Abstract
Background
Camrelizumab has shown promising survival benefits in treatment-naïve advanced non-small cell lung cancer (NSCLC) patients when used in combination with chemotherapy. However, its effectiveness and safety outside the clinical trial setting are largely unknown. Therefore, we conducted NOAH-LC-101, a prospective multicenter cohort study, to investigate the real-world effectiveness and safety of camrelizumab on a large cohort of advanced NSCLC patients in daily clinical practice.
Methods
All consecutive patients aged ≥18 years with confirmed advanced NSCLC scheduled for camrelizumab treatment were screened for inclusion at 43 hospitals in China. The primary outcome was progression-free survival (PFS). The secondary outcomes included overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety.
Results
Between August 2019 and February 2021, 403 patients were included. The median age of participants was 65 years (range, 27–87 years). There were 57 (14.1%) participants with an Eastern Cooperative Oncology Group performance status (ECOG PS) of ≥2. Most participants received camrelizumab in the second or later lines (68.7%) and plus chemotherapy (64.8%). The median PFS was 12.6 [95% confidence interval (CI): 10.7–17.0] months and median OS was 22.3 months [95% CI: 19.3–not reached (NR)]. The ORR was 28.8% (95% CI: 24.4–33.5%) and DCR was 79.9% (95% CI: 75.7–83.7%). Adverse events of any grade occurred in 348 (86.4%) participants. No new safety signals were identified. Reactive cutaneous capillary endothelial proliferation was observed in 75 (18.6%) patients, all of which were grade 1–2.
Conclusions
This study demonstrates the effectiveness and safety of camrelizumab in a large sample of real-world NSCLC patients. The results are generally consistent with those previously reported in pivotal clinical trials. This study supports the clinical use of camrelizumab in a broader patient population (ChiCTR1900026089).
Keywords: Non-small cell lung cancer (NSCLC), anti-PD-1, camrelizumab, real world
Highlight box.
Key findings
• This study demonstrates the effectiveness and safety of camrelizumab in a large sample of real-world non-small cell lung cancer (NSCLC) patients, with results that are generally consistent with those previously reported in pivotal clinical trials.
What is known and what is new?
• Camrelizumab is a humanized anti-PD-1 monoclonal antibody that has been approved by the National Medical Products Administration (NMPA) for the treatment of advanced NSCLC based on the CameL and CameL-sq trials.
• This study bridges the data gap on the effectiveness and safety of camrelizumab in NSCLC patients treated in daily clinical practice, especially those with older age, poor performance, and those with metastatic diseases (brain, liver, and adrenal gland).
What is the implication, and what should change now?
• This study supports the clinical use of camrelizumab in a broader NSCLC patient population.
Introduction
Although lung cancer has been re-ranked as the second most commonly diagnosed cancer, it has remained the leading cause of cancer death globally, with an estimated 2.2 million new cases and 1.8 million deaths in 2020 (1). As the major histological subtype, non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases. According to the latest cancer statistics in 2021, the mortality of patients with NSCLC has fallen sharply during the last decade, along with the steady decline in the incidence (2). The same trend was observed in the United States. The decreased mortality has been partially ascribed to treatment advances, particularly the successful application of targeted therapies (3).
In China, lung cancer currently ranks first in cancer burden and mortality (4). According to professor Chen from the National Cancer Center, China has been undergoing a cancer-related transition with an increasing burden of lung cancer and other cancers. Both the incidence and mortality of lung cancer showed an increasing trend between 2015 and 2020, with an estimated 0.79 and 0.82 million new cases and 0.63 and 0.72 million deaths, respectively (5). Novel treatment strategies are urgently needed to address the increasing tumor burden.
As a promising approach for targeting cancer, immunotherapy has revolutionized the treatment scenario for various cancers and become the fourth pillar for clinical cancer care. Although NSCLC was thought to have poor immunogenicity, it has consistently shown a high response to immune checkpoint inhibitors (ICIs), such as anti-programmed cell death 1 (PD-1) or its ligand (PD-L1) antibodies (6). Numerous anti-PD-1/PD-L1 inhibitors (e.g., pembrolizumab, nivolumab, atezolizumab, camrelizumab) are currently approved or their approval is underway for treatment of NSCLC in China (7). Camrelizumab is a Chinese developed humanized anti-PD-1 monoclonal antibody. It was firstly approved by the National Medical Products Administration (NMPA) for the treatment of relapsed or refractory classical Hodgkin lymphoma in 2019 (8). Since then, 7 new indications have been approved in China for the treatment of various solid tumors, including NSCLC. The approvals of camrelizumab for treatment of NSCLC were based primarily on the clinically meaningful improvement in progression-free survival (PFS) in 2 randomized multicenter phase III trials, the CameL and CameL-sq trials (9,10). Regrettably, traditional trials are generally conducted in a stringent research setting and a significant proportion of patients presenting in daily life are usually excluded or under-represented. Limited data are available on the effectiveness and safety of camrelizumab in NSCLC patients treated in daily clinical practice, especially those with older age, poor performance, or metastatic diseases (brain, liver, and adrenal gland).
