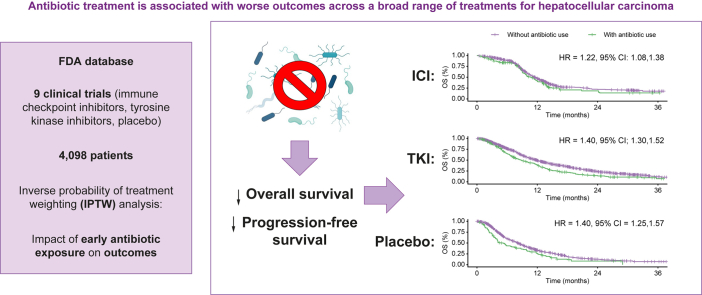
Abstract
Background & Aims
Immune checkpoint inhibitors (ICIs) alone or in combination with other ICIs or vascular endothelial growth factor pathway inhibitors are therapeutic options in unresectable/metastatic hepatocellular carcinoma (HCC). Whether antibiotic (ATB) exposure affects outcome remains unclear.
Methods
This study retrospectively analysed an FDA database including 4,098 patients receiving ICI (n = 842) either as monotherapy (n = 258) or in combination (n = 584), tyrosine kinase inhibitor (TKI) (n = 1,968), vascular endothelial growth factor pathway inhibitors (n = 480), or placebo (n = 808) as part of nine international clinical trials. Exposure to ATB within 30 days before or after treatment initiation was correlated with overall survival (OS) and progression-free survival (PFS) across therapeutic modality before and after inverse probability of treatment weighting (IPTW).
Results
Of 4,098 patients with unresectable/metastatic HCC, of which 39% were of hepatitis B aetiology and 21% were of hepatitis C aetiology, 83% were males with a median age of 64 years (range 18–88), a European Collaborative Oncology Group performance status of 0 (60%), and Child–Pugh A class (98%). Overall, ATB exposure (n = 620, 15%) was associated with shorter median PFS (3.6 months in ATB-exposed vs. 4.2 months; hazard ratio [HR] 1.29; 95% CI 1.22, 1.36) and OS (8.7 months in ATB-exposed vs. 10.6 months; HR 1.36; 95% CI 1.29, 1.43). In IPTW analyses, ATB was associated with shorter PFS in patients treated with ICI (HR 1.52; 95% CI 1.34, 1.73), TKI (HR 1.29; 95% CI 1.19, 1.39), and placebo (HR 1.23; 95% CI 1.11, 1.37). Similar results were observed in IPTW analyses of OS in patients treated with ICI (HR 1.22; 95% CI 1.08, 1.38), TKI (HR 1.40; 95% CI 1.30, 1.52), and placebo (HR 1.40; 95% CI 1.25, 1.57).
Conclusions
Unlike other malignancies where the detrimental effect of ATB may be more prominent in ICI recipients, ATB is associated with worse outcomes in this study across different therapies for HCC including placebo. Whether ATB is causally linked to worse outcomes through disruption of the gut–liver axis remains to be demonstrated in translational studies.
Impact and Implications
A growing body of evidence suggests the host microbiome, frequently altered by antibiotic treatment, as an important outcome predictor in the context of immune checkpoint inhibitor therapy. In this study, we analysed the effects of early antibiotic exposure on outcomes in almost 4,100 patients with hepatocellular carcinoma treated within nine multicentre clinical trials. Interestingly, early exposure to antibiotic treatment was associated with worse outcomes not only in patients treated with immune checkpoint inhibitors but also in those treated with tyrosine kinase inhibitors and placebo. This is in contrast to data published in other malignancies, where the detrimental effect of antibiotic treatment may be more prominent in immune checkpoint inhibitor recipients, highlighting the uniqueness of hepatocellular carcinoma given the complex interplay between cirrhosis, cancer, risk of infection, and the pleiotropic effect of molecular therapies for this disease.
Keywords: Antibiotics, Cancer immunotherapy, Targeted therapy, Immune checkpoint inhibitors, Hepatocellular carcinoma, Gut microbiota, Microbial dysbiosis
Graphical abstract
Highlights
-
•
Antibiotic treatment may impair ICI efficacy via modulation of the microbiome.
-
•
Early antibiotic exposure was associated with worse OS and PFS in HCC.
-
•
This effect was observed across a broad range of treatments including placebo.
-
•
Results were confirmed after inverse probability of treatment weighting (IPTW).
Introduction
The advent of effective systemic therapy options has reshaped the therapeutic landscape of advanced/unresectable hepatocellular carcinoma (HCC).1 Sequential use of tyrosine kinase inhibitor (TKI) therapy represented a breakthrough in an oncological diagnosis that had for a long time not benefited from the use of systemic therapy.2,3 More recently, clinical testing of monoclonal antibodies in the immune checkpoint inhibitor (ICI) class targeting the programmed cell death-1 (PD-1) receptor/ligand (PD-L1) and the cytotoxic T-cell lymphocyte associated antigen-4 (CTLA-4), two key drivers of anticancer immunity,4 has led to the understanding that a proportion of patients with HCC may respond to T-cell immune reconstitution.5 However, in the absence of predictive biomarkers capable of identifying patients who benefit from ICIs, demonstrating the clinical utility of monotherapy regimens has been particularly difficult, especially in view of late-stage failures in clinical development.6,7
Clinical testing of combinations of PD-1 pathway inhibitors with blockers of the vascular endothelial growth factor (VEGF) pathway, the CTLA-4 pathway, or TKIs have risen to prominence as therapies with the potential to generate radiologically measurable responses in over one-fourth of patients with advanced HCC.8 These combinations have also demonstrated survival benefit compared with sorafenib as illustrated in the IMbrave150 trial, which evaluated the combination of atezolizumab plus bevacizumab.9
As drug development in HCC continues at a rapid pace, considerable interest has been devoted to the investigation of mechanisms of response and resistance to ICI, in an effort to guide personalised therapy.10 The specific immune-mediated mechanism of action of ICIs has led to the study of the host immune status as a source of prognostic and predictive traits that may aid clinicians in formulating therapeutic decisions in the clinic. In recipients of ICI therapy, characteristics such as pretreatment BMI,11 the presence of a systemic pro-inflammatory status,12,13 the emergence of treatment-related adverse events14 are independently associated with outcomes from immunotherapy.
