Abstract
Neural tissue engineering (NTE) has made remarkable strides in recent years and holds great promise for treating several devastating neurological disorders. Selecting optimal scaffolding material is crucial for NET design strategies that enable neural and non-neural cell differentiation and axonal growth. Collagen is extensively employed in NTE applications due to the inherent resistance of the nervous system against regeneration, functionalized with neurotrophic factors, antagonists of neural growth inhibitors, and other neural growth-promoting agents. Recent advancements in integrating collagen with manufacturing strategies, such as scaffolding, electrospinning, and 3D bioprinting, provide localized trophic support, guide cell alignment, and protect neural cells from immune activity. This review categorises and analyses collagen-based processing techniques investigated for neural-specific applications, highlighting their strengths and weaknesses in repair, regeneration, and recovery. We also evaluate the potential prospects and challenges of using collagen-based biomaterials in NTE. Overall, this review offers a comprehensive and systematic framework for the rational evaluation and applications of collagen in NTE.
Keywords: Neural tissue engineering, Collagen scaffolds, Neural regeneration, 3D bioprinting
Graphical abstract
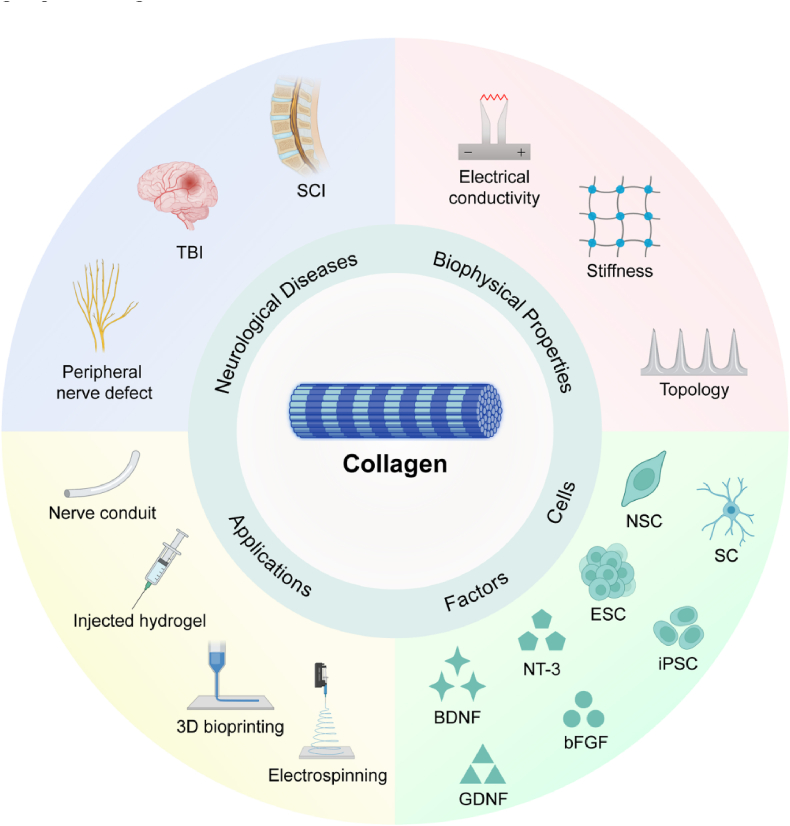
The review provides an overview of the design principles of collagen-based biomaterials, including their mechanical, biomedical, and electrical properties, which have gained increasing attention in recent years for neural regeneration, particularly in the adaptation of stem cells for the repair of neural injuries.

Highlights
-
•
The importance of collagen-based biomaterials in neural tissue engineering for repairing the central and peripheral nervous systems.
-
•
This review provides a comprehensive perspective of collagen-based biomaterials in neural tissue engineering, covering different manufacturing strategies, therapeutic potentials, challenges, and future prospects.
-
•
The review covers essential issues related to collagen-based biomaterials in neural tissue engineering, including characterization and application forms of collagen, current state-of-the-art technologies, application scenarios, challenges, and potential solutions, and prospects for treating neurological disorders and injury repair.
Abbreviations
- 3D
Three-dimensional
- BDNF
Brain derived neurotrophic factor
- bFGF
Basic fibroblast growth factor
- BMMCs
Bone marrow mononuclear cells
- CNS
Central nervous system
- CSPGs
Chondroitin sulfate proteoglycans
- DRG
Dorsal root ganglia
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- ESC
Embryonic stem cell
- FGF
Fibroblast growth factor
- GAG
Glycosaminoglycan
- GDNF
Glial cell derived neurotrophic factor
- HA
Hyaluronic acid
- HUVEC
Human umbilical vein endothelial cell
- IL-1α
Interleukin-1-alpha
- iPSC
induced pluripotent stem cell
- NIPS
Non-solvent induced phase separation
- NPC
Neural progenitor cell
- NSC
Neural stem cell
- NSPC
Neural stem/progenitor cell
- NT-3
Neurotrophin-3
- NTE
Neural tissue engineering
- OPC
Oligodendrocyte progenitor cell
- PCL:
Polycaprolactone
- PD
Parkinson's disease
- PNI
Peripheral nerve injury
- PNS
Peripheral nervous system
- SC
Schwann cell
- SCI
Spinal cord injury
- SDF-1α
Stromal-cell-derived factor 1α
- TBI
Traumatic brain injury
- TIPS
Thermally induced phase separation
- TNF-α:
Tumor necrosis factor-alpha
- VEGF
Vascular endothelial growth factor
1. Introduction
The mammalian nervous system is a highly complex physiological networks, consisting of the central nervous system (CNS) and peripheral nervous system (PNS). Damage to the nervous system significantly impacts medical care and the economy. The CNS, comprising the brain and spinal cord, exhibits a limited ability to regeneration due to the adult nervous system's inherent bias against neural differentiation and regeneration compared to the PNS [1]. In the CNS, the microenvironment that inhibits axon regeneration is more pronounced, and destroyed neuronal cell populations and disrupted synaptic connections tend to be irreversibly regenerative since mature neurons exhibit limited capability of axon outgrowth [2,3]. Medical treatments have failed to treat neuronal damage, which makes symptomatic relief the most reasonable solution for injury progression [4,5]. Axon regeneration in the CNS is adversely restricted after traumatic brain injury (TBI), spinal cord injury (SCI), and related circumstances involving fatal axonal disruption. On the contrary, PNS is prone to regenerate in certain cases to realize functional restoration, especially when the injury encompasses a relatively short distance [6]. When the damage occurs, an inhibitory microenvironment in the lesion sites will form to hinder axonal regeneration, in which case cell necrosis and synaptic disconnection are primarily irreversible since mature neurons tend to exhibit restricted regenerative potential in the CNS. Injury cascades of the CNS are further extensive to dimensional pathophysiological events such as ongoing demyelination, glial scar formation, ischemia, ionic dysregulation, and cystic cavitation [7]. In terms of the PNS, despite the optimistic regenerative capacity, functional restoration tends to be poor at the significant lesions in length (>10 mm) owing to the inadequate axonal bridging of distal targets [8]. Producing neurotrophins, and guiding axons toward their synaptic terminals by eradicating myelin debris, proliferative SC and macrophages restore neuronal function. However, the complication in CNS injury and regeneration is more pronounced. Wallerian degeneration takes place in the damage tract following the CNS injury. Nevertheless, since there was minimal macrophage recruitment after oligodendrocyte apoptosis, the degenerative myelin and axonal debris lasted significantly longer. Specific mechanism involved after the CNS and PNS injuries are summarized in Table 1.
Table 1.
Injury mechanism and confronted repair challenges of the PNS and the CNS.
| Scenario | Injury mechanism | Repairing challenges | Refs |
|---|---|---|---|
| PNS |
|
|
[8,44] |
| CNS |
|
|
[45,46] |
The extracellular matrix (ECM) plays a pivotal role in maintaining the architectural backbone and regulating cellular crosstalk in the nervous system. Dynamic changes in normal and injured nervous tissues of ECM compositions could be potential guidance for biomaterial design in NTE. For CNS, the constitution of a typical brain ECM is distinct in its presence of a hyaluronan backbone to which chondroitin sulfate proteoglycans from the lectican family and tenascins are linked [9], which are secreted by neurons, astrocytes, oligodendrocytes and microglia residing in brain. Additional ECM constituents in the basement membranes that support the blood-brain barrier comprise laminins, collagen, fibronectin, and heparan sulfate proteoglycans produced by neurons, glia, and endothelial cells [10]. In response to TBI, certain ECM composition levels and distributions would alter. For instance, chondroitin sulfate proteoglycans (CSPGs), comprising lecticans, phosphacan, and NG2, are the most studied proteoglycan in the nervous system. In the context of TBI, aggrecan and phosphate content decrease in the injury core, while neurocan, Versican-V2, NG-2, fibronectin, Tenascin-C, Tenascin-R and osteopontin levels are relatively elevated. Moreover, certain immunoreactive molecules are activated such as laminin, perlecan, glypicans and syndecans [11]. In SCI, dynamic changes of ECM have been prevalently examined for comprehending how ECM components affect axonal restoration. It is acknowledged that specific ECM compositions play an inhibitory role in neural regeneration, especially chondroitin sulfate glycosaminoglycan-chains (GAG-chains) on proteoglycans at the lesion site. Other typically discussed scar components encompass those from fibrotic scars with basement membrane-like composition, such as collagen, laminins, and fibronectin. Furthermore, such core ECM proteins degrade in response to damage, such as matrix metallopeptidases and elastases [12]. Recent research has focused on cathepsins, another catalytic ECM-related protein group, in the spinal cord as potential ECM regulators, which serves as an illustration of the undiscovered roles in ECM along with associated proteins in CNS injury [13]. As for the PNS, ECM, secreted by Schwann cells, resides in the form of the basal lamina, which is composed of collagen type IV, laminin, fibronectin, and nidogens [14,15]. In the degenerated peripheral nerves, CSPGs and upregulated myelin-associated inhibitors of regeneration might hinder axonal outgrowth. Unlike the CNS injury, the distal portion of axons and myelin detritus are lysed and eliminated due to the activation of Schwann cells and the recruitment of macrophages, paving the way for removing inhibitory factors [16]. Notably, Collagen I, III, IV, V, VI and laminin exhibit significantly elevated deposition in Schwann and perineurial cells during peripheral injuries and neuropathies [17].
Current medical interventions for treating neuronal damage are often inadequate and offer little more than symptomatic relief [18]. Even the most promising current strategies are limited in their ability to achieve complete damage repair or functional restoration due to a lack of understanding of neurodevelopment [19,20]. Animal models have limitations in representing human-specific features of neurodevelopment, and the use of human-induced pluripotent stem cells (hiPSCs) in a two-dimensional culture system fails to capture the complexity of the nervous system [[21], [22], [23], [24]]. Therefore, various strategies have emerged to assist in neural tissue engineering, including applying specific biomaterials and integrating cells and bioactive substances to repair damaged nerves [[25], [26], [27], [28], [29], [30], [31], [32]]. While these methods show promise, they require sophisticated architectural control and customization for more widespread application [33]. The fundamental principle of NTE is to create a biomimetic microenvironment that promotes neural tissue regeneration. Effective neural scaffolds in NTE should meet the following criteria: biocompatibility, controllable biodegradability, appropriate electrical conduction, an interconnected 3D porous architecture, and mimetic biophysical properties that aid in structural bridging and migratory cell guidance in lesion sites [34,35]. 3D scaffolds provide physical support for nerve regeneration, improving host tissue engraftment and enabling the regenerative tissue to attain cellular function [[36], [37], [38]].
The toolbox of conventional scaffold processing methods in tissue engineering includes gas foaming, freeze drying, melt moulding, electrospinning, phase separation, and rapid prototyping (RP) techniques, such as electrospinning and 3D bioprinting. Collagen, the most abundant protein in mammals, is a prevalent candidate for skin, cornea, cardiovascular, cartilage, and neural tissues for high biocompatibility and ease of manufacturing. In NTE, functionalized collagen electrospun mesh has been shown to promote the orientational migration of neural stem cells (NSCs) [39]. A tailored collagen scaffold also provided a robust platform for repair of spinal cord injury [40]. When integrated with additional bioactive components such as agarose [40], heparin sulfate [41], silk fibroin [42], and fibrin [43], it can have applications in drug screening and cancer modelling [33].
The processing techniques mentioned above have limitations in precisely altering scaffold geometry and inner channel architecture to guide cellular behaviours. In addition, these approaches restrict combined cellular participation in one-pot manufacturing, which post-cell-seeding processes could achieve. In contrast, 3D bioprinting offers the dominant advantages of customized scaffold dimensions and geometry involving various cell types, biomaterials, and growth factors. Furthermore, the selection of bioink in 3D bioprinting can regulate neuronal proliferation, differentiation, and migration in NTE. Although there are pros and cons to biofabricating neural tissues, this review will discuss collagen biosynthesis, extraction, and manufacturing approaches and evaluate current advancements in the utilization of collagen in NTE. This includes collagen scaffolds and bioprinting-mediated collagen processing in the context of neural injury repair, disease modeling, and drug screening based on neuroregenerative strategies (Fig. 1). Finally, we systematically review the present obstacles and possible future directions for collagen-based biomaterials for NTE. We aim to provide researchers with comprehensive knowledge and inspire thought-provoking ideas.
Fig. 1.
Schematic drawing of collagen-based therapeutical applications, applying diverse strategies to regenerate CNS and PNS. bFGF: basic fibroblast growth factor; NT-3: Neurotrophin-3; BDNF: Brain Derived Neurotrophic Factor; GDNF: Glial Cell Derived Neurotrophic Factor; ESC: Embryonic stem cell; iPSC: induced pluripotent stem cell; SC: Schwann cell; NSC: neural stem cell; TBI: traumatic brain injury; SCI: spinal cord injury. Created with BioRender.com.
2. Collagen
Selecting appropriate materials with favourable physical, chemical, and mechanical properties for neural repair is indispensable because the porosity, mechanical properties, biocompatibility, and biodegradation can be highly decisive in achieving ideal repair effects for the nervous system. Various biomaterials have been applied in NTE including synthetic polymers, polysaccharides, and proteins. Table 2 summarizes current applied materials in NTE and its relative advantages and disadvantages. Collagen, the most abundant protein in mammals, accounts for approximately 25–30% of the total protein composition. Decades of studies have identified 28 distinct types of collagen, each composed of homotrimers and heterotrimers formed by three polypeptide chains (referred to as α-chains) [47]. The characteristic structural feature of all collagens refers to the triple helix - a tight right-handed helix of three α-chains, each of which comprises at least one region characterized by the repeating amino acid motif Gly-X-Y, where X and Y can be any amino acid [48]. Notably, approximately 80–90% of the collagen in the body primarily consists of collagen I, II, and III, also known as fibril-forming collagen [49]. Collagen I is the most widely distributed collagen in skin, bone, tendon, lung, and the vasculature network. The distribution of collagen II is comparatively restricted to cartilage, while collagen III is predominantly found in elastic tissues such as lungs and blood vessels [50]. The intricate hierarchical architecture determines its versatility as a building block in tissue engineering. Adaptation can fulfill a given function at all levels, resulting in a wide range of properties.
Table 2.
Advantages and disadvantages of biomaterials in NTE.
| Polymer type | Polymer name | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Proteins | Collagen |
|
|
[18,51,52] |
| Gelatin |
|
|
[53,54] | |
| Polysaccharides | Hyaluronic acid |
|
|
[55,56] |
| Alginate |
|
|
[57,58] | |
| Chitosan |
|
|
[59,60] | |
| Synthetic polymers | PCL |
|
|
[61] |
| PLLA |
|
|
[62,63] | |
| PLGA |
|
|
[64,65] | |
| PPy |
|
|
[66] | |
| Carbon nanotubes |
|
|
[67] |
2.1. A glance of collagen: from biosynthesis to purification
In connective tissues, the fibroblast is responsible for most collagen production [68]. Our distinct processes are involved in the assembly of collagen fibrils after the transcription and translation of the procollagen α-chains are completed: (i) the importation of the rough endoplasmic reticulum, where triple-helical procollagen is sequentially formed after the modification of α-chains; (ii) the Golgi apparatus assists in the modification of procollagen, which is subsequently packaged into secretory vesicles; (iii) then extracellular cleavage of procollagen to generate collagen molecules; (iv) lysyl oxidase-catalyzed crosslinking of collagen molecules to reinforce their supramolecular structures [[69], [70], [71]]. Following the secretion of procollagen into the extracellular space, collagen-type-specific metalloproteinase enzymes cleave the amino- and carboxy-propeptides. Microfibrils of collagen characterize the extracellular matrix of connective tissues with a cross-striated structure [72]. Fibril-forming collagens are promising candidates for developing collagen-based biomaterials [73].
Collagen I is typically derived from pig, bovine, ovine skin, and tendon tissues for biomedical uses, whereas collagen II is mainly obtained from bovine, porcine, and chicken cartilaginous tissues. Rat-tail tendon-derived collagen was used in most preliminary collagen research due to its excellent purity and ease of extraction [74]. However, the covalently crosslinked fibrillar network restricts further applications despite the merits of excellent biocompatibility, easy availability, and high versatility. Various extraction and purification approaches are primarily carried out to tackle this challenge, including acidic solution, neutral salt, enzymatic, and alkali treatment. The main objective of these methods is to maximize the preservation of the supramolecular structure of collagen. A summary of the principles, advantages, and disadvantages of different collagen extraction methods is presented in Table 3.
Table 3.
Summary of distinct approaches of collagen extraction.
| Methods | Principles | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Acidic treatment | After lipid and protein removal, the sample is immersed in a diluted acidic solution, in which case, salt bonds between collagen molecules are broken, allowing the collagen to be solubilized. Acid-soluble collagen is obtained following salinization, dialysis, and other processes. |
|
|
[75,76] |
| Enzymatic treatment | The protease is used to restrictively cleave the terminal peptide of collagen. The remaining body part is soluble in neutral or acidic solutions, resulting in the extraction of enzyme-soluble collagen. |
|
|
[77,78] |
| Genetic engineering | The technology of designing, enzymatic cleavage, and splicing of human collagen genes with specific sequences, ligating vectors and then transferring them into engineered cells, and expressing the produced collagen and its analogs by fermentation. Different expression systems, such as E. coli, yeast, insect cells, and transgenic crops, are involved. |
|
|
[79] |
2.2. Typical characterization of collagen and collagen-based scaffolds
Collagen is a thermal-responsive biomaterial that can gel in-situ at physiological temperatures and neutral pH, making it suitable for use as an injectable hydrogel [80]. However, since the absence of lysyl oxidase in vitro, the mechanical strength of spontaneously self-assembled fibrils tends to be poor under physiological conditions. Hence, appropriate crosslinking approaches are utilized to enhance tissue-matched mechanical properties and enzymatically degradable transplantation scenarios in vivo. The basic principle of the crosslinking reaction involves modifying amine and carboxyl groups of collagen molecules, resulting in the formation of covalent bonds [68].