To bridge the data gap, the present study was prospectively designed to investigate the real-world effectiveness and safety of camrelizumab using a larger number of advanced NSCLC patients than in previous cohorts. The findings of our study may provide direct evidence of effectiveness and safety of camrelizumab in specific subsets of patients and thus aid in clinical decision-making and patient care. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-121/rc) (11).
Methods
Study design and patients
This was a prospective multicenter observational study conducted at 43 hospitals in the Jiangsu Province, an economically well-developed area in East China. All consecutive patients aged ≥18 years with histopathologically or cytologically confirmed advanced NSCLC who were scheduled for systemic camrelizumab treatment between 7 August 2019 and 2 February 2021 were screened for inclusion. Patients with incomplete data on major variables or receiving camrelizumab in the perioperative setting were excluded.
The study protocol was reviewed and approved by the Institutional Review Board of the Jiangsu Province Hospital (No. 2019-SR-331). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and local regulations. All participating hospitals/institutions were informed and agreed the study. Written informed consents were provided by all participants at the time of enrollment.
Procedures and data collection
All treatments were prescribed at the physicians’ discretion according to the institutional treatment protocol of each participating center. The recommended treatment regimens were camrelizumab plus chemotherapy or plus antiangiogenic therapy, or camrelizumab alone. Camrelizumab was recommended at a fixed dose of 200 mg intravenously for 30 min on day 1 of each 2/3-week cycle. The regimens for chemotherapy or antiangiogenic therapy were all at the discretion of the physicians according to the individual patient’s condition. Response assessment was recommended to be performed every 6 weeks according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1. Safety data were collected from the initiation of treatment until 28 days after the last dose. All patients were followed up by routine clinical visits along with telephone calls every 3 months. The data cutoff date was 13 August 2021.
De-identified patient data on demographic and clinicopathological characteristics, camrelizumab treatments, and outcomes were prospectively collected and entered in a dedicated electronic case report form (eCRF) by the trained specialized personnel at each participating center. The collected data were centrally reviewed and checked for accuracy and consistency.
Study outcomes
The primary outcome was progression-free survival (PFS) that was defined as the time interval from enrollment to the date of disease progression or death. Patients who were still alive without disease progression were censored at the last radiological assessment. The secondary outcomes included overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety. OS was defined as the time interval from enrollment to the date of death. Patients who were still alive were censored at the last contact or data cutoff date. ORR was defined as the percentage of patients achieving complete response (CR) or partial response (PR) to immunotherapy, whereas DCR was defined as the percentage of patients having CR, PR, or stable disease (SD). Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analysis
Statistical analyses were primarily descriptive. Continuous variables were expressed as mean ± standard deviation or median and interquartile range (IQR). Categorical variables were expressed as frequency and percentage. PFS and OS were estimated using the Kaplan-Meier method. Subgroup analyses was performed for ORR. Univariate and multivariate COX regression analysis was performed to identify potential predictive factors for PFS and OS. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics at camrelizumab initiation
Between 7 August 2019 and 2 February 2021, 480 consecutive lung cancer patients who were scheduled for camrelizumab treatment were screened for eligibility. Overall, 403 patients with advanced NSCLC receiving at least 1 dose of camrelizumab were prospectively included for analysis (Figure S1).
As shown in the Table 1, the median age of patients was 65 years (range, 27–87 years) and almost one third (31.0%) were aged 70 years or older. Most patients were males (75.7%), had non-squamous NSCLC (64.8%) and stage IV disease (82.1%). A total of 57 (14.1%) patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 2 or more. Brain metastases were present in 65 (16.1%) participants, liver metastases in 48 (11.9%) participants, and adrenal metastases in 34 (8.4%) participants. PD-L1 expression was assessed in 100 (24.8%) participants, of which 52 (52.0%) were positively stained. Epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) translocation were reported in 78 (43.6%) out of 179 tested patients.
