Abstract
Background
It remains unclear whether goblet cell numbers in offspring are altered by maternal nutritional status and/or early weaning. Herein, using a murine model, we clarified whether a low-protein (LP) diet during pregnancy and/or early weaning changes villus structures, goblet cell numbers, mucin intensity, and mucin mRNA expression in the mucosal layer throughout the intestines in mice offspring.
Methods
We examined villus-crypt structures and goblet cell numbers using hematoxylin-eosin staining. By performing alcian blue-PAS staining and RT-qPCR, we investigated mucin intensity in the mucosal layer and mRNA expressions of Muc2 and Muc4, respectively, in 17 (early weaning)-, 21 (normal weaning)- and 28-day old mice born from LP diet-fed mothers or those born from control diet-fed mothers during pregnancy.
Results
Dietary protein restriction reduced goblet cell numbers in throughout the intestine, particularly in the duodenum and jejunum, and mucin intensity in the mucosal layer at the border of the jejunum and colon. The LP diet increased villus height and decreased villus thickness throughout the small intestine and crypt depth and width in the cecum and colon.
Conclusions
Dietary protein restriction during pregnancy and/or early weaning decreased the number of goblet cells, mucin intensity in the mucosal layer, and the Muc2 and Muc4 mRNA expressions in the small and large intestines, and affected the villus and crypt structures in the small and large intestines in female offspring mice during and after weaning.
General significance
Dietary abnormalities in fetal and weaning periods affects intestinal function.
Keywords: Small intestine, Large intestine, Fetal malnutrition, Early weaning, Suckling period, Goblet cells
Highlights
-
•
Fetal protein restriction and early weaning in mice reduce intestinal mucin expression.
-
•
Fetal protein restriction and early weaning in mice suppress intestinal goblet cells.
-
•
Fetal protein restriction and early weaning in mice decrease intestinal villus thickness.
1. Introduction
The morphology and function of murine intestine develop rapidly during the suckling-weaning transition period, when the diet switches from milk to solid food [1]. The epithelial cells in the small intestine of mice develop into crypt-like structures at postnatal week 2, while villus structures in the small intestine develop and stabilize until postnatal week 3 [2]. Moreover, the proximal colon develops during suckling-weaning periods in rodent models [3,4].
Most of the small intestinal epithelial cells, as absorptive cells, participate in digestion and nutrient absorption. In addition, these cells prevent pathogen invasion by forming tight junctions with each other [5,6]. The goblet cells in the villi of the intestine participate in preventing the invading antigens from approaching the epithelial cells by secreting mucin. The mucus layer of the villi is essential for protecting the intestine from the external environment. Goblet cells constitute 4–12% of intestinal epithelial cells and occur at a higher density in the distal intestine [7,8].
Developmental environments during gestation and suckling-weaning transition periods may affect intestinal maturation and development after birth. For example, 16-week-old female mice born from mothers fed a high-fat diet (fat ratio: 34.9% [w/w], which corresponds to 60% energy) for 8 weeks before gestation and during gestation and lactation, showed decreased villus height, villi/crypt ratio, and number of goblet cells, and increased crypt depth in the ileum compared to female pups born from mothers fed a control diet (fat ratio: 4.3% [w/w], which corresponds to 10% energy) [9]. The crypt structure changes in 22 and 40 days old rats with intrauterine growth restriction [10]. As for the relationship between intestinal maturation and nutritional status during the weaning period, early weaned (6 days old) pigeons had decreased villus area throughout the small intestine, lower ileac ratio of villus height to crypt depth, and suppressed sucrase activity in the duodenum, at postnatal day 25, compared to normal weaning controls [11]. Weaning for 12 days increased villus height in the duodenum, and reduced the mucus thickness of the ileum in 34-day-old pigs [12]. However, it is unclear whether the number of goblet cells in the offspring is affected by maternal nutrition status and/or early weaning. In addition, none of the studies have assessed villus structural changes, including villus height, villus thickness, crypt depth, goblet cells, mucin intensity, and mRNA expression of mucins in the mucosal layer throughout the small and large intestines.
Thus, in the present study, we examined whether a low-protein diet during pregnancy and/or early weaning changes the villus structure, goblet cell numbers, mucin intensity, and mRNA expression of mucins in the mucosal layer.
2. Materials and methods
2.1. Animals
The experimental procedures used in the present study conformed to the guidelines of the Animal Usage Committee of the University of Yamanashi (approval no. A2-20).
ICR mice on the first day of pregnancy (a day after vaginal plug detection) were purchased from Japan SLC, Inc. (Shizuoka, Japan) (n = 20) and divided into two groups: a control AIN93G (AIN) diet group and a low-protein (LP) diet group. Mice were kept in cages under the following controlled conditions: temperature, 23 ± 2 °C; humidity, 50 ± 10%; 12-h light/dark cycle. There were no significant differences in body weights between the groups. Mice were fed the control AIN diet (20.5% protein, 63.6% carbohydrate, and 15.9% fat as kJ %), which is a standard rodent diet determined by the American Institute of Nutrition [13] and an LP diet (Supplemental Table 1). Compared to the AIN diet, the LP diet contained less protein and more carbohydrates (8.0% protein, 76.1% carbohydrate, and 15.9% fat as kJ %). Animals had free access to food and water throughout the study period. The LP diet group was switched to the AIN diet on day 17 of pregnancy. In our pilot experiment of feeding pregnant mice a low-protein diet until birth, the mother physically killed the pups as the mothers experienced significant stress due to the diet. Therefore, pregnant mothers were fed a low protein diet until the 17th day of pregnancy, thereafter, two days before the birth, mothers were fed the AIN93G diet. With this feeding protocol, killing of the pups by their mothers was not observed. The AIN diet group was continuously fed the AIN diet. We measured the body weight of the mice and the amount of feed consumed twice a week.
After birth, the mothers in both groups had free access to the AIN diet, and the litter was adjusted to 10 pups per cage for even body weight at 3 days of age. Each group (AIN and LP) was randomly divided into two groups (AIN-NW, AIN-EW and LP-NW, LP-EW) wherein the pups were weaned at 17 (early weaning, EW) or 21 (normal weaning, NW) days old (17 d: AIN n = 7, LP n = 13; 21 d: AIN-NW n = 5, AIN-EW n = 6, LP-NW n = 17, LP-EW n = 12). Weaning in rodents including mice begins from day 13 and concludes up until day 28, and laboratory mice usually complete weaning at 21 days [14]. Because completion of weaning at earlier time points makes it difficult for pups to access food and water and caused increased stress, we chose a middle time point (17 days) for the weaning period as the early weaning period. The pups were housed together with their mothers until weaning and were divided into males or females at 28 days of age (28 d: AIN-NW n = 5, AIN-EW n = 7, LP-NW n = 17, LP-EW n = 12). All pups were given access to the control diet and water. The design of the animal experiment is shown in Supplemental Fig. 1.