In tissue engineering applications of collagen-based biomaterials, immunogenicity is inevitably considered. Collagen is generally reckoned as a safe biomaterial for regenerative utilization due to low immunogenicity. Even though animal derivation may provoke an immune response, collagen is still categorized as a weak antigen [81]. Non-collagenous proteins [82], cells, and crosslinker residues mentioned may also trigger immune response [83]. Research has verified that terminal non-helical regions, known as telopeptides, might also cause an immunological response [84]. To minimize the immunological effects of collagen, telopeptides could be cleaved via proteolytic enzyme treatment, and an appropriate collagen source and extraction process can also be selected [52].
Collagen can promote cell adhesion and intracellular crosstalk with other cell types [85], leading to a cascade of cellular activity, including activating receptors as well as inducing gene transcriptions in dimensional signalling pathways in neural cells [86,87]. Regarding collagenous participation in neural cells in NTE, a previous study has demonstrated that embryo-derived neural stem cells cultured on collagen can differentiate into mature neurons with neuronal polarity and neurite outgrowth [88]. Furthermore, collagen 3D culture has also exhibited remarkable chemotropism in differentiated neurons [89].
3. Collagen-based biomaterials in the application of NTE
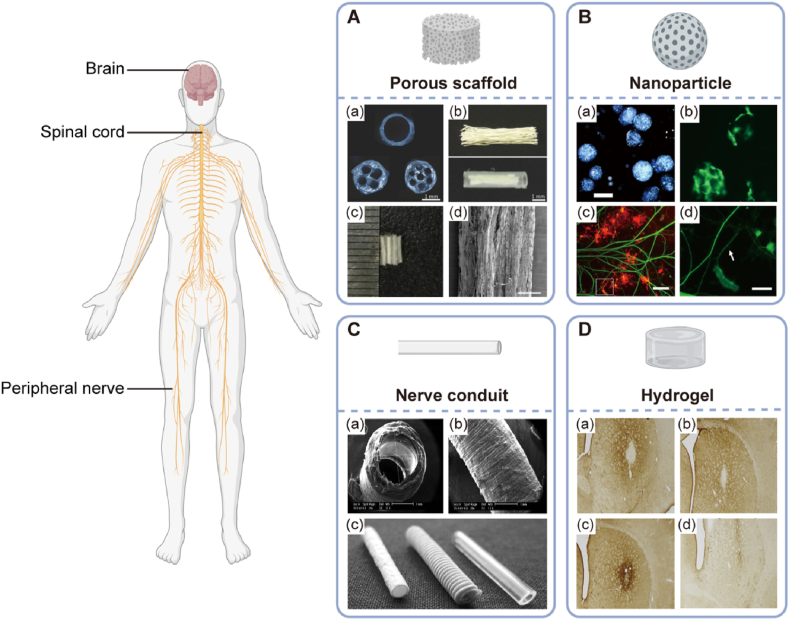
As one of the most competitive natural biomaterials, collagen beacons have a promising future in the therapeutic application of NTE, such as injury repair and functional restoration. Collagen-based biomaterials have been prevalent in coping with therapeutic dilemmas in NTE, such as neuroprotection and neuroregeneration. Collagen can be processed into scaffolds, microspheres, nerve conduits, and injectable hydrogels (Fig. 2). No matter what approach mentioned above should meet the following requirements: (i) providing structural bridging in lesion sites and cell-favourable microenvironment for neuronal proliferation and differentiation to facilitate axon outgrowth; (ii) releasing growth factors to stimulate neural cells to regenerate, especially in neurodegenerative diseases such as Alzheimer's, Parkinson's diseases, or spinal cord injury; (iii) encapsulate neural-related cells to accomplish therapeutic efficacy. This section will focus on the multitude of preparation formats of collagen comprising collagen scaffolds, fibrous or tubular conduits, micro/nanoparticles, and integrative collagen bioink for the regenerative solutions of NTE.
Fig. 2.
Forms of collagen-based biomaterials in CNS and PNS repair application. A: Collagen porous scaffold. (a) Multichannel collagen scaffolds as peripheral nerve conduits with 1, 4, and 7 channels, respectively. Reproduced with permission. [90] Copyright 2010, Elsevier. (b) Linear ordered collagen scaffold fibers within a silicon tube. Reproduced with permission. [91] Copyright 2011, Elsevier. (c and d) Modified collagen scaffold in acute spinal cord injury repair. Reproduced with permission. [92] Copyright 2017, Elsevier. B: Collagen microsphere-based cell carrier in oligodendrocyte progenitor cell growth and differentiation. (a) Fabricated collagen microspheres. Scale bar: 200 μm. (b) 3T3 cells expanded on collagen microspheres were labeled with Calcein. Scale bar: 200 μm. (c) Co-culture of dorsal ganglions (DRGs) with oligodendrocyte progenitor cells (OPCs). Scale bar: 200 μm. (d) Magnified image depicting oligodendrocytes (OLs) wrapping DRG neurites in an arrow. Scale bar: 50 μm. Reproduced with permission. [93] Copyright 2013, Springer. C: Nerve conduits in peripheral nerve repair. (a and b) Schematic overview of electrospun collagen-based peripheral nerve conduits. Scale bar: 1 mm. Reproduced with permission. [94] Copyright 2017, Wiley-VCH GmbH. (c) Commercial peripheral nerve conduit. Left to right: Collagen I conduit; polyglycolic acid conduit; poly (DL-lactid-ε-caprolactone) conduit (Neurolac; Polyganics BV, Groningen, The Netherlands). Reproduced with permission. [95] Copyright 2009 Georg Thieme Verlag KG. D: Collagen hydrogel in rat brain recovery. A striatal microgliosis response to the implanted cells encapsulated in collagen hydrogel was detectable in the host striatum. Reproduced with permission. [96] Copyright 2013, Elsevier.
3.1. Collagen scaffolds
Recent advancements in tissue engineering have led to the development of various techniques for fabricating collagen scaffolds. Freeze-drying processing was pioneered by Yannas et al. to create porous collagen-glycosaminoglycan scaffolds, which could be packed with therapeutically active chemicals that are released as the scaffold degrades [97]. Other customized features of scaffolds include thermal-responsiveness, pH-sensitivity, and controlled drug release in injury sites [98,99].
Collagen scaffolds have shown promising outcomes in CNS repair by preventing inflammation, excitotoxicity, and oxidative stress, shielding cholinergic neurons in Alzheimer's disease, and excluding toxic plaques from the brain [[100], [101], [102], [103], [104]]. In the case of SCI, conventional stem cell therapy is limited, and physical bridging is necessary for isolated cells to functionalize in the injury site. Collagen-based scaffolds integrated with NSCs have been successful in SCI repair by ameliorating neural outgrowth and axon reconnection, and these could be integrated with highly aligned micro-channeled scaffolds. Several studies have investigated the use of native collagen, dehydrothermally crosslinked collagen implants supplemented with laminin, or EGFR inhibitors to enhance the regenerative process of adult rat NSCs and neural progenitor cells (NPCs) for SCI healing [[105], [106], [107], [108]].
Collagen has been approved by the Food and Drug Administration (FDA) for clinical usage in peripheral nerve tissue repair, as it has been shown to promote nerve regeneration in various nerve scaffolds (NeuraGen [109] and Neuromaix [110]) and nerve cuffs (NeuroWrap and NeuroMend) with modulated degradation rates varying from months to years [101]. In addition, collagen scaffolds could be modified with functional proteins or growth factors to enhance nerve repair further. For instance, NGF combined with collagen scaffolds has successfully regenerated nerve gaps ranging from 5-mm to 35-mm in various animal models [6,[111], [112], [113]].
The selection of ideal cell sources for therapeutic neural regeneration is vital. Strategies for CNS regeneration emphasize on the transplantation of NSCs or differentiated neural cells integrated with growth factors and glial cells, which can foster neuronal differentiation in a permissive manner. As a result, the CNS and PNS responses to damage are noticeably different, and any therapeutic strategy must be tailored in accordance with this. Typically, stem cells, including iPSCs, ES, NSCs, NPCs, OPCs, and MSCs, are familiar cell sources in the CNS regeneration. In this context, stem cells are induced to differentiate in specific neuronal lineage for axonal outgrowth.
On the other hand, in PNS regeneration, SCs, astrocytes and MSCs are competitive candidates. SCs, glial cells in the PNS, are the most typically used cells, primarily aiming to support axons by releasing growth factors and isolating axons via forming the myelin sheath [114]. Besides, SCs can deposit extracellular components such as laminin and type IV collagen in addition to growth factors, which is conducive to PNI repair [115].
3.2. Manufacturing techniques of collagen-based biomaterials in NTE
Tissue engineering components, such as scaffolds and integrated technologies, have been established over time, with diverse methodologies being evolved to construct complex structures from a wide range of natural and synthetic polymers. The goal has generally been to create ideal scaffolds matching nervous tissue's physical, chemical, and mechanical features. These scaffolds should possess appropriate properties such as pores, fibres, and channels from nano to micro-scale, which are critical for NTE applications. The versatility of collagen primarily relies on different forms of collagen, including sponges, conduits, nano/microspheres, hydrogels, and nano/microfibers, which could be processed to meet specific requirements for the CNS and PNS repair. Notably, collagen is commonly fabricated as scaffolds via conventional methods (freeze drying, gas foaming, solvent casting, etc.), moulding and texturing methods (fiber mesh, photolithography, laser texturing, etc.) and rapid prototyping (microsphere sintering, 3D bioprinting, electrospinning, etc.). Conventional approaches for constructing collagen offer the advantages such as ease of operation, tunable pore size, and low cost. But they have limitations in achieving sophisticated architectures with customized sizes on the microscale and macroscale. In contrast, rapid prototyping (RP), also known as solid free-form fabrication [123], is categorized as 3D bioprinting, stereolithography, and selective laser sintering. Various processing strategies, including electrospinning, 3D bioprinting, and their combinations, have advanced significantly. These techniques facilitate control over scaffold uniformity and physical properties (i.e., porosity, hydrophilicity, hydrophobicity, and swelling ratio) and enable cell-biomaterial interaction within a favourable 3D microenvironment in a customized manner [124]. In this section, we reviewed current RP strategies in applying NTE. Table 4 summarizes the characteristics of the collagen-based manufacturing strategies used in NTE, the advantages and disadvantages of each method, and their current applications in NTE.
Table 4.
Scaffold manufacturing strategies and relative applications in NTE.
| Strategies | Preparation method | Advantages | Disadvantages | Application | Ref. |
|---|---|---|---|---|---|
| Freeze-drying | Polymers dissolved in a specific solvent | 1. Ease of operation 2. Maintenance of bioactivity without temperature elevation 3. Tunable porosity |
1. High energy cost 2. Endurable experimental duration 3. Compromised surface topology |
1. Repair of particular brain trauma 2. Collagen-based SCI repair 3. Nerve grafts in peripheral nerves |
[116] |
| Electrospinning | Solution of natural or synthetic polymers | 1. Efficacy in preparing micro-/nana-scales fibrous mesh 2. High porosity and surface area to volume ratio 3. Precise control of fibre alignment 4. Convenient post-processing |
1. Requirement of the high voltage field 2. Potential toxicity of the solvent |
1. Guidance for directional migration of NSCs 2. Sheet rolling process for fabricating nerve conduits in PNS repair |
[117] |
| Extrusion-based bioprinting | Cells encapsulated in homogenous, viscoelastic bioink | 1. Combination of multiple types of cells, bioink, and growth factors involved in 3D milieu 2. Customized size control |
1. Limited bioprinting resolution (about 100 μm) | 1. In vivo neural analysis 2. SCI repair |
[118,119] |
| Inkjet bioprinting | Dispersion of low-viscous droplets in a controllable manner | 1. High printing speed (1–10,000 droplets per second) 2. High resolution (50 μm) |
1. Low cell density (<106 cells/ml) | 1. Fabrication of functional neural constructs | [120,121] |
| Stereolithography | Biodegradable, biocompatible polymers | 1. 3D architectural integrity 2. High accuracy and resolution (25 μm) 3. No cost of possibly fabricating waste |
1. Resins with cytotoxic residues 2. Costly specialized equipment |
1. SCI repair | [122] |
3.3. 3D bioprinting of collagen-based bioinks
The field of neural tissue engineering is continuously incorporating innovative technologies that allow researchers to precisely control functional simulation at the cellular level in vitro and simulate the development, function, and regeneration of the nervous system with better precision. 3D bioprinting is an additive manufacturing process in tissue engineering and regenerative medicine. Computer-aided design models are employed to generate 3D constructs as a prototype of biofabricating tissue or organ constructs. Notably, 3D bioprinting is one of the most prominent TE technologies that employs biocompatible hydrogels to construct precisely architected scaffolds to facilitate cellular responses. Compared to other processing techniques, one of the dominant advantages lies in the spatially and temporally efficient collaboration of bioactive molecules, distinct cell types, and dimensional biomaterial, also known as bioink in this scenario. Lee et al. were the pioneered groups in implementing collagen-based biomaterials vis 3D bioprinting technique to fabricate neural constructs [125]. Currently, there are various bioprinting approaches for NTE, including inkjet bioprinting, stereolithography (SLA), laser-assisted bioprinting, and extrusion-based bioprinting, in which specific application requirements vary for distinct demands. However, for the specialized collagen-based NTE, inkjet bioprinting and extrusion-based bioprinting have garnered the most enthusiasm amongst 3D bioprinting techniques [126]. This section outlines the manufacturing fundamentals and applicable scenarios in functional simulation and damage repair of neural tissue engineering related to collagen-oriented bioprinting approaches.
Inkjet bioprinting utilizes thermal, piezoelectric, and electrostatic printing principles to propel bioink in a controlled manner, allowing for precise and consistent cell deposition in micrometre resolution [120]. The high resolution (50 μm) of inkjet bioprinting is a significant advantage of superiority [127]. Additionally, the nozzle diameter narrows down the volume per drop to 10 pL, implying the concentration for cell seeding could be relatively high (> 5 million cells/mL) [128]. Correspondingly, narrowed nozzle size may bring about nozzle clogging; hence, bioink with low viscosities is required, which can affect the mechanical properties of the fabricated constructs. Additionally, insufficient viscosity results in cell damage due to thermal or mechanical stress [[129], [130], [131]]. Xu et al. developed a composite bioink formulation consisting of collage I and fibrin, where NT2 cells were encapsulated to fabricate a 3D cellular sheet via inkjet bioprinting. The printed neural construct displayed physiological viability, as evidenced by immunostaining and patch-clamp analysis [132]. Lee et al. conducted a study on bioprinting a multilayered “ring-cross” 3D neural pattern using rat embryonic neurons and astrocytes embedded in crosslinked collagen through sodium bicarbonate [133]. Similarly, Y.B. Lee et al. developed an engineered neural bioprinting platform that utilized a hybrid bioink composed of collagen, fibrin, and vascular endothelial growth factor (VEGF) to encapsulate murine neural stem cells. The results demonstrated that bioprinting of VEGF-containing fibrin promoted supportively sustained growth factor (GF) release in the collagen scaffold [134].
Extrusion-based bioprinting is the most prevalent bioprinting methodology, which employs pneumatic or mechanical (piston or screw) dispensing mechanisms to form homogenous bioink strands [121]. This method involves utilizing a syringe to print viscous bioink in a layer-by-layer fashion, which is superior owing to permitting a variety of biomaterials encapsulated with distinct cell types and growth factors in one-pot [118]. However, the printing resolution is limited to approximately 100 μm [127]. Apart from that, nozzle diameters, correspondent shear stress, and applied pressure could affect cellular viability, which could be tackled by optimizing printing parameters [119]. Chen et al. encapsulated embryonic NSCs in collagen I, heparin sulfate loaded with basic fibroblast growth factor via extrusion-based bioprinting for neural in vivo analysis [135]. Jiang et al. developed rat NSCs-encapsulated collagen and silk fibroin bioink recipe for SCI repair, in which post-transplanted reduction of glial scars, axon outgrowth, and the functional restoration of the spinal cord was significantly observed [136] (Fig. 3A).
Fig. 3.
Integration of collagen with advanced technologies application for neural tissue engineering. A: 3D bioprinted collagen/heparin scaffold ameliorated SCI repair. (a) Photograph of collagen/heparin construct (3D-C/H). (b) SEM image of porous 3D-C/H. (c) Left: NSC neurosphere was counterstained with Nestin (red) and Hoechst (blue). Right: SEM image of NSCs cultured on 3D-C/H. Scale bar: 50 μm. (d) Histological analysis of HE staining at 8 weeks post-injury. Higher magnification of white boxes on the left was displayed in the lesion site. Scale bar: 50 μm. Reproduced with permission. [135] Copyright 2017, Wiley-VCH GmbH. B: Sponge-reinforced electrospun PLGA and collagen-based conduit in peripheral nerve repair. (a) Graphical depiction of fabricating process of the PLGA and collagen-based conduit with gelatin perfusion (Gel@PLGA/Col). (b) Cross-section of the fabricated collagen-based conduit. SEM images of SCs cultured on nanofibrous meshes are displayed (c). Reproduced with permission. [138] Copyright 2022, Elsevier. C: Growth factor loaded collagen hollow spheres for treatment platform in neuroregeneration. (a) Graphical abstract of collagen spheres in NGF releasing pattern. (b) Confocal images of NGF-loaded collagen microspheres. Collagen spheres, green; NGF, red. Reproduced with permission. [143] Copyright 2013, American Chemical Society.
3.4. Electrospinning of collagen-based nerve conduits
Electrospinning is a traditional manufacturing methodology that utilizes a high-voltage electric field to precisely control the deposition of natural and synthetic polymers onto a particular collecting substrate. This technique can fabricate fibrous meshes with customized fibre sizes ranging from several microns to 100 nm or less [137]. Due to remarkable efficiency, low cost, and ease of handling, electrospinning has been broadly applied in tissue engineering for the indispensable merit of manufacturing microfibers consistent with the scale of ECM. In this scenario, nano-sized fibers could be biophysical guidance for cell orientational migration. Electrospun collagen-based biomaterials take effect in two aspects: (i) providing topographical guidance as a biophysical cue for promoting aligned axonal outgrowth; (ii) functionalizing collagen scaffolds with neurotrophic biomolecules to preferably ameliorate traumatic injury. For instance, Li et al. originated an electrospinning formula where collagen was functionalized with stromal-cell-derived factor 1α (SDF-1α), creating a controllable biological gradient from the rostral and caudal ends. By optimizing electrospinning parameters, radially aligned collagen/polycaprolactone (PCL) hybrid scaffolds were obtained to guide the aligned migration of NSCs, providing a robust platform for effectively guiding nerve regeneration [39]. Electrospun nanofibers could be manufactured in sheet rolling fashion to prepare nerve conduits. Zhao et al. fabricated fluffy sponge-reinforced electrospun conduits for peripheral nerve repair. The Gel@PLGA/Col conduit exhibited promising nerve regeneration performance in a sciatic nerve defect implantation model with a large gap (Fig. 3B) [138]. When synthetic polymers are combined with aligned nanofibers, 3D nanofibrous nerve conduits with aligned nanofibers offer an optimum cell contact guidance (CCG) for axonal regeneration [139]. Besides, Kim et al. demonstrated that collagen fibres with extra topographical guidance promoted cell proliferation and neurite outgrowth along the fibres, reinstating neuronal regeneration in injury situations [140]. Liu et al. evaluated the feasibility of applying electrospun collagen I nanofibers for SCI repair. They found that neurites originated from dorsal root ganglia (DRG) migrated by the aligned pattern of electrospun collagen microfibers. These results implied that collagen could be conducive to infiltrating neuronal cells followed by neurofilament sprouting in the further strategic intervention of SCI [141]. Additionally, Zhang et al. constructed a 3D scaffold by employing an aligned electrospun fiber bundle as the inner core to dictate the aligned regeneration of axons biophysically, while sheathing collagen matrix in the outer layer to controllably release glial cell-derived neurotrophic factor (GDNF) and multiple microRNAs to rebuild neural circuitry functionally [142].