In addition to host factors, concomitant therapies may have a direct effect on the host immune function and have been studied for their ability to modify the efficacy of ICI or make patients more prone to developing toxicity from ICI therapy.15,16
Antibiotics (ATB) are among the class of drugs that exert immunomodulatory effects, for example, through perturbation of the gut microbiota.17 Use of broad-spectrum ATB can reduce gut bacterial diversity and foster the expansion of taxa that may negatively affect response to ICI.18,19 Data suggest that ATB exposure either before or early during the course of ICI therapy are associated with worse outcomes in recipients of ICI therapy in terms of objective response rate, progression-free survival (PFS), and overall survival (OS).[20], [21], [22], [23], [24] In indications other than HCC, it has been reported that the detrimental effect of ATB is most apparent in patients who receive ICI therapy as compared with those who receive cytotoxic chemotherapy25 or chemo-immunotherapy combinations,26 suggesting that ATB might exert a preconditioning effect on cancer-specific immune control.27
In patients with HCC the role of ATB exposure in influencing outcome from ICI is unclear.
Two previously published multicentre retrospective studies have reported conflicting results.28,29 However, significant heterogeneity exists among published studies, where the majority of patients were treated with PD-1 monotherapy across different treatment lines and with varying degrees of liver dysfunction, a factor that affects the prognosis of ICI recipients.30,31
To further evaluate the strength and direction of the relationship between ATB exposure and outcome from immunotherapy we performed a patient-level analysis of international clinical trials of systemic therapy (ICI and TKI) in advanced/unresectable HCC.
Patients and methods
Study population
An internal US FDA database was used to identify clinical trials submitted to the FDA between 2016 and 2019 to support marketing applications of systemic anticancer therapies for the treatment of patients with unresectable/metastatic HCC. Nine multicentre trials were included and consisted of treatment arms including placebo, TKI, VEGF inhibitors, and PD-1/PD-L1 inhibitors as monotherapy and in combination with CTLA-4 antagonists, VEGF pathway inhibitors, or TKI. The immunotherapy agents studied included anti-PD-1, anti-PD-L1, and anti-CTLA-4 agents. The TKIs and VEGF inhibitors studied were regorafenib, lenvatinib, sorafenib, cabozantinib, ramucirumab, and bevacizumab. The different trials studied patients in the first-line setting and beyond.
Endpoint definition
OS was defined as the time from randomisation (or date of enrolment for patients in single-arm studies) to death or last recorded follow-up. PFS was defined as the time from randomisation (or enrolment for single-arm studies) to progression or death. PFS was determined according to Response Evaluation Criteria in Solid Tumours (RECIST) v1.1 guidelines. For ATB exposure, concomitant medication records were screened to identify administration of any systemic ATB uses up to 30 days before treatment initiation. Patients who had ATB exposure, defined as exposure within 30 days before and 30 days after the initiation of any anticancer treatment, were of interest. Duration of exposure was recorded and categorised into more vs. less than 10 days. All clinical study-related procedures and data collection were indicated by the sponsors to have been conducted in accordance with the Declaration of Helsinki and in accordance with Good Clinical Practice.
Analysis methods
Demographic data were summarised using descriptive statistics. Categorical variables were summarised as proportions, and continuous variables were summarised using medians and ranges. For the analyses on OS and PFS, the Kaplan–Meier product limit method and log-rank tests were used to compare patients who had ATB exposure with patients who did not. Cox proportional hazard models were used to estimate the hazard ratio (HR) of patients who had ATB exposure compared with patients who did not. All PFS and OS analyses were conducted in all patients as well as by treatment type. Unless otherwise specified, the immunotherapy group included patients receiving PD-1/PD-L1 inhibitors as monotherapy and in combination with CTLA-4 antagonists, VEGF pathway inhibitors, or TKI. Because of the inconsistent association between ATB exposure and outcome in ICI recipients in our dataset and evidence in the literature suggesting that ICI combination therapies might be less susceptible to the detrimental effects of ATB, we also evaluated ICI combination regimens separately from ICI monotherapy.
Inverse probability of treatment weighting (IPTW) was used in attempt to achieve a balanced distribution of confounders across exposure groups. The weights were derived from a propensity score IPTW model that included the following baseline variables as covariates: age, race, sex, region, European Collaborative Oncology Group (ECOG) performance status, aetiology of chronic liver disease, presence of macrovascular invasion, presence of extrahepatic disease, and receipt of prior lines of treatment. The mean squared differences (MSDs) were calculated to measure the balance in observed baseline characteristics between groups of ATB exposure. The MSDs before and after IPTW were calculated for each covariate by ATB exposure group; both unadjusted and adjusted (by IPTW) PFS and OS analyses were conducted. To validate our findings, all statistical analyses were repeated after propensity score matching considering the same potential confounders as for IPTW. All statistical analyses were conducted using the SAS software (SAS Institute Inc., Cary, NC, USA).