Female offspring were killed by decapitation at 17, 21, or 28 days of age, and the duodenum, jejunoileum, and cecal colon were collected. At decapitation, the blood was collected using tubes containing heparin (CJ-2KL; TERUMO Corporation, Tokyo, Japan). The jejunoileum was divided into two segments of equal length, defined as the jejunum located towards the duodenum and the ileum on the other side. Plasma corticosterone levels were measured using the corticosterone EIA Kit (YK240) (Yanaihara Institute Inc., Shizuoka, Japan). We did not analyze the tissues of the male offspring in this study.
2.2. Histological analysis
The middle sections (1 cm) of the duodenum, jejunoileum, colon, and lower half of the cecum were immediately fixed with 4% paraformaldehyde and incubated at 4 °C for 24 h. Each tissue section was embedded in paraffin and stained with hematoxylin and eosin (H&E) or alcian blue-periodic acid-Schiff (PAS). The staining procedures were outsources to KAC Co., Ltd. (H&E, Shiga, Japan) and the Center for Life Science Research (alcian blue-PAS, Yamanashi, Japan). Stained tissues from the five intestinal sections were examined under a light microscope (CX41LF, Olympus Corp., Tokyo, Japan), and four images per mouse were captured for analysis. The villus height, crypt depth, and villus thickness of the duodenum and jejunoileum stained by H&E were measured in five crypt-villus segments per mouse, and the number of goblet cells was counted in 10 crypt-villus segments per mouse. The crypt depth, crypt thickness, and the number of goblet cells in the cecum and colon were measured in 20 crypt-villus segments per mouse. We chose villus-crypt segments to measure the duodenum, which can be observed from the top to the bottom of the villus-crypt. Regarding the cecum, segments were selected using a random number generator because the number of goblet cells and crypt depth in the villi of the cecum varied widely among villi. The intensity of the apical epithelial mucin of the mucosal layer in the jejunum and colon stained with alcian blue-PAS was quantified using the Image J software (Image Processing and Analysis in Java, NIH, Bethesda, MD). We did not assess the thickness of the mucosal layer because the tissues were too small. In the jejunum, we drew a line in the middle of the villi and measured the intensity at both ends of the apical membrane. In the colon, we drew a line from the lumen side to the enterocytes in the colon tissue and measured the intensity of the apical epithelial mucin layer. The background value was adjusted for each value. Four villi and crypts were measured per mouse.
2.3. Quantitative reverse transcription polymerase chain reaction (PCR)
Total RNA was extracted by following the method previously reported by Chomczynski and Sacchi [15]. The total RNA from the jejunum and colon (jejunum: 2287 ng; colon: 1675 ng) of ICR mice (17 d: AIN group, n = 7; LP group, n = 13; 21 d: AIN-NW group, n = 5; AIN-EW group, n = 6; LP-NW group, n = 17; LP-EW group, n = 12) reverse-transcribed into cDNA using SuperScript III reverse transcriptase (Invitrogen, Waltham, MA) according to the manufacturer's instructions. To quantitatively estimate the mRNA levels of each gene, we performed PCR amplification using the LightCycler 480 System (Roche Molecular Biochemicals, Penzberg, Germany). Real-time PCR for mucin encoding and the housekeeping genes Hprt (jejunum) and Rpl7 (colon) was performed using SYBR Green I (Roche Molecular Biochemicals). The housekeeping genes were selected as the gene with lesser variables among Hprt (jejunum) and Rpl7 (colon) in the jejunum and colon, respectively. The cycle threshold values for each gene were analyzed using the delta-delta method [16], which calculates the difference in one cycle threshold value as a two-fold difference between the signal for each gene and the signal for the housekeeping genes Hprt and Rpl7, respectively. The primer sequences used are listed in Supplementary Table 3.
2.4. Statistical analysis
Results are expressed as the mean ± the standard error of the mean (SEM). The Student's t-test was used to compare the data from AIN and LP male and female mice. An unpaired Tukey's test based on a two-way analysis of variance (two-way ANOVA) was used to compare female data (i.e., weaning period after birth). The significance level was set at p < 0.05.
3. Results
3.1. Body weights and basic characteristics of the offspring
The body weights of the offspring (male and female) at 3, 10, 13, and 17 days after birth were lower in the LP group than in the AIN group (Table 1). There were no significant differences in corticosterone concentrations among the groups at postnatal day 17 (PND17) and PND28. (Table 2). At PND17, body weight and ileum weight were lower in the LP group than in the AIN group. At PND21 and PND28, the body weight was lower in the LP-EW group than in the LP-NW group. A significant association was observed between duodenum weight and the timing of weaning at PND28. The dietary details of the mother and the offspring during the experiment are listed in Supplemental Table 2. The p-values obtained by two-way ANOVA are listed in Supplemental Table 4. Corticosterone concentrations were associated with maternal diet and the timing of weaning at PND21. A significant association was observed between duodenum weight and the timing of weaning at PND28.
Table 1.
Weight change of offspring before weaning.
| Female and male mice |
Body weight (g) |
||||||
|---|---|---|---|---|---|---|---|
| AIN |
LP |
||||||
| Number | 70 | 130 | |||||
| 3 days after birth | 3.13 | ± | 0.03 | 2.73 | ± | 0.04 | ** |
| 10 days after birth | 7.55 | ± | 0.07 | 6.67 | ± | 0.08 | ** |
| 13 days after birth | 8.97 | ± | 0.09 | 8.00 | ± | 0.10 | ** |
| 17 days after birth | 11.4 | ± | 0.14 | 9.71 | ± | 0.16 | ** |
Abbreviations: AIN, AIN-93G; LP, low-protein.
Values are expressed as the mean ± SEM for 70–130 animals.
**P < 0.01 vs. AIN-93G, according to the Student's t-test.
Table 2.