3.5. Collagen microspheres for the delivery of bioactive factors
Collagen could be processed into various configurations with therapeutic effects, such as membranes (e.g., DuraGen) and micro/nanospheres. Due to the satisfying surface-to-volume ratio, microspheres can encapsulate a greater quantity of medicine. Besides, the small size makes them easier to administer in vivo by stereotaxic surgery to a specific spot in the brain. In particular, collagen hollow microspheres have a capacity of stably and reproducibly loading growth factors involving nerve growth factor (NGF) [143] (Fig. 3C) or vascular endothelial growth factor (VEGF) [144]. In Parkinson's disease (PD), the loss of dopamine cells in the substantia nigra results in the degeneration of nigro-striatal axons. Hence, nigro-striatal regeneration route is of vital significance in PD, where collagen microspheres might serve as an effective platform for drug delivery. McRae et al. demonstrated that dopamine-loaded microspheres injected into the striatum successfully promoted neurofilament development in the striatum and functional restoration [145]. In a rat PD model, it has been recapitulated that intra-striatal implantation of GDNF-releasing microspheres increased dopaminergic neurofilament sprouting and motor function rehabilitation [146].
4. Fundamental design criteria in neural tissue engineering
One of the main challenges in neural tissue engineering (NTE) is to mimic the biological and mechanical properties of the native extracellular matrix (ECM) microenvironment. In the native tissue in vivo, cells are embedded in ECM, a highly dynamic, 3D fibrillary network comprising collagen, proteoglycans, glycoproteins, and a myriad of bioactive ligands. ECM offers an architectural scaffolding for the maintenance of tissue integrity and involves the crosstalk with residing cells, regulating diverse functions, including proliferation, migration, and differentiation, which is vital for cellular homeostasis [147]. Notably, collagen-based biomaterials are broadly employed in CNS and PNS repair as distinct scaffolds and hydrogels. Generally, parameters for therapeutic implanted biomaterials must comply with the following criteria: (i) mechanical properties that match the soft neural tissue (brain tissue is basically with a stiffness of approximately 0.5 kPa [148]) to avoid further damage to the surrounding host tissue after implantation. Scaffolds utilized for tissue bridging should support cell attachment, development, and differentiation; (ii) bioactive molecules that promote axonal regeneration and lesion bridging; (iii) electrical conductivity that enhances neural cell growth and differentiation. Besides, multi-strategies mediation for fabricating neural constitutes have been prevalent to meet dimensional requirements in diverse scales. For example, combining nanotexturing and micro-texturing can realize controlled neuron patterns in nano/micro scales [149]. Solvent casting/particulate leaching could be assisted with moulding techniques in PNI repair [150]. Furthermore, non-solvent-induced phase separation (NIPS) and microprinting could be synergized to construct nerve guides [151]. It is also reported that integrating injection moulding and thermally induced phase separation (TIPS) is effective in building nanofibrous scaffolds [63]. Besides, multi-microtubule chitosan conduits could be fabricated using novel molds and TIPS [152]. As for PNS and SCI repair, researchers constructed single-lumen and multiple-lumen nerve conduits loaded with NGF via the combination of moulding, gas foaming, and particulate leaching fabrication techniques [153]. This section will concisely elaborate on critical criteria in current designing requirements and the latest applicable instances of NTE.
4.1. Mechanical properties
Based on CNS, the brain and spinal cord are two of the most compliant tissues in the human body. Hence, implanted constructs must match the compliance of native neural tissues. Meanwhile, the endurable capability is required for bearing load forces in the motions of the spinal cord. Regarding mechanical properties, ideal transplants should be qualified to maintain structural integrity and matched conformation in the lesion cavities to eliminate potential cysts or fibrotic tissue formation. Previous studies have shown that growth cones exert traction force and respond to substrate rigidity-induced mechanical stress [154]. Soft substrates promoted neurite development and branching in CNS neuronal cells, while rigid substrates supported astrocyte growth [155]. Specifically, K Saha et al. revealed that under the mixed differentiation cultured conditions of NSCs with serum, softer gels (∼100–500 Pa) greatly favoured neurons, while stiffer gels (∼1–10 kPa) supported glial cultures [156]. Additional research discovered that spinal cord neurons on more rigid gels outspread more primary dendrites but shorter axons (30 kPa) [157]. The substrate stiffness-dependent axon outgrowth and traction forces of PNS (DRG) and CNS (hippocampal) neurons were identified to be distinct. Accordingly, neurite elongation from DRG neurons was maximal on substrates whose Young's modulus is approximately 1 kPa, whereas hippocampal neurites were independent of substrate stiffness [158].
Furthermore, immune-related mechanosensitivity of glial cells and foreign body reactions were investigated to confirm that compared to a substrate with a physiologic stiffness level (∼100 Pa), a substrate with a shear modulus of 10 kPa remarkably triggered the overexpression of inflammatory genes and proteins between primary rat microglial cells and astrocytes (∼100 Pa). Comparatively, a stiffer in situ implant induced considerably higher levels of glial cell activation and associated inflammation than a softer implant. Adjusting the mechanical stiffness of neural implants to counterpart native neural tissue may diminish negative responses and thus enhance biocompatibility, according to these findings [159].
The stiffness of the applied scaffolds must be finetuned to resemble native tissue and potentially activate suitable biological responses, such as adhesion, differentiation, or proliferation. Multiple strategies for differentiating stem cells into neural tissues have been reported, for instance, formulating a hydrogel recipe with an elastic modulus ranging from 1 to 10 kPa [160,161]. Besides, the elastic modulus of the spinal cord ranges between 200 and 600 kPa; thus, matching these variables can be advantageous for optimizing cellular biocompatibility and differentiating preferences [162]. Recent research aimed at inducing spinal cord axonal regeneration employed poly (2-hydroxyethyl methacrylate-co-methyl methacrylate) hydrogel tubes with elastic moduli precisely fine-tuned to counterpart the characteristics of the native tissue in vivo (400 kPa). The excellent elastic modulus of biomaterials for peripheral nerve regeneration is approximately 0.45 MPa [163]. The latest research has employed hydrogels with elastic moduli comparable to native tissues. Additionally, the elastic modulus of applied hydrogels could be flexibly tuned by controlling the degree of crosslinking [[164], [165], [166]].
4.2. Biochemical properties
Consideration of the biochemical properties of scaffolds is essential due to their direct interaction with cells, which can regulate cellular behaviours. In the context of NTE, modification of scaffolds with bioactive molecules or compounds specific to neural tissue has been widely applied. These modifications have been used to optimize the functionality of injected hydrogels and bioinks in 3D bioprinting, which can enhance tissue functionalization by promoting controllable cell growth and differentiation. Lately, various growth factors integrated into the functionalization of biomaterials in NTE, including neurotrophin-3 (NT-3), fibroblast growth factors (FGF), basic fibroblast growth factors (bFGF), epidermal growth factors (EGF), brain-derived neurotrophic factors (BDNF), and glial cell-derived neurotrophic factors (GDNF) [167,168]. Incorporating bioactive molecules or compounds boosts the crosstalk of encapsulated cells with stimuli, inducing tissue-specific cellular functions and mimicking the biochemical environment of native tissues. Various modification instances of collagen-based biomaterials have been recapitulated above. For example, Sharma et al. identified that integrating guggulsterone-encapsulated drug-releasing microspheres into the bioink could stimulate neuronal differentiation in 3D bioprinted constructs [169,170]. This study highlights the potential for further application of growth factors in combination with advanced manufacturing techniques for sophisticated control over cell behaviours mediated by microcarriers and 3D bioprinting.
4.3. Electrical conductivity
The use of electrical stimulation has been shown to promote neural regeneration, making the use of electrically conductive polymers in scaffold fabrication particularly attractive for NTE. The mechanical, biochemical, and electrical features of constructed conduits regulate the efficacy of neural regeneration. Transmitting electrical signals throughout the nervous system is the essence of neurons and has a profound impact on the efficacy of neural maturation and further functional performance [171]. To achieve the electrical conductivity of fabricated scaffolds, pre-blending and post-coating are two main strategies. For instance, for the aim to enhance electrical conductivity, researchers have integrated polypyrrole, a conductive polymer, into the composite scaffolds, which was accomplished either via direct inkjet printing of polypyrrole and collagen [172] or by employing a stereolithography-based 3D bioprinter to generate silk fibroin scaffolds which were successively coated with polypyrrole through electrospinning [173,174]. Joint investigations reflected on the neurite elongation, axonal regeneration, and remyelination resulting from the additives of conductive polymers. Therefore, the fabricating methodologies of considering electrical conductivity are pivotal in NTE. Although numerous in vitro approaches for electrical stimulation have been used, the clinical administration of electrical stimulation by applying conductive scaffolds has not yet succeeded. Moreover, exploiting the combined advantages of novel manufacturing techniques should be considered for precise control of well-designed neural regeneration [174].
5. Therapeutic applications of collagen-based biomaterials in neural tissue engineering
With the advent of tissue engineering methodologies, the management of neural nerve injuries involving the destruction of axonal tracts has undergone a paradigm shift. When conditions such as PNI, SCI, TBI, or neurodegenerative disorders occur, the intricate neural architecture is altered, resulting in neurite growth inhibition and the loss of long-distance guidance. Collagen has been identified as an excellent candidate for addressing these challenges in NTE. In addition, by incorporating dimensional manufacturing approaches, axonal regeneration is enhanced to achieve functional recovery resulting from neurodegenerative disorders. Collagen has been extensively applied in dimensional therapeutic treatment; hence, this section aims to investigate therapeutic instances categorized by SCI injury, PNI, and TBI, which might serve as potential references for further progress in NTE (Table 5).
Table 5.
Summary of collagen-based strategies in neural tissue engineering applications. (NA: Not applicable).
| Approach | Collagen-based formula | Crosslinking method | Cell type | Cell density | Cell viability | Application | Ref. |
|---|---|---|---|---|---|---|---|
| Drop-on-demand bioprinting | Agarose and collagen I | – | 1. MSCs 2. HUVECs 3. Human bone marrow-derived epithelial neuroblastoma immortalized cells |
1. MSCs: 1 × 106 cells mL−1 2. HUVECs: 3 × 106 cells mL−1 3. Neuroblastoma cells: 1 × 106 cells mL−1 |
NA | Cancer modeling | [175] |
| Electrohydrodynamic jet printing | Collagen I and fibrin | EDC, NHS, CaCl2 and thrombin | NSPCs | 3 × 105 cells mL−1 | NA | SCI repair | [176] |
| Extrusion-based bioprinting | Collagen I, heparin sulfate, and basic fibroblast growth factor | UV cross-linking | Embryonic NSCs | NA | In vivo analysis | SCI repair | [41] |
| Collagen and silk fibroin | Solidification | Rat NSCs | 1 × 107 cells mL−1 | NA | SCI repair | [136] | |
| Hydrogel injection | HA/heparin/collagen | Polyethylene glycol diacrylate | ES-derived NPCs | 1.4 × 107 cells mL−1 | NA | Stroke recovery | [177] |
| Collagen I sponge | – | Rat 14-day-old-embryo brain-derived NSCs | 1 × 105 cells mL−1 | NA | Rat cerebral ischemia | [178] | |
| Collagen I | 4S-StarPEG | MSCs | 1 × 107 cells mL−1 | >90% | Brain cell graft platform | [96] | |
| Microsphere | The porcine skin-derived collagen solution | Water-soluble carbodiimide | HUVECs | – | Capillary formation | Sustained release of VEGF | [144] |
| Collagen I | EDC | 1. OPCs 2. DRG |
1. 1.5 × 104 cells/well 2. NA |
∼90% | Neurite myelination | [93] | |
| Electrospinning | PCL/Collagen | – | 1. DRG explants 2. SCs |
NA | ∼90% | Nerve implants | [179] |
| PCL/Collagen | – | 1. Astrocytes 2. Human astrocytoma cell line |
NA | NA | CNS repair | [180] | |
| Tethered conduit | Collagen I | Thermal | 1. Human HUVECs 2. SCs |
1. 4 × 106 cells mL−1 2. 5 × 105 cells mL−1 |
NA | PNI repair | [181] |
5.1. Traumatic brain injury
Traumatic brain injury (TBI) is defined as short- or long-term damage to the brain resulting from external mechanical forces, such as accelerating, decelerating, and rotating forces, causing direct physical disruption of the neural tissues [185]. Loss of cerebral parenchyma after TBI is a substantial barrier to functional restoration. There is currently no clinically proven method of restoring cerebral parenchyma loss brought by TBI. A penetrative TBI (such as one caused by a gunshot or stabbing) causes an irregularly shaped lesion site in the brain which the natural healing procedures are inadequately to manage. The primary trauma results in significant tissue damage and swelling that may require a craniotomy to treat. Numerous growth inhibitory substances are released during this period, including particular proteoglycans and other compounds exposed due to the collapse of the myelin sheaths and surrounding supporting cells, all hindering the axon regeneration procedure. Therefore, scientists continuously examine which therapeutic interventions can aid the transition of the inflammatory response toward the reparative profile. Since the CNS is more intrinsically complicated than the PNS, the implanted biomaterial must shift the inhibitory microenvironment to pro-regenerative one [186]. Type I collagen plays a crucial role in the development of the dura and pia maters and has emerged as a promising candidate for interventions in brain injury [[187], [188], [189]]. As a hydrogel, collagen can retain a significant amount of water molecules. Hydrogels are highly compatible with the mechanical properties of soft nervous tissue and are, therefore, crucial for restoring the CNS [98,190]. Typically, collagen hydrogel gelates in physiological conditions, which forges a promising path of applying collagen as injectable hydrogels in TBI repair. Collagen fibres incorporated with alginate hydrogel were loaded with iPSCs-derived neurons, where neuronal maturation and the formation of neural networks were observed [191]. Moreover, collagen-chitosan scaffolds loaded with MSCs were formulated to facilitate motor functions in a rat TBI model [192]. And another research revealed that functional hyaluronate collagen scaffolds exhibited efficacy in inducing NSCs differentiation into functional neurons in restoring the TBI [193].
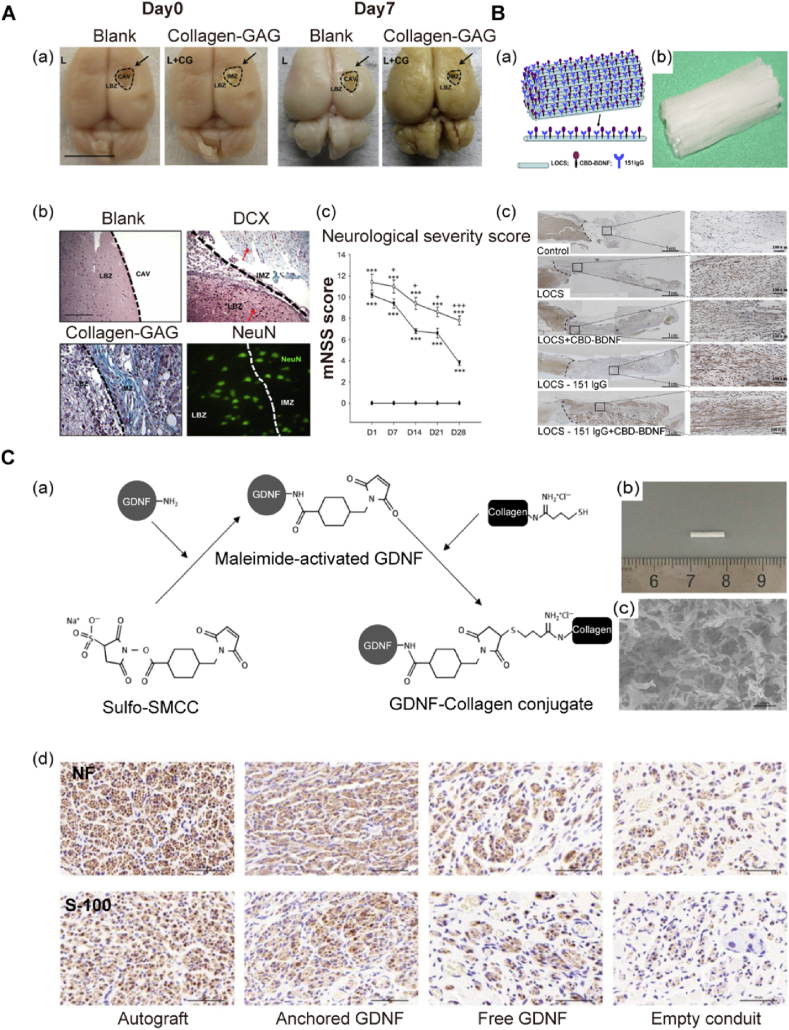
Studies have shown that the implantation of collagen glycosaminoglycan scaffold (collagen-GAG) post-TBI traumatized areas is significantly smaller compared with those in the blank group. Significant DCX+ and NeuN + cells validated the presence of migrating neuroblasts and mature neurons, contributing to the functional restoration of TBI (Fig. 4A) [182]. Hoban et al. investigated GDNF-overexpressing MSC in the encapsulation of collagen gel for intra-striatal transplantation, confirming that microglial cells are reduced in the graft zone and recruiting astrocytes without compromising cell viability and GDNF secretion [96]. Zhong et al. employed hyaluronan-heparin-collagen-polyethene glycol diacrylate composite hydrogels as a “cargo carrier” loading neural progenitor cells (NPCs) to the necrotic stroke cavity, resulting in significant cell viability compared to conventional cell delivery [177]. Marta et al. formulated a semi-interpenetrating network (semi-IPN) consisting of hyaluronic acid (HA), gelatin microparticles, and collagen; when loaded with Tat-Hsp70, a neuroprotective effect is observed in the dopaminergic destruction [194]. Moreover, ventral mesencephalon grafts encapsulated in GDNF-loaded collagen scaffold significantly elevated dopaminergic cell viability, striatal re-innervation, and functional regaining [195]. This technique potentially facilitates graft longevity for future stem cell transplants in cell replacement treatments for neurodegenerative illnesses, where collagen is an appropriate biomaterial choice.
Fig. 4.