Table 1. Baseline patient characteristics.
| Variables | Total (n=403) |
|---|---|
| Median age [IQR], years | 65 [57–71] |
| Age, n (%) | |
| <70 years | 278 (69.0) |
| ≥70 years | 125 (31.0) |
| Sex, n (%) | |
| Male | 305 (75.7) |
| Female | 98 (24.3) |
| Smoking history, n (%) | |
| Yes | 77 (19.1) |
| No | 144 (35.7) |
| Unknown | 182 (45.2) |
| ECOG performance status, n (%) | |
| 0–1 | 346 (85.9) |
| ≥2 | 57 (14.1) |
| Histology, n (%) | |
| Non-squamous cell carcinoma | 261 (64.8) |
| Squamous cell carcinoma | 136 (33.8) |
| Unspecified | 6 (1.5) |
| Clinical stage, n (%) | |
| III | 72 (17.9) |
| IV | 331 (82.1) |
| Metastatic diseases | |
| Brain | 65 (16.1) |
| Liver | 48 (11.9) |
| Adrenal gland | 34 (8.4) |
| PD-L1 expression, n (%) | |
| <1% | 48 (11.9) |
| ≥1% | 52 (12.9) |
| 1–49% | 32 (7.9) |
| ≥50% | 20 (5.0) |
| Unknown | 303 (75.2) |
| EGFR/ALK mutation, n (%) | |
| Positive | 78 (19.4) |
| Negative | 101 (25.1) |
| Unknown | 224 (55.6) |
IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand-1; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase.
Treatment patterns
Camrelizumab was administered to 31.3% (126/403) of participants in the first-line setting, 40.7% in the second-line setting, and 28.0% in the third- or later-line settings (Table 2). The median duration of camrelizumab treatment was 21.9 weeks (range, 12.1–35.3 weeks), whereas the median number of doses received was 6 (range, 1–24). Most (64.8%) participants received camrelizumab plus chemotherapy. At the date of data cutoff (13 August 2021), 118 (29.3%) patients were still undergoing camrelizumab treatment, whereas 285 (70.7%) patients had discontinued treatment. The primary reason for camrelizumab discontinuation was economic concerns (50.2%, 143/285), followed by disease progression (25.6%, 73/285), AEs (14.7%, 42/285), and death (9.5%, 27/285).
Table 2. Camrelizumab treatments.
| Variables | Total (n=403) |
|---|---|
| Treatment line, n (%) | |
| First line | 126 (31.3) |
| Second line | 164 (40.7) |
| Third or later line | 113 (28.0) |
| Duration of treatment, weeks, median (range) | 21.9 (12.1–35.3) |
| Treatment cycles, median [range] | 6 [1–24] |
| Treatment patterns, n (%) | |
| Camrelizumab monotherapy | 43 (10.7) |
| Camrelizumab plus chemotherapy | 261 (64.8) |
| Camrelizumab plus others* | 99 (24.6) |
*, others including anti-angiogenesis therapy or plus chemotherapy, or targeted therapy.
Effectiveness
After a median follow-up of 9.5 months (IQR, 6.4–12.6 months), 163 (40.5%) participants developed PD or died. The median PFS was 12.6 months [95% confidence interval (CI): 10.7–17.0] (Figure 1A) and the 12-month PFS rate was 50.1% (95% CI: 43.8–56.5%). The median OS was 22.3 [95% CI: 19.3– not reached (NR)] months (Figure 1B) and the 12-month OS rate was 73.4% (95% CI: 67.5–79.4%).
Figure 1.