Basic characteristics of offspring.
| Female mice |
17 days after birth |
|||||||
|---|---|---|---|---|---|---|---|---|
| AIN |
LP |
Interaction |
||||||
| Number | 7 | 13 | P | |||||
| Body weight (g) | 11.6 | ± | 0.480 | 9.97 | ± | 0.400 | * | |
| Duodenum weight (g) | 0.08 | ± | 0.008 | 0.07 | ± | 0.003 | ||
| Jejunum weight (g) | 0.23 | ± | 0.013 | 0.21 | ± | 0.008 | ||
| Ileum weight (g) | 0.14 | ± | 0.006 | 0.12 | ± | 0.005 | * | |
| Cecum weight (g) | 0.09 | ± | 0.040 | 0.04 | ± | 0.004 | ||
| Colon weight (g) | 0.11 | ± | 0.005 | 0.09 | ± | 0.005 | ||
| Serum corticosterone (ng/mL) | 4.61 | ± | 0.404 | 4.54 | ± | 0.450 | ||
| 21 days after birth |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIN |
LP |
Interaction |
||||||||||||
| NW |
EW |
NW |
EW |
P |
||||||||||
| Number | 5 | 6 | 17 | 12 | ||||||||||
| Body weight (g) | 15.1 | ± | 0.52 | 15.4 | ± | 0.51 | 13.9 | ± | 0.50 | 11.4 | ± | 0.92 | ## | |
| Duodenum weight (g) | 0.15 | ± | 0.009 | 0.14 | ± | 0.009 | 0.12 | ± | 0.005 | 0.11 | ± | 0.009 | ||
| Jejunum weight (g) | 0.35 | ± | 0.009 | 0.37 | ± | 0.009 | 0.34 | ± | 0.150 | 0.29 | ± | 0.023 | ||
| Ileum weight (g) | 0.16 | ± | 0.002 | 0.19 | ± | 0.002 | 0.17 | ± | 0.008 | 0.15 | ± | 0.012 | ||
| Cecum weight (g) | 0.06 | ± | 0.005 | 0.11 | ± | 0.005 | 0.06 | ± | 0.004 | 0.05 | ± | 0.005 | ||
| Colon weight (g) | 0.15 | ± | 0.007 | 0.16 | ± | 0.007 | 0.16 | ± | 0.005 | 0.14 | ± | 0.009 | ||
| Corticosterone (ng/mL) |
1.97 |
± |
0.413 |
3.71 |
± |
0.627 |
3.80 |
± |
0.429 |
2.60 |
± |
0.352 |
0.0096 |
|
| 28 days after birth |
||||||||||||||
| AIN |
LP |
Interaction | ||||||||||||
| NW |
EW |
NW |
EW |
P |
||||||||||
| Number |
5 |
7 |
17 |
12 |
||||||||||
| Body weight (g) | 25.8 | ± | 0.92 | 25.1 | ± | 0.72 | 24.3 | ± | 0.69 | 21.3 | ± | 1.76 | # | |
| Duodenum weight (g) | 0.17 | ± | 0.008 | 0.19 | ± | 0.009 | 0.20 | ± | 0.003 | 0.18 | ± | 0.012 | 0.019 | |
| Jejunum weight (g) | 0.53 | ± | 0.027 | 0.54 | ± | 0.028 | 0.51 | ± | 0.024 | 0.47 | ± | 0.039 | ||
| Ileum weight (g) | 0.28 | ± | 0.014 | 0.28 | ± | 0.019 | 0.28 | ± | 0.012 | 0.25 | ± | 0.015 | ||
| Cecum weight (g) | 0.08 | ± | 0.010 | 0.09 | ± | 0.009 | 0.13 | ± | 0.029 | 0.08 | ± | 0.006 | ||
| Colon weight (g) | 0.23 | ± | 0.012 | 0.21 | ± | 0.013 | 0.21 | ± | 0.008 | 0.19 | ± | 0.009 | ||
| Corticosterone (ng/mL) | 1.33 | ± | 0.173 | 2.45 | ± | 0.410 | 2.42 | ± | 0.173 | 1.98 | ± | 0.396 | ||
Abbreviations: AIN, AIN-93G; LP, low-protein, EW, early weaning; NW, normal weaning. Values are expressed as means ± SEM for 5–17 mice. Seventeen days after birth: *P < 0.05 vs. AIN-93G, according to the Student's t-test. Twenty-one and twenty-eight days after birth: #P < 0.05 vs. LP-NW, ##P < 0.01 vs. LP-NW according to the two-way analysis of variance (Tukey's test).
3.2. Histological analysis
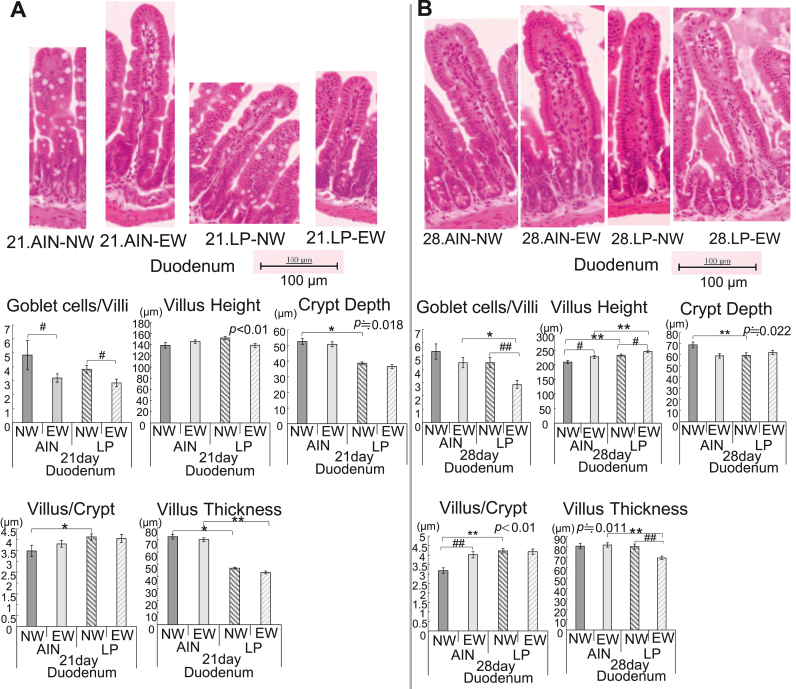
We performed H&E staining on intestinal tissue sections and measured the villus height, crypt depth, and villus thickness, and counted the number of goblet cells per villus in the duodenum, jejunum, ileum, cecum, and colon (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6 and Supplemental Figs. 4–22). At PND17, the number of goblet cells per villus, crypt depth, and the thickness in a villus of the duodenum were lower in the LP group than in the AIN group (Supplemental Fig. 2A). The number of goblet cells per villus in the AIN-EW and LP-EW groups at PND21 was lower than those in each NW group in the duodenum (Fig. 1). In addition, the number of goblet cells per villus and the thickness of the villus in the LP-EW group at PND28 were lower than those in each NW group in the duodenum (Fig. 1). The villus height in the AIN-EW and LP-EW groups and the ratio of villus/crypt in the AIN-EW groups were more than those in each NW group on PND28. The villus thickness at PND21 and PND28 was smaller and the number of goblet cells at PND28 in the LP-EW group were fewer, while the villus height at PND28 in the LP-EW group was higher than that in corresponding AIN group. In the duodenum, the crypt depth at PND21 and PND28 and the villus thickness at PND21 in LP-NW groups were smaller, while the villus thickness at PND21 and PND28 and villus height at PND28 in LP-NW were higher than those in the corresponding AIN group.