Results
Patients
Overall, 4,098 patients were pooled from nine selected clinical trials; among them, 1,968 (48%) received TKIs, 842 (21%) received ICI-based regimens, 480 (12%) received anti-VEGF monoclonal antibodies, and 808 (20%) received placebo. When considering antibiotic therapy exposure in the whole population, 620 patients (15%) had received ATB within the time frame of 30 days before and after first anticancer treatment or placebo was initiated, whereas the remaining 3,478 trial patients (85%) had not been exposed to ATB at all or had received them outside the time frame of interest. Rates of ATB exposure were similar across treatment types ranging from 12% of placebo to 16% of TKI and immunotherapy recipients. Duration of ATB treatment was less than or equal to 10 days in 281 of 620 exposed patients (45%) and comparable across types (Table S1).
Baseline characteristics
Baseline characteristics of the whole population are summarised in Table 1 following stratification by ATB exposure. In the overall population, 83% (n = 3,421) of patients were male, 46% (n = 1,879) were enrolled in Asia, and 54% (n = 2,218) were from the rest of the world. Leading aetiologic factors for chronic liver disease included 39% (n = 1,618) hepatitis B infection, 21% (n = 871) hepatitis C infection, 5% (n = 195) mixed infection, 6% (n = 241) alcohol use, 20% (n = 819) other causes, 2.8% (n = 115) unknown aetiologic factors, and 6% (n = 239) missing.
Table 1.
Baseline characteristics of clinical trial participants stratified according to antibiotic use.
| Variables | No antibiotic use n = 3,478 |
Antibiotic use n = 620 |
||
|---|---|---|---|---|
| Median/n | Range/% | Median/n | Range/% | |
| Age | 64 | 18–88 | 63 | 24–88 |
| Race | ||||
| Missing | 57 | 1.6 | 10 | 1.6 |
| Asian | 1,732 | 49.8 | 316 | 51.0 |
| Black | 59 | 1.7 | 14 | 2.3 |
| White | 1,432 | 41.2 | 239 | 38.5 |
| Not reported | 166 | 4.8 | 30 | 4.8 |
| Other | 32 | 0.9 | 11 | 1.8 |
| Sex | ||||
| Female | 538 | 15.5 | 139 | 22.4 |
| Male | 2,940 | 84.5 | 481 | 77.6 |
| Region | ||||
| Missing | 1 | 0.0 | 0 | 0.0 |
| Asia | 1,593 | 45.8 | 286 | 46.1 |
| ROW | 1,884 | 54.2 | 334 | 53.9 |
| ECOG performance status | ||||
| 0 | 2,109 | 60.6 | 347 | 56.0 |
| 1+ | 1,369 | 39.4 | 273 | 44.0 |
| Child–Pugh score | ||||
| Missing | 4 | 0.1 | 3 | 0.5 |
| A | 3435 | 98.8 | 601 | 96.9 |
| B | 39 | 1.1 | 16 | 2.6 |
| Aetiology of chronic liver disease | ||||
| Missing | 203 | 5.8 | 36 | 5.8 |
| Alcohol | 205 | 5.9 | 36 | 5.8 |
| HBV | 1,383 | 39.8 | 235 | 37.9 |
| HCV | 723 | 20.8 | 148 | 23.9 |
| Mixed | 166 | 4.8 | 29 | 4.7 |
| Other | 700 | 20.1 | 119 | 19.2 |
| Unknown | 98 | 2.8 | 17 | 2.7 |
| Prior systemic therapy | ||||
| Yes | 2,158 | 62.0 | 385 | 62.1 |
| No | 1,320 | 38.0 | 235 | 37.9 |
| Macrovascular invasion | ||||
| Missing | 2 | 0.1 | 1 | 0.2 |
| Absence | 2,495 | 71.7 | 431 | 69.5 |
| Presence | 981 | 28.2 | 188 | 30.3 |
| Extrahepatic spread | ||||
| Absence | 1,127 | 32.4 | 178 | 28.7 |
| Presence | 2,351 | 67.6 | 442 | 71.3 |
| Type of treatment | ||||
| ICI monotherapy | 225 | 6.5 | 33 | 5.3 |
| ICI combination | 482 | 13.9 | 102 | 16.5 |
| TKI monotherapy | 1,656 | 47.6 | 312 | 50.3 |
| VEGF inhibitors | 407 | 11.7 | 73 | 11.8 |
| Placebo | 708 | 20.4 | 100 | 16.1 |
ECOG, European Collaborative Oncology Group; ICI, immune checkpoint inhibitor; ROW, rest of the world; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
At baseline, Child–Pugh class was A in 4,036 patients (98%), and most trial participants carried a diagnosis of HCC with evidence of extrahepatic spread (n = 2,793, 68%), whereas macrovascular invasion was present in 1,169 patients (29%). At baseline, 2,543 patients (62%) had received at least one line of systemic anticancer therapy.
ATB exposure is associated with worse outcome in patients who receive systemic therapy in HCC
In total, 4,036 patients (98%) were eligible for analysis of ATB exposure in relation to OS and PFS outcomes, after exclusion of 62 patients with Child–Pugh class B (n = 55) and seven patients with missing Child–Pugh class information. Patients with Child–Pugh class B disease were not examined owing to their small sample size. In all treatment modalities (ICIs, TKIs, and placebo), unadjusted and adjusted (by IPTW) PFS and OS analyses were conducted to compare patients by ATB exposure groups.