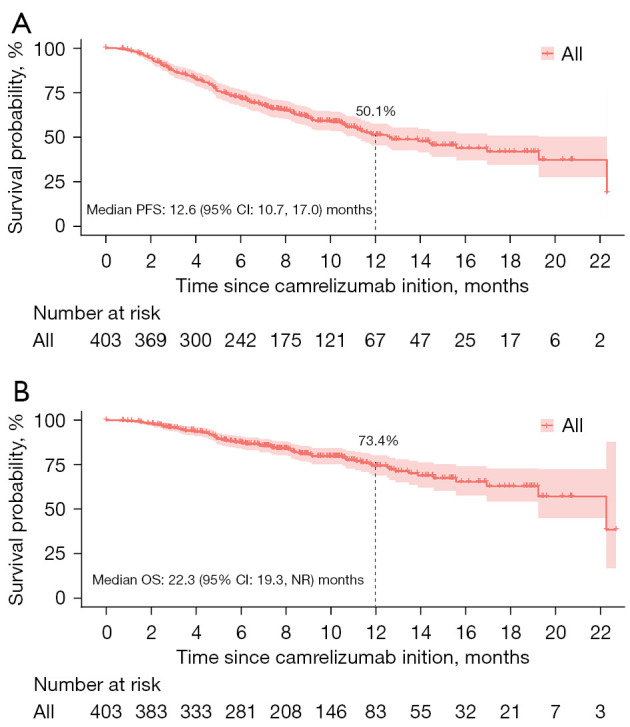
Kaplan-Meier analysis of PFS (A) and OS (B) in overall patients. PFS, progression-free survival; OS, overall survival; CI, confidential interval; NR, not reached.
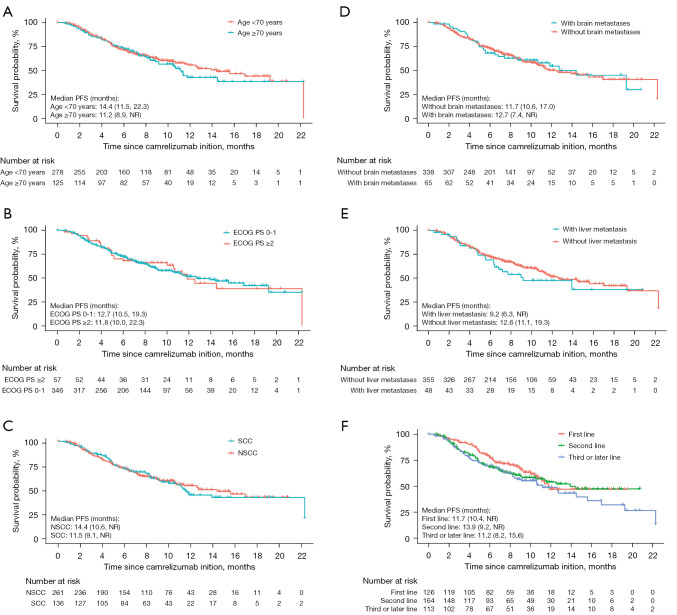
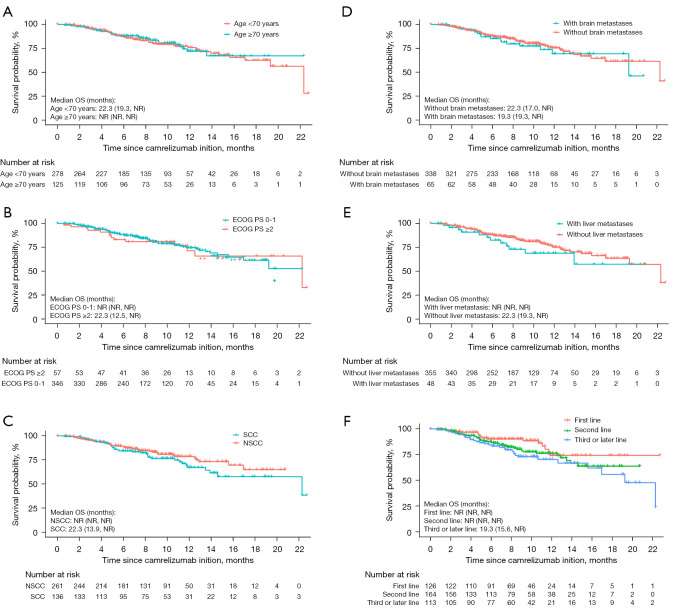
In the subgroup analysis, the median PFS was not markedly affected with respect to the patients with elder age (≥70 years), poor performance status (PS; ≥2), and those with brain or liver metastases (Figure 2A-2E). The data were not significantly influenced by camrelizumab treatment line (Figure 2F) and treatment patterns. As for OS, subgroup analyses showed no significant effect in specific subgroups of patients, including elderly patients (≥70 years), and those with an ECOG PS of 2 or more, or those with metastatic diseases (liver or brain) (Figure 3A-3E). Of note, patients receiving camrelizumab in the third- or later-line setting showed a significantly inferior OS when compared with their first-line counterparts [hazard ratio (HR) =1.995, 95% CI: 1.102–3.613, P=0.023, Figure 3F].