Fig. 1.
Villus-crypt structures and the number of goblet cells per villus in the duodenum of (A) 21-day-old and (B) 28-day-old female mice after birth. Each sample was stained with hematoxylin and eosin. Pregnant ICR mice received a control (AIN) diet or a low-protein diet starting at 1 d post-coitus until just after the birth of their offspring. Female mice were removed from their mothers at 17 or 21 days of age and then given free access to the AIN diets and water. The offspring were dissected at 17, 21, and 28 days after birth. We measured the number of goblet cells per villus, villus height, crypt depth, villus/crypt ratio, and villus thickness. Values are expressed as the mean ± SEM for 5–17 mice. #P < 0.05 vs. NW, ##P < 0.01 vs. NW, *P < 0.05 vs. AIN, and **P < 0.01 vs. AIN according to two-way analysis of variance (Tukey's test). Abbreviations: AIN, AIN-93G; LP, low protein; EW, early weaning; NW, normal weaning.
Fig. 2.
Villus-crypt structures and the number of goblet cells per villus in the jejunum of (A) 21-day-old and (B) 28-day-old female mice after birth. Each sample was stained with hematoxylin and eosin (H&E). Pregnant ICR mice received a control (AIN) diet or a low-protein diet starting at 1 d post-coitus until just after the birth of their offspring. Female mice were removed from their mothers at 17 or 21 days of age and then given free access to AIN diets and water. The offspring were dissected at 17, 21, and 28 days after birth. We measured the number of goblet cells per villus, villus height, crypt depth, villus/crypt ratio, and villus thickness. Values are expressed as the mean ± SEM for 5–17 mice. #P < 0.05 vs. NW, ##P < 0.01 vs. NW, *P < 0.05 vs. AIN, and **P < 0.01 vs. AIN according to the two-way analysis of variance (Tukey's test). Abbreviations: AIN, AIN-93G; LP, low protein; EW, early weaning; NW, normal weaning.
Fig. 3.
Villus-crypt structures and the number of goblet cells per villus in the ileum of (A) 21-day-old and (B) 28-day-old female mice after birth. Each sample was stained with hematoxylin and eosin (H&E). Pregnant ICR mice received a control (AIN) diet or a low-protein diet starting at 1 d post-coitus until just after the birth of their offspring. Female mice were removed from their mothers at 17 or 21 days of age and then given free access to AIN diets and water. The offspring were dissected at 17, 21, and 28 days after birth. We measured the number of goblet cells per villus, villus height, crypt depth, villus/crypt ratio, and villus thickness. Values are expressed as the mean ± SEM for 5–17 mice. #P < 0.05 vs. NW, ##P < 0.01 vs. NW, *P < 0.05 vs. AIN, and **P < 0.01 vs. AIN according to the two-way analysis of variance (Tukey's test). Abbreviations: AIN, AIN-93G; LP, low protein; EW, early weaning; NW, normal weaning.
Fig. 4.
Crypt structures and the number of goblet cells per crypt in the cecum of (A) 21-day-old and (B) 28-day-old female mice after birth. Each sample was stained with hematoxylin and eosin. Pregnant ICR mice received a control (AIN) diet or a low-protein diet starting at 1 d post-coitus until just after the birth of their offspring. Female mice were removed from their mothers at 17 or 21 days of age and then given free access to AIN diets and water. The offspring were dissected at 17, 21, and 28 days after birth. We measured the crypt depth, crypt width, and the number of goblet cells per crypt. Values are expressed as the mean ± SEM for 5–17 mice. #P < 0.05 vs. NW, ##P < 0.01 vs. NW, *P < 0.05 vs. AIN, and **P < 0.01 vs. AIN according to two-way analysis of variance (Tukey's test). Abbreviations: AIN, AIN-93G; LP, low protein; EW, early weaning; NW, normal weaning.
Fig. 5.
Crypt structures and the number of goblet cells per crypt in the colon of (A) 21-day-old and (B) 28-day-old female mice after birth. Each sample was stained with hematoxylin and eosin (H&E). Pregnant ICR mice received a control (AIN) diet or a low-protein diet starting at 1 d post-coitus until just after the birth of their offspring. Female mice were removed from their mothers at 17 or 21 days of age and then given free access to AIN diets and water. The offspring were dissected at 17, 21, and 28 days after birth. We measured the crypt depth, crypt width, and the number of goblet cells per crypt. Values are expressed as the mean ± SEM for 5–17 mice. #P < 0.05 vs. NW, ##P < 0.01 vs. NW, *P < 0.05 vs. AIN, and **P < 0.01 vs. AIN according to two-way analysis of variance (Tukey's test). Abbreviations: AIN, AIN-93G; LP, low protein; EW, early weaning; NW, normal weaning.
Fig. 6.
