was identified only among patients who had no prediagnosis psychiatric utilization and those who saw an outpatient psychiatrist (PUG score, 0 and 1). In contrast, those with more intense prediagnosis psychiatric utilization experienced no increased risk of suicide after diagnosis, suggesting that their risk of suicide is driven by their psychiatric disease, not their cancer diagnosis. In other words, these data suggest that the impact of a cancer diagnosis increases or invokes suicidal tendencies more prominently in patients at lower risk of suicide, likely because those at highest risk (PUG score, 2 or 3) are already at an elevated suicide risk level. As a corollary, we have identified a risk group (PUG score, 0 or 1) in whom targeted interventions may be directed to prevent suicide after a cancer diagnosis.
A particular at-risk population identified in previous studies are patients with advanced/metastatic disease.5,14,15,21,23,24,26,27 Stratifying by cancer stage, we found that a cancer diagnosis portended an increased risk of suicidal death compared with controls, supporting the association found in the overall cohort. Furthermore, when stratifying by cancer stage, patients with stage IV cancer had an increased risk of suicidal death (HR, 4.41; 95% CI, 3.05–6.33) compared with controls. Indeed, in a study of young patients (aged 20–40 years) with incurable or metastatic cancer, 22.6% of patients screened positive for suicidal ideation, and patients suffering from major depressive disorder on antidepressant medications had a 7-fold increased odds of suicidal ideation.49 Taken together, patients with advanced-stage cancer are likely to be depressed, to have suicidal ideation, and to be at significantly increased risk of suicidal death.
Assessing specific tumor sites, this study confirms previous reports suggesting increased suicidal risk among patients with lung,4,24–26 bladder,5,21,27 and head and neck cancers.4,8 There are several possible explanations for this particularly high suicidal risk. First, surgical treatment for head and neck cancer (extirpation with or without extensive neck lymphadenectomy) and bladder cancer (radical cystectomy with urinary diversion) is associated with significant morbidity and body dysmorphia. Certainly, this may lead to depression, decreased self-esteem, and suicidal ideation.50 Second, these 3 malignancies are all associated with cigarette smoking, which is an independent risk factor for suicide51–53; in addition, alcohol is a risk factor for head and neck cancer and suicide.54 Third, these malignancies are generally associated with advanced-stage disease that has poor survival, thus placing these patients at increased risk of suicidal death as we and others have shown.
There are several ways that members of the health care team can potentially decrease rates of suicidal death among patients with cancer. All patients with cancer should be routinely screened for distress, depression, and suicidal ideation and appropriately referred for urgent psychologic/psychiatric evaluation, as necessary. The National Comprehensive Cancer Network offers distress guidelines and provides a distress thermometer tool to identify at-risk patients.55 Among patients with prostate cancer (who are susceptible to developing long-term depression56), screening for depression and erectile dysfunction, in addition to suicidal ideation, has also been suggested.57 Second, particularly high-risk groups should be offered counseling or psychiatric referral regardless of suicidal ideation, in addition to smoking-cessation assistance when necessary. Third, patients with suicidal ideation should maintain a close alliance with their oncology team while also undergoing a complete psychiatric evaluation. Patients with advanced cancer who maintain a strong therapeutic alliance with their oncologist have better protection against suicidal ideation than other mental health interventions, including psychotropic medications.49 In addition, there is a benefit to ancillary services, such as lymphedema and stoma clinics, in providing assistance to patients who are coping with changes in body image after treatment. Ultimately, prospective trials evaluating the value of suicide-prevention interventions in high-risk individuals will help to further delineate the management of these patients.