Baseline characteristics in the various groups were found to be generally well balanced between the ATB-exposed and ATB-unexposed groups before and after weighting (Tables S2–S4). Exceptions are among TKI recipients where ATB was more frequently administered to female patients than to male patients (21 vs. 15%), and to patients who received prior systemic therapy for HCC (58 vs. 51%), and were less frequently administered to patients who had extrahepatic disease (65 vs. 71%). All variables were well balanced after weighting (Table S2).
Before weighting, in the overall patient population, ATB-exposed patients (n = 601) experienced worse PFS (median, 3.6 months in ATB-exposed patients vs. 4.2 months; HR 1.36; 95% CI 1.23, 1.50) and worse OS (median, 8.6 months in ATB-exposed patients vs. 10.8 months; HR 1.42; 95% CI 1.29, 1.57) than did their ATB-unexposed counterparts (n = 3,435). After IPTW, the survival estimates in ATB-exposed patients (n = 555) vs. ATB-unexposed patients (n = 3,171) were comparable with the unadjusted results for both PFS (median, 3.6 months in ATB-exposed patients vs. 4.2 months; HR 1.29; 95% CI 1.22, 1.36) and OS (median, 8.7 vs. 10.6 months; HR 1.36; 95% CI 1.23, 1.43).
ATB exposure is associated with worse efficacy and survival across various therapeutic modalities
To determine whether the effect of ATB exposure on PFS and OS seen in the overall patient population could be separately reproduced in patients treated with ICI, TKI, and placebo, we performed unmatched estimates for PFS in ATB-exposed vs. unexposed patients.
As shown in Table 2, worse PFS and OS in patients treated with TKIs and placebo was observed, whereas for the ICI group, median PFS (median, 8.3 months in ATB-exposed patients vs. 8.2 months; HR 1.24; 95% CI 0.97, 1.59) and OS (median, 10.7 months in ATB-exposed patients vs. 11.4 months; HR 1.24; 95% CI 0.98, 1.56) was not significantly different.
Table 2.
The relationship between antibiotic use and efficacy outcomes in patients with unresectable/advanced HCC.
| Unadjusted analyses |
Adjusted using IPTW∗ |
|||
|---|---|---|---|---|
| No antibiotic use | Antibiotic use | No antibiotic use | Antibiotic use | |
| All patients | ||||
| Sample size | ||||
| No. of patients | 3,435 | 601 | 3,171 | 555 |
| Effect size | 3,435 | 601 | 3,725.77 | 3,730.41 |
| PFS | ||||
| Median (95% CI), months | 4.2 (4.1, 4.5) | 3.6 (3.0, 4.0) | 4.2 (4.0, 4.4) | 3.6 (2.7, 4.0) |
| HR† (95% CI) | 1.36 (1.23, 1.50) | 1.29 (1.22,1.36) | ||
| OS | ||||
| Median (95% CI), months | 10.8 (10.4, 11.3) | 8.6 (7.9, 9.1) | 10.6 (10.3, 11.1) | 8.7 (7.8, 9.6) |
| HR† (95% CI) |
1.42 (1.29, 1.57) |
1.36 (1.29,1.43) |
||
| Tyrosine kinase inhibitor group | ||||
| Sample size | ||||
| No. of patients | 1,640 | 303 | 1,469 | 278 |
| Effect size | 1,640 | 303 | 1,746.74 | 1,752.47 |
| PFS | ||||
| Median (95% CI), months | 5.6 (5.5, 5.7) | 3.7 (3.6, 4.3) | 5.5 (5.4,5.6) | 3.9 (3.6,4.9) |
| HR (95% CI) | 1.40 (1.22, 1.61) | 1.29 (1.19,1.39) | ||
| OS | ||||
| Median (95% CI), months | 12.2 (11.6, 13.1) | 8.4 (7.1, 9.8) | 11.9 (11.1,12.5) | 8.8 (7.4,10.4) |
| HR (95% CI) |
1.51 (1.31, 1.73) |
1.40 (1.30,1.52) |
||
| Immunotherapy group | ||||
| Sample size | ||||
| No. of patients | 696 | 129 | 660 | 120 |
| Effect size | 696 | 129 | 779.56 | 788.67 |
| PFS | ||||
| Median (95% CI), months | 8.2 (7.6, 8.4) | 8.3 (6.8, 8.9) | 8.3 (7.6, 8.5) | 6.8 (5.4, 8.3) |
| HR† (95% CI) | 1.24 (0.97, 1.59) | 1.52 (1.34, 1.73) | ||
| OS | ||||
| Median (95% CI), months | 11.4 (10.6, 12.1) | 10.7 (9.1, 11.8) | 11.5 (10.7,12.3) | 10.7 (9.0,12.1) |
| HR† (95% CI) |
1.24 (0.98,1.56) |
1.22 (1.08,1.38) |
||
| Placebo group | ||||
| Sample size | ||||
| No. of patients | 697 | 97 | 675 | 92 |
| Effect size | 697 | 97 | 766.69 | 772.45 |
| PFS | ||||
| Median (95% CI), months | 1.87 (1.81, 1.93) | 1.61 (1.41, 1.84) | 1.9 (1.8,1.9) | 1.6 (1.4,1.9) |
| HR (95% CI) | 1.29 (1.03, 1.62) | 1.23 (1.11,1.37) | ||
| OS | ||||
| Median (95% CI), months | 8.2 (7.4, 8.9) | 4.4 (3.7, 6.6) | 8.1 (7.3,8.9) | 5.2 (3.7,7.7) |
| HR (95% CI) | 1.58 (1.25, 1.99) | 1.40 (1.25,1.57) | ||
ECOG, European Collaborative Oncology Group; HR, hazard ratio; IPTW, inverse probability of treatment weighting; OS, overall survival; PFS, progression-free survival; VEGF, vascular endothelial growth factor.