Characteristics of the mucosal layer. Mucin of the mucosal layer per villus in the jejunum of (A) 21-day-old and (B) 28-day-old female mice after birth. Mucin of the mucosa layer per crypt in the colon of (C) 21-day-old and (D) 28-day-old female mice after birth. Each sample was stained with Alcian Blue-PAS. Pregnant ICR mice received a control (AIN) diet or a low-protein diet starting at 1 d post-coitus until just after the birth of their offspring. Female mice were removed from their mothers at 17 or 21 days of age and then given free access to AIN diets and water. The offspring were dissected at 17, 21, and 28 days after birth. We measured the crypt depth, crypt width, and the number of goblet cells per crypt. Values are expressed as the mean ± SEM for 5–17 mice. #P < 0.05 vs. NW, ##P < 0.01 vs. NW, *P < 0.05 vs. AIN, and **P < 0.01 vs. AIN according to two-way analysis of variance (Tukey's test). Abbreviations: AIN, AIN-93G; LP, low protein; EW, early weaning; NW, normal weaning. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
At PND17, the number of goblet cells per villus, crypt depth, and villus thickness in the LP group was lower, while the villus height and ratio of villi per crypt in the jejunum in the LP group were higher than those in the AIN group (Supplemental Fig. 1B). In the jejunum, the number of goblet cells per villus in the AIN-EW and LP-EW groups at PND21 and PND28, villus thickness in the AIN-EW group at PND21 and PND28, villus height in the LP-EW group at PND21, villus/crypt in the LP-EW group, and crypt depth in AIN-EW at PND28 were smaller, while villus height and villi per crypt in the AIN-EW group at PND28, were higher compared with corresponding NW group (Fig. 2). In the jejunum, the number of goblet cells per villus at PND21 and PND28, crypt depth, and villus thickness at PND28 were lower, while the crypt depth at PND21 was higher in LP-NW compared with that in the LP-NW group. The villus/crypt in the jejunum at PND21 and the number of goblet cells at PND28 were smaller in LP-NW than in the corresponding AIN group.
In the ileum, the number of goblet cells and villus thickness in the LP group were lower at PND17 than those in the AIN group (Supplemental Fig. 1C). The villus thickness in the ileum in the AIN-EW and LP-EW groups at PND28 was smaller than that in the NW groups (Fig. 3). The villus height at PND21 and the number of goblet cells per villus at PND28, crypt depth at PND28, and villus thickness at PND28 were smaller in LP-NW than in the AIN groups. The crypt depth at PND21 and the ratio of villus/crypt at PND28 were higher in the LP-NW group than in corresponding AIN group in the ileum. The crypt depth and villus thickness in the ileum at PND28 were smaller in the LP-EW group than in the AIN-EW group. In the cecum, crypt depth, crypt width, and the number of goblet cells per crypt in the LP group were smaller at PND17 than those in the AIN group (Supplemental Fig. 2A). In the cecum, the crypt width at PND21 and PND28 and the crypt depth at PND28 in the AIN-EW and LP-EW groups, respectively, were smaller than those in the NW groups (Fig. 4). In addition, the crypt width at PND21 and PND28, the number of goblet cells at PND21, the crypt depth at PND28 in the LP-NW group, and the crypt width at PND28 were smaller in the cecum of LP-EW group than of the AIN groups.
In the colon, crypt depth, crypt width, and the number of goblet cells per crypt were smaller in the LP group than in the LP group at PND17 (Supplemental Fig. 2B). In the colon, the crypt width at PND21 and PND28, crypt depth, and the number of goblet cells at PND28 were smaller in the AIN-EW group than in the AIN-NW group (Fig. 5); furthermore, the number of goblet cells at PND21 and PND28 and the crypt width at PND28 were smaller in the LP-NW group than in the AIN-NW group.
Alcian blue-PAS staining was performed to measure the mucin layer of the epithelial mucosa in the jejunum and colon (Fig. 6). At PND17, the mucin layer intensity of the mucosa in the jejunum and colon of LP groups were smaller compared to the AIN groups (Supplemental Fig. 3C). At PND21 and PND28, the mucin layer was smaller in the jejunum of the AIN-EW and LP-NW groups than in those of the AIN-NW group, and it was smaller in the LP-EW group than in the AIN-EW group.
The P-values of interactions by two-way ANOVA are listed in Supplemental Table 4. Associations were observed between maternal diet and the timing of weaning villus height at PND21, crypt depth at PND21 and PND28, ratio of villus/crypt at PND28, duodenal villus thickness at PND28, and villus height, villus thickness, and intensity of the mucin layer at PND21 and PND28, crypt depth at PND21, jejunal ratio of villus/crypt at PND28, cecal crypt depth at PND21 and PND28, mucin layer intensity at PND21 and PND28, crypt depth, crypt width at PND21, and number of colon goblet cells at PND28.
3.3. mRNA expression in the jejunum and ileum of the offspring
At PND21 and PND28, the mRNA level of Muc2 in the jejunum was lower in the LP-EW group than that in the AIN-EW group (Fig. 7A). The Muc2 mRNA level in the colon at PND21 was lower in the AIN-EW group than that in the AIN-NW group (Fig. 7B). mRNA expression of other mucin (Muc3a) did not differ between groups. At PND21, mRNA levels of the aminopeptitase (Anpep) and solute carrier family 5 member 1 (Slc5a1), but not those of solute carrier family 2 member 2 (Slc2a2), Suclase-isomaltase (Sis), and Lactase (Lct), in the jejunum, were lower in the AIN-EW group than in the LP-EW group. At PND28, the Sis mRNA levels, but not of other genes were lower in the jejunum of the AIN-EW group than in that of the AIN-NW group (Supplemental Table 5). The p-values obtained by two-way ANOVA are listed in Supplemental Table 4. Associations were observed between maternal diet and the timing of weaning in context of Muc3 expression in the colon at PND21.
Fig. 7.
mRNA expression of mucin genes in the jejunum (A) and colon (B) of 21 and 28-d-old female mice. Values are expressed as the mean ± SEM for 5–17 mice. #P < 0.05 vs. NW, ##P < 0.01 vs. NW, *P < 0.05 vs. AIN, and **P < 0.01 vs. AIN according to two-way analysis of variance (Tukey's test). Abbreviations: AIN, AIN-93G; LP, low protein; EW, early weaning; NW, normal weaning.
4. Discussion
In this study, we demonstrated that LP restriction during pregnancy and early weaning resulted in decreased number of goblet cells, thickness of villus and crypts, mucin intensity in mucosal layer, and Muc2 and Muc4 mRNA expression in the small and large intestines of the pups. Several associations between fetal diet and weaning time points were observed, wherein most causative factors were protein-restricted diet during fetal period or early weaning. Overlapping of the factors was not observed because a single factor had tremendous impact; furthermore, synergistic associations were not observed.