Figure 2.
Kaplan-Meier analysis of PFS in specific subgroups of patients stratified by age (A), ECOG performance status (B), histology (C), brain metastases (D), liver metastasis (E), and camrelizumab treatment line (F). PFS, progression-free survival; NR, not reached; ECOG, Eastern Cooperative Oncology Group; PS, performance status; SCC, squamous cell carcinoma; NSCC, non-squamous cell carcinoma.
Figure 3.
Kaplan-Meier analysis of OS in specific subgroups of patients stratified by age (A), ECOG performance status (B), histology (C), brain metastases (D), liver metastases (E), and camrelizumab treatment lines (F). OS, overall survival; NR, not reached; ECOG, Eastern Cooperative Oncology Group; PS, performance status; SCC, squamous cell carcinoma; NSCC, non-squamous cell carcinoma.
In multivariate COX regression analysis, no factors were associated with PFS of patients (Table S1). By contrast, histology of non-squamous cell carcinoma was independently associated prolonged OS (vs squamous cell carcinoma, HR =0.559, 95% CI: 0.338–0.924, P=0.023), while increasing line of therapy was associated with shorter OS (vs. first line, second line, HR =1.981, 95% CI: 1.072–3.662, P=0.029; third or later line, HR =2.481, 95% CI: 1.285–4.790, P=0.007) (Table S2).
Among the 403 patients included, 350 participants had at least 1 post-baseline radiological assessment, including 116 (28.8%) participants with PR, 206 (51.1%) participants with SD, and 28 (6.9%) participants with PD (Table 3). When counting the remaining 53 participants who were not evaluable for tumor response as having PD, the resulting ORR was 28.8% (116/403, 95% CI: 24.4–33.5%) and DCR was 79.9% (322/403, 95% CI: 75.7–83.7%). There was no significant association of ORR with any patient characteristics (Figure S2). The ORR was decreased with the increasing line of camrelizumab treatments, that is, 50.0% (63/126) in patients receiving camrelizumab in the first-line setting, 23.2% (38/164) in those in the second-line setting, and 13.3% (15/113) in those in the third-line or beyond setting (Figure S3A). Meanwhile, the DCR was not obviously influenced by camrelizumab treatment line (first-line: 88.9%; second-line: 76.2%; third- or later-line: 75.2%). Additionally, both ORR and DCR were not markedly changed regarding camrelizumab treatment patterns (Figure S3B).
Table 3. Real world effectiveness of camrelizumab-based therapy.
| Variables | Total (n=403) |
|---|---|
| Best objective response, n (%) | |
| Complete response | 0 (0.0) |
| Partial response | 116 (28.8) |
| Stable disease | 206 (51.1) |
| Progressive disease | 28 (6.9) |
| Not evaluated | 53 (13.2) |
| Tumor response rate, % (95% CI) | 28.8 (24.4–33.5) |
| Disease control rate, % (95% CI) | 79.9 (75.7–83.7) |
CI, confidence interval.
Safety
AEs of any grade occurred in 348 (86.4%) patients (Table 4), most commonly anemia (58.1%), hypoalbuminemia (33.7%), and decreased white blood cell count (18.9%). Most AEs were mild (grade 1–2), and grade 3–4 AEs were observed in 51 (12.7%) participants. No new safety signal was identified. Reactive cutaneous capillary endothelial proliferation (RCCEP) was noted in 75 (18.6%) participants and all were grade 1–2. The AEs led to permanent discontinuation of camrelizumab in 42 (10.4%) participants.
Table 4. Adverse events (AEs).