The weights were derived from a propensity score (IPTW) model that included the following baseline variables as covariates: age, race, sex, region, ECOG performance status, aetiology of chronic liver disease, presence of macrovascular invasion, presence of extrahepatic disease, and receipt of prior lines of treatment.
HR is stratified by treatment group (tyrosine kinase inhibitor or immune checkpoint inhibitor alone or immune checkpoint inhibitor combination or placebo or VEGF for all patients, or immune checkpoint inhibitor alone or immune checkpoint inhibitor combination for immune checkpoint inhibitor group).
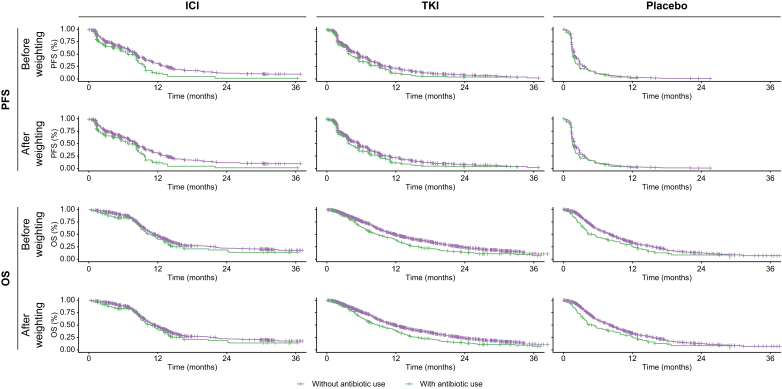
After adjusting for baseline differences using IPTW, estimates for PFS and OS showed a consistent worsening in ATB-exposed patients vs. ATB-unexposed counterparts. Fig. 1 illustrates Kaplan–Meier estimates of PFS and OS by ATB exposure groups across TKI-treated, ICI-treated, and placebo-treated cohorts.
Fig. 1.
Kaplan–Meier curves illustrating estimates for PFS and OS stratified according to antibiotic exposure before and after propensity score weighting in patients who received ICIs, TKIs, and placebo.
ICI, immune checkpoint inhibitor; OS, overall survival; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
Next, we evaluated ICI combination regimens separately from ICI monotherapy.
As shown in Table S5, the unadjusted analyses indicate an association between ATB exposure and worse PFS from ICI monotherapy regimens (median, 2.1 months in ATB-exposed patients vs. 4.0 months; HR 1.51; 95% CI 1.00, 2.28). Following IPTW, we found evidence of worse PFS and OS outcomes in ICI monotherapy and combination therapy for patients with ATB exposure (Fig. S1).
Comparable results were also observed after propensity score matching (Tables S6–S10).
Discussion
The liver immune microenvironment is naturally geared towards spontaneous immune suppression. As chronic liver disease worsens over time, increased intestinal permeability, bacterial overgrowth, or impaired clearance of microbial metabolites by Kupffer cells may increase the translocation of gut microbial species, leading to unopposed pro-inflammatory signalling within the liver and systemically.32,33 An altered intestinal homoeostasis not only is pathogenic in HCC but is intimately linked to the natural progression and prognosis of liver cancer.34 Although evidence gathered in other tumours has revealed an intimate relationship between diversity and taxonomic features of the gut microbiome and responsiveness to ICI, data on HCC are still preliminary.35
Similarly, the effect of ATB, a broad class of therapies associated with the highest disruption of and potential to induce long-lasting changes to the gut microbiome,36 has not been univocally confirmed to affect responsiveness and survival of patients with HCC treated with ICI.28,29
In this study, the first to evaluate the relationship between ATB exposure and outcomes in a large series of patients prospectively recruited to landmark clinical trials of unresectable/advanced HCC, we provide data that ATB exposure within 30 days of treatment initiation is associated with worse PFS and OS across patients treated with a wide variety of systemic therapies for HCC including TKI and ICI monotherapy and combinations.
In ICI recipients, the negative association between ATB and survival outcomes was preserved across ICI monotherapy and combinations including dual PD-1/VEGF pathway blockade, the novel standard of care in advanced HCC.10
In patients receiving immunotherapy, enrichment of certain stool bacterial species including Akkermansia, Ruminococcus, and Bifidobacteria is associated with a higher likelihood of response from ICI.19,37,38 The selective pressure of broad-spectrum ATB exposure on the gut microbiome leads to the expansion of Bacteroides and commensal Clostridia,39 which can in turn facilitate recruitment and activation of immunosuppressive cells including T-regulatory and myeloid-derived suppressor cells – all negatively associated with ICI response.40 One recent study suggested faecal microbial transplantation to restore sensitivity to PD-1 inhibition in patients with advanced melanoma,41 potentially highlighting the central role of the gut microbiome in driving adaptive resistance to ICI.
Although the relationship between ATB and outcomes is supported by mechanistic studies and analyses of clinical data in ICI recipients, the finding of a detrimental effect across multiple treatment arms including placebo in our study is in contrast with evolving evidence in oncological indications other than HCC, where the detrimental effect of ATB pretreatment on response and survival is restricted to ICI therapy and not to chemotherapy25 or targeted therapies unless prescribed after immune-modulating therapies.42 Previously published retrospective evidence in sorafenib recipients had suggested that receipt of broad-spectrum ATB therapy before sorafenib was associated with worse survival in patients with advanced HCC,43 concordantly with our study data.