Muc2 and Muc4 mRNA expression in the colon after weaning (PND22) are lower in mice born from mothers fed a protein-restricted diet (8% protein) during pregnancy than in those fed the control diet (20% protein) [10]. However, that study did not examine the changes in the number of goblet cells. Another study reported that the fetus born to mothers subjected to 30% diet restriction had a higher number of goblet cells in the jejunum compared with control mice [17]; however, the study did not examine whether a 30% diet restriction and protein restriction affected goblet cell number in the pups and the fetus. Therefore, this is the first study to show that fetal protein restriction reduces goblet cell numbers, mucin secretion in the mucosal layer, and Muc2 and Muc4 mRNA expression throughout the jejunum and colon in mouse offspring. In addition, we demonstrate for the first time that the mucin content in the epithelial mucus of the small and large intestines in pups was reduced due to protein restriction during pregnancy. Although mechanisms leading to reduced goblet cell number in the small intestine following early weaning, and decrease in mucin layer in pups following protein-restricted diet intake by mother during pregnancy remain unknown, a previous study reported reduced number of colon goblet cells in response to high-fat intake diet by 6-week-old mice for 16 weeks [18]. Furthermore, colon mucus layer of 25-week-old rat pups was decreased following 65% food restriction (control diet; A04, SAFE) during pregnancy [19]. Milk, including that of rodents, is rich in fats, which are mainly triglycerides [20]. We hypothesize that in our study, the decrease in goblet cell number, mucin layer, and Muc2 and Muc4 expression in the epithelial mucus was accompanied by an influx into the intestine of carbohydrates from the diet by force weaning.
In this study, we demonstrated that protein restriction during pregnancy and early weaning after birth enhanced villus height and reduced villus thickness in pups. As previously mentioned, the diet was changed by forced weaning with mainly from breast milk (high-fat diet) to solid food (high-carbohydrate diet). Breast milk is rich in fats [20] and carbohydrates, such as lactose [21], while solid foods are rich in carbohydrates, including starch. Consequently, increase in the enzyme activity of disaccharidases (e.g., maltase and sucrase) are accompanied by weaning progression [3]. Therefore, the increase in villus height and reduction in villus thickness may be caused by the inflow of starch or hydrolysates into the intestine. A recent study demonstrated that early weaning enhanced sucrase-isomaltase activity and villus height in the jejunum of rats [22]. It has also been reported that maltose, a starch hydrolysate, enhanced the protein expression of sucrase-isomaltase, whereas glucose did not, in the large intestine enterocyte-like Caco-2 cells [23]. However, whether starch and hydrolysates, including maltose, affect villus structure and whether changing from lactose to glucose during weaning affects villus structure are not clear. In addition, the reason why protein restriction during pregnancy enhanced villus height in pups during and after weaning remains unclear. These issues need to be clarified through further research.
Regarding the crypt structure of the large intestine, it has been reported that force-feeding of 20% casein for 3 days in rats recovering from surgery (to insert a silastic gastrostomy catheter) enhanced the crypt depth in the colon compared to force-feeding 2% casein [24]. These results indicate that proteins are essential for cell proliferation in the colon. In our study, the crypt depth and width in the colon of mice at weaning and 7 days after weaning were reduced by protein restriction during the pregnancy of their mother. A previous study demonstrated that crypt depth decreased in the colon of 12-month-old female mice fed on a high-fat diet (60%) for 14 weeks [25]. However, our results showed that crypt depth in the cecum and colon was reduced by early weaning, which cannot be explained by the dietary changes caused by weaning.
Corticosterone, a type of glucocorticoid, is one of the factors affecting intestinal structure. The development of gastrointestinal functions, such as maturation of the villus structure in the small intestine and cell proliferation of the stomach, are accompanied by increased levels of corticosterone during the weaning period in rats [14]. Moreover, these developments can be induced by intraperitoneal administration of a glucocorticoid agonist in rats [26,27]. In addition, protein restriction during pregnancy and early weaning may promote corticosterone secretion in response to stress in pups. For example, early weaning of mice enhanced stress due to separation from their mothers [28], and restraint stress in a special apparatus decreased the number of goblet cells in the colon of rats [29]. In our study, there were no changes in corticosterone concentrations in control mice and pups due to protein restriction during pregnancy of the mothers or mice that had undergone early weaning. However, the possibility of intestinal structural changes by altering glucocorticoid signals in the pups undergoing protein restriction during pregnancy and/or undergoing early weaning cannot be ruled out, because glucocorticoid receptor expression in the intestine and the sensitivity of glucocorticoids may change and blood corticosterone concentrations may increase due to the protein restriction of mothers prior to analyzing intestinal morphologies (17 days after birth in our study). Therefore, it is necessary to determine whether protein restriction during pregnancy affects blood corticosterone levels in offspring before PND17. To clarify the effects of diet or glucocorticoids, it is necessary to examine whether intestinal morphologies in early weaned mice differ between mice fed a high-fat diet and those fed a high-carbohydrate diet from the start of early weaning and whether stress during pregnancy and/or weaning rather than dietary changes affect intestinal morphology in mice.
Villus heights and ratios of villus to crypt in the duodenum was increased by protein restriction during fetal period and/or early weaning. In a mouse model, feeding a high-fat diet enhanced duodenal crypt-villus height and proliferation of mucosa, which are triggered by insulin resistance signaling [30]. In addition, GK rats, a model of type 2 diabetes, exhibited decreased crypt depth in the duodenum, but not villi, and thicker villi in the duodenum [31]. A previous study demonstrated that protein restriction in pregnant rats suppressed insulin secretion in the pups during weaning [32]. Gestational protein restriction led to peripheral insulin resistance in the pups [33]. In addition, early weaning in rats, which induces insulin secretion by early inflow of carbohydrates from solid food, led to obesity [34]. The changes of duodenal morphology induced by fetal protein restriction or early weaning may be caused by changes of insulin action. However, it should be examined in further works.