To our knowledge, this is the first population-based study to account for prediagnosis psychiatric care utilization while assessing the effect of a cancer diagnosis on suicidal death among patients with prevalent solid-organ malignancies. In addition, the universal coverage and systematic tracking of cancer diagnoses and relevant health services in Ontario allows for uniquely generalizable results, providing a representative spectrum of oncologic care delivered in Canada’s largest province over nearly 20 years. Third, the database for identifying suicidal death in Ontario is validated by coroner-confirmed suicides to capture from 95.9% to 98.8% of suicides.37
However, there are limitations. First, the ICES databases do not reliably capture oncology treatment, thus the effect of treatment was not accounted for when assessing the risk of suicide. Furthermore, complete stage data were not available. However, given the persistent effect of a cancer diagnosis on suicidal death when limiting analyses to patients with available stage data, a significant impact of treatment and complete stage data on outcomes is unlikely. Second, smoking is a known predictor of suicidal death51–53; however, it is not reliably recorded in ICES databases. Recent data have shown that the addition of these variables to the rich health administrative data in Ontario does not significantly change risk estimates, even for conditions that are known to be associated with smoking.58,59 Third, many factors that may influence suicidal risk are not available in the ICES databases. These include side effects of treatment, treatment effect (ie, response), psychological and physical symptoms, economic effect, family support, and education level. Finally, although we performed several sensitivity analyses to enhance the robustness of the results, there is a possibility of residual confounding. However, we identified that, to nullify the observed association between cancer diagnosis and suicidal death, such a confounder would need to be both strongly associated with suicidal death and have highly differential prevalence. Thus, the existence of such a confounder is unlikely, although not impossible. Finally, data on concomitant psychiatric medications are not available for our patient cohort, thus limiting our ability to account for psychiatric treatment effects.
Conclusion
Among adults with prevalent solid-organ malignancies, cancer is associated with an increased risk of suicide compared with the general population when accounting for precancer diagnosis psychiatric care utilization, particularly within the first 50 months after diagnosis. The specific factors underlying the observed associations remain to be elucidated and should be assessed in subsequent studies.
Supplementary Material
FUNDING SUPPORT
This study was funded by a Canadian Urological Association-Canadian Urologic Oncology Group-Astellas grant and by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care.
Footnotes
We thank Simon Chen and Ruth Croxford for their assistance with data acquisition and study design, respectively.
The opinions, results, and conclusions reported herein are those of the authors and are independent from the funding sources, and no endorsement by the Institute of Clinical Evaluative Sciences or the Ontario Ministry of Health and Long-Term Care is intended or should be concluded.
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.World Health Organization. Suicide Data Available at: www.who.int/mental_health/prevention/suicide/suicideprevent/en. Accessed March 15, 2018.
- 2.Zalsman G, Hawton K, Wasserman D, et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry 2016;3:646–659. [DOI] [PubMed] [Google Scholar]
- 3.Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA 2005;294:2064–2074. [DOI] [PubMed] [Google Scholar]
- 4.Misono S, Weiss NS, Fann JR, Redman M, Yueh B. Incidence of suicide in persons with cancer. J Clin Oncol 2008;26:4731–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klaassen Z, Jen RP, DiBianco JM, et al. Factors associated with suicide in patients with genitourinary malignancies. Cancer 2015; 121:1864–1872. [DOI] [PubMed] [Google Scholar]
- 6.Sugawara A, Kunieda E. Suicide in patients with gastric cancer: a population-based study. Jpn J Clin Oncol 2016;46:850–855. [DOI] [PubMed] [Google Scholar]
- 7.Dalela D, Krishna N, Okwara J, et al. Suicide and accidental deaths among patients with non-metastatic prostate cancer. BJU Int 2016;118:286–297. [DOI] [PubMed] [Google Scholar]
- 8.Kam D, Salib A, Gorgy G, et al. Incidence of suicide in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg 2015;141:1075–1081. [DOI] [PubMed] [Google Scholar]