Reasons and mechanisms explaining the shorter survival observed in patients treated with ATB and TKI are unknown. A significant component of the efficacy of sorafenib potentially resides in the capacity to improve the T-effector/T-regulatory cell ratio and augment the proportion of interferon-γ-secreting CD8+ T cells.44 Similarly, other molecularly targeted therapies such as lenvatinib45 and cabozantinib46 exert their pleiotropic antitumour effects by acting on lymphoid and myeloid constituents of the tumour microenvironment both alone and in synergy with concurrent PD-1 pathway inhibition. In addition, evidence from renal cell carcinoma suggests that VEGF pathway inhibitors can directly alter intestinal homoeostasis,18 highlighting how ATB may plausibly alter a complex bidirectional relationship between TKI therapy and survival by acting synergistically on composition and function of the gut microbiome.
In attempting to interpret potentially causal as opposed to purely associative links between ATB and outcome, a clinically important finding from our study is the demonstration of a detrimental effect of ATB on survival observed in patients who received placebo. Although it is possible that the postulated changes in the gut microbiome and immune function mediated by ATB preconditioning could have exerted their influence even in the absence of anticancer therapy, an alternative explanation that we should consider is whether ATB dosing before treatment might select for patients with uncontrolled infection, who are at higher risk for recurrent severe infections, or who have rapidly progressive disease before treatment allocation. Patients with cirrhosis carry a higher risk of bacterial infections compared with the general population,47 and both empirical and prophylactic ATB therapy is indicated in response to decompensation of chronic liver disease to improve survival outcomes.48 In the absence of adequately powered translational studies evaluating the effect of ATB therapy on stool samples and peripheral immune cells, support for a causative or an associative relationship between ATB and outcome remains speculative.
Although no direct causative role between ATB and outcome can be inferred from our retrospective analysis, the utilisation of prospectively accrued, geographically heterogeneous patient cohorts, carefully selected on the basis of stringent inclusion criteria and with careful documentation of response and survival outcomes lends significant credibility to the associations observed. In addition, use of methods such as IPTW that adjust for key clinicopathologic features of cirrhosis and HCC affords sufficient robustness to our survival estimates across ATB strata and reduces the risk of bias. Among important limitations to our study are the issues associated with retrospectively examining data from completed studies. These included missing information on the number and sizes of HCC lesions, baseline AFP levels, and BCLC stages in a proportion of patients, which did not allow us to consider these variables for matching. Moreover, the restriction of ATB exposure window to 30 days might have failed to capture clinically meaningful exposures that extended beyond 30 days from treatment initiation as well as the impact of ATB treatment later during the course of systemic therapy.20 In addition, the lack of information on indication (i.e. prophylactic vs. therapeutic), ATB class, and course length made further subgroup analyses impossible and might represent a limitation. Furthermore, we acknowledge significant heterogeneity in the studies selected for this analysis: the entirety of TKI studies included phase III trials powered on OS, whereas a number of ICI studies included phase I/II studies powered on safety outcomes and response rates, a finding that may have affected the maturity of OS and PFS data pooled for our analysis. The line of therapy was also heterogeneous across studies, and none of our OS estimates could be adjusted for post-study therapy. Finally, causes of death are not available, preventing further analyses on infection-related outcomes.
In conclusion, our study demonstrates that ATB exposure may be associated with worse PFS and OS in patients receiving ICI monotherapy as well as combinations. Although the positive association identified in our study does not prove causality, the hypothesis that an ATB-mediated gut dysbiosis may correlate with reduced antitumour efficacy is provocative and resonates with evolving clinical and translational knowledge in the field. The association between ATB exposure and outcomes from TKI therapy and placebo highlights the uniquely complex interplay between cirrhosis, cancer, risk of infection, and the pleiotropic effect of molecular therapies for HCC. To overcome the complexity of these distinctively intertwined relationships, prospective studies should investigate the role of gut microbial diversity as a determinant of outcome in patients with HCC undergoing systemic therapy. The rapid expansion of immunotherapy combinations, for which no predictive biomarker of benefit exists, highlights the importance of this stream of research as a pathway towards personalised medicine in HCC.10
Financial support
AD is supported by the NIHR Imperial BRC and by grant funding from EASL (Andrew Burroughs Fellowship) and Cancer Research UK (RCCPDB-Nov21/100008). DJP is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and the Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG 25697), and acknowledges grant support from the Cancer Treatment and Research Trust (CTRT) and infrastructural support by the Imperial Experimental Cancer Medicine Centre and the NIHR Imperial Biomedical Research Centre. AC is supported by the NIHR Imperial BRC.
Authors’ contributions
Study concept and design: DJP, LP, JS, PMK. Acquisition of data: XL, PMK, GW, JS. Analysis and interpretation of data: XL, PMK, DJP, LP, GW, AD, CAMF, BS, DRR, RP, MT, SC, SL, LF, AC. Drafting of the manuscript: DJP. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: XL, PMK. Obtained funding: DJP. Study supervision: DJP, LP.
Data availability statement
The data that support the findings of this study are available from the US FDA. Restrictions apply to the availability of these data, and employees of the FDA performed all raw data analyses.
Conflicts of interest
DJP received lecture fees from ViiV Healthcare, Bayer Healthcare, EISAI, BMS, and Roche; travel expenses from BMS and Bayer Healthcare; and consulting fees for Mina Therapeutics, DaVolterra, Mursla, IPSEN, Exact Sciences, Avamune, EISAI, Roche, and Astra Zeneca. DJP received research funding (to institution) from MSD, GSK, and BMS. BS received travel support from AbbVie, Gilead, and Ipsen. AC received consulting fees from MSD, Astra Zeneca, Roche, and BMS. He also received speaker fees from Novartis, Astra Zeneca, and EISAI. AD received educational grant support for conference attendance by Roche. There are no other personal or financial conflicts of interest to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100747.