Mice in which allergy was induced by feeding an amino acid-rich diet instead of a regular diet and subsequent feeding of ovalbumin after weaning decreased mucin secretion in the small intestine [35]. Another study demonstrated that Muc2-deficient mice suffered from colitis [4], ulcerative colitis, and Crohn's disease, which is caused by a decrease in the adherent mucus thickness in the left colon and rectum [36]. Comparison of signal intensity in stained tissue images was sometimes affected by variability, which is a limitation of our study. However, the decrease in the intensity of the mucin layer in the epithelial mucosa was consistent with visual observation, the number of goblet cells, the mRNA expressions of Muc2 and Muc4 in pups during and after weaning, and protein restriction of the mother and/or early weaning. In this study, we tried to detect protein expression of MUC2 and MUC4 by Western blot analysis, but specific signals could not be detected. Therefore, further studies are needed to determine whether MUC2 and MUC4 protein expression in the intestine is suppressed by protein restriction of the mother and/or early weaning.
To evaluate the digestion and nutrient absorption, we examined the mRNA expression of carbohydrate digestion and absorption-related genes such as Sis, Lactase (Lct), solute carrier family 2 member 2 (Slc2a2) and solute carrier family 5 member 1 (Slc5a1), protein digestion-related genes such as Anpep in the jejunum of 17-day, 21 day and 28-day old mice (Table S5). Expression of several genes such as Anpep and Slc5a1 at PND21 and Sis mRNA levels at PDN28 in the jejunum were lower in the AIN-EW group than in the AIN-NW group. Therefore, early weaning could reduce expression of genes related to carbohydrate and amino acid digestion and absorption in the jejunum of mice.
It remains unclear whether dietary protein restriction during pregnancy and/or early weaning affects the intestinal structures including goblet cell numbers and intestinal mucin expression, in male pups. A report demonstrated that male rats, but not female rats, that suffered malnutrition during the fetal period showed hypertensive symptoms during adulthood compared to controls [37]. Therefore, effects of dietary protein restriction during pregnancy and/or early weaning on intestinal structures in male pups may be different in female pups and needs separate evaluation.
5. Conclusions
Protein restriction during pregnancy and/or early weaning can change the villus and crypt structures, decrease the number of goblet cells, suppress mucin intensity in the mucosal layer, and downregulate Muc2 and Muc4 mRNA expressions in the epithelial mucosa of the small intestine (duodenum, jejunum, and ileum) and large intestine (cecum and colon) during the suckling to weaning period of female pups. Therefore, our results suggest that protein restriction during pregnancy and early weaning of pups impairs intestinal barrier function, which may cause various diseases, such as colitis and inflammatory bowel diseases.
Funding
The work presented was supported by the KAKENHI program of the Japan Society for the Promotion of Science (JP20H04103, JP17H01964) from the Ministry of Education, Culture, Sports, Science and Technology.
Data sharing
Not applicable.
CRediT authorship contribution statement
Haruka Adachi: did experiments and wrote the manuscript. Shiori Ishiyama: helped with experiments and drafting the manuscript. Kazuki Mochizuki: organized this study. All authors have approved the final article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2023.101475.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Miyata T., Minai Y., Haga M. Impaired growth of small intestinal epithelium by adrenalectomy in weaning rats. Acta Histochem. Cytoc. 2008;41:83–88. doi: 10.1267/ahc.08004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stappenbeck T.S., Wong M.H., Saam J.R., Mysorekar I.U., Gordon J.I. Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr. Opin. Cell Biol. 1998;10:702–709. doi: 10.1016/s0955-0674(98)80110-5. [DOI] [PubMed] [Google Scholar]
- 3.Koldovsky O., Dobiasova M., Hahn P., Kolinska J., Kraml J., Pacha J. Development of gastrointestinal functions. Physiol. Res. 1995;44:341–348. [PubMed] [Google Scholar]
- 4.Burger-van Paassen N., van der Sluis M., Bouma J., Korteland-van Male A.M., Lu P., Van Seuningen I., Boehm G., van Goudoever J.B., Renes I.B. Colitis development during the suckling-weaning transition in mucin Muc2-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G667–G678. doi: 10.1152/ajpgi.00199.2010. [DOI] [PubMed] [Google Scholar]
- 5.Luissint A.C., Parkos C.A., Nusrat A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology. 2016;151:616–632. doi: 10.1053/j.gastro.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C., Yin Y., Tu Q., Yang H. Glucose and amino acid in enterocyte: absorption, metabolism and maturation. Front. Biosci. 2018;23:1721–1739. doi: 10.2741/4669. [DOI] [PubMed] [Google Scholar]
- 7.Schneider H., Pelaseyed T., Svensson F., Johansson M.E.V. Study of mucin turnover in the small intestine by in vivo labeling. Sci. Rep. 2018;8:5760. doi: 10.1038/s41598-018-24148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng H., Leblond C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 9.Xue Y., Wang H., Du M., Zhu M.J. Maternal obesity induces gut inflammation and impairs gut epithelial barrier function in nonobese diabetic mice. J. Nutr. Biochem. 2014;25:758–764. doi: 10.1016/j.jnutbio.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanca-Berthon P., Michel C., Pagniez A., Rival M., Van Seuningen I., Darmaun D., Hoebler C. Intrauterine growth restriction alters postnatal colonic barrier maturation in rats. Pediatr. Res. 2009;66:47–52. doi: 10.1203/PDR.0b013e3181a2047e. [DOI] [PubMed] [Google Scholar]
- 11.Wen J.S., Xu Q.Q., Zhao W.Y., Hu C.H., Zou X.T., Dong X.Y. Effects of early weaning on intestinal morphology, digestive enzyme activity, antioxidant status, and cytokine status in domestic pigeon squabs (Columba livia) Poultry Sci. 2022;101 doi: 10.1016/j.psj.2021.101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang M., Laarveld B., Van Kessel A.G., Hamilton D.L., Estrada A., Patience J.F. Effect of segregated early weaning on postweaning small intestinal development in pigs. J. Anim. Sci. 1999;77:3191–3200. doi: 10.2527/1999.77123191x. [DOI] [PubMed] [Google Scholar]