- 9.Hultcrantz M, Svensson T, Derolf AR, et al. Incidence and risk factors for suicide and attempted suicide following a diagnosis of hematological malignancy. Cancer Med 2015;4:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsson S, Sandin F, Fall K, et al. Risk of suicide in men with low-risk prostate cancer. Eur J Cancer 2013;49:1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward KK, Roncancio AM, Plaxe SC. Women with gynecologic malignancies have a greater incidence of suicide than women with other cancer types. Suicide Life Threat Behav 2013;43:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alanee S, Russo P. Suicide in men with testis cancer. Eur J Cancer Care (Engl) 2012;21:817–821. [DOI] [PubMed] [Google Scholar]
- 13.Fang F, Fall K, Mittleman MA, et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med 2012;366:1310–1318. [DOI] [PubMed] [Google Scholar]
- 14.Mahdi H, Swensen RE, Munkarah AR, et al. Suicide in women with gynecologic cancer. Gynecol Oncol 2011;122:344–349. [DOI] [PubMed] [Google Scholar]
- 15.Turaga KK, Malafa MP, Jacobsen PB, Schell MJ, Sarr MG. Suicide in patients with pancreatic cancer. Cancer 2011;117:642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bill-Axelson A, Garmo H, Lambe M, et al. Suicide risk in men with prostate-specific antigen-detected early prostate cancer: a nationwide population-based cohort study from PCBaSe Sweden. Eur Urol 2010;57:390–395. [DOI] [PubMed] [Google Scholar]
- 17.Schairer C, Brown LM, Chen BE, et al. Suicide after breast cancer: an international population-based study of 723,810 women. J Natl Cancer Inst 2006;98:1416–1419. [DOI] [PubMed] [Google Scholar]
- 18.Muff Christensen ML, Yousaf U, Engholm G, Storm HH. Increased suicide risk among Danish women with non-melanoma skin cancer, 1971–1999. Eur J Cancer Prev 2006;15:266–268. [DOI] [PubMed] [Google Scholar]
- 19.Bowden MB, Walsh NJ, Jones AJ, Talukder AM, Lawson AG, Kruse EJ. Demographic and clinical factors associated with suicide in gastric cancer in the United States. J Gastrointest Oncol 2017;8:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson WG, Klaassen Z, Jen RP, Hughes WMT, Neal DE Jr, Terris MK. Analysis of suicide risk in patients with penile cancer and review of the literature. Clin Genitourin Cancer 2018;16:e257–e261. [DOI] [PubMed] [Google Scholar]
- 21.Klaassen Z, Goldberg H, Chandrasekar T, et al. Changing trends for suicidal death in patients with bladder cancer: a 40+ year population-level analysis. Clin Genitourin Cancer 2018;16:206–212.e1. [DOI] [PubMed] [Google Scholar]
- 22.Fang F, Keating NL, Mucci LA, et al. Immediate risk of suicide and cardiovascular death after a prostate cancer diagnosis: cohort study in the United States. J Natl Cancer Inst 2010;102:307–314. [DOI] [PubMed] [Google Scholar]
- 23.Samawi HH, Shaheen AA, Tang PA, Heng DYC, Cheung WY, Vickers MM. Risk and predictors of suicide in colorectal cancer patients: a Surveillance, Epidemiology, and End Results analysis. Curr Oncol 2017;24:e513–e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urban D, Rao A, Bressel M, Neiger D, Solomon B, Mileshkin L. Suicide in lung cancer: who is at risk? Chest 2013;144:1245–1252. [DOI] [PubMed] [Google Scholar]
- 25.Zaorsky NG, Zhang Y, Tuanquin L, Bluethmann SM, Park HS, Chinchilli VM. Suicide among cancer patients. Nat Commun 2019; 10:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saad AM, Gad MM, Al-Husseini MJ, et al. Suicidal death within a year of a cancer diagnosis: a population-based study. Cancer 2019; 125:972–979. [DOI] [PubMed] [Google Scholar]
- 27.Klaassen Z, DiBianco JM, Jen RP, et al. The impact of radical cystectomy and urinary diversion on suicidal death in patients with bladder cancer. J Wound Ostomy Continence Nurs 2016;43:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popovic D, Benabarre A, Crespo JM, et al. Risk factors for suicide in schizophrenia: systematic review and clinical recommendations. Acta Psychiatr Scand 2014;130:418–426. [DOI] [PubMed] [Google Scholar]
- 29.Costa Lda S, Alencar AP, Nascimento Neto PJ, et al. Risk factors for suicide in bipolar disorder: a systematic review. J Affect Disord 2015;170:237–254. [DOI] [PubMed] [Google Scholar]
- 30.Hawton K, Casanas ICC, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord 2013;147(1–3):17–28. [DOI] [PubMed] [Google Scholar]
- 31.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 32.Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juurlink DN, Preyra C, Croxford R. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study Toronto, ON, Canada: Institute of Clinical Evaluative Sciences; 2006. [Google Scholar]