| AEs | Total (n=403) | |
|---|---|---|
| All grade | Grade 3–4 | |
| Hematological AEs, n (%) | ||
| Anemia | 234 (58.1) | 22 (5.5) |
| White blood cell count decreased | 76 (18.9) | 10 (2.5) |
| Platelet count decreased | 61 (15.1) | 5 (1.2) |
| Neutrophil count decreased | 56 (13.9) | 9 (2.2) |
| Non-hematological AEs, n (%) | ||
| Hypoalbuminemia | 136 (33.7) | 0 (0.0) |
| Reactive cutaneous capillary endothelial proliferation | 75 (18.6) | 0 (0.0) |
| Elevated transaminase | 50 (12.4) | 2 (0.5) |
| Proteinuria | 35 (8.7) | 0 (0.0) |
| Fatigue | 30 (7.4) | 1 (0.2) |
| Nausea/vomiting | 27 (6.7) | 1 (0.2) |
| Fever | 26 (6.5) | 2 (0.5) |
| Hyperbilirubinemia | 23 (5.7) | 1 (0.2) |
| Immune-related pneumonitis | 18 (4.5) | 3 (0.7) |
| Decreased appetite | 17 (4.2) | 0 (0.0) |
| Rash | 16 (4.0) | 1 (0.2) |
| Pain | 14 (3.5) | 0 (0.0) |
| Creatinine increased | 12 (3.0) | 0 (0.0) |
| Wheezing and tightness in the chest | 11 (2.7) | 1 (0.2) |
| Cough | 10 (2.5) | 0 (0.0) |
Discussion
In this, the NOAH-LC-101 study, we used a large real-world NSCLC cohort to assess the effectiveness and safety of camrelizumab in a real-world setting. Unlike patients recruited based on stringent inclusion criteria in the previous pivotal trials, this study also included patients with advanced age, poor performance, and metastatic diseases. In addition, patients who received camrelizumab in the second or later line settings were also included. The findings of our study demonstrate the effectiveness and safety of camrelizumab in real-world NSCLC patients. The median PFS was 12.6 (95% CI: 10.7–17.0) months and median OS was 22.3 (95% CI: 19.3– NR) months. Camrelizumab was well tolerated and no new safety signal was noted in our study cohort.
In this prospective multicenter observational study, the effectiveness of camrelizumab was evaluated in 403 advanced NSCLC patients, with median follow-up time of 9.5 months (IQR, 6.4–12.6 months). The ORR was 28.8% (95% CI: 24.4–33.5%) and DCR was 79.9% (95% CI: 75.7–83.7%). In the CameL trial of camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naïve patients with advanced non-squamous NSCLC, the interim analysis showed an ORR of 60.5% (95% CI: 53.4–67.2%) in the camrelizumab plus chemotherapy group (9). Meanwhile, in the CameL-sq trial of camrelizumab or placebo plus carboplatin and paclitaxel in patients with previously untreated advanced squamous NSCLC, the results showed an ORR of 64.8% (95% CI: 57.6–71.5%) in the camrelizumab plus chemotherapy group (10). The ORR observed in the current study (50.0% in the first line; 23.2% in the second line; 13.3% in the third or subsequent line) was obviously lower than that reported in the CameL and CameL-sq trials. A possible explanation is the advanced settings of camrelizumab use in the real-world setting. Unlike the first-line CameL and CameL-sq trials, most (68.7%) of the patients in our study received camrelizumab in the second or later line settings. The increasing line of camrelizumab treatments was found to associate significantly with decreased ORR of patients. Also, some patient and treatment characteristics, such as brain and liver metastases, or camrelizumab treatment patterns, may have contributed to the comparatively lower ORR observed in our real-world patients.
Interestingly, despite the comparatively lower ORR in real-world NSCLC patients, the survival outcomes are generally consistent with those reported in the pivotal trials of camrelizumab. In the CameL trial, the median PFS was 11.3 months (95% CI: 9.6–15.4) (9) and median OS was 27.9 months (95% CI: 21.9– NR) (12). Accordingly, the 12-month PFS and OS rates were 49.6% (95% CI: 41.7–57.1%) and 74.9% (95% CI: 68.1–80.4%). In the CameL-sq trial, the median PFS was 8.5 (95% CI: 6.9–10.4) months (10), while median OS was 27.4 (95% CI: 22.1– NR) months (13). The estimated 12-month PFS and OS rates were 37.9% (95% CI: 30.7–45.0%) and 75.1% (95% CI: 68.2–80.7%), respectively (10). The PFS benefits observed in our study [median: 12.6 months (95% CI: 10.7–17.0%); 12-month rate: 50.1% (95% CI: 43.8–56.5%)] seem to be a little bit higher than those reported in the pivotal trials. However, slightly lower OS [median: 22.3 months (95% CI: 19.3– NR)] and 12-month OS rate [73.4% (95% CI: 67.5–79.4%)] were also noted. The somewhat higher PFS observed herein can be partially explained by difference in time interval for tumor monitoring and evaluation. In the CameL and CameL-sq trials, tumor imaging assessment was performed at an interval of 6 weeks during the study periods. In our daily-life practice, however, the time interval for imaging evaluation varied from 0.2 to 9.3 months and largely depended on clinical symptoms of disease progression, as well as other patient- or physician-related issues. Therefore, there may be a potential time difference of PFS. Despite that, the survival outcomes in our real-world patients were generally consistent with those of the pivotal trials.