Appropriate collagen-based biomaterials in neural tissue engineering. A: Collagen-based biomaterials ensure neurogenesis and functional restoration post-implantation. (a) Morphological repair effect in lesion site after collagen-GAG implantation. Scale bar: 1 mm. (b) Cell phenotypical identification in regenerated tissue. Scale bar: 75 μm. (c) Neurological severity was modulated post-collagen-GAG implantation. GAG (glycosaminoglycan), LBZ (lesion boundary zone), IMZ (intra-matrix zone), and CAV (cavity). Reproduced with permission. [182] Copyright 2012, Elsevier. B: Functionalized collagen scaffold in spinal cord injury repair. (a) Schematic illustration indicating functionalizing logic of the scaffold with neuroprotective efficacy. (b) Image of the scaffold with highly ordered, aligned microchannel. (c) The histological evaluation proved neural regeneration in the lesion cavity after scaffold implantation. Reproduced with permission. [183] Copyright 2010, Elsevier. C: Neurotrophic factor-immobilized collagen conduits in peripheral nerve regeneration. (a) Schematic drawing of functionalizing collagen with GDNF. (b–c) Morphological display of the collagen conduit. (d) Immunohistochemical evaluation of regenerated nerve at 6 and 12 weeks after injury. Nerve fibre (NF) and S-100 were stained to identify axons and Schwann cells. Scale bar: 50 μm. Reproduced with permission. [184] Copyright 2018, Elsevier.
Several challenges need to be resolved to maximize the potential of collagen-based biomaterials in TBI repair. Given biodegradation and dynamic tissue remodelling, longer-term research is also essential for a more profound knowledge of how transplanted and host cells interact with collagen. Besides, practical guidance and modulation of endogenous and transplanted cell fates in collagen-based scaffolds may facilitate cell survival and enhance TBI regeneration. Furthermore, the incorporation of anti-inflammatory growth factors in a controlled manner could be pivotal in modulating the post-injury microenvironment. Thus elevating the longevity and functionality of implanted cells would be achievable. Finally, in-depth comprehension of stem cell research, regeneration mechanisms of TBI, and crosstalk of diverse cell types with collagen-based biomaterials would be conducive to the enrichment of biomaterial design principles for biomimetic biofabrication for the CNS.
5.2. Spinal cord injury repair
Spinal cord injury (SCI) is a complex traumatic disorder frequently resulting in lifelong motor and sensory dysfunction, which is a tremendously catastrophic disability among CNS traumatic disorders. Pathological activities comprising axon disruption, glial scar formation, ongoing demyelination, cystic cavitation, cell necrosis, and the long-lasting immunological storm would hinder post-injury neuronal regeneration, which is commonly irreversible [7,196]. Current strategic interventions have been employed in treating SCI, but no effective solutions for regenerating axonal outgrowth and functional restoration exist. The failure of SCI repair lies in establishing an inhibitory microenvironment, glial formation, and demyelination around the lesion site, which fundamentally impairs further axonal regeneration. Synergistic collaboration of various methodologies has seen extensive repair solutions, composed of biomaterial scaffolds transplantation as a physical provision for axonal outgrowth, modulation of neurotoxicity and immune response, and introducing neurotrophic growth factor to ameliorate glial scar formation [[197], [198], [199]].
Collagen has been applied in SCI repair to provide a neural-favourable microenvironment for cell proliferation and differentiation. Reproducibility and versatility of collagen were achieved by various processing approaches. Han et al. formulated a modified collagen scaffold with neuroprotective proteins for SCI repair (Fig. 4B) [183]. Ma et al. loaded collagen sponge immobilized with (bFGF) encapsulating NSCs, identifying significantly accelerated proliferation, which could be a pioneered robust platform for growth factor-functionalized collagen-scaffold-based repair strategy targeted to SCI [200]. Liu et al. explored the role of topological cues in SCI repair. Electrospun collagen nanofibers were utilized for culturing rat astrocytes and DRGs, observing astrocytes and neurites form DRGs aligned along the nanofiber axes responsive to the topographical guidance cues. Consequently, the hemi-sectioned SCI rat model was utilized to rationalize the repair outcome of electrospun collagen microfiber, where neural fiber infiltration towards the scaffold was characterized, and acute inflammatory repones were significantly downregulated [201].
The chief obstacles in SCI interventional strategies are the inhibitory microenvironment post-injury, where it is more pronounced that NSCs are prone to differentiate into glial cell lines instead of neurons in the NSC-mediated repair approaches. In SCI repair strategies, functionalizing collagen scaffold with bioactive substances, such as neural-favourable growth factors (i.e., bFGF [202], NT-3 [203], BDNF [204], GDNF [205], etc.), the antagonist of myelin-inhibitors (i.e., cetuximab, Fab fragment of cetuximab [206], etc.), and therapeutic molecules (i.e., Taxol [207], paclitaxel [208], etc.) is a relatively superior treatment for SCI repair. Li et al. modified a collagen scaffold with cetuximab, an EGFR inhibitor, to regulate NSC differentiation in the SCI model [107]. Consequent research adopted the identical modifying method to ameliorate the SCI repair of dogs [108]. Notably, Hatami et al. revealed that human embryonic stem cells (hESCs)-derived neural progenitor cells (NPCs) loaded collagen scaffolds enhance the recovery of motor functions along with targeted migrations towards the lesion cavity [105]. Composite scaffolds functionalized with growth factors, such as collagen/chitosan, are also formulated. In a study pioneered by Liu et al., bFGF-loaded collagen/chitosan (CCS/bFGF) scaffolds transplanted into the T10 complete transection of the SCI rat model were identified to promote neural regeneration and axonal myelination at the lesion region [202]. Zou et al. investigated the potential of NSPC-based treatment in bridging lesion sites by transplanting exogenous neural stem progenitor cells (NSPCs) to replace necrotic cells. Human fetal brain and spinal cord-derived NSPCs were implanted through an aligned collagen sponge scaffold into a completely transected rat model. Comparing the two types of foreign cells, the spinal cord-derived neural stem progenitor cell-aligned collagen sponge scaffold (NSPC–ACSS) was noticeable in sustaining cell survival, neuronal maturation, and establishing a more constructive SCI milieu by suppressing inflammation and glial scar formation [209].
Recently, the role of biomaterials in regulating cell behaviours and modulating axonal inhibitory microenvironments has been getting more attention in NTE. Adhesive, stretchable, and SDF1α/paclitaxel spatiotemporal delivery collagen-fibrin (Col-FB) fibrous hydrogels were formulated to harness endogenous neural stem/progenitor cells for SCI repair. This aligned hybrid hydrogel also mimics the mechanical properties and topological features of the native spinal cords [176]. Besides, the covalent interaction between biomaterials and cells has also been integrated into SCI repair. Metabolic azido-labeled hNPCs conjugated on dibenzocyclooctyne-modified collagen fibres could significantly enhance cell adhesion, spreading, differentiation, and longitudinally aligned topological guidance cues in the drug-targeting context compared with non-covalent interaction [210].
Moreover, in the latest study, hNPCs were induced to differentiate into various dorsal and ventral neuronal cells on collagen scaffolds in vitro. Transplantation of human embryonic spinal cord-like tissues with dorsal and ventral neuronal characters into complete SCI models in rats and monkeys was verified to have significantly better therapeutic efficacy than undifferentiated hNPCs [211]. Furthermore, the synergistic incorporation of different cell sources is another promising fabrication window for enhanced SCI repair. Researchers have fabricated novel centimetre-scale human spinal cord neural tissue constructs with human spinal cord NPCs and astrocytes combined with a linearly ordered collagen scaffold. In this case, astrocytes could facilitate NPC adhesion and neurite outgrowth on the collagen scaffold, forming a linearly ordered spinal cord-like structure comprising mature neurons and glia cells [212].
In total, collagen scaffolds were generally functionalized to optimize binding specificity and efficacy with various bioactive and therapeutic compounds, such as growth factors and neurotrophic agents, or loaded with different cells, including human embryonic, mesenchymal, and neural stem cells. These scaffolds facilitated axonal regeneration by stimulating cell differentiation and reducing glial scar formation and astrogliosis. Moreover, functionalized collagen scaffolds enhance neuronal differentiation with neurite reconnection, displaying significantly elevated locomotor function, which reflected SCI recovery.
5.3. Peripheral nerve defect repair
Axonal regeneration is more pronounced in PNS than CNS following damages or diseases [52], in which Schwann cells (SCs) are essential for the functional repair post-injury, comprising proliferation, differentiation, migration, and potential remyelination. Additionally, immunological situations, such as recruitment and polarization of macrophages and controlled release of growth factors, are also crucial in the regenerative stages of PNS [213]. In response to peripheral injury, the endoneuria fibroblasts correspondingly secret collagen I, which aims to provide mechanical stabilization for further axonal regeneration [214,215]. Collagen has been demonstrated to be indispensable in PNS repair, positively regulating cellular behaviors of SCs and leading to rehabilitative conditions of PNS [216].
Ma et al. designed functionalized collagen conduits for peripheral nerve regeneration, facilitating sustained delivery of neurotrophic factors (Fig. 4C) [184]. Previous research has revealed that collagen V regulated SC adhesion and migration by functionalized N-terminal domain with heparin, facilitating cytoskeleton assembly and tyrosine phosphorylation of SCs [217,218]. Besides, the controlled release of collagen VI facilitates macrophage recruitment with the polarization of macrophages to the M2 phenotype, which was identified in the functional restoration route of sciatic nerve injury [219]. Given distinct processing approaches, collagen could be versatile in hydrogels, filaments, and films designed in multiple neural conduits. In vivo research employed dense, anisotropic collagen gel scaffolds encapsulated SCs demonstrating potential axonal regeneration and vascularization in repairing the 10 mm-gaping bridge of the rat sciatic nerves within 4 weeks [220]. As for collagen filaments in PNS repair, novel methodology of fabricating linear-ordered collagen (LOC) fibers [204] and functionalization of neurotrophins with the collagen-binding domain (CBD) or laminin-binding domain (LBD) [91] have seen significant success in restating neurite bridging and functional recovery in rodents and large animals [51,113,221,222]. Blending collagen with synthetic polymers or engineering topographical guiding cues could better boost axonal extension in a more controlled and reproducible manner [140]. As for the neural films, Zhuang et al. constructed a heterogeneous double-layered collagen film composed of loosely arranged collagen in the inner layer and compacted collagen fibres in the outer layer, which guaranteed intact maintenance of nerve conduits without the infiltration of foreign tissues [54]. Cerri et al. designed nerve conduits with stably gradient pores, collaboratively facilitating aligned outgrowth of neural cells, using a similar fabricating principle [223].
5.4. Drug screening and disease modelling
Traditionally, animal models have been employed to simulate the pathogenic invasion of neurological disorders. Nonetheless, these models do not faithfully recapitulate the complexity of human illness manifestations. Also, current in vitro disease models fail to elucidate dynamic crosstalk between normal cells and residing milieu. Particularly, a variety of neurological conditions afflict patients with diverse characteristics. For instance, glioblastoma (GBM) is the most prevalent malignant brain carcinoma in adults, whereas neuroblastoma (NB) is a pediatric brain tumor in the sympathetic nervous system. Recent advances in collagen-based hydrogels manufactured via 3D bioprinting have made breakthroughs in modelling pathological features in the process of tumorigenesis, especially in interpreting cell-cell and cell-matrix interactions. A recent study by Duarte Campos et al. [224] reveals that 3D bioprinted alginate-collagen I construct could serve as a platform in the NB disease model. Specifically, the scaffolds are loaded with NB cells, mesenchymal stromal cells, and human primary umbilical vein endothelial cells, followed by the physiological assessment of cellular behaviours. As a result, the NB cells formed Homer Wright-like rosettes, typical characteristics of NB tumours. Furthermore, cancer cells in collagen-based bioink maintain proliferative capabilities and secret Vimentin-rich matrices. Collective results could elucidate the platform's feasibility in precision medicine for cancer-targeted drug screening. Curtin et al. [225] formulated collagen-based scaffolds of two categories involving collagen-glycosaminoglycan (Coll-GAG) and collagen-nanohydroxyapatite (Coll-nHA). By applying two NB cell lines in the two composite collagen-based scaffolds, researchers identified the proliferation of NB cells exhibited >100-fold elevated resistance to cisplatin treatment compared with conventional 2D cultures, demonstrating the feasible functionality in the validation of miRNA-based gene delivery. Collectively, the research empowers the potential of collagen-based scaffolds in the chemotherapeutical evaluation and targeted therapies.
Liu et al. [226] displayed that ovarian cancer cell lines cultured in collagen I hydrogel scaffolds progressively transformed into multicellular spheroids with high cell viability and significantly increased expression of epithelial to mesenchymal transition (EMT) markers, including vimentin, fibronectin, and N-cadherin. In addition, the collagen-based culture system displayed a considerable activation of the Wnt/b-catenin and TGF-/Smad signalling pathways in the induction of EMT. Microfluidics device is a prevailing instrument for simulating cellular behaviour. Anguiano et al. [227] proposed a microfluidics device loaded with collagen-Matrigel hydrogels to investigate the migration of lung cancer cells in distinct microenvironments of carcinoma invasion. The collagen-based microfluidic apparatus increased lung cancer cells' migratory profiles and dynamics.
Consequently, collagen-based hydrogels integrated with a microfluidics system would benefit the research of cancer invasion mechanisms in diverse contexts and the identification of effective cancer-targeted medications. Besides, collagen-based biomaterials could be processed into fibrous scaffolds to model several cancer types. Murakami et al. [228] fabricated collagen IV-coated fibrous silica sheets to culture squamous carcinoma cells, exhibiting highly improved cell proliferation and invasive profiles. Fabrication of fibrous scaffolds is a pivotal tool for elucidating cell-ECM crosstalk in a biomimetic milieu, further facilitating the in-depth mechanistic research of tumour progression. Apart from the applications in solid tumours, collagen-based composite scaffolds are typically employed in hematologic malignancies research. Collagen I-coated PCL scaffolds were seeded by acute lymphoblastic leukaemia (ALL) Jurkat cells. In this case, elevated cell proliferation and the typical response of drug-resistive reactions to daunorubicin and cytarabine were observed compared with bare PCL scaffolds [229]. Collagen-coated porous PLGA microspheres (PPMS) were used as microcarriers in lung cancer cell line co-culture. Results collectively indicated cell adhesion and proliferation in the PPMS-mediated microcarrier co-culture system; besides, anti-cancer drugs, including doxorubicin, cisplatin, curcumin, paclitaxel, etoposide, and gemcitabine, were applied to evaluate the potential of responsive drug resistance [230]. Consequently, collagen-PPMS could be a robust in vitro platform for lung cancer modelling, especially in drug screening. Similarly, hydroxyapatite (nHA) nanoparticles orchestrated with collagen I have progressed in the osteosarcoma microenvironment's biomimicry [231].
In summary, collagen-based biomaterial modalities exert prominence on cancer modelling and anti-cancer drug screening while selecting appropriate 3D systems combined with advantageous techniques to address specific issues remains challenging in the field of NTE [232]. Moreover, the heterogeneity of patient-derived cells and tumour microenvironment might hinder the reproducibility of 3D scaffold systems for recapitulating the efficacy of cancer-targeted therapeutics.
6. Evaluation of collagen-based biomaterials for NTE
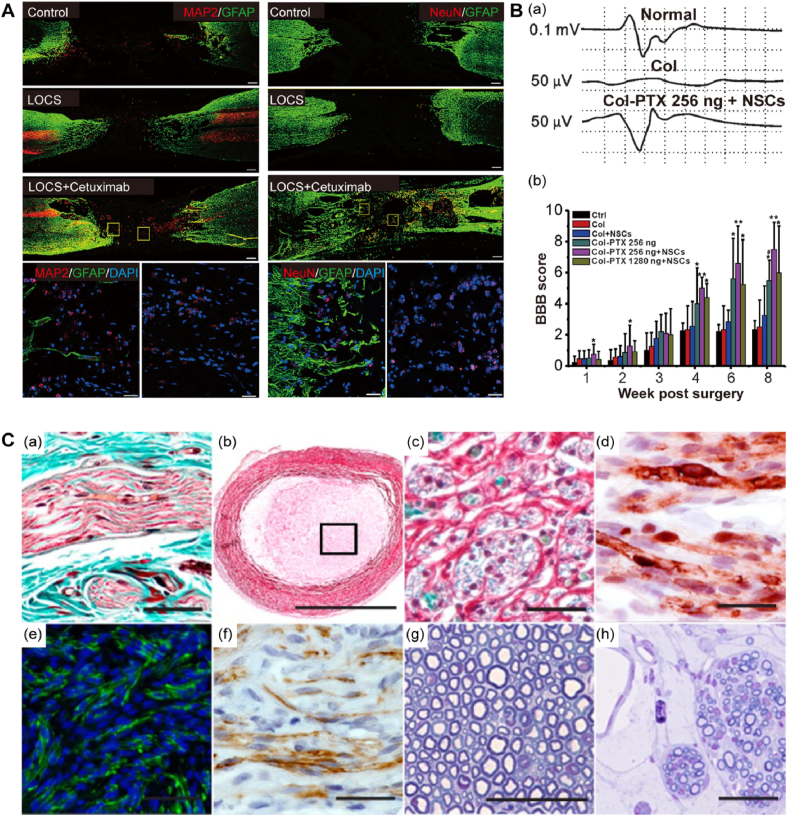
Successful application of collagen-based biomaterial systems in NTE requires technical characterising using different techniques. For functional restoration, neuronal reconnection is the crucial prerequisite for the evaluation basis. Moreover, as the nervous system regulates locomotor activities of the body, physiological assessment post collagen implantation plays an indispensable role in functional evaluation. Notably, immunochemistry is essentially utilized for the visualization of neuronal reconnection. In this context, biomarkers such as Tuj1, NeuN, and MAP2 can indicate neuronal differentiation and axon outgrowth, while GFAP is counterstained for characterizing glial scars in the lesion sites (Fig. 5A). More importantly, physiological analysis has been extensively adapted to assess the therapeutic efficacy of collagen transplantation. Li et al. validated that paclitaxel-liposomes modified collagen microchannel scaffold resulted in electrophysiological and locomotor recovery via electrophysiological analysis. Motor evoked potential results, and Basso, Beattie, and Bresnahan (BBB) locomotor scale method could be effective in evaluating hindlimb locomotion of SCI models (Fig. 5B) [233]. For peripheral nerve repair, Masson's trichrome method and toluidine blue staining serve as versatile tools in the histological evaluation of peripheral nerve regeneration. Immunofluorescence and immunohistochemical identification of regenerated axons are also applied in the quality assessment of peripheral nerve outgrowth (Fig. 5C) [234].
Fig. 5.