Contributor Information
David J. Pinato, Email: david.pinato@imperial.ac.uk.
Lorraine Pelosof, Email: Lorraine.Pelosof@fda.hhs.gov.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Fulgenzi C.A.M., D'Alessio A., Talbot T., Gennari A., Openshaw M.R., Demirtas C.O., et al. New frontiers in the medical therapy of hepatocellular carcinoma. Chemotherapy. 2022;67:164–172. doi: 10.1159/000521837. [DOI] [PubMed] [Google Scholar]
- 2.Finn R.S., Merle P., Granito A., Huang Y.H., Bodoky G., Pracht M., et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353–358. doi: 10.1016/j.jhep.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Haber P.K., Puigvehí M., Castet F., Lourdusamy V., Montal R., Tabrizian P., et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002–2020) Gastroenterology. 2021;161:879–898. doi: 10.1053/j.gastro.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Galluzzi L., Chan T.A., Kroemer G., Wolchok J.D., López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aat7807. [DOI] [PubMed] [Google Scholar]
- 5.Llovet J.M., Castet F., Heikenwalder M., Maini M.K., Mazzaferro V., Pinato D.J., et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 6.Yau T., Park J.W., Finn R.S., Cheng A.L., Mathurin P., Edeline J., et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhu A.X., Finn R.S., Edeline J., Cattan S., Ogasawara S., Palmer D., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 8.Shek D., Read S.A., Nagrial A., Carlino M.S., Gao B., George J., et al. Immune-checkpoint inhibitors for advanced hepatocellular carcinoma: a synopsis of response rates. Oncologist. 2021;26:e1216–e1225. doi: 10.1002/onco.13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 10.Muhammed A., D'Alessio A., Enica A., Talbot T., Fulgenzi C.A.M., Nteliopoulos G., et al. Predictive biomarkers of response to immune checkpoint inhibitors in hepatocellular carcinoma. Expert Rev Mol Diagn. 2022;22:253–264. doi: 10.1080/14737159.2022.2049244. [DOI] [PubMed] [Google Scholar]
- 11.Cortellini A., Bersanelli M., Buti S., Cannita K., Santini D., Perrone F., et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhammed A., Fulgenzi C.A.M., Dharmapuri S., Pinter M., Balcar L., Scheiner B., et al. The systemic inflammatory response identifies patients with adverse clinical outcome from immunotherapy in hepatocellular carcinoma. Cancers. 2021;14:186. doi: 10.3390/cancers14010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheiner B., Pomej K., Kirstein M.M., Hucke F., Finkelmeier F., Waidmann O., et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy – development and validation of the CRAFITY score. J Hepatol. 2022;76:353–363. doi: 10.1016/j.jhep.2021.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Pinato D.J., Marron T.U., Mishra-Kalyani P.S., Gong Y., Wei G., Szafron D., et al. Treatment-related toxicity and improved outcome from immunotherapy in hepatocellular cancer: evidence from an FDA pooled analysis of landmark clinical trials with validation from routine practice. Eur J Cancer. 2021;157:140–152. doi: 10.1016/j.ejca.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Hussain N., Naeem M., Pinato D.J. Concomitant medications and immune checkpoint inhibitor therapy for cancer: causation or association? Hum Vaccin Immunother. 2021;17:55–61. doi: 10.1080/21645515.2020.1769398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortellini A., Tucci M., Adamo V., Stucci L.S., Russo A., Tanda E.T., et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinato D.J., Gramenitskaya D., Altmann D.M., Boyton R.J., Mullish B.H., Marchesi J.R., et al. Antibiotic therapy and outcome from immune-checkpoint inhibitors. J Immunother Cancer. 2019;7:287. doi: 10.1186/s40425-019-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derosa L., Routy B., Fidelle M., Iebba V., Alla L., Pasolli E., et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol. 2020;78:195–206. doi: 10.1016/j.eururo.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 19.Derosa L., Routy B., Thomas A.M., Iebba V., Zalcman G., Friard S., et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28:315–324. doi: 10.1038/s41591-021-01655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derosa L., Hellmann M.D., Spaziano M., Halpenny D., Fidelle M., Rizvi H., et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29:1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinato D.J., Howlett S., Ottaviani D., Urus H., Patel A., Mineo T., et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5:1774–1778. doi: 10.1001/jamaoncol.2019.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinstock C., Maher V.E., Fernandes L.L., Tang S., Agrawal S., Brave M.H., et al. Impact of antibiotic use on clinical outcomes in patients with urothelial cancer receiving a programmed death protein 1 or programmed death ligand 1 (anti-PD-1/L1) antibody. J Clin Oncol. 2019;37:4557. doi: 10.1200/JCO.2019.37.15_suppl.4557. [DOI] [PubMed] [Google Scholar]
- 23.Weinstock C., Bandaru P., Fernandes L.L., Chang E., Girvin A., Tang S., et al. Impact of timing of antibiotic use on clinical outcomes in patients with urothelial cancer treated with immune checkpoint inhibitors (ICIs) J Clin Oncol. 2020;38(15_suppl) 5045–5045. [Google Scholar]
- 24.Maher V.E., Fernandes L.L., Weinstock C., Tang S., Agarwal S., Brave M., et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37:2730–2737. doi: 10.1200/JCO.19.00318. [DOI] [PubMed] [Google Scholar]