Certain special populations have been underrepresented in pivotal trials. Our study supported clinical use of camrelizumab in subsets of fragile patients, including elderly patients, those with poor PS (≥2), as well as those with brain, liver, or adrenal metastases. PS has long been established as the most powerful prognostic factor in advanced NSCLC patients (14). Patients with an ECOG PS of 2 or more are usually excluded from pivotal clinical trials of ICIs. Previous studies of other ICIs have indicated that survival outcomes of NSCLC patients undergoing immunotherapy were significantly affected by their ECOG PS (15-17). In a meta-analysis of real-world data, ECOG PS of 2 or more was confirmed to be an important prognostic determinant in NSCLC patients treated with ICIs (18). However, in the PePS2 trial, a single arm, phase 2 trial, pembrolizumab showed encouraging efficacy and safety profiles in treatment of NSCLC patients with a PS of 2 (19). Our study showed similar survival outcomes in patients undergoing camrelizumab treatment irrespective of ECOG PS. In addition to PS of patients, liver metastasis has been reported to have a negative impact on survival outcomes in patients treated with ICIs (20,21). In this study, the median PFS was found to be numerically lower in patients with liver metastases when compared with those without liver metastases (9.2 vs. 12.6 months), whereas the OS was not mature at the time of data analysis. Additionally, other patient characteristics, such as elder age and the presence of brain or adrenal metastases, were previously reported to have no significant effect on survival of NSCLC patients when undergoing immunotherapy (22,23). Consistent with the above-mentioned results, our study showed similar survival outcomes in patients with elder age, and brain or adrenal metastases, when compared with their corresponding counterparts. Taken together, the findings of our study support the effectiveness of camrelizumab in a broader NSCLC patient population.
In addition to the effectiveness, another major concern of camrelizumab use in the real-world setting is safety. In previous pivotal trials of camrelizumab, the most common treatment-related AEs reported were decreased neutrophil count, decreased white blood cell count, anemia, and RCCEP (9,10). In this study, AEs of any grade were observed in 86.4% of participants, most commonly anemia, hypoalbuminemia, and decreased white blood cell count. Notably, the incidence of RCCEP was obviously lower in our study than that reported in the pivotal trials, although almost all cases had mild symptoms (grade I or II). Overall, the safety profile of camrelizumab in the real-world setting was generally consistent with the previously established profile, with no new safety signals identified.
Of note, almost half (143/285) of the participants who discontinued camrelizumab treatment did so due to economic reasons. The median duration of camrelizumab treatment was 21.9 weeks and the median treatment cycle was 6 (range, 1–24), compared with 34.1 weeks (range, 0.1–90.1 weeks) in CameL trial and 12 (range, 1–32) cycles in CameL-sq trial (9,10). Therefore, we have a good reason to believe that, with the inclusion of camrelizumab in the National Medical Insurance Drug Catalog (3 December 2021), more patients will benefit from camrelizumab treatment.
The main strength of this study is the prospective multicenter design and the insights provided by the large number of real-life NSCLC patients. A limitation of the current study is that only patients receiving camrelizumab in the Jiangsu Province, a major economic province in Eastern China, were included for analysis. The results obtained in our study should be therefore interpreted with caution when applied to the general patient population in other areas. A previous phase II study showed greater benefits derived from camrelizumab among NSCLC patients with positive PD-L1 expression (24). Our study showed enhanced tumor response in patients with PD-L1 positive expression. Regrettably, no prognostic significance of PD-L1 expression was noted. Meanwhile, data on PD-L1 expression was not available for most (75.8%) of our patients, although it can be reasonably explained by the fact that camrelizumab was approved in NSCLC patients regardless of PD-L1 expression. Molecular biomarkers, such as PD-L1 expressions and EGFR/ALK mutation, may confound the results and data interpretation. Future studies are therefore needed to further explore the potential prognostic significance of PD-L1 expression in real-world NSCLC patients or in specific subgroups of patients (e.g., non-smokers). Nevertheless, our study provides valuable information about the real-world experience with camrelizumab in NSCLC patients and supports its effectiveness and safety in daily clinical practice.