Assessment of functional restoration in neural regeneration. A: Cetuximab-modified scaffold implantation (LOCS + cetuximab) facilitates neuronal regeneration in the lesion site of dogs at 9 months post-SCI injury. Confocal images displayed MAP2, NeuN-positive neuronal cells, and GFAP-positive related glial scar formation in the lesion site via immunostaining. Reproduced with permission. [108] Copyright 2017, Elsevier. B: Electrophysiological analysis revealed functional restoration for neural regeneration. (a) Motor-evoked potential reflects a functional recovery in spinal cord-injured rats. (b) Basso, Beattie, and Bresnahan (BBB) locomotor scale method reveals hindlimb locomotion of SCI models. Reproduced with permission. [233] Copyright 2018, Elsevier. C: Histological analysis as quality control in peripheral nerve regeneration. The longitudinal section of a peripheral nerve was stained with Masson's trichrome method (a). Scale bar: 50 μm. Cross-sectioned NeuraGen collagen conduit stained with the MCOLL with low (b, scale bar: 1 mm) and high magnification (c, scale bar: 100 μm). Collagen fibers are stained in red, the myelin in blue, and the nucleus darkly stained. (d) Schwann cells were characterized with S-100, featured nucleus, and cytoplasmatic positive reaction. Scale bar: 50 μm. Immunofluorescence image of neurofilament in green (e, scale bar: 100 μm) and regenerated axons in GAP-43 as brown (f, scale bar: 50 μm). Toluidine blue stained semithin cross-sectioned native nerves (g) and regenerative nerve tissue (h). Scale bar: 50 μm. Reproduced with permission. [234] Copyright 2014, Wolters Kluwer.
7. Future perspectives and challenges
Collagen is a biomaterial with various advantages over other candidates for use in neural tissue engineering (NTE), owing to its remarkable biocompatibility, biodegradability, versatility, reproducibility, and low immunogenicity. Collagen can be applicable in multiple forms, including hydrogel-involved injected formulation utilized in traumatic brain injury and stroke, lyophilized scaffolds employed in spinal cord injury repair and peripheral nerve conduits, as well as function-enhanced hydrogels developed in cancer modeling and drug screening. Typically, collagen-based biomaterials can be controllably processed via dimensional manufacturing techniques, such as 3D bioprinting and electrospinning. More significantly, specific functionalization is conducive to re-establishing a neural-favourable microenvironment. Collaborative efforts are exerted to realize the supreme goal of repair, regeneration, and recovery. As for the technological advancement in NTE, a scaffold-free 3D bioprinting approach, also known as the Kenzan bioprinting method, emerged as an effective tool in neural regeneration, which is applied in mimicking neuronal network formation [235,236], PNI nerve regeneration [237], and the understanding of neuropathology [238]. Notably, a review recapitulates the construction of 3D neural tissue models from the perspective of spheroids and bioprinting in distinct application scenarios [239]. However, this method is relatively restricted to the bioprinting resolution and the incorporation of bioactive factors in a controllable manner compared with scaffold-based bioprinting strategies. Basically, neural regeneration requires a favourable microenvironment assisted with the growth factor additives for cell outgrowth and migration. As for neural repair in the lesion site, the scaffold-based formulation provides mechanical support and biochemical cues to facilitate neural outgrowth against axonal inhibitory microenvironment. Despite the noteworthy progress made in collagen-based interventions in NTE, the ultimate goal of translating these products from bench to bedside has not yet been fully achieved. Standardized extraction and neutralization procedures have been established in experimental and commercial settings, but these processes can be costly and time-consuming. To further advance the use of collagen-based biomaterials in NTE, promising future perspectives include stem cell-based gene therapy, drug screening, brain-computer interfaces, 3D bioprinting, and stimuli-responsiveness. (Fig. 6). Investigation of these aspects can open a new chapter in NTE and confirm the therapeutic potential of collagen-based biomaterials.
Fig. 6.
Graphical diagram of promising future orientations of collagen-based biomaterials in NTE, including stem cell-based gene therapy, drug screening, brain-computer interface, 3D bioprinting, and stimuli-responsive alternatives.
The post-hydration stage of hydrogel formulation may compromise sufficient mechanical strength and structural maintenance for further translational applications in tissue engineering. Therefore, finding benign, inexpensive, and productive methodologies as alternatives presents a challenge. Currently, collagen extracted from bovine tendons, bovine skin, porcine skin, and rat tail tendons is the primary source of collagen. However, there is no specific evidence of the differences and potential impacts on encapsulated cell behaviours in the context of 3D culture. As for further translational research, possible heterogeneity resulting from diverse collagen sources should be eliminated via in-depth analysis. More significantly, collagen originating from animal sources may be risky for disease transmission. Bovine spongiform encephalopathy (BSE) induced by ruminant collagen for human consumption has been investigated as a featured example [240]. Also commonly employed in dental surgery are bovine-derived collagen grafts, which might pose hazardous transmission of prion to patients [241]. Besides, allergic reactions caused by multi-species-derived collagen products should be considered. Specific individuals may suffer from anaphylaxis after taking marine collagen supplements, which could be the same as collagen from other sources [242]. Gelatin, the primary ingredient in the measles, mumps, and rubella vaccinations, is a derivative of bovine-derived collagen I. It is reported that children with anaphylaxis exhibited sensitivity to the vaccines mentioned above, in which scenario bovine-derived gelatin could be responsible for the incidents [243].
Collagen is highly associated with clinical outcomes, making it a prevalent biomarker for various clinical applications, including a predictor of prognosis and recurrence; in diagnosis and serving as a drug carrier for targeted therapies [244]. Although collagen-based interventions in neurotrauma and neurodegenerative diseases have shown considerable promise in preclinical research, most applications are still in the preclinical phase, particularly for treating the central nervous system [245]. Clinical trials of collagen-based scaffolds are primarily in SCI repair (Table 6). Based on the completion of preclinical studies, a clinical study incorporating collagen scaffolds with MSCs to treat complete chronic SCI was conducted (NCT02352077), in which case, surgical debridement of the scar tissues was performed, followed by the MSCs-loaded collagen implantation. In a one-year follow-up, patients showed no significant side effects, primarily demonstrating collagen scaffolds' safety and efficacy. Locomotor functions of patients were partially restored in subsequent research. Other than that, collagen scaffolds loaded with MSCs in a complete acute SCI clinical study were conducted (NCT02510365). Some patients showed significant recovery of sensory and motor functions through one-year follow-up. As discussed above, collagen-based nerve conduits are the only FDA-approved products for PNS repair in clinical trials. For instance, NeuraGen proved significantly effective in PNS regeneration among 43% of patients [109]. Neuromaix exhibited remarkable performance in bridging nerves of long gaps in the first clinical trial [110].
Table 6.
Clinical trials on collagen-based scaffolds in NTE. (NA: Not applicable).
| ClinicalTrials.gov identifier | Transplant method | Injury site | Injury type | Phase | Country | Reference |
|---|---|---|---|---|---|---|
| NCT02510365 | Necrohemorrhagic tissues removal followed by transplanted with MSCs | Thoracic and cervical | Acute complete SCI | 1 | China | [246] |
| NCT02352077 | Scar tissues removal followed by transplanted with human umbilical cord MSCs or BMMCs | Thoracic and cervical | Chronic complete SCI | 1 | China | [247,248] |
| NCT02688049 | Scar tissues removal followed by transplanted with MSCs or NSCs | Thoracic and cervical | Chronic complete SCI | 1 and 2 | China | [249] |
| NCT02688062 | Scar tissues removal followed by transplanted with BMMCs | Thoracic | Chronic complete SCI | 1 and 2 | China | Clinicaltrials.gov |
| NCT01884376 | Implantation of Neuromaix during a diagnostic nerve biopsy | Sural nerve | PNI | NA | Germany | [250] |
| NCT01809002 | Implantation of bovine collagen-based nerve cuff | Peripheral nerve | PNI | 3 | America | Clinicaltrials.gov |
| NCT04353908 | Subcutaneous collagen injection of porcine origin | Facial nerve | PNI | NA | Italy | [251] |
From the perspective of technological advances, integrating current fabricating strategies is promising but challenging. The primary proposition during manufacturing is mimicking native neural tissue organization and microenvironment, which not only supports but also enhances tissue development and maturation. Pores, fibres, and channels are some of the key characteristics that emerge in neural tissue and are organized in diverse ways throughout the multiple aspects of the nervous system. Therefore, more effort is being expended in reproducing these natural architectural characteristics to mimic the local microenvironment in vitro. Notably, the integration of multi-techniques should meet the requirements to spatially pattern growth factors, neurotrophic factors, and bioactive proteins in a controlled manner. Furthermore, manipulating spatial distribution and temporal release profiles of bioactive factors can mimic the convoluted developmental profiles of native tissues, which is of particular interest in scaffold fabrication for NTE.
For particular circumstances in the CNS and PNS repair, collagen-based biomaterials should meet relatively meet native characteristics in brain, spinal cord, and peripheral nerve. However, since it is troublesome in CNS repair, the dimensional properties of collagen-based biomaterials should be carefully fine-tuned to match mechanical properties and biochemical requirements. Besides, due to the intrinsic complexity of the CNS, integrating diverse cell types is crucial such as OPC, astrocytes, and microglial. For PNI repair, more concentration should be put on the technological advancements in the topological scale in guiding axonal alignment and functional enhancement based on current research. More importantly, long-distance (>10 mm) lesion gap repair is another obstacle. Therefore, incorporating distinct cell types and modification strategies would be potential in PNS repair.
8. Concluding remarks
In recent decades, the development of therapeutic strategies to address the challenges of nerve regeneration in injury repair and disease restoration has been of significant interest. To this end, a wide range of interventional approaches have been developed to target the CNS and PNS, with collagen scaffolds functionalized with neurotrophic factors emerging as a promising approach to promoting axonal outgrowth and functional restoration. In this review, we have provided an overview of collagen's preparation methods and practical advantages, with a brief description of collagen characteristics. We have also systematically summarized the current applications of collagen in NTE, categorized by manufacturing approach.
Furthermore, we evaluate the criteria applicable to TBI, SCI, and PNS repair, highlighting collagen's potential benefits in specific disease scenarios. Finally, we discuss the promising future directions and challenges that must be addressed to advance NTE utilizing collagen-based biomaterials. As no up-to-date review has assessed the diverse applications of multi-manufactured collagen in NTE, we hope this review will inspire thought-provoking ideas in interdisciplinary fields and contributes to the development of novel approaches to neural tissue engineering.
Funding statement
The authors greatly acknowledge the financial support from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16020802), National Natural Science Foundation of China (T2222029, U21A20396 and 82272473), Beijing Institute for Stem Cell and Regenerative Medicine Project Incubation Found (2022FH110), CAS Engineering Laboratory for Intelligent Organ Manufacturing (KFJ-PTXM-039), CAS Project for Young Scientists in Basic Research (YSBR-012), the Natural Science Foundation of Beijing Municipality (Grant No. 7212020), the Science and Technology Planning Project of Beijing Municipal Education Commission (KM202110025013), and the Beijing Municipal Excellent Talents Project (2020A43).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ming-Zhu Zhang, Email: mingzhuzhang@mail.ccmu.edu.cn.
Qi Gu, Email: qgu@ioz.ac.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Madhusudanan P., Raju G., Shankarappa S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface. 2020;17 doi: 10.1098/rsif.2019.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramón y S. 1928. Cajal, Degeneration and Regeneration of the Nervous System. [Google Scholar]
- 3.Curcio M., Bradke F. Axon regeneration in the central nervous system: facing the challenges from the inside. Annu. Rev. Cell Dev. 2018;34:495–521. doi: 10.1146/annurev-cellbio-100617-062508. [DOI] [PubMed] [Google Scholar]
- 4.Manoli T., Schulz L., Stahl S., Jaminet P., Schaller H.E. Evaluation of sensory recovery after reconstruction of digital nerves of the hand using muscle-in-vein conduits in comparison to nerve suture or nerve autografting. Microsurgery. 2014;34:608–615. doi: 10.1002/micr.22302. [DOI] [PubMed] [Google Scholar]
- 5.Busch S.A., Silver J. The role of extracellular matrix in CNS regeneration. Curr. Opin. Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Aijie C., Xuan L., Huimin L., Yanli Z., Yiyuan K., Yuqing L., Longquan S. Nanoscaffolds in promoting regeneration of the peripheral nervous system. Nanomedicine. 2018;13:1067–1085. doi: 10.2217/nnm-2017-0389. [DOI] [PubMed] [Google Scholar]
- 7.Jeong H.-J., Yun Y., Lee S.-J., Ha Y., Gwak S.-J. Biomaterials and strategies for repairing spinal cord lesions. Neurochem. Int. 2021;144 doi: 10.1016/j.neuint.2021.104973. [DOI] [PubMed] [Google Scholar]
- 8.Pfister B.J., Gordon T., Loverde J.R., Kochar A.S., Mackinnon S.E., Cullen D.K. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011;39 doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 9.Rutka J.T., Apodaca G., Stern R., Rosenblum M. The extracellular matrix of the central and peripheral nervous systems: structure and function. J. Neurosurg. 1988;69:155–170. doi: 10.3171/jns.1988.69.2.0155. [DOI] [PubMed] [Google Scholar]
- 10.Almutairi M.M., Gong C., Xu Y.G., Chang Y., Shi H. Factors controlling permeability of the blood–brain barrier. Cell. Mol. Life Sci. 2016;73:57–77. doi: 10.1007/s00018-015-2050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George N., Geller H.M. Extracellular matrix and traumatic brain injury. J. Neurosci. Res. 2018;96:573–588. doi: 10.1002/jnr.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roll L., Faissner A. Influence of the extracellular matrix on endogenous and transplanted stem cells after brain damage. Front. Cell. Neurosci. 2014;8:219. doi: 10.3389/fncel.2014.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tica J., Bradbury E.J., Didangelos A. Combined transcriptomics, proteomics and bioinformatics identify drug targets in spinal cord injury. Int. J. Mol. Sci. 2018;19:1461. doi: 10.3390/ijms19051461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bannerman P.G., Mirsky R., Jessen K.R., Timpl R., Duance V.C. Light microscopic immunolocalization of laminin, type IV collagen, nidogen, heparan sulphate proteoglycan and fibronectin in the enteric nervous system of rat and Guinea pig. J. Neurocytol. 1986;15:733–743. doi: 10.1007/BF01625191. [DOI] [PubMed] [Google Scholar]
- 15.Bryan D.J., Litchfield C.R., Manchio J.V., Logvinenko T., Holway A.H., Austin J., Summerhayes I.C., Rieger-Christ K.M. Spatiotemporal expression profiling of proteins in rat sciatic nerve regeneration using reverse phase protein arrays. Proteome Sci. 2012;10:1–16. doi: 10.1186/1477-5956-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoll G., Müller H.W. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 1999;9:313–325. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massa R., Palumbo C., Panico M.B., Morello M., Bernardi G., Modesti A. Extracellular matrix remodeling in peripheral neuropathies. J. Peripher. Nerv. Syst. 2001;6:52. 52. [Google Scholar]
- 18.Gonzalez-Perez F., Cobianchi S., Heimann C., Phillips J.B., Udina E., Navarro X. Stabilization, rolling, and addition of other extracellular matrix proteins to collagen hydrogels improve regeneration in chitosan guides for long peripheral nerve gaps in rats. Neurosurgery. 2017;80:465–474. doi: 10.1093/neuros/nyw068. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X., Bhattacharyya A. Human models are needed for studying human neurodevelopmental disorders. Am. J. Hum. Genet. 2018;103:829–857. doi: 10.1016/j.ajhg.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keifer J., Summers C.H. Putting the "biology" back into "neurobiology": the strength of diversity in animal model systems for neuroscience research. Front. Syst. Neurosci. 2016;10 doi: 10.3389/fnsys.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Lullo E., Kriegstein A.R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 2017;18:573–584. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchi F., Malboubi M., Li Y., George J.H., Jerusalem A., Szele F., Thompson M.S., Ye H. Rapid and efficient differentiation of functional motor neurons from human iPSC for neural injury modelling. Stem Cell Res. 2018;32:126–134. doi: 10.1016/j.scr.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Abud E.M., Ramirez R.N., Martinez E.S., Healy L.M., Nguyen C.H.H., Newman S.A., Yeromin A.V., Scarfone V.M., Marsh S.E., Fimbres C., Caraway C.A., Fote G.M., Madany A.M., Agrawal A., Kayed R., Gylys K.H., Cahalan M.D., Cummings B.J., Antel J.P., Mortazavi A., Carson M.J., Poon W.W., Blurton-Jones M. iPSC-derived human microglia-like cells to study neurological diseases. Neuron. 2017;94:278–293.e9. doi: 10.1016/j.neuron.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo F.B., Freitas B.C., Pignatari G.C., Fernandes I.R., Sebat J., Muotri A.R., Beltrão-Braga P.C.B. Modeling the interplay between neurons and astrocytes in autism using human induced pluripotent stem cells. Biol. Psychiatr. 2018;83:569–578. doi: 10.1016/j.biopsych.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Joung D., Lavoie N.S., Guo S.Z., Park S.H., Parr A.M., McAlpine M.C. 3D printed neural regeneration devices. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201906237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnelly E.M., Lamanna J., Boulis N.M. Stem cell therapy for the spinal cord. Stem Cell Res. Ther. 2012;3:24. doi: 10.1186/scrt115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y., Saloupis P., Shaw S.J., Rickman D.W. Engraftment of adult neural progenitor cells transplanted to rat retina injured by transient ischemia. Investig. Ophthalmol. Vis. Sci. 2003;44:3194–3201. doi: 10.1167/iovs.02-0875. [DOI] [PubMed] [Google Scholar]
- 28.Park J.H., Kim D.Y., Sung I.Y., Choi G.H., Jeon M.H., Kim K.K., Jeon S.R. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012;70:1238–1247. doi: 10.1227/NEU.0b013e31824387f9. [DOI] [PubMed] [Google Scholar]