- 25.Cortellini A., Di Maio M., Nigro O., Leonetti A., Cortinovis D.L., Aerts J.G., et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortellini A., Ricciuti B., Facchinetti F., Alessi J.V.M., Venkatraman D., Dall'Olio F.G., et al. Antibiotic-exposed patients with non-small-cell lung cancer preserve efficacy outcomes following first-line chemo-immunotherapy. Ann Oncol. 2021;32:1391–1399. doi: 10.1016/j.annonc.2021.08.1744. [DOI] [PubMed] [Google Scholar]
- 27.Elkrief A., Derosa L., Kroemer G., Zitvogel L., Routy B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor? Ann Oncol. 2019;30:1572–1579. doi: 10.1093/annonc/mdz206. [DOI] [PubMed] [Google Scholar]
- 28.Cheung K.S., Lam L.K., Seto W.K., Leung W.K. Use of antibiotics during immune checkpoint inhibitor treatment is associated with lower survival in hepatocellular carcinoma. Liver Cancer. 2021;10:606–614. doi: 10.1159/000518090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fessas P., Naeem M., Pinter M., Marron T.U., Szafron D., Balcar L., et al. Early antibiotic exposure is not detrimental to therapeutic effect from immunotherapy in hepatocellular carcinoma. Liver Cancer. 2021;10:583–592. doi: 10.1159/000519108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Alessio A., Fulgenzi C.A.M., Nishida N., Schönlein M., von Felden J., Schulze K., et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child–Pugh A and B cirrhosis: a real-world study. Hepatology. 2022;76:1000–1012. doi: 10.1002/hep.32468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinato D.J., Kaneko T., Saeed A., Pressiani T., Kaseb A., Wang Y., et al. Immunotherapy in hepatocellular cancer patients with mild to severe liver dysfunction: adjunctive role of the ALBI grade. Cancers. 2020;12:1862. doi: 10.3390/cancers12071862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao X., Wang N., Qin W. Gut microbiota and hepatocellular carcinoma. Gastrointest Tumors. 2015;2:33–40. doi: 10.1159/000380895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinato D.J., Guerra N., Fessas P., Murphy R., Mineo T., Mauri F.A., et al. Immune-based therapies for hepatocellular carcinoma. Oncogene. 2020;39:3620–3637. doi: 10.1038/s41388-020-1249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwabe R.F., Greten T.F. Gut microbiome in HCC – mechanisms, diagnosis and therapy. J Hepatol. 2020;72:230–238. doi: 10.1016/j.jhep.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y., Wang T., Tu X., Huang Y., Zhang H., Tan D., et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7:193. doi: 10.1186/s40425-019-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez-Cobas A.E., Gosalbes M.J., Friedrichs A., Knecht H., Artacho A., Eismann K., et al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut. 2013;62:1591–1601. doi: 10.1136/gutjnl-2012-303184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gopalakrishnan V., Spencer C., Nezi L., Reuben A., Andrews M., Karpinets T., et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.L., et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spakowicz D., Hoyd R., Muniak M., Husain M., Bassett J.S., Wang L., et al. Inferring the role of the microbiome on survival in patients treated with immune checkpoint inhibitors: causal modeling, timing, and classes of concomitant medications. BMC Cancer. 2020;20:383. doi: 10.1186/s12885-020-06882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naqash A.R., Kihn-Alarcón A.J., Stavraka C., Kerrigan K., Maleki Vareki S., Pinato D.J., et al. The role of gut microbiome in modulating response to immune checkpoint inhibitor therapy in cancer. Ann Transl Med. 2021;9:1034. doi: 10.21037/atm-20-6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baruch E.N., Youngster I., Ben-Betzalel G., Ortenberg R., Lahat A., Katz L., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 42.Lalani A.A., Xie W., Braun D.A., Kaymakcalan M., Bosse D., Steinharter J.A., et al. Effect of antibiotic use on outcomes with systemic therapies in metastatic renal cell carcinoma. Eur Urol Oncol. 2020;3:372–381. doi: 10.1016/j.euo.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pomej K., Balcar L., Scheiner B., Semmler G., Meischl T., Mandorfer M., et al. Antibiotic therapy is associated with worse outcome in patients with hepatocellular carcinoma treated with sorafenib. J Hepatocell Carcinoma. 2021;8:1485–1493. doi: 10.2147/JHC.S317957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalathil S.G., Hutson A., Barbi J., Iyer R., Thanavala Y. Augmentation of IFN-γ+ CD8+ T cell responses correlates with survival of HCC patients on sorafenib therapy. JCI Insight. 2019;4 doi: 10.1172/jci.insight.130116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torrens L., Montironi C., Puigvehí M., Mesropian A., Leslie J., Haber P.K., et al. Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatology. 2021;74:2652–2669. doi: 10.1002/hep.32023. [DOI] [PubMed] [Google Scholar]
- 46.Esteban-Fabró R., Willoughby C.E., Piqué-Gili M., Montironi C., Abril-Fornaguera J., Peix J., et al. Cabozantinib enhances anti-PD1 activity and elicits a neutrophil-based immune response in hepatocellular carcinoma. Clin Cancer Res. 2022;28:2449–2460. doi: 10.1158/1078-0432.CCR-21-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernández J., Navasa M., Gómez J., Colmenero J., Vila J., Arroyo V., et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 48.Fernández J., Tandon P., Mensa J., Garcia-Tsao G. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology. 2016;63:2019–2031. doi: 10.1002/hep.28330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the US FDA. Restrictions apply to the availability of these data, and employees of the FDA performed all raw data analyses.