- 13.Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 14.Koldovsky O. Response of the gastrointestinal tract to premature weaning in experimental animals. Pediatrics. 1985;75:199–206. [PubMed] [Google Scholar]
- 15.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Srugo S.A., Bloise E., Nguyen T.T.N., Connor K.L. Impact of maternal malnutrition on gut barrier defense: implications for pregnancy health and fetal development. Nutrients. 2019;11 doi: 10.3390/nu11061375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina-Reyes E.I., Delgado-Buenrostro N.L., Diaz-Urbina D., Rodriguez-Ibarra C., Deciga-Alcaraz A., Gonzalez M.I., Reyes J.L., Villamar-Duque T.E., Flores-Sanchez M.L., Hernandez-Pando R., Mancilla-Diaz J.M., Chirino Y.I., Pedraza-Chaverri J. Food-grade titanium dioxide (E171) induces anxiety, adenomas in colon and goblet cells hyperplasia in a regular diet model and microvesicular steatosis in a high fat diet model. Food Chem. Toxicol. : an international J Publ. British Indust. Biol. Res. Assoc. 2020;146 doi: 10.1016/j.fct.2020.111786. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Oca P., Robles-Vera I., Sanchez-Roncero A., Escriva F., Perez-Vizcaino F., Duarte J., Alvarez C., Fernandez-Millan E. Gut DYSBIOSIS and altered barrier function precedes the appearance of metabolic syndrome in a rat model of nutrient-induced catch-up growth. J. Nutr. Biochem. 2020;81 doi: 10.1016/j.jnutbio.2020.108383. [DOI] [PubMed] [Google Scholar]
- 20.Miles E.A., Calder P.C. The influence of the position of palmitate in infant formula triacylglycerols on health outcomes. Nutr. Res. 2017;44:1–8. doi: 10.1016/j.nutres.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Gavalda-Navarro A., Hondares E., Giralt M., Mampel T., Iglesias R., Villarroya F. Fibroblast growth factor 21 in breast milk controls neonatal intestine function. Sci. Rep. 2015;5 doi: 10.1038/srep13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee P.C., Lebenthal E. Early weanling and precocious development of small intestine in rats: genetic, dietary or hormonal control. Pediatr. Res. 1983;17:645–650. doi: 10.1203/00006450-198308000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Cheng M.W., Chegeni M., Kim K.H., Zhang G., Benmoussa M., Quezada-Calvillo R., Nichols B.L., Hamaker B.R. Different sucrose-isomaltase response of Caco-2 cells to glucose and maltose suggests dietary maltose sensing. J. Clin. Biochem. Nutr. 2014;54:55–60. doi: 10.3164/jcbn.13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu Z., Ling P.R., Tahan S.R., Sierra P., Onderdonk A.B., Bistrian B.R. Protein and lipid refeeding changes protein metabolism and colonic but not small intestinal morphology in protein-depleted rats. J. Nutr. 1996;126:906–912. doi: 10.1093/jn/126.4.906. [DOI] [PubMed] [Google Scholar]
- 25.Xie Y., Ding F., Di W., Lv Y., Xia F., Sheng Y., Yu J., Ding G. Impact of a highfat diet on intestinal stem cells and epithelial barrier function in middleaged female mice. Mol. Med. Rep. 2020;21:1133–1144. doi: 10.3892/mmr.2020.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunin A.G., Nikolaev D.V. Effect of acute and chronic glucocorticoid treatments on epithelial cell proliferation in the esophagus and small intestine of rats. J. Gastroenterol. 1999;34:661–667. doi: 10.1007/s005350050316. [DOI] [PubMed] [Google Scholar]
- 27.Ghizoni H., Figueiredo P.M., Moisan M.P., Ogias D., Osaki L.H., Gama P. Regulation of corticosterone function during early weaning and effects on gastric cell proliferation. Nutrition. 2014;30:343–349. doi: 10.1016/j.nut.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Kreiker M., Perez K., Brown K.L. The effects of early weaning on Pavlovian fear conditioning in young rats. Dev. Psychobiol. 2021;63 doi: 10.1002/dev.22133. [DOI] [PubMed] [Google Scholar]
- 29.Perov Iu L., Evdokimov V.V. [Ultrastructure of the duodenal mucosa in rats under stress] Biulleten Eksp. Biol. Meditsiny. 1976;81:625–628. [PubMed] [Google Scholar]
- 30.Baldassano S., Amato A., Cappello F., Rappa F., Mule F. Glucagon-like peptide-2 and mouse intestinal adaptation to a high-fat diet. J. Endocrinol. 2013;217:11–20. doi: 10.1530/JOE-12-0500. [DOI] [PubMed] [Google Scholar]
- 31.Pereira J.N.B., Murata G.M., Sato F.T., Marosti A.R., Carvalho C.R.O., Curi R. Small intestine remodeling in male Goto-Kakizaki rats. Phys. Report. 2021;9 doi: 10.14814/phy2.14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bautista C.J., Bautista R.J., Montano S., Reyes-Castro L.A., Rodriguez-Pena O.N., Ibanez C.A., Nathanielsz P.W., Zambrano E. Effects of maternal protein restriction during pregnancy and lactation on milk composition and offspring development. Br. J. Nutr. 2019;122:141–151. doi: 10.1017/S0007114519001120. [DOI] [PubMed] [Google Scholar]
- 33.Blesson C.S., Chinnathambi V., Kumar S., Yallampalli C. Gestational protein restriction impairs glucose disposal in the gastrocnemius muscles of female rats. Endocrinology. 2017;158:756–767. doi: 10.1210/en.2016-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lima N.S., Moura E.G., Franco J.G., Pinheiro C.R., Pazos-Moura C.C., Cabanelas A., Carlos A.S., Nascimento-Saba C.C., de Oliveira E., Lisboa P.C. Developmental plasticity of endocrine disorders in obesity model primed by early weaning in dams. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2013;45:22–30. doi: 10.1055/s-0032-1323703. [DOI] [PubMed] [Google Scholar]
- 35.Paula-Silva J., Santiago A.F., Oliveira R.P., Rosa M.L., Carvalho C.R., Amaral J.F., Faria A.M. Effect of a protein-free diet in the development of food allergy and oral tolerance in BALB/c mice. Br. J. Nutr. 2015;113:935–943. doi: 10.1017/S0007114515000173. [DOI] [PubMed] [Google Scholar]
- 36.Pullan R.D., Thomas G.A., Rhodes M., Newcombe R.G., Williams G.T., Allen A., Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Rodriguez P., de Pablo A.L., Condezo-Hoyos L., Martin-Cabrejas M.A., Aguilera Y., Ruiz-Hurtado G., Gutierrez-Arzapalo P.Y., Ramiro-Cortijo D., Fernandez-Alfonso M.S., Gonzalez Mdel C., Arribas S.M. Fetal undernutrition is associated with perinatal sex-dependent alterations in oxidative status. J. Nutr. Biochem. 2015;26:1650–1659. doi: 10.1016/j.jnutbio.2015.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.