- 29.Yang R., Xu C., Wang T., Wang Y., Wang J., Quan D., Deng D.Y. PTMAc-PEG-PTMAc hydrogel modified by RGDC and hyaluronic acid promotes neural stem cells' survival and differentiation in vitro. RSC Adv. 2017;7:41098–41104. [Google Scholar]
- 30.Zhou X., Yang A., Huang Z., Yin G., Pu X., Jin J. Enhancement of neurite adhesion, alignment and elongation on conductive polypyrrole-poly (lactide acid) fibers with cell-derived extracellular matrix. Colloids Surf. B Biointerfaces. 2017;149:217–225. doi: 10.1016/j.colsurfb.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Xue C., Zhu H., Tan D., Ren H., Gu X., Zhao Y., Zhang P., Sun Z., Yang Y., Gu J. Electrospun silk fibroin-based neural scaffold for bridging a long sciatic nerve gap in dogs. J Tissue Eng Regen Med. 2018;12:e1143–e1153. doi: 10.1002/term.2449. [DOI] [PubMed] [Google Scholar]
- 32.Katoh H., Yokota K., Fehlings M.G. Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front. Cell. Neurosci. 2019;13:248. doi: 10.3389/fncel.2019.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadena M., Ning L., King A., Hwang B., Jin L., Serpooshan V., Sloan S.A. 3D bioprinting of neural tissues. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madhusudanan P., Reade S., Shankarappa S.A. Neuroglia as targets for drug delivery systems: a review. Nanomed. Nanotechnol. Biol. Med. 2017;13:667–679. doi: 10.1016/j.nano.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Gopalakrishnan A., Shankarappa S.A., Rajanikant G.K. Hydrogel scaffolds: towards restitution of ischemic stroke-injured brain. Transl. Stroke Res. 2019;10:1–18. doi: 10.1007/s12975-018-0655-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhu W., Castro N.J., Shen Y.-L., Zhang L.G. Elsevier; 2022. Nanotechnology and 3D/4D Bioprinting for Neural Tissue Regeneration, 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine; pp. 427–458. [Google Scholar]
- 37.Subramanian A., Krishnan U.M., Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: biomaterial mediated neural regeneration. J. Biomed. Sci. 2009;16:1–11. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doblado L.R., Martínez-Ramos C., Pradas M.M. Biomaterials for neural tissue engineering. Front. nanotechnol. 2021;3 [Google Scholar]
- 39.Li X., Li M., Sun J., Zhuang Y., Shi J., Guan D., Chen Y., Dai J. Radially aligned electrospun fibers with continuous gradient of SDF1α for the guidance of neural stem cells. Small. 2016;12:5009–5018. doi: 10.1002/smll.201601285. [DOI] [PubMed] [Google Scholar]
- 40.Zou Y., Ma D., Shen H., Zhao Y., Xu B., Fan Y., Sun Z., Chen B., Xue W., Shi Y., Xiao Z., Gu R., Dai J. Aligned collagen scaffold combination with human spinal cord-derived neural stem cells to improve spinal cord injury repair. Biomater. Sci. 2020;8:5145–5156. doi: 10.1039/d0bm00431f. [DOI] [PubMed] [Google Scholar]
- 41.Chen C., Zhao M.L., Zhang R.K., Lu G., Zhao C.Y., Fu F., Sun H.T., Zhang S., Tu Y., Li X.H. Collagen/heparin sulfate scaffolds fabricated by a 3D bioprinter improved mechanical properties and neurological function after spinal cord injury in rats. J. Biomed. Mater. Res. 2017;105:1324–1332. doi: 10.1002/jbm.a.36011. [DOI] [PubMed] [Google Scholar]
- 42.Chakraborty J., Mu X., Pramanick A., Kaplan D.L., Ghosh S. Recent advances in bioprinting using silk protein-based bioinks. Biomaterials. 2022;287 doi: 10.1016/j.biomaterials.2022.121672. [DOI] [PubMed] [Google Scholar]
- 43.Xu T., Gregory C.A., Molnar P., Cui X., Jalota S., Bhaduri S.B., Boland T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006;27:3580–3588. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 44.Jessen K.R., Mirsky R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016;594:3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Höke A., Brushart T. Introduction to special issue: challenges and opportunities for regeneration in the peripheral nervous system. Exp. Neurol. 2010;223:1–4. doi: 10.1016/j.expneurol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curcio M., Bradke F. Axon regeneration in the central nervous system: facing the challenges from the inside. Annu. Rev. Cell Dev. Biol. 2018;34:495–521. doi: 10.1146/annurev-cellbio-100617-062508. [DOI] [PubMed] [Google Scholar]
- 47.Mouw J.K., Ou G., Weaver V.M. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014;15:771–785. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brodsky B., Persikov A.V. Molecular structure of the collagen triple helix. Adv. Protein Chem. 2005;70:301–339. doi: 10.1016/S0065-3233(05)70009-7. [DOI] [PubMed] [Google Scholar]
- 49.Mienaltowski M.J., Birk D.E. Structure, physiology, and biochemistry of collagens. Adv. Exp. Med. Biol. 2014;802:5–29. doi: 10.1007/978-94-007-7893-1_2. [DOI] [PubMed] [Google Scholar]
- 50.Fratzl P. In: Collagen: Structure and Mechanics. Fratzl P., editor. Springer US; Boston, MA: 2008. Collagen: structure and mechanics, an introduction; pp. 1–13. [Google Scholar]
- 51.Cao J., Xiao Z., Jin W., Chen B., Meng D., Ding W., Han S., Hou X., Zhu T., Yuan B. Induction of rat facial nerve regeneration by functional collagen scaffolds. Biomaterials. 2013;34:1302–1310. doi: 10.1016/j.biomaterials.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 52.Li X., Zhang X., Hao M., Wang D., Jiang Z., Sun L., Gao Y., Jin Y., Lei P., Zhuo Y. The application of collagen in the repair of peripheral nerve defect. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.973301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu S., Sun L., Zhang H., Hu Q., Wang Y., Ramalingam M. High-resolution combinatorial 3D printing of gelatin-based biomimetic triple-layered conduits for nerve tissue engineering. Int. J. Biol. Macromol. 2021;166:1280–1291. doi: 10.1016/j.ijbiomac.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Zhuang H., Bu S., Hua L., Darabi M.A., Cao X., Xing M. Gelatin-methacrylamide gel loaded with microspheres to deliver GDNF in bilayer collagen conduit promoting sciatic nerve growth. Int. J. Nanomed. 2016;11:1383. doi: 10.2147/IJN.S96324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang Y., Walczak P., Bulte J.W. The survival of engrafted neural stem cells within hyaluronic acid hydrogels. Biomaterials. 2013;34:5521–5529. doi: 10.1016/j.biomaterials.2013.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y., Wei Y.T., Zu Z.H., Ju R.K., Guo M.Y., Wang X.M., Xu Q.Y., Cui F.Z. Combination of hyaluronic acid hydrogel scaffold and PLGA microspheres for supporting survival of neural stem cells. Pharm. Res. (N. Y.) 2011;28:1406–1414. doi: 10.1007/s11095-011-0452-3. [DOI] [PubMed] [Google Scholar]
- 57.Ansari S., Diniz I.M., Chen C., Sarrion P., Tamayol A., Wu B.M., Moshaverinia A. Human periodontal ligament-and gingiva-derived mesenchymal stem cells promote nerve regeneration when encapsulated in alginate/hyaluronic acid 3D scaffold. Adv. Healthc. Mater. 2017;6 doi: 10.1002/adhm.201700670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sitoci-Ficici K.H., Matyash M., Uckermann O., Galli R., Leipnitz E., Later R., Ikonomidou C., Gelinsky M., Schackert G., Kirsch M. Non-functionalized soft alginate hydrogel promotes locomotor recovery after spinal cord injury in a rat hemimyelonectomy model. Acta Neurochir. 2018;160:449–457. doi: 10.1007/s00701-017-3389-4. [DOI] [PubMed] [Google Scholar]
- 59.Li G., Xiao Q., McNaughton R., Han L., Zhang L., Wang Y., Yang Y. Nanoengineered porous chitosan/CaTiO3 hybrid scaffolds for accelerating Schwann cells growth in peripheral nerve regeneration. Colloids Surf. B Biointerfaces. 2017;158:57–67. doi: 10.1016/j.colsurfb.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 60.Wang S., Sun C., Guan S., Li W., Xu J., Ge D., Zhuang M., Liu T., Ma X. Chitosan/gelatin porous scaffolds assembled with conductive poly (3, 4-ethylenedioxythiophene) nanoparticles for neural tissue engineering. J. Mater. Chem. B. 2017;5:4774–4788. doi: 10.1039/c7tb00608j. [DOI] [PubMed] [Google Scholar]
- 61.Nectow A.R., Marra K.G., Kaplan D.L. Biomaterials for the development of peripheral nerve guidance conduits, Tissue Eng. Part B Rev. 2012;18:40–50. doi: 10.1089/ten.teb.2011.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li D., Pan X., Sun B., Wu T., Chen W., Huang C., Ke Q., Ei-Hamshary H.A., Al-Deyab S.S., Mo X. Nerve conduits constructed by electrospun P (LLA-CL) nanofibers and PLLA nanofiber yarns. J. Mater. Chem. B. 2015;3:8823–8831. doi: 10.1039/c5tb01402f. [DOI] [PubMed] [Google Scholar]
- 63.Sun C., Jin X., Holzwarth J.M., Liu X., Hu J., Gupte M.J., Zhao Y., Ma P.X. Development of channeled nanofibrous scaffolds for oriented tissue engineering. Macromol. Biosci. 2012;12:761–769. doi: 10.1002/mabi.201200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malinovskaya Y., Melnikov P., Baklaushev V., Gabashvili A., Osipova N., Mantrov S., Ermolenko Y., Maksimenko O., Gorshkova M., Balabanyan V. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int. J. Pharm. 2017;524:77–90. doi: 10.1016/j.ijpharm.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 65.Mir M., Ahmed N., ur Rehman A. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces. 2017;159:217–231. doi: 10.1016/j.colsurfb.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 66.Lee J.Y., Bashur C.A., Goldstein A.S., Schmidt C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30:4325–4335. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malarkey E.B., Fisher K.A., Bekyarova E., Liu W., Haddon R.C., Parpura V. Conductive single-walled carbon nanotube substrates modulate neuronal growth. Nano Lett. 2009;9:264–268. doi: 10.1021/nl802855c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parenteau-Bareil R., Gauvin R., Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863–1887. [Google Scholar]
- 69.Fratzl P., Misof K., Zizak I., Rapp G., Amenitsch H., Bernstorff S. Fibrillar structure and mechanical properties of collagen. J. Struct. Biol. 1998;122:119–122. doi: 10.1006/jsbi.1998.3966. [DOI] [PubMed] [Google Scholar]
- 70.Myllyharju J., Kivirikko K.I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Ricard-Blum S., Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol. Biol. 2005;53:430–442. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 72.Fan D., Takawale A., Lee J., Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:1–13. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Radhakrishnan S., Nagarajan S., Bechelany M., Kalkura S.N. In: Processes and Phenomena on the Boundary between Biogenic and Abiogenic Nature. Frank-Kamenetskaya O.V., Vlasov D.Y., Panova E.G., Lessovaia S.N., editors. Springer International Publishing; Cham: 2020. Collagen based biomaterials for tissue engineering applications: a review; pp. 3–22. [Google Scholar]
- 74.Sorushanova A., Delgado L.M., Wu Z., Shologu N., Kshirsagar A., Raghunath R., Mullen A.M., Bayon Y., Pandit A., Raghunath M., Zeugolis D.I. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv. Mater. 2019;31 doi: 10.1002/adma.201801651. [DOI] [PubMed] [Google Scholar]
- 75.Chen J., Li L., Yi R., Xu N., Gao R., Hong B. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus) LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;66:453–459. [Google Scholar]
- 76.Schmidt M., Dornelles R., Mello R., Kubota E., Mazutti M., Kempka A., Demiate I. Collagen extraction process. Int. Food Res. J. 2016;23:913. [Google Scholar]
- 77.Matinong A.M.E., Chisti Y., Pickering K.L., Haverkamp R.G. Collagen extraction from animal skin. Biology. 2022;11:905. doi: 10.3390/biology11060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mocan E., Tagadiuc O., Nacu V. Aspects of collagen isolation procedure. Curierul medical. 2011 [Google Scholar]
- 79.Wang T., Lew J., Premkumar J., Poh C.L., Win Naing M. Production of recombinant collagen: state of the art and challenges. Biol. Eng. 2017;1:18–23. [Google Scholar]
- 80.Yunoki S., Ohyabu Y., Hatayama H. Temperature-responsive gelation of type I collagen solutions involving fibril formation and genipin crosslinking as a potential injectable hydrogel. Int. J. Biomater. 2013;2013 doi: 10.1155/2013/620765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Furthmayr H., Timpl R. Immunochemistry of collagens and procollagens. Int. Rev. Connect. Tissue Res. 1976;7:61–99. doi: 10.1016/b978-0-12-363707-9.50008-3. [DOI] [PubMed] [Google Scholar]
- 82.DeLustro F., Condell R.A., Nguyen M.A., McPherson J.M. A comparative study of the biologic and immunologic response to medical devices derived from dermal collagen. J. Biomed. Mater. Res. 1986;20:109–120. doi: 10.1002/jbm.820200110. [DOI] [PubMed] [Google Scholar]
- 83.Speer D.P., Chvapil M., Eskelson C., Ulreich J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J. Biomed. Mater. Res. 1980;14:753–764. doi: 10.1002/jbm.820140607. [DOI] [PubMed] [Google Scholar]
- 84.Lynn A., Yannas I., Bonfield W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2004;71:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 85.Ahmed M.R., Jayakumar R. Peripheral nerve regeneration in RGD peptide incorporated collagen tubes. Brain Res. 2003;993:208–216. doi: 10.1016/j.brainres.2003.08.057. [DOI] [PubMed] [Google Scholar]
- 86.Wen F., Chang S., Toh Y., Teoh S., Yu H. Development of poly (lactic-co-glycolic acid)-collagen scaffolds for tissue engineering. Mater. Sci. Eng. C. 2007;27:285–292. [Google Scholar]
- 87.Jones J.M., Cohen R.L., Chambers D.A. Collagen modulates gene activation of plasminogen activator system molecules. Exp. Cell Res. 2002;280:244–254. doi: 10.1006/excr.2002.5644. [DOI] [PubMed] [Google Scholar]
- 88.Edelman D.B., Keefer E.W. A cultural renaissance: in vitro cell biology embraces three-dimensional context. Exp. Neurol. 2005;192:1–6. doi: 10.1016/j.expneurol.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 89.Najafi M.F., Zahri S., Vahedi F., Toosi L.E., Ariaee N. Which form of collagen is suitable for nerve cell culture? Neural Regen. Res. 2013;8:2165. doi: 10.3969/j.issn.1673-5374.2013.23.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao L., de Ruiter G.C., Wang H., Knight A.M., Spinner R.J., Yaszemski M.J., Windebank A.J., Pandit A. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials. 2010;31:5789–5797. doi: 10.1016/j.biomaterials.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 91.Cao J., Sun C., Zhao H., Xiao Z., Chen B., Gao J., Zheng T., Wu W., Wu S., Wang J. The use of laminin modified linear ordered collagen scaffolds loaded with laminin-binding ciliary neurotrophic factor for sciatic nerve regeneration in rats. Biomaterials. 2011;32:3939–3948. doi: 10.1016/j.biomaterials.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 92.Fan C., Li X., Xiao Z., Zhao Y., Liang H., Wang B., Han S., Li X., Xu B., Wang N., Liu S., Xue W., Dai J. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017;51:304–316. doi: 10.1016/j.actbio.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 93.Yao L., Phan F., Li Y. Collagen microsphere serving as a cell carrier supports oligodendrocyte progenitor cell growth and differentiation for neurite myelination in vitro. Stem Cell Res. Ther. 2013;4:109. doi: 10.1186/scrt320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mohamadi F., Ebrahimi-Barough S., Reza Nourani M., Ali Derakhshan M., Goodarzi V., Sadegh Nazockdast M., Farokhi M., Tajerian R., Faridi Majidi R., Ai J. Electrospun nerve guide scaffold of poly(ε-caprolactone)/collagen/nanobioglass: an in vitro study in peripheral nerve tissue engineering. J. Biomed. Mater. Res. 2017;105:1960–1972. doi: 10.1002/jbm.a.36068. [DOI] [PubMed] [Google Scholar]
- 95.Lohmeyer J.A., Siemers F., Machens H.-G., Mailänder P. The clinical use of artificial nerve conduits for digital nerve repair: a prospective cohort study and literature review. J. Reconstr. Microsurg. 2009;25:55–61. doi: 10.1055/s-0028-1103505. [DOI] [PubMed] [Google Scholar]
- 96.Hoban D.B., Newland B., Moloney T.C., Howard L., Pandit A., Dowd E. The reduction in immunogenicity of neurotrophin overexpressing stem cells after intra-striatal transplantation by encapsulation in an in situ gelling collagen hydrogel. Biomaterials. 2013;34:9420–9429. doi: 10.1016/j.biomaterials.2013.08.073. [DOI] [PubMed] [Google Scholar]
- 97.Yannas I., Orgill D., Silver J., Norregaard T., Zervas N., Schoene W. Regeneration of sciatic nerve across 15mm gap by use of a polymeric template. Adv. Polym. Sci. 1987:1–9. [Google Scholar]
- 98.Hoare T.R., Kohane D.S. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49:1993–2007. [Google Scholar]
- 99.Caliari S.R., Burdick J.A. A practical guide to hydrogels for cell culture. Nat. Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Foidl B.M., Ucar B., Schwarz A., Rebelo A.L., Pandit A., Humpel C. Nerve growth factor released from collagen scaffolds protects axotomized cholinergic neurons of the basal nucleus of Meynert in organotypic brain slices. J. Neurosci. Methods. 2018;295:77–86. doi: 10.1016/j.jneumeth.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 101.Ucar B., Humpel C. Collagen for brain repair: therapeutic perspectives. Neural Regen Res. 2018;13:595–598. doi: 10.4103/1673-5374.230273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bamberger M.E., Landreth G.E. Inflammation, apoptosis, and Alzheimer's disease. Neuroscientist. 2002;8:276–283. doi: 10.1177/1073858402008003013. [DOI] [PubMed] [Google Scholar]
- 103.Bramlett H.M., Dietrich W.D. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J. Cerebr. Blood Flow Metabol. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 104.Dexter D.T., Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013;62:132–144. doi: 10.1016/j.freeradbiomed.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 105.Hatami M., Mehrjardi N.Z., Kiani S., Hemmesi K., Azizi H., Shahverdi A., Baharvand H. Human embryonic stem cell-derived neural precursor transplants in collagen scaffolds promote recovery in injured rat spinal cord. Cytotherapy. 2009;11:618–630. doi: 10.1080/14653240903005802. [DOI] [PubMed] [Google Scholar]
- 106.Collagen scaffolds incorporating select therapeutic agents to facilitate a reparative response in a standardized hemiresection defect in the rat spinal cord. Tissue Eng. 2012;18:2158–2172. doi: 10.1089/ten.TEA.2011.0577. [DOI] [PubMed] [Google Scholar]
- 107.Li X., Xiao Z., Han J., Chen L., Xiao H., Ma F., Hou X., Li X., Sun J., Ding W., Zhao Y., Chen B., Dai J. Promotion of neuronal differentiation of neural progenitor cells by using EGFR antibody functionalized collagen scaffolds for spinal cord injury repair. Biomaterials. 2013;34:5107–5116. doi: 10.1016/j.biomaterials.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 108.Li X., Zhao Y., Cheng S., Han S., Shu M., Chen B., Chen X., Tang F., Wang N., Tu Y., Wang B., Xiao Z., Zhang S., Dai J. Cetuximab modified collagen scaffold directs neurogenesis of injury-activated endogenous neural stem cells for acute spinal cord injury repair. Biomaterials. 2017;137:73–86. doi: 10.1016/j.biomaterials.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 109.Wangensteen K.J., Kalliainen L.K. Collagen tube conduits in peripheral nerve repair: a retrospective analysis. Hand. 2010;5:273–277. doi: 10.1007/s11552-009-9245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bozkurt A., Claeys K.G., Schrading S., Rödler J.V., Altinova H., Schulz J.B., Weis J., Pallua N., van Neerven S.G. Clinical and biometrical 12-month follow-up in patients after reconstruction of the sural nerve biopsy defect by the collagen-based nerve guide Neuromaix. Eur. J. Med. Res. 2017;22:1–12. doi: 10.1186/s40001-017-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kehoe S., Zhang X., Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43:553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 112.Archibald S., Shefner J., Krarup C., Madison R. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J. Neurosci. 1995;15:4109–4123. doi: 10.1523/JNEUROSCI.15-05-04109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yao Y., Cui Y., Zhao Y., Xiao Z., Li X., Han S., Chen B., Fang Y., Wang P., Pan J. Efect of longitudinally oriented collagen conduit combined with nerve growth factor on nerve regeneration after dog sciatic nerve injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018;106:2131–2139. doi: 10.1002/jbm.b.34020. [DOI] [PubMed] [Google Scholar]