Conclusions
In conclusion, our study demonstrates the effectiveness and safety of camrelizumab in a larger sample of real-world NSCLC patients. The results are generally consistent with those reported in pivotal trials.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Xiaowei Ji (Department of Biostatistics, Hengrui Pharmaceutical Co. Ltd.) and Junyi Jiang (Department of Clinical Pharmacology, Xiangya Hospital, Central South University) for their assistance in statistical analysis and data interpretation. We also thank all the patients and hospital personals who participated in this study.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of the Jiangsu Province Hospital (No. 2019-SR-331). All participating hospitals/institutions were informed and agreed the study. Written informed consents were provided by all participants at the time of enrollment.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-121/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-121/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-121/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-121/coif). The authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020;383:640-9. 10.1056/NEJMoa1916623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. Journal of the National Cancer Center 2022;2:1-9. 10.1016/j.jncc.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783-91. 10.1097/CM9.0000000000001474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. 10.1016/S0140-6736(21)00312-3 [DOI] [PubMed] [Google Scholar]
- 7.Gan J, Huang Y, Fang W, et al. Research progress in immune checkpoint inhibitors for lung cancer in China. Ther Adv Med Oncol 2021;13:17588359211029826. 10.1177/17588359211029826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markham A, Keam SJ. Camrelizumab: First Global Approval. Drugs 2019;79:1355-61. 10.1007/s40265-019-01167-0 [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305-14. 10.1016/S2213-2600(20)30365-9 [DOI] [PubMed] [Google Scholar]
- 10.Ren S, Chen J, Xu X, et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J Thorac Oncol 2022;17:544-57. 10.1016/j.jtho.2021.11.018 [DOI] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 12.Zhou C, Chen G, Huang Y, et al. P79. 02 Updated OS and Time to Second Progression with First-Line Camrelizumab Plus Chemo vs Chemo for Advanced Non-Squamous NSCLC. J Thorac Oncol 2021;16:S645-6. 10.1016/j.jtho.2021.01.1181 [DOI] [Google Scholar]
- 13.Zhou C, Cheng Y, Chen J, et al. 3MO First-line camrelizumab plus carboplatin and paclitaxel for advanced squamous non-small cell lung cancer: Updated overall survival results from the phase III CameL-sq trial. Ann Oncol 2022;33:S28. 10.1016/j.annonc.2022.02.012 [DOI] [Google Scholar]
- 14.Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 2010;5:620-30. 10.1097/JTO.0b013e3181d2dcd9 [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto D, Yoshioka H, Kataoka Y, et al. Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: A multicenter retrospective cohort study. Lung Cancer 2018;119:14-20. 10.1016/j.lungcan.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 16.Facchinetti F, Mazzaschi G, Barbieri F, et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur J Cancer 2020;130:155-67. 10.1016/j.ejca.2020.02.023 [DOI] [PubMed] [Google Scholar]
- 17.Crinò L, Bidoli P, Delmonte A, et al. Italian Cohort of Nivolumab Expanded Access Program in Squamous Non-Small Cell Lung Cancer: Results from a Real-World Population. Oncologist 2019;24:e1165-71. 10.1634/theoncologist.2018-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dall'Olio FG, Maggio I, Massucci M, et al. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer 2020;145:95-104. 10.1016/j.lungcan.2020.04.027 [DOI] [PubMed] [Google Scholar]
- 19.Middleton G, Brock K, Savage J, et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet Respir Med 2020;8:895-904. 10.1016/S2213-2600(20)30033-3 [DOI] [PubMed] [Google Scholar]
- 20.Funazo T, Nomizo T, Kim YH. Liver Metastasis Is Associated with Poor Progression-Free Survival in Patients with Non-Small Cell Lung Cancer Treated with Nivolumab. J Thorac Oncol 2017;12:e140-1. 10.1016/j.jtho.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021;27:152-64. 10.1038/s41591-020-1131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao M, Zhou F, Hou L, et al. Efficacy of immune-checkpoint inhibitors in advanced non-small cell lung cancer patients with different metastases. Ann Transl Med 2021;9:34. 10.21037/atm-20-1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naltet C, Besse B. Immune checkpoint inhibitors in elderly patients treated for a lung cancer: a narrative review. Transl Lung Cancer Res 2021;10:3014-28. 10.21037/tlcr-20-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang JJ, Huang C, Fan Y, et al. Camrelizumab in different PD-L1 expression cohorts of pre-treated advanced or metastatic non-small cell lung cancer: a phase II study. Cancer Immunol Immunother 2022;71:1393-402. 10.1007/s00262-021-03091-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as