- 34.Williams JI, Young W. A summary of studies on the quality of health care administrative databases in Canada. In: Goel V, Williams JI, Anderson GM, et al. , eds. Patterns of Health Care in Ontario 2nd ed. Ottawa, ON, Canada: Canadian Medical Association; 1996: 339–345. [Google Scholar]
- 35.Robles SC, Marrett LD, Clarke EA, Risch HA. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol 1988;41:495–501. [DOI] [PubMed] [Google Scholar]
- 36.Iron K, Zagorski BM, Sykora K, Manuel DG. Living and Dying in Ontario: An Opportunity for Improved Health Information. ICES Investigative Report Toronto, ON, Canada: Institute of Clinical Evaluative Sciences; 2008. [Google Scholar]
- 37.Gatov E, Kurdyak P, Sinyor M, Holder L, Schaffer A. Comparison of vital statistics definitions of suicide against a coroner reference standard: a population-based linkage study. Can J Psychiatry 2018;63:152–160. doi: 10.1177/706743717737033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Johns Hopkins Bloomberg School of Public Health. The Johns Hopkins ACG Case-Mix System Reference Manual. Version 7.0 Baltimore, MD: The Johns Hopkins University Bloomberg School of Public Health; 2005. [Google Scholar]
- 39.Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care 2011;49:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228–1234. [Google Scholar]
- 41.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016; 133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med 1993;12:737–751. [DOI] [PubMed] [Google Scholar]
- 43.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923–936. [DOI] [PubMed] [Google Scholar]
- 44.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989;129:125–137. [DOI] [PubMed] [Google Scholar]
- 45.Budtz-Jorgensen E, Keiding N, Grandjean P, Weihe P. Confounder selection in environmental epidemiology: assessment of health effects of prenatal mercury exposure. Ann Epidemiol 2007;17:27–35. [DOI] [PubMed] [Google Scholar]
- 46.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998;54:948–963. [PubMed] [Google Scholar]
- 47.Kisely S, Sadek J, MacKenzie A, Lawrence D, Campbell LA. Excess cancer mortality in psychiatric patients. Can J Psychiatry 2008; 53:753–761. [DOI] [PubMed] [Google Scholar]
- 48.Kisely S, Crowe E, Lawrence D. Cancer-related mortality in people with mental illness. JAMA Psychiatry 2013;70:209–217. [DOI] [PubMed] [Google Scholar]
- 49.Trevino KM, Abbott CH, Fisch MJ, Friedlander RJ, Duberstein PR, Prigerson HG. Patient-oncologist alliance as protection against suicidal ideation in young adults with advanced cancer. Cancer 2014;120:2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider KL, Shenassa E. Correlates of suicide ideation in a population-based sample of cancer patients. J Psychosoc Oncol 2008;26: 49–62. [DOI] [PubMed] [Google Scholar]
- 51.Iwasaki M, Akechi T, Uchitomi Y, Tsugane S; Japan Public Health Center-Based Prospective Study on Cancer and Cardiovascular Disease (JPHC Study) Group. Cigarette smoking and completed suicide among middle-aged men: a population-based cohort study in Japan. Ann Epidemiol 2005;15:286–292. [DOI] [PubMed] [Google Scholar]
- 52.Tanskanen A, Tuomilehto J, Viinamaki H, Vartiainen E, Lehtonen J, Puska P. Smoking and the risk of suicide. Acta Psychiatr Scand 2000;101:243–245. [PubMed] [Google Scholar]
- 53.Sankaranarayanan A, Mancuso S, Castle D. Smoking and suicidality in patients with a psychotic disorder. Psychiatry Res 2014;215: 634–640. [DOI] [PubMed] [Google Scholar]
- 54.Huang CC, Hsiao JR, Lee WT, et al. Investigating the association between alcohol and risk of head and neck cancer in Taiwan. Sci Rep 2017;7:9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Comprehensive Cancer Network. Distress management. Clinical practice guidelines. J Natl Compr Canc Netw 2003; 1:344–374. [DOI] [PubMed] [Google Scholar]
- 56.Ravi P, Karakiewicz PI, Roghmann F, et al. Mental health outcomes in elderly men with prostate cancer. Urol Oncol 2014;32:1333–1340. [DOI] [PubMed] [Google Scholar]
- 57.Klaassen Z, Arora K, Wilson SN, et al. Decreasing suicide risk among patients with prostate cancer: implications for depression, erectile dysfunction, and suicidal ideation screening. Urol Oncol 2018;36:60–66. [DOI] [PubMed] [Google Scholar]
- 58.Nayan M, Hamilton RJ, Finelli A, Austin PC, Kulkarni GS, Juurlink DN. The value of complementing administrative data with abstracted information on smoking and obesity: a study in kidney cancer. Can Urol Assoc J 2017;11:167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gershon AS, Campitelli MA, Croxford R, et al. Combination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary disease. JAMA 2014;312:1114–1121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