- 114.Sebben A.D., Lichtenfels M., da Silva J.L. Peripheral nerve regeneration: cell therapy and neurotrophic factors. Rev Bras Ortop. 2011;46:643–649. doi: 10.1016/S2255-4971(15)30319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Höke A. Mechanisms of Disease: what factors limit the success of peripheral nerve regeneration in humans? Nat. Clin. Pract. Neurol. 2006;2:448–454. doi: 10.1038/ncpneuro0262. [DOI] [PubMed] [Google Scholar]
- 116.Lowe C.J., Reucroft I.M., Grota M.C., Shreiber D.I. Production of highly aligned collagen scaffolds by freeze-drying of self-assembled, fibrillar collagen gels. ACS Biomater. Sci. Eng. 2016;2:643–651. doi: 10.1021/acsbiomaterials.6b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maghdouri-White Y., Petrova S., Sori N., Polk S., Wriggers H., Ogle R., Ogle R., Francis M. Electrospun silk–collagen scaffolds and BMP-13 for ligament and tendon repair and regeneration, Biomed. Phys. Eng. Express. 2018;4 [Google Scholar]
- 118.Hospodiuk M., Dey M., Sosnoski D., Ozbolat I.T. The bioink: a comprehensive review on bioprintable materials. Biotechnol. Adv. 2017;35:217–239. doi: 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 119.Nair K., Gandhi M., Khalil S., Yan K.C., Marcolongo M., Barbee K., Sun W. Characterization of cell viability during bioprinting processes. Biotechnol. J. 2009;4:1168–1177. doi: 10.1002/biot.200900004. [DOI] [PubMed] [Google Scholar]
- 120.Zheng Q., Lu J., Chen H., Huang L., Cai J., Xu Z. Application of inkjet printing technique for biological material delivery and antimicrobial assays. Anal. Biochem. 2011;410:171–176. doi: 10.1016/j.ab.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 121.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 122.Koffler J., Zhu W., Qu X., Platoshyn O., Dulin J.N., Brock J., Graham L., Lu P., Sakamoto J., Marsala M., Chen S., Tuszynski M.H. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019;25:263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peltola S.M., Melchels F.P.W., Grijpma D.W., Kellomäki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008;40:268–280. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 124.Zhao P., Gu H., Mi H., Rao C., Fu J., Turng L.-s. Fabrication of scaffolds in tissue engineering: a review. Front. Mech. Eng. 2018;13:107–119. [Google Scholar]
- 125.Lee W.G., Demirci U., Khademhosseini A. Microscale electroporation: challenges and perspectives for clinical applications, Integr. Biol. 2009;1:242–251. doi: 10.1039/b819201d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee J.M., Suen S.K.Q., Ng W.L., Ma W.C., Yeong W.Y. Bioprinting of collagen: considerations, potentials, and applications. Macromol. Biosci. 2021;21 doi: 10.1002/mabi.202000280. [DOI] [PubMed] [Google Scholar]
- 127.Derakhshanfar S., Mbeleck R., Xu K., Zhang X., Zhong W., Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact. Mater. 2018;3:144–156. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O'Brien C.M., Holmes B., Faucett S., Zhang L.G. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng. B Rev. 2015;21:103–114. doi: 10.1089/ten.teb.2014.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226 doi: 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 130.Cui X., Boland T., D'Lima D.D., Lotz M.K. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formulation. 2012;6:149–155. doi: 10.2174/187221112800672949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim J.D., Choi J.S., Kim B.S., Chan Choi Y., Cho Y.W. Piezoelectric inkjet printing of polymers: stem cell patterning on polymer substrates. Polymer. 2010;51:2147–2154. [Google Scholar]
- 132.Xu T., Gregory C.A., Molnar P., Cui X., Jalota S., Bhaduri S.B., Boland T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006;27:3580–3588. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 133.Lee W., Pinckney J., Lee V., Lee J.H., Fischer K., Polio S., Park J.K., Yoo S.S. Three-dimensional bioprinting of rat embryonic neural cells. Neuroreport. 2009;20:798–803. doi: 10.1097/WNR.0b013e32832b8be4. [DOI] [PubMed] [Google Scholar]
- 134.Lee Y.B., Polio S., Lee W., Dai G., Menon L., Carroll R.S., Yoo S.S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 2010;223:645–652. doi: 10.1016/j.expneurol.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 135.Chen C., Zhao M.-l., Zhang R.-k., Lu G., Zhao C.-y., Fu F., Sun H.-t., Zhang S., Tu Y., Li X.-h. Collagen/heparin sulfate scaffolds fabricated by a 3D bioprinter improved mechanical properties and neurological function after spinal cord injury in rats. J. Biomed. Mater. Res. 2017;105:1324–1332. doi: 10.1002/jbm.a.36011. [DOI] [PubMed] [Google Scholar]
- 136.Jiang J.P., Liu X.Y., Zhao F., Zhu X., Li X.Y., Niu X.G., Yao Z.T., Dai C., Xu H.Y., Ma K., Chen X.Y., Zhang S. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neural Regen Res. 2020;15:959–968. doi: 10.4103/1673-5374.268974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matthews J.A., Wnek G.E., Simpson D.G., Bowlin G.L. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 138.Zhao R., Jiang L., Du J., Xu B., Li A., Wang W., Zhao S., Li X. Fluffy sponge-reinforced electrospun conduits with biomimetic structures for peripheral nerve repair. Compos. B Eng. 2022;230 [Google Scholar]
- 139.Ouyang Y., Huang C., Zhu Y., Fan C., Ke Q. Fabrication of seamless electrospun collagen/PLGA conduits whose walls comprise highly longitudinal aligned nanofibers for nerve regeneration. J. Biomed. Nanotechnol. 2013;9:931–943. doi: 10.1166/jbn.2013.1605. [DOI] [PubMed] [Google Scholar]
- 140.Hoffman-Kim D., Mitchel J.A., Bellamkonda R.V. Topography, cell response, and nerve regeneration. Annu. Rev. Biomed. Eng. 2010;12:203. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu T., Houle J.D., Xu J., Chan B.P., Chew S.Y. Nanofibrous collagen nerve conduits for spinal cord repair, Tissue. Eng. Part A. 2012;18:1057–1066. doi: 10.1089/ten.tea.2011.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang N., Lin J., Lin V.P.H., Milbreta U., Chin J.S., Chew E.G.Y., Lian M.M., Foo J.N., Zhang K., Wu W. A 3D fiber-hydrogel based non-viral gene delivery platform reveals that microRNAs promote axon regeneration and enhance functional recovery following spinal cord injury. Adv. Sci. 2021;8 doi: 10.1002/advs.202100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kraskiewicz H., Breen B., Sargeant T., McMahon S., Pandit A. Assembly of protein-based hollow spheres encapsulating a therapeutic factor. ACS Chem. Neurosci. 2013;4:1297–1304. doi: 10.1021/cn400080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nagai N., Kumasaka N., Kawashima T., Kaji H., Nishizawa M., Abe T. Preparation and characterization of collagen microspheres for sustained release of VEGF. J. Mater. Sci. Mater. Med. 2010;21:1891–1898. doi: 10.1007/s10856-010-4054-0. [DOI] [PubMed] [Google Scholar]
- 145.McRae A., Dahlström A. Transmitter-loaded polymeric microspheres induce regrowth of dopaminergic nerve terminals in striata of rats with 6-OH-DA induced parkinsonism. Neurochem. Int. 1994;25:27–33. doi: 10.1016/0197-0186(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 146.Jollivet C., Aubert-Pouessel A., Clavreul A., Venier-Julienne M.-C., Remy S., Montero-Menei C.N., Benoit J.-P., Menei P. Striatal implantation of GDNF releasing biodegradable microspheres promotes recovery of motor function in a partial model of Parkinson's disease. Biomaterials. 2004;25:933–942. doi: 10.1016/s0142-9612(03)00601-x. [DOI] [PubMed] [Google Scholar]
- 147.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Breuls R.G., Jiya T.U., Smit T.H. Scaffold stiffness influences cell behavior: opportunities for skeletal tissue engineering. Open Orthop. J. 2008;2:103. doi: 10.2174/1874325000802010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yiannakou C., Simitzi C., Manousaki A., Fotakis C., Ranella A., Stratakis E. Cell patterning via laser micro/nano structured silicon surfaces. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa71c6. [DOI] [PubMed] [Google Scholar]
- 150.Mi H.-Y., Jing X., Turng L.-S. Fabrication of porous synthetic polymer scaffolds for tissue engineering. J. Cell. Plast. 2015;51:165–196. [Google Scholar]
- 151.Hsu S.-h., Ni H.-C. Fabrication of the microgrooved/microporous polylactide substrates as peripheral nerve conduits and in vivo evaluation. Tissue Eng. 2009;15:1381–1390. doi: 10.1089/ten.tea.2008.0175. [DOI] [PubMed] [Google Scholar]
- 152.Ao Q., Wang A., Cao W., Zhang L., Kong L., He Q., Gong Y., Zhang X. Manufacture of multimicrotubule chitosan nerve conduits with novel molds and characterization in vitro. J. Biomed. Mater. Res. Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2006;77:11–18. doi: 10.1002/jbm.a.30593. [DOI] [PubMed] [Google Scholar]
- 153.Yang Y., De Laporte L., Rives C.B., Jang J.-H., Lin W.-C., Shull K.R., Shea L.D. Neurotrophin releasing single and multiple lumen nerve conduits. J. Contr. Release. 2005;104:433–446. doi: 10.1016/j.jconrel.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Franze K., Gerdelmann J., Weick M., Betz T., Pawlizak S., Lakadamyali M., Bayer J., Rillich K., Gögler M., Lu Y.-B. Neurite branch retraction is caused by a threshold-dependent mechanical impact. Biophys. J. 2009;97:1883–1890. doi: 10.1016/j.bpj.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Georges P.C., Miller W.J., Meaney D.F., Sawyer E.S., Janmey P.A. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys. J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saha K., Keung A.J., Irwin E.F., Li Y., Little L., Schaffer D.V., Healy K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang F.X., Yurke B., Firestein B.L., Langrana N.A. Neurite outgrowth on a DNA crosslinked hydrogel with tunable stiffnesses. Ann. Biomed. Eng. 2008;36:1565–1579. doi: 10.1007/s10439-008-9530-z. [DOI] [PubMed] [Google Scholar]
- 158.Koch D., Rosoff W.J., Jiang J., Geller H.M., Urbach J.S. Strength in the periphery: growth cone biomechanics and substrate rigidity response in peripheral and central nervous system neurons. Biophys. J. 2012;102:452–460. doi: 10.1016/j.bpj.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moshayedi P., Ng G., Kwok J.C., Yeo G.S., Bryant C.E., Fawcett J.W., Franze K., Guck J. The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials. 2014;35:3919–3925. doi: 10.1016/j.biomaterials.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 160.Engler A.J., Sweeney H.L., Discher D.E., Schwarzbauer J.E. Extracellular matrix elasticity directs stem cell differentiation. J. Musculoskelet. Neuronal Interact. 2007;7:335. [PubMed] [Google Scholar]
- 161.Lv H., Li L., Sun M., Zhang Y., Chen L., Rong Y., Li Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 2015;6:103. doi: 10.1186/s13287-015-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dalton P.D., Flynn L., Shoichet M.S. Manufacture of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) hydrogel tubes for use as nerve guidance channels. Biomaterials. 2002;23:3843–3851. doi: 10.1016/s0142-9612(02)00120-5. [DOI] [PubMed] [Google Scholar]
- 163.Sundback C.A., Shyu J.Y., Wang Y., Faquin W.C., Langer R.S., Vacanti J.P., Hadlock T.A. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–5464. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 164.Gu Q., Tomaskovic-Crook E., Lozano R., Chen Y., Kapsa R.M., Zhou Q., Wallace G.G., Crook J.M. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv Healthc Mater. 2016;5:1429–1438. doi: 10.1002/adhm.201600095. [DOI] [PubMed] [Google Scholar]
- 165.Huang C.-T., Kumar Shrestha L., Ariga K., Hsu S.-h. A graphene–polyurethane composite hydrogel as a potential bioink for 3D bioprinting and differentiation of neural stem cells. J. Mater. Chem. B. 2017;5:8854–8864. doi: 10.1039/c7tb01594a. [DOI] [PubMed] [Google Scholar]
- 166.Hsiao S.H., Hsu S.H. Synthesis and characterization of dual stimuli-sensitive biodegradable polyurethane soft hydrogels for 3D cell-laden bioprinting. ACS Appl. Mater. Interfaces. 2018;10:29273–29287. doi: 10.1021/acsami.8b08362. [DOI] [PubMed] [Google Scholar]
- 167.Oliveira S.L.B., Pillat M.M., Cheffer A., Lameu C., Schwindt T.T., Ulrich H. Functions of neurotrophins and growth factors in neurogenesis and brain repair. Cytometry. 2013;83A:76–89. doi: 10.1002/cyto.a.22161. [DOI] [PubMed] [Google Scholar]
- 168.Aloe L., Rocco M.L., Bianchi P., Manni L. Nerve growth factor: from the early discoveries to the potential clinical use. J. Transl. Med. 2012;10:239. doi: 10.1186/1479-5876-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sharma R., Smits I.P.M., De La Vega L., Lee C., Willerth S.M. 3D bioprinting pluripotent stem cell derived neural tissues using a novel fibrin bioink containing drug releasing microspheres. Front. Bioeng. Biotechnol. 2020;8:57. doi: 10.3389/fbioe.2020.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Agbay A., De La Vega L., Nixon G., Willerth S. Guggulsterone-releasing microspheres direct the differentiation of human induced pluripotent stem cells into neural phenotypes. Biomed. Mater. 2018;13 doi: 10.1088/1748-605X/aaaa77. [DOI] [PubMed] [Google Scholar]
- 171.Yu L., Leipzig N., Shoichet M. Promoting neuron adhesion and growth-Review. Mater. Today. 2008;11:36–43. [Google Scholar]
- 172.Weng B., Liu X., Shepherd R., Wallace G.G. Inkjet printed polypyrrole/collagen scaffold: a combination of spatial control and electrical stimulation of PC12 cells. Synth. Met. 2012;162:1375–1380. [Google Scholar]
- 173.Zhao Y.H., Niu C.M., Shi J.Q., Wang Y.Y., Yang Y.M., Wang H.B. Novel conductive polypyrrole/silk fibroin scaffold for neural tissue repair. Neural Regen Res. 2018;13:1455–1464. doi: 10.4103/1673-5374.235303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao Y., Liang Y., Ding S., Zhang K., Mao H.Q., Yang Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120164. [DOI] [PubMed] [Google Scholar]
- 175.Duarte Campos D.F., Bonnin Marquez A., O'Seanain C., Fischer H., Blaeser A., Vogt M., Corallo D., Aveic S. Exploring cancer cell behavior in vitro in three-dimensional multicellular bioprintable collagen-based hydrogels. Cancers. 2019;11 doi: 10.3390/cancers11020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen Z., Zhang H., Fan C., Zhuang Y., Yang W., Chen Y., Shen H., Xiao Z., Zhao Y., Li X., Dai J. Adhesive, stretchable, and spatiotemporal delivery fibrous hydrogels harness endogenous neural stem/progenitor cells for spinal cord injury repair. ACS Nano. 2022;16:1986–1998. doi: 10.1021/acsnano.1c06892. [DOI] [PubMed] [Google Scholar]
- 177.Zhong J., Chan A., Morad L., Kornblum H.I., Fan G., Carmichael S.T. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabilitation Neural Repair. 2010;24:636–644. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu H., Cao B., Feng M., Zhou Q., Sun X., Wu S., Jin S., Liu H., Lianhong J. Combinated transplantation of neural stem cells and collagen type I promote functional recovery after cerebral ischemia in rats. Anat. Rec.: Advan. Integrat. Anatomy Evolution. Biology. 2010;293:911–917. doi: 10.1002/ar.20941. [DOI] [PubMed] [Google Scholar]
- 179.Schnell E., Klinkhammer K., Balzer S., Brook G., Klee D., Dalton P., Mey J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28:3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 180.Gerardo-Nava J., Führmann T., Klinkhammer K., Seiler N., Mey J., Klee D., Möller M., Dalton P.D., Brook G.A. Human neural cell interactions with orientated electrospun nanofibers in vitro. Nanomedicine. 2009;4:11–30. doi: 10.2217/17435889.4.1.11. [DOI] [PubMed] [Google Scholar]
- 181.Muangsanit P., Roberton V., Costa E., Phillips J.B. Engineered aligned endothelial cell structures in tethered collagen hydrogels promote peripheral nerve regeneration. Acta Biomater. 2021;126:224–237. doi: 10.1016/j.actbio.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 182.Huang K.-F., Hsu W.-C., Chiu W.-T., Wang J.-Y. Functional improvement and neurogenesis after collagen-GAG matrix implantation into surgical brain trauma. Biomaterials. 2012;33:2067–2075. doi: 10.1016/j.biomaterials.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 183.Han Q., Jin W., Xiao Z., Ni H., Wang J., Kong J., Wu J., Liang W., Chen L., Zhao Y., Chen B., Dai J. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials. 2010;31:9212–9220. doi: 10.1016/j.biomaterials.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 184.Ma F., Xu F., Li R., Zheng Y., Wang F., wei N., zhong J., Tang Q., Zhu T., Wang Z., Zhu J. Sustained delivery of glial cell-derived neurotrophic factors in collagen conduits for facial nerve regeneration. Acta Biomater. 2018;69:146–155. doi: 10.1016/j.actbio.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 185.Chen W., Qi J., Feng F., Bao G., Wang T., Xiang M., Xie W.-f. Neuroprotective effect of allicin against traumatic brain injury via Akt/endothelial nitric oxide synthase pathway-mediated anti-inflammatory and anti-oxidative activities. Neurochem. Int. 2014;68:28–37. doi: 10.1016/j.neuint.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 186.Maclean F.L., Horne M.K., Williams R.J., Nisbet D.R. Biomaterial systems to resolve brain inflammation after traumatic injury. APL Bioeng. 2018;2 doi: 10.1063/1.5023709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aurand E.R., Lampe K.J., Bjugstad K.B. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci. Res. 2012;72:199–213. doi: 10.1016/j.neures.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pakulska M.M., Ballios B.G., Shoichet M.S. Injectable hydrogels for central nervous system therapy. Biomed. Mater. 2012;7 doi: 10.1088/1748-6041/7/2/024101. [DOI] [PubMed] [Google Scholar]
- 189.Hubert T., Grimal S., Carroll P., Fichard-Carroll A. Collagens in the developing and diseased nervous system. Cell. Mol. Life Sci. 2009;66:1223–1238. doi: 10.1007/s00018-008-8561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Khaing Z.Z., Thomas R.C., Geissler S.A., Schmidt C.E. Advanced biomaterials for repairing the nervous system: what can hydrogels do for the brain? Mater. Today. 2014;17:332–340. [Google Scholar]
- 191.Moxon S.R., Corbett N.J., Fisher K., Potjewyd G., Domingos M., Hooper N.M. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mater. Sci. Eng. C. 2019;104 doi: 10.1016/j.msec.2019.109904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yan F., Li M., Zhang H.-Q., Li G.-L., Hua Y., Shen Y., Ji X.-M., Wu C.-J., An H., Ren M. Collagen-chitosan scaffold impregnated with bone marrow mesenchymal stem cells for treatment of traumatic brain injury. Neural Regen. Res. 2019;14:1780. doi: 10.4103/1673-5374.257533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Duan H., Li X., Wang C., Hao P., Song W., Li M., Zhao W., Gao Y., Yang Z. Functional hyaluronate collagen scaffolds induce NSCs differentiation into functional neurons in repairing the traumatic brain injury. Acta Biomater. 2016;45:182–195. doi: 10.1016/j.actbio.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 194.Krishnan U.M. Biomaterials in the treatment of Parkinson's disease. Neurochem. Int. 2021;145 doi: 10.1016/j.neuint.2021.105003. [DOI] [PubMed] [Google Scholar]
- 195.Moriarty N., Pandit A., Dowd E. Encapsulation of primary dopaminergic neurons in a GDNF-loaded collagen hydrogel increases their survival, re-innervation and function after intra-striatal transplantation. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-15970-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Š. Kubinová, Soft and Rigid Scaffolds for Spinal Cord Injury Regeneration, Spinal Cord Injury (SCI) Repair Strategies, Elsevier2020, pp. 105-127.
- 197.Varma A.K., Das A., Wallace G., Barry J., Vertegel A.A., Ray S.K., Banik N.L. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem. Res. 2013;38:895–905. doi: 10.1007/s11064-013-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Führmann T., Anandakumaran P.N., Shoichet M.S. Combinatorial therapies after spinal cord injury: how can biomaterials help? Adv. Healthc. Mater. 2017;6 doi: 10.1002/adhm.201601130. [DOI] [PubMed] [Google Scholar]
- 199.Liu S., Schackel T., Weidner N., Puttagunta R. Biomaterial-supported cell transplantation treatments for spinal cord injury: challenges and perspectives. Front. Cell. Neurosci. 2018;11:430. doi: 10.3389/fncel.2017.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ma F., Xiao Z., Chen B., Hou X., Han J., Zhao Y., Dai J., Xu R. Accelerating proliferation of neural stem/progenitor cells in collagen sponges immobilized with engineered basic fibroblast growth factor for nervous system tissue engineering. Biomacromolecules. 2014;15:1062–1068. doi: 10.1021/bm500062n. [DOI] [PubMed] [Google Scholar]
- 201.Nanofibrous collagen nerve conduits for spinal cord repair, tissue. Eng. Part A. 2012;18:1057–1066. doi: 10.1089/ten.tea.2011.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu X.-Y., Liang J., Wang Y., Zhong L., Zhao C.-Y., Wei M.-G., Wang J.-J., Sun X.-Z., Wang K.-Q., Duan J.-H. Diffusion tensor imaging predicting neurological repair of spinal cord injury with transplanting collagen/chitosan scaffold binding bFGF. J. Mater. Sci. Mater. Med. 2019;30:1–17. doi: 10.1007/s10856-019-6322-y. [DOI] [PubMed] [Google Scholar]
- 203.Li X., Han J., Zhao Y., Ding W., Wei J., Li J., Han S., Shang X., Wang B., Chen B. Functionalized collagen scaffold implantation and cAMP administration collectively facilitate spinal cord regeneration. Acta Biomater. 2016;30:233–245. doi: 10.1016/j.actbio.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 204.Han Q., Sun W., Lin H., Zhao W., Gao Y., Zhao Y., Chen B., Xiao Z., Hu W., Li Y. Linear ordered collagen scaffolds loaded with collagen-binding brain-derived neurotrophic factor improve the recovery of spinal cord injury in rats. Tissue Eng. 2009;15:2927–2935. doi: 10.1089/ten.TEA.2008.0506. [DOI] [PubMed] [Google Scholar]
- 205.Cholas R., Hsu H.-P., Spector M. Collagen scaffolds incorporating select therapeutic agents to facilitate a reparative response in a standardized hemiresection defect in the rat spinal cord. Tissue Eng. 2012;18:2158–2172. doi: 10.1089/ten.TEA.2011.0577. [DOI] [PubMed] [Google Scholar]
- 206.Fan C., Li X., Xiao Z., Zhao Y., Liang H., Wang B., Han S., Li X., Xu B., Wang N. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017;51:304–316. doi: 10.1016/j.actbio.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 207.Fan C., Li X., Zhao Y., Xiao Z., Xue W., Sun J., Li X., Zhuang Y., Chen Y., Dai J. Cetuximab and Taxol co-modified collagen scaffolds show combination effects for the repair of acute spinal cord injury. Biomater. Sci. 2018;6:1723–1734. doi: 10.1039/c8bm00363g. [DOI] [PubMed] [Google Scholar]
- 208.Li X., Fan C., Xiao Z., Zhao Y., Zhang H., Sun J., Zhuang Y., Wu X., Shi J., Chen Y. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/β-catenin signaling for spinal cord injury repair. Biomaterials. 2018;183:114–127. doi: 10.1016/j.biomaterials.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 209.Zou Y., Ma D., Shen H., Zhao Y., Xu B., Fan Y., Sun Z., Chen B., Xue W., Shi Y. Aligned collagen scaffold combination with human spinal cord-derived neural stem cells to improve spinal cord injury repair. Biomater. Sci. 2020;8:5145–5156. doi: 10.1039/d0bm00431f. [DOI] [PubMed] [Google Scholar]
- 210.Liu W., Xu B., Zhao S., Han S., Quan R., Liu W., Ji C., Chen B., Xiao Z., Yin M., Yin Y., Dai J., Zhao Y. Spinal cord tissue engineering via covalent interaction between biomaterials and cells. Sci. Adv. 2023;9 doi: 10.1126/sciadv.ade8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xu B., Liu D., Liu W., Long G., Liu W., Wu Y., He X., Shen Y., Jiang P., Yin M., Fan Y., Shen H., Shi L., Zhang Q., Xue W., Jin C., Chen Z., Chen B., Li J., Hu Y., Li X., Xiao Z., Zhao Y., Dai J. Engineered human spinal cord-like tissues with dorsal and ventral neuronal progenitors for spinal cord injury repair in rats and monkeys. Bioact. Mater. 2023;27:125–137. doi: 10.1016/j.bioactmat.2023.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jin C., Wu Y., Zhang H., Xu B., Liu W., Ji C., Li P., Chen Z., Chen B., Li J., Wu X., Jiang P., Hu Y., Xiao Z., Zhao Y., Dai J. Spinal cord tissue engineering using human primary neural progenitor cells and astrocytes. Bioeng. Transl. Med. 2023;8 doi: 10.1002/btm2.10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Habre S.B., Bond G., Jing X.L., Kostopoulos E., Wallace R.D., Konofaos P. The surgical management of nerve gaps: present and future. Ann. Plast. Surg. 2018;80:252–261. doi: 10.1097/SAP.0000000000001252. [DOI] [PubMed] [Google Scholar]
- 214.Siironen J., Sandberg M., Vuorinen V., Röyttä M. Expression of type I and III collagens and fibronectin after transection of rat sciatic nerve. Reinnervation compared with denervation, Laboratory Investigation. J. Techn. Method. Pathol. 1992;67:80–87. [PubMed] [Google Scholar]
- 215.Koopmans G., Hasse B., Sinis N. The role of collagen in peripheral nerve repair. Int. Rev. Neurobiol. 2009;87:363–379. doi: 10.1016/S0074-7742(09)87019-0. [DOI] [PubMed] [Google Scholar]
- 216.Chen P., Cescon M., Megighian A., Ronaldo P. Collagen VI regulates peripheral nerve myelination and function. Faseb. J. 2014;28:1145–1156. doi: 10.1096/fj.13-239533. [DOI] [PubMed] [Google Scholar]
- 217.Chernousov M.A., Stahl R.C., Carey D.J. Schwann cell type V collagen inhibits axonal outgrowth and promotes Schwann cell migration via distinct adhesive activities of the collagen and noncollagen domains. J. Neurosci. 2001;21:6125–6135. doi: 10.1523/JNEUROSCI.21-16-06125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Erdman R., Stahl R.C., Rothblum K., Chernousov M.A., Carey D.J. Schwann cell adhesion to a novel heparan sulfate binding site in the N-terminal domain of α4 type V collagen is mediated by syndecan-3. J. Biol. Chem. 2002;277:7619–7625. doi: 10.1074/jbc.M111311200. [DOI] [PubMed] [Google Scholar]
- 219.Lv D., Zhou L., Zheng X., Hu Y. Sustained release of collagen VI potentiates sciatic nerve regeneration by modulating macrophage phenotype. Eur. J. Neurosci. 2017;45:1258–1267. doi: 10.1111/ejn.13558. [DOI] [PubMed] [Google Scholar]
- 220.Muangsanit P., Day A., Dimiou S., Ataç A.F., Kayal C., Park H., Nazhat S.N., Phillips J.B. Rapidly formed stable and aligned dense collagen gels seeded with Schwann cells support peripheral nerve regeneration. J. Neural. Eng. 2020;17 doi: 10.1088/1741-2552/abaa9c. [DOI] [PubMed] [Google Scholar]
- 221.Cui Y., Lu C., Meng D., Xiao Z., Hou X., Ding W., Kou D., Yao Y., Chen B., Zhang Z. Collagen scaffolds modified with CNTF and bFGF promote facial nerve regeneration in minipigs. Biomaterials. 2014;35:7819–7827. doi: 10.1016/j.biomaterials.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 222.Ma F., Xiao Z., Chen B., Hou X., Dai J., Xu R. Linear ordered collagen scaffolds loaded with collagen-binding basic fibroblast growth factor facilitate recovery of sciatic nerve injury in rats. Tissue Eng. 2014;20:1253–1262. doi: 10.1089/ten.tea.2013.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cerri F., Salvatore L., Memon D., Boneschi F.M., Madaghiele M., Brambilla P., Del Carro U., Taveggia C., Riva N., Trimarco A. Peripheral nerve morphogenesis induced by scaffold micropatterning. Biomaterials. 2014;35:4035–4045. doi: 10.1016/j.biomaterials.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Duarte Campos D.F., Bonnin Marquez A., O'Seanain C., Fischer H., Blaeser A., Vogt M., Corallo D., Aveic S. Exploring cancer cell behavior in vitro in three-dimensional multicellular bioprintable collagen-based hydrogels. Cancers. 2019;11:180. doi: 10.3390/cancers11020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Curtin C., Nolan J.C., Conlon R., Deneweth L., Gallagher C., Tan Y.J., Cavanagh B.L., Asraf A.Z., Harvey H., Miller-Delaney S., Shohet J., Bray I., O'Brien F.J., Stallings R.L., Piskareva O. A physiologically relevant 3D collagen-based scaffold–neuroblastoma cell system exhibits chemosensitivity similar to orthotopic xenograft models. Acta Biomater. 2018;70:84–97. doi: 10.1016/j.actbio.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 226.Liu M., Zhang X., Long C., Xu H., Cheng X., Chang J., Zhang C., Zhang C., Wang X. Collagen-based three-dimensional culture microenvironment promotes epithelial to mesenchymal transition and drug resistance of human ovarian cancer in vitro. RSC Adv. 2018;8:8910–8919. doi: 10.1039/c7ra13742g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Anguiano M., Castilla C., Maška M., Ederra C., Peláez R., Morales X., Munoz-Arrieta G., Mujika M., Kozubek M., Munoz-Barrutia A. Characterization of three-dimensional cancer cell migration in mixed collagen-Matrigel scaffolds using microfluidics and image analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Murakami S., Mukaisho K.-i., Iwasa T., Kawabe M., Yoshida S., Taniura N., Nakayama T., Noi M., Yamamoto G., Sugihara H. Application of “tissueoid cell culture system” using a silicate fiber scaffold for cancer research. Pathobiology. 2020;87:291–301. doi: 10.1159/000509133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Guo J., Zhao C., Yao R., Sui A., Sun L., Liu X., Wu S., Su Z., Li T., Liu S. 3D culture enhances chemoresistance of ALL Jurkat cell line by increasing DDR1 expression. Exp. Ther. Med. 2019;17:1593–1600. doi: 10.3892/etm.2019.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ding S.-L., Liu X., Zhao X.-Y., Wang K.-T., Xiong W., Gao Z.-L., Sun C.-Y., Jia M.-X., Li C., Gu Q., Zhang M.-Z. Microcarriers in application for cartilage tissue engineering: recent progress and challenges. Bioact. Mater. 2022;17:81–108. doi: 10.1016/j.bioactmat.2022.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tornín J., Villasante A., Solé-Martí X., Ginebra M.-P., Canal C. Osteosarcoma tissue-engineered model challenges oxidative stress therapy revealing promoted cancer stem cell properties. Free Radic. Biol. Med. 2021;164:107–118. doi: 10.1016/j.freeradbiomed.2020.12.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Unnikrishnan K., Thomas L.V., Ram Kumar R.M. Advancement of scaffold-based 3D cellular models in cancer tissue engineering: an update. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.733652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Li X., Fan C., Xiao Z., Zhao Y., Zhang H., Sun J., Zhuang Y., Wu X., Shi J., Chen Y., Dai J. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/β-catenin signaling for spinal cord injury repair. Biomaterials. 2018;183:114–127. doi: 10.1016/j.biomaterials.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 234.Carriel V., Garzón I., Alaminos M., Cornelissen M. Histological assessment in peripheral nerve tissue engineering. Neural Regen Res. 2014;9:1657–1660. doi: 10.4103/1673-5374.141798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kato-Negishi M., Tsuda Y., Onoe H., Takeuchi S. A neurospheroid network-stamping method for neural transplantation to the brain. Biomaterials. 2010;31:8939–8945. doi: 10.1016/j.biomaterials.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 236.Kato-Negishi M., Morimoto Y., Onoe H. Millimeter-sized neural building blocks for 3D heterogeneous neural network assembly. Adv Healthc Mater. 2013;2:1564–1570. doi: 10.1002/adhm.201300052. [DOI] [PubMed] [Google Scholar]
- 237.Zhang Q., Nguyen P.D., Shi S., Burrell J.C., Cullen D.K., Le A.D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018;8:6634. doi: 10.1038/s41598-018-24888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Choi Y.J., Park J., Lee S.-H. Size-controllable networked neurospheres as a 3D neuronal tissue model for Alzheimer's disease studies. Biomaterials. 2013;34:2938–2946. doi: 10.1016/j.biomaterials.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 239.Zhuang P., Sun A.X., An J., Chua C.K., Chew S.Y. 3D neural tissue models: from spheroids to bioprinting. Biomaterials. 2018;154:113–133. doi: 10.1016/j.biomaterials.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 240.Hazards E.P.o.B., Koutsoumanis K., Allende A., Bolton D.J., Bover-Cid S., Chemaly M., Davies R., De Cesare A., Herman L.M., Hilbert F. Potential BSE risk posed by the use of ruminant collagen and gelatine in feed for non-ruminant farmed animals. EFSA J. 2020;18 doi: 10.2903/j.efsa.2020.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kim Y., Nowzari H., Rich S.K. Risk of prion disease transmission through bovine-derived bone substitutes: a systematic review. Clin. Implant Dent. Relat. Res. 2013;15:645–653. doi: 10.1111/j.1708-8208.2011.00407.x. [DOI] [PubMed] [Google Scholar]
- 242.Wang H. A review of the effects of collagen treatment in clinical studies. Polymers. 2021;13 doi: 10.3390/polym13223868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sakaguchi M., Toda M., Ebihara T., Irie S., Hori H., Imai A., Yanagida M., Miyazawa H., Ohsuna H., Ikezawa Z. IgE antibody to fish gelatin (type I collagen) in patients with fish allergy. J. Allergy Clin. Immunol. 2000;106:579–584. doi: 10.1067/mai.2000.108499. [DOI] [PubMed] [Google Scholar]
- 244.Xu S., Xu H., Wang W., Li S., Li H., Li T., Zhang W., Yu X., Liu L. The role of collagen in cancer: from bench to bedside. J. Transl. Med. 2019;17:309. doi: 10.1186/s12967-019-2058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Boni R., Ali A., Shavandi A., Clarkson A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018;25:90. doi: 10.1186/s12929-018-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xiao Z., Tang F., Zhao Y., Han G., Yin N., Li X., Chen B., Han S., Jiang X., Yun C., Zhao C., Cheng S., Zhang S., Dai J. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with NeuroRegen scaffolds and mesenchymal stem cells. Cell Transplant. 2018;27:907–915. doi: 10.1177/0963689718766279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhao Y., Tang F., Xiao Z., Han G., Wang N., Yin N., Chen B., Jiang X., Yun C., Han W., Zhao C., Cheng S., Zhang S., Dai J. Clinical study of NeuroRegen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017;26:891–900. doi: 10.3727/096368917X695038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xiao Z., Tang F., Tang J., Yang H., Zhao Y., Chen B., Han S., Wang N., Li X., Cheng S., Han G., Zhao C., Yang X., Chen Y., Shi Q., Hou S., Zhang S., Dai J. One-year clinical study of NeuroRegen scaffold implantation following scar resection in complete chronic spinal cord injury patients. Sci. China Life Sci. 2016;59:647–655. doi: 10.1007/s11427-016-5080-z. [DOI] [PubMed] [Google Scholar]
- 249.Li J., Han S., Xiao Z., Dai J. Clinical studies on neural regeneration in traumatic spinal cord injury. Sci. Sin. Vitae. 2019;49:673–682. [Google Scholar]
- 250.Bozkurt A., Claeys K.G., Schrading S., Rödler J.V., Altinova H., Schulz J.B., Weis J., Pallua N., van Neerven S.G.A. Clinical and biometrical 12-month follow-up in patients after reconstruction of the sural nerve biopsy defect by the collagen-based nerve guide Neuromaix. Eur. J. Med. Res. 2017;22:34. doi: 10.1186/s40001-017-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lindsay R.W., Robinson M., Hadlock T.A. Comprehensive facial rehabilitation improves function in people with facial paralysis: a 5-year experience at the Massachusetts Eye and Ear Infirmary. Phys. Ther. 2010;90:391–397. doi: 10.2522/ptj.20090176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.